Abstract
Natural killer (NK) cells are critical for innate regulation of the acute phase of murine cytomegalovirus (MCMV) infection and have been reported to utilize perforin (Pfp)- and gamma interferon (IFN-γ)-dependent effector mechanisms in an organ-specific manner to regulate MCMV infection in the spleen and liver. In this study, we further examined the roles of NK cells, Pfp, and IFN-γ in innate immunity to MCMV infection. With the recently described NK cell-deficient (NKD) mouse, we confirmed previous findings that NK cells, but not NKT cells, are required for control of the acute phase of MCMV infection in spleen and liver cells. Interestingly, we found that Pfp and IFN-γ are each important for regulating MCMV replication in both the spleen and the liver. Moreover, NK cells can regulate MCMV infection in the spleens and livers of Pfp−/− mice in a Pfp-independent manner and can use an IFN-γ-independent mechanism to control MCMV infection in IFN-γ−/− mice. Thus, contrary to previous reports, NK cells utilize both Pfp and IFN-γ to control MCMV infection in the spleen and liver.
Natural killer (NK) cells are important for host innate immunity to viral infection prior to the development of a specific immune response (reviewed in reference 22). NK cell defects correlate with increased susceptibility to viral infections (7), and a human patient with NK cell deficiency was reported to suffer from severe and recurrent infections with herpesviruses (6). In mice, genetic loci conferring resistance to several viruses, including herpes simplex virus, ectromelia virus, and murine cytomegalovirus (MCMV), have been mapped to the NK gene complex (9, 17, 21, 38, 45). Moreover, multiple viruses use strategies for inhibiting NK cell activation and function (reviewed in reference 34), suggesting a critical role for NK cells in antiviral immunity.
Regulation of viral infection by NK cells has been most extensively studied, and most clearly demonstrated, with the betaherpesvirus cytomegalovirus. Human cytomegalovirus can establish a chronic infection that persists for the life of the host (37) and is a significant cause of morbidity and mortality in immunocompromised individuals. Because of its strict species specificity, studies of NK cell regulation of cytomegalovirus infection in vivo have used MCMV as a small-animal model. The importance of NK cells in resistance to MCMV infection has been demonstrated with a number of experimental systems, including infection of beige mice (4, 36, 50), in vivo depletion of NK cells (11, 12, 35, 48, 62), and transfer of NK cells into susceptible weanling mice (10, 13). In C57BL/6 (B6) mice, MCMV infection is regulated by a subset of NK cells expressing the Ly49H activating receptor (8, 16, 44), which recognizes the MCMV m157 protein (2, 52) and mediates Cmv-1-linked genetic resistance to MCMV (26, 28).
The primary effector mechanisms used by NK cells are cell-mediated cytotoxicity and cytokine secretion. NK cytotoxicity utilizes the granule exocytosis pathway consisting of the pore-forming protein perforin (Pfp) and a family of serine proteases known as granzymes (Gzms). Pfp−/− mice have increased susceptibility to retinitis and increased viral titers following MCMV infection (18), and MCMV replication in the salivary glands is regulated by Pfp, GzmA, and GzmB (43). Additionally, secretion of IFN-γ by NK cells is important during the acute phase of MCMV infection (24, 35, 54, 61). IFN-γ inhibits MCMV replication in vitro (23, 29, 42, 47) and protects against MCMV-induced lethality and pathogenesis in vivo (20, 24, 41). IFN-γ is also critical for efficient clearance of persistent viral replication (30, 41) and suppresses MCMV reactivation from latency (39, 41).
Interestingly, NK cells have been reported to use Pfp and IFN-γ in an organ-specific manner to regulate acute MCMV infection. In a study by Tay and Welsh, Pfp−/− mice had elevated viral titers in the spleen but not the liver, while IFN-γ depletion resulted in increased titers in the liver but not in the spleen (53). Moreover, depletion of NK cells in Pfp−/− mice effected increased viral titers only in the liver and not in the spleen. This previous study concluded that NK cells primarily utilize Pfp to regulate MCMV infection in the spleen but control infection in the liver with IFN-γ. In the present study, we further examined the roles of NK cells, Pfp, and IFN-γ in the regulation of MCMV infection in vivo. With NK cell-deficient (NKD) mice, which lack peripheral NK cells but retain normal NKT and T cell function (25), we confirmed the importance of NK cells, but not NKT cells, in conferring protection against acute MCMV infection. In contrast to the previous study (53), however, we did not observe organ specificity in the utilization of Pfp and IFN-γ by NK cells to regulate MCMV infection. Rather, we found that Pfp and IFN-γ were both important for control of viral replication in the spleen and liver, and depletion of NK cells from either Pfp−/− or IFN-γ−/− mice resulted in elevated viral titers in both the spleen and the liver. Thus, NK cells utilize both Pfp and IFN-γ to regulate the acute phase of MCMV infection in the spleen and the liver.
NK cells, but not NKT cells, are critical mediators of innate immunity to the acute phase of MCMV infection.
