Abstract
Background
Infections are an important cause of morbidity and mortality after burn injuries. Here, we describe the timeline of infections and pathogens after burns.
Methods
A retrospective study was performed in a large tertiary care burn center from 2004 through 2013. Analyses were performed on healthcare-associated infections (HAI) meeting Centers for Disease Control and Prevention criteria and on all positive cultures. Incidence rates (IR) per 1,000 days were calculated for specific HAI categories and pathogens, and across hospitalization time (week 1, 2–3 and 4+).
Results
Among 5,524 patients the median burn size was 4% of total body surface area (interquartile range, 2–10%). 7% of patients developed an HAI, of whom 33% had more than one HAI episode. Gram-positive bacteria were isolated earlier and Gram-negative later during hospitalization. Of 1,788 bacterial isolates, 44% met criteria for multi-drug resistance and 23% for extensive drug resistance. Bacteria tended to become increasingly resistant to antibiotics as time from admission increased.
Conclusions
We observed differences in infection type, pathogen and antibiotic resistant bacterium risk across time of hospitalization. These results may guide infection prevention in various stages of the post-burn admission.
Keywords: burn, intensive care unit, healthcare-associated infection, timing, bloodstream infection, pneumonia
Introduction
Burn injuries remain an important source of morbidity and mortality in the United States. An estimated 450,000 burn injuries require medical treatment, and 3,400 deaths are related to fires and burns per year in the US1. Burn patients are vulnerable to infections, and especially to infections with multidrug-resistant organisms2, 3. In the US, infections caused by resistant organisms add between $21 billion and $34 billion to health care costs annually, as compared to susceptible organisms4.
Outcomes after burn injuries vary; data from the National Burn Repository (NBR) shows high variability in mortality rates (2% to 12% hospital mortality) in high-volume centers, even after adjustment for burn severity5. This variability remains mostly unexplained, but is likely related to factors such as distribution of comorbidities including substance abuse, and infection rates. A burn injury results in an immunocompromised state; both through breakdown of the skin natural barrier function as well as through systemic mechanisms6–9. Unlike other immunocompromised host populations, few studies have been performed that describe the timeline of infections and infectious pathogens after burn injuries10. Here, we describe the timing of specific infections and pathogens during hospitalization in a large cohort of patients with burns.
Materials and Methods
Study Population and Design
All patients admitted to the burn unit of a large tertiary care referral burn center between 01/01/2004 and 12/31/2013 were included. Patients were identified through the North Carolina Jaycee Burn Center Registry, which includes data collected as part of participation in the National Burn Repository. Data were obtained from the NC Jaycee Burn Center Database, electronic health record, laboratory databases, and chart review. In addition, all data on healthcare-associated infections (HAI) was obtained from the Hospital Epidemiology database, which includes hospital-wide surveillance for all HAI defined by the Centers for Disease Control and Prevention (CDC) and performed in accordance with CDC criteria11.
The first hospital admission for each patient aged 18 years or older was reviewed. The data available from all data sources were merged and as needed additional medical record reviews were performed. Patients with missing discharge dates were excluded (n=36). This study was approved by the Institutional Review Board of the University of North Carolina.
Microbiology
All microbial isolates from all clinical cultures were considered “potential pathogens”. In addition, for viral pathogens, the results of nucleic acid amplification tests performed on blood, cerebrospinal fluid, or skin lesions were used as evidence of viral replication. No attempt was made in this study to distinguish colonization from infection. For bacterial isolates, the definitions outlined by Magiorakos et al. were used to define multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR) bacteria12. For Achromobacter sp. and Burkholderia spp. MDR, XDR, and PDR resistance was determined using Pseudomonas drug-resistance definitions. MDR status for Stenotrophomonas maltophilia and Streptococcus pneumoniae was determined by non-susceptibility to trimethoprim/sulfamethoxazole or penicillin, respectively. Enterobacteriaceae were further divided based on resistance to fluoroquinolones (FRE), presence of an extended spectrum beta-lactamase (ESBL) phenotype as per CDC guidelines (ESBL-E), and resistance to carbapenems (CRE).
Covariates
The revised Baux score was calculated for each patient, as described13. The Charlson Comorbidity Index was also calculated for each patient, as described14 Total burn surface area (TBSA) was potentially reported multiple times across data sources; if multiple TBSA values were identified, an average TBSA was computed and used for analysis. Burn mechanisms were reported as per NBR guidelines (contact, chemical, electrical, flame, radiation, scald, and other)5.
Statistical Analysis
Patients were followed from date of admission until death or hospital discharge. Incidence rates (IR) and 95% confidence intervals (CI) were calculated based on a Poisson distribution, and expressed as number of cases per 1,000 days. IRs and corresponding measures of precision were calculated for specific HAI categories and microbes. Time hospitalized was categorized by week of hospitalization, including Week 1 (0–7 days after admission for pathogens, 2–7 days after admission for HAIs), Weeks 2–3 (8–21 days after admission), and Weeks 4+ (22 or more days after admission). Differences in infection rates across hospitalization time intervals were compared using likelihood ratio tests from Poisson regression models. The 90-day cumulative incidence of specific HAIs and pathogens were calculated using Kaplan-Meier survival curves. Only patients at risk for HAIs as per CDC guidelines (i.e. hospitalized for ≥2 days) were included in HAI analyses.
