Abstract
Polycystic ovary syndrome (PCOS) is now recognized as an important metabolic as well as reproductive disorder conferring substantially increased risk for type 2 diabetes. Affected women have marked insulin resistance, independent of obesity. This article summarizes the state of the science since we last reviewed the field in the Endocrine Reviews in 1997. There is general agreement that obese women with PCOS are insulin resistant, but some groups of lean affected women may have normal insulin sensitivity. There is a post-binding defect in receptor signaling likely due to increased receptor and insulin receptor substrate-1 serine phosphorylation that selectively affects metabolic but not mitogenic pathways in classic insulin target tissues and in the ovary. Constitutive activation of serine kinases in the MAPK-ERK pathway may contribute to resistance to insulin's metabolic actions in skeletal muscle. Insulin functions as a co-gonadotropin through its cognate receptor to modulate ovarian steroidogenesis. Genetic disruption of insulin signaling in the brain has indicated that this pathway is important for ovulation and body weight regulation. These insights have been directly translated into a novel therapy for PCOS with insulin-sensitizing drugs. Furthermore, androgens contribute to insulin resistance in PCOS. PCOS may also have developmental origins due to androgen exposure at critical periods or to intrauterine growth restriction. PCOS is a complex genetic disease, and first-degree relatives have reproductive and metabolic phenotypes. Several PCOS genetic susceptibility loci have been mapped and replicated. Some of the same susceptibility genes contribute to disease risk in Chinese and European PCOS populations, suggesting that PCOS is an ancient trait.
-
I.
Background and Historical Perspective
-
A.
Reproduction and metabolism
-
B.
Experiments of nature—rare syndromes of extreme insulin resistance and hyperandrogenism
-
C.
Insulin resistance and PCOS
-
A.
-
II.
PCOS Reproductive Phenotype
-
A.
Clinical features
-
B.
Biochemical profile
-
C.
Polycystic ovaries
-
A.
-
III.
Diagnostic Criteria for PCOS
-
A.
Development of diagnostic criteria for PCOS
-
B.
National Institutes of Child Health and Human Development (NICHD)
-
C.
Rotterdam
-
D.
Androgen Excess Society (AES)
-
E.
Impact of diagnostic criteria on PCOS phenotypes
-
F.
Epidemiology
-
A.
-
IV.
PCOS Metabolic Phenotype
-
A.
Glucose tolerance
-
B.
Insulin resistance
-
C.
Other metabolic actions of insulin in PCOS
-
D.
Mitogenic actions of insulin in PCOS
-
E.
Insulin secretion in PCOS
-
F.
Insulin clearance in PCOS
-
G.
Obesity and PCOS
-
A.
-
V.
Mechanisms for the Association of Insulin Resistance and PCOS
-
A.
Insulin as a reproductive hormone
-
B.
Metabolic actions of androgens
-
C.
Genetic susceptibility to PCOS
-
D.
Developmental origins of PCOS
-
A.
-
VI.
Implications and Future Directions
I. Background and Historical Perspective
A. Reproduction and metabolism
The pathways linking reproductive function with metabolic cues are evolutionarily conserved traits that are present in Caenorhabditis elegans and Drosophila (1, 2). The reproductive features of polycystic ovary syndrome (PCOS) were noted by Hippocrates in the fifth century B.C. (3). The observation that signs of androgen excess were coupled with metabolic abnormalities, such as increased visceral fat, dates back to at least the 18th century. In 1765, Morgagni (4) reported detailed anatomic investigations in various conditions. He described a 74-yr-old woman with severe obesity and android aspect (valde obesa et virili aspectu). In 1921, Achard and Thiers (5) reported the coexistence of diabetes mellitus with clinical signs of androgen excess in a postmenopausal woman—the so-called “Achard-Thiers syndrome” or “diabetes of the bearded women” (diabète des femmes à barbe). Jean Vague (6) from the University of Marseille introduced the term “android obesity” to define the abdominal fat accumulation, which is the typical male pattern of body fat distribution, and started to explore the concept that this type of body adiposity was associated with increased diabetes and cardiovascular disease risk. Elegant studies by Kissebah et al. (7) documented that women with upper body obesity were insulin resistant. These women also had increased androgen production rates (8).
B. Experiments of nature—rare syndromes of extreme insulin resistance and hyperandrogenism
In the 1970s, several rare syndromes of extreme insulin resistance, acanthosis nigricans, and hyperandrogenism were described (9). The molecular mechanisms of insulin resistance in these syndromes involved reduced insulin binding to its receptor or defective receptor autophosphorylation due to insulin receptor mutations (Type A syndrome, Rabson-Mendenhall syndrome, Donohue syndrome, or leprechaunism) or insulin receptor autoantibodies (type B syndrome) (10–12). The phenotypically distinct disorders of familial lipodystrophy and extreme insulin resistance were also noted to be associated with signs and symptoms of hyperandrogenism (12–15). The common feature of these syndromes was profound hyperinsulinemia, which suggested for the first time that insulin might directly stimulate testosterone (T) production (9, 11).
C. Insulin resistance and PCOS
The original description of enlarged, smooth polycystic ovaries (PCO) is credited to Chereau in 1844 (16). In the 19th century, ovarian wedge resection became a recommended therapy (17), although Stein and Leventhal (18) first reported that the clinical features of menstrual regularity and infertility could be improved by removal of portions of both ovaries. As a result, the constellation of enlarged, sclerocystic ovaries frequently associated with hirsutism, menstrual irregularity, obesity, and infertility became known as the Stein-Leventhal syndrome (17, 19). In recent decades, PCOS has become the preferred terminology (17, 20). Until the 1980s, PCOS remained a poorly understood reproductive disorder (17, 19). In 1980, Burghen et al. (21) reported that women with PCOS had increased insulin responses during oral glucose tolerance testing that were not accounted for by obesity. Furthermore, women with typical PCOS had acanthosis nigricans, raising the possibility that they were insulin resistant, similar to women with the rare syndromes of extreme insulin resistance (22, 23). These observations launched a new field of study on the mechanisms for the association between insulin resistance and PCOS (Fig. 1).
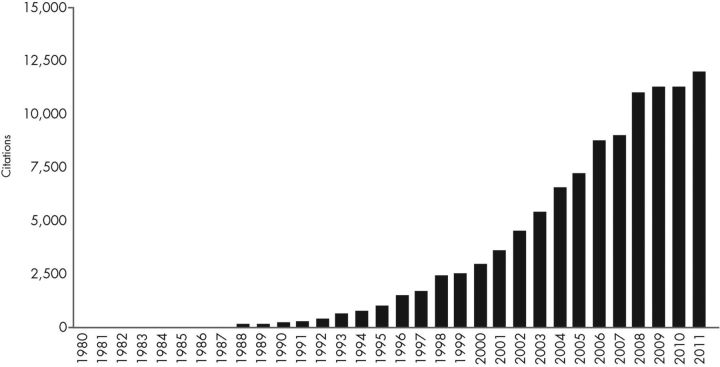
Figure 1.
A new field—PCOS and insulin resistance. The first article reporting an association between PCOS and hyperinsulinemia was published in 1980 (21). There are approximately 103,000 citations in a Web of Science (Thomson Reuters, New York, NY) Citation Report for 1980–2011 on the topics of PCOS or hyperandrogenism and hyperinsulinemia, insulin resistance, glucose intolerance, or diabetes mellitus. The annual citations have increased steadily from 1 in 1980 to approximately 12,000 in 2011. This figure was created from the Web of Science Citation Report.
II. PCOS Reproductive Phenotype (Fig. 2)
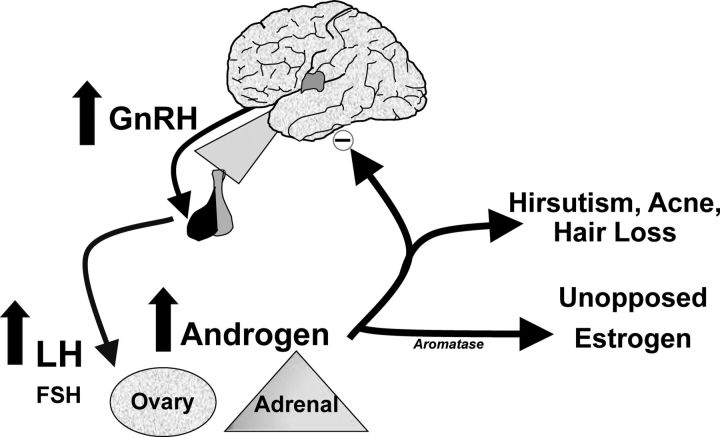
Figure 2.
Pathophysiology of the PCOS reproductive phenotype. There is increased frequency of pulsatile GnRH release that selectively increases LH secretion. LH stimulates ovarian theca cell T production. T is incompletely aromatized by the adjacent granulosa cells because of relative FSH deficiency. There are also constitutive increases in the activity of multiple steroidogenic enzymes in polycystic ovaries contributing to increased androgen production. Increased adrenal androgen production may also be present in PCOS. T acts in the periphery to produce signs of androgen excess, such as hirsutism, acne, and alopecia. T and androstenedione can also be aromatized extragonadally to estradiol and estrone, respectively, resulting in unopposed estrogen action on the endometrium. T feeds back on the hypothalamus to decrease the sensitivity to the normal feedback effects of estradiol and progesterone to slow GnRH pulse frequency. This figure is used with the permission of Andrea Dunaif.
A. Clinical features
Approximately 60% of women with PCOS are hirsute, the most common clinical sign of hyperandrogenemia (24). Acne and androgenic alopecia are other clinical signs of hyperandrogenemia (25–32). Acanthosis nigricans is a skin lesion characterized clinically by velvety, papillomatous, brownish-black, hyperkeratotic plaques, typically on the intertriginous surfaces and neck. However, acanthosis nigricans is diagnosed definitively by histological examination of the skin showing hyperkeratosis and papillomatosis, frequently with hyperpigmentation (33). It is evident on clinical examination in a substantial percentage of obese women with PCOS as well as in some lean affected women. However, it is present in the majority of obese women with PCOS and in obese control women by histological examination (33). Many lean women with PCOS also show histological evidence of acanthosis nigricans (33). Its severity is directly correlated with the degree of insulin resistance (33, 34).
Oligomenorrhea is defined as menstrual cycles that are longer than 35 d (usually fewer than eight cycles per year) and is a sign of anovulatory cycles (35). However, regular menstrual cycles do not exclude chronic anovulation, especially in women with clinical signs of androgen excess (24). Twenty to 50% of women with clinical hyperandrogenism and apparent eumenorrhea may have anovulation as documented by consecutive luteal serum progesterone levels in the follicular range (24). Therefore, ovulation should be assessed by measuring serum progesterone concentration during the luteal phase of the menstrual cycle in women with regular menses and androgenic signs or symptoms (24).
B. Biochemical profile
1. Sex hormones
Hyperandrogenemia is the biochemical hallmark of PCOS (24). Elevated circulating androgen levels are observed in 80–90% of women with oligomenorrhea (24, 36). Elevated levels of free T account for the vast majority of abnormal findings in the laboratory examination (24, 37). This finding reflects the fact that SHBG levels are typically decreased in PCOS due to the effects of T (38) and insulin (39) to decrease hepatic production of SHBG.
The measurement of total and free T levels is constrained by the available assay methods. Assays for total T lack precision and sensitivity in the female T range, including T levels typical of PCOS (40, 41). The accurate measurement of free T by equilibrium dialysis is technically challenging and costly, whereas direct measurement of free T is inaccurate (41, 42). Measurements of total T by RIA or liquid chromatography-mass spectrometry in a specialized endocrine laboratory are currently the best available methodologies (43). Free and biologically available T can be calculated from the concentrations of total T, SHBG, and albumin by using the affinity constants of T for these molecules (42). In practice, albumin is often not measured, and an assumed normal value is used in the calculation.
Whether the concurrent measurement of androstenedione increases the diagnosis of hyperandrogenemia is unclear (24, 37). Approximately 25% of women with PCOS will have elevated levels of dehydroepiandrosterone sulfate (DHEAS) (24), which may be the sole abnormality in circulating androgens in approximately 10% of these women (24).
Although the ovaries are the main source of increased androgens in PCOS (44), adrenal androgen excess is a common feature of the syndrome (24, 45). The prevalence of adrenal androgen excess is approximately 20% among white women and 30% among black women with PCOS using age- and race-adjusted normative values for circulating DHEAS levels (24, 45). Women with PCOS demonstrate increased secretion of adrenocortical precursor steroids basally and in response to ACTH stimulation including pregnenolone, 17-hydroxypregnenolone, dehydroepiandrosterone (DHEA), androstenedione, 11-deoxycortisol, and possibly cortisol (45, 46).
Estradiol levels are constantly in the early to midfollicular range without the normal midcycle increases (47, 48). Estrone levels are increased (47) because of extraglandular aromatization of increased circulating androstenedione levels (49). The decreased SHBG levels typical of PCOS result in increased non-SHBG bound or bioavailable estradiol as well as T levels (38, 50, 51).
2. Gonadotropins
Although PCOS is considered a part of the spectrum of normogonadotropic normoestrogenic anovulation (35), serum LH concentrations and the LH to FSH ratio are frequently elevated in affected women (52). FSH levels are normal to slightly suppressed and do not increase to threshold levels required during the early follicular phase of the menstrual cycle to stimulate normal follicular maturation (53). However, gonadotropin levels have never been included in any of the diagnostic criteria for PCOS because the characteristic derangements can escape detection on random blood samples because of the pulsatile nature of LH release (24, 54–56). Furthermore, LH levels may be lower in obese women with PCOS and may decrease after an ovulatory cycle in oligo-ovulatory affected women (56, 57).
C. Polycystic ovaries
PCO are characterized by an increase in antral follicles and ovarian stroma as well as by theca cell hyperplasia and ovarian cortical thickening (55, 58). Careful histological examination of PCO has revealed an excess of growing follicles, the number of which is 2- to 3-fold that of normal ovaries (58). A more recent study of ovarian cortical biopsies from normal and PCOS women (59) confirmed this observation, finding that the number of small, preantral follicles, both primordial and primary follicles, was substantially increased in anovulatory PCO compared with normal ovaries. In both ovulatory and anovulatory PCO, the proportion of early growing (primary) follicles is significantly increased, with a reciprocal decrease in the proportion of primordial follicles compared with normal ovaries (59). These differences are particularly striking in anovulatory PCO (59). There is decreased atresia of follicles from PCO in culture compared with those from normal ovaries (60). Markers of cell proliferation are significantly increased in granulosa cells from anovulatory PCO (61). Thus, it now appears that the gonadotropin-independent development of preantral follicles is disordered in PCOS (62). The excess of follicles could result from accelerated follicle growth and/or prolonged survival of small follicles in comparison to follicles from normal ovaries (59, 60, 62).
Theca cells from PCO secrete more androgens, basally and in response to LH and insulin (63), due to constitutive increases in the activity of multiple steroidogenic enzymes in these cells (64). Thus, enhanced ovarian androgen production in PCOS results from the combined effects of intrinsically increased thecal androgen secretion and increased responsiveness to trophic hormone stimulation. Whereas the increases in androgen production are found in theca cells isolated from ovulatory as well as anovulatory women with PCOS (63), granulosa cell steroidogenesis differs by ovulatory status (62, 65). Granulosa cells from ovulatory women with PCO are similar in terms of responses to FSH and estradiol production to those from normal women (65, 66). In contrast, granulosa cells isolated from some small-to-medium sized antral follicles obtained from anovulatory women with PCO showed increased estradiol production in response to FSH and premature responsiveness to LH (65, 66). These abnormalities may contribute to the arrest of follicular development. However, the arrest of antral follicle development in the otherwise normal follicle population is most likely accounted for by lower circulating FSH levels because FSH administration can produce normal follicular maturation and ovulation (62, 67, 68).
III. Diagnostic Criteria for PCOS (Table 1)
Table 1.
Diagnostic criteria for PCOS
| Criteria | |
|---|---|
| NICHD (54) | Both hyperandrogenism and chronic anovulation |
| Rotterdam (69, 70) | Two of the following: hyperandrogenism, chronic anovulation, and PCO |
| Androgen Excess Society (24, 71) | Hyperandrogenism plus ovarian dysfunction indicated by oligoanovulation and/or PCO |
All criteria require exclusion of other disorders: hyperprolactinemia, nonclassic congenital adrenal 21-hydroxylase deficiency, thyroid dysfunction, androgen-secreting neoplasms, and Cushing's syndrome.
A. Development of diagnostic criteria for PCOS
All of the diagnostic criteria for PCOS (24, 54, 69–71) have been based on expert opinion, the lowest level of evidence (72–75). None of these criteria were based on a formal consensus process (75, 76). In the United States, the National Institutes of Health (NIH) Consensus Development Program, administered by the Office of Medical Applications of Research, which has recently become part of the Office of Disease Prevention (http://consensus.nih.gov/), is a widely accepted consensus process (77, 78). These conferences have a “court” model where there is a presentation of evidence to a panel that functions as a jury (http://consensus.nih.gov/FAQs.htm#whatistheCDP). The panel is made up of individuals who are experts in their own fields but are not closely aligned with the subject. Thus, these Consensus Development Conferences permit an independent assessment of the issues in the field by an unbiased panel. An NIH conference on PCOS using this court model will be held in December 2012.
B. National Institutes of Child Health and Human Development (NICHD)
After a series of landmark studies in the 1980s identifying insulin resistance as a cardinal feature of the syndrome (21, 34, 79–81), the metabolic sequelae of the disorder began to be appreciated. This renaissance of interest in PCOS created a need for a better working definition of the syndrome; an issue of that was addressed at the 1990 NICHD Conference on PCOS (20). This conference was a meeting of experts who discussed various features of the syndrome. Participants were asked to vote on potential diagnostic features (Table 2); those receiving the most votes, hyperandrogenism and chronic anovulation, with the exclusion of secondary causes, became what are known as the NICHD or NIH criteria (54) and are often and inaccurately referred to as consensus criteria. The NICHD criteria did not include ovarian morphology because of the lack of specificity of this finding (54). It was clear at that time that 20–30% of women with regular menses and no androgenic symptoms had PCO on ovarian ultrasound examination (82). Many of these women did have elevated circulating T and/or LH levels (82). Furthermore, almost 10% of women with PCOS defined by NICHD criteria did not have PCO (83).
Table 2.
Percentage of participants agreeing on various criteria at 1990 NICHD PCOS conference (54)
| Definite or probable | Possible |
|---|---|
| Hyperandrogenemia, 64% | Insulin resistance, 69% |
| Exclusion of other etiologies, 60% | Perimenarchal onset, 62% |
| Exclusion of CAH, 59% | Elevated LH/FSH, 55% |
| Menstrual dysfunction, 52% | PCO by ultrasound, 52% |
| Clinical hyperandrogenism, 48% | Clinical hyperandrogenism, 52% |
| Menstrual dysfunction, 45% |
CAH, Congenital adrenal hyperplasia.
C. Rotterdam
In Europe, ovarian imaging was used for the diagnosis PCOS (27, 84, 85). Moreover, with the widespread use of assisted reproductive technologies, it became evident that women with PCO, even those who were reproductively normal, were hyperresponsive to exogenous gonadotropin stimulation and thus at risk for ovarian hyperstimulation syndrome (86–88). Accordingly, defining ovarian morphology became an essential component of infertility management (88). In 2003, another conference on diagnostic criteria was convened in Rotterdam (70). Despite being identified as a consensus conference, the recommendations were also based on expert opinion rather than a formal consensus process.
The result of the conference was that polycystic ovarian morphology on ultrasound examination was added to the NICHD diagnostic criteria (70). The Rotterdam criteria (69, 70) for the diagnosis of PCOS required the presence of two of the following findings, after the exclusion of disorders of the pituitary, ovary, or adrenals that could present in a manner similar to PCOS: 1) hyperandrogenism (clinical or biochemical); 2) chronic anovulation; and 3) PCO (Table 1). These criteria have extended the diagnosis to include two new groups of affected women: 1) PCO and hyperandrogenism without chronic anovulation; and 2) PCO and chronic anovulation without hyperandrogenism (71) (Fig. 3).
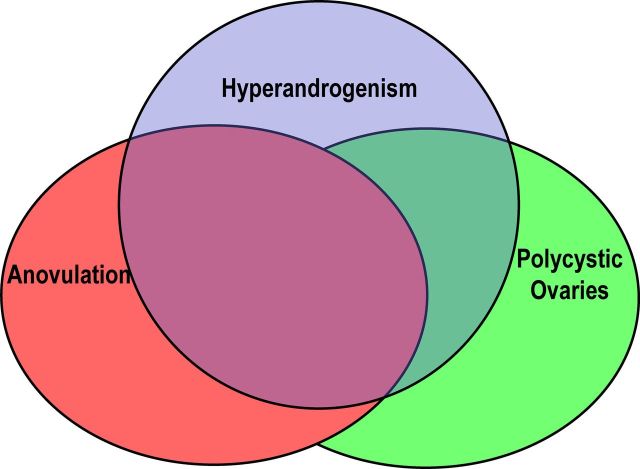
Figure 3.
