Abstract
Background & objectives:
Search for candidate genes for alcohol dependence (AD) has been inconsistent and inconclusive. Moreover, most of the research has been confined to a few specific ethnic groups. Hence, the aim of our study was to explore specific candidate genes for AD in north Indian male population.
Methods:
In this clinic-based genetic association study, 210 males with AD and 200 controls matched for age, gender and ethnicity were recruited from the clinic and the general population, respectively. Cases were diagnosed with Semi-structured Assessment for Genetics of Alcoholism-II (SSAGA-II). Single-nucleotide polymorphism genotyping was done by real-time quantitative-polymerase chain reaction (PCR) using Taq Man assay (ABI 7500) fast real-time PCR system.
Results:
Both at the genotypic level and at allelic frequency, Met158 variant of catechol-O-methyl transferase (COMT) showed significant increase in cases as compared to controls. The frequency of heterozygous genotype (A/G) of gamma-aminobutyric acid receptor A1 (GABRA1) was significantly lower in cases as compared to controls. Likewise, for GABRA2, the frequency of homozygous recessive genotype (G/G) was significantly higher in the control group. With respect to the 5-hydroxytryptamine (5HT) transporter long promoter region (5HTTLPR), cholinergic receptor muscarinic (CHRM2) and alcohol dehydrogenase 1B (ADH1B) genes, there was no significant difference between the cases and the controls. Aldehyde dehydrogenase (ALDH2) gene was found to be monomorphic in our study population.
Interpretation & conclusions:
Our study findings showed COMT polymorphism conferring risk and GABRA polymorphism as a protective genotype for Indian male with AD. Genes for alcohol metabolism, serotonin transporter and cholinergic receptor gene polymorphism were perhaps not contributory to AD for Indian population.
Keywords: Alcohol dependence, candidate gene, catechol-O-methyl transferase, gamma-aminobutyric acid, nicotinic cholinergic receptor, polymorphism
Alcohol dependence (AD) is a common and debilitating disorder and ranks among the leading causes of the global burden of disease1. It has been demonstrated that among those who drink alcohol, only a minority (~15%) eventually become dependent on it2. The role of genetics as measured by heritability index is around 40-60 per cent3. Being a multifaceted phenotype AD is likely to be influenced by multiple genes with small effects rather than being a single gene disorder4.
In the search for potential genetic risk factors for substance dependence, much work has focused on genes involved in alcohol metabolism and neurotransmitter regulation. For example, the associations with AD have been investigated for the catechol-O-methyl transferase (COMT), gamma-aminobutyric acid (GABA) and nicotinic cholinergic receptor genes4. Given the relevance of serotonin to alcohol use and abuse, the serotonin transporter gene has also received substantial research attention5. However, genetic polymorphism of the alcohol metabolizing enzymes in the liver is perhaps the most significant and yielding area of research till date4. The COMT gene contains multiple single-nucleotide polymorphisms, some of which are postulated to have clinical significance4. Most studies concentrated on the Val158Met polymorphism4. Although there are a few reports indicating a positive association with COMT polymorphisms and addiction6, the majority of the studies failed to detect such a link between them6. Moreover, the association has been demonstrated both with the high activity (or the Val158) and the low activity (or Met158) alleles7,8. Met polymorphic variant of COMT is associated with poor stress resilience and anxiety, whereas Val polymorphism has been linked with poor executive function, inattention, impulsivity which could be contributory for AD.
