Abstract
Phosducin (Pdc) is a conserved phosphoprotein that, when unphosphorylated, binds with high affinity to the complex of βγ-subunits of G protein transducin (Gtβγ). The ability of Pdc to bind to Gtβγ is inhibited through its phosphorylation at S54 and S73 within the N-terminal domain (Pdc-ND) followed by association with the scaffolding protein 14-3-3. However, the molecular basis for the 14-3-3-dependent inhibition of Pdc binding to Gtβγ is unclear. By using small-angle x-ray scattering, high-resolution NMR spectroscopy, and limited proteolysis coupled with mass spectrometry, we show that phosphorylated Pdc and 14-3-3 form a complex in which the Pdc-ND region 45−80, which forms a part of Pdc’s Gtβγ binding surface and contains both phosphorylation sites, is restrained within the central channel of the 14-3-3 dimer, with both 14-3-3 binding motifs simultaneously participating in protein association. The N-terminal part of Pdc-ND is likely located outside the central channel of the 14-3-3 dimer, but Pdc residues 20−30, which are also involved in Gtβγ binding, are positioned close to the surface of the 14-3-3 dimer. The C-terminal domain of Pdc is located outside the central channel and its structure is unaffected by the complex formation. These results indicate that the 14-3-3 protein-mediated inhibition of Pdc binding to Gtβγ is based on steric occlusion of Pdc’s Gtβγ binding surface.
Introduction
Phosducin (Pdc) is a highly conserved phosphoprotein that, when unphosphorylated, binds with high affinity to the complex of βγ-subunits of heterotrimeric G protein transducin (Gtβγ) (1, 2, 3). Although the Pdc gene is expressed in many tissues, it is most abundant in the retina and pineal gland (4). Based on that, Pdc has been thought to modulate phototransduction by sequestering Gtβγ from the Gtα-subunit of transducin during light adaptation (5, 6). On the other hand, experiments with Pdc knockout (Pdc−/−) rods revealed no changes in light adaptation in the absence of Pdc, but a significant reduction in sensitivity to light compared with wild-type rods. The reduced sensitivity to light is correlated with the lower expression of βγ-subunits, suggesting that Pdc plays a role in maintaining G protein expression rather than in adaptation mechanisms (7). Consequently, Pdc in its unphosphorylated form has been shown to regulate photoreceptor output independently of phototransduction by modulating transmission at the photoreceptor-to-ON-bipolar cell synapse (8). Moreover, Pdc has also been shown to participate in the modulation of sympathetic activity and blood pressure (9).
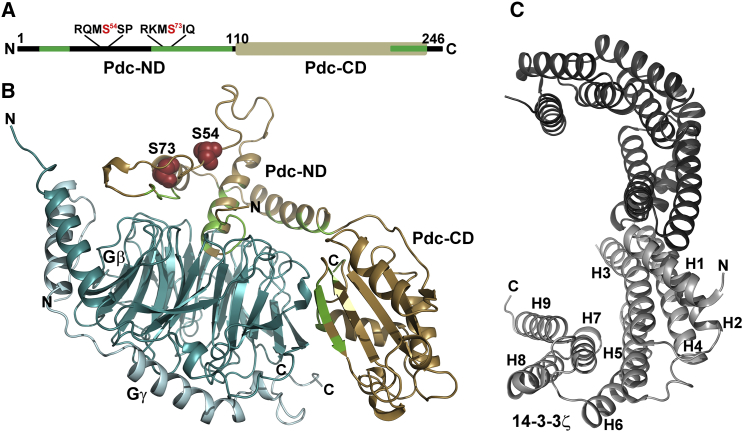
The Pdc molecule consists of two spatially and functionally separate domains (Fig. 1), with the N-terminal domain (Pdc-ND) being inherently unstructured and the C-terminal domain (Pdc-CD) possessing a thioredoxin-like fold (3, 10, 11). Upon binding to the Gtβγ complex, Pdc-ND undergoes a disorder-to-order transition into a mostly helical but still flexible structure that extensively interacts with Gtβγ using the same Gtβγ surfaces that are also involved in binding to Gtα:GDP, thus explaining the Pdc-dependent inhibition of the interaction between Gtβγ and Gtα (3). The ability of Pdc to bind to the Gtβγ complex is inhibited through phosphorylation at S54 and S73 within Pdc-ND by Ca2+/calmodulin-dependent protein kinase II and cAMP-dependent protein kinase, which disrupts part of Pdc’s interface with the Gtβγ complex (10) and induces binding of the scaffolding protein 14-3-3 (12, 13, 14). The 14-3-3 proteins are a family of abundant dimeric proteins (Fig. 1 C) that specifically recognize motifs containing phosphoserine or phosphothreonine residues (15). Through these binding interactions, 14-3-3 proteins regulate the function of their binding partners by a number of different mechanisms, including a direct structural change of the bound partner, physical occlusion of sequence-specific or structural features, and scaffolding that anchors the proteins in close proximity to each other (16, 17). Bioinformatics analysis revealed that the majority of 14-3-3 binding partners contain long, intrinsically disordered segments, and 14-3-3-binding motifs are frequently present within these disordered regions, which likely uncouples the binding strength from the specificity and renders these phosphorylation-dependent and highly specific interactions reversible (18, 19). Pdc is not an exception, as its N-terminal domain, where both phosphorylation sites and 14-3-3 binding motifs are located, is flexible and intrinsically disordered (10, 11).
Figure 1.
(A) Domain structure of Pdc. The positions of the 14-3-3 binding motifs are indicated by their sequences. (B) Crystal structure of the Pdc:Gtβγ complex (3). Phosphorylation sites S54 and S73 located within Pdc-ND are shown in red. Pdc regions involved in the interaction with Gtβγ are marked in green. Pdc-ND, N-terminal domain of Pdc; Pdc-CD, C-terminal domain of Pdc. (C) Crystal structure of the 14-3-3ζ protein dimer (35). Each monomer is shown in a different shade of gray. To see this figure in color, go online.
Although the interaction between 14-3-3 and phosphorylated Pdc (pPdc) is well established, its structural details and its role in the regulation of Pdc function remain elusive. For example, it is unclear whether all or part of the intrinsically disordered Pdc-ND becomes structured upon binding to 14-3-3, as has been observed in the Pdc:Gtβγ complex, or which regions of pPdc, apart from phosphosites pS54 and pS73, participate in binding to 14-3-3. The simultaneous phosphorylation of both S54 and S73 has been shown to be required for 14-3-3 binding to pPdc, indicating that the two 14-3-3 binding motifs simultaneously engage both ligand-binding grooves within the 14-3-3 dimer (13, 20). Since both 14-3-3 binding motifs are located within Pdc-ND, which constitutes the main part of Pdc’s interaction surface with Gtβγ, it is reasonable to speculate that the 14-3-3 protein would interact mainly with this domain and mask a significant portion of its surface. Indeed, recent fluorescence spectroscopy studies suggested that the 14-3-3 protein interacts mainly with regions from Pdc-ND (11, 20).
In addition to the hypothesis that complex formation blocks the interaction between Pdc and Gtβγ, it has been speculated that the 14-3-3 binding might protect the unstructured pPdc-ND from proteolytic degradation and/or dephosphorylation by virtue of its binding to the phosphorylated motifs. This would facilitate pPdc phosphorylation during dark adaptation and/or prolong the time that pPdc remains phosphorylated after light exposure (13). Moreover, 14-3-3 binding might also modulate interactions between pPdc and its other binding partners, such as CRX and SUG1, which have been suggested to interact with Pdc-CD (21, 22). Clearly, a more detailed structural view of the complex between pPdc and 14-3-3 is needed to better understand the role of 14-3-3 binding in the regulation of Pdc function. In this work, we used small-angle x-ray scattering (SAXS), high-resolution NMR spectroscopy, and limited proteolysis coupled with mass spectrometry (MS) to characterize the interaction between pPdc and the 14-3-3ζ protein. In addition, we investigated whether 14-3-3ζ binding slows down the dephosphorylation of pPdc at S54 and S73.
Materials and Methods
Preparation of full-length Pdc and Pdc-CD
Rat full-length Pdc (UniProt: P20942) Q52K mutant and Pdc-CD (sequence 110–246) were prepared as described previously (20). Isotopically labeled proteins for NMR experiments were expressed using the same procedure in minimal medium with [15N]NH4Cl and/or [13C]glucose as the sole nitrogen and carbon sources, respectively, and purified and phosphorylated as unlabeled Pdc and Pdc-CD. Phosphorylation was performed as described previously (20) and monitored by MS, which confirmed the stoichiometric phosphorylation of both S54 and S73.
