Abstract
Phosphoinositide-3 kinase (PI-3 kinase) and its downstream signaling molecules PDK-1 and Akt were analyzed in SK-N-SH and SK-N-BE(2) human neuroblastoma cell lines. When cells were stimulated with insulin, PI-3 kinase was activated in both cell lines, whereas the translocation of PDK-1 to the membrane fraction and phosphorylated Akt were observed only in SK-N-SH cells. Analyses of the insulin-mediated reactive oxygen species (ROS) generation and Phosphatase and Tensin homolog (PTEN) oxidation indicate that PTEN oxidation occurred in SK-N-SH cells, which can produce ROS, but not in SK-N-BE(2) cells, which cannot increase ROS in response to insulin stimulation. When SK-N-SH cells were pretreated with the NADPH oxidase inhibitor diphenyleneiodonium chloride before insulin stimulation, insulin-mediated translocation of PDK-1 to the membrane fraction and phosphorylation of Akt were remarkably reduced, whereas PI-3 kinase activity was not changed significantly. These results indicate that not only PI-3 kinase activation but also inhibition of PTEN by ROS is needed to increase cellular level of phosphatidylinositol 3,4,5-trisphosphate for recruiting downstream signaling molecules such as PDK-1 and Akt in insulin-mediated signaling. Moreover, the ROS generated by insulin stimulation mainly contributes to the inactivation of PTEN and not to the activation of PI-3 kinase in the PI-3 kinase/Akt pathway.
INTRODUCTION
Reactive oxygen species (ROS), and especially H2O2, are rapidly and transiently increased by a variety of external signals that include cytokines, peptide growth factors, and agonists of seven-transmembrane domain, or G protein-coupled receptors (GPCRs) (Ohba et al., 1994; Kimura et al., 1995; Krieger-Brauer and Kather, 1995; Sundaresan et al., 1995; Bae et al., 1997; Patterson et al., 1999). Previous studies have demonstrated that the binding of peptide growth factors to their specific receptors induces a transient increase in the intracellular concentration of ROS (Krieger-Brauer and Kather, 1995; Lo and Cruz, 1995; Sundaresan et al., 1996; Bae et al., 1997; Sattler et al., 1999). This growth factor-mediated transient increase of ROS is an integral constituent of downstream signal transduction (Finkel, 1998). The ROS-mediated modulation of growth factor signaling was achieved via the inactivation of PTPases through oxidation of the cysteine residue in the active site sequence motif (Zhang, 1998).
ROS generation in a variety of nonphagocytic cells is much lower than in phagocytes, although the ROS generation systems in nonphagocytic cells are considered similar (Babior, 1999; Bokoch and Diebold, 2002). ROS generation in phagocytes is catalyzed by NADPH oxidase, which consists of two transmembrane subunits, p22phox and gp91phox, and at least three cytosolic subunits, p47phox, p67phox, and Rac (Babior, 1999; Banfi et al., 2003). Recently, six gp91phox homologues (NOX1, NOX3, NOX4, NOX5, DUOX1, and DUOX2) have been identified in different mammalian tissue (Banfi et al., 2003). Although the mechanism of the growth factor-mediated ROS generation in nonphagocytotic cells has not been completely understood, several recent reports (Bae et al., 2000; Bokoch and Diebold, 2002) have demonstrated that the growth factor mediated-activation of phosphoinositide-3 kinase (PI-3 kinase) and Rac is needed for ROS generation.
The PI-3 kinase pathway is implicated in controlling a variety of cellular events, including proliferation, growth, and survival (Franke et al., 1997; Shepherd et al., 1998; Cantrell, 2001; Cantley, 2002). PI-3 kinase can be activated through tyrosine kinase receptors or GPCRs and generates phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] and phosphatidylinositol 3,4-bisphosphate (Stephens et al., 1993; Vanhaesebroeck et al., 1997). These 3-phosphorylated phosphatidylinositols (PIs) can recruit serine/threonine kinases such as PI-dependent kinases (PDKs) and Akt (PKB) via interactions with a pleckstrin homology (PH) domain in these proteins (Leevers et al., 1999). Once recruited, Akt is phosphorylated at two sites, Thr308 and Ser473, by one or more PDKs, and these phosphorylations activate the kinase and release it from the membrane (Alessi et al., 1997; Stokoe et al., 1997).
Phosphatase and Tensin homolog (PTEN), originally identified as a tumor suppressor gene mutated in a large percentage of human cancers, is considered a key negative regulator of PI-3 kinase (Cantley and Neel, 1999; Sulis and Parsons, 2003). Studies of phosphatidylinositol metabolism indicate that PTEN dephosphorylates PtdIns(3,4,5)P3 at the D3 position and reduces cellular levels of PtdIns(3,4,5)P3 (Maehama and Dixon, 1998; Myers et al., 1998). Because PTEN has a critical role in antagonizing PI-3 kinase signaling pathways, it might be expected that PTEN would be the target of complex control mechanisms. Only limited studies have addressed this issue, but evidence is now emerging for functional regulation of PTEN at the level of protein stability and localization, as well as transcription of the PTEN gene (Adey et al., 2000; Vazquez et al., 2000, 2001; Kurose et al., 2001; Bastola et al., 2002).
It has been demonstrated that the activity of PI-3 kinase, the level of 3-phosphorylated PIs, and the activity of Akt are increased in cells exposed to H2O2 (Konishi et al., 1997; Shaw et al., 1998; Sonoda et al., 1999; Tanaka et al., 1999; Qin et al., 2000). This H2O2-mediated activation of PI-3 kinase/Akt pathway is considered to be the result of reversible oxidation and inactivation of protein tyrosine phosphatase (PTP) family protein such as PTEN that have a critical cysteine residue in the active site (Denu and Tanner, 1998; Lee et al., 1998; Meng et al., 2002). Moreover, recent studies have indicated that inactivation by oxidation could be a physiological mechanism of regulation of PTP family proteins both by oxidative stress and by transiently increased ROS in cells stimulated with growth factors (Meng et al., 2002). Possible roles for the oxidation and inactivation of PTEN in regulating PI-3 kinase/Akt pathway have been suggested because this enzyme is a PTP family member. A recent report has revealed that PTEN can be oxidized and inactivated by formation of a disulfide bond between the active site Cys124 and Cys71in cells treated with H2O2 (Lee et al., 2002). It has been demonstrated that either H2O2 or endogenous ROS production in macrophages inactivates a fraction of PTEN and that this is associated with an oxidant-dependent activation of downstream signaling (Leslie et al., 2003). Moreover, the increase of cellular PtdIns(3,4,5)P3 levels and Akt activation by oxidative stress does not occur in cells lacking PTEN. These results suggest that the regulation of PTEN plays a pivotal role in PI-3 kinase/Akt signaling.
In this report, we have demonstrated that the generation ROS and the resultant inactivation of PTEN is needed for insulin-mediated Akt activation by using two neuroblastoma cell lines, SK-N-SH, which can produce ROS, and SK-N-BE(2), which cannot produce ROS. Moreover, the ROS generated in response to insulin stimulation primarily contributes to the inactivation of PTEN and not to the activation of PI-3 kinase.
MATERIALS AND METHODS
Materials
Antibodies specific for phosphotyrosine (PY20), phospho-Akt (Ser473), phospho-PTEN (Ser 380, Thr 382/383), PDK-1, and Akt were obtained from Cell Signaling Technology (Beverly, MA). Phosphotyrosine (4G10), p85 (regulatory subunit of PI-3 kinase), and PTEN antibodies were purchased from Upstate Biotechnology (Waltham, MA). Protein A- or G-conjugated Sepharose and enhanced chemiluminescence (ECL) reagent were purchased from Amersham Biosciences (Piscataway, NJ), and 2′,7′-dichlorodihydrofluorerscein diacetate (H2DCFDA) was from Molecular Probes (Eugene, OR). All the media, sera, and antibiotic-antimycotic mixtures were from Invitrogen (Carlsbad, CA), and all other reagents including Na3VO4, LY294002, diphenyleneiodonium chloride (DPI), N-acetylcysteine (NAC), phosphatidylinositol, insulin, and epidermal growth factor (EGF) were purchased from Sigma-Aldrich (St. Louis, MO). [γ-32P]ATP (1000 Ci/mmol) was obtained from PerkinElmer Life and Analytical Sciences (Boston, MA).
