Abstract
The “metabolic syndrome” (MetS) is a clustering of components that reflect overnutrition, sedentary lifestyles, and resultant excess adiposity. The MetS includes the clustering of abdominal obesity, insulin resistance, dyslipidemia, and elevated blood pressure and is associated with other comorbidities including the prothrombotic state, proinflammatory state, nonalcoholic fatty liver disease, and reproductive disorders. Because the MetS is a cluster of different conditions, and not a single disease, the development of multiple concurrent definitions has resulted. The prevalence of the MetS is increasing to epidemic proportions not only in the United States and the remainder of the urbanized world but also in developing nations. Most studies show that the MetS is associated with an approximate doubling of cardiovascular disease risk and a 5-fold increased risk for incident type 2 diabetes mellitus. Although it is unclear whether there is a unifying pathophysiological mechanism resulting in the MetS, abdominal adiposity and insulin resistance appear to be central to the MetS and its individual components. Lifestyle modification and weight loss should, therefore, be at the core of treating or preventing the MetS and its components. In addition, there is a general consensus that other cardiac risk factors should be aggressively managed in individuals with the MetS. Finally, in 2008 the MetS is an evolving concept that continues to be data driven and evidence based with revisions forthcoming.
I. Introduction
- II. Definitions
- A. Brief history: nomenclature of the metabolic syndrome
- B. Diverging definitions: a syndrome rooted in controversy
- III. Epidemiology
- A. Prevalence estimates according to definition
- B. Prevalence estimates by sex
- C. Prevalence estimates by race/ethnicity
- D. Prevalence estimates by age
- E. Prevalence estimates by socioeconomic status, tobacco, alcohol, and level of education
- F. Changes in prevalence following intervention
- IV. Pathophysiology
- A. Insulin resistance: a conceptual prologue
- B. Obesity as a “driving force” in the prevalence of insulin resistance
- C. Insulin resistance in adipose tissue
- D. Insulin resistance in the liver
- E. Insulin resistance in muscle
- F. Hypertension and insulin resistance
- G. Other contributors to insulin resistance (nocturnal FFA flux: sympathetic nervous system)
- H. Proinflammatory molecules, ER stress, and their roles in insulin resistance and the metabolic syndrome
- I. Animal models of the metabolic syndrome
- J. Genetic determinants of the metabolic syndrome in humans
- V. Risks of the Metabolic Syndrome
- A. Cardiovascular disease
- B. Type 2 diabetes mellitus
- VI. Associated Conditions
- A. Nonalcoholic fatty liver disease
- B. Polycystic ovarian syndrome
- C. Obstructive sleep apnea
- D. Hypogonadism
- E. Lipodystrophy
- F. Microvascular disease
- VII. Therapeutics
- A. Lifestyle modification
- B. Pharmaceutical therapy
- C. Bariatric surgery
VIII. Unanswered Questions
IX. Summary and Conclusions
I. Introduction
THE “METABOLIC SYNDROME” (MetS) is defined as a clustering of components that reflects the expanding waist lines of the world. Although steeped with controversy, the MetS has more than made its place in the medical literature of today with more than 24,000 citations now recorded in PubMed. Granted, many of the publications that occurred before May 2001, when the National Cholesterol Education Program’s Adult Treatment Panel III (NCEP:ATPIII) definition of the MetS was put forth, did not address the MetS as we know it today; yet, since May 2001 more than 15,000 articles have surfaced, averaging over 40 per week. In comparison with another endocrine syndrome we know so well, the world’s literature on Cushing’s syndrome is approximately 11,500 articles. Of course the number of publications or related citations on a topic fails to provide the final word on scientific merit; nevertheless no one can argue with the claim that this volume of literature over the past 7 yr documents an escalating level of interest and potential scientific and clinical importance of the MetS.
So why is there so much controversy, bickering, and confusion about the MetS? We believe it centers predominantly around two issues: 1) the definition; and 2) the ability or inability of the MetS as currently defined to predict coronary heart disease (CHD) or other forms of cardiovascular disease (CVD) or type 2 diabetes (T2D) in a way superlative to the risk factors we know so well. For CHD, these would include low-density lipoprotein (LDL) cholesterol (LDL-C), tobacco, family history, and others that are included in the definition of the syndrome, i.e., hypertension, low levels of high-density lipoprotein (HDL) cholesterol (HDL-C), and diabetes (if included as a component of the MetS). For T2D risk, this would simply be the fasting glucose concentration.
According to the NCEP:ATPIII panel, the primary purpose for identifying the MetS was to identify individuals at higher risk of CVD that extended beyond LDL-C and was obesity-related (1). The purpose of identifying these patients was to emphasize further the importance of a healthy lifestyle in reducing risk. Yet a syndrome is not a disease and cannot be viewed microscopically using hematoxylin and eosin. If one considers Cushing’s syndrome as an example, facial rounding, plethora, supraclavicular fullness, proximal myopathy, cutaneous wasting, central obesity, nephrolithiasis, hypertension, glucose intolerance, hyperandrogenism, oligomenorrhea, hypogonadism, osteoporosis, and neuropsychiatric disorders could all be part of the clinical presentation. Yet because only a clustering of these components is typically present, we teach our students, house staff, and fellows to consider Cushing’s syndrome when their patients demonstrate the presence of some but not all these components.
The purpose of this review is not to dwell on the controversy, but to acknowledge it. We then dissect the vast literature on the MetS highlighting the most important aspects of the epidemiology, pathophysiology, experimental models, and related clinical and population data. To conclude, some discussion of the conditions associated with the MetS and therapeutics follows. We are hopeful that this comprehensive review will not only inform the reader, but also challenge him/her to put the MetS into appropriate scientific and clinical perspective.
II. Definitions
A. Brief history: nomenclature of the metabolic syndrome
Although the term MetS has become widely used since its inception in 2001 by the NCEP:ATPIII (1), the concept of “clustering” metabolic disorders and CVD risk factors has been discussed in the scientific literature for many decades. In fact, recent reviews have noted that independent scientists published reports of the association between diabetes mellitus and hypertension as early as the 1920s (2), when Kylin (3) documented a connection between hypertension, hyperglycemia, and gout. While the primer for understanding visceral adiposity did not occur until nearly 30 yr later (4), by the early 1990s visceral obesity was fully appreciated as a component of the insulin resistance syndrome (5). In 1980, the seminal work of Margaret Albrink (6) focused on the relationship between obesity, hypertriglyceridemia, and hypertension (7). It was not until 1988 when Reaven (8), in his landmark Banting Lecture, coined the term “Syndrome X” to describe the proposed interrelationships between resistance to insulin-stimulated glucose uptake, hypertension, T2D, and CVD. During the ensuing 10 yr, Syndrome X and other terms were used to describe the clustering of cardiovascular and metabolic risk factors, including “deadly quartet” (9) and the “insulin resistance syndrome” (10, 11, 12).
B. Diverging definitions: a syndrome rooted in controversy
The clinical utility of identifying people with the MetS has raised concerns from many scientific groups. In particular, the use of the term “syndrome” was examined and discussed by the International Diabetes Federation (IDF) (13). The IDF described a syndrome as “a recognizable complex of symptoms and physical or biochemical findings for which a direct cause is not understood…the components coexist more frequently than would be expected by chance alone. When causal mechanisms are identified, the syndrome becomes a disease.” Although insulin resistance is present in a majority of people with the MetS, the IDF found insufficient evidence for a causal link between the two, a statement that agreed with the American Diabetes Association (ADA), which published its concerns about the lack of certainty regarding the causative pathogenesis of insulin resistance and its utility as a marker for CVD (14). In particular, the ADA emphasized the lack of clarity in the MetS definition and cautioned clinicians not to assume that the MetS is well characterized (14). Thus, the term syndrome in itself has sparked considerable controversy.
Overall, a combination of factors, such as improved methodologies and increased awareness of the comorbidity of cardiovascular and metabolic diseases, led to the notion that identifying such a syndrome could be predictive of CVD. Although there are divergent criteria for the identification of the MetS, they all tend to agree that the MetS core components include obesity [waist circumference (WC)], insulin resistance, dyslipidemia, and hypertension (13). The first formal definition of the MetS was put forth in 1998 by the World Health Organization (WHO) (15). This definition focused primarily on the presence of insulin resistance, identified by hyperinsulinemia, impaired glucose tolerance (IGT), or the diagnosis of T2D, which had to be present to make the diagnosis. In addition, two of the following also had to be present: dyslipidemia (reduced HDL-C and increased triglycerides), hypertension, and microalbuminuria (Table 1). Of interest, the earliest definition of hypertension was a blood pressure of at least 160/90 mm Hg, later revised to at least 140/90 mm Hg. According to the WHO, the primary purpose of identifying individuals with the MetS was to identify patients at high risk for developing CVD as well as nondiabetics at high risk for developing diabetes. The European Group for the Study of Insulin Resistance (EGIR) published a separate set of criteria shortly thereafter (12). The basic premise of the EGIR definition was that the MetS “is a syndrome of mild anomalies which, in combination, increase cardiovascular risk.” This definition, although similar to the WHO definition, did not include microalbuminuria (Table 1). The EGIR emphasized that the presence of microalbuminuria was not a requirement for one to have the MetS. In 2001, the NCEP:ATPIII published a new set of criteria based on common clinical measurements: WC, blood lipids, blood pressure, and fasting glucose (1, 16, 17). The NCEP:ATPIII definition differed from both the WHO and EGIR definitions in that the presence of “insulin resistance,” per se, was not a necessary criterion to make the diagnosis (Table 1). Again, the primary purpose of the NCEP:ATPIII definition of the MetS was to identify individuals at high risk for CVD that extended beyond the traditional cardiac risk factors.
TABLE 1.
Criteria for the MetS definitions
| WHO, 1998 (15 ) | EGIR, 1999 (12 ) | NCEP:ATPIII, 2001 (1 ) | AACE, 2003 (18 ) | IDF, 2006 (13 ) |
|---|---|---|---|---|
| High insulin levels, IFG or IGT, and two of the following: | Top 25% of the fasting insulin values among nondiabetic individuals and two of the following: | Three or more of the following: | IGT and two or more of the following: | Central obesity as defined by ethnic/racial, specific WC, and two of the following: |
| Abdominal obesity: WHR >0.9, BMI ≥30 kg/m2, WC > 37 inches | WC: ≥94 cm for men, ≥80 cm for women | WC: >40 inches for men, >35 inches for women | Triglycerides ≥150 mg/dl | Triglycerides ≥150 mg/dl |
| Lipid panel with triglycerides > 150 mg/dl, HDL-C <35 mg/dl | Triglycerides ≥2.0 mmol/liter and HDL-C <1.0 mg/dl | Triglycerides ≥150 mg/dl | HDL-C: <40 mg/dl for men, <50 mg/dl for women | HDL-C: <40 mg/dl for men, <50 mg/dl for women |
| BP >140/90 mm Hg | BP ≥140/90 mm Hg or antihypertensive medication | HDL-C: <40 mg/dl for men, <50 mg/dl for women | BP ≥130/85 mm Hg | BP ≥130/85 mm Hg |
| Fasting glucose ≥6.1 mmol/liter | BP ≥130/85 mm Hg | FPG ≥100 mg/dl | ||
| FPG ≥110 mg/dl1 |
Since these initial attempts to define the MetS, other groups, including the American Association of Clinical Endocrinologists (AACE) (18), have proposed working definitions to describe the interdependence of cardiovascular and metabolic diseases (Table 1). The AACE definition placed a greater focus on insulin resistance and excluded individuals with T2D. In their 2004 workshop, the IDF recognized the difficulties in identifying criteria for the MetS that were applicable across ethnic populations (13). Specifically, they argued that multiple definitions of the MetS led to difficulties in comparing data between studies and did not provide unified diagnostic criteria to identify the presence of the syndrome. The IDF definition emphasized central obesity as a necessary condition to make the diagnosis of the MetS (Table 1). The IDF also proposed a new set of criteria with ethnic/racial specific cutoffs. For example, WC ranges were specified for those from Europe, South Asia, China, Japan, ethnic South and Central America, sub-Saharan Africa, and the Eastern Mediterranean/Middle East. Moreover, a guide for measuring WC was suggested, and there was discussion to the effect that in Australia, when the body mass index (BMI) is greater than 30 kg/m2, a WC measurement is unnecessary. It is also notable that the most recent IDF criteria do not emphasize insulin resistance, but instead focus on fasting plasma glucose concentrations.
In general, until more evidence accumulates that elucidates the cause of the MetS and its impact on CVD and T2D incidence and outcomes, these controversies are unlikely to be resolved. However, identification of multiple components of the syndrome is undeniably an opportunity to encourage patients to make lifestyle changes that will attenuate their chances for CVD and metabolic disease later in life.
III. Epidemiology
The prevalence of the MetS is increasing throughout the world (19). Prevalence estimates of the MetS in the United States and around the world, however, are dependent on the definition that is used to determine inclusion as well as the composition (e.g., sex, age, race, and ethnicity) of the population being studied. Moreover, lifestyle habits and socioeconomic status (SES) appear to influence prevalence across sex, age, and race/ethnicity cohorts.
A. Prevalence estimates according to definition
Estimates of the prevalence of the MetS differ depending on the definition (NCEP:ATPIII, WHO, IDF, EGIR) being used to categorize individuals. The WHO and NCEP:ATPIII definitions are similar with respect to criteria for obesity, hypertension, and dyslipidemia. However, insulin resistance, IGT, and/or T2D as prerequisites of the WHO definition make this definition relatively more restrictive. The exclusion of people with T2D from the EGIR definition also makes its definition less inclusive. On the other hand, the IDF definition, which has central obesity as its prerequisite, may be relatively less restrictive than the NCEP:ATPIII definition. Prevalence estimates based on the original NCEP:ATPIII definition became more inclusive when the original criteria were revised to include the newer 2003 ADA-recommended cutoff for impaired fasting glucose (IFG) (i.e., ≤100 vs. 110 mg/dl). Clearly, whether or not epidemiological studies using the NCEP:ATPIII or WHO criteria include or exclude individuals with T2D impacts their prevalence estimates because the vast majority of T2D patients meet the minimum criteria for the MetS (20). Differences in the age-adjusted prevalence estimates using the various definitions of MetS within two National Health and Examination Survey (NHANES) cohorts (NHANES 1988–1994 and NHANES 1999–2002) are illustrated in Table 2 (21, 22, 23, 24). Prevalence estimates generally were: 1) higher when the NCEP:ATPIII definition was revised; 2) similar between WHO and NCEP:ATPIII; and 3) higher using IDF rather than NCEP:ATPIII criteria. Prevalence estimates also increased over time (across the two NHANES cohorts) from approximately 29% (1988–1994 cohort) to 35% (1999–2002 cohort). The cause of this apparent increase over such a short period of time is not known but was likely due to differences in WC and in the composition of the cohorts.
TABLE 2.
Age-adjusted prevalence according to MetS definition within NHANES (unadjusted for sex or race/ethnicity and including those with T2D)
MetS prevalence estimates were compared, using the WHO, NCEP:ATPIII, and IDF definitions, in middle-aged adults from the San Antonio Heart Study stratified by sex and race/ethnicity (Table 3) (25). The San Antonio data demonstrated lower prevalence estimates using the WHO criteria and higher prevalence estimates using the IDF criteria, relative to NCEP:ATPIII. These data further demonstrated an interaction between sex and race/ethnicity on MetS prevalence in middle-aged adults, such that MetS prevalence appeared to be higher in white, non-Hispanic men compared with women, whereas MetS prevalence was similar in Mexican-American men and women.
TABLE 3.
Prevalence according to MetS definition (stratified by sex and race/ethnicity and including those with T2D) in the San Antonio Heart Study (25 412 )
| n | WHO | ATPIII | IDF | |
|---|---|---|---|---|
| Women | ||||
| White, non-Hispanic | 506 | 12.1% | 16.8% | 24.7% |
| Mexican-American | 1171 | 27.3% | 30.9% | 38.5% |
| Men | ||||
| White, non-Hispanic | 422 | 18.8% | 24.0% | 28.4% |
| Mexican-American | 842 | 28.3% | 29.6% | 40.4% |
As is the case for studies conducted in the United States, studies from other countries have reported varying MetS prevalence rates depending on the definition used (Table 4). Estimates of prevalence using the IDF criteria are often slightly higher than when the NCEP:ATPIII definition of MetS is used within the same population (26, 27, 28, 29, 30, 31). However, both China and Iran seem to have lower prevalence rates when the IDF definition is used (32, 33). Other studies have found less consistent differences in MetS prevalence using the different definitions (34, 35, 36, 37, 38). Thus, definition-related differences in prevalence are not consistent among countries and may be attributed, in part, to the race-specific WC guidelines included in the IDF definition. Many global studies assessing MetS prevalence have included diabetic subjects in their sample population, which clearly impacts the number of people estimated to have MetS (27, 28, 29, 31, 35, 36, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52). Not surprisingly, the overall prevalence of the MetS often increases in parallel with increases in obesity (24, 41, 53).
TABLE 4.
Prevalence of the MetS among various countries (by definition and by sex where data were provided)
| Ref. | n | Age (yr) | ATPIII 2001 | WHO | IDF | EGIR | |
|---|---|---|---|---|---|---|---|
| Australia | 26 | 11,247 (8438 no DM) | ≥25 | 24.4% m, 19.9% w | 25.4% m, 18.2% w | 34.4% m, 27.2% w | 15.6% m, 11.3% w |
| Brazil | 56 | 1,242 | 40–74 | 25.9% m, 40.9% w | |||
| Cameroon | 99 | 1,573 | 24–742 | 0% m, 0% w | 4.9% m, 2% w | 0% m, 0% w | |
| Canada (non-Aboriginal) | 39 | 2,0581 | 18+ | 30.6% m, 29.2% w | |||
| Canada (Inuits) | 39 | 2381 | 18+ | 6.7% m, 18.8% w | |||
| China | 32 | 6,610 | ∼52 | 18.5% m, 15.7% w | 18.1% m, 22.4% w | 16.2% m, 19.0% w | 11.8% m, 12.2% w |
| China | 40 | 15,5401 | 35–74 | 9.8% m, 17.8% w | |||
| Denmark | 29 | 2,4931 | 41–72 | 18.6% m, 14.3% w | 23.8% m, 17.5% w | ||
| Finland | 34 | 2,182 | 24–39 | 13.0% m/w | 14.9% m/w | 9.8% m/w | |
| Finland | 35 | 2,0491 | 45–64 | 38.8% m, 22% w | |||
| France | 41 | 3,7701 | 30–64 | 11.0% m, 8.0% w | |||
| Greece | 59 | 2,282 | >18 | 25.2% m, 14.6% w | |||
| Greenland | 36 | 9171 | ≥35 | 13% m, 22% w | 20% m, 22% w | ||
| Hungary | 27 | 13,3831 | 30–60 | 6.7% m, 9.8% w | 14.9% m, 8.6% w | ||
| Southern India (Urban) | 28 | 2,3501 | 20+2 | 17.1% m, 19.4% w | 27.3% m, 19.7% w | 23.1% m, 28.2% w | |
| Northern India (Urban) | 57 | 300 | 20+ | 18.4% m, 30.9% w | |||
| Iran | 33 | 10,368 | 20+ | 24.0% m, 40.5% w | 17.0% m, 20.0% w | 21.0% m, 41.0% w | |
| Ireland | 42 | 8901 | 50–69 | 21.8% m, 21.5% w | 24.6% m, 17.8% w | ||
| Italy | 56 | 1,198 | 40–74 | 26.8% m, 23.7% w | |||
| Northern Jordan | 43 | 1,1211 | 25–85 | 28.7% m, 0.9% w | |||
| Mexico | 44 | 2,1581 | 20–69 | 28.5% m, 25.2% w | 13.4% m, 13.8% w | ||
| Oman | 45 | 1,4191 | 20–99 | 19.5% m, 23.0% w | |||
| Palestine | 46 | 9921 | 30–65 | 17.0% m/w | |||
| Peru | 47 | 1,8781 | 20–80 | 18.1% m/w | |||
| Philippines | 48 | 4,5411 | >20 | 14.3% m, 14.1% w | |||
| Russia | 49 | 1461 | 25–89 | 66.9% m/w | |||
| Slovakia | 50 | 6571 | ≥30 | 20.0% m/w | |||
| South Korea | 51 | 40,6981 | 20–82 | 5.2% m, 9.0% w | |||
| Spain | 30 | 2,540 | 35–64 | 22.0% m, 28.8% w | 27.3% m, 31.7% w | ||
| Sweden | 37 | 5,047 | 46–68 | 20.6% m/w | 21.9% m/w | 18.8% m/w | |
| Sweden | 24 | 1,007 | 45–69 | 14.8% m, 15.3% w | |||
| Sweden | 38 | 508 | 70 | 26.3% m, 19.2% w | |||
| Tunisia | 31 | 8631 | 40+ | 14.6% m, 30.8% w | 25.7% m, 30.8% w | 30.0% m, 55.8% w | |
| Turkey | 52 | 4,2591 | 20–90 | 28.0% m, 39.6% w | |||
| Turkey | 58 | 2,296 | 28+ | 32.2% m, 45.0% w | |||
| Taiwan | 55 | 5,936 | 20–80 | 18.3% m, 13.6% w | 16.1% m, 13.3% w |
DM, Diabetes mellitus; m, men; w, women.
T2D included.
Not age-adjusted.
B. Prevalence estimates by sex
In the United States, the age-adjusted prevalence of the MetS is somewhat different between women and men, but the directionality of that difference has been inconsistent across cohorts. In the NHANES 1988–1994 cohort, the prevalence of the MetS was lower in women than men (23.9 vs. 27.8%; n = 5775), whereas the prevalence was higher in women than men in the later 1999–2002 cohort (30.3 vs. 28.0%; n = 1514) (54). The age-adjusted prevalence increased dramatically in women over this timeframe but did not change in men. The reason for the increase in women is not clear, but it is likely that this was due, in part, to changes in the racial and ethnic composition of the female cohort. The relative impact of racial/ethnic composition on sex-related differences in MetS is illustrated in Table 5.
TABLE 5.
Age-adjusted prevalence of MetS by race/ethnicity and sex (using NCEP:ATPIII criteria, including those with T2D)
Although in many countries there is very little difference between rates of MetS among women and men, there are some countries that have noticeably greater numbers of women than men that meet the MetS criteria (30, 31, 33, 36, 39, 40, 43, 52, 55, 56, 57, 58), whereas others report greater prevalence in men (26, 35, 59). Because sex-related differences in MetS prevalence are not universal, differences between women and men within specific countries may be due, for example, to differing SES, work-related activities, and cultural views on body fat. Importantly, the development of the inclusion criteria for each of the definitions was based upon epidemiological data primarily from westernized countries. Although the definitions may still be used to estimate prevalence in any population, it remains unclear how each of the individual criteria may impact sex-specific prevalence rates within certain countries. For example, a WC of more than 88 cm in women from the United States may be a reasonable threshold indicative of higher than normal central adiposity and therefore increased risk of CVD and T2D, but that same waist threshold in an Arab or Asian nation might be too high. Indeed, the addition of the ethnicity-specific WC criteria to the IDF 2005 definition attempted to take this possibility into account, lowering the WC threshold from at least 88 cm to at least 80 cm for women of certain African, Arab, and Asian populations. However, apart from slightly shifting prevalence rates up or down in women and men, it is unclear whether making the WC criteria more inclusive in these populations more effectively captures those who are at greatest risk of CVD and T2D.
C. Prevalence estimates by race/ethnicity
Within the United States, sex-related differences in MetS prevalence are influenced by race and ethnicity-related differences (Table 5). For example, age-adjusted prevalence of MetS in the NHANES studies was lower in white, non-Hispanic women than men, whereas prevalence was higher in African-American women than men. Mexican-American women had a higher prevalence of MetS compared with men in the earlier NHANES 1988–1994 cohort, but the prevalence almost doubled in Mexican-American men in the later NHANES 1999–2002 cohort such that age-adjusted prevalence was lower in Mexican-American women than men. On the other hand, MetS prevalence was not different between Mexican-American men and women in the San Antonio Heart Study. Thus, sex-related differences in MetS prevalence appear to be largely dependent on the racial and ethnic composition of the cohort being studied. Comparison of MetS prevalence among cohorts from other countries further highlights the relative impact of racial/ethnic composition on sex-related differences in MetS (Table 4).
D. Prevalence estimates by age
1. MetS in older populations.
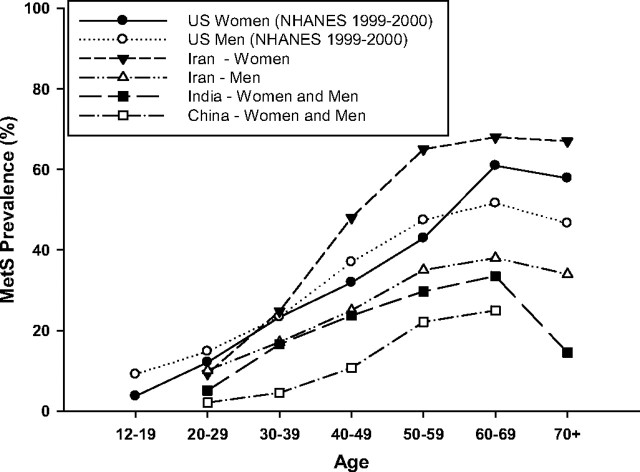
Not surprisingly, the MetS becomes more prevalent with each decade of life, increasing in parallel with age-related increases in obesity and, in particular, central adiposity (23, 60, 61). In the NHANES cohorts, MetS prevalence continued to increase with age into the sixth decade, with prevalence in women catching up to and then exceeding that in men after the age of 60 yr (62, 63) (Fig. 1). These trends suggest an interaction between age and sex on the prevalence of the MetS. The definition used to estimate prevalence, however, may influence this interaction. The Cardiovascular Health Study, which studied men and women over the age of 65 yr, observed higher MetS prevalence in women than men (37.4 vs. 32.1%) using the NCEP:ATPIII criteria, but lower prevalence in women than men (23.9 vs. 32.4%) using the WHO criteria (64). Nevertheless, MetS prevalence increases consistently with age between the ages of 12 to 60 yr in the United States (Fig. 1) and across the globe, independent of sex (27, 28, 44, 65).
Fig. 1.
Prevalence of the MetS across age groups and gender in various countries. The MetS prevalence continues to increase with age into the sixth decade, with prevalence in women catching up to and then exceeding that in men after the age of 60 yr in the United States and across the globe (27 28 44 55 65 ).
Studies that have compared age-related increases in prevalence among different definitions have observed variable prevalence estimates after the sixth or seventh decades (26, 28). Much of this variability in these later decades of life may be due to a survival effect, because those most susceptible to obesity-related mortality have likely died by this point (66). Finally, whether prevalence estimates plateau or drop off steeply after the age of 60 yr also varies according to the MetS definition being used (26, 28, 52).
2. MetS in younger populations.
Current literature supports the notion that the presence of the MetS in youth may be an important predictor of future risk for diabetes and CVD (67). Landmark studies from the Bogalusa Heart Study demonstrated that cardiovascular risk factors present in childhood are predictive of coronary artery disease in adulthood (68, 69). For example, LDL-C and BMI measured in childhood were found to predict carotid intima-media thickness in young adults (70). There is substantial evidence that obesity is the main determinant of insulin resistance in children (71) and that it increases the risk not only for the MetS in adulthood (72) but also for CVD and T2D later in life (73, 74, 75, 76).
A major problem with identifying the MetS in children and adolescents is that there are no established criteria in this population. In fact, a recent review found 27 publications that used 40 different definitions of the MetS in children and adolescents (77). The uniqueness of pediatric growth patterns, effects of hormonal changes of puberty on insulin sensitivity and lipid profile, and the impact of ethnic background on components of the syndrome make such criteria difficult to establish. The reported prevalence of MetS in youth, therefore, varies according to the age and population under study and the definition being used (Table 6) (71, 78, 79, 80, 81, 82). Cook et al. (79) estimated the prevalence of the MetS in 2430 U.S. adolescents using NHANES 1986–1994 data by modifying the NCEP:ATPIII definition, based on reference values for physiological parameters in youth. The overall prevalence was 4.2%, 6.1% in boys and 2.1% in girls, respectively. Among obese and overweight adolescents, prevalence of the MetS was 28.7 and 6.8%, respectively. Similar to adults, prevalence of individual components of the MetS differed by race/ethnicity (e.g., prevalence of elevated blood pressure was higher and prevalence of high triglyceride and of low HDL-C concentrations was lower in African-American youth compared with non-Hispanic white or Mexican-American youth). Using the same population and a similar definition but different cut points for hypertriglyceridemia and central obesity, de Ferranti et al. (80) obtained an overall prevalence estimate of 9.2%. In a population of 218 overweight Hispanic youth with a family history of T2D, the prevalence of the MetS ranged from 26 to 39%, depending on the definition used (83). Weiss et al. (82) assessed the impact of varying degrees of obesity on the prevalence of the MetS in 493 children and adolescents with BMI in the 97th percentile or above for age and gender. The prevalence of the MetS increased with the severity of obesity and reached approximately 50% in severely obese youngsters. Finally, Cook et al. (78) recently examined the prevalence of the MetS in 1,826 U.S. adolescents from the NHANES 1999–2002 survey using four definitions of the MetS previously used in this age group. They found that depending on the definition used, the prevalence varied between 2.0 and 9.4% in all teens and ranged between 12.4 and 44.2% in obese teens (78).
TABLE 6.
Prevalence of MetS in children and adolescents among various cohorts
| Population | Ref. | n | Criteria | Prevalence |
|---|---|---|---|---|
| NHANES (1988–1994), youths (12–19 yr old) | 79 | 2430 | ATP III (≥3): WC ≥90th %; IFG; TG ≥150 mg/dl; HDL <40 mg/dl; BP ≥90th % or medication | 4.2%; BMI ≥95th %, 28.7% |
| NHANES (1988–1994), youths (12–19 yr old) | 80 | 1960 | ATP III (≥3): WC ≥75th %; TG ≥70th % | 9.2% |
| Overweight Hispanic youth (BMI ≥85th %; 8–13 yr old) with family history of T2D | 81 | 126 | ATP III (≥3): HDL <10th %; IGT | 38% |
| Multiethnic/multiracial youths (4–20 yr old) | 82 | 490 | ATP III and WHO (≥3): BMI z-score ≥2; IGT; TG ≥95th %; HDL <5th %; BP ≥95th % | 38.7% in moderately obese; 49.7% in severely obese |
| NHANES (1999–2002), youths (12–19 yr old) | 78 | 1826 | Cook et al. (79 ) | 9.4% |
| Cruz et al. (81 ) | 2.0% | |||
| Weiss et al. (82 ) | 2.4% | |||
| Adult NCEP (1 ) | 5.8% |
BP, Blood pressure; TG, triglycerides.
These discrepancies clearly emphasize the need for a consensus definition of the MetS in younger individuals first to understand better the prevalence but also as a potential clinical tool in identifying at-risk individuals (67, 84). The IDF Task Force on the Epidemiology and Prevention of Diabetes has recently developed a definition primarily for those 10 yr and older but less than 16 yr of age (67). MetS can be diagnosed in this age group by abdominal obesity (≥90th percentile) and the presence of two or more other factors, including hypertriglyceridemia (≥1.7 mmol/liter), low HDL-C (≤1.03 mmol/liter), elevated blood pressure (≥130 mm Hg systolic or ≥85 mm Hg diastolic), or increased blood glucose (≥5.6 mmol/liter). For those less than 6 yr of age, there were insufficient data to make a recommendation. For children between the ages of 6 and 10 yr, they suggested that the MetS not be diagnosed but that a strong message regarding weight reduction should be made in those with abdominal obesity. Finally, they recommended that the adult criteria be used for those 16 yr or older.
Important to note in the study of the MetS in youth is that there are racial/ethnic differences in the prevalence of the MetS, as well as differences in the prevalence of individual components of the syndrome. For example, insulin resistance is greater among African-American compared with non- Hispanic white youth (85, 86, 87, 88). Goran et al. (85) reported that Mexican-American and African-American children were both more insulin resistant than non-Hispanic white children, to a comparable degree and independent of obesity, although underlying physiological compensatory mechanisms differed between African-American and Mexican-American youth. However, although African-American youth are more insulin-resistant, the prevalence of the MetS is lower in African-American youth (2%) than Hispanic (5.6%) and non-Hispanic white youth (4.8%) (79), suggesting that African-American youth may be less likely to develop obesity-related clustering of MetS components than non-Hispanic white youth.
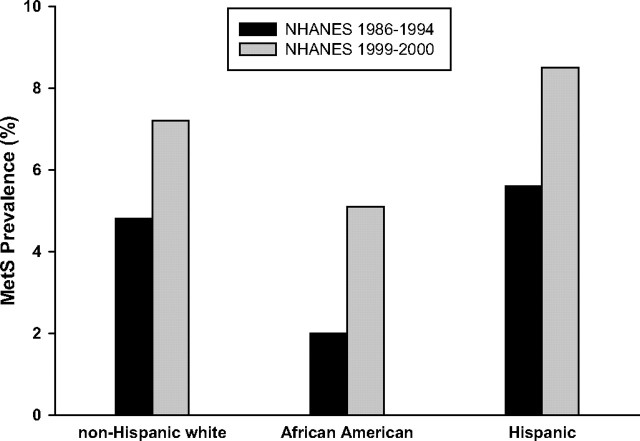
Duncan et al. (62) examined trends in the prevalence of MetS in U.S. adolescents aged 12–19 yr using NHANES data (1998–1994 and 1999–2000) and the pediatric definition developed by Cook (79). The overall prevalence of a MetS phenotype among U.S. adolescents increased from 4.2% in NHANES III (1988–1992) to 6.4% in NHANES 1999–2000 (P < 0.001). The trend was evident in both sexes and in all three major race/ethnic groups analyzed in this study (Fig. 2). Based on population-weighted estimates, the study estimated that more than 2 million U.S. adolescents currently have a MetS phenotype. The MetS was most frequent in obese adolescents, with a prevalence of 32.1%, compared with only 7.1% for overweight adolescents. Given the increasing prevalence of overweight and obesity in youth (89) and the strong relationship between obesity and the MetS, it is not surprising that the prevalence of MetS has increased over the past decade among U.S. adolescents.
Fig. 2.
The prevalence of the MetS among adolescents. Prevalence of the MetS among U.S. adolescents (age 12–19 yr) increased from 4.2% in NHANES III (1988–1992) to 6.4% in NHANES 1999–2000 (P < 0.001) in both sexes and in all three major race/ethnic groups analyzed (62 ).
Interestingly, the risk of developing T2D in youth with the MetS is currently unknown. This is partially attributed to the difficulty of diagnosing T2D in children and to the lack of a standard definition of the MetS definition in youth (67). Despite this, it is estimated that approximately 92% of the adolescent population that has T2D also has the MetS (90, 91). It has also recently been shown that the incidence of T2D in adulthood is increased 3-fold in those with the MetS as children (92). Further research is necessary to understand better the link between the MetS in youth and the development of T2D.
E. Prevalence estimates by socioeconomic status, tobacco, alcohol, and level of education
Few studies have evaluated the impact of SES outcomes on the prevalence of MetS. In the NHANES 1988–1994 cohort, multivariable adjusted odds ratios (OR) and 95% confidence intervals (CI) for the MetS were reported for select SES and lifestyle outcomes (23). Risk was increased in women (OR, 1.8; 95% CI, 1.2–2.6) and men (OR, 1.5; 95% CI, 1.1–2.2) who were current smokers compared with those who never smoked (23). In men only, the OR for MetS was increased (OR, 1.7; 95% CI, 1.2–2.5) in those who had heavy (>60% total calories) compared with moderate (40–60% total calories) carbohydrate intake, and the OR for MetS was increased for physical inactivity (OR, 1.4; 95% CI, 1.0–2.0) (23). In women only, the OR for MetS was decreased (OR, 0.8; 95% CI, 0.6–1.0) in those who reported regular (1 drink per day) compared with moderate (<1 drink per day) alcohol intake, and the OR was increased (OR, 1.5; 95% CI, 1.0–2.3) in those who reported a lower (≤$15,000/yr) compared with a higher (>$25,000/yr) household income (23). Level of education was not related to OR for MetS in women or men in this NHANES cohort (23). However, the association between SES and MetS within the United States appears to be confounded by race and ethnicity. Among African-American women and men (Pitt County Study, n = 1195), higher educational status (at least high school graduation) was associated with reduced risk of MetS (OR, 0.63; 95% CI, 0.48–0.83), compared with lower educational status (less than high school graduation) (93).
