ABSTRACT
Emerging insights have implicated the gut microbiota as an important factor in the maintenance of human health. Although nutrition research has focused on how direct interactions between dietary components and host systems influence human health, it is becoming increasingly important to consider nutrient effects on the gut microbiome for a more complete picture. Understanding nutrient-host-microbiome interactions promises to reveal novel mechanisms of disease etiology and progression, offers new disease prevention strategies and therapeutic possibilities, and may mandate alternative criteria to evaluate the safety of food ingredients. Here we review the current literature on diet effects on the microbiome and the generation of microbial metabolites of dietary constituents that may influence human health. We conclude with a discussion of the relevance of these studies to nutrition and public health and summarize further research needs required to realize the potential of exploiting diet-microbiota interactions for improved health.
KEYWORDS: fiber, gut microbiota, macronutrients, microbial metabolites, polyphenols, vegetarian
Diet as a major driver of microbial composition and function
The gut microbiota, which encompasses the trillions of organisms residing in the human gastrointestinal tract, is emerging as a major factor in the development of disease. These organisms assist with digestion, protect against invading organisms, and regulate metabolism and immunity.1,2 A disruption in these microbial functions has been associated with local and systemic disease and development of autoimmune disorders. Proposed mechanisms range from generation of bioactive metabolites to inducing systemic low-grade inflammation via Toll-Like receptors (TLR).3,4 While genetics, mode of delivery at birth, physical environment, age, stress, and other factors can influence the dynamics of the gut microbiota, diet may be the single most important driver of gut bacterial composition and function.5 In as little as 24-hourstransient changes in gut microbial composition and functional adaptations can be observed in response to dietary changes.6,7 In addition, habitual dietary patterns drive the establishment of stable, dominant microbial networks.7 These networks, or enterotypes, have become a convenient model for classifying an individual's gut bacterial profile, and may eventually serve as an indicator of disease risk or of microbial plasticity and the ability to respond to targeted dietary interventions. Dominance of plant or animal-based foods in the diet appears to be a predictor of enterotype classification, and these dietary patterns can typically be described as high fiber (plant-based foods) or high fat and protein (animal-based foods). Therefore, we have focused this review on the broad effects of these dietary patterns and their associated macronutrient composition on gut microbial community dynamics and function in adult humans. In addition, we will review interactions between the gut microbiota and phytochemicals. This is an exciting emergent area of research that may shed light on the widely variable bioavailability and bioactivity of these plant-based compounds. As we continue to uncover the importance of a healthy microbial community and the far reaching impacts of microbial dysbiosis on human health, the ability to optimize the gut microbiome through dietary strategies is critically needed.
Plant vs. animal-based diets
Numerous studies have shown that vegetarians compared with omnivores following a western diet exhibit a lower risk for various chronic diseases including obesity, hypertension, dyslipidemia, coronary heart disease, stroke, diabetes, and certain cancers. For example, non-Hispanic white, Hispanic, and African-American vegetarians compared with their same race/ethnicity omnivorous counterparts exhibit less obesity and hypertension and have more favorable lipid profiles.8-12 Longitudinal studies have shown vegetarians to exhibit lower mortality rates for cardiovascular disease and certain cancers in comparison to the general population.13,14 While vegetarians likely benefit from other health-related behaviors associated with lower disease risk, experimental studies that attempt to control for such confounders still show that changing from a western to a vegetarian dietary pattern reduces several disease risk factors.15 Identifying reasons for the lower disease burden among vegetarians has been the source of both study and speculation. Lower intakes of total fat, saturated fatty acids, dietary cholesterol, and animal proteins among vegetarians are thought to be important features of this dietary pattern linked to lower risk for chronic disease. However, there are many differences in the vegetarian and omnivorous dietary patterns beyond the distribution of these dietary macronutrients. In comparison to those consuming a western diet, vegetarians tend to ingest more dietary fiber, phytochemicals, potassium, magnesium, ascorbate, folate, and omega-6 polyunsaturated fatty acids, and have lower intakes of sodium, iron, and vitamin B-12.13 While these differences in dietary macro and micro-nutrient intakes can directly influence chronic disease risk, consumption of a plant-based versus western-type dietary pattern also results in distinct gut microbial communities,7 which in turn, via microbial metabolites may influence processes that are linked to chronic disease pathology.
While the effect of an exclusively vegetarian diet on the gut microbiota and microbial metabolites is not well-studied, animal models and human international studies have provided important insight into plant-based vs. animal-based dietary patterns. For example, among 60 mammalian species studied, microbiota clustered according to dietary patterns, and host bacterial diversity was highest among herbivores, followed by omnivores, and then by carnivores.16 Several observational studies of humans residing in different geographical areas with widely differing dietary patterns have also revealed important findings. The composition of the gut microbiota of children living in the African village of Burkina Faso was found to be distinct from that of children in Florence, Italy.17 While genetics, environment and other factors could account for these differences, the microbiota of unweaned children from both populations clustered together, suggesting that diet was the primary driver. The diets of the African children were largely vegetarian, with significant contributions of calories from millet and sorghum; this dietary pattern was associated with almost 50% greater enrichment of Bacteroidetes (Kruskil-Wallis, P = 4.80 × 10−4), including the unique appearance of the genera Prevotella and Xylanibacter, both of which are well equipped to harvest energy from indigestible plant polysaccharides. Likewise, Ou et al.18 found that among rural African (NA) adults compared with African Americans (AA), bacteria capable of polysaccharide degradation and fermentation to short chain fatty acids (acetate, propionate, and butyrate), including Prevotella (NA = 8.2 × 1010, AA = 3.5 × 1010; P = 0.011) and Clostridium clusters IV (NA = 5.1 × 109; AA = 2.9 × 109; P = 0.032) and XIVa (NA = 9.5 × 109, AA = 5.1 × 109; P = 0.049) were considerably more abundant. On the other hand, among the African Americans, who exhibited greater meat protein consumption, there was an abundance of bacteria capable of proteolytic fermentation with greater generation of branched chain fatty acids (BCFA) from amino acid degradation (isobutyrate: NA = 1.22, AA = 1.73; P = 0.02; isovaleric acid: NA = 0.33, AA = 1.49 ± 0.19 μmol/g feces; P = 0.0002; Mann-Whitney U test). Finally, in a third study, children and adults from the Amazonas State of Venezuela and from Malawi, where maize is eaten in large quantities, showed markedly greater fecal bacterial diversity compared with that of US children and adults (Americans∼1,200 OTUs compared with between 1,400–1,600 OTUs in Amerindians and Malawians), as well as up to 2.5x increase in amylase activity required for starch digestion, most likely the result of their higher maize intake.19 While these studies point to the important influence of dietary patterns on gut microbial composition, there are numerous non-dietary environmental factors that could also contribute to geographical differences in gut bacteria including hygiene and sanitation, soil composition, climate, etc. The nature of these international observational studies precludes controlling for such confounding factors.
Studies of vegetarians and non-vegetarians residing within similar geographic areas reduces confounding by other environmental factors. In general, these studies support findings from the international observational studies discussed above. Vegetarians compared with omnivores tend to exhibit greater bacterial diversity and richness and greater ratios of Prevotella to Bacteroides. For example, in a comparative study of vegans, lacto-ovo vegetarians, and omnivores, Zimmer et al.20 used culture-based methods and found lower counts of Bacteroides spp., Bifodobacterium spp, E. coli, and Enterobacteriaceae (P = 0.001, P = 0.002, P = 0.006 and P = 0.008, respectively) in vegans compared with the non-vegetarians. Vegans were also found to exhibit lower stool pH compared with non-vegetarians (P = 0.0001), presumably a reflection of greater production of short chain fatty acids via carbohydrate fermentation.
The gut microbiota composition changes rapidly in response to dietary adaptations. Using an experimental protocol, David et al.6 found rapid and consistent shifts, indicated by increased b-diversity compared with baseline (q < 0.05, Bonferroni-corrected Mann-Whitney U test), induced by 5-day ingestion of an animal-based diet, while the plant-based diet produced less profound effects. The animal diet was high in fat (69% of total calories) and protein (30% of total calories) and almost entirely void of carbohydrates, including dietary fiber. It induced gut bacterial taxonomic shifts and transcriptional responses characteristic of carnivorous mammals, with higher concentrations of bile-tolerant bacteria (presumably due to the extremely high fat intake known to increase bile acid secretion) and greater proteolytic activity and amino acid fermentation as determined by ∼2-fold higher fecal concentrations of short branched-chain fatty acids (isovalerate and isobutyrate). The opposite occurred on the plant-based diet (26 g fiber/1,000 kcal), with gut bacterial and transcriptome responses in line with herbivorous mammals (e.g. increases in fecal short chain fatty acids—acetate (∼2-fold higher) and butyrate (∼1.5-fold increase)—reflecting carbohydrate fermentation), although the magnitude of this response was less pronounced than that produced in the opposite direction by the animal-based diet. Note that these were extreme and abrupt dietary changes, which may have contributed to the somewhat discordant findings relative to a recent comparative study of vegans and omnivores by Wu et al.21 The latter found few dietary group differences in gut bacterial composition, and despite large differences in dietary intake of fermentable substrates between groups, i.e. higher indigestible carbohydrate in the vegans, there were no differences in fecal short chain fatty acids. They suggest that individuals residing in westernized communities are constrained by a more ‘restrictive’ gut microbiota with a limited ability to engage in energy harvest via fermentation of indigestible carbohydrates.
Foods of animal origin contain higher amounts of choline and L-carnitine, which have been linked to higher risk for cardiovascular disease as a result of their conversion to trimethylamines by gut bacteria, absorption into portal circulation, and conversion to trimethylamine N-oxides (TMAO) in the liver. While the mechanism(s) by which TMAO increases cardiovascular risk needs further clarification, it has been shown that TMAO reduces reverse cholesterol transport and bile acid synthesis22,23 potentially attenuating the normal route of intestinal cholesterol elimination. Vegans and lacto-ovo vegetarians have been shown to have negligible postprandial plasma TMAO concentrations in response to an L-carnitine meal challenge.23 Thus it appears that the lower CVD risk associated with a plant-based diet could result, in part, from lower circulating TMAO.
