Abstract
Objective:
To assess the results of an initial round of supplemental screening with hand-held bilateral breast ultrasound following a negative screening mammogram in asymptomatic women with dense breast tissue who are not at high risk for breast cancer.
Materials and Methods:
A retrospective, Health Insurance Portability and Accountability Act compliant, Institutional Research Board approved study was performed at a single academic tertiary breast center. Informed consent was waived. A systematic review of the breast imaging center database was conducted to identify and retrieve data for all asymptomatic women, who were found to have heterogeneously dense or extremely dense breast tissue on screening bilateral mammograms performed from July 1, 2010 through June 30, 2012 and who received a mammographic final assessment American College of Radiology's (ACR) Breast Imaging Reporting and Data System (BI-RADS) category 1 or BI-RADS category 2. Hand-held screening ultrasound was performed initially by a technologist followed by a radiologist. Chi-square and t-test were used and statistical significance was considered at P < 0.05.
Results:
A total of 1210 women were identified. Of these, 394 underwent the offered supplemental screening ultrasound. BI-RADS category 1 or 2 was assigned to 323 women (81.9%). BI-RADS category 3 was assigned to 50 women (12.9%). A total of 26 biopsies/aspirations were recommended and performed in 26 women (6.6%). The most common finding for which biopsy was recommended was a solid mass (88.5%) with an average size of 0.9 cm (0.5–1.7 cm). Most frequent pathology result was fibroadenoma (60.8%). No carcinoma was found.
Conclusion:
Our data support the reported occurrence of a relatively high number of false positives at supplemental screening with breast ultrasound following a negative screening mammogram in asymptomatic women with dense breast tissue, who are not at a high risk of developing breast cancer, and suggests that caution is necessary in establishing wide implementation of this type of supplemental screening for all women with dense breast tissue without considering other risk factors for breast cancer.
Keywords: Breast ultrasound, dense breasts, screening
Introduction
The sensitivity of screening mammography is decreased by the presence of dense breast tissue, as defined by the American College of Radiology's (ACR) Breast Imaging Reporting and Data System (BI-RADS).[1,2,3] In addition, some studies indicate that dense breast tissue increases breast cancer risk.[4,5] Published studies on hand-held breast ultrasound as a supplemental test to screening mammography in women with dense breast tissue report an incremental cancer detection rate of approximately 2–4/1000 examined women.[6,7,8,9,10,11,12,13,14,15,16,17,18] Breast cancers detected by supplemental ultrasound have been reported to be small invasive cancers, with a high proportion of node-negative cases.[6,7,8,9,10,11,12,13,14,15,16,17,18] However, these studies have important differences in methodology, including varied inclusion criteria and varied qualification of ultrasound performers.[6,7,8,9,10,11,12,13,14,15,16,17,18]
Recently, and mainly as a result of efforts by grassroots advocacy groups,[19] several states in the United States (US) have enacted legislation requiring that, following screening mammography, all women with dense breasts be informed of their breast tissue density and that supplemental screening tests, such as breast ultrasound, should be discussed with them by their providers.[19] In the US, this would entail supplemental screening of more than 40% of women over 40 years of age.[20]
The purpose of our study was to retrospectively assess the results of an initial round of supplemental screening with hand-held bilateral breast ultrasound performed consecutively by a technologist and a radiologist following a negative bilateral screening mammogram in asymptomatic women with dense breast tissue who were not at a high risk of breast cancer, as defined by the American College of Radiology (ACR) and the Society of Breast Imaging (SBI).[21]
Materials and Methods
A retrospective, Health Insurance Portability and Accountability Act (HIPAA)-compliant and Institutional Research Board (IRB)-approved study was performed. The need for informed consent was waived.
