Abstract
The mechanisms underlying induction of immune dysregulation and chronic fungal infection by a transient tumor necrosis factor alpha (TNF-α) deficiency remain to be defined. The objective of our studies was to determine the potential contribution of neutropenia and immature dendritic cells to the immune deviation. Administration of an anti-TNF-α monoclonal antibody at day 0 neutralized TNF-α only during the first week of a pulmonary Cryptococcus neoformans infection. Transient neutralization of TNF-α resulted in transient depression of interleukin-12 (IL-12), monocyte chemotactic protein 1 (MCP-1), and gamma interferon (IFN-γ) production but permanently impaired long-term clearance of the infection from the lungs even after the levels of these cytokines increased and a vigorous inflammatory response developed. Early neutrophil recruitment was defective in the absence of TNF-α. However, as demonstrated by neutrophil depletion studies, this did not account for the decrease in IL-12 and IFN-γ levels and did not play a role in establishing chronic pulmonary cryptococcal infection. Transient TNF-α neutralization also produced a deficiency in CD11c+ MHC II+ cells and IL-12 in the lymph nodes, potentially implicating a defect in mature dendritic cell trafficking. Transfer of cryptococcal antigen-pulsed immature dendritic cells into naïve mice prior to intratracheal challenge resulted in the development of a nonprotective immune response to C. neoformans that was similar to that observed in anti-TNF-α-treated mice (increased IL-4, IL-5, and IL-10 levels, pulmonary eosinophilia, and decreased clearance). Thus, stimulation of an antifungal response by immature dendritic cells can result in an immune deviation similar to that produced by transient TNF-α deficiency, identifying a new mechanism by which a chronic fungal infection can occur in an immunocompetent host.
The immunologic mechanisms underlying chronic fungal infections in otherwise healthy individuals remain unknown. However, an increasingly frequently reported side effect of immunotherapy with a monoclonal antibody (MAb) against tumor necrosis factor alpha (TNF-α) for rheumatoid arthritis is the subsequent development of fungal infections in these patients (24, 43, 46, 49, 51). In animal models, TNF-α is required to clear infections by Histoplasma, Blastomyces, Candida, Pneumocystis, Aspergillus, Paracoccidioides, and Cryptococcus species (2, 5, 6, 9, 15, 19, 23, 28, 29, 42). The development of T1-cell-mediated immunity is critical for controlling fungal infections, including infection by the ubiquitous encapsulated yeast Cryptococcus neoformans (7, 14).
Production of TNF-α is required for development of T1-cell-mediated immunity to C. neoformans infection (5, 15, 19). Continuous neutralization of TNF-α during the first 2 weeks of C. neoformans infection (via multiple doses of an anti-TNF-α antibody) reduces leukocyte recruitment by 80% and impairs clearance (19). Surprisingly, if a single injection of a TNF-α-neutralizing antibody is given at the time of infection, long-term clearance of C. neoformans still remains defective (19). TNF-α is required for the induction of interleukin 12 (IL-12) and gamma interferon (IFN-γ) (15) and for dendritic cell migration to initiate delayed-type hypersensitivity (DTH) responses (5). Other groups have also shown that IL-12 and IFN-γ are required for host defense against C. neoformans infection (11, 16, 52). Clinically, there have been reports of patients developing cryptococcosis following TNF-α antagonist therapy (46). The mechanisms underlying induction of immune dysregulation and chronic fungal infection by transient TNF-α deficiency remain to be defined.
In many infections, TNF-α is a proximal mediator for neutrophil chemotactic factor production (31, 41). Neutrophils are essential for host defense against several fungi including Candida albicans, Aspergillus fumigatus, and Paracoccidioides brasiliensis (14). In addition to being effective microbicidal cells, neutrophils can produce proinflammatory mediators (8). Depletion of neutrophils at the time of Candida infection results in a T2 response and renders mice susceptible to infection (39, 40). The early recruitment of neutrophils has also been shown to play a role in T1/T2 polarization during Legionella infection (44). In both of these infections, neutrophils can modulate developing immune responses through production of the proinflammatory cytokine IL-12.
TNF-α also promotes the maturation and migration of immature dendritic cells (imDC) from peripheral tissues to the draining lymph nodes, where T-cell clonal expansion is stimulated (3, 5, 10, 20). imDC capture and process antigens but express low levels of major histocompatibility complex class II (MHC II) and costimulatory molecules (CD40, CD80, CD86) on their surfaces. Mature DC present antigens, express high levels of MHC II, CD80, CD86, and CD40 on their surfaces, and produce high levels of IL-12 (3). While mature DC can stimulate polarized T-cell responses (Th1 or Th2), imDC have been proposed to induce regulatory T-cell responses (12, 21, 36, 48).
The objective of our present studies was to determine the potential contribution of neutropenia and imDC to the immune deviation that develops following transient TNF-α deficiency during C. neoformans infection. Our working hypothesis is that initiation of immune responses to fungi by imDC can lead to immune dysregulation and chronic fungal infection.
MATERIALS AND METHODS
Mice.
CBA/J mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Mice were housed under pathogen-free conditions in enclosed filter-top cages at University of Michigan Unit for Laboratory Animal Medicine facilities. Clean food and water were given ad libitum. The mice were handled and maintained by using microisolator techniques with daily veterinarian monitoring. Bedding from the mice was transferred weekly to cages of uninfected sentinel mice that were subsequently bled at weekly intervals and found to be negative for antibodies to mouse hepatitis virus, Sendai virus, and Mycoplasma pulmonis.
C. neoformans.
C. neoformans strain 52D was obtained from the American Type Culture Collection (ATCC 24067). For infection, yeast cells were grown to stationary phase (48 to 72 h) at 37°C in Sabouraud dextrose broth (1% neopeptone, 2% dextrose; Difco, Detroit, Mich.) on a shaker. The cultures were then washed in nonpyrogenic saline, counted on a hemocytometer, and diluted to 3.3 × 105 CFU/ml in sterile nonpyrogenic saline.
Intratracheal inoculation of C. neoformans.
Mice were inoculated as described previously (17). Briefly, a tuberculin syringe (Monoject, St. Louis, Mo.) was filled with the diluted C. neoformans culture. A 30-gauge needle was inserted into the tracheae of anesthetized mice, and 30 μl of inoculum was dispensed into the lungs (104 CFU). Aliquots of the inoculum were collected periodically to monitor the number of CFU delivered.
In vivo antibody administration.
C. neoformans-infected mice were given a single intraperitoneal (i.p.) injection of 0.25 mg of an anti-TNF-α MAb (MP6-XT3) at the time of infection. Neutrophils were depleted by i.p. injection of 0.25 mg of anti-Gr1 (RB6-8C5) on days 0, 2, 4, and 6 of infection. Sterile saline was given i.p. to control mice. Previous studies have shown no difference between saline and rat immunoglobulin G as a control for antibody administration (19).
CFU assay.
For lung CFU, small aliquots were collected from lung digests. Brains were excised, placed in 2 ml of sterile water, and homogenized. Ten-microliter aliquots were plated out on Sabouraud dextrose agar (Difco) plates in duplicate 10-fold dilutions and incubated at room temperature. C. neoformans colonies were counted 2 to 3 days later, and the number of CFU was calculated.
BAL.
Following euthanasia, mice were lavaged by cannulation of the trachea with polyethylene tubing (PE50) attached to a 25-gauge needle on a tuberculin syringe. The lungs were lavaged twice with 0.75 ml of phosphate-buffered saline. The fluid recovered (1.3 to 1.4 ml total) was spun at 1,500 rpm, and the supernatant was removed and stored at −20°C for further analysis. Levels of TNF-α, macrophage inflammatory protein 2 (MIP-2), KC, IL-12, and soluble TNF receptor type II (sTNFRII) in the bronchoalveolar lavage (BAL) fluid were measured by a sandwich enzyme-linked immunosorbent assay (ELISA) by following the manufacturer's instructions supplied with the cytokine-specific kits (OptEIA; PharMingen, San Diego, Calif.).
Preparation of lung leukocytes.