While there are some inconsistent findings among dietary studies and there is significant inter-individual variability among study participants within similar dietary patterns, taken together the observational and experimental studies comparing plant and animal-based diets suggest there are substrate-induced differences in gut microbial composition and metabolites. In particular, diet-induced differences in microbial metabolites such as SCFA, BCFA, secondary bile acids, and products of protein degradation all have the potential to modulate the host environment for disease prevention or promotion. Likewise, certain members of the microbial community can promote or attenuate immune responses and inflammation. Thus, microbiome differences resulting from these dietary patterns may contribute to the lower chronic disease risk seen with plant-based vs. western diets. As these differences between plant and animal-based diets are likely driven by differences in macronutrient (fat, protein, fiber) composition and phytochemical intake, these components are discussed in more depth in the following sections.
Dietary fats and protein
Few human studies have specifically examined the role of dietary fats and protein consumption on the composition and function of the gut microbiota. Increases in a particular macronutrient are typically associated with decreases in other macronutrients unless the diet is strictly controlled, and the combined effects of these dietary changes are responsible for the resulting alterations in gut microbial populations. Despite the difficulties of studying macronutrient effects in isolation, there is evidence to support that dietary fat and protein consumption elicit both compositional and functional changes to the gut microbiome. The partitioning of individuals into enterotypes appears to be driven by whether their primary dietary patterns include high complex carbohydrate (Prevotella) or high fat/protein (Bacteroides) consumption.7 More specifically, the Bacteroides enterotype was most strongly correlated with reports of frequent consumption of animal protein and saturated fat. Wu et al.7 implemented a short-term feeding study (CAFÉ study) where some individuals were randomized to a high fat/low fiber diet (38% fat, 35% carbohydrate, 27% protein) for 10 d while others were given a high fiber/low fat diet (13% fat, 69% carbohydrate, 18% protein). Although specific taxa changes varied between individuals, the high fat diet slowed intestinal transit time by as much as 3 d. Metagenomic analysis suggested that functional shifts, including greater protein export (P = 0.022) and lipoic acid metabolism (P = 0.045), were also associated with the high fat diet. As mentioned in the previous section, David et al.6 showed a rapid shift in gut microbial community composition and increased populations of Alistipes, Bilophila, and Bacteroides with 5-day consumption of a high fat/protein diet, which they hypothesized to be a result of increased bile secretion. Specific effects of fat on the gut microbiota will undoubtedly be dictated by the types of fats consumed, as was demonstrated in a recent rodent study. Animals fed lard showed increases in similar microbial genera as described in the previous human study and displayed signs of metabolic dysfunction, while animals fed fish oil showed increased levels of lactic acid bacteria and were protected from metabolic dysfunction.24
Diets high in fat interact in various ways with the gut microbiota to facilitate the translocation of bacterial lipopolysaccharides (LPS), which contribute to generation of chronic inflammation. LPS can be incorporated into lipid micelles formed during fat digestion, and certain gut microbes may be important in regulating this process. Studies in zebrafish suggest that gut bacteria in the phylum Firmicutes can increase the number and size of lipid droplets formed in the intestinal epithelia to facilitate lipid absorption.25 Bacterial modification can also alter activity and re-absorption of bile acids, which are important in fat digestion.26 Finally, these modified bile acids can act as agonists or antagonists of FXR, a central regulator of lipid transport and metabolism.27
The effects of high protein consumption (without concurrent high fat) on gut bacteria are not well studied, but are of increasing importance because of the current popularity of high protein diets. Obese men consuming a weight maintenance diet (85 g protein, 116 g fat, and 360 g carbohydrate/d) and two high protein weight loss diets (medium carbohydrate: 139 g protein, 82 g fat, 181 g carbohydrate/day; and low carbohydrate: 137 g protein, 142 g fat, 22 g carbohydrate/day) in a crossover design showed a marked decrease in total bacteria (maintenance diet = 10.52 log10/gram; high protein diets = 10.30 log10/gram) and reduced proportions of select butyrate producing organisms, which likely reflects the reduced carbohydrates rather than the increased protein.28 However, an examination of fecal metabolites from the high protein diets showed increased branch chain fatty acids (BCFA; ∼2.1 mmol/L in low carbohydrate diets vs 1.6 mmol/L in maintenance diet) Phenylacetic acid and N-nitroso compounds were about ∼3–5x higher in high protein diets, indicating a shift in microbial metabolism to protein fermentation. These metabolites, and the concurrent decrease in bacterial SCFAs resulting from reduced carbohydrate fermentation, may create a gut environment associated with inflammation and increased risk of colorectal cancer.29 Although these metabolite changes could indicate a negative impact of high protein diets on the gut microbiota, the overall influence of high protein diets may be dependent on other host factors. For example, elite professional athletes had lower inflammatory markers, improved metabolism, and greater microbial diversity compared with both low and high BMI control males and the increased bacterial diversity was positively correlated with protein consumption (correlation coefficients = 0.24–0.43).30 Decreased microbial diversity has been associated with colorectal cancer31 and other diseases.32 These seemingly opposing effects of high protein diets in the discussed studies suggest that the protein-diet interactions are modulated by factors such as host body composition and exercise intensity. The types and amounts of fats consumed in each of these studies were also likely important in the overall effects on the gut microbiota.
Carbohydrates and fiber
Carbohydrates as a chemical class have vastly different effects on gut microbiota, which is largely determined by the ability of the host to access the carbohydrate for energy. Relative to bacteria, humans possess a very limited number of enzymes for breakdown of dietary carbohydrates.33 These enzymes function to break down complex molecules with appropriate monomeric linkages into simple mono and di- saccharides, which are absorbed in the small intestine. Designation of a carbohydrate as ‘dietary fiber’ is made when polymers of three or more units pass undigested from the small intestine into the colonic environment.34,35 Gut microbiota rely on these recalcitrant fibers for energy, which they target for disassembly with a combined toolkit of thousands of enzymes.36-39 Dietary fiber as a combined chemical group represents one of the most diversely different chemical classes.40 Chemical structures include thousands of forms that vary in complexity and can be broken apart only with enzymes that pair appropriately with specific sugar composition, linkages and chain length.41 As such, dietary fiber represents enormous potential for modulation of gut microbiota based upon chemistry and accessibility of specific dietary fibers to particular microbial groups and consortia.42
Recent research by Sonnenburg et al. sought to clarify the importance of microbiota-accessible carbohydrates (MACs) to gut microbial communities through prolonged deprivation.43 Germ-free mice lacking resident microbiota were populated with a human fecal sample then assigned to either a high (LabDiet 5010) or low MAC (Harlan TD.86489) group. After seven weeks, mice on a low MAC diet had decreased abundance of 60% of the gut microbial taxa and nearly half of these taxa remained significantly less abundant six weeks after returning to a high MAC diet. The reduction of diversity primarily occurred in two bacterial taxa, Bacteroidales and Clostridiales. Further experiments in this same study revealed that the partial recovery of diversity when returning to the high MAC diet did not transfer to offspring, which experienced compounded diversity loss with each subsequent generation. Earlier investigations into effects of very low carbohydrate diets (approximately 20 g/day) on gut microbiota have suggested decreased abundance of butyrate-producing bacteria including Roseburia, Eubacterium rectale, and Bifidobacterium and a corresponding decrease in butyrate production.28,44,45 Increased intake of dietary fiber has not been shown to have a bifidogenic effect, but has been associated with an increase in gut microbial richness and/or diversity especially in individuals with reduced diversity initially.46,47 Additionally, dietary interventions that augment fiber intake have suggested altered gut microbial metabolism including increased glycan degradation47 that may be dependent on the chemical composition of the fiber being consumed.46 Similarly, overall gut bacterial gene diversity increased by 25% in obese and overweight subjects on a low calorie diet supplemented with soluble fiber.48
Long-term patterns of dietary fiber consumption can also shape the overall bacterial community type. As previously discussed, enterotype assignment to the Prevotella group is associated with high-fiber diets.7 Similarly, native rural Africans showed an enrichment of Prevotella spp relative to Bacteroides-dominated African Americans, who consume less resistant starch and more meat and fat.18 O'Keefe et al. studied effects on gut microbiota when fat and fiber content of native African and African American diets were swapped for 2 weeks,49 such that African Americans consumed high-fiber, low-fat rural African diets and vice versa. While changes in abundances of Bacteroides or Prevotella genera were not noted in either group, the high-fiber, low fat diets resulted in enrichment of bacterial genes for butyrate production and a decrease in genes for secondary bile acid synthesis.
Human microbiota changes associated with intake of specific dietary fibers with varying chemical characteristics were recently reviewed by Graf et al.50 and also by Hamaker et al.41 This survey of current literature supports the idea that dietary fibers of varying chemical composition induce different changes to gut microbiota. For example, resistant maltodextrin and hydrolyzed arabinoxylans were associated with increased Bacteroides51,52 after 21 or 24 d in two different crossover design studies in healthy adults. However, long chain inulin decreased Bacteroides and also Prevotella after 21 d in the same population type with a similar crossover design.53 Therefore, when interpreting results across studies involving increased intake of dietary fiber it's important to make note of the types of fibers or foods consumed by study participants.
Starches
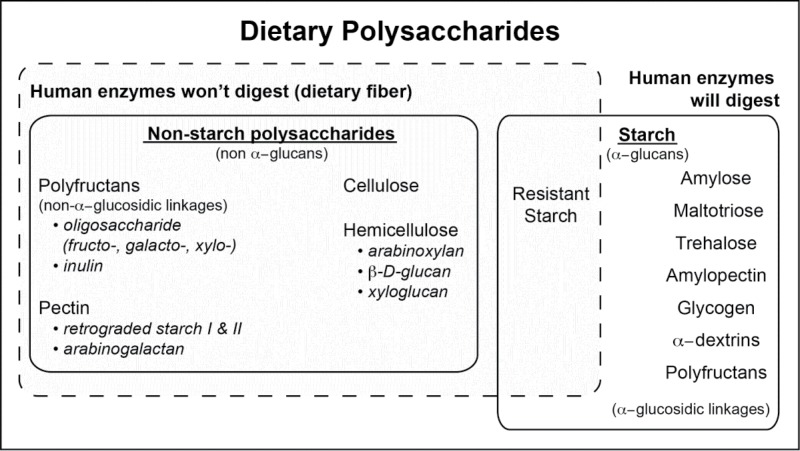
Dietary polysaccharides can be divided into starch and non-starch polysaccharides (Fig. 1). Humans are able to digest some starches, such as amylopectin and amylose, which starts digesting in the mouth with amylase found in saliva. Starches that cannot be broken down by human digestive enzymes enter the colon mostly unmodified from their ingested form and are subsequently fermented by gut microbiota. This component of dietary fiber is termed resistant starch and is categorized into four subtypes based on differing physicochemical properties. At least two human clinical trials investigating consumption of resistant starch have been conducted to date and both showed increases in Ruminococcus bromii and Eubacterium rectal.54,55 One in vitro study investigated cooperative degradation of resistant starch by co-culturing R. bromii, E. rectale, Bacteroides thetaiotaomicron, and Bifidobacterium adolescentis. The results highlight R. bromii as a keystone species that initiates degradation of resistant starch and produces byproducts that are more easily used by other gut species.56 Other results from this research varied widely and seemed to depend upon the type of resistant starch consumed.41,50
Figure 1.
