Abstract
Rheumatoid arthritis (RA) is the most common inflammatory arthropathy. The majority of evidence, derived from genetics, tissue analyses, models and clinical studies, points to an immune mediated etiology associated with stromal tissue dysregulation that together propogate chronic inflammation and articular destruction. A pre-RA phase lasting months to years, may be characterized by the presence of circulating autoantibodies, increasing concentration and range of inflammatory cytokines and chemokines and altered metabolism. Clinical disease onset comprises synovitis and systemic comorbidities affecting the vasculature, metabolism and bone. Targeted immune therapeutics, and aggressive treatment strategies have substantially improved clinical outcomes, and informed pathogenetic understanding, but no cure as yet exists. Herein we review recent data that support intriguing models of disease pathogenesis. They allude to the possibility of restoration of immunologic homeostasis and thus a state of tolerance associated with drug free remission. This target represents a bold vision for the future of RA therapeutics.
Immune dysregulation was first implicated in the pathogenesis of rheumatoid arthritis (RA) by the discovery of anti-immunoglobulin G (IgG) antibodies known as rheumatoid factors, initially by Erik Waaler and then more fully described by H. M. Rose in the 1940s. However, concepts of how immune responses contribute to disease have evolved dramatically over the last 50 years. Autoreactivity as a pivotal step dominates the conceptual landscape, although other mechanisms, both immunologic and tissue derived clearly contribute to disease pathogenesis. Thus, RA is characterized by evidence of disordered innate immunity, including immune complex-mediated complement activation, adaptive immune responses against ‘self’-antigens comprising predominantly post-translationally modified proteins, dysregulated cytokine networks, osteoclast and chondrocyte activation and imprinting of resident stromal cells that in turn develop semi-autonomous features that support disease progression (Arend & Firestein 2012; Firestein 2003).
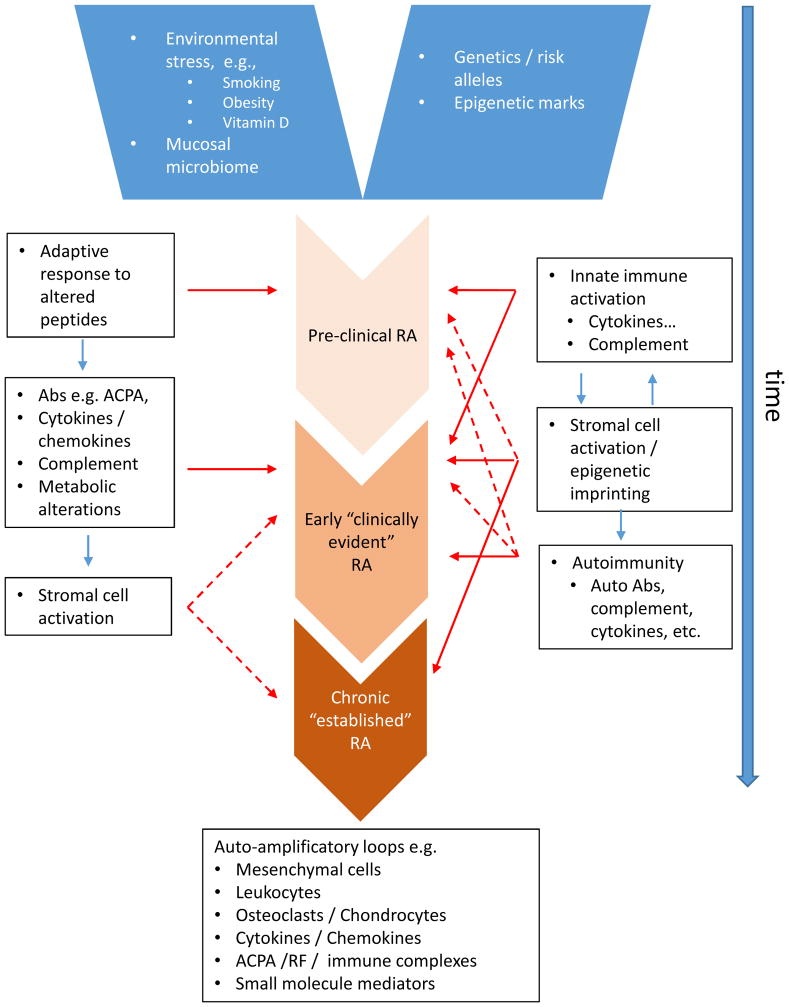
Based on substantial new data identifying the immunologic and metabolic events that precede onset of clinical disease, even by years (i.e., “pre-RA”), and the increasing impact of the application of the advanced molecular ‘omics’ revolution to disease investigation, an integrated hypothesis for disease pathology is emerging (Catrina et al 2016; Tan & Smolen 2016). In this review, we will posit that RA starts with a high-risk genetic background that, in combination with epigenomic marks that contribute to heritability and disease chronicity, and stochastic environmental exposures that create neo-epitopes, launches a cascade of events inducing synovitis and ultimately chronic destructive arthritis (Figure 1). Based on the diversity of clinical responses to highly targeted therapeutic agents, we propose that RA probably does not comprise a single entity. Rather, it is perhaps more appropriately considered a syndrome with a common clinical phenotype arising from diverse pathways operating variably, albeit often with overlap, in individual patients (Firestein, 2014).
Figure 1.
Depicting the sequence of events leading to the development of clinically detectable rheumatoid arthritis – at least two potential models are depicted. In model A, a pre-RA phase comprises early generation of autoantibodies (ACPAs) that can bind post-translationally modified self-proteins, particularly via citrullination. This is followed by amplification of the range of specificities of ACPA and by the elaboration of cytokines and chemokines, complement, and metabolic disturbance in the months prior to clinical development of disease. A transition event that requires a ‘second hit’, as yet poorly understood permits the development of synovitis. The latter is characterized by frank inflammation, stromal compartment changes and tissue modification leading to articular damage. In model B, which is not mutually exclusive, there is an early interaction between innate immune activation and stromal factors that lead to stromal cell alteration, including epigenetic modifications that initiate a cycle of inflammatory stromal mediated damage. Autoimmunity can arise as a result of these interactions that in turn can conrtibtue directly or in an amplification loop to disease perpetuation.
Genetics of Rheumatoid Arthritis
Genetic factors clearly play a role in RA risk, severity and progression. Monozygotic twins share RA on about 12–15% of occasions compared to 1% for the general population and around 2–5% for fraternal twins or other first-degree relatives. This relatively low concordance implicates many other factors, including those in the environment and the microbiome in pathogenesis. Note also that gene sequences are not the sole determinant of heritability, and epigenetic marks likely also contribute, especially for monozygotic twins (Kaminsky et al 2009).
The most important genetic risk allele for RA resides in the class II major histocompatibility (MHC) locus accounting for about 40% of the genetic influence. The odds ratio of developing RA in individuals with MHC class II HLA-DR4 alleles is about 5:1. This link between HLA-DR and RA was initially described in the 1970s with the observation that HLA-DR4 is present in 70% of RA patients, compared with about 30% of controls. A so called shared “susceptibility epitope” (SE) was identified in amino acids 70 through 74 in the third hypervariable region of the DRβ chain. The sequence associated with disease is generally glutamine-leucine-arginine-alanine-alanine (QKRAA), which is present in some DR4 and DR14, in addition to DR1β chains. The SE with the closest links to RA include DRB*0401, DRB*0404, DRB*0101, and DRB*1402. Over 90% of patients with RA express at least one of these variants (Weyand et al 1992). The SE is also associated with increased disease severity, such as extra-articular manifestations and progression of erosions.
The SE region predominantly faces away from the antigen-binding groove that binds processed peptides for presentation to T cells, which has raised some questions about their precise contributory role. RA-specific peptides that bind to QKRAA-containing molecules have been difficult to identify (Kirschmann et al 1995). This observation led to the notion that SE might also partially contribute by shaping the T cell repertoire in the thymus, altering intracellular HLA-DR trafficking and antigen loading, or serving as an autoantigen.
More recently, linkage disequilibrium and informatics-based studies to understand the role of the SE identified additional amino acids located in the base of the antigen binding groove as a likely explanation for antigen selection, especially leucine or valine variants at amino acid 11 (Raychaudhuri et al 2012). Serine at amino acid 11 confers decreased risk for RA. The risk alleles, especially deep within the cleft, could directly increase risk via presentation of arthritogenic antigens, such as citrullinated proteins. For example, citrullinated vimentin peptides bind much more avidly to the RA HLA-DR than does native protein; in contrast, other MHCs do not exhibit this differential binding (Hill et al 2003; Law et al 2012). Subsequent crystal studies have characterized in detail the nature of the citrullinated peptide binding to HLADRB1 (Scally S et al 2013). RA-associated alleles present citrullinated peptides efficiently to T cells, which, in turn, produce higher amounts of cytokines IL-17 and IFNγ than to native peptide. Adaptive immune responses to citrullinated peptides are also characterized by the presence of “anti-citrullinated peptide antibodies” (ACPAs), observed in 80–90% of RA patients.
Together these data support the notion that HLA-DR risk for RA is based, at least in part on the increased efficiency of antigen presentation for altered peptides rather than native proteins (van der Helm et al 2006). Citrullination of peptides in the presence of environmental stress is ubiquitous in mammalian cells and is not a unique feature of RA. Instead, production of antibodies recognizing citrullinated peptides differentiates individuals at risk. The emergence of numerous other post-translationally modified protein targets, e.g. via carbamylation or acetylation, recognized by autoantibodies in RA is consistent with the notion of altered presentation of post-translationally modified peptides; other families of altered peptides could be implicated in discrete subsets of patients.
Overall, genome wide association studies (GWAS) studies and meta-genomic analyses have identified over 100 single nucleotide polymorphisms (SNPs) and genes associated with ACPA+ RA beyond the HLA. RA-associated SNPs tend to cluster around immune genes (Okada et al 2014). The genetic architecture of RA has recently been extensively reviewed (Kim et al 2017). Briefly, individual SNPs usually provide modest contribution to risk with odds ratios typically in the 1.05 to 1.2-fold range, though this need not infer low functional impact. Combinations of these genes can potentially interact to increase risk. For example, a relatively rare combination of HLA-DR, PTPN22, and TRAF1-C5 SNPs increases risk over 40-fold (Firestein & Zvaifler 2002). While there are undoubtedly additional combinations or rare variants with high penetrance, the contribution of newly identified genes to overall risk is growing increasingly small. Notably, meta-analyses of patients with ACPA- RA show some differences from ACPA+ disease, consistent with the idea that ‘seronegative RA’ should be considered a distinct clinical pathologic entity.
