Abstract
Identifying the neurobiological mechanisms that underlie differential sensitivity to stress is critical for understanding the development and expression of stress-induced disorders, such as post-traumatic stress disorder (PTSD). Preclinical studies have suggested that rodents display different phenotypes associated with extinction of Pavlovian conditioned fear responses, with some rodent populations being resistant to extinction. An emerging literature also suggests a role for orexins in the consolidation processes associated with fear learning and extinction. To examine the possibility that the orexin system might be involved in individual differences in fear extinction, we used a Pavlovian conditioning paradigm in outbred Long-Evans rats. Rats showed significant variability in the extinction of cue-conditioned freezing and extinction recall, and animals were divided into groups based on their extinction profiles based on a median split of percent freezing behavior during repeated exposure to the conditioned cue. Animals resistant to extinction (high freezers) showed more freezing during repeated cue presentations during the within trial and between trial extinction sessions compared with the group showing significant extinction (low freezers), although there were no differences between these groups in freezing upon return to the conditioned context or during the conditioning session. Following the extinction recall session, activation of orexin neurons was determined using dual label immunohistochemistry for cFos in orexin positive neurons in the hypothalamus. Individual differences in the extinction of cue conditioned fear were associated with differential activation of hypothalamic orexin neurons. Animals showing poor extinction of cue-induced freezing (high freezers) had significantly greater percentage of orexin neurons with Fos in the medial hypothalamus than animals displaying significant extinction and good extinction recall (low freezers). Further, the freezing during extinction learning was positively correlated with the percentage of activated orexin neurons in both the lateral and medial hypothalamic regions. No differences in the overall density of orexin neurons or Fos activation were seen between extinction phenotypes. Although correlative, our results support other studies implicating a role of the orexinergic system in regulating extinction of conditioned responses to threat.
Keywords: Fear Extinction, Orexin/hypocretin, Hypothalamus, cFos, Individual Differences
1.0 Introduction
The formation of aversive memories following exposure to stressful or harmful stimuli is a natural and necessary process for survival. However, when fear memories take on excessive salience or extinction of learned fear is dysregulated, trauma or stress-related disorders, such as post-traumatic stress disorder (PTSD), can manifest. While somewhere between 50 and 84% of the general population will experience a traumatic event, most individuals are resilient to these stressors, and estimates suggest that somewhere around 10% of the population will go on to develop PTSD [1–4]. This suggests that there are individual neurobehavioral differences that contribute to either resiliency from or susceptibility to the long-term negative effects of stress. Animal models of fear extinction have emerged as a tenable way to understand the neurobiological, genetic, and epigenetic mechanisms that drive individual differences in risk and resilience following traumatic stress [5, 6]. Moreover, PTSD patients show impaired fear extinction learning and retention responses (see [5–9] for review), and emerging evidence suggests that individual differences in extinction of learned fear may predict susceptibility to stress disorders such as PTSD [10, 11].
Preclinical studies have described an analogous variability in extinction learning and recall among rodents [6, 12–17], making them a potentially useful model for examining the individual neurobiological differences that may underlie susceptibility to long term consequences of traumatic stress. In fear conditioning procedures animals are conditioned by pairing a neutral stimulus, such as a tone (the conditioned stimulus or CS) with an aversive stimulus such as a footshock (the US or unconditioned stimulus). The pairing of the CS and US enables both the context and the CS (the tone), even when the CS is presented in a novel context, to elicit a defensive response such as freezing [18]. Repeated re-exposure to the context or the CS in the absence of the US results in the extinction of the response [19]. A variety of studies have demonstrated key roles for plasticity in the amygdala, hippocampus and prefrontal cortex in driving these conditioned fear and extinction responses [5, 18, 19]. Moreover, studies suggest that extinction learning involves distinct neuronal populations and signaling processes from the original learning of the contextual or cue-conditioned responses [20, 21], and extinction of contextual or cued fear responses appears to involve prefrontal-amygdalar and prefrontal-hippocampal circuits (see [19, 22, 23]).
Another system that has emerged as an additional modulator of not only arousal and attention, but fear learning and extinction, is the orexin system [24–29]. The orexin/hypocretin family of neuropeptides were discovered in the late 1990s [30, 31] and has a well-established role as a physiological integrator in the control of sleeping and homeostatic regulation, as well as attention, arousal, and stress responses [25, 26, 32–35]). Two peptides, orexinA/hypocretin1 [OxA] and orexinB/hypocretin2 [OxB] are produced by the preproorexin gene, and act on two G protein-coupled receptors. The orexin/hypocretin 1 receptor [Ox1R/HcrtR1] is selective for OxA, while the orexin 2 receptor [Ox2R/HcrtR2] binds both OxA and OxB with high affinity [31]. Although restricted to the hypothalamus, orexin neurons have extensive projections throughout the central nervous system [36] and orexin receptors are found throughout the brain [26, 37–39]). Orexin projections are particularly dense to several brain regions known to be critical in fear learning and extinction, including the locus coeruleus (LC), amygdala, prefrontal cortex (PFC), and paraventricular thalamus (PVT) [36]. Orexin neurons also receive afferent projections from many of these same brain regions, in addition to projections from the brainstem [40, 41].
Several lines of evidence implicate orexins and orexin receptors in the threat circuit in mediating defensive responses in unconditioned behavioral tasks, as well as serving a modulatory role in the consolidation of fear memories and fear extinction. Activation of these neurons and orexin effects, however, seems to be more associated with stressors that induce arousal and attentional processes associated with environmental stimuli [42–44]. Administration of orexins or optogenetic stimulation of these neurons produce defensive responses or anxiogenic effects in several tasks [45–51]. Studies examining expression of immediate early genes, such as cfos, have demonstrated that orexin neurons are activated by anxiety-related or threat stimuli, or anxiogenic drugs such as FG-7142 or caffeine [25, 52, 53]. Acute unconditioned stressors [27, 33, 42, 54–57], as well as chronic unpredictable stress [58] and sodium lactate infusions that precipitate a panic-like state [59] induce activation of orexinergic neurons particularly in the dorsomedial or perifornical [60] hypothalamic regions. Activation of orexin neurons following exposure to a conditioned context has been seen in some, but not all studies [27, 42, 55], and extinction training with repeated exposures to the conditioned context or cues also activates orexin neurons [24, 61]. Pharmacological studies suggest that administration of OxA attenuates fear extinction [24], while orexin receptor antagonists or orexin receptor genetic ablation block the consolidation of fear learning and accelerate fear extinction [24, 26, 28, 29]. These studies have also demonstrated unique roles for Ox1 and Ox2 receptors in different brain areas in these processes [26]). Individual differences in expression of preproorexin mRNA following footshock are also correlated with freezing during re-exposure to the conditioned context, and these changes in preproorexin gene expression are more pronounced in animals that show enhanced response to novel sound cues after footshock stress [27]. Human studies have also demonstrated amygdalar orexin release is associated with emotional arousal [62].
Therefore, the aim of this study was to determine if there are correlations between activation of orexin neurons in the hypothalamus, and individual differences in extinction learning or recall. Using outbred Long-Evans rats, we examined neuronal activation using cfos (Fos) protein expression in OxA-positive neurons of the hypothalamus associated with individual differences in extinction of cue-induced conditioned freezing. Our results indicate animals showing poor extinction learning and recall (high freezers) had significantly more activation of orexin neurons in the medial hypothalamus than low freezers, suggesting that greater orexinergic activity is associated with resistance to fear memory extinction. Our results add to a growing literature suggesting the orexin system represents a target for understanding the neurobiological underpinnings of the individual differences in fear extinction, as well as susceptibility to the long term consequences of traumatic stress.
