Abstract
Eukaryotic cells use diverse cytoskeleton-dependent machineries to control inheritance and intracellular positioning of mitochondria. In particular, microtubules play a major role in mitochondrial motility in the filamentous fungus Neurospora crassa and in mammalian cells. We examined the role of two novel Unc104/KIF1-related members of the kinesin family, Nkin2 and Nkin3, in mitochondrial motility in Neurospora. The Nkin2 protein is required for mitochondrial interactions with microtubules in vitro. Mutant hyphae lacking Nkin2 show mitochondrial motility defects in vivo early after germination of conidiospores. Nkin3, a member of a unique fungal-specific subgroup of small Unc104/KIF1-related proteins, is not associated with mitochondria in wild-type cells. However, it is highly expressed and recruited to mitochondria in Δnkin-2 mutants. Mitochondria lacking Nkin2 require Nkin3 for binding to microtubules in vitro, and mitochondrial motility defects in Δnkin-2 mutants disappear with up-regulation of Nkin3 in vivo. We propose that mitochondrial transport is mediated by Nkin2 in Neurospora, and organelle motility defects in Δnkin-2 mutants are rescued by Nkin3. Apparently, a highly versatile complement of organelle motors allows the cell to efficiently respond to exogenous challenges, a process that might also account for the great variety of different mitochondrial transport systems that have evolved in eukaryotic cells.
INTRODUCTION
Mitochondria are ubiquitous and essential organelles of eukaryotic cells. As membrane-bounded organelles, they cannot be generated de novo, but they grow and divide from preexisting organelles (Nunnari and Walter, 1996). In many cell types, mitochondrial inheritance during the cell division cycle is coordinated by ordered partitioning mechanisms involving interactions with the cytoskeleton (Warren and Wickner, 1996). These interactions are also crucial to control the intracellular distribution of mitochondria, which are often positioned at intracellular sites of high demand for ATP (Bereiter-Hahn, 1990; Yaffe, 1999). However, the cellular machinery mediating mitochondrial motility and distribution is not well understood.
The molecular mechanisms used by eukaryotic cells to control mitochondrial transport are strikingly diverse (Yaffe, 1999; Westermann and Prokisch, 2002). Mitochondrial movement in budding yeast depends on the actin cytoskeleton (Hermann and Shaw, 1998; Jensen et al., 2000; Boldogh et al., 2001b). It is a matter of debate whether myosin-related motor proteins are involved (Itoh et al., 2002) or whether it is driven by actin polymerization involving the Arp2/3 complex (Boldogh et al., 2001a). In contrast, mitochondrial distribution in fission yeast is dependent on microtubules (Yaffe et al., 1996), but movement is apparently not mediated by kinesin-like motor proteins (Brazer et al., 2000; Weir and Yaffe, 2004). On the other hand, kinesin family members have been shown to be involved in microtubule-dependent mitochondrial transport in mammalian cells and Drosophila (Nangaku et al., 1994; Pereira et al., 1997; Khodjakov et al., 1998; Tanaka et al., 1998). Fundamental differences in the molecular machinery underlying mitochondrial motility can be found even in closely related organisms. The filamentous fungi Neurospora crassa and Aspergillus nidulans share many similarities in their life cycle and cell biological functions. However, Neurospora uses microtubule tracks for mitochondrial movement (Steinberg and Schliwa, 1993; Prokisch et al., 2000; Fuchs et al., 2002), whereas Aspergillus relies exclusively on microfilaments to mediate this process (Oakley and Rinehart, 1985; Suelmann and Fischer, 2000). Functional or evolutionary reasons that might account for these remarkable differences are not known.
The filamentous fungus N. crassa is an excellent model system to study microtubule-dependent mitochondrial motility. Cells grow very fast, at a speed of up to 1 μm/s (Kirchner et al., 1999), and in a polarized manner. This requires an efficient transport system for cell organelles, which is provided by microtubule tracks (Steinberg and Schliwa, 1993). Mitochondria show saltatory movement in vivo with a mean velocity of up to 2 μm/s (Steinberg and Schliwa, 1993). We recently reported that mitochondrial binding to microtubules is mediated by adenine nucleotidesensitive proteins on the organellar surface. These observations point to a possible involvement of kinesin-related motor proteins (Fuchs et al., 2002). However, conventional kinesin, Nkin, is not required for mitochondrial distribution and motility in Neurospora (Seiler et al., 1997), and the identity of molecular motors mediating this process remained obscure. To identify motor proteins involved in mitochondrial motility, we searched the Neurospora genome (Galagan et al., 2003) for genes encoding putative kinesin-related organelle motors. Functional analysis of two novel members of the Unc104/KIF1 subfamily revealed important roles of these proteins in mediating mitochondrial interactions with microtubules in vitro and in vivo.
MATERIALS AND METHODS
Plasmid Constructions
Standard methods were used for the manipulation of DNA. For construction of a plasmid for expression of Nkin3 fused to an N-terminal glutathione S-transferase (GST) moiety in Escherichia coli, the nkin-3 coding region was amplified from genomic Neurospora DNA by polymerase chain reaction (PCR) by using primers 5′ AAA AGC TTG GAT CCA TGT GTT TAT TCC ACC ACC and 5′ AAA GGA TCC GAA TTC TCA TAT GAT ATT AGT CCT CAC CC and cloned into the BamHI site of vector pETGEXCT (Sharrocks, 1994), yielding pFF10. For construction of a plasmid for expression of the C-terminal half of Nkin3 fused to an N-terminal GST moiety in E. coli, a DNA fragment covering 711 base pairs of the nkin-3 coding region was amplified from genomic Neurospora DNA by using primers 5′ AAA AGA TCT CGC ATT CGT ACC CGT GCC G and 5′ AAA GGA TCC GAA TTC TCA TAT GAT ATT AGT CCT CAC CC, digested with BglII and cloned into the BamHI site of vector pETGEXCT, yielding pFF11. For construction of a plasmid for expression of an Nkin2 fragment fused to a C-terminal hexahistidine tag in E. coli, a DNA fragment covering 885 base pairs of the nkin-2 coding region was amplified from genomic Neurospora DNA by using primers 5′ GGG CCA TGG AGG ACG CCA ATG CCC GCA and 5′ AAA AGA TCT GGC TTT CGC TTC AAG AGG ATG, and cloned into the NcoI and BglII sites of vector pQE60 (QIAGEN, Hilden, Germany), yielding pFF12. A plasmid for disruption of the nkin-2 gene, pFF15, was constructed in three consecutive cloning steps. First, a DNA fragment covering 2000 base pairs of the terminator region of the nkin-2 gene was amplified from genomic Neurospora DNA by using primers 5′ CAA CGT AGT AAC CCG TTG and 5′ AAT AGG CCG AAA TCG GCA GTG CAT TCA GGG ATA CCC AAT GGG, cloned into the PCR product cloning vector pCRII-TOPO (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions, yielding plasmid pFF13. Second, a cassette conferring resistance to hygromycin B was amplified from plasmid pCB1179 (Sweigard et al., 1997) by using primers 5′ AAA GCG GCC GCA GGG AAT AAG GGC GAC ACG G and 5′ AAA GCG GCC GCT GCC GAT TTC GGC CTA TTG G and cloned into the NotI site of pFF13, yielding plasmid pFF14. Third, a DNA fragment covering 2041 base pairs of the promoter region of the nkin-2 gene was amplified from genomic DNA by PCR by using primers 5′ GGG TCT AGA CGT CTG GAG TCT CGA CCG and 5′ GGG CTC GAG TCC GGA CGC GAT CAC AGC and cloned into the XbaI and XhoI sites of pFF14, yielding plasmid pFF15. pFF15 was linearized by restriction digestion with SacI before it was transformed into Neurospora.