Preparation of the 14-3-3 protein
C-terminally truncated 14-3-3ζΔC (residues 1–230 with a deleted disordered C-terminal loop) was prepared as described previously (23).
Labeling of 14-3-3ζΔC by 3-(2-iodoacetamido)-PROXYL
The 14-3-3ζΔC containing a single Cys residue at position 189 (24) was covalently modified by mixing the protein (190 μM) in 20 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM EDTA, and the paramagnetic label 3-(2-iodoacetamido)-PROXYL (IPSL; Sigma-Aldrich, Prague, Czech Republic) at a molar ratio of 1:20. It was incubated at 30°C for 3 h and then at 4°C overnight in the dark. The free unreacted label was removed by dialysis against 50 mM MES (pH 6.5) and 100 mM NaCl. The completeness of incorporation was verified by MS.
SAXS data collection and processing
SAXS data were collected on the European Molecular Biology Laboratory P12 beamline on the storage ring PETRA III (Deutsches Elektronen Synchrotron, Hamburg, Germany). The SAXS measurements were conducted in buffer containing 20 mM Tris-HCl (pH 7.5), 200 mM NaCl, 1 mM EDTA, and 2 mM dithiothreitol (DTT). The 14-3-3ζΔC concentrations were 4.3−11.1 mg.mL−1 and the pPdc:14-3-3ζΔC complex (1:2 stoichiometry) concentrations were 5.6−16.8 mg.mL−1. The forward scattering I(0) and the radius of gyration Rg were calculated using the Guinier approximation (25). The distance distribution functions P(r) and the maximum particle dimensions Dmax were obtained using the program GNOM (26). The excluded volume of the hydrated particle (the Porod volume, Vp) was calculated as reported by Porod (27). The Porod-Debye analysis was performed using the program Scatter (https://bl1231.als.lbl.gov/scatter/).
Ab initio modeling
The program DAMMIF (28) was used to calculate ab initio molecular envelopes. Multiple iterations of DAMMIF were averaged using the program DAMAVER (29). Calculated molecular envelopes were aligned to structural models using the program SUPCOMB (30). Theoretical scattering curves were calculated from structural models and fitted to experimental scattering data using CRYSOL (31) and FoXS (32).
All-atom modeling of the pPdc:14-3-3ζ complex
All-atom modeling of the pPdc:14-3-3ζ complex was performed using the AllosMod-FoXS method (33, 34). The starting model was generated by the AllosMod server using the crystal structures of 14-3-3ζ with bound phosphopeptide (35) and Pdc-CD (residues 107–246) (3). Pdc-ND (residues 1–106 with the His-tag sequence at the N-terminus) was modeled with both 14-3-3 binding motifs (containing phosphorylation sites S54 and S73, residues 51–57 and 69–76) restrained in the ligand-binding grooves of the 14-3-3ζ dimer, as has been observed in the crystal structure of the 14-3-3ζ:phosphopeptide complex. AllosMod simulations consisted of 30 runs (generating 101 models for each run) at temperatures between 300 and 500 K, with increased stability of secondary structure. The χ2 values were used to select the models that were the most consistent with the SAXS data. A similar approach was used to calculate alternate models of the complex where just one 14-3-3 binding motif (containing either S54 or S73) was restrained in the ligand-binding groove of 14-3-3ζ and the second one was left unbound.
Dephosphorylation of pPdc
pPdc in the absence and presence of 14-3-3ζΔC was dephosphorylated by either protein phosphatase 1 (PP1, catalytic subunit α-isoform from rabbit) or protein phosphatase 2A (PP2A, human catalytic subunit; Biomol, Hamburg, Germany). Dephosphorylation by PP1 (Sigma-Aldrich) with a specific activity of 10,327 units/mg was performed at 30°C in buffer containing 50 mM HEPES (pH 7.5), 100 mM NaCl, 2 mM DTT, 1 mM MnCl2, and 0.01% (v/v) NP-40. The reaction mixture contained 30 μM pPdc, 60 μM 14-3-3ζΔC (where needed), and PP1 in an optimized molar ratio of 1:1193 (enzyme/substrate). Dephosphorylation by PP2A with a specific activity of 9.5 units/mg was performed at 37°C in buffer containing 40 mM Tris-HCl (pH 8.4), 100 mM NaCl, 2 mM DTT, and 34 mM MgCl2. The reaction mixture contained 30 μM pPdc, 60 μM 14-3-3ζΔC (where needed), and PP2A in an optimized molar ratio of 1:36 (enzyme/substrate). Reactions were stopped after 0, 30, 90, 180, and 420 s by adding 100 mM β-glycerolphosphate (Sigma-Aldrich) and the pH was lowered to 2.5 by the addition of glycine-HCl buffer. Analysis was started by a digestion of sample solution containing 100 pmol of Pdc on either a pepsin or rhizopuspepsin column (36, 37). Generated peptides were trapped and desalted online on a Peptide MicroTrap (Optimize Technologies, Oregon City, OR) and separated on a C18 reversed-phase column (0.5 × 50 mm, Jupiter C18, Phenomenex, Torrance, CA) using a linear gradient of 10–25% solvent B in solvent A for 7 min (solvent A: 2% acetonitrile, 0.4% formic acid in water; solvent B: 95% acetonitrile, 5% water, 0.4% formic acid; Agilent 1200, Agilent Technologies, Waldbronn, Germany). The column was interfaced with the ESI source of a 15T FT-ICR mass spectrometer (SolariX XR, Bruker Daltonics, Bremen, Germany) operating in MS/MS mode. Peptides were identified by a MASCOT search against a database containing the sequence of rat Pdc (the only allowed partial modification was Ser/Thr phosphorylation). Data were analyzed using the program DataAnalysis version 4.2.
Limited proteolysis of Pdc
Limited proteolysis of pPdc in the absence and presence of 14-3-3ζΔC using either trypsin (Promega) or chymotrypsin (Sigma-Aldrich) was carried out in a buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, and 5 mM DTT. Cleavage by trypsin and chymotrypsin was performed on ice and at 25°C, respectively, with 30 μM pPdc, 60 μM 14-3-3ζΔC (where needed), and the optimized concentration of the proteinase. Reactions were stopped at fixed time intervals (1, 3, and 5 min) by boiling for 3 min, followed by acidification with trifluoroacetic acid to a final concentration of 0.03% (w/w). The samples were further processed by automated liquid chromatography (LC)-MS analysis on an Agilent LC 1200 (Agilent Technologies, Waldbronn, Germany) connected to a 15T FT-ICR mass spectrometer (SolariX XR, Bruker Daltonics). Each sample was desalted online using a Peptide CapTrap (Optimize Technologies) and separated on a reversed-phase column (0.2 × 150 mm, PLRP-S, Michrom Bioresources) using a linear gradient of 10–70% solvent B in solvent A for 3 min (solvent A: 2% acetonitrile, 0.2% formic acid in water; solvent B: 95% acetonitrile, 5% water, 0.2% formic acid). Peptides were identified by a MASCOT search against a database containing the sequence of rat Pdc (the only allowed partial modification was Ser/Thr phosphorylation) and by GPMAW (Lighthouse Data, Odense, Denmark) and mass searching in mMass (38). Data were analyzed using the program DataAnalysis version 4.2.
