Abstract
Obesity remains the most prevailing disorder in childhood males and females worldwide. Its high prevalence markedly predisposes children to insulin resistance, hypertension, hyperlipidemia and liver disorders while enhancing the risk of type 2 diabetes and cardiovascular diseases. In this review, the relationship of obesity with genetic and environmental factors will be described and the underlined causes will briefly be reported. As obesity in children constitutes an increasingly health concern, important potential biomarkers have been discussed for the diagnosis, treatment and follow-up of the wide range of overweight-related complications. Awareness about the applicability and limitations of these preventive and predictive biomarkers will intensify the research and medical efforts for new developments in order to efficiently struggle against childhood obesity.
Key words: pediatric obesity, insulin resistance, inflammation, oxidative stress, biomarkers
INTRODUCTION
The prevalence of childhood obesity is rapidly increasing and presents a major public health concern in developed and developing countries (1-4), and assessment of obesity is of utmost importance to paediatricians. However, there are varying definitions of obesity in children and adolescents, along with ethnic-specific variations in body fat content and distribution, which complicate this undertaking (5). Moreover, these divergences may explain prevalence dissimilarities associated with cardiometabolic diseases (CMD) (e.g. insulin resistance, hypertension, dyslipidemia and diabetes) in adulthood (6-11). In the context of epidemiological studies, body mass index (BMI, weight/height2) in adults is currently considered as a diagnostic test (separator variable) which is able to identify overweight (25 kg/m2) and obese (30 kg/m2) individuals and may predispose to increased CMD risk, morbidity and mortality (12, 13). However, no similar definite values can be used in childhood and adolescence because of the substantial changes in BMI, which occur naturally from birth to adulthood (14, 15), and because of the limited data in youth that relate BMI trajectory to cardiovascular events later in life. Age- and sex-specific BMI cut-offs were developed to define overweight and obese using different nationally representative age- and sex-specific data sets, following recommendations from the International Obesity Task Force (16, 17). International age- and sex-specific BMI cut-offs for overweight and obese girls and boys are illustrated in Figure 1. Applying this concept to BMI trajectory, Attard et al. (18) demonstrated that the odds for diabetes were 2.35 higher for those with a BMI of 30 kg/m2 relative to young male adults who had maintained a BMI of 23 kg/m2 over an average of 12 years. These data suggest there is potential for improving the ability to assess the effect of paediatric obesity on development of diseases at a later time point. Secular trends demonstrate that the prevalence has plateaued in some countries (19) or even decreased (20), but has continued to rise in others, independent of how overweight and obesity are defined in childhood (1, 21-23). The apparent contradiction could partially depend on the span of the retrospective studies and on the years included. Nevertheless, the present high number of young adults with the stigmata of the metabolic syndrome (MetS), and the related non-alcoholic fatty liver disease (NAFLD) justifies that it be considered a major world public health issue (24). This review briefly describes the various potential causes of obesity in youth and underscores the available biomarkers for associated conditions.
Figure 1.
International age- and sex-specific cut-off points for BMI for overweight and obesity
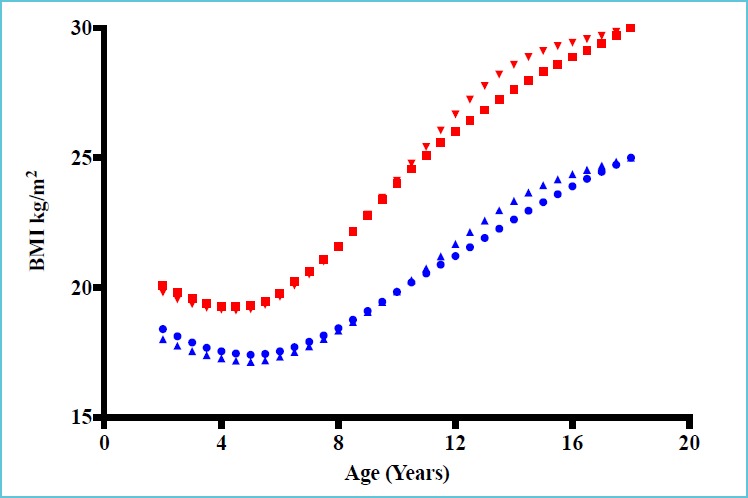
*Adapted with permission from data of Table 4 from Cole TJ et al. (16). Data obtained by averaging the national centiles.
BMI: Body Mass Index. Filled circles: curve for overweight boys; filled square: curve for obese boys; filled upward triangles: curve for overweight girls; filled downward triangles: curve for obese girls.
Definite BMI thresholds to identify an increased risk for CMD cannot be used in childhood and adolescence. Age- and sex-specific BMI cut-offs to define overweight and obesity and predict trajectory into adulthood should be utilized using different nationally representative age- and sex-specific data.
OBESITY AND LIFESTYLE
Lifestyle is broadly defined as the way or manner by which a person or a group of people lives. However, lifestyle can be influenced by a complex set of factors that are intertwined and can affect the quality of living and health (Figure 2). The socioeconomic position (SEP) stands out among these factors because it has a direct impact on the quality of nutrition and the living environment, including access to adequate physical activity facilities and education. Consequently, a comprehensive view must be adopted whenever addressing this topic but a majority of studies tend to focus in this area in a fragmented manner.
Figure 2.
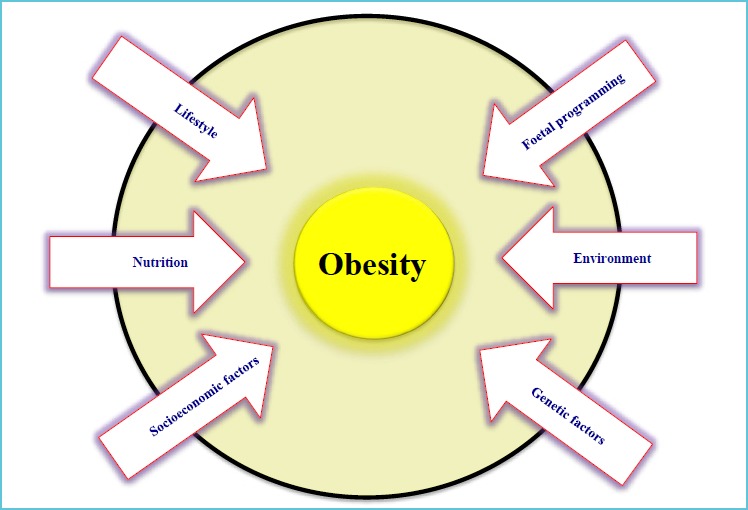
Factors involved in the development of obesity
One such study, based on self-reports, demonstrated that poor children in the United States have worse health compared to wealthy children. This difference in health status diverged further as the children aged; thereby suggesting the adult health gradient had its origins in childhood. However, other than family income no other factors were considered which could explain these results (25). SEP may also impact the quality of nutrition. Darmon et al. (26) reported that higher-quality diets consisting of whole grains, lean meats, fish, low-fat dairy products, fresh vegetables and fruits were associated with greater affluence, whereas energy-dense and nutrient-poor diets (refined grains, added fats) are preferentially consumed by persons of lower SEP. Likewise, in a systematic review, Cameron et al. (27) reported that children of lower SEP had a steeper weight gain trajectory initiating at birth and led to a greater prevalence of obesity in children and adults. Pre-pregnancy maternal BMI, diabetes, pre-pregnancy diet, smoking during pregnancy, low birth weight, breastfeeding initiation and duration, early introduction of solids, maternal and infant diet quality, and some aspects of the home food environment were among the early-life predictors of later obesity and amid links with SEP. Furthermore, lack of physical activity is an additional risk factor for developing obesity. A longitudinal study involving repeated 7-day physical activity recall questionnaires over a 5-year period demonstrated that greater fluctuations in physical activity led to an increase in body fat in adolescent girls and boys (28). An interventional study supported these conclusions, demonstrating interruption of sedentary time with brief moderate-intensity walking resulted in an improvement of short-term metabolic function in non-overweight children without increasing subsequent energy intake (29). Despite the difficulty in directly comparing studies because of the variety of environmental factors and defined end-points, systematic reviews consistently highlight that better and safer access to physical activity resources are directly related to increased leisure time physical activity in children and adolescents, which subsequently decreases the risk of developing obesity (30-34).
