Nuclear mispositioning in muscle is often associated with muscular diseases, but little is known about the mechanisms governing nuclear motion in these cells. A screen is presented for molecular motors involved in moving nuclei during myofiber differentiation.
Abstract
Nuclear positioning is a determining event in several cellular processes, such as fertilization, cell migration, and cell differentiation. The structure and function of muscle cells, which contain hundreds of nuclei, have been shown to rely in part on proper nuclear positioning. Remarkably, in the course of muscle differentiation, nuclear movements along the myotube axis might represent the event required for the even positioning of nuclei in the mature myofiber. Here we analyze nuclear behavior, time in motion, speed, and alignment during myotube differentiation and temporal interference of cytoskeletal microtubule-related motors. Using specific inhibitors, we find that nuclear movement and alignment are microtubule dependent, with 19 microtubule motor proteins implicated in at least one nuclear behavior. We further focus on Kif1c, Kif5b, kif9, kif21b, and Kif1a, which affect nuclear alignment. These results emphasize the different roles of molecular motors in particular mechanisms.
INTRODUCTION
The skeletal muscle fiber is a syncytium resulting from the fusion of hundreds of myoblasts (Abmayr and Pavlath, 2012). Muscle cells represent the main vertebrate cell type and are specialized to contract, and thus are responsible for voluntary movements. During muscle development or regeneration, nuclei are first found in the center of myotubes and then migrate to the periphery of mature skeletal myofibers (Bruusgaard et al., 2003). Proper nuclear positioning is required for muscle function (Metzger et al., 2012), and nuclear mispositioning is a hallmark of different muscle disorders, such as centronuclear myopathies (Jungbluth et al., 2008).
Multiple nuclear movement events occur during myofiber development to localize precisely each individual nucleus (Cadot et al., 2012; Metzger et al., 2012; Wilson and Holzbaur, 2012, 2015; Falcone et al., 2014). The first nuclear migration event in muscle development takes place just after fusion, when the nucleus from a fusing myoblast rapidly migrates toward the center of the myotube. This process is called centration (Cadot et al., 2012). Second, nuclei spread to become evenly spaced along the length of developing myotubes (Metzger et al., 2012), a step called spreading. These two events were proposed to rely on the microtubule (MT) network and on some motor proteins. Finally, as myotubes mature into myofibers, nuclei migrate from the center to the periphery of the fiber by a mechanism dependent on N-Wasp, an actin regulator, and amphiphysin-2, a BAR protein mutated in centronuclear myopathies. This nuclear migration is termed dispersion (Falcone et al., 2014). Nuclear positioning in cells appears to be a key mechanism required for multiple developmental processes in order to maintain functionally efficient muscle or brain (Dauer and Worman, 2009). These processes are described as being driven by MTs or actin (Gundersen and Worman, 2013). Even though recent advances in understanding nuclei position in developing muscle cells reveal an important role of MTs and some MT motors (kinesins and dyneins; Cadot et al., 2012; Metzger et al., 2012; Wilson and Holzbaur, 2012), the contribution of other MT motors to nuclei position during myofiber formation remains unexplored.
Here we present a comprehensive analysis of MT-related molecular motor involvement in nuclear spreading during myofiber development. We use time-lapse microscopy combined with a small interfering RNA (siRNA) screen targeting all MT-related molecular motors (kinesins and dyneins) of the mouse genome to identify their involvement. These results highlight the variable effects of molecular motors on particular mechanisms in the early phases of myofiber development, such as nuclear alignment, time in motion, and speed of nuclei inside myotubes.
RESULTS AND DISCUSSION
Nuclear movements contribute to nuclei alignment inside myotubes
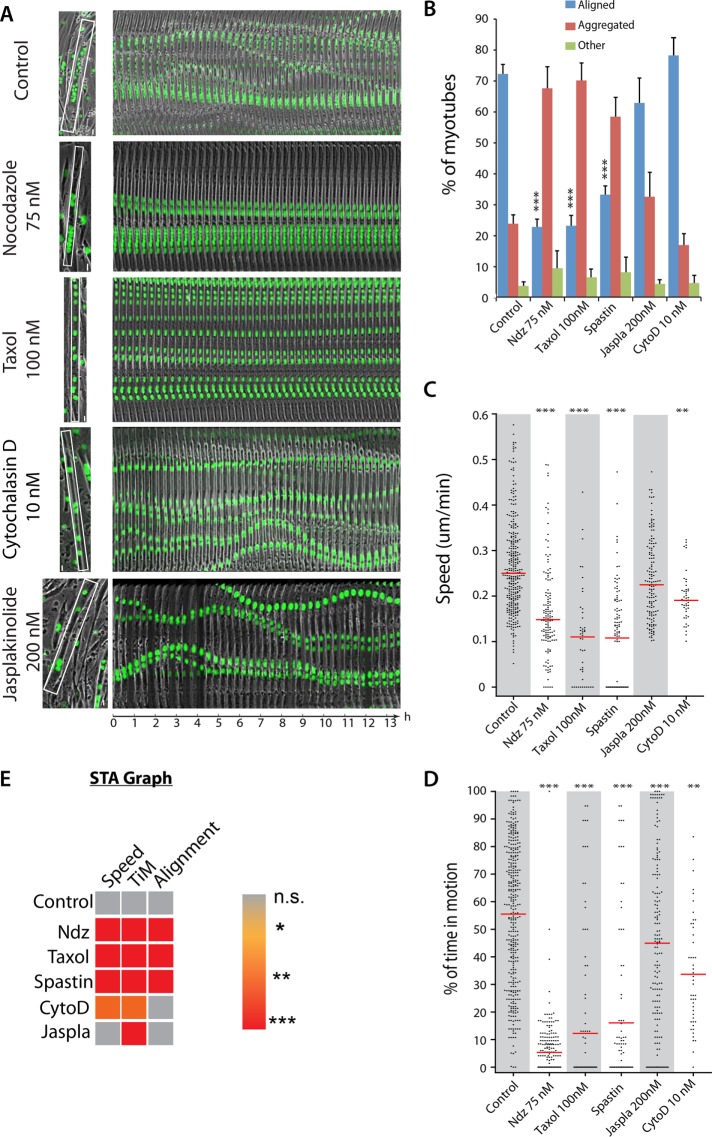
Nuclear movements occur in both primary differentiated muscle cells and C2C12s (Figure 1A; Englander and Rubin, 1987; Cadot et al., 2012; Metzger et al., 2012; Wilson and Holzbaur, 2012). We therefore used C2C12 muscle cells (Cadot et al., 2012), stably expressing green fluorescent protein (GFP)–histone H1, to quantify nuclear behaviors during myotube formation using time-lapse microscopy. Nuclei within myotubes follow different behaviors, suggesting that multiple forces are applied to nuclei, driving their displacement along developing myofibers. To better characterize these nuclear migrations, we tracked each nucleus inside myotubes during the first 4 d of myofiber development and analyzed nuclear movements using SkyPad (Cadot et al., 2014) to extract speed and percentage of time in motion. We also quantified nuclei distribution in myotubes to measure whether the resulting forces on each nucleus lead to a “nuclei alignment” organization (Metzger et al., 2012). Finally, we analyzed fusion index to correlate defects in nuclear behavior with differentiation. During differentiation, nuclei progressively reduce their movements (Supplemental Figure S1A), and after 4 d, nuclei are mostly spread along the myotube axis (Figures 1B and 2C; Metzger et al., 2012). Reduction of speed and time in motion during differentiation suggests that nuclear movement is not driven by a unique mechanism. We therefore decided to perform our analysis of nuclear movement between 60 and 75 h of differentiation, where nuclei inside myotubes move 55% of the time (±1.3) at a speed of 0.278 μm/min (± 0.1), similar to previously published results on nuclei speed (Figure 1C; Wilson and Holzbaur, 2012).
