Abstract
Baseline cue-dependent physiological reactivity may serve as an objective measure of posttraumatic stress disorder (PTSD) symptoms. Additionally, prior animal model and psychological studies would suggest that subjects with greatest symptoms at baseline may have the greatest violation of expectancy to danger when undergoing exposure based psychotherapy; thus treatment approaches which enhanced the learning under these conditions would be optimal for those with maximal baseline cue-dependent reactivity. However methods to study this hypothesis objectively are lacking. Virtual reality (VR) methodologies have been successfully employed as an enhanced form of imaginal prolonged exposure therapy for the treatment of PTSD.
Our goal was to examine the predictive nature of initial psychophysiological (e.g., startle, skin conductance, heart rate) and stress hormone responses (e.g., cortisol) during presentation of VR-based combat-related stimuli on PTSD treatment outcome. Combat veterans with PTSD underwent 6 weeks of VR exposure therapy combined with either D-cycloserine (DCS), alprazolam (ALP), or placebo (PBO). In the DCS group, startle response to VR scenes prior to initiation of treatment accounted for 76% of the variance in CAPS change scores, p < 0.001, in that higher responses predicted greater changes in symptom severity over time. Additionally, baseline cortisol reactivity was inversely associated with treatment response in the ALP group, p = 0.04. We propose that baseline cue-activated physiological measures will be sensitive to predicting patients’ level of response to exposure therapy, in particular in the presence of enhancement (e.g., DCS).
Keywords: Acoustic startle, Posttraumatic stress disorder, Virtual reality, Psychophysiology, Exposure therapy, Cortisol
1. Introduction
Clinical and empirical evidence strongly supports the use of prolonged exposure therapy as first-line intervention for reducing posttraumatic stress disorder (PTSD) symptom severity and for improving overall mental health (Bradley, Greene, Russ, Dutra, & Westen, 2005; DoD/VA PTSD Working Group, 2010; Institute of Medicine, 2007; Sherman, 1998; Steenkamp & Litz, 2013). According to the principles of Emotional Processing Theory (Foa & Kozak, 1986), prolonged exposure fosters a level of patient engagement that facilitates habituation to trauma-related cues and promotes the extinction of conditioned responses to fearful cues. Virtual reality methodologies have been successfully employed as an enhanced form of imaginal prolonged exposure therapy for the treatment of stressor-, trauma-, and anxiety-related disorders such as PTSD for more than a decade (Difede et al., 2007; Rizzo et al., 2010; Rothbaum, Hodges, Ready, Graap, & Alarcon, 2001). Virtual reality exposure (VRE) therapy is believed to foster engagement and elevate patient arousal (Robison-Andrew et al., 2014) through the inclusion of computer-generated simulations of trauma-related stimuli that span sensory modalities, including the ambient sights, sounds, smells, and tactile stimuli present during a traumatic event. In addition to its clinical impact, VR technology has also been used successfully to elicit robust fear reactions in patients with anxiety disorders (Diemer, Muhlberger, Pauli, & Zwanzger, 2014).
To date, the efficacy of prolonged exposure therapies, including those that are VR-based, has been largely indexed through the use of clinician-administered or self-report measures of patient symptom severity or within-session distress. More recently, psychophysiological measures have been employed as complementary assessment tools for use in traumatized populations presenting with PTSD symptoms (Costanzo et al., 2014; Griffin, Resick, & Galovski, 2012; Rabe, Dorfel, Zollner, Maercker, & Karl, 2006; Rhudy et al., 2010; Robison-Andrew et al., 2014; Rothbaum et al., 2014; Roy et al., 2013). The addition of psychophysiological indices, such as heart rate (HR), skin conductance (SC) and electromyography (EMG) responses to trauma-related cues can provide the potential prediction of treatment outcome, objective assessments of treatment outcome, and evidence of the underlying biological changes that accompany successful PTSD treatment.
Heightened psychophysiological reactivity in response to trauma cues has been observed in PTSD patients for several decades. More specifically, increased HR, SC, and startle EMG responses have repeatedly been found in chronic PTSD (Blanchard, Kolb, Gerardi, Ryan, & Pallmeyer, 1986; Blanchard, Kolb, Pallmeyer, & Gerardi, 1982; Buckley & Kaloupek, 2001; McTeague et al., 2010; Orr, Metzger, & Pitman, 2002), and have been a predictor of PTSD in acutely traumatized populations (Orr et al., 2012; Roy et al., 2013; Shalev et al., 2000). Pre- and post-treatment data suggest that exposure therapy reduces heart rate response to trauma related stimuli (Rabe et al., 2006). Similarly, EMG, HR, and SC in response to loud tones decreases after cognitive behavior therapy in those who show a positive treatment outcome (Griffin et al., 2012).
Exposure therapists have repeatedly shown that increased arousal is associated with treatment response. In fact, it is a core tenet of the theoretical underpinnings for exposure therapy in PTSD (Foa & Kozak, 1986). Many therapeutic resources and clinical supervision efforts are focused on better enabling clinicians to increase the emotional and physiological responses of clients/ patients early in the exposure therapy process (Foa, 2011). Although the theory is widely supported as evidenced by clinically significant effect sizes for exposure therapy in PTSD, investigations of the specific empirical links between psychophysiological indices of patient engagement in exposure therapy and positive treatment outcomes have only recently been initiated (Price et al., 2015). There is evidence from the literature supporting a link between biological reactivity pre-treatment with positive (Foa, Riggs, Massie, & Yarczower, 1995; Rauch et al., 2015) and negative (Yehuda et al., 2009) treatment outcomes.
More recent investigations have explored acoustic startle responses to trauma-related stimuli (termed trauma-potentiated startle) within the presentation of a virtual reality environment (Costanzo et al., 2014; Robison-Andrew et al., 2014; Roy et al., 2013). Robison-Andrew et al. (2014) reported that combat veterans who responded well to prolonged exposure therapy (defined as a >50% reduction in their CAPS score over the course of treatment) showed an initial increase in trauma-potentiated startle followed by a significant decrease in startle responses to trauma-related cues compared to veterans who did not respond to treatment. As in our aforementioned previous work, we examined participants’ startle response to cues specifically drawn from their collective traumatic experiences. Whereas the trauma-relevant cues depicted in the VR environment may not represent the exact nature of the index trauma experienced by the participant, it remains plausible that general combat-related stimuli produce robust arousal and psychophysiological reactivity in traumatized Veteran samples. While these physiological markers show promise as objective assessments of treatment efficacy, their predictive value has not been well studied.
