Abstract
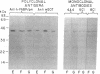
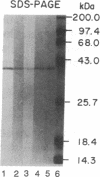
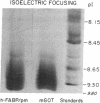
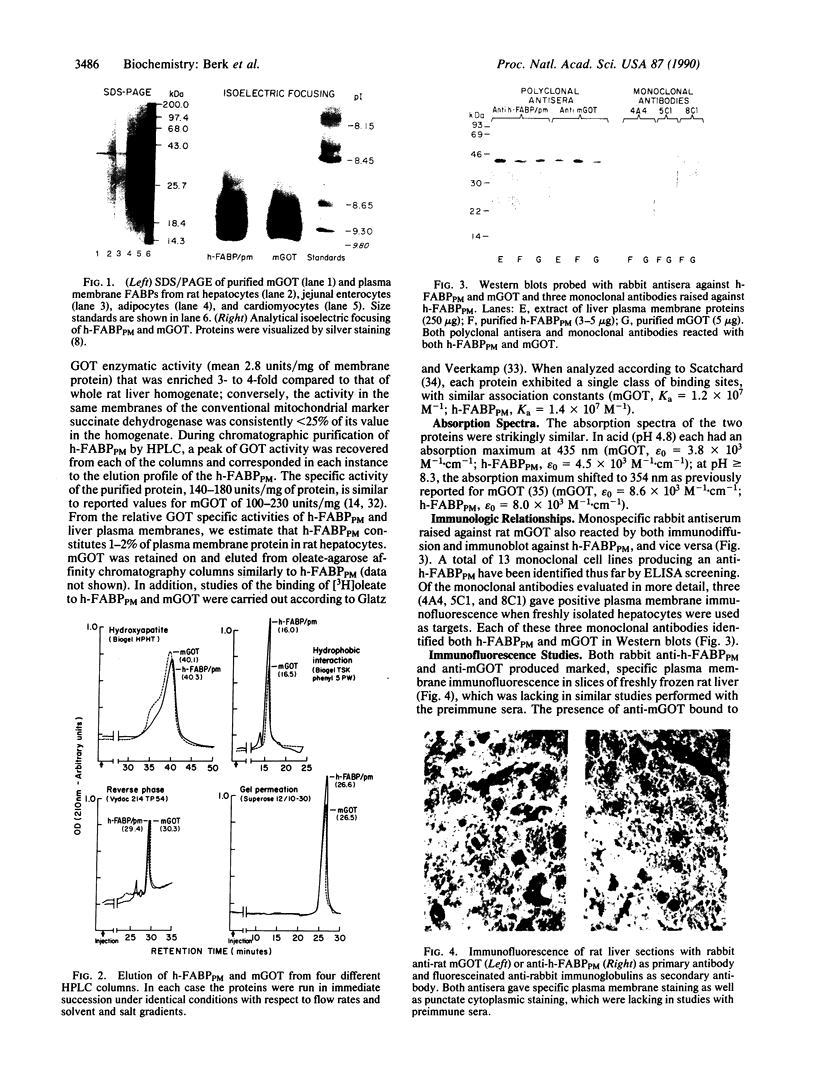
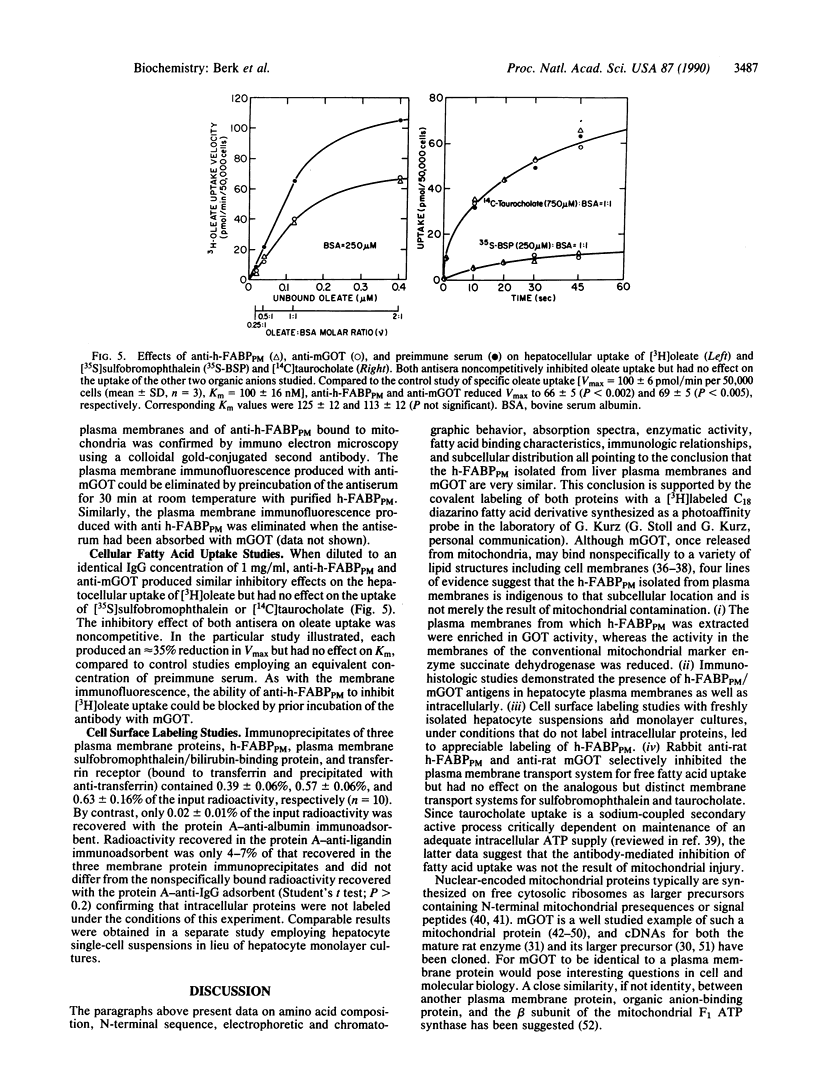
The hepatic plasma membrane fatty acid-binding protein (h-FABPPM) and the mitochondrial isoenzyme of glutamic-oxaloacetic transaminase (mGOT) of rat liver have similar amino acid compositions and identical amino acid sequences for residues 3-24. Both proteins migrate with an apparent molecular mass of 43 kDa on SDS/polyacrylamide gel electrophoresis, have a similar pattern of basic charge isomers on isoelectric focusing, are eluted similarly from four different high-performance liquid chromatographic columns, have absorption maxima at 435 nm under acid conditions and 354 nm at pH 8.3, and bind oleate with a Ka approximately 1.2-1.4 x 10(7) M-1. Sinusoidally enriched liver plasma membranes and purified h-FABPPM have GOT enzymatic activity; the relative specific activities (units/mg) of the membranes and purified protein suggest that h-FABPPM constitutes 1-2% of plasma membrane protein in the rat hepatocyte. Monospecific rabbit antiserum against h-FABPPM reacts on Western blotting with mGOT, and vice versa. Antisera against both proteins produce plasma membrane immunofluorescence in rat hepatocytes and selectively inhibit the hepatocellular uptake of [3H]oleate but not that of [35S]sulfobromophthalein or [14C]taurocholate. The inhibition of oleate uptake produced by anti-h-FABPPM can be eliminated by preincubation of the antiserum with mGOT; similarly, the plasma membrane immunofluorescence produced by either antiserum can be eliminated by preincubation with the other antigen. These data suggest that h-FABPPM and mGOT are closely related.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki N., Gerber M. A., Thung S. N., Chen M. L., Christman J. K., Price P. M., Flordellis C. S., Acs G. Ultrastructural studies of fibroblasts transfected with hepatitis B virus DNA. Hepatology. 1984 Jan-Feb;4(1):84–89. doi: 10.1002/hep.1840040115. [DOI] [PubMed] [Google Scholar]
- BOYD J. W. The intracellular distribution, latency and electrophoretic mobility of L-glutamate-oxaloacetate transaminase from rat liver. Biochem J. 1961 Nov;81:434–441. doi: 10.1042/bj0810434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass N. M. Function and regulation of hepatic and intestinal fatty acid binding proteins. Chem Phys Lipids. 1985 Aug 30;38(1-2):95–114. doi: 10.1016/0009-3084(85)90060-x. [DOI] [PubMed] [Google Scholar]
