Summary
During prostate development, basal and luminal cell lineages are generated through symmetric and asymmetric divisions of bipotent basal cells. However, the extent to which spindle orientation controls division symmetry or cell fate, and the upstream factors regulating this process, are still elusive. We report that GATA3 is expressed in both prostate basal progenitor and luminal cells and that loss of GATA3 leads to a mislocalization of PRKCZ, resulting in mitotic spindle randomization during progenitor cell division. Inherently proliferative intermediate progenitor cells accumulate, leading to an expansion of the luminal compartment. These defects ultimately result in a loss of tissue polarity and defective branching morphogenesis. We further show that disrupting the interaction between PRKCZ and PARD6B is sufficient to recapitulate the spindle and cell lineage phenotypes. Collectively, these results identify a critical role for GATA3 in prostate lineage specification, and further highlight the importance of regulating spindle orientation for hierarchical cell lineage organization.
Keywords: GATA3, prostate progenitor cells, atypical protein kinase C, aurothiomalate, spindle orientation, prostate development, par complex, cell polarity, lineage specification, epithelial stratification
Graphical Abstract
Highlights
-
•
Gata3 regulates prostate lineage specification and tissue architecture
-
•
Loss of Gata3 causes aPKC mislocalization and mitotic spindle randomization
-
•
aPKC-Par6 decoupling randomizes the spindle and perturbs lineage specification
-
•
Spindle regulation prevents progenitor cell accumulation and tissue hyperplasia
In this report, Bouchard and colleagues identify a role for the transcription factor Gata3 in mitotic spindle orientation and lineage specification of prostate progenitor cells through the subcellular localization of the polarity protein aPKC. Their results underscore the importance of mitotic spindle orientation for progenitor cell homeostasis and tissue architecture.
Introduction
The development of stratified epithelia requires precise coordination of morphogenetic and cell differentiation signals. The prostate epithelium is composed of secretory luminal cells surrounded by a layer of basal cells and interspersed by rare neuroendocrine cells. This epithelial bilayer is generated during development and maintained throughout adulthood (Marker et al., 2003). The hierarchy within and between these different cell types has recently been clarified both during development and in the adult prostate (Ousset et al., 2012, Wang et al., 2009, Wang et al., 2013, Wuidart et al., 2016), but much remains to be understood about the regulatory and molecular mechanisms of lineage specification.
During postnatal development of the prostate, the majority of prostatic bud growth and differentiation comes from progenitors within the basal lineage, contributing to the expansion and formation of both the basal and luminal lineages (Ousset et al., 2012, Pignon et al., 2013). Cell fate determination from basal progenitor cells is thought to rely largely on the orientation of cell division (Wang et al., 2014, Williams et al., 2014). Basal cells divide either symmetrically (parallel to the basement membrane), generating two daughter cells with a basal cell fate, or asymmetrically (perpendicular to the basement membrane), generating a basal and a luminal daughter cell (Wang et al., 2014). The transition from bipotent basal cells to the luminal fate can additionally proceed through the formation of “double-positive” (CK5+/CK8+) intermediate progenitor cells with unipotent luminal potential (Ousset et al., 2012, Xin et al., 2003). Once the prostate has formed, both basal and luminal cells are thought to be limited to unipotent divisions that maintain tissue homeostasis. Unipotent basal and luminal divisions also drive the majority of growth during regeneration of the tissue following castration (Choi et al., 2012, Liu et al., 2011). Evidence also exists for rare multipotent basal and luminal cells within the adult prostate (Choi et al., 2012, Liu et al., 2011, Wang et al., 2009, Wang et al., 2013, Xin et al., 2003).
Symmetric and asymmetric cell divisions are regulated by mitotic spindle orientation, which relies on the formation of a molecular bridge between the spindle poles and the lateral or apical membranes, respectively (Bergstralh and St Johnston, 2014). During asymmetric cell division, the spindle pole protein complex interacts with the apical polarity complex comprising the Par proteins Par3 (PARD3) and Par6 (PARD6B), and the protein kinase aPKC (PRKCZ and PRKCI) (Rodriguez-Boulan and Macara, 2014). During symmetric division, the dominant model is that PRKCZ prevents localization of the spindle pole to the apical membrane, leading to spindle pole attachment at the lateral membrane (Chatterjee and McCaffrey, 2014). These mechanisms have been proposed in several tissues, including the mammary gland, a system similar to the prostate. Intriguingly, very little is known about the regulation of these complexes (Ahmed and Macara, 2016).
We previously identified a role for the transcription factor Gata3 in prostate cancer (Nguyen et al., 2013). GATA3 expression is lost during cancer progression in both mouse and human prostates. In Pten-deficient mice, acute inactivation of Gata3 accelerates prostate cancer progression, while its sustained expression delays the transition to carcinoma (Nguyen et al., 2013). Gata3 is also important for the specification and maintenance of many epithelial tissues including the epidermis and mammary gland, and is a recognized tumor suppressor in breast cancer (Asselin-Labat et al., 2007, Dydensborg et al., 2009, Kaufman et al., 2003). However, the role that Gata3 plays during prostate development and in the generation and maintenance of epithelial polarity and homeostasis is poorly understood. Here, we show that Gata3 regulates epithelial progenitor cell division via atypical protein kinase C (PRKCZ) to control lineage commitment during prostate development. This function of Gata3 is achieved through precise regulation of spindle orientation in progenitor cells, disruption of which is sufficient to induce epithelial cell lineage and morphological defects.
Results
Gata3 Is Required for Branching Morphogenesis and Epithelial Homeostasis during Prostate Development
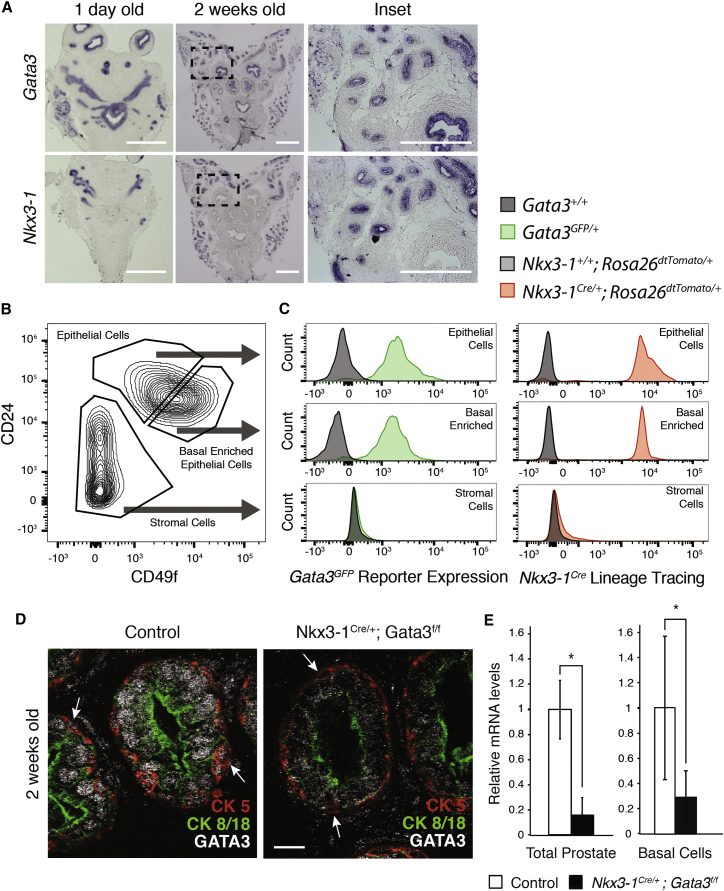
We have previously shown that the transcription factor GATA3 plays a role in prostate cancer progression (Nguyen et al., 2013). To assess its role during prostate development, we first determined its precise expression pattern. In situ hybridization revealed specific expression of Gata3 in the prostate epithelium (overlapping with Nkx3-1 expression), in the urothelium of the bladder and in the seminal vesicles, whereas the urogenital mesenchyme was negative for Gata3 (Figure 1A). To clarify which cell lineages expressed Gata3 at 2 weeks of age, we performed fluorescence-activated cell sorting (FACS) using the surface markers CD24 and CD49f on prostate tissue from Gata3GFP/+ knockin mice (Figure 1B). This confirmed that Gata3 is expressed in all epithelial cells, including a basal cell-enriched epithelial population (Figure 1B), which also expresses p63, CK5 and CK14 (Figure S1).