Lung leukocytes were isolated as previously described (18). Briefly, the lungs were excised, minced, and enzymatically digested for 30 min by using 15 ml of digestion buffer (RPMI, 5% fetal calf serum, antibiotics, 1 mg of collagenase/ml)/lung. Lung leukocytes were counted on a hemocytometer with trypan blue.
Cell differentials (neutrophils, macrophages, lymphocytes, eosinophils) were visually counted from Wright-Giemsa-stained samples of lung cell suspensions cytospun onto glass slides (Shandon Cytospin, Pittsburgh, Pa.). The absolute number of a leukocyte subset was equal to the percentage of that cell subset multiplied by the total number of leukocytes in the lungs.
Lung leukocyte culture.
Isolated leukocytes (from enzymatic digests) from individual mice were standardized to 15 × 106 cells/3 ml and cultured in complete medium without additional stimulation at 37°C under 5% CO2. Supernatants were harvested at 24 h and assayed for IFN-γ production by a sandwich ELISA according to the manufacturer's instructions supplied with the cytokine-specific kits (OptEIA; Pharmingen).
Generation of BMDC.
Bone marrow was harvested from femurs and tibias of 8- to 10-week-old CBA/J mice. Red blood cells were lysed on ice for 5 min. Bone marrow cells were cultured in RPMI 1640 (Gibco BRL) supplemented with 10% fetal calf serum, antibiotics, granulocyte-macrophage colony-stimulating factor (10 ng/ml), and IL-4 (10 ng/ml). On day 4, loosely adherent and nonadherent cells were harvested and layered onto a metrizamide gradient (14.5%). DC were collected, counted, and pulsed with cryptococcal mannoprotein (MP) alone (4 h) or MP plus lipopolysaccharide (LPS) (24 h). Following antigen pulsing, bone marrow-derived DC (BMDC) were washed twice with sterile phosphate-buffered saline. Pulsed BMDC (106) were injected into the spleens of naïve CBA/J mice 1 week prior to pulmonary C. neoformans infection.
Preparation of MP.
MP was prepared as outlined previously (35) and was kept sterile for BMDC pulsing (50 μg/ml).
Histology.
Following euthanasia and before removal, lungs were fixed by inflation with 1 ml of 10% neutral buffered formalin. The fixed lung specimens were dehydrated in 70% ethanol and paraffin embedded. Sections were cut, deparaffinized, stained with either hematoxylin and eosin, trichrome, or periodic acid-Schiff stain (PAS), and viewed by light microscopy.
Flow cytometric analysis.
Cell suspensions were made from draining lymph nodes harvested from mice at day 7 postinfection. CD11c+ cells were enriched by magnetic cell sorting with CD11c-conjugated magnetic beads and LS separation columns (Miltenyi Biotech, Auburn, Calif.). Enriched cells were stained with phycoerythrin-labeled CD11c (HL3) and fluorescein isothiocyanate (FITC)-labeled I-Ak (11-5.2). For BMDC analysis, cells were incubated with phycoerythrin-labeled CD11c (HL3) and FITC-labeled CD80 (16-10A1), CD86 (GL1), CD40 (HM40-3), or I-Ak (11-5.2) for 30 min on ice. The samples were washed in staining buffer and fixed in 2.5% paraformaldehyde in buffered saline. Stained samples were analyzed by flow cytometry.
Statistics.
The mean ± standard error (SE) was determined for each treatment group in the individual experiments. Statistical significance was calculated by using a t test with a significance level (P) of <0.05 for a single comparison.
RESULTS
Induction of a transient early TNF-α deficiency.
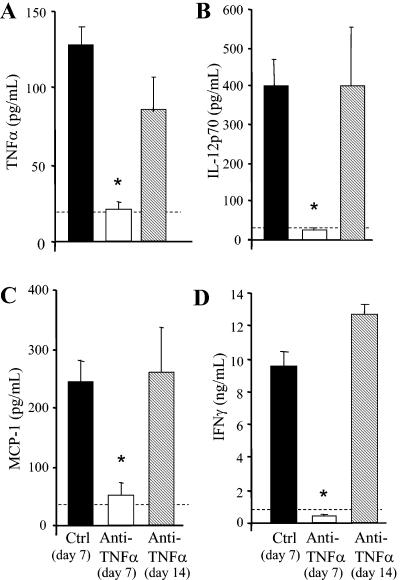
To determine the role of a transient early TNF-α deficiency in the establishment of a chronic pulmonary C. neoformans infection, mice were infected intratracheally with C. neoformans, and an anti-TNF-α MAb was administered by a single i.p. injection at the time of infection (day 0). TNF-α was measured by an ELISA using capture and detection antibodies that recognize different epitopes of the TNF-α protein. Treatment with the anti-TNF-α MAb at the time of infection significantly diminished the levels of TNF-α induced by C. neoformans through day 7 (Fig. 1A). Between days 7 and 14, TNF-α levels in the lavage fluid increased significantly in the anti-TNF-α-treated group of mice. Thus, administration of the anti-TNF-α MAb at day 0 neutralizes TNF-α only during the first week of infection (i.e., in the afferent phase of immunity).
FIG. 1.
Cytokine production in the lungs of anti-TNF-α-treated mice. (A-C) BAL fluid was collected on days 7 and 14 postinfection and was analyzed for TNF-α, IL-12, and MCP-1/CCL2 levels by ELISA. (D) Lung leukocytes were cultured for 24 h without additional stimulation, and supernatants were collected and analyzed by ELISA for IFN-γ levels. Cytokine levels in uninfected lungs are represented by the dashed line. Results are expressed as means ± SEs. n = 8 mice/group/time point from two separate experiments. *, P < 0.05 for comparisons with control mice.
Analysis of the long-term pulmonary inflammatory response that develops when TNF-α induction is delayed.
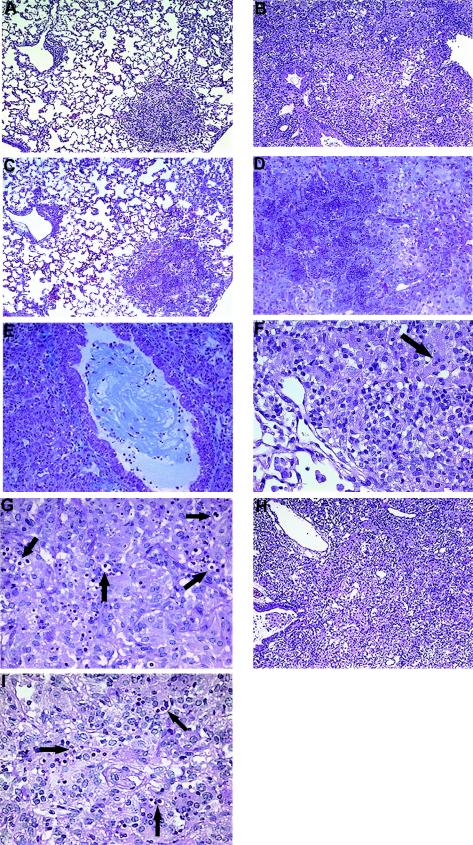
To determine how long the defect in host defense persisted following the transient TNF-α deficiency, histological analysis of the lungs was performed at week 7 postinfection. By this time point, control mice appeared healthy while mice treated with anti-TNF-α (day 0) had ruffled fur and were lethargic. The only discernible feature of the lungs of control-infected mice (relative to uninfected mice) was small foci of inflammation (Fig. 2A). Minimal numbers of cryptococci were detected in control-infected lungs, consistent with clearance of the infection (Fig. 2F). In contrast, there was significant consolidation of the airspaces (>60%) in anti-TNF-α-treated mice, characterized by large numbers of macrophages and neutrophils and a diffuse exudate (Fig. 2B). Evidence of a fibrotic response was also detected in anti-TNF-α-treated mice, characterized by significant collagen deposition in necrotic areas of the lung (Fig. 2D). In addition, many of the airways in anti-TNF-α-treated mice were obstructed by collagen (Fig. 2E). These fibrotic changes were not seen in control-infected animals (Fig. 2C). Large numbers of cryptococci were clearly evident in the lungs of anti-TNF-α-treated mice; the most striking feature was the number of intracellular cryptococci (Fig. 2G). Thus, delayed production of TNF-α following infection permanently impairs host defense (even after TNF-α levels rise), resulting in a chronic pulmonary inflammatory response that is unable to control the growth of the organism. Furthermore, this chronic inflammation induced a marked fibrotic response, further compromising the lungs of anti-TNF-α-treated mice.