NKD mice generated directly on the B6 background were recently described to be deficient in NK cells while retaining NKT and T cell function (25). NKD mice were the unexpected result of an attempt to generate Ly49A transgenic mice, and the genetic mechanism of NK cell deficiency in these mice is unclear. However, further characterization suggests that the NKD phenotype may be linked to insertion of the transgene into a gene encoding a transcription factor (S. Kim, D. Higuchi, A. R. French, and W. M. Yokoyama, unpublished data). NK cell deficiency in NKD mice is evidenced by a marked reduction in the number of peripheral NK cells, defective in vitro cytolytic activity, and impaired tumor cell killing in vivo. In contrast, NKT cells are present in near-normal numbers and retain the capacity for interleukin-4 and IFN-γ production upon anti-CD3 stimulation. The T cell compartment also appears unaffected, with normal numbers of total T cells or T cell subsets in lymphoid organs and normal T cell function as assessed by proliferation following anti-CD3 stimulation, cytotoxic activity, and skin allograft rejection. Thus, NKD mice have a selective deficiency in NK cells and have recently been used to examine the function of NK cells in innate immunity against Ebola virus (58).
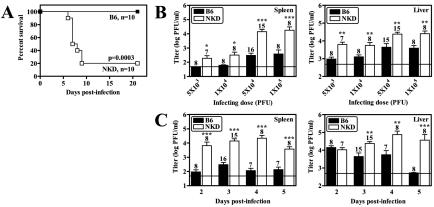
We used NKD mice to examine the effects of genetic deficiency in the NK cell compartment in the acute phase of MCMV infection. We found that NKD mice were significantly more susceptible to MCMV infection than B6 mice, with death occurring as early as 3 days postinfection (dpi) and 80% of the mice dying by 9 dpi. In contrast, B6 mice were completely resistant to MCMV-induced lethality at this dose (Fig. 1A) and appeared healthy throughout the course of the study. Additionally, NKD mice had elevated viral titers in both the spleen and the liver compared to B6 mice at 3 dpi with various doses of MCMV (Fig. 1B). This difference was especially apparent in the spleen, with more-than-10-fold increases observed in NKD mice relative to B6 controls at the two highest infecting doses. To test whether NK cell deficiency altered the kinetics of viral clearance, we determined the viral titers in the spleens and livers of infected mice at 2 to 5 dpi. NKD mice had 1- to 2-log increases in splenic viral titer compared to B6 mice at all of the time points tested (Fig. 1C). Significant differences in viral titer from the livers of NKD and B6 mice were only apparent beginning at 3 dpi, but by 5 dpi B6 mice had largely cleared the infection in the liver whereas NKD mice retained more than 104 PFU of virus. Thus, NKD mice exhibited heightened susceptibility to MCMV infection, as well as impaired control of viral replication following infection.
FIG. 1.
Increased susceptibility of NKD mice to MCMV infection. NKD mice were generated as previously described (25) and maintained in a specific-pathogen-free facility at the Washington University School of Medicine in accordance with all federal and university policies. B6 mice were purchased from Jackson Laboratories. The MCMV Smith strain was obtained from the American Type Culture Collection (VR-194, lot 10), and salivary gland-passaged virus stocks were generated and their titers were determined as previously described (24, 40). For all experiments, age- and sex-matched mice were infected intraperitoneally in Dulbecco modified Eagle medium containing 10% fetal calf serum. All of the data shown represent a minimum of two independent experiments. As no significant differences were observed between independent experiments, all of the data were pooled. (A) Mice infected with 105 PFU of MCMV were monitored for survival for 21 dpi. The number of mice analyzed and the P values are indicated. (B) Mice were infected with various doses of MCMV, and organs were harvested at 3 dpi and virus titers were determined by plaque assay as previously described (24). (C) Mice were infected with 5 × 104 PFU of MCMV, and organs were harvested for titer determination on various days postinfection. The number of mice analyzed is given above each bar, the limit of detection is denoted by the horizontal line, and statistically significant differences compared to B6 mice are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
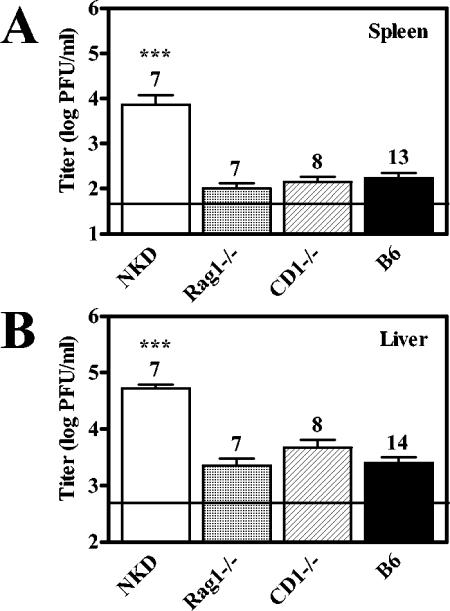
To confirm that the defects observed in NKD mice are due specifically to loss of NK cell function, we compared the viral titers of the spleens and livers of MCMV-infected NKD, Rag-1−/−, CD1−/−, and B6 mice. Rag-1−/− mice lack mature T, B, and NKT cells but have functional NK cells (32), while CD1−/− mice are selectively deficient in NKT cells (15, 31). As expected, NKD mice exhibited increased viral titers in the spleen and liver compared to B6 mice, whereas both Rag-1−/− and CD1−/− mice had viral titers equivalent to those of B6 mice (Fig. 2). Thus, control of this early phase of MCMV infection does not require T, B, or NKT cells, suggesting that the impairment of NKD mice in regulating MCMV infection is due specifically to loss of NK cell function. These results confirm, under our experimental conditions, previous findings on the importance of NK cells in regulating MCMV infection, thereby providing a basis for further examination of Pfp- and IFN-γ-dependent effector functions.
