Abstract
Background.
Immunodeficient individuals who excrete vaccine-derived polioviruses threaten polio eradication. Antivirals address this threat.
Methods.
In a randomized, blinded, placebo-controlled study, adults were challenged with monovalent oral poliovirus type 1 vaccine (mOPV1) and subsequently treated with capsid inhibitor pocapavir or placebo. The time to virus negativity in stool was determined.
Results.
A total of 144 participants were enrolled; 98% became infected upon OPV challenge. Pocapavir-treated subjects (n = 93) cleared virus a median duration of 10 days after challenge, compared with 13 days for placebo recipients (n = 48; P = .0019). Fifty-two of 93 pocapavir-treated subjects (56%) cleared virus in 2–18 days with no evidence of drug resistance, while 41 of 93 (44%) treated subjects experienced infection with resistant virus while in the isolation facility, 3 (3%) of whom were infected at baseline, before treatment initiation. Resistant virus was also observed in 5 placebo recipients (10%). Excluding those with resistant virus, the median time to virus negativity was 5.5 days in pocapavir recipients, compared with 13 days in placebo recipients (P < .0001). There were no serious adverse events and no withdrawals from the study.
Conclusions.
Treatment with pocapavir was safe and significantly accelerated virus clearance. Emergence of resistant virus and transmission of virus were seen in the context of a clinical isolation facility.
Clinical Trials Registration.
EudraCT 2011-004804-38.
Keywords: Polio, antiviral, pocapavir, poliovirus, clinical efficacy, drug resistance, virus challenge, transmission, clinical trial design, virus eradication.
(See the editorial commentary by Sutter et al on pages 333–4.)
As we approach the final stages of poliovirus eradication, challenges remain. Use of live oral poliovirus vaccines (OPVs) is being phased out globally as wild poliovirus disappears and should eliminate the source of circulating vaccine-derived polioviruses (VDPVs) capable of causing paralytic disease [1–3]. Cessation of OPV use will also stop generation of new cases of immunodeficiency-associated VDPVs (iVDPVs). Patients infected with iVDPVs have primary immune deficiencies that result in their inability to clear an OPV infection and can result in a chronic infection in which virus is excreted for years. Once OPV use stops, patients with iVDPV infection will represent the last reservoirs of poliovirus. Improved and expanded surveillance initiatives must identify these patients, and once they are identified, there must be a means of stopping virus excretion and resolving the underlying infection. Antiviral drugs represent the most plausible solution to this threat [4, 5]. Currently, there are no antipoliovirus drugs available.
Pocapavir (V-073) is an investigational drug that acts as a capsid inhibitor, preventing virion uncoating upon entry into the cell [5]. Pocapavir is particularly potent against polioviruses in laboratory tests [6, 7] and thus may have utility in the management of incidents of poliovirus infection during the eradication, certification, and posteradication periods. Pocapavir also exhibits activity against non–polio-associated enteroviruses and may be useful in treating serious enterovirus infections [8, 9]. Here, we report evaluation of pocapavir in humans challenged with monovalent oral poliovirus type 1 vaccine (mOPV1). The objectives of the study were to assess safety, pharmacokinetics (PK), and antiviral activity of pocapavir in healthy adult subjects.
METHODS
Study Design
We conducted this randomized, blinded, placebo-controlled study to evaluate therapeutic efficacy, safety, and PK of orally administered pocapavir at the Clinical Trial Center, Sahlgrenska University Hospital (Göteborg, Sweden). The study was sponsored by ViroDefense (Chevy Chase, MD), monitored by Quintiles (London, United Kingdom), and registered under the European Union Clinical Trials Register (EudraCT 2011-004804-38). The protocol (available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-004804-38/SE) and informed consent forms were reviewed and approved by the Göteborg Regional Ethical Review Board. This trial was conducted and essential study documentation archived in compliance with International Conference on Harmonization Guidelines and Good Clinical Practice.
Participants
Healthy Swedish volunteers 18–50 years old who had received the recommended Swedish childhood vaccination (4 injections of IPV) were screened for total and poliovirus-specific serum immunoglobulin A (IgA) antibody [10]. Subjects positive for total serum IgA (a measure of immune competency) and negative for poliovirus-specific IgA (an indicator that exposure to live poliovirus was unlikely and, therefore, that mOPV1 replication was likely) were enrolled in the study. Additional inclusion and exclusion criteria are provided in the protocol referenced above. Subjects attended a screening visit before the study start, where medical history was collected, vital signs were measured, and drug and alcohol screening, clinical laboratory testing, 12-lead echocardiography (ECG), and a complete physical examination were performed. All subjects provided written informed consent during the screening visit. Participants entered the clinic in groups of about 18 and were assigned to sleeping rooms, with 6 to a room (3 rooms total), and shared dining, entertainment, and lavatory facilities. Subjects remained isolated in their groups through the end of their 14-day dosing period.
Randomization
Allocation to treatment group (pocapavir [ie, active treatment] or placebo) was according to a SAS-generated randomization list. All clinical and nonclinical staff, including the investigator, laboratory, data management, virology, and serology staff, were blinded to treatment allocation until after database lock. However, during the study the following individuals were unblinded: the statistician preparing the randomization; staff at AAI Pharma and Penn Pharma, who labeled capsule bottles and kits; staff from the bioanalytical group at Charles River Laboratories; and quality assurance auditors, when necessary. The sponsor was also unblinded to assess early virology data.