FIGURE 1:
Role of MTs and actin in nuclear movement inside myotubes. (A) Frames from time-lapse two-channel movies (phase contrast and fluorescence) of differentiated GFP-H1-C2 cells in presence or absence of the indicated drugs (Supplemental Movie S1). The first frame of each representative 14-h movie shows a myotube with GFP-H1-C2 nuclei (green); the white rectangle corresponds to the region used to create the adjacent kymograph. (B) Histogram of nuclear distribution analysis in C2C12 myotubes: nontreated (Control), treated with 75 nM nocodazole (Ndz), 100 nM Taxol (Taxol), 200 nM jasplakinolide (Jaspla), or 10 nM cytochalasin D (CytoD), or transfected with GFP-spastin. Nuclear distribution is quantified as “aligned” if >70% of nuclei are aligned along the same axis; “aggregated” if >70% of nuclei do not align along the same axis; and “other” if nuclei are both aggregated and aligned in the same myotube. (C) Speed and (D) percentage of time in motion of nuclei inside C2C12 myotubes 2–3 d old in nontreated (Control), treated with 75 nM Ndz, 100 nM Taxol, 200 nM Jaspla, or 10 nM CytoD conditions or transfected with GFP-spastin. Red line indicates the median. Between 53 (CytoD) and 181 (Ctr) nuclei were monitored from three different experiments. Error bars, SEM. ***p < 0.001, **p < 0.01. (E) STA graph; color is representative of the p value after one-way analysis of variance (ANOVA) statistical test toward a decrease compared to control. ***p < 0.001, **p < 0.01, *p < 0.05.
FIGURE 2:
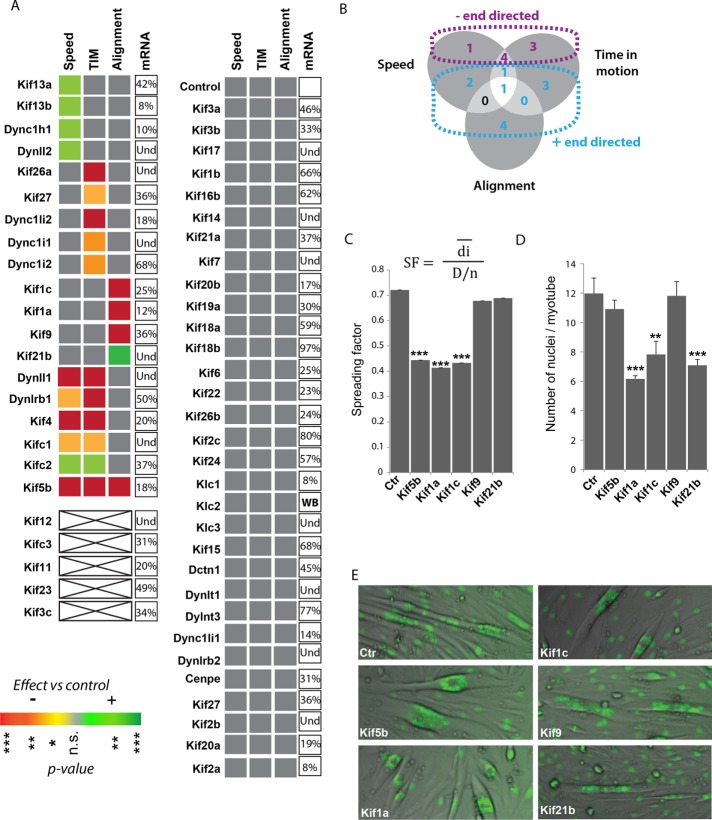
MT motor siRNA screen for nuclear movement and alignment in myotubes. (A) STA graph after silencing of kinesin or dynein members for nuclei inside myotubes after 3 d of differentiation. Colors are representative of the p values after one-way ANOVA statistical test toward a decrease (red) and increase (green) compared to control. ***p < 0.001, **p < 0.01, *p < 0.05. The percentage of remaining mRNA after silencing is also indicated. (B) Venn diagram to summarize the results obtained with siRNA screen, showing the distribution of genes implicated in one or more of the nuclear behaviors. Purple, proteins implicated in minus end–directed motion; blue, plus end–directed proteins. (C) Distribution of nuclei in myotubes and (D) number of nuclei per myotubes in five different conditions (Ctr, Kif5b, Kif1a, Kif1c, Kif9, and Kif21b siRNAs) after 88 h of differentiation. Spreading factor is the ratio between observed average distance and theoretical maximum distance between nuclei (41–76 myotubes). (E) Representative image of myotubes in each condition.
Nuclear movement and alignment are mainly MT dependent
We first investigated the contribution of actin and MT networks to nuclear behavior inside myotubes. Because these two networks are essential for fusion (Dobi et al., 2011; Cadot et al., 2012), we applied drugs affecting MTs (nocodazole and Taxol) and actin (cytochalasin D and jasplakinolide) 3 d after differentiation. After testing a range of concentrations, we used concentrations that did not affect global shape of myotubes but did impair cytoskeletal dynamics (Brown and Spudich, 1979; Schiff and Horwitz, 1980; Vasquez et al., 1997; Bubb et al., 2000; Rotsch and Radmacher, 2000). Surprisingly, some parameters, such as time in motion, are only slightly modified by actin-targeted drugs. Percentage of time in motion decreased from 55% in control to 34% in 25 nM cytochalasin D and to 45% in 200 nM jasplakinolide (Figure 1, A and D). However, other characteristics are more drastically affected, such as nuclei speed, which decreased to 0.20 μm/min in cytochalasin D– and 0.23 μm/min in jasplakinolide-treated myotubes, compared with 0.27 μm/min in controls (Figure 1C and Supplemental Movie S1). Of interest, drugs targeting the actin network did not affect the alignment of nuclei in myotubes (Figure 1C).
To hamper MT dynamics without affecting the overall shape of myotubes, we used drugs at low concentrations (75 nM nocodazole and 100 nM Taxol). Some parameters are highly modified during the first 24-h exposure, such as a dramatic decrease in the percentage of time in motion compared with control (Figure 1, A and D). Nuclear speed inside myotubes also decreases in nocodazole- and Taxol-treated myotubes (Figure 1C and Supplemental Movie S1A). To confirm MT involvement in maintaining these parameters, we overexpressed constitutively active spastin, a MT-severing protein (White et al., 2007). We previously found that expression of spastin, but not the non–MT-severing mutant, prevents nuclear movement after fusion (Cadot et al., 2012). We now found that ectopic expression of spastin in myotubes dramatically reduces nuclear motion and organization (Figure 1, B–D). To facilitate our readout, we represented the three measured parameters in an STA graph (speed, time in motion, and alignment), as represented in Figure 1E. These results describe the initial step of myofiber formation—when nuclei move extensively to establish an elongated myotube containing aligned nuclei. These results demonstrate the paramount role of dynamic MTs in nuclear movement within the myotube.