FIG. 2.
Photomicrographs of lungs from C. neoformans-infected, anti-TNF-α-treated, and imDC-treated mice at week 7. (A) Control C. neoformans-infected mouse. Note that only a small focus of inflammation is detected at week 7. (B) Anti-TNF-α-treated C. neoformans-infected mouse. Note the consolidation due to the overwhelming inflammatory response. (C) Control C. neoformans-infected lung demonstrating minimal collagen deposition. (D) Necrotic area in the lung of an anti-TNF-α-treated mouse demonstrating marked collagen deposition. (E) An airway in the lung of an anti-TNF-α-treated mouse significantly obstructed by collagen. (F) The presence of intracellular cryptococci (arrow) is minimal in the inflammatory sites in the lung of a control C. neoformans-infected mouse. (G) Detection of significant numbers of intracellular cryptococci (arrows) in the lung of an anti-TNF-α-treated mouse. (H) Lung of an imDC-treated C. neoformans-infected mouse. Note the consolidation due to the inflammatory response. (I) Detection of significant numbers of intracellular cryptococci (arrows) in the lung of an imDC-treated mouse. (A, B, H) Hematoxylin and eosin. Magnification, ×54. (C-E) Trichrome. Magnification, ×108. (F, G, I) PAS. Magnification, ×216.
Role of TNF-α in the induction of IL-12, MCP-1/CC chemokine ligand 2 (CCL2), and IFN-γ.
We next examined whether transient TNF-α deficiency caused a long-term deviation in the early expression of cytokines that drive T1 immunity to C. neoformans. Levels of IL-12 and MCP-1/CCL2 in BAL fluid from anti-TNF-α-treated mice were analyzed by ELISA. No detectable levels of IL-12 or MCP-1/CCL2 were observed at days 2 and 3 postinfection (data not shown). Neutralization of TNF-α at the time of infection markedly inhibited IL-12 and MCP-1/CCL2 induction at day 7 relative to that for control mice (Fig. 1B and C). IFN-γ levels in leukocytes isolated from the lungs of control and anti-TNF-α-treated mice were also assessed by an ELISA. At day 7, leukocytes from anti-TNF-α-treated mice produced very low levels of IFN-γ (Fig. 1D). By day 14, the levels of IL-12, MCP-1/CCL2, and IFN-γ had increased and were greater than or equal to the levels measured in control mice at day 7 (Fig. 1). The increase in TNF-α levels at day 14 in the treated mice was accompanied by a parallel increase in these other T1-promoting cytokines. Thus, transient TNF-α deficiency results in only a transient depression in the production of IL-12, MCP-1, and IFN-γ.
Effect of transient TNF-α deficiency on clearance of C. neoformans.
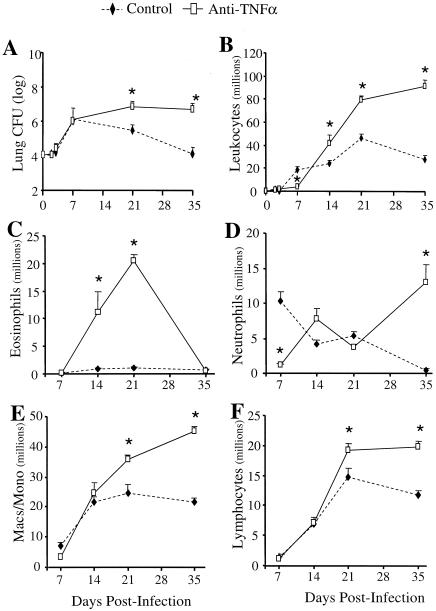
The pulmonary burden of C. neoformans during the course of infection was examined in order to determine whether the transient absence of TNF-α would impair the clearance of cryptococci from the lungs. In control mice, the cryptococcal burden in the lungs increased more than 100-fold from days 0 to 7 and then decreased progressively through days 21 and 35 (Fig. 3A). In contrast, numbers of CFU in the lung remained elevated in anti-TNF-α-treated mice at days 21 and 35 (Fig. 3A). Survival studies were not performed; however, the only mice that succumbed to infection during these experiments were from the anti-TNF-α group (data not shown). Therefore, long-term clearance of the infection from the lungs is impaired by the transient TNF-α deficiency during the first week of infection.
FIG. 3.
Pulmonary clearance and leukocyte recruitment in the lungs of C. neoformans-infected control mice and anti-TNF-α-treated mice. (A) CBA/J mice were infected intratracheally with 104 CFU and treated with an anti-TNF-α MAb on day 0. Total lung CFU were enumerated at days 2, 3, 7, 21, and 35 postinfection. (B) Lung leukocytes were isolated from individual lungs as described in Materials and Methods. Total leukocytes were enumerated, and recruited leukocytes were calculated. (C-F) Samples of individual lung leukocyte suspensions (days 7, 14, 21, and 35 postinfection) were cytospun onto slides and stained with Wright-Giemsa stain for visual quantification of leukocyte subsets. Results are expressed as the mean number per lung ± SE. n = 8 mice/group/time point from two separate experiments. *, P < 0.05 for comparisons with C. neoformans-infected mice. Macs/Mono, macrophages/monocytes.
Role of afferent-phase TNF-α production in leukocyte recruitment.
To determine the mechanism responsible for the impaired pulmonary clearance in anti-TNF-α-treated mice, leukocyte recruitment into the lungs was analyzed. Total lung leukocytes were isolated and counted after enzymatic dispersion of whole lungs as described in Materials and Methods. No cellular influx could be detected in control or anti-TNF-α-treated mice at day 2 or 3 postinfection. Recruitment of inflammatory cells into the lungs was first observed on day 7 post-C. neoformans infection. Control mice developed a vigorous pulmonary inflammatory response by day 21, which began to resolve by day 35 (Fig. 3B), paralleling clearance of the infection. Treatment of mice with an anti-TNF-α MAb at day 0 inhibited leukocyte recruitment into the lungs at day 7. There were 80% fewer leukocytes in the lungs of anti-TNF-α-treated mice than in those of C. neoformans-infected control animals. By day 14, leukocyte recruitment was evident in the lungs of anti-TNF-α-treated mice, and it continued to increase through day 35 (Fig. 3B). These data show that leukocyte recruitment is defective in the absence of TNF-α; however, subsequent production of TNF-α is accompanied by a chronic inflammatory infiltrate in the lungs.
