Abstract
Gram-negative bacteria possess an outer membrane layer which constrains uptake and secretion of solutes and polypeptides. To overcome this barrier, bacteria have developed several systems for protein secretion. The type V secretion pathway encompasses the autotransporter proteins, the two-partner secretion system, and the recently described type Vc or AT-2 family of proteins. Since its discovery in the late 1980s, this family of secreted proteins has expanded continuously, due largely to the advent of the genomic age, to become the largest group of secreted proteins in gram-negative bacteria. Several of these proteins play essential roles in the pathogenesis of bacterial infections and have been characterized in detail, demonstrating a diverse array of function including the ability to condense host cell actin and to modulate apoptosis. However, most of the autotransporter proteins remain to be characterized. In light of new discoveries and controversies in this research field, this review considers the autotransporter secretion process in the context of the more general field of bacterial protein translocation and exoprotein function.
INTRODUCTION
Until the 1960s, protein secretion through biological membranes was thought to be a relatively rare phenomenon. Furthermore, this view was governed by the assumption that where a protein was secreted it crossed the membrane via its own dedicated and specific mechanism. In the intervening years this view has radically changed, with the growing realization that both prokaryotic and eukaryotic cells secrete a prodigious array of proteins and possess protein translocation pathways which are surprisingly conserved throughout the phylogenetic kingdoms.
A surprising amount of information regarding bacterial protein secretion has been derived from the study of bacterial pathogenesis. Indeed, molecular and genetic investigations of bacteria have shown that pathogenic organisms may be differentiated from their nonpathogenic counterparts by the presence of genes encoding specific virulence determinants and that those proteinaceous virulence determinants, in the form of adhesins, toxins, enzymes, and mediators of motility, are usually secreted to or beyond the bacterial cell surface (139). Thus, by decorating their cell surfaces with such proteins, pathogens can directly or indirectly increase their ability to survive and multiply in the host. However, a facet often ignored by those studying bacterial pathogenesis and protein secretion is that many nonpathogenic organisms also secrete proteins which are adaptive to their life-styles; e.g., saprophytic bacteria may secrete cellulases or other degradative enzymes. However, in both cases, secretion of these factors is governed in large part by the structure of the bacterial cell envelope.
Gram-positive bacteria produce a single plasma membrane, the cytoplasmic membrane, followed by a thick cell wall layer. In contrast, gram-negative bacteria produce a double-membrane system consisting of a cytoplasmic membrane, also called the inner membrane, and an outer membrane which sandwich the peptidoglycan and periplasmic space between them. Protein secretion across the inner membrane of both gram-positive and gram-negative organisms generally follows the same routes, normally involving the Sec-dependent pathway (often referred to as the general secretory pathway [GSP]), although other routes have recently been identified, e.g., the Tat and signal recognition particle (SRP) pathways (96, 109, 116, 119, 208, 338, 419, 434). However, once across the inner membrane, the fate of the translocated proteins diverge. In gram-positive organisms, the proteins are either released into the extracellular environment or incorporated into the cell wall layer by virtue of one of several cell wall peptide-anchoring mechanisms (78, 319). In contrast, translocation across the gram-negative inner membrane results in release of the protein into the periplasmic space. Thus, proteins that are targeted for the cell surface or extracellular milieu must also cross the additional barrier to secretion formed by the outer membrane. To achieve translocation of these proteins to the cell surface and beyond, gram-negative bacteria have evolved several dedicated secretion systems, some of which bypass the Sec-dependent system and integrate both inner and outer membrane transport in a temporally linked fashion (see below).
In this review, we comprehensively describe the autotransporter secretion pathway, which is present in gram-negative organisms including animal and plant pathogens. We examine the roles of these proteins in the context of bacterial pathogenesis and highlight areas for future research. Readers are referred to more concise reviews of the autotransporter secretion pathway (101, 102, 203) and the roles of these proteins in pathogenesis (201).
SECRETION BY NUMBERS: PROTEIN SECRETION MECHANISMS IN GRAM-NEGATIVE BACTERIA
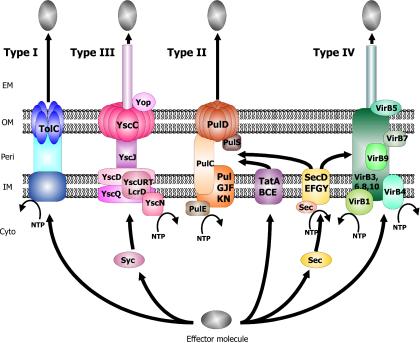
Recent progress in the molecular analysis of the protein secretion pathways of gram-negative bacteria has revealed the existence of at least five major mechanisms of protein secretion. These pathways are highly conserved throughout the gram-negative bacterial species and are functionally independent mechanisms with respect to outer membrane translocation; commonalities exist in the inner membrane transport steps of some systems. For better or worse, these pathways have been numbered type I, II, III, IV, and V. Several more extensive reviews of the gram-negative protein secretion systems have recently been published (4, 32, 64, 66, 147, 156, 264, 274, 294, 377, 395, 428, 429). Figure 1 gives an overview of the Type I, II, III and IV secretion pathways.
FIG. 1.
Schematic representation of the type I, II, III, and IV protein secretion systems. The type I pathway is exemplified by hemolysin A (HlyA) secretion in E. coli, the type III system is exemplified by Yop secretion in Yersinia, the type II system is exemplified by pullulanase secretion in Klebsiella oxytoca, and the type IV system is exemplified by the VirB system in A. tumefaciens. ATP hydrolysis by HlyB, YscN, SecA, and VirB11 is indicated. Secreted effector molecules are depicted as grey ovals. The type II and in some cases the type IV secretion systems utilize the cytoplasmic chaperone SecB, although the Tat export pathway has recently been implicated in the secretion of molecules via the type II pathway. Type III secretion also involves cytoplasmic chaperones (SycE); however, they do not interact with the Sec inner membrane translocon. The major structural proteins of each system are depicted in relation to their known or deduced position in the cell envelope. EM, extracellular milieu; OM, outer membrane; Peri, periplasm; IM, inner membrane; Cyto, cytoplasm.
At this juncture, it is worth noting that some confusion has existed in the literature regarding the nomenclature of the type IV and type V protein secretion pathways. Almost simultaneously, the type IV nomenclature appeared in several articles referring to both the autotransporter secretion pathway and the family of pathways exemplified by Agrobacterium tumefaciens T-DNA secretion. However, this matter has recently been resolved, with a consensus reached on the nomenclature of the pathways (160, 202). Thus, the A. tumefaciens and similar secretion pathways have retained the type IV terminology and the pathway used by the autotransporter and similar proteins has been termed the type V secretion system.
Type I Protein Secretion
The prototypical example of the type I secretion system (TOSS) is that required for secretion of Escherichia coli alpha-hemolysin (HlyA) (156). HlyA is a lipid modified protein with a repetitive domain composed of 11 to 17 9-amino-acid repeats which bind calcium and are thought to interact with host cells. This interaction stimulates the insertion of HlyA into the plasma membrane of eukaryotic cells, causing pore formation and release of the cytoplasmic contents. Other well-studied members of this group include the metalloprotease of Erwinia chrysanthemi (32, 160), the leukotoxin of Pasteurella haemolytica (345), and the adenylate cyclase of Bordetella pertussis (272).
Secretion of HlyA, and similar effector molecules, occurs in a Sec-independent manner and in a continuous process across both the inner and outer membranes. Furthermore, proteins secreted by TOSSs are not processed during secretion and do not form distinct periplasmic intermediates. Rather, the TOSS consists of three proteins: a pore-forming outer membrane protein, a membrane fusion protein (MFP), and an inner membrane ATP-binding cassette (ABC) protein (32). These proteins are represented in E. coli by TolC, HlyD and HlyB, respectively. Evidence suggests that the MFP (HlyD) and the ABC transporter (HlyB) in E. coli interact before binding of the effector (156). The secretion process begins when a secretion signal located within the C-terminal end of the secreted effector molecule interacts with the ABC transporter protein (224). In general, this signal sequence is specific and is recognized only by the dedicated ABC transporter. Once binding of the effector molecule occurs, interaction of HlyD with the outer membrane protein TolC is triggered, allowing secretion of the effector molecule to he external milieu (450). However, this model is somewhat controversial, since investigation of the E. chrysanthemi TOSS indicates that the ABC transporter and MFP interact only after binding of the effector molecule and subsequently MFP interacts with the pore-forming outer membrane protein (282). Nevertheless, it is apparent that in both cases the bridging formed by MFP and the outer membrane protein collapses after export of the effector molecule (485). ATP hydrolysis by the ABC transporter provides the impetus to drive secretion of the effector molecule across the cell envelope to the external milieu once bridging of all molecules has occurred. Hydrolysis of ATP is not required for interaction of the effector with the ABC protein or assembly of the TOSS complex (261, 262).
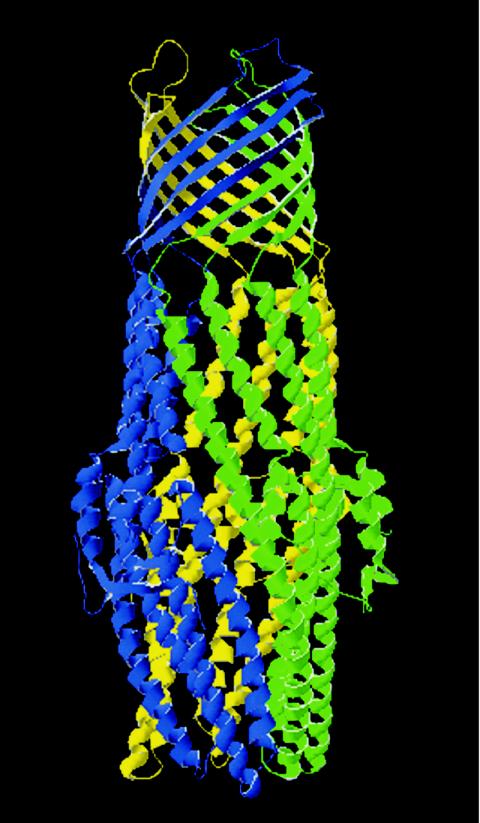
Recent data have indicated a possible structure for the TOSS complex. Resolution of the crystal structure demonstrated that TolC exists as a trimer and that the portion spanning the outer membrane formed a β-barrel structure (Fig. 2) (263). However, unlike the typical porins, each monomer of TolC contributes four β-strands to form a 12-strand antiparallel β-barrel. In addition to forming the β-barrel structure, TolC possesses a novel protein structure: the α-helical barrel. This extends from the integral outer membrane β-barrel structure into the periplasm (263). HlyD is also known to form a trimeric structure which projects from the inner membrane into the periplasm. Thus, it is easy to envisage a scenario where the monomers of HlyD and TolC, while forming their trimeric complexes, can extend to form a continuous channel across the inner and outer membranes at the appropriate time. Nevertheless, further investigations are required to fully appreciate the roles of each protein in the TOSS.
FIG. 2.
Structure of TolC. The structure of TolC was solved to 2.1-Å resolution. The functional TolC unit consists of three TolC monomers oligomerized into a regular structure embedded in the outer membrane and extending into the periplasm such that it forms a continuous solvent-accessible conduit. The portion of the trimeric molecule that is embedded in the outer membrane adopts a 12-strand β-barrel conformation consisting of four β-strands per monomer. The molecule is 140 Å long, the β-barrel is 40 Å long (spanning the outer membrane), and the remaining portion of the molecule adopts a 100 Å α-helical conformation spanning the periplasmic space. This α-helical domain consists of 12 α-helices, such that the α-helices extend from or connect to the periplasmic side of each β-strand. It is the α-helical domain that is predicted to interact with the MFP. Each monomer is highlighted in a separate color. Adapted from reference 263 and the Protein Data Bank (30).
Two-Step Type II Protein Secretion
The type II secretion pathway is often referred to as the main terminal branch (MTB) of the Sec-dependent GSP (428). The subunits of a type II secretion pathway identified in E. coli have been designated GspA to GspO (146). Unfortunately, this has served to confuse the general reader about the nomenclature of the type II secretion pathway and the GSP (100). Thus, it must be stressed here that type II secretion does not refer to the GSP in general but refers only to one of several branches, as described below. Recently, it has been suggested that the pathway be renamed the secreton-dependent pathway (516).
Secretion via the MTB is exemplified by pullulanase (PulA) secretion from Klebsiella oxytoca (478, 57). PulA is a starch-hydrolyzing lipoprotein which forms micelles once it has been secreted into the external environment (478). Since the discovery of the MTB in K. oxytoca, the pathway has been identified in a variety of other bacterial species exporting a range of proteins with diverse functions including the cholera toxin of Vibrio cholerae, exotoxin A of P. aeruginosa, and several cell wall-degrading enzymes (428, 429). Furthermore, analyses of the MTBs and type IV fimbrial systems at the genetic and structural levels have shown sufficient similarity between the two systems to suggest that the fimbrial biogenesis pathway be classed as a type II secretion pathway (34).
Secretion of substrate proteins via the MTB occurs in a two-step Sec-dependent fashion. Thus, all proteins secreted via a type II secretion pathway are synthesized with an N-terminal signal sequence which targets proteins for secretion to the Sec inner membrane secretion pathway (for in-depth reviews, see references 115; 245; and 509). The Sec machinery is composed of an ATPase, SecA, several integral inner membrane proteins (SecD, SecE, SecF, SecG, and SecY, and a signal peptidase (116, 338). SecB, a cytoplasmic chaperone, does not recognize the signal sequence but recognizes the mature part of the protein targeted for secretion and subsequently directs the protein to the Sec translocon (109). SecA provides the energy for transport through the translocon, and the signal peptidase cleaves the signal sequence, releasing the remainder of the protein into the periplasm. Evidence suggests that, once in the periplasm the remaining protein adopts a quasi-native state which is facilitated by certain chaperones such as disulfide bond isomerase, DsbA (486). These folding steps are necessary for translocation across the outer membrane.
Transport across the outer membrane requires an additional 12 to 16 accessory proteins, which are collectively referred to as the secreton (428, 429, 486). A consensus nomenclature has been defined by the letters A to O and S for homologous genes and their products (the Xcp proteins of P. aeruginosa are labelled P to Z). The organization of the loci encoding the MTB components is relatively conserved, and most of the genes are transcribed from a single operon in which several of the genes are overlapping (428, 429). Evidence from both the Klebsiella Pul system and the Erwinia Out system for pectinase secretion suggests that protein C contributes to a species-specific interaction defining the transport of specific substrate proteins (42, 290, 404, 405). Protein D belongs to a family of proteins termed secretins. These proteins are integral outer membrane proteins predicted to consist largely of transmembrane β-strands and form a β-barrel structure in the outer membrane, with 12 to 14 subunits forming a complex (34, 188, 257). Several possible configurations for the secretin complex have been proposed. The most popular of these include a complex where monomers of protein D oligomerize to form a ring with a central channel and a model reminiscent of the TolC structure, where each monomer contributes β-strands to form a single central channel (486). The C-terminal region is conserved among the secretins and is thought to be embedded in the outer membrane. It is interesting that homologues of the protein D secretin proteins have been found in the type III secretion pathways (see below) (486). Evidence suggests that protein E may act as a kinase regulating the secretion process by supplying energy to promote translocation and assembly of the pilin-like subunits, proteins G to K (430). No protein-protein interactions have been demonstrated for protein F (488). Proteins G to K possess homology to the type IV pili and are thought to form a pseudopilus (112, 264). Furthermore, these proteins are N-terminally processed and methylated by a prepilin peptidase, protein O (36, 390). A lipoprotein, protein S, has been demonstrated to play a role in stabilization of the outer membrane component protein D (428). Several other accessory proteins, which are not present in all MTB pathways, have a demonstrated requirement for substrate secretion in certain MTB systems. Evidence suggests that this may be related to the type of proteins being secreted or to substrate recognition by the MTB. In summary, the available evidence indicates that most if not all of the components of an MTB interact to form a multiprotein complex spanning both the inner and outer membranes (Fig. 1). Many more comprehensive reviews are available which describe the structure and function of the components of the MTB (428, 429).
The Injectisome: Type III Protein Secretion
The archetypal type III secretion system (TTSS) was first identified in pathogenic Yersinia spp. for the secretion of Yop proteins (326). Since this discovery, TTSS have been identified in several mammalian and plant pathogens including Salmonella enterica, Shigella flexneri, E. coli, Ralstonia solancearum, Pseudomonas syringae, and Chlamydia trachomatis (214, 395). Recently, TTSS have been the subject of considerable interest because of their primary role in the virulence of these animal and plant pathogens. Interestingly, detailed investigations have shown that TTSS and the gram-negative flagellar export apparatus are homologous, leading some investigators to classify the flagellar biosynthesis pathway as a TTSS (37, 307). Thus, while the mechanism for secretion of effector molecules is highly conserved in the TTSS, the effector molecules themselves are divergent and perform a variety of functions. Readers are referred to more in-depth reviews of the structure and function of TTSS, which cover the voluminous research available (4, 37, 64, 214, 307, 395).
Genes encoding components required for TTSS are generally located on a single plasmid or chromosomal locus. Comparison of the sequences demonstrates that the genes at these loci are conserved among different species, suggesting that these loci are inherited as a distinct genetic unit and that a common mechanism exists for the recognition and secretion of effector molecules (4, 64, 294, 395).
Like the TOSS, the TTSS translocate their effector molecules across the inner and outer membranes in a Sec-independent fashion. However, it is important to note that the Sec translocon, as in the case of TOSS, is required for secretion of structural components across the inner membrane. Controversy exists about the mechanism of effector molecule recognition and targeting to the TTSS. One hypothesis suggests that the signal resides in the 5′ mRNA, which may target the ribosome-RNA complex to the TTSS, permitting temporal coupling of translation and secretion (11). A second proposal suggests that the N-terminal 20 amino acids serve as a binding site for cytoplasmic chaperones which specifically target the effector molecules to the TTSS (295). Notwithstanding the differences in these hypotheses, it is apparent that the region encoding the first 20 amino acids (either the untranslated mRNA or the first 20 amino acids of the polypeptide) is essential for secretion and the process is highly regulated (214).
Once targeted to the cytoplasmic side of the TTSS, secretion of the effector molecule may occur without the formation of periplasmic intermediates, with secretion proceeding through a needle-like structure composed of approximately 20 different proteins (214, 269). Unfortunately, a description of the voluminous evidence to support the roles of specific proteins in the formation of the TTSS complex is beyond the scope of this review. Based on the current evidence, the placement of specific proteins in the TTSS complex is indicated in Fig. 1. However, it is worth reiterating that the TTSS possesses a secretin-like component homologous to those found in the MTB systems (486). This secretin-like protein has been demonstrated to form ring-shaped structures with large central pores, which facilitates secretion of effector molecules and stabilizes the needle-like complex formed by the other components of the TTSS (153). This complex spans both the inner and outer membranes, resembling a hypodermic needle. The formation of a needle-like structure is supported by the visualization of the S. enterica and S. flexneri TTSS by electron microscopy (269). These studies demonstrated a striking resemblance between the TTSS and the flagellar basal body, and since the TTSS were shown to form long pilus-like structures, it was assumed, through analogy to the flagellar system, that the pilus structure allows protein transport across the bacterial outer membrane to the external milieu (214, 307). This was supported by investigations showing structural and sequence similarities between the EspA pilus protein of the E. coli TTSS and the flagellar filament (89, 255).
Perhaps the most striking feature of TTSS is their ability to target effector proteins directly into eukaryotic cells. This phenomenon is triggered in some cases when the bacterium comes in contact with a eukaryotic cell; hence, secretion via the type III pathway has been termed “contact dependent” (545). The analogy between the TTSS and the hypodermic syringe, coupled with the ability of the systems to inject proteins directly into the host cytosol, has allowed the phrase “injectisome” to be coined for this protein secretion system. Nevertheless, investigators should be reminded that not all TTSS are contact dependent and some effector molecules secreted by TTSS are released into the external environment (75).
Type IV Protein and Nucleoprotein Secretion
The type IV protein secretion system (TFSS) is the least well understood gram-negative protein secretion pathway. The system is ancestrally related to the bacterial conjugation machinery, although some controversy exists over which system comes first on the evolutionary ladder. Nevertheless, it is clear that, like the TTSS, the unifying characteristic of the TFSS and the conjugative machinery is the ability to translocate protein molecules intercellularly, i.e., from one bacterium to another or from a bacterium to a eukaryotic host cell. The prototypical TFSS is that of the A. tumefaciens nucleoprotein T-DNA transfer system. However, several other systems, which are required for the full virulence of pathogenic bacteria, have been described, including the B. pertussis Ptl (pertussis toxin) system (130), the Dot/Icm system of Legionella pneumophila (546), and those of Brucella suis (41), Bartonella henselae (443), and Helicobacter pylori (18). Representatives of the conjugal DNA transfer systems include the IncF, IncP, and IncW plasmid transfer systems (Tra) (277). Thus, despite being a relatively poorly understood protein secretion system, the TFSS has been studied over several generations of researchers through its analogies to the Tra systems.
As in other secretion pathways, the effector molecules secreted by the TFSS have a wide variety of functions. Arguably, the best-characterized function is that of pertussis toxin, which belongs to the A-B5 toxin family (130, 481). Unlike the other type IV systems, the pertussis toxin is secreted to the extracellular milieu rather than directly into a host cell (481). The B domain (subunits S2 to S5) interacts with the host cell glycoprotein receptors and mediates translocation of the A domain (subunit S1) into the host cytosol. Once in the cytosol, the S1 subunit ribosylates the α subunits of G-proteins, interfering with signaling pathways (310). In contrast, the A. tumefaciens system secretes several effector molecules, VirD2, VirE2, and VirF, into the host cell cytosol (67, 212). VirD2 is secreted as a nucleoprotein complex; the protein remains covalently associated with a single-stranded copy of T-DNA (266). Once in the host cytosol, VirE2 interacts with the VirD2/T-DNA complex, mediating delivery of the T-DNA to the host cell nucleus and resulting in crown gall tumor formation. A role for the VirF protein has yet to be defined conclusively. Unlike the pertussis toxin, the Vir effector proteins are secreted separately through the TFSS.
Controversy exists over how the substrate molecules for TFSS pass through the inner membrane. For the A. tumefaciens protein-DNA complex, export is postulated to take place in a single continuous step from cytoplasm to the interior of the cell. In contrast, export of pertussis toxin, like the type II secretion pathway effector molecules, occurs in two steps; the toxin subunits are first translocated across the inner membrane via the Sec machinery and are subsequently targeted for export across the outer membrane (51, 52). Thus, one evolutionary branch of the TFSS appears to be a branch of the GSP whereas the division encompassing T-DNA transfer is not.
The process governing transfer across the outer membrane is best understood for the A. tumefaciens TFSS. The quantity of information to support the individual roles of separate proteins is too great to review here; however, it is worth mentioning the roles of a few major proteins. Transfer of T-DNA and assembly of the pilus requires VirA, VirB1 to VirB11, VirD1 to VirD4, VirE2, and VirG. VirB2 is the major pilus subunit, although it is not yet clear whether this pilus emanates from the inner or outer membrane. VirB4 and VirB11 are inner membrane proteins, and VirD4 is a cytoplasmic protein, all of which possess ATPase activity. It has been suggested that these proteins provide the energy for translocation of the effector molecules through the pilus structure in a fashion analogous to the TOSS and TTSS. The remaining proteins are variously associated with the inner and outer membranes and with each other; the proposed placement of each protein is indicated in Fig. 1. TFSS has been divided into two subclasses: type IVa corresponds to protein machinery containing VirB homologues of A. tumefaciens, and type IVb corresponds to functional secretion systems assembled from Tra homologues of the Incl Collb-P9 plasmid of Shigella flexneri (66). Several excellent reviews have covered the functions of each subunit in more detail (66, 111, 143, 274, 277, 448).
TYPE V PROTEIN SECRETION
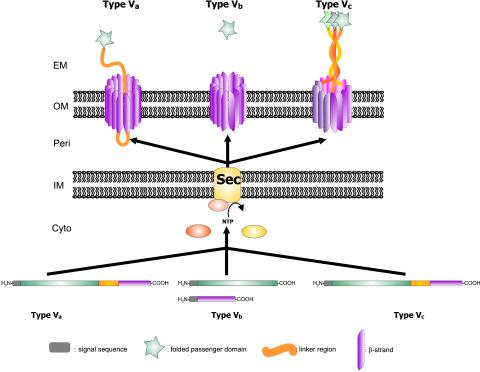
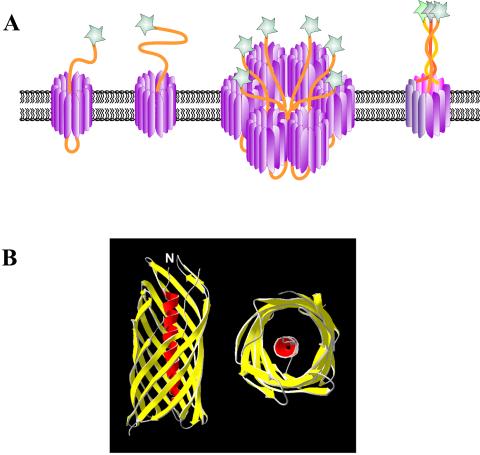
Perhaps the simplest protein secretion mechanisms are those which are included under the umbrella of type V secretion. This family of secreted proteins includes those secreted via the autotransporter system (type Va or AT-1), the two-partner secretion pathway (type Vb), and the recently described type Vc system (also temed AT-2) (99, 102). Proteins secreted via these pathways have similarities in their primary structures as well as striking similarities in their modes of biogenesis. Figure 3 provides a schematic overview of the type V secretion pathways.
FIG. 3.
Schematic overview of the type V secretion systems. The secretion pathway of the autotransporter proteins (type Va) is depicted at the bottom left of the diagram, the two-partner system (type Vb) is depicted in the center of the diagram, and the type Vc or AT-2 family is depicted on the right. The four functional domains of the proteins are shown: the signal sequence, the passenger domain, the linker region, and the β-domain. The autotransporter polyproteins are synthesized and generally exported through the cytoplasmic membrane via the Sec machinery. Interestingly, effector proteins with an unusual extended signal sequence, which purportedly mediates Srp-dependent export, are found in all three categories of type V secretion. Once through the inner membrane, the signal sequence is cleaved and the β-domain inserts into the outer membrane in a biophysically favored β-barrel structure that forms a pore in the outer membrane. After formation of the β-barrel, the passenger domain inserts into the pore and is translocated to the bacterial cell surface, where it may or may not undergo further processing.
Autotransporter Secretion Pathway (Type Va)
Pohlner et al. (401) were the first to describe and propose a model for type V secretion, elegantly elucidating the relationship between the gene encoding the gonococcal immunoglobulin A1 (IgA1) protease and its extracellular product. Thus, the DNA sequence of a cloned fragment (4.6 kb) revealed a single gene coding for a 169-kDa precursor of IgA1 protease; the precursor contained three functional domains: the N-terminal leader (which is assumed to initiate the inner membrane transport of the precursor), the extracellular IgA1 protease, and a C-terminal “helper” domain (which is required for secretion across the outer membrane). In this seminal work, the authors proposed a model in which the helper serves as a pore for the secretion of the protease domain through the outer membrane. IgA1 protease acquires an active conformation as its extracellular transport proceeds and is released as a proform from the membrane-bound helper by autoproteolysis. The soluble proform matures further into the functional 106-kDa IgA1 protease and a small stable α-protein. It is possible to find the active IgA1 protease in the culture medium from iga-transformed E. coli and S. enterica (185, 253), supporting the concept that the IgA1 protease precursor contains all the necessary determinants to direct the translocation of the protease from the periplasm into the medium, without the participation of accessory proteins (401). Since no energy coupling or accessory factor seemed to be required for the translocation process, and unlike the type I to IV systems, the molecules targeted for secretion were strictly dedicated to their covalently linked cognate β-domain, the proteins secreted in this fashion received the name of autotransporters.
Since this initial description, many more proteins which follow this pathway of secretion have been identified among the gram-negative bacteria (201). In all cases, the primary structure of the autotransporter protein is reminiscent of the IgA1 protease, consisting of a modular structure composed of three domains (231). These domains have been given different names throughout the literature. In this review, we use a consistent nomenclature throughout; thus, the domains are termed the signal sequence, the passenger domain, and the translocation unit. The signal sequence (also called the signal peptide or leader sequence) is present at the N-terminal end of the protein and allows targeting of the protein to the inner membrane for its further export into the periplasm (203). The next domain is the passenger domain (also called the α-domain, N-passenger domain, or N-domain), which confers the diverse effector functions of the various autotransporters. The last main domain, located at the C-terminal end of the protein, is the translocation unit (also called the β-domain, helper domain, C-domain, transporter domain, or autotransporter domain), consisting of a short linker region with an α-helical secondary structure and a β-core that adopts a β-barrel tertiary structure when embedded in the outer membrane (317, 364, 365, 475), facilitating translocation of the passenger domain through the outer membrane.
Inner membrane transport.
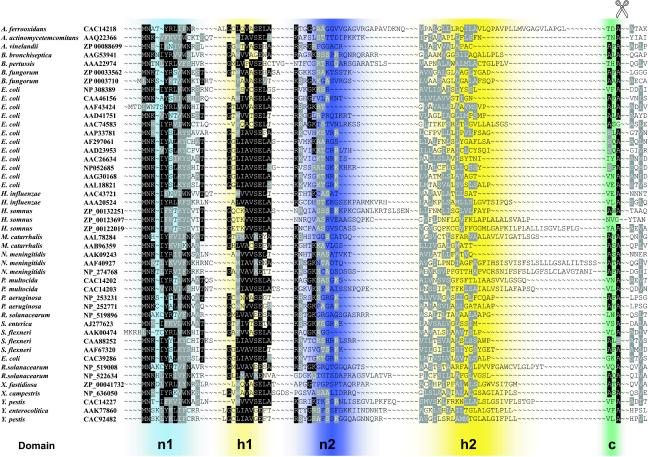
Bioinformatic analysis of the gonococcal IgA1 protease N terminus using SignalP (352) predicts that this domain functions as a signal sequence, allowing targeting of the protein to the inner membrane. Similarly, the N-terminal regions of all other autotransporter proteins display characteristics of the prototypical Sec-dependent signal sequence, for which SecB is presumed to act as the molecular chaperone (45, 203). These signal sequences possess (i) an n-domain with positively charged amino acids, (ii) an h-domain containing hydrophobic amino acids, and (iii) a c-domain with a consensus signal peptidase recognition site which often contains helix-breaking proline and glycine residues as well as uncharged and short lateral-chain residues at positions −3 and −1 that determine the site of signal sequence cleavage (315). However, some autotransporters, like AIDA-1, Pet, or Hbp, exhibit an unusually long signal sequence consisting of a C-terminus resembling a normal signal sequence with a hydrophobic core and a consensus signal peptidase cleavage site but presenting a conserved extension of the n-domain which contributes most to the variation in overall length (203). In silico analyses of completed bacterial genomes has revealed the presence of at least 80 proteins possessing these extended signal sequences across the breadth of the Proteobacteria (Fig. 4) (M. Desvaux and I. R. Henderson, unpublished observations). Interestingly, these extended signal sequences appear to be associated exclusively with proteins larger than 100 kDa. All of these atypical signal sequences, consisting of at least 42 amino acids, appear to consist of two charged domains (n1 and n2) and two hydrophobic domains (h1 and h2), in addition to the C-domain signal peptidase I recognition site (Fig. 4). While the n1 and h1 domains of these autotransporters are well conserved, the sequence of the n2 domain is variable and that of the following h2 domain even more so (203). The variability within the n2 and h2 domains is expected and consistent with Sec-dependent signal sequences, although, notably, the n2 domain contains an unusual number of charged amino acids (49). In contrast, the conservation within the n1 and h1 domains is highly unusual and is characterized by the presence of conserved aromatic amino acids in the n1 domain and a glutamate residue in the h1 domain (Fig. 4). Such sequence conservation is frequently indicative of the existence of a specialized biological function, suggesting that the extended n1-h1 portion of the signal sequence directs inner membrane export through a pathway different from the Sec system or that it recruits accessory proteins to work in tandem with the Sec pathway.
FIG. 4.
Structure and alignment of the extended signal sequences. The extended signal sequences belonging to autotransporter proteins from a wide range of gram-negative bacteria are depicted. Blue shading indicates the positions of the positively charged n1 and n2 domains. The positions of the hydrophobic h1 and h2 domains are denoted by yellow shading. The signal peptidase recognition sites are indicated by green shading. The n2, h2 and C-domains are characteristic of a typical signal sequence secreted via the Sec-dependent translocon in conjunction with the SecB chaperone. The in silico-predicted and/or empirically determined cleavage site between the signal sequence and the passenger domain is indicated. Conserved residues are highlighted.
It was speculated that the features of those unusually long signal sequences might serve to recruit accessory proteins, different from SecB, like the SRP, or to direct secretion via an alternative pathway such as the Tat pathway (203). In E. coli, protein export through the Sec translocon can use at least two pathways: (i) the pathway involving the molecular chaperone SecB, which keeps the preprotein in a translocation-competent state and targets it to SecA (413), and (ii) the SRP pathway, which permits a cotranslational translocation of the preprotein by targeting the ribosome-SRP complex directly to the translocon (208). Sijbrandi et al. (455) recently investigated the role of this signal sequence in Hbp. It was demonstrated that targeting and translocation through the inner membrane involved the targeting factor SRP and the Sec translocon. SecB was not required for the targeting of Hbp, but it could compensate for the absence of a functional SRP pathway to some extent. It was suggested that cotranslational translocation versus posttranslational translocation might prevent degradation or premature folding of Hbp in the cytoplasm. This study presents the first example of an extracellular protein targeted by SRP; previously in gram-negative bacteria SRP was associated only with insertion of proteins into the inner membrane (497). The authors speculated that the extended domain as a whole, i.e., n1-h1, and the hydrophobic region in particular might play an important role for the SRP recognition of the signal sequence. In fact, crystal structure analysis of the SRP has revealed an unusual RNA-protein interface that could interact with the hydrophobic core of the signal sequence (20). Controversially, the homologous EspP was found to be translocated across the inner membrane via the classical Sec pathway (476). However, recent investigations have shown that the truncated EspP signal sequence, i.e., ΔEsp lacking n1-h1 domains, fused to proteins normally targeted to the Sec apparatus by SecB leads to the rerouting of these proteins into the SRP pathway; therefore, the role of the extended region n1-h1 in secretion remains enigmatic (391). Furthermore, no evidence exists to support a role for this signal sequence in targeting to the Tat pathway (455; I. R. Henderson et al., unpublished observations) or for the involvement of other accessory factors.
Interestingly, several recent reports have described autotransporter proteins, namely, the H. pylori AlpA, B. pertussis SphB1, and N. meningitidis AspA/NalP proteins (82, 358, 508), with typical lipoprotein signal sequences which are indicative of processing by signal peptidase II. While investigations of AlpA demonstrated that it was processed as a lipoprotein when expressed in E. coli DH5α, no such processing could be demonstrated in wild-type H. pylori (358). An alternative hypothesis is that AlpA is processed further downstream by signal peptidase I. Indeed, the protein may be acylated and processed by signal peptidase II and may subsequently undergo processing by signal peptidase I prior to insertion into the outer membrane. Using a chimeric SphB1-β-lactamase fusion, Coutte et al. (83) demonstrated that SphB1, like AlpA, was processed as a lipoprotein in E. coli. However, such processing could not be demonstrated directly in the natural B. pertussis host. Nevertheless, growth in the presence of globomycin, a specific inhibitor of signal peptidase II, prevented localization of SphB1 in the outer membrane (83). Furthermore, the localization of AspA/NalP (also termed NalP) was also inhibited by treatment with globomycin (508). Interestingly, the presence of the lipoprotein signal sequence was found to be necessary for the functional localisation of SphB1 in the outer membrane, suggesting that it is required for anchoring SphB1 on the bacterial cell surface (83).
Periplasmic transit.
After export through the inner membrane, the autotransporter proprotein exists as a periplasmic intermediate. The original model of outer membrane secretion predicts that the first of the β-strands passes from the periplasmic space to the external surface, thus leaving the passenger domain temporarily extending into the periplasm (203). Using a reporter single-chain antibody as a reporter passenger domain, the secretion process has been investigated (510). It was concluded that the folding of the passenger domain takes place in the periplasm before, or at least simultaneously with its own translocation through the outer membrane. However, a recent investigation by Oliver et al. (365) revealed the presence of an intramolecular chaperone in BrkA, spanned by residues 606 to 702. This region corresponds to PD002475 in the ProDom database (76) and is found in many autotransporters but not in other proteins in the database. In the model derived from this study, the complete folding of the protein occurs on the surface of the bacteria (see the discussion of outer membrane transport below). This suggests that previous findings by Veiga et al. (510) may have been influenced by the study of a passenger domain unrelated to the autotransporters. Furthermore, it is unlikely that large molecules, such as the passenger domains, can pass through a pore of 2 nm while fully folded, and therefore the molecules must be maintained in a translocation-competent unfolded state in the periplasm.
Thus, the status of the autotransporter proteins in the periplasm remains controversial. Of particular interest is how such large proteins can maintain an unfolded, or partially folded, state and resist degradation by periplasmic proteases. For IcsA, the soluble periplasmic form of the autotransporter appears transient (46); hence, it was suggested that the β-barrel inserts rapidly into the outer membrane or may interact with a general or autotransporter-specific periplasmic chaperone (46). It was proposed that the chaperone activity of DegP was involved (410). It is worth mentioning here that the formation of disulfide bonds in the passenger domain in the presence of the periplasmic disulfide bond-forming enzyme DsbA decreases the efficiency of secretion of the passenger domain (46, 232, 499, 510). This suggests that the proprotein is accessible to periplasmic enzymes and that at least partial folding of the autotransporter may arise in the periplasm. However, it remains possible that the decrease in secretion efficiency is a result of the translocation of a nonnative passenger domain rather than the formation of disulfide bonds per se. Indeed, the IcsA protein forms disulfide bonds in the periplasm and is efficiently secreted via its native translocating unit (46). Nevertheless, the paucity of cysteine residues which contribute to disulfide bond formation is also a unifying characteristic of autotransporter passenger domains; thus, the secretion of proteins with secondary structure appears to be an exception rather than a rule (231).
β-Domain.
β-Barrels can have different topologies; the simplest of those theoretical topologies is the all-next-neighbor connection between adjacent strands, in which each β-strand aligns in an antiparallel fashion to form a β-sheet. The β-barrel architecture has been found in all integral outer membrane proteins whose structures have been solved (97); α-helical bundles have been found only in the cytoplasmic membrane (257, 444). Generally, it is assumed that this differentiation originates from the biogenesis of the outer membrane proteins, whose polypeptide chains have to cross the cytoplasmic membrane, where, if they contained hydrophobic α-helix-rich regions, they would be stuck. As with all hitherto known integral outer membrane proteins, the C-terminal domain of autotransporter proteins is predicted to consist of β-pleated sheets in the form of a β-barrel (303).
Generally, the C-terminal domains of autotransporters consist of 250 to 300 amino acid residues. The β-domain of autotransporters are all homologous but extremely diverse in sequence. Bioinformatic analyses of the C-terminal sequences of highly diverse autotransporters have permitted a consensus signature sequence to be proposed for this domain, which has been proven useful for the identification of new autotransporters from databases (203). While the exact shape of the β-barrels of various autotransporters is still subject to speculation, some general features can be determined from bioinformatic analysis. Using the AMPHI algorithm (223) or the WHAT and AveHAS programs in combination (543, 544), it was predicted that the β-domains of most autotransporters exhibit 14 antiparallel amphipathic strands consisting of 9 to 12 residues (303, 540).
In addition to the overall β-sheet structure, the β-domains share a consensus amino acid motif at the extreme C terminus, which represents the final spanning segment (231, 303). The terminal amino acid is always phenylalanine or tryptophan, preceded by alternating hydrophilic (charged or polar) and hydrophobic residues, i.e., (Y/V/I/F/W)-X-(F/W). This motif is also found in other gram-negative outer membrane proteins; in the case of PhoE, an E. coli porin, substitution or deletion of the C-terminal phenylalanine has a drastic effect on protein folding and stabilization of the monomer, resulting in an ineffective trimerization and outer membrane localization of the protein (95, 470). This observation has also been extended to the autotransporter proteins, since deletion of the final three amino acid residues of the H. influenzae Hap autotransporter, i.e., YSF, abolishes outer membrane localization of the protein (206). However, mutation of individual amino acids in the terminal three residues does not seem to affect its localization significantly.
By analogy to the mechanism of porin biogenesis (480), the current model proposes that the autotransporter proprotein spontaneously inserts into the outer membrane in a biophysically favoured β-barrel conformation as it interacts with the local nonpolar environment of the membrane (Fig. 3) (203). The first and last β-strands spontaneously form hydrogen bonds in an antiparallel fashion to close the ring conformation, permitting the establishment of a molecular pore. The alternating hydrophobic side chains of amino acids are embedded within the hydrophobic lipid bilayer, while the hydrophilic side chains project into an aqueous environment in the center of the barrel (507). However, investigations with other outer membrane proteins, like PhoE and OmpA, suggest that lipopolysaccharide (LPS) and the periplasmic chaperone Skp are required for efficient assembly into the outer membrane (95, 148). Recently, Bulieris et al. (50) proposed the first assisted-folding pathway of an integral membrane protein by using the example of OmpA. In this model, once OmpA is translocated into the periplasm, it binds to three molecules of Skp, which maintains OmpA in an unfolded state. This OmpA-Skp complex interacts with two to seven LPS molecules to form a folding-competent intermediate that facilitates insertion and folding of OmpA into the outer membrane lipid bilayer. It was suggested that the cochaperone function of Skp and LPS could be involved in the folding of a large number of outer membrane proteins (50). However, it also appeared that deletion of the skp gene does not entirely eliminate the presence of outer membrane proteins in the outer membrane, indicating this Skp-LPS assisted-folding pathway was certainly not the only mechanism by which outer membrane proteins insert and fold into the outer membrane.
