Abstract
Invasive grass weeds reduce farm productivity, threaten biodiversity, and increase weed control costs. Identification of invasive grasses from native grasses has generally relied on the morphological examination of grass floral material. DNA barcoding may provide an alternative means to identify co-occurring native and invasive grasses, particularly during early growth stages when floral characters are unavailable for analysis. However, there are no universal loci available for grass barcoding. We herein evaluated the utility of six candidate loci (atpF intron, matK, ndhK-ndhC, psbE—petL, ETS and ITS) for barcode identification of several economically important invasive grass species frequently found among native grasses in eastern Australia. We evaluated these loci in 66 specimens representing five invasive grass species (Chloris gayana, Eragrostis curvula, Hyparrhenia hirta, Nassella neesiana, Nassella trichotoma) and seven native grass species. Our results indicated that, while no single locus can be universally used as a DNA barcode for distinguishing the grass species examined in this study, two plastid loci (atpF and matK) showed good distinguishing power to separate most of the taxa examined, and could be used as a dual locus to distinguish several of the invasive from the native species. Low PCR success rates were evidenced among two nuclear loci (ETS and ITS), and few species were amplified at these loci, however ETS was able to genetically distinguish the two important invasive Nassella species. Multiple loci analyses also suggested that ETS played a crucial role in allowing identification of the two Nassella species in the multiple loci combinations.
Introduction
A variety of invasive grasses in Eastern Australia, including Nassella trichotoma (Nees) Hack. ex Arechav. (Serrated tussock), Nassella neesiana (Trin. & Rupr.) Barkworth (Chilean Needle Grass), Hyparrhenia hirta (L.) Stapf (Coolatai grass), Eragrostis curvula (Schrad.) Nees (African lovegrass), Chloris gayana Kunth (Rhodes grass) and others have caused serious damage to the environment and agricultural industry [1]. Both N. trichotoma and N. neesiana are native to South American countries (Argentina, Uruguay, Peru etc.), and have become naturalised weeds in Australia (particularly in eastern states such as New South Wales, Australian Capital Territory, Victoria and Tasmania)[2, 3]. They also occur as weeds in New Zealand, South Africa, USA and several European countries [4]. As these two weeds are capable of displacing palatable grasses from pastures, decreasing productivity of grazing livestock, and significantly degrading biodiversity in native grasslands, they have been listed as WONS (Weeds of National Significance in Australia)[4] requiring nationally coordinated control efforts. E. curvula is native to South Africa. As an invasive weed in Australia, New Zealand, and some parts of USA, it competes with native pasture species, reduces livestock productivity and posts fire hazard to environment [5]. Being a native in tropical and temperate Africa and the Mediterranean region, H. hirta has become naturalized in Australia, Mexico, the Caribbean and parts of South America [6]. It poses a major threat to natural biodiversity in stock routes, nature reserves and National Parks due to its tolerance to drought, fire and herbicide. Originated from Africa, C. gayana is a widely naturalised weed infesting pastures and native vegetation zones in Australia, New Zealand, USA, and several Pacific islands (Weeds of Australia, https://keyserver.lucidcentral.org/weeds/).
Identification of invasive and native grasses is crucial in weed management as misidentification of weeds may delay the control of invasive weeds while causing unnecessary eradication of native grasses with similar morphology[1]. Unfortunately, the five invasive grasses can be easily misidentified to a variety of native grasses in Australia. For examples, N. trichotoma and N. neesiana are both very similar in appearance to Austrostipa spp and in some cases similar to, Poa and Eragrostis species; E. curvula is alike to other tussock-like grasses; H. hirta is comparable to kangaroo grass (Themeda australis) and C. gayana is similar to feathertop Rhodes grass (Chloris virgata) (NSW WeedWise, http://weeds.dpi.nsw.gov.au/).
Weed abatement officers in eastern Australia and elsewhere generally identify grasses based mainly on visual examination of diagnostic floral characters present in mature specimens. As a result, grasses at earlier stages of development may not be readily identifiable, and in those instances, invasive grass infestations can go undetected during weed abatement surveys. DNA barcoding [7] provides a potential alternative method for distinguishing invasive grasses from native grasses without the dependency of suitable growth-stage samples. As a genetic based method, DNA barcoding can accurately and rapidly identify samples to species, even from trace amounts or degraded sample tissue [8, 9]. The success of DNA barcoding relies on the amplification of specific barcoding locus or loci in the genomes of the target species. The mitochondrial locus, COI (Cytochrome c Oxidase subunit I), has been successfully applied to provide species-level resolution in many animal species [10, 11]. In land plants, however, COI has proved to be less effective due to conservative rates of nucleotide substitution in land plant mitochondrial DNA [12].
The lack of universal barcoding locus in plants impelled the search for alternative DNA barcoding regions outside the mitochondrial genome [13–15]. As a result, the Consortium for the Barcode of Life (CBOL) recommended use of plastid coding genes maturase K (matK) and ribulose-1,5-bisphosphate carboxylase oxygenase (rbcL) as core plant barcodes[14] and the nuclear internal transcribed spacers 1–2 (ITS)[14] as supplementary loci. Nevertheless, finding more robust loci to increase the resolution in distinguishing particular groups of plant species (particularly higher plants) is still an ongoing process [16].
Syme, et al. (2013) [4] assessed the accuracy of standard barcoding loci (rbcL, matK and ITS) in a study aiming to test the efficiency of three sequence matching algorithms (BLAST, Neighbour Joining and Bayesian Likelihood) for genetic identification of stipoid grasses. They reported the best DNA barcode accuracy using ITS, partial accuracy using matK, and least accuracy using rbcL. Wang, et al. (2014) [1] screened 18 loci for the possibility of using DNA barcoding technology to identify invasive weeds and native grasses (up to 29 grass species) collected from eastern Australia. Based on PCR reliability and polymorphism levels, they advocated the use of matK and two other cpDNA loci [ndhK-ndhC intergenic spacer (referred as ndhK) and psbE—petL intergenic spacer (referred to here as as psbE)] as preferred grass DNA barcode targets over rbcL and ITS.
