Abstract
Regulatory T cells (Tregs) are a subset of CD4+ T cells with suppressive function and are critical for limiting inappropriate activation of T cells. Hence, the expansion of Tregs is an attractive strategy for the treatment of autoimmune diseases. Here, we demonstrate that the skin possesses the remarkable capacity to systemically expand Treg numbers by producing thymic stromal lymphopoietin (TSLP) in response to vitamin D receptor stimulation. An ~2-fold increase in the proportion and absolute number of Tregs was observed in mice treated topically but not systemically with the Vitamin D3 analog MC903. This expansion of Tregs was dependent on TSLP receptor signaling but not on VDR signaling in hematopoietic cells. However, TSLP receptor expression by Tregs was not required for their proliferation. Rather, skin-derived TSLP promoted Treg expansion through dendritic cells. Importantly, treatment of skin with MC903 significantly lowered the incidence of autoimmune diabetes in non-obese diabetic mice and attenuated disease score in experimental autoimmune encephalomyelitis. Together, these data demonstrate that the skin has the remarkable potential to control systemic immune responses and that Vitamin D-mediated stimulation of skin could serve as a novel strategy to therapeutically modulate the systemic immune system for the treatment of autoimmunity.
Keywords: Regulatory T cells, Thymic stromal lymphopoietin, Vitamin D, Immunotherapy, Tolerance
1. Introduction
Preservation of immunologic tolerance to self is a complex process that involves both central and peripheral mechanisms. The development and maintenance of regulatory T cells (Tregs) is pivotal in the induction of peripheral tolerance [1–3]. Tregs are a subset of CD4+ T cells with suppressive function and are critical for limiting inappropriate activation of T cells. Hence, the loss of Tregs leads to development of a widespread autoimmune syndrome in both mice and humans [4–8]. Furthermore, impaired homeostasis and function within the Treg subset underlies to a number of common autoimmune diseases such as type 1 diabetes (T1D), multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematous [9–12]. Moreover, treatment strategies that increase Treg numbers have been shown to be beneficial in limiting inflammation in multiple mouse models of autoimmunity [13–15]. Thus, understanding the cellular and molecular mechanisms by which Tregs expand is fundamentally and therapeutically important.
Tregs depend on a number of other cell types in order to develop in the thymus as well as survive and expand in the periphery. Two essential cell types are conventional T cells (Tconvs) and dendritic cells (DCs) that provide cytokines as well as cellular contacts for Tregs [16–18]. For example, Tconv-derived IL-2 drives the proliferation of Tregs in conjunction with costimulatory signals provided by DCs [17, 19, 20]. Hence, the exogenous administration of IL-2 or cytokines that augment DC numbers (e.g., GMCSF or FLT3L) leads to an expansion of Tregs in the periphery [17, 18, 20]. In addition to these known factors, there is a large, complex network of interactions between immune cells and other potentially unidentified cell types important in the maintenance of Tregs in both steady state and inflammatory environments.
The interaction between the immune system and epithelial barrier surfaces has been investigated by a number of studies examining the ability of skin and intestinal resident cells to alter local immune response [21]. Tissues such as these that interface with the external environment have a powerful ability to signal and instruct a local, compartmentalized immune response [22]. In addition to controlling local immune responses, the ability of remote tissues to alter systemic immune effects has recently been appreciated. For example, the presence of segmented filamentous bacteria in the intestine has been shown to play a role in the development of autoimmune diseases in the mouse at anatomical sites far from the segmented filamentous bacteria exposure [23, 24]. As another example, treatment of the skin with a vitamin D3 analogue induces abundant quantities of thymic stromal lymphopoeitin (TSLP) from keratinocytes [25], which in turn regulates systemic hematopoiesis of basophils in the bone marrow [26].
TSLP has traditionally been recognized as a potent promoter of T helper type 2 (Th2) cell-associated cytokine responses, but the role that it may play in regulatory T cell (Treg) development has been hinted by a number of studies. These reports demonstrate a positive role of TSLP in the development of thymic Tregs (tTregs) as well as conversion of Tconvs to Tregs in the periphery [27, 28]. These studies suggest that in addition to stimulating a Th2 response, TSLP might have immunomodulatory effects by augmenting Treg development or conversion. Thus, the production of TSLP from keratinocytes might represent a mechanism by which the skin can modulate the systemic immune responses through Tregs.
To test this notion, we investigated whether Vitamin D3-stimulated skin possesses the ability to alter Treg number or function. In this study, we show that the topical application of the Vitamin D3 analog MC903 leads to a large expansion of peripheral Tregs in a TSLP-dependent and DC-dependent manner. Importantly, topical MC903 treatment decreased the incidence of disease in the non-obese diabetic (NOD) mouse model of T1D. Together, this work demonstrates a remarkable ability of skin-derived TSLP to impact systemic immune responses by expanding Tregs. The capability to manipulate an easily accessible organ such as the skin provides a clear advantage in the development of clinical therapies for inflammatory diseases.
2. Materials and Methods
2.1 Mice
TSLP receptor knockout (TSLP-R KO), C56BL/6.Thy1.1, C56BL/6 Foxp3.GFP reporter, and C57BL/6.SJL Foxp3.GFP reporter mice were bred and maintained in our animal facility. RAG2-GFP reporter mice (RAG2-NGBAC-GFP) were described previously (76). CD11c-DTA mice were created by crossing CD11c-Cre mice with ROSA26-flox-STOP-DTA mice. All other mice were purchased from The Jackson Laboratory or Charles River. Mice were housed in pathogen-free conditions and treated in strict compliance with Institutional Animal Care and Use Committee regulations of the University of Pennsylvania.
2.2 Flow cytometry, cell sorting, and data analysis
Antibodies for flow cytometry were purchased from eBioscience (San Diego, CA), BD Bioscience (San Jose, CA), or Tonbo Bioscience (San Diego, CA). Flow cytometry was performed with an LSR II, FACSCanto, or a FACSCalibur. Cell sorting was performed with a FACSAria cell sorter (BD Biosciences) or MACS Cell Separation (Miltenyi Biotec; San Diego, CA). Data were analyzed with FlowJo software (FlowJo LLC, Ashland, OR) and Prism (GraphPad, La Jolla, CA).
2.3 MC903 treatment of mice
MC903 (Tocris Minneapolis, MN) was dissolved in 100% EtOH at a concentration of 200 µM. Mice were treated on both ears with 2 nmol/10–20 µI of MC903 or EtOH vehicle for 5–7 days. MC903 and EtOH treatment intraperitoneally consisted of injecting 4 nmol MC903 or an equivalent volume of EtOH diluted in 500 µL PBS for 5 days. Serum was collected from mice on Day 3 post EtOH or MC903 treatment and TSLP content was measured by ELISA using anti-TSLP capture antibody (2 µg/ml) and biotinylated anti-TSLP detection antibody (0.1 µg/ml) (R&D Systems, Minneapolis, MN). On the indicated days post treatment, mice were sacrificed and the blood and secondary lymphoid organs were analyzed for Tregs and DCs by flow cytometry.
In some experiments, ear skin tissue was obtained by separating the dermal sheets of the ear and digesting in 250 µg/ml LiberaseTL (Roche, Cat# 5401020001, Branchburg, NJ) and 10 µg/ml DNAse (Roche, Cat# 10104159001) for 90 min at 37°C. Digestion was quenched with complete media and the cells were filtered through cell strainers and single cell suspensions were analyzed by flow cytometry.
To measure Bromodeoxyuridine (BrdU) incorporation, mice were treated with EtOH or MC903 (2nmol/ear) topically from days 0–6. On the final day of topical treatment, mice were administered BrdU with an initial bolus of BrdU (2 mg in 200 µl PBS) i.p. and given drinking water containing BrdU (1 mg/mL) until the time of sacrifice 3 days later. Intracellular Foxp3 and BrdU staining was performed sequentially according to the manufacturer’s protocol (eBioscience and BD Bioscience respectively).
2.4 Generation of VDR KO bone marrow (BM) chimeras
C57BL/6.SJL mice were lethally irradiated with a split dose of 11 Gy and reconstituted with 4 × 106 MACS-purified T cell-depleted (CD90.2) bone marrow of either C57BL/6 or VDR KO origin. 9–10 weeks post reconstitution, mice were treated with EtOH or MC903 (2 nmol/ear) topically for 7 days and analyzed as described.
2.5 T cell adoptive transfers
For the pTreg conversion experiments, FACS-sorted Tconvs (CD45.2+Foxp3.GFP−) and Tregs (CD45.1+Foxp3.GFP+) were injected i.v. into lymphoreplete C57BL/6.Thy1.1 wildtype (WT) mice at a Tconv:Treg ratio of 3:1. Three days post transfer, mice were treated with ethanol (EtOH) or MC903 (2nmol/ear) topically from day 3–9 and analyzed on day 11. To test for the requirement of TSLP-R on Tregs, MACS-sorted CD4+ T cells from WT C57BL/6.SJL (CD45.1+) or TSLP-R KO (CD45.2+) mice were CFSE-labeled, mixed at a 1:1 ratio, and 10 × 106 cells were injected i.v. into WT C57BL/6.Thy1.1 host mice. Mice were treated with EtOH or MC903 (2nmol/ear) topically from day 3–7 and analyzed on day 11.