Recently, the involvement the surface antigen Omp85 challenged the idea of a spontaneous assembly of outer membrane proteins into the outer membrane (517). Omp85 is a highly conserved protein since homologues are present in all the complete genome sequences of gram-negative bacteria. Interestingly, the gene is located in close proximity to the skp gene and to the lpxA and lpxB genes involved in LPS biogenesis. This protein is essential for cell viability and for outer membrane protein assembly in mitochondria and bacteria and demonstrates sequence similarity to Toc75 of the chloroplast protein import machinery, suggesting a common evolutionary origin for all systems (155, 518). It has been shown that, following Omp85 depletion, unassembled forms of various outer membrane proteins, including autotransporters, accumulated. Thus, Omp85 could possess a general function in the assembly of outer membrane proteins, although the molecular mechanisms underlying this function remain to be elucidated. However, Genevrois et al. challenged this view by demonstrating that Omp85 was in fact involved in lipid export from the inner to the outer membrane (154). In this study, the localization of some outer membrane proteins was not impaired upon Omp85 depletion but degradation products were observed. Interestingly, the isolation of an omp85 knockout mutant in an LPS-deficient mutant, which would have definitively demonstrated that Omp85 was involved only in the LPS transport, was impossible, suggesting additional function(s) for Omp85. Thus, a concerted mechanism where Omp85 permits the phospholipid and LPS transport and assembly of the outer membrane as well as the assembly of outer membrane proteins cannot be completely ruled out and necessitates further investigations (518).
Translocation of the passenger domain.
Originally, it was proposed that the secretion of the unfolded passenger domain occurs through the hydrophilic channel formed by the monomeric β-barrel and that a globular conformation was not compatible with this mode of translocation (252-254). In support of this hypothesis, a recent study has demonstrated the existence of an ion channel in the β-domain of BrkA with an average conductance of 3.0 nS in 1 M KCl (449). However, recent investigations have challenged this long-held hypothesis. Several investigations suggested that the presence or absence of DsbA, the major periplasmic disulfide bond-forming catalyst in E. coli, did not make any difference to the stability and targeting of the passenger domain to the outer membrane (46, 499, 510). These investigations questioned whether a structured polypeptide could be translocated through the somewhat narrow hydrophilic channel of a β-barrel (355). Indeed, an alternative model of secretion was proposed after in vitro investigations of the secretion of gonococcal IgA1 protease using the β-domain embedded in multilamellar liposomes. This model suggests that the passenger domain is instead secreted through an oligomeric ring-shaped structure consisting of a minimum of six β-barrels and forming a central hydrophilic ∼2-nm-diameter pore (512). Nevertheless, these researchers also suggested that the oligomeric ring could consist of as many as 10 monomers and that the exact structure of the oligomeric ring remained speculative. Such a structure would be analogous to the outer membrane complexes found in other secretion systems, e.g., secretin or fimbrial ushers (459, 487, 540). This study has allowed the proposal of an alternative mechanism for autotransporter secretion through a 2-nm pore depicted as large enough to tolerate the passage of certain protein domains in a folded state (Fig. 5) (512). The use of different immunoglobulin domains, with distinct and defined folding properties, fused to the IgA1 protease β-domain tends to demonstrate that type Va secretion is compatible with folded passenger domains containing disulfide bonds following the action of periplasmic or intramolecular chaperones prior to the outer membrane translocation (511). However, based on the available evidence from a variety of different systems, which each offer only a partial view of the translocation process, several other hypotheses have been proffered for the outer membrane translocation step. These include scenarios where the export of the passenger domain occurs through a common channel shared by the different subunits assembled into a single complex and also where the passenger domain is not secreted through any pore but, rather, extends directly from the translocating unit into the extracellular milieu, in a manner analogous to the YadA-like proteins (Fig. 5) (422, 474).
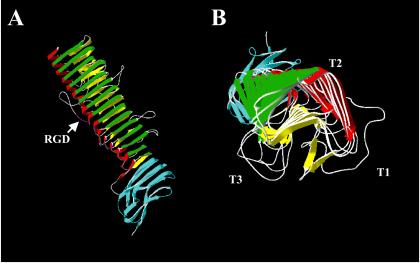
FIG. 5.
Alternative mechanisms of autotransporter protein secretion. (A) Several different hypotheses have been proffered for the mechanism of autotransporter protein biogenesis. From left to right are depicted the traditional view of secretion originally proposed by Pohlner et al. (401), in which the passenger domain passes through the pore of the β-barrel to the outside; a recently proposed structure where the passenger domain extends directly from the β-barrel into the extracellular milieu; the secretion pathway proposed by Veiga et al. (512), in which a central channel is formed through which the passenger domains are secreted to the outside, and the pathway proposed by Hoiczyk et al. (210) for the type Vc autotransporter family, in which several molecules contribute β-strands to make a large β-barrel pore through which the proteins are secreted to the external side of the outer membrane. (B) Crystal structure of the AspA/NalP translocating unit to 2.6 Å. A side view and a stereo-top view are depicted. The protein forms a 12-strand β-barrel structure characterized by short periplasmic turns and longer external loops. The barrel interior is highly hydrophilic due to the presence of charged amino acids. Within the barrel is embedded an α-helical region, which is attached to the first transmembrane β-strand such that the extreme N terminus of the protein, and to which it is presumed a native passenger domain would be attached, is located on the extracellular surface. Adapted from reference 366 and the Protein Data Bank (30).
However, recent evidence has shed light on the translocation process. On the basis of the sequence alignments published by Yen et al. (540) the AspA/NalP (or NalP) autotransporter translocator domain was estimated to consist of a 32-kDa 308-amino-acid protein. An in vitro refolded version of this domain demonstrated the same heat modifiability observed with the native form of the protein (366). Pore activity was measured in planar lipid bilayers, and two pore sizes were determined, 0.15 and 1.3 nS. The same protein was crystallized, and the structure was determined to a 2.6-Å resolution. As predicted from the data discussed above, the overall structure of AspA/NalP was characterized by a β-barrel and possessed a pore of 10 by 12.5 Å (366). Unlike the 14-strand structure predicted from bioinformatic analyses, the β-barrel consisted of only 12 antiparallel β-strands. The interior of the barrel was shown to be highly hydrophilic, with charged amino acids forming patches axially along the barrel wall in a manner reminiscent of the protein-translocating outer membrane pore formed by TolC (see “Type I protein secretion” above). The N-terminally located β-strand is connected to an α-helical stretch of residues which is located within the pore. The α-helix interacts with the interior of the barrel through salt bridges, hydrogen bonding, and van der Waals' forces (366). It is presumed that displacement of the α-helix from the pore, which may occur due to the detergent or salts used in the planar lipid bilayer experiments, would account for the 1.3-nS activity; the 0.15-nS activity would occur when the helical region is embedded in the pore (366). While these data strongly support the original model suggested by Pohlner et al. (401), it remains possible that the refolded protein does not adopt its native conformation. Alternatively, the passenger domain is secreted via a central pore, as proposed by Veiga et al. (512), and subsequently the α-helical region inserts into the β-barrel pore after secretion of the passenger domain. This latter theory has some support from the different measurements obtained for the pore size in planar lipid bilayers (366).
Oliver et al. (364, 365) recently discovered an autochaperone domain in BrkA. This well-conserved domain, found upstream of the translocation unit, is present within the passenger domains of several autotransporters, like AIDA-I, IgA1 protease, Ag43, Hap, and IcsA, but was not detected in some autotransporters such as TcfA and Hia (365). It was demonstrated that this domain is important for the folding of the BrkA passenger domain, probably by triggering or initiating correct folding of the passenger domain. In BrkA, this domain could act as an intramolecular building block (305). It was speculated that this domain might function as a general chaperone to scaffold the folding of any protein linked to it. This autochaperone domain is cleaved from the passenger domain of PrtS but not from BrkA, suggesting that the folding mechanism(s) may differ depending on the autotransporter (365).
Assuming that the model described by Pohlner et al. (401) is correct, the passenger domain forms a hairpin structure and appears to remain unfolded, or partially folded, as it travels through the channel. Indeed, the structure demonstrated for the β-barrel of AspA/NalP revealed a pore sufficiently wide to accommodate two extended polypeptide chains passing through the pore simultaneously (366). Then, as the passenger domain emerges from the β-domain channel, folding is triggered beginning vectorially from a C-terminal direction on the bacterial cell surface. Evidence that passenger folding can occur on the bacterial surface has been provided by studies of PrtS autotransporter (362). Interestingly, the passenger domains of the autotransporters demonstrate a high degree of β-helix structure, as predicted from the BetaWrap program (44), suggesting a structure analogous to pertactin (123) (see “Cluster 6: Bordetella autotransporters” below); thus, translocation of the passenger domains may actually be assisted by C-terminal winding of the β-helix. However, it was shown recently that while autotransporters lacking the autochaperone domain could be translocated and displayed on the cell surface, they did not fold into their native conformation (365). Unfortunately, the influence of the autochaperone domain deletion on the efficiency and kinetics of the passenger domain secretion is unknown.
Processing of autotransporters at the surface.
Once at the bacterial surface, several alternative processing steps have been demonstrated for autotransporters. First, the passenger domain may be processed and released into the extracellular milieu, e.g., Pet and EspP (49, 124). In contrast, for some autotransporters like Ag43 (373), AIDA-I (24) and the pertactins (279, 287), once the passenger domain is cleaved, it remains in contact with the bacterial surface via a noncovalent interaction with the β-domain. However, the passenger domain is not necessarily cleaved but may also remain intact as a large protein with a membrane-bound C-terminal domain and an N-terminal domain extending into the external milieu, e.g., Hia or Hsr (367, 465). Nevertheless, the cleavage between the passenger domain and the translocation unit can occur either well upstream of the linker region or within the predicted α-helical region, like BrkA (364). In this latter case, BrkA remains steadfastly associated with the bacterial outer membrane, raising the possibility that the linker region could also act as an anchor for the passenger domain.
Controversy still exists over how cleavage of the passenger domain from the translocation unit occurs, especially whether cleavage is a result of a membrane-bound protease or an autoproteolytic event. For IgA1, Hap, and, more recently, App, it has been demonstrated that the cleavage was the result of an autoprocessing event involving the integral serine protease active sites of the autotransporter, present in the passenger domain (206, 401, 445). AIDA-I is also thought to be autoprocessed, although no serine protease motif has been found (472). Passenger domains may also be processed at several sites and possibly by a variety of protease as observed with Hap, where the primary autocleavage site was mutated but the protein was still cleaved at alternative sites (206). Some autotransporters have been shown to undergo processing even after deletion of their serine protease motif, suggesting the action of alternative proteases (22, 409). It has been demonstrated that in S. flexneri, IcsA (also called VirG) is cleaved by another membrane protease, IcsP (also called SopA) (117, 451).
Interestingly, the IcsA autotransporter has the unusual characteristic of being localized to a single pole of the bacillus (166). The current model suggests that, on translocation into the outer membrane at one pole, IcsA, which is anchored in the membrane by its β-domain, diffuses laterally within the outer membrane such that some of it drifts down the sides of the bacillus towards the septum (463). IcsP, which is localized in the outer membrane, slowly cleaves IcsA at all sites on the bacterial surface, but since the insertion of IcsA is occurring exclusively at one pole, it results in a unipolar distribution of IcsA (463). This study raises the question of the fate of the β-barrel once cleavage of the passenger domain has occurred. By analogy to other outer membrane proteins, the β-barrel of autotransporters might be a rather stable structure (444). Results from cellular localization studies seem to corroborate the persistence of the β-barrel in the outer membrane (60). However, this question has not yet been satisfactorily addressed. One might speculate that accumulation of pore-forming structures in the outer membrane would be lethal for the bacteria and that some kind of degradation or regulation process must therefore occur. However, the degradation mechanism(s) of periplasmic and outer membrane proteins remains elusive and requires further investigation, although it might involve periplasmic enzymes such as DegP and DegQ or outer membrane proteases such as OmpT.
Energy of transport.
Comprehensive understanding of the energy requirements used to translocate proteins across the bacterial outer membrane has lagged behind the wide-ranging studies investigating inner membrane transport (378). Since neither ATP nor GTP occurs in the periplasm, hydrolysis of these molecules cannot be the driving force for the translocation of the passenger domain through the outer membrane. Moreover, there is still no evidence of the existence of a proton motive force (Δp) across the outer membrane. The presence of P-loop nucleotide motifs in several members of the autotransporter family has been reported (203). Proteins bearing a P-loop motif are not always ATP- or GTP-binding proteins, and even proteins binding ATP or GTP do not always possess this motif (433, 522). Subsequently, it was suggested that since autotransporters can be correctly targeted and exported in nonnative gram-negative bacterial backgrounds, e.g., gonococcal IgA1 protease in E. coli, no additional secretion function is required and thus the energy source driving translocation across the outer membrane is derived from the correct folding of the passenger domain on the bacterial cell surface (254). From the study by Veiga et al. (510), where a nonnative single-chain antibody reporter passenger domain was used, it appeared unlikely that the folding of the passenger domain on the cell surface would provide the energy required for the translocation through the outer membrane. Unfortunately, this set of experiments used a nonnative passenger domain lacking the intramolecular chaperone domain. Thus, a common sorting mechanism assisting the assembly and export of autotransporter proteins and involving the autochaperone domain may exist. However, as mentioned above, BrkA lacking the autochaperone domain is still secreted, suggesting that other factors provide the necessary driving force for secretion. In conclusion, the energy dependence of the autotransporter secretion is still controversial and speculative and further investigation is required to clarify this issue.
Evolution and distribution of autotransporter proteins.
While the translocation units of autotransporters are highly homologous, the passenger domains are very diverse. To date, all characterized passenger protein domains have been implicated in virulence by displaying enzymatic activity (protease, peptidase, lipase, and esterase); mediating actin-promoted bacterial motility; acting as adhesins, immunomodulatory proteins, toxins or cytotoxins, or, as more recently discovered, permitting the maturation of other virulence proteins (82, 201, 203, 540). The autotransporters are present only in the Bacteria kingdom and are most prevalent in the phylum Proteobacteria, including the α-, β-, γ-, and ɛ-Proteobacteria classes (540). Like the TTSS, the only other phylum in which autotransporters have also been identified is the phylum Chlamydiae, genera Chlamydia and Chlamydophila (198, 213). In most cases, multiple genes encoding autotransporters are found throughout the genome sequence of the microorganism, e.g., E. coli (530), Bordetella spp. (382), Yersinia pestis (98), Helicobacter pylori (313), Pseudomonas aeruginosa (306) and Neisseria (91). It has been suggested through genome sequence analysis that the passenger domain is not always covalently linked to the β-domain, since some open reading frames (ORFs) code either only for a passenger domain or a β-domain. However, these could simply be pseudogenes, since there is no experimental evidence that these proteins are expressed (540).
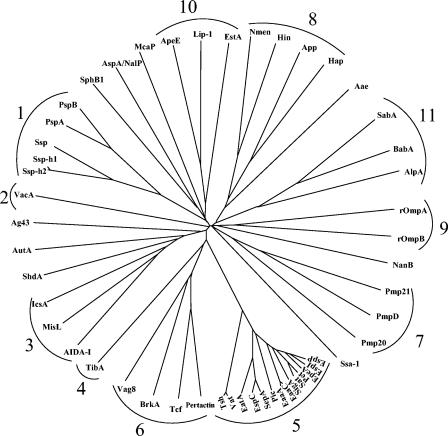
It has been proposed that autotransporter proteins could have evolved by domain shuffling (91, 303). Functionally novel autotransporters could arise by linking a new active passenger domain to a generic β-barrel domain. Phylogenetic analysis showed that horizontal transfer between distant organisms of the portions of the gene encoding the autotransporter β-domain was a rare evolutionary event; instead, close homologues appear to arise almost exclusively by speciation and late gene duplication events within a single organism. Such analyses have allowed Yen et al. to classify the autotransporters into 10 distinct phylogenetic clusters (540). However, reinvestigation of the phylogenetic relationship of the autotransporters reveals that the proteins can be grouped into at least 11 clusters (Fig. 6) (I. R. Henderson, unpublished data). However, it is clear that the functional passenger domains have spread by horizontal transfer (91, 201). For example, while the β-domains of the IgA1 proteases and the serine protease autotransporters of the Enterobacteriaceae (SPATE) have different evolutionary lineages (cluster 8 and 5, respectively), the functional passenger domains are evolutionarily related and belong to the same clan of serine proteases (Table 1, Fig. 6). These data suggest that most autotransporters have arisen through fusion events between passenger domains and β-domains.
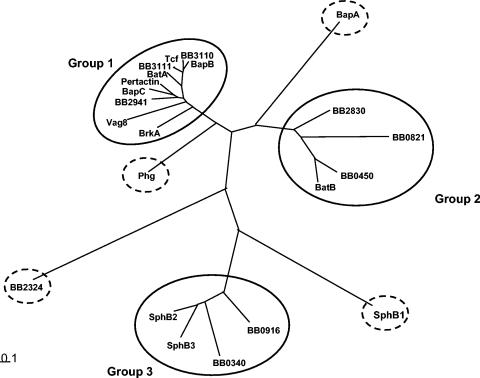
FIG. 6.
Phylogenetic tree of the autotransporter proteins. The CLUSTALX and TREE programs were used for multiple alignments and construction of a phylogenetic tree for the functionally characterized autotransporter proteins. The tree depicted in this figure is derived from analyses of the C-terminal translocating domains of the autotransporters. Proteins are clustered according to the pattern proposed by Yen et al. (540). A single additional cluster (cluster 11) is indicated.
TABLE 1.
Functionally characterized autotransporters
| Protein | Accession no. | Bacterial species | Mol wt | Significant functional motifs | Known function | Reference |
|---|---|---|---|---|---|---|
| Cluster 1 | ||||||
| Ssp | P09489 | S. marcescens | 112,345 | Peptidase S8A subtilisin-like serine protease, RGD cell attachment sequence | Protease | 537 |
| Ssp-h1 | BAA33455 | S. marcescens | 107,598 | Peptidase S8A subtilisin-like serine protease, RGD cell attachment sequence | None | 360 |
| Ssp-h2 | BAA11383 | S. marcescens | 107,766 | Peptidase S8A subtilisin-like serine protease, RGD cell attachment sequence | None | 360 |
| PspA | BAA36466 | P. fluorescens | 102,684 | Peptidase S8A subtilisin-like serine protease, RGD cell attachment sequence, P-loop ATP/GTP-binding site motif | None | 236 |
| PspB | BAA36467 | P. fluorescens | 106,789 | Peptidase S8A subtilisin-like serine protease, P-loop ATP/GTP-binding site motif | None | 236 |
| Ssa1 | AAA80490 | P. haemolytica | 103,585 | Peptidase S8A subtilisin-like serine protease | None | 169 |
| SphB1 | CAC44081 | B. pertussis | 109,801 | Peptidase S8A subtilisin-like serine protease | Proteolytic processing of secreted proteins | 82 |
| AspA/NalP | AAN71715 | N. meningitidis | 113,640 | Peptidase S8A subtilisin-like serine protease, P-loop ATP/GTP-binding site motif, prokaryotic membrane lipoprotein lipid attachment site | Proteolytic processing of secreted proteins | 508 |
| Cluster 2 | ||||||
| VacA | Q48247 | H. pylori | 139,761 | None | Vacuolating cytotoxin | 393 |
| Cluster 3 and 4 | ||||||
| AIDA-I | Q03155 | E. coli | 132,272 | None | Adherence | 24 |
| IcsA | AAA26547 | S. flexneri | 116,244 | None | Intracellular motility | 283 |
| MisL | AAD16954 | S. enterica | 101,216 | None | None | 35 |
| TibA | AAD41751 | E. coli | 101,112 | None | Adherence/invasion | 292 |
| Ag43 | P39180 | E. coli | 106,842 | RGD cell attachment sequence, aspartyl proteases active site, leucine zipper pattern, P-loop ATP/GTP-binding site motif | Biofilm formation | 204 |
| ShdA | AAD25110 | S. enterica | 207,033 | Hemglutinin repeat, P-loop ATP/GTP-binding site motif | Adherence, prolonged fecal shedding of bacteria | 249 |
| AutA | CAB89117 | N. meningitidis | 68,386 | RGD cell attachment sequence | None | 3 |
| Cluster 5 | ||||||
| Tsh | I54632 | E. coli | 148,227 | Peptidase S6 IgA endopeptidase, P-loop ATP/GTP-binding site motif | Hemaglobinase- and mucinase activity, hema binding | 409 |
| SepA | CAC05786 | S. flexneri | 145,938 | Peptidase S6 IgA endopeptidase, peptidase S1 chymotrypsin | Tissue invasion? | 22 |
| EspC | AAC44731 | E. coli | 140,861 | Peptidase S6 IgA endopeptidase | Enterotoxic activity | 461 |
| EspP | CAA66144 | E. coli | 141,758 | Peptidase S6 IgA endopeptidase, P-loop ATP/GTP-binding site motif | Cytotoxic activity | 49 |
| Pet | AAC26634 | E. coli | 139,769 | Peptidase S6 IgA endopeptidase, P-loop ATP/GTP-binding site motif | Enterotoxic and cytopathic toxin effects, cleavage of spectrin | 124 |
| Pic | AAD23953 | E. coli | 146,450 | Peptidase S6 IgA endopeptidase | Mucinase activity | 196 |
| SigA | AAF67320 | S. flexneri | 139,676 | Peptidase S6 IgA endopeptidase | Cytopathic activity | 5 |
| Sat | AAG30168 | E. coli | 140,043 | Peptidase S6 IgA endopeptidase, P-loop ATP/GTP-binding site motif | Vacuolating cytotoxin | 178 |
| Vat | AAO21903 | E. coli | 148,291 | Peptidase S6 IgA endopeptidase, P-loop ATP/GTP-binding site motif | Vacuolating cytotoxin | 384 |
| EpeA | AAL18821 | E. coli | 147,340 | Peptidase S6 IgA endopeptidase, P-loop ATP/GTP-binding site motif | Mucinolytic activity | 284 |
| EatA | AAO17297 | E. coli | 147,696 | Peptidase S6 IgA endopeptidase, peptidase S1 chymotrypsin clan, | None | 385 |
| EspI | CAC39286 | E. coli | 146,131 | Peptidase S6 IgA endopeptidase, P-loop ATP/GTP-binding site motif | Degradation of plasma proteins | 440 |
| EaaA | AAF63237 | E. coli | 141,678 | Peptidase S6 IgA endopeptidase | None | 431 |
| EaaC | AAF63038 | E. coli | 141,673 | Peptidase S6 IgA endopeptidase | None | 431 |
| Cluster 6 | ||||||
| Pertactin | P14283 | B. pertussis | 93,453 | RGD cell attachment sequence | Adherence | 61 |
| BrkA | AAA51646 | B. pertussis | 103,377 | RGD cell attachment sequence | Serum resistance | 132 |
| Tef | AAQ82668 | B. pertussis | 65,865 | RGD cell attachment sequence | Tracheal colonization | 141 |
| Vag8 | AAC31247 | B. pertussis | 94,910 | RGD cell attachment sequence | None | 140 |
| Cluster 7 | ||||||
| PmpD | O84818 | C. trachomatis | 160,748 | None | Adherence | 464 |
| Pmp20 | Q9Z812 | C. pneumoniae | 179,595 | Peptidase S1 chymotrypsin | Adherence | 234 |
| Pmp21 | Q9Z6U5 | C. pneumoniae | 170,866 | None | Adherence | 234 |
| Cluster 8 | ||||||
| IgA1 protease | NP_283693 | N. meningitidis | 196,351 | Peptidase S6 IgA endopeptidase | Degradation of IgA1 LAMP-1, and synaptobrevin | 300 |
| App | CAC14670 | N. meningitidis | 159,072 | Peptidase S6 IgA endopeptidase, peptidase S1 chymotrypsin, RGD cell attachment sequence | Adherence | 182 |
| IgA1 protease | P45386 | H. influenzae | 202,957 | Peptidase S6 IgA endopeptidase, P-loop ATP/GTP-binding site motif | Degradation of IgA1 | 406 |
| Hap | P45387 | H. influenzae | 155,440 | Peptidase S6 IgA endopeptidase, peptidase S1 chymotrypsin | Adherence, microcolony formation | 466 |
| Cluster 9 | ||||||
| rOmpA | P15921 | R. rickettsii | 224,333 | None | Adherence | 10 |
| rOmpB | Q53047 | R. rickettsii | 168,184 | None | Adherence | 163 |
| Cluster 10 | ||||||
| ApeE | AAC38796 | S. enterica | 69,862 | Lipolytic GDSL active-site enzyme | Hydrolysis of naphthyl esters | 58 |
| EstA | AAB61674 | P. aeruginosa | 69,609 | Lipolytic GDSL active-site enzyme | Lipolytic activity | 533 |
| Lip-1 | P40601 | X. luminescens | 70,717 | Lipolytic GDSL active-site enzyme, leucine zipper pattern | Hydrolysis of naphthyl acetate, Tween 80, and p-nitrophenylpalmitate | 523 |
| McaP | AAP97134 | M. catarrhalis | 71,484 | Lipolytic GDSL active-site enzyme | Hydrolysis of phophatidylcholine and lysophosphatidylcholine, and adherence | 489 |
| Cluster 11 | ||||||
| BabA | AAC38081 | H. pylori | 80,345 | None | Adherence to Lewisb blood group antigen | 215 |
| SabA | AAD06240 | H. pylori | 72,169 | None | Adherence to sialic Lewis antigen | 308 |
| AlpA | CAB05386 | H. pylori | 56,124 | Leucine zipper pattern | Adherence to Lewis x antigen | 358 |
| Unassigned | ||||||
| Aae | AAP21063 | A. actinomycetemcomitans | 100,609 | None | Adherence | 423 |
| NanB | AAG35309 | P. haemolytica | 119,791 | RGD cell attachment sequence sialidase | Sialidase activity | 329 |
Two-Partner Secretion Pathway (Type Vb)
In the bacterial two-partner secretion (TPS) pathway, as with the autotransporter secretion pathway, the passenger domain possesses a signal sequence that directs translocation across the inner membrane (195). After its export into the periplasm, the passenger domain inserts into an outer membrane pore formed by a β-barrel. Once at the surface of the bacterium, the passenger domain may undergo further proteolytic processing to achieve its physiological function (221). However, in contrast to the autotransporter pathway, where the protein is produced as a single polypeptide, the passenger domain (also called the exoprotein) and the pore-forming β-domain (also called the transporter domain) are translated as two separate proteins, respectively referred to as TpsA and TpsB family members (Fig. 3) (221). Compared to the autotransporter, the β-barrel topology seems different, as indicated by prediction of 19 amphipathic β-strands in TpsB instead of 14 for the autotransporter β-domain (176, 303, 540) and different conductance values in artificial bilayers (220, 259). Furthermore, TpsB proteins possess an additional level of complexity, as suggested by their involvement in passenger domain maturation into an active form (107, 221, 275, 439, 467). The genes for cognate exoprotein and transporter protein are generally organized in an operon (221). The TPS pathway implies specific recognition events between the passenger domain and the transporter domain. In TpsA, a conserved N-proximal domain, called the TPS domain, interacts specifically with TpsB to initiate the outer membrane translocation (172, 219, 441, 498). Like the autotransporter pathway, the TPS pathway appears to be dedicated to the secretion of very large proteins, i.e., >100 kDa (221). It has been suggested that the exoprotein transits to the periplasm in an unfolded conformation and folds progressively at the cell surface as it is translocated through the transporter domain (175). Thus, the translocation across both membranes seems coupled and the free energy of folding might be the driving force for the outer membrane translocation. Concomitantly with secretion, several passenger domains undergo further proteolytic processing (221). Interestingly, it has been demonstrated that the B. pertussis autotransporter SphB1 acted as a specific protease responsible for the bacterial surface maturation of FhaB, the filamentous haemagglutinin (FHA) secreted by the TPS pathway (82).
Thus, despite certain differences, the TPS pathway shares fundamental features with the autotransporters, the most important being the mode of secretion described above. Moreover, examination of the signal sequences of TpsA family members reveals that several of these proteins possess unusual signal sequences which are remarkably conserved with the extended signal sequence of autotransporters (Fig. 4) (195, 221). It can be hypothesized that such exoproteins are translocated through the inner membrane by the SRP-dependent pathway, as has been demonstrated for the Tsh/Hbp autotransporter (455). The third line of evidence supporting a connection with the autotransporter secretion pathway is derived from phylogenetic analyses, where it has been suggested that the shuffling of some passenger domains between the autotransporter and TPS pathways had occurred frequently in the course of evolution (195), even though the translocating units have apparently arisen independently (540). This is supported by the fact that the homologous passenger domains of the TPS and autotransporter pathways perform similar functions (195, 218). However, it is still difficult to discriminate between a divergent evolution from a common ancestor or a convergent evolution toward a similar solution for secretion of large proteins across the outer membrane (195, 202, 218). Interestingly, the efficient extracellular release of mature FHA depends on the presence of a C-terminal domain of FhaB, which is proteolytically removed in the course of secretion and acts as an intramolecular chaperone to assist secretion by preventing premature folding (414). These data highlight further similarity between the proteins secreted through the TPS pathway and the recent finding of an autochaperone domain in some autotransporters (365). From the similarities between the TPS and autotransporter pathways described above and the established fact that secretion in gram-negative bacteria is constrained by outer membrane translocation and not by the inner membrane pathways, Henderson et al. (195, 202) proposed that the TPS pathway should be grouped under the type V secretion umbrella as a distinct subgroup.
Type Vc
Recently, the members of the Oca (for “Oligomeric Coiled-coil Adhesins”) family have been described as a subfamily of surface-attached oligomeric autotransporters, with the Y. pestis YadA as the prototypical example (356, 422). YadA possesses six different domains: (i) an N-terminal signal sequence, (ii) head-D, (iii) neck-D, (iv) stalk-D, (v) linking-R, and (vi) a C-terminal region consisting of only four β-strands (210). Like the autotransporters, deletion of the C-terminal domain abolished outer membrane insertion of YadA (479) while the deletion of the linker region resulted in degradation of the whole protein (422). This region is proposed to form a β-barrel pore consisting of 12 β-strands after trimerization and confers an overall lollipop-like structure on the proteins displayed on the cell surface (210). TolC possesses an analogous β-barrel structure, although it also possesses α-helical regions which extend into the periplasmic space (Fig. 2) (263). Recent structural investigations have demonstrated that the H. influenzae Hia adhesin is a trimeric protein secreted in a manner analogous to YadA rather than the true autotransporters, as previously described (465, 474, 541). This type of trimeric conformation and mode of secretion has been proposed as an alternative model for autotransporter secretion system (see above) and has been designated the type Vc or AT-2 pathway (Fig. 3).
The passenger domains of the AT-2, two-partner, and autotransporter protein secretion systems correspond to the functional secreted effector molecule. To date, all of the functionally characterized passenger domains have been implicated in bacterial virulence (74, 82, 201, 203, 221, 422). While over 1,000 autotransporters can be identified in GenBank, relatively few have been characterized at the functional level. Critical aspects of the characterized autotransporters are listed in Table 1, and the functions of each are discussed in detail below. We have framed the discussion around the phylogenetic clusters identified in Fig. 6. For expediency, proteins of similar functional activity are discussed under the same heading.
CLUSTER 1: SUBTILASE FAMILY OF AUTOTRANSPORTERS
The subtilase group of autotransporters is part of an extensive family sharing a catalytic site characterized by a charge relay system resembling the trypsin family but having apparently evolved convergently. The sequence surrounding the residues involved in the catalytic triad (Asp, Ser, and His residues) is different from the analogous residues in the trypsin-like serine proteases (268). Since this family is best characterized for Bacillus subtilus, its members are referred to as the subtilases and are characterized by the conserved domain pfam00082 (314).
Ssp
Several subtilase autotransporters from different species have been identified. The first member was found in 1986 by Yanagida et al. (537) in Serratia marcescens, and its secretion system was elucidated almost at the same time that Pohlner et al. (401) proposed it for the IgA1 protease. Sequencing of the gene encoding this subtilase serine protease (Ssp) revealed that it contained an open reading frame encoding a 112-kDa protein. These authors found that the mature protein (41 kDa), which contains the catalytic triad (D49, H85, and S314), is the middle part of a proenzyme, which starts at A28 and finishes at D408. Interestingly, the secretion of the protease into the extracellular medium through the outer membrane of the E. coli host cell occurs in parallel with their growth (537). Additional data from the same group have shown that (i) the 112-kDa precursor is detected in an insoluble form in the periplasmic space of E. coli cells after isopropyl-β-d-thiogalactopyranoside (IPTG) induction of the expression of the gene under the control of the tac promoter, (ii) the mutated gene product lacking all the C-terminal domain was localized in the periplasmic space only, (iii) the mutant protein with no protease activity because of the change of the catalytic residue S341 to T341 was still secreted into the medium but with abnormal processing, (iv) the signal sequence was essential for the secretion of the mature protease of 66 kDa into the medium, and (v) cleavage of the mature extracellular Ssp from the β-domain occurred via an autocatalytic event and at several sites in the junction region between the passenger and β-domains. Furthermore, the N-terminal portion of the β-domain (S646 to G716), called the junction region of the Ssp precursor, was found to play a role in folding the mature protease domain in a manner analogous to the BrkA intramolecular chaperone domain described above. Finally, the extracellular transport of a foreign periplasmic protein, pseudoazurin of Alcaligenes faecalis, using the β-domain of Ssp was detected in E. coli (328, 361, 362, 452, 453).
Southern hybridization analysis with a DNA fragment encoding the β-domain of Ssp as a probe showed a wide distribution of nucleotide sequences encoding Ssp exporter-like proteins among Serratia species. Moreover, S. marcescens IFO 3046, from which the ssp gene had been cloned, was found to contain two ssp homologues, called ssp-h1 and ssp-h2 (360). ssp-h1 and ssp-h2 showed 55% identity in the amino acid sequence to Ssp. Furthermore, both genes are located in tandem on the genome, and the amino acid sequences of their products showed 81% identity to each other, suggesting that one of them was generated by gene duplication during evolution (360). E. coli JM105 containing the ssp-h1 gene produced a 53-kDa protein corresponding to the N-terminal portion and a 49-kDa protein corresponding to the C-terminal portion, both of which were rigidly integrated in the outer membrane. Furthermore, both ssp-h1 and ssp-h2 β domains, by using chimeric proteins, showed the ability to translocate the mature SSP part across the outer membrane into the medium, and the N-terminal part of the homologue was not translocated into the outer membrane without its C-terminal part.
PspA and PspB
Kawai et al. (236) found two ORFs (genes pspA and pspB) encoding proteins of 985 and 1036 amino acids, respectively, which were localized between the aprDEFPF33 and lipAPF33 genes of Pseudomonas fluorescens 33. PspA is 53% identical to PspB, and both are homologous to the S. marcescens subtilases. Three motifs for the active sites (D, H, S) of subtilases are also found in the PspA and PspB sequences. Interestingly, E. coli harboring pspA secretes a protein of 51 kDa into the supernatants from cultured media at 23°C, but when cultured at 37°C they produced only an insoluble protein of 51 kDa. In contrast, in Pseudomonas fluorescens, PspA is only detected in the lysates of cells cultured at 37°C but not at 23°C (236).
Ssa1
Despite the extensive investigation of the secretion of these subtilase family autotransporters, which started with Ssp in 1986, there is little information about the role of these proteins in vivo. Interestingly, these genes showed end-to-end similarity to the 100-kDa serotype-specific antigen 1 (Ssa1) of Mannheimia (Pasteurella) haemolytica (169, 360). Ssa1 appears to play a role in the pathogenicity of M. haemolytica. M. haemolytica strains have been grouped into 15 serotypes on the basis of standardized typing antisera raised in rabbits. In contrast to S. marcescens and P. fluorescens, M. haemolytica serotype 1 has been established as the primary agent responsible for acute lobar fibrinonecrotizing pleuropneumonia in cattle. Serotype 1 is rarely found in healthy cattle, but serotype 2 is cultured frequently from the upper respiratory tracts of healthy animals (169, 297). Interestingly, both serotypes possess the gene encoding serotype-specific antigen Ssa1 but M. haemolytica serotype 2 strains do not express serologically detectable levels of the Ssa1 protein. In addition, cattle showing resistance to pneumonic pasteurellosis possess antibodies to Ssa1 which appear to be protective (531).
SphB1
SphB1 is a newly described Bvg-regulated Bordetella autotransporter (82). It has 24% sequence identity to Ssp and is necessary for the surface maturation of a Bvg-regulated adhesin called filamentous hemagglutinin (see Two-Partner Secretion Pathway above). A serine protease (subtilase) motif is evident, and mutation of Ser412 to Ala inhibited the processing of FHA. In fact, the Ser-to-Ala mutation also prevented processing of SphB1 upstream of its β-domain, suggesting that SphB1 is autocatalytic. Since the β-domains of BrkA, pertactin, and Tcf were found in the Ser-to-Ala mutant, SphB1 is not responsible for their cleavage and is thought to have a function unique to FHA maturation (82). Like many other autotransporters, the passenger domain of SphB1 is attached to the bacterial surface, although a small amount was found shed in the supernatant. Anchoring of SphB1 to the bacterial surface is mediated by lipid modification of the N terminus of SphB1. Globomycin treatment or mutation of the cysteine residue in its conserved lipid modification site (Leu-Ala-Ala-Cys) abrogated the surface localization of SphB1, providing further evidence that SphB1 is indeed a lipoprotein (83). The ProDom domain PD011682 (76), which was associated with an intramolecular chaperone-like function in Ssp, is also present in SphB1; it is found in the area described as region IV, which bridges the subtilisin domain and the β-domain.
AspA/NalP
In addition to IgA1 protease, N. meningitidis appears to possess several autotransporter proteins. Recently, in a search for immunogenic virulence factors of N. meningitidis by using several molecular, immunological, and bioinformatic approaches, the Ala'Aldeen group has identified at least five autotransporter proteins including AutA, AutB, App, MspA, and AspA/NalP (3, 182, 495; D. A. Ala'Aldeen, unpublished data). AspA/NalP (for “Autotransported Serine Protease A” also called neisserial autotransported lipoprotein [NalP]) is a highly conserved, autotransported, 112-kDa subtilase, which was identified from the meningococcal genome sequence data by using bioinformatics techniques (495). This protein has significant N-terminal homology to the secreted subtilases from several organisms and contains a serine protease catalytic triad. The amino acid sequence of AspA/NalP is well conserved in meningococci. In N. gonorrhoeae, AspA/NalP appears to be a pseudogene. It also exhibits autocleavage activity, leading to the release of a 68-kDa fragment into the external milieu (495). The signal sequence contains a signal peptidase II cleavage motif, suggesting that AspA/NalP may be a lipoprotein. A well-characterized homologue of AspA/NalP, SphB1 in B. pertussis, is an important virulence determinant, which is responsible for the timely cleavage of the FHA precursor, leading to the secretion of mature FHA (82; see above). Interestingly, meningococci appear to possess the two genes (fhaB and fhaC) corresponding to B. pertussis fhaB and fhaC, which are secreted via the two-partner secretion pathway described above. AspA/NalP also shows homology to the serotype 1 antigen, Ssa1, from M. haemolytica A1, which causes a fibrinonecrotizing pleuropneumonia in cattle (297). The gene encoding AspA/NalP was cloned and expressed from meningococcal strains MC58 (B15:P1.16b) and H44/76; in the latter case, the protein was designated NalP and the crystal structure of the β-domain has been solved (see “β-domain” above). Anti-AspA/NalP antibodies were detected in patients' convalescent-phase sera, suggesting that AspA/NalP is expressed in vivo during infection and is immunogenic and cross-reactive. Rabbit polyclonal monospecific anti-AspA/NalP serum was used to probe whole-cell proteins from a panel of wild-type meningococcal strains and two AspA/NalP-mutant strains. Expression of the ca. 112-kDa precursor polypeptide was detected in 12 of 20 wild-type meningococcal strains examined, indicating that AspA/NalP expression is phase variable. Immunogold electron microscopy and cellular fractionation studies showed that the AspA/NalP precursor is transported to the outer membrane and remains surface exposed. Western blot experiments confirmed that smaller, ca. 68- or 70-kDa components of AspA/NalP (AspA68 and AspA70, respectively) are then secreted into the meningococcal culture supernatant. Site-directed mutagenesis of S426 abolished the secretion of both rAspA68 and rAspA70 in E. coli, confirming that AspA/NalP is an autocleaved autotransporter protein. Interestingly, like SphB1 (see Bordetella Autotransporters below), AspA/NalP alters the secretion of other bacterial proteins, namely, App, MspA, and IgA1 protease (508).
CLUSTERS 2 AND 11: HELICOBACTER PYLORI AUTOTRANSPORTERS
H. pylori is a gram-negative bacterium that colonizes the gastric mucosa of humans. Colonization with H. pylori is an important risk factor for the development of gastric and duodenal (peptic) ulcers, gastric adenocarcinoma, and gastric lymphoma (341). As in other complicated host-pathogen interactions, bacterial and host-related factors are important in the disease process. However, the exact mechanism by which H. pylori damages the human epithelial cell is not known. The bacterium expresses numerous virulence factors including the autotransporters VacA, BabA, SabA, and AlpA (201, 335, 408). Numerous other putative autotransporter proteins have been identified in the genome of H. pylori; however, they have not been well characterized (57).
VacA, the Vacuolating Cytotoxin
All H. pylori strains contain a copy of the vacA gene, which varies in length (3.9 kb ± 35 bp) among strains. Its transcriptional start point is located 119 bp upstream of the ATG start codon (144). VacA possesses features typical of an autotransporter protein, including a 33-amino-acid signal sequence, a ca. 90-kDa N-terminal passenger domain, and a 33-kDa C-terminal transporting unit (393). The 33-kDa C-terminal domain of VacA possesses the hallmarks of a β-barrel structure, and immunoblot experiments have confirmed that it is retained in the outer membrane of H. pylori (484).
Molecular and structural features.
Like many other autotransporters, the 90-kDa passenger domain is cleaved and secreted to the extracellular environment. Under nondenaturing conditions, the VacA passenger domains aggregate into large oligomeric complexes (84). Examination of the ultrastructure of rapidly frozen purified VacA, using deep-etch electron microscopy, demonstrated that the toxin assembles into large ring-form (flower-shaped) or flat-form complexes. The flower-shaped forms are about 30 nm in diameter and appear to consist of a central ring surrounded by six or seven “petals” (304). The flat-form complexes also consist of six or seven petals radiating from the center of the complex with a distinctive clockwise chirality but with no central ring. Atomic force microscopy imaging of purified VacA supports the deep-etch electron microscopy data and suggests that VacA is arranged in hexagonal central rings attached by connectors to peripheral domains (87). Following exposure to acidic or alkaline pH, the oligomeric complex dissociates into the 90-kDa monomeric units. This dissociation is probably important for the biological functionality of the protein; exposure of the purified oligomeric toxin to acidic or alkaline pH (activation) results in enhanced internalization of the toxin and a marked increase in its cytotoxic activity (85, 94, 321, 332, 535).