Methods
Cell Culture. SK-N-BE(2)C (Biedler et al., 1978) and SK-N-SH cells were cultured in DMEM supplemented with 10% calf serum in the presence of 5% CO2 at 37°C. Fresh media were supplied every 2 days. Insulin (100 nM) or H2O2 (1 mM) was added for appropriate periods, depending on the purpose of the experiment, to cells that had been incubated in serum-free media for the previous 12 h (serum-starved cells). To measure the effect of DPI, an inhibitor of NADPH oxidase, serum-starved SK-N-SH cells were incubated in DPI (10 μM) for 30 min, and then treated with insulin for 15 min. To inhibit PI-3 kinase activity, serum-starved cells were incubated in 10 μM LY294002 for 30 min before insulin stimulation.
Immunoprecipitation and Immunoblotting. Cells that had reached ∼80% confluence on 100-mm plastic dishes were serum starved, treated with insulin, and washed with ice-cold phosphate-buffered saline. The washed cells were lysed with 1 ml of ice-cold lysis buffer (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 1% NP-40, 100 μg/ml phenylmethylsulfonyl fluoride [PMSF], 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM Na3VO4, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, and 1 mM benzamidine). The cell lysate was centrifuged for 5 min at 13,000 × g, and the resulting supernatant (1 mg of protein) was incubated with an appropriate amount (∼5 μg) of antibodies at 4°C for 1 h. A 50-μl aliquot of protein A-Sepharose (10% suspension) was added and after a 30-min incubation, the antigen-antibody complexes were pelleted by a brief centrifugation. The pellet was washed three times with ice-cold lysis buffer by centrifugation at 13,000 × g for 30 s, dissolved in 20 μl of Laemmli's sample buffer, and fractionated on 10% SDS-polyacrylamide gels. The fractionated proteins were electrophoretically transferred to nitrocellulose. Antibody labeling of protein bands was detected with ECL reagents according to the manufacturer's protocol.
Measurement of PI-3 Kinase Activity. Anti-phosphotyrosine or anti-p85 immunoprecipitates were obtained from the cell lysates (1 mg) treated with insulin (100 nM), 1 mM H2O2, insulin plus DPI (10 μM), or insulin plus H2O2, for the indicated time depending on the purpose of the experiment. The pellet was washed three times with a buffer containing 20 mM Tris-Cl, pH 7.4, 100 mM NaCl, 10 mM MgCl2, and 0.5 mM EGTA. The pellet was incubated in 50 μl of reaction buffer containing 20 mM Tris-Cl, pH 7.4, 100 mM NaCl, 10 mM MgCl2, 0.5 mM EGTA, 100 μM phosphatidylinositol, 120 μM adenosine, 20 μM ATP, and 2 μCi of [γ-32P]ATP for 30 min at 25°C. The reaction was terminated by adding 100 μl of 1 N HCl. Phospholipids were extracted with 200 μl of chloroform/methanol (CHCl3/MeOH) (1:1), centrifuged at 10,000 × g for 1 min, and then the lower phase (organic phase) was transferred to a new tube. The organic phase was dried under nitrogen gas and resuspended in 20 μl of a CHCl3/MeOH (1:1) and resolved by thin layer chromatography (TLC) by using a silica gel plate coated with 1% potassium oxalate in a running buffer of CHCl3/MeOH/NH4OH/H2O (60:47:11.3:2). The 32P-labeled phosphatidylinositol phosphate (PIP) was visualized and quantitated using a PhosphorImager (BAS 2000; Fuji, Tokyo, Japan).
Translocation of PDK-1 to the Membrane Fraction. Cells treated with either insulin or H2O2 for 10 min were lysed in 100 μl of hypotonic buffer (20 mM Tris-Cl, pH 7.4, 20 mM β-mercaptoethanol, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 0.5 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mg/ml benzamidine) by repeated quick freezing and thawing by using liquid nitrogen, and then homogenized with a glass homogenizer after addition of 900 μl of same buffer. The homogenate was centrifuged at 3000 × g for 10 min to remove unbroken cells and nuclei. The supernatant was centrifuged for 30 min at 15,000 × g, and the pellet (50 μg of protein) was fractionated on a 10% SDS-polyacrylamide gel. Proteins were electrophoretically transferred to nitrocellulose. PDK-1 was visualized using an anti-PDK-1 antibody.
Measurement of ROS Generation. Intracellular ROS production was measured by a previously described method (Bae et al., 1997). Serum-starved cells were treated with insulin for the indicated time, and then washed with phenol red-free minimal essential medium and incubated in the dark for 5 min in DMEM containing 5 mM H2DCFH-DA at 37°C. Culture dishes were transferred to a confocal microscope (LSM 510; Carl Zeiss, Jena, Germany), and the production of ROS was measured by DCF fluorescence (DCFH, which is produced from DCFH-DA by intracellular esterases, is converted to the highly fluorescent dye DCF in the presence of H2O2). DCF fluorescence was detected using excitation at 488 nm and emission at 515-540 nm. To avoid photooxidation of DCFH, the fluorescent image was collected by a single rapid scan by using identical parameters (e.g., contrast and brightness) for all samples.
Analysis of Reduced and Oxidized Forms of PTEN by Nonreducing SDS-PAGE and Immunoblotting. After appropriate stimulation, cells were immediately frozen in liquid nitrogen, and lysed with 1 ml of ice-cold buffer containing 50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 1% NP-40, 50 mM N-ethylmaleimide (NEM), 100 μg/ml PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM Na3VO4, 1 mM EDTA, 1 mM EGTA, and 1 mM NaF. Cell lysates were centrifuged at 15,000 × g for 15 min. The resulting supernatant was fractionated on 10% nonreducing SDS-polyacrylamide gels followed by immunoblot assay by using the anti-PTEN antibody.
Measurement of PTEN Phosphatase Activity. Substrate for PTEN phosphatase activity assay was made as described previously (Walker et al., 2001) by using purified PI-3 kinase γ. PtdIns(3,4,5)P3 phosphatase assay was performed for 30 min at 25°C in 100 μl of 40 mM Tris-HCl, pH 8, containing immunoprecipitated PTEN, 10 mM dithiothreitol (DTT), 1 mM EDTA, 20 μl of lipid vesicle consisting of phosphatidylserine (0.05 mg/ml) and diacylglycerol (0.005 mg/ml), and PtdIns(3,4,5)P3 (0.025 mg/ml) containing ∼80,000 cpm of [32P]PtdIns(3,4,5)P3. The reaction was terminated by the addition of 200 μl of 1 N HCl and sequential addition of 200 μl of methanol and 200 μl of chloroform. Released [32P]Pi in the aqueous phase was pooled, and the radioactivity was counted. Anti-PTEN immunoprecipitates were obtained from the cell lysate (5 μg antibody/1 mg protein) that were prepared from the cells stimulated with either insulin or H2O2 by lysis in 1 ml of ice-cold lysis buffer containing 50 mM NEM to block free sulfhydryl groups in PTEN.
RESULTS
Insulin-mediated Activation of PI-3 Kinase Cascade
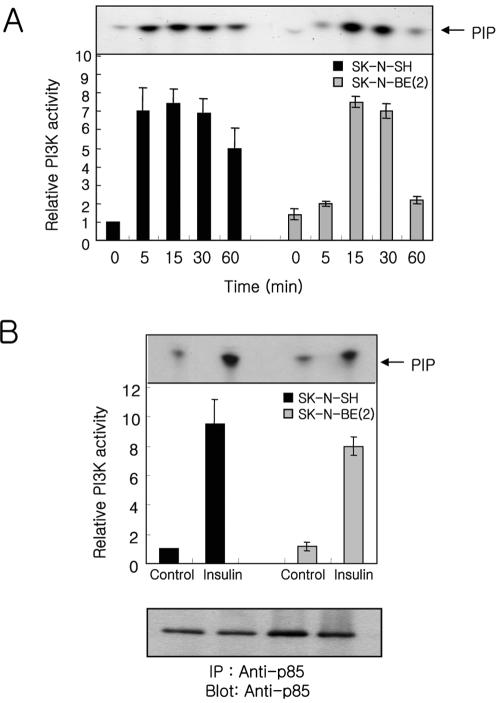
A previous report in our laboratory demonstrated that the tyrosine-phosphorylated components and the degree of tyrosine phosphorylation of each component in SK-N-BE(2) human neuroblastoma cells were relatively lower than those in PC12 cells in response to insulin or nerve growth factor (Hwang et al., 1995). To address whether the diminished levels of insulin-stimulated tyrosine phosphorylation observed in SK-N-BE(2) cells affected activation of PI-3 kinase cascade, we measured in vitro PI-3 kinase activity in anti-phosphotyrosine immunoprecipitates from insulin-stimulated SK-N-BE(2) and SK-N-SH cells (Figure 1A). An increase in PI-3 kinase activity occurred within 5 min after insulin stimulation, reached maximal level ∼15-30 min, and declined after 60 min in both SK-N-SH and SK-N-BE(2) cells. The insulin-mediated activation of PI-3 kinase always occurred more quickly and declined more slowly in SK-N-SH cells compared with SK-N-BE(2) cells, although the maximal level of activation was very similar between these two cell lines.