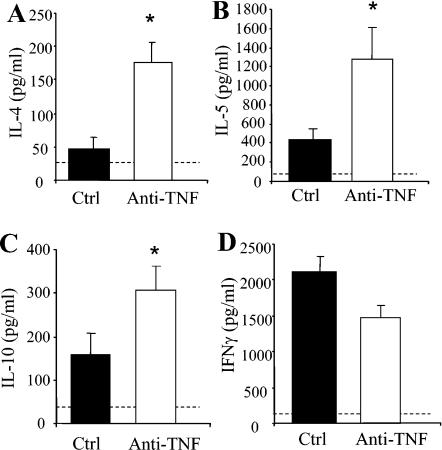
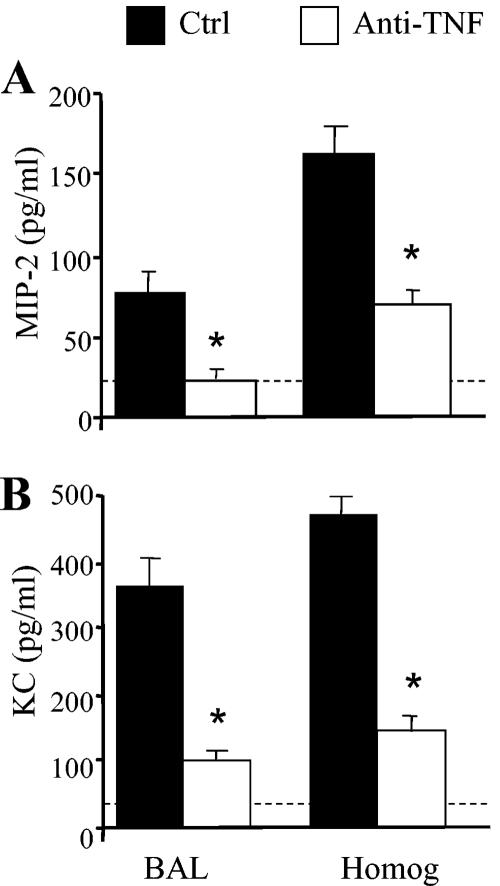
The leukocytic infiltrate was characterized at each time point to determine specific leukocyte populations affected by TNF-α neutralization. A transient eosinophilia developed in the lungs following afferent TNF-α neutralization (Fig. 3C). Whereas control mice had minimal eosinophils in their lungs at all time points, anti-TNF-α-treated mice had a significant influx of eosinophils into their lungs at days 14 and 21 (>20 million eosinophils at day 21) (Fig. 3C). Consistent with the pulmonary eosinophilia, production of IL-4, IL-5, and IL-10 by lung leukocytes was increased at day 14 in anti-TNF-α- but not control-treated mice (Fig. 4A to C). No significant difference in IFN-γ production was observed between the two groups of animals (Fig. 4D). Depletion of CD4 and CD8 T cells abolished eosinophil recruitment (21.3% in the group treated only with anti-TNF-α versus 1.1% in the anti-TNF-α-treated group depleted of CD4 and CD8 cells; P < 0.01). By day 35, the eosinophilia had resolved and the granulocytic infiltrate was predominantly neutrophils (Fig. 3D). This is in contrast to day 7, when there was virtually no recruitment of neutrophils in the lungs of anti-TNF-α-treated mice (Fig. 3D). Analysis of MIP-2 and KC in the BAL fluid and lung homogenates of anti-TNF-α-treated animals revealed a marked reduction in these CXC chemokines at day 7 postinfection (Fig. 5). Therefore, the absence of early neutrophil recruitment in anti-TNF-α-treated animals is likely due to the low levels of MIP-2 and KC.
FIG. 4.
Cytokine production in lungs of anti-TNF-α-treated mice at day 14 post-C. neoformans infection. Leukocytes were isolated from whole lungs at day 14 postinfection and were cultured for 24 h without additional stimulation. Supernatants were collected and assayed by ELISA for IL-4, IL-5, IL-10, and IFN-γ levels. Results are expressed as means ± SEs. n = 8 mice/group from two separate experiments. *, P < 0.05 for comparisons with C. neoformans-infected mice. Cytokine levels in uninfected lungs are represented by the dashed line.
FIG. 5.
Levels of MIP-2 and KC in lungs of control and anti-TNF-α-treated mice. BAL fluid was collected from control and anti-TNF-α-treated mice at day 7 postinfection. Lung homogenates were also prepared from both groups of mice at day 7. Levels of MIP-2 (A) and KC (B) were determined by ELISA. Results are expressed as means ± SEs. n = 5 mice/group. *, P < 0.05 for comparisons with C. neoformans-infected mice.
Macrophage and lymphocyte recruitment was enhanced in anti-TNF-α-treated mice at days 21 and 35 postinfection (Fig. 3E and F). T cells isolated from the lung-associated lymph nodes (day 7) and the lungs (day 14) of control and anti-TNF-α-treated mice were analyzed for T-cell receptor Vβ skewing during cryptococcal infection. No differences in T-cell Vβ usage in either the lymph nodes or the lung were observed between treatment groups (data not shown). Therefore, the development of chronic C. neoformans infection following transient depletion of TNF-α is not due to skewing of the T-cell repertoire. Overall, the recruitment data demonstrate that early TNF-α deficiency causes a transient pulmonary eosinophilia followed by an influx of neutrophils and large numbers of mononuclear cells as the infection progresses.
Effect of neutrophil depletion during the afferent phase of C. neoformans infection.
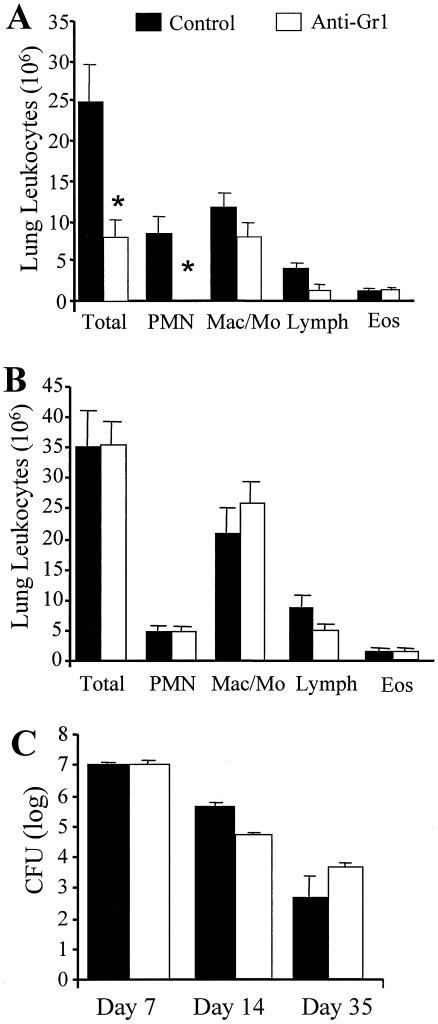
The most striking afferent cellular change was inhibition of neutrophil recruitment (Fig. 3D). We next examined whether this neutrophil deficit was responsible for the development of a chronic fungal infection in anti-TNF-α-treated mice. An anti-Gr1 antibody was injected into mice on days 0, 2, 4, and 6 of infection in order to deplete neutrophils throughout the first week of C. neoformans infection. Lung leukocyte recruitment was significantly reduced at day 7, a finding that could be attributed predominantly to the absence of neutrophils in these mice (Fig. 6A). By week 2, leukocyte recruitment was similar in control and anti-Gr1-treated mice. There were no differences in the proportions of neutrophils, monocytes/macrophages, lymphocytes, or eosinophils (Fig. 6B).
FIG. 6.
(A and B) Recruitment of lung leukocytes in neutrophil-depleted mice at days 7 (A) and 14 (B) post-C. neoformans infection. Mice were treated with an anti-Gr1 antibody on days 0, 2, 4, and 6. Samples of individual lung leukocyte suspensions were cytospun onto slides and stained with Wright-Giemsa stain for visual quantification of leukocyte subsets. Results are expressed as the mean number per lung ± SE. n = 8 mice/group/time point from two separate experiments. (C) CFU in the lungs of neutrophil-depleted mice were examined at days 7, 14, and 35 post-C. neoformans infection. Aliquots from lung digests were plated onto Sabouraud dextrose agar in 10-fold dilutions, and total numbers of CFU per lung were determined. Results are expressed as means ± SEs. n = 8 mice/group/time point from two separate experiments. *, P < 0.05 for comparisons with C. neoformans-infected control mice.