Here, we evaluated the chloroplast and nuclear loci (ITS, matK, ndhk and psbE), which were recommended by previous studies, on five major invasive grasses (N. trichotoma, N. neesiana, E. curvula, H. hirtai, C. gayana) and several co-occurring native grasses. We also tested two new loci, atpF intron (referred as atpF) and external transcribed spacer (ETS), which were reported to be effective in the genetic diversity study of Lolium perenne and other related grass species [17], and the phylogenetic studies of Poa [18]. We hope the availability of robust DNA barcoding loci will help weeds abatement officers to identify the five invasive weeds at early growth stages, which is important to Australian biosecurity (including border entry points for quarantine purposes) and weed control agencies as early detection in bio-surveillance for weed control will ensure an early intervention by control agencies.
Materials and methods
Sample collection
A total of 66 specimens representing five invasive grass species in Eastern Australia (Chloris gayana, Eragrostis curvula, Hyparrhenia hirta, Nassella neesiana, Nassella trichotoma) and seven native grass species (Austrostipa densiflora, Anthosachne scabra, Microlaena stipoides, Poa sieberiana, Rytidosperma caespitosum, Rytidosperma pallidum and Themeda triandra) were collected from the Australian Capital Territory, New South Wales, Queensland and Victoria in eastern Australia under the permit issued by Department of Primary Industry (Permit No. INT14/8307) (see S1 Table for collection information and GenBank accession numbers). The seven native grasses were selected for examination here, as each is morphologically similar to one or more of the invasive grasses and potentially affected by their presence in areas of Eastern Australia where they overlap. These samples included field sampled specimens (N = 62) and vouchered herbarium specimens from the Australian National Herbarium (N = 2) and the National Herbarium of Victoria (N = 2). Leaf samples (appropriately 0.3 cm2 in size) were preserved in > 70% EtOh and stored at the Wagga Wagga Agricultural Institute (NSW Department of Primary Industries) and allocated unique specimen identifiers (e.g. ww00001) for sample tracking. All specimen records and associated gene sequences have been submitted into the Barcode of Life Data systems (BOLD) [19].
DNA extraction, target loci PCR, and sequencing
In preparation for DNA extractions, leaf tissue (< 1 mg) of each specimen was incubated (55°C) overnight in 280 μl of DXT tissue digest reagent (QIAGEN, Doncaster, Australia) with 1% added proteinase K (Sigma—Aldrich). Genomic DNA was isolated from specimen digestions using a Corbett Research 1820 X-tractor Gene robot and associated DX buffers (Qiagen, Doncaster, Australia).
Loci specific forward and reverse primers used in Polymerase Chain Reaction (PCR) amplification are listed in (Table 1) and were modified by addition of 17 bp forward (GTAAAACGACGGCCAGT) and reverse (CAGGAAACAGCTATGAC) vector M-13 5’ tails to simplify subsequent sequencing.
Table 1. Loci targeted for PCR using sourced primers.
Forward (-F) and reverse (-R) primer directions indicated by suffix. Original primer sequences modified by addition of 17 bp vector M-13 5´ tails (tail sequences not shown here, refer to Materials and methods).
| Locus | Primer name | Primer sequence 5'-3' | Source |
|---|---|---|---|
| atpF intron | TeaCpSSR27FP | AATGCCGAATCGACGACCTA | [17] |
| TeaCpSSR27RP | CAATGGTCCCTCTACGCAAT | ||
| matK | 390-F | CGATCTATTCATTCAATATTTC | [20] |
| 1326-R | TCTAGCACACGAAAGTCGAAGT | ||
| 3F-Kimf | CGTACAGTACTTTTGTGTTTACGAG | ||
| 1R-Kimr | ACCCAGTCCATCTGGAAATCTTGGTTC | ||
| ndhK-ndhC | TeaCpSSR29FP | GGTACCAATCCATAACGATC | [17] |
| TeaCpSSR29RP | GCGCTAGTTTTTGTTGTTTT | ||
| psbE-petL | TeaCpSSR31FP | GGTCGTGGAATGCTTTTCTT | |
| TeaCpSSR31RP | TCCACGAATCTCAATGACCA | ||
| ETS | Rets4-F | TTGGCTACGCGAGCGCATGAG | [21] |
| 18S-R | AGACAAGCATATGACTACTGGCAGG | [22] | |
| ITS | 26SE | TAGAATTCCCCGGTTCGCTCGCCGTTAC | [23] |
| S3 | AACCTGCGGAAGGATCATTG | [24] | |
| ITS 5a–F | TATCATTTAGAGGAAGGAG | [25] | |
| ITS 4—R | GCATATCAATAAGCGGAGGA |
PCR (15 μl aliquots) for each locus (except ITS) contained Invitrogen™ reagents (1 × PCR buffer 2.9 mM MgCI2, 0.2 mM dNTPs, 0.4 U of Platinum Taq polymerase), 0.1 μM each primer, and 2 μl of specimen DNA. PCR thermal cycling consisted of 2 min at 95°C, 40 x [94°C (30s), 50°C (30s), 72°C (1 min)] and 72°C (5 min). All PCRs were conducted using an Eppendorf Mastercycler EP Gradient S Thermal Cycler.
ITS PCR modifications [18] included the addition of 0.75 μl of DMSO (100%) (Sigma—Aldrich) and 1 μl of specimen DNA in 15 μl reactions, with thermal cycling set as 95°C (5 min), 35 × [94°C (30 s), 55°C (30 s), 72°C (1 min)], and 72°C (5 min).
