Abstract
Overexpression of mitochondria-bound hexokinase II (HKII) in cancer cells plays an important role in their metabolic reprogramming and protects them against apoptosis, thereby facilitating their growth and proliferation. Here, we show that covalently coupling a peptide corresponding to the mitochondrial membrane–binding N-terminal 15 aa of HKII (pHK) to a short, penetration-accelerating sequence (PAS) enhances the cellular uptake, mitochondrial localization, and cytotoxicity of the peptide in HeLa cells. Further analysis revealed that pHK-PAS depolarized mitochondrial membrane potential, inhibited mitochondrial respiration and glycolysis, and depleted intracellular ATP levels. The effects of pHK-PAS were correlated with dissociation of endogenous full-length HKII from mitochondria and release of cytochrome c. Of significance, pHK-PAS treatment of noncancerous HEK293 cells resulted in substantially lower cytotoxicity. Thus, pHK-PAS effectively disrupted the mitochondria-HKII association in cancer cells, which led to mitochondrial dysfunction and, finally, apoptosis. Our results demonstrate the potential of the pHK-PAS cell-penetrating peptide as a novel therapeutic strategy in cancer.—Woldetsadik, A. D., Vogel, M. C., Rabeh, W. M., Magzoub, M. Hexokinase II–derived cell-penetrating peptide targets mitochondria and triggers apoptosis in cancer cells.
Keywords: ATP, cytochrome c, cytotoxicity, glycolysis, oxidative phosphorylation
Most cancer cells exhibit a high rate of glycolysis, which serves as their primary energy-generating pathway, irrespective of oxygen availability—a phenomenon termed the Warburg effect (1). In particular, malignant or rapidly proliferating cancer cells typically have glycolytic rates ≤200 times higher than those of their normal tissues of origin, even in the presence of oxygen. These high glycolytic rates are the result, in large part, of overexpression and increased activity of hexokinase II (HKII) (2, 3).
HKII is an enzyme that catalyzes ATP-dependent phosphorylation of glucose to produce glucose 6-phosphate, which is the first step of glycolysis (3, 4). HKII is abundant in embryonic tissues and, to a lesser extent, in certain adult tissues (e.g., adipose tissue, skeletal muscle, and cardiac muscle) (5); however, in highly aggressive cancer cells, expression levels of HKII are often >100-fold higher than those of normal cells (6, 7), and the increased HKII activity aids in survival and growth of these cells in the hypoxic conditions of neoplastic mass accrual (1, 3).
Among the 4 isoforms of mammalian hexokinase (HKI–HKIV), only HKI and HKII directly interact with mitochondria, both physically and functionally (4). HKII is the predominant isoform that is overexpressed in malignant tumors, where ≤70% of the enzyme is bound to the outer mitochondrial membrane (OMM) via interaction with the voltage-dependent anion channel (VDAC), the major channel for transport of ions and metabolites between mitochondria and the cytosol (5, 8, 9). Interaction with VDAC occurs via the N-terminal 15 aa of HKII, and this highly conserved hydrophobic domain at the N termini of HKI and HKII is both necessary and sufficient for mitochondrial binding (10). Binding to mitochondria gives HKII preferential access to mitochondria-generated ATP, which the enzyme selectively uses for glucose phosphorylation, even if extramitochondrial ATP is available, thereby directly coupling glycolysis to oxidative phosphorylation (oxphos) (4). Mitochondria-bound HKII is also less susceptible to inhibition by its glucose-6-phosphate product (3, 11). Thus, mitochondrial binding of HKII enables cancer cells to maintain a much greater rate of glycolysis.
Overexpression of HKII is required not only for tumor initiation and maintenance (12), but also for promotion of metastasis (13). The glucose-6-phosphate product of HKII-mediated phosphorylation of glucose is a metabolic intermediate precursor in most biosynthetic pathways and is therefore essential for generating nucleic acids, lipids, and proteins that are required for cell proliferation (14, 15). Moreover, high levels of mitochondria-bound HKII protect cancer cells against death by maintaining the integrity of the OMM and inhibiting release of key apoptogenic molecules, such as cytochrome c, from the intermembrane space (2, 7). Of significance, deleting HKII (12), silencing the HKII transcript (3), or dissociating HKII from mitochondria via disruption of the VDAC-HKII association have been shown to induce apoptosis in cancer cells (16–20).
In this work, we tested the ability of a peptide corresponding to the mitochondrial membrane–binding N-terminal 15 aa of HKII (pHK) to selectively dissociate HKII from mitochondria and induce apoptosis in cancer cells. To enhance the cellular uptake and efficacy of pHK, we covalently coupled the peptide to a short, penetration-accelerating segment (PAS; GKPILFF) (21). Attachment of PAS to cell-penetrating peptides (CPPs) has previously been shown to increase their cellular uptake (21–23). Our results demonstrate that pHK-PAS is a novel CPP with potent anticancer properties.
MATERIALS AND METHODS
Reagents
pHK, pHK-PAS, scrambled pKH (pHKscram)-PAS, and penetratin (pAntp)-PAS (sequences shown in Table 1) were synthesized by Selleck Chemicals (Houston, TX, USA) using standard Fmoc methods. PBS; DMSO; carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP); mitochondria isolation kit; heparin; sodium azide; 2-deoxy-d-glucose; and the endocytosis inhibitors chlorpromazine, methyl-β-cyclodextrin, filipin, nocodazole, and cytochalasin D were purchased from Sigma-Aldrich (St. Louis, MO, USA). Alexa Fluor 488 NHS Ester (succinimidylester), tetramethylrhodamine methyl ester (TMRM), 70 kDa neutral dextran-tetramethylrhodamine, cholera toxin subunit B (recombinant)–Alexa Fluor 555 conjugate, transferrin (from human serum)–Alexa Fluor 546 conjugate, Hoechst 33342, MitoTracker Red FM, wheat germ agglutinin–Alexa Fluor 594 conjugate (membrane marker), and dead cell apoptosis kit were all from Molecular Probes (Carlsbad, CA, USA). CellTiter 96 AQueous One Solution [MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] Cell Proliferation Assay and CellTiter-Glo (CTG) Luminescent Cell Viability Assay kits were from Promega (Madison, WI, USA). Bradford Protein Assay was procured from Bio-Rad (Hercules, CA, USA). Mouse monoclonal human HKII, VDAC1, cytochrome c, and β-actin Abs, as well as horseradish peroxidase–conjugated mouse IgG Ab, were purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
TABLE 1.
Primary sequences of HKII-derived and control peptides used in this study
| Peptide | Sequence |
|---|---|
| pHKa | MIASHLLAYFFTELN-amide |
| pHKA488b | A488-MIASHLLAYFFTELN-amide |
| pHK-PASc | MIASHLLAYFFTELNGKPILFF-amide |
| pHK-PASA488d | A488-MIASHLLAYFFTELNGKPILFF-amide |
| pHKscram-PASe | LINFEMFLATSLHYAGKPILFF-amide |
| pAntp-PASf | RQIKIWFQNRRMKWKKGKPILFF-amide |
aVDAC-binding N-terminal 15 aa of HKII. bAlexa 488-labeled pHK. cpHK coupled to PAS (GKPILFF). dAlexa 488-labeled pHK-PAS. epHKscram coupled to PAS. fpAntp coupled to PAS.
Peptide preparation
pHK, pHK-PAS, pHKscram-PAS, and pAntp-PAS were purified in house by reverse-phase high-performance liquid chromatography (HPLC;Waters 2535 Quaternary Gradient Module (QGM) HPLC; Waters, Milford, MA, USA), and purity was subsequently verified by using mass spectrometry [Agilent 6538 quadrupole time of flight liquid chromatography–mass spectrometry (QToF LC-MS) system; Agilent Technologies, Santa Clara, CA, USA]. After purification, peptides were lyophilized and stored at −20°C until needed. Peptide stock solutions were prepared in DMSO. Peptide concentrations were determined by absorbance measurements at 280 nm (ε = 5690 and 1280 M−1 ⋅ cm−1 for tryptophan and tyrosine, respectively) on a Lambda 25 UV/sis spectrophotometer (PerkinElmer, Waltham, MA, USA) using quartz cuvettes (1-cm path length).
Peptide labeling
Amine coupling was used to attach Alexa Fluor 488-succinimidylester to N termini of pHK and pHK-PAS [Alexa 488-labeled pHK (pHKA488) and pHK-PAS (pHK-PASA488)]. Lyophilized peptide (∼0.5 mg) was dissolved in 7 M guanidine hydrochloride (GuHCl), filtered through a Whatman 0.2 μm polytetrafluoroethylene (PTFE) syringe filter (GE Healthcare Life Sciences, Logan, UT, USA), and loaded onto a Vydac C-18 microspin column (The Nest Group, Southborough, MA, USA). The column was then washed with a 10% acetonitrile, 0.1% TFA solution, followed by MilliQ water (EMD Millipore, Billerica, MA, USA). Thereafter, dye solution (∼0.1 mg in 100 mM NaHCO3 buffer, pH 8.4) was added to the column, which was vortexed gently and incubated on a rotor for 2–4 h at room temperature. Finally, the column was washed again with 10% acetonitrile, 0.1% TFA, followed by MilliQ water, and the peptide was eluted with DMSO. Separation of labeled and unlabeled peptide was done by reverse-phase HPLC. The ratio of labeling was confirmed by mass spectrometry (QToF LC/MS), and concentration of labeled peptide was determined by using absorbance at 494 nm (ε = 73,000 M−1 ⋅ cm−1).
Cell lines
Human cervical HeLa cells [CCL-2; American Type Culture Collection (ATCC), Manassas, VA, USA] and HEK293 cells (CRL-1573; ATCC) were cultured in DMEM (Sigma-Aldrich) that was supplemented with 10% fetal bovine serum (FBS; GE Healthcare Life Sciences), 4 mM l-glutamine, and 1% penicillin/streptomycin (all from Sigma-Aldrich) in 5% CO2 at 37°C. CHO-K1 cells (CCL-61; ATCC) and mutant pgsA-745 cells (CRL-2242; ATCC) were cultured in Ham’s Nutrient Mixture F12 (Sigma-Aldrich) that was supplemented with 10% FBS, 4 mM l-glutamine, and 1% penicillin/streptomycin in 5% CO2 at 37°C. Once cells reached ∼95% confluence, they were split by using 0.25% trypsin-EDTA (Sigma-Aldrich) into fractions and propagated or used in experiments.
Quantification of peptide uptake
Fluorescence-activated cell sorting (FACS) was used to measure the cellular uptake of pHKA488 and pHK-PASA488. Cells (HeLa, CHO-K1, or pgsA-745) were seeded at a density of 2 × 104 cells/well in 500 μl of complete medium in 24-well plates. After culturing for 24 h, cells were washed with PBS at 37°C, and medium was replaced with serum-free medium that contained 5–50 μM pHKA488 or pHK-PASA488. Cells were then incubated for 30–180 min at 37°C. Subsequently, cells were washed 3 times with ice-cold PBS to remove the extracellular peptide, then treated with trypsin-EDTA (1 mg/ml) for 5 min to detach the cells and remove cell surface–bound peptide. Finally, cells were centrifuged (1000 g for 5 min at 4°C) and resuspended in 500 µl ice-cold PBS with 10% FBS. Data collection [10,000 cells/sample, gated on live cells by forward/side scatter and propidium iodide (PI) exclusion] was performed immediately after on a BD FACSAria III cell sorter (BD Biosciences, San Jose, CA, USA), and analysis was performed by using BD FACSDiva software (BD Biosciences).
