Abstract
Angiotensins are a group of hormonal peptides and include angiotensin II and angiotensin 1–7 produced by the renin angiotensin system. The biology, pharmacology and biochemistry of the receptors for angiotensins were extensively reviewed recently. In the review, the receptor nomenclature committee was not emphatic on designating MAS1 as the angiotensin 1–7 receptor on the basis of lack of classical G protein signalling and desensitization in response to angiotensin 1–7, as well as a lack of consensus on confirmatory ligand pharmacological analyses. A review of recent publications (2013–2016) on the rapidly progressing research on angiotensin 1–7 revealed that MAS1 and two additional receptors can function as ‘angiotensin 1–7 receptors’, and this deserves further consideration. In this review we have summarized the information on angiotensin 1–7 receptors and their crosstalk with classical angiotensin II receptors in the context of the functions of the renin angiotensin system. It was concluded that the receptors for angiotensin II and angiotensin 1–7 make up a sophisticated cross‐regulated signalling network that modulates the endogenous protective and pathogenic facets of the renin angiotensin system.
Abbreviations
- Ang
Angiotensin
- Ang(1–7)
angiotensin(1–7)
- AngII
octapeptide angiotensin (Asp1‐Arg2‐Val3‐Tyr4‐Ile5‐His6‐Pro7‐Phe8)
- AngIII
angiotensin 2–8 (Asp1‐Arg2‐Val3‐Tyr4‐Ile5‐His6‐Pro7)
- Angiotensin A
(Ala1‐Arg2‐Val3‐Tyr4‐Ile5‐His6‐Pro7‐Phe8)
- AngIV
angiotensin 3–8 (Val3‐Tyr4‐Ile5‐His6‐Pro7‐Phe8)
- RAS
renin‐angiotensin system
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | Enzymes b |
| AT1 receptor | ACE1 |
| AT2 receptor | ACE2 |
| B1 receptor | p70S6K |
| B2 receptor | Renin |
| MAS1 receptor | Other protein targets c |
| MRGPRD (MrgD) receptor | TNF‐α |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,cAlexander et al., 2015a,b,c).
Introduction
The classical renin angiotensin system (RAS) regulates (i) the production of the hormone angiotensin II (AngII) from angiotensinogen, which involves renin and angiotensin converting enzyme 1 (ACE1), and (ii) the initiation of the homeostatic physiological response to AngII, predominantly through the AngII type 1 (AT1) receptor (Karnik et al., 2015; Singh and Karnik, 2016). Both the production and cellular actions of this octapeptide hormone are critical body functions, because deletion of genes for either angiotensinogen or the AT1 receptors increases mortality in mice (Tsuchida et al., 1998; Doan et al., 2001). In humans, clinical application of RAS blockers does not necessarily lower AngII, instead, an increase in tissue and circulating AngII levels is observed, suggesting that their therapeutic effects are probably due to metabolized AngII products. The presumptive therapeutic messenger of RAS is the heptapeptide metabolite, Ang(1–7). Ang(1–7) has been characterized as an antagonist of the pathophysiological effects of AngII (Ferrario et al., 1991; Iyer et al., 1998). Subsequently, the mono‐carboxypeptidase ACE2 (Lemos et al., 2002; Ren et al., 2002) was shown to be the major enzyme producing Ang(1–7) in vivo from proteolysis of AngII. These discoveries led to the bifurcation of RAS with the addition of a new ACE2 arm (Figure 1), which produces Ang(1–7). ACE2 has been shown to cleave multiple peptide substrates and play a role in amino acid uptake in the gut. Moreover, ACE2‐knockout mice have been extensively used to elucidate the in vivo functions of Ang(1–7). Recent studies have demonstrated that increasing ACE2 in tissues up‐regulates levels of Ang(1–7), which are directly associated with a reduction in the progression of neuronal, renal and cardiovascular diseases including pulmonary arterial hypertension (PAH). Thus, the ACE2/Ang(1–7) axis may have evolved to counterbalance the pathophysiological effects of overactivation of the classical ACE1/AngII axis in vivo (Bradford et al., 2010; Ahmad et al., 2011; de Kloet et al., 2013; Jiang et al., 2014a,b). Characterization of receptor components of the ACE2/Ang(1–7) axis, however, is unfinished and currently in progress.
Figure 1.

The RAS including the novel ACE2/Ang(1–7) axis.
The finding that the GPCR, MAS (also called MAS1) mediates some of the actions of Ang(1–7) was a breakthrough in unravelling the body's response mechanism to Ang(1–7) (Santos et al., 2003). Initially MAS1 was discovered as a growth promoting GPCR (Young et al., 1986; Janssen et al., 1988; Dean and Boynton, 1990; van't Veer et al., 1993) and was erroneously proposed as a receptor for AngII and AngIII; but this assignment was later withdrawn (Jackson et al., 1988; Jackson and Hanley, 1989; McGillis et al., 1989; Sasamura et al., 1992). This time around, the investigations showed that Ang(1–7) elicited arachidonic acid release in MAS1‐transfected cells. Mas1 gene deleted mice are normal, but the binding of Ang(1–7) in their kidney slices is abolished, Ang(1–7)‐induced relaxation response in the aorta is lost and the antidiuretic action of Ang(1–7) after an acute water load is abolished (Santos et al., 2003). Mas1 gene‐null mouse tissues and organs respond poorly to Ang(1–7) (Walther et al., 1998; Fraga‐Silva et al., 2008; Santos et al., 2008; Mario et al., 2012). However, independent laboratories have reported a lack of MAS1 receptor signalling in response to Ang(1–7) and a lack of evidence for direct Ang(1–7) binding to the MAS1 receptor (Bikkavilli et al., 2006; Shemesh et al., 2008; Zhang et al., 2011; Tirupula et al., 2014a). There is evidence in the literature for additional receptors mediating the effects of Ang(1–7) (Figure 2). For example, the AT2 receptor was shown to bind Ang(1–7) (De Souza et al., 2004; Walters et al., 2005) and Ang(1–7) signals through AT2 receptors in some studies (Jones et al., 2011; Sipahi et al., 2011; Ohshima et al., 2014), although this is not replicated in all settings (Lautner et al., 2013; Mendonca et al., 2014; Tetzner et al., 2016). De‐orphanization efforts on the MAS1‐related GPCR (MRGPR) family have uncovered potential new receptors for consideration in the ACE2/Ang(1–7) pathway (Gembardt et al., 2008; Solinski et al., 2014). Functional studies indicate that the MRGPRD (MrgD) receptor mediates acute and persistent responses to Ang(1–7) and its metabolite alamandine in target tissues akin to those produced by MAS1 receptors (Habiyakare et al., 2014; Wilson et al., 2015; Tetzner et al., 2016).
Figure 2.
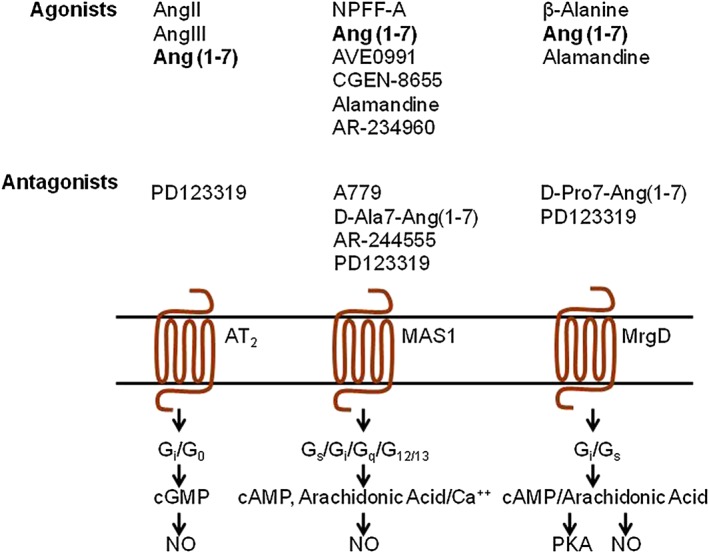
A current view of the three receptors proposed to signal in response to Ang(1–7). MrgD receptor is also known as MRGPRD receptor.
The confusing pharmacology and transduction modalities for MAS1, the awareness of ligand‐promiscuity among MRGPRs and evidence of MRGPRs responding to other angiotensin metabolites (Bader et al., 2014) have delayed a conclusive designation of MAS1 receptors and other candidate MRGPR receptors as the Ang(1–7) receptor by the IUPHAR/BPS subcommittee (Karnik et al., 2014; 2015; Singh and Karnik, 2016). In addition, the classification in Pharmacological Reviews (Davenport et al., 2013; Solinski et al., 2014) and the IUPHAR/BPS guide to pharmacology Database (http://www.guidetopharmacology.org/) have included MAS1 and MRGPRD (MrgD) in the list of ‘orphan’ GPCRs for which pairing with the endogenous ligand(s) is inconclusive. The aim of this review is to provide the present status of the field of candidate Ang(1–7) receptors, with focus on recent important findings and particularly the challenges for future research.
The progress in coupling Ang(1–7) functions with MAS1 receptors
MAS1 was originally identified as the oncogenic GPCR in skin cancer cell lines and is currently catalogued under a group of ∼40 orphan MRGPRs by the HUGO gene nomenclature committee and the IUPHAR/BP database (Monnot et al., 1991; Dong et al., 2001; Gembardt et al., 2008; Tirupula et al., 2014b). Tissue distribution of the MAS1 receptor is ubiquitous; it is abundantly expressed in the brain and testes whereas its expression is low in the heart, kidneys, lungs, vasculature, adipose tissue and skeletal muscle. The MAS1 receptor may regulate many vital body functions and its dysfunction may lead to various pathologies. There is emerging evidence suggesting an important role for Ang(1–7)’s interaction with MAS1 receptors in the function of the brain as well as that of multiple organs including the kidney, heart, vasculature, skeletal muscle, retina and the liver. MAS1 is a constitutively active GPCR; hence, changes in MAS1 expression levels could affect signalling pathways and thus may function without requiring a ligand. However, the MAS1 receptor is reported to bind various endogenous ligands including Ang(1–7), alamandine and neuropeptide FF (NPFF) and dimerize with other GPCRs (Figure 2). However, the physiological significance of these diverse mechanisms remains unclear. In this section, we summarize findings published in past 3 years ending August 2016 on the physiology and pathology involving MAS1 receptors linked to the ACE2/Ang(1–7) pathway (Bader, 2013; Solinski et al., 2014; Karnik et al., 2015). In reviewing recent functional studies on MAS1 receptors, we have tried to limit the inclusion of observations supported by a single publication. We have strived, in vain, to find unequivocal pharmacological evidence for a formal adoption of the MAS1 receptor as the Ang(1–7) receptor.
The promise of novel therapeutic approaches targeting the ACE2/Ang(1–7) axis appears to be the major impetus for the continuing interest in MAS1 functions in the brain for treatment of ischaemic stroke and cerebrovascular diseases (see Figure 1). Enhancing systemic ACE2/Ang(1–7) axis is thought to attenuate the development of hypertension and the pathological progress of atherosclerosis and prevent thrombogenic events, which may contribute to a reduced risk of ischaemic stroke, a reduction in cerebral infarct size and amelioration of neurological deficits; these are thought to be mediated through its antioxidative and anti‐inflammatory effects. Excellent reviews that highlight these topics are available (Bader, 2013; Jiang et al., 2013; Montezano et al., 2015; Silva and Pinheiro, 2015; Bennion et al., 2015a,b; Colafella et al., 2016; Machado‐Silva et al., 2016; Simoes and Teixeira, 2016)
The neuroprotective effects mediated by enhancing ACE2 or Ang(1–7) in ischaemic and haemorrhagic stroke have spurred interest in neuroprotective mechanisms of MAS1 in the clinical setting. The expression of ACE2 and MAS1 receptors are up‐regulated after acute cerebral ischaemic stroke in rats (Lu et al., 2013). Also a deficiency in MAS1 receptors has been found to exacerbate cerebral and systemic inflammation in mice (Oliveira‐Lima et al., 2015). In stroke‐prone spontaneously hypertensive rats, a haemorrhagic stroke model, centrally administered Ang(1–7) increased the survival of the rats, suggesting that the MAS1 receptor is a potential therapeutic target in haemorrhagic stroke (Sumners et al., 2013; Regenhardt et al., 2013a,b). When Ang(1–7) is injected into regions of the hypothalamus that control cardiovascular function, the BP is reduced. However, Ang(1–7) injection into the rostral ventrolateral medulla increases BP. It has been suggested that Ang(1–7) has a critical role in the control of baroreflex sensitivity and hypothalamic noradrenergic neurotransmission during the development of hypertension (Gironacci et al., 2014; Bennion et al., 2015a,b). Cerebral damage is enhanced in mice overexpressing human renin and angiotensinogen and experimental ischaemic stroke induced in brain slices from these mice in vitro by oxygen and glucose deprivation caused tissue swelling, which was associated with the production of ROS and cell death. When these mice were treated with Ang(1–7) the damaging effects were reduced and treatment with the MAS1 antagonist A‐779 eliminated this protective effect of Ang(1–7) (Regenhardt et al., 2013c; Zheng et al., 2014a). Infusion of Ang(1–7) induces cerebral ischaemic tolerance by promoting brain angiogenesis in a MAS1/eNOS‐dependent pathway which is reversed by the specific MAS1 antagonist A‐779 (Jiang et al., 2014a,b; Zheng et al., 2014b). The mortality rate due to rupture of intracranial aneurysms in mice is reduced by intracranial injection of Ang(1–7) (Pena Silva et al., 2014). IN diabetic rats Ang(1–7) treatment up‐regulates the expressions of GFAP and GDNF promoting neuron survival in the hippocampus, and this effect was blocked by treatment with A‐779 (Zhang et al., 2015a). In chronic neurogenic hypertension microglial activation and the production of pro‐inflammatory cytokines, IL‐1β and TNFα, and the anti‐inflammatory cytokine IL‐10 can be abolished by Ang(1–7). In contrast the MAS1 antagonist A‐779 enhances inflammation and microglial activation suggesting that ‘neurogenic’ hypertension may be targeted by MAS1 (Liu et al., 2015).
