Abstract
Epicardial fat is a unique adipose tissue located between the myocardium and the visceral layer of pericardium. This tissue is characterized by highly active fatty acid metabolism and highly expressed thermogenic genes. Epicardial fat and the underlying myocardium share the same microcirculation, suggesting a close and strong interaction between these two structures. Under physiological conditions, epicardial fat protects and supports the heart to exert its normal function. Many clinical studies have shown significant associations between increased amounts of epicardial fat and coronary artery disease (CAD). In patients with CAD, increased epicardial fat becomes inflammatory and may promote plaque development through secretion of proinflammatory cytokines and other mechanisms. Therefore, epicardial fat is a biomarker of cardiovascular risk and a potential therapeutic target for cardiovascular disease. Weight loss and pharmaceuticals can reduce epicardial fat and improve its protective physiological functions.
Keywords: epicardial fat, adipokine, inflammation, myocardium, coronary artery disease
Introduction: Location and Morphology of Epicardial Fat
Epicardial fat is defined as visceral fat that deposits within the heart.1 In the embryo stage, epicardial fat develops from brown adipose tissue. In adults, the macroscopic anatomy of epicardial adipose shows that it is situated between the myocardium and visceral layer of pericardium and commonly exists in the atrioventricular and interventricular grooves, whereas in juveniles it is found around the atrial free wall and two atrial appendages.1,2 Pericardial fat is located outside the visceral pericardium and on the external surface of the parietal pericardium. Although close in proximity, these two fat depots are very different (Table 1).3 Epicardial fat is vascularized by branches of the coronary arteries, while pericardial fat is vascularized by noncoronary arteries. Epicardial fat originates from splanchnopleuric mesoderm, while pericardial adipose is derived from the primitive thoracic mesenchyme. Notably, epicardial adipose has no fascia layer separating it from the underlying myocardium. This means these two essential components of the heart share the same microcirculation, suggesting a close and strong interaction between the two structures.3 Massive increases of epicardial fat could fill up the space between the visceral pericardium and myocardium surface and even cover the whole epicardium surface. Small amounts of epicardial fat can also grow inside myocardium and usually accompany the intramyocardial branch of coronary artery.4
Table 1.
Differences between epicardial and pericardial fat.

Physiological Functions of Epicardial Fat
The functional complexity of human epicardial fat is not fully elucidated. However, the role of epicardial fat within the heart can be generally distinguished by mechanical, metabolic, thermogenic, and endocrine/paracrine functions.
Mechanical Functions
Since epicardial fat commonly accompanies the main branches of coronary artery and is located in the atrioventricular or inter-ventricular groove as a cushion, it has the compressibility and elasticity that can mechanically protect the coronary artery against excessive distortion caused by artery pulse and myocardial contraction.5
Metabolic Functions
Epicardial fat has a higher rate of free fatty acid (FFA) release and uptake compared to subcutaneous and other visceral fat depots.6 It is well known that energy production in the heart is highly dependent on FFA oxidation. Epicardial fat is rich in saturated fatty acids, and this enrichment and increased metabolism of FFAs in epicardial fat sustains myocardium energy supplies, especially during periods of high demand. FFAs in epicardial fat might diffuse through the interstitial fluid into the myocardium. They also flow into the coronary artery bloodstream and are delivered to myocardium. Although epicardial fat was thought to be an energy supplier for myocardium, the exact degree of its contribution is unknown.
Thermogenic Functions
Brown adipose tissue contains mitochondria with large amounts of uncoupling protein-1 (UCP1) to generate heat in response to cold exposure. Sacks et al.7 found that expressions of UCP1 and its related genes are higher in epicardial fat than fat depots in other parts of the body, such as the abdomen, thighs, and subcutaneous tissue. These results suggested that epicardial fat could function similarly to brown adipose tissue, producing heat to protect the myocardium and coronary artery from hypothermia damage.