Study period and participants
A systematic review of the breast imaging center's database was performed to identify all asymptomatic women who were reported to have heterogeneously dense [Figure 1A and B] or extremely dense [Figure 2A and B] breast tissue, as defined by the BI-RADS Atlas,[1] on screening bilateral mammogram performed from July 1, 2010 through June 30, 2012 and who received a final assessment BIRADS category 1, negative or BIRADS category 2, benign.
Figure 1 (A and B).

(A) Bilateral Mammogram Cranio Caudal view-Heterogeneously dense breasts (B) Bilateral Mammogram Medio Lateral Oblique view-Heterogeneously dense breasts
Figure 2 (A and B).
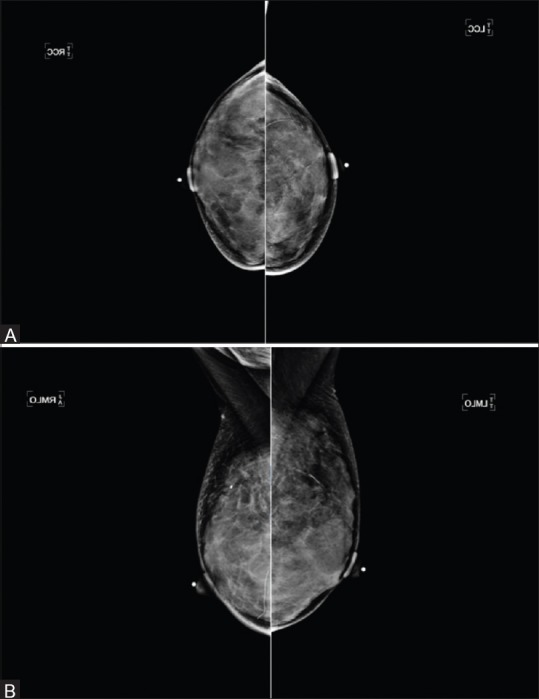
(A) Bilateral Mammogram Cranio Caudal view-Extremely dense breasts (B) Bilateral Mammogram Medio Lateral Oblique view-Extremely dense breasts
At the time of the study, the breast imaging center was initiating a policy by which a paragraph was added to the radiologist's mammogram report to the referring physician of women meeting the above criteria, stating “Given the dense breast tissue, which may lower the sensitivity of mammography, supplemental screening with breast ultrasound is offered.”
At our facility, women at high risk for breast cancer (20–25% or greater lifetime risk of breast cancer), as defined by the American Cancer Society,[10] are recommended to undergo supplemental screening with breast magnetic resonance imaging (MRI), and thus were not included in this study. Further, not included were women with a personal history of breast cancer, who at our institution undergo diagnostic, not screening mammography, for life.
Data collection
The following data was retrieved for all women included in the study: Age, race, family history of breast cancer, personal history of breast biopsy, personal history of biopsy-proven high risk lesion of the breast, age at menarche, age at menopause, parity, age of first pregnancy, and use of hormones (hormonal contraceptives and hormone replacement therapy).
In addition, for women who received the offered breast ultrasound, the following data was also retrieved based on the BI-RADS Atlas:[1] final assessment BI-RADS category of the breast ultrasound exam; if biopsy was recommended, descriptors of ultrasound findings (solid vs cystic, measurement of longest axis in cm, shape, margins, echogenicity, posterior acoustic features, orientation); and pathology results if biopsy was performed. Further, if the final assessment category of the initial screening breast ultrasound was BI-RADS 3, the results of the first two consecutive short-term follow-up ultrasounds were recorded. An interval follow-up increase in size of a mass by more than 20% in the longest axis was considered to warrant biopsy. The reference standard was biopsy result and mammogram at 12 months.
Breast imaging studies and interpretation
Every screening bilateral mammogram was obtained as 2D digital study on a Selenia®- Hologic® unit and was performed by one of six mammography technologists, all of whom are certified in mammography (registered by the American Registry of Radiologic Technologists), with an experience of 17, 11, 6, 6, 5, and 4 years, and was interpreted by one of the four board certified breast imagers, with 30, 11, 3, and 2 years of experience in breast imaging.