FIG. 2.
Regulation of the early acute phase of MCMV infection does not require T, B, or NKT cells. CD1−/− mice were a kind gift from Albert Bendelac, and Rag1−/− mice were purchased from Jackson Laboratories. Mice were infected with 5 × 104 PFU of MCMV, and organs were harvested for titer determination at 3 dpi as described in the legend to Fig. 1. The number of mice analyzed is given above each bar, the limit of detection is denoted by the horizontal line, and statistically significant differences are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Pfp and IFN-γ have important and nonredundant roles in regulating MCMV infection in both the spleen and the liver.
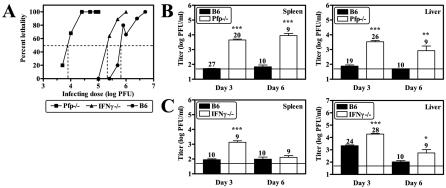
As Pfp and IFN-γ are two primary effector mechanisms utilized by NK cells, we assessed their roles in the regulation of MCMV infection with Pfp−/− and IFN-γ−/− mice. Comparison of percent lethality following MCMV infection of Pfp−/−, IFN-γ−/− (41), and B6 (41) mice revealed increased susceptibility to lethality following MCMV infection in both Pfp−/− and IFN-γ−/− mice, although the effect of Pfp deficiency was much more striking, with an approximately 2-log decrease in the 50% lethal dose compared to that for B6 mice (Fig. 3A). Thus, Pfp- and IFN-γ-dependent mechanisms are both important for protection against lethal MCMV infection.
FIG. 3.
Pfp and IFN-γ are important for protection against lethal MCMV infection. Pfp−/− and IFN-γ−/− mice were purchased from Jackson Laboratories. (A) Pfp−/− mice were infected with various doses of MCMV and monitored for survival as described in the legend to Fig. 1. Previously published data obtained with IFN-γ−/− and B6 mice are shown for comparison (41), and the 50% lethal dose is indicated by dotted lines. Mice were infected with 1 × 104 (B) or 7.5 × 104 (C) PFU of MCMV, and organs were harvested for titer determination at 3 or 6 dpi as described in the legend to Fig. 1. The number of mice analyzed is given above each bar, the limit of detection is denoted by the horizontal line, and statistically significant differences compared to B6 mice are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
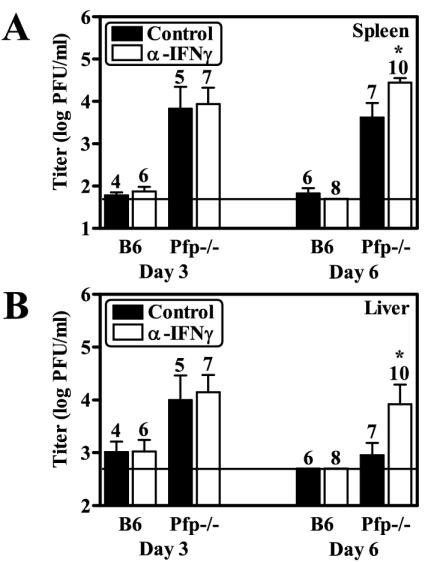
To test whether Pfp−/− and IFN-γ−/− mice have organ-specific impairment in control of MCMV replication, we examined viral titers in the spleens and livers of MCMV-infected Pfp−/− and IFN-γ−/− mice at 3 and 6 dpi. We found that Pfp−/− mice had significantly higher viral titers in both the spleen and the liver relative to B6 mice at both time points (Fig. 3B). Moreover, IFN-γ−/− mice also had significantly increased viral titers in both organs compared to B6 mice, although an increase in splenic viral titers was only observed at 3 dpi (Fig. 3C) and no effect of IFN-γ deficiency was observed with lower infecting doses (data not shown). To test whether the roles of Pfp and IFN-γ are redundant, we depleted IFN-γ in Pfp−/− mice and determined viral titers following MCMV infection. We found that depletion of IFN-γ resulted in significant increases in the viral titers in both the spleens and the livers of Pfp−/− mice at 6 dpi, although no effect was observed at 3 dpi (Fig. 4). Together, these data demonstrate that both Pfp and IFN-γ are important for controlling MCMV infection in the spleen and liver and that IFN-γ has a regulatory function that is independent of Pfp.