All analyses were performed using SAS software version 9.3 (SAS Inc., Cary, NC).
Results
Patient and Burn Characteristics
From 2004 to 2013, 5,524 adult patients with burn injuries were included in this study. The median age was 42.3 years (interquartile range [IQR] 29.7–54.8 years), 73% of patients were male, and 53% were white (Table 1). The most common mechanisms of burn were fire/flame (53%), and scald (31%) injury. In addition, 461 (8.4%) patients had inhalational injury upon admission. The median burn size as defined by percentage of total body surface area (%TBSA) was 4%TBSA (IQR 2% – 10%TBSA). The median revised Baux score was 49.9 (IQR 36.0–64.9). When individually calculated per patient, this corresponded to a median estimated predicted mortality of 0.54% (IQR 0.19%–1.54%)13.
Table 1.
Demographics and clinical characteristics of patients with burn injuries.
| Characteristic | |||
|---|---|---|---|
| All | 5,524 | ||
| Male | 4,011 (72.6) | ||
| Race/Ethnicity | |||
| White | 2,952 (53.4) | ||
| Black | 1,462 (26.5) | ||
| Hispanic | 309 (5.6) | ||
| Other | 801 (14.5) | ||
| Age, median (IQR) | 42.3 (29.7–54.8) | ||
| Charlson Comorbidity Index, median (IQR) | 1 (0–2) | ||
| Burn size (%TBSA*), median (IQR) | 4.0 (2.0–10.0) | ||
| Burn mechanism | |||
| Flame | 2,936 (53.2) | ||
| Scald | 1,703 (30.8) | ||
| Contact | 286 (5.2) | ||
| Chemical | 235 (4.3) | ||
| Electrical | 218 (4.0) | ||
| Radiation | 14 (0.3) | ||
| Other Burn | 107 (1.9) | ||
| Unknown | 25 (0.5) | ||
| Inhalation injury | 461 (8.4) | ||
| Revised Baux score, median (IQR)† | 49.9 (36.0–64.9) | ||
| Length of stay-in days, median (IQR) | 8 (2–14) | ||
| ICU admission | 1,832 (33.2) | ||
| Mechanical ventilation | 740 (13.4) | ||
| Disposition | |||
| Death | 243 (4.4) | ||
| Home | 4,903 (88.8) | ||
| Long term care facility | 248 (4.5) | ||
| Other‡ | 130 (2.4) | ||
All data are shown as n (%), unless otherwise noted.
TBSA total body surface area.
Revised Baux score defined as age (years) + %TBSA (+17 if inhalational injury is present).
Other disposition includes transfers to another hospital units, acute care facilities, mental health facilities or substance abuse programs, and unknown alive disposition. IQR: interquartile range
Hospitalizations
The median length of stay was 8 days (IQR 2–10 days), however, 595 (10.8%) of patients had a prolonged hospitalization of >30 days, and 124 (2.4%) of patients were in the hospital for >90 days. 1,832 (33.2%) patients were admitted to the intensive care unit. The overall hospital mortality rate was 4.4%.
Healthcare-associated infections
When analyzing the first specific HAI of its type per patient, a total of 631 HAI events were diagnosed among 383 (7%) patients over total follow-up time of 81,171 days (Table 2). Among patients with at least one HAI, 257 (67%) had 1, 73 (19%) 2, 29 (8%) 3, and 24 (6%) ≥4 HAI events during their hospitalization. The most common HAIs were bloodstream infections (25%), skin and soft tissue infections (19%), catheter-associated urinary tract infections (14%), and ventilator-associated pneumonia (VAP, 13%). The cumulative probability of being diagnosed with a HAI increased with additional time hospitalized, such that almost 50% of patients remaining hospitalized 80 days from admission had experienced at least one HAI. In general, skin and soft tissue infections were the first to occur after admission, with a median time from admission of 3 days (IQR 3–11), followed by respiratory infections, with a median time from admission of 30 days (IQR 14–66.5), and C. difficile infections, with a median time from admission of 35.5 days (IQR 9–77). Overall, bloodstream and urinary tract infections occurred later in the admission at a median of 41 days (IQR 15–76 days), and 41 days (IQR 12–73 days) after admission, respectively. The incidence rate of BSI increased from 1.27 (95% CI 0.84–1.93) in the first week after admission to 1.32 (95% CI 0.92–1.90) in weeks 2–3, and to 2.66 (95% CI 2.08–3.39) in week 4 and later, incident cases per 1,000 patient/days (Table 2). The 90-day cumulative incidence of skin and soft tissue infections, urinary tract infections, respiratory infections and bloodstream infections are shown in Figures 1A and 1B.
Table 2.