NMR data collection and analysis
NMR data were collected on Bruker Avance III 600, 850, and 950 MHz spectrometers equipped with a triple-resonance cryogenic probe at 20°C. The NMR samples were prepared in 50 mM MES (pH 6.5), 100 mM NaCl, 1 mM tris(2-carboxyethyl)phosphine, and 10% D2O. Unphosphorylated and phosphorylated full-length Pdc and Pdc-CD samples at 0.2–0.3 mM were used for assignment experiments. Three-dimensional (3D) and five-dimensional (5D) NMR experiments were performed using 600 and 950 MHz spectrometers to obtain resonance assignments of the full-length Pdc construct. 5D HN(CA)CONH and 5D HabCabCONH experiments were performed to obtain a sequential assignment of the disordered Pdc-ND. 3D HNCO HN(CA)CO, HNCA, HN(CO)CA, HNCACB, and CBCA(CO)NH experiments were performed to obtain a sequential assignment of the structured Pdc-CD. The data analysis provided a resonance assignment for 182 out of 246 residues (74% of the Pdc sequence). The effects of phosphorylation and 14-3-3ζΔC binding on Pdc were studied using 2D 1H-15N transverse relaxation optimized spectroscopy (TROSY) and 3D HNCA spectra acquired on samples containing 0.2 mM pPdc and 0.8 mM 14-3-3ζΔC. The program SSP (39) was used to analyze the secondary-structure motifs. Neighbor-corrected, random-coil chemical shifts (40) were used as reference values within the SSP program. The observed HA, CA, CB, C′, and N chemical shifts of Pdc or the CA chemical shifts of pPdc and the pPdc:14-3-3ζΔC complex were used in the SSP analysis, which provided the propensities for the presence of helical and extended secondary-structure motifs along the Pdc sequence. Paramagnetic relaxation enhancement (PRE) data were extracted from 1H-15N TROSY spectra acquired on 15N pPdc in complex with 14-3-3ζΔC specifically labeled on C189 by the IPSL spin label, with the spin label in an oxidized or reduced state. The IPSL spin label was reduced by adding a threefold molar excess of ascorbic acid. The sample was then incubated for 15 min and filtered before measurements were obtained. The NMR assignments of Pdc have been deposited in the Biological Magnetic Resonance Data Bank under accession number 26855.
Results and Discussion
Structural characterization of the pPdc:14-3-3ζ complex by SAXS
Since both 14-3-3 binding motifs of pPdc are located within the intrinsically disordered Pdc-ND, we decided to use SAXS, an established technique for structural analysis of flexible systems, to study the solution structure of the pPdc:14-3-3ζ complex. The complex was prepared by mixing pPdc (Q52K mutant phosphorylated at S54 and S73) with the 14-3-3ζΔC protein (14-3-3ζ missing its flexible C-terminal tail) in a 1:2 molar ratio at concentrations of 72–193 μM, which are well above the reported KD of 3 ± 0.5 μM (20).
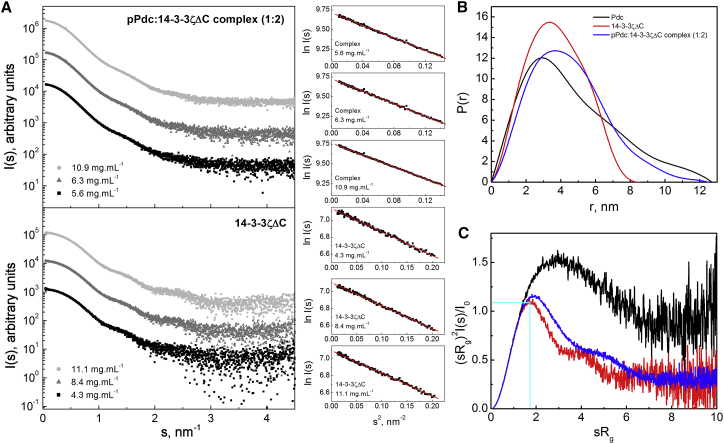
X-ray scattering data were collected for the pPdc:14-3-3ζΔC complex (1:2) and 14-3-3ζΔC alone at various protein concentrations (Fig. 2 and Fig. S1 in the Supporting Material; Table 1). Although the linearity of the Guinier plots confirmed the absence of aggregation in all samples (Fig. 2 A), the concentration dependence of the radius of gyration (Rg) and the Porod volume (Vp), as well as the decrease in scattering intensity at small angles (Fig. S1 B), suggested the presence of repulsive interparticle interactions in samples of both 14-3-3ζΔC and the pPdc:14-3-3ζΔC complex at high protein concentrations. Therefore, data collected for 14-3-3ζΔC at 4.3 and 8.4 mg.mL−1, and for the pPdc:14-3-3ζΔC complex at 5.6 and 6.3 mg.mL−1 were used for further structural analysis. The apparent molecular mass of the 14-3-3ζΔC dimer and the complex was estimated using the program SAXSMoW (41). The molecular mass of 57–60 kDa calculated using the SAXSMoW for 14-3-3ζΔC corresponds well with the expected molecular mass of its dimer (56.6 kDa). The molecular masses of 81–82 kDa calculated using SAXSMoW for the pPdc:14-3-3ζΔC complex are consistent with an expected 1:2 molar stoichiometry (theoretical molecular mass 86.9 kDa), thus corroborating previously reported results from analytical ultracentrifugation (20).
Figure 2.
SAXS analysis. (A) Scattering intensity as a function of the scattering vector s (s = 4πsin(θ)/λ, where 2θ is the scattering angle and λ is the wavelength) for the pPdc:14-3-3ζΔC complex mixed with a 1:2 molar stoichiometry (5.6–10.9 mg.mL−1) and the 14-3-3ζΔC protein (4.3–11.1 mg.mL−1). Scattering profiles shown in dark and light gray are offset by a factor of 10 and 100, respectively. Guinier plots are shown on the right side. (B) Distance distribution functions P(r) calculated from scattering data using the program GNOM (26). For the sake of comparison, a P(r) function calculated from previously published x-ray scattering data for Pdc alone is also shown (black) (11). (C) Normalized Kratky plots of 14-3-3ζΔC (red), Pdc (black), and the pPdc:14-3-3ζΔC complex (blue). Cyan lines mark the maximum at a value of 1.104 for sRg = 1.73, which is characteristic for the scattering data of compact globular proteins (42). To see this figure in color, go online.
Table 1.
Structural Parameters Determined from the SAXS Data
| c (mg.mL−1) | ca (μM) | Rg (Å)b | Rg (Å)c | Dmax (Å) | Vp (nm3)d | MMMoW (kDa)e,f | |
|---|---|---|---|---|---|---|---|
| Complex | 16.8 | 193 | 32.1 ± 0.5 | 32.9 ± 0.1 | 109 | 108 | 77.4 |
| 10.9 | 125 | 33.6 ± 0.1 | 34.1 ± 0.1 | 120 | 114 | 81.3 | |
| 6.3 | 72 | 34.3 ± 0.8 | 35.2 ± 0.1 | 125 | 118 | 82.4 | |
| 5.6 | 64 | 34.4 ± 0.8 | 35.1 ± 0.1 | 125 | 117 | 82.2 | |
| 14-3-3ζΔC | 11.1 | 196 | 28.2 ± 0.1 | 28.3 ± 0.1 | 84 | 85 | 56.6 |
| 8.4 | 148 | 28.5 ± 0.1 | 28.5 ± 0.1 | 85 | 87 | 57.8 | |
| 4.3 | 76 | 28.8 ± 0.1 | 28.8 ± 0.1 | 86 | 89 | 59.6 |
Molar concentration of the 14-3-3ζΔC dimer and the pPdc:14-3-3ζΔC complex with 1:2 molar stoichiometry.
Calculated using the Guinier approximation (25).
Calculated using the program GNOM (26).
Excluded volume of the hydrated particle (the Porod volume).
Molecular mass calculated from the experimental scattering data using the program SAXSMoW (41).
The theoretical molecular masses of the 14-3-3ζΔC dimer and the pPdc:14-3-3ζΔC complex (with 1:2 stoichiometry) are 56.6 and 86.9 kDa, respectively.
A comparison of the calculated distance distribution functions, P(r), shows longer intraparticle distances and a significantly larger maximum particle distance (Dmax) for the pPdc:14-3-3ζΔC complex compared with the 14-3-3ζΔC dimer alone, suggesting that its molecule is more extended (Fig. 2 B; Table 1). For the sake of comparison, Fig. 2 B also shows the P(r) function calculated from the previously published x-ray scattering data of Pdc alone at 4.2 mg.mL−1 (11). It can be noticed that the Dmax obtained for the complex (125 Å) is significantly larger than that determined for the 14-3-3ζΔC alone, indicating that the large part of pPdc is located outside the central channel of the 14-3-3 dimer. The normalized Kratky plots ((sRg)2I(s)/I(0) versus sRg) for 14-3-3ζΔC and the complex show similar bell-shaped curves with maxima at sRg values of 1.7 and 1.9, respectively (Fig. 2 C) (42). The scattering data for compact globular proteins in this plot exhibit a maximum value of 1.104 for sRg = 1.73 (marked by cyan lines in Fig. 2 C). A more gradual decrease of the curve toward zero at higher sRg and a higher maximum indicate increased flexibility of the polypeptide chain. Therefore, the comparison of these plots indicates increased flexibility in the molecule of the complex compared with 14-3-3ζΔC alone. However, the flexibility exhibited by the complex is significantly lower compared with Pdc alone, as the normalized Kratky plot of Pdc (11) shows an (sRg)2I(s)/I(0) maximum of ∼1.5 at an sRg of ∼2.9 (black traces in Fig. 2 C). Such a profile is consistent with the fact that the Pdc molecule consists of an intrinsically disordered Pdc-ND and a folded Pdc-CD. The increased flexibility of the complex compared with 14-3-3ζΔC alone was also corroborated by the Porod-Debye analysis, which revealed a clear Porod-Debye plateau for 14-3-3ζΔC, but not for the complex (Fig. S2) (43, 44).
