Abstract
BACKGROUND:
Knowledge regarding whether benzodiazepines and similarly acting non-benzodiazepines (Z-drugs) are associated with an increased risk of pneumonia among older adults is lacking. We sought to investigate this association among community-dwelling adults with Alzheimer disease, a condition in which both sedative/hypnotic use and pneumonia are common.
METHODS:
We obtained data on all community-dwelling adults with a recent diagnosis of Alzheimer disease in Finland (2005–2011) from the Medication use and Alzheimer disease (MEDALZ) cohort, which incorporates national registry data on prescriptions, reimbursement, hospital discharges and causes of death. Incident users of benzodiazepines and Z-drugs were identified using a 1-year washout period and matched with nonusers using propensity scores. The association with hospital admission or death due to pneumonia was analyzed with the Cox proportional hazards model and adjusted for use of other psychotropic drugs in a time-dependent manner.
RESULTS:
Among 49 484 eligible participants with Alzheimer disease, 5232 taking benzodiazepines and 3269 taking Z-drugs were matched 1:1 with those not taking these drugs. Collectively, use of benzodiazepines and Z-drugs was associated with an increased risk of pneumonia (adjusted hazard ratio [HR] 1.22, 95% confidence interval [CI] 1.05–1.42). When analyzed separately, benzodiazepine use was significantly associated with an increased risk of pneumonia (adjusted HR 1.28, 95% CI 1.07–1.54), whereas Z-drug use was not (adjusted HR 1.10, 95% CI 0.84–1.44). The risk of pneumonia was greatest within the first 30 days of benzodiazepine use (HR 2.09, 95% CI 1.26–3.48).
INTERPRETATION:
Benzodiazepine use was associated with an increased risk of pneumonia among patients with Alzheimer disease. Risk of pneumonia should be considered when weighing the benefits and risks of benzodiazepines in this population.
Alzheimer disease is the most common form of dementia, accounting for 60%–70% of all cases.1,2 In 2011, there were 35.6 million people with dementia globally, and the number is estimated to double every 20 years. Aging is the major risk factor for Alzheimer disease; thus, population aging is leading to major public health challenges related to dementia disorders. Dementia and Alzheimer disease result in frequent health care service utilization and substantial health care costs.3 Current drug therapy for Alzheimer disease has only modest efficacy.4 Behavioural and psychological symptoms of dementia are frequent5,6 and often treated with psychotropic drugs,7 although these symptoms are not approved indications for most of these drugs. Use of benzodiazepines and similarly acting non-benzodiazepines (Z-drugs) is frequent among those with Alzheimer disease, and many patients use these drugs long term,8 although clinical care guidelines recommend only short-term use.5,6
Incidence of pneumonia increases with age.9 Pneumonia is frequent and one of the leading causes of admission to hospital among adults with Alzheimer disease.10 Other risk factors include female sex, smoking, alcohol abuse, respiratory diseases and many other comorbidities.11 Dementia is also a risk factor for pneumonia-related death.12,13 Thus, it is important to recognize factors that increase the risk of pneumonia. Benzodiazepines are known to induce sedation, and more pronounced sedative effects are noticed among older adults because of pharmacodynamic changes.14 Sedation may lead to pneumonia by increased risk of aspiration.15
A nested case–control study in the United Kingdom evaluated benzodiazepine use and occurrence of pneumonia in people of all ages and found a 50% increased risk of pneumonia.16 However, another case–control study in the United States among older adults did not find an association between benzodiazepine use and pneumonia but reported an increased risk for opioid use.17 A recent population-based cohort study on benzodiazepine use found that it also was associated with adverse respiratory outcomes among older adults with chronic obstructive pulmonary disease (COPD).18
Our objective was to investigate whether benzodiazepine and Z-drug use is associated with risk of pneumonia among community-dwelling adults with a diagnosis of Alzheimer disease.
Methods
Setting
The Medication use and Alzheimer disease (MEDALZ) cohort included all community-dwelling adults with a diagnosis of Alzheimer disease (n = 70 718) between 2005 and 2011 in Finland.19 These participants were identified from the Special Reimbursement Register (maintained by the Social Insurance Institution of Finland) that includes people with a diagnosis of chronic disease according to a predefined protocol. The protocol for Alzheimer disease includes a computed tomography or magnetic resonance imaging scan and confirmation of the diagnosis by a neurologist or geriatrician. The Social Insurance Institution of Finland, which grants special reimbursement, ensures that diagnostic criteria are met. Diagnosis was based on criteria from the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA)20 and DSM-IV (Diagnostic and statistical manual of mental disorders. 4th ed. Washington: American Psychiatric Association; 1994).
Data sources
Data for the cohort were collected from several nationwide registers in Finland: Prescription Register (for drug use, 1995–2012), Special Reimbursement Register (for comorbidities, 1972–2012) and Hospital Discharge Register (for pneumonia diagnoses and comorbidities, 1972–2012); and socioeconomic data since 1970 and causes of death (2005–2012) from Statistics Finland. The Prescription Register includes information on purchases of reimbursed drugs that are classified according to the Anatomic Therapeutic Chemical classification system.21 Some small packages (i.e., 10–30 tablets) of benzodiazepines and related drugs are not reimbursed; therefore, they are not captured in the database. However, reimbursement status is a strong driver of prescribing, because drugs without reimbursement status are more expensive. Data on drug use were restricted to community-dwelling patients; drugs used during stays in hospital and public nursing homes are not recorded in the register.
Study design and participants
We modelled when drug use started and ended (i.e., drug use period) with a previously used method (Prescriptions to drug use periods, PRE2DUP).8,22 We based our model on sliding averages of daily dose, and we modelled each Anatomic Therapeutic Chemical classification system code separately for each participant according to purchase regularity, taking into account admissions to hospital, stockpiling of drugs and changing dose as described previously.8,22
We defined use of benzodiazepines and Z-drugs by the following Anatomic Therapeutic Chemical classification system codes: N05BA and N05CD for benzodiazepines and N05CF for Z-drugs. Benzodiazepines prescribed in this study included diazepam, nitrazepam, chlordiazepoxide, clobazam, oxazepam, alprazolam, lorazepam and temazepam, whereas Z-drugs included zopiclone and zolpidem. We modelled use of benzodiazepine and Z-drugs as “use of any of these drugs” by combining overlapping use periods of different drugs. Thus, patients were allowed to change from one drug to another if there were no breaks in drug availability. Similarly, we combined overlapping drug use periods when we modelled use of “any benzodiazepine” or “any Z-drug.”
