Abstract
Though cardioprotective, niacin monotherapy is limited by unpleasant cutaneous symptoms mimicking dermatitis: niacin-associated skin toxicity (NASTy). Niacin is prototypical of several emerging drugs suffering off-target rubefacient properties whereby agonizing the GPR109A receptor on cutaneous immune cells provokes vasodilation, prompting skin plethora and rubor, as well as dolor, tumor, and calor, and systemically, heat loss, frigor, chills, and rigors. Typically, NASTy effects are described by subjective patient-reported perception, at best semi-quantitative and bias-prone. Conversely, objective, quantitative, and unbiased methods measuring NASTy stigmata would facilitate research to abolish them, motivating development of several objective methods. In early drug development, such methods might better predict clinical tolerability in larger clinical trials. Measuring cutaneous stigmata may also aid investigations of vasospastic, ischemic, and inflammatory skin conditions. We present methods to measure NASTy physical stigmata to facilitate research into novel niacin mimetics/analogs, detailing characteristics of each technique following niacin, and how NASTy stigmata relate to symptom perception. We gave niacin orally and measured rubor by colorimetry and white-light spectroscopy, plethora by laser Doppler flowmetry, and calor/frigor by thermometry. Surprisingly, each stigma’s abruptness predicted symptom perception, whereas peak intensity did not. These methods are adaptable to study other rubefacient drugs or dermatologic and vascular disorders.
Keywords: hypolipidemic drugs, dyslipidemia, quantitation, flushing, white-light spectroscopy, laser Doppler flowmetry, niacin-associated skin toxicity
INTRODUCTION
Niacin lowers LDL-cholesterol and triglycerides, raises HDL-cholesterol (1–4), and 1 g thrice daily reduces morbidity from coronary heart disease (CHD) and mortality (5–7). Unfortunately, cutaneous toxicity limits tolerability. Cutaneous vasodilation causes the most visible manifestations, plethora and rubor. However, these may not be the most irritating symptoms. Importantly, several other, perhaps more noxious, symptoms present around the same time: skin burning, tingling, itching, warmth, and edema. Congruent with the skin’s role as a major thermoregulatory organ, vasodilatation-induced mobilization of core blood to the skin promotes heat loss to the environment, often followed by systemic symptoms, such as chills and rigors. Some symptoms correspond to cutaneous physical stigmata and easily lend themselves to objective measurement: plethora (hyperemia, i.e., increased blood flow), rubor (gross redness of the skin), calor (warmth), and tumor (edema). Massive heat loss presages an important systemic physical change to a vital sign: frigor praeter naturam, body temperature colder than normal (henceforth frigor). This cutaneous symptom complex is simplistically referred to as “flushing” (i.e., superficial rubor alone), but is more aptly named niacin-associated skin toxicity (NASTy) (8). NASTy effects are the main reason patients stop taking niacin, >25% within months (1, 9–11).
An extended-release (ER) niacin alternative promised to mitigate NASTy, but may have unwittingly done so at the expense of CHD benefits (see supplemental online material). This illustrates how NASTy frustrates efforts to use niacin as a cardioprotective strategy. Indeed, NASTy is not just a major problem for patients, but could also imperil entire development programs, motivating efforts to better understand it, and ultimately, abolish it. To wit, recent studies of the ER alternative show how much is at stake. Disappointingly, the ER alternative failed to demonstrate incremental cardiovascular benefits over background statin therapy in patients whose LDL-cholesterol was already optimized by the statin (12, 13). Major design flaws likely contributed to the ER alternative’s back-to-back failures in the AIM-HIGH and HPS2-THRIVE trials, as both enrolled populations whose lipids were already optimally treated at baseline, included patients who had been taking niacin chronically, and used relatively short exposures on trial (discussed in more detail in the supplemental online material) (14–17). Perhaps the most obvious problem with AIM-HIGH and HPS2-THRIVE was their use of a profoundly different niacin regimen. Importantly, both studies featured major changes to the established regimen, hoping to better cope with NASTy effects. For example, the alternative regimen substantially underdosed niacin, limiting the dose to no more than 2 g nightly, so, at best, the ER alternative provided a third less niacin than the established cardioprotective regimen, namely, 1 g thrice daily. Moreover, the ER alternative was limited to only the overnight fast, again to help the patients to better cope with NASTy effects. Though this minimizes NASTy by timing it with the sleeping hours, there is now evidence suggesting that dosing during the nocturnal fast foregoes the opportunity to limit postprandial lipidemia, perhaps further undermining efficacy (18). Though well-intended, it now appears that efforts to limit NASTy events by departing from the established cardioprotective regimen have been less helpful than hoped, inspiring a new round of efforts to suppress NASTy by other means.
In the meantime, the established cardioprotective regimen remains evidence-based monotherapy to prevent myocardial infarction and CHD death, especially in the statin-intolerant, statin-averse, and statin nonresponders. To clinicians, assurance of benefit depends on adhering to the established cardioprotective regimen, at the cost of increased cutaneous side effects, again highlighting the need for progress on mitigating NASTy. To developers, this presents an important opportunity to more skillfully exploit the niacin pathway. Indeed, despite the disappointing ER alternative, the niacin pathway remains a major target to suppress atherogenic lipoproteins beyond the capabilities of niacin itself. Several drug developers are testing a variety of niacin pro-drugs, metabolites, and niacin mimetics in phase I and phase II trials (19). Some of these have shown potent lipid-lowering effects in animal and early human studies, while avoiding dermal plethora, and hence show promise as better-tolerated replacements to niacin. If lipid suppression matches or surpasses that of niacin, and NASTy symptoms are minimized, such therapies might one day rival niacin’s established cardioprotective regimen, especially if they acquit themselves well in CHD outcomes trials. A major challenge to fully exploiting the niacin pathway is to mitigate NASTy effects enough to permit daytime dosing during the fed portion of the day, when NASTy symptoms are more noticeable. Preventing NASTy effects may also permit higher doses, enhancing efficacy beyond the range of the underachieving ER alternative, or even the established immediate-release niacin. Unfortunately, NASTy assessment has largely been described using subjective self-reported symptom perception scores rather than objective and quantitative measurements, leaving much to be desired.
MECHANISM OF NASTy AND THE NEED TO QUANTIFY NIACIN-INDUCED PHYSICAL STIGMATA
Niacin binds its receptor, GPR109A, in dermal immune cells (including Langerhans cells, keratinocytes, and macrophages) (20, 21), inducing vasodilatory eicosanoids, notably prostaglandins D2 and E2 (PGD2 and PGE2) (22–25), causing dermal vasodilation and plethora within minutes. Importantly, objective hyperemia demonstrated that mice lacking GPR109A did not “flush” after niacin and that PGD2 and PGE2 are major NASTy mediators, and elucidated the biphasic primary response (26). In humans, the incidence and severity of NASTy are usually assessed by subjective patient-reported questionnaires (27, 28), and vary widely. Though distinguishing placebo from niacin, these are not sensitive/quantitative enough to differentiate niacin doses (29), making them semi-quantitative at best. Using subjective scales, NASTy frequency and severity wanes over days to weeks, despite escalating doses (2, 11). Confusing matters further, perceived relief with repeated exposure apparently reflects acclimatization bias (30).
Concomitantly, adipocyte GPR109A drives niacin’s antilipolytic effect (31) via postreceptor pathways distinct from NASTy effects (32). This fostered vigorous ongoing attempts to develop partial/selective GPR109A-agonists preserving lipid-lowering while avoiding NASTy (19, 33). Though well-suited for use in large clinical trials, subjective scores are obviously unusable in preclinical animal experiments, and their high variability is not conducive to small early-phase human studies. In addition to involving small numbers of subjects, such studies are typically dose-ranging and, hence, unsuitable for the low-sensitivity subjective scales that cannot differentiate the relative severity of NASTy effects between doses (29). Importantly, quantitative hyperemia assessment has been integral to animal studies of GPR109A, helping to clarify receptor physiology (26, 34) and to identify numerous receptor agonists (32, 35), thus promoting therapeutic drug development. A similar tool is sorely needed in early-phase clinical studies to better predict clinical tolerability in larger clinical trials and to help to avoid the unintentional trade-off between tolerability and efficacy that apparently doomed the ER alternative.
A functional GPR109A assay is also indispensable to study receptor physiology. GPR109A function may vary, rendering niacin less effective in certain groups. As an extreme example, the vasodilatory effect of topically applied niacin is suppressed in schizophrenics, presenting a potential biomarker of schizophrenia (36), and variability in skin response predicts altered membrane fatty acid composition and metabolism (37). We found ER niacin ineffective in suppressing plasma triglycerides in African Americans after a fat load (18), suggesting a weaker antilipolytic effect of GPR109A agonism. Because rubor is a cardinal effect of GPR109A agonism, a quantitative NASTy assessment tool provides a functional GPR109A assay.
BEYOND NASTy: POTENTIAL APPLICATIONS OF OBJECTIVELY MEASURING PHYSICAL CHANGES TO THE SKIN
Topical niacin provokes intense inflammation involving endothelial cell swelling and perivascular neutrophilic and macrophage infiltration persisting >96 h (38). Similar changes accompany several inflammatory dermatologic disorders like psoriasis, atopic dermatitis, contact dermatitis, and rosacea. Niacin-mediated dermal response may therefore provide an easy and reliable model to study diverse dermatologic pathologies. Typically, severity, clinical course, and, most importantly, therapeutic response in such conditions are assessed using visual scales. More recently, therapeutic trials underscored the need for more objective quantitative tools. For instance, decreased cutaneous blood flow in patients with psoriasis after PUVA (photochemotherapy) therapy predicts clinical improvement (39). Another application is the SPF rating of sunscreens, based on a sunscreen’s ability to delay erythema by UV light. As a further example, monomethyl fumarate is an anti-psoriatic GPR109A agonist and, hence, recapitulates NASTy symptoms (21, 40). Further research into anti-psoriatic and NASTy sequelae necessitates objective/quantitative assessment. Beyond dermatology, tissue blood flow monitoring may outperform clinical assessment to predict surgical graft survival, assess burn wound depth and healing potential, and guide ischemic bowel resection (41–43). Furthermore, given easy accessibility, dermal microcirculation is ideal to study systemic vascular disorders like diabetic microangiopathy, vasculitis, Raynaud’s phenomenon, and atherosclerotic peripheral vascular disease (44). Hence, objective dermal blood flow and erythema assessment has protean applications in vasospastic and ischemic vascular disorders, as well as in inflammatory dermatologic disorders, both for physiological inquiry and therapeutic research.