Every supplemental bilateral breast ultrasound examination was obtained less than six months from the screening mammogram and was obtained on a dedicated breast ultrasound unit (GE LOGIC E9) with a high-resolution linear-array transducer (6–15 MHz) by one of the six technologists, two of whom are RDMS certified (Registered Diagnostic Medical Sonographer) and one of whom is ARRT certified (American Registry of Radiologic Technologists). Two of them with experience in breast sonography for 10 years each, one with 6 years, two with 5 years of experience each, and one with 1-year experience.
The breast ultrasound was performed in a standardized hand-held manner with overlapping scans in the radial and antiradial planes, extending from the nipple to the posterior breast tissue. If no abnormal findings were identified, images were documented in the 12-, 3-, 6-, and 9-o’clock positions, as well as in the retroareolar region. If any finding was present, images of each finding were obtained and measured in three dimensions.
Immediately after the breast ultrasound exam by the technologist, the exam was repeated by one of the four board-certified dedicated breast imaging radiologists. The same breast imaging radiologist interpreted the mammogram and ultrasound for each patient. The breast imager performed a repeat complete ultrasound scan of the breasts in real time, regardless of whether or not the technologist identified any abnormality and was not blinded to the results of the screening mammogram and the patient's history and was able to review the breast ultrasound images obtained by the technologist.
Ultrasound-guided procedures
All ultrasound-guided breast biopsies were performed either with a 14-gauge automated core biopsy needle (Achieve, Cardinal Health, Dublin, Ohio), or a 9 or 12-gauge vacuum-assisted core biopsy needle (Atec, Hologic, Bedford, MA). All cyst aspirations were performed with an 18 Gauge needle.
Statistical analysis
The Chi-square test was used for discrete data and the t-test for continuous data. Statistically significant difference was considered at P < 0.05. The statistical analysis was performed using MedCalc Statistical Software, Version 12.3.0 - © 1993-2012 MedCalc Software bvba - MedCalc Software, Broekstraat 52, 9030 Mariakerke, Belgium.
Results
During the study period, a total of 2469 asymptomatic women not at high risk of breast cancer and without a personal history of breast cancer and who received a final assessment BI-RADS category 1, negative, or BI-RADS category 2, benign, at screening bilateral mammogram were evaluated. Of these, 1210 (49%) were found to have heterogeneously dense or extremely dense breast tissue. Of these, 394 (32.5%) women underwent the offered supplemental screening bilateral breast ultrasound.
At the initial round of supplemental screening breast ultrasound, 323 women (81.9%) received a final assessment BI-RADS category 1, negative [Figure 3], or BI-RADS category 2, benign [Figure 4A and B] and were recommended to undergo routine yearly screening; whereas 50 women (12.9%) received a final assessment BI-RADS category 3, probably benign [Figure 5A–C] and were recommended to undergo short-term follow-up with breast ultrasound in 6 months [Table 1]. Two of the women who received a BI-RADS category 3 requested biopsy, which was performed. A BI-RADS category 4, suspicious [Figures 6A–C and 7A and B], with recommendation for biopsy was assigned to 19 women (4.8%) [Table 1]. A total of 21 women (5.3%) underwent an ultrasound-guided procedure as a result of the initial round of supplemental screening bilateral breast ultrasound. No BI-RADS category 5, highly suspicious, was assigned.
Figure 3.
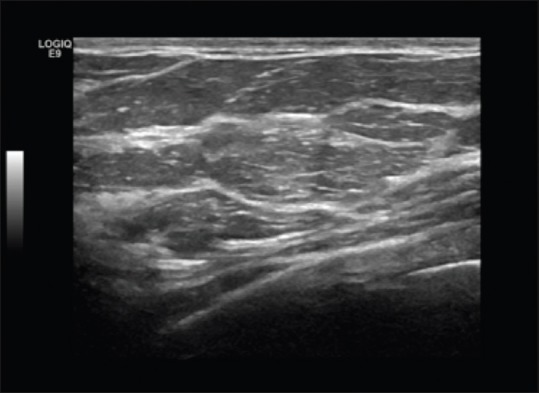
Bilateral screening breast ultrasound-Category BIRADS 1. Negative
Figure 4 (A and B).