Access to physical activity resources is directly related to higher leisure time physical activity in children and adolescents and decreases the risk of developing obesity.
OBESITY AND GENETIC/EPIGENETIC FACTORS
In addition to the risk factors previously discussed, genetic background and foetal programming through epigenetic modifications are equally important in the development of obesity and related diseases. There is also increasing evidence suggesting synergetic effects between gene variant loci involved in metabolic traits and dietary or lifestyle factors. Maes et al. (35) compiled data from more than 25,000 twin pairs and 50,000 biological and adoptive family members and reported that genetic components contribute 40-70% to the inter-individual variability in common obesity. Another study showed that parental obesity doubled the risk of adult obesity among both obese and non-obese children less than 10 years of age (36). Few studies have investigated the gene-environment interactions related to sedentary behaviour using large cohorts. The Identification and prevention of Dietary- and lifestyle-induced health EFfects In Children and infantS cohort (IDEFICS) used a subsample of 4406 participants to demonstrate that the fat mass and obesity-related gene (FTO) polymorphism (rs9939609) could explain ~9% of the obesity variance, thereby suggesting the FTO gene was sensitive to the social environment (37). To date, genome wide association studies (GWAS) have provided evidence for a number of gene variants associated with the development of obesity in the youth. Willer et al. (38), based on a cohort of 11 year-old children, demonstrated significant and consistent association between BMI and variant loci (SNPs) located in or near the trans-membrane protein-18 (TMEM18), potassium channel tetramerisation domain containing-15 (KCTD15) and glucosamine-6-phosphate deaminase-2 (GNPDA2) genes. The high brain and hypothalamic expression of these factors, together with FTO and the melanocortin-4 receptor (MC4R), independently associated with adiposity and insulin resistance (39), supports the argument for a neuronal foundation in obesity. Whether these loci are modulated under neuronal influence by the environment or lifestyle remains to be elucidated. Graff et al. (40) provided a partial answer by establishing a dose-dependent interaction leisure screen time (β = –0.014, 0.016, and 0.045/7h/week) with GNPDA2 (rs10938357) SNP in Afro-American subjects for 0, 1 and 2 risk alleles. They observed a similar interaction for the FLJ35779 (rs2112347) gene polymorphism (Figure 3). Although interactions are documented in each study, they are modest, and individually cannot explain the development of obesity or the onset of related diseases. Additional studies are required to probe the relationship between polymorphisms in multiple genes involved in energy management and the numerous environmental and lifestyle factors.
Figure 3.
Predicated BMI Z-score from model based coefficients per 7 hours/week of screen time in the presence of 0, 1 and 2 risk (T) FLJ35779 (rs2112347) alleles
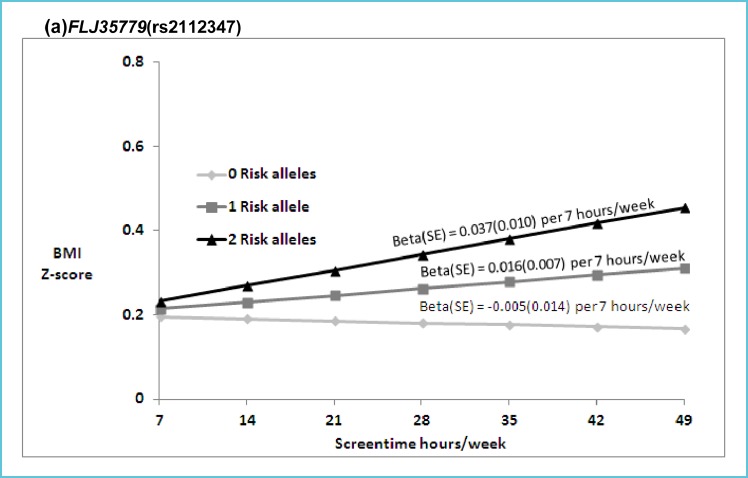
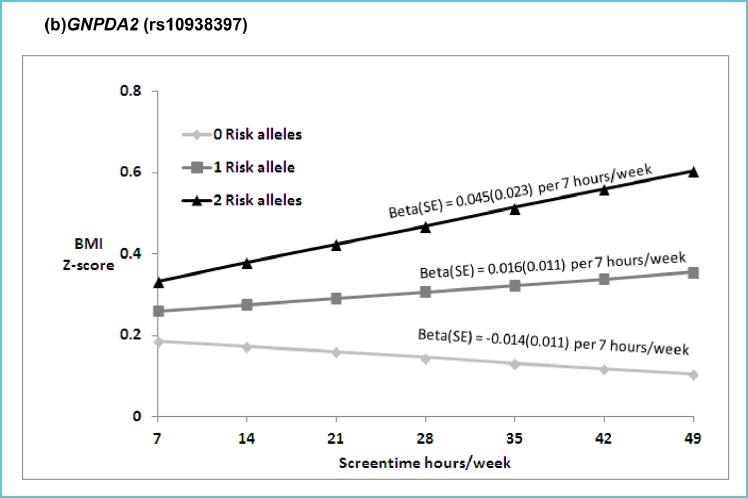
Legend: (p for interaction = 0.02) in EA (a), and 0, 1 and 2 risk (A) GNPDA2 (rs10938397) alleles, respectively (p for interaction = 0.03) in AA (b).
Abbreviations: BMI (body mass index), ST (hours per week of screen time), EA (European American), AA (African American).
Beta estimates are presented for the interaction model: Multivariable linear models of adolescent BMI Z scores regressed on SNP and ST (hr/wk), with SNP by ST interaction term, controlling for age, sex, current smoking (at least one cigarette every day for 30 days), geographic region, and self-reported heights and weights (n=39 EA, n= 12 AA), oversampling of highly educated African Americans (n=281; AA stratum only). Random intercepts allowed for individual, family and school with no sample weighting.
