Abstract
High density lipoproteins (HDL) represent a compositionally diverse population of particles in the circulation, containing a wide variety of lipids and proteins. Gene ontology functional analysis of the 96 commonly identified HDL binding proteins reveals that almost half of these proteins are either proteases or have known roles in protease regulation. Here, we discuss the activities of some of these proteins in regard to their roles in regulating proteases involved in inflammation, coagulation, and complement activation, particularly in the context of atherosclerosis. The overall goal of this review is to discuss potential functional roles of HDL in protease regulatory pathways based on current literature and known functions of HDL binding proteins and to promote the consideration of HDL as a global modulator of proteolytic equilibrium.
Keywords: high density lipoprotein, protease, protease inhibitor, SERPIN, coagulation, complement system, inflammation
1. Our current understanding of HDL function
High density lipoprotein (HDL) is most commonly referred to as the “good cholesterol”, because of an established inverse relationship between the cholesterol content of HDL particles and the risk for cardiovascular disease (CVD) (1). This relationship has withstood repeated evaluation in large scale population studies; however, there is a great deal of uncertainty about the nature of this relationship and whether HDL is “good” because it is directly protective against CVD or if the cholesterol content of HDL (HDL-C) is simply a biomarker of some other protective process, and in fact, has no direct causal relationship with CVD (2). While there is a great deal of functional data and some clinical data to support the hypothesis that HDL is playing a direct protective role against CVD, several recent studies indicate that pharmacological manipulation (3) or naturally occurring genetic mutations (4), which result in increased HDL-C, do not translate to a reduced risk of cardiovascular events. Many view these studies as a significant blow to the HDL field, suggesting a lack of importance of HDL in CVD; however, there is an alternative interpretation of these results. What these studies do clearly support is the concept that the cholesterol content of HDL is not necessarily directly linked to CVD and that the generic raising of HDL-C regardless of the mechanism may not be not an effective approach for reducing CVD. The lack of efficacy in these cholesterol centric approaches to modulating HDL might be better understood by an examination of the compositional diversity of HDL. HDL is a highly heterogeneous and dynamic set of lipoprotein particles, containing a wide variety of lipids and proteins (5, 6). The measurement of HDL-C is largely an indication of the cholesteryl ester content in the core of these particles and is not necessarily indicative of any functional capacity of HDL (7). Recent findings showing that the cholesterol efflux capacity of HDL is better than HDL-C in predicting CVD risk support the hypothesis that measurements of HDL function can outperform HDL-C (8, 9). The use of HDL function assays as a surrogate for HDL-C is complicated by several issues, including inherent difficulties in standardizing assays among multiple centers and the existence of multiple experimental models intended to measure the same function, for example in reverse cholesterol transport (10). As an alternative to functional activity measurements, the direct measurement of HDL bound components may be useful and would likely be easier to standardize and implement as a routine diagnostic test. The components of HDL that are likely responsible for its functionality are the surface exposed proteins and bioactive lipids and thus they may be likely to be stronger biomarkers of HDL-modifiable CVD risk.
The effects of current HDL-C manipulating therapies on the HDL proteome and lipidome have not been thoroughly studied. Perhaps there is a lack of interest in the effects of these compounds on the HDL proteome due to their general lack of clinical efficacy. Despite this, such studies may provide valuable information about the mechanism of efficacy, or lack thereof, for HDL targeting therapies. Despite recent failures of several CETP inhibitors, one compound in this class is still under evaluation in phase III trials, anacetrapib. Immunochemical composition analysis of HDL from patients taking anacetrapib has revealed some changes in the HDL protein cargo, including increased apoA-I and apoC-III with no change in apoA-II or apoE (11). The application of a shotgun proteomics approach to these samples may likely reveal more information about possible function or dysfunction in the HDL generated by anacetrapib. Niacin is another HDL-C raising agent that has undergone a lot of recent studies. The Coronary Drug Project first demonstrated a long-term cardiovascular benefit associated with high-dose (3 grams/day) niacin therapy (12). However, as demonstrated by the more recent AIM-High (13) and HSP2-THRIVE (14) studies, there appears to be no benefit from niacin when added to a statin regimen. Because most patients who would be considered for HDL raising are likely already taking a statin, there is currently diminished interest in pursuing niacin therapy for cardiovascular disease. But again, there may be lessons to be learned by studying the effect of niacin monotherapy on the HDL proteome. The effect of combination niacin/statin therapy on the HDL proteome has been reported and results in a shift of the HDL proteome in CAD patients toward that of healthy individuals (15). A comparison of the niacin only versus niacin/statin effects on the HDL proteome may provide insight into the lack of efficacy with combination therapy.
The importance of understanding the effect of HDL modifying therapies on the HDL proteome is further supported by the recent studies suggesting the existence of dysfunctional HDL. Under systemic inflammatory conditions, the HDL proteome can shift to a proinflammatory phenotype characterized by replacement of much of the apoA-I and many other minor HDL proteins with the acute phase proteins serum amyloid A 1/2 (SAA1/2) (16). The mechanism of action for this displacement is currently debated, however, binding of SAA1/2 to HDL has been demonstrated to affect HDL metabolism (17) and impair the activity of several cardioprotective functions of HDL (18, 19). The relationship between dysfunctional HDL and the protease regulator functions of HDL has not been reported but may yield interesting findings based on the high level of involvement of proteases/inhibitors in inflammatory pathways.
One reason why the protein and surface-lipid components of HDL have not already been developed into an effective metric for CVD risk is because the roles of these components in HDL function are not well understood. New analytical approaches for measuring the HDL proteome and lipidome are rapidly leading to a greater understanding of the potential role of these components of HDL. At this time, HDL is proposed to transport at least 96 different proteins (20) and over 200 different lipid species (6). Because of the relatively small size of HDL, 7–12 nm in diameter, not all of these different components can reside on every single HDL particle. In fact, it has been suggested that, based on the size and protein content of HDL, each individual HDL particle may only be able to accommodate 1–2 proteins in addition to its major structural components, apoA-I and apoA-II. Therefore, HDL exists as a diverse population of particles composed of many different possible combinations of proteins and lipids. It is unknown, however, whether these components assemble on HDL randomly or if somehow, protein-protein or protein-lipid interactions may orchestrate specific particle assemblies. The compositional and functional categorization of HDL subspecies is, therefore, an active and promising area of investigation, rich with potential for uncovering novel functions for HDL and for better understanding the relationship between HDL and CVD.
Many different functions for HDL have already been described, including the most well understood reverse cholesterol transport (RCT) pathway, the process by which HDL can remove cholesterol from peripheral cells and transport it to the liver where it can be converted to bile acids and excreted from the body. The initiating step in the RCT pathway is the efflux of cholesterol from cells, most notably macrophage foam cells within atherosclerotic lesions, via the interaction of HDL with cell membrane transporters, such as ABCA1, ABCG1, and SRB1 (21). In humans, the efflux capacity of HDL is negatively correlated with atherosclerotic CVD (9) and is the only function of HDL which has been evaluated for its relationship with CVD outcomes. A multitude of other functional properties of HDL have also been described and could potentially play equally important roles in the pathogenesis of CVD and other diseases. Some of these alternative HDL functions include effects on endothelial function, regulation of inflammation, glucose metabolism, and innate immune defense and have been previously reviewed (22). In this review, we propose a functional role of HDL as a broad-spectrum modulator of protease activity based on evidence from the HDL proteome and from recent HDL functional studies. This concept is supported by the early HDL proteome work of Vaisar et al., who initially noted the presence of numerous protease inhibitors on HDL (23). As we will discuss in more detail, the protease regulating role of HDL may have important implications in various physiological pathways related to atherogenesis, such as inflammation, coagulation, and complement activation.
