Summary
Immune-based interventions are promising strategies to achieve long-term cancer-free survival. Fasting was previously shown to differentially sensitize tumors to chemotherapy while protecting normal cells, including hematopoietic stem and immune cells, from its toxic side-effects. Here, we show that the combination of chemotherapy and a fasting-mimicking diet (FMD) increases the levels of bone marrow common lymphoid progenitor cells (CLP) and cytotoxic CD8+ tumor-infiltrating lymphocytes (TILs), leading to a major delay in breast cancer and melanoma progression. In breast tumors, this effect is partially mediated by the down-regulation of the stress-responsive enzyme heme oxygenase-1 (HO-1). These data indicate that FMD-cycles combined with chemotherapy can enhance T-cell-dependent targeted killing of cancer cells both by stimulating the hematopoietic system and by enhancing CD8+-dependent tumor-cytotoxicity.
Graphical Abstract
Introduction
Immune cells act as sentinels that recognize peptides originating from mutated genes and eliminate malignant and possibly pre-malignant cells (Rock and Shen, 2005). Cancer immunotherapy exploits this property of the immune system to recognize and eliminate cancer cells (Vesely et al., 2011; Zitvogel et al., 2008) by triggering the activation of inherent antitumor T cells (Pardoll, 2012; Wolchok et al., 2013) or through the reintroduction of engineered T cells into patients (Burns et al., 2010; Maude et al., 2014). The importance of a healthy immune system is underlined by the fact that immunosuppressed/immunocompromised subjects are at a higher risk for cancer (Zitvogel et al., 2006). Moreover, some traditional cytotoxic chemotherapeutics rely on the cooperation of the patient's immune system to eliminate cancer cells (Alizadeh et al., 2014; Arinaga et al., 1986; Bracci et al., 2014).
The immunosuppressive effect of some standard interventions, including radiotherapy and chemotherapy (Weinblatt et al., 1985; Weiner and Cohen, 2002), can compromise their therapeutic efficacy (Balow et al., 1975; Rasmussen and Arvin, 1982). Such therapeutic inefficacy and tumor resistance can also be caused by regulatory T cells (Tregs), which can suppress the lymphocytic activity through a mechanism mediated by heme oxygenase-1 (HO-1) (Choi et al., 2005; El Andaloussi and Lesniak, 2007). Conversely, some chemotherapeutics, such as anthracyclines, are known to stimulate the recognition of cancer cells by the immune system (Arinaga et al., 1986; Casares et al., 2005; Orsini et al., 1977), which may potentiate the effect of some immune-based therapies.
We have previously shown that a short-term starvation (STS) can selectively sensitize cancer cells to chemotherapeutics (differential stress-sensitization; DSS), while simultaneously protecting normal cells from its side effects (differential stress-resistance; DSR) (Lee et al., 2012; Raffaghello et al., 2008) via the insulin-like growth factor 1 (IGF-1) pathway (Lee et al., 2010). Recently, we also reported that STS promotes hematopoietic stem cell (HSC) self-renewal and reverses chemotherapy-induced immunosuppression (Cheng et al., 2014). Because water only STS is challenging for mice and cancer patients, we have developed a fasting-mimicking diet (FMD) that is low in calories, protein, and sugar (Brandhorst et al., 2015). This FMD reduces circulating IGF-1 and glucose, two major factors involved in DSR and DSS, to levels similar to those observed during STS (Brandhorst et al., 2015). Here, we tested the effect of FMD in combination with chemotherapy on the immune system, and on the immunogenicity of cancer cells.
Results
A Fasting-mimicking diet (FMD) alone or in combination with chemotherapy is as effective as short-term starvation (STS) in reducing tumor progression
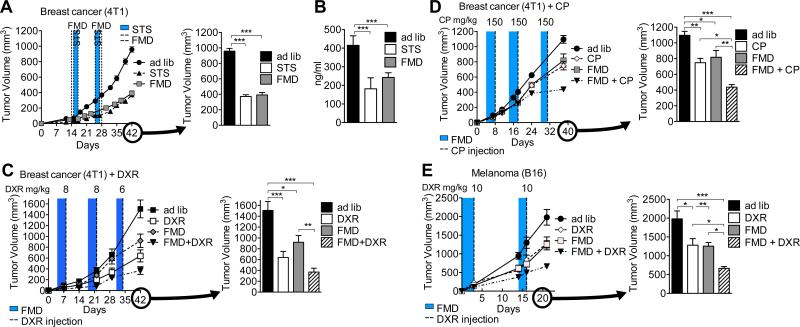
We have previously shown that STS is safe and effective in inducing DSS via IGF-1 signaling (Lee et al., 2012; Raffaghello et al., 2008; Safdie et al., 2009). Here we tested the efficacy of cycles of the FMD (Brandhorst et al., 2015) in inducing DSS in a syngeneic murine breast cancer (4T1) model (Figure S1). Four days of FMD feeding were as effective as two days of STS in retarding tumor growth and reducing circulating IGF-1 in the absence of chemotherapy (Figures 1A and 1B), and in sensitizing cancer cells to doxorubicin (DXR) and cyclophosphamide (CP) (Figures 1C, 1D, S2C, and S2D)(Lee et al., 2012; Lee et al., 2010; Raffaghello et al., 2008). Similar effects of the FMD were also observed in a murine melanoma (B16) model, in which mice were treated with DXR (Figure 1E). The combination of the FMD and DXR/CP had an additive effect on tumor suppression, causing a three-fold reduction in tumor volume compared to that observed in ad lib-fed counterparts (Figures 1C-1E).
Figure 1. A Fasting-mimicking diet (FMD) alone or in combination with chemotherapy is as effective as short-term starvation (STS) in reducing tumor progression.
(A-D) 12-week old female BALB/c mice were grafted with breast cancer (4T1) cells and subject to (A) multiple cycles of FMD or STS alone or to (C, D) a combination of FMD and the chemotherapy drugs (C) doxorubicin (DXR) or (D) cyclophosphamide (CP). (B) Circulating IGF-1 levels at the end of STS or FMD in (A) were measured. (E) 12-week old female C57BL/6 mice were grafted with melanoma (B16) cells and subject to multiple cycles of FMD alone or in combination with DXR. Tumor volume at multiple time-points (on the left) and immediately prior to euthanasia (on the right) are reported for each set of treatments. The animals receiving chemotherapy were injected at the end of each FMD cycle (shaded area). [(A, B) n=10, (C) n=13, (D) n=10, and (E) n=15]. Data represented as mean ± SEM. One-way ANOVA (Tukey post-analysis test) was performed. p-values <0.05, 0.01 and 0.001 are indicated as *, **, and ***, respectively. See also Figure S1.