*Reproduced with permission from: Graff et al. Pediatr Obes 2013;8:doi:10.1111/j.2047-6310.2013.00195x.
The epigenetic control of gene expression must also be considered in the understanding of the development of obesity. This concept stems from the early work of Barker et al. (41), who proposed that the tendency to store abdominal fat might be a persistent response to adverse conditions which initiated in the foetal life stage but persisted throughout infancy. A myriad of peer-reviewed publications have confirmed this initial hypothesis (42-46). Lee et al. (47) suggest there is a gene-foetal environment interaction, one of which occurs through in utero exposure to maternal cigarette smoking and leads to a preference in adolescence for moderately enhanced fatty foods by silencing the opioid receptor mu-1 gene (OPRM1) involved in the brain reward system. Small gestational age (SGA) is also well recognized and linked to an increased risk for rapid postnatal weight gain and subsequent development of obesity and chronic metabolic diseases later in life. The Auckland Birth weight Collaborative Study demonstrated that smoking, low pregnancy weight, maternal short stature, maternal diet, ethnic origin of mother and hypertension are all “environmental” risk factors for SGA (48). A subgroup of the cohort later established that polymorphic FTO (rs9939609, intron), KCNJ11 (rs5219, missense Lys23Glu), BDNF (rs925946, 9.2 kb upstream), PFKP (rs6602024, intron), PTER (rs10508503, 179 kb upstream) and SEC16B (rs10913469, intron) genes, were related to obesity, type 2 diabetes, and SGA which indicates the important interaction between genetic factors and foetal environment (49). Finally, a prospective singleton normal pregnancy cohort study demonstrated a direct relationship between the maternal adipokines, leptin (a satiety factor) and adiponectin (an insulin sensitizer). The study included 339 healthy women without pre-existing diabetes who were evaluated at 24-28 and 32-35 weeks of gestation and the cord blood (foetal compartment) assessed at birth (50). Foetal insulin sensitivity was negatively associated with cord blood leptin and positively with pro-insulin concentrations, suggesting the maternal impact on foetal adipokines may be an early life pathway in maternal-foetal transmission of the propensity to develop obesity and insulin resistance later in life. These examples provide compelling evidence on the role and impact of the foetal environment and development of chronic diseases later in life.
Parental obesity more than doubles the risk of adult obesity among obese and non-obese children.
Gene-environment interactions are modest, and individually are not able to explain the development of obesity and the onset of related diseases.
There are compelling evidence highlighting the role of foetal environment and development of chronic diseases later in life.
OBESITY AND MICROBIOTA
In addition to the above considerations, the gut microbiota may increasingly be shown to impact the course of metabolic diseases. This aspect is briefly reviewed. The synergistic relationship between the human body and the vast microbiotic environment present on all interfaces with the exterior, particularly the gut lumen, has become of major interest to the medical community. The microbiome cell number far outnumbers somatic or germ cells and represents a far more varied gene diversity than the human genome (51). The advent of high throughput genome sequencing technologies allowed the first meta-sequence of the human gut microbiome to be conducted, utilizing stool collected from 124 individuals, and characterized > 3X106 genes from approximately 1000 different microscopic species (52-54). An excellent review by Arora et al. (55) discusses the composition of the gut microbiota and its association with metabolic diseases. Figure 4, taken from this review, shows that 2 phyla, namely Firmicutes and Bacteriodetes, constitute healthy adult gut microbiota and their relative proportions differ among populations.
Figure 4.
Quantitative comparison of faecal microbiota in two healthy populations
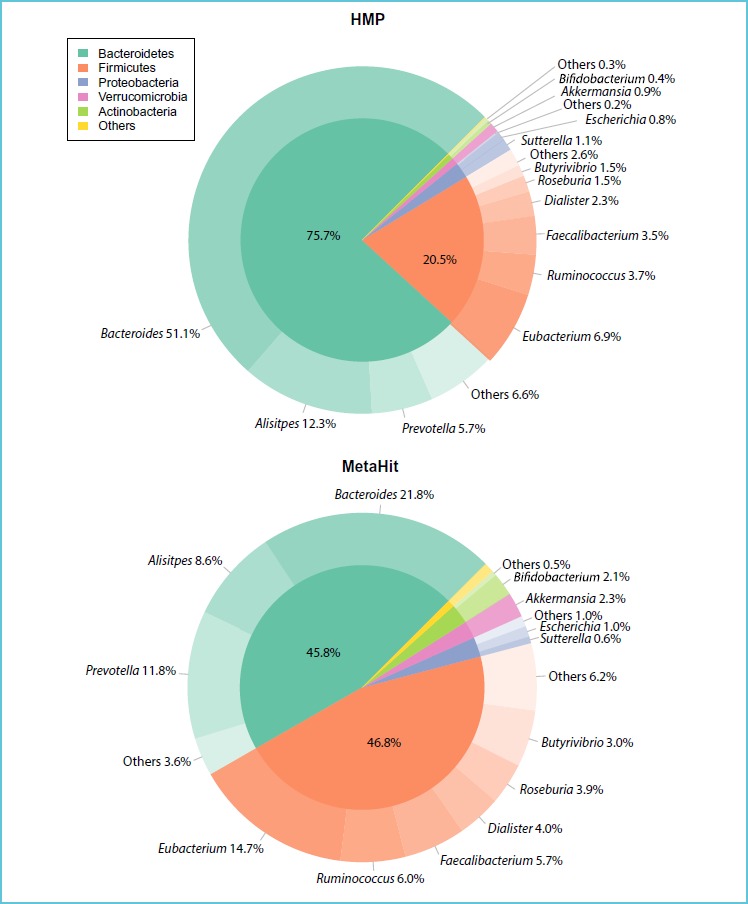
*Reproduced with permission from: Aurora T et al. J Intern Med 2016 Apr 12. doi: 10.1111/joim.12508.
Neonatal intestinal flora evolves according to its early environmental exposures, nutrition patterns (maternal or industrial milk), hygiene levels and therapeutic drug usage (56). Differences in intestinal flora patterns during the first six months of life may have potential impact and downstream consequences on the later development of chronic conditions such as type 2 diabetes and allergies (57, 58).
The gut microbiota has emerged as a new important player in the pathogenesis of obesity, potentially explained by the fact that each microbiotic species transforms the undigested and partially digested food into metabolites that may influence the physiological systems of the host. Therefore, a loss in diversity may lead to unwanted effects (55). This hypothesis is supported by the observation that composition of the gut microflora is globally less diverse in obese subjects, with a relative enrichment in Firmicutes and a impoverishment in Bacteroïdes (59). Moreover, detailed analysis of the flora in obese subjects reveals a bimodal distribution: those with a low gene count (LGC) characterised by the predominance of 5 pro-inflammatory bacteria and a less diversified metagenome, and those with a high gene count (HGC) with a high percentage of 4 anti-inflammatory bacteria genii (60). The LGC group presents with insulin-resistance, dyslipidemia and low-level infiltration of adipose tissue with pro-inflammatory cytokine secreting immunity cells. It has recently been established that levels of butyrate-producing bacteria are reduced in patients with type-2 diabetes, whereas levels of Lactobacillus sp. are increased, thus the reduction of butyrate-producing bacteria may be causally linked to type 2 diabetes. The causal relationship for these differences in humans remains to be elucidated but opens the way to possible treatment of obesity via dietary manipulation. For example, a low calorie regiment composed of plant fibres, proteins and low carbohydrates potentially increases the microbiota diversity (61). Interestingly, bariatric surgery also increases the gut microbiota diversity (62, 63). As each microbiotic species transforms the undigested and partially digested food into metabolites that may influence the physiological systems of the host, a loss in diversity may lead to unwanted effects.
