Abstract
The thermal decomposition of Nafion N117 membrane, a typical perfluorosulfonic acid membrane that is widely used in various chemical technologies, was investigated in this study. Structural identification of thermolysis products in water and methanol was performed using liquid chromatography-electrospray ionization-tandem mass spectrometry (LC/ESI-MS/MS). The fluoride release was studied using an ion-chromatography system, and the membrane thermal stability was characterized by thermogravimetric analysis. Notably, several types of perfluorinated compounds (PFCs) including perfluorocarboxylic acids were detected and identified. Based on these data, a thermolysis mechanism was proposed involving cleavage of both the polymer backbone and its side chains by attack of radical species. This is the first systematic report on the thermolysis products of Nafion by simulating its high-temperature operation and disposal process via incineration. The results of this study indicate that Nafion is a potential environmental source of PFCs, which have attracted growing interest and concern in recent years. Additionally, this study provides an analytical justification of the LC/ESI-MS/MS method for characterizing the degradation products of polymer electrolyte membranes. These identifications can substantially facilitate an understanding of their decomposition mechanisms and offer insight into the proper utilization and effective management on these membranes.
In recent decades, polymer electrolyte membranes (PEMs) have played an increasingly important role in numerous areas of chemical, biological and engineering applications, such as fuel cells, electrodialyzers and sensors1,2. In particular, PEM fuel cells, a promising zero-emission power source, have been extensively investigated owing to their great potential in supplying energy to automobiles, for stationary power generation and in portable electronic devices3. Previous research has shown that cell performance and lifetime were closely related to the characteristics of PEMs as observed under operating conditions4,5,6. As a typical perfluorosulfonic acid (PFSA) membrane, Nafion consists of a polytetrafluoroethylene (PTFE) backbone with perfluoroalkylether pendant chains terminating in sulfonic acid groups. Nafion has been widely employed in fuel cells because of its high proton conductivity, good chemical stability and high mechanical strength1,7,8. Recently, studies have primarily focused on its chemical degradation in fuel cells7,9,10,11, its utilization in modified electrodes12,13, and its use in the removal of environmental pollutants14,15. In particular, the chemical degradation of Nafion in PEM fuel cells can largely deteriorate cell performance and durability with decomposition mechanisms consistently attributed to the chemical attack by trace radical species such as hydroxyl radicals9,10,11.
In view of the increasing use of Nafion in various chemical technologies, it is important to establish waste treatment techniques for this PEM. As suggested by the manufacturer (DuPont), incineration is one of the current approaches for Nafion disposal16. According to the “trace chemistries of fire” hypothesis proposed by Crummett and Townsend17, almost all processes of combustion are incomplete and can cause the formation of a broad set of chemical products. This viewpoint can also be applied to thermolysis processes, of which numerous products are commonly generated and are highly influenced by operating conditions, such as oxygen supply and temperature programming18. Several studies have shown that the thermolysis of PTFE fluoropolymer can produce a variety of perfluorinated compounds (PFCs), such as environmentally persistent perfluorocarboxylic acids (PFCAs)19,20. These chemicals have increasingly attracted worldwide concerns due to their persistence in environmental matrices and potential negative impacts on living organisms21,22,23,24. Given the PTFE backbone in Nafion, it is reasonable to assume that some similar products can also be generated when it is thermally treated. Liquid chromatography/electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS) has been recognized as a powerful analytical tool for the precise identification of multiple components. In general, this method combines the high chromatographic resolution of LC with the high specificity and sensitivity of MS/MS25,26. Notably, this method has been used to characterize the decomposition products of a specific PEM in fuel cell water27,28 and to identify perfluorooctanoic acid (PFOA), a PFCA analogue of vital importance, released from commercial cookware under operating conditions29,30. Unfortunately, little is known regarding the compositional analysis and structural identification of Nafion thermolysis products using this method.
In the present work, we report on the thermolysis products of Nafion N117 (Fig. 1), a typical PFSA membrane, absorbed in water and methanol using a LC/ESI-MS/MS screening method. The release of F− ions during PEM thermolysis was studied with an ion-chromatography system. Additionally, thermal stability was characterized by thermogravametric analysis (TGA). The objectives of this study were to investigate the possible production of environmentally significant PFCs generated via N117 thermolysis and to propose thermal degradation mechanisms based on key products observed in previous studies and additional products detected in this work. To the best of our knowledge, this study is the first to report not only on the application of a LC/ESI-MS/MS method to characterize the thermolysis products of Nafion membrane but also on the potential impacts of its incineration or high-temperature operation. It is also noteworthy that the latter could potentially cause the release of PFC species that could contribute to environmental pollution and deterioration of membrane performance.
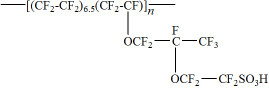
Figure 1. Chemical structure of Nafion N117 membrane.
Results
Thermogravametric analysis
The TGA curve of N117 following increasing temperatures is shown in Fig. 2 and is accompanied by enhanced presentations of certain transitions. Three main stages were observed with their temperature ranges recorded at 75–200°C, 275–420°C, and 420–600°C.
Figure 2. TGA curve of N117 membrane under nitrogen atmosphere (heating rate: 20°C min-1).
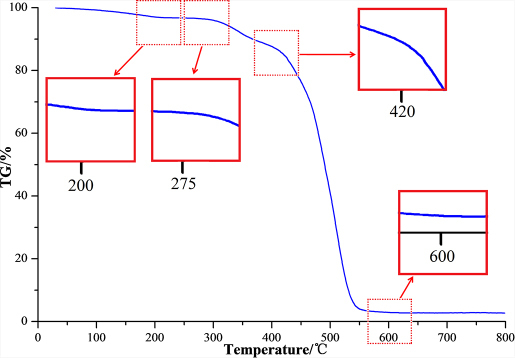
Insets represent the zoom-ins of the areas in red dashed-line boxes.
