Abstract
Abnormal tau phosphorylation occurs in several neurodegenerative disorders, including Alzheimer's disease (AD) and frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17). Here, we compare mechanisms of tau phosphorylation in mouse models of FTDP-17 and AD. Mice expressing a mutated form of human tau associated with FTDP-17 (tauV337M) showed age-related increases in exogenous tau phosphorylation in the absence of increased activation status of a number of kinases known to phosphorylate tau in vitro. In a “combined” model, expressing both tauV337M and the familial amyloid precursor protein AD mutation APPV717I in a CT100 fragment, age-dependent tau phosphorylation occurred at the same sites and was significantly augmented compared to “single” tauV337M mice. These effects were concomitant with increased activation status of mitogen-activated protein kinase (MAPK) family members (extracellular regulated kinases 1 and 2, p38, and c-Jun NH2-terminal kinase) but not glycogen synthase kinase-3αβ or cyclin-dependent kinase 5. The increase in MAPK activation was a discrete effect of APPV717I-CT100 transgene expression as near identical changes were observed in single APPV717I-CT100 mice. Age-dependent deficits in memory were also associated with tauV337M and APPV717I-CT100 expression. The data reveal distinct routes to abnormal tau phosphorylation in models of AD and FTDP-17 and suggest that in AD, tau irregularities may be linked to processing of APP C-terminal fragments via specific effects on MAPK activation status.
Abnormal phosphorylation of the microtubule-associated protein tau is a feature of several neurodegenerative disorders, including Alzheimer's disease (AD) and frontotemporal dementia with Parkinsonism associated with chromosome 17 (FTDP-17) (43). Recent efforts to discern the molecular pathology of tau dysfunction in these disorders have focused on the enzymes capable of phosphorylating tau, the so-called tau kinases, which include glycogen synthase kinase 3β (GSK-3β) (11), cyclin-dependent kinase 5 (cdk5) (11, 30), and the mitogen-activated protein kinase (MAPK) family members, extracellular regulated kinases 1 and 2 (ERK1/2) (10, 39), p38 (4), and the c-Jun NH2-terminal kinases (JNKs) (40). However, as most previous data have been obtained in vitro, often using cell-free systems or cell lines, the manner and extent to which any of these tau kinases may be causally implicated in the disease condition remains unknown. The present work, therefore, utilized transgenic mouse models of relevance to FTDP-17 and AD to examine tau phosphorylation and kinase function in intact brain tissue. The FTDP-17 model expressed the V337M (valine to methionine) tau mutation (38), which is associated in humans with severe behavioral and cognitive symptoms accompanied by widespread tau filamentous brain pathology (42, 46). For the AD model, we expressed the familial AD V717I (valine to isoleucine) “London” mutation (13) within a 100-amino-acid carboxy-terminal (C-terminal) fragment of the human amyloid precursor protein (APP-CT100), which encompasses the β amyloid (Aβ) sequence (6). The APPV717I substitution is associated with an aggressive early-onset dementia and a characteristic increase in the long amyloidogenic form of Aβ (Aβ1-42) in AD brain (36).
Our development of the FTDP-17 and AD models allowed us to address a number of additional questions by crossbreeding the single transgenic mice to produce a “combined” transgenic mouse. Hence, comparisons across the transgenic lines of the extent of tau phosphorylation and the activity status of kinases known to phosphorylate tau provided an analysis of any differences between the tauopathy models and also evidence of interactions between tau and APP C-terminal fragment metabolism. This latter possibility, which is of specific relevance to AD where tauopathy and amyloidopathy coexist, has been suggested by data obtained with neuronal cell lines showing that Aβ can activate GSK-3αβ and ERK1/2, both tau kinases (39, 48). However, the most compelling data derive from in vivo studies, where injection of Aβ fibrils into the brains of mice expressing an FTDP-17 mutation leads to increased numbers of neurofibrillary tangles (18). Mice expressing familial AD APP and FTDP-17 tau mutations in combination have also shown increased formation of tau-positive neuropathology compared to mice expressing only the tau mutation (31). To examine the evidence of possible cross talk between tau and APP C-terminal fragments in more detail, the present work focused on molecular aspects of tau phosphorylation. The work also used behavioral tests, including a task of olfactory memory, to assess the functional effects of any molecular changes identified.
MATERIALS AND METHODS
Transgenic mice.
Site-directed mutagenesis was used to introduce the V337M mutation into cDNA encoding the longest three-repeat human tau isoform and the V717I mutation into cDNA encoding the CT100 fragment of human APP. The mutated cDNA was subcloned into murine Thy1 genomic expression vectors (33) at an XhoI site. A Kozak consensus sequence had been introduced upstream of the initiation codon. Transgenic mice were produced by pronuclear injection of (C57BL/6J × CBA/Ca)F1 embryos. Founders were identified by PCR analysis of lysates from tail biopsies using the primer pairs 5′ GGTTTTTGCTGGAATCCTGG 3′ and 5′ GGAGTTCGAAGTGATGGAAG 3′ (tau) and 5′ TTCCGACATGACTCAGGATATGAAGTTC 3′ and 5′ CGTTCTGCTGCATCTTGGACAGG 3′ (CT100).
Founder animals were intercrossed with (C57BL/6J × CBA/Ca)F1 mice to establish lines. Hemizygous tauV337M and APPV717I-CT100 mice from the experimental lines chosen were mated to produce hemizygous tauV337M × APPV717I-CT100 combined, hemizygous tauV337M, hemizygous APPV717I-CT100, and wild-type animals.
In situ hybridization.
Brain sections from transgenic and littermate wild-type mice were cut on the sagittal plane at 12 μm and mounted on poly-l-lysine-coated slides. Slides were fixed in 4% paraformaldehyde, washed in phosphate-buffered saline, and stored in 95% ethanol at 4°C until use. Prior to hybridization, slides were washed in 1× saline sodium citrate (SSC) (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) with 20 mM β-mercaptoethanol, first at 20°C and then at 55°C, dehydrated in an ascending ethanol series, and dried. Brain sections were labeled with specific radiolabeled oligonucleotide probes hybridizing to unique sequences within both transgene mRNAs and endogenous tau and APP transcripts: 5′ CTTGGGCTCCCGGGTGGGTGGGGTTGGTTGGGACGGGGTGCGGGAG 3′ (human tau), 5′ CTTGGGCTCCCGGGTGGGCGGTGTTGGTAGGGATGGGGTGCGCGAG 3′ (mouse tau), 5′ CAGCATCACCAAGGTGAATGACGATCACTGTCGCTATGACAACACC 3′ (human APP-CT100), and 5′ CAACATCACCACGGTGATGACAATCACGGTTGCTATGACAACGCC 3′ (mouse APP).
Each probe was 3′ end labeled with terminal transferase and 35S-labeled d-ATP and used at 350,000 cpm/μl. Sections were hybridized overnight at 55°C in a 50% formamide hybridization mixture. The slides were washed in 2× SSC-0.1% sodium dodecyl sulfate (SDS), RNase treated (RNase, 10 μg/ml), and dehydrated in an ascending ethanol series. Labeled sections and radioactive standards (14C-labeled microstandards; Amersham, Little Chalfont, United Kingdom) were exposed to x-ray film for 25 days.
Immunohistochemical and histological studies.
Brain sections from transcardially perfused animals, cut in the sagittal plane at 30 μm, were stained with standard immunohistochemical techniques, following blocking of endogenous peroxidase activity (20% methanol, 1.5% hydrogen peroxide) and incubation in blocking solution (3% normal goat serum, 2 g of bovine serum albumin/liter, 0.1% Triton X-100) or MOM immunoglobulin blocking reagent (Vector Laboratories, Burlingame, Calif.). Free-floating sagittal sections were incubated overnight at 4°C with primary antibodies to APP (C-terminus specific; Chemicon), Aβ (amino acids 1 to 17; Chemicon), tau (T14 clone; Zymed) or phospho S202/T205 tau (AT8 epitope; Innogenetics). Goat anti-rabbit biotinylated secondary antibody (ABC Vectastain kit; Vector Laboratories) and reagents and biotinylated secondary antibody from a Vector MOM immunodetection kit (Vector Laboratories) were used, depending on the nature of the primary antibody. Immunoreactivity was identified by a diaminobenzidine tetrachloride reaction (Vector Laboratories). Congo red staining was performed with slide-mounted sagittal sections (15 μm) according to the methods of Wolman and Bubis (54). Bielschowsky staining was performed with slide-mounted sagittal sections (each, 15 μm). Slides were incubated in 20% silver nitrate solution for 2 h, washed in reducer (50 ml of formaldehyde, 200 ml of absolute alcohol, 750 ml of distilled water) for 3 min, followed by 10% alcohol for 2 min. Slides were placed in silver B solution (20 ml of 20% silver nitrate, 20 ml of 95% alcohol, and ammonia added until the precipitate formed was redissolved) for 2 min and then washed in a constant flow of reducer for 4 min. After being washed in distilled water, slides were toned with 0.1% gold chloride for 2 min, washed in distilled water, and fixed in 5% sodium thiosulphate.
Aβ1-40 and Aβ1-42 ELISA.