FMD in combination with doxorubicin (DXR) promotes accumulation of tumor-infiltrating lymphocytes (TILs) in the tumor bed
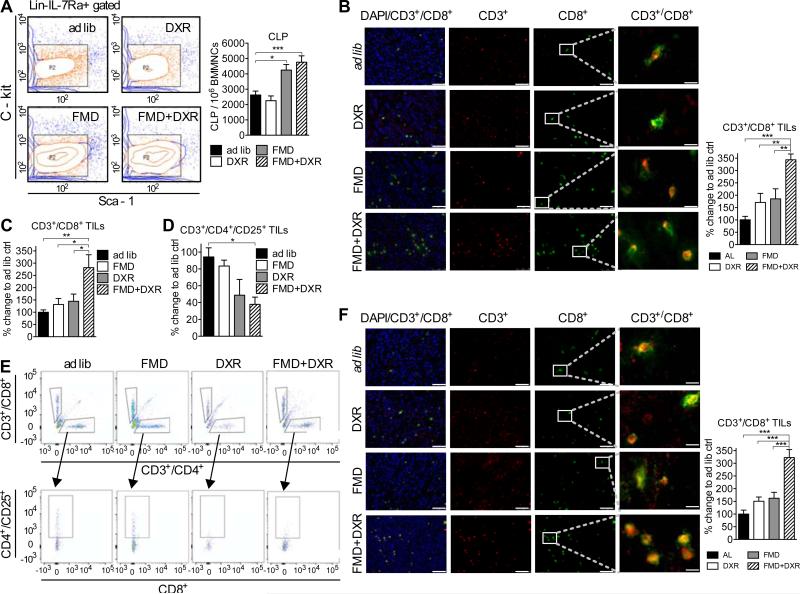
STS promotes HSC self-renewal and reverses chemotherapy-induced immunosuppression (Cheng et al., 2014). As previously reported (Cheng et al., 2014), three cycles of the FMD also caused a 33% increase in CD8+ circulating lymphocytes (Figure S2A) and a two-fold increase in common lymphoid progenitor cells (CLP) in the bone marrow (Figures 2A and S2B). This increase, likely due to HSC regeneration following re-feeding after STS (Cheng et al., 2014), raises the possibility that the immune system may be involved in mediating FMD-dependent DSS.
Figure 2. FMD in combination with doxorubicin (DXR) promotes accumulation of tumor-infiltrating lymphocytes (TILs) in the tumor bed.
(A) Bone marrow collected from BALB/c mice undergoing FMD/DXR treatments (see Figure S3A) was collected at the end of the experiment and analyzed with FACS (n=6) to assess the amount of Common Lymphoid Progenitors (CLP). (B) Breast cancer (4T1) tumor tissues collected at the end of the experiment (see Figure S3A) were analyzed using immunohistochemistry to assess CD3+/CD8+ TILs (n=8) (quantification on the right). (C, E) CD3+/CD8+ and (D, E) CD3+/CD4+/CD25+were also assessed by FACS analysis (n=7) in tumor tissue collected from a separate, equivalent experiment (see Figure 1C). (F) Melanoma (B16) tissue collected (see Figure 1E) was also processed to assess the levels of TILs (quantification on the right). Bar scale for DAPI, CD3 and CD8 is 75 μm. Bar scale for CD3+/CD8+ is 12.5 μm Data represented as mean ± SEM. The significance of the differences between experimental groups was determined by using one-way ANOVA (Tukey post-analysis test). p-values <0.05, 0.01 and 0.001 are indicated as *, **, and ***, respectively. See also Figure S2.
To evaluate whether FMD cycles could promote tumor immunogenicity, we measured the level of TILs after three cycles of FMD+DXR in mice. Only the combination of FMD+DXR significantly increased the number of CD3+/CD8+ TILs, as determined by both IHC (Figures 2B and S2E-S2J) and FACS analysis, (Figure 2C-2E) but there were no significant changes in the number of CD3-/CD8+ cells (Figure S2K). CD3+ cell infiltration increased after the first FMD cycle (Figure S2E-S2G) whereas CD8+ cell infiltration increased after the third FMD cycle, only in combination with DXR (Figure S2H-S2J). The cytotoxic activity of TILs was reflected by increased levels of granzyme-B and apoptosis in tumor cells, and by reduced tumor size (Figure S3). Similarly, higher levels of CD3+/CD8+ TILs were observed in C57BL/6 mice bearing melanoma (B16) that underwent FMD+DXR treatment (Figures 1E and 2F).
CD3+/CD8+ lymphocytes are key in FMD-mediated differential stress sensitization (DSS) to chemotherapy
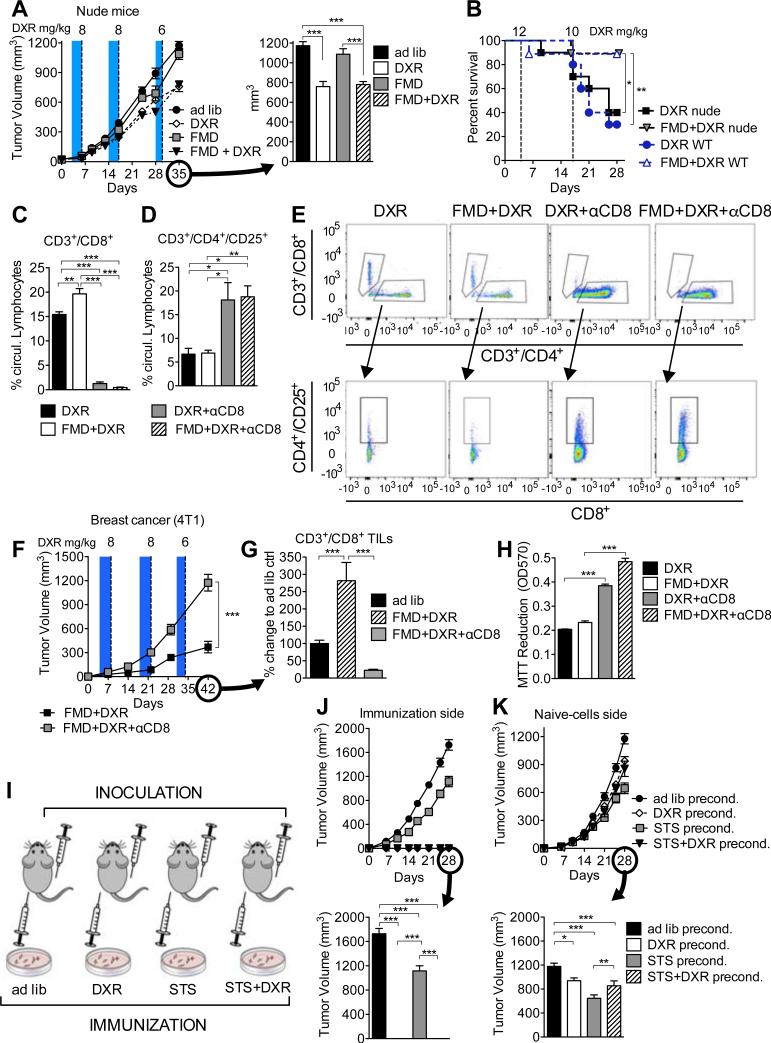
CD3+/CD8+ TILs are tumor-killing effector immune cells, which serve as an indicator of favorable prognosis (Andre et al., 2013; Issa-Nummer et al., 2013). To test whether CD3+/CD8+ TILs are required for FMD-induced tumor sensitization, we examined immunocompromised nude BALB/c mice that are athymic and therefore do not produce T lymphocytes. In contrast to the results in immunocompetent mice, the FMD was not effective in reducing tumor progression in nude mice (Figure 3A), and showed no changes in granzyme-B or cleaved-caspase-3 levels (Figure S4A-S4E). Nonetheless, the FMD successfully protected nude mice against DXR-dependent and tumor-independent toxicity (Figure 3B) (Raffaghello et al., 2008), implying that the FMD-induced protection of normal cells, unlike tumor sensitization, is not affected by T lymphocyte function.