Characterization of the thermolysis products of N117
The total ion current (TIC) chromatogram of NaOH absorption solution is illustrated in Fig. 3a. The peak assignments of some products are listed in Table 1 with their proposed structures, retention times, signal intensity and MS characteristics. As shown in Fig. 3a, there were many chromatographic peaks in the TIC. Specifically, deviations of 50n (n = 1, 2, 3, …) between the m/z values of certain products correspond to the “CF2” unit. After peak classification, four groups of chemical analogues were characterized in the NaOH absorption solution.
Figure 3. Total ion current (TIC) chromatograms of NaOH (a) and methanol (b) absorption solutions showing the various thermolysis products of N117, which were analyzed by LC/TOF-MS in a negative ion mode.
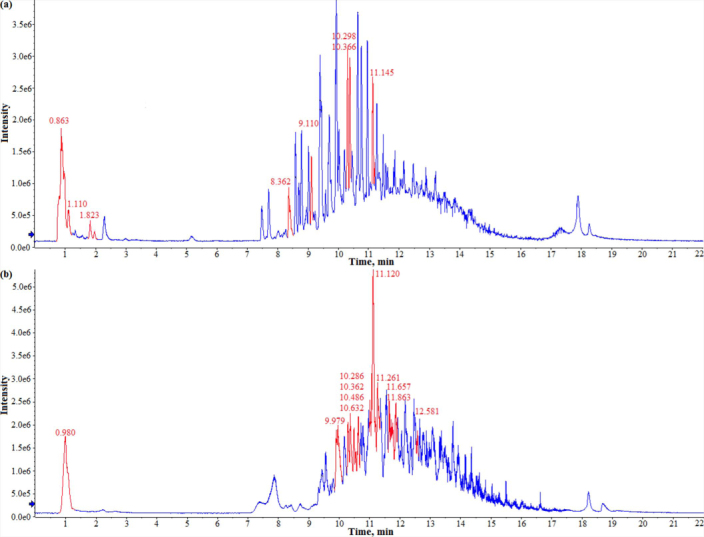
The peaks of some representative m/z values listed in Table 1 and 3 were highlighted in red.
Table 1. Peak assignments of different thermolysis products of N117 in NaOH absorption solution.
| Groups | Proposed structure | Observed m/z | Calculated m/z | Major MS/MS fragments (m/z) | RT (min) | Intensity (peak area) | m/z values of other analogues |
|---|---|---|---|---|---|---|---|
| 1 | CF3COO− | 112.9870 | 112.9856 | 68.9953, 45.0005 | 0.863 | 1.77×106 | 262.9738, 312.9721, 362.9674, 462.9630, 512.9618, 562.9593, 612.9575, 662.9552 |
| CF3(CF2)6COO− | 412.9658 | 412.9664 | 368.9759, 218.9851, 168.9885 | 10.366 | 1.94×106 | ||
| 2 | CF3(CF2)5CHFCOO− | 394.9780 | 394.9758 | 330.9707, 180.9837, 118.9896 | 10.298 | 3.14×106 | 294.9836, 344.9811, 444.9759, 494.9731, 594.9673, 644.9647, 694.9629, 744.9595 |
| CF3(CF2)8CHFCOO− | 544.9698 | 544.9663 | 480.9682, 330.9783, 168.9884 | 11.145 | 7.94×105 | ||
| 3 | CF3(CF2)4CF = CFCF2COO− | 424.9921 | 424.9664 | 380.9779, 230.9847, 192.9869 | 8.362 | 4.35×106 | 374.9818, 474.9754, 574.9961, 624.9673, 674.9550, 724.9635, 774.9514, 824.9579 |
| CF3(CF2)4CF = CF(CF2)3COO− | 524.9636 | 524.9600 | 480.9780, 330.9844, 292.9862 | 9.110 | 5.75×105 | ||
| 4 | CF3(CF2)3CF(COOH)COO− | 338.9692 | 338.9721 | 230.9825, 208.9806, 180.9861 | 1.100 | 5.24×105 | 438.9679, 488.9652, 538.9623, 588.9605, 638.9569, 688.9541, 738.9532, 788.9491 |
| CF3(CF2)4CF(COOH)COO− | 388.9697 | 388.9689 | 344.9791, 280.9850, 258.9827 | 1.823 | 8.32×105 | ||
| - | HSO4− | 96.9624 | 96.9601 | 79.9598 | 0.863 | 1.33×106 | - |
| - | CHF2SO3− | 130.9619 | 130.9620 | 79.9590 | 1.953 | 3.75×105 | - |
Group 1, represented by m/z 112.9870 and 412.9658, was tentatively identified as the PFCA analogues with the general structure of CF3(CF2)nCOO−. This structure was proposed according to their major MS/MS fragments (68.9953 and 45.0005 for m/z 112.9870; 368.9759, 218.9851 and 168.9885 for m/z 412.9658), while the structures of some representative m/z values are shown in Table 2. To further validate the generation of PFCAs, several products were subjected to the MS/MS analysis and compared with their possible standards. As shown in Supplementary Fig. S1 online, good agreement was commonly observed between these products and their standards. Additionally, based on some representative m/z values, group 2 (m/z 394.9780, 544.9698), 3 (m/z 424.9921, 524.9636) and 4 (m/z 338.9692, 388.9697) were also detected and largely classified into three different chemical analogues. Their respective structures were ambiguously proposed as CF3(CF2)nCHFCOO−, CF3(CF2)4CF = CF(CF2)nCOO− and CF3(CF2)nCF(COOH)COO− according to their major MS/MS fragments. In addition, these structures were further confirmed with minor discrepancies between the observed and calculated m/z values (Table 1).
