Abstract
Histone deacetylases (HDACs) have been extensively studied as drug targets in neurodegenerative diseases but less is known about their role in healthy neurons. We tested zinc-dependent HDACs using RNAi in Drosophila melanogaster and found memory deficits with RPD3 and HDAC6. We demonstrate that HDAC6 is required in both the larval and adult stages for normal olfactory memory retention. Neuronal expression of HDAC6 rescued memory deficits, and we demonstrate the N-terminal deacetylase (DAC) domain is required for this ability. This suggests that deacetylation of synaptic targets associated with the first DAC domain, such as the active zone scaffold Bruchpilot, are required for memory retention. Finally, electrophysiological experiments at the neuromuscular junction reveal that HDAC6 mutants exhibit a partial block of homeostatic plasticity, suggesting that HDAC6 may be required for the stabilization of synaptic strength. The learning deficit we observe in HDAC6 mutants could be a behavioral consequence of these synaptic defects.
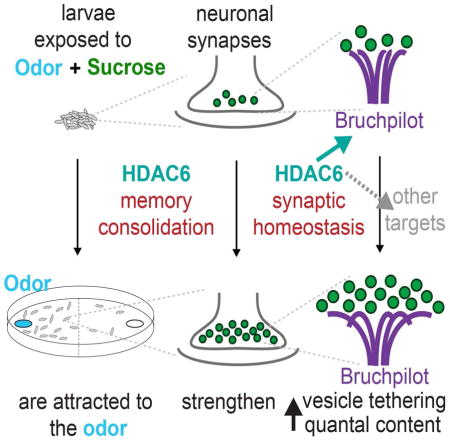
Graphical Abstract
Introduction
Neurodegenerative diseases are complex health issues that greatly affect the quality of human life. Among emerging treatments, drugs that serve as HDAC inhibitors (HDACi) are found to be quite effective (Fischer et al., 2010; Sleiman et al., 2009; Vecsey et al., 2007). Histone deacetylases (HDACs) are a highly conserved class of chromatin modifiers with the ability to dramatically impact gene expression. Deacetylation of lysine residues on histone tails promotes compacted chromatin structure and results in transcriptional repression of large regions of the genome. Treatment by HDAC inhibitors is thought to relieve this repression by promoting a more relaxed chromatin state. The resulting shift in gene expression that can ameliorate neuronal senescence and improve the inclusion and breakdown of harmful protein aggregates associated with many neurodegenerative disorders (Babenko et al., 2012; Qureshi and Mehler, 2011).
While many of these studies suggest a promising role for HDAC inhibitors in disease models, it is not clear how the loss of HDAC function affects healthy neurons. Commonly used HDAC inhibitors such as sodium butyrate and vorinostat (SAHA) target all zinc-dependent HDACs (up to 11 family members in mammals) regardless of their diverse roles in cellular processes (Bertrand, 2010). Despite their name, HDACs are able to target and regulate many non-histone proteins in both the nucleus and the cytoplasm and participate in a variety of cellular pathways (Bertrand, 2010; Cho et al., 2005). It is not fully understood how inhibition of HDACs impacts these aspects of cellular function.
The Drosophila model offers a relatively simple and effective approach to dissecting complex neuronal functions such as learning and memory. To examine the role of individual HDAC family members healthy brains, we performed RNAi knock-down of individual Drosophila HDACs and observed the effect on larval olfactory learning and memory. We find that pan-neuronal knock-down of RPD3 or HDAC6 causes a deficit in immediate-term memory. While RPD3 \ reported to be involved in courtship memory (Fitzsimons and Scott, 2011), HDAC6 has not yet been implicated in learning and memory.
HDAC6 is thought to reside predominantly in the cytoplasm, where it is involved in numerous cellular functions. It is known to deacetylate multiple targets including tubulin, cortactin, Hsp90 (DmHsp83) and Bruchpilot (Hubbert et al., 2002; Kovacs et al., 2005; Miskiewicz et al., 2014, Zhang et al., 2007). It is also able to bind ubiquitin and regulate protein degradation as well as serving as a molecular scaffold for many protein complexes (Valenzuela-Fernández et al., 2008). HDAC6 is both highly conserved and expressed in neurons in both flies and mammals, but is apparently not required for normal development (Du et al., 2010; Govindarajan et al., 2013). This suggests that it may have a role in higher functions of the mature brain.
In terms of neurodegenerative diseases, HDAC6 appears to have diverse roles depending on the disease model. It can be neuroprotective in some contexts, such as promoting alpha- synuclein inclusion in a Parkinson’s disease model (Du et al., 2010). Conversely, loss of HDAC6 can slow tau-induced axon degeneration and rescue cognitive deficits in Alzheimer’s disease models (Govindarajan et al., 2013; Xiong et al., 2013). These seemingly contradictory roles are likely due to HDAC6’s involvement in multiple cellular pathways, and loss of function can have various effects depending on the disease context.
While a great deal of work has been done examining HDAC6 function in diseased neurons, relatively little is known about its role in healthy neuronal function. Given its conservation across animal taxa and high expression level in the mammalian mid-brain structures, HDAC6 is likely indispensable for some important neuronal functions. We have employed the Drosophila larval model to further examine this. We show that the N-terminal deacetylase domain of HDAC6 is required for proper memory retention, that mutants show defects in homeostatic plasticity at the level of the synapse, and that the first Deacytylase domain of the protein is important in memory.