Figure 3. CD3+/CD8+ lymphocytes have a key role in FMD-mediated differential stress sensitization (DSS) to chemotherapy.
(A) The effect of FMD on breast tumor growth (4T1) in immunodeficient BALB/c nude mice (n=15) (see Figure S4A-E); (B) The survival of wild type (WT) and nude mice injected with high dose of DXR and undergoing ad lib or FMD regimen. (C-H) 4T1 breast tumor-bearing mice were treated with DXR, FMD+DXR alone or in combination with [.alpha]CD8 monoclonal antibody and (C-E) circulating levels of (C, E) CD3+/CD8+ and (D, E) CD3+/CD4+/CD25+ were determined by FACS (n=7). (F) Tumor volumes of FMD+DXR and FMD+DXR+αCD8 were measured at multiple time points, and (G) TILs were also assessed in tumor samples collected from the same animals. (H) Circulating lymphocytes from DXR, FMD+DXR, DXR+αCD8, and FMD+DXR+αCD8 these animals were collected and cultured ex vivo with 4T1 cells for 24 hours and viability was assessed by MTT reduction. (I) Mice were immunized by subcutaneous inoculation (in the left flank) with 4T1 breast cancer cells preconditioned in vitro with either ad lib- (2 g/L glucose+10% FBS) or STS-medium (0.5 g/L glucose+1% FBS) with or without doxorubicin (5 μM). 7 days after the immunization, the same animals were inoculated with naïve 4T1 cells in the right flank (n=10). (J, K) Tumor progression for the immunization (left) and the naïve (right) sides are reported. Data represented as mean ± SEM. One-way ANOVA (Tukey post-analysis test) and Log-rank (Mantel-Cox) test (survival) was performed. Comparisons between groups were performed with Student's t test. p-values <0.05, 0.01 and 0.001 are indicated as *, **, and ***, respectively. See also Figure S3.
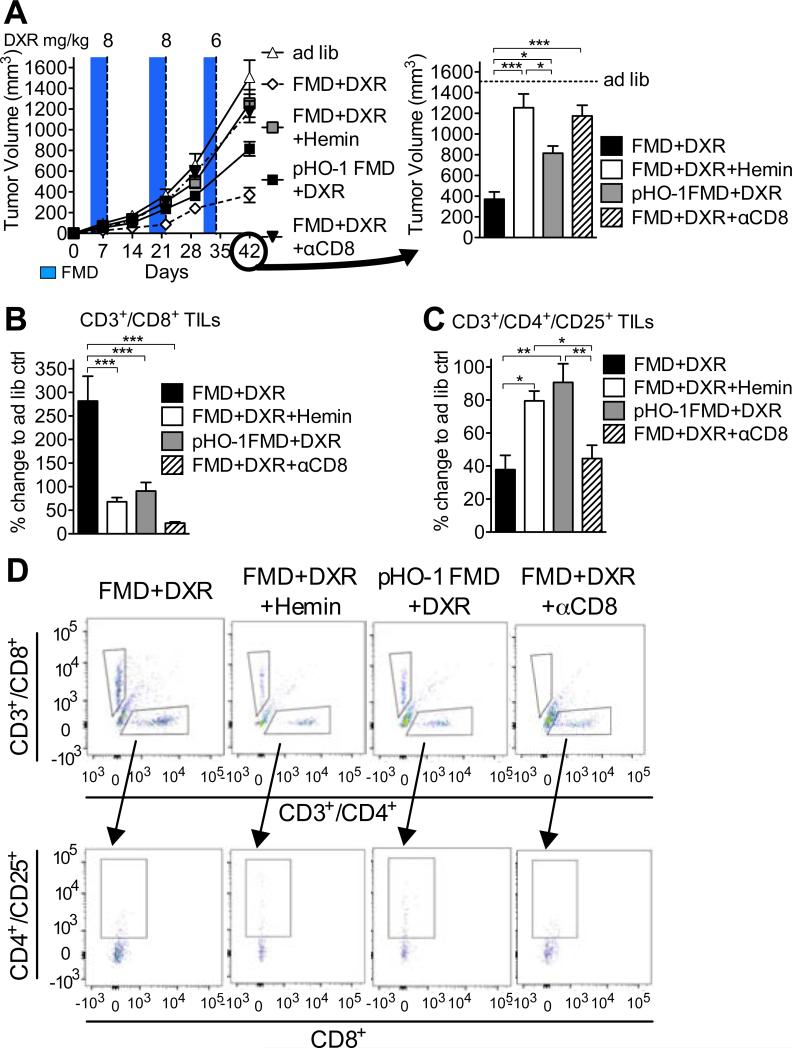
To directly test the role of TILs in mediating the FMD-dependent sensitization of tumors to DXR, we selectively depleted CD8+ T lymphocytes using a neutralizing monoclonal antibody (αCD8; clone YTS 169.4)(Carmi et al., 2015), or IgG (control) (Figures 3C and 3E). Notably, the depletion of CD8+ circulating cells was also associated with an increase in the number of regulatory T cells (Tregs)(Figures 3D and 3E). The antibody-dependent depletion of CD8+ T lymphocytes reversed the effects of FMD+DXR on the progression of grafted breast tumors (Figures 3F and 3G). Furthermore, lymphocytes isolated from αCD8 antibody-treated mice caused significantly less cytotoxicity to 4T1 cells in vitro compared to those from IgG-treated mice (Figure 3H).
These results indicate that activated TILs are important for the FMD-dependent sensitization of breast cancer cells to chemotherapeutics.