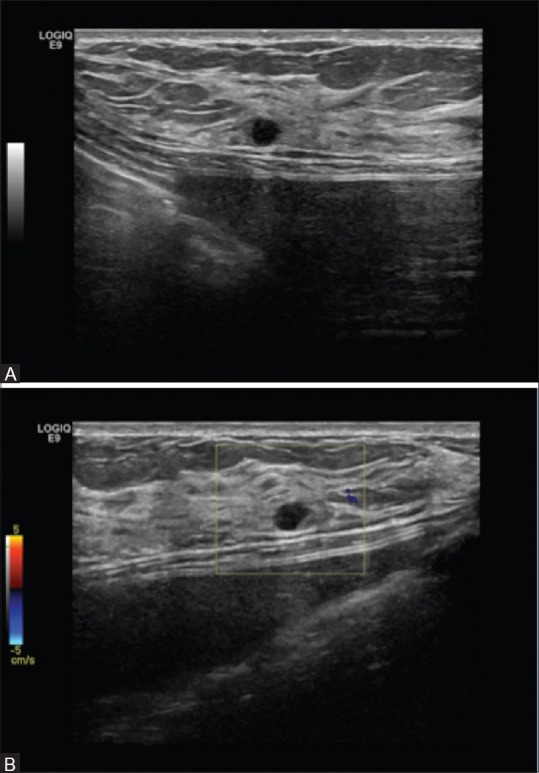
(A) Bilateral screening breast ultrasound showing a simple cyst in a patient with breast implants-Category BIRADS 2. Benign (B) Bilateral screening breast ultrasound showing a simple cyst with color doppler in the same patient with breast implants- Category BIRADS 2. Benign
Figure 5 (A-C).
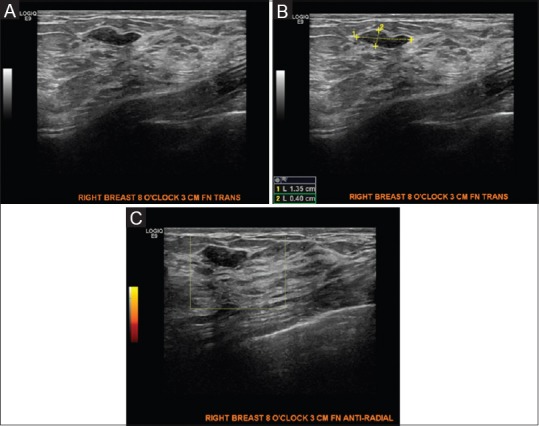
(A) Bilateral screening breast ultrasound showing a benign appearing mass, likely a fibroadenoma for which a six month follow up ultrasound was recommended- Category BIRADS 3. Probably Benign (B) Bilateral screening breast ultrasound showing a benign appearing mass, likely a fibroadenoma for which a six month follow up ultrasound was recommended- Category BIRADS 3. Probably Benign (C) Bilateral screening breast ultrasound showing a benign appearing mass, likely a fibroadenoma with color doppler for which a six month follow up ultrasound was recommended- Category BIRADS 3. Probably Benign
Table 1.
BI-RADS category at initial round of supplemental screening breast ultrasound and at recommended subsequent short-term follow-ups
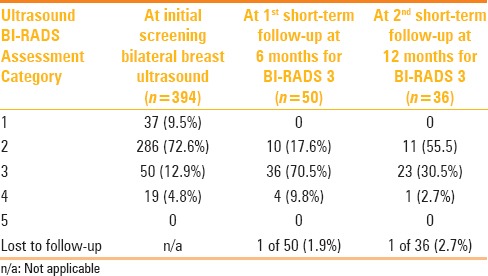
Figure 6 (A-C).