2.6 In vitro Treg assays
To test iTreg generation, FACS-sorted DCs (CD3ε−CD19−DX5−CD11c+IAbβ+) and Tconvs (CD4+Foxp3.GFP−) were plated at a 1:1 ratio. Culture medium consisted of T cell media (MEM-α supplemented with 10% FBS, 1% penicillin/streptomycin, 10 mM HEPES, and 1 × 10–5 M 2-mercaptoethanol) and contained anti-CD3 (1 µg/ml), IL-2 (100 U/ml), TGFβ (1 ng/ml), and/or TSLP (50 ng/ml). The expression of Foxp3 by CD4+ T cells was analyzed by flow cytometry 4 days after culture. For Treg/DC co-culture assays, FACS-sorted DCs and CFSE-labeled Tregs (CD90.2+CD8α−Foxp3.GFP+) were plated at a 1:1 ratio in T cell media containing IL-2 (0.5 – 50 U/ml) and/or TSLP (3 – 50 ng/ml) with or without CTLA4-Ig (20 µg/ml) or anti-PD-L1 antibody (20 µg/ml). CFSE labeling was performed by resuspending cells with PBS containing CFSE (5 mM) at 37°C followed by continuous shaking for 9 min. The reaction was then immediately quenched with 100% FBS, and the cells were washed before culture. CFSE dilution of Tregs was analyzed by flow cytometry 4 days after culture. For Treg suppression assays, Tregs were FACS-sorted (CD90.2+CD8α−Foxp3.GFP+) from EtOH and MC903 (2nmol/ear) topically treated WT C57BL/6.SJL Foxp3.GFP mice at day 8 and Tconvs (CD4+GFP−) from WT C57BL/6 Foxp3.GFP reporter mice. The Tconvs were CFSE-labeled and cultured at various ratios with Tregs in the presence of irradiated T cell-depleted feeder cells and soluble anti-CD3 (1 µg/ml). CFSE dilution of Tconvs was assessed by flow cytometry after 4 days in culture.
2.7 NOD T1D model
Female NOD mice were treated with EtOH or MC903 (2nmol/ear) topically three times a week, every other week, between 5 and 12 weeks of age or between 14 and 21 weeks of age. Blood glucose was measured weekly by tail blood using a Contour Blood Glucose monitor. Development of diabetes was determined by two consecutive blood glucose readings ≥ 250mg/dl. Insulitis scoring was performed on hematoxylin and eosin-stained pancreatic sections removed at week 10 and week 13 of age. The slides were blinded and a score of 0 to 4 was assigned based on islet infiltration, as previously described (77). Insulitis scores were graded as follows: grade 0, normal islets; grade 1, mild mononuclear infiltration (<25%) at the periphery; grade 2, 25–50% of the islets infiltrated; grade 3, >50% of the islets infiltrated; grade 4, islets completely infiltrated with no residual parenchyma remaining. More than 130 islets per group were analyzed and pooled from sections obtained from different mice.
2.8 Experimental autoimmune encephalitis (EAE) induction
EAE was induced as previously described [29]. Briefly, mice were immunized with an emulsion of MOG35–55 peptide (CS Bio, Menlo Park, USA) with complete Freund’s adjuvant (Difco; Detroit, MI) divided equally into 50 µl at each shoulder and flank. At the time of immunization and 48 hours later, mice were injected intraperitoneally with 200 ng of Pertussis toxin (Enzo; Farmingdale, NY) in PBS. Beginning seven days prior to immunization, mice were treated topically with 10 µl of vehicle (EtOH) or MC903 (2nmol/ear) three times per week. After the seventh dose, treatment was discontinued. Mice were scored according to a standard 5 point scale [30].
2.9 Statistics
Statistical analysis was performed by Student’s t test, paired t test, or chi-squared test as indicated in the Figure Legends using Prism computer software.
3. Results
3.1 Topical MC903 treatment increases systemic Treg numbers through TSLP signaling
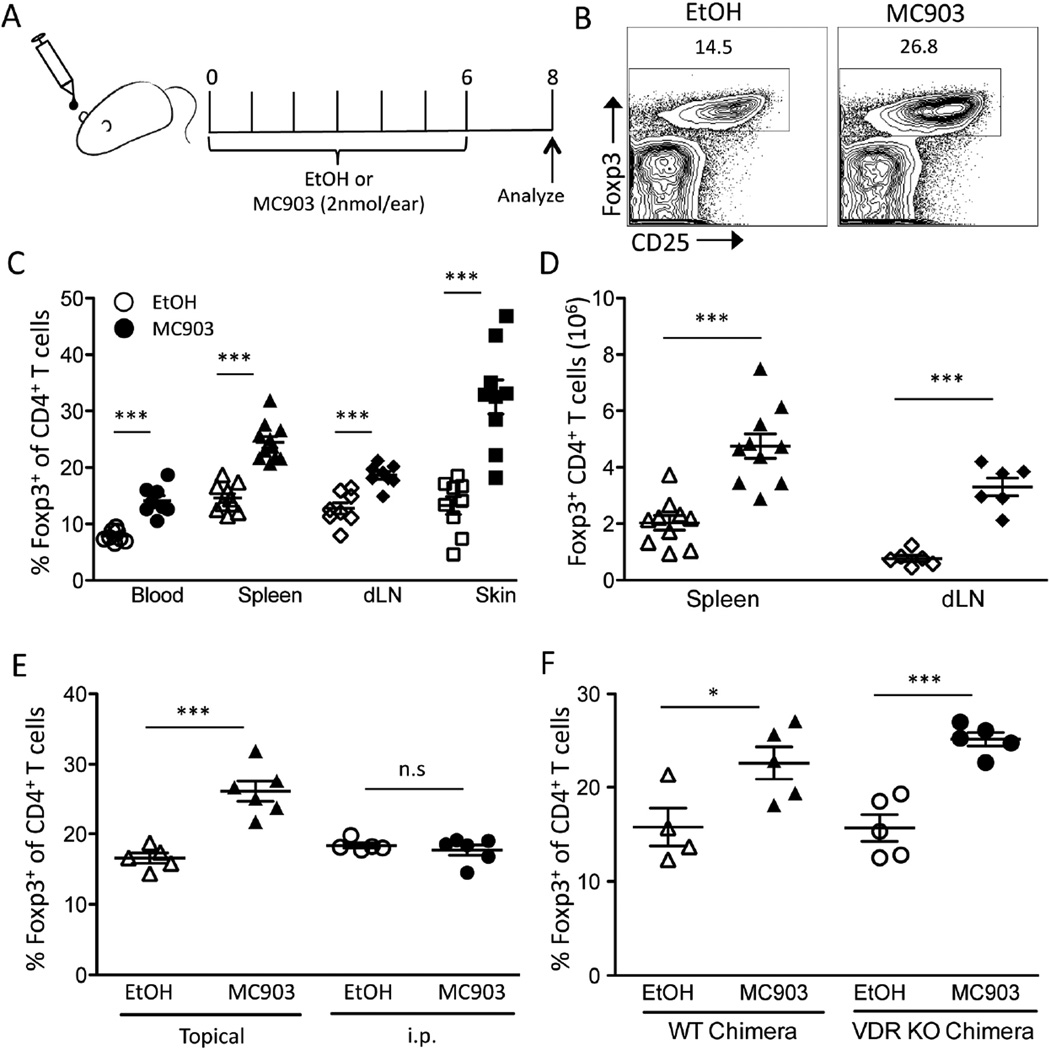
To test whether skin-derived TSLP affects peripheral Treg numbers, we stimulated the skin through the vitamin D receptor (VDR) using the vitamin D3 analog, MC903. WT mice were treated with topical MC903 or vehicle (EtOH) once a day on both ears for 7 days (Figure 1A). On day 8, the fraction of Tregs (% Foxp3+ of total CD4+ T cells) was significantly elevated in the spleen, blood, skin, and skin-draining cervical lymph nodes (dLN) (Figure 1, B and C). Additionally, the absolute number of Tregs was also significantly increased in the lymphoid organs of mice treated with MC903 compared to EtOH (Figure 1D).
Fig. 1.
Topical MC903 treatment increases Treg numbers independent of VDR expression on hematopoietic cells. (A) C57BL/6 mice were treated with ethanol (EtOH) or MC903 (2nmol/ear) once daily for 7 days. On day 8, mice were euthanized and analyzed. (B) Representative flow plots of live CD4+ cells from spleens of EtOH and MC903 treated mice at day 8. (C) The percent of Tregs of CD4+ T cells in blood, spleen, skin-draining lymph nodes (dLN) and skin at day 8 is plotted as mean ± SEM (n = 6–10 mice/group from 4 independent experiments). (D) Total Treg numbers in the spleen and dLN at day 8 are plotted as mean ± SEM (n = 6–10 mice/group from 3 independent experiments). (E) The percent of Tregs of CD4+ T cells at day 8 from mice treated with EtOH or MC903 topically or i.p. is plotted as mean ± SEM (n = 5–6 mice/group from 2 independent experiments). (F) WT and VDR KO BM chimeras were treated with EtOH or MC903. ) The percent of Tregs of CD4+ T cells in spleen on day 8 after treatment is plotted as mean ± SEM (n = 4–5/group from 2 independent experiments) *p<0.05 and ***p<0.001 by Student’s t test.
Vitamin D3 can exert effects directly on the immune system [31]. MC903 is relatively skin impermeable compared to vitamin D3 and has a significantly decreased half-life in circulation due to its inability to bind to vitamin D binding protein [32]. However, since mouse ear skin is thin, it was still possible that MC903 was leaking into the systemic circulation, and that MC903 directly increased Treg numbers by stimulating hematopoietic cells through their VDR. To test the possibility, we first compared the ability of topical vs. intraperitoneal (i.p.) MC903 administration to increase Treg numbers. Although topical MC903 treatment augmented Treg numbers systemically, no change was seen when the same amount of drug was injected i.p. (Figure 1E). Next, we created VDR KO bone marrow (BM) chimeras to test whether VDR signaling in hematopoietic cells was required for MC903 to increase Treg numbers. The fraction of Tregs was increased equally by topical MC903 treatment in WT and VDR KO BM chimeras (Figure 1F). These data suggest that MC903 acted on a skin-resident cell rather than on hematopoietic cells to systemically increase Treg numbers.