Intriguing individual non-MHC linkages are associated with peptidyl arginase deiminase (PADI) and PTPN22. PADI gene products are enzymes that convert arginine to citrulline, creating new potential antigens that bind to RA-associated MHC proteins. An extended PADI4 haplotype prolongs its mRNA stability and could, therefore, increase peptide citrullination (Suzuki et al 2003). Up to a 2-fold increase in risk of RA was observed with PADI4 SNPs in Asian and North American cohorts. The PTPN22 allele with an amino acid substitution (R620W) doubles the risk of developing ACPA+, but not ACPA- RA. This SNP is also associated with many other immune mediated diseases, including systemic lupus erythematosus (SLE) and type 1 diabetes (Rawlings et al 2015). The R620W allele is a gain of function change mutation in the PTPN22 protein, a phosphatase that regulates amongst other signal proteins, Lck and ZAP70. Thus, modified T cell receptor signaling could alter either the T cell repertoire in the thymus or immune responses, or tolerance in the periphery. The R620W allele however also positively regulates TLR induced type 1 IFN expression in myeloid cells via reduced efficiency of PTPN22W mediated TRAF3 ubiquitination and thus could amplify innate cytokine expression to promote disease (Wang et al 2013).
A number of additional genes involved with adaptive immunity have RA-associated SNPs in a various populations (Yamamoto et al 2015). In addition to PTPN22, the co-stimulation receptors, CTLA and CD28, have emerged from GWAS studies. Other genes associated with B cell function and/or antigen presentation such as BTLA (B- and T-lymphocyte attenuator), Fc receptors, and CD40 have been identified. Signal transduction genes and pathways that regulate immune function such as TRAF1-C5 and STAT4, cell migration (ELMO1) and fetal development (LBH) are also identified. Finally, multiple gene polymorphisms have been identified in cytokine or cytokine receptor coding loci, including TNF. This is especially relevant in light of the pivotal role of cytokines in synovitis described below and the consequent efficacy of targeted anti-cytokine, and cytokine receptor signal pathway therapeutics. In some cases, the causative mutations can alter expression or function. For instance, RA associated TNF promoter polymorphisms at positions -238 and -308 can potentially modulate TNF gene expression (Fonseca et al 2007). An IL-6 receptor polymorphism that is functionally implicated in the intensity of IL-6 signaling is also associated with RA (Ferriera et al 2013). Thus, the genetic clues to RA pathogenesis implicate inflammation, adaptive and innate immunity at the core of pathogenesis.
Interactions between genes and environment: RA as a ‘mucosal disease’?
RA is properly considered an immune-mediated disease with a strong genetic influence. However, its origins may involve the interface between external influences and the immune system, manifest especially at mucosal surfaces. Three sites have been associated particularly with RA namely, 1) the lungs, 2) the oral mucosa and 3) the gastrointestinal tract. Whereas precise mechanisms that enhance risk are not fully understood for each, it is likely that local tissue stress leads to post-translational modification of peptides with subsequent antibody formation serving as a common mechanism.
Epidemiologic/genetic studies, particularly those arising from impressive Swedish registries have highlighted the role of cigarette smoking as a prominent environmental risk factor for RA, pointing to a role for pulmonary mucosal biology in disease aetiology. The amount and duration of smoking are important, with the highest risk observed with greater than 20 pack-years of exposure. Risk declines slowly back to normal within 10 years of smoking cessation (Kallberg et al 2011), suggesting that effects are not solely toxin dependent but may also arise by altering immunologic function. Notably, other pulmonary exposures can replace smoking in increasing risk e.g., silica or textile dust. Nevertheless, mechanisms underlying the relationship with smoking are uncertain (Catrina AI et al 2014). Exposure to inhaled toxic chemicals in cigarette smoke can potentially increase PADI expression in the airway and increase protein citrullination (Vassalo et al 2014). Smoking related risk interacts in a synergistic manner with MHC risk alleles. The QKRAA-containing HLA chains alone modestly increase the likelihood of developing RA 4- to 6-fold, but this risk increases up to 20- to 40-fold when combined with smoking (Lundstrom et al 2009).
Thus in individuals carrying risk HLA alleles, such peptides could be more efficiently presented and ACPAs generated via subsequent T-B cell help. Alternatively, ‘innate’ B cell subsets could initiate responses to modified self-peptides, and thereafter present peptide to T cells. In this model, production of “autoantibodies” could result from “auto-reactive” T cell clones that are available to respond to modified peptides because they were not deleted during development since the altered proteins/peptides were not present in the thymus (Linn-Rasker et al 2006). A corollary to this would be that a major immunologic regulatory deficit in RA patients is a failure to regulate T cell recognition of post-translationally modified self-proteins across a range of specificities – as observed above, this is compatible with the expression of a relatively broad autoantibody spectrum in clinical disease.
Broncho-alveolar immunohistology shows high levels of citrullinated proteins in pulmonary macrophages and smoking increases such citrullinated proteins in broncho-alveolar lavage fluid (Makrygiannakis et al 2008). However citrullinated proteins can also be present in context of other diseases or even in clinically normal lungs (see Catrina AI et al 2014). Thus, risk from smoking might be more complex than via PADI induction, and may include promotion of epigenetic alterations notably DNA methylation with inflammatory potential (Tsaprouni et al 2014). Perhaps the location of citrullination and the stochastic combination of altered peptides, innate leukocyte subsets and mucosal inflammation conspire a permissive microenvironment for the creation of ACPAs. High-resolution CT studies demonstrate associations between ACPA formation and bronchial thickening in the pre-RA stages and support the idea that inflammatory responses are required (Reynisdottir et al 2014). More recent studies have shown signs of local inflammation in bronchial tissues of untreated RA patients (Reynisdottir et al 2016). Using B cell barcoding techniques, the frequency of circulating IgA plasmablasts was found to be elevated in at risk subjects without RA who are ACPA positive providing further evidence for a mucosal origin for at least a proportion of the autoimmunity detected in RA (Kinslow J et al 2016).
The relationship between the microbiome and RA has been suspected for many years and supported by pre-clinical studies. For example, arthritis susceptibility and severity in a variety of rodent strains is decreased when maintained germ-free or in environments with a restricted bacterial flora (Lui et al 2016). These data suggest that bacteria provide complex adjuvant functions that enhance autoimmunity, either directly (e.g., bacterial cell walls or lipopolysaccharides) or by critically altering the immunoregulatory mucosal environment. The latter could support particularly the generation of type 17 responses implicated in several animal models of RA (Miossec & Kolls 2012).
In humans, inflammation of the oral mucosa, particularly periodontitis, is associated with increased susceptibility to RA. The most common bacteria implicated in periodontitis is P. gingivalis, since it can express PADI. P. gingivalis could potentially citrullinate peptides in the oral mucosa that, in the context of inflammation, could promote ACPA generation (Wegner et al 2010). While this concept is attractive, a clear association between P. gingivalis compared other bacteria is not consistently observed in unbiased microbiome studies. More recent data suggest that P. gingivalis or other bacteria could act, instead, by ligating Toll-like receptor 2 (TLR2) (de Aquino et al 2014). This process could increase IL-1 production and stimulate local Th17 cell differentiation. An intriguing recent study identified that A. actinomycetemcomitans initiates hypercitrullination through the effect of a toxin, leukotoxin A (LtxA), mediated on neutrophils and was detected in RA patients’ oral microbiome wherein it could act as a bacterial trigger to disease (Konig et al 2016).
In the colon, Prevotella copri species were enriched in an unbiased analysis of the gastrointestinal microbiome in early RA patients. Bacteroides species were decreased in the same patients (Scher et al 2013). While not fully understood, the over-representation of Prevotella was not observed in chronic RA or in other forms of arthritis. Subsequent studies in the Chinese population have extended these observations to include novel microbial species, and demonstrated common expression in oral and GI mucosal sites in individuals that, remarkably, was correlated with ACPA levels and CRP. Moreover further alterations in the microbiome occurred upon therapeutic immune modification (Zhang et al 2015).
Epigenetics: Integrating environmental stress and the genome
Epigenetic modifications decorate genomic DNA and contribute to regulation of gene expression. The role of these marks had been widely viewed as key determinants of orderly gene expression during development as well as lineage-dependent RNA transcription throughout life. Their contribution to pathogenesis is now more clearly understood in many diseases, especially cancer. A variety of epigenetic mechanisms have been implicated in RA, including DNA methylation, histone modification, and microRNA expression. Because the science of epigenetics in autoimmunity is still relatively young, it is uncertain whether some or all of these disease-specific alterations are inherited, develop during life due to stochastic exposures, cause disease or are actually caused by disease. Regardless, stable epigenetic marks have been identified in RA that alter cell function and permanently imprint some lineages, most notably synovial fibroblast-like synoviocytes (FLS).
Most information on epigenetics in RA comes from studies of RA joint samples, including synovial tissue and FLS. Candidate gene approaches showed that the IL6, CXCL12, and EFNB1 promoters are hypomethylated in rheumatoid FLS while others, like DR3, EBF2, and IRX1 are hypermethylated (Karouzakis et al 2011). Genome-wide approaches have identified a large number of differentially methylated loci in RA FLS compared with normal or osteoarthritis (OA) FLS (Nakano et al 2013). The methylation pattern in RA FLS is stable over many cell passages in tissue culture and involves genes and pathways related to cell adhesion, matrix regulation, immune function, and cytokine signaling. While synovial cytokines can transiently alter the methylome by regulating DNA methyl transferase (DNMT) expression, the changes are reversible in culture and suggest that cytokines alone are not solely responsible for the ex vivo profile observed (Nakano et al 2013). The cells, therefore, appear to be “imprinted”, which could contribute particularly to the persistent aggressive phenotype of RA FLS.
The functional relevance of differential methylation was confirmed for several candidate genes identified via integrative analysis of various ‘omics platforms. For example, the development gene LBH was identified because it has several associated RA-associated SNPs and differentially methylated loci (DMLs) (Hammaker et al 2016). LBH regulates the cell cycle - a functional SNP in an LBH enhancer was identified adjacent to a DML in FLS, and modification of the enhancer methylation status altered LBH transcription (Ekwall et al 2015). In addition, a DML in PTPN11, the gene encoding the oncogene SHP2, led to discovery of a novel intron enhancer that regulates PTPN11 expression and contributes to high levels of SHP2 in RA FLS (Zhang et al 2016). In future, incorporating epigenetic marks into the systematic analysis of cell function should help identify key pathogenic genes and provides a rich source of potential therapeutic targets.