2. The HDL proteome is enriched with proteases and protease inhibitors
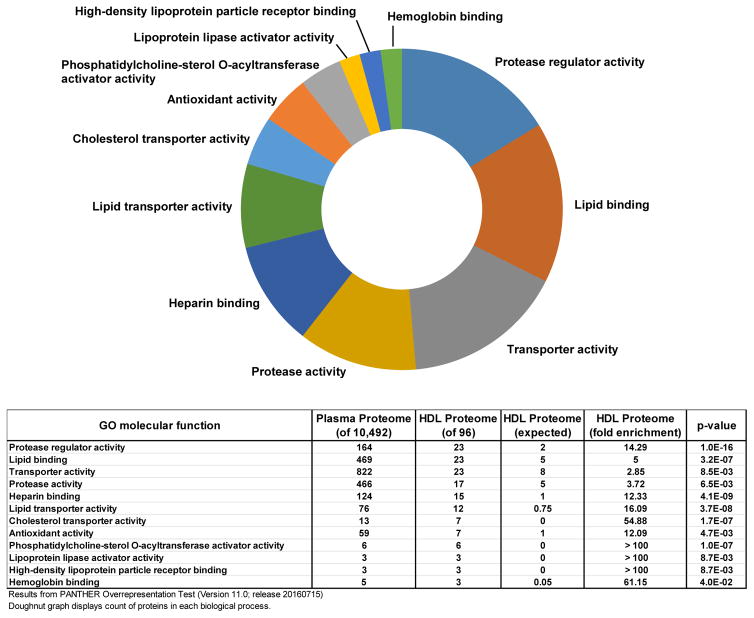
Over the last decade, modern mass spectrometry instruments have been employed to characterize the HDL proteome in healthy individuals and in those with various disease states (5). These early studies have provided overwhelming support for the compositional heterogeneity and dynamics of the HDL proteome. The W.S. Davidson laboratory maintains a database of HDL proteome studies, cataloging the identities of all the HDL associating proteins, with the current count up to 96 different proteins detected on HDL by at least 3 independent laboratories (Note: Actual HDL proteome database indicates 95 proteins on HDL but counts SAA1 and SAA2 as a single protein due to difficulty in distinction by mass spectrometry) (20). We used this database of commonly detected HDL associated proteins to perform a gene ontology (GO) molecular function enrichment test (24) to identify classes of proteins that are enriched on HDL compared to its surrounding milieu, the plasma (25). The enrichment of specific classes of proteins on HDL indicates that the HDL proteome is not simply a random sampling of plasma proteins, but rather a platform for the assembly of specific combinations of proteins with functional importance. A summary of the results of this analysis are presented in Fig. 1 and many of the known molecular functions associated with HDL are represented. While lipid binding and transporter activity make the top of the list as expected, one surprising finding of this analysis is that there is an equal number of proteins with molecular functions associated with protease regulator activity. Additionally, a large number of HDL bound proteins are known to have protease activity. The presence of significant numbers of both protease and protease regulator proteins on HDL suggests that HDL may be acting as a platform for the interaction of these classes of proteins.
Fig. 1. Gene ontology analysis of HDL proteome molecular function.
The HDL proteome was compared to the plasma proteome and a functional enrichment analysis for GO molecular functions was performed using Panther Overrepresentation Test (version 11.0). Selected molecular functions are displayed to reduce redundancy. The doughnut graph represents the counts of proteins from the HDL proteome in each molecular function category. The table below displays the numerical results of the enrichment test.
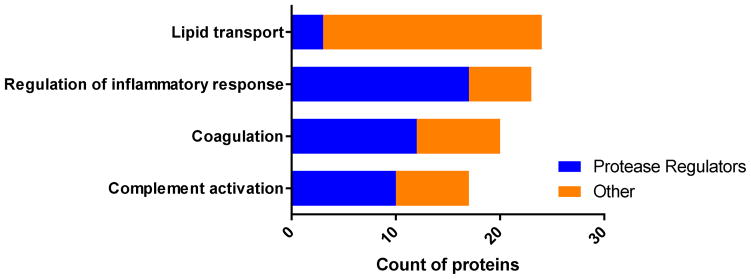
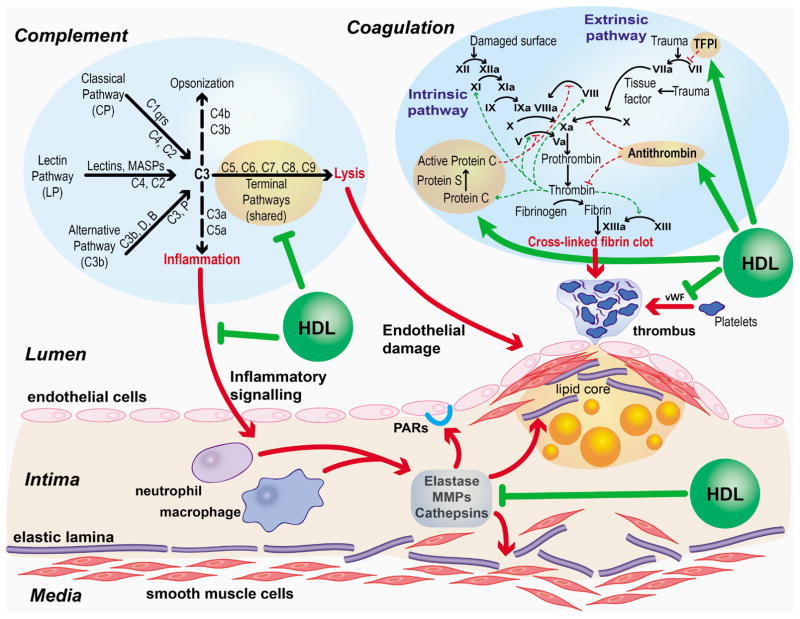
Many of the physiologic pathways and processes that HDL is known to modulate involve protease activity, such as inflammation, coagulation, and complement activation. A close examination of the HDL proteins involved in these pathways reveals that very few proteins with roles in lipid transport also have protease regulator function; however, a larger portion of the proteins with roles in inflammation, coagulation, and complement activation do have protease regulator function (Fig. 2). This suggests that HDL’s role in modulating these processes may involve protease regulation as a significant component. Fig. 3 highlights some of the potential pathways where HDL mediated protease regulation may play a role in atherosclerosis. It may be helpful to follow this figure as you read the next sections of this review.
Fig. 2. Analysis of protease regulating proteins associated with known HDL functions.
Proteins from the HDL proteome were assigned to the following GO biological processes: lipid transport, regulation of inflammatory response, coagulation, or complement activation. These protein groups were analyzed further for concurrent GO assignments in regulation of proteolysis. Within each of the four HDL functions presented the count of proteins which were identified as having protease regulator activity are represented in blue and all others are in orange.
Fig. 3. Roles for HDL mediated protease regulation in protection against atherosclerosis.
Complement activation, coagulation, and subendothelial inflammation all contribute to the progression of atherosclerosis through the action of proteolytic cascades (bold red arrows). HDL, by way of its protein cargo, may help to modulate these pathways at various steps (bold green arrows). Complement (112) and coagulation (113) pathway representations were adapted from original versions.
All of the proteases on HDL were classified as serine proteases (17 of 17) and the majority of protease inhibitors on HDL (15 of 23) are serine protease inhibitors (SERPINs). This unexpected enrichment of SERPINs on HDL was first pointed out by Vaisar et al. (23). The SERPINs are a superfamily of proteins with conserved structural elements, including a metastable reactive center loop, which acts as bait for the target protease. The interaction of a protease with its corresponding SERPIN results in irreversible inhibition of protease activity due to a dramatic conformational change in the protease/inhibitor complex (26). The SERPIN superfamily consists of 36 proteins, 27 of which are functional protease inhibitors, and they target a wide variety of proteases with established roles in inflammation, coagulation, and complement regulation (26). All of these processes have been implicated in CVD and, therefore, the existence of such an abundance of SERPINs on HDL is strongly suggestive of a mechanistic role for HDL in modulating protease activity.
3. Potential roles for protease regulation by HDL
3.1. Inflammation
3.1.1. Protease regulation in atherosclerosis
Atherosclerotic lesions are well known to be inflammatory environments, occurring in the subendothelial space of arteries. Within these lesions, activated neutrophils and macrophages secrete an array of cytokines, reactive oxygen species, and proteases, all of which promote atherosclerosis (27). Cytokines trigger proinflammatory signaling cascades in nearby cells, leading to the recruitment of circulating monocytes to the lesion. Reactive oxygen species generated in plaque oxidize low density lipoproteins (LDL), driving scavenger receptor mediated uptake by macrophages and leading to the formation of macrophage foam cells (28). Finally, proteases, which include serine proteases, matrix metalloproteases, and cathepsins, cause physical destruction of both soluble proteins and components of the extracellular matrix (ECM) and propagate proinflammatory signaling by generation of EMC fragments (16). They also promote the activation of protease activated receptors (PARs) found on almost all cells (29), which further amplifies inflammation. Protease inhibitors play an important role in counteracting the destructive activities of proteases, particularly during the resolution phase of inflammation and may play a protective role against atherosclerosis. For example, the myxoma virus protein Serp-1 is a serine protease inhibitor (SERPIN) with potent inhibitory activity against plasmin, urokinase-type plasminogen activator (uPA), and tissue-type plasminogen activator (tPA). In a mouse model of atherosclerosis (apoE−/−), administration of Serp-1 reduced atherosclerotic lesion progression and promoted a more stable plaque morphology (30).