- Behra R., Christen P. In vitro import into mitochondria of the precursor of mitochondrial aspartate aminotransferase. J Biol Chem. 1986 Jan 5;261(1):257–263. [PubMed] [Google Scholar]
- Beloqui O., Nunes R. M., Blades B., Berk P. D., Potter B. J. Depression of iron uptake from transferrin by isolated hepatocytes in the presence of ethanol is a pH-dependent consequence of ethanol metabolism. Alcohol Clin Exp Res. 1986 Aug;10(4):463–470. doi: 10.1111/j.1530-0277.1986.tb05125.x. [DOI] [PubMed] [Google Scholar]
- Crémel G., Filliol D., Waksman A. Simultaneous purification by affinity chromatography of rat liver mitochondrial aspartate aminotransferase and malate dehydrogenase and electrophoretic properties. Anal Biochem. 1985 Nov 1;150(2):332–336. doi: 10.1016/0003-2697(85)90519-6. [DOI] [PubMed] [Google Scholar]
- Fisher M. M., Bloxam D. L., Oda M., Phillips M. J., Yousef I. M. Characterization of rat liver cell plasma membranes. Proc Soc Exp Biol Med. 1975 Oct;150(1):177–184. doi: 10.3181/00379727-150-38998. [DOI] [PubMed] [Google Scholar]
- Furuya E., Yoshida Y., Tagawa K. Interaction of mitochondrial aspartate aminotransferase with negatively charged lecithin liposomes. J Biochem. 1979 May;85(5):1157–1163. [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Glatz J. F., Veerkamp J. H. A radiochemical procedure for the assay of fatty acid binding by proteins. Anal Biochem. 1983 Jul 1;132(1):89–95. doi: 10.1016/0003-2697(83)90429-3. [DOI] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Horio Y., Sakakibara R., Tanaka T., Taketoshi M., Obaru K., Shimada K., Morino Y., Wada H. Molecular cloning of rat mitochondrial glutamic oxaloacetic transaminase mRNA and regulation of its expression in regenerating liver. Biochem Biophys Res Commun. 1986 Jan 29;134(2):803–811. doi: 10.1016/s0006-291x(86)80492-2. [DOI] [PubMed] [Google Scholar]
- Huynh Q. K., Sakakibara R., Watanabe T., Wada H. Glutamic oxaloacetic transaminase isozymes from rat liver. Purification and physicochemical characterization. J Biochem. 1980 Jul;88(1):231–239. [PubMed] [Google Scholar]
- Huynh Q. K., Sakakibara R., Watanabe T., Wada M. Primary structure of mitochondrial glutamic oxaloacetic transaminase from rat liver : comparison with that of the pig heart isozyme. Biochem Biophys Res Commun. 1980 Nov 28;97(2):474–479. doi: 10.1016/0006-291x(80)90287-9. [DOI] [PubMed] [Google Scholar]