FIG. 4.
IFN-γ has a function in regulating MCMV infection that is independent of Pfp. For IFN-γ depletion studies, mice were injected with an endotoxin-free neutralizing antibody to IFN-γ diluted in saline (H22) (46) as previously described (24). Control mice were injected with either saline alone or an isotype-matched control antibody. As no significant differences were observed between mice given saline or the control antibody, the data from these two groups were pooled. At 2 days postinjection, mice were infected with 5 × 103 PFU of MCMV and the viral titers of their spleens (A) and livers (B) were determined at 3 and 6 dpi as described in the legend to Fig. 1. The number of mice analyzed is given above each bar, the limit of detection is denoted by the horizontal line, and statistically significant differences compared to control are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
NK cells use more than one effector mechanism to regulate MCMV infection in both the spleen and the liver.
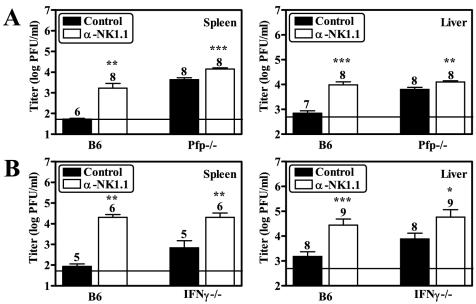
As our data suggested that both Pfp- and IFN-γ-dependent mechanisms regulate MCMV infection in the spleen and liver, we tested whether NK cells utilize more than one effector mechanism to control infection in these organs. To determine whether NK cells control MCMV infection with a Pfp-independent mechanism, we examined the effect of NK cell depletion on viral titers in Pfp−/− mice at 3 dpi. We found that NK cell depletion in Pfp−/− mice resulted in small, but statistically significant, increases in splenic and liver viral titers compared to those of mice treated with an isotype-matched control antibody (Fig. 5A). Additionally, we tested whether NK cells can regulate MCMV infection with an IFN-γ-independent mechanism by examining the effect of NK cell depletion on viral titers in IFN-γ−/− mice at 3 dpi. We found that NK cell depletion in IFN-γ−/− mice also resulted in significant increases in viral titers in both the spleen and the liver (Fig. 5B). These data demonstrate that NK cell regulation of MCMV infection in either the spleen or the liver does not rely upon a single effector mechanism and that Pfp and IFN-γ are not exclusively utilized in the spleen and liver, respectively.
FIG. 5.
NK cells utilize more than one effector mechanism to control MCMV infection in the spleen and liver. For NK cell depletion, mice were intraperitoneally injected with either 300 μg of antibody to NK1.1 (PK136; ATCC HB-191) or an isotype-matched control antibody (MAR 18.5; ATCC TIB-216). Depletion of NK cells was confirmed by 51Cr release assay as previously described (25). At 2 days postinjection, mice were infected with 5 × 103 PFU of MCMV and the viral titers of Pfp−/− (A) and IFN-γ−/− (B) mice were determined at 3 dpi as described in the legend to Fig. 1. The number of mice analyzed is given above each bar, the limit of detection is denoted by the horizontal line, and statistically significant differences compared to control are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Discussion.
In this study, we examined the roles of NK cells, Pfp, and IFN-γ in the regulation of the acute phase of MCMV infection in vivo. We found that NKD mice, which are specifically deficient in NK cells, have increased susceptibility to lethal MCMV infection, have elevated viral titers in the spleen and liver, and exhibit delayed clearance of the acute phase of infection. We further showed that control of the acute phase of MCMV infection does not require T, B, or NKT cells, which is consistent with the defect in NKD mice being due specifically to loss of NK cell function. Studies with Pfp−/− and IFN-γ−/− mice demonstrated that both Pfp and IFN-γ are important for control of MCMV infection in the spleen and liver and that IFN-γ has a function that is independent of Pfp. Moreover, we found that NK cells utilized more than one effector mechanism to regulate MCMV infection in the spleen and liver, which is contrary to previous reports that NK cells use Pfp and IFN-γ in an organ-specific manner. Cumulatively, our data indicate that NK cells utilize both Pfp- and IFN-γ-dependent mechanisms to regulate the acute phase of MCMV infection in the spleen and liver.