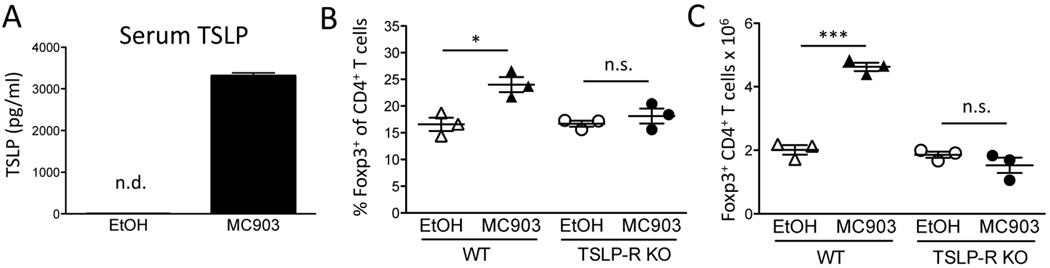
It has previously been shown that topical MC903 treatment induces TSLP production by keratinocytes, which is detectable in the circulation [33]. Consistent with these reports, TSLP was undetectable in the sera of EtOH-treated animals but found at high levels in MC903-treated mice (Figure 2A). To determine if TSLP was responsible for the MC903-mediated increase in Treg numbers, we treated WT and TSLP-R KO mice with EtOH or MC903 topically for 7 days. On day 8, we observed increases in both Treg percentages as well as total numbers in WT mice but not in the TSLP-R KO mice (Fig. 2, B and C). Together, these data suggest that VDR-stimulated skin possesses the ability to systemically increase Treg numbers by producing TSLP.
Fig. 2.
TSLP-R is required for MC903-mediated increases in Treg numbers. (A) C57BL/6 mice were treated with ethanol (EtOH) or MC903 (2nmol/ear) once daily. Serum TSLP was measured by ELISA on day 3 of treatment and plotted as mean ± SEM (n = 3 mice/group). n.d. = not detected. (B) The percent of splenic Tregs of CD4+ T cells and (C) total Treg numbers on day 8 after treatment of WT and TSLP-R KO mice is plotted as mean ± SEM (n = 3 mice/group) *p<0.05 and ***p<0.001 by Student’s t test.
3.2 MC903-induced TSLP augments the proliferation of pre-existing Tregs
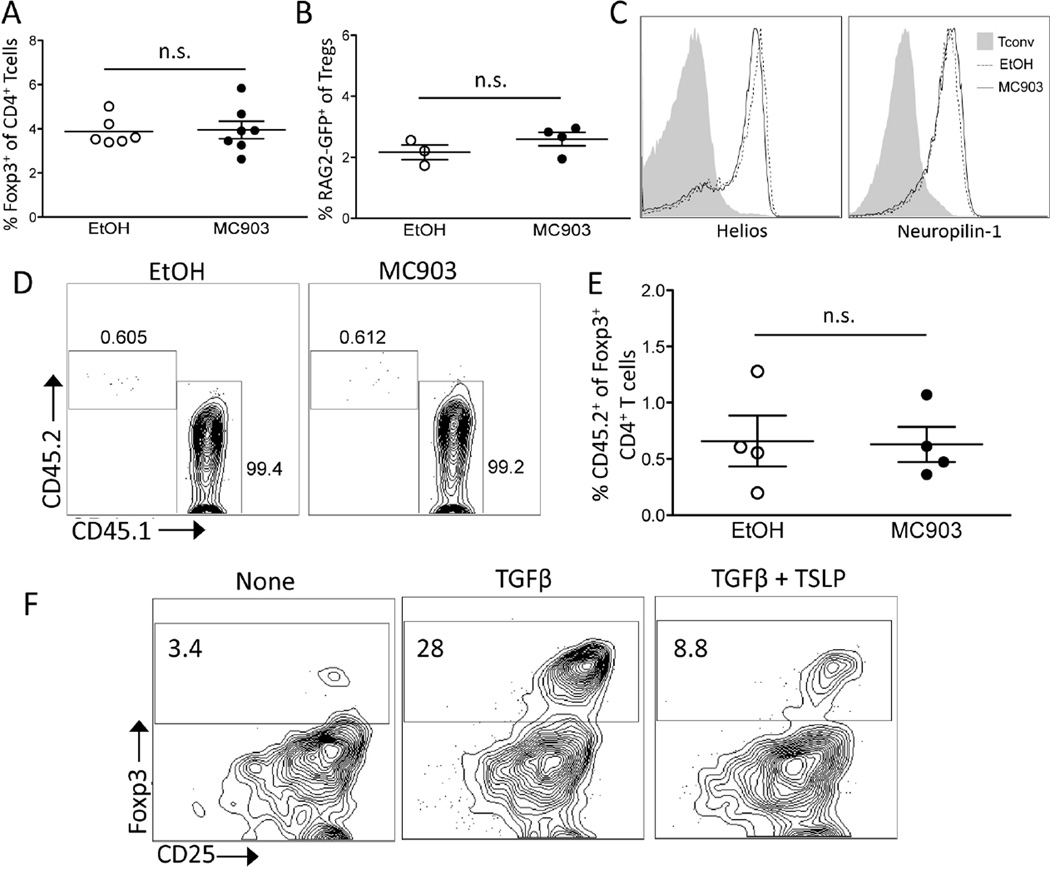
Given the potential role of TSLP in thymic generation [27] of Tregs (tTregs) and in the conversion of Tconvs into peripherally derived Tregs (pTreg) [28], we tested the contribution of these processes to the MC903-mediated increase in Treg numbers. We first examined the impact of topical MC903 treatment on the generation of tTregs by assessing Treg percentages within the thymus of mice. After 7 days of topical MC903 treatment, no increase in the percentage of Tregs in the thymus was seen (Figure 3A). To test whether thymic export of tTregs was increased with topical MC903 treatment, we utilized RAG2-GFP reporter mice to track recent thymic emigrants [34]. When RAG2-GFP mice were treated with EtOH or MC903 topically, we found no difference in the percentage of GFP+ Tregs (recent thymic emigrants) in the spleen at day 8 (Figure 3B), suggesting that the increase in Treg numbers was not due to thymic export of newly developed tTregs.
Fig. 3.
Augmentation of thymic generation/output or peripheral Treg conversion is not responsible for MC903-mediated increases in Treg numbers. (A) C57BL/6 mice were treated with ethanol (EtOH) or MC903 (2nmol/ear) once daily. The percent of splenic Tregs of single-positive (SP) CD4+ T cells in the thymus at day 8 is plotted as mean ± SEM (n = 6 mice/group from 2 independent experiments) (B) RAG2-GFP reporter mice were treated with EtOH or MC903 for 7 days and the percentage of GFP+CD4+CD25HI cells of all Tregs in the spleen on day 8 is plotted as mean ± SEM (n = 3–4/group). (C) Representative flow plots of helios and neuropilin-1 staining of Tregs in the spleen of mice on day 8 after treatment is shown. (D) FACS-sorted Tconvs (CD45.2+Foxp3.GFP−) and Tregs (CD45.1+Foxp3.GFP+) were transferred into C57BL/6.Thy1.1 mice and treated with EtOH or MC903 topically for 7 days. Representative flow plots gated on live, CD4+ Foxp3-GFP+ cells derived from Tconv (CD45.2+) and Treg (CD45.1+) transferred cells is shown. (E) The percent of Tregs derived from Tconv in EtOH and MC903 treated mice is plotted as mean ± SEM (n = 4 mice/group). (F) FACS-sorted DCs and CFSE-labeled Tconvs were co-cultured and stimulated with anti-CD3 and different combinations of TGFβ and TSLP as indicated. Representative flow plots gated on live, CD4+ T cells expressing Foxp3 and CD25 are shown. n.s. = not significant by Student’s t test.
Next, we tested whether topical MC903 treatment affected the generation of pTregs. We first examined the expression of helios and neuropilin-1, which have been shown to be expressed predominately by tTregs but not pTregs [35, 36]. The fraction of Tregs expressing helios and neuropilin-1 was similar between MC903 and EtOH-treated mice, suggesting that the generation of pTregs was unaltered by topical MC903 treatment (Figure 3C). We next utilized an adoptive transfer model to track the generation of pTregs from Tconvs. Congenically disparate Tconvs and Tregs were co-transferred into a lymphoreplete host, which were then topically treated with EtOH or MC903. At Day 8 of treatment, the contribution of the adoptively transferred Tconvs to the total donor Foxp3+ pool was small and was not increased in MC903 compared to EtOH-treated mice (Figure 3, D and E), suggesting that MC903 treatment did not increase the conversion of Tconvs into pTregs. Finally, we tested the effects of TSLP on inducible Treg (iTreg) formation and found that TSLP inhibited rather than augmented the generation of iTregs. Together, these data suggest that the conversion of Tconv to Foxp3+ pTregs is unlikely to contribute to the increase in Treg numbers observed in MC903-treated mice (Figure 3F).