Procedures
Baseline serologic data (for total IgA, poliovirus type 1–specific IgA, and serum neutralizing antibody) were obtained prior to entering the clinic [10]. Eight consecutive groups of 18 subjects in close succession reported to the facility on study day −1. The following day (day 0), all subjects received 1 vaccine dose (median cell culture infective dose [CCID50], 106) of mOPV1. On study day 1 or 3, subjects were randomly assigned at a ratio of 2:1 to initiate a 14-day course of treatment with either pocapavir or placebo. Clinical laboratory testing and 12-lead ECG were performed and vital signs measured during the dosing period. Blood samples were collected before delivery of the morning dose for determination of pocapavir trough concentrations, and repeat samples were collected on days 1 and 14 for determination of the PK profile. Adverse events were monitored daily and recorded in subject diaries. Using specified hygienic procedures, stool samples were collected from all subjects prior to mOPV1 challenge. During the 14-day dosing period, all stools from every subject were collected. Thereafter, stools were collected weekly for 4 weeks. Aliquots of all stool specimens were packaged in a designated area within the clinical unit for shipment to collaborating laboratories.
Subjects received placebo or 1600 mg pocapavir (8 capsules) per day according to the following 4 dosing regimens: once-daily dosing after consuming a high-fat meal (fat content, 60–75 g), starting 72 hours after mOPV1 exposure (the QD3HF group); twice-daily dosing (800 mg each) after a high-fat meal, starting 72 hours after mOPV1 exposure (the BID3HF group); once-daily dosing after a high-fat meal, starting 24 hours after mOPV1 exposure (the QD1HF group); and once-daily dosing after a standard meal (fat content, <25 g), starting 72 hours after mOPV1 exposure (the QD3STD group).
Chloroform-extracted stool samples were determined to be virus positive or negative, according to the presence of a virus-induced cytopathic effect on L20B cells [11]. Virus-positive samples were quantified by limiting dilution and, together with stool weight, were used to determine the level of daily and cumulative virus excretion during the in-clinic dosing period. Drug susceptibility was determined by analysis of a baseline stool sample obtained at baseline (defined as the first available stool specimen obtained after mOPV1 administration and before dosing initiation) and on the last day of virus positivity from each subject. Poliovirus-specific serum neutralization antibody titers were determined using standard methods [10] for samples obtained at baseline and on the last study day.
All clinical laboratory safety tests, including hematologic analysis (of white blood cell count and differential, red blood cells, hemoglobin, and platelets), serum biochemistry analysis (of sodium, potassium, creatinine, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, and bilirubin), and urinalysis (of protein, ketones, glucose, leukocytes, and red blood cells) were performed at the Clinical Chemistry Laboratory, Sahlgrenska University Hospital (Göteborg, Sweden). Drug levels in plasma were determined by a validated liquid chromatography/mass spectrometry method at Charles River Laboratories (Edinburgh, United Kingdom). PK, pharmacodynamic (PD), and statistical analyses were performed at Quintiles Biostatistics (Bloemfontein, South Africa, and Overland Park, KS). Virologic, serologic, nucleic acid sequencing and sequence analyses were performed at the Dutch National Institute for Public Health and the Environment (Bilthoven, the Netherlands). Virus quantification and susceptibility analyses were performed at the Centers for Disease Control and Prevention (Atlanta, GA).
Outcomes
The study’s primary efficacy measure was time from initiation of treatment to the first of 2 consecutive sampling days with no detection of virus in stool that were followed by no more than 2 nonconsecutive days with virus detection in stool during the remainder of the study. Safety and adverse events were assessed. The cumulative level of secreted virus during the in-clinic treatment period was a secondary end point. Drug susceptibility and poliovirus-specific serum neutralization antibody titers were determined. The PK profile of pocapavir after the first (day 1) and last (day 14) dose confirmed that drug exposure was at a level expected to have antiviral activity.
Statistical Analysis
Based on virologic data from the planned interim assessment of the first two groups of 18 subjects (clinic groups 1 and 2), a hazard ratio of 0.322 was observed between pocapavir recipients (n = 11) and placebo recipients (n = 11; P = .0119). As a result, cohort size was set at 24 subjects for pocapavir groups, resulting in 80% power to detect the difference between each cohort-matched treatment group, using a 2-sided log-rank test with a significance level of .05. PK parameters were derived by noncompartmental methods, using WinNonlin, version 5.2 (Pharsight, St. Louis, MO), or SAS, version 9.2 or higher (SAS Institute, Cary, NC). Log-transformed values of PK parameters were analyzed in an analysis of variance (ANOVA) model with treatment as a fixed effect. Geometric least squares means together with 2-sided 95% confidence intervals (CIs), stratified by treatment, and geometric least squares mean ratios for all cohort-matched treatment groups with 2-sided 90% CIs were presented for each PK parameter. Graphics were prepared with SAS, version 9.2 or higher; SigmaPlot 9.2 (Systat Software, San Jose, CA); or KaleidaGraph 4.5 (Synergy Software, Reading, PA).
Based on the qualitative results of virology, the times to negative virus cultures and detection of drug-susceptible virus for each subject were measured in days from both the day of initiation of treatment and the day of OPV receipt. Daily (24-hour) total levels of virus excreted were compared using a 1-way ANOVA model with treatment as a fixed effect. Cumulative levels of virus excreted during dosing days 1–14 were compared between pocapavir recipients and cohort-matched placebo recipients. A nonparametric estimate of survival function for the presence of virus was computed for each cohort of placebo recipients, each cohort of pocapavir recipients, pooled placebo recipients, and pooled pocapavir recipients, using Kaplan-Meier estimates. Comparisons between pocapavir recipients and cohort-matched placebo recipient and between pooled placebo recipients and pooled pocapavir recipients were conducted using Wilcoxon test. P values are presented. The SAS PROC LIFETEST was used for analysis of time to virus negativity in stool specimens. The hazard ratio (calculated as the ratio of the hazard for pooled data from pocapavir recipients versus the hazard for pooled data from placebo recipients) of time to virus negativity in stool specimens and the corresponding 95% CI were estimated using a Cox proportional hazard model with treatment as a model term. Least squares means together with corresponding 95% CIs are provided for each treatment for the difference (or ratio, for log-transformed data) between pocapavir recipients and cohort-matched placebo recipients and between pooled pocapavir recipients and pooled placebo recipients.