Rates of healthcare-associated infections stratified by week(s) after hospitalization for burn injury.
| Weeks after admission
|
|||||
|---|---|---|---|---|---|
| Overall | Week 1 | Weeks 2 – 3 | Weeks 4+ | p-value | |
|
| |||||
| Bloodstream infection | 1.82 (1.52–2.18) | 1.27 (0.84–1.93) | 1.32 (0.92–1.90) | 2.66 (2.08–3.39) | <0.001 |
|
| |||||
| Skin and soft tissue infection | 1.73 (1.45–2.07) | 5.86 (4.82–7.13) | 0.65 (0.39–1.10) | 0.14 (0.05–0.36) | <0.01 |
|
| |||||
| Ventilator-associated pneumonia | 1.20 (0.96–1.50) | 0.63 (0.35–1.14) | 1.17 (0.80–1.72) | 1.59 (1.18–2.14) | 0.01 |
|
| |||||
| Catheter-associated UTI | 1.06 (0.84–1.34) | 0.63 (0.35–1.14) | 0.72 (0.44–1.17) | 1.62 (1.21–2.18) | 0.001 |
|
| |||||
| Tracheobronchitis | 0.90 (0.70–1.15) | 0.40 (0.19–0.85) | 0.90 (0.58–1.39) | 1.20 (0.86–1.68) | 0.01 |
|
| |||||
| Non-device UTI | 0.43 (0.30–0.61) | 0.46 (0.23–0.92) | 0.36 (0.18–0.72) | 0.46 (0.27–0.78) | 0.83 |
|
| |||||
| Surgical site infection | 0.41 (0.29–0.59) | 0.06 (0.00–0.41) | 0.40 (0.21–0.77) | 0.62 (0.40–0.98) | <0.0001 |
|
| |||||
| C. difficile | 0.14 (0.08–0.26) | NA* | 0.18 (0.07–0.47) | 0.19 (0.09–0.43) | 0.06 |
|
| |||||
| Non-ventilator associated pneumonia | 0.13 (0.07–0.24) | 0.06 (0.00–0.41) | 0.09 (0.02–0.36) | 0.19 (0.09–0.43) | 0.37 |
|
| |||||
| Other infections† | 0.45 (0.32–0.64) | 0.17 (0.06–0.54) | 0.36 (0.18–0.72) | 0.69 (0.45–1.07) | 0.02 |
First infection per patient was included. Rates were calculated using Poisson regression and are expressed in incident cases per 1,000 patient/days. P-values were calculated using likelihood ratio tests comparing rates during various periods.
No C. difficile infections occurred during the first week after admission.
Other infections include cardiovascular infections, other respiratory infections, ear/eyes/nose/throat infections, and non-C. difficile gastrointestinal infections. UTI: urinary tract infection.
Figure 1.
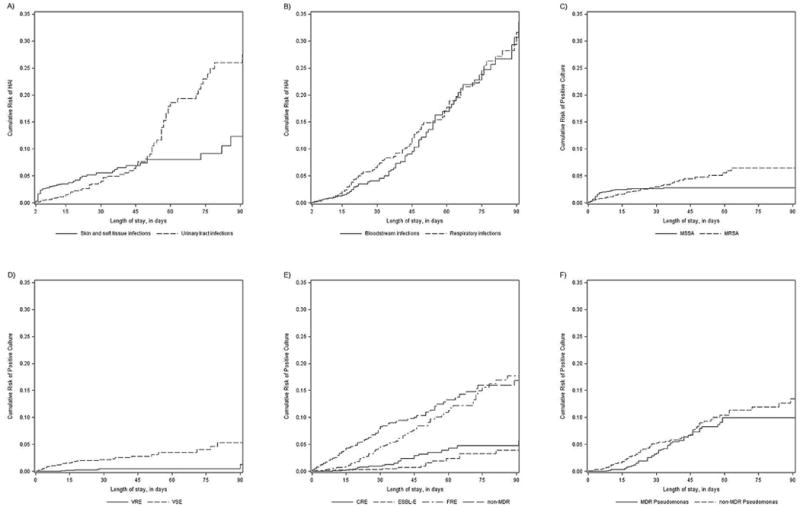
90-day cumulative incidences of A) skin and soft tissue infections and urinary tract infections, B) respiratory infections and bloodstream infections, C) methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus, D) vancomycin-susceptible Enterococcus sp. (VSE) and vancomycin-resistant Enterococcus sp. (VRE), E) non-multi-drug resistant (MDR) Enterobacteriaceae, fluoroquinolone-resistant Enterobacteriaceae (FRE), extended-spectrum-β-lactamase-producing Enterobacteriaceae (ESBL-E) and carbapenem-resistant Enterobacteriaceae (CRE) and F) non-MDR and MDR Pseudomonas sp.
Calculated using Kaplan-Meier estimates.
Potential Pathogens
The five most common pathogen classes were Enterobacteriaceae (37%), Staphylococcus aureus (26%), coagulase-negative Staphyloccocci (22%), Candida spp. (19%), and Pseudomonas spp. (17%). Overall, 774 (14%) patients had at least one potential pathogen reported from clinical cultures or viral PCR. 878 potential pathogens were recovered from these 774 patients; 298 (5%) had 1 potential pathogen isolated, 164 (3%) had 2 potential pathogens isolated, 97 (2%) had 3 potential pathogens isolated, and 215 (4%) had 4 or more potential pathogens isolated. Of note, two outbreaks with Acinetobacter baumannii occurred in the time periods 9/2007 to 4/2008 and 1/2009 to 9/201015.
