Abstract
Background:
Ovomucoid is the dominant allergen in hen's egg. Although several studies evaluated the utility of ovomucoid specific immunoglobulin E (sIgE) levels in predicting baked (e.g., muffin or cupcake) or raw egg food challenge outcomes, studies that evaluated ovomucoid sIgE as a predictor of cooked egg (e.g., scrambled or hard boiled) challenge outcomes are limited.
Objective:
To determine the relation of ovomucoid sIgE levels with cooked egg food challenge outcomes.
Methods:
A retrospective review of 44 children who underwent cooked egg food challenge and who had the ovomucoid sIgE level measured.
Results:
Thirty-six of 44 children (81.8%) passed cooked egg challenge. The ovomucoid sIgE level predicted cooked egg challenge outcome (passed median, <0.35 kU/L [range, <0.35–0.64 kU/L]; failed median, 0.40 kU/L [range, <0.35–3.13 kU/L]; p = 0.004). Ovomucoid sIgE levels correlated with egg white (EW) sIgE levels (Spearman correlation coefficient, 0.588; p < 0.001). Receiver operating characteristic curve analysis of ovomucoid and EW sIgE demonstrated areas under the curve of 0.711 and 0.766, respectively. No significant difference was observed among those immunologic parameters in their abilities to predict cooked egg challenge outcome (p = 0.559).
Conclusion:
The ovomucoid sIgE level may be helpful in predicting cooked egg challenge outcomes. However, our study did not support a role for ovomucoid sIgE replacing EW sIgE testing in evaluating egg allergy.
Keywords: Ovomucoid, egg, food allergy, oral food challenge, anaphylaxis, specific IgE, tolerance
Egg allergy is the second most common childhood food allergy, which affects 1–2% of children.1–3 Although most children will outgrow egg allergy, they are outgrowing egg allergies later than previously reported.4
Egg white (EW) contains >20 glycoproteins. Ovomucoid, the dominant allergen, is a glycoprotein with trypsin inhibitor activity, which bears multiple conformational and linear epitopes that can be bound by immunoglobulin E (IgE).5,6 The importance of ovomucoid as an allergen may be related to its stability against heat and proteolysis.7
Skin-prick testing (SPT) and blood specific IgE (sIgE) values are used to evaluate food sensitization and may predict the likelihood that an allergy has resolved.8 EW SPT and sIgE values are used to identify patients allergic or tolerant to cooked egg (e.g., scrambled, hard boiled)9 and baked egg (e.g., cake, muffin).10,11 Component-resolved diagnostics may more accurately diagnose food allergies.12 Whereas current tests measure sensitization to a group of proteins, component-resolved diagnostics measure sIgE levels to specific egg proteins, for example, ovomucoid.
Multiple studies evaluated the utility of ovomucoid sIgE in predicting food challenge outcomes to baked egg10,13–17 and raw egg.9,18–20 Few studies evaluated the importance of ovomucoid sIgE in predicting cooked egg tolerance.9,18,20,21 We sought to determine whether ovomucoid sIgE level predicted cooked egg tolerance.
METHODS
Study Design
A retrospective chart review was performed of patients who underwent cooked egg challenge and who had ovomucoid sIgE levels performed at Boston Children's Hospital from April 2010 to September 2011. EW SPT and sIgE, total IgE, clinical history, demographics, and food challenge outcomes were obtained through medical record review. A total of 1186 subjects had ovomucoid sIgE evaluation performed, and 54 underwent cooked egg challenges. We focused on subjects with sIgE level and SPTs performed ≤1 year before challenge. Forty-four subjects had ovomucoid sIgE level, 43 had EW sIgE level, and 42 had EW SPT performed in this time frame. Eight subjects were excluded due to negative sIgE values and SPT results, and no history of allergic reaction to egg. All the subjects analyzed had a history of allergic reaction to egg documented in the medical record by an allergist and/or egg sensitization determined by positive SPT or elevated sIgE value. The study was approved by the institutional review board of Boston Children's Hospital.