Anthracyclines can increase cancer cell immunogenicity and attract cytotoxic T lymphocytes (CTLs) to the tumor (Michaud et al., 2011). To test whether the FMD directly increases tumor immunogenicity, we cultured breast cancer cells (4T1) in FMD-like conditions (referred to as STS for in vitro experiments and consisting of reduced glucose/serum) and DXR prior to grafting them into immunocompetent mice. One week after grafting the pre-conditioned breast cancer cells, all mice were also grafted with “naïve” breast cancer cells on the other flank (Figure 3I). Although all mice were “immunized” by grafting the same number of live pre-conditioned breast cancer cells, DXR and STS+DXR groups did not develop tumors (Figures 3J and S4F). Consistent with previous studies (Casares et al., 2005; Inoue et al., 2014), “naïve” tumors on the other flank displayed retarded growth after 4 weeks in mice grafted with cells pre-cultured with DXR (Figure 3K). Notably, STS-preconditioning alone was more effective than STS+DXR, possibly because the less severe apoptotic effects of the STS allowed the immunogenic cancer cells to live longer and therefore provide a prolonged immunogenic stimuli after grafting in mice (Figures 3J, 3K, and S4F).
Collectively, these results indicate that FMD (i.e. STS in vitro) increased DXR-dependent tumor immunogenicity and expanded the pool of CD8+ TILs needed to effectively target tumors.
Heme oxygenase-1 (HO-1) is a mediator of FMD/STS-dependent Differential Stress Sensitization (DSS)
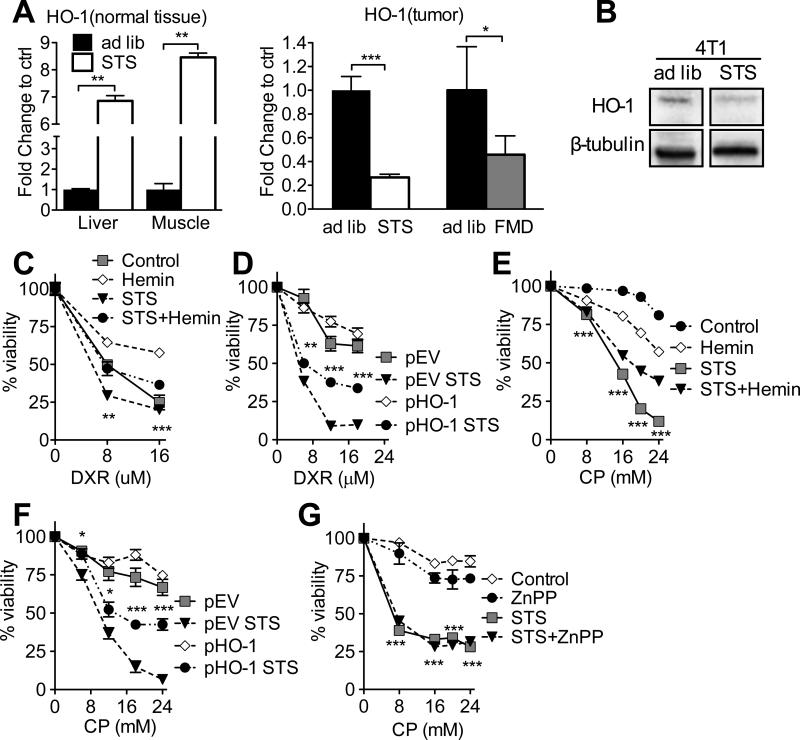
We performed microarray analysis to identify STS-dependent effects on the differential expression in normal and cancer cells of genes that may be relevant to TIL-dependent toxicity, and identified the inducible stress-responsive protein HO-1 as a potential candidate (Figure S5A). Thereafter, we confirmed that both STS and FMD reduced the expression of HO-1 in the grafted breast tumors (Figure 4A). On the contrary, STS differentially increased HO-1 expression in normal tissues (Figure 4A). Notably, the expression of Nrf2, an upstream regulator of HO-1, was also differentially reduced in the grafted breast tumors following STS (Figure S5B). Similar effects on HO-1 expression were also observed in vitro (Figures 4B, S5C, and S5D), specifically in the nucleus (Figure S5E). In fact, cancer cells have been reported to show increased levels of nuclear HO-1 independent of its enzymatic activity (Hsu et al., 2015).
Figure 4. Heme oxygenase-1 (HO-1) has a key role in mediating STS-dependent Differential Stress Sensitization (DSS).
(A) HO-1 expression levels of grafted 4T1 tumor and normal (liver and skeletal muscle) tissues collected from ad lib fed and mice undergoing STS or FMD regimen were analyzed with qRT-PCR. (B) HO-1 levels in 4T1 cells following 48 hours of in vitro STS were measured by Western blotting (n=3) (Blot was captured with Bio-Rad ChemiDoc, and unedited, representative bands are shown. (C, E, G) Viability of 4T1 cells was determined by MTT reduction following (C) DXR and hemin (10 M), (E) CP and hemin (10 M), and (G) CP and ZnPP (20 M). (D, F) Viability of 4T1 cell stably over-expressing HO-1 (pHO-1) or empty vector (pEV) was determined by MTT following (D) DXR and (F) CP under control and STS conditions. Data represented as mean ± SEM. The significance of the differences between experimental groups was determined by using one-way ANOVA (Tukey post-analysis test). Comparisons between groups were performed with Student's t test. p-values <0.05, 0.01 and 0.001 are indicated as *, **, and ***, respectively. See also Figure S5.
To test the role of HO-1 in FMD-mediated DSS, we cultured 4T1 cancer cells in normal or FMD-like conditions (referred to as STS for in vitro experiments and consisting of reduced glucose/serum) and exposed them to the HO-1 activator hemin or its inhibitor zinc protoporphyrin (ZnPP) in the presence of CP or DXR. The induction of HO-1 using hemin (Figures 4C, 4E, and S5F) or its overexpression by stably transfecting a pHO-1 construct (Suliman et al., 2007)(Figures S5G and S5H) in breast cancer cells (4T1) partially reversed the STS-induced sensitization to DXR and CP in vitro (Figures 4D and 4F). Conversely, the HO-1 inhibitor ZnPP sensitized breast cancer cells (4T1) to CP under normal conditions in vitro (Figure 4G). Thus, the down-regulation of HO-1 is important for FMD-dependent DSS.