2.0 Methods
2.1 Subjects
Adult male Long Evans rats (175–200g; Harlan, Indianapolis, IN) were singly housed and maintained on a 12-hour light dark cycle (lights on at 7 AM) with ad libitum access to food and water. After arrival in the vivarium, animals were habituated to daily handling for at least one week before the experiment. All procedures were approved by the University of South Carolina Institutional Animal Care and Use Committee. Twenty-three animals were tested in two cadres to provide tissue for immunohistochemical processing. Animals were tested in a cylindrical chamber for unconditioned freezing and other behaviors one week prior to fear conditioning (data not shown).
2.2 Fear Conditioning and Extinction
For examining conditioned fear and extinction, a protocol modified from Likhtik et al. (2008) was used [63]. For acquisition of conditioned fear, male Long-Evans rats (N=23) were placed in a shock box (Context A; Med Associates, Inc) within a sound-attenuating box containing a ventilation fan and a house light. Unconditioned freezing was recorded for 3 minutes. Rats were then conditioned with three 10 second tones (80 dB, 2 KHz) co-terminating with a 1 mA foot shock (1 sec) presented at 60 second intervals. The shock box chamber was cleaned with mild (7%) ammonium hydroxide solution between animals. On day two (24 hours post-acquisition) rats were returned to the shock box (Context A) for 8 minutes without tone or shock for assessing context-conditioned freezing. On day 3 (48 hours post-acquisition) to assess cue-conditioned freezing and extinction learning, animals were placed in a completely different context in a sound-attenuated box in a separate testing room (Context B) that consisted of a clear Plexiglas bowl with distinct visual and olfactory cues (20 μL lemon extract) compared to the conditioning context A, and context B was cleaned with 70% ethanol between animals (rather than ammonium hydroxide). After assessing unconditioned freezing in context B for 3 minutes, rats were presented with twenty 10 second tones (80 dB, 2 KHz) at 60 second intervals. This long trial of cue presentations was used to assess cue-conditioned freezing and within trial extinction of cue-induced fear behaviors [63]. On day 5 (96 hours post-acquisition) animals were again placed in Context B and presented with an additional twenty 10 second tones to assess extinction recall. In all trials, freezing behavior was assessed in one minute bins using Freezescan software (CleverSys, Inc, Reston VA), and parameters were set to detect freezing as the absence of movement other than breathing. Data are presented as the percent of freezing during each one minute bin, beginning at the initiation of each 10 second tone in trials having an auditory stimulus (trials A, C, and D). Thus, the data represent freezing during the entire trial, not just during the tone presentations.
2.3 Immunohistochemistry
Two hours after the start of the extinction recall trial, animals were deeply anesthetized using isoflurane (5%) inhalation. This time point was selected as optimal for seeing changes in Fos protein and assessing neuronal activation following a challenge [64]. Rats were transcardially perfused with ice cold 0.1M phosphate buffered followed by 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). Brains were removed from the skulls and post-fixed for 24 hours in 4% paraformaldehyde. Brains were transferred to 15% sucrose in 0.1M phosphate buffer and stored at 4°C until sectioning. Coronal brains sections (45μm) were cut on a microtome (Microm, Waldorf, Germany) and stored in anti-freezing solution (30% sucrose/30% ethylene glycol in 0.1M phosphate buffer) at −20°C until processing for immunohistochemistry.
Dual label immunohistochemistry for Fos and OxA was used to determine neuronal activation in orexinergic neurons in sections from hypothalamus as described previously [65]. Sections were washed in Tris-buffered saline prior to free-floating immunohistochemical processing. Two hypothalamic sections from each brain within each cadre of rats were processed together. Sections were agitated with rabbit anti-Fos antibody (1:8000; Millipore, Billerica, MA), followed by biotinylated donkey anti-rabbit secondary antibody (1:1000; Jackson ImmunoResearch, Inc, West Grove, PA) and horseradish peroxidase (HRP)-streptavidin (1:1600; Jackson ImmunoResearch, Inc). Labeling was visualized using nickel/cobalt-enhanced diaminobenzidine (DAB) to produce a blue-black precipitate confined to the nucleus of Fos immunoreactive neurons. For visualizing Fos in orexin neurons, sections were then incubated with goat anti-OxA antibody (1:1000; Santa Cruz, Dallas, TX), followed by unlabeled donkey anti-goat secondary antibody (1:200; Jackson ImmunoResearch, Inc) and goat peroxidase anti-peroxidase (1:250; Jackson ImmunoResearch, Inc). Labeling was visualized with DAB to produce a brown precipitate in OrexinA immunoreactive neurons.
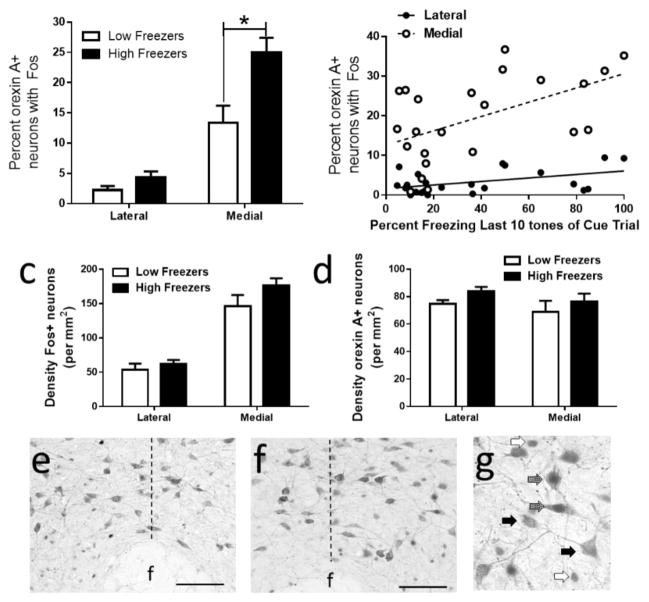
Analysis of cell counts was done using a number code for animals to insure the investigator was blinded to the animal’s treatment group. All sections were viewed at 10X-40X magnification under brightfield illumination (Nikon, Melville, NY). Images were acquired using a digital camera and immunoreactivity was quantified using Neurolucida MicroBright Field System (MBF Biosciences, Williston, VT). The number of Fos-positive, orexin A-positive, and dual labelled neurons were counted in two rostrocaudal sections of the lateral and medial hypothalamic regions defined by a vertical line bisecting the fornix as described in previous publications (see Figure 2; Bregma -2.80mm to −3.30mm) [65, 66]. Since the perifornical area is considered a transition zone between the medial and lateral hypothalamus it was not counted as a distinct region. Two sections from similar anterior-posterior planes were analyzed per hypothalamus, and counts from right and left of all sections/subregion within the hypothalamus were averaged for each animal. Results are expressed as average density of Fos positive, orexin positive, and dual-labeled neurons in the medial and lateral banks of hypothalamic orexin neurons based on area (mm2) of each subregion, as well as the percentage of orexin A-positive neurons containing Fos (total number of dual labeled neurons divided by total number of orexin A positive neurons).
Figure 2.
Activation of orexin neurons in the lateral and medial regions of the hypothalamus in high and low freezers. (a) High freezers with poor extinction recall had a significantly greater percentage of orexin A positive neurons expressing Fos in the medial hypothalamus than low freezers. (b) Mean percent freezing during the last ten tone presentations of within trial extinction was positively correlated with the density of dual labelled neurons in both the lateral and medial regions of the hypothalamus. No overall differences in Fos positive neuron density (c) or orexin A positive neuron density (d) between high and low freezers were observed. Representative photomicrographs showing Fos and orexin A immunoreactivity in the perifornical region of the hypothalamus in low (e) and high (f) freezers (20X; scale bar=100 microns) and high magnification (40X) of Fos positive (white arrows), orexin A positive (black arrows) and dual labelled neurons (gray arrows) in the medial hypothalamus. Dotted line represents the level of the fornix, separating medial and lateral hypothalamus. * indicates P<0.05.