We identified diagnoses of pneumonia from the Hospital Discharge and Causes of Death Registers, which represent hospital-treated pneumonia and pneumonia as a direct or underlying cause of death but excludes community-treated cases (not included in register-based data). We used the following ICD-10 (International Classifications of Diseases and Related Health Problems, 10th rev, World Health Organization, 1992) codes to define a diagnosis of pneumonia: J100, J110, J12, J13, J14, J15, J16, J18 and J690. Only the first recorded outcome (hospitalization or death due to pneumonia) for each person was considered after the date of the diagnosis of Alzheimer disease.
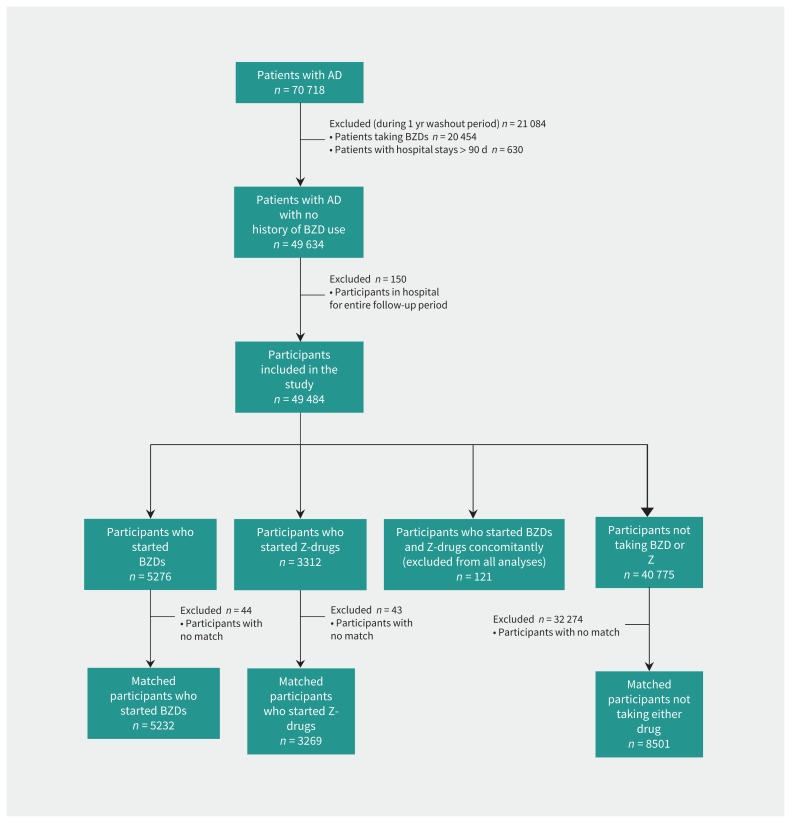
We excluded participants who were taking benzodiazepines or Z-drugs during the 1-year washout period before the date of diagnosis of Alzheimer disease, those who had more than 182 cumulative days in hospital and those who were in hospital for more than 90 days at the end of the washout period from all analyses. We also excluded participants who were in hospital or an institution during the entire follow-up period (Figure 1).
Figure 1:
Flow chart showing selection of participants for the study. AD = Alzheimer disease, BZD = benzodiazepine, Z-drug = non-benzodiazepine similar in activity to benzodiazepine, BZDR = benzodiazepine and/or Z-drug.
Statistical analysis
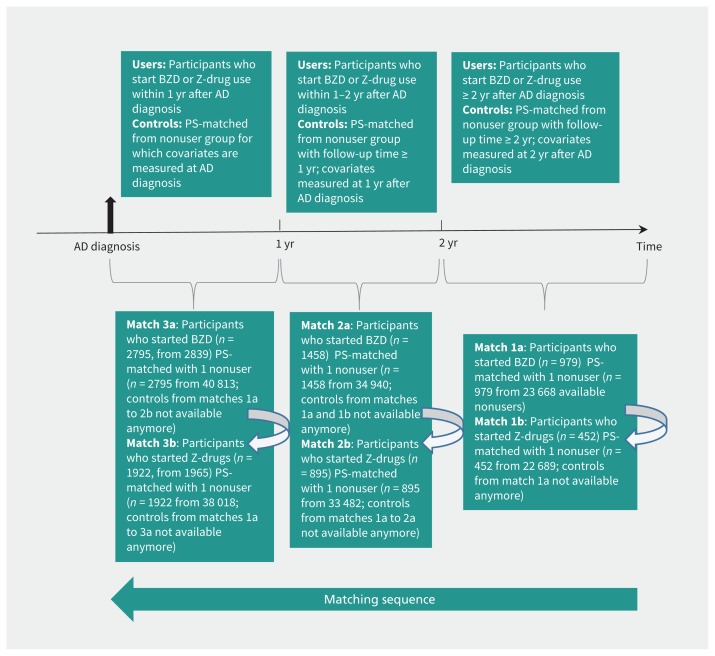
We conducted propensity score–matched analyses to compare participants who used benzodiazepines and Z-drugs with those who did not. Time since diagnosis of Alzheimer disease is a key factor that affects initiation of both benzodiazepines and Z-drugs, and risk of pneumonia. For this reason, we divided the follow-up time for the initiation of drug use into 3 sets by time passed since diagnosis: 0 to less than 1 year, 1 to less than 2 years and 2 or more years. We used logistic regression to derive 1 propensity score to predict initiation of benzodiazepine and another score to predict initiation of Z-drug within the prespecified time sets because initiators of these drugs may differ from each other in baseline covariates (Figure 2). Participants not taking these drugs were included in all time sets if censoring or an outcome event had not occurred before the start of the time set. More than 40 covariates (e.g., factors related to mental health that were strongly associated with use of benzodiazepines and Z-drugs, and markers of exacerbations of respiratory diseases) were included in the scores and measured before or at the start of the time sets. Descriptions of covariates are listed in Table 1, Table 2 and Appendix 1 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.160126/-/DC1).
Figure 2:
Process of propensity score matching for participants using benzodiazepines (BZDs) to those not using them and particpants using Z-drugs (non-benzodiazepines similar in activity to benzodiazepines) to those not using them, by time-since-diagnosis of Alzheimer disease (AD). PS = propensity score.