The levels of Aβ1-40 and Aβ1-42 in soluble and insoluble brain fractions in snap-frozen brain hemispheres were quantified by enzyme-linked immunosorbent assay (ELISA) as previously described (27). Brain samples were weighed and sonicated in phosphate-buffered saline containing 2% SDS with 50 mM protease inhibitor cocktail (Roche). Samples were centrifuged at 100,000 × g at 4°C for 1 h, and the supernatant (comprising the soluble fraction) was removed. Following the addition of 70% formic acid to the pellet, the samples were sonicated and centrifuged at 100,000 × g at 4°C for 1 h, and the supernatant (the insoluble fraction) was removed. Samples were assayed for human Aβ1-40 and Aβ1-42 with commercial ELISA kits (Biosource International, Camarillo, Calif.). Mouse monoclonal antibodies specific for the N terminus of human Aβ (the capture antibody) were coated onto wells in a microtiter plate. Brain samples and Aβ1-40 and Aβ1-42 standards of known concentration were pipetted into the wells, followed by a rabbit anti-mouse antibody specific for the amino acid sequence (amino acids 1 to 40 or 1 to 42) of Aβ (the detection antibody). The bound detection antibody was identified with an alkaline phosphatase-labeled donkey anti-rabbit antibody, producing a fluorescent signal. Comparison with the standard curve allowed the calculation of absolute values, in picomoles per gram of brain tissue.
Preparation of membrane protein.
Samples of freshly dissected brain tissue (between 100 to 150 mg [wet weight]) were placed into 2-ml capacity Lysing Marix D tubes (Q-Biogene, Bedfordshire, United Kingdom) containing 1 ml of ice-cold homogenization buffer (20 mM Tris-HCl, 1 mM EGTA, 1% Triton [pH 7.4]). The homogenization buffer contained a cocktail of protease (10 μg of leupeptin/ml, 1 μg of soya bean trypsin inhibitor/ml, 0.2 mg of bacitracin/ml, 1 mM aminoethylbenzylsulphonyl fluoride [AEBSF], 1 mM benzamidine hydrochloride) and phosphatase inhibitors (1 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 10 mM β-glycerophosphate, and 50 mM sodium fluoride). Lysates were prepared by vigorously shaking the tubes for three bursts of 20 s each with a ribolyser (Hybaid, Cambridge, United Kingdom). Homogenates were centrifuged at 4°C for 10 min at 100 × g to remove debris. Supernatants were recentrifuged for 60 min at 20,000 × g, and the pellets were resuspended in Tris-buffered saline at a final protein concentration of 4 mg/ml. The cytosolic fractions were also retained.
Quantitative Western blotting.
Sagittally dissected brain hemispheres, further dissected into forebrain (including olfactory bulbs, cerebral cortex, and hippocampus) and hindbrain (including pons, cerebellum, and brainstem) were homogenized in a cocktail of proteinase and phosphatase inhibitors containing 20 mM Tris-HCl (pH 7.4), 1 mM ethylene glycol-bis(2-aminoethyl)-N,N,N′,N′,-tetraacetic acid (EGTA), 1% Triton X-100, 1% pyrophosphate, 1% orthovanadate, 1% beta-glycophosphate, 5% NaF, and a protease inhibitor tablet (Roche, Lewes, United Kingdom). The isolated proteins were separated by standard SDS-polyacrylamide gel electrophoresis with 10% polyacrylamide gels unless otherwise stated. Following transfer onto nitrocellulose membrane, detection was made using a panel of primary antibodies to tau and APP: human tau (T14 clone; Zymed); human and mouse tau (BR133; kindly donated by M. Goedert); phospho-tau-S202/T205 (AT8 epitope) and phospho-tau-T231 (AT180 epitope) (Innogenetics); phospho-tau-S199, phospho-tau-S262, and phospho-tau-S422 (Biosource International); and human and mouse APP (C terminus) (Sigma). Blots were also probed with antibodies to the phosphorylated and/or total forms of ERK1/2, GSK-3αβ, p38, JNK, and p35. All antibodies were from Cell Signaling Technologies, with the exception of total ERK1/2 and p35 (Santa Cruz), GSK-3β (Transduction Laboratories), and phospho-GSK-3αβ-Y216/279 (Sigma). Blots were probed with peroxidase-labeled secondary antibodies (Amersham) and visualized with ECL-Plus (Amersham). The Western blots shown are a representative of at least three separate experiments and were quantified by detecting emitted chemiluminescence with the GeneGnome detection system (Syngene Bio Imaging). All data were normalized to the amount of β-actin staining and were expressed as means ± standard errors of the mean (SEM).
Behavioral testing.
Mice were tested on a rotarod apparatus to assess motor coordination and balance (Rotarod 7650 accelerating model; Ugo Basile, Biological Research Apparatus, Varese, Italy) (5). The mice received two training sessions of four trials at 10 to 15 rpm, sufficient to reach a baseline level of performance. The following day, mice were tested at six speeds ranging from 10 to 44 rpm. During training and test sessions, the latency to fall at each speed was recorded up to a maximum of 60 s. Locomotor activity testing took place in clear Perspex boxes containing two transverse infrared beams, spaced equally along the length of the box. The number of beam breaks during consecutive 120-min sessions over 3 days was recorded by a computer as described previously (24).
Olfactory memory functioning was assessed using the social transmission of food preferences (STFP) task (44). Assessment took place over a 7-day period during which normal home cage food was restricted. Demonstrator animals (120 days old; 129sv*C57BL/6J) were housed in the same holding rooms as the test mice and were maintained on the same food-restricted regime. In keeping with the Home Office project license conditions, no animal was permitted to lose more than 20% of free-feeding body weight. Any mouse exceeding this criterion was removed from the experiment and returned to ad libitum feeding. Two habituation sessions were undertaken on consecutive days in which the mice were permitted to freely explore the test arena for 30 min and a single food dispenser filled with 2 g of powdered standard laboratory chow was placed in the arena. A test of basic olfaction was undertaken prior to the STFP test, during which two food dispensers were placed into the test arena. One dispenser contained powdered standard laboratory chow, and the other contained powdered chow mixed with ginger (2%) or coriander (1%). Equal amounts of each food type were put into the dispensers, and the mice were allowed 30 min of free exploration. During the social interaction phase, demonstrator mice were given access to powdered chow mixed with one of four aromas (1% cocoa, 1% coffee, 1% nutmeg, and 0.1% cumin) for 2 h (the cued food). Food consumption was monitored, and demonstrator mice were only used in the ensuing social interaction if they had consumed more than 0.4 g of cued food. For the social interaction, test mice were placed in the arena with two demonstrator mice (sedated with 15 mg sodium of pentobarbitone-Sagatal/kg of body weight intraperitoneally), and the mice were left to interact for 5 min. In the 24-h memory test, the test mice were presented with a choice between two food dispensers, one containing powdered food mixed with the same aroma used in the social interaction (the cued food) and the other mixed with a novel aroma (uncued food). Following the 30 min of exploration and food consumption, the mice were returned to ad libitum food in the home cage. From the social interaction session, the number, latency, and duration of contacts with the demonstrator's mouth region was calculated. In sessions where food was present, the amount of food consumed was calculated, and the plain food (olfaction test) and cued food preferences (memory tests) were calculated from the total food consumed.
Statistics.
Quantitative data from the Aβ ELISA were analyzed by four-way analysis of variance (ANOVA) with the following factors: genotype (wild type, APPV717I-CT100, and combined tauV337M × APPV717I-CT100), age (6 and 12 months), species (Aβ1-40 and Aβ1-42), and fraction (soluble and insoluble). Quantitative data from the GeneGnome detection system assessing the level of mouse APP were analyzed by one-way ANOVA with the genotype factor (wild type, tauV337M, APPV717I-CT100, and tauV337M × APPV717I-CT100). Data examining levels of human tauV337M transgene and the tau phosphorylation epitopes in the tauV337M and combined tauV337M × APPV717I-CT100 mice were analyzed separately by Student's t test. This discrete manner of analysis was required because of potential differences in the affinities of the individual antibodies used to recognize the various tau phosphorylation sites. Similarly, the quantitative data examining the extent of endogenous tau kinase phosphorylation occurring in all four lines of mice were analyzed in terms of each separate tau kinase epitope. These data were compared using one-way ANOVA with the genotype factor (wild type, tauV337M, APPV717I-CT100, and tauV337M × APPV717I-CT100). Note that the age factor (6 and 12 months) was not used as a comparison in the Western analysis because tissue from these age groups was assessed separately of necessity (see above). Rotarod and locomotor activity data, assessed for mice that were 12 months old, were analyzed by separate two-way ANOVA with the following factors: speed (10, 15, 17.1, 21.8, 24, 26.1, and 44 rpm) and genotype (wild type, tauV337M, APPV717I-CT100, and tauV337M × APPV717I-CT100), and session (days 1, 2, or 3) and genotype (wild type, tauV337M, APPV717I-CT100, and tauV337M × APPV717I-CT100), respectively. The data from the STFP test of olfaction were analyzed by two-way ANOVA with the genotype (wild type, tauV337M, APPV717I-CT100, and combined tauV337M × APPV717I-CT100) and age (6 and 12 months) factors. The data quantifying the time spent in close proximity to the demonstrator's mouth regions during the STFP social interaction session was analyzed by two-way ANOVA with the genotype and age factors. The preference for the cued food and total amount of food consumed were assessed separately in the test of olfactory memory by two-way ANOVA with genotype and age factors. All data were analyzed with the SAS statistical package (SAS Institute, Inc., Cary, N.C.).
RESULTS
Characterization of the transgenic mouse lines.