Table 2. The proposed chemical structures of some representative m/z values detected in this study, and their possible cleavage sites (highlighted in red).
| m/z values | Molecular formula | Proposed chemical structures |
|---|---|---|
| 412.9658 | C8F15O2− | |
| 368.9759 | C7F15 | |
| 218.9851 | C4F9 | |
| 168.9885 | C3F7 | |
| 394.9780 | C8HF14O2− | |
| 330.9707 | C7F13 | |
| 180.9837 | C4F7 | |
| 118.9896 | C2F5 | |
| 96.9624 | HSO4− | |
| 79.9598 | SO3 | |
| 130.9619 | CHF2SO3− | |
| 79.9590 | SO3 |
Additionally, two deprotonated molecular [M-H]− ions at m/z 96.9624 and 130.9619 corresponded to the structures of HSO4− and CHF2SO3−. Their identifications were confirmed by comparison with the N117 structure and the observed MS/MS fragments (79.9598 and 79.9590, Table 2), corresponding to the loss of an OH unit [M-H− 17]− and a CHF2 unit [M-H− 51]−, respectively. In addition, sulfate ions (SO42-), together with F− ions, were also detected in the IC (see Supplementary Fig. S2 online), with their individual retention times at 6.853 and 3.037 min. Further validation was performed by comparing these data with the IC peaks of NaF (20mg L−1) and Na2SO4 (20mg L−1).
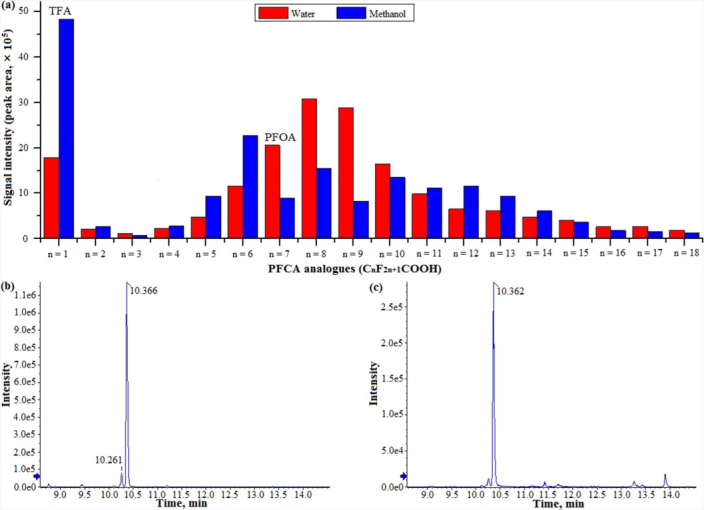
The TIC chromatogram of N117 thermolysis products absorbed with methanol is shown in Fig. 3b, and the peak assignments of some products are presented in Table 3. Their possible structures were ambiguously proposed based on the MS/MS fragments (see Supplementary Table S1 online) of two representative m/z values for each group (236.9847, 286.9848 for group 4; 252.9848, 352.9721 for group 5; 472.9934, 522.9896 for group 6; 340.9925, 390.9877 for group 7; 158.9915, 208.9867 for group 8). The detailed interpretations on the MS/MS fragments of five representative PFC analogues and the other products are illustrated in Supplementary Fig. S3 and Fig. S4 online, respectively. As for the formation of PFCA products, different analogues in NaOH and methanol absorption solutions and extracted ion chromatograms (EIC) corresponding to the deprotonated molecular ion [M-H]− of PFOA (m/z 412.97) are presented in Fig. 4. Notably, PFCAs with different chain lengths were commonly found, although their presence varied between the two different extraction solvents.
Table 3. Peak assignments of different thermolysis products of N117 in methanol absorption solution.
| Groups | Proposed structure | Observed m/z | Calculated m/z | Major MS/MS fragments (m/z) | RT (min) | Intensity (peak area) | m/z values of other analogues |
|---|---|---|---|---|---|---|---|
| 1 | CF3COO− | 112.9873 | 112.9856 | 68.9949, 44.9993 | 0.923 | 4.81×106 | 312.9878, 362.9899, 462.9922, 512.9930, 662.9994, 713.0036 |
| CF3(CF2)6COO− | 412.9717 | 412.9664 | 368.9806, 218.9876, 168.9904 | 10.362 | 8.42×105 | ||
| 2 | CF3(CF2)3CHFCOO− | 294.9905 | 294.9822 | 230.9909, 158.9882, 112.9850 | 11.109 | 8.08×106 | 244.9953,344.9981, 394.9987, 495.0019, 595.0047, 645.0054, 695.0098, 745.0105 |
| CF3(CF2)6CHFCOO− | 444.9944 | 444.9727 | 380.9920, 230.9915, 180.9902 | 10.286 | 7.96×105 | ||
| 3 | CF3(CF2)4CF = CF(CF2)2COO− | 474.9938 | 474.9632 | 430.9848, 280.9808, 230.9835 | 10.632 | 6.56×106 | 224.9881, 274.9885, 374.9907, 424.9921, 524.9946, 624.9987, 675.0003, 725.0028 |
| CF3(CF2)4CF = CF(CF2)4COO− | 574.9644 | 574.9568 | 530.9744, 380.9800, 330.9825 | 11.261 | 6.43×105 | ||
| 4 | CF3CF = CFCF = CFCOO− | 236.9847 | 236.9792 | 170.9888, 142.9928, 108.9902 | 0.980 | 5.24×105 | 336.9906, 386.9922, 436.9935, 687.0254 |
| CF3CF = CFCF = CFCF2COO− | 286.9848 | 286.9760 | 220.9881, 192.9917, 154.9932 | 9.979 | 8.32×105 | ||
| 5 | CF3CF = CFCF2COOCO− | 252.9848 | 252.9741 | 208.9844, 180.9592, 142.9922 | 11.006 | 9.09×105 | 302.9853, 453.0097, 503.0107, 553.0125, 603.0129, 503.0109, 553.0125, 653.0149 |
| CF3CF = CF(CF2)3COOCO− | 352.9721 | 352.9677 | 308.9998, 230.9866, 142.9924 | 10.486 | 9.61×105 | ||
| 6 | CF3(CF2)5CHFCOOCF2CO− | 472.9934 | 472.9676 | 394.9811, 308.9812, 242.9885 | 12.581 | 1.10×106 | 373.0142, 573.0012, 623.0195, 673.0138 |
| CF3(CF2)6CHFCOOCF2CO− | 522.9896 | 522.9644 | 444.9932, 428.9963, 358.9896 | 12.581 | 2.02×106 | ||
| 7 | CF3(CF2)3CHFCOOCH(OH)O− | 340.9925 | 340.9877 | 294.9863, 274.9785, 230.9881 | 11.120 | 8.23×105 | 290.9849, 491.0097, 591.0127, 641.0146, 691.0177 |
| CF3(CF2)4CHFCOOCH(OH)O− | 390.9877 | 390.9845 | 344.9841, 280.9861, 230.9982 | 11.657 | 2.20×106 | ||
| 8 | CF3CF = CFCO− | 158.9915 | 158.9875 | 92.9954 | 11.006 | 1.15×106 | 258.9938, 409.0153, 659.0071, 709.0101, 959.0201 |
| CF3CF = CFCF2CO− | 208.9867 | 208.9843 | 180.9911, 142.9937, 130.9936 | 11.863 | 6.98×105 | ||
| - | HSO4− | 96.9605 | 96.9601 | 79.9579 | 0.871 | 1.28×106 | - |
| - | CHF2SO3− | 130.9619 | 130.9620 | 79.9584 | 1.895 | 6.49×105 | - |
Figure 4. LC/ESI-MS/MS analysis of the PFCA analogues (CnF2n+1COOH, n = 1-18) in NaOH and methanol absorption solutions (a), and extracted ion chromatograms (EIC) corresponding to the deprotonated molecular ion [M-H]− of PFOA (m/z 412.97) in NaOH (b) and methanol (c) absorption solutions.