Antibodies
An antigen for Nkin2 was generated by expression of an Nkin2 fragment (amino acids 372-666) carrying a C-terminal hexahistidine tag in E. coli by using pFF12 (pQE60 expression system; QIAGEN) and purification on nickelnitrilotriacetic acid (Ni-NTA) according to the manufacturer's instructions. Soluble protein was eluted from a Ni-NTA column. Antigens for Nkin3 were generated by expression of either full-length Nkin3 or the C-terminal half fused to an N-terminal GST moiety in E. coli by using pFF10 and pFF11 (pETGEXCT expression system). Insoluble recombinant protein was purified as inclusion bodies. Antigens were injected into rabbits. Affinity purification of antibodies was according to published procedures (Harlow and Lane, 1988).
Strains, Growth Conditions, and Isolation of Neurospora Mutants
Standard genetic and microbiological techniques were used for the growth and manipulation of Neurospora strains (Davis and de Serres, 1970). Neurospora strains used were wild-type strain St. Lawrence 74A (Fungal Genetics Stock Center, Kansas City, KS), a wild-type strain expressing mitochondriatargeted green fluorescent protein (mtGFP) (Fuchs et al., 2002), and HP1, a parental strain used for sheltered disruption (Nargang et al., 1995). Neurospora was grown in Vogel's minimal medium under continuous aeration and illumination with white light at 25°C (Davis and de Serres, 1970). Transformation of Neurospora was carried out as described previously (Vollmer and Yanofsky, 1986; Staben et al., 1989).
A heterokaryotic nkin-2 mutant (Δnkin-2/nkin-2WT) was constructed by sheltered disruption, essentially as described previously (Nargang et al., 1995). Spheroplasts of HP1 were transformed with pFF15, and transformants were selected on minimal medium containing hygromycin. Isolates were purified by single-colony isolation on hygromycin-containing medium, genomic DNA was isolated, and transformants harboring a nucleus containing a targeted deletion of the nkin-2 gene were identified by Southern blotting. A strain containing a single integration of the disrupting DNA fragment was chosen for further analysis. Growth of this strain on minimal medium containing 0.2 mg/ml histidine and 400 μM p-fluoro-dl-phenyl-alanine (fpa) forced the disruptant-containing nucleus, as determined by Southern and Western blotting.
To isolate a homokaryotic Δnkin-2 mutant, the heterokaryotic strain was passaged three times on plates with selective medium forcing the disruptant-containing nucleus. Then, conidia were harvested and used to inoculate single colonies on plates containing minimal medium with 2% sorbose (instead of sucrose), 0.2 mg/ml histidine, and 10 μg/ml pantothenic acid. Cells from these colonies were transferred onto solid minimal medium supplemented with histidine and pantothenic acid and grown until conidia were formed. Conidia were then used to test the requirement of histidine and pantothenic acid. Isolates that could grow in the absence of pantothenic acid, but not in the absence of histidine, were homokaryotic and contained the Δnkin-2 allele. Genotypes were confirmed by Southern and Western blotting.
Cell Fractionation
Isolation of mitochondria, postmitochondrial supernatant, and cell extracts (Sebald et al., 1979), preparation of outer membrane vesicles (Mayer et al., 1993) and sucrose gradient purification of mitochondria (Rowley et al., 1994) were performed according to published procedures. For preparation of extracts of conidiospores, 30 OD600 units of cells were resuspended in 100 μl of SDS-PAGE sample buffer, glass beads were added, and the sample was incubated for 5 min at 95°C and vortexed for 5 min. Then, 400 μl of SDS-PAGE sample buffer was added, the sample was centrifuged for 10 min at 13,000 × g, and 15 μl of the supernatant was used for SDS-PAGE. Salt extraction of mitochondria was performed by resuspension of mitochondria in 250 mM sucrose, 1 mM EDTA, 10 mM MOPS/KOH, pH 7.2) (SEM) with 1 M KCl, incubation for 30 min on ice, and centrifugation for 12 min at 4°C at 13,000 × g. The supernatant was precipitated with trichloroacetic acid (TCA), and the mitochondrial pellet was resuspended in SEM.
Assay of Mitochondrial Binding to Microtubules
Mitochondrial binding to microtubules was assayed by flotation in sucrose gradients and by fluorescence microscopy as described previously (Fuchs et al., 2002). For antibody inhibition experiments, 500 ng of affinity-purified antibodies was added to 100 μg of mitochondria and incubated for 30 min on ice in 250-μl volume assay buffer in the absence of ATP (Fuchs et al., 2002). Then, mitochondria were reisolated by centrifugation for 12 min at 4°C at 13,000 × g, and 100 μg of microtubules was added. After an incubation for 30 min on ice, mitochondria were floated by sucrose gradient centrifugation (Fuchs et al., 2002). For microscopy, reisolation of mitochondria was omitted.
Microscopy
Staining of mitochondria and fluorescence microscopy was carried out as described previously (Prokisch et al., 2000; Fuchs et al., 2002). Mitochondrial motility was analyzed in intact cells by computer-enhanced phase contrast videomicroscopy. Under these conditions, mitochondria can be readily identified by their elongated shape (Steinberg and Schliwa 1993; Wu et al., 1998). Conidiospores were inoculated on microscope slides that were covered with a thin layer of minimal medium containing 0.2 mg/ml histidine and 15% gelatin. Spores were allowed to germinate and grow overnight at room temperature. Then, a few microliters of fresh medium was added, and the sample was covered with a coverslip. Mitochondrial movement was analyzed using a Zeiss Axiophot microscope equipped with a 100× oil immersion objective. Images were recorded with a Hamamatsu Orca ER 1394 camera and processed with SimplePCI software (Compix, Tualatin, OR).
RESULTS
Nkin2 and Nkin3 Are Novel Kinesin-related Motor Proteins of the Unc104/KIF1 Subfamily
The Neurospora genome harbors 10 genes that are predicted to encode kinesin-related motor proteins (Fuchs et al., 2002; Schoch et al., 2003; Xiang and Plamann, 2003; Borkovich et al., 2004). Two of these predicted proteins, Nkin2 (genome annotation number NCU06733.1) and Nkin3 (NCU03715.1), are closely related to members of the Unc104/KIF1 subfamily. Because this group contains many known organelle motors (Hirokawa, 1998), Nkin2 and Nkin3 are likely candidates for motor proteins mediating mitochondrial motility.