We still do not know when in the evolution of RA the epigenetic imprinting occurs. Recent data in FLS from patients with early RA (<1 year of symptoms) show that they also have an abnormal methylation pattern. However, the early RA methylome can be distinguished from late RA, especially for pathways related to cell growth and differentiation (Ai et al 2015). DNA methylation signatures in RA FLS probably occur very early or even antedate clinical disease. However, it is not static and can evolve over time based due to synovial inflammatory environment.
Perhaps even more intriguing, DNA methylation patterns, as well as transcriptomes, can vary between joints (Ai et al 2016). Early studies using histology and immunohistochemistry suggested that the various joints involved with RA are similar. However, recent studies show that the DNA methylation patterns and transcriptomes differ between RA hip and knee FLS (Ai et al 2016). Of interest, the pathways involved are related to therapeutic targets, including IL-6-JAK-STAT signaling. It is not clear if cells are imprinted elsewhere and then migrate to the joint, or whether they attain a local phenotype after arriving. These data provide a possible explanation why some joints improve in RA while others do not when patients are treated with a given targeted agent.
Sequencing studies suggest that the pattern of microRNA (mir) in RA compared with OA leukocytes and FLS exhibit global differences in expression. A growing number of mirs have been evaluated using a candidate gene approach. One example is mir124a, which is dysregulated in RA and can suppress cell cycle and chemokine gene expression. Increasing mir124a levels in RA FLS suppresses MCP-1 production (Nakamachi et al 2009). On the other hand, mir203 overexpression increases MMP and IL-6 expression (Stanczyk et al 2011), while mir155 knockdown decreases the same genes expression in RA FLS. Mir155 is also implicated in monocyte activation and cytokine production in part via regulation of TLR signaling (Kurowska-Stolarska et al 2012). Mir16, mir124a, mir202, mir134a, mir23, mir346, and mir15a and perhaps several others are differentially expressed in RA derived cells, although their pathogenic role still needs to be defined (Bottini & Firestein 2013).
There is considerably less genome-wide information on histone marks in RA. There are a variety of ways that histones can be modified, including methylation, acetylation and citrullination. Histone deacetylases, which remove acetyl groups and remodel chromatin, are expressed in RA FLS and can regulate their function (Huber et al 2007). For example, induced HDAC1 or 2 deficiency increases p53 tumor suppressor gene and matrix metalloproteinase 1 (MMP1) expression in RA synovial cells (Horiuchi et al 2009). Pro-inflammatory cytokines can increase HDAC1 expression in FLS and contribute to chromatin remodeling. HDAC inhibitors suppress adjuvant arthritis in rats and collagen-induced arthritis in mice, supporting the notion that histone modifications can participate in inflammatory joint disease (Joosten et al 2011).
Pre-RA: an evolving immunologic response?
As noted before, protein modification through citrullination is actually common, especially in the context of inflammation or stress. What is strikingly different in RA is the generation of antibodies against these epitopes, likely arising due to the unique properties of the binding pocket of RA-associated HLA-DR alleles and presumably other regulatory deficits (e.g. in T cells themselves) that permit emergence of autoreactivity over time. The teleologic explanation for induction of PADIs in normal immune responses is not known. PADIs have other potential functions that regulate cell migration and gene expression through citrullination of chemokines and histones (Christophorou et al 2014). PADI2, for example migrates to the nucleus and probably modifies many proteins involved in gene expression. Thus, protein citrullination could have a variety of functions that regulate homeostasis and cell responses to stress.
The presence of citrullinated peptides in the lungs or other mucosal sites could thus represent the “original sin” in a pathway that ultimately leads to inflammatory joint disease. ACPAs, along with other antibody systems are detected years or perhaps even decades before clinical disease is apparent. Over time, the serum levels of ACPAs gradually increase over time and reach a peak at the time of disease onset through the first year of symptoms. A concomitant rise in blood chemokines and cytokines and other altered metabolic parameters suggest an ongoing and gradually accelerating subclinical systemic inflammatory pathology (Deane et al 2010). APCA specificities at all stages are quite broad, and increase during the “pre-RA phase”. The specificity of antibodies varies widely but include citrullinated epitopes on fibrinogen, vimentin, fibronectin, collagen, enolase, histones and many others (Anzilotti et al 2006). There does not appear to be a single dominant specificity of ACPA, nor a particular pattern that predicts disease onset, phenotype or severity (Van Beers et al 2013). Notably, in patients with early undifferentiated inflammatory arthritis, ACPAs can predict individuals who will progress to RA.
In established disease, ACPAs are present in the serum of 80% to 90% of RA patients and are independently associated with greater disease activity, extra-articular manifestations and joint damage. The antibodies alone are not especially pathogenic and do not cause arthritis when injected into normal mice. However, they can exacerbate disease in mice with arthritis, perhaps because of the presence of citrullinated substrates that can ultimately lead to local complement fixation. They likely act together with rheumatoid factors (RFs), which are autoantibodies that bind to the Fc portion of IgG. ACPAs are more sensitive and specific for RA than RFs, with specificity approaching 90%. ACPAs are also present in nearly 20% of unaffected first-degree relatives and more than 10% of more distant relatives in Native American RA patients (El-gabalawy et al 2009). ACPAs and RFs are also produced by synovial tissue B cells and can be detected in synovial fluid, suggesting that they could play a role in local innate immunity activation and complement fixation. Evaluation of the phylogenetic tree of RF producing cells from the synovium suggests that affinity maturation occurs in the joint (Randen et al 1992).
A subset of patients with RA develops antibodies against other classes of modified proteins. For example, lysine and cyanate are converted to homocitrulline and can form neo-epitopes through non-enzymatic processes analogous to conversion of arginine to citrulline, giving rise to anti-carbamylated protein antibodies (ACarPs) present in a minority of patients with RA, including about 30% of ACPA- patients (Shi et al 2011). A variety of substrates are cambamylated and can lead to antibody formation with multiple specificities, such as fibronectin. As with ACPAs, they are observed about 40% of RA patients prior to the onset of clinical synovitis (Shi et al 2014). Like ACPAs, the ACarPs do not cause arthritis, but immunizing mice with carbamylated peptides is arthritogenic, and transferring lymphocytes from immunized animals can transfer disease to naïve mice (Mydel et al 2010). Autoantibodies that identify acetylated proteins have also been described (Juarez et al 2016). Finally there are data that challenge the notion of loss of tolerance to citrullination as a definitive early event – e.g. anti-native RA33 and citrullinated RA33 were detected as a continuum in a recent analysis with native reactivity dominant in earlier disease (Konig M et al 2016). Further sequential studies of detailed antibody profiles against the spectrum of the ‘citrullome’ in RA would be helpful in resolving these issues.
Evolution from a high risk state to clinical synovitis
The foregoing demonstrates that in high-risk individuals with a characteristic genetic (and possibly epigenetic) background, stochastic events especially at mucosal surfaces can lead to protein modification and ultimately antibody formation recognising altered peptides. The site of antibody production could either be at the site of stress, or in lymphoid organs defined by dendritic cells that process arthritogenic peptide at mucosal surfaces and migrate centrally to relevant draining lymph nodes. Lymph nodes in pre- and early RA are not normal (Rodríguez-Carrio et al 2016). By definition however, disease emerges in the joints. That ACPAs, rheumatoid RFs, ACarPs and perhaps other antibody systems do not typically lead to inflammatory joint disease per se suggests that a “second hit” is required, at least in model systems for tissue localization.
The nature of this second signal is not well defined but could comprise some combination of vascular, neuro-regulatory, microtrauma, or transient infection dependent pathways. In addition, there have long been suggestions that articular infections e.g. Epstein-Barr virus could further contribute (Tan & Smolen 2016). In animal models, the formation of immune complexes can trigger synovitis. The inciting complexes do not have to be specific for altered peptides but can actually be irrelevant; even immunocomplexes with antibody and horseradish peroxidase is sufficient in some mouse models. Thus, a variety of random tissue insults (e.g. microtrauma, viral infection) could lead to synovial vascular activation in the presence of the requisite size and charge of complexes that permits Fc receptor engagement in the synovium. Triggered synovial innate cells like mast cells could release vasoactive mediators and increases antibody access to the joint. Complement activation across this range of effector pathways could act to accelerate this response. Other mechanisms like Toll-like receptor engagement or triggering via various danger sensing molecules, could theoretically cause a similar cascade. Recent data suggest a potential role for ACPAs beyond immune complexes depending upon their chemical properties and cellular specificity. Intriguing studies suggest that ACPA recognition of osteoclast (OC) membrane epitopes e.g. citrullinated vimentin can promote osteoclastogenesis – thus an early route to articular involvement via systemic ACPA expression may be via local OC activation (Harre et al 2012). Moreover one consequence of ACPA OC binding is the induction of pain and release of IL-8 providing an enticing route to subsequent leukocyte recruitment and the imitation of a vicious cycle of articular inflammation (Krishnamurthy et al 2016). In addition ACPAs most likely to progress disease exhibit an altered glycosylation state (Rombouts et al 2015), though the functional consequences of these observations remains less well understood.
Once in the joint in established disease, autoantibodies may bind to antigen, fix complement, release chemotactic fragments and initiate a cascade of events that activates other resident cells, recruits new innate and adaptive immune cells and promotes stromal cell activation (Tan & Smolen 2016). These, in turn, can produce additional cytokines and chemokines to create a positive feedback loop and ultimately a self-perpetuating process with inadequate negative regulators required for termination (Figures 2 &3). The joint is uniquely susceptible to promote such a series of events. For example, the vasculature is already porous and facilitates ingress of proteins and cells. The synovial intimal lining, which forms the surface of the synovium and contains macrophage-like and fibroblast-like synoviocytes (FLS) are not an effective barrier because there are no tight junctions or an organized basement membrane. Once in the joint, antibodies can be displayed on cartilage to promote complement fixation. Murine models involving antibodies to type II collagen or glucose-6-phosphate isomerase are particularly effective at inducing synovitis because of their propensity to bind to these articular surfaces. The former is pathogenic because it is critical component of hyaline cartilage and the latter most likely adheres to negatively charged cartilage surfaces (Matsumoto et al 2002).
