This study is the first to use functional magnetic resonance-guided magnetic resonance spectroscopy to examine neurochemical mechanisms underlying functional reorganization in the primary somatosensory and motor cortices consequent to upper extremity amputation and its potential reversal through hand replantation or transplantation. We provide evidence for selective alteration of cortical neuronal integrity associated with amputation-related deafferentation that may not be reversible.
Keywords: unilateral hand amputees, allogeneic hand transplantation and hand replantation, functional MRI-guided 1H-MR spectroscopy, N-acetylaspartate, sensorimotor hand territory
Abstract
Deafferentation is accompanied by large-scale functional reorganization of maps in the primary sensory and motor areas of the hemisphere contralateral to injury. Animal models of deafferentation suggest a variety of cellular-level changes including depression of neuronal metabolism and even neuronal death. Whether similar neuronal changes contribute to patterns of reorganization within the contralateral sensorimotor cortex of chronic human amputees is uncertain. We used functional MRI-guided proton magnetic resonance spectroscopy to test the hypothesis that unilateral deafferentation is associated with lower levels of N-acetylaspartate (NAA, a putative marker of neuronal integrity) in the sensorimotor hand territory located contralateral to the missing hand in chronic amputees (n = 19) compared with the analogous hand territory of age- and sex-matched healthy controls (n = 28). We also tested whether former amputees [i.e., recipients of replanted (n = 3) or transplanted (n = 2) hands] exhibit NAA levels that are indistinguishable from controls, possible evidence for reversal of the effects of deafferentation. As predicted, relative to controls, current amputees exhibited lower levels of NAA that were negatively and significantly correlated with the time after amputation. Contrary to our prediction, NAA levels in both replanted and transplanted patients fell within the range of the current amputees. We suggest that lower levels of NAA in current amputees reflects altered neuronal integrity consequent to chronic deafferentation. Thus local changes in NAA levels may provide a means of assessing neuroplastic changes in deafferented cortex. Results from former amputees suggest that these changes may not be readily reversible through reafferentation.
NEW & NOTEWORTHY This study is the first to use functional magnetic resonance-guided magnetic resonance spectroscopy to examine neurochemical mechanisms underlying functional reorganization in the primary somatosensory and motor cortices consequent to upper extremity amputation and its potential reversal through hand replantation or transplantation. We provide evidence for selective alteration of cortical neuronal integrity associated with amputation-related deafferentation that may not be reversible.
extensive studies of animal models indicate that deafferenting injuries induce functional brain reorganization in the primary somatosensory and motor cortices contralateral to the injury (Kaas, 1991; Merzenich et al., 1984). This reorganization is characterized by a robust increase of cortical space occupied by spared sensory inputs or motor outputs at the expense of lost inputs/outputs and is attributable to several mechanisms operating over different timescales. Acute changes, apparent within minutes or hours of deafferentation, have been linked to the unmasking of existing yet functionally inhibited intracortical synapses and the modulation of synaptic efficacy (Jacobs and Donoghue, 1991; Kaas, 2000). Chronically deafferented animals exhibit additional synaptic plasticity as well as evidence for structural changes (Buonomano and Merzenich, 1998). Specifically, progressive neuronal degeneration, including withdrawal of the deafferented axons from the somatosensory cortex and changes in neuronal morphology [for reviews, see Feldman and Brecht (2005) and Holtmaat and Svoboda (2009)], loss and dysfunction of inhibitory neurons (Ralston et al., 1996; Welker et al., 1989), and structural changes in surviving neurons (Jones et al., 2002; Navarro et al., 2007; Schubert et al., 2013) have all been reported. It has been assumed that similar neural mechanisms underlie well-documented chronic postamputation cortical reorganization in humans. However, no direct evidence for this postulation has existed because of lack of a noninvasive means to evaluate the human brain at the cellular level. Likewise, whether such chronic changes can be reversed by restoration of afferent and efferent activity between the hand and brain remains unknown.
Here, we report the first attempt to address these issues in humans through functional MRI-guided proton magnetic resonance spectroscopy (1H-MRS) in a cohort of unilateral hand amputees and a small number of former amputees, i.e., individuals either who have undergone replantation of their own hands shortly after injury or who have received allogeneic hand transplants in subsequent years. 1H-MRS is uniquely able to measure microscopic neural state, by means of biomarkers related to neuronal and glial health, inflammation, and intracortical excitability/inhibition, in discrete regions of the human brain (Van Zijl and Barker, 1997). We specifically investigated whether there are indications of changes in neuronal integrity in the deafferented/deefferented hand territory, which, as will soon be described, was functionally defined using functional MRI (fMRI). This study builds on our prior studies in stroke (Cirstea et al., 2011, 2014; Craciunas et al., 2013) and literature on other neurological disorders [for review, see Moffett et al. (2007)] that established a relationship between a neuronal biomarker, N-acteylaspartate (NAA), and behaviorally relevant neurophysiological brain changes. Despite more than 50 years of research, the role of NAA, detected almost exclusively in neurons and their processes, remains controversial. Hypotheses include involvement in myelin synthesis, neuronal energetics, osmoregulation, and axonal-glial signaling. Thus NAA is considered a general biomarker of neuronal health, viability, and amount (Baslow 2003; Moffett et al., 2007).
To our knowledge, there exists only one other published study using 1H-MRS in similar populations (Ertem et al., 2005). Ertem et al. found a trend toward lower NAA levels in the contralateral vs. ipsilateral sensorimotor cortex at an average of 26 mo after replantation of the amputated hand in nine former amputees. This result was interpreted as evidence for the absence of dramatic alterations in neuronal integrity in the sensorimotor cortex contralateral to the replanted hand. Note that hand replantation must occur within hours of amputation. Therefore the question of whether such changes manifest during chronic amputation remains unknown, as does whether such long-standing alterations can be reversed following transplantation. Furthermore, it is possible that changes in NAA contralateral to the replanted hand could have been underestimated by comparing against the ipsilateral sensorimotor cortex. The reason for this is that unilateral amputation induces reorganizational changes in both cerebral hemispheres (Bogdanov et al., 2012; Hamzei et al., 2001; Knecht et al., 1996; Philip and Frey, 2014), and on the basis of work in animal models, these changes begin immediately (Calford and Tweedale, 1988). Nevertheless, the trend toward lower contralateral NAA in this initial report is suggestive of possible cortical neuronal-level changes associated with unilateral hand loss and serves as an important motivation for the current study.
Here, we used 1H-MRS to assess NAA levels in the functionally defined sensorimotor hand territories of chronic unilateral hand amputees (n = 19) and in several former amputees, i.e., hand replant (n = 3) and hand transplant (n = 2) recipients. Age- and sex-matched controls (n = 28) were evaluated to estimate the preamputation levels of NAA in the sensorimotor hand territories. On the basis of the aforementioned neurodegenerative cortical events reported after limb loss (Feldman and Brecht, 2005; Holtmaat and Svoboda, 2009; Ralston et al., 1996; Welker et al., 1989) and the observations from spinal cord injuries (Hains et al., 2003; Wrigley et al., 2009) and cervical spondylotic myelopathy (Kowalczyk et al., 2012), we predicted that the levels of NAA would be lower in the sensorimotor hand territory contralateral to the missing hand (former) in current amputees relative to healthy controls. On the basis of the well-proven dependence of the neural organization of the sensorimotor cortex on incoming activity (Holtmaat and Svoboda, 2009), we hypothesized that NAA levels in the sensorimotor hand territory contralateral to the replanted or transplanted hand in the former amputees should not differ from levels in healthy controls because of restoration of afferent and efferent activity.