The gut microbiota, a new player in the world of obesity and cardiometabolic diseases, is increasingly called upon to elucidate findings related to these diseases and may eventually impact their course and treatment.
BIOMARKERS
The status of metabolically healthy obese (MHO) individuals has been reported (64, 65) but obesity, particularly abdominal, remains a major risk factor for developing a series of complications (Figure 5) such as the metabolic syndrome, type 2 diabetes, early atherosclerosis and nonalcoholic fatty liver disease (NAFLD), the latter considered the hepatic manifestation of insulin resistance (66-68). Cellular redox potential imbalance, inflammatory processes and insulin resistance are central in the development of the complex chronic metabolic disturbances (Figure 6); hence measurement of related biomarkers to detect minor disturbances could help distinguish MHO from metabolically non-MHO individuals, and may result in establishing early primordial prevention programs. However, at the present time there is no international consensus as to the specific pathways that should preferentially be targeted in order to define the prevalence and severity of the conditions during childhood and adolescence.
Figure 5.
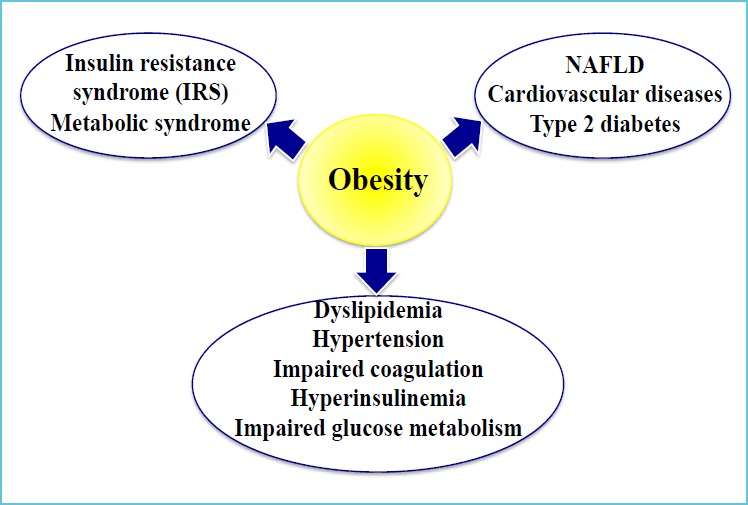
Impact of obesity on health status
Figure 6.
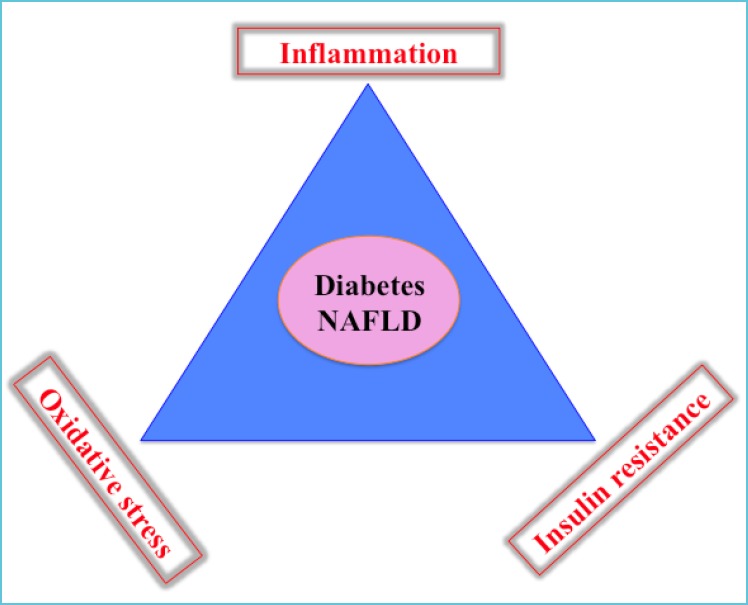
Cellular redox potential imbalance, inflammatory processes and insulin resistance in the development of diabetes and non-alcoholic liver disease (NAFLD)
IMAGING TECHNIQUES
In the last decade, utilization of ultrasonography, transient elastography and magnetic resonance imaging (MRI) has increased significantly. In the context of the present review these techniques, except for MRI, are not suitable for the detection of metabolic disturbances and are primarily used to evaluate the extent of liver damage. Although widely available, ultrasonography is unable to accurately detect or quantify early liver fatty acid infiltrations. Furthermore, this technique is prone to observer- and operator-dependent variability and its use in obese patients is subject of debate (69, 70). Transient elastography, based on the assessment of liver stiffness, has also been shown to be useful in presence of significant fibrosis and cirrhosis (71). Liver magnetic resonance imaging–estimated proton density fat fraction (PDFF) is more sensitive and favourably comparable to histopathology scores (72). This technology is currently restricted to tertiary care institutions, is expensive, and demands experienced staff. In summary, these imaging techniques are useful in detecting steatosis, but they are relatively inefficient in determining early stage liver damage. Biomarkers easily measured in central laboratories are therefore of utmost importance and should center on insulin resistance, inflammation and oxidative stress, as this triad is the signature of NAFLD.
INSULIN RESISTANCE
The term insulin resistance (IR) frequently refers to a physiological state characterized by a diminished biological response to insulin. More precisely, IR refers to a holistic reduction of glucose uptake in response to physiological insulin concentrations, primarily in muscle tissue. The optimal assessment of IR in children and adolescents remains controversial. Following the Consensus Conference on Childhood IR in 2010, experts highlighted: 1) the paucity of data regarding cut-offs to define insulin resistance; 2) poor performance of surrogate measures such as fasting plasma insulin; and 3) lack of justification for screening children, even obese children, because there are no accepted treatments for euglycemic IR (73). However, the development of robust methods for assessing insulin sensitivity (IS) in paediatric populations remains of great interest, particularly for epidemiological studies to monitor metabolic trajectory into adulthood.
The hyperinsulinemic-euglycemic clamp is the gold standard for determining total-body IS (73). However, it is not applicable in the context of population screening or routine clinical workup. In 2014 Brown and Yanovski (74) published an excellent review on this technique as well as surrogate measures and their pitfalls. The hyperinsulinemic-euglycemic clamp, as its name indicates, depends on repeated measures of both insulin and blood glucose, each having their own potential analytical pitfalls that may hinder inter-laboratory comparison (Table 1).
Table 1.