Neutrophil elastase (NE) is an interesting example of a potential HDL-modifiable protease involved in atherosclerosis. It is an abundant serine protease produced by both neutrophils and macrophages within atherosclerotic lesions (31). The primary target of NE is, as its name implies, elastin, a major structural component of the vessel wall and nearly all other elastic tissues of the body. Mutations that disrupt elastin structure result in an unstable plaque morphology and increased propensity for rupture (32). Breakdown of elastin results in the formation of desmosine, crosslinked fragments of elastin, and circulating levels of this fragment correlate with arterial stiffness and have been associated with cardiovascular risk and mortality in patients with chronic obstructive pulmonary disease (33). In vivo studies in mice have also demonstrated that chronic administration of elastin breakdown fragments results in increased atherosclerosis severity, likely involving the elastin receptor complex and PI3K signaling (34). While neutrophils are thought to be the primary source of NE in atherosclerotic lesions, cholesterol loading by incubation with acetylated LDL increases elastolytic activity associated with macrophages (35). In addition to physical damage to the extracellular matrix, NE can also propagate inflammation by activation of PARs. Cleavage of PAR-1 and PAR-2 by NE can stimulate secretion of an array of cytokines including IL-1β, IL-6, IL-8, and prostaglandin E2 (36, 37). Endothelial integrity can also be compromised by NE activation of endothelial PAR-2, resulting in increased permeability and inflammatory cell migration (38).
The body’s natural counter measure for excessive NE production in sites of inflammation is a SERPIN called alpha-1-antitrypsin (A1AT). This protein is produced primarily by the liver, but is secreted into the plasma, and is acute phase reactive, with plasma levels increasing up to 6-fold over its usual plasma concentration of 1–2 mg/mL. In the lung, bidirectional transport of A1AT across pulmonary capillary endothelial cells has been described (39), however, this process has not been studied in endothelial cells relevant to atherosclerosis. Although it is a relatively abundant plasma protein, A1AT might not readily cross the vascular endothelium of large arteries on its own because of reduced bulk phase transport and tissue specific effects on endothelial receptor expression (40). Hence the association of A1AT with HDL may provide a mechanism for transcytosis because HDL has been demonstrated to be actively transcytosed across vascular endothelial cells via mechanisms involving ABCA1 and SRB1 (41, 42). A1AT has been shown to bind to HDL in healthy humans (43), with the amount of HDL bound A1AT increasing significantly during treatment with the LDL lowering drug rosuvastatin (44). Isolated human HDL has potent anti-elastase activity (43) and HDL enriched with A1AT have been shown to reduce inflammatory cytokine production in elastase treated macrophages (44). Additionally, in vitro and ex vivo experiments show that HDL bound A1AT inhibits ECM degradation, detachment, and apoptosis of smooth muscle cells (43). The elevation of HDL bound A1AT during rosuvastatin treatment may provide some explanation for the pleiotropic effects of statins (i.e. lowering of CVD risk through mechanisms not related to lowering of LDL-C). Mechanistic studies of A1AT in atherosclerotic mouse models have not yet been reported, likely due to the lack of A1AT KO mouse models. Mouse models have proven difficult to generate due to gene duplication in mice resulting in the mouse having 3–6 copies of A1AT, depending on the strain. In the future, modern gene editing technologies such as the CRISPR/Cas9 system may simplify generation of these mice. In one previous report, an attempt to knockout one of these A1AT homologues resulted in embryonic lethality (45), an unexpected effect considering the survival of A1AT null humans (46). In addition, patients with A1AT deficiency are reported to have increased arterial stiffness, indicative of decreased vessel elasticity and an increased risk for CVD (47). Elevations in plasma A1AT were noted in patients following myocardial infarction and, in these patients, greater elevations were correlated with reduced mortality, further supporting a role for this SERPIN in protection against CVD (48).
Abdominal aortic aneurysm (AAA) is a vascular condition distinct from atherosclerosis but consisting of many similar pathophysiological hallmarks, such as inflammation in the vessel wall, oxidative stress, and proteolytic damage including degradation of vascular collagen and elastin (49). In fact, one of the most widely used models for in vivo study of AAA involves intraluminal injections of elastase in rodents (50). Interestingly, HDL has also been proposed to be associated with AAA. Patients with AAA have reduced amounts of HDL bound A1AT compared to healthy controls (43). This finding supports a potential mechanism whereby HDL bound A1AT may be involved in the preservation of vascular integrity, similar to the atherosclerosis scenario discussed above. Additionally, HDL isolated from these patients has impaired antioxidant activity (51). Total HDL and apoA-I levels have been demonstrated have a negative association with AAA occurrence and progression (52, 53). As further evidence of the ability of HDL to protect against AAA, HDL raising approaches have been used to mitigate AAA progression. Infusions of reconstituted HDL (54) and treatment with fenofibrate (55) have both shown improvement in AAA mouse models. Whether or not HDL mediated protection against AAA development involves HDL-mediated regulation of destructive proteolytic activity has not been directly evaluated but deserves investigation.
3.1.2. HDL regulates protease activity in the lung
A recent study in a mouse model of emphysema provides additional support to our hypothesis of HDL mediated co-transport of protease inhibitors to sites of inflammation. This study convincingly demonstrates that intravenous administration of A1AT, when pre-complexed with HDL, was more efficiently targeted and delivered to lung tissue than free A1AT or HDL administration alone (56). Additionally, A1AT-HDL resulted in a reduction of inflammatory markers and morphological damage and improved lung function in mice given intratracheal elastase (56). These results demonstrate that HDL effectively transports functional A1AT from the circulation across the lung endothelium. This targeting effect of HDL may have implications in therapy for humans with A1AT deficiency. The current standard of care for these patients is the infusion of free purified or recombinant A1AT; however, the efficacy of this approach, in terms of preservation of lung function, is relatively modest (57).
3.2. Coagulation
3.2.1. Coagulation and atherothrombosis
Coagulation is the body’s primary defense against blood loss and involves the aggregation of soluble factors at a site of injury to physically impede the movement of blood out of the circulatory system. The coagulation pathway begins with an initiating stimulus followed by a series of proteolytic events, which results in an amplifying effect and ultimately activation of thrombin, which then converts soluble fibrinogen to insoluble fibrin clots (58). This process is tightly regulated by protease activators and inhibitors at almost every step, and many of these regulatory proteins are known to bind to HDL or to be influenced by HDL bound proteins and lipids (58). Although many different HDL binding proteins have known functions in coagulation, the relationships between HDL and these proteins have only been investigated for a few.
Atherothrombosis occurs when an atherosclerotic lesion is disrupted, resulting in the formation of a thrombus at the luminal endothelium. Unstable thrombus formation in this manner greatly increases the risk of pathologic vascular events, such as myocardial infarction and stroke. In fact, atherothrombosis is responsible for two thirds of acute coronary syndromes (59). Risk factors for atherothrombosis include cigarette smoking, elevated LDL-C, and hyperglycemia (60). HDL participates in a variety of physiological pathways related to endothelial function and atherothrombosis (61). An association between HDL and thrombotic disease in humans has also been reported. Reduced HDL-C, HDL particle number, and apoA-I have been associated with venous thrombosis in men (62). Additional evidence indicates that HDL from patients with established cardiovascular disease has dramatically reduced anti-thrombotic properties (63). Early studies of the anticoagulant roles of HDL were primarily focused on the ability of HDL to prevent endothelial cell apoptosis, which is still considered an important aspect of this function. This function is, at least in part, related to the lipid cargo of HDL, namely sphingosine-1-phosphate (S1P). In the plasma S1P is carried almost exclusively on HDL and possesses potent anti-apoptotic activity (64, 65). Recent studies have emphasized additional anticoagulant mechanisms for HDL. Furthermore, the recent characterization of the HDL proteome suggests some novel potential mechanisms of action that have yet to be examined. In the following section, we will discuss the existing data supporting a relationship between HDL mediated protease regulation and coagulation and propose potential novel relationships based on evidence from the HDL proteome.
3.2.2. Regulation of coagulation by HDL
Intrinsic and extrinsic pathways
Thrombin is attracted to negatively charged lipid surfaces, such as those on lipoproteins. Therefore, this attraction can potentially facilitate the interaction of thrombin with functional modulators present on the lipoprotein surface. This may provide a way for the HDL associated proteins to modulate thrombin activation and activity. Similar logic can be applied to any of the components of the coagulation cascade. Intact isolated human lipoproteins can act as a suitable platform for the interaction of components of the intrinsic and extrinsic coagulation pathway. Assembly of these components (i.e. FXa, FVa, prothrombin) in the presence of human VLDL is known to result in efficient thrombin production (66). In contrast, the same assembly on HDL yields much slower rates of thrombin production, possibly due to the presence of endogenous inhibitory factors (66). By separation of HDL’s lipid and protein components, it was shown that the protein component of HDL can also directly inhibit tissue factor (TF) and FVIIa mediated activation of FX in the final step of the extrinsic pathway (67). The protein responsible for this function was later named lipoprotein associated coagulation inhibitor (68) and then renamed tissue factor pathway inhibitor (TFPI), a protein detected in recent MS based HDL proteome studies (69). Another HDL binding protein with known involvement in inhibition of FX activation is antithrombin-III. The functional relationship between HDL and antithrombin-III has not been reported; however, one intriguing study suggests that the consumption of antithrombin activity in serum is positively correlated with HDL-C (70). This association may suggest that HDL can facilitate the activity of antithrombin-III.