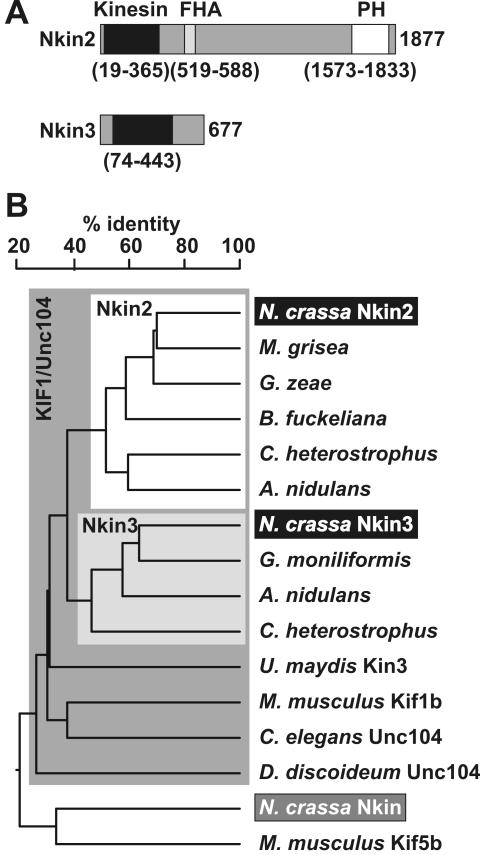
Nkin2 shares a conserved domain structure with other bona fide members of the Unc104/KIF1 subfamily (Figure 1A). It has an N-terminal kinesin motor domain, a forkhead associated (FHA) domain, which is thought to be involved in interactions with phosphopeptides (Durocher et al., 1999), and a C-terminal pleckstrin homology (PH) domain, which has been shown to play an important role in the interaction with cargo membranes (Klopfenstein et al., 2002). This conserved domain organization is also found, e.g., in the founding member of the subfamily, the Caenorhabditis elegans Unc104 protein, which serves as a vesicle transporter in neurons (Hall and Hedgecock, 1991).
Figure 1.
Nkin2 and Nkin3 are novel members of the Unc104/KIF1 subfamily of kinesin-related motor proteins. (A) Domain structure of Nkin2 and Nkin3. Homologies to known protein domains were revealed by searching the Pfam database (Bateman et al., 2002). Kinesin motor domains are depicted in black, FHA domains in light gray, and PH domains in white. Numbers indicate amino acid residues defining the borders of the predicted domains, and the C termini of the proteins, respectively. Protein domains are drawn to scale. (B) Homology tree of representative kinesin family members. Fungal homologues sharing the same domain structure with Nkin2 are highlighted by a white box. This group includes predicted proteins from Magnaporthe grisea (genome annotation number MG09255.4), Gibberella zeae (FG10189.1), Botryotinia fuckeliana (gene KLP8), Cochliobolus heterostrophus (KLP8), and Aspergillus nidulans (AN7547.2). Fungal homologues closely resembling Nkin3 are highlighted by a light gray box. This group includes predicted proteins from Gibberella moniliformis (KLP7), A. nidulans (AN6863.2), and Cochliobolus heterostrophus (KLP7). Other members of the KIF1/Unc104 family sharing the same domain structure with Nkin2 are Kin3 of Ustilago maydis (Wedlich-Söldner et al., 2002), KIF1B of Mus musculus (Nangaku et al., 1994), Unc104 of C. elegans (Otsuka et al., 1991), and Unc104 of D. discoideum (Pollock et al., 1999). Nkin (Steinberg and Schliwa, 1995) and Kif5b (Tanaka et al., 1998) are conventional kinesins. The tree was constructed using DNAMAN software (Lynnon BioSoft, Vaudreuil, Canada).
Nkin3 is a rather unusual kinesin family member because of its relatively low molecular mass (75.8 kDa). It has an N-terminal kinesin motor domain, and a C-terminal domain that does not share homology to any other known protein sequence motif (Figure 1A). Similar protein-coding sequences can be found in the genomes of related filamentous fungi. Based on extensive phylogenetic analysis it has been suggested that Nkin3 and its relatives constitute a unique fungal subgroup of “truncated” Unc104/KIF1-like proteins (Schoch et al., 2003). Cellular functions of members of this new subfamily are not known. Because Nkin3-related proteins belong to the Unc104/KIF1 family, it can be speculated that they act as organelle motors. However, members of this subgroup lack a PH domain, and possible mechanisms of binding to cellular membranes are unknown. A homology tree of putative Neurospora organelle motors and representative Unc104/KIF1 and conventional kinesin-like proteins is shown in Figure 1B.
Nkin2 Is Peripherally Associated with Mitochondria
We asked whether Nkin2 and Nkin3 are bound to mitochondria. Neurospora wild-type cells were fractionated by differential centrifugation into postmitochondrial supernatant (PMS) and a crude mitochondrial fraction. Then, mitochondria were further purified on a sucrose gradient, and outer membrane vesicles (OMVs) were prepared. All fractions were analyzed by immunoblotting by using specific antisera against Nkin2, Nkin3, and Nkin. Tom40 was used as a marker for the mitochondrial outer membrane, and the peroxisomal protein Pex14 was used as a marker for nonmitochondrial membranes (Figure 2A). Nkin3 and Nkin were found exclusively in the postmitochondrial supernatant, which contains cytosolic proteins and light cellular membranes. The fractionation behavior of Nkin2 was similar to the integral mitochondrial outer membrane protein, Tom40. It was highly enriched in purified mitochondria and outer membrane vesicles. In contrast, only trace amounts of Pex14 could be detected in these fractions. We suggest that a major fraction of Nkin2 is associated with mitochondria.
Figure 2.
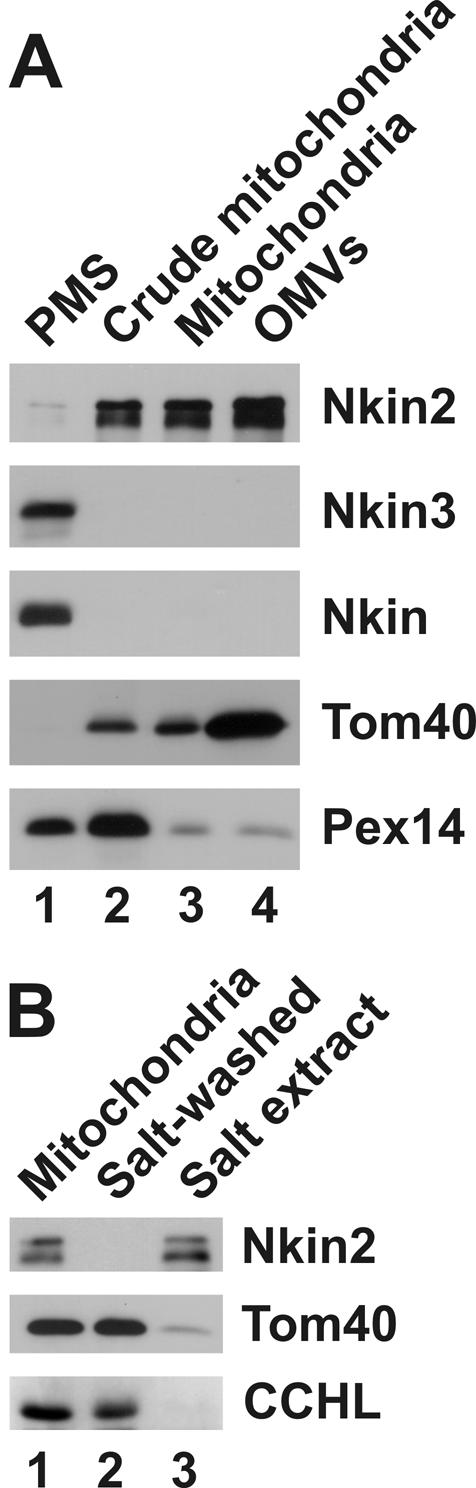
Nkin2 is peripherally associated with the mitochondrial outer membrane. (A) Subfractionation of cells. Wild-type cells were fractionated by differential centrifugation into PMS (lane 1) and a crude mitochondrial fraction (lane 2). Crude mitochondria were either further purified by sucrose gradient centrifugation (lane 3) or used for preparation of OMVs (lane 4). Fifty micrograms of protein of each fraction was analyzed by SDS-PAGE and immunoblotting by using specific antisera against Nkin2, Nkin3, and Nkin. The integral outer membrane protein, Tom40, served as a marker for mitochondria, and the peroxisomal protein, Pex14, served as a marker for nonmitochondrial membranes. (B) Salt-extraction of mitochondria. Isolated mitochondria were left untreated on ice (lane 1) or extracted with 1 M KCl and reisolated by centrifugation (lane 2). Proteins were precipitated from the salt extract with TCA (lane 3). Fifty micrograms of protein of each fraction was analyzed by SDS-PAGE and immunoblotting. Tom40 served as a marker for the mitochondrial outer membrane, and CCHL, a soluble protein of the intermembrane space, was used to control for integrity of the outer membrane.