Allergy Evaluation
SPTs were performed according to previously published methods22 by using the Multi-Test device (Alk-Abello, Round Rock, TX) and commercially prepared EW extract (Greer Laboratories, Lenoir, NC). Controls consisted of histamine (positive control) and normal saline solution (negative control). Wheal diameters were measured 15 minutes after SPT placement in a standard fashion.22 A wheal diameter ≥3 mm larger than the negative control was considered a positive result.8 Serum was analyzed for EW and ovomucoid sIgE value by ImmunoCAP fluorescence enzyme immunoassay (Thermo Scientific, Portage, MI). The lowest limit of detection was 0.35 kU/L, and the highest limit of detection was 100 kU/L.
Oral Challenge
Physician-supervised food challenges were performed as open challenges at Boston Children's Hospital. Blood sIgE was obtained ≤1 year before challenge; median, 2.64 months; and interquartile range, 0.61–4.68 months. SPT was done ≤1 year before challenge; median, 2.66 months; interquartile range, 0.39–4.93 months. The subjects were considered for challenge in the allergy clinic (AC) if the EW SPT wheal was ≤5 mm and EW sIgE level as ≤0.6 kU/L, based on previously suggested guidelines.23 The subjects were considered for challenge in the high-risk clinic (HRC) if levels were higher than these cutoffs. The subjects with anaphylaxis to egg within 2 years or unstable asthma were not recommended for challenges. The decision for ordering and determining challenge location was ultimately at the discretion of the ordering allergist.
Hard-boiled egg, scrambled egg, or egg powder was used for challenges. AC challenges were performed in standard fashion according to previously published methods,24–28 which consisted of increments every 15 minutes of 500 mg (1/12 egg), 1 g (1/6 egg) and 6.5 g (remainder of the egg plus an additional 1/3 egg), which totaled 8 g egg protein (1–1/3 egg). HRC challenges were performed in standard fashion according to previously published methods,22 which consisted of increments every 15 minutes of 100 mg (1/60 egg), 500 mg (1/12 egg), 1 g (1/6 egg), 2 g (1/3 egg), 4 g (2/3 egg), and 4 g (2/3 egg), which totaled 11.6 g egg protein (nearly 2 eggs). The subjects were monitored throughout and for 30–60 minutes after completion. Challenges were discontinued at the first objective sign of reaction,8 and treatment was initiated at the discretion of the supervising allergist.
Statistical Analysis
Median values were calculated for subject ages at the time of challenge and ovomucoid sIgE level. Prevalence rates of baseline characteristics were calculated. Challenge outcome was the criterion standard by which performance characteristics (sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV]) were calculated. PPV refers to a level above which a given percentage is likely that a patient will fail challenge. NPV refers to a level below which a given percentage is likely that a patient will pass.23 Receiver operator characteristic curve analysis was used to determine a threshold that would differentiate the subjects who were allergic or were tolerant. The relationship between sIgE and challenge outcome was analyzed by using logistic regression. Results from logistic regression were used to plot fitted predicted probability curves.
Continuous variables were analyzed by the Wilcoxon rank sum test. Dichotomous variables were analyzed by the Pearson χ2 or Fisher's exact tests, as appropriate. The strength of association between variables was analyzed by the Spearman correlation coefficient. An algorithm suggested by DeLong et al.29 was used to compare areas under receiver operator characteristic curves. A p value of <0.05 was considered statistically significant.
RESULTS
Outcomes of Food Challenges
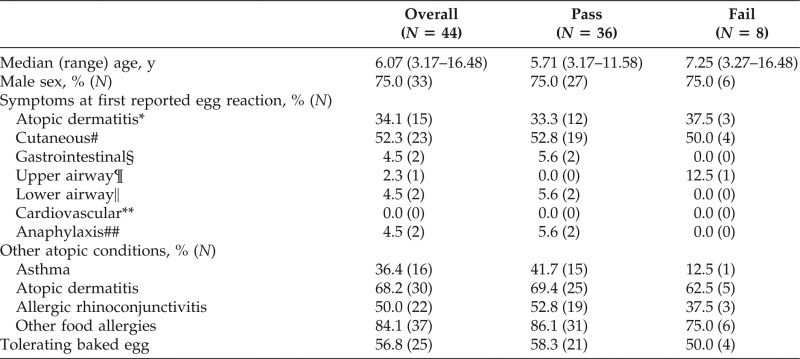
Thirty-six of 44 children (81.8%) passed and 8 of 44 (18.2%) had failed cooked egg challenges (Table 1). Age, sex, presence of atopic conditions, and other food allergies were not associated with challenge outcome. Consuming baked egg at the time of cooked egg challenge was not associated with outcome. Symptoms at first reported egg reaction did not differ among those who passed versus those with failed challenge.