Gamma-aminobutyric acid receptor A2 (GABRA2) has also been linked to alcoholism. Following systematic investigation by the Collaborative Study on the Genetics of Alcoholism (COGA), linkage disequilibrium has been established in chromosome 4 adjacent to the area for GABA receptor gene9. A significant association has been shown between multiple single-nucleotide polymorphisms (SNPs) in the GABRA2 gene and AD9. This result has been replicated in various studies10,11. Later, the association has also been extended to other clinical and electrophysiological intermediate phenotypes12. In a community based study, it has been argued, ‘The GABRA2 allelic associations found in clinical case-control studies have detectable but minor effects on DSM-defined AD in the general community’13. Genes involved in the cholinergic system, especially the cholinergic receptor muscarinic (CHRM2), are also observed to be associated with AD14. The association has been replicated by a large independent study15. However, in both the studies, association was perhaps mediated by the presence of depression. A relationship has been implicated between particular allelic variations of the serotonin transporter gene [5 hydroxytryptamine (HT) transporter long promoter region (5HTTLPR)] and AD16. However, the genes that have been associated with AD most consistently are those encoding the enzymes that metabolize alcohol. Polymorphisms have been demonstrated in alcohol metabolism carried out through the alcohol [alcohol dehydrogenase (ADH)] and the aldehyde dehydrogenase (ALDH) enzymes. ADH1B (ADH1B*2) allele has higher enzyme activity and metabolizes alcohol to acetaldehyde, whereas ALDH2*2 is a slow metabolizer of acetaldehyde. Hence, individuals having both the polymorphisms are likely to have increased aldehyde level following alcohol consumption and the lowest risk for AD17,18.
Although the underlying biological mechanism for addiction is expected to be the same across various population, the relative frequency of the allele in the population might affect the importance of a specific genotype. Considerable variation in the allele frequency of polymorphism of alcohol-metabolizing enzymes has been observed among different ethnic groups19,20. This study was carried out to identify specific candidate genes for AD in a north Indian male population.
Material & Methods
This was a clinic-based genetic association study including cases of AD and matched, screened controls unrelated to the cases. A total of 210 alcohol-dependent male patients, attending the Drug De-addiction & Treatment Centre, Department of Psychiatry, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India, were enrolled by convenient sampling. The control group included 200 non-related males from same ethnic background with exposure to alcohol but no alcohol use disorder. Those with a history of any other substance abuse/dependence other than nicotine were excluded. Also those with mental retardation, or having psychosis, bipolar disorder were excluded from the study. The study period was from September 2010 to August 2012. Cases (patients) were diagnosed by administration of a semi-structured interview by a trained clinician using a modified version of Semi-structured Assessment for Genetics of Alcoholism-II (SSAGA-II). This interview generated lifetime diagnosis of AD according to the International Classification of Diseases (ICD-10)21 as well as Diagnostic and Statistical Manual of Mental Disorders (DSM-IV)22. Controls were recruited from individuals who came for the purpose of genetic or drug studies in the department of Pharmacology and Experimental Medicine. All of them had used alcohol but those who were using it in pathological form (harmful use or dependent) were excluded from the study. Likewise, those who had used/abused any other substance were also excluded. Semi-structured instrument, SSAGA-II, was administered to all individuals for the assessment of alcohol use disorders. The control subjects were not blood-related with the cases. Family history of repetitive substance use was obtained from all the controls and those with a positive family history were excluded. Clinical details, including ethnicity, age at first use of alcohol, quantity of alcohol consumed (ml/day), duration of alcohol use, duration of AD and any other psychiatric or physical illness, were assessed and recorded.
All subjects gave written informed consent to participate in the study. The study protocol was approved by the ethics committee of the Institute.
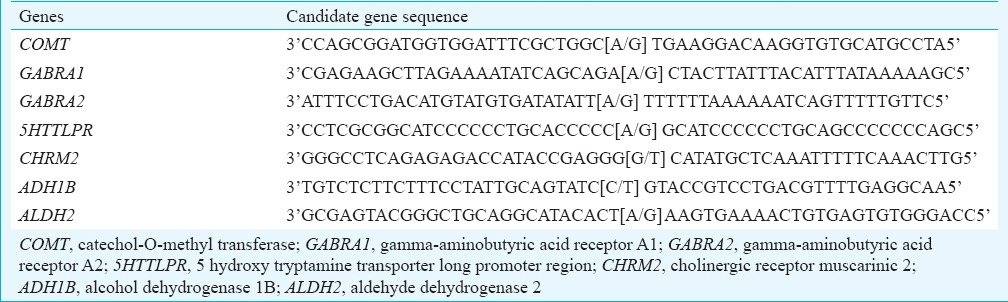
Genomic DNA extraction and genotyping: Intravenous blood samples (6 ml) were collected in sterile ethylenediaminetetraacetic acid (EDTA)-coated vacutainers and stored at -20°C until processing for DNA extraction was carried out. DNA was isolated from whole blood using the standard organic method (Phenol-chloroform-isoamyl). SNP genotyping for all the polymorphisms analyzed in the present study was done using ABI TaqMan assay kits (Life Technologies, India) by real-time quantitative-polymerase chain reaction (PCR) on ABI 7500 fast real-time PCR system, Singapore. The oligonucleotide sequences of the probes for different candidate genes studied are given in Table I. Oligonucleotides were custom designed and procured from Life Technologies (India).