Although there is a paucity of global epidemiological studies assessing the impact of SES on MetS prevalence, there are some data to suggest a similar relation between MetS and SES in other westernized nations as is seen within the United States (55, 56, 60, 61). An inverse association between level of education and risk of MetS has been observed in middle-aged Swedish women (94), as well as Finnish women and men (95). Among British civil servants (Whitehall II cohort, n = 7,013), women and men had a 2- to 3-fold increased risk of MetS if they were in the lowest (compared with the highest) employment grade quintile: women, OR, 2.8; 95% CI, 1.6–2.9; men, OR, 2.2; 95% CI, 1.6–2.9 (96). In these three European studies, adjusting for other behavioral risk factors (e.g., smoking and alcohol intake) did not alter the association between the SES outcomes and MetS (94, 95, 96). In Korea, the association between SES and MetS was evident only in women. Relative to women with lower education and income, Korean women with higher education and income levels had a lower risk for MetS (97). In Korean men, there was no significant relation between the prevalence of MetS with education or income levels (97). Furthermore, a separate Korean study demonstrated interactions between SES (i.e., education, income) and behavioral (i.e., smoking, alcohol intake, exercise) outcomes, suggesting that health behaviors differentially impact incidence of MetS across SES levels (98).
In addition to SES, urban vs. rural location may play a role in prevalence of MetS in developing nations. Abdul-Rahim et al. (46) found that Palestinians with T2D living in an urban area had a 17% greater likelihood of meeting NCEP:ATPIII MetS criteria than those living in a rural area. Furthermore, men in urban areas of Cameroon had a 7.3-fold greater risk of developing MetS, whereas women had a 5.9-fold increased risk (99). Similar trends have been observed in China and Russia (49, 100, 101). However, not all nations with developing economies exhibit differences in MetS prevalence between urban and rural areas (52, 102).
F. Changes in prevalence following intervention
Prevalence estimates of MetS may be modifiable by intervention. The Diabetes Prevention Program (DPP) conducted post hoc analyses to address this possibility by evaluating changes in the prevalence of MetS (NCEP:ATPIII 2001 criteria) after treatment with either lifestyle (diet + exercise-induced weight loss) or metformin (103). They evaluated the incidence of new MetS cases and resolution of existing MetS cases compared with placebo treatment in participants (n = 3234) of the DPP trial. Because IGT was a primary inclusion criterion for entrance into the DPP trial, the majority (53%) of the participants met the criteria for MetS at baseline (103). Incidence of MetS was reduced by 41% in the lifestyle group and by 17% in the metformin group compared with placebo (103). Among participants who met the MetS criteria at baseline, by 3 yr MetS resolved in 18% of placebo, 23% of metformin, and 38% of the lifestyle group (103). Whether these treatment effects apply to a non-IGT population remains unknown. However, in a small study of obese (BMI ≥30 kg/m2) older (age ≥65yr) women and men randomized to a similar lifestyle intervention (n = 17) or a control group (n = 10) for 26 wk, MetS resolved in 10 of 15 cases in the treatment group compared with no cases in the control group (103, 104). Whether reducing the incidence of MetS leads to a reduction in T2D and CVD-related morbidity and mortality remains unknown.
IV. Pathophysiology
As previously discussed, the primary purpose of identifying the MetS was to identify a clustering of features that were associated with increased CVD risk. As the term syndrome implies, a specific causative etiology to the MetS is not clear, nor was a common, unifying pathophysiological cause of the MetS necessarily intended. Nevertheless, abdominal adiposity and insulin resistance appear to be at the core of the pathophysiology of the MetS and its individual components. Thus, the purpose of this section is to review how abdominal adiposity and insulin resistance may contribute to the pathophysiology of the MetS.
A. Insulin resistance: a conceptual prologue
Insulin is a pleiotropic molecule that has effects on amino acid uptake, protein synthesis, proteolysis, adipose tissue triglyceride lipolysis, lipoprotein lipase activity, very low-density lipoprotein (VLDL) triglyceride secretion, muscle and adipose tissue glucose uptake, muscle and liver glycogen synthesis, and endogenous glucose production. Individuals are generally defined as insulin sensitive or insulin resistant by their response to an oral or iv glucose or insulin stimulus (105). Characteristics of the insulin-sensitive phenotype include a normal body weight (106) without abdominal or visceral obesity (5, 107), being moderately active (108), and consuming a diet low in saturated fats (109). Alternatively, insulin-resistant individuals demonstrate impaired glucose metabolism or tolerance by an abnormal response to a glucose challenge, elevated fasting glucose levels and/or overt hyperglycemia, or reductions in insulin action after iv administration of insulin (euglycemic clamp technique) with decreased insulin-mediated glucose clearance and/or reductions in the suppression of endogenous glucose production. In general, the characteristics of this phenotype are more likely to include being overweight or obese (106, 110), being sedentary (108), and consuming a diet high in total or saturated fats (109).
Insulin sensitivity, however, is not a simple dichotomy of being insulin sensitive or insulin resistant, but rather exists on a continuum. Moreover, the ability of the pancreas to secrete insulin in response to a glucose challenge may also reflect insulin resistance at the level of the β-cell. To define this, Bergman (111) proposed the disposition index, a quantitative measure that describes the relationship between β-cell sensitivity and insulin sensitivity (112). In metabolically normal individuals, changes in insulin sensitivity are accompanied by compensatory alterations in the response of the β-cell to glucose. In practice, disposition index is defined as the product of the insulin sensitivity index and β-cell function as measured by the acute insulin response to glucose, a relationship that is typically plotted as an inverse hyperbola. The movement along the continuum is more complicated than the model implies, and the molecular mechanism(s) by which insulin sensitivity and β-cell function are coregulated to create a homeostatic environment are not well understood.
B. Obesity as a “driving force” in the prevalence of insulin resistance
The worldwide increase in the prevalence of obesity in the recent decades is startling and is likely a cause of the rising incidence of insulin resistance and the MetS (7, 8, 113, 114, 115), as well as CVD and T2D (7). Although not all overweight or obese individuals are metabolically unhealthy, the majority are insulin resistant (116). Indeed, many experts assert that the MetS would never have been put forth if the obesity epidemic had not become the public health concern that it is today (113). In particular, the combination of obesity, physical inactivity, and consumption of an atherogenic diet is believed to lead to insulin resistance (117). In this state of insulin resistance, normoglycemia is initially maintained by a modest increase in β-cell mass and/or an increase in insulin secretory capacity (8, 118). Although the mechanism for this compensation is unclear, there is recent evidence supporting glucose signaling as a dominant force in this process (119); it is also acknowledged that genetic factors may be involved (120, 121). However, the loss of insulin secretory capacity in the natural history of T2D is likely an admixture of β-cell dysfunction in addition to reductions in β-cell mass (122). If the increasing β-cell function and/or mass is successful long-term as a compensatory mechanism to obesity and insulin resistance, T2D could be prevented for an undetermined amount of time, despite hyperinsulinemia as a consequence.
C. Insulin resistance in adipose tissue
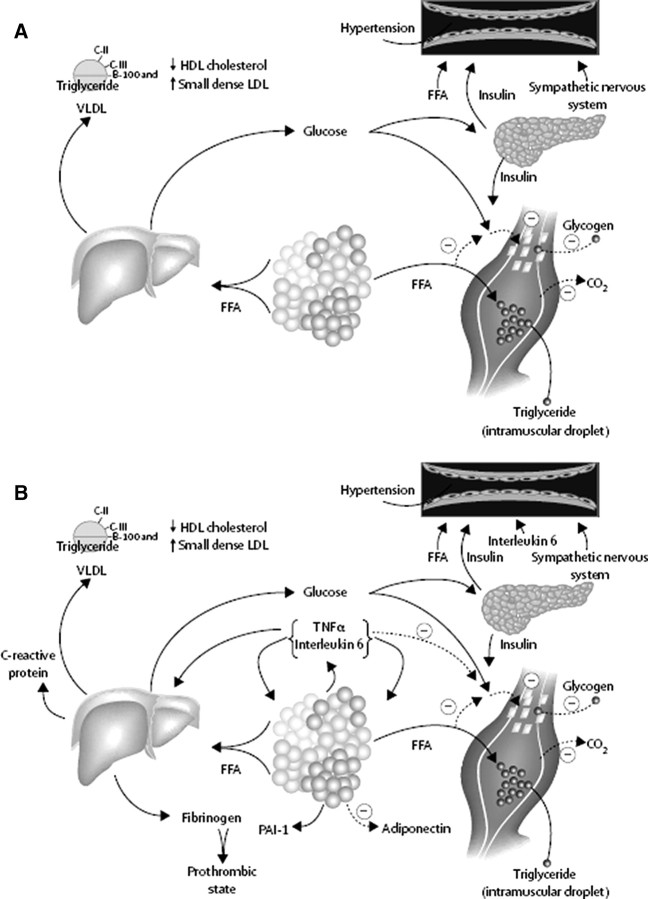
Adipose tissue insulin resistance appears to be important to the pathophysiology of the MetS (7, 8, 113, 123, 124). Specifically, a larger, expanded adipose tissue mass often results in an increased turnover of free fatty acids (FFAs) (125, 126) (Fig. 3). In the setting of insulin resistance and expanded adipose tissue triglyceride stores, the process of FFA mobilization (lipolysis) from stored adipose tissue triglyceride is accelerated (7, 127). Under normal conditions, insulin inhibits adipose tissue lipolysis; however, in the setting of insulin resistance, insulin is unable to properly suppress lipolysis, resulting in relatively more FFA being liberated into the plasma (113). Although it is well accepted that this process is mediated by hormone-sensitive lipase (HSL) (128), recent evidence points to adipose triglyceride lipase as playing an additional role; and collectively these two hormones account for 95% of triglyceride hydrolysis (129). In obese subjects, insulin resistance and hyperinsulinemia are strongly associated with decreased adipose triglyceride lipase and HSL mRNA and protein expression, an effect found to be independent of fat mass (130). There is also evidence supporting a genetic predisposition for insulin resistance and T2D linked to the HSL gene (131).
Fig. 3.
Pathophysiology of the Metabolic Syndrome and insulin resistance. A, FFA are released in abundance from an expanded adipose tissue mass. In the liver, FFA result in increased production of glucose and triglycerides and secretion of VLDL. Associated lipid/lipoprotein abnormalities include reductions in HDL-C and increased density of LDL. FFA also reduce insulin sensitivity in muscle by inhibiting insulin-mediated glucose uptake. Associated defects include a reduction in glucose partitioning to glycogen and increased lipid accumulation. Elevated circulating glucose and to some extent FFA increase pancreatic insulin secretion, resulting in hyperinsulinemia. Hyperinsulinemia may result in enhanced sodium reabsorption and increased sympathetic nervous system activity and may contribute to hypertension, as might increased levels of FFA. B, Superimposed and contributory to the insulin resistance produced by excessive FFA is the paracrine and endocrine effect of the proinflammatory state. Produced by a variety of cells in adipose tissue, including adipocytes and monocyte-derived macrophages, the enhanced secretion of IL-6 and TNF-α among others results in more insulin resistance and lipolysis of adipose tissue triglyceride stores, resulting in increased circulating FFA. IL-6 and other cytokines also are increased in the circulation and may enhance hepatic glucose production, the production of VLDL by the liver, and insulin resistance in muscle. Cytokines and FFA also increase the production of fibrinogen and PAI-1 by the liver, complementing the overproduction of PAI-1 by adipose tissue. This results in a prothrombotic state. Reductions in the production of the antiinflammatory and insulin-sensitizing cytokine adiponectin are also associated with the metabolic syndrome and insulin resistance. [Reproduced from R.H. Eckel et al.: Lancet 365:1415–1428, 2005 (113 ) with permission from Elsevier.]
Not only does insulin resistance appear to cause FFA to rise, but elevated FFA levels also appear to cause insulin resistance. Substantial evidence has accumulated to suggest that the visceral depot contributes to increased FFA turnover and insulin resistance (5, 132, 133, 134, 135, 136). Specifically, visceral adipocytes are more sensitive to catecholamine-stimulated lipolysis than sc adipocytes (137). Because the venous drainage of the visceral adipose tissue depot is directly into the portal system (136), it has been hypothesized that in visceral obesity the liver is bathed with fatty acids and consequently becomes insulin resistant (“portal theory”) (113, 138).
Another manner in which adipose tissue contributes to the pathophysiology of the MetS is through the excessive release of proinflammatory cytokines. The source of these cytokines in adipose tissue is debated, with controversy surrounding the relative roles of adipocytes vs. monocyte-derived macrophages (139, 140). Since the earliest report of the presence of monocyte-derived macrophages in human adipose tissue by Ferrante and colleagues (141), it is now clear that larger fat cells also produce more cytokines (142). Not only are circulating cytokines from adipose tissue important to insulin action in other tissues such as the liver or skeletal muscle, but paracrine effects of the cytokines may also modify insulin action locally in adipose tissue (143, 144).
D. Insulin resistance in the liver
The liver plays a major role in substrate metabolism. Increases in FFA flux have been shown in numerous models to impair hepatic insulin action (138). This includes increases in hepatic glucose output, the synthesis of proinflammatory cytokines, and major changes in lipoprotein metabolism. In the liver, the increased FFA flux must be oxidized or stored. Insulin, under normal physiological conditions, increases the gene expression of a number of enzymes central to triglyceride biosynthesis (145), but also reduces VLDL triglyceride and apolipoprotein (apo) B production and secretion, an effect largely attributable to reductions in adipose tissue lipolysis (146). Another intrahepatic effect of insulin is to enhance apo B degradation (147). In the liver of insulin-resistant patients, FFA flux is high, triglyceride synthesis and storage are increased, and excess triglyceride is secreted as VLDL (148).
For the most part, it is believed that the dyslipidemia associated with insulin resistance is a direct consequence of increased VLDL secretion by the liver (147). This may explain why increases in plasma apo B are variably associated with the MetS in the absence of increases in LDL-C (149). In addition to overproduction of VLDL by the liver, alterations in lipoprotein lipase (LPL) have been associated with the MetS. In a large family-based population of Mexican-Americans who were genotyped at six polymorphisms in the LPL gene that define the most common haplotypes in the population, specific LPL haplotypes showed linkage to glucoregulatory aspects of insulin action (150). Other reports linking the LPL gene to the MetS have been published (120, 151, 152). Although there is also evidence that an inverse relationship exists between pre-heparin LPL mass and insulin resistance or the MetS (153, 154), there is little evidence at present that reductions in post-heparin LPL activity (the active enzyme) occur in the MetS (155, 156). This may be a consequence of the tissue-specific regulation of LPL by insulin (157).
Hypertriglyceridemia is typically associated with reductions in HDL-C. This in part relates to the transfer of cholesteryl ester from the core of triglyceride-rich lipoproteins to HDL-C, a process catalyzed by cholesteryl ester transfer protein (CETP) (7, 113, 158). This generates a smaller, triglyceride-rich HDL-C that is a better substrate for hepatic lipase, which results in a particle that is more rapidly cleared by the kidney (159). CETP gene polymorphisms influence plasma CETP activity and plasma HDL-C concentrations, a relationship that in several reports has been associated with the presence of abdominal obesity and some features of the insulin resistance syndrome (160, 161, 162). In the setting of hypertriglyceridemia, LDL-C particles are also triglyceride-enriched, small, and dense. Evidence supports an association of small dense LDL-C with CVD (163, 164). Of interest, the MetS has been associated with increased CETP mass in men, and possibly reduced LDL-C particle diameter in addition to reduced HDL-C (165). As previously noted, hepatic lipase plus another member of the lipase gene family, endothelial lipase, have moderate to substantial phospholipase activities, respectively, and are important in HDL-C catabolism. Moreover, endothelial lipase is linked to the proinflammatory state (166). Evidence also relates increases in hepatic lipase to CVD events (167).
Hepatic steatosis is related not only to insulin resistance but also to the MetS. This includes simple deposition of excessive hepatic fatty acids as triglycerides in the liver as well as a more advanced and inflammatory lesion, nonalcoholic steatohepatitis (NASH) (168). Recent studies emphasize the role of insulin resistance, oxidative stress, lipid peroxidation, and cytokines in the development of NASH. At present, therapies of hepatic steatosis directed at improving insulin action implicate the importance of insulin resistance in the etiology of excessive hepatic fat accumulation (169).
E. Insulin resistance in muscle
In muscle, increased plasma FFA disrupt the glucose-fatty acid cycle (125, 170, 171). The predominant defect in insulin action in skeletal muscle relates to an inhibitory effect of this increase in plasma FFA on insulin-mediated glucose transport (172, 173, 174). It has also been hypothesized that triglyceride accumulation in skeletal muscle plays a direct role in the etiology of insulin resistance (124). There is also evidence that the degree of whole body insulin sensitivity is inversely correlated with im triglyceride content (124, 175) (Fig. 3). Yet, muscle triglyceride may only be a marker of other related mediators of insulin resistance in muscle, e.g., ceramide (176). Moreover, after insulin sensitivity is improved by exercise or weight reduction, muscle triglyceride content changes little if at all (177, 178).
F. Hypertension and insulin resistance
The relationship between insulin resistance and hypertension has been established (179, 180, 181) and relates to several potentially different mechanisms. First, it is important to note that insulin is a vasodilator when given iv to people of normal weight (182), with secondary effects on sodium reabsorption in the kidney (183). Evidence indicates that sodium reabsorption is increased in whites but not Africans or Asians with the MetS (184). In the setting of insulin resistance, the vasodilatory effect of insulin can be lost (185), but the renal effect on sodium reabsorption preserved (186). Fatty acids themselves can mediate relative vasoconstriction (187). Moreover, the infusion of fatty acids into the portal vein activates the sympathetic nervous system and elevates blood pressure in rodents (188). Insulin also increases the activity of the sympathetic nervous system (189), an effect that might also be preserved in the setting of insulin resistance (190). However, when assessed by concentrations of fasting insulin or the homeostatic model assessment (HOMA) (191), insulin resistance contributes only modestly to the increased prevalence of hypertension in the MetS (192). Because adipose tissue is a source of angiotensinogen (193), it is not a surprise to note the association of hyperaldosteronism with hypertension and the MetS (194). Recent evidence also suggests that elevations in adipocyte-derived resistin and leptin may contribute to the pathogenesis of hypertension in patients with insulin resistance (195, 196).
G. Other contributors to insulin resistance (nocturnal FFA flux: sympathetic nervous system)
In response to any kind of stress, emotional and physical, lipolysis is stimulated via β1 receptors, thus liberating FFA from adipose tissue (197). It has further been demonstrated that in the setting of obesity, sympathetic nervous system activation is exaggerated, adding to the rise in FFA concentration (198, 199, 200). In diet-induced obese canines, there is evidence of an apparent pulsatile release of FFA from the visceral depot and a consequential, sustained elevation of nocturnal FFA in response to a moderate fat feeding (138, 201). Whether or not a similar response occurs in humans remains unclear, but it is possible that these fatty acid patterns, diet- and physiologically-induced, play a causal role in the development of insulin resistance and the MetS.
H. Proinflammatory molecules, ER stress, and their roles in insulin resistance and the metabolic syndrome
1. Proinflammatory molecules.
It is well documented that the MetS is associated with an elevated inflammatory state (202). This is evidenced by the presence of elevated concentrations of inflammatory molecules including C-reactive protein (CRP), TNFα, plasma resistin, IL-6, and IL-18 (203, 204, 205, 206, 207), consistent with the increase in adipose tissue mass characteristic of the MetS. Conversely, as is seen in obesity, levels of the antiinflammatory adipokine adiponectin are depressed in the MetS (204, 205, 207). In addition, as the number of the MetS components an individual exhibits increases, inflammatory markers, including CRP (208, 209), TNFα (205), IL-18 (210), and plasminogen activator inhibitor-1 (PAI-1) activity (211) also increase. Individual inflammatory markers are also associated with singular components of the MetS, as detailed below.
CRP is a general marker of inflammation, making it suitable to assess in individuals with the MetS. Elevated levels of CRP are associated with increased WC (208), insulin resistance (212), BMI (213, 214), and hyperglycemia (204, 208) and are increased with the number of the MetS components. In addition, it has been demonstrated that regardless of the presence or degree of the MetS in an individual, CRP levels independently predicted the occurrence of future CVD events (209). Because the MetS has been linked with a greater chance of future CVD events (215), CRP levels may be an important independent predictor of unfavorable outcomes in the MetS.
TNFα mRNA is expressed to a significantly greater degree in the adipose tissue of obese humans in comparison with those who are lean. This difference is abated with weight loss, thereby supporting the observations of elevated TNFα in the MetS (216). The degree of TNFα mRNA adipose tissue expression is positively correlated with plasma insulin, indicating that the amount of TNFα present in adipose tissue may be related to insulin resistance (216). Plasma TNFα is also positively associated with fasting insulin and insulin resistance (HOMA), as well as body weight, WC, and triglycerides; a negative association exists between plasma TNFα and HDL-C (205). TNFα neutralization has differential effects on critical adipokines and body composition indices; thus, it improves inflammatory markers and total adiponectin in patients with the MetS without improving insulin sensitivity (217).
Resistin is expressed in adipocytes and, most notably, inflammatory cells in humans (218). It has been linked to obesity, T2D, inflammation, and atherosclerosis, although the results of animal and human studies have been at variance. Serum resistin is highly heritable and has some common genetic background with traits related to insulin resistance, reinforcing the hypothesis that this adipokine may play a pathogenic role in insulin resistance-related abnormalities, including the MetS, T2D, and CVD (219). Elevated resistin levels in the MetS have been observed, and plasma resistin is positively associated with WC (203), systolic blood pressure (203, 208), and triglycerides (203, 208), whereas it is negatively associated with HDL-C (203, 208). Resistin shows significant BMI-dependent associations with insulin resistance and factors linked with obesity and inflammation in patients with T2D (220).
IL-1β genetic variants are associated with measures of chronic inflammation and risk for the MetS, and genetic influences are more evident among subjects with low (n-3) polyunsaturated fatty acid (PUFA) intake (221). IL-1β reduces insulin receptor substrate-1 (IRS-1) expression at a transcriptional level through an ERK-dependent mechanism and at a posttranscriptional level independently of ERK activation. By targeting IRS-1, IL-1β is capable of impairing insulin signaling and action and may thus participate in concert with other cytokines in the development of insulin resistance in adipocytes (222).
IL-6 is released by both adipose tissue and skeletal muscle in humans (92, 223) and, despite its role as both an inflammatory and an antiinflammatory molecule, has been shown to be positively associated with BMI, fasting insulin, and the development of T2D (224, 225) and negatively associated with HDL-C (226). Elevated IL-6 correlates temporally with increases in AMP kinase activity in multiple tissues (227) and has potential systemic impact on both glucose and lipid metabolism (228). The detriment in insulin signaling mediated by IL-6 is thought to occur at the level of IRS-1 because myotubes incubated with IL-6 have demonstrated a reversal of IRS-1 tyrosine phosphorylation induced by insulin (229).
IL-10 is a major antiinflammatory cytokine that has been associated with insulin resistance, obesity, MetS, and T2D (230, 231). IL-10 gene polymorphisms are also identified in the polycystic ovary syndrome (232). Serum IL-10 levels are significantly correlated with IL-6, CRP, and TNF-α levels, but not with adiponectin in healthy individuals. However, IL-10 is significantly correlated with adiponectin, especially in the subjects with the MetS. Thus, IL-10 may be involved in the inflammatory network of the MetS (207, 233).
The pleiotropic proinflammatory cytokine IL-18 plays a role in the inflammatory cascade, promoting both TNFα and IL-6 production (234). It is positively associated with BMI, WC, triglycerides, systolic and diastolic blood pressure, fasting glucose and insulin, and negatively associated with HDL-C in a nondiabetic Australian population (210). The GC genotype of the IL-18 −137 G/C polymorphism and the circulating IL-18 levels are independently associated with raised blood pressure, and fasting IL-18 levels are associated with the other metabolic risk factors for CVD in normal-weight and obese black South African women (235). A common IL-18 haplotype is associated with higher BMI in individuals with T2D and CVD (236). An inverse correlation between IL-18 and the antiatherogenic adipokine adiponectin has been reported in obesity, insulin resistance, CVD, and the MetS (237). IL-18 suppresses adiponectin expression in 3T3-L1 adipocytes via a novel signal transduction pathway involving ERK1/2-dependent nuclear factor of activated T-cells, cytoplasmic, cacineurin dependent 4 (NFATc4) phosphorylation (238). A report from the large population-based Dallas Heart Study showed that in univariate analysis, IL-18 levels were associated with traditional CVD risk factors and particularly with components of the MetS. In multivariate analyses, IL-18 remained associated with multiple components of the MetS but not with coronary artery calcium or aortic plaque (239).
Visfatin (also known as pre-B-cell colony-enhancing factor) is an adipokine that is highly expressed in visceral fat. Plasma visfatin has been reported to correlate with the degree of visceral adiposity in humans (240) and has been proposed as a surrogate marker for visceral fat accumulation in obese children (241, 242). There is a significant association between plasma visfatin and visceral visfatin mRNA expression (243). Associations between circulating visfatin and characteristics of the MetS, therefore, may be directly related to an expanded visceral adipose mass or as a result of increased expression of visfatin in visceral adipose tissue. Plasma visfatin levels are elevated in individuals with the MetS (244) and are associated with several components of the MetS. Serum visfatin is positively associated with BMI (245), and visfatin mRNA expression in visceral adipose tissue is associated with BMI and percent body fat (243). Interestingly, results from the recent PIOSTAT study suggest that although visfatin has been postulated as a good marker of the MetS, insulin resistance and CVD risk factors are not associated with visfatin levels, and regulation of visfatin secretion occurs through biochemical pathways independent from those influenced by pioglitazone or simvastatin (246).
The antiinflammatory molecule, adiponectin, is negatively associated with body weight (205), WC (205), triglycerides (204, 205, 247), BMI (247), fasting insulin (205), insulin resistance (HOMA) (204, 205), and systolic and diastolic blood pressure (247), whereas a positive association exists between adiponectin and HDL-C (204, 205, 247, 248). Adiponectin is a powerful inducer of other proinflammatory cytokine (IL-1β, IL-6, IL-8, and TNF-α) production by adipose tissues and macrophages (249). Transgenic mice that express human adiponectin in their liver show significantly decreased weight gain associated with less fat accumulation and smaller adipocytes in both visceral and sc adipose tissues. These mice also have increased energy expenditure, longer life span, and reduced morbidity and mortality when fed a high-calorie diet (250). Of note, high molecular weight (HMW) adiponectin exhibits a significant association with central fat distribution, whereas low molecular weight adiponectin does not (251). Additionally, the HMW/total adiponectin ratio been shown to have a greater power to predict the presence of both insulin resistance and the MetS in comparison with total plasma adiponectin (252). Therefore, in addition to total adiponectin levels, measurement of HMW adiponectin may also be valuable for the prediction of the MetS.
Interestingly, in contrast to disorders typically associated with excess adiposity, adiponectin levels are elevated in classic chronic inflammatory/autoimmune diseases, i.e., rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, type 1 diabetes, and cystic fibrosis (253). In these patients, adiponectin levels correlate positively rather than negatively with inflammatory markers. In the MetS, however, plasma adiponectin levels are reduced (254, 255). Finally, of great interest at present is whether the primary proinflammatory defect in adipose tissue in the MetS is the large adipocyte, insulin resistance, the infiltration and activity of monoctye-derived macrophages, or the reduction in adiponectin synthesis and secretion.
2. Endoplasmic reticulum (ER) stress.
The ER is responsible for the folding of unfolded proteins delivered to its lumen. Under stress conditions (ER stress), however, unfolded proteins can accumulate in the ER lumen, activating the unfolded protein response. As a result, ER chaperone gene transcription is up-regulated to increase protein-folding capacity and reduce the ER stress (256). Despite this mechanism being in place, ER stress can increase the activity of the serine/threonine kinase c-Jun N-terminal kinase (154). An increase in the activity of c-Jun N-terminal kinase can lead to serine phosphorylation of IRS-1, down-regulating insulin signaling and possibly contributing to the development of insulin resistance (257). Stimuli that contribute to ER stress may therefore also be indirectly promoting the development of components of the MetS such as insulin resistance (258).
I. Animal models of the metabolic syndrome
Animal models can be helpful in further understanding the potential pathophysiology of the MetS. Murine models in particular have become quite useful tools in recent years because the entire mouse genome is now sequenced, and a large number of transgenic and knockout models are readily available. There are a number of limitations with these models, however, that must be considered. Rodent lipid physiology, for example, is significantly different compared with humans. Rodents carry most of their cholesterol in HDL, not LDL; thus, a low level of HDL-C is an unusual finding. Blood pressure is usually not measured in these models, again limiting the use of the “human” clinical definition of the MetS. Nevertheless, there remains much to be learned from animal models that may be applicable to mechanisms of the MetS in humans.
1. Mouse models.
Historically, there were a number of murine models that exhibited many of the components of the MetS, i.e., leptin-deficient ob/ob and leptin-resistant db/db mice (259, 260). More recently, when ob/ob mice were crossed with the LDL-receptor-deficient mouse, the features of the MetS including obesity, dyslipidemia, hypertension, insulin resistance, and IGT, and/or diabetes plus hypercholesterolemia resulted in more oxidative stress and atherosclerosis (261, 262).
A number of less-well known polygenic mouse models have a mixture of components of the MetS and its associated diseases. Some of these features are summarized in Table 7. It is worth noting that mice with different genetic backgrounds have a variable propensity to develop the MetS in response to changes in diet composition (263, 264). For instance, when C57Bl/6 (B6) and 129S6/SvEvTac (129) mice were placed on a low-fat or high-fat diet for 18 wk, the 129 strain developed features of the MetS, notably obesity, hyperinsulinemia, and glucose intolerance only on the high-fat diet, whereas the B6 strain developed these features on both diets (265).
TABLE 7.
Mouse models of the MetS
| Gene | KO/Tg | Tissue | Strain background | Sex | Age | Diet environment | Phenotypes (MetS-related) | Phenotypes (others) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obesity or WAT | Glucose tolerance/ insulin sensitivity | Lipids (TG/FFA) | Blood pressure | ||||||||||||
| ApoE | KO | Whole body | Apo100 and ob/ob (C57BL/6J) | M | 7–16 wk | Chow | BW ↑ | ↓ | ↑ | ↑ | Atherosclerosis | 269 | |||
| 11-β -HSD1 | Tg | WAT | FVB | M | 9–18 wk | Chow and HF | WAT ↑ | ↓ | ↑ | ↑ | Intra AT and portal corticosterone ↑ | 270 271 | |||
| LDLr | KO | Whole body | Apo100 and ob/ob (C57BL/6J) | M | 7–16 wk | Chow | BW ↑ | ↓ | ↑ | ↑ | Atherosclerosis | 269 | |||
| eNOS | KO | Whole body | C57BL/6J | M | 10–12 wk | Chow and HF | ↓ | ↑ | ↑ | Leptin ↑, uric acid ↑, fibrinogen ↑ | 272 | ||||
| 11-β -HSD1 | Tg | Liver | C57BL/6J | M | 24 wk | Chow and HF | ↓ | ↑ | ↑ | Fatty liver | 270 273 | ||||
| STAMP-2 | KO | Whole body | C57BL/6J | M | 2–5 months | Chow | WAT ↑ | ↓ | ↑ | Fatty liver, V-WAT inflammation | 274 | ||||
| PLSCR3 | KO | Whole body | C57BL6J and 129SvEvBRD | M | 2–6 months | Chow | WAT ↑ | ↓ | ↑ | Leptin ↑, adiponectin ↓ | 275 276 | ||||
| ATM | KO | Whole body | ApoE−/− (C57BL/6J) | M and F | Chow and HF | WAT ↑ on HF | ↓ (HF) | ↑ (HF) | apoB48 ↑, adiponectin ↓, atherosclerosis | 277 278 | |||||
| V1aR (vasopressin receptor 1a) | KO | Whole body | C57BL/6J and 129sv | M | 8–10 wk | Chow and HF | BW ↑ on HF | ↓ | ↓ | Blood volume ↓, hepatic insulin resistance, ketone bodies ↑ | 279 280 281 | ||||
| Lepr | KO | SF-1 neurons of VMH | C57BL/6J | M and F | 20 wk | HF and LF | BW ↑ on HF, WAT ↑ on LF | ↓ | ↑ | Hepatosteatosis, leptin ↑ | 282 | ||||
| PKC-λ | KO | Muscle | C57BL/6J and FVB | M and F | 5 months | Chow and LF | WAT ↑ | ↓ | ↑ | Hepatosteatosis, Islet b cell hyperplasia | 283 284 | ||||
| Aromatase (ArKO) | KO | Whole body | C57BL/6J | M and F | 10 wk to 3 months | Chow | BW ↑, WAT ↑ | ↓ | ↑ | Hepatic steatosis, adipocyte hyperplasia and hypertrophy | 285 286 287 | ||||
| FGFR4 | KO | Whole body | 129Sv-C57BL/6 | M and F | 4–6 months | Chow and HF | WAT ↑ | ↓ | ↑ | Fatty liver ↓ on HF | 288 | ||||
| NEIL1 | KO | Whole body | C57 BL/6J | M and F | 6–10 months | Chow | BW ↑ | ↓ | ↑ | Leptin ↑ fatty liver, kidney defect | 289 | ||||
| IL-6 | KO | Whole body | C57BL/6J | M and F | 3–9 months | Chow, exercise | BW ↑, WAT ↑ | ↓ | ↑ (FFA) | Leptin ↑, AMPK ↓ at rest, exercise effect ↓ | 227 290 291 | ||||
| PPARγ | KI | DN hetero | 129Sv/J | M and F | 3–11 months | Chow and HF | BW ↑ on HF | ↓ HF | ↑ | Reduced muscle glucose uptake on HF, WAT/BAT morphology change | 292 293 | ||||
| Mas | KO | Whole body | FVB/N | M | 9 wk | Chow | ↓ | ↑ | Leptin ↑ | 294 | |||||
| (Continued) | |||||||||||||||
TABLE 7A.
(Continued)
| Gene | KO/Tg | Tissue | Strain background | Sex | Age | Diet environment | Phenotypes (MetS-related) | Phenotypes (others) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obesity or WAT | Glucose tolerance/ insulin sensitivity | Lipids (TG/FFA) | Blood pressure | ||||||||||||
| LPL | Tg | Skeletal muscle | FVB/N | M | 4–6 months | Chow and HF | ↓ | ↑ | ND | Fiber-type switching in skeletal muscle, cold-induced thermogenesis ↑ | 295 296 297 | ||||
| Wrn | KO | Whole body | C57BL/6J | M | 6–10 months | HFS | BW ↑ | ↓ | Fatty liver, pancreatic islet hyperplasia | 298 | |||||
| 4E-BP1/4E-BP2 | KO | Whole body | BALB/c | M | 24 wk | Chow and HF | BW ↑, WAT ↑ | ↓ HF | Adipocyte size ↑, liver TG ↑, leptin ↑ | 299 | |||||
| MC4-R | KO | Whole body | C57BL/6J | M and F | 3–24 wk | Chow and HF | WAT ↑ | ↓ | Fatty liver, leptin ↑, food intake ↑ | 300 301 302 303 | |||||
| Pomc | KO | Neuron | C57BL/6J 129 × 1;129S6 | M and F | 6–30 wk | Chow | BW ↑ | ↓ | Defect in adrenal function | 304 | |||||
| Pomc | KO/Tg (tg in pituitary) | Neuron | C57BL/6J DBA/2, 129 × 1;129S6 | M and F | 6–30 wk | Chow | BW ↑, WAT ↑ | ↓ | Leptin ↑, food intake ↑, oxygen consumption ↑ | 305 | |||||
| NPY | Tg | Noradrenergic neurons | C57BL/6J | M and F | 3–6 months | Chow | WAT ↑, BW- | ↓ | Ghrelin ↑, no hyperphagia, liver TG ↑ | 306 | |||||
| ALMS1 | KO | Whole body | NOD and C57BL/6J | M and F | 4 months | Chow and HF | BW ↑ | ↓ | Islet hyperplasia, islet cysts, male sterile, Alstrom syndrome, steatohepatitis on HF, diabetes | 307 308 309 | |||||
| ERα | KO (RNAi) | VMH | C57BL/6J or Swiss–Webster mice | F | 12–30 wk | Phytoestrogen-free diet | BW ↑, WAT ↑ | ↓ | Hyperphagia, energy expenditure ↓ | 310 | |||||
| FABP5 (mall) | Tg | WAT | C57BL/6J | M | 24 wk | HF | ↓ | 311 | |||||||
| KK/Ta | M | 10–22 wk | HF | V-WAT ↑ | ↓ | Fatty liver, adiponectin ↓, TNF-α ↑ | 312 | ||||||||
| BALB/c | F | 11–13 wk | Repeated stress | ↓ | ↑ | Lean body mass ↓, leptin ↑, acidosis ↑, cortisol ↑, thyroid ↓, aa turnover ↓ | 313 | ||||||||
| NZBWF1 | F | 36 wk | Chow | BW ↑, V-WAT ↑ | ↓ | SLE, leptin ↑, macrophage infiltration in WAT | 314 | ||||||||
| NZO | M | 6 wk | Chow | BW ↑ | ↓ | ↑ | 315 | ||||||||
| SMXA5, (SM/J&A/J) | M | 20 wk | Chow and HF | BW ↑ | ↓ | ↑ | Enlarged islets | 316 317 318 | |||||||
| A(vy) Agouti viable yellow (B6) | M | 2–8 months | Chow | BW ↑, WAT ↑ | Leptin ↑, Agouti-colored coat | 319 | |||||||||
KO, Knockout; Tg, transgenic; HF, high fat; LF, low fat; BW, body weight; WAT, white adipose tissue; TG, triglycerides; ND, not determined; AMPK, AMP kinase; SLE, systemic lupus erythematosus; RNAi, RNA interference; SF-1, steroidogenic factor 1; HFS, high-fat and high-sugar diet; VMH, ventromedial hypothalamus; M, male; F, female; AT, adipose tissue; aa, amino acid; V-WAT, visceral white adipose tissue; DN hetero, heterozygous dominant negative; KI, knock-in.