Caveats in assessing insulin sensitivity
| Variable | Issue |
|---|---|
| Measurement of insulin | Wide inter-inter method (laboratory) variations Variation in standardization among methods |
| Measurement of plasma glucose | Pre-analytical quality of blood specimens Glycolysis at room temperature NaF inhibits enolase, a late glycolytic enzyme |
| Patient preparation | Poor assessment of patient’s nutritional status Elevation of post-prandial glucose in malnutrition and low carbohydrate diets |
Reliable interpretation of hyperinsulinemic-euglycemic clamp studies is also dependent upon normal inter-individual biological differences such as insulin clearance rates and time required to reach a steady state. Alternative methods include the insulin tolerance test (ITT), the hyperglycemic clamp, the insulin-modified or frequently sampled intravenous glucose tolerance test (FSIGT) and the more frequently used oral glucose tolerance test (OGTT) (74).
FASTING INSULIN AND THE HOMA-IR
Assessment of IR or IS is frequently conducted using single measurements due to ease of availability and simplicity. Measurement of fasting insulin concentrations are considered representative of insulin hepatic sensitivity (low concentrations) or resistance (high concentrations). In theory, this information is valuable and may alert clinicians to eventual liver function impairment but there are issues around defining an abnormal elevated fasting insulin concentration because the data on reference values in fasting insulinemia are scarce. In addition, the lack of standardization or harmonization between different insulin assays hampers direct comparison between laboratories, peer-reviewed publications, and impedes coherent measures for treatment guidelines. This was highlighted in 2007 by the IFCC Working Group on Standardization of Insulin Assays, in an evaluation of 12 commercial insulin methods (75). The within-assay CVs ranged from 3.7% to 39.0% and between assay CVs from 12% to 66% (75). In 2009 the working group reported that 4 out of 10 insulin assays, when re-calibrated with a purified recombinant insulin preparation, had ≥ 95% of the 39 individual donor sera results within 32% of the target value assigned by an isotope dilution-mass spectrometry assay. In addition, 7 of 10 assays had a bias >15% in 36 to 100% of individual samples. The consensus group concluded that agreement between assays would improve using an international reference material and a higher order mass spectrometry method (76). Subsequent high-throughput mass spectrometry immunoassays have been developed to quantitate human intact insulin as well as insulin analogs, which may allow an accurate definition of insulinemia to be determined (77, 78). Accurate measurement of plasma insulin is of paramount importance for establishing comparable Homeostasis Model Assessment of IR (HOMA-IR) reference values across laboratories, although variation between ethnic populations may be a confounding factor that should be taken into consideration. At the present time HOMA-IR cut-offs are still highly method dependent. Table 2 illustrates the distribution of published cut-off points for defining IR, and confirm the warning of Wallace et al. (79): “The HOMA model has become a widely used clinical and epidemiological tool and, when used appropriately, it can yield valuable data. However, as with all models, the primary input data need to be robust, and the data need to be interpreted carefully.” To address this issue, the IFCC (http://www.ifcc.org/ifcc-scientific-division/sd-working-groups/wg-sia/), in collaboration with the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD), has created the Working group on Standardisation of Insulin Assays (WG-SIA) with the mandate of improving the standardization of assays for insulin by the development of a candidate reference method based on liquid chromatography-tandem mass spectrometry, and of a lyophilized recombinant human insulin preparation as primary reference material.
Table 2.
Cut-off points for defining insulin resistance (IR)
| Insulin measurement | Population Studied | Age (years) | Gender | HOMA-IR 95th percentile | Ref |
|---|---|---|---|---|---|
| Immunoassay (Access, Beckman Coulter) |
French Canadian | 9 13 16 |
M/F | 1.88/2.07 3.28/3.86 3.31/3.10 |
(76) |
| Fluoroimmunoassay (AutoDelfia, Pharmacia) |
Brazilian | 10-19 | M/F | >2.93 | (77) |
| Chemiluminescence Immunoassay (Immulite, Siemens) |
American | 11-14 | M/F | ≥2.7 | (78) |
| Chemiluminescence Immunoassay (Cobas, Roche Diagnostics) |
Spanish | 8-18 | M/F | ≥3.6 | (79) |
Although insulin resistance is a well-recognized clinical entity, there are currently no internationally accepted definition of its expression in children and adolescents. One well-characterized definition requires the presence of three or more factors which can be age-adjusted to define hyperinsulinaemia: Overweight, high systolic blood pressure, hypertriglyceridemia, low HDL-cholesterol and impaired fasting plasma glucose (84).
Data on normal reference intervals for fasting insulinemia are scarce.
Lack of standardized or harmonized insulin assays hampers comparison between laboratories and impedes coherent measures for treatment guidelines.
Distinguishing MHO young patients from those unhealthy bears a major clinical importance as they are, for reasons that are yet to be defined, resistant to develop CMD; hence follow-up and treatment differ (64). Low-grade inflammation and cellular redox potential imbalance are, together with insulin resistance, key-role players in the development of the non-healthy state in obese subjects.
INFLAMMATION
Inflammation is the second cause in the development of CMD and NAFLD related to paediatric obesity. A number of biomarkers have been identified but primarily in the context of clinical trials, thus their specificity, sensitivity and predictive values have yet to be defined for screening and diagnostic purposes. C-Reactive Protein (CRP), a member of the pantraxin family involved in plaque instability, is the most commonly utilized inflammatory biomarker. Although the sensitivity of CRP is generally high, the specificity is low, particularly in the setting of potential low-grade inflammation. Nevertheless, discrete elevation in circulating CRP concentrations has been associated in the definition of the metabolic syndrome (84, 85). Its advantage resides in its wide accessibility by central laboratories. However, as for any other biomarkers, well-defined age-, sex- and ethnicity-adjusted reference values or thresholds have to be defined if they are to be used for clinical purposes. The analytical sensitivity, even for the high-sensitivity CRP (hsCRP) test, however, limits the definition of reference ranges. One European population-based study reported that 44% of the 9855 children tested exhibited serum CRP concentrations below the detection limit (0.2 mg/l) and confirmed our observation (85) to the effect that obesity influenced serum CRP concentrations (86).
C-Reactive Protein (CRP) is the most commonly utilized biomarker of inflammation. The specificity of CRP is questionable, particularly in the setting of low-level inflammation.
Well-defined age-, sex- and ethnicity-adjusted reference values or thresholds have to be defined if they are to be used for clinical purposes.
Visceral adipose tissue per se and its resident macrophages contribute importantly to systemic inflammation by secreting adipokines and pro- and anti-inflammatory cytokines. Indeed, clinical studies have consistently shown elevated blood concentrations of pro-inflammatory cytokines such as IL-6, IL-8, TNFα, PAI-1, resistin and amylin in overweight and obese insulin-resistant youth (87-90). However, this relationship does not imply unanimity. A recent report has noted that the relationship between pro-inflammatory and metabolic markers commonly observed in adults and pubertal adolescents is reversed in healthy black and white children before puberty, which warrants questions as to whether these inverse relationships modify the trajectory later in life (91). Population-based studies focused on evaluating pro-inflammatory and metabolic markers to determine which bio-markers constitute sensitive and specific tools in the context of a diagnosis of insulin resistance would be valuable.