Results
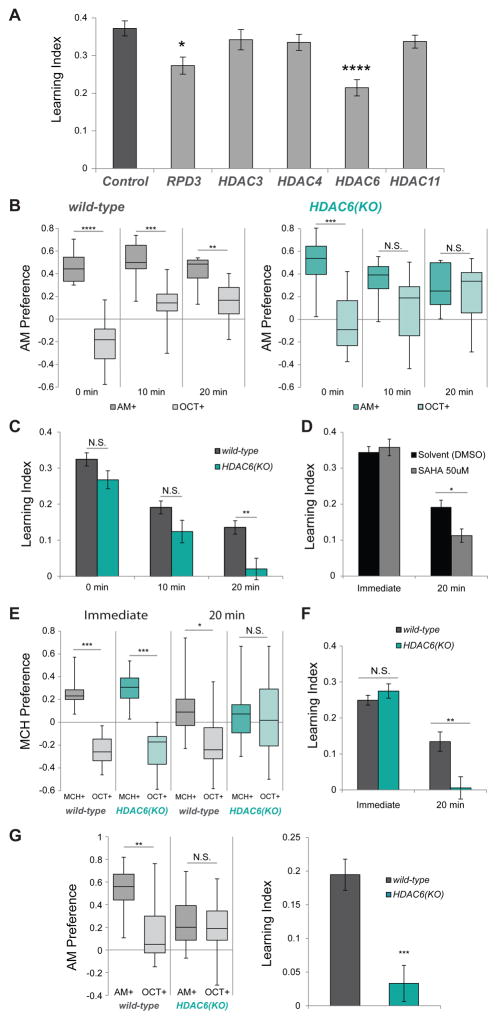
In order to investigate the role of individual HDACs in complex neuronal functions, we chose to employ a Drosophila larval olfactory learning and memory model developed previously (Gerber and Hendel, 2006; Gerber and Stocker, 2007; Hendel et al., 2005; Scherer et al., 2003). In this associative conditioning assay, one of two odors is presented to animals on a sucrose plate and a second odor on a plain agarose plate. This training is repeated for a total of three repetitions before animals are tested for their preference between the two odors. The reciprocal training is also performed and the resulting preference indexes are used to calculate a learning index (Figure S1A, (Scherer et al., 2003)). Because some HDAC mutants (such as RPD3 and HDAC3) are embryonic lethal, we chose an RNAi approach to knock down individual HDACs in post-mitotic neurons using the Elav-Gal4 driver and transgenic RNAi constructs from the TRiP collection (Table S1). Using this approach, we tested all 5 Zinc-dependent HDACs and found that knock-down of RPD3 and HDAC6 resulted in a reduction, but not a complete loss of larval immediate-term memory (Figure 1A). RPD3 has previously been implicated in long-term courtship suppression memory (Fitzsimons and Scott, 2011), but HDAC6 has not yet been characterized for memory deficits in Drosophila, and we went on to investigate this in further detail.
Figure 1. HDAC6 is required for retention of olfactory memory.
A. Larval learning indexes for control and HDAC-RNAi animals after training. All larvae tested are UAS-DCR2/+; Elav- Gal4/{UAS-RNAi}attP2 with RNAi constructs from the TRiP collection. Control larvae have an empty attP2 site derived from the TRiP donor line. (N=12–14). B. Larval preference for AM vs OCT (1 = all prefer AM; -1 = all prefer OCT) in wild-type and HDAC6(KO) larva 0 minutes, 10 minutes and 20 minutes after training. (N=10–12) (Box and whisker plots represent minimum, 1st quartile, median, 3rd quartile and maximum) C. Larval learning Indexes calculated from the AM+ and OCT+ preferences shown in (A). D. Immediate and 20 minute learning indexes of larvae treated with either solvent (DMSO) or 50 uM HDAC inhibitor (SAHA). (N = 12,12) E. Adult preference for MCH vs OCT (1 = all prefer MCH; −1 = all prefer OCT) in wild-type and HDAC6(KO) adult flies in the immediate and 20 minute tests. (N=9–12) F. Adult learning Indexes calculated from the MCH+ and OCT+ preferences shown in (B). G. (left) Preference for AM vs OCT in wild-type and HDAC6(KO) larva after 2 minute cold anesthesia applied directly after training. (N=12) (right) Larval learning Indexes calculated using these AM+ and OCT+ preferences. Error bars represent +- s.e.m. 2-tailed Mann-Whitney U test was used for statistical analysis. * p < 0.05; ** p < 0.01; *** p < 0.001; ****p < 0.0001
Interestingly, HDAC6 null (HDAC6(KO)) animals are adult-viable and apparently healthy (Du et al., 2010) so we examined them for memory deficits. We find that HDAC6(KO) larvae have immediate-term learning comparable to that of wild-type. However, while wild-type animals can retain some learning 10 and 20 minutes after training, HDAC6 mutants perform poorly in 10 and 20-minute retention tests (Figure 1B). In the 20 minute memory test, HDAC6(KO) larvae show a clear deficit compared with wild-type (Figure 1C). Feeding wild-type animals with the HDAC inhibitor SAHA also caused a similar effect: Immediate-term memory appeared unaffected, but memory assayed after 20 mins was significantly reduced (Figure 1D). SAHA is known to affect at least three Drosophila HDACs (RPD3, HDAC3 and HDAC6) (Cho et al., 2005), so we also tested larvae that had been fed with a putatively more specific HDAC6 inhibitor, BRD9757 (Wagner et al., 2013). Inhibitor-fed larva showed a significant reduction in immediate-term memory similar to the RNAi knockdown of HDAC6. However, we did not observe a reduction in the 20-minute memory at this concentration and the feeding-based method of treatment (Figure S2A).
We also examined adult animals for memory deficits using a comparable appetitive olfactory conditioning paradigm (Figure S1B, (Krashes and Waddell, 2008)). As with the larvae, adult HDAC6(KO) mutants have normal immediate-term memory but poor 20-minute memory retention (Figure 1E–F), suggesting a similar role for HDAC6 in both larval and adult neurons.