PCR products stained with SYBR® safe DNA Gel Stain (Invitrogen) were examined under UV light in a Bio—Rad Universal Hood II following electrophoresis through a 1.5% agarose gel, and in the presence of 1 kb size markers and negative controls. Successful PCR products were sequenced at the Brisbane, Queensland node of the Australian Genome Research Facility (AGRF).
Data analysis
Forward and reverse sequence chromatograms at each locus were assembled and checked for signal quality using SeqMan (DNA STAR package, DNAStar Inc., Madison, WI, USA). At each locus, specimen consensus sequences were exported into BioEdit [26] for alignment using ClustalW [27] with default parameters. The aligned sequences were also manually edited in BioEdit to remove primer reads.
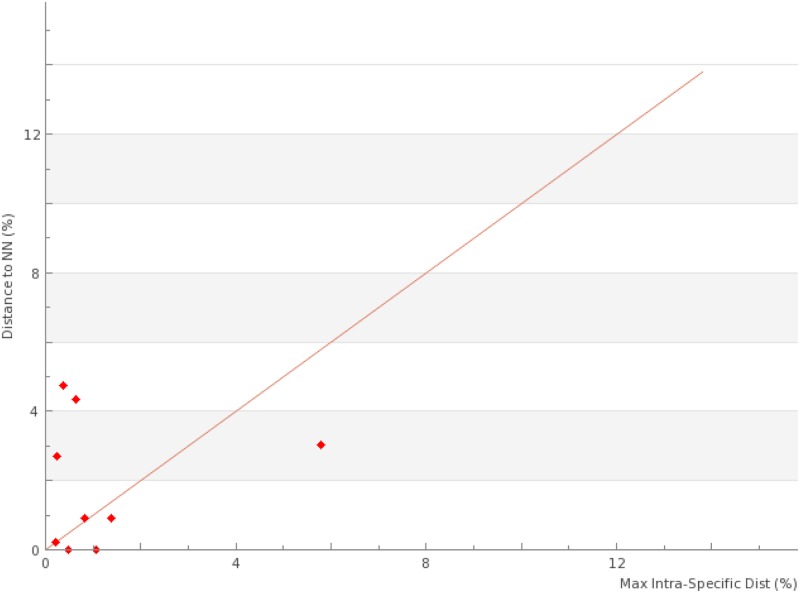
DNA barcode gap analysis was determined at each locus by plotting maximum intraspecific distance (Dintra) against minimum nearest neighbour distance (DNN). Intra and inter-specific pairwise genetic distances used in barcode gap analyses were generated at BOLD and adjusted by the Kimura 2-parameter (K2P) model of nucleotide evolution.
At each locus, the nearest neighbour minimum interspecific p–distances (DNN) was plotted against maximum intraspecific p–distances (Dintra) to determine presence or absence of a DNA barcode gap among species (Fig 1).
Fig 1. Maximum percent intraspecific distance (Dintra), and percent distance to nearest genetic neighbour species for six loci across five invasive and seven native grass species.
A: atpF; B: psbE; C: matK; D: ETS; E: ndhK; F: ITS.
Species monophyly was tested at each locus using both Neighbor-joining (NJ) and Maximum Likelihood (ML) tree construction methods. K2P pairwise distances used in NJ tree constructions were computed in MEGA 6.0 [28]. ML trees were constructed using PhyML 3.1 [29], incorporating a GTR nucleotide substitution model (plus Gamma distribution). Bootstrap replication (N = 1000) was used to assess confidence of NJ clusters and ML clades.
The same phylogenetic analyses were also performed on multi locus combinations (di, tri, tetra and penta) of the six loci to determine if any locus combination outperformed single loci for resolving species monophyly.
Results
Sequence characteristics of the six loci
While only one primer set was tested for each of four loci (ETS, atpF, ndhK and psbE), two primer sets were applied to amplify the ITS and matK sequences across the 12 species (Table 1). PCR success rate varied across different loci and grass species (Table 2). atpF was the only locus that successfully amplified all tested grass species. High rates of PCR success were also apparent at matK and ndhK. ITS had the least PCR success, with only three of the twelve grass species (C. gayana, E. curvula and N. trichotoma) being successfully amplified at this locus (Table 2). The aligned sequence matrix of ETS was 491 nucleotides (nt) in length with 314 parsimony informative sites and 325 variable sites (Table 3), which represents the highest percentage value of the parsimony informative or variable nucleotide sites against the total length (66% and 68% respectively). The shortest alignment (356 nt) was at ndhK, which also had the least amount of parsimony informative and variable sites against the total length (11% and 15%) respectively. DNA barcode gap minimum interspecific p—distances [represented as nearest neighbor distance (DNN)] and maximum intraspecific p—distances (Dintra) among the tested species across the six loci were presented in Table 4 and in Fig 1.
Table 2. PCR success rate of each locus against each species tested (Invasive grasses are indicated with *).
| Species (specimen No.) | atpF | ETS | ITS | matK | ndhK | psbE |
|---|---|---|---|---|---|---|
| Austrostipa densiflora (2) | 100% | 100% | 0% | 100% | 100% | 0% |
| *Chloris gayana (3) | 100% | 67% | 100% | 0% | 33% | 0% |
| Anthosachne scaber (4) | 75% | 0% | 0% | 100% | 25% | 75% |
| *Eragrostis curvula (18) | 39% | 0% | 100% | 78% | 89% | 33% |
| *Hyparrhenia hirta (6) | 100% | 33% | 0% | 33% | 83% | 83% |
| Microlaena stipoides (3) | 100% | 0% | 0% | 100% | 67% | 0% |
| *Nassella neesiana (12) | 100% | 92% | 0% | 83% | 92% | 92% |
| *Nassella trichotoma (8) | 100% | 100% | 13% | 75% | 88% | 75% |
| Poa sieberiana (2) | 100% | 0% | 0% | 100% | 0% | 0% |
| Rytidosperma caespitosum (2) | 100% | 0% | 0% | 100% | 0% | 0% |
| Rytidosperma pallidum (4) | 100% | 0% | 0% | 100% | 0% | 0% |
| Themeda triandra (2) | 100% | 0% | 0% | 100% | 0% | 0% |
Table 3. Information used to evaluate the utility of the six loci.
