Abstract
The centisome 63 type III secretion system (T3SS-1) encoded by Salmonella pathogenicity island 1 (SPI1) mediates invasion of epithelial cells by Salmonella enterica serotype Typhimurium. Characterization of mutants lacking individual genes has revealed that T3SS-1 secreted proteins (effectors) SopE2 and SopB are required for invasion while the SipA protein accelerates entry into cells. Here we have revisited the question of which T3SS-1 effectors contribute to the invasion of epithelial cells by complementing a strain lacking all of the effector genes that are required to cause diarrhea in a calf (a sipA sopABDE2 mutant). Introduction of either the cloned sipA, the cloned sopB, or the cloned sopE2 gene increased the invasiveness of the sipA sopABDE2 mutant for nonpolarized HT-29 cells. However, a contribution of sopA or sopD to invasion was not apparent when invasion assays were performed with the nonpolarized colon carcinoma cell lines T84 and HT-29. In contrast, introduction of either the sopA, the sopB, the sopD, or the sopE2 gene increased the invasiveness of the sipA sopABDE2 mutant for polarized T84 cells. Furthermore, introduction of a plasmid carrying sipA and sopB increased the invasiveness of the sipA sopABDE2 mutant for polarized T84 cells significantly compared to the introduction of plasmids carrying only sipA or sopB. We conclude that SipA, SopA, SopB, SopD, and SopE2 contribute to S. enterica serotype Typhimurium invasion of epithelial cells in vitro.
Invasion of epithelial cells (11) and induction of diarrhea in calves (30, 35) are governed by the centisome 63 type III secretion system (T3SS-1) of Salmonella enterica serotype Typhimurium. T3SS-1 secretes proteins, termed effectors, across the inner and outer membranes of the bacterial cell. Some of the secreted effectors, including SipA (SspA), SipB (SspB), SipC (SspC), SptP, and AvrA, are encoded by genes located on SPI1 at centisome 63 of the bacterial chromosome (17, 20, 22-24). The remaining effectors, including SopA, SopB(SigD), SopD, SopE, SopE2, SlrP, and SspH1, are encoded by genes that are scattered around the S. enterica serotype Typhimurium chromosome (1, 16, 19, 25, 28, 33, 36). Upon secretion from the bacterial cell, the SipB, SipC, and SipD proteins are thought to form a complex in the eukaryotic membrane that is required for translocation of the remaining effectors into the host cell cytoplasm (3, 10, 12, 17, 37). The T3SS-1-mediated secretion and translocation of effectors trigger the formation of ruffles in the host cell membrane that results in bacterial internalization (9, 13). Characterization of strains carrying mutations in T3SS-1 effector genes suggests that in most S. enterica serotype Typhimurium isolates invasion of epithelial cell lines is mediated by translocation of SopB and SopE2 into host cells (26, 43). A small fraction of S. enterica serotype Typhimurium isolates carry in addition a bacteriophage (SopEΦ) encoding the effector SopE, which also contributes to bacterial internalization (16, 18, 26). Strains carrying mutations in both sopB and sopE2 (and isolates carrying SopEΦ in addition to a mutation in sopE) are not able to invade nonpolarized epithelial cell lines (26, 43). Inactivation of sipA causes a short (5-min) delay during invasion of S. enterica serotype Typhimurium into epithelial cell lines, but equal numbers of the wild type and the sipA mutant are recovered at later times from gentamicin protection assays (21, 44). T3SS-1 effectors other than SipA, SopB, SopE, and SopE2 have not been implicated in the mediation of bacterial invasion.
Bacterial strains with mutations in individual effector genes have also been used to determine which T3SS-1 effectors contribute to the ability of S. enterica serotype Typhimurium to cause diarrhea in calves. Strains carrying mutations in slrP, sptP, avrA, or sspH1 cause fluid accumulation and neutrophil infiltration in bovine ligated ileal loops at levels similar to those of wild-type S. enterica serotype Typhimurium (41). In contrast, mutations in either sipA, sopA, sopB, sopD, sopE2, or sopE (in isolates carrying SopEΦ) cause a significant reduction in fluid accumulation and neutrophil influx (40, 41). However, the molecular mechanisms by which SipA, SopA, SopB, SopD, SopE2, and SopE contribute to inflammation are unknown.
S. enterica serotype Typhimurium causes a neutrophil influx in the bovine intestinal mucosa by inducing the expression of neutrophil chemoattractants (i.e., interleukin-8 and GROα) in epithelial cells in a T3SS-1-dependent manner (39). Studies with colon carcinoma cell lines show that the release of chemoattractants from epithelial cells is triggered through recognition of bacterial molecular patterns (i.e., flagella) by Toll-like receptors located on their basolateral membrane (i.e., TLR5) (14, 38). One hypothesis is that T3SS-1-mediated invasion of epithelial cells in the bovine mucosa is required for subsequent recognition of bacterial molecular patterns by Toll-like receptors that are located in cellular compartments not accessible from the intestinal lumen. A correlate of this hypothesis is that all of the effectors previously implicated in eliciting inflammation in vivo (41) should also contribute to the invasion of epithelial cells in vitro. The goal of this study was to test this correlate.
Analysis of effectors is complicated by their partial functional redundancy, which may mask the role of an effector in epithelial invasion when the respective mutant is analyzed (26, 43). Previous studies on the role of effectors in epithelial invasion relied on an the analysis of mutants that were still expressing several of the effectors required for fluid accumulation and inflammation in the calf model. In this study, we have taken an alternate approach by using a strain background lacking the expression of all of the effectors required for fluid accumulation and inflammation in the calf model. This approach allowed us to test the contribution of individual effectors in the absence of proteins with functional redundancy. Furthermore, we compared the invasiveness in nonpolarized and polarized colon carcinoma cell lines to ensure that we would be able to identify those effectors that may only be required for invasion of cells containing an apical membrane brush border.
MATERIALS AND METHODS
Bacterial strains, tissue culture cells, and culture conditions.