Features of PCOS. The diagnostic criteria for PCOS (Table 1) include two or more of these features: hyperandrogenism (blue circle), anovulation (pink circle), and PCO (green circle), resulting in several PCOS phenotypes depending on the diagnostic criteria applied (Table 3). This figure is used with the permission of Andrea Dunaif.
D. Androgen Excess Society (AES)
The Rotterdam Criteria do not discriminate between the cardinal features of PCOS, placing equal diagnostic importance on PCO, chronic anovulation, and hyperandrogenism (24, 71). In 2006, an expert panel of the AES recommended criteria that hyperandrogenism be considered as an essential component of PCOS (71). These criteria require the combination of biochemical or clinical hyperandrogenism with chronic anovulation or PCO (24, 71) (Table 1). Nevertheless, these AES criteria included the additional phenotype of hyperandrogenism, ovulatory cycles and PCO (71) (Table 3).
Table 3.
PCOS phenotypes according to diagnostic criteria applied
| HA and Anov | HA and PCO | Anov and PCO | HA | PCO | Anov | |
|---|---|---|---|---|---|---|
| NICHD | + | − | − | − | − | − |
| Rotterdam | + | + | + | − | − | − |
| AES | + | + | − | − | − | − |
HA, Hyperandrogenism; Anov, chronic anovulation.
E. Impact of diagnostic criteria on PCOS phenotypes (Table 3)
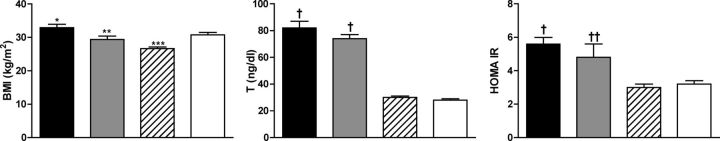
Even before Rotterdam, studies (34, 89) had suggested that these additional subgroups differed metabolically from the group with classic PCOS identified by the NICHD criteria (Fig. 4). Women with ovulatory cycles and hyperandrogenemia (34) or PCO (89) had normal insulin sensitivity. Furthermore, ovarian morphology did not correlate with the severity of symptoms in PCOS (90, 91). The hyperandrogenic woman with PCO but documented normal ovulation was recognized as a distinct phenotype of PCOS by both the Rotterdam criteria and the AES criteria (24, 70, 71) (Table 3). It has been suggested that this ovulatory form of PCOS may represent a transitional, intermediate stage between normality and the classic anovulatory form of PCOS. Women with this phenotype are often leaner than those with classic PCOS (92–94). In addition, they have milder metabolic abnormalities or may even be metabolically normal (92, 95–103). This PCOS group may potentially convert to classic PCOS under the influence of environmental factors like weight gain (104). However, there have been no longitudinal studies to follow the natural course of women with ovulatory PCOS.
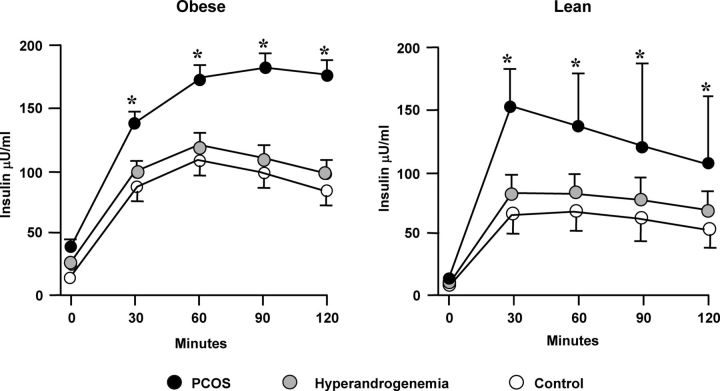
Figure 4.
Insulin responses basally and after a 40 g/m2 oral glucose load in obese and lean PCOS women (black circles), ovulatory hyperandrogenic (HA) women (gray circles), and age- and weight-comparable ovulatory control women (white circles). Insulin responses are significantly increased only in PCOS women (P < 0.001 obese PCOS vs. obese HA and obese control; P < 0.01 lean PCOS vs. lean HA and control), suggesting that hyperinsulinemia is a unique feature of PCOS and not hyperandrogenic states in general. [Adapted from A. Dunaif et al.: Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab 65:499–507, 1987 (34), with permission. © The Endocrine Society.]
The anovulatory woman with normal androgen levels and PCO is a second distinct phenotype of PCOS according to the Rotterdam criteria (24, 71) (Table 3). Women in this group most often have normal insulin sensitivity (97–100). Women with ovulatory cycles and PCO but no hyperandrogenism do not fulfill NICHD, Rotterdam, or AES criteria for PCOS (Table 3). However, these groups of nonhyperandrogenic women with PCO may have subtle endocrine aberrations, like higher LH and lower SHBG levels (82, 92, 97, 99). Moreover, they may have hyperandrogenic responses to GnRH analog (GnRHa) testing, despite normal androgen levels at baseline (95, 105). Women with isolated PCO are at increased risk to develop ovarian hyperstimulation during ovulation induction, analogous to women with hyperandrogenic forms of PCOS (88). PCO from ovulatory women do have abnormalities in folliculogenesis (62) and constitutive increases in theca cell androgen production (62, 63). Taken together, these findings suggest that PCO have constitutive increases in androgen biosynthesis and responsiveness to gonadotropins in the absence of ovulatory disturbances (62, 106).
However, a follow-up study of eumenorrheic women with the isolated PCO has shown that this ultrasound finding is unstable and irreproducible across the reproductive period (91). Women with PCO at baseline did not demonstrate any tendency to develop PCOS during the follow-up arguing against the hypothesis that PCO could represent an early, preclinical stage in the natural continuum of PCOS (91). The prevalence of PCO is also age-related and decreases in frequency with increasing age (103). There appears to be a genetic susceptibility to PCO because they are highly heritable in affected sister pairs (107).
F. Epidemiology
PCOS is now recognized as one of the most common endocrinopathies in women of reproductive age with a prevalence of 4–10% for the NICHD defined form (108–111). These prevalence estimates for PCOS using the NICHD criteria are remarkably consistent across racial and ethnic groups (108, 109, 111–113). This observation suggests that PCOS is an ancient evolutionary trait that was present before humans migrated out of Africa. The recent confirmation in European PCOS cohorts (114, 115) of two gene loci identified in a genome-wide association of Han Chinese women with PCOS (116) supports this hypothesis (the genetics of PCOS is discussed later in Section V.C.). There is, however, variation in the phenotypes of PCOS in many ethnic/racial groups, such as Latinas (117, 118), African-Americans (119), Icelanders (120) Sri Lankans (93), Koreans (121), and Chinese (100). However, a recent study comparing Black and White women with PCOS found no differences in reproductive features and mild differences in metabolic features (119).
PCOS is the most common cause of normogonadotropic anovulation, accounting for 55–91% of the entire World Health Organization-II (WHO-II) cohort (35). The prevalence of PCOS is higher using the 2003 Rotterdam criteria because it includes additional phenotypes (70) (Table 3). The Rotterdam-PCOS group was 1.5 times larger than the group classified as NICHD PCOS among women with normogonadotropic anovulation (35). Although PCOS is commonly associated with obesity, there is no evidence that the prevalence of PCOS is increasing with the increasing prevalence of obesity (122).
IV. PCOS Metabolic Phenotype
A. Glucose tolerance (Fig. 5)
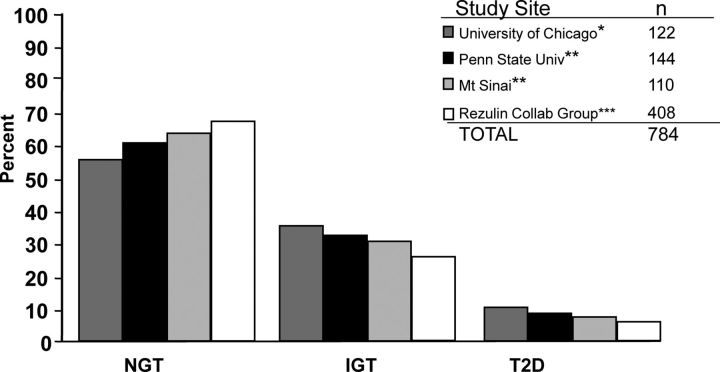
Figure 5.
Prevalence of glucose intolerance and T2D in PCOS. The prevalence of IGT and T2D in four large multiethnic PCOS cohorts is substantially increased. The true prevalence of diabetes was likely underestimated in these studies because diagnosed women with type 1 or type 2 diabetes were not included in the cohorts. NGT, Normal glucose tolerance.* [The University of Chicago data were reported by D. A. Ehrmann et al.: Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 22:141–146, 1999 (124), with permission. © American Diabetes Association.** The Penn State University and Mt. Sinai data were reported by R. S. Legro et al.: Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84:165–169, 1999 (123), with permission. © The Endocrine Society.*** The Rezulin (troglitazone) Collaborative Group data were reported by R. Azziz et al.: Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab 86:1626–1632, 2001 (392), with permission. © The Endocrine Society.] The figure is used with the permission of David Ehrmann.
Despite the fact that hyperinsulinemia reflecting some degree of peripheral insulin resistance was well recognized in PCOS by the mid 1980s, glucose tolerance was not systematically investigated until 1987 (49). This study reported that obese women with PCOS had significantly increased glucose levels during an oral glucose tolerance test (OGTT) compared with age- and weight-comparable reproductively normal control women. However, obese ovulatory hyperandrogenemic women had OGTT-glucose responses similar to control women, suggesting that derangements in glucose homeostasis were a feature of the anovulatory PCOS phenotype (i.e., NICHD criteria PCOS) rather than hyperandrogenemia per se. Twenty percent of the obese women with PCOS met criteria for impaired glucose tolerance (IGT) or type 2 diabetes (T2D) (34). Conversely, there were no significant differences in OGTT- glucose responses in lean women with PCOS compared with age- and weight-comparable reproductively normal control women. This study also suggested that metabolic features varied by PCOS phenotype, a finding that has been confirmed with investigation of the Rotterdam PCOS phenotypes (discussed in Section III.E. and reviewed in Ref. 101): women with NICHD PCOS are at the greatest metabolic risk. Accordingly, differing diagnostic criteria for PCOS will affect the results of metabolic investigations. The majority of the studies assessing glucose tolerance and insulin resistance have used the NICHD criteria for the diagnosis of PCOS.
The prevalence of IGT and T2D in U.S. women with PCOS has been assessed in three large cross-sectional studies in racially and ethnically diverse cohorts (123–125) (Fig. 5). The prevalence was 23–35% for IGT and 4–10% for T2D in these studies. Furthermore, prevalence rates of IGT and T2D did not change in a subgroup analysis limited to non-Hispanic white women (123). The prevalence rate of IGT in PCOS was 3-fold higher than the population prevalence rate in women of similar age from the National Health and Nutrition Survey (NHANES) II and twice the prevalence rate in age- and weight-comparable reproductively normal control women (123). The prevalence rate of undiagnosed T2D was 7.5- to 10-fold higher than the prevalence rate in NHANES II women of similar age (123, 124); none of the control women had T2D. Moreover, these studies likely underestimated the prevalence of diabetes mellitus in PCOS because they excluded women with diagnosed type 1 or type 2 diabetes (123–125).
Dysglycemia (fasting glucose ≥ 100 mg/dl, and/or 2-h postchallenge glucose ≥ 140 mg/dl) was mainly evident in post-glucose challenge glucose levels (Fig. 6), and the prevalence of dysglycemia increased with body mass index (BMI), being highest in obese affected women (i.e., BMI ≥ 30 kg/m2) (123, 125). However, even lean women with PCOS had increased rates of IGT and T2D (123). A first-degree relative with T2D increased risk for dysglycemia (123, 125). The majority of women in these studies were in their third and fourth decades of life; however, the prevalence rates of IGT and T2D were similarly increased in U.S. adolescents with PCOS (126).
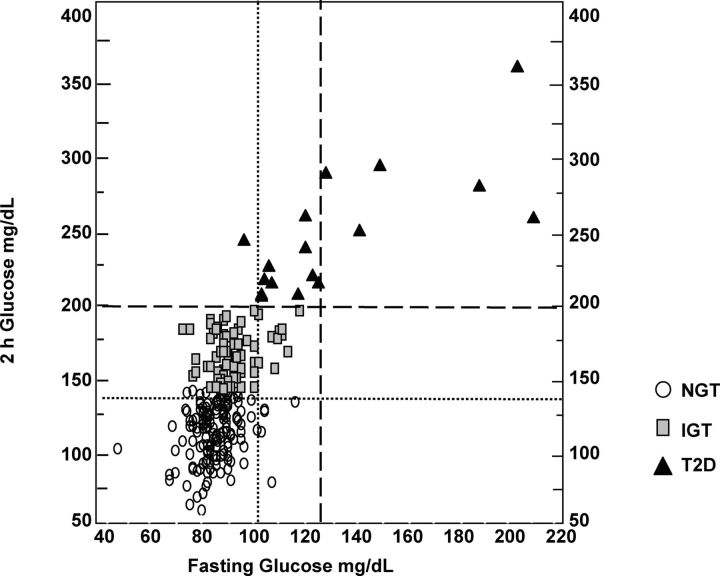
Figure 6.
Fasting and post-challenge dysglycemia in PCOS. The individual fasting and 2-h post-75 g oral glucose challenge glucose data from 254 women with PCOS are shown. The dotted vertical line is the fasting glucose threshold for impaired fasting glucose (100 mg/dl), the dashed vertical line is the fasting glucose threshold for diabetes (T2D) (126 mg/dl), the dotted horizontal line is the post-challenge glucose threshold for IGT (140 mg/dl), and the horizontal dashed line is the post-challenge glucose threshold (200 mg/dl) for T2D, according to the American Diabetes Association criteria (158). Most women with PCOS have post-challenge rather than fasting dysglycemia. NGT, Normal glucose tolerance. [Adapted from R. S. Legro et al.: Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84:165–169, 1999 (123), with permission. © The Endocrine Society.]
Prevalence rates of dysglycemia are elevated in non-U.S. women with PCOS but not to the same magnitude as those in U.S. women with PCOS. The prevalence rates of IGT and T2D were 15.7 and 2.5%, respectively, which was higher than the estimated rates in the general population in an Italian PCOS cohort (127). A telephone interview study of Dutch women with PCOS found a significant increase in diagnosed T2D compared with population prevalence estimates (128). Another European study (129) did not show increased prevalence rates of IGT and T2D in women with NICHD PCOS from Spain compared with age-, BMI-, and ethnicity-comparable control women. The reasons for these discrepant findings, apart for racial/ethnic differences, are unclear. Although the prevalence of obesity is higher and its severity is greater in U.S. PCOS populations (123, 124), such differences alone cannot account for differing rates of dysglycemia, which persist between European and U.S. PCOS cohorts in comparable BMI categories (123, 127, 129). Other factors, such as diet (130, 131) and race/ethnicity (118, 132), may contribute to higher prevalence rates of dysglycemia among U.S. women with PCOS.
A recent meta-analysis (133) reviewed more than 2000 studies of glucose tolerance in PCOS from which only 30 full-text studies were assessed for the final analysis. The increased prevalence of IGT and T2D in women with PCOS compared with women without PCOS, in both BMI- and non-BMI-matched studies, was confirmed. In the meta-analysis, the odds ratios (OR) and confidence intervals (CI) were significantly increased: IGT—OR, 2.48; 95% CI, 1.63–3.77; BMI-matched studies, OR, 2.54; 95% CI, 1.44–4.47; and T2D—OR, 4.43; 95% CI, 4.06–4.82; BMI-matched studies, OR, 4.00; 95% CI, 1.97–8.10. This meta-analysis confirms that the risk for IGT and T2D is increased in PCOS. PCOS is now recognized as a diabetes risk factor by the American Diabetes Association (134). Nevertheless, the magnitude of risk is unclear because most studies have been cross-sectional, relatively small, and lacking concurrently studied control women (135). Furthermore, differences in diagnostic criteria for PCOS, race/ethnicity, and BMI have led to variable risk estimates among PCOS cohorts (133, 135).
Large cross-sectional and prospective population-based studies are needed to accurately estimate the magnitude of T2D risk in PCOS. A recent prospective study in an Italian PCOS cohort confirmed an increased risk for T2D (594). However, some insights can be provided by prospective cohort studies that have used self-reported menstrual irregularity (136, 137) and/or hirsutism (138) as surrogate markers for PCOS. Among reproductive-age women with oligomenorrhea, as many as 90% may have PCOS, depending on the diagnostic criteria applied (35, 36, 139, 140). Furthermore, women with self-reported oligomenorrhea and/or hirsutism have reproductive and metabolic features of PCOS (138, 141), particularly those with both clinical findings (141). In Pima Indians (136) and in the Nurses Health Study II (137), the risk for T2D was significantly increased in women with menstrual irregularity. In the Nurses Health Study (137), a multivariate analysis adjusting for multiple confounders, including BMI at age 18, race, physical activity, first-degree relative with diabetes, smoking, and oral contraceptive use, found the relative risk for diabetes was 1.82 (95% CI, 1.35–2.44) in women with long or irregular menstrual cycles at ages 18–22 yr. The risk was increased by obesity but remained significant in lean women with irregular menses (137). This association was not confirmed in a relatively small U.S. prospective cohort study (142), but it was supported in a more recent and larger Dutch study (143). Several studies in postmenopausal women with a history of PCOS and/or PCO are consistent with an ongoing increased risk for T2D (144–148). These data suggest that PCOS increases the risk for T2D across a woman's lifespan.
There have been very few follow-up studies to assess conversion rates from normal glucose tolerance to IGT and from IGT to T2D. The conversion rate from normal to IGT or from IGT to T2D in PCOS has been estimated to range from 2.5 to 3.6% annually over a period of 3–8 yr (133, 149–152). These conversion rates are lower than in the general population of individuals with IGT who convert to T2D at rates of approximately 7% annually (153–155). This discrepancy likely represents an underestimate in conversion rates in PCOS because the studies have been limited by small sample size (133, 149–152).
Women with PCOS most commonly have postprandial dysglycemia (123, 125), which reflects peripheral, primarily skeletal muscle, insulin resistance (156) rather than fasting dysglycemia (Fig. 6), which reflects increased endogenous glucose production (156). Therefore, 2-h postchallenge glucose values are optimal for the diagnosis of IGT and T2D in PCOS (123, 125, 135) and the AES position statement (157) has recommended screening of all women with PCOS with a 75-g OGTT. The optimal time period for repeat OGTT is uncertain. A hemoglobin A1c value between 5.7 and 6.4% has been recommended by the American Diabetes Association (158) for predicting increased diabetes risk. A recent study (159) that assessed the utility of hemoglobin A1c to detect IGT and diabetes in PCOS found that this test had low sensitivity when compared with OGTT assessment of glucose tolerance. This discrepancy in diagnostic accuracy may be because affected women have mainly post-glucose challenge rather than fasting dysglycemia (123, 125).
B. Insulin resistance
1. Insulin action in vivo
Insulin acts to regulate glucose homeostasis by stimulating glucose uptake by insulin-responsive target tissues, adipocytes, and skeletal and cardiac muscle, as well as by suppressing hepatic glucose production (160, 161). Insulin also suppresses lipolysis, resulting in a decrease in circulating free fatty acid levels (162), which may mediate the action of insulin on hepatic glucose production (163–165). Insulin resistance has traditionally been defined as a decreased ability of insulin to mediate these metabolic actions on glucose uptake, glucose production, and/or lipolysis, resulting in a requirement for increased amounts of insulin to achieve a given metabolic action (166). Accordingly, insulin resistance is characterized by increased circulating insulin levels, basally and in response to a glucose load, if pancreatic β-cell function is intact (166, 167). Insulin has other metabolic as well as mitogenic and reproductive actions (discussed in Sections IV.D. and V.A. and in Refs. 12, 168, and 169), but it is unknown whether isolated defects in these pathways would provoke compensatory hyperinsulinemia.
The “gold standard” for assessing metabolic insulin resistance in vivo is the hyperinsulinemic, euglycemic glucose clamp technique (167, 170). This technique quantitatively assesses insulin action on whole-body glucose uptake by infusing a desired dose of insulin and maintaining euglycemia using a variable glucose infusion where the rate is adjusted based on frequent arterialized blood glucose determinations and a negative feedback principle (167, 170). At steady state, the amount of glucose that is infused equals the amount of glucose taken up by the peripheral tissues, and it can be used as a measure of peripheral sensitivity to insulin, known as insulin-mediated glucose disposal (IMGD) or M (167, 170). In lean, normal individuals, skeletal muscle accounts for about 85% of IMGD (160). As fat mass increases, it accounts for a larger amount of IMGD (160). Endogenous glucose production, which reflects both hepatic and renal glucose production (167, 170–172), can be determined by the infusion of isotopically labeled glucose at baseline and during the euglycemic clamp (173, 174). The suppression of hepatic glucose production can be assessed by determining the decrease in endogenous glucose production in response to insulin (173, 174).