Table I.
Sequences of oligonucleotide probes against polymorphic regions of various candidate genes of alcoholism
Statistical analysis: Statistical analysis between cases and controls with different genotypic profiles for different candidate gene polymorphisms was performed using the SPSS software (version 12.0, SPSS Inc., Chicago, IL, USA). Discrete and continuous variables between cases and controls were compared using Pearson's Chi-square test and unpaired t test as appropriate. The genetic association of alcoholism with different candidate genes in relation to polymorphic prevalence was evaluated using logistic regression to calculate odds ratio (OR) and 95 per cent confidence intervals (CIs) after adjustment for potential confounders. Power analysis was performed using Quanto (Version 1.0) (http://www.hydra.usc.edu/gxe). Genotype distributions were tested for deviation from the Hardy–Weinberg Equilibrium proportions using the HWSIM program23.
Results
Comparison of cases and controls with respect to their socio-demographic parameters demonstrated that there was no significant difference in locality, religion and family type between these two groups. However, the controls were found to be more educated (P<0.001) compared to the alcohol-dependent cases. The mean age of patients was 41.5±8.5 yr (39.2±4.73 yr for controls) and the average amount of alcohol consumed/day was 722.9±490.41 ml/day. Mean age at first use of alcohol was 22.5±6.1 yr and duration of alcohol use was 18.5±8.3 yr; duration of AD was 10.7±8.03 yr in cases.
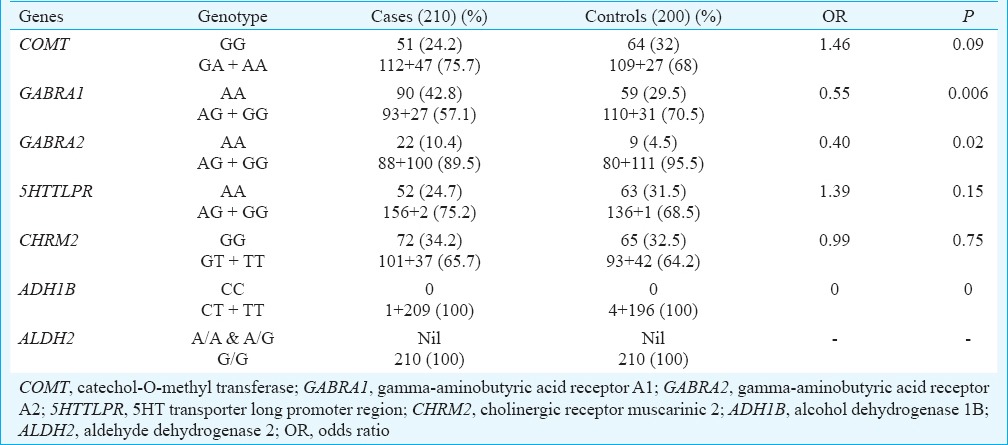
The genotype and allele frequencies of studied genes (COMT, GABRA1, GABRA2, 5’HTTLPR, CHRM2, ALDH2 & ADH1B) in cases and controls are shown in (Tables II and III), respectively. At the genotypic level, the frequency of homozygous recessive genotype (A/A) of COMT showed significant increase in cases as compared to controls and was associated with risk of alcohol addiction [Homozygous recessive genotype (A/A) OR, 2.18; 95% CI 1.19-3.97; P=0.01]. The frequency of heterozygous genotype (A/G) of GABRA1 was significantly lower in cases as compared to controls and was associated with decreased risk of alcoholism [Heterozygous genotype (A/G) OR, 0.55; 95% CI 0.36-0.85; P=0.007]. In GABRA2, the frequency of homozygous recessive genotype (G/G) was significantly lower in cases as compared to controls and was associated with decreased risk of alcohol addiction [Homozygous genotype (G/G) OR, 0.36; 95% CI 0.16-0.83; P=0.01] (Table II). At the allelic level, the frequency of variant allele (A) in COMT gene was found to be significantly higher in cases as compared to controls, (COMT gene OR 0.71; P=0.02) indicating an increased risk of alcohol addiction in our population. The variant allele (G) frequency of GABRA1 and GABRA2 genes was significantly lower in cases as compared to controls and was associated with decreased risk of alcoholism (GABRA1 gene OR 0.71; P=0.02; GABRA2 gene OR 0.70; P=0.03) (Table III). No significant association of 5’HTTLPR, CHRM2 and ADH1B genes was found with the risk of alcoholism at both genotypic and allelic levels between the cases and control groups (Tables II & III). ALDH2 gene was found to be monomorphic in our study population. Table IV represents the dominant mode of inheritance of candidate genes. The genotype frequencies of GABRA1 and GABRA2 genes were found to be significantly associated with decreased risk of alcoholism. (GABRA1 gene OR, 0.55; P=0.006, GABRA2 gene OR, 0.40; P =0.02).