Nkin2 was less enriched in outer membrane vesicles than Tom40 (Figure 2A, lane 4). A possible explanation is that Nkin2 is only peripherally associated with the mitochondrial outer membrane, resulting in the loss of a certain amount of protein during outer membrane vesicles preparation. Furthermore, it cannot be excluded that a subfraction of Nkin2 also might be associated with other membranous organelles. To investigate the nature of the association of Nkin2 with mitochondrial membranes more directly, mitochondria were washed with 1 M KCl and reisolated. High salt treatment released Nkin2 into the supernatant, whereas Tom40 and the soluble intermembrane space protein cytochrome c heme lyase (CCHL) remained in the organellar pellet (Figure 2B). We conclude that Nkin2 is a peripheral membrane protein that binds to mitochondrial outer membrane lipids and/or receptor proteins involving ionic interactions.
Nkin2 Is Required for Binding of Mitochondria to Microtubules
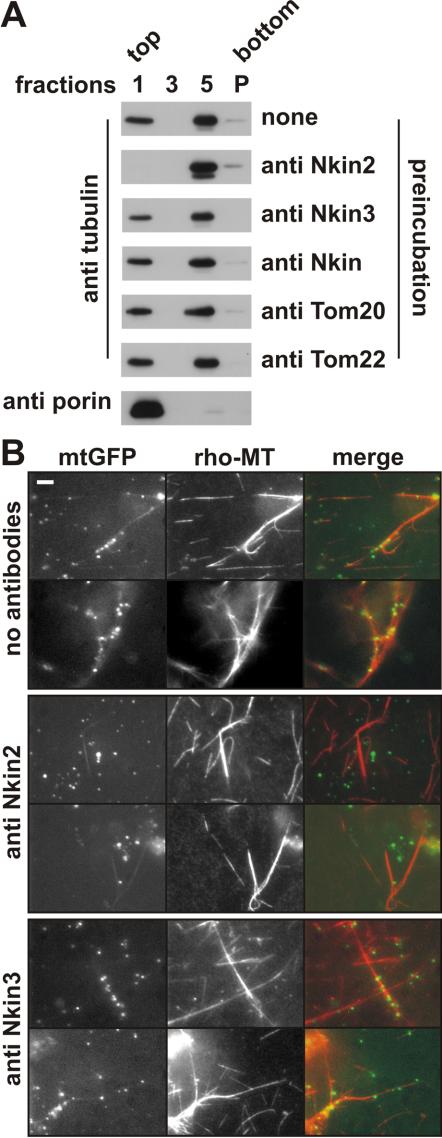
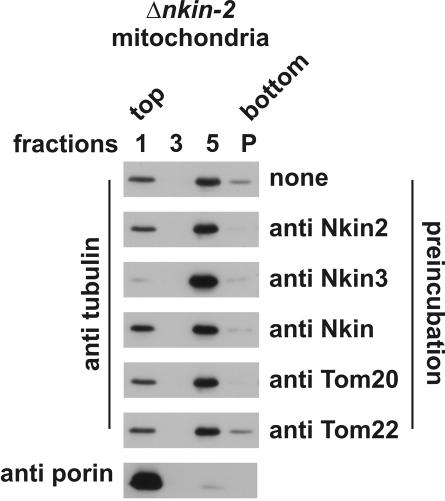
The mitochondrial association points to a possible role of Nkin2 as a mitochondrial motor protein. To investigate whether Nkin2 is required for mitochondrial binding to microtubules we used an assay that allows this interaction to be monitored in vitro. Isolated mitochondria were incubated with taxol-stabilized microtubules, floated in a sucrose density gradient, and fractions harvested from the gradient were analyzed by immunoblotting. Flotation of microtubules with mitochondria indicates a specific interaction, which is adenine nucleotide sensitive and mediated by peripherally associated mitochondrial proteins (Fuchs et al., 2002). To investigate whether Nkin2 is required for this interaction, mitochondria were preincubated with antibodies directed against Nkin2 before microtubules were added. This treatment abolished binding of mitochondria to microtubules (Figure 3A). Preincubation of mitochondria with antibodies against other kinesin-related proteins, Nkin and Nkin3, or nonrelated outer membrane proteins, Tom20 and Tom22, had no effect compared with nontreated mitochondria (Figure 3A).
Figure 3.
Antibodies against Nkin2 inhibit mitochondrial binding to microtubules in vitro. (A) Flotation assay. Isolated mitochondria were preincubated in the absence or presence of the antibodies indicated on the right-hand side. Then, mitochondria were reisolated by centrifugation, incubated with taxol-stabilized microtubules in the absence of ATP and floated in a sucrose density gradient. Fractions were harvested from the gradient, and proteins were precipitated and analyzed by SDS-PAGE and immunoblotting by using antibodies against the proteins indicated on the left-hand side. Only fractions 1 (containing floated mitochondria as controlled by blotting against porin), 3 (from the middle of the gradient), and 5 (containing nonfloated proteins) are shown. P, pellet from the bottom of the gradient. (B) Visual assay. Mitochondria were isolated from a Neurospora strain expressing mtGFP and preincubated in the absence or presence of the indicated antibodies. Then, rhodamine-labeled microtubules (rho-MT) were added, incubation was continued in the absence of ATP, and samples were analyzed by fluorescence microscopy. Bar, 5 μm.
To corroborate the observation that Nkin2 antibodies inhibit mitochondrial binding to microtubules, we used a microscopic in vitro assay that allows the visualization of the behavior of individual organelles (Fuchs et al., 2002). Mitochondria were isolated from a strain expressing mtGFP. These mitochondria were preincubated with antibodies against Nkin2 or Nkin3, or left untreated, before rhodaminelabeled microtubules were added. Binding of mitochondria to microtubules was visualized by fluorescence microscopy. Again, Nkin2 antibodies efficiently inhibited the interaction of mitochondria with microtubules, whereas Nkin3 antibodies had no effect (Figure 3B). We conclude that Nkin2 plays a crucial and specific role in mediating mitochondrial binding to microtubules.