Like the IgA1 proteases the VacA passenger domain is further processed after secretion, separating into two subdomains i.e., p33, a 33-kDa N-terminal subdomain, and p55, the 55-kDa C-terminal region (351). H. pylori mutant strains, constructed with in-frame deletions in the p33 subdomain, express truncated VacA proteins which are secreted but which fail to oligomerize and lack detectable cytotoxic activity (415, 514). A VacA mutant, lacking most of the p33 subdomain, has been characterized in detail and found to form water-soluble dimers that have an ultrastructural appearance similar to that of the peripheral petals of VacA oligomers (415). This suggests that the peripheral petals of VacA oligomers correspond to the p55 subdomain. The two subdomains can be separated by limited proteolysis or by slow autodegradation during prolonged storage (85, 304). The cleavage site, which contains multiple charged amino acids, is thought to form a surface-exposed loop which is likely to add flexibility to the overall structure, although it does not appear to affect the level of cytotoxic activity (53).
Vacuolating activity.
The most striking feature of VacA is its ability to induce the formation of prominent intracellular vacuoles in cultured cells. The current model suggests that oligomeric VacA binds to the plasma membrane on the apical portion of the epithelial cell. The oligomerized p55 subdomains act as the host cell receptor-binding domain (152). At least three different proteins have been implicated as the receptor for the p55 subdomain (149, 374, 446); however, several groups have demonstrated that VacA binds to lipid rafts and glycosylphospahatidylinositol (GPI)-anchored proteins, suggesting that VacA interacts with cholesterol-rich microdomains (270, 416, 442).
After initial binding, VacA inserts into the plasma membrane, forming anion-selective channels (477). Eventually, the toxin is internalized by endocytosis and forms a pore located in a membrane-delimited vacuole. These vacuoles contain both late endosomal and lysosomal markers and appear to result from successive fusion between internal membranes with the limiting membrane of late endosomal compartments (333). The influx of anions alters the permeability of the vacuoles, eventually leading to water flux and vesicle swelling, thus forming the large vacuoles characteristic of VacA activity.
The p33 subdomain is considered the enzymatically active part of VacA, which becomes exposed to the host cell cytosol and influences the regulation of endosome-endosome fusion. Experiments with epithelial cell lines transfected with plasmid constructs encoding various lengths of the functional domain of VacA showed that the p33 subdomain is fully active when associated with the intersubdomain loop and a small fragment of p55. Loss of the remainder of the p55 C-terminal subdomain has no deleterious effect on the vacuolating activity of the molecule (92, 539). Furthermore, the loss of the first 6 or 10 N-terminal amino acids of the p33 subdomain led to partial or total loss of vacuolating activity, respectively (92, 539). The loss of amino acids 6 to 27 rendered the VacA molecule inactive but structurally unchanged (525). Further, point mutations at proline 9 or glycine 14, using alanine scanning mutagenesis, abolished VacA activity (538).
Host cell vacuolation may slowly but eventually lead to the death of primary human gastric epithelial cells (458). It is not known if this process reflects events occurring in vivo, although animal work clearly points to a loss of epithelial cells (285). Various studies have shown an association between vacuolating activity of the organism and peptic ulcer disease. H. pylori strains that express vacuolating cytotoxic activity in vitro are more commonly (but not absolutely) associated with disease than are noncytotoxic strains. In addition to direct damage to gastric epithelial cells, VacA is thought to contribute to the capacity of H. pylori to colonize and persist in the human gastric mucosa.
Other functions.
While the hallmark of VacA activity is its ability to produce vacuolization in cultured cells, it demonstrates properties of a multifunctional protein. In addition to causing the formation of intracellular vacuoles, VacA interferes with the process of antigen presentation (334), increases the permeability of polarized epithelial monolayers (380), acts as a urea permease that promotes urea diffusion across epithelia (491), produces enterotoxic effects in Ussing chambers (174), acts as a hexameric chloride channel in mammalian membranes (217), interacts with a cellular protein associated with intermediate filaments (93), and induces apoptosis (151, 388). Indeed, examination of the recent evidence suggests that induction of apoptosis may be the primary role of VacA (38), although the contribution of such activity to the life-style of H. pylori remains enigmatic.
Epidemiology of H. pylori infection.
VacA is highly conserved in terms of its distribution among H. pylori population; however, despite the importance of VacA for virulence and its structure-function relationship, there are extensive variations in its DNA and amino acid sequences. This sequence variation is most marked in a ca. 800-bp central region, termed the “mid-region,” which covers a large portion of the p55 subdomain coding area, and the “signal region,” which encompasses the distal region of the signal sequence and a small part of the p33 subdomain. The remainder of the p33 subdomain is relatively well conserved between alleles (14, 15), although there is evidence of recombination (170, 471).
Phylogenetic analysis of the variable mid-region has enabled the classification of vacA into two allelic families, termed type m1 and type m2 (14, 15). H. pylori strains with either allele are widespread in most human populations examined, although type m2 alleles are rare among the Japanese (16, 216, 536). Analysis of H. pylori isolates from unrelated individuals indicates that recombination within the alleles is common (471), but the main families have remained relatively intact (15, 379, 524). This suggests that various in vivo selective forces favor the preservation of these structures.
Two main signal region allelic families are recognized, termed s1 (sla, slb, and slc) and s2. Cleavage of the signal sequence in type s1 and type s2 VacA occurs at different locations (16). After cleavage, the secreted type s2 VacA is left with a hydrophilic amino terminus, and several studies have suggested that type s2 VacA proteins are relatively noncytotoxic in vitro compared to type s1 VacA proteins (280). Indeed, McClain et al. (320) reported that a 12-amino-acid hydrophilic N-terminal segment, which is present in type s2 but absent from type s1 VacA proteins, diminishes the capacity of VacA to form membrane channels and induce cytotoxic effects.
Interestingly, VacA molecules encoded by type s1/m1 vacA alleles are associated with a high level of vacuolating cytotoxin activity (14), and are active on a wide variety of epithelial cell lines. In contrast, other forms of VacA (e.g., type s1/m2) are associated with a lower or absent vacuolating activity. While these remain active against primary gastric epithelial cells and RK-13 cells, they are minimally active on HeLa cells (376). This difference in cell type specificity may be due to differences in the binding capacity of the p55 (binding) subdomains (376). These data are supported by examination of chimeric s2/m1 VacA constructs, which lacked activity and inhibited the vacuolating activity of wild-type s1/m1 VacA, presumably through formation of hetero-oligomeric complexes (281, 320).
The classification of vacA alleles according to families, particularly according to type s1 or type s2, seems to correlate with the risk for clinical disease. Numerous studies have concluded that peptic ulceration occurs more commonly among patients infected with H. pylori strains containing a type s1 vacA allele, although this association is less apparent in many Asian countries than in Europe and the Americas (375). To account for the association of certain vacA genotypes with peptic ulcer disease in Western countries, several possible explanations have been suggested. First, the type s1 allele is associated more frequently with the presence of other virulence factors that play a role in the pathogenesis of peptic ulcer disease than is the type s2 allele, e.g., the cag PAI and the BabA adhesin (14, 157, 359, 389, 425). This suggests that multiple bacterial factors contribute to ulcerogenesis. Second, H. pylori strains with the type s1 allele produce higher levels of VacA than do strains with type s2 allele (144). Finally, the type s1 VacA proteins are more cytotoxic than the type s2 proteins and therefore may cause greater gastric epithelial damage, thereby contributing to the pathogenesis of peptic ulceration (14, 144, 159, 280).
It is interesting that most studies have not shown an association between the mid-region alleles and disease, although two studies have shown an association of type m1 strains with gastric adenocarcinoma (239, 506). The latter disease is highly prevalent in Japan, where nearly all the prevalent strains are of this vacA genotype. This homogeneity suggests that vacA genotype and phenotype are not potentially useful markers of more pathogenic strains within Japan.
BabA, SabA, and AlpA
In addition to VacA, H. pylori expresses a number of other autotransporter proteins. These can be predicted in silico based on the deduced molecular features of ORFs. Two H. pylori autotransporter proteins, BabA and BabB, have recently been characterized (215). BabA was the first to be fully characterized. This 78-kDa outer membrane protein was successfully cross-linked to the human Lewisb (α-1,3/4-difucosylated) blood group antigens expressed on gastric epithelial cells (39, 40, 215). Thus, BabA2 is thought to act as a potent adhesin that not only mediates attachment of the organism to human gastric epithelium and protects it from gastric acidity and peristalsis but also contributes to the specific tropism of H. pylori.
It is interesting that all members of a subset of strains expressing BabA bound to Lewisb-coated microtiter dishes whereas organisms which did not express the protein did not adhere (157). In vitro adherence assays revealed that H. pylori bound in a lineage-specific manner to gastric surface mucous cells mediated by fucosylated blood group antigens (128). Moreover, studies with transgenic mice expressing the human Lewisb epitope in gastric epithelial cells indicated that Lewisb functions as a receptor for a H. pylori adhesin and mediates its attachment to gastric pit and surface mucus cells (129). Attachment of H. pylori to gastric epithelial cells in such transgenic mice resulted in the development of chronic gastritis and gastric atrophy (177).
The expression of BabA is probably phase variable, and the gene contains a repeat motif which undergoes frequent deletion and slip-strand mispairing. This phase variation can be significant in helping the organism avoid host defence mechanisms.
BabA and BabB are members of a paralogous family of outer membrane proteins, in which the members have significant N- and C-terminal similarity (490), suggesting possibilities for increased chances of recombination and enhanced capability of antigenic variation; babA and babB are almost identical in their 5′ and 3′ regions, with most of their sequence divergence being in their passenger domain mid-regions (7, 215). The sequence polymorphism of babA has been studied in detail, and several allelic types have been recognized. One specific allelic type, named babA2, appears to be associated with clinical disease (see below).
Pride et al (407) examined the relationships between babA and other members of this family of proteins and the diversity that exists within babA and babB. Eleven family members within a single H. pylori strain were classified as babA paralogues. These were predicted to encode proteins with substantial N- and C-terminal similarity to each other. In their central regions, most are less than 54% related to one another. Examining the babA and babB central regions in 42 H. pylori strains from different geographic locales, they also identified five different allele groups of babA (AD1 to AD5) and three different allele groups of babB (BD1 to BD3). Phylogenetic analysis revealed that the allelic groupings of babA and babB are independent of one another and that, for both, geographic variation is present.
Studies with transgenic mice expressing the Lewisb epitope have shown that such attachment can alter disease outcome. Gerhard et al. (157) demonstrated that the babA2 gene is of high clinical relevance in Western countries and would be a useful marker to identify patients who are at higher risk for specific H. pylori-related diseases. They studied the presence of the babA2 gene encoding the adhesin in clinical isolates from a German population by using PCR and reverse transcription-PCR. The presence of babA2 was significantly associated with duodenal ulcer and adenocarcinoma. The presence of babA2, vacAs1, and cagA in the genotype (“triple-positive” strains) showed a highly significant correlation to the prevalence of ulcer and adenocarcinoma. In contrast, however, Mizushima et al. (330) investigated the presence of babA2 and cagA in 179 Japanese clinical isolates by PCR and Southern blot analysis and looked for correlations with various clinical outcomes. The results indicate that babA2 status is not of high clinical relevance in Japan and that Japanese strains are different from those infecting Western populations.
A recent investigation of a babA null mutant found that this strain bound to the gastric mucosa of an H. pylori-infected patient with gastritis (308). Analysis of the glycosphingolipids bound to the surface of the babA mutant revealed that this strain could bind sialyl-dimeric Lewisx antigen. Utilizing the same “retagging” method that was used to identify BabA, Mahdavi et al. (308) identified SabA as the adhesin responsible for binding the sialic Lewis antigen. This adhesin belongs to the same paralogous family as BabA. Like BabA, SabA appears to be phase variable and possesses an apparently nonfunctional homologue, SabB. Interestingly, these researchers demonstrated that H. pylori was able to modulate the expression of the sialic Lewisx antigen on the surface of the host epithelial cell, causing an up regulation during persistent infection. Subsequent analyses demonstrated that SabA was also capable of mediating the binding to sialic Lewisa antigens, which is particularly noteworthy when one considers that this is an established tumor antigen and marker of gastric dysplasia (308).
Recently, two paralogous autotransporter genes, alpA and alpB, were identified. Using several molecular techniques, Odenbreit et al. (358) demonstrated that these genes were organized in an operon and that expression of both genes was required for a specific adherence of H. pylori to human gastric tissue sections. Subsequently, these investigators revealed that the adherence mediated by AlpA and AlpB was independent of the Lewisx antigen (357). Interestingly, AlpA possesses a lipoprotein signal sequence; however, acylation of the protein could be demonstrated only in E. coli and not in the wild-type H. pylori background (see “Inner membrane transport” above).
CLUSTERS 3 AND 4: AIDA AUTOTRANSPORTER FAMILY
The largest subfamily of autotransporters is defined by the AidA conserved domain COG3468 (representing 644 residues spanning the passenger and β-domains) and consists of members from a diverse range of animal and plant pathogens including E. coli, S. enterica, Y. pestis, N. meningitidis, Pasteurella multocida, A. tumefaciens, Mesorhizobium loti, and Brucella melitensis (314). This subfamily, which is composed of more than 55 proteins, possesses some of the best-characterized autotransporter proteins including the S. flexneri mediator of motility IcsA, the major phase-variable E. coli outer membrane protein antigen 43, and the diffuse adhering E. coli adhesin AIDA-I, from which this family derives its name. Despite the fact that this subfamily contains such well-characterized proteins, most members remain uncharacterized. Interestingly, the passenger domains of these autotransporter proteins display homology to wide array of proteins secreted via other protein secretion pathways, e.g., the B. pertussis FHA secreted via the TPS pathway (see above) (195) and the V. cholerae RtxA protein secreted by a type 1 pathway (289).
AIDA-I
Classical enteropathogenic E. coli (EPEC) strains were originally classified by serotype; however, over the past two decades it has become clear that the classical EPEC strains were composed of several different pathotypes of E. coli as defined by the pattern of adherence manifested in HeLa cell culture assays. Today, only strains displaying a localized pattern of adherence are referred to as EPEC, with diffuse-adhering E. coli (DAEC) and enteroaggregative E. coli (EAEC) being recognized as separate distinct pathotypes with differing virulence factors and mechanisms of mediating diarrheal disease (346).
One of the classical EPEC strains that displayed a diffuse adhering phenotype was E. coli strain 2787 (O12:H27), which was isolated from a patient with infantile diarrhea (25). Since the genes responsible for the localized phenotype were found on a large plasmid, Benz and Schmidt (25) hypothesized that the factor responsible for conferring the diffuse-adhering phenotype might also be located on an extrachromosomal element. By transforming E. coli C600 with recombinant clones containing fragments of the E. coli strain 2787 plasmids and examining the transformants for a diffuse-adhering pattern in the HeLa cell culture assay, these researchers were able to define the region responsible for the diffuse pattern of adherence as a 6-kb fragment from one of the large virulence plasmids (25, 26). This 6-kb fragment was shown to encode two proteins with predicted molecular masses of 44.8 and 132 kDa, termed Aah and AIDA-I, respectively. Several lines of evidence supported the theory that the AIDA-I protein was responsible for the diffuse-adhering phenotype, including the following: (i) mutant strains had a diffuse-adhering negative phenotype, (ii) specific antisera to AIDA-I inhibited bacterial attachment to HeLa cells, and (iii) electron microscopy demonstrated that the protein was surface localized (29).
Subsequent investigations revealed that the 132-kDa protein was processed posttranslationally in a manner consistent with secretion via the autotransporter pathway; thus, the AIDA-I protein was processed into a passenger domain with a predicted molecular mass of 79.5 kDa and a β-domain of 47.5 kDa (24, 27, 472). Interestingly, the passenger domain possessed several characteristics reminiscent of the E. coli surface protein antigen 43 (see below), including the facts that (i) after processing it remains associated with the cell surface through a noncovalent interaction, (ii) it can be released from the cell surface by heating to 60°C; (iii) it displays an aberrant migration pattern on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing conditions, migrating with an observed molecular mass higher than the predicted molecular mass (∼100 kDa in the case of AIDA-I); and (iv) it possesses a highly repetitive structure consisting of 31 conserved repeats composed of 19 to 21 amino acids each (24). Furthermore, purification of AIDA-I using gel filtration columns revealed that the purified AIDA-I passenger domain aggregates together, eluting in the 450- to 600-kDa range and suggesting a pentameric or hexameric structure (271). In silico analyses of the AIDA-I protein suggested that the passenger domain possessed a significant amount of β-strand structure. Furthermore, empirical data from circular dichroism spectra indicate that β-strands are as the major structural motifs of the AIDA-I passenger domain (271). These data are redolent of the B. pertussis protein pertactin, which also contains repetitive stretches and represents the largest β-stranded helix known (see below) (123). Interestingly, such repetitive structures appear to be a characteristic of proteins associated with adhesive or receptor functions, e.g., mycobacterial heparin-binding haemagglutinin adhesin (392), matrix-binding proteins of Staphylococcus aureus (190), and FHA of B. pertussis (233).
In an attempt to define the role of Aah, Benz, and Schmidt made mutations in the aah gene (28). In the absence of Aah, the ability of AIDA-I to confer the DA phenotype on E. coli C600 was abolished. In addition, Western blot experiments revealed that AIDA-I migrated with an observed molecular mass of ∼80 kDa, similar to the predicted value for the passenger domain, although the ability of the antisera to detect AIDA-I was severely diminished. These data suggested that during or after secretion, AIDA-I undergoes further posttranslational processing which is dependent on Aah. In silico examination of the Aah protein revealed that it was homologous to TibC, a protein involved in the glycosylation of another autotransporter protein termed TibA (292). In addition, both proteins had similarity to the consensus sequence of heptosyltransferases (28). For a long time, protein glycosylation was thought to be restricted to the realm of eukaryotes since it was assumed that prokaryotes lacked the necessary complexity and machinery for glycosylation to occur. However, research in recent years has demonstrated that glycosylation is a frequent occurrence in archaea and eubacteria and that these glycosylated proteins often play vital roles in the life-styles of the various organisms that synthesize these moieties (325, 331). Utilizing a glycan detection assay, it was demonstrated that the mature AIDA-I protein possessed a carbohydrate modification. The carbohydrate composition was determined to be 10.7% and consisted solely of monosaccharide heptose residues at a ratio of 19:1 (heptose molecules to AIDA-I). The position or motif at which glycosylation occurs remains to be determined, but intuitively the region most likely to be glycosylated is the repetitive region, perhaps the conserved VXNSGG (28). The occurrence of the glycosylation phenomena for AIDA-I and TibA suggests that this may be a much more widespread phenomenon than previously thought and that such a modification may occur frequently among the autotransporter proteins. Interestingly, the TibC and Aah proteins were demonstrated to be functionally interchangeable, which suggests another level of functionality, in that these proteins may be able to glycosylate other proteins within the cell (337).
Using HeLa cells as a model system, the receptor for AIDA-I was recently identified (271). Protease treatment and periodate oxidation of HeLa cells revealed that the receptor was proteinaceous. Biotinylation in conjunction with coimmunoprecipitation experiments utilizing specific anti-AIDA-I antisera revealed that the receptor was a 119-kDa protein. Treatment of HeLa cells with various glycosidases revealed that the receptor protein was N glycosylated. A variety of methods were utilized to no avail in an attempt to extract the glycoprotein receptor from HeLa cells, indicating that the protein does not have a loose association with the cell surface. In addition, treatment with GPI-anchor specific phopholipase (PI-PLC) suggests that the receptor is not a GPI-anchored protein. The cumulative data suggest that the receptor for AIDA-I is an integral membrane glycoprotein (termed gp119) whose identity remains elusive to date (271).
Although AIDA-I is the prototypical member of this subfamily of autotransporters, and it is from this protein that the family derives its name, it has a surprisingly sparse distribution among the diffuse-adhering strains of E. coli (25, 29, 435, 436). Such data, coupled with the fact that AIDA-I-specific antisera recognize protein species from other DAEC strains which do not hybridize with an AIDA-I gene probe, suggests that the DAEC pathotype of E. coli may be more complex than previously thought and that other autotransporter proteins homologous to AIDA-I may have the ability to confer a diffuse-adhering pattern of adherence on E. coli. Interestingly, a recent study demonstrated a relationship between the presence of AIDA-I, F18, and stx2e in E. coli isolates from pigs diagnosed with edema disease and postweaning diarrhea (354).
Antigen 43
During the 1970s there was intense interest in the architecture of the inner and outer membranes of E. coli. In what could be considered the first proteomic experiments, several researchers attempted to identify the complement of proteins present in each membrane by using a variety of techniques such as SDS-PAGE and crossed immunoelectrophoresis (Fig. 7). It was in one such study of the E. coli cell envelope that the protein antigen 43 (Ag43) was discovered (371, 372). The terminology for this protein arises from the fact that antigen 43 was the 43rd antigen in the crossed immunoelectrophoresis profile of membrane vesicles prepared from E. coli (Fig. 7). As such, antigen 43 was one of the first autotransporters identified, however, it was not until much later that its mode of biogenesis was uncovered (204). In the intervening period, much time was invested in the biochemical characterization of this protein; Owen and coworkers (54, 370) demonstrated that antigen 43 existed as a bipartite complex of two proteins (termed α43 and β43) which could be resolved under denaturing conditions. These subunit proteins were found to exist in a stoichiometric fashion at a ratio of 1:1, and the interaction between the two proteins was noncovalent. Further investigation revealed that α43 was a surface-localized protein which could be released from the cell surface on brief heating to 60°C. In contrast, β43 was an integral outer membrane protein. Later, Henderson and Owen (204) demonstrated that the α43 and β43 subunits represented the passenger domain and β-domain of an autotransporter, respectively.
FIG. 7.
Phase-variable expression of antigen 43. (A) Crossed-immunoelectrophoresis profile of Triton X-100-EDTA-extracted E. coli membrane vesicles. This represents the first resolution of the antigen 43 complex. (B) Coomassie blue-stained SDS-PAGE profile of outer membrane proteins from antigen 43-expressing and nonexpressing variants of E. coli. (C and D) Fluorescence microscopy of antigen 43 phase ON (Ag43+) and phase OFF (Ag43−) populations of E. coli, respectively. (E) Expression of antigen 43 induces bacteria to autoaggregate and thus to settle to the bottom of the growth vessel. The identity of antigen 43, its α43 and β43 subunits, and expressing and nonexpressing variants are depicted by arrowheads and appropriate labels. (Panels A, B, and E, copyright Peter Owen and Mary Meehan.)
For many years the function of antigen 43 (Ag43) remained elusive. Although the presence of two RGD motifs, which are associated with binding of the homologous FHA protein to integrins, suggested a role in adhesion, no definitive adherence phenotype could be demonstrated (13, 204). Recently, Henderson et al. (199) demonstrated that expression of Ag43 was responsible for a flat, frizzy, and irregular colonial morphology whereas cells not expressing antigen 43 formed glossy, circular colonies. This variation is distinct from the similar transitions associated with smooth and rough forms of LPS (199) and from expression of type 1 fimbriae (191). Furthermore, antigen 43 expression was also shown to mediate autoaggregation of bacterial cells by mediating a cell-cell interaction (199); indeed, Ag43-mediated aggregation was blocked by type 1 fimbrial expression, presumably by steric hindrance (191). Interestingly, expression of Ag43 in other species allowed the formation of inter- and intraspecies cell aggregation (250, 251). Recent investigations have demonstrated that the ability of Ag43 to mediate cell-cell interactions contributes to E. coli biofilm formation in minimal media (88), and microarray experiments have revealed that Ag43 expression was significantly higher within the biofilm than at any stage of planktonic growth (437). Interestingly, Ag43 was also demonstrated to facilitate λ, P1, and T4 phage infection of E. coli (150), raising the possibility that phage therapy could be used for certain E. coli-mediated diseases (526).
Unlike other members of the AIDA subfamily, Ag43 appears to have a ubiquitous distribution in E. coli, existing in the same form as prototypical Ag43 described above or as one of several immunologically cross-reactive proteins with slightly different molecular weights (6, 373, 421). Furthermore, many strains were found to possess multiple copies of Ag43. Significantly, multiple copies were found associated only with pathogenic isolates of E. coli (421). Recently, Torres et al. (493) described the Ag43 homologue (Cah) of E. coli O157:H7. The homologue demonstrated 69% identity to that described by Henderson and Owen (204); however, most of the difference could be attributed to a small truncation around the area of α43 and β43 cleavage. Furthermore, these researchers revealed that Cah was a calcium-binding protein which also mediated autoaggregation and biofilm formation (493). Electrospray mass spectrometry analysis of the purified Ag43 passenger domain revealed that, unlike Cah, Ag43 was not postranslationally modified or bound by any cofactors (204).
In contrast to other characterized autotransporters, Ag43 demonstrates a complex epigenetic and heritable phase variation mechanism which involves the alternative activation and repression of agn43 transcription (200) (Fig. 7). Such phase-variable expression has been noted for other surface-expressed proteins, although the mechanisms of regulation are quite varied (205); however, all give rise to a phenotypically heterogeneous population. The rate of variation was calculated to be 2.2 × 10−3 Ag43+→Ag43− and 1 × 10−3 Ag43−→Ag43+ (373). Screening of various mutations in a variety of regulatory genes revealed that all cells in an oxyR mutant background expressed Ag43. OxyR is a positive global regulator of genes transcribed under conditions of oxidative stress. In contrast, all cells in a dam mutant population had an Ag43− phenotype (204). Further investigations revealed that OxyR acts as a repressor, binding to a region downstream of the agn43 transcriptional start site in an area which encompasses three 5′-GATC-3′ sites (180, 521). This binding prevents the transcription of agn43 and results in an Ag43− phenotype. The 5′-GATC-3′ sites are susceptible to DNA methylation by Dam (deoxyadenosine methylase). Methylation of these sites was shown to prevent OxyR binding, and thus agn43 transcription could occur, resulting in an Ag43+ phenotype (521). Moreover, Ag43 phase variation was found to be coordinately regulated with type 1 fimbrial expression (437).
IcsA
IcsA is one of the most notorious autotransporter proteins, being an absolute requirement for Shigella-mediated diarrhea. The wealth of information generated about the mechanism of IcsA action is too capacious to review in detail here; however, it is worth noting some of the seminal characteristics of IcsA action.
IcsA was first described by Makino et al. (309) and annotated as VirG. These researchers found that mutants with a Tn5 insertion in a region on the large virulence plasmid were avirulent and, while capable of initially invading and multiplying in epithelial cells, were unable to move intercellularly and to infect adjacent cells. The mutation was localized to a 4-kb gene, and sequences hybridizing to this region were found in all Shigella and enteroinvasive E. coli strains (283, 309). Sequencing revealed that this gene possessed the characteristic features of an autotransporter (283). Further evidence for the essential nature of icsA in the pathogenesis of Shigella infection arose from human and animal experiments which indicated that icsA mutants were greatly attenuated (31, 265, 283, 432) and caused smaller ulcerations and abscesses in the intestinal mucosa (432).
In contrast to other autotransporters, which show an equal distribution of expression over the cell surface, expression of IcsA is localized to the old pole of the bacterium (63, 417). Once the molecule is inserted at the old pole, the passenger domain is cleaved from the bacterial cell surface by a specific protease, IcsP (451). In addition, once expressed at the pole, IcsA undergoes lateral diffusion such that the level of IcsA forms a gradient with the highest density at the pole and decreasing in density as it moves away from the pole (417). The region of IcsA responsible for directing the polar localization of the protein was localized to the passenger domain (118). However, recent data suggest that while IcsA is located predominantly on polar regions of the cell, it is also located laterally but the O-antigen side chains mask the laterally located molecules (339, 503).
Polar localization of IcsA results in actin accumulation at the pole of the organism. Actin forms the scaffold of the supportive structures of the cell, providing three-dimensional structure and permitting cells to adopt different shapes and certain types of movement (165). Accumulation of actin by IcsA leads to intracellular motility of Shigella spp. and formation of an actin tail (Fig. 8). In addition, this motility allows intercellular movement, by allowing the cells to breach the membranes separating adjacent cells. That IcsA is essential and sufficient for such motility has been demonstrated by several experiments showing that (i) mutants with mutations in icsA did not develop actin tails, (ii) E. coli strains expressing IcsA condense actin and demonstrate motility, and (iii) inert particles coated with IcsA exhibit actin-based motility (167, 256). The region of IcsA responsible for the actin-based motility was localized to the passenger domain of the protein.
FIG. 8.
IcsA-mediated Shigella motility. Fluorescence microscopy demonstrating that IcsA (red label) is expressed preferentially at one pole of the bacterium, where it mediates actin (green label) polymerization and formation of the characteristic comet tails. The actin comet tails are indicated by white arrowheads, and the Shigella bacterium is indicated by a black arrowhead. (Copyright Marcia B. Goldberg.)
Recently, the mechanism by which IcsA accumulates actin at the old pole, thus causing motility, has been reviewed in detail (165). In summary, IcsA appears to recruit a protein termed N-WASP to the bacterial cell surface, disrupting intramolecular bonds and thereby activating the N-WASP proteins. The activated N-WASP protein stimulates actin assembly via the Arp2/3 complex. In addition to N-WASP and the Arp2/3 complex, actin tail assembly requires the VASP, vinculin, profiling, cofilin, and Cdc42 proteins. The exact role of these proteins in actin tail assembly and motility remains to be determined (165).
TibA, ShdA, and MisL
The remaining three characterized members of the Aida subfamily of autotransporters include TibA from enterotoxigenic E. coli (ETEC) and ShdA and MisL from S. enterica. ETEC strains are capable of adhering to and invading cells derived from the human ileocecum and colon. Two distinct chromosomal loci (tia and tib) which are responsible for the invasion have been identified in ETEC (121, 122). Interestingly, when screened against a panel of pathogenic and nonpathogenic E. coli strains and other members of the Enterobacteriaceae, a tib probe hybridized only with ETEC strains, detecting the tib locus in 30% of ETEC strains and, surprisingly, showing a direct correlation with the presence of the plasmid-encoded fimbrial adhesin CFA/I (292). In silico analyses of the tib locus revealed two genes necessary for the invasion phenotype, e.g., tibA and tibC (291, 292). As mentioned above, the protein encoded by the tibC gene demonstrated homology to the RfaQ heptosyltransferase whereas TibA demonstrated homology to AIDA-I and possessed all the features of an autotransporter protein. In contrast to AIDA-I, TibA possesses two repetitive regions; one region is similar to AIDA-I, but the second shows a high level of proline residues like the pertactin protein (292). Such proline-rich regions have been implicated in weak nonstoichiometric binding activity (534). Expression and mutagenesis studies of the tibA gene demonstrated that it encoded a 104-kDa membrane protein which was directly responsible for the ability of ETEC to invade cells; chromosomal deletion mutants invaded cells at 15% of the wild-type level, and the full level of invasion could be restored by trans-complementation of the mutant (122). Enzyme-linked immunosorbent assays with purified TibA demonstrated that the protein was capable of binding to cultured HCT8 ileocecal cells in a specific and saturable manner and that this binding could be inhibited by anti-TibA polyclonal antibodies (291). Mutagenesis studies combined with periodate oxidation analyses of TibA and TibC demonstrated that TibC played a role in the glycosylation of TibA; thus, TibA was the first glycoprotein to be described in E. coli (291, 292). Furthermore, abolition of glycosylation also abolished the ability of TibA to direct adherence and invasion (291). The current dogma suggests that enterotoxins are the primary agents responsible for the water loss associated with ETEC infections. Thus, one may hypothesize that the role of TibA is to provide intimate contact with host cells in order to allow effective delivery of the toxin. Alternatively, TibA may contribute to ETEC pathogenesis through an undefined mechanism.
In a recent study, Kingsley et al. (249) identified an S. enterica Mud-Cam mutant in a chick virulence assay. Characterization of this mutant revealed that the insertion was present in a gene termed shdA and that the locus carrying shdA was inserted in the xseA-yfgK region relative to the E. coli K-12 chromosome (249). Sequencing of this region and flanking DNA revealed a pathogenicity island (PAI) of 25 kb, recently designated the CS54 island, which contained several genes including shdA, ratA, ratB, sivI, and sivH (247). In silico analyses of shdA demonstrated that the protein encoded by this gene possesses all the features of an autotransporter protein and has homology to AIDA-I, TibA, and another S. enterica autotransporter protein termed MisL (see below) (249). Like AIDA-I and TibA, ShdA possesses imperfect amino acid repeats in the passenger domain. However, ShdA contains three distinct repeats, three copies of a 102-amino-acid repeat, nine copies of a 63-amino-acid repeat, and four copies of a proline- and glycine-rich 12-amino-acid repeat which, as mentioned previously, is a feature reminiscent of other adhesins (249). To assess the role of ShdA in virulence, a well-defined S. enterica servor Typhimurium mutant was constructed by allelic exchange and analyzed in the BALB/c murine model of infection. Results of these experiments demonstrated that ShdA is essential for S. enterica serovar Typhimurium colonization of the cecum and that inactivation of shdA causes a reduction in bacterial number and the length of time for which bacteria are shed in murine feces (248, 249). Interestingly, the cecum appears to represent the main reservoir for S. enterica serovar Typhimurium during infection in mice (248). Recently, Kingsley et al. demonstrated that an ShdA-glutathione S-transferase fusion bound fibronectin in vitro and that this binding was dose dependent and partially inhibited by antifibronectin antibodies (248). Furthermore, using fluorescence microscopy and antibodies to the S. enterica serovar Typhimurium O-antigen, ShdA, and fibronectin, these investigators demonstrated that all three factors colocalized in the murine cecum, strongly suggesting that fibronectin is a receptor for ShdA binding (248). However, antifibronectin antibodies did not completely abolish binding of ShdA, leaving the possibility that other receptors may also play a role in ShdA-mediated adherence.
Interestingly, Southern hybridizations revealed that shdA was associated only with S. enterica subspecies I; serotypes of S. enterica subspecies II to VII and S. bongori do not possess shdA (249). Serotypes derived from subspecies I differ from the other serotypes with regard to animal reservoir since members of subspecies I are associated with disease in warm-blooded animals while the other serotypes are associated with disease in reptiles. The prevalence of shdA is in contrast to previously identified virulence factors such as SPI1, SPI2, agf, lpf, and fim, all of which show a distribution outside of the subspecies I lineage (249). Furthermore, virulence factors which are known to be restricted to subspecies I show a limited distribution within the lineage whereas shdA appears to be universally (97%) distributed (249). These data suggest that acquisition of shdA occurred at the time of expansion of the S. enterica host range into warm-blooded animals. Indeed, shdA may be responsible for the persistence of S. enterica within warm-blooded animals and the food chain.
MisL is also encoded by S. enterica subspecies I, but in contradistinction to ShdA, MisL also shows a limited distribution among strains of other lineages (35). MisL is encoded on the SPI-3 PAI, which contains genes required for intramacrophage survival, virulence in mice, and growth in low-Mg2+ media (35). While MisL demonstrates homology to AIDA-I, ShdA, and several other adhesins, little knowledge has been gained about the function of this protein. An S. enterica serovar Typhimurium misL-lac fusion did not demonstrate any expression under conditions of laboratory growth. Furthermore, a misL mutant exhibited wild-type levels of invasion of epithelial cells and survival within macrophages. In addition, a misL mutant was as efficient as the parental strain in its ability to cause a lethal infection in the BALB/c murine model (35). These data led researchers to designate MisL a pseudogene. However, it remains possible that MisL could be involved in other aspects of infection such as persistence or perhaps host specificity. Indeed, MisL could also play a role in the S. enterica life-style which is unrelated to infection.
AutA and AutB
AutA, a 68.3-kDa protein, was identified as a putative autotransporter protein by using a combined genetic and immunological approach designed to detect potent CD4+ T-cell-stimulating proteins (3). AutA, which shows homology to AIDA-I, was shown to be a strong T-cell-stimulating protein, both for T cells taken from patients convalescing from meningococcal disease and for healthy adults. Furthermore, AutA is constitutively expressed and was expressed in all 22 strains examined, which belonged to different serogroups. Importantly, antibodies were demonstrable in all patients who were infected with different meningococcal strains. Thus, AutA is consistently expressed in vivo and may play a role in the pathogenesis of N. meningitidis. In addition, as a vaccine component it is possible that AutA not only may act as a helper T-cell-stimulating carrier of other vaccine targets but also may be a helpful primary target for neutralization of its potential role in meningococcal virulence (3).
AutB, which shows homology to AutA and appears to have been transferred to pathogenic neisseriae from a virulent strain of H. influenzae, is predicted to be a phase-variable outer membrane protein due to the presence of tetranucleotide repeats at the 5′ end of the gene. Although a strain survey suggested that AutB may not be expressed in meningococci (and may be a pseudogene) (3), further evaluation is required to clarify this and exclude expression of this protein in a subset of strains or in vivo during infection.
CLUSTERS 5 AND 8: SERINE PROTEASE AUTOTRANSPORTERS
IgA1 Proteases
Specific immunologic defense on mucosal surfaces is mediated primarily by immunoglobulins of the IgA isotype. IgA appears to be involved in several defense mechanisms, including the inhibition of microbial adherence and the neutralization of bacterial toxins and viruses. At the beginning of the 1970s, Plaut and coworkers found that IgA is degraded by some bacterial enzymes (323, 396-400). Later, this event was implicated as an evasion mechanism of immune responses (398). The bacterial enzymes were called IgA1 proteases, since they were first reported to attack only immunoglobulins of the human IgA1 isotype, cleaving one of the peptide bonds lying within the replicated hinge region segment of the IgA1 heavy chain to yield intact Fabα and Fcα fragments (240, 401). Human IgA2 proteins are resistant to these enzymes because the hinge region of their heavy chains has a primary sequence deletion that involves most of the protease-susceptible peptide sequence found in IgA1.
IgA1 proteases are extracellular proteolytic enzymes produced by major bacterial pathogens including N. gonorrhoea and N. meningitidis, H. influenzae, Streptococcus pneumoniae, S. sanguis, and some members of the resident oral and pharyngeal microflora (240, 260, 396, 406). Three different classes of proteases are represented among the IgA1 proteases: the IgA1 proteases of Haemophilus and Neisseria are genetically related serine proteases (300), those of S. sanguis and S. pneumoniae are metalloproteases (162), whereas studies using specific inhibitors indicate that the IgA1 protease produced by Prevotella melaninogenica is a cysteine protease (340). Since IgA1 proteases are all postproline endopeptidases but clearly belong to three different families, it appears that the cleavage of human IgA1 is a property that has evolved among bacteria through convergent evolution following at least three independent lines (406).
IgA1 proteases from N. gonorrhoeae and N. meningitidis are inhibited by human secretory IgA and serum (161). This last observation could be related to the fact that both proteases are genetically related serine proteases. Halter et al. (185) cloned the gene encoding the IgA protease of N. gonorrhoeae MS11. This clone secretes IgA protease to a similar extent to the parental MS11 strain. These authors identified a 105-kDa protein as the extracellular form of gonococcal IgA1 protease, but by exonucleolytic digestion of the cloned insert they obtained a fragment of 4.6 kb which could not be shortened further without loss of IgA protease expression and, as mentioned previously, was later demonstrated to contain the region encoding the translocating unit (401).
H. influenzae, N. meningitidis, and N. gonorrhoeae are all major causes of morbidity and mortality, which have in common the secretion of IgA1 proteases. The IgA1 proteases of the three species are all serine protease autotransporters (17), which specifically attack one of several Pro-Ser or Pro-Thr peptide bonds within the duplicated octapeptide in the hinge region of IgA1 (243, 343) and possess the consensus sequence GDSGSPLF, where S is the active-site serine characteristic of serine proteases (17).
The exact peptide bond cleaved by IgA1 proteases of the three species differs among strains. While H. influenzae strains cleave either the Pro231-Ser232 (type 1 protease) or the Pro235-Thr236 (type 2 protease) bonds in human IgA1, Neisseria IgA proteases cleave Pro235-Thr236 (type 2 protease) or Pro237-Ser238 (type 1 protease). Cloning and sequencing of the IgA1 protease gene (iga) from N. meningitidis showed an overall structure equivalent to that of iga genes from N. gonorrhoeae and H. influenzae, although no region corresponding to the gonococcal α-peptide was evident (300). Moreover, the epitopes that are recognized by enzyme-neutralizing antibodies show a remarkable heterogeneity among H. influenzae (243, 301) and N. meningitidis IgA1 proteases (299).
Cleavage of intact IgA1 (or S-IgA1) results in the loss of Fcα-mediated secondary effector functions, such as inhibition of adherence (184), despite the retention of antigen-binding activity by the Fabα fragments (312). Further, it has been hypothesized that Fabα fragments may simultaneously block the binding of intact (functional) antibodies of IgA or other isotypes to bacterial epitopes (241) and thereby inhibit complement activation and bacteriolysis (225, 426). Plaut et al. (399) have found that milk antibodies neutralizing serine-type IgA proteases interfered with the autoproteolytic mechanism of release of these enzymes and that IgA1 protease molecules consequently persisting on the cell surface provided for antibody-mediated agglutination of the bacteria. Thus, cell-bound and secreted IgA1 protease may play distinct roles in vivo. Several other lines of indirect evidence for a role in pathogenesis have been reported: nonpathogenic species of Neisseria and Haemophilus lack IgA1 protease activity (242, 342); the products of IgA1 cleavage have been found in the cerebrospinal fluid of patients with bacterial meningitis, in vaginal washings from patients with gonorrhea, and in other secretions from individuals infected with known IgA protease-producing bacteria (2, 244).