In addition to the primary hypotheses concerning NAA, we tested several secondary predictions involving four other neurochemicals: myo-inositol (mIn, considered a putative glial marker), glutamate-glutamine (Glx, a marker of glutamatergic neurotransmission), choline (Cho, a marker of cellular membrane turnover), and creatine (Cr, central energy marker of both neurons and astrocytes). In the contralateral (former hand) territory of the current amputees, we expected to find 1) higher mIn, based on the relationship between neuronal cell death/depression and reactive gliosis (Ding et al., 2000); 2) higher Glx, particularly in those who experience pain due to the hyperexcitability of nociceptive pathways (Knecht et al., 1996); and 3) no Cho or Cr changes (based on the slim chance of altered cell membrane integrity or changes in brain energetics in this chronic stage). As for NAA, we expected to find no significant changes in these secondary neurochemicals in former amputees compared with controls. For the reasons described for NAA above, we also explored the neurochemical profile of hand territory ipsilateral (intact) to the amputation.
MATERIALS AND METHODS
Participants.
Patients tested were 19 current, unilateral, upper limb amputees [8 women, age 45.6 ± 15.7 (SD) yr] and 5 former unilateral amputees; 3 of the former amputees received autologous hand replantation (all men, 53.7 ± 11.9 yr), and 2 underwent allogeneic hand transplantation (men, 44.5 ± 7.8 yr). Controls were 28 healthy individuals matched for sex, age, and hand dominance (11 women, 42.0 ± 12.6 yr). All participants completed the study after providing written informed consent in accordance with the Institutional Review Board at the University of Missouri, which approved this study.
As summarized in Tables 1 and 2, all patients experienced a traumatic unilateral transradial (below elbow, 52%) or transhumeral (above elbow, 48%) amputation as an adult at least 2 yr before participating in our study. Four amputees (21%) lost their left hand. The average time elapsed since the amputation was 13.8 ± 13.2 yr (Table 1). In the transplanted patients, the mean time between the injury and surgical transplantation was 7.8 ± 7.4 yr (Table 2). Hand replantation generally occurred within 6 h of amputation.
Table 1.
Demographic and clinical details of current amputees
| Sex/Age, yr | Years Since Amputation | Level of Amputation | Side of Amputation | Prosthesis Use | NPS | SF-MPQ | PLP Bursts | RLP | Pain Medication |
|---|---|---|---|---|---|---|---|---|---|
| F/37 | 4 | BE | R | + | 54 | 17 | 8 | — | — |
| M/42 | 21 | AE | R | — | 58 | 22 | 10 | — | — |
| M/62 | 36 | AE | R | — | — | — | — | 5 | — |
| F/46 | 3 | BE | R | + | — | — | — | 5 | Neurontin |
| M/32 | 14 | BE | R | + | — | — | — | — | — |
| F/67 | 2 | AE | R | + | 12 | 1 | 6 | 2 | Neurontin |
| F/31 | 7 | AE | R | + | 23 | 5 | 5 | — | Tylenol, Oxycodone, Flexeril |
| F/43 | 6 | AE | R | — | 40 | 26 | 8 | — | Hydrocodone, Fentanyl patch, Cymbalta |
| M/56 | 3 | AE | R | + | 24 | 5 | 3 | — | Aleve |
| M/52 | 11 | BE | L | + | 17 | 3 | 6 | — | Citalopram |
| F/64 | 13 | AE | R | + | 25 | 0 | 6 | — | — |
| M/20 | 6 | BE | L | + | 16 | 2 | 9 | — | — |
| F/38 | 38 | BE | L | — | — | — | — | 2 | Klonopin |
| M/29 | 7 | BE | R | + | 22 | 11 | 10 | — | — |
| M/65 | 45 | BE | R | + | 14 | 4 | 9 | — | Aleve |
| M/44 | 5 | AE | R | + | 53 | 18 | 10 | 4 | — |
| M/42 | 20 | BE | L | + | 51 | 19 | 10 | — | — |
| M/74 | 8 | BE | R | + | — | — | — | 7 | Neurotin |
| F/22 | 2 | AE | R | + | 19 | 4 | 9 | — | — |
F, female; M, male; L, left; R, right; AE, above elbow; BE, below elbow; PLP, phantom limb pain; RLP, residual limb pain; NPS, Neuropathic Pain Scale; SF-MPQ, short-form McGill Pain Questionnaire. PLP bursts and RLP were evaluated on a scale ranging from 0 = no pain to 10 = severe pain.
Table 2.
Demographic and clinical details of the former amputees
| Sex/Age, yr | Years Since Amputation | Years Between Amputation and Transplantation | Years Since Replantation/Transplantation | Side/Level of Amputation | Prosthesis Use | NPS | SF-MPQ | Pain Bursts | Pain Medication |
|---|---|---|---|---|---|---|---|---|---|
| Replanted participants | |||||||||
| M/62 | 7 | — | 7 | L/BE | — | 5 | 2 | 2 | Aleve |
| M/59 | 0.4 | — | 0.4 | L/BE | — | 27 | 0 | 10 | Neurontin |
| M/40 | 6 | — | 6 | L/BE | — | 20 | 4 | 9 | Tylenol |
| Transplanted participants | |||||||||
| M/39 | 17 | 13 | 4 | L/BE | + (before) | 3 | 0 | 0 | |
| M/50 | 9 | 2.6 | 6.5 | R/BE | + (before) | 30 | 6 | 10 | |
F, female; M, male; L, left; R, right; BE, below elbow; NPS, Neuropathic Pain Scale; SF-MPQ, short-form McGill Pain Questionnaire. Pain bursts were evaluated on a scale ranging from 0 = no pain to 10 = severe pain.
Patients and healthy individuals were excluded if there was a prior history of brain injury, neurological disease, major psychiatric disorder, substance/alcohol abuse or dependence, or MRI contraindications. There were no group differences for age (control vs. amputee, P = 0.4), sex (39% female in control vs. 42% in amputee), or hand dominance [all participants were right hand dominant, Edinburgh Handedness Inventory (Oldfield, 1971)].
Clinical evaluation.
Given evidence implicating cortical sensorimotor reorganization in postamputation pain (Flor et al., 2006, 2013), we carefully differentiated phantom limb pain (PLP) vs. residual limb pain (RLP) in our participants and explored whether these symptoms were related to the neurochemical profile of the former hand territory. PLP in current amputees and pain in the replanted/transplanted hand of former amputees were assessed with the Neuropathic Pain Scale [NPS; Galer and Jensen (1997)] and short form of the McGill Pain Questionnaire (SF-MPQ; Melzack (1987)]. The intensity of PLP or replanted/transplanted hand pain bursts was assessed on a scale ranging from 0 = no pain to 10 = severe pain. A similar scale (ranging from 0 to 10) was also used to assess the intensity of RLP in current amputees. These data are summarized in Tables 1 and 2. These tables also include data regarding medications in current and former amputees and the frequency of prosthesis use in current amputees.
Neuroimaging methods.