Figure 1.
Gata3 Is Expressed in Basal Cells during Prostate Development
(A) In situ hybridization of Gata3 and Nkx3-1 mRNA in newborn (1 day old) and postnatal (2 weeks old) prostate tissue. Insets show detection of mRNA in epithelial cells but not in surrounding stromal cells. Scale bars, 0.5 mm.
(B) Representative fluorescence-activated cell sorting (FACS) plot of prostate stromal, epithelial, and basal enriched cell populations from 2-week-old prostate tissue by CD24 and CD49f.
(C) Expression levels of endogenous Gata3GFP and Nkx3-1Cre activated Rosa26dtTomato lineage tracing reporters in the basal cell-enriched populations from 2-week-old prostate tissue. Wild-type and Gata3GFP/+ mice, and Nkx3-1Cre/+;Rosa26dtTomato/+ and Nkx3-1+/+;Rosa26dtTomato/+ mice were used, respectively.
(D) Immunohistochemistry against GATA3 protein in luminal (CK8/18+) and basal (CK5+) epithelial cells. Arrows indicate expression of GATA3 in basal cells. Scale bar, 5 μm.
(E) qRT-PCR detection of Gata3 mRNA in total and FACS enriched basal cells from control and Nkx3-1Cre/+;Gata3flox/flox mice. Expression levels displayed are relative to control tissue and corrected on housekeeping Ppia expression levels. Representative images and quantifications are from four control and three Nkx3-1Cre/+;Gata3flox/flox prostates and independent sorted populations. ∗p < 0.05.
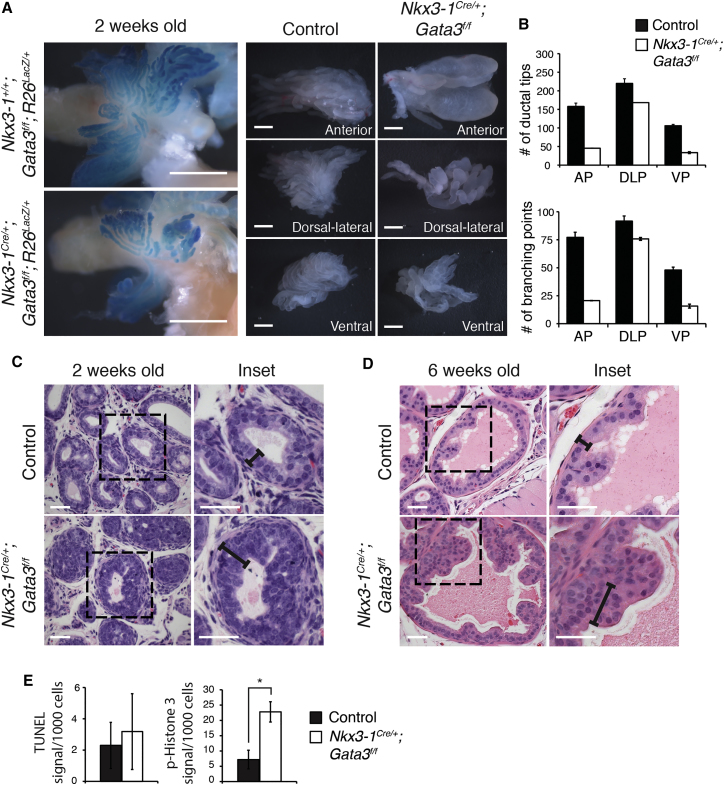
To assess the functional role of Gata3 during prostate development, we used the Nkx3-1Cre knockin mouse line in combination with a Gata3 conditional knockout allele (Gata3f/f) (Grote et al., 2006, Thomsen et al., 2008) (Figure S1B). Although it is later restricted to the luminal compartment, Nkx3-1Cre is expressed in both basal and luminal lineages during early development and efficiently activated the Rosa26dtTomato lineage tracer allele in the basal enriched CD24+;CD49f+ cell population at 2 weeks of age (Figure 1C) (Wu et al., 2011). Exon 4 of Gata3 is also deleted by Nkx3-1Cre in both lineages at 2 weeks of age, leading to a loss of GATA3 protein in basal and luminal cells (Figures 1D and 1E). To visualize branching morphogenesis of the developing prostate, we took advantage of the Rosa26RLacZ reporter allele (Soriano, 1999), which was effectively activated in the prostate epithelium of Nkx3-1Cre;R26RLacZ mice. At 2 weeks of age, Gata3-deficient prostates were smaller than control prostates and harbored less complex branching patterns, with significantly fewer ductal tips and branching points (Figures 2A and 2B). Similar smaller prostates and defective ductal architecture were also observed at 6 weeks of age (Figure S2). Cross-sections from 2- to 6-week-old Gata3-deficient prostates showed hyperplasia of the epithelial compartment as compared to control animals (Figures 2C and 2D). This was associated with an increase in proliferation (phospho-histone H3 marks), but no significant changes in cell death (TUNEL) (Figure 2E). Together, these results point to an important role for Gata3 in prostate development.
Figure 2.
Gata3 Is Required for Branching Morphogenesis and Prostate Epithelial Homeostasis
(A) Ductal architecture of control and Nkx3-1Cre/+;Gata3flox/flox prostates and individual lobes at 2 weeks of age as shown by β-galactosidase staining. Scale bars, 1 mm.
(B) Quantification of the number of prostate ducts and branch points in control and Nkx3-1Cre/+;Gata3flox/flox prostates.
(C) H&E staining of developing (2-week-old) prostate sections in control and Nkx3-1Cre/+;Gata3flox/flox mice. Scale bars, 20 μm.
(D) H&E staining of developing (6-week-old) prostate sections in control and Nkx3-1Cre/+;Gata3flox/flox mice. Black bars indicate the thickness of the epithelial layer. Scale bars, 20 μm.
(E) Quantification of proliferating (phospho-histone H3-positive cells) and apoptotic (TUNEL staining) cells in control and Nkx3-1Cre/+;Gata3flox/flox tissue at P14. ∗p < 0.05.
Representative images and quantifications are from three control and Nkx3-1Cre/+;Gata3flox/flox prostates, except in (B), where error bars represent SE from two control and Nkx3-1Cre/+;Gata3flox/flox prostates.