To elucidate the cellular internalization pathways of pHK and pHK-PAS, HeLa cells were preincubated for 1 h at 4°C in serum-free DMEM, pretreated for 1 h at 37°C with 10 mM sodium azide and 6 mM 2-deoxy-d-glucose in serum- and glucose-free DMEM, or pretreated for 30 min at 37°C in serum-free DMEM with the following drugs: 0–25 μg/ml heparin, 10 µM chlorpromazine, 5 mM methyl-β-cyclodextrin, 4 µM filipin; 10 µM nocodazole, or 10 µM cytochalasin D. After addition of 25 µM pHKA488 or pHK-PASA488, cells were maintained for 2 h at 4°C or in the presence of inhibitors at 37°C. Thereafter, cells were washed 3 times with ice-cold PBS, trypsinized, centrifuged, and resuspended in 500 µl ice-cold PBS with 10% FBS, and fluorescence was measured by FACS. Cells that were treated with peptide without inhibitors at 37°C were used as control, and cells that were treated with vehicle alone served as background. Uptake efficiency was determined by the ratio of fluorescence of cells treated with peptide under different inhibition conditions to control cells.
Intracellular imaging and colocalization
Cells (HeLa, CHO-K1, or pgsA-745) were seeded at a density of 2 × 104 cells/well in 500 μl of complete medium in 4-chambered 35-mm glass bottom Cellview cell culture dishes (Greiner Bio-One, Monroe, NC, USA). After culturing for 24 h, medium was replaced with phenol red– and serum-free medium that contained 25 μM pHK or pHK-PAS, and incubated for 2 h. For some experiments, HeLa cells were preincubated for 1 h at 4°C in serum-free DMEM, pretreated for 1 h at 37°C with 10 mM sodium azide and 6 mM 2-deoxy-d-glucose in serum- and glucose-free DMEM, or pretreated for 30 min at 37°C with 10 µM cytochalasin D in serum-free DMEM. After addition of 25 µM pHKA488 or pHK-PASA488, cells were maintained for 2 h at 4°C or in the presence of inhibitors at 37°C. Thirty minutes before imaging, medium was replaced with fresh medium that contained organelle markers (5 μg/ml Hoechst 33342 and 50 nM MitoTracker Red) or vehicle. Finally, immediately before imaging, medium was once again replaced with fresh medium to remove any extracellular markers. Imaging was performed on an Olympus Fluoview FV-1000 confocal laser scanning microscope using a ×63 Plan-Apo/1.3 NA oil immersion objective with differential interference contrast capability. Image processing was done using Fiji image processing software (Fiji/ImageJ, National Institutes of Health, Bethesda, MD, USA) (24).
Cell viability and toxicity assays
Cell viability and toxicity was measured by using the following: 1) MTS assay, which measures reduction of the tetrazolium compound MTS to soluble formazan, by dehydrogenase enzymes, in living cells (25, 26); 2) CTG assay, which involves the addition of a single reagent directly to cells in culture and results in cell lysis and generation of a luminescent signal proportional to the amount of ATP present (27); 3) dead cell apoptosis assay, in which Alexa Fluor 488–conjugated annexin V is used as a sensitive probe to detect exposed phosphatidylserine in apoptotic cells (28), and red-fluorescent PI, a membrane impermeant nucleic acid binding dye, assesses plasma membrane integrity and distinguishes between apoptosis and necrosis (29).
HeLa or HEK293 cells were seeded at a density of 2 × 104 cells/well in 100 μl of complete DMEM in standard (for MTS) or white opaque-walled (for CTG) 96-well plates. After culturing for 24 h, medium was replaced with medium that contained pHK, pHK-PAS, pHKscram-PAS, or pAntp-PAS at the desired concentration, and cells were incubated for the indicated duration at 37°C. Thereafter, medium was replaced with fresh medium, and 20 μl MTS reagent was added to each well. MTS reagent was incubated for 4 h at 37°C, and absorbance of the soluble formazan product (λ = 490 nm) of MTS reduction was measured on a Synergy H1MF Multi-Mode microplate reader (BioTek, Winooski, VT, USA), with a reference wavelength of 650 nm to subtract background. For CTG assay, plates were first allowed to equilibrate to room temperature for 30 min, at which point 100 μl CTG reagent—prepared beforehand by dissolving CTG substrate in CTG buffer—was added to each well, and contents were mixed for 2 min on an orbital shaker to induce cell lysis. Plates were then incubated for 10 min at room temperature, and luminescence was measured on a Synergy H1MF Multi-Mode microplate reader. Wells that were treated with peptide-free carrier were used as control, and wells with medium alone served as a blank. MTS reduction or intracellular ATP levels were determined from the ratio of the absorbance or luminescence, respectively, of treated wells to control wells.
For the dead cell apoptosis assay, HeLa cells were treated with 50 μM pHK or pHK-PAS for 24 h at 37°C. Cells were subsequently washed with ice-cold PBS, harvested by trypsinization, centrifuged, and resuspended in 1× annexin binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4) to a density of ∼1 × 106 cells/ml. Cells were then stained with 5 µl Alexa Fluor 488–conjugated annexin V and 0.1 µg PI per 100 µl of cell suspension for 15 min at room temperature. Immediately after, fluorescence was measured by using FACS, and fractions of live (annexin V−/PI−), early, and late apoptotic (annexin V+/PI− and annexin V+/PI+, respectively), as well as necrotic (annexin V−/PI+) cells were determined.
Measurement of mitochondrial membrane potential
HeLa or HEK293 cells were seeded at a density of 2 × 104 cells/well in 24-well plates, cultured for 24 h, then treated with 25 or 50 μM pHK or pHK-PAS, or 50 μM pHKscram-PAS or pAntp-PAS, for 24 h at 37°C. Thereafter, cells were washed with PBS and incubated with the mitochondrial membrane potential (ΔΨm) probe TMRM (200 nM) (30) in complete DMEM for 20 min at 37°C. Finally, cells were washed 3 times with ice-cold PBS, trypsinized, centrifuged, and resuspended in 500 µl ice-cold PBS with 10% FBS, and TMRM fluorescence was measured by FACS. Cells that were treated with uncoupling agent, FCCP (10 µM), were used as positive controls for ΔΨm dissipation, and cells that were treated with vehicle alone served as negative controls.
Measurement of cellular metabolic activities
The oxygen consumption rate (OCR) and extracellular acidification rate (ECAR), which are indicators for oxphos and glycolysis, respectively, were measured on a Seahorse XFp Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA, USA). HeLa cells were seeded at a density of 2 × 104 cells/well in 8-well Seahorse XFp Miniplates, cultured in complete DMEM for 24 h, then treated with 25 μM pHK or pHK-PAS for 24 h at 37°C.
Oxphos was assessed by using the Seahorse XF Mito Stress Test Kit. Culture medium was removed and cells were rinsed twice with 300 μl assay medium (Seahorse XF Base Medium supplemented with 1 mM pyruvate, 2 mM glutamine, and 10 mM glucose, pH 7.4), that was prewarmed to 37°C, and, finally, 150 μl of assay medium was added to each well. Plates were then incubated at 37°C with CO2 for 1 h before assay. OCR was measured for 20 min to establish a baseline rate, followed by sequential addition of 1.0 μM FCCP, an uncoupling agent that induces maximal oxygen consumption by cytochrome c oxidase (complex IV), and a mixture of 1.0 μM rotenone and 1.0 μM antimycin A, which inhibit complexes I and III, respectively, and effectively shut down mitochondrial respiration. The change in OCR from the baseline rate in response to FCCP was used to calculate spare respiratory capacity, whereas the difference between FCCP-induced OCR and the value after addition of rotenone/antimycin A was used to calculate maximal respiration.
Glycolytic function was assessed by using the Seahorse XF Glycolysis Stress Kit. Culture medium was removed and cells were rinsed twice with 300 μl of assay medium (Glycolysis Stress Test Base Medium supplemented with 2 mM l-glutamine, pH 7.4) that was prewarmed to 37°C, and, finally, 150 μl of assay medium was added to each well. Plates were then incubated at 37°C with CO2 for 1 h before assay. ECAR was measured for 20 min to establish a baseline rate, followed by sequential addition of 10 mM glucose and 1.0 μM oligomycin, an ATP synthase (complex V) inhibitor. Changes in ECAR from the baseline rate in response to glucose and oligomycin were used to calculate glycolysis and glycolytic capacity, respectively.
Cell fractionation and Western blot analysis
pHK-PAS–treated HeLa and HEK293 cells were washed with ice-cold PBS, harvested by trypsinization, and centrifuged (1000 g for 5 min at 4°C). Cell pellets were resuspended in 150 μl isotonic isolation buffer (250 mM sucrose, 20 mM HEPES, pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM sodium EDTA, 1 mM dithiothreitol, 10 mM PMSF, 10 µM leupeptin, and 10 µM aprotinin). After chilling on ice for 3 min, cells were disrupted by 40 strokes of a glass homogenizer, and lysis was verified by phase-contrast light microscopy. The homogenate was centrifuged twice at 1500 g for 30 min at 4°C to remove intact cells and nuclei. The mitochondria-enriched fraction was then pelleted by centrifugation at 12,000 g for 30 min at 4°C. To isolate cytosolic protein, supernatant was removed and filtered through 0.2 µm followed by 0.1 µm Ultrafree MC filters (Millipore, Billerica, MA, USA). The protein content of mitochondria-enriched and cytosolic fractions was determined by Bradford assay. Samples (2 µg/lane) were electrophoresed on a 10–12% SDS polyacrylamide gel, transferred to a nitrocellulose membrane, and incubated overnight with mouse anti-human HKII, VDAC1, or cytochrome c (1:1000) Abs or anti–β-actin Ab (1:2000), followed by incubation for 3 h with horseradish peroxidase–conjugated mouse IgG Ab (1:10,000). Samples were then visualized by using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA, USA). Densitometry of each band was quantified by using Fiji image processing software (24).
Statistical analysis
Confidence intervals in this work represent sd across at least 3 independent trials. Statistical analysis was performed by using Prism 6.0 software (GraphPad Software, La Jolla, CA, USA). Statistical significance between 2 groups was assessed by unpaired Student’s t test, and among ≥3 groups by 2-way ANOVA, followed by Bonferroni's post hoc test. A value of P < 0.05 was considered statistically significant.