The overexpression of ACE2 in the brain was found to activate central MAS1‐induced spontaneous postsynaptic inhibitory currents, indicative of presynaptic GABA release onto pyramidal neurons, which reduced anxiety‐like behaviour in mice; the MAS1 antagonist A‐779 eliminated this effect. Furthermore, centrally administering A‐779 abolished the anxiolytic phenotype in ACE2‐null mice (Oscar et al., 2014; Fontes et al., 2015; Wang et al., 2016). Indeed, activation of the brain Ang(1–7)/MAS1 axis in hypertensive transgenic (mRen2)27 rats lowered BP and attenuated cardiac remodelling by improving the autonomic balance (Kangussu et al., 2015). In female rats, central Ang(1–7) protects against aldosterone/NaCl‐induced hypertension, by decreasing heart rate and renal sympathetic nerve activity (Xue et al., 2013). In Sprague–Dawley rats, Ang(1–7) injected into the nucleus tractus solitarii (NTS) decreases BP, heart rate and renal sympathetic nerve activity through the generation of NO (Wu et al., 2015).
Ang(1–7) mediated MAS1 activation has been evaluated as an antinociceptive intervention. Treatment with Ang(1–7) significantly attenuated bone pain suggesting it could be used in the relief of excruciating pain in advanced stage cancer patients (Forte et al., 2016). Intraplantar peripheral hyperalgesia induced by PGE2 was reversed by a low‐dose of Ang(1–7) (Costa et al., 2014; Nemoto et al., 2014; Castor et al., 2015).
In animal studies and in small human subgroups, treatments selectively targeting different components of the RAS are changing the perspectives for the prevention of the pathophysiology of heart failure and coronary atherothrombosis. In renal arteries from diabetic patients, ex vivo Ang(1–7) treatment reduced oxidative stress (Zhang et al., 2015b). The central ACE2/Ang(1–7) axis is a key player in the regulation of sympathetic outflow in chronic heart failure (Zucker et al., 2014), the risk of adverse cardiovascular events in response to acute emotional stress (Martins Lima et al., 2013; Xing et al., 2014), and also cellular hypertrophy and myofibroblast transformation (Alzayadneh and Chappell, 2014). Vascular sympathetic modulation mediated through the ACE2/Ang(1‐) axis is well‐documented (Rabello Casali et al., 2016). These studies indicate that Ang(1–7) is involved in producing NO through MAS1, which contributes to the variations in heart rate in the autonomic modulation of arterial pressure and cardiovascular regulation (Abwainy et al., 2015; da Silva et al., 2015; Zhang et al., 2015c). An increased expression of the ACE2/Ang(1–7) axis is observed in hyperthyroidism‐induced cardiac hypertrophy (Diniz et al., 2015) and in a pressure overloaded rat model (Liang et al., 2015a,b). In an acute atrial tachycardia canine model, Ang(1–7)/MAS1/PI3K/Akt/NO/atrial natriuretic peptide prevented acute electrical remodelling (Zhao et al., 2014). Cardioprotection by Ang(1–7) against AngII‐induced cardiac remodelling through inhibition of cardiomyocyte autophagy is also reported (Lin et al., 2015). Pharmacological blockade or genetic deletion of MAS1 attenuates physiological cardiac hypertrophy that can occur during pregnancy (Carmos‐Silva et al., 2016). Treatment with Ang(1–7) reduces inflammation in carotid atherosclerotic plaques (Fraga‐Silva et al., 2014). Long‐term administration of Ang(1–7) prevents inflammation and pathological remodelling of the heart and lung in a mouse model of type 2 diabetes (Hao et al., 2015; Papinska et al., 2016). Ang(1–7) reverses hyperglycaemia and its consequences in an animal model of type 2 diabetes (Santos et al., 2013). Ang(1–7) regulates insulin secretion through a MAS‐dependent cAMP signalling pathway (Sahr et al., 2016).
Analysis of muscular expression of various components of the RAS in patients with achalasia has shown the existence of local RAS in human oesophageal and skeletal muscle. In the epithelium of patients with healthy and acid reflux‐exposed human oesophageal mucosae, the expression of RAS components, particularly ACE2, ACE, AT1 and AT2 receptors and MAS1, was reported to vary (Bjorkman et al., 2012). In patients with achalasia, a shift in receptor physiology from AT1 receptor to MAS1 receptor is reported (Casselbrant et al., 2014). The healing of pre‐existing gastric ulcers is associated with an up‐regulation of Ang(1–7), MAS1, NO, prostaglandins and pro‐inflammatory cytokines and a decreased expression of AT1 receptors has been observed during the local vascular and metabolic effects associated with gastric ulcer healing (Pawlik et al., 2016). The gastric mucosal protection mechanism involves a similar scenario (Brzozowski, 2014; Lu et al., 2014). After the induction of experimental colitis, the colonic expression of ACE2, Ang1–7 and MAS1 was enhanced and daily Ang(1–7) treatment significantly reduced colitis, whereas administration of A‐779 significantly increased colitis (Khajah et al., 2016).
In skeletal muscle, RAS activation induces insulin resistance and impairs glucose uptake, which is counterbalanced by actions of the ACE2/ANG(1–7)/MAS1 axis (Henriksen and Prasannarong, 2012). Ang(1–7) affects muscle microvasculature and enhances insulin's metabolic action via the MAS1 receptor (Fu et al., 2014). Chronic oral administration of Ang(1–7) has been shown to improve skeletal muscle insulin sensitivity, and autonomic and locomotor phenotypes in muscular dystrophy (Echeverria‐Rodriguez et al., 2013; Gomes‐Santos et al., 2014; Sabharwal et al., 2014; Morales et al., 2014a,b). Endotoxin‐induced skeletal muscle wasting can be prevented by Ang(1–7) through a p38 MAPK‐dependent mechanism. Ang(1–7) prevents the decrease in the diameter of myofibres and myotubes, and decrease in muscle strength induced by LPS. These effects were reversed by the MAS1 receptor antagonist A‐779 (Cabello‐Verrugio et al., 2015; Morales et al., 2015). Ang(1–7) reduces myonuclear apoptosis during recovery from AngII‐induced skeletal muscle atrophy in mice (Meneses et al., 2014), and attenuates signalling involving TGF‐β both in vitro and in vivo (Morales et al., 2014a,b). The oral administration of Ang(1–7) in a mouse model of Duchenne muscular dystrophy (DMD) normalized skeletal muscle architecture, decreased local fibrosis and improved muscle functions. These effects are mediated through inhibition of TGF‐β Smad signalling (Acuna et al., 2013). Ang(1–7) could contribute to weight gain in this model (Schuchard et al., 2015).
Recent reviews on kidney diseases discuss new research elucidating the role of ACE1 and ACE2 in haemodialysis patients, which may aid the development of targeted therapies that slow the progression of chronic kidney and cardiovascular diseases (Carey, 2015; Malik and Raizada, 2015; Oparil and Schmieder, 2015; Te Riet et al., 2015). The anti‐inflammatory effects of Ang(1–7) could ameliorate high fat diet‐induced renal injury. The administration of Ang(1–7) significantly improved the inflammatory status, down regulated LDLr, SREBP2 and SCAP, and then, decreased the deposits of lipid in the kidney and improved renal injury (Olivon et al., 2015; Zheng et al., 2015b). An up‐regulation in the expression of ACE2/AT2 receptors and MAS1 receptors in diabetic mice is associated with the renoprotective effects of candesartan in db/db mice suggesting that the ACE‐2/Ang(1–7) axis can be a therapeutic target for diabetes‐induced hypertension and renal damage (Callera et al., 2016; Padda et al., 2016). The endothelial progenitors may be dysfunctional in diabetic mice. In patients with diabetes, Ang(1–7) overexpression by lentiviral delivery restored both the in vitro vaso‐reparative functions and the in vivo homing efficiency of the peripheral blood CD34+ cells to areas of ischaemia compared with those of nondiabetic controls. In a cohort of patients who had higher expression of ACE2/MAS1 receptors than diabetic patients, the development of CD34+ cell dysfunction was reduced. Thus, activating the ACE2/Ang(1–7)/MAS1 receptor axis could overcome endothelial dysfunction and enhance the reparative function of endothelial progenitors (Jarajapu et al., 2012; Singh et al., 2015).
The expression of MAS1 receptors and other components of the renin‐angiotensin system were shown in the human eye, suggesting that MAS1 receptors may have a role in physiological and pathological processes in the eye and in the retina (Vaajanen et al., 2015). The expression and cellular localization of MAS1 receptors in the adult and developing mouse retina is also documented (Prasad et al., 2014) the activation of intrinsic ACE2 has been shown to have anti‐glaucomatous effects (Foureaux et al., 2013). Several independent reports suggest that deletion of ACE2 leads to vascular diseases (Thomas et al., 2010; Thatcher et al., 2011; Sahara et al., 2013; Rabelo et al., 2016). Furthermore, the MAS axis may be an interesting therapeutic target for multiple sclerosis and atherosclerosis (Hammer et al., 2016) and hypothermia (Souza et al., 2014). Activation of MAS could be an important target for the chronic hepatic and metabolic alterations during atherosclerosis (Silva et al., 2013). An impairment of the Ang(1–7)/Mas receptor pathway may lead to a worsening of the pathophysiological changes in asthma (Magalhaes et al., 2016).
Experimental model studies
Central Ang(1–7) treatment of hypertensive (mRen2)27 transgenic rats attenuates the anxiety and depression‐like behaviour in these animals (Almeida‐Santos et al., 2016; Wang et al., 2016). In a chronic constriction injury rat model, intrathecal administration of Ang(1–7) relieves, whereas A‐779 aggravates injury‐induced neuropathic pain (Zhao et al., 2015). The MAS1‐null mice may serve as an animal model to study post‐traumatic stress disorder. Ang(1–7)/MAS1 axis deficits may accelerate memory extinction in the MAS1‐null mice (Lazaroni et al., 2015). In a mouse model of Alzheimer's disease, i.c.v. infusion of Ang(1–7) ameliorated the cognitive impairments and memory dysfunction (Uekawa et al., 2016). In models of Alzheimer's and Parkinson's diseases, Ang(1–7) activity correlates with tau hyperphosphorylation (Wright et al., 2013; Jiang et al., 2015). The Ang(1–7)/MAS1 axis affects the proliferation of the population of doublecortin positive cells within the dentate gyrus and the piriform cortex. A deficiency of MAS1 in the null mice increases the number of DCX‐positive young neurons, suggesting that blockade of MAS1 might be beneficial in stimulating neurogenesis in adults (Freund et al., 2013).
Ang(1–7) counteracted the inflammation involved in vascular remodelling and haemorrhagic stroke in cerebral micro vessels by affecting the NFкB‐mediated expression of TNF‐α, MCP‐1 and IL‐8 (Bihl et al., 2015). The cardiomyopathy and diastolic dysfunction in diabetic (db/db) mice is ameliorated by Ang(1–7) (Mori et al., 2014). Long‐term administration of Ang(1–7) prevents heart and lung dysfunction in db/db mice (Papinska et al., 2016). In Ren‐2 transgenic rats exposed to chronic hypoxia, intrapulmonary activation of the ACE2/Ang(1–7)/MAS1 axis attenuates pulmonary hypertension (Hampl et al., 2014). In an acute lung injury model, Ang(1–7) attenuates lung fibrosis (Chen et al., 2013). The ACE2/Ang(1–7)/MAS1 axis inhibits the MAPK/NF‐κB pathway to protect against lung fibrosis (Meng et al., 2013). In a rat model of neonatal hyperoxia‐induced lung injury, MAS1 and AT2 receptor agonists attenuate cardiopulmonary disease (Wagenaar et al., 2013). In a model of chronic allergic lung inflammation, Ang(1–7) attenuates lung inflammation, airway remodelling and hyperresponsiveness (Magalhaes et al., 2015; Murugan et al., 2015). Deletion of the MAS1 gene significantly increased intimal proliferation, increasing the aortic intima : media ratio, which promotes atherogenesis (Alsaadon et al., 2015).
The role of RAS has been examined in models of liver disease model, so as to evaluate it as a target for the treatment and prevention of liver dysfunction (Moreira de Macedo et al., 2014). The ACE2/Ang(1–7)/MAS1 axis activates Akt signalling to ameliorate hepatic steatosis. Deletion of the ACE2 gene aggravates liver steatosis and insulin resistance, increases the expression of hepatic lipogenic genes and decreases the expression of fatty acid oxidation genes in mice (Cao et al., 2016). In ACE2 knockout mice, Ang(1–7) alleviates insulin resistance and oxidative stress (Cao et al., 2014).
In a model of acute reflux oesophagitis, Ang(1–7) exerts a protective effect on the oesophagus through the involvement of NO, sensory nerve hypoxia‐inducible factor‐1α and pro‐inflammatory cytokines IL‐1β and TNF‐α (Pawlik et al., 2015). In an acetic acid‐induced gastric ulcer model, Ang(1–7) accelerates the healing of pre‐existing gastric ulcers by increasing the macro‐ and microcirculation, which increases gastric tissue oxygenation. Blockade of MAS1 receptors by A‐779 abolishes this healing effect of Ang(1–7) mediated by PGs and NO, and increases the expression of the antioxidizing enzyme SOD 2 and cytokines IL‐1β and TNF‐α (Pawlik et al., 2016). Ang(1–7) attenuates disuse atrophy in skeletal muscle. The classical RAS is activated in the immobilized hind limb of C57BL6 mice and Ang(1–7) treatment increased anti‐atrophic signalling by IGF‐1/IGFR‐1/Akt/p70S6K and FoxO3. These effects of Ang(1–7) were not observed in MAS1 knockout mice (Morales et al., 2016). Ang1–7 restored muscle strength in dystrophic muscles through the inhibition of TGF‐β signalling in a mouse model of Duchenne muscular dystrophy (Morales et al., 2016).