OXIDATIVE STRESS
Oxidative stress is often a neglected cause of paediatric obesity-related morbidities, and no biomarkers have been successfully validated yet for routine clinical use. To our knowledge there are no clinical research studies demonstrating that circulating concentrations of malonyldialdehyde (MDA), Hydroxynonenal (HNE), advanced glycation end-products (AGEs) and 8-hydroxy-2-deoxyguanosine (8-OH-dG), which are surrogate markers for lipids, proteins and deoxyribonucleic acid damages respectively, are effective diagnostic tools for CMD in childhood and adolescence.
In an observational study performed on 35 children between the ages of 12 and 18 years, Khelishadi et al. (92) reported that the age- and sex-adjusted changes in ox-LDL, waist circumference, CRP, MDA and body fat mass had the highest correlations with changes in coronary intima media thickness. More recently, in a population-based study, Galan-Chilet et al. (93) demonstrated a positive association of selenium at plasma concentrations above ~110 μg/L for 8-oxo-dG, but an inverse association with GSSG/GSH and MDA. They further identified potential risk genotypes associated with increased levels of oxidative stress markers with high selenium levels.
CONCLUSIONS
There is currently no single biomarker which can adequately define obesity-related CMD risk in paediatrics or adults. Prospective clinical trials should focus on devising a score based on well-characterized and appropriately validated biomarkers.
REFERENCES
- 1.Broyles S, Katzmarzyk PT, Srinivasan SR, Chen W, Bouchard C, Freedman DS, et al. The pediatric obesity epidemic continues unabated in Bogalusa, Louisiana. Pediatrics. 2010;125(5):900-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brune M, Hochberg Z. Secular trends in new childhood epidemics: insights from evolutionary medicine. BMC medicine. 2013;11:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta N, Shah P, Nayyar S, Misra A. Childhood obesity and the metabolic syndrome in developing countries. Indian journal of pediatrics. 2013;80 Suppl 1:S28-S37. [DOI] [PubMed] [Google Scholar]
- 4.Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM. Childhood obesity and cardiovascular dysfunction. Journal of the American College of Cardiology. 2013;62(15):1309-1319. [DOI] [PubMed] [Google Scholar]
- 5.Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. The American journal of clinical nutrition. 2012;96(4):714-726. [DOI] [PubMed] [Google Scholar]
- 6.Dulloo AG, Jacquet J, Solinas G, Montani JP, Schutz Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. International journal of obesity (2005). 2010;34 Suppl 2:S4-S17. [DOI] [PubMed] [Google Scholar]
- 7.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet (London, England). 2006;368(9548):1681-1688. [DOI] [PubMed] [Google Scholar]
- 8.Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension. 2011;57(6):1101-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maskarinec G, Grandinetti A, Matsuura G, Sharma S, Mau M, Henderson BE, et al. Diabetes prevalence and body mass index differ by ethnicity: the Multiethnic Cohort. Ethnicity & disease. 2009;19(1):49-55. [PMC free article] [PubMed] [Google Scholar]
- 10.Liska D, Dufour S, Zern TL, Taksali S, Cali AM, Dziura J, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PloS one. 2007;2(6):e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azuma K, Kadowaki T, Cetinel C, Kadota A, El-Saed A, Kadowaki S, et al. Higher liver fat content among Japanese in Japan compared with non-Hispanic whites in the United States. Metabolism: clinical and experimental. 2009;58(8):1200-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Interim Report of the Commission on Ending Childhood Obesity. Geneva, Switzerland: World Health Organization, 2015. [Google Scholar]
- 13.World Health Organization. Obesity: preventing and managing the global epidemic Report of a WHO Consultation (WHO Technical Report Series 894). Geneva, Switzerland: World Health Organization, 2000. [PubMed] [Google Scholar]
- 14.Rolland-Cachera MF, Sempe M, Guilloud-Bataille M, Patois E, Pequignot-Guggenbuhl F, Fautrad V. Adiposity indices in children. The American journal of clinical nutrition. 1982;36(1):178-184. [DOI] [PubMed] [Google Scholar]
- 15.de Onis M, Garza C, Onyango AW, Rolland-Cachera MF. [WHO growth standards for infants and young children]. Archives de pediatrie : organe officiel de la Societe francaise de pediatrie. 2009;16(1):47-53. [DOI] [PubMed] [Google Scholar]
- 16.Bellizzi MC, Dietz WH. Workshop on childhood obesity: summary of the discussion. The American journal of clinical nutrition. 1999;70(1):173s-175s. [DOI] [PubMed] [Google Scholar]
- 17.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ (Clinical research ed). 2000;320(7244):1240-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attard SM, Herring AH, Howard AG, Gordon-Larsen P. Longitudinal trajectories of BMI and cardiovascular disease risk: the national longitudinal study of adolescent health. Obesity (Silver Spring, Md). 2013;21(11):2180-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olds T, Maher C, Zumin S, Peneau S, Lioret S, Castetbon K, et al. Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2011;6(5-6):342-360. [DOI] [PubMed] [Google Scholar]
- 20.Sjoberg A, Lissner L, Albertsson-Wikland K, Marild S. Recent anthropometric trends among Swedish school children: evidence for decreasing prevalence of overweight in girls. Acta paediatrica (Oslo, Norway : 1992). 2008;97(1):118-123. [DOI] [PubMed] [Google Scholar]
- 21.Wabitsch M, Moss A, Kromeyer-Hauschild K. Unexpected plateauing of childhood obesity rates in developed countries. BMC medicine. 2014;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meszaros Z, Meszaros J, Volgyi E, Sziva A, Pampakas P, Prokai A, et al. Body mass and body fat in Hungarian schoolboys: differences between 1980-2005. Journal of physiological anthropology. 2008;27(5):241-325. [DOI] [PubMed] [Google Scholar]
- 23.Zong XN, Li H. Secular trends in prevalence and risk factors of obesity in infants and preschool children in 9 Chinese cities, 1986-2006. PloS one. 2012;7(10):e46942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50(8):1844-1850. [DOI] [PubMed] [Google Scholar]
- 25.Case AL, D., Paxson D. Economic Status and Health in Childhood: The Origins of the Gradient. Amer Econ Rev. 2002;92(5):1308-1334. [DOI] [PubMed] [Google Scholar]
- 26.Darmon N, Drewnowski A. Does social class predict diet quality? The American journal of clinical nutrition. 2008;87(5):1107-1117. [DOI] [PubMed] [Google Scholar]
- 27.Cameron AJ, Spence AC, Laws R, Hesketh KD, Lioret S, Campbell KJ. A Review of the Relationship Between Socioeconomic Position and the Early-Life Predictors of Obesity. Current obesity reports. 2015;4(3):350-362. [DOI] [PubMed] [Google Scholar]
- 28.Belanger M, O’Loughlin J, Karp I, Barnett TA, Sabiston CM. Physical activity fluctuations and body fat during adolescence. Pediatric obesity. 2012;7(1):73-81. [DOI] [PubMed] [Google Scholar]