Prolonged exposure therapy represents one of the most effective treatments for PTSD (group, 2010) and can be characterized as a clinical homolog of laboratory fear extinction learning (Rothbaum & Davis, 2003). D-cycloserine (DCS), an N-methyl-d-aspartate (NMDA) glutamate receptor partial agonist (Dravid et al., 2010), has improved the short- and long-term efficacy of exposure therapy when administered as a single dose just prior to treatment for several anxiety disorders (Guastella, Lovibond, Dadds, Mitchell, & Richardson, 2007; Kushner et al., 2007; Otto et al., 2010; Ressler et al., 2004; Rothbaum et al., 2014; Wilhelm et al., 2008). However, DCS has also been shown to be ineffective under specific conditions with some patient populations (e.g., cognitive behavioral therapy for social phobia Hofmann et al., 2013); for review see (Rodrigues et al., 2014). Nevertheless, acute administration of DCS has been shown to be effective at facilitating emotional learning in humans (e.g., behavioral exposure therapy Rothbaum & Davis, 2003), and fear extinction in rodent models of fear- and anxiety-based psychopathologies (e.g., Ledgerwood, Richardson, & Cranney, 2003, 2004, 2005; Richardson, Ledgerwood, & Cranney, 2004; Walker, Ressler, Lu, & Davis, 2002). Benzodiazepines, which increase g-aminobutyric acid (GABA) activity, have been used clinically to treat PTSD (Lund, Bernardy, Vaughan-Sarrazin, Alexander, & Friedman, 2013) but their effect on exposure therapies for PTSD remains largely unknown. There is evidence that the use of benzodiazepines is contraindicated for exposure therapy due to the potential to reduce engagement and arousal resulting in a lack of improvement, and, in turn, increase the likelihood of patient dropout (van Minnen, Arntz, & Keijsers, 2002; van Minnen, Harned, Zoellner, & Mills, 2012).
In addition to psychophysiological responses, alterations in stress hormones have long been a target of investigation in PTSD (Heim et al., 2000). However, findings on baseline cortisol levels have been mixed, and a recent meta-analysis based on a large number of patients and controls concluded that there are no consistent differences between PTSD and controls (Meewisse, Reitsma, De Vries, Gersons, & Olff, 2007). A much more promising approach is measuring cortisol reactivity to a stress challenge. As part of the hypothalamic-pituitary-adrenal axis (HPA)-mediated stress hormone cascade, glucocorticoid receptors (GR) in the hypothalamus regulate cortisol release and higher GR sensitivity has been proposed as a consistent alteration in PTSD (Yehuda, 2009). To our knowledge, few studies have conducted a randomized clinical trial for PTSD with cortisol reactivity as an outcome measure. For example, one study examined treatment effects on cortisol response to a cognitive challenge and found that 12-month open label SSRI treatment reduced reactivity in women with PTSD (Vermetten et al., 2006).
In addition to the above background, it is hypothesized that subjects with the greatest within-session extinction will respond most robustly to D-cycloserine enhancement of exposure therapy (Guastella, Lovibond, Dadds, Mitchell, & Richardson, 2007; Hofmann et al., 2006; Ressler et al., 2004). How might we predict, prior to treatment, which subjects may have the most robust within-session extinction learning? Animal models have begun to suggest that prediction error may be required to explain neural plasticity in extinction learning (Delamater & Westbrook, 2014). Historically, learning theory suggests that extinction is enhanced by increased attentional salience of the conditional stimulus (Mackintosh, 1965, 1975; Pearce & Hall, 1980). Furthermore, a number of human studies have shown that greater expectancy violation may be among the most important variables involved in exposure therapy (reviewed in Craske, Treanor, Conway, Zbozinek, & Vervliet, 2014). This seems to occur across learning paradigms including auditory attentional processing (Vachon, Hughes, & Jones, 2012), contextual learning (Ferdinand, Mecklinger, & Opitz, 2015), and exposure therapy for social phobia (Price, Mehta, Tone, & Anderson, 2011). Thus, we hypothesized that subjects with the highest baseline levels of cue-dependent biological reactivity would have the greatest likelihood for expectancy violation with VR-based exposure therapy, and thus those with the most robust DCS response.
The purpose of the present study was to examine the predictive nature of psychophysiological and cortisol responses during presentation of combat-related stimuli in a VR environment on PTSD treatment outcome in a population of combat veterans from Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF). We examined both immediate treatment outcomes and sustained treatment effects at 6 months in patients receiving VRE therapy in combination with DCS, a benzodiazepine (alprazolam; ALP), or placebo (PBO). The neural processes underlying mammalian fear extinction learning and human emotional learning through exposure therapy appear to use similar molecular and cellular mechanisms (e.g., glutamatergic NMDA receptor systems; (Davis & Myers, 2002; Davis, Ressler, Rothbaum, & Richardson, 2006). Ongoing translational work from our laboratories has shown that psychophysiological reactivity serves as a reliable, objective measure of learned (or extinguished) fear. This work coupled with more recent findings suggest the use of psychophysiological reactivity as an index of emotional engagement. As such, we hypothesized that psychophysiological reactivity to trauma reminders, would be predictive of enhanced extinction and thus better treatment outcomes in the DCS group, but not in the other treatment groups. Our hypothesized effects also consider the potential for benzodiazepines to reduce arousal levels (see van Minnen et al., 2002) and the intentional “under-dosing” of prolonged exposure to minimize floor effects (ie., mask the enhancing effects of DCS). All groups received only 6 sessions of exposure therapy (as opposed to a full regimen of 10–15 sessions, (Foa, Hembree, & Rothbaum, 2007).
2. Materials and methods
The results presented here were collected as part of a large-scale investigation of the treatment efficacy of DCS, ALP, or PBO administered in combination with VRE therapy in a population of combat veterans from OIF/OEF (Rothbaum et al., 2014). The parent study was a double-blind, placebo-controlled study that included a baseline screening assessment, six VRE treatment visits, and follow-up assessments at 3, 6, and 12 months post-treatment. Participants were randomly assigned using a 1:1:1 ratio to one of three conditions: VRE plus 50 mg DCS, VRE plus 0.25 mg ALP, or VRE plus PBO. Study staff members and patients were blind to treatment group.
2.1. Participants
Participants reviewed and signed an informed consent form approved by the Emory University Institutional Review Board and the Atlanta VAMC Research and Development Committee. Participants enrolled in this study met criteria for PTSD per DSM-IV criteria with index trauma(s) related to combat experience in Iraq and/or Afghanistan. Exclusion criteria included the following: lifetime history of psychosis, bipolar disorder, current risk of suicide, current alcohol or drug dependence, pregnancy, and the current use of psychotropic or endocrine treatments that could confound the study data (e.g., benzodiazepines, opioids, glucocorticoids). Veterans with mild traumatic brain injury were enrolled in the study. Due to the inclusion of a benzodiazepine as a psychiatric drug control condition, participants were required to discontinue long-acting benzodiazepines for 1 month and short-acting benzodiazepines for 2 weeks prior to the initial screening visit. Individuals taking psychotropic drugs that were not considered exclusionary were required to be on a stable dose for at least 2 weeks prior to study enrollment and to maintain a stable dose throughout the course of their participation. The current study included 50 participants who had pre-treatment psychophysiological data, completed 6 weeks of treatment and a 6-month follow-up visit. The mean age of the study population was 36.14 (range 23–55 years of age) and the sample was 94% male and 6% female. Per participant self-report, the race/ethnicity distribution was 38% Caucasian/non Hispanic, 52% African American/non Hispanic, 6% Hispanic, 2% Asian, and 2% other. Of the 50 patients who had complete data, 13 were in the DCS group, 20 were in the ALP group, and 17 were in the PBO group. Table 1 shows the demographic data, trauma history and pre-treatment symptom severity for the three groups. There were no differences between the groups in any measures.
Table 1.
