Significance
How individuals identify self (clonemates) in heterogeneous populations is a fundamental biological question. Here, we propose a mechanism by which Myxococcus xanthus, an aggregative multicellular bacterium, uses a cell surface adhesin to recognize clonemates. Cells selectively bind to other individuals that bear identical or nearly identical receptors. The social outcome of recognition includes bulk sharing of private cellular goods between cells. We reveal the malleable nature of this receptor that provides recognition selectivity among wild populations of myxobacteria. This is an example where single residue substitution can govern the decision for how microbes physically and socially interact.
Keywords: kin recognition, outer membrane exchange, Myxococcus xanthus
Abstract
The ability to recognize close kin confers survival benefits on single-celled microbes that live in complex and changing environments. Microbial kinship detection relies on perceptible cues that reflect relatedness between individuals, although the mechanisms underlying recognition in natural populations remain poorly understood. In myxobacteria, cells identify related individuals through a polymorphic cell surface receptor, TraA. Recognition of compatible receptors leads to outer membrane exchange among clonemates and fitness consequences. Here, we investigated how a single receptor creates a diversity in recognition across myxobacterial populations. We first show that TraA requires its partner protein TraB to function in cell–cell adhesion. Recognition is shown to be traA allele-specific, where polymorphisms within TraA dictate binding selectivity. We reveal the malleability of TraA recognition, and seemingly minor changes to its variable region reprogram recognition outcomes. Strikingly, we identify a single residue (A/P205) as a molecular switch for TraA recognition. Substitutions at this position change the specificity of a diverse panel of environmental TraA receptors. In addition, we engineered a receptor with unique specificity by simply creating an A205P substitution, suggesting that modest changes in TraA can lead to diversification of new recognition groups in nature. We hypothesize that the malleable property of TraA has allowed it to evolve and create social barriers between myxobacterial populations and in turn avoid adverse interactions with relatives.
Kin recognition is a fundamental biological property of a diverse range of communal living organisms. Mammals (1), plants (2), invertebrates (3), and more recently, unicellular organisms (4) are known to distinguish kin from nonkin based on their genetic relatedness. Kin recognition allows close relatives to form social groups and to conduct behaviors that are beyond the abilities of the individual (5). Such behaviors include increased fitness in obtaining food and assembling societies to protect and nurture offspring. Kin recognition also allows individuals to aggregate and build multicellular organisms. Because microbes are social (6, 7) and genetically tractable, they allow for the investigation of the molecular basis of kin recognition, cooperative behaviors, and how social interactions might evolve.
Myxococcus xanthus is a soil-dwelling Gram-negative bacterium that transitions between individual and multicellular life (8–10). Individuals move by gliding motility to forage for food and to identify proximal kin. Their behaviors in motility, predation, and development are also coordinated in multicellular aggregates. Multicellularity in myxobacteria is exemplified by fruiting body development, where thousands of cells aggregate into mounds that erect into fruits wherein a subset of cells differentiate into spores. To assemble multicellular communities, cells must discriminate kin to ensure their structures contain like individuals. Previously, we described a system where M. xanthus recognize self upon physical contact (4, 11). This self-recognition system, termed outer membrane exchange (OME), involves bidirectional transfer of large quantities of private cellular goods between kin (12, 13). OME is thought to aid cells in the transition toward multicellularity by helping establish outer membrane (OM) homeostasis in a population. OM homeostasis in turn leads to cellular repair and building a society where all individuals contribute toward multicellular life (14). For example, OME was originally discovered based on its ability to repair a subset of gliding motility mutants through extracellular complementation (11, 15). This occurs by the transfer of missing motility proteins to a mutant from another cell that makes the corresponding wild-type proteins. Rescue is transient, as DNA is not exchanged, and following rounds of cell division the transferred proteins are diluted and turned over. In addition, OME can repair motility, development, cell permeability, and viability defects associated with OM damage caused by various lipopolysaccharide (LPS) mutants (14). Here, cellular repair occurs by a healthy donor population replenishing wild-type LPS to the damaged population. This form of wound healing provides obvious benefits to the damaged cells. Additionally, the whole population benefits when their fitness depends on a quorum or threshold population size to perform multicellular tasks such as fruiting body development (16).
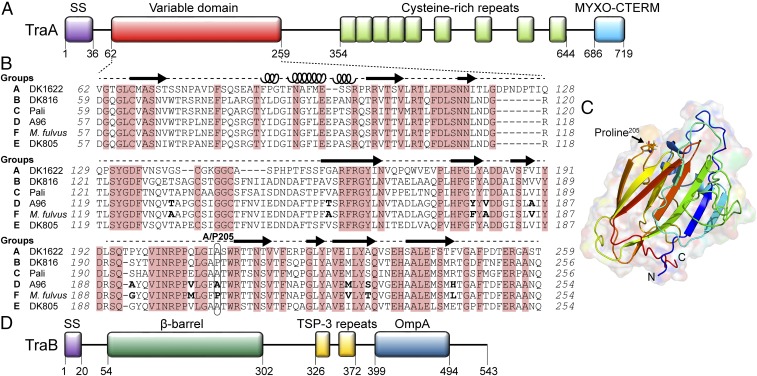
To ensure that bulk sharing of cellular goods occurs among self (clonemates), myxobacteria use a polymorphic cell surface receptor, TraA, which governs self-recognition (4). Across natural isolates, we found that TraA contains a variable domain that correlates with recognition specificity (Fig. 1A). That is, compatible OME partners contain identical or nearly identical TraA receptors. The specificity of OME is reprogrammed simply by experimentally swapping traA alleles among isolates. These findings suggest self-recognition occurs through homotypic interactions between TraA receptors. TraA functions with its partner protein, TraB, where OME requires both cells to contain TraA/B (11). Overexpression of TraA/B causes tight cell–cell binding, suggesting they function as adhesins (11, 14). TraA/B are also hypothesized to function as fusogens, which leads to the bilateral sharing of OM components between engaged cells (9, 11, 14).
Fig. 1.
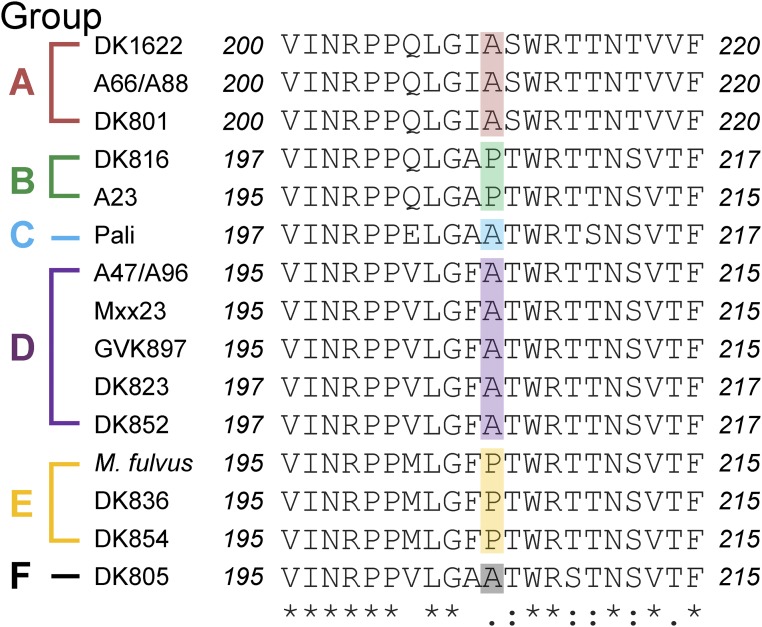
TraA contains a variable domain that determines recognition specificity. (A) Domain architecture of TraA: Type I signal sequence (SS); Cys-rich region and MYXO-CTERM are a putative stalk and a sorting tag, respectively (11). (B) Sequence alignment of the TraA variable domain from six recognition groups. Conserved residues are shaded red. Amino acid differences between the TraAA96 and TraAM. fulvus are in bold. Predicted secondary structures by I-TASSER (22) are indicated (loops, α-helices; arrows, β-strands). (C) Modeled 3D structure of the TraAM. fulvus variable domain by I-TASSER where the highest C-score was selected. (D) Domain architecture of TraB; Thrombospondin type 3 (TSP-3, Pfam02412). β-Barrel prediction was made with BOCTOPUS2 (32).
Because TraA is a specificity determinant, it functions as a greenbeard or kind recognition determinant. That is, TraA recognizes other cells that bear an identical allele and confers the preferential social behavior of exchanging private goods. Although traA is a greenbeard gene, OME also involves discrimination against kin because other loci impact OME. Our recent findings suggest that toxins encoded on a prophage are transferred by OME and partnering cells lacking cognate antitoxins are killed (17). Thus, TraA binding leads to self-recognition and the exchange of toxins further discriminates self from nonself. These combined functions are thought to facilitate the assembly of communities composed of highly related individuals.