In patients who had at least one positive culture, the time to first positive culture with any potential pathogen was 4 days (IQR 2–11 days). Streptococci spp., S. aureus, Enterococcus spp. and other Gram-positive pathogens were generally the first to be found after admission, with median times to isolation of 2 days (IQR 1–3 days), 3 days (IQR 2–8 days), 9 days (IQR 3–33 days), and 6.5 days (IQR 2–29 days), respectively. In contrast, Enterobacteriaceae, Pseudomonas sp., and Acinetobacter sp. tended to occur later during hospitalization at a median of 11.5 days (IQR 4–26 days), 18days (IQR 9–36 days), and 26 days (IQR 14–59 days), respectively.
Antibiotic Resistance
Antibiotic resistance was assessed for the following bacteria: Enterococcus sp., S. aureus, S. pneumoniae, Achromobacter sp., Acinetobacter sp., Burkholderia sp., Enterobacteriaceae, Pseudomonas sp., and S. maltophilia. Among these pathogens, 1,788 bacterial isolates derived from 543 unique patients were tested for antibiotic susceptibility; 793 (44%) met the criteria for MDR, and 416 (23%) met the criteria for XDR. No isolates met the criteria for PDR. MDR isolates tended to be isolated later in the hospitalization as compared to non-MDR bacterial isolates (Table 3); the median time from admission to first MDR isolation was 38 days (IQR 17–77 days), as compared to 11 days (IQR 3–44 days) for non-MDR isolates. This trend towards isolation later in the hospital course was seen in all major pathogen classes (Table 3). Among Gram-positive pathogens, the median time from admission to first positive culture for methicillin-resistant S. aureus (MRSA) was 11.5 days (IQR 3–33 days), as compared to 3 days (IQR 2–5 days) for methicillin-susceptible S. aureus (MSSA).
Table 3.
Rates of bacteria, fungi and viruses recovered in various periods after hospitalization for burn injury.
| Weeks after admission
|
||||||
|---|---|---|---|---|---|---|
| Overall | Week 1 | Weeks 2 – 3 | Weeks 4+ | p-value | ||
|
| ||||||
| Gram positive aerobic bacteria | ||||||
|
| ||||||
| S. aureus | ||||||
|
| ||||||
| MRSA | 1.03 (0.83–1.28) | 1.38 (1.00–1.90) | 1.04 (0.69–1.57) | 0.69 (0.45–1.08) | 0.04 | |
|
| ||||||
| MSSA | 1.29 (1.06–1.58) | 3.04 (2.44–3.77) | 0.61 (0.35–1.05) | 0.11 (0.04–0.34) | <0.0001 | |
|
| ||||||
| Coagulase-negative Staphylococcus spp. |
2.79 (2.42–3.21) | 2.43 (1.90–3.09) | 3.80 (3.05–4.73) | 1.99 (1.51–2.61) | <0.001 | |
|
| ||||||
| Enterococcus spp. | ||||||
|
| ||||||
| VRE | 0.10 (0.05–0.20) | 0.04 (0.00–0.26) | 0.13 (0.04–0.41) | 0.13 (0.05–0.34) | 0.39 | |
|
| ||||||
| VSE | 0.92 (0.73–1.17) | 1.45 (1.06–1.99) | 0.82 (0.52–1.31) | 0.50 (0.30–0.84) | 0.001 | |
|
| ||||||
| Streptococcus spp. | 1.20 (0.98–1.47) | 3.12 (2.51–3.86) | 0.14 (0.05–0.43) | 0.18 (0.07–0.43) | <0.0001 | |
|
| ||||||
| Other gram-positive aerobic bacteria | 0.27 (0.18–0.42) | 0.41 (0.23–0.74) | 0.18 (0.07–0.48) | 0.22 (0.11–0.47) | 0.26 | |
|
| ||||||
| Gram negative aerobic bacteria | ||||||
|
| ||||||
| Enterobacteriaceae | ||||||
|
| ||||||
| Carbapenem resistant | 0.43 (0.31–0.61) | 0.04 (0.00–0.26) | 0.40 (0.21–0.77) | 0.82 (0.55–1.23) | <0.0001 | |
|
| ||||||
| ESBL-phenotype | 0.29 (0.19–0.43) | 0.26 (0.12–0.54) | 0.09 (0.02–0.36) | 0.46 (0.27–0.78) | 0.03 | |
|
| ||||||
| fluoroquinolone resistant | 1.40 (1.15–1.69) | 0.52 (0.31–0.88) | 1.04 (0.69–1.56) | 2.61 (2.05–3.30) | <0.0001 | |
|
| ||||||
| other (“susceptible”) | 2.57 (2.23–2.97) | 2.81 (2.24–3.52) | 2.86 (2.23–3.68) | 2.06 (1.57–2.71) | 0.14 | |
|
| ||||||
| Pseudomonas spp. | ||||||
|
| ||||||