Table 1:
Characteristics of participants taking benzodiazepines and those not taking benzodiazepines before and after propensity score matching
| Characteristic | No. of participants (before PS matching), n (%) | Standardized difference before PS matching, %* | No. of patients (after PS matching), n (%) | Standardized difference after PS matching, %* | ||
|---|---|---|---|---|---|---|
| No BZD use n = 40 775 |
BZD use n = 5276 |
No BZD use n = 5232 |
BZD use n = 5232 |
|||
| Sex | 1.2 | 1.6 | ||||
| Male | 15 271 (37.5) | 1945 (36.9) | 1889 (36.1) | 1928 (36.9) | ||
| Female | 25 504 (62.6) | 3331 (63.1) | 3343 (63.9) | 3304 (63.1) | ||
| Age, yr | ||||||
| < 65 | 1472 (3.6) | 248 (4.7) | 5.5 | 178 (3.4) | 217 (4.2) | 3.9 |
| 65–74 | 7807 (19.2) | 1066 (20.2) | 2.7 | 875 (16.7) | 904 (17.3) | 1.5 |
| 75–84 | 22 508 (55.2) | 2779 (52.7) | 5.1 | 2832 (54.1) | 2733 (52.2) | 3.8 |
| ≥ 85 | 8988 (22.0) | 1183 (22.4) | 0.9 | 1347 (25.8) | 1378 (26.3) | 1.3 |
| Socioeconomic position | ||||||
| High | 14 127 (34.7) | 1654 (31.4) | 7.0 | 1716 (32.8) | 1644 (31.4) | 3.0 |
| Medium | 23 698 (58.1) | 3138 (59.5) | 2.8 | 3030 (57.9) | 3109 (59.4) | 3.1 |
| Low | 2471 (6.1) | 417 (7.9) | 7.2 | 407 (7.8) | 413 (7.9) | 0.4 |
| Unknown | 479 (1.2) | 67 (1.3) | 0.9 | 79 (1.5) | 66 (1.3) | 2.1 |
| Drug use ever before index date | ||||||
| Antipsychotic | 3837 (9.4) | 1255 (23.8) | 39.4 | 2193 (41.9) | 1994 (38.1) | 7.8 |
| Benzodiazepine or Z-drug | 8136 (20.0) | 1649 (31.3) | 26.1 | 1809 (34.6) | 1615 (30.9) | 7.9 |
| Antidepressant | 10 341 (25.4) | 1855 (35.2) | 21.4 | 2316 (44.3) | 2176 (41.6) | 5.4 |
| Drug use at index date | ||||||
| Antineoplastic drug | 1363 (3.3) | 150 (2.8) | 2.9 | 171 (3.3) | 167 (3.2) | 0.4 |
| Opioid | 1100 (2.7) | 198 (3.8) | 6.0 | 295 (5.6) | 277 (5.3) | 1.5 |
| Antipsychotic | 2408 (5.9) | 1283 (24.3) | 53.2 | 2011 (38.4) | 2040 (39.0) | 1.1 |
| Antidepressant | 5462 (13.4) | 1195 (22.7) | 24.3 | 1553 (29.7) | 1493 (28.5) | 2.5 |
| Proton pump inhibitor | 3976 (9.8) | 666 (12.6) | 9.1 | 903 (17.3) | 857 (16.4) | 2.4 |
| Antiepileptic | 1405 (3.5) | 239 (4.5) | 5.5 | 310 (5.9) | 294 (5.6) | 1.3 |
| Cardiovascular drug | 29 903 (73.3) | 3841 (72.8) | 1.2 | 4045 (77.3) | 3935 (75.2) | 4.9 |
| Comorbidity | ||||||
| Cardiovascular disease | 19 633 (48.2) | 2567 (48.7) | 1.0 | 2694 (51.5) | 2567 (49.1) | 4.9 |
| Chronic heart failure | 11 332 (27.8) | 1452 (27.5) | 0.6 | 1586 (30.3) | 1518 (29.0) | 2.9 |
| Cardiac arrhythmia | 2748 (6.7) | 360 (6.8) | 0.3 | 362 (6.9) | 363 (6.9) | 0.1 |
| Hypertension | 12 005 (29.4) | 1574 (29.8) | 0.9 | 1680 (32.1) | 1632 (31.2) | 2.0 |
| Stroke | 3631 (8.9) | 489 (9.3) | 1.3 | 337 (6.4) | 104 (2.0) | 1.3 |
| Diabetes | 5430 (13.3) | 571 (10.8) | 7.7 | 641 (12.3) | 591 (11.3) | 3.0 |
| Complicated diabetes | 6631 (16.3) | 708 (13.4) | 8.0 | 778 (14.9) | 709 (13.6) | 0.0 |
| Peripheral vascular disease | 2005 (4.9) | 247 (4.7) | 1.1 | 258 (4.9) | 249 (4.8) | 0.8 |
| Substance abuse | 775 (1.9) | 190 (3.6) | 10.4 | 189 (3.6) | 203 (3.9) | 1.4 |
| Previous hospital-treated fracture | 7103 (17.4) | 951 (18.0) | 1.6 | 1085 (20.7) | 1067 (20.4) | 0.9 |
| Any tumour | 5178 (12.7) | 662 (12.6) | 0.5 | 668 (12.8) | 660 (12.6) | 0.5 |
| Anemia | 2695 (6.6) | 311 (5.9) | 3.0 | 345 (6.6) | 321 (6.1) | 1.9 |
| Fluid and electrolyte disorder | 1053 (2.6) | 188 (3.6) | 5.7 | 201 (3.8) | 197 (3.8) | 0.4 |
| Epilepsy | 808 (2.0) | 106 (2.0) | 0.2 | 135 (2.6) | 125 (2.4) | 1.2 |
| Respiratory comorbidity | ||||||
| Asthma/COPD | 3208 (7.9) | 425 (8.1) | 0.7 | 418 (8.0) | 424 (8.1) | 0.4 |
| Any pulmonary disease | 4302 (10.6) | 572 (10.8) | 0.9 | 591 (11.3) | 570 (10.9) | 1.3 |
| Previous pneumonia 1 yr before diagnosis of Alzheimer disease | 978 (2.4) | 105 (2.0) | 2.8 | 105 (2.0) | 104 (2.0) | 0.1 |
| Hospital admission for asthma/COPD before (≤ 1 yr) index date | 629 (1.5) | 102 (1.9) | 3.0 | 116 (2.2) | 114 (2.2) | 0.3 |
| Antibiotic or oral corticosteroid use before (≤ 30 d) index date | 4334 (10.6) | 624 (11.8) | 3.8 | 827 (15.8) | 760 (14.5) | 3.6 |
| Theophylline use before (≤ 180 d) index date | 331 (0.8) | 58 (1.1) | 3.0 | 50 (1.0) | 58 (1.1) | 1.5 |
| β-Agonist use before (≤ 180 d) index date | 1777 (4.4) | 230 (4.4) | 0.0 | 227 (4.3) | 225 (4.3) | 0.2 |
| Use of inhaled anticholinergic before (≤ 180 d) index date | 527 (1.3) | 78 (1.5) | 1.6 | 77 (1.5) | 83 (1.6) | 0.9 |
| Use of inhaled corticosteroid before (≤ 180 d) index date | 1395 (3.4) | 169 (3.2) | 1.2 | 170 (3.3) | 148 (2.8) | 2.5 |
| Combined inhaled corticosteroid/long-acting β-agonist use before (≤ 180 d) index date | 1457 (3.6) | 181 (3.4) | 0.8 | 180 (3.4) | 196 (3.8) | 1.6 |
| Mental health–related comorbidity | ||||||
| Schizophrenia | 447 (1.1) | 100 (1.9) | 6.6 | 103 (2.0) | 100 (1.9) | 0.4 |
| Depression or bipolar disorder | 806 (2.0) | 164 (3.1) | 7.2 | 194 (3.7) | 163 (3.1) | 3.3 |
| Psychosis | 2044 (5.0) | 510 (9.7) | 17.9 | 552 (10.6) | 513 (9.8) | 2.5 |
| Any admission to hospital for mental or behavioural disorders before (≤ 1 yr) index date | 1535 (3.8) | 330 (6.3) | 11.4 | 2452 (46.9) | 2380 (45.5) | 2.8 |
| Any psychiatric hospital admission before (≤ 1 yr) diagnosis of Alzheimer disease | 6985 (17.1) | 1456 (27.6) | 25.3 | 364 (7.0) | 327 (6.3) | 2.9 |
Note: BZD = benzodiazepine, COPD = chronic obstructive pulmonary disease, PS = propensity score, Z-drug = nonbenzodiazepine drug with similar activity to benzodiazepine.