Protective Secreting Factors
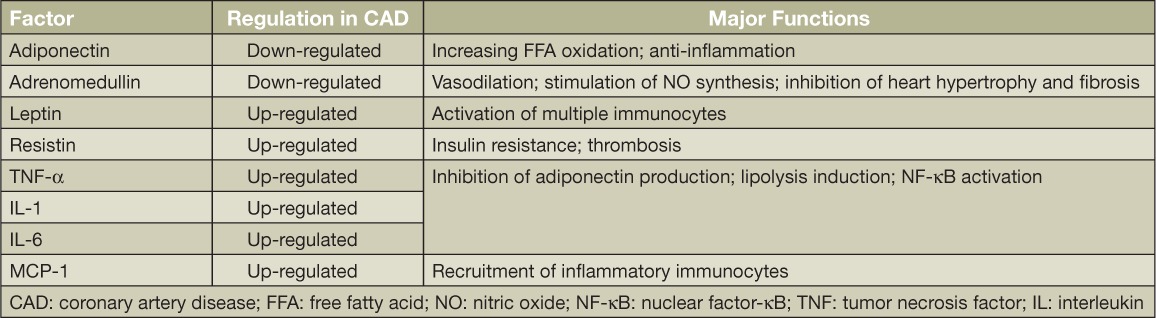
Growing evidence demonstrates that human epicardial fat can produce multiple bioactive cytokines. The secretomes are so multitudinous that they make epicardial fat uniquely different from other fat depots. These cytokines are involved in the regulation of endothelial function, coagulation, and inflammation. In fact, a number of bioactive molecules secreted from epicardial fat can either protect or adversely affect the myocardium and coronary arteries (Table 2).1 Under normal physiological conditions, epicardial adipose produces anti-inflammation or antiatherosclerotic cytokines, such as adiponectin and adrenomedullin, to exert its cardioprotective functions.8,9 Adiponectin is a secreting factor produced only by adipocytes and has been described as having antidiabetic, antiatherogenic, antioxidative, and anti-inflammatory properties.8 Adiponectin activates the adenosine monophosphate-activated protein kinase (AMPK) pathway, leading to increased fatty acid oxidation and reduced lipid deposits in cardiac muscle.9 In addition, adiponectin inhibits production of inflammatory factors and maintains an anti-inflammatory microenvironment in the cardiovascular system.10 Unlike adiponectin, adrenomedullin is produced in a variety of organs including the kidneys, lungs, and heart, and it exerts many cardioprotective actions including vasodilation, natriuresis, antiapoptosis, and stimulation of nitric oxide production.11 In disease conditions, epicardial fat can be harmful, producing inflammatory factors that are discussed later in this review.
Table 2.
Major bioactive secreting factors released from epicardial fat.
Clinical Implication of Epicardial Fat
Epicardial Fat Measurement
Detection and quantification of epicardial fat require a variety of useful imaging techniques, including 2-dimensional (2D) echocardiography, magnetic resonance imaging (MRI), and non-contrast computed tomography (CT).12,13 Though the epicardial fat volume is superior to thickness as a clinical index, it is technically more difficult to measure. The thickness of epicardial fat tissue can be visualized and calculated with standard 2D echocardiographic methods that are noninvasive and reliable. Epicardial fat is generally identified as the echo-free space between the outer wall of the myocardium and the visceral layer of pericardium, and its thickness is measured between the epicardial surface and the parietal pericardium in at least two locations on the right ventricular free wall. There is some controversy over which stage in the cardiac cycle is most suitable for echocardiographic measurement. Some recommend that measurement be taken during systole to prevent possible deformation by epicardial fat compression during diastole, while others suggest measurement in diastole to coincide with other imaging modalities (CT and MRI).12,14,15
Since fat tissue generates a strong MRI signal, both thickness and volume of epicardial fat can be easily measured with this modality. At the end of diastole, the epicardial fat is contoured in each short axis; therefore, it is in this stage that the thickness and total volume of epicardial fat can be measured and calculated manually using the modified Simpson method.15,16
Noncontrast CT provides a more sensitive and accurate measurement of epicardial fat thickness and volume, but it is more expensive and cumbersome.12,14 Epicardial fat measurements are taken in the right ventricular free wall and around the main coronary arteries, and the acquired images are reconstructed in 3D slices with 2-mm to 3-mm thickness.15,17,18 These higher-resolution images provide more detail but need more technical care and require longer radiation exposure for the patient. Several studies used the semiautomated technique for measuring the volume of epicardial fat, and software has been developed more recently to automate this process.17–20
Clinical Correlations among Epicardial Fat, Obesity, and Heart Disease
Epicardial fat has a direct relationship with obesity and body mass index.19 Most studies report an association between increased amounts of epicardial fat and metabolic syndrome, which is characterized by inflammation, hypertension, and disturbances in insulin sensitivity.16 Epicardial fat thickness and volume have subsequently been associated with multiple independent cardiac risk factors, such as high fasting glucose, high levels of C-reactive protein, low HDL, carotid intima-media thickness, and others.21 However, when adjusted for visceral adipose tissue, many of these associations are diminished or absent; even so, epicardial fat thickness and volume are still directly associated with coronary artery disease and some cancers.15 The magnitude of the association is quite variable, even nonexistent in some studies, which could be attributed to differences in coronary artery disease severity among individuals and to the research methods used.22