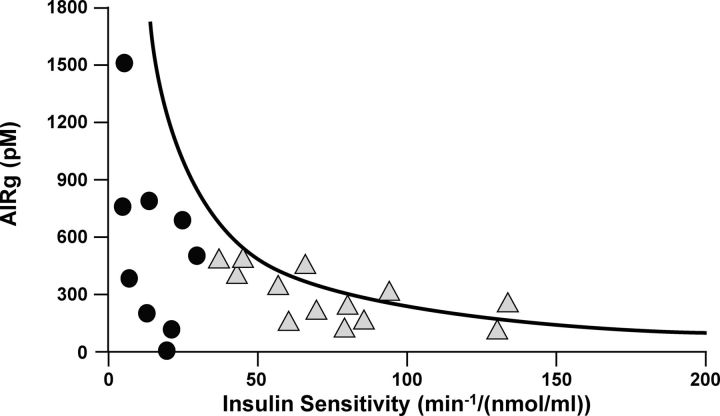
Whole-body insulin sensitivity can also be accurately measured in subjects without diabetes using the frequently sampled iv glucose tolerance test (FSIGT) with minimal model analysis (167). The minimal model determines insulin sensitivity (sensitivity index), which reflects insulin action to stimulate glucose uptake as well as to suppress glucose production (167). The acute insulin response to glucose (AIRg) is also determined from the FSIGT data. The disposition index (DI), the product of AIRg and insulin sensitivity, assesses insulin secretion in the context of insulin sensitivity and is a robust parameter of pancreatic β-cell function (161, 175) that will be discussed in Section IV.E. It is possible to model hepatic glucose production with the administration of isotopically labeled glucose during the FSIGT (176, 177), but this measurement is rarely performed because the tracer is expensive and the model is complex. The standard FSIGT is substantially easier and less expensive to perform than the clamp, although it is still an investigational procedure that requires frequent blood sampling.
The FSIGT provides quantitative, reproducible measurements of insulin sensitivity in individuals without T2D; in patients with diabetes, it may not be possible to differentiate between very low insulin sensitivity values (178, 179). The FSIGT also provides a simultaneous assessment of insulin secretion (161, 178). The euglycemic clamp provides a quantitative, reproducible measurement of insulin action across a spectrum of insulin sensitivities and can be used in patients with T2D (167, 170). Endogenous, primarily hepatic, glucose production can also be assessed during the clamp (170, 178). The measurement of insulin secretion requires a separate, hyperglycemic clamp study (170, 178, 180). The glucose clamp procedure requires highly trained personnel and specialized equipment (167, 170). It is also substantially more expensive to perform than the FSIGT (167).
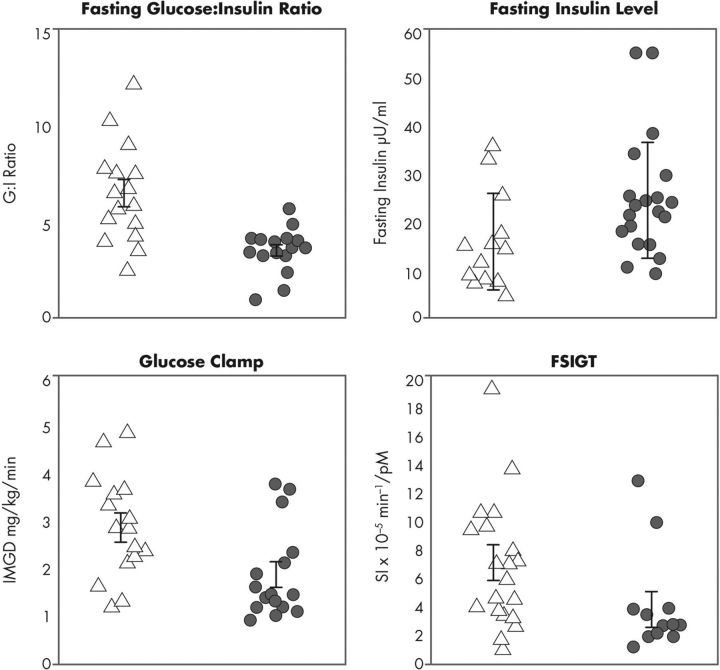
Because of the complexity and expense of the clamp and the FSIGT, there has been a desire to use fasting parameters of glucose homeostasis as surrogate measures of insulin resistance. These measures include homeostatic model assessment (181), fasting glucose:insulin ratio (182), and quantitative insulin sensitivity check index (183). They are all based on fasting glucose and insulin levels and essentially provide identical information (184). Fasting glucose levels reflect endogenous glucose production (156), an index of hepatic rather than peripheral insulin action (156). Fasting insulin levels reflect not only insulin sensitivity but also insulin secretion and clearance (184). Accordingly, fasting insulin levels will not provide accurate information on insulin sensitivity in individuals with β-cell dysfunction (184). OGTT-derived parameters of insulin action have also been shown to be insensitive to large changes in insulin sensitivity (184). Although fasting measures (185) and OGTT-derived parameters (186) may correlate with clamp or FSIGT measures of insulin sensitivity, they lack precision for quantitatively measuring insulin resistance in the general population (184). These measures have been found to be similarly imprecise for the assessment of insulin sensitivity in women with PCOS (187).
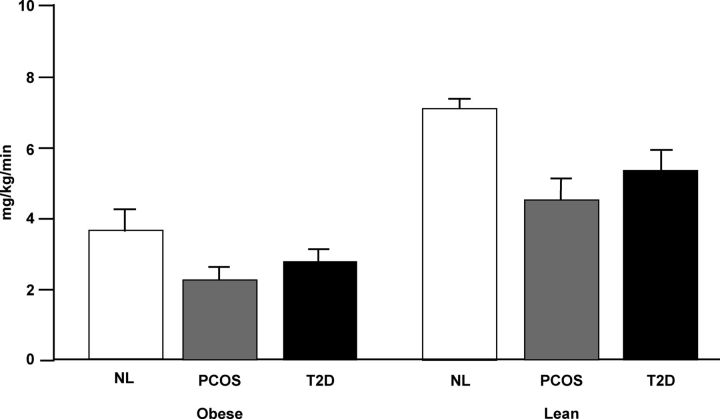
PCOS women have an increased prevalence of obesity (19, 122, 188), and women with upper as opposed to lower body obesity have an increased frequency of hyperandrogenism (189). Androgens can also increase visceral fat mass in women (190). Muscle is the major site of insulin-mediated glucose use (160), and androgens can increase muscle mass (191). Thus, potential changes in lean body (primarily muscle) and fat mass as well as in fat distribution should be considered to accurately assess insulin action in PCOS (81, 192). In 1989 (81), it was shown that IMGD measured by euglycemic clamp was significantly and substantially decreased (∼35–40%) in women with PCOS compared with age- and body composition-comparable reproductively normal control women (81, 192) (Fig. 7). The decrease in IMGD in PCOS was of a similar magnitude to that reported in T2D (160) (Fig. 7). Furthermore, IMGD was significantly decreased per kilogram of fat free, primarily muscle, mass (160). IMGD was also significantly decreased in lean PCOS women, all of whom had normal glucose tolerance.
Figure 7.
Decreased IMGD in PCOS. IMGD at steady-state insulin levels of 100 μU/ml is significantly decreased by 35–40% in women with PCOS (gray bars), independent of obesity, compared with age- and weight-matched control women (NL, open bars). This decrease is similar in magnitude to that reported in T2D (open bars) (160). This figure is used with the permission of Andrea Dunaif.
Body fat topography, upper compared with lower body, can affect insulin sensitivity (7), with increases in upper body and visceral fat being associated with decreased insulin sensitivity (7, 193). The study of Dunaif et al. (81) did not control for this parameter, and some subsequent studies have suggested that increases in upper body obesity, assessed by waist and hip measurements and adipocyte size, are associated with insulin resistance in PCOS (192, 194–197). However, visceral fat mass accurately quantified by magnetic resonance imaging (MRI) (197, 198) or by computerized tomography (199) does not differ in women with PCOS compared with BMI-matched control women. Thus, the study of PCOS and control women of comparable BMI appears to be sufficient to control for the confounding effects of obesity as well as of fat distribution on insulin sensitivity.
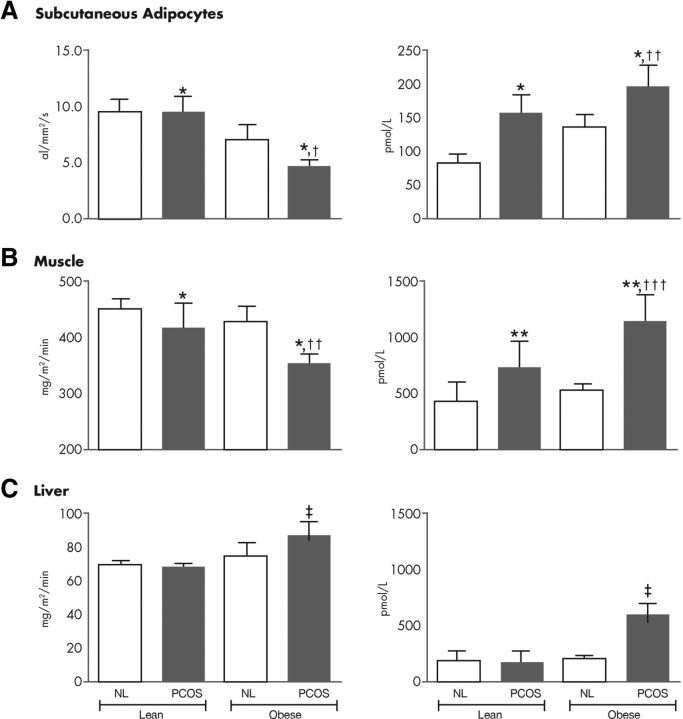
Insulin has concentration-dependent saturable actions that can be examined in vivo using sequential multiple insulin dose euglycemic clamp studies (200). The concentration required for a half-maximal (ED50) response defines insulin sensitivity and usually reflects insulin receptor binding or phosphorylation, whereas the maximal biological effect is defined as insulin responsiveness and usually reflects postreceptor events, for example, translocation of the GLUT4 glucose transporter for IMGD (166). Dose-response studies have indicated that the ED50 insulin for glucose uptake was significantly increased and that maximal rates of IMGD were significantly decreased in lean and in obese women with PCOS women (192) (Fig. 8). It appears, however, that body fat has a more pronounced negative effect on insulin sensitivity in women with PCOS (201, 202). Basal endogenous glucose production and the ED50 insulin for suppression of endogenous glucose production were significantly increased only in obese PCOS women (81, 192) (Fig. 8). This synergistic negative effect of obesity and PCOS on endogenous glucose production is an important factor in the pathogenesis of glucose intolerance (34, 81, 123, 192).
Figure 8.
Insulin action in isolated sc adipocytes and in vivo. The dose-response of insulin-stimulated glucose uptake was determined in isolated sc adipocytes in vitro and in vivo during sequential multiple insulin dose euglycemic glucose clamp studies. Maximal rates of glucose uptake (insulin responsiveness) in isolated sc adipocytes are depicted in vitro (A, left) and in vivo, which reflects primarily skeletal muscle glucose uptake (B, left). Rates of postabsorptive endogenous glucose production (EGP) (C, left) and its suppression by insulin were also assessed during the euglycemic glucose clamp study. The ED50 insulin (insulin sensitivity) for stimulation of glucose uptake and suppression of EGP are depicted in the graphs on the right (A, sc adipocytes in vitro; B, in vivo; C, EGP). Women with PCOS, gray bars; normal control women (NL), open bars. A two-way ANOVA with PCOS and obesity as factors was applied: *, P < 0.01 PCOS groups vs. NL groups; †, P < 0.05 obese groups vs. lean groups; ††, P < 0.01 obese groups vs. lean groups; †††, P < 0.001 obese groups vs. lean groups; ‡, P < 0.05 interaction PCOS and obesity. [Adapted from data published in A. Dunaif et al.: Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes 41:1257–1266, 1992 (192), with permission. © American Diabetes Association.]
Many subsequent studies using euglycemic glucose clamps or FSIGTs have confirmed that women with PCOS have profound resistance to the action of insulin to stimulate glucose uptake (for example, see Refs. 201 and 203–209). There is general consensus that obese women with PCOS are insulin resistant (24). However, several studies have failed to demonstrate insulin resistance in lean women with PCOS (for example, see Refs. 205, 210, and 211) using the highly sensitive euglycemic glucose clamp technique. Some of these conflicting results can be accounted for by differences in the diagnostic criteria for PCOS that resulted in the inclusion of women with ovulatory cycles and hyperandrogenism who have minimal to absent evidence for insulin resistance (see discussion in Section III.E. of diagnostic criteria and Refs. 34, 89, 96, 98, and 101). However, it is also possible that racial/ethnic differences in insulin action (118, 132) or environmental factors such as diet (130, 131) contributed to these discrepant findings.
Attempts to quantitate the prevalence of insulin resistance in PCOS are limited by the methods used to determine insulin sensitivity. Prevalence rates of insulin resistance have been reported from 44 to 70% (187, 212–216) using surrogate markers, which lack sensitivity and specificity (184, 187). Even when insulin resistance is assessed using the euglycemic glucose clamp, it is clear that some women with PCOS have normal insulin sensitivity (81) (Fig. 9). Thus, defects in insulin action on glucose metabolism are not a universal feature of the syndrome. Indeed, two of the PCOS phenotypes identified with the Rotterdam criteria (Table 3)—hyperandrogenism and PCO with ovulatory cycles, and anovulation and PCO without hyperandrogenism—have modest (217) or absent (99) evidence for insulin resistance using surrogate markers. Nevertheless, it remains possible that there is increased sensitivity to the reproductive actions of insulin in PCOS because hyperandrogenism and anovulation improve during metformin treatment in women with PCOS without evidence for insulin resistance (218). Alternatively, these improvements may be related to a direct action of metformin on steroidogenesis (219).
Figure 9.
Fasting and dynamic measures of insulin resistance. Fasting measure of insulin sensitivity, the glucose:insulin ratio (185) and insulin levels are shown in the top graphs. Dynamic measures of insulin sensitivity, the euglycemic glucose clamp determined IMGD, and sensitivity index (SI) assessed by minimal model analysis of FSIGT are shown in the bottom graphs. For all measures of insulin action, there is considerable overlap between control (open triangles) and PCOS (gray circles) women. The data have been previously published (81, 185, 301) and were adapted for use in this figure, which is used with the permission of Andrea Dunaif.
2. Cellular and molecular mechanisms of insulin resistance (Fig. 10)
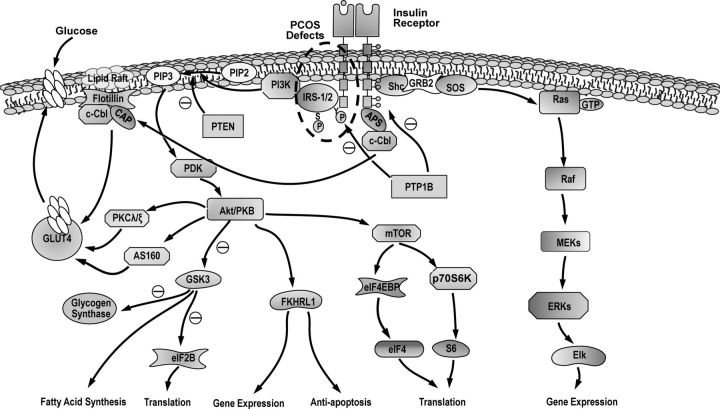
Figure 10.
Insulin receptor signaling pathways. The insulin receptor is a heterotetramer consisting of two α, β dimers linked by disulfide bonds. The α-subunit contains the ligand binding domain, and the β-subunit contains a ligand-activated tyrosine kinase. Tyrosine autophosphorylation increases the receptor's intrinsic tyrosine kinase activity, whereas serine phosphorylation inhibits it. The tyrosine-phosphorylated insulin receptor phosphorylates intracellular substrates, such as IRS 1–4, Shc, and APS, initiating signal transduction pathways mediating the pleiotropic actions of insulin. The major pathway for the metabolic actions of insulin is mediated through activation of PI3-K and Akt/PKB, resulting in the translocation for the insulin responsive glucose transporter, GLUT4, from intracellular vesicles to the plasma membrane. Insulin activation of PI3-K and Akt/PKB also leads to serine phosphorylation of GSK3, resulting in inhibition of its kinase activity. The inhibition of GSK3 results in dephosphorylation of glycogen synthase increasing glycogen synthesis. The Ras-ERK/MAPK pathway regulates gene expression. Insulin modulates protein synthesis and degradation via mTOR, which is activated via PI3-K and Akt/PKB. The mTOR pathway is also important in nutrient sensing. Insulin-stimulated inhibition of GSK3 via PI3-K and Akt/PKB also results in dephosphorylation of eIF2B increasing protein synthesis. Insulin signaling can be terminated by dephosphorylation of the receptor by tyrosine phosphatases, such as PTP1B, or dephosphorylation of PI3-K by PTEN. Serine phosphorylation of the insulin receptor and IRSs can also decrease insulin signaling and may be mediated by serine kinases in the insulin signaling pathway providing a feedback mechanism to terminate insulin action. There is a post-binding defect in insulin signaling in PCOS affecting metabolic but not mitogenic pathways (see Fig. 11 for details). The signaling steps that are compromised in PCOS are circled with a dotted line. Signaling steps downstream of these abnormalities may also be compromised. SOS, Son-of-sevenless. This figure is used with the permission of Andrea Dunaif.
a. Molecular mechanisms of insulin action.
Insulin has multiple cellular actions beyond the regulation of glucose uptake (220). It has other anabolic effects to increase storage of lipids and proteins as well as to promote cell growth and differentiation (220). Insulin acts on cells by binding to its cell surface receptor (221, 222). The insulin receptor is a heterotetramer made up of two α,β dimers linked by disulfide bonds (223). Each α,β dimer is the product of one gene (224, 225). The α-subunit is extracellular and contains the ligand-binding domain; it also inhibits the intrinsic kinase activity of the β-subunit (220, 222). The β-subunit spans the membrane, and the cytoplasmic portion contains intrinsic protein tyrosine kinase activity, which is activated further by ligand-mediated autophosphorylation (226). The insulin receptor shares substantial structural homology the IGF-I receptor and the insulin-related receptor (220). The α,β dimer of the insulin receptor can assemble with similar dimers of the IGF-I receptor or insulin-related receptor to form hybrid receptors (227).
Ligand binding induces autophosphorylation of the insulin receptor on specific tyrosine residues and further activation of its intrinsic kinase activity (228–230). The activated insulin receptor then tyrosine-phosphorylates intracellular substrates, such as insulin receptor substrates (IRS) 1–4, src homolog and collagen homolog (Shc), and APS [adapter protein with a PH and homology 2 (SH2) domain], to initiate signal transduction (222, 231, 232). The IRS are phosphorylated on specific motifs, and these phosphorylated sites then bind signaling molecules, such as the SH2 domain of phosphatidylinositol 3-kinase (PI3-K) or the adaptor molecule, Nck (220, 222, 233), leading to activation of downstream signaling pathways.
Insulin stimulates glucose uptake by increasing the translocation of the insulin-responsive glucose transporter, GLUT4, from intracellular vesicles to the cell surface (222, 232). This pathway is mediated by activation of PI3-K, which then phosphorylates membrane phospholipids and phosphatidylinositol 4,5-bisphosphate, leading to activation of the 3-phosphoinositide-dependent protein kinases (PDK-1 and PDK-2) (220, 232). These kinases activate the serine/threonine kinases Akt/protein kinase B (PKB) and atypical protein kinase C λ and ζ, (PKCλ/ζ). Akt/PKB transmits the signal by phosphorylation of its 160-kDa substrate, AS160 (220, 232). Both of these pathways stimulate the translocation of GLUT4 to the cell surface (220, 232). Glycogen synthase activity is constitutively inhibited via phosphorylation by glycogen synthase kinase-3 (GSK3) (220, 232). Activation of Akt/PKB also results in the serine phosphorylation and inactivation of GSK3, allowing glycogen synthase activity to increase and resulting in glycogen synthesis (220, 232, 234).
Insulin stimulates cell growth and differentiation through the MAPK-ERK (220, 232) pathway (220, 235). This pathway is activated by insulin receptor-mediated phosphorylation of Shc or IRS, leading to association with Grb2 and Son-of-sevenless resulting in Ras activation (220). This activation stimulates a cascade of serine/threonine kinase resulting in the stepwise stimulation of Raf, MAPK kinase (MEK) and MAPK-ERK1/2. ERK1/2 translocates to the nucleus and phosphorylates transcription factors to initiate cell growth and differentiation (220). This so-called mitogenic pathway can be disrupted without affecting the metabolic actions of insulin and vice versa (220, 236–238). As a result, insulin resistance can be selective and affect only metabolic but not mitogenic pathways of insulin action (236, 239, 240). Insulin regulates protein synthesis and degradation via mammalian target of rapamycin (mTOR) (220), which is activated via PI3-K. The mTOR pathway is also important in nutrient sensing (241). Insulin-stimulated inhibition of GSK3 via PI3-K and Akt/PKB also results in dephosphorylation of eukaryotic initiation factor 2B (eIF2B) activating protein synthesis (234).