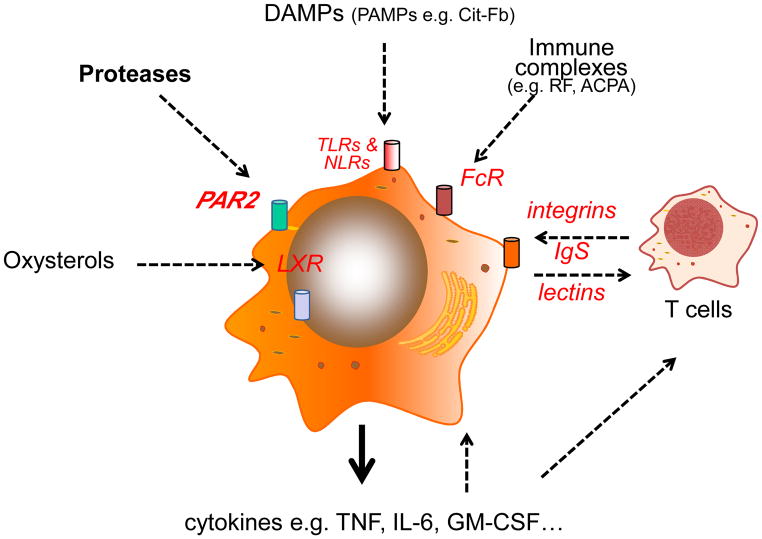
Figure 2.
Myeloid cells, particularly macrophages are central to the perpetuation of disease pathogenesis. Pathways that can activate macrophages in RA synovitis are shown that promote cytokine release – IL-6 and TNF in particular, and probably GM-CSF, are of central importance as defined by the clear therapeutic benefits achieved upon their inhibition.
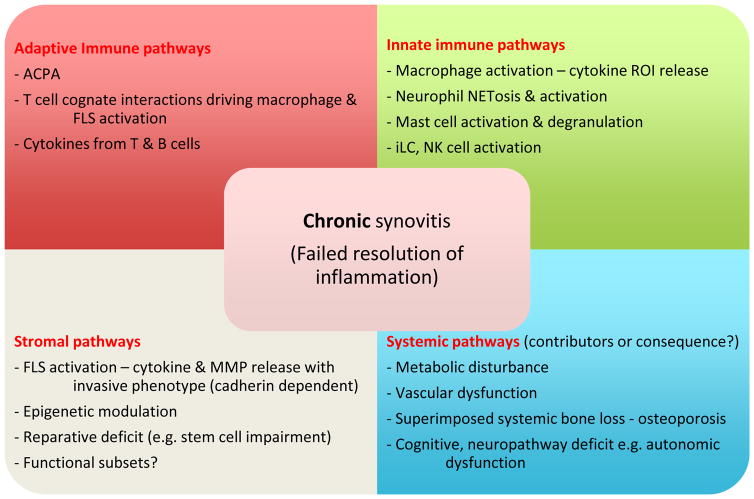
Figure 3.
Mechanisms that drive the chronicity of RA. Distinct pathways combine to mediate failed resolution of disease – these include adaptive, innate and stromal components with additional impact of the systemic features of disease. The latter may be both consequence of the chronic inflammation or could promote the chronicity e.g. via creation of pro-inflammatory lipid profiles, promotion of bone remodeling and secondary osteoarthritis.
One of the important lessons of this prolonged and step-wise pre-RA process is that the sequence of events does not require autoimmunity against native proteins in the earliest stages. The immune response could be more properly considered a normal appropriate adaptive immune response to altered antigens that are viewed as “foreign” because they were not generated during thymic development and pathogenic T cells could not be deleted. The high-risk individuals with ‘unfortunate’ HLA-DR alleles happen to bind to these modified proteins more avidly than the native molecule. Later, perhaps through epitope spreading, more traditional autoimmunity develops and leads to production of antibodies commonly detected in chronic RA that bind to collagens and many other native proteins.
Considering an early role for stromal cells as important pathogenic factors?
Compelling data implicate adaptive immune responses in the pathogenesis of RA, but alternative hypotheses propose that stromal elements also can play a critical role. Early pioneering studies by Gay and colleagues showed that FLS assume an autonomous aggressive phenotype that is maintained for months after removal from the patient (Muller-Ladner, et al 1997). These data and the destructive potential of fibroblasts led to the notion that FLS are imprinted or permanently altered in RA and could either initiate or exacerbate disease (Firestein, 1996, Firestein and Bottini, 2013). Numerous mechanisms for “partial transformation” have been implicated, including abnormal DNA methylation (see below), somatic mutations in various genes (e.g., p53, mitochondria, vimentin), defective PTEN expression, and high levels of sentrin (Pap et al, 2000, Franz et al 2000, Firestein et al 1997, and reviewed in Firestein and Bottini, 2013). In addition, activated FLS can play an active role in antigen presentation and contribute to local adaptive immune responses (Tran et al 2007). Finally, the synovial fibroblasts are not a uniform population but segregate into different phenotypes based, in part, on their cytokine profiles (Croft et al, 2016). The role of mesenchymal cells in disease initiation and progression represents an interesting therapeutic opportunity because no approved agents currently target these cells (see Figure 1 for alternate models of contribution of stromal cells and autoimmunity in a notional hierarchy – note that these are not mutually exclusive).
Pathologic characteristics of clinical synovitis
The histologic appearances of RA synovitis have been extensively characterized. Normal synovial membrane is relatively acellular comprising scattered macrophages within a stromal tissue curated by FLS and sparse blood vessels. Its primary function is to lubricate and nourish the articular surface, which lacks a blood supply. During the pre-RA phase, the synovial appearance is not markedly altered; biopsy studies or ACPA+ arthralgia are remarkably benign, with no obvious leukocyte infiltrates, nor transcriptional changes (de Hair et al 2014). However with the onset of clinically evident articular swelling (including that detected by MRI or power-Doppler ultrasound imaging) the synovium assumes a rather different appearance (reviewed by McInnes & Schett 2011). The lining layer, normally 2–3 cells thickness expands up to 10–12 cells depth, comprising macrophages and FLS. Underlying this is a dense interstitial cellular infiltrate containing cells characteristic of innate, e.g., macrophages, dendritic cells, mast cells, natural killer (NK) cells, innate lymphoid cells, and of adaptive responses e.g. B and T lymphocytes, and plasma cells. This is associated with significant neovascularization, with some lymphangiogenesis.
The immunologic properties of RA synovium are now well characterized. Synovitis studies relied originally on arthroplasty-derived tissues, that were usually by definition end stage disease, but have been expanded by the use of synovial biopsies obtained via arthroscopy or ultrasound methodologies facilitating investigation of all stages of disease (Kelly et al 2015). Macrophages, mainly of M1 phenotype, are potent contributors to effector pathology by virtue of release of cytokines (e.g. TNF, IL-1, IL-6), chemokines, MMPs, vasoactive peptides, oxygen and nitrogen intermediates. Numerous activation pathways of macrophages have been described including TLR ligands, soluble proteases (via PAR2), cholesterol derivatives and immune complexes (Figure 2). The latter, containing RF and ACPA, act via FcgR1 and FcgR3 that are highly expressed on synovial macrophages. Coincident binding of ACPAs to soluble citrullinated TLR ligands e.g. cit-Fibronectin may enhance these effects – by this model ACPAs can bind both TLR and FcR on the same cell to optimize activation (Sokolove et al 2011) leading to high levels of cytokine release. The glycosylation status, particularly sialylation, of ACPAs also alters their capacity to promote inflammation (Rombouts et al 2015). Cognate interaction with memory T cells is a further intriguing activation pathway – acting via a non-antigen dependent manner, the cytokine rich synovial milieu, particularly IL-15, IL-7 and IL-6, can induce integrin and Ig-superfamily dependent interactions between CD45RO+ memory T cells and macrophages to drive subsequent macrophage cytokine and chemokine release (McInnes & Schett 2007)
Most interest in the contribution of T cells has however focused on their antigen-driven role in initiation and perpetuation of disease. CD45RO T cells are abundant in synovial membrane and exhibit an activated phenotype. Fascinating studies have established that RA peripheral blood CD45RO naïve T cells exhibit unique anabolic metabolic features characterized by low glucose metabolism, lower icATP levels associated with PFKFB3 deficiency that may play a role in their relative senescence and likely therefore their chronic activation state (Zhen et al 2013; Yang et al 2016). Synovial biopsies from very early RA contain expanded T cell clones that likely represent enrichment for self-reactive (or least altered-peptide reactive) clones – this feature disappears with disease progression presumably reflecting dilution of these clones by secondarily recruited polyclonal memory T cells (Klarenbeek et al 2012). The differentiation state of the synovial T cell is also uncertain. Murine arthritis models strongly suggest a pivotal role for T helper 17 (Th17) cells (Miossec & Kolls 2012: Lubberts 2015). Human data have rather challenged this proposition though no firm conclusions can yet be drawn as the critical pre-RA phase could well be Th17 driven but not manifest in the effector stages of disease amenable to direct examination. Nevertheless, one report suggests that cytokine profiles in lavage fluid from early RA are paradoxically rich in IL-4, IL-13 and IL-15, whereas those T cells detected in established disease have features of both Th1 and Th17 (Raza et al 2005). A recent study in which citrullinated tetramer responses were explored in RA patients found most T cells that were cit-specific were of Th1 phenotype (Snir et al 2011). IL-17 targeting therapeutics including IL-17A and IL-17RA inhibitors have as yet not been particularly effective in established RA trials despite remarkable responses being achieved in psoriasis (Langley et al 2014) and to some extent spondyloarthropathies (McInnes et al 2015).
Future studies using single cell based sequencing methodologies and the availability of detailed transcriptional definitions of T cells are underway to facilitate characterization of T cell subset differentiation in the phases of RA development. Understanding those factors that define T cell differentiation in RA or perhaps other cell lineages that could contribute to the evolution of this unique disease might critically important because the detection of Tregs in synovium has also been extensively documented - clearly their functional impact is insufficient, or they may have a functional deficit. Some evidence suggests that inadequate Treg function is characteristic of RA and is corrected by TNF blocker therapy (Byng-Maddick et al 2015). However, it is not clear if this defect is primary or secondary. Re-establishing local T cell homeostatic balance via expansion or recovery of Treg function is an attractive approach to future therapeutics particularly as we seek restoration of tolerance as the clinical target (Rossetti et al 2016; Benham et al 2015).
Clearly autoantibody production is a hallmark of RA. B cell lineages at each stage of development are detected in synovium, include the presence of mature plasma cells. The synovium provides a cytokine rich milieu for B cell survival, e.g., via BAFF, APRIL, IL-6 expression. A particular role for FLS (which are the primary source of IL-6 in the RA synovium) in maintaining this milieu has recently emerged exemplifying the tissue dependency of the articular immune response (Bombardieri et al 2011). In a proportion of tissues there are ectopic lymphoid structures (ELS) that have been associated with more rapid erosive progression. Prior studies demonstrated that ELS in RA tissue contain the enzymatic machinery necessary for antigen-driven selection, differentiation, somatic hypermutation and class switching (Pitzalis et al 2014). Recently single cell sequencing of synovial B cells suggests extensive self-reactivity particularly to citrullinated histones H2A and H2B and other citrullinated self-proteins – neutrophil could form a focal event permitting expansion of such clones (Corsiero et al 2015). Thus, in addition to antigen presentation, autoantibody formation and release, B cells should be considered important sources of local antigen presentation, and of cytokine release into the milieu.