Demographic, trauma history and pre-treatment PTSD symptom severity between the three treatment groups, d-cyloserine (DCS), alprazolam (ALP), and placebo (PBO). There were no differences in any variables. Abbreviations: CTQ = Childhood Trauma Questionnaire, DRRI = Deployment Risk and Resilience Inventory, CAPS = Clinician Administered PTSD Scale.
| DCS (n = 13) | ALP (n = 20) | PBO (n = 17) | ||
|---|---|---|---|---|
| Demographics | ||||
| Sex (N, % male) | 13 (100.0%) | 19 (95.0%) | 15 (88.2%) | p = 0.39 |
| Age (M, SE) | 36.15 (2.49) | 37.5 (2.11) | 34.73 (2.31) | p = 0.68 |
| Childhood trauma | ||||
| CTQ (M, SE) | 36.54 (3.50) | 36.39 (2.98) | 39.20 (3.26) | p = 0.79 |
| Combat trauma | ||||
| DRRI (M, SE) | 9.36 (0.89) | 10.77 (0.75) | 9.36 (0.83) | p = 0.36 |
| PTSD symptoms | ||||
| CAPS (M, SE) | 92.77 (5.56) | 90.89 (4.72) | 86.73 (5.17) | p = 0.71 |
2.2. Treatment description
Six sessions of treatment were included in the current study. The first session was a 90-min introductory period during which time the implementation and rationale for the virtual reality treatment regimen was discussed. This initial session was followed by five weekly, 90-min sessions of VRE therapy provided by a doctoral level clinician. Thirty minutes prior to each VRE session, the patient completed questionnaires and took a study pill under supervision of a study staff member. Following the guidelines for traditional, standardized exposure therapy, patients were encouraged to focus on their most distressing traumatic memories during the VRE sessions (Foa et al., 2007). During VRE treatment, the therapist viewed and controlled the VR environment via a computer monitor while the patient experienced the VR environment through an integrated VR system that included auditory, visual, olfactory, and tactile stimulation. The therapist presented stimuli that closely matched what was being described by the patient. Continued exposure to trauma-related stimuli was encouraged until within-session anxiety diminished (approximately 30–45 min). Anxiety ratings were collected every 5 min through administration of a Subjective Units of Discomfort scale (SUDs; 0 = no anxiety, 100 = high anxiety). The VRE session concluded with a 15–20 min period for processing the experience that included the active integration of newly acquired associations (often termed extinction learning in laboratory models; Rothbaum & Davis, 2003).
Treatment was administered according to previous published work (Rothbaum et al., 2014). For VRE treatment sessions, patients viewed the visual virtual imagery through a head-mounted display (eMagin Z800, eMagin Corp., Bellevue, WA) that was coupled to a head tracker and stereo earphones. The virtual Iraq and Afghanistan environments changed in a naturalistic way in response to head and body movement. The participant used a handheld video game style controller to navigate through each environment. Environments included either driving a Humvee on a desert road alone or in a convoy or walking on foot through a Middle Eastern city similar to those experienced in Iraq and Afghanistan. As mentioned previously, trigger stimuli were of four modalities: auditory (e.g., weapons fire, explosions), visual (e.g., smoke, time of day), olfactory (e.g., spices, burning rubber), and tactile (vehicular vibrations, nearby explosions).
2.3. Clinical assessments
All assessments occurred when the participants were free of study medication. The initial study visit was a pre-treatment baseline screening assessment that included administration of the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995) to determine PTSD symptom severity. This assessment was completed by a master’s level clinician who focused on the most traumatic combat experience as identified by the patient. The presence of comorbid Axis I disorders was determined using the Mini International Neuropsychiatric Interview (MINI; Sheehan, Lecrubier, & Sheehan, 1998). Complementary self-report measures were also administered including the PTSD Symptom Scale (PSS; Foa, Riggs, Dancu, & Rothbaum, 1993). Inter-rater reliability was measured via videotaping of assessment interviews with ten percent of taped CAPS and MINI interviews randomly selected for evaluation of reliability. This subset of diagnostic interviews was reviewed by a master’s-level clinician and the agreement for the primary diagnosis across assessments was 100%. Trauma history was assessed using the Childhood Trauma Questionnaire (CTQ, Bernstein and Fink) and the combat exposure subscale of the Deployment Risk and Resilience Inventory (DRRI).
2.3.1. Psychophysiological assessment
Psychophysiological assessments were completed after the interviews and at pre-, and post-treatment, and at 6 months follow-up visits. Psychophysiological responses were acquired using the Biopac MP150 data acquisition system (Biopac Systems, Inc., Goleta, CA). Heart rate was based on R-peaks detected from electrocardiogram (ECG) recordings from two 5-mm Ag/AgCl electrodes placed on the upper right torso and left wrist. Skin conductance was measured using the galvanic skin response (GSR) module of Biopac, with two 5-mm Ag/AgCl electrodes placed on the middle fingers of the non-dominant hand. Startle responses were measured through EMG activity of the orbicularis oculi muscle using two 5-mm Ag/AgCl electrodes. Similar to our previous reports (Norrholm et al., 2011), one electrode was placed 1 cm below the pupil and another placed 1 cm below the lateral canthus of the right eye. EMG startle response was assessed in response to an acoustic white noise burst of 40 ms at 108 dB. ECG, SC and EMG responses were sampled at 1000 Hz, amplified, digitized and filtered using Biopac. The data were exported for further analysis using Mindware software (Mindware Technologies, Gahanna, OH). The EMG signal was acquired with a gain of 5000, and bandpass filtered at 28 and 500 Hz, according to guidelines for human startle measurement (Blumenthal et al., 2005). Peak startle amplitude within 20–200 ms after acoustic probe onset was used as the startle response measurement.
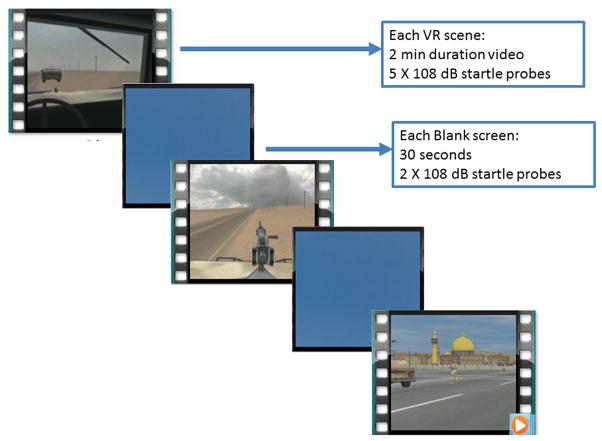
During assessments, participants viewed the standardized VR clips using an eMagin Z800 head mounted display and audio stimuli were presented binaurally with headphones. Stimulus presentation occurred though the use of SuperLab 4.0 for Windows (Cedrus Corp., San Pedro, CA). Unlike during the VR treatment sessions, participants were not able to navigate in the VR scenes or tailor them to their own specific experiences. This was done in order for all participants to view the same stimuli with the same duration during assessment. The scenes that were used for assessment were those most commonly experienced by OIF and OEF soldiers. The psychophysiology assessment sessions consisted of three 2-min VR scenes (Roy et al., 2010) separated by presentation of a 30-sec blank blue screen (Blank Screen), see Fig. 1A. Two scenes were presented from the perspective of a soldier in either a gun turret (Turret) or Humvee cabin (Cabin) as their convoy encounters improvised explosive devices (IEDs), gunfire, and insurgent ambushes. The third scene (City) was shown from the perspective of a soldier on foot patrol walking through an Iraqi city marketplace who encounters explosions, civilians, and rocket propelled grenades. The blank screen and VR scenes were not counterbalanced, but alternated through the session. Startle probes were presented at times in the VR scenes when ambient noise was absent or minimal. Two startle probes were presented prior to the onset of VR scenes, and during each blank screen. Five startle probes were presented during each VR scenes, during periods of low level background sounds. A total of 25 startle probes was delivered. The time between startle probes was between 9 and 22 s as in our previous work (Norrholm et al., 2006).