OVERVIEW OF HIGH-RESOLUTION NASTy STIGMATA MEASUREMENTS
To measure NASTy physical stigmata, we focused on three signs that lend themselves to high-frequency measurement: 1) rubor (gross erythema of the skin, including, but not limited to, erythema from hyperperfusion); 2) plethora (i.e., hyperemia: red corpuscle engorgement of dermal vessels from a hastened blood flow rate); and 3) calor (warmth of the skin from mobilizing core blood to the surface or more directly from the local inflammatory response). The last is tightly related to a fourth systemic sign: 4) frigor (a drop in core body temperature per rectum, whose measurement is normally conducted simultaneously with skin temperature). Importantly, rubor, plethora, calor, and frigor progress over distinct time courses. Thus, investigators should carefully consider their differences when designing experiments, to choose the stigma(ta) best suited to the physiology and select an appropriate observation interval, lest they “miss the action.” The distinction between rubor and plethora merits elaboration: Even though plethora is an important cause of rubor, it is not the only cause. For example, in the setting of erythrocyte engorgement, if the accompanying dermal inflammation, edema, and leukocyte/monocyte infiltration were also accompanied by increased vascular permeability and erythrocyte extravasation, the latter might contribute to rubor, allowing rubor to persist even after plethora abates. Consistent with this, we have observed sustained rubor persisting long after plethora resolved. For all four signs, high-frequency sampling provides high-resolution time course curves, which could prove especially helpful for investigations into NASTy mechanisms. Though some devices measure as often as 40 Hz, we typically condense the data to more manageable frequencies (e.g., 1 Hz). By contrast, subjective perception scores are typically assessed hourly or every other hour, providing relatively low-resolution time course curves. The objective techniques are also fairly nonobtrusive, as the sensors are placed on the skin and, ideally, remain in place throughout the experiment. Though it is technically possible to objectively measure tumor, comparable sampling frequencies are not yet feasible and measurements are more intrusive.
MEASURING RUBOR BY CLINICAL COLORIMETRY
Rubor or erythema is a cardinal manifestation of NASTy, as well as dermal inflammation, and is quantifiable using tristimulus colorimetry. Following diverse insults, rubor intensity by tristimulus colorimetry correlated with both subjective rubor and plethora intensity by laser Doppler flowmetry (LDF) (45, 46). Using red, green, and blue light-emitting photodiodes, the colorimeter sensor detects the relative intensity of each color reflected from skin. The erythema index quantifies the degree of cutaneous rubor (supplemental Appendix) (47).
MEASURING RUBOR BY WHITE-LIGHT SPECTROSCOPY
Spectroscopy measures specific colors as changes in incident energy to assess the quality/quantity of matter. Hence, specific cutaneous pigments are quantifiable by measuring incident light of different wavelengths reflected from skin. A recent advance in reflectance spectrophotometry, white-light reflectance spectroscopy (WLS), illuminates skin with the entire visible light spectrum, allowing simultaneous measurements of hundreds of wavelengths, eliminating scatter artifact, unlike narrow-bandwidth spectrophotometers. This is especially well-suited to measure intensely pigmented compounds, such as hemoglobin. WLS-derived changes in total hemoglobin predict improved mesenteric blood flow after revascularization of ischemic bowel (48). Simultaneous LDF and WLS also predicted vascular compromise in >150 surgical flaps well in advance of clinically detectable changes (49). We adapted WLS to assess total dermal hemoglobin to measure hemoglobin-specific rubor. We prefer WLS over traditional pulse oximetry because WLS penetrates only the very superficial layers of the skin, avoiding artifact from denser tissues and larger arteries.
MEASURING PLETHORA BY LDF
Principally, LDF uses magnitude and frequency distribution of wavelength shift of laser light (specifically red shift) to derive red blood cell (RBC) flux (erythrocyte concentration × velocity) to measure skin perfusion or plethora. While not measuring absolute blood flow in milliliters per minute per gram of tissue, LDF quantifies tissue engorgement in perfusion units (PU), where 1 PU = 10 mV of flux (50, 51). Hence, LDF continuously quantifies relative changes in blood flow. In alcohol-induced facial plethora, a 1.8-fold increase in RBC flux had high sensitivity, specificity, and positive predictive value for clinical symptoms (52).
MEASURING CALOR/FRIGOR BY CONTACT THERMOMETRY
Skin temperature has been used clinically to assess blood flow for centuries. Metabolically generated heat is transmitted to skin largely by convection via blood, hence rapid changes in skin temperature reliably reflect altered blood flow. Surface thermometry in surgical free flaps revealed that free flap temperature correlated with capillary blood flow (53). In the present context, Wilkin (54) found that malar calor predicted investigator-rated rubor from various provocations, including niacin. Specifically, peak calor by malar temperature predicted observed rubor, but the sensitivity, specificity, and predictive value of a positive test was markedly improved by Wilkin’s malar thermal circulation index (ΔMTCI) (supplemental Fig. S1). Moreover, the ΔMTCI correlated strongly with plethora per increased flux by LDF following alcohol-provoked flushing (52).
In addition to measuring calor as a “poor man’s” colorimeter or flowmeter, thermometry can also be used to track frigor, the drop in core body temperature following systemic heat loss, itself a vitally important physical sign. Typically, after calor peaks, niacin drops core body temperature 0.6–1.0°C (1–2°F) per rectum. Though this degree of frigor seldom crosses the threshold delimiting frank hypothermia, it is associated with several unsettling symptoms, including chills, rigors, and a feeling of internal illness. The transition from dermal calor to core frigor is rapid, and some individuals even perceive calor and frigor simultaneously. Curiously, one subject complained that he felt as if he was “on fire and freezing at the same time.” Measuring frigor presents some advantages over calor. To wit, calor assessment is inherently more variable, as the skin, being a major thermoregulatory organ, readily reacts to sudden changes in either core or ambient temperature as an adaptive response. By contrast, as endothermic homeotherms, our core temperature is under tight homeostatic control to keep it within a narrow range. Thus, changes to core temperature are more apt to represent signal than noise, as signs under elaborate homeostatic control are relatively more resistant to perturbation. We use rectal temperature to assess core temperature, lest hyperperfusion of the head and neck and even axillae influence core measurements in their vicinity. Unlike skin temperature, rectal temperature also minimizes interaction with ambient temperatures, and is therefore less susceptible to environmental bias. Thus, even though calor and frigor may represent two sides of the same coin, frigor may present a more robust measurement.
OBJECTIVE MEASUREMENTS OF PHYSICAL STIGMATA PREDICT SUBJECTIVE SYMPTOM PERCEPTION
Some of the aforementioned techniques have been used for decades to quantify physical changes of the skin in diverse settings. In the context of NASTy, LDF and thermometry have been employed to study topically applied, and sometimes, systemic niacin (supplemental Table S1), while colorimetry has, so far, been adapted only to topical niacin. As a much more recent innovation, WLS has not yet been applied in this context. Though both LDF and thermometry can predict NASTy as a binary occurrence, we know of only one study, by Parson et al. (55), that directly assessed objective measurement of plethora (by LDF) against a semi-quantitative symptom perception scale. It is unclear how rubor and calor/frigor would perform against such symptom scores. This is important, because NASTy is a composite of several signs and symptoms, varying by the relative contribution of the diverse eicosanoids that surge after niacin influencing each symptom, and how various stigmata relate to symptom perception remains poorly understood. Moreover, a semi-quantitative score, the Flushing Symptom Questionnaire, failed to detect changes in NASTy severity between 1 and 2 g of niacin (29), even though objective measurements by thermometry have consistently shown a dose-dependent increase in calor with escalating niacin (or acipimox) doses (56–58). This underscores the need for objective quantitative tools, especially when agonists/agents with relatively small differences in potency at GPR109A are to be compared. Furthermore, at best, hourly subjective scales only provide a coarse profile of the time course of drug effects. Finally, several natural food components have been touted to attenuate NASTy based on very small sample sizes using semi-quantitative scales (59, 60). Short of much larger and costlier clinical trials, the efficacy of such measures might be confirmed/refuted more readily by objective NASTy quantification techniques, with relatively smaller sample sizes.
Against this backdrop, we developed objective high-resolution techniques to measure NASTy stigmata and more fully characterize several aspects of the underlying dermal and systemic responses. These include optical techniques to measure rubor (colorimetry and WLS) and plethora (LDF), and direct contact techniques to measure calor/frigor (contact thermometry), complementing subjective perception by symptom surveys.
MATERIALS AND METHODS
Rubor by clinical colorimetry
We used a prototype device by cyberDERM Inc., Broomall, PA (supplemental Fig. S3) based on the same sensors as the DSM II colorimeter (Cortex Technology, Hadsund, Denmark). The prototype consists of control hardware coupled to four separate red, green, blue (RGB) color sensors. Sensor output is displayed via a Windows-based VB.net GUI software. Each sensor has three light-emitting diodes (LEDs), red, green, and blue, and a wide-spectrum photosensitive diode. During measurement, the sensor cycles through each LED, and the photodiode picks up the corresponding value of the selected diode’s reflective light. This cycle repeats fast enough to create an illusion of a white light-emitting LED, when it is, in fact, three separate LEDs turning on and off rapidly.
Sensors pass RGB values to the control board, where they are then sent across a USB connection to the computer to be adjusted and calculated into usable RGB/erythema index values. From there, the GUI software saves the RGB data into a .csv file and displays them graphically. We used a sampling frequency of 2 Hz.
Rubor by WLS
We used a moorVMS-OXYTM (MoorInstruments Inc., Wilmington, DE) with the OP1-1000 probe having the emitting and receiving fibers separated by 1 mm (supplemental Fig. S4). Each cable has an outside diameter of 3 mm. The device measures as often as 40 Hz using white light, with a measurement depth of 1 mm below the skin surface. Measurements are in arbitrary units (AUs) rather than absolute hemoglobin concentration.
Plethora by LDF
We used a moorVMS-LDFTM (MoorInstruments Inc.) laser Doppler monitor with the VP1T probe to continuously record dermal RBC flux (supplemental Fig. S5). The probe has a light-emitting fiber and a light-detecting fiber, each 0.5 mm in diameter and separated by 0.5 mm. The maximal recording frequency is 40 Hz using a wavelength of 785 ± 10 nm. The wavelength of light and fiber separation is important because these largely determine sampling depth. Like WLS, measurements are in AUs.
Calor/frigor by thermometry
We used the MadgeTech (Warner, NH) OctTemp 8-channel temperature data logger (supplemental Fig. S6) paired with Thermometrics RTD thermistor probes to measure and record malar, core body (rectal), and ambient temperatures simultaneously, sampling temperature at 4 Hz. Thermistors were custom built by Thermometrics (Northridge, CA) (supplemental Fig. S7).