RESULTS
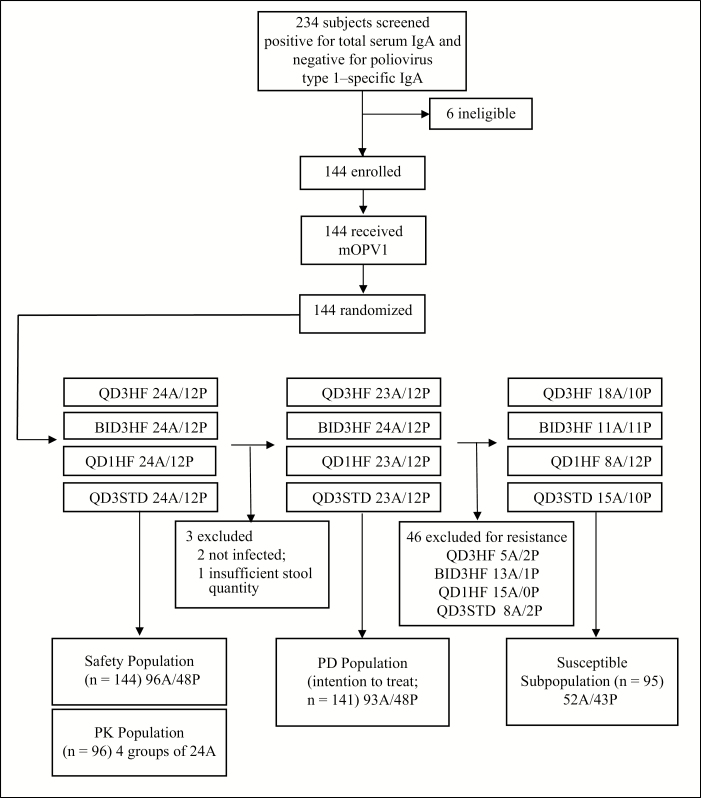
Between 10 January and 18 December 2012, healthy nonsmoking male and female Swedish subjects who were 18–50 years old and received IPV during childhood were evaluated for study eligibility. Of 234 volunteers screened, 228 (97%) were negative for poliovirus type 1–specific IgA; all were positive for total serum IgA, thus excluding IgA immunodeficiency. One hundred forty-four volunteers were enrolled, challenged with mOPV1, and randomly assigned at a ratio of 2:1 to receive pocapavir or placebo allocated into 4 treatment cohorts (Figure 1).
Figure 1.
Study profile. Subjects received placebo or 1600 mg of pocapavir (8 capsules) per day according to the following 4 dosing regimens: once-daily dosing after consuming a high-fat meal (fat content, 60–75 g), starting 72 hours after monovalent oral poliovirus type 1 vaccine (mOPV1) exposure (QD3HF); twice-daily dosing (800 mg each) after a high-fat meal, starting 72 hours after mOPV1 exposure (BID3HF); once-daily dosing after a high-fat meal, starting 24 hours after mOPV1 exposure (QD1HF); and once-daily dosing after a standard meal (fat content, <25 g) starting 72 hours after mOPV1 exposure (QD3STD). Abbreviations: A, subjects in the active (pocapavir)–treatment group; IgA, immunoglobulin A; P, subjects in the placebo group; PD, pharmacodynamic; PK, pharmacokinetic.
No significant differences in baseline characteristics were noted among cohorts (Table 1). There were no deaths and no drug-related serious adverse events. All subjects were poliovirus negative before entering the clinic. All completed the study, and all were negative for virus in stool specimens on the last study day. Baseline serum neutralizing antibody titers ranged from 1:4 to 1:128 (median, 1:8). There was a substantial rise (≥4-fold; median, 16-fold) in serum neutralizing antibody titer between the prestudy time point and the last study day (day 43–45) after mOPV1 challenge for 138 of the 144 serum pairs (96%). Of the remaining 6, 4 became infected upon OPV challenge and 2 appeared uninfected. The latter were excluded from efficacy analyses. One additional subject was excluded for insufficient stool sampling, resulting in a PD population of 141.
Table 1.