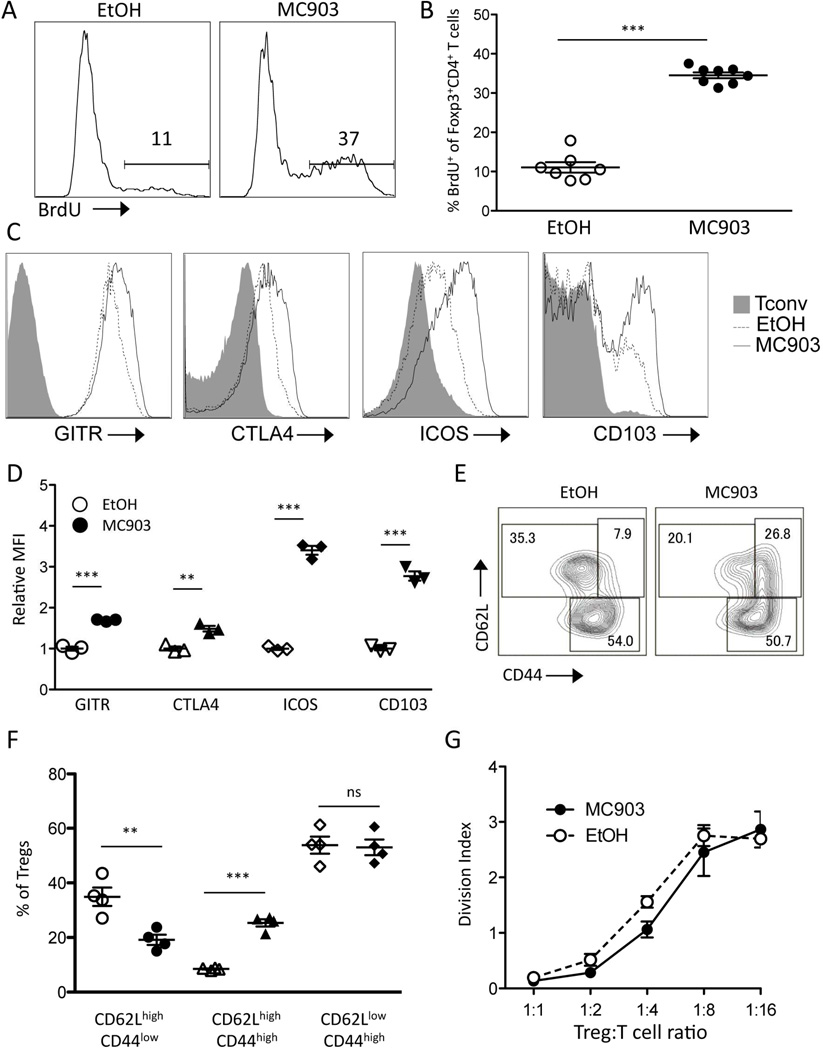
Given that thymic generation and peripheral conversion of Tregs were unlikely contributors to MC903-mediated Treg expansion, we hypothesized that MC903 treatment promoted the proliferation of pre-existing Tregs. To measure the proliferation of the Tregs, MC903 and EtOH-treated mice were pulsed with BrdU for 3 days to determine BrdU uptake by Tregs. Tregs from MC903-treated animals incorporated significantly more BrdU than Tregs from EtOH-treated mice (Figure 4, A and B), suggesting that MC903-mediated Treg expansion was due to increased proliferation of pre-existing Tregs.
Fig. 4.
Topical MC903 treatment augments Treg proliferation and expression of activation markers but not suppressive function. (A) C57BL/6 mice were treated with EtOH and MC903 (2nmol/ear) for 8 days and administered BrdU for the last 3 days of treatment. Representative histogram plots and (B) the percent of Tregs incorporating BrdU at day 9 plotted as mean ± SEM (n = 7–8 mice/group of two independent experiments) is shown. (C) Representative histogram plots of phenotypic surface markers (GITR, CTLA4, ICOS, and CD103) expressed by Tregs and (D) their MFI relative to EtOH control in the spleen at day 8 plotted as mean ± SEM (n = 3 mice/group) is shown. (E) Representative density plots of CD62L and CD44 expressed by Tregs and (F) the % of Tregs within each subset in the spleen at day 8 is plotted as mean ± SEM (n = 4 mice/group) is shown. (G) The ability of Tregs from MC903 or EtOH-treated mice to suppress anti-CD3-stimulated Tconv division at different Treg:Tconv ratios plotted as mean ± SEM of triplicate cultures is shown. **p<0.01 and ***p<0.001 by Student’s t test.
We phenotypically and functionally analyzed the expanded Tregs from MC903-treated mice. Tregs can be divided into subsets known as central and effector, with the central subset being quiescent and the effector subset being more proliferative [37]. Along with the observed increase in proliferation, we found that the resulting Tregs in MC903-treated mice exhibited an effector phenotype as evidenced by increased expression of the markers GITR, CTLA4, ICOS, and CD103 (Figure 4, C and D). Effector Tregs are characterized by high expression of CD44 and low levels of CD62L. Although there was no difference in the fraction of CD62Llo Tregs, the activation marker CD44 was expressed on Tregs at significantly higher level in MC903 compared EtOH-treated mice (Fig. 4, E and F). Despite the activated effector phenotype of the Tregs in MC903 mice, they were no more suppressive than Tregs from an EtOH-treated mouse on a per-cell basis as determined by an in vitro Tconv proliferation suppression assay (Figure 4G).
3.3 MC903-induced TSLP expands Tregs indirectly by a dendritic cell-dependent mechanism
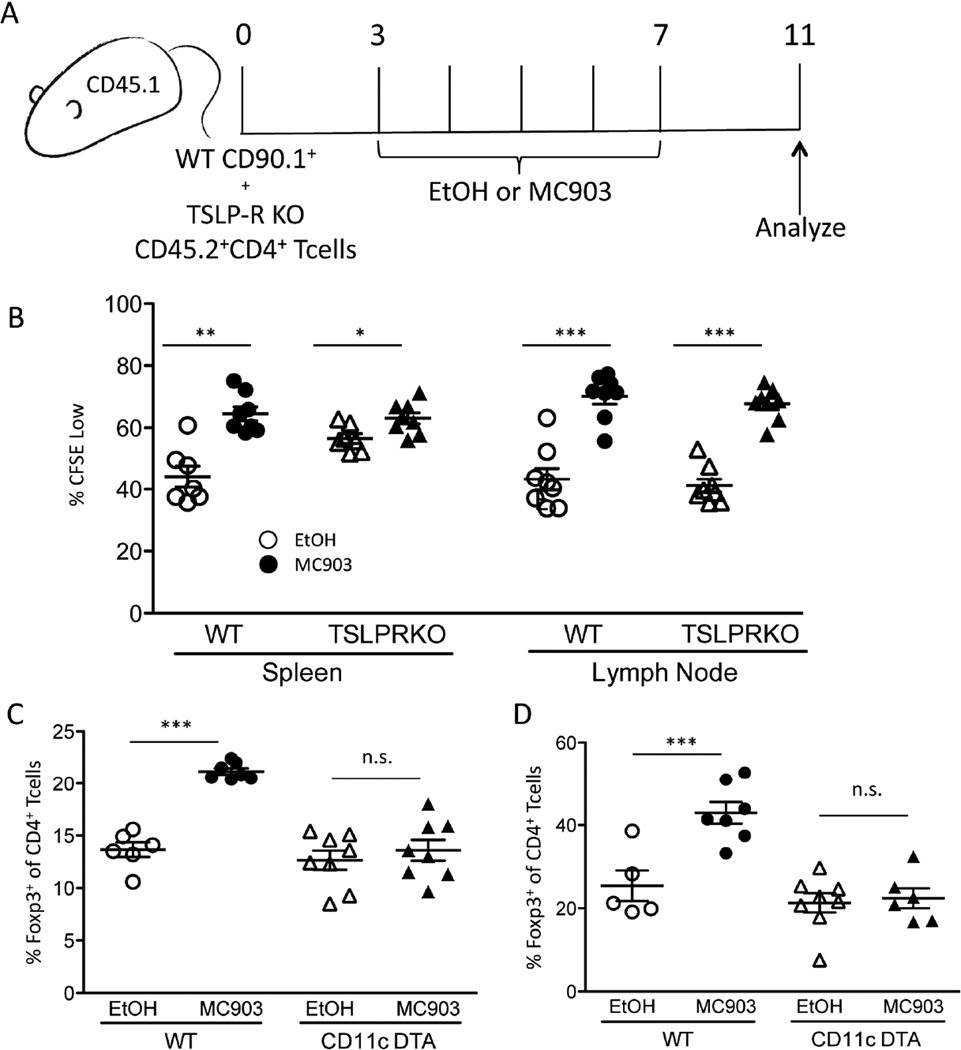
Expression of TSLP is largely restricted to epithelial cells, while expression of the TSLP receptor has been found in many immune cell types including Tregs [38]. To test whether TSLP-R signaling is required by Tregs for MC903-mediated expansion, CFSE-labeled WT and TSLP-R KO CD4+ T cells were adoptively transferred into a congenically disparate WT host and subsequently treated with EtOH or MC903 for 7 days (Figure 5A). Compared to EtOH treatment, MC903 treatment significantly augmented CFSE dilution of Tregs. Surprisingly, both WT and TSLP-R KO Tregs diluted CFSE to a similar extent, suggesting that TSLP-R signaling by Tregs was not required for MC903-mediated proliferation (Figure 5C). These data suggest that the effect of TSLP on Treg proliferation is mediated by another TSLP-responsive cell type.
Fig. 5.
MC903-derived TSLP indirectly expands Tregs by a dendritic cell-dependent mechanism. (A) CFSE-labeled WT (CD90.1+) and TSLP-R KO (CD45.2+) CD4+ T cells were transferred into CD45.1+ mice and treated with EtOH or MC903 (2nmol/ear) for 5 days and analyzed 4 days later. (B) The percent of Tregs in the spleen and lymph node at day 11 that are CFSE low is plotted as mean ± SEM (n = 8/group from 2 independent experiments) (C) WT and CD11c-DTA mice were treated with EtOH or MC903 (2nmol/ear) and Tregs were measured in the spleen and (D) skin at day 8. Summary data are plotted as mean ± SEM (n=5–8/group from two independent experiments). *p<0.05, **p<0.01, ***p<0.001, and n.s. = not significant by Student’s t test.