Despite these functions ascribed to the IgA1 protease, its role in pathogenesis remains enigmatic. A recent human challenge study showed that an iga mutant was not impaired in its ability to initiate an infection in the human male urethra (228). However, since infection in this study was terminated at the onset of urethritis, a role for IgA1 protease in initial colonization, in establishing invasive complications such as pelvic inflammatory disease, or in contributing to reinfection of a previously exposed host cannot be ruled out. Another report showed that the secretion of this protease seems not to be required for the adherence and invasion of epithelial cells by N. gonorrhoeae in vitro (72). On the other hand, IgA1 protease activity can be inhibited by antibodies (161), and antibodies to IgA1 protease and inhibitory antibodies have been detected in serum and secretions from patients infected with IgA1 protease-producing organisms (48, 103). Hedges et al. (193) examined the levels of these opposing factors (IgA1 protease and inhibitory antibodies) in genital tract secretions and sera from women infected with N. gonorrhoeae, and their results suggested that cleavage of IgA1 by gonoccocal IgA1 protease within the lumen of the lower genital tract is unlikely to be a significant factor in the pathogenesis of infection by N. gonorrhoeae.
It had been repeatedly reported that this group of enzymes is specific for a single substrate, the hinge region of human IgA1 (397). However, the first description of a substrate other than human IgA1 for the gonococcal enzyme was its autoproteolytic event, since once exposed on the extracellular side of the outer membrane, the proenzyme releases itself via an autoproteolytic event. Finally in the fluid phase, IgA1 protease makes another cleavage in its own sequence to release the α-peptide (12 or 15 kDa). Shoberg and Mulks (454) examined the susceptibility of isolated outer and cytoplasmic membranes of N. gonorrhoeae to IgA1 protease in an in vitro assay system using both wild-type gonococcal strains and IgA1 protease-deficient variants. These authors found that the gonococcal IgA1 protease is capable of hydrolyzing several proteins in the outer and cytoplasmic membranes and further indicated that this effect is neither strain nor species limited.
Binscheck et al. (33) reported a second target of the IgA1 protease in eukaryotic cells, synaptobrevin II. Like tetanus toxin, the IgA protease cleaved synaptobrevin II, a fusion complex-forming protein associated with vesicles (438), although at a different site of the molecule, but unlike tetanus toxin, it does not cleave cellubrevin. When IgA1 protease was introduced by electroporation into chromaffin cells, an inhibition of the exocytosis similar to that shown for tetanus toxin was observed (33). However, the IgA protease is nontoxic for nerve cells under physiological conditions, and this potential target is restricted to defined intracellular compartments that may not naturally be accessible by gonococci.
Several years ago, two independent groups reported that Neisseria type 2 IgA1 protease cleaves the lysosomal/phagosomal membrane protein LAMP-1 (192, 288). Lysosomal/phagosomal membranes of mammalian cells are coated by highly conserved glycoproteins (LAMPs) that are thought to protect the membranes from degradation. It has been shown that intracellular N. gonorrhoeae resides in the same compartment as the human LAMP-1 (192) and that this membrane protein contains an IgA1-like hinge region with potential cleavage sites for the neisserial type 1 and type 2 IgA1 proteases (192, 288). Neisserial type 2 IgA1 protease cleaves purified LAMP-1 in vitro, and, unlike its wild-type isogenic parent, an iga mutant of N. gonorrhoeae cannot affect LAMP-1 turnover and its growth in epithelial cells is dramatically reduced (288). Interestingly, IgA1 protease is much more active on LAMP-1 in cell lysates than on purified LAMP-1. Furthermore, Hauck and Meyer (192) demonstrated that the secreted enzyme can be active in an acidic phagosomal microenvironment and that the IgA1 protease is capable of cleaving glycosylated human LAMP-1. Many intracellular bacteria have developed various means of modifying their phagosomal environments in order to avoid lysosome killing. Thus, IgA protease cleavage of LAMP-1 promotes intracellular survival of pathogenic Neisseria spp. More recently, Hopper et al. (211) determined that the IgA1 protease also affects the trafficking of N. gonorrhoeae across polarized T84 epithelial monolayers. They found that N. gonorrhoeae infection of T84 monolayers also results in IgA1 protease-mediated reduction of LAMP-1 and that iga mutants were impaired in their ability to traverse polarized T84 monolayers. These data strongly suggest that the IgA1 protease plays a role in N. gonorrhoeae transepithelial trafficking.
Novel functions of the native IgA1 protease related to the immune system have been reported. Lorenzen et al. (302) studied the immunogenic and immunomodulatory properties of the neisserial IgA1 protease. They found that IgA1 protease acts like a modulin by stimulating the release of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-8 from peripheral blood mononuclear cells but not the regulatory cytokine IL-10. The capacity of IgA1 protease to elicit such cytokine responses in monocytes is enhanced in the presence of T lymphocytes, and its proteolytic activity is not required for this induction. Interestingly, these proinflammatory cytokines have been detected in local secretions and sera during mucosal infection with N. gonorrhoeae (193). It has been suggested that the induction of proinflammatory cytokines may contribute to the characteristic inflammatory reaction caused by the pathogenic Neisseria spp. (302). TNF-α plays a pivotal role in protection against parasitic, viral, and bacterial infections, Beck and Meyer (21) therefore investigated the possible role of neisserial IgA1 protease in TNF-α-dependent signaling processes. They found an inhibition of TNF-α-mediated apoptosis of the human myelomonocytic cell line U937 by IgA1 protease. Furthermore, they also found that IgA1 protease specifically cleaved TNF receptor II (TNF-RII) on the surface of intact cells whereas TFN-RI was not affected by the enzyme (21). The modulation of programmed cell death is very important in the pathophysiology of inflammation and infection (69), and it has been shown that TNF receptor II activates NF-κB and induces apoptosis (189).
As mentioned previously, the gonococcal iga encodes a secreted α-protein, which is cleaved from the C-terminal end of the passenger domain after release into the extracellular milieu. Among the greater IgA1 protease family, the pathogenic Neisseria species appear to be unique with regard to the production of extracellular α-protein (402). The physical linkage of IgA1 protease and α-protein suggests a functional relationship of the two precursor components. It has been reported that α-protein is essential neither for extracellular transport nor for the proteolytic activity of IgA1 protease. Pohlner et al. (402) have reported that the amino acid sequences are reminiscent of nuclear localization signals (NLS) of viral and eukaryotic proteins in that the α-protein is functional in transfected eukaryotic cells. More importantly, these authors showed that native purified α-protein is capable of entering certain human primary cells from the exterior via an endocytic route and accumulates in the nuclei. Additionally, neisserial α-protein shares several features with eukaryotic transcription factors; the authors therefore propose that α-protein, probably in concert with IgA1 protease, functions in the modulation of host cell properties.
H. influenzae Adhesion and Penetration Protein (Hap)
Nontypeable H. influenzae is a common commensal organism in the human upper respiratory tract and an important cause of localized nasopharyngeal diseases such as otitis media, sinusitis, bronchitis, and pneumonia, in contrast to encapsulated strains, which cause systemic disease such as septicemia, meningitis, and suppurative arthritis (494). Adherence of nontypeable H. influenzae to mammalian cells depends on the presence of several different factors: (i) a minority of strains express the adhesin Hia (H. influenzae adhesin), (ii) most isolates express the adhesins HMW1 and HMW2 (high-molecular-weight surface-exposed proteins), and (iii) all strains contain the hap gene.
Hap is a surface protein associated with the capacity for intimate interaction with, attachment to, and entry into cultured epithelial cells. This protein was identified as an additional adhesin in mutants deficient in expression of HMW1 and HMW2 or Hia (466). Hap is synthesized as a 155-kDa protein which is localized to the outer membrane, but it is also associated with the production of a 110-kDa extracellular protein (the passenger domain) and a second outer membrane protein of about 45 kDa (the translocating unit). Hap has significant homology to the IgA protease of H. influenza (60% identity and 80% similarity). This homology includes the region identified as the catalytic site of the IgA1 proteases, which in Hap is represented by GDSGSPMF (466). Like the IgA1 proteases, the passenger domain directs autoproteolytic cleavage via its intrinsic serine protease activity, releasing the passenger domain into the culture supernatant; the autoproteolytic cleavage occurs primarily at the peptide bond between Leu1036 and Asn1037 (206); however, this cleavage event is not complete, much of the secreted Hap remains uncleaved and cell associated; there are therefore two forms of Hap, cell associated and extracellular.
In addition to harboring serine protease activity, the Hap passenger domain possesses the adhesive activities responsible for bacterial-epithelial cell interaction and bacterial aggregation. Hendrixson and St Geme (207) found that the uncleaved cell-associated form of Hap mediates adherence to cultured epithelial cells and promotes bacterial aggregation and microcolony formation, whereas more recent data demonstrated an interaction between Hap and extracellular matrix proteins (135, 137, 138). Adherence and aggregation are augmented by secretory leukocyte protease inhibitor, a soluble protein component of respiratory secretions that inhibits Hap autoproteolysis. These data suggest that secretory leukocyte protease inhibitor, whose primary function is to protect host epithelium, potentiates properties that facilitate bacterial colonization. These data also indicate that Hap serine protease activity and release of the mature 110-kDa Hap protein are not essential for Hap to promote interaction with cultured epithelial cells (207). In contrast to the role of secretory leukocyte protease inhibitor, Qiu et al. (411) have found that human milk lactoferrin is able to inactivate Hap. Previous studies had already demonstrated that human milk lactoferrin is protective against H. influenzae colonization and disease. Human milk lactoferrin efficiently extracts the IgA1 protease proprotein from the bacterial outer membrane and specifically degrades Hap and abolishes Hap-mediated adherence. Extraction of IgA1 protease and degradation of Hap were localized to the N-lobe of the bilobed lactoferrin molecule and were inhibited by serine protease inhibitors, suggesting that the lactoferrin N-lobe may contain serine protease activity (411).
Autoproteolytic cleavage of Hap seems counterintuitive for a protein which promotes successful colonization and bacterial aggregation. However, it is possible that release serves one of several functions. Release may facilitate evasion of the immune responses directed at Hap. Alternatively, autoproteolytic cleavage of Hap at several sites on the same molecule may release bioactive peptides, as has been documented for IgA1 protease (402). Free Hap may also interact with specific mucosal IgA antibodies to avoid bacterial opsonization and enhance phagocytic removal. On the other hand, shedding of an adhesive domain from the cell surface may allow individual organisms to disperse from microcolonies or epithelial cells and then migrate within the respiratory tract. A further possibility is that release of Hap facilitates the degradation of target host proteins such as tissue components or immune system effectors. Interestingly, the first experiments to find a Hap-immune system interaction have shown that Hap does not induce proinflammatory cytokines from human respiratory epithelial cells but that immunization with the purified protein does protect mice against nasopharyngeal colonization with wild-type organisms (68, 86).
N. meningitidis App and MspA Autotransporter Proteins
App is a highly conserved and constitutively expressed 160-kDa protein, is localized in the outer membrane, and is partly secreted by autocleavage (182). The outer membrane-localized precursor is surface accessible, and antibodies raised against recombinant App exhibit bactericidal activity against the homologous strain (182). Recently, Serruto et al. (445) have confirmed that App is an important virulence determinant, which mediates adhesion to epithelial cells (but not endothelial cells). Indeed, App is the first nonpilus protein to be shown to possess adhesive properties in the presence of the polysaccharide capsule. Analogous to the Hap protein in H. influenzae, autocleavage of App may assist in the detachment and dispersal of meningococci, allowing colonization/invasion of neighboring epithelial cells, a phenomenon also suggested for B. pertussis (81, 136). Taken together, these data strongly suggest that App plays an important (and possibly indispensable) role in the early stages of meningococcal pathogenesis in the nasopharynx. A specific and separate role for the secreted fragments of App is also possible; as has been shown in the case of the IgA1 protease, App also possesses a helix-rich putative α-protein located between the passenger domain and the β-domain. One RGD motif is present in the App protein from the group A strains Z3515 and Z2491; however, it was not conserved in the sequences of the group B strains SD or MC58 or in the group C strain Z4181. Since these motifs in autotransporters are usually present in pairs, a single RDG motif within the App of strain Z2491 might be fortuitous. A serine protease motif, GDSGP, is also present and conserved in the App proteins from all five meningococcal App sequences (182).
When whole-cell meningococcal lysates of strain MC58 were probed with a rabbit anti-App antiserum, strong bands of 175 and 100 kDa and weaker bands of ca. 160 and ca. 140 kDa were detected. The Hap protein has been shown to be cleaved at several sites (206). It is likely, therefore, that the App-derived products observed in meningococcal supernatants also result from autocatalytic cleavage at alternatives sites; however, the precise cleavage sites within App have not been determined. It is interesting that App also possesses a putative NLS motif, consisting of five or six positively charged residues preceded by a proline residue. In App (from strain MC58), the putative NLS motif (932PRRRSRR938) is located upstream of the predicted N-terminal autocleavage site (956F↓NTL959). The native α-protein from IgA1 protease, which contains NLS motifs, was demonstrated to enter human cells and accumulate in the nuclei, potentially influencing the regulation of host cell functions (402). In addition, the arginine-rich region in App may act as a signal for the correct localization of the downstream autocleavage sites (182). Interestingly, a recent study has demonstrated that Hap is cleaved by lactoferrin (long known for its role in mucosal defense) following RRSR or RSRR motifs; these motifs are also present in App and are clustered immediately downstream of the putative NLS motif. The authors therefore postulate that lactoferrin may also cleave App and, as with Hap, reduce the biological effectiveness of the molecule. An effective antibody response to App may similarly lead to functional neutralization of the protein and thus contribute to protection against invasive disease. The latter underlines the importance of a functional assessment of App in relation to vaccine potential. Finally, analogous to AutA, App was also shown to strongly stimulate helper T cells (182).
Proteins of the size expected for App, including the secreted form, were specifically detected by the anti-App antiserum in a range of meningococcal isolates. In addition, the sera from patients convalescing from invasive meningococcal disease contained antibodies that recognized recombinant App, which provides evidence that App is both expressed during infection and a B-cell immunogen. Moreover, antiserum raised against denatured recombinant App was capable of killing meningococci of the homologous strain in the presence of the complement (182). These authors also have shown that App is a strong T-cell stimulant in several unrelated individuals, inducing proliferative responses of the same magnitude as those elicited by outer membrane vesicle vaccine for human volunteers (504). Nevertheless, App is conserved, is expressed during infection and carriage, and stimulates B and T cells, and antibodies to App are bacteriocidal (182).
MspA (meningococcal serine protease A), a recently identified and characterized autotransporter protein (D. P. J. Turner and D. A. Ala'Aldeen, unpublished data), shows 34% overall homology to App at the amino acid level. Like App, MspA possesses a chymotrypsin serine protease active site and an identical putative autocleavage site (870F↓NTL873). No function has been assigned to MspA.
Serine Protease Autotransporters of the Enterobacteriaceae
Members of the SPATE family are proteins from E. coli and Shigella spp., which, like the IgA1 proteases and Hap, possess a consensus serine protease motif (203). Since 1994, investigators have described SPATE members in several pathotypes of E. coli, although their full contributions to pathogenesis are unknown. However, these proteins possess several common features: (i) unlike the IgA1 proteases, none of the SPATE family has been shown to cleave IgA1, (ii) the serine protease motif of SPATE proteins does not play a role in autoprocessing, (iii) each SPATE member is among the predominant secreted proteins of their respective pathogens, (iv) no SPATE has yet been identified in a nonpathogenic organism, and (v) SPATE are highly immunogenic proteins. Interestingly, despite their high levels of homology, the SPATE proteins demonstrate distinct substrate specificities (113).
Tsh.
In 1994, the first SPATE was described as a temperature-sensitive haemagglutinin (Tsh) in avian-pathogenic E. coli (APEC), which causes disseminated infections in birds. Cosmid screening and subcloning identified a 140-kDa secreted protein, which confers on E. coli K-12 a temperature-sensitive hemagglutination phenotype that is best expressed when cells are grown at 26°C (409). Conjugation and hybridization experiments revealed that tsh gene is located on a ColV-type plasmid, near the colicin V genes, in many of the APEC strains studied by Dozois et al. (108). The deduced amino acid sequence encoded by tsh was found to contain homology to four serologically distinct H. influenzae iga genes and with N. gonorrhoeae IgA1 protease. Furthermore, the region of Tsh homologous to the serine-type IgA1 proteases is limited mainly to the approximately 900 N-terminal residues of Tsh (409). Further studies have shown that E. coli K-12 containing the recombinant tsh gene produces two proteins, a 106-kDa extracellular protein and a 33-kDa outer membrane protein. The Tsh passenger domain contains the 7-amino-acid serine protease motif that includes the active site serine (S259), found also in the secreted domain of the IgA1 protease. However, site-directed mutagenesis of this motif did not abolish the hemagglutinin activity or extracellular secretion of Tsh. Moreover, the passenger domain did not cleave human or chicken IgA and did not show proteolytic activity in a casein-based assay (460).
Correlation of Tsh expression and hemagglutination activity appears to be a very complex phenomenon, influenced by strain and environmental conditions. Nevertheless, for both APEC and recombinant E. coli K-12 strains containing the tsh gene, it was only the whole bacterial cells and not the cell-free supernatant that could confer hemagglutinin activity (460). In addition to the temperature-sensitive phenotype, haemagglutination activity is absent after growth of Tsh-positive E. coli χ7122 strain on agar supplemented with 0.15 M NaCl (460). Maurer et al. (318) examined the occurrence of ambient-temperature-regulated Tsh among avian and mammalian E. coli isolates. Tsh was present in approximately 46% of clinical avian E. coli isolates but was not detected among commensal E. coli strains isolated from healthy broiler chicken. In a prevalence study, Dozois et al. (108) found that of the tsh-positive APEC isolates, 90.6% belonged to the highest-virulence class. Furthermore, they found that experimental inoculation of chicken with χ7122 and an isogenic tsh mutant demonstrated that Tsh may contribute to the development of lesions within the air sacs of birds but is not required for subsequent generalized infection manifesting as perihepatitis, pericarditis, and septicemia.
Recently, a novel heme-binding protein, termed the hemoglobin-binding protease (Hbp), was characterized in an E. coli strain isolated from a wound infection (369). Hbp is 99.9% identical to Tsh, demonstrating two nonsynonymous changes in the amino acid sequence of the passenger domains, neither of which appears to be present in a domain affecting the function of the protein. Like the tsh gene, hbp is located on a ColV-type plasmid, near the colicin V genes (108). Hbp scavenges heme from its environment, interacts specifically with human hemoglobin, degrades it, and subsequently binds the released heme (369). However, purified Hbp is unable to induce hemagglutination activity. The reason for the apparent difference between Hbp and Tsh in hemagglutination activity remains unclear but may be due to different culture conditions. It seems that only under very specific conditions can hemagglutination activity by Tsh be detected. More recent results suggest that the synergy of abscess formation by E. coli and Bacteroides fragilis can be partly explained by the capacity of B. fragilis to intercept Hbp and iron from heme and to overcome the iron restrictions imposed by the host (368).
SepA.
In 1995, Benjelloun-Touimi et al. described a Tsh homologue designated SepA (for Shigella extracellular protein), which is the major extracellular protein of S. flexneri (22). Investigation of the proteolytic activity of SepA utilizing a wide range of synthetic peptides found that SepA hydrolyzed several of these substrates and that the activity was inhibited by the serine protease inhibitor phenylmethylsulfonyl fluoride (PMSF) (23). Several SepA-hydrolyzed peptides were described as specific substrates for cathepsin G, a serine protease produced by polymorphonuclear leukocytes that was proposed to play a role in inflammation. However, unlike capthesin G, SepA degraded neither fibronectin nor angiotensin I and had no effect on the aggregation of human platelets.
The presence of sepA on the virulence plasmid, as well as the recognition of SepA by the sera of monkeys infected with Shigella, suggested that SepA might be involved in Shigella pathogenicity (22). However, construction and phenotypic characterization of a sepA mutant suggested that SepA is required neither for entry into cultured cells nor for intracellular dissemination. Nevertheless, the sepA mutant demonstrated a reduced ability to induce both mucosal atrophy and tissue inflammation in the rabbit ligated ileal loop model, indicating that SepA may play a role in tissue invasion, although this hypothesis remains to be elucidated (22).
EspC.
In 1996, Stein et al. (461) described EspC (for “EPEC-secreted protein C”), the major exported protein in the supernatants of the prototypical EPEC strain E2368/69. However, the construction of a deletion in espC by allelic exchange showed that EspC is not necessary for mediating EPEC-induced signal transduction in HeLa epithelial cells and does not play a role in adherence or invasion of tissue culture cells (461).
Interestingly, genes homologous to espC also exist in other pathogenic bacteria which form attaching and effacing (A/E) lesions. However, secretion of a 110-kDa protein by these strains was not detected. The presence of genes homologous to espC in the pathogenic strains of bacteria that cause A/E lesions but not in nonpathogenic strains implies that EspC may play a role in the virulence of A/E-inducing pathogens. A possible function in EPEC virulence is supported by the fact that EspC is a highly immunogenic protein. Human serum colleted from a volunteer 28 days after infection with EPEC strongly recognized EspC, while serum colleted prior to infection did not (226). Moreover, S-IgA antibodies purified from Mexican women, which inhibit the adherence of EPEC to cells, strongly reacted with a 110-kDa protein, among other proteins. The molecular size of this protein and its reactivity with specific anti-EspC antiserum suggest that it is EspC (311).
Recently, it was demonstrated that expression of espC was under the control of several extragenic regulators. While expression of EspC is unaffected by mutations in the genes required for assembly of the EPEC type III secretion apparatus encoded in the locus of enterocyte effacement (LEE), expression of EspC is coregulated with secretion of type III secreted proteins (237). This regulation was influenced by temperature, CO2 and a positive transcriptional regulator, PerA (168, 183). In addition, Elliott et al. (120) have found that a LEE-encoded regulator (Ler), part of the Per-mediated regulatory cascade, is essential for the expression of EspC as well as multiple LEE genes.
The function of EspC in EPEC is unknown; however, it was determined that the espC gene is located within a second EPEC PAI at 60 min on the chromosome of E. coli. It was also shown that EspC is an enterotoxin, as indicated by rises in short-circuit current and potential difference in rat jejunal tissue mounted in Ussing chambers. In addition, preincubation with antiserum against the homologous Pet enterotoxin of EAEC eliminated EspC enterotoxin activity (324). Recently, investigations have demonstrated that coincubation of high concentrations of EspC with epithelial cells in vitro, and over a prolonged period, induces cytopathic effects similar to those of Pet (348; see below).
EspP.
In 1997, almost simultaneously Brunder et al. (49) described EspP (for “extracellular serine protease plasmid-encoded”) and Djafari et al. (106) described PssA (for “protease secreted by STEC”) in EHEC O157:H7 and Shiga-toxin producing E. coli (STEC) O26:H−, respectively. These proteins are identical, are encoded on the large virulence plasmids, and represent the major extracellular proteins of both organisms. Similar to other SPATE, the genes (espP and pssA) gave rise to processed 104-kDa mature extracellular proteins.
Hybridization experiments and immunoblot analysis of clinical EHEC isolates showed that EspP is widespread among EHEC of the serogroup O157 and that it also exists in serogroup O26, while antibodies against PssA from STEC O26:H− detected PssA in three human STEC isolates (O157:H7, O157:H−, and O157:H−) and in strains 332 (O26:H+) and 570/89 (O111:H−). On the other hand, both proteins are highly immunogenic and have been detected in sera from patients (49, 106). Interestingly, different biological activities were described for EspP and PssA. EspP is a protease capable of cleaving pepsin A and human coagulation factor V (when present in serum but not in its purified form), suggesting that degradation of factor V could contribute to the mucosal hemorrhage observed in patients with hemorrhagic colitis (49), whereas, PssA-showed serine protease activity in a casein-based assay and is cytotoxic for Vero cells, suggesting a functional importance during infection of the mucosal cell layer by the bacterial pathogen (106).
Pet.
In 1998, a heat-labile enterotoxin from EAEC was described as a secreted 108-kDa protein encoded in the EAEC virulence plasmid; it was recognized by sera from children who were infected during an outbreak of diarrhea in a pediatric ward of a Mexican hospital (125, 349). The genetic characterization of this high-molecular-mass heat-labile toxin demonstrated that it represented the passenger domain of a 140-kDa autotransporter precursor which was termed Pet (124). Several lines of evidence support a role for this protein in mediating enterotoxic activity. Thus, this enterotoxin produced tissue damage characterized by increased mucus release, cell exfoliation, and crypt abscesses in rat jejunum (349). Furthermore, purified Pet raised the short-circuit current and decreased the electrical resistance of rat jejunum mounted in an Ussing chamber, an effect that is indicative of mucosal damage (349). Finally, in vitro organ experiments with human intestinal mucosa demonstrated that a mutant with a null mutation in the pet gene was unaffected in its ability to adhere to colonic tissue but was unable to elicit the characteristic mucosal changes observed with the wild-type parental strain (Fig. 9). The effects were restored by trans-complementation of the pet gene (196, 197).
FIG. 9.
Activity of the EAEC Pet toxin. (A and B) Internalization of Pet (A) and a serine protease motif mutant (Pet S260I) (B) into HEp-2 cells. HEp-2 cells were treated with either Pet or Pet S260I for 1 h. The actin cytoskeleton is labeled with green, and Pet or Pet S2601 is labeledwith red. Note the perinuclear localization of Pet or Pet S260I inside the cells. (C and D) Effect of Pet (C) or Pet S260I (D) on fodrin redistribution in HEp-2 cells. HEp-2 cells were treated with either Pet or Pet S260I for 3 h. The actin cytoskeleton is labeled with blue, Pet or Pet S260I is labeled with red, and fodrin is labeled with green. Note that Pet but not Pet S260I causes cytoskeletal damage and fodrin redistribution (arrows), and it is possible to detect a delayed interaction between Pet S260I and fodrin due to inability to cleave it (yellow dots). (E and F) Scanning electron photomicrographs of in vitro-cultured human colonic tissues infected with the EAEC strain 042 (E) and the pet mutant strain J1F1 (F). For the tissue shown in panel E, the surface of the colon is markedly abnormal, as manifested by increased crypt apertures (white arrowhead), prominent mucosal crevices (white arrow), goblet cell pitting (black arrowhead in a circle), and rounding of epithelial cells (black arrow in a circle).
While investigating its mode of action, Navarro-Garcia et al. (350) found that Pet induces temperature-, time-, and dose-dependent cytopathic effects on HEp-2 and HT29/C1 cells. Furthermore, Pet appears to be a cytoskeleton-altering toxin since it induces contraction of the cytoskeleton, loss of actin stress fibers, and release of the cellular focal contacts in cell monolayers, followed by complete cell rounding and detachment. Pet cytotoxicity and enterotoxicity depend on Pet serine protease activity, since both effects are inhibited by PMSF and are not induced by Pet S260I, which is mutated in a predicted serine protease motif and thereby lacks in vitro protease activity on zymogram gels (350).
Recently, it has been reported that Pet enters HEp-2 cells and that this internalization is required for the induction of cytopathic effects and is mediated by a domain within the central region of the passenger protein (114, 347, 513). Further, Pet trafficking appears to occur through a retrograde transport mechanism since brefeldin A prevents the cytopathic and cytoskeletal effects observed when cells are treated with native Pet. Moreover, while native Pet and Pet S260I can be observed by confocal microscopy inside the epithelial cells and while recent data obtained using alanine scanning-linker mutagenesis have shown that the proteolytic site was necessary for toxicity, only native Pet is able to produce the cytoskeletal effects, indicating that the target protein for Pet is an intracellular moiety (114, 347). Indeed, Pet is capable of cleaving spectrin of erythrocyte ghosts, purified spectrin, and fodrin (nonerythroid spectrin) from HEp-2 cells. This processing effect was inhibited by PMSF and was not observed for the Pet S260I mutant (513). In further support of this work, Sui et al. (473) demonstrated spectrin condensation when the Pet functional domain was expressed intracellularly. In fact, experiments in Navarro-Garcia's laboratory show that Pet is able to cleave recombinant fodrin fused to glutathione S-transferase (109 kDa), and this effect correlated with dose-dependent redistribution of fodrin in HEp-2 cells. Moreover, Pet has an affinity for fodrin as demonstrated by overlay and colocalization experiments (55). Pet is able to cleave fodrin between M1198 and V1199 in helix C and inside of the calmodulin-binding domain. Such effects and fodrin redistribution in HEp-2 cells were prevented by using the Pet S260I mutant or PMSF (56). Fodrin cleavage as the primary mode of action could account for the cellular changes induced by Pet toxin, since fodrin is one of the main protein components of the focal adhesion complex. In addition, several membrane channels are linked to the fodrin network (104), and therefore cleavage of fodrin could explain the enterotoxic effects of Pet as well (Fig. 9).
Pic.
A second SPATE member was identified in EAEC. This protein was recognized by the sera from the same children who were infected during an outbreak of diarrhea in a paediatric ward of a Mexican hospital and which were used to identify Pet (125, 349). In contrast to Pet, Pic is localized in the EAEC chromosome. Henderson et al. (196) found that Pic was identical to a protein termed Shmu (Shigella mucinase), which is encoded on the Shigella she PAI (412). Interestingly, the pic gene has an unique characteristic among the autotransporter proteins since within the pic (she) gene there are two oppositely oriented genes, set1B and set1A (196, 412), in tandem, which encode the 7- and 20-kDa subunits of the 55-kDa ShET1 toxin (131). The sequences flanking pic are different in EAEC and S. flexneri; in the case of S. flexneri, the she PAI contains another SPATE, SigA, which is similar to Pet (see below).
Pic catalyzes gelatin degradation; degradation can be abolished by disruption of the predicted proteolytic active site. Functional analysis of Pic implicates this factor in mucinase activity, serum resistance, and hemagglutination. Phenotypes identified for Pic suggest that it is involved in the early stages of pathogenesis and most probably promotes intestinal colonization (196). Recently, a homologue, PicU (96% identity at the amino acid level), with similar functions has been characterized in uropathogenic E. coli (381). Subsequent investigations by Heimer et al. (194) revealed that picU was expressed during experimental infection in the mouse model of urinary tract infection. However, cochallenge of wild-type organisms and the picU mutant in the mouse model suggested that PicU-encoding strains had no competitive advantage in colonization (194). Nevertheless, since PicU is a secreted protease, it is possible that protein secreted by the wild-type strains may complement the picU deficiency of the mutant strains. Indeed, challenge of mice separately with wild-type and picU mutant strains revealed that the wild type colonized the bladder to a greater extent and displayed higher levels of neutrophil infiltration into the lumen, epithelium, and submucosa. Unfortunately, while these data showed a trend, they were not statistically significant (P = 0.2) (194). In addition, it should not be discounted that loss of PicU function may be complemented by the homologous Vat and Sat serine protease autotransporters (see the discussions of these proteins). Thus, the role of PicU in uropathogenic E. coli infection remains enigmatic.
SigA.
An initial report showed that the she PAI, which contains the pic (she) gene, also contains a gene encoding a second IgA protease-like homologue, sigA, lying 3.6 kb downstream and in an inverted orientation with respect to pic (412). Functional analysis showed that SigA is a secreted temperature-regulated serine protease capable of degrading casein. Performing similar experiments to those used with Pet revealed that SigA is cytopathic for HEp-2 cells, suggesting that it may be a cell-altering toxin with a role in the pathogenesis of Shigella infections. Furthermore, SigA was at least partly responsible for the ability of S. flexneri to stimulate fluid accumulation in ligated rabbit ileal loops (5).
Sat.
Uropathogenic E. coli CFT073, a strain cultured from blood and urine of a patient with acute pyelonephritis, expresses a 107-kDa protein named Sat (for “secreted autotransporter toxin”), which is expressed more often by E. coli strains associated with the clinical syndrome of acute pyelonephritis than by fecal strains (178). Like Pet, Sat exhibited serine protease activity and displayed cytopathic activity on Vero primary kidney, HK-2 bladder, and HEp-2 cells lines (178). Further investigations revealed that Sat triggered the vacuolation of both human bladder (CRL-1749) and kidney (CRL-1573) cell lines. Using a mouse model of transurethral infection, Guyer et al. demonstrated that a sat mutant was similar to wild-type E. coli CFT073 in colonization of the urine, bladder, and kidney tissue. In contrast, in mice infected with wild-type E. coli CFT073, dissolution of the glomerular membrane and vacuolation of proximal tubule cells could be observed, while no such effects were seen in kidney sections from mice infected with a sat null mutant, suggesting that Sat contributes to the pathogenesis of urinary tract infection by eliciting damage to the kidneys during upper urinary tract infection (179).
Vat.
Recently, it was reported that certain APEC strains induced vacuolating cytotoxic activity similar to that induced by the H. pylori autotransporter protein VacA (427) (see the following sections). Subsequently, Parreira and Gyles (384) identified a gene encoding a serine protease autotransporter protein (Vat), which was responsible for the vacuolating activity of a strain of E. coli (Ec222) that had been isolated from a septicemic chicken. Further investigations revealed that this gene was required for the virulence of E. coli Ec222, since a null mutant was avirulent in several animal models of infection. Interestingly, Heimer et al. (194) demonstrated by reverse transcription-PCR that vat was expressed in mice during experimental urinary tract infection; however, the contribution of Vat to infection in this model was not reported.
Vat is encoded on a PAI which is inserted adjacent to the thrW tRNA gene. The presence of vat at this position has been demonstrated for uropathogenic E. coli strain CFT073 and the neonatal meningitis strain E. coli RS218 (530; N. J. Parham and I. R. Henderson, unpublished data). Interestingly, all of these strains are associated with septicemia, suggesting that Vat may be required for E. coli to enter or survive within the bloodstream. Indeed, recent data have demonstrated the presence of Vat at a higher frequency in invasive strains of E. coli than in strains isolated from patients with cystitis or intestinal infections (Parham and Henderson, unpublished).
Others.
Several other SPATE have been identified in a variety of E. coli but have been subjected to only partial characterization. Recently, Sandt and Hill (431) discovered a family of four cell surface proteins (EibA and EibC to EibE) which could bind IgG in a strain of E. coli (ECOR-9) isolated from a healthy child. Further investigation revealed that these proteins belonged to the type Vc class of secreted protein exemplified by YadA and Hia (see above). Further investigations revealed that two of these genes (eibA and eibC) were linked to genes encoding two SPATE proteins termed EaaA and EaaC, respectively (431). Unfortunately, a function was not determined for these highly homologous proteins (99.4% identity); however, because of their association with immunoglobulin-binding proteins and their obvious homology to the IgA1 proteases, it is tempting to speculate that they play a role in degradation of the bound immunoglobulins.
Interestingly, in two different studies members of the SPATE family were found to be associated with unique virulence factors of LEE-negative STEC. Thus, Schmidt et al. (440) identified EspI on a chromosomal PAI inserted at the selC tRNA locus in E. coli O91:H− strain 4797/97 and Leyton et al. (284) identified EpeA on the large virulence plasmid of E. coli O113:H21 strain EH41. It is worth noting that EspI described by Schmidt et al. (440) is distinct from the type III secreted effector molecule of the same name which was recently described by Mundy et al. (344) in Citrobacter rodentium. Both EspI and EpeA were assessed by in vitro assays for functional activity. Schmidt et al. (440) revealed that EspI did not degrade high-density or low-density lipoproteins, IgA1, hemoglobin, haptoglobin, α2-macroglobulin, thrombin, lactoferrin, transferrin, bovine serum albumin, or collagen type 3. However, purified EspI could degrade pepsin, and this activity could be inhibited by PMSF. Furthermore, coincubation of human plasma with purified EspI revealed that it was capable of degrading apolipoprotein A-I. EspI demonstrates a high level of homology to EpeA (58% identity) (284). EpeA and EspI were unable to induce cytopathic effects on HeLa cells even after prolonged incubation (284). In vitro assays for protein function illustrated that, like Pic, EpeA could act as a mucinase and possessed the ability to degrade gelatin and pepsin. Further investigations are needed to fully assess the contribution of EspI and EpeA to the pathogenesis of LEE-negative STEC.
Recently, a second autotransporter of ETEC (EatA) was described (385). In vitro studies demonstrated that EatA possesses the same specificity as SepA for p-nitroanilide-conjugated oligopeptides, cleaving peptides which have been identified as substrates for cathepsin G. Site-directed mutagenesis of the residues within the predicted serine protease catalytic triad abolished the ability of EatA to cleave the oligopeptides. Comparison of the parent strain with an isogenic eatA mutant, in the in vivo rabbit ileal loop model of infection, indicated that there were no differences at 16 h postinfection in the fluid accumulation elicited by each strain. In contrast, fluid accumulation was not as pronounced with the eatA mutant at 7 h postinfection compared to that for the wild type, even though similar numbers of bacteria could be recovered from the loops, indicating that the differences were not due to bacterial numbers or an altered survival rate. Furthermore, at 7 h postinfection the eatA mutant did not demonstrate foci of mucosal destruction and leukocyte infiltration characteristic of the wild-type organism (385). These data suggest that while EatA is not absolutely required for infection, it may act to accelerate ETEC virulence.
CLUSTER 6: BORDETELLA AUTOTRANSPORTERS
The genus Bordetella, which belongs to the β-subclass of the Proteobacteria, is composed of eight species: B. pertussis, B. parapertussis, B. bronchiseptica, B. avium, B. holmesii, B. hinzii, B. trematum, and B. petrii (515). With the exception of B. petrii, all are associated with colonization or infection of humans, animals, or birds (158). B. pertussis, B. parapertussis, and B. bronchiseptica are the best characterized and the most closely related phylogenetically, and it has been suggested that they be considered subspecies instead of separate species (80, 158, 505). Each of the three subspecies is associated with respiratory infections. B. pertussis is a highly contagious human pathogen, the etiologic agent of whooping cough. B. parapertussis causes a similar illness in humans, albeit in smaller numbers, and certain strains infect only sheep (505, 542). B. bronchiseptica colonizes a variety of mammals and can, given appropriate circumstances, cause kennel cough, atrophic rhinitis, and bronchopneumonia in dogs, pigs, and rabbits, respectively (80). B. bronchiseptica infections in humans occur rarely. Details on Bordetella virulence factors, their regulation, and their role in pathogenesis have been recently reviewed (80, 238, 298). In general, successful colonization and infection is dependent on the production of an array of adhesins and toxins which are regulated by a histidine kinase system called Bvg (79, 529). Included in this arsenal are several autotransporter proteins. Only a handful of the Bordetella autotransporters have been functionally characterized to date. In terms of Bvg regulation, brkA, prn (pertactin), sphb1, tcf, and vag8 are positively regulated by Bvg whereas bapA, phg, sphb2, and sphb3 are unaffected by the Bvg system (12, 298).
Identification of Autotransporters in the Bordetella Genomes
The sequencing and comparative analyses of the genomes of B. pertussis strain Tohama I, B. parapertussis strain 12822, and B. bronchiseptica strain RB50 (382) have provided a unique opportunity to examine the genomes of these closely related subspecies for their complement of autotransporters. Analysis of these genomes has uncovered an inventory of 22 known or putative autotransporter proteins (Table 2). These include pertactin (47, 61), BrkA (132, 133), Tcf (141), Vag8 (140), Phg (accession number AJ009835), and SphB1 (82), as well as a number of previously identified but as yet uncharacterized proteins (382) and nine novel putative autotransporters including a putative adhesin and a putative lipase (Table 2). While most of the autotransporter ORFs are intact, some are pseudogenes and still others are absent altogether in one or more of the strains analyzed. Thus, these strains of B. pertussis, B. parapertussis, and B. bronchiseptica have the capacity to express 12, 16, and 20 putative functional autotransporters, respectively. The dozen pseudogenes present in the three strains have resulted from mutations producing frameshifts and an in-frame stop codon, and by disruption by the insertion elements IS481 and IS1001 (Table 2). Frameshift mutations found in brkA and tcf, and the absence of tcf in B. parapertussis, probably explain why they have eluded detection in B. parapertussis and B. bronchiseptica in previous studies (141; S. Pleasance and R. C. Fernandez, unpublished data). However, not all mutations result in pseudogenes. For example, approximately one-quarter of the passenger domain of BatB is deleted in B. parapertussis.
TABLE 2.
Bordetella autotransporter proteins
| Known or predicted Bordetella auto- transporter | ORF designation and no. of residuesa
|
Accession no.b | Reference(s) or best BLAST hit of passenger | Bvg reg- ulationc | Known (predicted) signal peptided | Notable motifse | ||
|---|---|---|---|---|---|---|---|---|
| Bp | Bpa | Bb | ||||||
| Pertactin | BP1054, 910 | BPP1150, 922 | BB1366, 916 | P14283 | 47, 61 | + | 34 | RGD (×2) |
| BrkA | BP3494, 1,010 | BPP0867, (173) | BB0961, (127) | AAA51646 | 132 | + | 42 | RGD (×2) |
| SphB1f | BP0216, 1,039 | BPP0417, 1,043 | BB0419, 1,039 | CAC44081 | 82, 83 | + | No | Serine protease, lipoprotein |
| Tcf | BP1201, 647 | BB3291, (145) | CAA08832 | 141 | + | (39) | RGD | |
| Vag8 | BP2315, 915 | BPP2415, 915 | BB1864, 915 | AAC31247 | 140 | + | 37 | RGD |
| Phg | BP1767, 418 | BPP1998, 415 | BB2246, 415 | CAB38010 | None | 0 | (35) | None |
| SphB2 | BP1660, 1,024 | BPP2745, (193) | BB2741, 1,025 | 12 | 0 | (37) | None | |
| SphB3 | BP1110, 1,076 | BPP2053, 1,076 | BB2301, 1,076 | 12 | 0 | (29) | Thiol protease | |
| BapA (AidB)g | BP2224, 903 | BPP2251, (295) [I] | BB1649, 903 | CAC14165 | AIDA-1 | 0 | (30)h | None |
| BapB | BP1200, (119) [I] | BPP1815, 605 | BB3292, 613 | CAC14166i | None | ND | (41) | None |
| BapC (BatE)g | BP2738, (102) | BPP2591, (100) | BB2033, 998 | CAC14167 | Pertactin | ND | (45) | RGD (×2) |
| BatA | BP1793, (53) [I] | BPP2022, 538 | BB2270, 536 | None | ND | (33) | None | |
| BatB | BP0529, 2,300 | BPP0452, 1,769 | BB0452, 2,300 | ShdA | ND | (38) | RGD (×2), P-loop, aspartyl protease | |
| Novel | BP0775, 340 | BPP0337, 340 | BB0340, 340 | None | ND | ? | None | |
| Novel | BP1344, 866 | BPP2678, (717) | BB2830, 866 | Ag43, AIDA-1, BapA | ND | (36) | RGD (×2) | |
| Novel | BP1610, (124) | BPP2975, 937 | BB2941, 937 | Pertactin | ND | 32h | None | |
| Novel | BPP1617, 491 | BB3111, 497 | None | ND | (33) | None | ||
| Novel | BPP0449, 1,616 | BB0450, 2,152 | ShdA | ND | (40)h | P-loop, lipase | ||
| Novel | BPP0735, 996 | BB0821, 999 | YapE | ND | (30) | RGD | ||
| Novel | BPP0822, 1,196 | BB0916, 1,196 | SSP-h1 | ND | (23)h | RGD | ||
| Novel | BPP1618, 519 | BB3110, 705 | Tcf | ND | BP1618 (33), BB3110 (54) | None | ||
| Novel | BP2627, (178) | BPP1256, 718 | BB2324, 718 | (34) | Lipoprotein | |||
ORF designation refers to genome sequence annotation. Bp, B. pertussis; Bpa, B. parapertussis; Bb, B. bronchiseptica. ORFs in bold type have been analyzed. When the number of amino acid residues is in parentheses, the ORF is truncated due to frameshift or insertion of a stop codon. Disruption by an insertion element is indicated by [I].