Semi-quantitative NASTy symptom perception: the flushing assessment tool
The flushing assessment tool (FAST©) was developed to provide detailed and semi-quantitative assessment of individual NASTy symptoms of redness, warmth, tingling, and itching, as well as the overall troublesomeness of these symptoms (27). The symptoms are quantified on a 10 point scale where a score of 1–3 is rated as mild, 4–6 as moderate, and 7–10 as severe. Subjects rated their perception hourly after dosing immediate-release niacin 500 mg.
Stepwise instructions on the application of the aforementioned NASTy assessment tools are detailed in the supplemental Appendix for interested readers. Study subjects were examined under various protocols approved by the University of Pennsylvania IRB, having undergone informed consent.
RESULTS
General characteristics of niacin-induced dermal and systemic responses
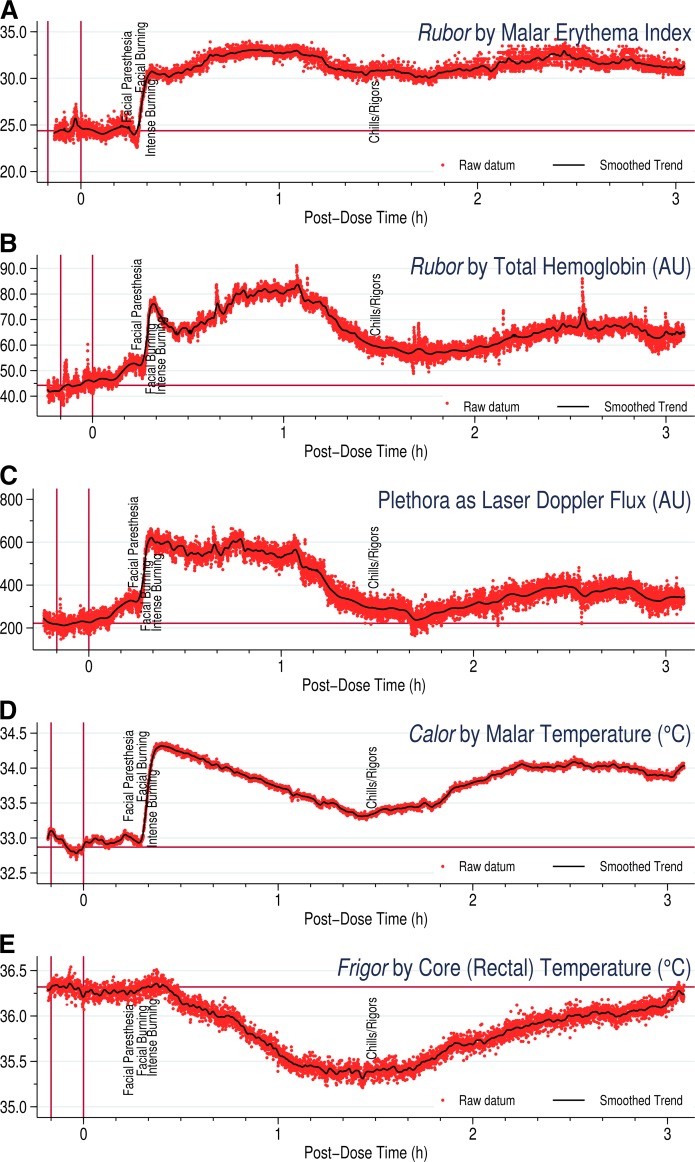
Figure 1 depicts simultaneous colorimetry, WLS, LDF, and thermometry following 2,000 mg immediate-release niacin. Typically, the skin undergoes abrupt physical changes 15–20 min after ingesting niacin, and can take an hour or more to hit the peak. The sharp increase in the physical stigmata coincides with subjective complaints, such as facial paresthesia and burning.
Fig. 1.
Objective physical measurements following ingestion of 2,000 mg immediate-release niacin. A: Rubor as the increase in malar skin erythema index by colorimetry. B: Rubor as the increase in total hemoglobin in the malar dermal capillaries by WLS, expressed in AUs. C: Plethora as RBC flux changes by LDF, expressed in AUs. D: Calor as change in malar skin temperature by contact thermometry. E: Frigor as change in core body temperature per rectum by contact thermometry. Note that WLS and LDF do not measure actual hemoglobin concentrations or absolute blood flow in milliliters per minute per gram. Red dots indicate actual measurements of the relevant parameter and the black line indicates the graph obtained after smoothing, as detailed in the supplemental Appendix.
Plethora by LDF differs from rubor in measuring one of the major mechanistic contributors to the latter, namely, skin blood vessel engorgement assessed from the motion of blood flow. In contrast to rubor, plethora usually abates by 90–120 min after niacin, when it returns to baseline, though it may rise several times thereafter. Strikingly, plethora may return between 4 and 8 h after the one-time niacin exposure and need not follow the time course of rubor.
Calor by malar thermometry typically reaches peak quickly, then descends fairly linearly over the next hour or so without quite hitting baseline. Interestingly, when calor reaches peak, the core (rectal) temperature starts to decline, and the linear drop in malar temperature coincides with the evolution of frigor. As rectal temperature reaches its minimum, subjective complaints of chills and rigors become most noticeable, and both core and malar temperature start to climb again.
Rubor by clinical colorimetry
Following 2,000 mg niacin, the colorimetric response was most distinct, showing nearly constant rubor throughout the measurement period (Fig. 1). On clinical colorimetry, superficial rubor persisted for several hours with the erythema index varying between the initial increment and peak without approaching baseline up to 3 h after niacin was dosed, and even as the plethora was waning by LDF about 90 min after niacin, the erythema index remained elevated. This may implicate other rubefacient skin changes beyond vascular engorgement. Histologically, NASTy is accompanied by perivascular inflammation around dermal capillaries and venules (38), and grossly by cutaneous edema. This inflammatory response may also involve greater vascular permeability and epidermal RBC extravasation, prolonging superficial rubor. A discrepancy between LDF and superficial colorimetry may arise from the fact that the flux index is determined by both RBC concentration and velocity. Hence, the lethargic or static extravasated RBCs would contribute much less toward motion flux as assessed by Doppler red shift. Moreover, interstitial edema could also compress superficial blood vessels, and thereby impact measurement depth. Interestingly, persistent superficial rubor is also seen with WLS, but at a lesser magnitude. Though rubor does not return to baseline on WLS, the signal is much lower than peak clinical colorimetry, suggesting that WLS is less sensitive at capturing superficial rubor, that is, gross erythema visible at the skin surface rather than just beneath the skin. Perhaps the number of extravasated RBCs is sufficient to cause superficial erythema grossly, but is less readily detected at the depth sensed by WLS. Similar to LDF, the WLS probe measures to a depth of 1 mm, and edema could impact measurements by increasing the distance between probe and extravasated hemoglobin. As an alternative explanation for this discrepancy, perhaps above a certain level of tissue hemoglobin, colorimetry cannot discern severity of further redness, implying a flat dose-response curve above a certain level of tissue hemoglobin. In essence, that would suggest that colorimetry has a lower resolution than WLS in detecting incremental changes in rubor above a certain threshold. In any case, superficial colorimetry may be the preferred method where gross rubor is of primary interest.
Rubor by WLS
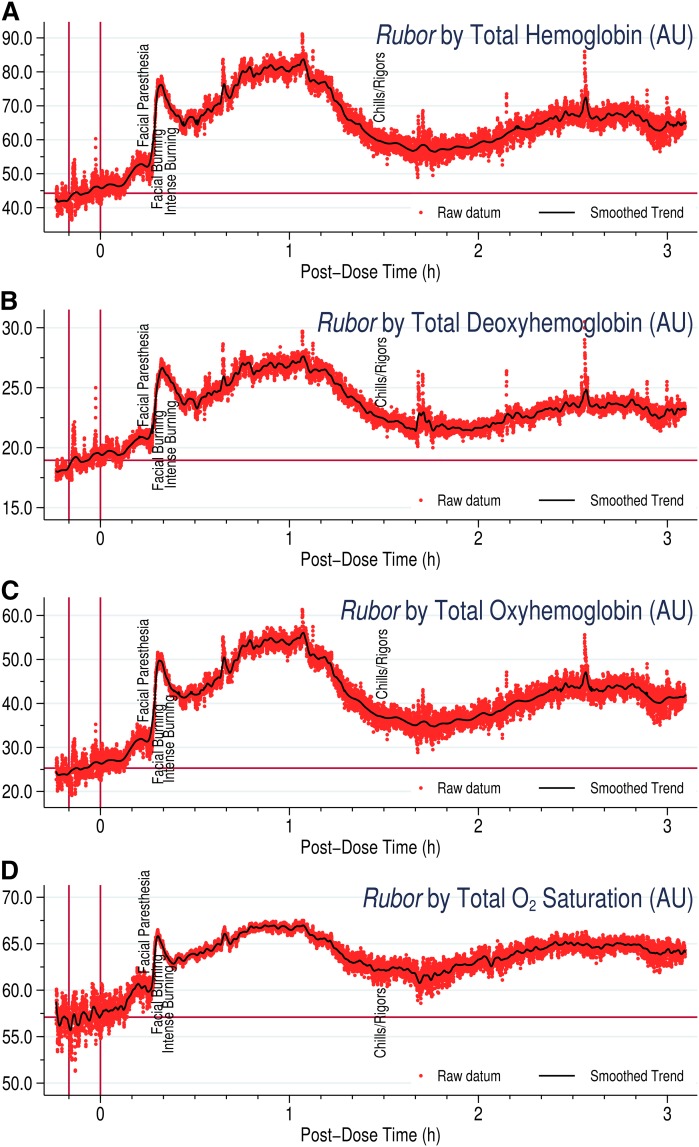
Figure 2 demonstrates separate hemoglobin fractions assessed by WLS to measure rubor. Like LDF, measurements are in AUs, not actual hemoglobin concentrations. Even so, changes in total hemoglobin were largely additive of the component deoxygenated and oxygenated hemoglobins. Apart from scale, the graphs for each hemoglobin parameter were nearly superimposable. This is not surprising, as pure hyperemia should not alter relative hemoglobin fractions. Total oxygen saturation yielded a similar pattern. The sustained rubor seen with colorimetry was less apparent by the WLS approach, and between 1.5 and 3 h, the device recorded colors in the hemoglobin range between the peak and baseline. This suggests that WLS may not record rubor as efficiently in later hours, as discussed above, compared with clinical colorimetry, though both indicate sustained rubor for several hours.