Table II.
Genotype frequency of candidate genes
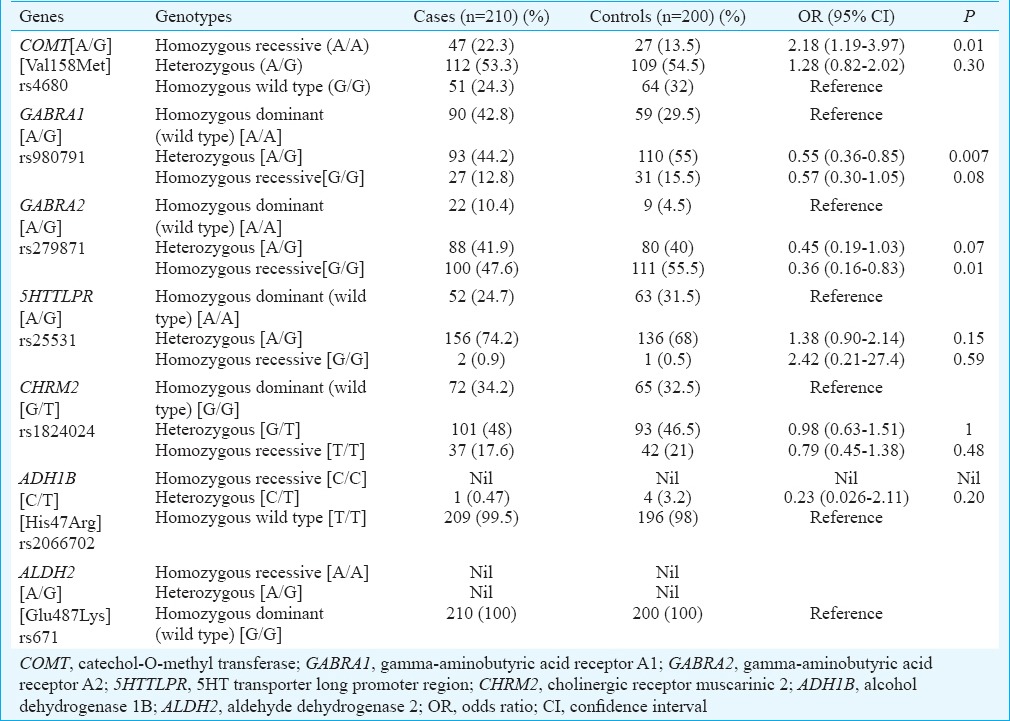
Table III.