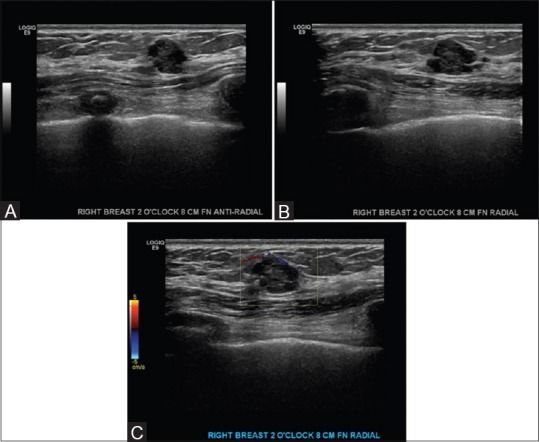
(A) Bilateral screening breast ultrasound showing a round, heterogenous mass with mixed solid and cystic components and irregular margins. Anti radial plane. Biopsy was recommended and performed. Results showed benign papilloma- Category BIRADS 4. Suspicious (B) Bilateral screening breast ultrasound showing a round, heterogenous mass with mixed solid and cystic components and irregular margins. Radial plane. Biopsy was recommended and performed. Results showed benign papilloma- Category BIRADS 4. Suspicious (C) Bilateral screening breast ultrasound showing a round, heterogenous mass with mixed solid and cystic components and irregular margins. With color doppler. Biopsy was recommended and performed. Results showed benign papilloma- Category BIRADS 4. Suspicious
Figure 7 (A and B).
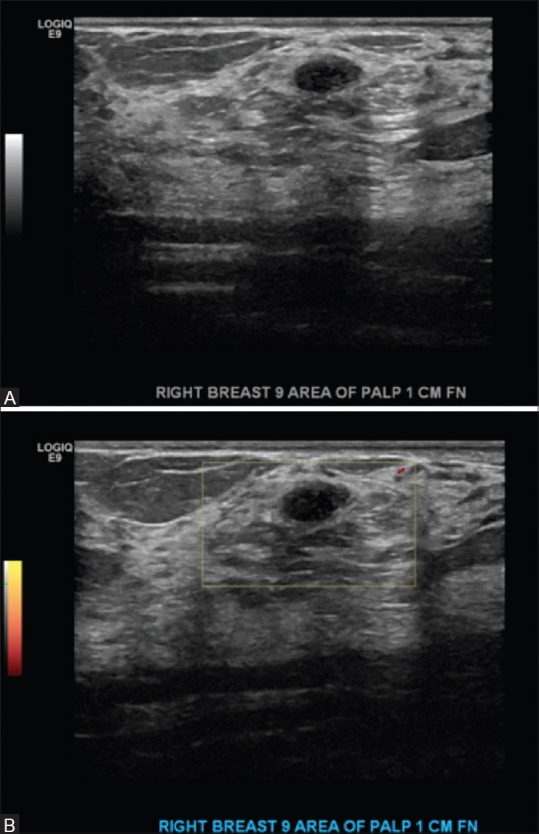
(A) Bilateral screening breast ultrasound showing a palpable mass in the right breast 9:00 axis. Biopsy was performed because it was palpable. Results showed fibroadenoma- Category BIRADS 4. Suspicious (B) Bilateral screening breast ultrasound showing a palpable mass in the right breast 9:00 axis with color doppler. Biopsy was performed because it was palpable. Results showed fibroadenoma- Category BIRADS 4. Suspicious
As a result of the first two consecutive short-term follow-ups with breast ultrasound recommended to patients who were assigned a BI-RADS category 3, five more biopsies were recommended and performed; four biopsy recommendations were generated at the first short-term follow-up cycle and one at the second short-term follow-up cycle [Table 1]. All of them were due to interval increase in size of the initial finding. Overall, a total of 26 women (6.6%) were recommended a biopsy, which was performed in all of them.