Expression of both tauV337M and APPV717I-CT100 transgenes was under the control of the brain-specific modified mouse Thy-1 promoter (51). Following initial reverse transcription-PCR and Western blot analysis to confirm that expression of the transgenes had been successful and was limited to brain tissue, hemizygotes from the tauV337M and APPV717I-CT100 lines were cross-bred to produce wild type, tauV337M hemizygotes, APPV717I-CT100 hemizygotes, and tauV337M × APPV717I-CT100 combined hemizygotes, according to Mendelian ratios. In situ hybridization data obtained with sagittal brain sections indicated a degree of similarity in terms of expression patterns of the human tauV337M and human APPV717I-CT100 transgenes (Fig. 1). In particular, high levels of expression of both transgenes were found throughout the cortex, hippocampus, and a number of hindbrain regions including the pons. By contrast, very little expression was evident in the striatum. Qualitative differences in transgene expression were observed in cerebellum, with substantial levels of APPV717I-CT100 and minimal levels of tauV337M. Importantly, no evidence of interactions between the transgenes was identified in terms of gross differences in levels or localization when the combined line was compared to either of the single transgenics. Furthermore, exogenous transgene expression had no effects on the levels of endogenous (i.e., murine) transcripts for APP and tau. Immunohistochemical assessment of the transgene protein products revealed that the spatial distribution of APP-CT100 in both the APPV717I-CT100 and combined mice was similar and widespread throughout the brain (Fig. 2A and B). Immunostaining for APP-CT100 (Fig. 2A) was most intense in the cortex and hippocampus, whereas very little staining was visible in the striatum. In the tauV337M hemizygote and combined tauV337M × APPV717I-CT100 mice, immunostaining for the human tau mutated protein was especially evident in neurones of the cortex, hippocampus, pons, and brainstem (Fig. 2B).
FIG. 1.
Spatial distribution of APPV717I-CT100 and tauV337M transgene mRNA. Sagittal brain sections (each, 12 μm) from wild-type (WT), APPV717I-CT100 (CT100), tauV337M (Tau), and tauV337M× APPV717I-CT100 (COM) mice were analyzed by in situ hybridization with radiolabeled probes specific to human APP-CT100 (A), mouse APP (B), human tau (C), and mouse tau (D) mRNA. The data are representative of four independent samples per group.
FIG. 2.
Spatial distribution of APPV717I-CT100 and tauV337M transgene protein products. Free-floating sagittal sections (each, 30 μm; 6-month-old mice) were immunohistochemically stained with an anti-APP (C-terminal) antibody (A) or human-specific anti-tau T14 antibody (B). The sections shown from the various brain areas (as labeled) are from APPV717I-CT100 (A) or tauV337M (B) mice. The level of staining did not differ between APPV717I-CT100 or tauV337M and the combined mice (not shown). A section from the prefrontal cortex (wild-type littermate) is representative of the level of staining throughout wild type littermate brains. Scale bar, 100 μm. The data are representative of four independent samples per group. (C) The levels of exogenous human APP-CT100 and tau protein relative to mouse APP and tau were assessed by Western blotting. Protein from forebrain tissue extracts of wild-type (WT) mice and transgenic mice expressing human APPV717I-CT100 alone (CT100), human tauV337M alone (Tau), or both transgenes (COM) was separated on a 15% polyacrylamide gel and transferred onto nitrocellulose. The blot was probed with an antibody which recognizes both mouse and human APP within the C terminus. The running position of molecular mass marker proteins is shown (left panel). To establish that the CT100 fragment was localized to the plasma membrane, samples of cytosolic and membrane proteins were analyzed by SDS-15% polyacrylamide gel electrophoresis. Either β-actin or Gαo was used to assess protein loading, and detection was made with an anti-APP (C-terminal) antibody. Whole-tissue extracts were also analyzed with antibodies to β-actin to show consistency of protein loading and antibodies which recognize either human tau or both human and mouse tau (BR133) (center panel). Quantification of the levels of human and mouse tau was undertaken with the GeneGnome detection system (right panel).
Western analysis of mouse brain protein using an antibody that cross-reacts with human and mouse APP identified a strong immunoreactive product of approximately 85 kDa, consistent with the predicted size of mouse APP, which was uniform in intensity between wild type and transgenic mice (Fig. 2C). Quantification of the chemiluminescence using the GeneGnome detection system revealed no effect of genotype on the level of mouse APP protein. The mean values as a percentage of wild type, where the value of the wild type was set at 100%, were as follows. For forebrain, the values were wild type, 100% ± 9.14%; APPV717I-CT100, 101.84% ± 0.79%; tauV337M, 96.65% ± 23.18%; tauV337M× APPV717I-CT100, 86.38% ± 11.05%; F3,11, 0.2396 (not significant [n.s.]). For the hindbrain, the values were wild type, 100% ± 8.86%; APPV717I-CT100, 126% ± 28.10%; tauV337M, 113% ± 28.26%; tauV337M× APPV717I-CT100, 138% ± 28.36%; F3,11, 0.44 (n.s.). Immunoreactivity with a molecular weight expected for the APP-CT100 fragment could only be detected in the APPV717I-CT100 and combined tauV337M × APPV717I-CT100 mice (Fig. 2C). Quantification of the chemiluminescence detected was difficult due to the weak nature of the signal obtained for the APP-CT100 fragment. However, visual inspection suggested that human APP-CT100 transgene protein expression was approximately 20% of that of mouse APP in both APPV717I-CT100 and combined tauV337M × APPV717I-CT100 transgenic mice. The APP-CT100 fragment could not be detected in a cytosolic extract of brain tissue but was copurified with other membrane-associated proteins (Fig. 2C). When the phosphorylation-independent T14 antibody, which is selective for human tau, was used, immunoreactivity of the correct molecular mass (55 to 60 kDa) could only be detected in the tauV337M and combined tauV337M × APPV717I-CT100 mice (Fig. 2C). Quantification of the chemiluminescence detected using the phosphorylation-independent BR133 antibody, which recognized both mouse and human tau, showed that twice as much human tau was expressed in the tauV337M and combined transgenic animals as compared to the endogenous levels of mouse tau (Fig. 2C). There was no significant difference in the level of human tau protein between the tauV337M and combined tauV337M × APPV717I-CT100 mice, and the level of mouse tau protein was unaffected by transgene expression.
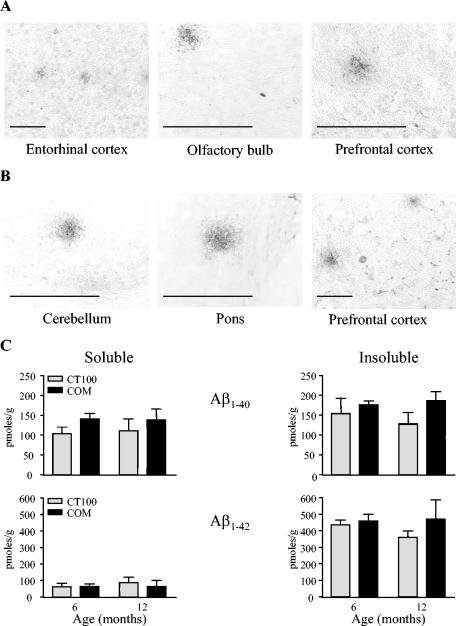
Expression of the APPV717I-CT100 transgene led to the production of Aβ and the deposition of diffuse, noncongophilic extracellular Aβ plaques from 6 months of age in both the APPV717I-CT100 (Fig. 3A) and combined tauV337M × APPV717I-CT100 (Fig. 3B) mice. Immunohistochemistry using Aβ-specific antibodies revealed a widespread distribution of accumulations in the brain parenchyme and cerebral blood vessels. Staining for Aβ was most intense in the cortex and hippocampal regions but was also found in other brain regions, including the olfactory bulbs and hindbrain. The data from a quantitative ELISA assay are shown in Fig. 3C. Reflecting the specificity of the antibodies used in the ELISA to human Aβ species, very low levels were identified in brain tissue taken from wild-type mice; as a result, the data obtained in APPV717I-CT100 and combined tauV337M × APPV717I-CT100 tissues were normalized to that of the wild type. Both human Aβ1-40 and Aβ1-42 species were identified within the brains of APPV717I-CT100 and combined tauV337M × APPV717I-CT100 mice from 6 months of age onward. No effect of genotype (F1,48 = 2.21 [n.s.]) on levels of either species of Aβ was identified in the soluble and insoluble brain fractions, indicating no effect of the tauV337M transgene on levels of Aβ. In addition, no main effect of age (F1,48 = 0.11 [n.s.]) or interaction between genotype and age was identified (F1,48 = 0.28 [n.s.]) in terms of levels of Aβ. A comparison of the particular Aβ species present in the brain samples revealed that significantly more Aβ1-42 than Aβ1-40 was present in both soluble and insoluble brain fractions from the APPV717I-CT100 and combined tauV337M × APPV717I-CT100 mice (effect of species; F1,48 = 23.06, P < 0.0001). In addition, there was an effect or fraction, representing the fact that significantly more Aβ of both species was found in the insoluble fraction (F1,48 = 75.37; P < 0.0001); an interaction between species and fraction was also identified, relating to the high levels of Aβ1-42 found to be present in the insoluble brain fraction (F1,48 = 51.19; P < 0.0001), which formed the major species of Aβ found in the brains of the APPV717I-CT100 and combined tauV337M × APPV717I-CT100 mice.
FIG. 3.