Down-regulation of HO-1 in the tumor by FMD/STS is necessary for the decrease of Tregs and for the immune-dependent targeting of cancer cells
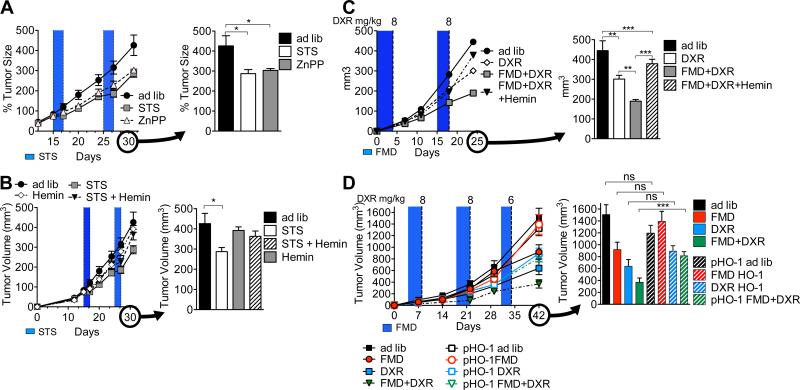
HO-1 is known to confer protection against oxidative damage and apoptosis (Chen et al., 2004), promote tumor progression (Hirai et al., 2007; Liu et al., 2011; Metz et al., 2010), and have immuno-modulatory roles that affect various T cell subpopulations including Tregs (Brusko et al., 2005; Xia et al., 2008). In mice, ZnPP (40 mg/kg/day; IP) treatment was as effective as STS in retarding breast tumor progression (4T1) (Figure 5A). In agreement with the in vitro results, concurrent hemin treatment (30 mg/kg/day; IP) reversed STS-induced retardation of breast tumors (4T1) whereas hemin alone did not show significant responses (Figure 5B). Both the induction and overexpression of HO-1 (by hemin or stable transfection, respectively), and the CD8+ CTL depletion reversed the FMD-induced (i) sensitization of breast tumors (4T1) to DXR (Figures 5C, 5D, and 6A) and (ii) accumulation of CD3+/CD8+ TILs (Figures 6B and 6D). Interestingly, HO-1 induction/over-expression also increased CD3+/CD4+/CD25+ Tregs and, to a much smaller extent, CD3+/CD8+/CD25+ Tregs in the tumor (Figures 6C, 6D, and S5K). Similar effects were observed after CP treatment (Figure S6). As expected, CD8+ CTL depletion alone was not associated with increased levels of tumor-associated Tregs (Figure 6C). In mammalian cells, HO-1 is a rate-limiting enzyme catalyzing the degradation of heme to produce carbon monoxide (CO), free iron, and biliverdin (Maines, 1988). Biliverdin can have antioxidative effects (Abraham and Kappas, 2008; Otterbein et al., 2000), but its treatment alone was not sufficient to reverse the FMD-dependent DSS (Figures S5I and S5J), suggesting a more complex involvement of the HO-1 pathway. Because HO-1-dependent increase of CO has been shown to interfere with angiogenesis (Ahmad et al., 2015), metastasis (Tertil et al., 2015), and tumor growth (Wegiel et al., 2013) it will be important to further study the potential role of CO in the antitumor effects of the FMD.
Figure 5. Down-regulation of HO-1 in the tumor during FMD/STS is required for the targeted attack of cancer cells.
(A-C) 4T1 tumor-bearing mice were treated with (A) ZnPP (40 mg/kg/day; IP; n=7), (B) STS and hemin (30 mg/kg/day; IP; n=7), and (C) FMD and hemin and DXR. (D) Mice bearing 4T1 tumors stably over-expressing HO-1 (pHO-1), or empty vector (pEV) were treated with DXR under ad lib or FMD regimens (n=10-15) (see Figure 1C). Data represented as mean ± SEM. One-way ANOVA (Tukey post-analysis test) was performed. p-values <0.05, 0.01 and 0.001 are indicated as *, **, and ***, respectively. See also Figure S6.
Figure 6. Up-regulation or overexpression of HO-1 lead to decreased infiltration of TILs and accumulation of Tregs in the tumor bed.
Mice bearing 4T1 or 4T1-pHO-1 breast tumors under FMD were treated with DXR and either hemin (30 mg/kg/day; IP) or CD8+ specific neutralizing monoclonal antibodies (αCD8; clone YTS 169.4) (n=13-15) (see Figures 3F and 5D). (A) Tumor volumes were measured at multiple time-points (left) and immediately prior to euthanasia (right). (B-D) CD3+/CD8+ (CTL) and CD3+/CD4+/CD25+ (Treg)lymphocytes from the tumor bed was analyzed by FACS. Data represented as mean ± SEM. One-way ANOVA (Tukey post-analysis test) was performed. p-values <0.05, 0.01 and 0.001 are indicated as *, **, and ***, respectively.
These results indicate that reduced HO-1 expression in tumors mediates, in part, the anticancer effect of FMD by increasing CD8+ TIL-dependent cytotoxicity, which may be facilitated by down-regulation of Tregs.
Discussion
We provide evidence that the FMD-dependent DSS is, in part, mediated by CD8+ TIL-dependent killing of cancer cells, associated with an increase in lymphoid progenitor cells.
Immunological approaches are among the most promising treatments with the potential to result in cancer free survival. However, immunotherapies are expensive and often effective only in a subset of patients. Here we show that fasting can be substituted with a low calorie and protein FMD which provides high nourishment and is effective in inducing the targeted killing of cancer cells. The efficacy of the FMD can be enhanced when administered in combination with various chemotherapeutics. The efficacy of fasting in inducing DSS both in in vitro and in vivo models has been extensively reported in the recent years and has been proposed to be mediated, in part, by the reduction of circulating IGF-1 and glucose levels (Lee et al., 2012; Lee et al., 2010; Raffaghello et al., 2008). Our study shows that T cells play a key role in the killing of breast cancer cells during FMD/STS. Another important component of the antitumor activity triggered by FMD/STS appears to be its effect on the repopulation of the bone marrow with CLPs (Cheng et al., 2014). Our work extended from the systemic benefits of the FMD on the immune system to those promoting a targeted killing of cancer cells. Because of the strong association between the presence of CD3+/CD8+ TILs in the tumor bed and positive outcome of the cancer treatment (Andre et al., 2013; Issa-Nummer et al., 2013), our results are of potential therapeutic relevance as they show the efficacy of a safe and inexpensive regimen in improving the efficacy of cancer therapy by stimulating T cell-dependent cytotoxicity. Notably, FMD and STS are likely to contribute to the killing of cancer cells both by immune-dependent and immune-independent mechanisms, although the effects on 4T1 cells appear to be mostly dependent on the activity of T cells.
HO-1 expression, which is often elevated in tumors, has been shown to be involved in the pathogenesis of several types of cancers (Liu et al., 2004). Here, we show that FMD/STS causes a reduction in HO-1 expression in cancer but not in normal cells. Thus, under FMD conditions, cancer but not normal cells are sensitized to chemotherapy, in part by differential regulation of HO-1. Moreover, HO-1 down-regulation in cancer cells can void their ability to activate Tregs and overcome in situ immunoattack (Otterbein et al., 2000). In agreement with an involvement of Tregs in HO-1-dependent regulation of DSS, the activation of HO-1 in mice rendered the FMD ineffective in inducing an immune-based attack of cancer cells, while its pharmacological inhibition was sufficient to reduce tumor progression in the absence of an FMD treatment.