Gata3 Deficiency Leads to Disorganization of Epithelial Polarity and an Accumulation of Intermediate Progenitor Cells
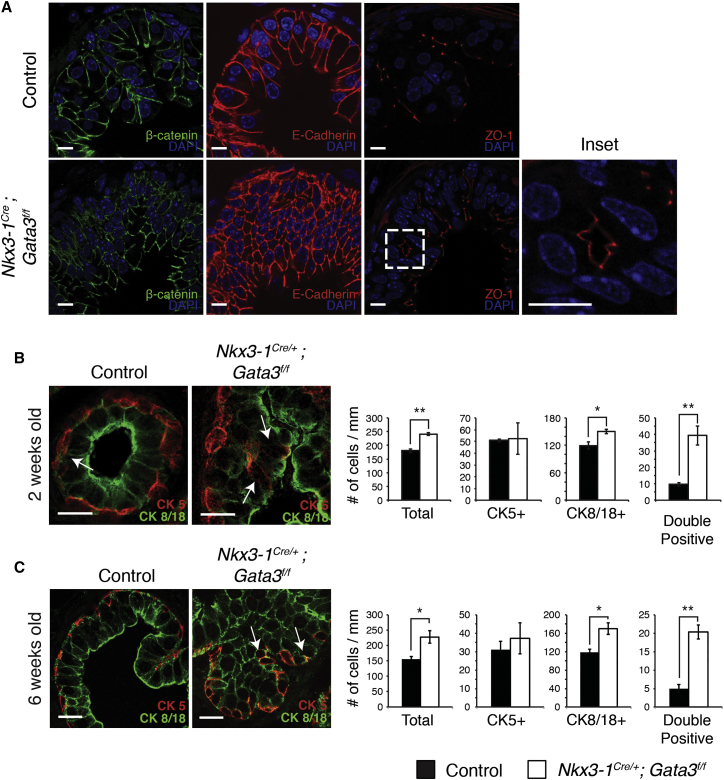
We next assessed whether disrupted prostate epithelial architecture induced by loss of Gata3 affected apical/basal cell polarity. In wild-type prostate epithelial cells, E-cadherin localizes to the basal-lateral domain and ZO-1 marks tight junctions on the apical domain (Figure 3A). Interestingly, hyperplastic regions of Gata3-deficient prostates showed an accumulation of non-polarized cells, evidenced by expression of E-cadherin throughout the cell membrane (Figure 3A). Gata3-deficient luminal cells maintained ZO-1 localization and apical-basal expression of E-cadherin, indicating that they retained the ability to form a polarized epithelial layer. This staining further revealed the formation of ectopic lumens within hyperplastic regions of the epithelium (Figure 3A, inset).
Figure 3.
Loss of Gata3 Disrupts Prostate Epithelial Polarity and Increases the Double-Positive Progenitor Cell Population
(A) Immunofluorescence staining of basolateral (B-catenin and E-cadherin) and apical (ZO-1) markers in control and Nkx3-1Cre/+;Gata3flox/flox prostate tissue.
(B) Immunofluorescence staining of basal (CK5) and luminal (CK8/18) cell markers in 2-week-old control and Nkx3-1Cre/+;Gata3flox/flox prostate and quantification of the number of single-positive basal (CK5+), luminal (CK8/18+), and double-positive intermediate progenitor (CK5+; CK8/18+) cells per millimeter of ductal circumference.
(C) Immunofluorescence staining of basal (CK5) and luminal (CK8/18) cell markers in 6-week-old control and Nkx3-1Cre/+;Gata3flox/flox prostate and quantification of the number of single-positive basal (CK5+), luminal (CK8/18+), and double-positive intermediate progenitor (CK5+; CK8/18+) cells per millimeter of ductal circumference. Arrows in (B and C) indicate double-positive cells.
∗p < 0.05, ∗∗p < 0.01. Representative images and quantifications are from four control and Nkx3-1Cre/+;Gata3flox/flox prostates. Error bars represent SE from three (B) and four (C) control and Nkx3-1Cre/+;Gata3flox/flox prostates. Scale bars, 10 μm.
In the absence of major defects in cellular apical-basal polarity within the luminal lineage, the observed epithelial defects could be caused by an effect on lineage specification from progenitor cells. To identify cell lineage defects within the prostate, we assessed the number of single-positive basal cells (CK5+), single-positive luminal cells (CK8/18+), or double-positive intermediate progenitor cells (CK5+; CK8/18+) in both control and Gata3-deficient 2-week-old prostate tissue by immunohistochemistry (IHC). This analysis revealed that epithelial hyperplasia was caused by an increase in the number of intermediate progenitor and luminal cells, while the number of single-positive basal cells was not significantly affected (Figure 3B). Analysis of 6-week-old prostates revealed a similar accumulation of intermediate progenitor cells (Figure 3C). In addition, double-positive cells were found throughout both the basal and luminal compartments in Gata3-deficient prostates, in contrast to their typical basal location in control tissue. Together, these results identify a crucial role for Gata3 in establishing tissue architecture and lineage specification during prostate development.
Gata3 Deficiency Leads to Spindle Orientation Defects
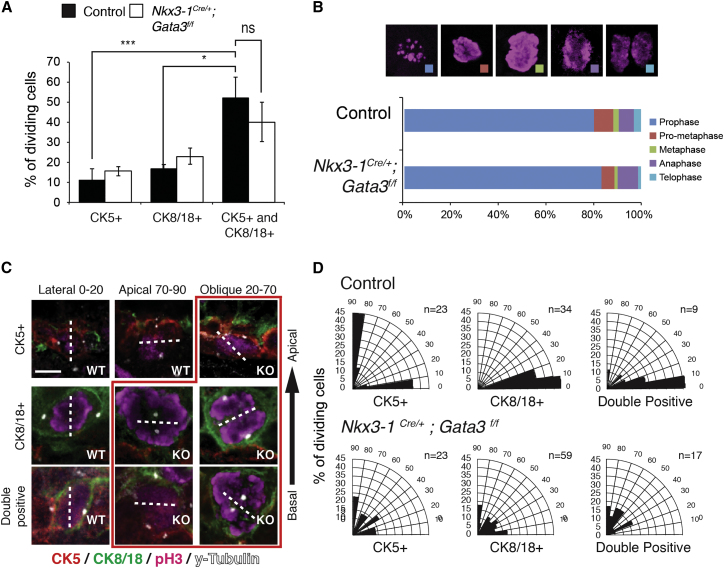
These changes in cell lineage composition raised the intriguing possibility that the hyperplasia of Gata3 mutant prostates may be caused by the increase in the population of intermediate progenitor cells rather than an increase in the proliferation rate of Gata3-deficient cells. To test this we measured the number of proliferative basal, luminal, and double-positive cells, controlling for the size of each population. This analysis revealed a 3- to 5-fold higher proliferation rate of intermediate progenitors relative to single-positive cells in wild-type prostates (Figure 4A). However, the proliferation rate and cell-cycle progression of Gata3-deficient cells were unchanged compared with controls (Figures 4A and 4B). This important result shows that the hyperplastic phenotype, and increased luminal cell numbers, results from an increase in intermediate progenitor specification rather than a defect in proliferation control of Gata3-deficient prostatic epithelial cells.
Figure 4.
Loss of Gata3 Randomizes Spindle Orientation in Basal Progenitor Cells without Affecting Their Proliferation Rate
(A) Percentage of dividing cells in each epithelial lineage in control and Nkx3-1Cre/+;Gata3flox/flox prostate tissue.
(B) Analysis of the phases of mitosis in control and Nkx3-1Cre/+;Gata3flox/flox prostate tissue.
(C) Mitotic spindle orientation was measured in basal (CK5+), luminal (CK8/18+), and double-positive cells (CK5+; CK8/18+) of control and Nkx3-1Cre/+;Gata3flox/flox (red box) prostate tissue by γ-tubulin (spindle poles) and phospho-histone H3 (dividing cells) immunofluorescence staining.
(D) Quantification of spindle orientations in the three cell types in control and Nkx3-1Cre/+;Gata3flox/flox prostate.