GenBank accession number, if known.
Regulation by the Bvg two-component system. +, activated genes; 0, not activated; ND, not determined.
Values represent numbers of amino acids in the signal sequence; values in parentheses are derived from predictions based on SignalP (http://www.cbs.dtu.dk/services/SignalP-2.0/).
Based on PROSITE patterns (http://ca.expasy.org/prosite/).
Number of residues does not match the genome annotation.
Alternate name for this putative autotransporter.
Weakly predicted.
Accession number annotation does not take into account the presence of the insertion element.
There are three examples of ORFs encoding autotransporters in tandem. While BPP0449/BB0450 has overall high sequence identity to BPP0452/BB0452 (batB), suggesting a gene duplication, BPP1617/BB3111 and BPP1618/BB3110 found in tandem have highly similar translocating units but with different passenger domains. This is also seen with tcf (BB3291) and bapB (BB3292). Interestingly, the translocating units of tcf, bapB, BPP1617/BB3111, and BPP1618/BB3110 are all highly homologous.
One ORF (BP0775/BPP0337/BB0340) may encode a passengerless translocator; however, two additional amino acids are present following the canonical terminal aromatic amino acid present in autotransporters, and the putative signal sequence, which is predicted with low probability, is more reminiscent of a linker region. Thus, it remains to be determined whether this is a bona fide autotransporter. Of the proteins whose predicted translated product includes the β-domain, BapA, BPP0822/BB0916, BPP2975/BB2941, and BPP0449/BB0450 are weakly predicted to have signal sequences (Table 2), and it remains to be seen whether they are surface expressed. Although SphB1 does not have a predicted signal peptidase I cleavage site, it has a lipoprotein consensus sequence and recently has been shown to be a lipoprotein (83; see above). The predicted signal sequences of the rest of the autotransporters range in size from 30 to 54 amino acids, in keeping with the general theme of unusually long signal sequences in autotransporter proteins.
Phylogenetic analyses of the amino acid sequences of the β-domains of the Bordetella autotransporters have suggested that they fall into three primary groups, designated 1, 2, and 3, that correspond to the function of their passenger domains (Pleasance and R. Fernandez, unpublished). Half of the proteins are found in cluster 1, and this group includes pertactin, BrkA, Tcf, and Vag8. A group of proteins that appear to be adhesins related to the E. coli AIDA-I (25) autotransporter are found within group 2, and known or predicted proteases are segregated in group 3. Phg, BapA, and SphB1 appear to be outliers within groups 1, 2, and 3, respectively (Fig. 10). The one exception to this classification is BP2627/BPP1256/BB2324, which most closely resembles conserved ORFs in A. tumefaciens and Chlamydophila pneumoniae.
FIG. 10.
Phylogenetic relationships of the transporter domains of known and predicted B. bronchiseptica autotransporters. The predicted transporter domains of B. bronchiseptica autotransporters were aligned using CLUSTALW and subjected to distance matrix and neighbor-joining methods by using the PHYLIP package. The resulting phylogenetic tree was visualized with TreeView. Bootstrap values are for 100 replicates.
Pertactin
The archetypal Bordetella autotransporter is pertactin (47, 61). Pertactin was implicated as an adhesin when Chinese hamster ovary (CHO) and HeLa cells were used as the experimental system (278), but a direct role for pertactin-mediated adherence has not been observed in human bronchial (NC1-H292) and laryngeal (HEp-2) epithelial cell lines (418, 502). Still, pertactin is an important component of the current acellular pertussis vaccines (65, 209, 468), even though its exact role in pathogenesis remains to be definitively defined. Pertactin, formerly called P.69, is synthesized as a 93-kDa precursor in B. pertussis. Following cleavage of a 34-amino-acid signal sequence, pertactin is processed after amino acid 597 to yield a passenger domain of 60.3 kDa that migrates as a 69-kDa protein on SDS-polyacrylamide gels. Additional processing can occur after amino acid 577 to produce a 67-kDa passenger domain (61). The protease responsible for cleavage of the passenger moiety from the β-domain has not been identified. Although it is cleaved, the pertactin passenger remains associated with the bacterial surface in a manner analogous to antigen 43 and AIDA-I (see above).
The crystal structure of the passenger domain of pertactin (1DAB) has been solved to 2.5 Å (123) and comprises a right-handed three-strand parallel β helix that adopts a kidney or “V” shape in cross-section (Fig. 11). Typically, the three β-strands in parallel β-helix proteins are designated PB1, PB2, and PB3 and are separated by T1, T2, and T3 turns (227). Unlike the T2 turn at the base of the “V,” the T1 and T3 turns are heterogeneous in size. In the β-helix structure, PB1 forms the indentation in the “V.” This groove is the site where other β-helix structures (e.g., the tail spike protein of phage P22) interact with their cognate substrates (462). No such interactions have been noted for pertactin; it is possible that the large T1 loop of pertactin (Arg260-Gly294) may occlude this site (123, 227). Indeed, the only domain in pertactin thus far associated with adherence is the RGD motif found in the T1 loop (278). This T1 loop comprises a proline-rich repeat, GGXXP5, designated “region 1,” which is polymorphic in different clinical isolates and vaccine strains (336). Antibodies to this region have been found in patients with pertussis and are associated with protection in an animal model (246). The passenger domain of pertactin is the largest β-helix described to date, having 16 rungs or coils. Stacking of internal residues is a defining feature of β-helix proteins, and in pertactin the stacks are composed of aliphatic residues and are quite striking (123). Toward the C terminus of pertactin, the β-helix structure changes to a two-strand β-roll with internal aromatic stacks. The β-roll then returns to a three-strand structure, which leads into a β-sandwich. As noted by Jenkins and Pickersgill (227), the β-helix structure of pertactin is capped by a β-hairpin at its C terminus. Val472-Leu566, which comprises the C-terminal region of this pertactin structure, is conserved in several autotransporters and promotes folding of the passenger domain in a related autotransporter, BrkA (351). Not evident in the pertactin structure (1DAB) is the second proline-rich region (PQP5) that follows the β-hairpin. This region is associated with eliciting protective immunity in a mouse aerosol model (62).
FIG. 11.
Crystal structure of the B. pertussis protein pertactin. The structure of pertactin was solved to a resolution of 2.5 Å (123). (A) Side view of pertactin, with the N terminus located to the top. (B) End view of pertactin looking from the N terminus toward the C terminus. This protein consists of 16 parallel β-strands arranged in a left-handed helix from which several loops extend. T1, T2, and T3 represent the turns between the three strands of the β-helix. The RGD motif is shown in purple in T1. The region in blue represents a conserved region that is present in many autotransporters and has been shown to mediate folding of the passenger domain (351). This is the largest β-helix known. Structurally, this molecule is related to the tail spike proteins of P22 phage and to the pectin lyases and methylesterases (227). Adapted from reference 123 and the Protein Data Bank (30).
A recent report (293) has implicated pertactin as the major tropism determinant for the newly described B. bronchiseptica Bvg-plus tropic phage BPP-1. BPP-1 does not plaque efficiently on bvg or prn (pertactin) mutants of B. bronchiseptica but can do so if pertactin is expressed either under the usual Bvg-plus conditions or ectopically in a Bvg-minus background. The BPP-1 phage can switch tropism by using a Bordetella reverse transcriptase (Brt) mechanism that transcribes mutated copies of the major tropism determinant (Mtd) protein, a protein associated with tail fiber formation. For example, the BMP-1 phage is specific for Bvg-minus cells by virtue of a mutated Mtd protein. Similarly, the BIP-1 phage infects Bvg-plus and -minus bacteria indiscriminately. The receptors for BMP-1 and BIP-1 have not been determined.
BrkA, TcfA, and Vag8
BrkA protects B. pertussis from killing by the classical complement pathway (132). Although the mechanism of protection has not been elucidated, BrkA acts at an early step in the complement activation pathway (19), possibly by recruiting a complement resistance factor present in serum (133). However, a recent study demonstrated that anti-BrkA antibodies could boost the existing bactericidal capacity of human serum against B. pertussis by neutralizing the activity of BrkA (363). BrkA also plays a role in adherence, since mutants with mutations in BrkA bind less efficiently to epithelial (HeLa) and fibroblast (MRC-5) cell lines (127, 132). Previous 50% lethal-dose studies have shown that a brkA mutant was impaired in its capacity to kill infant mice (528), showing that BrkA is an important B. pertussis virulence factor. BrkA is synthesized as a 103-kDa protein that is processed into a 73-kDa passenger domain and a 30-kDa β-domain. N-terminal sequencing of the 73-kDa passenger domain has determined the signal sequence to be 42 amino acids long (364). Cleavage of BrkA after amino acid 731 yields the outer membrane-resident β-domain (187). Although having a function distinct from pertactin, BrkA has 28% sequence identity to pertactin over its passenger domain and 54% identity over the β-domain (132). Like pertactin, the 73-kDa passenger of BrkA is often seen as a doublet (132, 171). Since it has sequence identity to pertactin at the amino acid residues comprising the processing sites of pertactin, it is likely that BrkA is processed similarly, although the protease has yet to be identified. Processed BrkA is steadfastly associated with the bacterial surface.
Tcf (tracheal colonization factor) is a proline-, glycine-, and serine-rich protein that, although predicted to be a 68-kDa protein, migrates at 90 kDa on SDS-polyacrylamide gels (141). It is predicted to have a 39-amino-acid signal sequence and can be found both associated with the bacterial cell surface and as a secreted protein. Tcf mutants have a 10-fold-reduced capacity for tracheal colonization as measured in a mouse aerosol model. The mechanisms, contributing to the colonization properties of Tcf have not been elucidated; however, an RGD motif might suggest a role in adherence.
Vag8 is a somewhat unusual Bordetella autotransporter. Unlike the other characterized Bordetella autotransporters, Vag8 is not cleaved away from its β-domain. Vag8 is a 95-kDa protein that has a 37-amino-acid signal sequence (187). Despite its similarity to pertactin, Tcf, and BrkA, a phenotype attributed to virulence has yet to be associated with Vag8 (140).
CLUSTER 7: CHLAMYDIAL AUTOTRANSPORTERS
Members of the Chlamydiaceae are obligate intracellular pathogens that replicate within membrane-bound vacuoles. The recent completion of five chlamydial genome sequences has led to the division of the Chlamydiaceae into two genera; Chlamydia (Chlamydia trachomatis, Chlamydia suis, and Chlamydia muridarum), and Chlamydophila (Chlamydophila pneumoniae, Chlamydophila abortus, Chlamydophila psittaci, Chlamydophila pecorum, Chlamydophila felis, and Chlamydophila caviae) (77, 126). However, despite these phylogenetic differences, all the species demonstrate commonalities in their life cycles, virulence traits, and modes of host and tissue tropism (77, 186, 469). Indeed, all members of the Chlamydiaceae possess a developmental life cycle that alternates between the infectious extracellular form termed the elementary body (EB) and the metabolically active form termed the reticulate body (RB); the EB develops into the RB after infection (186). Furthermore, examination of the completed genomes revealed the existence of a family of genes encoding polymorphic outer membrane proteins (POMPs), which are unequally distributed throughout the different species. Recent evidence has demonstrated that these proteins are secreted via the autotransporter pathway (198). Thus, Chlamydiaceae is the only family of bacteria outside of the Proteobacteriaceae known to possess autotransporters.
Although the POMPs are widespread and numerous (C. trachomatis possesses 21 pomp genes), a definitive function for these proteins remains to be determined. However, several lines of investigation suggest that these proteins play a role in virulence. First, several investigations of different organisms have demonstrated that the extracellular infective form, the EB, expresses POMPs during the infective process (173, 387, 483, 501). In silico analyses demonstrated that these POMPS are homologous to the adhesins AIDA-I and filamentous haemagglutinin (see above) and, like these adhesins, possess internal repetitive amino acid sequences (198). Indeed, Vretou et al. (519) provided some recent evidence to suggest that the passenger domains of the POMPs exist as a β-helix pyramid, similar to the structure of the pertactin autotransporter protein. Several of these proteins also possess functional motifs associated with virulence, including the RGD cell attachment sequence and the subtilase serine protease motif, both of which are associated with the functions of several autotransporter proteins (see above). Furthermore, recent investigations have also demonstrated that, like the E. coli AIDA-I and TibA adhesins, several of the POMPs are glycosylated (520). In AIDA-I and TibA, this glycosylation is required for the adhesive ability of the molecule. Such data suggest that the EB expresses the POMPs to allow entry into the host cell. Indeed, recent evidence demonstrated that antibodies against the C. pneumoniae PmpD protein blocked the ability of EBs to infect tissue culture cells (527).
Recently, Rocha et al. have demonstrated that several of these POMPs undergo reversible phase variation by slipped-strand mispairing (420). Phase variation has been demonstrated for immunogenic surface proteins throughout the Proteobacteriaeceae and has been noted for several autotransporter proteins including the neisserial AspA/NalP protein and antigen 43 from the AIDA-I family of autotransporters (see above). Moreover, examination of the role of the POMPs in triggering an immune response recently demonstrated that Pmp20 and Pmp21 from C. pneumoniae could increase the production of the inflammatory mediators IL-6, IL-8 and monocyte chemoattractant protein 1 in a dose-dependent fashion in cultured human endothelial cells. Furthermore, this induction was due to the activation of the nuclear factor κB pathway (353). It was suggested that by interaction of the POMPs with the endothelium, the POMPs contribute to vascular injury and thus to the development and progression of atherosclerotic lesions, which are associated with infection by several organisms from the Chlamydiaceae. No doubt the true function of the autotransporter POMPs will be revealed by future investigations.
CLUSTER 9: RICKETTSIAL AUTOTRANSPORTERS
Rickettsiae are obligate intracellular organisms that infect arthropods and vertebrates, causing typhus and spotted fevers in humans. The spotted fever group rickettsiae are further differentiated from the typhus group by the presence of 190- and 120-kDa outer membrane proteins called rickettsial outer membrane proteins rOmpA and rOmpB, respectively.
The rOmpB protein of R. rickettsii is encoded by a gene with the capacity to code for a protein of about 168 kDa (163). However, like other autotransporters, a polypeptide of this size is not detected on purified rickettsiae; indeed, N-terminal sequencing of a 32-kDa, heat-modifiable outer membrane protein revealed that it is encoded by the 3′ end of the rompB gene, suggesting that the rOmpB protein is processed from a large precursor to yield the mature 120-kDa rOmpB protein and the 32-kDa peptide, which remain noncovalently associated (163). The rompA gene sequence predicts a 217-kDa protein, with a unique pattern of 13 tandemly repeated units of 72 to 75 amino acids each in the N-terminal end of the protein and greater than 75% identity between repeat units (10). rOmpB does not possess any sequence similarity to the amino acid repeats found in the rOmpA protein; however, rOmpA and rOmpB do show homology within their respective C-terminal 320 amino acids (>36% identity), a region that may include a membrane anchor in the last 10 amino acids of each protein (181, 403).
rOmpA is a minor constituent of the rickettsial cell surface, but important biological functions have been demonstrated or implicated for similar repeat domains in proteins of other prokaryotes and eukaryotes, such as antigenic structure or variation, opsonic properties, carbohydrate- and other ligand-binding activities, and organelle structure. For rOmpA from R. rickettsii, a monoclonal antibody to this region is protective against experimental infection. In addition, recombinant rOmpA antigen serves as an effective immunogen in mice and guinea pigs challenged with virulent R. rickettsii (322). The repeat unit domain has been hypothesized to be involved in rickettsial adhesion (286, 496). The length of the repeat regions is variable among species of spotted fever group rickettsiae (145) and has been used extensively in an effort to determine the evolutionary status of the spotted fever group of Rickettsiales (418, 424, 532).
The rOmpB protein is the most abundant surface protein on rickettsiae, and it is interesting because of its surface localization, strong immunogenicity, and reactivity with monoclonal antibodies that protect mice against lethal rickettsial challenge (9). Antigenically, rOmpB displays species- and serotype-specific antigenic properties but also displays group- and genus-specific activity (9). Ultrastructural analyses of the rickettsial outer membrane have been interpreted as evidence for a paracrystalline surface array, or S layer, on rickettsiae (457). Immunization with native rOmpB of R. typhi completely protects mice (90) and partially protects guinea pigs (43) against a lethal dose of R. typhi.
Recently, Díaz-Montero et al. (105) have shown that immunization with each of three fragments (rompA4999-6710, rompB1550-2738, and rompB2459-4123) conferred a degree of protection on immunized mice against virulent rickettsial challenge. Protection was achieved when DNA immunizations were followed by booster immunizations with the homologous recombinant protein. Proliferation and gamma interferon secretion were detected after in vitro stimulation of lymphocytes from immunized animals with whole R. conorii antigen.
CLUSTER 10: LIPASES AND ESTERASES
EstA
Pseudomonas aeruginosa is a soil bacterium that is an important opportunistic human pathogen which secretes a variety of proteins into the extracellular medium. Three of these are lipolytic enzymes, including two extracellular phospholipase C proteins and a lipase (222). Lipases are carboxylesterases that have the ability to hydrolyze long-chain acylglycerols, whereas esterases hydrolyze ester substrates with short-chain fatty acids. However, it should be emphasized that lipases are perfectly capable of hydrolysing esterase substrates. Wilhelm et al. found that a lipase-negative deletion mutant of P. aeruginosa PAO1 still showed extracellular lipolytic activity toward short-chain p-nitrophenylesters (533). The sequence responsible for this phenotype revealed an ORF of 1,941 bp, encoding an esterase EstA. The product of estA is a 69.5-kDa protein, which possesses all the features of an autotransporter protein. Expression of estA in P. aeruginosa and E. coli and subsequent cell fractionation revealed that the enzyme was associated with the cellular membranes and can be released by trypsin treatment. These data indicate that the enzyme is located in the outer membrane serine and the esterase/lipase catalytic domain is exposed to the surface (533). This catalytic site is characterized by the consensus motif GDSL, where S represents the active serine residue. In fact, the absolute esterase activity was about 10-fold higher in the overexpressing strain, but in both strains more than 95% of the esterase activity was detected in the crude membrane fraction.
ApeE
Interestingly, the estA gene showed high similarity to apeE encoding an outer membrane esterase from S. enterica serovar Typhimurium (58) and to lip-1 encoding a lipase from Xenorhabdus (Photorhabdus) luminescens (523), both of which are putative autotransporter proteins. Mutations at the apeA (now designated tesA) locus of S. enterica serovar Typhimurium lead to loss of a periplasmic enzyme originally identified by its ability to hydrolyze the chromogenic substrate N-acetylphenylalanine β-naphthyl ester (NAPNE) (327). However, isolation of pseudorevertants with the ability to hydrolyze NAPNE led to the identification of mutations in the apeR locus, which encodes a negative regulator of the transcription of the membrane hydrolase ApeE (70). The predicted product of apeE is a 69.9-kDa protein, which is processed to a 67-kDa species by removal of a signal sequence (25 amino acid residues). Amino acid sequence analysis of ApeE indicates that, like EstA, it is a member of the GDSL family of serine esterases/lipases. The Salmonella esterase catalyzes the hydrolysis of variety of fatty acid naphthyl esters and of C6 to C16 fatty acid p-nitrophenyl esters but not peptide bonds (58). Interestingly, one method for identifying Salmonella spp. in the clinical laboratory involves the use of methyl umbelliferyl caprylate (MUCAP), a substrate that fluoresces on hydrolysis of the ester bond (1), and the apeE gene product is the enzyme in Salmonella uniquely responsible for the hydrolysis of this substrate (58). Recently, it has been found that mutations in apeR, the regulatory locus of apeE, affect the pstSCAB-phoU high-affinity phosphate transport operon. Furthermore, expression of apeE was induced by phosphate limitation, and this induction required the phoBR phosphate regulatory system (71).
Lip-1
ApeE is most similar to a lipase secreted by the entomopathogenic bacterium Photorhabdus luminescens. The P. luminescens Lip-1 lipase is able to hydrolyze Tween 80, a water-soluble oleic acid ester of a polyoxyalkylene derivative of sorbitan (523). In fact, ApeE cleaves Tween 80 but not as efficiently as Lip-1 does. This could be due to a lower activity against this substrate or to the difference in localization of the two enzymes; Lip-1 is secreted into the culture medium, whereas ApeE activity is membrane bound (58). The lip-1 sequence revealed a translation product of 645 amino acids, from which a hydrophobic signal sequence of 24 amino acids is removed during processing, and a β-domain consistent with an autotransporter mode of secretion. Furthermore, the lipase gene was cloned from P. luminescens on the basis of the activity of the gene product on Tween 80, p-nitrophenylpalmitate, and naphthyl acetate. The gene was efficiently expressed in E. coli under the control of its own promoter (523).
McaP
A recent report described the identification, using a plasmid library and transposon mutagenesis-based approach, of a novel adhesin (McaP) in M. catarrhalis (489). Sequence analysis revealed that McaP was a member of the GDSL family of lipases/esterases and had all the hallmarks of an autotransporter protein. Investigation of enzymatic activity revealed that McaP was an esterase and was capable of cleaving phosphatidylcholine and lysophosphatidylcholine in a manner analogous to the Moraxella bovis phopholipase B. Interestingly, McaP displayed activity toward tributyrin, a substance which is used diagnostically to differentiate between M. catarrhalis and Neisseria spp. Surprisingly, expression of the gene product in E. coli increased adherence to a range of epithelial cells in vitro (489). Thus, the precise role of this protein in mediating disease or colonization of the host remains to be determined. Interestingly, the other major adhesins of M. catarrhalis include UspA1, UspA2, and Hag, which all appear to be members of the type Vc family of proteins (73, 210, 273, 386).
OTHER AUTOTRANSPORTER PROTEINS
Adhesins
In a recent report, Rose et al. (423) described an in silico analysis of the Actinobacillus actinomycetemcomitans genome for the presence of autotransporters. Using this approach, these researchers identified Aae, which demonstrated C-terminal homology to H. influenzae Hap adhesin. However, the N-terminal passenger domain did not possess significant homology to any proteins listed in GenBank. Sequencing of the gene from several isolates revealed allelic diversity that was dependent on the presence of amino acid repeats located within the passenger domain. A null aae mutant showed a marked reduction in adherence to KB cells, suggesting a role in adhesion. In agreement with a role for Aae in adhesion, Rose et al. (423) were able to demonstrate a specific interaction between the Aae protein and epithelial cells. Like the Hap autotransporter protein, Aae is cleaved on the bacterial cell surface by the action of lactoferrin. These results were compatible with previous investigations which demonstrated that lactoferrin decreased the efficiency of A. actinomycetemcomitans adhesion to fibroblasts and basement membrane proteins (8, 134).
The advent of genome sequencing has revealed many potential virulence factors from a variety of bacterial sources. Perhaps some of the most widespread homologous virulence determinants are the putative autotransporter adhesin molecules, the vast majority of which remain uncharacterized. These molecules have been found across the Proteobacteriaceae, including human pathogens such as Y. pestis (383) and plant pathogens such as Xylella fastidiosa (456). A recent report described the existence of a putative autotransporter adhesin in the animal pathogen Mannheimia haemolytica (296) which was recognized by antisera to the H. influenzae HMW1 protein; HMW1 is secreted by the two-partner pathway. However, a definitive function was not ascribed to this secreted protein.
A family of previously uncharacterized autotransporter proteins in Bartonella spp. (Iba, for “inducible Bartonella autotransporter”) were recently described (447). Bartonella spp. establish a hemotropic infection in mammals. After initial infection, the bacteria are cleared from the bloodstream but survive in an as yet undetermined primary niche. From this niche, the bloodstream in synchronously seeded with bacteria every 4 to 5 days. These bacteria adhere to and subsequently invade mature erythrocytes. Using differential fluorescence induction, Seubert et al. (447) demonstrated that transcription of the Iba family of autotransporters was up regulated during endothelial infection in vitro. Interestingly, using an animal model of Bartonella infection, at least one gene (ibaB) was shown to be fully activated at the onset of hemotropic infection but immediately down regulated after erythrocyte invasion, suggesting a role for this protein in adhering to the erythrocyte. However, definitive functions for these proteins remain to be determined.
Other descriptions of autotransporters involved in adhesion can be divined from global mutagenesis, proteomic, and transcriptomic studies. Thus, it appears that A. tumefaciens possesses an essential autotransporter adhesin that remains uncharacterized (235), P. aeruginosa possesses an autotransporter that contributes to Caenorhabditis elegans killing and Arabidopsis leaf colonization (482), and M. catarrhalis possesses an autotransporter, McaP, which appears to play a dual role as a lipase and an adhesin (489; see above).
Sialidases
P. multocida is a mucosal pathogen and a normal inhabitant of upper respiratory systems of many animals, is commonly a secondary pathogen in upper respiratory infections, and is a common cause of human soft tissue infections that result from animal bites. Most isolates of P. multocida produce sialidase activity (110), which may contribute to colonization of the respiratory tract (492) or the production of lesions in an active infection (164). Mizan et al. (329) cloned and sequenced a sialidase gene, nanH, from a fowl cholera isolate of P. multocida. NanH (80 kDa) exhibited significant amino acid sequence homology to many microbial sialidases. However, insertional inactivation of nanH resulted in a mutant strain that was not deficient in sialidase production but exhibited reduced enzyme activity and growth rate on 2,3′-sialyllactose compared with the wild type. As a result, the authors identified a second gene, which differed from nanH in DNA sequence and substrate specificity but possessed sialidase activity. The derived amino acid sequence of a 3,210-bp ORF contained an N-terminal signal sequence, exhibited homology to sialidase proteins, and demonstrated all the hallmarks of an autotransporter. Since the DNA sequence showed no significant homology (20%) to the nanH gene, this sialidase gene was designated nanB, NanB is a 120-kDa protein. The sialidase domain resided within the N-terminal 510 amino acids, which exhibited approximately 50% homology to Streptococcus pneumoniae NanA and the large clostridial sialidase proteins, suggesting that NanB belongs to the large sialidase family. NanB contained a FRIP motif and four aspartate boxes, which are expected sialidase motifs. This group of sialidases, however, appeared not to contain the conserved tryptophan residues expected to occupy the hydrophobic pocket of the active enzyme (329). Nevertheless, NanB demonstrated activity on both 2,3′- and 2,6′-sialyllactose, while NanH demonstrated activity only on 2,3′-sialyllactose. Neither enzyme liberated sialic acid from colominic acid (2,8′-sialyllactose). Recombinant E. coli containing the sialidase genes was able to utilize several sialoconjugants when they were provided as sole carbon sources in minimal medium. These data suggest that sialidases have a nutritional function and may contribute to the ability of P. multocida to colonize and persist on vertebrate mucosal surfaces (329).
FUTURE DIRECTIONS
Type V secretion systems are protein translocation machines dedicated to the secretion of single specific bacterial effector molecules. Since the initial description of the IgA1 proteases over 15 years ago, little research has been invested in elucidating the secretion mechanism of these molecules. This has been redressed in the last several years, with multiple groups studying the details of the separate inner and outer membrane translocation events. While some investigations appear to have confirmed the initial model of secretion, others have cast doubt on it, and thus several important questions about the structure and function of the pathway remain to be answered. Does the translocating unit remain as a monomer or oligomerize to form a large central channel? Do the translocating units permit translocation of the polypeptide through the β-barrel, via a large central channel, or through an alternative pathway? What is the energy force driving the secretion of the passenger domain? Are there accessory factors involved in periplasmic transit and assembly into the outer membrane? What is the nature of the factors involved in processing the proteins on the cell surface? Are intrinsic factors, such as the autochaperone domain, necessary for the secretion of all autotransporters? It appears from the current research strategies of several laboratories that the next few years will undoubtedly shed light on these quandaries, permitting a fuller view of the secretion mechanism and perhaps revealing novel variations on the type V secretion theme.
The advent of genomics has spawned a new chapter in the study of proteins secreted via the type V pathway. Determination of the genomic sequences has allowed a rapid expansion of the family and has demonstrated that autotransporter proteins represent the largest family of secreted polypeptides in gram-negative bacteria. However, despite the identification of several hundred autotransporter proteins through in silico analyses, only a relatively small number have been characterized. It is likely future investigations will reveal that many of these proteins play essential roles in the pathogenesis of infectious diseases. Nevertheless, the number of uncharacterized proteins awaiting investigation will ensure plenty of opportunity for research in decades to come.
Perhaps the most interesting facet of autotransporter research will be its application to therapeutics and biotechnology. Indeed, several passenger domains are candidates for subunit or multicomponent vaccines. The incorporation of these proteins into such vaccines is spurred on by the demonstration that pertactin contributes to the protective effect of the whooping cough vaccine (65). Indeed, Pizza et al. (394), using a rational genomic approach to designing vaccines, identified NhhA as one of their candidate proteins for a meningococcal vaccine. The seminal work on secretion has also demonstrated the potential biotechnological exploitation of the autotransporter secretion pathway, namely, by displaying heterologous proteins on the bacterial surface (453). The possible applications of this autodisplay system are numerous and include (i) exposure of antigenic determinants for vaccine development (142, 267, 316), (ii) expression of peptide libraries for epitope mapping or antibody specificity test (258), (iii) display of receptor or ligand for binding assays or purification (499, 500), (iv) functional domain analyses of a heterologous protein (59, 499, 500), and (v) bioconversion by expressing enzymatic activity on the bacterial surface (229, 230, 276). In conclusion, further understanding of the autotransporter secretion pathway and the functions of the passenger domains will facilitate a richer view of the mechanisms and evolution of bacterial pathogenesis and provide important practical applications for the medical and biotechnological communities.
Acknowledgments
We extend our sincere gratitude to Roy Chaudhuri, Marcia Goldberg, Peter Owen, Mary Meehan, and Adrian Canizalez-Roman for aid in preparing figures. We thank Steve Pleasance for providing the analysis of the Bordetella autotransporters and Clasien Oomen for access to her prepublication data. We also thank Mark Pallen for his suggestions and critical reading of the manuscript.
Work in our laboratories was supported by grants from the Natural Sciences and Engineering Research Council of Canada (to R.C.F.), by grant 30004M from CONACYT, Mexico (to F.N.-G.), and by grants 81/D14955 and 81/P14130 from the British Biotechnology and Biological Sciences Research Council (to I.R.H.).
REFERENCES
- 1.Aguirre, P. M., J. B. Cacho, L. Folgueira, M. Lopez, J. Garcia, and A. C. Velasco. 1990. Rapid fluorescence method for screening Salmonella spp. from enteric differential agars. J. Clin. Microbiol. 28:148-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahl, T., and J. Reinholdt. 1991. Detection of immunoglobulin A1 protease-induced Fab alpha fragments on dental plaque bacteria. Infect. Immun. 59:563-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ait-Tahar, K., K. G. Wooldridge, D. P. Turner, M. Atta, I. Todd, and D. A. Ala'Aldeen. 2000. Auto-transporter A protein of Neisseria meningitidis: a potent CD4+ T-cell and B-cell stimulating antigen detected by expression cloning. Mol. Microbiol. 37:1094-1105. [DOI] [PubMed] [Google Scholar]
- 4.Aldridge, P., and K. T. Hughes. 2001. How and when are substrates selected for type III secretion? Trends Microbiol. 9:209-214. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hasani, K., I. R. Henderson, H. Sakellaris, K. Rajakumar, T. Grant, J. P. Nataro, R. Robins-Browne, and B. Adler. 2000. The sigA gene which is borne on the she pathogenicity island of Shigella flexneri 2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect. Immun. 68:2457-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Hasani, K., K. Rajakumar, D. Bulach, R. Robins-Browne, B. Adler, and H. Sakellaris. 2001. Genetic organization of the she pathogenicity island in Shigella flexneri 2a. Microb. Pathog. 30:1-8. [DOI] [PubMed] [Google Scholar]
- 7.Alm, R. A., J. Bina, B. M. Andrews, P. Doig, R. E. Hancock, and T. J. Trust. 2000. Comparative genomics of Helicobacter pylori: analysis of the outer membrane protein families. Infect. Immun. 68:4155-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alugupalli, K. R., and S. Kalfas. 1997. Characterization of the lactoferrin-dependent inhibition of the adhesion of Actinobacillus actinomycetemcomitans, Prevotella intermedia and Prevotella nigrescens to fibroblasts and to a reconstituted basement membrane. Apmis 105:680-688. [DOI] [PubMed] [Google Scholar]
- 9.Anacker, R. L., R. E. Mann, and C. Gonzales. 1987. Reactivity of monoclonal antibodies to Rickettsia rickettsii with spotted fever and typhus group rickettsiae. J. Clin. Microbiol. 25:167-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson, B. E., G. A. McDonald, D. C. Jones, and R. L. Regnery. 1990. A protective protein antigen of Rickettsia rickettsii has tandemly repeated, near-identical sequences. Infect. Immun. 58:2760-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson, D. M., and O. Schneewind. 1997. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science 278:1140-1143. [DOI] [PubMed] [Google Scholar]
- 12.Antoine, R., S. Alonso, D. Raze, L. Coutte, S. Lesjean, E. Willery, C. Locht, and F. Jacob-Dubuisson. 2000. New virulence-activated and virulence-repressed genes identified by systematic gene inactivation and generation of transcriptional fusions in Bordetella pertussis. J. Bacteriol. 182:5902-5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arico, B., S. Nuti, V. Scarlato, and R. Rappuoli. 1993. Adhesion of Bordetella pertussis to eukaryotic cells requires a time-dependent export and maturation of filamentous hemagglutinin. Proc. Natl. Acad. Sci. USA 90:9204-9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atherton, J. C., P. Cao, R. M. Peek, Jr., M. K. Tummuru, M. J. Blaser, and T. L. Cover. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270:17771-17777. [DOI] [PubMed] [Google Scholar]
- 15.Atherton, J. C., T. L. Cover, R. J. Twells, M. R. Morales, C. J. Hawkey, and M. J. Blaser. 1999. Simple and accurate PCR-based system for typing vacuolating cytotoxin alleles of Helicobacter pylori. J. Clin. Microbiol. 37:2979-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atherton, J. C., P. M. Sharp, T. L. Cover, G. Gonzalez-Valencia, R. M. Peek, Jr., S. A. Thompson, C. J. Hawkey, and M. J. Blaser. 1999. Vacuolating cytotoxin (vacA) alleles of Helicobacter pylori comprise two geographically widespread types, m1 and m2, and have evolved through limited recombination. Curr. Microbiol. 39:211-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachovchin, W. W., A. G. Plaut, G. R. Flentke, M. Lynch, and C. A. Kettner. 1990. Inhibition of IgA1 proteinases from Neisseria gonorrhoeae and Hemophilus influenzae by peptide prolyl boronic acids. J. Biol. Chem. 265:3738-3743. [PubMed] [Google Scholar]
- 18.Backert, S., Y. Churin, and T. F. Meyer. 2002. Helicobacter pylori type IV secretion, host cell signalling and vaccine development. Keio J. Med. 51(Suppl. 2):6-14. [DOI] [PubMed] [Google Scholar]
- 19.Barnes, M. G., and A. A. Weiss. 2001. BrkA protein of Bordetella pertussis inhibits the classical pathway of complement after C1 deposition. Infect. Immun. 69:3067-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batey, R. T., R. P. Rambo, L. Lucast, B. Rha, and J. A. Doudna. 2000. Crystal structure of the ribonucleoprotein core of the signal recognition particle. Science 287:1232-1239. [DOI] [PubMed] [Google Scholar]
- 21.Beck, S. C., and T. F. Meyer. 2000. IgA1 protease from Neisseria gonorrhoeae inhibits TNFalpha-mediated apoptosis of human monocytic cells. FEBS Lett. 472:287-292. [DOI] [PubMed] [Google Scholar]
- 22.Benjelloun-Touimi, Z., P. J. Sansonetti, and C. Parsot. 1995. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol. Microbiol. 17:123-135. [DOI] [PubMed] [Google Scholar]
- 23.Benjelloun-Touimi, Z., M. S. Tahar, C. Montecucco, P. J. Sansonetti, and C. Parsot. 1998. SepA, the 110 kDa protein secreted by Shigella flexneri: two-domain structure and proteolytic activity. Microbiology 144:1815-1822. [DOI] [PubMed] [Google Scholar]
- 24.Benz, I., and M. A. Schmidt. 1992. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol. Microbiol. 6:1539-1546. [DOI] [PubMed] [Google Scholar]
- 25.Benz, I., and M. A. Schmidt. 1989. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect. Immun. 57:1506-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benz, I., and M. A. Schmidt. 1990. Diffuse adherence of enteropathogenic Escherichia coli strains. Res. Microbiol. 141:785-786. [DOI] [PubMed] [Google Scholar]
- 27.Benz, I., and M. A. Schmidt. 1993. Diffuse adherence of enteropathogenic Escherichia coli strains—processing of AIDA-I. Zentbl. Bakteriol. 278:197-208. [DOI] [PubMed] [Google Scholar]
- 28.Benz, I., and M. A. Schmidt. 2001. Glycosylation with heptose residues mediated by the aah gene product is essential for adherence of the AIDA-I adhesin. Mol. Microbiol. 40:1403-1413. [DOI] [PubMed] [Google Scholar]
- 29.Benz, I., and M. A. Schmidt. 1992. Isolation and serologic characterization of AIDA-I, the adhesin mediating the diffuse adherence phenotype of the diarrhea-associated Escherichia coli strain 2787 (O126:H27). Infect. Immun. 60:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berman, H. M., T. Battistuz, T. N. Bhat, W. F. Bluhm, P. E. Bourne, K. Burkhardt, Z. Feng, G. L. Gilliland, L. Iype, S. Jain, P. Fagan, J. Marvin, D. Padilla, V. Ravichandran, B. Schneider, N. Thanki, H. Weissig, J. D. Westbrook, and C. Zardecki. 2002. The Protein Data Bank. Acta Crystallogr. Ser. D 58:899-907. [DOI] [PubMed] [Google Scholar]
- 31.Bernardini, M. L., J. Mounier, H. d'Hauteville, M. Coquis-Rondon, and P. J. Sansonetti. 1989. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc. Natl. Acad. Sci. USA 86:3867-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binet, R., S. Letoffe, J. M. Ghigo, P. Delepelaire, and C. Wandersman. 1997. Protein secretion by Gram-negative bacterial ABC exporters—a review. Gene 192:7-11. [DOI] [PubMed] [Google Scholar]
- 33.Binscheck, T., F. Bartels, H. Bergel, H. Bigalke, S. Yamasaki, T. Hayashi, H. Niemann, and J. Pohlner. 1995. IgA protease from Neisseria gonorrhoeae inhibits exocytosis in bovine chromaffin cells like tetanus toxin. J. Biol. Chem. 270:1770-1774. [DOI] [PubMed] [Google Scholar]
- 34.Bitter, W., M. Koster, M. Latijnhouwers, H. de Cock, and J. Tommassen. 1998. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol. Microbiol. 27:209-219. [DOI] [PubMed] [Google Scholar]
- 35.Blanc-Potard, A. B., F. Solomon, J. Kayser, and E. A. Groisman. 1999. The SPI-3 pathogenicity island of Salmonella enterica. J. Bacteriol. 181:998-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bleves, S., R. Voulhoux, G. Michel, A. Lazdunski, J. Tommassen, and A. Filloux. 1998. The secretion apparatus of Pseudomonas aeruginosa: identification of a fifth pseudopilin, XcpX (GspK family). Mol. Microbiol. 27:31-40. [DOI] [PubMed] [Google Scholar]
- 37.Blocker, A., K. Komoriya, and S. Aizawa. 2003. Type III secretion systems and bacterial flagella: insights into their function from structural similarities. Proc. Natl. Acad. Sci. USA 100:3027-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boquet, P., V. Ricci, A. Galmiche, and N. C. Gauthier. 2003. Gastric cell apoptosis and H. pylori: has the main function of VacA finally been identified? Trends Microbiol. 11:410-413. [DOI] [PubMed] [Google Scholar]
- 39.Boren, T., P. Falk, K. A. Roth, G. Larson, and S. Normark. 1993. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262:1892-1895. [DOI] [PubMed] [Google Scholar]
- 40.Boren, T., S. Normark, and P. Falk. 1994. Helicobacter pylori: molecular basis for host recognition and bacterial adherence. Trends Microbiol. 2:221-228. [DOI] [PubMed] [Google Scholar]
- 41.Boschiroli, M. L., S. Ouahrani-Bettache, V. Foulongne, S. Michaux-Charachon, G. Bourg, A. Allardet-Servent, C. Cazevieille, J. P. Lavigne, J. P. Liautard, M. Ramuz, and D. O'Callaghan. 2002. Type IV secretion and Brucella virulence. Vet. Microbiol. 90:341-348. [DOI] [PubMed] [Google Scholar]
- 42.Bouley, J., G. Condemine, and V. E. Shevchik. 2001. The PDZ domain of OutC and the N-terminal region of OutD determine the secretion specificity of the type II out pathway of Erwinia chrysanthemi. J. Mol. Biol. 308:205-219. [DOI] [PubMed] [Google Scholar]
- 43.Bourgeois, A. L., and G. A. Dasch. 1981. The species-specific surface protein antigen of Rickettsia typhi: immunogenicity and protective efficacy in guinea pigs, p. 71-80. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, Inc., New York, N.Y.
