Abstract
Background: Metabolic syndrome (MetS) is frequent in patients with chronic obstructive pulmonary disease (COPD). Systemic inflammation plays an important role in both COPD and MetS. The aim of this study was to assess the frequency of MetS in COPD patients and to evaluate the status of systemic inflammation in COPD patients with MetS and those without MetS. Methods: This cross-sectional study included 98 consecutive stable COPD patients. The MetS was defined using the criteria of the International Diabetes Federation. Components of MetS and markers of systemic inflammation: C-reactive protein (CRP), fibrinogen, and leukocyte count were measured. All patients underwent spirometry. The staging of COPD was made according to the Global initiative for chronic obstructive lung disease (GOLD) criteria. Results: MetS was present in 37.8 % COPD patients. The frequencies of MetS in patients with GOLD stages I, II, III, and IV were 33.3 %, 48.8 %, 31.6 %, and 23.1 %, respectively. MetS frequencies were not significantly different between GOLD stages. The multivariate logistic regression analysis revealed leukocyte count and CRP level as significant independent predictors of the presence of Mets in COPD patients (OR =1.321, 95%CI: 1.007-1.628, p =0.009 and OR =1.184, 95%CI: 1.020-1.376, p =0.027 respectively). Conclusions: This study shows that MetS is frequent in patients with COPD. Systemic inflammatory markers are higher in COPD patients with MetS than in patients without MetS. These findings suggest that physicians should screen COPD patients for associated MetS and elevated circulatory inflammatory markers. Management of these disorders should reduce the risk of cardiovascular morbidity and mortality in these patients. Hippokratia 2016, 20(2):110-114
Keywords: Chronic obstructive pulmonary disease, metabolic syndrome, inflammatory markers
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex and progressive disease and one of the main causes of morbidity and mortality worldwide. COPD is characterized not only by airway inflammation but also by systemic inflammation. The precise relationship between these two inflammatory processes is still unknown. Systemic inflammation is responsible for a significant amount of comorbidity in COPD patients1-3. Fabbri et al considered COPD as a part of the chronic systemic inflammatory syndrome4.
Metabolic syndrome (MetS) is a complex of interrelated medical disorders that increase the risk of developing an atherosclerotic cardiovascular disease and type 2 diabetes. These risk factors are abdominal obesity, elevated blood glucose, hypertension and dyslipidemia [elevated triglycerides and low levels of high-density lipoprotein (HDL) cholesterol]5.
The prevalence of MetS in COPD patients varies from 21 % to more than 50 %. Previously conducted studies reported that MetS is 1.3-1.5 times more prevalent in COPD patients than in people with normal lung function. Obesity, physical inactivity, cigarette smoking, corticosteroid use, as well as inflammation, oxidative stress, and hypoxia, are mechanisms responsible for the development of MetS in COPD patients6-8.
It is well-known that systemic inflammation plays a key role in both COPD and MetS9. Coexistence of COPD and MetS intensifies systemic inflammation. Various studies showed that systemic inflammation is more severe in COPD patients with MetS than in those without MetS7,10,11.
Increased circulating cytokines, chemokines, and acute-phase proteins as well as abnormalities in circulating cells represent the evidence of systemic inflammation in patients with COPD12,13. Multiple studies have demonstrated that there is a relationship between systemic inflammation and metabolic derangements in patients with COPD3,14. The circulating inflammatory markers which are increasingly evaluated in COPD patients are C-reactive protein (CRP), fibrinogen, and leukocytes15.
It is important to emphasize that both MetS and COPD increase the risk of cardiovascular morbidity and mortality. Low-grade inflammation, a hallmark of COPD and MetS, is included in all phases of atherosclerosis16-19.
The aim of this study was to investigate the frequency of MetS in patients with COPD and to assess the status of systemic inflammation in COPD patients with MetS and those without MetS.
Material and methods
This cross-sectional study included 98 consecutive COPD patients admitted to the outpatient ward, at the Clinic for Pulmonology, of the Clinical Centre of Serbia, Belgrade, during the period March-December 2015. The study was approved by the Ethical Committee of the Medical Faculty of the University of Belgrade (number: 29/V-20, 20/05/2015) and all participants signed an informed consent. The diagnosis of COPD and classification of patients were made according to the Global initiative for chronic obstructive lung disease (GOLD) criteria20. Inclusion criteria were a diagnosis of COPD, stable state of disease (no exacerbations and no medication change in the preceding six weeks), while exclusion criteria were considered the presence of an inflammatory comorbidity (e.g. rheumatologic diseases, vasculitis, inflammatory bowel disease), acute infections (all acute infections e.g. infections of the respiratory, urogenital, gastrointestinal tract and skin, within six weeks before enrolement to the study), respiratory diseases other than COPD, and steroid treatment.