Table 1.
Characteristics of study population
*In relation to egg ingestion or allergy evaluated and exclusion recommended in the setting of atopic dermatitis.
#Hives, angioedema, rash, or pruritus.
§Abdominal pain, vomiting, or diarrhea.
¶Rhinoconjunctivitis, oral pruritus, tongue swelling, or stridor.
‖Wheezing or coughing.
**Hypotension or lethargy.
##Defined by clinical criteria from the Second Symposium on Anaphylaxis (from Ref. 30).
Failed Cooked Egg Challenges
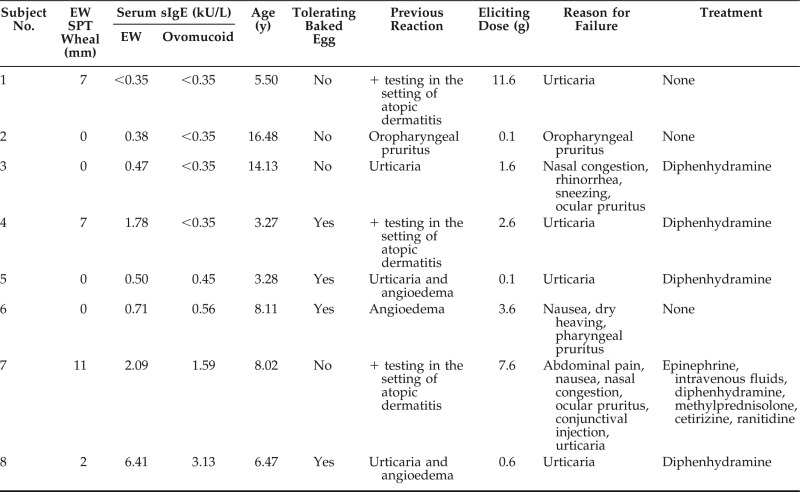
The subjects with failed challenge are described in Table 2. The four subjects with failed challenge and with negative ovomucoid sIgE value manifested minor symptoms that resolved with diphenhydramine or that self-resolved. Subject nos. 6 and 7 had anaphylaxis30 and had two of the highest ovomucoid sIgE levels. No subject with failed challenge had negative testing results to all parameters analyzed (ovomucoid sIgE value, EW sIgE value, and EW SPT). All failed challenges occurred in the HRC.
Table 2.
Failed cooked egg challenges
Predictive Value of Ovomucoid sIgE Level
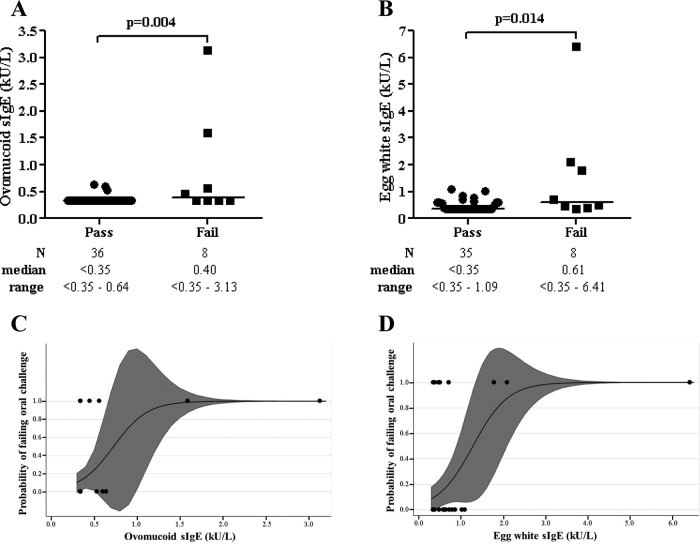
Ovomucoid and EW sIgE levels were correlated (Spearman correlation coefficient = 0.588; p < 0.001). The ovomucoid sIgE level was lower in the subjects who passed compared with those with failed challenge (median, passed = <0.35 kU/L and failed = 0.40 kU/L; z = 1.27; p = 0.004) (Fig. 1A). The EW sIgE level was lower in the subjects who passed, compared with those with failed challenge (median, passed = <0.35 kU/L and failed = 0.61 kU/L; z = 2.09; p = 0.014) (Fig. 1B). Probability curves for passing based on sIgE levels were generated (Fig. 1C, D). The total IgE value did not differ between the subjects who passed versus those with failed challenge. When the subjects without a history of IgE-mediated symptoms to egg were excluded from analysis, ovomucoid sIgE level was still lower in the subjects who passed compared with those with failed challenge (p = 0.027).