Cloning of T3SS-1 effector genes was performed with Escherichia coli strain DH5α, which has been described previously (15). The S. enterica serotype Typhimurium strains used in this study are listed in Table 1. Strains were cultured aerobically at 37°C in Luria-Bertani (LB) broth supplemented with antibiotics as appropriate at the following concentrations: carbenicillin at 100 mg/liter, chloramphenicol at 30 mg/liter, tetracycline at 20 mg/liter, kanamycin at 60 mg/liter, and nalidixic acid at 50 mg/liter. For invasion assays with tissue explants, tissue culture cells, or bovine ligated ileal loops, each strain was grown overnight at 37°C in 4 ml of LB broth in a roller. A volume of 0.04 ml of this overnight culture was used for inoculation of 4 ml of LB broth, and bacteria were grown at 37°C for 3 h in a roller. Subsequently, this culture was used as the inoculum and numbers of CFU were determined by plating serial 10-fold dilutions on LB plates.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Source or reference |
|---|---|---|
| S. enterica serotype Typhimurium strains | ||
| IR715 | Nalidixic acid-resistant derivative of wild-type isolate ATCC 14028 | 29 |
| ZA21 | IR715 ΔsipA ΔsopA sopB::MudJ sopD::pEP182.5 sopE2::pSB1039 | 41 |
| ZA20 | IR715 ΔsopA sopB::MudJ sopD::pEP182.5 sopE2::pSB1039 | 41 |
| AJB75 | IR715 invA::TnphoA | 2 |
| CAS152 | ATCC 14028 StrrphoN::Tn10dCm ΔsipB | 31 |
| CAS108 | ATCC 14028 StrrphoN::Tn10dCm ΔsipC | 31 |
| Plasmids | ||
| pCR2.1 | Cloning vector | Invitrogen |
| pWSK29 | Cloning vector | 34 |
| pGFP-UV | GFP expression vector | Clontech Laboratories Inc. |
| pMR15 | pWSK29 carrying sopD gene | This study |
| pMR17 | pWSK29 carrying sopE2 gene | This study |
| pMR26 | pWSK29 carrying sopB gene | This study |
| pMR28 | pWSK29 carrying sopA gene | This study |
| pMR29 | pWSK29 carrying sipA gene | This study |
| pMR31 | pWSK29 carrying sipA and sopABDE2 genes | This study |
| pPW1 | pWSK29 carrying sipA and sopB genes | This study |
| pMR18 | pWSK29 carrying sopAE2 genes | This study |
| pMR24 | pWSK29 carrying sipA and sopBD genes | This study |
The human colon carcinoma cell line HT-29 has been described previously (8) and was obtained from the American Type Culture Collection (ATCC). HT-29 cells were grown in McCoy's 5a medium with 1.5 mM l-glutamine (Gibco) supplemented with 10% fetal calf serum. For invasion assays, cells were seeded at 2.5 × 105 cells/well in 24-well plates and the invasion assay was performed on the following day. The human colon carcinoma cell line T84 has been described previously (6) and was obtained from ATCC. T84 cells were grown in DMEM/F12 medium (Gibco) containing 1.2 g of sodium bicarbonate per liter, 2.5 mM l-glutamine, 15 mM HEPES, and 0.5 mM sodium pyruvate (Gibco) supplemented with 10% fetal calf serum. For nonpolarized T84 invasion assays, cells were seeded as described above for HT-29 cells. To polarize T84 cells, 0.5 ml of medium containing 4 × 105 cells/well was seeded on the apical compartment in 12-mm-diameter Transwell plates (polycarbonate membrane with a pore size of 0.4 μm; Corning Costar) and 1.5 ml of medium was added to the basolateral compartment. The medium was changed every other day, and the transepithelial electrical resistance (TER) was measured after a week. When the cells reached a TER of at least 1,500 Ω/cm2, they were incubated overnight in fresh medium and the invasion assay was performed on the following day.
Construction of plasmids.
The primers used for amplification of the sipA, sopA, sopB, sopD, and sopE2 genes from S. enterica serotype Typhimurium strain IR715 by PCR are listed in Table 2. All PCR products were initially cloned into pCR2.1, and amplification of the correct genes was confirmed by sequence analysis. A DNA fragment containing the sopA gene and its promoter region was cloned into the ClaI site of the low-copy-number vector pWSK29 to give rise to plasmid pMR28 (Table 1). A DNA fragment containing the sopE2 gene and its promoter region was cloned into the EcoRI site of pWSK29 to give rise to plasmid pMR17. The sopA gene was cloned into the ClaI site of plasmid pMR17 to give rise to plasmid pMR18. A DNA fragment containing the sopD gene and its promoter region was cloned into the SacI site of pWSK29 to give rise to plasmid pMR15. The sipA gene is located in the sipBCDA operon and was amplified without its promoter and cloned directionally behind the lac promoter of pWSK29 with BamHI and XbaI to give rise to plasmid pMR29. The sopB gene was amplified with its promoter but without a termination loop and cloned into pWSK29 with NotI and XbaI to give rise to plasmid pMR26. The sipA gene was introduced in the same orientation and downstream of the sopB stop codon into plasmid pMR26 to give rise to plasmid pPW1. The sopB and sipA genes were excised from pPW1 with NotI BamHI and cloned into pMR15 to give rise to plasmid pMR24. The sopA and sopE2 genes were excised from pMR18 and cloned into XhoI-BamHI-digested pMR24 to give rise to plasmid pMR31.
TABLE 2.
Primers used in this study
| Primer | Sequencea | Engineered restriction endonuclease site | Tm (°C) |
|---|---|---|---|
| SipA-1 | GGATCCTCATCGCATCTTTCCC | BamHI | 54.4 |
| SipA-2 | GCTCTAGAGCAAGGATAACAGAAGAGG | XbaI | 58.3 |
| SopA-1 | ATCGATGGACTGTCATTGAATAATCAGC | ClaI | 59.6 |
| SopA-2 | ATCGATGGTTGAGGCTGGACTAC | ClaI | 60 |
| SopB-1 | GCTCTAGACCTCAAGACTCAAGATG | XbaI | 58.3 |
| SopB-2 | GCGGCCGCTACGCAGGAGTAAATCGGTG | NotI | 60.4 |
| SopD-1 | GAGCTCACGACCATTTGCGGCG | SacI | 59.5 |
| SopD-2 | GAGCTCCGAGACACGCTTCTTCG | SacI | 56.9 |
| SopE2-1 | TACTACCATCAGGAGG | 54.4 | |
| SopE2-2 | GAATGTTTTATGTGACGCAG | 56.3 |
The restriction endonuclease sites engineered into the primer sequences are underlined.
Tm, midpoint temperature.
Tissue culture invasion assays.
Invasion assays were performed by using protocols for gentamicin protection assays described previously (32). In brief, colon carcinoma cells were seeded as described above and infected with S. enterica serotype Typhimurium strains at approximately 107 CFU/well. The bacteria were incubated for 1 h at 37°C in 5% CO2 to allow invasion. Each well was washed five times with sterile phosphate-buffered saline (PBS) (KCl at 2.7 mM, KH2PO4 at 1.8 mM, NaCl at 140 mM, Na2HPO4 at 10 mM, pH 7.4) to remove extracellular bacteria, and medium containing gentamicin at a concentration of 0.1 mg/ml was added for a 90-min incubation at 37°C in 5% CO2. After three washes with PBS, the cells were lysed with 0.5 ml of 1% Triton X-100, the lysates were transferred to sterile tubes, and each well was rinsed with 0.5 ml of PBS. Tenfold serial dilutions were plated to count the intracellular bacteria. During assays with polarized T84 cells, cells were washed with Dulbecco's PBS 14040 (CaCl2 at 100 mg/liter, KCl at 200 mg/liter, KH2PO4 at 200 mg/liter, MgCl2 · 6H2O at 100 mg/liter, NaCl at 8 g/liter, Na2HPO4 · 7H2O at 2.16 g/liter) (Gibco) instead of PBS and TER was measured before infection and 1 and 2.5 h after infection with a voltmeter (Millipore-ERS resistance meter; Millipore, Bedford, Mass.).