Discussion
To become commercialized in various chemical applications, Nafion-related technologies such as PEM fuel cells must be able to function within a wide temperature range and under temperature cycling conditions7. Currently, a number of studies have been conducted to design fuel cells that can operate above 100°C to enhance electrochemical kinetics, simplify cooling systems, facilitate water management, and improve system CO tolerance3,5,7. However, operation of these fuel cells for an extended time at high temperatures can accelerate membrane degradation and inevitably leads to concerns regarding the thermal stability and response of the Nafion electrolyte. Furthermore, the waste disposal of Nafion components via incineration may produce undesirable thermal decomposition products. Thus, the current study was conducted to systematically investigate the thermolysis of Nafion membranes by TGA, LC/ESI-MS/MS and IC analysis.
Currently, TGA has been commonly utilized as an effective tool for studying the thermolysis of chemicals6,31,32. Specifically, the thermal stability of PFSA membranes (e.g., Nafion) has been previously studied by this method6,33,34. This study has consistently shown three stages of thermal degradation in Nafion. These stages correspond to the loss of water, the cleavage of C-S bond, and the decomposition of the perfluorinated matrix, respectively. In the present research, these three stages were also observed during N117 thermolysis. These results were largely in accordance with several previous reports6,34. A detailed analysis of the thermal decomposition process of N117 is presented in the following sections.
To better analyze the possible thermolysis products of N117, two different solvents were used in parallel for the products absorption. The two solvents were water [polarity index (P) = 9, dipole moment (DM) = 1.85], with NaOH dissolved in it, and methanol (P = 5.1, DM = 1.70), thus yielding two different absorption solutions. In a recent study, Kaur et al.35 adopted five different extraction solvents of various polarity or “extraction strengths” to initiate the chemical characterization of the combustion products of chlorogenic acid. Furthermore, several previous studies have also used water and/or methanol to extract PFOA from PTFE fluoropolymer36,37 and the surface of commercial cookware30. In our study, more thermolysis products were detected in methanol than those in NaOH solution, which may be attributed to the higher extraction strength of methanol.
Among these thermolysis products, several new bonds, such as C-H, C = O and C = C, were introduced into the proposed structures when compared to the structure of N117. Generally, these bonds have been reported to exist in the main chain of Nafion as undesired byproducts of the manufacturing process38,39. On the other hand, Danilczuk et al.40 reported the formation of C-H and C = O bonds during Nafion degradation in the fuel cell by 2D spectral-spatial FTIR. Additionally, the formation of C = C bonds has been suggested experimentally and theoretically in alkaline treated polyvinylidene fluoride (PVDF) membrane41,42. This may be equally applied to Nafion thermolysis given their structural similarity. Hydroxyl groups were partially incorporated into N117 thermolysis products, contributing to their structural diversity. In a previous study, hydroxyl groups were also observed in PVDF membranes treated with alkaline solutions42.
Notably, in this work, PFCA analogues of chain lengths ranging from C1 to C18 were readily observed during N117 thermolysis. In the recent past, PFCAs have been ubiquitously detected and characterized as persistent, bioaccumulative, and toxic (PBT) pollutants21,22,23,24. Thus, increasing attention has been focused on understanding the behavior and fate of these analogues in multiple environmental matrices. In an effort to reduce the environmental impact of PFCA analogues, systematic studies are required to elucidate their potential environmental sources. Previous studies have suggested that PFCA emission during manufacturing had the greatest environmental impact43,44. Meanwhile, the formation of PFCAs from some potential precursors such as fluorotelomer alcohols45, polyfluoroalkyl phosphates46, and polyfluorinated amides47, has also been confirmed. The current study suggests that the thermolysis process of N117 could also be a potential environmental source of PFCAs, as validated by comparing the MS/MS data against standards (see Supplementary Fig. S1 online). Specifically, trifluoroacetic acid (TFA) was detected as the major analogue. Additionally, the amount of long-chain PFCAs (n > 8) was found to decrease with the increasing chain lengths. These observations coincided well with the product composition of PTFE thermolysis20. TFA, one of the major pollutants in rainwater48, has also been observed in fuel cell degradation tests49 and Nafion degradation in subcritical water with zerovalent metals8. Notably, long-chain PFCAs have gained specific interest by some global regulatory communities50,51. They have been regarded as vPvB chemicals (very persistent and very bioaccumulative) due to their degradation resistance and high accumulation potentials52. Together with PFOA, they were included in the Candidate List of Substances of Very High Concern under the European chemical regulation, REACH53.