Baseline Characteristics of Subjects, by Cohort and Treatment Group
| Characteristic | QD3HF | BID3HF | QD1HF | QD3STD | Overall | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 12) | Pocapavir (n = 24) | Placebo (n = 12) | Pocapavir (n = 24) | Placebo (n = 12) | Pocapavir (n = 24) | Placebo (n = 12) | Pocapavir (n = 24) | Placebo (n = 48) | Pocapavir (n = 96) | |
| Race | ||||||||||
| Asian | 0 | 0 | 0 | 0 | 0 | 0 | 2 (17) | 0 | 2 (4) | 0 |
| White | 12 (100) | 24 (100) | 12 (100) | 24 (100) | 12 (100) | 24 (100) | 10 (83) | 24 (100) | 46 (96) | 96 (100) |
| Sex | ||||||||||
| Female | 2 (17) | 8 (33) | 2 (17) | 7 (29) | 4 (33) | 8 (33) | 3 (25) | 9 (38) | 11 (23) | 32 (33) |
| Male | 10 (83) | 16 (67) | 10 (83) | 17 (71) | 8 (67) | 16 (67) | 9 (75) | 15 (63) | 37 (77) | 64 (67) |
| Age, y | 24.1 ± 3.85 | 24.4 ± 3.82 | 28.3 ± 5.63 | 27 ± 5.39 |
23.3 ± 2.05 | 25.4 ± 2.72 |
24.5 ± 3.26 | 24.4 ± 3.98 |
25.0 ± 4.27 | 25.3 ± 4.10 |
| Height, cm |
180.8 ± 6.51 | 176.8 ± 9.34 | 180.4 ± 8.61 | 179.1 ± 10.39 | 173.2 ± 8.52 | 179.1 ± 8.35 | 176.9 ± 8.66 | 175.5 ± 9.81 | 177.8 ± 8.45 | 177.6 ± 9.47 |
| Weight, kg |
76.6 ± 12.13 | 74.0 ± 13.83 | 83.2 ± 14.36 | 76.2 ± 15.10 | 69.5 ± 6.41 | 75.0 ± 11.62 | 74.6 ± 12.03 | 70.5 ± 11.06 | 76.0 ± 12.29 | 73.9 ± 12.90 |
| BMIa
|
23.4 ± 2.58 | 23.5 ± 3.06 |
25.5 ± 3.03 | 23.5 ± 2.83 |
23.3 ± 1.55 | 23.4 ± 2.45 |
24.0 ± 3.28 | 22.8 ± 2.30 |
24.0 ± 2.75 | 23.3 ± 2.66 |
Data are no. (%) of subjects or mean value ± SD. Subjects received placebo or 1600 mg of pocapavir (8 capsules) per day according to the following 4 dosing regimens: once-daily dosing after consuming a high-fat meal (fat content, 60–75 g), starting 72 hours after monovalent oral poliovirus type 1 vaccine (mOPV1) exposure (QD3HF); twice-daily dosing (800 mg each) after a high-fat meal, starting 72 hours after mOPV1 exposure (BID3HF); once-daily dosing after a high-fat meal, starting 24 hours after mOPV1 exposure (QD1HF); and once-daily dosing after a standard meal (fat content, <25 g) starting 72 hours after mOPV1 exposure (QD3STD).
aBody mass index (BMI) is calculated as the weight in kilograms divided by the height in meters squared.
Subjects received placebo or 1600 mg of pocapavir daily. Study variables for the four 36-member cohorts included once-daily dosing versus split dosing, dosing initiation on study day 1 versus study day 3 after OPV administration, or receipt of treatment after consuming either a high-fat or standard meal.
The PK profile of pocapavir was determined on dosing days 1 and 14 (Table 2). The mean concentration time profiles were similar among dosing cohorts (Supplementary Figure S1). There were no significant differences in pocapavir exposure (a measured by peak concentration [Cmax] and area under the curve [AUC] values) between dosing days 1 and day 14 within each cohort. Cmax and AUC levels were approximately 2-fold higher when pocapavir was administered after high-fat meals (ie, in the QD3HF and QD1HF groups), compared with administration with a standard meal (in the QD3STD group), or as a split dose for the twice-daily high fat group, compared with the once-daily high-fat groups. Drug levels for all dosing regimens were well above the mean effective in vitro antiviral inhibitory concentration (EC50), defined as 10 ng/mL or 0.024 µM.
Table 2.
Pharmacokinetic Characteristics Among Subjects Who Received Pocapavir, by Cohort and Dosing Day
| QD3HF (n = 24) | QD1HF (n = 24) | QD3STD (n = 24) | BID3HF (n = 24) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Day 1 | Day 14 | Day 1 | Day 14 | Day 1 | Day 14 | Day 1 (Morning) | Day 1 (Evening) | Day 1 (Combined) | Day 14 (Morning) | Day 14 (Evening) | Day 14 (Combined) |
| Cmax | ||||||||||||
| Geometric mean, ng/mL | 2860 | 2680 | 2710 | 3110 | 1410 | 1340 | 1440 | 1730 | … | 1730 | 1540 | … |
| Coefficient of variation, % | 47.6 | 33.8 | 44.8 | 45.9 | 42.4 | 40.5 | 46.9 | 33.1 | … | 46.6 | 31.7 | … |
| AUC | ||||||||||||
| Geometric mean, ng•h/mL | 15400 | 16700 | 15500 | 17700 | 7850 | 8170 | 6000 | 10300 | 16500 | 9000 | 9430 | 18600 |
| Coefficient of variation, % | 47.6 | 31.8 | 42.4 | 28.2 | 42.3 | 50.3 | 39.3 | 33.7 | 31.7 | 39.1 | 28.5 | 31.3 |
| Tmax, h, median | 4.98 | 4.97 | 4.97 | 4.97 | 4.96 | 4.96 | 4.99 | 5.99 | … | 4.98 | 6 | … |
| Cmin | ||||||||||||
| Geometric mean, ng/mLa | 75.5 | 130 | 85 | 154 | 39.1 | 71.6 | 191 | 701 | … | 337 | 453 | … |
| Coefficient of variation, % | 88.2 | 56 | 70 | 32.3 | 46.7 | 53.4 | 98.5 | 87.3 | … | 56.5 | 57.4 | … |
Subjects received 1600 mg of pocapavir (8 capsules) per day according to the following 4 dosing regimens: once-daily dosing after consuming a high-fat meal (fat content, 60–75 g), starting 72 hours after monovalent oral poliovirus type 1 vaccine (mOPV1) exposure (QD3HF); twice-daily dosing (800 mg each) after a high-fat meal, starting 72 hours after mOPV1 exposure (BID3HF); once-daily dosing after a high-fat meal, starting 24 hours after mOPV1 exposure (QD1HF); and once-daily dosing after a standard meal (fat content, <25 g) starting 72 hours after mOPV1 exposure (QD3STD).
Abbreviations: AUC, area under the curve (0 − τ); Cmax, peak concentration; Cmin, trough concentration; Tmax, time to maximum concentration.
aMeasured at 24 hours for once-daily cohorts and at 12 hours for the twice-daily cohort (before receipt of the next dose).