MRI/1H-MRS data were collected on a Siemens 3T Trio MR system using an eight-channel birdcage head coil within a 24-h period after clinical evaluation. High-resolution T1-weighted anatomical images were acquired parallel to the anterior commissure-posterior commissure axis with a magnetization-prepared rapid gradient-echo (MPRAGE) sequence [repetition time (TR) = 1,900 ms; echo time (TE) = 3.2 ms; flip angle = 9°; matrix size, 256 × 215; in-plane resolution, 0.9 × 0.9 mm; 176 contiguous axial slices; thickness, 0.9 mm; field of view (FOV) = 230 × 201 mm; scan time, 5.53 min].
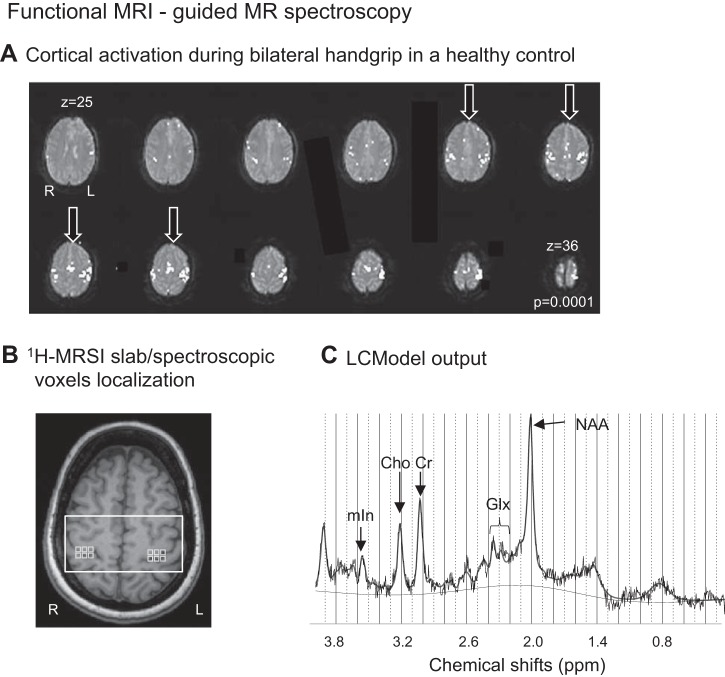
To accurately identify our region of interest (ROI; the sensorimotor hand territories in both hemispheres), we used a gradient echo blood oxygen level-dependent scan (BOLD; TR, 2,500 ms; TE, 30 ms; matrix size, 64 × 64; 36 contiguous axial slices; in-plane resolution, 4 × 4 mm; thickness, 4 mm; no skip; FOV, 256 × 256 mm; scan time, 3 min) to functionally define these territories. Two alternating conditions were repeated: movement condition (25 s), where participants were visually cued to flex and extend the fingers of both hands (only the intact hand in amputees), and rest condition (25 s), where participants were motionless. This task was designed on the basis of evidence of bilateral sensorimotor activation exhibited by movement (or stimulation) of the intact hand/limb in deafferented patients (Bogdanov et al., 2012) and animals (Pelled et al., 2007), respectively. BOLD data were analyzed using the scanner analysis software to guide the 1H-MRS imaging (1H-MRSI) slab positioning. Specifically, our task-related activations were mainly located on the middle genu of the central sulcus (or omega shape structure) and directly opposite of this structure on the anterior face of the postcentral gyrus (Bogdanov et al., 2012; Cirstea et al., 2011; Ward et al., 2003; Yousry et al., 1997), a well-known area to represent the hand sensorimotor function (Yousry et al., 1997). Four slices corresponding to the larger activated sensorimotor hand areas were selected by visual inspection (see Fig. 1A) and then used to identify the corresponding coincident T1-weighted image, on which the 1H-MRSI slab was centered. Thus the 1H-MRSI slab spanned the central sulcus and extended rostrally into the precentral gyrus and caudally into the postcentral gyrus (see Fig. 1B). The slab orientation was parallel to the anterior commissure-posterior commissure line.
Fig. 1.
A: motor-related cortical activation during a handgrip task executed with both hands in a healthy control. The arrows indicate the four anatomical slices used to select the corresponding coincident T1-weighted image on which the 1H-MRS imaging slab was centered. R, right; L, left. B: 1H-MRSI slab (white rectangle) and MRS voxels (light gray squares) were positioned on axial T1-weighted MR image on the basis of the anatomical landmarks of the sensorimotor hand territory. C: LCModel output from one MRS voxel located in left sensorimotor cortex in a healthy control shows distinct peaks corresponding to NAA (at 2.02 ppm), Glx (2.05–2.50 ppm), Cr (3.02 ppm), Cho (3.22 ppm), and mIn (3.56 ppm) and a signal-to-noise ratio of 16.
1H-MRSI acquisition.
1H-MRSI was performed with a point-resolved spectroscopy sequence, both with and without water suppression (PRESS; TR, 1,700 ms; TE, 30 ms, matrix size, 16 × 16; thickness, 15 mm; interpolated in-plane resolution, 5 × 5 mm2; FOV, 160 mm; spectral width, 1,200 Hz, scan time, 6.53 min). Chemical spectra were obtained via suppressing the water signal by means of a chemical shift-selective saturation pulse with a bandwidth of 50 Hz. The signal of unsuppressed water was also acquired. Scalp lipid artifact was minimized with eight 30-mm outer voxel suppression bands prescribed around the 1H-MRS excitation volume. Automated, followed by manual, shimming was performed to achieve a width at half height of the water peak below 20 Hz (Cirstea et al., 2011).
1H-MRSI data postprocessing.
1H-MRSI data were reconstructed using the scanner software. A Hamming filter implemented by the vendor software was applied before spatial reconstruction, which resulted in an increased effective voxel size by 30%. The final effective voxel size was 19.4 mm estimated with the full width at half maximum (FWHM) of the point spread function reflecting a circular k-space sampling and the Hamming filter. Neurochemical concentrations were quantified via LCModel (version 6.3-1H, linear combination of model spectra). We used PRESS basis set [including aspartate, creatine, GABA, glucose, glutamine, glutamate, glycerophosphocholine, phosphocholine, mIn, NAA, N-acetylaspartylglutamate, scyllo-inositol, taurine, and guanine; Provencher (2001)] and water as an internal concentration reference (Gasparovic et al., 2006). Figure 1C shows an MRS spectrum in the range of 0.2–4.0 ppm, including our target neurochemical, NAA, and four secondary neurochemicals that were also included for exploratory purposes: mIn, Glx, Cho, and Cr (see Table 3).
Table 3.