2.4 Statistical Analyses
Freezing during each trial was analyzed as percent freezing in each one-minute bin, objectively calculated by the FreezeScan software (Clever Sys, Inc, Reston VA). For reliability and scientific rigor, data were collected from two separate cadres of rats run at separate times during the year, and data were grouped for final analysis. Analysis of the behavioral data in the 23 animals during the extinction trial suggested that the data were not normally distributed (Shapiro-Wilk normality test, P<0.004). A frequency distribution analyzing the average percent freezing during the last 10 tone presentations of the first extinction trial suggested distinct groups of subjects within the population, similar to differences in extinction learning/recall seen by other groups [12, 13]. Therefore, for analysis purposes, animals were divided into high and low freezers based on a median split of the average percent freezing duration during the last 10 minutes of the cue trial (within trial extinction) on day 3 of the protocol. This extinction learning trial was chosen (rather than extinction recall) for the median split since it will permit comparison of changes immediately following extinction learning (after the cue trial) in future studies, as well as extinction recall (in present study), using the same method of segregating the animals based on extinction behaviors. Animals with an average freezing duration greater than the median (23%) during this period were classified as high freezers (poor extinction), while animals with an average freezing duration less than the median during this period were classified as low freezers (good extinction). Freezing behavior during each trial was recorded as percent freezing per one minute time bin and high and low freezers were compared by one-way analysis of variance (ANOVA; high versus low freezing) with repeated measures. If main effects were seen in ANOVA, comparisons at each time bin were done using a post-hoc Bonferroni analysis. Densities of Fos+, orexin+, and dual-labeled neurons in the lateral versus medial hypothalamus, and percentage of activated orexin neurons, were compared in high and low freezers using a one-way ANOVA (high versus low freezers) with subregion as a repeated measure, with Bonferroni post-hoc analyses to determine the source of main effects. Average freezing behaviors during separate trials (acquisition, context exposure, cue presentation, and extinction recall) were correlated with densities of Fos+, orexin+, and dual-labeled neurons in the lateral and medial hypothalamus to assess the overall relationship between neuronal activation and freezing behaviors. All significance levels were set to P<0.05.
3.0 Results
3.1 Individual Differences in Fear Extinction
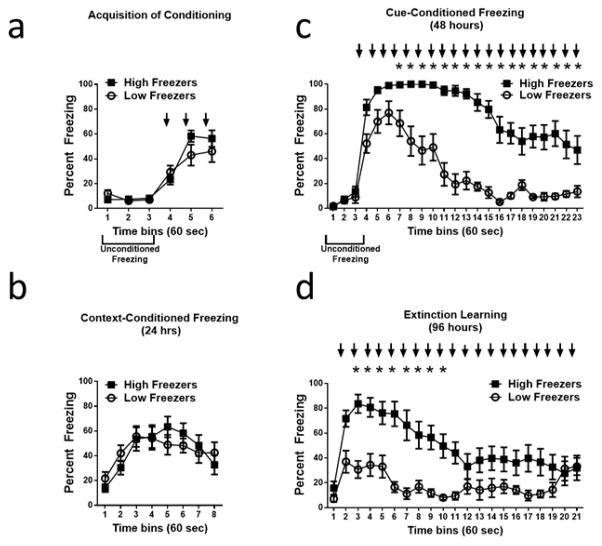
As seen by others in rat strains [12, 13, 17], Long Evans rats showed significant individual differences in the extinction of cue-conditioned freezing. For analysis purposes, animals were divided into high and low freezing groups based on a median split of the average percent freezing observed during the last 10 minutes of the cue (within trial extinction) session. Using this distribution, Figure 1 shows percent freezing during acquisition (a), context re-exposure (b), cue re-exposure (c) and extinction recall (d) trials in high and low freezing groups. Overall, high freezers showed significantly more freezing behavior than low freezers during cue exposure (panel c; F[1,462]=70.5; p<0.0001). Both groups showed significant changes in freezing behavior over time (F[22,462]=36.1; p<0.0001), and a significant interaction (F[22,462]=6.9; p<0.0001) was detected by 2-way repeated measures ANOVA. Post-hoc Bonferroni analysis indicated high and low groups started to diverge significantly after the presentation of the third tone (time bin 7) until the end of the trial. Similar differences were seen in freezing behaviors between high and low freezers during the extinction recall trial (panel d), with high freezers expressing significantly more freezing behavior in response to cue presentation than low freezers (F[1,420]=14.3; p<0.005). Two-way repeated measures ANOVA also detected a significant change in freezing over time (F[20,420]=9.1; p<0.0001) and a significant interaction (F[20,420]=4.19; p<0.0001) for the extinction recall trial. Post-hoc analysis indicated freezing in high and low groups differed significantly in response to tones 2–9; the response to the first tone was not statistically different due to large variations particularly in the low freezing group (see Figure 1d). Although significant differences were seen in extinction of cue-induced freezing and extinction recall in high and low freezing groups, no significant group differences were detected in freezing behaviors during acquisition of cue-conditioned fear (panel a) or context re-exposure to the conditioning context (panel b). Two-way ANOVA did detect significant changes in freezing behaviors over time during both acquisition (F[5,105]=47.7; p<0.0001) and context re-exposure (F[7,147]=11.7; p<0.0001) trials. In addition, the first three minutes of the cue trial in the novel arena (Context B) did not show any difference between these groups in unconditioned freezing responses (F[1,42] = 0.24, p=0.63 for group difference).
Figure 1.
Individual differences in freezing behavior during fear conditioning and extinction. Animals were divided into high and low freezing groups based on a median split of the mean percent freezing during the last ten tone presentations during the extinction learning trial. High (poor extinction) and low (normal extinction) freezing groups show no differences in percent freezing during acquisition of conditioned fear (a) or context re-exposure (b). During the within-trial extinction test (c), high and low freezers showed similar freezing to the initial three tone presentations (at arrows), indicating similar cue-conditioned freezing, but high freezers had significantly higher percent freezing than low freezers for subsequent tones indicating divergent within trial extinction. During the extinction recall test forty-eight hours later (d), high freezers still showed significantly higher percent freezing than low freezers during the initial tones. Tone presentations are represented by the arrows. Mean ± S.E.M of N= 11 low freezers and N=12 high freezers, in a total group of 23 rats. * indicates P<0.05.
3.2 Individual differences in Neuronal Activation
Dual-label immunohistochemistry was used to examine neuronal activation of OxA positive neurons in medial and lateral hypothalamus after extinction recall in animals showing high or low freezing during the last 10 tones of extinction training (cue trial, day 3). There was a high correlation (r=0.71, R2 =0.5, P=0.0002) between freezing during the last ten tones of extinction training (cue trial) and the first 10 tones of extinction recall. High freezers had a significantly higher percentage of Fos-labeled OxA-positive neurons in the medial hypothalamus, as compared to low freezers (Figure 2a). ANOVA showed that there was significant main effect of group (high versus low freezers; F[1,21]=8.43; p<0.009) and subregion (F[1,21]=117; p<0.0001), as well as an interaction (F[1,1]=10.6; p<0.0004). Post-hoc Bonferroni analysis indicated that the percent of orexin neurons with Fos differed between high and low groups in the medial but not lateral hypothalamus. In addition, the percentage of Fos-labeled OxA-positive neurons was positively correlated with percent freezing behavior during the last 10 minutes of the cue trial in both the medial (r=0.53; R2=0.29; p<0.01) and lateral (r=0.45; R2=0.21; p<0.05) hypothalamic regions (Figure 2b). The density of dual Fos+/OxA+ neurons was also positively correlated with percent freezing behavior during the last 10 minutes of the cue trial in both the medial (r=0.51; R2=0.26; p<0.05) and lateral (r=0.53; R2=0.28; p<0.01) hypothalamic regions (data not shown). In the medial region, there was also a correlation of the density of dual labeled Fos-OxA neurons with freezing during the first five tones of extinction recall (r=0.43, R2=0.19; P=0.04), but this was not significant in the lateral hypothalamus (r=0.35, R2=0.12; P=0.10). Neither overall Fos density (Figure 2c; F[1,21]=2.2; p=0.16 for high-low comparison) nor overall orexin neuron density (Figure 2d; F[1,21]=1.6; p<0.22 for high-low comparison) were significantly different in high and low freezing animals in either subregion region. The density of Fos+ neurons was greater in the medial compared to the lateral hypothalamus (F[1,21]=168; p<0.0001), but there was no difference in the density of OxA+ neurons between the two subregions (F[1,21]=3.34; p=0.08). Although there was no difference between the number of OxA+ neurons between high and low freezers in lateral (289±15 versus 270±18) or medial (349±27 versus 319±36) hypothalamus (F[1,21]=0.7, P=0.42), there were more total OxA+ neurons in medial than lateral subregions (F[1,21]=8.7, P=0.007). Representative photomicrographs showing dual Fos- and OxA-positive staining in the medial and lateral regions of the hypothalamus in low (Figure 2e) and high freezers (Figure 2f), and an enlargement (Figure 2g) showing single labeled Fos neurons (white arrows), single labeled OxA neurons (black arrows), and dual labeled Fos/OxA neurons (gray arrows).