The insulin signal can be terminated by dephosphorylation of proximal signaling molecules. Multiple tyrosine phosphatases, such as protein tyrosine phosphatase 1B (PTP1B), can dephosphorylate the insulin receptor to terminate the insulin signal (220, 232). Phosphatase and tension homolog deleted on chromosome 10 (PTEN), a lipid phosphatase, decreases PI3-K signaling by dephosphorylating lipid signaling molecules (232, 242, 243). Serine phosphorylation of the insulin receptor and IRS can also inhibit insulin signaling (220, 244–246). It has been postulated that PKC-mediated serine phosphorylation of the insulin receptor is important in the pathogenesis of hyperglycemia-induced insulin resistance (246–248) and that the mechanism of TNF-α-mediated insulin resistance is serine phosphorylation of IRS-1 (249). Furthermore, it has been suggested that a number of serine/threonine kinases in the insulin signaling pathway, such as PI3-K, Akt/PKB and GSK3, can serine phosphorylate the insulin receptor and/or IRS-1 to attenuate signaling providing a feedback mechanism to terminate insulin action (220, 246).
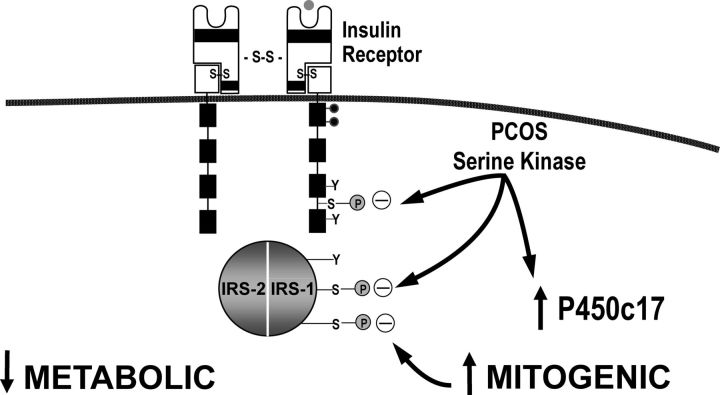
b. Molecular defects in PCOS (Fig. 11).
Figure 11.
Insulin signaling defects in PCOS. There is a post-binding defect in insulin signaling in PCOS resulting in marked decreases in insulin sensitivity (see Fig. 8). There is a more modest defect in insulin responsiveness. The signaling defect is due to serine phosphorylation of the insulin receptor and IRS-1 secondary to intracellular serine kinases. This results in decreased insulin-mediated activation of PI3-K and resistance to the metabolic actions of insulin. There is constitutive activation of kinases in the ERK/MAPK mitogenic pathway in PCOS, and these kinases contribute to inhibitory serine phosphorylation of IRS-1 in PCOS skeletal muscle. Serine phosphorylation of P450c17 increases its activity, and it has been postulated that the same kinase may inhibit insulin signaling and increase androgen production in PCOS. S-S, Disulfide bond; Y, tyrosine; S, serine; P, phosphate. This figure is used with the permission of Andrea Dunaif.
The cellular and molecular mechanisms of insulin action in PCOS have been characterized in cultured skin fibroblasts, which are not classic insulin target tissues (222). Defects in fibroblast insulin action that persist in cells that have been removed from the in vivo environment for many passages suggest that the changes are the result of mutations in genes regulating these pathways (10, 250). Consistent with this hypothesis, decreases in insulin receptor binding or autophosphorylation in cultured skin fibroblasts have reflected mutations in the insulin receptor gene in patients with the syndromes of extreme insulin resistance (10, 12).
Insulin action has also been examined in the classic insulin target tissues for glucose uptake, adipocytes and skeletal muscle (160, 222). The size of sc adipocytes isolated from both lean and obese women with PCOS was increased (192, 197). Insulin receptor number and/or receptor affinity was similar to control women in isolated sc adipocytes (192, 251). However, decreased insulin receptor β-subunit abundance has been reported in homogenates of omental adipose tissue from women with PCOS (252). The most striking and consistent defect in adipocyte insulin action in PCOS was a marked increase in the ED50 for insulin-mediated glucose uptake (192, 206, 251), indicating a decrease in insulin sensitivity, when compared with isolated adipocytes from appropriately weight-comparable reproductively normal control women (Fig. 8). The decrease in insulin sensitivity suggested that there was a defect in insulin receptor binding or phosphorylation (166).
Most studies have also found less striking, but significant, decreases in maximal rates of insulin-stimulated glucose transport (192, 253), insulin responsiveness, suggesting a decrease in post-receptor events (192, 251) (Fig. 8). Significant decreases in the abundance of GLUT4 glucose transporters in sc adipocytes from women with PCOS most likely accounted for the decrease in insulin responsiveness (252, 254). However, a recent study (206) failed to find decreases in insulin responsiveness or GLUT4 abundance in sc adipocytes isolated from women with PCOS, despite the fact the euglycemic clamp studies in these PCOS subjects showed decreased insulin responsiveness for IMGD consistent with a postbinding defect in insulin action. The reasons for these discrepant results in isolated sc adipocytes are unclear because both studies used the same diagnostic criteria (NICHD) for PCOS and contained control women of comparable BMI (206, 254). Similar defects in adipocyte insulin action have been reported in T2D and in obesity but are ameliorated by control of hyperglycemia and hyperinsulinemia as well as by weight-reduction, suggesting acquired rather than intrinsic defects (255–257). In contrast, in PCOS such defects can occur in the absence of obesity and glucose intolerance (192, 254). Moreover, these abnormalities are not significantly correlated with sex hormone levels (55, 117).
There have been no differences in abundance of downstream signaling proteins, IRS-1, Akt/PKB 1/2, PKCζ, c-Cbl-associated protein, or cbl or in activation of Akt/PKB at maximal insulin doses in PCOS adipocytes (206). However, one study suggested abnormalities in the phosphorylation of GSK3β, which is a substrate for Akt/PKB, in PCOS adipocytes (253). These studies are constrained by the fact that signaling protein abundance and basal phosphorylation may be unaltered, but insulin-stimulated activation may still be defective in insulin-resistant states (220). Studies using maximally stimulating doses of insulin may fail to detect alterations in insulin sensitivity (232). Akt/PKB activation may be normal despite substantial insulin resistance (232, 258). Furthermore, downstream signaling events may be decreased if there are defects in signaling at the level of the insulin receptor (220).
Insulin receptor function in PCOS was investigated in receptors isolated from cultured skin fibroblasts. Consistent with findings in isolated adipocytes (192, 251), there was no change in insulin binding or receptor affinity compared with control women (69). However, insulin receptor basal autophosphorylation was markedly increased with minimal further insulin-stimulated autophosphorylation in receptors isolated from approximately 50% of PCOS fibroblasts (259). Insulin-dependent receptor tyrosine autophosphorylation was significantly decreased (259, 260). Insulin-independent receptor serine phosphorylation was markedly increased (259), and these receptors had reduced intrinsic tyrosine kinase activity, suggesting that serine phosphorylation inhibited normal receptor signaling (259). Although fibroblasts are not a classic insulin target tissue for glucose uptake, insulin receptors isolated from skeletal muscle biopsies from women with PCOS had similar abnormalities in phosphorylation, suggesting that this defect was physiologically relevant (259).
Isolating insulin receptors from lysates of PCOS skin fibroblasts by immunopurification before insulin-stimulated autophosphorylation corrected constitutive increases in receptor serine phosphorylation (259). Furthermore, mixing lysates from PCOS skin fibroblasts with purified human insulin receptors resulted in increased receptor serine phosphorylation (259). Taken together, these findings suggested that a serine kinase extrinsic to the insulin receptor was responsible for the abnormal pattern of receptor phosphorylation (259). These findings were supported by an independent group of investigators (260) who confirmed significant decreases in PCOS skin fibroblast insulin receptor autophosphorylation. Furthermore, they demonstrated that decreased receptor autophosphorylation could be corrected immunocapture of the insulin receptor before insulin stimulation, consistent with the presence of a factor extrinsic to the receptor as the cause of the defect (260). Most importantly, serine kinase inhibitors corrected the phosphorylation defect, supporting the role of a serine kinase extrinsic to the insulin receptor as the cause of decreased receptor autophosphorylation (260).
This defect in the early steps of the insulin signaling pathway may cause the insulin resistance in a subpopulation of women with PCOS (Fig. 11). Increased insulin-independent serine phosphorylation in PCOS insulin receptors appears to be a unique disorder of insulin action because other insulin-resistant states, such as obesity, T2D, Type A syndrome, and leprechaunism, do not exhibit this abnormality (222, 255, 259). In approximately 50% of PCOS women, insulin receptor phosphorylation in receptors isolated from skin fibroblasts was similar to control women (259), despite the fact that these women had comparable severity of insulin resistance as that found in affected women with abnormal insulin receptor phosphorylation. This observation suggests that a defect downstream of insulin receptor phosphorylation, such as phosphorylation of IRS-1 or activation of PI3-K, was responsible for insulin resistance in some PCOS women (220, 222, 259).
Studies of insulin signaling in vivo have shown a significant decrease in insulin-mediated IRS-1-associated PI3-K activation in serial skeletal muscle biopsies obtained during a euglycemic clamp study in association with decreased IMGD in women with PCOS (261). The abundance of the insulin receptor, IRS-1, and the p85 subunit of PI3-K was unchanged, consistent with an abnormality in insulin receptor and/or post-receptor phosphorylation events (261). The abundance of IRS-2 was increased, suggesting a change to compensate for decreased signaling via IRS-1 (261). Analogous to in vitro studies, the signaling changes occurred rapidly and were evident in biopsies at 15- and 30-min time points during each insulin dose, but the changes had returned to baseline by 90 min of each infusion (261). This study confirmed that there is a physiologically relevant defect in rapid insulin receptor-mediated signaling in the major insulin target tissue for IMGD, skeletal muscle.
Højlund et al. (262), however, did not find differences in insulin-stimulated IRS-1-associated PI3-K activity in skeletal muscle biopsies taken after 3 h of insulin infusion, despite significant decreases in IMGD in women with PCOS. Nevertheless, this finding is consistent with the time course of these signaling changes determined in the previous study of Dunaif et al. (261). These authors did find signaling abnormalities in the activation of Akt/PKB and its downstream target for GLUT4 translocation, AS160 (262) (Fig. 10). The decrease in insulin receptor-mediated IRS-1 phosphorylation and PI3-K activation identified in PCOS skeletal muscle (263) could account for these changes because these signaling events are downstream in the pathway of insulin-stimulated glucose uptake (232) (Fig. 10). In contrast, Ciaraldi et al. (206) failed to find changes in Akt/PKB activation in skeletal muscle biopsies from PCOS women taken after 3 h of insulin infusion, despite significant decreases in IMGD in affected women. However, they used maximally stimulating doses of insulin, whereas Højlund et al. (262) used physiological doses of insulin. Accordingly, changes in the sensitivity of Akt/PKB activation to insulin could have escaped detection in the Ciaraldi study (206).
It is possible to isolate myoblasts from human skeletal muscle biopsies, culture these cells in vitro, and differentiate them into myotubes (264–266). This culture system has been used to investigate whether the defects in insulin action in PCOS skeletal muscle are the result of the in vivo hormonal environment or reflect intrinsic abnormalities (206, 267–269). Cultured myotubes from women with PCOS had a distinctive phenotype: despite similar population doublings, they had an increase in markers of differentiation compared with myotubes from control women (267). Insulin action findings in PCOS myotubes have been conflicting. Corbould et al. (267) found that basal and insulin-stimulated glucose transport was increased in PCOS compared with control myotubes, but the increments in glucose transport were similar in both groups. GLUT1 abundance was increased in PCOS myotubes and correlated with the increases in basal, non-insulin-mediated glucose transport, whereas GLUT4 abundance was unchanged in PCOS compared with control myotubes (267). In contrast, Ciaraldi et al. (206) found that both basal and maximal insulin-stimulated glucose transport were decreased in another study of PCOS myotubes. PCOS myotube GLUT4 abundance did not differ in PCOS and control myotubes in this study (206). Eriksen et al. (269, 270) found no significant changes in glucose transport in PCOS myotubes, although there was a trend toward higher basal rates of glucose transport in PCOS myotubes. Insulin action on other metabolic parameters, such as glycogen synthesis and lipid uptake, also did not differ in PCOS compared with control myotubes (270).
The most comprehensive study of insulin signaling in PCOS myotubes by Corbould et al. (267) found no differences in insulin receptor β-subunit abundance or tyrosine phosphorylation. However, the abundance of IRS-1 was increased in PCOS myotubes. When normalized for IRS-1 abundance, PI3-K activity was decreased in PCOS myotubes. Furthermore, phosphorylation of the IRS-1 inhibitory serine 312 was increased in PCOS myotubes. IRS-2-associated PI3-K activity was also decreased in PCOS myotubes. These findings suggest that there are intrinsic abnormalities in insulin signaling in PCOS myotubes, despite the fact that glucose transport is not compromised (267). It is possible that these abnormalities confer increased susceptibility to circulating factors that induce insulin resistance, such as free fatty acids or TNF-α (267).
Ciaraldi et al. (206) found no changes in IRS-1, Akt/PKB 1/2, PKCζ, c-Cbl-associated protein, or cbl protein expression in PCOS myotubes, analogous to their findings in skeletal muscle biopsies. They only examined activation of Akt/PKB at maximal insulin doses and did not detect any changes in PCOS compared with control myotubes, despite the fact that this group reported decreased basal and insulin-stimulated glucose transport in these PCOS myotubes (206). Activation of PI3-K was not examined, and as discussed in this Section, decreases in the sensitivity of Akt/PKB activation by insulin could have escaped detection by the use of only maximally stimulating doses of insulin (232).
In addition to insulin signaling defects, it has also been suggested that mitochondrial dysfunction may contribute to insulin resistance in PCOS skeletal muscle. In T2D, decreased numbers of skeletal muscle mitochondria have been reported (271). Skeletal muscle biopsies from women with PCOS have shown decreased expression of genes involved in mitochondrial oxidative metabolism (272). Furthermore, pioglitazone-mediated improvements in insulin sensitivity were associated with increased expression of genes involved in mitochondrial phosphorylation pathways in these affected women (273). However, there were no differences in mitochondrial number or function in cultured myotubes from women with PCOS (269). These findings suggest that changes in mitochondrial oxidative gene expression in PCOS skeletal muscle are not a primary defect.
C. Other metabolic actions of insulin in PCOS
There have been limited studies of insulin action lipid homeostasis in PCOS. Fasting free fatty acid levels have been increased (274) or unchanged (262) in obese women with PCOS compared with control women of similar weight. There has been decreased insulin-mediated suppression of lipid oxidation during euglycemic clamp studies in obese women with PCOS (262). However, lipid uptake and oxidation did not differ from control in PCOS myotubes (270).
Alterations in catecholamine regulation of lipolysis have been reported in PCOS. There was decreased sensitivity to catecholamine-stimulated lipolysis in adipocytes isolated from the sc fat depot of lean women with PCOS, which may favor increased fat cell size (275). In contrast, adipocytes isolated from the visceral fat depot of lean women with PCOS had increased catecholamine-stimulated lipolysis (276). The cellular mechanisms of this defect, alterations in protein kinase A subunit expression and decreases in hormone sensitive lipase, differed from those in visceral adipocytes in subjects with the metabolic syndrome (276). This increase in catecholamine-stimulated lipolysis may contribute to hepatic insulin resistance by increasing portal free fatty acid delivery to the liver (163). Insulin action to suppress lipolysis was similar in visceral adipocytes from lean women with PCOS and control women (276). There are no reports of insulin action on protein turnover in PCOS.
D. Mitogenic actions of insulin in PCOS
Insulin's mitogenic actions on cell growth and differentiation can be regulated by the MAPK-ERK 1/2 pathway independently of insulin's metabolic action (220) (Fig. 10). The metabolic pathway can be disrupted without altering the mitogenic pathway (220). Such so-called selective insulin resistance has been found in cultured skin fibroblasts from patients with extreme insulin resistance (236). A similar selective defect in insulin action was found in cultured skin fibroblasts from women with PCOS (277). Both insulin- and IGF-I-stimulated glycogen synthesis were significantly decreased in PCOS fibroblasts, whereas thymidine incorporation was similar to that in control fibroblasts (277).
Euglycemic clamp studies in subjects with T2D have demonstrated decreased metabolic signaling via PI3-K with preserved mitogenic signaling via MAPK-ERK1/2 in skeletal muscle biopsies (240). In skeletal muscle biopsies from women with PCOS, MAPK-ERK1/2 was constitutively activated (268). This alteration persisted in cultured PCOS myotubes where MAPK-ERK1/2 was activated basally and in response to insulin, whereas MEK activation was increased only in response to insulin (268). The activity of p21 Ras was significantly decreased, and the abundance of Raf-1 was increased, suggesting that the alteration of signaling began at this molecule. Pharmacological inhibition of MEK1/2 inhibited MAPK-ERK1/2 activation, reduced IRS-1 serine 312 phosphorylation, and enhanced IRS-1-associated PI3-K activation (268). These findings suggested that activation of MAPK-ERK1/2 contributed to serine phosphorylation of IRS-1 and diminished metabolic signaling in PCOS myotubes. MAPK-ERK1/2 may be a serine kinase contributing to the increased serine phosphorylation of IRS-1 and, perhaps, the insulin receptor in PCOS (55, 259, 268), although myotube insulin receptor serine phosphorylation was not directly examined. Furthermore, these findings suggest that a primary activation of mitogenic signaling pathways produces metabolic insulin resistance by serine phosphorylating proximal metabolic signaling molecules, such as IRS-1 (268) (Fig. 11).
A recent study in skeletal muscle biopsies from women with PCOS confirmed the constitutive activation of MAPK-ERK1/2 (278). This study also reported that insulin-stimulated activation of MAPK-ERK1/2 was decreased in PCOS skeletal muscle biopsies. However, the biopsies were performed 15–20 min after a bolus dose of insulin, which is cleared rapidly (279), as part of an insulin tolerance test rather than during the continuous infusion of insulin as part of a euglycemic clamp study. The clearance of insulin is also altered in insulin-resistant states (279, 280). Therefore, differences in the kinetics of the insulin bolus in PCOS compared with control women could have confounded the results. Moreover, a counterregulatory hormone response due to insulin-induced hypoglycemia could also confound the results (281). Nevertheless, the findings are consistent with the hypothesis that constitutive activation of MAPK-ERK1/2 impairs metabolic signaling in PCOS via serine phosphorylation of IRS-1.
In summary, the major defect in insulin action in PCOS is a post-binding defect in the early steps of insulin signal transduction (Figs. 10 and 11). This defect is present in the two main target tissues for insulin-stimulated glucose uptake: adipocytes (192, 251) and skeletal muscle (259, 263). Furthermore, in at least some tissues, such as skin fibroblasts (277) and ovarian granulosa-lutein cells (see in Section V.A. and Ref. 282), insulin resistance in PCOS is selective, affecting metabolic but not other actions of insulin (Figs. 10 and 11). However, both metabolic and mitogenic pathways may be compromised in PCOS skeletal muscle (278).
The post-binding defect in insulin signaling appears to be secondary to increased inhibitory serine phosphorylation of the insulin receptor and IRS-1. Our group (259) and Li et al. (260) have provided evidence that autophosphorylation can be normalized after immunopurification of the insulin receptor. This observation suggests that a kinase extrinsic to the insulin receptor causes the increased receptor serine phosphorylation. This hypothesis is supported by the finding of Li et al. (260) that decreased PCOS skin fibroblast insulin receptor autophosphorylation can be ameliorated by serine kinase inhibitors. In skeletal muscle, two groups (268, 278) have shown that kinases in the MAPK-ERK1/2 mitogenic pathway are constitutively activated. The activation of these kinases contributes to serine phosphorylation of IRS-1 and inhibition of metabolic signaling (268). Two studies suggest that metabolic insulin resistance does not persist in cultured myotubes from women with PCOS (267, 270), whereas another (206) has found persistent defects in glucose uptake. However, some of the abnormalities in insulin signaling are present in passaged myotubes, suggesting the interaction of the in vivo environment with intrinsic defects to produce insulin resistance in vivo (267).
In 1995, Miller and colleagues (283) reported that serine phosphorylation of human cytochrome P450c17, a key regulatory enzyme for ovarian and adrenal androgen biosynthesis with both 17α-hydroxylase and C17, 20 lyase activities, increases its C17,20 lyase activity (283). Thus, this posttranslational modification could result in increased androgen production (283). This observation led to the hypothesis that the same factor that serine-phosphorylates the insulin receptor causing insulin resistance also serine-phosphorylates P450c17 causing hyperandrogenism. Accordingly, some cases of PCOS could be caused by an activating mutation in a serine kinase (283–285) resulting in both hyperandrogenism and insulin resistance (Fig. 11).