A superposition of the filtered average ab initio molecular envelope of 14-3-3ζΔC with its crystal structure (35) shows a correct reproduction of the 14-3-3 molecular shape (Fig. S3 A; the ab initio shape reconstruction parameters and statistics are listed in Table S1). The theoretical scattering curve calculated from the crystal structure fits the experimental data well, with χ2 = 0.75 (Fig. S3 C), and the Rg calculated from the crystal structure (29.3 Å) is consistent with experimental values. The filtered average molecular envelope of the pPdc:14-3-3ζΔC complex is more extended and asymmetric compared with 14-3-3ζΔC alone, with one end containing more mass than the other (Fig. S4). However, it is necessary to emphasize that the validity of such an ab initio reconstruction for highly flexible proteins is uncertain, as just one conformation is used to describe a flexible molecule (45). Thus, a more appropriate approach based on conformational sampling using the AllosMod-FoXS method (33, 34) was used to model the solution structure of the pPdc:14-3-3ζΔC complex against the SAXS data.
The 14-3-3 protein interacts mainly with Pdc-ND
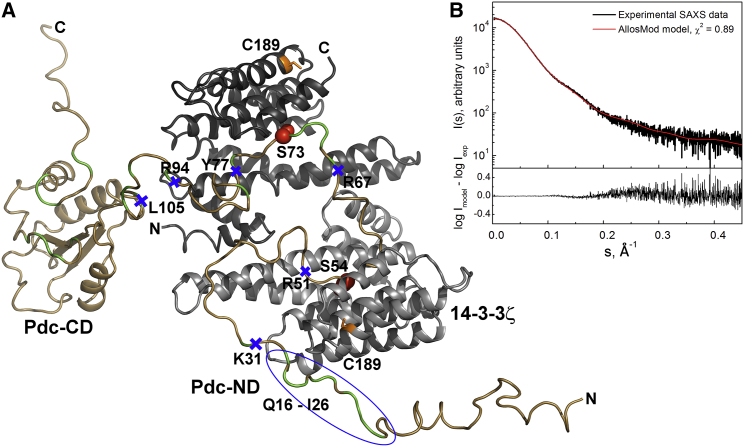
The starting models for AllosMod-FoXS simulations were prepared using the crystal structures of 14-3-3ζ with bound phosphopeptide (35) and Pdc-CD (3) as described in the Materials and Methods section. Pdc-ND was modeled either with both 14-3-3 binding motifs containing S54 and S73 docked into the ligand-binding grooves of the 14-3-3ζ dimer or with just one motif docked, leaving the second one free outside the 14-3-3 groove. The superimposition of 15 models of the complex with the lowest value of χ2 from the simulation in which both 14-3-3 binding motifs of pPdc were docked is shown in Fig. S5. Although all of these models show a similar overall conformation of the pPdc chain, they differ significantly in the conformation of the N-terminal segment, which is located outside the central channel of 14-3-3. The best-scoring model fitted the experimental SAXS data with χ2 = 0.89 (Figs. 3 and S5), and its theoretical Rg value of 35.1 Å is consistent with the experimental values (Table 1). In this model, Pdc-ND residues 45−80 containing both 14-3-3 binding motifs are restrained within the central channel of the 14-3-3ζ dimer, the N-terminal segment of Pdc-ND is outside the central channel of 14-3-3ζ with residues ∼20−30 being located close to the surface of 14-3-3ζ, and Pdc-CD is positioned outside the central channel close to the N-terminus of one 14-3-3ζ chain (Fig. 3). In addition, this model shows a reasonable agreement with the ab initio molecular envelope of the complex (Fig. S6). However, as mentioned above, the ab initio reconstruction for flexible proteins is inaccurate, and it is important to recognize that this comparison is just illustrative.
Figure 3.
(A) The best-scoring model of the pPdc:14-3-3ζ complex calculated using the AllosMod-FoXS simulation (33, 34). The proteolytic cleavage sites are labeled by blue crosses. The 14-3-3ζ residue C189, used as an attachment site for the IPSL spin label in PRE NMR measurements, is shown in orange. The region where a significant relaxation enhancement was observed in the presence of the oxidized spin label is marked by a blue ellipse. Residues directly involved in Gtβγ binding are shown in green (3). (B) Comparison of the calculated scattering curve of the best-scoring AllosMod model of the complex (red line) with the experimental scattering data of the complex at 5.6 mg.mL−1 (black line). Theoretical scattering curves were calculated and fitted to experimental data using FoXS (32). To see this figure in color, go online.
The AllosMod models calculated from the starting structures where just one of the two 14-3-3 binding motifs was docked into the ligand-binding groove of the 14-3-3ζ dimer were unable to reproduce the SAXS data correctly. Examples of such models that fitted the experimental SAXS data with χ2 values of 4.88 (model with a docked S54-containing motif) and 6.00 (model with a docked S73-containing motif) are shown in Fig. S7. Furthermore, the Rg values calculated from these two models (41.1 and 42.2 Å, respectively) are significantly larger than the experimental values. Thus, only the model in which both 14-3-3 binding motifs of pPdc were simultaneously restrained within the central channel of the 14-3-3ζ dimer reproduced the experimental SAXS data correctly. This is in a good agreement with previous suggestions that the simultaneous phosphorylation of both S54 and S73 is required for Pdc binding to 14-3-3, as well as an efficient inhibition of pPdc binding to Gtβγ in intact retinas (13, 20), and that the 14-3-3 protein interacts mainly with Pdc-ND (11, 20). On the other hand, it is necessary to keep in mind that for flexible systems, the SAXS data reflect a conformational average over the entire ensemble, and thus we cannot exclude the possibility that a minor population of the pPdc:14-3-3ζΔC complex exists in the single-site bound state.