We speculated that the poor memory retention observed in HDAC6 mutants might be due to a consolidation deficit. In Drosophila adult olfactory associative conditioning, observed learning is supported by genetically distinct memory phases: Short-term memory is consolidated to more intermediate-term phases via two pathways: amnesiac-dependent anesthesia-sensitive memory (ASM) and radish-dependent anesthesia-resistant memory (ARM) (Folkers et al., 1993; Krashes and Waddell, 2008; Margulies et al., 2005; McGuire et al., 2005). These previous studies show that applying cold anesthesia to the animals after training and prior to testing disrupts the ASM portion of the memory trace, leaving only ARM observable. In order to test ARM in larvae, we anesthetized the animals for 2 minutes in ice water directly after training and allowed them to recover on a plain agarose plate for 3 minutes before testing their odor preference. Wild-type animals retain some memory after cold anesthesia, but HDAC6 mutant larvae do not (Figure 1G). We believe this larval cold-shock resistant memory is similar to the ARM memory that has been observed in adult aversive and appetitive assays (Folkers et al., 1993; Krashes and Waddell, 2008). These results indicate that HDAC6 mutants are likely deficient in the ARM consolidation pathway.
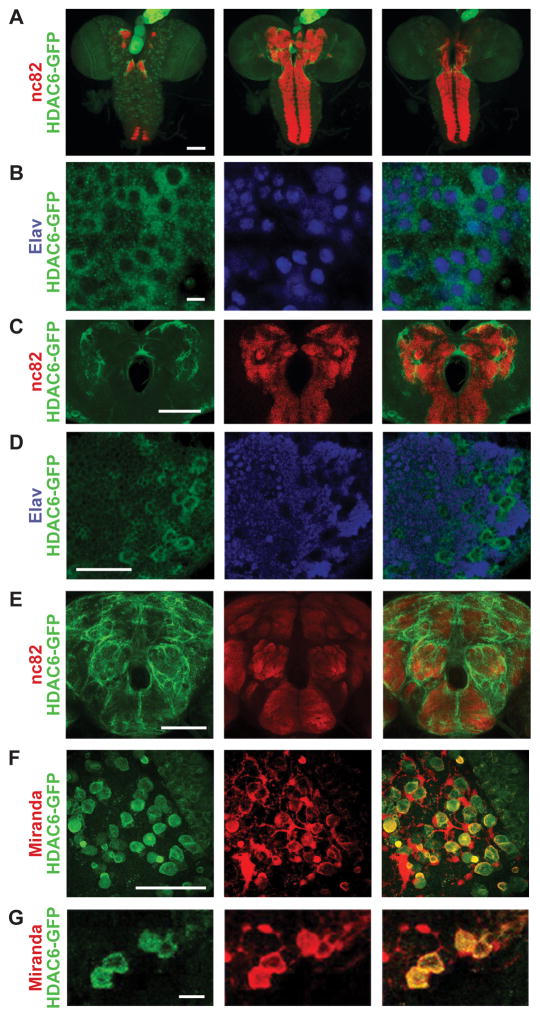
To further understand its role in neurons, we went on to characterize HDAC6 expression and localization in the nervous system in order to determine its potential targets. While many members of the HDAC family are known to regulate gene expression through deacetylation of lysine residues on histone tails, HDACs also have many non-histone targets. This is especially relevant since HDAC6 is thought to be primarily cytoplasmic, as observed in mammalian neurons and Drosophila cell lines (Cho et al., 2005; Govindarajan et al., 2013). However, its sub-cellular localization pattern has not been characterized in vivo in flies. To examine this, we used a transgenic line expressing a C-terminally GFP-tagged HDAC6 under control of endogenous promoter sequences (Sarov et al., 2015) and stained for GFP expression in the larval central nervous system (CNS). HDAC6-GFP is ubiquitously present in the larval CNS. It appears to be highly expressed in the ring gland (Figure 2A) as well as the imaginal discs (not shown). Most GFP signal is observed in the soma outside of the nucleus in neuronal (Figure 2B bottom arrow) as well as non-neuronal cells (Figure 2B middle arrow). In a few cells, it can be observed localizing to the nucleus (Figure 2B top arrow). GFP signal is also observable in the neuropil regions labeled by nc82 (anti-Bruchpilot), although it is more diffuse in both larval and adult brains (Figure 2C,E). HDAC6-GFP is most visible in axonal tracts surrounding neuropil regions (Figure 2E). While observable in most cells, HDAC6 appears to be most abundant in larger non-elav labeled cells that are likely neuroblasts (Figure 2D). Co-staining with antibody against the neuroblast marker Miranda (Callan et al., 2010; Mollinari et al., 2002) shows that HDAC6-GFP does indeed co-localize with Miranda, most noticeably in the GMC during asymmetric cell division from the neuroblast (Figure 2F,G).
Figure 2. HDAC6 primarily localizes to the cytoplasm in neurons and neuroblasts.
Images showing whole mount antibody staining from an HDAC6-GFP transgenic animal (VK00033{HDAC6-2XTY1-SGFP-V5-preTEV-BLRP-3XFLAG}) where anti-GFP (labeling HDAC6-GFP) is always shown in green.. A. The larval central nervous system (CNS) shown as Z-stacks in three portions going from more dorsal (left), medial (middle) to ventral (right) with anti-Bruchpilot (nc82) is shown in red; B. Larval ventral nerve cord (VNC) with anti-Elav (labeling neuronal nuclei) is shown in blue. White arrows indicate example cell bodies: Neuron with nuclear HDAC6 signal (top); non-neuronal cell with cytoplasmic HDAC6 (middle); Neuron with cytoplasmic HDAC6 (bottom). C. Larval central brain neuropil region with anti-Bruchpilot (nc82) is shown in red. D. Larval optic lobe with anti-Elav (labeling neuronal nuclei) is shown in blue. White arrows indicate putative neuroblasts. E. Adult central nervous system (CNS) with anti-Bruchpilot (nc82) is shown in red; anti-GFP (labeling HDAC6-GFP) is shown in green. F. Larval optic lobe with anti-Miranda (labeling dividing neuroblasts) is shown in red. White arrows indicate putative neuroblasts undergoing asymmetrical cell division with both Miranda and HDAC6 potentially accumulating in the newly forming daughter cell. G. Close-up section of the larval optic lobe showing expression of HDAC6-GFP (green) in anti-Miranda labeled neuroblasts (red). Scale bars correspond to 100μm: (A, E); 50μm (C, D, F); 10μm (B, G).