We recorded for each study participant the demographic characteristics, medical history, smoking status (current smokers, former smokers defined as those who had stopped smoking ≥1 year, non-smokers) and the number of pack-years (years of smoking x number of daily smoked cigarettes / 20). We measured blood pressure, weight, height, and waist circumference. Venous blood samples were obtained for analysis of glucose, triglyceride, cholesterol [total, HDL, low-density lipoprotein (LDL)], CRP, fibrinogen, and leukocyte count. Patients also underwent pulmonary function tests.
The MetS was assessed according to criteria of the International Diabetes Federation (IDF): waist circumference ≥94 cm for Europid men and ≥80 cm for Europid women; plus any two of the following four factors: triglyceride levels ≥1.7 mmol/L, or specific treatment for this lipid abnormality, HDL cholesterol levels of <1.03 mmol/L in men, and <1.29 mmol/L in women, or specific treatment for this lipid abnormality; systolic blood pressure ≥130 mm Hg, or diastolic blood pressure ≥85 mm Hg, or treatment of previously diagnosed hypertension; and fasting plasma glucose level of ≥ 5.6 mmol/L, or previously diagnosed type 2 diabetes21.
Anthropometric assessment
The height and weight of the study participants were measured in light clothes and without shoes, and body mass index (BMI) was calculated by dividing weight by height squared (kg/m2). Waist circumference was determined using a tapeline at the midpoint between the lowest rib and the iliac crest.
Blood pressure measurements
Blood pressure was measured by a sphygmomanometer (Omron MX3 Plus, Omron, Japan) according to the American Heart Association recommendations22.
Blood sampling and analyses
A venous blood sample was collected from each patient after a 12-hour fasting. Glucose level was measured using the hexokinase/glucose-6-phosphate dehydrogenase method. Triglyceride and total cholesterol were determined by enzymatic methods and HDL cholesterol by the direct homogeneous assay. All the measurements were performed on the analyzer Beckman Coulter (USA). LDL cholesterol was calculated using the Friedewald equation. In order to evaluate the CRP level, the immunoturbidimetric method was applied (analyzer Beckman Coulter, USA). For the analysis of fibrinogen we used clotting-based test (BCS XP coagulation analyzer, Siemens Healthcare Diagnostics, Germany). The leukocyte count was measured using hematology analyzer (Beckman Coulter LH 750, USA).
Pulmonary function testing
Standard spirometry was performed (spirometer: Masterscreen Pneumo, Viasys Healthcare, Germany). Procedures for lung function testing were applied according to the European Respiratory Society guidelines23. The forced vital capacity (FVC), forced expiratory volume in the first second (FEV1) and FEV1/FVC ratio were obtained. The staging of COPD was made using GOLD criteria (spirometric classification of COPD severity based on post-bronchodilator FEV1) as stage I (mild): FEV1/FVC <0.70; FEV1 ≥80 % predicted; stage II (moderate): FEV1/FVC <0.70; 50% ≤ FEV1 <80% predicted; stage III (severe): FEV1/FVC <0.70; 30% ≤ FEV1 <50% predicted; stage IV (very severe): FEV1/FVC <0.70; FEV1 <30% predicted20.
Statistical analysis
The statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) software for Windows, version 15.0 (SPSS Inc., Chicago, IL, USA). Values are expressed as the mean [± standard deviation (SD)] and as number (percentage, %). Normality of distribution was assessed using the Kolmogorov- Smirnov test. To assess the difference between groups we used the Student t-test for variables with normal distribution and the Mann-Whitney test for variables without normal distribution. A multivariate logistic regression analysis was conducted to identify independent predictors of MetS presence. p values less than 0.05 were considered significant.