Treg proliferation is largely dependent on contacts with DCs. Since DCs also express TSLP-R (49), we tested whether DCs were necessary for the expansion of Tregs with topical MC903 treatment. DC-deficient (CD11c-DTA mice) and WT littermates were treated with EtOH or MC903 topically for 7 days and on day 8, Tregs were assessed in the spleen and locally within the skin of the mice. While WT mice showed a significant increase in the proportion of Tregs at both locations, there was no increase observed in CD11c-DTA mice (Figure 5, C and D). This demonstrated a requirement for DCs in MC903-induced Treg expansion.
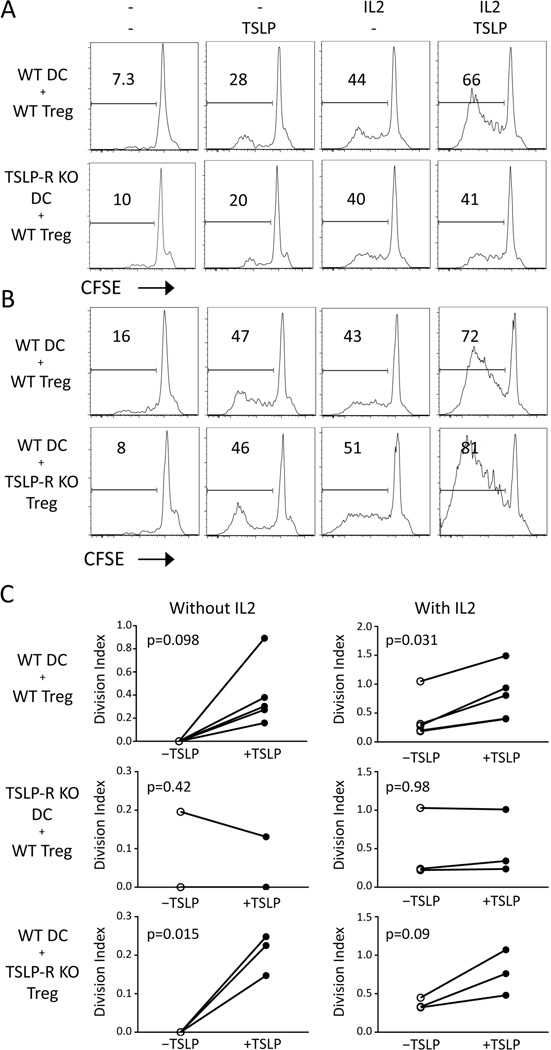
To further test the role of DCs in TSLP-mediated Treg expansion, we co-cultured purified DCs and Tregs in the presence or absence of TSLP and/or IL-2. The addition of TSLP alone induced a small amount of proliferation by Tregs. Moreover, TSLP amplified Treg proliferation by DCs in the presence of IL-2 (Figure 6A). Similar effects were seen at a range of physiological IL-2 and TSLP concentrations (Supplementary Fig. 1). This effect was dependent on the expression of TSLP-R on DCs, but was independent of TSLP-R expression by Tregs (Figure 6, B and C). Altogether, these data suggest that TSLP-sensing DCs are responsible for Treg expansion by topical MC903 treatment.
Fig. 6.
TSLP stimulates Treg proliferation in vitro through dendritic cells. (A) CFSE-labeled WT Tregs were co-cultured with dendritic cells of either WT or TSLP-R KO origin for 96 hours with TSLP and/or IL-2. (B) CFSE-labeled WT or TSLP-R KO Tregs were co-cultured with WT dendritic cells for 96 hours with TSLP and/or IL-2. Representative FACS plots are gated on CD4+Foxp3+ Tregs. (C) The division index of Tregs is plotted in the presence (right plots) or absence (left plots) of IL-2 with (closed circles) or without (open circles) TSLP is plotted across multiple experiments. Statistical analysis was performed by paired t test.
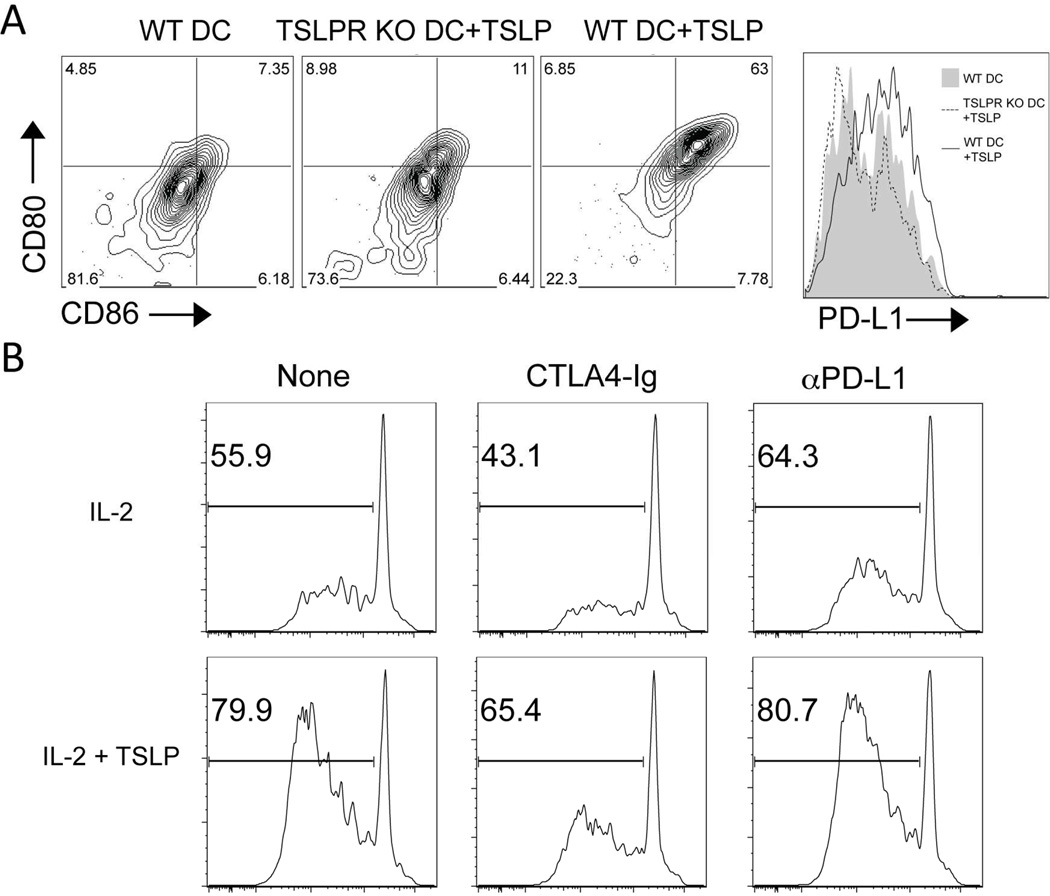
We have previously shown that DCs are required for Treg proliferation, because they provide co-stimulatory signals through CD80 and CD86 [19, 39]. These signals in conjunction with IL-2 are sufficient to promote Treg proliferation. Thus, we examined whether MC903 treatment upregulated co-stimulatory molecule expression by DCs. The fraction and absolute number of DCs was increased in draining lymph nodes but not in the spleen of MC903 compared to EtOH-treated mice after 3 days of treatment. Moreover, the expression of CD80, CD86, and PD-L1 were significantly increased on lymph node DCs (Supplementary Figure 2, A-D). Splenic DCs showed an increase in CD86 and PD-L1 expression but not CD80 (Supplementary Figure 2, E-H). Similarly, a 24− hour TSLP treatment of cultured DCs also induced higher levels of CD80, CD86, and PD-L1 in WT but not in TSLP-R KO DCs (Figure 7, C and D). The upregulation of CD80 and CD86 appeared to be in part responsible for the augmented proliferation, as the co-stimulatory blockade with CTLA4-Ig but not PD-L1, inhibited TSLP-augmented Treg proliferation (Figure 7E). These data suggest that elevated CD80/86 expression by DCs plays a role in TSLP-augmented Treg proliferation.
Fig. 7.
TSLP-mediated upregulation of CD80 and CD86 is partially responsible for the ability of TSLP-stimulated DCs to augment Treg proliferation. (A) Representative plots of CD80, CD86, and PD-L1 expression by WT or TSLP-R KO DCs treated with or without TSLP for 24 hours is shown. (D) CFSE-labeled WT Tregs were co-cultured with WT dendritic cells for 96 hours with IL-2 and/or TSLP with or without CTLA4-Ig or anti-PD-L1 antibody. The % of CFSElo Tregs is indicated in the histogram plots. One representative of 3 independent experiments is shown.