Identification of MT motors involved in nuclear spreading in myotubes
Next we sought to identify the MT-related molecular motors implicated in nuclear movement behavior. We performed an siRNA screen with three different sequences for each known kinesin and cytoplasmic dynein subunit (56 proteins; Table 1). STA parameters were measured in three different experiments on an area of 3.79 mm², corresponding to a single field of view under the microscope at a 4× magnification. We also quantified the fusion index to monitor the effect of protein silencing on the fusion process (Supplemental Figure S1C). At 36 h after silencing, cells were induced to differentiate into myotubes. Nuclear trajectories were analyzed using SkyPad (Cadot et al., 2014) from 60 to 75 h after the induction of differentiation (Supplemental Figure S2, A and B). At this time, the initial fusion events and their associated nuclear movements (centration) are terminated, and myotubes contain enough nuclei to be monitored for their displacements. The last frame of each movie was used to quantify nuclei alignment in myotubes and fusion index (Supplemental Figures S1C and S2C). The data were compiled in an STA graph, allowing an easy overview of the effect of all kinesins and dynein members on nuclear movement behavior (Figure 2A). In our conditions, cells divide at least once before differentiation. Therefore depletion of motors implicated in the cell division process leads to massive cell death: Kif11 (Eg5), which is an establisher of the bipolar spindle (Blangy et al., 1995), Kif23 (MKLP-1), and Kif12, which is known to be essential for cytokinesis (Lakshmikanth et al., 2004; Zhu et al., 2005). Depletion of two other kinesins leads to failure in differentiation: Kifc3, responsible for Golgi positioning and integration (Xu et al., 2002), and Kif3c, a kinesin whose function remains to be elucidated (Yang et al., 2001). We were still able to quantify the fusion index for the remaining cells, but the nuclei number per myotube (two to five) was too low to allow correct quantification of the other STA parameters. Of interest, dynein motor inhibition does not lead to any gross cell fusion defects (Figure 2A and Supplemental Figure S1C). Furthermore, we did not find any correlation between defects in nuclei behavior and fusion index. For example, Kif5b or Kif20a depletion affects uniquely nuclear movement or fusion index, respectively. It is important to note that efficiency of knockdown might have affected the functional readouts. We therefore measured the percentage of remaining mRNA for most targets by Taqman (Figure 2A) or at the protein level by Western blot (Supplemental Figure S1B).
TABLE 1:
siRNA sequences and Taqman probes used for each molecular motor.
| RefSeq accession number | Gene symbol | Sense siRNA sequence | Antisense siRNA sequence | Taqman assay ID |
|---|---|---|---|---|
| NM_008440 | Kif1a | GGACAUCAACUAUGCCUCUtt | AGAGGCAUAGUUGAUGUCCtc | Mm00492863_m1 |
| GGAAACAGAGAAGAUCAUUtt | AAUGAUCUUCUCUGUUUCCtt | |||
| CCAAGUCCUUCAUCGAAUAtt | UAUUCGAUGAAGGACUUGGtc | |||
| NM_207682 | Kif1b | CGGGCUGAUUCAACUGGUGtt | CACCAGUUGAAUCAGCCCGtt | Mm00801827_m1 |
| CCUCAAUGAAGACCCAUUAtt | UAAUGGGUCUUCAUUGAGGtt | |||
| GGAUGGAAUUACAAGGGUUtt | AACCCUUGUAAUUCCAUCCtt | |||
| NM_153103 | Kif1c | CCUUCGACUAUUCUUACUGtt | CAGUAAGAAUAGUCGAAGGtg | Mm00462184_m1 |
| GGAAACAGAGAAGAUCAUAtt | UAUGAUCUUCUCUGUUUCCtg | |||
| CCAUGUUUUCCGCUUCAAUtt | AUUGAAGCGGAAAACAUGGtt | |||
| NM_008442 | Kif2a | GGGAAUUUAUGCAUUAGCAtt | UGCUAAUGCAUAAAUUCCCtt | Mm00515233_m1 |
| CGCAGAUCAAUUUUCAUAGtt | CUAUGAAAAUUGAUCUGCGtt | |||
| GCUCCUAAUGAAAUGGUUUtt | AAACCAUUUCAUUAGGAGCtg | |||
| NM_134471 | Kif2c | GGAGGUACCACAAAAGGCAtt | UGCCUUUUGUGGUACCUCCtt | Mm00728630_s1 |
| GGCAAAGAGAUUGACAUUGtt | CAAUGUCAAUCUCUUUGCCtt | |||
| GCAGAAAUUAACAAGUCUCtt | GAGACUUGUUAAUUUCUGCtc | |||
| NM_008443 | Kif3a | GGGCGACACAAGGUUUUUGtt | CAAAAACCUUGUGUCGCCCtc | Mm01288585_m1 |
| GGGACCAAGCAGGUAAAAAtt | UUUUUACCUGCUUGGUCCCtt | |||
| CCGUAAUUGAUUCUUUACUtt | AGUAAAGAAUCAAUUACGGtc | |||
| NM_008444 | Kif3b | GGAUUUGUCUUCUUUUGUCtt | GACAAAAGAAGACAAAUCCtt | Mm00492891_m1 |
| GGUGGUAGAUGCGGAUGUGtt | CACAUCCGCAUCUACCACCtt | |||
| GGGUUUCAAUGGCACAAUUtt | AAUUGUGCCAUUGAAACCCtg | |||
| NM_008445 | Kif3c | GGAGAAUCCUGAAACAGGGtt | CCCUGUUUCAGGAUUCUCCtt | Mm00492900_m1 |
| GGAAGAUGAUAACAACAACtt | GUUGUUGUUAUCAUCUUCCtc | |||
| CCGGCUAUCUUUGAGAUGGtt | CCAUCUCAAAGAUAGCCGGtg | |||
| NM_008446 | Kif4 | GGUGGUGGUUGGUAAUGAUtt | AUCAUUACCAACCACCACCtg | Mm00492908_m1 |
| GGAAGAGGUCUUUAAUACAtt | UGUAUUAAAGACCUCUUCCtg | |||
| GGAUACAAUGCAACUGUCCtt | GGACAGUUGCAUUGUAUCCtt | |||
| NM_177052 | Kif6 | CCUGGCAGAUGGAUUCGUAtt | UACGAAUCCAUCUGCCAGGtc | Mm00723857_m1 |
| GCUUCAACCCGGUCACACUtt | AGUGUGACCGGGUUGAAGCtt | |||
| CGAAUGUGGCUAUGACCUGtt | CAGGUCAUAGCCACAUUCGtt | |||
| NM_010628 | Kif9 | GGACUUGGUUUAUGAAACAtt | UGUUUCAUAAACCAAGUCCtg | Mm00495130_m1 |
| GCAUCGACAUCCACUUGAAtt | UUCAAGUGGAUGUCGAUGCtt | |||
| GGCUUGUCAGUGCAUCUCAtt | UGAGAUGCACUGACAAGCCtt | |||