Figure 1.
Insulin stimulation increases PI-3 kinase activity in SKN-SH and SK-N-BE(2) cells. Cells treated with insulin (100 nM) for the indicated time, were lysed and immunoprecipitated with either anti-phosphotyrosine (PY20, 5 μg) (A) or anti-p85 (B). Kinase activity was measured as described under Materials and Methods by using phosphatidyl inositol as substrate, and the product PIP was resolved by TLC. Incorporation of 32P into PIP was imaged and quantitated using a PhosphorImager. Each bar represents the quantitated average ± SE for three independent experiments normalized to the value of unstimulated SK-N-SH cells. The anti-p85 blot (B) was obtained from the protein A beads used for PI-3 kinase activity assay.
To confirm the observed insulin-mediated increase of PI-3 kinase activity in anti-phosphotyrosine immunoprecipitates sufficiently reflects the change of PI-3 kinase activity in the cell, we measured PI-3 kinase activity in anti-p85 immunoprecipitates from insulin-stimulated SK-N-SH and SK-NBE(2) cells for 15 min (Figure 1B). The insulin-mediated increase of PI-3 kinase activity in both cells seemed very similar with that observed in anti-phosphotyrosine immunoprecipitates. These results indicate that PI-3 kinase can be activated in SK-N-BE(2) cells by insulin stimulation.
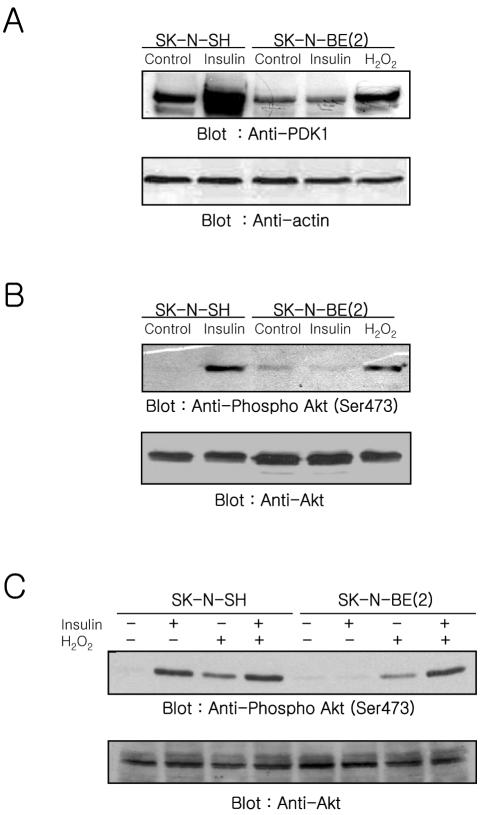
Next, we measured whether PDK-1 can be recruited to the membrane fraction (Figure 2A) and whether Akt can be activated by insulin stimulation (Figure 2B). Pilot experiments that measured the time-dependent change of Akt phosphorylation revealed that the phosphorylation of Akt was observed within 5 min after insulin stimulation and maintained up to 30 min in SK-N-SH cells but did not occur in SK-N-BE(2) cells (our unpublished data). Based on these data, we examined the translocation of PDK-1 to the membrane fraction and Akt phosphorylation in the cells stimulated with insulin for 10 min. The translocation of PDK-1 to the membrane fraction was observed when SK-N-SH cells were stimulated with insulin. In contrast, the insulin-mediated translocation of PDK-1 to the membrane fraction was not observed in SK-N-BE(2) cells (Figure 2A). The lack of membrane association of PDK-1 in insulin stimulated SK-NBE(2) cells could be due either to a defect in the PI-3 kinase/PtdIns(3,4,5)P3 generation pathway or to a defect in the ability of PDK-1 to bind PtdIns(3,4,5)P3. To address this possibility, we applied H2O2, which is known to recruit PDK-1 to the plasma membrane and to activate Akt, to SK-N-BE(2) cells. The translocation of PDK-1 to the membrane fraction was apparent when SK-N-BE(2) cells were stimulated with H2O2, although the amount of PDK-1 found in the membrane fraction was less than that observed in insulin-stimulated SK-N-SH cells (Figure 2A). We measured the phosphorylation status of Akt in whole cell lysates from insulin-stimulated cells by using an antibody specific for Akt phosphorylated at Ser473 (Figure 2B). In agreement with PDK-1 translocation to the membrane fraction, stimulation of cells with insulin revealed that the phosphorylation of Akt increased in SK-N-SH cells but not in SK-N-BE(2) cells. An increased phosphorylation of Akt also was observed in SK-N-BE(2) cells in response to exogenous H2O2 (Figure 2B).
Figure 2.
Effect of insulin or H2O2 on the PDK-1 recruitment to the membrane fraction (A) and Akt activation (B and C) in SK-N-SH and SK-N-BE(2) cells. Membrane fractions (A) or whole cell lysates (B and C) (50 μg of protein) prepared from cells treated with insulin (100 nM), H2O2 (1 mM), or insulin plus H2O2 for 10 min were analyzed with anti-PDK-1 (A), or anti-phospho-Akt (Ser473) (B and C). The same blots were reprobed with anti-actin (A) and anti-Akt (B and C), respectively, to confirm equal protein loading.
Although the increase of PDK-1 recruitment to the membrane fraction and Akt phosphorylation occurred in SK-NBE(2) cells stimulated with exogenous H2O2, the level of increase was lower than that in SK-N-SH cells stimulated with insulin. This could be due to not enough activation of PI-3 kinase. To address this possibility, we compared the level of phosphorylated Akt in both cells treated with insulin, H2O2, or insulin plus H2O2 (Figure 2C). Akt phosphorylation was increased in insulin-, H2O2-, or insulin plus H2O2-stimulated SK-N-SH cells. The level of Akt phosphorylation in insulin-stimulated cells was higher than that in H2O2-stimulated cells. But, Akt phosphorylation was not increased further by stimulation with insulin plus H2O2 compared with insulin alone. The comparison of Akt phosphorylation between SK-N-SH and SK-N-BE(2) cells revealed that insulin stimulation along with exogenous H2O2 restored the level of Akt phosphorylation in SK-N-BE(2) cells comparable with that in insulin- or insulin plus H2O2-stimulated SK-N-SH cells. The increase of Akt phosphorylation in exogenous H2O2-stimulated SK-N-SH cells was very similar to that in SK-N-BE(2) cells.
These results indicate that the lack of PDK-1 translocation to the membrane fraction and Akt activation in insulin stimulated SK-N-BE(2) cells is due to a defect in PI-3 kinase's ability to produce PtdIns(3,4,5)P3 and thereby recruit PDK-1/Akt to the plasma membrane. These results suggest that activation of PI-3 kinase is not sufficient to increase PtdIns(3,4,5)P3 level to recruit PDK-1 and Akt in insulin-stimulated SK-N-BE(2) cells.