NAA and secondary neurochemical levels and spectroscopic voxels' brain tissue composition in two groups: controls-current amputees and former amputees
| Secondary Neurochemicals, mM |
||||||
|---|---|---|---|---|---|---|
| Participants | NAA, mM | mIn | Glx | Cho | Cr | Brain Tissue, % |
| Hand territory in left hemisphere in controls vs. hand territory contralateral to injury | ||||||
| Controls and current amputees | ||||||
| Controls | 8.4 ± 1.3 (7.9, 8.9) | 5.4 ± 0.8 (5.0, 5.7) | 10.1 ± 1.8 (9.4, 10.8) | 1.4 ± 0.3 (1.3, 1.5) | 4.2 ± 1.1 (3.8, 4.6) | 90.8 ± 11.0 (87.4, 94.1) |
| Current amputees | 7.4 ± 0.7 (6.8, 7.9) | 5.1 ± 0.7 (4.7, 5.5) | 9.8 ± 1.4 (8.9, 10.6) | 1.4 ± 0.2 (1.2, 1.5) | 4.2 ± 1.0 (3.8, 4.7) | 87.0 ± 10.8 (83.1, 91.1) |
| ANOVA* | F1,47 = 10.9, P = 0.002 | F1,47 = 1.3, P = 0.3 | F1,47 = 0.5, P = 0.5 | F1,47 = 0.02, P = 0.9 | F1,47 = 0.01, P = 0.97 | F1,47 = 1.3, P = 0.3 |
| Former amputees | ||||||
| Replanted 1 | 7.3 | 4.9 | 10.7 | 1.2 | 5.1 | 88.6 |
| Replanted 2 | 8.2 | 5.9 | 13.1 | 1.1 | 4.6 | 80.9 |
| Replanted 3 | 6.9 | 5.5 | 9.2 | 1.3 | 4.1 | 84.0 |
| Transplanted 1 | 7.5 | 4.9 | 9.9 | 1.7 | 4.0 | 94.7 |
| Transplanted 2 | 7.9 | 5.5 | 12.4 | 1.2 | 4.7 | 87.8 |
| Hand territory in right hemisphere in controls vs. hand territory ipsilateral to injury | ||||||
| Controls and current amputees | ||||||
| Controls | 8.8 ± 1.4 (8.3, 9.3) | 5.5 ± 0.8 (5.1, 5.9) | 10.5 ± 2.0 (9.8, 11.2) | 1.5 ± 0.3 (1.3, 1.6) | 4.4 ± 0.8 (3.9, 4.8) | 93.1 ± 5.7 (89.8, 96.5) |
| Current amputees | 9.1 ± 1.4 (8.6, 9.7) | 5.5 ± 1.1 (5.1, 5.9) | 10.9 ± 1.9 (10.1, 11.7) | 1.5 ± 0.4 (1.3, 1.6) | 4.4 ± 1.2 (3.9, 4.8) | 92.3 ± 6.2 (88.3, 96.3) |
| ANOVA* | F1,47 = 0.7, P = 0.4 | F1,47 = 0.1, P = 0.8 | F1,47 = 0.4, P = 0.5 | F1,47 = 0.0, P = 0.9 | F1,47 = 0.0, P = 0.98 | F1,47 = 0.2, P = 0.6 |
| Former amputees | ||||||
| Replanted 1 | 7.7 | 4.9 | 8.8 | 1.4 | 4.5 | 96.5 |
| Replanted 2 | 8.3 | 6.2 | 14.0 | 1.4 | 5.4 | 77.9 |
| Replanted 3 | 7.2 | 5.7 | 7.9 | 1.6 | 3.9 | 96.3 |
| Transplanted 1 | 8.4 | 4.9 | 8.2 | 1.9 | 5.6 | 100.0 |
| Transplanted 2 | 8.0 | 6.1 | 8.0 | 1.5 | 3.3 | 99.4 |
Values for controls and current amputees are means ± SD (95% confidence interval); values for former amputees are individual levels.
ANOVA between current amputee and control groups.
Custom MATLAB (2014; MathWorks, Natick, MA) programs were used to coregister the quantitative MRS output (from LCModel) on each individual's T1-weighted anatomical image. T1-weighted images were segmented into gray matter, white matter, and cerebrospinal fluid using Statistical Parametric Mapping 8 (SPM8; Wellcome Trust Center for Neuroimaging, University College London, London, United Kingdom). Volumes of gray matter and white matter per MRSI voxels were calculated using the segmented T1-weighted images by incorporating the point spread function of MRSI and the slice selection profile (Weber-Fahr et al., 2002).
The MRS voxels were selected from a similar sensorimotor territory in all participants using the anatomical landmarks [Fig. 1B; Yousry et al. (1997)] as well as the presence of functional activation. Only the spectroscopic voxels with the following criteria (Cirstea et al., 2011, 2014; Craciunas et al., 2013) were included into data analysis: signal-to-noise ratio [which is defined by the LCModel analysis program as the ratio between the difference between the maximum in the acquired spectrum and baseline and 2 times root-mean-square of residuals (Freeman, 1988)] >10, FWHM of the neurochemicals <20 Hz, and brain tissue (gray matter and white matter) volume >75%. A similar number of spectroscopic voxels was selected for all participants (left hemisphere: controls, 6 ± 1 voxels; current amputees, 6 ± 1; transplanted, 5 ± 0; replanted, 4 ± 1; right hemisphere: controls, 5 ± 1; current amputees, 5 ± 1; transplanted, 4 ± 0; replanted, 4 ± 0).
From each MRS voxel, any neurochemical with Cramér-Rao lower bounds values <15% were included into data analysis. MR spectra with obvious lipid signal contribution or artifacts (double peaks, etc.) were discarded. The neurochemical concentrations were then corrected for brain tissue volume obtained from the corresponding MRS voxel as follows: c = cLCModel × [1/Vbrain], where c is the corrected concentration, cLCModel is absolute concentration from LCModel (mmol/kg wet wt, mM), and Vbrain is the brain tissue (gray matter and white matter) volume. Finally, for each ROI, we averaged (mean ± SD) the corrected neurochemical absolute concentrations and the brain tissue volumes from the selected MRS voxels.
For purposes of averaging, data from the four amputees and one transplant recipient who had lost their left hands were left-right flipped so that the “left” hemisphere was contralateral to the affected limb. This same manipulation was done to data from five age- and sex-matched controls.
Statistical analysis of MRS data.
Statistical analyses were performed using SPSS Statistics (version 23.0; Chicago, IL). NAA (and mIn, Glx, Cho, and Cr) absolute concentrations were extracted from one ROI (primary sensorimotor hand area) in two hemispheres (left/contralateral to the missing hand and right/ipsilateral) and two groups (control and current amputee), and the means and SDs were computed. Smirnov normality tests revealed that neurochemical levels were normally distributed in control and amputee groups (P > 0.05 for all). Data were then analyzed via a 2 (groups: amputee vs. control) × 2 (hemispheres) × 1 (ROI) measures analysis of variance (mixed ANOVA). Significant effects were further evaluated with Bonferroni-corrected t-tests. Given the small sample sizes of former amputees, we presented and discussed the individual participant data relative to the healthy or current amputee range (95% confidence interval). Spearman correlation analysis was performed in the current amputees to investigate the relationships between NAA (and secondary neurochemicals), time after amputation, and pain scores.
RESULTS
1H-MRSI data quality.
Signal-to-noise ratios for all spectra in each group were within recommended tolerances (left hemisphere: controls, 16.8 ± 2.5 from LCModel; amputees, 13.9 ± 2.5; replanted, 12.8 ± 1.5; transplanted, 15.1 ± 02.7; right hemisphere: controls, 18.1 ± 2.6; amputees, 16.3 ± 2.5; replanted, 15.2 ± 2.9; transplanted, 16.1 ± 3.4). The FWHM of all neurochemicals included in the data analysis was <12.3 Hz in all participants, reflecting high spectral resolution [Jansen et al. (2006); left hemisphere: controls, 5.5 ± 1.4 Hz; current amputees, 6.0 ± 1.6 Hz; replanted, 6.0 ± 0.2 Hz; transplanted, 5.7 ± 11.1 Hz; right hemisphere: controls, 5.0 ± 9.8 Hz, current amputees, 5.8 ± 1.1 Hz; replanted, 5.0 ± 0.6 Hz; transplanted, 6.2 ± 0.9 Hz].