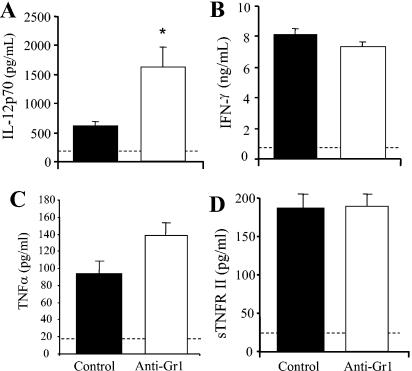
Transient neutropenia did not interfere with the development of protective immunity to pulmonary C. neoformans infection. Clearance of C. neoformans at day 7 postinfection was not compromised in the absence of neutrophils (Fig. 6C). IL-12p70 and IFN-γ production was not inhibited in anti-Gr1-treated mice (Fig. 7A and B). Levels of IL-12p70 actually increased in these mice, potentially contributing to the slightly increased clearance of the infection between weeks 1 to 2 in neutrophil-depleted mice (Fig. 6C). In addition, levels of TNF-α and sTNFRII in the lungs of control and anti-Gr1-treated mice were similar (Fig. 7C and D). Levels of T2-type cytokines (IL-4, IL-5, and IL-10) were not elevated in the lungs of anti-Gr1-treated mice (data not shown). By week 5, clearance was observed in both groups of mice, and the difference between the groups was not significant (Fig. 6C). These data demonstrate that the inhibition of early neutrophil influx following TNF-α neutralization did not account for the decrease in IL-12p70 and IFN-γ levels (Fig. 1) or play a role in establishing a chronic pulmonary cryptococcal infection (Fig. 2).
FIG. 7.
Levels of IL-12p70, IFN-γ, TNF-α, and sTNFRII in neutrophil-depleted mice at day 7 post-C. neoformans infection. (A, C, D) Mice were treated with an anti-Gr1 antibody on days 0, 2, 4, and 6. BAL fluid was collected from control and anti-Gr1-treated mice at day 7 postinfection. Levels of IL-12p70 and sTNFRII were determined by ELISA. (B) Leukocytes were isolated from whole lungs at day 7 postinfection and were cultured for 24 h without additional stimulation. Supernatants were collected and assayed for IFN-γ by ELISA. Results are expressed as means ± SEs. n = 10 mice/group from two separate experiments. *, P < 0.05 for comparisons with C. neoformans-infected control mice. Dashed line indicates cytokine levels in uninfected mice.
Effect of DC maturation on the protective immune response to C. neoformans.
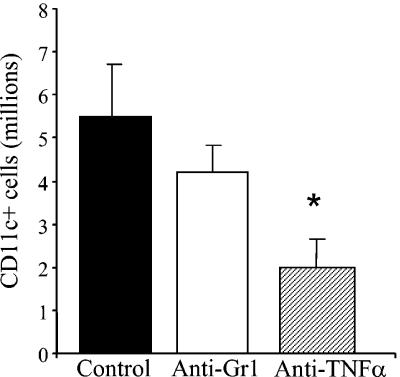
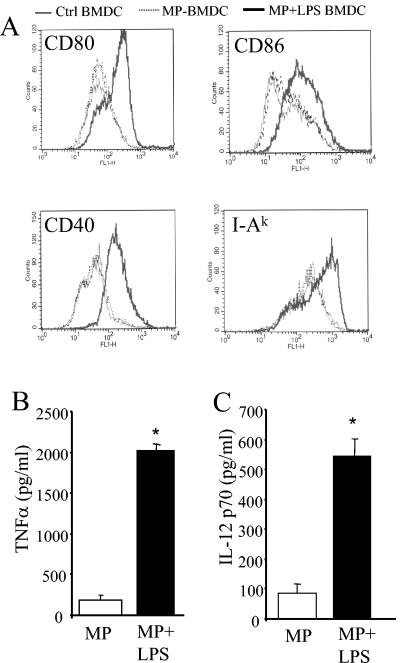
Analysis of the lung-associated lymph nodes of anti-TNF-α-treated mice revealed a significant reduction in the number of CD11c+ cells relative to that in control-infected mice (Fig. 8). In addition, IL-12 levels were significantly lower in the lymph nodes of anti-TNF-α-treated mice at day 7 postinfection (data not shown). Therefore, to determine whether imDC could be involved in the development of chronic fungal infection, BMDC were pulsed with MP and injected into naïve mice. BMDC were pulsed either with MP alone (4 h) or with MP plus LPS (24 h), and their phenotype was characterized prior to injection. These BMDC were CD11c+ CD11b+ myeloid-type DC as determined by flow cytometry analysis. Maturation was determined by expression of CD80, CD86, CD40, and MHC II on the surface. As shown in Fig. 9A, expression of these markers was significantly increased on DC pulsed with MP plus LPS. DC pulsed with MP alone displayed a phenotype identical to that of unpulsed DC. Furthermore, induction of TNF-α and IL-12 production was observed only for MP-plus-LPS-pulsed DC (Fig. 9B and C). Based on these data, we concluded that MP-pulsed BMDC exhibit an immature phenotype whereas MP-plus-LPS-pulsed BMDC exhibit a mature phenotype.
FIG. 8.
Analysis of CD11c+ cells from lymph nodes of control-, anti-TNF-α-, and anti-Gr1-treated mice at day 7. CD11c+ cells were enriched from lymph node cell suspensions by magnetic cell sorting. To calculate the number of CD11c+ cells, the percentage of CD11c+ cells was determined by flow cytometry and multiplied by the total number of cells. *, P < 0.05 for comparisons with C. neoformans-infected control mice.
FIG. 9.
Characterization of mannoprotein-pulsed BMDC. (A) BMDCwere pulsed with MP (4 h) or MP plus LPS (24 h). Cells were stained with FITC-conjugated antibodies specific for CD80, CD86, CD40, and I-Ak and were analyzed by flow cytomety. Expression of CD80, CD86, CD40, and I-Ak was analyzed on CD11c+cells. (B, C) Supernatants were collected from pulsed BMDC cultures and were assayed for TNF-α and IL-12 p70 by ELISA. *, P < 0.05 for comparisons with MP-pulsed BMDC.
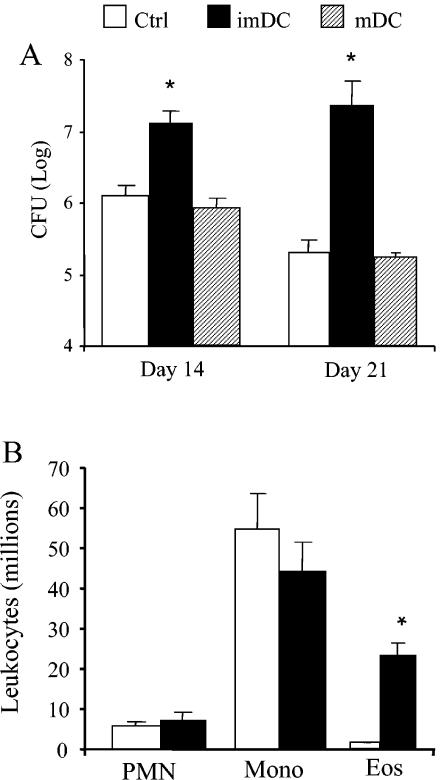
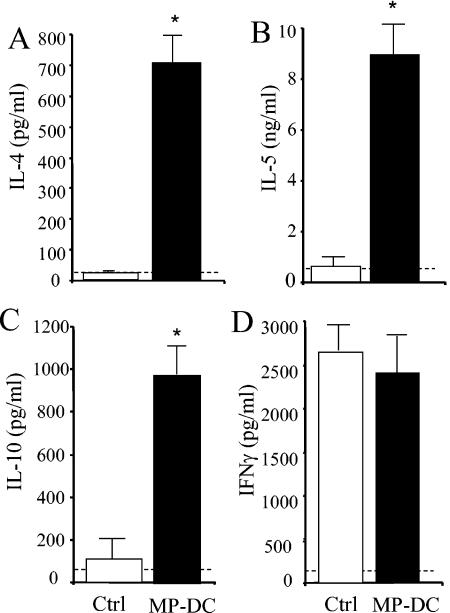
We next evaluated whether transfer of MP-pulsed imDC would alter the immune response to a pulmonary cryptococcal infection. CBA/J mice were intratracheally infected with C. neoformans 7 days after transfer of MP-pulsed imDC, and the immune response was examined at day 14 postinfection. The pulmonary cryptococcal burden was significantly higher in mice with MP-imDC than in control-infected animals at days 14 and 21 (Fig. 10A). In contrast, numbers of CFU in the lungs of mice receiving MP-pulsed mature DC were similar to those for control-infected mice, demonstrating that maturation of the DC could reverse the immune deviation but did not augment clearance. Recruitment of leukocytes into the lungs was increased in the MP-imDC group. Differential analysis of leukocyte subsets revealed eosinophilia in the lungs of MP-imDC mice, whereas minimal numbers of eosinophils were detected in those of control-infected mice (Fig. 10B). Recruitment of neutrophils and mononuclear cells (i.e., macrophages and lymphocytes) was similar in both groups. The cytokine profile in the lungs at day 14 postinfection was also examined. While IFN-γ levels were similar in control and MP-imDC mice, levels of IL-4, IL-5, and IL-10 were all significantly elevated in MP-imDC animals during C. neoformans infection (Fig. 11). Therefore, transfer of MP-pulsed imDC prior to pulmonary infection results in up-regulation of the T2 response, preventing the development of protective immunity to C. neoformans.