Results
A total number of 98 COPD patients were evaluated. The mean age of patients was 62.7 ± 7.3 years; males were 64.3 % of the participants. Of all patients, 44.9 % were current smokers and 55.1 % were former smokers, and the mean pack-year index was 52.2 ± 40.2. The mean post-bronchodilator FEV1 (% of predicted) was 38.50 ± 14.55. The percentages of patients with GOLD stages I, II, III, IV were 6.1, 41.8, 38.8, and 13.3 %, respectively. The mean COPD duration was 6.1 ± 4.6 years.
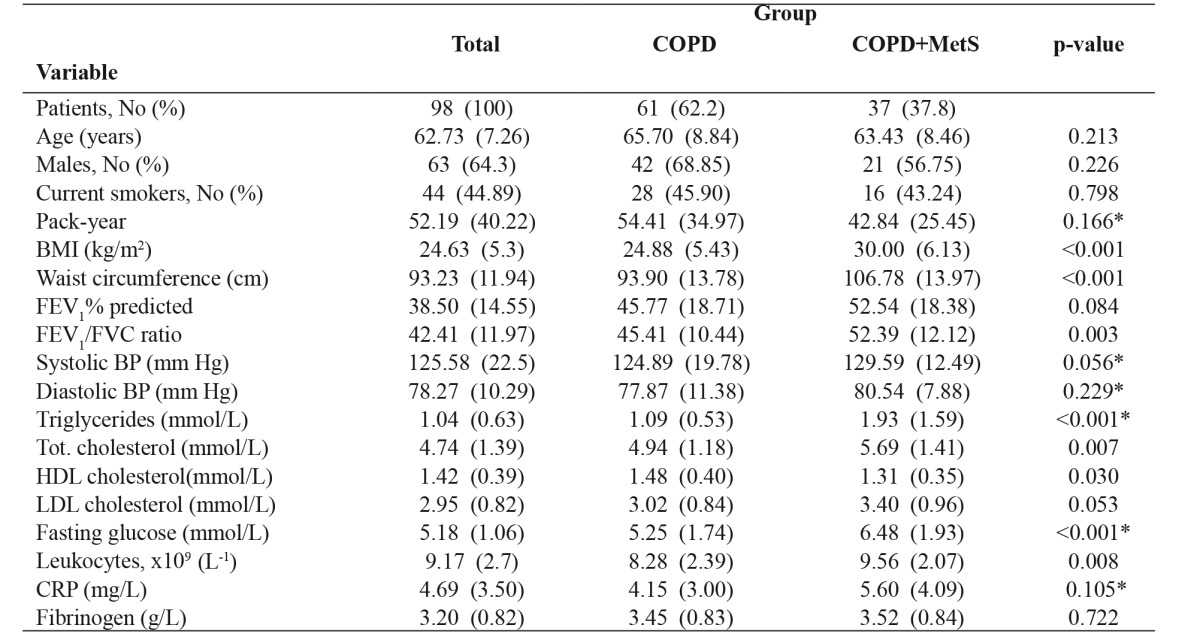
MetS was diagnosed in 37 (37.8 %) COPD patients. The frequencies of MetS in patients with GOLD stages I, II, III, IV were 33.3 %, 48.8 %, 31.6 %, and 23.1 %, respectively. MetS frequencies were not significantly different between the GOLD stages. The characteristics of COPD patients with MetS and COPD patients without MetS are presented in Table 1.
Table 1. Characteristics of the 98 consecutive stable chronic obstructive pulmonary disease (COPD) patients with metabolic syndrome (MetS) and without MetS that were included in this cross-sectional study.

Values are given as number (percentage, %) or mean (standard deviation-SD), COPD: chronic obstructive pulmonary disease, MetS: metabolic syndrome, p-value: COPD group versus COPD+MetS group, BMI: body mass index, FEV1: forced expiratory volume in one second, FVC: forced vital capacity, BP: blood pressure, HDL: high-density lipoprotein, LDL: low-density lipoprotein, CRP: C-reactive protein, *: Mann-Whitney test for independent samples
No significant differences were noticed between the groups according to age, gender, smoking status, the intensity of smoking, COPD duration, and FEV1. Fasting plasma glucose, triglycerides, total cholesterol, BMI and waist circumference were significantly higher in the group with MetS (p <0.001, p <0.001, p =0.007, p <0.001, and p <0.001, respectively).