Values > 10% were considered to indicate meaningful imbalance between groups.
Table 2:
Characteristics of participants taking Z-drugs and those not taking Z-drugs before and after propensity score matching
| Characteristic | No. of participants (before PS matching), n (%) | Standardized difference before PS matching, % | No. of participants (after PS matching), n (%) | Standardized difference after PS matching, %* | ||
|---|---|---|---|---|---|---|
| No Z-drug use n = 40 775 |
Z-drug use n = 3312 |
No Z-drug use n = 3269 |
Z-drug use n = 3269 |
|||
| Sex | 2.6 | 1.9 | ||||
| Male | 15 271 (37.5) | 1200 (36.2) | 1161 (35.5) | 1190 (36.4) | ||
| Female | 25 504 (62.6) | 2112 (63.8) | 2108 (64.5) | 2079 (63.6) | ||
| Age, yr | ||||||
| < 65 | 1472 (3.6) | 111 (3.4) | 1.4 | 96 (2.9) | 100 (3.1) | 0.7 |
| 65–74 | 7807 (19.2) | 644 (19.4) | 0.8 | 523 (16.0) | 546 (16.7) | 1.9 |
| 75–84 | 22 508 (55.2) | 1888 (57.0) | 3.6 | 1799 (55.0) | 1841 (56.3) | 2.6 |
| ≥ 85 | 8988 (22.0) | 669 (20.2) | 4.5 | 851 (26.0) | 782 (23.9) | 4.9 |
| Socioeconomic position | ||||||
| High | 14 127 (34.7) | 1156 (34.9) | 0.5 | 1160 (35.5) | 1140 (34.9) | 1.3 |
| Medium | 23 698 (58.1) | 1892 (57.1) | 2.0 | 1851 (56.6) | 1866 (57.1) | 0.9 |
| Low | 2471 (6.1) | 225 (6.8) | 3.0 | 219 (6.7) | 224 (6.9) | 0.6 |
| Unknown | 479 (1.2) | 39 (1.2) | 0.0 | 39 (1.2) | 39 (1.2) | 0.0 |
| Drug use ever before index date | ||||||
| Antipsychotic | 3837 (9.4) | 474 (14.3) | 15.2 | 699 (21.4) | 682 (20.9) | 1.3 |
| Benzodiazepine or Z-drug | 8136 (20.0) | 1235 (37.3) | 39.1 | 1227 (37.5) | 1196 (36.6) | 2.0 |
| Antidepressant | 10 341 (25.4) | 1155 (34.9) | 20.8 | 1356 (41.5) | 1271 (38.9) | 5.3 |
| Drug use at index date | ||||||
| Antineoplastic drug | 1363 (3.3) | 106 (3.2) | 0.8 | 128 (3.9) | 114 (3.5) | 2.3 |
| Opioid | 1100 (2.7) | 124 (3.7) | 5.9 | 192 (5.9) | 174 (5.3) | 2.4 |
| Antipsychotic | 2408 (5.9) | 393 (11.9) | 21.1 | 540 (16.5) | 560 (17.1) | 1.6 |
| Antidepressant | 5462 (13.4) | 645 (19.5) | 16.5 | 753 (23.0) | 747 (22.9) | 0.4 |
| Proton pump inhibitor | 3976 (9.8) | 459 (13.9) | 12.8 | 597 (18.3) | 572 (17.5) | 2.0 |
| Antiepileptic | 1405 (3.5) | 169 (5.1) | 8.2 | 198 (6.1) | 193 (5.9) | 0.6 |
| Cardiovascular drug | 29 903 (73.3) | 2528 (76.3) | 6.9 | 2564 (78.4) | 2559 (78.3) | 0.4 |
| Comorbidity | ||||||
| Cardiovascular disease | 19 633 (48.2) | 1652 (49.9) | 3.5 | 1622 (49.6) | 1633 (50.0) | 0.7 |
| Chronic heart failure | 11 332 (27.8) | 917 (27.7) | 0.2 | 941 (28.8) | 928 (28.4) | 0.9 |
| Cardiac arrhythmia | 2748 (6.7) | 256 (7.7) | 3.8 | 223 (6.8) | 254 (7.8) | 3.7 |
| Hypertension | 12 005 (29.4) | 1004 (30.3) | 1.9 | 1030 (31.5) | 1012 (31.0) | 1.2 |
| Stroke | 3631 (8.9) | 312 (9.4) | 1.8 | 226 (6.9) | 51 (1.6) | 2.4 |
| Diabetes | 5430 (13.3) | 403 (12.2) | 3.4 | 409 (12.5) | 423 (12.9) | 1.3 |
| Complicated diabetes | 6631 (16.3) | 529 (15.9) | 1.0 | 516 (15.8) | 524 (16.0) | 0.0 |
| Peripheral vascular disease | 2005 (4.9) | 185 (5.6) | 3.0 | 184 (5.6) | 183 (5.6) | 0.1 |
| Substance abuse | 775 (1.9) | 96 (2.9) | 6.5 | 103 (3.2) | 104 (3.2) | 0.2 |
| Previous hospital-treated fracture | 7103 (17.4) | 635 (19.2) | 4.5 | 670 (20.5) | 697 (21.3) | 2.0 |
| Any tumour | 5178 (12.7) | 461 (13.9) | 3.6 | 491 (15.0) | 461 (14.1) | 2.6 |
| Anemia | 2695 (6.6) | 246 (7.4) | 3.2 | 238 (7.3) | 252 (7.7) | 1.6 |
| Fluid and electrolyte disorders | 1053 (2.6) | 97 (2.9) | 2.1 | 102 (3.1) | 97 (3.0) | 0.9 |
| Epilepsy | 808 (2.0) | 61 (1.8) | 1.0 | 61 (1.9) | 65 (2.0) | 0.9 |
| Respiratory comorbidity | ||||||
| Asthma/COPD | 3208 (7.9) | 284 (8.6) | 2.6 | 278 (8.5) | 283 (8.7) | 0.6 |
| Any pulmonary disease | 4302 (10.6) | 355 (10.7) | 0.5 | 352 (10.8) | 357 (10.9) | 0.5 |
| Previous pneumonia 1 yr before diagnosis of Alzheimer disease | 978 (2.4) | 60 (1.8) | 4.1 | 52 (1.6) | 59 (1.8) | 1.7 |
| Admission to hospital for asthma/COPD before (≤ 1 yr) index date | 629 (1.5) | 67 (2.0) | 3.6 | 72 (2.2) | 69 (2.1) | 0.6 |
| Antibiotic or oral corticosteroid use before (≤ 30 d) index date | 4334 (10.6) | 383 (11.6) | 3.0 | 476 (14.6) | 467 (14.3) | 0.8 |
| Theophylline use before (≤ 180 d) index date | 331 (1.0) | 19 (0.6) | 2.9 | 16 (0.5) | 19 (0.6) | 1.3 |