Taken together, these results indicate that FMD can enhance the efficacy of chemotherapy by enhancing tumor immunogenicity, in part by an HO-1-dependent mechanism, which promotes the recruitment of cytotoxic CD8+ T cells to the tumor bed and reduces tumor-associated Tregs (Figure 7). Thus, FMD cycles have potential of clinical application based on the enhancement of T cell-mediated cytotoxicity to cancer cells in the presence or absence of chemotherapy, but may also increase the efficacy of antibodies or other immunological strategies being developed for the treatment of a wide variety of tumors.
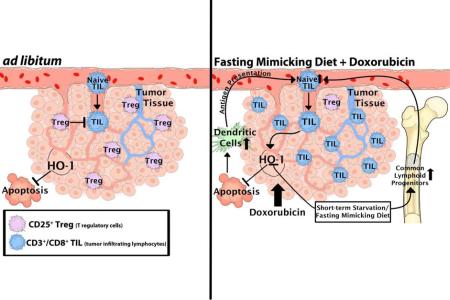
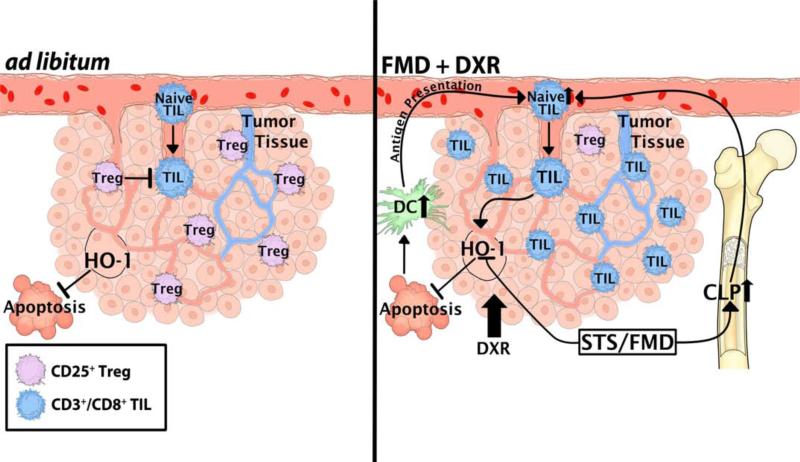
Figure 7. A model of FMD-dependent DSS in tumors.
Two scenarios describing the rearrangement of tumor and immune system under different dietary interventions are shown. FMD+DXR increases the immunogenicity of tumors in an HO-1-dependent manner, and increases the recruitment of CD3+/CD8+ cytotoxic lymphocytes to the tumor bed (TILs), leading to the targeted attack of cancer cells. Such increase of CD3+/CD8+ TILs is accompanied by the reduction of CD3+/CD4+/CD25+ Tregs in the tumor bed. The latter scenario is also associated with an increase of CLP in the bone marrow and of circulating CD3+/CD8+ cytotoxic lymphocytes. Dendritic cells (DC) can recognize DXR-induced apoptotic tumor cells stimulate circulating CD3+/CD8+ CTLs, which, in our model, can be augmented by FMD cycles.
Experimental Procedures
Animals
12-week old BALB/c (wild type) and BALB/c-nude mice were purchased from Simonsen Laboratories (USA) or from Envigo (USA). 12-week old C57BL/6 female mice were purchased from Charles River Laboratories International, Inc (USA). The animals were housed under specific pathogen-free conditions with 12h day/light cycles. All the experiments were performed according to procedures approved by USC's Institutional Animal Care and Use Committee.
Diet
Mice were maintained on irradiated TD.7912 rodent chow (Harlan Teklad). In brief, this diet contains 3.75 kcal/g of digestible energy with calories supplied by protein, carbohydrate and fat in a percent ratio of 25: 58: 17. Food was provided ad lib. On average, mice in the control group consumed 14.9 kcal/day (or 3.9 g/day). Our experimental FMD diet is based on a nutritional screen that identified ingredients allowing high nourishment during periods of low calorie consumption (Brandhorst et al., 2013). Prior to supplying the FMD diet, animals were transferred into fresh cages to avoid feeding on residual chow and coprophagy. The FMD diet consists of two different components designated as day 1 diet and day 2-4 diet that were fed in this order respectively. The day 1 diet contains 1.88 kcal/g and was designed to adapt the mouse to a period of low caloric intake during the subsequent feeding days. The day 2-4 diet is identical on all feeding days and contains 0.36 kcal/g. The day 1 and day 2-4 diets were fed as the average intake (~4 g) of the ad lib fed control group every two weeks. Due to the different caloric densities of the supplied FMD diet, mice in this cohort had a ~50% reduction in consumed calories on day 1 and consumed 9.7% of the control cohort on days 2 to 4. Mice consumed all the supplied food on each day of the FMD regimen and showed no signs of food aversion. After the end of the day 2-4 diet, we supplied TD.7912 chow ad lib for 10 days before starting another FMD cycle.
STS
(In vivo) Animals underwent complete food deprivation with free access to water for a total of 48 to 60 hours to allow a 20% bodyweight loss. In order to avoid dehydration, the mice were fed a low caloric hydrogel, which encouraged water consumption. During STS regimen, mice were individually housed in a clean cage to reduce cannibalism and coprophagy. Body weight was measured immediately before, during and after fasting. (In vitro) FMD-like conditions in cell culture models are referred to as STS (0.5 g/L glucose with 1% FBS).
Cancer cell lines and tumor cell injection
MCF7 human breast adenocarcinoma (gift from Amy Lee – University of Southern California), 4T1-luc murine breast cancer (SibTech, Inc.) and B16 murine melanoma (gift from Noah Craft, UCLA) cell lines were cultured in high glucose (4.5g/L) Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS (Thermo Fisher Scientific Inc.) at 37°C and 5% CO 2. To establish a subcutaneous cancer mouse model, we injected female BALB/c mice or female C57BL/6 mice with 4T1 breast cancer cells or B16 melanoma cells, respectively. Before injection, cells in log phase of growth were harvested and suspended in phosphate-buffered saline (PBS) at 2 × 106 cells/ml, and 100 μl (2 × 105 cells per mouse) were injected subcutaneously in the flank. Body weight was determined periodically, and tumor size was measured with a digital vernier caliper. Tumor volume was calculated with the following equation: tumor volume (mm3) = (length × width x height) / 2, where the height, length and width are in millimeters.