Fig. 1.
Panel A. Schematic illustration of the virtual reality (VR) session presented to assess baseline and trauma-potentiated acoustic startle responses. Three 2-min VR video clips were presented with 30-sec blank blue screen images interleaved between the VR clips. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.3.2. Cortisol assays
Saliva for cortisol was collected using Salivette collection system. When possible, sampling occurred between 11:00a.m. and 2:00 PM at each visit. In four cases cortisol was collected between 9:30a.m. and 10:30a.m., and in four cases samples were collected between 3:00p.m. and 4:00p.m.. There were no difference in sampling times between groups, and collection time was not associated with cortisol levels (p > 0.1). Samples were collected at three points during each assessment visit. The first sample was collected immediately prior to viewing the Virtual Iraq scenes, immediately after the 15-min VR assessment, and 15 min after the end of the scenes. The samples were immediately transferred to a −80° freezer. Saliva cortisol was measured by a chemiluminescent immunoassay available on the Beckman Access analyzer. Prior to assay, the saliva was thawed and centrifuged. The assay was calibrated against the European GC/MS reference method. All samples were run in duplicate and three levels of quality control were processed in every assay run. The detection limit for the salivary assay was 0.1 nmol/L. The inter-assay and the intra-assay CVs were under 10%.
2.4. Data analysis
Startle response was measured as the peak EMG amplitude 20–200 ms following the onset of the acoustic startle probe. The ECG data were analyzed as average HR in beats per minute. Electrodermal activity analyses captured skin conductance levels. Startle, HR, and SC data were averaged across the three VR scenes and compared to Blank screens with a repeated measures analysis of variance (RM-ANOVA) with the factor of Stimulus type (VR vs. Blank screen) in order to see if exposure to VR combat scenes increased responding.
In order to test whether psychophysiological reactivity changed with treatment, we compared startle, HR, and SC at 3 time points (pre-, post-treatment and at 6 months) using a RM-ANOVA. In addition, the psychophysiological reactivity to VR scenes at pre-treatment was used as predictors of change in PTSD symptoms at post-treatment and at 6 months follow-up assessments. Change in PTSD symptoms was calculated by subtracting the CAPS scores at post-treatment and 6 months from the pre-treatment scores. Linear regression analyses were used to analyze whether EMG startle, HR, or SC, data during VR scenes predicted change scores within each treatment group (DCS, ALP, PBO).
Cortisol levels were analyzed using RM-ANOVA testing the effects of the VR scenes across time points. Within-subjects variables included VR sample (3 levels: baseline, post-VR, and 15 min post-VR) and Time (3 levels: pre-treatment, post-treatment, and 6 months follow-up). Stepwise linear regression analyses were performed to examine the relationship between cortisol change score and CAPS change after controlling for baseline levels of cortisol and initial CAPS score. Cortisol levels and change scores were Z-score transformed to normalize the distribution of the data. All analyses were performed using SPSS 20.0 for Windows. Alpha level was set at 0.05, and the Greenhouse-Geisser correction was used for repeated measures analyses.
3. Results
3.1. Psychophysiological reactivity
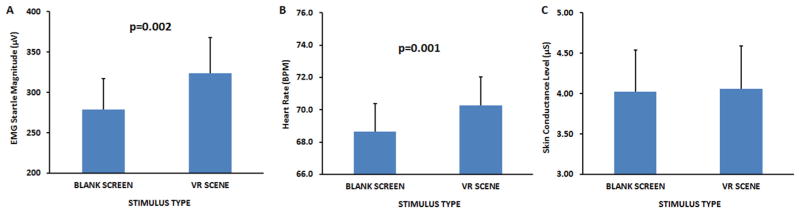
For pre-treatment testing, a RM-ANOVA of VR scenes compared to the neutral blue screen revealed a significant increase in startle response, F(1,49) = 10.64, p = 0.002 and HR, F(1,49) = 12.12, p = 0.001, but not SC, F(1,49) < 1 during VR. Fig. 2A and B shows increased responses to startle and HR, and Fig. 2C shows SC. However, none of the measures were correlated with CAPS or PSS scores at pre-treatment.
Fig. 2.
Acoustic startle, heart rate, and skin conductance responses (mean ± SEM) assessed prior to initiation of virtual reality exposure (VRE) therapy in the presence of d-cyloserine (DCS), alprazolam (ALP), or placebo (PBO). Acoustic startle (panel A) and heart rate (panel B), but not skin conductance (panel C), responses were elevated in the presence of the combat-related virtual reality clips as compared to blank blue screen. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Changes in reactivity across treatment conditions in this dataset have already been published elsewhere (Rothbaum et al., 2014); here we briefly show that these indices changed with treatment. Specifically, startle EMG responses to VR scenes decreased across time points, F(2,62) = 6.76, p = 0.006, with a significant linear association from pre-treatment to 6 months (p = 0.007). In addition there was a significant interaction of condition and time on startle responses, F(4,62) = 3.88, p < 0.05. Follow-up analyses within each treatment condition found that startle response during the VR scenes were reduced only in the DCS group, F(2,12) = 6.24, p = 0.01. Likewise, SC responses decreased after treatment, F(2,70) = 5.13, p = 0.01, with the quadratic pattern of association having higher significance (p = 0.01), in that SC responses did not continue to decrease from post-treatment to 6 months follow-up. In contrast, HR responses did not show any significant changes across treatment timepoints. The SC and HR measures did not show interaction effect of condition by time.
Next we examined whether startle, SC, or HR during VR scenes were predictive of the change in CAPS scores from pre to post-treatment using a linear regression within each treatment group. We examined both immediate CAPS change (pre-to post-treatment) and sustained CAPS change (pre-treatment to 6 month follow-up). In the DCS group, startle and HR showed trends in predicting immediate CAPS change (p = 0.08 and p = 0.06, respectively), but SC did not. When CAPS change was examined as percent change from pre-treatment baseline, startle response was significantly predictive of change (F(1,12) = 8.13, p < 0.05), but HR and SC were not. None of the variables predicted change in the ALP and PBO groups.
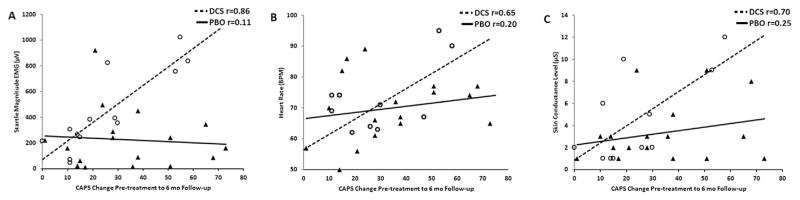
However, we found a stronger prediction when we examined the change in CAPS scores from pre-treatment to 6 months follow-up. In the DCS group, startle response to VR scenes prior to treatment accounted for 76% of the variance in CAPS change scores, (F(1,12) = 34.92, p < 0.001), in that higher responses predicted greater changes in symptom severity (i.e. better outcome). The same was true when CAPS was analyzed as percent change from pre-treatment to 6 months follow-up (F(1,12) = 18.80, p = 0.001. Neither regression was significant for either the ALP or PBO groups (both p’s > 0.1). Fig. 3A shows the CAPS change score at 6 months follow-up and the pre-treatment startle response for DCS and PBO groups. Higher HR and SC during VR scenes also predicted better 6 months change scores (R2 = 0.47, F(1,12) = 6.46, p = 0.03 and R2 = 0.50, F(1,12) = 9.82, p = 0.01, respectively; see Fig. 3B and C). The results were partially replicated using percent change in CAPS as the dependent variable (HR R2 = 0.37, p = 0.06 and SC R2 = 0.38, p < 0.05, respectively). As was the case with startle, the ALP and PBO groups did not show significant effects. The regression analysis in the DCS group remained significant after controlling for initial CAPS scores at pre-treatment (startle, R2 = 0.69, p < 0.001; HR, R2 = 0.43, p = 0.04; and SC, R2 = 0.51, p = 0.01).