Finally it is vital to consider the role of the local stromal tissues in synovium. FLS have been long recognized to contribute to the matrix deposition and tissue remodeling that characterizes RA (Zvaifler & Firestein 1994). They exhibit anchorage independence, migratory activity (potentially even between joints), local proliferation and release high levels of MMP and TIMPs (Tissue inhibitors of metalloproteinases), cytokines and chemokines together with collagens and other ECM components (Filer 2013). They appear to be particularly dependent upon cadherin 11 for their effector function (Kiener et al 2009). As such they should be considered a critical component of the RA inflammatory response rather than as responding bystanders. Other studies suggest the existence of FLS subsets although the functional significance of these remains uncertain. Further complexity is emerging since FLS exhibit distinct features even across different diarthrodial joints e.g. hips and knees described above. FLS may also migrate across anatomically discrete articular sites (Lefevre et al 2009). Presumably reflecting the epigenetic changes described above FLS thus play a critical role in recruiting, retaining, activating and sustaining leukocytes e.g. via type 1 interferons, CXCL12 (SDF-1) in the synovium (e.g. Perlman et al 2003). Finally, in combination with endothelial cells they may become permissive for ELS formation (Pitzalis et al 2014) further enhancing an ‘autoreactive-prone’ articular microenvironment.
Articular destruction, often reflected in radiographic evidence of bone erosion, is a further hallmark of RA. Mechanisms driving damage are reviewed elsewhere (McInnes & Schett 2007). Briefly, FLS drive damage primarily via MMP (particularly MMP1 & MMP3) mediates direct cartilage damage or by promoting chondrocyte activation and tissue catabolism through cytokine release in concert with macrophages. IL-1, TNF and IL-17 are particularly relevant in this respect. Bone damage arises from more complex interactions that sustain osteoclast precursor maturation mediated particularly via RANKL, M-CSF and TNF pathways. Recent insights into the participation of sclerostin, DKK, ACPAs and other osteoclast and osteoblast co-regulators suggest the intriguing possibility of promoting bone healing – this has not yet been possible on the basis of existing therapeutics (Diarra et al 2007: McInnes & Schett 2011). Other factors in the synovial milieu including ACPAs are also implicated via their ability to bind directly to osteoclasts and promote their differentiation and activation.
One further development of note is the recent observation by some groups of synovial tissue micro-heterogeneity in several cohort studies. Thus, subsets of patients can potentially be defined by myeloid, lymphoid and stromal rich synovial pathotypes (Dennis et al 2014). The longer-term functional implications of these subsets are currently unclear but carry the potential to offer subtype specific therapeutics in due course – clinical trials are underway to test this hypothesis (Astorri et al 2015; Kelly et al 2015). If confirmed this would suggest that distinct pathologic processes can induce pathotypes that are pathologically discrete, and of functional importance in identifying dominant effector pathways and tissue responses.
Lessons from therapeutic targeting as to the hierarchy of pathways and heterogeneity of responses
The utility of immune targeting in RA represents a major advance in disease management (Smolen et al 2016; Smolen & Aletaha 2015). Thus inhibitors of TNF and IL-6 are particularly potent in delivering clinical responses. Co-stimulatory blockade with abatacept (CTLA4:Ig) is similarly effective, as is depletion of CD20 lineages using rituximab (Nam et al 2014; Schiff et al 2014). Notably, those blocking for example IL-17, IL-1, BAFF, IL-21 and IL-20 appear less effective. More recently small molecule inhibitors of the JAK pathways, e.g. tofacinitib (targeting JAK1 and JAK3), and baricitinib (JAK1 and JAK2) have demonstrated considerable efficacy, especially if used in early disease (Genovese et al 2016: Lee et al 2014).
Immune therapeutics have taught us much (Table): (i) critical immune checkpoints or functional nodes must exist within the complex synovial lesion that are amenable to blockade and promote collapse of the majority of the lesion; (ii) upstream immune dysregulation apparently manifests primarily in cytokine (especially TNF and IL-6) mediated effector mechanisms; and (iii) murine models, despite offering exquisite mechanistic insight and proof of possibility, poorly reflect the complexity of the human RA state and therefore the therapeutic potential of any given agent. Moreover the discrete effects of cytokine inhibitors across the wider range of chronic inflammatory diseases is increasingly pointing to a new molecular disease taxonomy that can be therapeutically defined – RA might represent a TNF and IL-6 dependent axis whereas psoriasis might reflect more TNF, IL-23 and IL-17 dependent pathways.
Table.
The major drug classes that have been employed in the treatment or investigation of RA and that have been informative for pathogenesis studies are depicted herein.
| Immune Target | Drugs | Key modes of action |
|---|---|---|
| TNF | Adalimumab Etanercept Golimumab Certolizumab Infliximab Biosimilars |
Reduced endothelial, stromal cell & chondrocyte activation Reduced osteoclast differentiation/activation Modified cellular migration Reduced cytokine expression e.g. IL-6 Reduced metabolic/CVD risk |
| IL-6 | Tocilizumab Multiple Ab in development |
Reduced endothelial, stromal cell activation Reduced osteoclast activation Reduced cytokine/chemokine expression Altered lipid metabolism |
| Co-stimulation (CD28 - CD80/86) | Abatacept | Reduced T cell activation Diminished dendritic cell activation, cytokine production and antigen presentation Reduced osteoclast activation |
| B cell depletion (anti-CD20) | Rituximab | Depletion of CD20+ B cell lineages, sparing plasma cells. Reduced cytokine production Reduced antigen presentation |
| JAK inhibitors | Tofacitinib Baricitinib Multiple compounds in development |
Inhibition of pivotal cytokine receptors with predictable downstream effects. JAK1, JAK2 and JAK3 thus far targeted |
| IL-1 | Anakinra | Decreased endothelial cell, stromal cell activation Decreased cellular migration Altered T cell differentiation Innate immunity activation |
| Other cytokines (GM-CSF, IL-17, and others) | Multiple | Multiple |
The diversity of responses to highly targeted agents like cytokine inhibition, B cell depletion, or co-stimulation blockade suggest that RA is not a single disease entity. Instead, it is a syndrome with a common clinical presentation but multiple pathogenic pathways, any one of which (or combination) might be relevant for a particular patient (Firestein 2014). One of the key challenges going forward will be how we can stratify patients so that we can make better treatment selections. Thus far there are no studies that have separated responder RA populations to distinct interventions. A variety of molecular and cellular profiling approaches in peripheral blood have not been successful as yet. As noted above, preliminary studies suggest that synovial histologic patterns might correlate with responses to therapy, although other careful assessments of synovial structure and biomarkers have not demonstrated sufficient power for clinical utility (Dennis et al 2014). These in turn could presage the advent of biomarkers that enrich for response. We also need to begin considering more robust clinical trial instruments to take into account joint-specific differences in clinical responses.
Kinetically distinct phases of immune pathway utilization as RA progresses also should be considered – in essence we use all immune therapeutics across the various phases of RA development without consideration to the dominant pathogenesis at that time point (McInnes et al 2016). Immunologic adaptation is a characteristic of chronic infectious responses and it seems plausible that similar alterations in effector pathways will evolve as damage accrues in patients. Should we now consider ‘pathogenesis-appropriate’ timing of therapeutic interventions? For example, could there be a role for immune tolerance inducing therapies in very early RA, or even pre-RA? This supposes that pre-RA can be identified in a meaningful population and at a stage when immune homeostasis or re-programming can be achieved, and that molecular outcome measures can be derived to define therapeutic success. Various therapeutics are being explored in this respect, including B cell depletion or co-stimulatory blockade in high risk populations. The PRAIRI study administered a single dose of rituximab to people at high risk of developing RA – formal reporting of outcome is awaited (NTR2442 Netherlands Trial Register). A similar study is ongoing in which abatacept is being given to high risk pre-RA (APIPRA Study; ISRCTN46017566). Finally a broader approach to identification of pre-RA comprises the StopRA study in which hydroxychloroquine is being given to health subjects with an elevated ACPA (NCT02603146). Finally, exciting cell transfer clinical trials using tolerogenic dendritic cells, with thus far encouraging results in terms of immune function modulation are also ongoing (Benham et al 2015).
Processes driving co-morbidities
RA is associated with increased cardiovascular risk, metabolic syndrome, psychosocial deficit, including psychiatric disease, osteoporosis, and increased cancer rates. The mechanisms underlying these are increasingly understood to depend on cytokines and other circulating immune moieties, primarily using immune therapeutics as molecular scalpels. For example, IL-6 and to some extent TNF directly regulate cholesterol metabolism, such that active RA is associated with paradoxical low cholesterol levels that are replenished with effective therapy – the latter appears to operate via direct IL-6 mediated, STAT3 dependent modulation of cholesterol ester catabolism (Charles-Shoeman et al 2015: Robertson et al 2014). Similarly the atherosclerotic lesion and RA synovium exhibit remarkable similarities leading to the suggestion that TNF and IL-6 driven pathways may enhance the progression of vascular disease. TNF inhibition in patients leads to reduced vascular dysfunction and to improve vascular mortality commensurate with this notion (McKellar et al 2009). Moreover RA atherosclerotic plaques exhibit exaggerated cytokine expression compared to non-RA controls (Ahmed et al 2016). Similarly circulating TNF, RANKL and IL-1 are implicated in systemic osteoporosis (McInnes & Schett 2011), and TNF has been implicated in the hippocampal dysfunction that likely underlies depression in RA (Krishnadas et al 2016).