RESULTS
Our overall approach was to correlate the cellular internalization of HKII-derived peptides, pHK and pHK-PAS (sequences shown in Table 1), with their cytotoxic effects. Specifically, we used FACS and confocal fluorescence microscopy to assess cellular uptake, internalization pathways, and intracellular localization of pHK and pHK-PAS. We then used established measures of cell viability, mitochondrial function, and cellular metabolic activities to evaluate the impact of the peptides. Finally, we related these observations to the mitochondrial binding of endogenous full-length HKII. This work was performed by using 4 complementary cell lines: HeLa and CHO-K1, 2 cancer cell lines that have established use in studies of HKII function, localization, and therapeutic targeting (16, 31–33); pgsA-745, a mutant CHO-K1–derived cell line with a defect in xylosyltransferase activity that inhibits production of heparan sulfate and chondroitin sulfate proteoglycans (34); and HEK-293, a noncancerous cell line (16).
PAS enhances pHK cellular uptake
Uptake of pHKA488 and pHK-PASA488 in HeLa cells was quantified by using FACS. To overcome overestimation of cellular uptake by FACS as a result of its inability to discriminate between membrane-associated and internalized peptide, cells were treated with trypsin to remove cell surface–bound peptide (35). Confocal microscopy was used to verify that all extracellular and membrane-bound peptide was successfully removed (Supplemental Fig. S1A).
For both pHKA488 and pHK-PASA488, internalization began immediately upon addition of peptides to cells and reached a plateau at 2 h (Fig. 1A); however, the amount of pHKII-PAS taken up by cells at 30 min was ∼3-fold of that of pHK, which indicated that attachment of the PAS segment to pHK accelerated the peptide’s internalization. Moreover, at all peptide concentrations tested, the amount of pHK-PASA488 taken up by cells at 2 h significantly exceeded that of pHKA488 (Fig. 1B). Enhancement in uptake of pHK-PASA488 relative to pHKA488 ranged from 2.9-fold at 5 μM to 3.5-fold at 50 μM; thus, covalently coupling PAS to pHK substantially enhanced the peptide’s cellular uptake.
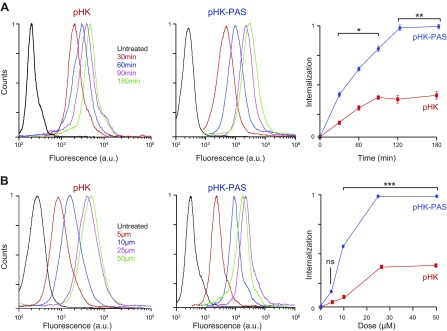
Figure 1.
Quantification of cellular uptake of pHK and pHK-PAS. A) Time course of peptide internalization. HeLa cells were incubated with 25 µM pHKA488 (left) or pHK-PASA488 (middle) in serum-free DMEM for 30–180 min. B) Dose dependence of peptide internalization. HeLa cells were incubated with 5–50 µM pHKA488 (left) or pHK-PASA488 (middle) in serum-free DMEM for 2 h. After peptide incubation, cells were washed 3 times with ice-cold PBS, trypsinized, centrifuged, and resuspended in ice-cold PBS with 10% FBS, and fluorescence was measured by FACS. Peptide internalization was determined by subtraction of background signal (cells treated with vehicle alone) from fluorescence intensities of peptide-treated cells and plotted relative to the maximum fluorescence intensity observed (right). (Error bars lie within the symbol for some data points.) ns, nonsignificant (P > 0.05). *P < 0.01, **P < 0.001, ***P < 0.0001 compared with pHK.
Cellular uptake of pHK-PAS is partially mediated by interactions with cell-surface heparan sulfate proteoglycans
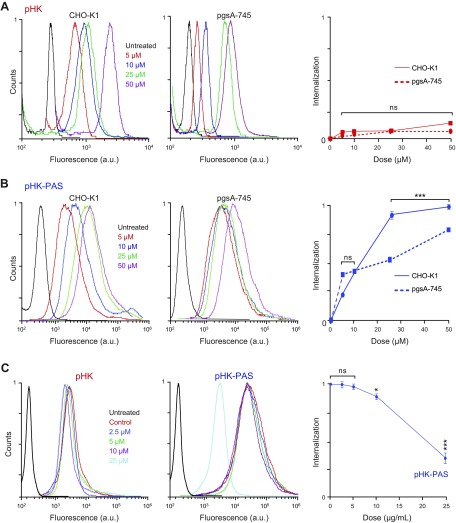
Uptake of several CPPs, including the TAT peptide (derived from the HIV-1 transcription-activating protein), oligoarginine, and pAntp (from the Antennapedia homeodomain, a Drosophila transcription factor), was reported to be initiated by strong binding to anionic cell-surface proteoglycans, in particular, heparan sulfate (35–37). To determine whether binding to cell-surface proteoglycans is also a prerequisite for internalization of pHK and pHK-PAS, we measured the uptake of peptides in wild type CHO-K1 and proteoglycan-deficient pgsA-745 cells (Fig. 2).
Figure 2.
Role of cell-surface proteoglycans in cellular uptake pHK and pHK-PAS. A, B) Wild-type CHO-K1 (left) and proteoglycan-deficient pgsA-745 (middle) cells were incubated with 5–50 µM pHKA488 (A) or pHK-PASA488 (B) in serum-free DMEM for 2 h. C) Effect of free heparin on peptide internalization. HeLa cells were pretreated for 30 min at 37°C in serum-free DMEM with extracellular heparin (0–25 μg/ml), then treated with 25 µM pHKA488 (left) or pHK-PASA488 (middle) and maintained for 2 h at 37°C in the presence of inhibitor and peptide. After peptide incubation, cells were washed 3 times with ice-cold PBS, trypsinized, centrifuged, and resuspended in ice-cold PBS with 10% FBS, and fluorescence was measured by FACS. Peptide internalization was determined by subtraction of background signal (cells treated with vehicle alone) from fluorescence intensities of peptide-treated cells and plotted relative to the maximum fluorescence intensity observed (right). (Error bars lie within the symbol for some data points.) ns, nonsignificant (P > 0.05). *P < 0.01, ***P < 0.0001 compared with controls.
Consistent with observations in HeLa cells, coupling of PAS to pHK substantially enhanced the peptide’s internalization in CHO-K1 cells, with the uptake of pHK-PASA488 ranging from 4-fold to 7-fold of that of pHKA488 (Fig. 2A, B). pHKA488 exhibited similar uptake in the 2 cell lines at all peptide concentrations, which indicated that cellular uptake of the peptide was not dependent on binding to cell-surface proteoglycans (Fig. 2A). For pHK-PASA488, cellular uptake was comparable between the 2 cell lines at low peptide concentrations (<10 μM), whereas at 25 and 50 μM pHK-PASA488, uptake in the mutant pgsA-745 cells was 64 ± 3 and 80 ± 3%, respectively, of that of the uptake in the parent CHO-K1 cells. This suggested that binding to cell-surface proteoglycans contributed to the cellular uptake of pHK-PAS.
To ascertain whether the decreased pHK-PASA488 uptake in the proteoglycan-deficient cells was a result of the absence of heparan sulfate specifically, HeLa cells were treated with pHK-PASA488 in the presence of increasing concentrations of the acidic polysaccharide, heparin, a highly sulfated analog of heparan sulfate (Fig. 2C). Free heparin in the extracellular medium competes with cell-surface heparan sulfate proteoglycans for binding to CPPs, thereby inhibiting any potential heparan sulfate–mediated cell-surface interaction and subsequent internalization of peptides. This heparin competition assay has been widely used to determine the role of cell-surface heparan sulfate proteoglycans in the cellular uptake of a wide range of CPPs (35, 38, 39). Uptake of pHK-PASA488 was unaffected by the presence of free heparin up to a concentration of 5 μg/ml; however, at higher heparin concentrations of 10 and 25 μg/ml, pHK-PAsA488 uptake was inhibited to 90 ± 4 and 38 ± 8%, respectively, of that of controls. Taken together, our data suggests that uptake of the pHK-PAS CPP is partially mediated by interaction with cell-surface heparan sulfate proteoglycans.
pHK-PAS cellular uptake occurs by both macropinocytosis and energy-independent mechanisms
To establish whether cellular internalization of pHK and pHK-PAS was by an active, energy-dependent process, such as endocytosis, or an energy-independent mechanism (e.g., pore formation), as has been reported for some CPPs (40), the uptake of peptides in HeLa cells was quantified at 4°C, where all energy-dependent uptake processes are inhibited. Lowering the temperature to 4°C decreased cellular uptake of pHKA488 and pHK-PASA488 to 8 ± 1 and 17 ± 4%, respectively, of controls at 37°C (Fig. 3A). Likewise, depleting the cellular ATP pool by preincubation of cells with sodium azide and deoxyglucose (34, 41) led to a reduction in cellular internalization of pHKA488 and pHK-PASA488 to 31 ± 3 and 47 ± 1%, respectively, of controls (Fig. 3A). Confocal images confirmed that uptake of peptides was reduced, but not abolished, at 4°C (Supplemental Fig. S1B) or in ATP-depleted cells (Supplemental Fig. S1C). Thus, although low temperature and ATP depletion diminished the amount of pHKA488 and pHK-PASA488 taken up by cells appreciably, significant uptake of both peptides was observed under these conditions. Our results, therefore, indicated that the cellular internalization of HKII-derived peptides occurs by both energy-dependent and -independent uptake mechanisms.
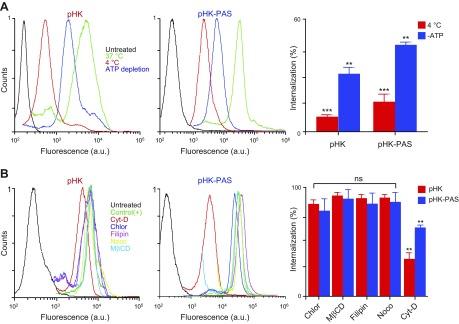
Figure 3.
Determination of cellular uptake mechanisms of pHK and pHK-PAS. A) Effects of low temperature and energy depletion on peptide internalization. HeLa cells were preincubated for 1 h at 4°C in serum-free DMEM or pretreated for 1 h at 37°C with 10 mM sodium azide and 6 mM 2-deoxy-d-glucose in serum- and glucose-free DMEM to deplete cellular ATP. pHKA488 (25 µM; left) or pHK-PASA488 (25 µM; middle) was then added, and cells were maintained for 2 h at 4°C or in the presence of sodium azide/2-deoxy-d-glucose at 37°C. B) Effects of endocytosis inhibitors on peptide internalization. HeLa cells were treated for 30 min at 37°C in serum-free DMEM with the following: 10 µM chlorpromazine (Chlor; clathrin-dependent endocytosis), 5 mM methyl-β-cyclodextrin (MβCD; lipid raft–mediated endocytosis), 4 µM filipin (Filip; caveolae-dependent endocytosis), 10 µM nocodazole (Nocod; microtubule polymerization), or 10 µM cytochalasin D (Cyto D; macropinocytosis). Cells were then treated with 25 µM pHKA488 (left) or pHK-PASA488 (middle) and maintained for 2 h at 37°C in the presence of inhibitors and peptides. Thereafter, cells were washed 3 times with ice-cold PBS, trypsinized, centrifuged, and resuspended in ice-cold PBS with 10% FBS, and fluorescence was measured by FACS. Cells that were treated with peptide without inhibitors at 37°C were used as control, and cells that were treated with vehicle alone served as background. Uptake efficiencies (right) were determined from the ratio of fluorescence of cells treated with peptide under different inhibition conditions to control cells. ns, nonsignificant (P > 0.05). **P < 0.001, ***P < 0.0001 compared with controls at 37°C.