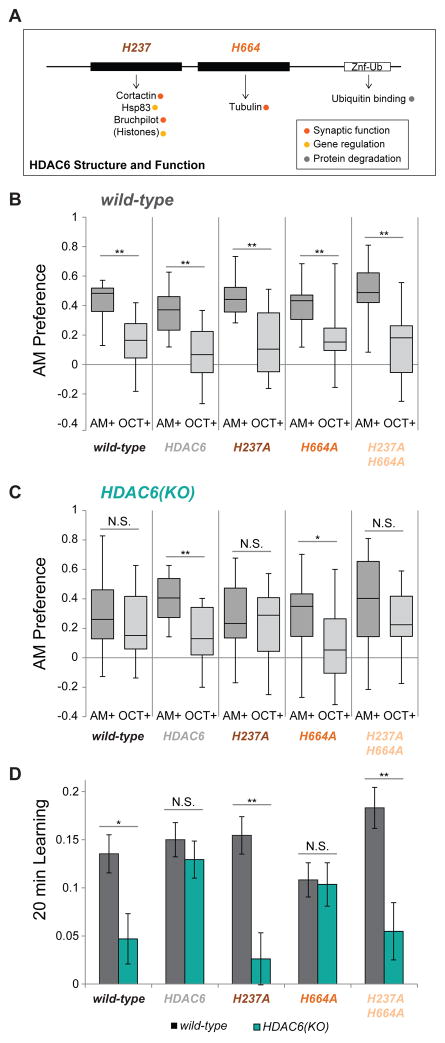
Although HDAC6 can deacetylate histone peptides in vitro (Cho et al., 2005), it appears primarily to be localized to the cytoplasm in neurons, indicating that non-histone targets may be important during learning and memory. HDAC6 is the only HDAC family member with two deacetylase (DAC) domains as well as a ubiquitin binding domain (Figure 3A, (Du et al., 2010), reviewed in (Boyault et al., 2007)). The second DAC domain is thought to be devoted exclusively to tubulin deacetylation (Hubbert et al., 2002), while the first DAC domain is associated with all other targets, including Bruchpilot (flies), cortactin (mammals) and potentially histones (Figure 3A, (Kovacs et al., 2005; Liu et al., 2012; Miskiewicz et al., 2014; Zhang et al., 2007)). In order to understand which DAC domains are important for memory retention, we performed pan-neuronal (elav-Gal4) rescue of HDAC6 in an HDAC6 mutant background using a fully functional HDAC6 construct as well as constructs lacking DAC activity in the first domain (H237A), second domain (H664A) or both domains (H237A.H664A). HDAC6 is located on the first chromosome, so HDAC6(KO) females crossed to wild-type males produce a larval population comprised of heterozygous females and HDAC6 null males. This population performed poorly in the 20 minute memory assay, so we expressed the various HDAC6 transgenes in this HDAC6 “reduced” background by crossing HDAC6(KO) females to elav- Gal4>UAS-HDAC6 males and testing the resulting progeny.
Figure 3. The deacetylase activity of HDAC6’s first domain is required for normal memory retention.
A. Schematic showing the functional domains of HDAC6. Critical active site residues for the deacetylase (DAC) domains are shown above and known targets of HDAC6 are shown below. Adapted from (Du et al., 2010), reviewed in (Boyault et al., 2007). B. Larval preference for AM vs OCT (1 = all prefer AM; −1 = all prefer OCT) after 20 minutes in wild-type and HDAC6 overexpression controls. (N = 10 – 12) (Box and whisker plots represent minimum, 1st quartile, median, 3rd quartile and maximum) C. Larval preference for AM vs OCT for HDAC6 overexpression in the HDAC6 reduced background after 20 minutes. (N= 12 – 16) C. Larval 20 minute learning Indexes calculated from the AM+ and OCT+ preferences shown in (B,C). Error bars represent +- s.e.m. 2-tailed Mann-Whitney U test was used for statistical analysis. * p < 0.05; ** p < 0.01
Overexpression of the various HDAC6 constructs in a wild-type background did not produce any 20 minute memory deficits (Figure 3B). When overexpressed in the HDAC6 “reduced” background, only wild-type HDAC6 and HDAC6.H664A (the first DAC domain intact) were able to rescue the 20 minute memory deficit (Figure 3C–D). Rescue using constructs lacking activity in the first DAC domain (H237A constructs) did not restore 20 minute learning behavior memory and still showed a learning deficit compared with overexpression controls (Fig 3C–D). Previously published data demonstrate that the HDAC6.H237A transgene does produce protein with a functional second DAC domain (Xiong et al., 2013).(Xiong et al., 2013). This indicates that activity in the first DAC domain is most critical for 20 minute memory retention suggesting that HDAC6’s tubulin deacetylase activity is dispensable in this context and other first domain targets are most important.