| NM_010615 | Kif11 | CCAUUUAAUCUGGCAGAGCtt | GCUCUGCCAGAUUAAAUGGtc | Mm01204225_m1 |
| GCUUGUUAAAAUUGGAAAGtt | CUUUCCAAUUUUAACAAGCtc | |||
| GGUCUACUGAUAUAAUCAAtt | UUGAUUAUAUCAGUAGACCtc | |||
| NM_010616 | Kif12 | CCUGGCUAUUAGAUCGCGUtt | ACGCGAUCUAAUAGCCAGGtg | Mm00802885_m1 |
| CCUGAGUCUCGGUUCACAAtt | UUGUGAACCGAGACUCAGGtt | |||
| CCUUCACCUGGCUAUUAGAtt | UCUAAUAGCCAGGUGAAGGtc | |||
| NM_010617 | Kif13a | GGUAUCGUAUAUGGAGAUCtt | GAUCUCCAUAUACGAUACCtc | Mm00660179_m1 |
| GGGAAAUAAGUCUCGAACGtt | CGUUCGAGACUUAUUUCCCtc | |||
| GCUGGAGAAUAAGCUAAUUtt | AAUUAGCUUAUUCUCCAGCtt | |||
| NM_010620 | Kif15 | GGAGUCUGUAUUCUCAACAtt | UGUUGAGAAUACAGACUCCtg | Mm01341275_m1 |
| GCGGUUAUAAUGGGACCAUtt | AUGGUCCCAUUAUAACCGCtc | |||
| GCAAACCUCAAUCUUGAAAtt | UUUCAAGAUUGAGGUUUGCtt | |||
| NM_010623 | Kif17 | GGCAGUGGGAAGUCUUUCAtt | UGAAAGACUUCCCACUGCCtg | Mm00456740_m1 |
| GGAGGCCACCAAAAUUAACtt | GUUAAUUUUGGUGGCCUCCtt | |||
| CCCUGAUGAAUAAGGACUCtt | GAGUCCUUAUUCAUCAGGGtg | |||
| NM_139303 | Kif18a | GGCGGUGCAGUUCUGUAAAtt | UUUACAGAACUGCACCGCCtt | Mm01327661_m1 |
| GCCAAUCCUUCAUAGUUUUtt | AAAACUAUGAAGGAUUGGCtt | |||
| CGUGCUUAAACUUACUCCAtt | UGGAGUAAGUUUAAGCACGtt | |||
| NM_028547 | Kif2b | CCAAUGAACUAGUUUACCATT | UGGUAAACUAGUUCAUUGGAT | Mm01308520_s1 |
| CGACAAUACGAAUUCGGGATT | UCCCGAAUUCGUAUUGUCGTT | |||
| GCUCCAAUCACUACGAGAATT | UUCUCGUAGUGAUUGGAGCTT | |||
| NM_010626 | Kif7 | GGAGAACGGCUCAAAGAGATT | UCUCUUUGAGCCGUUCUCCGG | Mm01320530_m1 |
| GCCUGGAGAUUGAUAGCAATT | UUGCUAUCAAUCUCCAGGCGT | |||
| CAACAGCAAAAGAUCCUGATT | UCAGGAUCUUUUGCUGUUGCT | |||
| NM_001081177 | Kif13b | AGACGGGCAUUGUACGGUATT | UACCGUACAAUGCCCGUCUTG | Mm01314840_m1 |
| GGCUAGAAGUAACAUCUGATT | UCAGAUGUUACUUCUAGCCTC | |||
| CCAUCUCCCAUGGUGGUUATT | UAACCACCAUGGGAGAUGGAG | |||
| NM_001081258 | Kif14 | CAGGGAUGCUGUUCGGAUATT | UAUCCGAACAGCAUCCCUGCA | Not available |
| CCUCUGUUCGAGUUCGUAATT | UUACGAACUCGAACAGAGGTA | |||
| GGAAAGUCCUAUACGAUGATT | UCAUCGUAUAGGACUUUCCAG | |||
| NM_001081133 | Kif16b | GUACAUAAUUCAACAUAUATT | UAUAUGUUGAAUUAUGUACAT | Mm01327880_m1 |
| CACUUAGAGAAAUACCUCATT | UGAGGUAUUUCUCUAAGUGTG | |||
| GGAUUUGGAUUUAAUAAUATT | UAUUAUUAAAUCCAAAUCCAA | |||
| NM_001102615 | Kif19a | GAAGGAGUCCUACACCAAATT | UUUGGUGUAGGACUCCUUCCT | Mm01244862_m1 |
| CAACUAUCGGGACAGCAAATT | UUUGCUGUCCCGAUAGUUGAT | |||
| CAAUCUAUCUAGCAGCACATT | UGUGCUGCUAGAUAGAUUGTC | |||
| NM_183046 | Kif20b | CAACGGUAGAAGUAAGUAATT | UUACUUACUUCUACCGUUGAT | Mm01205010_m1 |
| CCAACGAUCUAAGUGCAAATT | UUUGCACUUAGAUCGUUGGTT | |||
| GCGAAUAAUUUGCAUGAUATT | UAUCAUGCAAAUUAUUCGCCA | |||
| NM_001097621 | Kif26a | UGACGAGUUUGAUGCUUAUTT | AUAAGCAUCAAACUCGUCATT | Mm01339746_m1 |
| GCCCUGAACGUAUGUCGAATT | UUCGACAUACGUUCAGGGCCT | |||
| AGAUCAAGGUGUACGAAAUTT | AUUUCGUACACCUUGAUCUCA | |||
| NM_009004 | Kif20a | GGACCUGUUGUCAGACUGCtt | GCAGUCUGACAACAGGUCCtt | Mm00436226_m1 |
| GGUGAAAGUUUACCUUCGAtt | UCGAAGGUAAACUUUCACCtt | |||
| CGUACACCAUUCAAGGUACtt | GUACCUUGAAUGGUGUACGtt | |||
| NM_016705 | Kif21a | GGAUAUUGCCAGUAAUUAAtt | UUAAUUACUGGCAAUAUCCtc | Mm00497322_m1 |
| CCCAGUGCAUCGAAAAGCUtt | AGCUUUUCGAUGCACUGGGtg | |||
| CGAAGAGAUCAGUAAUAUGtt | CAUAUUACUGAUCUCUUCGtt | |||
| NM_019962 | Kif21b | GGAAAAAGUUCCAAAAGAAtt | UUCUUUUGGAACUUUUUCCtg | Not available |
| GGAGAAGAUGCUGUCUUGCtt | GCAAGACAGCAUCUUCUCCtt | |||
| GGCUGCUCAAAAAGAACAUtt | AUGUUCUUUUUGAGCAGCCtg | |||
| NM_145588 | Kif22 | GGAAGUCUAUGUAGGUUCAtt | UGAACCUACAUAGACUUCCtg | Mm01139072_m1 |
| GGGCAGAAUGCCAGUGUACtt | GUACACUGGCAUUCUGCCCtt | |||
| GGAAAACUCUACCUUAUUGtt | CAAUAAGGUAGAGUUUUCCtt | |||
| NM_024245 | Kif23 | GGGCUAUCGACUCAACAGAtt | UCUGUUGAGUCGAUAGCCCtc | Mm00458527_m1 |
| GGAAAAAGAGCAAAUUACUtt | AGUAAUUUGCUCUUUUUCCtg | |||
| GCAUAGGGUCAUUUCAAGCtt | GCUUGAAAUGACCCUAUGCtg | |||
| NM_024241 | Kif24 | GCCAAGAGGACAUUUGGCAtt | UGCCAAAUGUCCUCUUGGCtg | Mm01211351_m1 |
| CCAGCAUUCACCUGACAAAtt | UUUGUCAGGUGAAUGCUGGtg | |||
| GCAUGUGGUACAGAUAGCUtt | AGCUAUCUGUACCACAUGCtt | |||
| NM_177757 | Kif26b | GGAGAGAGAUGAAAUUUGAtt | UCAAAUUUCAUCUCUCUCCtt | Not available |
| CCUUCGAGACCUGUUGUCUtt | AGACAACAGGUCUCGAAGGtt | |||
| GCUCUCAGCAAAAACCGAGtt | CUCGGUUUUUGCUGAGAGCtt | |||
| NM_175214 | Kif27 | GGAUCUCUACUUCUAUAAGtt | CUUAUAGAAGUAGAGAUCCtt | Mm00723524_m1 |
| GGCCAUGUUGCAUCAGUUGtt | CAACUGAUGCAACAUGGCCtc | |||
| NM_053173 | Kifc1 | GGCUAAUAAGAAGUGAAGUtt | ACUUCACUUCUUAUUAGCCtg | Mm03011779_m1 |
| GGAACUGAAGGGCAAUAUCtt | GAUAUUGCCCUUCAGUUCCtg | |||
| GGCCAUUAACAGCAGUCUGtt | CAGACUGCUGUUAAUGGCCtg | |||
| NM_010630 | Kifc2 | GGAGGAACAGAGAGUUUGGtt | CCAAACUCUCUGUUCCUCCtc | Mm00495161_m1 |
| GGUCAACCUUUAAAAAACAtt | UGUUUUUUAAAGGUUGACCtg | |||
| GCUGAGUAGACUUCGUCUGtt | CAGACGAAGUCUACUCAGCtc | |||
| NM_010631 | Kifc3 | GGGCAUGUAUAUAAUGUUCtt | GAACAUUAUAUACAUGCCCtg | Mm00516085_m1 |
| CGACUACAAUGGGCUCAAGtt | CUUGAGCCCAUUGUAGUCGtt | |||
| GGUUAAUAGCAACAACCAGtt | CUGGUUGUUGCUAUUAACCtc | |||
| NM_008451 | Klc2 | GGCGGUGAUCCAGGGUUUAtt | UAAACCCUGGAUCACCGCCtt | Mm00492945_m1 |
| GGGUUUAGAGACCCUGAGAtt | UCUCAGGGUCUCUAAACCCtg | |||
| GGUGGUAGAACUGUUAAAAtt | UUUUAACAGUUCUACCACCtt | |||
| NM_146182 | Klc3 | CAAAUGUGGCCAAGACUAAtt | UUAGUCUUGGCCACAUUUGgg | Mm00461422_m1 |