The inflammatory profile of patients with COPD and MetS differs from that of patients with COPD without MetS. Leukocyte count was significantly elevated in COPD patients with MetS in comparison with those who did not have MetS (p =0.008). CRP level was also higher in the group of COPD patients with MetS than in the group without MetS, but the difference is not significant (p =0.105). There were no significant differences between the groups as regards the fibrinogen level, though greater values of this inflammatory marker were recorded in COPD patients with MetS (p =0.722).
The multivariate logistic regression model revealed leukocyte count and CRP level as significant independent predictors of the presence of MetS in COPD patients (Table 2).
Table 2. Logistic regression model of the presence of metabolic syndrome in chronic obstructive pulmonary disease (COPD) patients.
COPD: chronic obstructive pulmonary disease, OR: odds ratio, CI: confidence interval
Discussion
The main findings of the current study were the following: more than 37 % of COPD patients had MetS, and the level of systemic inflammation was higher in COPD patients with MetS in comparison with COPD patients without MetS.
The prevalence of MetS in COPD patients is highly variable between studies. The prevalence depends on the criteria used to diagnose MetS and the study inclusion criteria. Also, it depends on the country/ethnicity studied. In the research carried out in Germany by Wats et al, IDF criteria were applied and the prevalence was estimated at 47.5 %7. On the other hand, Minas et al performed a study in Greece, using Adult Treatment Panel III criteria and excluding patients with diabetes, cardiovascular disease, and other comorbidities. They found the prevalence of Mets 21 %6. Studies conducted in China (Lam et al) and Japan (Funakoshi et al) revealed that 22.6 % and 23 % of COPD patients had MetS, respectively24,25. In the study performed by Hosny et al in Egypt, MetS was present in 40 % of COPD patients26. The similar prevalence was reported by Akpinar et al from Turkey and Diez-Manglano et al from Spain at 44.6 % and 42.9 %, respectively9,27. Stanciu et al from Romania showed that 48.1 % COPD patients had associated MetS11. Mekov et al from Bulgaria found a relatively low prevalence of 25 %28. In the research of Breyer et al MetS was detected in 57 % of COPD patients (relatively higher prevalence in comparison with other reports)29.
The frequency of MetS in COPD patients observed in our study (37.8 %) is notably higher (almost double) than frequencies in many other studies (e.g. Minas et al6, Lam et al24, Funakoshi et al25). On the other hand, the frequency in our study is much lower than in Breyer’s29.
MetS is less frequent in patients with severe form of COPD. This is a consequence of weight loss that often occurs in patients with advanced disease. Various studies show that the MetS is more common in younger patients and the earlier stages of COPD (GOLD I-II). It is suggested that these patients may constitute a specific COPD phenotype which indicates higher risk of diabetes and cardiovascular diseases and requires a closer follow up6.
The following reports support these observations. Thus, in the study of Wats et al the frequencies of MetS in GOLD stages I-IV were 50 %, 53 %, 37 %, and 44 % respectively7. Akpinar et al reported the distribution of the prevalence of MetS between GOLD stages as follows: 38.5 %, 52.8 %, 30 %, and 33.3 %9. In the study of Diez-Manglano all patients were in GOLD II, III,IV stages, and the frequencies of MetS were 51.2 %, 41.2 %, and 25.5 %, respectively27. In the Canadian study of Marquis et al the frequency of MetS in patients with COPD was 47 %, and the frequency decreased to about 10 % at GOLD stages III and IV30. In the study of Alpaydin et al performed in Turkey, MetS was assessed in 44 % COPD patients. The autors found significantly different MetS prevalence in COPD patients in different GOLD stages: the highest prevalence was observed in stage II (59 %), and the lowest one in stage IV (4.5 %), thus MetS was more frequent in the early stages of the disease31.
Our findings that MetS is more common in GOLD stages I and II (33.3 % and 48.8 %, respectively) than in GOLD stages III and IV (31.6 % and 23.1 %, respectively) are in line with the results of previously mentioned studies. In our study, the frequency of MetS was the highest in COPD patients in GOLD stage II, as observed in studies of Wats, Akpinar, Diez-Manglano and Alpaydin7,9,27,31.
The second main finding of this study is that the level of systemic inflammation is higher in COPD patients with MetS in comparison with COPD patients without MetS. Wats et al showed that in COPD patients coexisting MetS was associated with increased levels of systemic inflammatory markers. COPD patients with MetS had significantly higher levels of high-sensitivity CRP (hsCRP) and interleukin-6 compared with patients without MetS, but fibrinogen levels did not differ between the groups. Multivariate linear regression analysis revealed MetS to be an independent predictor of hsCRP level and interleukin-6, but not of fibrinogen level7.