Measurement of Insulin-mediated ROS Generation
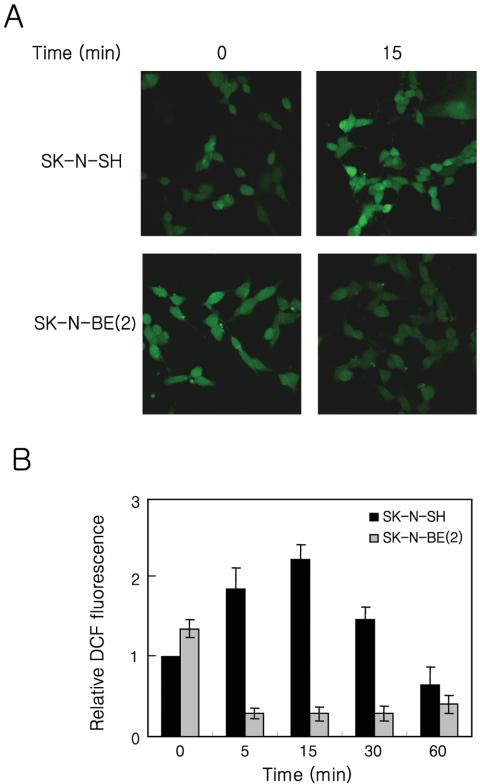
The reported roles of ROS on the growth factor-mediated signaling and the observed increase of PDK-1 recruitment to the plasma membrane and Akt phosphorylation in SK-NBE(2) cells stimulated with H2O2 suggest that the defect in insulin-mediated signaling could be related to ROS generation. To address this possibility, we analyzed whether insulin can induce the transient generation of ROS in SK-N-SH and SK-N-BE(2) cells (Figure 3). Cells loaded with the redox-sensitive fluorophore H2DCFH-DA were treated with insulin, and the production of ROS was measured by DCF fluorescence with a confocal microscope. In SK-N-SH cells, the DCF fluorescence rapidly increased, reached a maximal level within 15 min, and then declined in response to insulin stimulation. In contrast, an insulin-mediated increase of DCF fluorescence was not detected in SK-N-BE(2) cells. Instead, DCF fluorescence was decreased by insulin stimulation. Application of other growth factors, such as EGF or platelet-derived growth factor (PDGF), produced similar results (our unpublished data). However, the basal DCF fluorescence in SK-N-BE(2) cells was always higher than that in SK-N-SH cells. These results indicated not only that the SK-N-BE(2) cell line cannot generate ROS in response to insulin stimulation but also that the lack of insulin-mediated ROS production could be responsible for the defective signaling in these cells.
Figure 3.
Effect of insulin on ROS generation in SK-N-SH and SK-N-BE(2)C cells. The generation of ROS was assayed on the basis of DCF fluorescence as described in Materials and Methods. Confocal micrographs (A) of DCF fluorescence from the SK-N-SH and SK-NBE(2) cells either unstimulated or stimulated with insulin (100 nM) for 15 min. Time course of the ROS generation by insulin in SKN-SH (black bar) and SK-N-BE(2) (gray bar) cells. The relative fluorescence value of each time point was normalized to the value of unstimulated SK-N-SH cells. Each bar represents the average ± SE of three independent experiments.
Insulin-mediated Modification of PTEN and PTEN Activity
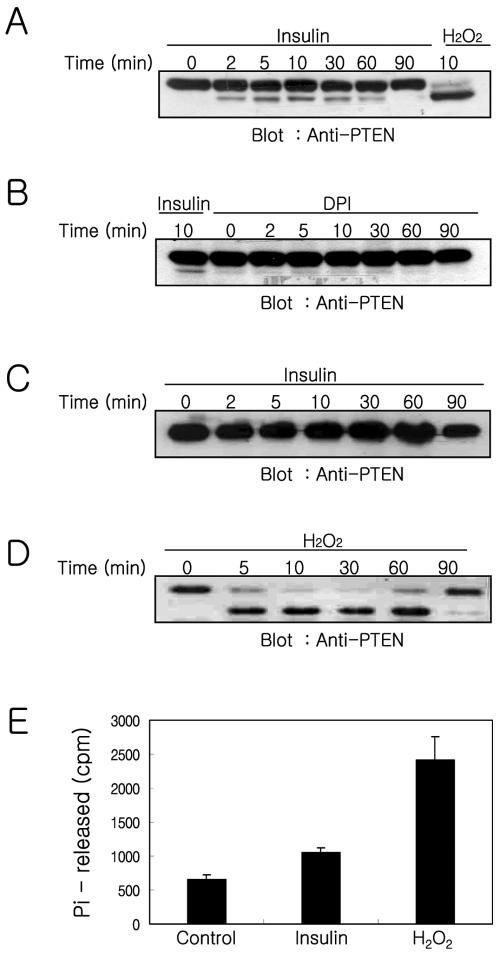
Studies of phosphatidylinositol metabolism indicate that PTEN has a critical role as a negative regulator of PI-3 kinase because PTEN dephosphorylates PtdIns(3,4,5)P3 at the D3 position and reverses the action of PI-3 kinase (Maehama and Dixon, 1998; Myers et al., 1998). Recent studies indicate that PTEN can be inactivated by H2O2, and this inactivation was correlated with a shift in the electrophoretic mobility of PTEN consistent with its oxidation both in vitro and in cultured cells (Lee et al., 2002). Moreover, either H2O2 or endogenous ROS production in macrophages inactivates a fraction of PTEN (Leslie et al., 2003). Our results, along with these earlier observations suggest that the inactivation of PTEN by endogenously produced ROS is required to produce a net increase in PtdIns(3,4,5)P3 levels in response to insulin stimulation. To address this possibility, we examined PTEN oxidation in insulin-treated SK-N-SH and SK-N-BE(2) cells by using nonreducing SDS-PAGEs and immunoblot analysis as described previously (Lee et al., 2002). Both SK-N-SH and SK-N-BE(2) cells were incubated for various periods of time with insulin or H2O2. Cell lysates were prepared with a buffer containing NEM to block free sulfhydryl groups, and lysates were then subjected to immunoblot by using anti-PTEN antibodies (Figure 4). When SK-N-SH cells were treated with insulin, the oxidized form of PTEN, based on the increased electrophoretic mobility, occurred 2 min after insulin stimulation. Oxidized PTEN increased with time of incubation, reached a maximum at 10 min, and decreased gradually thereafter. In addition, application of DPI before insulin stimulation of the cells abolished the oxidized form of PTEN that occurred in response to insulin stimulation (Figure 4A). Only a minor portion of PTEN (∼10% at a maximal level) occurred in the oxidized form in insulin-treated cells. However, the majority of PTEN was observed to be in the oxidized form when cells were treated with 1 mM H2O2 for 10 min. In contrast, the oxidized form of PTEN was not observed in SK-N-BE(2) cells treated with insulin, even when double the amount of protein was loaded on the gel (Figure 4B). However, application of 1 mM H2O2 in SK-N-BE(2) cells induced oxidation of the majority of PTEN within 5 min, and oxidized PTEN was maintained up to 60 min and then returned to the reduced form after 90 min.
Figure 4.
Oxidation of PTEN and recovery of PTEN activity from oxidative inactivated PTEN in the cells stimulated either insulin or H2O2. The oxidation of PTEN was analyzed in SK-N-SH cells stimulated with insulin (A) or treated with DPI (10 μM) for 30 min before insulin stimulation (B) and in SK-N-BE(2) cells stimulated with insulin (C) or H2O2 (D). SK-N-SH and SK-N-BE(2) cells were stimulated for the indicated times with insulin (100 nM), DPI (10 μM) plus insulin, or H2O2 (1 mM) and processed by the procedure described in Materials and Methods. Proteins, 200 μg (A and B), 400 μg (C), or 50 μg (D), were fractionated on a 10% nonreducing SDS-PAGE. The redox state of PTEN was analyzed using anti-PTEN antibody immunoblotting to identify shifts in PTEN mobility. The recovery of PTEN phosphatase activity from oxidative inactivated PTEN was measured in anti-PTEN immunoprecipitates from the cells stimulated with insulin or H2O2 for 15 min (E). Free cysteine residues of PTEN were blocked with irreversible alkylating reagent NEM immediately after cells were stimulated with insulin or H2O2 as described in Materials and Methods. The oxidized fraction of PTEN in anti-PTEN immunoprecipitates was converted to active form by reducing with DTT. The phosphatase activity was measured using 32P-labeled PtdIns(3,4,5)P3 as substrates. Each bar represents the average ± SE of the two independent experiments.
To address whether the oxidized PTEN in insulin-stimulated SK-N-SH cells is correlated with the oxidative inhibition of PTEN activity, we examined phosphatase activity in PTEN immunoprecipitates from the cells stimulated with insulin or H2O2 (Figure 4E). Previous reports (Lee et al., 2002; Meng et al., 2002; Leslie et al., 2003) demonstrated that PTEN is reversibly inactivated by oxidation, like other members of PTP family, and the oxidized cysteine residues in PTEN are protected from alkylation. To measure the portion of inactivated PTEN activity by oxidation, cells were lyzed in a buffer containing NEM immediately after stimulation to prevent further oxidation of PTEN and to block free sulfhydryl groups in the unoxidized fraction of PTEN before anti-PTEN immunoprecipitation. Thus, the phosphatase activity in anti-PTEN immunoprecipitates reflects the fraction of inactivated PTEN by oxidation in the cells because their oxidation is reversible. The released [32P]phosphate was 661 ± 68, 1049 ± 80, or 2415 ± 352 cpm in the anti-PTEN immunoprecipitates from the cells unstimulated, stimulated with insulin, or stimulated with 1 mM exogenous H2O2, respectively. To measure what fraction of total PTEN is oxidized by insulin stimulation, the phosphatase activities of PTEN from insulin- or H2O2-stimulated cells were compared after subtracting the basal level of phosphatase activity observed from unstimulated cells. The phosphatase activity of H2O2-stimulated cells is considered as total PTEN activity, because almost all PTEN in cells was oxidized by H2O2. About 16% of the total PTEN is oxidized by insulin stimulation. The higher value of insulin-mediated PTEN oxidation observed in the phosphatase activity assay (∼16%) compared with the value observed in the gel-shift assay (∼10%) may reflect the difference of sensitivity between these two methods, or it may reflect the fact that intermediate form of oxidized PTEN, which did not form intramolecular disulfide bonds, is included in the phosphatase activity assay.