- 44.Bradley, P., L. Cowen, M. Menke, J. King, and B. Berer. 2001. BETAWRAP: successful prediction of parallel beta-helices from primary sequences reveals an association with many microbial pathogens. Proc. Natl. Acad. Sci. USA 98:14819-14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brandon, L. D., N. Goehring, A. Janakiraman, A. W. Yan, T. Wu, J. Beckwith, and M. B. Goldberg. 2003. IcsA, a polarly localized autotransporter with an atypical signal peptide, uses the Sec apparatus for secretion, although the Sec apparatus is circumferentially distributed. Mol. Microbiol. 50:45-60. [DOI] [PubMed] [Google Scholar]
- 46.Brandon, L. D., and M. B. Goldberg. 2001. Periplasmic transit and disulfide bond formation of the autotransported Shigella protein IcsA. J. Bacteriol. 183:951-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brennan, M. J., Z. M. Li, J. L. Cowell, M. E. Bisher, A. C. Steven, P. Novotny, and C. R. Manclark. 1988. Identification of a 69-kilodalton nonfimbrial protein as an agglutinogen of Bordetella pertussis. Infect. Immun. 56:3189-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brooks, G. F., C. J. Lammel, M. S. Blake, B. Kusecek, and M. Achtman. 1992. Antibodies against IgA1 protease are stimulated both by clinical disease and asymptomatic carriage of serogroup A Neisseria meningitidis. J. Infect. Dis. 166:1316-1321. [DOI] [PubMed] [Google Scholar]
- 49.Brunder, W., H. Schmidt, and H. Karch. 1997. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol. Microbiol. 24:767-778. [DOI] [PubMed] [Google Scholar]
- 50.Bulieris, P. V., S. Behrens, O. Holst, and J. H. Kleinschmidt. 2003. Folding and insertion of the outer membrane protein OmpA is assisted by the chaperone Skp and by lipopolysaccharide. J. Biol. Chem. 278:9092-9099. [DOI] [PubMed] [Google Scholar]
- 51.Burns, D. L. 1999. Biochemistry of type IV secretion. Curr. Opin. Microbiol. 2:25-29. [DOI] [PubMed] [Google Scholar]
- 52.Burns, D. L. 2003. Type IV transporters of pathogenic bacteria. Curr. Opin. Microbiol. 6:29-34. [DOI] [PubMed] [Google Scholar]
- 53.Burroni, D., P. Lupetti, C. Pagliaccia, J. M. Reyrat, R. Dallai, R. Rappuoli, and J. L. Telford. 1998. Deletion of the major proteolytic site of the Helicobacter pylori cytotoxin does not influence toxin activity but favors assembly of the toxin into hexameric structures. Infect. Immun. 66:5547-5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caffrey, P., and P. Owen. 1989. Purification and N-terminal sequence of the alpha subunit of antigen 43, a unique protein complex associated with the outer membrane of Escherichia coli. J. Bacteriol. 171:3634-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Canizalez-Roman, A., J. Luna, R. Mondragon, and F. Navarro-Garcia. 2001. Abstr. 101st Gen. Mee. Am. Soc. Microbiol. 2001, abstr. B-217, 2001.
- 56.Canizalez-Roman, A., and F. Navarro-Garcia. 2003. Fodrin CaM-binding domain cleavage by Pet from enteroaggregative Escherichia coli leads to actin cytoskeletal disruption. Mol. Microbiol. 48:947-958. [DOI] [PubMed] [Google Scholar]
- 57.Cao, P., and T. L. Cover. 2002. Two different families of hopQ alleles in Helicobacter pylori. J. Clin. Microbiol. 40:4504-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carinato, M. E., P. Collin-Osdoby, X. Yang, T. M. Knox, C. A. Conlin, and C. G. Miller. 1998. The apeE gene of Salmonella typhimurium encodes an outer membrane esterase not present in Escherichia coli. J. Bacteriol. 180:3517-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casali, N., M. Konieczny, M. A. Schmidt, and L. W. Riley. 2002. Invasion activity of a Mycobacterium tuberculosis peptide presented by the Escherichia coli AIDA autotransporter. Infect. Immun. 70:6846-6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charles, I., N. Fairweather, D. Pickard, J. Beesley, R. Anderson, G. Dougan, and M. Roberts. 1994. Expression of the Bordetella pertussis P.69 pertactin adhesin in Escherichia coli: fate of the carboxy-terminal domain. Microbiology 140:3301-3308. [DOI] [PubMed] [Google Scholar]
- 61.Charles, I. G., G. Dougan, D. Pickard, S. Chatfield, M. Smith, P. Novotny, P. Morrissey, and N. F. Fairweather. 1989. Molecular cloning and characterization of protective outer membrane protein P.69 from Bordetella pertussis. Proc. Natl. Acad. Sci. USA 86:3554-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Charles, I. G., J. L. Li, M. Roberts, K. Beesley, M. Romanos, D. J. Pickard, M. Francis, D. Campbell, G. Dougan; M. J. Brennan, et al. 1991. Identification and characterization of a protective immunodominant B cell epitope of pertactin (P.69) from Bordetella pertussis. Eur. J. Immunol. 21:1147-1153. [DOI] [PubMed] [Google Scholar]
- 63.Charles, M., M. Perez, J. H. Kobil, and M. B. Goldberg. 2001. Polar targeting of Shigella virulence factor IcsA in Enterobacteriacae and Vibrio. Proc. Natl. Acad. Sci. USA 98:9871-9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng, L. W., and O. Schneewind. 2000. Type III machines of Gram-negative bacteria: delivering the goods. Trends Microbiol. 8:214-220. [DOI] [PubMed] [Google Scholar]
- 65.Cherry, J. D., J. Gornbein, U. Heininger, and K. Stehr. 1998. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 16:1901-1906. [DOI] [PubMed] [Google Scholar]
- 66.Christie, P. J., and J. P. Vogel. 2000. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 8:354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Citovsky, V., J. Zupan, D. Warnick, and P. Zambryski. 1992. Nuclear localization of Agrobacterium VirE2 protein in plant cells. Science 256:1802-1805. [DOI] [PubMed] [Google Scholar]
- 68.Clemans, D. L., R. J. Bauer, J. A. Hanson, M. V. Hobbs, J. W. St Geme III, C. F. Marrs, and J. R. Gilsdorf. 2000. Induction of proinflammatory cytokines from human respiratory epithelial cells after stimulation by nontypeable Haemophilus influenzae. Infect. Immun. 68:4430-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen, J. J., R. C. Duke, V. A. Fadok, and K. S. Sellins. 1992. Apoptosis and programmed cell death in immunity. Annu. Rev. Immunol. 10:267-293. [DOI] [PubMed] [Google Scholar]
- 70.Collin-Osdoby, P., and C. G. Miller. 1994. Mutations affecting a regulated, membrane-associated esterase in Salmonella typhimurium LT2. Mol. Gen. Genet. 243:674-680. [DOI] [PubMed] [Google Scholar]
- 71.Conlin, C. A., S. L. Tan, H. Hu, and T. Segar. 2001. The apeE gene of Salmonella enterica serovar Typhimurium is induced by phosphate limitation and regulated by phoBR. J. Bacteriol. 183:1784-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cooper, M. D., Z. A. McGee, M. H. Mulks, J. M. Koomey, and T. L. Hindman. 1984. Attachment to and invasion of human fallopian tube mucosa by an IgA1 protease-deficient mutant of Neisseria gonorrhoeae and its wild-type parent. J. Infect. Dis. 150:737-744. [DOI] [PubMed] [Google Scholar]
- 73.Cope, L. D., E. R. Lafontaine, C. A. Slaughter, C. A. Hasemann, Jr., C. Aebi, F. W. Henderson, G. H. McCracken, Jr., and E. J. Hansen. 1999. Characterization of the Moraxella catarrhalis uspA1 and uspA2 genes and their encoded products. J. Bacteriol. 181:4026-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cope, L. D., S. E. Thomas, J. L. Latimer, C. A. Slaughter, U. Muller-Eberhard, and E. J. Hansen. 1994. The 100 kDa haem:haemopexin-binding protein of Haemophilus influenzae: structure and localization. Mol. Microbiol. 13:863-873. [DOI] [PubMed] [Google Scholar]
- 75.Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 76.Corpet, F., F. Servant, J. Gouzy, and D. Kahn. 2000. ProDom and ProDom-CG: tools for protein domain analysis and whole genome comparisons. Nucleic Acids Res. 28:267-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corsaro, D., M. Valassina, and D. Venditti. 2003. Increasing diversity within chlamydiae. Crit. Rev. Microbiol. 29:37-78. [DOI] [PubMed] [Google Scholar]
- 78.Cossart, P., and R. Jonquieres. 2000. Sortase, a universal target for therapeutic agents against gram-positive bacteria? Proc. Natl. Acad. Sci. USA 97:5013-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cotter, P. A., and V. J. DiRita. 2000. Bacterial virulence gene regulation: an evolutionary perspective. Annu. Rev. Microbiol. 54:519-565. [DOI] [PubMed] [Google Scholar]
- 80.Cotter, P. A., and J. F. Miller. 2001. Bordetella, p. 619-674. In E. A. Groisman (ed.), Principles of bacterial pathogenesis. Academic Press, Inc., San Diego, Calif.
- 81.Coutte, L., S. Alonso, N. Reveneau, E. Willery, B. Quatannens, C. Locht, and F. Jacob-Dubuisson. 2003. Role of adhesin release for mucosal colonization by a bacterial pathogen. J. Exp. Med. 197:735-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coutte, L., R. Antoine, H. Drobecq, C. Locht, and F. Jacob-Dubuisson. 2001. Subtilisin-like autotransporter serves as maturation protease in a bacterial secretion pathway. EMBO J. 20:5040-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coutte, L., E. Willery, R. Antoine, H. Drobecq, C. Locht, and F. Jacob-Dubuisson. 2003. Surface anchoring of bacterial subtilisin important for maturation function. Mol. Microbiol. 49:529-539. [DOI] [PubMed] [Google Scholar]
- 84.Cover, T. L., and M. J. Blaser. 1992. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J. Biol. Chem. 267:10570-10575. [PubMed] [Google Scholar]
- 85.Cover, T. L., P. I. Hanson, and J. E. Heuser. 1997. Acid-induced dissociation of VacA, the Helicobacter pylori vacuolating cytotoxin, reveals its pattern of assembly. J. Cell Biol. 138:759-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cutter, D., K. W. Mason, A. P. Howell, D. L. Fink, B. A. Green, and J. W. St Geme III. 2002. Immunization with Haemophilus influenzae Hap adhesin protects against nasopharyngeal colonization in experimental mice. J. Infect. Dis. 186:1115-1121. [DOI] [PubMed] [Google Scholar]
- 87.Czajkowsky, D. M., H. Iwamoto, T. L. Cover, and Z. Shao. 1999. The vacuolating toxin from Helicobacter pylori forms hexameric pores in lipid bilayers at low pH. Proc. Natl. Acad. Sci. USA 96:2001-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Danese, P. N., L. A. Pratt, S. L. Dove, and R. Kolter. 2000. The outer membrane protein, antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol. Microbiol. 37:424-432. [DOI] [PubMed] [Google Scholar]
- 89.Daniell, S. J., E. Kocsis, E. Morris, S. Knutton, F. P. Booy, and G. Frankel. 2003. 3D structure of EspA filaments from enteropathogenic Escherichia coli. Mol. Microbiol. 49:301-308. [DOI] [PubMed] [Google Scholar]
- 90.Dasch, G. A., A. L. Bourgeois, and F. M. Rollwagen. 1999. The surface protein antigen of Rickettsia typhi: in vitro and in vivo immunogenicity and protective efficacy in mice, p. 116-122. In D. Raolt and P. Brouqui (ed.), Rickettsiae and rickettsial diseases at the turn of the third millenium. Elsevier, Paris, France.
- 91.Davis, J., A. L. Smith, W. R. Hughes, and M. Golomb. 2001. Evolution of an autotransporter: domain shuffling and lateral transfer from pathogenic Haemophilus to Neisseria. J. Bacteriol. 183:4626-4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Bernard, M., D. Burroni, E. Papini, R. Rappuoli, J. Telford, and C. Montecucco. 1998. Identification of the Helicobacter pylori VacA toxin domain active in the cell cytosol. Infect. Immun. 66:6014-6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Bernard, M., M. Moschioni, G. Napolitani, R. Rappuoli, and C. Montecucco. 2000. The VacA toxin of Helicobacter pylori identifies a new intermediate filament-interacting protein. EMBO J. 19:48-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Bernard, M., E. Papini, V. de Filippis, E. Gottardi, J. Telford, R. Manetti, A. Fontana, R. Rappuoli, and C. Montecucco. 1995. Low pH activates the vacuolating toxin of Helicobacter pylori, which becomes acid and pepsin resistant. J. Biol. Chem. 270:23937-23940. [DOI] [PubMed] [Google Scholar]
- 95.de Cock, H., K. Brandenburg, A. Wiese, O. Holst, and U. Seydel. 1999. Non-lamellar structure and negative charges of lipopolysaccharides required for efficient folding of outer membrane protein PhoE of Escherichia coli. J. Biol. Chem. 274:5114-5119. [DOI] [PubMed] [Google Scholar]
- 96.De Gier, J. W., Q. A. Valent, G. Von Heijne, and J. Luirink. 1997. The E. coli SRP: preferences of a targeting factor. FEBS Lett. 408:1-4. [DOI] [PubMed] [Google Scholar]
- 97.Delcour, A. H. 2002. Structure and function of pore-forming beta-barrels from bacteria. J. Mol. Microbiol. Biotechnol. 4:1-10. [PubMed] [Google Scholar]
- 98.Deng, W., V. Burland, G. Plunkett, 3rd, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 184:4601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Desvaux, M., N. J. Parham, and I. R. Henderson. 2003. Le système de sécrétion de type V chez les bactéries Gram-négatives. Biofutur 237:34-37. [Google Scholar]
- 100.Desvaux, M., N. J. Parham, A. Scott-Tucker, and I. R. Henderson. 2004. The general secretory pathway: a general misnomer? Trends Microbiol. 12:306-309. [DOI] [PubMed] [Google Scholar]
- 101.Desvaux, M., N. J. Parham, and I. R. Henderson. 2004. The autotransporter secretion system. Res. Microbiol. 155:53-60. [DOI] [PubMed] [Google Scholar]
- 102.Desvaux, M., N. J. Parham, and I. R. Henderson. 2004. Type V protein secretion: simplicity gone awry? Curr. Issues Mol. Biol. 6:111-124. [PubMed] [Google Scholar]
- 103.Devenyi, A. G., A. G. Plaut, F. J. Grundy, and A. Wright. 1993. Post-infectious human serum antibodies inhibit IgA1 proteinases by interaction with the cleavage site specificity determinant. Mol. Immunol. 30:1243-1248. [DOI] [PubMed] [Google Scholar]
- 104.Dhermy, D. 1991. The spectrin super-family. Biol. Cell. 71:249-254. [PubMed] [Google Scholar]
- 105.Diaz-Montero, C. M., H. M. Feng, P. A. Crocquet-Valdes, and D. H. Walker. 2001. Identification of protective components of two major outer membrane proteins of spotted fever group rickettsiae. Am. J. Trop. Med. Hyg. 65:371-378. [DOI] [PubMed] [Google Scholar]
- 106.Djafari, S., F. Ebel, C. Deibel, S. Kramer, M. Hudel, and T. Chakraborty. 1997. Characterization of an exported protease from Shiga toxin-producing Escherichia coli. Mol. Microbiol. 25:771-784. [DOI] [PubMed] [Google Scholar]
- 107.Domenighini, M., D. Relman, C. Capiau, S. Falkow, A. Prugnola, V. Scarlato, and R. Rappuoli. 1990. Genetic characterization of Bordetella pertussis filamentous haemagglutinin: a protein processed from an unusually large precursor. Mol. Microbiol. 4:787-800. [DOI] [PubMed] [Google Scholar]
- 108.Dozois, C. M., M. Dho-Moulin, A. Bree, J. M. Fairbrother, C. Desautels, and R. Curtiss III. 2000. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the Tsh genetic region. Infect. Immun. 68:4145-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Driessen, A. J. 2001. SecB, a molecular chaperone with two faces. Trends Microbiol. 9:193-196. [DOI] [PubMed] [Google Scholar]
- 110.Drzeniek, R., W. Scharmann, and E. Balke. 1972. Neuraminidase and N-acetylneuraminate pyruvate-lyase of Pasteurella multocida. J. Gen. Microbiol. 72:357-368. [DOI] [PubMed] [Google Scholar]
- 111.Duckely, M., and B. Hohn. 2003. The VirE2 protein of Agrobacterium tumefaciens: the Yin and Yang of T-DNA transfer. FEMS Microbiol. Lett. 223:1-6. [DOI] [PubMed] [Google Scholar]
- 112.Durand, E., A. Bernadac, G. Ball, A. Lazdunski, J. N. Sturgis, and A. Filloux. 2003. Type II protein secretion in Pseudomonas aeruginosa: the pseudopilus is a multifibrillar and adhesive structure. J. Bacteriol. 185:2749-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dutta, P. R., R. Cappello, F. Navarro-Garcia, and J. P. Nataro. 2002. Functional comparison of serine protease autotransporters of Enterobacteriaceae. Infect. Immun. 70:7105-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dutta, P. R., B. Q. Sui, and J. P. Nataro. 2003. Structure-function analysis of the enteroaggregative E. coli plasmid encoded toxin autotransporter using scanning linker mutagenesis. J. Biol. Chem. 278:39912-39920. [DOI] [PubMed] [Google Scholar]
- 115.Economou, A. 2002. Bacterial secretome: the assembly manual and operating instructions. Mol. Membr. Biol. 19:159-169. [DOI] [PubMed] [Google Scholar]
- 116.Economou, A. 1999. Following the leader: bacterial protein export through the Sec pathway. Trends Microbiol. 7:315-320. [DOI] [PubMed] [Google Scholar]
- 117.Egile, C., H. d'Hauteville, C. Parsot, and P. J. Sansonetti. 1997. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol. Microbiol. 23:1063-1073. [DOI] [PubMed] [Google Scholar]
- 118.Egile, C., T. P. Loisel, V. Laurent, R. Li, D. Pantaloni, P. J. Sansonetti, and M. F. Carlier. 1999. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 146:1319-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eichler, J., and R. Moll. 2001. The signal recognition particle of Archaea. Trends Microbiol. 9:130-136. [DOI] [PubMed] [Google Scholar]
- 120.Elliott, S. J., V. Sperandio, J. A. Giron, S. Shin, J. L. Mellies, L. Wainwright, S. W. Hutcheson, T. K. McDaniel, and J. B. Kaper. 2000. The locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE- and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 68:6115-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Elsinghorst, E. A., and D. J. Kopecko. 1992. Molecular cloning of epithelial cell invasion determinants from enterotoxigenic Escherichia coli. Infect. Immun. 60:2409-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Elsinghorst, E. A., and J. A. Weitz. 1994. Epithelial cell invasion and adherence directed by the enterotoxigenic Escherichia coli tib locus is associated with a 104-kilodalton outer membrane protein. Infect. Immun. 62:3463-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Emsley, P., I. G. Charles, N. F. Fairweather, and N. W. Isaacs. 1996. Structure of Bordetella pertussis virulence factor P.69 pertactin. Nature 381:90-92. [DOI] [PubMed] [Google Scholar]
- 124.Eslava, C., F. Navarro-Garcia, J. R. Czeczulin, I. R. Henderson, A. Cravioto, and J. P. Nataro. 1998. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect. Immun. 66:3155-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eslava, C., J. M. Villaseca, M. R. Morales, A. Navarro, and A. Cravioto. 1993. Abstr. 93rd Gen. Mee. Am. Soc. Microbiol. 1993, abstr. B-105, 1993.
- 126.Everett, K. D., R. M. Bush, and A. A. Andersen. 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 49:415-440. [DOI] [PubMed] [Google Scholar]
- 127.Ewanowich, C. A., A. R. Melton, A. A. Weiss, R. K. Sherburne, and M. S. Peppler. 1989. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect. Immun. 57:2698-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Falk, P., K. A. Roth, T. Boren, T. U. Westblom, J. I. Gordon, and S. Normark. 1993. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage-specific tropism in the human gastric epithelium. Proc. Natl. Acad. Sci. USA 90:2035-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Falk, P. G., L. Bry, J. Holgersson, and J. I. Gordon. 1995. Expression of a human alpha-1,3/4-fucosyltransferase in the pit cell lineage of FVB/N mouse stomach results in production of Leb-containing glycoconjugates: a potential transgenic mouse model for studying Helicobacter pylori infection. Proc. Natl. Acad. Sci. USA 92:1515-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Farizo, K. M., T. Huang, and D. L. Burns. 2000. Importance of holotoxin assembly in Ptl-mediated secretion of pertussis toxin from Bordetella pertussis. Infect. Immun. 68:4049-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fasano, A., F. R. Noriega, D. R. Maneval, Jr., S. Chanasongcram, R. Russell, S. Guandalini, and M. M. Levine. 1995. Shigella enterotoxin 1: an enterotoxin of Shigella flexneri 2a active in rabbit small intestine in vivo and in vitro. J. Clin. Investig 95:2853-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fernandez, R. C., and A. A. Weiss. 1994. Cloning and sequencing of a Bordetella pertussis serum resistance locus. Infect. Immun. 62:4727-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fernandez, R. C., and A. A. Weiss. 1998. Serum resistance in bvg-regulated mutants of Bordetella pertussis. FEMS Microbiol. Lett. 163:57-63. [DOI] [PubMed] [Google Scholar]
- 134.Fine, D. H., and D. Furgang. 2002. Lactoferrin iron levels affect attachment of Actinobacillus actinomycetemcomitans to buccal epithelial cells. J. Periodontol. 73:616-623. [DOI] [PubMed] [Google Scholar]
- 135.Fink, D. L., A. Z. Buscher, B. Green, P. Fernsten, and J. W. St Geme, 3rd. 2003. The Haemophilus influenzae Hap autotransporter mediates microcolony formation and adherence to epithelial cells and extracellular matrix via binding regions in the C-terminal end of the passenger domain. Cell Microbiol. 5:175-186. [DOI] [PubMed] [Google Scholar]
- 136.Fink, D. L., L. D. Cope, E. J. Hansen, and J. W. St Geme III. 2001. The Haemophilus influenzae Hap autotransporter is a chymotrypsin clan serine protease and undergoes autoproteolysis via an intermolecular mechanism. J. Biol. Chem. 276:39492-39500. [DOI] [PubMed] [Google Scholar]
- 137.Fink, D. L., B. A. Green, and J. W. St Geme III 2002. The Haemophilus influenzae Hap autotransporter binds to fibronectin, laminin, and collagen IV. Infect. Immun. 70:4902-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fink, D. L., and J. W. St Geme III. 2003. Chromosomal expression of the Haemophilus influenzae Hap autotransporter allows fine-tuned regulation of adhesive potential via inhibition of intermolecular autoproteolysis. J. Bacteriol. 185:1608-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Finn, T. M., and D. F. Amsbaugh. 1998. Vag8, a Bordetella pertussis bvg-regulated protein. Infect. Immun. 66:3985-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Finn, T. M., and L. A. Stevens. 1995. Tracheal colonization factor: a Bordetella pertussis secreted virulence determinant. Mol. Microbiol. 16:625-634. [DOI] [PubMed] [Google Scholar]
- 142.Fischer, W., R. Buhrdorf, E. Gerland, and R. Haas. 2001. Outer membrane targeting of passenger proteins by the vacuolating cytotoxin autotransporter of Helicobacter pylori. Infect. Immun. 69:6769-6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fischer, W., R. Haas, and S. Odenbreit. 2002. Type IV secretion systems in pathogenic bacteria. Int. J. Med. Microbiol. 292:159-168. [DOI] [PubMed] [Google Scholar]
- 144.Forsyth, M. H., and T. L. Cover. 1999. Mutational analysis of the vacA promoter provides insight into gene transcription in Helicobacter pylori. J. Bacteriol. 181:2261-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fournier, P. E., V. Roux, and D. Raoult. 1998. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int. J. Syst. Bacteriol. 48:839-849. [DOI] [PubMed] [Google Scholar]
- 146.Francetic, O., and A. P. Pugsley. 1996. The cryptic general secretory pathway (gsp) operon of Escherichia coli K-12 encodes functional proteins. J. Bacteriol. 178:3544-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Frankel, G., A. D. Phillips, L. R. Trabulsi, S. Knutton, G. Dougan, and S. Matthews. 2001. Intimin and the host cell—is it bound to end in Tir(s)? Trends Microbiol. 9:214-218. [DOI] [PubMed] [Google Scholar]
- 148.Freudl, R., H. Schwarz, Y. D. Stierhof, K. Gamon, I. Hindennach, and U. Henning. 1986. An outer membrane protein (OmpA) of Escherichia coli K-12 undergoes a conformational change during export. J. Biol. Chem. 261:11355-11361. [PubMed] [Google Scholar]
- 149.Fujikawa, A., D. Shirasaka, S. Yamamoto, H. Ota, K. Yahiro, M. Fukada, T. Shintani, A. Wada, N. Aoyama, T. Hirayama, H. Fukamachi, and M. Noda. 2003. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat. Genet. 33:375-381. [DOI] [PubMed] [Google Scholar]
- 150.Gabig, M., A. Herman-Antosiewicz, M. Kwiatkowska, M. Los, M. S. Thomas, and G. Wegrzyn. 2002. The cell surface protein Ag43 facilitates phage infection of Escherichia coli in the presence of bile salts and carbohydrates. Microbiology 148:1533-1542. [DOI] [PubMed] [Google Scholar]
- 151.Galmiche, A., J. Rassow, A. Doye, S. Cagnol, J. C. Chambard, S. Contamin, V. de Thillot, I. Just, V. Ricci, E. Solcia, E. Van Obberghen, and P. Boquet. 2000. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 19:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Garner, J. A., and T. L. Cover. 1996. Binding and internalization of the Helicobacter pylori vacuolating cytotoxin by epithelial cells. Infect. Immun. 64:4197-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gauthier, A., J. L. Puente, and B. B. Finlay. 2003. Secretin of the enteropathogenic Escherichia coli type III secretion system requires components of the type III apparatus for assembly and localization. Infect. Immun. 71:3310-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Genevrois, S., L. Steeghs, P. Roholl, J.J. Letesson, and P. van der Ley. 2003. The Omp85 protein of Neisseria meningitidis is required for lipid export to the outer membrane. EMBO J. 22:1780-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gentle, I., K. Gabriel, P. Beech, R. Waller, and T. Lithgow. 2004. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J. Cell Biol. 164:19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gentschev, I., G. Dietrich, and W. Goebel. 2002. The E. coli alpha-hemolysin secretion system and its use in vaccine development. Trends Microbiol. 10:39-45. [DOI] [PubMed] [Google Scholar]
- 157.Gerhard, M., N. Lehn, N. Neumayer, T. Boren, R. Rad, W. Schepp, S. Miehlke, M. Classen, and C. Prinz. 1999. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc. Natl. Acad. Sci. USA 96:12778-12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gerlach, G., F. von Wintzingerode, B. Middendorf, and R. Gross. 2001. Evolutionary trends in the genus Bordetella. Microbes Infect. 3:61-72. [DOI] [PubMed] [Google Scholar]
- 159.Ghiara, P., M. Marchetti, M. J. Blaser, M. K. Tummuru, T. L. Cover, E. D. Segal, L. S. Tompkins, and R. Rappuoli. 1995. Role of the Helicobacter pylori virulence factors vacuolating cytotoxin, CagA, and urease in a mouse model of disease. Infect. Immun. 63:4154-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ghigo, J. M., and C. Wandersman. 1992. Cloning, nucleotide sequence and characterization of the gene encoding the Erwinia chrysanthemi B374 PrtA metalloprotease: a third metalloprotease secreted via a C-terminal secretion signal. Mol. Gen. Genet. 236:135-144. [DOI] [PubMed] [Google Scholar]
- 161.Gilbert, J. V., A. G. Plaut, B. Longmaid, and M. E. Lamm. 1983. Inhibition of bacterial IgA proteases by human secretory IgA and serum. Ann. N. Y. Acad. Sci. 409:625-636. [DOI] [PubMed] [Google Scholar]
- 162.Gilbert, J. V., A. G. Plaut, and A. Wright. 1991. Analysis of the immunoglobulin A protease gene of Streptococcus sanguis. Infect. Immun. 59:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gilmore, R. D., Jr., W. Cieplak, Jr., P. F. Policastro, and T. Hackstadt. 1991. The 120 kilodalton outer membrane protein (rOmp B) of Rickettsia rickettsii is encoded by an unusually long open reading frame: evidence for protein processing from a large precursor. Mol. Microbiol. 5:2361-2370. [DOI] [PubMed] [Google Scholar]
- 164.Godoy, V. G., M. M. Dallas, T. A. Russo, and M. H. Malamy. 1993. A role for Bacteroides fragilis neuraminidase in bacterial growth in two model systems. Infect. Immun. 61:4415-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Goldberg, M. B. 2001. Actin-based motility of intracellular microbial pathogens. Microbiol. Mol. Biol. Rev. 65:595-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goldberg, M. B., O. Barzu, C. Parsot, and P. J. Sansonetti. 1993. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J. Bacteriol. 175:2189-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Goldberg, M. B., and J. A. Theriot. 1995. Shigella flexneri surface protein IcsA is sufficient to direct actin-based motility. Proc. Natl. Acad. Sci. USA 92:6572-6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gomez-Duarte, O. G., and J. B. Kaper. 1995. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect. Immun. 63:1767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gonzalez, C. T., S. K. Maheswaran, and M. P. Murtaugh. 1995. Pasteurella haemolytica serotype 2 contains the gene for a noncapsular serotype 1-specific antigen. Infect. Immun. 63:1340-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gottke, M. U., C. A. Fallone, A. N. Barkun, K. Vogt, V. Loo, M. Trautmann, J. Z. Tong, T. N. Nguyen, T. Fainsilber, H. H. Hahn, J. Korber, A. Lowe, and R. N. Beech. 2000. Genetic variability determinants of Helicobacter pylori: influence of clinical background and geographic origin of isolates. J. Infect. Dis. 181:1674-1681. [DOI] [PubMed] [Google Scholar]
- 171.Gotto, J. W., T. Eckhardt, P. A. Reilly, J. V. Scott, J. L. Cowell, T. N. Metcalf III, K. Mountzouros, J. J. Gibbons, Jr., and M. Siegel. 1993. Biochemical and immunological properties of two forms of pertactin, the 69,000-molecular-weight outer membrane protein of Bordetella pertussis. Infect. Immun. 61:2211-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Grass, S., and J. W. St Geme III. 2000. Maturation and secretion of the non-typable Haemophilus influenzae HMW1 adhesin: roles of the N-terminal and C-terminal domains. Mol. Microbiol. 36:55-67. [DOI] [PubMed] [Google Scholar]
- 173.Grimwood, J., L. Olinger, and R. S. Stephens. 2001. Expression of Chlamydia pneumoniae polymorphic membrane protein family genes. Infect. Immun. 69:2383-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Guarino, A., M. Bisceglia, R. B. Canani, M. C. Boccia, G. Mallardo, E. Bruzzese, P. Massari, R. Rappuoli, and J. Telford. 1998. Enterotoxic effect of the vacuolating toxin produced by Helicobacter pylori in Caco-2 cells. J. Infect. Dis. 178:1373-1378. [DOI] [PubMed] [Google Scholar]
- 175.Guedin, S., E. Willery, C. Locht, and F. Jacob-Dubuisson. 1998. Evidence that a globular conformation is not compatible with FhaC-mediated secretion of the Bordetella pertussis filamentous haemagglutinin. Mol. Microbiol. 29:763-774. [DOI] [PubMed] [Google Scholar]
- 176.Guedin, S., E. Willery, J. Tommassen, E. Fort, H. Drobecq, C. Locht, and F. Jacob-Dubuisson. 2000. Novel topological features of FhaC, the outer membrane transporter involved in the secretion of the Bordetella pertussis filamentous hemagglutinin. J. Biol. Chem. 275:30202-30210. [DOI] [PubMed] [Google Scholar]
- 177.Guruge, J. L., P. G. Falk, R. G. Lorenz, M. Dans, H. P. Wirth, M. J. Blaser, D. E. Berg, and J. I. Gordon. 1998. Epithelial attachment alters the outcome of Helicobacter pylori infection. Proc. Natl. Acad. Sci. USA 95:3925-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guyer, D. M., I. R. Henderson, J. P. Nataro, and H. L. Mobley. 2000. Identification of Sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 38:53-66. [DOI] [PubMed] [Google Scholar]
- 179.Guyer, D. M., S. Radulovic, F. E. Jones, and H. L. Mobley. 2002. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect. Immun. 70:4539-4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Haagmans, W., and M. van der Woude. 2000. Phase variation of Ag43 in Escherichia coli: Dam-dependent methylation abrogates OxyR binding and OxyR-mediated repression of transcription. Mol. Microbiol. 35:877-887. [DOI] [PubMed] [Google Scholar]
- 181.Hackstadt, T., R. Messer, W. Cieplak, and M. G. Peacock. 1992. Evidence for proteolytic cleavage of the 120-kilodalton outer membrane protein of rickettsiae: identification of an avirulent mutant deficient in processing. Infect. Immun. 60:159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hadi, H. A., K. G. Wooldridge, K. Robinson, and D. A. Ala'Aldeen. 2001. Identification and characterization of App: an immunogenic autotransporter protein of Neisseria meningitidis. Mol. Microbiol. 41:611-623. [DOI] [PubMed] [Google Scholar]
- 183.Haigh, R., T. Baldwin, S. Knutton, and P. H. Williams. 1995. Carbon dioxide regulated secretion of the EaeB protein of enteropathogenic Escherichia coli. FEMS Microbiol. Lett. 129:63-67. [DOI] [PubMed] [Google Scholar]
- 184.Hajishengallis, G., E. Nikolova, and M. W. Russell. 1992. Inhibition of Streptococcus mutans adherence to saliva-coated hydroxyapatite by human secretory immunoglobulin A (S-IgA) antibodies to cell surface protein antigen I/II: reversal by IgA1 protease cleavage. Infect. Immun. 60:5057-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Halter, R., J. Pohlner, and T. F. Meyer. 1984. IgA protease of Neisseria gonorrhoeae: isolation and characterization of the gene and its extracellular product. EMBO J. 3:1595-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hammerschlag, M. R. 2002. The intracellular life of chlamydiae. Semin. Pediatr. Infect. Dis. 13:239-248. [DOI] [PubMed] [Google Scholar]
- 187.Hamstra, H. J., B. Kuipers, D. Schijf-Evers, H. G. Loggen, and J. T. Poolman. 1995. The purification and protective capacity of Bordetella pertussis outer membrane proteins. Vaccine 13:747-752. [DOI] [PubMed] [Google Scholar]
- 188.Hardie, K. R., A. Seydel, I. Guilvout, and A. P. Pugsley. 1996. The secretin-specific, chaperone-like protein of the general secretory pathway: separation of proteolytic protection and piloting functions. Mol. Microbiol. 22:967-976. [DOI] [PubMed] [Google Scholar]
- 189.Haridas, V., B. G. Darnay, K. Natarajan, R. Heller, and B. B. Aggarwal. 1998. Overexpression of the p80 TNF receptor leads to TNF-dependent apoptosis, nuclear factor-kappa B activation, and c-Jun kinase activation. J. Immunol. 160:3152-3162. [PubMed] [Google Scholar]
- 190.Hartford, O., D. McDevitt, and T. J. Foster. 1999. Matrix-binding proteins of Staphylococcus aureus: functional analysis of mutant and hybrid molecules. Microbiology 145:2497-2505. [DOI] [PubMed] [Google Scholar]
- 191.Hasman, H., M. A. Schembri, and P. Klemm. 2000. Antigen 43 and type 1 fimbriae determine colony morphology of Escherichia coli K-12. J. Bacteriol. 182:1089-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hauck, C. R., and T. F. Meyer. 1997. The lysosomal/phagosomal membrane protein h-lamp-1 is a target of the IgA1 protease of Neisseria gonorrhoeae. FEBS Lett. 405:86-90. [DOI] [PubMed] [Google Scholar]
- 193.Hedges, S. R., M. S. Mayo, L. Kallman, J. Mestecky, E. W. Hook, 3rd, and M. W. Russell. 1998. Evaluation of immunoglobulin A1 (IgA1) protease and IgA1 protease-inhibitory activity in human female genital infection with Neisseria gonorrhoeae. Infect. Immun. 66:5826-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Heimer, S. R., D. A. Rasko, C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2004. Autotransporter genes pic and tsh are associated with Escherichia coli strains that cause acute pyelonephritis and are expressed during urinary tract infection. Infect. Immun. 72:593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Henderson, I. R., R. Cappello, and J. P. Nataro. 2000. Autotransporter proteins, evolution and redefining protein secretion. Trends Microbiol. 8:529-532. [DOI] [PubMed] [Google Scholar]
- 196.Henderson, I. R., J. Czeczulin, C. Eslava, F. Noriega, and J. P. Nataro. 1999. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 67:5587-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Henderson, I. R., S. Hicks, F. Navarro-Garcia, W. P. Elias, A. D. Philips, and J. P. Nataro. 1999. Involvement of the enteroaggregative Escherichia coli plasmid-encoded toxin in causing human intestinal damage. Infect. Immun. 67:5338-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Henderson, I. R., and A. C. Lam. 2001. Polymorphic proteins of Chlamydia spp.—autotransporters beyond the Proteobacteria. Trends Microbiol. 9:573-578. [DOI] [PubMed] [Google Scholar]
- 199.Henderson, I. R., M. Meehan, and P. Owen. 1997. Antigen 43, a phase-variable bipartite outer membrane protein, determines colony morphology and autoaggregation in Escherichia coli K-12. FEMS Microbiol. Lett. 149:115-120. [DOI] [PubMed] [Google Scholar]
- 200.Henderson, I. R., M. Meehan, and P. Owen. 1997. A novel regulatory mechanism for a novel phase-variable outer membrane protein of Escherichia coli. Adv. Exp. Med. Biol. 412:349-355. [DOI] [PubMed] [Google Scholar]
- 201.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Henderson, I. R., J. P. Nataro, J. B. Kaper, T. F. Meyer, S. K. Farrand, D. L. Burns, B. B. Finlay, and J. W. St Geme III. 2000. Renaming protein secretion in the gram-negative bacteria. Trends Microbiol. 8:352. [PubMed] [Google Scholar]
- 203.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6:370-378. [DOI] [PubMed] [Google Scholar]
- 204.Henderson, I. R., and P. Owen. 1999. The major phase-variable outer membrane protein of Escherichia coli structurally resembles the immunoglobulin A1 protease class of exported protein and is regulated by a novel mechanism involving Dam and oxyR. J. Bacteriol. 181:2132-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Henderson, I. R., P. Owen, and J. P. Nataro. 1999. Molecular switches—the ON and OFF of bacterial phase variation. Mol. Microbiol. 33:919-932. [DOI] [PubMed] [Google Scholar]
- 206.Hendrixson, D. R., M. L. de la Morena, C. Stathopoulos, and J. W. St Geme III. 1997. Structural determinants of processing and secretion of the Haemophilus influenzae hap protein. Mol. Microbiol. 26:505-518. [DOI] [PubMed] [Google Scholar]
- 207.Hendrixson, D. R., and J. W. St Geme III. 1998. The Haemophilus influenzae Hap serine protease promotes adherence and microcolony formation, potentiated by a soluble host protein. Mol. Cell 2:841-850. [DOI] [PubMed] [Google Scholar]
- 208.Herskovits, A. A., E. S. Bochkareva, and E. Bibi. 2000. New prospects in studying the bacterial signal recognition particle pathway. Mol. Microbiol. 38:927-939. [DOI] [PubMed] [Google Scholar]
- 209.Hewlett, E. L., and S. A. Halperin. 1998. Serological correlates of immunity to Bordetella pertussis. Vaccine 16:1899-1900. [DOI] [PubMed] [Google Scholar]
- 210.Hoiczyk, E., A. Roggenkamp, M. Reichenbecher, A. Lupas, and J. Heesemann. 2000. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 19:5989-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hopper, S., B. Vasquez, A. Merz, S. Clary, J. S. Wilbur, and M. So. 2000. Effects of the immunoglobulin A1 protease on Neisseria gonorrhoeae trafficking across polarized T84 epithelial monolayers. Infect. Immun. 68:906-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Howard, E. A., J. R. Zupan, V. Citovsky, and P. C. Zambryski. 1992. The VirD2 protein of A. tumefaciens contains a C-terminal bipartite nuclear localization signal: implications for nuclear uptake of DNA in plant cells. Cell 68:109-118. [DOI] [PubMed] [Google Scholar]
- 213.Hsia, R. C., Y. Pannekoek, E. Ingerowski, and P. M. Bavoil. 1997. Type III secretion genes identify a putative virulence locus of Chlamydia. Mol. Microbiol. 25:351-359. [DOI] [PubMed] [Google Scholar]
- 214.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ilver, D., A. Arnqvist, J. Ogren, I. M. Frick, D. Kersulyte, E. T. Incecik, D. E. Berg, A. Covacci, L. Engstrand, and T. Boren. 1998. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 279:373-377. [DOI] [PubMed] [Google Scholar]
- 216.Ito, Y., T. Azuma, S. Ito, H. Miyaji, M. Hirai, Y. Yamazaki, F. Sato, T. Kato, Y. Kohli, and M. Kuriyama. 1997. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J. Clin. Microbiol. 35:1710-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Iwamoto, H., D. M. Czajkowsky, T. L. Cover, G. Szabo, and Z. Shao. 1999. VacA from Helicobacter pylori: a hexameric chloride channel. FEBS Lett. 450:101-104. [DOI] [PubMed] [Google Scholar]