FIG. 10.
Pulmonary clearance and leukocyte recruitment following C. neoformans infection. (A) Aliquots from day-14 and day-21 lung digests from both treatment groups were plated onto Sabouraud dextrose agar in 10-fold dilutions, and total numbers of CFU per lung were determined. Results are expressed as means ± SEs. n = 8 mice/group/time point from two separate experiments. (B) Samples of individual lung leukocyte suspensions were cytospun onto slides and stained with Wright-Giemsa stain for visual quantification of leukocyte subsets. Results are expressed as the mean number per lung ± SE. n = 8 mice/group/time point from two separate experiments. *, P < 0.05 for comparisons with C. neoformans-infected control mice. mDC, mature DC; PMN, polymorphonuclear leukocytes; Mono, mononuclear cells; Eos, eosinophils.
FIG. 11.
Cytokine profile in the lungs of MP-DC recipient mice at day 14 post-C. neoformans infection. Leukocytes were isolated from whole lungs at day 14 postinfection and were cultured for 24 h without additional stimulation. Supernatants were collected and assayed for IL-4, IL-5, IL-10, and IFN-γ by ELISA. Results are expressed as means ± SEs. n = 8 mice/group from two separate experiments. *, P < 0.05 for comparisons with C. neoformans-infected control mice. Cytokine levels in uninfected lungs are represented by dashed lines.
To determine the long-term effects of MP-imDC transfer on the host response to C. neoformans infection, histological analysis of the lungs was performed at week 7 postinfection. As shown in Fig. 2H (compared to Fig. 2A), an overwhelming inflammatory response resulted in significant consolidation of the airspaces of imDC recipient mice but not in control mice. The cellular infiltrate in imDC recipient mice was characterized by large numbers of macrophages and neutrophils. Large numbers of cryptococci were also observed in the lungs of imDC recipient mice (Fig. 2I). The magnitudes of the inflammatory responses and cryptococcal burdens in the lungs of imDC-recipient mice were similar to those found in the lungs of anti-TNF-α-treated mice (Fig. 2B and G). Thus, like early transient anti-TNF-α treatment, transfer of imDC also results in chronic fungal infection and inflammation.
DISCUSSION
These are the first reported studies to find the modulation of an immune response to fungal infection by imDC. Our data demonstrate that injection of MP-pulsed imDC inhibited protective T1 immunity to a pulmonary cryptococcal infection. The immune response in animals receiving the imDC was shifted toward a T2-type response characterized by elevated IL-4, IL-5, and IL-10 levels and eosinophilia in the lungs. These studies have also shown that the absence of TNF-α during the afferent phase of the immune response to C. neoformans results in chronic fungal infection in the lungs.
TNF-α is required for the induction of IL-12, IFN-γ, and MCP-1/CCL2 in the lungs following C. neoformans infection. It has recently been demonstrated that TNF-α neutralization at the time of infection alters the IFN-γ/IL-4 ratio in the lung-associated lymph nodes at day 7, causes a rise in serum immunoglobulin E levels, and prevents the development of C. neoformans-specific delayed-type hypersensitivity (15). IL-12, in turn, is required for the induction of IFN-γ in the lungs during C. neoformans infection. MCP-1/CCL2 does not play a role in the afferent phase of the immune response but is a key efferent-phase mediator of T1 immunity to C. neoformans (18, 45). Altogether, if TNF-α production is delayed, a dysregulated host response develops in the lungs (even after TNF-α, IL-12, IFN-γ, and MCP-1/CCL2 levels rise) due to a shift in the T1/T2 balance of the immune response. The host defense against the infection is permanently impaired, leading to a chronic C. neoformans infection.
In our present study, MP-pulsed DC failed to produce IL-12 and were phenotypically immature based on low expression of CD80, CD86, CD40, and MHC II. We injected our imDC directly into the spleen because the absolute number of FITC+ MHC II+ cells following intranasal FITC administration was greatest in the spleen (50). Transfer of these imDC prior to C. neoformans infection disrupts the T1/T2 balance in the lungs, resulting in pulmonary eosinophilia, elevated T2 cytokine levels, and increased cryptococcal burden. A major function of imDC is to induce tolerance (4); therefore, one interpretation of our data is that immature MP-pulsed DCs are inducing tolerance to the C. neoformans antigen. As a result of this unresponsiveness, a chronic pulmonary infection develops. imDC can also induce T cells with regulatory properties (21, 36, 48). IL-10-producing DC induce pathogen-specific T-regulatory cells that inhibit protective T1 responses in the respiratory tract (26). Furthermore, injection of antigen-pulsed imDC leads to inhibition of effector T-cell function (12). Based on this evidence, we hypothesize that injection of MP-pulsed imDC induces T-regulatory cells in our model, leading to immune deviation and chronic infection. Future studies will address the involvement of regulatory T cells during C. neoformans infection.
The parallels between the MP-pulsed DC model and the anti-TNF-α model are striking. Both groups of animals develop nonprotective immunity to C. neoformans, resulting in impaired clearance from the lungs. Inflammatory stimuli are crucial for DC maturation and subsequent induction of naïve T cells to develop into effector cells. Several lines of evidence demonstrate the importance of TNF-α in promoting the maturation of DC. For example, maturation of wild-type BMDC is inhibited by administration of an anti-TNF-α antibody (38). DC isolated from the bone marrow of TNF-α−/− mice remain in an immature phenotype (38), and similar findings have been described for TNF LTα LTβ triple-knockout mice (1). TNF-α also plays a central role in DC migration to draining lymph nodes. Injection of TNF-α into tissue significantly increased the migration of DC to the nodes (25). This increase in antigen-presenting DC correlated with the magnitude and quality of the T-cell immune response (25). Analysis of the lung-associated lymph nodes of anti-TNF-α-treated mice revealed a reduction in the number of CD11c+ cells relative to that for control-infected mice (Fig. 8). In addition, IL-12 levels were significantly lower in the lymph nodes of anti-TNF-α-treated mice at day 7 postinfection (data not shown). The reduction in CD11c+ antigen-presenting cells and the cytokine milieu in the lymph node following TNF-α neutralization likely contribute to the immune deviation reported for these mice (15, 19). Based on the evidence given above, we speculate that modulation of immunity by imDC is one potential mechanism for the development of chronic fungal infection in anti-TNF-α-treated mice. Anti-TNF-α-treated mice are immunologically “normal” after the first week of infection but are no longer able to clear the infection. Therefore, a critical early window for TNF-α production exists and is required in order for the host to develop protective immunity against the fungus.
The early influx of neutrophils into the lungs does not play a role in the development of a protective T1 response to C. neoformans infection. Early recruitment of neutrophils is required for the development of protective immunity to C. albicans, Legionella pneumophila, and Mycobacterium avium, because neutrophils are a major source of early IL-12 during these infections (37, 39, 44). Our present studies show that neutrophil depletion did not compromise the development of T1 protective immunity or reduce the number of CD11c+ cells in the lymph nodes (Fig. 8), unlike TNF-α neutralization. Recently, Mednick and colleagues reported that neutropenia early in the course of C. neoformans infection changes the inflammatory response to benefit the host (27). Levels of IL-10, IL-4, and IL-12 in the lungs were elevated in neutropenic animals, and IFN-γ production was similar to that of control mice, at day 7 (27). We have also shown that levels of IL-12 are elevated and those of IFN-γ are unchanged in the lungs of neutrophil-deficient mice at day 7 post-C. neoformans infection. Therefore, neutropenic mice are able to control a cryptococcal infection. The early absence of neutrophils does not contribute to the development of chronic C. neoformans infection, because T1 immunity still develops in neutrophil-depleted animals.