4. 0 Discussion
Like other rodent studies, we show Long-Evans rats extinguish cue conditioned fear at variable rates, and we demonstrate that these individual differences in the extinction of cue conditioned fear are associated with differential activation of hypothalamic orexin neurons. Animals showing poor extinction of cue-induced freezing (high freezers) had a significantly greater percentage of orexin neurons with Fos in the medial hypothalamus than animals displaying significant extinction and good extinction recall (low freezers). Further, the percent of freezing during extinction learning was positively correlated with the percentage of activated orexin neurons in both the lateral and medial hypothalamic regions. Although correlative, our results support other studies implicating a role of the orexinergic system in regulating extinction of conditioned responses to threat [24, 26, 61]. These individual differences appear to be related to differential extinction learning and recall processes, since the groups with distinct extinction learning profiles showed similar acquisition of fear learning, similar contextual fear responses, and similar freezing during the initial cue presentations.
Individual differences in fear extinction have been seen in outbred rats or mice, animals selectively breed for fear behaviors, and between inbred strains of rodents (see [6] for review). Quirk and colleagues showed a bimodal distribution in extinction recall, with high fear animals showing greater freezing during recall (50–60%) as opposed to <50% freezing in the low fear group [14, 15]. Similarly, Herry et al. (2004) showed low and high recovery groups during extinction recall, although these animals did not show differences in extinction learning [16]. LeDoux and colleagues demonstrated a similar level of variation in outbred Sprague Dawley rats, with a divergence during extinction training and recall [12, 17]. In their initial study they used the top 20 percent and lower 20 percent as cutoffs for their reactivity groups, but subsequent evaluation using a Latent Class Growth Analysis (LCGA) yielded three groups of rats characterized as rapidly extinguishing, slow extinguishing, and failing to extinguish [12, 17]. A comparison of the extinction curves during twenty tone presentations during extinction learning in these two studies look remarkably like our data with Long Evans rats, with the Failure to Extinguish group representing ~10% of their population appearing highly analogous to our “high freezers” [17]. Another comparison using Long Evans rats by Shumake et al. (2014) indicated their robust learners were freezing 25% or less during extinction recall, while poor learners were freezing 75% or more [13]. These results are similarly analogous to ours, although we utilized a simple median split (at ~23% freezing) rather than freezing cutoffs for defining groups, and used the last cue presentations during extinction learning rather than recall [13]. Our results appear quite similar to these previous studies, since rats showed differences during extinction learning and recall, but not in acquisition of learned fear or contextually-conditioned freezing. The study by Shumake and colleagues (2014) indicated these innate differences were moderately heritable and associated with the level of ultrasonic vocalizations during acquisition [13]. This could suggest that vocalizations will provide a unique method of identifying extinction resistant phenotypes before extinction trials, but that remains to be determined.
Several previous studies have also demonstrated that variable levels of extinction learning and recall are associated with differences in neuronal activation using immediate early genes (IEG) and neuronal responses, particularly in the prefrontal cortex and amygdala. In general these studies demonstrate that impaired extinction is associated with decreased IEG expression in the infralimbic prefrontal cortex as well as regions of the amygdala, including the lateral, basolateral, and lateral central nuclei and some of the intercalated cell clusters. Other regions, such as the prelimbic cortex are hyper-activated in animals with poor extinction [6, 16, 67–69]. Further, animals with different fear phenotypes during extinction recall also show physiological differences in the prelimbic or infralimbic cortex [14–16]. Although our studies did not demonstrate an overall difference in Fos activation in the medial or lateral hypothalamus (Figure 2c), we instead found a differential activation of a specific neuronal population, namely orexin neurons, associated with variability in extinction learning and recall. Further, given the fact that less than 30% of the orexin neurons in the region were dual-labeled with Fos following this extinction trial, this may have diminished our ability to detect an overall increase in Fos density if the other, non-orexinergic populations were not differentially activated during extinction recall, as orexin neurons comprise only a minority of neurons in this area. It is also possible that low freezers have higher neuronal activation in another neuronal population than high freezers, such as the neurotensin/corticotropin releasing factor (CRF) phenotype that are distinct from orexin positive neurons, yielding no net change in overall Fos density.
Other studies using Fos as a marker of neuronal activation have indicated orexinergic neurons in the hypothalamus are activated by various types of anxiety-related or threat stimuli, and especially following procedures such as extinction or habituation that reduce conditioned fear. Acute unconditioned stressors including novelty, restraint, immobilization, cold, and footshock [27, 33, 42, 54–57], as well as chronic unpredictable stress [58], anxiety-provoking drugs [52], and sodium lactate infusions that induce a panic-like state [59] show activation of orexin neurons, particularly in the dorsomedial or perifornical (PeF) hypothalamic regions (but see [42]). The discrepancies might be due to differences between mice and rats, and/or differences in the length and intensity of the stressor. Some studies have demonstrated activation of orexin neurons in the medial hypothalamus, as well as increased OxA in the cerebrospinal fluid, following exposure to a fearful context [27, 42], although other studies have failed to see activation of orexin neurons associated with exposure to a conditioned context [55]. In addition, the study by Chen and colleagues (2014) showed similar increases in Fos activation of orexin neurons in the dorsomedial, but not lateral, hypothalamus after exposure to both the conditioned context and a novel chamber. A recent report also showed that orexin neurons in the dorsomedial and PeF, but not lateral, hypothalamus were activated during suppression of freezing behavior induced by both extinction training with repeated cue presentations and habituation to the unconditioned stimulus, which in this case was a loud (120 dB) white noise burst [61]. Similarly, orexin neurons in both the lateral hypothalamus and the dorsomedial-PeF regions were increased after repeated exposure to the context during extinction trials; interestingly more activation was seen with higher numbers of extinction trials (2 versus 5) [24]. Our studies similarly demonstrate that orexin neurons are activated after an extinction trial, although we saw higher levels of activation in animals that were resistant to extinction and showed enhanced freezing responses despite repeated cue presentations. This may have been related to differences in cued versus context extinction processes, or a different pattern of extinction training, since our studies used longer extinction trials with more within trial presentations of the cue. Others, however have demonstrated that contextual fear conditioning with a robust shock paradigm enhances the expression of preproorexin mRNA for at least 14 days, and that this increase is correlated with freezing in the context. Furthermore, the increase in preproorexin mRNA is seen in a population of rats that freezes significantly more to a novel sound stimulus (high responders) compared to low responders to the novel tone, or non-shocked controls [27]. Like our studies, this suggests that divergent changes in the orexin system may be associated with individual differences in freezing behaviors in response to tones (either novel or conditioned).