| multidrug-resistant | 0.90 (0.71–1.14) | 0.04 (0.00–0.26) | 0.81 (0.51–1.28) | 1.85 (1.40–2.44) | <0.0001 | |
|
| ||||||
| not multidrug-resistant | 1.46 (1.21–1.76) | 1.45 (1.06–1.98) | 1.82 (1.34–2.48) | 0.87 (0.58–1.31) | 0.01 | |
|
| ||||||
| Acinetobacter spp. | ||||||
|
| ||||||
| multidrug-resistant | 0.78 (0.60–1.00) | 0.07 (0.02–0.30) | 0.81 (0.51–1.28) | 1.44 (1.06–1.96) | <0.0001 | |
|
| ||||||
| not multidrug-resistant | 0.19 (0.11–0.31) | 0.26 (0.12–0.54) | 0.13 (0.04–0.42) | 0.16 (0.07–0.39) | 0.56 | |
|
| ||||||
| Other gram-negative aerobic bacteria | 3.34 (2.93–3.81) | 3.60 (2.95–4.40) | 2.67 (2.05–3.46) | 3.03 (2.41–3.81) | 0.18 | |
|
| ||||||
| Anaerobic bacteria | 0.22 (0.14–0.35) | 0.26 (0.12–0.54) | 0.13 (0.04–0.41) | 0.25 (0.13–0.51) | 0.54 | |
|
| ||||||
| Fungi | ||||||
|
| ||||||
| Candida sp. | 2.31 (1.98–2.69) | 1.04 (0.72–1.51) | 3.52 (2.81–4.41) | 2.24 (1.74–2.88) | <0.0001 | |
|
| ||||||
| Other yeasts | 0.09 (0.04–0.18) | 0.04 (0.00–0.26) | 0.13 (0.04–0.41) | 0.10 (0.03–0.30) | 0.47 | |
|
| ||||||
| Molds | 1.26 (1.03–1.54) | 1.72 (1.29–2.29) | 1.05 (0.70–1.58) | 0.89 (0.60–1.31) | 0.02 | |
|
| ||||||
| Viruses | ||||||
|
| ||||||
| Cytomegalovirus | 1.40 (1.16–1.70) | 0.11 (0.04–0.34) | 0.98 (0.65–1.50) | 2.67 (2.14–3.31) | <0.0001 | |
|
| ||||||
| Herpes simplex virus | 0.70 (0.54–0.92) | 0.26 (0.12–0.54) | 1.58 (1.13–2.20) | 0.44 (0.25–0.75) | <0.0001 | |
|
| ||||||
| Other viruses | 0.11 (0.06–0.21) | 0.04 (0.00–0.26) | 0* | 0.25 (0.13–0.51) | <0.01 | |
For each pathogen class, the first per patient was included. Rates were calculated using Poisson regression and are expressed in incident cases per 1,000 patient/days.
No ‘other’ viral infections occurred during weeks 2 – 3 after admission; MRSA: methicillin-resistant S. aureus; MSSA: methicillin-susceptible S. aureus; VRE: vancomycin-resistant Enterococcus spp.; VSE: vancomycin-susceptible Enterococcus spp.; ESBL: Extended spectrum beta-lactamase
As Gram-positive organisms were overall less common later during admission, the incidence rates of both MRSA and MSSA decreased over time since admission. However, in week 1 MSSA was more common with an incidence rate of 3.04 (95% CI 2.44–3.77), as compared to MRSA with 1.38 (95% CI 1.00–1.90) incident cases per 1,000 patient/days, respectively. In contrast, in the time period of 4 weeks and later after admission, MSSA became significantly less common as MRSA with MSSA rates decreasing to 0.11 (95% CI 0.04–0.34), while MRSA rates had a less marked decrease to 0.69 (95% CI 0.45–1.08) incident cases per 1,000 patient/days, respectively (Table 3). The cumulative incidence of MRSA and MSSA are shown in Figure 1c.
In Enterococci, the median time from admission to first positive culture was 27.5 days (IQR 13–90 days) for vancomycin-resistant Enterococci (VRE) as compared to 7 days (IQR 3–33 days) for vancomycin-susceptible Enterococci (VSE). The cumulative incidence of VRE and VSE are shown in Figure 1d.
Similarly, for Enterobacteriaceae, the median time to first positive culture was 12 days (IQR 4–29 days) for fluoroquinolone-susceptible isolates, 44 days (IQR 21–82 days) for FRE, 52 days (IQR 8–90 days) for ESBL-E, and 59 days (IQR 34–98 days) for CRE. Rates of fluoroquinolone-susceptible Enterobacteriaceae were not different during the three analyzed time periods. In contrast, for all 3 resistance phenotypes of Enterobacteriaceae, incidence rates increased with increasing time after admission (Table 3). The cumulative incidence of FRE, ESBL-E, CRE, and susceptible Enterobacteriaceae are shown in Figure 1e.