Fig. 2.
Rubor, as represented by changes in (A) total, (B) oxygenated, and (C) deoxygenated hemoglobin fractions along with (D) total oxygen saturation, by WLS, following 2,000 mg immediate-release niacin. The typical early minor peak preceding the later major peak for rubor seems to be most prominent and well-defined during WLS.
Plethora by LDF
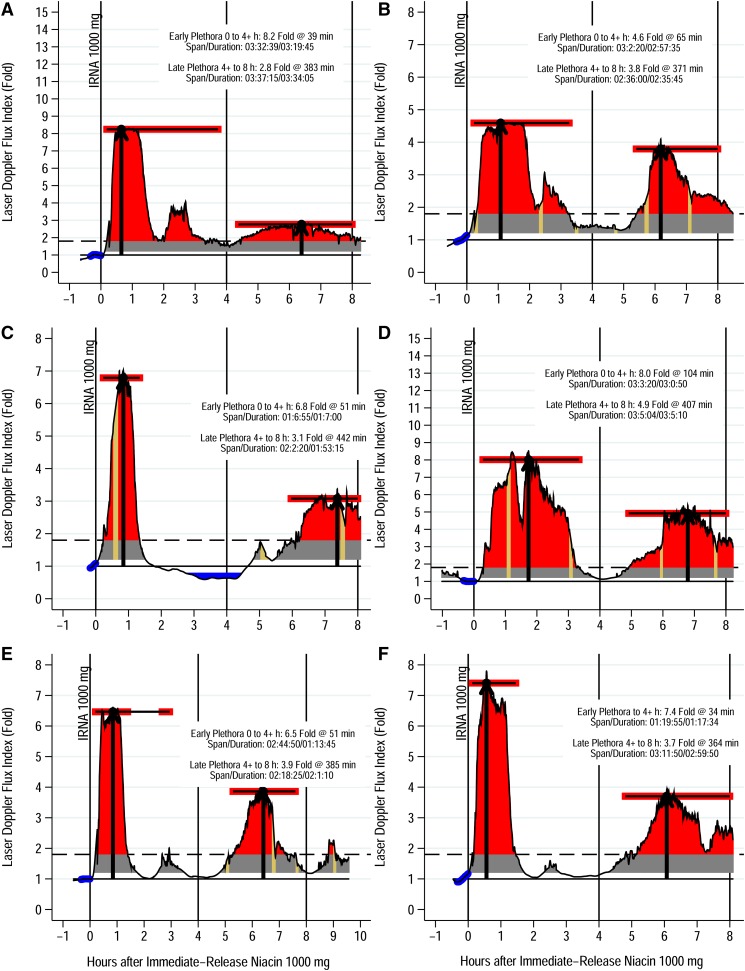
Within 15–20 min, niacin induces clinically significant plethora, that is, flux index >1.8 on LDF (52). Peaking within an hour to a flux index 6- to 8-fold baseline, plethora typically falls below the perception threshold 2 h postingestion (Fig. 3). Fascinatingly, a rebound often occurs 4–6 h later, with the flux index rising 2–4 times baseline, in one case approaching the primary peak. Given the extremely short plasma half-life of prostaglandins, of the order of minutes, this rebound suggests additional pathways mediating NASTy beyond PGD2 and PGE2. Niacin may activate the capsaicin receptor, TRPV1, in dermal macrophages, binding the receptor intracellularly. Interestingly, TRPV1 inhibition mitigates dermal hyperemia post niacin (61, 62). Perhaps secondary hyperemia follows TRPV1 activation after niacin presents intracellularly, explaining the several-hour delay. This could also explain why COX-inhibition and/or PGD2 receptor blockade fail to abolish NASTy. A more subtle feature seen with WLS and flux index (Figs. 1A, B; 2D) is an early sharp narrow minor peak preceding a broader major peak. This minor peak occurs within minutes of niacin dosing. Though much briefer and often less pronounced than the major peak, the minor peak still exceeds the perception threshold. Benyó et al. (26) observed this in mice by LDF, showing that the minor peak was significantly attenuated in mice lacking the PGD2 receptor, whereas mice lacking the PGE2 receptor had a diminished slightly delayed major peak. This implies that flux from the two prostaglandins superimposes as a biphasic response within primary hyperemia, PGD2 mediating the very early response and PGE2 mediating the later more pronounced phase. These findings support the concept that precise measurement of niacin-induced NASTy stigmata could also facilitate mechanistic studies to probe the underlying inflammatory response mediating NASTy signs and symptoms. For example, the early minor peak is more prominent as rubor by WLS compared with plethora by LDF; accordingly, WLS may prove advantageous over LDF for experiments attempting to deconvolute the curve or otherwise elucidate the mechanism of the NASTy response.
Fig. 3.
Plethora by LDF of six representative subjects after 1,000 mg immediate-release niacin (IRNA). Laser Doppler flux is from the malar eminence spanning at least 8 h postdose. Predose readings were taken as that day’s baseline, and are depicted as predose values highlighted in blue. Data were rescaled by dividing each value by the baseline, thus converting to fold changes where 1 represents the baseline. Plethora onset is when flux first exceeds 1.8-fold baseline (dashed horizontal line) and resolution time when it returned below 1.8-fold baseline following the primary peak. The duration of plethora is simply the resolution time minus the onset time. The perfusion area under the curve (AUC) may also be calculated (red area). The area where flux index is <1.8 is subtracted to derive a plethora incremental AUC (iAUC). Further details of graph analysis are discussed in the supplemental Appendix. As an example, (A) shows an 8.2-fold increase in flux over baseline 39 min after taking niacin, this subject’s peak flux index, indicating plethora at the malar eminence (first black arrow). Tan regions indicate spans with no valid readings, which for example occur when the subject leaves the bedside to go to the restroom. The curve for these brief intervals is simply interpolated. Some subjects, usually men, have no such interpolations because they urinated at the bedside. In this study, we arbitrarily divided the 8 h period into two 4 h periods. Typically, subjects have a major hyperemia episode during the early period (i.e., first 4 h) followed by a lesser episode in the late period (i.e., second 4 h), all from a single dose given at 0 h. For each 4 h interval, we present the flux index at the peak with the heavy black arrow. Patient characteristics: age (mean ± SD), 25 ± 7 years; sex, two male/four female; ethnicity, four African American/two Caucasian; BMI (mean ± SD), 25 ± 2.4 kg/m2; baseline lipids (mean ± SD): total cholesterol, 142 ± 30 mg/dl; triglyceride, 66 ± 22 mg/dl; LDL, 71 ± 13 mg/dl; HDL, 57 ± 19 mg/dl. All subjects were healthy volunteers without any history of cardiovascular disease, hepatic or renal disease, or diabetes mellitus and were not on any antihypertensive, lipid-lowering, anti-inflammatory, or vasoactive medications.
Calor/frigor by contact thermometry
The bottom two panels in Fig. 1 and supplemental Fig. S2 present surface calor and core frigor following 2,000 mg immediate-release niacin. Niacin clearly induces striking frigor, involving a rapid and substantial drop in rectal temperature. Massive heat loss likely explains the severe chills and rigors some subjects feel after niacin, underscoring the systemic nature of NASTy and the profound shortcomings of the innocuous metonym, flushing. Notably, thermometry could not discern the bimodal nature of the primary peak. This is intuitive, because temperature changes in response to changes in blood flow would have some lag time, and rapid fluctuations in blood flow may go undetected. In essence, thermometry is not as “nimble” as the other three techniques. This is also evident in Fig. 1, where thermometry lags other techniques by several minutes before starting to show changes in surface temperature. Malar, core, and ambient temperatures are combined as Wilkin’s ΔMTCI, and an example calculation is presented in supplemental Fig. S1.
Objective physical measurements predict subjective symptom perception
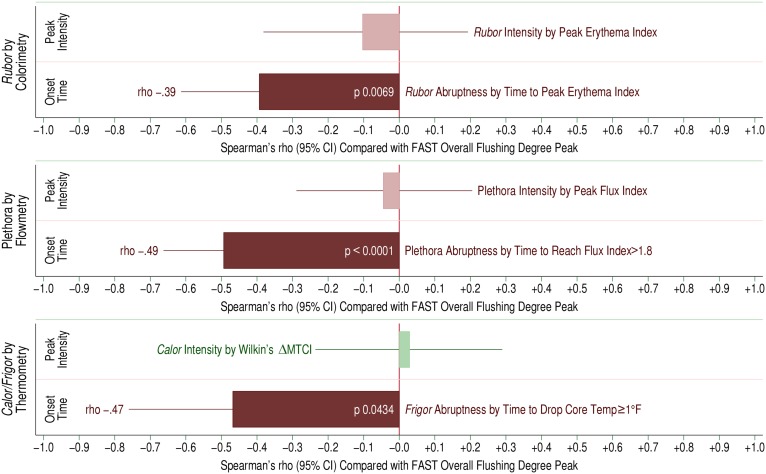
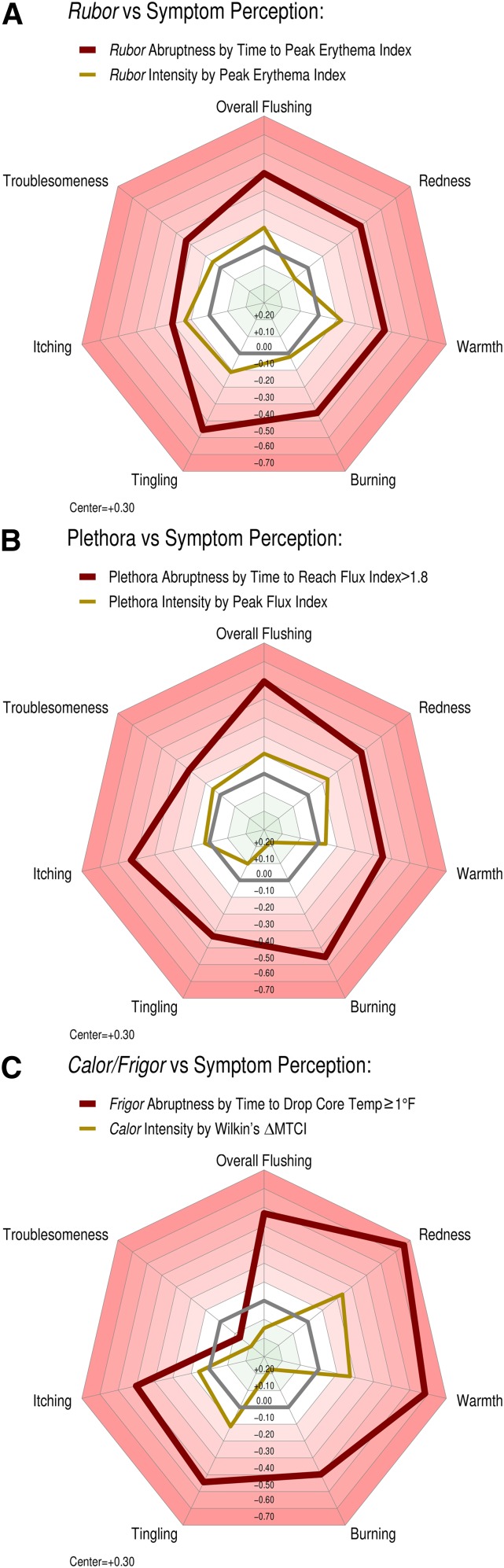
Figure 4 depicts the relationship between peak overall symptom perception by hourly FAST survey and objective physical measurements after 500 mg immediate-release niacin (n = 68 exposures). Specifically, we correlated peak flushing symptom perception per FAST against rubor by colorimetry, plethora by flowmetry, and calor/frigor by thermometry. Interestingly, we found that symptom severity had a strong inverse correlation with time to response for each objective measurement in a dose-response manner, rather than the magnitude of the peak response. In other words, subjects with more rapid responses felt worse than those with a more leisurely time course. In contrast, the peak intensity of rubor or plethora was entirely unrelated to symptom perception. Notably, the thermometric parameter that correlated best with symptom severity was a systemic sign rather than a dermal sign: the abruptness of frigor, assessed as the time it took to drop body temperature 1°F (0.6°C). In other words, subjects experiencing a precipitous drop in core body temperature felt worse than those with a gradual drop. These findings imply that subjects are quite sensitive to how fast NASTy presents, and at that, in a dose-responsive manner. However, above a certain threshold of perceived intensity, they appear entirely incapable of distinguishing further degrees of rubor, plethora, or calor. In pharmacodynamic terms, this implies that the time to effect drives symptom perception, whereas the height or depth of the “concentration” effect is entirely dissociated.