Concluding remarks
RA represents a remarkable example of successful immunologic interrogation of tissue culture cells, pre-clinical models and human translational studies that has delivered enormous therapeutic advances. The penumbra of these advances has also benefited many other immune-mediated disease, such as psoriasis, spondyloarthropathies, inflammatory bowel disease (IBD) and inflammatory eye disease. It has taught us much about immune pathogenetic mechanisms in the context of human autoimmunity and chronic inflammation, not least in unraveling the complex multi-hit sequence of events that ultimately overcome immune homeostasis to manifest as tissue destructive disease. Despite such successes there remains much unmet need, not least in the current absence of the capacity to predict, prevent and cure the disease or even predict which patients respond to a particular therapy. It is clear that further pathogenetic discovery must drive such progress and lead ultimately to the delivery of agents with homeostatic activity, used within a precision medicine-based therapeutic culture..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ai R, Whitaker JW, Boyle DL, Tak PP, Gerlag DM, Wang W, Firestein GS. DNA Methylome Signature in Synoviocytes From Patients With Early Rheumatoid Arthritis Compared to Synoviocytes From Patients With Longstanding Rheumatoid Arthritis. Arthritis Rheumatol. 2015;67(7):1978–80. doi: 10.1002/art.39123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai R, Hammaker D, Boyle DL, Morgan R, Walsh AM, Fan S, Firestein GS, Wang W. Joint-specific DNA methylation and transcriptome signatures in rheumatoid arthritis identify distinct pathogenic processes. Nat Commun. 2016 Jun 10;7:11849. doi: 10.1038/ncomms11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Hollan I, Curran SA, Kitson SM, Riggio MP, Mikkelsen K, Almdahl SM, Aukrust P, McInnes IB, Goodyear CS. Proatherogenic Cytokine Microenvironment in the Aortic Adventitia of Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2016 Jun;68(6):1361–6. doi: 10.1002/art.39574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzilotti C, Merlini G, Pratesi F, et al. Antibodies to viral citrullinated peptide in rheumatoid arthritis. J Rheumatol. 2006;33:647. [PubMed] [Google Scholar]
- Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nat Rev Rheumatol. 2012;8(10):573–86. doi: 10.1038/nrrheum.2012.134. [DOI] [PubMed] [Google Scholar]
- Astorri E, Nerviani A, Bombardieri M, Pitzalis C. Towards a stratified targeted approach with biologic treatments in rheumatoid arthritis: role of synovial pathobiology. Curr Pharm Des. 2015;21(17):2216–24. doi: 10.2174/1381612821666150310145758. [DOI] [PubMed] [Google Scholar]
- Benham H, Nel HJ, Law SC, Mehdi AM, Street S, Ramnoruth N, Pahau H, Lee BT, Ng J, Brunck ME, Hyde C, Trouw LA, Dudek NL, Purcell AW, O’Sullivan BJ, Connolly JE, Paul SK, Lê Cao KA, Thomas R. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci Transl Med. 2015 Jun 3;7(290):290ra87. doi: 10.1126/scitranslmed.aaa9301. [DOI] [PubMed] [Google Scholar]
- Bombardieri M, Kam NW, Brentano F, Choi K, Filer A, Kyburz D, McInnes IB, Gay S, Buckley C, Pitzalis C. A BAFF/APRIL-dependent TLR3-stimulated pathway enhances the capacity of rheumatoid synovial fibroblasts to induce AID expression and Ig class-switching in B cells. Ann Rheum Dis. 2011 Oct;70(10):1857–65. doi: 10.1136/ard.2011.150219. [DOI] [PubMed] [Google Scholar]
- Bottini N, Firestein GS. Epigenetics in rheumatoid arthritis: a primer for rheumatologists. Curr Rheumatol Rep. 2013;15:372. doi: 10.1007/s11926-013-0372-9. [DOI] [PubMed] [Google Scholar]
- Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9:24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byng-Maddick R, Ehrenstein MR. The impact of biological therapy on regulatory T cells in rheumatoid arthritis. Rheumatology (Oxford) 2015 May;54(5):768–75. doi: 10.1093/rheumatology/keu487. [DOI] [PubMed] [Google Scholar]
- Catrina AI, Joshua V, Klareskog L, Malmström V. Mechanisms involved in triggering rheumatoid arthritis Immunol Rev. 2016 Jan;269(1):162–74. doi: 10.1111/imr.12379. [DOI] [PubMed] [Google Scholar]
- Catrina AI, Ytterberg AJ, Reynisdottir G, Malmström V, Klareskog L. Lungs, joints and immunity against citrullinated proteins in rheumatoid arthritis. Nat Rev Rheumatol. 2014 Nov;10(11):645–53. doi: 10.1038/nrrheum.2014.115. [DOI] [PubMed] [Google Scholar]
- Charles-Schoeman C, Fleischmann R, Davignon J, Schwartz H, Turner SM, Beysen C, Milad M, Hellerstein MK, Luo Z, Kaplan IV, Riese R, Zuckerman A, McInnes IB. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 2015 Mar;67(3):616–25. doi: 10.1002/art.38974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophorou MA, Castelo-Branco G, Halley-Stott RP, Oliveira CS, Loos R, Radzisheuskaya A, Mowen KA, Bertone P, Silva JC, Zernicka-Goetz M, Nielsen ML, Gurdon JB, Kouzarides T. Citrullination regulates pluripotency and histone H1 binding to chromatin. Nature. 2014;507(7490):104–8. doi: 10.1038/nature12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsiero E, Bombardieri M, Carlotti E, Pratesi F, Robinson W, Migliorini P, Pitzalis C. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2015-208356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft AP, Naylor AJ, Marshall JL, Hardie DL, Zimmermann B, Turner J, Desanti G, Adams H, Yemm AI, Müller-Ladner U, Dayer JM, Neumann E, Filer A, Buckley CD. Rheumatoid synovial fibroblasts differentiate into distinct subsets in the presence of cytokines and cartilage. Arthritis Res Ther. 2016 Nov 18;18(1):270. doi: 10.1186/s13075-016-1156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aquino SG, Abdollahi-Roodsaz S, Koenders MI, et al. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J Immunol. 2014;192:4103. doi: 10.4049/jimmunol.1301970. [DOI] [PubMed] [Google Scholar]
- de Hair MJ, van de Sande MG, Ramwadhdoebe TH, Hansson M, Landewé R, van der Leij C, Maas M, Serre G, van Schaardenburg D, Klareskog L, Gerlag DM, van Baarsen LG, Tak PP. Features of the synovium of individuals at risk of developing rheumatoid arthritis: implications for understanding preclinical rheumatoid arthritis. Arthritis Rheumatol. 2014 Mar;66(3):513–22. doi: 10.1002/art.38273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007 Feb;13(2):156–63. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- Deane KD, O’Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, Gilliland WR, Edison JD, Norris JM, Robinson WH, Holers VM. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 2010 Nov;62(11):3161–72. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G, Jr, Holweg CT, Kummerfeld SK, Choy DF, Setiadi AF, Hackney JA, Haverty PM, Gilbert H, Lin WY, Diehl L, Fischer S, Song A, Musselman D, Klearman M, Gabay C, Kavanaugh A, Endres J, Fox DA, Martin F, Townsend MJ. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther. 2014;16(2):R90. doi: 10.1186/ar4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwall AK, Whitaker JW, Hammaker D, Bugbee WD, Wang W, Firestein GS. The Rheumatoid Arthritis Risk Gene LBH Regulates Growth in Fibroblast-like Synoviocytes. Arthritis Rheumatol. 2015;67(5):1193–202. doi: 10.1002/art.39060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gabalawy HS, Robinson DB, Hart D, et al. Immunogenetic risks of anti-cyclical citrullinated peptide antibodies in a North American Native population with rheumatoid arthritis and their first-degree relatives. J Rheumatol. 2009;6:1130. doi: 10.3899/jrheum.080855. [DOI] [PubMed] [Google Scholar]
- Ferreira RC, Freitag DF, Cutler AJ, et al. Functional IL6R 358Ala allele impairs classical IL-6 receptor signaling and influences risk of diverse inflammatory diseases. PLoS Genet. 2013;9:e1003444. doi: 10.1371/journal.pgen.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filer A. The fibroblast as a therapeutic target in rheumatoid arthritis. Curr Opin Pharmacol. 2013 Jun;13(3):413–9. doi: 10.1016/j.coph.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39:1781–90. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- Firestein GS, Echeverri F, Yeo M, Zvaifler NJ, Green DR. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci USA. 1997;94:10895–900. doi: 10.1073/pnas.94.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein GS, Zvaifler NJ. How important are T cells in chronic rheumatoid synovitis?: II. T cell-independent mechanisms from beginning to end. Arthritis Rheum. 2002;46:298. doi: 10.1002/art.502. [DOI] [PubMed] [Google Scholar]
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003 May 15;423(6937):356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- Firestein GS. The disease formerly known as rheumatoid arthritis. Arthritis Res Ther. 2014;16(3):114. doi: 10.1186/ar4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca JE, Cavaleiro J, Teles J, Sousa E, Andreozzi VL, Antunes M, Amaral-Turkman MA, Canhão H, Mourão AF, Lopes J, Caetano-Lopes J, Weinmann P, Sobral M, Nero P, Saavedra MJ, Malcata A, Cruz M, Melo R, Braña A, Miranda L, Patto JV, Barcelos A, da Silva JC, Santos LM, Figueiredo G, Rodrigues M, Jesus H, Quintal A, Carvalho T, da Silva JA, Branco J, Queiroz MV. Contribution for new genetic markers of rheumatoid arthritis activity and severity: sequencing of the tumor necrosis factor-alpha gene promoter. Arthritis Res Ther. 2007;9(2):R37. doi: 10.1186/ar2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JK, Pap T, Hummel KM, Nawrath M, Aicher WK, Shigeyama Y, Müller-Ladner U, Gay RE, Gay S. Expression of sentrin, a novel antiapoptotic molecule, at sites of synovial invasion in rheumatoid arthritis. Arthritis Rheum. 2000;43:599–607. doi: 10.1002/1529-0131(200003)43:3<599::AID-ANR17>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Genovese MC, Kremer J, Zamani O, Ludivico C, Krogulec M, Xie L, Beattie SD, Koch AE, Cardillo TE, Rooney TP, Macias WL, de Bono S, Schlichting DE, Smolen JS. Baricitinib in Patients with Refractory Rheumatoid Arthritis. N Engl J Med. 2016 Mar 31;374(13):1243–52. doi: 10.1056/NEJMoa1507247. [DOI] [PubMed] [Google Scholar]