Dashed lines indicate axis of cytokinesis. ∗p < 0.05, ∗∗∗p < 0.001; ns, not significant. Quantifications are from four control and Nkx3-1Cre/+;Gata3flox/flox prostates. Error bars in (A) represent SE from four control and Nkx3-1Cre/+;Gata3flox/flox prostate replicates. n values in (D) represent the total number of cell divisions quantified per cell type. Scale bars, 5 μm.
Given the correlation between spindle orientation and prostate lineage specification (Wang et al., 2014), we reasoned that the increase in double-positive intermediate progenitors could be secondary to a defect in the regulation of oriented cell division in basal cells. To identify dividing cells and the orientation of their spindle poles, we co-stained prostates for CK5 and CK8/18 (basal and luminal markers, respectively), γ-tubulin (spindle poles), and phospho-histone H3 to mark mitotic cells (mother cells during metaphase/anaphase or daughter cells during telophase). This analysis in control prostate tissues at 2 weeks of age confirmed that basal cells divide both vertically (basal-luminal) or horizontally (within the cell layer), whereas luminal cells only divide horizontally (Figures 4C and 4D). Strikingly, in the absence of Gata3, both the basal and luminal cell division angles were randomized (Figures 4C and 4D). In addition, γ-tubulin staining revealed a centrosome positioning defect in basal cells during interphase, which is a marker of polarization within these cells (Figure S3). Together, these results indicate that Gata3 is required for mitotic spindle orientation and that spindle randomization may lead to lineage specification defects.
Gata3 Is Necessary for Normal PRKCZ Subcellular Localization in Basal and Luminal Cells
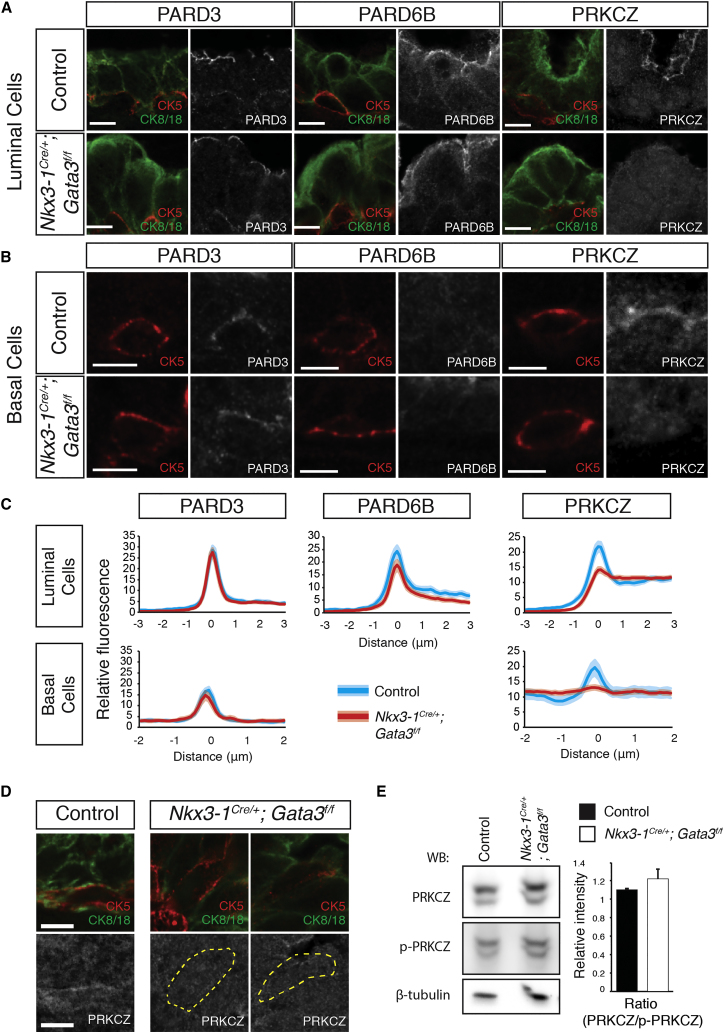
The apically localized Par complex is critical for the establishment and maintenance of epithelial cell polarity and for mitotic spindle orientation during symmetric/asymmetric cell division (Rodriguez-Boulan and Macara, 2014). We determined the subcellular localization of Par complex components PARD3, PARD6B, and PRKCZ by IHC. PARD3 and PARD6B were specifically localized to the apical domains in both wild-type and Gata3 null luminal cells (Figure 5A). PARD3 was also detected in basal cells, and was localized to the apical membrane in both control and Gata3 null tissue (Figure 5B), while the PARD6B isoform was not detected in basal cells (Figure 5B). Importantly, PRKCZ, which localized to the apical membrane of both luminal and basal cells in control ducts, failed to do so in Gata3-deficient prostates (Figures 5A and 5B). Measurements of fluorescence across the apical membrane (from apical side to nucleus) revealed a significant decrease in apical accumulation of PRKCZ in both cell types, leading to a diffuse staining in the cytoplasm (Figure 5C). To confirm this result and rule out any phenotypic contribution of Nkx3-1 heterozygosity in Nkx3-1Cre/+;Gata3f/f mice, we derived embryonic day 18.5 urogenital sinuses (UGS) by chemical rescue of germline Gata3 mutant embryos (Kaufman et al., 2003, Lim et al., 2000) and implanted them under the kidney capsule of immunodeficient mice for 2 weeks (Figure S4A). Immunohistochemical analysis of UGS derivative tissues showed normal PARD3 and PARD6B expression but mislocalization of PRKCZ in both prostate and seminal vesicle epithelial cells (Figures S4D–S4E).
Figure 5.
Gata3 Controls PRKCZ Localization in Prostate Basal and Luminal Cells
(A) Immunofluorescence of PARD3, PARD6B, and PRKCZ in luminal cells of control and Nkx3-1Cre/+;Gata3flox/flox prostates.
(B) Immunofluorescence of PARD3, PARD6B, and PRKCZ in basal cells of control and Nkx3-1Cre/+;Gata3flox/flox prostates.
(C) Quantification of PARD3, and PRKCZ fluorescence intensity across the apical membrane in luminal and basal cells measured from the lumen (−3/2) to the nucleus (+3/2), and quantification of PARD6B localization in luminal cells.
(D) Example immunofluorescence images of CK5, CK8/18, and PRKCZ staining in double-positive intermediate cells. Dashed lines indicate the periphery of double-positive cells.
(E) Western blot of PRKCZ and T-560 phosphorylated PRKCZ from control and Nkx3-1Cre/+;Gata3flox/flox mice.
Images are representative of results from five control or Nkx3-1Cre/+;Gata3flox/flox prostates, and quantification is from three control and Nkx3-1Cre/+;Gata3flox/flox prostates (thickness of lighter red and blue bands represent SD from the mean). Scale bars, 5 μm.
To determine whether the mislocalized PRKCZ is still active in Gata3-deficient prostates, we next assessed the amount of total and phosphorylated PRKCZ (phosphoT560) by western blot. This analysis revealed no differences in total or active PRKCZ between control and Gata3 null prostates (Figure 5D), indicating that Gata3 regulates PRKCZ localization without affecting its activation potential.
We next assessed the transcriptional consequences of Gata3 deficiency by microarray analysis of laser captured prostate epithelial tissue from control and Gata3-deficient prostates (Figure S5, Table S1, and Data S1). This analysis revealed major effects on the secretory function of the prostate (secreted, signal, disulfide bond, extracellular matrix, glycoprotein), and on genes involved in immunity, most likely reflective of the known functions of GATA3 (Figure S5B). However, no obvious mediators of PRKCZ subcellular localization were identified.