Here we sought to understand how one receptor gives rise to many, potentially hundreds, of different recognition groups among natural myxobacterial isolates. To elucidate the molecular basis of TraA recognition, we first define the roles of TraA and TraB in cell–cell adhesion. Second, by chimeric allele analysis and site-directed mutagenesis, we precisely map key specificity determinants within TraA and surprisingly reveal that single residue substitutions alter recognition specificity. This finding represents a rare example where a single residue change reprograms social interactions. We suggest that the malleable nature of the TraA receptor has facilitated the evolution of social diversity within the Myxococcales order.
Results
TraA Requires TraB to Form Functional Cell Surface Adhesins.
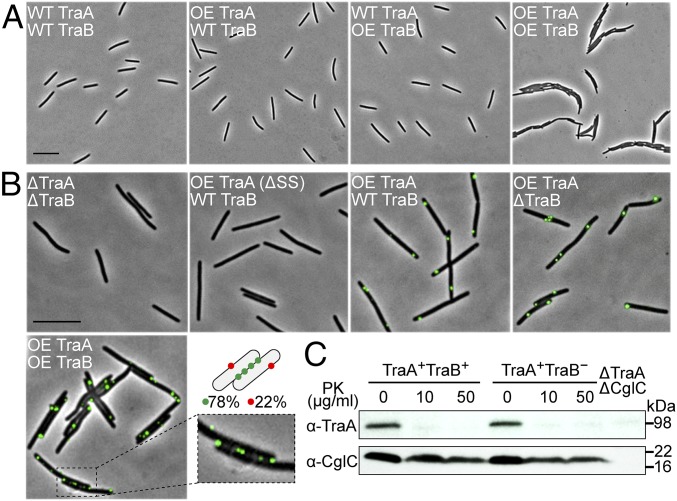
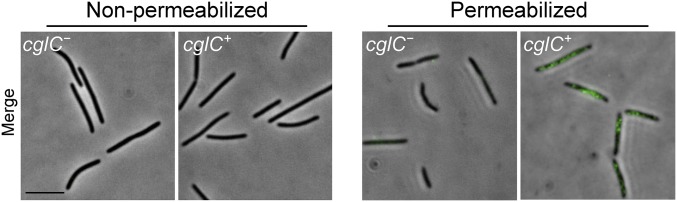
We previously showed that overexpression of TraA/B causes tight cell–cell adhesion during liquid growth (14) (Fig. S1). We suspect weaker adhesions also form when TraA/B are expressed at wild-type levels. To clearly delineate the roles of each protein in adhesion, we tested whether overexpression of either TraA or TraB was sufficient for cell adhesion. Notably, cell–cell adhesion only occurred when TraA/B were both overexpressed (Fig. 2A). In our model of OME, TraA serves as a receptor that recognizes clonemates by homotypic interactions, whereas the role of TraB is poorly understood. Sequence analysis predicts that TraB contains an N-terminal OM β-barrel and a C-terminal OmpA domain that presumably binds peptidoglycan (11) (Fig. 1D). Because of its domain features and because the traAB genes overlap in an operon, we speculated that TraB might facilitate the transport of TraA to the cell surface analogous to two-partner secretion systems (18). However, as shown in Fig. 2B, TraA was detected on the cell surface in a ΔtraB mutant by live-cell immunofluorescence. As an experimental control, CglC, a lipoprotein that localizes to the inner leaflet of OM (19), was probed in the same manner and was not detected on the surface (Fig. S2). In a separate approach, a ΔtraB mutant was treated with proteinase K (PK), and TraA was susceptible to proteolysis as found in the traB+ strain (Fig. 2C). We conclude that TraA transport occurs independently of TraB. Notably, the majority of TraA receptors distribute along cell–cell adhesion junctions when TraB was co-overexpressed (Fig. 2B, Inset), suggesting TraB assists TraA to function in adhesion.
Fig. S1.
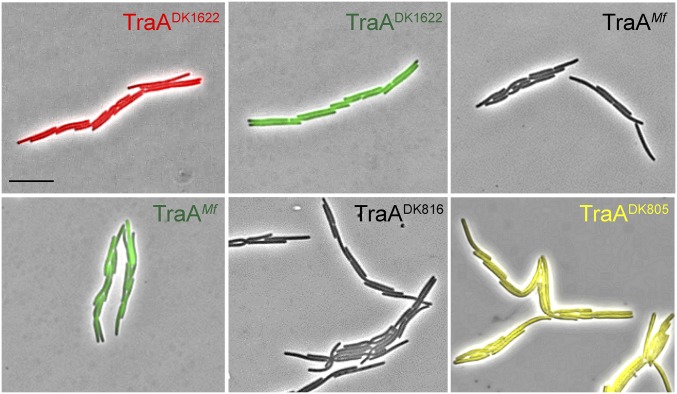
Overexpression of different TraA/B adhesins causes self-clumping. Cultures were grown in shake flasks, and wet mounts were directly prepared. Strain details (Left to Right): traABDK1622 mCherry (DW1463), traABDK1622 sfGFP (DW2201), traABMf nonlabeled (DW2206), traABMf sfGFP (DW2207), traADK816traBDK1622 nonlabeled (DW2208), and traADK805traBDK1622 EYFP (DW2209). (Scale bar, 2 μm.)
Fig. 2.
TraA requires TraB to form a functional adhesin. (A) Wet mounts of indicated strains taken directly from shake flask cultures. Relevant properties shown: wild type (WT) and overexpression (OE). Strains from Left to Right are DK8601, DW2202, DW2203, and DW1463. (Scale bars: A and B, 2 μm.) (B) Cell surface localization of TraA. Immunofluorescent labeling with α-TraA serum was done on live, nonpermeabilized cells. Relevant properties are shown. Strains (Left to Right) are DW1483, DW2204 (signal sequence deletion, ΔSS), DW2202, DW2205, and DW1463. Inset shows TraA foci along a cell–cell junction. Among cells that clumped, 78% (379 out of 485) of the TraA foci localized along cell–cell junctions. (C) Protease accessibility assay of TraA in traB+ (DW2202) or traB− (DW2205) backgrounds. Live cells were treated with indicated concentrations of PK and analyzed by immunoblot with α-TraA serum. The same samples were also probed with α-CglC serum as a negative control. A ΔtraA ΔcglC strain (DW2220) was used as an immunoblot control.
Fig. S2.
Immunofluorescence labeling of a control OM protein requires cell permeablization. CglC, a lipoprotein localized to the inner leaflet of the OM (19), was not detected following the immunofluorescence procedure used to detect TraA on live cells (Left; compare with Fig. 2B). CglC was detected when cells were permeabilized before immunofluorescence labeling (Right). Strains are DW1466 (cglC−) and DK8601 (cglC+). (Scale bar, 2 μm.)
Cell–Cell Adhesion Is traA Allele-Specific.