The study’s primary end point was time from initiation of treatment to virus negativity in stool specimens, as measured by virus culture. The daily status (virus positive or virus negative) of all stool samples was determined. For virus-positive samples, specimens obtained at baseline and on the last day of virus positivity were evaluated for drug susceptibility. If samples were found to have drug-resistant virus, additional samples from that subject were evaluated to determine when resistance first appeared.
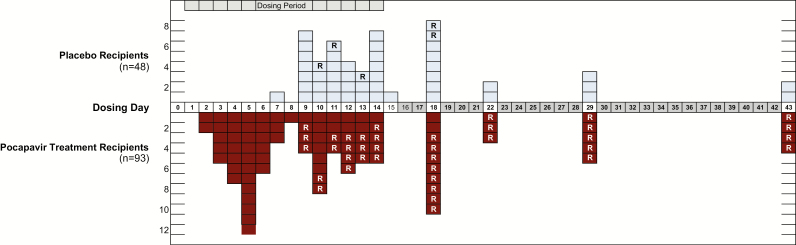
Figure 2 presents the day after dose initiation on which the primary end point was achieved for each subject in the PD population. The median duration of virus excretion among all placebo-treated subjects combined (n = 48) was 13 days and that for all pocapavir-treated subjects combined (n = 93) was 10 days (P = .0019). However, when individual cohort-matched pocapavir and placebo recipients were analyzed, the difference was significant only for the QD3HF cohort (median, 7 vs 13.5 days; P = .0039; Table 3A).
Figure 2.
Primary efficacy measure. Number of subjects in the pharmacodynamic population achieving the end point (virus negativity), by day after initiation of treatment. Abbreviations: R, detection of resistant virus.
Table 3.
Summary of Primary and Secondary Efficacy Measures for the Pharmacodynamic (PD) Population and Susceptible Subpopulation, by Cohort and Treatment Group
| Efficacy Result, by Population | QD3HF | BID3HF | QD1HF | QD3STD | Overall | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pocapavir | Placebo | P | Pocapavir | Placebo | P | Pocapavir | Placebo | P | Pocapavir | Placebo | P | Pocapavir | Placebo | P | |
| PD population | |||||||||||||||
| Subjects, no. | 23 | 12 | 24 | 12 | 23 | 12 | 23 | 12 | 93 | 48 | |||||
| Subjects with drug-resistant virus, no. (%) | 5 (22) | 2 (17) | 12 (50) | 1 (8) | 15 (65) | 0 (0) | 8 (35) | 2 (17) | 41 (44) | 5 (10) | |||||
| Primary: time to virus clearance, d, median (95% CI) | 7(5.0–10.0) | 13.5(9.0–18.0) | .0039 | 10(5.0–12.0) | 11.5(9.0–18.0) | .1221 | 14(12.0–18.0) | 14(11.0–18.0) | .7168 | 10(6.0–18.0) | 13(9.0–22.0) | .1714 | 10(9.0–12.0) | 13(11.0–14.0) | .0019 |
| Secondary: percentage reduction in excreted virus (95% CI)a | 84.9 (32.45–6.62) | … | 50.26(−120.09 – 88.76) | … | 43.48(−152.79–87.36) | … | 75.5 (−9.58–94.52) | … | … | … | |||||
| Least squares mean (CCID50 × 106) (95% CI) | 65.8(27.37–58.17) | 435.82(129.41–1467.75) | .0138 | 106.76(45.24–251.94) | 214.62(63.73–722.79) | .3547 | 192.79(80.20–463.43) | 341.07(101.27–1148.63) | .4526 | 291.8(121.39–701.44) | 1190.91(353.62–4010.73) | .0655 | … | … | |
| Susceptible subpopulation | |||||||||||||||
| Subjects, no. | 18 | 10 | 11 | 11 | 8 | 12 | 15 | 10 | 52 | 43 | |||||
| Primary: time to virus clearance, d, median (95% CI) | 6(4.0–8.0) | 12(9.0–14.0) | .0012 | 5(3.0–7.0) | 12(9.0–18.0) | .0003 | 7(4.0–14.0) | 14(11.0–18.0) | .0128 | 6(5.0–10.0) | 13.5(7.0–22.0) | .0017 | 5.5(5.0–7.0) | 13(11.0–14.0) | <.0001 |
| Secondary: percentage reduction in excreted virus (95% CI)a | 94.3(70.79–8.87) | … | 79.17(−26.48–96.23) | … | 76.29(−55.50–96.38) | … | 87.68(33.74–97.71) | … | … | … | |||||
| Least squares mean (CCID50 × 106) | 29.89(11.32–78.95) | 519.74(141.22–1912.79) | .0008 | 48.52(14.01–168.05) | 222.28(64.17–769.91) | .0887 | 80.87(18.84–347.11) | 341.07(64.17–769.91) | .1319 | 159.19(54.94–461.27) | 1291.87(351.03–4754.42) | .0153 | … | … | |
Subjects received placebo or 1600 mg of pocapavir (8 capsules) per day according to the following 4 dosing regimens: once-daily dosing after consuming a high-fat meal (fat content, 60–75 g), starting 72 hours after monovalent oral poliovirus type 1 vaccine (mOPV1) exposure (QD3HF); twice-daily dosing (800 mg each) after a high-fat meal, starting 72 hours after mOPV1 exposure (BID3HF); once-daily dosing after a high-fat meal, starting 24 hours after mOPV1 exposure (QD1HF); and once-daily dosing after a standard meal (fat content, <25 g) starting 72 hours after mOPV1 exposure (QD3STD).
Abbreviations: CCID50, median cell culture infective dose; CI, confidence interval.
aCompared with cohort-matched placebo recipients.