Fig. 4.
Dose-response relationships between objective physical stigmata and subjective symptom perception scoring, specifically, peak “overall flushing” by the FAST survey, following 500 mg immediate-release niacin. The relationship between physical stigmata with subjective symptom perception was assessed by Spearman’s rho, graphed as the width of the colored bar. The line at the end of the bar indicates the 95% confidence interval for rho. The upper bar in each panel involves a continuous measure of peak intensity (comparable to c-max on typical pharmacodynamics analysis), while the lower bar involves a continuous measure of time to onset or time to event (similar to time-of-max/min). Top panel: Relationship of peak rubor, measured as the erythema index by colorimetry (upper bar) and time to peak rubor (lower bar) with overall flushing by FAST survey. Middle panel: Relationship of peak plethora by laser Doppler, measured as peak flux index (upper bar) and time to reach a flux index of 1.8 (lower bar) with peak overall flushing by FAST. Flux index of 1.8 has been determined to correlate with observed rubor. Lower panel: Relationship of peak calor by ΔMTCI (upper bar) and time to frigor, core body temperature fall by 1°F (~0.6°C) (lower bar) with peak overall flushing by FAST. Study population characteristics (68 tracings among 17 subjects receiving immediate-release niacin 500 mg): age, 38.1 ± 13.0 years; sex, eight male/nine female; ethnicity, seven African American/nine Caucasian/one Hispanic; BMI (mean ± SD), 25.8 ± 5.8 kg/m2; baseline lipids (mean ± SD): total cholesterol, 183 ± 35 mg/dl; triglyceride, 74.4 ± 39.1 mg/dl; LDL, 98.9 ± 33.4 mg/dl, HDL, 68.9 ± 17.5 mg/dl. All subjects were healthy without any history of cardiovascular disease, diabetes mellitus, or renal or hepatic disease. No subject was taking any vasoactive medications including anti-hypertensives, anti-histaminics, selective serotonin reuptake inhibitors, nonsteroidal anti-inflammatory drugs, oral steroids, or leukotriene inhibitors.
Beyond overall symptom perception, we tested correlations between the aforementioned objective parameters from colorimetry, LDF, and thermometry, and the individual NASTy symptoms of redness, warmth, tingling, burning, itching, and troublesomeness (Fig. 5, supplemental Fig. S8). Again, the celerity of objective physical stigmata was a uniformly stronger negative predictor of all individual component NASTy symptoms than their intensity across the three modalities. LDF and thermometry predicted a somewhat broader list of symptoms than colorimetry. This suggests that the onset of rubor is less noticeable to patients than plethora and frigor. Though intuitive, it is also intriguing that subjects perceiving the greatest degree of subjective warmth and redness suffered the most precipitous drops in core temperature. This accords with the concept that subjects with the severest vasodilatation should offload body heat to the environment fastest, thus rapidly dropping core temperature.
Fig. 5.
Radar plots representing the relationships between objective physical stigmata with seven individual components of NASTy symptom perception by FAST survey following 500 mg immediate-release niacin. Correlation was assessed by Spearman’s rho (see also supplemental Fig. S8). Regular heptagons: The regular heptagons each represent a level of Spearman’s rho, with more intense red shades corresponding to greater inverse relationships. The center of each plot represents a rho of +0.30, with a progressive decrement of 0.1 with each encompassing heptagon. The heavy gray regular heptagon represents a rho of 0, that is, no relationship. Irregular heptagons: The outer heavy red irregular heptagon in each panel represents time to onset, and the inner golden heptagon depicts peak intensity. Similar to overall flushing, celerity of each objective sign correlated with individual perceived symptoms much more strongly than the peak intensity, indicated by the small irregular heptagon that seldom differs from a Spearman’s rho of 0. Strikingly, without exception, the heptagon representing peak intensity is completely surrounded by that representing time to onset, and by a wide margin, consistent with the latter’s prowess as a predictor. A: Correlations between peak rubor and time to peak rubor by colorimetry and NASTy symptom perception. B: Correlations between peak plethora and time to plethora onset (i.e., time to when flux index first exceeded 1.8 and peak flux index) by flowmetry and NASTy symptom perception. C: Correlation between peak calor (ΔMTCI) and time to frigor (time to reach a 1°F (~0.6°C) fall in core body temperature) by thermometry and NASTy symptom perception. The population was the same as that used to derive data for Fig. 4. For study population characteristics, please refer to Fig. 4.
DISCUSSION
We present a robust, multi-modality, and objective suite of methods to quantify NASTy physical stigmata. We also present some preliminary and, to our knowledge, novel results obtained during the course of our investigations with niacin to objectively quantify NASTy. Though there have been previous reports of LDF and thermometry after topical and systemic niacin (supplemental Table S1), and colorimetry after topical niacin, to our knowledge, we have used colorimetry for the first time after systemic niacin and WLS in either circumstance (systemic or topical niacin). Perhaps more importantly, few investigators have objectively assessed dermal effects of niacin while simultaneously using a semi-quantitative symptom perception scale, and tested the correlation between the two.
We present several novel findings. First, and most obviously, our results across the three modalities suggest NASTy symptoms are best predicted by the rapidity of stigmata onset, rather than their peak intensity. The latter would not be expected if there was a linear dose/response relationship between peak intensity of the physical stigmata and symptom perception. One reason we found no relationship might simply be that the dose/response curve is indeed a curve rather than a straight line, and our subjects turned out to be on a flat region of the dose/response curve. If so, further increases in the signs’ intensity would not translate to increased symptom perception. In any case, the absence of a relationship indicates that above a certain level of rubor by colorimetry, plethora by laser Doppler, or calor by thermometry, subjects do not perceive greater degrees of cutaneous symptoms. On the other hand, subjects perceived symptoms more severely with more rapid onset of rubor, plethora, or frigor. Second, a limitation of ΔMTCI is that it applies only to steady states, and is hence unusable for dynamic NASTy assessment; helpfully, this is offset by our novel use of thermometry to measure the rapidity of frigor, a major drop in core body temperature. Analogously, Pontiroli et al. (58) studied the effect of escalating doses of the GPR109A agonist, acipimox, on dermal plethora, finding that the rate of rise in malar temperature correlated most strongly with flushing symptom perception, as assessed by a 10 cm visual analog scale. Together with our data, these results suggest that a parameter incorporating the dimension of time will prove more informative than one limited to only height/depth of an outcome’s intensity. Third, we demonstrate that the time course of physical stigmata from several techniques can reliably predict NASTy symptom perception. These findings are novel in that rather than testing objective techniques against NASTy symptoms as a dichotomous variable (yes/no), we correlated them with subjective semi-quantitative symptom perception. However, given the drawbacks of these subjective scales, as discussed in previous sections, these objective methods offer a valid and robust alternative for use in pharmacodynamic and mechanistic studies and in drug development. This is especially topical for niacin analogs, several of which have entered phase I and phase II human trials, with several others in the pipeline. These methods are quite feasible in small early phase dose-ranging studies to quantify the relative propensity of these novel agents to provoke NASTy adverse events, and a robust continuous outcome may be better suited to distinguish not only niacin from its mimetics, but also different doses of the same mimetic. All techniques need not be applied simultaneously, just the one(s) best suited to the specific experiment.
Another group correlated NASTy symptoms to one of the four stigmata described, providing an illuminating point of comparison. Parson et al. (55) studied the effect of 325 mg aspirin (ASA) on NASTy symptoms and plethora (using LDF) in hairy and glabrous skin after 1 g immediate-release niacin. Interestingly, they found that maximum forearm plethora over 90 min post niacin predicted overall flushing perception by the FAST survey, as well as the component NASTy symptoms of redness, warmth, tingling, and itching. Our findings differ from theirs in that we found a much stronger correlation between symptom perception and rapidity of stigmata development, rather than plethora intensity, the latter failing to predict symptoms whatsoever in our experiment. The reasons why their findings could differ from ours include differences in sample size, niacin dose, LDF devices, and body site assessed (malar eminence vs. forearm). Moreover, they administered the FAST survey only once at the end of the monitoring period, as symptoms were resolving; whereas, we conducted the survey hourly in real time, providing several inputs throughout the monitoring period. Perhaps the longer interval between the physical stigmata and symptom perception scoring renders the ratings more susceptible to recall error than our shorter interval. We believe our overall findings to be more robust because we had consistent results from several stigmata across three different objective modalities. We also used half the dose of niacin, finding that only the time to effect predicted symptom perception, affording no role for the peak effect of the physical stigmata. Perhaps the peak effect only predicts symptom perception at gram quantities of niacin. If so, this would commend time to effect as the more robust parameter across a broader dose range. Because niacin is usually started at 500 mg daily, our experiment featuring that dose may be more important for studies concerned with NASTy effects of niacin initiation. On the other hand, studies of gram doses by both groups may better inform mechanistic studies.