| Items | ETS | ITS | atpF | matK | ndhK | psbE |
|---|---|---|---|---|---|---|
| No. of primers screened | 1 | 2 | 1 | 2 | 1 | 1 |
| Aligned length (bp) | 491 | 687 | 457 | 838 | 356 | 687 |
| Informative sites/variable sites | 314/325 | 140/243 | 95/114 | 168/195 | 38/54 | 396/401 |
| No. of Indels | 70 | 48 | 82 | 6 | 10 | 74 |
Table 4. Percent distance of maximum percent intraspecific distance (Dintra) and nearest genetic neighbour species (DNN) across five invasive and seven native grass species (N/A: not available for single specimen).
| Locus | Species | Dintra | Nearest species | DNN |
|---|---|---|---|---|
| matK | Anthosachne scabra | 0.9 | Rytidosperma pallidum | 0.38 |
| Austrostipa densiflora | 0.12 | Nassella trichotoma | 1.43 | |
| Eragrostis curvula | 1.28 | Hyparrhenia hirta | 4.49 | |
| Hyparrhenia hirta | 0.56 | Eragrostis curvula | 4.49 | |
| Microlaena stipoides | 1.28 | Austrostipa densiflora | 6.21 | |
| Nassella neesiana | 0.66 | Nassella trichotoma | 0.11 | |
| Nassella trichotoma | 0.97 | Nassella neesiana | 0.11 | |
| Poa sieberiana | 0.45 | Anthosachne scabra | 4.84 | |
| Rytidosperma caespitosum | 9.71 | Rytidosperma pallidum | 0 | |
| Rytidosperma pallidum | 9.45 | Rytidosperma caespitosum | 0 | |
| Themeda triandra | 6.47 | Rytidosperma pallidum | 0 | |
| ETS | Austrostipa densiflora | 0.77 | Nassella neesiana | 7.09 |
| Chloris gayana | 0 | Hyparrhenia hirta | 27.31 | |
| Hyparrhenia hirta | 0.7 | Chloris gayana | 27.31 | |
| Nassella neesiana | 0.21 | Nassella trichotoma | 2.92 | |
| Nassella trichotoma | 0.21 | Nassella neesiana | 2.92 | |
| ITS | Chloris gayana | 0.87 | Eragrostis curvula | 16.74 |
| Eragrostis curvula | 5.21 | Chloris gayana | 16.74 | |
| Nassella trichotoma | N/A | Eragrostis curvula | 20.7 | |
| atpF | Anthosachne scabra | 0.25 | Austrostipa densiflora | 2.7 |
| Austrostipa densiflora | 0.2 | Nassella trichotoma | 0.22 | |
| Chloris gayana | 0 | Eragrostis curvula | 3.03 | |
| Eragrostis curvula | 5.8 | Chloris gayana | 3.03 | |
| Hyparrhenia hirta | 0.38 | Eragrostis curvula | 4.73 | |
| Microlaena stipoides | 0.63 | Nassella neesiana | 4.34 | |
| Nassella neesiana | 1.05 | Nassella trichotoma | 0 | |
| Nassella trichotoma | 0.48 | Nassella neesiana | 0 | |
| Poa sieberiana | 0 | Themeda triandra | 2.45 | |
| Rytidosperma caespitosum | 0.83 | Rytidosperma pallidum | 0.9 | |
| Rytidosperma pallidum | 1.38 | Rytidosperma caespitosum | 0.9 | |
| Themeda triandra | 0 | Poa sieberiana | 2.45 | |
| ndhK | Anthosachne scabra | N/A | Austrostipa densiflora | 2.13 |
| Austrostipa densiflora | 0 | Nassella neesiana | 0.26 | |
| Chloris gayana | N/A | Eragrostis curvula | 2.53 | |
| Eragrostis curvula | 2.79 | Chloris gayana | 2.53 | |
| Hyparrhenia hirta | 0.75 | Eragrostis curvula | 3.2 | |
| Microlaena stipoides | 0.82 | Eragrostis curvula | 3.37 | |
| Nassella neesiana | 2.83 | Nassella trichotoma | 0 | |
| Nassella trichotoma | 4.85 | Nassella neesiana | 0 | |
| psbE | Anthosachne scabra | 0.47 | Nassella neesiana | 42.41 |
| Eragrostis curvula | 0.63 | Hyparrhenia hirta | 5.16 | |
| Hyparrhenia hirta | 1.16 | Eragrostis curvula | 5.16 | |
| Nassella neesiana | 2.07 | Nassella trichotoma | 0 | |
| Nassella trichotoma | 0.29 | Nassella neesiana | 0 |
ETS and ITS were the only loci to show evidence of a clear DNA barcode gap, as exemplified by the absence of overlap between Dintra and DNN. In contrast these two measures overlapped at each of the remaining four cpDNA loci, indicating instances where more variation was present within particular species than between their nearest genetic neighbor species.
Among the six loci, ETS showed good distinguishing power to separate all targeted invasive grasses (except for E. curvula where it failed to amplify) as clear barcode gaps separating maximum intraspecific and minimum interspecific distances were identified between the invasive species (Fig 1D). While the remaining loci differed in their abilities to separate different weeds species, they shared the same feature that they failed to distinguish two important invasive grasses N. neesiana and N. trichotoma (Fig 1A, 1B, 1C, 1E and 1F).