Fig. 3.
Correlations between pre-treatment acoustic startle (panel A), heart rate (panel B), and skin conductance (panel C) responses and PTSD symptom change in the groups receiving DCS or PBO. Higher degrees of startle, heart rate, and skin conductance predicted better 6-month change scores in PTSD symptoms as measured by CAPS interview.
3.2. Cortisol levels
Cortisol data were available for 45 individuals at all 3 assessment visits (pre-treatment, post-treatment, and 6 month follow-up). As with the psychophysiological variables, cortisol reactivity to VR scenes changed across treatment time points (RM ANOVA Time × VR interaction, F(4,152) = 2.43, p = 0.05). The contrast term for VR samples indicated that the difference between cortisol collected at baseline and 15 min post-VR decreased from pre-to post-treatment (F(1,38) = 5.23, p < 0.05).
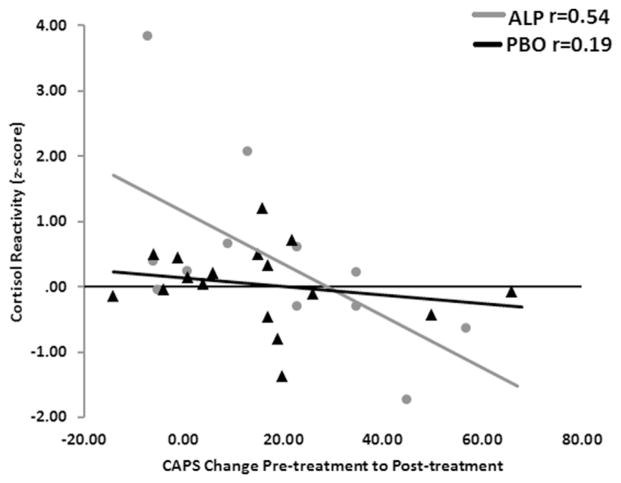
Next we examined whether cortisol levels prior to treatment predicted changes in PTSD symptoms immediately after treatment and at 6 months follow-up. Linear regression analyses of baseline cortisol levels revealed a trend association with treatment response, in that higher baseline cortisol predicted greater changes in CAPS scores immediately post treatment only in the ALP group (F(1,16) = 4.14, p = 0.06). The same trend was found using percent CAPS change (F(1,16) = 3.86, p = 0.07). Cortisol reactivity (the difference between cortisol 15 min after VR scenes and baseline cortisol) had an opposite prediction to treatment response, in that higher reactivity was significantly associated with worse outcome. In the ALP group, cortisol reactivity prior to treatment accounted for 27% of the variance in change in CAPS scores (F(1,16) = 5.56, p = 0.03, see Fig. 4), and in percent CAPS change (F(1,16) = 6.64, p = 0.02). This association remained significant after controlling for baseline cortisol levels (R2 = 36%, p = 0.04). In the DCS group, there was a trend for the same prediction, but the sample size was smaller (F(1,9) = 4.13, p = 0.08). Neither baseline nor reactive levels of cortisol predicted treatment response in the PBO group. In addition, sustained changes in CAPS scores (pre-treatment to 6 month follow-up), were not predicted by cortisol levels.
Fig. 4.
Correlations between pre-treatment cortisol reactivity to VR scenes and PTSD symptom change in the groups receiving DCS or ALP. In the ALP group, higher cortisol reactivity was associated with worse treatment outcome when comparing PTSD symptoms at the pre- and post-treatment timepoints.
4. Discussion
In the present study, startle responses to VR scenes prior to treatment accounted for 76% of the variance in PTSD symptom reduction from baseline to 6-month follow-up (as measured via change in CAPS score) in the group that received DCS just prior to VR exposure therapy. A prior publication reported that the patients receiving DCS displayed markedly greater changes in trauma-potentiated startle responses when assessed pre- and post-VR exposure treatment as compared to groups receiving ALP or PBO (Rothbaum et al., 2014). The present paper focuses on pre-treatment psychophysiological responses, including SC, HR in addition to startle EMG as predictors of clinical outcomes separately for each treatment condition. It has been suggested that increased engagement with exposure therapy can be predictive of better treatment outcome (Robison-Andrew et al., 2014). An objective measure of engagement may be one’s psychophysiological response in the presence of trauma-related cues. DCS has shown effectiveness as an adjunct treatment in concert with prolonged exposure (with some notable exceptions and experimental caveats cited in the literature, e.g., Rodrigues et al., 2014). It is important to note that the current study “under-dosed” the exposure therapy, inasmuch as only 5 VR exposure sessions were used. Therefore, we expected minimal reductions in fear in the placebo group as compared to the group that received DCS; effects similar to those observed by Ressler et al. (2004) who compared “under-dosed” VR exposure therapy with and without adjunct DCS. The results of the present study suggest that a higher level of engagement coupled with the cognitive enhancing properties of DCS may lead to more substantial improvements in patient symptom severity.
There is currently a significant void in the literature with respect to objective biomarkers that are predictive of PTSD treatment efficacy. The results of the current study suggest that the inclusion of psychophysiological assessments prior to and over the course of treatment and follow-up may prove to be an invaluable tool for monitoring therapeutic progress. Our findings represent one of the first demonstrations of the predictive value of the acoustic startle response with respect to PTSD treatment outcome. There have been several investigations showing strong associations between acoustic startle-based assessments and PTSD symptom severity (e.g., Jovanovic et al., 2009; Norrholm et al., 2011) as well as studies illustrating the potential predictive nature of acoustic startle response and PTSD vulnerability in the aftermath of a traumatic experience (Pole et al., 2009). We now extend these earlier findings to show the potential clinical utility of assessing baseline and trauma-potentiated startle over the course of treatment.
This study also showed that VR exposure therapy attenuated cortisol response to a trauma-relevant scene. Attenuation of the cortisol response with PTSD treatment was also observed by Vermetten et al. (2006); however, the current study expanded this finding to a placebo-controlled RCT, and demonstrated the effect of treatment after 6 weeks, rather than 12 months as in the previous study. The results suggest that neuroendocrine biomarkers may serve as objective measures of treatment response.
Finally, higher cortisol reactivity prior to starting treatment was predictive of poorer treatment outcome. It is possible that individual variability in HPA axis response increases treatment resistance. Such individual variability may be due to genotype. For example, FKBP5 risk polymorphisms are associated with increased glucocorticoid sensitivity and higher PTSD symptoms (Binder et al., 2008). Alternatively, since the cortisol change score was derived by subtracting sample values collected 15 min after the end of the VR scenes from the pre-VR sample values, it is possible the individual differences are due to slower recovery of the cortisol response and more glucocorticoid resistance. While a limitation of the current study, future studies should include a later sample collection time to examine whether cortisol recovery is predictive of treatment outcome. It is important to note that baseline cortisol levels were associated with treatment response in the opposite direction from cortisol reactivity, i.e. higher baseline was associated with better outcome, further indicating that GR sensitivity may be a unique predictor relative to baseline HPA measures.
