Abstract
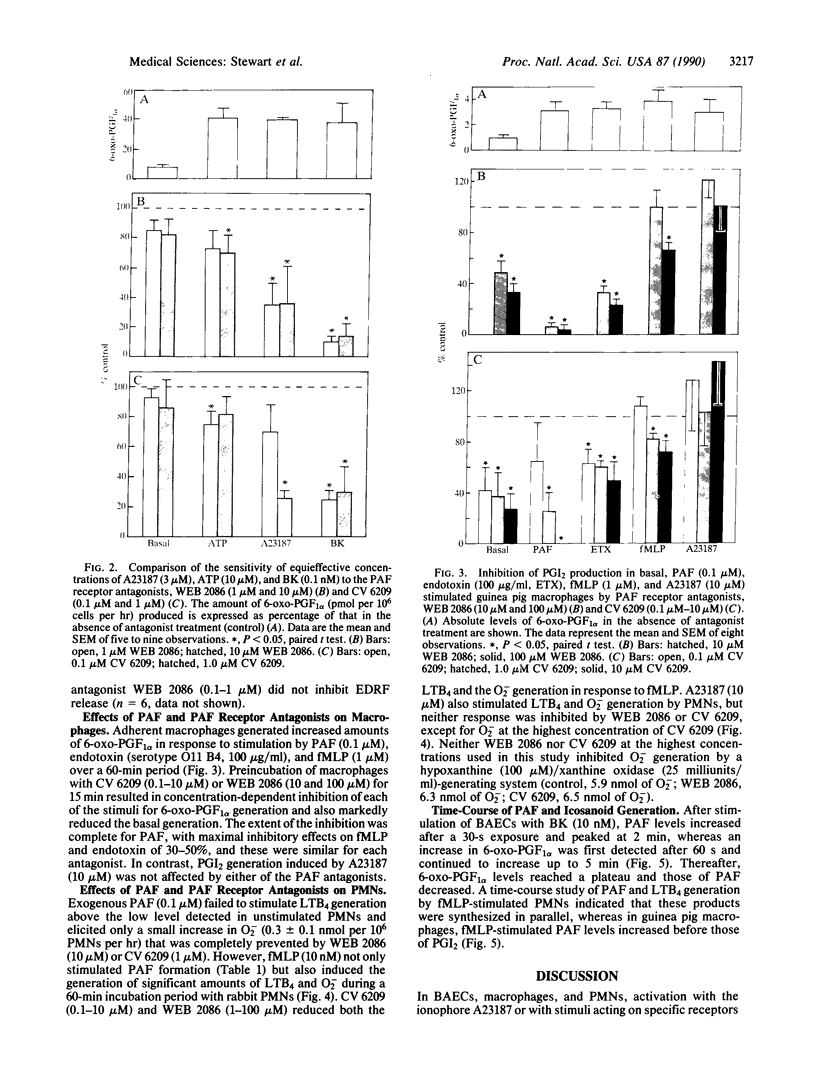
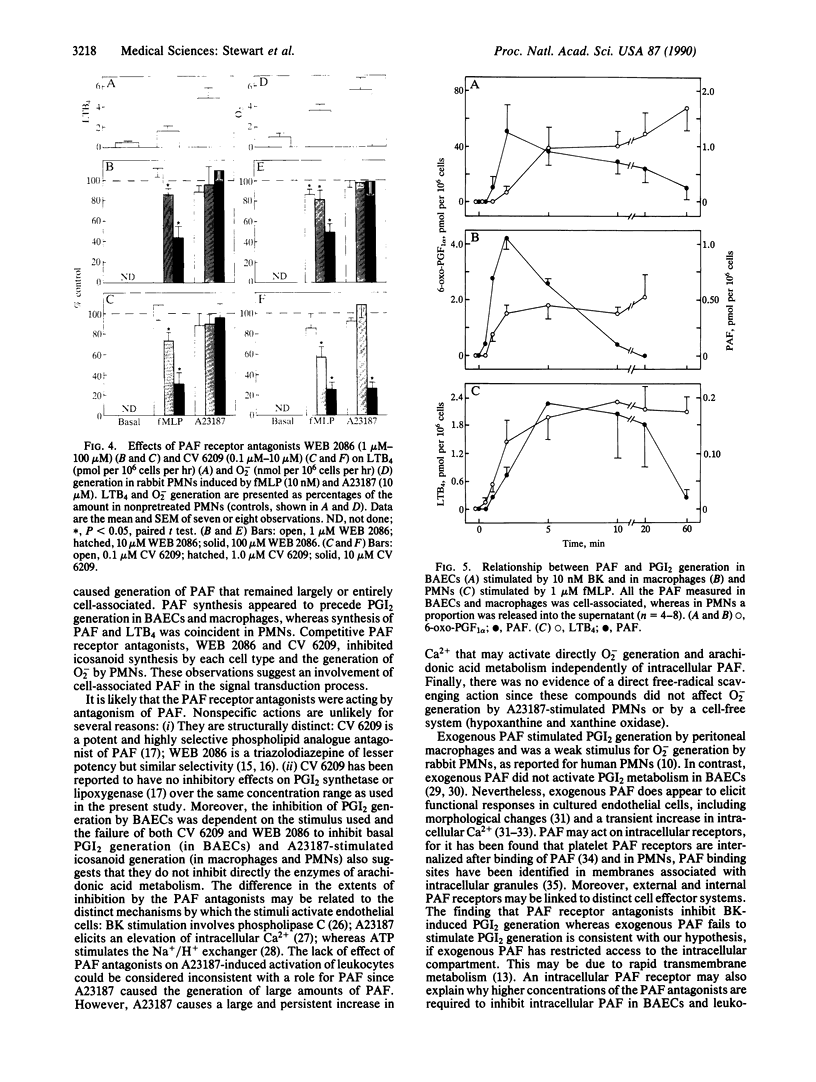
Platelet-activating factor (PAF) is generated by endothelial cells, polymorphonuclear leukocytes, and macrophages after activation by appropriate receptor agonists, but much of the PAF remains intracellular. We have investigated whether PAF formation is important for the subsequent generation of icosanoids and superoxide anions by these cells. The generation of prostacyclin and leukotriene B4 were measured by radioimmunoassay, superoxide anion was measured by reduction of cytochrome c, and PAF was measured by bioassay. In each cell type, PAF formation preceded or accompanied icosanoid generation. Bradykinin-induced prostacyclin generation in endothelial cells was markedly reduced by the PAF receptor antagonists WEB 2086 or CV 6209. In guinea pig adherent macrophages in vitro, basal prostacyclin generation and that induced by endotoxin and fMet-Leu-Phe were inhibited by either WEB 2086 (1-100 microM) or CV 6209 (0.1-10 microM). In isolated rabbit polymorphonuclear leukocytes, fMet-Leu-Phe stimulated the generation of both leukotriene B4 and superoxide anion. WEB 2086 and CV 6209 caused concentration-dependent inhibition of both these markers of leukocyte activation. These observations lead us to suggest that PAF may be a second messenger in leukocytes and endothelial cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessin P., Bonnet J., Apffel D., Soulard C., Desgroux L., Pelas I., Benveniste J. Acute circulatory collapse caused by platelet-activating factor (PAF-acether) in dogs. Eur J Pharmacol. 1983 Jan 21;86(3-4):403–413. doi: 10.1016/0014-2999(83)90190-5. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Sedlak B. J., Rafelson M. E., Jr Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975 Dec 15;34(3):825–839. [PubMed] [Google Scholar]
- Bourgain R. H., Maes L., Braquet P., Andries R., Touqui L., Braquet M. The effect of 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine (paf-acether) on the arterial wall. Prostaglandins. 1985 Aug;30(2):185–197. doi: 10.1016/0090-6980(85)90184-4. [DOI] [PubMed] [Google Scholar]