| β-Agonist use before (≤ 180 d) index date | 1777 (4.4) | 175 (5.3) | 4.3 | 173 (5.3) | 176 (5.4) | 0.4 |
| Use of inhaled anticholinergic before (≤ 180 d) index date | 527 (1.3) | 41 (1.2) | 0.5 | 42 (1.3) | 44 (1.4) | 0.5 |
| Use of inhaled corticosteroid before (≤ 180 d) index date | 1395 (3.4) | 136 (4.1) | 3.6 | 119 (3.6) | 119 (3.6) | 0.0 |
| Combined inhaled corticosteroid/ long-acting β-agonist use before (≤ 180 d) index date | 1457 (3.6) | 126 (3.8) | 1.2 | 135 (4.1) | 134 (4.1) | 0.2 |
| Mental health–related comorbidity | ||||||
| Schizophrenia | 447 (1.1) | 39 (1.2) | 0.8 | 44 (1.4) | 39 (1.2) | 1.4 |
| Depression or bipolar disorder | 806 (2.0) | 100 (3.0) | 6.7 | 102 (3.1) | 100 (3.1) | 0.4 |
| Psychosis | 2044 (5.0) | 249 (7.5) | 10.4 | 249 (7.6) | 242 (7.4) | 0.8 |
| Any admission to hospital for mental or behavioural disorders before (≤ 1 yr) index date | 1535 (3.8) | 157 (4.7) | 4.8 | 956 (29.2) | 973 (29.8) | 1.1 |
| Any psychiatric hospital admission before (≤ 1 yr) diagnosis of Alzheimer disease | 6985 (17.1) | 645 (19.5) | 6.1 | 160 (4.9) | 154 (4.7) | 0.9 |
Note: COPD = chronic obstructive pulmonary disease, PS = propensity score, Z-drug = non-benzodiazepine drug with similar activity to benzodiazepine.
Values > 10% were considered to indicate meaningful imbalance between groups.
Based on the estimated propensity scores, we conducted 1:1 nearest neighbour matching without replacement using 0.2 as a caliper width within the time sets, starting with those participants initiating use 2 or more years since the date of diagnosis.23 We obtained 6 sets of matches that were merged together to form the matched study population, which excluded 44 participants who started benzodiazepines and 43 participants who started Z-drugs because they did not have a match. We evaluated similarity of matched patients taking these drugs and those not taking them using standardized difference, and we considered values greater than 10% to indicate meaningful imbalance between the groups.24
We defined the index date as the start date of the time set when participants were matched with those not using the drugs (i.e., date of diagnosis, 1 yr after diagnosis or 2 yr after diagnosis). Follow-up for outcome events started at the index date for participants not using the drugs and at the initiation of use for those using the drugs. The follow-up ended when the participant was admitted to hospital or an institution for more than 90 days, upon death, discontinuation of use of benzodiazepines and Z-drugs, upon a switch between or concomitant use of benzodiazepines and Z-drugs, outcome of interest (pneumonia) or the end of study follow-up (Dec. 31, 2012), whichever occurred first.
We evaluated the effect of incident benzodiazepine use on risk of pneumonia using a Cox proportional hazards model, taking into account matching between those participants taking benzodiazepines and those not taking them. We also analyzed how the risk of pneumonia varied by length of benzodiazepine and Z-drug use by using a time-dependent classification of duration of use — 30 days or less, 31 to 180 days, 181 to 365 days, 366 to 1095 days and more than 1095 days of use — and adding it as a time-dependent covariate in the main model.
To minimize the impact of other psychotropic drugs (antipsychotics, antidepressants and opioids) on the risk of pneumonia, we constructed a model with use of psychotropic drugs as a time-dependent covariate (instead of use at the index date). We conducted drug-substance–specific and higher versus lower dose analyses among those participants who initiated with monotherapy (n = 8382).
We performed secondary analyses by treating death due to causes other than pneumonia and a hospital stay of more than 90 days as competing risks for pneumonia in cause-specific hazards models.25 In this method, we considered competing risks that prevent occurrence of pneumonia as censoring events. Cause-specific hazard ratios (HRs) for both pneumonia and competing events were modelled separately, by applying the main Cox model for the event of interest and censoring all other observations. Proportional hazards assumptions were met for all the models. All the analyses were restricted to the first 1500 days of follow-up because of sparse data.