In addition to several groups of chemical analogues, CHF2SO3− and SO42- were also observed as two single products during N117 thermolysis. Specifically, the formation of CHF2SO3− was also found in Nafion degradation using zerovalent metals in subcritical water8, suggesting the possible linkage of its pendant chain. Previous research on Nafion degradation has also indicated the generation of SO42- and F− ions whether under in situ (fuel cell operation) or ex situ (Fenton test) conditions9,54,55. Recently, Yu et al.11, using quantum mechanical calculations, proposed two mechanisms for OH• attack on Nafion polymer that also involved the formation of these two ions. Notably, SO42- and F− ions were observed by solution analysis of Nafion after being heated at 433 K for 12 h54, which is consistent with results from the current study on N117 thermal decomposition.
It is noteworthy to mention that in this study several different thermolysis products, especially the PFCs, were detected and identified after the solution analysis. These new-found substances may also be generated under the high-temperature conditions found during the operation of PEM fuel cells, and they have the potential to impact cell performance and lifetime. Regarding the researchers who focus on extending the cell durability, the possible effects induced by these products should be systematically evaluated and effectively avoided. Additionally, it is well established that PFCs, such as those produced during Nafion disposal, will significantly contribute to pollution on a global scale. In addition to the PFCAs, several previously unreported PFCs such as groups 3–5 were also observed as the thermolysis products of Nafion fluoropolymer. Moreover, some long-chain PFCs, which have been reported to be more bioaccumulative than short-chain analogues21,56, were also found in this investigation. Although most efforts thus far have focused on PFCAs, it is also of vital importance to assess and understand the environmental disposition and toxicity of some other PFCs.
Despite the increasing interest in elucidating the chemical degradation of Nafion (see Supplementary text online), a limited amount of research has been conducted to systematically understand the possible mechanisms of Nafion thermolysis. In this study, a mechanism was tentatively proposed as involving the cleavage of both polymer backbone and its side chains by attack of radical species. Samms et al.34 and Wilkie et al.57 proposed a thermal degradation mechanism of Nafion at higher temperatures that included an initial C-S bond cleavage to yield sulphur dioxide, OH• radicals, and a carbon-based radical. In our work, a variety of thermolysis products of N117 were identified. Relevant to the PFCA analogues generated, Ellis et al.19 reported several pathways in the thermal decomposition of PTFE polymer that involved the formation of carbene radicals, longer-chain diradicals, and a series of reactions with other species. These proposed pathways may also be adapted to N117 thermolysis given the shared PTFE backbone. Alternately, C = C bonds in the products may form from an attack of OH• radicals on the unintentionally introduced C-H defects present in the main chains (eq 1).
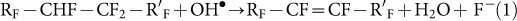 |
In addition, the common detection of HSO4− and CHF2SO3− in different absorption solutions indicated cleavage of the Nafion side chain. The formation of these species was also found accompanying chemical degradation8,54,55. In general, thermally produced radicals can react with the pendant chain under high temperatures, leading to the production of •CF(CF3)OC2F4SO3−. This species has also been reported in Nafion degradation using zerovalent metals in subcritical water8. Subsequent to its production, this radical can be cleaved to generate CF3COF, and further hydrolysed to form TFA (eq 2-3).
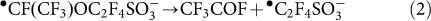 |
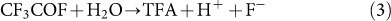 |
Recently, radical reactions involving the Nafion side chain have been theoretically confirmed by frontier orbital theory55. In this study, the calculated highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) showed a wide distribution of the electron cloud around the side chain. This phenomenon may also indicate potential reaction sites preferred by radicals generated during N117 thermolysis. Although detailed mechanisms on the formation of some species, such as CHF2SO3−, remain unclear, the obtained results regarding these thermolysis products can help to elucidate a more accurate mechanism of Nafion thermal degradation. Recently, two investigations also utilized the LC/ESI-MS/MS method to determine the degradation products of sulfonated polyarylether membrane in fuel cell water27,28. Together with the current work, these studies collectively indicate the potential this method has for characterizing the products of PEM decomposition, which may be triggered by attack of radical species generated under realistic operating conditions.
Conclusions
The thermal degradation of Nafion was investigated by mimicking the conditions of high-temperature operation typical of several chemical applications, and the waste disposal process via incineration. By analyzing two different absorption solutions, multiple groups of related thermal decomposition products were identified and their structures were proposed. These results indicated the structural diversity of these thermolysis products, which allowed for proposing thermal degradation mechanisms. With regards to technical applications, such as PEM fuel cells, these thermally generated Nafion products may potentially hinder cell performance and lifetime. With respect to the environment, the production of numerous PFCs, such as PFCAs, during Nafion disposal will contribute to pollution on a global scale. It is important to note that this study has identified a potential source of PFCs, which will continue as a class of emerging POPs representing a long-term challenge to scientists in the foreseeable future. Additionally, this study showed that the LC/ESI-MS/MS screening method is a powerful and effective tool for the detailed elucidation of the decomposition mechanisms of the current-use PEMs in chemical technologies.
Methods
Chemicals
Nafion N117 membranes (basic weight: 360g m−2, typical thickness: 183μm) were obtained from Shanghai Hesen Electric Co., Ltd (Shanghai, China). The chemical structure is presented in Fig. 1. No pretreatment was performed on this membrane prior to its use. The LC/MS grade solvents were used for the chromatographic separation (water and acetonitrile) and thermolytic absorption (methanol). Together with the additive formic acid, they were supplied from Merck (Darmstadt, Germany) and utilized without further purification. PFCA analogues (see Supplementary Table S2 online for their structures) were obtained from J&K Chemical Co., Ltd. (Shanghai, China). Sodium hydroxide (NaOH), sodium fluoride (NaF) and sodium sulfate (Na2SO4) were purchased from Nanjing Chemical Reagent Co., Ltd. (Nanjing, China). Aqueous solutions were prepared with ultrapure water (> 18.2MΩ cm), obtained from a Milli-Q Plus system (Millipore, Bedford, MA, USA).