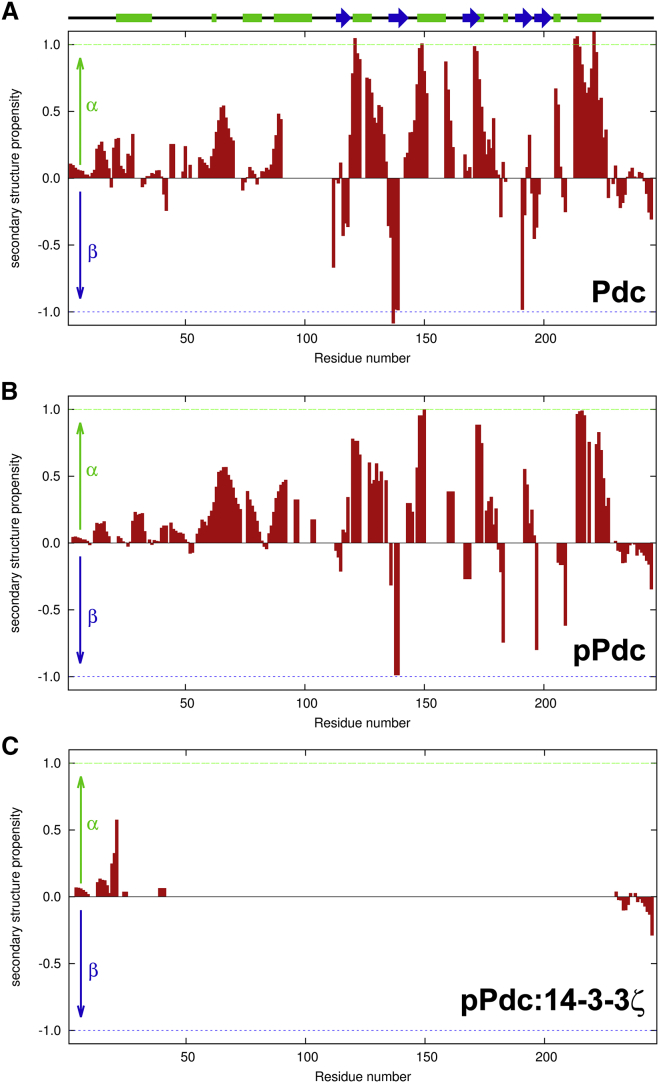
To further investigate the nature of the interaction between pPdc and 14-3-3ζΔC, we next used NMR spectroscopy to monitor conformational changes of Pdc upon its phosphorylation and 14-3-3ζΔC binding. A comparison of the 1H-15N heteronuclear single quantum coherence (HSQC) spectra of full-length Pdc and Pdc-CD revealed that the pattern of signals of the Pdc-CD construct (Fig. S8, black) overlays the signals of full-length Pdc (Fig. S8, red). The signals of Pdc-CD in the HSQC spectrum are well dispersed, which is typical for proteins that possess a unique, well-defined conformation in solution. Analysis of the assigned chemical shifts confirmed the presence of secondary-structure motifs that correspond well with the secondary structures observed in the crystal structure of the Pdc:Gtβζ complex (PDB: 2TRC) (Fig. 4 A). On the other hand, the signals of Pdc-ND show very low dispersion in the HSQC spectrum of full-length Pdc (Fig. S8, red), which is a characteristic feature of polypeptide chains that do not form a single, well-defined structure and are disordered in solution. We performed two 5D NMR experiments (5D HN(CA)CONH and 5D HabCabCONH) to obtain the resonance assignment of Pdc-ND. The obtained chemical shifts allowed us to determine the presence of secondary-structure motifs along the Pdc-ND sequence. Analysis of the secondary-structure propensities determined by the program SSP revealed the presence of two compact regions in which all residues had an SSP value of >0.1. These regions span residues 61−70 and 84−90, and reveal a propensity to form helical structure in the intrinsically disordered Pdc-ND. The rest of the N-terminal domain, on the other hand, does not show significant propensities for secondary-structure elements (Fig. 4 A). The two regions with helical propensities stretching residues 61−70 and 84−90 were also present after Pdc phosphorylation (Fig. 4 B). The fact that the amplitudes of the secondary-structure propensities in these regions are very similar for both Pdc and pPdc suggests that phosphorylation does not shift the conformational equilibrium toward the formation of a stable helix in the regions adjacent to the phosphorylation sites. However, the SSP values reveal an increase in helical propensity for residues 71−72 and 76−80 upon phosphorylation, which results in an extension of the region with a helical propensity located toward the N-terminus (residues 61−80). We speculate that increased helicity might be important for bringing the two phosphoserines of pPdc to the distance corresponding to spacing of the binding sites on 14-3-3. In the presence of 14-3-3ζΔC, the pPdc:14-3-3ζΔC complex has a molecular mass of >80 kDa. As a result, the majority of the pPdc signals are broadened beyond the detection limit in the 3D NMR spectra due to the slow tumbling of the relatively large complex. Only signals of residues 1−35, 39−41, and 230−246 were detected in the 3D TROSY-HNCA spectrum (Fig. 4 C). The secondary CA chemical shifts extracted from the TROSY-HNCA spectrum suggest that these residues remain unstructured after complex formation and likely do not participate in the interaction between pPdc and 14-3-3.
Figure 4.
(A) Secondary-structure propensities of Pdc alone as determined from CA, CB, CO, N, and HA chemical shifts. The positive values indicate a propensity to form helical structure, and the negative values indicate the presence of extended structures. The cylinders and arrows at the top depict the presence of secondary-structure motifs in the structure of Pdc in the Pdc:Gtβγ complex (3). (B) Secondary-structure propensities of pPdc. (C) Secondary CA chemical shift extracted from the 3D HNCA spectrum of the pPdc:14-3-3ζΔC (1:2) complex. To see this figure in color, go online.
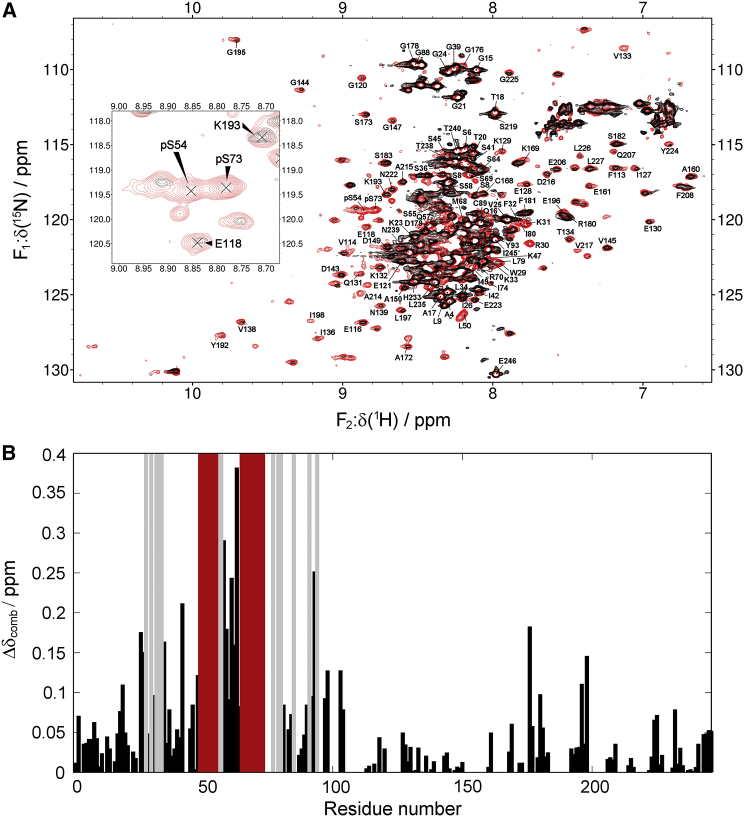
Next, the chemical-shift changes of pPdc in the fingerprint 2D 1H-15N TROSY spectra upon binding to 14-3-3ζΔC were monitored (Fig. 5 B). The combined chemical-shift difference of the 1H and 15N chemical shifts of pPdc in the absence and presence of 14-3-3ζΔC did not reveal any significant changes in the structured Pdc-CD domain upon 14-3-3ζΔC binding. On the other hand, signals of the residues positioned in the proximity of phosphoserines pS54 and pS73 were not observed in the 2D 1H-15N TROSY spectrum of the complex, and the largest chemical-shift perturbations were observed for the residues in the direct neighborhood of this region. Assignment of additional residues in Pdc-ND could not be transferred to the 2D 1H-15N TROSY spectrum of the complex due to the significant degeneracy of the resonance frequencies of amino acids in the N-terminal domain. Therefore, the complete set of residues that are involved in the interaction with 14-3-3ζΔC cannot be directly determined from these data. However, signals of residues 58–64 are still observable in the 2D 1H-15N TROSY spectrum of the pPdc:14-3-3ζΔC complex. The chemical-shift perturbation in this region is largest among all residues in the pPdc sequence, but the presence of the signals in the spectrum suggests that the motional state of these residues is at least partially decoupled from the slow tumbling of the 14-3-3ζΔC dimer in solution (Fig. 5 B). Therefore, we observe two regions around the phosphorylation sites (residues 49−55 and 65−73) for which a continuous stretch of five or more residues is missing in the 1H-15N TROSY spectrum of the complex (Fig. 5 B). In addition, there are two regions in the Pdc-ND sequence that are not assigned upon complex formation (residues 28−34 and 77−94). However, these regions do not comprise a continuous stretch of residues missing in the spectrum of the complex, and the chemical-shift perturbation of the assigned residues is in correspondence with the rest of the Pdc sequence. Therefore, the structure of this complex should be considered as an ensemble of conformers where the pPdc chain is likely being held by two phosphorylated motifs within the central channel of the 14-3-3ζ dimer.
Figure 5.