Apolipoprotein H (apoH) is an HDL binding protein with multiple anti-coagulant activities, ranging from inhibition of platelet aggregation (71) to direct inhibitory effects on factors of the intrinsic pathway (72). However, the role of apoH is complicated as it has also been proposed to have procoagulant properties under certain conditions by preventing binding of activated protein C (APC) to a lipid surface by competitive inhibition (73). Additionally, some patients with antiphospholipid syndrome produce autoantibodies against apoH. These antibodies bind lipid bound apoH and further inhibit APC activity resulting in a procoagulant effect (74).
In addition to carrying inhibitors of procoagulant proteases, HDL and apoA-I can inhibit coagulation by sequestering procoagulant anionic phospholipids. Phosphatidylserine and other anionic lipids can be actively transported from apoB containing lipoproteins to HDL via the action of phospholipid transfer protein (PLTP) and, once incorporated into the HDL particle, they no longer promote coagulation by activation of the prothrombinase complex (75). The mechanism for this activity appears to be sequestration of PS into the smaller sized HDL particles, which do not provide enough surface area for assembly of the prothrombinase complex, specifically by excluding FVa (75). PLTP may also have direct antithrombotic properties by interacting with and inhibiting the activation of FXIIa in the intrinsic pathway of coagulation (76).
Protein C pathway
A major regulator of coagulation, and one of the most studied in terms of interaction with HDL, is the protein C pathway. Protein C, in the presence of its cofactor, protein S, forms activated protein C (APC), a serine protease that inactivates Factors Va and VIIIa, thus acting as an anti-coagulant. Patients with deficiency in protein C are at increased risk of thrombosis (77). An interaction between protein C and HDL has been described whereby purified human HDL promotes the anti-coagulant activity of protein C (78). This effect was greater in larger HDL2 subfractions than smaller, denser fractions of HDL, namely HDL3 (58). Additionally, there was a positive correlation between plasma apoA-I and the capacity for APC mediated inhibition of coagulation (78). The mechanism for this activity may involve several components of the HDL particle, including negatively charged lipids, apoA-I, and apoC-III (58, 79). Further experimentation may reveal roles for additional HDL bound proteins in modulation of the protein C pathway.
Von Willebrand factor (vWF)
Another major player in coagulation pathways is vWF. This large glycoprotein is secreted from activated endothelial cells and self-associates to form long sticky strands which bind to platelets and promote the formation of thrombi. Studies in hypercholesterolemic rabbits support a role for vWF in platelet binding and thrombosis on atherosclerotic lesions (80). Several stimuli have been implicated in vWF release from endothelial cells such as inflammatory cytokines (81) and proteases, including thrombin (82) and elastase (83). Recently, HDL has been implicated in the function of vWF. In vitro studies demonstrate the ability of HDL to block self-association of vWF and prevent platelet adhesion to stimulated endothelial cells (84). Additionally, lipid-free apoA-I can block the binding of circulating soluble vWF multimers to adherent vWF fibers (84). These observations were consistent with in vivo experiments in a mouse model of thrombotic microangiopathy where HDL was able to reduce thrombocytopenia when co-injected with vWF (84). Human data provides additional support for a role of HDL in vWF adhesion. Patients with two conditions that cause systemic endothelial activation (i.e. sepsis and thrombotic thrombocytopenic purpura) display reduced plasma apoA-I and increased active vWF (84). The results of several prospective cardiovascular outcome studies indicate that although vWF generally demonstrates an association with CVD events, this association is often lost after correction for other known risk factors (85–88). This supports the hypothesis that vWF is a modifiable risk factor whose ultimate association with atherosclerotic disease is dependent on its interaction with other components, possibly HDL.
Fibrinolysis
Even after a clot has formed, HDL may still be able provide protection from thrombosis. The physical structure of fibrin clots on atherosclerotic plaque is related to risk of atherothrombotic events (89). Increased clot density, reduced permeability, and susceptibility to lysis are associated with increased risk (89). In a study of 136 individuals, plasma apoA-I and HDL-C showed a positive correlation with clot permeability and a negative correlation with clot lysis time, indicating a more favorable clot phenotype in individuals with higher HDL (90). This study reports that individuals with HDL-C > 55 mg/dL had about 20% improvement in both of these parameters compared to the rest of the study population (90). The mechanism behind this relationship has not been reported but may involve HDL carried proteins. For example, plasminogen is commonly detected on HDL. Plasminogen is a major player in the fibrinolysis pathway. Proteolytic cleavage of plasminogen by various proteases, some of which are also detected on HDL (e.g. kallikrein) results in the active form, plasmin, capable of dissolving fibrin clots. This connection might support a hypothesis where HDL is acting as a platform for activation of plasmin by facilitating the interaction of plasminogen and kallikrein. To add another level of complexity, HDL also carries several plasmin inhibitors (e.g. alpha-2-antiplasmin and alpha-2-macroglobulin). This may suggest some level of dynamic regulation of plasmin activity by HDL.
3.3. Complement
3.3.1. Complement in innate immunity and atherosclerosis
Another potential role for protease inhibitors on HDL may be in the regulation of the complement system. The traditional role of the complement system in human physiology is in innate immunity, by promoting antibody binding and opsonization or by direct killing of invading pathogens (91). Similar to the coagulation pathway, the complement pathway involves a highly regulated series of proteolytic activities. Activation of complement by the classical, alternative, or lectin pathways initiates a cascade of proteolytic events that result in the generation of numerous proinflammatory cytokines and the formation of the membrane attack complex (91). Over 30 different proteins have been implicated in the complement pathway and at least 17 of these proteins have been found to bind to HDL, supporting the concept of HDL involvement in complement regulation.
In addition to the well-studied innate immune functions of complement, there is evidence that complement proteins may also be involved in the pathogenesis of CVD. C3 is one of the most abundant proteins of the complement system (1.3 mg/mL in plasma) and has been associated with previous myocardial infarction (92) and as a risk factor for coronary heart disease, independent of other risk factors and acute phase proteins (93). The C5 cleavage fragment, C5a, has also been associated with increased cardiovascular risk (94). Additionally, complement protein concentrations are increased within and along the luminal endothelium of atherosclerotic lesions, including C3, C4, C5a, and the terminal C5b-9 complex (95–97). It has been proposed that these components are involved in disruption of the vascular endothelium (98, 99) and activation of intra-lesion phagocytosis (95). The complicated role of the complement system in atherosclerosis has been reviewed previously (100).
3.3.2. Regulation of complement activity by HDL
HDL carries many proteins that are known inhibitors of complement. Clusterin, commonly known as apoJ in the lipoprotein field (alternative names include: complement lysis inhibitor and SP-40, 40), forms a complex with apoA-I in circulation and can inhibit the cytolytic activity of the C5b-9 complex (101, 102). Factor H is a complement regulatory protein with binds to apoE on HDL particles and regulates activation of the alternative pathway (103). All of these complexes represent minor fractions of the total HDL population, yet may be contributing in major ways to suppress inflammation in the body. Even the most abundant HDL proteins apoA-I and apoA-II have been shown to have increased binding affinity to endothelial cells treated with C5b-9 (104). This binding is specifically dependent on the presence of polymeric C9 and both apoA-I and apoA-II have been shown to interfere with the formation large C9 polymers and their insertion into cell membranes (105), thus protecting endothelial cells from complement directed cell death. Preservation of endothelial integrity in this manner may also contribute to the antithrombotic effects of HDL discussed above, since endothelial disruption is trigger for atherothrombosis.
Patients with familial hypoalphalipoproteinaemia, a condition marked by low plasma levels of apoA-I and HDL-C and associated with increased risk for atherosclerosis, have elevated plasma concentrations of complement proteins C3 and C4, as well as, C reactive protein and the soluble cell adhesion molecule ICAM-1 (106). Similarly, Tangier disease patients, who have a mutation in ATP binding cassette transporter A1 (ABCA1) and, therefore, also have low HDL (107), have increase complement activity (108). High cholesterol concentrations in atherosclerotic plaque result in the formation cholesterol crystals, which have been shown to trigger inflammasome activation via a complement dependent mechanism (109). Reconstituted HDL (rHDL) binds to cholesterol crystals and inhibits crystal induced activation of complement and also prevents crystal induced production of inflammatory cytokines in whole blood (110, 111). These studies provide convincing evidence that a lack of HDL can result in excessive complement activation, and highlight the importance of HDL in suppressing complement induced inflammatory activities. Other specific mechanisms for how HDL may modulate complement activity have not been systematically examined. Perhaps HDL can act by sequestering proinflammatory cleavage fragments (e.g. C3a and C5a) or by preventing proteolytic generation of these fragments.