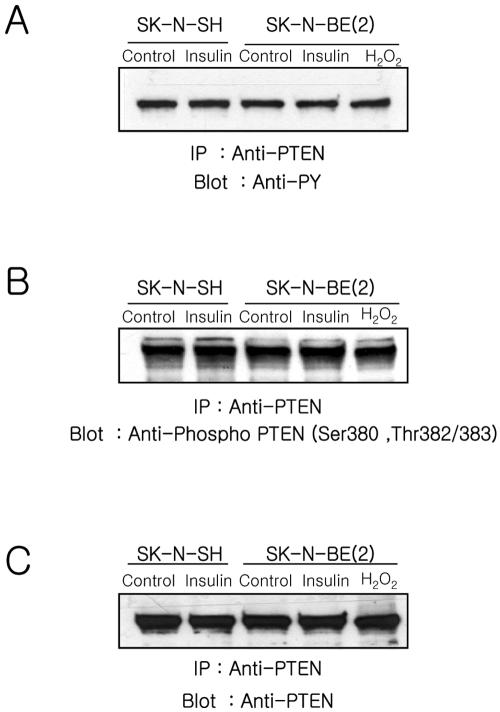
A recent report demonstrated that activated Src reduces PTEN activity to in micelles, promotes Akt translocation to the plasma membrane, and increases tyrosine phosphorylation of PTEN (Lu et al., 2003). To test whether the status of PTEN phosphorylation was changed, we studied PTEN phosphorylation in anti-PTEN immunoprecipitates from insulin-treated cells by using anti-phosphotyrosine (4G10) and an antibody specific Ser/Thr phosphorylated PTEN (Figure 5). The level of tyrosine phosphorylated (Figure 5A) and Ser/Thr phosphorylated (Figure 5B) PTEN did not change in either cell type in response to insulin or H2O2 treatment for 15 min. Similar amounts of PTEN were loaded on the gel as confirmed by reblotting with anti-PTEN (Figure 5C).
Figure 5.
Analyses of the phosphorylation status of PTEN. Cells were stimulated with insulin (100 nM) or H2O2 (1 mM) for 10 min, lysed, and immunoprecipitated using anti-PTEN (5 μg) as described in Materials and Methods. The anti-PTEN immunoprecipitates were fractionated on a 10% SDS-PAGE, transferred to nitrocellulose, and blotted with anti-phosphotyrosine (4G10) (A), anti-phospho-PTEN (B), and anti-PTEN.
These results indicate that when cells were stimulated with insulin, PTEN can be oxidized and inactivated by endogenously produced ROS in SK-N-SH cells, but not in SK-N-BE(2) cells. Moreover, the modification of PTEN by phosphorylation is not related to in this early event of PI-3 kinase/Akt signaling.
Effect of DPI and H2O2 on PI-3 Kinase Activity and Akt Phosphorylation
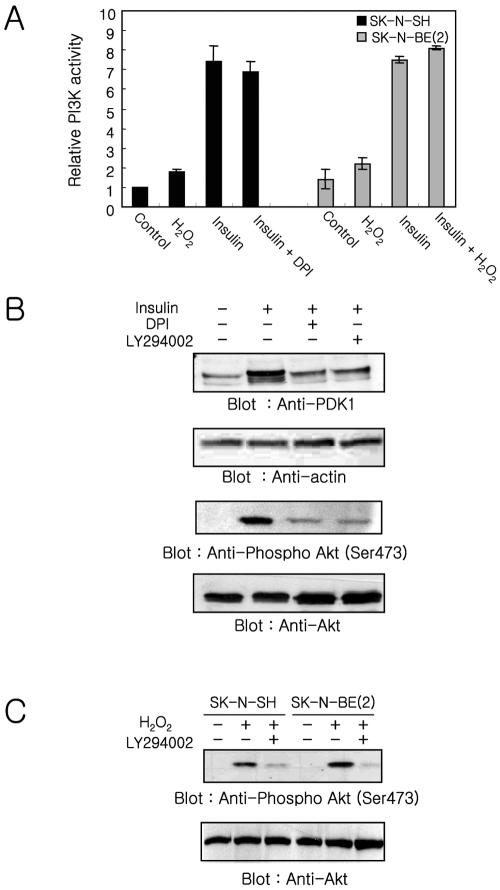
SK-N-BE(2) cells, which have a defective response to insulin, are unable to generate ROS and inactivate PTEN. SK-N-SH cells, which respond normally to insulin, have increased ROS and oxidized, inactivated PTEN. These dada suggest that the PTEN inactivation via oxidation together with the activation of PI-3 kinase are both required to increase the cellular PtdIns(3,4,5)P3 level. To address this possibility and to confirm that the defect in PI-3 kinase/Akt signaling observed in SK-N-BE(2) is due to lack of ROS generation, we examined the effect of DPI, an inhibitor of NADPH oxidase, on the PI-3 kinase activity and Akt phosphorylation in SKN-SH cells stimulated with insulin (Figure 6A). The PI-3 kinase activity was increased 1.8 ± 0.2- and 1.6 ± 0.3-fold in SK-N-SH and SK-N-BE(2) cells, respectively, in response to 1 mM H2O2. When SK-N-SH cells were pretreated with DPI before insulin stimulation, the increase of PI-3 kinase activity (7.0 ± 0.5-fold) was almost identical with that (7.5 ± 0.8-fold) in the cells treated with insulin alone. Moreover, the increase of PI-3 kinase activity in SK-N-BE(2) was very similar in cells stimulated with insulin alone (5.4 ± 0.2) or insulin plus 1 mM H2O2 (5.8 ± 0.1). The lower increase of PI-3 kinase activity in SK-N-BE(2) cells compared with SKN-SH cells in response to insulin stimulation is due to higher basal activity of PI-3 kinase in SK-N-BE(2) cells.
Figure 6.
PI-3 kinase/Akt pathway regulation. (A) Effect of the NADPH oxidase inhibitor DPI and exogenous H2O2 on PI-3 kinase/Akt pathway. The PI-3 kinase activity was measured in anti-phosphotyrosine immunoprecipitates from cells treated for 10 min with insulin, H2O2, insulin plus DPI, or insulin plus H2O2. Each bar represents the mean value ± SE of three independent experiments normalized to the value of unstimulated SK-N-SH cells. (B) Effect of DPI and LY294002 on the insulin-mediated translocation of PDK-1 and Akt activation in SK-N-SH cells. Membrane fractions and lysates were prepared from cells stimulated with insulin, insulin plus DPI, or insulin plus LY294002. Samples were fractionated (50 μg of protein) by 10% SDS-PAGE and transferred to nitrocellulose. PDK-1 and activated Akt were visualized using anti-PDK-1 and anti-phospho-Akt. The same blots were reprobed with anti-actin and anti-Akt, respectively, to confirm equal protein loading. (C) Effect of PI-3 kinase inhibitor on the Akt activation in response to exogenous H2O2. Proteins (50 μg) from cells stimulated with H2O2 or H2O2 plus LY294002, were fractionated on two 10% SDS-PAGs and transferred to nitrocellulose. One blot was used for visualizing phosphorylated Akt by using anti-phospho-Akt, and the other blot was used for visualizing Akt by using anti-Akt to confirm equal protein loading.
In contrast, the amount of PDK-1 in the membrane fraction markedly decreased in SK-N-SH cells pretreated with DPI or LY294002 before insulin stimulation, compared with levels in the cells stimulated with insulin alone (Figure 6B). Consistent with the recruitment of PDK-1 to the membrane fraction, phosphorylated Akt also was decreased in the SKN-SH cells pretreated with DPI or LY294002 before insulin stimulation (Figure 6B). An increase of Akt phosphorylation in response to exogenous H2O2 was abolished by LY294002 in both SK-N-SH and SK-N-BE(2) cells (Figure 6C). Moreover, the examination of phosphorylated Akt in human embryonic kidney 293T cells stimulated with insulin or epidermal growth factor (EGF) revealed that the level of either insulin- or EGF-mediated Akt phosphorylation was decreased by DPI pretreatment (our unpublished data).