For a number of years, the Jackson Laboratory has carried out a comprehensive assessment of genetic susceptibility to the MetS in inbred mice when challenged with a high-fat, high-cholesterol diet (266). A high-throughput protocol was set up to evaluate female and male mice from 43 inbred strains for 10 traits including all the major criteria of MetS while mice consumed the diet for 18 wk. A few strains of mice developed a phenotype with a plethora of metabolic abnormalities remarkably similar to the human MetS (strains CAST/EiJ, CBA/J, and MSM/Ms). Other strains had a more limited phenotype, i.e., severe obesity (AKR/J and KK/HIJ) vs. protection from obesity (WSB/EiJ); severe dyslipidemia (MOLF/EiJ) vs. no dyslipidemia (CZECHII/EiJ for males and D2 for females); and severe insulin resistance (KK/HIJ) vs. being spared from insulin resistance (A/J). Overall, the discrepant phenotypes within the same environmental exposures may prove useful in dissecting the genetic and related molecular mechanisms underlying the MetS and its components (267).
Many other murine models of the MetS have resulted from modifications of single genes. Although some of these models have been summarized in a previous review on the MetS (268), it is our intent in this review to provide an extensive list of the murine models of the MetS and characterize each of the models over the period of phenotypic development. The mouse models listed in Table 7 are characterized by the number of MetS components they demonstrate: glucose intolerance/insulin resistance; abnormalities in lipids (increased triglycerides and/or FFA); and increased blood pressure. Models with all three components are listed in Table 7 first (269, 270, 271, 272, 273), followed by those with two (227, 274, 275, 276, 277, 278, 279, 280, 281, 282, 283, 284, 285, 286, 287, 288, 289, 290, 291, 292, 293, 294, 295, 296, 297), and finally those with only insulin resistance (298, 299, 300, 301, 302, 303, 304, 305, 306, 307, 308, 309, 310, 311). The final mouse grouping is animals with a polygenic background that meet the criteria for the MetS as stated (312, 313, 314, 315, 316, 317, 318, 319). Details for each of the models including murine strain, gender, age, environmental exposure including diet, and components of the MetS phenotype plus related structural and/or functional abnormalities when present are all provided.
2. Rat models.
A number of models of the MetS have been identified in rats. The Zucker fatty rat was among the first identified (320). Subsequently, a number of studies have been published to examine the impact of diet on the phenotypic development of the MetS (321, 322, 323, 324, 325, 326). Wistar Ottawa Karlsburg W rats (WOKW) develop all components of the MetS. Genetic analysis of this rat model has identified potential major quantitative trait loci (QTL) for glucose metabolism on chromosome 3, dyslipidemia on chromosomes 4 and 17, and obesity on chromosomes 1 and 5 (327). Moreover, the severe insulin resistance predominant in epididymal adipose tissue of these rats was associated with a 10-fold decrease in adipocyte adiponectin gene expression and decreased peroxisome proliferator-activated receptor (PPAR)-λ gene expression, but increased FOX01 gene expression compared with control rats (328). Moreover, the MetS in WOKW rats was associated with impaired coronary vasodilatation due to altered adrenoceptor sensitivity (329).
Another example of the MetS in rats is the corpulent (JCR:LA-cp) rat that like db/db in mice is a homozygous mutation in the leptin receptor (330). These rats are obese, insulin resistant, and hypertriglyceridemic. JCR:LA-cp rats, however, are prone to atherosclerosis (331, 332, 333) and also appear to be a good model to study the contribution of postprandial lipemia to the atherosclerotic process. In these rats, lymphatic chylomicron apoB48, chylomicron size, fasting and postprandial plasma apo B48 area under the curve are all elevated (334).
The Prague hereditary hypertriglyceridemic (hHTG) rat was developed as a model of hypertriglyceridemia. Although these rats are not obese, they are hypertensive, insulin resistant, and glucose intolerant (335, 336). Using F2 hybrids, several QTL have been identified for hypertension and hypertriglyceridemia (337). Another model of the MetS in rats that includes hypertension is the Lyon hypertensive rat (LH). These rats also have obesity, dyslipidemia, and an increased insulin/glucose ratio. This rat strain has been used to identify linkage of body weight, blood pressure, and renal, metabolic, and endocrine phenotypes (338). This is a renin-dependent model of hypertension in which low-dose (nonantihypertensive) angiotensin-converting-enzyme inhibitor therapy affords significant and durable renal protection. A total genome scan in the offspring of an F2 intercross between the hypertensive and normotensive Lyon strains has identified a series of QTL for the MetS, body weight, blood pressure, lipid metabolism, and renal function (339, 340). Other hypertensive rat models of the MetS include SHR/NDmc-cp(cp/cp) (341) and SHROB (spontaneously hypertensive, obese rat) (342).
3. Other animal models.
Of interest to pet owners and veterinarians alike is the fact that obesity in dogs and cats has increased in recent years (343), and dogs in particular are models of the MetS. The canine obesity model closely recapitulates the relationship between human visceral adiposity and insulin resistance. The work of Bergman et al. (138) supports the portal theory of insulin resistance, in which FFA from visceral adipose tissue directly enter the liver and unfavorably modify insulin action. Sympathetic nervous system hyperactivity in this model of obesity may also contribute to excessive FFA release, hypertension, and insulin resistance. As noted previously, a nocturnal increase in plasma FFA levels may account for both insulin resistance and compensatory hyperinsulinemia.
Obesity is common in cats and is a risk factor for diabetes. The prevalence of diabetes has increased concomitantly with the increase in obesity, and diabetes is now seen in approximately 0.5–1% of cats (344). Cats develop a form of diabetes that is similar to T2D in humans, characterized by islet amyloid accumulation and loss of β-cell mass (345). From more recent studies in felines, it appears that glucose metabolism in cats is similar to that in humans; however, lipid metabolism is quite different (346). This may explain why the MetS in cats is less frequent than in dogs and is not frequently studied.
4. Primate models.
Historically, nonhuman primates (NHPs) have been used for a variety of studies on diabetes mellitus. Spontaneous, natural forms of diabetes have been well documented in several species, and diabetes has also been induced in NHPs with drugs and diets. Hyperglycemia and impaired glucose clearance are also commonly associated with hyperlipidemia in primates (347).
Old World NHPs first develop obesity and then insulin resistance. Like humans, when either a relative or absolute deficiency in insulin production occurs, fasting glucose concentrations increase, and diabetes follows. NHPs with diabetes have detrimental changes in plasma lipid and lipoprotein metabolism and concentrations, lipoprotein composition, and glycation of proteins, all of which likely contribute to an accelerated rate of atherosclerosis development. The prevalence and heritability of obesity and risk factors associated with the MetS in a pedigreed colony of Vervet monkeys has been reported recently (348). Vervet monkeys demonstrated obesity, insulin resistance, and associated changes in plasma lipids even while consuming a low-fat (chow) diet. Female monkeys were at a higher risk for central obesity and dyslipidemia.
Rhesus monkeys have also been used to study metabolic diseases. During healthy aging, rhesus monkeys demonstrate decreases in insulin sensitivity and reductions in HDL-C. In monkeys that develop diabetes, significant decreases in glucose tolerance were evident by middle age (age, ∼14 yr), with elevations in fasting insulin and then a progressive decline in insulin secretion with aging (349). With aging, rhesus monkeys with diabetes also demonstrated dyslipidemia and shifts in lipoprotein particle size and number as measured by nuclear magnetic resonance (350). In humans, Bjorntorp and Rosmond (351) were among the first to suggest that the MetS was caused by stress. In a model of early-life stress using variable foraging demand, food insecurity was imposed on NHP mothers for 16 wk, initiated when their nursing offspring were 3–5 months of age. Although variable foraging demand does not restrict food availability or the infant’s growth, this modification in nutrient access during rearing, however, did result in a range of neurobiological abnormalities in the offspring including greater weight gain, BMI, abdominal circumference, and glucagon-like peptide-1, and decreased glucose disposal rates during a hyperinsulinemic-euglycemic clamp (352).
J. Genetic determinants of the metabolic syndrome in humans
Increasing evidence suggests that there is genetic basis for the MetS. As with most complex traits, however, the associations are quite weak, and the replication of findings has been poor. Some recently published reviews have summarized the results from multiple genome-wide scans involving different cohorts (353, 354, 355). From these studies, familial aggregation is most evident for the individual components of the MetS; however, some studies suggest that specific genes, such as those that encode for 11β-hydroxysteroid dehydrogenase, adiponectin, and the β3-adrenergic receptor, may also predispose to the development of the MetS (356, 357, 358). Furthermore, it has been suggested that the risk of the MetS may be modified by dietary fatty acid composition (359).
Of the five subphenotypes defining MetS, all are known to have strong genetic components (typically 50–80% of population variation). For example, in a study among a population of 163 individuals from Yucatan, Mexico, which has a high prevalence of obesity, T2D, and dyslipidemia, a polymorphism in the insulin gene was associated with the presence of at least one abnormality related to the MetS (360).
A genome-wide scan for glucose homeostasis in subjects without diabetes has been carried out as part of the Insulin Resistance Atherosclerosis Study (IRAS) family study (361). Significant evidence for linkage of insulin sensitivity, disposition index, and acute insulin response to glucose to different regions on chromosome 11 and 12 was observed. These results provide impetus for future positional cloning of QTL to identify the genetic determinants of the MetS.
Another study investigated the heritability of determinants of the MetS among healthy Arabs of the Oman Family Study (362). Results from this study indicated that weight, BMI, and HDL-C level were under significant genetic influence, whereas other determinants such as insulin resistance, abdominal obesity, diastolic blood pressure, and triglyceride levels seemed to be more environmentally driven.
The prevalence rates of T2D and CHD in the Ontario Oji-Cree tribe are among the highest in the world. Studying 515 adult and 115 adolescent Oji-Cree subjects revealed that increased WC and depressed HDL-C were the most prevalent MetS components, whereas increased blood pressure was the least prevalent (363). Furthermore, different functional polymorphisms in candidate genes were found to associate with the MetS in adults (AGT T174M, GNB3 825C>T, APOC3-455T>C) and more so than in adolescents (FABP2 A54T). In a separate multiethnic study, the APOC3-455T>C promoter polymorphism was also found to be associated with an approximately 2-fold increased risk of the MetS (364).
Studies defining genetic predispositions have typically focused on older populations with MetS. A recent study in younger populations (365) found a much stronger association of the PPAR-α L162V locus and triglycerides in males than previously reported in older and less healthy populations. Specifically, the V allele increased triglycerides by 78% (P = 0.004), and this single polymorphism accounted for 3.8% of all variation in serum triglycerides (P = 0.0037).
The Kiel Obesity Prevention Study (KOPS) examined the common genetic background that contributes to the clustering between insulin resistance, central obesity, and other MetS traits. Their findings suggested that a common genetic background contributed to the clustering of different MetS components and central obesity or insulin resistance. Common genetic influences favor central obesity as a major characteristic linking other traits (366).
A most recent report focused on the endothelial nitric oxide synthase (eNOS) gene and its role in MetS. Previous studies suggested that endothelium-derived nitric oxide facilitates skeletal muscle glucose uptake, and eNOS null mice present with many phenotypes of the MetS including insulin resistance, hypertension, and hypertriglyceridemia (367). By using haplotype tagging single nucleotide polymorphism analysis, it was suggested that genetic variation at the eNOS locus was associated with features of MetS (368). Similar single nucleotide polymorphism analysis in different populations also revealed lipid (LPIN1) gene variants (369) and polymorphism at the IL6ST (gp130) locus (370) to be associated with the MetS.
V. Risks of Metabolic Syndrome
A. Cardiovascular disease
One of the primary observations regarding the clustering of metabolic disorders was the association of these features with increased CVD risk. It is well accepted and established that multiple risk factors confer greater risk than a single risk factor. In fact, the findings that led to the development of the Framingham Risk Score (FRS) are based on this observation. The NCEP:ATPIII emphasized that the risk for CVD can be further reduced by the modification of risk factors beyond LDL lowering (1). Thus the MetS was identified as a clustering of factors that further increase the risk for CVD.
The vast majority of studies have found that patients with the MetS have more CVD and are at increased risk for developing CVD (29, 371, 372, 373, 374, 375, 376, 377, 378, 379, 380, 381, 382, 383, 384, 385, 386). A recent meta-analysis by Gami et al. (387) that included 36 different reports found that the overall relative risk for incident CVD events and death for individuals with the MetS was 1.78 (95% CI, 1.58–2.00). Despite the power of the large sample size of this meta-analysis, there are some concerns of confounding due to the inclusion criteria of the selected studies. A frequently quoted study examining this issue is from the Kuopio Ischemic Heart Disease Risk Factor Study. Finnish men without CVD were followed for approximately 11 yr, and those with the MetS were three to four times more likely to die of CHD, 2.6 to three times more likely to die of CVD, and two times more likely to die from all causes (371). In another report, U.S. adults without prior CVD from the NHANES were followed for approximately 13 yr. For those with the MetS, the risk factor-adjusted proportional-hazards regression for CHD mortality was doubled (375). Using the Framingham database, the age-adjusted relative risks for CVD and CHD in men with the MetS were 2.88 and 2.54, respectively, with those in women being slightly lower (2.25 and 1.54, respectively) (388). Scandinavian men and women with the MetS from the Botnia Study had a 3-fold increase in risk for CHD and stroke (372), whereas the age-adjusted risk of fatal CVD in men and nonfatal CVD in women was increased 2-fold in Dutch adults in the Hoorn Study (382). The Atherosclerosis Risk in Communities (ARIC) study, which included more minorities, also found a relative risk of CHD of 1.5 and 2 in men and women with the MetS, respectively (379). The presence of the MetS in patients with preexisting CHD is also associated with an increased risk for CVD events and mortality (375, 389). In fact, the MetS is associated with greater risk of CVD in patients with preexisting CHD compared with those without known CHD (RR, 2.68 vs. 1.94) (387). Obese individuals and those with preexisting diabetes also have a doubling of CVD risk when the MetS is present (377, 390). McNeil et al. (391) found that older individuals (mean age, 72 yr) with the MetS were 20–30% more likely to experience a CVD event than those without. Finally, as one would expect, the more components or features of the MetS that are present, the greater the CVD risk (373, 384, 392).
There are just a few exceptions to these findings. The Casale Monferrato Study, a study in an Italian cohort of older individuals with T2D, found that the MetS did not predict CVD above and beyond the risks attributed to T2D (393). The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER), another study of older individuals aged 70–82 yr, also failed to show an association between the MetS and increased risk of CVD (394). WC, however, was not measured in PROSPER, and HDL-C and diastolic blood pressure (modestly) were the only components that predicted incident CVD; neither BMI, nor systolic blood pressure, LDL-C, or triglycerides were associated with CVD risk. Because the prevalence of MetS plateaus in the 60s, this may not be a study that resolves the MetS/CVD outcome controversy. A study in a cohort of nondiabetic American Indians, The Strong Heart Study, also found no association between either insulin sensitivity or MetS and incident CVD (395). Event rates were quite low overall in this cohort, as has been shown in other studies of American Indians, potentially impacting the power to detect differences. Finally, in a study of individuals with known stable CHD, the MetS was associated with increased total mortality and CVD mortality in women but did not appear to be associated with excess risk of CVD mortality in men (396). Again, WC was not measured in this study, which may have attenuated the relationship especially in men. The mean BMI of those with the MetS was actually lower (27 kg/m2) than the inclusion criteria (>30 kg/m2) described in the methods, suggesting further methodological problems with the identification of those with or without the MetS. Overall, though, it appears that there is greater evidence supporting the association between the MetS and CVD risk, even in very high-risk populations.
An important question that arises regarding the association between the MetS and CVD is whether the CVD risk in the MetS is greater than the sum of the risk of the individual risk factors. This question has been reviewed and debated elsewhere (14, 397). The Framingham experience has certainly long suggested that multiple risk factors increase CVD risk more than the sum of the individual risk factors (398). A recent meta-analysis found that the risk for CVD is still increased in people with the MetS, even after controlling for the component risk factors (RR, 1.54; 95% CI, 1.32–1.79) (387). Conversely, there are several studies that suggest that the risk of the MetS is not greater than the sum of its parts (373, 379, 382, 399). Another important issue is whether the MetS offers greater prediction of CVD risk than previously established risk assessments such as the FRS (44). Analysis of the ARIC study suggested that the MetS did not improve CHD risk prediction beyond that predicted by the FRS (379). Wannamethee et al. (383) also found the FRS to be a better predictor of CHD and stroke than the MetS. On the other hand, post hoc analysis of the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) showed that individuals with the MetS had increased risk for major coronary events irrespective of their FRS (374).
The ability of the MetS to predict the incidence of CVD may differ according to how the MetS is defined. The 10-yr CVD risk in the Hoorn Study was assessed using different definitions of the MetS. The NCEP:ATPIII definition was associated with a 2-fold increase in CVD, whereas the risk was slightly less using the WHO, EGIR, and AACE definitions (382). The NCEP:ATPIII definition was also associated with a 2-fold increased risk for CVD compared with the IDF definition when several American and European cohorts were examined (400). The meta-analysis by Gami et al. (387) found the WHO definition to be associated with slightly greater risk than the NCEP:ATPIII definition (2.06 vs. 1.67). On the other hand, the NCEP, WHO, and IDF MetS definitions all showed similar CVD risk in follow-up of the San Antonio Heart Study (25).
B. Type 2 diabetes mellitus
The prevalence of T2D has tripled in the last 30 yr (401). Currently, diabetes afflicts more than 20 million people in the United States (402). T2D is a complex disease caused by both environmental and genetic factors. It is marked by chronically elevated blood glucose concentrations, which result from defects in insulin production, insulin action, or a combination of both. Although insulin resistance is considered the hallmark of prediabetes, defects in insulin secretion are regarded as the key pathophysiological characteristic of T2D. Although T2D is a heterogeneous disease, most patients with T2D have insulin resistance and the MetS before onset of T2D (403). In fact, insulin resistance, hyperinsulinemia, dyslipidemia, and obesity precede the progression to T2D in 75 to 85% of patients (404).
Numerous studies have examined the ability of the MetS to predict T2D. The presence of the MetS increases the risk (115, 405) and is highly predictive of new-onset T2D (394, 406, 407). The risk for incident T2D is up to five times higher in individuals with the MetS compared with those without the syndrome (397, 408). Interestingly, the presence of both the MetS and insulin resistance has an additive effect because these patients exhibit a 6- to 7-fold increased risk for T2D (385).
The ability of the MetS to predict the incidence of T2D differs according to how the MetS is defined (408). The NCEP:ATPIII and IDF definitions consider elevated fasting plasma glucose as an essential, but not required, criterion for defining the presence of MetS. The WHO definition, however, requires the presence of IFG and/or IGT. The effect of varying definitions of the MetS on the risk for T2D may be significant because the risk for T2D conferred by either IFG or IGT is higher than that conferred by other individual components of the syndrome (409). Furthermore, IFG and IGT have been shown to predict the development of diabetes, independent of other components of the MetS (268). The Hoorn study found that in patients without the MetS, 33% of those with IFG only and 64.5% of those with the combination of IFG and IGT developed diabetes over a 5.8–6.5 yr follow-up (410). Additionally, Hanson et al. (407) found that hyperinsulinemia was the strongest predictor of diabetes incidence. This led many investigators to question whether the syndrome’s ability to predict diabetes is due to a single factor (i.e., insulin resistance) or whether it represents an additive effect of multiple metabolic abnormalities.
A number of major studies have published data on the effectiveness of the MetS to predict the incidence of diabetes. The Insulin Resistance Atherosclerosis Study found that IDF and NCEP:ATPIII MetS definitions predicted incidence of diabetes as well as the WHO definition, despite the first two not requiring the use of an oral glucose tolerance test or a measure of insulin resistance (411). Laaksonen et al. (406) compared the WHO and the NCEP:ATPIII definitions in a cohort of Finnish men and found that the WHO definition was the most sensitive of the definitions because it detected over four fifths of prevalent and two thirds of incident cases of diabetes, all with good specificity (0.78–0.80). Subjects in the Framingham Offspring Study demonstrated that the presence of the MetS (as defined by NCEP:ATPIII criteria) accounted for approximately half of the new diabetes cases over an 8-yr follow-up period (388). The San Antonio Heart Study evaluated NCEP:ATPIII and a modified-WHO (without an oral glucose tolerance test) definition and found that the presence of IGT alone and the NCEP definition had similar and greater sensitivities for identifying patients at risk for T2D than the modified WHO definition (52, 53, and 43%, respectively. Combining IGT with the NCEP and modified WHO definitions increased the sensitivity 71 and 66%, respectively (412).
The ability of the MetS to predict diabetes risk has also been compared with the Diabetes Predicting Model and the FRS. Stern et al. (413) found that, in the San Antonio Heart Study population, the MetS was inferior to the Diabetes Predicting Model for determining incidence of diabetes. On the other hand, the FRS, which was developed to predict the risk of CHD, was inferior to the MetS for predicting incidence of diabetes in men from the British Regional Health Study (383).
Finally, the presence of the MetS in women with gestational diabetes mellitus (GDM) substantially increases the risk of developing T2D. GDM alone significantly increases a woman’s risk for subsequently developing T2D (414, 415). The conversion of GDM into T2D varies between 6 and 92%, depending on diagnostic criteria, racial/ethnic background of the subject sample, and duration of surveillance (416). The presence of the MetS further increases the progression from GDM into T2D. In a 9.8-yr follow-up study, 481 women with prior GDM who were treated with diet alone were compared with 1000 age-matched control women. The prevalence of the MetS was three times higher in the prior GDM group when compared with controls, and this persisted after adjustment for age and BMI. As much as 67% of the GDM women were glucose intolerant vs. only 19% in the control group, and the prevalence of the MetS was double in the prior GDM group vs. the control group (417). These findings are in line with a similar study by Verma et al. (418), in which the prevalence of the MetS was three times higher in GDM women vs. controls after an 11-yr follow-up.
VI. Associated Conditions
There are a number of conditions associated with the MetS that deserve brief attention here. Some of these conditions are directly associated with the underlying excess adiposity and insulin resistance associated with the MetS.
A. Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) includes a range of pathological features from mild steatosis to NASH to cirrhosis. A presumptive diagnosis of NAFLD can be made in patients with elevated liver enzymes and/or fatty liver by imaging in the absence of other causes of liver disease, although a definitive diagnosis can only be made by liver biopsy (419). The prevalence of NAFLD ranges from 3 to 36% of the general population, depending on how it is defined (420, 421). It has been suggested that 95% of obese individuals and up to 70% of those with T2D have some form of NAFLD (420). The prevalence of NAFLD is also increased in children with obesity and insulin resistance (422). In addition, the presence of NAFLD is a strong predictor of the MetS (421), and liver fat correlates to all of the components of the MetS (423). In those patients with the MetS, liver fat content is significantly increased up to 4-fold higher than those without the MetS (423), and the incidence of NAFLD has shown to be increased 4-fold in men and 11-fold in women with the MetS (424).
Fat deposition in the liver has been shown to be primarily due to an increased influx of fatty acids to the liver, most likely as a result of the increased lipolysis associated with obesity and insulin resistance and as a result of increased hepatic de novo lipogenesis (425). Reduced fatty acid oxidation and mitochondrial dysfunction and decreased export of fat further contribute to the accumulation of liver fat (420, 426). In addition, a number of transcription factors and adipokines appear to play an important role in the development of NAFLD (427). For example, in certain models of obesity, sterol regulatory element-binding protein 1c is up-regulated, potentially resulting in increased conversion of glucose to fatty acids and triglycerides (426). PPAR-α, a nuclear receptor important in fatty acid uptake and oxidation, has been shown to be underexpressed in animal models of NAFLD (427). Adiponectin concentrations are reduced in individuals with NAFLD, and the administration of adiponectin reverses NAFLD in experimental models (428). Although insulin resistance is associated with fat accumulation in the liver, fat accumulation in the liver appears to result in hepatic insulin resistance, suggesting a “dynamic” process (426). Furthermore, it has been shown that pure hepatic insulin resistance, as demonstrated in the liver insulin receptor knockout mouse model, is sufficient “to produce the dyslipidemia and increased risk of atherosclerosis associated with the MetS” (429). NAFLD, which may be a result of insulin resistance but may also be a cause of insulin resistance, may then be central to the pathophysiology of the MetS. Finally, it is less clear why some individuals with NAFLD progress to NASH and potentially to cirrhosis. Genetic factors are likely important, as are the roles of cytokines such as TNF-α and oxidative stress (420, 426).
B. Polycystic ovarian syndrome
Polycystic ovarian syndrome (PCOS) is a clinical syndrome that is associated with anovulation, androgen excess, and insulin resistance. Not only do women with PCOS suffer from problems with fertility and clinical stigmata of androgen excess, but they also suffer from consequences of insulin resistance, such as a significant risk for the development of T2D (430) and CVD risk factors (431). There is, therefore, significant overlap between PCOS and the MetS. There have been debates on whether PCOS may in fact be in the continuum with the MetS. The MetS is common in women, especially obese women, with PCOS (432). The prevalence of PCOS is also rising, with rates reported as high as 28% in overweight/obese women (433). The pathophysiology of the PCOS, like the MetS, is also unclear and highly debated. The ovary, hypothalamic-pituitary axis, and insulin resistance all are thought to have a role in this condition (434). The insulin resistance and obesity that are frequently found in women with PCOS have been implicated in significant risk for CVD and metabolic disorders. Greater than two thirds of women with PCOS have some degree of glucose intolerance and are therefore at high risk for developing diabetes if they do not already have it (430, 435). Women with PCOS clearly have a higher prevalence of CVD risk factors (436, 437). Although it is not clear whether women with PCOS have a greater risk for CVD events, they certainly have evidence of greater risk for subclinical CVD (436, 438).
C. Obstructive sleep apnea
Obstructive sleep apnea (OSA) is a potentially serious consequence of obesity and is associated with increasing BMI. There is also an association between insulin resistance and OSA (439, 440). OSA has also been shown to be associated with increased inflammation (441) and reduced adiponectin concentrations (442, 443). Individuals with OSA are more likely to have the features of the MetS than those without OSA, even when adjusted for obesity (444, 445, 446). In addition, disordered sleep in general is associated with weight gain and insulin resistance (439, 447, 448, 449, 450). Some have even suggested that OSA should be considered as a manifestation of the MetS (448).
D. Hypogonadism
As women with PCOS are at greater risk for the MetS, there is also a relationship between the MetS and male gonadal and erectile dysfunction. Men with the MetS appear to have a greater prevalence of hypogonadism (451, 452). Conversely, hypogonadism is a risk factor for the development of the MetS and T2D (453). In addition, features of the MetS improve with testosterone replacement (454). The MetS has also been shown to be independently associated with a greater prevalence of erectile dysfunction (455, 456, 457, 458).
The prevalence of MetS increases in women after the menopause, but whether this is the result of aging per se or the result of changing hormonal milieu is unclear. Furthermore, it is unclear whether the menopause-related increases in insulin resistance and dyslipidemia are the result of estrogen deficiency directly or occur secondary to increases in abdominal adiposity (459). Indeed, increases in abdominal adiposity may precede changes in insulin action and dyslipidemia, because visceral fat begins to increase during the menopausal transition (460). Nevertheless, estrogen replacement in postmenopausal women has been shown to improve many components of the MetS, such as abdominal adiposity, HDL-C, and fasting glucose (461, 462, 463, 464) and to lower the incidence of T2D (462, 465, 466). Thus, declines in sex steroids likely contribute to the increased prevalence of the MetS in women after menopause.
E. Lipodystrophy
Lipodystrophies are inherited or acquired disorders characterized by the loss of selective adipose tissue depots. The pathogenesis of the lipodystrophies is complex and has been recently reviewed elsewhere (467, 468). These patients, especially those with partial and generalized forms of lipodystrophy, generally have severe insulin resistance and often share the features of the MetS, putting these individuals at risk for T2M, dyslipidemia, NAFLD, and CVD. Highly active antiviral therapy for the treatment of HIV-infected patients has been associated with the development of severe metabolic disturbances and “acquired” lipodystrophy (469, 470). These metabolic disturbances include hypertriglyceridemia, low HDL-C, and insulin resistance reminiscent of the MetS. The prevalence for the MetS in these patients, however, is actually lower than in the general population due in part to the low WC in this population (469).
F. Microvascular disease
Although the MetS is clearly associated with CVD, it is less clear whether patients with the MetS are at greater risk for microvascular disease independent of diabetes. Approximately 8–10% of individuals with IFG and IGT but without diabetes, most of whom have the MetS, have retinopathy (471, 472). The MetS has also been shown to be associated with an increased risk of chronic kidney disease (473, 474, 475) and microalbuminuria (476, 477, 478). Moreover, the MetS has been found to be associated with increased risk for neuropathy (479). For those individuals with diabetes, the MetS appears to be associated with increased risk for all types of microvascular disease (480, 481), although a recent post hoc analysis of the United Kingdom Perspective Diabetes Study did not find the MetS to be associated with a greater risk for microvascular disease in individuals with T2D (482).
VII. Therapeutics
A discussion on the therapeutic options for managing the MetS must be prefaced with the understanding that there are no randomized controlled trials published to help guide specific recommendations for managing the MetS. In addition, because it is unclear whether there is a unifying pathophysiological mechanism resulting in the MetS, it is unclear whether the MetS can be treated in and of itself. The following discussion will therefore center on treating the individual components of the MetS, with the overall goals of reducing the risk for or preventing CVD and T2D as outlined in Table 8. Nevertheless, concentrating therapeutic efforts on treating the excess adiposity and insulin resistance associated with the MetS may provide the most overall success in attaining these goals. In addition, certain therapeutic options may impact more than one component of the MetS. The following discussion will be divided into lifestyle modification, pharmaceutical therapy, and surgery.
TABLE 8.
Therapy of MetS risk factors
| Therapeutic target | Goals and recommendations |
|---|---|
| Abdominal obesity | 5–10% Weight loss or weight maintenance |
| Lifestyle modification with diet and increased physical activity | |
| Pharmacological weight loss therapy | |
| Bariatric surgery | |
| Insulin resistance/hyperglycemia | Prevention or delay of progression to type 2 diabetes |
| Lifestyle modification and weight loss as described above | |
| Pharmacotherapy | |
| Treatment of diabetes | |
| Appropriate glycemic control | |
| Metabolic dyslipidemia | |
| Primary target: LDL-C | LDL-C lowering as per NCEP:ATPIII goals (see Table 9) |
| Secondary target: non-HDL-C | If TG ≥200 mg/dl, lower non-HDL-C to <30 mg/dl plus the LDL-C goal |
| Tertiary target: HDL-C | If HDL-C <40 mg/dl in men or <50 mg/dl in women, consider therapy for HDL-C raising |
| Elevated blood pressure | Goal BP is <140/90 mm Hg (<130/80 mm Hg if diabetes or CKD present) |
| Prothrombotic state | Consider low-dose aspirin for high-risk patients |
| Proinflammatory state | No specific goals; treat all of the above risk factors |
TG, Triglycerides; BP, blood pressure.
A. Lifestyle modification
1. Diet.
It is well established that weight loss is beneficial for treating all of the components of the MetS, including excessive adiposity, dyslipidemia, hypertension, insulin resistance, and hyperglycemia (483). The magnitude of weight loss need not be drastic; the Finnish Diabetes Prevention Study showed that lifestyle intervention with modest weight loss significantly reduced the prevalence of the MetS (OR, 0.62; 95% CI, 0.40–0.95) compared with the control group (484). A 41% reduction in the incidence of the MetS was also seen with the intensive lifestyle intervention of the DPP (485). In addition, a weight loss as small as 5–10% of body weight can significantly reduce triglycerides and increase HDL-C (486). Furthermore, both hypertensive individuals and individuals at risk for developing hypertension can see a significant reduction in blood pressure with a modest weight loss (487, 488, 489). Fasting blood glucose, insulin, and hemoglobin A1c can also be decreased with modest weight loss (490); interestingly, a 7-d negative energy balance without measurable weight loss has also been shown to improve insulin sensitivity (491). Notably, the DPP demonstrated that weight loss was the number 1 predictor of reduction in the incidence of diabetes (492). In fact, for every kilogram of weight loss, the risk of diabetes development was decreased by 16%. A decrease in caloric intake is an avenue by which to promote a chronic negative energy balance resulting in weight loss. Although the macronutrient classification of the eliminated calories is of lesser importance when addressing overall energy balance, the type of macronutrients habitually consumed can influence the health of the individual with MetS.
a. Carbohydrate.
Currently, the United States Department of Agriculture (USDA) and the Institute of Medicine (IOM) recommend a carbohydrate intake of 45–65% of total caloric intake (USDA, 2005). This recommendation is appropriate for most populations because total carbohydrate consumption has not been shown to be associated with the development of T2D or the MetS (493, 494, 495). Due in part to the recent rise in the popularity of low-carbohydrate diets, there has been interest in the effect of carbohydrate intake on serum lipid levels. Investigations into this question have consistently reported that carbohydrate intake is positively associated with total cholesterol, LDL-C, and triglycerides and negatively associated with HDL-C (496, 497). In addition, lower carbohydrate diets have been associated with improved carbohydrate metabolism in those with insulin resistance and/or T2D (498). Although weight loss has been shown to be greater with lower carbohydrate diets in the short term (499, 500, 501), the effects on long-term weight loss have been mixed (502, 503, 504, 505).
Dietary carbohydrate can be placed into two categories: simple and complex. It is the latter that should comprise the bulk of the carbohydrate intake, whereas simple carbohydrates, especially in the form of added sugars, should be limited (USDA, 2005). Common sources of added sugars in the diet include soft drinks, cakes, cookies, pies, fruit drinks, dairy desserts, and candy (506). Although added sugars are chemically identical to naturally occurring simple sugars (e.g., sugars found in fruit), concern is warranted regarding the lack of nutrients found in foods laden with added sugars. It has been shown that individuals who consume a greater percentage of calories as added sugars consume significantly less vitamins and minerals (507).
The glycemic index has received considerable attention in terms of classifying which carbohydrates are “good” or “bad” for disease risk. Low glycemic index foods (i.e., those that are minimally processed) have been shown to improve components of the MetS including hyperlipidemia and hyperglycemia (508), whereas a higher glycemic index has been shown to be positively associated with insulin resistance and MetS prevalence (495). Therefore, a diet high in complex, unrefined carbohydrates with an emphasis on fiber (14 g/1000 calories consumed daily) and low in added sugars (<25% of caloric intake) is recommended for individuals with or at risk for the MetS. This type of diet was recommended for participants in the lifestyle intervention group of the DPP (i.e., high carbohydrate, low fat); participants decreased their percentage fat intake by an average of 6.6% over a 1-yr period (492). This dietary change contributed to weight loss, which, as previously noted, was the primary predictor of the decrease in diabetes incidence in the study. Moreover, a lower glycemic load was associated with a reduced risk of CVD in the Nurses’ Health Study (509). Interestingly, though, diet soda but not “regular” soda was found to be a predictor of the MetS in the ARIC study (510).
b. Protein.
Data regarding appropriate protein intakes for patients with the MetS are sparse. The ARIC study, however, recently found that meat intake was associated with MetS incidence (510). With the exception of patients with nephropathy, a protein intake within the recommendations for the general population is acceptable: a protein intake of 10–35% of total caloric intake is recommended by the IOM (http://health.gov/dietaryguidelines/dga2005/report/HTML/D1_Tables.htm).
c. Fat.
Since NHANES 1971, the average percentage fat intake in the United States has decreased from 36.9 to 32.8% in men and from 36.1 to 32.8% in women (511), thus bringing fat intake within the recommended range of intake (i.e., 20–35%; USDA/IOM). Despite these reductions, there has been a marked increase in obesity and the MetS over the same time period (512). Like carbohydrate, it may be the type of fats that are consumed, rather than the total amount, that has a greater effect on components of the MetS. Several studies have shown no effect of increased fat intake (20 to 40% of caloric intake) on insulin sensitivity (513, 514, 515, 516), although some conflicting results have been reported (517). Interestingly, it has been shown that obese insulin-resistant women lost more weight on a 16-wk high-fat (40%), low-carbohydrate (40%) diet, whereas obese insulin-sensitive women lost more weight on a low-fat (20%), high-carbohydrate diet (60%) (518). Therefore, the degree of insulin resistance may determine what macronutrient composition is most appropriate to promote weight loss.