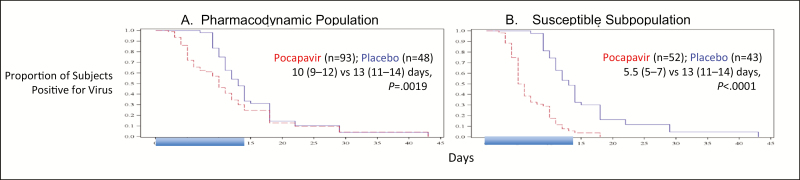
Kaplan-Meier analysis of the combined PD population (Figure 3A) and each individual cohort (Supplementary Figure S2A) revealed an early separation of the placebo and pocapavir curves, followed by a narrowing of the gap toward the end of the dosing period.
Figure 3.
Kaplan-Meier plots of time in days from initiation of treatment to negativity for virus excretion for all pocapavir recipients and all placebo recipients in the pharmacokinetic population (A) and the susceptible subpopulation (B). Shaded days denote the dosing period. Ranges in parentheses denote 95% confidence intervals.
Similarly, the secondary measure of drug efficacy, the reduction relative to the placebo group in the amount of infectious poliovirus excreted in stool during the 14-day dosing period, was significant for only the QD3HF cohort (84.9%; P = .0138; Table 3A and Supplementary Figure S3A). Noteworthy, excretion of the mOPV1 strain by these IPV recipients was quite robust. The mean peak virus titers among the 48 placebo recipients ranged from 4.58 to 7.94 log CCID50 per gram of stool (mean, 6.25 log CCID50 per gram of stool).
The drug susceptibility of virus in stool samples at baseline (ie, after OPV administration and before any treatment) for all subjects in the PD population was determined. Of the 141 subjects composing the PD population, 138 harbored virus susceptible to pocapavir prior to initiation of drug treatment (mean EC50, 0.024 µM), consistent with the susceptibility of the input mOPV1 strain (EC50, 0.017 µM) [7]. Surprisingly, however, 3 subjects had baseline virus resistant to pocapavir (EC50, >1.0 µM). When stool samples from the last virus-positive day were tested for drug susceptibility, an unexpectedly high proportion was positive for resistant virus. Among the 138 subjects excreting initially susceptible viruses, 43 (31%) had a stool sample from at least 1 day that tested positive for resistant virus. Of these, 38 were from the pocapavir treatment groups and 5 subjects were from placebo groups. The time of first appearance of resistant virus in pocapavir recipients ranged from day 2 to day 13 (mean, day 4; n = 38), and the time for the 5 placebo subjects ranged from day 2 to day 10 (mean, day 6). Resistant virus maintained its presence in subsequent daily stool samples until achievement of end point. The duration of resistant-virus excretion ranged from 2 to 27 days (mean, 13 days) for pocapavir recipients and from 1 to 12 days (mean, 7 days) for placebo recipients.
The molecular basis for pocapavir resistance was established previously from cell culture experiments [12]. Variant viruses with reduced susceptibility to pocapavir can be isolated from drug-susceptible virus populations by propagating virus in the presence of drug. Resistance is associated with one of two single amino acid substitutions: the isoleucine residue (I) at position 194 in capsid protein VP1 is replaced with phenylalanine or methionine (I194F/M) or the alanine at position 24 in VP3 is replaced with valine (A24V). Of the 46 subjects harboring resistant virus (including 3 with baseline samples positive for resistant virus), 32 subjects (70%) had VP1 (I194F), 10 (22%) had VP3 (A24V), and 2 (4%) had both. Two subjects (4%) with resistant virus lacked the recognized resistance markers. The distribution of resistant virus-harboring subjects across the 8 clinic groups (listed in chronological order 1 through 8) was generally uniform (Table 4). All clinic groups experienced secondary infections with both categories of drug-resistant virus.
Table 4.
Distribution, by Clinic Group and by Pocapavir Resistance Marker, of Subjects Harboring Pocapavir-Resistant Virus
| Variable | Subjects Evaluated, No. | Subjects With Resistant Virus | Cohort(s) | |||
|---|---|---|---|---|---|---|
| No. and Marker a | Total, No. (% b) | |||||
| Baseline | Placebo Group | Pocapavir Group | ||||
| Clinic group | ||||||
| 1 | 18 | 0 | 1, VP1 | 3, VP1; 2, VP3; 1, unknown | 7 (15) | QD3HF, BID3HF |
| 2 | 16 | 0 | 1, VP1 | 2, VP1; 1, VP3 | 4 (9) | QD3HF, BID3HF |
| 3 | 19 | 1, VP1 | 0 | 3, VP1; 1, VP3 | 5 (11) | QD3HF, BID3HF |
| 4 | 18 | 0 | 1, VP1 | 2, VP1; 1, VP3; 1, unknown | 5 (11) | QD3HF, BID3HF |
| 5 | 17 | 1, VP1 | 0 | 5, VP1; 1, VP3 | 7 (15) | QD1HF |
| 6 | 18 | 1, VP1+VP3 | 0 | 5, VP1; 2, VP3 | 8 (17) | QD1HF |
| 7 | 17 | 0 | 0 | 1, VP1; 1, VP3 | 2 (4) | QD3STD |
| 8 | 18 | 0 | 2, VP1 | 4, VP1; 1, VP3; 1, VP1+VP3 | 8 (17) | QD3STD |
| Markera | Subjects With Marker, No. or No. (%) | … | ||||
| VP1 | … | 2 | 5 | 25 | 32 (70) | … |
| VP3 | … | 0 | 0 | 10 | 10 (22) | … |
| VP1+VP3 | … | 1 | 0 | 1 | 2 (4) | … |
| Unknown | … | 0 | 0 | 2 | 2 (4) | … |
| Total | … | 3 (2) | 5 (3) | 38 (26) | 46 (33) | … |
Clinic groups (in chronological order 1 through 8) were comprised of approximately 18 cohabitating subjects each. Subjects received placebo or 1600 mg of pocapavir (8 capsules) per day according to the following 4 dosing regimens: once-daily dosing after consuming a high-fat meal (fat content, 60–75 g), starting 72 hours after monovalent oral poliovirus type 1 vaccine (mOPV1) exposure (QD3HF); twice-daily dosing (800 mg each) after a high-fat meal, starting 72 hours after mOPV1 exposure (BID3HF); once-daily dosing after a high-fat meal, starting 24 hours after mOPV1 exposure (QD1HF); and once-daily dosing after a standard meal (fat content, <25 g) starting 72 hours after mOPV1 exposure (QD3STD).
aMarkers for pocapavir resistance are as follows: VP1, I194F; and VP3, A24V.
bPercentages were calculated using the number of all subjects with resistant virus as the denominator.