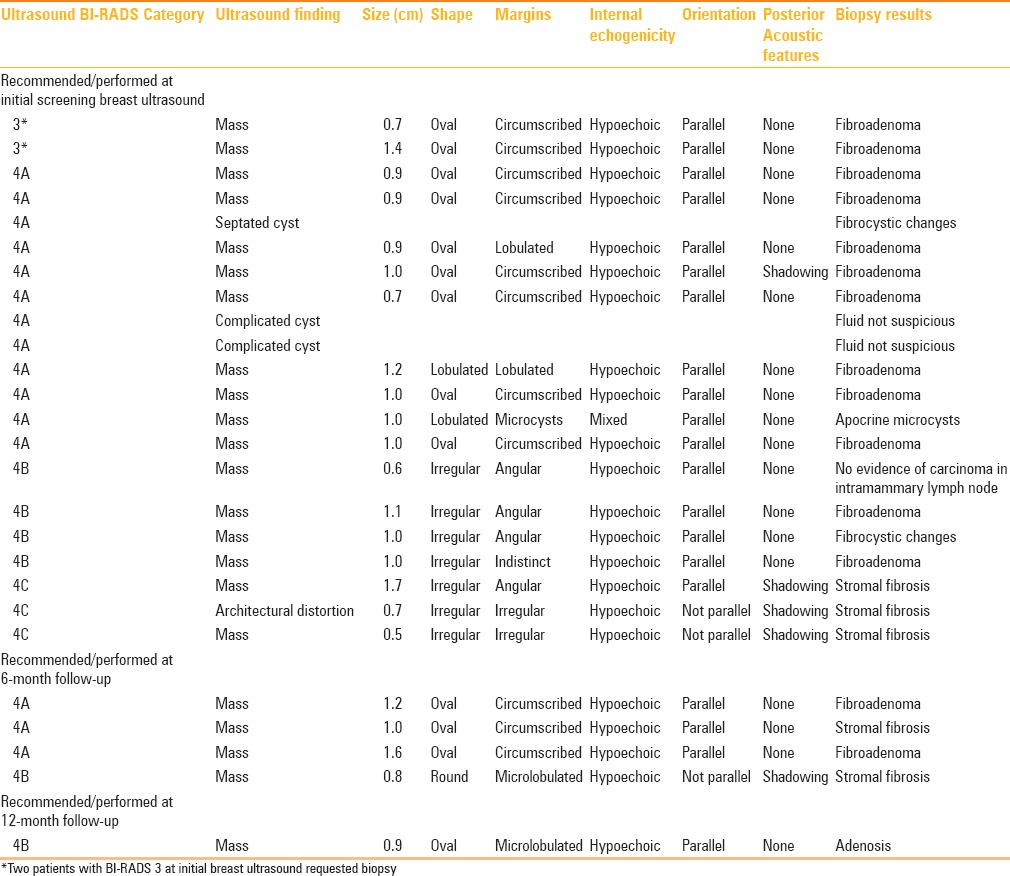
The most common ultrasound finding for which biopsy was recommended and performed was a solid mass (88.5%) with an average size of 0.9 cm (range: 0.5–1.7 cm) [Table 2]. The most frequent pathology result was fibroadenoma (60.8%). Fine needle aspiration of a complicated cyst in two patients (11.5%) was performed and resulted in complete resolution of the cyst with nonsuspicious fluid [Table 2]. No carcinoma was found at biopsy. Moreover, no interval carcinoma was found at 12-month follow-up mammogram.
Table 2.