The following section describes the relative advantages/disadvantages of each technique to help investigators choose the one most suited for particular circumstances. Beyond lipidology, with minor variations, these methods can also be adapted to study diverse dermatologic, vasospastic, and ischemic disorders. There are certain limitations and advantages of each tool to aid appropriate use in specific circumstances. Many subject-related, device-related, and environmental factors might influence dermal blood flow and affect validity and reproducibility (supplemental Table S2).
Environmental factors
As the predominant thermoregulator, dermal perfusion is very sensitive to ambient and core body temperatures. In highly vascular areas like the fingertips or face, widely-varying ambient temperature influences baseline and peak flux. Variations widen at temperatures ≥28°C (63). For skin surface temperature to accurately reflect blood flow, core body heat reaching the skin by direct conduction, i.e., tissue conductance, must remain constant. Mean body conductance remains constant until ambient temperatures reach 26–28°C (64), underscoring the need to maintain constant and neutral ambient temperatures (∼21–24°C). Moreover, ΔMTCI does not account for perspiration-induced heat loss and temperatures between 21 and 24°C seldom stimulate perspiration. Finally, ΔMTCI is only valid under steady state conditions, hence, usually of utility only when comparisons of maximum and baseline blood flow are of interest; whereas, the other three modalities can be used for continuous real-time blood flow assessment.
Dermal heat loss can also occur via convection, and exposure of skin to cold air can significantly decrease cutaneous blood flow (65). Therefore, direct exposure to air drafts, such as air conditioning vents, open windows, and direct sunlight or heating sources are best minimized. Bright sunlight can also impact colorimetry measurements. Interestingly, mental stress and mathematical calculations may influence cutaneous blood flow, especially in areas with rich baseline blood flow; thus, physical stigmata should be assessed in a quiet environment without excessive audio-visual stimuli (66, 67).
Subject-related factors
There are considerable regional differences in dermal blood flow, with flow to face, palms, and soles exceeding that to the trunk, arms, or legs (68) by as much as 6- to 10-fold (69). Thus, comparisons of plethora or rubor are best made between the same body part, irrespective of laterality. There are also significant local differences in blood flow, promoting significant variability when LDF or WLS probes are moved even a few millimeters, due to the structural anatomy of the dermal microcirculation (70). Dermal capillaries are supplied by ascending arterioles about 1.5–7 mm apart, while LDF and WLS probes measure blood flow in an approximately 1 mm2 area. Hence, whether the ascending arteriole is centered underneath the probe will significantly influence baseline flow, up to 2- to 4-fold (71). Hence, repeated removal and reapplication of LDF and WLS probes should be minimized and measurement sites marked. Prolonged measurements over several hours necessitate probe removal for subject movements, like using the bathroom, a problem easily overcome with probe-holders that remain fixed to the skin when the probe is removed.
Excluding neonates, age, sex, and race do not influence LDF measurements. Because age >66 years and race impact rubor assessment, colorimetry may be less suited to older or racially diverse populations (72). Although the WLS device accounts for melanin, heavy skin pigmentation can interfere with readings. Meals seem not to affect rubor or plethora measurements, but coffee, alcohol, nicotine, or vasoactive drugs immediately before or during assessment should be avoided, as should anti-inflammatories (73). Deep inspiration, valsalva, postural changes, and hyperventilation also influence dermal blood flow. Anemia does not seem to influence LDF measurements. Obviously, with WLS and colorimetry, anemia will give lower baseline and peak readings without impacting comparisons between changes in blood flow. Areas with inflammatory lesions or rash should not be used, unless the lesion itself is of interest. Perimenopausal women might warrant exclusion from investigations of dermal hyperemia. Baseline blood flow and vasomotor reactivity likely varies with the phase of the menstrual cycle and should be accounted for when measuring during different phases (74, 75).
Device-related factors
LDF is majorly limited by artifact due to motion of light emitting and receiving fibers relative to each other, and to skin, both impacting measurement depth. Incorporating emitting and receiving fibers in the same cable and probe, use of smaller caliber fibers, and use of probe holders all help to reduce motion artifact. Nevertheless, subject movement during LDF is best minimized (76). This applies less to thermometry and colorimetry, but excessive motion increases internal heat generation and may also cause perspiration and peripheral vasodilatation. Hence, in studies where significant motion is inevitable or a part of the experiment, thermometry or colorimetry is preferable.
Uniform measurement depth is critical during WLS and LDF, because dermal hyperperfusion is largely a function of the superficial dermal microcirculation, ∼1–1.5 mm below the skin surface (70). Measurement depth is largely a function of WLS and LDF device parameters, chiefly, wavelength of incident light, optical fiber diameter, and emitting and detecting fiber separation (77, 78). Hence, valid comparisons necessitate the same probe configuration in all subjects and careful reporting of these parameters to ensure reproducibility. Probe pressure on the skin influences dermal blood flow via a neurogenic vasomotor reflex, with light pressure causing vasodilatation and moderate to heavy pressure decreasing blood flow (79). Even slight pressure can blanch the skin and affect colorimetry measurements. Thus, excessive probe pressure can influence colorimetry, WLS, and LDF measurements, but not thermometry. Importantly, due to small areas of assessment, LDF and WLS have an inherent tendency toward intra-individual variability that is less apparent with colorimetry or thermometry. Severe dermal reactions can present with significant dermal edema, which may affect WLS/LDF measurements by compressing superficial capillaries and increasing the distance between the probe and dermal capillaries.
Alternative applications
NASTy assessment techniques are easily adapted to specific circumstances. Obviously, colorimetry is most suited for circumstances where measuring skin color or pigmentation alone without blood flow assessment is the goal. The device can be customized to assess any pigment of interest, for example, melanin or bilirubin. Due to high sampling rate and quick response to blood flow alterations, WLS, LDF, and colorimetry are most suited for ultra-short duration studies (minutes to hours), whereas thermometry may be too “sluggish”. In skin disorders like psoriasis, where lesions are typically patchy, though widespread, only same body sites should be compared inter- and intra-individually. When spot measurements are required, for example, in assessing response to therapy during outpatient visits, 10–12 one-minute measurements could be made over a single lesion and averaged for comparison between visits, as in disorders like acne rosacea. In psoriasis, particularly due to scaling of lesions, colorimetry may not be reliable, and blood flow assessment techniques like LDF or WLS are probably more appropriate. An adaptation of the laser Doppler, 2D laser Doppler imaging, can be applied when large areas of skin need to be assessed intermittently over periods of weeks to months (51). The latter, however, cannot be used to monitor rapid changes in blood flow due to slow image acquisition and is semi-quantitative. Fuller characterization of these techniques by defining parameters that correlate best with clinical symptoms and with the eicosanoid surge should be a focus of future research. Our NASTy assessment methodology is by no means exhaustive of all objective skin assessment tools. Alternative physical techniques that hold promise and warrant study to quantify NASTy-induced dermal plethora include: laser Doppler perfusion imaging, photoplethysmography-derived perfusion index, laser speckle contrast imaging, and trans-cutaneous oxygen tension measurement.
SUMMARY
In summary, we present a more robust methodology than the subjective patient-reported questionnaire to quantify NASTy physical stigmata, with potential applications in further investigations of niacin, and especially a host of novel chemical entities exploiting this venerable therapeutic pathway. These techniques, either in combination or individually, may also be applied in diverse investigations both in the field of dermatology and of vascular medicine. Though preliminary, our novel findings of correlations between objective physical stigmata and subjective symptom perception highlight advantages of the former over the latter, and hopefully provide a frame of reference for future investigations, especially the importance of the rapidity of stigmata onset. We also outline some principles that should aid choice of the most suitable technique in distinct circumstances, as well as general guidelines and precautions for appropriate use of each technique.
Supplementary Material
Acknowledgments
The authors acknowledge Lee Wildman from cyberDERM, Inc. for technical assistance and the loan of the clinical colorimeter; Tim Brown from MoorInstruments Inc. for technical support and the loan of the WLS device; Thermometrics for design assistance and the manufacture of the RTD thermistors; Jacob Levy for assistance in editing the manuscript; and Rahma Warsi and Laura Pollan for collecting data from study participants. The authors accord special thanks to Rhoda Collick, RN, Eric Nelson, Joane Burke, RN, and Diane Levy, RN, from the Presbyterian Hospital Clinical and Translational Research Center, Philadelphia, and above all, the many research volunteers who participated in the clinical studies, graciously allowing deep probing into the physical response to niacin exposure with all of its discomforts.