Immunization with in vitro pre-conditioned cancer cells
4T1-luc murine breast cancer cells (SibTech, Inc.) were pre-treated in vitro by 48h culture in normal conditions (2.0 g/L glucose supplemented with 10% FBS) or “fasting-mimicking conditions” (i.e. STS; 0.5 g/L glucose supplemented with 1% FBS) at 37°C and 5% CO2. For those cells undergoing doxorubicin pre-conditioning, the drug was added to the medium to a final concentration of 5μM for the 24h preceding the tumor implantation. To induce a “tumor vaccination”, the in vitro pre-conditioned cells were injected subcutaneously into the left flank of 12-week-old female BALB/c mice. In the specific, cancer cells pre-conditioned with normal and “fasting-mimicking” medium were inoculated in ad lib and FMD mice respectively. Cells pre-treated in vitro with DXR under the same conditions described above were injected in mice belonging to the DXR and FMD+DXR groups. One week after the implantation of pre-treated 4T1 tumor cells, the mice were subject to implantation of untreated 4T1 cells in the right flank. Before injection, cells in log phase of growth were harvested, analyzed with trypan blue staining and suspended in phosphate-buffered saline (PBS) at 2 × 106 living cells/ml, then 100 μl (2 × 105 cells per mouse) were injected subcutaneously in the mouse. It is important to clarify that for this specific experiment, all the mice were ad lib fed for the whole time and the only difference between one group and the other was the different pre-conditioning of the cancer cells inoculated. All cells were grown to 70% confluency and only live cells, determined by trypan blue exclusion, were used for subcutaneous grafting. Equal number of live cells was injected into each mouse.
Generation of pHO-1 4T1 cells
4T1-luc murine breast cancer (SibTech, Inc.) cells were transfected with HO-1 plasmid (Suliman et al., 2007), or its parental empty vector, DNA using Lipofectamine 3000. Following incubation at 37°C for 48h, the transfected cells were incubated in selection medium containing G418. The selection step was carried on for 7 days and the selecting medium was replaced 48h. Upon seeding, single colonies were grown, selected and the expended. The effectiveness of transfection was confirmed with PCR and western blot analyses in some of the clones.
Quantitative PCR
Relative transcript expression levels were measured using RT2 qPCR Primer Assay for Hmox1 (Qiagen Inc).
Sub-cellular fraction isolation
For the isolation of cellular fraction, following culture in control and STS medium condition, 4T1 cells were trypsinized, washed in ice-cold PBS and processed using a Qproteome Mitochondria Isolation Kit following manufacturer recommendations (Qiagen Inc.).
Statistical Analyses
ImageJ software was used for the quantification of the signal and GraphPad Prism v5.0c was used for the graphic representation and statistical analysis of the data. The significance of the differences between groups in mouse experiments was determined by using Analysis Of Variance (ANOVA) analysis in GraphPad Prism v5.0c. Tukey test was used in the post analysis and the differences were considered significant if the p value was ≤0.05. Comparisons between groups were done with Student's t test using GraphPad Prism v.5.0c. All the statistical analyses were two-sided and p values ≤0.05 were considered significant.
Supplementary Material
Significance.
Cancer immunotherapy is a promising intervention for the targeted killing of tumor cells. However, tumor-localized immunosuppression induced by cancer cells (immunoresistance) often leads to limited T cell-mediated cytotoxicity. Moreover, chemotherapy- and radiotherapy-induced immunosuppression can further reduce the efficacy of immune-based treatments. Here we show that a fasting-mimicking diet (FMD) increases the levels of common lymphoid progenitor cells (CLPs) and of circulating CD8+ lymphocytes. The combination of the FMD and doxorubicin increased the number of cytotoxic CD8+ tumor-infiltrating lymphocytes (TILs). The FMD-induced recruitment of TILs was mediated by heme oxygenase-1 (HO-1) and was significantly correlated with a lower number of regulatory T cells in the tumor bed. Thus, the FMD is a promising intervention to potentiate immune-based and standard cancer therapies.
Highlights.
The FMD is as effective as fasting in sensitizing tumors to chemotherapy.
FMD cycles promote the enrichment of common lymphoid progenitor cells (CLP)
FMD/doxorubicin increase tumor immunogenicity and tumor-infiltrating lymphocytes
HO-1 plays a key role in the FMD-induced chemosensitization of breast cancer cells
Acknowledgments
This study was funded by NIH grant P01 AG034906 to V.D.L. and subcontract HHSN261201200051C from NCI to M.W. R.d.C and A.M.M. were funded by the Intramural Research Program of the National Institute on Aging, NIH. We thank William Wood, Elin Lehrmann and Yongqing Zhang for assistance with microarray analyses.
V.D.L. and T.M. have equity interest in L-Nutra, a company developing medical food.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
S.D.B. and C.L. performed most of the experiments and analyses together with C.W.C., A.M.M., R.D.C., R.B., M.C., B.M., and S.B; S.D.B., C.L. and V.D.L. conceived the project; D.B.S., C.L., V.D.L., M.W. and T.M. designed the experiments; S.D.B., C.L., and V.D.L wrote the paper.
Accession number: Microarray data shown in this study have been deposited to Gene Expression Omnibus (GEO), accession number GSE28556.