4. Conclusion
HDL is a well-documented carrier of a variety of proteases and protease inhibitors, with known functions related to anti-inflammation, coagulation, and complement activation. Currently, there is limited in vivo evidence supporting a physiological role for protease regulation in HDL biology or atherosclerosis prevention. The relatively large proportion of HDL bound proteins with protease regulator function, especially SERPINs, is surprising and strongly suggests a new paradigm whereby HDL plays an important role in protease regulation. Although this has not been a central focus of HDL research, numerous studies provide support for this type of role for HDL in various processes related to atherosclerosis. It seems likely that the interaction of HDL with the various proteases and protease inhibitors is important for two reasons: 1. Transport, movement of these proteins from the plasma compartment to tissues where they perform their activity or 2. Facilitation, HDL is acting as a platform for the assembly and activation of functionally related proteins, involved in various proteolytic processes. Further work is needed in this promising area of research to gain a more thorough understanding how the HDL proteome modulates proteases involved in CVD and to demonstrate the biological relevance of such activities. Targeted in vitro studies utilizing reconstituted HDL, with incorporation of various proteins of interest, should be performed to evaluate the effect of HDL binding on the known activities these proteins and in vivo studies will be necessary to confirm biological relevance to atherosclerotic disease. We encourage investigators from all fields to browse the HDL proteome (20) and to develop new hypotheses to explain how these proteins may be interacting on the surface of HDL to drive specific functions.
Highlights.
The high density lipoprotein (HDL) proteome is significantly enriched in proteins with protease regulator function.
HDL may act as a regulator of protease activity in biological process, including inflammation, coagulation, and complement activation.
Protease regulator activities may contribute to HDL mediated protection against atherosclerosis.
Acknowledgments
The authors acknowledge Maureen Sampson for illustration of Fig. 3.
Footnotes
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kannel WB, Castelli WP, Gordon T. Cholesterol in the prediction of atherosclerotic disease. New perspectives based on the Framingham study. Ann Intern Med. 1979;90:85–91. doi: 10.7326/0003-4819-90-1-85. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett J, Predazzi IM, Williams SM, Bush WS, Kim Y, Havas S, Toth PP, Fazio S, Miller M. Is Isolated Low High-Density Lipoprotein Cholesterol a Cardiovascular Disease Risk Factor? New Insights From the Framingham Offspring Study. Circulation. Cardiovascular quality and outcomes. 2016;9:206–212. doi: 10.1161/CIRCOUTCOMES.115.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosmas CE, DeJesus E, Rosario D, Vittorio TJ. CETP Inhibition: Past Failures and Future Hopes. Clinical Medicine Insights. Cardiology. 2016;10:37–42. doi: 10.4137/CMC.S32667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart AFR, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MSS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki MLL, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AHH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WMM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GRR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NPP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet (London, England) 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah AS, Tan L, Long JL, Davidson WS. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J Lipid Res. 2013;54:2575–2585. doi: 10.1194/jlr.R035725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kontush A, Lhomme M, Chapman MJ. Unraveling the complexities of the HDL lipidome. Journal of lipid research. 2013;54:2950–2963. doi: 10.1194/jlr.R036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos-Gallego CG. HDL: Quality or quantity? Atherosclerosis. 2015;243:121–123. doi: 10.1016/j.atherosclerosis.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. The New England journal of medicine. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos-Gallego CG, Giannarelli C, Badimon JJ. Experimental models for the investigation of high-density lipoprotein-mediated cholesterol efflux. Curr Atheroscler Rep. 2011;13:266–276. doi: 10.1007/s11883-011-0177-0. [DOI] [PubMed] [Google Scholar]
- 11.Krauss RM, Wojnooski K, Orr J, Geaney JC, Pinto CA, Liu Y, Wagner JA, Luk JM, Johnson-Levonas AO, Anderson MS, Dansky HM. Changes in lipoprotein subfraction concentration and composition in healthy individuals treated with the CETP inhibitor anacetrapib. Journal of lipid research. 2012;53:540–547. doi: 10.1194/jlr.M018010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 13.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 14.Group HT, Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. The New England journal of medicine. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 15.Green PS, Vaisar T, Pennathur S, Kulstad JJ, Moore AB, Marcovina S, Brunzell J, Knopp RH, Zhao XQ, Heinecke JW. Combined statin and niacin therapy remodels the high-density lipoprotein proteome. Circulation. 2008;118:1259–1267. doi: 10.1161/CIRCULATIONAHA.108.770669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenson RS, Brewer HB, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, Kontush A, Tall AR, Webb NR. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nature reviews. Cardiology. 2016;13:48–60. doi: 10.1038/nrcardio.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MHH, de Beer MC, Wroblewski JM, Charnigo RJ, Ji A, Webb NR, de Beer FC, van der Westhuyzen DR. Impact of individual acute phase serum amyloid A isoforms on HDL metabolism in mice. Journal of lipid research. 2016;57:969–979. doi: 10.1194/jlr.M062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han CY, Tang C, Guevara ME, Wei H, Wietecha T, Shao B, Subramanian S, Omer M, Wang S, O'Brien KD, Marcovina SM, Wight TN, Vaisar T, de Beer MC, de Beer FC, Osborne WR, Elkon KB, Chait A. Serum amyloid A impairs the antiinflammatory properties of HDL. The Journal of clinical investigation. 2016;126:266–281. doi: 10.1172/JCI83475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaisar T, Tang C, Babenko I, Hutchins P, Wimberger J, Suffredini AF, Heinecke JW. Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. Journal of lipid research. 2015;56:1519–1530. doi: 10.1194/jlr.M059089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson WS. The HDL Proteome Watch. 2015 doi: 10.1016/j.bbalip.2021.159072. http://homepages.uc.edu/~davidswm/HDLproteome.html. [DOI] [PMC free article] [PubMed]
- 21.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–2555. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 22.Gordon SM, Hofmann S, Askew DS, Davidson WS. High density lipoprotein: it's not just about lipid transport anymore. Trends Endocrinol Metab. 2011;22:9–15. doi: 10.1016/j.tem.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mi H, Poudel S, Muruganujan A, Casagrande JT, Thomas PD. PANTHER version 10: expanded protein families and functions, and analysis tools. Nucleic acids research. 2016:44. doi: 10.1093/nar/gkv1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nanjappa V, Thomas JK, Marimuthu A, Muthusamy B, Radhakrishnan A, Sharma R, Ahmad Khan A, Balakrishnan L, Sahasrabuddhe NA, Kumar S, Jhaveri BN, Sheth KV, Kumar Khatana R, Shaw PG, Srikanth SM, Mathur PP, Shankar S, Nagaraja D, Christopher R, Mathivanan S, Raju R, Sirdeshmukh R, Chatterjee A, Simpson RJ, Harsha HC, Pandey A, Prasad TS. Plasma Proteome Database as a resource for proteomics research: 2014 update. Nucleic acids research. 2014;42:65. doi: 10.1093/nar/gkt1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law RH, Zhang Q, McGowan S, Buckle AM, Silverman GA, Wong W, Rosado CJ, Langendorf CG, Pike RN, Bird PI, Whisstock JC. An overview of the serpin superfamily. Genome biology. 2006;7:216. doi: 10.1186/gb-2006-7-5-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libby P, Ridker PM, Hansson GKK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 29.Simon DI, Jain MK. Targeting proteases in atherosclerosis: hitting the nail with the hammer. Circulation. 2011;124:2480–2482. doi: 10.1161/CIRCULATIONAHA.111.069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bot I, von der Thusen JH, Donners MM, Lucas A, Fekkes ML, de Jager SC, Kuiper J, Daemen MJ, van Berkel TJ, Heeneman S, Biessen EA. Serine protease inhibitor Serp-1 strongly impairs atherosclerotic lesion formation and induces a stable plaque phenotype in ApoE−// −mice. Circ Res. 2003;93:464–471. doi: 10.1161/01.RES.0000090993.01633.D4. [DOI] [PubMed] [Google Scholar]