3.4 Topical MC903 treatment decreases the incidence of diabetes in NOD mice
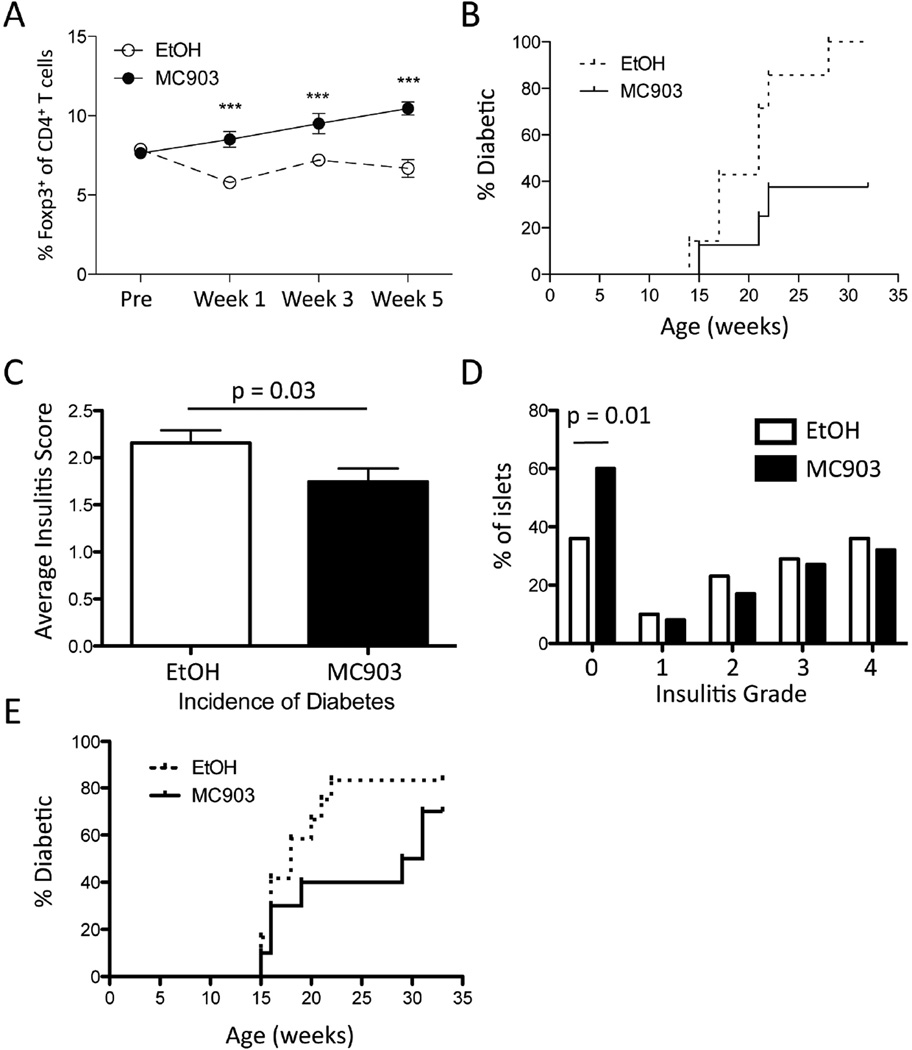
We next tested whether MC903-mediated Treg expansion could display benefit in the amelioration of autoimmune disease. We first examined the impact of topical MC903 treatment in the non-obese diabetic (NOD) model of T1D, since increases in Treg numbers have been shown to delay the onset of disease, while depletion of Tregs accelerates disease progression [14, 15, 28, 40]. Female NOD mice were treated with MC903 using a slightly modified treatment schedule. Since continuous daily application of MC903 to ear skin of mice elicits an atopic dermatitis-like disease, mice were given EtOH or MC903 three times a week, every other week. This allowed for continuous treatment of the mice over a prolonged period with little to no evidence of atopic dermatitis-like disease. Although NOD mice exhibited ~50% of the number of circulating Tregs compared to B6 mice at baseline, we found that Tregs in the peripheral blood remained ~2 fold elevated in MC903 compared to EtOH-treated NOD mice using this treatment protocol (Figure 8A). These effects correlated with a significantly decreased incidence of diabetes in MC903 compared to EtOH treated mice (Figure 8B). Histologic analysis of pancreata taken at 12 weeks of age showed an overall decrease in mean insulitis score in mice treated with MC903 compared to EtOH (Figure 8C). Moreover, the number of unaffected islets was significantly higher in the treatment group (Figure 8D). Since almost all NOD mice will exhibit insulitis at 14 weeks of age [41], we treated a cohort of NOD mice starting at 14 weeks of age to test the impact of MC903 treatment in NOD mice with existing insulitis. Although the data were not statistically significant (p = 0.08), we found that MC903 treatment started at this time point exhibited a trend towards delaying the onset of T1D (Figure 8E).
Fig. 8.
Long-term topical MC903 treatment increases Tregs in NOD mice and decreases the incidence of diabetes. (A) Female NOD mice were treated with EtOH or 2nmol/ear MC903 three times a week every other week between 5–12 weeks of age. The percent of circulating Tregs of CD4+ T cells at baseline, Week 1, Week 3, and Week 5 from mice treated with EtOH or MC903 topically is plotted as mean ± SEM (n = 7–8 mice/group). ***p<0.001 by unpaired, two-tailed Student’s t test. (B) The fraction of EtOH and MC903-treated NOD mice developing diabetes (two consecutive blood glucose readings ≥250 mg/dl) is plotted over time (n = 7–8 mice/group). p = 0.015 by Mantel-Cox test. (C) Insulitis scoring was performed on pancreatic specimens taken from 2 experiments (week 10 and week 13 of age; n = 7–8 mice/group/experiment). A score of 0 to 4 was assigned based on islet infiltration. Summary data from >130 islets per group are shown. The average insulitis score and the (D) percent of islets scored in each grading category is plotted. (E) Female NOD mice were treated with EtOH or 2nmol/ear MC903 three times a week every other week between 14–21 weeks of age. The fraction of EtOH and MC903-treated NOD mice developing diabetes (two consecutive blood glucose readings ≥250 mg/dl) is plotted over time (n = 10–12 mice/group). p = 0.22 by Mantel-Cox test. Statistical analysis was performed by Student’s t test (C) or by chi-squared test (D).
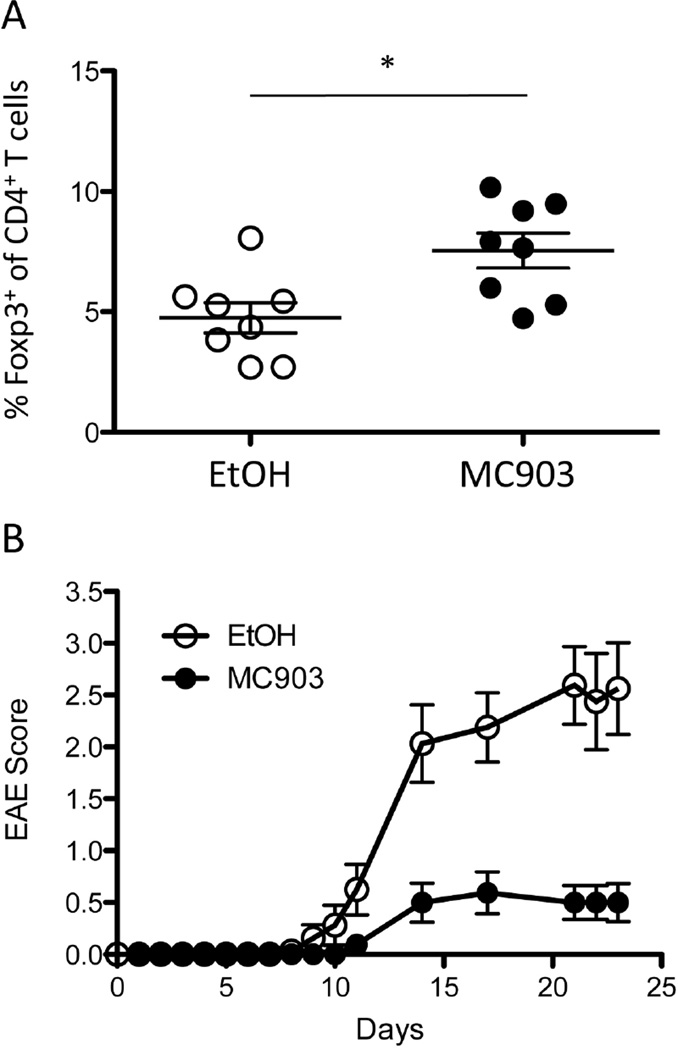
We next tested the impact of MC903 treatment in another autoimmune disease model, EAE, which has also been shown to be alleviated by Treg expansion [13]. It has been shown that Tregs could be unstable under the acute inflammatory conditions in EAE [42], so it was important to examine whether MC903 could still increase Treg numbers in this setting. Similar to NOD mice, topical treatment of MC903 resulted in an increase in the fraction of circulating Tregs (Figure 9A). This again correlated with significantly reduced disease scores in MC903 compared to EtOH-treated mice (Figure 9B). Together, these data demonstrate that topical treatment with MC903 may represent a novel strategy to expand Tregs and to treat inflammatory diseases that can be alleviated by Treg expansion.
Fig. 9.
MC903 treatment promotes Treg expansion and attenuates disease in EAE-induced mice. Mice were treated with MC903 or vehicle (EtOH) prior to and subsequently after the induction of active EAE with MOG35–55. (A) The frequency of circulating FoxP3+ Tregs in MC903-treated mice (filled circles) and EtOH controls (open circles; n = 8 per group, p = 0.04 by Student’s t test) is shown. (B) Clinical scores for mice treated with MC903 (filled circles) and EtOH (open circles) are shown. The graph is inclusive of two separate experiments (n = 8 mice per group per experiment).
4. Discussion
There is a recognized importance of non-immune cells within local tissue to produce factors critical in regulating immune responses. In particular, epithelial cells have been linked to the production of a number of cytokines that are known to play roles in local immune activation. Additionally, intestinal signals driven by the presence of segmented filamentous bacteria have been found to be important in the activation of not only local responses but also influence the outcome of systemic disease such as EAE and autoimmune arthritis in sites far removed from SFB exposure [23, 24]. Similar to the gut, the skin also possesses the ability to alter immune responses in more than just the local tissue. For example, treatment of the skin of mice with MC903 promotes hematopoiesis of basophils from the bone marrow in a TSLP-dependent manner [26].
In this report, we demonstrate that the skin also has the ability to control Treg numbers both locally and systemically through the elaboration of TSLP. Although TSLP-R deficiency does not lead to decreases in Treg numbers at baseline, tTreg generation can be enhanced with TSLP [27, 43, 44]. Additionally, although TSLP signaling by DCs was not an absolute requirement for iTreg generation [45], more iTregs were found when naïve Tconvs were cultured with DCs that developed in conditions containing TSLP [28]. Intriguingly, however, our data suggest that neither thymic generation/export nor peripheral conversion of Tregs was responsible for MC903-mediated Treg expansion. Rather, based on CFSE dilution and BrdU incorporation studies, we found that MC903-derived TSLP promoted the proliferation of pre-existing Tregs.