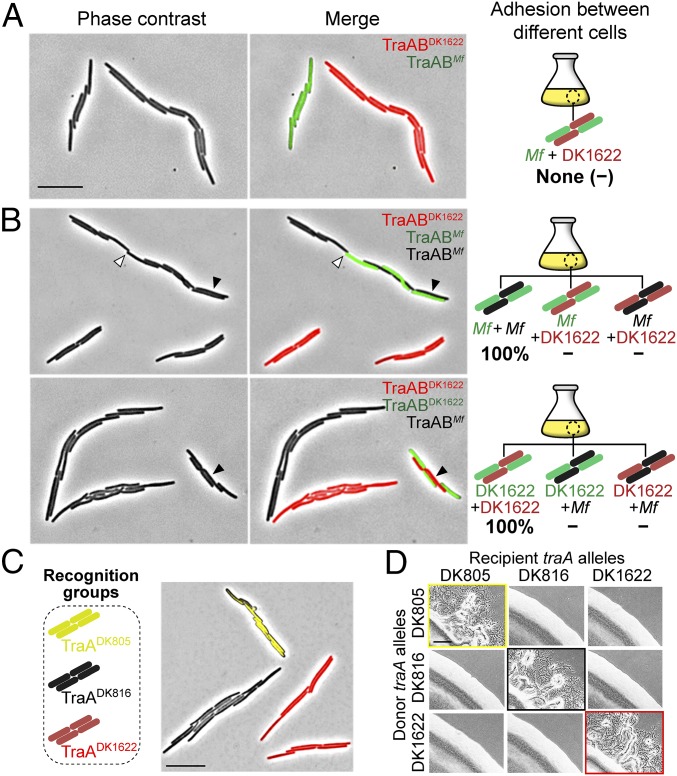
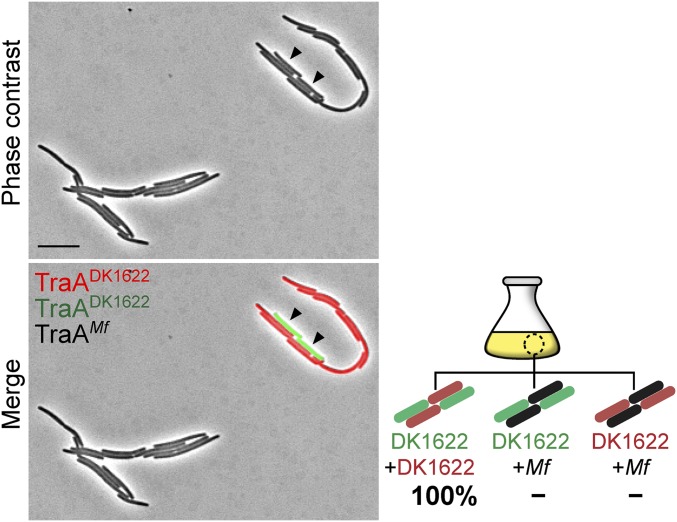
TraA contains a variable [a.k.a. PA14-like (11), Pfam07691] domain (Fig. 1A), which we suggest contains the specificity determinants (4). To test our model that TraA receptors from opposing cell membranes directly interact (9), we asked whether such interactions promoted self-adhesion. Here we overexpressed the Myxococcus fulvus HW-1 (20) traAB operon in a ΔtraAB M. xanthus strain. As expected, this strain formed cell–cell adhesions in liquid culture, similar to cells that overexpress the M. xanthus (DK1622) traAB operon (Fig. S1). The traABM. fulvus strain labeled with sfGFP was then cocultured with a traABDK1622 strain labeled with mCherry. In support of a homotypic interaction model, we found that cells only adhere to ones bearing identical receptors (Fig. 3A). To confirm that cell–cell adhesion was not the result of aberrant cell division from TraA/B overexpression, we cocultured three strains to test for selective binding. As shown in Fig. 3B, when traABM. fulvus (sfGFP), traABM. fulvus (nonlabeled), and traABDK1622 (mCherry) strains were cocultured at a 1:1:1 ratio, interstrain binding occurred and represented ∼30% of total cell clusters. One hundred one interstrain clusters were examined from three independent experiments, and coaggregation was only found between traABM. fulvus cells, whereas traABDK1622 cells only bound to themselves. Similarly, in separate tripartite coculture experiments, traABDK1622 (mCherry) and traABDK1622 (sfGFP) cells were found to only cluster with one another and not traABM. fulvus (nonlabeled) cells (Fig. 3B; 103 interstrain clusters analyzed). To test the role of TraB in binding selectivity, we constructed a strain overexpressing traAM. fulvustraBDK1622 (nonlabeled) and cocultured it with traABDK1622 (mCherry) and traABDK1622 (sfGFP) cells. Binding only occurred between cells harboring the same TraA receptors (Fig. S3), suggesting TraB is not involved in specificity. In addition, we expressed traA alleles from two other recognition groups (4) in an isogenic ΔtraA strain that overexpressed traBDK1622. The resulting strains, traADK805 (EYFP) and traADK816 (nonlabeled), formed cell clusters in monocultures (Fig. S1). These strains were then cocultured with the traABDK1622 (mCherry) strain, which belongs to a third recognition group. As shown in Fig. 3C, the expression of different TraA proteins, but identical TraB proteins, led to distinct social clusters. Consistent with this, in an extracellular complementation (stimulation) assay, which monitors the transfer of OM lipoproteins and restoration of motility to a mutant by OME, motility rescue (emergent flares) only occurred between donor and recipient strains harboring identical TraA receptors (Fig. 3D). We conclude that TraA serves as the sole specificity determinant, whereas TraB plays an essential role in adhesion and OME.
Fig. 3.
Cell–cell adhesion is traA allelic-specific. (A) A traABDK1622 strain (mCherry) was cocultured with a traABM. fulvus strain (sfGFP). (B) Coincubation of three traAB strains: traABM. fulvus (sfGFP), traABM. fulvus (nonlabeled), and traABDK1622 (mCherry) (Upper); traABDK1622 (sfGFP), traABDK1622 (mCherry), and traABM. fulvus (nonlabeled) (Lower). Shown are pole-to-pole adhesion (open triangle) and side-by-side adhesion (filled triangle). Schematics of all possible interstrain adhesions are shown. Percent of each cell cluster is indicated (−, not detected). (C) Coincubation of strains expressing TraA receptors that belong to three different recognition groups. Strains all express traBDK1622 and are labeled (EYFP, mCherry, and nonlabeled) as indicated. (D) OME among cells expressing different traA alleles assessed by stimulation. Boxed panels highlight OME as detected by the rescue of motility defects. The recipient strains are nonmotile and stimulatable (ΔcglC Δtgl), whereas the nonmotile donors (aglB1 ΔpilA) are not stimulatable. Table S1 for strain details. (Scale bars: A and C, 2 µm; and D, 200 µm.)
Fig. S3.
TraB does not confer self-recognition specificity. Coculture of three strains (DW1463, DW2201, and DW2242) that expressed different TraA receptors and identical TraBDK1622 protein led to selective binding. Strains are labeled as indicated. Interstrain binding (filled triangle). Schematic of all possible partner bindings are shown. One hundred interstrain clusters from three independent experiments were evaluated, and the percent of each partner pair is indicated (−, not detected). (Scale bar, 2 μm.)
Chimeric Allele Analysis Defines a Specificity Region in TraA.
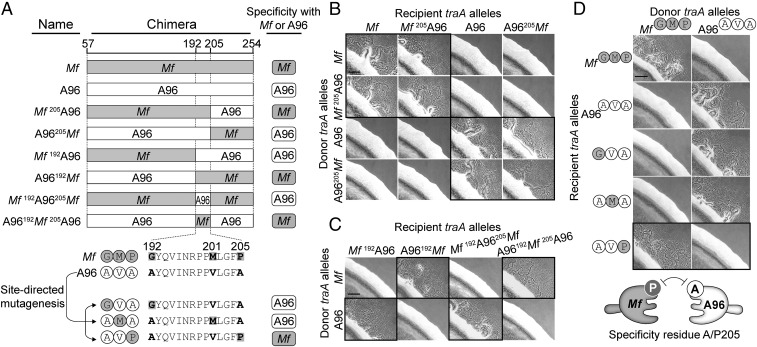
To elucidate the molecular basis of TraA recognition, we sought to identify a subregion(s) within the variable domain that governs specificity. To this end, a series of chimeric traA alleles were constructed (Fig. 4A). Initially, we examined the traA alleles from environmental isolates M. fulvus HW-1 and M. xanthus A96. These two alleles are not compatible, and yet they only contain 11 amino acid differences between their variable regions (Fig. 1B). The first chimera fusion site between traAM. fulvus (Mf) and traAA96 (A96) was engineered after residue 205 (Fig. 4A), and the two resulting chimeras (Mf205A96 and A96205Mf) were transformed into ΔtraA donor and recipient strains. As judged by stimulation assays, both chimeras were functional in self-recognition (Fig. 4B). The Mf205A96 chimera was also found to interact exclusively with the Mf allele, whereas the A96205Mf chimera interacted exclusively with the A96 allele. In other words, the chimeras recognized their parental alleles only when their N termini were identical. To further define the region involved in specificity, two additional chimeras, Mf192A96 and A96192Mf, were made in which the fusion site was moved upstream by 14 residues (Fig. 4A). Notably, these chimeras swapped recognition specificity; stimulation only occurred when the chimeras and parental alleles shared identical C termini (Fig. 4C). These combined results indicate that a small region (residues 192–205) plays a critical role in specificity. To confirm this, we created two more chimeras (Mf192A96205Mf and A96192Mf205A96), in which residues 192–205 from traAA96 and traAMf alleles were swapped into the parental traAMf and traAA96 backbones, respectively (Fig. 4A). Strikingly, Mf192A96205Mf now only recognized traAA96 (Fig. 4C). Similarly, A96192Mf205A96 recognized traAMf instead of traAA96. We conclude that residues 192–205 play a key role in determining recognition specificity between these receptors.
Fig. 4.
A single residue (A/P205) governs the specificity of TraAMf–TraAA96 recognition. (A) Schematics of chimeric variable domains made between TraAMf (gray) and TraAA96 (white). The C-terminal region (not involved in specificity) is not shown. Chimeric junction sites are indicated. Bottom illustration shows mutagenesis strategy on a traA allele (pPC11) containing the TraAA96 subregion. Recognition compatibilities of the mutants with parental alleles are shown at the right (corresponding stimulation data shown in B–D). (B) Stimulation assays among chimeric and parental traA alleles. Mixtures that show recognition are highlighted with black frames. (C) Same as B but with different traA chimeras. (D) Stimulation assays of point mutants against parental alleles. The black-bordered micrographs show that a A205P substitution changes specificity. Table S1 for plasmids/strain details. Cartoon summarizes the A/P205 differences that underlie incompatibility. (Scale bar, 200 μm.)
Single Amino Acid Substitution (A/P205) Changes Cell–Cell Recognition.
There are only three amino acid differences within the described specificity region between TraAMf and TraAA96 (Fig. 4A). To test the roles of each residue, we conducted site-directed mutagenesis. Here, the specificity region from TraAA96 was used as a template and the original residues were replaced individually to the corresponding residues from TraAMf (Fig. 4A). The 192A→G or 201V→M substitutions led to recognition with the TraAA96 parental receptor (Fig. 4D). In contrast, the 205A→P mutant recognized TraAMf rather than the parental receptor. Combined with the above results, we surprisingly found that a single residue acts as a specificity determinant between TraAMf and TraAA96.