- Hammaker D, Whitaker JW, Maeshima K, Boyle DL, Ekwall AH, Wang W, Firestein GS. Limb Bud and Heart Development Gene Transcription is Regulated by the Interplay of an Enhancer Risk Allele and DNA Methylation in Rheumatoid Arthritis. Arthritis Rheumatol. 2016 doi: 10.1002/art.39746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, Jakobsson PJ, Baum W, Nimmerjahn F, Szarka E, Sarmay G, Krumbholz G, Neumann E, Toes R, Scherer HU, Catrina AI, Klareskog L, Jurdic P, Schett G. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest. 2012;122(5):1791–802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171:538. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- Horiuchi M, Morinobu A, Chin T, Sakai Y, Kurosaka M, Kumagai S. Expression and function of histone deacetylases in rheumatoid arthritis synovial fibroblasts. J Rheumatol. 2009;36:1580–9. doi: 10.3899/jrheum.081115. [DOI] [PubMed] [Google Scholar]
- Huber LC, Brock M, Hemmatazad H, et al. Histone deacetylase/acetylase activity in total synovial tissue derived from rheumatoid arthritis and osteoarthritis patients. Arthritis Rheum. 2007;56:1087–93. doi: 10.1002/art.22512. [DOI] [PubMed] [Google Scholar]
- Hueber W1, Tomooka BH, Batliwalla F, Li W, Monach PA, Tibshirani RJ, Van Vollenhoven RF, Lampa J, Saito K, Tanaka Y, Genovese MC, Klareskog L, Gregersen PK, Robinson WH. Blood autoantibody and cytokine profiles predict response to anti-tumor necrosis factor therapy in rheumatoid arthritis. Arthritis Res Ther. 2009;11(3):R76. doi: 10.1186/ar2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten LA, Leoni F, Meghji S, Mascagni P. Inhibition of HDAC activity by ITF2357 ameliorates joint inflammation and prevents cartilage and bone destruction in experimental arthritis. Mol Med. 2011;17:391–6. doi: 10.2119/molmed.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez M, Bang H, Hammar F, Reimer U, Dyke B, Sahbudin I, Buckley CD, Fisher B, Filer A, Raza K. Identification of novel antiacetylated vimentin antibodies in patients with early inflammatory arthritis. Ann Rheum Dis. 2016;75:1099–107. doi: 10.1136/annrheumdis-2014-206785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källberg H, Ding B, Padyukov L, et al. EIRA Study Group: Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. 2011;70:508. doi: 10.1136/ard.2009.120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky ZA, Tang T, Wang SC, Ptak C, Oh GH, Wong AH, Feldcamp LA, Virtanen C, Halfvarson J, Tysk C, McRae AF, Visscher PM, Montgomery GW, Gottesman II, Martin NG, Petronis A. DNA methylation profiles in monozygotic and dizygotic twins. Nat Genet. 2009;41(2):240–5. doi: 10.1038/ng.286. [DOI] [PubMed] [Google Scholar]
- Karouzakis E, Rengel Y, Jüngel A, et al. DNA methylation regulates the expression of CXCL12 in rheumatoid arthritis synovial fibroblasts. Genes Immun. 2011;12:643–52. doi: 10.1038/gene.2011.45. [DOI] [PubMed] [Google Scholar]
- Kelly S, Humby F, Filer A, Ng N, Di Cicco M, Hands RE, Rocher V, Bombardieri M, D’Agostino MA, McInnes IB, Buckley CD, Taylor PC, Pitzalis C. Ultrasound-guided synovial biopsy: a safe, well-tolerated and reliable technique for obtaining high-quality synovial tissue from both large and small joints in early arthritis patients. Ann Rheum Dis. 2015 Mar;74(3):611–7. doi: 10.1136/annrheumdis-2013-204603. [DOI] [PubMed] [Google Scholar]
- Kiener HP, Niederreiter B, Lee DM, Jimenez-Boj E, Smolen JS, Brenner MB. Cadherin 11 promotes invasive behavior of fibroblast-like synoviocytes. Arthritis Rheum. 2009 May;60(5):1305–10. doi: 10.1002/art.24453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Bang SY, Lee HS, Bae SC. Update on the genetic architecture of rheumatoid arthritis. Nat Rev Rheumatol. 2017 Jan;13(1):13–24. doi: 10.1038/nrrheum.2016.176. [DOI] [PubMed] [Google Scholar]
- Kinslow JD, Blum LK, Deane KD, Demoruelle MK, Okamoto Y, Parish MC, Kongpachith S, Lahey LJ, Norris JM, Robinson WH, Holers VM. Elevated IgA Plasmablast Levels in Subjects at Risk of Developing Rheumatoid Arthritis. Arthritis Rheumatol. 2016 Oct;68(10):2372–83. doi: 10.1002/art.39771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschmann DA, Duffin KL, Smith CE, Welply JK, Howard SC, Schwartz BD, Woulfe SL. Naturally processed peptides from rheumatoid arthritis associated and non-associated HLA-DR alleles. J Immunol. 1995;155(12):5655–62. [PubMed] [Google Scholar]
- Klarenbeek PL, de Hair MJ, Doorenspleet ME, van Schaik BD, Esveldt RE, van de Sande MG, Cantaert T, Gerlag DM, Baeten D, van Kampen AH, Baas F, Tak PP, de Vries N. Inflamed target tissue provides a specific niche for highly expanded T-cell clones in early human autoimmune disease. Ann Rheum Dis. 2012 Jun;71(6):1088–93. doi: 10.1136/annrheumdis-2011-200612. [DOI] [PubMed] [Google Scholar]
- Konig MF, Abusleme L, Reinholdt J, Palmer RJ, Teles RP, Sampson K, Rosen A, Nigrovic PA, Sokolove J, Giles JT, Moutsopoulos NM, Andrade F. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016;8(369):369ra176. doi: 10.1126/scitranslmed.aaj1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig MF, Giles JT, Nigrovic PA, Andrade F. Antibodies to native and citrullinated RA33 (hnRNP A2/B1) challenge citrullination as the inciting principle underlying loss of tolerance in rheumatoid arthritis. Ann Rheum Dis. 2016 Nov;75(11):2022–2028. doi: 10.1136/annrheumdis-2015-208529. [DOI] [PubMed] [Google Scholar]
- Krishnadas R, Nicol A, Sassarini J, Puri N, Burden AD, Leman J, Combet E, Pimlott S, Hadley D, McInnes IB, Cavanagh J. Circulating tumour necrosis factor is highly correlated with brainstem serotonin transporter availability in humans. Brain Behav Immun. 2016 Jan;51:29–38. doi: 10.1016/j.bbi.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy A, Joshua V, Haj Hensvold A, Jin T, Sun M, Vivar N, Ytterberg AJ, Engström M, Fernandes-Cerqueira C, Amara K, Magnusson M, Wigerblad G, Kato J, Jiménez-Andrade JM, Tyson K, Rapecki S, Lundberg K, Catrina SB, Jakobsson PJ, Svensson C, Malmström V, Klareskog L, Wähämaa H, Catrina AI. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann Rheum Dis. 2016 Apr;75(4):721–9. doi: 10.1136/annrheumdis-2015-208093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E, Tsai TF, Wasel N, Tyring S, Salko T, Hampele I, Notter M, Karpov A, Helou S, Papavassilis C ERASURE Study Group; FIXTURE Study Group. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014 Jul 24;371(4):326–38. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- Law SC, Street S, Yu CH, et al. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res Ther. 2012;14:R118. doi: 10.1186/ar3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, Koncz T, Krishnaswami S, Wallenstein GV, Zang C, Zwillich SH, van Vollenhoven RF ORAL Start Investigators. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014 Jun 19;370(25):2377–86. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- Lefevre S, et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nature Medicine. 2009;15:1414–1420. doi: 10.1038/nm.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn-Rasker SP, van der Helm-van Mil AH, van Gaalen FA, et al. Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann Rheum Dis. 2006;65:366. doi: 10.1136/ard.2005.041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubberts E. The IL-23-IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol. 2015 Jul;11(7):415–29. doi: 10.1038/nrrheum.2015.53. [DOI] [PubMed] [Google Scholar]
- Liu X, Zeng B, Zhang J, Li W, Mou F, Wang H, Zou Q, Zhong B, Wu L, Wei H, Fang Y. Role of the Gut Microbiome in Modulating Arthritis Progression in Mice. Sci Rep. 2016;6:30594. doi: 10.1038/srep30594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Longo FJ, Oliver-Miñarro D, de la Torre I, et al. Association between anti-cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61:419. doi: 10.1002/art.24390. [DOI] [PubMed] [Google Scholar]
- Lundström E, Källberg H, Alfredsson L, et al. Gene-environment interaction between the DRB1 shared epitope and smoking in the risk of anti-citrullinated protein antibody-positive rheumatoid arthritis: all alleles are important. Arthritis Rheum. 2009;60:1597. doi: 10.1002/art.24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima K, Stanford SM, Hammaker D, Sacchetti C, Zeng LF, Ai R, Zhang V, Boyle DL, Aleman Muench GR, Feng GS, Whitaker JW, Zhang ZY, Wang W, Bottini N, Firestein GS. Abnormal PTPN11 enhancer methylation promotes rheumatoid arthritis fibroblast-like synoviocyte aggressiveness and joint inflammation. JCI Insight. 2016;1(7) doi: 10.1172/jci.insight.86580. pii: e86580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrygiannakis D, Hermansson M, Ulfgren AK, Nicholas AP, Zendman AJ, Eklund A, Grunewald J, Skold CM, Klareskog L, Catrina AI. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis. 2008;67(10):1488–92. doi: 10.1136/ard.2007.075192. [DOI] [PubMed] [Google Scholar]
- Matsumoto I, Maccioni M, Lee DM, Maurice M, Simmons B, Brenner M, Mathis D, Benoist C. How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nat Immunol. 2002;3(4):360–5. doi: 10.1038/ni772. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Buckley CD, Isaacs JD. Cytokines in rheumatoid arthritis - shaping the immunological landscape. Nat Rev Rheumatol. 2016 Jan;12(1):63–8. doi: 10.1038/nrrheum.2015.171. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, van der Heijde D, Landewé R, Conaghan PG, Gottlieb AB, Richards H, Pricop L, Ligozio G, Patekar M, Mpofu S FUTURE 2 Study Group. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015 Sep 19;386(9999):1137–46. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011 Dec 8;365(23):2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007 Jun;7(6):429–42. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol. 2009 Jun;6(6):410–7. doi: 10.1038/nrcardio.2009.57. [DOI] [PubMed] [Google Scholar]
- Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012 Oct;11(10):763–76. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- Müller-Ladner U, Kriegsmann J, Franklin BN, Matsumoto S, Geiler T, Gay RE, Gay S. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1997;149:1607–15. [PMC free article] [PubMed] [Google Scholar]
- Mydel P, Wang Z, Brisslert M, Hellvard A, Dahlberg LE, Hazen SL, Bokarewa M. Carbamylation-dependent activation of T cells: a novel mechanism in the pathogenesis of autoimmune arthritis. J Immunol. 2010;184:6882–90. doi: 10.4049/jimmunol.1000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamachi Y, Kawano S, Takenokuchi M, et al. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 2009;60:1294. doi: 10.1002/art.24475. [DOI] [PubMed] [Google Scholar]