Kidney injury caused by unilateral ureteral obstruction (UUO) in a Sprague–Dawley rat model of nephropathy was attenuated by Ang(1–7). Renal tubulointerstitial apoptosis, expression of profibrotic proteins, pro‐apoptotic proteins and (TGF)‐β1/Smad signalling, were reduced by Ang(1–7) treatment. Treatment with A‐779 and MAS1 receptor siRNA enhanced Ang II‐induced apoptosis and fibrosis (Kim et al., 2015). Ang(1–7) normalizes renal ACE2 and MAS1 receptor expression in Type 1 diabetic Akita mice (Shi et al., 2014). Ang(1–7) attenuates the progression of streptozotocin‐induced renal injury, and this is accompanied by a decreased expression of collagen IV, TGF‐β1, VEGF, NOX4, p47phox, PKCα and PKCβ1, and the phosphorylation of Smad3 (Zhang et al., 2014). Ang(1–7) modulates renal vascular resistance through inhibition of p38 MAPK in apolipoprotein E‐deficient mice (Potthoff et al., 2013).
Confusing evidence
The results of the studies summarized above support the identification of MAS1 as the receptor mediating the in vivo effects of Ang(1–7). This designation is primarily based on the loss of Ang(1–7)‐mediated functional responses in MAS1‐null animal models and the antagonism of MAS1‐regulated physiology by the specific antagonist, A‐779 (Santos et al., 1994; 2003). In some studies, surrogate MAS1‐specific agonists AVE0991 (Wiemer et al., 2002) and CGEN‐856S (Shemesh et al., 2008) and another antagonist and D‐Pro7‐Ang(1–7) have been used, but not regularly. However, a direct assessment of the functional effects of Ang(1–7) in different vascular tissues in humans, rats and various disease models has raised some concerns regarding the pairing of Ang(1–7) with the MAS1 receptor. In a recent study, the therapeutic potential of Ang(1–7) for the treatment of PAH was found to be limited as opposed to the huge benefits reported in other models of cardiovascular diseases. The effectiveness of Ang(1–7) and a stable, cyclic analogue, cAng(1–7), was found to be limited in a rat experimental monocrotaline (MCT) model of PAH. Treatment reduced right ventricular systolic pressure, but this reduction was <50% and not significant (Breitling et al., 2015).
In human mammary artery (HMA) samples obtained from patients undergoing coronary revascularization surgery, MAS1 expression was confirmed in endothelium. Also, acute Ang(1–7) treatment attenuates the AngII‐induced constriction of HMA explants. Unexpectedly, pre‐incubation with the specific MAS1 antagonist A‐779 did not abolish this effect of AngII. Furthermore, the Ang(1–7) effect observed in HMA was not altered by the AT2 receptor antagonist PD123177, indicating a pharmacologically distinct receptor in HMA (Mendonca et al., 2014).
In the cirrhotic rat liver Ang(1–7) induces a vasodilator response and the activation of endothelial NOS (eNOS) by phosphorylation. This effect of Ang(1–7) was not blocked by MAS1‐selective antagonist A‐779 or by blockade of AT1, AT2 and bradykinin B2 receptors. Unexpectedly, the observed Ang(1–7) effects were not reproduced by MAS1‐selective agonist AVE0991. However, the D‐Pro7‐Ang(1–7) analogue antagonized all the functional effects of Ang(1–7). These findings suggest that the Ang(1–7)‐induced beneficial effects in cirrhosis are mediated by receptors that are pharmacologically distinct from the MAS1 receptor (Herath et al., 2012). In the splanchnic vessels of patients and rat cirrhosis model similar observations were reported (Grace et al., 2013). Pharmacological evidence for the existence of a distinct Ang(1–7) receptor was obtained in the aorta from Sprague–Dawley rats, also the Ang(1–7)‐induced vasodilator effect was not blocked by A‐779, but was specifically abolished by D‐Pro7‐Ang(1–7) (Silva et al., 2007). Altogether, these findings indicate the existence of an Ang(1–7) receptor that is not sensitive to A‐779.
MAS1‐related GPCR MRGPRD (MrgD) coupling with Ang(1–7)/alamandine
Characterization of the MAS1‐related GPCR (MRGPR) subfamily may help better understand the confusion as regards Ang(1–7) pairing with the MAS1 receptor. More than 40 members of this receptor family have been identified in multiple species. They are expressed in sensory neurons, and thought to be involved in the perception of itch, pain and pathological pain (Solinski et al., 2014; Tiwari et al., 2016). Most MRGPR are orphan receptors. However, de‐orphanization efforts have led to new receptors pairing with the ACE2/Ang(1–7) pathway (Figure 2). For instance, while screening an assortment of MRGPRs for arachidonic acid release in response to angiotensin metabolites, Gembardt et al. discovered that in addition to the MAS1 receptor, Ang IV [Ang(3–8)] stimulated AA release in MRGPRD (MrgD) and MRGPRX1 (Mrg1) transfected cells. AngIII activated MAS1 and MRGPRX2 receptors. This initial study lead to further characterization of the MRGPR family of GPCRs as potential targets of Ang(1–7) and related peptides (Gembardt et al., 2008).
The MRGPRD receptor may mediate NO dependent vasodilator effects of a relatively new heptapeptide, alamandine, which structurally differs from Ang(1–7) with an Ala replacing the N‐terminal Asp and is thought to be produced in vivo from Ang A by the action of ACE2 (Lautner et al., 2013). Ang A has Ala1 instead of Asp1 in AngII. It was first identified in human plasma. Alamandine was identified in rats, mice and humans, where it is thought to produce the same effects as Ang(1–7), but by interacting with the MRGPRD receptor (Mendoza‐Torres et al., 2015). The alamandine‐induced effects have been shown to be abolished by D‐Pro‐Ang(1–7), and the AT2 receptor antagonist PD123319. The binding of alamandine to the MRGPRD receptor is blocked by the MRGPRD agonist β‐alanine, D‐Pro7‐Ang(1–7) and PD123319, but not by the MAS1 antagonist A‐779. Direct radioligand binding and rigorous pharmacological analysis data were provided to support the claim that MRGPRD is the alamandine receptor (Habiyakare et al., 2014; Wilson et al., 2015). MRGPRD or MRGPRD‐like receptors may explain the findings that in certain circumstances Ang(1–7) effects are not blocked by A‐779 (Etelvino et al., 2014; Solinski et al., 2014; Villela et al., 2014a,b).
A recent study independently claimed that MRGPRD is the receptor for Ang(1–7) in addition to alamandine and the AT2 receptor antagonist, PD123319 (Lautner et al., 2013; Tetzner et al., 2016). Using the cAMP readout assay (instead of arachdonic acid release assay), the authors showed functional activation of MAS1 receptors by Ang(1–7) in transfected HEK293 cells, contradicting data from previous independent studies (Bikkavilli et al., 2006; Shemesh et al., 2008; Zhang et al., 2011; Tirupula et al., 2014a). Furthermore, the authors presented data showing that MRGPRD functions as a receptor for Ang(1–7). In their analysis, Ang(1–7) failed to increase cAMP levels in primary mesangial cells obtained from MAS1 and MRGPRD double knockout mice. The haemodynamic response to Ang(1–7) was also impaired in MRGPRD‐null mice. In this study, the Ang‐(1–7) stimulated arachidonic acid release was modest compared to the cAMP response in MRGPRD‐transfected heterologous cells. Furthermore, the authors demonstrated polypharmacology of PD123319, which blocks the signalling by MAS1 and MRGPRD receptors as well as from the AT2 receptor.
AT2 receptor coupling with Ang(1–7)
Reports from multiple laboratories have highlighted an Ang(1–7)/AT2 receptor axis as a new protective arm of the RAS (Gaspari et al., 2012). AT2 receptor‐dependent protective effects of Ang(1–7) against aneurysmal subarachnoid haemorrhage development in mice is reported. The protective effect of Ang(1–7) was not observed in AT2 receptor knockout mice. Furthermore, the protective effects of Ang(1–7) were blocked by the AT2 receptor antagonist PD123319, but not by the MAS1 receptor antagonist A‐779. These findings indicate that protective effect of Ang(1–7) against aneurysmal rupture is mediated by AT2 receptors (Shimada et al., 2015). AT2 receptor expression is up‐regulated in many disease states suggesting a role for this receptor in disease (Gaspari et al., 2012). In the apolipoprotein E−/− mouse model of atherosclerosis, Ang(1–7) can act via the AT2 receptors to exert vasoprotective and atheroprotective effects and these effects of Ang(1–7) can be abolished by either the AT2 receptor antagonist PD123319 or A‐779 (Pernomian et al., 2015). AT2 receptor stimulation by Ang(1–7) has been shown to lower BP in adult normotensive rats (Walters et al., 2005). Furthermore, the effects of Ang(1–7) on vascular remodelling and its ability to inhibit neointimal growth are attenuated in AT2 receptor knockout mice compared with wild‐type mice. Surprisingly, Ang(1–7)‐dependent attenuation of neointimal formation after vessel injury in the MAS1‐null mice requires an increased expression of AT2 receptors (Ohshima et al., 2014). In a mouse model of UUO, the administration of Ang(1–7) caused injury in obstructed kidneys compared with controls and increased macrophage infiltration. In mice with MAS1 gene deletion, the delivery of exogenous Ang(1–7) worsens kidney damage. Surprisingly, an infusion of Ang(1–7), but not A‐779 further increased apoptosis and macrophage influx in obstructed kidneys from MAS1‐null mice compared with untreated mice (Zimmerman et al., 2015). Could these effects be due to an unknown receptor or are mediated by the AT2 receptor? Taken together, these findings suggest that the AT2 receptor is a an important mediator of effects attributed to the ACE2/Ang(1–7) axis.
Pharmacology
The suggestion that Ang(1–7) is the physiological agonist for the MAS1 receptor is based on the loss of physiological effects of Ang(1–7) and reduced [125I]–Ang(1–7) binding to kidney sections in MAS1‐null mice (Santos et al., 2003; Pinheiro et al., 2004a,b). Generally, specificity and a dissociation constant for the ligand or receptor density are not measured in these types of experiments. Despite impressive advances made through functional characterization supporting MAS1 receptors coupling to Ang(1–7), attempts at pharmacological analysis of MAS1 receptors in tissue membrane preparations have shown that [125/127I]–Ang(1–7) binding is not specific. Furthermore, in competitive displacement experiments, Ang(1–7) is less potent than other angiotensin metabolites, leading to the suggestion that either the radioligand binding sites in tissue membrane preparations are non‐specific or the isotope modifies Ang(1–7) in such a way as to affect the high‐affinity interaction (Conti, 2016). Additional radioligand development could help resolve this problem.
Concentration‐response studies in MAS1 as well as MAS1‐GFP transfected cells have been used to estimate an IC50 for the Ang(1–7) antagonist, D‐Ala7‐Ang(1–7) and K d values of [125I]–Ang(1–7) (Santos et al., 2003; Gironacci et al., 2011). The binding specificity of fluorescently labelled Ang(1–7) has been studied in CHO cells (Pinheiro et al., 2004a,b; Savergnini et al., 2010; Jankowski et al., 2011), platelets (Fraga‐Silva et al., 2008) and Leydig cells (Leal et al., 2009). Also the MAS1 receptor mediated signalling has been found to be stimulated by several other peptides such as NPFF, alamandine, Ang III, Ang IV, angioprotectin, CGEN‐857 and P61/P33 and CGEN‐856. Some of the peptides have also been found to elicit a response through the MRGPRD receptor, but additional systematic studies are warranted (Dong et al., 2001; Bikkavilli et al., 2006; Canals et al., 2006; Gembardt et al., 2008; Shemesh et al., 2008; Savergnini et al., 2010; Jankowski et al., 2011; Zhang et al., 2012; Savergnini et al., 2013; Tirupula et al., 2014a; Lee et al., 2015). In transfected HEK293 cells, the MAS1 receptor was found to activate Gq/11‐PKC signalling without ligand stimulation (Canals et al., 2006). Several ligands that modulate MAS1 receptor signalling by other G‐proteins and receptor desensitization have been described. These include peptide ligands such as MBP7 (Bikkavilli et al., 2006), NPFF, AngIII, AngIV and the small nonpeptide agonist AR234960 and inverse agonist AR244555 (Zhang et al., 2011; Tirupula et al., 2014a). G protein mediated cAMP signalling by MAS1 and MRGPRD receptors in response to stimulation by peptides and non‐peptide ligands have been recently reported (Tirupula et al., 2014a; Klein et al., 2015; Tetzner et al., 2016). Ang(1–7) has been shown to increase arachidonic acid levels but, it does not modulate Gq‐PLC signalling in MAS1‐expressing cells (Bikkavilli et al., 2006; Shemesh et al., 2008; Zhang et al., 2011; Tirupula et al., 2014a) leading to the concept of functional selectivity of ligands interacting with MAS1 receptors (Tirupula et al., 2014a).