These results together with the data obtained from SK-NBE(2) cells indicate that both activation of PI-3 kinase as well as inhibition of PTEN by endogenously produced ROS are needed to increase cellular PtdIns(3,4,5)P3 levels enough to recruit downstream target molecules such as PDK-1 and Akt to the membrane fraction via their PH domains. Moreover, the ROS generated by insulin stimulation mainly contributes to the inactivation of PTEN and not to the activation of PI-3 kinase.
DISCUSSION
A large body of compelling evidence demonstrates that peptide growth factors induce a transient increase in the intracellular concentration of ROS, especially H2O2 (Ohba et al., 1994; Kimura et al., 1995; Krieger-Brauer and Kather, 1995; Sundaresan et al., 1995; Bae et al., 1997; Patterson et al., 1999). This growth factor-mediated transient increase of ROS is considered an integral component of downstream signal transduction (Finkel, 1998). In this report, we have shown that the activation of PI-3 kinase as well as inactivation of PTEN, via oxidation by transiently increased ROS, are both needed for insulin-mediated Akt activation by using two different neuroblastoma cell lines, SK-N-SH and SK-N-BE(2), which can and cannot produce ROS, respectively, in response to insulin stimulation. Moreover, the insulin-mediated increase of ROS contributes mainly to the inactivation of PTEN and not to the activation of PI-3 kinase to cause a net increase cellular PtdIns(3,4,5)P3 levels.
Analysis of PI-3 kinase/Akt pathway in insulin-stimulated SK-N-BE(2) cells, which cannot increase insulin-mediated cellular ROS levels, demonstrates that recruitment of PDK-1 to the membrane fraction and activation of Akt do not occur despite PI-3 kinase activation. Moreover, translocation of PDK-1 to the membrane fraction and activation of Akt can be induced by application of exogenous H2O2 to this cell line. These results indicate that the insulin-mediated activation of PI-3 kinase is not sufficient to produce the levels of cellular PtdIns(3,4,5)P3 needed to recruit PDK-1 and activate Akt and suggest that the transient increase of ROS may play a pivotal role in increasing PtdIns(3,4,5)P3 levels. Consistent with this possibility, our data demonstrate that inhibition of insulin-mediated ROS production in SKN-SH cells by using DPI results in a decrease of PDK-1 translocation to the membrane fraction and reduced Akt activation without significant effect on PI-3 kinase activity, although several reports showed that the subcellular localization of PDK-1 seem to be growth factor insensitive (Yamada et al., 2002; Kim et al., 2003) and that PDK-1 is activated through a PI-3 kinase-independent pathway (Kim et al., 2003). However, a recent report demonstrated that Akt cannot be activated by insulin-like growth factor-1 in homozygous knockin embryonic stem cells expressing a form of PDK-1 with a mutation in its PH domain that abolishes PtdIns(3,4,5)P3 binding, without affecting catalytic activity (McManus et al., 2004).
A previous report has revealed that PTEN was reversibly oxidized and inactivated in the cells stimulated with H2O2 through the formation of a disulfide bond between Cys 124 at the active site and Cys 71 (Lee et al., 2002). Moreover, endogenous oxidant production in macrophages inactivates a fraction of the cellular PTEN, and this is associated with an oxidant-dependent activation of downstream signaling (Leslie et al., 2003). These results, together with our observations identifying the insulin-mediated oxidized form of PTEN, translocation of PDK-1 to the membrane fraction, and Akt activation in SK-N-SH cells, but not in the SK-N-BE(2) or SK-N-SH cells preincubated with DPI, strongly suggest that insulin-stimulated ROS generation plays a key role in the PI-3 kinase/Akt pathway through the reversible oxidation and inactivation of PTEN. Although only a small fraction of PTEN (<10%) is oxidized in insulin-stimulated SK-N-SH cells this might be enough to increase cellular PtdIns(3,4,5)P3 levels to recruit PDK-1 and Akt. The relevant fraction of PTEN may be closely associated with the signaling complex where ROS generation most likely regulates the PtdIns(3,4,5)P3 level in the insulin-mediated signaling. In fact, a previous study has revealed that only ∼10% of PTEN was oxidized in stimulated macrophages (Leslie et al., 2003). It has been demonstrated that PtdIns(3,4,5)P3 and the NADPH oxidase complex are found in the plasma membrane (Babior, 1999) and that the activated PDGF receptor recruits PI-3 kinase to the plasma membrane (Nave et al., 1996; Clark et al., 1998). Thus, it is likely that ROS is produced near activated receptors and that the PTEN localized at these sites can be the target of oxidative inactivation.
In contrast to PDGF, studies on the localization of insulin-mediated signaling complex have revealed that the majority of IRS-1 and PI-3 kinase occur in the low-density membrane fractions that contain GLUT4 vesicles (Nave et al., 1996; Inoue et al., 1998). However, a more detailed evaluation of the components in the low-density fractions revealed that PI-3 kinase and IRS-1 are associated with a cytoskeletal structure that is distinct from the microsomal membrane (Clark et al., 1998). In addition, a recent report has demonstrated that plasma membrane-associated IRS-1 is needed for insulin-mediated full activation of Akt in a cell-free system (Murata et al., 2003). These results suggest that IRS-1 and PI-3 kinase must be in close opposition to the cell surface and accessible to the insulin receptor and other signaling molecules. Moreover, it has been revealed that the insulin receptor possesses a conserved tyrosine autophosphorylation site (YxxM motif) in the C terminus its β-subunit and recruits PI-3 kinase directly to this site (Levy-Toledano et al., 1994; Van Horn et al., 1994).
Our data revealed that SK-N-BE(2) cells, which cannot increase ROS, and SK-N-SH cells, in which ROS generation was inhibited using DPI, are not able to activate Akt in response to insulin, whereas PI-3 kinase activation is normal in these cells. It is difficult to rule out the possibility that our data derived from the results of the oxidative inhibition of the protein phosphatase or of other lipid phosphatases, which control the redox-dependant PI metabolism. However, our data together with a previous report that has demonstrated that PTEN-null glioblastoma cells increase cellular PtdIns(3,4,5)P3 levels and activate Akt in response to PDGF stimulation but not exogenous H2O2 (Leslie et al., 2003), indicate that ROS generated by insulin stimulation mainly contributes to the inactivation of PTEN and not to the activation of PI-3 kinase. In fact, we also observed that Akt is activated by either insulin or PDGF stimulation but not by exogenous H2O2 in PTEN-null glioblastoma cells (U87MG) (our unpublished data).
It has been postulated that the increased production of intracellular oxidants may contribute to enhancing tyrosine phosphorylation-dependent signaling in response to growth factors by transiently suppressing activities of the members of PTP family (Denu and Tanner, 1998; Lee et al., 1998; Meng et al., 2002). However, it is not clear how widely this can be applied across a variety of tyrosine phosphorylation-dependent signaling pathways. The examination of PI-3 kinase activity has demonstrated that the increase of PI-3 kinase activity in both SK-N-SH and SK-N-BE(2) cells is much lower in response to exogenous H2O2 (<2-fold) than insulin (>7-fold) as noted in a previous report (Van der Kaay et al., 1999). This result suggests that exogenous oxidant is less effective than insulin stimulation in the activation of PI-3 kinase. However, it has been demonstrated that application of exogenous H2O2 activates receptor tyrosine kinases such as EGF receptors which in turn activate PI-3 kinase, resulting in production of PtdIns(3,4,5)P3 and in the activation of Akt (Knebel et al., 1996; Wang et al., 2000). Our data show that the insulin-mediated increase of PI-3 kinase activity is independent of ROS production and suggest that PI-3 kinase is not the major target of ROS. Moreover, these results suggest that the binding motifs of the p85 regulatory subunit of PI-3 kinase located in the insulin receptor or IRS are more resistant to the member of PTP family, the known target of the reversible inactivation by ROS, than other tyrosine phosphorylation sites.
A role for ROS in insulin-mediated signaling has been suggested by the observation that insulin stimulation induces the rapid production of ROS and that H2O2 can mimic some of the action of insulin (Czech et al., 1974; Krieger-Brauer et al., 1997). Recent reports (Mahadev et al., 2001a; Mahadev et al., 2001b) have demonstrated that insulin-stimulated ROS generation modulated the steady-state tyrosine phosphorylation of the insulin receptor and its cellular substrates by reversible inhibition of PTPases. In addition, the transient increase of ROS in response to insulin regulates the activity of PI-3 kinase/Akt and cellular glucose transport. Our results demonstrating that Akt is activated in SK-N-SH, which can produce ROS, but not in SK-N-BE(2) cells, which cannot produce ROS in response to insulin stimulation, indicate the importance of ROS generation in insulin-mediated signaling. However, why SK-N-BE(2) cells decrease ROS levels in response to insulin stimulation is difficult to explain until the growth factor-mediated ROS generation system in this cell line is completely understood.