Humans only possess digestive enzymes for breakdown of a subset of dietary polysaccharides, which can be divided into starches and non-starch polysaccharides. Recalcitrant components are defined as dietary fiber and provide substrates for gut microbial fermentation in the colon.
Non-starch polysaccharides
Dietary fiber that does not fit within the definition of starch is termed non-starch polysaccharides and includes polyfructans, cellulose, hemicellulose, and pectins. These fibers are typically complex and diverse with some categories having both soluble and insoluble components. Oligosaccharides that resist human digestion are made up of three to ten monosaccharide units, are frequently grouped together as polyfructans, and are named according to the dominant sugar type. Galactooligosaccharides (GOS) are best known for being an important component of human breast milk.57 Clinical trials investigating GOS consumption in infants58 adults,52,59 and older (age > 50) populations all showed increased Bifidobacterium, which was replicated in vitro.28 Some results suggest that Bacteroides and Clostridium may also be reduced with GOS, while F. prausnitzii may be increased. However, these results could not be confirmed across studies due to inter-individual variability and inconsistency of technical approaches.50,60 Fructooligosaccharides vary by fructan chain lengths from two up to nine, which can greatly influence study outcomes.50 In vitro co-culture experiments, including different species and strains of Bifidobacterium and butyrate-producing Roseburia spp, suggest that the species and strains present in the microbial community are also important.61,62 Bacterial cross-feeding likely plays a role in FOS degradation and further research is needed to determine how these relationships determine bifidogenic and/or butyrogenic outcomes. Xylo-oligosaccharides, predominated by xylose monomers, have also shown bifidogenic and butyrogenic effects relative to resistant maltodextrin 63 Longer chains of fructooligosaccharides contain chains of up to sixty fructose monomers and are classified as inulin. Most studies involving inulin included co-administration of other fibers.64-66 However, one human clinical trial involving consumption of 10 g/day longer chain inulin extracted from globe artichoke (Cynara scolymus) increased Bifidobacterium spp (2.8-fold; P < 0.05), Lactobacilli/Enterococci (2.4-fold; P < 0.01), and Atopobium spp (2.8-fold; P < 0.05) and decreased Bacteroides/Prevotella spp (1.77-fold; P < 0.05).53 Results from in vitro studies 67-69 and animal models 70-72 suggest that cross-feeding of the gut microbial community occurs during inulin degradation and suggest that E. rectale may be a primary degrader and that some butyrate-producing bacteria (R. intestinalis and A. caccae) can utilize smaller inulin fragments.72
Cellulose and hemi-cellulose
Cellulose has a particularly recalcitrant structure involving hydrogen bonding and microfibrils that interact with proteins and pectin to limit fermentation.73,74 Fermentability of dietary celluloses in the human gut varies by both food source75 and gut bacterial composition76 Gut bacteria that degrade cellulose can be divided into methane-producers, predominately Bacteroidetes, and non-methane producers, predominately Firmicutes. The species that degrade cellulose during digestion depends upon the specific cellulose structure being consumed and may include Clostridium spp, Eubacterium spp, Ruminococcus spp77-79 and Bacteroides spp80 Both cellulose and hemi-cellulose are components of most plant cell walls and hemi-celluloses can be further divided into arabinoxylans, xyloglucans, β-glucans, glucomannans, and galactomannans.41 The molecular size of hemi-celluloses plays a role in their effects on the gut bacterial community. As such, these components are often hydrolyzed to smaller and more consistently sized component fragments before supplementing. Two human studies have examined effects of consumption of hydrolyzed arabinoxylans, which form arabinoxylooligosaccharides. Both studies had a crossover design and were conducted over a period of 3 weeks, but effects on gut microbiota were different. One study, which used fluorescent in situ hybridization (FISH) to investigate microbial changes, showed increased Eubacterium rectale, Roseburia/Eubacterium, Faecalibacterium prausnitzii, and Bacteroides spp The other study used real time polymerase chain reaction (RT-PCR) and found increases in Bifidobacterium with neither study finding decreases in any of the targeted bacterial groups. Relative to arabinoxylans, limited research has been done investigating effects of β-glucans on gut microbiota. One in vitro study found that hydrolyzed β-glucans favored growth of the Bacteroides-Prevotella group.81 So far, animal models have primarily been used to investigate pectin influence on gut microbiota. Studies supplementing 6.5–7% pectin in rats increased abundance of Bacteroides spp after 3 weeks of citrus pectin,82 but increased abundance of Anaeroplasma, Anaerostipes, and Roseburia with apple pectin.83 Consuming apple pectin also decreased abundance of Alistipes and Bacteroides spp83 These results suggest that the structure of pectins is also a determinate of the effect on gut microbiota and that it varies according to the food source.82,83
Whole grains
The human diet rarely, if ever, includes chemically distinct fiber structures, but rather includes a complex mix of these structures as a part of the food we eat. As such, it is important to consider the effect of consuming these fibers in unique forms and combinations found in various foods and food products. Studies involving various cereal grains in humans show them to be bifidogenic. For example, two different crossover design studies lasting for three weeks showed increased Bifidobacterium spp, one using whole grain maize53 and the other using whole grain wheat cereal.84 Consuming maize cereal also increased abundance of Atobium spp and wheat cereal increased abundance of Lactobacilli/Enterococci. Two different studies, one supplementing with whole grain barley 54 and the other with heat-stabilized rice bran85 observed increased abundance of Bifidobacterium. A follow-up randomized clinical trial supplementing heat-stabilized rice bran (30 g/day) in colorectal cancer survivors did not find increased abundance of Bifidobacteria, but rather saw the largest increases in Bacteroides (20-fold increase; P = 0.043) and Lachnobacterium (23-fold increase; P = 0.039)46 among many other changes, and may have reflected lower baseline populations of Bifidobacterium spp in this population. Bifidogenic effects were also observed in a study where healthy adults consumed apples daily for 2 weeks86 or in different study where bananas were consumed daily for 60 d.87 Decreases in abundance of the Clostridium group were noted in studies supplementing rice bran,46 chickpeas,88 apples,86 mushrooms89 and low flavonoid fruit and vegetables.90 In contrast, increased abundance of Clostridium was observed in one human study supplementing wheat bran53 after three weeks and another supplementing navy bean powder46 after four weeks. The types of fibers contained in these foods combined with a summary of changes on gut microbiota listed in Supplemental Table 1.
Identifying effects of dietary fiber on gut microbiota is complicated by differences in chemical structures, other food components, host variation, and study methodologies. Regardless, some similar patterns are observed across studies including frequent increases in Bifidobacterium spp, Ruminococcus spp, Eubacterium spp and Faecalibacterium prausnitzii. The metabolites produced by fermentation of these fibers/whole foods often dictate whether observed microbial changes will impart positive or negative health effects as well as overall state of energy balance in the host. For example, SCFAs are a major by-product of fiber fermentation, and although they are critical to intestinal health and can positively influence host metabolism, they can also contribute to increased energy harvest from the diet.91 Additionally, polyphenols and other phytochemicals can be bound in ligno-cellulosic matrices and bacterial fermentation may alter their bioavailability and bioactivity, as discussed in the following section. Future research should focus on more detailed characterization of specific fibers found in whole plant foods and associations with microbial changes including these products of microbial metabolism.
Phytochemicals
“Phytochemical” is a term used to describe chemical compounds naturally occurring in plants which don't satisfy the definition of macro or micro-nutrients. In addition to the indigestible polysaccharides previously discussed, plants provide phytochemicals that include phenolic compounds, terpenoids, glucosinolates, chlorophylls, βlains, amines and other chemical compounds. One class of phytochemicals that has been studied extensively is the polyphenols. Phenolic/polyphenolic compounds are the second most abundant class of plant secondary metabolites and they act as antibiotics and antioxidants, as well as modulating numerous host processes via action as signaling molecules. While they are generally poorly absorbed in the host gastrointestinal tract, interactions with gut bacteria, particularly hydrolysis of glycosides, may increase their bioavailability.92 This section will be devoted to the findings on phenolic compounds and their impact on gut microbiota and metabolome, and will include findings from pre-clinical and in vitro studies due to the paucity of human intervention studies to date.
Monophenols and aromatic acids
Thymol and carvacrol, isomeric monoterpene phenols found in thyme have been studied for their potential antibiotic activities. Studies in broiler chickens indicate that thymol and carvacrol reduce the numbers of Campylobacter jejuni,93 and Salmonella, spp, Escherichia coli and Clostridium perfringens.94-97 Moreover, these studies reported that commensal bacteria were largely unaffected by these compounds. In vitro studies have demonstrated that thymol and carvacrol are not metabolized by human gut microbiota, suggesting they are likely to be absorbed in the upper gastrointestinal tract 98 or may be excreted in the feces.