Depletion of Nkin2 Results in an Increase of Nkin3 Levels and the Recruitment of Nkin3 to Mitochondria
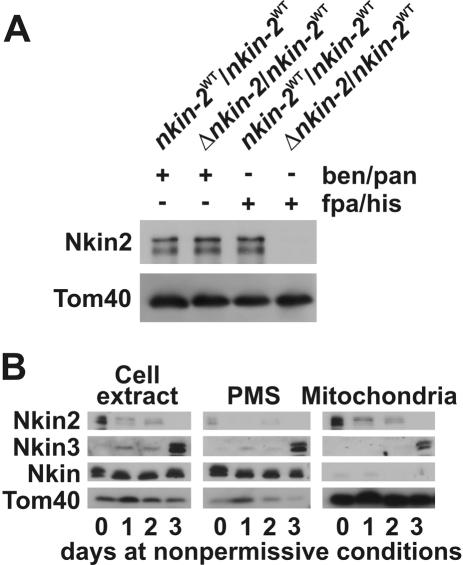
To examine the role of Nkin2 in vivo, we constructed a null mutant by using the “sheltered disruption” method (Nargang et al., 1995), which takes advantage of the fact that Neurospora has multinucleate cells. In brief, the nkin-2 gene was deleted via homologous recombination in a heterokaryotic strain. The resulting mutant harbors two kinds of nuclei, one with a null allele (Δnkin-2) and another one with a wild-type allele (nkin-2WT). These nuclei contain selectable markers that enable the shift of nuclear ratios in heterokaryotic cells. Growth on medium containing benomyl and pantothenic acid favors propagation of the nucleus containing the nkin-2WT allele. Under these conditions, Nkin2 protein levels in cell extracts are normal (Figure 4A). In contrast, growth of heterokaryotic cells on medium containing fpa and histidine forces the Δnkin-2 nucleus to predominate in the growing mycelium and thus leads to depletion of the Nkin2 protein (Figure 4A). These conditions allow the phenotype of cells lacking Nkin2 to be studied. Surprisingly, cells depleted of Nkin2 did not show any obvious phenotype. The growth behavior of cells depleted of Nkin2 was normal, and mitochondrial distribution, morphology, and motility resembled wild-type cells (our unpublished observations).
Figure 4.
Nkin3 is up-regulated and recruited to mitochondria in the absence of Nkin2 in heterokaryotic cells. (A) Depletion of Nkin2 in heterokaryotic cells. The HP1 wild-type strain (nkin-2WT/nkin- 2WT) and a heterokaryotic mutant strain (Δnkin-2/nkin-2WT) were grown for 3 d in liquid minimal medium supplemented either with benomyl and pantothenic acid (ben/pan) or p-fluoro-DL-phenylalanine and histidine (fpa/his). Equal amounts of cell extracts were analyzed by SDS-PAGE and immunoblotting. (B) Up-regulation and mitochondrial association of Nkin3 upon depletion of Nkin2. Heterokaryotic Δnkin-2/nkin-2WT cells were grown for the indicated time periods in liquid minimal medium supplemented with fpa/his. Total cell extracts, PMSs, and mitochondria were prepared, and equal amounts of protein were analyzed by SDS-PAGE and immunoblotting.
Intriguingly, the level of Nkin3 present in mutant cells was strongly elevated after depletion of Nkin2. The Nkin3 protein is barely visible in immunoblots of total cell extracts of wild-type cells. However, after 3 d growth in the presence of fpa and histidine, the time required to completely deplete Nkin2 from the heterokaryon, Nkin3 was visible as a strong band (Figure 4B). Levels of Nkin and Tom40 were not changed under these conditions. When cells were fractionated into postmitochondrial supernatant and mitochondria, a significant portion of Nkin3 was recovered with mitochondria (Figure 4B). Again, this effect was specific for Nkin3, and Nkin was not affected. This suggests that Nkin3 levels are increased and the protein is recruited to mitochondria when Nkin2 levels become limiting.
Nkin3 Is Up-Regulated during Growth of Cells in the Absence of Nkin2
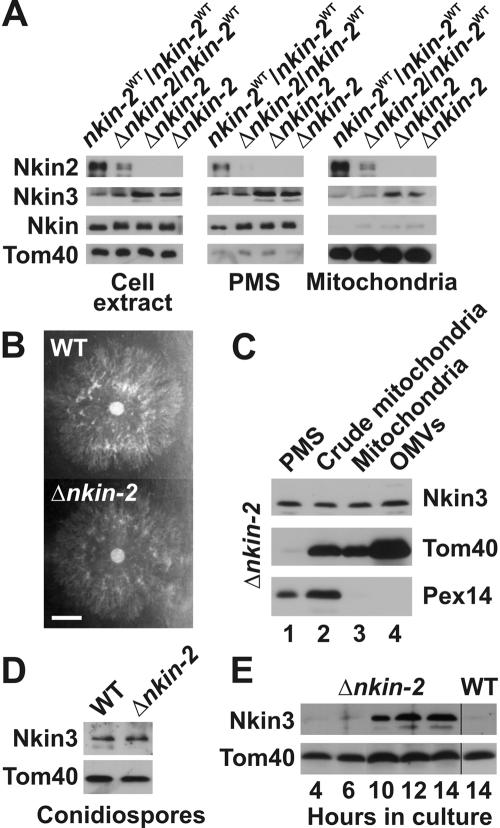
Next, we isolated a homokaryotic mutant containing exclusively the Δnkin-2 nucleus. In brief, the heterokaryotic strain was grown under conditions favoring propagation of the nucleus harboring the Δnkin-2 allele. Conidiospores were harvested from this culture and plated on sorbose medium to obtain single colonies. These were then screened for clones lacking the markers of the nkin-2WT nucleus. Absence of the wild-type allele in homokaryotic Δnkin-2 strains was confirmed by Southern blot analysis (our unpublished observations) and immunoblotting (Figure 5A). Similar to heterokaryotic strains, homokaryotic mutants did not show any growth defects (Figure 5B). Mitochondria resembled the wild type under most conditions, and motility defects could be observed only in newly germinated hyphae (see below).
Figure 5.
Nkin3 is up-regulated and binds to mitochondria in growing homokaryotic cells lacking Nkin2. (A) Up-regulation and mitochondrial association of Nkin3 in homokaryotic Δnkin-2 mutants. The HP1 wild-type strain (nkin-2WT/nkin-2WT), a heterokaryotic mutant (Δnkin-2/nkin-2WT), and two independently isolated homokaryotic mutants (Δnkin-2) were grown for 16 h in fpa/his-containing medium. Then, cells were analyzed as in Figure 4B. (B) Growth of Δnkin-2 cells. Wild-type (WT) and Δnkin-2 cells were inoculated in the middle of a petri dish containing Vogel's minimal medium agar and grown over night at 25°C. Bar, 1 cm. (C) Mitochondrial association of Nkin3 in Δnkin-2 cells. Δnkin-2 cells were subfractionated and analyzed as in Figure 2A. (D) Nkin3 level in conidiospores. Cell extracts were prepared from conidiospores of the homokaryotic Δnkin-2 strain and its isogenic wild-type (WT) and analyzed by SDS-PAGE and immunoblotting. (E) Up-regulation of Nkin3 in newly germinated Δnkin-2 cells. Conidiospores of the homokaryotic Δnkin-2 strain were allowed to germinate and grow for the indicated time periods. Then, total cell extracts were prepared and analyzed by SDS-PAGE and immunoblotting. For comparison, a cell extract of a 14 h old wild-type culture is shown.