Evidence points toward the type of fat that is consumed having an effect on insulin sensitivity. Saturated fat has consistently been shown to be positively associated with fasting insulin levels (517, 519, 520). The substitution of unsaturated fats for saturated fats in the diet has been shown either to have no effect on (521, 522, 523, 524, 525) or to improve (109, 526, 527, 528) insulin sensitivity. Given the observed association between saturated fat intake and insulin levels, it is prudent to recommend a reduction in saturated fat intake (<7% of caloric intake) and in increase in the unsaturated fatty acids, specifically linoleic (5–10% of caloric intake) and α-linolenic (0.7–1.6% of caloric intake), as is promoted by the 2005 USDA Dietary Guidelines. These guidelines are also applicable in the case of CVD because investigators began researching this relationship as early as the 1960s. Both serum cholesterol and overall CVD risk have been shown to be improved by type of dietary fat, i.e., a reduction in saturated fat and an increase in unsaturated fat, more so than total fat intake (529, 530, 531, 532). The Nurses’ Health Study investigators reported that a 5% increase in saturated fat intake was associated with a 17% increase in coronary risk, whereas monounsaturated and polyunsaturated fat intakes were inversely related to coronary disease (533).
d. Sodium.
In addition to the effects of diet on weight loss, other “diet-related” lifestyle modifications can have a significant impact on blood pressure regulation (534). A clear positive association has been shown between sodium intake and blood pressure, with excessive sodium intake associated with hypertension (535, 536). In addition, sodium restriction has been shown to be an important strategy in the prevention and treatment of hypertension (536, 537, 538, 539). The Dietary Approaches to Stop Hypertension (DASH) diet showed that lower sodium intake reduced blood pressure in patients with high-normal blood pressure and mild hypertension (539). Furthermore, sodium restriction has also been associated with reduced CVD events (540) and congestive heart failure (541). Guidelines therefore recommend that daily sodium intake should be restricted to no more than 65–100 mmol (534, 542). In addition to sodium restriction, increased potassium intake has also been shown to improve blood pressure, especially in the setting of high sodium intake (543). Guidelines have recommended the intake of foods enriched with potassium, such as fruit and vegetables, with a goal of 90–120 mmol of potassium per day (534, 544).
In summary, dietary intake clearly has an impact on all of the components of the MetS. Although each case should be treated individually, it is prudent to recommend a diet low in saturated fat, higher in unsaturated fats, high in complex carbohydrates, and low in sodium.
2. Physical activity.
A lifestyle intervention designed to increase physical activity and decrease, or possibly maintain, body weight is another important approach for global CVD risk modification. Higher cardiorespiratory fitness (i.e., aerobic capacity) and increased self-reported physical activity have been shown to be inversely related to CVD mortality and to incidence of IGT and T2D (545, 546, 547). Although it is difficult to separate out the effect of exercise, independent of weight loss, increased physical activity appears to reduce CVD risk and incidence of T2D (546, 548, 549, 550). Thus, it should not be surprising that physical activity has been shown to predict incidence of MetS in a dose-dependent manner; lower levels of activity increased incidence of MetS and higher levels of physical activity protected against the development of MetS (551, 552). Furthermore, the odds of having the MetS were almost doubled in adults reporting no moderate or vigorous physical activity compared with those reporting engaging in at least 150 min/wk (553). Higher cardiorespiratory fitness also predicted lower incidence of MetS in middle-aged women and men followed for an average of 5.7 yr (554). Physical activity and cardiorespiratory fitness likely protect against development of the MetS through their effects on each of the individual components. Exercise is particularly effective at reducing insulin resistance and has also been shown to improve dyslipidemia and hypertension, albeit to varying degrees. Whether or not physical activity is accompanied by a change in body weight (particularly abdominal adiposity) is an important mediator in its ability to modify each of the components.
a. Aerobic exercise and abdominal adiposity.
When negative energy balance (i.e., caloric deficit) and weight loss are the same between groups of individuals undergoing dietary restriction, either with or without exercise, fat mass (whole-body and abdominal) is reduced to the same extent (555). However, even in the absence of weight loss, exercise has been shown to reduce visceral adipose tissue (556, 557, 558). A recent systematic review of the literature supports a dose-response effect of aerobic exercise volume on visceral adiposity, but the ability of exercise to reduce visceral adipose tissue was less robust in those with metabolic disorders (e.g., T2D, dyslipidemia) (559). Thus, it remains unclear whether a dose-response of exercise on central adiposity holds true in MetS. Nevertheless, during weight maintenance (i.e., energy balance), regular exercise appears to play an important role in abdominal fat loss (556, 557, 558) and prevention of weight regain in those who have successfully lost weight (560, 561).
b. Aerobic exercise and insulin resistance.
Insulin resistance has generally been considered to be an important underlying pathology of the MetS. Although there are no definitive criteria for categorizing an individual as insulin resistant or insulin sensitive, the majority (∼78%) of people who have the MetS are relatively more insulin resistant (i.e., upper tertile of steady-state plasma glucose during insulin suppression) (562). Exercise improves glucose homeostasis by enhancing glucose transport and insulin action in working skeletal muscle. Not only does muscle contraction stimulate uptake of glucose through non-insulin-dependent mechanisms during exercise, but sensitivity to insulin-mediated glucose uptake is greatly improved immediately after exercise (563, 564). Although a single bout of aerobic exercise will normally not acutely improve glucose tolerance in an insulin-resistant individual with T2DM, glucose uptake during exercise is increased, and glucose and insulin response to a meal immediately after exercise is improved (565). However, in obese and T2D individuals, this acute effect of exercise on insulin-stimulated glucose uptake does not appear to persist beyond 24 h after the last bout of exercise (566). Additionally, repeated bouts of exercise that are accompanied by improvements in cardiorespiratory fitness (i.e., aerobic exercise training), but no change in body weight, do not appear to improve insulin-mediated glucose uptake beyond the effect of the last bout of exercise (567). Thus, for continued benefit of exercise on insulin action, an individual would need to follow the AHA and American College of Sports Medicine recommendation to exercise at least 30 min/d most days of the week (568). There is evidence to suggest that aerobic exercise training may need to be accompanied by weight loss for a persistent effect on glucose tolerance and insulin action beyond the immediate postexercise effects (569, 570).
c. Aerobic exercise and dyslipidemia.
Current NCEP:ATPIII recommendations maintain LDL-C reduction as the primary treatment goal for CVD risk reduction, but also recommend that therapeutic lifestyle changes (e.g., physical activity, weight management) be implemented in those individuals with the MetS with the aim of treating elevated triglycerides and low HDL-C (571). Although aerobic exercise training has generally been shown to increase HDL-C and to decrease triglycerides, results are mixed particularly for an effect on LDL-C (572, 573, 574, 575, 576). The variable results of previous studies are impacted by the characteristics of the cohort being studied, including: baseline lipid/lipoprotein profile, degree of overweight/obesity (and changes in body composition over time), age, sex, and disease (e.g., T2D). Nevertheless, beneficial effects of exercise training on lipids and lipoproteins are routinely observed and may have additional impact when combined with dietary modification and weight loss (577). Furthermore, exercise has an acute effect on postprandial triglyceride excursions, possibly providing additional antiatherogenic protection (578).
d. Aerobic exercise and hypertension.
A recent meta-analyses of randomized, controlled trials studying the effect of aerobic exercise on blood pressure suggests that exercise reduces systolic and diastolic blood pressure by approximately 3.8 and 2.6 mm Hg, respectively (579). Although the effect of aerobic exercise on blood pressure is small, and not routinely observed in all studies, there may be added benefit when combined with dietary modification (i.e., DASH diet) and/or weight loss (580).
e. Resistance exercise and the metabolic syndrome.
As with cardiorespiratory fitness, greater muscle strength has been associated with decreased risk of developing MetS in men, suggesting that there may be a role for resistance training in the prevention of MetS (581). This association between resistance training and the MetS, however, is attenuated after adjusting for cardiorespiratory fitness (581), emphasizing the interrelatedness of the two functional measures. Nevertheless, resistance exercise has been shown to improve many of the individual components of MetS. Although resistance training has little effect on lipids, it can improve glycemic control and insulin sensitivity (582, 583) and may reduce blood pressure (584). Furthermore, resistance training may indirectly affect metabolic improvements through reductions in abdominal fat (582, 583). Prospective lifestyle interventions designed to evaluate the combined effects of resistance and aerobic exercise on MetS will add important information to this area (585).
B. Pharmaceutical therapy
1. Excess adiposity.
Central adiposity is a core component of the MetS and may be one of the key elements in the pathophysiology responsible for the development of the MetS and its components. It therefore seems logical to target weight loss aggressively. As discussed above, lifestyle interventions resulting in modest weight loss can result in significant clinical benefits. Lifestyle modification, however, is too often met with failure and frustration. The National Institutes of Health guidelines for the treatment of obesity recommend consideration of pharmaceutical therapy for weight loss for individuals with a BMI of at least 30 kg/m2 or for those with a BMI of at least 27 kg/m2 and comorbidities associated with their excess weight. The majority of patients who meet the criteria for the MetS will therefore meet the criteria for considering pharmaceutical weight loss therapy.
It is beyond the scope of this paper to review pharmacotherapy for weight loss, and this topic has been reviewed elsewhere recently (586, 587, 588, 589). Currently, only sibutramine and orlistat are Food and Drug Administration approved for long-term use. Studies have shown that pharmacological therapy for weight loss results in improvements in the individual components of the MetS (586). In addition, a 4-yr randomized controlled study of orlistat showed a significant reduction in the progression to diabetes in high-risk individuals (590). The long-term benefits of these agents in reducing CV risk in those with the MetS, however, have not yet been clearly established. Nevertheless, these agents should be considered as a potential valid treatment option.
2. Insulin resistance/hyperglycemia.
Insulin resistance is another core component of the MetS that potentially deserves specific attention when discussing pharmacotherapy. As discussed above, weight loss and lifestyle modification independent of weight loss can lead to clinically meaningful improvements in insulin sensitivity and should be considered the primary therapeutic options for treating insulin resistance. The difficulties and frustrations associated with weight loss efforts and lifestyle modification have driven the demand for using pharmaceutical agents that target insulin resistance more directly. The exact role of using these agents, however, is less clear. There are now several randomized, controlled trials showing that agents that target insulin resistance can help prevent the progression to T2D in individuals with IGT. It must be remembered, however, that these studies have not directly targeted individuals with the MetS. It is unclear whether these agents truly prevent the progression to T2D or simply treat glucose intolerance or mild hyperglycemia. In addition, it is unclear from these studies whether these agents improve CVD outcomes. Therefore, as with weight loss medications, the goals for the use of agents targeting insulin resistance must be kept clear.
Metformin, which has a primary mechanism of action of reducing hepatic glucose production, has been shown to reduce the progression of diabetes from IGT by approximately 31% in the DPP, of which 53% had the MetS (591). Incidence of the MetS was also reduced by 17% in the metformin-treated group of the DPP, which was driven primarily by improvements in WC and fasting glucose (485). Other cardiac risk factors, however, did not improve with metformin to the same degree as with the intensive lifestyle intervention (592). Long-term follow-up would suggest that metformin is in fact treating IGT and not necessarily “preventing” progression to T2D (593). From a safety perspective, though, no concerns emerged with 5 yr of follow-up. Now that metformin is available in generic form, the cost may not be prohibitive although no formal cost effectiveness studies have been performed. Despite the potential attractiveness of this agent for patients with the MetS, there are still many unresolved issues. What dose should be used? How long should this therapy last: indefinitely? Does metformin reduce the risk for CVD outcomes? The United Kingdom Diabetes Prospective Study (UKPDS) and its 10-yr follow-up report suggests the answer is “yes,” but this outcome was measured in patients with T2D, not the MetS (594, 595).
Thiazolidinediones have been shown to improve insulin sensitivity. There are now several studies showing their potential for “preventing” T2D in high-risk individuals (596, 597, 598). Of note, the TRIPOD study showed that troglitazone could slow the progression to T2D in high-risk women with recent history of GDM (597). There appeared to be a lasting effect of troglitazone on slowing diabetes progression even when the agent was discontinued. More recently, the DREAM study showed that rosiglitazone also slowed the progression to T2D in patients with IGT by about 60% (598). Again, these studies were not performed in patients with the MetS specifically, and more recently there has been concern regarding potential increased risk in CVD outcomes, especially with rosiglitazone (599, 600, 601). Although thiazolidinediones increase body weight, they are associated with a reduction in waist-to-hip ratio and improvements in other components of the MetS, including blood pressure, triglycerides, HDL-C, and liver-related transaminases (598).
In the STOP-NIDDM trial, acarbose, a drug that affects carbohydrate absorption and is approved for the treatment of T2D, was also shown to reduce the progression to T2D in individuals with IGT (602). This trial also showed that acarbose treatment was in fact associated with reduced CVD and hypertension (603). The main limitation of the use of this agent is its poor patient tolerability.
3. Dyslipidemia.
The “metabolic” dyslipidemia is characterized by elevated concentrations of triglycerides, low levels of HDL-C, and small, dense LDL-C particles. Dyslipidemia, especially elevated LDL-C, is a major modifiable risk factor for CVD, and proper management has been shown to significantly reduce CVD events and deaths (604). This has prompted the guidelines to recommend reaching appropriate LDL-C concentrations as the primary goal. Although it is necessary to state the importance of implementing therapeutic lifestyle changes in patients with the MetS (e.g., increased physical activity and decreased saturated fat and cholesterol intake), a portion of MetS patients will require drug therapy to achieve lipid goals.
a. Treatment goals.
The NCEP:ATPIII guidelines have identified elevated LDL-C as the primary target of cholesterol-lowering therapy, after which other components of dyslipidemia should be addressed (1). The LDL-C goal is dependent upon a person’s absolute risk for CHD, meaning the higher the risk, the lower the goal as outlined in Table 9. The majority of MetS patients will be of moderately high to high risk. The guidelines recommend that LDL-C goals should be set at less than 130 mg/dl with the option of targeting less than 100 mg/dl in moderately high-risk individuals. Target goals should be set at an LDL-C less than 100 mg/dl in high-risk patients with the option of aiming for less than 70 mg/dl in the “very high-risk” patient (605). The NCEP:ATPIII guidelines recommend setting a secondary lipid goal for non-HDL-C. Specifically, for individuals with triglycerides of at least 200 mg/dl, after achieving LDL-C goals, the goal should be to decrease non-HDL-C (LDL-C + VLDL-C). The goal for the non-HDL-C is 30 mg/dl greater than LDL-C (1). A primary lipid target to reach this goal will be either further LDL-C lowering or triglyceride lowering. Perhaps another secondary goal should reflect atherogenic particle number instead. Atherogenic particle number can be estimated by non-HDL-C (recommended by the NCEP:ATPIII as discussed above), LDL particle number, or apo B. It is beyond the scope of this review to discuss the relative merits of each of these biomarkers as targets for lipid management in the MetS. A tertiary lipid goal should be for HDL-C. Although the NCEP:ATPIII guidelines classify low HDL-C as less than 40 mg/dl in both men and women, there is no specific target goal for HDL-C because there is insufficient evidence to specify a therapy goal. Similarly, because the guidelines classify hypertriglyceridemia as triglyceride concentrations of at least 150 mg/dl, no specific triglyceride goals have been set.
TABLE 9.
Goals for LDL-C lowering (605 )
| Risk category | LDL-C goals | Recommendations |
|---|---|---|
| Lower risk | <160 mg/dl | Lifestyle modification |
| 0–1 Major risk factor | Consider pharmacotherapy if LDL-C ≥190 mg/dl after lifestyle modification | |
| 10-yr risk <10% | ||
| Moderate risk | <130 mg/dl | Lifestyle modification |
| ≥2 major risk factors | Consider pharmacotherapy if LDL-C ≥160 mg/dl after lifestyle modification | |
| 10-yr risk <10% | ||
| Moderately high risk | <130 mg/dl | Lifestyle modification |
| ≥2 Major risk factors | Optional <100 mg/dl | Consider pharmacotherapy if LDL-C ≥130 mg/dl or optionally ≥100 mg/dl after lifestyle modification |
| 10-yr risk 10–20% | ||
| High risk | <100 mg/dl | Lifestyle modification |
| CHD or CHD risk equivalents | Optional <70 mg/dl | Consider pharmacotherapy if LDL-C ≥100 mg/dl or optionally ≥70 mg/dl after lifestyle modification |
b. Statins.
Because LDL-C lowering is the primary treatment goal of the metabolic dyslipidemia, the use of LDL-C-lowering agents such as the 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase inhibitors or statins has become the standard first-line therapy. Due to their minimal drug-drug interactions and side effects, statins are considered to be the most effective class of drugs for reducing LDL-C concentrations (604). Depending on the dose and the specific type of statin used, LDL-C reductions of 15 to 60 mg/dl are observed (606). Statins increase HDL-C by 5–10%, with greater increases seen in individuals with lower HDL-C and elevated triglycerides (607, 608, 609), and reduce triglyceride concentrations by 7–30% primarily with moderate to high doses (607, 608, 609). Non-lipid-lowering or pleiotropic effects of statins have also been implicated in their beneficial effects on inflammation, endothelial function, and CVD events (610, 611). Statins are highly effective in decreasing CVD mortality and morbidity, but despite the number of primary and secondary prevention trials showing benefits in reducing CVD outcomes with statin therapy, there are no published trials to date that have specifically targeted patients with the MetS. The study that most resembles a primary prevention trial of CVD in patients with the MetS is the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm trial (612). In this study, individuals with hypertension and at least one other CVD risk factor were randomized to atorvastatin or placebo. Regardless of their baseline lipids, the treatment arm showed a significant reduction in CVD events. Post hoc analysis of the Treating to New Targets study found that intensive lowering of LDL-C in patients with both CHD and the MetS was associated with a reduction in major CVD events (613). Thus, at present the evidence strongly supports attention directed toward lowering LDL-C before addressing the lipid components of the MetS (614).
c. Other LDL-C lowering agents.
Bile acid sequestrants (BAS) and cholesterol absorption inhibitors (CAI) lower LDL-C by decreasing absorption of intestinal bile acids and cholesterol, respectively. BAS result in 15 to 30% reductions in LDL-C (615). The only clinically available CAI, ezetimibe, has been shown to result in 15–25% reductions in LDL-C (616). Although BAS and CAI are both effective as monotherapy, greater benefits are obtained when used in combination with statins, an effect that may be due to their complementary mechanisms of action (617, 618, 619). BAS have been shown to reduce the risk for major coronary events (620), whereas ezetimibe has only been shown to reduce potential cardiac risk in patients with the MetS (620, 621). The recent Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) study, although not an outcome study, demonstrated no effect of ezetimibe on carotid intima media thickness in patients with familial hypercholesterolemia despite a 17% lower level of LDL-C (622).
d. Fibrates.
Fibrates are an effective therapy for reducing serum triglyceride concentrations and therefore non-HDL-C. Fibrates decrease triglycerides by 25–50%, with greater reductions in individuals that are hypertriglyceridemic (623). Fibrates also increase HDL-C by 5–15% and reduce LDL-C by 0–30%, although LDL-C may be increased in patients with low HDL-C and elevated triglycerides (604). The decrease in triglycerides may transform small, dense LDL-C into more normal-sized LDL-C (624). The Helsinki Heart Study, a primary prevention study, found that fibrate therapy with gemfibrozil significantly reduced the incidence of CVD (625, 626), but this was not specifically in patients with the MetS. The Veterans Affairs High-Density Lipoprotein Intervention Trial (VA-HIT), a secondary prevention study, also showed that gemfibrozil significantly reduced CVD, especially in obese individuals with diabetes (627). Other clinical fibrate trials, such as the Bezafibrate Infarction Prevention (BIP) and the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) studies, however, have found less favorable effects (628, 629); therefore the evidence for cardioprotection is not as robust as it is for statins. Combination therapy of fibrates plus statins in high-risk individuals attains the target for non-HDL-C better than statins alone. However, no clinical trials to date have compared statins against the combination of statins plus fibrates on CHD outcomes (604, 629). The potential benefits of combining these agents must be weighed against the increased risk of myopathy (630). Post hoc analyses of some of these fibrate studies, however, suggest that individuals with the MetS may derive greater benefits than those without the MetS (631).
e. Niacin.
Niacin has favorable effects on essentially all of the abnormalities of the metabolic dyslipidemia. It is considered the most effective agent for raising HDL-C (15 to 35%) and increasing HDL particle size (632). Niacin significantly lowers triglycerides (20 to 50%) and LDL-C (5–25%) (604). Niacin also causes beneficial changes in lipoprotein subclasses because it has been shown to reduce the proportion of small, dense LDL particles while increasing large, more buoyant LDL particles and larger HDL particles (633, 634, 635). Combination therapy of niacin and a statin produces greater effects on lipid levels than does either agent given alone (636). The primary limitations for the use of niacin include flushing (most often associated with immediate-release niacin) and hyperglycemia (637, 638, 639, 640).
f. Omega-3 fatty acids.
Supplementation with marine omega-3 PUFAs may be indicated in MetS patients presenting with combined dyslipidemia. Two to 4 g of omega-3 PUFAs per day have been shown to reduce fasting and postprandial serum triglycerides by 20 to 40%, an effect that is most profound in individuals with elevated levels of triglycerides (641, 642, 643). The long-chain PUFAs, eicosapentaenoic acid and docosahexaenoic acid, appear to be equally effective in reducing serum triglyceride concentrations (644). Omega-3 PUFAs have little or no effect on HDL-C (645, 646) but can lead to 5–10% elevations in LDL-C levels (647). Studies examining the use of statins and omega-3 PUFA in treating combined dyslipidemia have found significant reductions in serum triglycerides without adverse affects on LDL-C (648, 649). In the JELIS study, the addition of eicosapentaenoic acid to a statin therapy significantly decreased the incidence of primary end points of major coronary events (e.g., sudden cardiac death and fatal or nonfatal myocardial infarction) (650). Additional benefits of high-dose omega-3 PUFAs for patients with the MetS are improvements in inflammatory state (651), decreased platelet aggregation (652), reductions in blood pressure (653), enhanced endothelial function (654), and potential antiarrhythmic effects (655). Despite these benefits, the NCEP:ATPIII guidelines recommend that more definitive clinical trials are necessary before recommending high intakes of omega-3 PUFAs (1).
4. Elevated blood pressure/hypertension.
Management of elevated blood pressure and hypertension is another key target in CVD risk reduction in the MetS patient, although there are no clear guidelines for blood pressure management specific to this population. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure has recommended that the target blood pressure should be less than 140/90 mm Hg in those without diabetes or chronic kidney disease (CKD) and less than 130/80 mm Hg for those with diabetes or CKD (656). As with management of dyslipidemia, the primary therapeutic intervention for blood pressure management should be lifestyle modification, as discussed above, but many patients will require pharmacological therapy to reach blood pressure goals. It has been proposed that angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers should be the first-line classes of agents in the MetS, especially in the setting of diabetes or CKD (657, 658). Certainly these classes of agents have been shown to be effective in reducing the incidence of albuminuria or progression of nephropathy in patients with diabetes (659). Although a number of trials have shown that ACE inhibitors and angiotensin receptor blockers may reduce the risk for diabetes (660), a more recent study designed to examine this issue directly found that the ACE inhibitor ramipril did not prevent the progression of diabetes in persons with IFG or IGT (661). The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) showed that treatment with a thiazide-type diuretic in patients with the MetS results in superior CVD outcomes compared with treatment with calcium channel blockers, α-blockers, or ACE inhibitors despite the less favorable metabolic profile associated with thiazide diuretics (662, 663). The ALLHAT and the UKPDS have shown that agents such as thiazide diuretics and beta-blockers lower the risk for CVD events even in patients with diabetes (664, 665). These agents, however, have also been associated with increased risk for diabetes (663, 664). Certainly the majority of patients who need antihypertensive therapy will likely need more than one agent for proper blood pressure control (542).
5. Prothrombotic state.
The MetS is associated with elevated levels of coagulation factors such as fibrinogen and PAI-1 (666). Although low-dose aspirin is frequently recommended to patients with MetS (667, 668), there are no specific studies of the use of aspirin or other antiplatelet agents for the primary prevention of CVD in individuals with the MetS specifically. Long-term use of aspirin therapy has been advocated in the secondary prevention of CVD (669), and some have recommended aspirin in high-risk patients with the MetS, especially those with CVD (670). Until there are more data, however, the use of aspirin in the primary prevention of CVD should remain as an “individual clinical judgment” (669).
6. Proinflammatory state.
The MetS is associated with elevated markers of inflammation. Elevated levels of CRP, a systemic marker of inflammation, have been shown to be associated with greater risk for CVD in patients with MetS (209). There are, however, no currently recommended direct therapies targeting inflammation. Lifestyle modification and weight loss result in reduced CRP concentrations (671, 672), as does the treatment of the other associated comorbidities such as dyslipidemia, elevated blood pressure, and insulin resistance/hyperglycemia (673, 674).
C. Bariatric surgery
Perhaps the most promising treatment of multiple risk factors within the MetS in the context of severe obesity lies in bariatric surgery. The data supporting the safety and efficacy of bariatric surgery have been reviewed previously (675). Titles in the literature reflect optimism about its role in resolving many health risk factors and in attenuating at least a portion of the epidemics of obesity, diabetes (676, 677), and perhaps the MetS (678). Bariatric surgery has been found to be associated with the improvement and resolution of multiple comorbidities associated with obesity, including hypertension, T2D, NAFLD, OSA, cardiopulmonary failure, CVD, arthritis, PCOS, dyslipidemia (exclusive of hypercholesterolemia), hyperuricemia, and infertility (676, 679, 680, 681, 682, 683, 684, 685, 686, 687, 688). The seminal study of Pories et al. (676) highlighted the resolution of aberrances in glucose metabolism (IGT, T2D) over a 14-yr follow-up period. This observation was substantiated in the Swedish Obese Subjects study in which a decrease in the incidence of T2D was seen at 2 yr (including a reversal of previously diagnosed diabetes and prevention of new onset diabetes). In an extension of the study of Pories and colleagues, the reduction in incidence of T2D was from 31.8 to 8.6%, whereas the incidence increased from 56.4 to 87.5% in the control group (689). In a recent meta-analysis of 22,094 morbidly obese patients who underwent bariatric surgery, T2D resolved in 76.8% of cases and improved in 86.0% of cases (680). In a more recent study, patients with T2D randomized to laparoscopic adjustable gastric banding had a greater than 5-fold increase in “remission” of their diabetes as compared with conventional diabetes therapy (690). More specifically to the MetS, improvements in the metabolic profile have also been documented as an effect of the surgery (677, 682, 691, 692), presumably due to the redistribution of adiposity (693). Most recently, 10-yr follow-up data from the Swedish Obese Subjects study (n = 4047 severely obese subjects) showed an overall reduction in mortality in patients who had the surgery compared with the control group (694). And in a recent retrospective cohort study (n = 7925), mortality in the surgery group was reduced by 40% over 7.1 yr, particularly from deaths due to CVD, diabetes, and cancer (695). Thus, bariatric surgery appears to be beneficial on many levels in treating numerous risk factors of the MetS in severely obese people who qualify for the surgery, and it should be considered as a valid treatment option for the MetS patient.
VIII. Unanswered Questions
Despite the great interest in the MetS, many unanswered questions remain. There has been much debate and controversy over whether there is a unifying pathogenesis of the MetS. Although abdominal adiposity and insulin resistance appear to be at the core of the development of the MetS, it is still not clear whether all of the components are directly related to these conditions. How should the MetS best be defined is another important question that deserves considerable attention. As is evident from the number of different definitions proposed by different groups (NCEP:ATPIII, WHO, IDF, AACE, etc.), there is not a consensus on how best to define the MetS. This issue is important to resolve for a number of different reasons. Without a consensus definition, it is difficult to perform research on the MetS, and this creates confusion for the clinicians. Should a marker for the prothrombotic state or inflammation be added? In addition, whatever the components, should they all be considered equal in their importance or risk prediction? This is reflected by some definitions requiring some, but not all, components to be present. In addition, whether the MetS should reflect more of a continuum of risk as opposed to a “present” or “absent” situation has also been debated (696). In fact, it is unclear whether these cutpoints should be based on biological or statistical endpoints. How many of the factors should actually be present to make the diagnosis also needs better scientific rationale. How should the MetS best be treated? Beyond favorable lifestyle modification, most would agree that treating each of the individual components of the MetS is important. It is less clear, however, what the goals of treatment for the individual components should be for patients with the MetS. For example, should lipid or blood pressure goals be treated more aggressively than IGT in those with the MetS? It is also unclear whether targeting a more unifying pathophysiological process such as abdominal adiposity or insulin resistance should be recommended at this time.
IX. Summary and Conclusions
The MetS is a clustering of components or risk factors associated with an increased risk for CVD and T2D. A consensus definition of the MetS has been difficult to develop, but it is an important consideration for the groups involved and continues to be a “work in progress.” Although the prevalence of the MetS depends on the definition used and population studied, it has clearly been increasing globally. The MetS is not exclusive to adults. In fact, the prevalence of the MetS in younger populations is increasing in parallel with childhood obesity. This will likely be associated with increased risk for CVD and T2D in adulthood. Most studies show that the MetS is associated with an approximate doubling of CVD risk and that the risk for incident T2D is more than five times higher in individuals with the MetS compared with those without the syndrome. In addition, the MetS is associated with a number of other comorbidities such as NAFLD, sleep disorders, reproductive tract disorders, and microvascular disease.
Specific guidelines for the treatment of the MetS and/or its components have not yet been established. In addition, because it is unclear whether there is a unifying pathophysiological mechanism resulting in the MetS, it is unclear whether the MetS can be treated in and of itself. It is our opinion that lifestyle modification and weight loss should be at the core of treating or preventing the MetS and its components. It is well established that weight loss with diet and physical activity is beneficial for treating all of the components of the MetS, including excessive adiposity, dyslipidemia, hypertension, insulin resistance, and hyperglycemia. In addition, there is some consensus on treating the individual components of the MetS with the overall goals of reducing the risk for or preventing CVD and T2D. Nevertheless, concentrating therapeutic efforts on treating the excess adiposity and insulin resistance associated with the MetS may provide the most overall success in attaining these goals. In addition, pharmacotherapy and surgery for weight loss also have an important role. Pharmacotherapy targeting a number of the components of the MetS, in addition to aggressive management of LDL-C and therapy for the prothrombotic state, has also been generally accepted as appropriate management of these high-risk patients. There is more controversy over pharmacotherapy targeting insulin resistance and hyperglycemia. Finally, a number of unanswered questions regarding the MetS remain, including questions regarding the definition, pathogenesis, and treatment of the MetS.
Footnotes
Disclosure Statement: T.L.H., R.C.L., A.J.S., N.R.S., R.E.V.P., and H.W. have nothing to disclose. M.-A.C. has received lecture fees from Merck, Schering-Plough, Sanofi-Aventis, Lilly, and Takeda. D.D. has consulted for Merck. R.H.E. has consulted for Sanofi-Aventis and Merck, received lecture fees from the Cardiometabolic Health Congress and the Committee on Cardiovascular and Metabolic Diseases, and received grant support from Sanofi-Aventis.
First Published Online October 29, 2008
Abbreviations: ACE, Angiotensin-converting enzyme; apo, apolipoprotein; BAS, bile acid sequestrants; BMI, body mass index; CAI, cholesterol absorption inhibitor; CETP, cholesteryl ester transfer protein; CHD, coronary heart disease; CI, confidence interval; CKD, chronic kidney disease; CRP, C-reactive protein; CVD, cardiovascular disease; eNOS, endothelial nitric oxide synthase; ER, endoplasmic reticulum; FFA, free fatty acid; GDM, gestational diabetes mellitus; HDL, high-density lipoprotein; HDL-C, HDL cholesterol; HMW, high molecular weight; HOMA, homeostatic model assessment; HSL, hormone-sensitive lipase; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; IRS-1, insulin receptor substrate-1; LDL, low-density lipoprotein; LDL-C, LDL cholesterol; LPL, lipoprotein lipase; MetS, metabolic syndrome; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NHP, nonhuman primate; OR, odds ratio; OSA, obstructive sleep apnea; PAI-1, plasminogen activator inhibitor-1; PCOS, polycystic ovarian syndrome; PPAR, peroxisome proliferator-activated receptor; PUFA, polyunsaturated fatty acid; QTL, quantitative trait loci; SES, socioeconomic status; T2D, type 2 diabetes; VLDL, very low-density lipoprotein; WC, waist circumference.