ROI brain tissue (gray matter and white matter) volumes were similar between groups and hemispheres.
Similar brain tissue volumes (from SPM8) within ROI were found in each group (left vs. contralateral hemisphere to the missing hand: 90.8 ± 0.1 vs. 87.0 ± 0.1%, P = 0.3; right vs. ipsilateral hemisphere: 93.1 ± 0.05 vs. 92.3 ± 0.1%, P = 0.6). Similar volumes were also found between hemispheres in controls (left vs. right, P = 0.2) and current amputees (contralateral vs. ipsilateral, P = 0.1). The brain tissue volumes for both left and right ROIs in former amputees fell within a 95% confidence interval of either group (Table 3).
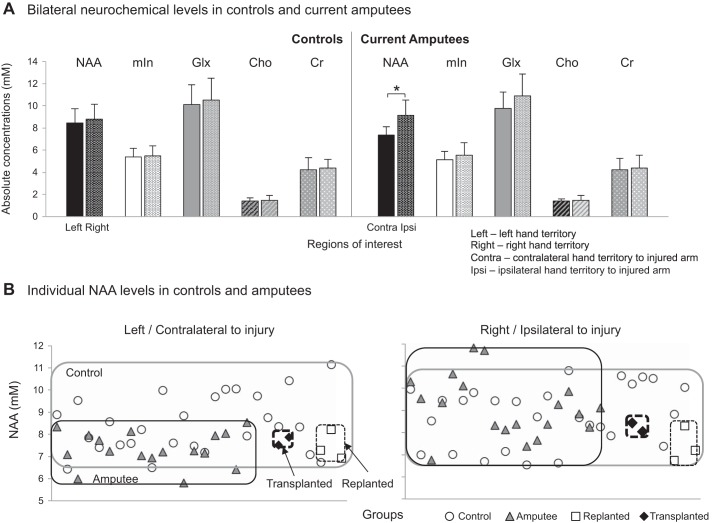
Healthy controls show similar interhemispheric levels of NAA and secondary neurochemicals.
Similar to our previous work (Cirstea et al., 2011), there were no significant differences in any of the neurochemical measures between left and right sensorimotor hand territories in healthy controls (NAA, F1,56 = 0.9, P = 0.3; mIn, F1,56 = 0.1, P = 0.7; Glx, F1,56 = 0.5, P = 0.5; Cho, F1,56 = 0.2, P = 0.7; Cr, F1,56 = 0.3, P = 0.6; Fig. 2A and Table 3). No correlations were found between age and the neurochemical levels (P > 0.5 for both left and right ROIs; for NAA, left, r = −0.01, P = 0.95; right, r = −0.06, P = 0.75).
Fig. 2.
A: mean + SD levels of NAA (black), mIn (white), Glx (light gray), Cho (patterned-line gray), and Cr (patterned-dot gray) in left and right hand sensorimotor territories in control group and in contralateral and ipsilateral territories in current amputee group. In amputees, NAA levels are significantly lower in contralateral vs. ipsilateral hand territory (P < 0.001). B: individual NAA levels in controls (○) and current (gray triangles) and former (replanted, □; transplanted, ◆) amputees in both left/contralateral and right/ipsilateral territories. The gray rectangle delimits data for all controls, the black rectangle delimits current amputees, the thin dashed rectangle delimits replanted, and the bold dashed rectangle delimits transplanted.
Current amputees exhibit lower contralateral NAA levels, unaltered ipsilateral NAA levels, and unaltered secondary neurochemicals in either contralateral or ipsilateral hand territories.
Contrary to controls, amputees displayed significantly lower NAA in the hand territory contralateral vs. ipsilateral to the missing hand (7.4 ± 0.7 vs. 9.1 ± 1.4 mM, F1,38 = 24.9, P < 0.001; Fig. 2A). This result was specific to NAA and was not found for mIn, Glx, Cho, or Cr (F1,38 = 1.8, P = 0.2; F1,38 = 4.1, P = 0.05; F1,38 = 0.3, P = 0.6; and F1,38 = 0.1, P = 0.7; respectively).
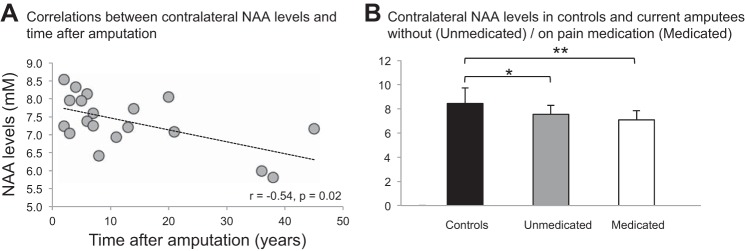
As predicted, relative to controls, the current amputees exhibited significantly lower NAA in the hand territory contralateral to the missing hand (controls, 8.4 ± 1.3 mM vs. amputees, 7.4 ± 0.7 mM, −12.8% difference, F1,47 = 10.9, P = 0.002; Table 3 and Fig. 2B). The mean of the control group fell with 95% certainty within the range of 7.9–8.9 mM while that of the current amputees fell within the range of 6.8–7.9 mM (Table 3). We also examined the effect of time on NAA levels and found a significant inverse relationship between contralateral NAA and years postinjury (r = −0.54, P = 0.02; Fig. 3A). Notably, this relationship persisted after controlling for age (r = −0.51, P = 0.03). This result may reflect a central neurodegenerative phenomenon associated with postamputation deafferentation [see reviews Feldman and Brecht (2005) and Holtmaat and Svoboda (2009)].
Fig. 3.
A: correlation between NAA levels in the contralateral hand sensorimotor territory and time after injury. Line indicates the statistically significant r (P < 0.05). B: mean + SD levels of NAA in left territory in control group (black) and contralateral territory in current amputees without (unmedicated, n = 10, gray) and with pain medication (medicated, n = 9, white). Note that NAA is significantly lower in both subgroups relative to control group (unmedicated, P = 0.01; medicated, P = 0.001). *P < 0.05, **P < 0.01.
NAA levels in the hand territory ipsilateral to the amputation did not differ from those detected in controls (Table 3 and Fig. 2).
We also failed to detect differences between current amputees and controls in mIn, Glx, Cho, or Cr in either the contralateral or ipsilateral hand territories (Table 3).
Contralateral NAA levels in current amputees were not significantly correlated with pain characteristics.
Our next series of analyses investigated whether NAA in the hand territory contralateral to the amputation is related to the pain exhibited by our participants. Although the correlations between NAA and PLP scores were of moderate strength (burst pain, r = 0.44, P = 0.07; NPS, r = 0.43, P = 0.07; SF-MPQ, r = 0.46, P = 0.06), they did not reach statistical significance. A nonsignificant correlation was also found between NAA and the RLP intensity (r = −0.34, P = 0.16).