We performed all statistical analyses using SAS statistical software, version 9.3 (SAS Institute Inc.).
Data were de-identified before submission to the research team and, by Finnish law, no ethics committee approval was required.
Results
After applying exclusion criteria, we included 49 484 participants (mean age 80 [SD 7] yr, 62.7% were women; Figure 1) in our study cohort. From this cohort, we matched 5232 participants taking benzodiazepines (Table 1) and 3269 taking Z-drugs (Table 2) with those not taking these drugs. After propensity score matching, we found no significant differences for those taking the drugs and those who were not in terms of comorbidities included. The median follow-up time for participants taking benzodiazepines was 134 (interquartile range [IQR] 85–422) days and 638 (IQR 306–1095) days for those not taking benzodiazepines. Corresponding values for participants taking or not taking Z-drugs were 100 (IQR 39–295) and 683 (IQR 366–1145) days, respectively.
During the period of benzodiazepine and Z-drug use, 8.10 pneumonias per 100 person-years (95% confidence interval [CI] 8.03–8.16) were diagnosed (Table 3). Use of benzodiazepines and Z-drugs was associated with an increased risk of pneumonia among participants with Alzheimer disease (adjusted HR 1.22, 95% CI 1.05–1.42) (Table 3). Benzodiazepine use was associated with a increased risk of pneumonia (adjusted HR 1.28, 95% CI 1.07–1.54), whereas use of Z-drugs was not (adjusted HR 1.10, 95% CI 0.84–1.44).
Table 3:
Risk of pneumonia using propensity score matching for risk of pneumonia among participants taking and not taking benzodiazepines and Z-drugs
| Comparison | No. of participants included | No. of cases of pneumonia | Person-years of follow-up | No. of diagnoses of pneumonia per 100 person-years (95% CI) | PS-matched HR (95% CI) |
PS-matched and time-dependent adjustment for other psychotropic drugs HR (95% CI)*,†,‡ |
|---|---|---|---|---|---|---|
| Any benzodiazepine and Z-drug use | ||||||
| No use of benzodiazepines or Z-drugs | 8501 | 1135 | 17 967 | 6.32 (6.28–6.35) | Ref. | Ref. |
| Use of benzodiazepines or Z-drugs | 8501 | 566 | 6993 | 8.10 (8.03–8.16) | 1.24 (1.07–1.43) | 1.22 (1.05–1.42) |
| Benzodiazepine use | ||||||
| No use of benzodiazepines or Z-drugs | 5232 | 713 | 10 846 | 6.57 (6.53–6.62) | Ref. | Ref. |
| Use of benzodiazepines | 5232 | 394 | 4629 | 8.51 (8.43–8.60) | 1.31 (1.10–1.56) | 1.28 (1.07–1.54) |
| Interval of benzodiazepine use, d | ||||||
| 0–30 | 5232 | 46 | 421 | 10.92 (10.60–11.23) | 2.09 (1.26–3.48) | |
| 31–180 | 4856 | 124 | 1398 | 8.87 (8.71–9.02) | 1.27 (0.96–1.67) | |
| 181–365 | 2207 | 77 | 930 | 8.28 (8.09–8.46) | 1.16 (0.80–1.69) | |
| 366–1095 | 1468 | 117 | 1534 | 7.63 (7.49–7.76) | 1.23 (0.85–1.76) | |
| ≥ 1096 | 322 | 30 | 360 | 8.33 (8.04–8.64) | 1.40 (0.44–4.41) | |
| Z-drug use | ||||||
| No use of benzodiazepines or Z-drugs | 3269 | 422 | 7121 | 5.92 (5.87–5.98) | Ref. | |
| Use of Z-drugs | 3269 | 172 | 2363 | 7.28 (7.17–7.39) | 1.11 (0.86–1.43) | 1.10 (0.84–1.44) |
| Interval of Z-drug use, d | ||||||
| 0–30 | 3269 | 24 | 257 | 9.34 (9.27–9.41) | 1.60 (0.84–3.05) | |
| 31–180 | 2719 | 51 | 665 | 7.67 (7.46–7.88) | 0.88 (0.59–1.32) | |
| 181–365 | 1044 | 29 | 440 | 6.60 (6.36–6.84) | 1.14 (0.64–2.02) | |
| 366–1095 | 708 | 53 | 794 | 6.67 (6.43–6.91) | 1.19 (0.67–2.13) | |
| ≥ 1096 | 189 | 15 | 216 | 6.95 (6.76–7.13) | 1.75 (0.51–5.98) | |
Note: CI = confidence interval, HR = hazard ratio, PS = propensity score, Z-drug = non-benzodiazepine drug with similar activity to benzodiazepine.
Main analyses in time-dependent model, HR (95%CI): antipsychotic use, 1.18 (0.90–1.54); antidepressant use, 0.79 (0.62–1.02); and opioid use, 2.23 (1.50–3.31).
Benzodiazepine analyses in time-dependent model, HR (95% CI): antipsychotic use, 1.22 (0.88–1.68); antidepressant use, 0.83 (0.61–1.14); and opioid use, 2.09 (1.32–3.35).
Analyses of Z-drugs in time-dependent model, HR (95%CI): antipsychotic use, 1.10 (0.67–1.81); antidepressant use, 0.71 (0.46–1.11); and opioid use, 2.60 (1.25–5.42).
We found results similar to the main analysis for time-dependent adjustment of other psychotropic drugs (Table 3). Among those particpants taking benzodiazepines, the increased risk was only observed at the start of use (first 30 d, HR 2.09 [95% CI 1.26–3.48]). To further explore possible prevalent user bias, we conducted additional analyses separately categorizing those participants with the shortest duration of use (≤ 7 d). We found that the risk of pneumonia among participants taking benzodiazepines was attenuated and no longer statistically significant for the first 7 days of use (adjusted HR 1.83, 95% CI 0.68–4.96) but was still significant between 8 and 30 days of use (adjusted HR 2.06, 95% CI 1.14–3.75). Risk of pneumonia was not affected by type of drug, and higher doses were associated with a larger HR than lower doses; however, in both cases, CIs were wide, which limited our conclusions (Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.160126/-/DC1).
In the secondary analyses, participants taking benzodiazepine and Z-drugs had an increased risk of death due to other causes and stays in hospital of more than 90 days (HR 1.13, 95% CI 1.03–1.25) (Table 4).