References
- 1.2001 Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 2.Sarafidis PA, Nilsson PM 2006. The metabolic syndrome: a glance at its history. J Hypertens 24:621–626 [DOI] [PubMed] [Google Scholar]
- 3.Kylin ES 1923. Hypertonie-Hyperglykamie-Hyperurikamiesyndrome. Zentralblatt fur innere Medizin 44
- 4.Vague J 1947. La differenciation sexuelle, facteur determinant des formes de l’obesite. Presse Med 30 339–340 [PubMed]
- 5.Despres JP 1993. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition 9:452–459 [PubMed] [Google Scholar]
- 6.Albrink MJ, Krauss RM, Lindgrem FT, von der GJ, Pan S, Wood PD 1980. Intercorrelations among plasma high density lipoprotein, obesity and triglycerides in a normal population. Lipids 15:668–676 [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg HN 2000. Insulin resistance and cardiovascular disease. J Clin Invest 106:453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reaven GM 1988. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37:1595–1607 [DOI] [PubMed] [Google Scholar]
- 9.Kaplan NM 1989. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med 149:1514–1520 [DOI] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Ferrannini E 1991. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 14:173–194 [DOI] [PubMed] [Google Scholar]
- 11.Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP 1992. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes 41:715–722 [DOI] [PubMed] [Google Scholar]
- 12.Balkau B, Charles MA 1999. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med 16:442–443 [DOI] [PubMed] [Google Scholar]
- 13.Alberti KG, Zimmet P, Shaw J 2006. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23:469–480 [DOI] [PubMed] [Google Scholar]
- 14.Kahn R, Buse J, Ferrannini E, Stern M 2005. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 28:2289–2304 [DOI] [PubMed] [Google Scholar]
- 15.Alberti KG, Zimmet PZ 1998. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15:539–553 [DOI] [PubMed] [Google Scholar]
- 16.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P 2003. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 17.Shaw JE, Zimmet PZ, Alberti KG 2006. Point: impaired fasting glucose: the case for the new American Diabetes Association criterion. Diabetes Care 29:1170–1172 [DOI] [PubMed] [Google Scholar]
- 18.Bloomgarden ZT 2003. American Association of Clinical Endocrinologists (AACE) consensus conference on the insulin resistance syndrome: 25–26 August 2002, Washington, DC. Diabetes Care 26:1297–1303 [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM 2008. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 28:629–636 [DOI] [PubMed] [Google Scholar]
- 20.Marchesini G, Forlani G, Cerrelli F, Manini R, Natale S, Baraldi L, Ermini G, Savorani G, Zocchi D, Melchionda N 2004. WHO and ATPIII proposals for the definition of the metabolic syndrome in patients with type 2 diabetes. Diabet Med 21:383–387 [DOI] [PubMed] [Google Scholar]
- 21.Ford ES, Giles WH, Dietz WH 2002. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287:356–359 [DOI] [PubMed] [Google Scholar]
- 22.Ford ES, Giles WH 2003. A comparison of the prevalence of the metabolic syndrome using two proposed definitions. Diabetes Care 26:575–581 [DOI] [PubMed] [Google Scholar]
- 23.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB 2003. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 163:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollman G, Kristenson M 2007. The prevalence of the metabolic syndrome and its risk factors in a middle-aged Swedish population—mainly a function of overweight? Eur J Cardiovasc Nurs 7:21–26 [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo C, Williams K, Hunt KJ, Haffner SM 2007. The National Cholesterol Education Program—Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care 30:8–13 [DOI] [PubMed] [Google Scholar]
- 26.Cameron AJ, Magliano DJ, Zimmet PZ, Welborn T, Shaw JE 2007. The metabolic syndrome in Australia: prevalence using four definitions. Diabetes Res Clin Pract 77:471–478 [DOI] [PubMed] [Google Scholar]
- 27.Csaszar A, Kekes E, Abel T, Papp R, Kiss I, Balogh S 2006. Prevalence of metabolic syndrome estimated by International Diabetes Federation criteria in a Hungarian population. Blood Press 15:101–106 [DOI] [PubMed] [Google Scholar]
- 28.Deepa M, Farooq S, Datta M, Deepa R, Mohan V 2007. Prevalence of metabolic syndrome using WHO, ATPIII and IDF definitions in Asian Indians: the Chennai Urban Rural Epidemiology Study (CURES-34). Diabetes Metab Res Rev 23:127–134 [DOI] [PubMed] [Google Scholar]
- 29.Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C, Madsbad S 2007. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: a population-based study. J Am Coll Cardiol 49:2112–2119 [DOI] [PubMed] [Google Scholar]
- 30.Lorenzo C, Serrano-Rios M, Martinez-Larrad MT, Gonzalez-Sanchez JL, Seclen S, Villena A, Gonzalez-Villalpando C, Williams K, Haffner SM 2006. Geographic variations of the International Diabetes Federation and the National Cholesterol Education Program-Adult Treatment Panel III definitions of the metabolic syndrome in nondiabetic subjects. Diabetes Care 29:685–691 [DOI] [PubMed] [Google Scholar]
- 31.Harzallah F, Alberti H, Ben Khalifa F 2006. The metabolic syndrome in an Arab population: a first look at the new International Diabetes Federation criteria. Diabet Med 23:441–444 [DOI] [PubMed] [Google Scholar]
- 32.Chien KL, Lee BC, Hsu HC, Lin HJ, Chen MF, Lee YT 2008. Prevalence, agreement and classification of various metabolic syndrome criteria among ethnic Chinese: a report on the hospital-based health diagnosis of the adult population. Atherosclerosis 196:764–771 [DOI] [PubMed] [Google Scholar]
- 33.Zabetian A, Hadaegh F, Azizi F 2007. Prevalence of metabolic syndrome in Iranian adult population, concordance between the IDF with the ATPIII and the WHO definitions. Diabetes Res Clin Pract 77:251–257 [DOI] [PubMed] [Google Scholar]
- 34.Mattsson N, Ronnemaa T, Juonala M, Viikari JS, Raitakari OT 2007. The prevalence of the metabolic syndrome in young adults. The Cardiovascular Risk in Young Finns Study. J Intern Med 261:159–169 [DOI] [PubMed] [Google Scholar]
- 35.Ilanne-Parikka P, Eriksson JG, Lindstrom J, Hamalainen H, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Rastas M, Salminen V, Aunola S, Sundvall J, Valle T, Lahtela J, Uusitupa M, Tuomilehto J 2004. Prevalence of the metabolic syndrome and its components: findings from a Finnish general population sample and the Diabetes Prevention Study cohort. Diabetes Care 27:2135–2140 [DOI] [PubMed] [Google Scholar]
- 36.Jorgensen ME, Bjerregaard P, Gyntelberg F, Borch-Johnsen K 2004. Prevalence of the metabolic syndrome among the Inuit in Greenland. A comparison between two proposed definitions. Diabet Med 21:1237–1242 [DOI] [PubMed] [Google Scholar]
- 37.Nilsson PM, Engstrom G, Hedblad B 2007. The metabolic syndrome and incidence of cardiovascular disease in non-diabetic subjects: a population-based study comparing three different definitions. Diabet Med 24:464–472 [DOI] [PubMed] [Google Scholar]
- 38.Gause-Nilsson I, Gherman S, Kumar Dey D, Kennerfalk A, Steen B 2006. Prevalence of metabolic syndrome in an elderly Swedish population. Acta Diabetol 43:120–126 [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Hanley AJ, Young TK, Harris SB, Zinman B 2006. Characteristics and prevalence of the metabolic syndrome among three ethnic groups in Canada. Int J Obes (Lond) 30:669–676 [DOI] [PubMed] [Google Scholar]
- 40.Gu D, Reynolds K, Wu X, Chen J, Duan X, Reynolds RF, Whelton PK, He J 2005. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet 365:1398–1405 [DOI] [PubMed] [Google Scholar]
- 41.Hillier TA, Fagot-Campagna A, Eschwege E, Vol S, Cailleau M, Balkau B 2006. Weight change and changes in the metabolic syndrome as the French population moves towards overweight: the D.E.S.I.R. cohort. Int J Epidemiol 35:190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villegas R, Creagh D, Hinchion R, O'Halloran D, Perry IJ 2004. Prevalence and lifestyle determinants of the metabolic syndrome. Ir Med J 97:300–303 [PubMed] [Google Scholar]
- 43.Khader Y, Bateiha A, El-Khateeb M, Al-Shaikh A, Ajlouni K 2007. High prevalence of the metabolic syndrome among Northern Jordanians. J Diabetes Complications 21:214–219 [DOI] [PubMed] [Google Scholar]
- 44.Aguilar-Salinas CA, Rojas R, Gomez-Perez FJ, Valles V, Rios-Torres JM, Franco A, Olaiz G, Rull JA, Sepulveda J 2004. High prevalence of metabolic syndrome in Mexico. Arch Med Res 35:76–81 [DOI] [PubMed] [Google Scholar]
- 45.Al-Lawati JA, Mohammed AJ, Al-Hinai HQ, Jousilahti P 2003. Prevalence of the metabolic syndrome among Omani adults. Diabetes Care 26:1781–1785 [DOI] [PubMed] [Google Scholar]
- 46.Abdul-Rahim HF, Husseini A, Bjertness E, Giacaman R, Gordon NH, Jervell J 2001. The metabolic syndrome in the West Bank population: an urban-rural comparison. Diabetes Care 24:275–279 [DOI] [PubMed] [Google Scholar]
- 47.Medina-Lezama J, Zea-Diaz H, Morey-Vargas OL, Bolanos-Salazar JF, Munoz-Atahualpa E, Postigo-Macdowall M, Corrales-Medina F, Valdivia-Ascuna Z, Cuba-Bustinza C, Paredes-Diaz S, Villalobos-Tapia P, Chirinos-Pacheco J, Goldberg RB, Chirinos JA 2007. Prevalence of the metabolic syndrome in Peruvian Andean Hispanics: The PREVENCION study. Diabetes Res Clin Pract 78:270–281 [DOI] [PubMed] [Google Scholar]
- 48.Tanchoco CC, Cruz AJ, Duante CA, Litonjua AD 2003. Prevalence of metabolic syndrome among Filipino adults aged 20 years and over. Asia Pac J Clin Nutr 12:271–276 [PubMed] [Google Scholar]
- 49.Jones ED, Ivanov LL, Wallace DC, Von Cannon L 2006. Examining the metabolic syndrome in Russia. Int J Nurs Pract 12:260–266 [DOI] [PubMed] [Google Scholar]
- 50.Vozarova de Courten B, de Courten M, Hanson RL, Zahorakova A, Egyenes HP, Tataranni PA, Bennett PH, Vozar J 2003. Higher prevalence of type 2 diabetes, metabolic syndrome and cardiovascular diseases in gypsies than in non-gypsies in Slovakia. Diabetes Res Clin Pract 62:95–103 [DOI] [PubMed] [Google Scholar]
- 51.Lee WY, Park JS, Noh SY, Rhee EJ, Kim SW, Zimmet PZ 2004. Prevalence of the metabolic syndrome among 40,698 Korean metropolitan subjects. Diabetes Res Clin Pract 65:143–149 [DOI] [PubMed] [Google Scholar]
- 52.Kozan O, Oguz A, Abaci A, Erol C, Ongen Z, Temizhan A, Celik S 2007. Prevalence of the metabolic syndrome among Turkish adults. Eur J Clin Nutr 61:548–553 [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Mi J, Shan XY, Wang QJ, Ge KY 2007. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 31:177–188 [DOI] [PubMed] [Google Scholar]
- 54.Ford ES 2005. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care 28:2745–2749 [DOI] [PubMed] [Google Scholar]
- 55.Hwang LC, Bai CH, Chen CJ 2006. Prevalence of obesity and metabolic syndrome in Taiwan. J Formos Med Assoc 105:626–635 [DOI] [PubMed] [Google Scholar]
- 56.Leite ML, Nicolosi A, Firmo JO, Lima-Costa MF 2007. Features of metabolic syndrome in non-diabetic Italians and Brazilians: a discriminant analysis. Int J Clin Pract 61:32–38 [DOI] [PubMed] [Google Scholar]
- 57.Gupta R, Deedwania PC, Gupta A, Rastogi S, Panwar RB, Kothari K 2004. Prevalence of metabolic syndrome in an Indian urban population. Int J Cardiol 97:257–261 [DOI] [PubMed] [Google Scholar]
- 58.Onat A, Ceyhan K, Basar O, Erer B, Toprak S, Sansoy V 2002. Metabolic syndrome: major impact on coronary risk in a population with low cholesterol levels—a prospective and cross-sectional evaluation. Atherosclerosis 165:285–292 [DOI] [PubMed] [Google Scholar]
- 59.Panagiotakos DB, Pitsavos C, Chrysohoou C, Skoumas J, Tousoulis D, Toutouza M, Toutouzas P, Stefanadis C 2004. Impact of lifestyle habits on the prevalence of the metabolic syndrome among Greek adults from the ATTICA study. Am Heart J 147:106–112 [DOI] [PubMed] [Google Scholar]
- 60.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL 1998. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord 22:39–47 [DOI] [PubMed] [Google Scholar]
- 61.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP 2001. The continuing epidemics of obesity and diabetes in the United States. JAMA 286:1195–1200 [DOI] [PubMed] [Google Scholar]
- 62.Duncan GE, Li SM, Zhou XH 2004. Prevalence and trends of a metabolic syndrome phenotype among U.S. adolescents, 1999–2000. Diabetes Care 27:2438–2443 [DOI] [PubMed] [Google Scholar]
- 63.Ford ES, Giles WH, Mokdad AH 2004. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care 27:2444–2449 [DOI] [PubMed] [Google Scholar]
- 64.Scuteri A, Najjar SS, Morrell CH, Lakatta EG 2005. The metabolic syndrome in older individuals: prevalence and prediction of cardiovascular events: the Cardiovascular Health Study. Diabetes Care 28:882–887 [DOI] [PubMed] [Google Scholar]
- 65.Cameron AJ, Shaw JE, Zimmet PZ 2004. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am 33:351–375 [DOI] [PubMed] [Google Scholar]
- 66.Elia M 2001. Obesity in the elderly. Obes Res 9(Suppl 4):244S–248S [DOI] [PubMed]
- 67.Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S 2007. The metabolic syndrome in children and adolescents. Lancet 369:2059–2061 [DOI] [PubMed] [Google Scholar]
- 68.Berenson GS, Srinivasan SR, Bao W, Newman III WP, Tracy RE, Wattigney WA 1998. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 338:1650–1656 [DOI] [PubMed] [Google Scholar]
- 69.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS 2003. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA 290:2271–2276 [DOI] [PubMed] [Google Scholar]
- 70.Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, Jarvisalo MJ, Uhari M, Jokinen E, Ronnemaa T, Akerblom HK, Viikari JS 2003. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA 290:2277–2283 [DOI] [PubMed] [Google Scholar]
- 71.Caprio S 2002. Insulin resistance in childhood obesity. J Pediatr Endocrinol Metab 15(Suppl 1):487–492 [PubMed] [Google Scholar]
- 72.Sun SS, Liang R, Huang TT, Daniels SR, Arslanian S, Liu K, Grave GD, Siervogel RM 2008. Childhood obesity predicts adult metabolic syndrome: the Fels Longitudinal Study. J Pediatr 152:191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davis PH, Dawson JD, Riley WA, Lauer RM 2001. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: the Muscatine Study. Circulation 104:2815–2819 [DOI] [PubMed] [Google Scholar]
- 74.Mahoney LT, Burns TL, Stanford W, Thompson BH, Witt JD, Rost CA, Lauer RM 1996. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine Study. J Am Coll Cardiol 27:277–284 [DOI] [PubMed] [Google Scholar]
- 75.1990 Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking. A preliminary report from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. JAMA 264:3018–3024 [DOI] [PubMed] [Google Scholar]
- 76.Baker JL, Olsen LW, Sorensen TI 2007. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med 357:2329–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ford ES, Li C 2008. Defining the metabolic syndrome in children and adolescents: will the real definition please stand up? J Pediatr 152:160–164 [DOI] [PubMed] [Google Scholar]
- 78.Cook S, Auinger P, Li C, Ford ES 2008. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. J Pediatr 152:165–170 [DOI] [PubMed] [Google Scholar]
- 79.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH 2003. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med 157:821–827 [DOI] [PubMed] [Google Scholar]
- 80.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N 2004. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation 110:2494–2497 [DOI] [PubMed] [Google Scholar]
- 81.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI 2004. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab 89:108–113 [DOI] [PubMed] [Google Scholar]
- 82.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S 2004. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350:2362–2374 [DOI] [PubMed] [Google Scholar]
- 83.Shaibi GQ, Goran MI 2008. Examining metabolic syndrome definitions in overweight Hispanic youth: a focus on insulin resistance. J Pediatr 152:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang TT 2008. Finding thresholds of risk for components of the pediatric metabolic syndrome. J Pediatr 152:158–159 [DOI] [PubMed] [Google Scholar]
- 85.Goran MI, Bergman RN, Cruz ML, Watanabe R 2002. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care 25:2184–2190 [DOI] [PubMed] [Google Scholar]
- 86.Gower BA, Nagy TR, Trowbridge CA, Dezenberg C, Goran MI 1998. Fat distribution and insulin response in prepubertal African American and white children. Am J Clin Nutr 67:821–827 [DOI] [PubMed] [Google Scholar]
- 87.Gower BA, Nagy TR, Goran MI 1999. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes 48:1515–1521 [DOI] [PubMed] [Google Scholar]
- 88.Arslanian S, Suprasongsin C, Janosky JE 1997. Insulin secretion and sensitivity in black versus white prepubertal healthy children. J Clin Endocrinol Metab 82:1923–1927 [DOI] [PubMed] [Google Scholar]
- 89.Ogden CL, Flegal KM, Carroll MD, Johnson CL 2002. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 288:1728–1732 [DOI] [PubMed] [Google Scholar]
- 90.Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, Imperatore G, Williams DE, Bell RA, Wadwa RP, Palla SL, Liu LL, Kershnar A, Daniels SR, Linder B 2006. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for diabetes in youth study. Diabetes Care 29:1891–1896 [DOI] [PubMed] [Google Scholar]
- 91.Duncan GE 2006. Prevalence of diabetes and impaired fasting glucose levels among US adolescents: National Health and Nutrition Examination Survey, 1999–2002. Arch Pediatr Adolesc Med 160:523–528 [DOI] [PubMed] [Google Scholar]
- 92.Morrison JA, Friedman LA, Wang P, Glueck CJ 2008. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr 152:201–206 [DOI] [PubMed] [Google Scholar]
- 93.Lucove JC, Kaufman JS, James SA 2006. Association between adult and childhood socioeconomic status and prevalence of the metabolic syndrome in African Americans: the Pitt County Study. Am J Public Health 97:234–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wamala SP, Lynch J, Horsten M, Mittleman MA, Schenck-Gustafsson K, Orth-Gomer K 1999. Education and the metabolic syndrome in women. Diabetes Care 22:1999–2003 [DOI] [PubMed] [Google Scholar]
- 95.Silventoinen K, Pankow J, Jousilahti P, Hu G, Tuomilehto J 2005. Educational inequalities in the metabolic syndrome and coronary heart disease among middle-aged men and women. Int J Epidemiol 34:327–334 [DOI] [PubMed] [Google Scholar]
- 96.Brunner EJ, Marmot MG, Nanchahal K, Shipley MJ, Stansfeld SA, Juneja M, Alberti KG 1997. Social inequality in coronary risk: central obesity and the metabolic syndrome. Evidence from the Whitehall II study. Diabetologia 40:1341–1349 [DOI] [PubMed] [Google Scholar]
- 97.Park MJ, Yun KE, Lee GE, Cho HJ, Park HS 2007. A cross-sectional study of socioeconomic status and the metabolic syndrome in Korean adults. Ann Epidemiol 17:320–326 [DOI] [PubMed] [Google Scholar]
- 98.Paek KW, Chun KH, Jin KN, Lee KS 2006. Do health behaviors moderate the effect of socioeconomic status on metabolic syndrome? Ann Epidemiol 16:756–762 [DOI] [PubMed] [Google Scholar]
- 99.Fezeu L, Balkau B, Kengne AP, Sobngwi E, Mbanya JC 2006. Metabolic syndrome in a sub-Saharan African setting: central obesity may be the key determinant. Atherosclerosis 193:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feng Y, Hong X, Li Z, Zhang W, Jin D, Liu X, Zhang Y, Hu FB, Wei LJ, Zang T, Xu X, Xu X 2006. Prevalence of metabolic syndrome and its relation to body composition in a Chinese rural population. Obesity (Silver Spring) 14:2089–2098 [DOI] [PubMed] [Google Scholar]
- 101.Weng X, Liu Y, Ma J, Wang W, Yang G, Caballero B 2007. An urban-rural comparison of the prevalence of the metabolic syndrome in eastern China. Public Health Nutr 10:131–136 [DOI] [PubMed] [Google Scholar]
- 102.Lim S, Jang HC, Lee HK, Kimm KC, Park C, Cho NH 2006. A rural-urban comparison of the characteristics of the metabolic syndrome by gender in Korea: the Korean Health and Genome Study (KHGS). J Endocrinol Invest 29:313–319 [DOI] [PubMed] [Google Scholar]
- 103.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S 2005. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 142:611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Villareal DT, Miller III BV, Banks M, Fontana L, Sinacore DR, Klein S 2006. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr 84:1317–1323 [DOI] [PubMed] [Google Scholar]
- 105.Pacini G 2006. The hyperbolic equilibrium between insulin sensitivity and secretion. Nutr Metab Cardiovasc Dis 16 S22–S27 [DOI] [PubMed]
- 106.Bravata DM, Wells CK, Concato J, Kernan WN, Brass LM, Gulanski BI 2004. Two measures of insulin sensitivity provided similar information in a U.S. population. J Clin Epidemiol 57:1214–1217 [DOI] [PubMed] [Google Scholar]
- 107.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ 1996. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes 45:633–638 [DOI] [PubMed] [Google Scholar]
- 108.Mayer-Davis EJ, D'Agostino Jr R, Karter AJ, Haffner SM, Rewers MJ, Saad M, Bergman RN 1998. Intensity and amount of physical activity in relation to insulin sensitivity: the Insulin Resistance Atherosclerosis Study. JAMA 279:669–674 [DOI] [PubMed] [Google Scholar]
- 109.Vessby B, Unsitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, Nalsen C, Berglund L, Louheranta A, Rasmussen BM, Calvert GD, Maffetone A, Pedersen E, Gustafsson IB, Storlien LH 2001. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU Study. Diabetologia 44:312–319 [DOI] [PubMed] [Google Scholar]
- 110.St Onge MP, Janssen I, Heymsfield SB 2004. Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes Care 27:2222–2228 [DOI] [PubMed] [Google Scholar]
- 111.Bergman RN 1989. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes 38:1512–1527 [DOI] [PubMed] [Google Scholar]
- 112.Bergman RN, Ader M, Huecking K, Van Citters G 2002. Accurate assessment of β-cell function: the hyperbolic correction. Diabetes 51(Suppl 1):S212–S220 [DOI] [PubMed]
- 113.Eckel RH, Grundy SM, Zimmet PZ 2005. The metabolic syndrome. Lancet 365:1415–1428 [DOI] [PubMed] [Google Scholar]
- 114.Grundy SM 2005. Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol 25:2243–2244 [DOI] [PubMed] [Google Scholar]
- 115.Eckel RH, Grundy SM, Zimmet PZ 2005. The metabolic syndrome. Lancet 365:1415–1428 [DOI] [PubMed] [Google Scholar]
- 116.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Haring HU 2008. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 168:1609–1616 [DOI] [PubMed] [Google Scholar]
- 117.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith Jr SC, Spertus JA, Costa F 2005. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev 13:322–327 [PubMed] [Google Scholar]
- 118.Weir GC, Bonner-Weir S 2007. A dominant role for glucose in β-cell compensation of insulin resistance. J Clin Invest 117:81–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, Tsutsumi S, Tsubamoto Y, Hashimoto S, Eto K, Nakamura A, Noda M, Tobe K, Aburatani H, Nagai R, Kadowaki T 2007. Glucokinase and IRS-2 are required for compensatory β cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest 117:246–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goodarzi MO, Taylor KD, Guo X, Hokanson JE, Haffner SM, Cui J, Chen YD, Wagenknecht LE, Bergman RN, Rotter JI 2007. Haplotypes in the lipoprotein lipase gene influence fasting insulin and discovery of a new risk haplotype. J Clin Endocrinol Metab 92:293–296 [DOI] [PubMed] [Google Scholar]
- 121.Bonnycastle LL, Willer CJ, Conneely KN, Jackson AU, Burrill CP, Watanabe RM, Chines PS, Narisu N, Scott LJ, Enloe ST, Swift AJ, Duren WL, Stringham HM, Erdos MR, Riebow NL, Buchanan TA, Valle TT, Tuomilehto J, Bergman RN, Mohlke KL, Boehnke M, Collins FS 2006. Common variants in maturity-onset diabetes of the young genes contribute to risk of type 2 diabetes in Finns. Diabetes 55:2534–2540 [DOI] [PubMed] [Google Scholar]
- 122.Wajchenberg BL 2007. β-Cell failure in diabetes and preservation by clinical treatment. Endocr Rev 28:187–218 [DOI] [PubMed] [Google Scholar]
- 123.Reaven GM 1995. The fourth musketeer—from Alexandre Dumas to Claude Bernard. Diabetologia 38:3–13 [DOI] [PubMed] [Google Scholar]
- 124.McGarry JD 2002. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51:7–18 [DOI] [PubMed] [Google Scholar]
- 125.Randle PJ, Garland PB, Hales CN, Newsholme EA 1963. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–789 [DOI] [PubMed] [Google Scholar]
- 126.Randle PJ 1998. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 14:263–283 [DOI] [PubMed] [Google Scholar]
- 127.Kahn BB, Flier JS 2000. Obesity and insulin resistance. J Clin Invest 106:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kraemer FB, Shen WJ 2002. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res 43:1585–1594 [DOI] [PubMed] [Google Scholar]
- 129.Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R 2006. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 281:40236–40241 [DOI] [PubMed] [Google Scholar]
- 130.Jocken JW, Langin D, Smit E, Saris WH, Valle C, Hul GB, Holm C, Arner P, Blaak EE 2007. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocrinol Metab 92:2292–2299 [DOI] [PubMed] [Google Scholar]
- 131.Klannemark M, Orho M, Langin D, Laurell H, Holm C, Reynisdottir S, Arner P, Groop L 1998. The putative role of the hormone-sensitive lipase gene in the pathogenesis of type II diabetes mellitus and abdominal obesity. Diabetologia 41:1516–1522 [DOI] [PubMed] [Google Scholar]
- 132.Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, Adams PW 1982. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 54:254–260 [DOI] [PubMed] [Google Scholar]
- 133.Bjorntorp P 1991. Metabolic implications of body fat distribution. Diabetes Care 14:1132–1143 [DOI] [PubMed] [Google Scholar]
- 134.Kahn SE, Prigeon RL, Schwartz RS, Fujimoto WY, Knopp RH, Brunzell JD, Porte Jr D 2001. Obesity, body fat distribution, insulin sensitivity and Islet β-cell function as explanations for metabolic diversity. J Nutr 131:354S–360S [DOI] [PubMed] [Google Scholar]
- 135.Pouliot MC, Despres JP, Nadeau A, Moorjani S, Prud’Homme D, Lupien PJ, Tremblay A, Bouchard C 1992. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes 41:826–834 [DOI] [PubMed] [Google Scholar]
- 136.Arner P 1998. Not all fat is alike. Lancet 351:1301–1302 [DOI] [PubMed] [Google Scholar]
- 137.Large V, Arner P 1998. Regulation of lipolysis in humans. Pathophysiological modulation in obesity, diabetes, and hyperlipidaemia. Diabetes Metab 24:409–418 [PubMed] [Google Scholar]
- 138.Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, Richey JM, Ader M 2007. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med 120:S3–S8 [DOI] [PubMed]
- 139.de Luca C, Olefsky JM 2008. Inflammation and insulin resistance. FEBS Lett 582:97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gustafson B, Hammarstedt A, Andersson CX, Smith U 2007. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol 27:2276–2283 [DOI] [PubMed] [Google Scholar]
- 141.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW 2003. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Skurk T, Alberti-Huber C, Herder C, Hauner H 2007. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 92:1023–1033 [DOI] [PubMed] [Google Scholar]
- 143.Sopasakis VR, Sandqvist M, Gustafson B, Hammarstedt A, Schmelz M, Yang X, Jansson PA, Smith U 2004. High local concentrations and effects on differentiation implicate interleukin-6 as a paracrine regulator. Obes Res 12:454–460 [DOI] [PubMed] [Google Scholar]
- 144.Hube F, Hauner H 1999. The role of TNF-α in human adipose tissue: prevention of weight gain at the expense of insulin resistance? Horm Metab Res 31:626–631 [DOI] [PubMed] [Google Scholar]
- 145.Gonzalez-Baro MR, Lewin TM, Coleman RA 2007. Regulation of triglyceride metabolism. II. Function of mitochondrial GPAT1 in the regulation of triacylglycerol biosynthesis and insulin action. Am J Physiol Gastrointest Liver Physiol 292:G1195–G1199 [DOI] [PMC free article] [PubMed]
- 146.Lewis GF, Uffelman KD, Szeto LW, Steiner G 1993. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes 42:833–842 [DOI] [PubMed] [Google Scholar]
- 147.Ginsberg HN, Zhang YL, Hernandez-Ono A 2005. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res 36:232–240 [DOI] [PubMed] [Google Scholar]
- 148.Lewis GF, Steiner G 1996. Acute effects of insulin in the control of VLDL production in humans. Implications for the insulin-resistant state. Diabetes Care 19:390–393 [DOI] [PubMed] [Google Scholar]
- 149.Sniderman AD, Faraj M 2007. Apolipoprotein B, apolipoprotein A-I, insulin resistance and the metabolic syndrome. Curr Opin Lipidol 18:633–637 [DOI] [PubMed] [Google Scholar]
- 150.Goodarzi MO, Guo X, Taylor KD, Quinones MJ, Saad MF, Yang H, Hsueh WA, Rotter JI 2004. Lipoprotein lipase is a gene for insulin resistance in Mexican Americans. Diabetes 53:214–220 [DOI] [PubMed] [Google Scholar]
- 151.Miller M, Rhyne J, Chen H, Beach V, Ericson R, Luthra K, Dwivedi M, Misra A 2007. APOC3 promoter polymorphisms C-482T and T-455C are associated with the metabolic syndrome. Arch Med Res 38:444–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Komurcu-Bayrak E, Onat A, Poda M, Humphries SE, Acharya J, Hergenc G, Coban N, Can G, Erginel-Unaltuna N 2007. The S447X variant of lipoprotein lipase gene is associated with metabolic syndrome and lipid levels among Turks. Clin Chim Acta 383:110–115 [DOI] [PubMed] [Google Scholar]
- 153.Hanyu O, Miida T, Kosuge K, Ito T, Soda S, Hirayama S, Wardaningsih E, Fueki Y, Obayashi K, Aizawa Y 2007. Preheparin lipoprotein lipase mass is a practical marker of insulin resistance in ambulatory type 2 diabetic patients treated with oral hypoglycemic agents. Clin Chim Acta 384:118–123 [DOI] [PubMed] [Google Scholar]
- 154.Saiki A, Oyama T, Endo K, Ebisuno M, Ohira M, Koide N, Murano T, Miyashita Y, Shirai K 2007. Preheparin serum lipoprotein lipase mass might be a biomarker of metabolic syndrome. Diabetes Res Clin Pract 76:93–101 [DOI] [PubMed] [Google Scholar]
- 155.Goodarzi MO, Wong H, Quinones MJ, Taylor KD, Guo X, Castellani LW, Antoine HJ, Yang H, Hsueh WA, Rotter JI 2005. The 3′ untranslated region of the lipoprotein lipase gene: haplotype structure and association with post-heparin plasma lipase activity. J Clin Endocrinol Metab 90:4816–4823 [DOI] [PubMed] [Google Scholar]
- 156.Sattar N, Tan CE, Han TS, Forster L, Lean ME, Shepherd J, Packard CJ 1998. Associations of indices of adiposity with atherogenic lipoprotein subfractions. Int J Obes Relat Metab Disord 22:432–439 [DOI] [PubMed] [Google Scholar]
- 157.Eckel RH 1989. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med 320:1060–1068 [DOI] [PubMed] [Google Scholar]
- 158.Murakami T, Michelagnoli S, Longhi R, Gianfranceschi G, Pazzucconi F, Calabresi L, Sirtori CR, Franceschini G 1995. Triglycerides are major determinants of cholesterol esterification/transfer and HDL remodeling in human plasma. Arterioscler Thromb Vasc Biol 15:1819–1828 [DOI] [PubMed] [Google Scholar]
- 159.Lewis GF, Rader DJ 2005. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res 96:1221–1232 [DOI] [PubMed] [Google Scholar]
- 160.Vohl MC, Lamarche B, Pascot A, Leroux G, Prud’homme D, Bouchard C, Nadeau A, Despres JP 1999. Contribution of the cholesteryl ester transfer protein gene TaqIB polymorphism to the reduced plasma HDL-cholesterol levels found in abdominal obese men with the features of the insulin resistance syndrome. Int J Obes Relat Metab Disord 23:918–925 [DOI] [PubMed] [Google Scholar]
- 161.Edwards KL, Talmud PJ, Newman B, Krauss RM, Austin MA 2001. Lipoprotein candidate genes for multivariate factors of the insulin resistance syndrome: a sib-pair linkage analysis in women twins. Twin Res 4:41–47 [DOI] [PubMed] [Google Scholar]
- 162.Sandhofer A, Tatarczyk T, Laimer M, Ritsch A, Kaser S, Paulweber B, Ebenbichler CF, Patsch JR 2008. The Taq1B-variant in the cholesteryl ester-transfer protein gene and the risk of metabolic syndrome. Obesity (Silver Spring) 16:919–922 [DOI] [PubMed] [Google Scholar]
- 163.Sniderman AD, Marcovina SM 2006. Apolipoprotein A1 and B. Clin Lab Med 26:733–750 [DOI] [PubMed] [Google Scholar]
- 164.Austin MA, King MC, Vranizan KM, Krauss RM 1990. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation 82:495–506 [DOI] [PubMed] [Google Scholar]
- 165.Sandhofer A, Kaser S, Ritsch A, Laimer M, Engl J, Paulweber B, Patsch JR, Ebenbichler CF 2006. Cholesteryl ester transfer protein in metabolic syndrome. Obesity (Silver Spring) 14:812–818 [DOI] [PubMed] [Google Scholar]
- 166.Lamarche B, Paradis ME 2007. Endothelial lipase and the metabolic syndrome. Curr Opin Lipidol 18:298–303 [DOI] [PubMed] [Google Scholar]
- 167.Zambon A, Brown BG, Deeb SS, Brunzell JD 2001. Hepatic lipase as a focal point for the development and treatment of coronary artery disease. J Investig Med 49:112–118 [DOI] [PubMed] [Google Scholar]
- 168.Jiang J, Torok N 2008. Nonalcoholic steatohepatitis and the metabolic syndrome. Metab Syndr Relat Disord 6:1–7 [DOI] [PubMed] [Google Scholar]
- 169.Khashab M, Chalasani N 2007. Use of insulin sensitizers in NASH. Endocrinol Metab Clin North Am 36:1067–1087; xi [DOI] [PubMed] [Google Scholar]
- 170.Frayn KN 2003. Metabolic regulation: a human perspective. Oxford, UK: Blackwell Science
- 171.Schalch DS, Kipnis DM 1965. Abnormalities in carbohydrate tolerance associated with elevated plasma nonesterified fatty acids. J Clin Invest 44:2010–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Boden G, Chen X, Ruiz J, White JV, Rossetti L 1994. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest 93:2438–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI 1999. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 103:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Roden M, Krssak M, Stingl H, Gruber S, Hofer A, Furnsinn C, Moser E, Waldhausl W 1999. Rapid impairment of skeletal muscle glucose transport/phosphorylation by free fatty acids in humans. Diabetes 48:358–364 [DOI] [PubMed] [Google Scholar]
- 175.Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH 1997. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46:983–988 [DOI] [PubMed] [Google Scholar]
- 176.Summers SA 2006. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 45:42–72 [DOI] [PubMed] [Google Scholar]
- 177.Bruce CR, Kriketos AD, Cooney GJ, Hawley JA 2004. Disassociation of muscle triglyceride content and insulin sensitivity after exercise training in patients with type 2 diabetes. Diabetologia 47:23–30 [DOI] [PubMed] [Google Scholar]
- 178.Sato F, Tamura Y, Watada H, Kumashiro N, Igarashi Y, Uchino H, Maehara T, Kyogoku S, Sunayama S, Sato H, Hirose T, Tanaka Y, Kawamori R 2007. Effects of diet-induced moderate weight reduction on intrahepatic and intramyocellular triglycerides and glucose metabolism in obese subjects. J Clin Endocrinol Metab 92:3326–3329 [DOI] [PubMed] [Google Scholar]
- 179.Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S 1987. Insulin resistance in essential hypertension. N Engl J Med 317:350–357 [DOI] [PubMed] [Google Scholar]
- 180.Bonora E, Capaldo B, Perin PC, Del Prato S, De Mattia G, Frittitta L, Frontoni S, Leonetti F, Luzi L, Marchesini G, Marini MA, Natali A, Paolisso G, Piatti PM, Pujia A, Solini A, Vettor R, Bonadonna RC 2007. Hyperinsulinemia and insulin resistance are independently associated with plasma lipids, uric acid and blood pressure in non-diabetic subjects. The GISIR database. Nutr Metab Cardiovasc Dis 18:624–631 [DOI] [PubMed] [Google Scholar]
- 181.Laakso M, Sarlund H, Mykkanen L 1989. Essential hypertension and insulin resistance in non-insulin-dependent diabetes. Eur J Clin Invest 19:518–526 [DOI] [PubMed] [Google Scholar]
- 182.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD 1994. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 94:1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ 1975. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest 55:845–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Barbato A, Cappuccio FP, Folkerd EJ, Strazzullo P, Sampson B, Cook DG, Alberti KG 2004. Metabolic syndrome and renal sodium handling in three ethnic groups living in England. Diabetologia 47:40–46 [DOI] [PubMed] [Google Scholar]