Importantly, pain scores were not significantly correlated with time after amputation (P varied between 0.2 and 0.9) or age (P varied between 0.5 and 0.8).
Pain medication does not have an effect on contralateral NAA levels in current amputees.
Because 47% of current amputees are pain medicated, we also investigated medication effects on NAA levels. Since we expected a generalized effect, we investigated bilateral NAA levels in two subgroups of patients: pain medicated (n = 9; 55% female; age, 52.4 ± 14.3 yr; time postamputation, 13.7 ± 16.1 yr) vs. unmedicated (n = 10; 30% female; age, 39.4 ± 14.9 yr; time postamputation, 12.8 ± 10.5 yr). We found that interhemispheric differences in NAA levels persisted in both medicated (7.1 ± 0.7 mM in contralateral vs. 9.4 ± 1.5 mM in ipsilateral territory, paired t-test, P < 0.001) and unmedicated (7.5 ± 0.7 vs. 8.9 ± 1.2 mM, P = 0.001) subgroups. Furthermore, relative to controls, both medicated and unmedicated subgroups displayed significantly lower levels of NAA contralaterally (8.4 ± 1.3 mM in controls vs. 7.1 ± 0.7 mM, P = 0.001, and vs. 7.5 ± 0.7 mM, P = 0.01, respectively; Fig. 3B). Because the medicated subgroup is significantly older than controls (42.0 ± 12.6 yr, P = 0.04), we also explored the effects of age on contralateral NAA levels but found no significant correlation (r = −0.1, P = 0.8). Overall, these findings are consistent with the lack of a relationship between NAA changes in the former hand territory and use of pain medication.
Former amputees demonstrate NAA and secondary neurochemical levels comparable with current amputees.
Contrary to our hypothesis that after reafferentation the neuronal state would be similar to its status before injury, the NAA levels of two (out of 3) immediate hand replant patients and both allogeneic hand transplant patients fell within the range of the current amputees (Table 3 and Fig. 2B). This was also the case for mIn but not for Glx, Cho, and Cr, which fell within the control range (Table 3). Although the ipsilateral NAA, mIn, Glx, and Cr in most former amputees fell out of either group range, the ipsilateral Cho fell within the range of either group (Table 3).
The minimum sample size of former amputees needed to detect a difference of 1.0 mM in mean NAA levels (with a power of 80% and confidence of 95%) was calculated to be 16. We expected the power to be lower for the former amputees (who are rare), and thus we interpret these results with caution.
DISCUSSION
To our knowledge, this is the first study to provide evidence for alteration of cortical neuronal integrity associated with postamputation deafferentation/deefferentation. As predicted, compared with controls, amputees exhibited lower levels of NAA—a putative biomarker of neuronal integrity (Moffett et al., 2007)—in the sensorimotor hand territory located contralateral to the missing hand. NAA levels were further negatively associated with the time after amputation. Notably, no such differences were detected in any of the other neurochemicals that were assessed, indicating that these effects are quite selective in nature. Below, we discuss these findings in detail, as well as their implications and the limitations of our study.
Lower levels of NAA in the sensorimotor hand territory contralateral to amputation are associated with time after amputation.
Consistent with our predictions, we found lower levels of NAA in the former hand territory relative to the analogous territory in age- and sex-matched healthy controls (see Table 3). The lower levels of NAA reported here (by 12.8%) are of similar magnitude to MRS findings previously reported in other central nervous system pathologies, e.g., stroke [by 14.2% in M1; Cirstea et al. (2011)], systemic lupus erythematosus [by 15.9%; Sabet et al. (1998)], multiple sclerosis [by 12.3%; Sastre-Garriga et al. (2005)], and traumatic brain injury [by 16.1%; Capizzano et al. (2010)]. Consequently, we consider the differences in NAA levels reported in amputees compared with controls to be reliable.
Lower levels of NAA are thought to be indicative of neuronal metabolic downregulation and/or neuronal death [for review, see Moffett et al. (2007)]. We hypothesized that one major cue responsible for these neuronal changes is the progressive neuronal degenerative events caused by the loss of the afferent input from and possibly a reduction in efferent output to one hand. These reductions in activity are known to be associated with a variety of cellular-level metabolic (i.e., abnormalities in mitochondrial function) and morphological (i.e., shrinkage in body cell size, reduction in number of synaptic buttons, incomplete endings, loss of synapses, decreased metabolic activity, or even neuronal death) changes in cortical neurons [for reviews, see Feldman and Brecht (2005) and Holtmaat and Svoboda (2009)]. Likewise, loss of functional inhibitory interneurons may also contribute (Ralston et al., 1996; Welker et al., 1989). In addition, the negative relationship between NAA levels and time since injury suggests that these neuronal alterations may continue over years postamputation. This is consistent with evidence indicating progressive changes in cortical map organization years after amputation (Qi et al., 2000) or deafferenting nerve injury (Cusick, 1996).
A number of previous studies using fMRI reported a positive relationship between cortical reorganization in the former hand territory and PLP (Bolognini et al., 2013; Hsu and Cohen, 2013; Moseley and Flor, 2012), while other studies found an inverse (Makin et al., 2013) or no relationship (Makin et al., 2015). We failed to find a significant relationship between the intensity or duration of PLP and neuronal-level measurements in this territory. We also failed to find a significant relationship between the intensity of RLP and NAA levels. However, the nature of these relationships is unclear: Do these cellular-level changes somehow contribute to the maintenance of pain, or are they responses to chronic nociceptive inputs to primary sensorimotor cortex? Obviously, more work is needed to understand the nature of these relationships.
Thus the current NAA results support previous findings in animal models (Feldman and Brecht, 2005; Holtmaat and Svoboda, 2009) and humans (Freund et al., 2011; Makin et al., 2013; Preissler et al., 2013) by revealing altered neuronal state in the former hand territory probably resulting from deafferentation-related degenerative events.
It is important to note that the differences that we detected between amputees and controls were selective to NAA and not evident in mIn, Glx, Cho, or Cr (Table 3). Furthermore, no relationships were found between the secondary neurochemicals in this territory and pain ratings. This is consistent with the hypothesis that years and even decades after hand amputation, there is a low probability of gliosis/demyelination/inflammation/changes in brain energy homeostasis in this territory as suggested by earlier work in hand replant recipients (Ertem et al., 2005). Although Glx was not significantly different from controls, we found a trend toward lower levels in the former hand territory compared with ipsilateral territory (P = 0.05). Therefore, we cannot rule out that subtle changes in glutamatergic or GABA-ergic neurotransmission may be present (Bak et al., 2006). Because we quantified Glx, a composite of glutamate, glutamine, GABA, and other metabolites, the sensitivity of our method to detect an effect on each component of Glx has been limited.
Despite evidence for bilateral functional changes in the sensorimotor cortices following unilateral hand amputation (Bogdanov et al., 2012; Elbert and Rockstroh, 2004; Elbert et al., 1997; Frey et al., 2008), we failed to detect any evidence for alterations in levels of NAA (and secondary neurochemicals) in the ipsilateral (intact) hand territory relative to controls. Therefore, regardless of the mechanism underlying ipsilateral functional reorganization, the neurochemical profile is not altered, and thus this territory could be used as a control area in future chronic amputee 1H-MRS studies.
NAA levels in the sensorimotor hand territory contralateral to former amputation are comparable with those in current amputees.