- Braquet P., Touqui L., Shen T. Y., Vargaftig B. B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987 Jun;39(2):97–145. [PubMed] [Google Scholar]
- Brock T. A., Gimbrone M. A., Jr Platelet activating factors alters calcium homeostasis in cultured vascular endothelial cells. Am J Physiol. 1986 Jun;250(6 Pt 2):H1086–H1092. doi: 10.1152/ajpheart.1986.250.6.H1086. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Camussi G., Aglietta M., Braquet P., Bosia A., Pescarmona G., Sanavio F., D'Urso N., Marchisio P. C. Human endothelial cells are target for platelet-activating factor. I. Platelet-activating factor induces changes in cytoskeleton structures. J Immunol. 1987 Oct 1;139(7):2439–2446. [PubMed] [Google Scholar]
- Camussi G., Aglietta M., Coda R., Bussolino F., Piacibello W., Tetta C. Release of platelet-activating factor (PAF) and histamine. II. The cellular origin of human PAF: monocytes, polymorphonuclear neutrophils and basophils. Immunology. 1981 Feb;42(2):191–199. [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Aglietta M., Malavasi F., Tetta C., Piacibello W., Sanavio F., Bussolino F. The release of platelet-activating factor from human endothelial cells in culture. J Immunol. 1983 Nov;131(5):2397–2403. [PubMed] [Google Scholar]
- Casals-Stenzel J., Muacevic G., Weber K. H. Pharmacological actions of WEB 2086, a new specific antagonist of platelet activating factor. J Pharmacol Exp Ther. 1987 Jun;241(3):974–981. [PubMed] [Google Scholar]
- Casals-Stenzel J. Protective effect of WEB 2086, a novel antagonist of platelet activating factor, in endotoxin shock. Eur J Pharmacol. 1987 Mar 17;135(2):117–122. doi: 10.1016/0014-2999(87)90602-9. [DOI] [PubMed] [Google Scholar]
- Clark M. A., Bomalaski J. S., Conway T. M., Wartell J., Crooke S. T. Differential effects of aspirin and dexamethasone on phospholipase A2 and C activities and arachidonic acid release from endothelial cells in response to bradykinin and leukotriene D4. Prostaglandins. 1986 Nov;32(5):703–708. doi: 10.1016/0090-6980(86)90192-9. [DOI] [PubMed] [Google Scholar]