- 185.Tooke JE, Hannemann MM 2000. Adverse endothelial function and the insulin resistance syndrome. J Intern Med 247:425–431 [DOI] [PubMed] [Google Scholar]
- 186.Kuroda S, Uzu T, Fujii T, Nishimura M, Nakamura S, Inenaga T, Kimura G 1999. Role of insulin resistance in the genesis of sodium sensitivity in essential hypertension. J Hum Hypertens 13:257–262 [DOI] [PubMed] [Google Scholar]
- 187.Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P 2003. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 52:2882–2887 [DOI] [PubMed] [Google Scholar]
- 188.Grekin RJ, Vollmer AP, Sider RS 1995. Pressor effects of portal venous oleate infusion. A proposed mechanism for obesity hypertension. Hypertension 26:193–198 [DOI] [PubMed] [Google Scholar]
- 189.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL 1991. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 87:2246–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Egan BM 2003. Insulin resistance and the sympathetic nervous system. Curr Hypertens Rep 5:247–254 [DOI] [PubMed] [Google Scholar]
- 191.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- 192.Hanley AJ, Karter AJ, Festa A, D'Agostino Jr R, Wagenknecht LE, Savage P, Tracy RP, Saad MF, Haffner S 2002. Factor analysis of metabolic syndrome using directly measured insulin sensitivity: the Insulin Resistance Atherosclerosis Study. Diabetes 51:2642–2647 [DOI] [PubMed] [Google Scholar]
- 193.Kim S, Soltani-Bejnood M, Quignard-Boulange A, Massiera F, Teboul M, Ailhaud G, Kim JH, Moustaid-Moussa N, Voy BH 2006. The adipose renin-angiotensin system modulates systemic markers of insulin sensitivity and activates the intrarenal renin-angiotensin system. J Biomed Biotechnol 2006:27012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vogt B, Bochud M, Burnier M 2007. The association of aldosterone with obesity-related hypertension and the metabolic syndrome. Semin Nephrol 27:529–537 [DOI] [PubMed] [Google Scholar]
- 195.Takata Y, Osawa H, Kurata M, Kurokawa M, Yamauchi J, Ochi M, Nishida W, Okura T, Higaki J, Makino H 2008. Hyperresistinemia is associated with coexistence of hypertension and type 2 diabetes. Hypertension 51:534–539 [DOI] [PubMed] [Google Scholar]
- 196.Bernal-Mizrachi C, Weng S, Li B, Nolte LA, Feng C, Coleman T, Holloszy JO, Semenkovich CF 2002. Respiratory uncoupling lowers blood pressure through a leptin-dependent mechanism in genetically obese mice. Arterioscler Thromb Vasc Biol 22:961–968 [DOI] [PubMed] [Google Scholar]
- 197.Coppack SW, Jensen MD, Miles JM 1994. In vivo regulation of lipolysis in humans. J Lipid Res 35:177–193 [PubMed] [Google Scholar]
- 198.Landsberg L 2001. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why). J Hypertens 19:523–528 [DOI] [PubMed] [Google Scholar]
- 199.Tentolouris N, Liatis S, Katsilambros N 2006. Sympathetic system activity in obesity and metabolic syndrome. Ann NY Acad Sci 1083:129–152 [DOI] [PubMed] [Google Scholar]
- 200.Vgontzas AN, Bixler EO, Chrousos GP 2003. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med 254:32–44 [DOI] [PubMed] [Google Scholar]
- 201.Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN 2007. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab 292:E1590—E1598 [DOI] [PubMed]
- 202.Sutherland JP, McKinley B, Eckel RH 2004. The metabolic syndrome and inflammation. Metab Syndr Relat Disord 2:82–104 [DOI] [PubMed] [Google Scholar]
- 203.Norata GD, Ongari M, Garlaschelli K, Raselli S, Grigore L, Catapano AL 2007. Plasma resistin levels correlate with determinants of the metabolic syndrome. Eur J Endocrinol 156:279–284 [DOI] [PubMed] [Google Scholar]
- 204.Bahia L, Aguiar LG, Villela N, Bottino D, Godoy-Matos AF, Geloneze B, Tambascia M, Bouskela E 2006. Relationship between adipokines, inflammation, and vascular reactivity in lean controls and obese subjects with metabolic syndrome. Clinics 61:433–440 [DOI] [PubMed] [Google Scholar]
- 205.Xydakis AM, Case CC, Jones PH, Hoogeveen RC, Liu MY, Smith EO, Nelson KW, Ballantyne CM 2004. Adiponectin, inflammation, and the expression of the metabolic syndrome in obese individuals: the impact of rapid weight loss through caloric restriction. J Clin Endocrinol Metab 89:2697–2703 [DOI] [PubMed] [Google Scholar]
- 206.Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, Desouza CA 2006. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity (Silver Spring) 14:2127–2131 [DOI] [PubMed] [Google Scholar]
- 207.Choi KM, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG, Kim NH, Choi DS, Baik SH 2007. Serum adiponectin, interleukin-10 levels and inflammatory markers in the metabolic syndrome. Diabetes Res Clin Pract 75:235–240 [DOI] [PubMed] [Google Scholar]
- 208.Gonzalez AS, Guerrero DB, Soto MB, Diaz SP, Martinez-Olmos M, Vidal O 2006. Metabolic syndrome, insulin resistance and the inflammation markers C-reactive protein and ferritin. Eur J Clin Nutr 60:802–809 [DOI] [PubMed] [Google Scholar]
- 209.Ridker PM, Buring JE, Cook NR, Rifai N 2003. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14,719 initially healthy American women. Circulation 107:391–397 [DOI] [PubMed] [Google Scholar]
- 210.Hung J, McQuillan BM, Chapman CM, Thompson PL, Beilby JP 2005. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol 25:1268–1273 [DOI] [PubMed] [Google Scholar]
- 211.Mertens I, Verrijken A, Michiels JJ, Van der Planken M, Ruige JB, Van Gaal LF 2006. Among inflammation and coagulation markers, PAI-1 is a true component of the metabolic syndrome. Int J Obes (Lond) 30:1308–1314 [DOI] [PubMed] [Google Scholar]
- 212.Deepa R, Velmurugan K, Arvind K, Sivaram P, Sientay C, Uday S, Mohan V 2006. Serum levels of interleukin 6, C-reactive protein, vascular cell adhesion molecule 1, and monocyte chemotactic protein 1 in relation to insulin resistance and glucose intolerance—the Chennai Urban Rural Epidemiology Study (CURES). Metabolism 55:1232–1238 [DOI] [PubMed] [Google Scholar]
- 213.Guldiken S, Demir M, Arikan E, Turgut B, Azcan S, Gerenli M, Tugrul A 2007. The levels of circulating markers of atherosclerosis and inflammation in subjects with different degrees of body mass index: soluble CD40 ligand and high-sensitivity C-reactive protein. Thromb Res 119:79–84 [DOI] [PubMed] [Google Scholar]
- 214.Saito I, Yonemasu K, Inami F 2003. Association of body mass index, body fat, and weight gain with inflammation markers among rural residents in Japan. Circ J 67:323–329 [DOI] [PubMed] [Google Scholar]
- 215.Clearfield MB 2005. C-reactive protein: a new risk assessment tool for cardiovascular disease. J Am Osteopath Assoc 105:409–416 [PubMed] [Google Scholar]
- 216.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM 1995. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J Clin Invest 95:2409–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lo J, Bernstein LE, Canavan B, Torriani M, Jackson MB, Ahima RS, Grinspoon SK 2007. Effects of TNF-α neutralization on adipocytokines and skeletal muscle adiposity in the metabolic syndrome. Am J Physiol Endocrinol Metab 293:E102–E109 [DOI] [PMC free article] [PubMed]
- 218.Yang RZ, Huang Q, Xu A, McLenithan JC, Eisen JA, Shuldiner AR, Alkan S, Gong DW 2003. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun 310:927–935 [DOI] [PubMed] [Google Scholar]
- 219.Menzaghi C, Coco A, Salvemini L, Thompson R, De Cosmo S, Doria A, Trischitta V 2006. Heritability of serum resistin and its genetic correlation with insulin resistance-related features in nondiabetic Caucasians. J Clin Endocrinol Metab 91:2792–2795 [DOI] [PubMed] [Google Scholar]
- 220.Mojiminiyi OA, Abdella NA 2007. Associations of resistin with inflammation and insulin resistance in patients with type 2 diabetes mellitus. Scand J Clin Lab Invest 67:215–225 [DOI] [PubMed] [Google Scholar]
- 221.Shen J, Arnett DK, Peacock JM, Parnell LD, Kraja A, Hixson JE, Tsai MY, Lai CQ, Kabagambe EK, Straka RJ, Ordovas JM 2007. Interleukin 1β genetic polymorphisms interact with polyunsaturated fatty acids to modulate risk of the metabolic syndrome. J Nutr 137:1846–1851 [DOI] [PubMed] [Google Scholar]
- 222.Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF 2007. Interleukin-1β-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 148:241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B 2003. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil 24:113–119 [DOI] [PubMed] [Google Scholar]
- 224.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP 1997. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab 82:1313–1316 [DOI] [PubMed] [Google Scholar]
- 225.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM 2001. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286:327–334 [DOI] [PubMed] [Google Scholar]
- 226.Zuliani G, Volpato S, Ble A, Bandinelli S, Corsi AM, Lauretani F, Paolisso G, Fellin R, Ferrucci L 2007. High interleukin-6 plasma levels are associated with low HDL-C levels in community-dwelling older adults: the In Chianti study. Atherosclerosis 192:384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ruderman NB, Keller C, Richard AM, Saha AK, Luo Z, Xiang X, Giralt M, Ritov VB, Menshikova EV, Kelley DE, Hidalgo J, Pedersen BK, Kelly M 2006. Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes 55(Suppl 2):S48–S54 [DOI] [PubMed]
- 228.Glund S, Krook A 2008. Role of interleukin-6 signalling in glucose and lipid metabolism. Acta Physiol (Oxf) 192:37–48 [DOI] [PubMed] [Google Scholar]
- 229.Rieusset J, Bouzakri K, Chevillotte E, Ricard N, Jacquet D, Bastard JP, Laville M, Vidal H 2004. Suppressor of cytokine signaling 3 expression and insulin resistance in skeletal muscle of obese and type 2 diabetic patients. Diabetes 53:2232–2241 [DOI] [PubMed] [Google Scholar]
- 230.Ye JH, Li ZZ, Li Y, Li F, Yan L, Cheng H, Fu ZZ 2006. [Relationship between serum interleukin-10 and insulin resistance in metabolic syndrome]. Nan Fang Yi Ke Da Xue Xue Bao 26:428–430 [PubMed] [Google Scholar]
- 231.Chang YH, Huang CN, Wu CY, Shiau MY 2005. Association of interleukin-10 A-592C and T-819C polymorphisms with type 2 diabetes mellitus. Hum Immunol 66:1258–1263 [DOI] [PubMed] [Google Scholar]
- 232.Karadeniz M, Erdogan M, Zengi A, Tamsel S, Berdeli A, Saygili F, Yilmaz C 2008. Polymorphism of the interleukin-10 gene in polycystic ovary syndrome. Int J Immunogenet 35:119–123 [DOI] [PubMed] [Google Scholar]
- 233.Nishida M, Moriyama T, Sugita Y, Yamauchi-Takihara K 2007. Interleukin-10 associates with adiponectin predominantly in subjects with metabolic syndrome. Circ J 71:1234–1238 [DOI] [PubMed] [Google Scholar]
- 234.Okamura H, Tsutsui H, Kashiwamura S, Yoshimoto T, Nakanishi K 1998. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol 70:281–312 [DOI] [PubMed] [Google Scholar]
- 235.Evans J, Collins M, Jennings C, van der Merwe L, Soderstrom I, Olsson T, Levitt NS, Lambert EV, Goedecke JH 2007. The association of interleukin-18 genotype and serum levels with metabolic risk factors for cardiovascular disease. Eur J Endocrinol 157:633–640 [DOI] [PubMed] [Google Scholar]
- 236.Thompson SR, Sanders J, Stephens JW, Miller GJ, Humphries SE 2007. A common interleukin 18 haplotype is associated with higher body mass index in subjects with diabetes and coronary heart disease. Metabolism 56:662–669 [DOI] [PubMed] [Google Scholar]
- 237.Straczkowski M, Kowalska I, Nikolajuk A, Otziomek E, Adamska A, Karolczuk-Zarachowicz M, Gorska M 2007. Increased serum interleukin-18 concentration is associated with hypoadiponectinemia in obesity, independently of insulin resistance. Int J Obes (Lond) 31:221–225 [DOI] [PubMed] [Google Scholar]
- 238.Chandrasekar B, Patel DN, Mummidi S, Kim JW, Clark RA, Valente AJ 2008. Interleukin-18 suppresses adiponectin expression in 3T3–L1 adipocytes via a novel signal transduction pathway involving ERK1/2-dependent NFATc4 phosphorylation. J Biol Chem 283:4200–4209 [DOI] [PubMed] [Google Scholar]
- 239.Zirlik A, Abdullah SM, Gerdes N, MacFarlane L, Schonbeck U, Khera A, McGuire DK, Vega GL, Grundy S, Libby P, de Lemos JA 2007. Interleukin-18, the metabolic syndrome, and subclinical atherosclerosis: results from the Dallas Heart Study. Arterioscler Thromb Vasc Biol 27:2043–2049 [DOI] [PubMed] [Google Scholar]
- 240.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I 2005. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 307:426–430 [DOI] [PubMed] [Google Scholar]
- 241.Haider DG, Holzer G, Schaller G, Weghuber D, Widhalm K, Wagner O, Kapiotis S, Wolzt M 2006. The adipokine visfatin is markedly elevated in obese children. J Pediatr Gastroenterol Nutr 43:548–549 [DOI] [PubMed] [Google Scholar]
- 242.Araki S, Dobashi K, Kubo K, Kawagoe R, Yamamoto Y, Kawada Y, Asayama K, Shirahata A 2008. Plasma visfatin concentration as a surrogate marker for visceral fat accumulation in obese children. Obesity (Silver Spring) 16:384–388 [DOI] [PubMed] [Google Scholar]
- 243.Berndt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR, Stumvoll M, Bluher M 2005. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes 54:2911–2916 [DOI] [PubMed] [Google Scholar]
- 244.Filippatos TD, Derdemezis CS, Gazi IF, Lagos K, Kiortsis DN, Tselepis AD, Elisaf MS 2008. Increased plasma visfatin levels in subjects with the metabolic syndrome. Eur J Clin Invest 38:71–72 [DOI] [PubMed] [Google Scholar]
- 245.Sandeep S, Velmurugan K, Deepa R, Mohan V 2007. Serum visfatin in relation to visceral fat, obesity, and type 2 diabetes mellitus in Asian Indians. Metabolism 56:565–570 [DOI] [PubMed] [Google Scholar]
- 246.Pfutzner A, Hanefeld M, Lubben G, Weber MM, Karagiannis E, Kohler C, Hohberg C, Forst T 2007. Visfatin: a putative biomarker for metabolic syndrome is not influenced by pioglitazone or simvastatin treatment in nondiabetic patients at cardiovascular risk—results from the PIOSTAT study. Horm Metab Res 39:764–768 [DOI] [PubMed] [Google Scholar]
- 247.Lee HS, Lee M, Joung H 2007. Adiponectin represents an independent risk factor for hypertension in middle aged Korean women. Asia Pac J Clin Nutr 16:10–15 [PubMed] [Google Scholar]
- 248.Altinova AE, Toruner F, Bukan N, Yasar DG, Akturk M, Cakir N, Arslan M 2007. Decreased plasma adiponectin is associated with insulin resistance and HDL cholesterol in overweight subjects. Endocr J 54:221–226 [DOI] [PubMed] [Google Scholar]
- 249.Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, Gravanis A, Margioris AN 2006. Peripheral factors in the metabolic syndrome: the pivotal role of adiponectin. Ann NY Acad Sci 1083:185–195 [DOI] [PubMed] [Google Scholar]
- 250.Otabe S, Yuan X, Fukutani T, Wada N, Hashinaga T, Nakayama H, Hirota N, Kojima M, Yamada K 2007. Overexpression of human adiponectin in transgenic mice results in suppression of fat accumulation and prevention of premature death by high-calorie diet. Am J Physiol Endocrinol Metab 293:E210–E218 [DOI] [PubMed]
- 251.Lara-Castro C, Fu Y, Chung BH, Garvey WT 2007. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol 18:263–270 [DOI] [PubMed] [Google Scholar]
- 252.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T 2006. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care 29:1357–1362 [DOI] [PubMed] [Google Scholar]
- 253.Fantuzzi G 2008. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol 121:326–330 [DOI] [PubMed] [Google Scholar]
- 254.Salmenniemi U, Ruotsalainen E, Pihlajamaki J, Vauhkonen I, Kainulainen S, Punnonen K, Vanninen E, Laakso M 2004. Multiple abnormalities in glucose and energy metabolism and coordinated changes in levels of adiponectin, cytokines, and adhesion molecules in subjects with metabolic syndrome. Circulation 110:3842–3848 [DOI] [PubMed] [Google Scholar]
- 255.Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF 2003. Adiponectin and protection against type 2 diabetes mellitus. Lancet 361:226–228 [DOI] [PubMed] [Google Scholar]
- 256.Iwawaki T, Akai R, Kohno K, Miura M 2004. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat Med 10:98–102 [DOI] [PubMed] [Google Scholar]
- 257.Aguirre V, Uchida T, Yenush L, Davis R, White MF 2000. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem 275:9047–9054 [DOI] [PubMed] [Google Scholar]
- 258.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461 [DOI] [PubMed] [Google Scholar]
- 259.Coleman DL, Hummel KP 1969. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol 217:1298–1304 [DOI] [PubMed] [Google Scholar]
- 260.Coleman DL 1973. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia 9:294–298 [DOI] [PubMed] [Google Scholar]
- 261.Mertens A, Verhamme P, Bielicki JK, Phillips MC, Quarck R, Verreth W, Stengel D, Ninio E, Navab M, Mackness B, Mackness M, Holvoet P 2003. Increased low-density lipoprotein oxidation and impaired high-density lipoprotein antioxidant defense are associated with increased macrophage homing and atherosclerosis in dyslipidemic obese mice: LCAT gene transfer decreases atherosclerosis. Circulation 107:1640–1646 [DOI] [PubMed] [Google Scholar]
- 262.Verreth W, De Keyzer D, Pelat M, Verhamme P, Ganame J, Bielicki JK, Mertens A, Quarck R, Benhabiles N, Marguerie G, Mackness B, Mackness M, Ninio E, Herregods MC, Balligand JL, Holvoet P 2004. Weight-loss-associated induction of peroxisome proliferator-activated receptor-α and peroxisome proliferator-activated receptor-γ correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation 110:3259–3269 [DOI] [PubMed] [Google Scholar]
- 263.Demigne C, Bloch-Faure M, Picard N, Sabboh H, Besson C, Remesy C, Geoffroy V, Gaston AT, Nicoletti A, Hagege A, Menard J, Meneton P 2006. Mice chronically fed a westernized experimental diet as a model of obesity, metabolic syndrome and osteoporosis. Eur J Nutr 45:298–306 [DOI] [PubMed] [Google Scholar]
- 264.Barbosa CR, Albuquerque EM, Faria EC, Oliveira HC, Castilho LN 2007. Opposite lipemic response of Wistar rats and C57BL/6 mice to dietary glucose or fructose supplementation. Braz J Med Biol Res 40:323–331 [DOI] [PubMed] [Google Scholar]
- 265.Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR 2005. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes 54:1314–1323 [DOI] [PubMed] [Google Scholar]
- 266.Svenson KL, Von Smith R, Magnani PA, Suetin HR, Paigen B, Naggert JK, Li R, Churchill GA, Peters LL 2007. Multiple trait measurements in 43 inbred mouse strains capture the phenotypic diversity characteristic of human populations. J Appl Physiol 102:2369–2378 [DOI] [PubMed] [Google Scholar]
- 267.Polotsky VY 2007. Mouse model of the metabolic syndrome: the quest continues. J Appl Physiol 102:2088–2089 [DOI] [PubMed] [Google Scholar]
- 268.Eckel RH 2007. Mechanisms of the components of the metabolic syndrome that predispose to diabetes and atherosclerotic CVD. Proc Nutr Soc 66:82–95 [DOI] [PubMed] [Google Scholar]
- 269.Lloyd DJ, McCormick J, Helmering J, Kim KW, Wang M, Fordstrom P, Kaufman SA, Lindberg RA, Veniant MM 2008. Generation and characterization of two novel mouse models exhibiting the phenotypes of the metabolic syndrome: Apob48−/−Lepob/ob mice devoid of ApoE or Ldlr. Am J Physiol Endocrinol Metab 294:E496–E505 [DOI] [PubMed]
- 270.Morton NM, Seckl JR 2008. 11β-hydroxysteroid dehydrogenase type 1 and obesity. Front Horm Res 36:146–164 [DOI] [PubMed] [Google Scholar]
- 271.Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS 2001. A transgenic model of visceral obesity and the metabolic syndrome. Science 294:2166–2170 [DOI] [PubMed] [Google Scholar]
- 272.Cook S, Hugli O, Egli M, Vollenweider P, Burcelin R, Nicod P, Thorens B, Scherrer U 2003. Clustering of cardiovascular risk factors mimicking the human metabolic syndrome X in eNOS null mice. Swiss Med Wkly 133:360–363 [DOI] [PubMed] [Google Scholar]
- 273.Paterson JM, Morton NM, Fievet C, Kenyon CJ, Holmes MC, Staels B, Seckl JR, Mullins JJ 2004. Metabolic syndrome without obesity: hepatic overexpression of 11β-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc Natl Acad Sci USA 101:7088–7093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wellen KE, Fucho R, Gregor MF, Furuhashi M, Morgan C, Lindstad T, Vaillancourt E, Gorgun CZ, Saatcioglu F, Hotamisligil GS 2007. Coordinated regulation of nutrient and inflammatory responses by STAMP2 is essential for metabolic homeostasis. Cell 129:537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wiedmer T, Zhao J, Li L, Zhou Q, Hevener A, Olefsky JM, Curtiss LK, Sims PJ 2004. Adiposity, dyslipidemia, and insulin resistance in mice with targeted deletion of phospholipid scramblase 3 (PLSCR3). Proc Natl Acad Sci USA 101:13296–13301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mutch DM, O'Maille G, Wikoff WR, Wiedmer T, Sims PJ, Siuzdak G 2007. Mobilization of pro-inflammatory lipids in obese Plscr3-deficient mice. Genome Biol 8:R38 [DOI] [PMC free article] [PubMed]
- 277.Wu D, Yang H, Xiang W, Zhou L, Shi M, Julies G, Laplante JM, Ballard BR, Guo Z 2005. Heterozygous mutation of ataxia-telangiectasia mutated gene aggravates hypercholesterolemia in apoE-deficient mice. J Lipid Res 46:1380–1387 [DOI] [PubMed] [Google Scholar]
- 278.Schneider JG, Finck BN, Ren J, Standley KN, Takagi M, Maclean KH, Bernal-Mizrachi C, Muslin AJ, Kastan MB, Semenkovich CF 2006. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab 4:377–389 [DOI] [PubMed] [Google Scholar]
- 279.Hiroyama M, Aoyagi T, Fujiwara Y, Birumachi J, Shigematsu Y, Kiwaki K, Tasaki R, Endo F, Tanoue A 2007. Hypermetabolism of fat in V1a vasopressin receptor knockout mice. Mol Endocrinol 21:247–258 [DOI] [PubMed] [Google Scholar]
- 280.Hiroyama M, Aoyagi T, Fujiwara Y, Oshikawa S, Sanbe A, Endo F, Tanoue A 2007. Hyperammonaemia in V1a vasopressin receptor knockout mice caused by the promoted proteolysis and reduced intrahepatic blood volume. J Physiol 581:1183–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Aoyagi T, Birumachi J, Hiroyama M, Fujiwara Y, Sanbe A, Yamauchi J, Tanoue A 2007. Alteration of glucose homeostasis in V1a vasopressin receptor-deficient mice. Endocrinology 148:2075–2084 [DOI] [PubMed] [Google Scholar]
- 282.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL 2008. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 149:2138–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower Jr WR, Nimal S, Choi CS, Kim S, Shulman GI, Kahn CR, Braun U, Leitges M 2007. Muscle-specific knockout of PKC-λ impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest 117:2289–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Beguinot F, Formisano P 2008. Atypical protein kinase C dysfunction and the metabolic syndrome. Trends Endocrinol Metab 19:39–41 [DOI] [PubMed] [Google Scholar]
- 285.Misso ML, Murata Y, Boon WC, Jones ME, Britt KL, Simpson ER 2003. Cellular and molecular characterization of the adipose phenotype of the aromatase-deficient mouse. Endocrinology 144:1474–1480 [DOI] [PubMed] [Google Scholar]
- 286.Misso ML, Hewitt KN, Boon WC, Murata Y, Jones ME, Simpson ER 2005. Cholesterol feeding prevents adiposity in the obese female aromatase knockout (ArKO) mouse. Horm Metab Res 37:26–31 [DOI] [PubMed] [Google Scholar]
- 287.Jones ME, McInnes KJ, Boon WC, Simpson ER 2007. Estrogen and adiposity—utilizing models of aromatase deficiency to explore the relationship. J Steroid Biochem Mol Biol 106:3–7 [DOI] [PubMed] [Google Scholar]
- 288.Huang X, Yang C, Luo Y, Jin C, Wang F, McKeehan WL 2007. FGFR4 prevents hyperlipidemia and insulin resistance but underlies high-fat diet induced fatty liver. Diabetes 56:2501–2510 [DOI] [PubMed] [Google Scholar]
- 289.Vartanian V, Lowell B, Minko IG, Wood TG, Ceci JD, George S, Ballinger SW, Corless CL, McCullough AK, Lloyd RS 2006. The metabolic syndrome resulting from a knockout of the NEIL1 DNA glycosylase. Proc Natl Acad Sci USA 103:1864–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO 2002. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8:75–79 [DOI] [PubMed] [Google Scholar]
- 291.Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB 2004. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun 320:449–454 [DOI] [PubMed] [Google Scholar]
- 292.Freedman BD, Lee EJ, Park Y, Jameson JL 2005. A dominant negative peroxisome proliferator-activated receptor-γ knock-in mouse exhibits features of the metabolic syndrome. J Biol Chem 280:17118–17125 [DOI] [PubMed] [Google Scholar]
- 293.Gray SL, Dalla Nora E, Vidal-Puig AJ 2005. Mouse models of PPAR-γ deficiency: dissecting PPAR-γ’s role in metabolic homeostasis. Biochem Soc Trans 33:1053–1058 [DOI] [PubMed] [Google Scholar]
- 294.Santos SH, Fernandes LR, Mario EG, Ferreira AV, Porto LC, Alvarez-Leite JI, Botion LM, Bader M, Alenina N, Santos RA 2008. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes 57:340–347 [DOI] [PubMed] [Google Scholar]
- 295.Jensen DR, Schlaepfer IR, Morin CL, Pennington DS, Marcell T, Ammon SM, Gutierrez-Hartmann A, Eckel RH 1997. Prevention of diet-induced obesity in transgenic mice overexpressing skeletal muscle lipoprotein lipase. Am J Physiol 273:R683–R689 [DOI] [PubMed]
- 296.Ferreira LD, Pulawa LK, Jensen DR, Eckel RH 2001. Overexpressing human lipoprotein lipase in mouse skeletal muscle is associated with insulin resistance. Diabetes 50:1064–1068 [DOI] [PubMed] [Google Scholar]
- 297.Jensen DR, Knaub LA, Konhilas JP, Leinwand LA, MacLean PS, Eckel RH 2008. Increased thermoregulation in cold-exposed transgenic mice overexpressing lipoprotein lipase in skeletal muscle: an avian phenotype? J Lipid Res 49:870–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Moore G, Knoblaugh S, Gollahon K, Rabinovitch P, Ladiges W 2008. Hyperinsulinemia and insulin resistance in Wrn null mice fed a diabetogenic diet. Mech Ageing Dev 129:201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Le Bacquer O, Petroulakis E, Paglialunga S, Poulin F, Richard D, Cianflone K, Sonenberg N 2007. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest 117:387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F 1997. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88:131–141 [DOI] [PubMed] [Google Scholar]
- 301.Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD 2001. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 4:605–611 [DOI] [PubMed] [Google Scholar]
- 302.Weide K, Christ N, Moar KM, Arens J, Hinney A, Mercer JG, Eiden S, Schmidt I 2003. Hyperphagia, not hypometabolism, causes early onset obesity in melanocortin-4 receptor knockout mice. Physiol Genomics 13:47–56 [DOI] [PubMed] [Google Scholar]
- 303.Ellacott KL, Murphy JG, Marks DL, Cone RD 2007. Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling. Endocrinology 148:6186–6194 [DOI] [PubMed] [Google Scholar]
- 304.Smart JL, Low MJ 2003. Lack of proopiomelanocortin peptides results in obesity and defective adrenal function but normal melanocyte pigmentation in the murine C57BL/6 genetic background. Ann NY Acad Sci 994:202–210 [DOI] [PubMed] [Google Scholar]
- 305.Smart JL, Tolle V, Low MJ 2006. Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice. J Clin Invest 116:495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Ruohonen ST, Pesonen U, Moritz N, Kaipio K, Roytta M, Koulu M, Savontaus E 2008. Transgenic mice overexpressing neuropeptide Y in noradrenergic neurons: a novel model of increased adiposity and impaired glucose tolerance. Diabetes 57:1517–1525 [DOI] [PubMed] [Google Scholar]
- 307.Arsov T, Silva DG, O'Bryan MK, Sainsbury A, Lee NJ, Kennedy C, Manji SS, Nelms K, Liu C, Vinuesa CG, de Kretser DM, Goodnow CC, Petrovsky N 2006. Fat aussie–a new Alstrom syndrome mouse showing a critical role for ALMS1 in obesity, diabetes, and spermatogenesis. Mol Endocrinol 20:1610–1622 [DOI] [PubMed] [Google Scholar]
- 308.Arsov T, Larter CZ, Nolan CJ, Petrovsky N, Goodnow CC, Teoh NC, Yeh MM, Farrell GC 2006. Adaptive failure to high-fat diet characterizes steatohepatitis in Alms1 mutant mice. Biochem Biophys Res Commun 342:1152–1159 [DOI] [PubMed] [Google Scholar]
- 309.Collin GB, Cyr E, Bronson R, Marshall JD, Gifford EJ, Hicks W, Murray SA, Zheng QY, Smith RS, Nishina PM, Naggert JK 2005. Alms1-disrupted mice recapitulate human Alstrom syndrome. Hum Mol Genet 14:2323–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S 2007. Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA 104:2501–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Maeda K, Uysal KT, Makowski L, Gorgun CZ, Atsumi G, Parker RA, Bruning J, Hertzel AV, Bernlohr DA, Hotamisligil GS 2003. Role of the fatty acid binding protein mal1 in obesity and insulin resistance. Diabetes 52:300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Akagiri S, Naito Y, Ichikawa H, Mizushima K, Takagi T, Handa O, Kokura S, Yoshikawa T 2008. A mouse model of metabolic syndrome; increase in visceral adipose tissue precedes the development of fatty liver and insulin resistance in high-fat diet-fed male KK/Ta mice. J Clin Biochem Nutr 42:150–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Depke M, Fusch G, Domanska G, Geffers R, Volker U, Schuett C, Kiank C 2008. Hypermetabolic syndrome as a consequence of repeated psychological stress in mice. Endocrinology 149:2714–2723 [DOI] [PubMed] [Google Scholar]
- 314.Ryan MJ, McLemore Jr GR, Hendrix ST 2006. Insulin resistance and obesity in a mouse model of systemic lupus erythematosus. Hypertension 48:988–993 [DOI] [PubMed] [Google Scholar]
- 315.Ortlepp JR, Kluge R, Giesen K, Plum L, Radke P, Hanrath P, Joost HG 2000. A metabolic syndrome of hypertension, hyperinsulinaemia and hypercholesterolaemia in the New Zealand obese mouse. Eur J Clin Invest 30:195–202 [DOI] [PubMed] [Google Scholar]
- 316.Anunciado RV, Horio F, Ohno T, Tanaka S, Nishimura M, Namikawa T 2000. Characterization of hyperinsulinemic recombinant inbred (RI) strains (SMXA-5 and SMXA-9) derived from normoinsulinemic SM/J and A/J mice. Exp Anim 49:83–90 [DOI] [PubMed] [Google Scholar]
- 317.Kobayashi M, Io F, Kawai T, Nishimura M, Ohno T, Horio F 2004. SMXA-5 mouse as a diabetic model susceptible to feeding a high-fat diet. Biosci Biotechnol Biochem 68:226–230 [DOI] [PubMed] [Google Scholar]
- 318.Kumazawa M, Kobayashi M, Io F, Kawai T, Nishimura M, Ohno T, Horio F 2007. Searching for genetic factors of fatty liver in SMXA-5 mice by quantitative trait loci analysis under a high-fat diet. J Lipid Res 48:2039–2046 [DOI] [PubMed] [Google Scholar]
- 319.Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ 2008. Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology 149:2798–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Cleary MP, Vasselli JR, Greenwood MR 1980. Development of obesity in Zucker obese (fafa) rat in absence of hyperphagia. Am J Physiol 238:E284–E292 [DOI] [PubMed]
- 321.Shirouchi B, Nagao K, Inoue N, Furuya K, Koga S, Matsumoto H, Yanagita T 2008. Dietary phosphatidylinositol prevents the development of nonalcoholic fatty liver disease in Zucker (fa/fa) rats. J Agric Food Chem 56:2375–2379 [DOI] [PubMed] [Google Scholar]
- 322.de Nigris F, Balestrieri ML, Williams-Ignarro S, D'Armiento FP, Fiorito C, Ignarro LJ, Napoli C 2007. The influence of pomegranate fruit extract in comparison to regular pomegranate juice and seed oil on nitric oxide and arterial function in obese Zucker rats. Nitric Oxide 17:50–54 [DOI] [PubMed] [Google Scholar]
- 323.Doyon C, Samson P, Lalonde J, Richard D 2007. Effects of the CRF1 receptor antagonist SSR125543 on energy balance and food deprivation-induced neuronal activation in obese Zucker rats. J Endocrinol 193:11–19 [DOI] [PubMed] [Google Scholar]
- 324.Davis J, Higginbotham A, O'Connor T, Moustaid-Moussa N, Tebbe A, Kim YC, Cho KW, Shay N, Adler S, Peterson R, Banz W 2007. Soy protein and isoflavones influence adiposity and development of metabolic syndrome in the obese male ZDF rat. Ann Nutr Metab 51:42–52 [DOI] [PubMed] [Google Scholar]
- 325.Galisteo M, Sanchez M, Vera R, Gonzalez M, Anguera A, Duarte J, Zarzuelo A 2005. A diet supplemented with husks of Plantago ovata reduces the development of endothelial dysfunction, hypertension, and obesity by affecting adiponectin and TNF-α in obese Zucker rats. J Nutr 135:2399–2404 [DOI] [PubMed] [Google Scholar]
- 326.Kim KS, Seo EK, Lee YC, Lee TK, Cho YW, Ezaki O, Kim CH 2000. Effect of dietary Platycodon grandiflorum on the improvement of insulin resistance in obese Zucker rats. J Nutr Biochem 11:420–424 [DOI] [PubMed] [Google Scholar]
- 327.Kovacs P, van den Brandt J, Kloting I 2000. Genetic dissection of the syndrome X in the rat. Biochem Biophys Res Commun 269:660–665 [DOI] [PubMed] [Google Scholar]
- 328.Kloting N, Bluher M, Kloting I 2006. The polygenetically inherited metabolic syndrome of WOKW rats is associated with insulin resistance and altered gene expression in adipose tissue. Diabetes Metab Res Rev 22:146–154 [DOI] [PubMed] [Google Scholar]
- 329.Grisk O, Frauendorf T, Schluter T, Kloting I, Kuttler B, Krebs A, Ludemann J, Rettig R 2007. Impaired coronary function in Wistar Ottawa Karlsburg W rats—a new model of the metabolic syndrome. Pflugers Arch 454:1011–1021 [DOI] [PubMed] [Google Scholar]
- 330.Brindley DN, Russell JC 2002. Animal models of insulin resistance and cardiovascular disease: some therapeutic approaches using JCR:LA-cp rat. Diabetes Obes Metab 4:1–10 [DOI] [PubMed] [Google Scholar]
- 331.Misra T, Gilchrist JS, Russell JC, Pierce GN 1999. Cardiac myofibrillar and sarcoplasmic reticulum function are not depressed in insulin-resistant JCR:LA-cp rats. Am J Physiol 276:H1811–H1817 [DOI] [PubMed]
- 332.Brunner F, Wolkart G, Pfeiffer S, Russell JC, Wascher TC 2000. Vascular dysfunction and myocardial contractility in the JCR:LA-corpulent rat. Cardiovasc Res 47:150–158 [DOI] [PubMed] [Google Scholar]
- 333.Clark TA, Pierce GN 2000. Cardiovascular complications of non-insulin-dependent diabetes: the JCR:LA-cp rat. J Pharmacol Toxicol Methods 43:1–10 [DOI] [PubMed] [Google Scholar]
- 334.Vine DF, Takechi R, Russell JC, Proctor SD 2007. Impaired postprandial apolipoprotein-B48 metabolism in the obese, insulin-resistant JCR:LA-cp rat: increased atherogenicity for the metabolic syndrome. Atherosclerosis 190:282–290 [DOI] [PubMed] [Google Scholar]
- 335.Zicha J, Pechanova O, Cacanyiova S, Cebova M, Kristek F, Torok J, Simko F, Dobesova Z, Kunes J 2006. Hereditary hypertriglyceridemic rat: a suitable model of cardiovascular disease and metabolic syndrome? Physiol Res 55(Suppl 1):S49–S63 [DOI] [PubMed]
- 336.Kadlecova M, Hojna S, Bohuslavova R, Hubacek JA, Zicha J, Kunes J 2006. Apolipoprotein A5 and hypertriglyceridemia in Prague hypertriglyceridemic rats. Physiol Res 55:373–379 [DOI] [PubMed] [Google Scholar]
- 337.Ueno T, Tremblay J, Kunes J, Zicha J, Dobesova Z, Pausova Z, Deng AY, Sun YL, Jacob HJ, Hamet P 2004. Rat model of familial combined hyperlipidemia as a result of comparative mapping. Physiol Genomics 17:38–47 [DOI] [PubMed] [Google Scholar]
- 338.Sassard J 2006. [Human essential hypertension and genetic hypertension in rats: the Lyon model]. Bull Acad Natl Med 190:111–119; discussion, 119–121 [PubMed] [Google Scholar]
- 339.Bilusic M, Bataillard A, Tschannen MR, Gao L, Barreto NE, Vincent M, Wang T, Jacob HJ, Sassard J, Kwitek AE 2004. Mapping the genetic determinants of hypertension, metabolic diseases, and related phenotypes in the Lyon hypertensive rat. Hypertension 44:695–701 [DOI] [PubMed] [Google Scholar]
- 340.Gilibert S, Kwitek AE, Hubner N, Tschannen M, Jacob HJ, Sassard J, Bataillard AP 2008. The effects of chromosome 17 on features of the metabolic syndrome in the Lyon hypertensive (Lh) rat. Physiol Genomics 33:212–217 [DOI] [PubMed] [Google Scholar]
- 341.Harikai N, Hashimoto A, Semma M, Ichikawa A 2007. Characteristics of lipolysis in white adipose tissues of SHR/NDmc-cp rats, a model of metabolic syndrome. Metabolism 56:847–855 [DOI] [PubMed] [Google Scholar]
- 342.Ernsberger P, Johnson JL, Rosenthal T, Mirelman D, Koletsky RJ 2007. Therapeutic actions of allylmercaptocaptopril and captopril in a rat model of metabolic syndrome. Am J Hypertens 20:866–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Laflamme DP 2006. Understanding and managing obesity in dogs and cats. Vet Clin North Am Small Anim Pract 36:1283–1295, vii [DOI] [PubMed] [Google Scholar]