Amyloid-β plaque formation. Free-floating sagittal sections (each, 30 μm; 6-month-old mice) were immunohistochemically stained with human-specific anti-Aβ antibody (amino acids 1 to 17). Staining (both intracellular and extracellular) was widespread across the brain. The sections shown are from APPV717I-CT100 (A) and combined (B) mice, in which diffuse non-congophilic plaques are clearly visible. No plaques were visible in the brains of littermate wild-type mice (not shown). Scale bar, 100 μm. The immunohistochemical data are representative of four independent samples per group. The levels of human Aβ1-40 and Aβ1-42 present in SDS-soluble (soluble) and formic acid-extracted (insoluble) brain fractions from APPV717I-CT100 (grey bars) and combined (black bars) mice aged 6 and 12 months were analyzed by ELISA (C). Background wild-type levels were subtracted from each sample group and consisted of less than 10% of the total value obtained in each instance. The data are expressed as picomoles per gram of frozen brain sample weight and are the means ± SEM for four independent samples per group. Note the change of scale between the Aβ1-40 and Aβ1-42 ordinates.
Staining for phosphorylated tau in sections taken from 6-month-old tauV337M and combined tauV337M × APPV717I-CT100 mice, using the AT8 (S202/T205) phospho-specific antibody, provided evidence of widespread phosphorylation of tau protein (Fig. 4). The spatial staining patterns in tauV337M (Fig. 4A) and combined tauV337M× APPV717I-CT100 mice (Fig. 4B) were very similar, with the highest levels of immunoreactivity present in the cortex, hippocampus, accessory olfactory bulb, and hindbrain. Silver staining for the presence of neurofibrillary tangles in 12-month-old tauV337M and combined tauV337M × APPV717I-CT100 mice by the Bielschowsky method was localized in the brainstem, with staining identified in the pons and medulla (Fig. 4C and D). No specific staining could be identified in hippocampal or cortical regions of the brain.
FIG. 4.
Phospho-tau (AT8; S202/T205) and Bielschowsky staining. Free-floating sagittal sections (each, 30 μm; 6-month-old mice) were immunohistochemically stained with the AT8 anti-phospho S202/T205 antibody for phosphorylated tau. Intracellular neuronal staining was widespread across the brains of tauV337M (A) and combined (B) mice, but was particularly localized in the regions shown. Bielschowsky silver staining revealed the presence of neurofibrillary tangles in the brainstem of 12-month-old combined (C) and tauV337M (D) mice but not in the hippocampus. No staining was seen in the brains of littermate wild-type mice by either AT8 immunohistochemistry or Bielschowsky staining (E). Scale bar, 100 μm. The data are representative of four independent samples per group.
Site-specific phosphorylation of tau.
The extent and nature of human tau phosphorylation in the tauV337M and combined mice were examined in protein extracts of fore- and hindbrain by using quantitative Western blotting and a panel of tau phosphorylation-specific antibodies selective for discrete sites known to be phosphorylated in FTDP-17 and AD. A total of 25 potential sites for phosphorylation (40) have been identified to date in human tau, many of which are serine or threonine residues that are immediately followed in sequence by proline. Several proline-directed kinases, including GSK-3αβ, cdk5/p25, ERK1, ERK2, p38, and JNK, phosphorylate tau at these sites in cell-free systems, although with different efficiencies (2). Members of the MAPK family (ERK1, ERK2, p38, and JNK) show essentially equivalent substrate specificities towards tau and can phosphorylate S422 (40) as well as S202 and T205, the epitope of the AT8 monoclonal antibody, which can also be phosphorylated by GSK-3αβ (15). In contrast, S199 has been shown to be an effective site for phosphorylation by GSK-3αβ and cdk5 but does not appear to be a selective substrate for the MAPKs (25, 40). Phosphorylation of T231 can be brought about by both MAPKs and GSK-3αβ (40); phosphorylation of this site in tau, as well as S262 (which is not a suitable substrate for either enzyme family), is required for maximal inhibition of its binding to microtubules (41).
Analysis of equivalent amounts of protein from the wild-type and transgenic mice with a phosphorylation-independent antibody specific to human tau confirmed that the level of human tau protein translation in forebrain and hindbrain samples was similar in both the tauV337M and combined mice at 6 or 12 months of age (Fig. 5A and B). In contrast, the extent of human tau phosphorylation varied substantially and was dependent on genotype and age. At 6 months of age (Fig. 5A), phosphorylation at the S202/T205 (AT8) and T231 (AT180) epitopes was detected in both the tauV337M and combined tauV337M × APPV717I-CT100 mice. Phosphorylation of endogenous mouse tau was not observed in APPV717I-CT100 mice with these antibodies. Further examination of the blots showed that phosphorylation at both epitopes and in both fore- and hindbrain samples was markedly elevated in the combined tauV337M × APPV717I-CT100 transgenic mice in comparison to the single tauV337M mice. Subsequent quantification of the phosphorylation at the S202/T205 site using the GeneGnome detection system (Fig. 5C) confirmed a significant (approximately twofold) difference between the combined and single tauV337M transgenic mice. Quantification of the T231 epitope was not possible, due to low absolute levels of signal. A similar pattern of phosphorylation at the S202/T205 and T231 sites was seen with mice at 12 months of age in that the blot data (Fig. 5B) indicated augmented phosphorylation at these epitopes in the combined mouse compared to the tauV337M genotype. Again, the signal from the T231 epitope was not sufficient for accurate quantification by the GeneGnome detection system, but the S202/T205 data showed significant increases in the combined mouse compared to the tauV337M genotype (Fig. 5D). Phosphorylation at S422, an additional site phosphorylated by the MAPKs in AD and FTDP-17 brain (20), was barely detectable in mice examined at 6 months of age, but by 12 months of age marked immunoreactivity was present in tauV337M and combined tauV337M × APPV717I-CT100 mice (Fig. 5B). As with the S202/T205 and T231 sites, quantification of the blots showed that this change was more evident in the combined mice than in the tauV337M mice in both fore- and hindbrain samples (Fig. 5D). In marked contrast, phosphorylation at the GSK-3αβ- and cdk5-sensitive site, S199, was completely indifferent to either age or genotype. Instead, phosphorylation at this site was detected at 6 and 12 months of age and no difference in phosphorylation levels was identified between the combined tauV337M × APPV717I-CT100 and single tauV337M mice (Fig. 5A and B). Quantification of the chemiluminescence confirmed this finding. For 6-month-old forebrain, data were as follows: tauV337M, 24% ± 2.1%; tauV337M× APPV717I-CT100, 23.9% ± 1.2%. For 6-month-old hindbrain, data were as follows: tauV337M, 22.9% ± 1.4%; tauV337M× APPV717I-CT100, 23% ± 2.8%. For 12-month-old forebrain, data were as follows: tauV337M, 27% ± 5.5%; tauV337M× APPV717I-CT100, 26% ± 1.9%. For 12-month-old hindbrain, data were as follows: tauV337M, 25% ± 0.5%; tauV337M× APPV717I-CT100, 25% ± 1.4%. Furthermore, the immunoreactivity observed was common to both human and endogenous mouse tau. Phosphorylation at S262 (a site not phosphorylated by either the MAPKs or GSK-3αβ) (3, 14) was characterized by very low levels of immunoreactivity at both 6 and 12 months of age that was common to the endogenous mouse tau across all genotypes but was effectively absent in human tau in the combined tauV337M × APPV717I-CT100 and tauV337M mice. This negative finding was of note, as phosphorylation of tau at this non-proline-directed kinase epitope is a key pathological marker that has been shown to have the effect of strongly reducing the binding of tau to microtubules (3) and that forms a major component of paired helical filament tau extracted from AD brain (21). Failure to see changes at the S262 site may indicate a limitation of the models; alternatively, such changes may become manifest at later time points. Taken together, the data indicated highly selective effects of the human tauV337M mutation and an augmentation of tau phosphorylation at the same sites in the combined tauV337M× APPV717I-CT100 mouse, the latter indicating an effect of the mutated carboxy-terminal fragment of human APP (APPV717I-CT100) on tau phosphorylation.
FIG. 5.
Phosphorylation of human tau. Lysates extracted from the forebrain and hindbrain of wild-type mice (WT) and transgenic mice expressing human APPV717I-CT100 alone (CT100), human tauV337M alone (Tau), or both transgenes (COM) were analyzed by Western blotting at 6 (A) and 12 (B) months of age. The extent of phosphorylation was determined with a panel of phospho-dependent antibodies specific to particular epitopes. In all cases, equivalent amounts of protein were assessed, and the chemiluminescence emitted from identically sized areas on the nitrocellulose membrane was quantified (C and D) with the GeneGnome imaging system following normalization to the amount of β-actin in each sample. Note that a distinction between the phosphorylation of human mutated tauV337M (h) and endogenous mouse (m) tau at S199 could be made as a result of the retarded electrophoretic mobility of the human form. (C and D) For the quantitative data shown, mean values ± SEM for three independent samples per group for the relevant transgenic lines were expressed as percentages of the mean control WT value, which was set at 0%. Note the change of scale between forebrain and hindbrain ordinates. As noted in Materials and Methods, the data from individual antibodies were analyzed separately, due to potential differences in antibody affinities. The key statistical comparisons showing increased levels of phosphorylation at MAPK consensus sites in the combined tauV337M × APPV717I-CT100 line are shown by the joined or dotted lines, where *** indicates COM values statistically significant from those obtained for Tau (P < 0.001) and * indicates COM values statistically significant from those obtained for Tau (P < 0.05), by Student's t test.
Changes in kinase activity.