Thermogravimetric analysis (TGA)
The thermal stability of N117 was characterized using a Perkin-Elmer TGA 4000 instrument (Perkin-Elmer Inc., Wellesley, MA, USA). TGA test was conducted with a specimen weighting approximately 20mg at a constant heating rate of 20°C min−1 over a temperature range of 30–800°C under nitrogen atmosphere.
Thermolysis procedure
Approximately 0.5g N117 pieces were heated in specialized pyrolysis equipment (AZ-HC-06a, Tianjin Aozhan Technology Co., Ltd, Tianjin, China). The operating parameters including the atmosphere, heating temperatures and running time were set according to the literature16 with a slight modification, while the related thermolysis data are cited in Supplementary Table S3 online. Specifically, the atmosphere was air, and flow rate was set as 13mL min−1. The N117 sample was heated in a stainless steel tube at 10°C min−1 to 200°C, and then 5°C min−1 to 600°C, where the temperature was maintained for an additional 20 minutes, for a total run time of approximately 117 minutes. The products released from N117 thermolysis were absorbed using 0.5L 0.05mol L−1 NaOH solution and methanol. Afterwards, Nafion residues were immersed in these solutions and shaken for 20 min for an adequate products elusion. These absorption solutions were then filtered through 0.22μm membrane filter, and the filtrate was collected for subsequent analysis.
LC/ESI-MS/MS analysis
HPLC was carried out on an Agilent Infinity 1260 series LC system (Agilent Technologies, Santa Clara, CA, USA). The system was equipped with G1379B degasser, G1312B binary pump, and G1329B autosampler. Chromatographic separation was performed at a flow rate of 300μL min−1 using a Thermo BDS Hypersil C18 column (2.1mm × 100mm, particle size 2.4μm) (Thermo Fisher Scientific, Waltham, MA) that was maintained at 30°C. The mobile phase consisted of 0.3% formic acid in water (A) and acetonitrile (B). The linear gradient was initially 90% A, which was held isocratic for 2 min, decreased to 10% in 9 min, which was held under this condition for 2.5 min, and returned back to the starting condition in 0.5 min followed by 8 min equilibration. The injection volume was 10μL, and elution time was 22 min for each sample.
Mass spectrometric analysis was performed with a Q-TOF MS (TripleTOF 5600, AB Sciex, Concord, ON, Canada) operating in the negative ion mode using an electrospray ion source. Mass range of TOF MS was m/z 50-1000. The other parameters were set as follows: curtain gas, 35 (arbitrary units); ion source gas 1, 55 (arbitrary units); ion source gas 2, 55 (arbitrary units); temperature, 550°C; ionspray voltage floating, -4500kV; declustering potential, -80V; collision energy, −10eV. The MS/MS experiments were conducted in product ion scan mode at variable collision energies (20–50eV). The data were acquired in a data-dependent mode that used criteria from the previous MS scan to select the target precursor ions that would be submitted to MS/MS fragmentation. The final chemical structure of an unknown compound was identified based on the accurate mass measurements of the parent ions and fragments obtained from the MS/MS experiments. The high-resolution LC-MS/MS data were acquired using Analyst TF 1.6 (AB Sciex) and processed using PeakView 1.2 (AB Sciex).
Ion chromatography analysis (ICA)
F− ions released from N117 thermolysis were studied using an ion-chromatography system (ICS-1000, Dionex, USA). This system was equipped with an autosampler (injection volume: 25μL), a pump, a degasser, a guard column, and a separation column (Dionex IonPac AS 12A, 4mm i.d × 200mm, USA) operating at 30°C. The mobile phase was 20mM KOH and flow rate was set at 1.0mL min−1. Data acquisition and processing were carried out using Chromeleon 6.80 software (Dionex Corporation, Sunnyvale, CA, USA).
Supplementary Material
Supplementary Information
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (No. 41071319, 21377051), the Major Science and Technology Program for Water Pollution Control and Treatment of China (No. 2012ZX07506-001) and the Scientific Research Foundation of Graduate School of Nanjing University (2013CL08).
Footnotes
The authors declare no competing financial interests.
Author Contributions M.B.F., L.S.W. and Z.Y.W. designed the project. M.B.F., P.S. and Z.B.W. performed the experiments and analyzed the data. M.B.F. wrote the manuscript. R.J.Q. revised the manuscript. All authors reviewed the paper.