Studies have found epicardial fat to be 22% thicker in patients ages 65 years and older, implying that it increases with age.20 There is no consensus in the literature on the impact of gender on epicardial fat. Some literature has suggested that epicardial fat is more associated with risk factors in women than in men.23 While data is lacking with regard to epicardial fat volume and ethnicity, there is speculation of an association; for example, African American men have less central obesity than Caucasian men and have been shown to have less epicardial fat, although they are more insulin resistant.18,24
Epicardial Fat in the Pathogenesis of Heart Diseases
The mechanism by which epicardial fat can influence heart function and structure continues to be an active research topic. Increased epicardial fat leads to additional mass on both ventricles that can increase the work demands on the heart and result in left ventricular hypertrophy.25 Moreover, epicardial fat thickness is positively correlated with myocardial lipid content and may affect cardiomyocyte function.26 In addition to these mechanical and metabolic mechanisms, fat tissue-derived secreting factors were recently identified as major players linking abnormal epicardial fat to heart dysfunction. Like other visceral adipose tissue, epicardial fat serves as an endocrine and paracrine organ and plays an important role in regulating myocardial function. In this section, we will focus on the inflammatory processes and factors in epicardial fat and their roles in CAD.
The first evidence indicating the inflammatory state of human epicardial fat was provided in 2003 by Mazurek et al.27 Since then, many studies have shown elevated inflammation with epicardial fat in terms of leukocyte infiltration and inflammatory gene expression. Macrophages are one of the major effector cells mediating adipose inflammation. Hirata et al. found that patients with advanced CAD have more inflammatory M1 (classically activated) macrophages but fewer anti-inflammatory M2 (alternatively activated) macrophages in epicardial fat compared to subjects without CAD.28 The transition of macrophages from an anti-inflammatory M2 state to an activated M1 state suggests a macrophage-mediated inflammation in epicardial fat.
In addition to macrophages, other immunocytes, including T lymphocytes and mast cells, have been identified in epicardial fat and may be associated with inflammatory states in patients with CAD.27 These infiltrated leukocytes respond to innate inflammatory signals through Toll-like receptors (TLRs) and produce inflammatory factors. TLRs recognize endogenous ligands such as lipopolysaccharide and saturated fatty acids, leading to translocation of nuclear factor-κB into the nucleus and activation of c-Jun N-terminal kinase with subsequent transcription of inflammatory cytokines such as interleukin (IL)-1, IL-6, tumor necrosis factor alpha, and monocyte chemoattractant protein-1.29 In obese patients with critical CAD, expression and secretion of these factors are upregulated in epicardial fat and are associated with infiltration of leukocytes.27,30,31
Under obese conditions, adipocytes also produce inflammatory adipokines and can be viewed as integral components of the immune system.32 For example, obesity induces overexpression of major histocompatibility complex class II molecules in adipocytes and makes them act as antigen-presenting cells to instigate T-cell activation in adipose tissue.33 Adipocytes possess machinery including TLRs to produce inflammatory cytokines. They also release inflammatory adipokines, such as resistin and leptin, to promote epicardial adipose inflammation in patients with CAD.34 Parallel to the inflammatory state of epicardial fat, secretion of anti-inflammatory adipokines from epicardial fat is decreased in patients with CAD,35 further suggesting increased epicardial adipose inflammation in CAD development. Thus, the inflammatory process within epicardial fat results in paracrine or vasocrine secretion of epicardial inflammatory cytokines, in turn creating the metabolic and inflammatory milieu that promotes CAD.