To examine if a concomitant increase in the activity status of endogenous MAPKs could be observed in those transgenic models where increases in tau phosphorylation had been detected, the activities of the major proline-directed kinases known to phosphorylate tau were assessed by Western blot analysis. As activity of the MAPK family and GSK-3αβ is controlled by their phosphorylation at specific sites (8, 10), phospho-specific antibodies were again used to assay the activity status of ERK1/2, p38, JNK, and GSK-3αβ in 6- and 12-month-old animals. The levels and phosphorylation status of the MAPKs are shown in Fig. 6. While the absolute levels of the kinases were equivalent across all mouse models, the pattern of kinase phosphorylation was dependent on genotype and age, as was found to be the case with tau phosphorylation. The tauV337M mutation alone had no effect on the phosphorylation status of any of the MAPKs, revealing dissociations between the impact of this manipulation on tau phosphorylation and cognate tau kinase function. In contrast, the combined tauV337M × APPV717I-CT100 mice did show activations of the MAPKs, but in an age-related manner. Thus, at 6 months of age (Fig. 6A), there were detectable increases in the phosphorylation and hence activity status of ERK1/2 and p38. At 12 months of age (Fig. 6B), ERK1/2 phosphorylation had returned to wild-type baseline levels, but JNK phosphorylation was now elevated and p38 phosphorylation levels remained high. The increased activation status of MAPKs in the combined tauV337M × APPV717I-CT100 mice appeared to be a discrete effect of APPV717I-CT100 transgene expression, as near-identical changes in MAPK phosphorylation were observed in the single APPV717I-CT100 transgenic mouse line. Thus, statistical quantification of the phosphorylation of ERK1/2 at 6 months (Fig. 6C) showed the approximately 50% increase in the APPV717I-CT100 and combined mice to be closely comparable. Similarly, at 12 months the levels of JNK phosphorylation were not significantly different between the APPV717I-CT100 and combined tauV337M × APPV717I-CT100 mice but were approximately 2.5-fold higher than levels for wild-type littermates (note that quantification of p38 chemiluminescence by the GeneGnome detection system was not possible due to low signals). There were, in addition, no obvious distinctions between the qualitative changes observed in the kinase activity status across the transgenic mice lines for samples taken from forebrain and hindbrain. Consistent with the previous findings indicating no change in tau phosphorylation at the S199 site, the activity status of GSK-3αβ and/or cdk5 was indifferent to either genotype or age (Fig. 7). GSK-3 consists of α and β isoforms, which are critical downstream elements of the PI3K/Akt cell survival pathway (8). Their activities are inhibited by Akt-mediated phosphorylation at S21 of GSK-3α and S9 of GSK-3β. It has also been reported that phosphorylation at Y279/216 of GSK-3αβ can enhance the activity status of these kinases once the inhibition by dephosphorylation at S21/9 is alleviated (19). However, as illustrated in Fig. 7, no change in the phosphorylation status of either site for GSK-3αβ could be detected across the transgenic models at 6 and 12 months of age. To monitor the activity of cdk5, the relative ratio of p35 to p25 in brain samples was determined (30). An increase in the activity of cdk5 (as would be indicated by the loss of p35 expression with a concomitant increase in p25 levels) was not observed for any of the mouse lines at either 6 or 12 months of age.
FIG. 6.
Changes in the activity of MAPK family members. Lysates extracted from the forebrain and hindbrain of wild-type mice (WT) and transgenic mice expressing human APPV717I-CT100 alone (CT100), human tauV337M alone (Tau) or both transgenes (COM) were analyzed by Western blotting at 6 (A) and 12 (B) months of age with phospho-specific (-P) and hence activity-dependent antibodies to members of the MAPK family. Quantification using the GeneGnome imaging system was normalized to the amount of β-actin in each forebrain and hindbrain sample and is shown for the total levels (open bars) and phosphorylated forms (hatched bars) of ERK1/2 and of the JNKs at 6 (C) and 12 (D) months of age. Values are expressed as percentages of the mean WT value ± SEM for three independent samples per group (the mean WT values was set as 100%). Comparison of kinase activities by ANOVA showed a significant main effect of genotype on ERK1/2 activity in mice at 6 months of age in both forebrain (F3,11 = 80.60; P < 0.0001) and hindbrain (F3,11 = 13.69; P < 0.01). Post hoc analysis using Tukey's test showed that this was due to increased activity specifically in the APPV717I-CT100 and combined mice. Further analysis indicated no difference between the activation levels of ERK1/2 in these two lines in either fore- or hindbrain samples (joined lines). At 12 months of age, comparison by ANOVA of kinase activities showed a significant main effect of genotype on JNK activity in both forebrain (F3,11 = 28.71, P < 0.0001) and hindbrain (F3,11 = 30.23, P < 0.0001), which post hoc analysis using Tukey's test again showed to be due to increased activity specifically in the APPV717I-CT100 and combined mice. As with ERK1/2, further analysis indicated no difference between the activation levels of JNK in these two lines in either fore- or hindbrain samples (joined lines).
FIG. 7.
GSK-3αβ and cdk5 activities are unaffected in transgenic mice expressing human mutant APPV717I-CT100 and/or tauV337M. Protein lysates extracted from the forebrain and hindbrain of wild-type mice (WT) and transgenic mice expressing human APPV717I-CT100 alone (CT100), human tauV337M alone (Tau), or both transgenes (COM) at 6 (A) and 12 (B) months of age were analyzed by Western blotting. GSK-3β expression remained unchanged between samples from all mouse models, as did the phosphorylation status of GSK-3αβ at S21/9 (GSK-PS) and Y279/216 (GSK-PY). The antibody to the total form of GSK-3 only recognizes the β isoform. The ratio of p25 to p35 also remained consistent, suggesting that the activity of cdk5 was unaffected by transgene expression. Consistency of protein loading was substantiated by the levels of β-actin (not shown). The chemiluminescence emitted for GSK-3αβ was quantified using the GeneGnome imaging system and normalized to the amount of β-actin in each sample. Values for forebrain and hindbrain samples at 6 (C) and 12 (D) months are expressed as a percentage of the mean WT value ± SEM for three independent samples per group. The WT value was set at 100%. Quantification is shown for the total levels of GSK-3β (open bars) and for GSK-3αβ forms phosphorylated at S21/9 (hatched bars) and Y216/279 (grey bars).
Motoric function of transgenic mice.
In previous work examining tau FTDP-17 mutations in mice (using the P301L mutation driven by the mouse prion promoter), an association between transgene expression and profound motor disturbances leading to hindlimb paralysis was observed, with symptoms emerging from as early as 4.5 months of age (32). Assessments carried out using the rotarod and locomotor activity apparatus indicated no such motoric deficits in the tauV337M and combined tauV337M × APPV717I-CT100 mice when measured at 3, 6, and 12 months. The 12-month data for the rotarod and locomotor activity performance is shown in Fig. 8A and B. For the rotarod data, there was a main effect of speed (F6,196 = 30.42; P < 0.0001) but no main effects or interactions involving genotype (F3,196 = 1.64 [n.s.] and F18,196 = 0.83 [n.s.], respectively). For the locomotor activity data, there were no main effects or interactions involving session (F2,84 = 1.51 [n.s.]) or genotype (F3,84 = 1.18 [n.s.]). There were also negative data for several other behavioral tests including the SHIRPA protocol, novelty place preference, and prepulse inhibition of acoustic startle (data not shown). Additionally, the general health of all the mouse lines was good, and there was no evidence of any departure from Mendelian ratios indicative of transgene effects on prenatal and/or perinatal lethality.
FIG. 8.
Assessment of motor coordination and locomotor activity. Latency to fall from rotarod apparatus (A) and infrared beam breaks made in locomotor activity cages (B) in 12-month-old mice. The rotarod data were from the test day following 2 training days, as detailed in Materials and Methods. Data are the mean values for each mouse line (± SEM) for eight animals per group. Memory functioning in 6- and 12-month-old mice was assessed with the STFP olfactory memory paradigm. To assess basic olfactory functioning, mice were presented with a choice between familiar plain food and the same food mixed with a novel odor, and the percentage of mice with a preference for the familiar plain food was calculated (C). In the social interaction session, test mice were able to interact with sedated demonstrator mice that had consumed powdered food mixed with one of four possible odors (cued food) less than 1 h previously. The data show the time spent by the observer mice in close proximity to the mouths of the demonstrator mice, which has been shown to be critical to the learning process (D). At 24 h following the social interaction, the test mice were presented with a choice between the cued food eaten by the demonstrator mice and a novel food with an odor not previously encountered (uncued food). The data show the total amount of food consumed (E) and the percentage of mice with a preference for the cued food (F). The data are the means ± SEM for 12 animals per group.
Cognitive deficits in transgenic mice.