Although studies of NK cell regulation of MCMV infection have used several experimental systems, interpretation of these studies has been complicated by the fact that none of these models are specifically and completely deficient in NK cell function. For example, CD3ɛ transgenic mice are impaired in both the NK and T cell compartments (57), whereas beige mice, which lack NK cytolytic function, also have defects in cytolysis in other cells and may retain other NK cell functions (3, 5). Furthermore, depletion of NK cells may additionally deplete other cell populations that express NK cell surface markers. Our observations that NKD mice are, as expected, highly susceptible to MCMV infection and lethality suggests that they can be an additional useful system for in vivo studies of NK cell regulation of MCMV infection. Although the molecular mechanism for NK cell deficiency in NKD mice is not clear, T and NKT cells from NKD mice appear normal by multiple assays (25). NKD mice are therefore a more specific experimental model for NK cell deficiency than has previously been described. The use of NKD mice in this study confirmed previous findings that NK cells have a critical regulatory role during the acute phase of MCMV infection in the spleen and liver. Our studies also corroborate, with a different experimental system, previous reports that NKT cells are not required for regulation of the acute phase of MCMV infection (56). However, as NKT cells are involved in the regulation of infection with multiple viruses (reviewed in reference 51), secrete cytokines that activate NK cells (14, 19), and can indirectly inhibit MCMV replication through potentiation of NK cell antiviral function (56), NKT cells may contribute to NK cell regulation of MCMV infection by modulating NK cell activity.
Although Tay and Welsh have reported that NK cells regulate MCMV infection with Pfp in the spleen and IFN-γ in the liver (53), our studies demonstrated increased viral titers in both the spleen and the liver in Pfp−/− and IFN-γ−/− mice, indicating that both Pfp and IFN-γ regulate MCMV replication in each organ. Moreover, depletion of NK cells from either Pfp−/− or IFN-γ−/− mice resulted in increased viral titers in both the spleen and the liver, suggesting that NK cells use more than one effector mechanism to control MCMV infection in each organ. Other studies examining the role of these effector mechanisms in controlling MCMV infection have also produced conflicting results—a study by Orange and Biron found that Pfp deficiency has no effect on viral titers in the liver (33), while studies by van Dommelen et al. reveal an IFN-γ-independent mechanism for regulating MCMV infection in the liver and suggest the importance of Pfp in that organ (56).
One possible explanation for these disparities are differences in experimental parameters. Notably, the studies by Tay et al. and Orange et al. both utilized Pfp−/− mice on a mixed B6/129 background, whereas van Dommelen et al. used Pfp−/− and IFN-γ−/− mice on the BALB/c background and our studies used mice on the B6 background. The background strain is an important factor, as the NK cell-mediated regulation of MCMV infection differs significantly between mice of different genetic backgrounds (reviewed in reference 27). Additionally, we found that IFN-γ deficiency resulted in increased viral titers in the liver, but not in the spleen, at 6 dpi with 7.5 × 104 PFU of MCMV. Although this appears to reflect tissue-specific regulation of MCMV infection by IFN-γ, viral titers increased in both the spleens and the livers of IFN-γ−/− mice at 3 dpi, suggesting instead that these differences in regulation are kinetic rather than tissue specific. Thus, the effects of Pfp and IFN-γ on MCMV replication in the spleen and liver may vary depending on the mouse strain, the infecting dose, and the time of assay. Nonetheless, the data presented here clearly demonstrate that NK-mediated regulation of MCMV infection does not exclusively utilize Pfp in the spleen and IFN-γ in the liver and that both effector mechanisms are important for control of MCMV infection in each organ.
As IFN-γ has been reported to enhance NK cell cytotoxic activity (59, 60), it is possible that the roles of IFN-γ and Pfp in regulating MCMV infection are linked. The observation that depletion of IFN-γ resulted in increased viral titers in the spleens and livers of Pfp−/− mice does not preclude a role for IFN-γ in potentiating NK cell killing but demonstrates that IFN-γ has a function in controlling MCMV infection that is independent of Pfp. This Pfp-independent function of IFN-γ may involve direct antiviral activity and/or modulation of the inflammatory response. Although Pfp and IFN-γ are clearly important for NK cell regulation of MCMV infection, other cytokines secreted by NK cells, such as tumor necrosis factor alpha (TNF-α), may also be involved in regulating MCMV infection. TNF-α can directly, or synergistically with IFN-γ, inhibit MCMV replication in vitro (29, 55). TNF-α has also been reported to limit MCMV replication and pathology (24, 33) and enhance the protective effects of IFN-γ against lethal infection (1). However, other studies have found no effect of TNF-α on the acute phase of MCMV infection in vivo (49, 55) and NK cells are not required for production of TNF-α during MCMV infection (33, 35). It is therefore unclear whether TNF-α secretion constitutes a significant mechanism for NK-mediated regulation of MCMV infection.
Acknowledgments
H.W.V. was supported by grant R01 HL60090 from the NIH. J.L. was supported by NIH training grant 5-T32-AI07163-26. Work in the Yokoyama laboratory is supported by the Howard Hughes Medical Institute, the Barnes-Jewish Hospital Research Foundation, and grants from the NIH.
We thank Darren Kreamalmeyer for expert assistance in the breeding and maintenance of mice. We thank the members of the laboratories of H.W.V. and W.M.Y. for helpful discussions and comments on this work.