Studies suggest that functional subdivisions within the orexin neurons of the hypothalamus, particularly in the medial-lateral dimension, may mediate the diverse array of neurobiological phenomena associated with this neuropeptide system [32]. For example, the lateral hypothalamic orexin neurons preferentially regulate reward and motivated behaviors, including food and drug seeking behaviors, while the PeF-dorsomedial orexin neurons respond during stress and arousal functions [70, 71]. Thus, cues associated with drugs or food reward, or reinstatement of drug or alcohol seeking behaviors, significantly increased Fos accumulation in orexin neurons of the lateral hypothalamus, but cause little change in dorsomedial regions [66, 70, 72–76]. Further, both context-induced reinstatement of alcohol seeking, and home-cage ethanol preference, were correlated with the activation of orexin neurons in the lateral hypothalamus and PeF regions; interestingly the amount of cue-induced reinstatement of alcohol seeking was not correlated with activation of orexin neurons in the hypothalamus [72]. Orexin neurons in the dorsomedial and/or PeF regions of the hypothalamus are preferentially activated during active periods [76] and by various types of anxiety-related or threat stimuli, including unconditioned acute stressors [27, 42, 54–56], chronic stressors [58], sodium lactate infusions [59], anxiogenic drugs [52], or conditioned stimuli [27, 42, 61]. Further, many of these pharmacological and activation studies suggest that orexin neurons, particularly in the dorsomedial hypothalamus, respond preferentially to stressors that induce responses associated with arousal and attentional processes toward salient cues in the environment [42]). Like Furlong et al. (2016,) our results suggested the greatest difference in activation of orexinergic neurons associated with our extinction recall phenotypes was seen in the medial portions of the hypothalamus, although significant correlations between freezing behavior and neuron activation were seen in both the medial and lateral portions of the hypothalamus. Extinction of sucrose conditioned responding was also associated with activation in the dorsomedial and PeF regions of the hypothalamus [77], but extinction to the context activated the dorsomedial-PeF and lateral subregions of the hypothalamic orexin neurons [24]. Thus, this proposed functional segregation may not be absolute as suggested by the positive correlation between freezing behavior during the extinction trial and neuronal activation of orexin neurons in the lateral region, but it may be related to extinction processes engaging not only stress-arousal brain areas but also brainstem nuclei receiving projections from lateral hypothalamic areas. Similarly, correlations between Fos activation of orexin neurons in the dorsomedial region was correlated with context-induced reinstatement of alcohol seeking, but not home-cage ethanol preference [72]. In addition, since the PeF segment seems to be the transition zone it does not appear that further segregation of the activation into three (rather than two) subregions would have altered our results. Moreover, studies in humans examining orexin release in the amygdala using microdialysis suggested that orexin release was associated with transitions in emotional state, not just “arousal” per se [62].
Studies suggest that activation of the orexin system promotes behavioral, endocrine and autonomic responses associated with both conditioned and unconditioned forms of stress, and these effects may be more pronounced with familiar stressors [59, 78]. Administration of OxA or OxB i.c.v. induced stress-like behavioral responses including locomotion, face washing or chewing, grooming, rearing, freezing, and burrowing, as well as increased release of corticosterone [33, 47, 50, 57, 79], activation of CRF-containing neurons of the paraventricular nucleus and central amygdala [56], and cardiovascular responses associated with sympathetic activation [43]. Intracerebroventricular administration of OxA or OxB also decreased startle responses, but did not alter light-potentiated startle responses, suggesting that orexins may increase alarm, arousal or alerting responses, while decreasing unconditioned behavioral responses [80]. In fact, i.c.v. administration of OxA, but not OxB, attenuated extinction during repeated exposure to either the conditioned context or cues [24]. Our results similarly suggest that activation of orexin neurons, presumably enhancing orexin release in key brain sites, could be attenuating extinction processes in our poor extinguishing (high freezing) rats.
Pharmacological, lesion or genetic ablation studies also suggest that the orexin system regulates distinct physiological and behavioral aspects of conditioning processes, with a greater role for Ox1R in the consolidation of cued fear learning and extinction, while both Ox1 and Ox2 receptor systems affect contextual fear learning [24, 26, 29]. Large neurotoxic lesions of the PeF hypothalamus attenuated contextually conditioned freezing, ultrasonic vocalizations (USVs), and cardiovascular (heart rate and blood pressure) changes, but not the responses to restraint stress [81]; unfortunately these studies were confounded somewhat by the loss of both orexin and melanin concentrating hormone (MCH) neurons in this region. In addition, Ox1R knockout mice showed reduced freezing during acquisition and expression of both cue and context dependent conditioning [24, 28], as well as decreases in neuronal activation in the LC and lateral amygdala [28], while Ox2R knockout mice only showed deficits in contextual fear responses [28]. Differences were not seen in USVs in these knockout animals [28], suggesting a divergent regulation of distinct physiological and behavioral aspects of conditioning processes, as well as differential regulation of Ox1 and Ox2 receptor systems in conditioning processes associated with cues versus context. In support of this, systemic administration of the dual Ox1R/Ox2R antagonist almorexant decreased some cardiovascular measures, but not the behavioral freezing or USVs, associated with contextual fear and locomotion in a novel environment, and did not alter measures associated with restraint or cold stress [42]. Acute administration of almorexant also decreased fear-potentiated startle, but not baseline startle [82], although chronic (3 week) administration of the dual antagonist attenuated generalized avoidance behavior but not avoidance to the similar, conditioned environment [83]. Studies using the Ox1R antagonist SB334867 demonstrated that administration prior to training, but not post-training or pre-testing, reduced cue-conditioned freezing [29]. In contrast, post-training administration of SB334867 given immediately, but not four hours, after training decreased both contextual and cued fear responses [24]. Although post-training administration of the OX2R antagonist TCS OX2 29 reduced contextual fear responses [24], no effects of selective Ox2R antagonists are seen in cued fear responses [24, 29], reinforcing the distinct regulation via Ox1R, but not Ox2R, in cued fear learning. More relevant to our current results, administration of SB334867, but not TCS OX2 29, immediately after extinction trials accelerated extinction to both the context and cues in mice, and similar effects of the Ox1R antagonist were seen in a strain of extinction resistant mice (I129I/svlmj) [24]. Administration of SB334867 similarly impaired both acquisition and extinction in an appetitive conditioning protocol. Interestingly, the antagonist given before the initial extinction trial failed to affect behavior during the extinction learning phase, but impaired extinction recall during the second trial, further suggesting a role of OX1 receptors in consolidation of extinction learning with appetitive cues [84]. Taken together with neuronal activation of orexin neurons (see above), these results suggest that the orexin system is engaged during consolidation of conditioned fear responses, as well as extinction learning, and that cued fear learning and extinction rely more on orexin actions via the Ox1R system. Future pharmacological intervention studies will be needed to determine if orexins or orexin antagonists can modulate the extinction phenotypes in Long Evans rats.
Although we found correlations between activation of orexin neurons and freezing behavior during extinction training or recall, the specific role of orexinergic neurons in extinction learning processes will require additional studies. Evidence suggests the orexin system participates in behavioral responding during high arousal, aversive situations, since orexin neurotransmission is associated with stressors and administration of orexin can induce a variety of stress-like behavioral and physiological effects [25, 33, 85]. Thus activation of these neurons may merely reflect the behavioral output itself, and differing levels of activity versus freezing during the twenty minute extinction recall session. This is indeed a possibility, since single unit recording studies of orexin neurons in freely moving animals indicate that firing rates are elevated by exploration, grooming, and (to a lesser degree) sound stimuli [44]. However, since all animals are exposed to the same twenty tones, and more active behaviors (i.e., less freezing) were correlated with less neuronal activation, our results are not likely a reflection of the behavioral output alone. It is similarly unlikely that activation of orexin neurons just induces freezing, since administration of OxA or OxB increased face washing, grooming, and burrowing behaviors (e.g., active behaviors) rather than freezing [50, 76], although injections into the PVT can induce freezing in certain contexts [47] and increased freezing during a defensive burying task induced by sodium lactate infusion (which activates orexin neurons) could be attenuated by SB334867 in the BNST [59]. Alternatively, the differential activation of orexin neurons may be a consequence of the presumably higher level of stress responses in animals showing poor extinction, and high freezing behaviors. Orexinergic neurons are contacted by CRF terminals in asymmetric, presumably excitatory synapses, and perfusion of hypothalamic slices with CRF depolarizes orexin neurons and enhances firing rates [54]. The neuronal activation of orexin neurons by restraint or footshock stress is also ablated in CRF receptor1 knockout mice [54]. Thus, activation of orexin neurons could be indirectly related to the activation of the HPA axis, but that will require further studies.