Chlorogenic acid, an aromatic acid, is present in high quantities in coffee and tart cherries. As with many other phenolic compounds, Bifidobacterium and, to a lesser extent, Lactobacillus have been reported as potentially involved in bioconversion of chlorogenic acid.99-102
Flavonoids
A detailed review of bacterial species involved in flavonoid conversion has been published recently.103 Among the flavonols, quercetin has been the most extensively studied. It appears that a wide array of bacteria (E. coli, Bacteroides fragilis and several lactic acid bacteria) are able to degrade quercetin.104 Furthermore, studies in rats indicated that quercetin intake relieved the gut microbial dysbiosis induced by high fat diet consumption, possibly leading to reduced weight gain.105
Isoflavonoids
Isoflavones have received some attention for their purported health benefits and their natural abundance in soy and other Fabaceae. Isoflavones have been shown to be converted by gut bacteria to equol, which reportedly has more potent health benefits than the parent compounds.106 Specifically, Aldercreutzia equolifaciens, Slackia isoflavoniconvertens, Slackia equolifaciens, and Lactococcus garvieae have been identified as equol producers from daidzein, but only S. isoflavoniconvertens has been shown to produce equol from daidzein and genistein in vivo.107 Daidzein can also be converted to O-desmethylangolensin, and this metabotype has been shown to be potentially associated with obesity.108,109 Isoflavones are mostly present in glycoside forms, but aglycone forms are released by gut bacteria, which may increase their bioavailability in host tissues. In particular, the less common deglycosylation of isoflavone C-glycoside (puerarin and apitregin) is performed by Streptocococcus and Enterococcus strains.
Stilbenoids
Resveratrol is naturally found in several foods (peanuts, grapes, berries), but has mainly attracted interest as purportedly being responsible for the cardio-protective effects of red wine. However, there is currently insufficient evidence to support health claims. 110,111 Early and recent reports indicate that resveratrol stimulates growth of Bifidobacterium and Lactobacillus in mice gut microbiota.112,113 Moreover, Slackia equolifaciens and Adlercreutzia equolifaciens, have also been shown to produce dihydroresveratrol from trans-resveratrol.114
Tannins
Urolithins were the first microbial metabolites of dietary polyphenols, namely ellagitannins, to be attributed with more health beneficial properties than their parent polyphenol.115-117 A study showed that elagitannins from pomegranate stimulates Bifidobacterium and Lactobacillus (while inhibiting growth of Bacteroides, Clostridia and Enterobacteriaceae) in stool slurries, but those genera did not produce urolithins.118 In the companion in vivo study, it was found that Akkermansia are drastically more abundant in individuals producing urolithins compared with non-producers suggesting a role for this genus in the breakdown of ellagitannins.119 However, the specific bacterial genera and metabolic pathways involved in conversion of elagitannins to urolithins are still unknown.120
The other group of tannins that has been studied extensively is proanthocyanidins. Again, there is little information as to which groups of bacteria are involved in proanthocyanidin bioconversion, but Bifidobacterium and Lactobacillus increases have been reported in response to pure or anthocyanidin-rich fruit fermentation in vitro and in animal models.121,122 Diverse microbial metabolites from proanthocyanidins have been reported including phloroglucinol and benzoic acid derivatives,121 gallic, syringic and coumaric acids.123
Polyphenol-rich foods
A significant portion of the research on the effect of dietary polyphenols on gut microbiota and metabolome has been conducted with polyphenol-rich foods rather than specific polyphenol classes. Tea contains a mixture of polyphenolic compounds dominated by catechins. There is ample evidence that tea polyphenols exert strong antibacterial (pathogens), antitoxin and antiviral effects.92,124 There is less information on potential positive modulation of gut microbiota, but there are reports of bifidogenic effects,124 as well as reversal of diet-induced dysbiosis.125,126 However, it was also reported that long-term consumption of green tea did not alter the human gut microbiota,127 suggesting that the polyphenolic profile of the tea determines the gut microbiota response. Common metabolites from tea catechins include conjugated catechins, valerolactones, valeric acids and other phenolic acids.124,128,129 Coffee has been shown in vitro to stimulate Bifidobacterium,100,130,131 and to lead to the production of dihydrocaffeic acid, dihydroferulic acid, and 3-(3′-hydroxyphenyl)propionicacid.99 Cocoa and chocolate consumption have also been shown to increase Lactobacillus and Bifidobacterium counts in animal models,132,133 and human intervention studies.134,135
Berries including strawberries, raspberries, and blackcurrants have all been shown to increase Lactobacillus and Bifidobacterium levels and lead to the production of elagitannins or proanthocyanidins derivatives.136-138 Pomegranate appears to have a very similar impact to red berries on the gut microbiota and metabolome.118,119,139-141 Freeze-dried mango pulps prevented decreased Bifidobacterium counts observed in the cecal microbiota of mice subjected to a high-fat diet.142 Increase in Lactobacillus and Bifidobacterium counts were also observed in mice that were fed apples enriched in flavonoids; 122 however, this effect could arguably be due to the high amount of dietary fiber as previously discussed. Citrus contains large amounts of hesperetin, naringenin, and ferulic acid. These compounds are fermented to different hydroxyphenyl propionic acids by human gut microbiota in vitro 143 and in vivo.144,145
Understanding the interactions between dietary phytochemical compounds is a burgeoning area of study that promises to increase our understanding of the beneficial health effects of consuming plant-based foods. The reviewed studies clearly indicate bi-directional effects between dietary phytochemicals and gut microbial communities. Because of their poor bioavailability, the health relevance of many polyphenols has been questioned, but their effect on the intestinal environment and gut microbiota is worth further examination. The microbial metabolites of these compounds may have additional bioactivities that have not yet been explored and their interaction with the gut bacteria may increase host access to the aglycones and other metabolites. Properly controlled human intervention studies and observational studies incorporating valid phytochemical biomarkers are needed to determine the importance of these interactions in human health.
Conclusion
Diet is arguably one of the most important forces shaping the gut microbiota. Dietary interventions and targeted nutritional therapies, such as medical foods and dietary supplements, hold great promise for preventing and treating microbiota-associated diseases. However, much research is needed before these possibilities can be fully realized. Effects of specific nutrients, such as differential effects of mono- vs. polyunsaturated fats or various types of fiber, need to be assessed in clinical models. Also, while the enterotype concept is useful for classifying baseline gut microbiota populations, how these enterotypes influence disease risk and response to diet needs to be clarified before enterotyping can have clinical relevance. Individuals are likely to show varying responses to gut-targeted therapies depending on their baseline microbiota characteristics. Human genetic polymorphisms are also emerging as an important factor in determining diet and microbiota interactions, with recent research revealing that the fucosyltransferase 2 (FUC2) gene in women, which is referred to as “secretor” status, influences oligosaccharide content of the breastmilk and subsequent colonization of the microbiota of their infants.146
The effects of non-nutrient dietary components are another area that need additional study. Phytochemical-microbiota interactions may be an important contributor to the health promoting properties of many plant-based foods. Another important consideration is the impact of “novel” food ingredients on the gut microbiota. For example, artificial sweeteners were introduced a few decades ago and are generally considered attractive alternatives to sugar for individuals with diabetes or those trying to lose weight. However, recent studies suggest that these compounds may unfavorably alter the gut microbiota in ways that encourage insulin resistance and weight gain.147 Findings such as these suggest there may be a need to reassess the criteria used to evaluate the safety of food additives and ingredients. These and other questions regarding the microbiota-host-diet interactions are likely to be answered in the near future with a new emphasis in the field on integrating “omics” data sets and sophisticated gene and metabolite modeling for a systems-level approach.
Supplementary Material
Abbreviations
- BCFA
branched chain fatty acids
- FOS
fructo-oligosaccharide
- GOS
galacto-oligosaccharides
- LPS
lipopolysaccharide
- MAC
microbiota accessible carbohydrates
- SCFA
Short chain fatty acids
- TLR
Toll-like receptor
- TMAO
trimethylamine oxide
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
TLW is supported by funding from the International Life Sciences Institute (ILSI-North America) and Colorado Agriculture Experiment Station.