Fluorescence microscopy assay.
HT-29 cells were seeded onto coverslips in a 24-well plate. An invasion assay was performed as described above. After treatment with gentamicin, the monolayers were washed three times with PBS and incubated with 3% formaldehyde for 20 min. After three washes with PBS, the coverslips were incubated with 1% goat serum in PBS for 1 h. The cells were subsequently incubated for 45 min with a rabbit anti-O4 antibody (Difco) at a dilution 1:250 in 2% goat serum and then washed three times with PBS. The coverslips were then stained with the secondary antibody (Alexa Fluor 594, goat anti-rabbit immunoglobulin G; Molecular Probes) and Hoechst nuclear stain, washed three times with PBS, and then mounted on slides. Fluorescence analysis was performed with a Nikon Eclipse E800 microscope. Three random fields for each coverslip were selected, and pictures were taken on the 4′,6′-diamidino-2-phenylindole (DAPI) and Texas red channels with a digital camera (DS L-1; Nikon). For those strains expressing green fluorescent protein (GFP), the fluorescein isothiocyanate fluorescence was analyzed as well.
Analysis of protein secretion.
Bacteria were grown under SPI-1-inducing conditions as described above. The cells were pelleted by centrifugation, and 2 ml of supernatant was collected for each sample. The supernatants were then filtered (0.45-μm pore size), and the proteins were precipitated with 25% trichloroacetic acid by high-speed centrifugation (14,000 × g for 30 min). The pellet was washed in cold acetone and resuspended in PBS. Four independent extractions for each sample were added together to minimize differences in protein recovery from sample to sample. The proteins were than boiled in sodium dodecyl sulfate (SDS) for 5 min, and an aliquot of each sample was separated by SDS-10% polyacrylamide gel electrophoresis (SDS-PAGE).
Animal experiments.
Four male Holstein calves, 4 to 5 weeks of age, weighing 45 to 55 kg, were used. They were fed milk replacer twice a day and water ad libitum. The calves were clinically healthy before the experiment and were culture negative for fecal excretion of Salmonella serotypes. Detection of Salmonella serotypes in fecal swabs was performed by enrichment in tetrathionate broth (Difco) and streaking on brilliant green agar (BBL).
Bovine ligated ileal loop surgery has been described previously (27). In brief, the calves were fasted for 24 h prior to the surgery. Anesthesia was induced with propofol (PropoFlo; Abbott Laboratories, Chicago, Ill.), followed by placement of an endotracheal tube and maintenance with isoflurane (Isoflo; Abbott Laboratories) for the duration of the experiment. A laparotomy was performed, the ileum was exposed, and loops with lengths ranging from 6 to 9 cm were ligated, leaving 1-cm loops between them. The loops were infected by intraluminal injection of 3 ml of a suspension of either sterile LB broth or S. enterica serotype Typhimurium strains in LB broth containing approximately 109 CFU. The loops were returned to the abdominal cavity. Samples for bacteriologic culture were collected at 1 h after infection with a 3.5-mm biopsy punch and incubated in PBS containing 0.1 mg of gentamicin per liter for 90 min. Tissue samples were homogenized in PBS, serially diluted, and plated onto LB agar plates containing appropriate antibiotics for enumeration of CFU. Data on bacterial CFU were normalized to the length of the ligated loop and the number of CFU present in the inoculum prior to statistical analysis.
Ex vivo tissue explants.
Uninfected tissue from loops infected with sterile LB broth (see above) was collected with a 3.5-mm biopsy punch and placed on sterile Whatman paper with the mucosal side oriented upward. Invasion of these tissue explants was investigated with a micro-Snapwell system as described previously (7). In brief, circular Plexiglas pieces with a 12-mm diameter and a 3-mm-diameter central hole (Rohm & Haas Co., Philadelphia, Pa.) were washed in 100% ethanol, air dried, and sterilized overnight under UV light. Tissue samples were sandwiched between two Plexiglas pieces and inserted into Costar Snapwell plates (Costar Corning Inc., Acton, Mass.). The luminal side of the tissue sample (containing a surface area of 7 mm2) was infected with S. enterica serotype Typhimurium (approximately 107 CFU/well) and incubated for 1 h at 37°C and 5% CO2 to allow bacterial invasion. One hour after infection, samples were washed three times with 0.5 ml of PBS and incubated with gentamicin for 90 min. Tissue samples were homogenized in PBS, serially diluted, and plated onto LB agar plates containing nalidixic acid (50 μg/ml) for counting of CFU. Data on bacterial CFU recovered from tissue were normalized to bacterial numbers present in the inoculum prior to statistical analysis.
Statistical analysis.
For analysis of percentages, the data were transformed logarithmically. Geometric means were determined, and the statistical significance of differences was calculated with parametric tests. A two-tailed Student t test was used to determine whether introduction of plasmids into the sipA sopABDE2 mutant resulted in significant changes in the invasiveness of the resulting strain.
RESULTS
Proteins secreted by S. enterica serotype Typhimurium strains.
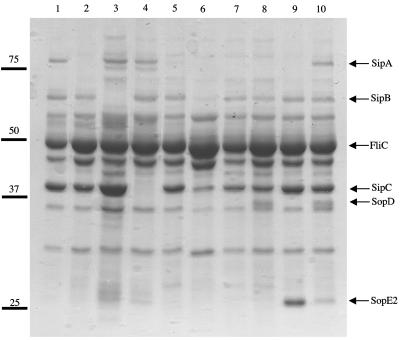
The contribution of effector genes to invasion was assessed by introducing them (sipA, sopA, sopB, sopD, and/or sopE2), cloned on a low-copy-number plasmid (pWSK29, about six copies per cell), into a sipA sopABDE2 mutant (ZA21). The strain used by our laboratory to study enteropathogenicity in the calf model, S. enterica serotype Typhimurium ATCC 14028, does not carry SopEΦ. Thus, we did not include the sopE gene (carried by SopEΦ) in our analysis. Proteins were isolated from culture supernatants and analyzed by SDS-PAGE to evaluate the secretion of T3SS-1 effectors in the bacterial supernatant (Fig. 1). Bands with apparent sizes of 68 (SipB) and 40 (SipC) kDa were absent only from the culture supernatants of a sipB mutant (CAS152) and a sipC mutant (CAS108), respectively. The proteins SipB and SipC were secreted at equal levels in ZA21 complemented with various plasmids (pMR15, pMR17, pMR26, pMR29, and pMR31), except in a strain carrying the cloned sopA gene (pMR28), in which expression was reduced. A large band (approximately 80 kDa) was visible in culture supernatants of strains IR715 (wild type), CAS152, CAS108, and ZA21(pMR31), suggesting that this band represented SipA. The presence of an approximately 30-kDa band present in culture supernatants of strains ZA21(pMR17) and ZA21(pMR31) suggested that this protein represented SopE2. Similarly, the presence of a 45-kDa band in culture supernatants of strains ZA21(pMR15) and ZA21(pMR31) suggested that this band represented SopD. We were not able to visualize the expression of SopB and SopA in culture supernatants of S. enterica serotype Typhimurium strain ATCC 14028 by SDS-PAGE.