Down-regulation of TNF-α is likely a virulence mechanism used by fungi to evade host defenses. TNF-α is required for protective immunity against the fungi Histoplasma capsulatum, Blastomyces dermatitidis, C. albicans, Pneumocystis carinii, A. fumigatus, and Paracoccidioides immitis (2, 9, 28, 29, 42). Fungal virulence factors, such as production of polysaccharide capsule, melanin, or prostaglandin-like molecules, can down-regulate TNF-α production by leukocytes (17, 30, 32, 47). Production of prostaglandin E and prostaglandin D lipids appears to be a common trait of all pathogenic fungi including C. neoformans (33). In agreement with these observations, a number of highly virulent isolates of C. neoformans do not induce early TNF-α production following infection (22, 34). Inhibition of TNF-α production by the virulence factor W-1 of B. dermatitidis has also been reported (13). Thus, fungi produce a number of virulence factors capable of down-regulating TNF-α production by the host, thereby promoting chronic infection.
The mechanism underlying chronic fungal infection in healthy individuals remains an enigma. Often, the terms “immunologic unresponsiveness, ” “anergy, ” or “immune dysregulation” are used to describe the host response. Our studies suggest that interruption of key early signals may underlie such susceptibility in otherwise healthy individuals. Thus, the timing of cytokine signals is more important than the simple presence of a cytokine during infection in determining whether a protective host response develops. While most of the concepts about the importance of TNF-α in antifungal host defense in humans have been extrapolated from animal models of fungal infection, the increasing use of the anti-TNF-α MAb infliximab has confirmed the critical role of this inflammatory mediator in antifungal host defense. There are numerous reports of both reactivation and primary fungal infections in rheumatoid arthritis patients being treated with anti-TNF-α therapy, including infections due to Histoplasma, Aspergillus, Pneumocystis, and Cryptococcus (24, 43, 46, 49, 51). Thus, even though TNF-α is a mediator of inflammatory diseases, it is also a critical mediator of protective immunity against fungal infections. Delayed induction of early TNF-α production due to virulence factors, secondary infections, immunotherapy, or trauma may promote fungal infection by (i) induction of an ineffective cellular immune response (shift in the T1/T2 balance) or (ii) induction of regulatory T cells by imDC. These results provide a starting point for studying the regulatory mechanisms underlying cryptococcal infections in immunocompetent patients. Furthermore, our findings have broader implications for understanding mechanisms by which chronic fungal infections develop in immunocompetent patients.
Acknowledgments
This work was supported in part by the National Heart, Lung, and Blood Institute (R01-HL65912 and R01-HL63670 [to G.B.H.], R01-HL51082 [to G.B.T.], and T32-HL07749 [to A.C.H.]), the Burroughs-Wellcome Fund (to G.B.H.), and a Department of Veterans Affairs Merit Grant (to G.B.T.).
Editor: T. R. Kozel
REFERENCES
- 1.Abe, K., F. O. Yarovinsky, T. Murakami, A. N. Shakhov, A. V. Tumanov, D. Ito, L. N. Drutskaya, K. Pfeffer, D. V. Kuprash, K. L. Komschlies, and S. A. Nedospasov. 2003. Distinct contributions of TNF and LT cytokines to the development of dendritic cells in vitro and their recruitment in vivo. Blood 101:1477-1483. [DOI] [PubMed] [Google Scholar]
- 2.Allendoerfer, R., and G. J. Deepe. 1998. Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J. Immunol. 160:6072-6082. [PubMed] [Google Scholar]
- 3.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. Liu, B. Pulendran, and K. Palucka. 2001. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Bauman, S., G. B. Huffnagle, and J. Murphy. 2003. Effects of tumor necrosis factor alpha on dendritic cell accumulation in lymph nodes draining the immunization site and the impact on the anticryptococcal cell-mediated immune response. Infect. Immun. 71:68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brieland, J., C. Jackson, F. Menzel, D. Loebenberg, A. Cacciapuoti, J. Halpern, S. Hurst, T. Muchamuel, R. Debets, R. Kastelein, T. Churakova, J. Abrams, R. Hare, and A. O'Garra. 2001. Cytokine networking in lungs of immunocompetent mice in response to inhaled Aspergillus fumigatus. Infect. Immun. 69:1554-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington, D.C.
- 8.Cassatella, M. A. 1995. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today 16:21-25. [DOI] [PubMed] [Google Scholar]
- 9.Chen, W., E. A. Havell, and A. G. Harmsen. 1992. Importance of endogenous tumor necrosis factor alpha and gamma interferon in host resistance against Pneumocystis carinii infection. Infect. Immun. 60:1279-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cumberbatch, M., and I. Kimber. 1995. Tumor necrosis factor-alpha is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology 84:31. [PMC free article] [PubMed] [Google Scholar]
- 11.Decken, K., G. Kohler, K. Palmer-Lehmann, A. Wunderlin, F. Mattner, J. Magram, M. K. Gately, and G. Alber. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 66:4994-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhodapkar, M., R. Steinman, J. Krasovsky, C. Munz, and N. Bhardwaj. 2001. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkel-Jimenez, B., M. Wuthrich, T. Brandhorst, and B. S. Klein. 2001. The WI-1 adhesin blocks phagocyte TNF-α production, imparting pathogenicity on Blastomyces dermatitidis. J. Immunol. 166:2665-2673. [DOI] [PubMed] [Google Scholar]
- 14.Herring, A. C., and G. B. Huffnagle. 2001. Innate immunity and fungal infections, p. 127-137. In A. S. Stefan, H. E. Kaufmann, and Rafi Ahmed (ed.), Immunology of infectious diseases. American Society for Microbiology, Washington, D.C.