References
- [1].Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN. Role of the normal gut microbiota. World J Gastroenterol 2015; 21:8787-803; PMID:26269668; http://dx.doi.org/ 10.3748/wjg.v21.i29.8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Consortium THMP Structure, function and diversity of the healthy human microbiome. Nature 2012; 486:207-14; PMID:22699609; http://dx.doi.org/ 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Marchesi JR, Adams DH, Fava F, Hermes GDA, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, et al.. The gut microbiota and host health: A new clinical frontier. Gut 2016; 65:330-9; PMID:26338727; http://dx.doi.org/ 10.1136/gutjnl-2015-309990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Richards JL, Yap YA, McLeod KH, Mackay CR, Marino E. Dietary metabolites and the gut microbiota: An alternative approach to control inflammatory and autoimmune diseases. Clin Trans Immunology 2016; 5:e82; http://dx.doi.org/ 10.1038/cti.2016.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Voreades N, Kozil A, Weir T. Diet and the development of the human intestinal microbiome. Front Microbiol 2014; 5:494; PMID:25295033; http://dx.doi.org/ 10.3389/fmicb.2014.00494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al.. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505:559-63; PMID:24336217; http://dx.doi.org/ 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al.. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011; 334:105-8; PMID:21885731; http://dx.doi.org/ 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alexander H, Lockwood LP, Harris MA, Melby CL. Risk factors for cardiovascular disease and diabetes in two groups of Hispanic Americans with differing dietary habits. J Am Coll Nutr 1999; 18:127-36; PMID:10204828; http://dx.doi.org/ 10.1080/07315724.1999.10718840 [DOI] [PubMed] [Google Scholar]
- [9].Melby CL, Goldflies DG, Hyner GC, Lyle RM. Relation between vegetarian/nonvegetarian diets and blood pressure in black and white adults. Am J Public Health 1989; 79:1283-8; PMID:2764208; http://dx.doi.org/ 10.2105/AJPH.79.9.1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Melby CL, Goldflies DG, Toohey ML. Blood pressure differences in older black and white long-term vegetarians and nonvegetarians. J Am Coll Nutr 1993; 12:262-9; PMID:8409080; http://dx.doi.org/ 10.1080/07315724.1993.10718308 [DOI] [PubMed] [Google Scholar]
- [11].Melby CL, Toohey ML, Cebrick J. Blood pressure and blood lipids among vegetarian, semivegetarian, and nonvegetarian African Americans. Am J Clin Nutr 1994; 59:103-9; PMID:8279389 [DOI] [PubMed] [Google Scholar]
- [12].Toohey ML, Harris MA, DeWitt W, Foster G, Schmidt WD, Melby CL. Cardiovascular disease risk factors are lower in African-American vegans compared to lacto-ovo-vegetarians. J Am Coll Nutr 1998; 17:425-34; PMID:9791838; http://dx.doi.org/ 10.1080/07315724.1998.10718789 [DOI] [PubMed] [Google Scholar]
- [13].Orlich MJ, Singh PN, Sabate J, Jaceldo-Siegl K, Fan J, Knutsen S, Beeson WL, Fraser GE. Vegetarian dietary patterns and mortality in adventist health study 2. JAMA Intern Med 2013; 173:1230-8; PMID:23836264; http://dx.doi.org/ 10.1001/jamainternmed.2013.6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the adventist health study-2. Nutr Metab Cardiovasc Dis 2013; 23:292-9; http://dx.doi.org/ 10.1016/j.numecd.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bloomer RJ, Kabir MM, Canale RE, Trepanowski JF, Marshall KE, Farney TM, Hammond KG. Effect of a 21 day Daniel Fast on metabolic and cardiovascular disease risk factors in men and women. Lipids Health Dis 2010; 9:94; PMID:20815907; http://dx.doi.org/ 10.1186/1476-511X-9-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ley RE, Hamady M, Lozupone C, Turnbaugh P, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, et al.. Evolution of mammals and their gut microbes. Science 2008; 320:1647-51; PMID:18497261; http://dx.doi.org/ 10.1126/science.1155725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 2010; 107:14691-6; PMID:20679230; http://dx.doi.org/ 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, Gaskins HR, O'Keefe SJ. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr 2013; 98:111-20; PMID:23719549; http://dx.doi.org/ 10.3945/ajcn.112.056689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al.. Human gut microbiome viewed across age and geography. Nature 2012; 486:222-7; PMID:22699611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zimmer J, Lange B, Frick JS, Sauer H, Zimmermann K, Schwiertz A, Rusch K, Klosterhalfen S, Enck P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr 2012; 66:53-60; PMID:21811294; http://dx.doi.org/ 10.1038/ejcn.2011.141 [DOI] [PubMed] [Google Scholar]
- [21].Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, Chehoud C, Albenberg LG, Nessel L, Gilroy E, et al.. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016; 65:63-72; PMID:25431456; http://dx.doi.org/ 10.1136/gutjnl-2014-308209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wilson A, McLean C, Kim RB. Trimethylamine-N-oxide: A link between the gut microbiome, bile acid metabolism, and atherosclerosis. Curr Opin Lipidol 2016; 27:148-54; PMID:26959704; http://dx.doi.org/ 10.1097/MOL.0000000000000274 [DOI] [PubMed] [Google Scholar]
- [23].Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al.. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013; 19:576-85; PMID:23563705; http://dx.doi.org/ 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani Patrice D, Bäckhed F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab 2015; 22:658-68; PMID:26321659; http://dx.doi.org/ 10.1016/j.cmet.2015.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Semova I, Carten Juliana D, Stombaugh J, Mackey Lantz C, Knight R, Farber Steven A, Rawls JF. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe; 12:277-88; PMID:22980325; http://dx.doi.org/ 10.1016/j.chom.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 2006; 47:241-59; PMID:16299351; http://dx.doi.org/ 10.1194/jlr.R500013-JLR200 [DOI] [PubMed] [Google Scholar]
- [27].Qi Y, Jiang C, Cheng J, Krausz KW, Li T, Ferrell JM, Gonzalez FJ, Chiang JY. Bile acid signaling in lipid metabolism: Metabolomic and lipidomic analysis of lipid and bile acid markers linked to anti-obesity and anti-diabetes in mice. Biochim Biophys Acta 2015; 1851:19-29; PMID:24796972; http://dx.doi.org/ 10.1016/j.bbalip.2014.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Russell WR, Gratz SW, Duncan SH, Holtrop G, Ince J, Scobbie L, Duncan G, Johnstone AM, Lobley GE, Wallace RJ, et al.. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr 2011; 93:1062-72; PMID:21389180; http://dx.doi.org/ 10.3945/ajcn.110.002188 [DOI] [PubMed] [Google Scholar]
- [29].Sheflin AM, Whitney AK, Weir TL. Cancer-promoting effects of microbial dysbiosis. Curr Oncol Rep 2014; 16(10):406; PMID:25123079; http://dx.doi.org/ 10.1007/s11912-014-0406-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Clarke SF, Murphy EF, O'Sullivan O, Lucey AJ, Humphreys M, Hogan A, Hayes P, O'Reilly M, Jeffery IB, Wood-Martin R, et al.. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014; 63:1913-20; PMID:25021423; http://dx.doi.org/ 10.1136/gutjnl-2013-306541 [DOI] [PubMed] [Google Scholar]
- [31].Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, Goedert JJ, Hayes RB, Yang L. Human gut microbiome and risk of colorectal cancer. J Natl Cancer Inst 2013; 105:1907-11; PMID:24316595; http://dx.doi.org/ 10.1093/jnci/djt300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Scher JU, Ubeda C, Artacho A, Attur M, Isaac S, Reddy SM, Marmon S, Neimann A, Brusca S, Patel T, et al.. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol 2015; 67:128-39; PMID:25319745; http://dx.doi.org/ 10.1002/art.38892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Martens EC. Microbiome: Fibre for the future. Nature 2016; 529:158-9; PMID:26762451; http://dx.doi.org/ 10.1038/529158a [DOI] [PubMed] [Google Scholar]
- [34].Camire M, Cho S, Craig S, Devrie J, Gordon D, Jones J, et al.. The definition of dietary fiber. Cereal Foods World 2001; 46:112-24 [Google Scholar]
- [35].Commission CA Report of the 30th session of the codex committee on nutrition and foods for special dietary uses. Cape Town, South Africa: ALINORM, 2008. [Google Scholar]
- [36].Cuskin F, Lowe EC, Temple MJ, Zhu Y, Cameron EA, Pudlo NA, Porter NT, Urs K, Thompson AJ, Cartmell A, et al.. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 2015; 517:165-9; PMID:25567280; http://dx.doi.org/ 10.1038/nature13995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol 2013; 11:497-504; PMID:23748339; http://dx.doi.org/ 10.1038/nrmicro3050 [DOI] [PubMed] [Google Scholar]
- [38].Larsbrink J, Rogers TE, Hemsworth GR, McKee LS, Tauzin AS, Spadiut O, et al.. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature 2014; 506:498-502; PMID:24463512; http://dx.doi.org/ 10.1038/nature12907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, et al.. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol 2011; 9:e1001221; PMID:22205877; http://dx.doi.org/ 10.1371/journal.pbio.1001221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Davidson MH, McDonald A. Fiber: Forms and functions. Nutr Res 1998; 18:617-24; http://dx.doi.org/ 10.1016/S0271-5317(98)00048-7 [DOI] [Google Scholar]
- [41].Hamaker BR, Tuncil YE. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J Mol Biol 2014; 426:3838-50; PMID:25088686; http://dx.doi.org/ 10.1016/j.jmb.2014.07.028 [DOI] [PubMed] [Google Scholar]
- [42].Ze X, Le Mougen F, Duncan SH, Louis P, Flint HJ. Some are more equal than others: the role of “keystone” species in the degradation of recalcitrant substrates. Gut Microbes 2013; 4:236-40; PMID:23549436; http://dx.doi.org/ 10.4161/gmic.23998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016; 529:212-5; PMID:26762459; http://dx.doi.org/ 10.1038/nature16504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Brinkworth GD, Noakes M, Clifton PM, Bird AR. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br J Nutr 2009; 101:1493-502; PMID:19224658; http://dx.doi.org/ 10.1017/S0007114508094658 [DOI] [PubMed] [Google Scholar]