Although the ROS generation in a variety of nonphagocytic cells is much lower than in phagocytes, the ROS generation system itself is thought to be similar (Bokoch and Diebold, 2002). Although the mechanism of the growth factor-mediated ROS generation in nonphagocytic cells is still rather poorly understood, several recent reports (Bae et al., 2000; Bokoch and Diebold, 2002) have indicated that growth factor mediated-activation of PI-3 kinase and Rac is needed for ROS generation. However, a recent report has shown that the insulin-mediated ROS production is independent of PI-3 kinase activity in 3T3-L1 adipocytes (Mahadev et al., 2001a). Our results demonstrating that SK-N-BE(2) cells do not produce ROS in response to EGF or PDGF, although this cell line has receptors for these growth factors (our unpublished data), suggest that the defect in the ROS generation system in SK-N-BE(2) cells could be located at a common point shared by both insulin and EGF signaling pathway.
In conclusion, the data in this report show that the generation of ROS has a pivotal role in insulin-mediated activation of PI-3 kinase/Akt signaling through the reversible oxidation and inactivation of PTEN, which occurs independently of PI-3 kinase activation. Moreover, our results suggest that there must be a signal amplification mediated by the transiently increased ROS. Insulin-stimulated ROS production oxidizes and inactivates PTEN, thereby allowing PI-3 kinase to increase the cellular level of PtdIns(3,4,5)P3 enough to recruit PDK and Akt.
Supplementary Material
Acknowledgments
This work was supported by Korea Science and Engineering Foundation grants and a BK21 grant, Ministry of Education. We thank Dr. Nick R. Leslie (University of Dundee, Dundee, United Kingdom) for providing purified PI3-kinase γ.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-05-0369. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-05-0369.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Adey, N. B., Huang, L., Ormonde, P. A., Baumgard, M. L., Pero, R., Byreddy, D. V., Tavtigian, S. V., and Bartel, P. L. (2000). Threonine phosphorylation of the MMAC1/PTEN PDZ binding domain both inhibits and stimulates PDZ binding. Cancer Res. 60, 35-37. [PubMed] [Google Scholar]
- Alessi, D. R., James, S. R., Downes, C. P., Holmes, A. B., Gaffney, P. R., Reese, C. B., and Cohen, P. (1997). Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 7, 261-269. [DOI] [PubMed] [Google Scholar]
- Babior, B. M. (1999). NADPH oxidase: an update. Blood 93, 1464-1476. [PubMed] [Google Scholar]
- Bae, Y. S., Kang, S. W., Seo, M. S., Baines, I. C., Tekle, E., Chock, P. B., and Rhee, S. G. (1997). EGF-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272, 217-221. [PubMed] [Google Scholar]
- Bae, Y. S., Sung, J. Y., Kim, O. S., Kim, Y. J., Hur, K. C., Kazlauskas, A., and Rhee, S. G. (2000). PDGF-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase. J. Biol. Chem. 275, 10527-10531. [DOI] [PubMed] [Google Scholar]
- Banfi, B., Clark, R. A., Steger, K., and Krause, K. H. (2003). Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 278, 3510-3513. [DOI] [PubMed] [Google Scholar]
- Bastola, D. R., Pahwa, G. S., Lin, M. F., and Cheng, P. W. (2002). Downregulation of PTEN/MMAC/TEP1 expression in human prostate cancer cell line DU145 by growth stimuli. Mol. Cell. Biochem. 236, 75-81. [DOI] [PubMed] [Google Scholar]
- Biedler, J. L., Roffler-Tarlov, S., Schachner, M., and Freedman, L. S. (1978). Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 38, 3751-3757. [PubMed] [Google Scholar]
- Bokoch, G. M., and Diebold, B. A. (2002). Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood 100, 2692-2696. [DOI] [PubMed] [Google Scholar]
- Cantley, L. C. (2002). The phosphoinositide 3-kinase pathway. Science 296, 1655-1657. [DOI] [PubMed] [Google Scholar]
- Cantley, L. C., and Neel, B. G. (1999). New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 96, 4240-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell, D. A. (2001). Phosphoinositide 3-kinase signalling pathways. J. Cell Sci. 114, 1439-1445. [DOI] [PubMed] [Google Scholar]
- Clark, S. F., Martin, S., Carozzi, A. J., Hill, M. M., and James, D. E. (1998). Intracellular localization of phosphatidylinositide 3-kinase and insulin receptor substrate-1 in adipocytes: potential involvement of a membrane skeleton. J. Cell Biol. 140, 1211-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech, M. P., Lawrence, J. C., Jr., and Lynn, W. S. (1974). Evidence for the involvement of sulfhydryl oxidation in the regulation of fat cell hexose transport by insulin. Proc. Natl. Acad. Sci. USA 71, 4173-4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denu, J. M., and Tanner, K. G. (1998). Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry 37, 5633-5642. [DOI] [PubMed] [Google Scholar]
- Finkel, T. (1998). Oxygen radicals and signaling. Curr. Opin. Cell. Biol. 10, 248-253. [DOI] [PubMed] [Google Scholar]
- Franke, T. F., Kaplan, D. R., and Cantley, L. C. (1997). PI3K: downstream AKTion blocks apoptosis. Cell 88, 435-437. [DOI] [PubMed] [Google Scholar]
- Hwang, J. J., Kwon, J. H., Lee, K. Y., and Hur, K. C. (1995). Effet of nerve growth factor, insulin, and extracellular proteins on the neurite outgrowth of SK-N-BE(2) human neuroblastoma cells. Mol. Cell 5, 501-507. [Google Scholar]
- Inoue, G., Cheatham, B., Emkey, R., and Kahn, C. R. (1998). Dynamics of insulin signaling in 3T3-L1 adipocytes. Differential compartmentalization and trafficking of insulin receptor substrate (IRS)-1 and IRS-2. J. Biol. Chem. 273, 11548-11555. [DOI] [PubMed] [Google Scholar]
- Kim, D. W., et al. (2003). RET/PTC (rearranged in transformation/papillary thyroid carcinomas) tyrosine kinase phosphorylates and activates phosphoinositide-dependent kinase 1 (PDK1): an alternative phosphatidylinositol 3-kinase-independent pathway to activate PDK1. Mol. Endocrinol. 17, 1382-1394. [DOI] [PubMed] [Google Scholar]
- Kimura, T., Okajima, F., Sho, K., Kobayashi, I., and Kondo, Y. (1995). Thyrotropin-induced hydrogen peroxide production in FRTL-5 thyroid cells is mediated not by adenosine 3′,5′-monophosphate, but by Ca2+ signaling followed by phospholipase-A2 activation and potentiated by an adenosine derivative. Endocrinology 136, 116-123. [DOI] [PubMed] [Google Scholar]
- Knebel, A., Rahmsdorf, H. J., Ullrich, A., and Herrlich, P. (1996). Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 15, 5314-5325. [PMC free article] [PubMed] [Google Scholar]
- Konishi, H., Matsuzaki, H., Tanaka, M., Takemura, Y., Kuroda, S., Ono, Y., and Kikkawa, U. (1997). Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett. 410, 493-498. [DOI] [PubMed] [Google Scholar]
- Krieger-Brauer, H. I., and Kather, H. (1995). The stimulus-sensitive H2O2-generating system present in human fat-cell plasma membranes is multireceptor-linked and under antagonistic control by hormones and cytokines. Biochem. J. 307, 543-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Brauer, H. I., Medda, P. K., and Kather, H. (1997). Insulin-induced activation of NADPH-dependent H2O2 generation in human adipocyte plasma membranes is mediated by Galphai2. J. Biol. Chem. 272, 10135-10143. [DOI] [PubMed] [Google Scholar]
- Kurose, K., Zhou, X. P., Araki, T., Cannistra, S. A., Maher, E. R., and Eng, C. (2001). Frequent loss of PTEN expression is linked to elevated phosphorylated Akt levels, but not associated with p27 and cyclin D1 expression, in primary epithelial ovarian carcinomas. Am. J. Pathol. 158, 2097-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. R., Kwon, K. S., Kim, S. R., and Rhee, S. G. (1998). Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with EGF. J. Biol. Chem. 273, 15366-15372. [DOI] [PubMed] [Google Scholar]