Signalling
Ang(1–7)‐ or AVE0991‐activated MAS1 receptors have been demonstrated to couple to many downstream signalling pathways (see Figure 2) including activation of phospholipase A2, release of arachidonic acid, calcium‐independent activation of NOS, activation of phosphatidylinositol 3‐kinase/Akt, MAP kinases, RhoA, and cAMP/PKA in human mesangial cells and MAS1 transfected CHO and COS cells (Santos et al., 2003; Sampaio et al., 2007a, 2007b; Gembardt et al., 2008; Zimpelmann and Burns, 2009; Liu et al., 2011; Lopez Verrilli et al., 2011; Verano‐Braga et al., 2012; Savergnini et al., 2013; Than et al., 2013). In early studies in MAS1 transfected cells, treatment with Ang(1–7) or AVE099 did not produce a G protein coupled calcium, IP3 and cAMP signalling response (Bikkavilli et al., 2006; Shemesh et al., 2008). However, this observation was disputed by findings in the kidney, where Ang(1–7) treatment increased cAMP levels and activated PKA through Gαs activation by MAS1 receptors. These signals could be inhibited using A‐779 (Magaldi et al., 2003; Liu et al., 2011). The MAS1 receptor was also shown to constitutively couple to Gαi, Gαq and Gα12/13 proteins (Zohn et al., 1998; Whitehead et al., 2001; Booden et al., 2002; Chen and Ikeda, 2004; Dias‐Peixoto et al., 2008; Shemesh et al., 2008; Singh et al., 2010; Zhang et al., 2011; Gomes et al., 2012; Tirupula et al., 2014a). Conventional G protein signalling by MAS1 receptors was reported upon stimulation with neuropeptide FF (Dong et al., 2001; Tirupula et al., 2014a), MBP7 (Bikkavilli et al., 2006), the angiotensin metabolites, AngIII and AngIV (Gembardt et al., 2008; Tirupula et al., 2014a), novel non‐peptide ligands, AR234960, AR244555 and AR305352 (Zhang et al., 2011; Tirupula et al., 2014a) and CGEN‐856/P61 (Shemesh et al., 2008; Savergnini et al., 2010). Thus, this atypical G protein response may be due to the functional selectivity of the MAS1 receptor, which allows different ligands to activate different signalling pathways (Tirupula et al., 2014a). Calcium‐independent activation of NOS, and Gi/o‐mediated cAMP signalling is reported for Ang(1–7) engaged with AT2 and MRGPRD receptors. However, additional validation studies are required to elucidate the ligand selectivity of different modes of signalling in these receptors.
Heterodimeric interactions of AT1 and MAS1 receptors was reported in transfected cells. MAS1‐AT1 receptor hetero oligomerization resulted in the altered trafficking of AT1 receptors in transfected cells (Kostenis et al., 2005; Canals et al., 2006; Santos et al., 2007). The physiological effects of Ang(1–7) treatment in mice may involve MAS1 receptors interacting with AT1 and AT2 receptors (Castro et al., 2005). The MAS1 receptor was shown to function as a physiological antagonist of the AT1 receptor by altering the response to AngII in the mice (Von Bohlen und Halbach et al., 2000; Kostenis et al., 2005; Rakusan et al., 2010). Thus, the interaction of MAS1 receptors with AT1 and AT2 receptors may be a mechanism for the MAS1 receptor in RAS pathophysiology (Villela et al., 2014a). In experimental disease models Ang(1–7)/MAS1 signalling suppress AngII‐induced pathogenesis. For example, AngII‐induced ROS overproduction and apoptosis in cerebral endothelial cells (Xiao et al., 2015), phosphorylation of c‐Src and MAPKs, leading to production of TGF‐β1 and collagen in cardiac fibroblasts (Meng et al., 2014; Tao et al., 2014; Zheng et al., 2015a), muscle atrophy of gastrocnemius (Cisternas et al., 2014), pancreatic cell damage (Wang et al., 2014), ICAM‐1, VCAM‐1, and MCP‐1 expression in HUVECs (Liang et al., 2015a,b) is suppressed by Ang(1–7) activated MAS1 receptors.
Similarly, receptor heterodimer dependent crosstalk between the bradykinin B2 receptor and MAS1 receptor was implicated in vasorelaxation responses. Microvessels in MAS1‐deficient mice lacked the capacity to relax in response to both Ang(1–7) and bradykinin (Peiro et al., 2013). Fluorescence resonance energy transfer analysis showed that the B2 receptor and MAS receptor formed a constitutive heteromer, leading to potentiation of agonist binding to both receptors. B2 receptor or MAS1 receptor antagonists promoted heteromer dissociation. Agonist stimulation promoted internalization of the heteromer into early endosomes. B2 receptor‐MAS1 receptor hetromers have been shown in human glomerular endothelial cells by proximity ligation assay (Cerrato et al., 2016).
G protein independent signalling scaffold proteins have been shown to interact with the MAS receptor C‐terminal tail in a tissue specific manner (Tirupula et al., 2015). This mechanism may explain the signalling events that were demonstrated to be not associated with the generation of G protein mediated second messengers. The MAS receptor has been shown to interact with postsynaptic density 95 (PSD95), a novel binding protein. MAS interacts with the PDZ1–2 domain of PSD95 through engaging the last four amino acids [ETVV (Glu‐Thr‐Val‐Val)] of MAS‐CT. This interaction with PSD95 enhanced the expression and proteolysis stability of MAS receptors in cells (Bian et al., 2013). An agonist driven ‘MAS‐signalosome’ model has also been proposed as molecular mechanism of MAS receptor function. Identifying the hierarchy of interactions of ‘signalosome’ components with MAS receptors will be a necessary step in the future to fully understand the physiological and pathological functions of this enigmatic receptor (Tirupula et al., 2015).
Conclusion
Initially, the pairing of Ang(1–7) with the MAS1 receptor generated huge excitement, in part, due to the lack of a reasonable alternative target for Ang(1–7) at the time. However, now three Ang(1–7) receptor candidates have been proposed by independent groups, all of which meet some of the criteria used by IUPHAR in their de‐orphanization efforts. Firstly, the findings that Ang(1–7) stimulates the MAS1, MRGPRD and AT2 receptors with potencies consistent with physiological function have been independently established. Secondly, the distribution of ACE2 – the enzyme producing Ang(1–7) at physiologically significant concentrations – is anatomically co‐localized with endogenous MAS1, MRGPRD and AT2 receptors, which supports the local production and action of Ang(1–7). Thirdly, genetic modification affecting each of these three receptors alters the physiological response to Ang(1–7) in experimental models. The Ang(1–7)/MAS1 receptor axis currently seems to be most studied beneficial counter‐regulator of the effects of activation of the renin‐angiotensin system in several neurological and cardiovascular diseases. In comparison, the Ang(1–7)/MRGPRD and Ang(1–7)/AT2 receptor pairing require extensive further evaluation. A recent paper has proposed an Ang(1–7)/AT1 receptor biased signalling mechanism (Galandrin et al., 2016), but this needs independent confirmation. Improving the validity of the pharmacological tests, including saturation, competitive radioligand binding and signalling assays, which may further support and strengthen the Ang(1–7) pairing with each of these GPCRs would be most helpful. The criteria that selective agonists should mimic and antagonists should selectively block the signals of the endogenous ligand through each of these GPCRs are not uniformly established for all putative ligands. The recent discovery of an AT2 receptor‐selective antagonist PD123319, which inhibits the action of Ang(1–7) mediated through MAS1 and MRGPRD receptors, is a case in point for the confusing state of molecular pharmacology of Ang(1–7) receptors. However, the existence of multiple receptors responding to Ang(1–7) may have evolved for tissue‐specific sensitive effects. A specific Ang(1–7) receptor axis may mediate a particular effect, which depends on the concentration of one or other of the receptors in a tissue. Until the unique distinctions of each receptor pairing with Ang(1–7) emerges, the adoption of these receptors strictly as Ang(1–7) receptors by the nomenclature committee may well be provisional.
Conflict of interest
The authors declare no conflicts of interest.
Methodology
Compiler note
We performed a systematic computerized literature search of the PubMed database, Scopus and Web of Science (last search: 16 September 2016) to identify all published articles on MAS1 from 1 January 2013 to 16 September 2016. The syntax/key words used in the literature search are MAS1 or MAS1 receptor or Ang(1–7) receptor. The reference lists all articles we recovered and those of relevant review articles were also cross‐referenced.
Acknowledgements
This work was supported in part by National Institutes of Health Grants, HL115964 and HL132351 to S. K. and National Research Service Award, HL007914 to H. U.
Karnik, S. S. , Singh, K. D. , Tirupula, K. , and Unal, H. (2017) Significance of angiotensin 1–7 coupling with MAS1 receptor and other GPCRs to the renin‐angiotensin system: IUPHAR Review 22. British Journal of Pharmacology, 174: 737–753. doi: 10.1111/bph.13742.
This paper, written by members of the International Union of Basic and Clinical Pharmacology Committee on Receptor Nomenclature and Drug Classification (NC‐IUPHAR) subcommittees for the angiotensin receptors, confirms the existing nomenclature for these receptors and reviews our current understanding of their structure, pharmacology and functions, and their likely physiological roles in health and disease. More information on these receptor families can be found in the Concise Guide to PHARMACOLOGY (http://onlinelibrary.wiley.com/doi/10.1111/bph.12445/abstract); for classical angiotensin receptors, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=6.; and for MAS1, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=150.
References
- Abwainy A, Babiker F, Akhtar S, Benter IF (2015). Endogenous angiotensin‐(1‐7)/Mas receptor/NO pathway mediates the cardioprotective effects of pacing postconditioning. Am J Physiol Heart Circ Physiol 310: H104–H112. [DOI] [PubMed] [Google Scholar]
- Acuna MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Bader M et al. (2013). Restoration of muscle strength in dystrophic muscle by angiotensin‐1‐7 through inhibition of TGF‐beta signaling. Hum Mol Genet 23: 1237–1249. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM (2011). Chymase‐dependent generation of angiotensin II from angiotensin‐(1‐12) in human atrial tissue. PLoS One 6: e28501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The concise guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The concise guide to PHARMACOLOGY 2015/16: enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: overview. Br J Pharmacol 172: 5729–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida‐Santos AF, Kangussu LM, Moreira FA, Santos RA, Aguiar DC, Campagnole‐Santos MJ (2016). Anxiolytic‐ and antidepressant‐like effects of angiotensin‐(1‐7) in hypertensive transgenic (mRen2)27 rats. Clin Sci (Lond) 130: 1247–1255. [DOI] [PubMed] [Google Scholar]
- Alsaadon H, Kruzliak P, Smardencas A, Hayes A, Bader M, Angus P et al. (2015). Increased aortic intimal proliferation due to MasR deletion in vitro. Int J Exp Pathol 96: 183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzayadneh EM, Chappell MC (2014). Angiotensin‐(1‐7) abolishes AGE‐induced cellular hypertrophy and myofibroblast transformation via inhibition of ERK1/2. Cell Signal 26: 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader M (2013). ACE2, angiotensin‐(1‐7), and Mas: the other side of the coin. Pflugers Arch 465: 79–85. [DOI] [PubMed] [Google Scholar]
- Bader M, Alenina N, Andrade‐Navarro MA, Santos RA (2014). MAS and its related G protein‐coupled receptors, Mrgprs. Pharmacol Rev 66: 1080–1105. [DOI] [PubMed] [Google Scholar]
- Bennion DM, Haltigan EA, Irwin AJ, Donnangelo LL, Regenhardt RW, Pioquinto DJ et al. (2015a). Activation of the neuroprotective angiotensin‐converting enzyme 2 in rat ischemic stroke. Hypertension 66: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennion DM, Haltigan E, Regenhardt RW, Steckelings UM, Sumners C (2015b). Neuroprotective mechanisms of the ACE2‐angiotensin‐(1‐7)‐Mas axis in stroke. Curr Hypertens Rep 17: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian W, Sun L, Yang L, Li JF, Hu J, Zheng S et al. (2013). Stabilization of the angiotensin‐(1‐7) receptor Mas through interaction with PSD95. Biochem J 453: 345–356. [DOI] [PubMed] [Google Scholar]
- Bihl JC, Zhang C, Zhao Y, Xiao X, Ma X, Chen Y et al. (2015). Angiotensin‐(1‐7) counteracts the effects of Ang II on vascular smooth muscle cells, vascular remodeling and hemorrhagic stroke: role of the NFsmall ka, CyrillicB inflammatory pathway. Vascul Pharmacol 73: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikkavilli RK, Tsang SY, Tang WM, Sun JX, Ngai SM, Lee SS et al. (2006). Identification and characterization of surrogate peptide ligand for orphan G protein‐coupled receptor mas using phage‐displayed peptide library. Biochem Pharmacol 71: 319–337. [DOI] [PubMed] [Google Scholar]
- Bjorkman E, Edebo A, Casselbrant A, Helander HF, Bratlie SO, Vieth M et al. (2012). The renin‐angiotensin system in the esophageal mucosa of healthy subjects and patients with reflux disease. Scand J Gastroenterol 48: 147–159. [DOI] [PubMed] [Google Scholar]
- Booden MA, Siderovski DP, Der CJ (2002). Leukemia‐associated Rho guanine nucleotide exchange factor promotes G alpha q‐coupled activation of RhoA. Mol Cell Biol 22: 4053–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford CN, Ely DR, Raizada MK (2010). Targeting the vasoprotective axis of the renin‐angiotensin system: a novel strategic approach to pulmonary hypertensive therapy. Curr Hypertens Rep 12: 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling S, Krauszman A, Parihar R, Walther T, Friedberg MK, Kuebler WM (2015). Dose‐dependent, therapeutic potential of angiotensin‐(1‐7) for the treatment of pulmonary arterial hypertension. Pulm Circ 5: 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzozowski T (2014). Role of renin‐angiotensin system and metabolites of angiotensin in the mechanism of gastric mucosal protection. Curr Opin Pharmacol 19: 90–98. [DOI] [PubMed] [Google Scholar]
- Cabello‐Verrugio C, Morales MG, Rivera JC, Cabrera D, Simon F (2015). Renin‐angiotensin system: an old player with novel functions in skeletal muscle. Med Res Rev 35: 437–463. [DOI] [PubMed] [Google Scholar]