For patients with Pseudomonas spp., the median time from admission to first MDR Pseudomonas sp. isolation was 52.5 days (IQR 28–106 days), as compared to 22 days (IQR 11–51 days) for non- MDR Pseudomonas spp. The incidence rate of non-MDR Pseudomonas spp. decreased significantly (1.46 [95% CI 1.21–1.76] in week 1 to 0.87 [95% CI 0.58–1.31] in weeks 4 and after, incident cases per 1,000 patient/days, respectively), while the incidence rate of MDR Pseudomonas spp. increased (0.04 [95% CI 0.00–0.26] in week 1 to 1.85 [95% CI 1.40–2.44] in weeks 4 and after, incident cases per 1,000 patient/days, respectively). The cumulative incidence of MDR Pseudomonas sp. and non-MDR Pseudomonas sp. are shown in Figure 1e.
Discussion
In this study, we have provided a comprehensive evaluation of common healthcare-associated infections and potential pathogens in order to provide a framework for a timeline of infections after hospitalization for burn injuries. We found that skin and soft tissue infections occurred early during admission, whereas respiratory tract infections and bloodstream infections represent later complications after burns. These data specifically represent healthcare-associated infections that met criteria from the CDC. Therefore, no patients who presented with a delayed presentation of an infected burn would be included in the skin and soft tissue infection group. Regarding specific pathogens, we found a predominance of Gram-positive pathogens early on during hospitalization, shifting to Gram-negative bacteria later during hospitalization. As expected, antibiotic-resistant pathogens tended to be isolated later during hospitalization as well. The findings of a number of smaller studies (n ranging from 51 to 94 patients) showed similar trends16–18. For instance, in a study on VAP in a Belgian burn unit, MDR organisms increased from 9% in early-onset infection to 32% in late infection17. In addition, in a 6-year study from a military burn unit, the percentages of 4 major pathogens – A. baumannii, P. aeruginosa, K. pneumoniae, and S. aureus – that were susceptible to main antibiotic classes were compared between the first 5 days and days 15–30 of hospitalization. Similar to our findings, they observed that for all pathogen-antibiotic combinations, the susceptibility rates were lower as patients were admitted longer10.
In several ways, patients with burns are similar to other immunocompromised patients such as recipients of solid organ or hematopoietic stem cell transplants. In those populations, timelines of expected infections during specific periods of exposures and immunosuppression have been well-described and put into clinical use19, 20. In addition to interfering with the local immune barrier of the skin, burns have a variety of central immunomodulatory effects. Amongst others, reported effects include a skew towards production of Th17 T cells, alterations in the expression of immune signaling genes, increased Toll-like receptor-4 expression, and dendritic cell dysfunction6–9. Therefore, we propose that in the burn population – similar to other immunocompromised populations – knowledge of when certain infections are likely to occur will be beneficial in guiding empiric treatment as well as preventative strategies. In addition, infection preventionists who care for burn populations may use these data to evaluate not just the overall incidence of specific infections and MDR organisms, but also their incidence as it relates to the timing of the admission.
Infections are an important threat to the healing process of burn patients. In addition to immune alterations, patients have several risk factors for healthcare-associated infections. These risk factors include prolonged exposure to the hospital environment, large open wounds both from the primary burn site as well as from graft sites, inhalational injury, and frequent use of invasive devices such as intravenous and arterial catheters, endotracheal tubes, tracheostomies, and indwelling urinary catheters. We have previously reported on trends over time of healthcare-associated infections in our burn unit21–23. Specifically, we noted that the rates of catheter-associated bloodstream infections (CLABSI) decreased during the last decade, whereas respiratory tract infections remained more stable. The decrease in CLABSI rates likely resulted from a bundled approach of several interventions21. A controversial component of this bundle is the practice of frequent line changes which has not been shown to be beneficial in other intensive care settings. Current guidelines from the Healthcare Infection Control Practices Advisory Committee (HICPAC) recommend against scheduled line changes as a means of decreased CLABSI rates24. However, it should be noted that these recommendations are based on two small older trials that did not include burn patients25, 26. Therefore, these recommendations may or may not apply to current patients with burn injuries. A recommended component is the use of chlorhexidine for bathing, which is employed at our burn unit. In a small study that used historical controls, the introduction of daily chlorhexidine bathing decreased rates of VAP and CLABSI to zero27.
Antibiotic usage is very common in the burn population28. This antibiotic exposure combined with other risk factors increase the risk for acquisition of multidrug-resistant bacteria. In our cohort, almost half of pathogens met criteria for multi-drug resistance, with more than half of those MDR organisms being in the extensively drug resistant category. The rise of MDR organisms is an extremely worrisome finding that has been reported in other burn centers worldwide29–31. Infections with MDR organisms are associated with increased time to effective therapy, increased mortality, increased length of stay and increased health care costs, as compared to infections with susceptible bacteria32, 33. These risks are especially important in burn patients, given their prolonged hospitalizations and risk for recurrent infections.