To determine whether the energy-dependent uptake mechanism of the peptides involved a specific endocytic pathway, cells were pretreated with endocytosis inhibitors (Fig. 3B). As a control, we verified the efficacy of the endocytic inhibitors, at the doses used, by determining their effect on the cellular uptake of established endocytosis markers: transferrin–Alexa Fluor 546 (clathrin-mediated endocytosis), cholera toxin B subunit–Alexa Fluor 555 (caveolae-dependent endocytosis), and 70-kDa neutral dextran-tetramethylrhodamine (micropinocytosis; Supplemental Fig. S2A) (36, 43, 44). In addition, we confirmed that all inhibitors did not affect cell viability at the concentrations used (Supplemental Fig. S2B).
First, we inhibited clathrin-mediated endocytosis, the best-characterized endocytosis pathway, by using chlorpromazine, which inhibits clathrin-coated pit formation (45). Treatment with chlorpromazine had a negligible effect on the cellular uptake of pHKA488 and pHK-PASA488. Next, cells were treated with methyl-β-cyclodextrin to remove cholesterol from the plasma membrane, which disrupts several lipid raft lipid–mediated endocytic pathways, including caveolae-dependent endocytosis and lipid raft–dependent macropinocytosis (46–48). Disruption of lipid rafts by methyl-β-cyclodextrin had little effect on the cellular uptake of peptides, which indicated that internalization is lipid raft independent. This was confirmed by treating cells with filipin, an inhibitor of lipid raft–mediated caveolae endocytosis (46, 49), which again had a negligible effect on the uptake of peptides. Likewise, treatment with nocodazole, an inhibitor of microtubule polymerization, did not inhibit the uptake of pHKA488 and pHK-PASA488. Conversely, treatment with cytochalasin D, a specific inhibitor of F-actin elongation that is involved in macropinocytosis (50), reduced the amount of internalized pHKA488 and pHK-PASA488 to 36 ± 5 and 64 ± 2%, respectively, of controls (Fig. 3B and Supplemental Fig. S1D). Taken together, these results show that cellular uptake of pHKA488 and pHK-PASA488 occurs by both energy-independent mechanisms and macropinocytosis.
pHK-PAS localizes to mitochondria
Intracellular localization of pHKA488 and pHK-PASA488 after uptake was determined by using confocal fluorescence microscopy. HeLa, CHO-K1, and pgsa-745 cells were incubated with 25 μM pHKA488 and pHK-PASA488 for 2 h at 37°C (Fig. 4). Nucleus and mitochondria were costained with Hoechst 33342 and MitoTracker Red, respectively.
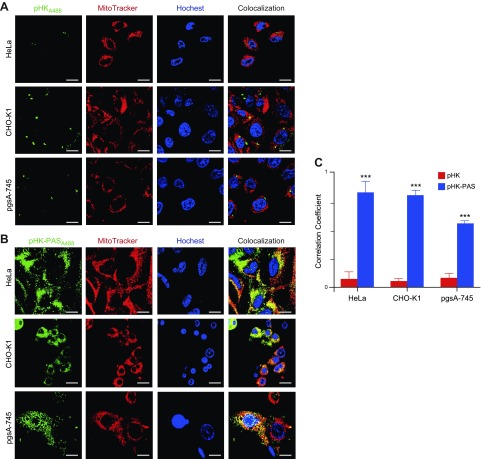
Figure 4.
Intracellular localization of pHK and pHK-PAS. A, B) Colocalization of pHKA488 (A) and pHK-PASA488 (B) with mitochondria in HeLa (top), CHO-K1 (middle), and pgsA-745 (lower) cells. Cells were incubated with 25 µM pHKA488 or pHK-PASA488 in phenol red– and serum-free medium for 2 h, then stained with organelle markers (5 μg/ml Hoechst 33342 and 50 nM MitoTracker Red) for 30 min before confocal imaging. Scale bars, 10 µM. C) Pearson’s correlation coefficient for pHKA488 (red bars) and pHK-PASA488 (blue bars) in HeLa, CHO-K1, and pgsA-745 cells, respectively. Pearson’s correlation coefficient measures pixel-by-pixel covariance in the signal level of 2 images and is a useful means of evaluating colocalization (72). ***P < 0.0001 compared with the correlation coefficient of pHKA488 in the same cell line.
In agreement with FACS data (Figs. 1 and 2), pHKA488 showed weak uptake in all 3 cell lines (Fig. 4A), and very little colocalization with mitochondria was observed (Fig. 4C). In contrast, pHK-PASA488 exhibited strong uptake in both HeLa and CHO-K1 cells, and the peptide was distributed throughout the cytosol (Fig. 4B). OF interest, pHK-PASA488 showed strong colocalization with mitochondria in both cell lines (Fig. 4C). In proteoglycan-deficient pgsa-745 cells, pHK-PASA488 internalization was somewhat reduced compared with that of parent CHO-K1 cells (Fig. 4B), and colocalization with mitochondria was weaker (Fig. 4C). These results are in agreement with the FACS data (Fig. 2) and confirm that uptake of pHK-PAS is partially mediated by interaction with cell-surface heparan sulfate proteoglycans. Thus, pHK-PAS CPP exhibits substantially greater mitochondrial localization compared with pHK.
pHK-PAS exhibits selective cytotoxicity against cancer cells
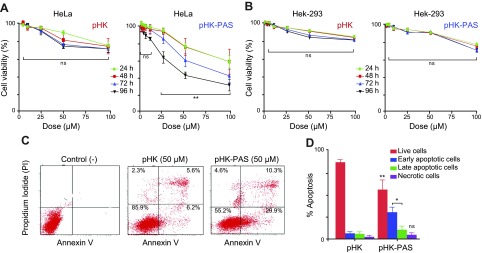
The cytotoxic effects of HKII-derived peptides were assessed by using the MTS assay, which measures reduction of the tetrazolium compound MTS in living cells. In HeLa cells, treatment with pHK resulted in a small decrease in cell viability. At the highest pHK concentration tested (100 μM), MTS response at 96 h incubation decreased to 70 ± 7% of that of controls using protein-free carrier (Fig. 5A). Conversely, treatment of HeLa cells with pHK-PAS showed a significantly greater effect on cell viability. pHK-PAS diminished MTS reduction in a dose-dependent manner, which resulted in an MTS response of 73 ± 4 and 54 ± 8% at 24 h for 50 and 100 μM, respectively (Fig. 5A). Inhibition of cell viability by pHK-PAS was also time dependent, with MTS response for 50 and 100 μM pHK-PAS decreasing further to 55 ± 8 and 35 ± 8%, respectively, at 96 h of incubation. Thus, coupling of PAS to pHK substantially enhanced the cytotoxic effects of the peptide in cancer cells.
Figure 5.
Complementary measures of the cytotoxic effects of pHK and pHK-PAS. A, B) Dose- and time-dependent inhibition of MTS reduction. HeLa (A) and HEK293 (B) cells were treated with the indicated concentrations of pHK (left) or pHK-PAS (right) for the indicated durations. Cells that were treated with peptide-free carrier were used as control. The percent viability was determined form the ratio of the absorbance of treated cells to control cells. (Error bars lie withing the symbol for some data points.) C, D) Detection of apoptosis and necrosis. C) FACS analysis of annexin V/PI staining of HeLa cells that were either untreated (control; left) or treated with 50 μM pHK (middle) or pHK-PAS (right) for 24 h. The bottom left quadrant (annexin V−/PI−) represents live cells; bottom right (annexin V+/PI−), early apoptotic cells; top right (annexin V+/PI+), late apoptotic cells; and top left (annexin V−/PI+), necrotic cells. D) A summary of the incidence of early and late apoptosis and necrosis in HeLa cells that were treated with pHK (left bars) or pHK-PAS (right bars) determined from FACS analysis of annexin V/PI staining in panel C. ns, nonsignificant (P > 0.05). *P < 0.01, **P < 0.001 compared with pHK.
To determine whether the observed cytotoxicity of pHK-PAS in HeLa cells (Fig. 5A) is mediated by the pHK segment of the peptide, we tested 2 variants of pHK-PAS: a peptide with a pHKscram sequence, denoted pHKscram-PAS, and a peptide in which the pHK sequence was replaced with the CPP pAntp [from the Antennapedia homeodomain, a Drosophila transcription factor (51)], denoted pAntp-PAS (23). Treatment of HeLa cells with 10–100 μM pHKscram-PAS resulted in negligible inhibition of MTS reduction (Supplemental Fig. S3A). Likewise, pAntp-PAS did not affect the MTS response of HeLa cells at all peptide concentrations tested (Supplemental Fig. S3B). Taken together, these results demonstrate that the cytotoxicity of pHK-PAS in cancer cells is a result, specifically, of the pHK segment of the peptide.
In noncancerous HEK293 cells, treatment with pHK again resulted in a small decrease in cell viability, with MTS response for 100 μM peptide at 96 h decreasing to 84 ± 2% (Fig. 5B). Of significance, treatment with pHK-PAS led to substantially lower inhibition of MTS reduction in HEK293 cells compared with HeLa cells at all peptide concentrations and incubations times (Fig. 5B). For instance, MTS response in HEK293 cells for 50 and 100 μM pHK-PAS was 91 ± 1 and 76 ± 2%, respectively, at 24 of incubation and 90 ± 1 and 70 ± 2%, respectively, at 96 h of incubation. These results suggest that pHK-PAS exhibited selective cytotoxicity against cancer cells.