Contrary to our expectations that NAA levels in the hand territory contralateral to the replanted/transplanted hand should not differ from levels in controls, but consistent with earlier results (Ertem et al., 2005), we found a trend of lower NAA in former amputees. Prior functional MRI data indicate that amputation-related reorganizational changes persist in former amputees long after hand transplantation (Frey et al., 2008) or replantation (Eickhoff et al., 2008). The persistent changes in NAA may echo these effects at the cellular level. However, because of our small sample, this preliminary result should be interpreted cautiously. Further work with larger sample sizes is warranted.
Limitations.
The current study has several limitations. The first limitation is the borderline significance between NAA levels in the former hand territory and phantom pain ratings (P varied between 0.05 and 0.07) for the group of current amputees; a larger sample size would allow for more reliable results concerning these relationships. Second, neurochemical concentrations depend on the brain tissue sampled. A brain tissue volume-corrected concentration was used. Third, there were no significant differences in brain tissue volume between groups (Table 3). This is compatible with a recent study showing that an artificial peripheral input may have a normalizing effect on the sensory cortex volume (Herrera-Rincon et al., 2012). Indeed, 79% of our current amputees regularly use prostheses to perform daily activities. Fourth, 47% of our current amputees were on pain medication. This could potentially alter neurochemical levels, and therefore the between-group differences could have been changed or masked. However, we do not attribute our results to pain medication use since we did not find a bilateral NAA alteration (as would be expected), and NAA levels in the former hand territory of those who do not take any kind of pain medication were significantly lower relative to controls (Fig. 3B). Finally, the variance of time since amputation is relatively large. Because we examined the amputees years and even decades after unilateral hand loss, we have no hint from the present data of the possible time course of NAA changes in this population.
Conclusions.
In summary, we found that over years and even decades after unilateral hand amputation, neuronal integrity is altered in the former sensorimotor hand territory. Local changes in NAA levels may provide a means of assessing neuroplasticity in a deafferented cortex.
GRANTS
This work was supported by United States Army Medical Research Acquisition Activity Grant W81XWH-09-2-0114 and National Institute of Neurological Disorders and Stroke Grant NS083377 to S. H. Frey.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.M.C. conceived and designed research; C.M.C. and H.P. performed experiments; C.M.C., I.-Y.C., P.L., and H.P. analyzed data; C.M.C. and S.H.F. interpreted results of experiments; C.M.C. prepared figures; C.M.C. drafted manuscript; C.M.C., I.-Y.C., P.L., C.L.K., and S.H.F. edited and revised manuscript; C.M.C., I.-Y.C., P.L., H.P., C.L.K., and S.H.F. approved final version of manuscript.
REFERENCES
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem 98: 641–653, 2006. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res 28: 941–953, 2003. doi: 10.1023/A:1023250721185. [DOI] [PubMed] [Google Scholar]
- Bogdanov S, Smith J, Frey SH. Former hand territory activity increases after amputation during intact hand movements, but is unaffected by illusory visual feedback. Neurorehabil Neural Repair 26: 604–615, 2012. doi: 10.1177/1545968311429687. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Spandri V, Olgiati E, Fregni F, Ferraro F, Maravita A. Long-term analgesic effects of transcranial direct current stimulation of the motor cortex on phantom limb and stump pain: a case report. J Pain Symptom Manage 46: e1–e4, 2013. doi: 10.1016/j.jpainsymman.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci 21: 149–186, 1998. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature 332: 446–448, 1988. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- Capizzano AA, Jorge RE, Robinson RG. Limbic metabolic abnormalities in remote traumatic brain injury and correlation with psychiatric morbidity and social functioning. J Neuropsychiatry Clin Neurosci 22: 370–377, 2010. doi: 10.1176/jnp.2010.22.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirstea CM, Brooks WM, Craciunas SC, Popescu EA, Choi IY, Lee P, Bani-Ahmed A, Yeh HW, Savage CR, Cohen LG, Nudo RJ. Primary motor cortex in stroke: a functional MRI-guided proton MR spectroscopic study. Stroke 42: 1004–1009, 2011. doi: 10.1161/STROKEAHA.110.601047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirstea CM, Savage CR, Nudo RJ, Cohen LG, Yeh HW, Choi IY, Lee P, Craciunas SC, Popescu EA, Bani-Ahmed A, Brooks WM. Handgrip-related activation in the primary motor cortex relates to underlying neuronal metabolism after stroke. Neurorehabil Neural Repair 28: 433–442, 2014. doi: 10.1177/1545968313516868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craciunas SC, Brooks WM, Nudo RJ, Popescu EA, Choi IY, Lee P, Yeh HW, Savage CR, Cirstea CM. Motor and premotor cortices in subcortical stroke: proton magnetic resonance spectroscopy measures and arm motor impairment. Neurorehabil Neural Repair 27: 411–420, 2013. doi: 10.1177/1545968312469835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick CG. Extensive cortical reorganization following sciatic nerve injury in adult rats versus restricted reorganization after neonatal injury: implications for spatial and temporal limits on somatosensory plasticity. Prog Brain Res 108: 379–390, 1996. doi: 10.1016/S0079-6123(08)62553-4. [DOI] [PubMed] [Google Scholar]
- Ding M, Haglid KG, Hamberger A. Quantitative immunochemistry on neuronal loss, reactive gliosis and BBB damage in cortex/striatum and hippocampus/amygdala after systemic kainic acid administration. Neurochem Int 36: 313–318, 2000. doi: 10.1016/S0197-0186(99)00139-4. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Dafotakis M, Grefkes C, Shah NJ, Zilles K, Piza-Katzer H. Central adaptation following heterotopic hand replantation probed by fMRI and effective connectivity analysis. Exp Neurol 212: 132–144, 2008. doi: 10.1016/j.expneurol.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Elbert T, Rockstroh B. Reorganization of human cerebral cortex: the range of changes following use and injury. Neuroscientist 10: 129–141, 2004. doi: 10.1177/1073858403262111. [DOI] [PubMed] [Google Scholar]
- Elbert T, Sterr A, Flor H, Rockstroh B, Knecht S, Pantev C, Wienbruch C, Taub E. Input-increase and input-decrease types of cortical reorganization after upper extremity amputation in humans. Exp Brain Res 117: 161–164, 1997. [DOI] [PubMed] [Google Scholar]
- Ertem K, Alkan A, Sarac K, Onal C, Bostan H, Yologlu S, Bora A. Magnetic resonance spectroscopy study of proton metabolite level changes in sensorimotor cortex after upper limb replantation-revascularization. Magn Reson Imaging 23: 105–109, 2005. doi: 10.1016/j.mri.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science 310: 810–815, 2005. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Flor H, Diers M, Andoh J. The neural basis of phantom limb pain. Trends Cogn Sci 17: 307–308, 2013. doi: 10.1016/j.tics.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Flor H, Nikolajsen L, Staehelin Jensen T. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci 7: 873–881, 2006. doi: 10.1038/nrn1991. [DOI] [PubMed] [Google Scholar]
- Freeman R. A Handbook of Nuclear Magnetic Resonance. Edinburgh: Longman, 1988. [Google Scholar]