REFERENCES
- 1.Anderson, K. P., Y. S. Lie, M. A. Low, and E. H. Fennie. 1993. Effects of tumor necrosis factor-alpha treatment on mortality in murine cytomegalovirus-infected mice. Antiviral Res. 21:343-355. [DOI] [PubMed] [Google Scholar]
- 2.Arase, H., E. S. Mocarski, A. E. Campbell, A. B. Hill, and L. L. Lanier. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296:1323-1326. [DOI] [PubMed] [Google Scholar]
- 3.Baetz, K., S. Isaaz, and G. M. Griffiths. 1995. Loss of cytotoxic T lymphocyte function in Chediak-Higashi syndrome arises from a secretory defect that prevents lytic granule exocytosis. J. Immunol. 154:6122-6131. [PubMed] [Google Scholar]
- 4.Bancroft, G. J., G. R. Shellam, and J. E. Chalmer. 1981. Genetic influences on the augmentation of natural killer (NK) cells during murine cytomegalovirus infection: correlation with patterns of resistance. J. Immunol. 126:988-994. [PubMed] [Google Scholar]
- 5.Barbosa, M. D., Q. A. Nguyen, V. T. Tchernev, J. A. Ashley, J. C. Detter, S. M. Blaydes, S. J. Brandt, D. Chotai, C. Hodgman, R. C. Solari, M. Lovett, and S. F. Kingsmore. 1996. Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature 382:262-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biron, C. A., K. S. Byron, and J. L. Sullivan. 1989. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320:1731-1735. [DOI] [PubMed] [Google Scholar]
- 7.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 8.Brown, M. G., A. O. Dokun, J. W. Heusel, H. R. Smith, D. L. Beckman, E. A. Blattenberger, C. E. Dubbelde, L. R. Stone, A. A. Scalzo, and W. M. Yokoyama. 2001. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science 292:934-937. [DOI] [PubMed] [Google Scholar]
- 9.Brown, M. G., J. Zhang, Y. Du, J. Stoll, W. M. Yokoyama, and A. A. Scalzo. 1999. Localization on a physical map of the NKC-linked Cmv1 locus between Ly49b and the Prp gene cluster on mouse chromosome 6. J. Immunol. 163:1991-1999. [PubMed] [Google Scholar]
- 10.Bukowski, J. F., J. F. Warner, G. Dennert, and R. M. Welsh. 1985. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. J. Exp. Med. 161:40-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukowski, J. F., and R. M. Welsh. 1985. Inability of interferon to protect virus-infected cells against lysis by natural killer (NK) cells correlates with NK cell-mediated antiviral effects in vivo. J. Immunol. 135:3537-3541. [PubMed] [Google Scholar]
- 12.Bukowski, J. F., B. A. Woda, and R. M. Welsh. 1984. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J. Virol. 52:119-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukowski, J. F., H. Yang, and R. M. Welsh. 1988. Antiviral effect of lymphokine-activated killer cells: characterization of effector cells mediating prophylaxis. J. Virol. 62:3642-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carnaud, C., D. Lee, O. Donnars, S. H. Park, A. Beavis, Y. Koezuka, and A. Bendelac. 1999. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163:4647-4650. [PubMed] [Google Scholar]
- 15.Chen, Y. H., N. M. Chiu, M. Mandal, N. Wang, and C. R. Wang. 1997. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity 6:459-467. [DOI] [PubMed] [Google Scholar]
- 16.Daniels, K. A., G. Devora, W. C. Lai, C. L. O'Donnell, M. Bennett, and R. M. Welsh. 2001. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med. 194:29-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delano, M. L., and D. G. Brownstein. 1995. Innate resistance to lethal mousepox is genetically linked to the NK gene complex on chromosome 6 and correlates with early restriction of virus replication by cells with an NK phenotype. J. Virol. 69:5875-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dix, R. D., E. R. Podack, and S. W. Cousins. 2003. Loss of the perforin cytotoxic pathway predisposes mice to experimental cytomegalovirus retinitis. J. Virol. 77:3402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eberl, G., and H. R. MacDonald. 2000. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur. J. Immunol. 30:985-992. [DOI] [PubMed] [Google Scholar]
- 20.Fennie, E. H., Y. S. Lie, M. A. Low, P. Gribling, and K. P. Anderson. 1988. Reduced mortality in murine cytomegalovirus infected mice following prophylactic murine interferon-gamma treatment. Antiviral Res. 10:27-39. [DOI] [PubMed] [Google Scholar]
- 21.Forbes, C. A., M. G. Brown, R. Cho, G. R. Shellam, W. M. Yokoyama, and A. A. Scalzo. 1997. The Cmv1 host resistance locus is closely linked to the Ly49 multigene family within the natural killer cell gene complex on mouse chromosome 6. Genomics 41:406-413. [DOI] [PubMed] [Google Scholar]
- 22.French, A. R., and W. M. Yokoyama. 2003. Natural killer cells and viral infections. Curr. Opin. Immunol. 15:45-51. [DOI] [PubMed] [Google Scholar]