References
- Iwai Y., Hiroki A., Tamada M. & Yamanishi T. Radiation deterioration in mechanical properties and ion exchange capacity of Nafion N117 swelling in water. J. Membrane Sci. 322, 249–255 (2008). [Google Scholar]
- Iwai Y., Hiroki A. & Tamada M. Radiation-induced crosslinking of Nafion® N117CS membranes. J. Membrane Sci. 369, 397–403 (2011). [Google Scholar]
- Devanathan R. Recent developments in proton exchange membranes for fuel cells. Energ. Environ. Sci. 1, 101–119 (2008). [Google Scholar]
- Collier A., Wang H. J., Yuan X. Z., Zhang J. J. & Wilkinson D. P. Degradation of polymer electrolyte membranes. Int. J. Hydrogen Energ. 31, 1838–1854 (2006). [Google Scholar]
- Wu J. F. et al. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 184, 104–119 (2008). [Google Scholar]
- Mu S. C. et al. Degradation behaviors of perfluorosulfonic acid polymer electrolyte membranes for polymer electrolyte membrane fuel cells under varied acceleration conditions. J. Appl. Polym. Sci. 129, 1586–1592 (2013). [Google Scholar]
- Rodgers M. P., Bonville L. J., Kunz H. R., Slattery D. K. & Fenton J. M. Fuel cell perfluorinated sulfonic acid membrane degradation correlating accelerated stress testing and lifetime. Chem. Rev. 112, 6075–6103 (2012). [DOI] [PubMed] [Google Scholar]
- Hori H., Murayama M., Sano T. & Kutsuna S. Decomposition of perfluorinated ion-exchange membrane to fluoride ions using zerovalent metals in subcritical water. Ind. Eng. Chem. Res. 49, 464–471 (2010). [Google Scholar]
- Chen C., Levitin G., Hess D. W. & Fuller T. F. XPS investigation of Nafion® membrane degradation. J. Power Sources 169, 288–295 (2007). [Google Scholar]
- Ghassemzadeh L., Kreuer K. D., Maier J. & Müller K. Chemical degradation of Nafion membranes under mimic fuel cell conditions as investigated by solid-state NMR spectroscopy. J. Phys. Chem. C 114, 14635–14645 (2010). [Google Scholar]
- Yu T. H. et al. Mechanism for degradation of Nafion in PEM fuel cells from quantum mechanics calculations. J. Am. Chem. Soc. 133, 19857–19863 (2011). [DOI] [PubMed] [Google Scholar]
- Yeh M. H. et al. A low-cost counter electrode of ITO glass coated with a graphene/Nafion® composite film for use in dye-sensitized solar cells. Carbon 50, 4192–4202 (2012). [Google Scholar]
- Qiu Y. Y., Qu X. J., Dong J., Ai S. Y. & Han R. X. Electrochemical detection of DNA damage induced by acrylamide and its metabolite at the graphene-ionic liquid-Nafion modified pyrolytic graphite electrode. J. Hazard. Mater. 190, 480–485 (2011). [DOI] [PubMed] [Google Scholar]
- Lien H. L. & Zhang W. X. Removal of methyl tert-butyl ether (MTBE) with Nafion. J. Hazard. Mater. 144, 194–199 (2007). [DOI] [PubMed] [Google Scholar]
- Nasef M. M. & Yahaya A. H. Adsorption of some heavy metal ions from aqueous solutions on Nafion 117 membrane. Desalination 249, 677–681 (2009). [Google Scholar]
- DuPont Fuel Cells. . Safe Handling and Use of Perfluorosulfonic Acid Products (Technical Information). (2009). [Google Scholar]
- Crummet W. B. & Townsend D. I. The trace chemistries of fire hypothesis: review and update. Chemosphere 13, 777–788 (1984). [Google Scholar]
- Wichmann H., Heitmann K., Annur S., Vogt R. & Bahadir M. A. Emission and burnt smell characteristics from combustion experiments with defined materials. Chemosphere 88, 650–654 (2012). [DOI] [PubMed] [Google Scholar]
- Ellis D. A., Mabury S. A., Martin J. W. & Muir D. C. G. Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Nature 412, 321-324 (2001). [DOI] [PubMed] [Google Scholar]
- Ellis D. A., Martin J. W., Muir D. C. G. & Mabury S. A. The use of 19F NMR and mass spectrometry for the elucidation of novel fluorinated acids and atmospheric fluoroacid precursors evolved in the thermolysis of fluoropolymers. Analyst 128, 756–764 (2003). [DOI] [PubMed] [Google Scholar]
- Lindstrom A. B., Strynar M. J. & Libelo E. L. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 45, 7954–7961 (2011). [DOI] [PubMed] [Google Scholar]
- Houde M., De Silva A. O., Muir D. C. G. & Letcher R. J. Monitoring of perfluorinated compounds in aquatic biota: An updated review. Environ. Sci. Technol. 45, 7962–7973 (2011). [DOI] [PubMed] [Google Scholar]
- Cheng X. G. & Klaassen C. D. Perfluorocarboxylic acids induce cytochrome P450 enzymes in mouse liver through activation of PPAR-α and CAR transcription factors. Toxicol. Sci. 106, 29–36 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulhaq M., Örn S., Carisson G., Morrison D. A. & Norrgren L. Locomotor behavior in zebrafish (Danio rerio) larvae exposed to perfluoroalkyl acids. Aquat. Toxicol. 144-145, 332–340 (2013). [DOI] [PubMed] [Google Scholar]
- Hossain M. B., Rai D. K., Brunton N. P., Martin-Diana A. B. & Barry-Ryan C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agr. Food Chem. 58, 10576–10581 (2010). [DOI] [PubMed] [Google Scholar]
- Jäntti S. E. et al. Steroid and steroid glucuronide profiles in urine during pregnancy determined by liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal. Chim. Acta 802, 56–66 (2013). [DOI] [PubMed] [Google Scholar]
- Zedda M., Tuerk J., Teutenberg T., Peil S. & Schmidt T. C. A strategy for the systematic development of a liquid chromatographic mass spectrometric screening method for polymer electrolyte membrane degradation products using isocratic and gradient phase optimized liquid chromatography. J. Chromatogr. A 1216, 8910–8917 (2009). [DOI] [PubMed] [Google Scholar]
- Zedda M., Tuerk J., Peil S. & Schmidt T. C. Determination of polymer electrolyte membrane (PEM) degradation products in fuel cell water using electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Sp. 24, 3531–3538 (2010). [DOI] [PubMed] [Google Scholar]
- Powley C. R., Michalczyk M. J., Kaiser M. A. & Buxton L. M. Determination of perfluorooctanoic acid (PFOA) extractable from the surface of commercial cookware under simulated cooking conditions by LC/MS/MS. Analyst 130, 1299–1302 (2005). [DOI] [PubMed] [Google Scholar]