- Callera GE, Antunes TT, Correa JW, Moorman D, Gutsol A, He Y et al. (2016). Differential renal effects of candesartan at high and ultra‐high doses in diabetic mice‐potential role of the ACE2/AT2R/Mas axis. Biosci Rep 36. doi:10.1042/BSR20160344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals M, Jenkins L, Kellett E, Milligan G (2006). Up‐regulation of the angiotensin II type 1 receptor by the MAS proto‐oncogene is due to constitutive activation of Gq/G11 by MAS. J Biol Chem 281: 16757–16767. [DOI] [PubMed] [Google Scholar]
- Cao X, Yang F, Shi T, Yuan M, Xin Z, Xie R et al. (2016). Angiotensin‐converting enzyme 2/angiotensin‐(1‐7)/Mas axis activates Akt signaling to ameliorate hepatic steatosis. Sci Rep 6: 21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Yang FY, Xin Z, Xie RR, Yang JK (2014). The ACE2/Ang(1‐7)/Mas axis can inhibit hepatic insulin resistance. Mol Cell Endocrinol 393: 30–38. [DOI] [PubMed] [Google Scholar]
- Carey RM (2015). The intrarenal renin‐angiotensin system in hypertension. Adv Chronic Kidney Dis 22: 204–210. [DOI] [PubMed] [Google Scholar]
- Carmos‐Silva C, Almeida JF, Macedo LM, Melo MB, Pedrino GR, Santos FF et al. (2016). Mas receptor contributes to pregnancy‐induced cardiac remodeling. Clin Sci (Lond) . doi:10.1042/CS20160095. [DOI] [PubMed] [Google Scholar]
- Casselbrant A, Kostic S, Lonroth H (2014). The muscular expression of RAS in patients with achalasia. J Renin Angiotensin Aldosterone Syst 16: 578–586. [DOI] [PubMed] [Google Scholar]
- Castor MG, Santos RA, Duarte ID, Romero TR (2015). Angiotensin‐(1‐7) through Mas receptor activation induces peripheral antinociception by interaction with adrenoreceptors. Peptides 69: 80–85. [DOI] [PubMed] [Google Scholar]
- Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP (2005). Evidence for a functional interaction of the angiotensin‐(1‐7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension 46: 937–942. [DOI] [PubMed] [Google Scholar]
- Cerrato BD, Carretero OA, Janic B, Grecco HE, Gironacci MM (2016). Heteromerization Between the Bradykinin B2 Receptor and the Angiotensin‐(1‐7) Mas Receptor: Functional Consequences . Hypertension 68: 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ikeda SR (2004). Modulation of ion channels and synaptic transmission by a human sensory neuron‐specific G‐protein‐coupled receptor, SNSR4/mrgX1, heterologously expressed in cultured rat neurons. J Neurosci 24: 5044–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yang Y, Huang Y, Pan C, Liu L, Qiu H (2013). Angiotensin‐(1‐7) attenuates lung fibrosis by way of Mas receptor in acute lung injury. J Surg Res 185: 740–747. [DOI] [PubMed] [Google Scholar]
- Cisternas F, Morales MG, Meneses C, Simon F, Brandan E, Abrigo J et al. (2014). Angiotensin‐(1‐7) decreases skeletal muscle atrophy induced by angiotensin II through a Mas receptor‐dependent mechanism. Clin Sci (Lond) 128: 307–319. [DOI] [PubMed] [Google Scholar]
- Colafella KM, Hilliard LM, Denton KM (2016). Epochs in the depressor/pressor balance of the renin‐angiotensin system. Clin Sci (Lond) 130: 761–771. [DOI] [PubMed] [Google Scholar]
- Conti FF (2016). Characterization of 125‐I‐angiotensin (1‐7) binding to rat kidney. FASEB J 30 (1) Supplement 765.7. [Google Scholar]
- Costa AC, Romero TR, Pacheco DF, Perez AC, Savernini A, Santos RR et al. (2014). Participation of AT1 and Mas receptors in the modulation of inflammatory pain. Peptides 61: 17–22. [DOI] [PubMed] [Google Scholar]
- da Silva AR, Fraga‐Silva RA, Stergiopulos N, Montecucco F, Mach F (2015). Update on the role of angiotensin in the pathophysiology of coronary atherothrombosis. Eur J Clin Invest 45: 274–287. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Alexander SP, Sharman JL, Pawson AJ, Benson HE, Monaghan AE et al. (2013). International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein‐coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol Rev 65: 967–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Krause EG, Shi PD, Zubcevic J, Raizada MK, Sumners C (2013). Neuroimmune communication in hypertension and obesity: a new therapeutic angle? Pharmacol Ther 138: 428–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza AM, Lopes AG, Pizzino CP, Fossari RN, Miguel NC, Cardozo FP et al. (2004). Angiotensin II and angiotensin‐(1‐7) inhibit the inner cortex Na + −ATPase activity through AT2 receptor. Regul Pept 120: 167–175. [DOI] [PubMed] [Google Scholar]
- Dean NM, Boynton AL (1990). Angiotensin II causes phosphatidylinositol turnover and increases 1,2‐diacylglycerol mass but is not mitogenic in rat liver T51B cells. Biochem J 269: 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias‐Peixoto MF, Santos RA, Gomes ER, Alves MN, Almeida PW, Greco L et al. (2008). Molecular mechanisms involved in the angiotensin‐(1‐7)/Mas signaling pathway in cardiomyocytes. Hypertension 52: 542–548. [DOI] [PubMed] [Google Scholar]
- Diniz GP, Senger N, Carneiro‐Ramos MS, Santos RA, Barreto‐Chaves ML (2015). Cardiac ACE2/angiotensin 1‐7/Mas receptor axis is activated in thyroid hormone‐induced cardiac hypertrophy. Ther Adv Cardiovasc Dis 10: 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan TN, Gletsu N, Cole J, Bernstein KE (2001). Genetic manipulation of the renin‐angiotensin system. Curr Opin Nephrol Hypertens 10: 483–491. [DOI] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ (2001). A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106: 619–632. [DOI] [PubMed] [Google Scholar]
- Echeverria‐Rodriguez O, Del Valle‐Mondragon L, Hong E (2013). Angiotensin 1‐7 improves insulin sensitivity by increasing skeletal muscle glucose uptake in vivo. Peptides 51: 26–30. [DOI] [PubMed] [Google Scholar]
- Etelvino GM, Peluso AA, Santos RA (2014). New components of the renin‐angiotensin system: alamandine and the MAS‐related G protein‐coupled receptor D. Curr Hypertens Rep 16: 433. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Brosnihan KB, Diz DI, Jaiswal N, Khosla MC, Milsted A et al. (1991). Angiotensin‐(1‐7): a new hormone of the angiotensin system. Hypertension 18: III126–III133. [DOI] [PubMed] [Google Scholar]
- Fontes MA, Martins Lima A, Santos RA (2015). Brain angiotensin‐(1‐7)/Mas axis: A new target to reduce the cardiovascular risk to emotional stress. Neuropeptides 56: 9–17. [DOI] [PubMed] [Google Scholar]
- Forte BL, Slosky LM, Zhang H, Arnold MR, Staatz WD, Hay M et al. (2016). Angiotensin‐(1‐7)/Mas receptor as an antinociceptive agent in cancer‐induced bone pain. Pain . doi:10.1097/j.pain.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foureaux G, Nogueira JC, Nogueira BS, Fulgencio GO, Menezes GB, Fernandes SO et al. (2013). Antiglaucomatous effects of the activation of intrinsic Angiotensin‐converting enzyme 2. Invest Ophthalmol Vis Sci 54: 4296–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga‐Silva RA, Pinheiro SV, Goncalves AC, Alenina N, Bader M, Santos RA (2008). The antithrombotic effect of angiotensin‐(1‐7) involves mas‐mediated NO release from platelets. Mol Med 14: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga‐Silva RA, Savergnini SQ, Montecucco F, Nencioni A, Caffa I, Soncini D et al. (2014). Treatment with angiotensin‐(1‐7) reduces inflammation in carotid atherosclerotic plaques. Thromb Haemost 111: 736–747. [DOI] [PubMed] [Google Scholar]
- Freund M, Walther T, von Bohlen Und Halbach O (2013). Effects of the angiotensin‐(1‐7) receptor Mas on cell proliferation and on the population of doublecortin positive cells within the dentate gyrus and the piriform cortex. Eur Neuropsychopharmacol 24: 302–308. [DOI] [PubMed] [Google Scholar]
- Fu Z, Zhao L, Aylor KW, Carey RM, Barrett EJ, Liu Z (2014). Angiotensin‐(1‐7) recruits muscle microvasculature and enhances insulin's metabolic action via mas receptor. Hypertension 63: 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandrin S, Denis C, Boularan C, Marie J, M'Kadmi C, Pilette C et al. (2016). Cardioprotective angiotensin‐(1‐7) peptide acts as a natural‐biased ligand at the angiotensin II type 1 receptor. Hypertension. doi:10.1161/HYPERTENSIONAHA.116.08118. [DOI] [PubMed] [Google Scholar]
- Gaspari TA, Vinh A, Jones ES, Widdop RE (2012). Ganging up on angiotensin II type 1 receptors in vascular remodeling. Hypertension 60: 17–19. [DOI] [PubMed] [Google Scholar]
- Gembardt F, Grajewski S, Vahl M, Schultheiss HP, Walther T (2008). Angiotensin metabolites can stimulate receptors of the Mas‐related genes family. Mol Cell Biochem 319: 115–123. [DOI] [PubMed] [Google Scholar]
- Gironacci MM, Adamo HP, Corradi G, Santos RA, Ortiz P, Carretero OA (2011). Angiotensin (1‐7) induces MAS receptor internalization. Hypertension 58: 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gironacci MM, Cerniello FM, Longo Carbajosa NA, Goldstein J, Cerrato BD (2014). Protective axis of the renin‐angiotensin system in the brain. Clin Sci (Lond) 127: 295–306. [DOI] [PubMed] [Google Scholar]
- Gomes ER, Santos RA, Guatimosim S (2012). Angiotensin‐(1‐7)‐mediated signaling in cardiomyocytes. Int J Hypertens 2012: 493129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes‐Santos IL, Fernandes T, Couto GK, Ferreira‐Filho JC, Salemi VM, Fernandes FB et al. (2014). Effects of exercise training on circulating and skeletal muscle renin‐angiotensin system in chronic heart failure rats. PLoS One 9: e98012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace JA, Klein S, Herath CB, Granzow M, Schierwagen R, Masing N et al. (2013). Activation of the MAS receptor by angiotensin‐(1‐7) in the renin‐angiotensin system mediates mesenteric vasodilatation in cirrhosis. Gastroenterology 145: 874‐884.e5. [DOI] [PubMed] [Google Scholar]
- Habiyakare B, Alsaadon H, Mathai ML, Hayes A, Zulli A (2014). Reduction of angiotensin A and alamandine vasoactivity in the rabbit model of atherogenesis: differential effects of alamandine and Ang(1‐7). Int J Exp Pathol 95: 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampl V, Herget J, Bibova J, Banasova A, Huskova Z, Vanourkova Z et al. (2014). Intrapulmonary activation of the angiotensin‐converting enzyme type 2/angiotensin 1‐7/G‐protein‐coupled Mas receptor axis attenuates pulmonary hypertension in Ren‐2 transgenic rats exposed to chronic hypoxia. Physiol Res 64: 25–38. [DOI] [PubMed] [Google Scholar]
- Hammer A, Yang G, Friedrich J, Kovacs A, Lee DH, Grave K et al. (2016). Role of the receptor Mas in macrophage‐mediated inflammation in vivo. Proc Natl Acad Sci U S A 113: 14109–14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao P, Yang J, Liu Y, Zhang M, Zhang K, Gao F et al. (2015). Combination of angiotensin‐(1‐7) with perindopril is better than single therapy in ameliorating diabetic cardiomyopathy. Sci Rep 5: 8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen EJ, Prasannarong M (2012). The role of the renin‐angiotensin system in the development of insulin resistance in skeletal muscle. Mol Cell Endocrinol 378: 15–22. [DOI] [PubMed] [Google Scholar]
- Herath CB, Mak K, Burrell LM, Angus PW (2012). Angiotensin‐(1‐7) reduces the perfusion pressure response to angiotensin II and methoxamine via an endothelial nitric oxide‐mediated pathway in cirrhotic rat liver. Am J Physiol Gastrointest Liver Physiol 304: G99–108. [DOI] [PubMed] [Google Scholar]
- Iyer SN, Chappell MC, Averill DB, Diz DI, Ferrario CM (1998). Vasodepressor actions of angiotensin‐(1‐7) unmasked during combined treatment with lisinopril and losartan. Hypertension 31: 699–705. [DOI] [PubMed] [Google Scholar]
- Jackson TR, Hanley MR (1989). Tumor promoter 12‐O‐tetradecanoylphorbol 13‐acetate inhibits mas/angiotensin receptor‐stimulated inositol phosphate production and intracellular Ca2+ elevation in the 401L‐C3 neuronal cell line. FEBS Lett 251: 27–30. [DOI] [PubMed] [Google Scholar]
- Jackson TR, Blair LA, Marshall J, Goedert M, Hanley MR (1988). The mas oncogene encodes an angiotensin receptor. Nature 335: 437–440. [DOI] [PubMed] [Google Scholar]
- Jankowski V, Tolle M, Santos RA, Gunthner T, Krause E, Beyermann M et al. (2011). Angioprotectin: an angiotensin II‐like peptide causing vasodilatory effects. FASEB J 25: 2987–2995. [DOI] [PubMed] [Google Scholar]
- Janssen JW, Steenvoorden AC, Schmidtberger M, Bartram CR (1988). Activation of the mas oncogene during transfection of monoblastic cell line DNA. Leukemia 2: 318–320. [PubMed] [Google Scholar]
- Jarajapu YP, Bhatwadekar AD, Caballero S, Hazra S, Shenoy V, Medina R et al. (2012). Activation of the ACE2/angiotensin‐(1‐7)/Mas receptor axis enhances the reparative function of dysfunctional diabetic endothelial progenitors. Diabetes 62: 1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q et al. (2014a). Angiotensin‐converting enzyme 2 and angiotensin 1‐7: novel therapeutic targets. Nat Rev Cardiol 11: 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Gao L, Lu J, Zhang YD (2013). ACE2‐Ang(1‐7)‐Mas axis in brain: a potential target for prevention and treatment of ischemic stroke. Curr Neuropharmacol 11: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Yu JT, Zhu XC, Zhang QQ, Tan MS, Cao L et al. (2014b). Angiotensin‐(1‐7) induces cerebral ischaemic tolerance by promoting brain angiogenesis in a Mas/eNOS‐dependent pathway. Br J Pharmacol 171: 4222–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Zhang YD, Zhou JS, Zhu XC, Tian YY, Zhao HD et al. (2015). Angiotensin‐(1‐7) is reduced and inversely correlates with tau hyperphosphorylation in animal models of Alzheimer's disease. Mol Neurobiol 53: 2489–2497. [DOI] [PubMed] [Google Scholar]
- Jones ES, Del Borgo MP, Kirsch JF, Clayton D, Bosnyak S, Welungoda I et al. (2011). A single beta‐amino acid substitution to angiotensin II confers AT2 receptor selectivity and vascular function. Hypertension 57: 570–576. [DOI] [PubMed] [Google Scholar]
- Kangussu LM, Guimaraes PS, Nadu AP, Melo MB, Santos RA, Campagnole‐Santos MJ (2015). Activation of angiotensin‐(1‐7)/Mas axis in the brain lowers blood pressure and attenuates cardiac remodeling in hypertensive transgenic (mRen2)27 rats. Neuropharmacology 97: 58–66. [DOI] [PubMed] [Google Scholar]
- Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM et al. (2015). International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin receptors: interpreters of pathophysiological angiotensinergic stimuli [corrected]. Pharmacol Rev 67: 754–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik SS, Bernstein KE, Kemp J, Eguchi S, Escher E, Hunyady L et al. (2014). Angiotensin receptors. IUPHAR/BPS Guide to PHARMACOLOGY. Available at: http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=6 (accessed 30/01/2017).