FIG. 1.
Analysis of proteins secreted by S. enterica serotype Typhimurium into the culture supernatant. Proteins were separated by SDS-PAGE and stained with Coomassie blue. Positions (bars) and molecular weights (103) of standard proteins are shown on the left. Lanes: 1, IR715 (wild type); 2, ZA21 (sipA sopABDE2 mutant); 3, CAS152 (sipB mutant); 4, CAS108 (sipC mutant); 5, ZA21(pMR29) (sipA sopABDE2 mutant complemented with sipA); 6, ZA21(pMR28) (sipA sopABDE2 mutantcomplemented with sopA); 7, ZA21(pMR28) (sipA sopABDE2 mutant complemented with sopB); 8, ZA21(pMR15) (sipA sopABDE2 mutant complemented with sopD); 9, ZA21(pMR17) (sipA sopABDE2 mutant complemented with sopE2); 10, ZA21(pMR31) (sipA sopABDE2 mutant complemented with sipA sopABDE2).
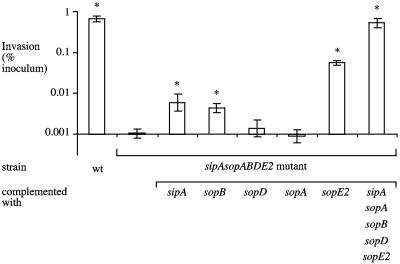
SipA, SopE2, and SopB contribute to the invasion of nonpolarized HT-29 cells.
Invasiveness of S. enterica serotype Typhimurium strains for the human colon carcinoma cell line HT-29 was determined with nonpolarized cells and a standard gentamicin protection assay. Wild-type S. enterica serotype Typhimurium was recovered in 600-fold higher numbers from HT-29 cells than the sipA sopABDE2 mutant was (Fig. 2). The invasion defect of the sipA sopABDE2 mutant could be fully restored by introducing the cloned sipA and sopABDE2 genes on a plasmid. Introduction of the cloned sopD or sopA gene did not increase the invasiveness of the sipA sopABDE2 mutant. However, introduction of the cloned sipA, sopB, or sopE2 gene on a plasmid significantly increased the invasiveness of the sipA sopABDE2 mutant for HT-29 cells (P < 0.05). While it has been shown previously that a mutation in sipA results in a brief (5-min) delay in the invasion of tissue culture cells (21, 44), this is the first report demonstrating that the sipA gene mediates the invasion of human cell lines.
FIG. 2.
Invasion of nonpolarized HT-29 cells by S. enterica serotype Typhimurium. Data are plotted as geometric means from at least four independent assays (bars) ± the standard errors. Asterisks indicate that differences between the invasiveness of the sipA sopABDE2 mutant and that of other strains for HT-29 cells were statistically significant (P < 0.05). wt, wild type.
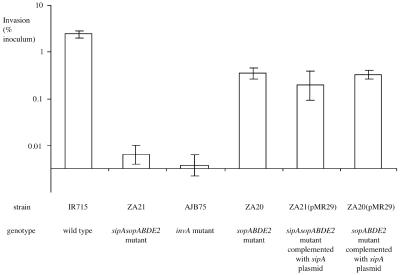
To determine whether introduction of sipA expressed from a heterologous promoter and cloned on a low-copy-number plasmid would alter the outcome of an invasion assay, we compared the invasiveness of the sipA sopABDE2 mutant (ZA21) with that of a sopABDE2 mutant (ZA20, which carries a chromosomal copy of sipA) and with that of derivatives of these strains carrying the cloned sipA gene (pMR29). There was no significant difference in invasiveness among strains ZA20, ZA21(pMR29), and ZA20(pMR29), suggesting that both the chromosomal and plasmid-encoded copies of sipA similarly effected entry of S. enterica serotype Typhimurium into HT-29 cells (Fig. 3). ZA20, ZA21(pMR29), and ZA20(pMR29) were recovered in significantly higher numbers (P < 0.01) from HT-29 cells than from either the sipA sopABDE2 mutant (ZA21) or strain AJB75, which carries a defective form of T3SS-1 because of a mutation in invA. There was no significant difference in the invasiveness for HT-29 cells of strains ZA21 (sipA sopABDE2) and AJB75 (invA). Strains AJB75, ZA20, ZA21, ZA21(pMR29), and ZA20(pMR29) were recovered in significantly lower numbers from HT-29 cells than wild-type S. enterica serotype Typhimurium (IR715) was.
FIG. 3.
Invasion of nonpolarized HT-29 cells by S. enterica serotype Typhimurium. Data are plotted as geometric means from at least four independent assays (bars) ± the standard errors.
To ensure that the results of the gentamicin protection assay were not influenced by differences between strains in the formation of antibiotic-resistant aggregates of extracellular bacteria, we analyzed extracellular bacteria by fluorescence microscopy. In a first experiment, HT-29 cells infected with the wild type (IR715) carrying a plasmid encoding GFP (pGFP-UV) were treated with gentamicin for 90 min and then fixed without being permeabilized. Extracellular bacteria were labeled with anti-lipopolysaccharide (LPS) antibodies (rabbit anti-O4 and goat anti-rabbit Alexa Fluor 594; red fluorescence) but did not show green fluorescence, presumably because GFP was not stable for 90 min after extracellular bacteria were killed by addition of gentamicin. Bacteria exhibiting green fluorescence were not labeled by the anti-LPS antibody, suggesting an intracellular location. The experiment shown in Fig. 2 was then repeated, and extracellular bacteria were fluorescently labeled with anti-LPS antibody as described above. No differences in the appearance (i.e., clumping) or numbers of extracellular bacteria were noted when three microscopic fields were examined for each strain (data not shown). These data showed that the 600-fold difference in the numbers of viable bacteria recovered from gentamicin protection assays with HT-29 cells (Fig. 2) could not be explained by the presence of different numbers of extracellular bacteria, suggesting that the differences were due to different numbers of intracellular bacteria.
SopE2 contributes to invasion of nonpolarized T84 cells.