To ascertain whether pHK-PAS–induced cancer cell death occurs via apoptosis or necrosis, we used Alexa Fluor 488–conjugated annexin V/PI staining, an established method for the detection of apoptotic cells (52). Treatment of HeLa cells with pHK resulted in a small number of cells undergoing early and late apoptosis (6 ± 1 and 5 ± 2%, respectively, of that of controls that used protein-free carrier; Fig. 5C, D). In contrast, exposure to pHK-PAS increased the population of both early and late apoptotic cells to 30 ± 5 and 10 ± 4%, respectively. These results demonstrate that pHK-PAS–induced cancer cell death occurs primarily via apoptosis.
pHK-PAS depolarizes mitochondrial membrane potential and depletes intracellular ATP levels
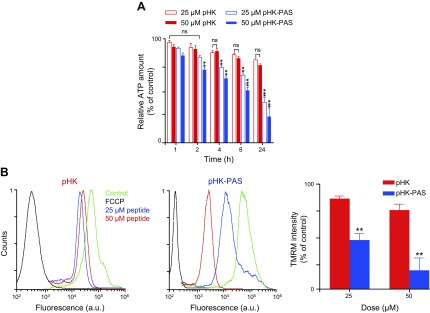
To investigate the mechanism of apoptosis in cancer cells induced by HKII-derived peptides, we first measured the effect of the peptides on intracellular ATP levels by using the CTG assay (Fig. 6A). The CTG assay generates a luminescent signal that is proportional to the amount of ATP present (27). Treatment of HeLa cells with pHK resulted in a small decrease in intracellular ATP levels at 24 h of incubation (75 ± 2% of controls at 50 μM). A comparable decrease in ATP levels was observed after treatment of HeLa cells with pHK-PAS for only 2 h (82 ± 2 and 71 ± 2% at 25 and 50 μM, respectively), and ATP levels continued to decrease with prolonged exposure to the peptide. After 24 h of incubation, ATP levels in HeLa cells were severely depleted to 39 ± 5 and 26 ± 8% at 25 and 50 μM pHK-PAS, respectively. Conversely, exposure to 50 μM pHK-PAS for 24 h did not significantly decrease intracellular ATP levels in HEK293 cells (Supplemental Fig. S3C). Thus, whereas pHK-PAS strongly decreased intracellular ATP levels in cancer cells, the peptide did not affect ATP levels in noncancerous cells.
Figure 6.
Effects of pHK and pHK-PAS on intracellular ATP levels and mitochondrial membrane potential. A) Dose- and time-dependent decrease in intracellular ATP levels. HeLa cells were treated with pHK (red bars) or pHK-PAS (blue bars) for the indicated durations. Two peptide concentrations, 25 (open bars) or 50 µM (filled bars), were used. ATP levels were then measured by using the CTG assay. Cells that were treated with peptide-free carrier were used as control. Relative ATP levels were determined from the ratio of the luminescence of treated cells to control cells. ns, nonsignificant (P > 0.05). **P < 0.001, ***P < 0.0001 compared with controls. B) ΔΨm depolarization of the inner mitochondrial membrane. HeLa cells were treated with 25 or 50 µM pHK (left) or pHK-PAS (middle) for 24 h, and fluorescence of the ΔΨm probe TMRM was measured by using FACS. Cells that were treated with the uncoupling agent, FCCP (10 µM), were used as positive controls for ΔΨm dissipation, and cells that were treated with vehicle alone served as negative controls. TMRM fluorescence intensity is plotted as a percentage of controls (right). **P < 0.001 compared with pHK.
We next examined the effect of pHK-PAS on ΔΨm, which is critical for maintaining the physiologic function of the respiratory chain to generate ATP. Changes in ΔΨm upon exposure of cells to pHK-PAS were monitored by using TMRM, an established fluorescent probe for measuring ΔΨm (Fig. 6B) (29). The cell-permeant TMRM accumulates in active mitochondria and its fluorescence intensity changes in proportion to changes in ΔΨm. Cells that were treated with the protonophore, FCCP, an uncoupling agent that facilitates uninhibited movement of protons across the inner mitochondrial membrane and effectively depolarizes ΔΨm, served as a positive control. Treatment of HeLa cells with 50 μM pHK resulted in a small decrease in TMRM fluorescence to 77 ± 5% of controls, which indicated that the peptide had little effect on ΔΨm. In contrast, exposure of HeLa cells to 50 μM pHK-PAS dramatically decreased TMRM fluorescence to 19 ± 10% of controls, which is indicative of substantial depolarization of ΔΨm; however, treatment of HEK293 cells with 50 μM pHK-PAS did not significantly decrease TMRM fluorescence (Supplemental Fig. S3C), which indicated that the peptide did not depolarize ΔΨm in noncancerous cells. Taken together, our results show that treatment with pHK-PAS selectively depolarizes ΔΨm and depletes intracellular ATP levels in cancer cells.
As a control, we measured the effects of pHK-PAS variants on intracellular ATP levels and ΔΨm in HeLa cells. Treatment of HeLa cells with 50 μM pHKscram-PAS resulted in a negligible decrease in intracellular ATP levels and ΔΨm (Supplemental Fig. S3A). Similarly, 50 μM pAntp-PAS did not significantly reduce intracellular ATP levels or ΔΨm in HeLa cells (Supplemental Fig. S3B). These results confirmed that depolarization of ΔΨm and depletion of intracellular ATP levels by pHK-PAS in cancer cells were mediated by the pHK segment of the peptide.
pHK-PAS inhibits mitochondrial bioenergetic function and glycolysis
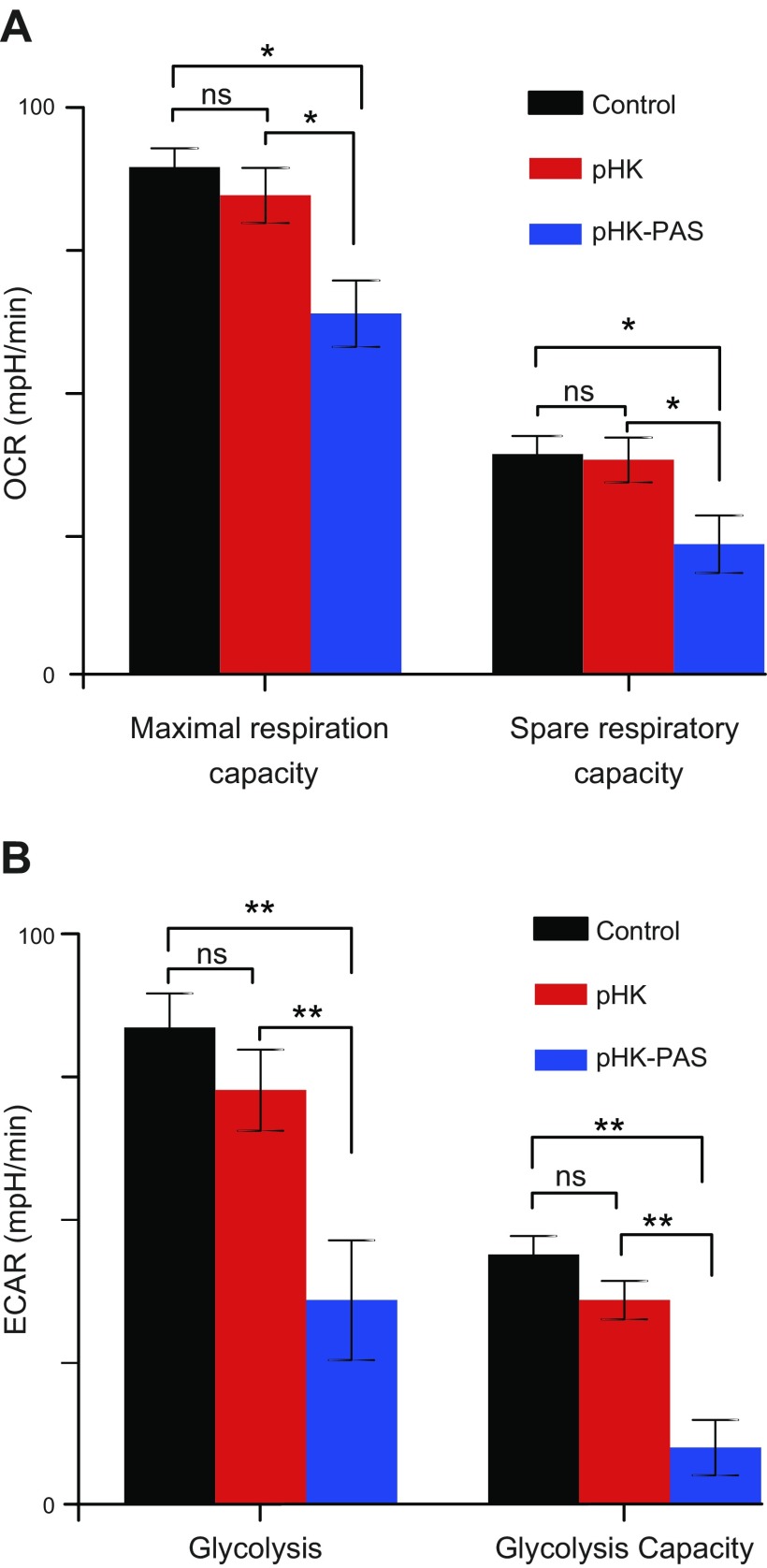
We probed the effect of pHK and pHK-PAS on cellular metabolic activities by monitoring mitochondrial respiration and glycolytic function. HeLa cells were treated with 25 μM pHK or pHK-PAS for 24 h at 37°C, and OCR and ECAR were measured (53). As actively respiring mitochondria consume oxygen, OCR provides as a measure of substrate flux through the oxphos pathway, whereas ECAR reflects the net production and extrusion of protons into the extracellular medium after conversion of glucose to lactate during glycolysis.
Changes in OCR as a result of sequential addition of FCCP and rotenone/antimycin A were used to determine maximal respiration and spare respiratory capacity (Fig. 7A). Depletion of ΔΨm by FCCP results in uninhibited electron flow through the mitochondrial respiratory chain, which leads to maximal oxygen consumption by cytochrome c oxidase (complex IV) (54). The combination of rotenone, a complex I inhibitor, and antimycin A, a complex III inhibitor, effectively shuts down oxphos (54, 55). The difference between OCR values because of FCCP and rotenone/antimycin A gives the maximal respiration (55). In control cells, maximal respiration was 96 ± 3 pmol/min, with a similar value (90 ± 5 pmol/min) obtained in pHK-treated cells. Conversely, in pHK-PAS–treated cells, maximal respiration was reduced significantly to 63 ± 6 pmol/min. The difference between the FCCP-stimulated OCR and the basal OCR yields an estimate of the spare respiratory capacity, which is a measure of the ability of cells to respond to increased ATP demand (55). Treatment with pHK did not adversely affect the spare respiratory capacity of cells; however, treatment with pHK-PAS decreased the spare respiratory capacity to 18 ± 4 pmol/min. These results show that pHK-PAS leads to severe impairment of mitochondrial respiration.
Figure 7.
Effects of pHK and pHK-PAS on cellular metabolic activities. A) Changes in mitochondrial bioenergetic function. HeLa cells were treated with 25 μM pHK (red bars) or pHK-PAS (blue bars) for 24 h at 37°C. Cells were then subjected to sequential addition of 1.0 μM FCCP and a mixture of 1.0 μM rotenone and 1.0 μM antimycin A, and the OCR was measured on a Seahorse XFp Extracellular Flux Analyzer. Spare respiratory capacity (right bars) was determined from the change in OCR from the baseline rate in response to FCCP, and maximal respiration (left bars) from the difference between the FCCP-induced OCR and the value after addition of rotenone and antimycin A. B) Changes in glycolytic function. HeLa cells were treated with 25 μM pHK (red bars) or pHK-PAS (blue bars) for 24 h at 37°C, followed by sequential addition of 10 mM glucose and 1.0 μM oligomycin, and the ECAR was measured on a Seahorse XFp Extracellular Flux Analyzer. Glycolysis (left bars) and glycolytic capacity (right bars) were determined from changes in ECAR from the baseline rate in response to glucose and oligomycin, respectively. ns, nonsignificant (P > 0.05). *P < 0.05, **P < 0.001.