Also in homokaryotic Δnkin-2 strains, high levels of Nkin3 were found (Figure 5A). On subcellular fractionation of Δnkin-2 cells, a significant fraction of Nkin3 was associated with mitochondria and could be recovered with outer membrane vesicles (Figure 5C). We asked whether Nkin3 is constitutively up-regulated in cells lacking Nkin2 or whether this is an adaptation of actively growing cells. To discriminate between these possibilities, we first analyzed Nkin3 levels in cell extracts of wild-type and Δnkin-2 conidiospores. Intriguingly, spores lacking Nkin2 contained only basal levels of Nkin3 (Figure 5D). However, when conidiospores were germinated in liquid culture, a strong increase of Nkin3 was observed in vegetatively growing cells after 6-10 h (Figure 5E). We conclude that up-regulation of Nkin3 is a response of growing hyphae to limiting levels of Nkin2.
Mitochondria Lacking Nkin2 Require Nkin3 for Binding to Microtubules
The observations that Nkin3 is recruited to mitochondria in cells lacking Nkin2 and that the Δnkin-2 mutant does not show severe defects in mitochondrial distribution, point to the possibility that Nkin3 overexpression might compensate for the loss of Nkin2 function. Therefore, we tested whether Nkin3 antibodies would interfere with binding of Δnkin-2 mitochondria to microtubules in vitro. Remarkably, deletion of the nkin-2 gene made mitochondria sensitive to the addition of Nkin3 antibodies in the microtubule flotation assay. Although Nkin3 antibodies had no effect on wild-type mitochondria (compare Figure 3A), they efficiently inhibited binding of Δnkin-2 mitochondria to microtubules (Figure 6). As expected, Nkin2 antibodies did not interfere with the interaction of mutant mitochondria with microtubules. We conclude that Nkin3 mediates mitochondrial binding to microtubules in the absence of Nkin2.
Figure 6.
Antibodies against Nkin3 inhibit binding of Δnkin-2 mitochondria to microtubules in vitro. Mitochondria were isolated from a Δnkin-2 mutant and subjected to the microtubule flotation assay as in Figure 3A.
Mitochondrial Dynamics Is Compromised by Deletion of the nkin-2 Gene
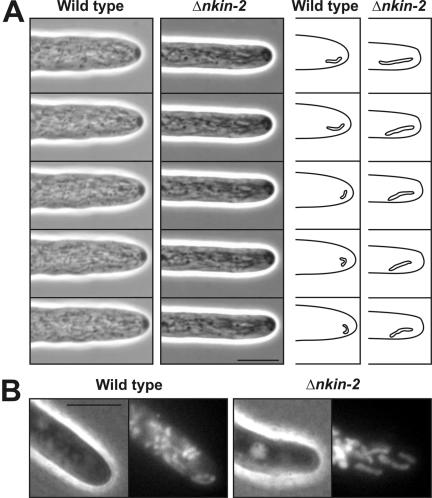
We examined the role of Nkin2 in mitochondrial motility by computer-enhanced videomicroscopy in live cells. Wild-type and Δnkin-2 conidia were allowed to germinate, and the behavior of mitochondria was observed in growing hyphae. Wild-type mitochondria were seen as numerous small, snake-like organelles that showed a complex and dynamic behavior. Continuous movement, fusion and fission resulted in frequent shape changes (Supplemental Movie 1). Within a few seconds, the spatial arrangement of the organelles was reorganized in wild-type cells, and individual mitochondria were seen that moved significantly faster than the growth speed of the hyphal tip (Figure 7A). In contrast, mutant cells that were examined a few hours after germination of conidiospores showed several defects in mitochondrial morphology and behavior (Supplemental Movie 2). In average, mutant mitochondria were longer than wild-type mitochondria. Almost all mutant mitochondria were oriented parallel to the longitudinal axis of the hypha, whereas wild-type mitochondria moved more freely in the cell. Most strikingly, forward movement of mitochondria in mutant cells was not faster than the growth speed of the hyphal tip. Apparently, mitochondria were moved forward with the growing cell, probably in a passive manner, and their spatial arrangement changed much slower than in wild-type cells (Figure 7A). Very similar changes of mitochondrial distribution and morphology were seen when mitochondria were stained with the membrane potential-sensitive dye rhodamine B hexyl ester. Mitochondria were larger, less numerous and more oriented along the longitudinal axis in Δnkin-2 cells (Figure 7B). We conclude that Nkin2 is required for normal mitochondrial distribution and motility. It should be noted that it was not possible to exactly measure the velocity of mitochondrial movement and statistically analyze their behavior, because the mitochondria frequently left the focal plane, and it was difficult to track the movement of individual organelles for more than a few seconds. Mitochondrial motility defects could not be observed any more when cells were allowed to grow for more than 10 h. This is the time required for up-regulation of Nkin3 (compare Figure 5C). We propose that mitochondrial motility defects seen in recently germinated cells are rescued by Nkin3 as growth of hyphae proceeds.
Figure 7.
Cells lacking Nkin2 show altered mitochondrial motility and morphology. (A) Wild-type and Δnkin-2 conidiospores were allowed to germinate and grow over night on medium-covered microscope slides. Then, mitochondrial behavior in growing hyphae was analyzed by computer-enhanced phase contrast videomicroscopy (2 images per second). The complete datasets are available in two movies covering 30 s of hyphal growth (see Supplemental Movie 1 for behavior of wild-type mitochondria and movie 2 for Δnkin-2 mitochondria). They are representative examples of 10 independent experiments. Differences in cellular growth rate are due to variations between individual cells. Right, representative individual organelles near the hyphal tip have been highlighted. Bar, 5 μm. (B) Wild-type and Δnkin-2 cells were grown for 7 h after germination of conidiospores, stained with 10 μg/ml rhodamine B hexyl ester, and analyzed by phase contrast (left) and fluorescence (right) microscopy. Bar, 5 μm.
DISCUSSION
Here, we show that Nkin2, a novel kinesin-related protein, mediates mitochondrial interactions with microtubules in Neurospora. Intriguingly, a related protein, KIF1B, has been shown to play a similar role in murine cells. KIF1B colocalizes with mitochondria in vivo, is enriched in mitochondrial fractions, and is able to transport mitochondria along microtubules in vitro (Nangaku et al., 1994). Nkin2 and KIF1B belong to the same group of evolutionarily conserved motor proteins, the Unc104/KIF1 family. Thus, mitochondrial transport is mediated by closely related motors in organisms as distantly related as Neurospora and mice.