- [45].Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol 2007; 73:1073-8; PMID:17189447; http://dx.doi.org/ 10.1128/AEM.02340-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sheflin AM, Borresen EC, Kirkwood JS, Boot CM, Whitney AK, Lu S, Brown RJ, Broeckling CD, Ryan EP, Weir TL. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors. Mol Nutr Food Res 2016; PMID:27461523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tap J, Furet JP, Bensaada M, Philippe C, Roth H, Rabot S, Lakhdari O, Lombard V, Henrissat B, Corthier G, et al.. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ Microbiol 2015; 17:4954-64; PMID:26235304; http://dx.doi.org/ 10.1111/1462-2920.13006 [DOI] [PubMed] [Google Scholar]
- [48].Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, lmeida M, Quinquis B, Levenez F, Galleron N, et al.. Dietary intervention impact on gut microbial gene richness. Nature 2013; 500:585-8; PMID:23985875; http://dx.doi.org/ 10.1038/nature12480 [DOI] [PubMed] [Google Scholar]
- [49].O'Keefe SJ, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, Posma JM, Kinross J, Wahl E, Ruder E, et al.. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun 2015; 6:6342; http://dx.doi.org/ 10.1038/ncomms7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Graf D, Di Cagno R, Fåk F, Flint HJ, Nyman M, Saarela M, Watzl B. Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis 2015; 26:26164; ISSN: 1651-2235; PMID:25656825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Baer DJ, Stote KS, Henderson T, Paul DR, Okuma K, Tagami H, Kanahori S, Gordon DT, Rumpler WV, Ukhanova M, et al.. The metabolizable energy of dietary resistant maltodextrin is variable and alters fecal microbiota composition in adult men. J Nutr 2014; 144:1023-9; PMID:24744316; http://dx.doi.org/ 10.3945/jn.113.185298 [DOI] [PubMed] [Google Scholar]
- [52].Walton GE, van den Heuvel EG, Kosters MH, Rastall RA, Tuohy KM, Gibson GR. A randomised crossover study investigating the effects of galacto-oligosaccharides on the faecal microbiota in men and women over 50 years of age. Br J Nutr 2012; 107:1466-75; PMID:21910949; http://dx.doi.org/ 10.1017/S0007114511004697 [DOI] [PubMed] [Google Scholar]
- [53].Costabile A, Klinder A, Fava F. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. Brit J Nutr 2008; 99:110-120; PMID:17761020; http://dx.doi.org/ 10.1017/S0007114507793923 [DOI] [PubMed] [Google Scholar]
- [54].Martínez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, Louk JA, Rose DJ, Kyureghian G, Peterson DA, et al.. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J 2013; 7:269-80; http://dx.doi.org/ 10.1038/ismej.2012.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A, et al.. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 2010; 5:220-30; PMID:20686513; http://dx.doi.org/ 10.1038/ismej.2010.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. The ISME J 2012; 6:1535-43; PMID:22343308; http://dx.doi.org/ 10.1038/ismej.2012.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Macfarlane G, Steed H, Macfarlane S. Bacterial metabolism and health‐related effects of galacto‐oligosaccharides and other prebiotics. J Appl Microbiol 2008; 104:305-44; PMID:18215222 [DOI] [PubMed] [Google Scholar]
- [58].Vivatvakin B, Mahayosnond A, Theamboonlers A, Steenhout PG, Conus NJ. Effect of a whey-predominant starter formula containing LCPUFAs and oligosaccharides (FOS/GOS) on gastrointestinal comfort in infants. Asia Pacific Journal of Clinical Nutrition 2010; 19:473-80; PMID:21147707 [PubMed] [Google Scholar]
- [59].Vulevic J, Juric A, Tzortzis G, Gibson GR. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J Nutr 2013; 143:324-31; PMID:23303873; http://dx.doi.org/ 10.3945/jn.112.166132 [DOI] [PubMed] [Google Scholar]
- [60].Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res 2013; 69:52-60; PMID:23147033; http://dx.doi.org/ 10.1016/j.phrs.2012.10.020 [DOI] [PubMed] [Google Scholar]
- [61].Falony G, Calmeyn T, Leroy F, De Vuyst L. Coculture fermentations of Bifidobacterium species and Bacteroides thetaiotaomicron reveal a mechanistic insight into the prebiotic effect of inulin-type fructans. Appl Environ Microbiol 2009; 75:2312-9; PMID:19251883; http://dx.doi.org/ 10.1128/AEM.02649-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Scott KP, Martin JC, Chassard C, Clerget M, Potrykus J, Campbell G, Mayer CD, Young P, Rucklidge G, Ramsay AG, et al.. Substrate-driven gene expression in Roseburia inulinivorans: importance of inducible enzymes in the utilization of inulin and starch. Proc Natl Acad Sci USA 2011; 108:4672-9; PMID:20679207; http://dx.doi.org/ 10.1073/pnas.1000091107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lecerf J-M, Dépeint F, Clerc E, Dugenet Y, Niamba CN, Rhazi L, Cayzeele A, Abdelnour G, Jaruga A, Younes H, et al.. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr 2012; 108:1847-58; PMID:22264499; http://dx.doi.org/ 10.1017/S0007114511007252 [DOI] [PubMed] [Google Scholar]
- [64].Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, Bindels LB, de Vos WM, Gibson GR, Thissen JP, et al.. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013; 62:1112-21; PMID:23135760; http://dx.doi.org/ 10.1136/gutjnl-2012-303304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].García-Peris P, Velasco C, Lozano M, Moreno Y, Paron L, de la Cuerda C, Bretón I, Camblor M, García-Hernández J, Guarner F, et al.. Effect of a mixture of inulin and fructo-oligosaccharide on Lactobacillus and Bifidobacterium intestinal microbiota of patients receiving radiotherapy: a randomised, double-blind, placebo-controlled trial. Nutr Hosp 2012; 27:1908-15; PMID:23588438 [DOI] [PubMed] [Google Scholar]
- [66].Waitzberg DL, Pereira CA, Logullo L, Jacintho TM, Almeida D, Silva M, Matos de Miranda Torrinhas RS. Microbiota benefits after inulin and partially hydrolized guar gum supplementation–a randomized clinical trial in constipated women. Nutr Hosp 2012; 27:123-9; PMID:22566311 [DOI] [PubMed] [Google Scholar]
- [67].Grootaert C, Van den Abbeele P, Marzorati M, Broekaert WF, Courtin CM, Delcour JA, Verstraete W, Van de Wiele T. Comparison of prebiotic effects of arabinoxylan oligosaccharides and inulin in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol Ecol 2009; 69:231-42; PMID:19508502; http://dx.doi.org/ 10.1111/j.1574-6941.2009.00712.x [DOI] [PubMed] [Google Scholar]
- [68].Rossi M, Corradini C, Amaretti A, Nicolini M, Pompei A, Zanoni S, Matteuzzi D. Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study of pure and fecal cultures. Appl Environ Microbiol 2005; 71:6150-8; PMID:16204533; http://dx.doi.org/ 10.1128/AEM.71.10.6150-6158.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Van de Wiele T, Boon N, Possemiers S, Jacobs H, Verstraete W. Inulin‐type fructans of longer degree of polymerization exert more pronounced in vitro prebiotic effects. J ApplMicrobiol 2007; 102:452-60; PMID:17241351 [DOI] [PubMed] [Google Scholar]
- [70].Juśkiewicz J, Zduńczyk Z, Frejnagel S. Caecal parameters of rats fed diets supplemented with inulin in exchange for sucrose. Archives of Animal Nutrition 2007; 61:201-10; PMID:17578262; http://dx.doi.org/ 10.1080/17450390701297735 [DOI] [PubMed] [Google Scholar]
- [71].Sakaguchi E, Sakoda C, Toramaru Y. Caecal fermentation and energy accumulation in the rat fed on indigestible oligosaccharides. Br J Nutr 1998; 80:469-76; PMID:9924269 [PubMed] [Google Scholar]
- [72].Van den Abbeele P, Gérard P, Rabot S, Bruneau A, El Aidy S, Derrien M, Kleerebezem M, Zoetendal EG, Smidt H, Verstraete W, et al.. Arabinoxylans and inulin differentially modulate the mucosal and luminal gut microbiota and mucin‐degradation in humanized rats. Environ Microbiol 2011; 13:2667-80; PMID:21883787; http://dx.doi.org/ 10.1111/j.1462-2920.2011.02533.x [DOI] [PubMed] [Google Scholar]
- [73].Leschine SB. Cellulose degradation in anaerobic environments. AnnRev Microbiol 1995; 49:399-426; http://dx.doi.org/ 10.1146/annurev.mi.49.100195.002151 [DOI] [PubMed] [Google Scholar]
- [74].Slavin JL, Brauer PM, Marlett JA. Neutral detergent fiber, hemicellulose and cellulose digestibility in human subjects. J Nutr 1981; 111:287-97; PMID:6257867 [DOI] [PubMed] [Google Scholar]
- [75].Van Soest PJ. Dietary fibers: their definition and nutritional properties. Am J Clin Nutr 1978; 31:S12-S20; PMID:707360 [DOI] [PubMed] [Google Scholar]
- [76].Chassard C, Delmas E, Robert C, Bernalier-Donadille A. The cellulose-degrading microbial community of the human gut varies according to the presence or absence of methanogens. FEMS Microbiol Ecol 2010; 74:205-13; PMID:20662929; http://dx.doi.org/ 10.1111/j.1574-6941.2010.00941.x [DOI] [PubMed] [Google Scholar]
- [77].Montgomery L. Isolation of human colonic fibrolytic bacteria. Lett Appl Microbiol 1988; 6:55-7; http://dx.doi.org/ 10.1111/j.1472-765X.1988.tb01214.x [DOI] [Google Scholar]
- [78].Robert C, Bernalier-Donadille A. The cellulolytic microflora of the human colon: evidence of microcrystalline cellulose-degrading bacteria in methane-excreting subjects. FEMS Microbiol Ecol 2003; 46:81-9; PMID:19719585; http://dx.doi.org/ 10.1016/S0168-6496(03)00207-1 [DOI] [PubMed] [Google Scholar]
- [79].Wedekind K, Mansfield H, Montgomery L. Enumeration and isolation of cellulolytic and hemicellulolytic bacteria from human feces. Appl Environ Microbiol 1988; 54:1530-5; PMID:3415224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Betian H, Linehan B, Bryant M, Holdeman L. Isolation of a cellulolytic bacteroides sp. from human feces. Appl Environ Microbiol 1977; 33:1009-10; PMID:869523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hughes SA, Shewry PR, Gibson GR, McCleary BV, Rastall RA. In vitro fermentation of oat and barley derived β-glucans by human faecal microbiota. FEMS Microbiol Ecol 2008; 64:482-93; PMID:18430007; http://dx.doi.org/ 10.1111/j.1574-6941.2008.00478.x [DOI] [PubMed] [Google Scholar]