To assess the impact of the molecular changes identified on cognition, memory functioning was assayed using the STFP paradigm, an olfactory memory task that uses conspecifics as a source of information to guide mice in their food choice. Olfactory-based behaviors can be confounded by general anosmia; therefore, the task incorporated a test of basic olfactory discrimination in addition to the olfactory memory component of the task (Fig. 8C). Mice were presented with a choice between familiar plain powdered chow and powdered chow with a novel aroma. The data demonstrates a strong preference for the familiar food that was unaffected by either genotype (F3,88 = 0.09 [n.s.]) or age (F1,88 = 0.137 [n.s.]). In addition, there were no effects of genotype or age on the total amount of food consumed during the task (data not shown). These data were consistent with the typical food neophobia response in rodents and suggested the presence of intact olfactory functioning in all experimental groups. Wild-type male demonstrator mice that had also undergone food restriction were fed the cued powdered chow, which had one of four possible odors (cocoa, coffee, nutmeg, and cumin), for 2 h, then sedated, and placed in an arena containing the test mice for 5 min. The behavior of the test mice during the ensuing social interaction was monitored by video. The time spent by the test mice in close contact with the mouth of the demonstrator has been shown by previous work to be a key factor in the learning process. Analysis revealed no effects or interactions involving genotype (F3,88 = 0.13 [n.s.]) or age (F1,88 = 0.005 [n.s.]) on the amount of time spent by the test mice in contact with the mouth of the demonstrator mice (Fig. 8D). Twenty-four hours after the demonstrator-observer social interaction, the test mice underwent a discrimination task to examine the extent to which food preference was determined by the memory of the cued food encountered on the breath of the demonstrator mice. Mice were presented with a choice between equal quantities of the cued food and a novel scented food, neither of which had been previously experienced by the mouse. While 6-month-old mice of all genotypes exhibited a robust and continuing preference for the cued food in the memory test, at 12 months of age the tauV337M, APPV717I-CT100, and combined tauV337M × APPV717I-CT100 groups failed to demonstrate a food preference (Fig. 8F). This pattern of data was reflected by a significant main effect of genotype (F3,88 = 4.17; P < 0.01) and age (F1,88 = 7.02; P < 0.001). In addition, the effects of genotype and age on cued food preference were not associated with concomitant changes in total food consumption (Fig. 8E) (main effect of genotype, F3,88 = 0.32 [n.s.]; effect of age, F1,88 = 2.39 [n.s.]). Taken together, the data obtained in the STFP task suggest specific age-related effects of the tauV337M and APPV717I-CT100 transgenes on olfactory memory function, leading to deficits in this aspect of cognitive functioning in the tauV337M, APPV717I-CT100, and combined tauV337M × APPV717I-CT100 mice.
DISCUSSION
We have utilized novel transgenic mouse models for FTDP-17 and AD to examine differential mechanisms of tau phosphorylation and putative molecular links between abnormal tau and APP C-terminal fragment processing. Analyses of the tauV337M mouse by immunohistochemistry revealed evidence of accumulations of phosphorylated tau, and Bielschowsky silver staining indicated the presence of neurofibrillary tangles in specific brain regions. Expression of the APPV717I-CT100 transgene was associated with deposition of Aβ in the form of diffuse, noncongophilic extracellular plaques from 6 months of age. As in humans bearing the APPV717I mutation (36), a preponderance of the Aβ1-42 isoform was identified. The combined tauV337M × APPV717I-CT100 mice showed very similar spatial patterns of Aβ burden, extracellular plaque formation, and phospho-tau and neurofibrillary tangle formation when compared to the single transgenic strains. Additionally, neither the tauV337M nor the combined tauV337M × APPV717I-CT100 mice showed evidence of motoric impairment or sickness up to 12 months of age. This is in marked contrast to mice expressing other FTDP-17 mutations, which begin to show profound motor disturbances from 4 to 5 months of age onward (32). The reason(s) for the absence of motoric deficits is at present unclear (though it is likely to be related to the nature of the specific tau mutation and/or promoter construct in determining the extent of hindbrain damage) (1, 32, 49), but this outcome has obvious advantages for the present work, where we were able for the first time to examine progressive, age-related effects on cognitive function in these models.
To compare the nature and extent of tau phosphorylation in the transgenic lines, we utilized a quantitative Western blotting approach using phospho-specific antibodies, which enabled a parallel analysis of tau kinase activity status and the degree of tau phosphorylation at consensus sites. Our data provide evidence for the existence of distinct mechanisms mediating the abnormal phosphorylation of tau in FTDP-17 and AD. In the first situation, associated with the FTDP-17 tauV337M mutation, human tau phosphorylation occurred at MAPK consensus sites in the absence of concomitant change in kinase activity status. In the second situation, related to the presence of human APPV717I-CT100, augmented tau phosphorylation at these same sites was identified in combination with specific MAPK activations. The observation of tau phosphorylation in the FTDP-17 model without any accompanying change in tau kinase activities may be explained by a possible effect of the V337M mutation on the conformation of tau. Hence, putative structural modifications within the tau protein may have allowed greater access to an unaltered basal level of MAPK activity and facilitated a hierarchical phosphorylation cascade (26).
The mechanism(s) underlying the phosphorylation of tau in the FTDP-17 tauV337M mouse without concomitant changes in kinase activity can be contrasted with the influence of the APPV717I-CT100 fragment on the phosphorylation status of the MAPKs. Here, as evidenced by data from the combined tauV337M× APPV717I-CT100 mice, the hyperphosphorylation of human tau at MAPK consensus sites was accompanied by activations of members of the MAPK family, specifically, elevations of ERK, p38, and JNK phosphorylation at 6 and 12 months of age. As this pattern of MAPK activations was mirrored precisely in the single APPV717I-CT100 mice, we conclude that the expression of APPV717I-CT100 or a subsequently produced fragment of the APP-CT100 transgene was responsible for the MAPK activation. The exact identity of the APP C-terminal fragment(s) stimulating MAPK activity remains unknown. Many C-terminal fragments of APP have been shown to be neurotoxic (28, 45), including Aβ and APP-CT100, both of which are present in our mouse model. Gotz and colleagues showed increases in neurofibrillary tangles following injections of Aβ fibrils into the brain of P301L mice (18). However, in our work, while the pattern of tau phosphorylation in the tauV337M × APPV717I-CT100 mice was age related, the levels of brain Aβ remained relatively constant over age, suggesting in turn that Aβ may not be the only APP C-terminal fragment capable of influencing tau phosphorylation.
The data also suggest that members of the MAPK family may have different contributions to play in tau phosphorylation over time. In our model, ERK activation status increased at early stages and subsequently declined in accord with data obtained using the Tg2576 Swedish mutant APP transgenic mouse (7). It was of particular note that phosphorylation at tau S422, a marker for tau neuropathology (20) was not detectable until 12 months of age and that this change coincided with an age-dependent increase in JNK activity. These data were especially interesting, not only because they recapitulate the involvement of this tau phosphorylation site in a mouse model but also because the tight temporal relationship suggests the possibility of a specific causal linkage between phosphorylation of tau at S422 and JNK activity occurring in vivo. The extent to which these data bear on the time course of similar molecular changes in AD remains an important question.
Somewhat surprisingly given the previous evidence obtained in cell-free systems and neuronal cell lines, the present studies did not corroborate a role for GSK-3αβ in the hyperphosphorylation of tau. We did detect the presence of tau phosphorylation at S199, which is a consensus site for GSK-3αβ, but this was at levels that were unchanged between transgenic and wild-type animals, suggesting that in this case phosphorylation at S199 was related to the normal physiological regulation of tau. In addition, a lack of change in the activity of GSK-3αβ was identified by assessing its phosphorylation at the regulatory sites S21/9 and Y279/216. Our negative findings are consistent with in vivo data involving the use of the GSK-3αβ inhibitor, lithium, which failed to prevent tau hyperphosphorylation in rabbits injected with aggregated Aβ1-42 (12). Furthermore, it is of significance to the present study that work by colleagues at the Babraham Institute has provided equivalent data, indicating increases in activity of the MAPKs but not GSK3-αβ in studies of postmortem brain tissue from human AD subjects (47). Specifically, an increase in the activity of both p38 and JNKs was observed in both the transgenic mice and postmortem brain tissue. In addition, a similar pattern of tau phosphorylation was also identified. However, it would be premature to exclude a role for GSK-3αβ in the pathological phosphorylation of tau in vivo, since it is possible that it may act via mechanisms that have not been investigated in the present study. In particular, Yuan et al. (56) have demonstrated that Ser9-phosphorylated GSK-3β can phosphorylate tau by forming a complex with the phosphoserine binding protein 14-3-3 in transfected cells. It is therefore possible that in our model, tau may also be phosphorylated by GSK-3β via this mechanism. Moreover, GSK-3αβ may feature in AD progression by its involvement in other pathways. For example, a recent report has shown evidence that lithium reduces Aβ production both in cultured cells and in the brains of mice that overproduce Aβ peptides through the inhibition of GSK-3α (37). In addition, lithium has been shown to block the activation of a c-Jun stress response to trophic withdrawal via inhibition of GSK-3αβ (22). A further possibility that our work has not investigated is that the pattern of tau phosphorylation may have involved, in part, changes in the activity of protein phosphatases. In this regard, it is of note that the activities of both PP1 and PP2A have been shown to be decreased in AD brain (17) and that a number of the FTDP-17 mutations, including V337M, induce a decrease in the binding affinity of tau for PP2A in vivo (16).
The cognitive effects of the molecular changes identified were assessed using the ethobiological STFP olfactory memory paradigm, which has been previously utilized in mice overexpressing the neuropeptide galanin, shown to be up-regulated in AD (44, 55). The data indicated age-related effects of both the tauV337M and APPV717I-CT100 transgenes on memory functioning. Thus, tauV337M, APPV717I-CT100, and combined tauV337M × APPV717I-CT100 mice all demonstrated olfactory memory functioning not significantly different from that of wild-type littermates at 6 months of age, but at 12 months of age all three transgenic lines showed significant memory impairment. The impairment in memory functioning was found to occur in the absence of differences in basic olfactory functioning or overall food consumption and was, as noted previously, not confounded by changes in sensorimotor functions. In addition, during the STFP assay no differences were identified in the time spent by the test mice in contact with the conspecific, indicating that memory impairment was unlikely to be the result of prior effects on learning. The data failed to provide any evidence to suggest that memory impairment in the combined tauV337M × APPV717I-CT mice was greater than in mice expressing the single tauV337M and APPV717I-CT100 transgenes. In fact, revealing such an effect may be problematic using this task due to floor effects insofar as all transgenic groups were close to chance performance. However, cognitive dissociations between the transgenic models may emerge by using different behavioral tests. There are a number of reports of cognitive effects in transgenic mouse models for dementia, often involving the Morris water maze (e.g., references 23, 35, 50, and 53). The present data extend these findings into the nonspatial olfactory domain, which has not previously been investigated in FAD or FTDP-17 transgenic models.