(A) 1H-15N TROSY spectrum of pPdc in the absence (red) and presence (black) of 14-3-3ζΔC. For better clarity, overlapping signals in the center part of the spectrum are not labeled. The TROSY spectrum corresponding to the region of phosphoserine resonances is depicted in detail in the inset. The pPdc:14-3-3ζΔC molar ratio was 1:2. The overall numbers of 164 and 136 signals were assigned in the case of pPdc and the pPdc:14-3-3ζΔC complex, respectively. (B) Combined chemical-shift difference calculated from the chemical shifts observed in the 1H-15N TROSY spectra of pPdc and pPdc:14-3-3ζΔC. Combined chemical-shift differences (Δδcomb) were calculated as . The compact stretch of five or more residues that were present in the pPdc spectrum, but were not observed in the 1H-15N TROSY spectrum of the pPdc:14-3-3ζΔC complex, is highlighted in red, and the isolated residues missing in the spectrum are depicted in gray. To see this figure in color, go online.
14-3-3ζ protein binding slows down the proteolysis of Pdc-ND
To verify the AllosMod model of the pPdc:14-3-3ζΔC complex, we next performed limited proteolysis of the complex to analyze the accessibility of various regions within the pPdc molecule in the absence and presence of the 14-3-3ζΔC protein. Limited proteolysis was performed using trypsin or chymotrypsin and the products of cleavage reactions were identified by LC-MS. The cleavage kinetics was followed by analyzing time-dependent changes in the abundance of six detected peptides containing the N-terminal part of pPdc up to the corresponding cleavage site (K31, R51, R67, Y77, R94, and L105) (Fig. S9). Due to the possible influence of the phosphate group on the ionization efficiency of the peptides, we compared peptides ending at K31 and R51 from all three states of Pdc-ND, whereas for the other cleavage products we performed a direct comparison only for the phosphorylated version of Pdc-ND (free and in complex with 14-3-3ζΔC). It can be noticed that the cleavage of pPdc at residues K31, R51, R67, and Y77 was slower in the presence of 14-3-3ζΔC compared with pPdc alone. On the other hand, the cleavage site at R94 was protected to a lesser extent, and almost no difference in the cleavage kinetics in the absence or presence of 14-3-3ζΔC was observed for the site at residue L105. These results are consistent with the AllosMod model of the complex (Fig. 3 A, cleavage sites marked by blue crosses) and confirm that Pdc-ND region 30–80, which contains many residues involved in the interaction with Gtβγ (shown in green in Fig. 3 A), is masked by the 14-3-3ζ dimer. The protection of the cleavage site at position 31, which is predicted to be outside the central channel of the 14-3-3ζ dimer, indicates that this region is located close to the surface of 14-3-3ζ. This is consistent with our previous observation that 14-3-3ζ binding affects the fluorescence properties of W29 and reduces the flexibility of the segment containing this tryptophan residue (20). In addition, the slower pPdc cleavage at residue R51 compared with unphosphorylated Pdc (compare the green and red traces in the top-right panel in Fig. S9) is consistent with our previous finding that phosphorylation at S54 and S73 affects the conformational behavior of the N-terminal half of Pdc-ND (11, 20).
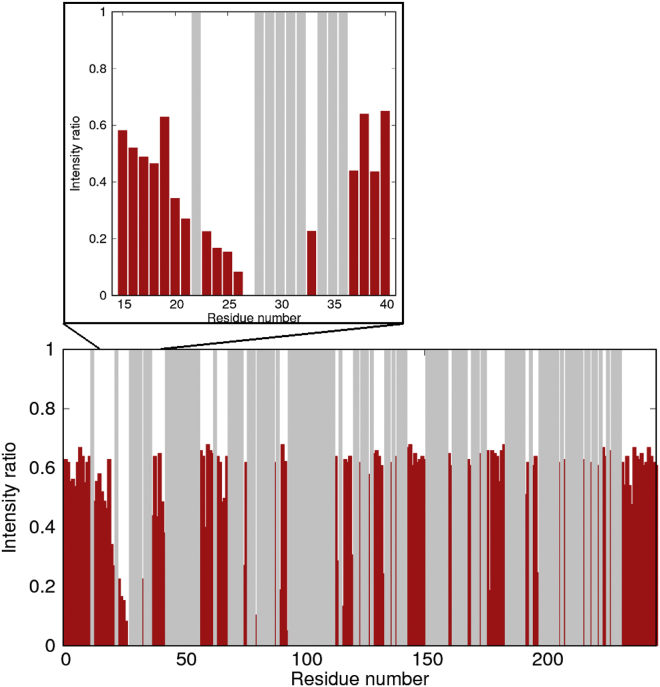
The N-terminal part of bound pPdc is located in proximity to C189 of 14-3-3ζ
To further characterize the binding interface between 14-3-3ζΔC and pPdc, we obtained PRE NMR data for pPdc bound to 14-3-3ζΔC. The introduction of the paramagnetic label increased the relaxation rate of nearby nuclei, enabling us to obtain additional structural information. 1H-15N TROSY spectra of 15N-labeled pPdc in the presence of 14-3-3ζΔC specifically labeled at C189 with the IPSL spin probe in an oxidized or reduced form were acquired. The ratios of peak intensities for individual amino acids of pPdc in the spectra obtained for samples with the label in the oxidized or reduced form revealed that the N-terminal region of bound pPdc (residues 16−26) is located in close proximity to C189 of 14-3-3ζΔC (Fig. 6). This is consistent with the SAXS-based AllosMod model of the complex (Fig. 3), where pPdc residues ∼45−80 containing both phosphorylated 14-3-3 binding motifs are restrained within the central channel of the 14-3-3ζ dimer, and residues 1–44 are located outside the central channel, with residues ∼20−30 being close to the loop between helices H7 and H8 and the C-terminus of helix H9. Residue C189 containing IPSL is located at the N-terminus of helix H8 close to the H7-H8 loop (shown in orange in Fig. 3 A). The proximity of this region, which contains many residues that are directly involved in the interaction with Gtβγ (Fig. 1) (3), to the surface of 14-3-3ζ is further corroborated by the protection of the cleavage site at position 31 upon 14-3-3ζΔC binding (Fig. S9).
Figure 6.
PREs of 15N pPdc in complex with 14-3-3ζΔC single C189. The IPSL spin label was attached to C189 of the 14-3-3ζΔC protein and a 1H-15N TROSY spectrum was acquired with IPSL in an oxidized or reduced state. The bars indicate the ratios of peak intensities for individual amino acids in the pPdc sequence in the spectra acquired on samples with the label in the oxidized or reduced form, respectively. The gray background shows the amino acid residues for which data are not available from either spectrum. A zoomed view of the region corresponding to the Pdc sequence 15−40 is shown in the upper panel. The intensity ratios do not converge to one even in the case of residues that are not affected by the presence of the IPSL label, due to the fact that a lower concentration of pPdc:14-3-3ζΔC complex was used in the sample with the IPSL label in the oxidized state as compared with the sample with IPSL in the reduced state. To see this figure in color, go online.
14-3-3 binding slows down the dephosphorylation of pS54 and pS73
pPdc has been shown to be dephosphorylated in response to light by type 1 and 2A protein phosphatases (PP1 and PP2A), and it has been speculated that 14-3-3 binding might protect pPdc from dephosphorylation by virtue of its binding to the phosphorylated motifs (13, 46). To test this hypothesis, we compared time-dependent dephosphorylation of pS54 and pS73 by PP1 and PP2A in the absence and presence of 14-3-3ζΔC. For this purpose we used an LC-MS setup, which is commonly employed in hydrogen-deuterium exchange coupled with MS (47). Proteins treated by phosphatases for various times were collected and directly injected onto an immobilized protease column. After rapid online digestion and desalting, LC separation coupled with MS detection was done. Extracted ion chromatograms were then plotted for selected peptides containing motifs with S54 and S73. The obtained abundance profiles revealed slower dephosphorylation of both phosphoresidues for pPdc bound to 14-3-3ζΔC compared with pPdc alone (Fig. S10), suggesting that 14-3-3ζ binding protects both the phosphorylated motifs and 14-3-3 binding sites of pPdc against dephosphorylation. Moreover, both sites were protected to a similar extent, suggesting that both phosphorylated motifs are involved in pPdc binding to 14-3-3ζ, in agreement with SAXS and NMR measurements. These results indicate that protecting pPdc against dephosphorylation might be another role of 14-3-3 binding, as has already been suggested (13) and observed for other 14-3-3 binding partners, including ataxin-1 (48), Cdc25 (49), and SRp38 (50).