In addition, other hormones (such as DHEA) or hormone metabolites (such as allopregnanolone) may be interacting with the cortisol response to contribute to treatment resistance (Rasmusson et al., 2006). Interestingly, treatment drug did not impact the association between cortisol response and treatment outcome, suggesting that the individual neuroendocrine risk factors may not be sensitive to DCS interventions. Thus the DCS effect may be specific to factors that determine how stressful the perception of the traumatic VR scenes is but not the glucocorticoid response to that perception.
Interestingly, cortisol and psychophysiological reactivity to trauma-related stimuli (i.e. VR scenes) differed in their predictive value in three important ways: 1) they had opposite predictions in terms of direction, 2) they predicted different post-treatment timepoints, and 3) they were predictive in different drug treatment groups.
With regard to the first point, higher cortisol reactivity predicted poorer outcome, wheareas greater psychophysiological reactivity predicted better outcome. Several studies have found that exposure therapy is more effective when patients are more engaged in the trauma memories (Jaycox, Foa, & Morral, 1998). Increased psychophysiological activity may be indicative of higher engagement with the stimuli and activation of the sympathetic nervous system (SNS). Given increases in the autonomic measures, SCL and HR, SNS activation seems highly likely. In addition, the startle reflex can be modulated by norepinephrine, which is part of the fight-or-flight SNS response to fear eliciting stimuli. On the other hand, cortisol reactivity is part of the HPA axis, and may be related to neuroendocrine coping mechanisms governed by negative feedback signaled by GR. It is possible that heightened GR sensitivity may be related to poor stress hormone regulation during traumatic stimulation rather than higher engagement with the VR environment.
The psychophysiological responses predicted better sustained treatment outcome at the 6 month follow-up visit in the DCS group, while cortisol reactivity data predicted immediate treatment outcome in the ALP group. DCS is known to enhance extinction (Davis et al., 2006; Vervliet, 2008). Therefore, higher early engagement may facilitate learning during VR exposure therapy with better long-term gains. On the other hand, higher cortisol reactivity to stressors may have overwhelmed the beneficial effects of engagement in VR stimuli, and that the addition of benzodiazepines provided enough “numbing” to allow for better engagement. In any case, these effects appear to be temporary, as they were not associated with lower PTSD symptoms 6 months after treatment. Importantly, the drugs were given ONLY during individual treatment sessions and the assessments were performed free of study drugs; therefore there was no direct contribution to the predictors.
Better understanding of the potential augmentation of cognitive behavioral strategies and the evaluation of biological predictors of treatment outcome will emerge as studies move forward in this area. The current results, taken together with several reports from the literature (for review see Foa, Gillihan, & Bryant, 2013), suggest that increased arousal (often referred to as engagement) during early exposure therapy can foster more positive treatment outcomes. There is some evidence that decreased arousal over the course of several weeks of treatment can increase outcome (Telch et al., 2014). Future examination of the time course, psychophysiological index, and time since trauma will enable clinical investigators to focus on the most promising biological markers for exposure based treatment measures (see discussion by Tuerk, 2014).
The current analyses were part of a treatment study and not a psychophysiological study per se. There is a rather large body of literature, including sample sizes of varies size and demographics that support a strong association between psychophysiological reactivity and PTSD (e.g., for meta analysis see Pole et al., 2009). There continues to be a compelling rationale to include physiological measures in behavioral treatment outcome studies. Unfortunately, these studies can be costly and time-consuming. However, these measures can be interwoven into clinical studies as in the current study. Further reasoning for doing so rests in the observations that positive treatment outcomes can be assessed by reductions in objectively measured arousal (Lindauer et al., 2008). Lastly, several translational studies using these methods have included sample sizes of in this range and have been appropriately powered to detect significant differences (e.g., Norrholm et al., 2014).
In summary, this study suggests that psychophysiological responses may serve as biomarkers that predict treatment outcome. Specifically, these measures are likely to capture the patients’ level of engagement with the exposure therapy, which in turn predicts better treatment response.
Acknowledgments
We thank Kemp Anderson, Cliffe Kwon, Taylor Warren, Alexander McCarthy, Ilana Olin, and Cole Youngner for their assistance in the performance of study procedures, analysis of study data, and in the preparation of this manuscript.
Footnotes
Disclosures and financial support
Dr. Rothbaum is a consultant to and owns equity in Virtually Better, Inc., which creates virtual environments; however, Virtually Better did not create the Virtual Iraq environment tested in this study; the terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict of interest policies. Dr. Rothbaum also has funding from Department of Defense Clinical Trial Grant W81XWH-10-1-1045 (“Enhancing Exposure Therapy for PTSD: Virtual Reality and Imaginal Exposure With a Cognitive Enhancer”), from NIMH grant U19 MH-069056 (“The Emory-MSSM-GSK-NIMH Collaborative Mood and Anxiety Disorders Initiative”), from NIMH grant R01 MH-70880 (“A Cognitive Enhancer May Facilitate Behavioral Exposure Therapy”), from NIMH grant R01 MH-094757 (“Prospective Determination of Psychobiological Risk Factors for Posttraumatic Stress”), from a Brain and Behavior Research Foundation (NARSAD) Distinguished Investigator Grant (“Optimal Dose of Early Intervention to Prevent PTSD”), and from the McCormick Foundation (“BraveHeart: MLB’s Welcome Back Veterans Southeast Initiative”); she has received previous support from Transcept Pharmaceuticals (“A Multi-Center, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study to Evaluate the Efficacy and Safety of Low-Dose Ondansetron for Adjunctive Therapy in Adult Patients With Obsessive-Compulsive Disorder Who Have Not Adequately Responded to Treatment With a Serotonin Reuptake Inhibitor”); she receives royalties from Oxford University Press, Guilford, American Psychiatric Publishing, and Emory University; and she received one advisory board payment from Genentech. Drs. Ressler and Davis are founding members of Extinction Pharmaceuticals/Therapade Technologies, which seek to develop D-cycloserine and other compounds for use to augment the effectiveness of psychotherapy; they have received no equity or income from this relationship within the last 3 years; the terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict of interest policies. Dr. Duncan has received research support from the Posit Science Corporation and grant support from NIMH and the National Institute on Drug Abuse. The remaining authors report no financial relationships with commercial interests.
Clinicaltrials.gov identifier: NCT00356278.
VA Acknowledgments: This material is the result of work supported with resources and the use of facilities at the Atlanta VA Medical Center, Decatur, GA. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. The following authors are employed by the Atlanta VAMC (Decatur, GA): Dr. Seth D. Norrholm (Program Analyst, Mental Health Service Line), Dr. Bekh Bradley (Chief of the Mental Health Service Line), and Dr. Erica Duncan (Staff Psychiatrist).