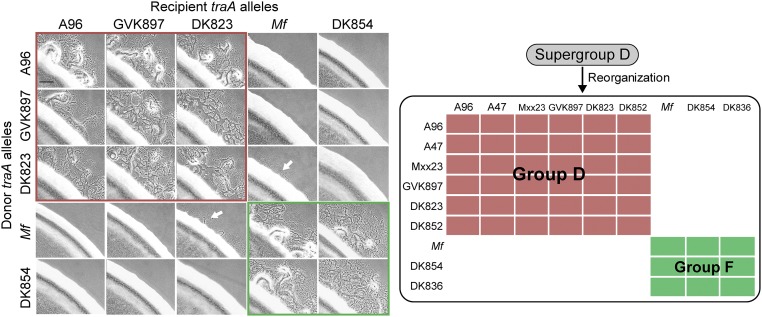
According to our previous finding (4), M. fulvus and A96 were assigned to the recognition “supergroup D.” Supergroup D was loosely defined because in a few cases promiscuous interactions among natural isolates were detected in an assay that monitors the transfer of fluorescent lipids. Here, we sought to clarify these recognition outcomes, in terms of group members containing an A or P at the position that corresponded to residue 205 in TraAA96 or TraAMf (Fig. 1B). OME was reevaluated in stimulation assays using isogenic ΔtraA background strains. Notably, we found that the original supergroup D members were now divided into two groups: members containing A205 (renamed D) and members containing P205 (renamed F) (Fig. S4). We suspect that the promiscuous interactions observed in prior experiments were due, in part, to the interstrain killing between natural isolates and/or toxicity associated with lipophilic dye staining. Those complications were avoided in stimulation assays with isogenic strains. In addition, poor stimulation was observed when traADK823 (containing A) cells were mixed with traAMf (containing P) cells, suggesting crosstalk between these receptors (Fig. S4). Taken together, these results suggest that the residue A/P205 serves as a predictive feature for recognition specificity.
Fig. S4.
Reassignment of supergroup D into two recognition groups: D and F. Interactions between indicated TraA receptors were assessed in isogenic backgrounds by stimulation assays (Left). Panels with red borders show group D members, whereas the green borders show group F members. These results were combined with lipophilic dye transfer results (4) and summarized (Right). All group D members contain an A that corresponds to residue 205 in TraAA96, whereas all group F members contain a P at this position. Alleles that were not analyzed here include receptors with identical variable domains (traAA47 and traAA96; traADK852 and traADK823) or very similar and compatible variable domains (traAMxx23 and traAA96 contain four amino acid differences, whereas traADK836 and traADK854 contain three amino acid differences). White arrows indicate poor cross-talk between receptors. (Scale bar, 200 μm.)
A/P Substitution Changes Recognition Specificity in Other traA Alleles.
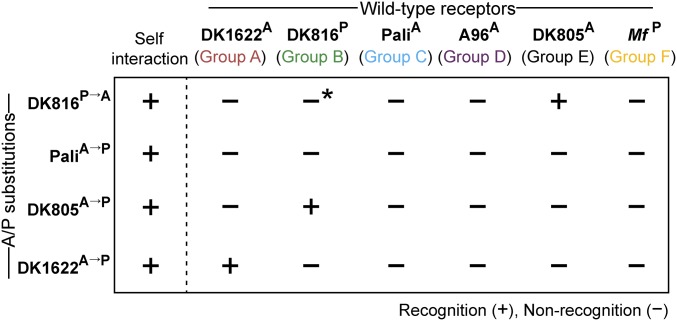
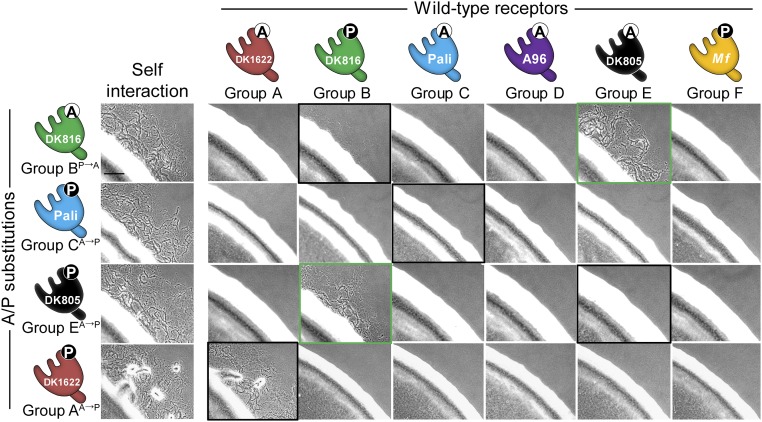
The discovery of A/P205 as a specificity determinant prompted us to study other recognition groups: A, B, C, and E. Notably, this A/P residue is ubiquitously found in all 17 traA alleles that we characterized (Fig. S5), suggesting this residue plays a broad role in recognition. To test this, site-directed mutagenesis was done on representative traA alleles from these groups where the original A or P residue was changed to P or A, respectively. All of the mutant alleles were functional as judged by robust self-recognition (Fig. 5 and Fig. S6). Interestingly, the P→A substitution in TraADK816 (group B) resulted in poor interaction with the parental TraADK816 receptor, whereas the A→P substitutions in TraAPali (group C) and TraADK805 (group E) completely abolished interactions with their parental receptors (Fig. 5 and Fig. S6). In contrast, the A→P mutation in TraADK1622 (group A) led to recognition with its parental receptor with little change in activity (Fig. 5 and Fig. S6), indicating the A/P residue is not critical in group A recognition. Taken together, we conclude that the A/P205 residue plays a key role in determining specificity among members of groups B, C, D, E, and F.
Fig. S5.
Sequence alignment of the A/P subregion from 17 traA alleles. The recognition group designations are shown at the left, and A/P residues are color coded.
Fig. 5.
Single residue substitutions (A/P) change recognition specificity in a panel of traA alleles. Shown are substitutions on representative traA alleles from four different recognition groups where the original A or P was changed as indicated (superscript). Mutants were tested for recognition against strains expressing different wild-type TraA receptors and scored for stimulation (*, poor stimulation). Fig. S6 for experimental data.
Fig. S6.
A/P substitutions change recognition specificity of TraA receptors from diverse environmental isolates. These experimental stimulation data are schematically summarized in Fig. 5. Donor and recipient strain sets are isogenic and described in Table S1. Black borders highlight mixtures between mutants and their parental traA alleles. Green borders indicate a switch in recognition specificity. (Scale bar, 200 μm.)
Unique TraA Recognition Receptor Engineered by Single Amino Acid Substitution.
We next evaluated the role of A/P205 in intergroup discrimination. To this end, the interactions between the above A/P point mutants and representative traA alleles from all six groups were assessed. We considered three possible outcomes: A/P substitutions overcome recognition barriers between receptors from different groups, resulting in (i) crosstalk, (ii) mutant receptors with changed specificities, and (iii) receptors with unique specificities. As shown in Fig. 5 and Fig. S6, traADK805 (A→P) recognized traADK816 (containing P) but not its parental allele. In the reciprocal experiment, traADK816 (P→A) interacted robustly with traADK805 (containing A) and exhibited poor recognition with its parental allele. These results suggest A/P acts as a major specificity determinant between traADK816 (group B) and traADK805 (group E). Interestingly, the A→P substitution in traAPali (group C) created a receptor that only interacted with itself and not with its parental allele or any other allele (Fig. 5 and Fig. S6), thus generating a receptor with a unique specificity.
The variable domain in TraA shows distant homology to the PA14 domain found in Flo5 from yeast (11). Flo5 is a cell adhesin that mediates flocculation, and the crystal structure of its PA14 domain is known (21). Using this structure as a template, a computational model for the variable domain of TraADK1622 was previously made (11). Here, a model of the variable domain from TraAM.fulvus was made with I-TASSER (Fig. 1C) (22), using multiple templates including Flo1, Epa1, Epa9, and Cea1 adhesins from various yeast strains as well as the protective antigen (PA) from anthrax toxin. This analysis suggests that A/P205 resides within a loop between two β-strands (Fig. 1 B and C). We propose that this loop acts as a recognition switch and A/P205 substitutions alter its conformation and hence specificity of recognition.
Discussion
OME in myxobacteria is a unique platform for cells to interact and survive as multicellular entities (9, 23). A polymorphic receptor, TraA, allows individuals to recognize clonemates and exchange their private goods. Here we show that TraA requires TraB to form an active adhesin for self-recognition. In the absence of TraB, TraA localizes to the cell surface but does not function in cell–cell binding. TraA contains a predicted sorting motif, MYXO-CTERM, which is analogous to the cell wall-sorting tag LPXTG in Gram-positive bacteria (11) and may contribute to its cell surface display. TraB, with a predicted OM β-barrel and an OmpA cell wall binding domain, may help anchor TraA to the cell envelop during adhesion and OME. We also found that heterologous expression of different TraA–TraB protein combinations were functional, and the specificity of cell–cell recognition was determined by TraA. We suspect that TraB interacts with TraA in regions that are conserved between these proteins.