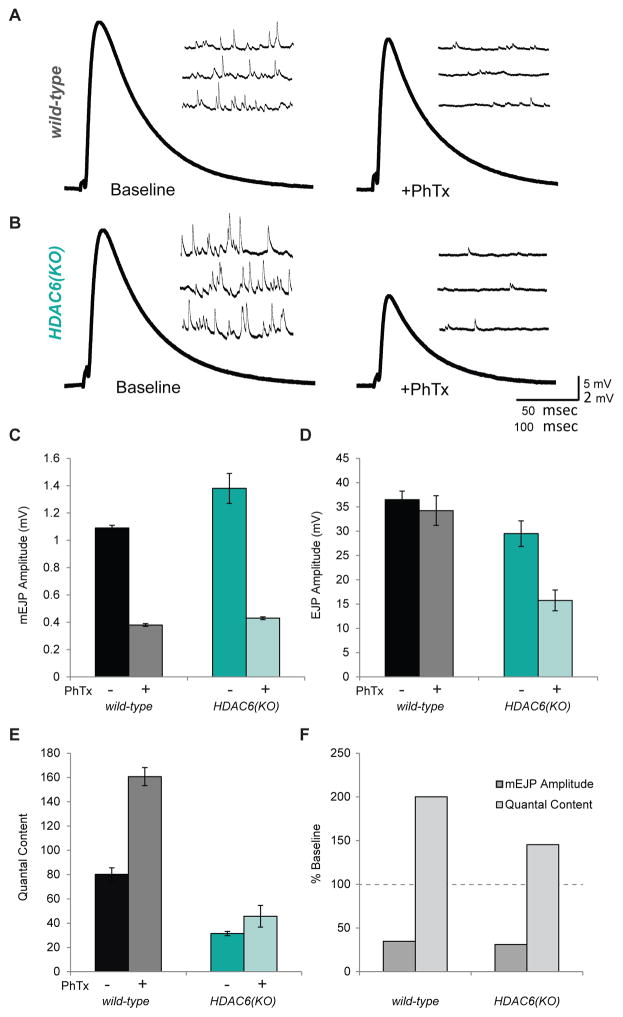
In Drosophila, the only confirmed target of HDAC6’s first domain is Bruchpilot, an important component of the presynaptic active zone. Multiple Bruchpilot fibers form umbrella- shaped T-bar structures at active zones with their N-termini anchored to Cacophony calcium channels and the C-termini fanning into the cytoplasm where they contact and tether vesicles (Fouquet et al., 2009; Kittel et al., 2006; Wagh et al., 2006). In HDAC6 mutants, the C-terminal region of Bruchpilot becomes hyperacetylated, presumably by its acetyltransferase ELP3, resulting in narrower T-bar structures which contact fewer vesicles. Overexpression of HDAC6 results in expanded T-bar tops and increased vesicle tethering (Miskiewicz et al., 2014; Miśkiewicz et al., 2011). Because gain or loss of HDAC6 function is known to correlate with increased or decreased synaptic transmission at the neuromuscular junction (Miskiewicz et al., 2014), we speculated that HDAC6 may modulate synaptic transmission during synaptic plasticity processes such as those occurring during learning and memory. In order to investigate whether or not HDAC6 is involved in modulating synaptic function, we chose to examine its role in homeostatic synaptic plasticity using the neuromuscular junction (NMJ) as a model synapse. Reducing sensitivity of the post-synaptic muscle to presynaptic glutamate by genetic loss or pharmacological perturbation of one of the glutamate receptors is known to induce a compensatory increase in presynaptic release in motor neurons, restoring synaptic strength to wild-type levels (Davis and Müller, 2015; DiAntonio et al., 1999; Petersen et al., 1997). This type of homeostatic plasticity can also be observed with pharmacological treatment by philanthotoxin (PhTx), which acutely blocks postsynaptic glutamate receptors and dramatically reduces the postsynaptic sensitivity to glutamate release (Frank et al., 2006). Following PhTx treatment, evoked response (EJP) in wild-type animals is able to recover to baseline levels after approximately 10 minutes because of a homeostatic increase in presynaptic release (quantal content)(Figure 4A). However, in HDAC6(KO) animals, EJP amplitudes do not fully recover following PhTx application, indicating that these mutants are not able to respond normally to the homeostatic challenge (Figure 4B). The miniature excitatory junctional potentials (mEJP) and EJP amplitudes can be used to estimate quantal content (the number of vesicles released per stimulus) before and after PhTx treatment. In wild-type animals, PhTx causes an approximate doubling of the quantal content, which restores evoked response to baseline levels. In contrast, HDAC6(KO) animals show only a small increase in quantal content that fails to restore EJP amplitudes to baseline levels (Figure 4E,F). This indicates that HDAC6(KO) mutants do not have the capacity to fully express homeostatic plasticity.
Figure 4. Homeostatic synaptic plasticity is disrupted in HDAC6 mutants.
