The case of a patient with the rare “8p11 (eight‐p‐eleven) myeloproliferative syndrome” with CEP‐FGFR1 rearrangement is reported.
Keywords: CEP110‐FGFR1 fusion protein, Eosinophilia, Acute myeloid leukemia, Imatinib, Dasatinib
Abstract
This brief communication reports on a patient with an exceedingly rare “8p11 (eight‐p‐eleven) myeloproliferative syndrome” (EMS) with CEP110‐FGFR1 rearrangement who responded to treatment with the multi‐tyrosine kinase inhibitor (TKI) dasatinib. Dasatinib improved quality of life substantially by increasing blood counts and reducing the need for transfusions. This report demonstrates that the second‐generation TKI may provide a therapeutic option for elderly and frail EMS patients who cannot be offered aggressive therapy, including allogeneic hematopoietic cell transplantation.
Background
Besides “classical” myeloproliferative neoplasms (MPN), the World Health Organization 2016 classification of tumors of hematopoietic and lymphoid tissues/myeloid neoplasms and acute leukemia incorporates uncommon MPN variants with distinct genetic abnormalities as “myeloid/lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB, or FGFR1” [1]. Within these, the subset of “8p11 (eight‐p‐eleven) myeloproliferative syndromes” (EMS) is defined as acquired hematopoietic stem cell disorders characterized by reciprocal translocation of the FGFR1 gene on chromosome 8p11 to at least three different partner genes at chromosomal regions 6q27, 13q12, or 9q33 [2, 3]. The CEP110‐FGFR1 fusion translates into a protein that acts as a constitutively active oncogenic tyrosine kinase [4]. EMS may manifest with concomitant lymphoblastic lymphoma and frequently transforms into acute myeloid leukemia (AML) [5]. Since the first description of the translocation t(8;9)(p11q33) in 1983 [6] and the identification of the resulting CEP110‐FGFR1 fusion in 2000 [3], to our knowledge, only 14 EMS patients with this fusion have been reported. Today, this disease subgroup is considered refractory to tyrosine kinase inhibitors (TKI) and is thus afflicted with a particularly poor prognosis [1].
Case Report
Here, we report on a 64‐year‐old woman who presented in 2004 with fatigue, weakness, dizziness, anemia (Hb 99 g/L), and leukocytosis (33 x 109/L; 28% neutrophils, 26% eosinophils, 2% basophils, 15% monocytes, 16% myeloid progenitors). The bone marrow (BM) was hypercellular, with 53% eosinophils and 3% blasts. Molecular and cytogenetic analysis confirmed the diagnosis of EMS with translocation t(8;9)(p11;q33) and CEP110‐FGFR1 fusion transcript. A lymphoproliferative malignancy was ruled out. With no response to 1 month of prednisone, treatment with imatinib was initiated (100 mg/d, gradual increase to 400 mg/d). At the time of diagnosis, imatinib was the only TKI available, but little data were available regarding its efficacy in patients with the CEP110‐FGFR1 fusion transcript. After 9 months, imatinib was tapered and maintained at 100 mg/d for almost 3 years of stabilized disease. However, in 2007, disease progressed into AML with multi‐lineage myelodysplasia and 23% and 65% blasts in blood and BM, respectively. The CEP110‐FGFR1 fusion transcript remained detectable (Fig. 1A). Two cycles of standard induction chemotherapy (“3 + 7” daunorubicin/cytarabine) resulted in a complete morphological remission of the AML. In 2013, after 6 years of remission of both AML and EMS, the patient became symptomatic again. Diagnostic work‐up revealed an ongoing remission for AML but relapse of EMS with detectable CEP110‐FGFR1 transcripts, anemia (Hb 89 g/L), thrombocytopenia (19 × 109/L), and pronounced eosinophilia (12.8 × 109/L; Fig. 1A, 1B). Re‐initiation of imatinib treatment reduced eosinophil counts by 50%; however, anemia and thrombocytopenia persisted. The patient required weekly red blood cell transfusions. With availability of novel TKIs, treatment was switched to dasatinib (50 mg p.o. every second day), a second‐generation TKI that is also known to inhibit SRC family tyrosine kinases [7]. Off‐label treatment with ponatinib was not a therapeutic alternative for this then 73‐year‐old patient as she was suffering from cardiovascular comorbidities (carotid artery stenosis). Dasatinib treatment restored platelet counts and hemoglobin to subnormal levels, and after 7 months, no more transfusions were required (Fig. 1B). Eosinophil counts normalized within 3 months. Despite detectable CEP110‐FGFR1 at all times, this elderly and frail patient benefited clinically from this oral treatment for >9 months. Ultimately, dasatinib therapy was complicated by pleural effusions and thus had to be decreased in dose and finally stopped. At this point, the patient refused further transfusions, developed pancytopenia (but no evidence of AML relapse), and died 3 months later of pneumonia.