Figure 1.
Egg challenge outcome based on sIgE level. (A, B) Ovomucoid (n = 44) and EW (n = 43) sIgE levels grouped by challenge outcomes; data points represent individual subjects; medians are indicated by horizontal lines. (C, D) Estimated probability curves for failed challenges at a given ovomucoid (n = 44) and EW (n = 43) sIgE level derived from logistic regression; data points represent individual subjects; shaded regions indicate 95% confidence limits (these regions can sometimes be outside the parameter space, i.e., >1 or <0).
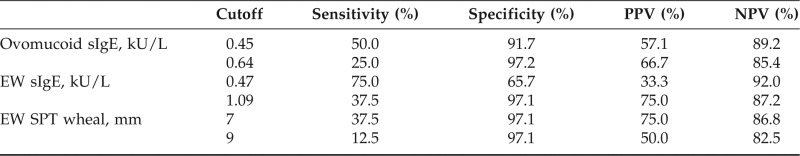
We could not identify a >90% predictive value for passing challenges (>90% NPV) for the ovomucoid sIgE level. The highest NPV established was 89.2% for ovomucoid sIgE level of 0.45 kU/L (Table 3). No subject with an ovomucoid sIgE level of >0.64 kU/L passed. A >95% specificity, proposed by some as a decision point above which challenge should not be considered,14 was established for an ovomucoid sIgE level of 0.64 kU/L. The highest NPV values and >95% specificities for EW sIgE and SPT are included in Table 3 for comparison. Receiver operator characteristic curve analysis for ovomucoid and EW sIgE levels revealed areas under the curve (AUC) of 0.711 and 0.766, respectively. There was no significant difference between the AUCs (p = 0.559).
Table 3.
Performance characteristics of immunologic parameters at various cutoff values
DISCUSSION
We found that measurement of ovomucoid sIgE level may predict cooked egg challenge outcomes but that it was not superior to EW sIgE level. This study was one of the few that evaluated the utility of ovomucoid sIgE value in predicting cooked egg challenge outcomes. Although the subjects with lower ovomucoid sIgE levels were more likely to pass, 50% of those with failed challenge had undetectable ovomucoid sIgE values. No subject with both negative EW SPT and sIgE values failed. Although EW SPT results did not predict challenge outcome in our study, the correlation between the EW SPT result and challenge outcome is variable in the literature, and this may have also been influenced by sample size.31
Other groups analyzed the utility of the ovomucoid sIgE level in predicting cooked egg challenge outcomes. Dieguez et al.9 challenged 157 Spanish children ages 1–16 years with a history of egg allergy by using a protocol that culminated in eating a 2-minute cooked egg. The median ovomucoid sIgE was 0.71 kU/L in the subjects with persistent egg allergy compared with <0.35 kU/L in the subjects who were tolerant (p < 0.0001). AUCs for EW and ovomucoid were not significantly different, consistent with our results. They determined that ovomucoid an sIgE level of 1 kU/L corresponded to >90% PPV of failing cooked egg challenge, similar to our >90% PPV at 1.59 kU/L. Vazquez-Ortiz et al.18 challenged 85 Spanish children ages 5–18 years with a history of egg allergy by using a protocol of EW boiled at 90°C for 10 minutes. Consistent with our findings, the ovomucoid sIgE level correlated with challenge outcome, and AUCs for EW and ovomucoid sIgE were not significantly different. The investigators proposed a negative decision point, defined as the cutoff level with 95% sensitivity, for ovomucoid sIgE level of 0.23 kU/L (PPV, 67.6%; NPV, 83.3%) and a positive decision point, defined as the cutoff level with 95% specificity, for an ovomucoid sIgE level of 3.74 kU/L (PPV, 92.2%; NPV, 65.2%). Their negative decision point is similar to our 89.2% NPV of an ovomucoid sIgE level of 0.45 kU/L. Their positive decision point is higher than the highest ovomucoid sIgE level in our study, which likely reflects differences in study populations.