- 23.Gribaudo, G., S. Ravaglia, A. Caliendo, R. Cavallo, M. Gariglio, M. G. Martinotti, and S. Landolfo. 1993. Interferons inhibit onset of murine cytomegalovirus immediate-early gene transcription. Virology 197:303-311. [DOI] [PubMed] [Google Scholar]
- 24.Heise, M. T., and H. W. Virgin. 1995. The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J. Virol. 69:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, S., K. Iizuka, H. L. Aguila, I. L. Weissman, and W. M. Yokoyama. 2000. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc. Natl. Acad. Sci. USA 97:2731-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, S. H., S. Girard, D. Macina, M. Busa, A. Zafer, A. Belouchi, P. Gros, and S. M. Vidal. 2001. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat. Genet. 28:42-45. [DOI] [PubMed] [Google Scholar]
- 27.Lee, S. H., J. R. Webb, and S. M. Vidal. 2002. Innate immunity to cytomegalovirus: the Cmv1 locus and its role in natural killer cell function. Microbes Infect. 4:1491-1503. [DOI] [PubMed] [Google Scholar]
- 28.Lee, S. H., A. Zafer, Y. de Repentigny, R. Kothary, M. L. Tremblay, P. Gros, P. Duplay, J. R. Webb, and S. M. Vidal. 2003. Transgenic expression of the activating natural killer receptor Ly49H confers resistance to cytomegalovirus in genetically susceptible mice. J. Exp. Med. 197:515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucin, P., S. Jonjic, M. Messerle, B. Polic, H. Hengel, and U. H. Koszinowski. 1994. Late phase inhibition of murine cytomegalovirus replication by synergistic action of interferon-gamma and tumor necrosis factor. J. Gen. Virol. 75:101-110. [DOI] [PubMed] [Google Scholar]
- 30.Lucin, P., I. Pavic, B. Polic, S. Jonjic, and U. H. Koszinowski. 1992. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J. Virol. 66:1977-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendiratta, S. K., W. D. Martin, S. Hong, A. Boesteanu, S. Joyce, and L. VanKaer. 1997. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity 6:469-477. [DOI] [PubMed] [Google Scholar]
- 32.Mombaerts, P., J. Iacomini, R. S. Johnson, K. Herrup, S. Tonegawa, and V. E. Papaioannou. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869-877. [DOI] [PubMed] [Google Scholar]
- 33.Orange, J. S., and C. A. Biron. 1996. Characterization of early IL-12, IFN-α/β, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J. Immunol. 156:4746-4756. [PubMed] [Google Scholar]
- 34.Orange, J. S., M. S. Fassett, L. A. Koopman, J. E. Boyson, and J. L. Strominger. 2002. Viral evasion of natural killer cells. Nat. Immunol. 3:1006-1012. [DOI] [PubMed] [Google Scholar]
- 35.Orange, J. S., B. Wang, C. Terhorst, and C. A. Biron. 1995. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 182:1045-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papadimitriou, J. M., G. R. Shellam, and J. E. Allan. 1982. The effect of the beige mutation on infection with murine cytomegalovirus: histopathologic studies. Am. J. Pathol. 108:299-309. [PMC free article] [PubMed] [Google Scholar]
- 37.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2706. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 38.Pereira, R. A., A. Scalzo, and A. Simmons. 2001. Cutting edge: a NK complex-linked locus governs acute versus latent herpes simplex virus infection of neurons. J. Immunol. 166:5869-5873. [DOI] [PubMed] [Google Scholar]
- 39.Polic, B., H. Hengel, A. Krmpotic, J. Trgovcich, I. Pavic, P. Lucin, S. Jonjic, and U. H. Koszinowski. 1998. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J. Exp. Med. 188:1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollock, J. L., R. M. Presti, S. Paetzold, and H. W. Virgin. 1997. Latent murine cytomegalovirus infection in macrophages. Virology 227:168-179. [DOI] [PubMed] [Google Scholar]
- 41.Presti, R. M., J. L. Pollock, A. J. Dal Canto, A. K. O'Guin, and H. W. Virgin. 1998. Interferon-gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J. Exp. Med. 188:577-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Presti, R. M., D. L. Popkin, M. Connick, S. Paetzold, and H. W. Virgin. 2001. Novel cell type-specific antiviral mechanism of interferon gamma action in macrophages. J. Exp. Med. 193:483-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riera, L., M. Gariglio, G. Valente, A. Mullbacher, C. Museteanu, S. Landolfo, and M. M. Simon. 2000. Murine cytomegalovirus replication in salivary glands is controlled by both perforin and granzymes during acute infection. Eur. J. Immunol. 30:1350-1355. [DOI] [PubMed] [Google Scholar]
- 44.Scalzo, A. A., N. A. Fitzgerald, C. R. Wallace, A. E. Gibbons, Y. C. Smart, R. C. Burton, and G. R. Shellam. 1992. The effect of the Cmv-1 resistance gene, which is linked to the natural killer cell gene complex, is mediated by natural killer cells. J. Immunol. 149:581-589. [PubMed] [Google Scholar]