- Jaussi R., Behra R., Giannattasio S., Flura T., Christen P. Expression of cDNAs encoding the precursor and the mature form of chicken mitochondrial aspartate aminotransferase in Escherichia coli. J Biol Chem. 1987 Sep 15;262(26):12434–12437. [PubMed] [Google Scholar]
- Jaussi R., Cotton B., Juretić N., Christen P., Schümperli D. The primary structure of the precursor of chicken mitochondrial aspartate aminotransferase. Cloning and sequence analysis of cDNA. J Biol Chem. 1985 Dec 25;260(30):16060–16063. [PubMed] [Google Scholar]
- Joh T., Nomiyama H., Maeda S., Shimada K., Morino Y. Cloning and sequence analysis of a cDNA encoding porcine mitochondrial aspartate aminotransferase precursor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6065–6069. doi: 10.1073/pnas.82.18.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATUNUMA N., MATSUZAWA T., HUZINO A. Differences between the transaminases in mitochondria and soluble fraction. II. Glutamic-oxaloacetic transaminase. J Vitaminol (Kyoto) 1962 Mar 10;8:74–79. doi: 10.5925/jnsv1954.8.74. [DOI] [PubMed] [Google Scholar]
- Marra E., Doonan S., Saccone C., Quagliariello E. Studies of the selective permeation of radioactively labelled aspartate aminotransferase isozymes into mitochondria in vitro. Eur J Biochem. 1978 Feb;83(2):427–435. doi: 10.1111/j.1432-1033.1978.tb12109.x. [DOI] [PubMed] [Google Scholar]
- Mattingly J. R., Jr, Rodriguez-Berrocal F. J., Gordon J., Iriarte A., Martinez-Carrion M. Molecular cloning and in vivo expression of a precursor to rat mitochondrial aspartate aminotransferase. Biochem Biophys Res Commun. 1987 Dec 31;149(3):859–865. doi: 10.1016/0006-291x(87)90487-6. [DOI] [PubMed] [Google Scholar]
- Obaru K., Nomiyama H., Shimada K., Nagashima F., Morino Y. Cloning and sequence analysis of mRNA for mouse aspartate aminotransferase isoenzymes. J Biol Chem. 1986 Dec 25;261(36):16976–16983. [PubMed] [Google Scholar]
- Okuda H., Nunes R., Vallabhajosula S., Strashun A., Goldsmith S. J., Berk P. D. Studies of the hepatocellular uptake of the hepatobiliary scintiscanning agent 99mTc-DISIDA. J Hepatol. 1986;3(2):251–259. doi: 10.1016/s0168-8278(86)80035-6. [DOI] [PubMed] [Google Scholar]
- Pavé-Preux M., Ferry N., Bouguet J., Hanoune J., Barouki R. Nucleotide sequence and glucocorticoid regulation of the mRNAs for the isoenzymes of rat aspartate aminotransferase. J Biol Chem. 1988 Nov 25;263(33):17459–17466. [PubMed] [Google Scholar]