False positive results by ultrasound BI-RADS category, descriptors and biopsy results
Discussion
Our study includes only asymptomatic women with dense breast tissue who are not at high risk for breast cancer, which represent the majority of women with dense breast tissue who undergo screening mammography for breast cancer. Some prior studies on supplemental screening with breast ultrasound for dense breast tissue have only included women at a high risk for breast cancer, whereas others have included symptomatic women and unilateral breast ultrasound obtained in women with known mammographic abnormalities in the contralateral breast or even in a different quadrant of the ipsilateral breast.[6,7,8,9,10,11,12,13,14,15,16,17,18] Moreover, it has been pointed out that there may be methodological flaws in the numerous studies which have previously suggested a link between breast density and the risk of breast cancer. This may be in part because of the problem of trying to extract 3D information from 2D images as stated by Kopans.[22]
Prior studies on this topic have differences in the qualification of the ultrasound exam performers, with some performed only by the radiologist, some only by the technologist, and some by both.[6,7,8,9,10,11,12,13,14,15,16,17,18] Performing the supplemental hand-held screening breast ultrasound consecutively by two performers, first by a technologist and then by the interpreting radiologist, as in our study, likely serves to elucidate most mammographically occult findings; however, it is time consuming and represents a burden on the already limited healthcare resources in a busy clinical practice.
An important methodological similarity between our study and some of the prior studies is using the biopsy results and the results of a 1-year follow-up as a reference standard to assess for false negative results, including the occurrence of interval cancers.[10,11,12,13]
Participants in our study were slightly younger (mean age of 47.3 years) as compared to those in other studies with a mean age of participants ranging from 51.2 to 55.2 years.[10,12,16,17,18]
Our results have several similarities to the results of previous studies, including the proportion of subjects with BI-RADS categories 1, 2, and 3, the biopsy rate, and that fibroadenoma and stromal fibrosis accounted for most pathology findings.[6,9] Moreover, in concurrence with prior studies was the small number of the ultrasound findings that required further evaluation with biopsy, which ultimately is in concordance with the expected small number of the mammographically occult abnormalities that were found with supplemental breast ultrasound.[6,7,8,9,10,11,12,13,14,15,16,17,18]
Limitations of our study include a small population size, which is likely responsible for the fact that no carcinoma was found. There is only one published study in which the population size is smaller than ours and in which additional breast carcinoma was found with supplemental ultrasound. However, unlike our study, that study included participants with a personal history of breast cancer.[13]
Conclusion
In conclusion, our results confirm some of the reported disadvantages of performing supplemental screening with breast ultrasound, in particular the high false-positive rate and a relatively high rate of short interval follow-up, and support the expressed opinions that caution should be exercised when recommending supplemental screening with hand-held bilateral breast ultrasound for all asymptomatic women with dense breast tissue without taking into account other risk factors, the expected large number of women who would undergo this additional test, and the added costs to the health care system.[23,24,25,26,27]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to acknowledge medical students Mohammed Ezuddin, Jessica Schmidtman and Rachel Franklyn for their help with data collection.