A. Representative electrophysiological traces showing evoked excitatory junctional potentials (EJP) and mini excitatory junctional potentials (mEJPs) from wild-type larva before and after PhTx treatment. B. Representative traces from HDAC6(KO) larva before and after PhTx treatment. C. Average mEJP amplitudes in wild-type and HDAC6(KO) larva with and without PhTx treatment. D. Average EJP amplitudes in wild-type and HDAC6(KO) larva with and without PhTx treatment. E. Estimated quantal content in wild-type and HDAC6(KO) larva with and without PhTx treatment. F. mEJP amplitude and quantal content in wild-type and HDAC6(KO) larva following PhTx treatment shown as percent base line. (N = 10–14) G. Model for HDAC6’s role in synaptic plasticity: in wild-type animals, HDAC6 may deacetylate BRP C- terminal domains to increase vesicle tethering during synaptic strengthening. This would result in increased quantal content and increased transmission at these synapses. In HDAC6 mutant animals, BRP would not be deacetylated during synaptic strengthening. This may result in a limited increase in quantal content and a deficit in plasticity. Error bars represent +- s.e.m
These findings would also suggest that acetylation levels of Bruchpilot may be modulated by HDAC6 and the opposing acetyltransferase (ELP3) at key synapses during learning and memory. Because loss of HDAC6 results in a reduction of vesicle tethering by Bruchpilot (Miskiewicz et al., 2014), it follows that reduction in overall levels of Bruchpilot may also impair learning. Unfortunately, because even hypomorphic alleles of BRP result in reduced synaptic transmission and motor deficits (Hallermann et al., 2010) analysis of BRP mutants in a behavior assay is not possible. However, RNAi knock-down of BRP in adult mushroom bodies has been shown to result in an ARM deficit (Knapek et al., 2011a). When we knock-down BRP in larval mushroom bodies, we do observe a small reduction in immediate-term memory without compromising sucrose or odor naïve behaviors (Figure S2B). However, we note that this could also be due to deficits in synaptic transmission in these neurons, which is also known to impact immediate-term memory (Pauls et al., 2010). Another interesting possibility is that the ELP3 acetyltransferase activity may be genetically opposed to HDAC6 deacetylase activity. In order to test for genetic interaction, we examined appetitive learning in larva, which have lost one copy of ELP3 in the HDAC6 reduced background. However, loss of one copy of ELP3 did not rescue 20-minute or cold-shock memory deficits (Figure S3). ELP3 heterozygous mutants appear to display a 20-minute memory deficit but we did not examine this further (Figure S3B). Overexpression of HDAC6 in a wild-type background was also not observed to impact memory performance (Figure 3C–D). This may indicate that the interplay between HDAC6 and ELP3 may be less important for behavioral plasticity than the presence of absence of HDAC6 itself.
Discussion
The study of HDACs in complex neuronal functions has primarily focused on their role as chromatin modifiers and modulators of gene expression. However, we are beginning to understand that HDAC family members have non-histone targets and are likely involved in diverse cellular processes. Here we find that HDAC6, a primarily cytoplasmic deacetylase is involved in retention and consolidation of associative olfactory memory. HDAC6 mutants appear to have normal immediate-term memory but the learned response decays rapidly over the course of 20 minutes. Furthermore, appetitive memory in HDAC6 mutant larvae is highly sensitive to cold anesthesia, suggesting that HDAC6 is involved in anesthesia resistant consolidation of memory (ARM). While outside the scope of this study, it will be interesting to further understand the genetic interactions between HDAC6 and other known Drosophila memory pathways.
Relatively little is known about the molecular mechanisms underlying anesthesia resistant memory. The only known classical mutant deficient in ARM is radish (Folkers et al., 1993), but more recently, knock-down of Bruchpilot in the adult mushroom bodies has also been observed to result in an ARM deficit suggesting that consolidation to ARM may be directly related to changes at active zones (Knapek et al., 2011). HDAC6 is thought to be Bruchpilot’s only deacetylase and loss or gain of HDAC6 function is known to alter Bruchpilot’s C-terminal acetylation levels and ability to tether vesicles and regulate synaptic transmission (Miskiewicz et al., 2014). In the context of what we observe behaviorally, it’s possible that HDAC6 is required biochemically during memory consolidation to deacetylate BRP and strengthen key synapses. It has not escaped our attention that post-translational modification of BRP could drive rapid structural changes to active zones similar to those observed during synaptic homeostasis at the fly NMJ (Tsurudome et al., 2010; Weyhersmüller et al., 2011) (Figure 4G).
Furthermore, when we employed a homeostatic model of presynaptic strengthening to examine rapid, PhTx-induced plasticity using NMJ electrophysiology, we observe that HDAC6 mutants are unable to fully compensate for the homeostatic challenge. The fundamental mechanisms underlying plasticity at the NMJ could be analogous to those required to affect plasticity in CNS synapses, such as those involved in learning and memory. Our findings also provide a connection between synaptic and behavioral plasticity. We would like to note, however, that it is possible that the synaptic mechanisms in central neurons use different mechanisms than the NMJ. Although it is not currently feasible to identify synapses involved in olfactory memory or record from them in central neurons, future circuit mapping studies may help to resolve this question.
HDAC6 is highly conserved from flies to mammals and it is thought to interact with similar targets across taxa. While mammals do not have a direct ortholog of Bruchpilot, it is a member of the ELKS/CAST family of active zone proteins. It is conceivable that HDAC6 may target these or other proteins in mammals and participate in modulation of synaptic plasticity. In addition, HDAC6 has other substrates and many additional interaction partners. It has the potential to regulate cytoskeleton dynamics, affect gene expression and influence protein degradation. All of these interactions could also be important for healthy neuronal function.
Materials and Methods
Fly stocks
Unless otherwise stated, all Drosophila used in this study were reared on standard cornmeal-dextrose media at 25C. A white1118 backcrossed to Canton-S (wCS) line was used as the wild-type control for all behavior experiments. A complete list of mutant and transgenic lines can be found in Table S1.
SAHA and BRD9757 treatments
SAHA (Sigma-Aldrich) was dissolved at 50mM in DMSO and diluted 1:1000 into molten fly food for a final concentration of 50uM. Larvae were grown in treated media from egg to third instar (about 5 days) before being used for behavior experiments. BRD9757 (Sigma-Aldrich) treated food was prepared in a similar manner, but larvae were given a second dose of the inhibitor (50 uL of 50mM solution per 50mL culture) 24 hours prior to behavior experiments. DMSO at 1:1000 in food was used as a control in both cases.
Larval appetitive olfactory learning assay
See Figure S1A. The larval learning assay in this study was adapted from a well-established protocol (Scherer et al., 2003).
Adult appetitive olfactory learning assay
See Figure S1B. The adult learning assay in this study was adapted from a well-established protocol (Krashes and Waddell, 2008).