Our study has several limitations. It is a retrospective study, and specimens were collected based on clinical need rather than as part of a prospective research strategy. Nonetheless, our findings are representative of a “real-world” experience in a large burn center. In addition, the determination of healthcare-associated infections was made in real-time by infection preventionists following standardized definitions. Another limitation is that this study was conducted in a single burn center. It is possible that these findings may not completely translate to other centers. However, the observed patterns of shifts during hospitalization from Gram-positives to Gram-negatives and to increasing antimicrobial resistance are intuitive, biologically plausible, and were also found in smaller studies performed at other centers16–18. Finally, we have evaluated all pathogens that were recovered from clinical cultures, without attempting to determine infection vs. colonization, which was outside of the scope of the current study.
Conclusions
We have analyzed in detail the timeline of infections during hospitalization for a burn injury. Skin and soft tissue infections tend to occur early in hospitalization, followed by respiratory tract infections and even later bloodstream infections. Gram-positives are more common in the first days of hospitalization, whereas Gram-negative bacteria predominate during later phases. Importantly, multidrug resistant pathogens tend to occur later as well. These findings may have important implications for the empiric treatment and diagnostics of patients with burns who are suspected of having an infection.
Highlights.
Seven percent of patients with burn injuries develop a hospital-acquired infection
Gram-positive bacteria predominate early during the hospitalization after burn injury, whereas Gram-negative bacteria become more common later during the admission.
Bacterial pathogens isolated from burn patients tend to become resistant to an increasing number of antibiotics as time from admission increases.
Acknowledgments
The authors would like to thank Ali Fokar for his assistance with data management.
Funding:
The project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001111. SWJ is supported by K08GM109106-02 (NIGMS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
D.v.D.: Actavis, Tetraphase, Sanofi-Pasteur, Astellas, Medimmune: Advisory Board. Steris Inc., Scynexis: Research funding
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest:
All other authors: no conflicts reported
References
- 1.American Burn Association. Burn Incidence and Treatment in the United States: 2012 Fact Sheet. 2012 http://wwwameriburnorg/resources_factsheetphp.
- 2.Azzopardi EA, Azzopardi SM, Boyce DE, Dickson WA. Emerging gram-negative infections in burn wounds. Journal of burn care & research : official publication of the American Burn Association. 2011;32:570–6. doi: 10.1097/BCR.0b013e31822ac7e6. [DOI] [PubMed] [Google Scholar]
- 3.Mason ST, Esselman P, Fraser R, Schomer K, Truitt A, Johnson K. Return to work after burn injury: a systematic review. Journal of burn care & research : official publication of the American Burn Association. 2012;33:101–9. doi: 10.1097/BCR.0b013e3182374439. [DOI] [PubMed] [Google Scholar]
- 4.Spellberg B, Blaser M, Guidos RJ, Boucher HW, Bradley JS, Eisenstein BI, et al. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis. 2011;52(Suppl 5):S397–428. doi: 10.1093/cid/cir153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessey PQ, Casavant CW, Edelman LS, Kemalyan NA, Klein MB, Lentz CW, et al. National Burn Repository 2012 Report. American Burn Association, National Burn Repository; 2012. http://www.ameriburn.org/resources_publications.php. [Google Scholar]
- 6.Neely CJ, Maile R, Wang MJ, Vadlamudi S, Meyer AA, Cairns BA. Th17 (IFNgamma- IL17+) CD4+ T cells generated after burn injury may be a novel cellular mechanism for postburn immunosuppression. The Journal of trauma. 2011;70:681–90. doi: 10.1097/TA.0b013e31820d18a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore CB, Medina MA, van Deventer HW, O’Connor BP, Cameron S, Taxman DJ, et al. Downregulation of immune signaling genes in patients with large surface burn injury. Journal of burn care & research : official publication of the American Burn Association. 2007;28:879–87. doi: 10.1097/BCR.0b013e318159a41e. [DOI] [PubMed] [Google Scholar]
- 8.Cairns B, Maile R, Barnes CM, Frelinger JA, Meyer AA. Increased Toll-like receptor 4 expression on T cells may be a mechanism for enhanced T cell response late after burn injury. The Journal of trauma. 2006;61:293–8. doi: 10.1097/01.ta.0000228969.46633.bb. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 9.Bohannon J, Cui W, Sherwood E, Toliver-Kinsky T. Dendritic cell modification of neutrophil responses to infection after burn injury. Journal of immunology. 2010;185:2847–53. doi: 10.4049/jimmunol.0903619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keen EF, 3rd, Robinson BJ, Hospenthal DR, Aldous WK, Wolf SE, Chung KK, et al. Prevalence of multidrug-resistant organisms recovered at a military burn center. Burns : journal of the International Society for Burn Injuries. 2010;36:819–25. doi: 10.1016/j.burns.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Weber DJ, Sickbert-Bennett EE, Brown V, Rutala WA. Completeness of surveillance data reported by the National Healthcare Safety Network: an analysis of healthcare-associated infections ascertained in a tertiary care hospital, 2010. Infect Control Hosp Epidemiol. 2012;33:94–6. doi: 10.1086/663344. [DOI] [PubMed] [Google Scholar]