- 344.Panciera DL, Thomas CB, Eicker SW, Atkins CE 1990. Epizootiologic patterns of diabetes mellitus in cats: 333 cases (1980–1986). J Am Vet Med Assoc 197:1504–1508 [PubMed] [Google Scholar]
- 345.O'Brien TD, Hayden DW, Johnson KH, Fletcher TF 1986. Immunohistochemical morphometry of pancreatic endocrine cells in diabetic, normoglycaemic glucose-intolerant and normal cats. J Comp Pathol 96:357–369 [DOI] [PubMed] [Google Scholar]
- 346.Hoenig M 2006. The cat as a model for human nutrition and disease. Curr Opin Clin Nutr Metab Care 9:584–588 [DOI] [PubMed] [Google Scholar]
- 347.Howard Jr CF 1982. Nonhuman primates as models for the study of human diabetes mellitus. Diabetes 31:37–42 [DOI] [PubMed] [Google Scholar]
- 348.Kavanagh K, Fairbanks LA, Bailey JN, Jorgensen MJ, Wilson M, Zhang L, Rudel LL, Wagner JD 2007. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity (Silver Spring) 15:1666–1674 [DOI] [PubMed] [Google Scholar]
- 349.Tigno XT, Gerzanich G, Hansen BC 2004. Age-related changes in metabolic parameters of nonhuman primates. J Gerontol A Biol Sci Med Sci 59:1081–1088 [DOI] [PubMed] [Google Scholar]
- 350.Ding SY, Tigno XT, Hansen BC 2007. Nuclear magnetic resonance-determined lipoprotein abnormalities in nonhuman primates with the metabolic syndrome and type 2 diabetes mellitus. Metabolism 56:838–846 [DOI] [PubMed] [Google Scholar]
- 351.Bjorntorp P, Rosmond R 1999. Hypothalamic origin of the metabolic syndrome X. Ann NY Acad Sci 892:297–307 [DOI] [PubMed] [Google Scholar]
- 352.Kaufman D, Banerji MA, Shorman I, Smith EL, Coplan JD, Rosenblum LA, Kral JG 2007. Early-life stress and the development of obesity and insulin resistance in juvenile bonnet macaques. Diabetes 56:1382–1386 [DOI] [PubMed] [Google Scholar]
- 353.Pollex RL, Hegele RA 2006. Genetic determinants of the metabolic syndrome. Nat Clin Pract Cardiovasc Med 3:482–489 [DOI] [PubMed] [Google Scholar]
- 354.Sale MM, Woods J, Freedman BI 2006. Genetic determinants of the metabolic syndrome. Curr Hypertens Rep 8:16–22 [DOI] [PubMed] [Google Scholar]
- 355.Teran-Garcia M, Bouchard C 2007. Genetics of the metabolic syndrome. Appl Physiol Nutr Metab 32:89–114 [DOI] [PubMed] [Google Scholar]
- 356.Draper N, Echwald SM, Lavery GG, Walker EA, Fraser R, Davies E, Sorensen TI, Astrup A, Adamski J, Hewison M, Connell JM, Pedersen O, Stewart PM 2002. Association studies between microsatellite markers within the gene encoding human 11β-hydroxysteroid dehydrogenase type 1 and body mass index, waist to hip ratio, and glucocorticoid metabolism. J Clin Endocrinol Metab 87:4984–4990 [DOI] [PubMed] [Google Scholar]
- 357.Heid IM, Wagner SA, Gohlke H, Iglseder B, Mueller JC, Cip P, Ladurner G, Reiter R, Stadlmayr A, Mackevics V, Illig T, Kronenberg F, Paulweber B 2006. Genetic architecture of the APM1 gene and its influence on adiponectin plasma levels and parameters of the metabolic syndrome in 1,727 healthy Caucasians. Diabetes 55:375–384 [DOI] [PubMed] [Google Scholar]
- 358.Kawamura T, Egusa G, Okubo M, Imazu M, Yamakido M 1999. Association of β3-adrenergic receptor gene polymorphism with insulin resistance in Japanese-American men. Metabolism 48:1367–1370 [DOI] [PubMed] [Google Scholar]
- 359.Phillips C, Lopez-Miranda J, Perez-Jimenez F, McManus R, Roche HM 2006. Genetic and nutrient determinants of the metabolic syndrome. Curr Opin Cardiol 21:185–193 [DOI] [PubMed] [Google Scholar]
- 360.Sanchez-Corona J, Flores-Martinez SE, Machorro-Lazo MV, Galaviz-Hernandez C, Moran-Moguel MC, Perea FJ, Mujica-Lopez KI, Vargas-Ancona L, Laviada-Molina HA, Fernandez V, Pardio J, Arroyo P, Barrera H, Hanson RL 2004. Polymorphisms in candidate genes for type 2 diabetes mellitus in a Mexican population with metabolic syndrome findings. Diabetes Res Clin Pract 63:47–55 [DOI] [PubMed] [Google Scholar]
- 361.Rich SS, Bowden DW, Haffner SM, Norris JM, Saad MF, Mitchell BD, Rotter JI, Langefeld CD, Wagenknecht LE, Bergman RN 2004. Identification of quantitative trait loci for glucose homeostasis: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes 53:1866–1875 [DOI] [PubMed] [Google Scholar]
- 362.Bayoumi RA, Al-Yahyaee SA, Albarwani SA, Rizvi SG, Al-Hadabi S, Al-Ubaidi FF, Al-Hinai AT, Al-Kindi MN, Adnan HT, Al-Barwany HS, Comuzzie AG, Cai G, Lopez-Alvarenga JC, Hassan MO 2007. Heritability of determinants of the metabolic syndrome among healthy Arabs of the Oman family study. Obesity (Silver Spring) 15:551–556 [DOI] [PubMed] [Google Scholar]
- 363.Pollex RL, Hanley AJ, Zinman B, Harris SB, Khan HM, Hegele RA 2006. Metabolic syndrome in aboriginal Canadians: prevalence and genetic associations. Atherosclerosis 184:121–129 [DOI] [PubMed] [Google Scholar]
- 364.Pollex RL, Ban MR, Young TK, Bjerregaard P, Anand SS, Yusuf S, Zinman B, Harris SB, Hanley AJ, Connelly PW, Huff MW, Hegele RA 2007. Association between the −455T>C promoter polymorphism of the APOC3 gene and the metabolic syndrome in a multi-ethnic sample. BMC Med Genet 8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Uthurralt J, Gordish-Dressman H, Bradbury M, Tesi-Rocha C, Devaney J, Harmon B, Reeves EK, Brandoli C, Hansen BC, Seip RL, Thompson PD, Price TB, Angelopoulos TJ, Clarkson PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Gordon PM, Hoffman EP 2007. PPARα L162V underlies variation in serum triglycerides and subcutaneous fat volume in young males. BMC Med Genet 8:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Bosy-Westphal A, Onur S, Geisler C, Wolf A, Korth O, Pfeuffer M, Schrezenmeir J, Krawczak M, Muller MJ 2007. Common familial influences on clustering of metabolic syndrome traits with central obesity and insulin resistance: the Kiel obesity prevention study. Int J Obes (Lond) 31:784–790 [DOI] [PubMed] [Google Scholar]
- 367.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I 2006. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension 47:1108–1116 [DOI] [PubMed] [Google Scholar]
- 368.Gonzalez-Sanchez JL, Martinez-Larrad MT, Saez ME, Zabena C, Martinez-Calatrava MJ, Serrano-Rios M 2007. Endothelial nitric oxide synthase haplotypes are associated with features of metabolic syndrome. Clin Chem 53:91–97 [DOI] [PubMed] [Google Scholar]
- 369.Wiedmann S, Fischer M, Koehler M, Neureuther K, Riegger G, Doering A, Schunkert H, Hengstenberg C, Baessler A 2008. Genetic variants within the LPIN1 gene, encoding lipin, are influencing phenotypes of the metabolic syndrome in humans. Diabetes 57:209–217 [DOI] [PubMed] [Google Scholar]
- 370.Gottardo L, De Cosmo S, Zhang YY, Powers C, Prudente S, Marescotti MC, Trischitta V, Avogaro A, Doria A 2008. A polymorphism at the IL6ST (gp130) locus is associated with traits of the metabolic syndrome. Obesity (Silver Spring) 16:205–210 [DOI] [PubMed] [Google Scholar]
- 371.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT 2002. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288:2709–2716 [DOI] [PubMed] [Google Scholar]
- 372.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L 2001. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24:683–689 [DOI] [PubMed] [Google Scholar]
- 373.Sattar N, Gaw A, Scherbakova O, Ford I, O'Reilly DS, Haffner SM, Isles C, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J 2003. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation 108:414–419 [DOI] [PubMed] [Google Scholar]
- 374.Girman CJ, Rhodes T, Mercuri M, Pyorala K, Kjekshus J, Pedersen TR, Beere PA, Gotto AM, Clearfield M 2004. The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Am J Cardiol 93:136–141 [DOI] [PubMed] [Google Scholar]
- 375.Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR 2004. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 110:1245–1250 [DOI] [PubMed] [Google Scholar]
- 376.Olijhoek JK, van der Graaf Y, Banga JD, Algra A, Rabelink TJ, Visseren FL 2004. The metabolic syndrome is associated with advanced vascular damage in patients with coronary heart disease, stroke, peripheral arterial disease or abdominal aortic aneurysm. Eur Heart J 25:342–348 [DOI] [PubMed] [Google Scholar]
- 377.Alexander CM, Landsman PB, Teutsch SM, Haffner SM 2003. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 52:1210–1214 [DOI] [PubMed] [Google Scholar]
- 378.Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS 2004. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation 109:42–46 [DOI] [PubMed] [Google Scholar]
- 379.McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, Ballantyne CM, Heiss G 2005. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care 28:385–390 [DOI] [PubMed] [Google Scholar]
- 380.Solymoss BC, Bourassa MG, Lesperance J, Levesque S, Marcil M, Varga S, Campeau L 2003. Incidence and clinical characteristics of the metabolic syndrome in patients with coronary artery disease. Coron Artery Dis 14:207–212 [DOI] [PubMed] [Google Scholar]
- 381.Turhan H, Yasar AS, Basar N, Bicer A, Erbay AR, Yetkin E 2005. High prevalence of metabolic syndrome among young women with premature coronary artery disease. Coron Artery Dis 16:37–40 [DOI] [PubMed] [Google Scholar]
- 382.Dekker JM, Girman C, Rhodes T, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ 2005. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation 112:666–673 [DOI] [PubMed] [Google Scholar]
- 383.Wannamethee SG, Shaper AG, Lennon L, Morris RW 2005. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med 165:2644–2650 [DOI] [PubMed] [Google Scholar]
- 384.Hong Y, Jin X, Mo J, Lin HM, Duan Y, Pu M, Wolbrette DL, Liao D 2007. Metabolic syndrome, its preeminent clusters, incident coronary heart disease and all-cause mortality—results of prospective analysis for the Atherosclerosis Risk in Communities study. J Intern Med 262:113–122 [DOI] [PubMed] [Google Scholar]
- 385.Meigs JB, Rutter MK, Sullivan LM, Fox CS, D'Agostino Sr RB, Wilson PW 2007. Impact of insulin resistance on risk of type 2 diabetes and cardiovascular disease in people with metabolic syndrome. Diabetes Care 30:1219–1225 [DOI] [PubMed] [Google Scholar]
- 386.Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K 2004. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med 164:1066–1076 [DOI] [PubMed] [Google Scholar]
- 387.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM 2007. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol 49:403–414 [DOI] [PubMed] [Google Scholar]
- 388.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB 2005. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 112:3066–3072 [DOI] [PubMed] [Google Scholar]
- 389.Daly CA, Hildebrandt P, Bertrand M, Ferrari R, Remme W, Simoons M, Fox KM 2007. Adverse prognosis associated with the metabolic syndrome in established coronary artery disease. Data from the EUROPA trial. Heart 93:1406–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB 2006. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 91:2906–2912 [DOI] [PubMed] [Google Scholar]
- 391.McNeill AM, Katz R, Girman CJ, Rosamond WD, Wagenknecht LE, Barzilay JI, Tracy RP, Savage PJ, Jackson SA 2006. Metabolic syndrome and cardiovascular disease in older people: the Cardiovascular Health Study. J Am Geriatr Soc 54:1317–1324 [DOI] [PubMed] [Google Scholar]
- 392.Klein BE, Klein R, Lee KE 2002. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care 25:1790–1794 [DOI] [PubMed] [Google Scholar]
- 393.Bruno G, Merletti F, Biggeri A, Bargero G, Ferrero S, Runzo C, Prina Cerai S, Pagano G, Cavallo-Perin P 2004. Metabolic syndrome as a predictor of all-cause and cardiovascular mortality in type 2 diabetes: the Casale Monferrato Study. Diabetes Care 27:2689–2694 [DOI] [PubMed] [Google Scholar]
- 394.Sattar N, McConnachie A, Shaper AG, Blauw GJ, Buckley BM, de Craen AJ, Ford I, Forouhi NG, Freeman DJ, Jukema JW, Lennon L, Macfarlane PW, Murphy MB, Packard CJ, Stott DJ, Westendorp RG, Whincup PH, Shepherd J, Wannamethee SG 2008. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet 371:1927–1935 [DOI] [PubMed] [Google Scholar]
- 395.Resnick HE, Jones K, Ruotolo G, Jain AK, Henderson J, Lu W, Howard BV 2003. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease in nondiabetic American Indians: the Strong Heart Study. Diabetes Care 26:861–867 [DOI] [PubMed] [Google Scholar]
- 396.Kragelund C, Kober L, Faber J, Steffensen R, Hildebrandt P 2007. Metabolic syndrome and mortality in stable coronary heart disease: relation to gender. Int J Cardiol 121:62–67 [DOI] [PubMed] [Google Scholar]
- 397.Grundy SM 2006. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol 47:1093–1100 [DOI] [PubMed] [Google Scholar]
- 398.Kannel WB, Larson M 1993. Long-term epidemiologic prediction of coronary disease. The Framingham experience. Cardiology 82:137–152 [DOI] [PubMed] [Google Scholar]
- 399.Golden SH, Folsom AR, Coresh J, Sharrett AR, Szklo M, Brancati F 2002. Risk factor groupings related to insulin resistance and their synergistic effects on subclinical atherosclerosis: the Atherosclerosis Risk in Communities Study. Diabetes 51:3069–3076 [DOI] [PubMed] [Google Scholar]
- 400.Assmann G, Guerra R, Fox G, Cullen P, Schulte H, Willett D, Grundy SM 2007. Harmonizing the definition of the metabolic syndrome: comparison of the criteria of the Adult Treatment Panel III and the International Diabetes Federation in United States American and European populations. Am J Cardiol 99:541–548 [DOI] [PubMed] [Google Scholar]
- 401.Gregg EW, Cadwell BL, Cheng YJ, Cowie CC, Williams DE, Geiss L, Engelgau MM, Vinicor F 2004. Trends in the prevalence and ratio of diagnosed to undiagnosed diabetes according to obesity levels in the U.S. Diabetes Care 27:2806–2812 [DOI] [PubMed] [Google Scholar]
- 402.2005 Prevention CfDCa. National diabetes fact sheet: general information and national estimates on diabetes in the United States. Atlanta: Health and Human Services, Centers for Disease Control and Prevention
- 403.Reaven GM 2005. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med 47:201–210 [PubMed] [Google Scholar]
- 404.Lebovitz HE 1999. Type 2 diabetes: an overview. Clin Chem 45:1339–1345 [PubMed] [Google Scholar]
- 405.Grundy SM, Brewer Jr HB, Cleeman JI, Smith Jr SC, Lenfant C 2004. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol 24:e13–e18 [DOI] [PubMed]
- 406.Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA 2002. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol 156:1070–1077 [DOI] [PubMed] [Google Scholar]
- 407.Hanson RL, Imperatore G, Bennett PH, Knowler WC 2002. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes 51:3120–3127 [DOI] [PubMed] [Google Scholar]
- 408.Ford ES, Li C, Sattar N 2008. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care 31:1898–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Bonora E, Targher G, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, Gemma L, Santi L, Bonadonna RC, Muggeo M 2004. The metabolic syndrome is an independent predictor of cardiovascular disease in type 2 diabetic subjects. Prospective data from the Verona Diabetes Complications Study. Diabet Med 21:52–58 [DOI] [PubMed] [Google Scholar]
- 410.de Vegt F, Dekker JM, Jager A, Hienkens E, Kostense PJ, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ 2001. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: the Hoorn Study. JAMA 285:2109–2113 [DOI] [PubMed] [Google Scholar]
- 411.Hanley AJ, Karter AJ, Williams K, Festa A, D'Agostino Jr RB, Wagenknecht LE, Haffner SM 2005. Prediction of type 2 diabetes mellitus with alternative definitions of the metabolic syndrome: the Insulin Resistance Atherosclerosis Study. Circulation 112:3713–3721 [DOI] [PubMed] [Google Scholar]
- 412.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM 2003. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio Heart Study. Diabetes Care 26:3153–3159 [DOI] [PubMed] [Google Scholar]
- 413.Stern MP, Williams K, Gonzalez-Villalpando C, Hunt KJ, Haffner SM 2004. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care 27:2676–2681 [DOI] [PubMed] [Google Scholar]
- 414.Pyorala M, Miettinen H, Halonen P, Laakso M, Pyorala K 2000. Insulin resistance syndrome predicts the risk of coronary heart disease and stroke in healthy middle-aged men: the 22-year follow-up results of the Helsinki Policemen Study. Arterioscler Thromb Vasc Biol 20:538–544 [DOI] [PubMed] [Google Scholar]
- 415.Jovanovic L, Pettitt DJ 2001. Gestational diabetes mellitus. JAMA 286:2516–2518 [DOI] [PubMed] [Google Scholar]
- 416.Kim C, Newton KM, Knopp RH 2002. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 25:1862–1868 [DOI] [PubMed] [Google Scholar]
- 417.Lauenborg J, Mathiesen E, Hansen T, Glumer C, Jorgensen T, Borch-Johnsen K, Hornnes P, Pedersen O, Damm P 2005. The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab 90:4004–4010 [DOI] [PubMed] [Google Scholar]
- 418.Verma A, Boney CM, Tucker R, Vohr BR 2002. Insulin resistance syndrome in women with prior history of gestational diabetes mellitus. J Clin Endocrinol Metab 87:3227–3235 [DOI] [PubMed] [Google Scholar]
- 419.Clark JM, Diehl AM 2003. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA 289:3000–3004 [DOI] [PubMed] [Google Scholar]
- 420.Bloomgarden ZT 2005. Second World Congress on the Insulin Resistance Syndrome: insulin resistance syndrome and nonalcoholic fatty liver disease. Diabetes Care 28:1518–1523 [DOI] [PubMed] [Google Scholar]
- 421.Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, Cha BS 2004. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med 164:2169–2175 [DOI] [PubMed] [Google Scholar]
- 422.Bloomgarden ZT 2007. Nonalcoholic fatty liver disease and insulin resistance in youth. Diabetes Care 30:1663–1669 [DOI] [PubMed] [Google Scholar]
- 423.Kotronen A, Westerbacka J, Bergholm R, Pietilainen KH, Yki-Jarvinen H 2007. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab 92:3490–3497 [DOI] [PubMed] [Google Scholar]
- 424.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, Kato T, Okuda J, Ida K 2005. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 143:722–728 [DOI] [PubMed] [Google Scholar]
- 425.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ 2005. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Mendez-Sanchez N, Arrese M, Zamora-Valdes D, Uribe M 2007. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int 27:423–433 [DOI] [PubMed] [Google Scholar]
- 427.Shulman AI, Mangelsdorf DJ 2005. Retinoid x receptor heterodimers in the metabolic syndrome. N Engl J Med 353:604–615 [DOI] [PubMed] [Google Scholar]
- 428.Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A, de Iasio R, Gentilcore E, Natale S, Cassader M, Rizzetto M, Pasquali R, Marchesini G 2005. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab 90:3498–3504 [DOI] [PubMed] [Google Scholar]
- 429.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR 2008. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab 7:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J 1999. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 22:141–146 [DOI] [PubMed] [Google Scholar]
- 431.Cho LW, Atkin SL 2007. Cardiovascular risk in women with polycystic ovary syndrome. Minerva Endocrinol 32:263–273 [PubMed] [Google Scholar]
- 432.Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN 2006. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 91:48–53 [DOI] [PubMed] [Google Scholar]
- 433.Alvarez-Blasco F, Botella-Carretero JI, San Millan JL, Escobar-Morreale HF 2006. Prevalence and characteristics of the polycystic ovary syndrome in overweight and obese women. Arch Intern Med 166:2081–2086 [DOI] [PubMed] [Google Scholar]
- 434.Legro RS 2007. A 27-year-old woman with a diagnosis of polycystic ovary syndrome. JAMA 297:509–519 [DOI] [PubMed] [Google Scholar]
- 435.Legro RS, Kunselman AR, Dodson WC, Dunaif A 1999. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84:165–169 [DOI] [PubMed] [Google Scholar]
- 436.Talbott EO, Guzick DS, Sutton-Tyrrell K, McHugh-Pemu KP, Zborowski JV, Remsberg KE, Kuller LH 2000. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol 20:2414–2421 [DOI] [PubMed] [Google Scholar]
- 437.Orio Jr F, Palomba S, Spinelli L, Cascella T, Tauchmanova L, Zullo F, Lombardi G, Colao A 2004. The cardiovascular risk of young women with polycystic ovary syndrome: an observational, analytical, prospective case-control study. J Clin Endocrinol Metab 89:3696–3701 [DOI] [PubMed] [Google Scholar]
- 438.Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy 2nd PF, Fitzpatrick LA 2003. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab 88:2562–2568 [DOI] [PubMed] [Google Scholar]
- 439.Tasali E, Van Cauter E 2002. Sleep-disordered breathing and the current epidemic of obesity: consequence or contributing factor? Am J Respir Crit Care Med 165:562–563 [DOI] [PubMed] [Google Scholar]
- 440.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL 2002. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 165:677–682 [DOI] [PubMed] [Google Scholar]
- 441.Peled N, Kassirer M, Shitrit D, Kogan Y, Shlomi D, Berliner AS, Kramer MR 2007. The association of OSA with insulin resistance, inflammation and metabolic syndrome. Respir Med 101:1696–1701 [DOI] [PubMed] [Google Scholar]
- 442.Zhang XL, Yin KS, Wang H, Su S 2006. Serum adiponectin levels in adult male patients with obstructive sleep apnea hypopnea syndrome. Respiration 73:73–77 [DOI] [PubMed] [Google Scholar]
- 443.Masserini B, Morpurgo PS, Donadio F, Baldessari C, Bossi R, Beck-Peccoz P, Orsi E 2006. Reduced levels of adiponectin in sleep apnea syndrome. J Endocrinol Invest 29:700–705 [DOI] [PubMed] [Google Scholar]
- 444.Parish JM, Adam T, Facchiano L 2007. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med 3:467–472 [PMC free article] [PubMed] [Google Scholar]
- 445.Gami AS, Somers VK 2004. Obstructive sleep apnoea, metabolic syndrome, and cardiovascular outcomes. Eur Heart J 25:709–711 [DOI] [PubMed] [Google Scholar]
- 446.Gruber A, Horwood F, Sithole J, Ali NJ, Idris I 2006. Obstructive sleep apnoea is independently associated with the metabolic syndrome but not insulin resistance state. Cardiovasc Diabetol 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Cizza G, Skarulis M, Mignot E 2005. A link between short sleep and obesity: building the evidence for causation. Sleep 28:1217–1220 [DOI] [PubMed] [Google Scholar]
- 448.Vgontzas AN, Bixler EO, Chrousos GP 2005. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev 9:211–224 [DOI] [PubMed] [Google Scholar]
- 449.Wolk R, Somers VK 2007. Sleep and the metabolic syndrome. Exp Physiol 92:67–78 [DOI] [PubMed] [Google Scholar]
- 450.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS 2002. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 165:670–676 [DOI] [PubMed] [Google Scholar]
- 451.Corona G, Mannucci E, Petrone L, Balercia G, Paggi F, Fisher AD, Lotti F, Chiarini V, Fedele D, Forti G, Maggi M 2007. NCEP-ATPIII-defined metabolic syndrome, type 2 diabetes mellitus, and prevalence of hypogonadism in male patients with sexual dysfunction. J Sex Med 4:1038–1045 [DOI] [PubMed] [Google Scholar]
- 452.Chen RY, Wittert GA, Andrews GR 2006. Relative androgen deficiency in relation to obesity and metabolic status in older men. Diabetes Obes Metab 8:429–435 [DOI] [PubMed] [Google Scholar]
- 453.Kalyani RR, Dobs AS 2007. Androgen deficiency, diabetes, and the metabolic syndrome in men. Curr Opin Endocrinol Diabetes Obes 14:226–234 [DOI] [PubMed] [Google Scholar]
- 454.Saad F, Gooren LJ, Haider A, Yassin A 2008. A dose response study of testosterone on sexual dysfunction and on features of the metabolic syndrome using testosterone gel and parenteral testosterone undecanoate. J Androl 29:102–105 [DOI] [PubMed] [Google Scholar]
- 455.Heidler S, Temml C, Broessner C, Mock K, Rauchenwald M, Madersbacher S, Ponholzer A 2007. Is the metabolic syndrome an independent risk factor for erectile dysfunction? J Urol 177:651–654 [DOI] [PubMed] [Google Scholar]
- 456.Demir T, Demir O, Kefi A, Comlekci A, Yesil S, Esen A 2006. Prevalence of erectile dysfunction in patients with metabolic syndrome. Int J Urol 13:385–388 [DOI] [PubMed] [Google Scholar]
- 457.Bansal TC, Guay AT, Jacobson J, Woods BO, Nesto RW 2005. Incidence of metabolic syndrome and insulin resistance in a population with organic erectile dysfunction. J Sex Med 2:96–103 [DOI] [PubMed] [Google Scholar]
- 458.Bal K, Oder M, Sahin AS, Karatas CT, Demir O, Can E, Gumus BH, Ozer K, Sahin O, Esen AA 2007. Prevalence of metabolic syndrome and its association with erectile dysfunction among urologic patients: metabolic backgrounds of erectile dysfunction. Urology 69:356–360 [DOI] [PubMed] [Google Scholar]
- 459.Carr MC 2003. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88:2404–2411 [DOI] [PubMed] [Google Scholar]
- 460.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR 2008. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 32:949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Mackey RH, Kuller LH, Sutton-Tyrrell K, Evans RW, Holubkov R, Matthews KA 2005. Hormone therapy, lipoprotein subclasses, and coronary calcification: the Healthy Women Study. Arch Intern Med 165:510–515 [DOI] [PubMed] [Google Scholar]
- 462.Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E 2003. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/Progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 138:1–9 [DOI] [PubMed] [Google Scholar]
- 463.Espeland MA, Stefanick ML, Kritz-Silverstein D, Fineberg SE, Waclawiw MA, James MK, Greendale GA 1997. Effect of postmenopausal hormone therapy on body weight and waist and hip girths. Postmenopausal Estrogen-Progestin Interventions Study Investigators. J Clin Endocrinol Metab 82:1549–1556 [DOI] [PubMed] [Google Scholar]
- 464.Gambacciani M, Ciaponi M, Cappagli B, De Simone L, Orlandi R, Genazzani AR 2001. Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormonal replacement therapy. Maturitas 39:125–132 [DOI] [PubMed] [Google Scholar]
- 465.Bonds DE, Lasser N, Qi L, Brzyski R, Caan B, Heiss G, Limacher MC, Liu JH, Mason E, Oberman A, O'Sullivan MJ, Phillips LS, Prineas RJ, Tinker L 2006. The effect of conjugated equine oestrogen on diabetes incidence: the Women’s Health Initiative Randomised Trial. Diabetologia 49:459–468 [DOI] [PubMed] [Google Scholar]
- 466.Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV 2004. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 47:1175–1187 [DOI] [PubMed] [Google Scholar]
- 467.Garg A 2004. Acquired and inherited lipodystrophies. N Engl J Med 350:1220–1234 [DOI] [PubMed] [Google Scholar]
- 468.Monajemi H, Stroes E, Hegele RA, Fliers E 2007. Inherited lipodystrophies and the metabolic syndrome. Clin Endocrinol (Oxf) 67:479–484 [DOI] [PubMed] [Google Scholar]
- 469.Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A 2007. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care 30:113–119 [DOI] [PubMed] [Google Scholar]
- 470.Falasca K, Ucciferri C, Manzoli L, Mancino P, Pizzigallo E, Conti P, Vecchiet J 2007. Metabolic syndrome and cardiovascular risk in HIV-infected patients with lipodystrophy. Int J Immunopathol Pharmacol 20:519–527 [DOI] [PubMed] [Google Scholar]
- 471.Diabetes Prevention Program Research Group 2007. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med 24:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Tyrberg M, Melander A, Lovestam-Adrian M, Lindblad U 2007. Retinopathy in subjects with impaired fasting glucose: the NANSY-Eye baseline report. Diabetes Obes Metab 10:646–651 [DOI] [PubMed] [Google Scholar]
- 473.Rashidi A, Ghanbarian A, Azizi F 2007. Are patients who have metabolic syndrome without diabetes at risk for developing chronic kidney disease? Evidence based on data from a large cohort screening population. Clin J Am Soc Nephrol 2:976–983 [DOI] [PubMed] [Google Scholar]
- 474.Kurella M, Lo JC, Chertow GM 2005. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol 16:2134–2140 [DOI] [PubMed] [Google Scholar]
- 475.Ninomiya T, Kiyohara Y, Kubo M, Yonemoto K, Tanizaki Y, Doi Y, Hirakata H, Iida M 2006. Metabolic syndrome and CKD in a general Japanese population: the Hisayama Study. Am J Kidney Dis 48:383–391 [DOI] [PubMed] [Google Scholar]
- 476.Klausen KP, Parving HH, Scharling H, Jensen JS 2007. The association between metabolic syndrome, microalbuminuria and impaired renal function in the general population: impact on cardiovascular disease and mortality. J Intern Med 262:470–478 [DOI] [PubMed] [Google Scholar]
- 477.Lin CC, Liu CS, Li TC, Chen CC, Li CI, Lin WY 2007. Microalbuminuria and the metabolic syndrome and its components in the Chinese population. Eur J Clin Invest 37:783–790 [DOI] [PubMed] [Google Scholar]
- 478.Diamantopoulos EJ, Andreadis EA, Tsourous GI, Katsanou PM, Georgiopoulos DX, Nestora KC, Raptis SA 2006. Early vascular lesions in subjects with metabolic syndrome and prediabetes. Int Angiol 25:179–183 [PubMed] [Google Scholar]
- 479.Gordon Smith A, Robinson Singleton J 2006. Idiopathic neuropathy, prediabetes and the metabolic syndrome. J Neurol Sci 242:9–14 [DOI] [PubMed] [Google Scholar]
- 480.Bonadonna RC, Cucinotta D, Fedele D, Riccardi G, Tiengo A 2006. The metabolic syndrome is a risk indicator of microvascular and macrovascular complications in diabetes: results from Metascreen, a multicenter diabetes clinic-based survey. Diabetes Care 29:2701–2707 [DOI] [PubMed] [Google Scholar]
- 481.Krentz AJ, Clough G, Byrne CD 2007. Interactions between microvascular and macrovascular disease in diabetes: pathophysiology and therapeutic implications. Diabetes Obes Metab 9:781–791 [DOI] [PubMed] [Google Scholar]
- 482.Cull CA, Jensen CC, Retnakaran R, Holman RR 2007. Impact of the metabolic syndrome on macrovascular and microvascular outcomes in type 2 diabetes mellitus: United Kingdom Prospective Diabetes Study 78. Circulation 116:2119–2126 [DOI] [PubMed] [Google Scholar]
- 483.Pasanisi F, Contaldo F, de Simone G, Mancini M 2001. Benefits of sustained moderate weight loss in obesity. Nutr Metab Cardiovasc Dis 11:401–406 [PubMed] [Google Scholar]
- 484.Ilanne-Parikka P, Eriksson JG, Lindstrom J, Peltonen M, Aunola S, Hamalainen H, Keinanen-Kiukaanniemi S, Laakso M, Valle TT, Lahtela J, Uusitupa M, Tuomilehto J 2008. Effect of lifestyle intervention on the occurrence of metabolic syndrome and its components in the Finnish Diabetes Prevention Study. Diabetes Care 31:805–807 [DOI] [PubMed] [Google Scholar]
- 485.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S 2005. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program Randomized Trial. Ann Intern Med 142:611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Van Gaal LF, Wauters MA, De Leeuw IH 1997. The beneficial effects of modest weight loss on cardiovascular risk factors. Int J Obes Relat Metab Disord 21(Suppl 1):S5–S9 [PubMed]
- 487.Stamler R, Stamler J, Grimm R, Gosch FC, Elmer P, Dyer A, Berman R, Fishman J, Van Heel N, Civinelli J, McDonald A 1987. Nutritional therapy for high blood pressure. Final report of a four-year randomized controlled trial—the Hypertension Control Program. JAMA 257:1484–1491 [DOI] [PubMed] [Google Scholar]
- 488.Langford HG, Blaufox MD, Oberman A, Hawkins CM, Curb JD, Cutter GR, Wassertheil-Smoller S, Pressel S, Babcock C, Abernethy JD, Hotchkiss J, Tyler M 1985. Dietary therapy slows the return of hypertension after stopping prolonged medication. JAMA 253:657–664 [PubMed] [Google Scholar]
- 489.Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger Jr WH, Kostis JB, Kumanyika S, Lacy CR, Johnson KC, Folmar S, Cutler JA 1998. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA 279:839–846 [DOI] [PubMed] [Google Scholar]
- 490.Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D 1987. Long-term effects of modest weight loss in type II diabetic patients. Arch Intern Med 147:1749–1753 [PubMed] [Google Scholar]
- 491.Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M 1993. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 77:1287–1293 [DOI] [PubMed] [Google Scholar]
- 492.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J 2006. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 29:2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Lundgren H, Bengtsson C, Blohme G, Isaksson B, Lapidus L, Lenner RA, Saaek A, Winther E 1989. Dietary habits and incidence of noninsulin-dependent diabetes mellitus in a population study of women in Gothenburg, Sweden. Am J Clin Nutr 49:708–712 [DOI] [PubMed] [Google Scholar]
- 494.Marshall JA, Hamman RF, Baxter J 1991. High-fat, low-carbohydrate diet and the etiology of non-insulin-dependent diabetes mellitus: the San Luis Valley Diabetes Study. Am J Epidemiol 134:590–603 [DOI] [PubMed] [Google Scholar]
- 495.McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PW, Jacques PF 2004. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 27:538–546 [DOI] [PubMed] [Google Scholar]
- 496.Ma Y, Li Y, Chiriboga DE, Olendzki BC, Hebert JR, Li W, Leung K, Hafner AR, Ockene IS 2006. Association between carbohydrate intake and serum lipids. J Am Coll Nutr 25:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Liese AD, Gilliard T, Schulz M, D'Agostino Jr RB, Wolever TM 2007. Carbohydrate nutrition, glycaemic load, and plasma lipids: the Insulin Resistance Atherosclerosis Study. Eur Heart J 28:80–87 [DOI] [PubMed] [Google Scholar]
- 498.Harber MP, Schenk S, Barkan AL, Horowitz JF 2005. Alterations in carbohydrate metabolism in response to short-term dietary carbohydrate restriction. Am J Physiol Endocrinol Metab 289:E306–E312 [DOI] [PubMed]
- 499.Brehm BJ, Seeley RJ, Daniels SR, D'Alessio DA 2003. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab 88:1617–1623 [DOI] [PubMed] [Google Scholar]
- 500.Yancy Jr WS, Olsen MK, Guyton JR, Bakst RP, Westman EC 2004. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med 140:769–777 [DOI] [PubMed] [Google Scholar]
- 501.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L 2003. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 348:2074–2081 [DOI] [PubMed] [Google Scholar]
- 502.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC 2007. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA 297:969–977 [DOI] [PubMed] [Google Scholar]
- 503.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ 2005. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 293:43–53 [DOI] [PubMed] [Google Scholar]
- 504.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S 2003. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 348:2082–2090 [DOI] [PubMed] [Google Scholar]
- 505.Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams M, Gracely EJ, Samaha FF 2004. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med 140:778–785 [DOI] [PubMed] [Google Scholar]
- 506.Guthrie JF, Morton JF 2000. Food sources of added sweeteners in the diets of Americans. J Am Diet Assoc 100:43–51; quiz, 49–50 [DOI] [PubMed] [Google Scholar]
- 507.Lewis CJ, Park YK, Dexter PB, Yetley EA 1992. Nutrient intakes and body weights of persons consuming high and moderate levels of added sugars. J Am Diet Assoc 92:708–713 [PubMed] [Google Scholar]
- 508.Jenkins DJ, Kendall CW, Augustin LS, Franceschi S, Hamidi M, Marchie A, Jenkins AL, Axelsen M 2002. Glycemic index: overview of implications in health and disease. Am J Clin Nutr 76:266S–273S [DOI] [PubMed] [Google Scholar]
- 509.Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, Hu FB 2006. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med 355:1991–2002 [DOI] [PubMed] [Google Scholar]
- 510.Lutsey PL, Steffen LM, Stevens J 2008. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation 117:754–761 [DOI] [PubMed] [Google Scholar]
- 511.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM 2004. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 291:2847–2850 [DOI] [PubMed] [Google Scholar]
- 512.Ford ES, Giles WH, Mokdad AH 2004. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care 27:2444–2449 [DOI] [PubMed] [Google Scholar]
- 513.Borkman M, Campbell LV, Chisholm DJ, Storlien LH 1991. Comparison of the effects on insulin sensitivity of high carbohydrate and high fat diets in normal subjects. J Clin Endocrinol Metab 72:432–437 [DOI] [PubMed] [Google Scholar]
- 514.Garg A, Grundy SM, Unger RH 1992. Comparison of effects of high and low carbohydrate diets on plasma lipoproteins and insulin sensitivity in patients with mild NIDDM. Diabetes 41:1278–1285 [PubMed] [Google Scholar]