Mislocalization of PRKCZ Is Sufficient to Cause Spindle Orientation and Lineage Specification Defects
To assess whether the misregulation of the polarity regulator PRCKZ was sufficient to generate spindle orientation defects, we made use of the small molecule aurothiomalate (ATM), which inhibits the interaction between PRKCZ and PARD6B by specifically binding to the PB1 domain of PRKCZ (Erdogan et al., 2006). Treatment with ATM has been successfully used to disrupt asymmetric cell division in T cells (Oliaro et al., 2010). We initially used Caco-2 cells, which are a well-established model of epithelial morphogenesis and form highly polarized cysts that exhibit strictly planar/symmetric divisions, which are reliant upon PRKCZ (Durgan et al., 2011). Strikingly, ATM treatment led to a randomization of mitotic spindle orientation and a decrease in the formation of hollow cysts accompanied by an increase in the incidence of cysts with multiple lumens (Figure S6). These results suggest that apical PRKCZ-PARD6B interaction is important for proper mitotic spindle orientation and epithelial morphogenesis.
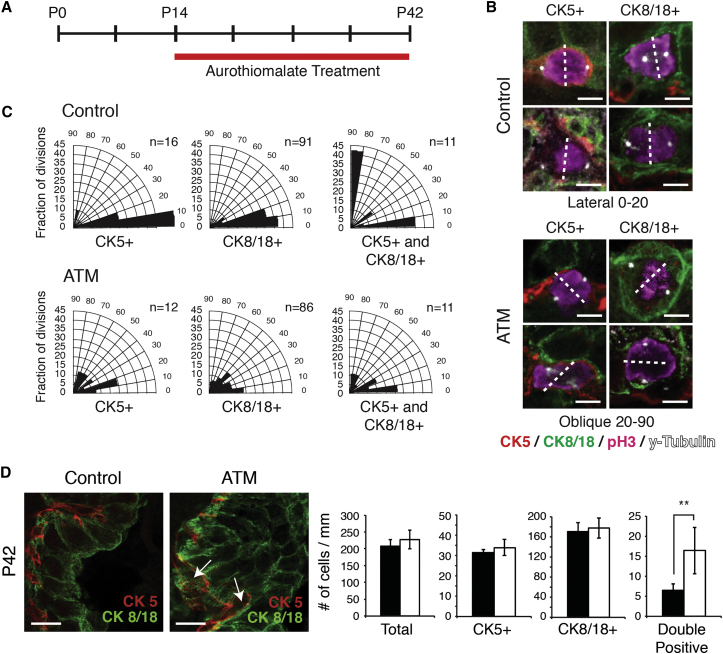
We next asked whether the PRKCZ-PARD6B interaction was necessary for mitotic spindle regulation and lineage specification in vivo. To this end, we treated adolescent male mice with ATM for 4 weeks during prostate development (Figure 6A). At 6 weeks of age, control and ATM-treated prostates were dissected, and both spindle orientation and epithelial lineage specification were analyzed. As expected, both basal and luminal cells of control animals divided strictly symmetrically, parallel to the duct, consistent with their established unipotency at this stage (Figures 6B and 6C) (Wuidart et al., 2016). Strikingly, ATM treatment was sufficient to randomize the mitotic spindle in both basal and luminal cells (Figures 6B and 6C), and randomization of the mitotic spindle during prostate development caused a significant increase in the population of double-positive progenitor cells compared with control prostates (Figure 6D). These results demonstrate that spindle orientation is required for proper lineage specification from basal prostate epithelial cells, and highlight the critical importance of PRKCZ regulation during prostate development (Figure 7).
Figure 6.
Disruption of PRKCZ-PARD6B Interaction Increases the Population of Double-Positive Intermediate Progenitor Cells in the Developing Prostate
(A) Timeline of ATM administration to adolescent male mice.
(B) Representative examples of spindle orientations in control or ATM-treated prostates at 6 weeks old. Dashed lines indicate axis of cytokinesis.
(C) Quantification of mitotic spindle orientation measured in basal (CK5+), luminal (CK8/18+), and double-positive cells (CK5+; CK8/18+) in control and ATM-treated prostate tissue. Spindle orientation was revealed by γ-tubulin (spindle poles) and phospho-histone H3 (dividing cells) immunofluorescence staining.
(D) Immunofluorescence of control and ATM-treated prostates, and quantification of the number of single-positive basal (CK5+), luminal (CK8/18+), and double-positive progenitor (CK5+; CK8/18+) (arrows) cells in control and ATM-treated prostates. ∗∗p < 0.01.
Representative images and quantifications are from four control and four ATM-treated prostates. n values in (C) represent the total number of cell divisions quantified per cell type. Scale bars, 5 μm (B) and 10 μm (D).
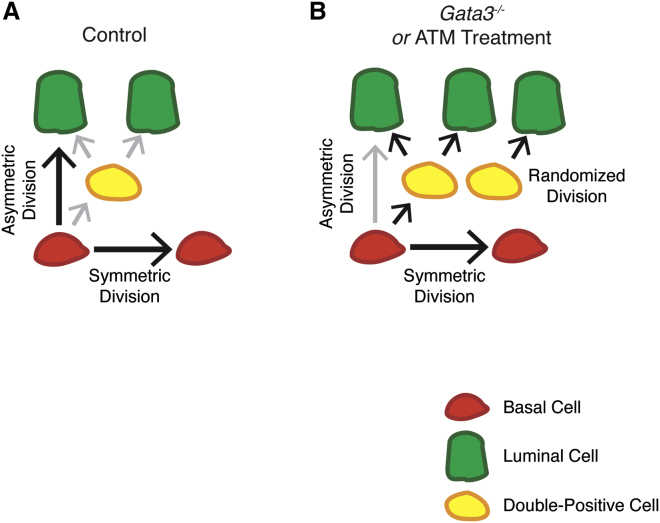
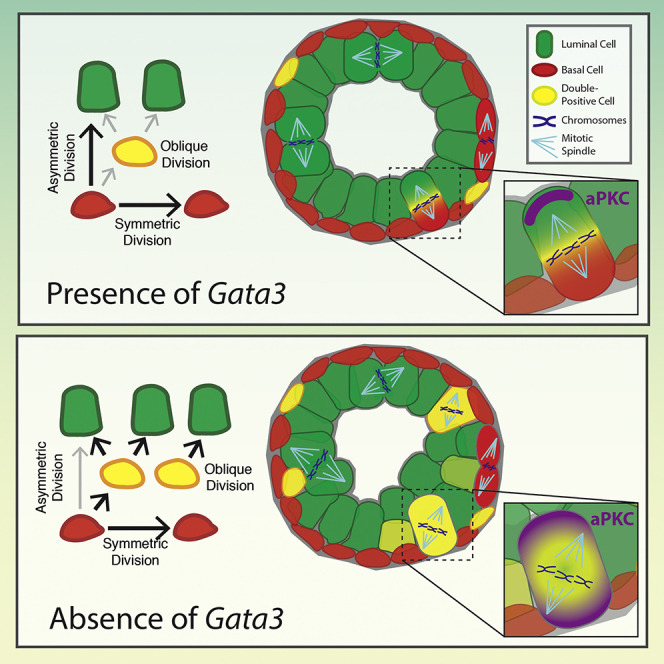
Figure 7.
Control of Lineage Specification and Cell-Type Stratification during Prostate Development
(A) During normal prostate development, basal (red) cells generate both the basal and luminal lineages using symmetric and asymmetric cell divisions. In addition, double-positive cells (yellow) are generated from basal cells and contribute to the formation of the luminal lineage (green).