Statistical Analysis
Statistical comparisons for behavior experiments were performed using a 2-tailed Mann-Whitney U test available at the VassarStats website interface (http://vassarstats.net/utest.html).
Immunohistochemistry
Brain tissue antibody staining was performed as previously described (Callan et al., 2010). The primary antibodies used were mouse anti-nc82 (1:10, DSHB), rat anti-elav-7E8A10 (1:5, DSHB), rabbit anti-GFP (1:500, Invitrogen) and rat anti-Miranda (1:100, Abcam 197788). The secondary antibodies used were goat anti-rabbit-488, goat anti-rat-568 and goat anti-mouse-647 (1:200, Invitrogen). Images were taken using a Leica SP-5 inverted confocal microscope and processed using Image J. Supplementary images were taken using a Nikon A1R confocal microscope and processed using Image J.
Electrophysiology
NMJ electrophysiology was similar as previously described (Dickman and Davis, 2009). Average mEJP, EJP, and quantal content were calculated for each genotype with corrections for nonlinear summation (Martin, 1955). Recordings were rejected if the Vrest was above −60 mV, if the Rin was less than 5MΩ, or if either measurement deviated by more than 20% during the experiment. Larvae were incubated with or without philanthotoxin-433 (Sigma; 20 μM) resuspended in HL3 for 10 mins.
Additional details are available in SUPPLEMENTAL EXPERIMENTAL PROCEDURES
Supplementary Material
Acknowledgments
This work was supported by funds to A.R. from NIH (NINDS) grant R21NS085765. The funding agency had no role in design of experiments or its interpretation.
Footnotes
Author contributions: S.P. conceived the experiments, planned and carried out behavior and immunohistochemistry experiments and wrote the manuscript. B.K. planned and carried out electrophysiology experiments. D.D. contributed to discussions and edited the manuscript. A.R. supervised the project and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babenko O, Kovalchuk I, Metz GA. Epigenetic programming of neurodegenerative diseases by an adverse environment. Brain Res. 2012;1444:96–111. doi: 10.1016/j.brainres.2012.01.038. [DOI] [PubMed] [Google Scholar]
- Bertrand P. Inside HDAC with HDAC inhibitors. Eur J Med Chem. 2010;45:2095–2116. doi: 10.1016/j.ejmech.2010.02.030. [DOI] [PubMed] [Google Scholar]
- Boyault C, Sadoul K, Pabion M, Khochbin S. HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene. 2007;26:5468–5476. doi: 10.1038/sj.onc.1210614. [DOI] [PubMed] [Google Scholar]
- Callan MA, Cabernard C, Heck J, Luois S, Doe CQ, Zarnescu DC. Fragile X protein controls neural stem cell proliferation in the Drosophila brain. Hum Mol Genet. 2010;19:3068–3079. doi: 10.1093/hmg/ddq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Griswold A, Campbell C, Min KT. Individual histone deacetylases in Drosophila modulate transcription of distinct genes. Genomics. 2005;86:606–617. doi: 10.1016/j.ygeno.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Davis GW, Müller M. Homeostatic Control of Presynaptic Neurotransmitter Release. Annu Rev Physiol. 2015;77:251–270. doi: 10.1146/annurev-physiol-021014-071740. [DOI] [PubMed] [Google Scholar]
- Davis GW, DiAntonio A, Petersen SA, Goodman CS. Postsynaptic PKA Controls Quantal Size and Reveals a Retrograde Signal that Regulates Presynaptic Transmitter Release in Drosophila. Neuron. 1998;20:305–315. doi: 10.1016/s0896-6273(00)80458-4. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Petersen SA, Heckmann M, Goodman CS. Glutamate Receptor Expression Regulates Quantal Size and Quantal Content at the Drosophila Neuromuscular Junction. J Neurosci. 1999;19:3023–3032. doi: 10.1523/JNEUROSCI.19-08-03023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman DK, Davis GW. The schizophrenia susceptibility gene dysbindin controls synaptic homeostasis. Science. 2009;326:1127–1130. doi: 10.1126/science.1179685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Liu X, Chen X, Song M, Yan Y, Jiao R, Wang C-c. Drosophila Histone Deacetylase 6 Protects Dopaminergic Neurons against -Synuclein Toxicity by Promoting Inclusion Formation. Mol Biol Cell. 2010;21:2128–2137. doi: 10.1091/mbc.E10-03-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Mungenast A, Tsai LH. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;31:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Fitzsimons HL, Scott MJ. Genetic modulation of Rpd3 expression impairs long-term courtship memory in Drosophila. PLoS One. 2011;6:e29171. doi: 10.1371/journal.pone.0029171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkers E, Drain P, Quinn WG. Radish, a Drosophila mutant deficient in consolidated memory. Proc Natl Acad Sci U S A. 1993;90:8123–8127. doi: 10.1073/pnas.90.17.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquet W, Owald D, Wichmann C, Mertel S, Depner H, Dyba M, Hallermann S, Kittel RJ, Eimer S, Sigrist SJ. Maturation of active zone assembly by Drosophila Bruchpilot. J Cell Biol. 2009;186:129–145. doi: 10.1083/jcb.200812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber B, Hendel T. Outcome expectations drive learned behaviour in larval Drosophila. Proc Biol Sci. 2006;273:2965–2968. doi: 10.1098/rspb.2006.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber B, Stocker RF. The Drosophila larva as a model for studying chemosensation and chemosensory learning: a review. Chem Senses. 2007;32:65–89. doi: 10.1093/chemse/bjl030. [DOI] [PubMed] [Google Scholar]