Conclusions
Our data suggest that pPdc and 14-3-3ζ form a complex in which the Pdc-ND region containing phosphorylation sites S54 and S73 is likely restrained within the central channel of the 14-3-3ζ dimer, with both 14-3-3 binding motifs simultaneously participating in protein association (Fig. 3), thus corroborating previous studies by our group as well as others (13, 20). Our structural model also suggests that the N-terminal segment of Pdc-ND is located outside the central channel of the 14-3-3ζ dimer, with residues 20−30 being positioned close to the 14-3-3ζ surface. This is consistent with our previous fluorescence spectroscopy measurements, which suggested that 14-3-3ζ interacts mainly with Pdc-ND, including the region containing tryptophan residue W29 (11, 20). Previous experiments also indicated a transient interaction between 14-3-3ζ and Pdc-CD consistent with the proposed location of Pdc-CD outside the central channel of the 14-3-3ζ dimer, where it can adopt various positions with respect to 14-3-3. Furthermore, previous results from hydrogen-deuterium exchange coupled with MS analysis indicated that the surface of 14-3-3ζ helices H6 and H8 outside the central channel interacts with pPdc (11). According to our model, these regions likely interact with the N-terminal segment of Pdc-ND and possibly also the C-terminal tail of Pdc-CD. Since the main part of the Gtβγ binding surface of Pdc consists of Pdc-ND regions 17−32, 67−72, and 94−105 (Fig. 1) (3), it is reasonable to assume that the mechanism by which 14-3-3ζ inhibits interaction between Pdc and Gtβγ is based on 14-3-3 competing with Gtβγ for binding to Pdc. Nonphosphorylated Pdc binds Gtβγ with a KD of 17–110 nM (5, 51, 52), and this interaction is inhibited by phosphorylation of Pdc at S54 and S73, which disrupts part of Pdc’s interface with the Gtβγ complex and creates two 14-3-3 binding motifs (10, 12, 13, 20). Phosphorylation decreases the binding affinity of Pdc for Gtβγ by a factor of ∼3−100, depending on the number of phosphorylated residues in the Pdc molecule and the presence of 14-3-3 (13, 14, 53). The binding affinity of 14-3-3ζ for pPdc (phosphorylated at S54 and S73) is in the low micromolar range (11, 20), and the complex formation masks the Gtβγ binding regions of Pdc-ND, thus blocking its interaction with Gtβγ.
Author Contributions
M.K., J.N., and P.M. conducted most of the experiments and analyzed the results. P.M. performed LC-MS experiments and data analysis and interpretation. J.N. performed NMR experiments and data analysis and interpretation. V.O. and T.O. conceived the idea for the project, analyzed the results, and wrote the manuscript.
Acknowledgments
This work was supported by the Czech Science Foundation (project 16-02739S), the Grant Agency of Charles University (project 793913), and the Czech Academy of Sciences (research project RVO: 67985823 of the Institute of Physiology). P.M. received support from LQ1604 NPU II (MEYS) and CZ.1.05/1.1.00/02.0109 BIOCEV (ERDF/MEYS). Czech Infrastructure for Integrative Structural Biology (CIISB) project LM2015043, funded by MEYS CR, supported the measurements at the Jozef Dadok National NMR Centre at CEITEC MU and at the MS facility of BioCeV.
Editor: Jill Trewhella.
Footnotes
Ten figures and one table are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30251-5.
Contributor Information
Veronika Obsilova, Email: veronika.obsilova@fgu.cas.cz.
Tomas Obsil, Email: obsil@natur.cuni.cz.
Supporting Material
References
- 1.Lee R.H., Lieberman B.S., Lolley R.N. A novel complex from bovine visual cells of a 33,000-dalton phosphoprotein with beta- and gamma-transducin: purification and subunit structure. Biochemistry. 1987;26:3983–3990. doi: 10.1021/bi00387a036. [DOI] [PubMed] [Google Scholar]
- 2.Bauer P.H., Müller S., Lohse M.J. Phosducin is a protein kinase A-regulated G-protein regulator. Nature. 1992;358:73–76. doi: 10.1038/358073a0. [DOI] [PubMed] [Google Scholar]
- 3.Gaudet R., Bohm A., Sigler P.B. Crystal structure at 2.4 angstroms resolution of the complex of transducin betagamma and its regulator, phosducin. Cell. 1996;87:577–588. doi: 10.1016/s0092-8674(00)81376-8. [DOI] [PubMed] [Google Scholar]
- 4.Danner S., Lohse M.J. Phosducin is a ubiquitous G-protein regulator. Proc. Natl. Acad. Sci. USA. 1996;93:10145–10150. doi: 10.1073/pnas.93.19.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida T., Willardson B.M., Bitensky M.W. The phosphorylation state of phosducin determines its ability to block transducin subunit interactions and inhibit transducin binding to activated rhodopsin. J. Biol. Chem. 1994;269:24050–24057. [PubMed] [Google Scholar]
- 6.Lee R.H., Ting T.D., Ho Y.K. Regulation of retinal cGMP cascade by phosducin in bovine rod photoreceptor cells. Interaction of phosducin and transducin. J. Biol. Chem. 1992;267:25104–25112. [PubMed] [Google Scholar]
- 7.Krispel C.M., Sokolov M., Burns M.E. Phosducin regulates the expression of transducin betagamma subunits in rod photoreceptors and does not contribute to phototransduction adaptation. J. Gen. Physiol. 2007;130:303–312. doi: 10.1085/jgp.200709812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrmann R., Lobanova E.S., Arshavsky V.Y. Phosducin regulates transmission at the photoreceptor-to-ON-bipolar cell synapse. J. Neurosci. 2010;30:3239–3253. doi: 10.1523/JNEUROSCI.4775-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beetz N., Harrison M.D., Hein L. Phosducin influences sympathetic activity and prevents stress-induced hypertension in humans and mice. J. Clin. Invest. 2009;119:3597–3612. doi: 10.1172/JCI38433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudet R., Savage J.R., Sigler P.B. A molecular mechanism for the phosphorylation-dependent regulation of heterotrimeric G proteins by phosducin. Mol. Cell. 1999;3:649–660. doi: 10.1016/s1097-2765(00)80358-5. [DOI] [PubMed] [Google Scholar]
- 11.Kacirova M., Kosek D., Obsil T. Structural characterization of phosducin and its complex with the 14-3-3 protein. J. Biol. Chem. 2015;290:16246–16260. doi: 10.1074/jbc.M115.636563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano K., Chen J., Bitensky M.W. Rethinking the role of phosducin: light-regulated binding of phosducin to 14-3-3 in rod inner segments. Proc. Natl. Acad. Sci. USA. 2001;98:4693–4698. doi: 10.1073/pnas.071067198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thulin C.D., Savage J.R., Willardson B.M. Modulation of the G protein regulator phosducin by Ca2+/calmodulin-dependent protein kinase II phosphorylation and 14-3-3 protein binding. J. Biol. Chem. 2001;276:23805–23815. doi: 10.1074/jbc.M101482200. [DOI] [PubMed] [Google Scholar]
- 14.Lee B.Y., Thulin C.D., Willardson B.M. Site-specific phosphorylation of phosducin in intact retina. Dynamics of phosphorylation and effects on G protein beta gamma dimer binding. J. Biol. Chem. 2004;279:54008–54017. doi: 10.1074/jbc.M405669200. [DOI] [PubMed] [Google Scholar]
- 15.Muslin A.J., Tanner J.W., Shaw A.S. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84:889–897. doi: 10.1016/s0092-8674(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 16.Tzivion G., Avruch J. 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J. Biol. Chem. 2002;277:3061–3064. doi: 10.1074/jbc.R100059200. [DOI] [PubMed] [Google Scholar]
- 17.Obsil T., Obsilova V. Structural basis of 14-3-3 protein functions. Semin. Cell Dev. Biol. 2011;22:663–672. doi: 10.1016/j.semcdb.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Bustos D.M. The role of protein disorder in the 14-3-3 interaction network. Mol. Biosyst. 2012;8:178–184. doi: 10.1039/c1mb05216k. [DOI] [PubMed] [Google Scholar]