(B) Upon loss of Gata3 or treatment with ATM, spindle orientation is randomized, leading to an increase in the number of intermediate progenitor cells. Increased intermediate progenitor cells contribute to an expanded luminal layer and prostate epithelial hyperplasia.
Discussion
Basal prostate epithelial cells divide either symmetrically to expand the basal progenitor cell pool, or asymmetrically to generate double-positive intermediate progenitor cells or differentiated luminal cells (Ousset et al., 2012, Wang et al., 2013, Wuidart et al., 2016). However, the molecular determinants underlying this process and the consequences of altering spindle orientation in the prostate are currently unknown. In this report we show that loss of Gata3 leads to tissue hyperplasia by accumulation of a poorly polarized cell layer at the interphase between the basal and luminal cells, largely composed of intermediate progenitor cells. This disruption in tissue architecture results from a defect in mitotic spindle orientation secondary to mislocalization of the polarity protein PRKCZ from PARD3/PARD6B. Accordingly, disruption of the PRKCZ-PARD6B interaction by ATM alone was sufficient to disrupt spindle orientation and lineage specification. Together, these results underscore the critical importance of regulating spindle orientation within progenitor cells to generate and maintain stable tissue architecture composed of stratified basal and luminal cell layers.
The most striking phenotype observed in Gata3-deficient prostates is a complete randomization of the mitotic spindle in both basal and luminal cells. In other systems, orientation of the mitotic spindle is recognized as the primary endogenous mechanism to regulate symmetric versus asymmetric cell divisions and control the inheritance of cellular fates within the resulting daughter cells (Knoblich, 2010, Morin and Bellaiche, 2011, Neumuller and Knoblich, 2009). This developmental program and its regulatory machinery are largely conserved during evolution, but little is known of the role of spindle and polarity proteins during development and homeostasis (Pearson et al., 2011, Win and Acevedo-Duncan, 2008, Zhang et al., 2016). Evidence from lineage tracing analysis and measurements of the mitotic spindle during lineage specification strongly support the presence of bipotent basal progenitor cells able to expand the basal compartment via laterally oriented divisions, or generate differentiated luminal cells via apical-oriented divisions (Ousset et al., 2012, Wang et al., 2014). Generation of luminal cells is partially accomplished through the formation of intermediate progenitors with a “double-positive” (CK5+; CK8/18+) signature. In contrast, luminal cells appear to be strictly unipotent and only capable of planar-oriented divisions during development and homeostasis. Importantly, this differentiation process occurs in a highly regulated manner such that prostatic ducts maintain stratified and single-cell thick layers of basal and luminal cells following mitotic events. In Gata3-deficient embryos, mitotic spindle randomization does not overtly affect the basal progenitor pool or the polarity of differentiated non-proliferative cells lining the lumen. Instead, it affects the relative allocation of the different lineages by accumulation of intermediate progenitor cells at the interphase between the differentiated basal and luminal cell compartments. These progenitor cells, along with oblique and apical divisions of luminal cells, disrupt tissue architecture by generating a surplus of luminal cells. Although we cannot exclude the possibility that some double-positive cells arise from aberrant luminal cell divisions, the fact that we observe spindle randomization in basal cell progenitors indicates disruption of the normal specification process in those cells.
We also find that PRKCZ fails to accumulate normally at the apical membrane in Gata3 mutant embryos, even though its expression and activation levels are maintained. PRKCZ is part of the apical Par complex comprising PARD3 and PARD6B and acts as a key regulator of mitotic spindle orientation in other systems (Durgan et al., 2011, Guilgur et al., 2012, Niessen et al., 2013). Of interest, the subcellular redistribution of PRKCZ is found in the presence of normal PARD3 and PARD6B apical expression, suggesting a decoupling of PRKCZ from the Par complex. Very few examples of such a decoupling phenotype have been documented so far. Only loss of Patj (Inadl) in MDCK cells or Dlg5 during mouse lung development have been associated with a mislocalization of PRKCZ, possibly through PARD3 and PARD6B protein misexpression, which is not observed upon Gata3 loss (Adachi et al., 2009, Nechiporuk et al., 2013, Shin et al., 2005). The detailed mechanisms by which loss of Gata3 leads to PRKCZ mislocalization will require further investigation.
Using ATM, a small molecule that affects the interaction between PRKCZ and the Par complex (Erdogan et al., 2006), we showed that PRKCZ decoupling from PARD6B is sufficient to cause mitotic spindle orientation and epithelial defects. This is in line with the known role of PRKCZ as a kinase for spindle pole proteins (Hao et al., 2010). Phosphorylation by apical PRKCZ has been proposed to prevent spindle machinery from interacting with the apical domain, thereby preventing asymmetric cell division (Chatterjee and McCaffrey, 2014, Hao et al., 2010). Together, our results suggest a model by which the mislocalization of PRKCZ by loss of Gata3 leads to spindle randomization by ectopic inhibition of the interaction between astral microtubules and the cell cortex (Figure 7).
The epithelial hyperplasia resulting from spindle randomization is likely to be an important underlying cause of the branching morphogenesis defects observed in Gata3-deficient prostates. Hyperplastic tissue remained epithelial in this system as evidenced by the maintenance of the epithelial markers E-cadherin, ZO-1, and Par complex components, suggesting that the role of Gata3 in prostate progenitor cells is different from the regulation of epithelial-mesenchymal transition reported in metastatic prostate cancer cells (Jiang et al., 2016, Wang et al., 2015).
An interesting consequence of these findings is that they provide a mechanism by which tissue hyperplasia occurs in the absence of cellular transformation. It is likely that this intermediate population generated by aberrant spindle orientation defects constitutes an epithelial cell population prone to oncogenic growth and dissemination upon transformation. Spindle orientation defects may therefore contribute to the hyperplastic and tumor progression phenotypes observed in the adult prostates and in other systems such as the skin and mammary gland in the absence of Gata3 (Kaufman et al., 2003, Kouros-Mehr et al., 2006, Kouros-Mehr et al., 2008, Nguyen et al., 2013). Together, this work highlights the critical importance of regulating mitotic spindle orientation in progenitor cells to control the stepwise cellular differentiation process and maintain tissue architecture and homeostasis.
Experimental Procedures
Mice
All experimental mice were kept in a C57BL/6 background. Nkx3-1Cre (Thomsen et al., 2008), Gata3flox, Gata3GFP (Grote et al., 2006), Rosa26LacZ (R26R) (Soriano, 1999), and Rosa26dtTomato (Madisen et al., 2010) mice were described previously. Immunodeficient SCID-beige mice were obtained from Charles River and kept in pathogen-free conditions. All animal procedures were approved by McGill University Animal Care Committee according to the Canadian Council on Animal Care guidelines for use of laboratory animals in biological research. Genotyping primers used are listed in Table S2. All histological analyses were performed on dorsal-lateral prostates.
In Situ Hybridization and β-Galactosidase Staining
Gata3 and Nkx3-1 probe sequences have been previously described (Bhatia-Gaur et al., 1999, George et al., 1994). RNA probes for in situ hybridization were synthesized using T7 or SP6 RNA polymerase following the manufacturer’s specifications (Roche). Tissues for in situ hybridization were fixed in 4% paraformaldehyde (PFA), passed through a sucrose gradient, embedded in optimal cutting temperature (OCT) medium, and sectioned at 12 μm. β-Galactosidase activity was detected by X-gal staining of whole-mount prostate tissue. In brief, whole prostates were fixed in Beta-Gal fixation solution, washed, stained using Beta-Gal staining solution for 1 hr at 37°C, and fixed in 4% PFA for 30 min.
qRT-PCR
Total RNA was extracted from control (n = 6) or Nkx3-1Cre/+;Gata3flox/flox (n = 6) total or sorted prostate cells using an RNeasy mini kit (Qiagen) and reverse transcribed with Moloney murine leukemia virus (Invitrogen) according to the manufacturer’s protocol. Real-time qPCR was performed using Green-2-go Mastermix (BioBasic) on a Realplex2 Mastercycler (Eppendorf). All primers used are listed in Table S2.