Figure 1.
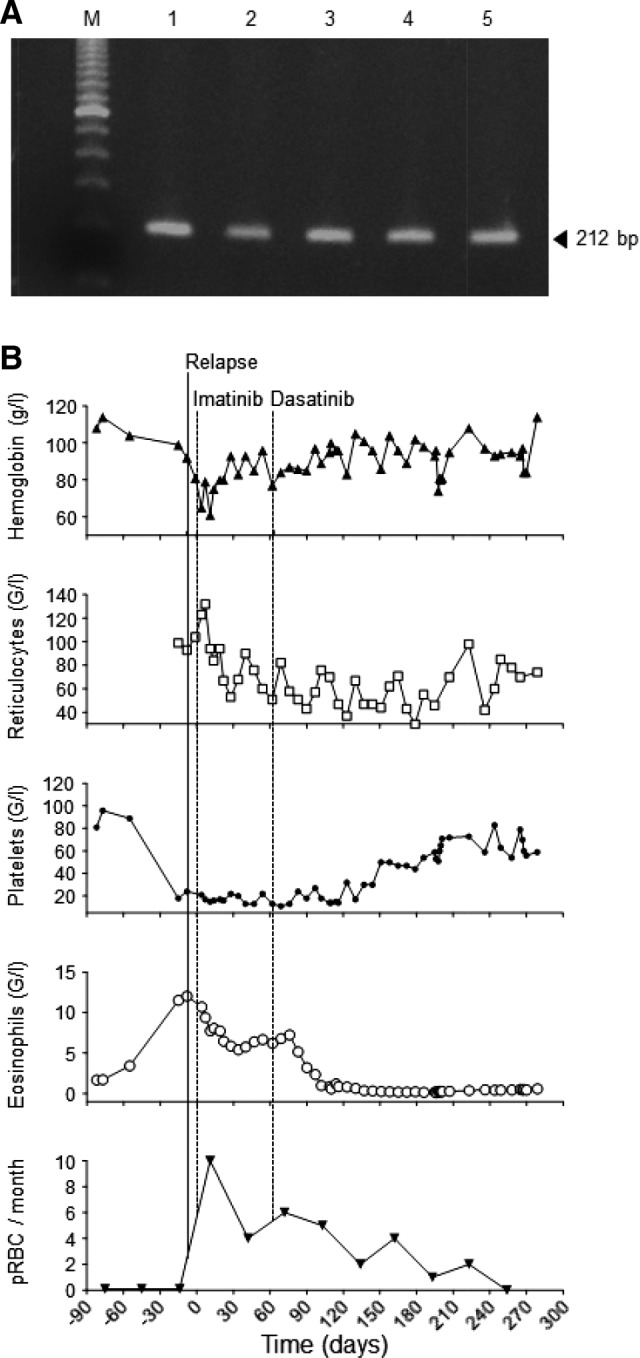
Dynamics of the disease as assessed by CEP110‐FGFR1, hemogram, and number of transfusions. (A): Gel electrophoresis of the CEP110‐FGFR1 polymerase chain reaction products (212 bp). Lane M, 100 bp ladder as a size marker; lane 1, peripheral blood (PB) at diagnosis of 8p11 myeloproliferative syndrome; lane 2, bone marrow (BM) at diagnosis of AML; lanes 3 and 4, PB and BM at relapse, respectively; lane 5, positive control. (B): Hemoglobin, reticulocyte, platelet, and eosinophil counts before, during, and after treatment with imatinib and dasatinib. Numbers of pRBC transfusions per month are displayed in the bottom panel. The solid line represents relapse of disease. Day 0 (dotted line) indicates start with imatinib. Day 63 (dotted line) indicates start of dasatinib treatment.