Since the behavioral effects of orexin activation appear to be threat-specific [42, 43, 80, 81], orexin influences on freezing during extinction are likely to depend on actions in distinct brain regions and might contribute to the individual differences in extinction observed in our study. Presumably acting via Ox1R, the heightened orexin activity might regulate extinction learning or expression via projections to multiple sites known to be key in the fear extinction circuit, such as the amygdala, prefrontal cortex, basal forebrain cholinergic system, PVT, or brainstem nuclei such as the LC. Conversely, orexin neurons also have reciprocal connections with regions like the amygdala that could serve to activate them during extinction trials and act as a feedback loop [41]. This would be supported by studies in humans showing that narcoleptics with cataplexy (and therefore reduced orexinergic activity), have reduced aversive conditioning responses accompanied by reduced amygdalar activity and attenuated amygdala-PFC coupling during conditioning [86]. Two elegant studies have implicated orexin projections to the LC, acting via Ox1R to excite noradrenergic projections to the lateral amygdala, in the consolidation of cued threat learning [28, 29]. Studies examining differential Fos activation during early (trial 2) and late (trial 5) extinction following SB334867 administration also implicated orexin effects in the infralimbic PFC and basolateral amygdala [24], and microinjection of SB334867 into the basolateral amygdala, but not the infralimbic cortex or dorsal hippocampus, accelerated extinction learning to the context [24]. The ability of both OxA and OxB to depolarize and induce bursting in neurons of the medial central amygdala, an output region for conditioned fear responses, also suggests direct orexin effects mediated via Ox2 receptors could influence freezing behaviors [87]. However, it is also possible that orexin activity might facilitate arousal or attentional processes associated with extinction learning/recall, particularly in response to a cue [25]. These attentional effects might be mediated via orexin effects in the basal forebrain cholinergic system, or direct modulation of attentional processes in regions like the prefrontal cortex [25]. OxB activates basal forebrain cholinergic neurons [88], and cholinergic regulation influences both consolidation of fear memories and extinction [89]. OxA injections into the basal forebrain or the PFC enhance acetylcholine release in the PFC, and either intrabasalis or i.c.v. Ox A attenuates deficits in attentional processes [25, 90–93]. Systemic and intrabasalis inhibition of Ox1 receptors disrupts attentional performance [94]. Further, antagonists of the OX1, but not OX2 receptor, decrease firing and reduce the power of gamma oscillations of putative pyramidal neurons in the prelimbic prefrontal cortex, suggesting a direct effect of orexins in regulating activity and potentially synchronous activity of the prefrontal cortex in attentional processes [95]. Although the PVT receives very dense orexin projections, and Ox1R antagonists in the PVT can prevent stress induced increases in hypothalamic Fos activation with stressors [96], microinjection of a dual orexin receptor antagonist into the PVT has no effect on either cue or context conditioned freezing behavior [97]. Thus, the exact nature and neurobiological effects of orexin activation in mediating these individual differences in fear extinction will require additional more mechanistic studies aimed at these distinct brain regions.
A better understanding of individual differences extinction learning and recall has implications for a variety of anxiety disorders, but particularly PTSD and panic disorders [26, 71, 85]. PTSD is characterized by four symptom clusters, including re-experiencing, avoidance, negative cognitions and mood, and arousal/reactivity. Re-experiencing refers to having spontaneous memories, recurrent dreams, or flashbacks of the traumatic event, or psychological or physical distress associated with cues related to the event, suggesting fear learning and extinction may provide a useful model for understanding the risk and resilience factors for PTSD. While somewhere between 50 and 84% of the general population will experience a traumatic event, most individuals are resilient to these stressors, and estimates suggest that somewhere around 10% of the population will go on to develop PTSD [1–4]. Moreover, PTSD patients show impaired fear extinction learning and retention responses (see [5–9] for review) and emerging evidence suggests that individual differences in extinction of learned fear may predict susceptibility to PTSD. In a study of Dutch soldiers, reduced extinction learning prior to deployment was associated with increased PTSD symptom severity after returning home [10]. Similar results were seen in a study of firefighters, with reduced extinction learning in cadets associated with more severe PTSD symptoms after 24 months of active duty [11]. In addition, studies have demonstrated low OxA levels in cerebrospinal fluid and plasma in PTSD patients compared to normal controls, and a negative association between orexin concentrations and more severe symptoms in these combat veterans with PTSD [98]. In contrast, panic patients with anxiety symptoms show elevated CSF orexin concentrations [59]. The positive correlation between CSF and blood levels of orexin suggest this may also serve as a biomarker for these disorders [98].
Conclusions
Long-Evans rats exhibit distinct phenotypes in cued fear extinction that are associated with differential activation of hypothalamic orexin neurons. Animals showing poor extinction of cue-induced freezing had significantly greater activation of orexin neurons in the medial hypothalamus than animals displaying significant extinction. Further, freezing during extinction learning was positively correlated with the percentage of activated orexin neurons in both the lateral and medial hypothalamic regions. Although correlative, our results support other studies implicating a role of the orexin system in regulating extinction of conditioned responses to threat. An enhanced understanding of the role of orexins and their receptors in individual differences in fear extinction may provide novel insights into the neurobiological underpinnings of risk or resilience to traumatic stress, and offer new pharmacologic targets for PTSD or panic disorders.
Highlights.
Outbred rats show individual differences in cued fear extinction
Orexin neurons in the medial hypothalamus are activated during fear extinction
Greater activation of orexin neurons in hypothalamus is correlated with freezing during fear extinction
Acknowledgments
Support
This work was supported by the Veterans Administration [VA Merit Award 1101 BX001374 to MAW], the National Institutes of Health [NIA grant RO1AG050518 to JRF], and the University of South Carolina [ASPIRE I award to ACS].