- Bononi M. & Tateo F. Identification of perfluorooctanoic acid release from commercial coated cooking pans by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Am. J. Agri. Biol. Sci. 2, 191–194 (2007). [Google Scholar]
- Damartzis,. Th.,. Vamvuka D., Sfakiotakis S. & Zabaniotou A. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresource Technol. 102, 6230–6238 (2011). [DOI] [PubMed] [Google Scholar]
- Lee H. J. et al. Application of TGA techniques to analyze the compositional and structural degradation of PEMFC MEAs. Polym. Degrad. Stabil. 97, 1010–1016 (2012). [Google Scholar]
- Surowiec J. & Bogoczek R. Studies on the thermal stability of the perfluorinated cation-exchange membrane Nafion-417. J. Therm. Anal. Calorim. 33, 1097–1102 (1988). [Google Scholar]
- Samms S. R., Wasmus S. & Savinell R. F. Thermal stability of Nafion® in simulated fuel cell environments. J. Electrochem. Soc. 143, 1498–1504 (1996). [Google Scholar]
- Kaur N., Lacasse M., Fürtös A., Waldron K. C. & Morin A. Sequential fractionation with concurrent chemical and toxicological characterization of the combustion products of chlorogenic acid. J. Chromatogr. A 1216, 4703–4712 (2009). [DOI] [PubMed] [Google Scholar]
- Larsen B. S., Kaiser M. A., Botelho M., Wooler G. R. & Buxton L. W. Comparison of pressurized solvent and reflux extraction methods for the determination of perfluorooctanoic acid in polytetrafluoroethylene polymers using LC-MS-MS. Analyst 130, 59–62 (2005). [DOI] [PubMed] [Google Scholar]
- Larsen B. S., Kaiser M. A., Botelho M. A., Bachmura S. F. & Buxton L. W. Efficient “total” extraction of perfluorooctanoate from polytetrafluoroethylene fluoropolymer. Analyst 131, 1105–1108 (2006). [DOI] [PubMed] [Google Scholar]
- Curtin D. E., Lousenberg R. D., Henry T. J., Tangeman P. C. & Tisack M. E. Advanced materials for improved PEMFC performance and life. J. Power Sources 131, 41–48 (2004). [Google Scholar]
- Yu T. H. et al. The effect of different environments on Nafion degradation: Quantum mechanics study. J. Membrane Sci. 437, 276–285 (2013). [Google Scholar]
- Danilczuk M., Lancucki L., Schlick S., Hamrock S. J. & Haugen G. M. In-depth profiling of degradation processes in a fuel cell: 2D spectral-spatial FTIR spectra of Nafion membranes. ACS Macro Lett. 1, 280–285 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang S. C., Shen J., Qiu X. P., Weng D. S. & Zhu W. T. ESR and vibrational spectroscopy study on poly(vinylidene fluoride) membranes with alkaline treatment. J. Power Sources 153, 234–238 (2006). [Google Scholar]
- Zhao X. D., Song L. Z., Fu J., Tang P. & Liu F. Experimental and DFT investigation of surface degradation of polyvinylidene fluoride membrane in alkaline solution. Surf. Sci. 605, 1005–1015 (2011). [Google Scholar]
- Armitage J. et al. Modeling global-scale fate and transport of perfluorooctanoate emitted from direct sources. Environ. Sci. Technol. 40, 6969–6975 (2006). [DOI] [PubMed] [Google Scholar]
- Prevedouros K., Cousins I. T., Buck R. C. & Korzeniowski S. H. Source, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 40, 32–44 (2006). [DOI] [PubMed] [Google Scholar]
- Styler S. A., Myers A. L. & Donaldson D. J. Heterogeneous photooxidation of fluorotelomer alcohols: A new source of aerosol-phase perfluorinated carboxylic acids. Environ. Sci. Technol. 47, 6358–6367 (2013). [DOI] [PubMed] [Google Scholar]
- Lee H., D’eon J. & Mabury S. A. Biodegradation of polyfluoroalkyl phosphates as a source of perfluorinated acids to the environment. Environ. Sci. Technol. 44, 3305–3310 (2010). [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Wallington T. J. & Mabury S. A. Atmospheric oxidation of polyfluorinated amides: Historical source of perfluorinated carboxylic acids to the environment. Environ. Sci. Technol. 47, 4317–4324 (2013). [DOI] [PubMed] [Google Scholar]
- Taniyasu S. et al. Analysis of trifluoroacetic acid and other short-chain perfluorinated acids (C2–C4) in precipitation by liquid chromatography–tandem mass spectrometry: comparison to patterns of long-chain perfluorinated acids (C5–C18). Anal. Chim. Acta 619, 221–230 (2008). [DOI] [PubMed] [Google Scholar]
- Chen C. & Fuller T. F. The effect of humidity on the degradation of Nafion® membrane. Polym. Degrad. Stabil. 94, 1436–1447 (2009). [Google Scholar]
- USEPA,. United States Environmental Protection Agency. . Long-chain perfluorinated chemicals (PFCs) action plan. (2009) Available at: http://www.epa.gov/opptintr/existingchemicals/pubs/pfcs_action_plan1230_09.pdf. (Cited: 11st December 2010) [Google Scholar]
- OECD,. Organisation for Economic Co-operation and Development. . OECD portal on perfluorinated chemicals. (2011) Available at: http://www.oecd.org/site/0,3407,en_21571361_44787844_1_1_1_1_1,00.html. (Cited: 8th February 2011) [Google Scholar]
- Wang Z. Y., Cousins I. T., Scheringer M. & Hungerbühler K. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ. Int. 60, 242–248 (2013). [DOI] [PubMed] [Google Scholar]
- ECHA,. European Chemical Agency. . Candidate list of substances of very high concern for authorization. (2013) Available at: http://echa.europa.eu/web/guest/candidate-list-table. (Accessed: 14th September 2013) [Google Scholar]
- Akiyama Y. et al. Study on degradation process of polymer electrolyte by solution analysis. J. Power Sources 195, 5915–5921 (2010). [Google Scholar]
- Uegaki R., Akiyama Y., Tojo S., Honda Y. & Nishijima S. Radical-induced degradation mechanism of perfluorinated polymer electrolyte membrane. J. Power Sources 196, 9856–9861 (2011). [Google Scholar]
- Buck R. C. et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Intergr. Environ. Assess. Manag. 7, 513–541 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie C. A., Thomsen J. R. & Mittleman M. L. Interaction of poly(methyl methacrylate) and nafions. J. Appl. Polym. Sci. 42, 901–909 (1991). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