Abbreviations: pRBC, packed red blood cell.
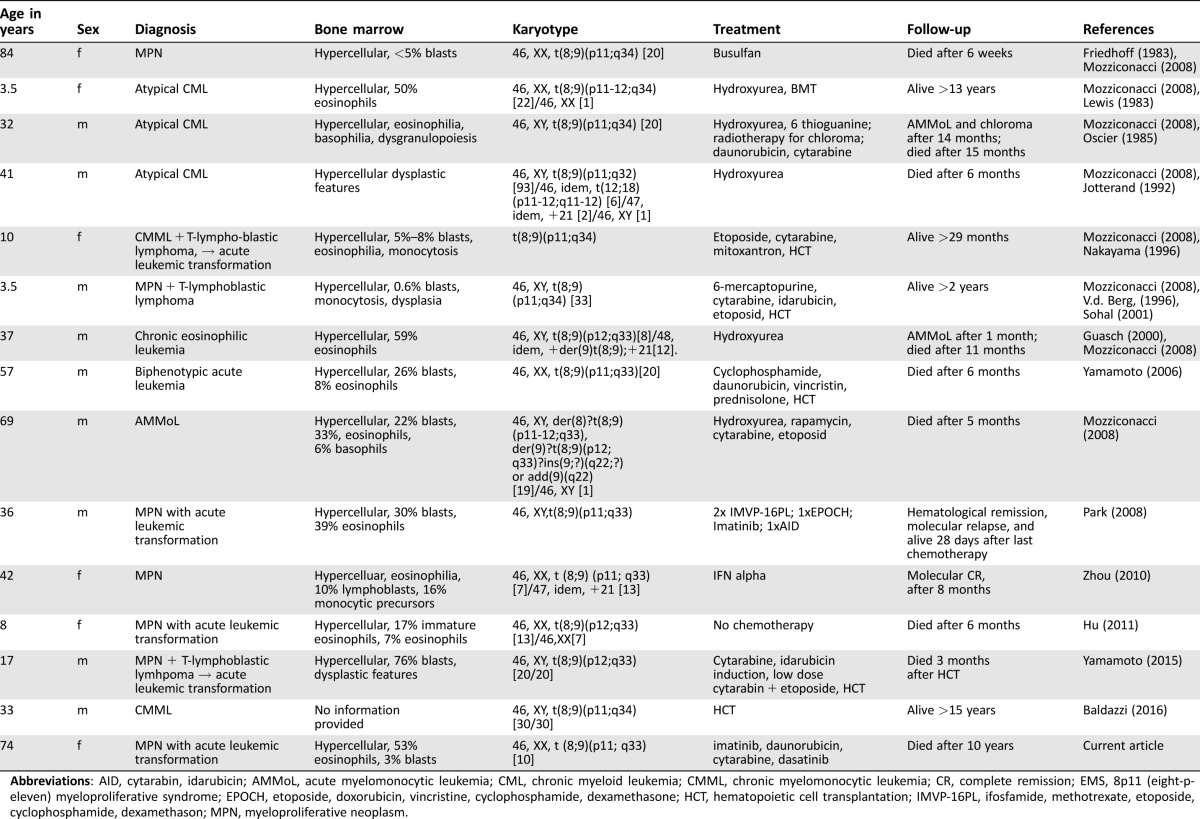
In contrast to MPN with alterations involving PDGFRA/B, those with rearranged FGFR1 (EMS) are considered refractory to imatinib and other TKIs [1], [4], [5], [8]. EMS with CEP110‐FGFR1 in particular represent highly aggressive subsets of MPN for which no established therapeutic strategies exist. Table 1 displays a comprehensive overview of published reports, characteristics, treatments, and outcomes of EMS patients. Of 14 previously reported EMS patients, 8 died within 15 months after diagnosis [2], [3], [4], [6], [8], [9], [10], [11]. Six patients underwent allogeneic hematopoietic cell transplantation (HCT), and four of these were long‐term survivors, suggesting this aggressive strategy as the only curative treatment option [5]. In one case, molecular remission was achieved after 8 months following single‐agent IFNα treatment [10]. In another patient, molecular relapse was confirmed 28 days post combination chemotherapy, but no information on outcome and survival was provided. Within the published literature, reports on treatment with TKIs are scarce. Recently, the third‐generation TKI ponatinib has been demonstrated to inhibit phosphorylation levels of various FGFR1‐fusion kinases and their downstream effector functions, resulting in cell growth inhibition and cell death in vitro [12]. Induced pluripotent stem cells generated from a patient with EMS with CEP110‐FGFR1 formed reduced colony‐forming unit numbers when treated with CHIR258 (dovitinib), PKC 412 (midostaurin), and ponatinib, but not with imatinib, and similar effects were observed in vitro for this patient's blood cells [2]. Finally, a partial response to ponatinib has recently been reported in a 47‐year‐old patient with BCR‐FGFR1‐positive mixed‐phenotype leukemia who was subsequently rescued with repeat inductions with methotrexate and cytarabine followed by allogeneic HCT [13].
Table 1. Comprehensive review of the literature with characteristics of 14 previously reported EMS patients.
Abbreviations: AID, cytarabin, idarubicin; AMMoL, acute myelomonocytic leukemia; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; CR, complete remission; EMS, 8p11 (eight‐p‐eleven) myeloproliferative syndrome; EPOCH, etoposide, doxorubicin, vincristine, cyclophosphamide, dexamethasone; HCT, hematopoietic cell transplantation; IMVP‐16PL, ifosfamide, methotrexate, etoposide, cyclophosphamide, dexamethason; MPN, myeloproliferative neoplasm.