Boyano Martinez et al.20 studied Spanish children ages 11–24 months with a history of egg allergy and administered challenges to boiled EW to 56 subjects, followed by raw EW. The ovomucoid sIgE level was higher in subjects with failed boiled EW and/or raw challenges, compared with those who passed (1.68 kU/L and <0.35 kU/L, respectively, p < 0.002). The ovomucoid sIgE level was higher in the subjects with failed boiled EW challenges compared with those who passed boiled EW challenges but failed raw EW challenges (1.83 kU/L and 0.18 kU/L, respectively, p < 0.05). This study did not compare the utility of the ovomucoid sIgE level versus other tests in predicting challenge outcomes. Haneda et al.21 studied 100 Japanese children ages 12–23 months without previous egg exposure and challenged to boiled EW. The median ovomucoid sIgE level was 8.12 kU/L in the subjects with failed challenge compared with 1.00 kU/L in the subjects who passed (p < 0.01). The investigators proposed that the ovomucoid sIgE level had a better predictive value than the EW sIgE level; direct comparison of the performance characteristics was not performed. Their results cannot be directly compared with our results because their population consisted of children who had not previously ingested egg. In addition, these two studies20,21 consisted of much younger patient populations, and predictive values of sIgE level and SPT result for egg allergy are dependent on patient age, especially in children <2 years of age.32
Our study was novel and important because it was the first, to our knowledge, that described the utility of ovomucoid sIgE in predicting cooked egg challenge outcomes in a North American population. Previous studies were conducted in Spanish and Japanese populations. Major egg allergens vary by geographic region: ovomucoid is the predominant egg allergen in North America and Japan, but ovalbumin is the major allergen in Spain.33 Because results from different regions may not be generalizable to other populations, our study provided novel findings that informed the predictive value of ovomucoid sIgE in North American children.
We investigated cooked egg in forms and amounts typically ingested in the Western diet. A limitation of our study is the use of different protocols for the AC and HRC challenges. The AC challenges were deemed low risk based on SPT and sIgE, and were allocated less time with the three doses given. We gave a typical serving size portion (8 g) for a child. In the HRC, the patients had potentially greater risk of reaction based on SPT and sIgE, and, therefore, received more doses and a higher total dose. Despite this limitation, no child who passed a cooked egg challenge in the AC or HRC developed allergic symptoms with subsequent egg ingestion at home. The fact that some subjects were consuming baked egg may have altered their SPT and sIgE levels.34 Due to the retrospective nature of our study, we did not have information available about the length of time that the subjects were consuming baked egg, but this would be an important question to address in future studies. The different egg preparations (e.g., hard-boiled, scrambled, egg powder) used in challenges may have had different specific protein contents and conformations that could have affected challenge outcomes. Escudero et al.35 showed that the protein composition, allergenicity, and egg food challenge outcomes by using raw versus dehydrated EW were equivalent. It may have been beneficial to perform food challenges to raw egg, but this was beyond the scope of our study. However, at follow-up visits, none of the subjects reported difficulty incorporating other forms of egg into their diets.
Another limitation of our study was the small patient population, viz. having only eight challenge failures. The performance characteristics of ovomucoid sIgE values at various cutoffs (Table 3) are based on this small number of failures. The predictability of outcomes for ovomucoid sIgE values may be enhanced by a larger sample size, and future larger studies are needed to confirm these findings. Although we were able to calculate NPV and PPV for ovomucoid sIgE values, the NPV and PPV depend on prevalence. Other clinical settings may yield different prevalence proportions due to differences in screening or selection of patients. The decision for food challenges was based on EW sIgE and SPT levels, which may have biased ovomucoid sIgE levels. Our study and others showed that ovomucoid sIgE level correlates with the EW sIgE level, so the ovomucoid decision points in our study are likely still valid.10,21 Future studies that investigate the predictive value of ovomucoid sIgE independent of EW sIgE value and SPT results could be done to verify our findings. Another limitation of our study was the retrospective design. Nevertheless, our study added the novel role of ovomucoid sIgE to other routinely obtained diagnostic markers to further understand predictors of food challenge outcomes, in a practical clinical setting, and may inform future, larger prospective studies.