- 29.Belcher BR, Berrigan D, Papachristopoulou A, Brady SM, Bernstein SB, Brychta RJ, et al. Effects of Interrupting Children’s Sedentary Behaviors With Activity on Metabolic Function: A Randomized Trial. The Journal of clinical endocrinology and metabolism. 2015;100(10):3735-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng J, Glass TA, Curriero FC, Stewart WF, Schwartz BS. The built environment and obesity: A systematic review of the epidemiologic evidence. Health & Place. 2010;16(2):175-190. [DOI] [PubMed] [Google Scholar]
- 31.Ding D, Sallis JF, Kerr J, Lee S, Rosenberg DE. Neighborhood environment and physical activity among youth a review. American journal of preventive medicine. 2011;41(4):442-455. [DOI] [PubMed] [Google Scholar]
- 32.Ding D, Gebel K. Built environment, physical activity, and obesity: What have we learned from reviewing the literature? Health & Place. 2012;18(1):100-105. [DOI] [PubMed] [Google Scholar]
- 33.Datar A, Nicosia N, Shier V. Parent perceptions of neighborhood safety and children’s physical activity, sedentary behavior, and obesity: evidence from a national longitudinal study. American journal of epidemiology. 2013;177(10):1065-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prins RG, Kamphuis CB, van Empelen P, Beenackers MA, Brug J, Mackenbach JP, et al. Explaining socio-demographic differences in disengagement from sports in adolescence. European journal of public health. 2013;23(5):811-816. [DOI] [PubMed] [Google Scholar]
- 35.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behavior genetics. 1997;27(4):325-351. [DOI] [PubMed] [Google Scholar]
- 36.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. The New England journal of medicine. 1997;337(13):869-873. [DOI] [PubMed] [Google Scholar]
- 37.Foraita R, Gunther F, Gwozdz W, Reisch LA, Russo P, Lauria F, et al. Does the FTO gene interact with the socioeconomic status on the obesity development among young European children? Results from the IDEFICS study. International journal of obesity (2005). 2015;39(1):1-6. [DOI] [PubMed] [Google Scholar]
- 38.Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nature genetics. 2009;41(1):25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chambers JC, Elliott P, Zabaneh D, Zhang W, Li Y, Froguel P, et al. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nature genetics. 2008;40(6):716-718. [DOI] [PubMed] [Google Scholar]
- 40.Graff M, North KE, Richardson AS, Young KM, Mohlke KL, Lange LA, et al. Screen time behaviours may interact with obesity genes, independent of physical activity, to influence adolescent BMI in an ethnically diverse cohort. Pediatric obesity. 2013;8(6):e74-e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Law CM, Barker DJ, Osmond C, Fall CH, Simmonds SJ. Early growth and abdominal fatness in adult life. Journal of epidemiology and community health. 1992;46(3):184-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leduc L, Levy E, Bouity-Voubou M, Delvin E. Fetal programming of atherosclerosis: possible role of the mitochondria. European journal of obstetrics, gynecology, and reproductive biology. 2010;149(2):127-130. [DOI] [PubMed] [Google Scholar]
- 43.Lane RH. Fetal programming, epigenetics, and adult onset disease. Clinics in perinatology. 2014;41(4):815-831. [DOI] [PubMed] [Google Scholar]
- 44.Desai M, Jellyman JK, Ross MG. Epigenomics, gestational programming and risk of metabolic syndrome. International journal of obesity (2005). 2015;39(4):633-641. [DOI] [PubMed] [Google Scholar]
- 45.Alexander BT, Dasinger JH, Intapad S. Fetal programming and cardiovascular pathology. Comprehensive Physiology. 2015;5(2):997-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rolland-Cachera MF, Akrout M, Peneau S. Nutrient Intakes in Early Life and Risk of Obesity. Int J Environ Res Public Health. 2016;13(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee KW, Abrahamowicz M, Leonard GT, Richer L, Perron M, Veillette S, et al. Prenatal exposure to cigarette smoke interacts with OPRM1 to modulate dietary preference for fat. Journal of psychiatry & neuroscience : JPN. 2015;40(1):38-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson JM, Clark PM, Robinson E, Becroft DM, Pattison NS, Glavish N, et al. Risk factors for small-for-gestational-age babies: The Auckland Birthweight Collaborative Study. Journal of paediatrics and child health. 2001;37(4):369-375. [DOI] [PubMed] [Google Scholar]
- 49.Morgan AR, Thompson JM, Murphy R, Black PN, Lam WJ, Ferguson LR, et al. Obesity and diabetes genes are associated with being born small for gestational age: results from the Auckland Birthweight Collaborative study. BMC medical genetics. 2010;11:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo ZC, Nuyt AM, Delvin E, Fraser WD, Julien P, Audibert F, et al. Maternal and fetal leptin, adiponectin levels and associations with fetal insulin sensitivity. Obesity (Silver Spring, Md). 2013;21(1):210-216. [DOI] [PubMed] [Google Scholar]
- 51.Greenhalgh K, Meyer KM, Aagaard KM, Wilmes P. The human gut microbiome in health: establishment and resilience of microbiota over a lifetime. Environmental microbiology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huttehower C GD, Knight R, Abubucker S, Badger JH, Chinwalla AT, et al. For the Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Methé BA NK, Pop M, Creasy HH, Giglio MG, Huttenhower C., et al. For the Human Microbiome Project Consortsium;. A framework for human microbiome research. Nature. 2012;486(7402):215-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arora T, Backhed F. The gut microbiota and metabolic disease: current understanding and future perspectives. Journal of internal medicine. 2016. [DOI] [PubMed] [Google Scholar]
- 56.Cong X, Xu W, Janton S, Henderson WA, Matson A, McGrath JM, et al. Gut Microbiome Developmental Patterns in Early Life of Preterm Infants: Impacts of Feeding and Gender. PloS one. 2016;11(4):e0152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Debré PLG. Intestinal microbiota. Bull Acad Natle Méd. 2014;198(9):1667-1684. [PubMed] [Google Scholar]
- 59.Angelakis E, Armougom F, Million M, Raoult D. The relationship between gut microbiota and weight gain in humans. Future microbiology. 2012;7(1):91-109. [DOI] [PubMed] [Google Scholar]
- 60.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541-546. [DOI] [PubMed] [Google Scholar]
- 61.Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World journal of gastroenterology. 2014;20(43):16079-16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aron-Wisnewsky J, Dore J, Clement K. The importance of the gut microbiota after bariatric surgery. Nature reviews Gastroenterology & hepatology. 2012;9(10):590-598. [DOI] [PubMed] [Google Scholar]
- 63.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bluher S, Schwarz P. Metabolically healthy obesity from childhood to adulthood - Does weight status alone matter? Metabolism: clinical and experimental. 2014;63(9):1084-1092. [DOI] [PubMed] [Google Scholar]