- 45.Scalzo, A. A., P. A. Lyons, N. A. Fitzgerald, C. A. Forbes, W. M. Yokoyama, and G. R. Shellam. 1995. Genetic mapping of Cmv1 in the region of mouse chromosome 6 encoding the NK gene complex-associated loci Ly49 and musNKR-P1. Genomics 27:435-441. [DOI] [PubMed] [Google Scholar]
- 46.Schreiber, R. D., L. J. Hicks, A. Celada, N. A. Buchmeier, and P. W. Gray. 1985. Monoclonal antibodies to murine gamma interferon which differentially modulate macrophage activation and antiviral activity. J. Immunol. 134:1609-1618. [PubMed] [Google Scholar]
- 47.Schut, R. L., G. Gekker, S. Hu, C. C. Chao, C. Pomeroy, M. C. Jordan, and P. K. Peterson. 1994. Cytomegalovirus replication in murine microglial cell cultures: suppression of permissive infection by interferon-gamma. J. Infect. Dis. 169:1092-1096. [DOI] [PubMed] [Google Scholar]
- 48.Shanley, J. D. 1990. In vivo administration of monoclonal antibody to the NK 1.1 antigen of natural killer cells: effect on acute murine cytomegalovirus infection. J. Med. Virol. 30:58-60. [DOI] [PubMed] [Google Scholar]
- 49.Shanley, J. D., E. Goff, R. J. Debs, and S. J. Forman. 1994. The role of tumor necrosis factor-alpha in acute murine cytomegalovirus infection in BALB/c mice. J. Infect. Dis. 169:1088-1091. [DOI] [PubMed] [Google Scholar]
- 50.Shellam, G. R., J. E. Allan, J. M. Papadimitriou, and G. J. Bancroft. 1981. Increased susceptibility to cytomegalovirus infection in beige mutant mice. Proc. Natl. Acad. Sci. USA 78:5104-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skold, M., and S. M. Behar. 2003. Role of CD1d-restricted NKT cells in microbial immunity. Infect. Immun. 71:5447-5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith, H. R., J. W. Heusel, I. K. Mehta, S. Kim, B. G. Dorner, O. V. Naidenko, K. Iizuka, H. Furukawa, D. L. Beckman, J. T. Pingel, A. A. Scalzo, D. H. Fremont, and W. M. Yokoyama. 2002. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. USA 99:8826-8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tay, C. H., and R. M. Welsh. 1997. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J. Virol. 71:267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tay, C. H., L. Y. Yu, V. Kumar, L. Mason, J. R. Ortaldo, and R. M. Welsh. 1999. The role of LY49 NK cell subsets in the regulation of murine cytomegalovirus infections. J. Immunol. 162:718-726. [PubMed] [Google Scholar]
- 55.van der Meer, J. W., R. H. Rubin, M. Pasternack, D. N. Medearis, P. Lynch, and C. A. Dinarello. 1989. The in vivo and in vitro effects of interleukin-1 and tumor necrosis factor on murine cytomegalovirus infection. Biotherapy 1:227-231. [DOI] [PubMed] [Google Scholar]
- 56.van Dommelen, S. L., H. A. Tabarias, M. J. Smyth, and M. A. Degli-Esposti. 2003. Activation of natural killer (NK) T cells during murine cytomegalovirus infection enhances the antiviral response mediated by NK cells. J. Virol. 77:1877-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, B., C. Biron, J. She, K. Higgins, M. J. Sunshine, E. Lacy, N. Lonberg, and C. Terhorst. 1994. A block in both early T lymphocyte and natural killer cell development in transgenic mice with high-copy numbers of the human CD3E gene. Proc. Natl. Acad. Sci. USA 91:9402-9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warfield, K. L., J. G. Perkins, D. L. Swenson, E. M. Deal, C. M. Bosio, M. J. Aman, W. M. Yokoyama, H. A. Young, and S. Bavari. 2004. Role of natural killer cells in innate protection against lethal Ebola virus infection. J. Exp. Med. 200:169-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weigent, D. A., M. P. Langford, W. R. Fleischmann, Jr., and G. J. Stanton. 1983. Potentiation of lymphocyte natural killing by mixtures of alpha or beta interferon with recombinant gamma interferon. Infect. Immun. 40:35-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weigent, D. A., J. G. Stanton, and H. M. Johnson. 1983. Interleukin 2 enhances natural killer cell activity through induction of gamma interferon. Infect. Immun. 41:992-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welsh, R. M., J. O. Brubaker, M. Vargas Cortes, and C. L. O'Donnell. 1991. Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency. The stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. J. Exp. Med. 173:1053-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Welsh, R. M., P. L. Dundon, E. E. Eynon, J. O. Brubaker, G. C. Koo, and C. L. O'Donnell. 1990. Demonstration of the antiviral role of natural killer cells in vivo with a natural killer cell-specific monoclonal antibody (NK 1.1). Nat. Immun. Cell Growth Regul. 9:112-120. [PubMed] [Google Scholar]