Special Issue in Honor of Dr. Randall R. Sakai: We feel this contribution to Physiology and Behavior is particularly fitting to honor Randall as a cherished colleague and friend, since his research career rested at the intersection of physiological homeostasis, ingestive behaviors, and stress responses. First, it suggests how the hypothalamic peptide orexin, noted for its role as a physiological regulator generally, and in feeding behaviors in particular, might be involved in regulating extinction to conditioned stimuli. In addition, Randall always believed control groups were critical, and this research on individual differences in fear learning and extinction evolved from our work showing the most variability in our control groups. Randall was an excellent scientist and remarkable mentor, and we are all indebted to how his research, his support, and his insight has not only advanced the field but shaped us as scientists.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein RB, Smith SM, Chou SP, Saha TD, Jung J, Zhang H, Pickering RP, Ruan WJ, Huang B, Grant BF. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Soc Psychiatry Psychiatr Epidemiol. 2016;51:1137–1148. doi: 10.1007/s00127-016-1208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Psychiatric comorbidity of full and partial posttraumatic stress disorder among older adults in the United States: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Am J Geriatr Psychiatry. 2012;20:380–390. doi: 10.1097/JGP.0b013e31820d92e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes A, Singewald N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013;36:23–31. doi: 10.1016/j.tins.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kutlu MG, Gould TJ. Nicotine modulation of fear memories and anxiety: Implications for learning and anxiety disorders. Biochem Pharmacol. 2015;97:498–511. doi: 10.1016/j.bcp.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lommen MJ, Engelhard IM, Sijbrandij M, van den Hout MA, Hermans D. Pre-trauma individual differences in extinction learning predict posttraumatic stress. Behav Res Ther. 2013;51:63–67. doi: 10.1016/j.brat.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Guthrie RM, Bryant RA. Extinction learning before trauma and subsequent posttraumatic stress. Psychosom Med. 2006;68:307–311. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- 12.Bush DE, Sotres-Bayon F, LeDoux JE. Individual differences in fear: isolating fear reactivity and fear recovery phenotypes. J Trauma Stress. 2007;20:413–422. doi: 10.1002/jts.20261. [DOI] [PubMed] [Google Scholar]
- 13.Shumake J, Furgeson-Moreira S, Monfils MH. Predictability and heritability of individual differences in fear learning. Anim Cogn. 2014;17:1207–1221. doi: 10.1007/s10071-014-0752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur J Neurosci. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- 17.Galatzer-Levy IR, Bonanno GA, Bush DE, Ledoux JE. Heterogeneity in threat extinction learning: substantive and methodological considerations for identifying individual difference in response to stress. Front Behav Neurosci. 2013;7:55. doi: 10.3389/fnbeh.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 19.Baldi E, Bucherelli C. Brain sites involved in fear memory reconsolidation and extinction of rodents. Neurosci Biobehav Rev. 2015;53:160–190. doi: 10.1016/j.neubiorev.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Tronson NC, Schrick C, Guzman YF, Huh KH, Srivastava DP, Penzes P, Guedea AL, Gao C, Radulovic J. Segregated populations of hippocampal principal CA1 neurons mediating conditioning and extinction of contextual fear. J Neurosci. 2009;29:3387–3394. doi: 10.1523/JNEUROSCI.5619-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 22.Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rozeske RR, Valerio S, Chaudun F, Herry C. Prefrontal neuronal circuits of contextual fear conditioning. Genes Brain Behav. 2015;14:22–36. doi: 10.1111/gbb.12181. [DOI] [PubMed] [Google Scholar]
- 24.Flores A, Valls-Comamala V, Costa G, Saravia R, Maldonado R, Berrendero F. The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology. 2014;39:2732–2741. doi: 10.1038/npp.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fadel J, Burk JA. Orexin/hypocretin modulation of the basal forebrain cholinergic system: Role in attention. Brain Res. 2010;1314:112–123. doi: 10.1016/j.brainres.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flores A, Saravia R, Maldonado R, Berrendero F. Orexins and fear: implications for the treatment of anxiety disorders. Trends Neurosci. 2015;38:550–559. doi: 10.1016/j.tins.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Wang H, Lin Z, Li S, Li Y, Bergen HT, Vrontakis ME, Kirouac GJ. Orexins (hypocretins) contribute to fear and avoidance in rats exposed to a single episode of footshocks. Brain Struct Funct. 2014;219:2103–2118. doi: 10.1007/s00429-013-0626-3. [DOI] [PubMed] [Google Scholar]
- 28.Soya S, Shoji H, Hasegawa E, Hondo M, Miyakawa T, Yanagisawa M, Mieda M, Sakurai T. Orexin receptor-1 in the locus coeruleus plays an important role in cue-dependent fear memory consolidation. J Neurosci. 2013;33:14549–14557. doi: 10.1523/JNEUROSCI.1130-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sears RM, Fink AE, Wigestrand MB, Farb CR, de Lecea L, Ledoux JE. Orexin/hypocretin system modulates amygdala-dependent threat learning through the locus coeruleus. Proc Natl Acad Sci U S A. 2013;110:20260–20265. doi: 10.1073/pnas.1320325110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1–696. doi: 10.1016/s0092-8674(02)09256-5. [DOI] [PubMed] [Google Scholar]
- 32.Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17:1298–1303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berridge CW, Espana RA, Vittoz NM. Hypocretin/orexin in arousal and stress. Brain Res. 2010;1314:91–102. doi: 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 35.Carter ME, Schaich Borg J, de Lecea L. The brain hypocretins and their receptors: mediators of allostatic arousal. Curr Opin Pharmacol. 2009;9:39–45. doi: 10.1016/j.coph.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 38.Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- 39.Kilduff TS, de Lecea L. Mapping of the mRNAs for the hypocretin/orexin and melanin-concentrating hormone receptors: networks of overlapping peptide systems. J Comp Neurol. 2001;435:1–5. doi: 10.1002/cne.1189. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Furlong TM, Vianna DM, Liu L, Carrive P. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci. 2009;30:1603–1614. doi: 10.1111/j.1460-9568.2009.06952.x. [DOI] [PubMed] [Google Scholar]
- 43.Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- 44.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res. 2005;1044:116–121. doi: 10.1016/j.brainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Hsu DT, Kirouac GJ, Zubieta JK, Bhatnagar S. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front Behav Neurosci. 2014;8:73. doi: 10.3389/fnbeh.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ. Changes in emotional behavior produced by orexin microinjections in the paraventricular nucleus of the thalamus. Pharmacol Biochem Behav. 2010;95:121–128. doi: 10.1016/j.pbb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 48.Lungwitz EA, Molosh A, Johnson PL, Harvey BP, Dirks RC, Dietrich A, Minick P, Shekhar A, Truitt WA. Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol Behav. 2012;107:726–732. doi: 10.1016/j.physbeh.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heydendael W, Sengupta A, Beck S, Bhatnagar S. Optogenetic examination identifies a context-specific role for orexins/hypocretins in anxiety-related behavior. Physiol Behav. 2014;130:182–190. doi: 10.1016/j.physbeh.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ida T, Nakahara K, Katayama T, Murakami N, Nakazato M. Effect of lateral cerebroventricular injection of the appetite-stimulating neuropeptide, orexin and neuropeptide Y, on the various behavioral activities of rats. Brain Res. 1999;821:526–529. doi: 10.1016/s0006-8993(99)01131-2. [DOI] [PubMed] [Google Scholar]
- 51.Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun. 2000;270:318–323. doi: 10.1006/bbrc.2000.2412. [DOI] [PubMed] [Google Scholar]
- 52.Johnson PL, Samuels BC, Fitz SD, Federici LM, Hammes N, Early MC, Truitt W, Lowry CA, Shekhar A. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol Behav. 2012;107:733–742. doi: 10.1016/j.physbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A. Orexin, stress, and anxiety/panic states. Prog Brain Res. 2012;198:133–161. doi: 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu L, Onaka T, Sakurai T, Yada T. Activation of orexin neurones after noxious but not conditioned fear stimuli in rats. Neuro Report. 2002;13:1351–1353. doi: 10.1097/00001756-200207190-00027. [DOI] [PubMed] [Google Scholar]