Akpinar et al noticed higher CRP levels in COPD patients with MetS. This observation indicates that the presence of MetS in COPD patients is associated with more intensive systemic inflammation9. Stanciu et al revealed higher levels of serum TNF-alpha and hsCRP, but lower adiponectin level in COPD patients with MetS than in those without11. The research of the Hosny et al detected statistically significant correlation between serum IL-6 level and the frequency of MetS in the COPD patient group26. In the study of Yasar et al, carried out in Turkey, MetS prevalence in COPD patients was 45 %. The patients with COPD and MetS had significantly higher leukocytes and CRP levels than patients with COPD alone (p <0.001, p <0.001, respectively)32. Our finding of greater values of CRP, fibrinogen and leukocyte count in COPD patients with MetS compared with those without MetS is in line with all above mentioned studies.
The limitations of our study were the small sample size and its cross-sectional design. These limitations make it difficult to adequately describe causal relationships of detected associations. Furthermore, this study was performed in a single center. Further prospective studies are needed for better understanding of MetS components and systemic inflammatory profile in patients with COPD.
Conclusion
The present study shows that MetS is frequent in patients with COPD. Systemic inflammatory markers are elevated in COPD patients with MetS in comparison with patients without MetS. Some of the investigated inflammatory markers are independent predictors of presence of the MetS in COPD patients. These findings suggest that physicians should screen COPD patients for associated MetS and elevated circulatory inflammatory markers. Management of these disorders should reduce the risk of cardiovascular morbidity and mortality in patients with COPD.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Rabe KF, Wedzicha JA, Wouters EFM. Introduction. Rabe KF, Wedzicha JA, Wouters EFM (eds). COPD and Comorbidity. European Respiratory Society, Sheffield. 2013:ix–x. [Google Scholar]
- 2.Agusti A, Soriano JB. COPD as a systemic disease. COPD. 2008;5:133–138. doi: 10.1080/15412550801941349. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ. Chronic obstructive pulmonary disease: effects beyond the lungs. PLoS Med. 2010;7:e1000220. doi: 10.1371/journal.pmed.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet. 2007;370:797–799. doi: 10.1016/S0140-6736(07)61383-X. [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 6.Minas M, Kostikas K, Papaioannou AI, Mystridou P, Karetsi E, Georgoulias P, et al. The association of metabolic syndrome with adipose tissue hormones and insulin resistance in patients with COPD without co-morbidities. COPD. 2011;8:414–420. doi: 10.3109/15412555.2011.619600. [DOI] [PubMed] [Google Scholar]
- 7.Watz H, Waschki B, Kirsten A, Müller KC, Kretchmar G, Meyer T, et al. The metabolic syndrome in patients with chronic bronchitis and COPD: frequency and associated consequences for systemic inflammation and physical inactivity. Chest. 2009;136:1039–1046. doi: 10.1378/chest.09-0393. [DOI] [PubMed] [Google Scholar]
- 8.Wells CE, Baker EH. Metabolic syndrome and diabetes mellitus in COPD. Rabe KF, Wedzicha JA, Wouters EFM (eds). COPD and Comorbidity. European Respiratory Society, Sheffield. 2013:117–134. [Google Scholar]
- 9.Akpınar EE, Akpınar S, Ertek S, Sayın E, Gülhan M. Systemic inflammation and metabolic syndrome in stable COPD patients. Turbek Toraks. 2012;60:230–237. [PubMed] [Google Scholar]
- 10.Poulain M, Doucet M, Drapeau V, Fournier G, Tremblay A, Poirier P, et al. Metabolic and inflammatory profile in obese patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2008;5:35–41. doi: 10.1177/1479972307087205. [DOI] [PubMed] [Google Scholar]
- 11.Stanciu S, Marinescu R, Iordache M, Dumitrescu S, Mureşan M, Bogdan MA. Are systemic inflammatory profiles different in patients with COPD and metabolic syndrome as compared to those with COPD alone? Rom J Intern Med. 2009;47:381–386. [PubMed] [Google Scholar]