Monophyly tests of species based on phylogenetic trees
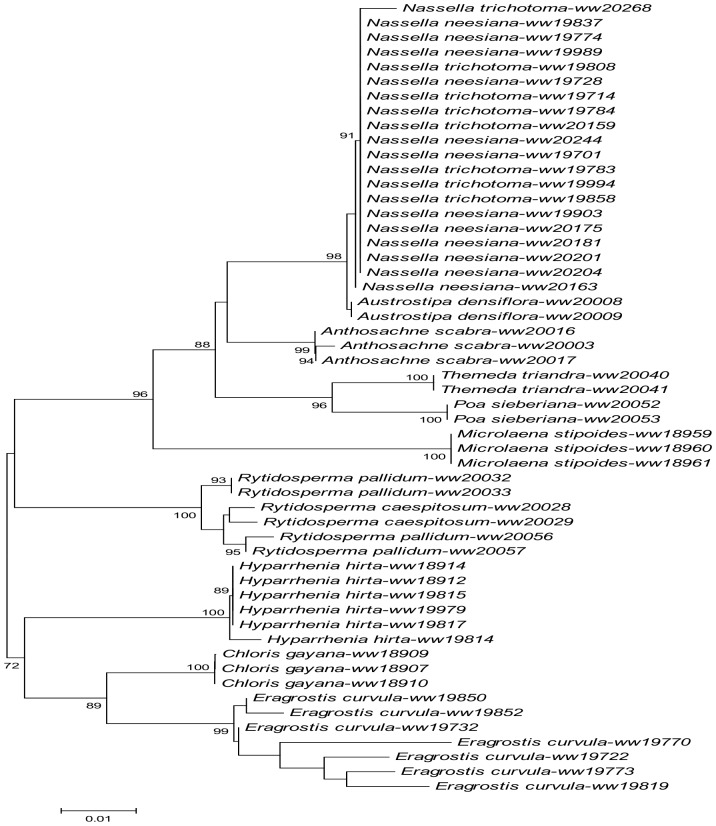
For psbE, the monophyly of two invasive grasses, H. hirta and E. curvula, were well supported (95% and 99% bootstrap support) by the NJ analysis. Although the genus Nassella was supported as monophyletic, species within the genus (N. neesiana and N. trichotoma) were paraphyletic. All four invasive grasses which amplified at this gene were clearly distinguished from a single native species (A. scabra) (Fig 2B). Similar results were obtained from the ML tree of psbE (Fig 3B).
Fig 2. NJ trees inferred from six loci.
A: atpF, B: psbE, C: matK, D: ETS, E: ndhK, F: ITS. Bootstrap support > 70% (N = 1000 replications) for clusters as reported. Scale bars indicate proportion of differences under a K2P model.
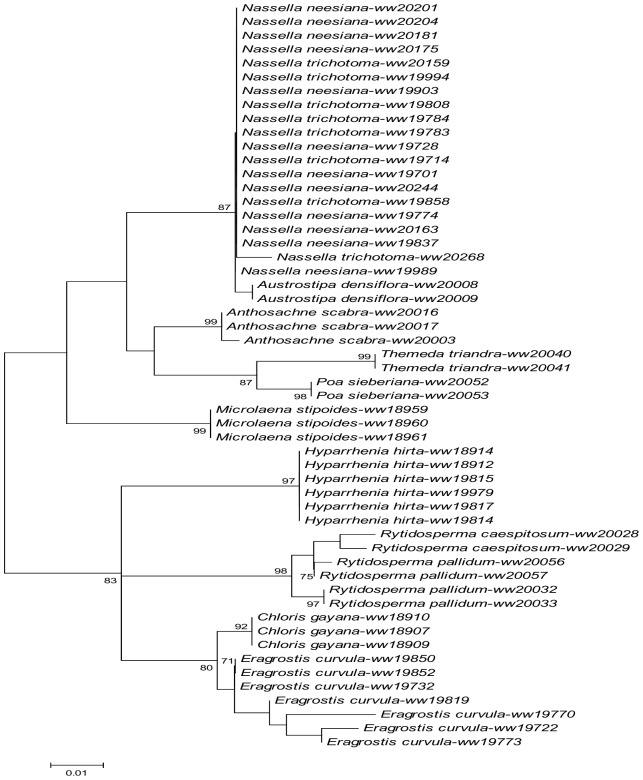
Fig 3. ML trees inferred from six loci.
A: atpF, B: psbE, C: matK, D: ETS, E: ndhK, F: ITS. Bootstrap supports > 70% (N = 1000 replications) for clades as reported. Scale bars report proportion of differences under a GTR model.
The higher PCR success rates in atpF (Table 2) made it possible to examine the distinguishing power of this locus across a wider range of grass species. Both NJ and ML trees of atpF supported the monophyly of three invasive species (C. gayana, E. curvula, H. hirta) and four native species (M. stipoides, A. scabra, T. triandra, P. Sieberiana), which means the majority of the examined taxa was distinguished by this locus. Although atpF failed to separate two invasive Nasella species (N. neesiana and N. trichotoma), it confirmed the monophyly of the Nassella genus and separated this genus from the native stipa species A. densiflora (Figs 2A and 3A). Similar results were obtained from the ndhK data (Figs 2E and 3E) but species coverage dropped slightly in the NJ and ML trees of ndhK (C. gayana and A. scabra were each represented by a single specimen, and T. triandra and P. sieberiana were not represented at all).
Similar to atpF, matK distinguished the invasive grasses of H. hirta and E. curvula from the tested native grasses (A. scabra, P. sieberiana, T. triandra, M. stipoides, R. pallidum and R. caespitosum). The monophyly of these native grasses were also confirmed except for the T. triandra, R. pallidum and R. caespitosum (Figs 2C and 3C). In addition, both NJ and ML trees of matK provided weak support to the monophyly of N. trichotoma and N. neesiana, and strong support for the separation of native stipa species (A. densiflora) from the two invasive Nassella species.