There has been considerable interest in identifying a common kinase that could cause serine phosphorylation affecting insulin signaling and steroidogenesis. A screen of serine kinases in an adrenal cell line did not find evidence that MAPK-ERK1/2 increases P450c17 activity (286). Conversely, this pathway may inhibit P450c17 in theca cells (see in Section V.A. and Ref. 287). In adrenal cells, a Rho-associated, coiled-coil containing protein kinase 1 was identified as a potential factor regulating the phosphorylation of P450c17 (286). This kinase can also serine-phosphorylate IRS-1 and inhibit insulin signaling (288). However, attempts to prove that the same kinase can serine-phosphorylate P450c17 and the insulin receptor by transfecting P450c17 into skin fibroblasts from women with PCOS with high insulin receptor serine phosphorylation have failed (289). However, because fibroblasts are not steroidogenic cells, they may have lacked essential cofactors for serine phosphorylation of P450c17 (289).
E. Insulin secretion in PCOS
It is now generally accepted that pancreatic β-cell dysfunction is required for the development of T2D (290, 291). The high prevalence of dysglycemia in PCOS suggests the presence of defects in insulin secretion as well as action. Pancreatic β-cell insulin secretion increases to compensate for peripheral insulin resistance (290). Dysglycemia develops when the β-cell is no longer able to secrete sufficient amounts of insulin to meet the increased requirements (161, 175). Accordingly, insulin secretion must be examined in the context of peripheral insulin sensitivity rather than in isolation (292). Under normal circumstances, this relationship is a constant, hyperbolic function (175, 292) (Fig. 12). It can be quantitated by the DI, the product of insulin sensitivity and insulin secretion (175). DI is highly heritable (293), associated with specific genetic loci (294, 295) and the most powerful predictor of diabetes risk (296).
Figure 12.
β-Cell dysfunction in PCOS. Under normal circumstances, there is a compensatory increase in insulin secretion when insulin sensitivity decreases. This hyperbolic relationship is known as the DI. The majority of women with PCOS fall below the normal curve determined in concurrently studied age- and weight-comparable control women as well as normative data in the literature (292), which places them at increased risk for T2D. DI is decreased independent of obesity. Insulin secretion was determined as AIRg and insulin sensitivity by minimal model analysis of FSIGT glucose and insulin data. Circles, Obese PCOS; triangles, lean PCOS. [Adapted from A. Dunaif and D. T. Finegood: β-Cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab 81:942–947, 1996 (301) with permission. © The Endocrine Society.]
There is no “gold standard” method for assessing insulin secretion as there is for insulin resistance (178). However, DI with insulin secretion assessed by AIRg after an iv glucose bolus and insulin sensitivity assessed by euglycemic clamp (297) or FSIGT (296) have been shown to predict the future development of T2D, indicating the physiological relevance of DI as an assessment of β-cell function. Evidence for β-cell dysfunction in PCOS was first provided by the elegant studies of Ehrmann, Polonsky and colleagues (298), who demonstrated defects in β-cell entrainment to an oscillatory glucose infusion and decreased meal-related insulin secretory responses (299). These defects were more pronounced in PCOS women who have a first-degree relative with T2D, suggesting that such women may be at particularly high risk to develop glucose intolerance (300). Consistent with these findings, DI was significantly decreased in both lean and obese women with PCOS compared with weight-comparable control women (301) (Fig. 12). Taken together, these observations suggest that there is a defect in glucose-stimulated insulin secretion in PCOS, independent of obesity. Furthermore, this abnormality is found as early as adolescence in girls with PCOS and IGT (302). Increased basal secretion rates of insulin (299) contribute to the fasting hyperinsulinemia that is characteristic of obese women with PCOS (21, 34).
There are reports of increased glucose-stimulated insulin secretion in PCOS (201, 205, 303, 304), but these studies have not examined insulin secretion in the context of insulin sensitivity and/or have included women in whom the diagnosis was made on the basis of ovarian morphological changes rather than endocrine criteria. Another study (305) suggesting increased β-cell function in PCOS was limited by the use of a fasting parameter of β-cell function, homeostasis model assessment of β-cell function (306), which reflects basal and not glucose-stimulated insulin secretion and is also confounded by alterations in insulin clearance (184). Furthermore, β-cell function was not corrected for insulin sensitivity in this study (305).
Correlations between circulating T levels and parameters of insulin secretion in women with PCOS have led to the suggestion that androgens play a role in β-cell dysfunction (307). Androgen administration to female mice produces evidence for oxidative stress and increased susceptibility to streptozotocin-induced β-cell failure (308). Short-term methyltestosterone administration to normal women significantly reduced IMGD without changing insulin secretion, suggesting that there may have been a lack of β-cell compensation (309). The possibility that T plays a role in β-cell dysfunction in women with PCOS merits further investigation. A family history of T2D is associated with more pronounced defects in insulin secretion in PCOS (298). In PCOS women with dysglycemia, a marker of β-cell dysfunction, a fasting proinsulin:insulin ratio, is associated with the T2D susceptibility variants in the transcription factor 7-like 2 (TCF7L2) gene (310), suggesting that variation in T2D susceptibility genes contributes to decreased insulin secretion in PCOS as it does in the general population (294). Furthermore, β-cell dysfunction is heritable in the families of women with PCOS (311).
F. Insulin clearance in PCOS
Hyperinsulinemia can result from decreases in insulin clearance as well as from increases in insulin secretion (184, 280). Indeed, because insulin clearance is receptor-mediated, decreased insulin clearance is usually present in insulin-resistant states because of intrinsic or acquired decreases in receptor number and/or function (280, 312). Accordingly, women with PCOS would be expected to have decreases in insulin clearance. Direct measurement of posthepatic insulin clearance during euglycemic clamp studies has been normal in PCOS (81, 132). However, circulating insulin:C-peptide molar ratios were increased in PCOS, suggesting decreased hepatic extraction of insulin (313, 314). Furthermore, direct measurement of hepatic insulin clearance in non-PCOS hyperandrogenic women has found it to be decreased (315). Similarly, women with PCOS have had decreased hepatic insulin extraction by model analysis of C-peptide levels (299). Therefore, in PCOS, fasting hyperinsulinemia is the result of a combination of increased basal insulin secretion and decreased hepatic insulin clearance.
G. Obesity and PCOS
Obesity is a common feature of the PCOS. Indeed, in the United States, the prevalence of obesity (BMI ≥ 30 kg/m2) in women with PCOS is approximately 80% (108, 123, 124). Outside the United States, the prevalence of obesity in affected women is approximately 50% in most studies (84, 104). Differences in the diagnostic criteria for PCOS account for some of this difference in prevalence rates. However, even when comparable diagnostic criteria are applied, both the prevalence and severity of obesity are lower in women with PCOS outside the United States. This observation suggests that environmental factors, such as lifestyle, contribute to the presence of obesity in PCOS (130, 316). PCOS is also common in women with obesity; in a European series of overweight and obese women presenting for weight management, 25% had PCOS (317).
Many studies have suggested that women with PCOS have increased abdominal body fat distribution, regardless of BMI, a conclusion based on anthropometric measurements such as waist circumference or waist:hip ratios (reviewed in Refs. 318 and 319). This conclusion is consistent with the observation that women with upper body obesity defined by anthropometric assessments have increased androgen production rates (8). However, when visceral adipose tissue has been accurately assessed by MRI (197, 198) or computerized axial tomography (199), it has not differed in women with PCOS compared with reproductively normal control women of comparable BMI, even in the presence of increased waist:hip ratios in affected women (197). Nevertheless, sc adipocyte size is increased in both lean and obese women with PCOS (192, 197).
There also appear to be functional abnormalities in PCOS adipose tissue. Ek et al. (275, 276) reported that the lipolytic effect of catecholamines was decreased in sc adipocytes but increased in visceral adipocytes isolated from nonobese women with PCOS. These changes could lead to decreased fat mobilization from the sc depot enlarging fat cell size (320) and enhanced free fatty acid release from the visceral depot, increasing portal free fatty acid delivery to the liver and thereby contributing to hepatic insulin resistance (321). Furthermore, both a meta-analysis (322) and the large study of Mannerås-Holm et al. (197), which controlled accurately for body fat distribution quantitated by MRI, reported lower circulating levels of the insulin-sensitizing adipokine, adiponectin (323), in women with PCOS compared with reproductively normal women, after controlling for BMI. This observation suggests that decreased adiponectin secretion by PCOS adipose tissue contributes to insulin resistance.
It is now well accepted that obesity is associated with chronic low-grade inflammation (324), which may contribute to insulin resistance by the actions of inflammatory adipocytokines, such as TNF-α (325, 326). Increased circulating levels of TNF-α, high-sensitivity C-reactive protein, and IL, which suggest the presence of inflammation, have been reported in PCOS (327, 328). However, these studies are constrained by small sample size and heterogeneity in diagnostic criteria (329). Furthermore, no increases in adipose tissue macrophage content have been reported in PCOS compared with control women of similar weight and fat distribution (197). Recently, decreased expression of genes involved in inflammatory pathways was found in sc adipose tissue from nonobese women with PCOS (330). Accordingly, the contribution of chronic inflammation to insulin resistance in PCOS remains controversial (329).
By whatever mechanisms, it is clear that obesity plays an important role in the expression of the metabolic features of PCOS (284, 331, 332). Hepatic insulin resistance is present only in obese women with PCOS (192). The prevalence of dysglycemia increases with increasing BMI (123, 124). Nevertheless, obesity alone cannot account for PCOS because there are many lean affected women (79, 81, 84). Furthermore, the majority of obese women are reproductively normal (317, 333), and the current obesity epidemic has not been accompanied by an increase in the prevalence of PCOS (122). Finally, defects in insulin sensitivity (81, 192) and secretion (301) are present in lean women with PCOS.
Despite the magnitude of the problem, there are remarkably few studies investigating the pathogenesis of obesity in PCOS. Food intake has not been assessed in women with PCOS. There are conflicting data on energy expenditure, with one study finding a decrease (334) and another reporting no change (335). Leptin levels do not differ in PCOS (336). However, insulin resistance (55, 337) or decreased meal-stimulated glucagon-like peptide (338) or cholecystokinin (339) could result in decreased satiety in PCOS. Decreased subjective satiety has been reported in one study of obese women with PCOS (340), but not in another from the same investigators (341). Furthermore, significantly decreased meal-stimulated cholecystokinin levels did not correlate with satiety ratings in obese PCOS, whereas a significant positive correlation was present in obese control women (339). There was also a trend toward increased cravings for sweets in PCOS in this study (339). Several studies have reported an association between bulimia and PCOS with positive correlations between binge eating and androgen levels (342–344). Thus, there are very preliminary data to suggest alterations in satiety in PCOS.
V. Mechanisms for the Association of Insulin Resistance and PCOS
A. Insulin as a reproductive hormone
Hyperandrogenemia and ovulatory disturbances are commonly encountered in the syndromes of extreme insulin resistance when they occur in premenopausal women (12, 55). There are a number of distinct molecular mechanisms of insulin resistance in these disorders that result in substantial hyperinsulinemia (10, 12, 55). This observation has led to the hypothesis that hyperinsulinemia causes hyperandrogenemia and anovulation (9). Similarly, the finding of significant positive correlations between insulin and androgen levels in PCOS has suggested that insulin also contributes to hyperandrogenism in affected women (21). There is now an extensive body of evidence demonstrating direct ovarian actions of insulin on steroidogenesis as well as the importance of the insulin signaling pathway in the control of ovulation.
Insulin receptors are present in normal and polycystic human ovaries (345–347). The IGF-I receptor is a tyrosine kinase that shares considerable structural and functional homology with the insulin receptor (348). The IGF-I receptor is also present in the ovary, and its ligand, IGF-I, is synthesized by the ovary (346, 347). Insulin can bind to and activate the IGF-I receptor, and IGF-I can bind to and activate the insulin receptor (348, 349). The affinity of the IGF-I receptor for insulin is considerably less than it is for IGF-I and vice versa (349). However, α, β dimers of the insulin and IGF-I receptor can assemble together to form hybrid heterotetramers, which can bind insulin and IGF-I with similar affinity (350, 351). Accordingly, some insulin actions on the ovary may be mediated by the IGF-I or hybrid insulin-IGF-I receptors (352). Nevertheless, studies using specific anti-insulin receptor antibodies indicate that insulin action on steroidogenesis in granulosa and theca cells isolated from normal and polycystic ovaries is mediated via the insulin receptor (353–355). In addition, in granulosa cells from anovulatory PCO, increased insulin levels in synergy with LH may trigger premature LH receptor expression in a subpopulation of small follicles leading to premature granulosa terminal differentiation and the arrest of follicular growth that may contribute to anovulation in this subgroup (65, 282, 356, 357).
Ovarian insulin action on steroidogenesis is preserved, despite resistance to insulin's metabolic actions in PCOS (282, 358). Indeed, in granulosa-lutein cells isolated from ovaries of women with classic PCOS, insulin action on glucose metabolism is significantly decreased, whereas insulin action on steroidogenesis is unchanged compared with granulosa-lutein cells from control women (282). This observation suggests that in PCOS there is selective insulin resistance in the ovary as there is in skeletal muscle and in skin fibroblasts (268, 277, 282).
Studies examining the insulin-signaling pathways modulating ovarian steroidogenesis have been conflicting. In normal theca cells, insulin in synergy with LH activates 17α-hydroxylase activity of P450c17, a key enzyme in the regulation of androgen biosynthesis encoded by CYP17, via PI3-K signaling; inhibition of MAPK-ERK1/2 signaling had no effect on 17α-hydroxylase activity (355). In contrast, McAllister and colleagues (287) suggested that MAPK-ERK1/2 signaling inhibits P450c17 mRNA expression and activity. Furthermore, they found decreased phosphorylation of MEK1/2 and MAPK-ERK1/2 in PCOS compared with control cultured theca cells in association with increased P450c17 expression (287). These findings are the opposite of those in PCOS skeletal muscle where MEK1/2 and MAPK-ERK1/2 phosphorylation are increased (268). Consistent with findings in PCOS skeletal muscle, PCOS theca, but not granulosa, cells have increased expression of IRS-1 and IRS-2 (359). In normal human granulosa cells, GLUT4 translocation is regulated by PI3-K activation of Akt/PKB (152), as it is in the classic insulin target tissues, fat and skeletal muscle (232). There are no published studies of the insulin-signaling pathways regulating granulosa cell steroidogenesis in PCOS.
Insulin action on theca cell androgen production is evident only at supraphysiological insulin concentrations (354). Furthermore, it appears that theca cells from women with PCOS are more responsive to the androgen-stimulating actions of insulin than those from control women (354). Under physiological circumstances, insulin most likely acts as a co-gonadotropin to increase LH-induced androgen synthesis in theca cells (355, 360–362) as well as to enhance FSH-induced estrogen production and LH-induced luteinization in granulosa cells (362). Insulin can also enhance GnRH-mediated LH and FSH release from cultured rat pituitary cells (363). Furthermore, female mice with hyperinsulinemia secondary to diet-induced obesity have increased basal and GnRH-stimulated LH release (364).
Human studies have confirmed that insulin can increase circulating androgen levels in women with PCOS. Insulin infusion during euglycemic clamp studies increased androgen levels (81, 365), without altering gonadotropin secretion (366), suggesting a direct effect on steroidogenesis. Consistent with these studies, prolonged infusion of moderately supraphysiological levels of insulin using euglycemic clamps during dexamethasone suppression of adrenal androgen secretion increased GnRH-stimulated secretion of both androstenedione and progesterone compared with saline infusions in the same PCOS women (367). T levels were increased 24 h after the insulin infusion was stopped. There were no significant differences in gonadotropin levels between the insulin and saline groups (367).
Suppressing insulin levels with diazoxide resulted in a decrease in circulating T levels in women with PCOS, independent of alterations in LH release (368). SHBG levels also increased with suppression of insulin levels by diazoxide (39), consistent with an important role for insulin as a negative regulator of SHBG production (39, 369). Indeed, insulin rather than sex steroids appears to be the major regulator of SHBG production (370). These effects of altering insulin levels were seen only in women with PCOS (366, 368, 371) and not in normal women (366, 371). This observation suggests that polycystic ovarian changes (e.g., theca cell hyperplasia) and/or disordered gonadotropin secretion (e.g., increased LH levels) are a prerequisite for these reproductive actions of insulin, consistent with the studies in isolated theca cells discussed in this Section where insulin has a substantially greater effect on T production in theca cells isolated from women with PCOS (354).
An extensive body of literature indicates that lowering insulin levels with the insulin- sensitizing drugs (ISDs), metformin (372) and the thiazolidinediones (TZDs) (373), can reduce circulating androgen levels, increase SHBG levels, and restore ovulatory menstrual cycles in women with PCOS (reviewed in Refs. 374–378). Abnormalities in apparent 17,20-lyase activity have improved in parallel with reduced circulating insulin levels, consistent with insulin-mediated stimulation of this enzyme (379). Estrogen levels have also been reported to decrease during ISD therapy in PCOS (373, 378), suggesting that insulin has direct stimulatory effects on multiple steroidogenic pathways. Some of the effects of ISDs to lower circulating androgen levels are most likely secondary to direct effects of these agents on steroidogenesis. TZDs directly inhibit theca cell steroidogenesis (380–382). The preponderance of the data suggests that metformin also has direct effects to inhibit theca cell steroidogenesis (219, 380, 383, 384). ISD therapy ameliorates but does not completely normalize circulating androgen levels in PCOS (374, 378).
The effects of insulin on adrenal androgen production have been less clear. Acute insulin infusions decreased DHEAS levels in men as well as in women (361, 385). When insulin levels are chronically lowered, however, circulating DHEA and DHEAS levels rise in normal men but not in normal women (386), which appears to be secondary to insulin-mediated increases in DHEA and DHEAS clearance in men but not women (387). Lowering insulin levels with ISDs has resulted in decreases in DHEAS levels in PCOS women (373, 388). This insulin effect appears to be a direct action of insulin to increase adrenal sensitivity to ACTH in hyperandrogenic women (389, 390).
Human studies of insulin effects of gonadotropin secretion are conflicting. Dunaif and Graf (366) reported that high-dose insulin infusion did not acutely alter LH pulses or GnRH sensitivity in women with PCOS or in control women, but mean gonadotropin levels were lower on the day after the infusion in affected women. Tosi et al. (367) found no differences in GnRH-stimulated gonadotropin levels during prolonged infusion (17 h) of moderately high doses of insulin in women with PCOS. However, Lawson et al. (391) reported that acute infusion of a range of insulin doses, including doses similar to those used by Dunaif and Graf (366), decreased pituitary responsiveness to GnRH in women with PCOS but not in control women. Chronic lowering of insulin levels with the ISDs, troglitazone (373) and metformin (379), decreased circulating LH levels. However, a much larger trial with troglitazone (392) reported no changes in circulating LH levels or LH:FSH ratios over a range of troglitazone doses. Eagleson et al. (393) found that LH pulse amplitude and mean LH levels increased after approximately 1 month on metformin in women with PCOS, but not in control women. These latter findings could be accounted for by the amelioration of insulin-mediated suppression of pituitary responsiveness to GnRH (391). Furthermore, it is possible that the more severe hyperinsulinemia seen in obese PCOS women contributes to the inverse relationship between BMI and LH levels in PCOS (56). Lawson et al. (391) confirmed the inverse relationship between BMI and LH levels and found that the addition of insulin to the model improved the prediction of LH levels in PCOS. In normal women in the latter study, insulin levels (but not BMI) were significantly inversely correlated with LH levels.
Despite the disparate effects of insulin and ISDs on gonadotropin secretion, improving insulin sensitivity with ISDs consistently restores ovulatory menstrual cycles in women with PCOS (55, 375, 394). There is a dose-response effect of TZDs on ovulatory function in PCOS (392), whereas such an effect has not been investigated for metformin. These observations suggest that insulin resistance contributes to anovulation in PCOS. Some of the human studies discussed in this Section (391, 393) suggest that hyperinsulinemia/insulin resistance-mediated reductions in pituitary sensitivity to GnRH contribute to anovulation in PCOS.
Genetic manipulation of the insulin receptor and IRS-2 in mice has confirmed the importance of insulin action in the control of reproduction. Deletion of IRS-2 results in anovulation and obesity in female mice (395). Tissue-specific disruption of the neuronal insulin receptor results in diet-sensitive obesity, disrupted LH release, and impaired ovarian follicle maturation, suggesting that central nervous system (CNS) insulin signaling is important for normal reproduction (396). Consistent with this hypothesis, disruption of both the insulin and leptin receptors in hypothalamic proopiomelanocortin neurons results in decreased fertility and increased circulating T levels in female mice (397). In contrast, disruption of the pituitary insulin receptor is protective against the effects of diet-induced obesity to increase LH release and produce infertility in female mice (364). This observation suggests that insulin signaling in the pituitary is necessary for obesity-mediated disruption of reproduction. Female mice with less profound genetic disruption of the insulin receptor that was not associated with obesity had normal fertility despite subtle alterations in hypothalamic-pituitary-gonadal function as well as increased pregnancy loss (398).