| GGAGGCUAGCCCAAGAGAAtt | UUCUCUUGGGCUAGCCUCCgt | |||
| AUGGAAAACGUGGACGUUAtt | UAACGUCCACGUUUUCCAUag | |||
| NM_001025360 | Klc1 | GGAGGAGAAGAAACACCUGtt | CAGGUGUUUCUUCUCCUCCtc | Mm00492936_m1 |
| CCUAGCAGUACUGUACGGUtt | ACCGUACAGUACUGCUAGGtt | |||
| GGGAUCAGAACAAGUAUAAtt | UUAUACUUGUUCUGAUCCCtg | |||
| NM_197959 | Kif18b | CGAGCGGAUGCUGGUAUUUtt | AAAUACCAGCAUCCGCUCGtc | Mm01253048_g1 |
| GCCGAGCAGUUACUUGAGAtt | UCUCAAGUAACUGCUCGGCtg | |||
| CCUACGAGGAUACUUACAAtt | UUGUAAGUAUCCUCGUAGGtc | |||
| NM_173762 | Cenpe | GGUUCAAGAACUUAAGACAtt | UGUCUUAAGUUCUUGAACCtt | Mm00620549_m1 |
| GGAAAUGCUCAAAGAUUUUtt | AAAAUCUUUGAGCAUUUCCtc | |||
| GGAUUACUGAUCUCCAAAAtt | UUUUGGAGAUCAGUAAUCCtt | |||
| NM_030238 | Dync1h1 | GGAGAAAGAAUUCAUUUCCtt | GGAAAUGAAUUCUUUCUCCtc | Mm00466548_m1 |
| GCUCCUGUGAUUGAUGCAGtt | CUGCAUCAAUCACAGGAGCtc | |||
| GGAGGUUAUGUUUAAAACUtt | AGUUUUAAACAUAACCUCCtc | |||
| NM_010063 | Dync1i1 | GGAAGAGGAGAGGAAGAAGtt | CUUCUUCCUCUCCUCUUCCtt | Mm01135515_m1 |
| CCCAAAAUUGGUCAUGAUUtt | AAUCAUGACCAAUUUUGGGtt | |||
| GGAAGAAAAACAGCAGAUCtt | GAUCUGCUGUUUUUCUUCCtc | |||
| NM_010064 | Dync1i2 | CCAUUCUACGAGAAUUGUAtt | UACAAUUCUCGUAGAAUGGtc | Mm01333946_m1 |
| GCAGAUUAACAUCUUCUUUtt | AAAGAAGAUGUUAAUCUGCtc | |||
| GGAAAGGAAAAAAAAGGAAtt | UUCCUUUUUUUUCCUUUCCtc | |||
| NM_007835 | Dctn1 | CCACAUCAAGUUCACCCAGtt | CUGGGUGAACUUGAUGUGGtc | Mm01184845_m1 |
| GGAAGUAUUUCACAUGUGAtt | UCACAUGUGAAAUACUUCCtt | |||
| CCUGGAAACAUCAUGUAGUtt | ACUACAUGAUGUUUCCAGGtc | |||
| NM_019682 | Dynll1 | GGAGUUUGACAAGAAGUACtt | GUACUUCUUGUCAAACUCCtt | Mm00850282_g1 |
| GGUGGCCAUUCUUCUGUUCtt | GAACAGAAGAAUGGCCACCtg | |||
| GGACUGCAUCCAAAUUCCAtt | UGGAAUUUGGAUGCAGUCCtt | |||
| NM_009342 | dynlt1 | GGAACCACAUGACUUCAGCtt | GCUGAAGUCAUGUGGUUCCtg | Not available |
| GGCAGUACCACUUGUCUUAtt | UAAGACAAGUGGUACUGCCtg | |||
| GGUGCUAAAAACUCAAGUCtt | GACUUGAGUUUUUAGCACCtt | |||
| NM_025975 | Dynlt3 | GCAUAGUGGAACAGUCUAUtt | AUAGACUGUUCCACUAUGCtt | Mm00458834_m1 |
| GGCCAUUAACUUAAGUUUGtt | CAAACUUAAGUUAAUGGCCtt | |||
| GCCCGUAUGGAUUUCACACtt | GUGUGAAAUCCAUACGGGCtc | |||
| NM_025947 | Dynlrb1 | CCUUCCUUCGAAUUCGCUCtt | GAGCGAAUUCGAAGGAAGGtt | Mm00508951_m1 |
| GGCUUUGGAAUGAGAGCUUtt | AAGCUCUCAUUCCAAAGCCtg | |||
| GGCAUUCCCAUCAAGAGCAtt | UGCUCUUGAUGGGAAUGCCtt | |||
| NM_026556 | Dynll2 | GGCCAUGGAGAAGUACAACtt | GUUGUACUUCUCCAUGGCCtg | Not available |
| GGACAUUGCUGCCUAUAUCtt | GAUAUAGGCAGCAAUGUCCtt | |||
| GGUCUGAAGUAUAGCAAUGtt | CAUUGCUAUACUUCAGACCtg | |||
| NM_029297 | dylnrb2 | GGAAUAUCUUCUGAUUGUCtt | GACAAUCAGAAGAUAUUCCtt | Mm00466467_m1 |
| CGAAUCCAUGAUAUUGUACtt | GUACAAUAUCAUGGAUUCGtg | |||
| CGACCUGACUUUUCUUAGGtt | CCUAAGAAAAGUCAGGUCGtt | |||
| NM_146229 | dync1li1 | CCGGCUAAGAAAGACAAUUtt | AAUUGUCUUUCUUAGCCGGtc | Mm01353886_m1 |
| GCUACAGUCUCUUUUAGCAtt | UGCUAAAAGAGACUGUAGCtt | |||
| GCCUUGGACUGCUUUGGAUtt | AUCCAAAGCAGUCCAAGGCtt | |||
| NM_001013380 | dync1li2 | GCAGGUUAAGUAGCUGACAtt | UGUCAGCUACUUAACCUGCtt | Dync1li2 |
| CGUGCUGACUCAUAACCUGtt | CAGGUUAUGAGUCAGCACGtt | |||
| CGUAGCACUUCUUCUCUUAtt | UAAGAGAAGAAGUGCUACGtt |
We found eight minus end–directed motors or associated proteins and 11 plus end–directed motors involved in at least one nuclear movement behavior (Figure 2B), suggesting a high degree of complexity for the regulation of nuclear positioning in muscle cells. Several motors affect only one nuclear behavior; depletion of members of the kinesin-3 family (Kif13a, Kif13b), the dynein heavy chain (Dync1h1), and the dynein light chain LC8-type2 (Dynll2) solely increase nuclear speed. Kif13a and Kif13b are mainly involved in endosome trafficking (Delevoye et al., 2009, 2014). However, recent evidence demonstrated that the Drosophila homologue of Kif13b, khc73, is implicated, together with dynein heavy chain, in spindle positioning by anchoring MTs to the cell cortex (Lu and Prehoda, 2013). We previously showed that dynein is important in nuclear centration movement, probably through its localization at the nuclear envelope (Cadot et al., 2012). In contrast, others found that depletion of dynein reduces nuclear speed in myotubes (Wilson and Holzbaur, 2012). These differences may stem from the fact that their measurements were done 168 h after differentiation, when nuclear movement characteristics (speed and percentage of time in motion) could be different from those in our analysis (60 h after differentiation). Because Dynll2 is involved in the formation of the dynein complex (Rapali et al., 2011), its identification as playing a role in nuclear speed is in agreement with previous reports and emphasizes the connection between dynein and Kif13b.
The time in motion of nuclei was reduced exclusively after silencing of Kif26a, Kif27, Dync1i1, Dync1i2, or Dync1li2. Little is known about the function of Kif26a and Kif27, whereas Dync1i1, Dync1i2, and Dync1li2 are cargo adapters for dynein. Their absence could thus induce a delay in the recruitment of motors involved in moving the nucleus.