- 65.Prince RL, Kuk JL, Ambler KA, Dhaliwal J, Ball GD. Predictors of metabolically healthy obesity in children. Diabetes care. 2014;37(5):1462-1468. [DOI] [PubMed] [Google Scholar]
- 66.Cook S, Kavey RE. Dyslipidemia and pediatric obesity. Pediatric clinics of North America. 2011;58(6):1363-1373, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finn P. Dyslipidemia in Overweight and Obese School-Aged Children. NASN school nurse (Print). 2015;30(5):255-257. [DOI] [PubMed] [Google Scholar]
- 68.Ali O, Cerjak D, Kent JW, Jr., James R, Blangero J, Zhang Y. Obesity, central adiposity and cardiometabolic risk factors in children and adolescents: a family-based study. Pediatric obesity. 2014;9(3):e58-e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. Journal of hepatology. 2009;51(3):433-445. [DOI] [PubMed] [Google Scholar]
- 70.Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR American journal of roentgenology. 2007;189(6):W320-W323. [DOI] [PubMed] [Google Scholar]
- 71.Alkhouri N, Sedki E, Alisi A, Lopez R, Pinzani M, Feldstein AE, et al. Combined paediatric NAFLD fibrosis index and transient elastography to predict clinically significant fibrosis in children with fatty liver disease. Liver international : official journal of the International Association for the Study of the Liver. 2013;33(1):79-85. [DOI] [PubMed] [Google Scholar]
- 72.Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267(2):422-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levy-Marchal C, Arslanian S, Cutfield W, Sinaiko A, Druet C, Marcovecchio ML, et al. Insulin resistance in children: consensus, perspective, and future directions. The Journal of clinical endocrinology and metabolism. 2010;95(12):5189-5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown RJ, Yanovski JA. Estimation of insulin sensitivity in children: methods, measures and controversies. Pediatric diabetes. 2014;15(3):151-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marcovina S, Bowsher RR, Miller WG, Staten M, Myers G, Caudill SP, et al. Standardization of insulin immunoassays: report of the American Diabetes Association Workgroup. Clinical chemistry. 2007;53(4):711-716. [DOI] [PubMed] [Google Scholar]
- 76.Miller WG, Thienpont LM, Van Uytfanghe K, Clark PM, Lindstedt P, Nilsson G, et al. Toward standardization of insulin immunoassays. Clinical chemistry. 2009;55(5):1011-1018. [DOI] [PubMed] [Google Scholar]
- 77.Oran PE, Jarvis JW, Borges CR, Sherma ND, Nelson RW. Mass spectrometric immunoassay of intact insulin and related variants for population proteomics studies. Proteomics Clinical applications. 2011;5(7-8):454-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Der Gugten JG, Wong S, Holmes DT. Quantitation of Insulin Analogues in Serum Using Immunoaffinity Extraction, Liquid Chromatography, and Tandem Mass Spectrometry. Methods in molecular biology (Clifton, NJ). 2016;1378:119-130. [DOI] [PubMed] [Google Scholar]
- 79.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes care. 2004;27(6):1487-1495. [DOI] [PubMed] [Google Scholar]
- 80.Allard P, Delvin EE, Paradis G, Hanley JA, O’Loughlin J, Lavallee C, et al. Distribution of fasting plasma insulin, free fatty acids, and glucose concentrations and of homeostasis model assessment of insulin resistance in a representative sample of Quebec children and adolescents. Clinical chemistry. 2003;49(4):644-649. [DOI] [PubMed] [Google Scholar]
- 81.Rocco ER, Mory DB, Bergamin CS, Valente F, Miranda VL, Calegare BF, et al. Optimal cutoff points for body mass index, waist circumference and HOMA-IR to identify a cluster of cardiometabolic abnormalities in normal glucose-tolerant Brazilian children and adolescents. Arquivos brasileiros de endocrinologia e metabologia. 2011;55(8):638-645. [DOI] [PubMed] [Google Scholar]
- 82.Bindler RC, Daratha KB. Relationship of weight status and cardiometabolic outcomes for adolescents in the TEAMS study. Biological research for nursing. 2012;14(1):65-70. [DOI] [PubMed] [Google Scholar]
- 83.de Onis M, Martinez-Costa C, Nunez F, Nguefack-Tsague G, Montal A, Brines J. Association between WHO cutoffs for childhood overweight and obesity and cardiometabolic risk. Public health nutrition. 2013;16(4):625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lambert M, Paradis G, O’Loughlin J, Delvin EE, Hanley JA, Levy E. Insulin resistance syndrome in a representative sample of children and adolescents from Quebec, Canada. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2004;28(7):833-841. [DOI] [PubMed] [Google Scholar]
- 85.Lambert M, Delvin EE, Paradis G, O’Loughlin J, Hanley JA, Levy E. C-reactive protein and features of the metabolic syndrome in a population-based sample of children and adolescents. Clinical chemistry. 2004;50(10):1762-1768. [DOI] [PubMed] [Google Scholar]
- 86.Schlenz H, Intemann T, Wolters M, Gonzalez-Gil EM, Nappo A, Fraterman A, et al. C-reactive protein reference percentiles among pre-adolescent children in Europe based on the IDEFICS study population. International journal of obesity (2005). 2014;38 Suppl 2:S26-S31. [DOI] [PubMed] [Google Scholar]
- 87.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87-91. [DOI] [PubMed] [Google Scholar]
- 88.Lambert M, O’Loughlin J, Delvin EE, Levy E, Chiolero A, Paradis G. Association between insulin, leptin, adiponectin and blood pressure in youth. Journal of hypertension. 2009;27(5):1025-1032. [DOI] [PubMed] [Google Scholar]
- 89.Kim J, Bhattacharjee R, Kheirandish-Gozal L, Khalyfa A, Sans Capdevila O, Tauman R, et al. Insulin sensitivity, serum lipids, and systemic inflammatory markers in school-aged obese and nonobese children. International journal of pediatrics. 2010;2010:846098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Izadpanah A, Barnard RJ, Almeda AJ, Baldwin GC, Bridges SA, Shellman ER, et al. A short-term diet and exercise intervention ameliorates inflammation and markers of metabolic health in overweight/obese children. American journal of physiology Endocrinology and metabolism. 2012;303(4):E542-E550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zabaleta J, Velasco-Gonzalez C, Estrada J, Ravussin E, Pelligrino N, Mohler MC, et al. Inverse correlation of serum inflammatory markers with metabolic parameters in healthy, Black and White prepubertal youth. International journal of obesity (2005). 2014;38(4): 563-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kelishadi R, Hashemi M, Mohammadifard N, Asgary S, Khavarian N. Association of changes in oxidative and proinflammatory states with changes in vascular function after a lifestyle modification trial among obese children. Clinical chemistry. 2008;54(1):147-153. [DOI] [PubMed] [Google Scholar]
- 93.Galan-Chilet I, Tellez-Plaza M, Guallar E, De Marco G, Lopez-Izquierdo R, Gonzalez-Manzano I, et al. Plasma selenium levels and oxidative stress biomarkers: A gene–environment interaction population-based study. Free Radical Biology and Medicine. 2014;74:229-236. [DOI] [PubMed] [Google Scholar]