- Potter B. J., Stump D., Schwieterman W., Sorrentino D., Jacobs L. N., Kiang C. L., Rand J. H., Berk P. D. Isolation and partial characterization of plasma membrane fatty acid binding proteins from myocardium and adipose tissue and their relationship to analogous proteins in liver and gut. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1370–1376. doi: 10.1016/s0006-291x(87)80283-8. [DOI] [PubMed] [Google Scholar]
- Reichen J., Blitzer B. L., Berk P. D. Binding of unconjugated and conjugated sulfobromophthalein to rat liver plasma membrane fractions in vitro. Biochim Biophys Acta. 1981 Jan 8;640(1):298–312. doi: 10.1016/0005-2736(81)90554-x. [DOI] [PubMed] [Google Scholar]
- Roise D., Schatz G. Mitochondrial presequences. J Biol Chem. 1988 Apr 5;263(10):4509–4511. [PubMed] [Google Scholar]
- Sakakibara R., Huynh Q. K., Nishida Y., Watanabe T., Wada H. In vitro synthesis of glutamic oxaloacetic transaminase isozymes of rat liver. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1781–1788. doi: 10.1016/s0006-291x(80)80105-7. [DOI] [PubMed] [Google Scholar]
- Sakakibara R., Kamisaki Y., Wada H. Import of a putative precursor of rat liver mitochondrial glutamic oxaloacetic transaminase into mitochondria. Biochem Biophys Res Commun. 1981 Sep 16;102(1):235–242. doi: 10.1016/0006-291x(81)91512-6. [DOI] [PubMed] [Google Scholar]
- Sannia G., Abrescia P., Colombo M., Giardina P., Marino G. In vitro synthesis of precursor forms of pig heart aspartate aminotransferase isozymes. Biochem Biophys Res Commun. 1982 Mar 30;105(2):444–449. doi: 10.1016/0006-291x(82)91454-1. [DOI] [PubMed] [Google Scholar]
- Schwieterman W., Sorrentino D., Potter B. J., Rand J., Kiang C. L., Stump D., Berk P. D. Uptake of oleate by isolated rat adipocytes is mediated by a 40-kDa plasma membrane fatty acid binding protein closely related to that in liver and gut. Proc Natl Acad Sci U S A. 1988 Jan;85(2):359–363. doi: 10.1073/pnas.85.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger P., Jaussi R., Christen P. Cell-free synthesis of a putative precursor of mitochondrial aspartate aminotransferase with higher molecular weight. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1256–1260. doi: 10.1016/0006-291x(80)90554-9. [DOI] [PubMed] [Google Scholar]