Cells form distinct kin groups in liquid according to which TraA receptor they express. The ability of a single gene to identify others that bear the same allele and to confer preferential social behaviors is a hallmark of a greenbeard gene (24, 25). This is an example demonstrating that single allele variation (i.e., homotypic receptor) within a bacterium governs selective cell–cell binding. We hypothesize that selective adhesion with clonemates in heterogeneous myxobacterial populations facilitates cooperative behaviors among close relatives. In future studies, we will address the roles of TraA/B recognition and adhesion during the formation of social groups on solid surfaces, where cells glide and form structured communities.
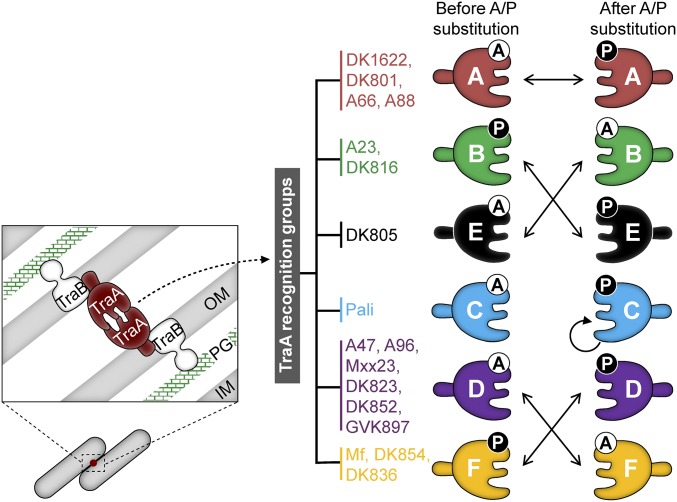
We show that TraA is a malleable protein where chimeric alleles and amino acid substitutions are tolerated and lead to changes in self-recognition (Fig. 6). We think these qualities are important for the evolution of diversity in TraA recognition. This platform allows new recognition alleles to be created by spontaneous mutations or horizontal gene transfer that results in homologous recombination between traA alleles. Previously, we alluded to this latter possibility by suggesting rearrangements occurred between ancestral traA alleles (4). With changes in traA sequences and concurrent changes in recognition, populations will in turn diversify, ensuring that private good exchange only occurs with clonemates or strains that share recognition specificity. The diversification of large populations into distinct social groups also provides a selective advantage to adapt to changing environments (5, 10). For example, a homogenous population, in terms of TraA recognition, exposes itself to exploitation by selfish elements. One such element is Mx-alpha, which resembles a defective prophage and is hypervariable (17, 26, 27). Mx-alpha may harbor toxins that are transferred by OME and kill nonclonemates lacking cognate immunity. TraA polymorphisms create distinct recognition groups that prevent OME and lethal encounters from occurring between sympatric individuals. These social barriers, in turn, block exploitation by Mx-alpha.
Fig. 6.
Summary for how A/P205 substitutions influence TraA recognition. TraA functions with TraB to form an active adhesin wherein TraA determines the specificity of cell–cell recognition (Left). Inner membrane, IM; outer membrane, OM; peptidoglycan, PG. Right shows 17 TraA receptors are divided into six recognition groups. Solid arrows indicate how A/P205 substitutions reprogram recognition.
We identified a single residue in TraA that plays a key role in specificity. For example, swapping A/P205 residues between traADK816 and traADK805 led to changes in recognition from the parental allele to the other allele. These findings are striking given that there are 42 amino acid differences between their variable domains (Fig. 1B). However, simply sharing an A or P205 residue is not sufficient for recognition (e.g., TraAA96 vs. TraADK805, TraAMf vs. TraADK816), demonstrating other residues contribute toward specificity. Strikingly, an A→P substitution within traAPali created an allele that only recognized itself; it did not recognize its parental receptor or other receptors. This highlights the importance of A/P205 and suggests that TraA may undergo diversification and form novel recognition groups in nature. Given the high levels of heterogeneity of myxobacterial communities and traA alleles (4, 28, 29), we suspect the traAPali A→P allele is compatible with undiscovered receptors in nature. For group A receptors, which are divergent from the other five groups (Fig. 1B) (4), substitutions in A/P205 did not alter specificity. We suspect that the divergent sequences of group A members likely result in structural differences that will need to be elucidated elsewhere.
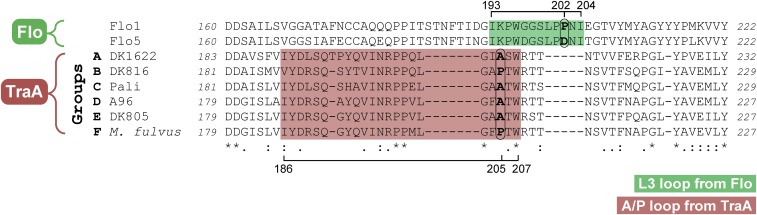
Given that A/P205 plays a key role in specificity, we hypothesize that this residue acts as a molecular recognition switch, where an A or P at this position changes the conformation of the loop. In support of this, a single residue was recently shown to determine the conformational position of an analogous loop (21, 30). Here, the Flo1 and Flo5 adhesins from Saccharomyces cerevisiae contain a distantly homologous PA14 domain (11). Crystallographic studies revealed both structures contain a loop, L3, that is flexible and adopts different conformations due to single residue variations: P in Flo1 and D in Flo5 (30). These different L3 conformations result in different mannose-binding affinities. From sequence alignments (Fig. S7), L3 from Flo1/5 corresponds with the predicted A/P205 loop in TraA. By extension, we suggest that this loop in TraA switches conformations based on the presence of an A or P residue, which consequently governs self-recognition.
Fig. S7.
Sequence alignment of the subregions from the Flo and TraA proteins. The L3 loop in Flo and the predicted A/P loop in TraA are shaded green and red, respectively. Residues that determine the loop conformations are in bold and circled. The alignment of the complete PA14 (Flo5) and variable (TraADK1622) domains was previously shown (11).
The interaction of different TraA receptors does not always result in a clear OME outcomes. In a few cases, a weak response between different receptors is observed (Fig. S4). These promiscuous interactions represent crosstalk between receptors. In our system, crosstalk may arise from receptor overexpression by the strong heterologous pilA promoter. In nature, crosstalk might be selected against because OME between nonclonemates is lethal (17). In other molecular recognition systems, crosstalk also occurs. For example, in two-component signaling systems, histidine kinases (HKs) may interact with more than one response regulator (RR) (31). Depending on the selective conditions, this crosstalk might lead to the diversification of the HK–RR interfaces and the evolution of new specificity pairs. Similar evolutionary forces may apply to TraA–TraA interactions.
Materials and Methods
Bacterial strains, plasmids, and growth conditions are described in SI Materials and Methods and Tables S1 and S2.
Table S1.