References
- Binder EB, Bradley RG, Liu W, Epstein M, Deveau T, Mercer KB, … Ressler KJ. Association of FKBP5 polymorphisms and child abuse with risk of posttraumatic stress disorder symptoms in adults. Journal of the American Medical Association. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Kolb LC, Gerardi RJ, Ryan DH, Pallmeyer TP. Cardiac response to relevant stimuli as an adjunctive tool for diagnosing post-traumatic stress disorder in Vietnam veterans. Behavior Therapy. 1986;17:592–606. [Google Scholar]
- Blanchard EB, Kolb LC, Pallmeyer TP, Gerardi RJ. A psychophysiological study of post-traumatic stress disorder in Vietnam veterans. Psychiatric Quarterly. 1982;54:220–229. doi: 10.1007/BF01064817. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A. Committee report: guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. The American Journal of Psychiatry. 2005;162(2):214–227. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Kaloupek DG. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosomatic Medicine. 2001;63(4):585–594. doi: 10.1097/00006842-200107000-00011. [DOI] [PubMed] [Google Scholar]
- Costanzo ME, Leaman S, Jovanovic T, Norrholm SD, Rizzo AA, Taylor P, et al. Psychophysiological response to virtual reality and sub-threshold PTSD symptoms in recently deployed military. Psychosomatic Medicine. 2014;76(9):670–677. doi: 10.1097/PSY.0000000000000109. [DOI] [PubMed] [Google Scholar]
- Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B. Maximizing exposure therapy: an inhibitory learning approach. Behaviour Research and Therapy. 2014;58:10–23. doi: 10.1016/j.brat.2014.04.006. http://dx.doi.org/10.1016/j.brat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Myers KM. The role of glutamate and gamma-aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biological Psychiatry. 2002;52(10):998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biological Psychiatry. 2006;60(4):369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Delamater AR, Westbrook RF. Psychological and neural mechanisms of experimental extinction: a selective review. Neurobiology of Learning and Memory. 2014;108:38–51. doi: 10.1016/j.nlm.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diemer J, Muhlberger A, Pauli P, Zwanzger P. Virtual reality exposure in anxiety disorders: impact on psychophysiological reactivity. The World Journal of Biological Psychiatry. 2014;15(6):427–442. doi: 10.3109/15622975.2014.892632. [DOI] [PubMed] [Google Scholar]
- Difede J, Cukor J, Jayasinghe N, Patt I, Jedel S, Spielman L, Hoffman HG. Virtual reality exposure therapy for the treatment of posttraumatic stress disorder following September 11, 2001. The Journal of Clinical Psychiatry. 2007;68(11):1639–1647. [PubMed] [Google Scholar]
- Dravid SM, Burger PB, Prakash A, Geballe MT, Yadav R, Le P, … Traynelis SF. Structural determinants of D-cycloserine efficacy at the NR1/NR2C NMDA receptors. The Journal of Neuroscience. 2010;30(7):2741–2754. doi: 10.1523/JNEUROSCI.5390-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinand NK, Mecklinger A, Opitz B. Learning context modulates the processing of expectancy violations. Brain Research. 2015;1629:72–84. doi: 10.1016/j.brainres.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Foa EB. Prolonged exposure therapy: past, present, and future. Depress Anxiety. 2011;28(12):1043–1047. doi: 10.1002/da.20907. [DOI] [PubMed] [Google Scholar]
- Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychological Science Public Interest. 2013;14(2):65–111. doi: 10.1177/1529100612468841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Hembree E, Rothbaum BO. Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences, therapist guide. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychological Bulletin. 1986;99(1):20–35. [PubMed] [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing posttraumatic stress disorder. Journal of Traumatic Stress. 1993;6(4):459–473. [Google Scholar]
- Foa EB, Riggs DS, Massie ED, Yarczower M. The impact of fear activation and anger on the efficacy of exposure treatment for posttraumatic stress disorder. Behaviour Therapy. 1995;26(3):487–499. [Google Scholar]
- Griffin MG, Resick PA, Galovski TE. Does physiologic response to loud tones change following cognitive-behavioral treatment for posttraumatic stress disorder? Journal of Traumatic Stress. 2012;25(1):25–32. doi: 10.1002/jts.21667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of d-cycloserine on extinction and fear conditioning in humans. Behaviour Research and Therapy. 2007;45(4):663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, … Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association. 2000;284(5):592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JAJ, Simon NM, Pollack MH, Eisenmenger K, … Otto MW. Augmentation of exposure therapy with d-cycloserine for social anxiety disorder. Archives of General Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA, Rosenfield D, Simon N, Otto MW, Meuret AE, … Pollack MH. D-Cycloserine as an augmentation strategy with cognitive-behavioral therapy for social anxiety disorder. The American Journal of Psychiatry. 2013;170(7):751–758. doi: 10.1176/appi.ajp.2013.12070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Treatment of PTSD: An assessment of the evidence. Washington, D.C: 2007. [Google Scholar]
- Jaycox LH, Foa EB, Morral AR. Influence of emotional engagement and habituation on exposure therapy for PTSD. Journal of Consulting Clinical Psychology. 1998;66(1):185–192. doi: 10.1037//0022-006x.66.1.185. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos A, Myers KM, … Duncan EJ. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Research. 2009;167(1–2):151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, … Foa EB. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biological Psychiatry. 2007;62(8):835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behavioral Neuroscience. 2003;117(2):341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behavioral Neuroscience. 2004;118(3):505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biological Psychiatry. 2005;57(8):841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Booij J, Habraken JB, van Meijel EP, Uylings HB, Olff M, … Gersons BP. Effects of psychotherapy on regional cerebral blood flow during trauma imagery in patients with post-traumatic stress disorder: a randomized clinical trial. Psychological Medicine. 2008;38(4):543–554. doi: 10.1017/S0033291707001432. [DOI] [PubMed] [Google Scholar]
- Lund BC, Bernardy NC, Vaughan-Sarrazin M, Alexander B, Friedman MJ. Patient and facility characteristics associated with benzodiazepine prescribing for veterans with PTSD. Psychiatric Service. 2013;64(2):149–155. doi: 10.1176/appi.ps.201200267. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. Overtraining, reversal, and extinction in rats and chicks. Journal of Comparative and Physiological Psychology. 1965;59:31–36. doi: 10.1037/h0021620. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. Blocking of conditioned suppression: role of the first compound trial. Journal of Experimental Psychology: Animal Behavior Process. 1975;1(4):335–345. doi: 10.1037//0097-7403.1.4.335. [DOI] [PubMed] [Google Scholar]
- Management of posttraumatic stress working group. VA/DoD clinical practice guideline for management of post-traumatic stress. Washington, DC: 2010. [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM. Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biological Psychiatry. 2010;67(4):346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, De Vries GJ, Gersons BPR, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. The British Journal of Psychiatry. 2007;191(5):387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- van Minnen A, Arntz A, Keijsers GP. Prolonged exposure in patients with chronic PTSD: predictors of treatment outcome and dropout. Behaviour and Research Therapy. 2002;40(4):439–457. doi: 10.1016/s0005-7967(01)00024-9. [DOI] [PubMed] [Google Scholar]