In addition to inflammation, oxidative stress also plays a significant role in the development and progression of CAD. Epicardial fat in subjects with CAD shows higher mRNA expression of genes involved in oxidative stress and higher levels of reactive oxygen species (ROS) products than subcutaneous fat,36 suggesting a higher oxidative stress in epicardial fat. Since ROS induces chronic inflammation, the higher oxidative stress could activate inflammatory signals in epicardial fat and contribute to the development and progression of CAD.
Conclusions: Therapeutic Implications and Future Directions
Epicardial fat is a special visceral fat depot with unique anatomical and functional features. It is a primary source of biomolecules that act as a local gland to regulate the heart through paracrine or vasocrine mechanisms. The thickness and volume of epicardial fat can be measured by using echocardiography, CT, or MRI. It is well established that epicardial fat volume and thickness is significantly associated with the degree and severity of metabolic syndrome and CAD. This association and the simplicity of imaging techniques have enabled the use of epicardial fat measurements as a predictive biomarker of cardiometabolic diseases.
The pharmaceutical value of epicardial fat in cardiovascular diseases is still unclear. Despite strong clinical correlations, there is still no direct evidence that inflammatory epicardial fat exacerbates CAD. Since mice have no epicardial fat, the vast majority of studies on epicardial fat have been performed in humans. These clinical studies are cross-sectional and correlative and have not yet clarified the causative role of epicardial fat in CAD. A larger mammalian animal model of epicardial fat must be developed to investigate whether and how increased epicardial adipose fat promotes CAD. Studies focusing on the detailed mechanisms associated with epicardial fat and its causative role will also provide direct evidence to support the idea that epicardial fat is a valuable cardiovascular therapeutic target.
Many factors, including weight loss and pharmaceutical treatments, can decrease the thickness of epicardial fat. Weight loss by caloric restriction,37 exercise,38 or bariatric surgery39 in obese patients leads to significant reductions (9%–32%) in epicardial fat thickness. Pharmaceutical treatments associated with thinner epicardial fat target either lipid metabolism or glucose homeostasis. In a 2-year study of 145 patients with coronary artery stenosis, statins were shown to improve lipid profiles and also significantly decrease epicardial fat thickness.40 Another study performed in type 2 diabetes patients showed that insulin replacement therapy resulted in a reduction of epicardial fat thickness.41 It is still unknown whether the decrease of epicardial fat volume following weight loss or pharmaceutical treatment is associated with changes in the inflammatory state of epicardial fat. Future studies that determine the effect of these interventions on epicardial fat-related inflammatory cells and molecules are warranted.
Key Points
Epicardial fat has multiple functions to support a healthy heart.
An increased amount of epicardial fat is associated with both obesity and coronary artery disease.
Inflammatory epicardial fat promotes the development of coronary artery disease.
Conflict of Interest Disclosure
Dr. Hamilton's work is supported by Houston Methodist Foundation grants from Stedman-West Foundation, Cary Wilson, and Patrick Studdert; Dr. Deng receives funding from AHA grant 14SDG18970097.