Our study was important because it was one of the few that analyzed the utility of the ovomucoid sIgE value as a predictor of cooked egg challenge outcomes. Of the eight subjects with failed challenge, five had a negative SPT results to EW but elevated sIgE levels to ovomucoid and/or EW. One subject with failed challenge had a negative sIgE value to both ovomucoid and EW but a positive EW SPT result. Obtaining both SPT results and sIgE levels may be useful before performing cooked egg challenges to help determine which subjects are good candidates for challenge.
We defined novel decision points based on the ovomucoid sIgE value that may be useful in predicting outcomes of cooked egg challenges. Compared with the EW sIgE level, the ovomucoid sIgE level was a useful but not a superior predictor of cooked egg challenge outcome. Although there is a recent focus on using component-resolved diagnostics to possibly more accurately diagnose food allergies,12 our study did not support a role for ovomucoid sIgE testing to replace traditional EW sIgE testing in evaluating egg allergy.
Footnotes
This research was supported by National Institutes of Health (NIH) grants R01 AI 073964, U01 AI 110397, and K24 AI 106822 (PI, W. Phipatanakul) and NIH grant K23 AI 104780 (PI, W.J. Sheehan). This work was conducted with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic health care centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers, or the NIH
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol 125:S116–S125, 2010. [DOI] [PubMed] [Google Scholar]
- 2. Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 58:427–443, xi, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hasan SA, Wells RD, Davis CM. Egg hypersensitivity in review. Allergy Asthma Proc 34:26–32, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol 120:1413–1417, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Benede S, Lopez-Fandino R, Reche M, et al. Influence of the carbohydrate moieties on the immunoreactivity and digestibility of the egg allergen ovomucoid. PLoS One 8:e80810, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nowak-Wegrzyn A, Fiocchi A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr Opin Allergy Clin Immunol 9:234–237, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Caubet JC, Kondo Y, Urisu A, Nowak-Wdgrzyn A. Molecular diagnosis of egg allergy. Curr Opin Allergy Clin Immunol 11:210–215, 2011. [DOI] [PubMed] [Google Scholar]
- 8. NIAID-Sponsored Expert Panel, Boyce JA, Assa'ad A, et al. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 126:S1–S58, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dieguez MC, Cerecedo I, Muriel A, et al. Utility of diagnostic tests in the follow-up of egg-allergic children. Clin Exp Allergy 39:1575–1584, 2009. [DOI] [PubMed] [Google Scholar]
- 10. Bartnikas LM, Sheehan WJ, Larabee KS, et al. Ovomucoid is not superior to egg white testing in predicting tolerance to baked egg. J Allergy Clin Immunol Pract 1:354–360, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cortot CF, Sheehan WJ, Permaul P, et al. Role of specific IgE and skin-prick testing in predicting food challenge results to baked egg. Allergy Asthma Proc 33:275–281, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Incorvaia C, Rapetti A, Aliani M, et al. Food allergy as defined by component resolved diagnosis. Recent Pat Inflamm Allergy Drug Discov 8:59–73, 2014. [DOI] [PubMed] [Google Scholar]
- 13. Lemon-Mule H, Sampson HA, Sicherer SH, et al. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol 122:977–983.e1, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Ando H, Moverare R, Kondo Y, et al. Utility of ovomucoid-specific IgE concentrations in predicting symptomatic egg allergy. J Allergy Clin Immunol 122:583–588, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Urisu A, Ando H, Morita Y, et al. Allergenic activity of heated and ovomucoid-depleted egg white. J Allergy Clin Immunol 100:171–176, 1997. [DOI] [PubMed] [Google Scholar]
- 16. Caubet JC, Bencharitiwong R, Moshier E, et al. Significance of ovomucoid- and ovalbumin-specific IgE/IgG(4) ratios in egg allergy. J Allergy Clin Immunol 129:739–747, 2012. [DOI] [PubMed] [Google Scholar]