- 12.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 13.Osler T, Glance LG, Hosmer DW. Simplified estimates of the probability of death after burn injuries: extending and updating the baux score. The Journal of trauma. 2010;68:690–7. doi: 10.1097/TA.0b013e3181c453b3. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Kanamori H, Parobek CM, Weber DJ, van Duin D, Rutala WA, Cairns BA, et al. Next-Generation Sequencing and Comparative Analysis of Sequential Outbreaks Caused by Multidrug-Resistant Acinetobacter baumannii at A Large Academic Burn Center. Antimicrob Agents Chemother. 2015 doi: 10.1128/AAC.02014-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wibbenmeyer L, Williams I, Ward M, Xiao X, Light T, Latenser B, et al. Risk factors for acquiring vancomycin-resistant Enterococcus and methicillin-resistant Staphylococcus aureus on a burn surgery step-down unit. Journal of burn care & research : official publication of the American Burn Association. 2010;31:269–79. doi: 10.1097/BCR.0b013e3181d0f479. [DOI] [PubMed] [Google Scholar]
- 17.Brusselaers N, Logie D, Vogelaers D, Monstrey S, Blot S. Burns, inhalation injury and ventilator-associated pneumonia: value of routine surveillance cultures. Burns : journal of the International Society for Burn Injuries. 2012;38:364–70. doi: 10.1016/j.burns.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Erol S, Altoparlak U, Akcay MN, Celebi F, Parlak M. Changes of microbial flora and wound colonization in burned patients. Burns : journal of the International Society for Burn Injuries. 2004;30:357–61. doi: 10.1016/j.burns.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Fishman JA, Rubin RH. Infection in organ-transplant recipients. The New England journal of medicine. 1998;338:1741–51. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 20.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:1143–238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Duin D, Jones SW, Dibiase L, Schmits G, Lachiewicz A, Hultman CS, et al. Reduction in Central Line-Associated Bloodstream Infections in Patients with Burns. Infect Control Hosp Epidemiol. 2014;35:1066–8. doi: 10.1086/677165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lachiewicz AM, van Duin D, DiBiase LM, Jones SW, Carson S, Rutala WA, et al. Rates of hospital-associated respiratory infections and associated pathogens in a regional burn center, 2008–2012. Infect Control Hosp Epidemiol. 2015;36:601–3. doi: 10.1017/ice.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber DJ, van Duin D, DiBiase LM, Hultman CS, Jones SW, Lachiewicz AM, et al. Healthcare-associated infections among patients in a large burn intensive care unit: incidence and pathogens, 2008–2012. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2014;35:1304–6. doi: 10.1086/678067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52:e162–93. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uldall PR, Merchant N, Woods F, Yarworski U, Vas S. Changing subclavian haemodialysis cannulas to reduce infection. Lancet. 1981;1:1373. doi: 10.1016/s0140-6736(81)92553-8. [DOI] [PubMed] [Google Scholar]
- 26.Eyer S, Brummitt C, Crossley K, Siegel R, Cerra F. Catheter-related sepsis: prospective, randomized study of three methods of long-term catheter maintenance. Critical care medicine. 1990;18:1073–9. [PubMed] [Google Scholar]
- 27.Popp JA, Layon AJ, Nappo R, Richards WT, Mozingo DW. Hospital-acquired infections and thermally injured patients: chlorhexidine gluconate baths work. American journal of infection control. 2014;42:129–32. doi: 10.1016/j.ajic.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Rex S. Burn injuries. Current opinion in critical care. 2012;18:671–6. doi: 10.1097/MCC.0b013e328359fd6e. [DOI] [PubMed] [Google Scholar]
- 29.Diederen BM, Wardle CL, Krijnen P, Tuinebreijer WE, Breederveld RS. Epidemiology of clinically relevant bacterial pathogens in a burn center in the Netherlands between 2005 and 2011. Journal of burn care & research : official publication of the American Burn Association. 2015;36:446–53. doi: 10.1097/BCR.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 30.Japoni A, Alborzi A, Kalani M, Nasiri J, Hayati M, Farshad S. Susceptibility patterns and cross-resistance of antibiotics against Pseudomonas aeruginosa isolated from burn patients in the South of Iran. Burns : journal of the International Society for Burn Injuries. 2006;32:343–7. doi: 10.1016/j.burns.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Wibbenmeyer LA, Kealey GP, Latenser BA, Diekema DJ, Williams IM, Coffman SL, et al. Emergence of the USA300 strain of methicillin-resistant Staphylococcus aureus in a burn-trauma unit. Journal of burn care & research : official publication of the American Burn Association. 2008;29:790–7. doi: 10.1097/BCR.0b013e3181848b8f. [DOI] [PubMed] [Google Scholar]
- 32.Vardakas KZ, Rafailidis PI, Konstantelias AA, Falagas ME. Predictors of mortality in patients with infections due to multi-drug resistant Gram negative bacteria: the study, the patient, the bug or the drug? J Infect. 2013;66:401–14. doi: 10.1016/j.jinf.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 33.Tedja R, Nowacki A, Fraser T, Fatica C, Griffiths L, Gordon S, et al. The impact of multidrug resistance on outcomes in ventilator-associated pneumonia. Am J Infect Control. 2014;42:542–5. doi: 10.1016/j.ajic.2013.12.009. [DOI] [PubMed] [Google Scholar]