Allele frequency of candidate genes
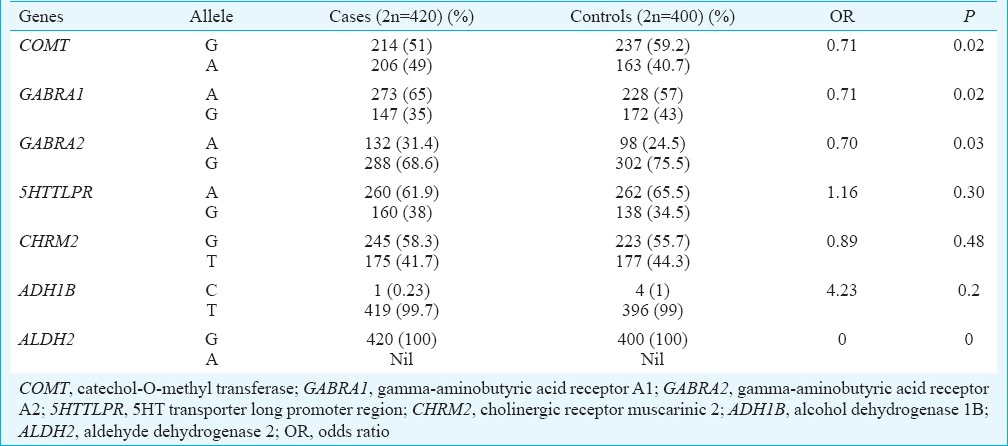
Table IV.
Genotype frequency of candidate genes using dominant model
Discussion
The present study examined the association of AD with genetic polymorphisms in various receptors, enzymes responsible for the metabolism of neurotransmitters and alcohol metabolizing enzymes. Our study revealed a genetic association of AD with the polymorphic variant of COMT, GABRA1 and A2.
Results demonstrated that from the standpoint of both genotype and allelic frequency of the COMT gene, the Met158 and the A allele were more frequently encountered in the AD group as compared to the control group. This finding translates to the lower activity of COMT enzyme which metabolizes dopamine, norepinephrine and other catecholamines in the brain, especially in the prefrontal cortex where the dopamine transporters are less expressed. Thus, in the presence of Met158 allele, there would be a higher level of dopamine in the brain. It has been shown that the Met allele impairs stress resilience and increased anxiety24,25,26. This allele is also found to be associated with pain-stress response, decreasing pain threshold and increased amygdala activity to negative stimuli26. All these psychological and intermediate biological phenotypes could add up to the vulnerability toward AD. Results from studies exploring the association between COMT and addiction are mixed. One study failed to find evidence for an association6; while others indicated Val158 and Met158 as the risk alleles6. Studies on late-onset AD and Finnish social drinkers revealed an association of Met158 allele8,27. Although in the present study, the age of onset of AD was not examined, from an earlier study conducted in the same clinic, the proportion of late-onset AD was observed to be much higher than the early onset AD28.
A significant association of AD was found with GABA receptor gene polymorphism. The preponderance of G/G genotype of GABRA2 in the controls indicated that individuals homozygous for the G/G allele had a low risk for AD. Li et al29 found the polymorphic variant of GABRA2 (rs567926) more common in controls than the alcohol dependent group. Our results were in contrast with the study which showed a positive association of G/G genotype of GABRA2 with AD and other intermediate electrophysiological or clinical traits12. Moreover, the association was seemingly more meaningful for the severe form of AD30. In our study, although the percentage difference between the alcohol-dependent group and the control was relatively small, this could not be explained by sampling because of rigorous selection criteria employed and a reasonably good sample size. The effect of GABRA2 polymorphism is found to be mediated by the effect of alcohol in an individual or the common underlying externalizing disorders comprising illicit drug use, conduct disorder and antisocial personality disorder9,10. In our study, we did not assess for these externalizing disorders. In the light of this indirect and complex interaction of GABRA and AD, it is difficult to explain the inverse association demonstrated. Moreover, the evidence for the strength of association available so far was strongest in Americans as opposed to the Europeans alluding to the possibility of ethnic variation11. The diverse ethnic background of the present study subjects could potentially explain the divergent result. In our study, GABRA1 polymorphism was also found to have a protective role from AD. Although studies failed to demonstrate an association between GABRA1 and AD4, a study sponsored by COGA has found some association with DSMIII-derived AD, alcoholic blackouts and age of the first drunkenness31 GABRA1 polymorphism was less in AD group as compared to the controls in our study. Polymorphic variant of GABA receptor is expected to have reduced functioning. Reduced functionality of the subtypes of these receptors (GABRA1/A2) is likely to alter the effect of alcohol in an individual, eventually resulting in lesser reinforcement and lessen the tendency to seek alcohol.