A few motors affect two types of nuclei behavior without altering others. Kif4 knockdown before differentiation leads to a decrease in speed and time in motion. Kif4 is known to be involved in the alignment of MTs in Xenopus (Mitchison et al., 2013) and might have the same activity during myotube formation. Depletion of Kifc1, Kifc2, Dynlrb1, or Dynll1 affects both nuclei speed and time in motion but does not perturb nuclei alignment (Figure 2A). Kifc1, also known as HSET, is as a centrosome-clustering protein in cancers with supernumerary centrosomes (Chavali et al., 2016) but is also capable of transporting the MT minus-end protein γ-tubulin (Lecland and Lüders, 2014). Because there is a relocalization of MT nucleation toward the nuclear envelope and a disappearance of centrosomes during differentiation (Tassin et al., 1985), Kifc1 might affect these events and thereby perturb normal nuclear positioning.
Unexpectedly, knockdown of kinesin light chain 1, 2, or 3 did not effect nuclear movement or organization. As previously suggested, however, redundancy of Klc1 and 2 may require double knockdown of these proteins to observe an effect on nuclear aggregation (Wilson and Holzbaur, 2015).
As expected, only knockdown of the motor protein Kif5b altered the three types of nuclear behavior (Figure 2A). Kif5b was already implicated in nuclear rotation, movement, and positioning in muscle cells (Metzger et al., 2012; Wilson and Holzbaur, 2012, 2015). Kif5b was also identified as the kinesin responsible for basally directing nuclear movement in neurons and thus contributing to neurogenesis (Tsai et al., 2010).
Motors affecting nuclear alignment
Because nuclear alignment might depend on a complex mechanism, we decided to focus our attention on the five motor proteins whose depletion affects nuclei alignment: Kif5b, Kif1c, Kif1a, Kif9, and Kif21b. Kif9 couples centrosomes to nuclei (Tikhonenko et al., 2013). Kif1a and Kif1c are mutated in spastic paraplegia (Caballero Oteyza et al., 2014; Dor et al., 2014; Citterio et al., 2015), and Kif1a is associated with interkinetic nuclear movement in the developing brain (Tsai et al., 2010; Carabalona et al., 2016). Finally, Kif21b knockdown increases nuclear alignment in myotubes.
We therefore further analyzed these phenotypes by measuring the spreading factor, that is, the ratio between the average observed distances between nuclei and the theoretically optimal distance. We found a similar reduction of this ratio in Kif5b-, Kif1a-, and Kif1c-knockdown myotubes (Figure 2C). Unexpectedly, this factor was nearly unchanged in myotubes depleted for Kif9 or Kif21b. Kif9-silenced myotubes display aggregated nuclei, but the phenotype is different from the one observed in Kif5b- or Kif1a-depleted myotubes. In the kif9 siRNA cohort, nuclei form distinct clusters along the length of the myotube, yielding a higher average distance between nuclei than in Kif5b-knockdown myotubes (Figure 2, C and E, and Supplemental Figure S2D). Concerning Kif21b depletion, the increase in the percentage of myotubes with aligned nuclei does not imply an increase in nuclear spreading.
To understand whether these motors affect differentiation, we measured, besides the fusion index (Supplemental Figure S1CD), the number of nuclei per myotube, which represents myotube size (Figure 2D). Kif5b or Kif9 depletion shows an increase in the fusion index while maintaining a normal number of nuclei per myotube. This suggests that even if kif5b and kif9 affect nuclear positioning, they do not interfere with the myogenic program. On the other hand, Kif1a or Kif1c depletion does not affect the fusion index but affects the number of nuclei per myotube. The most plausible explanation is that this result stems from a reduction in the number of cells available for fusion. Kif1a or Kif1c must therefore have an effect on the cycle of division before differentiation. Nevertheless, both motors remain interesting candidates for nuclear aggregation. Finally, Kif21b depletion decreases both the fusion index and the number of nuclei per myotube, suggesting a dual role, one for nuclear alignment and one for differentiation.
MT motors could affect nuclear movement and positioning by modifying MT organization or dynamics. To answer this question, we analyzed the orientation and length of EB1 comets in myotubes over a period of 200 s after depletion of a few motors (Supplemental Figure S1, D and E). This quantification of the MT growing plus ends allowed us to estimate the dynamics of the MTs and their organization. Most MTs grow parallel to the longest axis of the myotube (Supplemental Figure S1D). Only slight modifications in comet orientation were found after depletion of Kif5b or Dynlrb1, which could correlate with the observed differences in nuclear behavior. Surprisingly, the lengths of the EB1 comets were significantly modified after depletion of several motors without any correlation to their individual effects on nuclear movement (Supplemental Figure S1E). We hypothesize that normal nuclear movement might rely on different aspects—an oriented MT network, dynamic MTs, and molecular motors—to produce the necessary forces.