FACS Sorting and Analysis
Prostate tissue was dissected in cold PBS and 2% fetal bovine serum, minced, and digested at 37°C for 3 hr in collagenase/hyaluronidase solution (StemCell Technologies), followed by 5 min in 0.25% trypsin/EDTA and 10 min in 5 U/mL of dispase II with 0.1 mg/mL DNase I (Roche). The digested cells were passed through a 27-gauge needle and filtered through a 70-μm cell strainer. Single cells were stained on ice for 30 min with antibodies from BioLegend: CD45 (30-F11), Ter119 (TER-119), CD31 (MEC13.3), CD49f (GoH3), and CD24 (M1/69) (Wang et al., 2013). Fixable Viability dye (eBioscience) was used to select viable cells. For intracellular staining, cells were stained with CK5 antibody (Poly19055, BioLegend) and anti-rabbit Alexa Fluor 488 (Life Technologies) secondary antibody using a BD Cytofix/Cytoperm Kit (BD Biosciences). FACS analysis and sorting was performed on a BD Fortessa and Aria Fusion apparatus (BD Biosciences). Unstained cells, single fluorochrome-stained cells, and cells stained as FMO (fluorescence minus one) were used to set up the machine and gating strategy. Data were analyzed using FlowJo software.
Immunohistochemistry
Immunofluorescence staining was performed on freshly frozen tissue embedded in OCT and sectioned to obtain 10- to 15-μm thick sections as described by Nguyen et al. (2013). Antibodies and dilutions used are listed in Table S3. Five mice of each genotype were used in IHC experiments.
Aurothiomalate Administration
Three control and four 2-week-old mice were injected intraperitoneally once daily with 80 mg/kg ATM in PBS (Sigma-Aldrich) for 4 weeks to inhibit the PRKCZ-PARD6B interaction in vivo. Solutions were light protected and stored at −20°C.
Microscopy and Image Analysis
H&E and bright-field whole-mount images were acquired with an Axioplan 2 microscope (Zeiss). Immunofluorescence images were acquired using an LSM3, LSM710, LSM780, or LSM800 confocal microscope (Zeiss). For quantification and spindle orientation analysis, images were captured using a spectral detector and linear unmixing was performed using Zen software (Zeiss).
Additional materials and methods are detailed in Supplemental Experimental Procedures.
Author Contributions
M.E.R.S., A.H.T.N., M.T., S.V., and M.B. performed experiments. N.R.B. and M.P. contributed to microarray analysis. M.E.R.S., A.H.T.N., and M.B. conceived the project. M.E.R.S. and M.B. wrote the manuscript.
Acknowledgments
We are grateful to members of the Bouchard laboratory for critical reading of the manuscript. We would like to thank the McGill Advanced BioImaging Facility (ABIF) for their technical support with spectral unmixing and confocal microscopy, as well as the Flow Cytometry platform of the McGill University Life Sciences Complex for cell sorting. This work was supported by grants from the Canadian Institutes of Health Research (CIHR; MOP-130460) and the Cancer Research Society (CRS, Canada) to M.B. M.B. holds a Senior Research Scholar Award from the Fonds de la Recherche du Québec-Santé (FRQS). M.E.R.S. was supported by a Graduate Studentship from Prostate Cancer Canada, and a Lloyd-Carr Harris Graduate Studentship from McGill University. A.H.T.N. was supported by a studentship from the CIHR and the McGill Integrated Cancer Research Training Program (MICRTP). M.T. was supported by a Dr. Gerald B. Price Fellowship (Cancer Research Society), and MICRTP and FRSQ postdoctoral fellowships.
Published: March 9, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, four tables, and one data file and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.02.004.
Supplemental Information
References
- Adachi M., Hamazaki Y., Kobayashi Y., Itoh M., Tsukita S., Furuse M., Tsukita S. Similar and distinct properties of MUPP1 and Patj, two homologous PDZ domain-containing tight-junction proteins. Mol. Cell Biol. 2009;29:2372–2389. doi: 10.1128/MCB.01505-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.M., Macara I.G. Mechanisms of polarity protein expression control. Curr. Opin. Cell Biol. 2016;42:38–45. doi: 10.1016/j.ceb.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Labat M.L., Sutherland K.D., Barker H., Thomas R., Shackleton M., Forrest N.C., Hartley L., Robb L., Grosveld F.G., van der Wees J. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat. Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- Bergstralh D.T., St Johnston D. Spindle orientation: what if it goes wrong? Semin. Cell Dev. Biol. 2014;34:140–145. doi: 10.1016/j.semcdb.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia-Gaur R., Donjacour A.A., Sciavolino P.J., Kim M., Desai N., Young P., Norton C.R., Gridley T., Cardiff R.D., Cunha G.R. Roles for Nkx3.1 in prostate development and cancer. Genes. Dev. 1999;13:966–977. doi: 10.1101/gad.13.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S.J., McCaffrey L. Emerging role of cell polarity proteins in breast cancer progression and metastasis. Breast Cancer. 2014;6:15–27. doi: 10.2147/BCTT.S43764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi N., Zhang B., Zhang L., Ittmann M., Xin L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell. 2012;21:253–265. doi: 10.1016/j.ccr.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgan J., Kaji N., Jin D., Hall A. Par6B and atypical PKC regulate mitotic spindle orientation during epithelial morphogenesis. J. Biol. Chem. 2011;286:12461–12474. doi: 10.1074/jbc.M110.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dydensborg A.B., Rose A.A., Wilson B.J., Grote D., Paquet M., Giguere V., Siegel P.M., Bouchard M. GATA3 inhibits breast cancer growth and pulmonary breast cancer metastasis. Oncogene. 2009;28:2634–2642. doi: 10.1038/onc.2009.126. [DOI] [PubMed] [Google Scholar]
- Erdogan E., Lamark T., Stallings-Mann M., Lee J., Pellecchia M., Thompson E.A., Johansen T., Fields A.P. Aurothiomalate inhibits transformed growth by targeting the PB1 domain of protein kinase Ciota. J. Biol. Chem. 2006;281:28450–28459. doi: 10.1074/jbc.M606054200. [DOI] [PubMed] [Google Scholar]
- George K.M., Leonard M.W., Roth M.E., Lieuw K.H., Kioussis D., Grosveld F., Engel J.D. Embryonic expression and cloning of the murine GATA-3 gene. Development. 1994;120:2673–2686. doi: 10.1242/dev.120.9.2673. [DOI] [PubMed] [Google Scholar]
- Grote D., Souabni A., Busslinger M., Bouchard M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development. 2006;133:53–61. doi: 10.1242/dev.02184. [DOI] [PubMed] [Google Scholar]
- Guilgur L.G., Prudencio P., Ferreira T., Pimenta-Marques A.R., Martinho R.G. Drosophila aPKC is required for mitotic spindle orientation during symmetric division of epithelial cells. Development. 2012;139:503–513. doi: 10.1242/dev.071027. [DOI] [PubMed] [Google Scholar]