- 19.Collins M.O., Yu L., Choudhary J.S. Phosphoproteomic analysis of the mouse brain cytosol reveals a predominance of protein phosphorylation in regions of intrinsic sequence disorder. Mol. Cell. Proteomics. 2008;7:1331–1348. doi: 10.1074/mcp.M700564-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Rezabkova L., Kacirova M., Obsil T. Structural modulation of phosducin by phosphorylation and 14-3-3 protein binding. Biophys. J. 2012;103:1960–1969. doi: 10.1016/j.bpj.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu X., Craft C.M. Modulation of CRX transactivation activity by phosducin isoforms. Mol. Cell. Biol. 2000;20:5216–5226. doi: 10.1128/mcb.20.14.5216-5226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X., Craft C.M. Interaction of phosducin and phosducin isoforms with a 26S proteasomal subunit, SUG1. Mol. Vis. 1998;4:13. [PubMed] [Google Scholar]
- 23.Obsilova V., Herman P., Obsil T. 14-3-3zeta C-terminal stretch changes its conformation upon ligand binding and phosphorylation at Thr232. J. Biol. Chem. 2004;279:4531–4540. doi: 10.1074/jbc.M306939200. [DOI] [PubMed] [Google Scholar]
- 24.Silhan J., Obsilova V., Obsil T. 14-3-3 protein C-terminal stretch occupies ligand binding groove and is displaced by phosphopeptide binding. J. Biol. Chem. 2004;279:49113–49119. doi: 10.1074/jbc.M408671200. [DOI] [PubMed] [Google Scholar]
- 25.Guinier A. La diffraction des rayons X aux très faibles angles: applications à l’etude des phénomènes ultra-microscopies. Ann. Phys. (Paris) 1939;12:161–237. [Google Scholar]
- 26.Svergun D.I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Cryst. 1992;25:495–503. [Google Scholar]
- 27.Porod G. General theory. In: Glatter O., Kratky O., editors. Small-Angle X-Ray Scattering. Academic Press; London: 1982. pp. 17–51. [Google Scholar]
- 28.Franke D., Svergun D.I. DAMMIF, a program for rapid ab-initio shape determination in small-angle scattering. J. Appl. Crystallogr. 2009;42:342–346. doi: 10.1107/S0021889809000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volkov V.V., Svergun D.I. Uniqueness of ab initio shape determination in small-angle scattering. J. Appl. Cryst. 2003;36:860–864. doi: 10.1107/S0021889809000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozin M.B., Svergun D.I. Automated matching of high- and low-resolution structural models. J. Appl. Cryst. 2001;34:33–41. [Google Scholar]
- 31.Svergun D., Barberato C., Koch M.H.J. CRYSOL—a program to evaluate x-ray solution scattering of biological macromolecules from atomic coordinates. J. Appl. Cryst. 1995;28:768–773. [Google Scholar]
- 32.Schneidman-Duhovny D., Hammel M., Sali A. Accurate SAXS profile computation and its assessment by contrast variation experiments. Biophys. J. 2013;105:962–974. doi: 10.1016/j.bpj.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinkam P., Pons J., Sali A. Structure-based model of allostery predicts coupling between distant sites. Proc. Natl. Acad. Sci. USA. 2012;109:4875–4880. doi: 10.1073/pnas.1116274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneidman-Duhovny D., Hammel M., Sali A. FoXS: a web server for rapid computation and fitting of SAXS profiles. Nucleic Acids Res. 2010;38:W540–W544. doi: 10.1093/nar/gkq461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rittinger K., Budman J., Yaffe M.B. Structural analysis of 14-3-3 phosphopeptide complexes identifies a dual role for the nuclear export signal of 14-3-3 in ligand binding. Mol. Cell. 1999;4:153–166. doi: 10.1016/s1097-2765(00)80363-9. [DOI] [PubMed] [Google Scholar]
- 36.Wang L., Pan H., Smith D.L. Hydrogen exchange-mass spectrometry: optimization of digestion conditions. Mol. Cell. Proteomics. 2002;1:132–138. doi: 10.1074/mcp.m100009-mcp200. [DOI] [PubMed] [Google Scholar]
- 37.Rey M., Man P., Pelosi L. Recombinant immobilized rhizopuspepsin as a new tool for protein digestion in hydrogen/deuterium exchange mass spectrometry. Rapid Commun. Mass Spectrom. 2009;23:3431–3438. doi: 10.1002/rcm.4260. [DOI] [PubMed] [Google Scholar]
- 38.Strohalm M., Kavan D., Havlícek V. mMass 3: a cross-platform software environment for precise analysis of mass spectrometric data. Anal. Chem. 2010;82:4648–4651. doi: 10.1021/ac100818g. [DOI] [PubMed] [Google Scholar]
- 39.Marsh J.A., Singh V.K., Forman-Kay J.D. Sensitivity of secondary structure propensities to sequence differences between alpha- and gamma-synuclein: implications for fibrillation. Protein Sci. 2006;15:2795–2804. doi: 10.1110/ps.062465306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamiola K., Acar B., Mulder F.A. Sequence-specific random coil chemical shifts of intrinsically disordered proteins. J. Am. Chem. Soc. 2010;132:18000–18003. doi: 10.1021/ja105656t. [DOI] [PubMed] [Google Scholar]
- 41.Fischer H., Neto M.D., Craievich A.F. Determination of the molecular weight of proteins in solution from a single small-angle X-ray scattering measurement on a relative scale. J. Appl. Cryst. 2010;43:101–109. [Google Scholar]
- 42.Receveur-Brechot V., Durand D. How random are intrinsically disordered proteins? A small angle scattering perspective. Curr. Protein Pept. Sci. 2012;13:55–75. doi: 10.2174/138920312799277901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rambo R.P., Tainer J.A. Characterizing flexible and intrinsically unstructured biological macromolecules by SAS using the Porod-Debye law. Biopolymers. 2011;95:559–571. doi: 10.1002/bip.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reyes F.E., Schwartz C.R., Rambo R.P. Methods for using new conceptual tools and parameters to assess RNA structure by small-angle X-ray scattering. Methods Enzymol. 2014;549:235–263. doi: 10.1016/B978-0-12-801122-5.00011-8. [DOI] [PubMed] [Google Scholar]
- 45.Bernadó P., Svergun D.I. Structural analysis of intrinsically disordered proteins by small-angle X-ray scattering. Mol. Biosyst. 2012;8:151–167. doi: 10.1039/c1mb05275f. [DOI] [PubMed] [Google Scholar]
- 46.Pagh-Roehl K., Lin D., Burnside B. Phosducin and PP33 are in vivo targets of PKA and type 1 or 2A phosphatases, regulators of cell elongation in teleost rod inner-outer segments. J. Neurosci. 1995;15:6475–6488. doi: 10.1523/JNEUROSCI.15-10-06475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadek A., Mrazek H., Man P. Aspartic protease nepenthesin-1 as a tool for digestion in hydrogen/deuterium exchange mass spectrometry. Anal. Chem. 2014;86:4287–4294. doi: 10.1021/ac404076j. [DOI] [PubMed] [Google Scholar]
- 48.Lai S., O’Callaghan B., Orr H.T. 14-3-3 binding to ataxin-1(ATXN1) regulates its dephosphorylation at Ser-776 and transport to the nucleus. J. Biol. Chem. 2011;286:34606–34616. doi: 10.1074/jbc.M111.238527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Margolis S.S., Walsh S., Kornbluth S. PP1 control of M phase entry exerted through 14-3-3-regulated Cdc25 dephosphorylation. EMBO J. 2003;22:5734–5745. doi: 10.1093/emboj/cdg545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi Y., Manley J.L. A complex signaling pathway regulates SRp38 phosphorylation and pre-mRNA splicing in response to heat shock. Mol. Cell. 2007;28:79–90. doi: 10.1016/j.molcel.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 51.Xu J., Wu D., Simon M.I. The N terminus of phosducin is involved in binding of beta gamma subunits of G protein. Proc. Natl. Acad. Sci. USA. 1995;92:2086–2090. doi: 10.1073/pnas.92.6.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schröder S., Lohse M.J. Inhibition of G-protein betagamma-subunit functions by phosducin-like protein. Proc. Natl. Acad. Sci. USA. 1996;93:2100–2104. doi: 10.1073/pnas.93.5.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen F., Lee R.H. Phosducin and betagamma-transducin interaction I: effects of post-translational modifications. Biochem. Biophys. Res. Commun. 1997;233:370–374. doi: 10.1006/bbrc.1997.6460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.