- Govindarajan N, Rao P, Burkhardt S, Sananbenesi F, Schlüter OM, Bradke F, Lu J, Fischer A. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO Mol Med. 2013;5:52–63. doi: 10.1002/emmm.201201923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallermann S, Kittel RJ, Wichmann C, Weyhersmuller A, Fouquet W, Mertel S, Owald D, Eimer S, Depner H, Schwarzel M, et al. Naked Dense Bodies Provoke Depression. J Neurosci. 2010;30:14340–14345. doi: 10.1523/JNEUROSCI.2495-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendel T, Michels B, Neuser K, Schipanski A, Kaun K, Sokolowski MB, Marohn F, Michel R, Heisenberg M, Gerber B. The carrot, not the stick: appetitive rather than aversive gustatory stimuli support associative olfactory learning in individually assayed Drosophila larvae. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:265–279. doi: 10.1007/s00359-004-0574-8. [DOI] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- Keene AC, Krashes MJ, Leung B, Bernard JA, Waddell S. Drosophila dorsal paired medial neurons provide a general mechanism for memory consolidation. Curr Biol. 2006;16:1524–1530. doi: 10.1016/j.cub.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Knapek S, Sigrist S, Tanimoto H. Bruchpilot, a synaptic active zone protein for anesthesia-resistant memory. J Neurosci. 2011;31:3453–3458. doi: 10.1523/JNEUROSCI.2585-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JJ, Murphy PJM, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone- dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis- dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci. 2008;28:3103–3113. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Peng L, Seto E, Huang S, Qiu Y. Modulation of histone deacetylase 6 (HDAC6) nuclear import and tubulin deacetylase activity through acetylation. J Biol Chem. 2012;287:29168–29174. doi: 10.1074/jbc.M112.371120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies C, Tully T, Dubnau J. Deconstructing Memory in Drosophila. Curr Biol. 2005;15:R700–R713. doi: 10.1016/j.cub.2005.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR. A further study of the statistical composition of the end-plate potential. J Physiol. 1955;130:114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Deshazer M, Davis RL. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog Neurobiol. 2005;76:328–347. doi: 10.1016/j.pneurobio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Miskiewicz K, Jose LE, Yeshaw WM, Valadas JS, Swerts J, Munck S, Feiguin F, Dermaut B, Verstreken P. HDAC6 is a Bruchpilot deacetylase that facilitates neurotransmitter release. Cell Rep. 2014;8:94–102. doi: 10.1016/j.celrep.2014.05.051. [DOI] [PubMed] [Google Scholar]
- Miśkiewicz K, Jose LE, Bento-Abreu A, Fislage M, Taes I, Kasprowicz J, Swerts J, Sigrist S, Versées W, Robberecht W, et al. ELP3 controls active zone morphology by acetylating the ELKS family member Bruchpilot. Neuron. 2011;72:776–788. doi: 10.1016/j.neuron.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Mollinari C, Lange B, González C. Miranda, a protein involved in neuroblast asymmetric division, is associated with embryonic centrosomes of Drosophila melanogaster. Biol Cell. 2002;94:1–13. doi: 10.1016/s0248-4900(02)01181-4. [DOI] [PubMed] [Google Scholar]
- Pauls D, Selcho M, Gendre N, Stocker RF, Thum AS. Drosophila larvae establish appetitive olfactory memories via mushroom body neurons of embryonic origin. J Neurosci. 2010;30:10655–10666. doi: 10.1523/JNEUROSCI.1281-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic Analysis of Glutamate Receptors in Drosophila Reveals a Retrograde Signal Regulating Presynaptic Transmitter Release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Advances in epigenetics and epigenomics for neurodegenerative diseases. Curr Neurol Neurosci Rep. 2011;11:464–473. doi: 10.1007/s11910-011-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov M, Barz C, Jambor H, Hein MY, Schmied C, Suchold D, Stender B, Janosch S, Vikas VKJ, Krisnan RT, et al. A genome-wide resource for the analysis of protein localisation in Drosophila. Cold Spring Harbor Labs Journals. 2015 doi: 10.7554/eLife.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S, Stocker RF, Gerber B. Olfactory learning in individually assayed Drosophila larvae. Learn Mem. 2003;10:217–225. doi: 10.1101/lm.57903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman SF, Basso M, Mahishi L, Kozikowski AP, Donohoe ME, Langley B, Ratan RR. Putting the “HAT” back on survival signalling: the promises and challenges of HDAC inhibition in the treatment of neurological conditions. Expert Opin Investig Drugs. 2009;18:573–584. doi: 10.1517/13543780902810345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurudome K, Tsang K, Liao EH, Ball R, Penney J, Yang JS, Elazzouzi F, He T, Chishti A, Lnenicka G, et al. The Drosophila miR-310 cluster negatively regulates synaptic strength at the neuromuscular junction. Neuron. 2010;68:879–893. doi: 10.1016/j.neuron.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela-Fernández A, Cabrero JR, Serrador JM, Sánchez-Madrid F. HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 2008;18:291–297. doi: 10.1016/j.tcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Dürrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Wagner FF, Olson DE, Gale JP, Kaya T, Weïwer M, Aidoud N, Thomas M, Davoine EL, Lemercier BC, Zhang YL, et al. Potent and Selective Inhibition of Histone Deacetylase 6 (HDAC6) Does Not Require a Surface-Binding Motif. J Med Chem. 2013;56:1772–1776. doi: 10.1021/jm301355j. [DOI] [PubMed] [Google Scholar]
- Weyhersmüller A, Hallermann S, Wagner N, Eilers J. Rapid active zone remodeling during synaptic plasticity. J Neurosci. 2011;31:6041–6052. doi: 10.1523/JNEUROSCI.6698-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Zhao K, Wu J, Xu Z, Jin S, Zhang YQ. HDAC6 mutations rescue human tau-induced microtubule defects in Drosophila. Proc Natl Acad Sci U S A. 2013;110:4604–4609. doi: 10.1073/pnas.1207586110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.