- 17. Alessandri C, Zennaro D, Scala E, et al. Ovomucoid (Gal d 1) specific IgE detected by microarray system predict tolerability to boiled hen's egg and an increased risk to progress to multiple environmental allergen sensitisation. Clin Exp Allergy 42:441–450, 2012. [DOI] [PubMed] [Google Scholar]
- 18. Vazquez-Ortiz M, Pascal M, Jimenez-Feijoo R, et al. Ovalbumin-specific IgE/IgG4 ratio might improve the prediction of cooked and uncooked egg tolerance development in egg allergic children. Clin Exp Allergy 44:579–588, 2014. [DOI] [PubMed] [Google Scholar]
- 19. Marriage DE, Erlewyn-Lajeunesse M, Unsworth DJ, Henderson AJ. Unscrambling egg allergy: The diagnostic value of specific IgE concentrations and skin prick tests for ovomucoid and egg white in the management of children with hen's egg allergy. ISRN Allergy 2012:627545, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boyano Martinez T, Garcia-Ara C, Diaz-Pena JM, et al. Validity of specific IgE antibodies in children with egg allergy. Clin Exp Allergy 31:1464–1469, 2001. [DOI] [PubMed] [Google Scholar]
- 21. Haneda Y, Kando N, Yasui M, et al. Ovomucoids IgE is a better marker than egg white-specific IgE to diagnose boiled egg allergy. J Allergy Clin Immunol 129:1681–1682, 2012. [DOI] [PubMed] [Google Scholar]
- 22. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: An updated practice parameter. Ann Allergy Asthma Immunol 100:S1–S148, 2008. [DOI] [PubMed] [Google Scholar]
- 23. Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol 100:444–451, 1997. [DOI] [PubMed] [Google Scholar]
- 24. Bock SA, Sampson HA, Atkins FM, et al. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: A manual. J Allergy Clin Immunol 82:986–997, 1988. [DOI] [PubMed] [Google Scholar]
- 25. Morisset M, Moneret-Vautrin DA, Kanny G, et al. Thresholds of clinical reactivity to milk, egg, peanut and sesame in immunoglobulin E-dependent allergies: Evaluation by double-blind or single-blind placebo-controlled oral challenges. Clin Exp Allergy 33:1046–1051, 2003. [DOI] [PubMed] [Google Scholar]
- 26. Sicherer SH. Food allergy: When and how to perform oral food challenges. Pediatr Allergy Immunol 10:226–234, 1999. [DOI] [PubMed] [Google Scholar]
- 27. Taylor SL, Hefle SL, Bindslev-Jensen C, et al. A consensus protocol for the determination of the threshold doses for allergenic foods: How much is too much? Clin Exp Allergy 34:689–695, 2004. [DOI] [PubMed] [Google Scholar]
- 28. Williams LW, Bock SA. Skin testing and food challenges in allergy and immunology practice. Clin Rev Allergy Immunol 17:323–338, 1999. [DOI] [PubMed] [Google Scholar]
- 29. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44:837–845, 1988. [PubMed] [Google Scholar]
- 30. Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: Summary report–second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med 47:373–380, 2006. [DOI] [PubMed] [Google Scholar]
- 31. Mehl A, Niggemann B, Keil T, et al. Skin prick test and specific serum IgE in the diagnostic evaluation of suspected cow's milk and hen's egg allergy in children: Does one replace the other? Clin Exp Allergy 42:1266–1272, 2012. [DOI] [PubMed] [Google Scholar]
- 32. Komata T, Soderstrom L, Borres MP, et al. The predictive relationship of food-specific serum IgE concentrations to challenge outcomes for egg and milk varies by patient age. J Allergy Clin Immunol 119:1272–1274, 2007. [DOI] [PubMed] [Google Scholar]
- 33. Dang TD, Mills CE, Allen KJ. Determination of the clinical egg allergy phenotypes using component-resolved diagnostics. Pediatr Allergy Immunol 25:639–643, 2014. [DOI] [PubMed] [Google Scholar]
- 34. Leonard SA, Sampson HA, Sicherer SH, et al. Dietary baked egg accelerates resolution of egg allergy in children. J Allergy Clin Immunol 130:473–480.e1, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Escudero C, Sanchez-Garcia S, Rodriguez del Rio P, et al. Dehydrated egg white: An allergen source for improving efficacy and safety in the diagnosis and treatment for egg allergy. Pediatr Allergy Immunol 24:263–269, 2013. [DOI] [PubMed] [Google Scholar]