Plasmids and strains used in this study
| Plasmids/strains | Relevant features* | Source |
| Plasmids | ||
| pDP22 | pSWU19 Mx8 attP cassette, PpilA, Kmr | (4) |
| pDP23 | pDP22 PpilA-traADK816, Kmr | (4) |
| pDP24 | pDP22 PpilA-traAA96, Kmr | (4) |
| pDP25 | pDP22 PpilA-traAMf, Kmr | (4) |
| pDP26 | pDP22 PpilA-traAPali, Kmr | (4) |
| pDP27 | pDP22 PpilA-traADK1622, Kmr | (4) |
| pDP28 | pBJ114 ΔtraA cassette, galK Kmr | (4) |
| pDP35 | pBJ114 ΔtraAB cassette, galK Kmr | This study |
| pDP36 | pDP22 PpilA-traBDK1622, Kmr | This study |
| pPC1 | pKSAT PpilA-SSOM-sfGFP, Smr | (14) |
| pPC2 | pKSAT PpilA-EYFP, Smr | This study |
| pPC3 | pDP22 PpilA-traADK1622 ΔSS, Kmr | This study |
| pPC4 | pDP22 PpilA-traABMf, Kmr | This study |
| pPC5 | pDP22 PpilA-traADK816traBDK1622, Kmr | This study |
| pPC6 | pDP22 PpilA-traADK805traBDK1622, Kmr | This study |
| pPC7 | pDP22 PpilA-traAMf205A96, Kmr | This study |
| pPC8 | pDP22 PpilA-traAA96205Mf, Kmr | This study |
| pPC9 | pDP22 PpilA-traAMf192A96, Kmr | This study |
| pPC10 | pDP22 PpilA-traAA96192Mf, Kmr | This study |
| pPC11 | pDP22 PpilA-traAMf192A96205Mf, Kmr | This study |
| pPC12 | pDP22 PpilA-traAA96192Mf205A96, Kmr | This study |
| pPC13 | pDP22 PpilA-traAMf192A96205Mf (A192G), Kmr | This study |
| pPC14 | pDP22 PpilA-traAMf192A96205Mf (V201M), Kmr | This study |
| pPC15 | pDP22 PpilA-traAMf192A96205Mf (A205P), Kmr | This study |
| pPC16 | pDP22 PpilA-traADK805, Kmr | This study |
| pPC17 | pDP22 PpilA-traADK816 (P205A), Kmr | This study |
| pPC18 | pDP22 PpilA-traAPali (A205P), Kmr | This study |
| pPC19 | pDP22 PpilA-traADK805 (A205P), Kmr | This study |
| pPC20 | pDP22 PpilA-traADK1622 (A205P), Kmr | This study |
| pPC21 | pDP22 PpilA-traAGVK897, Kmr | This study |
| pPC22 | pDP22 PpilA-traADK823, Kmr | This study |
| pPC23 | pDP22 PpilA-traADK854, Kmr | This study |
| pPC24 | pDP22 PpilA-traAMftraBDK1622, Kmr | This study |
| Strains | ||
| DH5α | E. coli cloning strain | Lab collection |
| DK1622 | A+S+, WT | (17) |
| DK8601 | A–S–, aglB1 (aglQ1) ΔpilA::tc, Tcr | (36) |
| DW1463 | A–S–, DK8601(pXW6, pDP21), Kmr, Tcr, Smr | (11) |
| DW1466 | A–S–, DK1622 Δtgl::tc ΔcglC (markerless), Tcr | (11) |
| DW1467 | A–S–, DK8601 ΔtraA (markerless), Tcr | (4) |
| DW1468 | A–S–, DW1467 (pDP23), Kmr, Tcr | (4) |
| DW1469 | A–S–, DW1467 (pDP24), Kmr, Tcr | (4) |
| DW1470 | A–S–, DW1467 (pDP25), Kmr, Tcr | (4) |
| DW1471 | A–S–, DW1467 (pDP26), Kmr, Tcr | (4) |
| DW1483 | A–S–, DK8601 ΔtraAB (markerless), Tcr | This study |
| DW2201 | A–S–, DK8601(pPC1, pDP21), Kmr, Tcr, Smr | (14) |
| DW2202 | A–S–, DK8601 (pDP27), Kmr, Tcr | This study |
| DW2203 | A–S–, DK8601 (pDP36), Kmr, Tcr | This study |
| DW2204 | A–S–, DK1467 (pPC3), Kmr, Tcr | This study |
| DW2205 | A–S–, DW1483 (pDP27), Kmr, Tcr | This study |
| DW2206 | A–S–, DW1483 (pPC4), Kmr, Tcr | This study |
| DW2207 | A–S–, DW2206 (pPC1), Kmr, Tcr, Smr | This study |
| DW2208 | A–S–, DW1483 (pPC5), Kmr, Tcr | This study |
| DW2209 | A–S–, DW1483 (pPC2, pPC6), Kmr, Tcr, Smr | This study |
| DW2210 | A–S–, DW1467 (pPC7), Kmr, Tcr | This study |
| DW2211 | A–S–, DW1467 (pPC8), Kmr, Tcr | This study |
| DW2212 | A–S–, DW1467 (pPC16), Kmr, Tcr | This study |
| DW2213 | A–S–, DW1467 (pPC17), Kmr, Tcr | This study |
| DW2214 | A–S–, DW1467 (pPC18), Kmr, Tcr | This study |
| DW2215 | A–S–, DW1467 (pPC19), Kmr, Tcr | This study |
| DW2216 | A–S–, DW1467 (pPC20), Kmr, Tcr | This study |
| DW2217 | A–S–, DW1467 (pPC21), Kmr, Tcr | This study |
| DW2218 | A–S–, DW1467 (pPC22), Kmr, Tcr | This study |
| DW2219 | A–S–, DW1467 (pPC23), Kmr, Tcr | This study |
| DW2220 | A–S–, DW1466 ΔtraA (markerless), Tcr | This study |
| DW2221 | A–S–, DW2220 (pDP23), Kmr, Tcr | This study |
| DW2222 | A–S–, DW2220 (pDP24), Kmr, Tcr | This study |
| DW2223 | A–S–, DW2220 (pDP25), Kmr, Tcr | This study |
| DW2224 | A–S–, DW2220 (pDP26), Kmr, Tcr | This study |
| DW2225 | A–S–, DW2220 (pPC7), Kmr, Tcr | This study |
| DW2226 | A–S–, DW2220 (pPC8), Kmr, Tcr | This study |
| DW2227 | A–S–, DW2220 (pPC9), Kmr, Tcr | This study |
| DW2228 | A–S–, DW2220 (pPC10), Kmr, Tcr | This study |
| DW2229 | A–S–, DW2220 (pPC11), Kmr, Tcr | This study |
| DW2230 | A–S–, DW2220 (pPC12), Kmr, Tcr | This study |
| DW2231 | A–S–, DW2220 (pPC13), Kmr, Tcr | This study |
| DW2232 | A–S–, DW2220 (pPC14), Kmr, Tcr | This study |
| DW2233 | A–S–, DW2220 (pPC15), Kmr, Tcr | This study |
| DW2234 | A–S–, DW2220 (pPC16), Kmr, Tcr | This study |
| DW2235 | A–S–, DW2220 (pPC17), Kmr, Tcr | This study |
| DW2236 | A–S–, DW2220 (pPC18), Kmr, Tcr | This study |
| DW2237 | A–S–, DW2220 (pPC19), Kmr, Tcr | This study |
| DW2238 | A–S–, DW2220 (pPC20), Kmr, Tcr | This study |
| DW2239 | A–S–, DW2220 (pPC21), Kmr, Tcr | This study |
| DW2240 | A–S–, DW2220 (pPC22), Kmr, Tcr | This study |
| DW2241 | A–S–, DW2220 (pPC23), Kmr, Tcr | This study |
| DW2242 | A–S–, DW1467 (pPC24), Kmr, Tcr | This study |
Fusion sites for chimera construction are highlighted in bold (superscript).
Table S2.
Primers used in this study
| Primer name | Sequence, 5′–3′* |
| pSWU19-EcoRI-PpilA-F | AGGAAACAGCTATGACCATGATTACGAATTCCGTCATGTTGGACGAGGT |
| pSWU19-XbaI-HindIII-TraA-R | TGCATGCCTGCAGGTCGACTCTAGAAAGCTTGAAGAGCTGCACGTTGAAG |
| Mf205A96-R | GTAACGCTGTTGGTGGTTCGCCAC |
| Mf205A96-F | GTGGCGAACCACCAACAGCGTTAC |
| A96205Mf-R | Same as Mf 205A96-R |
| A96205Mf-F | Same as Mf 205A96-F |
| Mf192A96-R | GTGGCCGGTTGATGACTTGGTAGGCCTGGCTGCGGTCGTAGAT |
| Mf192A96-F | GCCTACCAAGTCATCAACCGG |
| A96192Mf-R | GTGGCCGATTGATGACCTGATATCCTTGGCTGCGGTCGTAGATG |
| A96192Mf-F | GGATATCAGGTCATCAATCGGC |
| Mf192A96205Mf-R | Same as Mf 205A96-R |
| Mf192A96205Mf-F | Same as Mf 205A96-F |
| A96192Mf 205A96-R | Same as Mf 205A96-R |
| A96192Mf 205A96-F | Same as Mf 205A96-F |
| Mf192A96205Mf (A192G)-R | GTGGCCGGTTGATGACTTGGTATCCCTGGCTGCGGTCGTAGAT |
| Mf192A96205Mf (A192G)-F | GGATACCAAGTCATCAACCGGCCAC |
| Mf192A96205Mf (V201M)-R | TGGTTCGCCACGTCGCGAATCCGAGCATCGGTGGCCGGTTGATGAC |
| Mf192A96205Mf (V201M)-F | CTCGGATTCGCGACGTGG |
| Mf192A96205Mf (A205P)-R | GTAACGCTGTTGGTGGTTCGCCACGTTGGGAATCCGAGCACCGGTGG |
| Mf192A96205Mf (A205P)-F | Same as Mf 205A96-F |
| DK1622 (A→P)-R | TGGTCGTGCGCCAGGACGGGATTCCGAG |
| DK1622 (A→P)-F | CCACCGCAGCTCGGAATCCCGTCCTGGCGCACGA |
| DK816 (P→A)-R | GTCCGCCAGGTGGCCGCTCCTAGCTGGGGCG |
| DK816 (P→A)-F | AGCTAGGAGCGGCCACCTGGCGGACGACC |
| Pali (A→P)-R | GCCAAGTGGGCGCACCTAGCTCAGGCGG |
| Pali (A→P)-F | CCTGAGCTAGGTGCGCCCACTTGGCGGACGAGCA |
| DK805 (A→P)-R | GCCAGGTGGGCGCACCTAGCACTGGTGGG |
| DK805 (A→P)-F | CCAGTGCTAGGTGCGCCCACCTGGCGGTCGACCAAC |
| TraA-RBS-XbaI-F | GACGACTCTAGAGGAAACCAAGAATAGAAATAGAAAGGAGAATTAGTGGGAGATATCCCTCATTG |
| TraA-HindIII-R | GACGACAAGCTTGAAGAGCTGCACGTTGAAG |
| TraB-RBS- XbaI-F | GACGACTCTAGAGGATCGACGCCTATATAGACGGGAGATATAATGAAGCCCTCGCACCTG |