Table 4:
Cause-specific hazard ratios, by competing outcomes
| Outcome | HR (95% CI) | ||
|---|---|---|---|
| Benzodiazepine and Z-drug use | Benzodiazepine use only | Z-drug use only | |
| Pneumonia risk | 1.24 (1.07–1.43) | 1.31 (1.10–1.56) | 1.11 (0.86–1.43) |
| Death due to other causes and hospital stays > 90 d | 1.13 (1.03–1.25) | 1.15 (1.02–1.29) | 1.09 (0.91–1.30) |
Note: CI = confidence interval, HR = hazard ratio.
Interpretation
Use of benzodiazepines and Z-drugs was associated with an increased risk of pneumonia among patients with Alzheimer disease. The risk was highest at the start of treatment, and benzodiazepine use was associated with an increased risk of pneumonia, whereas the association with Z-drug use did not reach significance. However, the results are not sufficient to support a conclusion that Z-drugs would be safer than benzodiazepines for patients with Alzheimer disease, because our study was not designed for a direct comparison of the these groups. Furthermore, differing physician attitudes toward benzodiazepine and Z-drug prescribing26 would complicate comparisons between drug groups. Both drugs have similar mechanisms of action and adverse effects,27 which often are pronounced in older patients because of age-related changes in pharmacokinetics and pharmacodynamics.14,28,29 Benzodiazepines and Z-drugs have prolonged elimination half-lives in older patients, which may lead to accumulation of the drug.
One possible explanation for the increased risk of pneumonia associated with benzodiazepine use may be related to the more pronounced sedative effects of these drugs in this population. Sedation may increase risk of aspiration,15 which may lead to pneumonia. In addition, sedation is more pronounced at the start of use before tolerance develops. This supports the results of duration of use indicating that the higher risk is only at the start of drug use.
An increased risk of pneumonia is an important finding to consider in treatment of patients with Alzheimer disease. Benzodiazepines and Z-drugs are frequently prescribed for this population, and long-term use is typical.8,30 Pneumonia often leads to admission to hospital,10 and patients with dementia are at increased risk of death related to pneumonia.12,13 Although our findings need further research and confirmation, the increased risk of pneumonia should be considered when benzodiazepines and Z-drugs are prescribed to older adults with Alzheimer disease.
Our results concur with a large case–control study on benzodiazepine use and pneumonia in the United Kingdom that reported a 50% increase in the risk among patients of all ages.16 In our study, benzodiazepine use was associated with a 30% increased risk of pneumonia. Another smaller case–control study found no association between benzodiazepine use and pneumonia among older adults, which may have been related to the small sample size for this study.17
Some researchers have suggested that benzodiazepines and Z-drugs may suppress the immune system and consequently induce adverse respiratory effects.17,31 In 2014, a population-based cohort study in Ontario reported that benzodiazepine use was associated with respiratory exacerbations and visits to the emergency department for COPD or pneumonia among older adults with COPD.18 A study involving a cohort of patients with asthma in the UK showed increased odds of exacerbations and death among those patients taking benzodiazepines.31
Limitations
We investigated the association of benzodiazepine and Z-drug use with a diagnosis of pneumonia in a large nationwide cohort that included patients with a clinically verified diagnosis of Alzheimer disease. For this reason, our results are generalizable to community-dwelling older adults with Alzheimer disease. We restricted analyses to incident use because prevalent users are more likely to have developed tolerance to drug effects. We also restricted the analyses to incident pneumonia cases to exclude the possibility of multiple admissions to hospital because of the same occurrence of pneumonia. With propensity-score matched design and by taking into account time-since-diagnosis, we were able to control for the main measured confounders. However, as in all observational studies, unmeasured confounders may still exist. For example, register-based data lack information on smoking, which is a predictor of pneumonia.32 The covariates we selected (e.g., psychotropic drug use, asthma/COPD, previous pneumonia, lower socioeconomic status and history of substance abuse) likely captured some of the variation in smoking status. We also conducted analyses to reduce the impact of other psychotropic drugs on risk of pneumonia by adjusting for their time-dependent use.
The participants in our study were either admitted to hospital with pneumonia or died of pneumonia. We did not include cases of pneumonia treated through home care. Therefore, our results represent the most severe infections. The limitations also include lack of information on indications for drug use (including presence and severity of behavioural symptoms) and severity of Alzheimer disease, both of which may affect the start of drug use and incidence of pneumonia. We controlled for time-since-diagnosis as a crude measure of severity.
Drug use was based on data recorded in the Prescription Register in Finland. Benzodiazepine use based on data from the Prescription Register was previously validated in a population-based intervention study.33 Our study may underestimate benzodiazepine use, because smaller packages of these drugs were not reimbursed. We used a mathematical modelling method (PRE2DUP) to construct drug-use periods.22 This allowed us to use time-dependent exposure in our analyses.
Conclusion
Benzodiazepine use was associated with a 30% increase in the relative risk of pneumonia among patients with Alzheimer disease compared with patients not using the drug. Benefits and risks of the use of benzodiazepines should be carefully considered for patients with Alzheimer disease and include risk of pneumonia. Accumulating evidence that use of benzodiazepines and Z-drugs is associated with respiratory adverse effects suggests that future studies examine the possible pharmacologic mechanisms.
See related article at www.cmaj.ca/lookup/doi/10.1503/cmaj.170193
Footnotes
Competing interests: Heidi Taipale, Antti Tanskanen and Jari Tiihonen have participated in research projects funded by Janssen with grants paid to the institution where they were employed. Jari Tiihonen received consultant fees from Lundbeck, Organon, Janssen-Cilag, Eli Lilly, AstraZeneca, F. Hoffman-La Roche and Bristol-Myers Squibb. He also received fees for expert opinions from Bristol-Myers Squibb and GlaxoSmithKline; lecture fees from Janssen-Cilag, Bristol-Myers Squibb, Eli Lilly, Pfizer, Lundbeck, GlaxoSmithKline, AstraZeneca and Novartis; and a grant from the Stanley Foundation. He is a member of advisory board in AstraZeneca, Janssen-Cilag, and Otsuka. Marjaana Koponen has received personal research grants from Oy H. Lundbeck Ab foundation. No other conflicts of interest were declared.
This article has been peer reviewed.
Contributors: All of the authors participated in the design of the study and interpretation of data. Heidi Taipale, Marjaana Koponen, Antti Tanskanen, Anna-Maija Tolppanen, Jari Tiihonen and Sirpa Hartikainen acquired the data. Heidi Taipale, Marjaana Koponen, Anna-Maija Tolppanen, Reijo Sund and Piia Lavikainen analyzed the data. All of the authors contributed, drafted and revised the article, approved the final version to be published and agreed to be accountable for all aspects of the work.