Footnotes
Abbreviations:
- AU
- arbitrary unit
- CHD
- coronary heart disease
- ER
- extended-release
- FAST
- flushing assessment tool
- LDF
- laser Doppler flowmetry
- LED
- light-emitting diode
- ΔMTCI
- malar thermal circulation index
- NASTy
- niacin-associated skin toxicity
- PGD2
- prostaglandin D2
- PGE2
- prostaglandin E2
- RBC
- red blood cell
- RGB
- red, green, blue
- WLS
- white-light spectroscopy
This work was supported by National Institutes of Health Grants SCCOR P50-HL-083799 (D.J.R.), 5-K12-RR-017625-05 (R.L.D.), and K23HL091130 (R.L.D.); and National Center for Research Resources Grant UL1RR024134. Other support included a Junior Investigator Pilot Grant (R.L.D.) by the University of Pennsylvania Institutes for Diabetes, Obesity, and Metabolism and Translational Medicine and Therapeutics, with additional support by an investigator-initiated grant from Abbvie Inc. (R.L.D.) and American Heart Association Grants 0725480U and 09SDG2180013 (R.L.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. R.L.D. applied techniques described herein in an early phase human experiment of a novel synthetic niacin analog developed by Merck & Co. as the local Principal Investigator of a research protocol funded by Merck. R.L.D. has consulted for Catabasis Pharmaceuticals, Inc., the maker of two novel niacin pro-drugs, and demonstrated the techniques described herein for use in early phase human experiments. R.L.D. has demonstrated the techniques described herein to Abbvie, Inc. (formerly Abbott Laboratories), the maker of extended-release niacin, and Arisaph Pharmaceuticals, Inc., the maker of a novel synthetic niacin analog. G.L.G. (cyberDERM, Inc.) developed the clinical colorimeter used in this work, with plans to market it commercially. R.L.D. was PI of the University of Pennsylvania and Philadelphia Veterans Affairs Medical Center sites for the AIM-HIGH trial. H.G. participated as research associate at the University of Pennsylvania and Philadelphia Veterans Affairs Medical Center sites for the AIM-HIGH trial.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Birjmohun R. S., Hutten B. A., Kastelein J. J. P., and Stroes E. S. G.. 2005. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: a meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 45: 185–197. [DOI] [PubMed] [Google Scholar]
- 2.Capuzzi D. M., Guyton J. R., Morgan J. M., Goldberg A. C., Kreisberg R. A., Brusco O. A., and Brody J.. 1998. Efficacy and safety of an extended-release niacin (Niaspan): a long-term study. Am. J. Cardiol. 82(12A): 74U–81U. [DOI] [PubMed] [Google Scholar]
- 3.Elam, M. B., Hunninghake D. B., Davis K. B. , R. Garg, Johnson C., Egan D., Kostis J. B., Sheps D. S., and Brinton E. A.. 2000. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients. JAMA. 284: 1263–1270. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg A., Alagona P., Capuzzi D., Guyton J., Morgan J. M., Rodgers J., Sachson R., and Samuel P.. 2000. Multiple-dose efficacy and safety of an extended-release form of niacin in the management of hyperlipidemia. Am. J. Cardiol. 85: 1100–1105. [DOI] [PubMed] [Google Scholar]
- 5.Canner P. L., Berge K. G., Wenger N. K., Stamler J., Friedman L., Prineas R. J., and Friedewald W.. 1986. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J. Am. Coll. Cardiol. 8: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 6.Carlson L. A., and Rosenhamer G.. 1988. Reduction of mortality in the Stockholm Ischaemic Heart Disease Secondary Prevention Study by combined treatment with clofibrate and nicotinic acid. Acta Med. Scand. 223: 405–418. [DOI] [PubMed] [Google Scholar]
- 7.CDP. 1975. Clofibrate and niacin in coronary heart disease. JAMA. 231: 360–381. [PubMed] [Google Scholar]
- 8.Dunbar R. L., and Gelfand J. M.. 2010. Seeing red: flushing out instigators of niacin-associated skin toxicity. J. Clin. Invest. 120: 2651–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamal-Bahl S., Watson D. J., and Ambegaonkar B. M.. 2009. Patients’ experiences of niacin-induced flushing in clinical practice: a structured telephone interview. Clin. Ther. 31: 130–140. [DOI] [PubMed] [Google Scholar]
- 10.Mills E., Prousky J., Raskin G., Gagnier J., Rachlis B., Montori V. M., and Juurlink D.. 2003. The safety of over-the-counter niacin. A randomized placebo-controlled trial. BMC Clin. Pharmacol. 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg A. C. 1998. Clinical trial experience with extended-release niacin (Niaspan): dose-escalation study. Am. J. Cardiol. 82(12A): 35U–38U. [DOI] [PubMed] [Google Scholar]
- 12.Boden W. E., Probstfield J. L., Anderson T., and Chaitman B. R.. 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255–2267. [DOI] [PubMed] [Google Scholar]
- 13.Landray, M. J., R. Haynes, J. C. Hopewell, Parish S. , T. Aung, Tomson J., Wallendszus K., Craig M., Jiang L., Collins R. , et al.. 2014. Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med. 371: 203–212. [DOI] [PubMed] [Google Scholar]
- 14.Dunbar R. L., and Goel H.. 2016. Niacin alternatives for dyslipidemia: fool’s gold or gold mine? Part I: alternative niacin regimens. Curr. Atheroscler. Rep. 18: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholls S. J. 2012. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve. Clin. J. Med. 79: 38–43. [DOI] [PubMed] [Google Scholar]
- 16.Bloomgarden Z., and Handelsman Y.. 2012. Did AIM-HIGH aim too low? J. Diabetes. 4: 1–2. [DOI] [PubMed] [Google Scholar]
- 17.Brinton E. A. 2012. Search and rescue for hypotheses surviving AIM-HIGH, the niacin therapy earthquake: Still problematic after the primary publication. J. Clin. Lipidol. 6: 312–317. [DOI] [PubMed] [Google Scholar]
- 18.Usman M. H. U., Qamar A., Gadi R., Lilly S., Goel H., Hampson J., Mucksavage M. L., Nathanson G. A., Rader D. J., and Dunbar R. L.. 2012. Extended-release niacin acutely suppresses postprandial triglyceridemia. Am. J. Med. 125: 1026–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goel H., and Dunbar R. L.. 2016. Niacin alternatives for dyslipidemia: fool’s gold or gold mine? Part II: novel niacin mimetics. Curr. Atheroscler. Rep. 18: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyers C. D., Liu P., Kamanna V. S., and Kashyap M. L.. 2007. Nicotinic acid induces secretion of prostaglandin D2 in human macrophages: an in vitro model of the niacin flush. Atherosclerosis. 192: 253–258. [DOI] [PubMed] [Google Scholar]
- 21.Hanson J., Gille A., Zwykiel S., Lukasova M., Clausen B. E., Ahmed K., Tunaru S., Wirth A., and Offermanns S.. 2010. Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J. Clin. Invest. 120: 2910–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrow J. D., Parsons W. G., and Roberts L. J.. 1989. Release of markedly increased quantities of prostaglandin D2 in vivo in humans following the administration of nicotinic acid. Prostaglandins. 38: 263–274. [DOI] [PubMed] [Google Scholar]
- 23.Soga T., Kamohara M., Takasaki J., Matsumoto S., Saito T., Ohishi T., Hiyama H., Matsuo A., Matsushime H., and Furuichi K.. 2003. Molecular identification of nicotinic acid receptor. Biochem. Biophys. Res. Commun. 303: 364–369. [DOI] [PubMed] [Google Scholar]
- 24.Tunaru S., Kero J., Schaub A., Wufka C., Blaukat A., and Pfeffer K. O. S.. 2003. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 9: 352–355. [DOI] [PubMed] [Google Scholar]
- 25.Wise A., Foord S. M., Fraser N. J., Barnes A. A., Elshourbagy N., Eilert M., Ignar D. M., Murdock P. R., Steplewski K., Green A., et al. 2003. Molecular identification of high and low affinity receptors for nicotinic acid. J. Biol. Chem. 278: 9869–9874. [DOI] [PubMed] [Google Scholar]
- 26.Benyó, Z., Gille A., Kero J., Csiky M., Suchánková M. C., Nüsing R. M., Moers A., Pfeffer K., and Offermanns S.. 2005. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J. Clin. Invest. 115: 3634–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawata A. K., Revicki D. A., Thakkar R., Jiang P., Krause S., Davidson M. H., Punzi H. A., and Padley R. J.. 2009. Flushing assessment tool (FAST) psychometric properties of a new measure assessing flushing symptoms and clinical impact of niacin therapy. Clin. Drug Investig. 29: 215–229. [DOI] [PubMed] [Google Scholar]
- 28.Paolini J. F., Mitchel Y. B., Reyes R., Thompson-Bell S., Yu Q., Lai E., Watson D. J., Norquist J. M., McCrary Sisk C., and Bays H. E.. 2008. Measuring flushing symptoms with extended-release niacin using the flushing symptom questionnaire: results from a randomised placebo-controlled clinical trial. Int. J. Clin. Pract. 62: 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norquist J. M., Watson D. J., Yu Q., Paolini J. F., McQuarrie K., and Santanello N. C.. 2007. Validation of a questionnaire to assess niacin-induced cutaneous flushing. Curr. Med. Res. Opin. 23: 1549–1560. [DOI] [PubMed] [Google Scholar]
- 30.Lai E., De Lepeleire I., Crumley T. M., Liu F., Wenning L. A., Michiels N., Vets E., O’Neill G., Wagner J. A., and Gottesdiener K.. 2007. Suppression of niacin-induced vasodilation with an antagonist to prostaglandin D2 receptor subtype 1. Clin. Pharmacol. Ther. 81: 849–857. [DOI] [PubMed] [Google Scholar]
- 31.Gille A., Bodor E. T., Ahmed K., and Offermanns S.. 2008. Nicotinic acid: pharmacological effects and mechanisms of action. Annu. Rev. Pharmacol. Toxicol. 48: 79–106. [DOI] [PubMed] [Google Scholar]
- 32.Richman J. G., Kanemitsu-Parks M., Gaidarov I., Cameron J. S., Griffin P., Zheng H., Guerra N. C., Cham L., Maciejewski-Lenoir D., Behan D. P., et al. 2007. Nicotinic acid receptor agonists differentially activate downstream effectors. J. Biol. Chem. 282: 18028–18036. [DOI] [PubMed] [Google Scholar]
- 33.Wanders D., and Judd R. L.. 2011. Future of GPR109A agonists in the treatment of dyslipidaemia. Diabetes Obes. Metab. 13: 685–691. [DOI] [PubMed] [Google Scholar]