- Sorrentino D., Stump D., Potter B. J., Robinson R. B., White R., Kiang C. L., Berk P. D. Oleate uptake by cardiac myocytes is carrier mediated and involves a 40-kD plasma membrane fatty acid binding protein similar to that in liver, adipose tissue, and gut. J Clin Invest. 1988 Sep;82(3):928–935. doi: 10.1172/JCI113700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Berk P. D. Hepatocellular influx of [14C]oleate reflects membrane transport rather than intracellular metabolism or binding. Proc Natl Acad Sci U S A. 1986 May;83(10):3086–3090. doi: 10.1073/pnas.83.10.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W. Fatty acid uptake by isolated rat heart myocytes represents a carrier-mediated transport process. J Clin Invest. 1988 Mar;81(3):844–852. doi: 10.1172/JCI113393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Gerber M. A., Glezerov V., Thung S. N., Kochwa S., Berk P. D. Physicochemical and immunohistological studies of a sulfobromophthalein- and bilirubin-binding protein from rat liver plasma membranes. J Clin Invest. 1983 Jun;71(6):1796–1805. doi: 10.1172/JCI110935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Lotz G., Strohmeyer G., Berk P. D. Identification, isolation, and partial characterization of a fatty acid binding protein from rat jejunal microvillous membranes. J Clin Invest. 1985 Mar;75(3):1068–1076. doi: 10.1172/JCI111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Berk P. D. Hepatocellular uptake of oleate is energy dependent, sodium linked, and inhibited by an antibody to a hepatocyte plasma membrane fatty acid binding protein. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3584–3588. doi: 10.1073/pnas.83.11.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Borchard F., Kochwa S., Berk P. D. Isolation and partial characterization of a fatty acid binding protein in rat liver plasma membranes. Proc Natl Acad Sci U S A. 1985 Jan;82(1):4–8. doi: 10.1073/pnas.82.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thung S. N., Gerber M. A., Kasambalides E. J., Gilja B. K., Keh W., Gerlich W. H. Demonstration of pre-S polypeptides of hepatitis B virus in infected livers. Hepatology. 1986 Nov-Dec;6(6):1315–1318. doi: 10.1002/hep.1840060615. [DOI] [PubMed] [Google Scholar]
- Watts C. Rapid endocytosis of the transferrin receptor in the absence of bound transferrin. J Cell Biol. 1985 Feb;100(2):633–637. doi: 10.1083/jcb.100.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R. Mitochondrial function in the heart. Annu Rev Physiol. 1979;41:485–506. doi: 10.1146/annurev.ph.41.030179.002413. [DOI] [PubMed] [Google Scholar]