References
- 1.Fratiglioni L, Launer LJ, Andersen K, et al. Incidence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000;54:S10–5. [PubMed] [Google Scholar]
- 2.World Health Organization and Alzheimer’s Disease International. Dementia: a public health priority. Geneva: World Health Organization; 2012. [Google Scholar]
- 3.Alzheimer’s Association. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement 2011;7:208–44. [DOI] [PubMed] [Google Scholar]
- 4.Lanctôt KL, Herrmann N, Yau KK, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 2003;169:557–64. [PMC free article] [PubMed] [Google Scholar]
- 5.Rabins PV, Blacker D, Rovner BW, et al. APA Work Group on Alzheimer’s Disease and other Dementias. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Second edition. Am J Psychiatry 2007; 164(Suppl):5–56. [PubMed] [Google Scholar]
- 6.Memory disorders: current care summary [in Finnish with English summary]. Helsinki (Finland): Finnish Medical Society Duodecim; 2010. Available: www.kaypahoito.fi/web/english/guidelineabstracts/guideline?id=ccs00081 (accessed 2017 Jan. 6). [Google Scholar]
- 7.Ballard C, Corbertt A. Management of neuropsychiatric symptoms in people with dementia. CNS Drugs 2010;24:729–39. [DOI] [PubMed] [Google Scholar]
- 8.Taipale H, Koponen M, Tanskanen A, et al. Long-term use of benzodiazepines and related drugs among community-dwelling individuals with and without Alzheimer’s disease. Int Clin Psychopharmacol 2015;30:202–8. [DOI] [PubMed] [Google Scholar]
- 9.Millett ER, Quint JK, Smeeth L, et al. Incidence of community-acquired lower respiratory tract infections and pneumonia among older adults in the United Kingdom: a population-based study. PLoS One 2013;8:e75131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudolph JL, Zanin NM, Jones RN, et al. Hospitalization in community-dwelling persons with Alzheimer’s disease: frequency and causes. J Am Geriatr Soc 2010;58:1542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres A, Peetermans WE, Viegi G, et al. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax 2013;68:1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamuz J, Viasus D, Jiménez-Martinez E, et al. Incidence, timing and risk factors associated with 1-year mortality after hospitalization for community-acquired pneumonia. J Infect 2014;68:534–41. [DOI] [PubMed] [Google Scholar]
- 13.Foley NC, Affoo RH, Martin RE. A systematic review and meta-analysis examining pneumonia-associated mortality in dementia. Dement Geriatr Cogn Disord 2015;39:52–67. [DOI] [PubMed] [Google Scholar]
- 14.Trifirò G, Spina E. Age-related changes in pharmacodynamics: focus on drugs acting on central nervous and cardiovascular systems. Curr Drug Metab 2011;12:611–20. [DOI] [PubMed] [Google Scholar]
- 15.Loeb M, McGeer A, McArthur M, et al. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med 1999;159:2058–64. [DOI] [PubMed] [Google Scholar]
- 16.Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax 2013;68:163–70. [DOI] [PubMed] [Google Scholar]
- 17.Dublin S, Walker RL, Jackson ML, et al. Use of opioids or benzodiazepines and risk of pneumonia in older adults: a population-based case-control study. J Am Geriatr Soc 2011;59:1899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J 2014;44:332–40. [DOI] [PubMed] [Google Scholar]
- 19.Tolppanen AM, Taipale H, Koponen M, et al. Cohort profile: the Finnish Medication and Alzheimer’s disease (MEDALZ) study. BMJ Open 2016;6:e012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- 21.Structure and principles: the Anatomical Therapeutic Chemical classification system. Oslo (Norway): WHO Collaborating Centre for Drug Statistics Methodology; 2011. Available: https://www.whocc.no/atc/structure_and_principles/ (accessed 2015 May 27). [Google Scholar]
- 22.Tanskanen A, Taipale H, Koponen M, et al. From prescription drug purchases to drug use periods — a second generation method (PRE2DUP). BMC Med Inform Decis Mak 2015;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanehart RE, Rodriguez de Gil P, Kim ES, et al. Propensity score analysis and assessment of propensity score approaches using SAS® procedures. Proceedings of the SAS Global Forum 2012 Conference; 2012 Apr. 22–25; Orlando, Florida Cary, North Carolina: SAS Institute Inc; 2012. [Google Scholar]
- 24.Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf 2008;17:1202–17. [DOI] [PubMed] [Google Scholar]
- 25.Andersen PK, Geskus RB, de Witte T, et al. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 2012;41:861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann F. Perceptions of German GPs on benefits and risks of benzodiazepines and Z-drugs. Swiss Med Wkly 2013;143:w13745. [DOI] [PubMed] [Google Scholar]
- 27.Wortelboer U, Cohrn S, Rodenbeck A, et al. Tolerability of hypnosedatives in older patients. Drugs Aging 2002;19:529–39. [DOI] [PubMed] [Google Scholar]
- 28.Turnheim K. When drug therapy gets old: pharmacokinetics and pharmacodynamics in the elderly. Exp Gerontol 2003;38:843–53. [DOI] [PubMed] [Google Scholar]
- 29.Bowie MW, Slattum PW. Pharmacodynamics in older adults: a review. Am J Geriatr Pharmacother 2007;5:263–303. [DOI] [PubMed] [Google Scholar]
- 30.Taipale H, Koponen M, Tanskanen A, et al. High prevalence of psychotropic drug use among persons with and without Alzheimer’s disease in Finnish nationwide cohort. Eur Neuropsychopharmacol 2014;24:1729–37. [DOI] [PubMed] [Google Scholar]
- 31.Nakafero G, Sanders RD, Nguyen-Van-Tam JS, et al. Association between benzodiazepine use and exacerbations and mortality in patients with asthma: a matched case-control and survival analysis using the United Kingdom Clinical Practice Research Datalink. Pharmacoepidemiol Drug Saf 2015;24:793–802. [DOI] [PubMed] [Google Scholar]
- 32.Almirall J, González CA, Balanzó X, et al. Proportion of community-acquired pneumonia cases attributable to tobacco smoking. Chest 1999;116:375–9. [DOI] [PubMed] [Google Scholar]
- 33.Rikala M, Hartikainen S, Sulkava R, et al. Validity of the Finnish Prescription Register for measuring psychotropic drug exposures among elderly Finns: a population-based intervention study. Drugs Aging 2010;27:337–49. [DOI] [PubMed] [Google Scholar]