REFERENCES
- Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacological reviews. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Hewett PW, Fujisawa T, Sissaoui S, Cai M, Gueron G, Al-Ani B, Cudmore M, Ahmed SF, Wong MK, et al. Carbon monoxide inhibits sprouting angiogenesis and vascular endothelial growth factor receptor-2 phosphorylation. Thromb Haemost. 2015;113:329–337. doi: 10.1160/TH14-01-0002. [DOI] [PubMed] [Google Scholar]
- Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer research. 2014;74:104–118. doi: 10.1158/0008-5472.CAN-13-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre F, Dieci MV, Dubsky P, Sotiriou C, Curigliano G, Denkert C, Loi S. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:28–33. doi: 10.1158/1078-0432.CCR-11-2701. [DOI] [PubMed] [Google Scholar]
- Arinaga S, Akiyoshi T, Tsuji H. Augmentation of the generation of cell-mediated cytotoxicity after a single dose of adriamycin in cancer patients. Cancer research. 1986;46:4213–4216. [PubMed] [Google Scholar]
- Balow JE, Hurley DL, Fauci AS. Cyclophosphamide suppression of established cell-mediated immunity. Quantitative vs. qualitative changes in lymphocyte populations. The Journal of clinical investigation. 1975;56:65–70. doi: 10.1172/JCI108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell death and differentiation. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell metabolism. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, Wei M, Hwang S, Morgan TE, Longo VD. Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Exp Gerontol. 2013 doi: 10.1016/j.exger.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusko TM, Wasserfall CH, Agarwal A, Kapturczak MH, Atkinson MA. An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J Immunol. 2005;174:5181–5186. doi: 10.4049/jimmunol.174.9.5181. [DOI] [PubMed] [Google Scholar]
- Burns WR, Zhao Y, Frankel TL, Hinrichs CS, Zheng Z, Xu H, Feldman SA, Ferrone S, Rosenberg SA, Morgan RA. A high molecular weight melanoma-associated antigen-specific chimeric antigen receptor redirects lymphocytes to target human melanomas. Cancer research. 2010;70:3027–3033. doi: 10.1158/0008-5472.CAN-09-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmi Y, Spitzer MH, Linde IL, Burt BM, Prestwood TR, Perlman N, Davidson MG, Kenkel JA, Segal E, Pusapati GV, et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature. 2015;521:99–104. doi: 10.1038/nature14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. The Journal of experimental medicine. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheadle C, Vawter MP, Freed WJ, Becker KG. Analysis of microarray data using Z score transformation. The Journal of molecular diagnostics : JMD. 2003;5:73–81. doi: 10.1016/S1525-1578(10)60455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GG, Liu ZM, Vlantis AC, Tse GM, Leung BC, van Hasselt CA. Heme oxygenase-1 protects against apoptosis induced by tumor necrosis factor-alpha and cycloheximide in papillary thyroid carcinoma cells. J Cell Biochem. 2004;92:1246–1256. doi: 10.1002/jcb.20157. [DOI] [PubMed] [Google Scholar]
- Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, Da Sacco S, Mirisola M, Quinn DI, Dorff TB, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell stem cell. 2014;14:810–823. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BM, Pae HO, Jeong YR, Kim YM, Chung HT. Critical role of heme oxygenase-1 in Foxp3-mediated immune suppression. Biochemical and biophysical research communications. 2005;327:1066–1071. doi: 10.1016/j.bbrc.2004.12.106. [DOI] [PubMed] [Google Scholar]
- El Andaloussi A, Lesniak MS. CD4+ CD25+ FoxP3+ T-cell infiltration and heme oxygenase-1 expression correlate with tumor grade in human gliomas. Journal of neurooncology. 2007;83:145–152. doi: 10.1007/s11060-006-9314-y. [DOI] [PubMed] [Google Scholar]
- Hirai K, Sasahira T, Ohmori H, Fujii K, Kuniyasu H. Inhibition of heme oxygenase-1 by zinc protoporphyrin IX reduces tumor growth of LL/2 lung cancer in C57BL mice. Int J Cancer. 2007;120:500–505. doi: 10.1002/ijc.22287. [DOI] [PubMed] [Google Scholar]
- Hsu FF, Yeh CT, Sun YJ, Chiang MT, Lan WM, Li FA, Lee WH, Chau LY. Signal peptide peptidase-mediated nuclear localization of heme oxygenase-1 promotes cancer cell proliferation and invasion independent of its enzymatic activity. Oncogene. 2015;34:2360–2370. doi: 10.1038/onc.2014.166. [DOI] [PubMed] [Google Scholar]
- Inoue S, Setoyama Y, Odaka A. Doxorubicin treatment induces tumor cell death followed by immunomodulation in a murine neuroblastoma model. Experimental and therapeutic medicine. 2014;7:703–708. doi: 10.3892/etm.2014.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa-Nummer Y, Darb-Esfahani S, Loibl S, Kunz G, Nekljudova V, Schrader I, Sinn BV, Ulmer HU, Kronenwett R, Just M, et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer--a substudy of the neoadjuvant GeparQuinto trial. PloS one. 2013;8:e79775. doi: 10.1371/journal.pone.0079775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Volsky DJ. PAGE: parametric analysis of gene set enrichment. BMC bioinformatics. 2005;6:144. doi: 10.1186/1471-2105-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Science translational medicine. 2012;4:124ra127. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer research. 2010;70:1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liang Y, Zheng T, Yang G, Zhang X, Sun Z, Shi C, Zhao S. Inhibition of heme oxygenase-1 enhances anti-cancer effects of arsenic trioxide on glioma cells. J Neurooncol. 2011;104:449–458. doi: 10.1007/s11060-010-0513-1. [DOI] [PubMed] [Google Scholar]
- Liu ZM, Chen GG, Ng EK, Leung WK, Sung JJ, Chung SC. Upregulation of heme oxygenase-1 and p21 confers resistance to apoptosis in human gastric cancer cells. Oncogene. 2004;23:503–513. doi: 10.1038/sj.onc.1207173. [DOI] [PubMed] [Google Scholar]
- Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1988;2:2557–2568. [PubMed] [Google Scholar]
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz R, Duhadaway JB, Rust S, Munn DH, Muller AJ, Mautino M, Prendergast GC. Zinc protoporphyrin IX stimulates tumor immunity by disrupting the immunosuppressive enzyme indoleamine 2,3-dioxygenase. Mol Cancer Ther. 2010;9:1864–1871. doi: 10.1158/1535-7163.MCT-10-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- Orsini F, Pavelic Z, Mihich E. Increased primary cell-mediated immunity in culture subsequent to adriamycin or daunorubicin treatment of spleen donor mice. Cancer research. 1977;37:1719–1726. [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nature medicine. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. Immunology beats cancer: a blueprint for successful translation. Nature immunology. 2012;13:1129–1132. doi: 10.1038/ni.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L, Arvin A. Chemotherapy-induced immunosuppression. Environmental health perspectives. 1982;43:21–25. doi: 10.1289/ehp.824321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunological reviews. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, Cohen P, Longo VD. Fasting and cancer treatment in humans: A case series report. Aging. 2009;1:988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. The Journal of clinical investigation. 2007;117:3730–3741. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tertil M, Golda S, Skrzypek K, Florczyk U, Weglarczyk K, Kotlinowski J, Maleszewska M, Czauderna S, Pichon C, Kieda C, et al. Nrf2-heme oxygenase-1 axis in mucoepidermoid carcinoma of the lung: Antitumoral effects associated with down-regulation of matrix metalloproteinases. Free radical biology & medicine. 2015;89:147–157. doi: 10.1016/j.freeradbiomed.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annual review of immunology. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- Wegiel B, Gallo D, Csizmadia E, Harris C, Belcher J, Vercellotti GM, Penacho N, Seth P, Sukhatme V, Ahmed A, et al. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer research. 2013;73:7009–7021. doi: 10.1158/0008-5472.CAN-13-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, Trentham DE. Efficacy of low-dose methotrexate in rheumatoid arthritis. The New England journal of medicine. 1985;312:818–822. doi: 10.1056/NEJM198503283121303. [DOI] [PubMed] [Google Scholar]
- Weiner HL, Cohen JA. Treatment of multiple sclerosis with cyclophosphamide: critical review of clinical and immunologic effects. Mult Scler. 2002;8:142–154. doi: 10.1191/1352458502ms790oa. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. The New England journal of medicine. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia ZW, Zhong WW, Meyrowitz JS, Zhang ZL. The role of heme oxygenase-1 in T cell-mediated immunity: the all encompassing enzyme. Current pharmaceutical design. 2008;14:454–464. doi: 10.2174/138161208783597326. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nature reviews Immunology. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nature reviews Immunology. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.