- 31.Dollery CM, Owen CA, Sukhova GK, Krettek A, Shapiro SD, Libby P. Neutrophil elastase in human atherosclerotic plaques: production by macrophages. Circulation. 2003;107:2829–2836. doi: 10.1161/01.CIR.0000072792.65250.4A. [DOI] [PubMed] [Google Scholar]
- 32.Van der Donckt C, Van Herck JL, Schrijvers DM, Vanhoutte G, Verhoye M, Blockx I, Van Der Linden A, Bauters D, Lijnen HR, Sluimer JC, Roth L, Van Hove CE, Fransen P, Knaapen MW, Hervent ASS, De Keulenaer GW, Bult H, Martinet W, Herman AG, De Meyer GR. Elastin fragmentation in atherosclerotic mice leads to intraplaque neovascularization, plaque rupture, myocardial infarction, stroke, and sudden death. European heart journal. 2015;36:1049–1058. doi: 10.1093/eurheartj/ehu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabinovich RA, Miller BE, Wrobel K, Ranjit K, Williams MC, Drost E, Edwards LD, Lomas DA, Rennard SI, Agustí A, Tal-Singer R, Vestbo J, Wouters EF, John M, van Beek EJ, Murchison JT, Bolton CE, MacNee W, Huang JT behalf of of to Investigators, o. Circulating desmosine levels do not predict emphysema progression but are associated with cardiovascular risk and mortality in COPD. The European respiratory journal. 2016;47:1365–1373. doi: 10.1183/13993003.01824-2015. [DOI] [PubMed] [Google Scholar]
- 34.Gayral S, Garnotel R, Castaing-Berthou A, Blaise S, Fougerat A, Berge E, Montheil A, Malet N, Wymann MP, Maurice P, Debelle L, Martiny L, Martinez LO, Pshezhetsky AV, Duca L, Laffargue M. Elastin-derived peptides potentiate atherosclerosis through the immune Neu1-PI3Kγ pathway. Cardiovascular research. 2014;102:118–127. doi: 10.1093/cvr/cvt336. [DOI] [PubMed] [Google Scholar]
- 35.Rouis M, Nigon F, Lafuma C, Hornebeck W, Chapman MJ. Expression of elastase activity by human monocyte-macrophages is modulated by cellular cholesterol content, inflammatory mediators, and phorbol myristate acetate. Arteriosclerosis. 1990;10:246–255. doi: 10.1161/01.atv.10.2.246. [DOI] [PubMed] [Google Scholar]
- 36.Asokananthan N, Graham PT, Fink J. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. Journal of Immunology. 2002;168:3577–3585. doi: 10.4049/jimmunol.168.7.3577. [DOI] [PubMed] [Google Scholar]
- 37.Alfaidi M, Wilson H, Daigneault M, Burnett A, Ridger V, Chamberlain J, Francis S. Neutrophil elastase promotes interleukin-1β secretion from human coronary endothelium. The Journal of biological chemistry. 2015;290:24067–24078. doi: 10.1074/jbc.M115.659029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chin AC, Lee WY, Nusrat A, Vergnolle N, Parkos CA. Neutrophil-mediated Activation of Epithelial Protease-Activated Receptors-1 and -2 Regulates Barrier Function and Transepithelial Migration. The Journal of Immunology. 2008;181:5702–5710. doi: 10.4049/jimmunol.181.8.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lockett AD, Brown M, Santos-Falcon N, Rush NI, Oueini H, Oberle AJ, Bolanis E, Fragoso MA, Petrusca DN, Serban KA, Schweitzer KS, Jr, RG, Campos M, Petrache I. Active Trafficking of Alpha 1 Antitrypsin across the Lung Endothelium. PLoS One. 2014:9. doi: 10.1371/journal.pone.0093979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuma PL, Hubrd AL. Transcytosis: Crossing Cellular Barriers. Physiological Reviews. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 41.Rohrer L, Cavelier C, Fuchs S, Schluter MA, Volker W, von Eckardstein A. Binding, internalization and transport of apolipoprotein A-I by vascular endothelial cells. Biochim Biophys Acta. 2006;1761:186–194. doi: 10.1016/j.bbalip.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Rohrer L, Ohnsorg PM, Lehner M, Landolt F, Rinninger F, von Eckardstein A. High-density lipoprotein transport through aortic endothelial cells involves scavenger receptor BI and ATP-binding cassette transporter G1. Circ Res. 2009;104:1142–1150. doi: 10.1161/CIRCRESAHA.108.190587. [DOI] [PubMed] [Google Scholar]
- 43.Ortiz-Munoz G, Houard X, Martin-Ventura JL, Ishida BY, Loyau S, Rossignol P, Moreno JA, Kane JP, Chalkley RJ, Burlingame AL, Michel JB, Meilhac O. HDL antielastase activity prevents smooth muscle cell anoikis, a potential new antiatherogenic property. FASEB J. 2009;23:3129–3139. doi: 10.1096/fj.08-127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon SM, McKenzie B, Kemeh G, Sampson M, Perl S, Young NS, Fessler MB, Remaley AT. Rosuvastatin Alters the Proteome of High Density Lipoproteins: Generation of alpha-1-antitrypsin Enriched Particles with Anti-inflammatory Properties. Mol Cell Proteomics. 2015;14:3247–3257. doi: 10.1074/mcp.M115.054031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang D, Wang W, Dawkins P, Paterson T, Kalsheker N, Sallenave JMM, Houghton AM. Deletion of Serpina1a, a murine α1-antitrypsin ortholog, results in embryonic lethality. Experimental lung research. 2011;37:291–300. doi: 10.3109/01902148.2011.554599. [DOI] [PubMed] [Google Scholar]
- 46.Frazier GC, Siewertsen MA, Hofker MH, Brubacher MG, Cox DW. A null deficiency allele of alpha 1-antitrypsin, QOludwigshafen, with altered tertiary structure. The Journal of clinical investigation. 1990;86:1878–1884. doi: 10.1172/JCI114919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duckers JM, Shale DJ, Stockley RA, Gale NS, Evans BA, Cockcroft JR, Bolton CE. Cardiovascular and musculskeletal co-morbidities in patients with alpha 1 antitrypsin deficiency. Respir Res. 2010;11:173. doi: 10.1186/1465-9921-11-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilutz H, Siegel Y, Paran E, Cristal N, Quastel MR. Alpha 1-antitrypsin in acute myocardial infarction. Br Heart J. 1983;49:26–29. doi: 10.1136/hrt.49.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nature reviews cardiology. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 50.Azuma J, Maegdefessel L, Kitagawa T, Dalman RL, McConnell MV, Tsao PS. Assessment of elastase-induced murine abdominal aortic aneurysms: comparison of ultrasound imaging with in situ video microscopy. Journal of biomedicine & biotechnology. 2011;2011:252141. doi: 10.1155/2011/252141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delbosc S, Diallo D, Dejouvencel T, Lamiral Z, Louedec L, Martin-Ventura JLL, Rossignol P, Leseche G, Michel JBB, Meilhac O. Impaired high-density lipoprotein anti-oxidant capacity in human abdominal aortic aneurysm. Cardiovascular research. 2013;100:307–315. doi: 10.1093/cvr/cvt194. [DOI] [PubMed] [Google Scholar]
- 52.Singh K, Bønaa KH, Jacobsen BK. Prevalence of and Risk Factors for Abdominal Aortic Aneurysms in a Population-based Study The Tromsø Study. American journal of …. 2001 doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- 53.Burillo E, Lindholt JS, Molina-Sanchez P, Jorge I, Martinez-Pinna R, Blanco-Colio LM, Tarin C, Torres-Fonseca MM, Esteban M, Laustsen J, Ramos-Mozo P, Calvo E, Lopez JA, Vega de Ceniga M, Michel JB, Egido J, Andres V, Vazquez J, Meilhac O, Martin-Ventura JL. ApoA-I/HDL-C levels are inversely associated with abdominal aortic aneurysm progression. Thromb Haemost. 2015:113. doi: 10.1160/TH14-10-0874. [DOI] [PubMed] [Google Scholar]
- 54.Torsney E, Pirianov G, Charolidi N. Elevation of plasma high-density lipoproteins inhibits development of experimental abdominal aortic aneurysms. 2012 doi: 10.1161/ATVBAHA.112.00009. [DOI] [PubMed] [Google Scholar]