- 12.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Eeden SF, Sin DD. Chronic obstructive pulmonary disease: a chronic systemic inflammatory disease. Respiration. 2008;75:224–238. doi: 10.1159/000111820. [DOI] [PubMed] [Google Scholar]
- 14.Skyba P, Ukropec J, Pobeha P, Ukropcova B, Joppa P, Kurdiova T, et al. Metabolic phenotype and adipose tissue inflammation in patients with chronic obstructive pulmonary disease. Mediators Inflamm. 2010;2010:173498. doi: 10.1155/2010/173498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Bunjhoo H, Xiong W, Xu Y, Yang D. Association between C-reactive protein concentration and chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Int Med Res. 2012;40:1629–1635. doi: 10.1177/030006051204000501. [DOI] [PubMed] [Google Scholar]
- 16.Devaraj S, Singh U, Jialal I. Human C-reactive protein and the metabolic syndrome. Curr Opin Lipidol. 2009;20:182–189. doi: 10.1097/MOL.0b013e32832ac03e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowe GD, Pepys MB. C-reactive protein and cardiovasculare disease: weighing the evidence. Curr Atheroscler Rep. 2006;8:421–428. doi: 10.1007/s11883-006-0040-x. [DOI] [PubMed] [Google Scholar]
- 18.Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Resp J. 2006;28:1245–1257. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal R, Zaheer MS, Ahmad Z, Akhtar J. The relationship between C-reactive protein and prognostic factors in chronic obstructive pulmonary disease. Multidiscip Respir Med. 2013;8:63. doi: 10.1186/2049-6958-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of COPD. Updated 2015. Available at: www.goldcopd.com. last accessed: 03/01/2016.
- 21.Alberti KG, Zimmer P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 22.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 23.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yemault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- 24.Lam KB, Jordan RE, Jiang CQ, Thomas GN, Miller MR, Zhang WS, et al. Airflow obstruction and metabolic syndrome: the Guangzhou Biobank Cohort Study. Eur Respir J. 2010;35:317–323. doi: 10.1183/09031936.00024709. [DOI] [PubMed] [Google Scholar]
- 25.Funakoshi Y, Omori H, Mihara S, Marubayashi T, Katoh T. Association between airflow obstruction and the metabolic syndrome and its components in Japanese men. Intern Med. 2010;49:2093–2099. doi: 10.2169/internalmedicine.49.3882. [DOI] [PubMed] [Google Scholar]
- 26.Hosny H, Abdel-Hafiz H, Moussa H, Soliman A. Metabolic syndrome and systemic inflammation in patients with chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc. 2013;62:85–89. [Google Scholar]
- 27.Diez-Manglano J, Barquero-Romero J, Almagro P, Cabrera FJ, López García F, Montero L, et al. Working Group on COPD; Spanish Society of Internal Medicine. COPD patients with and without metabolic syndrome: clinical and functional differences. Intern Emerg Med. 2014;9:419–425. doi: 10.1007/s11739-013-0945-7. [DOI] [PubMed] [Google Scholar]
- 28.Mekov E, Slavova Y, Tsakova A, Genova M, Kostadinov D, Minchev D, et al. Metabolic syndrome in hospitalized patients with chronic obstructive pulmonary disease. PeerJ. 2015;3:e1068. doi: 10.7717/peerj.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breyer MK, Spruit MA, Hanson CK, Franssen FM, Vanfleteren LE, Groenen MT, et al. Prevalence of metabolic syndrome in COPD patients and its consequences. PLoS One. 2014;9:e98013. doi: 10.1371/journal.pone.0098013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marquis K, Maltais F, Duguay V, Bezeau AM, LeBlanc P, Jobin J, et al. The metabolic syndrome in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2005;25:226–232. doi: 10.1097/00008483-200507000-00010. discussion 233-234. [DOI] [PubMed] [Google Scholar]
- 31.Ozgen Alpaydin A, Konyar Arslan I, Serter S, Sakar Coskun A, Celik P, Taneli F, et al. Metabolic syndrome and carotid intima-media thickness in chronic obstructive pulmonary disease. Multidiscip Respir Med. 2013;8:61. doi: 10.1186/2049-6958-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasar Z, Buyuksirin M, Ucsular FD, Kargı A, Erdem F, Talay F, et al. Is an elevated neutrophil-to-lymphocyte ratio a predictor of metabolic syndrome in patients with chronic obstructive pulmonary disease? Eur Rev Med Pharmacol Sci. 2015;19:956–962. [PubMed] [Google Scholar]