The clinical scenario described here was different, as our patient was 64 years old at diagnosis and 73 years old when relapsed with EMS after AML, suffering from cardiovascular and other comorbidities that precluded her from treatment with ponatinib and consolidation with allogeneic HCT. Stable disease was initially maintained for 3 years under treatment with imatinib. Possibly, additional cryptic molecular aberrations were present that may have functioned as therapeutic targets of imatinib and thereby induced the unexpected response to the first‐generation TKI. Later, activity of dasatinib was observed at a time when the disease was already refractory to imatinib. The explanation for the inhibitory activity of the second‐generation TKI dasatinib is most likely due to its capacity to block SRC family kinases [7]. SRC family kinases have been reported to play a role in malignant cell signaling [14]. Moreover, it is known that FGFR1‐fusion transcripts can signal through SRC‐kinases [7], [12]. We postulate that dasatinib can exert inhibitory activity in FGFR1‐rearranged diseases such as EMS with CEP110‐FGFR1 rearrangements by blocking SRC‐kinases.
For the first time, we report on a patient with relapsed, imatinib‐refractory EMS who has been treated successfully with dasatinib to achieve a hematological remission for more than 9 months. We demonstrate that the oral multi‐TKI dasatinib may provide a therapeutic option for elderly and frail EMS patients who cannot be offered allogeneic HCT.
Contributed equally.
Author Contributions
Conception/Design: Marc Wehrli, Antonia M.S. Müller, Jeroen S. Goede
Provision of study material or patients: Heinrich H. Gattiker, Markus G. Manz, Antonia M.S. Müller, Jeroen S. Goede
Collection and/or assembly of data: Marc Wehrli, Elisabeth Oppliger Leibundgut, Heinrich H. Gattiker, Antonia M.S. Müller, Jeroen S. Goede
Data analysis and interpretation: Marc Wehrli, Elisabeth Oppliger Leibundgut, Antonia M.S. Müller, Jeroen S. Goede
Manuscript writing: Marc Wehrli, Elisabeth Oppliger Leibundgut, Markus G. Manz, Antonia M.S. Müller, Jeroen S. Goede
Final approval of manuscript: Marc Wehrli, Elisabeth Oppliger Leibundgut, Heinrich H. Gattiker, Markus G. Manz, Antonia M.S. Müller, Jeroen S. Goede
Disclosures
The authors indicated no financial relationships.