Owing to the high incidence of secondary infections and virus transmission among study participants, analysis of the PD population may substantially underestimate the potential response to pocapavir. To investigate the magnitude of this effect, we created a susceptible subpopulation, in which subjects harboring resistant viruses were excluded (Figure 1). When the time to virus negativity was determined for the susceptible subpopulation, the pocapavir groups in all cohorts achieved the primary end point in significantly shorter times as compared to their matched placebo recipients (Table 3B). The time to virus negativity among all pocapavir recipients in the susceptible subpopulation was 5.5 days, compared with 13 days among all placebo recipients in the susceptible subpopulation (P < .0001). For the Kaplan-Meier analysis of the susceptible subpopulation, the early separation between the placebo and pocapavir curves was maintained for all pocapavir recipients and placebo recipients (Figure 3B) and for each cohort individually (Supplementary Figure S2B).
Similarly, improvement in the secondary measure of drug efficacy, the amount of infectious poliovirus excreted in stool during the dosing period, was observed, with percentage reductions in total excreted virus ranging from 44% to 85% for the PD population and from 76% to 94% for the susceptible subpopulation (Table 3A and 3B). Dosing initiation either 1 day or 3 days after OPV challenge revealed little difference in outcome (Supplementary Figures S2B and S3B).
Administration of pocapavir to healthy adults was safe and well tolerated. Detailed safety data are provided in Supplementary Tables S1 and S2. The incidence of adverse events was similar across pocapavir groups and was either lower or the same as that in the cohort-matched placebo groups. The most frequently reported adverse event in subjects receiving pocapavir was headache. The majority of adverse events were considered of mild intensity and resolved by the end of the study. There was 1 adverse event of severe intensity, which was judged unrelated to pocapavir and due to influenza.
There were no clinically significant safety laboratory, vital sign, or 12-lead ECG findings during the study. Three subjects (2 receiving pocapavir and 1 receiving placebo) had alanine aminotransferase values >3 times the upper limit of normal and aspartate aminotransferase values >2 times the upper limit of normal between 7 and 18 days after initiation of dosing. None had increased bilirubin levels during the study, and values for all returned to normal before completion of the study.
DISCUSSION
The objective of this OPV challenge model study was to assess the antipoliovirus activity of pocapavir in healthy, adult IPV recipients. Ninety-eight percent of the study participants became infected with the mOPV1 strain and shed substantial amounts of virus, indicating that this population of IPV recipients was fully susceptible to infection with the mOPV1 strain. The drug was well tolerated, and plasma levels achieved with all dosing regimens were well above those predicted to be active. The antipoliovirus effectiveness of pocapavir, as determined by a reduction in time to cessation of virus shedding in the intention-to-treat (ie, PD) population, was significant (10 vs 13 days; P = .0019). However, owing to the unexpectedly high rate of resistance, efficacy may be underestimated. Indeed, when subjects harboring drug-resistant viruses were excluded from analysis, the median time to end point was 5.5 days in the pocapavir group and 13 days in the placebo group. In each clinic group, resistant viruses representing each of the 2 amino acid substitutions associated with drug resistance were identified. This might suggest that at least 2 drug selection events occurred in each group. However, the existence of 3 subjects harboring resistant virus prior to dosing, coupled with the resistance observed in placebo recipients, suggest that both intraclinic and interclinic group transmission were occurring. In fact, this would be anticipated because of the high within-household transmissibility of polioviruses [13] and the complete susceptibility (to resistant virus) of the study population.
The data collected in this study are insufficient to determine the contribution to resistance due to drug selection or virus transmission. However, in the case of another well-studied enterovirus capsid inhibitor, pleconaril, the frequency of drug-selected resistance ranged from 3% to 11% [14]. Regardless of their origin, the resistant viruses identified in the clinic bore the same single amino acid changes shown to confer resistance in the laboratory. These amino acid changes have been shown to attenuate virus replicative and pathogenic characteristics in mice and virion stability in the environment [12,15].
There currently are no treatments available that address the threat posed by iVDPVs, either to the infected individual’s risk of paralytic or fatal disease or to the community as a continuing source of poliovirus transmission. Intravenous immunoglobulin is often used in the management of B-cell–immunodeficient patients and, for those infected with poliovirus, may provide a temporary diminution in virus shedding in some cases [16]. Additionally, poliovirus-specific monoclonal antibodies show promise [17]. However, we know of no controlled clinical studies with immunoglobulin or antibodies for the treatment of poliovirus infections. Clearly, the parameters of this study differ substantially from those anticipated for the primary target patient population: the healthy, immunocompetent adult recipients of IPV in this study, who were sequestered for 2 weeks in a high-density household environment after receipt of a high dose of challenge virus on day 0, are distinct from nonimmune iVDPV-infected patients who are persistently excreting poliovirus due to a primary immunodeficiency and receiving individual care. With poliovirus eradication on the horizon, there is an urgency to evaluate pocapavir in iVDPV-infected individuals to assess drug resistance in this population and, if an issue, to explore multiple drug combination therapy to manage resistance [18,19].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank all study participants and staff at the Clinical Trial Center, in particular Sari Huusko, Liselott Lisjö, and Susanne Colebring; Walter Dowdle, for his efforts in establishing the Poliovirus Antiviral Initiative; to the Poliovirus Antiviral Initiative steering team members, for encouragement and advice; Peter Wright (Poliovirus Antiviral Initiative steering team), for his review of the manuscript, comments, and suggestions; Marion Koopmans, for constructive discussions; Ron Altena, Bas van der Veer, Edin Jusic, and Gokhan Uslu (Dutch National Institute for Public Health and the Environment) and Larin McDuffie (Centers for Disease Control and Prevention), for technical assistance; and Julie R. Garon, for writing, formatting, and editorial support.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the Task Force for Global Health.