- 15.Herring, A. C., J. Lee, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2002. Induction of interleukin-12 and gamma interferon requires tumor necrosis factor alpha for protective T1-cell-mediated immunity to pulmonary Cryptococcus neoformans infection. Infect. Immun. 70:2959-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoag, K. A., M. F. Lipscomb, A. A. Izzo, and N. E. Street. 1997. IL-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant CB-17 mice. Am. J. Respir. Cell Mol. Biol. 17:733-739. [DOI] [PubMed] [Google Scholar]
- 17.Huffnagle, G. B., G. H. Chen, J. L. Curtis, R. A. McDonald, R. M. Strieter, and G. B. Toews. 1995. Downregulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J. Immunol. 155:3507-3516. [PubMed] [Google Scholar]
- 18.Huffnagle, G. B., R. M. Strieter, T. J. Standiford, R. A. McDonald, M. D. Burdick, S. L. Kunkel, and G. B. Toews. 1995. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J. Immunol. 155:4790-4797. [PubMed] [Google Scholar]
- 19.Huffnagle, G. B., G. B. Toews, M. D. Burdick, M. B. Boyd, K. S. McAllister, R. A. McDonald, S. L. Kunkel, and R. M. Strieter. 1996. Afferent phase production of TNF-α is required for the development of protective T cell immunity to Cryptococcus neoformans. J. Immunol. 157:4529-4536. [PubMed] [Google Scholar]
- 20.Jonuleit, H., J. Knop, and A. Enk. 1996. Cytokines and their effects on maturation, differentiation, and migration of dendritic cells. Arch. Dermatol. Res. 289:1. [DOI] [PubMed] [Google Scholar]
- 21.Jonuleit, H., E. Schmitt, G. Schuler, J. Knop, and A. H. Enk. 2000. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192:1213-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawakami, K., X. Qifeng, M. Tohyama, M. H. Qureshi, and A. Saito. 1996. Contribution of tumor necrosis factor-alpha (TNF-α) in host defence mechanism against Cryptococcus neoformans. Clin. Exp. Immunol. 106:468-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolls, J., D. Lei, C. Vazquez, G. Odom, W. Summer, S. Nelson, and J. Shellito. 1997. Exacerbation of murine Pneumocystis carinii infection by adenoviral-mediated gene transfer of a TNF inhibitor. Am. J. Respir. Cell Mol. Biol. 16:112-118. [DOI] [PubMed] [Google Scholar]
- 24.Lee, J. H., N. R. Slifman, S. K. Gershon, E. T. Edwards, W. D. Schwieterman, J. N. Siegel, R. P. Wise, S. L. Brown, J. N. Udall, Jr., and M. M. Braun. 2002. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 46:2565-2570. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Fontecha, A., S. Sebastiani, U. E. Hopken, M. Uguccioni, M. Lipp, A. Lanzavecchia, and F. Sallusto. 2003. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGuirk, P., C. McCann, and K. H. G. Mills. 2002. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 195:221-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mednick, A. J., M. Feldmesser, J. Rivera, and A. Casadevall. 2003. Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur. J. Immunol. 33:1744-1753. [DOI] [PubMed] [Google Scholar]
- 28.Mehrad, B., R. M. Strieter, and T. J. Standiford. 1999. Role of TNF-α in pulmonary host defense in murine invasive aspergillosis. J. Immunol. 162:1633-1640. [PubMed] [Google Scholar]
- 29.Mencacci, A., E. Cenci, G. Del Sero, C. F. d'Ostiani, P. Mosci, C. Montagnoli, A. Bacci, F. Bistoni, V. F. J. Quesniaux, B. Ryffel, and L. Romani. 1998. Defective co-stimulation and impaired Th1 development in tumor necrosis factor/lymphotoxin-α double-deficient mice infected with Candida albicans. Int. Immunol. 10:37-48. [DOI] [PubMed] [Google Scholar]
- 30.Murphy, J., A. Zhou, and S. C. Wong. 1997. Direct interactions of human natural killer cells with Cryptococcus neoformans inhibit granulocyte-macrophage colony-stimulating factor and tumor necrosis factor alpha production. Infect. Immun. 65:4564-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Netea, M. G., L. J. van Tits, J. H. Curfs, F. Amiot, J. F. Meis, J. W. van der Meer, and B. J. Kullberg. 1999. Increased susceptibility of TNF-α lymphotoxin-alpha double knockout mice to systemic candidiasis through impaired recruitment of neutrophils and phagocytosis of Candida albicans. J. Immunol. 163:1498-1505. [PubMed] [Google Scholar]
- 32.Noverr, M. C., S. M. Phare, G. B. Toews, M. J. Coffey, and G. B. Huffnagle. 2001. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 69:2957-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noverr, M. C., G. B. Toews, and G. B. Huffnagle. 2002. Production of prostaglandins and leukotrienes by pathogenic fungi. Infect. Immun. 70:400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olszewski, M. A., G. B. Huffnagle, T. R. Traynor, R. A. McDonald, and G. B. Toews. 2001. MIP-1α/CCL3 regulatory effects on the development of immunity to Cryptococcus neoformans depend on expression of early inflammatory cytokines. Infect. Immun. 69:6256-6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orendi, J., A. Verheul, N. De Vos, M. Visser, H. Snippe, and R. Cherniak. 1997. Mannoproteins of Cryptococcus neoformans induce proliferative response in human peripheral blood mononuclear cells (PBMC) and enhance HIV-1 replication. Clin. Exp. Immunol. 107:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palucka, K., and J. Banchereau. 2002. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr. Opin. Immunol. 14:420-431. [DOI] [PubMed] [Google Scholar]
- 37.Petrofsky, M., and L. E. Bermudez. 1999. Neutrophils from Mycobacterium avium-infected mice produce TNF-α, IL-12, and IL-1β and have a putative role in early host response. Clin. Immunol. 91:354-358. [DOI] [PubMed] [Google Scholar]
- 38.Ritter, U., A. Meissner, J. Ott, and H. Korner. 2003. Analysis of the maturation process of dendritic cells deficient for TNF and lymphotoxin-α reveals an essential role for TNF. J. Leukoc. Biol. 74:216-222. [DOI] [PubMed] [Google Scholar]
- 39.Romani, L., F. Bistoni, and P. Puccetti. 1997. Initiation of T-helper cell immunity to Candida albicans by IL-12: the role of neutrophils. Chem. Immunol. 68:110-135. [DOI] [PubMed] [Google Scholar]
- 40.Romani, L., A. Mencacci, E. Cenci, P. Puccetti, and F. Bistoni. 1996. Neutrophils and the adaptive immune responses to Candida albicans. Res. Immunol. 147:512-518. [DOI] [PubMed] [Google Scholar]
- 41.Schelenz, S., D. A. Smith, and G. J. Bancroft. 1999. Cytokine and chemokine responses following pulmonary challenge with Aspergillus fumigatus: obligatory role of TNF-α and GM-CSF in neutrophil recruitment. Med. Mycol. 37:183-194. [DOI] [PubMed] [Google Scholar]
- 42.Souto, J. T., F. Figueiredo, A. Furlanetto, K. Pfeffer, M. A. Rossi, and J. S. Silva. 2000. Interferon-gamma and tumor necrosis factor-alpha determine resistance to Paracoccidioides brasiliensis infection in mice. Am. J. Pathol. 156:1811-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tai, T. L., K. P. O'Rourke, M. McWeeney, C. M. Burke, K. Sheehan, and M. Barry. 2002. Pneumocystis carinii pneumonia following a second infusion of infliximab. Rheumatology 41:951-952. [DOI] [PubMed] [Google Scholar]
- 44.Tateda, K., T. A. Moore, J. Deng, M. W. Newstead, X. Zeng, A. Matsukawa, M. S. Swanson, K. Yamaguchi, and T. J. Standiford. 2001. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J. Immunol. 166:3355-3361. [DOI] [PubMed] [Google Scholar]
- 45.Traynor, T. R., A. C. Herring, W. A. Kuziel, G. B. Toews, and G. B. Huffnagle. 2002. Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J. Immunol. 168:4659-4666. [DOI] [PubMed] [Google Scholar]
- 46.True, D. G., M. Penmetcha, and S. J. Peckham. 2002. Disseminated cryptococcal infection in rheumatoid arthritis treated with methotrexate and infliximab. J. Rheumatol. 29:1561-1563. [PubMed] [Google Scholar]
- 47.Vecchiarelli, A., C. Retini, D. Pietrella, C. Monari, C. Tascini, T. Beccari, and T. R. Kozel. 1995. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1β secretion from human monocytes. Infect. Immun. 63:2919-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wakkach, A., N. Fournier, V. Brun, J.-P. Breittmayer, F. Cottrez, and H. Groux. 2003. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity 18:605-617. [DOI] [PubMed] [Google Scholar]
- 49.Warris, A., A. Bjorneklett, and P. Gaustad. 2001. Invasive pulmonary aspergillosis associated with infliximab therapy. N. Engl. J. Med. 344:1099-1100. [PubMed] [Google Scholar]
- 50.Wolvers, D. A. W., C. J. J. Coenen-de Roo, R. E. Mebius, M. J. F. van der Cammen, F. Tirion, A. M. M. Miltenburg, and G. Kraal. 1999. Intranasally induced immunological tolerance is determined by characteristics of the draining lymph nodes: studies with OVA and human cartilage gp-39. J. Immunol. 162:1994-1998. [PubMed] [Google Scholar]
- 51.Wood, K. L., C. A. Hage, K. S. Knox, M. B. Kleiman, A. Sannuti, R. B. Day, L. J. Whea, and H. L. I. Twigg. 2003. Histoplasmosis after treatment with anti-tumor necrosis factor-alpha therapy. Am. J. Respir. Crit. Care Med. 167:1279-1282. [DOI] [PubMed] [Google Scholar]
- 52.Yuan, R., A. Casadevall, J. Oh, and M. D. Scharff. 1997. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc. Natl. Acad. Sci. USA 94:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]