Changes in ECAR in response to sequential addition of glucose and oligomycin were used to determine glycolysis and glycolytic capacity, respectively (Fig. 7B). Addition of glucose to control cells resulted in an increase in ECAR of 85 ± 5 mph/min. A similar increase in ECAR of 75 ± 6 mph/min was observed in pHK-treated cells upon addition of glucose. In contrast, cells that were treated with pHK-PAS showed a much lower increase in ECAR (38 ± 1 mph/min) in response of glucose. Oligomycin inhibits ATP synthase (complex V), and the disruption of mitochondrial ATP production shifts energy production to glycolysis, with the subsequent increase in ECAR revealing the maximum glycolytic capacity of cells (53). Addition of oligomycin to control cells resulted in a maximal ECAR of 40 ± 3 mph/min, with a similar response of 37 ± 3 mph/min recorded in pHK-treated cells; however, in pHK-PAS–treated cells, maximal ECAR was substantially lower (10 ± 5 mph/min). Thus, pHK-PAS strongly inhibited glycolytic function.
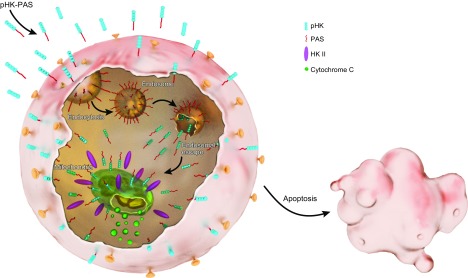
pHK-PAS displaces endogenous HKII from the mitochondrial membrane and triggers release of cytochrome c
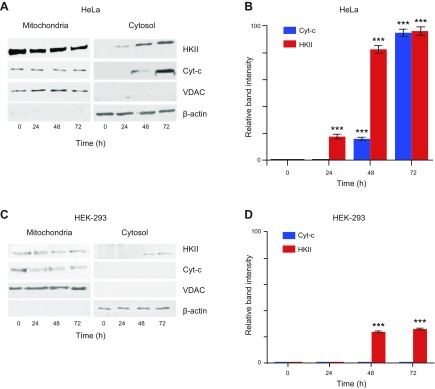
Finally, we established whether the observed pHK-PAS–induced effects in cancer cells were a result, specifically, of CPP displacing endogenous full-length HKII from the mitochondrial membrane. HeLa cells were treated with 50 μM pHK-PAS for 24–72 h, and immunoblotting was used to determine the amount of endogenous HKII, VDAC, and cytochrome c in mitochondria-enriched and cytosolic fractions at different time points.
Treatment of HeLa cells with pHK-PAS decreased HKII levels in the mitochondria-enriched fraction and concomitantly increased HKII levels in the cytosolic fraction in a time-dependent manner (Fig. 8A, B). Similarly, cytochrome c levels in the cytosolic fraction increased with pHK-PAS incubation time (Fig. 8A, B). In contrast, VDAC levels remained constant in the mitochondria-enriched fraction, and the protein was completely absent from the cytosolic fraction at all time points (Fig. 8A). Taken together, our results demonstrate that pHK-PAS specifically displaces HKII from mitochondria in cancer cells without affecting localization of VDAC to the OMM, and this disruption of the HKII–VDAC complex triggers release of cytochrome c to the cytosol and apoptosis.
Figure 8.
Effects of pHK-PAS on HKII and cytochrome c localization. HeLa and HEK293 cells were treated with 50 µM pHK-PAS for 24–72 h. Thereafter, cells were harvested and mitochondria-enriched, and cytosolic fractions were isolated. Samples were normalized for protein content and electrophoresed on a 10–12% SDS polyacrylamide gel, then transferred to a nitrocellulose membrane; incubated overnight with mouse anti-human HK II, VDAC, or cytochrome c Abs, followed by 3 h with horseradish peroxidase–conjugated mouse IgG Ab; and finally visualized by using SuperSignal West Pico Chemiluminescent Substrate. A, C) Immunoblots of HKII, VDAC1, and cytochrome c (Cyt-c) in the mitochondria-enriched (left) and cytosolic fractions (right) of control (t = 0) and pHK-PAS-treated (24–72 h) HeLa (A) and HEK293 (C) cells. VDAC and β-actin were used as loading controls for the mitochondria-enriched and cytosolic fractions, respectively. B, D) Changes in the HKII and cytochrome c content of the cytosolic fraction of control and pHK-PAS–treated HeLa (B) and HEK293 (D) cells determined by densitometric quantification of the band intensities in panels A and C, respectively. ***P < 0.0001 compared with controls.
We also examined whether pHK-PAS similarly displaced HKII from mitochondria in noncancerous HEK293 cells, where HKII expression is approximately 10% of that in HeLa cells (Supplemental Fig. S4). Treatment of HEK293 cells with 50 μM pHK-PAS for 24–72 h again resulted in a time-dependent decrease in mitochondria-associated HKII levels, coupled with an increase in cytosolic HKII levels (Fig. 8C, D). However, unlike in HeLa cells, the competitive dissociation of HKII from mitochondria in HEK293 cells was not accompanied by a significant release of cytochrome c to the cytosol (Fig. 8C, D). This is consistent with cell viability data that showed that pHK-PAS was substantially less toxic to HEK293 cells compared with HeLa cells (Fig. 5). Thus, pHK-PAS–mediated displacement of HKII from mitochondria in noncancerous cells does not lead to apoptosis.
DISCUSSION
A key characteristic of many cancers—in particular, highly aggressive cancers—is overexpression of HKII, a phenotype that is detected clinically by positron emission tomography (2, 3). Elevated levels of HKII enable cancer cells to maintain a much higher rate of glycolysis, which serves as their primary energy-generating pathway and produces the glucose-derived metabolic intermediates that are necessary for synthesis of nucleotides, fatty acids, and amino acids essential for growth and proliferation (1, 3, 14, 15). Furthermore, the increased association of HKII with the OMM inhibits apoptosis in cancer cells (2, 7) and is associated with chemoresistance (56, 57), thereby increasing the potential for metastasis.
Given its role in cancer cell survival, growth, and proliferation, as well as its low expression levels in normal adult tissues, HKII constitutes an attractive cancer therapy target. Treatment with compounds that detach HKII from mitochondria, such as clotrimazole, 3-bromopyruvate, and methyl jasmonate, has been shown to induce apoptosis in cancer cells in vitro and in vivo (17, 19, 30); however, these compounds also seem to interact with other molecular targets and cause nonspecific cytotoxicity independent of their activity on HKII (30, 58, 59).
A promising strategy has been to use pHK, a peptide that corresponds to the VDAC-binding N-terminal 15 aa of HKII, to selectively dissociate HKII from mitochondria to inhibit activity of the enzyme (60) and trigger apoptosis (16). As pHK is poorly cell permeable (Figs. 1 and 2), this has necessitated the use of various delivery strategies to increase the peptide’s cellular uptake. One such approach has been to couple pHK to CPPs, such as pAntp, TAT, or oligoarginine (16, 17, 31); however, given that the cellular uptake of most CPPs occurs primarily by endocytosis (41), a drawback of this approach is the endolysosomal entrapment and proteolytic degradation of coupled pHK. Recently, multiwalled carbon nanotubes were used as carriers for the cellular delivery of conjugated pHK (61). Although useful as drug delivery platforms, carbon nanotubes currently suffer from several limitations, including significant intrinsic cytotoxicity and variability in size, morphology, and purity, as well as poor drug loading, retention, or release (62, 63).
As an alternative approach, we covalently coupled pHK to a short PAS (GKPILFF) (21). pHK-PAS exhibited a 3- to 7-fold-greater cellular uptake compared with pHK (Figs. 1 and 2). Of interest, attachment of PAS to oligoarginine or pAntp resulted in a comparable enhancement in cellular uptake of these CPPs (21–23). Internalization of pHK-PAS occurs by both macropinocytosis and energy-independent mechanisms (Fig. 3B). Macropinocystosis is the primary cellular uptake route for a number of CPPs, including TAT, oligoarginine, and pAntp (23, 36, 64, 65). For these highly cationic CPPs, cell-surface binding via electrostatic interactions with negatively charged cell-surface heparan sulfate proteoglycans is a prerequisite for macropinocytosis (36–38). Internalization of the more hydrophobic pHK-PAS is less dependent on heparan sulfate proteoglycans (Fig. 2B, C), and only ∼40% of the peptide is taken up by macropinocytosis (Fig. 3B). The remainder of pHK-PAS enters cells by an ATP-independent mechanism (Fig. 3A) that does not require binding to heparan sulfate proteoglycans, which suggests that the initial interaction for this mechanism is mediated by other cell surface components. Energy-independent uptake (i.e., direct translocation across the plasma membrane) has also been observed for hydrophobic CPPs (41, 66). The molecular details of direct translocation by CPPs are as yet unclear, and several mechanisms have been proposed, including transient pore formation, membrane carpeting, and inverted micelle formation (41). As part of our follow-up to this study, we are currently investigating the energy-independent internalization mechanism of pHK-PAS. Thus, cellular uptake of pHK-PAS occurs by both macropinocytosis and energy-independent processes, and the utilization of multiple internalization mechanisms accounts for enhanced CPP properties of the peptide.
After uptake by cancer cells, pHK-PAS localizes to the cytosol (Fig. 4B). In the case of uptake by macropinocytosis, the PAS sequence facilitates rapid escape of the peptide from macropinosomes to the cytosol (21–23), whereas translocation across the membrane provides the peptide with direct access to the cytosol. Once in the cytosol, pHK-PAS accumulates at the mitochondrial membrane (Fig. 4B, C), where it binds to VDAC via the pHK segment of the peptide (10), competitively displacing endogenous full-length HKII in the process (Fig. 8A, B). Disruption of the VDAC–HKII interaction leads to ΔΨm depolarization (Fig. 6B), inhibition of mitochondrial respiration and glycolysis (Fig. 7), depletion of intracellular ATP levels (Fig. 6A), release of cytochrome c (Fig. 8A, B) and, finally, apoptosis (Fig. 5C, D). This proposed model for the action of pHK-PAS in cancer cells is strongly supported by control experiments with variants of the peptide, pHKscram-PAS and pAntp-PAS (Supplemental Fig. S3A, B), which demonstrated that the effects of pHK-PAS in cancer cells are mediated by the specific displacement of HKII from mitochondria by the pHK segment of CPP.