References
- 1.Breast Imaging Reporting and Data System. 4th ed. Reston: American College of Radiology; 2003. [Google Scholar]
- 2.Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168–75. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 3.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 4.Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: Development and validation of a new predictive model. Ann Intern Med. 2008;148:337–47. doi: 10.7326/0003-4819-148-5-200803040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, et al. Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–83. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan SS. Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology. 2001;221:641–9. doi: 10.1148/radiol.2213010364. [DOI] [PubMed] [Google Scholar]
- 7.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and US and evaluation of factors that influence them: An analysis of 27,825 patient evaluations. Radiology. 2002;225:165–75. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 8.Crystal P, Strano SD, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. AJR Am J Roentgenol. 2003;181:177–82. doi: 10.2214/ajr.181.1.1810177. [DOI] [PubMed] [Google Scholar]
- 9.Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151–63. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394–404. doi: 10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corsetti V, Houssami N, Ghirardi M, Ferrari A, Speziani M, Bellarosa S, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: Interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47:1021–6. doi: 10.1016/j.ejca.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: Initial experience with Connecticut Public Act 09-41. Radiology. 2012;265:59–69. doi: 10.1148/radiol.12120621. [DOI] [PubMed] [Google Scholar]
- 13.Leong LC, Gogna A, Pant R, Ng FC, Sim LS. Supplementary breast ultrasound screening in Asian women with negative but dense mammograms-A pilot study. Ann Acad Med Singapore. 2012;41:432–9. [PubMed] [Google Scholar]
- 14.Youk JH, Kim EK, Kim MJ, Kwak JY, Son EJ. Performance of hand-held whole-breast ultrasound based on BI-RADS in women with mammographically negative dense breast. Eur Radiol. 2011;21:667–75. doi: 10.1007/s00330-010-1955-8. [DOI] [PubMed] [Google Scholar]
- 15.Brancato B, Bonardi R, Catarzi S, Iacconi C, Risso G, Taschini R, et al. Negligible advantages and excess costs of routine addition of breast ultrasonography to mammography in dense breasts. Tumori. 2007;93:562–6. doi: 10.1177/030089160709300608. [DOI] [PubMed] [Google Scholar]
- 16.Girardi V, Tonegutti M, Ciatto S, Bonetti F. Breast ultrasound in 22,131 asymptomatic women with negative mammography. Breast. 2013;22:806–9. doi: 10.1016/j.breast.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Parris T, Wakefield D, Frimmer H. Real world performance of screening breast ultrasound following enactment of Connecticut Bill 458. Breast J. 2013;19:64–70. doi: 10.1111/tbj.12053. [DOI] [PubMed] [Google Scholar]
- 18.Weigert J, Steenbergen S. The Connecticut experiment: The role of ultrasound in the screening of women with dense breasts. Breast J. 2012;18:517–22. doi: 10.1111/tbj.12003. [DOI] [PubMed] [Google Scholar]
- 19.Are you Dense? Advocacy. D.E.N.S.E. ® State Efforts. 2014. [Last accessed on 2015 Aug 30]. Available from: http://www.areyoudenseadvocacy.org/dense/
- 20.Sprague BL, Gangnon RE, Burt V, Trentham-Dietz A, Hampton JM, Wellman RD, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American College of Radiology. ACR Appropriateness Criteria®. Breast cancer screening. 2012. [Last accessed on 2015 Aug 30]. Available from: https://acsearch.acr.org/docs/70910/Narrative/
- 22.Kopans DB. Basic physics and doubts about relationship between mammographically determined tissue density and breast cancer risk. Radiology. 2008;246:348–53. doi: 10.1148/radiol.2461070309. [DOI] [PubMed] [Google Scholar]
- 23.Lee CH. Radiologic Screening for Breast Cancer: Current Controversies. Curr Radiol Rep. 2014;2:34. [Google Scholar]
- 24.Position Statements. ACR Statement on Reporting Breast Density in Mammography Reports and Patient Summaries. April 24. 2012. [Last accessed on 2015 Aug 21]. Available from: http://www.acr.org/About-Us/Media-Center/Position-Statements/Position-Statements-Folder/Statement-on-Reporting-Breast-Density-in-Mammography-Reports-and-Patient-Summaries .
- 25.Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, et al. International Agency for Research on Cancer Handbook Working Group. Breast-cancer screening--viewpoint of the IARC Working Group. N Engl J Med. 2015;372:2353–8. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- 26.Sprague BL, Stout NK, Schechter C, van Ravesteyn NT, Cevik M, Alagoz O, et al. Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162:157–66. doi: 10.7326/M14-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerlikowske K, Zhu W, Tosteson AN, Sprague BL, Tice JA, Lehman CD, et al. Breast Cancer Surveillance Consortium. Identifying women with dense breasts at high risk for interval cancer: A cohort study. Ann Intern Med. 2015;162:673–81. doi: 10.7326/M14-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]