Two nuclear loci, ITS and ETS, were tested for their potential in distinguishing invasive grasses from native grasses in present study. While the PCR success rate for these two loci were relatively lower than that of the plasmid loci, the available sequence data from these two loci provided good distinguishing power in identifying different grass species. The NJ and ML trees of ITS clearly supported the monophyly of E. curvula and C. gayana whilst its counterparts of ETS provided strong support for the monophyly of N. neesiana, N. trichotoma, C. gayana and H. hirta (100% in both NJ and ML) (Figs 2D, 2F, 3D and 3F). The NJ and ML trees of ETS also separated the native stipa species (A. densiflora) from the invasive Nassella species with strong bootstrap support (100%).
Multiple loci analysis
Four ETS related two loci combinations (ETS—matK, ETS—ndhK, ETS–psbE and ETS–atpF) strongly confirmed the monophyly of N. neesiana and N. trichotoma, which is an improvement relative to the results of single locus (matK, ndhK, psbE and atpF). On the contrary, matK related two loci combinations (except for matK—psbE) remain weak in distinguishing N. neesiana from N. trichotoma, although some combinations (matK—atpF, matK—ndhK, matK—psbE) were effective in confirming the monophyly of E. curvula, H. hirta and several native grass species. Monophyly of E. curvula was confirmed by all ITS related two loci combinations except for ETS—ITS (Table 5) (Trees not show).
Table 5. Overview of the multiple loci combinations analyses.
| Combination of loci | Shared Specimens Number | Confirmed monophyletic species | Number of loci |
|---|---|---|---|
| ETS—matK | 17 | N. trichotoma, N. neesiana, A. densiflora | Di |
| ETS—ndhK | 21 | N. trichotoma, N. neesiana, H. hirta, A. densiflora | Di |
| ETS—psbE | 17 | N. trichotoma, N. neesiana, H. hirta | Di |
| ETS—atpF | 25 | N. trichotoma, N. neesiana, H. hirta, A. densiflora, C.gayana | Di |
| ITS—matK | 15 | E. curvula, N. trichotoma | Di |
| ITS—ndhK | 18 | E. curvula, N. trichotoma, C.gayana | Di |
| ITS—psbE | 7 | E. curvula, N. trichotoma | Di |
| ITS—atpF | 11 | E. curvula, C. gayana, N. trichotoma | Di |
| matK—ndhK | 35 | E. curvula, H. hirta, M. stipoides, A. densiflora | Di |
| matK—psbE | 26 | N. neesiana, N. trichotoma, H. hirta, E. curvula, A. scabra | Di |
| matK—atpF | 34 | E. curvula, H. hirta, A. densiflora, A. scabra, P. sieberiana, M. stipoides | Di |
| ndhK—psbE | 27 | E. curvula, H. hirta | Di |
| ndhK—atpF | 35 | E. curvula, H. hirta, M. stipoides, C. gayana, A. scabra | Di |
| psbE—atpF | 26 | H. hirta, E. scabe, E.curvula | Di |
| atpF—ndhK—psbE | 22 | H. hirta | Tri |
| ETS—atpF—ndhK | 21 | N. trichotoma, N. neesiana, A.densiflora, H. hirta | Tri |
| ETS—matK—atpF | 17 | N. trichotoma, N. neesiana, A.densiflora | Tri |
| ETS—ndhK—psbE | 16 | N. trichotoma, N. neesiana | Tri |
| matK—atpF—ndhK | 24 | E. curvula, M. stipoides, A. densiflora | Tri |
| matK—ndhK—psbE | 22 | E. curvula | Tri |
| ndhK—ITS—matK | 14 | E. curvula | Tri |
| psbE—matK—atpF | 20 | A. scabra, H. hirta | Tri |
| ETS—matK—atpF—ndhK | 17 | N. trichotoma, N. neesiana, A. densiflora | Tetra |
| ETS—atpF—ndhK—psbE | 16 | N. trichotoma, N. neesiana | Tetra |
| ETS—matK—atpF—ndhK—psbE | 14 | N. trichotoma, N. neesiana | Penda |
Similar results were obtained from other combinations (tri, tetra and penta) of the six loci. Two Nassella invasive grasses, N. neesiana and N. trichotoma, were clearly distinguished by loci combinations consisting the ETS locus (three tri combinations: ETS—atpF—ndhK, ETS—matK—atpF, and ETS—ndhK—psbE; two tetra combinations: ETS—matK—atpF—ndhK and ETS—atpF—ndhK—psbE; and one penta combination: ETS—matK—atpF—ndhK—psbE). In addition, multiple loci combinations consisting of ITS failed to separate all grasses species except for E. curvula (Table 5) (Trees not show).
Discussion
In present study, we tested six loci for their utility as DNA barcode targets to distinguish five invasive grass species from seven native grasses which frequently co-occur in eastern Australia. Among these, matK is recommended as one of two core loci by CBOL [14] for plant DNA barcoding. Our results (DNA barcode gap analysis, NJ and ML phylogenetic analyses) indicated that matK was suitable for distinguishing invasive H. hirta and E. curvula from native grasses (M. stipoides, A. scabra, T. triandra, P. sieberiana), but provided no or weak support for distinguishing N. neesiana from N. trichotoma, which are two important invasive grasses.
Similar results were obtained from ndhK, but the length of this locus (356 bp) is shorter than the recommended DNA barcode length [30]. The relatively lower PCR success rate of ndhK also limits its use as a general DNA barcode locus across the surveyed species. In contrast highest PCR success was achieved at atpF, and most of the examined taxa were distinguished (except for the two Nassella species) by this locus, indicating its potentials as a promising DNA barcode locus for the grasses of concern.
The remaining chloroplast locus, psbE, had longer sequence length (687 bp) and a high proportion (57.6%) of informative sites, but low PCR success rates across the examined species, which limits its utility as a general DNA barcode for grasses. As evidenced at other plastid loci, psbE provided no resolution in distinguishing N. neesiana from N. trichotoma.