- van Minnen A, Harned MS, Zoellner L, Mills K. Examining potential contraindications for prolonged exposure therapy for PTSD. European Journal of Psychotraumatology. 2012:3. doi: 10.3402/ejpt.v3i0.18805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Glover EM, Stevens JS, Fani N, Galatzer-Levy IR, Bradley B, … Jovanovic T. Fear load: the psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. International Journal of Psychophysiology. 2014;98(2 Pt 2):270–275. doi: 10.1016/j.ijpsycho.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biological Psychiatry. 2011;69(6):556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, et al. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learning and Memory. 2006;13(6):681–685. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Macklin ML, Pineles SL, Chang Y, Pitman RK. Predicting post-trauma stress symptoms from pre-trauma psychophysiologic reactivity, personality traits and measures of psychopathology. Biology Mood Anxiety Disorder. 2012;2(1):8. doi: 10.1186/2045-5380-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Pitman RK. Psychophysiology of post-traumatic stress disorder. Psychiatric Clinics of North America. 2002;25(2):271–293. doi: 10.1016/s0193-953x(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, … Pollack MH. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biological Psychiatry. 2010;67(4):365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian conditioning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87(6):532–552. [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, Marmar CR. Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biological Psychiatry. 2009;65(3):235–240. doi: 10.1016/j.biopsych.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M, Maples JL, Jovanovic T, Norrholm SD, Heekin M, Rothbaum BO. An investigation of outcome expectancies as a predictor of treatment response for combat veterans with ptsd: comparison of clinician, self-report, and biological measures. Depress Anxiety. 2015;32(6):392–399. doi: 10.1002/da.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M, Mehta N, Tone EB, Anderson PL. Does engagement with exposure yield better outcomes? Components of presence as a predictor of treatment response for virtual reality exposure therapy for social phobia. Journal of Anxiety Disorder. 2011;25(6):763–770. doi: 10.1016/j.janxdis.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe S, Dorfel D, Zollner T, Maercker A, Karl A. Cardiovascular correlates of motor vehicle accident related posttraumatic stress disorder and its successful treatment. Applied Psychophysiology and Biofeedback. 2006;31(4):315–330. doi: 10.1007/s10484-006-9027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, … Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biological Psychiatry. 2006;60(7):704–713. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Rauch SA, King AP, Abelson J, Tuerk PW, Smith E, Rothbaum BO, … Liberzon I. Biological and symptom changes in posttraumatic stress disorder treatment: a randomized clinical trial. Depress Anxiety. 2015;32(3):204–212. doi: 10.1002/da.22331. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, … Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Archives of General Psychiatry. 2004;61(11):1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Davis JL, Williams AE, McCabe KM, Bartley EJ, Byrd PM, et al. Cognitive-behavioral treatment for chronic nightmares in trauma-exposed persons: assessing physiological reactions to nightmare-related fear. Journal of Clinical Psychology. 2010;66(4):365–382. doi: 10.1002/jclp.20656. [DOI] [PubMed] [Google Scholar]
- Richardson R, Ledgerwood L, Cranney J. Facilitation of fear extinction by D-cycloserine: theoretical and clinical implications. Learning and Memory. 2004;11(5):510–516. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- Rizzo AS, Difede J, Rothbaum BO, Reger G, Spitalnick J, Cukor J, et al. Development and early evaluation of the Virtual Iraq/Afghanistan exposure therapy system for combat-related PTSD. Annals of the New York Academy of Sciences. 2010;1208:114–125. doi: 10.1111/j.1749-6632.2010.05755.x. [DOI] [PubMed] [Google Scholar]
- Robison-Andrew EJ, Duval ER, Nelson CB, Echiverri-Cohen A, Giardino N, Defever A, … Rauch SA. Changes in trauma-potentiated startle with treatment of posttraumatic stress disorder in combat Veterans. Journal of Anxiety Disorder. 2014;28(4):358–362. doi: 10.1016/j.janxdis.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Rodrigues H, Figueira I, Lopes A, Goncalves R, Mendlowicz MV, Coutinho ES, et al. Does d-cycloserine enhance exposure therapy for anxiety disorders in humans? A meta-analysis. PLoS One. 2014;9(7):e93519. doi: 10.1371/journal.pone.0093519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Annals of the New York Academy of Sciences. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Hodges LF, Ready D, Graap K, Alarcon RD. Virtual reality exposure therapy for Vietnam veterans with posttraumatic stress disorder. The Journal of Clinical Psychiatry. 2001;62(8):617–622. doi: 10.4088/jcp.v62n0808. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, … Ressler KJ. A randomized, double-blind evaluation of d-Cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan war veterans. The American Journal of Psychiatry. 2014;171(6):640–648. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MJ, Costanzo ME, Jovanovic T, Leaman S, Taylor P, Norrholm SD, et al. Heart rate response to fear conditioning and virtual reality in sub-threshold PTSD. Studies in Health Technology and Informatics. 2013;191:115–119. [PubMed] [Google Scholar]
- Roy MJ, Francis J, Friedlander J, Banks-Williams L, Lande R, Taylor P, et al. Improvement of cerebral function with treatment of posttraumatic stress disorder. Annals of the New York Academy of Sciences. 2010;1208:142–149. doi: 10.1111/j.1749-6632.2010.05689.x. [DOI] [PubMed] [Google Scholar]
- Shalev A, Peri T, Brandes D, Freedman S, Orr S, Pitman R. Auditory startle response in trauma survivors with posttraumatic stress disorder: a prospective study. American Journal of Psychiatry. 2000;157(2):255–261. doi: 10.1176/appi.ajp.157.2.255. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry. 1998;1959:22–33. [PubMed] [Google Scholar]
- Sherman JJ. Effects of psychotherapeutic treatments for PTSD: a meta-analysis of controlled clinical trials. Journal of Traumatic Stress. 1998;11(3):413–435. doi: 10.1023/A:1024444410595. [DOI] [PubMed] [Google Scholar]
- Steenkamp MM, Litz BT. Psychotherapy for military-related post-traumatic stress disorder: review of the evidence. Clinical Psychology Review. 2013;33:45–53. doi: 10.1016/j.cpr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Telch MJ, Bruchey AK, Rosenfield D, Cobb AR, Smits J, Pahl S, et al. Effects of post-session administration of methylene blue on fear extinction and contextual memory in adults with claustrophobia. The American Journal of Psychiatry. 2014;171(10):1091–1098. doi: 10.1176/appi.ajp.2014.13101407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk PW. Starting from something: augmenting exposure therapy and methods of inquiry. The American Journal of Psychiatry. 2014;171(10):1034–1037. doi: 10.1176/appi.ajp.2014.14070880. [DOI] [PubMed] [Google Scholar]
- Vachon F, Hughes RW, Jones DM. Broken expectations: violation of expectancies, not novelty, captures auditory attention. Journal of Experimental Psychology Learning, Memory, Cognition. 2012;38(1):164–177. doi: 10.1037/a0025054. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Schmahl C, De Kloet C, Southwick SM, Charney DS, et al. Alterations in stress reactivity after long-term treatment with paroxetine in women with posttraumatic stress disorder. Annals of the New York Academy of Sciences. 2006;1071(1):184–202. doi: 10.1196/annals.1364.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet B. Learning and memory in conditioned fear extinction: effects of D-cycloserine. Acta Psychologica (Amst) 2008;127(3):601–613. doi: 10.1016/j.actpsy.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. Journal of Neuroscience. 2002;22(6):2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, … Rauch SL. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. The American Journal of Psychiatry. 2008;165(3):335–341. doi: 10.1176/appi.ajp.2007.07050776. quiz 409. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Annals of the New York Academy of Sciences. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. (Glucocorticoids and Mood Clinical Manifestations, Risk Factors, and Molecular Mechanisms) [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Sarapas C, Makotkine I, Andrew R, Seckl JR. Cortisol metabolic predictors of response to psychotherapy for symptoms of PTSD in survivors of the World Trade Center attacks on September 11, 2001. Psychoneuroendocrinology. 2009;34(9):1304–1313. doi: 10.1016/j.psyneuen.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]