It was of interest that, while molecular changes in both APP-CT100 and tau (in terms of Aβ production and deposition and abnormal tau phosphorylation) were found to be present in mice at 6 months of age, no memory deficits were found in either the tauV337M, APPV717I-CT100, or tauV337M× APPV717I-CT100 mice until 12 months of age. These findings would suggest that early changes in molecular amyloid and tau neuropathology in the brain were present in advance of cognitive deficits. It is also of note that, although evidence of neurofibrillary tangles was identified in tauV337M and tauV337M × APPV717I-CT100 at 12 months of age, these were confined to the brainstem and as such were unlikely to have had effects on cognitive functioning (at least in terms of the known brain circuitry mediating the STFP task). In addition, although diffuse Aβ plaque formation was identified, there was no evidence of congophilic dense-cored plaques in the APPV717I-CT100 or tauV337M × APPV717I-CT100 mice. As such, it would appear that in the models described here, there was a dissociation between neurofibrillary tangle and/or congophilic plaque formation and cognitive decline, as has been found to be the case in several other dementia models (9, 29, 35). Hence, the precise mechanism(s) by which the changes observed at the molecular level may induce cognitive impairment, as indexed by the STFP, is undefined as yet and may involve abnormal changes in neuronal plasticity and/or actual cell death.
To conclude, we have obtained data that suggest distinct molecular routes to abnormal tau phosphorylation in models of FTDP-17 and AD and in addition provide evidence that tau irregularities in AD may be linked to the processing of APP C-terminal fragments via specific effects on MAPK function. Our findings highlight a major role for MAPK signaling pathways, key mediators of both neuronal plasticity (34) and cell death (52), in the hyperphosphorylation of tau. On this basis, interventions using selective MAPK inhibitors may in theory be capable of ameliorating the progression of neurofibrillary pathology in diseases such as AD.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council United Kingdom, a Royal Society Joint Grant (12150 to P.C.E. and Y.-H.S.), a National Creative Research Initiative Grant (for the years 2000 to 2003 to Y.-H.S.) from MOST, and the Alzheimer's Research Trust United Kingdom. S.L.L. was the Peacock Trust/Alzheimer's Research Trust Scholar.
We gratefully acknowledge M. Goedert (Medical Research Council Laboratory of Molecular Biology, Cambridge, United Kingdom) for supplying the tauV337M mice and M. G. Spillantini for assistance with tau immunohistochemical staining (Brain Repair Centre, University of Cambridge, Cambridge, United Kingdom).
REFERENCES
- 1.Allen, B., E. Ingram, M. Takao, M. J. Smith, R. Jakes, K. Virdee, H. Yoshida, M. Holzer, M. Craxton, P. C. Emson, C. Atzori, A. Migheli, R. A. Crowther, B. Ghetti, M. G. Spillantini, and M. Goedert. 2002. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J. Neurosci. 22:9340-9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderton, B. H., J. Betts, W. P. Blackstock, J. P. Brion, S. Chapman, J. Connell, R. Dayanandan, J. M. Gallo, G. Gibb, D. P. Hanger, M. Hutton, E. Kardalinou, K. Leroy, S. Lovestone, T. Mack, C. H. Reynolds, and M. Van Slegtenhorst. 2001. Sites of phosphorylation in tau and factors affecting their regulation. Biochem. Soc. Symp. 67:73-80. [DOI] [PubMed] [Google Scholar]
- 3.Biernat, J., N. Gustke, G. Drewes, E. M. Mandelkow, and E. Mandelkow. 1993. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11:153-163. [DOI] [PubMed] [Google Scholar]
- 4.Buée-Scherrer, V., and M. Goedert. 2002. Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases in intact cells. FEBS Lett. 515:151-154. [DOI] [PubMed] [Google Scholar]
- 5.Carter, R. J., L. A. Lione, T. Humby, L. Mangiarini, A. Mahal, G. P. Bates, S. B. Dunnett, and A. J. Morton. 1999. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J. Neurosci. 19:3248-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Jonghe, C., C. Esselens, S. Kumar-Singh, K. Craessaerts, S. Serneels, F. Checler, W. Annaert, C. Van Broeckhoven, and B. De Strooper. 2001. Pathogenic APP mutations near the gamma-secretase cleavage site differentially affect Aβ secretion and APP C-terminal fragment stability. Hum. Mol. Genet. 10:1665-1671. [DOI] [PubMed] [Google Scholar]
- 7.Dineley, K. T., M. Westerman, D. Bui, K. Bell, K. H. Ashe, and J. D. Sweatt. 2001. Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: in vitro and in vivo mechanisms related to Alzheimer's disease. J. Neurosci. 21:4125-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doble, B. W., and J. R. Woodgett. 2003. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116:1175-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodart, J. C., H. Meziane, C. Mathis, K. R. Bales, S. M. Paul, and A. Ungerer. 1999. Behavioral disturbances in transgenic mice overexpressing the V717F beta-amyloid precursor protein. Behav. Neurosci. 113:982-990. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer, I., R. Blanco, M. Carmona, R. Ribera, E. Goutan, B. Puig, M. J. Rey, A. Cardozo, F. Vinals, and T. Ribalta. 2001. Phosphorylated map kinase (ERK1, ERK2) expression is associated with early tau deposition in neurons and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer's disease, Pick's disease, progressive supranuclear palsy, and corticobasal degeneration. Brain Pathol. 11:144-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaherty, D. B., J. P. Soria, H. G. Tomasiewicz, and J. G. Wood. 2000. Phosphorylation of human tau protein by microtubule-associated kinases: GSK-3β and cdk5 are key participants. J. Neurosci. Res. 62:463-472. [DOI] [PubMed] [Google Scholar]
- 12.Ghribi, O., M. M. Herman, and J. Savory. 2003. Lithium inhibits Aβ-induced stress in endoplasmic reticulum of rabbit hippocampus but does not prevent oxidative damage and tau phosphorylation. J. Neurosci. Res. 71:853-862. [DOI] [PubMed] [Google Scholar]
- 13.Goate, A., M. C. Chartier-Harlin, M. Mullan, J. Brown, F. Crawford, L. Fidani, L. Giuffra, A. Haynes, N. Irving, L. James, et al. 1991. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 349:704-706. [DOI] [PubMed] [Google Scholar]
- 14.Godemann, R., J. Biernat, E. Mandelkow, and E. M. Mandelkow. 1999. Phosphorylation of tau protein by recombinant GSK-3β: pronounced phosphorylation at select Ser/Thr-Pro motifs but no phosphorylation at Ser262 in the repeat domain. FEBS Lett. 454:157-164. [DOI] [PubMed] [Google Scholar]
- 15.Goedert, M., R. Jakes, and E. Vanmechelen. 1995. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci. Lett. 189:167-169. [DOI] [PubMed] [Google Scholar]
- 16.Goedert, M., S. Satumtira, R. Jakes, M. J. Smith, C. Kamibayashim C. L. White III, and E. Sontag. 2000. Reduced binding of protein phosphatase 2A to tau protein with frontotemporal dementia and parkinsonism linked to chromosome 17 mutations. J. Neurochem. 75:2155-2162. [DOI] [PubMed] [Google Scholar]
- 17.Gong, C. X., T. J. Singh, I. Grundke-Iqbal, and K. Iqbal. 1993. Phosphoprotein phosphatase activities in Alzheimer disease brain. J. Neurochem. 61:921-927. [DOI] [PubMed] [Google Scholar]
- 18.Gotz, J., F. Chen, J. van Dorpe, and R. M. Nitsch. 2001. Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Aβ 42 fibrils. Science 293:1491-1495. [DOI] [PubMed] [Google Scholar]
- 19.Harwood, A. J. 2000. Signal transduction: life, the universe and development. Curr. Biol. 10:R116-R119. [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa, M., R. Jakes, R. A. Crowther, V. M. Lee, Y. Ihara, and M. Goedert. 1996. Characterization of mAb AP422, a novel phosphorylation-dependent monoclonal antibody against tau protein. FEBS Lett. 384:25-30. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa, M., M. Morishima-Kawashima, K. Takio, M. Suzuki, K. Titani, and Y. Ihara. 1992. Protein sequence and mass spectrometric analyses of tau in the Alzheimer's disease brain. J. Biol. Chem. 267:17047-17054. [PubMed] [Google Scholar]
- 22.Hongisto, V., N. Smeds, S. Brecht, T. Herdegen, M. J. Courtney, and E. T. Coffey. 2003. Lithium blocks the c-Jun stress response and protects neurons via its action on glycogen synthase kinase 3. Mol. Cell. Biol. 23:6027-6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsiao, K., P. Chapman, S. Nilsen, C. Eckman, Y. Harigaya, S. Younkin, F. Yang, and G. Cole. 1996. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274:99-102. [DOI] [PubMed] [Google Scholar]