We studied the invasiveness of S. enterica serotype Typhimurium strains for the human colon carcinoma cell line T84. Wild-type S. enterica serotype Typhimurium was recovered in approximately 100-fold higher numbers from nonpolarized T84 cells than the sipA sopABDE2 mutant was (Fig. 4). The invasion defect of the sipA sopABDE2 mutant could be fully restored by introducing the cloned sipA sopABDE2 genes on a plasmid. Introduction of the cloned sopE2 gene but not introduction of the cloned sipA, sopB, sopD, or sopA gene significantly increased the invasiveness of the sipA sopABDE2 mutant for nonpolarized T84 cells. Comparison of results obtained with nonpolarized HT-29 cells (Fig. 2) and nonpolarized T84 cells (Fig. 4) suggested that differences between human colon carcinoma cell lines significantly influence the mechanisms available for S. enterica serotype Typhimurium entry into host cells.
FIG. 4.
Invasion of nonpolarized T84 cells by S. enterica serotype Typhimurium. Data are plotted as geometric means from at least four independent assays (bars) ± the standard errors. Asterisks indicate that differences between the invasiveness of the sipA sopABDE2 mutant and that of other strains for T84 cells were statistically significant (P < 0.05). wt, wild type.
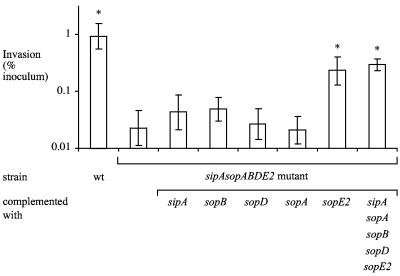
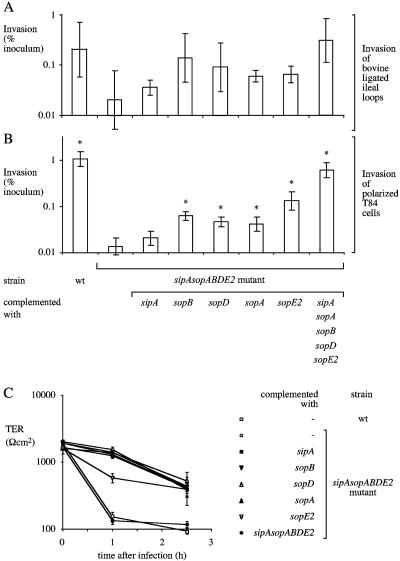
SopA, SopD, SopE2, and SopB contribute to invasion of polarized T84 cells.
Polarization of epithelial cell lines changes the distribution of factors required for S. enterica serotype Typhimurium invasion (4, 5). We reasoned that polarized colon carcinoma cells may more closely resemble epithelial cells in the intestinal mucosa with regard to actin architecture and the presence in their apical membrane of factors required for invasion and may thus be a better model for identifying genes that contribute to mucosal invasion. Wild-type S. enterica serotype Typhimurium was recovered in approximately 100-fold higher numbers from polarized T84 cells than the sipA sopABDE2 mutant was. The invasion defect of the sipA sopABDE2 mutant could be complemented by introducing the cloned sipA sopABDE2 genes on a plasmid (Fig. 5B). Introduction of the cloned sopE2, sopB, sopD, or sopA gene significantly increased the invasiveness of the sipA sopABDE2 mutant for polarized T84 cells (Fig. 5B). This is the first report showing that sopD and sopA are involved in the invasion of host cells by S. enterica serotype Typhimurium. Our data demonstrate that polarization of epithelial cells dramatically alters the requirements for S. enterica serotype Typhimurium invasion, thereby providing a possible explanation for why the contribution of SopD and SopA to invasion has not previously been observed in studies with nonpolarized cells.
FIG. 5.
Invasion of S. enterica serotype Typhimurium in bovine ligated ileal loops (A) and polarized T84 colon carcinoma cells (B). Data are plotted as geometric means from at least four independent assays (bars) ± the standard errors. Asterisks indicate that differences between the invasiveness of the sipA sopABDE2 mutant and that of other strains for polarized T84 cells were statistically significant (P < 0.05). (C) TER during invasion of polarized T84 cells. Data are shown as means ± the standard deviation. wt, wild type.
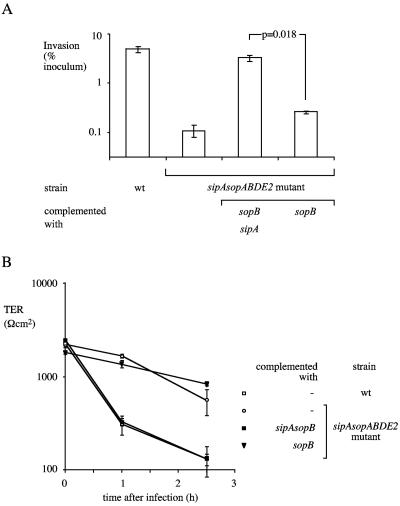
SipA and SopB synergistically promote invasion and loss of TER.
Introduction of the cloned sipA gene did not significantly increase the invasiveness of the sipA sopABDE2 mutant for polarized T84 cells (Fig. 5B). However, introduction of a plasmid carrying sipA and sopB significantly increased the invasiveness of the sipA sopABDE2 mutant for polarized T84 cells compared to the introduction of each individual effector gene (Fig. 6A). These data suggested that SipA acts in concert with SopB during the invasion of polarized T84 cells. A synergism between sipA and sopB was also observed during the invasion of nonpolarized HT-29 cells. Complementation of the sipA sopABDE2 mutant with a plasmid carrying sopB and sipA resulted in 100-fold greater invasion of nonpolarized HT-29 cells than complementation with either sipA or sopB alone (data not shown). In summary, our data provide evidence for contributions of sipA, sopE2, sopB, sopD, and sopA to the invasion of colon carcinoma cells.
FIG. 6.
Invasion of polarized T84 cells by S. enterica serotype Typhimurium (A). Data are plotted as geometric means from CFU recovered from three different wells (bars) ± the standard errors. (B) TER during invasion of polarized T84 cells. Data are shown as means ± the standard deviation. wt, wild type.
Infection of polarized T84 cells with wild-type S. enterica serotype Typhimurium caused a rapid loss of TER compared to infection with the sipA sopABDE2 mutant (Fig. 5C and 6B). Similarly, introduction of a plasmid containing sipA and sopB (Fig. 6B) or a plasmid containing all five effector genes (Fig. 5C) into the sipA sopABDE2 mutant resulted in rapid loss of TER. Introduction of individual effector genes into the sipA sopABDE2 mutant resulted in no decrease (in the case of sipA, sopB, sopD, or sopA) or only a small decrease (in the case of sopE2) in TER (Fig. 5C).
Assays with bovine intestinal tissue are less well suited for assessing the invasiveness of S. enterica serotype Typhimurium than tissue culture models are.