Unc104/KIF1-related proteins function as organelle transporters in a variety of organisms from fungi to mammals. For example, the Dictyostelium homologue DdUnc104 serves as a vesicle transporter, but it is not required for mitochondrial transport (Pollock et al., 1999). Unc104 and KIF1A are examples for specialized transporters of neuronal vesicles in C. elegans (Hall and Hedgecock, 1991) and mice (Yonekawa et al., 1998), and Kin3 is a motor mediating the transport of early endosomes in the basidiomycetous fungus Ustilago maydis (Wedlich-Söldner et al., 2002). Apparently, the major cellular functions of Unc104/KIF1 proteins vary between organisms, and, conversely, not all cell types rely on Unc104/KIF1 family members to mediate mitochondrial motility. The involvement of conventional kinesins in mitochondrial transport is similarly diverse between organisms. Disruption of the gene encoding mouse conventional kinesin heavy chain, kif5B, results in perinuclear clustering of mitochondria (Tanaka et al., 1998), and a specific form of kinesin light chain is associated with mitochondria in cultured mammalian cells (Khodjakov et al., 1998). Mutants lacking conventional kinesin in the filamentous fungus Nectria hematococca display a withdrawal of mitochondria from the growing hyphal tip (Wu et al., 1998). Thus, conventional kinesin and its close relatives are important for mitochondrial distribution in certain mammalian and fungal cell types. In contrast, conventional kinesin, Nkin, is not involved in mitochondrial transport in Neurospora. Mitochondria show wild-type-like behavior in nkin knockout mutants (Seiler et al., 1997), and here we show that Nkin is not localized to mitochondria. Obviously, the involvement of a particular type of motor protein in mitochondrial motility in different organisms does not reflect the evolutionary relatedness of these species.
How can we reconcile the observations that diverse organisms harbor similar sets of highly conserved kinesin-related proteins, but often rely on different motors— or even on different cytoskeletal systems—to perform the same cellular function? A possible explanation is that eukaryotic cells are equipped with a kit of organelle motors that is, at least in part, redundant and allows rapid responses to physiological and environmental challenges. We observed an astonishingly fast adaptation of mutant cells to the depletion of Nkin2. Within a few hours, expression of Nkin3 was highly increased, and this normally nonmitochondrial motor protein was recruited to mitochondria. Mitochondrial motility defects were manifest only in a small time window a few hours after germination of conidiospores. This effect was seen in several independently isolated mutants and did not require any second mutagenesis event. Thus, it reflects an inherent capacity of the organism to adapt to a mutagenic challenge. Both Nkin2 and Nkin3 are able to mediate mitochondrial binding to microtubules in vitro—Nkin2 on wild-type mitochondria and Nkin3 on Δnkin-2 mitochondria. If two different motor proteins have the same intrinsic capability, it is likely that only subtle changes are required to favor one over the other to execute a particular cellular function. These changes conceivably occurred many times during evolution and might account for the variability of organellar transport machineries that we observe in eukaryotic cells.
Our current study shows that mitochondrial binding to microtubules in Neurospora depends on Nkin2, a protein strikingly similar to the murine mitochondrial motor KIF1B. It will be interesting to see in the future whether organellar transport systems in highly polarized mammalian cells, such as neurons (Brown, 2003), have this and other conserved features in common with the fast growing hyphae of filamentous fungi. Our second major finding is that Nkin3, a member of a fungal-specific subgroup of small Unc104/KIF1-related proteins, mediates mitochondrial motility in the absence of Nkin2. This implies that the cellular transport machinery is remarkably robust against mutagenic challenges. Furthermore, it shows that Nkin3 has the capacity to act as an organelle motor, even though its normal cellular function remains unknown. With the identification of the motor proteins involved, we can now address further fundamental questions, such as the identity of putative mitochondrial receptors for motor proteins and regulatory factors that are required to tune mitochondrial motor activity.
Supplementary Material
Acknowledgments
We thank Walter Neupert for continuous support, Gabi Ludwig for technical assistance; Hannes Herrmann for critical comments on the manuscript; Manfred Schliwa, Günther Woehlke and members of these laboratories for many stimulating discussions and exchange of reagents; Lucia Driller and Ralph Gräf for help with videomicroscopy; Frank Nargang for advice with mutant construction and comments on the manuscript; and Ralf Erdmann for antibodies against Pex14. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 413/B3 and We 2174/3-1) and the Friedrich-Baur-Stiftung.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-05-0413. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-05-0413.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Bateman, A., et al. (2002). The Pfam protein families database. Nucleic Acids Res. 30, 276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter-Hahn, J. (1990). Behavior of mitochondria in the living cell. Int. Rev. Cytol. 122, 1-63. [DOI] [PubMed] [Google Scholar]
- Boldogh, I. R., Yang, H.-C., Nowakowski, W. D., Karmon, S. L., Hays, L. G., Yates III, J. R., and Pon, L. A. (2001a). Arp2/3 complex and actin dynamics are required for actin-based mitochondrial motility in yeast. Proc. Natl. Acad. Sci. USA 98, 3162-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh, I. R., Yang, H.-C., and Pon, L. A. (2001b). Mitochondrial inheritance in budding yeast. Traffic 2, 368-374. [DOI] [PubMed] [Google Scholar]
- Borkovich, K. A., et al. (2004). Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol. Mol. Biol. Rev. 68, 1-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazer, S. C., Williams, H. P., Chappell, T. G., and Cande, W. Z. (2000). A fission yeast kinesin affects Golgi membrane recycling. Yeast 16, 149-166. [DOI] [PubMed] [Google Scholar]
- Brown, A. (2003). Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J. Cell Biol. 160, 817-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, R. H., and de Serres, F. J. (1970). Genetic and microbial research techniques for Neurospora crassa. Methods Enzymol. 17A, 79-143. [Google Scholar]
- Durocher, D., Henckel, J., Fersht, A. R., and Jackson, S. P. (1999). The FHA domain is a modular phosphopeptide recognition motif. Mol. Cell 4, 387-394. [DOI] [PubMed] [Google Scholar]
- Fuchs, F., Prokisch, H., Neupert, W., and Westermann, B. (2002). Interaction of mitochondria with microtubules in the filamentous fungus Neurospora crassa. J. Cell Sci. 115, 1931-1937. [DOI] [PubMed] [Google Scholar]
- Galagan, J. E., et al. (2003). The genome sequence of the filamentous fungus Neurospora crassa. Nature 422, 859-868. [DOI] [PubMed] [Google Scholar]
- Hall, D. H., and Hedgecock, E. M. (1991). Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65, 837-847. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1988). Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Hermann, G. J., and Shaw, J. M. (1998). Mitochondrial dynamics in yeast. Annu. Rev. Cell Dev. Biol. 14, 265-303. [DOI] [PubMed] [Google Scholar]