- Khajah MA, Fateel MM, Ananthalakshmi KV, Luqmani YA (2016). Anti‐inflammatory action of angiotensin 1‐7 in experimental colitis. PLoS One 11: e0150861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Kim IJ, Bae EH, Ma SK, Lee J, Kim SW (2015). Angiotensin‐(1‐7) attenuates kidney injury due to obstructive nephropathy in rats. PLoS One 10: e0142664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Herath CB, Schierwagen R, Grace J, Haltenhof T, Uschner FE et al. (2015). Hemodynamic effects of the non‐peptidic angiotensin‐(1‐7) agonist AVE0991 in liver cirrhosis. PLoS One 10: e0138732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostenis E, Milligan G, Christopoulos A, Sanchez‐Ferrer CF, Heringer‐Walther S, Sexton PM et al. (2005). G‐protein‐coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation 111: 1806–1813. [DOI] [PubMed] [Google Scholar]
- Lautner RQ, Villela DC, Fraga‐Silva RA, Silva N, Verano‐Braga T, Costa‐Fraga F et al. (2013). Discovery and characterization of alamandine: a novel component of the renin‐angiotensin system. Circ Res 112: 1104–1111. [DOI] [PubMed] [Google Scholar]
- Lazaroni TL, Bastos CP, Moraes MF, Santos RS, Pereira GS (2015). Angiotensin‐(1‐7)/Mas axis modulates fear memory and extinction in mice. Neurobiol Learn Mem 127: 27–33. [DOI] [PubMed] [Google Scholar]
- Leal MC, Pinheiro SV, Ferreira AJ, Santos RA, Bordoni LS, Alenina N et al. (2009). The role of angiotensin‐(1‐7) receptor Mas in spermatogenesis in mice and rats. J Anat 214: 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Evans MA, Chu HX, Kim HA, Widdop RE, Drummond GR et al. (2015). Effect of a selective mas receptor agonist in cerebral ischemia in vitro and in vivo. PLoS One 10: e0142087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos VS, Cortes SF, Silva DM, Campagnole‐Santos MJ, Santos RA (2002). Angiotensin‐(1‐7) is involved in the endothelium‐dependent modulation of phenylephrine‐induced contraction in the aorta of mRen‐2 transgenic rats. Br J Pharmacol 135: 1743–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Li Y, Han Z, Xue J, Zhang Y, Jia S et al. (2015a). ACE2‐Ang (1‐7) axis is induced in pressure overloaded rat model. Int J Clin Exp Pathol 8: 1443–1450. [PMC free article] [PubMed] [Google Scholar]
- Liang B, Wang X, Zhang N, Yang H, Bai R, Liu M et al. (2015b). Angiotensin‐(1‐7) attenuates angiotensin II‐induced ICAM‐1, VCAM‐1, and MCP‐1 expression via the MAS receptor through suppression of P38 and NF‐kappaB pathways in HUVECs. Cell Physiol Biochem 35: 2472–2482. [DOI] [PubMed] [Google Scholar]
- Lin L, Liu X, Xu J, Weng L, Ren J, Ge J et al. (2015). Mas receptor mediates cardioprotection of angiotensin‐(1‐7) against Angiotensin II‐induced cardiomyocyte autophagy and cardiac remodelling through inhibition of oxidative stress. J Cell Mol Med 20: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GC, Oudit GY, Fang F, Zhou J, Scholey JW (2011). Angiotensin‐(1‐7)‐induced activation of ERK1/2 is cAMP/protein kinase A‐dependent in glomerular mesangial cells. Am J Physiol Renal Physiol 302: F784–F790. [DOI] [PubMed] [Google Scholar]
- Liu M, Shi P, Sumners C (2015). Direct anti‐inflammatory effects of angiotensin‐(1‐7) on microglia. J Neurochem 136: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Verrilli MA, Rodriguez Fermepin M, Longo Carbajosa N, Landa S, Cerrato BD, Garcia S et al. (2011). Angiotensin‐(1‐7) through Mas receptor up‐regulates neuronal norepinephrine transporter via Akt and Erk1/2‐dependent pathways. J Neurochem 120: 46–55. [DOI] [PubMed] [Google Scholar]
- Lu CL, Wang Y, Yuan L, Li Y, Li XY (2014). The angiotensin‐converting enzyme 2/angiotensin (1‐7)/Mas axis protects the function of pancreatic beta cells by improving the function of islet microvascular endothelial cells. Int J Mol Med 34: 1293–1300. [DOI] [PubMed] [Google Scholar]
- Lu J, Jiang T, Wu L, Gao L, Wang Y, Zhou F et al. (2013). The expression of angiotensin‐converting enzyme 2‐angiotensin‐(1‐7)‐Mas receptor axis are upregulated after acute cerebral ischemic stroke in rats. Neuropeptides 47: 289–295. [DOI] [PubMed] [Google Scholar]
- Machado‐Silva A, Passos‐Silva D, Santos RA, Sinisterra RD (2016). Therapeutic uses for angiotensin‐(1‐7). Expert Opin Ther Pat 26: 669–678. [DOI] [PubMed] [Google Scholar]
- Magaldi AJ, Cesar KR, de Araujo M, Simoes e silva AC, Santos RA (2003). Angiotensin‐(1‐7) stimulates water transport in rat inner medullary collecting duct: evidence for involvement of vasopressin V2 receptors. Pflugers Arch 447: 223–230. [DOI] [PubMed] [Google Scholar]
- Magalhaes GS, Rodrigues‐Machado MG, Motta‐Santos D, Silva AR, Caliari MV, Prata LO et al. (2015). Angiotensin‐(1‐7) attenuates airway remodelling and hyperresponsiveness in a model of chronic allergic lung inflammation. Br J Pharmacol 172: 2330–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes GS, Rodrigues‐Machado MG, Motta‐Santos D, Alenina N, Bader M, Santos RA et al. (2016). Chronic allergic pulmonary inflammation is aggravated in angiotensin‐(1‐7) Mas receptor knockout mice. Am J Physiol Lung Cell Mol Physiol 311: L1141–L1148. [DOI] [PubMed] [Google Scholar]
- Malik U, Raizada V (2015). Some aspects of the renin‐angiotensin‐system in hemodialysis patients. Kidney Blood Press Res 40: 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mario EG, Santos SH, Ferreira AV, Bader M, Santos RA, Botion LM (2012). Angiotensin‐(1‐7) Mas‐receptor deficiency decreases peroxisome proliferator‐activated receptor gamma expression in adipocytes. Peptides 33: 174–177. [DOI] [PubMed] [Google Scholar]
- Martins Lima A, Xavier CH, Ferreira AJ, Raizada MK, Wallukat G, Velloso EP et al. (2013). Activation of angiotensin‐converting enzyme 2/angiotensin‐(1‐7)/Mas axis attenuates the cardiac reactivity to acute emotional stress. Am J Physiol Heart Circ Physiol 305: H1057–H1067. [DOI] [PubMed] [Google Scholar]
- McGillis JP, Sudduth‐Klinger J, Harrowe G, Mitsuhashi M, Payan DG (1989). Transient expression of the angiotensin II receptor: a rapid and functional analysis of a calcium‐mobilizing seven‐transmembrane domain receptor in COS‐7 cells. Biochem Biophys Res Commun 165: 935–941. [DOI] [PubMed] [Google Scholar]
- Mendonca L, Mendes‐Ferreira P, Bento‐Leite A, Cerqueira R, Amorim MJ, Pinho P et al. (2014). Angiotensin‐(1‐7) modulates angiotensin II‐induced vasoconstriction in human mammary artery. Cardiovasc Drugs Ther 28: 513–522. [DOI] [PubMed] [Google Scholar]
- Mendoza‐Torres E, Oyarzun A, Mondaca‐Ruff D, Azocar A, Castro PF, Jalil JE et al. (2015). ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension. Ther Adv Cardiovasc Dis 9: 217–237. [DOI] [PubMed] [Google Scholar]
- Meneses C, Morales MG, Abrigo J, Simon F, Brandan E, Cabello‐Verrugio C (2014). The angiotensin‐(1‐7)/Mas axis reduces myonuclear apoptosis during recovery from angiotensin II‐induced skeletal muscle atrophy in mice. Pflugers Arch 467: 1975–1984. [DOI] [PubMed] [Google Scholar]
- Meng Y, Li T, Zhou GS, Chen Y, Yu CH, Pang MX et al. (2014). The angiotensin‐converting enzyme 2/angiotensin (1‐7)/Mas axis protects against lung fibroblast migration and lung fibrosis by inhibiting the NOX4‐derived ROS‐mediated RhoA/Rho kinase pathway. Antioxid Redox Signal 22: 241–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Yu CH, Li W, Li T, Luo W, Huang S et al. (2013). Angiotensin‐converting enzyme 2/angiotensin‐(1‐7)/Mas axis protects against lung fibrosis by inhibiting the MAPK/NF‐kappaB pathway. Am J Respir Cell Mol Biol 50: 723–736. [DOI] [PubMed] [Google Scholar]
- Monnot C, Weber V, Stinnakre J, Bihoreau C, Teutsch B, Corvol P et al. (1991). Cloning and functional characterization of a novel mas‐related gene, modulating intracellular angiotensin II actions. Mol Endocrinol 5: 1477–1487. [DOI] [PubMed] [Google Scholar]
- Montezano AC, Nguyen A, Touyz RM (2015). Chapter 23 – Mas signaling: resolved and unresolved issues. The Protective Arm of the Renin Angiotensin System (RAS) Functional Aspects and Therapeutic Implications, pp. 169–179.