In summary, investigation of the association between insulin resistance and PCOS has revealed that insulin is a reproductive as well as a metabolic hormone. It functions as a co-gonadotropin through its cognate receptor to modulate ovarian steroidogenesis. This action is preserved despite resistance to the metabolic actions of insulin in the periphery as well as in the ovary, an example of selective insulin resistance. Insulin signaling in the CNS also appears to be critical for ovulation. Human studies have confirmed that hyperinsulinemia augments androgen production in PCOS. Intrinsic abnormalities in steroidogenesis appear to be necessary for this insulin action to be manifested because lowering insulin levels does not affect circulating androgen levels in normal women. Insulin is also a major regulator of SHBG production. Reducing insulin resistance can also restore ovulatory menstrual cycles. These insights have led to an important therapeutic modality for PCOS with ISDs (374, 375).
B. Metabolic actions of androgens
Men are more insulin resistant than premenopausal women when glucose utilization is expressed as a function of muscle mass rather than total body mass (399, 400). Male adipocytes are less sensitive than female adipocytes to insulin-stimulated glucose uptake (401). These differences appear to be mediated by sex steroids because prolonged T administration to female-to-male transsexuals has resulted in significant decreases in IMGD in euglycemic clamp studies, particularly at lower insulin doses (402). However, a subsequent report (403) in female-to-male transsexuals did not find a decrease in insulin sensitivity during T administration. Administration of supraphysiological amounts of DHEA, which also results in T elevations because DHEA is a T prehormone, has produced mild hyperinsulinemia in women, but it had no effect on insulin sensitivity in men (404, 405). These differences in insulin action are modest, however, and do not approach the degree of impairment in insulin sensitivity observed in PCOS (81, 192).
Androgens can produce insulin resistance by direct effects on skeletal muscle and adipose tissue insulin action, by altering adipokine secretion, and by increasing visceral adiposity. T-treated castrated female rats had an increase in the number of less insulin-sensitive type II b skeletal muscle fibers (406) and an inhibition of muscle glycogen synthase activity (407). In cultured rat myotubes, T increased serine phosphorylation of Akt/PKB, mTOR ribosomal S6-kinase and IRS-1, which may contribute to insulin resistance (408). Administration of the nonaromatizable androgen, dihydrotestosterone, to peripubertal rats increased visceral fat accumulation and reduced insulin sensitivity (409). In human preadipocytes, T treatment, acting via the androgen receptor, resulted in decreased metabolic but not mitogenic actions of insulin (410). This effect was mediated by impaired insulin-mediated phosphorylation of PKCζ, independent of PI3-K activation (410). T had depot-specific effects on catecholamine-stimulated lipolysis, decreasing sensitivity in human sc but not visceral adipocytes (411, 412). Circulating levels of the insulin-sensitizing adipokine, high molecular weight adiponectin, are higher in women than in men (413). T treatment directly decreased secretion of high molecular weight adiponectin in rat adipocytes (413).
In human studies, administration of a nonaromatizable synthetic androgen to normal postmenopausal women increased visceral adiposity (190). There is a sex difference in this androgen action because androgen reduces visceral adiposity in men (414, 415) but increases it in female-to-male transsexuals (416). However, some of this sex difference may be dose-related because low-dose T administration after suppression of endogenous T secretion with GnRHa increases visceral fat in men (417).
There are conflicting data regarding the impact of decreasing circulating androgen levels or antagonizing androgen action in women with PCOS. In studies where ovarian androgen production was suppressed with a GnRHa (418) or with laparoscopic ovarian cautery (419), or where androgen action was blocked with an antiandrogen (420), there were no improvements in peripheral or hepatic insulin sensitivity assessed by euglycemic clamp. In contrast, other investigators have reported modest improvements in insulin sensitivity in PCOS during androgen suppression with GnRHa or during antiandrogen therapy (421–423). Such changes were apparent in less insulin-resistant, less obese, or lean PCOS women (421–423). However, in obese women with PCOS, prolonged treatment with the antiandrogen, flutamide, in combination with weight loss, decreased visceral adiposity and improved insulin sensitivity compared with diet alone (424, 425). Weight loss accounted for the major improvements in these endpoints, whereas antiandrogen treatment had modest but significant effects. Another study in which androgens were lowered by laparoscopic ovarian drilling did show a partial reversal of insulin signaling defects in visceral fat specimens (252).
In summary, androgens can produce insulin resistance in women. Furthermore, decreasing circulating androgen levels or blocking androgen action can improve insulin sensitivity in women with PCOS. Androgens can directly alter insulin action in the classic target tissues, skeletal muscle, and adipocytes. Androgens can also increase visceral adiposity and reduce the secretion of the major insulin-sensitizing adipokine, adiponectin. However, these androgen effects on insulin action are modest. This observation suggests that additional factors contribute to insulin resistance in PCOS.
C. Genetic susceptibility to PCOS
The possibility that there might be a genetic susceptibility to PCOS and its associated insulin resistance has been suggested by several observations. First, families with multiple affected women have been reported (426–428). Second, the phenotypic similarity between PCOS and the rare syndromes of extreme insulin resistance and hyperandrogenism suggested that insulin receptor mutations might also be present in PCOS (117, 429). Third, defects in insulin action persist in cultured cells, suggesting that they are genetically determined (259, 430). Fourth, the fact that insulin resistance could not entirely account for reproductive dysfunction and vice versa suggests that additional factors contributed to the pathogenesis of PCOS (55).
Familial clustering of PCOS (431, 432) suggesting a genetic susceptibility to the disorder is now well documented in PCOS (reviewed in Refs. 433 and 434). Twin studies have shown heritability of 79% for PCOS with a correlation of 0.71 between monozygotic twins and 0.38 between dizygotic twins (435), consistent with a major influence of genetic factors in PCOS. Although some studies have suggested that there is an autosomal dominant mode of inheritance (436), these studies have been limited by a lack of prospective design, a failure to examine all first-degree relatives, and the fact that only reproductive-age women can be phenotyped for PCOS (432, 437). PCOS is more likely a complex genetic disease with at least several susceptibility genes (433, 434, 438, 439).
The intermediate reproductive phenotype of hyperandrogenemia aggregates in PCOS families (432). About 40% of reproductive-age sisters are affected, but there is phenotypic heterogeneity. Some sisters have classic NICHD PCOS with hyperandrogenemia and oligomenorrhea, whereas others have hyperandrogenemia with regular menses. Brothers of women with PCOS have elevations in the adrenal androgen, DHEAS (440), a marker of male androgen excess because testicular androgen production is tightly regulated by T feedback on the hypothalamus (441). This observation suggests that they have the same defect in androgen biosynthesis as their proband sisters with PCOS (440).
Hyperandrogenemia is the major underlying reproductive phenotype in PCOS families, and this finding has been replicated in other populations (437, 442) (Fig. 13). Affected sisters with either of these hyperandrogenemia phenotypes have insulin resistance (443) (Fig. 13), metabolic syndrome, and elevated low-density lipoprotein levels (444). Furthermore, mothers (445) and brothers (446) also have defects in glucose homeostasis and circulating lipid levels. Therefore, reproductive and metabolic abnormalities are tightly associated in PCOS families, suggesting that they are causally related, have a common pathogenesis, or reflect closely linked genetic traits. Franks et al. (107) have confirmed that hyperandrogenemia and hyperinsulinemia are heritable traits in the sisters of women with PCOS. In addition, several reproductive phenotypes can occur in reproductive-age sisters (107, 432), suggesting that some of the phenotypic heterogeneity of PCOS reflects variable expression of the same gene because sisters would be expected to share the same genetic basis for the disorder. Factors that may contribute to phenotypic variation within families include obesity and insulin resistance (432) (Fig. 14). There may also be additional environmental factors or genes that modify the phenotypic expression of PCOS.
Figure 13.
Two affected phenotypes in sisters of women with PCOS. Approximately 40% of the sisters of women with PCOS have hyperandrogenemia with similar T elevations (middle graph). About half of these affected women fulfill NICHD criteria for PCOS; the remaining hyperandrogenemic (HA) sisters have regular menses and normal fertility, suggesting that their menstrual cycles are ovulatory. Sisters with the PCOS phenotype are heavier (left graph) and more insulin resistant [significantly increased homeostasis model assessment of insulin resistance (HOMA IR), right graph] than sisters with the HA phenotype and sisters with normal androgen levels and regular menses (unaffected, UA). Black bars, PCOS; gray bars, HA; hatched bars, UA; open bars, reproductively normal control women. One-way ANOVA was applied, *, P < 0.05 vs. HA and UA; **, P < 0.05 vs. PCOS and HA; ***, P < 0.05 vs. PCOS, HA, and UA; †, P < 0.05 vs. UA and control; ††, P < 0.05 vs. control. [Adapted from R. S. Legro et al.: Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab 87:2128–2133, 2002 (443), with permission. © The Endocrine Society.]
Figure 14.
Determinants of PCOS phenotypes. The affected sisters of women with PCOS have either highly irregular menstrual cycles characteristic of chronic anovulation or regular cycles consistent with ovulation. Circulating T levels are similar in both affected phenotypes, PCOS and hyperandrogenemia. Both affected groups also have PCO. Possible factors that determine the ovulatory status of affected sisters are obesity, insulin resistance, additional modifier genes, and environmental factors such as lifestyle or diet. This figure is used with the permission of Andrea Dunaif.
Because hyperandrogenemia and markers of insulin resistance are tightly associated in PCOS families (107, 432, 437, 443, 445, 447), mapping genes for the reproductive phenotype may also identify susceptibility genes for insulin resistance. There have been a number of attempts to find susceptibility genes for PCOS (434). Early studies focused on screening the insulin receptor gene for mutations because of the phenotypic similarities between PCOS and the Type A syndrome. Analysis of the exons of the insulin receptor gene in a limited number of women with PCOS and insulin resistance did not show any mutations (117, 448, 449). Several studies (450–452) have reported that polymorphisms in exon 17 of the insulin receptor gene, which encodes part of the tyrosine kinase domain, were associated with PCOS.
There have been numerous case-control studies investigating other PCOS candidate genes. Most studies have been limited by small sample size (434). Furthermore, they assume that the cases and controls are perfectly matched, which is rarely true because of racial and ethnic population stratification (434). In addition, in most studies, a limited number of candidate gene variants have been investigated, and variants associated with PCOS may have escaped detection. This was the case in studies of the T2D susceptibility gene, TCF7L2 (453, 454). There was no association between the TCF7L2 T2D susceptibility single nucleotide polymorphisms (SNPs), rs7903146 and rs12255372, and PCOS (453). However, when SNPs spanning the entire gene were investigated (310), a novel region associated with the PCOS reproductive phenotype, rs11196236 and rs11196229, was mapped that was not in linkage disequilibrium with the T2D susceptibility region. Finally, few findings have been replicated in separate cohorts, which has now become an accepted standard for validation of genetic analyses of complex traits (434, 455). A meta-analysis of small case-control studies (456) suggested that a variant in IRS-1 was associated with PCOS. A recent case-control study of candidate genes in the insulin metabolic signaling pathway (457) did replicate an association between PCOS and a SNP in the insulin receptor gene.
Linkage studies to identify novel genetic susceptibility loci have been constrained by the shortage of large multiplex families and the fact that PCOS-affected status can only be determined in reproductive-age women; non-reproductive-age women and men cannot be assigned a PCOS phenotype (434, 458). In a linkage analysis where postmenopausal-affected status was assigned based on reproductive history and male-affected status was assigned based on premature balding (459, 460), associations were found with CYP11a and with the insulin gene VNTR. However, these findings were not replicated in subsequent analyses (461, 462). Moreover, it has become clear that, despite a number of analytical modifications, linkage mapping studies simply have not been as successful for complex diseases as they were for Mendelian disorders (438, 455).
The transmission disequilibrium test (TDT), a type of family-based association testing, has been successfully used with a candidate gene approach to map PCOS susceptibility genes (434). The TDT examines association in the presence of linkage by assessing transmission of alleles from parents to affected offspring (458). This approach obviates the need for multiplex families and controls for population stratification because analyses are performed within the family unit (434, 463). Using the TDT approach, an allele of a dinucleotide repeat D19S884 on chromosome 19p13.2 was linked and associated with the PCOS reproductive phenotype (464, 465). These findings were replicated in an independent sample of PCOS families (466). In a case-control study, Tucci et al. (467) also found evidence for association between PCOS and D19S884. However, two other case-control studies (468, 469), which tested for association between PCOS and D19S884 in Caucasian women of European ancestry, did not find evidence for association between D19S884 and PCOS. It should be noted that all of these case-control studies were limited by relatively small sample sizes.
D19S884 is a microsatellite marker that had been selected for mapping the insulin receptor but mapped to intron 55 of the fibrillin-3 (FBN3) gene located approximately 1 Mb centromeric to the insulin receptor gene on chromosome 19p13.2 (464, 466, 470). The allele was also associated with evidence for insulin resistance in women with PCOS and for pancreatic β-cell dysfunction in brothers, suggesting a sex difference in the associated metabolic phenotypes (470). Fibrillins are extracellular matrix macromolecules important in connective tissue architecture (471). FBN3 is homologous to FBN1, which encodes fibrillin-1 and is mutated in Marfan syndrome (472), and FBN2, which encodes fibrillin-2 and is mutated in congenital contractural arachnodactyly (473). FBN3 expression levels are highest in fetal tissues, specifically in the brain, lung, kidney, and aorta (474). FBN3 is transcribed in cows, pigs, and chickens but not in mice and rats, making traditional knockout experiments uninformative for FBN3 (474). Fibrillins bind TGFβ and modulate signaling via this pathway (473, 475–478). The gene-specific functions of the fibrillins may be determined by their temporal and spatial expression pattern rather than an inherent difference in protein function (473, 479).
Linkage studies (464) in PCOS-affected sib pairs have implicated follistatin as another PCOS susceptibility gene. Follistatin also functions as an extracellular modulator of the bioavailability of members of the TGFβ signaling family (464, 480). Moreover, follistatin and fibrillins share unique homology in the tertiary structure of their TGFβ binding domains (481). It has not been possible to replicate the association between follistatin and PCOS in small studies, which have also been constrained by failure to comprehensively examine the entire gene for variation (482, 483).
Nevertheless, these observations suggest that genes in the TGFβ signaling family are intriguing candidate genes for PCOS (Fig. 15). The family includes ligands, extracellular antagonists, receptors, and signaling molecules (Table 4) (476, 477, 480, 484–488). Many of the pathways modulated by this signaling family are important in the control of folliculogenesis, including follistatin-activin (480, 489, 490). Members of this family also play a role in pathways regulating metabolism. Follistatin and activin are important in pancreatic islet development (491–493). Myostatin is a key negative regulator of skeletal muscle mass (494, 495) and may also play a role in adipogenesis (496–499). Follistatin can antagonize myostatin as well as activin action (500–502). Furthermore, genetic deletion of follistatin-like 3 gene, whose product also antagonizes activin and myostatin action, in mice produces a metabolic phenotype (503). Accordingly, genetic variation in genes in the TGFβ signaling family is a common pathway that could account for reproductive and metabolic phenotypes in PCOS (470) (Fig. 15).
Figure 15.
TGFβ signaling family. The TGFβ signaling family regulates cell proliferation and differentiation in diverse biological processes including reproduction, cancer progression, extracellular matrix formation, inflammation, metabolism, and development of bone, skeletal muscle, and fat. The family consists of extracellular antagonists that bind TGFβ ligands and modulate their biological availability. The receptors in this family are serine/threonine kinases. Ligands bind to type II receptors, which then recruit and phosphorylate the type I receptor, initiating signaling through phosphorylation of intracellular Smads. Fibrillins and follistatin are extracellular antagonists of ligands in the TGFβ signaling family. A variant within an intron in the fibrillin-3 gene has been linked and associated with PCOS. The follistatin gene has been linked with PCOS in an affected sib pair study. The fibrillin-3 variant is also associated with a metabolic phenotype in women with PCOS and their brothers. These observations suggest that genes in the TGFβ signaling family are candidate genes for PCOS.
Table 4.
Members of TGFβ signaling family
| Ligands |
| Inhibins, activins, myostatin, BMPs, GDFs, AMH, TGFβ1-3, nodal |
| Extracellular antagonists |
| Follistatin, follistatin-like 3, fibrillins, noggin, chordin, DAN/cereberus |
| Type I receptors |
| ACVR1, ACVR1B, ACVR1C, BMPR1A, BMPR1B, TGFβR1 |
| Type II receptors |
| ACVRL1, ACVR2A, ACVR2B, BMPR2, AMHR2, TGFβR2 |
| Type III receptors |
| TGFβR3, ENG, IgSF |
| Intracellular modulators of signaling |
| Smad1-8, Smurf1-2, SARA, TRAP1, BAMBI, Gsc |
ACVRs, Activin receptors; ACVRL1, activin A receptor type II-like 1; AMH, anti-Mullerian hormone; BAMBI, BMP and activin membrane-bound inhibitor homolog; BMPs, bone morphogenic proteins; DAN, differential screening-selected gene aberrative in neuroblastoma; ENG, endoglin; Gsc, goosecoid; GDFs, growth differentiation factors; IgSF, Ig superfamily; SARA, Smad anchor for receptor activation; Smads, Mothers against decapentaplegic homolog; R, receptor; TRAP-1, TGFβ receptor associated protein-1.
Recent studies support a role for fibrillin-3 in the pathogenesis of PCOS. Examination of fibrillin expression in normal and polycystic ovaries from adult women (504) found significantly decreased fibrillin-3 expression in the perifollicular stroma of follicles in morphological transition from primordial to primary follicles in PCO, a stage at which folliculogenesis is disrupted in PCOS (see Section II.C. and Refs. 61, 62, and 107). There were no differences in fibrillin-1 or -2 expression in PCO compared with normal ovaries (504). Studies of human fetal ovaries found that fibrillin-3 as well as components of the TGFβ signaling pathway are expressed in ovarian stroma during development (505).
Genome-wide association studies (GWAS) have been extensively used since the publication of the human haplotype map (HapMap) in 2005 to localize susceptibility genes for complex traits, such as macular degeneration, Crohn's disease, obesity, and T2D (506–512). GWAS depend fundamentally on the widely accepted notion that gene regions containing variation affecting a phenotype can be identified through the indirect relationship between the contributing variation and nearby variation that is in linkage disequilibrium with the contributing variation (513–515). Furthermore, GWAS permit an unbiased interrogation of the entire genome for novel disease susceptibility loci and are, unlike candidate gene approaches, hypothesis generating (455). Population stratification can be controlled for in GWAS by the use of ancestry-specific markers (455, 516, 517).
However, it is now clear that the identification of disease susceptibility loci requires many thousands of individuals (counting GWAS and replication case-control cohorts) and an increasing density of markers (512, 518, 519). Furthermore, many of the loci identified confer very small increases in disease risk and, for traits such as T2D and obesity, loci discovered thus far when taken together do not account for the observed heritability (520, 521). This so-called “missing heritability” (520) may reflect the fact that rare rather than common variants contribute to complex diseases (520, 522). Nevertheless, GWAS has been important for implicating novel biological pathways in disease pathogenesis (523).
The first GWAS of PCOS was published online in December 2010 (116). It was conducted in Han Chinese with PCOS diagnosed by the Rotterdam criteria and contained a discovery sample of 744 PCOS cases and 895 controls. The replication study included two independent cohorts: 2840 PCOS cases and 5012 controls, and 498 PCOS and 780 controls. There was strong evidence for association with meta-analysis P values of 10−19 to 10−23; the proposed threshold for genome-wide significance is 5 × 10−8 (524, 525) between PCOS and loci on chromosomes 2p16.3 (OR, 0.71), 2p21 (OR, 0.67), and 9q33.3 (OR, 1.34).
Several known genes located nearby the most significant SNP at 2p16.3 are GTF2A1L (TFIIA-α and β-like factor) and LHCGR. GTF2A1L is highly expressed in adult testis and may play a role in spermatogenesis (526). LHCGR encodes the receptor for LH and human chorionic gonadotropin and is a highly plausible PCOS candidate gene (434, 464). The strongest evidence for association at 2p21 was with THADA, a gene originally identified in thyroid adenomas (527). The region on chromosome 9q33.3 associated with PCOS was located within DENND1A, which encodes a domain differentially expressed in normal and neoplastic cells that can bind to and negatively regulate endoplasmic reticulum aminopeptidase-1 (528). Nevertheless, confirmation that the signals reflect variation in these genes and not other genes in linkage disequilibrium with these loci requires further genetic analyses.
GWAS are currently under way in PCOS cohorts of European and of Korean ancestry. However, recent studies in European PCOS cohorts have replicated some of the Han Chinese PCOS GWAS signals (114, 115). Both of these studies had large sample sizes of carefully phenotyped women with PCOS as well as control women, used appropriate statistical methods including adjustment of P values for multiple testing, and included one or more replication populations of affected women, which is now a required component for these types of genetic association studies. In the first study, Goodarzi et al. (114) replicated the association of the chromosome 2p21 THADA and chromosome 9p33.3 DENND1A susceptibility loci. Welt et al. (115) also replicated the association between variants in DENND1A and PCOS. This study found a significant association between one variant in DENND1A and T levels, supporting the hypothesis of Legro et al. (432) that hyperandrogenemia was the trait most likely to have a genetic basis in PCOS. Furthermore, another variant in DENND1A was significantly associated with hyperandrogenism and irregular menses but not with polycystic ovarian morphology in the European populations studied (115). This observation suggests that there is genetic susceptibility for PCOS diagnosed by the NICHD rather than the Rotterdam criteria. The finding that the same susceptibility genes contribute to disease risk in Chinese and European PCOS populations suggests that PCOS is an ancient trait present in ancestral populations before their migration out of Africa approximately 40,000 to 60,000 yr ago (529, 530), although genetic exchanges between populations may have occurred until as recently as 20,000 yr ago (531).