- 56.Sakamoto F, Yamada S, Ueta Y. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul Pept. 2004;118:183–191. doi: 10.1016/j.regpep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 57.Espana RA, Valentino RJ, Berridge CW. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience. 2003;121:201–217. doi: 10.1016/s0306-4522(03)00334-8. [DOI] [PubMed] [Google Scholar]
- 58.Nollet M, Gaillard P, Minier F, Tanti A, Belzung C, Leman S. Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology. 2011;61:336–346. doi: 10.1016/j.neuropharm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 59.Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castro MG, Morrison E, Perone MJ, Brown OA, Murray CA, Ahmed I, Perkins AV, Europefinner G, Lowenstein PR, Linton EA. Corticotrophin-releasing hormone receptor type 1 - generation and characterization of polyclonal antipeptide antibodies and their localization in pituitary cells and cortical neurones in vitro. J Neuroendocrin. 1996;8:521–531. doi: 10.1046/j.1365-2826.1996.04866.x. [DOI] [PubMed] [Google Scholar]
- 61.Furlong TM, Richardson R, McNally GP. Habituation and extinction of fear recruit overlapping forebrain structures. Neurobiol Learn Mem. 2016;128:7–16. doi: 10.1016/j.nlm.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Blouin AM, Fried I, Wilson CL, Staba RJ, Behnke EJ, Lam HA, Maidment NT, Karlsson KAE, Lapierre JL, Siegel JM. Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat Commun. 2013;4:1547. doi: 10.1038/ncomms2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pasumarthi RK, Fadel J. Activation of orexin/hypocretin projections to basal forebrain and paraventricular thalamus by acute nicotine. Brain Res Bull. 2008;77:367–373. doi: 10.1016/j.brainresbull.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fadel J, Bubser M, Deutch AY. Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci. 2002;22:6742–6746. doi: 10.1523/JNEUROSCI.22-15-06742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Busti D, Geracitano R, Whittle N, Dalezios Y, Manko M, Kaufmann W, Satzler K, Singewald N, Capogna M, Ferraguti F. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J Neurosci. 2011;31:5131–5144. doi: 10.1523/JNEUROSCI.6100-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lehner M, Wislowska-Stanek A, Taracha E, Maciejak P, Szyndler J, Skorzewska A, Turzynska D, Sobolewska A, Hamed A, Bidzinski A, Plaznik A. The expression of c-Fos and colocalisation of c-Fos and glucocorticoid receptors in brain structures of low and high anxiety rats subjected to extinction trials and re-learning of a conditioned fear response. Neurobiol Learn Mem. 2009;92:535–543. doi: 10.1016/j.nlm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 70.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 71.Yeoh JW, Campbell EJ, James MH, Graham BA, Dayas CV. Orexin antagonists for neuropsychiatric disease: progress and potential pitfalls. Front Neurosci. 2014;8:36. doi: 10.3389/fnins.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moorman DE, James MH, Kilroy EA, Aston-Jones G. Orexin/hypocretin neuron activation is correlated with alcohol seeking and preference in a topographically specific manner. Eur J Neurosci. 2016;43:710–720. doi: 10.1111/ejn.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosci. 2010;30:4626–4635. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 75.Pasumarthi RK, Reznikov LR, Fadel J. Activation of orexin neurons by acute nicotine. Eur J Pharmacol. 2006;535:172–176. doi: 10.1016/j.ejphar.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 76.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology (Berl) 2013;226:155–165. doi: 10.1007/s00213-012-2902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Staples LG, Cornish JL. The orexin-1 receptor antagonist SB-334867 attenuates anxiety in rats exposed to cat odor but not the elevated plus maze: an investigation of Trial 1 and Trial 2 effects. Horm Behav. 2014;65:294–300. doi: 10.1016/j.yhbeh.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 79.Ida T, Nakahara K, Kuroiwa T, Fukui K, Nakazato M, Murakami T, Murakami N. Both corticotropin releasing factor and neuropeptide Y are involved in the effect of orexin (hypocretin) on the food intake in rats. Neurosci Lett. 2000;293:119–122. doi: 10.1016/s0304-3940(00)01498-1. [DOI] [PubMed] [Google Scholar]
- 80.Singareddy R, Uhde T, Commissaris R. Differential effects of hypocretins on noise-alone versus potentiated startle responses. Physiol Behav. 2006;89:650–655. doi: 10.1016/j.physbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Furlong T, Carrive P. Neurotoxic lesions centered on the perifornical hypothalamus abolish the cardiovascular and behavioral responses of conditioned fear to context but not of restraint. Brain Res. 2007;1128:107–119. doi: 10.1016/j.brainres.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 82.Steiner MA, Lecourt H, Jenck F. The brain orexin system and almorexant in fear-conditioned startle reactions in the rat. Psychopharmacology (Berl) 2012;223:465–475. doi: 10.1007/s00213-012-2736-7. [DOI] [PubMed] [Google Scholar]
- 83.Viviani D, Haegler P, Jenck F, Steiner MA. Orexin neuropeptides contribute to the development and persistence of generalized avoidance behavior in the rat. Psychopharmacology (Berl) 2015;232:1383–1393. doi: 10.1007/s00213-014-3769-x. [DOI] [PubMed] [Google Scholar]
- 84.Keefer SE, Cole S, Petrovich GD. Orexin/hypocretin receptor 1 signaling mediates Pavlovian cue-food conditioning and extinction. Physiol Behav. 2016;162:27–36. doi: 10.1016/j.physbeh.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shekhar A. Many faces of orexin/hypocretin. Prog Brain Res. 2012;198:1–4. doi: 10.1016/B978-0-444-59489-1.00019-7. [DOI] [PubMed] [Google Scholar]
- 86.Ponz A, Khatami R, Poryazova R, Werth E, Boesiger P, Schwartz S, Bassetti CL. Reduced amygdala activity during aversive conditioning in human narcolepsy. Ann Neurol. 2010;67:394–398. doi: 10.1002/ana.21881. [DOI] [PubMed] [Google Scholar]
- 87.Bisetti A, Cvetkovic V, Serafin M, Bayer L, Machard D, Jones BE, Muhlethaler M. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience. 2006;142:999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 88.Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, Muhlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- 89.Wilson MA, Fadel JR. Cholinergic regulation of fear learning and extinction. Journal of Neuroscience Research. 2016 doi: 10.1002/jnr.23840. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zajo KN, Fadel JR, Burk JA. Orexin A-induced enhancement of attentional processing in rats: role of basal forebrain neurons. Psychopharmacology (Berl) 2016;233:639–647. doi: 10.1007/s00213-015-4139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frederick-Duus D, Guyton MF, Fadel J. Food-elicited increases in cortical acetylcholine release require orexin transmission. Neuroscience. 2007;149:499–507. doi: 10.1016/j.neuroscience.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 92.Fadel J, Pasumarthi R, Reznikov LR. Stimulation of cortical acetylcholine release by orexin A. Neuroscience. 2005;130:541–547. doi: 10.1016/j.neuroscience.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 93.Fadel J, Frederick-Duus D. Orexin/hypocretin modulation of the basal forebrain cholinergic system: insights from in vivo microdialysis studies. Pharmacol Biochem Behav. 2008;90:156–162. doi: 10.1016/j.pbb.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 94.Boschen KE, Fadel JR, Burk JA. Systemic and intrabasalis administration of the orexin-1 receptor antagonist, SB-334867, disrupts attentional performance in rats. Psychopharmacology (Berl) 2009;206:205–213. doi: 10.1007/s00213-009-1596-2. [DOI] [PubMed] [Google Scholar]
- 95.He C, Chen QH, Ye JN, Li C, Yang L, Zhang J, Xia JX, Hu ZA. Functional inactivation of hypocretin 1 receptors in the medial prefrontal cortex affects the pyramidal neuron activity and gamma oscillations: An in vivo multiple-channel single-unit recording study. Neuroscience. 2015;297:1–10. doi: 10.1016/j.neuroscience.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 96.Heydendael W, Sharma K, Iyer V, Luz S, Piel D, Beck S, Bhatnagar S. Orexins/hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology. 2011;152:4738–4752. doi: 10.1210/en.2011-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dong X, Li Y, Kirouac GJ. Blocking of orexin receptors in the paraventricular nucleus of the thalamus has no effect on the expression of conditioned fear in rats. Front Behav Neurosci. 2015;9:161. doi: 10.3389/fnbeh.2015.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Strawn JR, Pyne-Geithman GJ, Ekhator NN, Horn PS, Uhde TW, Shutter LA, Baker DG, Geracioti TD., Jr Low cerebrospinal fluid and plasma orexin-A (hypocretin-1) concentrations in combat-related posttraumatic stress disorder. Psychoneuroendocrinology. 2010;35:1001–1007. doi: 10.1016/j.psyneuen.2010.01.001. [DOI] [PubMed] [Google Scholar]