IL-2-induced STAT5 phosphorylation is a critical signaling pathway for Treg proliferation [46]. Similar to IL-2, TSLP transmits its signal through the phosphorylation of STAT5 [47] and thus, we predicted that TSLP acted directly on Tregs to promote their proliferation. In addition, TSLP can act directly on CD4+ T cells to augment their proliferation [48]. Surprisingly, however, we found that MC903-driven Treg expansion did not require TSLP-R expression by Tregs. Instead, we found that TSLP promoted Treg proliferation by acting on DCs, since Treg numbers were not augmented by MC903 treatment in mice lacking DCs. Moreover, TSLP significantly augmented Treg proliferation when Tregs were co-culture with WT but not TSLP-R KO DCs.
There has been long-standing interest in harnessing the potential of Vitamin D as a therapeutic agent for immune modulation. Epidemiological studies have associated Vitamin D deficiency with a variety of autoimmune diseases including T1D [49–51]. The impact of Vitamin D deficiency on immune cells has been thought to be direct, as numerous studies have shown that Vitamin D can directly suppress the activation of immune cells such as T cells, DCs, monocytes/macrophages, and B cells [31]. Moreover, oral or parenteral treatment of mice with Vitamin D or its analogs results in improved disease outcomes in a variety of autoimmune mouse models including multiple sclerosis, arthritis, and T1D [52–54]. However, clinical trials to investigate the effects of oral or intravenous Vitamin D supplementation in autoimmune patients have been met with limited success [49]. In T1D, Vitamin D supplementation has been shown to provide modest benefit in reducing the amount of insulin needed for glucose control [55]. The effects of Vitamin D supplementation on autoimmune diseases may be ineffective or modest at best, because of the limited dose of Vitamin D that can be administered systemically before serious side effects such as hypercalcemia appear.
As opposed to the prevailing notion that Vitamin D derives its effects by direct action on all target cells, immunomodulation by Vitamin D may not require systemic exposure. Our data demonstrate that topical but not systemic treatment with MC903 increases Treg numbers. Moreover, Treg numbers were increased in MC903-treated VDR KO BM chimeras, suggesting that VDR signaling by hematopoietic cells was not required for Treg expansion. Thus, VDR signaling in the skin alone appears sufficient to harness the immunomodulatory potential of Vitamin D. Restricting VDR signaling to the skin alone can be potentially accomplished by using MC903, which has low systemic absorption through the skin. Moreover, low-calcemic Vitamin D analogs such as MC903 do not readily cause hypercalcemia, because these drugs do not bind to Vitamin D binding protein in the circulation, rendering the systemic half-life of MC903 short [32]. Thus, MC903 is optimal for treatment of skin diseases such as psoriasis. However, our data suggest that topical MC903 treatment has the potential not only to improve localized skin diseases but also to affect systemic inflammation by the elaboration of TSLP. Thus, topical MC903 application could represent a novel strategy for skin-mediated immunomodulation in treatment of systemic inflammatory disorders.
Indeed, our data support the notion that a systemic autoimmune disease can be treated with topical MC903 application. Topical MC903 treatment of NOD mice enhanced Treg numbers and reduced the incidence of T1D. Histology and diabetes incidence curves suggest that MC903 treatment at an early age has a beneficial effect to prevent insulitis from developing, however, once established, the kinetics of disease appear similar to control littermates. In addition to preventing T1D, topical MC903 treatment also showed a trend toward delaying the onset of T1D even after the development of insulitis (week 14 of age). Changes in Treg numbers have been implicated in both the prevention and the enhancement of the progression of T1D in the NOD mouse model. Depletion of Tregs leads to a significantly faster onset of disease as measured by blood glucose level [40]. Alternatively, increasing Tregs through either adoptive transfer or treatment with agents to increase Tregs in vivo leads to protection from diabetes [14, 15, 28]. In addition to augmenting Treg numbers, MC903 treatment may also be protective in T1D by promoting Th2 responses and skewing of the immune response away from the Th1 inflammation that drives diabetes [56, 57]. Although our modified MC903 treatment protocol (every other day/every other week) did not result in any overt Th2-driven atopic dermatitis-like disease, it is still possible that some Th2 skewing contributed to MC903-mediated protection against T1D in NOD mice. In addition to protection against T1D, we also found that topical MC903 treatment increased circulating Treg numbers and protected against disease in EAE-induced mice. Tregs have been previously shown to lose Foxp3 and convert to effector T cells in the inflammatory environment of EAE [42]. Despite this, we found that Tregs were elevated in MC903-treated mice with EAE, suggesting that MC903 could provide benefit even under acute inflammatory processes.
Topical MC903 treatment will not be beneficial in every auto-inflammatory disease setting. For example, if there is an inherent defect in Treg function, merely increasing the number of Tregs by MC903 treatment may not be sufficient to alleviate disease. We were surprised to find that there was no significant difference in the ability of Tregs to suppress Tconv proliferation in vitro, suggesting that MC903 treatment did not increase the function of Tregs. However, many markers that are associated with increased function of Tregs were upregulated (GITR, CTLA4, CD103, ICOS) and the Tregs adopted an activated phenotype (CD44hi) in MC903-treated mice. Thus, it is possible that the Tregs from MC903-treated mice are more functional in vivo. It is also important to note that while tTreg numbers were increased by MC903 treatment, the generation of iTregs was reduced by TSLP in vitro. Thus, MC903 treatment may also not be effective in diseases such as colitis or bone marrow transplant-associated graft-versus-host disease (GVHD), where the peripheral conversion of Tconvs to Tregs contributes to disease protection [58, 59].
Our data bear implications on how Vitamin D production in the skin upon UV exposure (sunlight or therapeutic) can modulate both local and systemic immune responses. UVB converts 7-dehydro-cholesterol into pre-Vitamin D3, which consequently will enrich Vitamin D3 in the skin. Although it is widely believed that conversion of pre-Vitamin D3 to active Vitamin D3 requires the liver and the kidney, skin keratinocytes express the necessary enzymes to convert pre-Vitamin D3 into active Vitamin D3 [60]. Thus, active Vitamin D3 can be produced locally in the skin, potentially leading to TSLP-mediated Treg expansion. This could provide a mechanistic explanation as to how phototherapy is beneficial in the treatment of inflammatory skin diseases such as cutaneous GVHD and psoriasis. Although the amount of Vitamin D3 produced locally upon natural sunlight exposure may be less compared to topical MC903 treatment, Tregs may still be increased at local sites of sunlight exposure. This could be beneficial in maintaining tolerance to environmental antigens and commensals encountered by the skin.
5. Conclusions
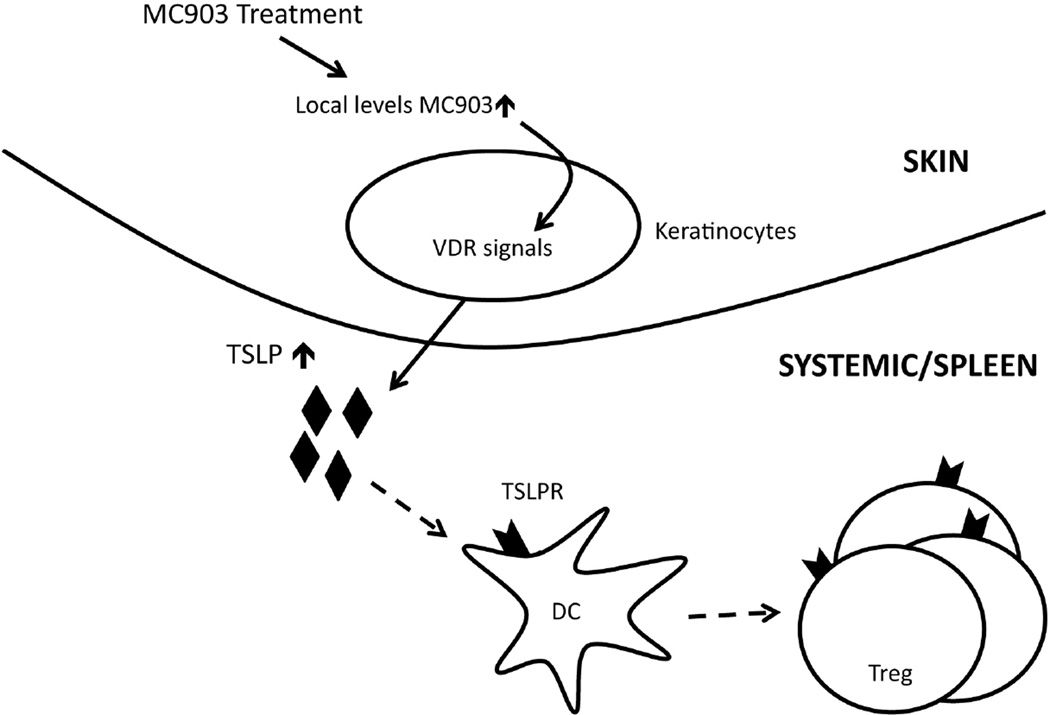
In summary, our data present a model in which VDR stimulation of the skin can increase Treg numbers both locally and systemically through the production of TSLP (Figure 8). Although it has previously shown that TSLP can be induced by VDR stimulation of skin, our report is the first to propose the concept that skin-derived TSLP can have systemic immunomodulatory effects by expanding Tregs. This is of particular importance as we begin to recognize the strong ability of local tissue responses, in places such as the skin, to alter the immune cells outside of the immediate environment. Moreover, there is great potential in creating therapies that take advantage of the biology of the skin to produce the intermediary cytokines needed for altering both local and systemic immune responses.
Supplementary Material
Fig. 10.
Model of MC903-mediated Treg expansion. MC903 activates the VDR of keratinocytes to induce systemically available TSLP. Circulating TSLP acts on DCs in secondary lymphoid organs, which drives the systemic proliferation of Tregs.