| TraB-HindIII-R | GACGACAAGCTTGGAGTTCTTCACCTCGGACTC |
| ΔtraA-Upstr-HindIII-F | CACACAAAGCTTGGGTGCTCTTCGAGGTGAT |
| ΔtraA-Upstr-EcoRI-PstI-R | CACACAGAATTCATCCTGCAGTGGTGAAGGAGCTACGGGA |
| ΔtraB-Dwnstr-PstI-F | CACACACTGCAGGAGGACAAGAAGCGCAAG |
| ΔtraB-Dwnstr-EcoRI-R | CACACAGAATTCCTCAATTCGCTGGACGTG |
| TraA ΔSS-RBS-XbaI-F | GACGACTCTAGAGGAAACCAAGAATAGAAATAGAAAGGAGAATTAATGCAGCCAGAGCCAGGCGAA |
| TraA (Mf)-RBS-XbaI-F | GACGACTCTAGAGGAAACCAAGAATAGAAATAGAAAGGAGAATTAATGGACGATATCCCTCATTCT |
| TraB (Mf)-HindIII-R | GACGACAAGCTTAGCTTGAGTCACTTCGCG |
| TraA-Upstr-R | ATATCTCCCATTAATTCTCCTTTCTATTTCTATTCTTGGTTTCC |
| TraA (different alleles)-F | AGAAAGGAGAATTAATGGGAGATATCCCTCATTGTTGCGG |
| TraA (different alleles)-R | ACGATGAGGAACAGGTGCGAGGGCTTCATCGAGCAGGGCGCCG |
| TraA (Mf)-R | ACGATGAGGAACAGGTGCGAGGGCTTCATCGGGCGGTGCGCCGGGTGCGGAGGAGCACGAA |
| TraB (DK1622)-F | AGCCCTCGCACCTGTTCC |
| TraB (DK1622)-HindIII-R | TGCATGCCTGCAGGTCGACTCTAGAAAGCTTGGAGTTCTTCACCTCGGACTC |
| pKSAT-HindIII-PpilA-F | ATGAGGATCGTTTCATATGAAGCTTCGTCATGTTGGACGAGGTC |
| pKSAT-PpilA-R | CTTGCTCACCATGGGGGTCCTCAGAGAAGG |
| EYFP-F | TCTGAGGACCCCCATGGTGAGCAAGGGCGAG |
| EYFP-EcoRI-R | TGGATCCCCCGGGCTGCAGGAATTCTTACTTGTACAGCTCGTCCATGCC |
Restriction sites are underlined; codons that are subjected to site-directed mutagenesis are in bold.
Cell–Cell Adhesion Assay.
Strains were grown to midlog phase and were mixed in indicated combinations at a density of 1 × 107 cells per mL. Cocultures were incubated overnight with vigorous shaking, and cell suspensions were directly mounted on glass slides and observed by phase contrast/fluorescence microscopy.
Stimulation Assay.
Cells were grown to midlog phase, harvested, and resuspended to the calculated density of ∼2.5 × 109 cells per mL. The nonmotile donor cells (DW1467 background) could not be stimulated, whereas the recipient cells (DW2220 background) could be stimulated, were mixed at 1:1 ratio, and 5 µL of the mixtures were spotted onto casitone agar containing 2 mM CaCl2. To test specificity of recognition, the endogenous traA gene was deleted and heterologous traA alleles were expressed from the Mx8 attB locus (Table S1). The plates were incubated at 33 °C overnight. Colony edges were imaged by phase contrast microscopy with a 10× objective lens.
Immunological Methods.
Protease accessibility assay was essentially done as in ref. 19 with a few modifications. TraA immunofluorescence was performed as previously described (4). SI Materials and Methods for details.
Additional details are in SI Materials and Methods.
SI Materials and Methods
Bacterial Strains and Growth Conditions.
Bacterial strains and plasmids used in this study are listed in Table S1. M. xanthus was routinely cultured in the dark at 33 °C with shaking in CTT medium (1% casitone, 10 mM Tris–HCl, pH 7.6, 1 mM KH2PO4, 8 mM MgSO4) to midlog phase. For 1/2 CTT (stimulation assays), casitone was reduced to 0.5%. TPM buffer (10 mM Tris–HCl, pH 7.6, 1 mM KH2PO4, 8 mM MgSO4) was used to wash cells. Escherichia coli was grown at 37 °C with shaking in LB medium. We added 1.5% (wt/vol) agar to the above media to prepare plates. Antibiotics were added as necessary: 50 µg/mL kanamycin (Km) for both M. xanthus and E. coli, 50 µg/mL streptomycin (Sm) for E. coli, and 300 µg/mL Sm for M. xanthus.
Plasmid and Strain Construction.
Primers used in this study are listed in Table S2. To create traA-, traB-, or traAB-containing plasmids, various alleles with a synthetic ribosomal binding site (RBS) (33) were PCR-amplified from genomic DNA of Myxococcus isolates (4, 29), and the amplicons were cloned downstream of the pilA promoter (PpilA) in pDP22 (between XbaI and HindIII). To create pPC3 (traADK1622 ΔSS), primers were designed that removed the signal sequence and the amplicon was subcloned into pDP22. For heterologous expression plasmids (pPC5, 6, and 24), fragments of the PpilA, the respective traA alleles with a synthetic RBS, and the traBDK1622 gene were first independently amplified. The resulting three amplicons, containing ∼25-bp overlaps between adjacent DNA fragments, were cloned into a linearized pDP22 vector (EcoRI and XbaI digestion) in Gibson Assembly Master Mix (New England Biolabs). To create pPC2, the PpilA and EYFP fragments were PCR-amplified and cloned into a linearized pXW6 vector (digested with HindIII and EcoRI) through Gibson assembly. To create traA chimeric alleles, various subregions were PCR-amplified from specific traA alleles, and the amplicons containing ∼25-bp overlaps were ligated within the linearized pDP22 vector (EcoRI and XbaI) through Gibson assembly to generate pPC7–12. Site-directed mutagenesis was done by dividing traA into two fragments by PCR, and ∼25-bp overlaps containing the desired mutation were designed into the primer sequences, and the amplified fragments were ligated with linearized pDP22 vector (EcoRI and XbaI) through Gibson assembly to generate pPC13–15 and -17–20. All cloning was done in DH5α, and the plasmids were verified by colony PCR and if necessary by DNA sequencing. Verified plasmids were electroporated into M. xanthus and selected by antibiotic resistance. The cloning vectors pDP22 and pKSAT were integrated into the genome at the Mx8 attachment site.
Markerless in-frame deletion of traA was constructed in DW1466 (Δtgl::tc ΔcglC). Briefly, pDP28, containing a traA deletion cassette and a positive–negative (Kmr-galK) selection cassette, was electroporated into DW1466. Homologous recombinants were first selected by Kmr, and then plasmid excision was counterselected on 1% galactose plates. This markerless deletion of traA generated DW2220, which was verified by PCR with flanking primers. To create a traAB deletion cassette (pDP35), the upstream and downstream regions of traAB were PCR amplified from DK1622 genomic DNA and ligated into pBJ114 using appropriate restriction sites. Markerless in-frame deletion of traAB in DK8601 was made in a similar manner as described to create DW1483.
Protease Accessibility Assay.
Live M. xanthus cells were treated with PK (New England Biolabs) at indicated concentrations for 10 min. The reaction was stopped by addition of complete protease inhibitor mixture EDTA-free (Roche). Cells were washed, mixed with SDS sample buffer, and boiled to prepare lysates for immunoblotting. Primary rabbit antibodies used were α-TraA serum (1:50,000 dilution) and α-CglC serum (1:15,000 dilution). A secondary HRP-conjugated goat anti-rabbit antibody (1:15,000 dilution, Pierce) was used for detection. To generate α-CglC serum, the CglC peptide EDLICVRDQDYPRA was synthesized and conjugated to the keyhole limpet hemocyanin (KLH) carrier protein that was used for immunization (Peptibody, Inc.).
Microscopy and Immunofluorescence.