References
- 1. Arber DA, Orazi A, Hasserjian R et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391–2405. [DOI] [PubMed] [Google Scholar]
- 2. Yamamoto S, Otsu M, Matsuzaka E et al. Screening of drugs to treat 8p11 myeloproliferative syndrome using patient‐derived induced pluripotent stem cells with fusion gene CEP110‐FGFR1. PLoS One 2015;10:e0120841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guasch G, Mack GJ, Popovici C et al. FGFR1 is fused to the centrosome‐associated protein CEP110 in the 8p12 stem cell myeloproliferative disorder with t(8;9)(p12;q33). Blood 2000;95:1788–1796. [PubMed] [Google Scholar]
- 4. Mozziconacci MJ, Carbuccia N, Prebet T et al. Common features of myeloproliferative disorders with t(8;9)(p12;q33) and CEP110‐FGFR1 fusion: Report of a new case and review of the literature. Leuk Res 2008;32:1304–1308. [DOI] [PubMed] [Google Scholar]
- 5. Jackson CC, Medeiros LJ, Miranda RN. 8p11 myeloproliferative syndrome: A review. Hum Pathol 2010;41:461–476. [DOI] [PubMed] [Google Scholar]
- 6. Friedhoff F, Rajendra B, Moody R et al. Novel reciprocal translocation between chromosomes 8 and 9 found in a patient with myeloproliferative disorder. Cancer Genet Cytogenet 1983;9:391–394. [DOI] [PubMed] [Google Scholar]
- 7. Ren M, Qin H, Ren R et al. Src activation plays an important key role in lymphomagenesis induced by FGFR1 fusion kinases. Cancer Res 2011;71:7312–7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park TS, Song J, Kim JS et al. 8p11 myeloproliferative syndrome preceded by t(8;9)(p11;q33), CEP110/FGFR1 fusion transcript: Morphologic, molecular, and cytogenetic characterization of myeloid neoplasms associated with eosinophilia and FGFR1 abnormality. Cancer Genet Cytogenet 2008;181:93–99. [DOI] [PubMed] [Google Scholar]
- 9. Yamamoto K, Kawano H, Nishikawa S et al. A biphenotypic transformation of 8p11 myeloproliferative syndrome with CEP1/FGFR1 fusion gene. Eur J Haematol 2006;77:349–354. [DOI] [PubMed] [Google Scholar]
- 10. Zhou L, Fu W, Yuan Z et al. Complete molecular remission after interferon alpha treatment in a case of 8p11 myeloproliferative syndrome. Leuk Res 2010;34:e306–e307. [DOI] [PubMed] [Google Scholar]
- 11. Baldazzi C, Luatti S, Paolini S et al. FGFR1 and KAT6A rearrangements in patients with hematological malignancies and chromosome 8p11 abnormalities: Biological and clinical features. Am J Hematol 2016;91:E14–E16. [DOI] [PubMed] [Google Scholar]
- 12. Ren M, Qin H, Ren R et al. Ponatinib suppresses the development of myeloid and lymphoid malignancies associated with FGFR1 abnormalities. Leukemia 2013;27:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khodadoust MS, Luo B, Medeiros BC et al. Clinical activity of ponatinib in a patient with FGFR1‐rearranged mixed‐phenotype acute leukemia. Leukemia 2016;30:947–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mathisen MS, Kantarjian HM, Cortes J et al. Practical issues surrounding the explosion of tyrosine kinase inhibitors for the management of chronic myeloid leukemia. Blood Rev 2014;28:179–187. [DOI] [PubMed] [Google Scholar]