- 34.Walters R. W., Shukla A. K., Kovacs J. J., Violin J. D., Dewire S. M., Lam C. M., Chen J. R., Muehlbauer M. J., Whalen E. J., and Lefkowitz R. J.. 2009. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J. Clin. Invest. 119: 1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boatman P. D., Schrader T. O., Kasem M., Johnson B. R., Skinner P. J., Jung J. K., Xu J., Cherrier M. C., Webb P. J., Semple G., et al. 2010. Potent tricyclic pyrazole tetrazole agonists of the nicotinic acid receptor (GPR109a). Bioorg. Med. Chem. Lett. 20: 2797–2800. [DOI] [PubMed] [Google Scholar]
- 36.Ross B. M., Hughes B., Turenne S., Seeman M., and Warsh J. J.. 2004. Reduced vasodilatory response to methylnicotinate in schizophrenia as assessed by laser Doppler flowmetry. Eur. Neuropsychopharmacol. 14: 191–197. [DOI] [PubMed] [Google Scholar]
- 37.Berger G. E., Smesny S., Schäfer M. R., Milleit B., Langbein K., Hipler U. C., Milleit C., Klier C. M., Schlögelhofer M., Holub M., et al. 2016. Niacin skin sensitivity is increased in adolescents at ultra-high risk for psychosis. PLoS One. 11: e0148429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.English J. S. C., Winkelmann R. K., Louback B., Greaves M. W., and Macdonald D. M.. 1987. The cellular inflammatory response in nicotinate skin reactions. Br. J. Dermatol. 116: 341–349. [DOI] [PubMed] [Google Scholar]
- 39.Suh D. H., Kwon T. E., Kim S. D., Park S. B., Kwon O. S., Eun H. C., and Youn J. I.. 2001. Changes of skin blood flow and color on lesional and control sites during PUVA therapy for psoriasis. J. Am. Acad. Dermatol. 44: 987–994. [DOI] [PubMed] [Google Scholar]
- 40.Tang H., Lu J. Y. L., Zheng X., Yang Y., and Reagan J. D.. 2008. The psoriasis drug monomethylfumarate is a potent nicotinic acid receptor agonist. Biochem. Biophys. Res. Commun. 375: 562–565. [DOI] [PubMed] [Google Scholar]
- 41.Hedén P. G., Hamilton R., Arnander C., and Jurell G.. 1985. Laser Doppler surveillance of the circulation of free flaps and replanted digits. Microsurgery. 6: 11–19. [DOI] [PubMed] [Google Scholar]
- 42.Yeong E. K., Mann R., Goldberg M., Engrav L., and Heimbach D.. 1996. Improved accuracy of burn wound assessment using laser Doppler. J. Trauma. 40: 956–961. [DOI] [PubMed] [Google Scholar]
- 43.Redaelli C. A., Schilling M. K., and Buchler M. W.. 1998. Intraoperative laser Doppler flowmetry: a predictor of ischemic injury in acute mesenteric infarction. Dig. Surg. 15: 55–59. [DOI] [PubMed] [Google Scholar]
- 44.Schabauer A. M., and Rooke T. W.. 1994. Cutaneous laser Doppler flowmetry: applications and findings. Mayo Clin. Proc. 69: 564–574. [DOI] [PubMed] [Google Scholar]
- 45.Serup J., and Agner T.. 1990. Colorimetric quantification of erythema–a comparison of two colorimeters (Lange Micro Color and Minolta Chroma Meter CR-200) with a clinical scoring scheme and laser-Doppler flowmetry. Clin. Exp. Dermatol. 15: 267–272. [DOI] [PubMed] [Google Scholar]
- 46.Lahti A., Kopola H., Harila A., Myllylä R., and Hannuksela M.. 1993. Assessment of skin erythema by eye, laser Doppler flowmeter, spectroradiometer, two-channel erythema meter and Minolta chroma meter. Arch. Dermatol. Res. 285: 278–282. [DOI] [PubMed] [Google Scholar]
- 47.Diffey B. L., Oliver R. J., and Farr P. M.. 1984. A portable instrument for quantifying erythema induced by ultraviolet radiation. Br. J. Dermatol. 111: 663–672. [DOI] [PubMed] [Google Scholar]
- 48.Friedland S., Benaron D., Coogan S., Sze D. Y., and Soetikno R.. 2007. Diagnosis of chronic mesenteric ischemia by visible light spectroscopy during endoscopy. Gastrointest. Endosc. 65: 294–300. [DOI] [PubMed] [Google Scholar]
- 49.Hölzle F., Rau A., Loeffelbein D. J., Mücke T., Kesting M. R., and Wolff K. D.. 2010. Results of monitoring fasciocutaneous, myocutaneous, osteocutaneous and perforator flaps: 4-year experience with 166 cases. Int. J. Oral Maxillofac. Surg. 39: 21–28. [DOI] [PubMed] [Google Scholar]
- 50.Cracowski J-L., Minson C. T., Salvat-Melis M., and Halliwill J. R.. 2006. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol. Sci. 27: 503–508. [DOI] [PubMed] [Google Scholar]
- 51.Humeau A., Steenbergen W., Nilsson H., Strömberg T.. 2007. Laser Doppler perfusion monitoring and imaging: novel approaches. Med. Biol. Eng. Comput. 45: 421–435. [DOI] [PubMed] [Google Scholar]
- 52.Wilkin J. K. 1986. Quantitative assessment of alcohol-provoked flushing. Arch. Dermatol. 122: 63–65. [PubMed] [Google Scholar]
- 53.Kraemer R., Lorenzen J., Knobloch K., Papst S., Kabbani M., Koennecker S., and Vogt P. M.. 2011. Free flap microcirculatory monitoring correlates to free flap temperature assessment. J. Plast. Reconstr. Aesthet. Surg. 64: 1353–1358. [DOI] [PubMed] [Google Scholar]
- 54.Wilkin J. K. 1982. A quantitative, non-invasive method for the characterization of flushing reactions. J. Invest. Dermatol. 78: 276–279. [DOI] [PubMed] [Google Scholar]
- 55.Parson H. K., Harati H., Cooper D., and Vinik A. I.. 2013. Role of prostaglandin D2 and the autonomic nervous system in niacin-induced flushing. J. Diabetes. 5: 59–67. [DOI] [PubMed] [Google Scholar]
- 56.Aberg G., and Svedmyr N.. 1971. Thermographic registration of flush. Arzneimittelforschung. 21: 795–796. [PubMed] [Google Scholar]
- 57.Wilkin J. K., Wilkin O., Kapp R., Donachie R., Chernosky M. E., and Buckner J.. 1982. Aspirin blocks nicotinic acid-induced flushing. Clin. Pharmacol. Ther. 31: 478–482. [DOI] [PubMed] [Google Scholar]
- 58.Pontiroli A. E., Fattor B., Pozza G., Pianezzola E., Benedetti M., and Musatti L.. 1992. Acipimox-induced facial skin flush: frequency, thermographic evaluation and relationship to plasma acipimox level. Eur. J. Clin. Pharmacol. 43: 145–148. [DOI] [PubMed] [Google Scholar]
- 59.Kalogeromitros D., Makris M., Chlica C., Aggelides X., Kempuraj D., and Theoharides T. C.. 2008. A quercetin containing supplement reduces niacin-induced flush in humans. Int. J. Immunopathol. Pharmacol. 21: 509–514. [DOI] [PubMed] [Google Scholar]
- 60.Moriarty P. M., Backes J., Dutton J. A., He J., Ruisinger J. F., and Schmelzle K.. 2013. Apple pectin for the reduction of niacin-induced flushing. J. Clin. Lipidol. 7: 140–146. [DOI] [PubMed] [Google Scholar]
- 61.Ma L., Lee B. H., Mao R., Cai A., Jia Y., Clifton H., Schaefer S., Xu L., and Zheng J.. 2014. Nicotinic acid activates the capsaicin receptor TRPV1: Potential mechanism for cutaneous flushing. Arterioscler. Thromb. Vasc. Biol. 34: 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clifton H. L., Inceoglu B., Ma L., Zheng J., and Schaefer S.. 2015. TRPV1 channels are involved in niacin-induced cutaneous vasodilation in mice. J. Cardiovasc. Pharmacol. 65: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roustit M., Blaise S., Millet C., and Cracowski J. L.. 2010. Reproducibility and methodological issues of skin post-occlusive and thermal hyperemia assessed by single-point laser Doppler flowmetry. Microvasc. Res. 79: 102–108. [DOI] [PubMed] [Google Scholar]
- 64.Hertzman A. 1953. Am. J. Phys. Med. 32: 233–251. [PubMed] [Google Scholar]
- 65.Nilsson A. L. 1987. Blood flow, temperature, and heat loss of skin exposed to local radiative and convective cooling. J. Invest. Dermatol. 88: 586–593. [DOI] [PubMed] [Google Scholar]
- 66.Elam M., and Wallin B. G.. 1987. Skin blood flow responses to mental stress in man depend on body tempereture. Acta Physiol. Scand. 129: 429–431. [DOI] [PubMed] [Google Scholar]
- 67.Wilkin J. K. 1987. Cognitive activity and cutaneous blood flow. Arch. Dermatol. 123: 1503–1506. [PubMed] [Google Scholar]
- 68.Tur E., Tur M., Maibach H. I., and Guy R. H.. 1983. Basal perfusion of the cutaneous microcirculation: measurements as a function of anatomic position. J. Invest. Dermatol. 81: 442–446. [DOI] [PubMed] [Google Scholar]
- 69.Hertzman A. B., and Randall W. C.. 1948. Regional differences in the basal and maximal rates of blood flow in the skin. J. Appl. Physiol. 1: 234–241. [DOI] [PubMed] [Google Scholar]
- 70.Braverman I. M. 2000. The cutaneous microcirculation. J. Investig. Dermatol. Symp. Proc. 5: 3–9. [DOI] [PubMed] [Google Scholar]
- 71.Braverman I. M., Keh A., and Goldminz D.. 1990. Correlation of laser Doppler wave patterns with underlying microvascular anatomy. J. Invest. Dermatol. 95: 283–286. [DOI] [PubMed] [Google Scholar]
- 72.Fullerton A., Fischer T., Lahti A., Wilhelm K. P., Takiwaki H., and Serup J.. 1996. Guidelines for measurement of skin colour and erythema. A report from the Standardization Group of the European Society of Contact Dematitis. Contact Dermatitis. 35: 1–10. [DOI] [PubMed] [Google Scholar]
- 73.Bircher A., de Boer E. M., Agner T., Wahlberg J. E., and Serup J.. 1994. Guidelines for measurement of cutaneous blood flow by laser Doppler flowmetry. A report from the Standardization Group of the European Society of Contact Dematitis. Contact Dermatitis. 30: 65–72. [DOI] [PubMed] [Google Scholar]
- 74.Harvell J., Hussona-Saeed I., and Maibach H. I.. 1992. Changes in transdermal water loss and cutaneous blood flow during the menstrual cycle. Contact Dermatitis. 27: 294–301. [DOI] [PubMed] [Google Scholar]
- 75.Agner T., Damm P., and Skouby S. O.. 1991. Menstrual cycle and skin reactivity. J. Am. Acad. Dermatol. 24: 566–570. [DOI] [PubMed] [Google Scholar]
- 76.Leahy M. J., de Mul F. F., Nilsson G. E., and Maniewski R.. 1999. Principles and practice of the laser-Doppler perfusion technique. Technol. Health Care. 7: 143–162. [PubMed] [Google Scholar]
- 77.Jakobsson A., and Nilsson G. E.. 1993. Prediction of sampling depth and photon pathlength in laser Doppler flowmetry. Med. Biol. Eng. Comput. 31: 301–307. [DOI] [PubMed] [Google Scholar]
- 78.Fredriksson I., Larsson M., and Strömberg T.. 2009. Measurement depth and volume in laser Doppler flowmetry. Microvasc. Res. 78: 4–13. [DOI] [PubMed] [Google Scholar]
- 79.Fromy B., Abraham P., and Saumet J. L.. 1998. Non-nociceptive capsaicin-sensitive nerve terminal stimulation allows for an original vasodilatory reflex in the human skin. Brain Res. 811: 166–168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.