- Dusting G. J., Read M. A., Stewart A. G. Endothelium-derived relaxing factor released from cultured cells: differentiation from nitric oxide. Clin Exp Pharmacol Physiol. 1988 Feb;15(2):83–92. doi: 10.1111/j.1440-1681.1988.tb01049.x. [DOI] [PubMed] [Google Scholar]
- Grigorian G. Y., Ryan U. S. Platelet-activating factor effects on bovine pulmonary artery endothelial cells. Circ Res. 1987 Sep;61(3):389–395. doi: 10.1161/01.res.61.3.389. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Moncada S., Palmer R. M. Bioassay of prostacyclin and endothelium-derived relaxing factor (EDRF) from porcine aortic endothelial cells. Br J Pharmacol. 1986 Apr;87(4):685–694. doi: 10.1111/j.1476-5381.1986.tb14586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D., Needham L. A. Thrombin-stimulated elevation of human endothelial-cell cytoplasmic free calcium concentration causes prostacyclin production. Biochem J. 1988 Apr 1;251(1):243–249. doi: 10.1042/bj2510243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Hwang S. B. Identification of a second putative receptor of platelet-activating factor from human polymorphonuclear leukocytes. J Biol Chem. 1988 Mar 5;263(7):3225–3233. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvin-Marche E., Ninio E., Beaurain G., Tence M., Niaudet P., Benveniste J. Biosynthesis of Paf-acether (platelet-activating factor). VII. Precursors of Paf-acether and acetyl-transferase activity in human leukocytes. J Immunol. 1984 Aug;133(2):892–898. [PubMed] [Google Scholar]
- Kitazono T., Takeshige K., Cragoe E. J., Jr, Minakami S. Intracellular pH changes of cultured bovine aortic endothelial cells in response to ATP addition. Biochem Biophys Res Commun. 1988 May 16;152(3):1304–1309. doi: 10.1016/s0006-291x(88)80427-3. [DOI] [PubMed] [Google Scholar]
- Korth R., Benveniste J. BN 52021 displaces [3H]paf-acether from, and inhibits its binding to intact human platelets. Eur J Pharmacol. 1987 Oct 27;142(3):331–341. doi: 10.1016/0014-2999(87)90071-9. [DOI] [PubMed] [Google Scholar]