- 218.Jacob-Dubuisson, F., R. Antoine, and C. Locht. 2000. Autotransporter proteins, evolution and redefining protein secretion: response. Trends Microbiol. 8:533-534. [DOI] [PubMed] [Google Scholar]
- 219.Jacob-Dubuisson, F., C. Buisine, E. Willery, G. Renauld-Mongenie, and C. Locht. 1997. Lack of functional complementation between Bordetella pertussis filamentous hemagglutinin and Proteus mirabilis HpmA hemolysin secretion machineries. J. Bacteriol. 179:775-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Jacob-Dubuisson, F., C. El-Hamel, N. Saint, S. Guedin, E. Willery, G. Molle, and C. Locht. 1999. Channel formation by FhaC, the outer membrane protein involved in the secretion of the Bordetella pertussis filamentous hemagglutinin. J. Biol. Chem. 274:37731-37735. [DOI] [PubMed] [Google Scholar]
- 221.Jacob-Dubuisson, F., C. Locht, and R. Antoine. 2001. Two-partner secretion in Gram-negative bacteria: a thrifty, specific pathway for large virulence proteins. Mol. Microbiol. 40:306-313. [DOI] [PubMed] [Google Scholar]
- 222.Jaeger, K. E., S. Ransac, B. W. Dijkstra, C. Colson, M. van Heuvel, and O. Misset. 1994. Bacterial lipases. FEMS Microbiol. Rev. 15:29-63. [DOI] [PubMed] [Google Scholar]
- 223.Jahnig, F. 1990. Structure predictions of membrane proteins are not that bad. Trends Biochem. Sci. 15:93-95. [DOI] [PubMed] [Google Scholar]
- 224.Jarchau, T., T. Chakraborty, F. Garcia, and W. Goebel. 1994. Selection for transport competence of C-terminal polypeptides derived from Escherichia coli hemolysin: the shortest peptide capable of autonomous HlyB/HlyD-dependent secretion comprises the C-terminal 62 amino acids of HlyA. Mol. Gen. Genet. 245:53-60. [DOI] [PubMed] [Google Scholar]
- 225.Jarvis, G. A., and J. M. Griffiss. 1991. Human IgA1 blockade of IgG-initiated lysis of Neisseria meningitidis is a function of antigen-binding fragment binding to the polysaccharide capsule. J. Immunol. 147:1962-1967. [PubMed] [Google Scholar]
- 226.Jarvis, K. G., J. A. Giron, A. E. Jerse, T. K. McDaniel, M. S. Donnenberg, and J. B. Kaper. 1995. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 92:7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jenkins, J., and R. Pickersgill. 2001. The architecture of parallel beta-helices and related folds. Prog. Biophys. Mol. Biol. 77:111-175. [DOI] [PubMed] [Google Scholar]
- 228.Johannsen, D. B., D. M. Johnston, H. O. Koymen, M. S. Cohen, and J. G. Cannon. 1999. A Neisseria gonorrhoeae immunoglobulin A1 protease mutant is infectious in the human challenge model of urethral infection. Infect. Immun. 67:3009-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Jose, J., R. Bernhardt, and F. Hannemann. 2002. Cellular surface display of dimeric Adx and whole cell P450-mediated steroid synthesis on E. coli. J. Biotechnol. 95:257-268. [DOI] [PubMed] [Google Scholar]
- 230.Jose, J., R. Bernhardt, and F. Hannemann. 2001. Functional display of active bovine adrenodoxin on the surface of E. coli by chemical incorporation of the [2Fe-2S] cluster. Chembiochem 2:695-701. [DOI] [PubMed] [Google Scholar]
- 231.Jose, J., F. Jahnig, and T. F. Meyer. 1995. Common structural features of IgA1 protease-like outer membrane protein autotransporters. Mol. Microbiol. 18:378-380. [DOI] [PubMed] [Google Scholar]
- 232.Jose, J., J. Kramer, T. Klauser, J. Pohlner, and T. F. Meyer. 1996. Absence of periplasmic DsbA oxidoreductase facilitates export of cysteine-containing passenger proteins to the Escherichia coli cell surface via the Iga beta autotransporter pathway. Gene 178:107-110. [DOI] [PubMed] [Google Scholar]
- 233.Kajava, A. V., N. Cheng, R. Cleaver, M. Kessel, M. N. Simon, E. Willery, F. Jacob-Dubuisson, C. Locht, and A. C. Steven. 2001. Beta-helix model for the filamentous haemagglutinin adhesin of Bordetella pertussis and related bacterial secretory proteins. Mol. Microbiol. 42:279-292. [DOI] [PubMed] [Google Scholar]
- 234.Kalman, S., W. Mitchell, R. Marathe, C. Lammel, J. Fan, R. W. Hyman, L. Olinger, J. Grimwood, R. W. Davis, and R. S. Stephens. 1999. Comparative genomes of Chlamydia pneumoniae and C. trachomatis. Nat. Genet. 21:385-389. [DOI] [PubMed] [Google Scholar]
- 235.Kang, H. W., I. G. Wirawan, and M. Kojima. 1992. Isolation and genetic analysis of an Agrobacterium tumefaciens avirulent mutant with a chromosomal mutation produced by transposon mutagenesis. Biosci. Biotechnol. Biochem. 56:1924-1928. [DOI] [PubMed] [Google Scholar]
- 236.Kawai, E., A. Idei, H. Kumura, K. Shimazaki, H. Akatsuka, and K. Omori. 1999. The ABC-exporter genes involved in the lipase secretion are clustered with the genes for lipase, alkaline protease, and serine protease homologues in Pseudomonas fluorescens no. 33. Biochim. Biophys. Acta 1446:377-382. [DOI] [PubMed] [Google Scholar]
- 237.Kenny, B., and B. B. Finlay. 1995. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc. Natl. Acad. Sci. USA 92:7991-7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kerr, J. R., and R. C. Matthews. 2000. Bordetella pertussis infection: pathogenesis, diagnosis, management, and the role of protective immunity. Eur. J. Clin. Microbiol. Infect. Dis. 19:77-88. [DOI] [PubMed] [Google Scholar]
- 239.Kidd, M., A. J. Lastovica, J. C. Atherton, and J. A. Louw. 1999. Heterogeneity in the Helicobacter pylori vacA and cagA genes: association with gastroduodenal disease in South Africa? Gut 45:499-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kilian, M., J. Mestecky, R. Kulhavy, M. Tomana, and W. T. Butler. 1980. IgA1 proteases from Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Streptococcus sanguis: comparative immunochemical studies. J. Immunol. 124:2596-2600. [PubMed] [Google Scholar]
- 241.Kilian, M., J. Mestecky, and M. W. Russell. 1988. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol. Rev. 52:296-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kilian, M., J. Mestecky, and R. E. Schrohenloher. 1979. Pathogenic species of the genus Haemophilus and Streptococcus pneumoniae produce immunoglobulin A1 protease. Infect. Immun. 26:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kilian, M., and B. Thomsen. 1983. Antigenic heterogeneity of immunoglobulin A1 proteases from encapsulated and non-encapsulated Haemophilus influenzae. Infect. Immun. 42:126-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kilian, M., B. Thomsen, T. E. Petersen, and H. Bleeg. 1983. Molecular biology of Haemophilus influenzae IgA1 proteases. Mol. Immunol. 20:1051-1058. [DOI] [PubMed] [Google Scholar]
- 245.Kim, J., and D. A. Kendall. 2000. Sec-dependent protein export and the involvement of the molecular chaperone SecB. Cell Stress Chaperones 5:267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.King, A. J., G. Berbers, H. F. van Oirschot, P. Hoogerhout, K. Knipping, and F. R. Mooi. 2001: Role of the polymorphic region 1 of the Bordetella pertussis protein pertactin in immunity. Microbiology 147:2885-2895. [DOI] [PubMed] [Google Scholar]
- 247.Kingsley, R. A., A. D. Humphries, E. H. Weening, M. R. De Zoete, S. Winter, A. Papaconstantinopoulou, G. Dougan, and A. J. Baumler. 2003. Molecular and phenotypic analysis of the CS54 island of Salmonella enterica serotype Typhimurium: identification of intestinal colonization and persistence determinants. Infect. Immun. 71:629-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kingsley, R. A., R. L. Santos, A. M. Keestra, L. G. Adams, and A. J. Baumler. 2002. Salmonella enterica serotype Typhimurium ShdA is an outer membrane fibronectin-binding protein that is expressed in the intestine. Mol. Microbiol. 43:895-905. [DOI] [PubMed] [Google Scholar]
- 249.Kingsley, R. A., K. van Amsterdam, N. Kramer, and A. J. Baumler. 2000. The shdA gene is restricted to serotypes of Salmonella enterica subspecies I and contributes to efficient and prolonged fecal shedding. Infect. Immun. 68:2720-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kjaergaard, K., M. A. Schembri, H. Hasman, and P. Klemm. 2000. Antigen 43 from Escherichia coli induces inter- and intraspecies cell aggregation and changes in colony morphology of Pseudomonas fluorescens. J. Bacteriol. 182:4789-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kjaergaard, K., M. A. Schembri, C. Ramos, S. Molin, and P. Klemm. 2000. Antigen 43 facilitates formation of multispecies biofilms. Environ. Microbiol. 2:695-702. [DOI] [PubMed] [Google Scholar]
- 252.Klauser, T., J. Kramer, K. Otzelberger, J. Pohlner, and T. F. Meyer. 1993. Characterization of the Neisseria Iga beta-core. The essential unit for outer membrane targeting and extracellular protein secretion. J. Mol. Biol. 234:579-593. [DOI] [PubMed] [Google Scholar]
- 253.Klauser, T., J. Pohlner, and T. F. Meyer. 1990. Extracellular transport of cholera toxin B subunit using Neisseria IgA protease beta-domain: conformation-dependent outer membrane translocation. EMBO J. 9:1991-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Klauser, T., J. Pohlner, and T. F. Meyer. 1992. Selective extracellular release of cholera toxin B subunit by Escherichia coli: dissection of Neisseria Iga beta-mediated outer membrane transport. EMBO J. 11:2327-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kocks, C., J. B. Marchand, E. Gouin, H. d'Hauteville, P. J. Sansonetti, M. F. Carlier, and P. Cossart. 1995. The unrelated surface proteins ActA of Listeria monocytogenes and IcsA of Shigella flexneri are sufficient to confer actin-based motility on Listeria innocua and Escherichia coli respectively. Mol. Microbiol. 18:413-423. [DOI] [PubMed] [Google Scholar]
- 257.Koebnik, R., K. P. Locher, and P. Van Gelder. 2000. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol. Microbiol. 37:239-253. [DOI] [PubMed] [Google Scholar]
- 258.Konieczny, M. P., M. Suhr, A. Noll, I. B. Autenrieth, and M. A. Schmidt. 2000. Cell surface presentation of recombinant (poly-) peptides including functional T-cell epitopes by the AIDA autotransporter system. FEMS Immunol. Med. Microbiol. 27:321-332. [DOI] [PubMed] [Google Scholar]
- 259.Konninger, U. W., S. Hobbie, R. Benz, and V. Braun. 1999. The haemolysin-secreting ShlB protein of the outer membrane of Serratia marcescens: determination of surface-exposed residues and formation of ion-permeable pores by Sh1B mutants in artificial lipid bilayer membranes. Mol. Microbiol. 32:1212-1225. [DOI] [PubMed] [Google Scholar]
- 260.Kornfeld, S. J., and A. G. Plaut. 1981. Secretory immunity and the bacterial IgA proteases. Rev. Infect. Dis. 3:521-534. [DOI] [PubMed] [Google Scholar]
- 261.Koronakis, E., C. Hughes, I. Milisav, and V. Koronakis. 1995. Protein exporter function and in vitro ATPase activity are correlated in ABC-domain mutants of HlyB. Mol. Microbiol. 16:87-96. [DOI] [PubMed] [Google Scholar]
- 262.Koronakis, V., C. Hughes, and E. Koronakis. 1991. Energetically distinct early and late stages of HlyB/HlyD-dependent secretion across both Escherichia coli membranes. EMBO J. 10:3263-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Koronakis, V., A. Sharff, E. Koronakis, B. Luisi, and C. Hughes. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405:914-919. [DOI] [PubMed] [Google Scholar]
- 264.Koster, M., W. Bitter, and J. Tommassen. 2000. Protein secretion mechanisms in Gram-negative bacteria. Int. J. Med. Microbiol. 290:325-331. [DOI] [PubMed] [Google Scholar]
- 265.Kotloff, K. L., F. Noriega, G. A. Losonsky, M. B. Sztein, S. S. Wasserman, J. P. Nataro, and M. M. Levine. 1996. Safety, immunogenicity, and transmissibility in humans of CVD 1203, a live oral Shigella flexneri 2a vaccine candidate attenuated by deletions in aroA and virG. Infect. Immun. 64:4542-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Koukolikova-Nicola, Z., and B. Hohn. 1993. How does the T-DNA of Agrobacterium tumefaciens find its way into the plant cell nucleus? Biochimie 75:635-638. [DOI] [PubMed] [Google Scholar]
- 267.Kramer, U., K. Rizos, H. Apfel, I. B. Autenrieth, and C. T. Lattemann. 2003. Autodisplay: development of an efficacious system for surface display of antigenic determinants in Salmonella vaccine strains. Infect. Immun. 71:1944-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Krem, M. M., and E. Di Cera. 2001. Molecular markers of serine protease evolution. EMBO J. 20:3036-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galan, and S. I. Aizawa. 1998. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280:602-605. [DOI] [PubMed] [Google Scholar]
- 270.Kuo, C. H., and W. C. Wang. 2003. Binding and internalization of Helicobacter pylori VacA via cellular lipid rafts in epithelial cells. Biochem. Biophys. Res. Commun. 303:640-644. [DOI] [PubMed] [Google Scholar]
- 271.Laarmann, S., and M. A. Schmidt. 2003. The Escherichia coli AIDA autotransporter adhesin recognizes an integral membrane glycoprotein as receptor. Microbiology 149:1871-1882. [DOI] [PubMed] [Google Scholar]
- 272.Ladant, D., and A. Ullmann. 1999. Bordetella pertussis adenylate cyclase: a toxin with multiple talents. Trends Microbiol. 7:172-176. [DOI] [PubMed] [Google Scholar]
- 273.Lafontaine, E. R., L. D. Cope, C. Aebi, J. L. Latimer, G. H. McCracken, Jr., and E. J. Hansen. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 182:1364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lai, E. M., and C. I. Kado. 2000. The T-pilus of Agrobacterium tumefaciens. Trends Microbiol. 8:361-369. [DOI] [PubMed] [Google Scholar]
- 275.Lambert-Buisine, C., E. Willery, C. Locht, and F. Jacob-Dubuisson. 1998. N-terminal characterization of the Bordetella pertussis filamentous haemagglutinin. Mol. Microbiol. 28:1283-1293. [DOI] [PubMed] [Google Scholar]
- 276.Lattemann, C. T., J. Maurer, E. Gerland, and T. F. Meyer. 2000. Autodisplay: functional display of active beta-lactamase on the surface of Escherichia coli by the AIDA-I autotransporter. J. Bacteriol. 182:3726-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lawley, T. D., W. A. Klimke, M. J. Gubbins, and L. S. Frost. 2003. F factor conjugation is a true type IV secretion system. FEMS Microbiol. Lett. 224:1-15. [DOI] [PubMed] [Google Scholar]
- 278.Leininger, E., C. A. Ewanowich, A. Bhargava, M. S. Peppler, J. G. Kenimer, and M. J. Brennan. 1992. Comparative roles of the Arg-Gly-Asp sequence present in the Bordetella pertussis adhesins pertactin and filamentous hemagglutinin. Infect. Immun. 60:2380-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Leininger, E., M. Roberts, J. G. Kenimer, I. G. Charles, N. Fairweather, P. Novotny, and M. J. Brennan. 1991. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc. Natl. Acad. Sci. USA 88:345-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Letley, D. P., and J. C. Atherton. 2000. Natural diversity in the N terminus of the mature vacuolating cytotoxin of Helicobacter pylori determines cytotoxin activity. J. Bacteriol. 182:3278-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Letley, D. P., A. Lastovica, J. A. Louw, C. J. Hawkey, and J. C. Atherton. 1999. Allelic diversity of the Helicobacter pylori vacuolating cytotoxin gene in South Africa: rarity of the vacA sla genotype and natural occurrence of an s2/ml allele. J. Clin. Microbiol. 37:1203-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Letoffe, S., P. Delepelaire, and C. Wandersman. 1996. Protein secretion in gram-negative bacteria: assembly of the three components of ABC protein-mediated exporters is ordered and promoted by substrate binding. EMBO J. 15:5804-5811. [PMC free article] [PubMed] [Google Scholar]
- 283.Lett, M. C., C. Sasakawa, N. Okada, T. Sakai, S. Makino, M. Yamada, K. Komatsu, and M. Yoshikawa. 1989. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the virG protein and determination of the complete coding sequence. J. Bacteriol. 171:353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Leyton, D. L., J. Sloan, R. E. Hill, S. Doughty, and E. L. Hartland. 2003. Transfer region of pO113 from enterohemorrhagic Escherichia coli: similarity with R64 and identification of a novel plasmid-encoded autotransporter, EpeA. Infect. Immun. 71:6307-6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Li, H., I. Kalies, B. Mellgard, and H. F. Helander. 1998. A rat model of chronic Helicobacter pylori infection. Studies of epithelial cell turnover and gastric ulcer healing. Scand. J. Gastroenterol. 33:370-378. [DOI] [PubMed] [Google Scholar]
- 286.Li, H., and D. H. Walker. 1998. rOmpA is a critical protein for the adhesion of Rickettsia rickettsii to host cells. Microb. Pathog. 24:289-298. [DOI] [PubMed] [Google Scholar]
- 287.Li, L. J., G. Dougan, P. Novotny, and I. G. Charles. 1991. P. 70 pertactin, an outer-membrane protein from Bordetella parapertussis: cloning, nucleotide sequence and surface expression in Escherichia coli. Mol. Microbiol. 5:409-417. [DOI] [PubMed] [Google Scholar]
- 288.Lin, L., P. Ayala, J. Larson, M. Mulks, M. Fukuda, S. R. Carlsson, C. Enns, and M. So. 1997. The Neisseria type 2 IgA1 protease cleaves LAMP1 and promotes survival of bacteria within epithelial cells. Mol. Microbiol. 24:1083-1094. [DOI] [PubMed] [Google Scholar]
- 289.Lin, W., K. J. Fullner, R. Clayton, J. A. Sexton, M. B. Rogers, K. E. Calia, S. B. Calderwood, C. Fraser, and J. J. Mekalanos. 1999. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc. Natl. Acad. Sci. USA 96:1071-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lindeberg, M., G. P. Salmond, and A. Collmer. 1996. Complementation of deletion mutations in a cloned functional cluster of Erwinia chrysanthemi out genes with Erwinia carotovora out homologues reveals OutC and OutD as candidate gatekeepers of species-specific secretion of proteins via the type II pathway. Mol. Microbiol. 20:175-190. [DOI] [PubMed] [Google Scholar]
- 291.Lindenthal, C., and E. A. Elsinghorst. 2001. Enterotoxigenic Escherichia coli TibA glycoprotein adheres to human intestine epithelial cells. Infect. Immun. 69:52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lindenthal, C., and E. A. Elsinghorst. 1999. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect. Immun. 67:4084-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Liu, M., R. Deora, S. R. Doulatov, M. Gingery, F. A. Eiserling, A. Preston, D. J. Maskell, R. W. Simons, P. A. Cotter, J. Parkhill, and J. F. Miller. 2002. Reverse transcriptase-mediated tropism switching in Bordetella bacteriophage. Science 295:2091-2094. [DOI] [PubMed] [Google Scholar]
- 294.Lloyd, S. A., A. Forsberg, H. Wolf-Watz, and M. S. Francis. 2001. Targeting exported substrates to the Yersinia TTSS: different functions for different signals? Trends Microbiol. 9:367-371. [DOI] [PubMed] [Google Scholar]
- 295.Lloyd, S. A., M. Norman, R. Rosqvist, and H. Wolf-Watz. 2001. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol. Microbiol. 39:520-531. [DOI] [PubMed] [Google Scholar]
- 296.Lo, R. Y. 2001. Genetic analysis of virulence factors of Mannheimia (Pasteurella) haemolytica A1. Vet. Microbiol. 83:23-35. [DOI] [PubMed] [Google Scholar]
- 297.Lo, R. Y., C. A. Strathdee, P. E. Shewen, and B. J. Cooney. 1991. Molecular studies of Ssal, a serotype-specific antigen of Pasteurella haemolytica A1. Infect. Immun. 59:3398-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Locht, C., R. Antoine, and F. Jacob-Dubuisson. 2001. Bordetella pertussis, molecular pathogenesis under multiple aspects. Curr. Opin. Microbiol. 4:82-89. [DOI] [PubMed] [Google Scholar]
- 299.Lomholt, H., K. Poulsen, D. A. Caugant, and M. Kilian. 1992. Molecular polymorphism and epidemiology of Neisseria meningitidis immunoglobulin A1 proteases. Proc. Natl. Acad. Sci. USA 89:2120-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lomholt, H., K. Poulsen, and M. Kilian. 1995. Comparative characterization of the iga gene encoding IgA1 protease in Neisseria meningitidis, Neisseria gonorrhoeae and Haemophilus influenzae. Mol. Microbiol. 15:495-506. [DOI] [PubMed] [Google Scholar]
- 301.Lomholt, H., L. van Alphen, and M. Kilian. 1993. Antigenic variation of immunoglobulin A1 proteases among sequential isolates of Haemophilus influenzae from healthy children and patients with chronic obstructive pulmonary disease. Infect. Immun. 61:4575-4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lorenzen, D. R., F. Dux, U. Wolk, A. Tsirpouchtsidis, G. Haas, and T. F. Meyer. 1999. Immunoglobulin A1 protease, an exoenzyme of pathogenic neisseriae, is a potent inducer of proinflammatory cytokines. J. Exp. Med. 190:1049-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Loveless, B. J., and M. H. Saier, Jr. 1997. A novel family of channel-forming, autotransporting, bacterial virulence factors. Mol. Membr. Biol. 14:113-123. [DOI] [PubMed] [Google Scholar]
- 304.Lupetti, P., J. E. Heuser, R. Manetti, P. Massari, S. Lanzavecchia, P. L. Bellon, R. Dallai, R. Rappuoli, and J. L. Telford. 1996. Oligomeric and subunit structure of the Helicobacter pylori vacuolating cytotoxin. J. Cell Biol. 133:801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ma, B., C. J. Tsai, and R. Nussinov. 2000. Binding and folding: in search of intramolecular chaperone-like building block fragments. Protein Eng. 13:617-627. [DOI] [PubMed] [Google Scholar]
- 306.Ma, Q., Y. Zhai, J. C. Schneider, T. M. Ramseier, and M. H. Saier. 2003. Protein secretion systems of Pseudomonas aeruginosa and P. fluorescens. Biochim. Biophys. Acta 1611:223-233. [DOI] [PubMed] [Google Scholar]
- 307.Macnab, R. M. 1999. The bacterial flagellum: reversible rotary propellor and type III export apparatus. J. Bacteriol. 181:7149-7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Mahdavi, J., B. Sonden, M. Hurtig, F. O. Olfat, L. Forsberg, N. Roche, J. Angstrom, T. Larsson, S. Teneberg, K. A. Karlsson, S. Altraja, T. Wadstrom, D. Kersulyte, D. E. Berg, A. Dubois, C. Petersson, K. E. Magnusson, T. Norberg, F. Lindh, B. B. Lundskog, A. Arnqvist, L. Hammarstrom, and T. Boren. 2002. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Makino, S., C. Sasakawa, K. Kamata, T. Kurata, and M. Yoshikawa. 1986. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell 46:551-555. [DOI] [PubMed] [Google Scholar]
- 310.Malaisse, W. J., M. Svoboda, S. P. Dufrane, F. Malaisse-Lagae, and J. Christophe. 1984. Effect of Bordetella pertussis toxin on ADP-ribosylation of membrane proteins, adenylate cyclase activity and insulin release in rat pancreatic islets. Biochem. Biophys. Res. Commun. 124:190-196. [DOI] [PubMed] [Google Scholar]
- 311.Manjarrez-Hernandez, H. A., S. Gavilanes-Parra, E. Chavez-Berrocal, A. Navarro-Ocana, and A. Cravioto. 2000. Antigen detection in enteropathogenic Escherichia coli using secretory immunoglobulin A antibodies isolated from human breast milk. Infect. Immun. 68:5030-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Mansa, B., and M. Kilian. 1986. Retained antigen-binding activity of Fab alpha fragments of human monoclonal immunoglobulin A1 (IgA1) cleaved by IgA1 protease. Infect. Immun. 52:171-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Marais, A., G. L. Mendz, S. L. Hazell, and F. Megraud. 1999. Metabolism and genetics of Helicobacter pylori: the genome era. Microbiol. Mol. Biol. Rev. 63:642-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Marchler-Bauer, A., J. B. Anderson, C. DeWeese-Scott, N. D. Fedorova, L. Y. Geer, S. He, D. I. Hurwitz, J. D. Jackson, A. R. Jacobs, C. J. Lanczycki, C. A. Liebert, C. Liu, T. Madej, G. H. Marchler, R. Mazumder, A. N. Nikolskaya, A. R. Panchenko, B. S. Rao, B. A. Shoemaker, V. Simonyan, J. S. Song, P. A. Thiessen, S. Vasudevan, Y. Wang, R. A. Yamashita, J. J. Yin, and S. H. Bryant. 2003. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 31:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Martoglio, B., and B. Dobberstein. 1998. Signal sequences: more than just greasy peptides. Trends Cell Biol. 8:410-415. [DOI] [PubMed] [Google Scholar]
- 316.Maurer, J., J. Jose, and T. F. Meyer. 1997. Autodisplay: one-component system for efficient surface display and release of soluble recombinant proteins from Escherichia coli. J. Bacteriol. 179:794-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Maurer, J., J. Jose, and T. F. Meyer. 1999. Characterization of the essential transport function of the AIDA-I autotransporter and evidence supporting structural predictions. J. Bacteriol. 181:7014-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Maurer, J. J., T. P. Brown, W. L. Steffens, and S. G. Thayer. 1998. The occurrence of ambient temperature-regulated adhesins, curli, and the temperature-sensitive hemagglutinin tsh among avian Escherichia coli. Avian Dis. 42:106-118. [PubMed] [Google Scholar]
- 319.Mazmanian, S. K., H. Ton-That, and O. Schneewind. 2001. Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol. Microbiol. 40:1049-1057. [DOI] [PubMed] [Google Scholar]
- 320.McClain, M. S., P. Cao, and T. L. Cover. 2001. Amino-terminal hydrophobic region of Helicobacter pylori vacuolating cytotoxin (VacA) mediates transmembrane protein dimerization. Infect. Immun. 69:1181-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.McClain, M. S., W. Schraw, V. Ricci, P. Boquet, and T. L. Cover. 2000. Acid activation of Helicobacter pylori vacuolating cytotoxin (VacA) results in toxin internalization by eukaryotic cells. Mol. Microbiol. 37:433-442. [DOI] [PubMed] [Google Scholar]
- 322.McDonald, G. A., R. L. Anacker, and K. Garjian. 1987. Cloned gene of Rickettsia rickettsii surface antigen: candidate vaccine for Rocky Mountain spotted fever. Science 235:83-85. [DOI] [PubMed] [Google Scholar]
- 323.Mehta, S. K., A. G. Plaut, N. J. Calvanico, and T. B. Tomasi, Jr. 1973. Human immunoglobulin A: production of an Fc fragment by an enteric microbial proteolytic enzyme. J. Immunol. 111:1274-1276. [PubMed] [Google Scholar]
- 324.Mellies, J. L., F. Navarro-Garcia, I. Okeke, J. Frederickson, J. P. Nataro, and J. B. Kaper. 2001. espC pathogenicity island of enteropathogenic Escherichia coli encodes an enterotoxin. Infect. Immun. 69:315-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Messner, P. 1997. Bacterial glycoproteins. Glycoconj. J. 14:3-11. [DOI] [PubMed] [Google Scholar]
- 326.Michiels, T., P. Wattiau, R. Brasseur, J. M. Ruysschaert, and G. Cornelis. 1990. Secretion of Yop proteins by yersiniae. Infect. Immun. 58:2840-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Miller, C. G., C. Heiman, and C. Yen. 1976. Mutants of Salmonella typhimurium deficient in an endoprotease. J. Bacteriol. 127:490-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Miyazaki, H., N. Yanagida, S. Horinouchi, and T. Beppu. 1989. Characterization of the precursor of Serratia marcescens serine protease and COOH-terminal processing of the precursor during its excretion through the outer membrane of Escherichia coli. J. Bacteriol. 171:6566-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Mizan, S., A. Henk, A. Stallings, M. Maier, and M. D. Lee. 2000. Cloning and characterization of sialidases with 2-6′ and 2-3′ sialyl lactose specificity from Pasteurella multocida. J. Bacteriol. 182:6874-6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Mizushima, T., T. Sugiyama, Y. Komatsu, J. Ishizuka, M. Kato, and M. Asaka. 2001. Clinical relevance of the babA2 genotype of Helicobacter pylori in Japanese clinical isolates. J. Clin. Microbiol. 39:2463-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Moens, S., and J. Vanderleyden. 1997. Glycoproteins in prokaryotes. Arch. Microbiol. 168:169-175. [DOI] [PubMed] [Google Scholar]
- 332.Molinari, M., C. Galli, M. de Bernard, N. Norais, J. M. Ruysschaert, R. Rappuoli, and C. Montecucco. 1998. The acid activation of Helicobacter pylori toxin VacA: structural and membrane binding studies. Biochem. Biophys. Res. Commun. 248:334-340. [DOI] [PubMed] [Google Scholar]
- 333.Molinari, M., C. Galli, N. Norais, J. L. Telford, R. Rappuoli, J. P. Luzio, and C. Montecucco. 1997. Vacuoles induced by Helicobacter pylori toxin contain both late endosomal and lysosomal markers. J. Biol. Chem. 272:25339-25344. [DOI] [PubMed] [Google Scholar]
- 334.Molinari, M., M. Salio, C. Galli, N. Norais, R. Rappuoli, A. Lanzavecchia, and C. Montecucco. 1998. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J. Exp. Med. 187:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Montecucco, C., and M. de Bernard. 2003. Molecular and cellular mechanisms of action of the vacuolating cytotoxin (VacA) and neutrophil-activating protein (HP-NAP) virulence factors of Helicobacter pylori. Microbes Infect. 5:715-721. [DOI] [PubMed] [Google Scholar]
- 336.Mooi, F. R., H. van Oirschot, K. Heuvelman, H. G. van der Heide, W. Gaastra, and R. J. Willems. 1998. Polymorphism in the Bordetella pertussis virulence factors P. 69/pertactin and pertussis toxin in The Netherlands: temporal trends and evidence for vaccine-driven evolution. Infect. Immun. 66:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Moormann, C., I. Benz, and M. A. Schmidt. 2002. Functional substitution of the TibC protein of enterotoxigenic Escherichia coli strains for the autotransporter adhesin heptosyltransferase of the AIDA system. Infect. Immun. 70:2264-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Mori, H., and K. Ito. 2001. The Sec protein-translocation pathway. Trends Microbiol. 9:494-500. [DOI] [PubMed] [Google Scholar]
- 339.Morona, R., and L. Van Den Bosch. 2003. Lipopolysaccharide O antigen chains mask IcsA (VirG) in Shigella flexneri. FEMS Microbiol. Lett. 221:173-180. [DOI] [PubMed] [Google Scholar]
- 340.Mortensen, S. B., and M. Kilian. 1984. A rapid method for the detection and quantitation of IgA protease activity by macrobore gel-permeation chromatography. J. Chromatogr. 296:257-262. [DOI] [PubMed] [Google Scholar]
- 341.Moss, S. F., and S. Sood. 2003. Helicobacter pylori. Curr. Opin. Infect. Dis. 16:445-451. [DOI] [PubMed] [Google Scholar]
- 342.Mulks, M. H., and A. G. Plaut. 1978. IgA protease production as a characteristic distinguishing pathogenic from harmless neisseriaceae. N. Engl. J. Med. 299:973-976. [DOI] [PubMed] [Google Scholar]
- 343.Mulks, M. H., A. G. Plaut, H. A. Feldman, and B. Frangione. 1980. IgA proteases of two distinct specificities are released by Neisseria meningitidis. J. Exp. Med. 152:1442-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Mundy, R., L. Petrovska, K. Smollett, N. Simpson, R. K. Wilson, J. Yu, X. Tu, I. Rosenshine, S. Clare, G. Dougan, and G. Frankel. 2004. Identification of a novel Citrobacter rodentium type III secreted protein, EspI, and roles of this and other secreted proteins in infection. Infect. Immun. 72:2288-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Narayanan, S. K., T. G. Nagaraja, M. M. Chengappa, and G. C. Stewart. 2002. Leukotoxins of gram-negative bacteria. Vet. Microbiol. 84:337-356. [DOI] [PubMed] [Google Scholar]
- 346.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Navarro-Garcia, F., A. Canizalez-Roman, J. Luna, C. Sears, and J. P. Nataro. 2001. Plasmid-encoded toxin of enteroaggregative Escherichia coli is internalized by epithelial cells. Infect. Immun. 69:1053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Navarro-Garcia, F., A. Canizalez-Roman, B. Q. Sui, J. P. Nataro, and Y. Azamar. 2004. The serine protease motif of EspC from enteropathogenic Escherichia coli produces epithelial damage by a mechanism different from that of Pet toxin from enteroaggregative E. coli. Infect. Immun. 72:3609-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Navarro-Garcia, F., C. Eslava, J. M. Villaseca, R. Lopez-Revilla, J. R. Czeczulin, S. Srinivas, J. P. Nataro, and A. Cravioto. 1998. In vitro effects of a high-molecular-weight heat-labile enterotoxin from enteroaggregative Escherichia coli. Infect. Immun. 66:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Navarro-Garcia, F., C. Sears, C. Eslava, A. Cravioto, and J. P. Nataro. 1999. Cytoskeletal effects induced by Pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect. Immun. 67:2184-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Nguyen, V. Q., R. M. Caprioli, and T. L. Cover. 2001. Carboxy-terminal proteolytic processing of Helicobacter pylori vacuolating toxin. Infect. Immun. 69:543-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Nielsen, H., S. Brunak, and G. von Heijne. 1999. Machine learning approaches for the prediction of signal peptides and other protein sorting signals. Protein Eng. 12:3-9. [DOI] [PubMed] [Google Scholar]
- 353.Niessner, A., C. Kaun, G. Zorn, W. Speidl, Z. Turel, G. Christiansen, A. S. Pedersen, S. Birkelund, S. Simon, A. Georgopoulos, W. Graninger, R. de Martin, J. Lipp, B. R. Binder, G. Maurer, K. Huber, and J. Wojta. 2003. Polymorphic membrane protein (PMP) 20 and PMP 21 of Chlamydia pneumoniae induce proinflammatory mediators in human endothelial cells in vitro by activation of the nuclear factor-kappaB pathway. J. Infect. Dis. 188:108-113. [DOI] [PubMed] [Google Scholar]
- 354.Niewerth, U., A. Frey, T. Voss, C. Le Bouguenec, G. Baljer, S. Franke, and M. A. Schmidt. 2001. The AIDA autotransporter system is associated with F18 and stx2e in Escherichia coli isolates from pigs diagnosed with edema disease and postweaning diarrhea. Clin. Diagn. Lab. Immunol. 8:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Nikaido, H. 1994. Porins and specific diffusion channels in bacterial outer membranes. J. Biol. Chem. 269:3905-3908. [PubMed] [Google Scholar]
- 356.Nummelin, H., M. C. Merckel, J. C. Leo, H. Lankinen, M. Skurnik, and A. Goldman. 2004. The Yersinia adhesin YadA collagen-binding domain structure is a novel left-handed parallel beta-roll. EMBO J. 23:701-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Odenbreit, S., G. Faller, and R. Haas. 2002. Role of the AlpAB proteins and lipopolysaccharide in adhesion of Helicobacter pylori to human gastric tissue. Int. J. Med. Microbiol. 292:247-256. [DOI] [PubMed] [Google Scholar]
- 358.Odenbreit, S., M. Till, D. Hofreuter, G. Faller, and R. Haas. 1999. Genetic and functional characterization of the alpAB gene locus essential for the adhesion of Helicobacter pylori to human gastric tissue. Mol. Microbiol. 31:1537-1548. [DOI] [PubMed] [Google Scholar]
- 359.Ogura, K., S. Maeda, M. Nakao, T. Watanabe, M. Tada, T. Kyutoku, H. Yoshida, Y. Shiratori, and M. Omata. 2000. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J. Exp. Med. 192:1601-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Ohnishi, Y., T. Beppu, and S. Horinouchi. 1997. Two genes encoding serine protease homologues in Serratia marcescens and characterization of their products in Escherichia coli. J. Biochem. (Tokyo) 121:902-913. [DOI] [PubMed] [Google Scholar]
- 361.Ohnishi, Y., and S. Horinouchi. 1996. Extracellular production of a Serratia marcescens serine protease in Escherichia coli. Biosci. Biotechnol. Biochem. 60:1551-1558. [DOI] [PubMed] [Google Scholar]
- 362.Ohnishi, Y., M. Nishiyama, S. Horinouchi, and T. Beppu. 1994. Involvement of the COOH-terminal pro-sequence of Serratia marcescens serine protease in the folding of the mature enzyme. J. Biol. Chem. 269:32800-32806. [PubMed] [Google Scholar]
- 363.Oliver, D. C., and R. C. Fernandez. 2001. Antibodies to BrkA augment killing of Bordetella pertussis. Vaccine 20:235-241. [DOI] [PubMed] [Google Scholar]
- 364.Oliver, D. C., G. Huang, and R. C. Fernandez. 2003. Identification of secretion determinants of the Bordetella pertussis BrkA autotransporter. J. Bacteriol. 185:489-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Oliver, D. C., G. Huang, E. Nodel, S. Pleasance, and R. C. Fernandez. 2003. A conserved region within the Bordetella pertussis autotransporter BrkA is necessary for folding of its passenger domain. Mol. Microbiol. 47:1367-1383. [DOI] [PubMed] [Google Scholar]
- 366.Oomen, C. J., P. Van Ulsen, P. Van Gelder, M. Feijen, J. Tommassen, and P. Gros. 2004. Structure of the translocator domain of a bacterial autotransporter. EMBO J. 23:1257-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.O'Toole, P. W., J. W. Austin, and T. J. Trust. 1994. Identification and molecular characterization of a major ring-forming surface protein from the gastric pathogen Helicobacter mustelae. Mol. Microbiol. 11:349-361. [DOI] [PubMed] [Google Scholar]
- 368.Otto, B. R., S. J. van Dooren, C. M. Dozois, J. Luirink, and B. Oudega. 2002. Escherichia coli hemoglobin protease autotransporter contributes to synergistic abscess formation and heme-dependent growth of Bacteroides fragilis. Infect. Immun. 70:5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Otto, B. R., S. J. van Dooren, J. H. Nuijens, J. Luirink, and B. Oudega. 1998. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli strain EB1. J. Exp. Med. 188:1091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Owen, P., P. Caffrey, and L. G. Josefsson. 1987. Identification and partial characterization of a novel bipartite protein antigen associated with the outer membrane of Escherichia coli. J. Bacteriol. 169:3770-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Owen, P., and H. R. Kaback. 1979. Antigenic architecture of membrane vesicles from Escherichia coli. Biochemistry 18:1422-1426. [DOI] [PubMed] [Google Scholar]
- 372.Owen, P., and H. R. Kaback. 1978. Molecular structure of membrane vesicles from Escherichia coli. Proc. Natl. Acad. Sci. USA 75:3148-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Owen, P., M. Meehan, H. de Loughry-Doherty, and I. Henderson. 1996. Phase-variable outer membrane proteins in Escherichia coli. FEMS Immunol. Med. Microbiol. 16:63-76. [DOI] [PubMed] [Google Scholar]
- 374.Padilla, P. I., A. Wada, K. Yahiro, M. Kimura, T. Niidome, H. Aoyagi, A. Kumatori, M. Anami, T. Hayashi, J. Fujisawa, H. Saito, J. Moss, and T. Hirayama. 2000. Morphologic differentiation of HL-60 cells is associated with appearance of RPTPbeta and induction of Helicobacter pylori VacA sensitivity. J. Biol. Chem. 275:15200-15206. [DOI] [PubMed] [Google Scholar]
- 375.Pagliaccia, C., M. de Bernard, P. Lupetti, X. Ji, D. Burroni, T. L. Cover, E. Papini, R. Rappuoli, J. L. Telford, and J. M. Reyrat. 1998. The m2 form of the Helicobacter pylori cytotoxin has cell type-specific vacuolating activity. Proc. Natl. Acad. Sci. USA 95:10212-10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Pai, R., T. L. Cover, and A. S. Tarnawski. 1999. Helicobacter pylori vacuolating cytotoxin (VacA) disorganizes the cytoskeletal architecture of gastric epithelial cells. Biochem. Biophys. Res. Commun. 262:245-250. [DOI] [PubMed] [Google Scholar]
- 377.Pallen, M. J., R. R. Chaudhuri, and I. R. Henderson. 2003. Genomic analysis of secretion systems. Curr. Opin. Microbiol. 6:519-527. [DOI] [PubMed] [Google Scholar]
- 378.Palmen, R., A. J. Driessen, and K. J. Hellingwerf. 1994. Bioenergetic aspects of the translocation of macromolecules across bacterial membranes. Biochim. Biophys. Acta 1183:417-451. [DOI] [PubMed] [Google Scholar]
- 379.Pan, Z. J., D. E. Berg, R. W. van der Hulst, W. W. Su, A. Raudonikiene, S. D. Xiao, J. Dankert, G. N. Tytgat, and A. van der Ende. 1998. Prevalence of vacuolating cytotoxin production and distribution of distinct vacA alleles in Helicobacter pylori from China. J. Infect. Dis. 178:220-226. [DOI] [PubMed] [Google Scholar]
- 380.Papini, E., B. Satin, N. Norais, M. de Bernard, J. L. Telford, R. Rappuoli, and C. Montecucco. 1998. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J. Clin. Investig. 102:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Parham, N. J., U. Srinivasan, M. Desvaux, B. Foxman, C. F. Marrs, and I. R. Henderson. 2004. PicU, a second serine protease autotransporter of uropathogenic Escherichia coli. FEMS Microbiol. Lett. 230:73-83. [DOI] [PubMed] [Google Scholar]