Our study failed to observe an association of 5HTTLPR, CHRM2 and ADH1B or ALDH2 polymorphism and AD. In the study population, ALDH2 was found to be monomorphic, and ADH1B allele was found to be present at very low frequency. Hence, the difference between the two groups could not be calculated. In a study from Indian tribal population, ADHIB was observed to be monomorphic32. For ALDH2, literature from India indicates the preponderance of the monomorphic genotype, like the Caucasians, Africans and Americans19,33. Another study from the northern part of the country has also demonstrated high frequency of ALDH2*2 (G/G) in alcohol-dependent male subjects34. The monomorphic wild-type genes for the alcohol metabolizing enzymes in both study groups indicated a lack of underlying protection for the development of AD in the Indian population. Hence, the role of other genetic polymorphism would become more important for our population. There was no association found between SNP in 5HTTLPR and AD. Perhaps genetic polymorphism of 5HTTLPR could predict a specific subtype of AD, only in exposure to explicit environmental variables such as stress in the early life or presence of internalizing symptoms such as anxiety and depression, which are likely to interact and compound the effect of the polymorphism4,16.
In our study the cases were selected from a tertiary care clinic, thus, the full spectrum of alcohol use disorder was difficult to obtain. Hence, our study had a limited scope of generalizability. In this study, only males with AD were considered, further limiting its generalization. AD is a polygenic disorder and there are multiple genes with small effect sizes4. We examined only a few of the allelic polymorphism among many which were shown to be associated with AD. Despite all these limitations, our study findings implicated COMT polymorphism conferring risk and GABRA polymorphism as a protective genotype for Indian male with AD. These results highlight the importance of candidate gene association studies in diverse ethnic populations and are expected to encourage further research in this area from India.
Acknowledgment
We would like to acknowledge the Indian Council of Medical Research for funding support.
Footnotes
Conflicts of Interest: None.
References
- 1.World Health Organization. Global health risks: Mortality and burden of disease attributable to selected major risks. Geneva: WHO; 2009. [Google Scholar]
- 2.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 3.Enoch MA, Goldman D. Problem drinking and alcoholism: diagnosis and treatment. Am Fam Physician. 2002;65:441–8. [PubMed] [Google Scholar]
- 4.Ducci F, Goldman D. The genetic basis of addictive disorders. Psychiatr Clin North Am. 2012;35:495–519. doi: 10.1016/j.psc.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabakoff B, Hoffman PL. Neurobiology of alcohol. In: Galanter M, Kleber HD, editors. Textbook of substance abuse treatment. Arlington, VA: American Psychiatric Publishing; 2004. pp. 111–28. [Google Scholar]
- 6.Tammimäki AE, Männistö PT. Are genetic variants of COMT associated with addiction? Pharmacogenet Genomics. 2010;20:717–41. doi: 10.1097/FPC.0b013e328340bdf2. [DOI] [PubMed] [Google Scholar]
- 7.Vandenbergh DJ, Rodriguez LA, Miller IT, Uhl GR, Lachman HM. High-activity catechol-O-methyltransferase allele is more prevalent in polysubstance abusers. Am J Med Genet. 1997;74:439–42. [PubMed] [Google Scholar]
- 8.Tiihonen J, Hallikainen T, Lachman H, Saito T, Volavka J, Kauhanen J, et al. Association between the functional variant of the catechol-O-methyltransferase (COMT) gene and type 1 alcoholism. Mol Psychiatry. 1999;4:286–9. doi: 10.1038/sj.mp.4000509. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal A, Edenberg HJ, Foroud T, Bierut LJ, Dunne G, Hinrichs AL, et al. Association of GABRA2 with drug dependence in the collaborative study of the genetics of alcoholism sample. Behav Genet. 2006;36:640–50. doi: 10.1007/s10519-006-9069-4. [DOI] [PubMed] [Google Scholar]