- Lerner R., Lindström P., Palmblad J. Platelet activating factor and leukotriene B4 induce hyperpolarization of human endothelial cells but depolarization of neutrophils. Biochem Biophys Res Commun. 1988 Jun 16;153(2):805–810. doi: 10.1016/s0006-291x(88)81167-7. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Pohl U., Mülsch A., Busse R. Differential role of extra- and intracellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br J Pharmacol. 1988 Sep;95(1):189–196. doi: 10.1111/j.1476-5381.1988.tb16564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre T. M., Zimmerman G. A., Satoh K., Prescott S. M. Cultured endothelial cells synthesize both platelet-activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate. J Clin Invest. 1985 Jul;76(1):271–280. doi: 10.1172/JCI111957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencia-Huerta J. M., Roubin R., Morgat J. L., Benveniste J. Biosynthesis of platelet-activating factor (PAF)acether). III. Formation of PAF-acether from synthetic substrates by stimulated murine macrophages. J Immunol. 1982 Aug;129(2):804–808. [PubMed] [Google Scholar]
- Muid R. E., Twomey B., Dale M. M. The effect of inhibition of both diacylglycerol metabolism and phospholipase A2 activity on superoxide generation by human neutrophils. FEBS Lett. 1988 Jul 4;234(1):235–240. doi: 10.1016/0014-5793(88)81342-5. [DOI] [PubMed] [Google Scholar]
- Parente L., Flower R. J. Hydrocortisone and 'macrocortin' inhibit the zymosan-induced release of lyso-PAF from rat peritoneal leucocytes. Life Sci. 1985 Apr 1;36(13):1225–1231. doi: 10.1016/0024-3205(85)90266-8. [DOI] [PubMed] [Google Scholar]
- Pinckard R. N., Farr R. S., Hanahan D. J. Physicochemical and functional identity of rabbit platelet-activating factor (PAF) released in vivo during IgE anaphylaxis with PAF released in vitro from IgE sensitized basophils. J Immunol. 1979 Oct;123(4):1847–1857. [PubMed] [Google Scholar]