- 55.Krishna SM, Seto SW, Moxon JV, Rush C. Fenofibrate increases high-density lipoprotein and sphingosine 1 phosphate concentrations limiting abdominal aortic aneurysm progression in a mouse …. The American journal of …. 2012 doi: 10.1016/j.ajpath.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 56.Moreno JA, Ortega-Gomez A, Rubio-Navarro A, Louedec L, Ho-Tin-Noe B, Caligiuri G, Nicoletti A, Levoye A, Plantier L, Meilhac O. High-density lipoproteins potentiate alpha1-antitrypsin therapy in elastase-induced pulmonary emphysema. Am J Respir Cell Mol Biol. 2014;51:536–549. doi: 10.1165/rcmb.2013-0103OC. [DOI] [PubMed] [Google Scholar]
- 57.Chapman KR, Stockley RA, Dawkins C, Wilkes MM, Navickis RJ. Augmentation therapy for alpha1 antitrypsin deficiency: a meta-analysis. COPD. 2009;6:177–184. doi: 10.1080/15412550902905961. [DOI] [PubMed] [Google Scholar]
- 58.Griffin JH, Fernández JA, Deguchi H. Plasma lipoproteins, hemostasis and thrombosis. Thrombosis and haemostasis. 2001;86:386–394. [PubMed] [Google Scholar]
- 59.Viles-Gonzalez JF, Fuster V, Badimon JJ. Atherothrombosis: a widespread disease with unpredictable and life-threatening consequences. European heart journal. 2004;25:1197–1207. doi: 10.1016/j.ehj.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Rauch U, Osende JI, Fuster V, Badimon JJ, Fayad Z, Chesebro JH. Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences. Annals of internal medicine. 2001;134:224–238. doi: 10.7326/0003-4819-134-3-200102060-00014. [DOI] [PubMed] [Google Scholar]
- 61.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circulation research. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 62.Deguchi H, Pecheniuk NM, Elias DJ, Averell PM, Griffin JH. High-density lipoprotein deficiency and dyslipoproteinemia associated with venous thrombosis in men. Circulation. 2005;112:893–899. doi: 10.1161/CIRCULATIONAHA.104.521344. [DOI] [PubMed] [Google Scholar]
- 63.Holy EW, Besler C, Reiner MF, Camici GG, Manz J, Beer JH, Luscher TF, Landmesser U, Tanner FC. High-density lipoprotein from patients with coronary heart disease loses anti-thrombotic effects on endothelial cells: impact on arterial thrombus formation. Thromb Haemost. 2014;112:1024–1035. doi: 10.1160/TH13-09-0775. [DOI] [PubMed] [Google Scholar]
- 64.Kontush A, Therond P, Zerrad A, Couturier M, Négre-Salvayre A, de Souza JA, Chantepie S, Chapman MJ. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: relevance to antiapoptotic and antioxidative activities. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1843–1849. doi: 10.1161/ATVBAHA.107.145672. [DOI] [PubMed] [Google Scholar]
- 65.Santos-Gallego CG, Vahl TP, Goliasch G, Picatoste B, Arias T, Ishikawa K, Njerve IU, Sanz J, Narula J, Sengupta PP, Hajjar RJ, Fuster V, Badimon JJ. Sphingosine-1-Phosphate Receptor Agonist Fingolimod Increases Myocardial Salvage and Decreases Adverse Postinfarction Left Ventricular Remodeling in a Porcine Model of Ischemia/Reperfusion. Circulation. 2016;133:954–966. doi: 10.1161/CIRCULATIONAHA.115.012427. [DOI] [PubMed] [Google Scholar]
- 66.Moyer MP, Tracy RP, Tracy PB, van't Veer C, Sparks CE, Mann KG. Plasma lipoproteins support prothrombinase and other procoagulant enzymatic complexes. Arteriosclerosis, thrombosis, and vascular biology. 1998;18:458–465. doi: 10.1161/01.atv.18.3.458. [DOI] [PubMed] [Google Scholar]
- 67.Carson SD. Plasma high density lipoproteins inhibit the activation of coagulation factor X by factor VIIa and tissue factor. FEBS letters. 1981;132:37–40. doi: 10.1016/0014-5793(81)80422-x. [DOI] [PubMed] [Google Scholar]
- 68.Broze GJ, Warren LA, Novotny WF, Higuchi DA, Girard JJ, Miletich JP. The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action. Blood. 1988;71:335–343. [PubMed] [Google Scholar]
- 69.Rezaee F, Casetta B, Levels JH, Speijer D, Meijers JC. Proteomic analysis of high-density lipoprotein. Proteomics. 2006;6:721–730. doi: 10.1002/pmic.200500191. [DOI] [PubMed] [Google Scholar]
- 70.Winter JH, Bennett B, McTaggart F, Douglas AS. Lipoprotein fractions and antithrombin III consumption during clotting. Thrombosis and haemostasis. 1982;47:236–238. [PubMed] [Google Scholar]
- 71.Nimpf J, Bevers EM, Bomans PH, Till U, Wurm H, Kostner GM, Zwaal RF. Prothrombinase activity of human platelets is inhibited by beta 2-glycoprotein-I. Biochimica et biophysica acta. 1986;884:142–149. doi: 10.1016/0304-4165(86)90237-0. [DOI] [PubMed] [Google Scholar]
- 72.Schousboe I. beta 2-Glycoprotein I: a plasma inhibitor of the contact activation of the intrinsic blood coagulation pathway. Blood. 1985;66:1086–1091. [PubMed] [Google Scholar]
- 73.Mori T, Takeya H, Nishioka J, Gabazza EC, Suzuki K. beta 2-Glycoprotein I modulates the anticoagulant activity of activated protein C on the phospholipid surface. Thrombosis and haemostasis. 1996;75:49–55. [PubMed] [Google Scholar]
- 74.Izumi T, Pound ML, Su Z, Iverson GM, Ortel TL. Anti-beta(2)-glycoprotein I antibody-mediated inhibition of activated protein C requires binding of beta(2)-glycoprotein I to phospholipids. Thrombosis and haemostasis. 2002;88:620–626. [PubMed] [Google Scholar]
- 75.Oslakovic C, Krisinger MJ, Andersson A, Jauhiainen M, Ehnholm C, Dahlbäck B. Anionic phospholipids lose their procoagulant properties when incorporated into high density lipoproteins. The Journal of biological chemistry. 2009;284:5896–5904. doi: 10.1074/jbc.M807286200. [DOI] [PubMed] [Google Scholar]
- 76.Deguchi H, Wolfbauer G, Cheung MC, Banerjee Y, Elias DJ, Fernández JAA, Albers JJ, Griffin JH. Inhibition of thrombin generation in human plasma by phospholipid transfer protein. Thrombosis journal. 2015;13:24. doi: 10.1186/s12959-015-0054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Griffin JH, Evatt B, Zimmerman TS, Kleiss AJ, Wideman C. Deficiency of protein C in congenital thrombotic disease. The Journal of clinical investigation. 1981;68:1370–1373. doi: 10.1172/JCI110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Griffin JH, Kojima K, Banka CL, Curtiss LK, Fernández JA. High-density lipoprotein enhancement of anticoagulant activities of plasma protein S and activated protein C. The Journal of clinical investigation. 1999;103:219–227. doi: 10.1172/JCI5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernandez JA, Deguchi H, Banka CL, Witztum JL, Griffin JH. Re-evaluation of the anticoagulant properties of high-density lipoprotein-brief report. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:570–572. doi: 10.1161/ATVBAHA.114.304938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Theilmeier G, Michiels C, Spaepen E, Vreys I, Collen D, Vermylen J, Hoylaerts MF. Endothelial von Willebrand factor recruits platelets to atherosclerosis-prone sites in response to hypercholesterolemia. Blood. 2002;99:4486–4493. doi: 10.1182/blood.v99.12.4486. [DOI] [PubMed] [Google Scholar]
- 81.Paleolog EM, Crossman DC, McVey JH, Pearson JD. Differential regulation by cytokines of constitutive and stimulated secretion of von Willebrand factor from endothelial cells. Blood. 1990;75:688–695. [PubMed] [Google Scholar]
- 82.Levine JD, Harlan JM, Harker LA, Joseph ML, Counts RB. Thrombin-mediated release of factor VIII antigen from human umbilical vein endothelial cells in culture. Blood. 1982;60:531–534. [PubMed] [Google Scholar]
- 83.Chignard M, Balloy V, Renesto P. Leucocyte elastase-mediated release of von Willebrand factor from cultured endothelial cells. The European respiratory journal. 1993;6:791–796. [PubMed] [Google Scholar]
- 84.Chung DW, Chen J, Ling M, Fu X, Blevins T, Parsons S, Le J, Harris J, Martin TR, Konkle BA, Zheng Y, López JAA. High-density lipoprotein modulates thrombosis by preventing von Willebrand factor self-association and subsequent platelet adhesion. Blood. 2016;127:637–645. doi: 10.1182/blood-2014-09-599530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morange PE, Simon C, Alessi MC, Luc G, Arveiler D, Ferrieres J, Amouyel P, Evans A, Ducimetiere P, Juhan-Vague I, Group P. Endothelial cell markers and the risk of coronary heart disease: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) study. Circulation. 2004;109:1343–1348. doi: 10.1161/01.CIR.0000120705.55512.EC. [DOI] [PubMed] [Google Scholar]