Potential conflicts of interest. M. S. C. and J. R. H. are employees of the drug-developer ViroDefense, sponsor of pocapavir, and report grants from the Task Force for Global Health during the conduct of this study. K. B., E. D., and H. A. report grants to the Dutch National Institute for Public Health and the Environment from the Task Force for Global Health during this study. M. A. M. is an employee of the Task Force for Global Health, the funder of this study, and reports grants from the Bill and Melinda Gates Foundation during the conduct of this study. All other authors report no potential conflicts.
References
- 1. Burns CC, Diop OM, Sutter RM, Kew OM. Vaccine-derived polioviruses. J Infect Dis 2014; 210 (suppl 1):S283–93. [DOI] [PubMed] [Google Scholar]
- 2. Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol 2005; 59:587–635. [DOI] [PubMed] [Google Scholar]
- 3. Li L, Ivanova O, Driss N, et al. Poliovirus excretion among persons with primary immune deficiency disorders: summary of a seven-country study series. J Infect Dis 2014; 210 (suppl 1):S368–72. [DOI] [PubMed] [Google Scholar]
- 4. Collett MS, Neyts J, Modlin JF. A case for developing antiviral drugs against polio. Antiviral Res 2008; 79:179–87. [DOI] [PubMed] [Google Scholar]
- 5. Katz SL, Andino R, Joseph-McCarthy J, Modlin JF, Nathanson N, Whitley RJ, Wimmer E. Exploring the role of antiviral drugs in the eradication of polio: workshop report. Washington, DC: National Academies Press, 2006. http://www.nap.edu/catalog/11599. Accessed 7 July 2016. [Google Scholar]
- 6. Buontempo PJ, Cox S, Wright-Minogue J, et al. SCH 48973: a potent, broad-spectrum, antienterovirus compound. Antimicrob Agents Chemother 1997; 41:1220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oberste MS, Moore D, Anderson B, Pallansch MA, Pevear DC, Collett MS. In vitro antiviral activity of V-073 against polioviruses. Antimicrob Agents Chemother 2009; 53:4501–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torres-Torres S, Myers AL, Klatte JM, et al. First use of investigational antiviral drug pocapavir (V-073) for treating neonatal enteroviral sepsis. Pediatr Infect Dis J 2015; 34:52–4. [DOI] [PubMed] [Google Scholar]
- 9. Bearden D, Collett M, Quan PL, Costa-Carvalho BT, Sullivan KE. Enteroviruses in X-linked agammaglobulinemia: update on epidemiology and therapy. J Allergy Clin Immunol Pract 2016; 4:1059–65. [DOI] [PubMed] [Google Scholar]
- 10. Herremans TM, Reimerink JH, Buisman AM, Kimman TG, Koopmans MP. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J Immunol 1999; 162:5011–8. [PubMed] [Google Scholar]
- 11. Polio laboratory manual. 4th ed Geneva, Switzerland: World Health Organization, 2004. http://apps.who.int/iris/bitstream/10665/68762/1/WHO_IVB_04.10.pdf. Accessed 7 July 2016. [Google Scholar]
- 12. Liu H-M, Roberts JA, Moore D, et al. Characterization of poliovirus variants selected for resistance to the antiviral compound V-073. Antimicrob Agents Chemother 2012; 56:5568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fine PEM, Carneiro IAM. Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative. Am J Epidemiol 1999; 150:1001–21. [DOI] [PubMed] [Google Scholar]
- 14. Pevear DC, Hayden FG, Demenczuk TM, Barone LR, McKinlay MA, Collett MS. Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses. Antimicrob Agents Chemother 2005; 49:4492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kouiavskaia D, Dragunsky EM, Liu H- M, Oberste MS, Collett MS, Chumakov KM. Immunological and pathogenic properties of poliovirus variants selected for resistance to antivral drug V-073. Antivir Ther 2011; 16:999–1004. [DOI] [PubMed] [Google Scholar]
- 16. McKinney RE, Katz SL, Wilfert CM. Chronic enteroviral meningoencephalitis in agammaglobulinemic patients. Rev Infect Dis 1987; 9:334–56. [DOI] [PubMed] [Google Scholar]
- 17. Chen Z, Chumakov K, Dragunsky E, et al. Chimpanzee-human monoclonal antibodies for treatment of chronic poliovirus excretors and emergency postexposure prophylaxis. J Virol 2011; 85:4354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rhoden E, Liu H-M, Wang-Chern S, Oberste MS. Anti-poliovirus activity of protease inhibitor AG-7404, and assessment of in vitro activity in combination with antiviral capsid inhibitor compounds. Antiviral Res 2013; 98:186–91. [DOI] [PubMed] [Google Scholar]
- 19. McKinlay MA, Collett MS, Hincks JR, et al. Progress in the development of poliovirus antiviral agents and their essential role in reducing risks that threaten eradication. J Infect Dis 2014; 210(suppl 1):S447–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.