Among the nuclear loci, ITS has been frequently reported as a potentially useful locus for plant DNA barcoding [31, 32], including its use for identification of stipoid grasses [4]. However, we experienced difficulties in amplifying this locus across multiple grass species. Our previous study [1] reported 75% fungal contamination rates among sequenced PCR products when using non-specific and universal ITS primers (ITS 5aF—ITS4R). Similarly fungal contamination with ITS primers has also been reported by Hollingsworth et al. [33]. However, in the present study, we have successfully eliminated the fungal contamination using the new primer set of ITS 26SE—ITS S3 together with modified PCR cycling conditions described previously[18]. Nevertheless, PCR and sequencing success rates using this new primer set remained low (average 20.5%), and the limited number of retrieved sequences failed to distinguish between N. neesiana from N. trichotoma, despite its success in distinguishing E. curvula and C. gayana.
The other nuclear locus, ETS, outperformed ITS in many ways, including the relatively higher PCR and sequencing success rate, and the power to distinguish three invasive grass species (N. neesiana, N. trichotoma and H. hirta). This locus could be a promising marker for grass DNA barcoding if more robust primers are designed for this locus to increase its PCR success rate (particularly for E. curvula).
Results of our PCR screening and DNA barcode gap analyses indicated no single locus can be universally used as a DNA barcode for distinguishing the grass species examined here. Loci examined either failed to amplify a portion of species or to resolve genetic limits among the species. Greater species resolution was in some cases obtained when combined loci analyses were employed. For example, matK confirmed the monophyly of multiple grass species except for N. neesiana and N. trichotoma, whilst ETS confirmed the monophyly of N. neesiana and N. trichotoma but failed to amplify E. curvula. When the DNA sequences of these two loci were concatenated and jointly analyzed, species monophyly of N. trichotoma, N. neesiana and A. densiflora were confirmed (Table 5). We noticed that the monophyly of N. neesiana and N. trichotoma were confirmed by all ETS related loci combinations (except for those combinations consisting both ETS and ITS), but were not confirmed by other loci combinations without the component of ETS. This suggests that ETS plays a crucial role in allowing identification of the two Nassella species in the multiple loci combinations, and could be a useful 2nd locus in combined analyses to improve accuracy of invasive species identifications.
In summary, the present studies evaluated the distinguishing power of six loci (ETS, ITS, atpF, matK, ndhK and psbE) for DNA barcoding of five invasive weeds and seven native grasses, which co-occur in eastern Australia. Among the four plastid loci, atpF and matK showed higher PCR rates and better distinguishing power than the remaining loci, making them suitable for further consideration as promising DNA barcodes of the targeted grass species. Among the two nuclear loci, ETS showed better potential as a DNA barcode for the separation of two invasive Nassella species. We conclude that a dual locus DNA barcode combination of atpF and matK may be used to genetically distinguish several prominent invasive grass species present in eastern Australia from co-occurring native grasses often mistaken for the invasive types. Furthermore, use of the ETS locus as a DNA barcode for genetic separation and identification of the two Nassella spp. may provide some application in future screening of those two WONs species; arguably further optimization of this locus may also allow it to be used for DNA barcode assay and identification of a broader assemblage of native and invasive grass species in Australia.
Supporting information
All specimens are housed at WWAI unless otherwise indicated; N/A—indicates no sequence was obtained.
(DOCX)
Acknowledgments
This study was funded by the NSW Weeds Action Program (WAP). Mr Adam Shephard, Mr Peter Deane, Mr Malcolm Ross, Mr Jeremy Crocker and other Council Weeds Officers provided assistances in field sampling. We thank Dr Pina Milne and Mr Wayne Gebert from the National Herbarium of Victoria for providing herbarium samples (MEL 2375629A and MEL 2330503A). Native grasses were provided by Mr Ian Chivers (Native Seeds Nursery in Melbourne) and Dr Peter Orchard (Charles Sturt University).
Data Availability
All DNA sequences files are available from the GenBank database (accession number(s) KX281066 - KX281111;KX290952 - KX291002;KX360607 - KX360637;KX434529 - KX434573;KY079282 - KY079335).
Funding Statement
This work was supported by NSW Weeds Action Programs (WAP).
References
- 1.Wang AS, Gopurenko D, Wu H, Stanton R, Lepschi B. DNA barcoding for identification of exotic grass species present in eastern Australia. In: Proceedings of the 19th Australasian Weeds Conference—Science, Community and Food Security: the Weed Challenge, 1–4 September, Hobart, Tasmania, ed. M. Baker. (Tasmanian Weed Society, Hobart). 2014; pp 444–447.