- Lee, S. R., Yang, K. S., Kwon, J., Lee, C., Jeong, W., and Rhee, S. G. (2002). Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 277, 20336-20342. [DOI] [PubMed] [Google Scholar]
- Leevers, S. J., Vanhaesebroeck, B., and Waterfield, M. D. (1999). Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr. Opin. Cell Biol. 11, 219-225. [DOI] [PubMed] [Google Scholar]
- Leslie, N. R., Bennett, D., Lindsay, Y. E., Stewart, H., Gray, A., and Downes, C. P. (2003). Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 22, 5501-5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Toledano, R., Taouis, M., Blaettler, D. H., Gorden, P., and Taylor, S. I. (1994). Insulin-induced activation of phosphatidyl inositol 3-kinase. Demonstration that the p85 subunit binds directly to the COOH terminus of the insulin receptor in intact cells. J. Biol. Chem. 269, 31178-31182. [PubMed] [Google Scholar]
- Lo, Y. Y., and Cruz, T. F. (1995). Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J. Biol. Chem. 270, 11727-11730. [DOI] [PubMed] [Google Scholar]
- Lu, Y., et al. (2003). Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J. Biol. Chem. 278, 40057-40066. [DOI] [PubMed] [Google Scholar]
- Maehama, T., and Dixon, J. E. (1998). The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375-13378. [DOI] [PubMed] [Google Scholar]
- Mahadev, K., Wu, X., Zilbering, A., Zhu, L., Lawrence, J. T., and Goldstein, B. J. (2001a). Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J. Biol. Chem. 276, 48662-48669. [DOI] [PubMed] [Google Scholar]
- Mahadev, K., Zilbering, A., Zhu, L., and Goldstein, B. J. (2001b). Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J. Biol. Chem. 276, 21938-21942. [DOI] [PubMed] [Google Scholar]
- McManus, E. J., Collins, B. J., Ashby, P. R., Prescott, A. R., Murray-Tait, V., Armit, L. J., Arthur, J. S., and Alessi, D. R. (2004). The in vivo role of PtdIns(3,4,5)P3 binding to PDK1 PH domain defined by knockin mutation. EMBO J. 23, 2071-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, T. C., Fukada, T., and Tonks, N. K. (2002). Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 9, 387-399. [DOI] [PubMed] [Google Scholar]
- Murata, H., Hresko, R. C., and Mueckler, M. (2003). Reconstitution of phosphoinositide 3-kinase-dependent insulin signaling in a cell-free system. J. Biol. Chem. 278, 21607-21614. [DOI] [PubMed] [Google Scholar]
- Myers, M. P., Pass, I., Batty, I. H., Van der Kaay, J., Stolarov, J. P., Hemmings, B. A., Wigler, M. H., Downes, C. P., and Tonks, N. K. (1998). The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc. Natl. Acad. Sci. USA 95, 13513-13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave, B. T., Haigh, R. J., Hayward, A. C., Siddle, K., and Shepherd, P. R. (1996). Compartment-specific regulation of phosphoinositide 3-kinase by PDGF and insulin in 3T3-L1 adipocytes. Biochem. J. 318, 55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba, M., Shibanuma, M., Kuroki, T., and Nose, K. (1994). Production of hydrogen peroxide by transforming growth factor-beta 1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J. Cell Biol. 126, 1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, C., Ruef, J., Madamanchi, N. R., Barry-Lane, P., Hu, Z., Horaist, C., Ballinger, C. A., Brasier, A. R., Bode, C., and Runge, M. S. (1999). Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. Evidence that p47(phox) may participate in forming this oxidase in vitro and in vivo. J. Biol. Chem. 274, 19814-19822. [DOI] [PubMed] [Google Scholar]
- Qin, S., Stadtman, E. R., and Chock, P. B. (2000). Regulation of oxidative stress-induced calcium release by phosphatidylinositol 3-kinase and Bruton's tyrosine kinase in B cells. Proc. Natl. Acad. Sci. USA 97, 7118-7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler, M., Winkler, T., Verma, S., Byrne, C. H., Shrikhande, G., Salgia, R., and Griffin, J. D. (1999). Hematopoietic growth factors signal through the formation of reactive oxygen species. Blood 93, 2928-2935. [PubMed] [Google Scholar]
- Shaw, M., Cohen, P., and Alessi, D. R. (1998). The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem. J. 336, 241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd, P. R., Withers, D. J., and Siddle, K. (1998). Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem. J. 333, 471-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda, Y., Watanabe, S., Matsumoto, Y., Aizu-Yokota, E., and Kasahara, T. (1999). FAK is the upstream signal protein of the phosphatidylinositol 3-kinase-Akt survival pathway in hydrogen peroxide-induced apoptosis of a human glioblastoma cell line. J. Biol. Chem. 274, 10566-10570. [DOI] [PubMed] [Google Scholar]
- Stephens, L. R., Jackson, T. R., and Hawkins, P. T. (1993). Agonist-stimulated synthesis of phosphatidylinositol(3,4,5)-trisphosphate: a new intracellular signalling system? Biochim. Biophys. Acta 1179, 27-75. [DOI] [PubMed] [Google Scholar]
- Stokoe, D., Stephens, L. R., Copeland, T., Gaffney, P. R., Reese, C. B., Painter, G. F., Holmes, A. B., McCormick, F., and Hawkins, P. T. (1997). Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277, 567-570. [DOI] [PubMed] [Google Scholar]
- Sulis, M. L., and Parsons, R. (2003). PTEN: from pathology to biology. Trends Cell Biol. 13, 478-483. [DOI] [PubMed] [Google Scholar]
- Sundaresan, M., Yu, Z. X., Ferrans, V. J., Irani, K., and Finkel, T. (1995). Requirement for generation of H2O2 for PDGF signal transduction. Science 270, 296-299. [DOI] [PubMed] [Google Scholar]
- Sundaresan, M., Yu, Z. X., Ferrans, V. J., Sulciner, D. J., Gutkind, J. S., Irani, K., Goldschmidt-Clermont, P. J., and Finkel, T. (1996). Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem. J. 318, 379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, K., et al. (1999). Evidence that a phosphatidylinositol 3,4,5-trisphosphate-binding protein can function in nucleus. J. Biol. Chem. 274, 3919-3922. [DOI] [PubMed] [Google Scholar]
- Van der Kaay, J., Beck, M., Gray, A., and Downes, C. P. (1999). Distinct phosphatidylinositol 3-kinase lipid products accumulate upon oxidative and osmotic stress and lead to different cellular responses. J. Biol. Chem. 274, 35963-35968. [DOI] [PubMed] [Google Scholar]
- Van Horn, D. J., Myers, M. G., Jr., and Backer, J. M. (1994). Direct activation of the phosphatidylinositol 3′-kinase by the insulin receptor. J. Biol. Chem. 269, 29-32. [PubMed] [Google Scholar]
- Vanhaesebroeck, B., Leevers, S. J., Panayotou, G., and Waterfield, M. D. (1997). Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem. Sci. 22, 267-272. [DOI] [PubMed] [Google Scholar]
- Vazquez, F., Grossman, S. R., Takahashi, Y., Rokas, M. V., Nakamura, N., and Sellers, W. R. (2001). Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J. Biol. Chem. 276, 48627-48630. [DOI] [PubMed] [Google Scholar]
- Vazquez, F., Ramaswamy, S., Nakamura, N., and Sellers, W. R. (2000). Phosphorylation of the PTEN tail regulates protein stability and function. Mol. Cell. Biol. 20, 5010-5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, S. M., Downes, C. P., and Leslie, N. R. (2001). TPIP: a novel phosphoinositide 3-phosphatase. Biochem. J. 360, 277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., McCullough, K. D., Franke, T. F., and Holbrook, N. J. (2000). EGF receptor-dependent Akt activation by oxidative stress enhances cell survival. J. Biol. Chem. 275, 14624-14631. [DOI] [PubMed] [Google Scholar]
- Yamada, T., Katagiri, H., Asano, T., Tsuru, M., Inukai, K., Ono, H., Kodama, T., Kikuchi, M., and Oka, Y. (2002). Role of PDK1 in insulin-signaling pathway for glucose metabolism in 3T3-L1 adipocytes. Am. J. Physiol. 282, E1385-E1394. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. Y. (1998). Protein-tyrosine phosphatases: biological function, structural characteristics, and mechanism of catalysis. Crit. Rev. Biochem. Mol. Biol. 33, 1-52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.