The mechanism by which dissociation of HKII from VDAC leads to mitochondrial dysfunction and apoptosis in cancer cells remains controversial. Some studies have proposed that upon displacement of HKII from the mitochondrial membrane, proapoptotic Bax translocates to the mitochondria, where it interacts with the unoccupied VDAC to form a large pore that exhibits conductance levels that are 4- and 10-fold greater than those of VDAC and Bax channels, respectively, but that lacks the ion selectivity of individual channels (16, 67). Moreover, unlike either the VDAC or Bax channels, the VDAC–Bax pore is large enough to allow passage of cytochrome c to the cytosol. Other studies have suggested that disruption of the VDAC–HKII interaction results in the opening of the mitochondrial permeability transition pore (PTP) independent of Bax (31, 68). PTP is a pathalogic channel that is hypothesized to be a multiprotein complex that consists of cyclophilin D in the matrix, adenine nucleotide translocator in the inner membrane, and VDAC in the outer membrane (2, 69). The opening of PTP allows an influx of solutes of <1.5 kDa into the matrix, which results in rapid ΔΨm depolarization and matrix swelling, followed by cristae unfolding and breaches in the OMM that are permeable to proteins (70, 71). Irrespective of the mechanism, it is clear that targeted displacement of HKII from the mitochondrial membrane by pHK-PAS transmits a potent death signal in cancer cells.
Of importance, pHK-PAS is considerably less toxic to noncancerous HEK293 cells compared with HeLa cells (Fig. 5A). Our results are in line with a recent study that showed that delivery of the N-terminal 15 aa of HKII by multiwalled carbon nanotubes resulted in substantially greater toxicity in MCF-7 human breast cancer cells and HCT116 human colon carcinoma cells compared with noncancerous IMR-90 human fetal lung fibroblasts (61). It should be noted that significant toxicity was also reported in IMR-90 cells, which is likely the result of the inherent cytotoxicity of the carbon nanotube delivery platform (62, 63). Here, the small apparent decrease in MTS response of HEK293 cells that were treated with higher peptide concentrations of pHK-PAS (Fig. 5B) can be attributed to nonspecific perturbation of cells by the peptide, which has been reported for a number of other hydrophobic CPPs (40). However, this effect is not statistically significant, as evidenced by the lack of depletion of intracellular ATP levels or depolarization of ΔΨm in these cells upon exposure to the peptide (Supplemental Fig. S3C).
The markedly differing responses of HeLa and HEK293 cells to pHK-PAS can be explained by the different characteristics of the 2 cell types. Whereas cancer cells, such as HeLa, express >60-fold higher levels of HKII compared with normal tissue (16), HKII expression in noncancerous HEK293 cells is ∼10-fold lower than in HeLa cells (Supplemental Fig. S4). In cancer cells, elevated levels of mitochondria-associated HKII serve to inhibit apoptosis, thereby allowing these cells to continue to grow and proliferate; noncancerous cells, on the other hand, do not utilize their lower levels of mitochondria-bound HKII as protection against apoptosis (2, 7, 16, 31). Dissociation of HKII from mitochondria in noncancerous cells, therefore, does not lead to apoptosis, as illustrated by the lack of cytochrome c release in HEK293 cells (Fig. 8C, D). Moreover, whereas elevated levels of mitochondria-associated HKII are needed to maintain the high glycolytic rates that are necessary for energy production and biomolecule synthesis in rapidly dividing cancer cells, noncancerous cells rely on oxphos as their primary energy-generating pathway (1, 3, 14, 15). Thus, dissociation of HKII from mitochondria, which inhibits the enzyme’s activity (60), is far less detrimental to noncancerous cells compared with cancer cells.
In summary (Fig. 9), pHK-PAS exhibits enhanced cellular uptake and mitochondrial localization compared with pHK in cancer cells. Upon binding to mitochondria, pHK-PAS displaces endogenous full-length HKII. Disruption of the HKII–VDAC interaction in cancer cells results in ΔΨm depolarization, inhibition of mitochondrial respiration and glycolysis, depletion of intracellular ATP levels, release of cytochrome c, and, finally, apoptosis. Of significance, pHK-PAS is considerably less toxic to noncancerous HEK293 cells, in which displacement of HKII from mitochondria does not lead to apoptosis, indicating that the peptide exhibits selective HKII-mediated cytotoxicity against cancer cells. Taken together, our results demonstrate that pHK-PAS is a novel CPP with potent anticancer properties.
Figure 9.
Proposed model of pHK-PAS action. Coupling of PAS to pHK enhances the peptide’s cellular uptake. This uptake occurs by both macropinocytosis and energy-independent mechanisms (translocation across the plasma membrane). In the case of uptake by macropinocytosis, the PAS sequence facilitates escape of the peptide from macropinosomes to the cytosol, whereas translocation provides the peptide with direct access to the cytosol. Once in the cytosol, pHK-PAS accumulates at the mitochondrial membrane, where it binds to VDAC and displaces endogenous full-length HKII in the process. The disruption of the HKII–VDAC interaction leads to ΔΨm depolarization, inhibition of mitochondrial respiration and glycolysis, depletion of intracellular ATP levels, release of cytochrome c, and, finally, apoptosis.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Khulood Alawadi [Department of Laboratories, New York University (NYU) Abu Dhabi] for preparing the graphic illustration (Fig. 9). Confocal imaging experiments were carried out by using the Core Technology Platform resources at NYU Abu Dhabi. This work was supported by a start-up fund from NYU Abu Dhabi (to M.M.). The authors declare no competing financial interests.
Glossary
- ΔΨm
mitochondrial membrane potential
- CPP
cell-penetrating peptide
- CTG
CellTiter-Glo
- ECAR
extracellular acidification rate
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- FCCP
carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone
- HKII
hexokinase II
- HPLC
high-performance liquid chromatography
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt
- OCR
oxygen consumption rate
- OMM
outer mitochondrial membrane
- oxphos
oxidative phosphorylation
- pAntp
penetratin
- PAS
penetration-accelerating sequence
- pHK
peptide corresponding to the mitochondrial membrane-binding N-terminal 15 aa of HKII
- pHKA488
Alexa 488-labeled pHK; pHK-PASA488, Alexa 488-labeled pHK-PAS
- pHKscram
scrambled pHK
- PI
propidium iodide
- PTP
permeability transition pore
- TMRM
tetramethylrhodamine methyl ester
- VDAC
voltage-dependent anion channel
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. D. Woldetsadik, W. M. Rabeh, and M. Magzoub conceived of the research; A. D. Woldetsadik and M. Magzoub designed experiments; A. D. Woldetsadik and M. C. Vogel performed the experiments; and A. D. Woldetsadik and M. Magzoub analyzed the data and wrote the manuscript.
REFERENCES
- 1.Vander Heiden M. G., Cantley L. C., Thompson C. B. (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathupala S. P., Ko Y. H., Pedersen P. L. (2006) Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 25, 4777–4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathupala S. P., Ko Y. H., Pedersen P. L. (2009) Hexokinase-2 bound to mitochondria: cancer’s stygian link to the “Warburg effect” and a pivotal target for effective therapy. Semin. Cancer Biol. 19, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robey R. B., Hay N. (2006) Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 25, 4683–4696 [DOI] [PubMed] [Google Scholar]
- 5.Wilson J. E. (2003) Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J. Exp. Biol. 206, 2049–2057 [DOI] [PubMed] [Google Scholar]
- 6.Mathupala S. P., Rempel A., Pedersen P. L. (2001) Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J. Biol. Chem. 276, 43407–43412 [DOI] [PubMed] [Google Scholar]
- 7.Pedersen P. L., Mathupala S., Rempel A., Geschwind J. F., Ko Y. H. (2002) Mitochondrial bound type II hexokinase: a key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention. Biochim. Biophys. Acta 1555, 14–20 [DOI] [PubMed] [Google Scholar]
- 8.Nakashima R. A., Mangan P. S., Colombini M., Pedersen P. L. (1986) Hexokinase receptor complex in hepatoma mitochondria: evidence from N,N′-dicyclohexylcarbodiimide-labeling studies for the involvement of the pore-forming protein VDAC. Biochemistry 25, 1015–1021 [DOI] [PubMed] [Google Scholar]
- 9.Arora K. K., Pedersen P. L. (1988) Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J. Biol. Chem. 263, 17422–17428 [PubMed] [Google Scholar]
- 10.Sui D., Wilson J. E. (1997) Structural determinants for the intracellular localization of the isozymes of mammalian hexokinase: intracellular localization of fusion constructs incorporating structural elements from the hexokinase isozymes and the green fluorescent protein. Arch. Biochem. Biophys. 345, 111–125 [DOI] [PubMed] [Google Scholar]
- 11.Pedersen P. L. (2008) Voltage dependent anion channels (VDACs): a brief introduction with a focus on the outer mitochondrial compartment’s roles together with hexokinase-2 in the “Warburg effect” in cancer. J. Bioenerg. Biomembr. 40, 123–126 [DOI] [PubMed] [Google Scholar]
- 12.Patra K. C., Wang Q., Bhaskar P. T., Miller L., Wang Z., Wheaton W., Chandel N., Laakso M., Muller W. J., Allen E. L., Jha A. K., Smolen G. A., Clasquin M. F., Robey R. B., Hay N. (2013) Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 24, 213–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Botzer L. E., Maman S., Sagi-Assif O., Meshel T., Nevo I., Yron I., Witz I. P. (2016) Hexokinase 2 is a determinant of neuroblastoma metastasis. Br. J. Cancer 114, 759–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lunt S. Y., Vander Heiden M. G. (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 [DOI] [PubMed] [Google Scholar]
- 15.Schulze A., Harris A. L. (2012) How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 491, 364–373 [DOI] [PubMed] [Google Scholar]
- 16.Pastorino J. G., Shulga N., Hoek J. B. (2002) Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J. Biol. Chem. 277, 7610–7618 [DOI] [PubMed] [Google Scholar]
- 17.Majewski N., Nogueira V., Bhaskar P., Coy P. E., Skeen J. E., Gottlob K., Chandel N. S., Thompson C. B., Robey R. B., Hay N. (2004) Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol. Cell 16, 819–830 [DOI] [PubMed] [Google Scholar]
- 18.Machida K., Ohta Y., Osada H. (2006) Suppression of apoptosis by cyclophilin D via stabilization of hexokinase II mitochondrial binding in cancer cells. J. Biol. Chem. 281, 14314–14320 [DOI] [PubMed] [Google Scholar]
- 19.Fulda S., Galluzzi L., Kroemer G. (2010) Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 9, 447–464 [DOI] [PubMed] [Google Scholar]