- [82].Dongowski G, Lorenz A, Proll J. The degree of methylation influences the degradation of pectin in the intestinal tract of rats and in vitro. J Nutr 2002; 132:1935-44; PMID:12097673 [DOI] [PubMed] [Google Scholar]
- [83].Licht TR, Hansen M, Bergström A, Poulsen M, Krath BN, Markowski J, et al.. Effects of apples and specific apple components on the cecal environment of conventional rats: Role of apple pectin. BMC Microbiology 2010; 10:13 doi: 10.1186/1471-2180-10-13; PMID:20089145; http://dx.doi.org/ 10.1186/1471-2180-10-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Carvalho-Wells AL, Helmolz K, Nodet C, Molzer C, Leonard C, McKevith B, et al.. Determination of the in vivo prebiotic potential of a maize-based whole grain breakfast cereal: A human feeding study. Brit J Nutr 2010; 104:1353-6; PMID:20487589; http://dx.doi.org/ 10.1017/S0007114510002084 [DOI] [PubMed] [Google Scholar]
- [85].Sheflin AM, Borresen EC, Wdowik MJ, Rao S, Brown RJ, Heuberger AL, et al.. Pilot dietary intervention with heat-stabilized rice bran modulates stool microbiota and metabolites in healthy adults. Nutrients 2015; 7:1282-300; PMID:25690418; http://dx.doi.org/ 10.3390/nu7021282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Shinohara K, Ohashi Y, Kawasumi K, Terada A, Fujisawa T. Effect of apple intake on fecal microbiota and metabolites in humans. Anaerobe 2010; 16:510-5; PMID:20304079; http://dx.doi.org/ 10.1016/j.anaerobe.2010.03.005 [DOI] [PubMed] [Google Scholar]
- [87].Mitsou E, Kougia E, Nomikos T, Yannakoulia M, Mountzouris K, Kyriacou A. Effect of banana consumption on faecal microbiota: A randomised, controlled trial. Anaerobe 2011; 17:384-7; PMID:21524710; http://dx.doi.org/ 10.1016/j.anaerobe.2011.03.018 [DOI] [PubMed] [Google Scholar]
- [88].Fernando W, Hill J, Zello G, Tyler R, Dahl W, Van Kessel A. Diets supplemented with chickpea or its main oligosaccharide component raffinose modify faecal microbial composition in healthy adults. Beneficial Microbes 2010; 1:197-207; PMID:21831757; http://dx.doi.org/ 10.3920/BM2009.0027 [DOI] [PubMed] [Google Scholar]
- [89].Varshney J. The beneficial effects of white button mushrooms on the gut health [master's thesis]. [State College]: The Pennsylvania State University; 2012. [Google Scholar]
- [90].Klinder A, Shen Q, Heppel S, Lovegrove JA, Rowland I, Tuohy KM. Impact of increasing fruit and vegetables and flavonoid intake on the human gut microbiota. Food Funct 2016; 7:1788-96; PMID:26757793; http://dx.doi.org/ 10.1039/C5FO01096A [DOI] [PubMed] [Google Scholar]
- [91].den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 2013; 54:2325-40; PMID:23821742; http://dx.doi.org/ 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].van Duynhoven J, Vaughan EE, Jacobs DM, Kemperman RA, van Velzen EJJ, Gross G, et al.. Metabolic fate of polyphenols in the human superorganism. ProcNatl Acad Sci USA 2011; 108:4531-8; http://dx.doi.org/ 10.1073/pnas.1000098107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Thibodeau A, Fravalo P, Yergeau E, Arsenault J, Lahaye L, Letellier A. Chicken caecal microbiome modifications induced by campylobacter jejuni colonization and by a non-antibiotic feed additive. PLoS One 2015; 10:e0131978; PMID:26161743; http://dx.doi.org/ 10.1371/journal.pone.0131978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Du E, Gan L, Li Z, Wang W, Liu D, Guo Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. Anim Sci Biotechnol 2015; 6:58, 015-0055-7; ; http://dx.doi.org/ 10.1186/s40104-015-0055-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Thapa D, Losa R, Zweifel B, Wallace RJ. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology 2012; 158:2870-7; PMID:22878397; http://dx.doi.org/ 10.1099/mic.0.061127-0 [DOI] [PubMed] [Google Scholar]
- [96].Thapa D, Louis P, Losa R, Zweifel B, Wallace RJ. Essential oils have different effects on human pathogenic and commensal bacteria in mixed faecal fermentations compared with pure cultures. Microbiology 2015; 161:441-9; PMID:25500493; http://dx.doi.org/ 10.1099/mic.0.000009 [DOI] [PubMed] [Google Scholar]
- [97].Tiihonen K, Kettunen H, Bento MH, Saarinen M, Lahtinen S, Ouwehand AC, Schulze H, Rautonen N. The effect of feeding essential oils on broiler performance and gut microbiota. Br Poult Sci 2010; 51:381-92; PMID:20680873; http://dx.doi.org/ 10.1080/00071668.2010.496446 [DOI] [PubMed] [Google Scholar]
- [98].Mosele JI, Martin-Pelaez S, Macia A, Farras M, Valls RM, Catalan U, et al.. Study of the catabolism of thyme phenols combining in vitro fermentation and human intervention. J Agric Food Chem 2014; 62:10954-61; PMID:25339317; http://dx.doi.org/ 10.1021/jf503748y [DOI] [PubMed] [Google Scholar]
- [99].Ludwig IA, Paz de Pena M, Concepcion C, Alan C. Catabolism of coffee chlorogenic acids by human colonic microbiota. Biofactors 2013; 39:623-32; PMID:23904092; http://dx.doi.org/ 10.1002/biof.1124 [DOI] [PubMed] [Google Scholar]
- [100].Mills CE, Tzounis X, Oruna-Concha MJ, Mottram DS, Gibson GR, Spencer JP. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br J Nutr 2015; 113:1220-7; PMID:25809126; http://dx.doi.org/ 10.1017/S0007114514003948 [DOI] [PubMed] [Google Scholar]
- [101].Tomas-Barberan F, Garcia-Villalba R, Quartieri A, Raimondi S, Amaretti A, Leonardi A, Rossi M. In vitro transformation of chlorogenic acid by human gut microbiota. Mol Nutr Food Res 2014; 58:1122-31; PMID:24550206; http://dx.doi.org/ 10.1002/mnfr.201300441 [DOI] [PubMed] [Google Scholar]
- [102].Raimondi S, Anighoro A, Quartieri A, Amaretti A, Tomas-Barberan FA, Rastelli G, Rossi M. Role of bifidobacteria in the hydrolysis of chlorogenic acid. Microbiology Open 2015; 4:41-52; PMID:25515139; http://dx.doi.org/ 10.1002/mbo3.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Braune A, Blaut M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016; 7:216-34; PMID:26963713; http://dx.doi.org/ 10.1080/19490976.2016.1158395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zhang Z, Peng X, Li S, Zhang N, Wang Y, Wei H. Isolation and identification of quercetin degrading bacteria from human fecal microbes. PLoS One 2014; 9:e90531; PMID:24594786; http://dx.doi.org/ 10.1371/journal.pone.0090531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Etxeberria U, Arias N, Boque N, Macarulla MT, Portillo MP, Martinez JA, Milagro FI. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J Nutr Biochem 2015; 26:651-60; PMID:25762527; http://dx.doi.org/ 10.1016/j.jnutbio.2015.01.002 [DOI] [PubMed] [Google Scholar]
- [106].Setchell KD, Equol CC. History, chemistry, and formation. J Nutr 2010; 140:1355S-62S; PMID:20519412; http://dx.doi.org/ 10.3945/jn.109.119776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Matthies A, Loh G, Blaut M, Braune A. Daidzein and genistein are converted to equol and 5-hydroxy-equol by human intestinal slackia isoflavoniconvertens in gnotobiotic rats. J Nutr 2012; 142:40-6; PMID:22113864; http://dx.doi.org/ 10.3945/jn.111.148247 [DOI] [PubMed] [Google Scholar]
- [108].Frankenfeld CL, Atkinson C, Wahala K, Lampe JW. Obesity prevalence in relation to gut microbial environments capable of producing equol or O-desmethylangolensin from the isoflavone daidzein. Eur J Clin Nutr 2014; 68:526-30; PMID:24569543; http://dx.doi.org/ 10.1038/ejcn.2014.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Reverri EJ, Slupsky CM, Mishchuk DO, Steinberg FM. Metabolomics reveals differences between three daidzein metabolizing phenotypes in adults with cardiometabolic risk factors. Mol Nutr Food Res 2016; PMID:27364093 [DOI] [PubMed] [Google Scholar]
- [110].Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Tomás-Barberán FA, García-Conesa MT, Espín JC. Resveratrol in primary and secondary prevention of cardiovascular disease: A dietary and clinical perspective. Ann N Y Acad Sci 2013; 1290:37-51; PMID:23855464; http://dx.doi.org/ 10.1111/nyas.12150 [DOI] [PubMed] [Google Scholar]
- [111].Nunez-Sanchez MA, Gonzalez-Sarrias A, Romo-Vaquero M, Garcia-Villalba R, Selma MV, Tomas-Barberan FA, García-Conesa MT, Espín JC. Dietary phenolics against colorectal cancer–from promising preclinical results to poor translation into clinical trials: Pitfalls and future needs. Mol Nutr Food Res 2015; 59:1274-91; PMID:25693744; http://dx.doi.org/ 10.1002/mnfr.201400866 [DOI] [PubMed] [Google Scholar]
- [112].Larrosa M, Yanez-Gascon MJ, Selma MV, Gonzalez-Sarrias A, Toti S, Ceron JJ, Tomás-Barberán F, Dolara P, Espín JC. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J Agric Food Chem 2009; 57:2211-20; PMID:19228061; http://dx.doi.org/ 10.1021/jf803638d [DOI] [PubMed] [Google Scholar]
- [113].Chen ML, Yi L, Zhang Y, Zhou X, Ran L, Yang J, et al.. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio 2016; 7(2):e02210-15; http://dx.doi.org/ 10.1128/mBio.02210-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bode LM, Bunzel D, Huch M, Cho GS, Ruhland D, Bunzel M, Bub A, Franz CM, Kulling SE. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am J Clin Nutr 2013; 97:295-309; PMID:23283496; http://dx.doi.org/ 10.3945/ajcn.112.049379 [DOI] [PubMed] [Google Scholar]
- [115].Espin JC, Larrosa M, Garcia-Conesa MT, Tomás-Barberán F. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: The evidence so far. Evid Based Complement Alternat Med 2013; 2013:270418; PMID:23781257; http://dx.doi.org/ 10.1155/2013/270418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Tulipani S, Urpi-Sarda M, García-Villalba R, Rabassa M, López-Uriarte P, Bulló M, Jáuregui O, Tomás-Barberán F, Salas-Salvadó J, Espín JC, et al.. Urolithins are the main urinary microbial-derived phenolic metabolites discriminating a moderate consumption of nuts in free-living subjects with diagnosed metabolic syndrome. J Agric Food Chem 2012; 60:8930-40; PMID:22631214; http://dx.doi.org/ 10.1021/jf301509w [DOI] [PubMed] [Google Scholar]