- 515.Sarkkinen E, Schwab U, Niskanen L, Hannuksela M, Savolainen M, Kervinen K, Kesaniemi A, Uusitupa MI 1996. The effects of monounsaturated-fat enriched diet and polyunsaturated-fat enriched diet on lipid and glucose metabolism in subjects with impaired glucose tolerance. Eur J Clin Nutr 50:592–598 [PubMed] [Google Scholar]
- 516.Bisschop PH, de Metz J, Ackermans MT, Endert E, Pijl H, Kuipers F, Meijer AJ, Sauerwein HP, Romijn JA 2001. Dietary fat content alters insulin-mediated glucose metabolism in healthy men. Am J Clin Nutr 73:554–559 [DOI] [PubMed] [Google Scholar]
- 517.Mayer EJ, Newman B, Quesenberry Jr CP, Selby JV 1993. Usual dietary fat intake and insulin concentrations in healthy women twins. Diabetes Care 16:1459–1469 [DOI] [PubMed] [Google Scholar]
- 518.Cornier MA, Donahoo WT, Pereira R, Gurevich I, Westergren R, Enerback S, Eckel PJ, Goalstone ML, Hill JO, Eckel RH, Draznin B 2005. Insulin sensitivity determines the effectiveness of dietary macronutrient composition on weight loss in obese women. Obes Res 13:703–709 [DOI] [PubMed] [Google Scholar]
- 519.Maron DJ, Fair JM, Haskell WL 1991. Saturated fat intake and insulin resistance in men with coronary artery disease. The Stanford Coronary Risk Intervention Project Investigators and Staff. Circulation 84:2020–2027 [DOI] [PubMed] [Google Scholar]
- 520.Parker DR, Weiss ST, Troisi R, Cassano PA, Vokonas PS, Landsberg L 1993. Relationship of dietary saturated fatty acids and body habitus to serum insulin concentrations: the Normative Aging Study. Am J Clin Nutr 58:129–136 [DOI] [PubMed] [Google Scholar]
- 521.Heine RJ 1993. Dietary fish oil and insulin action in humans. Ann NY Acad Sci 683:110–121 [DOI] [PubMed] [Google Scholar]
- 522.Uusitupa M, Schwab U, Makimattila S, Karhapaa P, Sarkkinen E, Maliranta H, Agren J, Penttila I 1994. Effects of two high-fat diets with different fatty acid compositions on glucose and lipid metabolism in healthy young women. Am J Clin Nutr 59:1310–1316 [DOI] [PubMed] [Google Scholar]
- 523.Schwab U, Uusitupa M, Karhapaa P, Makimattila S, Rasanen M, Makinen E, Laakso M 1993. Effects of two fat-modified diets on glucose and lipid metabolism in healthy subjects. Ann NY Acad Sci 683:279–280 [DOI] [PubMed] [Google Scholar]
- 524.Fasching P, Ratheiser K, Schneeweiss B, Rohac M, Nowotny P, Waldhausl W 1996. No effect of short-term dietary supplementation of saturated and poly- and monounsaturated fatty acids on insulin secretion and sensitivity in healthy men. Ann Nutr Metab 40:116–122 [DOI] [PubMed] [Google Scholar]
- 525.Lovejoy JC, Smith SR, Champagne CM, Most MM, Lefevre M, DeLany JP, Denkins YM, Rood JC, Veldhuis J, Bray GA 2002. Effects of diets enriched in saturated (palmitic), monounsaturated (oleic), or trans (elaidic) fatty acids on insulin sensitivity and substrate oxidation in healthy adults. Diabetes Care 25:1283–1288 [DOI] [PubMed] [Google Scholar]
- 526.Summers LK, Fielding BA, Bradshaw HA, Ilic V, Beysen C, Clark ML, Moore NR, Frayn KN 2002. Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia 45:369–377 [DOI] [PubMed] [Google Scholar]
- 527.Xiao C, Giacca A, Carpentier A, Lewis GF 2006. Differential effects of monounsaturated, polyunsaturated and saturated fat ingestion on glucose-stimulated insulin secretion, sensitivity and clearance in overweight and obese, non-diabetic humans. Diabetologia 49:1371–1379 [DOI] [PubMed] [Google Scholar]
- 528.Budohoski L, Panczenko-Kresowska B, Langfort J, Zernicka E, Dubaniewicz A, Ziemlanski S, Challiss RA, Newsholme EA 1993. Effects of saturated and polyunsaturated fat enriched diet on the skeletal muscle insulin sensitivity in young rats. J Physiol Pharmacol 44:391–398 [PubMed] [Google Scholar]
- 529.Keys A, Parlin RW 1966. Serum cholesterol response to changes in dietary lipids. Am J Clin Nutr 19:175–181 [DOI] [PubMed] [Google Scholar]
- 530.Dayton S, Pearce ML 1969. Diet high in unsaturated fat. A controlled clinical trial. Minn Med 52:1237–1242 [DOI] [PubMed] [Google Scholar]
- 531.Turpeinen O, Karvonen MJ, Pekkarinen M, Miettinen M, Elosuo R, Paavilainen E 1979. Dietary prevention of coronary heart disease: the Finnish Mental Hospital Study. Int J Epidemiol 8:99–118 [DOI] [PubMed] [Google Scholar]
- 532.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N 1999. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 99:779–785 [DOI] [PubMed] [Google Scholar]
- 533.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC 1997. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 337:1491–1499 [DOI] [PubMed] [Google Scholar]
- 534.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM 2006. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 47:296–308 [DOI] [PubMed] [Google Scholar]
- 535.Dahl LK 1972. Salt and hypertension. Am J Clin Nutr 25:231–244 [DOI] [PubMed] [Google Scholar]
- 536.Johnson AG, Nguyen TV, Davis D 2001. Blood pressure is linked to salt intake and modulated by the angiotensinogen gene in normotensive and hypertensive elderly subjects. J Hypertens 19:1053–1060 [DOI] [PubMed] [Google Scholar]
- 537.MacGregor GA, Markandu ND, Sagnella GA, Singer DR, Cappuccio FP 1989. Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet 2:1244–1247 [DOI] [PubMed] [Google Scholar]
- 538.1997 Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med 157:657–667 [PubMed] [Google Scholar]
- 539.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller 3rd ER, Simons-Morton DG, Karanja N, Lin PH 2001. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 344:3–10 [DOI] [PubMed] [Google Scholar]
- 540.He J, Ogden LG, Vupputuri S, Bazzano LA, Loria C, Whelton PK 1999. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA 282:2027–2034 [DOI] [PubMed] [Google Scholar]
- 541.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK 2002. Dietary sodium intake and incidence of congestive heart failure in overweight US men and women: first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med 162:1619–1624 [DOI] [PubMed] [Google Scholar]
- 542.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, Jones DW, Materson BJ, Oparil S, Wright Jr JT, Roccella EJ 2003. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42:1206–1252 [DOI] [PubMed] [Google Scholar]
- 543.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ 1997. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 277:1624–1632 [DOI] [PubMed] [Google Scholar]
- 544.2006 American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of hypertension. Endocr Pract 12:193–222 [PubMed] [Google Scholar]
- 545.Ardern CI, Katzmarzyk PT, Janssen I, Church TS, Blair SN 2005. Revised Adult Treatment Panel III guidelines and cardiovascular disease mortality in men attending a preventive medical clinic. Circulation 112:1478–1485 [DOI] [PubMed] [Google Scholar]
- 546.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN 2000. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 132:605–611 [DOI] [PubMed] [Google Scholar]
- 547.Oguma Y, Shinoda-Tagawa T 2004. Physical activity decreases cardiovascular disease risk in women: review and meta-analysis. Am J Prev Med 26:407–418 [DOI] [PubMed] [Google Scholar]
- 548.Yates T, Khunti K, Bull F, Gorely T, Davies MJ 2007. The role of physical activity in the management of impaired glucose tolerance: a systematic review. Diabetologia 50:1116–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl III HW, Blair SN 1997. Reduction in cardiovascular disease risk factors: 6-month results from Project Active. Prev Med 26:883–892 [DOI] [PubMed] [Google Scholar]
- 550.King DE, Mainous III AG, Geesey ME 2007. Turning back the clock: adopting a healthy lifestyle in middle age. Am J Med 120:598–603 [DOI] [PubMed] [Google Scholar]
- 551.Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA 2002. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care 25:1612–1618 [DOI] [PubMed] [Google Scholar]
- 552.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wareham NJ 2005. Physical activity energy expenditure predicts progression toward the metabolic syndrome independently of aerobic fitness in middle-aged healthy Caucasians: the Medical Research Council Ely Study. Diabetes Care 28:1195–1200 [DOI] [PubMed] [Google Scholar]
- 553.Ford ES, Kohl III HW, Mokdad AH, Ajani UA 2005. Sedentary behavior, physical activity, and the metabolic syndrome among U.S. adults. Obes Res 13:608–614 [DOI] [PubMed] [Google Scholar]
- 554.LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN 2005. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation 112:505–512 [DOI] [PubMed] [Google Scholar]
- 555.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E 2007. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab 92:865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, Nguyen-Duy TB, Lee S, Kilpatrick K, Hudson R 2004. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res 12:789–798 [DOI] [PubMed] [Google Scholar]
- 557.Janssen I, Fortier A, Hudson R, Ross R 2002. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care 25:431–438 [DOI] [PubMed] [Google Scholar]
- 558.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I 2000. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 133:92–103 [DOI] [PubMed] [Google Scholar]
- 559.Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I 2007. A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int J Obes (Lond) 31:1786–1797 [DOI] [PubMed] [Google Scholar]
- 560.Wing RR, Hill JO 2001. Successful weight loss maintenance. Annu Rev Nutr 21:323–341 [DOI] [PubMed] [Google Scholar]
- 561.Hill JO, Wyatt HR 2005. Role of physical activity in preventing and treating obesity. J Appl Physiol 99:765–770 [DOI] [PubMed] [Google Scholar]
- 562.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G 2003. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med 139:802–809 [DOI] [PubMed] [Google Scholar]
- 563.Jessen N, Goodyear LJ 2005. Contraction signaling to glucose transport in skeletal muscle. J Appl Physiol 99:330–337 [DOI] [PubMed] [Google Scholar]
- 564.Holloszy JO 2005. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol 99:338–343 [DOI] [PubMed] [Google Scholar]
- 565.Larsen JJ, Dela F, Kjaer M, Galbo H 1997. The effect of moderate exercise on postprandial glucose homeostasis in NIDDM patients. Diabetologia 40:447–453 [DOI] [PubMed] [Google Scholar]
- 566.Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ 2000. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest 105:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Segal KR, Edano A, Abalos A, Albu J, Blando L, Tomas MB, Pi-Sunyer FX 1991. Effect of exercise training on insulin sensitivity and glucose metabolism in lean, obese, and diabetic men. J Appl Physiol 71:2402–2411 [DOI] [PubMed] [Google Scholar]
- 568.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A 2007. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 116:1081–1093 [DOI] [PubMed] [Google Scholar]
- 569.Dengel DR, Pratley RE, Hagberg JM, Rogus EM, Goldberg AP 1996. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol 81:318–325 [DOI] [PubMed] [Google Scholar]
- 570.Ryan AS, Nicklas BJ, Berman DM 2006. Aerobic exercise is necessary to improve glucose utilization with moderate weight loss in women. Obesity (Silver Spring) 14:1064–1072 [DOI] [PubMed] [Google Scholar]
- 571.Stone NJ, Bilek S, Rosenbaum S 2005. Recent National Cholesterol Education Program Adult Treatment Panel III update: adjustments and options. Am J Cardiol 96:53E–59E [DOI] [PubMed] [Google Scholar]
- 572.Kelley GA, Kelley KS, Tran ZV 2005. Exercise, lipids, and lipoproteins in older adults: a meta-analysis. Prev Cardiol 8:206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Kelley GA, Kelley KS, Vu TZ 2005. Aerobic exercise, lipids and lipoproteins in overweight and obese adults: a meta-analysis of randomized controlled trials. Int J Obes (Lond) 29:881–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Kelley GA, Kelley KS 2007. Effects of aerobic exercise on lipids and lipoproteins in adults with type 2 diabetes: a meta-analysis of randomized-controlled trials. Public Health 121:643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, Suzuki E, Shimano H, Yamamoto S, Kondo K, Ohashi Y, Yamada N, Sone H 2007. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med 167:999–1008 [DOI] [PubMed] [Google Scholar]
- 576.Halverstadt A, Phares DA, Wilund KR, Goldberg AP, Hagberg JM 2007. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metabolism 56:444–450 [DOI] [PubMed] [Google Scholar]
- 577.Stefanick ML, Mackey S, Sheehan M, Ellsworth N, Haskell WL, Wood PD 1998. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. N Engl J Med 339:12–20 [DOI] [PubMed] [Google Scholar]
- 578.Katsanos CS 2006. Prescribing aerobic exercise for the regulation of postprandial lipid metabolism: current research and recommendations. Sports Med 36:547–560 [DOI] [PubMed] [Google Scholar]
- 579.Whelton SP, Chin A, Xin X, He J 2002. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 136:493–503 [DOI] [PubMed] [Google Scholar]
- 580.Bacon SL, Sherwood A, Hinderliter A, Blumenthal JA 2004. Effects of exercise, diet and weight loss on high blood pressure. Sports Med 34:307–316 [DOI] [PubMed] [Google Scholar]
- 581.Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN 2005. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc 37:1849–1855 [DOI] [PubMed] [Google Scholar]
- 582.Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, Roubenoff R, Tucker KL, Nelson ME 2002. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care 25:2335–2341 [DOI] [PubMed] [Google Scholar]
- 583.Ibanez J, Izquierdo M, Arguelles I, Forga L, Larrion JL, Garcia-Unciti M, Idoate F, Gorostiaga EM 2005. Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care 28:662–667 [DOI] [PubMed] [Google Scholar]
- 584.Cornelissen VA, Fagard RH 2005. Effect of resistance training on resting blood pressure: a meta-analysis of randomized controlled trials. J Hypertens 23:251–259 [DOI] [PubMed] [Google Scholar]
- 585.Balducci S, Zanuso S, Massarini M, Corigliano G, Nicolucci A, Missori S, Cavallo S, Cardelli P, Alessi E, Pugliese G, Fallucca F 2007. The Italian Diabetes and Exercise Study (IDES): design and methods for a prospective Italian multicentre trial of intensive lifestyle intervention in people with type 2 diabetes and the metabolic syndrome. Nutr Metab Cardiovasc Dis 18:585–595 [DOI] [PubMed] [Google Scholar]
- 586.Bray GA, Greenway FL 2007. Pharmacological treatment of the overweight patient. Pharmacol Rev 59:151–184 [DOI] [PubMed] [Google Scholar]
- 587.DeWald T, Khaodhiar L, Donahue MP, Blackburn G 2006. Pharmacological and surgical treatments for obesity. Am Heart J 151:604–624 [DOI] [PubMed] [Google Scholar]
- 588.Cerulli J, Lomaestro BM, Malone M 2007. Update on the pharmacotherapy of obesity. 1998. Ann Pharmacother 41:1505–1517 [DOI] [PubMed] [Google Scholar]
- 589.Eckel RH 2008. Clinical practice. Nonsurgical management of obesity in adults. N Engl J Med 358:1941–1950 [DOI] [PubMed] [Google Scholar]
- 590.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L 2004. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 27:155–161 [DOI] [PubMed] [Google Scholar]
- 591.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM 2002. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Ratner R, Goldberg R, Haffner S, Marcovina S, Orchard T, Fowler S, Temprosa M 2005. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care 28:888–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.2003 Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care 26:977–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.1998 Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352:854–865 [PubMed] [Google Scholar]
- 595.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA 2008. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 596.Knowler WC, Hamman RF, Edelstein SL, Barrett-Connor E, Ehrmann DA, Walker EA, Fowler SE, Nathan DM, Kahn SE 2005. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 54:1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP 2002. Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 598.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR 2006. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 599.Nissen SE, Wolski K 2007. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356:2457–2471 [DOI] [PubMed] [Google Scholar]
- 600.Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ 2007. Rosiglitazone evaluated for cardiovascular outcomes—an interim analysis. N Engl J Med 357:28–38 [DOI] [PubMed] [Google Scholar]
- 601.McGuire DK, Inzucchi SE 2008. New drugs for the treatment of diabetes mellitus. Part I: Thiazolidinediones and their evolving cardiovascular implications. Circulation 117:440–449 [DOI] [PubMed] [Google Scholar]
- 602.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M 2002. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 359:2072–2077 [DOI] [PubMed] [Google Scholar]
- 603.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M 2003. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 290:486–494 [DOI] [PubMed] [Google Scholar]
- 604.2002 Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106:3143–3421 [PubMed] [Google Scholar]
- 605.Grundy SM, Cleeman JI, Merz CN, Brewer Jr HB, Clark LT, Hunninghake DB, Pasternak RC, Smith Jr SC, Stone NJ 2004. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110:227–239 [DOI] [PubMed] [Google Scholar]
- 606.Bakker-Arkema RG, Davidson MH, Goldstein RJ, Davignon J, Isaacsohn JL, Weiss SR, Keilson LM, Brown WV, Miller VT, Shurzinske LJ, Black DM 1996. Efficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. JAMA 275:128–133 [PubMed] [Google Scholar]
- 607.1998 Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 339:1349–1357 [DOI] [PubMed] [Google Scholar]
- 608.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto Jr AM 1998. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 279:1615–1622 [DOI] [PubMed] [Google Scholar]
- 609.Wissler RW 1994. New insights into the pathogenesis of atherosclerosis as revealed by PDAY. Pathobiological Determinants of Atherosclerosis in Youth. Atherosclerosis 108 Suppl:S3–S20 [DOI] [PubMed]
- 610.Davignon J 2004. Beneficial cardiovascular pleiotropic effects of statins. Circulation 109:III39–III43 [DOI] [PubMed]
- 611.McFarlane SI, Muniyappa R, Francisco R, Sowers JR 2002. Clinical review 145: pleiotropic effects of statins: lipid reduction and beyond. J Clin Endocrinol Metab 87:1451–1458 [DOI] [PubMed] [Google Scholar]
- 612.Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J 2003. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 361:1149–1158 [DOI] [PubMed] [Google Scholar]
- 613.Deedwania P, Barter P, Carmena R, Fruchart JC, Grundy SM, Haffner S, Kastelein JJ, LaRosa JC, Schachner H, Shepherd J, Waters DD 2006. Reduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets study. Lancet 368:919–928 [DOI] [PubMed] [Google Scholar]
- 614.Eckel RH 2007. Treating dyslipidemia of the metabolic syndrome: where’s the evidence? Nat Clin Pract Endocrinol Metab 3:437. [DOI] [PubMed] [Google Scholar]
- 615.Turley SD, Dietschy JM 2003. The intestinal absorption of biliary and dietary cholesterol as a drug target for lowering the plasma cholesterol level. Prev Cardiol 6:29–33, 64 [DOI] [PubMed] [Google Scholar]
- 616.Dujovne CA, Ettinger MP, McNeer JF, Lipka LJ, LeBeaut AP, Suresh R, Yang B, Veltri EP 2002. Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am J Cardiol 90:1092–1097 [DOI] [PubMed] [Google Scholar]
- 617.Simons L, Tonkon M, Masana L, Maccubbin D, Shah A, Lee M, Gumbiner B 2004. Effects of ezetimibe added to on-going statin therapy on the lipid profile of hypercholesterolemic patients with diabetes mellitus or metabolic syndrome. Curr Med Res Opin 20:1437–1445 [DOI] [PubMed] [Google Scholar]
- 618.Mikhailidis DP, Sibbring GC, Ballantyne CM, Davies GM, Catapano AL 2007. Meta-analysis of the cholesterol-lowering effect of ezetimibe added to ongoing statin therapy. Curr Med Res Opin 23:2009–2026 [DOI] [PubMed] [Google Scholar]
- 619.Mandeville WH, Arbeeny C 1999. Bile acid sequestrants: their use in combination with other lipid-lowering agents. IDrugs 2:237–242 [PubMed] [Google Scholar]
- 620.1984 The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 251:365–374 [PubMed] [Google Scholar]
- 621.Sampalis JS, Bissonnette S, Habib R, Boukas S 2007. Reduction in estimated risk for coronary artery disease after use of ezetimibe with a statin. Ann Pharmacother 41:1345–1351 [DOI] [PubMed] [Google Scholar]
- 622.Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, Visseren FL, Sijbrands EJ, Trip MD, Stein EA, Gaudet D, Duivenvoorden R, Veltri EP, Marais AD, de Groot E 2008. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 358:1431–1443 [DOI] [PubMed] [Google Scholar]
- 623.Leaf DA, Connor WE, Illingworth DR, Bacon SP, Sexton G 1989. The hypolipidemic effects of gemfibrozil in type V hyperlipidemia. A double-blind, crossover study. JAMA 262:3154–3160 [PubMed] [Google Scholar]
- 624.Eisenberg S, Gavish D, Oschry Y, Fainaru M, Deckelbaum RJ 1984. Abnormalities in very low, low and high density lipoproteins in hypertriglyceridemia. Reversal toward normal with bezafibrate treatment. J Clin Invest 74:470–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V, Mäenpää H, Mälkonen, Mänttäri M, Norola S, Pasternack A, Pikkarainen J, Romo M, Sjöblom T, Nikkilä EA 1987. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 317:1237–1245 [DOI] [PubMed] [Google Scholar]
- 626.1978 A co-operative trial in the primary prevention of ischaemic heart disease using clofibrate. Report from the Committee of Principal Investigators. Br Heart J 40:1069–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J 1999. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 341:410–418 [DOI] [PubMed] [Google Scholar]
- 628.2000 Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation 102:21–27 [DOI] [PubMed] [Google Scholar]
- 629.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesaniemi YA, Sullivan D, Hunt D, Colman P, d’Emden M, Whiting M, Ehnholm C, Laakso M 2005 Effects of long-term fenofibrate therapy on cardiovascular events in 9795. people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366:1849–1861 [DOI] [PubMed] [Google Scholar]
- 630.Pierce LR, Wysowski DK, Gross TP 1990. Myopathy and rhabdomyolysis associated with lovastatin-gemfibrozil combination therapy. JAMA 264:71–75 [PubMed] [Google Scholar]
- 631.Barter PJ, Rye KA 2008. Is there a role for fibrates in the management of dyslipidemia in the metabolic syndrome? Arterioscler Thromb Vasc Biol 28:39–46 [DOI] [PubMed] [Google Scholar]
- 632.Kashyap ML, McGovern ME, Berra K, Guyton JR, Kwiterovich PO, Harper WL, Toth PD, Favrot LK, Kerzner B, Nash SD, Bays HE, Simmons PD 2002. Long-term safety and efficacy of a once-daily niacin/lovastatin formulation for patients with dyslipidemia. Am J Cardiol 89:672–678 [DOI] [PubMed] [Google Scholar]
- 633.Morgan JM, Capuzzi DM, Baksh RI, Intenzo C, Carey CM, Reese D, Walker K 2003. Effects of extended-release niacin on lipoprotein subclass distribution. Am J Cardiol 91:1432–1436 [DOI] [PubMed] [Google Scholar]
- 634.Pan J, Lin M, Kesala RL, Van J, Charles MA 2002. Niacin treatment of the atherogenic lipid profile and Lp(a) in diabetes. Diabetes Obes Metab 4:255–261 [DOI] [PubMed] [Google Scholar]
- 635.Pan J, Van JT, Chan E, Kesala RL, Lin M, Charles MA 2002. Extended-release niacin treatment of the atherogenic lipid profile and lipoprotein (a) in diabetes. Metabolism 51:1120–1127 [DOI] [PubMed] [Google Scholar]
- 636.Hunninghake DB, McGovern ME, Koren M, Brazg R, Murdock D, Weiss S, Pearson T 2003. A dose-ranging study of a new, once-daily, dual-component drug product containing niacin extended-release and lovastatin. Clin Cardiol 26:112–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Mills E, Prousky J, Raskin G, Gagnier J, Rachlis B, Montori VM, Juurlink D 2003. The safety of over-the-counter niacin. A randomized placebo-controlled trial [ISRCTN18054903]. BMC Clin Pharmacol 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Goldberg A, Alagona Jr P, Capuzzi DM, Guyton J, Morgan JM, Rodgers J, Sachson R, Samuel P 2000. Multiple-dose efficacy and safety of an extended-release form of niacin in the management of hyperlipidemia. Am J Cardiol 85:1100–1105 [DOI] [PubMed] [Google Scholar]
- 639.Grundy SM, Vega GL, McGovern ME, Tulloch BR, Kendall DM, Fitz-Patrick D, Ganda OP, Rosenson RS, Buse JB, Robertson DD, Sheehan JP 2002. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the assessment of diabetes control and evaluation of the efficacy of niaspan trial. Arch Intern Med 162:1568–1576 [DOI] [PubMed] [Google Scholar]
- 640.McKenney JM, Proctor JD, Harris S, Chinchili VM 1994. A comparison of the efficacy and toxic effects of sustained- vs immediate-release niacin in hypercholesterolemic patients. JAMA 271:672–677 [PubMed] [Google Scholar]
- 641.Pownall HJ, Brauchi D, Kilinc C, Osmundsen K, Pao Q, Payton-Ross C, Gotto Jr AM, Ballantyne CM 1999. Correlation of serum triglyceride and its reduction by omega-3 fatty acids with lipid transfer activity and the neutral lipid compositions of high-density and low-density lipoproteins. Atherosclerosis 143:285–297 [DOI] [PubMed] [Google Scholar]
- 642.Weber P, Raederstorff D 2000. Triglyceride-lowering effect of omega-3 LC-polyunsaturated fatty acids—a review. Nutr Metab Cardiovasc Dis 10:28–37 [PubMed] [Google Scholar]
- 643.Weintraub MS, Zechner R, Brown A, Eisenberg S, Breslow JL 1988. Dietary polyunsaturated fats of the W-6 and W-3 series reduce postprandial lipoprotein levels. Chronic and acute effects of fat saturation on postprandial lipoprotein metabolism. J Clin Invest 82:1884–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Mori TA, Woodman RJ 2006. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care 9:95–104 [DOI] [PubMed] [Google Scholar]
- 645.Harris WS, Dujovne CA, Zucker M, Johnson B 1988. Effects of a low saturated fat, low cholesterol fish oil supplement in hypertriglyceridemic patients. A placebo-controlled trial. Ann Intern Med 109:465–470 [DOI] [PubMed] [Google Scholar]
- 646.Nikkila M 1991. Influence of fish oil on blood lipids in coronary artery disease. Eur J Clin Nutr 45:209–213 [PubMed] [Google Scholar]
- 647.Harris WS 1997. n-3 Fatty acids and serum lipoproteins: human studies. Am J Clin Nutr 65:1645S–1654S [DOI] [PubMed] [Google Scholar]
- 648.Contacos C, Barter PJ, Sullivan DR 1993. Effect of pravastatin and omega-3 fatty acids on plasma lipids and lipoproteins in patients with combined hyperlipidemia. Arterioscler Thromb 13:1755–1762 [DOI] [PubMed] [Google Scholar]
- 649.Durrington PN, Bhatnagar D, Mackness MI, Morgan J, Julier K, Khan MA, France M 2001. An omega-3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia. Heart 85:544–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K 2007. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369:1090–1098 [DOI] [PubMed] [Google Scholar]
- 651.Simopoulos AP 2002. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr 21:495–505 [DOI] [PubMed] [Google Scholar]
- 652.von Schacky C, Fischer S, Weber PC 1985. Long-term effects of dietary marine omega-3 fatty acids upon plasma and cellular lipids, platelet function, and eicosanoid formation in humans. J Clin Invest 76:1626–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Ueshima H, Stamler J, Elliott P, Chan Q, Brown IJ, Carnethon MR, Daviglus ML, He K, Moag-Stahlberg A, Rodriguez BL, Steffen LM, Van Horn L, Yarnell J, Zhou B 2007. Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure: INTERMAP study. Hypertension 50:313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Meydani M 2000. Omega-3 fatty acids alter soluble markers of endothelial function in coronary heart disease patients. Nutr Rev 58:56–59 [DOI] [PubMed] [Google Scholar]
- 655.Kris-Etherton PM, Harris WS, Appel LJ 2002. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106:2747–2757 [DOI] [PubMed] [Google Scholar]
- 656.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, Jones DW, Materson BJ, Oparil S, Wright Jr JT, Roccella EJ 2003. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289:2560–2572 [DOI] [PubMed] [Google Scholar]
- 657.Bestermann W, Houston MC, Basile J, Egan B, Ferrario CM, Lackland D, Hawkins RG, Reed J, Rogers P, Wise D, Moore MA 2005. Addressing the global cardiovascular risk of hypertension, dyslipidemia, diabetes mellitus, and the metabolic syndrome in the southeastern United States. Part II: treatment recommendations for management of the global cardiovascular risk of hypertension, dyslipidemia, diabetes mellitus, and the metabolic syndrome. Am J Med Sci 329:292–305 [DOI] [PubMed] [Google Scholar]
- 658.Israili ZH, Lyoussi B, Hernandez-Hernandez R, Velasco M 2007. Metabolic syndrome: treatment of hypertensive patients. Am J Ther 14:386–402 [DOI] [PubMed] [Google Scholar]
- 659.Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, Mustonen J 2004. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 351:1952–1961 [DOI] [PubMed] [Google Scholar]
- 660.Abuissa H, Jones PG, Marso SP, O'Keefe Jr JH 2005. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J Am Coll Cardiol 46:821–826 [DOI] [PubMed] [Google Scholar]
- 661.Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, Diaz R, Avezum A, Lanas F, Probstfield J, Fodor G, Holman RR 2006. Effect of ramipril on the incidence of diabetes. N Engl J Med 355:1551–1562 [DOI] [PubMed] [Google Scholar]
- 662.Wright Jr JT, Harris-Haywood S, Pressel S, Barzilay J, Baimbridge C, Bareis CJ, Basile JN, Black HR, Dart R, Gupta AK, Hamilton BP, Einhorn PT, Haywood LJ, Jafri SZ, Louis GT, Whelton PK, Scott CL, Simmons DL, Stanford C, Davis BR 2008. Clinical outcomes by race in hypertensive patients with and without the metabolic syndrome: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med 168:207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Black HR, Davis B, Barzilay J, Nwachuku C, Baimbridge C, Marginean H, Wright Jr JT, Basile J, Wong ND, Whelton P, Dart RA, Thadani U 2008. Metabolic and clinical outcomes in nondiabetic individuals with the metabolic syndrome assigned to chlorthalidone, amlodipine, or lisinopril as initial treatment for hypertension: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Diabetes Care 31:353–360 [DOI] [PubMed] [Google Scholar]
- 664.2002 Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 288:2981–2997 [DOI] [PubMed] [Google Scholar]
- 665.1998 Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study Group. BMJ 317:713–720 [PMC free article] [PubMed] [Google Scholar]
- 666.Schneider DJ 2005. Abnormalities of coagulation, platelet function, and fibrinolysis associated with syndromes of insulin resistance. Coron Artery Dis 16:473–476 [DOI] [PubMed] [Google Scholar]
- 667.Sowers JR 2003. Recommendations for special populations: diabetes mellitus and the metabolic syndrome. Am J Hypertens 16:41S–45S [DOI] [PubMed] [Google Scholar]
- 668.Shields TM, Hennekens CH 2004. Management of metabolic syndrome: aspirin. Endocrinol Metab Clin North Am 33:577–593, vii [DOI] [PubMed] [Google Scholar]
- 669.Hennekens CH, Dyken ML, Fuster V 1997. Aspirin as a therapeutic agent in cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 96:2751–2753 [DOI] [PubMed] [Google Scholar]
- 670.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith Jr SC, Spertus JA, Costa F 2005. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev 13:322–327 [PubMed] [Google Scholar]
- 671.Selvin E, Paynter NP, Erlinger TP 2007. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med 167:31–39 [DOI] [PubMed] [Google Scholar]
- 672.van Dielen FM, Buurman WA, Hadfoune M, Nijhuis J, Greve JW 2004. Macrophage inhibitory factor, plasminogen activator inhibitor-1, other acute phase proteins, and inflammatory mediators normalize as a result of weight loss in morbidly obese subjects treated with gastric restrictive surgery. J Clin Endocrinol Metab 89:4062–4068 [DOI] [PubMed] [Google Scholar]
- 673.Devaraj S, Rogers J, Jialal I 2007. Statins and biomarkers of inflammation. Curr Atheroscler Rep 9:33–41 [DOI] [PubMed] [Google Scholar]
- 674.Prasad K 2006. C-reactive protein (CRP)-lowering agents. Cardiovasc Drug Rev 24:33–50 [DOI] [PubMed] [Google Scholar]
- 675.Fisher BL, Schauer P 2002. Medical and surgical options in the treatment of severe obesity. Am J Surg 184:9S–16S [DOI] [PubMed] [Google Scholar]
- 676.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM 1995. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 222:339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Madan AK, Orth W, Ternovits CA, Tichansky DS 2006. Metabolic syndrome: yet another co-morbidity gastric bypass helps cure. Surg Obes Relat Dis 2:48–51 [DOI] [PubMed] [Google Scholar]
- 678.Nunes JP 2007. The risk factor association syndrome as a barisystemic syndrome: a view on obesity and the metabolic syndrome. Med Hypotheses 68:541–545 [DOI] [PubMed] [Google Scholar]
- 679.Sugerman HJ, Wolfe LG, Sica DA, Clore JN 2003. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 237:751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K 2004. Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 681.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H 2004. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 682.Alexandrides TK, Skroubis G, Kalfarentzos F 2007. Resolution of diabetes mellitus and metabolic syndrome following Roux-en-Y gastric bypass and a variant of biliopancreatic diversion in patients with morbid obesity. Obes Surg 17:176–184 [DOI] [PubMed] [Google Scholar]
- 683.Mattar SG, Velcu LM, Rabinovitz M, Demetris AJ, Krasinskas AM, Barinas-Mitchell E, Eid GM, Ramanathan R, Taylor DS, Schauer PR 2005. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg 242:610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Peiser J, Ovnat A, Uwyyed K, Lavie P, Charuzi I 1985. Cardiac arrhythmias during sleep in morbidly obese sleep-apneic patients before and after gastric bypass surgery. Clin Cardiol 8:519–521 [DOI] [PubMed] [Google Scholar]
- 685.Mathier MA, Ramanathan RC 2007. Impact of obesity and bariatric surgery on cardiovascular disease. Med Clin North Am 91:415–431, x–xi [DOI] [PubMed] [Google Scholar]
- 686.Sampalis JS, Sampalis F, Christou N 2006. Impact of bariatric surgery on cardiovascular and musculoskeletal morbidity. Surg Obes Relat Dis 2:587–591 [DOI] [PubMed] [Google Scholar]
- 687.Eid GM, Cottam DR, Velcu LM, Mattar SG, Korytkowski MT, Gosman G, Hindi P, Schauer PR 2005. Effective treatment of polycystic ovarian syndrome with Roux-en-Y gastric bypass. Surg Obes Relat Dis 1:77–80 [DOI] [PubMed] [Google Scholar]
- 688.Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, Sancho J, San Millan JL 2005. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 90:6364–6369 [DOI] [PubMed] [Google Scholar]
- 689.MacDonald Jr KG, Long SD, Swanson MS, Brown BM, Morris P, Dohm GL, Pories WJ 1997. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg 1:213–220 [DOI] [PubMed] [Google Scholar]
- 690.Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M 2008. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 299:316–323 [DOI] [PubMed] [Google Scholar]
- 691.Wolf AM, Beisiegel U, Kortner B, Kuhlmann HW 1998. Does gastric restriction surgery reduce the risks of metabolic diseases? Obes Surg 8:9–13 [DOI] [PubMed] [Google Scholar]
- 692.Gasteyger C, Suter M, Calmes JM, Gaillard RC, Giusti V 2006. Changes in body composition, metabolic profile and nutritional status 24 months after gastric banding. Obes Surg 16:243–250 [DOI] [PubMed] [Google Scholar]
- 693.Folli F, Pontiroli AE, Schwesinger WH 2007. Metabolic aspects of bariatric surgery. Med Clin North Am 91:393–414, x [DOI] [PubMed] [Google Scholar]
- 694.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM 2007. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357:741–752 [DOI] [PubMed] [Google Scholar]
- 695.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC 2007. Long-term mortality after gastric bypass surgery. N Engl J Med 357:753–761 [DOI] [PubMed] [Google Scholar]
- 696.Beaser RS, Levy P 2007. Metabolic syndrome: a work in progress, but a useful construct. Circulation 115:1812–1818; discussion, 1818 [DOI] [PubMed] [Google Scholar]