- Freund P, Weiskopf N, Ward NS, Hutton C, Gall A, Ciccarelli O, Craggs M, Friston K, Thompson AJ. Disability, atrophy and cortical reorganization following spinal cord injury. Brain 134: 1610–1622, 2011. doi: 10.1093/brain/awr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey SH, Bogdanov S, Smith JC, Watrous S, Breidenbach WC. Chronically deafferented sensory cortex recovers a grossly typical organization after allogenic hand transplantation. Curr Biol 18: 1530–1534, 2008. doi: 10.1016/j.cub.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology 48: 332–338, 1997. doi: 10.1212/WNL.48.2.332. [DOI] [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med 55: 1219–1226, 2006. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- Hains BC, Black JA, Waxman SG. Primary cortical motor neurons undergo apoptosis after axotomizing spinal cord injury. J Comp Neurol 462: 328–341, 2003. doi: 10.1002/cne.10733. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Liepert J, Dettmers C, Adler T, Kiebel S, Rijntjes M, Weiller C. Structural and functional cortical abnormalities after upper limb amputation during childhood. Neuroreport 12: 957–962, 2001. doi: 10.1097/00001756-200104170-00019. [DOI] [PubMed] [Google Scholar]
- Herrera-Rincon C, Torets C, Sanchez-Jimenez A, Avendaño C, Panetsos F. Chronic electrical stimulation of transected peripheral nerves preserves anatomy and function in the primary somatosensory cortex. Eur J Neurosci 36: 3679–3690, 2012. doi: 10.1111/ejn.12000. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10: 647–658, 2009. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Hsu E, Cohen SP. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res 6: 121–136, 2013. doi: 10.2147/JPR.S32299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science 251: 944–947, 1991. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Jansen JF, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology 240: 318–332, 2006. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- Jones EG, Woods TM, Manger PR. Adaptive responses of monkey somatosensory cortex to peripheral and central deafferentation. Neuroscience 111: 775–797, 2002. doi: 10.1016/S0306-4522(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Plasticity of sensory and motor maps in adult mammals. Annu Rev Neurosci 14: 137–167, 1991. doi: 10.1146/annurev.ne.14.030191.001033. [DOI] [PubMed] [Google Scholar]
- Kaas JH. The reorganization of somatosensory and motor cortex after peripheral nerve or spinal cord injury in primates. Prog Brain Res 128: 173–179, 2000. doi: 10.1016/S0079-6123(00)28015-1. [DOI] [PubMed] [Google Scholar]
- Knecht S, Henningsen H, Elbert T, Flor H, Höhling C, Pantev C, Taub E. Reorganizational and perceptional changes after amputation. Brain 119: 1213–1219, 1996. doi: 10.1093/brain/119.4.1213. [DOI] [PubMed] [Google Scholar]
- Kowalczyk I, Duggal N, Bartha R. Proton magnetic resonance spectroscopy of the motor cortex in cervical myelopathy. Brain 135: 461–468, 2012. doi: 10.1093/brain/awr328. [DOI] [PubMed] [Google Scholar]
- Makin TR, Scholz J, Filippini N, Henderson Slater D, Tracey I, Johansen-Berg H. Phantom pain is associated with preserved structure and function in the former hand area. Nat Commun 4: 1570, 2013. doi: 10.1038/ncomms2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin TR, Scholz J, Henderson Slater D, Johansen-Berg H, Tracey I. Reassessing cortical reorganization in the primary sensorimotor cortex following arm amputation. Brain 138: 2140–2146, 2015. doi: 10.1093/brain/awv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R. The short-form McGill Pain Questionnaire. Pain 30: 191–197, 1987. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol 224: 591–605, 1984. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 81: 89–131, 2007. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley GL, Flor H. Targeting cortical representations in the treatment of chronic pain: a review. Neurorehabil Neural Repair 26: 646–652, 2012. doi: 10.1177/1545968311433209. [DOI] [PubMed] [Google Scholar]
- Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol 82: 163–201, 2007. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pelled G, Chuang KH, Dodd SJ, Koretsky AP. Functional MRI detection of bilateral cortical reorganization in the rodent brain following peripheral nerve deafferentation. Neuroimage 37: 262–273, 2007. doi: 10.1016/j.neuroimage.2007.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip BA, Frey SH. Compensatory changes accompanying chronic forced use of the nondominant hand by unilateral amputees. J Neurosci 34: 3622–3631, 2014. doi: 10.1523/JNEUROSCI.3770-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissler S, Feiler J, Dietrich C, Hofmann GO, Miltner WH, Weiss T. Gray matter changes following limb amputation with high and low intensities of phantom limb pain. Cereb Cortex 23: 1038–1048, 2013. doi: 10.1093/cercor/bhs063. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14: 260–264, 2001. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Qi HX, Stepniewska I, Kaas JH. Reorganization of primary motor cortex in adult macaque monkeys with long-standing amputations. J Neurophysiol 84: 2133–2147, 2000. [DOI] [PubMed] [Google Scholar]
- Ralston HJ III, Ohara PT, Meng XW, Wells J, Ralston DD. Transneuronal changes of the inhibitory circuitry in the macaque somatosensory thalamus following lesions of the dorsal column nuclei. J Comp Neurol 371: 325–335, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Sabet A, Sibbitt WL Jr, Stidley CA, Danska J, Brooks WM. Neurometabolite markers of cerebral injury in the antiphospholipid antibody syndrome of systemic lupus erythematosus. Stroke 29: 2254–2260, 1998. doi: 10.1161/01.STR.29.11.2254. [DOI] [PubMed] [Google Scholar]
- Sastre-Garriga J, Ingle GT, Chard DT, Ramió-Torrentà L, McLean MA, Miller DH, Thompson AJ. Metabolite changes in normal-appearing gray and white matter are linked with disability in early primary progressive multiple sclerosis. Arch Neurol 62: 569–573, 2005. doi: 10.1001/archneur.62.4.569. [DOI] [PubMed] [Google Scholar]
- Schubert V, Lebrecht D, Holtmaat A. Peripheral deafferentation-driven functional somatosensory map shifts are associated with local, not large-scale dendritic structural plasticity. J Neurosci 33: 9474–9487, 2013. doi: 10.1523/JNEUROSCI.1032-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zijl PC, Barker PB. Magnetic resonance spectroscopy and spectroscopic imaging for the study of brain metabolism. Ann NY Acad Sci 820, 75–96, 1997. doi: 10.1111/j.1749-6632.1997.tb46190.x. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain 126: 1430–1448, 2003. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Fahr W, Ende G, Braus DF, Bachert P, Soher BJ, Henn FA, Büchel C. A fully automated method for tissue segmentation and CSF-correction of proton MRSI metabolites corroborates abnormal hippocampal NAA in schizophrenia. Neuroimage 16: 49–60, 2002. doi: 10.1006/nimg.2002.1057. [DOI] [PubMed] [Google Scholar]
- Welker E, Soriano E, Van der Loos H. Plasticity in the barrel cortex of the adult mouse: effects of peripheral deprivation on GAD-immunoreactivity. Exp Brain Res 74: 441–452, 1989.Exp Brain Res 77October1989, 666 10.1007/BF00249620. [DOI] [PubMed] [Google Scholar]
- Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, Siddall PJ, Henderson LA. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex 19: 224–232, 2009. doi: 10.1093/cercor/bhn072. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120: 141–157, 1997. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]