To assess the contribution of effector genes to invasion of the bovine ileal mucosa, ligated ileal loops were infected with bacterial strains and collected 1 h after infection. Bacterial numbers recovered from biopsy punches of each loop were determined after tissue had been incubated with gentamicin to kill extracellular bacteria. Overall, the relative contribution of effector genes to the invasion of polarized T84 cells (Fig. 5B) was similar to that seen in bovine ligated ileal loops (Fig. 5A). Wild-type S. enterica serotype Typhimurium was recovered in higher numbers from the ileal mucosa than the sipA sopABDE2 mutant was (Fig. 5A). This invasion defect was complemented by introducing the cloned sipA sopABDE2 genes into the sipA sopABDE2 mutant on a plasmid (P < 0.05). The sipA sopABDE2 mutant complemented with either sipA, sopA, sopB, sopD, or sopE2 invaded at higher levels than the sipA sopABDE2 mutant did but at lower levels than wild-type S. enterica serotype Typhimurium did. These differences were not statistically significant, which was mainly due to two technical difficulties. The first limitation was a large variation between the numbers of bacteria recovered from the mucosae of different animals. The second limitation was the fact that the wild type was only recovered in about 10-fold higher numbers from tissue than the sipA sopABDE2 mutant was, leaving a relatively small window for detecting the contributions of individual effector genes.
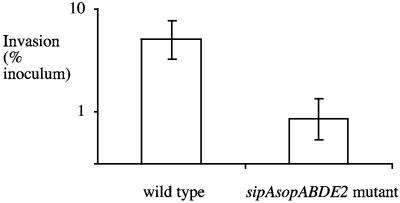
Much larger differences between the wild type and the sipA sopABDE2 mutant were observed when invasion was studied in tissue culture (i.e., IR715 was recovered in 50- to 600-fold higher numbers than ZA21 was) (Fig. 2, 4, and 5B) than when it was studied in bovine ligated loops (i.e., IR715 was recovered in 10-fold higher numbers than ZA21 was) (Fig. 5A). It is possible that differences in the invasiveness of the wild type (IR715) relative to that of the sipA sopABDE2 mutant (ZA21) are due to different growth conditions encountered in tissue culture compared to the milieu of the ligated ileal loop. To test this possibility, we performed invasion assays with bovine ileal biopsy tissue explants under conditions used in tissue culture invasion assays. Tissue samples were sandwiched between Plexiglas inserts and inserted into Snapwell plates to limit bacterial exposure to the mucosal surface. Importantly, this method allowed us to perform invasion assays with bovine tissue under conditions identical to those used for infection of polarized T84 cells. In contrast to invasion assays performed with polarized T84 cells (Fig. 5C), the TER remained constant in invasion assays performed with bovine tissue explants (data not shown). Wild-type S. enterica serotype Typhimurium was recovered in approximately sixfold higher numbers from the ileal mucosa than the sipA sopABDE2 mutant was (Fig. 7). The results in Fig. 7 show that the invasiveness of the wild type (IR715) relative to that of the sipA sopABDE2 mutant in tissue explants was much closer to that observed in ligated ileal loops (10-fold higher than that of the sipA sopABDE2 mutant) than to that observed in tissue culture (50- to 600-fold higher than that of the sipA sopABDE2 mutant), even though the explants and polarized T84 cells were infected under similar conditions. This difference in the invasiveness of S. enterica serotype Typhimurium for bovine tissue compared to that for human cell lines may result from differences in the mechanism of invasion (i.e., invasion efficiency may differ between tissue and tissue culture) or from differences in bacterial recovery (i.e., tissue may provide better protection from gentamicin-mediated killing). However, our data do indicate that the differences between explants and polarized T84 cells were not due to differences in culture conditions.
FIG. 7.
Invasion of bovine small intestinal tissue explants by S. enterica serotype Typhimurium. Data are plotted as geometric means from two independent assays (bars) ± the standard errors.
DISCUSSION
The goal of this study was to determine whether all of the T3SS-1 effectors previously implicated in eliciting fluid accumulation and inflammation in the calf model of enterocolitis (41) are also required for invasion of epithelial cells in vitro. Previous studies that relied heavily on an analysis of bacterial invasion of nonpolarized cell lines by strains lacking individual effectors faced limitations due to genetic redundancy in bacterial invasion mechanisms and the absence of a brush border on host cells. In this study we used an alternate approach of complementing a strain lacking all of the effector genes required to elicit fluid accumulation and inflammation in the calf (a sipA sopABDE2 mutant) by introducing individual effector genes cloned on a low-copy-number plasmid (pWSK29).
Mutational analysis has shown that wild-type S. enterica serotype Typhimurium is 2 times more invasive for nonpolarized human colon carcinoma (Int-407) cells (42) and 10 times more invasive for hamster (CHO) cells and human larynx carcinoma (HeLa) cells (19) than a sopB mutant is. Consistent with these reports, we show that introduction of the cloned sopB gene into the sipA sopABDE2 mutant increased its invasiveness for nonpolarized human colon carcinoma (HT-29) cells fourfold (Fig. 2). Wild-type S. enterica serotype Typhimurium has been shown to be 1.2 times more invasive for nonpolarized simian kidney fibroblast (COS7) cells (28) and fourfold more invasive for HeLa cells (1) than a sopE2 mutant is. Our data demonstrate that introduction of the cloned sopE2 gene into the sipA sopABDE2 mutant increased its invasiveness 50-fold for HT-29 cells (Fig. 2) and 10-fold for T84 cells (Fig. 5). Wild-type S. enterica serotype Typhimurium has been shown to be as invasive as a sipA mutant for canine kidney (MDCK) cells when bacteria are recovered 15 min after infection or at subsequent time points (21). In contrast, we show that introduction of the cloned sipA gene into the sipA sopABDE2 mutant increased its invasiveness for HT-29 cells sixfold (Fig. 2). Thus, the contribution of sipA and sopE2 to the invasion of nonpolarized cells could be demonstrated more clearly by complementation of a sipA sopABDE2 mutant than by previous characterization of strains in which one gene was inactivated.
Studying invasion of tissue was complicated by the fact that differences in the invasiveness of the strains tested were small and difficult to demonstrate given the relatively large variation observed during animals experiments. Thus, the bovine ligated ileal loop model and the bovine tissue explant model are less well suited for analyzing bacterial invasion than tissue culture models are. However, analysis of S. enterica serotype Typhimurium interaction with the bovine mucosa can provide insights into aspects of host-pathogen interaction that appear not to be modeled in tissue culture. For example, we did not observe loss of TER in infected tissue explants while polarized T84 monolayers rapidly lost their TER during invasion.