- Morales MG, Abrigo J, Acuna MJ, Santos RA, Bader M, Brandan E et al. (2016). Angiotensin‐(1‐7) attenuates disuse skeletal muscle atrophy in mice via its receptor, Mas. Dis Model Mech 9: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales MG, Abrigo J, Meneses C, Cisternas F, Simon F, Cabello‐Verrugio C (2014a). Expression of the Mas receptor is upregulated in skeletal muscle wasting. Histochem Cell Biol 143: 131–141. [DOI] [PubMed] [Google Scholar]
- Morales MG, Abrigo J, Meneses C, Simon F, Cisternas F, Rivera JC et al. (2014b). The Ang(1‐7)/Mas‐1 axis attenuates the expression and signalling of TGF‐beta1 induced by AngII in mouse skeletal muscle. Clin Sci (Lond) 127: 251–264. [DOI] [PubMed] [Google Scholar]
- Morales MG, Olguin H, Di Capua G, Brandan E, Simon F, Cabello‐Verrugio C (2015). Endotoxin‐induced skeletal muscle wasting is prevented by angiotensin‐(1‐7) through a p38 MAPK‐dependent mechanism. Clin Sci (Lond) 129: 461–476. [DOI] [PubMed] [Google Scholar]
- Moreira de Macedo S, Guimaraes TA, Feltenberger JD, Sousa Santos SH (2014). The role of renin‐angiotensin system modulation on treatment and prevention of liver diseases. Peptides 62: 189–196. [DOI] [PubMed] [Google Scholar]
- Mori J, Patel VB, Abo Alrob O, Basu R, Altamimi T, Desaulniers J et al. (2014). Angiotensin 1‐7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ Heart Fail 7: 327–339. [DOI] [PubMed] [Google Scholar]
- Murugan D, Lau YS, Lau CW, Mustafa MR, Huang Y (2015). Angiotensin 1‐7 protects against angiotensin II‐induced endoplasmic reticulum stress and endothelial dysfunction via Mas receptor. PLoS One 10: e0145413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto W, Ogata Y, Nakagawasai O, Yaoita F, Tadano T, Tan‐No K (2014). Angiotensin (1‐7) prevents angiotensin II‐induced nociceptive behaviour via inhibition of p38 MAPK phosphorylation mediated through spinal Mas receptors in mice. Eur J Pain 18: 1471–1479. [DOI] [PubMed] [Google Scholar]
- Ohshima K, Mogi M, Nakaoka H, Iwanami J, Min LJ, Kanno H et al. (2014). Possible role of angiotensin‐converting enzyme 2 and activation of angiotensin II type 2 receptor by angiotensin‐(1‐7) in improvement of vascular remodeling by angiotensin II type 1 receptor blockade. Hypertension 63: e53–e59. [DOI] [PubMed] [Google Scholar]
- Oliveira‐Lima OC, Pinto MC, Duchene J, Qadri F, Souza LL, Alenina N et al. (2015). Mas receptor deficiency exacerbates lipopolysaccharide‐induced cerebral and systemic inflammation in mice. Immunobiology 220: 1311–1321. [DOI] [PubMed] [Google Scholar]
- Olivon VC, Aires RD, Santiago LB, Ramalho LZ, Cortes SF, Lemos VS (2015). Mas receptor overexpression increased Ang(1‐7) relaxation response in renovascular hypertensive rat carotid. Peptides 71: 250–258. [DOI] [PubMed] [Google Scholar]
- Oparil S, Schmieder RE (2015). New approaches in the treatment of hypertension. Circ Res 116: 1074–1095. [DOI] [PubMed] [Google Scholar]
- Oscar CG, Muller‐Ribeiro FC, de Castro LG, Martins Lima A, Campagnole‐Santos MJ, Santos RA et al. (2014). Angiotensin‐(1‐7) in the basolateral amygdala attenuates the cardiovascular response evoked by acute emotional stress. Brain Res 1594: 183–189. [DOI] [PubMed] [Google Scholar]
- Padda RS, Shi Y, Lo CS, Zhang SL, Chan JS (2016). Angiotensin‐(1‐7): a novel peptide to treat hypertension and nephropathy in diabetes? J Diabetes Metab 6. doi: 10.4172/2155-6156.1000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papinska AM, Soto M, Meeks CJ, Rodgers KE (2016). Long‐term administration of angiotensin (1‐7) prevents heart and lung dysfunction in a mouse model of type 2 diabetes (db/db) by reducing oxidative stress, inflammation and pathological remodeling. Pharmacol Res 107: 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlik MW, Kwiecien S, Pajdo R, Ptak‐Belowska A, Brzozowski B, Krzysiek‐Maczka G et al. (2015). Esophagoprotective activity of angiotensin‐(1‐7) in experimental model of acute reflux esophagitis. Evidence for the role of nitric oxide, sensory nerves, hypoxia‐inducible factor‐1alpha and proinflammatory cytokines. J Physiol Pharmacol 65: 809–822. [PubMed] [Google Scholar]
- Pawlik MW, Kwiecien S, Ptak‐Belowska A, Pajdo R, Olszanecki R, Suski M et al. (2016). The renin‐angiotensin system and its vasoactive metabolite angiotensin‐(1‐7) in the mechanism of the healing of preexisting gastric ulcers. The involvement of Mas receptors, nitric oxide, prostaglandins and proinflammatory cytokines. J Physiol Pharmacol 67: 75–91. [PubMed] [Google Scholar]
- Peiro C, Vallejo S, Gembardt F, Palacios E, Novella S, Azcutia V et al. (2013). Complete blockade of the vasorelaxant effects of angiotensin‐(1‐7) and bradykinin in murine microvessels by antagonists of the receptor Mas. J Physiol 591: 2275–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena Silva RA, Kung DK, Mitchell IJ, Alenina N, Bader M, Santos RA et al. (2014). Angiotensin 1‐7 reduces mortality and rupture of intracranial aneurysms in mice. Hypertension 64: 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernomian L, do Prado AF, Gomes MS, da Silva CH, Gerlach RF, de Oliveira AM (2015). MAS receptors mediate vasoprotective and atheroprotective effects of candesartan upon the recovery of vascular angiotensin‐converting enzyme 2‐angiotensin‐(1‐7)‐MAS axis functionality. Eur J Pharmacol 764: 173–188. [DOI] [PubMed] [Google Scholar]
- Pinheiro SV, Simoes e Silva AC, Sampaio WO, de Paula RD, Mendes EP, Bontempo ED et al. (2004a). Nonpeptide AVE 0991 is an angiotensin‐(1‐7) receptor Mas agonist in the mouse kidney. Hypertension 44: 490–496. [DOI] [PubMed] [Google Scholar]
- Pinheiro SV, Simoes e Silva AC, Sampaio WO, de Paula RD, Mendes EP, Bontempo ED et al. (2004b). Nonpeptide AVE 0991 is an angiotensin‐(1‐7) receptor Mas agonist in the mouse kidney. Hypertension 44: 490–496. [DOI] [PubMed] [Google Scholar]
- Potthoff SA, Fahling M, Clasen T, Mende S, Ishak B, Suvorava T et al. (2013). Angiotensin‐(1‐7) modulates renal vascular resistance through inhibition of p38 mitogen‐activated protein kinase in apolipoprotein E‐deficient mice. Hypertension 63: 265–272. [DOI] [PubMed] [Google Scholar]
- Prasad T, Verma A, Li Q (2014). Expression and cellular localization of the Mas receptor in the adult and developing mouse retina. Mol Vis 20: 1443–1455. [PMC free article] [PubMed] [Google Scholar]
- Rabello Casali K, Ravizzoni Dartora D, Moura M, Bertagnolli M, Bader M, Haibara A et al. (2016). Increased vascular sympathetic modulation in mice with Mas receptor deficiency. J Renin Angiotensin Aldosterone Syst 17 1470320316643643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabelo LA, Todiras M, Nunes‐Souza V, Qadri F, Szijarto IA, Gollasch M et al. (2016). Genetic deletion of ACE2 induces vascular dysfunction in C57BL/6 mice: role of nitric oxide imbalance and oxidative stress. PLoS One 11: e0150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakusan D, Burgelova M, Vaneckova I, Vanourkova Z, Huskova Z, Skaroupkova P et al. (2010). Knockout of angiotensin 1‐7 receptor Mas worsens the course of two‐kidney, one‐clip Goldblatt hypertension: roles of nitric oxide deficiency and enhanced vascular responsiveness to angiotensin II. Kidney Blood Press Res 33: 476–488. [DOI] [PubMed] [Google Scholar]
- Regenhardt RW, Bennion DM, Sumners C (2013b). Cerebroprotective action of angiotensin peptides in stroke. Clin Sci (Lond) 126: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenhardt RW, Desland F, Mecca AP, Pioquinto DJ, Afzal A, Mocco J et al. (2013c). Anti‐inflammatory effects of angiotensin‐(1‐7) in ischemic stroke. Neuropharmacology 71: 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenhardt RW, Mecca AP, Desland F, Ritucci‐Chinni PF, Ludin JA, Greenstein D et al. (2013a). Centrally administered angiotensin‐(1‐7) increases the survival of stroke‐prone spontaneously hypertensive rats. Exp Physiol 99: 442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Garvin JL, Carretero OA (2002). Vasodilator action of angiotensin‐(1‐7) on isolated rabbit afferent arterioles. Hypertension 39: 799–802. [DOI] [PubMed] [Google Scholar]
- Sabharwal R, Cicha MZ, Sinisterra RD, De Sousa FB, Santos RA, Chapleau MW (2014). Chronic oral administration of Ang(1‐7) improves skeletal muscle, autonomic and locomotor phenotypes in muscular dystrophy. Clin Sci (Lond) 127: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara M, Ikutomi M, Morita T, Minami Y, Nakajima T, Hirata Y et al. (2013). Deletion of angiotensin‐converting enzyme 2 promotes the development of atherosclerosis and arterial neointima formation. Cardiovasc Res 101: 236–246. [DOI] [PubMed] [Google Scholar]
- Sahr A, Wolke C, Maczewsky J, Krippeit‐Drews P, Tetzner A, Drews G et al. (2016). The angiotensin‐(1‐7)/Mas axis improves pancreatic beta‐cell function in vitro and in vivo. Endocrinology 157: 4677–4690. [DOI] [PubMed] [Google Scholar]
- Sampaio WO, Henrique de Castro C, Santos RA, Schiffrin EL, Touyz RM (2007a). Angiotensin‐(1‐7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension 50: 1093–1098. [DOI] [PubMed] [Google Scholar]
- Sampaio WO, Souza dos Santos RA, Faria‐Silva R, da Mata Machado, LT , Schiffrin EL, Touyz RM (2007b). Angiotensin‐(1‐7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt‐dependent pathways. Hypertension 49: 185‐192. [DOI] [PubMed] [Google Scholar]
- Santos EL, Reis RI, Silva RG, Shimuta SI, Pecher C, Bascands JL et al. (2007). Functional rescue of a defective angiotensin II AT1 receptor mutant by the Mas protooncogene. Regul Pept 141: 159–167. [DOI] [PubMed] [Google Scholar]
- Santos RA, Campagnole‐Santos MJ, Baracho NC, Fontes MA, Silva LC, Neves LA et al. (1994). Characterization of a new angiotensin antagonist selective for angiotensin‐(1‐7): evidence that the actions of angiotensin‐(1‐7) are mediated by specific angiotensin receptors. Brain Res Bull 35: 293–298. [DOI] [PubMed] [Google Scholar]
- Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I et al. (2003). Angiotensin‐(1‐7) is an endogenous ligand for the G protein‐coupled receptor Mas. Proc Natl Acad Sci U S A 100: 8258–8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SH, Fernandes LR, Mario EG, Ferreira AV, Porto LC, Alvarez‐Leite JI et al. (2008). Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes 57: 340–347. [DOI] [PubMed] [Google Scholar]
- Santos SH, Giani JF, Burghi V, Miquet JG, Qadri F, Braga JF et al. (2013). Oral administration of angiotensin‐(1‐7) ameliorates type 2 diabetes in rats. J Mol Med (Berl) 92: 255–265. [DOI] [PubMed] [Google Scholar]
- Sasamura H, Hein L, Krieger JE, Pratt RE, Kobilka BK, Dzau VJ (1992). Cloning, characterization, and expression of two angiotensin receptor (AT‐1) isoforms from the mouse genome. Biochem Biophys Res Commun 185: 253–259. [DOI] [PubMed] [Google Scholar]
- Savergnini SQ, Beiman M, Lautner RQ, de Paula‐Carvalho V, Allahdadi K, Pessoa DC et al. (2010). Vascular relaxation, antihypertensive effect, and cardioprotection of a novel peptide agonist of the MAS receptor. Hypertension 56: 112–120. [DOI] [PubMed] [Google Scholar]
- Savergnini SQ, Ianzer D, Carvalho MB, Ferreira AJ, Silva GA, Marques FD et al. (2013). The novel Mas agonist, CGEN‐856S, attenuates isoproterenol‐induced cardiac remodeling and myocardial infarction injury in rats. PLoS One 8: e57757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchard J, Winkler M, Stolting I, Schuster F, Vogt FM, Barkhausen J et al. (2015). Lack of weight gain after angiotensin AT1 receptor blockade in diet‐induced obesity is partly mediated by an angiotensin‐(1‐7)/Mas‐dependent pathway. Br J Pharmacol 172: 3764–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh R, Toporik A, Levine Z, Hecht I, Rotman G, Wool A et al. (2008). Discovery and validation of novel peptide agonists for G‐protein‐coupled receptors. J Biol Chem 283: 34643–34649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Lo CS, Padda R, Abdo S, Chenier I, Filep JG et al. (2014). Angiotensin‐(1‐7) prevents systemic hypertension, attenuates oxidative stress and tubulointerstitial fibrosis, and normalizes renal angiotensin‐converting enzyme 2 and Mas receptor expression in diabetic mice. Clin Sci (Lond) 128: 649–663. [DOI] [PubMed] [Google Scholar]
- Shimada K, Furukawa H, Wada K, Wei Y, Tada Y, Kuwabara A et al. (2015). Angiotensin‐(1‐7) protects against the development of aneurysmal subarachnoid hemorrhage in mice. J Cereb Blood Flow Metab 35: 1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva ACS, Pinheiro SVB (2015). Chapter 29 – Mas in the kidney. The Protective Arm of the Renin Angiotensin System (RAS) Functional Aspects and Therapeutic Implications, pp. 207–212.