D. Developmental origins of PCOS
1. Prenatal androgen excess
Another hypothesis for the pathogenesis of the reproductive and metabolic features of PCOS is fetal programming (532, 533). Prenatal exposure to androgens by T administration to pregnant rhesus macaques, sheep, or rodents can produce most of the phenotypic features of PCOS in the female offspring, including ovarian and adrenal hyperandrogenism, disordered gonadotropin secretion, anovulation, obesity, insulin resistance, and pancreatic β-cell dysfunction (532, 534–537). Increased LH and androstenedione levels can be detected in female prenatally androgenized rhesus offspring in late gestation and early infancy (538). In addition, prenatally androgenized male offspring have reproductive and metabolic phenotypes (539–542) similar to the phenotypes that have been described in the brothers of women with PCOS (427, 440, 442, 446, 447, 470, 543).
Human evidence supporting this hypothesis comes from the observation that perinatal exposure to endogenously elevated androgens secondary to androgen-secreting neoplasms or congenital adrenal hyperplasia can permanently alter gonadotropin secretion in girls (44, 544). Although maternal androgen levels are elevated in PCOS pregnancies (545), it is unlikely that these androgens reach the fetus because placental aromatase acts as a barrier to maternal androgens (546, 547). Nevertheless, the finding that hyperandrogenemia is the underlying reproductive defect in PCOS families (432) suggests that genetic variation leading to fetal ovarian androgen production may program features of PCOS. Consistent with this line of reasoning, there is evidence that the fetal ovary is steroidogenically active because it expresses P450c17 (548). However, there were no increases in umbilical cord androgen levels in the female infants of women with PCOS compared with infants of control women (547), although another study using less sensitive and specific techniques for measuring T levels did find elevated levels in the cord blood of female infants of affected women (549). In a large prospective study, neither maternal nor umbilical cord androgen levels predicted the later development of PCOS (550). Nevertheless, umbilical cord androgen levels reflect fetal exposure in late gestation, and it is possible that there are androgen elevations earlier in gestation that contribute to the pathogenesis of PCOS.
2. Intrauterine growth restriction
The fetal origins or Barker hypothesis (551, 552) proposes that intrauterine growth restriction as evidenced by low birth weight causes insulin resistance, cardiovascular disease, and other features of the insulin resistance syndrome (553–555). According to this hypothesis, decreased fetal nutrition results in decreased fetal insulin secretion and growth (552, 556). Insulin resistance is a compensatory mechanism that further decreases fetal nutrient use: the “thrifty” phenotype (551, 556–558). Extensive animal studies support the long-term impact of the fetal environment on the adult animal, known as fetal programming (555, 556). Many epidemiological studies in humans support the association between low birth weight and metabolic diseases (554, 555, 559, 560). Fetal overnutrition, as occurs in gestational diabetes resulting in large for gestational age infants, can also program adverse metabolic consequences later in life (561–565).
Intrauterine growth restriction leading to low birth weight has been hypothesized to initiate developmental pathways leading to PCOS (566, 567). Increased maternal T levels during gestation were associated with lower birth weights in a random sample of parous women followed prospectively through pregnancy (568). Women with PCOS have higher androgen levels during pregnancy than control women (545) and may have an increased prevalence of small for gestational age infants (569). Girls to premature pubarche, considered to be a precursor of PCOS (570–572), have been reported to have an increased risk of being small for gestational age (567, 573). Young women with a history of low birth weight had an increased prevalence of symptoms of PCOS, evidence for insulin resistance, and higher androgen levels (574, 575) compared with control women with normal birth weights.
Arguing against an influence of intrauterine growth restriction on the later development of PCOS, young women with a history of low birth weight had no difference in symptoms of PCOS (576) or in circulating androgen levels (577) when compared with control women with normal birth weights. Neither women with PCOS (578, 579) nor their first-degree relatives (579) had lower birth weights or an increased prevalence of being small for gestational age compared to concurrently studied control women or to population normative data. Furthermore, there was no association between reproductive or metabolic phenotypes and birth weight in women with PCOS or in their first-degree male or female relatives (579). In a small prospective study, the infants of women with PCOS were not smaller for gestational age than those of control women (547). However, the infants of affected women had an increased prevalence of being large for gestational age compared with those of control women (547). In a large prospective cohort study, there was no association between birth weight and the later development of PCOS (550).
3. Childhood precursors of PCOS
The daughters of women with PCOS have elevations in T levels compared with the daughters of normal women beginning with the onset of puberty (580, 581). The PCOS daughters also had higher anti-Mullerian hormone levels, a marker of follicle number (582), and increased ovarian volume (583). A subset of PCOS daughters had evidence for early adrenarche (584). Furthermore, girls with premature adrenarche have an increased risk for PCOS (571). Leptin, but not insulin, levels were increased in the cord blood of PCOS compared with control offspring (585). Hyperinsulinemia and lower adiponectin levels were present before puberty in daughters of women with PCOS (580, 581). The sons of women with PCOS were heavier than the sons of control women during infancy and childhood (∼6 yr old) (543). PCOS sons had higher urinary T levels than control sons in early puberty (586). These data suggest that PCOS begins early in life and that metabolic changes precede reproductive abnormalities.
4. Summary
In summary, although animal studies provide compelling evidence that prenatal androgen exposure can program the reproductive and metabolic features of PCOS in adult offspring, human data to suggest that prenatal androgen excess plays a role in the development of PCOS are lacking. There are studies to suggest an association between low birth weight and PCOS. However, several large studies, including prospective analyses, do not find an association between birth weight and the development of PCOS. Studies of the daughters of women with PCOS suggest that hyperinsulinemia and hypoadiponectinemia occur before the onset of puberty (580, 581). These observations suggest that PCOS does have its origins very early in life.
VI. Implications and Future Directions
PCOS is a major metabolic as well as reproductive disorder that is associated with increased diabetes risk across a woman's life span. Affected women have a unique disorder of insulin action secondary to decreased insulin receptor signaling likely caused by serine hyperphosphorylation of the receptor and IRS-1. Enhanced intracellular serine kinase activity produces this phosphorylation. The insulin resistance in PCOS is selective, affecting metabolic but not mitogenic pathways both in classic insulin target tissues and in the ovary. Indeed, there is constitutive activation of mitogenic pathways in skeletal muscle in PCOS, and serine kinases in this pathway contribute to increased phosphorylation of IRS-1 and inhibition of metabolic signaling.
The expansion of the diagnostic criteria for PCOS has added additional phenotypes to the diagnosis. Careful study of these phenotypes has indicated that the affected women at high risk for insulin resistance are those who fulfill the original NICHD diagnostic criteria. This insight is particularly important with respect to metabolic disease risk, which is substantially higher in the NICHD subgroup. Although not reviewed here (reviewed in 587), these women with PCOS are at increased risk for the spectrum of disorders associated with insulin resistance, including metabolic syndrome (588, 589), endothelial dysfunction (590), nonalcoholic fatty liver disease (591), gestational diabetes (592, 593), and pregnancy-induced hypertension (592, 593).
The association between insulin resistance and PCOS has led to the discovery that insulin is an important reproductive hormone and that insulin signaling in the CNS is critical for ovulation. This insight was directly translated into a novel therapy for PCOS with ISDs. Androgens also have important effects on insulin sensitivity and secretion. Furthermore, the possibility that developmental exposure to androgens could contribute to PCOS has received considerable attention because striking phenocopies of the syndrome can be produced by prenatal androgen administration to nonhuman primates, sheep, and rodents.
The most exciting advance in the field since it was last reviewed in 1997 is the recognition that PCOS is a highly heritable complex genetic trait. Metabolic as well as reproductive phenotypes have now been described in male, postmenopausal female, and prepubertal first-degree relatives. Several intriguing susceptibility loci have been mapped for the PCOS reproductive phenotype. The loci have implicated novel biological pathways in the pathogenesis of the disorder, such as the TGFβ signaling family. Further analysis of these loci as well as additional loci discovered in GWAS should continue to provide novel biological insights into the pathogenesis of PCOS and the mechanisms of its phenotypic heterogeneity.
Acknowledgments
The authors thank all the volunteers with PCOS and their families who have generously given of their time to advance our understanding of this field. The authors also thank Drs. Margrit Urbanek and Richard S. Legro who have been long-term collaborators on much of the author's (A.D.) work cited herein. The critical input of Dr. Richard N. Bergman is gratefully acknowledged.
This work was supported by National Institutes of Health Grants P50 HD044405, R01 DK073411, and R01 HD057223 (to A.D.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AIRg
- Acute insulin response to glucose
- BMI
- body mass index
- CI
- confidence interval
- CNS
- central nervous system
- DHEA
- dehydroepiandrosterone
- DHEAS
- DHEA sulfate
- DI
- disposition index
- eIF2B
- eukaryotic initiation factor 2B
- FSIGT
- frequently sampled iv glucose tolerance test
- GLUT
- glucose transporter
- GnRHa
- GnRH analog
- GSK3
- glycogen synthase kinase-3
- GWAS
- genome-wide association studies
- IGT
- impaired glucose tolerance
- IMGD
- insulin-mediated glucose disposal
- IRS
- insulin receptor substrate
- ISD
- insulin-sensitizing drug
- MEK
- MAPK kinase
- MRI
- magnetic resonance imaging
- mTOR
- mammalian target of rapamycin
- OGTT
- oral glucose tolerance test
- OR
- odds ratio
- PCO
- polycystic ovaries
- PCOS
- polycystic ovary syndrome
- PI3-K
- phosphatidylinositol 3-kinase
- PKB
- protein kinase B
- PKC
- protein kinase C
- PTEN
- phosphatase and tension homolog deleted on chromosome 10
- PTP1B
- protein tyrosine phosphatase 1B
- Shc
- src homolog and collagen homolog
- SNP
- single nucleotide polymorphism
- T
- testosterone
- TCF7L2
- transcription factor 7-like 2 (gene)
- T2D
- type 2 diabetes
- TDT
- transmission disequilibrium test
- TZD
- thiazolidinedione.
References
- 1. Finch CE , Ruvkun G. 2001. The genetics of aging. Annu Rev Genomics Hum Genet 2:435–462 [DOI] [PubMed] [Google Scholar]
- 2. Kenyon C. 2010. A pathway that links reproductive status to lifespan in Caenorhabditis elegans. Ann NY Acad Sci 1204:156–162 [DOI] [PubMed] [Google Scholar]
- 3. Hanson AE. 1975. Hippocrates: Diseases of Women 1. Signs (Chic) 1:567–584 [DOI] [PubMed] [Google Scholar]
- 4. Morgagni J. 1765. De Sedibus et Causis Morborum per Anatomen Indagata (The seats and causes of diseases investigated by anatomy). Tomus primus editio secunda ed. Patavii: Sumptibus Remondinianis [Google Scholar]
- 5. Achard EC , Thiers J. 1921. Le virilisme et son association à l'insuffisance glycolytique (diabète des femmes à barbe). Bull Acad Natl Med (Paris) 86:51–66 [Google Scholar]
- 6. Vague J. 1947. La différentiation sexuelle. Facteur determinant des formes de l'obesité. Presse Med 55:339–340 [PubMed] [Google Scholar]
- 7. Kissebah AH , Vydelingum N , Murray R , Evans DJ , Hartz AJ , Kalkhoff RK , Adams PW. 1982. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 54:254–260 [DOI] [PubMed] [Google Scholar]
- 8. Kirschner MA , Samojlik E , Drejka M , Szmal E , Schneider G , Ertel N. 1990. Androgen-estrogen metabolism in women with upper body versus lower body obesity. J Clin Endocrinol Metab 70:473–479 [DOI] [PubMed] [Google Scholar]
- 9. Kahn CR , Flier JS , Bar RS , Archer JA , Gorden P , Martin MM , Roth J. 1976. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med 294:739–745 [DOI] [PubMed] [Google Scholar]
- 10. Taylor SI , Cama A , Accili D , Barbetti F , Quon MJ , de la Luz Sierra M , Suzuki Y , Koller E , Levy-Toledano R , Wertheimer E. 1992. Mutations in the insulin receptor gene. Endocr Rev 13:566–595 [DOI] [PubMed] [Google Scholar]
- 11. Musso C , Cochran E , Moran SA , Skarulis MC , Oral EA , Taylor S , Gorden P. 2004. Clinical course of genetic diseases of the insulin receptor (Type A and Rabson-Mendenhall syndromes): a 30-year prospective. Medicine (Baltimore) 83:209–222 [DOI] [PubMed] [Google Scholar]
- 12. Semple RK , Savage DB , Cochran EK , Gorden P , O'Rahilly S. 2011. Genetic syndromes of severe insulin resistance. Endocr Rev 32:498–514 [DOI] [PubMed] [Google Scholar]
- 13. Garg A , Peshock RM , Fleckenstein JL. 1999. Adipose tissue distribution pattern in patients with familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab 84:170–174 [DOI] [PubMed] [Google Scholar]
- 14. Garg A. 2000. Lipodystrophies. Am J Med 108:143–152 [DOI] [PubMed] [Google Scholar]
- 15. Garg A. 2004. Acquired and inherited lipodystrophies. N Engl J Med 350:1220–1234 [DOI] [PubMed] [Google Scholar]
- 16. Chereau A. 1844. Mémoires pour servir à l'étude des malades ovaires. Paris: Fortin, Masson, Cie [Google Scholar]
- 17. Goldzieher J. 2002. Historical perspectives. In: , Chang RJ , Heindel J , Dunaif A, eds. Polycystic ovary syndrome. New York: Marcel Dekker, Inc.; 1–14 [Google Scholar]
- 18. Stein I , Leventhal M. 1935. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol 29:181–191 [Google Scholar]
- 19. Goldzieher JW , Green JA. 1962. The polycystic ovary. I. Clinical and histologic features. J Clin Endocrinol Metab 22:325–338 [DOI] [PubMed] [Google Scholar]
- 20. Givens JR , Wild RA. 1992. Historical Overview of the Polycystic Ovary. In: , Dunaif A , Givens JR , Haseltine FP , Merriam GR. Polycystic ovary syndrome. Cambridge, MA: Blackwell Scientific Publications, Inc; 3–15 [Google Scholar]
- 21. Burghen GA , Givens JR , Kitabchi AE. 1980. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab 50:113–116 [DOI] [PubMed] [Google Scholar]
- 22. Dunaif A , Hoffman AR , Scully RE , Flier JS , Longcope C , Levy LJ , Crowley WF. 1985. Clinical, biochemical, and ovarian morphologic features in women with acanthosis nigricans and masculinization. Obstet Gynecol 66:545–552 [PubMed] [Google Scholar]
- 23. Flier JS , Eastman RC , Minaker KL , Matteson D , Rowe JW. 1985. Acanthosis nigricans in obese women with hyperandrogenism. Characterization of an insulin-resistant state distinct from the Type A and B syndromes. Diabetes 34:101–107 [DOI] [PubMed] [Google Scholar]
- 24. Azziz R , Carmina E , Dewailly D , Diamanti-Kandarakis E , Escobar-Morreale HF , Futterweit W , Janssen OE , Legro RS , Norman RJ , Taylor AE , Witchel SF, Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess PCOS Society 2009. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91:456–488 [DOI] [PubMed] [Google Scholar]
- 25. Cunliffe WJ , Gould DJ. 1979. Prevalence of facial acne vulgaris in late adolescence and in adults. Br Med J 1:1109–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Futterweit W , Dunaif A , Yeh HC , Kingsley P. 1988. The prevalence of hyperandrogenism in 109 consecutive female patients with diffuse alopecia. J Am Acad Derm 19:831–836 [DOI] [PubMed] [Google Scholar]
- 27. Conway GS , Honour JW , Jacobs HS. 1989. Heterogeneity of the polycystic ovary syndrome: clinical, endocrine and ultrasound features in 556 patients. Clin Endocrinol (Oxf) 30:459–470 [DOI] [PubMed] [Google Scholar]
- 28. O'Driscoll JB , Mamtora H , Higginson J , Pollock A , Kane J , Anderson DC. 1994. A prospective study of the prevalence of clear-cut endocrine disorders and polycystic ovaries in 350 patients presenting with hirsutism or androgenic alopecia. Clin Endocrinol (Oxf) 41:231–236 [DOI] [PubMed] [Google Scholar]
- 29. Deplewski D , Rosenfield RL. 2000. Role of hormones in pilosebaceous unit development. Endocr Rev 21:363–392 [DOI] [PubMed] [Google Scholar]
- 30. Dalgard F , Svensson A , Holm JØ , Sundby J. 2004. Self-reported skin morbidity in Oslo. Associations with sociodemographic factors among adults in a cross-sectional study. Br J Dermatol 151:452–457 [DOI] [PubMed] [Google Scholar]
- 31. Galobardes B , Davey Smith G , Jefferys M , McCarron P. 2005. Has acne increased? Prevalence of acne history among university students between 1948 and 1968. The Glasgow Alumni Cohort Study. Br J Dermatol 152:824–825 [DOI] [PubMed] [Google Scholar]
- 32. Essah PA , Wickham EP , Nunley JR , Nestler JE. 2006. Dermatology of androgen-related disorders. Clin Dermatol 24:289–298 [DOI] [PubMed] [Google Scholar]
- 33. Dunaif A , Green G , Phelps RG , Lebwohl M , Futterweit W , Lewy L. 1991. Acanthosis nigricans, insulin action, and hyperandrogenism: clinical, histological, and biochemical findings. J Clin Endocrinol Metab 73:590–595 [DOI] [PubMed] [Google Scholar]
- 34. Dunaif A , Graf M , Mandeli J , Laumas V , Dobrjansky A. 1987. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab 65:499–507 [DOI] [PubMed] [Google Scholar]
- 35. Broekmans FJ , Knauff EA , Valkenburg O , Laven JS , Eijkemans MJ , Fauser BC. 2006. PCOS according to the Rotterdam consensus criteria: change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG 113:1210–1217 [DOI] [PubMed] [Google Scholar]
- 36. Hull MG. 1987. Epidemiology of infertility and polycystic ovarian disease: endocrinological and demographic studies. Gynecol Endocrinol 1:235–245 [DOI] [PubMed] [Google Scholar]
- 37. Wild RA , Umstot ES , Andersen RN , Ranney GB , Givens JR. 1983. Androgen parameters and their correlation with body weight in one hundred thirty-eight women thought to have hyperandrogenism. Am J Obstet Gynecol 146:602–606 [DOI] [PubMed] [Google Scholar]
- 38. Moll GW , Rosenfield RL. 1979. Testosterone binding and free plasma androgen concentrations under physiological conditions: characterization by flow dialysis technique. J Clin Endocrinol Metab 49:730–736 [DOI] [PubMed] [Google Scholar]
- 39. Nestler JE , Powers LP , Matt DW , Steingold KA , Plymate SR , Rittmaster RS , Clore JN , Blackard WG. 1991. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab 72:83–89 [DOI] [PubMed] [Google Scholar]
- 40. Rosner W , Auchus RJ , Azziz R , Sluss PM , Raff H. 2007. Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society Position Statement. J Clin Endocrinol Metab 92:405–413 [DOI] [PubMed] [Google Scholar]
- 41. Vesper HW , Botelho JC. 2010. Standardization of testosterone measurements in humans. J Steroid Biochem Mol Biol 121:513–519 [DOI] [PubMed] [Google Scholar]