Our data provide evidence for a synergistic action between SopB and SipA during the invasion of HT-29 and T84 cells (Fig. 6A). A synergistic action of T3SS-1 effectors during host cell invasion has previously been reported for SopB, SopE, and SopE2 (26, 42). Wild-type S. enterica serotype Typhimurium was shown to be 100-fold more invasive for nonpolarized COS7 cells than a sopB sopE sopE2 mutant and 600-fold more invasive than a strain carrying a defective form of T3SS-1 (invG mutant) (26). These and similar results obtained with Int-407 cells led to the conclusion that SopB, SopE, and SopE2 are the only effectors required for invasion of epithelial cells (26, 42). Although this conclusion may be correct for nonpolarized COS7 and Int-407 cells, our data show that different combinations of effectors contributed to the invasion of nonpolarized T84 and HT-29 cells (Table 3). While SopB, SipA, and SopE2 enhanced the invasiveness of the sipA sopABDE2 mutant for nonpolarized HT-29 cells (Fig. 2), only introduction of the cloned sopE2 gene significantly enhanced the invasiveness of the sipA sopABDE2 mutant for nonpolarized T84 cells (Fig. 4). Thus, caution should be taken in generalizing results obtained with individual nonpolarized cell lines.
TABLE 3.
Contribution of T3SS-1 effectors to invasion of different cell lines
| Cell line and culture condition | S. enterica serotype Typhimurium strain | Effector(s) contributing to invasion | Reference(s) |
|---|---|---|---|
| Polarized T84 | ATCC 14028 | SopB, SopD, SopA, SopE2, SipAa | This study |
| Nonpolarized T84 | ATCC 14028 | SopE2 | This study |
| Nonpolarized HT29 | ATCC 14028 | SipA, SopB, SopE2 | This study |
| Nonpolarized Int407 | SL1344 | SopB, SopE, SopE2 | 42 |
| Nonpolarized HeLa | ATCC 14028, F98 | SopB, SopE2 | 1, 19 |
| Nonpolarized CHO | ATCC 14028 | SopB | 19 |
| Nonpolarized COS7 | SL1344 | SopB, SopE, SopE2 | 26, 42 |
A contribution of SipA can only be detected in the presence of SopB.
Perhaps the most significant result of this study is that the contribution of some T3SS-1 effectors to invasion was only apparent when polarized epithelial cells were used. This finding is highly relevant because polarized human colon carcinoma cells are thought to resemble epithelial cells encountered by S. enterica serotype Typhimurium in the intestinal mucosa more closely than other cell culture models do. Comparison of the general pattern of invasion observed in bovine ligated ileal loops with that observed in polarized T84 cells supported this view (Fig. 5A and B). Polarization of cells has previously been shown to affect host cell signaling pathways activated by S. enterica serotype Typhimurium during bacterial entry. While S. enterica serotype Typhimurium activates the GTPases Rac1 and CDC42 when entering nonpolarized MDCK cells, only Rac1 is activated when the bacterium invades the apical membrane of polarized MDCK cells (4). A likely explanation for this observation is that SopE, a nucleotide exchange factor for Rac1 (16), acts locally at sites underlying bacterial contact and that Rac1 is more abundant in apical membrane compartments than CDC42 is (4).
Importantly, previous studies with nonpolarized cells did not provide evidence for a contribution of SopA and SopD to bacterial invasion (26, 36). In contrast, our data show that the sopD and sopA genes contributed to the invasion of polarized but not nonpolarized T84 cells (Fig. 5B). This is the first report implicating these effectors in bacterial entry into mammalian cells and suggests that at least six T3SS-1 effectors, including SipA, SopA, SopB, SopD, SopE, and SopE2, are involved in epithelial invasion (Table 3). It could be speculated that SopA and SopD may only be required during invasion of the brush border of an epithelial cell. However, it is difficult to study the underlying mechanisms because the cellular targets of SopA and SopD have not been identified.
Acknowledgments
We thank Anna Sapone and Alessio Fasano for help in setting up micro-Snapwell invasion assays and Renée M. Tsolis, Josely Figueiredo, Jairo Nunes, Tamara Gull, Carlos Rossetti, and Andrea Humphries for assistance during calf surgery.
Work in A.B.'s laboratory is supported by USDA/NRICGP grant 2002-35204-12247 and Public Health Service grants AI40124 and AI44170. H.A.-P. is supported by Public Health Service grant AI52250.
Editor: F. C. Fang
REFERENCES
- 1.Bakshi, C. S., V. P. Singh, M. W. Wood, P. W. Jones, T. S. Wallis, and E. E. Galyov. 2000. Identification of SopE2, a Salmonella secreted protein which is highly homologous to SopE and involved in bacterial invasion of epithelial cells. J. Bacteriol. 182:2341-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bäumler, A. J., R. M. Tsolis, P. J. Valentine, T. A. Ficht, and F. Heffron. 1997. Synergistic effect of mutations in invA and lpfC on the ability of Salmonella typhimurium to cause murine typhoid. Infect. Immun. 65:2254-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collazo, C. M., and J. E. Galán. 1997. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol. Microbiol. 24:747-756. [DOI] [PubMed] [Google Scholar]
- 4.Criss, A. K., D. M. Ahlgren, T. S. Jou, B. A. McCormick, and J. E. Casanova. 2001. The GTPase Rac1 selectively regulates Salmonella invasion at the apical plasma membrane of polarized epithelial cells. J. Cell Sci. 114:1331-1341. [DOI] [PubMed] [Google Scholar]
- 5.Criss, A. K., and J. E. Casanova. 2003. Coordinate regulation of Salmonella enterica serovar Typhimurium invasion of epithelial cells by the Arp2/3 complex and Rho GTPases. Infect. Immun. 71:2885-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dharmsathaphorn, K., J. A. McRoberts, K. G. Mandel, L. D. Tisdale, and H. Masui. 1984. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am. J. Physiol. 246:G204-G208. [DOI] [PubMed] [Google Scholar]
- 7.El Asmar, R., P. Panigrahi, P. Bamford, I. Berti, T. Not, G. V. Coppa, C. Catassi, and A. Fasano. 2002. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 123:1607-1615. [DOI] [PubMed] [Google Scholar]
- 8.Fogh, J., and G. Trempe. 1975. New human cell lines, p. 115-141. In J. Fogh (ed.), Human cells in vitro. Plenum Publishing Corp., New York, N.Y.