- Hao Y., Du Q., Chen X., Zheng Z., Balsbaugh J.L., Maitra S., Shabanowitz J., Hunt D.F., Macara I.G. Par3 controls epithelial spindle orientation by aPKC-mediated phosphorylation of apical Pins. Curr. Biol. 2010;20:1809–1818. doi: 10.1016/j.cub.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Chen Y., Du E., Yang K., Zhang Z., Qi S., Xu Y. GATA3-driven expression of miR-503 inhibits prostate cancer progression by repressing ZNF217 expression. Cell Signal. 2016;28:1216–1224. doi: 10.1016/j.cellsig.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Kaufman C.K., Zhou P., Pasolli H.A., Rendl M., Bolotin D., Lim K.C., Dai X., Alegre M.L., Fuchs E. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes. Dev. 2003;17:2108–2122. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich J.A. Asymmetric cell division: recent developments and their implications for tumour biology. Nat. Rev. Mol. Cell Biol. 2010;11:849–860. doi: 10.1038/nrm3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H., Slorach E.M., Sternlicht M.D., Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–1055. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H., Bechis S.K., Slorach E.M., Littlepage L.E., Egeblad M., Ewald A.J., Pai S.Y., Ho I.C., Werb Z. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13:141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K.C., Lakshmanan G., Crawford S.E., Gu Y., Grosveld F., Engel J.D. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat. Genet. 2000;25:209–212. doi: 10.1038/76080. [DOI] [PubMed] [Google Scholar]
- Liu J., Pascal L.E., Isharwal S., Metzger D., Ramos Garcia R., Pilch J., Kasper S., Williams K., Basse P.H., Nelson J.B. Regenerated luminal epithelial cells are derived from preexisting luminal epithelial cells in adult mouse prostate. Mol. Endocrinol. 2011;25:1849–1857. doi: 10.1210/me.2011-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker P.C., Donjacour A.A., Dahiya R., Cunha G.R. Hormonal, cellular, and molecular control of prostatic development. Dev. Biol. 2003;253:165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Morin X., Bellaiche Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev. Cell. 2011;21:102–119. doi: 10.1016/j.devcel.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Nechiporuk T., Klezovitch O., Nguyen L., Vasioukhin V. Dlg5 maintains apical aPKC and regulates progenitor differentiation during lung morphogenesis. Dev. Biol. 2013;377:375–384. doi: 10.1016/j.ydbio.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumuller R.A., Knoblich J.A. Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes. Dev. 2009;23:2675–2699. doi: 10.1101/gad.1850809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A.H., Tremblay M., Haigh K., Koumakpayi I.H., Paquet M., Pandolfi P.P., Mes-Masson A.M., Saad F., Haigh J.J., Bouchard M. Gata3 antagonizes cancer progression in Pten-deficient prostates. Hum. Mol. Genet. 2013;22:2400–2410. doi: 10.1093/hmg/ddt088. [DOI] [PubMed] [Google Scholar]
- Niessen M.T., Scott J., Zielinski J.G., Vorhagen S., Sotiropoulou P.A., Blanpain C., Leitges M., Niessen C.M. aPKClambda controls epidermal homeostasis and stem cell fate through regulation of division orientation. J. Cell Biol. 2013;202:887–900. doi: 10.1083/jcb.201307001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliaro J., Van Ham V., Sacirbegovic F., Pasam A., Bomzon Z., Pham K., Ludford-Menting M.J., Waterhouse N.J., Bots M., Hawkins E.D. Asymmetric cell division of T cells upon antigen presentation uses multiple conserved mechanisms. J. Immunol. 2010;185:367–375. doi: 10.4049/jimmunol.0903627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousset M., Van Keymeulen A., Bouvencourt G., Sharma N., Achouri Y., Simons B.D., Blanpain C. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat. Cell Biol. 2012;14:1131–1138. doi: 10.1038/ncb2600. [DOI] [PubMed] [Google Scholar]
- Pearson H.B., Perez-Mancera P.A., Dow L.E., Ryan A., Tennstedt P., Bogani D., Elsum I., Greenfield A., Tuveson D.A., Simon R. SCRIB expression is deregulated in human prostate cancer, and its deficiency in mice promotes prostate neoplasia. J. Clin. Invest. 2011;121:4257–4267. doi: 10.1172/JCI58509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignon J.C., Grisanzio C., Geng Y., Song J., Shivdasani R.A., Signoretti S. p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia. Proc. Natl. Acad. Sci. USA. 2013;110:8105–8110. doi: 10.1073/pnas.1221216110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Macara I.G. Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 2014;15:225–242. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K., Straight S., Margolis B. PATJ regulates tight junction formation and polarity in mammalian epithelial cells. J. Cell Biol. 2005;168:705–711. doi: 10.1083/jcb.200408064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Thomsen M.K., Butler C.M., Shen M.M., Swain A. Sox9 is required for prostate development. Dev. Biol. 2008;316:302–311. doi: 10.1016/j.ydbio.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Wang X., Kruithof-de Julio M., Economides K.D., Walker D., Yu H., Halili M.V., Hu Y.P., Price S.M., Abate-Shen C., Shen M.M. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.A., Mitrofanova A., Bergren S.K., Abate-Shen C., Cardiff R.D., Califano A., Shen M.M. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat. Cell Biol. 2013;15:274–283. doi: 10.1038/ncb2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhu H.H., Chu M., Liu Y., Zhang C., Liu G., Yang X., Yang R., Gao W.Q. Symmetrical and asymmetrical division analysis provides evidence for a hierarchy of prostate epithelial cell lineages. Nat. Commun. 2014;5:4758. doi: 10.1038/ncomms5758. [DOI] [PubMed] [Google Scholar]
- Wang L., Song G., Tan W., Qi M., Zhang L., Chan J., Yu J., Han J., Han B. MiR-573 inhibits prostate cancer metastasis by regulating epithelial-mesenchymal transition. Oncotarget. 2015;6:35978–35990. doi: 10.18632/oncotarget.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.E., Ratliff L.A., Postiglione M.P., Knoblich J.A., Fuchs E. Par3-mInsc and Galphai3 cooperate to promote oriented epidermal cell divisions through LGN. Nat. Cell Biol. 2014;16:758–769. doi: 10.1038/ncb3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win H.Y., Acevedo-Duncan M. Atypical protein kinase C phosphorylates IKKalphabeta in transformed non-malignant and malignant prostate cell survival. Cancer Lett. 2008;270:302–311. doi: 10.1016/j.canlet.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Wu X., Xu K., Zhang L., Deng Y., Lee P., Shapiro E., Monaco M., Makarenkova H.P., Li J., Lepor H. Differentiation of the ductal epithelium and smooth muscle in the prostate gland are regulated by the Notch/PTEN-dependent mechanism. Dev. Biol. 2011;356:337–349. doi: 10.1016/j.ydbio.2011.05.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuidart A., Ousset M., Rulands S., Simons B.D., Van Keymeulen A., Blanpain C. Quantitative lineage tracing strategies to resolve multipotency in tissue-specific stem cells. Genes. Dev. 2016;30:1261–1277. doi: 10.1101/gad.280057.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L., Ide H., Kim Y., Dubey P., Witte O.N. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc. Natl. Acad. Sci. USA. 2003;100:11896–11903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Zhao H., Ji Z., Zhang C., Zhou P., Wang L., Chen Q., Wang J., Zhang P., Chen Z. Shp2 promotes metastasis of prostate cancer by attenuating the PAR3/PAR6/aPKC polarity protein complex and enhancing epithelial-to-mesenchymal transition. Oncogene. 2016;35:1271–1282. doi: 10.1038/onc.2015.184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.