- 10.Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, et al. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29:493–8. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- 11.Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:104–9. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- 12.Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–14. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lind PA, Macgregor S, Agrawal A, Montogomery GW, Heath AC, Maritn NG, et al. The role of GABRA2 in alcohol dependence, smoking, and illicit drug use in an Australian population sample. Alcohol Clin Exp Res. 2008;32:1721–31. doi: 10.1111/j.1530-0277.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13:1903–11. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- 15.Luo X, Kranzler HR, Zuo L, Wang S, Blumberg HP, Gelernter J. CHRM2 gene predisposes to alcohol dependence, drug dependence and affective disorders: results from an extended case-control structured association study. Hum Mol Genet. 2005;14:2421–32. doi: 10.1093/hmg/ddi244. [DOI] [PubMed] [Google Scholar]
- 16.McHugh RK, Hofmann SG, Asnaani A, Sawyer AT, Otto MW. The serotonin transporter gene and risk for alcohol dependence: a meta-analytic review. Drug Alcohol Depend. 2010;108:1–6. doi: 10.1016/j.drugalcdep.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- 18.Eng MY, Luczak SE, Wall TL. ALDH2, ADH1B, and ADH1C genotypes in Asians: a literature review. Alcohol Res Health. 2007;30:22–7. [PMC free article] [PubMed] [Google Scholar]
- 19.Whitfield JB. Alcohol dehydrogenase and alcohol dependence: variation in genotype-associated risk between populations. Am J Hum Genet. 2002;71:1247–50. doi: 10.1086/344287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CC, Lu RB, Chen YC, Wang MF, Chang YC, Li TK, et al. Interaction between the functional polymorphisms of the alcohol-metabolism genes in protection against alcoholism. Am J Hum Genet. 1999;65:795–807. doi: 10.1086/302540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. International statistical classification of diseases and related health problems. Geneva: WHO; 1992. [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, DSM-IV-TR. 5th ed. Arlington: American Psychiatric Association Publishing; 2000. [Google Scholar]
- 23.Cubells JF, Kobayashi K, Nagatsu T, Kidd KK, Kidd JR, Calafell F, et al. Population genetics of a functional variant of the dopamine beta-hydroxylase gene (DBH) Am J Med Genet. 1997;74:374–9. doi: 10.1002/(sici)1096-8628(19970725)74:4<374::aid-ajmg7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 24.Enoch MA, Xu K, Ferro E, Goldman D. Genetic origins of anxiety in women: a role for a functional catechol-O-methyltransferase polymorphism. Psychiatr Genet. 2003;13:33–41. doi: 10.1097/00041444-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–43. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 26.Smolka MN, Schumann G, Wrase J, Grüsser SM, Flor H, Mann K, et al. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25:836–42. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kauhanen J, Hallikainen T, Tuomainen TP, Koulu M, Karvonen MK, Salonen JT, et al. Association between the functional polymorphism of catechol-O-methyltransferase gene and alcohol consumption among social drinkers. Alcohol Clin Exp Res. 2000;24:135–9. [PubMed] [Google Scholar]
- 28.Ghosh A, Malhotra S, Basu D. A study of childhood disruptive disorders and attention deficit hyperactive disorder in alcohol dependent subjects. (MD Thesis) Chandigarh: Postgraduate Institute of Medical Education & Research; 2011. [Google Scholar]
- 29.Li D, Sulovari A, Cheng C, Zhao H, Kranzler HR, Gelernter J. Association of gamma-aminobutyric acid A receptor a2 gene (GABRA2) with alcohol use disorder. Neuropsychopharmacology. 2014;39:907–18. doi: 10.1038/npp.2013.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36:577–90. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- 31.Dick DM, Plunkett J, Wetherill LF, Xuei X, Goate A, Hesselbrock V, et al. Association between GABRA1 and drinking behaviors in the collaborative study on the genetics of alcoholism sample. Alcohol Clin Exp Res. 2006;30:1101–10. doi: 10.1111/j.1530-0277.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- 32.Reddy BM, Reddy ANS, Nagaraja T. Single nucleotide polymorphisms of the alcohol dehydrogenase genes among the 28 caste and tribal populations of India. Int J Hum Genet. 2006;6:309–16. [Google Scholar]
- 33.Goedde HW, Agarwal DP, Fritze G, Meier-Tackmann D, Singh S, Beckmann G, et al. Distribution of ADH2 and ALDH2 genotypes in different populations. Hum Genet. 1992;88:344–6. doi: 10.1007/BF00197271. [DOI] [PubMed] [Google Scholar]
- 34.Vaswani M, Prasad P, Kapur S. Association of ADH1B and ALDH2 gene polymorphisms with alcohol dependence: a pilot study from India. Hum Genomics. 2009;3:213–20. doi: 10.1186/1479-7364-3-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]