- 24.Humby, T., F. M. Laird, W. Davies, and L. S. Wilkinson. 1999. Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur. J. Neurosci. 11:2813-2823. [DOI] [PubMed] [Google Scholar]
- 25.Ishiguro, K., K. Sato, M. Takamatsu, J. Park, T. Uchida, and K. Imahori. 1995. Analysis of phosphorylation of tau with antibodies specific for phosphorylation sites. Neurosci. Lett. 202:81-84. [DOI] [PubMed] [Google Scholar]
- 26.Jicha, G. A., C. Weaver, E. Lane, C. Vianna, Y. Kress, J. Rockwood, and P. Davies. 1999. cAMP-dependent protein kinase phosphorylations on tau in Alzheimer's disease. J. Neurosci. 19:7486-7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawarabayashi, T., L. H. Younkin, T. C. Saido, M. Shoji, K. H. Ashe, S. G. Younkin. 2001. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J. Neurosci. 21:372-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, S. H., and Y. H. Suh. 1996. Neurotoxicity of a carboxyl-terminal fragment of the Alzheimer's amyloid precursor protein. J. Neurochem. 67:1172-1182. [DOI] [PubMed] [Google Scholar]
- 29.Kumar-Singh, S., I. Dewachter, D. Moechars, U. Lubke, C. De Jonghe, C. Ceuterick, F. Checler, A. Naidu, B. Cordell, P. Cras, C. Van Broeckhoven, and F. Van Leuven. 2000. Behavioral disturbances without amyloid deposits in mice overexpressing human amyloid precursor protein with Flemish (A692G) or Dutch (E693Q) mutation. Neurobiol. Dis. 7:9-22. [DOI] [PubMed] [Google Scholar]
- 30.Lee, M. S., Y. T. Kwon, M. Li, J. Peng, R. M. Friedlander, and L. H. Tsai. 2000. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405:360-364. [DOI] [PubMed] [Google Scholar]
- 31.Lewis, J., D. W. Dickson, W. L. Lin, L. Chisholm, A. Corral, G. Jones, S. H. Yen, N. Sahara, L. Skipper, D. Yager, C. Eckman, J. Hardy, M. Hutton, and E. McGowan. 2001. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293:1487-1491. [DOI] [PubMed] [Google Scholar]
- 32.Lewis, J., E. McGowan, J. Rockwood, H. Melrose, P. Nacharaju, M. Van Slegtenhorst, K. Gwinn-Hardy, M. Paul Murphy, M. Baker, X. Yu, K. Duff, J. Hardy, A. Corral, W. L. Lin, S. H. Yen, D. W. Dickson, P. Davies, and M. Hutton. 2000. Neurofibrillary tangles, amytrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 25:402-405. [DOI] [PubMed] [Google Scholar]
- 33.Luthi, A., H. Van der Putten, F. M. Botteri, I. M. Mansuy, M. Meins, U. Frey, G. Sansig, C. Portet, M. Schmutz, M. Schroder, C. Nitsch, J. P. Laurent, and D. Monard. 1997. Endogenous serine protease inhibitor modulates epileptic activity and hippocampal long-term potentiation. J. Neurosci. 17:4688-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzucchelli, C., C. Vantaggiato, A. Ciamei, S. Fasano, P. Pakhotin, W. Krezel, H. Welzl, D. P. Wolfer, G. Pages, O. Valverde, A. Marowsky, A. Porrazzo, P. C. Orban,R. Maldonado, M. U. Ehrengruber, V. Cestari, H. P. Lipp, P. F. Chapman, J. Pouyssegur, and R. Brambilla. 2002. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron 34:807-820. [DOI] [PubMed] [Google Scholar]
- 35.Moechars, D., I. Dewachter, K. Lorent, D. Reverse, V. Baekelandt, A. Naidu, I. Tesseur, K. Spittaels, C. V. Haute, F. Checler, E. Godaux, B. Cordell, and F. Van Leuven. 1999. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J. Biol. Chem. 274:6483-6492. [DOI] [PubMed] [Google Scholar]
- 36.Mullan, M., S. Tsuji, T. Miki, T. Katsuya, S. Naruse, K. Kaneko, T. Shimizu, T. Kojima, I. Nakano, T. Ogihara, et al. 1993. Clinical comparison of Alzheimer's disease in pedigrees with the codon 717 Val→Ile mutation in the amyloid precursor protein gene. Neurobiol. Aging 14:407-419. [DOI] [PubMed] [Google Scholar]
- 37.Phiel, C. J., C. A. Wilson, V. M. Lee, and P. S. Klein. 2003. GSK-3α regulates production of Alzheimer's disease amyloid-beta peptides. Nature 423:435-439. [DOI] [PubMed] [Google Scholar]
- 38.Poorkaj, P., T. D. Bird, E. Wijsman, E. Nemens, R. M. Garruto, L. Anderson, A. Andreadis, W. C. Wiederholt, M. Raskind, and G. D. Schellenberg. 1998. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 43:815-825. [DOI] [PubMed] [Google Scholar]
- 39.Rapoport, M., and A. Ferreira. 2000. PD98059 prevents neurite degeneration induced by fibrillar β-amyloid in mature hippocampal neurons. J. Neurochem. 74:125-133. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds, C. H., J. C. Betts, W. P. Blackstock, A. R. Nebreda, and B. H. Anderton. 2000. Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3β. J. Neurochem. 74:1587-1595. [DOI] [PubMed] [Google Scholar]
- 41.Sengupta, A., J. Kabat, M. Novak, Q. Wu, I. Grundke-Iqbal, and K. Iqbal. 1998. Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch. Biochem. Biophys. 357:299-309. [DOI] [PubMed] [Google Scholar]
- 42.Spillantini, M. G., R. A. Crowther, and M. Goedert. 1996. Comparison of the neurofibrillary pathology in Alzheimer's disease and familial presenile dementia with tangles. Acta Neuropathol. (Berlin) 92:42-48. [DOI] [PubMed] [Google Scholar]
- 43.Spillantini, M. G., and M. Goedert. 1998. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 21:428-433. [DOI] [PubMed] [Google Scholar]
- 44.Steiner, R. A., J. G. Hohmann, A. Holmes, C. C. Wrenn, G. Cadd, A. Jureus, D. K. Clifton, M. Luo, M. Gutshall, S. Y. Ma, E. J. Mufson, and J. N. Crawley. 2001. Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer's disease. Proc. Natl. Acad. Sci. USA 98:4184-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suh, Y. H., and F. Checler. 2002. Amyloid precursor protein, presenilins, and alpha-synuclein: molecular pathogenesis and pharmacological applications in Alzheimer's disease. Pharmacol. Rev. 54:469-525. [DOI] [PubMed] [Google Scholar]
- 46.Sumi, S. M., T. D. Bird, M. D. Nochlin, and M. A. Raskind. 1992. Familial presenile dementia with psychosis associated with cortical neurofibrillary tangles and degeneration of the amygdala. Neurology 42:120-127. [DOI] [PubMed] [Google Scholar]
- 47.Swatton, J. E., L. A. Sellers, R. L. M. Faull, A. Holland, S. Iritani, and S. Bahn. 2004. Increased MAP kinase activity in Alzheimer's and Down syndrome but not in schizophrenia human brain. Eur. J. Neurosci. 19:2711-2719. [DOI] [PubMed] [Google Scholar]
- 48.Takashima, A., T. Honda, K. Yasutake, G. Michel, O. Murayama, M. Murayama, K. Ishiguro, and H. Yamaguchi. 1998. Activation of tau protein kinase I/glycogen synthase kinase-3β by amyloid beta peptide (25-35) enhances phosphorylation of tau in hippocampal neurons. Neurosci. Res. 31:317-323. [DOI] [PubMed] [Google Scholar]
- 49.Tanemura, K., T. Akagi, M. Murayama, N. Kikuchi, O. Murayama, T. Hashikawa, Y. Yoshiike, J. M. Park, K. Matsuda, S. Nakao, X. Sun, S. Sato, H. Yamaguchi, and A. Takashima. 2001. Formation of filamentous tau aggregations in transgenic mice expressing V337M human tau. Neurobiol. Dis. 8:1036-1045. [DOI] [PubMed] [Google Scholar]
- 50.Tatebayashi, Y., T. Miyasaka, D. H. Chui, T. Akagi, K. Mishima, K. Iwasaki, M. Fujiwara, K. Tanemura, M. Murayama, K. Ishiguro, E. Planel, S. Sato, T. Hashikawa, and A. Takashima. 2002. Tau filament formation and associative memory deficit in aged mice expressing mutant (R406W) human tau. Proc. Natl. Acad. Sci. USA 99:13896-13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vidal, M., R. Morris, F. Grosveld, and E. Spanopoulou. 1990. Tissue-specific control elements of the Thy-1 gene. EMBO J. 9:833-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vila, M., and S. Przedborski. 2003. Targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci. 4:365-375. [DOI] [PubMed] [Google Scholar]
- 53.Westerman, M. A., D. Cooper-Blacketer, A. Mariash, L. Kotilinek, T. Kawarabayashi, L. H. Younkin, G. A. Carlson, S. G. Younkin, and K. H. Ashe. 2002. The relationship between Aβ and memory in the Tg2576 mouse model of Alzheimer's disease. J. Neurosci. 22:1858-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolman, M., and J. J. Bubis. 1965. The cause of green polarization colour of amyloid stained with Congo red. Histochemie 4:35-36. [DOI] [PubMed] [Google Scholar]
- 55.Wrenn, C. C., A. P. Harris, M. C. Saavedra, and J. N. Crawley. 2003. Social transmission of food preference in mice: methodology and application to galanin-overexpressing transgenic mice. Behav. Neurosci. 117:21-31. [PubMed] [Google Scholar]
- 56.Yuan, Z., A. Agarwal-Mawal, and H. K. Paudel. 2004. 14-3-3 binds to and mediates phosphorylation of microtubule-associated Tau protein by Ser9-phosphorylated GSK3β in the brain. J. Biol. Chem. 279:26105-26114. [DOI] [PubMed] [Google Scholar]