References
- 1. Iacobellis G, Corradi D, Sharma AM.. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005. October; 2( 10): 536– 43. [DOI] [PubMed] [Google Scholar]
- 2. Marchington JM, Mattacks CA, Pond CM.. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B. 1989; 94( 2): 225– 32. [DOI] [PubMed] [Google Scholar]
- 3. Iacobellis G. Epicardial and pericardial fat: close, but very different. Obesity (Silver Spring). 2009. April; 17( 4): 625; author reply 626–7. [DOI] [PubMed] [Google Scholar]
- 4. Corradi D, Maestri R, Callegari S, . et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol. 2004. Nov-Dec; 13( 6): 313– 6. [DOI] [PubMed] [Google Scholar]
- 5. Prati F, Arbustini E, Labellarte A, . et al. Eccentric atherosclerotic plaques with positive remodelling have a pericardial distribution: a permissive role of epicardial fat? Eur Heart J. 2003; 24: 329– 36. [DOI] [PubMed] [Google Scholar]
- 6. Pezeshkian M, Noori M, Najjarpour-Jabbari H, . et al. Fatty acid composition of epicardial and subcutaneous human adipose tissue. Metab Syndr Relat Disord. 2009. April; 7( 2): 125– 31. [DOI] [PubMed] [Google Scholar]
- 7. Sacks HS, Fain JN, Holman B, . et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009. September; 94( 9): 3611– 5. [DOI] [PubMed] [Google Scholar]
- 8. Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012. September; 55( 9): 2319– 26. [DOI] [PubMed] [Google Scholar]
- 9. Fang X, Palanivel R, Cresser J, . et al. An APPL1-AMPK signaling axis mediates beneficial metabolic effects of adiponectin in the heart. Am J Physiol Endocrinol Metab. 2010. November; 299( 5): E721– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ouchi N, Parker JL, Lugus JJ, Walsh K.. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011. February; 11( 2): 85– 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong HK, Cheung TT, Cheung BM.. Adrenomedullin and cardiovascular diseases. JRSM Cardiovasc Dis. 2012. August; 1( 5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelson AJ, Worthley MI, Psaltis PJ, . et al. Validation of cardiovascular magnetic resonance assessment of pericardial adipose tissue volume. J Cardiovasc Magn Reson. 2009. May 5; 11: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009. December; 22( 12): 1311– 9; quiz 1417–8. [DOI] [PubMed] [Google Scholar]
- 14. Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015. June; 11( 6): 363– 71. [DOI] [PubMed] [Google Scholar]
- 15. Mookadam F, Goel R, Alharthi MS, Jiamsripong P, Cha S.. Epicardial fat and its association with cardiovascular risk: a cross-sectional observational study. Heart Views. 2010. October; 11( 3): 103– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iacobellis G, Ribaudo MC, Assael F, . et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003. November; 88( 11): 5163– 8. [DOI] [PubMed] [Google Scholar]
- 17. Doesch C, Haghi D, Fluchter S, . et al. Epicardial adipose tissue in patients with heart failure. J Cardiovasc Magn Reson. 2010. July 12; 12: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bertaso AG, Bertol D, Duncan BB, Foppa M.. Epicardial fat: definition, measurements and systematic review of main outcomes. Arquivos brasileiros de cardiologia. 2013. July; 101( 1): e18– e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forouzandeh F, Chang SM, Muhyieddeen K, . et al. Does quantifying epicardial and intrathoracic fat with noncontrast computed tomography improve risk stratification beyond calcium scoring alone? Circ Cardiovasc Imaging. 2013. January; 6: 58– 66. [DOI] [PubMed] [Google Scholar]
- 20. Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P.. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis. 2010. May; 210( 1): 150– 4. [DOI] [PubMed] [Google Scholar]
- 21. Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab. 2005. November; 90( 11): 6300– 2. [DOI] [PubMed] [Google Scholar]
- 22. Nelson MR, Mookadam F, Thota V, . et al. Epicardial fat: an additional measurement for subclinical atherosclerosis and cardiovascular risk stratification? J Am Soc Echocardiogr. 2011. March; 24( 3): 339– 45. [DOI] [PubMed] [Google Scholar]