For immunofluorescence labeling of live cells, M. xanthus was first grown to midlog phase, and 5 × 108 cells were harvested and washed with TPM. Cells were then blocked with TPM containing 2% BSA for 30 min. Primary α-TraA serum (1:5,000 dilution) or α-CglC serum (1:1,500 dilution) was then added, and cell suspensions were incubated for 45 min. Cells were then pelleted and resuspended in 150 µL blocking solution (TPM with 2% BSA) containing 1 µL of secondary antibody (Alexa Fluor 488-conjugated donkey anti-rabbit IgG; Jackson ImmunoResearch) for a 45-min incubation in the dark. Following primary and secondary antibody incubations, cells were washed four times in TPM. All incubations were done at room temperature with gentle end-to-end rocking. In the case of CglC, immunofluorescence was also done on fixed and permeabilized cells as essentially described (34) with the following modifications: Cells were fixed with 1.6% paraformaldehyde and 0.025% glutaraldehyde for 10 min. After washing in PBS buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4), cells were permeabilized with GTE buffer (50 mM glucose, 10 mM EDTA, 20 mM Tris, pH 7.5) supplemented with 0.2 µg/mL lysozyme for 5 min on a poly-l-lysine–coated diagnostic slide. Cells were then blocked in PBS containing 2% BSA for 20 min. Primary α-CglC serum (1:1,000 dilution) was added, and cells were incubated for 2 h at room temperature. Secondary Alexa Fluor 488 conjugated donkey anti-rabbit IgG (1:150 dilution) was then added for a 1-h incubation.
Fluorescent microscopy was done with mounts on glass slides and observed under a Nikon E800 phase contrast/fluorescence microscope equipped with a 100× or 60× oil objective lens and FITC or Texas Red filter sets. Some microscopy experiments were done with a Zeiss fluorescence microscope equipped with a 100× oil objective lens and 46HE and 63HE filter sets. SlowFade Antifade Reagents (Thermo Fisher Scientific) were added to the slide before immunofluorescence imaging on fixed and permeabilized cells.
Alignment and 3D Modeling.
Multiple sequence alignments were generated with MUSCLE (35). The 3D structure of TraAM. fulvus variable domain was made with I-TASSER (22), and a model with the highest C-score was selected.
Acknowledgments
We thank Darshan Pathak for reagents, Grant Bowman for use of a microscope, and Chris Vassallo for helpful comments. This work was supported by National Institutes of Health Grant GM101449 (to D.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700315114/-/DCSupplemental.
References
- 1.Green JP, et al. The genetic basis of kin recognition in a cooperatively breeding mammal. Curr Biol. 2015;25(20):2631–2641. doi: 10.1016/j.cub.2015.08.045. [DOI] [PubMed] [Google Scholar]
- 2.Karban R, Shiojiri K. Self-recognition affects plant communication and defense. Ecol Lett. 2009;12(6):502–506. doi: 10.1111/j.1461-0248.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- 3.Karadge UB, Gosto M, Nicotra ML. Allorecognition proteins in an invertebrate exhibit homophilic interactions. Curr Biol. 2015;25(21):2845–2850. doi: 10.1016/j.cub.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pathak DT, Wei X, Dey A, Wall D. Molecular recognition by a polymorphic cell surface receptor governs cooperative behaviors in bacteria. PLoS Genet. 2013;9(11):e1003891. doi: 10.1371/journal.pgen.1003891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wall D. Kin recognition in bacteria. Annu Rev Microbiol. 2016;70(1):143–160. doi: 10.1146/annurev-micro-102215-095325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West SA, Diggle SP, Buckling A, Gardner A, Griffin AS. The social lives of microbes. Annu Rev Ecol Evol Syst. 2007;38(1):53–77. [Google Scholar]
- 7.Strassmann JE, Gilbert OM, Queller DC. Kin discrimination and cooperation in microbes. Annu Rev Microbiol. 2011;65(1):349–367. doi: 10.1146/annurev.micro.112408.134109. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz-Dorado J, Marcos-Torres FJ, García-Bravo E, Moraleda-Muñoz A, Pérez J. Myxobacteria: Moving, killing, feeding, and surviving together. Front Microbiol. 2016;7(781):781. doi: 10.3389/fmicb.2016.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao P, Dey A, Vassallo CN, Wall D. How myxobacteria cooperate. J Mol Biol. 2015;427(23):3709–3721. doi: 10.1016/j.jmb.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velicer GJ, Vos M. Sociobiology of the myxobacteria. Annu Rev Microbiol. 2009;63(1):599–623. doi: 10.1146/annurev.micro.091208.073158. [DOI] [PubMed] [Google Scholar]
- 11.Pathak DT, et al. Cell contact-dependent outer membrane exchange in myxobacteria: Genetic determinants and mechanism. PLoS Genet. 2012;8(4):e1002626. doi: 10.1371/journal.pgen.1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nudleman E, Wall D, Kaiser D. Cell-to-cell transfer of bacterial outer membrane lipoproteins. Science. 2005;309(5731):125–127. doi: 10.1126/science.1112440. [DOI] [PubMed] [Google Scholar]
- 13.Wei X, Pathak DT, Wall D. Heterologous protein transfer within structured myxobacteria biofilms. Mol Microbiol. 2011;81(2):315–326. doi: 10.1111/j.1365-2958.2011.07710.x. [DOI] [PubMed] [Google Scholar]
- 14.Vassallo C, et al. Cell rejuvenation and social behaviors promoted by LPS exchange in myxobacteria. Proc Natl Acad Sci USA. 2015;112(22):E2939–E2946. doi: 10.1073/pnas.1503553112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodgkin J, Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci USA. 1977;74(7):2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassallo CN, Wall D. Tissue repair in myxobacteria: A cooperative strategy to heal cellular damage. BioEssays. 2016;38(4):306–315. doi: 10.1002/bies.201500132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dey A, et al. Sibling rivalry in Myxococcus xanthus is mediated by kin recognition and a polyploid prophage. J Bacteriol. 2016;198(6):994–1004. doi: 10.1128/JB.00964-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob-Dubuisson F, Locht C, Antoine R. Two-partner secretion in Gram-negative bacteria: A thrifty, specific pathway for large virulence proteins. Mol Microbiol. 2001;40(2):306–313. doi: 10.1046/j.1365-2958.2001.02278.x. [DOI] [PubMed] [Google Scholar]
- 19.Jakobczak B, Keilberg D, Wuichet K, Søgaard-Andersen L. Contact- and protein transfer-dependent stimulation of assembly of the gliding motility machinery in Myxococcus xanthus. PLoS Genet. 2015;11(7):e1005341. doi: 10.1371/journal.pgen.1005341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z-F, et al. Genome sequence of the halotolerant marine bacterium Myxococcus fulvus HW-1. J Bacteriol. 2011;193(18):5015–5016. doi: 10.1128/JB.05516-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veelders M, et al. Structural basis of flocculin-mediated social behavior in yeast. Proc Natl Acad Sci USA. 2010;107(52):22511–22516. doi: 10.1073/pnas.1013210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9(1):40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wall D. Molecular recognition in myxobacterial outer membrane exchange: Functional, social and evolutionary implications. Mol Microbiol. 2014;91(2):209–220. doi: 10.1111/mmi.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawkins R. The Selfish Gene. Oxford Univ Press; Oxford, UK: 1976. [Google Scholar]
- 25.Hamilton WD. The genetical evolution of social behaviour. II. J Theor Biol. 1964;7(1):17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- 26.Starich T, Zissler J. Movement of multiple DNA units between Myxococcus xanthus cells. J Bacteriol. 1989;171(5):2323–2336. doi: 10.1128/jb.171.5.2323-2336.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wielgoss S, et al. A barrier to homologous recombination between sympatric strains of the cooperative soil bacterium Myxococcus xanthus. ISME J. 2016;10(10):2468–2477. doi: 10.1038/ismej.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z-H, Jiang D-M, Li P, Li Y-Z. Exploring the diversity of myxobacteria in a soil niche by myxobacteria-specific primers and probes. Environ Microbiol. 2005;7(10):1602–1610. doi: 10.1111/j.1462-2920.2005.00852.x. [DOI] [PubMed] [Google Scholar]
- 29.Vos M, Velicer GJ. Genetic population structure of the soil bacterium Myxococcus xanthus at the centimeter scale. Appl Environ Microbiol. 2006;72(5):3615–3625. doi: 10.1128/AEM.72.5.3615-3625.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goossens KVY, et al. Molecular mechanism of flocculation self-recognition in yeast and its role in mating and survival. MBio. 2015;6(2):e00427-15. doi: 10.1128/mBio.00427-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Podgornaia AI, Laub MT. Determinants of specificity in two-component signal transduction. Curr Opin Microbiol. 2013;16(2):156–162. doi: 10.1016/j.mib.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Hayat S, Peters C, Shu N, Tsirigos KD, Elofsson A. Inclusion of dyad-repeat pattern improves topology prediction of transmembrane β-barrel proteins. Bioinformatics. 2016;32(10):1571–1573. doi: 10.1093/bioinformatics/btw025. [DOI] [PubMed] [Google Scholar]
- 33.Salis HM, Mirsky EA, Voigt CA. Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol. 2009;27(10):946–950. doi: 10.1038/nbt.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leonardy S, Freymark G, Hebener S, Ellehauge E, Søgaard-Andersen L. Coupling of protein localization and cell movements by a dynamically localized response regulator in Myxococcus xanthus. EMBO J. 2007;26(21):4433–4444. doi: 10.1038/sj.emboj.7601877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wall D, Kaiser D. Alignment enhances the cell-to-cell transfer of pilus phenotype. Proc Natl Acad Sci USA. 1998;95(6):3054–3058. doi: 10.1073/pnas.95.6.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]