Highlights.
-
(1)
Vitamin D3-treated skin produces TSLP that systemically increases Treg numbers.
-
(2)
Skin-derived TSLP systemically expands pre-existing Tregs.
-
(3)
TSLP-mediated Treg expansion is not direct and requires DCs.
-
(4)
Topical Vitamin D3 may be useful in treatment of autoimmunity and inflammation.
Acknowledgments
We thank Mariko Okumura for technical support. This work was in part supported by grants from the National Institutes of Health (R01HL107589, R01HL111501 – Kambayashi; R01NS083678 – Wu) and the American Asthma Foundation.
Abbreviations used in this article
- BM
bone marrow
- BrdU
bromodeoxyuridine
- DC
dendritic cell
- EtOH
ethanol
- EAE
experimental autoimmune encephalitis
- iTreg
inducible Treg
- NOD
non-obese diabetic
- pTreg
peripherally derived Tregs
- T1D
type 1 diabetes (T1D)
- Treg
regulatory T cell
- TSLP
thymic stromal lymphopoietin
- TSLP-R KO
TSLP receptor knockout
- tTregs
thymic Tregs
- VDR
vitamin D receptor
- WT
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Taku Kambayashi has a patent application pending relating to the use of MC903 in treatment of inflammatory disorders.
Author Contributions
TML, AS, TY, VSH, BSK, MCS, AA, GSW, and TK designed and conducted experiments. AN and DA provided valuable reagents and designed experiments. WJL provided valuable reagents. TML, VSH, GSW, and TK acquired data, analyzed data, and wrote the manuscript.
References
- 1.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annual review of immunology. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 2.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nature genetics. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 5.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nature genetics. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 6.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature immunology. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. Journal of immunology. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 8.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nature genetics. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 9.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 10.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. The Journal of experimental medicine. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. The Journal of experimental medicine. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horwitz DA. Regulatory T cells in systemic lupus erythematosus: past, present and future. Arthritis research & therapy. 2008;10:227. doi: 10.1186/ar2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. The Journal of experimental medicine. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szanya V, Ermann J, Taylor C, Holness C, Fathman CG. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. Journal of immunology. 2002;169:2461–2465. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- 15.Lepault F, Gagnerault MC. Characterization of peripheral regulatory CD4+ T cells that prevent diabetes onset in nonobese diabetic mice. Journal of immunology. 2000;164:240–247. doi: 10.4049/jimmunol.164.1.240. [DOI] [PubMed] [Google Scholar]
- 16.Almeida AR, Zaragoza B, Freitas AA. Indexation as a novel mechanism of lymphocyte homeostasis: the number of CD4+CD25+ regulatory T cells is indexed to the number of IL-2-producing cells. Journal of immunology. 2006;177:192–200. doi: 10.4049/jimmunol.177.1.192. [DOI] [PubMed] [Google Scholar]
- 17.Swee LK, Bosco N, Malissen B, Ceredig R, Rolink A. Expansion of peripheral naturally occurring T regulatory cells by Fms-like tyrosine kinase 3 ligand treatment. Blood. 2009;113:6277–6287. doi: 10.1182/blood-2008-06-161026. [DOI] [PubMed] [Google Scholar]
- 18.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. The Journal of experimental medicine. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou T, Caton AJ, Koretzky GA, Kambayashi T. Dendritic cells induce regulatory T cell proliferation through antigen-dependent and -independent interactions. Journal of immunology. 2010;185:2790–2799. doi: 10.4049/jimmunol.0903740. [DOI] [PubMed] [Google Scholar]
- 20.Zou T, Satake A, Ojha P, Kambayashi T. Cellular therapies supplement: the role of granulocyte macrophage colony-stimulating factor and dendritic cells in regulatory T-cell homeostasis and expansion. Transfusion. 2011;(51 Suppl 4):160S–168S. doi: 10.1111/j.1537-2995.2011.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunological reviews. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belkaid Y, Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nature immunology. 2013;14:646–653. doi: 10.1038/ni.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2011;(108 Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Hener P, Frossard N, Kato S, Metzger D, Li M, et al. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1536–1541. doi: 10.1073/pnas.0812668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, et al. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 28.Besin G, Gaudreau S, Menard M, Guindi C, Dupuis G, Amrani A. Thymic stromal lymphopoietin and thymic stromal lymphopoietin-conditioned dendritic cells induce regulatory T-cell differentiation and protection of NOD mice against diabetes. Diabetes. 2008;57:2107–2117. doi: 10.2337/db08-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin S, Walz KA, Archambault AS, Sim J, Bollman BP, Koenigsknecht-Talboo J, et al. Apolipoprotein E mediation of neuro-inflammation in a murine model of multiple sclerosis. Journal of neuroimmunology. 2014;271:8–17. doi: 10.1016/j.jneuroim.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Racke MK. Experimental autoimmune encephalomyelitis (EAE) Current protocols in neuroscience. 2001 doi: 10.1002/0471142301.ns0907s14. Chapter 9:Unit9 7. [DOI] [PubMed] [Google Scholar]
- 31.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nature reviews Immunology. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kissmeyer AM, Binderup L. Calcipotriol (MC 903): pharmacokinetics in rats and biological activities of metabolites. A comparative study with 1,25(OH)2D3. Biochemical pharmacology. 1991;41:1601–1606. doi: 10.1016/0006-2952(91)90160-7. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Hener P, Zhang Z, Ganti KP, Metzger D, Chambon P. Induction of thymic stromal lymphopoietin expression in keratinocytes is necessary for generating an atopic dermatitis upon application of the active vitamin D3 analogue MC903 on mouse skin. The Journal of investigative dermatology. 2009;129:498–502. doi: 10.1038/jid.2008.232. [DOI] [PubMed] [Google Scholar]
- 34.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nature immunology. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 35.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. Journal of immunology. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. The Journal of experimental medicine. 2012;209:1723–1742. doi: 10.1084/jem.20120914. S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunological reviews. 2014;259:40–59. doi: 10.1111/imr.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He R, Geha RS. Thymic stromal lymphopoietin. Annals of the New York Academy of Sciences. 2010;1183:13–24. [Google Scholar]
- 39.Satake A, Schmidt AM, Archambault A, Leichner TM, Wu GF, Kambayashi T. Differential targeting of IL-2 and T cell receptor signaling pathways selectively expands regulatory T cells while inhibiting conventional T cells. Journal of autoimmunity. 2013;44:13–20. doi: 10.1016/j.jaut.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 41.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annual review of immunology. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 42.Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity. 2013;39:949–962. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazzucchelli R, Hixon JA, Spolski R, Chen X, Li WQ, Hall VL, et al. Development of regulatory T cells requires IL-7Ralpha stimulation by IL-7 or TSLP. Blood. 2008;112:3283–3292. doi: 10.1182/blood-2008-02-137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JY, Lim YM, Park MJ, Min SY, Cho ML, Sung YC, et al. Murine thymic stromal lymphopoietin promotes the differentiation of regulatory T cells from thymic CD4(+)CD8(−)CD25(−) naive cells in a dendritic cell-independent manner. Immunology and cell biology. 2008;86:206–213. doi: 10.1038/sj.icb.7100127. [DOI] [PubMed] [Google Scholar]
- 45.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. The Journal of experimental medicine. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal period. The Journal of experimental medicine. 2005;201:769–777. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isaksen DE, Baumann H, Trobridge PA, Farr AG, Levin SD, Ziegler SF. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. Journal of immunology. 1999;163:5971–5977. [PubMed] [Google Scholar]
- 48.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. Journal of immunology. 2007;178:6720–6724. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 49.Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmunity reviews. 2012;12:127–136. doi: 10.1016/j.autrev.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. Jama. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 51.Littorin B, Blom P, Scholin A, Arnqvist HJ, Blohme G, Bolinder J, et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS) Diabetologia. 2006;49:2847–2852. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 52.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. The Journal of nutrition. 1998;128:68–72. doi: 10.1093/jn/128.1.68. [DOI] [PubMed] [Google Scholar]
- 53.Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia. 1994;37:552–558. doi: 10.1007/BF00403372. [DOI] [PubMed] [Google Scholar]
- 54.Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. The Journal of clinical investigation. 1991;87:1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pitocco D, Crino A, Di Stasio E, Manfrini S, Guglielmi C, Spera S, et al. The effects of calcitriol and nicotinamide on residual pancreatic beta-cell function in patients with recent-onset Type 1 diabetes (IMDIAB XI) Diabetic medicine : a journal of the British Diabetic Association. 2006;23:920–923. doi: 10.1111/j.1464-5491.2006.01921.x. [DOI] [PubMed] [Google Scholar]
- 56.Rapoport MJ, Jaramillo A, Zipris D, Lazarus AH, Serreze DV, Leiter EH, et al. Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. The Journal of experimental medicine. 1993;178:87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chatenoud L, Salomon B, Bluestone JA. Suppressor T cells--they’re back and critical for regulation of autoimmunity! Immunological reviews. 2001;182:149–163. doi: 10.1034/j.1600-065x.2001.1820112.x. [DOI] [PubMed] [Google Scholar]
- 58.Haribhai D, Lin W, Edwards B, Ziegelbauer J, Salzman NH, Carlson MR, et al. A central role for induced regulatory T cells in tolerance induction in experimental colitis. Journal of immunology. 2009;182:3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sawamukai N, Satake A, Schmidt AM, Lamborn IT, Ojha P, Tanaka Y, et al. Cell-autonomous role of TGFbeta and IL-2 receptors in CD4+ and CD8+ inducible regulatory T-cell generation during GVHD. Blood. 2012;119:5575–5583. doi: 10.1182/blood-2011-07-367987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bikle DD, Nemanic MK, Whitney JO, Elias PW. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry. 1986;25:1545–1548. doi: 10.1021/bi00355a013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.