- Hirokawa, N. (1998). Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279, 519-526. [DOI] [PubMed] [Google Scholar]
- Itoh, T., Watabe, A., Toh-e, A., and Matsui, Y. (2002). Complex formation with Ypt11p, a rab-type small GTPase, is essential to facilitate the function of Myo2p, a class V myosin, in mitochondrial distribution in Saccharomyces cerevisiae. Mol. Cell. Biol. 22, 7744-7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, R. E., Aiken Hobbs, A. E., Cerveny, K. L., and Sesaki, H. (2000). Yeast mitochondrial dynamics: fusion, division, segregation, and shape. Microsc. Res. Tech. 51, 573-583. [DOI] [PubMed] [Google Scholar]
- Khodjakov, A., Lizunova, E. M., Minin, A. A., Koonce, M. P., and Gyoeva, F. K. (1998). A specific light chain of kinesin associates with mitochondria in cultured cells. Mol. Biol. Cell 9, 333-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner, J., Woehlke, G., and Schliwa, M. (1999). Universal and unique features of kinesin motors: insights from a comparison of fungal and animal conventional kinesins. Biol. Chem. 380, 915-921. [DOI] [PubMed] [Google Scholar]
- Klopfenstein, D. R., Tomishige, M., Stuurman, N., and Vale, R. D. (2002). Role of phosphatidylinositol(4,5)bisphosphate organization in membrane transport by the Unc104 kinesin motor. Cell 109, 347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, A., Lill, R., and Neupert, W. (1993). Translocation and insertion of precursor proteins into isolated outer membranes of mitochondria. J. Cell Biol. 121, 1233-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangaku, M., Sato-Yoshitake, R., Okada, Y., Noda, Y., Takemura, R., Yamazaki, H., and Hirokawa, N. (1994). KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell 79, 1209-1220. [DOI] [PubMed] [Google Scholar]
- Nargang, F. E., Künkele, K. P., Mayer, A., Ritzel, R. G., Neupert, W., and Lill, R. (1995). `Sheltered disruption' of Neurospora crassa MOM22, an essential component of the mitochondrial protein import complex. EMBO J. 14, 1099-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari, J., and Walter, P. (1996). Regulation of organelle biogenesis. Cell 84, 389-394. [DOI] [PubMed] [Google Scholar]
- Oakley, B. R., and Rinehart, J. E. (1985). Mitochondria and nuclei move by different mechanisms in Aspergillus nidulans. J. Cell Biol. 101, 2392-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka, A. J., Jeyaprakash, A., Garcia-Anoveros, J., Tang, L. Z., Fisk, G., Hartshorne, T., Franco, R., and Born, T. (1991). The C. elegans unc-104 gene encodes a putative kinesin heavy chain-like protein. Neuron 6, 113-122. [DOI] [PubMed] [Google Scholar]
- Pereira, A. J., Dalby, B., Stewart, R. J., Doxsey, S. J., and Goldstein, L. S. (1997). Mitochondrial association of a plus end-directed microtubule motor expressed during mitosis in Drosophila. J. Cell Biol. 136, 1081-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock, N., de Hostos, E. L., Turck, C. W., and Vale, R. D. (1999). Reconstitution of membrane transport powered by a novel dimeric kinesin motor of the Unc104/KIF1A family purified from Dictyostelium. J. Cell Biol. 147, 493-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokisch, H., Neupert, W., and Westermann, B. (2000). Role of MMM1 in maintaining mitochondrial morphology in Neurospora crassa. Mol. Biol. Cell 11, 2961-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley, N., Prip-Buus, C., Westermann, B., Brown, C., Schwarz, E., Barrell, B., and Neupert, W. (1994). Mdj1p, a novel chaperone of the DnaJ family, is involved in mitochondrial biogenesis and protein folding. Cell 77, 249-259. [DOI] [PubMed] [Google Scholar]
- Schoch, C. L., Aist, J. R., Yoder, O. C., and Gillian Turgeon, B. (2003). A complete inventory of fungal kinesins in representative filamentous ascomycetes. Fungal Genet. Biol. 39, 1-15. [DOI] [PubMed] [Google Scholar]
- Sebald, W., Neupert, W., and Weiss, H. (1979). Preparation of Neurospora crassa mitochondria. Methods Enzymol. 55, 144-148. [DOI] [PubMed] [Google Scholar]
- Seiler, S., Nargang, F. E., Steinberg, G., and Schliwa, M. (1997). Kinesin is essential for cell morphogenesis and polarized secretion in Neurospora crassa. EMBO J. 16, 3025-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks, A. D. (1994). A T7 expression vector for producing N- and C-terminal fusion proteins with glutathione S-transferase. Gene 138, 105-108. [DOI] [PubMed] [Google Scholar]
- Staben, C., Jensen, B., Singer, M., Pollock, J., Schechtman, M., Kinsey, J., and Selker, E. (1989). Use of bacterial Hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet. Newsl. 36, 79-81. [Google Scholar]
- Steinberg, G., and Schliwa, M. (1993). Organelle movements in the wild type and wall-less fz;sg;os-1 mutants of Neurospora crassa are mediated by cytoplasmic microtubules. J. Cell Sci. 106, 555-564. [DOI] [PubMed] [Google Scholar]
- Steinberg, G., and Schliwa, M. (1995). The Neurospora organelle motor: a distant relative of conventional kinesin with unconventional properties. Mol. Biol. Cell 6, 1605-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suelmann, R., and Fischer, R. (2000). Mitochondrial movement and morphology are dependent on an intact actin cytoskeleton in Aspergillus nidulans. Cell Motil. Cytoskeleton 45, 42-50. [DOI] [PubMed] [Google Scholar]
- Sweigard, J., Chumley, F., Carroll, A., Farrall, L., and Valent, B. (1997). A series of vectors for fungal transformation. Fungal Genet. Newsl. 44, 52-53. [Google Scholar]
- Tanaka, Y., Kanai, Y., Okada, Y., Nonaka, S., Takeda, S., Harada, A., and Hirokawa, N. (1998). Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell 93, 1147-1158. [DOI] [PubMed] [Google Scholar]
- Vollmer, S., and Yanofsky, C. (1986). Efficient cloning of genes of Neurospora crassa. Proc. Natl. Acad. Sci. USA 83, 4869-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, G., and Wickner, W. (1996). Organelle inheritance. Cell 84, 395-400. [DOI] [PubMed] [Google Scholar]
- Wedlich-Söldner, R., Straube, A., Friedrich, M. W., and Steinberg, G. (2002). A balance of KIF1A-like kinesin and dynein organizes early endosomes in the fungus Ustilago maydis. EMBO J. 21, 2946-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir, B. A., and Yaffe, M. P. (2004). Mmd1p, a novel, conserved protein essential for normal mitochondrial morphology and distribution in the fission yeast Schizosaccharomyces pombe. Mol. Biol. Cell 15, 1656-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann, B., and Prokisch, H. (2002). Mitochondrial dynamics in filamentous fungi. Fungal Genet. Biol. 36, 91-97. [DOI] [PubMed] [Google Scholar]
- Wu, Q., Sandrock, T. M., Turgeon, B. G., Yoder, O. C., Wirsel, S. G., and Aist, J. R. (1998). A fungal kinesin required for organelle motility, hyphal growth, and morphogenesis. Mol. Biol. Cell 9, 89-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, X., and Plamann, M. (2003). Cytoskeleton and motor proteins in filamentous fungi. Curr. Opin. Microbiol. 6, 628-633. [DOI] [PubMed] [Google Scholar]
- Yaffe, M. P. (1999). The machinery of mitochondrial inheritance and behavior. Science 283, 1493-1497. [DOI] [PubMed] [Google Scholar]
- Yaffe, M. P., Harata, D., Verde, F., Eddison, M., Toda, T., and Nurse, P. (1996). Microtubules mediate mitochondrial distribution in fission yeast. Proc. Natl. Acad. Sci. USA 93, 11664-11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekawa, Y., Harada, A., Okada, Y., Funakoshi, T., Kanai, Y., Takei, Y., Terada, S., Noda, T., and Hirokawa, N. (1998). Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J. Cell Biol. 141, 431-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.