- Silva DM, Vianna HR, Cortes SF, Campagnole‐Santos MJ, Santos RA, Lemos VS (2007). Evidence for a new angiotensin‐(1‐7) receptor subtype in the aorta of Sprague–Dawley rats. Peptides 28: 702–707. [DOI] [PubMed] [Google Scholar]
- Silva AR, Aguilar EC, Alvarez‐Leite JI, da Silva RF, Arantes RM, Bader M et al. (2013). Mas receptor deficiency is associated with worsening of lipid profile and severe hepatic steatosis in ApoE‐knockout mice. Am J Physiol Regul Integr Comp Physiol 305: R1323–R1330. [DOI] [PubMed] [Google Scholar]
- Simoes ESAC, Teixeira MM (2016). ACE inhibition, ACE2 and angiotensin‐(1‐7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res 107: 154–162. [DOI] [PubMed] [Google Scholar]
- Singh A, Boyer JL, Der CJ, Zohn IE (2010). Transformation by a nucleotide‐activated P2Y receptor is mediated by activation of Galphai, Galphaq and Rho‐dependent signaling pathways. J Mol Signal 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DK, Karnik SS (2016). Angiotensin receptors: structure, function, signaling and clinical applications. J Cell Signal 1: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Joshi S, Guo L, Baker MB, Li Y, Castellano RK et al. (2015). ACE2/Ang(1‐7)/Mas axis stimulates vascular repair‐relevant functions of CD34+ cells. Am J Physiol Heart Circ Physiol 309: H1697–H1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipahi S, Hur E, Demirtas S, Kocayigit I, Bozkurt D, Tamer A et al. (2011). Body composition monitor measurement technique for the detection of volume status in peritoneal dialysis patients: the effect of abdominal fullness. Int Urol Nephrol 43: 1195–1199. [DOI] [PubMed] [Google Scholar]
- Solinski HJ, Gudermann T, Breit A (2014). Pharmacology and signaling of MAS‐related G protein‐coupled receptors. Pharmacol Rev 66: 570–597. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza LL, Duchene J, Todiras M, Azevedo LC, Costa‐Neto CM, Alenina N et al. (2014). Receptor MAS protects mice against hypothermia and mortality induced by endotoxemia. Shock 41: 331–336. [DOI] [PubMed] [Google Scholar]
- Sumners C, Horiuchi M, Widdop RE, McCarthy C, Unger T, Steckelings UM (2013). Protective arms of the renin‐angiotensin‐system in neurological disease. Clin Exp Pharmacol Physiol 40: 580–588. [DOI] [PubMed] [Google Scholar]
- Tao X, Fan J, Kao G, Zhang X, Su L, Yin Y et al. (2014). Angiotensin‐(1‐7) attenuates angiotensin II‐induced signalling associated with activation of a tyrosine phosphatase in Sprague–Dawley rats cardiac fibroblasts. Biol Cell 106: 182–192. [DOI] [PubMed] [Google Scholar]
- Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH (2015). Hypertension: renin‐angiotensin‐aldosterone system alterations. Circ Res 116: 960–975. [DOI] [PubMed] [Google Scholar]
- Tetzner A, Gebolys K, Meinert C, Klein S, Uhlich A, Trebicka J et al. (2016). G‐Protein‐coupled receptor MrgD is a receptor for angiotensin‐(1‐7) involving adenylyl cyclase, cAMP, and phosphokinase A. Hypertension 68: 185–194. [DOI] [PubMed] [Google Scholar]
- Than A, Leow MK, Chen P (2013). Control of adipogenesis by the autocrine interplays between angiotensin 1‐7/Mas receptor and angiotensin II/AT1 receptor signaling pathways. J Biol Chem 288: 15520–15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher SE, Zhang X, Howatt DA, Lu H, Gurley SB, Daugherty A et al. (2011). Angiotensin‐converting enzyme 2 deficiency in whole body or bone marrow‐derived cells increases atherosclerosis in low‐density lipoprotein receptor−/− mice. Arterioscler Thromb Vasc Biol 31: 758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MC, Pickering RJ, Tsorotes D, Koitka A, Sheehy K, Bernardi S et al. (2010). Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res 107: 888–897. [DOI] [PubMed] [Google Scholar]
- Tirupula KC, Desnoyer R, Speth RC, Karnik SS (2014a). Atypical signaling and functional desensitization response of MAS receptor to peptide ligands. PLoS One 9: e103520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirupula KC, Karnik SS, Thomas W, Eguchi S, Vanderheyden P (2014b). Class A orphans: MAS1. Last modified on 05/03/2014. IUPHAR/BPS Guide to PHARMACOLOGY, Available at: http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=150 (accessed 30/01/2017).
- Tirupula KC, Zhang D, Osbourne A, Chatterjee A, Desnoyer R, Willard B et al. (2015). MAS C‐terminal tail interacting proteins identified by mass spectrometry‐based proteomic approach. PLoS One 10: e0140872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, He S, Zhang T, Raja SN, Dong X, Guan Y (2016). Mas‐related G protein‐coupled receptors offer potential new targets for pain therapy. Adv Exp Med Biol 904: 87–103. [DOI] [PubMed] [Google Scholar]
- Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H et al. (1998). Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest 101: 755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uekawa K, Hasegawa Y, Senju S, Nakagata N, Ma M, Nakagawa T et al. (2016). Intracerebroventricular infusion of angiotensin‐(1‐7) ameliorates cognitive impairment and memory dysfunction in a mouse model of Alzheimer's disease. J Alzheimers Dis 53: 127–133. [DOI] [PubMed] [Google Scholar]
- Vaajanen A, Kalesnykas G, Vapaatalo H, Uusitalo H (2015). The expression of Mas‐receptor of the renin‐angiotensin system in the human eye. Graefes Arch Clin Exp Ophthalmol 253: 1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van't Veer LJ, van der Feltz MJ, van den Berg‐Bakker CA, Cheng NC, Hermens RP, van Oorschot DA et al. (1993). Activation of the mas oncogene involves coupling to human alphoid sequences. Oncogene 8: 2673–2681. [PubMed] [Google Scholar]
- Verano‐Braga T, Schwammle V, Sylvester M, Passos‐Silva DG, Peluso AA, Etelvino GM et al. (2012). Time‐resolved quantitative phosphoproteomics: new insights into Angiotensin‐(1‐7) signaling networks in human endothelial cells. J Proteome Res 11: 3370–3381. [DOI] [PubMed] [Google Scholar]
- Villela D, Leonhardt J, Patel N, Joseph J, Kirsch S, Hallberg A et al. (2014a). Angiotensin type 2 receptor (AT2R) and receptor Mas: a complex liaison. Clin Sci (Lond) 128: 227–234. [DOI] [PubMed] [Google Scholar]
- Villela DC, Passos‐Silva DG, Santos RA (2014b). Alamandine: a new member of the angiotensin family. Curr Opin Nephrol Hypertens 23: 130–134. [DOI] [PubMed] [Google Scholar]
- Von Bohlen und Halbach O, Walther T, Bader M, Albrecht D (2000). Interaction between Mas and the angiotensin AT1 receptor in the amygdala. J Neurophysiol 83: 2012–2021. [DOI] [PubMed] [Google Scholar]
- Wagenaar GT, Laghmani el H, Fidder M, Sengers RM, de Visser YP, de Vries L et al. (2013). Agonists of MAS oncogene and angiotensin II type 2 receptors attenuate cardiopulmonary disease in rats with neonatal hyperoxia‐induced lung injury. Am J Physiol Lung Cell Mol Physiol 305: L341–L351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters PE, Gaspari TA, Widdop RE (2005). Angiotensin‐(1‐7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension 45: 960–966. [DOI] [PubMed] [Google Scholar]
- Walther T, Balschun D, Voigt JP, Fink H, Zuschratter W, Birchmeier C et al. (1998). Sustained long term potentiation and anxiety in mice lacking the Mas protooncogene. J Biol Chem 273: 11867–11873. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu R, Qi H, Wang Y, Cui L, Wen Y et al. (2014). The ACE2‐angiotensin‐(1‐7)‐Mas axis protects against pancreatic cell damage in cell culture. Pancreas 44: 266–272. [DOI] [PubMed] [Google Scholar]
- Wang L, de Kloet AD, Pati D, Hiller H, Smith JA, Pioquinto DJ et al. (2016). Increasing brain angiotensin converting enzyme 2 activity decreases anxiety‐like behavior in male mice by activating central Mas receptors. Neuropharmacology 105: 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead IP, Zohn IE, Der CJ (2001). Rho GTPase‐dependent transformation by G protein‐coupled receptors. Oncogene 20: 1547–1555. [DOI] [PubMed] [Google Scholar]
- Wiemer G, Dobrucki LW, Louka FR, Malinski T, Heitsch H (2002). AVE 0991, a nonpeptide mimic of the effects of angiotensin‐(1‐7) on the endothelium. Hypertension 40: 847–852. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Cruz‐Diaz N, Marshall AC, Pirro NT, Su Y, Gwathmey TM et al. (2015). An angiotensin‐(1‐7) peptidase in the kidney cortex, proximal tubules, and human HK‐2 epithelial cells that is distinct from insulin‐degrading enzyme. Am J Physiol Renal Physiol 308: F594–F601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JW, Kawas LH, Harding JW (2013). A role for the brain RAS in Alzheimer's and Parkinson's diseases. Front Endocrinol (Lausanne) 4: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZT, Ren CZ, Yang YH, Zhang RW, Sun JC, Wang YK et al. (2015). The PI3K signaling‐mediated nitric oxide contributes to cardiovascular effects of angiotensin‐(1‐7) in the nucleus tractus solitarii of rats. Nitric Oxide 52: 56–65. [DOI] [PubMed] [Google Scholar]
- Xiao X, Zhang C, Ma X, Miao H, Wang J, Liu L et al. (2015). Angiotensin‐(1‐7) counteracts angiotensin II‐induced dysfunction in cerebral endothelial cells via modulating Nox2/ROS and PI3K/NO pathways. Exp Cell Res 336: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Lu J, Li J (2014). Role of angiotensin‐(1‐7) and Mas‐R‐nNOS pathways in amplified neuronal activity of dorsolateral periaqueductal gray after chronic heart failure. Neurosci Lett 563: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Zhang Z, Johnson RF, Guo F, Hay M, Johnson AK (2013). Central endogenous angiotensin‐(1‐7) protects against aldosterone/NaCl‐induced hypertension in female rats. Am J Physiol Heart Circ Physiol 305: H699–H705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D, Waitches G, Birchmeier C, Fasano O, Wigler M (1986). Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell 45: 711–719. [DOI] [PubMed] [Google Scholar]
- Zhang T, Li Z, Dang H, Chen R, Liaw C, Tran TA et al (2012). Inhibition of Mas G‐protein signaling improves coronary blood flow, reduces myocardial infarct size, and provides long‐term cardioprotection. Am J Physiol Heart Circ Physiol 302: H299–311. [DOI] [PubMed] [Google Scholar]
- Zhang D, Xiao Q, Luo H, Zhao K (2015a). Effects of angiotensin‐(1‐7) on hippocampal expressions of GFAP and GDNF and cognitive function in rats with diabetes mellitus. Nan Fang Yi Ke Da Xue Xue Bao 35: 646–651. [PubMed] [Google Scholar]
- Zhang Y, Liu J, Luo JY, Tian XY, Cheang WS, Xu J et al. (2015b). Upregulation of angiotensin (1‐7)‐mediated signaling preserves endothelial function through reducing oxidative stress in diabetes. Antioxid Redox Signal 23: 880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li B, Wang B, Zhang J, Wu J, Morgan T (2015c). Alteration of cardiac ACE2/Mas expression and cardiac remodelling in rats with aortic constriction. Chin J Physiol 57: 335–342. [DOI] [PubMed] [Google Scholar]
- Zhang K, Meng X, Li D, Yang J, Kong J, Hao P et al. (2014). Angiotensin(1‐7) attenuates the progression of streptozotocin‐induced diabetic renal injury better than angiotensin receptor blockade. Kidney Int 87: 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Li Z, Dang H, Chen R, Liaw C, Tran TA et al. (2011). Inhibition of Mas G‐protein signaling improves coronary blood flow, reduces myocardial infarct size, and provides long‐term cardioprotection. Am J Physiol Heart Circ Physiol 302: H299–H311. [DOI] [PubMed] [Google Scholar]
- Zhao J, Liu E, Li G, Qi L, Li J, Yang W (2014). Effects of the angiotensin‐(1‐7)/Mas/PI3K/Akt/nitric oxide axis and the possible role of atrial natriuretic peptide in an acute atrial tachycardia canine model. J Renin Angiotensin Aldosterone Syst 16: 1069–1077. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Qin Y, Liu T, Hao D (2015). Chronic nerve injury‐induced Mas receptor expression in dorsal root ganglion neurons alleviates neuropathic pain. Exp Ther Med 10: 2384–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Li G, Chen S, Bihl J, Buck J, Zhu Y et al. (2014a). Activation of the ACE2/Ang(1‐7)/Mas pathway reduces oxygen–glucose deprivation‐induced tissue swelling, ROS production, and cell death in mouse brain with angiotensin II overproduction. Neuroscience 273: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Li GZ, Chen SZ, Wang JJ, Olson JE, Xia HJ et al. (2014b). Angiotensin converting enzyme 2/Ang(1‐7)/mas axis protects brain from ischemic injury with a tendency of age‐dependence. CNS Neurosci Ther 20: 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Tang L, Huang W, Yan R, Ren F, Luo L et al. (2015b). Anti‐inflammatory effects of Ang(1‐7) in ameliorating HFD‐induced renal injury through LDLr‐SREBP2‐SCAP pathway. PLoS One 10: e0136187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Yang Y, Song R, Yang X, Liu H, Ma Q et al. (2015a). Ang(1‐7) promotes the migration and invasion of human renal cell carcinoma cells via Mas‐mediated AKT signaling pathway. Biochem Biophys Res Commun 460: 333–340. [DOI] [PubMed] [Google Scholar]
- Zimmerman DL, Zimpelmann J, Xiao F, Gutsol A, Touyz R, Burns KD (2015). The effect of angiotensin‐(1‐7) in mouse unilateral ureteral obstruction. Am J Pathol 185: 729–740. [DOI] [PubMed] [Google Scholar]
- Zimpelmann J, Burns KD (2009). Angiotensin‐(1‐7) activates growth‐stimulatory pathways in human mesangial cells. Am J Physiol Renal Physiol 296: F337–F346. [DOI] [PubMed] [Google Scholar]
- Zohn IE, Symons M, Chrzanowska‐Wodnicka M, Westwick JK, Der CJ (1998). Mas oncogene signaling and transformation require the small GTP‐binding protein Rac. Mol Cell Biol 18: 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker IH, Xiao L, Haack KK (2014). The central renin‐angiotensin system and sympathetic nerve activity in chronic heart failure. Clin Sci (Lond) 126: 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]