- 23. Shmilovich H, Dey D, Cheng VY, . et al. Threshold for the upper normal limit of indexed epicardial fat volume: derivation in a healthy population and validation in an outcome-based study. Am J Cardiol. 2011. December 1; 108( 11): 1680– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salami SS, Tucciarone M, Bess R, . et al. Race and epicardial fat: the impact of anthropometric measurements, percent body fat and sex. Ethn Dis. 2013. Summer; 23( 3): 281– 5. [PubMed] [Google Scholar]
- 25. Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F.. Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol. 2004. October 15; 94( 8): 1084– 7. [DOI] [PubMed] [Google Scholar]
- 26. Malavazos AE, Di Leo G, Secchi F, . et al. Relation of echocardiographic epicardial fat thickness and myocardial fat. Am J Cardiol. 2010. June 15; 105( 12): 1831– 5. [DOI] [PubMed] [Google Scholar]
- 27. Mazurek T, Zhang L, Zalewski A, . et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003. November 18; 108( 20): 2460– 6. [DOI] [PubMed] [Google Scholar]
- 28. Hirata Y, Tabata M, Kurobe H, . et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol. 2011. July 12; 58( 3): 248– 55. [DOI] [PubMed] [Google Scholar]
- 29. Baker AR, Harte AL, Howell N, . et al. Epicardial adipose tissue as a source of nuclear factor-kB and c-Jun N-terminal kinase mediated inflammation in patients with coronary artery disease. J Clin Endocrinol Metab. 2009. January; 94( 1): 261– 7. [DOI] [PubMed] [Google Scholar]
- 30. Baker AR, Silva NF, Quinn DW, . et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006. January 13; 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kremen J, Dolinkova M, Krajickova J, . et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006. November; 91( 11): 4620– 7. [DOI] [PubMed] [Google Scholar]
- 32. Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005. May 13; 96( 9): 939– 49. [DOI] [PubMed] [Google Scholar]
- 33. Deng T, Lyon CJ, Minze LJ, . et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013. March 5; 17( 3): 411– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Golia E, Limongelli G, Natale F, . et al. Adipose tissue and vascular inflammation in coronary artery disease. World J Cardiol. 2014. July 26; 6( 7): 539– 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iacobellis G, Pistilli D, Gucciardo M, . et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005. March 21; 29( 6): 251– 5. [DOI] [PubMed] [Google Scholar]
- 36. Salgado-Somoza A, Teijeira-Fernandez E, Fernandez AL, Gonzalez-Juanatey JR, Eiras S.. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am J Physiol Heart Circ Physiol. 2010. July; 299( 1): H202– 9. [DOI] [PubMed] [Google Scholar]
- 37. Iacobellis G, Singh N, Wharton S, Sharma AM.. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring). 2008. July; 16( 7): 1693– 7. [DOI] [PubMed] [Google Scholar]
- 38. Kim MK, Tomita T, Kim MJ, Sasai H, Maeda S, Tanaka K.. Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol (1985). 2009. January; 106( 1): 5– 11. [DOI] [PubMed] [Google Scholar]
- 39. Willens HJ, Byers P, Chirinos JA, Labrador E, Hare JM, de Marchena E.. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am J Cardiol. 2007. May 1; 99( 9): 1242– 5. [DOI] [PubMed] [Google Scholar]
- 40. Park JH, Park YS, Kim YJ, . et al. Effects of statins on the epicardial fat thickness in patients with coronary artery stenosis underwent percutaneous coronary intervention: comparison of atorvastatin with simvastatin/ezetimibe. J Cardiovasc Ultrasound. 2010. December; 18( 4): 121– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Elisha B, Azar M, Taleb N, Bernard S, Iacobellis G, Rabasa-Lhoret R.. Body Composition and Epicardial Fat in Type 2 Diabetes Patients Following Insulin Detemir Versus Insulin Glargine Initiation. Horm Metab Res. 2016. January; 48( 1): 42– 7. [DOI] [PubMed] [Google Scholar]