- [117].Tomas-Barberan FA, Gonzalez-Sarrias A, Garcia-Villalba R, Nunez-Sanchez MA, Selma MV, Garcia-Conesa MT, Espín JC. Urolithins, the rescue of ‘old’ metabolites to understand a ‘new’ concept: Metabotypes as a nexus between phenolic metabolism, microbiota dysbiosis and host health status. Mol Nutr Food Res 2016. [DOI] [PubMed] [Google Scholar]
- [118].Li Z, Summanen PH, Komoriya T, Henning SM, Lee RP, Carlson E, Heber D, Finegold SM. Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe 2015; 34:164-8; PMID:26051169; http://dx.doi.org/ 10.1016/j.anaerobe.2015.05.012 [DOI] [PubMed] [Google Scholar]
- [119].Li Z, Henning SM, Lee R-P, Lu Q-Y, Summanen PH, Thames G, Corbett K, Downes J, Tseng CH, Finegold SM, et al.. Pomegranate extract induces ellagitannin metabolite formation and changes stool microbiota in healthy volunteers. Food & Function 2015; 6:2487-95; PMID:26189645; http://dx.doi.org/ 10.1039/C5FO00669D [DOI] [PubMed] [Google Scholar]
- [120].Garcia-Munoz C, Vaillant F. Metabolic fate of ellagitannins: Implications for health, and research perspectives for innovative functional foods. Crit Rev Food Sci Nutr 2014; 54; PMID:24580560; http://dx.doi.org/ 10.1080/10408398.2011.644643 [DOI] [PubMed] [Google Scholar]
- [121].Faria A, Fernandes I, Norberto S, Mateus N, Calhau C. Interplay between anthocyanins and gut microbiota. J Agric Food Chem 2014; 62:6898-902; PMID:24915058; http://dx.doi.org/ 10.1021/jf501808a [DOI] [PubMed] [Google Scholar]
- [122].Espley RV, Butts CA, Laing WA, Martell S, Smith H, McGhie TK, Zhang J, Paturi G, Hedderley D, Bovy A, et al.. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J Nutr 2014; 144:146-54; PMID:24353343; http://dx.doi.org/ 10.3945/jn.113.182659 [DOI] [PubMed] [Google Scholar]
- [123].Hanske L, Engst W, Loh G, Sczesny S, Blaut M, Braune A. Contribution of gut bacteria to the metabolism of cyanidin 3 glucoside in human microbiota-associated rats. Br J Nutr 2013; 109:1433-41; PMID:22906731; http://dx.doi.org/ 10.1017/S0007114512003376 [DOI] [PubMed] [Google Scholar]
- [124].van Duynhoven J, Vaughan EE, van Dorsten F, Gomez-Roldan V, de Vos R, Vervoort J, van der Hooft JJ, Roger L, Draijer R, Jacobs DM. Interactions of black tea polyphenols with human gut microbiota: Implications for gut and cardiovascular health. Am J Clin Nutr 2013; 98:1631S-41S; PMID:24172295; http://dx.doi.org/ 10.3945/ajcn.113.058263 [DOI] [PubMed] [Google Scholar]
- [125].Seo DB, Jeong HW, Cho D, Lee BJ, Lee JH, Choi JY, et al.. Fermented green tea extract alleviates obesity and related complications and alters gut microbiota composition in diet-induced obese mice. J Med Food 2015; 18:549-56; PMID:25764354; http://dx.doi.org/ 10.1089/jmf.2014.3265 [DOI] [PubMed] [Google Scholar]
- [126].Foster MT, Gentile CL, Cox-York K, Wei Y, Wang D, Estrada AL, Reese L, Miller T, Pagliassotti MJ, Weir TL. Fuzhuan tea consumption imparts hepatoprotective effects and alters intestinal microbiota in high saturated fat diet-fed rats. Mol Nutr Food Res 2016; 60:1213-20; PMID:26890069; http://dx.doi.org/ 10.1002/mnfr.201500654 [DOI] [PubMed] [Google Scholar]
- [127].Janssens PL, Penders J, Hursel R, Budding AE, Savelkoul PH, Westerterp-Plantenga MS. Long-term green tea supplementation does not change the human gut microbiota. PLoS One 2016; 11:e0153134; PMID:27054321; http://dx.doi.org/ 10.1371/journal.pone.0153134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].van Duynhoven J, van der Hooft JJ, van Dorsten FA, Peters S, Foltz M, Gomez-Roldan V, Vervoort J, de Vos RC, Jacobs DM. Rapid and sustained systemic circulation of conjugated gut microbial catabolites after single-dose black tea extract consumption. J Proteome Res 2014; 13:2668-78; PMID:24673575; http://dx.doi.org/ 10.1021/pr5001253 [DOI] [PubMed] [Google Scholar]
- [129].Clarke KA, Dew TP, Watson RE, Farrar MD, Osman JE, Nicolaou A, Rhodes LE, Williamson G. Green tea catechins and their metabolites in human skin before and after exposure to ultraviolet radiation. J Nurt Biochem 2016; 27:203-10; http://dx.doi.org/ 10.1016/j.jnutbio.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Jaquet M, Rochat I, Moulin J, Cavin C, Bibiloni R. Impact of coffee consumption on the gut microbiota: A human volunteer study. Int J Food Microbiol 2009; 130:117-21; PMID:19217682; http://dx.doi.org/ 10.1016/j.ijfoodmicro.2009.01.011 [DOI] [PubMed] [Google Scholar]
- [131].Nakayama T, Oishi K. Influence of coffee (coffea arabica) and galacto-oligosaccharide consumption on intestinal microbiota and the host responses. FEMS Microbiol Lett 2013; 343:161-8; PMID:23551139; http://dx.doi.org/ 10.1111/1574-6968.12142 [DOI] [PubMed] [Google Scholar]
- [132].Massot-Cladera M, Perez-Berezo T, Franch A, Castell M, Pérez-Cano FJ. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch Biochem Biophys 2012; 527:105-12; PMID:22663919; http://dx.doi.org/ 10.1016/j.abb.2012.05.015 [DOI] [PubMed] [Google Scholar]
- [133].Jang S, Sun J, Chen P, Lakshman S, Molokin A, Harnly JM, Vinyard BT, Urban JF Jr, Davis CD, Solano-Aguilar G. Flavanol-enriched cocoa powder alters the intestinal microbiota, tissue and fluid metabolite profiles, and intestinal gene expression in pigs. J Nutr 2016; 146:673-80; PMID:26936136; http://dx.doi.org/ 10.3945/jn.115.222968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Tzounis X, Rodriguez-Mateos A, Vulevic J, Gibson GR, Kwik-Uribe C, Spencer JP. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am J Clin Nutr 2011; 93:67-72; http://dx.doi.org/ 10.3945/ajcn.110.000075 [DOI] [PubMed] [Google Scholar]
- [135].Martin FP, Montoliu I, Nagy K, Moco S, Collino S, Guy P, Redeuil K, Scherer M, Rezzi S, Kochhar S. Specific dietary preferences are linked to differing gut microbial metabolic activity in response to dark chocolate intake. J Proteome Res 2012; 11:6252-63; PMID:23163751; http://dx.doi.org/ 10.1021/pr300915z [DOI] [PubMed] [Google Scholar]
- [136].Puupponen-Pimiä R, Seppänen-Laakso T, Kankainen M, Maukonen J, Törrönen R, Kolehmainen M, Leppänen T, Moilanen E, Nohynek L, Aura AM, et al.. Effects of ellagitannin-rich berries on blood lipids, gut microbiota, and urolithin production in human subjects with symptoms of metabolic syndrome. Mol Nutr Food Res 2013; 57:2258-63; http://dx.doi.org/ 10.1002/mnfr.201300280 [DOI] [PubMed] [Google Scholar]
- [137].Truchado P, Larrosa M, García-Conesa MT, Cerdá B, Vidal-Guevara ML, Tomás-Barberán FA, Espín JC. Strawberry processing does not affect the production and urinary excretion of urolithins, ellagic acid metabolites, in humans. J Ag Food Chem 2012; 60:5749-54; http://dx.doi.org/ 10.1021/jf203641r [DOI] [PubMed] [Google Scholar]
- [138].Jakobsdottir G, Blanco N, Xu J, Ahrn S, et al.. Formation of short-chain fatty acids, excretion of anthocyanins, and microbial diversity in rats fed blackcurrants, blackberries, and raspberries. J Nutr Met 2013; 202534; http://dx.doi.org/ 10.1155/2013/202534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Neyrinck AM, Van Hee VF, Bindels LB, De Backer F, Cani PD, Delzenne NM. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: Potential implication of the gut microbiota. Br J Nutr 2013; 109:802-9; PMID:22676910; http://dx.doi.org/ 10.1017/S0007114512002206 [DOI] [PubMed] [Google Scholar]
- [140].Nuñez-Sánchez MA, García-Villalba R, Monedero-Saiz T, García-Talavera NV, Gómez-Sánchez MB, Sánchez-Álvarez C, García-Albert AM, Rodríguez-Gil FJ, Ruiz-Marín M, Pastor-Quirante FA, et al.. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol Nutr Food Res 2014; 58:1199-211; http://dx.doi.org/ 10.1002/mnfr.201300931 [DOI] [PubMed] [Google Scholar]
- [141].Bialonska D, Ramnani P, Kasimsetty SG, Muntha KR, Gibson GR, Ferreira D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int J Food Microbiol 2010; 140:175-82; PMID:20452076; http://dx.doi.org/ 10.1016/j.ijfoodmicro.2010.03.038 [DOI] [PubMed] [Google Scholar]
- [142].Ojo B, El-Rassi GD, Payton ME, Perkins-Veazie P, Clarke S, Smith BJ, Lucas EA. Mango supplementation modulates gut microbial dysbiosis and short-chain fatty acid production independent of body weight reduction in C57BL/6 mice fed a high-fat diet. J Nutr 2016; PMID:27358411 [DOI] [PubMed] [Google Scholar]
- [143].Pereira-Caro G, Borges G, Ky I, Ribas A, Calani L, Del Rio D, Clifford MN, Roberts SA, Crozier A. In vitro colonic catabolism of orange juice (poly)phenols. Mol Nutr Food Res 2015; 59:465-75; PMID:25545994; http://dx.doi.org/ 10.1002/mnfr.201400779 [DOI] [PubMed] [Google Scholar]
- [144].Vallejo F, Larrosa M, Escudero E, Zafrilla MP, Cerda B, Boza J, García-Conesa MT, Espín JC, Tomás-Barberán FA. Concentration and solubility of flavanones in orange beverages affect their bioavailability in humans. J Agric Food Chem 2010; 58:6516-24; PMID:20441150; http://dx.doi.org/ 10.1021/jf100752j [DOI] [PubMed] [Google Scholar]
- [145].Pereira-Caro G, Ludwig IA, Polyviou T, Malkova D, García A, Moreno-Rojas JM, Crozier A. Identification of plasma and urinary metabolites and catabolites derived from orange juice (Poly)phenols: Analysis by high-performance liquid chromatography–high-resolution mass spectrometry. J Agr Food Chem 2016; 64:5724-35; PMID:27339035; http://dx.doi.org/ 10.1021/acs.jafc.6b02088 [DOI] [PubMed] [Google Scholar]
- [146].Lewis ZT, Totten SM, Smilowitz JT, Popovic M, Parker E, Lemay DG, Van Tassell ML, Miller MJ, Jin YS, German JB, et al.. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 2015; 3:1-21; PMID:25621171; http://dx.doi.org/ 10.1186/s40168-015-0071-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, et al.. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014; 514:181-6; PMID:25231862 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.