- 9.Frances, C. L., T. A. Ryan, B. D. Jones, S. J. Smith, and S. Falkow. 1993. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364:639-642. [DOI] [PubMed] [Google Scholar]
- 10.Fu, Y., and J. E. Galán. 1998. The Salmonella typhimurium tyrosine phosphatase SptP is translocated into host cells and disrupts the actin cytoskeleton. Mol. Microbiol. 27:359-368. [DOI] [PubMed] [Google Scholar]
- 11.Galán, J. E., and R. Curtiss III. 1989. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. USA 86:6383-6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galyov, E. E., M. W. Wood, R. Rosqvist, P. B. Mullan, P. R. Watson, S. Hedges, and T. S. Wallis. 1997. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol. Microbiol. 25:903-912. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-delPortillo, F., and B. B. Finlay. 1994. Salmonella invasion of nonphagocytic cells induces formation of macropinosomes in the host cell. Infect. Immun. 62:4641-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 15.Grant, S. G. N., J. Jessee, F. R. Bloom, and D. Hanahan. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. USA 87:4645-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardt, W. D., L. M. Chen, K. E. Schuebel, X. R. Bustelo, and J. E. Galán. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93:815-826. [DOI] [PubMed] [Google Scholar]
- 17.Hardt, W. D., and J. E. Galán. 1997. A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc. Natl. Acad. Sci. USA 94:9887-9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardt, W. D., H. Urlaub, and J. E. Galán. 1998. A substrate of the centisome 63 type III protein secretion system of Salmonella typhimurium is encoded by a cryptic bacteriophage. Proc. Natl. Acad. Sci. USA 95:2574-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong, K. H., and V. L. Miller. 1998. Identification of a novel Salmonella invasion locus homologous to Shigella ipgDE. J. Bacteriol. 180:1793-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hueck, C. J., M. J. Hantman, V. Bajaj, C. Johnston, C. A. Lee, and S. I. Miller. 1995. Salmonella typhimurium secreted invasion determinants are homologous to Shigella Ipa proteins. Mol. Microbiol. 18:479-490. [DOI] [PubMed] [Google Scholar]
- 21.Jepson, M. A., B. Kenny, and A. D. Leard. 2001. Role of sipA in the early stages of Salmonella typhimurium entry into epithelial cells. Cell. Microbiol. 3:417-426. [DOI] [PubMed] [Google Scholar]
- 22.Kaniga, K., D. Trollinger, and J. E. Galán. 1995. Identification of two targets of the type III secretion system encoded in inv and spa loci of Salmonella typhimurium that share homology to IpaD and IpaA proteins. J. Bacteriol. 177:7078-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaniga, K., S. Tucker, D. Trollinger, and J. E. Galán. 1995. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J. Bacteriol. 177:3965-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaniga, K., J. Uralil, J. B. Bliska, and J. E. Galán. 1996. A secreted tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium. Mol. Microbiol. 21:633-641. [DOI] [PubMed] [Google Scholar]
- 25.Miao, E. A., C. A. Scherer, R. M. Tsolis, R. A. Kingsley, L. G. Adams, A. J. Bäumler, and S. I. Miller. 1999. Salmonella typhimurium leucine-rich repeat proteins are targeted to the SPI1 and SPI2 type III secretion systems. Mol. Microbiol. 34:850-864. [DOI] [PubMed] [Google Scholar]
- 26.Mirold, S., K. Ehrbar, A. Weissmüller, R. Prager, H. Tschäpe, H. Rüssmann, and W.-D. Hardt. 2001. Salmonella host cell invasion emerged by acquisition of a mosaic of separate genetic elements, including Salmonella pathogenicity island 1 (SPI1), SPI5, and sopE2. J. Bacteriol. 183:2348-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos, R. L., R. M. Tsolis, S. Zhang, T. A. Ficht, A. J. Bäumler, and L. G. Adams. 2001. Salmonella-induced cell death is not required for enteritis in calves. Infect. Immun. 69:4610-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stender, S., A. Friebel, S. Linder, M. Rohde, S. Mirold, and W. D. Hardt. 2000. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol. Microbiol. 36:1206-1221. [DOI] [PubMed] [Google Scholar]
- 29.Stojiljkovic, I., A. J. Bäumler, and F. Heffron. 1995. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J. Bacteriol. 177:1357-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsolis, R. M., L. G. Adams, T. A. Ficht, and A. J. Bäumler. 1999. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 67:4879-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsolis, R. M., L. G. Adams, M. J. Hantman, C. A. Scherer, T. Kimborough, R. A. Kingsley, T. A. Ficht, S. I. Miller, and A. J. Bäumler. 2000. SspA is required for lethal Salmonella enterica serovar Typhimurium infections in calves but is not essential for diarrhea. Infect. Immun. 68:3158-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsolis, R. M., A. J. Bäumler, and F. Heffron. 1995. Role of Salmonella typhimurium Mn-superoxide dismutase (SodA) in protection against early killing by J774 macrophages. Infect. Immun. 63:1739-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsolis, R. M., S. M. Townsend, E. A. Miao, S. I. Miller, T. A. Ficht, L. G. Adams, and A. J. Bäumler. 1999. Identification of a putative Salmonella enterica serotype Typhimurium host range factor with homology to IpaH and YopM by signature-tagged mutagenesis. Infect. Immun. 67:6385-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 35.Watson, P. R., E. E. Galyov, S. M. Paulin, P. W. Jones, and T. S. Wallis. 1998. Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle. Infect. Immun. 66:1432-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood, M. W., M. A. Jones, P. R. Watson, A. M. Siber, B. A. McCormick, S. Hedges, R. Rosqvist, T. S. Wallis, and E. E. Galyov. 2000. The secreted effector protein of Salmonella dublin, SopA, is translocated into eukaryotic cells and influences the induction of enteritis. Cell. Microbiol. 2:293-303. [DOI] [PubMed] [Google Scholar]
- 37.Wood, M. W., R. Rosqvist, P. B. Mullan, M. H. Edwards, and E. E. Galyov. 1996. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol. Microbiol. 22:327-338. [DOI] [PubMed] [Google Scholar]
- 38.Zeng, H., A. Q. Carlson, Y. Guo, Y. Yu, L. S. Collier-Hyams, J. L. Madara, A. T. Gewirtz, and A. S. Neish. 2003. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J. Immunol. 171:3668-3674. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, S., L. G. Adams, J. Nunes, S. Khare, R. M. Tsolis, and A. J. Bäumler. 2003. Secreted effector proteins of Salmonella enterica serotype Typhimurium elicit host-specific chemokine profiles in animal models of typhoid fever and enterocolitis. Infect. Immun. 71:4795-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, S., R. L. Santos, R. M. Tsolis, S. Mirold, W.-D. Hardt, L. G. Adams, and A. J. Bäumler. 2002. Phage mediated horizontal transfer of the sopE1 gene increases enteropathogenicity of Salmonella enterica serotype Typhimurium for calves. FEMS Microbiol. Lett. 217:243-247. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, S., R. L. Santos, R. M. Tsolis, S. Stender, W.-D. Hardt, A. J. Bäumler, and L. G. Adams. 2002. SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves infected with Salmonella enterica serotype Typhimurium. Infect. Immun. 70:3843-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou, D., L. M. Chen, L. Hernandez, S. B. Shears, and J. E. Galán. 2001. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol. Microbiol. 39:248-259. [DOI] [PubMed] [Google Scholar]
- 43.Zhou, D., and J. Galán. 2001. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect. 3:1293-1298. [DOI] [PubMed] [Google Scholar]
- 44.Zhou, D., M. S. Mooseker, and J. E. Galán. 1999. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science 283:2092-2095. [DOI] [PubMed] [Google Scholar]