The nucleus represents the biggest organelle in the cell, and these findings illustrate the complexity required to move as well as organize the distribution of multiple nuclei in a large muscle cell. Several interesting candidates remain to be explored for their specific role, such as the proteins affecting two parameters of nuclear behavior. This opens new routes of research toward understanding intracellular organization.
MATERIALS AND METHODS
Cell line
C2C12 cells were cultured in DMEM with 10% fetal bovine serum (Invitrogen) and antibiotics (penicillin at 100 U/ml and streptomycin at 100 μg/ml) and were plated on 0.1% gelatin-coated dishes or acid-washed coverslips for 1–2 d before differentiation. Differentiation was induced by switching to DM (DMEM with 1% horse serum).
Chemicals and plasmids
Drugs were applied on 48-h-differentiated C2C12 cells and directly time-lapse recorded during 10 h. STA parameters were quantified during this time period. Taxol was from Calbiochem (580555), nocodazole from Sigma-Aldrich (M1404), cytochalasin D from Calbiochem (250255), and jasplakinolide from Santa Cruz Biotechnology (sc-202191A). YFP-Δ-227-spastin (C-terminus truncation) was a gift from Brett Lauring (Columbia University, New York).
siRNA transfection
Cells were transfected with three different siRNAs per gene using RNAiMAX, following the manufacturer’s instructions (Life Technologies), 2 d before differentiation. For spastin cDNA transfection, we used Lipofectamine 2000 (Life Technologies), and cells were switched to DM 12 h after transfection. siRNAs were obtained from Life Technologies (primers used are listed in Table 1).
Microscopy
Epifluorescence images were acquired using a Nikon Ti microscope equipped with a CoolSNAP HQ2 camera (Roper Scientific) and an XY-motorized stage (Nikon) driven by MetaMorph (Molecular Devices). Multipositioning images were stitched with MetaMorph. For live imaging, we used an incubator to maintain cultures at 37°C and 5% CO2 (Okolab).
Quantification of nuclear movement and positioning inside myotubes
We used the SkyPad algorithm as previously described (Cadot et al., 2014) to quantify speed and time in motion. Briefly, nuclei centroids positions were manually picked every 15 min over 15 h, and coordinates were transferred to an Excel spreadsheet before running the SkyPad algorithm. For nuclear positioning, we identified three possible situations: nuclei are spread along the myotube axis (aligned), nuclei are clustered together (aggregated), and there is a mix of both (other). To calculate the spreading factor, we measured the nuclei interdistances and the length of the myotube. The spreading factor is the observed average interdistance over the optimal interdistance, that is, the myotube length divided by the number of nuclei.
Quantification of fusion index and number of nuclei per myotubes
The fusion index was calculated as percentage of nuclei found inside myotubes (more than two nuclei) over the total number of nuclei present in the same area (3.79 mm2) at the end of each movie, 88 h after switching to differentiation medium.
Validation of siRNA knockdown by Western blot and reverse transcription quantitative PCR
At 48 h after silencing, cells were lysed in 1% SDS in phosphate-buffered saline (PBS) and passed through Qiashredder columns. Equal amounts of proteins were loaded on 4–12% gradient gels, followed by transfer on nitrocellulose membrane using the iBlot apparatus (Life Technologies). Primary antibodies were from Bethyl (Kif1b, Klc2), BD (Dcnt1), and Santa Cruz Biotechnology (kif5b). mRNAs from C2C12 cells were isolated with the RNeasy 96 Kit (Qiagen), and cDNA was prepared with the High-Capacity cDNA Reverse Transcription Kit (Life Technologies). Quantitative PCR (qPCR) analyses were performed using Taqman Gene Expression assay (Life Technologies; see Table 1) in a StepOne Plus system (Applied Biosystems). For Kif5b, Kif1a, and Kif26b, mRNAs from C2C12 cells were isolated with the RNeasy Micro Kit (Qiagen), and cDNA was prepared with the Transcriptor First-Strand cDNA Synthesis Kit (Roche). qPCR was performed using a CyberGreen Kit in a LightCycler 480 II system (Roche), using the following primers: Kif5b-F, 5′-GGAGGCAAGCAGTCGTAAAC-3′; Kif5b-R, 5′-TCTAGTGTTGGGAAGCAGCA-3′; Kif1a-F, 5′-GAAGACTCCCTCCCCTGTTC-3′, Kif1a-R, 5′-ATCTCTCCACCGTGTCCTTG-3′, Kif26b-F, 5′-TGGGGAACCATTCGAAATTA, Kif26b-R, 5′-AGGACCTGCTCCAAGTCAAA-3′; Hprt1-F, 5′-GTTAAGCAGTACAGCCCCAAA-3′; and Hprt1-R, 5′-AGGGCATATCCAACAACAAACTT-3′.
Statistics
A Gaussian distribution of averaged speeds of each nucleus was tested using the D’Agostino and Pearson omnibus normality test for each condition (GraphPad software); it appeared that the distribution was not Gaussian, and so the statistical significance between conditions was measured using the Mann–Whitney test for non-Gaussian distributions. A normal Student’s t test was used otherwise.
Supplementary Material
Acknowledgments
We thank members of the Gomes lab for helpful discussions and W. Roman for correcting the manuscript. B.C. was supported initially by a Fondation pour la Recherche Médicale fellowship. V.G. was supported initially by a Region Ile-de-France fellowship. This work was supported by Muscular Dystrophy Association, INSERM Avenir Programme, and Agence Nationale de la Recherche grants to E.R.G. and Association Institut de Myologie and Agence Nationale de la Recherche grants to B.C.
Abbreviations used:
- GFP
green fluorescent protein
- MT
microtubules
- siRNA
small interfering RNA.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E16-06-0405) on February 8, 2017.