- 86.Whincup PH, Danesh J, Walker M, Lennon L, Thomson A, Appleby P, Rumley A, Lowe GD. von Willebrand factor and coronary heart disease: prospective study and meta-analysis. European heart journal. 2002;23:1764–1770. doi: 10.1053/euhj.2001.3237. [DOI] [PubMed] [Google Scholar]
- 87.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. The New England journal of medicine. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 88.Vischer UM. von Willebrand factor, endothelial dysfunction, and cardiovascular disease. Journal of thrombosis and haemostasis : JTH. 2006;4:1186–1193. doi: 10.1111/j.1538-7836.2006.01949.x. [DOI] [PubMed] [Google Scholar]
- 89.Undas A, Ariëns RA. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:99. doi: 10.1161/ATVBAHA.111.230631. [DOI] [PubMed] [Google Scholar]
- 90.Za˛bczyk M, Hońdo Ł, Krzek M, Undas A. High-density cholesterol and apolipoprotein AI as modifiers of plasma fibrin clot properties in apparently healthy individuals. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2013;24:50–54. doi: 10.1097/MBC.0b013e32835a083c. [DOI] [PubMed] [Google Scholar]
- 91.Sarma JV, Ward PA. The complement system. Cell and tissue research. 2011;343:227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muscari A, Massarelli G, Bastagli L, Poggiopollini G, Tomassetti V, Drago G, Martignani C, Pacilli P, Boni P, Puddu P. Relationship of serum C3 to fasting insulin, risk factors and previous ischaemic events in middle-aged men. European heart journal. 2000;21:1081–1090. doi: 10.1053/euhj.1999.2013. [DOI] [PubMed] [Google Scholar]
- 93.Onat A, Uzunlar B, Hergenç G, Yazici M, Sari I, Uyarel H, Can G, Sansoy V. Cross-sectional study of complement C3 as a coronary risk factor among men and women. Clinical science (London, England : 1979) 2005;108:129–135. doi: 10.1042/CS20040198. [DOI] [PubMed] [Google Scholar]
- 94.Speidl WS, Exner M, Amighi J, Kastl SP, Zorn G, Maurer G, Wagner O, Huber K, Minar E, Wojta J, Schillinger M. Complement component C5a predicts future cardiovascular events in patients with advanced atherosclerosis. European heart journal. 2005;26:2294–2299. doi: 10.1093/eurheartj/ehi339. [DOI] [PubMed] [Google Scholar]
- 95.Hollander W, Colombo MA, Kirkpatrick B, Paddock J. Soluble proteins in the human atherosclerotic plaque. With spectral reference to immunoglobulins, C3-complement component, alpha 1-antitrypsin and alpha 2-macroglobulin. Atherosclerosis. 1979;34:391–405. doi: 10.1016/0021-9150(79)90064-9. [DOI] [PubMed] [Google Scholar]
- 96.Vlaicu R, Niculescu F, Rus HG, Cristea A. Immunohistochemical localization of the terminal C5b-9 complement complex in human aortic fibrous plaque. Atherosclerosis. 1985;57:163–177. doi: 10.1016/0021-9150(85)90030-9. [DOI] [PubMed] [Google Scholar]
- 97.Speidl WS, Kastl SP, Hutter R, Katsaros KM, Kaun C, Bauriedel G, Maurer G, Huber K, Badimon JJ, Wojta J. The complement component C5a is present in human coronary lesions in vivo and induces the expression of MMP-1 and MMP-9 in human macrophages in vitro. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:35–44. doi: 10.1096/fj.10-156083. [DOI] [PubMed] [Google Scholar]
- 98.Benzaquen LR, Nicholson-Weller A, Halperin JA. Terminal complement proteins C5b-9 release basic fibroblast growth factor and platelet-derived growth factor from endothelial cells. The Journal of experimental medicine. 1994;179:985–992. doi: 10.1084/jem.179.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Christiansen VJ, Sims PJ, Hamilton KK. Complement C5b-9 increases plasminogen binding and activation on human endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:164–171. doi: 10.1161/01.atv.17.1.164. [DOI] [PubMed] [Google Scholar]
- 100.Speidl WS, Kastl SP, Huber K, Wojta J. Complement in atherosclerosis: friend or foe? Journal of thrombosis and haemostasis : JTH. 2011;9:428–440. doi: 10.1111/j.1538-7836.2010.04172.x. [DOI] [PubMed] [Google Scholar]
- 101.James RW, Hochstrasser AC, Borghini I, Martin B, Pometta D, Hochstrasser D. Characterization of a human high density lipoprotein-associated protein, NA1/NA2. Identity with SP-40, 40, an inhibitor of complement-mediated cytolysis. Arteriosclerosis and thrombosis : a journal of vascular biology/American Heart Association. 1991;11:645–652. doi: 10.1161/01.atv.11.3.645. [DOI] [PubMed] [Google Scholar]
- 102.Jenne DE, Lowin B, Peitsch MC, Böttcher A, Schmitz G, Tschopp J. Clusterin (complement lysis inhibitor) forms a high density lipoprotein complex with apolipoprotein A-I in human plasma. The Journal of biological chemistry. 1991;266:11030–11036. [PubMed] [Google Scholar]
- 103.Haapasalo K, van Kessel K, Nissilä E, Metso J, Johansson T, Miettinen S, Varjosalo M, Kirveskari J, Kuusela P, Chroni A, Jauhiainen M, van Strijp J, Jokiranta TS. Complement Factor H Binds to Human Serum Apolipoprotein E and Mediates Complement Regulation on High Density Lipoprotein Particles. The Journal of biological chemistry. 2015;290:28977–28987. doi: 10.1074/jbc.M115.669226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hamilton KK, Sims PJ. The terminal complement proteins C5b-9 augment binding of high density lipoprotein and its apolipoproteins A-I and A-II to human endothelial cells. The Journal of clinical investigation. 1991;88:1833–1840. doi: 10.1172/JCI115504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hamilton KK, Zhao J, Sims PJ. Interaction between apolipoproteins A-I and A-II and the membrane attack complex of complement. Affinity of the apoproteins for polymeric C9. The Journal of biological chemistry. 1993;268:3632–3638. [PubMed] [Google Scholar]
- 106.Sampietro T, Bigazzi F, Dal Pino B, Rossi G, Chella E, Lusso S, Puntoni M, Tuoni M, Bionda A. Up regulation of C3, C4, and soluble intercellular adhesion molecule-1 co-expresses with high sensitivity C reactive protein in familial hypoalphalipoproteinaemia: further evidence of inflammatory activation. Heart (British Cardiac Society) 2004;90:1438–1442. doi: 10.1136/hrt.2003.017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Serfaty-Lacrosniere C, Civeira F, Lanzberg A, Isaia P, Berg J, Janus ED, Smith MP, Pritchard PH, Frohlich J, Lees RS. Homozygous Tangier disease and cardiovascular disease. Atherosclerosis. 1994;107:85–98. doi: 10.1016/0021-9150(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 108.Choi-Miura NH, Sakamoto T, Ohtaki S, Nakamura H, Ishizawa S, Takagi Y, Gomi K, Tomita M. Elevated complement activities of sera from patients with high density lipoprotein deficiency (Tangier disease): the presence of normal level of clusterin and the possible implication in the atherosclerosis. Clinical and experimental immunology. 1993;93:242–247. doi: 10.1111/j.1365-2249.1993.tb07973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Samstad EO, Niyonzima N, Nymo S, Aune MH, Ryan L, Bakke SS, Lappegård KT, Brekke OLL, Lambris JD, Damås JK, Latz E, Mollnes TE, Espevik T. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. Journal of immunology (Baltimore, Md. : 1950) 2014;192:2837–2845. doi: 10.4049/jimmunol.1302484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thacker SG, Zarzour A, Chen Y, Alcicek MS, Freeman L, Sviridov DO, Demosky S, Remaley AT. High Density Lipoprotein reduces inflammation from cholesterol crystals by inhibiting inflammasome activation. Immunology. 2016 doi: 10.1111/imm.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Niyonzima N, Samstad EO, Aune MH, Ryan L, Bakke SS, Rokstad AM, Wright SD, Damås JK, Mollnes TE, Latz E, Espevik T. Reconstituted High-Density Lipoprotein Attenuates Cholesterol Crystal-Induced Inflammatory Responses by Reducing Complement Activation. Journal of immunology (Baltimore, Md. : 1950) 2015;195:257–264. doi: 10.4049/jimmunol.1403044. [DOI] [PubMed] [Google Scholar]
- 112.IDF. Complement Deficiencies. 2013 http://primaryimmune.org/about-primary-immunodeficiencies/specific-disease-types/complement-deficiencies/
- 113.DJ Coagulation full. 2007 https://commons.wikimedia.org/w/index.php?curid=1983833/