- 2.Weller SL, Florentine SK, Sillitoe JF, Grech CJ, McLaren DA. An investigation of the effects of stage of ensilage on Nassella neesiana seeds, for reducing seed viability and injury to livestock. Scientific reports. 2016;6:22345 10.1038/srep22345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayres L, Leech F. Serrated tussock—identification and control, Primefact.2006; 44, NSW DPI, Orange
- 4.Syme AE, Udovicic F, Stajsic V, Murphy DJ. A test of sequence-matching algorithms for a DNA barcode database of invasive grasses. DNA Barcodes. 2013; 1: 19–26. [Google Scholar]
- 5.Campbell M. Effects of age on the germination of African lovegrass seed, Grasslands Society Newsletter, 2002;17(1) [Google Scholar]
- 6.CRC for Australian Weed Management. Coolatai grass (Hyparrhenia hirta) Weed Management Guide. 2007;9 p. CSIRO & Bureau of Meteorology (2007) Climate change in Australia Technical Report, - http://climatechangeinaustralia.com.au/
- 7.Hebert PDN, Cywinska A, Ball SL, DeWaard JR. Biological identifications through DNA barcodes. Proceedings of the Royal Society B.2003; 270: 313–321. 10.1098/rspb.2002.2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.deWaard JR, Mitchell A, Keena MA, Gopurenko D, Boykin LM, Armstrong KF, et al. Towards a Global Barcode Library for Lymantria (Lepidoptera: Lymantriinae) Tussock Moths of Biosecurity Concern. PLoS One. 2010; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahadani P, Ghosh SK. DNA Barcoding: A tool for species identification from herbal juices. In. DNA Barcodes. 2013; 35–38 p. [Google Scholar]
- 10.Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proceedings of the National Academy of Sciences of the United States of America. 2004; 101: 14812–14817. 10.1073/pnas.0406166101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith MA, Woodley NE, Janzen DH, Hallwachs W, Hebert PDN. DNA barcodes reveal cryptic host-specificity within the presumed polyphagous members of a genus of parasitoid flies (Diptera: Tachinidae). Proceedings of the National Academy of Sciences of the United States of America. 2006;103: 3657–3662. 10.1073/pnas.0511318103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe KH, Li WH, Sharp PM. Rates of Nucleotide Substitution Vary Greatly among Plant Mitochondrial, Chloroplast, and Nuclear DNAs. Proceedings of the National Academy of Sciences of the United States of America. 1987; 84:9054–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chase MW, Cowan RS, Hollingsworth PM, van den Berg C, Madrinan S, Petersen G, et al. A proposal for a standardised protocol to barcode all land plants. Taxon. 2007; 56: 295–299. [Google Scholar]
- 14.CBOL Plant Working Group. A DNA barcode for land plants. Proceedings of the National Academy of Sciences of the United States of America. 2009; 106: 12794–12797. 10.1073/pnas.0905845106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kress WJ, Wurdack KJ, Zimmer EA, Weigt LA, Janzen DH. Use of DNA barcodes to identify flowering plants. Proceedings of the National Academy of Sciences of the United States of America. 2005; 102: 8369–8374. 10.1073/pnas.0503123102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong WP, Xu C, Li CH, Sun JH, Zuo YJ, Shi S, et al. ycf1, the most promising plastid DNA barcode of land plants. Scientific Reports. 2015; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diekmann K, Hodkinson TR, Barth S. New chloroplast microsatellite markers suitable for assessing genetic diversity of Lolium perenne and other related grass species. Annals of Botany. 2012; 110: 1327–1339. 10.1093/aob/mcs044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birch JL, Cantrill DJ, Walsh NG, Murphy DJ. Phylogenetic investigation and divergence dating of Poa (Poaceae, tribe Poeae) in the Australasian region. Biological Journal of the Linnean Society. 2014; 175: 523–552. [Google Scholar]
- 19.Ratnasingham S, Hebert PD. Bold: The Barcode of Life Data System (http://www.barcodinglife.org). Molecular Ecology Notes. 2007; 7: 355–364. 10.1111/j.1471-8286.2007.01678.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford CS, Ayres KL, Toomey N, Haider N, Stahl JV, Kelly LJ, et al. Selection of candidate coding DNA barcoding regions for use on land plants. Biological Journal of the Linnean Society. 2009; 159: 1–11. [Google Scholar]
- 21.Gillespie LJ, Soreng RJ, Jacobs SWL. Phylogenetic relationships of Australian Poa (Poaceae: Poinae), including molecular evidence for two new genera, Saxipoa and Sylvipoa. Australian Systematic Botany. 2009; 22: 413–436. [Google Scholar]
- 22.Starr JR, Harris SA, Simpson DA. Potential of the 5 ' and 3 ' ends of the intergenic spacer (IGS) of rDNA in the Cyperaceae: New sequences for lower-level phylogenies in sedges with an example from Uncinia pers. International Journal of Plant Sciences. 2003; 164: 213–227. [Google Scholar]
- 23.Sun Y, Skinner DZ, Liang GH, Hulbert SH. Phylogenetic Analysis of Sorghum and Related Taxa Using Internal Transcribed Spacers of Nuclear Ribosomal DNA. Theoretical and Applied Genetics. 1994; 89: 26–32. 10.1007/BF00226978 [DOI] [PubMed] [Google Scholar]
- 24.Kass E, Wink M. Molecular phylogeny and phylogeography of Lupinus (Leguminosae) inferred from nucleotide sequences of the rbcL gene and ITS 1+2 regions of rDNA. Plant Systematics and Evolution. 1997; 208: 139–167. [Google Scholar]
- 25.Baldwin BG. Phylogenetic utility of the internal transcribed spacers of nuclear ribosomal DNA in plants: an example from the compositae. Molecular Phylogenetics and Evolution. 1992; 1: 3–16. [DOI] [PubMed] [Google Scholar]
- 26.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic acids symposium series. 1999; 41: 95–98. [Google Scholar]
- 27.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007; 23: 2947–2948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- 28.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2013; 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guindon S, Delsuc F, Dufayard JF, Gascuel O. Estimating maximum likelihood phylogenies with PhyML. Methods in Molecular Biology. 2009; 537: 113–137. 10.1007/978-1-59745-251-9_6 [DOI] [PubMed] [Google Scholar]
- 30.Kress WJ, Erickson DL. DNA barcodes: genes, genomics, and bioinformatics. Proceedings of the National Academy of Sciences of the United States of America. 2008; 105: 2761–2762. 10.1073/pnas.0800476105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kress WJ, Erickson DL. A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS One. 2007; 2: e508 10.1371/journal.pone.0000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang JQ, Meng SY, Wen J, Rao GY. DNA barcoding of rhodiola (crassulaceae): a case study on a group of recently diversified medicinal plants from the qinghai-tibetan plateau. PLoS One. 2015; 10: e0119921 10.1371/journal.pone.0119921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollingsworth PM, Graham SW, Little DP. Choosing and Using a Plant DNA Barcode. PLoS One. 2011; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All specimens are housed at WWAI unless otherwise indicated; N/A—indicates no sequence was obtained.
(DOCX)
Data Availability Statement
All DNA sequences files are available from the GenBank database (accession number(s) KX281066 - KX281111;KX290952 - KX291002;KX360607 - KX360637;KX434529 - KX434573;KY079282 - KY079335).