- 20.Levy A. G., Zage P. E., Akers L. J., Ghisoli M. L., Chen Z., Fang W., Kannan S., Graham T., Zeng L., Franklin A. R., Huang P., Zweidler-McKay P. A. (2012) The combination of the novel glycolysis inhibitor 3-BrOP and rapamycin is effective against neuroblastoma. Invest. New Drugs 30, 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takayama K., Nakase I., Michiue H., Takeuchi T., Tomizawa K., Matsui H., Futaki S. (2009) Enhanced intracellular delivery using arginine-rich peptides by the addition of penetration accelerating sequences (Pas). J. Control. Release 138, 128–133 [DOI] [PubMed] [Google Scholar]
- 22.Takayama K., Hirose H., Tanaka G., Pujals S., Katayama S., Nakase I., Futaki S. (2012) Effect of the attachment of a penetration accelerating sequence and the influence of hydrophobicity on octaarginine-mediated intracellular delivery. Mol. Pharm. 9, 1222–1230 [DOI] [PubMed] [Google Scholar]
- 23.Rezgui R., Blumer K., Yeoh-Tan G., Trexler A. J., Magzoub M. (2016) Precise quantification of cellular uptake of cell-penetrating peptides using fluorescence-activated cell sorting and fluorescence correlation spectroscopy. Biochim. Biophys. Acta 1858, 1499–1506 [DOI] [PubMed] [Google Scholar]
- 24.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. (2012) Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barltrop J. A., Owen T. C., Cory A. H., Cory J. G. (1991) 5-(3-carboxymethoxyphenyl)-2-(4,5-dimethylthiazolyl)-3-(4-sulfophenyl)tetrazolium, inner salt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans As cell-viability indicators. Bioorg. Med. Chem. Lett. 1, 611–614 [Google Scholar]
- 26.Berridge M. V., Tan A. S. (1993) Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 303, 474–482 [DOI] [PubMed] [Google Scholar]
- 27.Crouch S. P. M., Kozlowski R., Slater K. J., Fletcher J. (1993) The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 160, 81–88 [DOI] [PubMed] [Google Scholar]
- 28.Koopman G., Reutelingsperger C. P., Kuijten G. A., Keehnen R. M., Pals S. T., van Oers M. H. (1994) Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84, 1415–1420 [PubMed] [Google Scholar]
- 29.Elmore S. (2007) Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35, 495–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehrenberg B., Montana V., Wei M. D., Wuskell J. P., Loew L. M. (1988) Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys. J. 53, 785–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiara F., Castellaro D., Marin O., Petronilli V., Brusilow W. S., Juhaszova M., Sollott S. J., Forte M., Bernardi P., Rasola A. (2008) Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS One 3, e1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.John S., Weiss J. N., Ribalet B. (2011) Subcellular localization of hexokinases I and II directs the metabolic fate of glucose. PLoS One 6, e17674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calmettes G., Weiss J. N. (2013) A quantitative method to track protein translocation between intracellular compartments in real-time in live cells using weighted local variance image analysis. PLoS One 8, e81988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esko J. D., Stewart T. E., Taylor W. H. (1985) Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82, 3197–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richard J. P., Melikov K., Vives E., Ramos C., Verbeure B., Gait M. J., Chernomordik L. V., Lebleu B. (2003) Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 278, 585–590 [DOI] [PubMed] [Google Scholar]
- 36.Wadia J. S., Stan R. V., Dowdy S. F. (2004) Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 10, 310–315 [DOI] [PubMed] [Google Scholar]
- 37.Nakase I., Tadokoro A., Kawabata N., Takeuchi T., Katoh H., Hiramoto K., Negishi M., Nomizu M., Sugiura Y., Futaki S. (2007) Interaction of arginine-rich peptides with membrane-associated proteoglycans is crucial for induction of actin organization and macropinocytosis. Biochemistry 46, 492–501 [DOI] [PubMed] [Google Scholar]
- 38.Åmand H. L., Rydberg H. A., Fornander L. H., Lincoln P., Nordén B., Esbjörner E. K. (2012) Cell surface binding and uptake of arginine- and lysine-rich penetratin peptides in absence and presence of proteoglycans. Biochim. Biophys. Acta 1818, 2669–2678 [DOI] [PubMed] [Google Scholar]
- 39.Zhou J., Liu W., Pong R.-C., Hao G., Sun X., Hsieh J.-T. (2012) Analysis of oligo-arginine cell-permeable peptides uptake by prostate cells. Amino Acids 42, 1253–1260 [DOI] [PubMed] [Google Scholar]
- 40.Pan R., Xu W., Ding Y., Lu S., Chen P. (2016) Uptake mechanism and direct translocation of a new CPP for siRNA delivery. Mol. Pharm. 13, 1366–1374 [DOI] [PubMed] [Google Scholar]
- 41.Magzoub M., Gräslund A. (2004) Cell-penetrating peptides: [corrected] from inception to application. Q. Rev. Biophys. 37, 147–195 [DOI] [PubMed] [Google Scholar]
- 42.Manceur A. P., Driscoll B. D., Sun W., Audet J. (2009) Selective enhancement of the uptake and bioactivity of a TAT-conjugated peptide inhibitor of glycogen synthase kinase-3. Mol. Ther. 17, 500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orlandi P. A., Fishman P. H. (1998) Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141, 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Roy C., Wrana J. L. (2005) Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell Biol. 6, 112–126 [DOI] [PubMed] [Google Scholar]
- 45.Wang L. H., Rothberg K. G., Anderson R. G. (1993) Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123, 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson R. G. W. (1998) The caveolae membrane system. Annu. Rev. Biochem. 67, 199–225 [DOI] [PubMed] [Google Scholar]
- 47.Nichols B. J., Lippincott-Schwartz J. (2001) Endocytosis without clathrin coats. Trends Cell Biol. 11, 406–412 [DOI] [PubMed] [Google Scholar]
- 48.Liu N. Q., Lossinsky A. S., Popik W., Li X., Gujuluva C., Kriederman B., Roberts J., Pushkarsky T., Bukrinsky M., Witte M., Weinand M., Fiala M. (2002) Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J. Virol. 76, 6689–6700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schnitzer J. E., Oh P., Pinney E., Allard J. (1994) Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 127, 1217–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sampath P., Pollard T. D. (1991) Effects of cytochalasin, phalloidin, and pH on the elongation of actin filaments. Biochemistry 30, 1973–1980 [DOI] [PubMed] [Google Scholar]
- 51.Joliot A., Pernelle C., Deagostini-Bazin H., Prochiantz A. (1991) Antennapedia homeobox peptide regulates neural morphogenesis. Proc. Natl. Acad. Sci. USA 88, 1864–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Engeland M., Nieland L. J., Ramaekers F. C., Schutte B., Reutelingsperger C. P. (1998) Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 31, 1–9 [DOI] [PubMed] [Google Scholar]
- 53.Wu M., Neilson A., Swift A. L., Moran R., Tamagnine J., Parslow D., Armistead S., Lemire K., Orrell J., Teich J., Chomicz S., Ferrick D. A. (2007) Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol. Cell Physiol. 292, C125–C136 [DOI] [PubMed] [Google Scholar]
- 54.Jekabsons M. B., Nicholls D. G. (2004) In situ respiration and bioenergetic status of mitochondria in primary cerebellar granule neuronal cultures exposed continuously to glutamate. J. Biol. Chem. 279, 32989–33000 [DOI] [PubMed] [Google Scholar]
- 55.Dranka B. P., Benavides G. A., Diers A. R., Giordano S., Zelickson B. R., Reily C., Zou L., Chatham J. C., Hill B. G., Zhang J., Landar A., Darley-Usmar V. M. (2011) Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic. Biol. Med. 51, 1621–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lyon R. C., Cohen J. S., Faustino P. J., Megnin F., Myers C. E. (1988) Glucose metabolism in drug-sensitive and drug-resistant human breast cancer cells monitored by magnetic resonance spectroscopy. Cancer Res. 48, 870–877 [PubMed] [Google Scholar]
- 57.Kaplan O., Navon G., Lyon R. C., Faustino P. J., Straka E. J., Cohen J. S. (1990) Effects of 2-deoxyglucose on drug-sensitive and drug-resistant human breast cancer cells: toxicity and magnetic resonance spectroscopy studies of metabolism. Cancer Res. 50, 544–551 [PubMed] [Google Scholar]
- 58.Flescher E. (2007) Jasmonates in cancer therapy. Cancer Lett. 245, 1–10 [DOI] [PubMed] [Google Scholar]
- 59.Ganapathy-Kanniappan S., Vali M., Kunjithapatham R., Buijs M., Syed L. H., Rao P. P., Ota S., Kwak B. K., Loffroy R., Geschwind J. F. (2010) 3-bromopyruvate: a new targeted antiglycolytic agent and a promise for cancer therapy. Curr. Pharm. Biotechnol. 11, 510–517 [DOI] [PubMed] [Google Scholar]
- 60.Ahn K. J., Kim J., Yun M., Park J. H., Lee J. D. (2009) Enzymatic properties of the N- and C-terminal halves of human hexokinase II. BMB Rep. 42, 350–355 [DOI] [PubMed] [Google Scholar]
- 61.Yoong S. L., Lau W. L., Liu A. Y., Prendergast D., Ho H. K., Yu V. C. K., Lee C., Ang W. H., Pastorin G. (2015) Mitochondria-acting hexokinase II peptides carried by short-length carbon nanotubes with increased cellular uptake, endosomal evasion, and enhanced bioactivity against cancer cells. Nanoscale 7, 13907–13917 [DOI] [PubMed] [Google Scholar]
- 62.Sajid M. I., Jamshaid U., Jamshaid T., Zafar N., Fessi H., Elaissari A. (2016) Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 501, 278–299 [DOI] [PubMed] [Google Scholar]
- 63.Alshehri R., Ilyas A. M., Hasan A., Arnaout A., Ahmed F., Memic A. (2016) Carbon nanotubes in biomedical applications: factors, mechanisms, and remedies of toxicity. J. Med. Chem. 59, 8149–8167 [DOI] [PubMed] [Google Scholar]
- 64.Nakase I., Niwa M., Takeuchi T., Sonomura K., Kawabata N., Koike Y., Takehashi M., Tanaka S., Ueda K., Simpson J. C., Jones A. T., Sugiura Y., Futaki S. (2004) Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol. Ther. 10, 1011–1022 [DOI] [PubMed] [Google Scholar]
- 65.Dupont E., Prochiantz A., Joliot A. (2011) Penetratin story: an overview. In Cell-Penetrating Peptides (Langel Ü., ed.), pp. 21–29, Humana Press, New York: [Google Scholar]
- 66.Madani F., Lindberg S., Langel U., Futaki S., Gräslund A. (2011) Mechanisms of cellular uptake of cell-penetrating peptides. J. Biophys. 2011, 414729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shimizu S., Ide T., Yanagida T., Tsujimoto Y. (2000) Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J. Biol. Chem. 275, 12321–12325 [DOI] [PubMed] [Google Scholar]
- 68.Sun L., Shukair S., Naik T. J., Moazed F., Ardehali H. (2008) Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Mol. Cell. Biol. 28, 1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halestrap A. P., McStay G. P., Clarke S. J. (2002) The permeability transition pore complex: another view. Biochimie 84, 153–166 [DOI] [PubMed] [Google Scholar]
- 70.Bernardi P., Krauskopf A., Basso E., Petronilli V., Blachly-Dyson E., Di Lisa F., Forte M. A. (2006) The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 273, 2077–2099 [DOI] [PubMed] [Google Scholar]
- 71.Rasola A., Bernardi P. (2007) The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis 12, 815–833 [DOI] [PubMed] [Google Scholar]
- 72.Dunn K. W., Kamocka M. M., McDonald J. H. (2011) A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 300, C723–C742 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.