- 382.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeno-Tarraga, L. Temple, K. James, B. Harris, M. A. Quail, M. Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35:32-40. [DOI] [PubMed] [Google Scholar]
- 383.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523-527. [DOI] [PubMed] [Google Scholar]
- 384.Parreira, V. R., and C. L. Gyles. 2003. A novel pathogenicity island integrated adjacent to the thrW tRNA gene of avian pathogenic Escherichia coli encodes a vacuolating autotransporter toxin. Infect. Immun. 71:5087-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Patel, S. K., J. Dotson, K. P. Allen, and J. M. Fleckenstein. 2004. Identification and molecular characterization of EatA, an autotransporter protein of enterotoxigenic Escherichia coli. Infect. Immun. 72:1786-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Pearson, M. M., E. R. Lafontaine, N. J. Wagner, J. W. St Geme III and E. J. Hansen. 2002. A hag mutant of Moraxella catarrhalis strain O35E is deficient in hemagglutination, autoagglutination, and immunoglobulin D-binding activities. Infect. Immun. 70:4523-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Pedersen, A. S., G. Christiansen, and S. Birkelund. 2001. Differential expression of Pmp10 in cell culture infected with Chlamydia pneumoniae CWL029. FEMS Microbiol. Lett. 203:153-159. [DOI] [PubMed] [Google Scholar]
- 388.Peek, R. M., Jr., M. J. Blaser, D. J. Mays, M. H. Forsyth, T. L. Cover, S. Y. Song, U. Krishna, and J. A. Pietenpol. 1999. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 59:6124-6131. [PubMed] [Google Scholar]
- 389.Peek, R. M., Jr., G. G. Miller, K. T. Tham, G. I. Perez-Perez, X. Zhao, J. C. Atherton, and M. J. Blaser. 1995. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab. Investig. 73:760-770. [PubMed] [Google Scholar]
- 390.Pepe, J. C., and S. Lory. 1998. Amino acid substitutions in PilD, a bifunctional enzyme of Pseudomonas aeruginosa: Effect on leader peptidase and N-methyltransferase activities in vitro and in vivo. J. Biol. Chem. 273:19120-19129. [DOI] [PubMed] [Google Scholar]
- 391.Peterson, J. H., C. A. Woolhead, and H. D. Bernstein. 2003. Basic amino acids in a distinct subset of signal peptides promote interaction with the signal recognition particle. J. Biol. Chem. 278:46155-46162. [DOI] [PubMed] [Google Scholar]
- 392.Pethe, K., M. Aumercier, E. Fort, C. Gatot, C. Locht, and F. D. Menozzi. 2000. Characterization of the heparin-binding site of the mycobacterial heparin-binding hemagglutinin adhesin. J. Biol. Chem. 275:14273-14280. [DOI] [PubMed] [Google Scholar]
- 393.Phadnis, S. H., D. Ilver, L. Janzon, S. Normark, and T. U. Westblom. 1994. Pathological significance and molecular characterization of the vacuolating toxin gene of Helicobacter pylori. Infect. Immun. 62:1557-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Arico, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 395.Plano, G. V., J. B. Day, and F. Ferracci. 2001. Type III export: new uses for an old pathway. Mol. Microbiol. 40:284-293. [DOI] [PubMed] [Google Scholar]
- 396.Plaut, A. G. 1983. The IgA1 proteases of pathogenic bacteria. Annu. Rev. Microbiol. 37:603-622. [DOI] [PubMed] [Google Scholar]
- 397.Plaut, A. G. 1978. Microbial IgA proteases. N. Engl. J. Med. 298:1459-1463. [DOI] [PubMed] [Google Scholar]
- 398.Plaut, A. G., J. V. Gilbert, M. S. Artenstein, and J. D. Capra. 1975. Neisseria gonorrhoeae and Neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science 190:1103-1105. [DOI] [PubMed] [Google Scholar]
- 399.Plaut, A. G., J. Qiu, F. Grundy, and A. Wright. 1992. Growth of Haemophilus influenzae in human milk: synthesis, distribution, and activity of IgA protease as determined by study of iga+ and mutant iga− cells. J. Infect. Dis. 166:43-52. [DOI] [PubMed] [Google Scholar]
- 400.Plaut, A. G., R. Wistar, Jr., and J. D. Capra. 1974. Differential susceptibility of human IgA immunoglobulins to streptococcal IgA protease. J. Clin. Investig. 54:1295-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Pohlner, J., R. Halter, K. Beyreuther, and T. F. Meyer. 1987. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 325:458-462. [DOI] [PubMed] [Google Scholar]
- 402.Pohlner, J., U. Langenberg, U. Wolk, S. C. Beck, and T. F. Meyer. 1995. Uptake and nuclear transport of Neisseria IgA1 protease-associated alpha-proteins in human cells. Mol. Microbiol. 17:1073-1083. [DOI] [PubMed] [Google Scholar]
- 403.Policastro, P. F., and T. Hackstadt. 1994. Differential activity of Rickettsia rickettsii opmA and ompB promoter regions in a heterologous reporter gene system. Microbiology 140:2941-2919. [DOI] [PubMed] [Google Scholar]
- 404.Possot, O. M., M. Gerard-Vincent, and A. P. Pugsley. 1999. Membrane association and multimerization of secreton component pulC. J. Bacteriol. 181:4004-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Possot, O. M., G. Vignon, N. Bomchil, F. Ebel, and A. P. Pugsley. 2000. Multiple interactions between pullulanase secretion components involved in stabilization and cytoplasmic membrane association of PulE. J. Bacteriol. 182:2142-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Poulsen, K., J. Reinholdt, C. Jespersgaard, K. Boye, T. A. Brown, M. Hauge, and M. Kilian. 1998. A comprehensive genetic study of streptococcal immunoglobulin A1 proteases: evidence for recombination within and between species. Infect. Immun. 66:181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Pride, D. T., R. J. Meinersmann, and M. J. Blaser. 2001. Allelic variation within Helicobacter pylori babA and babB. Infect. Immun. 69:1160-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Prinz, C., N. Hafsi, and P. Voland. 2003. Helicobacter pylori virulence factors and the host immune response: implications for therapeutic vaccination. Trends Microbiol. 11:134-138. [DOI] [PubMed] [Google Scholar]
- 409.Provence, D. L., and R. Curtiss III. 1994. Isolation and characterization of a gene involved in hemagglutination by an avian-pathogenic Escherichia coli strain. Infect. Immun. 62:1369-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Purdy, G. E., M. Hong, and S. M. Payne. 2002. Shigella flexneri DegP facilitates IcsA surface expression and is required for efficient intercellular spread. Infect. Immun. 70:6355-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Qiu, J., D. R. Hendrixson, E. N. Baker, T. F. Murphy, J. W. St Geme III, and A. G. Plaut. 1998. Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae. Proc. Natl. Acad. Sci. USA 95:12641-12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Rajakumar, K., C. Sasakawa, and B. Adler. 1997. Use of a novel approach, termed island probing, identifies the Shigella flexneri she pathogenicity island which encodes a homolog of the immunoglobulin A protease-like family of proteins. Infect. Immun. 65:4606-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Randall, L. L., and S. J. Hardy. 2002. SecB, one small chaperone in the complex milieu of the cell. Cell. Mol. Life Sci. 59:1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Renauld-Mongenie, G., J. Cornette, N. Mielcarek, F. D. Menozzi, and C. Locht. 1996. Distinct roles of the N-terminal and C-terminal precursor domains in the biogenesis of the Bordetella pertussis filamentous hemagglutinin. J. Bacteriol. 178:1053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Reyrat, J. M., S. Lanzavecchia, P. Lupetti, M. de Bernard, C. Pagliaccia, V. Pelicic, M. Charrel, C. Ulivieri, N. Norais, X. Ji, V. Cabiaux, E. Papini, R. Rappuoli, and J. L. Telford. 1999. 3D imaging of the 58 kDa cell binding subunit of the Helicobacter pylori cytotoxin. J. Mol. Biol. 290:459-470. [DOI] [PubMed] [Google Scholar]
- 416.Ricci, V., A. Galmiche, A. Doye, V. Necchi, E. Solcia, and P. Boquet. 2000. High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis. Mol. Biol. Cell 11:3897-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Robbins, J. R., D. Monack, S. J. McCallum, A. Vegas, E. Pham, M. B. Goldberg, and J. A. Theriot. 2001. The making of a gradient: IcsA (VirG) polarity in Shigella flexneri. Mol. Microbiol. 41:861-872. [DOI] [PubMed] [Google Scholar]
- 418.Roberts, M., N. F. Fairweather, E. Leininger, D. Pickard, E. L. Hewlett, A. Robinson, C. Hayward, G. Dougan, and I. G. Charles. 1991. Construction and characterization of Bordetella pertussis mutants lacking the vir-regulated P.69 outer membrane protein. Mol. Microbiol. 5:1393-1404. [DOI] [PubMed] [Google Scholar]
- 419.Robinson, C., and A. Bolhuis. 2001. Protein targeting by the twin-arginine translocation pathway. Nat. Rev. Mol. Cell. Biol. 2:350-356. [DOI] [PubMed] [Google Scholar]
- 420.Rocha, E. P., O. Pradillon, H. Bui, C. Sayada, and E. Denamur. 2002. A new family of highly variable proteins in the Chlamydophila pneumoniae genome. Nucleic Acids Res. 30:4351-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Roche, A., J. McFadden, and P. Owen. 2001. Antigen 43, the major phase-variable protein of the Escherichia coli outer membrane, can exist as a family of proteins encoded by multiple alleles. Microbiology 147:161-169. [DOI] [PubMed] [Google Scholar]
- 422.Roggenkamp, A., N. Ackermann, C. A. Jacobi, K. Truelzsch, H. Hoffmann, and J. Heesemann. 2003. Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA. J. Bacteriol. 185:3735-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Rose, J. E., D. H. Meyer, and P. M. Fives-Taylor. 2003. Aae, an autotransporter involved in adhesion of Actinobacillus actinomycetemcomitans to epithelial cells. Infect. Immun. 71:2384-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Roux, V., P. E. Fournier, and D. Raoult. 1996. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 34:2058-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Rudi, J., C. Kolb, M. Maiwald, D. Kuck, A. Sieg, P. R. Galle, and W. Stremmel. 1998. Diversity of Helicobacter pylori vacA and cagA genes and relationship to VacA and CagA protein expression, cytotoxin production, and associated diseases. J. Clin. Microbiol. 36:944-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Russell, M. W., J. Reinholdt, and M. Kilian. 1989. Anti-inflammatory activity of human IgA antibodies and their Fab alpha fragments: inhibition of IgG-mediated complement activation. Eur. J. Immunol. 19:2243-2249. [DOI] [PubMed] [Google Scholar]
- 427.Salvadori, M. R., T. Yano, H. E. Carvalho, V. R. Parreira, and C. L. Gyles. 2001. Vacuolating cytotoxin produced by avian pathogenic Escherichia coli. Avian Dis. 45:43-51. [PubMed] [Google Scholar]
- 428.Sandkvist, M. 2001. Biology of type II secretion. Mol. Microbiol. 40:271-283. [DOI] [PubMed] [Google Scholar]
- 429.Sandkvist, M. 2001. Type II secretion and pathogenesis. Infect. Immun. 69:3523-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Sandkvist, M., M. Bagdasarian, S. P. Howard, and V. J. DiRita. 1995. Interaction between the autokinase EpsE and EpsL in the cytoplasmic membrane is required for extracellular secretion in Vibrio cholerae. EMBO J. 14:1664-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Sandt, C. H., and C. W. Hill. 2000. Four different genes responsible for nonimmune immunoglobulin-binding activities within a single strain of Escherichia coli. Infect. Immun. 68:2205-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Sansonetti, P. J., J. Arondel, A. Fontaine, H. d'Hauteville, and M. L. Bernardini. 1991. OmpB (osmo-regulation) and icsA (cell-to-cell spread) mutants of Shigella flexneri: vaccine candidates and probes to study the pathogenesis of shigellosis. Vaccine 9:416-422. [DOI] [PubMed] [Google Scholar]
- 433.Saraste, M., P. R. Sibbald, and A. Wittinghofer. 1990. The P-loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem. Sci. 15:430-434. [DOI] [PubMed] [Google Scholar]
- 434.Sargent, F. 2001. A marriage of bacteriology with cell biology results in twin arginines. Trends Microbiol. 9:196-198. [DOI] [PubMed] [Google Scholar]
- 435.Scaletsky, I. C., M. Z. Pedroso, C. A. Oliva, R. L. Carvalho, M. B. Morais, and U. Fagundes-Neto. 1999. A localized adherence-like pattern as a second pattern of adherence of classic enteropathogenic Escherichia coli to HEp-2 cells that is associated with infantile diarrhea. Infect. Immun. 67:3410-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Scaletsky, I. C., M. Z. Pedroso, and R. M. Silva. 1999. Phenotypic and genetic features of Escherichia coli strains showing simultaneous expression of localized and diffuse adherence. FEMS Immunol. Med. Microbiol. 23:181-188. [DOI] [PubMed] [Google Scholar]
- 437.Schembri, M. A., and P. Klemm. 2001. Coordinate gene regulation by fimbriae-induced signal transduction. EMBO J. 20:3074-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Schiavo, G., F. Benfenati, B. Poulain, O. Rossetto, P. Polverino de Laureto, B. R. DasGupta, and C. Montecucco. 1992. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 359:832-835. [DOI] [PubMed] [Google Scholar]
- 439.Schiebel, E., H. Schwarz, and V. Braun. 1989. Subcellular location and unique secretion of the hemolysin of Serratia marcescens. J. Biol. Chem. 264:16311-16320. [PubMed] [Google Scholar]
- 440.Schmidt, H., W. L. Zhang, U. Hemmrich, S. Jelacic, W. Brunder, P. I. Tarr, U. Dobrindt, J. Hacker, and H. Karch. 2001. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect. Immun. 69:6863-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Schonherr, R., R. Tsolis, T. Focareta, and V. Braun. 1993. Amino acid replacements in the Serratia marcescens haemolysin ShlA define sites involved in activation and secretion. Mol. Microbiol. 9:1229-1237. [DOI] [PubMed] [Google Scholar]
- 442.Schraw, W., Y. Li, M. S. McClain, F. G. van der Goot, and T. L. Cover. 2002. Association of Helicobacter pylori vacuolating toxin (VacA) with lipid rafts. J. Biol. Chem. 277:34642-34650. [DOI] [PubMed] [Google Scholar]
- 443.Schulein, R., and C. Dehlo. 2002. The VirB/VirD4 type IV secretion system of Bartonella is essential for establishing intraerythrocytic infection. Mol. Microbiol. 46:1053-1067. [DOI] [PubMed] [Google Scholar]
- 444.Schulz, G. E. 2002. The structure of bacterial outer membrane proteins. Biochim. Biophys. Acta 1565:308-317. [DOI] [PubMed] [Google Scholar]
- 445.Serruto, D., J. Adu-Bobie, M. Scarselli, D. Veggi, M. Pizza, R. Rappuoli, and B. Arico. 2003. Neisseria meningitidis App: a new adhesin with autocatalytic serine protease activity. Mol. Microbiol. 48:323-334. [DOI] [PubMed] [Google Scholar]
- 446.Seto, K., Y. Hayashi-Kuwabara, T. Yoneta, H. Suda, and H. Tamaki. 1998. Vacuolation induced by cytotoxin from Helicobacter pylori is mediated by the EGF receptor in HeLa cells. FEBS Lett. 431:347-350. [DOI] [PubMed] [Google Scholar]
- 447.Seubert, A., R. Schulein, and C. Dehio. 2002. Bacterial persistence within erythrocytes: a unique pathogenic strategy of Bartonella spp. Int. J. Med. Microbiol. 291:555-560. [DOI] [PubMed] [Google Scholar]
- 448.Sexton, J. A., and J. P. Vogel. 2002. Type IVB secretion by intracellular pathogens. Traffic 3:178-185. [DOI] [PubMed] [Google Scholar]
- 449.Shannon, J. L., and R. C. Fernandez. 1999. The C-terminal domain of the Bordetella pertussis autotransporter BrkA forms a pore in lipid bilayer membranes. J. Bacteriol. 181:5838-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Sharff, A., C. Fanutti, J. Shi, C. Calladine, and B. Luisi. 2001. The role of the TolC family in protein transport and multidrug efflux. From stereochemical certainty to mechanistic hypothesis. Eur. J. Biochem. 268:5011-5026. [DOI] [PubMed] [Google Scholar]
- 451.Shere, K. D., S. Sallustio, A. Manessis, T. G. D'Aversa, and M. B. Goldberg. 1997. Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol. Microbiol. 25:451-462. [DOI] [PubMed] [Google Scholar]
- 452.Shikata, S., K. Shimada, H. Kataoka, S. Horinouchi, and T. Beppu. 1992. Detection of large COOH-terminal domains processed from the precursor of Serratia marcescens serine protease in the outer membrane of Escherichia coli. J. Biochem. (Tokyo) 111:627-632. [DOI] [PubMed] [Google Scholar]
- 453.Shimada, K., Y. Ohnishi, S. Horinouchi, and T. Beppu. 1994. Extracellular transport of pseudoazurin of Alcaligenes faecalis in Escherichia coli using the COOH-terminal domain of Serratia marcescens serine protease. J. Biochem. (Tokyo) 116:327-334. [DOI] [PubMed] [Google Scholar]
- 454.Shoberg, R. J., and M. H. Mulks. 1991. Proteolysis of bacterial membrane proteins by Neisseria gonorrhoeae type 2 immunoglobulin A1 protease. Infect. Immun. 59:2535-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Sijbrandi, R., M. L. Urbanus, C. M. ten Hagen-Jongman, H. D. Bernstein, B. Oudega, B. R. Otto, and J. Luirink. 2003. Signal recognition particle (SRP)-mediated targeting and Sec-dependent translocation of an extracellular Escherichia coli protein. J. Biol. Chem. 278:4654-4659. [DOI] [PubMed] [Google Scholar]
- 456.Simpson, A. J., F. C. Reinach, P. Arruda, F. A. Abreu, M. Acencio, R. Alvarenga, L. M. Alves, J. E. Araya, G. S. Bala, C. S. Baptista, M. H. Barros, E. D. Bonaccorsi, S. Bordin, J. M. Bove, M. R. Briones, M. R. Bueno, A. A. Camargo, L. E. Camargo, D. M. Carraro, H. Carrer, N. B. Colauto, C. Colombo, F. F. Costa, M. C. Costa, C. M. Costa-Neto, L. L. Coutinho, M. Cristofani, E. Dias-Neto, C. Docena, H. El-Dorry, A. P. Facincani, A. J. Ferreira, V. C. Ferreira, J. A. Ferro, J. S. Fraga, S. C. Franca, M. C. Franco, M. Frohme, L. R. Furlan, M. Garnier, G. H. Goldman, M. H. Goldman, S. L. Gomes, A. Gruber, P. L. Ho, J. D. Hoheisel, M. L. Junqueira, E. L. Kemper, J. P. Kitajima, J. E. Krieger, E. E. Kuramae, F. Laigret, M. R. Lambais, L. C. Leite, E. G. Lemos, M. V. Lemos, S. A. Lopes, C. R. Lopes, J. A. Machado, M. A. Machado, A. M. Madeira, H. M. Madeira, C. L. Marino, M. V. Marques, E. A. Martins, E. M. Martins, A. Y. Matsukuma, C. F. Menck, E. C. Miracca, C. Y. Miyaki, C. B. Monteriro-Vitorello, D. H. Moon, M. A. Nagai, A. L. Nascimento, L. E. Netto, A. Nhani, Jr., F. G. Nobrega, L. R. Nunes, M. A. Oliveira, M. C. de Oliveira, R. C. de Oliveira, D. A. Palmieri, A. Paris, B. R. Peixoto, G. A. Pereira, H. A. Pereira, Jr., J. B. Pesquero, R. B. Quaggio, P. G. Roberto, V. Rodrigues, M. R. A. J. de, V. E. de Rosa, Jr., R. G. de Sa, R. V. Santelli, H. E. Sawasaki, A. C. da Silva, A. M. da Silva, F. R. da Silva, W. A. da Silva, Jr., J. F. da Silveira, et al. 2000. The genome sequence of the plant pathogen Xylella fastidiosa. The Xylella fastidiosa Consortium of the Organization for Nucleotide Sequencing and Analysis. Nature 406:151-157. [DOI] [PubMed] [Google Scholar]
- 457.Sleytr, U. B., and P. Messner. 1983. Crystalline surface layers on bacteria. Annu. Rev. Microbiol. 37:311-339. [DOI] [PubMed] [Google Scholar]
- 458.Smoot, D. T., J. H. Resau, M. H. Earlington, M. Simpson, and T. L. Cover. 1996. Effects of Helicobacter pylori vacuolating cytotoxin on primary cultures of human gastric epithelial cells. Gut 39:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Stathopoulos, C., D. R. Hendrixson, D. G. Thanassi, S. J. Hultgren, J. W. St Geme III, and R. Curtiss III. 2000. Secretion of virulence determinants by the general secretory pathway in gram-negative pathogens: an evolving story. Microbes Infect. 2:1061-1072. [DOI] [PubMed] [Google Scholar]
- 460.Stathopoulos, C., D. L. Provence, and R. Curtiss, 3rd. 1999. Characterization of the avian-pathogenic Escherichia coli hemagglutinin Tsh, a member of the immunoglobulin A protease-type family of autotransporters. Infect. Immun. 67:772-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Stein, M., B. Kenny, M. A. Stein, and B. B. Finlay. 1996. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J. Bacteriol. 178:6546-6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Steinbacher, S., U. Baxa, S. Miller, A. Weintraub, R. Seckler, and R. Huber. 1996. Crystal structure of phage P22 tailspike protein complexed with Salmonella sp. O-antigen receptors. Proc. Natl. Acad. Sci. USA 93:10584-10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Steinhauer, J., R. Agha, T. Pham, A. W. Varga, and M. B. Goldberg. 1999. The unipolar Shigella surface protein IcsA is targeted directly to the bacterial old pole: IcsP cleavage of IcsA occurs over the entire bacterial surface. Mol. Microbiol. 32:367-377. [DOI] [PubMed] [Google Scholar]
- 464.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 465.St Geme, J. W., III, and D. Cutter. 2000. The Haemophilus influenzae Hia adhesin is an autotransporter protein that remains uncleaved at the C terminus and fully cell associated. J. Bacteriol. 182:6005-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.St Geme, J. W., III, M. L. de la Morena, and S. Falkow. 1994. A Haemophilus influenzae IgA protease-like protein promotes intimate interaction with human epithelial cells. Mol. Microbiol. 14:217-233. [DOI] [PubMed] [Google Scholar]
- 467.St Geme, J. W., III, and S. Grass. 1998. Secretion of the Haemophilus influenzae HMW1 and HMW2 adhesins involves a periplasmic intermediate and requires the HMWB and HMWC proteins. Mol. Microbiol. 27:617-630. [DOI] [PubMed] [Google Scholar]
- 468.Storsaeter, J., H. O. Hallander, L. Gustafsson, and P. Olin. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16:1907-1916. [DOI] [PubMed] [Google Scholar]
- 469.Stothard, D. R., G. A. Toth, and B. E. Batteiger. 2003. Polymorphic membrane protein H has evolved in parallel with the three disease-causing groups of Chlamydia trachomatis. Infect. Immun. 71:1200-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Struyve, M., M. Moons, and J. Tommassen. 1991. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J. Mol. Biol. 218:141-148. [DOI] [PubMed] [Google Scholar]
- 471.Suerbaum, S., J. M. Smith, K. Bapumia, G. Morelli, N. H. Smith, E. Kunstmann, I. Dyrek, and M. Achtman. 1998. Free recombination within Helicobacter pylori. Proc. Natl. Acad. Sci. USA 95:12619-12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Suhr, M., I. Benz, and M. A. Schmidt. 1996. Processing of the AIDA-I precursor: removal of AIDAc and evidence for the outer membrane anchoring as a beta-barrel structure. Mol. Microbiol. 22:31-42. [DOI] [PubMed] [Google Scholar]
- 473.Sui, B. Q., P. R. Dutta, and J. P. Nataro. 2003. Intracellular expression of the plasmid-encoded toxin from enteroaggregative Escherichia coli. Infect. Immun. 71:5364-5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Surana, N. K., D. Cutter, S. J. Barenkamp, and J. W. St Geme, 3rd. 2004. The Haemophilus influenzae Hia autotransporter contains an unusually short trimeric translocator domain. J. Biol. Chem. 279:14679-14685. [DOI] [PubMed] [Google Scholar]
- 475.Suzuki, T., M. C. Lett, and C. Sasakawa. 1995. Extracellular transport of VirG protein in Shigella. J. Biol. Chem. 270:30874-30880. [DOI] [PubMed] [Google Scholar]
- 476.Szabady, R., and H. Bernstein. 2003. Abstr. 103rd Gen. Meet. Am. Soc. Microbiol. 2003, abstr. B-426, 2003.
- 477.Szabo, I., S. Brutsche, F. Tombola, M. Moschioni, B. Satin, J. L. Telford, R. Rappuoli, C. Montecucco, E. Papini, and M. Zoratti. 1999. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. EMBO J. 18:5517-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Takizawa, N., and Y. Murooka. 1985. Cloning of the pullulanase gene and overproduction of pullulanase in Escherichia coli and Klebsiella aerogenes. Appl. Environ. Microbiol. 49:294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Tamm, A., A. M. Tarkkanen, T. K. Korhonen, P. Kuusela, P. Toivanen, and M. Skurnik. 1993. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol. Microbiol. 10:995-1011. [DOI] [PubMed] [Google Scholar]
- 480.Tamm, L. K., A. Arora, and J. H. Kleinschmidt. 2001. Structure and assembly of beta-barrel membrane proteins. J. Biol. Chem. 276:32399-32402. [DOI] [PubMed] [Google Scholar]
- 481.Tamura, M., K. Nogimori, S. Murai, M. Yajima, K. Ito, T. Katada, M. Ui, and S. Ishii. 1982. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry 21:5516-5522. [DOI] [PubMed] [Google Scholar]
- 482.Tan, M. W., L. G. Rahme, J. A. Sternberg, R. G. Tompkins, and F. M. Ausubel. 1999. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc. Natl. Acad. Sci. USA 96:2408-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Tanzer, R. J., D. Longbottom, and T. P. Hatch. 2001. Identification of polymorphic outer membrane proteins of Chlamydia psittaci 6BC. Infect. Immun. 69:2428-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Telford, J. L., P. Ghiara, M. Dell'Orco, M. Comanducci, D. Burroni, M. Bugnoli, M. F. Tecce, S. Censini, A. Covacci, Z. Xiang, et al. 1994. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J. Exp. Med. 179:1653-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Thanabalu, T., E. Koronakis, C. Hughes, and V. Koronakis. 1998. Substrate-induced assembly of a contiguous channel for protein export from E. coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J. 17:6487-6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Thanassi, D. G. 2002. Ushers and secretins: channels for the secretion of folded proteins across the bacterial outer membrane. J. Mol. Microbiol. Biotechnol. 4:11-20. [PubMed] [Google Scholar]
- 487.Thanassi, D. G., E. T. Saulino, M. J. Lombardo, R. Roth, J. Heuser, and S. J. Hultgren. 1998. The PapC usher forms an oligomeric channel: implications for pilus biogenesis across the outer membrane. Proc. Natl. Acad. Sci. USA 95:3146-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Thomas, J. D., P. J. Reeves, and G. P. Salmond. 1997. The general secretion pathway of Erwinia carotovora subsp. carotovora: analysis of the membrane topology of OutC and OutF. Microbiology 143:713-720. [DOI] [PubMed] [Google Scholar]
- 489.Timpe, J. M., M. M. Holm, S. L. Vanlerberg, V. Basrur, and E. R. Lafontaine. 2003. Identification of a Moraxella catarrhalis outer membrane protein exhibiting both adhesin and lipolytic activities. Infect. Immun. 71:4341-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, J. C. Venter, et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 491.Tombola, F., L. Morbiato, G. Del Giudice, R. Rappuoli, M. Zoratti, and E. Papini. 2001. The Helicobacter pylori VacA toxin is a urea permease that promotes urea diffusion across epithelia. J. Clin. Investig. 108:929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Tong, H. H., L. E. Blue, M. A. James, and T. F. DeMaria. 2000. Evaluation of the virulence of a Streptococcus pneumoniae neuraminidase-deficient mutant in nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect. Immun. 68:921-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Torres, A. G., N. T. Perna, V. Burland, A. Ruknudin, F. R. Blattner, and J. B. Kaper. 2002. Characterization of Cah, a calcium-binding and heat-extractable autotransporter protein of enterohaemorrhagic Escherichia coli. Mol. Microbiol. 45:951-966. [DOI] [PubMed] [Google Scholar]
- 494.Turk, D. C. 1984. The pathogenicity of Haemophilus influenzae. J. Med. Microbiol. 18:1-16. [DOI] [PubMed] [Google Scholar]
- 495.Turner, D. P., K. G. Wooldridge, and D. A. Ala'Aldeen. 2002. Autotransported serine protease A of Neisseria meningitidis: an immunogenic, surface-exposed outer membrane, and secreted protein. Infect. Immun. 70:4447-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Uchiyama, T. 1999. Role of major surface antigens of Rickettsia japonica in the attachment to host cells, p. 182-188. In D. Raoult and P. Brouqui (ed.), Rickettsiae and rickettsial diseases at the turn of the third millenium. Elsevier, Paris, France.
- 497.Ulbrandt, N. D., J. A. Newitt, and H. D. Bernstein. 1997. The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell 88:187-196. [DOI] [PubMed] [Google Scholar]
- 498.Uphoff, T. S., and R. A. Welch. 1990. Nucleotide sequencing of the Proteus mirabilis calcium-independent hemolysin genes (hpmA and hpmB) reveals sequence similarity with the Serratia marcescens hemolysin genes (shlA and shlB). J. Bacteriol. 172:1206-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Valls, M., S. Atrian, V. de Lorenzo, and L. A. Fernandez, 2000. Engineering a mouse metallothionein on the cell surface of Ralstonia eutropha CH34 for immobilization of heavy metals in soil. Nat. Biotechnol. 18:661-665. [DOI] [PubMed] [Google Scholar]
- 500.Valls, M., V. de Lorenzo, R. Gonzalez-Duarte, and S. Atrian. 2000. Engineering outer-membrane proteins in Pseudomonas putida for enhanced heavy-metal bioadsorption. J. Inorg. Biochem. 79:219-223. [DOI] [PubMed] [Google Scholar]
- 501.Vandahl, B. B., A. S. Pedersen, K. Gevaert, A. Holm, J. Vandekerckhove, G. Christiansen, and S. Birkelund. 2002. The expression, processing and localization of polymorphic membrane proteins in Chlamydia pneumoniae strain CWL029. BMC Microbiol. 2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.van den Berg, B. M., H. Beekhuizen, R. J. Willems, F. R. Mooi, and R. van Furth. 1999. Role of Bordetella pertussis virulence factors in adherence to epithelial cell lines derived from the human respiratory tract. Infect. Immun. 67:1056-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Van den Bosch, L., and R. Morona. 2003. The actin-based motility defect of a Shigella flexneri rmlD rough LPS mutant is not due to loss of IcsA polarity. Microb. Pathog. 35:11-18. [DOI] [PubMed] [Google Scholar]
- 504.van der Voort, E. R., H. van Dijken, B. Kuipers, J. van der Biezen, P. van der Ley, J. Meylis, I. Claassen, and J. Poolman. 1997. Human B- and T-cell responses after immunization with a hexavalent PorA meningococcal outer membrane vesicle vaccine. Infect. Immun. 65:5184-5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.van der Zee, A., H. Groenendijk, M. Peeters, and F. R. Mooi. 1996. The differentiation of Bordetella parapertussis and Bordetella bronchiseptica from humans and animals as determined by DNA polymorphism mediated by two different insertion sequence elements suggests their phylogenetic relationship. Int. J. Syst. Bacteriol. 46:640-647. [DOI] [PubMed] [Google Scholar]
- 506.van Doorn, L. J., C. Figueiredo, R. Sanna, S. Pena, P. Midolo, E. K. Ng, J. C. Atherton, M. J. Blaser, and W. G. Quint. 1998. Expanding allelic diversity of Helicobacter pylori vacA. J. Clin. Microbiol. 36:2597-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Van Gelder, P., F. Dumas, I. Bartoldus, N. Saint, A. Prilipov, M. Winterhalter, Y. Wang, A. Philippsen, J. P. Rosenbusch, and T. Schirmer. 2002. Sugar transport through maltoporin of Escherichia coli: role of the greasy slide. J. Bacteriol. 184:2994-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.van Ulsen, P., L. van Alphen, J. ten Hove, F. Fransen, P. van der Ley, and J. Tommassen. 2003. A neisserial autotransporter NalP modulating the processing of other autotransporters. Mol. Microbiol. 50:1017-1030. [DOI] [PubMed] [Google Scholar]
- 509.van Wely, K. H., J. Swaving, R. Freudl, and A. J. Driessen. 2001. Translocation of proteins across the cell envelope of Gram-positive bacteria. FEMS Microbiol. Rev. 25:437-454. [DOI] [PubMed] [Google Scholar]
- 510.Veiga, E., V. de Lorenzo, and L. A. Fernandez. 1999. Probing secretion and translocation of a beta-autotransporter using a reporter single-chain Fv as a cognate passenger domain. Mol. Microbiol. 33:1232-1243. [DOI] [PubMed] [Google Scholar]
- 511.Veiga, E., V. de Lorenzo, and L. A. Fernandez. 2004. Structural tolerance of bacterial autotransporters for folded passenger protein domains. Mol. Microbiol. 52:1069-1080. [DOI] [PubMed] [Google Scholar]
- 512.Veiga, E., E. Sugawara, H. Nikaido, V. de Lorenzo, and L. A. Fernandez. 2002. Export of autotransported proteins proceeds through an oligomeric ring shaped by C-terminal domains. EMBO J. 21:2122-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Villaseca, J. M., F. Navarro-Garcia, G. Mendoza-Hernandez, J. P. Nataro, A. Cravioto, and C. Eslava. 2000. Pet toxin from enteroaggregative Escherichia coli produces cellular damage associated with fodrin disruption. Infect. Immun. 68:5920-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Vinion-Dubiel, A. D., M. S. McClain, D. M. Czajkowsky, H. Iwamoto, D. Ye, P. Cao, W. Schraw, G. Szabo, S. R. Blanke, Z. Shao, and T. L. Cover. 1999. A dominant negative mutant of Helicobacter pylori vacuolating toxin (VacA) inhibits VacA-induced cell vacuolation. J. Biol. Chem. 274:37736-37742. [DOI] [PubMed] [Google Scholar]
- 515.von Wintzingerode, F., A. Schattke, R. A. Siddiqui, U. Rosick, U. B. Gobel, and R. Gross. 2001. Bordetella petrii sp. nov., isolated from an anaerobic bioreactor, and emended description of the genus Bordetella. Int. J. Syst. Evol. Microbiol. 51:1257-1265. [DOI] [PubMed] [Google Scholar]
- 516.Voulhoux, R., G. Ball, B. Ize, M. L. Vasil, A. Lazdunski, L. F. Wu, and A. Filloux. 2001. Involvement of the twin-arginine translocation system in protein secretion via the type II pathway. EMBO J. 20:6735-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Voulhoux, R., M. P. Bos, J. Geurtsen, M. Mols, and J. Tommassen. 2003. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299:262-265. [DOI] [PubMed] [Google Scholar]
- 518.Voulhoux, R., and J. Tommassen. 2004. Omp85, an evolutionarily conserved bacterial protein involved in outer-membrane-protein assembly. Res. Microbiol. 155:129-135. [DOI] [PubMed] [Google Scholar]
- 519.Vretou, E., P. Giannikopoulou, D. Longbottom, and E. Psarrou. 2003. Antigenic organization of the N-terminal part of the polymorphic outer membrane proteins 90, 91A, and 91B of Chlamydophila abortus. Infect. Immun. 71:3240-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Vretou, E., P. Giannikopoulou, and E. Psarrou. 2001. Polymorphic outer-membrane proteins of Chlamydophila abortus are glycosylated. Microbiology 147:3303-3310. [DOI] [PubMed] [Google Scholar]
- 521.Waldron, D. E., P. Owen, and C. J. Dorman. 2002. Competitive interaction of the OxyR DNA-binding protein and the Dam methylase at the antigen 43 gene regulatory region in Escherichia coli. Mol. Microbiol. 44:509-520. [DOI] [PubMed] [Google Scholar]
- 522.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Wang, H., and B. C. Dowds. 1993. Phase variation in Xenorhabdus luminescens: cloning and sequencing of the lipase gene and analysis of its expression in primary and secondary phases of the bacterium. J. Bacteriol. 175:1665-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Wang, H. J., C. H. Kuo, A. A. Yeh, P. C. Chang, and W. C. Wang. 1998. Vacuolating toxin production in clinical isolates of Helicobacter pylori with different vacA genotypes. J. Infect. Dis. 178:207-212. [DOI] [PubMed] [Google Scholar]
- 525.Weel, J. F., R. W. van der Hulst, Y. Gerrits, P. Roorda, M. Feller, J. Dankert, G. N. Tytgat, and A. van der Ende. 1996. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J. Infect. Dis. 173:1171-1175. [DOI] [PubMed] [Google Scholar]
- 526.Wegrzyn, G., and M. S. Thomas. 2002. Modulation of the susceptibility of intestinal bacteria to bacteriophages in response to Ag43 phase variation—a hypothesis. Med. Sci. Monit. 8:HY15-HY18. [PubMed] [Google Scholar]
- 527.Wehrl, W., V. Brinkmann, P. R. Jungblut, T. F. Meyer, and A. J. Szczepek. 2004. From the inside out—processing of the chlamydial autotransporter PmpD and its role in bacterial adhesion and activation of human host cells. Mol. Microbiol. 51:319-334. [DOI] [PubMed] [Google Scholar]
- 528.Weiss, A. A., and M. S. Goodwin. 1989. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect. Immun. 57:3757-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Weiss, A. A., and E. L. Hewlett. 1986. Virulence factors of Bordetella pertussis. Annu. Rev. Microbiol. 40:661-686. [DOI] [PubMed] [Google Scholar]
- 530.Welch, R. A., V. Burland, G. Plunkett, 3rd, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Weldon, S. K., D. A. Mosier, K. R. Simons, R. C. Craven, and A. W. Confer. 1994. Identification of a potentially important antigen of Pasteurella haemolytica. Vet. Microbiol. 40:283-291. [DOI] [PubMed] [Google Scholar]
- 532.Weller, S. J., G. D. Baldridge, U. G. Munderloh, H. Noda, J. Simser, and T. J. Kurtti. 1998. Phylogenetic placement of rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. J. Clin. Microbiol. 36:1305-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Wilhelm, S., J. Tommassen, and K. E. Jaeger. 1999. A novel lipolytic enzyme located in the outer membrane of Pseudomonas aeruginosa. J. Bacteriol. 181:6977-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Williamson, M. P. 1994. The structure and function of proline-rich regions in proteins. Biochem. J. 297:249-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Yahiro, K., T. Niidome, M. Kimura, T. Hatakeyama, H. Aoyagi, H. Kurazono, K. Imagawa, A. Wada, J. Moss, and T. Hirayama. 1999. Activation of Helicobacter pylori VacA toxin by alkaline or acid conditions increases its binding to a 250-kDa receptor protein-tyrosine phosphatase beta. J. Biol. Chem. 274:36693-36699. [DOI] [PubMed] [Google Scholar]
- 536.Yamaoka, Y., T. Kodama, O. Gutierrez, J. G. Kim, K. Kashima, and D. Y. Graham. 1999. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J. Clin. Microbiol. 37:2274-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Yanagida, N., T. Uozumi, and T. Beppu. 1986. Specific excretion of Serratia marcescens protease through the outer membrane of Escherichia coli. J. Bacteriol. 166:937-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Ye, D., and S. R. Blanke. 2000. Mutational analysis of the Helicobacter pylori vacuolating toxin amino terminus: identification of amino acids essential for cellular vacuolation. Infect. Immun. 68:4354-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Ye, D., D. C. Willhite, and S. R. Blanke. 1999. Identification of the minimal intracellular vacuolating domain of the Helicobacter pylori vacuolating toxin. J. Biol. Chem. 274:9277-9282. [DOI] [PubMed] [Google Scholar]
- 540.Yen, M. R., C. R. Peabody, S. M. Partovi, Y. Zhai, Y. H. Tseng, and M. H. Saier. 2002. Protein-translocating outer membrane porins of Gram-negative bacteria. Biochim. Biophys. Acta 1562:6-31. [DOI] [PubMed] [Google Scholar]
- 541.Yeo, H. J., S. E. Cotter, S. Laarmann, T. Juehne, J. W. St Geme, and G. Waksman. 2004. Structural basis for host recognition by the Haemophilus influenzae Hia autotransporter. EMBO J. 23:1245-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Yuk, M. H., U. Heininger, G. Martinez de Tejada, and J. F. Miller. 1998. Human but not ovine isolates of Bordetella parapertussis are highly clonal as determined by PCR-based RAPD fingerprinting. Infection 26:270-273. [DOI] [PubMed] [Google Scholar]
- 543.Zhai, Y., and M. H. Saier, Jr. 2001. A web-based program (WHAT) for the simultaneous prediction of hydropathy, amphipathicity, secondary structure and transmembrane topology for a single protein sequence. J. Mol. Microbiol. Biotechnol. 3:501-502. [PubMed] [Google Scholar]
- 544.Zhai, Y., and M. H. Saier, Jr. 2001. A web-based program for the prediction of average hydropathy, average amphipathicity and average similarity of multiply aligned homologous proteins. J. Mol. Microbiol. Biotechnol. 3:285-286. [PubMed] [Google Scholar]
- 545.Zierler, M. K., and J. E. Galan. 1995. Contact with cultured epithelial cells stimulates secretion of Salmonella typhimurium invasion protein InvJ. Infect. Immun. 63:4024-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Zink, S. D., L. Pedersen, N. P. Cianciotto, and Y. Abu-Kwaik. 2002. The Dot/Icm type IV secretion system of Legionella pneumophila is essential for the induction of apoptosis in human macrophages. Infect. Immun. 70:1657-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]