REFERENCES
- Abmayr SM, Pavlath GK. Myoblast fusion: lessons from flies and mice. Development. 2012;139:641–656. doi: 10.1242/dev.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangy A, Lane HA, d’ Hérin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Brown SS, Spudich JA. Cytochalasin inhibits the rate of elongation of actin filament fragments. J Cell Biol. 1979;83:657–662. doi: 10.1083/jcb.83.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruusgaard JC, Liestøl K, Ekmark M, Kollstad K, Gundersen K. Number and spatial distribution of nuclei in the muscle fibres of normal mice studied in vivo. J Physiol. 2003;551:467–478. doi: 10.1113/jphysiol.2003.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb MR, Spector I, Beyer BB, Fosen KM. Effects of jasplakinolide on the kinetics of actin polymerization an explanation for certain in vivo observations. J Biol Chem. 2000;275:5163–5170. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- Caballero Oteyza A, Battaloğlu E, Ocek L, Lindig T, Reichbauer J, Rebelo AP, Gonzalez MA, Zorlu Y, Ozes B, Timmann D, et al. Motor protein mutations cause a new form of hereditary spastic paraplegia. Neurology. 2014;82:2007–2016. doi: 10.1212/WNL.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadot B, Gache V, Gomes ER. Fast, multi-dimensional and simultaneous kymograph-like particle dynamics (SkyPad) analysis. PLoS One. 2014;9:e89073. doi: 10.1371/journal.pone.0089073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadot B, Gache V, Vasyutina E, Falcone S, Birchmeier C, Gomes ER. Nuclear movement during myotube formation is microtubule and dynein dependent and is regulated by Cdc42, Par6 and Par3. EMBO Rep. 2012;13:741–749. doi: 10.1038/embor.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabalona A, Hu DJ-K, Vallee RB. KIF1A inhibition immortalizes brain stem cells but blocks BDNF-mediated neuronal migration. Nat Neurosci. 2016;19:253–262. doi: 10.1038/nn.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavali PL, Chandrasekaran G, Barr AR, Tátrai P, Taylor C, Papachristou EK, Woods CG, Chavali S, Gergely F. A CEP215-HSET complex links centrosomes with spindle poles and drives centrosome clustering in cancer. Nat Commun. 2016;7:11005. doi: 10.1038/ncomms11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citterio A, Arnoldi A, Panzeri E, Merlini L, D’Angelo MG, Musumeci O, Toscano A, Bondi A, Martinuzzi A, Bresolin N, Bassi MT. Variants in KIF1A gene in dominant and sporadic forms of hereditary spastic paraparesis. J Neurol. 2015;262:2684–2690. doi: 10.1007/s00415-015-7899-9. [DOI] [PubMed] [Google Scholar]
- Dauer WT, Worman HJ. The nuclear envelope as a signaling node in development and disease. Dev Cell. 2009;17:626–638. doi: 10.1016/j.devcel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Delevoye C, Hurbain I, Tenza D, Sibarita J-B, Uzan-Gafsou S, Ohno H, Geerts WJC, Verkleij AJ, Salamero J, Marks MS, Raposo G. AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J Cell Biol. 2009;187:247–264. doi: 10.1083/jcb.200907122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevoye C, Miserey-Lenkei S, Montagnac G, Gilles-Marsens F, Paul-Gilloteaux P, Giordano F, Waharte F, Marks MS, Goud B, Raposo G. Recycling endosome tubule morphogenesis from sorting endosomes requires the kinesin motor KIF13A. Cell Rep. 2014;6:445–454. doi: 10.1016/j.celrep.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobi KC, Metzger T, Baylies MK. Characterization of early steps in muscle morphogenesis in a Drosophila primary culture system. Fly (Austin) 2011;5:68–75. doi: 10.4161/fly.5.2.15031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor T, Cinnamon Y, Raymond L, Shaag A, Bouslam N, Bouhouche A, Gaussen M, Meyer V, Durr A, Brice A, et al. KIF1C mutations in two families with hereditary spastic paraparesis and cerebellar dysfunction. J Med Genet. 2014;51:137–142. doi: 10.1136/jmedgenet-2013-102012. [DOI] [PubMed] [Google Scholar]
- Englander LL, Rubin LL. Acetylcholine receptor clustering and nuclear movement in muscle fibers in culture. J Cell Biol. 1987;104:87–95. doi: 10.1083/jcb.104.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone S, Roman W, Hnia K, Gache V, Didier N, Lainé J, Auradé F, Marty I, Nishino I, Charlet-Berguerand N, et al. N-WASP is required for amphiphysin-2/BIN1-dependent nuclear positioning and triad organization in skeletal muscle and is involved in the pathophysiology of centronuclear myopathy. EMBO Mol Med. 2014:6, 1455–1475. doi: 10.15252/emmm.201404436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, Worman HJ. Nuclear positioning. Cell. 2013;152:1376–1389. doi: 10.1016/j.cell.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungbluth H, Wallgren-Pettersson C, Laporte J. Centronuclear (myotubular) myopathy. Orphanet J Rare Dis. 2008;3:26. doi: 10.1186/1750-1172-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmikanth GS, Warrick HM, Spudich JA. A mitotic kinesin-like protein required for normal karyokinesis, myosin localization to the furrow, and cytokinesis in Dictyostelium. Proc Natl Acad Sci USA. 2004;101:16519–16524. doi: 10.1073/pnas.0407304101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecland N, Lüders J. The dynamics of microtubule minus ends in the human mitotic spindle. Nat Cell Biol. 2014;16:770–778. doi: 10.1038/ncb2996. [DOI] [PubMed] [Google Scholar]
- Lu MS, Prehoda KE. A NudE/14-3-3 pathway coordinates dynein and the kinesin Khc73 to position the mitotic spindle. Dev Cell. 2013;26:369–380. doi: 10.1016/j.devcel.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger T, Gache V, Xu M, Cadot B, Folker ES, Richardson BE, Gomes ER, Baylies MK. MAP and kinesin-dependent nuclear positioning is required for skeletal muscle function. Nature. 2012;484:120–124. doi: 10.1038/nature10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Nguyen P, Coughlin M, Groen AC. Self-organization of stabilized microtubules by both spindle and midzone mechanisms in Xenopus egg cytosol. Mol Biol Cell. 2013;24:1559–1573. doi: 10.1091/mbc.E12-12-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapali P, Szenes Á, Radnai L, Bakos A, Pál G, Nyitray L. DYNLL/LC8: a light chain subunit of the dynein motor complex and beyond. FEBS J. 2011;278:2980–2996. doi: 10.1111/j.1742-4658.2011.08254.x. [DOI] [PubMed] [Google Scholar]
- Rotsch C, Radmacher M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophys J. 2000;78:520–535. doi: 10.1016/S0006-3495(00)76614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin AM, Maro B, Bornens M. Fate of microtubule-organizing centers during myogenesis in vitro. J Cell Biol. 1985;100:35–46. doi: 10.1083/jcb.100.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonenko I, Magidson V, Graf R, Khodjakov A, Koonce MP. A kinesin-mediated mechanism that couples centrosomes to nuclei. Cell Mol Life Sci. 2013;70:1285–1296. doi: 10.1007/s00018-012-1205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J-W, Lian W-N, Kemal S, Kriegstein A, Vallee RB. An unconventional kinesin and cytoplasmic dynein are responsible for interkinetic nuclear migration in neural stem cells. Nat Neurosci. 2010;13:1463–1471. doi: 10.1038/nn.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez R, Howell B, Yvon A, Wadsworth P, Cassimeris L. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol Biol Cell. 1997;8:973–985. doi: 10.1091/mbc.8.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SR, Evans KJ, Lary J, Cole JL, Lauring B. Recognition of C-terminal amino acids in tubulin by pore loops in Spastin is important for microtubule severing. J Cell Biol. 2007;176:995–1005. doi: 10.1083/jcb.200610072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MH, Holzbaur ELF. Opposing microtubule motors drive robust nuclear dynamics in developing muscle cells. J Cell Sci. 2012;125:4158–4169. doi: 10.1242/jcs.108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MH, Holzbaur ELF. Nesprins anchor kinesin-1 motors to the nucleus to drive nuclear distribution in muscle cells. Development. 2015;142:218–228. doi: 10.1242/dev.114769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Takeda S, Nakata T, Noda Y, Tanaka Y, Hirokawa N. Role of KIFC3 motor protein in Golgi positioning and integration. J Cell Biol. 2002;158:293–303. doi: 10.1083/jcb.200202058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Roberts EA, Goldstein LS. Functional analysis of mouse kinesin motor Kif3C. Mol Cell Biol. 2001;21:5306–5311. doi: 10.1128/MCB.21.16.5306-5311.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Bossy-Wetzel E, Jiang W. Recruitment of MKLP1 to the spindle midzone/midbody by INCENP is essential for midbody formation and completion of cytokinesis in human cells. Biochem J. 2005;389:373–381. doi: 10.1042/BJ20050097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.