- Roubin R., Dulioust A., Haye-Legrand I., Ninio E., Benveniste J. Biosynthesis of paf-acether: VIII: Impairment of paf-acether production in activated macrophages does not depend upon acetyltransferase activity. J Immunol. 1986 Mar 1;136(5):1796–1802. [PubMed] [Google Scholar]
- Salmon J. A. A radioimmunoassay for 6-keto-prostaglandin F1alpha. Prostaglandins. 1978 Mar;15(3):383–397. doi: 10.1016/0090-6980(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Salmon J. A., Simmons P. M., Palmer R. M. A radioimmunoassay for leukotriene B4. Prostaglandins. 1982 Aug;24(2):225–235. doi: 10.1016/0090-6980(82)90148-4. [DOI] [PubMed] [Google Scholar]
- Sisson J. H., Prescott S. M., McIntyre T. M., Zimmerman G. A. Production of platelet-activating factor by stimulated human polymorphonuclear leukocytes. Correlation of synthesis with release, functional events, and leukotriene B4 metabolism. J Immunol. 1987 Jun 1;138(11):3918–3926. [PubMed] [Google Scholar]
- Stewart A. G., Dusting G. J. Characterization of receptors for platelet-activating factor on platelets, polymorphonuclear leukocytes and macrophages. Br J Pharmacol. 1988 Aug;94(4):1225–1233. doi: 10.1111/j.1476-5381.1988.tb11642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashita Z., Imura Y., Takatani M., Tsushima S., Nishikawa K. CV-6209, a highly potent antagonist of platelet activating factor in vitro and in vivo. J Pharmacol Exp Ther. 1987 Jul;242(1):263–268. [PubMed] [Google Scholar]
- Worthen G. S., Seccombe J. F., Clay K. L., Guthrie L. A., Johnston R. B., Jr The priming of neutrophils by lipopolysaccharide for production of intracellular platelet-activating factor. Potential role in mediation of enhanced superoxide secretion. J Immunol. 1988 May 15;140(10):3553–3559. [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Prescott S. M. Thrombin stimulates the adherence of neutrophils to human endothelial cells in vitro. J Clin Invest. 1985 Dec;76(6):2235–2246. doi: 10.1172/JCI112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
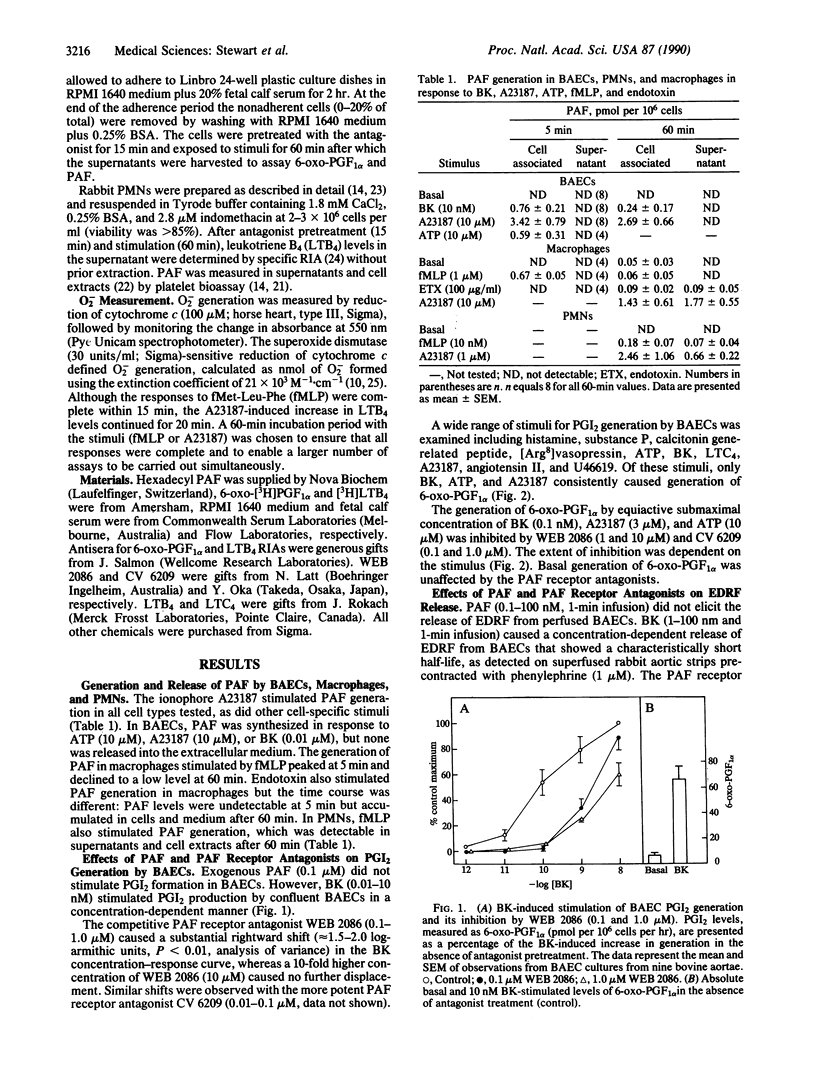
- de Nucci G., Gryglewski R. J., Warner T. D., Vane J. R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]