- Nakano K, Boyle DL, Firestein GS. Regulation of DNA methylation in rheumatoid arthritis synoviocytes. J Immunol. 2013;190:1297–303. doi: 10.4049/jimmunol.1202572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Whitaker JW, Boyle DL, Wang W, Firestein GS. DNA methylome signature in rheumatoid arthritis. Ann Rheum Dis. 2013;72:110–7. doi: 10.1136/annrheumdis-2012-201526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JL, Ramiro S, Gaujoux-Viala C, Takase K, Leon-Garcia M, Emery P, Gossec L, Landewe R, Smolen JS, Buch MH. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2014 Mar;73(3):516–28. doi: 10.1136/annrheumdis-2013-204577. [DOI] [PubMed] [Google Scholar]
- Okada Y, Wu D, Trynka G, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap T, Franz JK, Hummel KM, Jeisy E, Gay R, Gay S. Activation of synovial fibroblasts in rheumatoid arthritis: lack of Expression of the tumour suppressor PTEN at sites of invasive growth and destruction. Arthritis Res. 2000;2:59–64. doi: 10.1186/ar69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman H, Bradley K, Liu H, Cole S, Shamiyeh E, Smith RC, et al. IL-6 and matrix metalloproteinase-1 are regulated by the cyclin-dependent kinase inhibitor p21 in synovial fibroblasts. J Immunol. 2003 Jan 15;170(2):838–45. doi: 10.4049/jimmunol.170.2.838. [DOI] [PubMed] [Google Scholar]
- Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014 Jul;14(7):447–62. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- Randen I, Brown D, Thompson KM, Hughes-Jones N, Pascual V, Victor K, Capra JD, Førre O, Natvig JB. Clonally related IgM rheumatoid factors undergo affinity maturation in the rheumatoid synovial tissue. J Immunol. 1992;148(10):3296–301. [PubMed] [Google Scholar]
- Rawlings DJ, Dai X, Buckner JH. The role of PTPN22 risk variant in the development of autoimmunity: finding common ground between mouse and human. J Immunol. 2015;194(7):2977–84. doi: 10.4049/jimmunol.1403034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri S, Sandor C, Stahl EA, et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet. 2012;44:291. doi: 10.1038/ng.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza K, Falciani F, Curnow SJ, Ross EJ, Lee CY, Akbar AN, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7(4):R784–95. doi: 10.1186/ar1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju A, Engström M, Grunewald J, Nyren S, Eklund A, Klareskog L, Sköld CM, Catrina AI. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol. 2014 Jan;66(1):31–9. doi: 10.1002/art.38201. [DOI] [PubMed] [Google Scholar]
- Reynisdottir G, Olsen H, Joshua V, Engström M, Forsslund H, Karimi R, Sköld CM, Nyren S, Eklund A, Grunewald J, Catrina AI. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann Rheum Dis. 2016;75:1722–7. doi: 10.1136/annrheumdis-2015-208216. [DOI] [PubMed] [Google Scholar]
- Robertson J, Peters MJ, McInnes IB, Sattar N. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat Rev Rheumatol. 2013 Sep;9(9):513–23. doi: 10.1038/nrrheum.2013.91. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Carrio J, Hähnlein JS, Ramwadhdoebe TH, Semmelink JF, Choi IY, van Lienden KP, Maas M, Gerlag DM, Tak PP, Geijtenbeek TB, van Baarsen LG. Altered Innate Lymphoid Cells subsets in human lymph node biopsies during the at risk and earliest phase of rheumatoid arthritis. Arthritis Rheumatol. 2016 Jul 18; doi: 10.1002/art.39811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts Y, Ewing E, van de Stadt LA, Selman MH, Trouw LA, Deelder AM, Huizinga TW, Wuhrer M, van Schaardenburg D, Toes RE, Scherer HU. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis. 2015 Jan;74(1):234–41. doi: 10.1136/annrheumdis-2013-203565. [DOI] [PubMed] [Google Scholar]
- Rossetti M, Spreafico R, Consolaro A, Leong JY, Chua C, Massa M, Saidin S, Magni-Manzoni S, Arkachaisri T, Wallace CA, Gattorno M, Martini A, Lovell DJ, Albani S. TCR repertoire sequencing identifies synovial Treg cell clonotypes in the bloodstream during active inflammation in human arthritis. Ann Rheum Dis. 2016 Jun 16; doi: 10.1136/annrheumdis-2015-208992. pii: annrheumdis-2015–208992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scally SW, Petersen J, Law SC, Dudek NL, Nel HJ, Loh KL, Wijeyewickrema LC, Eckle SB, van Heemst J, Pike RN, McCluskey J, Toes RE, La Gruta NL, Purcell AW, Reid HH, Thomas R, Rossjohn J. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J Exp Med. 2013;210(12):2569–82. doi: 10.1084/jem.20131241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Knevel R, Suwannalai P, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci U S A. 2011;108:17372–7. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, van de Stadt LA, Levarht EW, et al. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann Rheum Dis. 2014;73:780–3. doi: 10.1136/annrheumdis-2013-204154. [DOI] [PubMed] [Google Scholar]
- Schiff M, Weinblatt ME, Valente R, van der Heijde D, Citera G, Elegbe A, Maldonado M, Fleischmann R. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis. 2014 Jan;73(1):86–94. doi: 10.1136/annrheumdis-2013-203843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol. 2015 May;11(5):276–89. doi: 10.1038/nrrheum.2015.8. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016 Oct 22;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- Snir O, Rieck M, Gebe JA, Yue BB, Rawlings CA, Nepom G, Malmström V, Buckner JH. Identification and functional characterization of T cells reactive to citrullinated vimentin in HLA-DRB1*0401-positive humanized mice and rheumatoid arthritis patients. Arthritis Rheum. 2011 Oct;63(10):2873–83. doi: 10.1002/art.30445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fc γ receptor. Arthritis Rheum. 2011 Jan;63(1):53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk J, Ospelt C, Karouzakis E, et al. Altered expression of microRNA-203 in rheumatoid arthritis synovial fibroblasts and its role in fibroblast activation. Arthritis Rheum. 2011;63(2):373–381. doi: 10.1002/art.30115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Yamada R, Chang X, et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet. 2003;34:395. doi: 10.1038/ng1206. [DOI] [PubMed] [Google Scholar]
- Tan EM, Smolen JS. Historical observations contributing insights on etiopathogenesis of rheumatoid arthritis and role of rheumatoid factor. J Exp Med. 2016 Sep 19;213(10):1937–50. doi: 10.1084/jem.20160792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CN, Davis MJ, Tesmer LA, Endres JL, Motyl CD, Smuda C, Somers EC, Chung KC, Urquhart AG, Lundy SK, Kovats S, Fox DA. Presentation of arthritogenic peptide to antigen-specific T cells by fibroblast-like synoviocytes. Arthritis Rheum. 2007 May;56(5):1497–506. doi: 10.1002/art.22573. [DOI] [PubMed] [Google Scholar]
- Tsaprouni LG, Yang TP, Bell J, Dick KJ, Kanoni S, Nisbet J, Viñuela A, Grundberg E, Nelson CP, Meduri E, Buil A, Cambien F, Hengstenberg C, Erdmann J, Schunkert H, Goodall AH, Ouwehand WH, Dermitzakis E, Spector TD, Samani NJ, Deloukas P. Cigarette smoking reduces DNA methylation levels at multiple genomic loci but the effect is partially reversible upon cessation. Epigenetics. 2014 Oct;9(10):1382–96. doi: 10.4161/15592294.2014.969637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helm-van Mil AH, Verpoort KN, Breedveld FC, et al. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 2006;54:1117. doi: 10.1002/art.21739. [DOI] [PubMed] [Google Scholar]
- Vassallo R, Luckey D, Behrens M, Madden B, Luthra H, David C, Taneja V. Cellular and humoral immunity in arthritis are profoundly influenced by the interaction between cigarette smoke effects and host HLA-DR and DQ genes. Clin Immunol. 2014;152(1–2):25–35. doi: 10.1016/j.clim.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shaked I, Stanford SM, Zhou W, Curtsinger JM, Mikulski Z, Shaheen ZR, Cheng G, Sawatzke K, Campbell AM, Auger JL, Bilgic H, Shoyama FM, Schmeling DO, Balfour HH, Jr, Hasegawa K, Chan AC, Corbett JA, Binstadt BA, Mescher MF, Ley K, Bottini N, Peterson EJ. The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type 1 interferon-dependent immunity. Immunity. 2013;39(1):111–22. doi: 10.1016/j.immuni.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K, Kinloch A, Culshaw S, Potempa J, Venables PJ. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010 Sep;62(9):2662–72. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand CM, Hicok KC, Conn DL, Goronzy JJ. The influence of HLA-DRB1 genes on disease severity in rheumatoid arthritis. Ann Intern Med. 1992;117:801. doi: 10.7326/0003-4819-117-10-801. [DOI] [PubMed] [Google Scholar]
- van Beers JJ, Willemze A, Jansen JJ, Engbers GH, Salden M, Raats J, Drijfhout JW, van der Helm-van Mil AH, Toes RE, Pruijn GJ. ACPA fine-specificity profiles in early rheumatoid arthritis patients do not correlate with clinical features at baseline or with disease progression. Arthritis Res Ther. 2013 Oct 1;15(5):R140. doi: 10.1186/ar4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Okada Y, Suzuki A, Kochi Y. Genetics of rheumatoid arthritis in Asia--present and future. Nat Rev Rheumatol. 2015;11(6):375–9. doi: 10.1038/nrrheum.2015.7. [DOI] [PubMed] [Google Scholar]
- Yang Z, Fujii H, Mohan SV, Goronzy J, Weyand CM. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med. 2013 Sep 23;210(10):2119–34. doi: 10.1084/jem.20130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Shen Y, Oishi H, Matteson EL, Tian L, Goronzy JJ, Weyand CM. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci Transl Med. 2016 Mar 23;8(331):331ra38. doi: 10.1126/scitranslmed.aad7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, Wu X, Li J, Tang L, Li Y, Lan Z, Chen B, Li Y, Zhong H, Xie H, Jie Z, Chen W, Tang S, Xu X, Wang X, Cai X, Liu S, Xia Y, Li J, Qiao X, Al-Aama JY, Chen H, Wang L, Wu QJ, Zhang F, Zheng W, Li Y, Zhang M, Luo G, Xue W, Xiao L, Li J, Chen W, Xu X, Yin Y, Yang H, Wang J, Kristiansen K, Liu L, Li T, Huang Q, Li Y, Wang J. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015 Aug;21(8):895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- Zvaifler NJ, Boyle D, Firestein GS. Early synovitis--synoviocytes and mononuclear cells. Semin Arthritis Rheum. 1994 Jun;23(6 Suppl 2):11–6. doi: 10.1016/0049-0172(94)90080-9. [DOI] [PubMed] [Google Scholar]