- 42. Vermeulen A , Verdonck L , Kaufman JM. 1999. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- 43. Legro RS , Schlaff WD , Diamond MP , Coutifaris C , Casson PR , Brzyski RG , Christman GM , Trussell JC , Krawetz SA , Snyder PJ , Ohl D , Carson SA , Steinkampf MP , Carr BR , McGovern PG , Cataldo NA , Gosman GG , Nestler JE , Myers ER , Santoro N , Eisenberg E , Zhang M , Zhang H, Reproductive Medicine Network 2010. Total testosterone assays in women with polycystic ovary syndrome: precision and correlation with hirsutism. J Clin Endocrinol Metab 95:5305–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ehrmann DA , Barnes RB , Rosenfield RL. 1995. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev 16:322–353 [DOI] [PubMed] [Google Scholar]
- 45. Yildiz BO , Azziz R. 2007. The adrenal and polycystic ovary syndrome. Rev Endocr Metab Disord 8:331–342 [DOI] [PubMed] [Google Scholar]
- 46. Lachelin GC , Barnett M , Hopper BR , Brink G , Yen SS. 1979. Adrenal function in normal women and women with the polycystic ovary syndrome. J Clin Endocrinol Metab 49:892–898 [DOI] [PubMed] [Google Scholar]
- 47. DeVane GW , Czekala NM , Judd HL , Yen SS. 1975. Circulating gonadotropins, estrogens, and androgens in polycystic ovarian disease. Am J Obstet Gynecol 121:496–500 [DOI] [PubMed] [Google Scholar]
- 48. Baird DT , Corker CS , Davidson DW , Hunter WM , Michie EA , Van Look PF. 1977. Pituitary-ovarian relationships in polycystic ovary syndrome. J Clin Endocrinol Metab 45:798–801 [DOI] [PubMed] [Google Scholar]
- 49. MacDonald PC , Rombaut RP , Siiteri PK. 1967. Plasma precursors of estrogen. I. Extent of conversion of plasma δ-4-androstenedione to estrone in normal males and nonpregnant normal, castrate and adrenalectomized females. J Clin Endocrinol Metab 27:1103–1111 [DOI] [PubMed] [Google Scholar]
- 50. Moll GW , Rosenfield RL , Helke JH. 1981. Estradiol-testosterone binding interactions and free plasma estradiol under physiological conditions. J Clin Endocrinol Metab 52:868–874 [DOI] [PubMed] [Google Scholar]
- 51. Lobo RA , Granger L , Goebelsmann U , Mishell DR. 1981. Elevations in unbound serum estradiol as a possible mechanism for inappropriate gonadotropin secretion in women with PCO. J Clin Endocrinol Metab 52:156–158 [DOI] [PubMed] [Google Scholar]
- 52. Rebar R , Judd HL , Yen SS , Rakoff J , Vandenberg G , Naftolin F. 1976. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest 57:1320–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hillier SG. 1994. Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod 9:188–191 [DOI] [PubMed] [Google Scholar]
- 54. Zawadzki JK , Dunaif A. 1992. Diagnostic criteria for polycystic ovary syndrome; towards a rational approach. In: , Dunaif A , Givens JR , Haseltine F , Merriam G, eds. Polycystic ovary syndrome. Boston: Blackwell Scientific; 377–384 [Google Scholar]
- 55. Dunaif A. 1997. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18:774–800 [DOI] [PubMed] [Google Scholar]
- 56. Taylor AE , McCourt B , Martin KA , Anderson EJ , Adams JM , Schoenfeld D , Hall JE. 1997. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab 82:2248–2256 [DOI] [PubMed] [Google Scholar]
- 57. Arroyo A , Laughlin GA , Morales AJ , Yen SS. 1997. Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. J Clin Endocrinol Metab 82:3728–3733 [DOI] [PubMed] [Google Scholar]
- 58. Hughesdon PE. 1982. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis.” Obstet Gynecol Surv 37:59–77 [DOI] [PubMed] [Google Scholar]
- 59. Webber LJ , Stubbs S , Stark J , Trew GH , Margara R , Hardy K , Franks S. 2003. Formation and early development of follicles in the polycystic ovary. Lancet 362:1017–1021 [DOI] [PubMed] [Google Scholar]
- 60. Webber LJ , Stubbs SA , Stark J , Margara RA , Trew GH , Lavery SA , Hardy K , Franks S. 2007. Prolonged survival in culture of preantral follicles from polycystic ovaries. J Clin Endocrinol Metab 92:1975–1978 [DOI] [PubMed] [Google Scholar]
- 61. Stubbs SA , Stark J , Dilworth SM , Franks S , Hardy K. 2007. Abnormal preantral folliculogenesis in polycystic ovaries is associated with increased granulosa cell division. J Clin Endocrinol Metab 92:4418–4426 [DOI] [PubMed] [Google Scholar]
- 62. Franks S , Stark J , Hardy K. 2008. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update 14:367–378 [DOI] [PubMed] [Google Scholar]
- 63. Gilling-Smith C , Willis DS , Beard RW , Franks S. 1994. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab 79:1158–1165 [DOI] [PubMed] [Google Scholar]
- 64. Nelson VL , Legro RS , Strauss JF , McAllister JM. 1999. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol 13:946–957 [DOI] [PubMed] [Google Scholar]
- 65. Willis DS , Watson H , Mason HD , Galea R , Brincat M , Franks S. 1998. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J Clin Endocrinol Metab 83:3984–3991 [DOI] [PubMed] [Google Scholar]
- 66. Mason HD , Willis DS , Beard RW , Winston RM , Margara R , Franks S. 1994. Estradiol production by granulosa cells of normal and polycystic ovaries: relationship to menstrual cycle history and concentrations of gonadotropins and sex steroids in follicular fluid. J Clin Endocrinol Metab 79:1355–1360 [DOI] [PubMed] [Google Scholar]
- 67. Erickson GF , Magoffin DA , Garzo VG , Cheung AP , Chang RJ. 1992. Granulosa cells of polycystic ovaries: are they normal or abnormal? Hum Reprod 7:293–299 [DOI] [PubMed] [Google Scholar]
- 68. White DM , Polson DW , Kiddy D , Sagle P , Watson H , Gilling-Smith C , Hamilton-Fairley D , Franks S. 1996. Induction of ovulation with low-dose gonadotropins in polycystic ovary syndrome: an analysis of 109 pregnancies in 225 women. J Clin Endocrinol Metab 81:3821–3824 [DOI] [PubMed] [Google Scholar]
- 69. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2004. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19–25 [DOI] [PubMed] [Google Scholar]
- 70. Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group 2004. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Hum Reprod 19:41–47 [DOI] [PubMed] [Google Scholar]
- 71. Azziz R , Carmina E , Dewailly D , Diamanti-Kandarakis E , Escobar-Morreale HF , Futterweit W , Janssen OE , Legro RS , Norman RJ , Taylor AE , Witchel SF, Androgen Excess Society 2006. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 91:4237–4245 [DOI] [PubMed] [Google Scholar]
- 72. Kane RL. 1995. Creating practice guidelines: the dangers of over-reliance on expert judgment. J Law Med Ethics 23:62–64 [DOI] [PubMed] [Google Scholar]
- 73. Woolf SH. 2000. Evidence-based medicine and practice guidelines: an overview. Cancer Control 7:362–367 [DOI] [PubMed] [Google Scholar]
- 74. Atkins D , Best D , Briss PA , Eccles M , Falck-Ytter Y , Flottorp S , Guyatt GH , Harbour RT , Haugh MC , Henry D , Hill S , Jaeschke R , Leng G , Liberati A , Magrini N , Mason J , Middleton P , Mrukowicz J , O'Connell D , Oxman AD , Phillips B , Schünemann HJ , Edejer TT , Varonen H , Vist GE , Williams JW , Zaza S. 2004. Grading quality of evidence and strength of recommendations. BMJ 328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Swiglo BA , Murad MH , Schünemann HJ , Kunz R , Vigersky RA , Guyatt GH , Montori VM. 2008. A case for clarity, consistency, and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinol Metab 93:666–673 [DOI] [PubMed] [Google Scholar]
- 76. Rycroft-Malone J. 2001. Formal consensus: the development of a national clinical guideline. Qual Health Care 10:238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wortman PM , Vinokur A , Sechrest L. 1988. Do consensus conferences work? A process evaluation of the NIH Consensus Development Program. J Health Polit Policy Law 13:469–498 [DOI] [PubMed] [Google Scholar]
- 78. Portnoy B , Miller J , Brown-Huamani K , DeVoto E. 2007. Impact of the National Institutes of Health Consensus Development Program on stimulating National Institutes of Health-funded research, 1998 to 2001. Int J Technol Assess Health Care 23:343–348 [DOI] [PubMed] [Google Scholar]
- 79. Chang RJ , Nakamura RM , Judd HL , Kaplan SA. 1983. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab 57:356–359 [DOI] [PubMed] [Google Scholar]
- 80. Shoupe D , Kumar DD , Lobo RA. 1983. Insulin resistance in polycystic ovary syndrome. Am J Obstet Gynecol 147:588–592 [DOI] [PubMed] [Google Scholar]
- 81. Dunaif A , Segal KR , Futterweit W , Dobrjansky A. 1989. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 38:1165–1174 [DOI] [PubMed] [Google Scholar]
- 82. Polson DW , Adams J , Wadsworth J , Franks S. 1988. Polycystic ovaries—a common finding in normal women. Lancet 1:870–872 [DOI] [PubMed] [Google Scholar]
- 83. Balen AH , Laven JS , Tan SL , Dewailly D. 2003. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update 9:505–514 [DOI] [PubMed] [Google Scholar]
- 84. Balen AH , Conway GS , Kaltsas G , Techatrasak K , Manning PJ , West C , Jacobs HS. 1995. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod 10:2107–2111 [DOI] [PubMed] [Google Scholar]
- 85. Franks S. 1995. Polycystic ovary syndrome. N Engl J Med 333:853–861 [DOI] [PubMed] [Google Scholar]
- 86. MacDougall MJ , Tan SL , Jacobs HS. 1992. IVF and the ovarian hyperstimulation syndrome. Hum Reprod 7:597–600 [DOI] [PubMed] [Google Scholar]
- 87. Swanton A , Storey L , McVeigh E , Child T. 2010. IVF outcome in women with PCOS, PCO and normal ovarian morphology. Eur J Obstet Gynecol Reprod Biol 149:68–71 [DOI] [PubMed] [Google Scholar]
- 88. Fauser BC , Diedrich K , Devroey P. 2008. Predictors of ovarian response: progress towards individualized treatment in ovulation induction and ovarian stimulation. Hum Reprod Update 14:1–14 [DOI] [PubMed] [Google Scholar]
- 89. Robinson S , Kiddy D , Gelding SV , Willis D , Niththyananthan R , Bush A , Johnston DG , Franks S. 1993. The relationship of insulin insensitivity to menstrual pattern in women with hyperandrogenism and polycystic ovaries. Clin Endocrinol (Oxf) 39:351–355 [DOI] [PubMed] [Google Scholar]
- 90. Legro RS , Chiu P , Kunselman AR , Bentley CM , Dodson WC , Dunaif A. 2005. Polycystic ovaries are common in women with hyperandrogenic chronic anovulation but do not predict metabolic or reproductive phenotype. J Clin Endocrinol Metab 90:2571–2579 [DOI] [PubMed] [Google Scholar]
- 91. Murphy MK , Hall JE , Adams JM , Lee H , Welt CK. 2006. Polycystic ovarian morphology in normal women does not predict the development of polycystic ovary syndrome. J Clin Endocrinol Metab 91:3878–3884 [DOI] [PubMed] [Google Scholar]
- 92. Welt CK , Gudmundsson JA , Arason G , Adams J , Palsdottir H , Gudlaugsdottir G , Ingadottir G , Crowley WF. 2006. Characterizing discrete subsets of polycystic ovary syndrome as defined by the Rotterdam criteria: the impact of weight on phenotype and metabolic features. J Clin Endocrinol Metab 91:4842–4848 [DOI] [PubMed] [Google Scholar]
- 93. Wijeyaratne CN , Seneviratne Rde A , Dahanayake S , Kumarapeli V , Palipane E , Kuruppu N , Yapa C , Seneviratne Rde A , Balen AH. 2011. Phenotype and metabolic profile of South Asian women with polycystic ovary syndrome (PCOS): results of a large database from a specialist endocrine clinic. Hum Reprod 26:202–213 [DOI] [PubMed] [Google Scholar]
- 94. Wild RA , Carmina E , Diamanti-Kandarakis E , Dokras A , Escobar-Morreale HF , Futterweit W , Lobo R , Norman RJ , Talbott E , Dumesic DA. 2010. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab 95:2038–2049 [DOI] [PubMed] [Google Scholar]
- 95. Chang PL , Lindheim SR , Lowre C , Ferin M , Gonzalez F , Berglund L , Carmina E , Sauer MV , Lobo RA. 2000. Normal ovulatory women with polycystic ovaries have hyperandrogenic pituitary-ovarian responses to gonadotropin-releasing hormone-agonist testing. J Clin Endocrinol Metab 85:995–1000 [DOI] [PubMed] [Google Scholar]
- 96. Carmina E , Chu MC , Longo RA , Rini GB , Lobo RA. 2005. Phenotypic variation in hyperandrogenic women influences the findings of abnormal metabolic and cardiovascular risk parameters. J Clin Endocrinol Metab 90:2545–2549 [DOI] [PubMed] [Google Scholar]
- 97. Dewailly D , Catteau-Jonard S , Reyss AC , Leroy M , Pigny P. 2006. Oligoanovulation with polycystic ovaries but not overt hyperandrogenism. J Clin Endocrinol Metab 91:3922–3927 [DOI] [PubMed] [Google Scholar]
- 98. Diamanti-Kandarakis E , Panidis D. 2007. Unravelling the phenotypic map of polycystic ovary syndrome (PCOS): a prospective study of 634 women with PCOS. Clin Endocrinol (Oxf) 67:735–742 [DOI] [PubMed] [Google Scholar]
- 99. Barber TM , Wass JA , McCarthy MI , Franks S. 2007. Metabolic characteristics of women with polycystic ovaries and oligo-amenorrhoea but normal androgen levels: implications for the management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 66:513–517 [DOI] [PubMed] [Google Scholar]
- 100. Zhang HY , Zhu FF , Xiong J , Shi XB , Fu SX. 2009. Characteristics of different phenotypes of polycystic ovary syndrome based on the Rotterdam criteria in a large-scale Chinese population. BJOG 116:1633–1639 [DOI] [PubMed] [Google Scholar]
- 101. Moran L , Teede H. 2009. Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod Update 15:477–488 [DOI] [PubMed] [Google Scholar]
- 102. Goverde AJ , van Koert AJ , Eijkemans MJ , Knauff EA , Westerveld HE , Fauser BC , Broekmans FJ. 2009. Indicators for metabolic disturbances in anovulatory women with polycystic ovary syndrome diagnosed according to the Rotterdam consensus criteria. Hum Reprod 24:710–717 [DOI] [PubMed] [Google Scholar]
- 103. Johnstone EB , Rosen MP , Neril R , Trevithick D , Sternfeld B , Murphy R , Addauan-Andersen C , McConnell D , Pera RR , Cedars MI. 2010. The polycystic ovary post-Rotterdam: a common, age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab 95:4965–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kiddy DS , Hamilton-Fairley D , Bush A , Short F , Anyaoku V , Reed MJ , Franks S. 1992. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 36:105–111 [DOI] [PubMed] [Google Scholar]
- 105. Mortensen M , Ehrmann DA , Littlejohn E , Rosenfield RL. 2009. Asymptomatic volunteers with a polycystic ovary are a functionally distinct but heterogeneous population. J Clin Endocrinol Metab 94:1579–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jonard S , Dewailly D. 2004. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update 10:107–117 [DOI] [PubMed] [Google Scholar]
- 107. Franks S , Webber LJ , Goh M , Valentine A , White DM , Conway GS , Wiltshire S , McCarthy MI. 2008. Ovarian morphology is a marker of heritable biochemical traits in sisters with polycystic ovaries. J Clin Endocrinol Metab 93:3396–3402 [DOI] [PubMed] [Google Scholar]
- 108. Knochenhauer ES , Key TJ , Kahsar-Miller M , Waggoner W , Boots LR , Azziz R. 1998. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 83:3078–3082 [DOI] [PubMed] [Google Scholar]
- 109. Diamanti-Kandarakis E , Kouli CR , Bergiele AT , Filandra FA , Tsianateli TC , Spina GG , Zapanti ED , Bartzis MI. 1999. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab 84:4006–4011 [DOI] [PubMed] [Google Scholar]
- 110. Carmina E , Lobo RA. 1999. Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab 84:1897–1899 [DOI] [PubMed] [Google Scholar]
- 111. Azziz R , Woods KS , Reyna R , Key TJ , Knochenhauer ES , Yildiz BO. 2004. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749 [DOI] [PubMed] [Google Scholar]
- 112. Asunción M , Calvo RM , San Millán JL , Sancho J , Avila S , Escobar-Morreale HF. 2000. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab 85:2434–2438 [DOI] [PubMed] [Google Scholar]
- 113. Kumarapeli V , Seneviratne Rde A , Wijeyaratne CN , Yapa RM , Dodampahala SH. 2008. A simple screening approach for assessing community prevalence and phenotype of polycystic ovary syndrome in a semi-urban population in Sri Lanka. Am J Epidemiol 168:321–328 [DOI] [PubMed] [Google Scholar]
- 114. Goodarzi MO , Jones MR , Li X , Chua AK , Garcia OA , Chen YD , Krauss RM , Rotter JI , Ankener W , Legro RS , Azziz R , Strauss JF , Dunaif A , Urbanek M. 2012. Replication of association of DENND1A and THADA variants with polycystic ovary syndrome in European cohorts. J Med Genet 49:90–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Welt CK , Styrkarsdottir U , Ehrmann DA , Thorleifsson G , Arason G , Gudmundsson JA , Ober C , Rosenfield RL , Saxena R , Thorsteinsdottir U , Crowley WF , Stefansson K. 2012. Variants in DENND1A are associated with polycystic ovary syndrome in women of European ancestry. J Clin Endocrinol Metab 97:E1342–E1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chen ZJ , Zhao H , He L , Shi Y , Qin Y , Shi Y , Li Z , You L , Zhao J , Liu J , Liang X , Zhao X , Zhao J , Sun Y , Zhang B , Jiang H , Zhao D , Bian Y , Gao X , Geng L , Li Y , Zhu D , Sun X , Xu JE , Hao C , Ren CE , Zhang Y , Chen S , Zhang W , Yang A , Yan J , Li Y , Ma J , Zhao Y. 2011. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet 43:55–59 [DOI] [PubMed] [Google Scholar]
- 117. Sorbara LR , Tang Z , Cama A , Xia J , Schenker E , Kohanski RA , Poretsky L , Koller E , Taylor SI , Dunaif A. 1994. Absence of insulin receptor gene mutations in three insulin-resistant women with the polycystic ovary syndrome. Metabolism 43:1568–1574 [DOI] [PubMed] [Google Scholar]
- 118. Goodarzi MO , Quiñones MJ , Azziz R , Rotter JI , Hsueh WA , Yang H. 2005. Polycystic ovary syndrome in Mexican-Americans: prevalence and association with the severity of insulin resistance. Fertil Steril 84:766–769 [DOI] [PubMed] [Google Scholar]
- 119. Ladson G , Dodson WC , Sweet SD , Archibong AE , Kunselman AR , Demers LM , Williams NI , Coney P , Legro RS. 2011. Racial influence on the polycystic ovary syndrome phenotype: a black and white case-control study. Fertil Steril 96:224–229.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Welt CK , Arason G , Gudmundsson JA , Adams J , Palsdóttir H , Gudlaugsdóttir G , Ingadóttir G , Crowley WF. 2006. Defining constant versus variable phenotypic features of women with polycystic ovary syndrome using different ethnic groups and populations. J Clin Endocrinol Metab 91:4361–4368 [DOI] [PubMed] [Google Scholar]
- 121. Park HR , Choi Y , Lee HJ , Oh JY , Hong YS , Sung YA. 2007. Phenotypic characteristics according to insulin sensitivity in non-obese Korean women with polycystic ovary syndrome. Diabetes Res Clin Pract 77(Suppl 1):S233–S237 [DOI] [PubMed] [Google Scholar]
- 122. Yildiz BO , Knochenhauer ES , Azziz R. 2008. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab 93:162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Legro RS , Kunselman AR , Dodson WC , Dunaif A. 1999. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84:165–169 [DOI] [PubMed] [Google Scholar]
- 124. Ehrmann DA , Barnes RB , Rosenfield RL , Cavaghan MK , Imperial J. 1999. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 22:141–146 [DOI] [PubMed] [Google Scholar]
- 125. Ehrmann DA , Kasza K , Azziz R , Legro RS , Ghazzi MN, for the PCOS/Troglitazone Study Group 2005. Effects of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome. J Clin Endocrinol Metab 90:66–71 [DOI] [PubMed] [Google Scholar]
- 126. Palmert MR , Gordon CM , Kartashov AI , Legro RS , Emans SJ , Dunaif A. 2002. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab 87:1017–1023 [DOI] [PubMed] [Google Scholar]
- 127. Gambineri A , Pelusi C , Manicardi E , Vicennati V , Cacciari M , Morselli-Labate AM , Pagotto U , Pasquali R. 2004. Glucose intolerance in a large cohort of Mediterranean women with polycystic ovary syndrome: phenotype and associated factors. Diabetes 53:2353–2358 [DOI] [PubMed] [Google Scholar]
- 128. Elting MW , Korsen TJ , Bezemer PD , Schoemaker J. 2001. Prevalence of diabetes mellitus, hypertension and cardiac complaints in a follow-up study of a Dutch PCOS population. Hum Reprod 16:556–560 [DOI] [PubMed] [Google Scholar]
- 129. Luque-Ramírez M , Alpañés M , Escobar-Morreale HF. 2010. The determinants of insulin sensitivity, β-cell function, and glucose tolerance are different in patients with polycystic ovary syndrome and in women who do not have hyperandrogenism. Fertil Steril 94:2214–2221 [DOI] [PubMed] [Google Scholar]
- 130. Essah PA , Nestler JE , Carmina E. 2008. Differences in dyslipidemia between American and Italian women with polycystic ovary syndrome. J Endocrinol Invest 31:35–41 [DOI] [PubMed] [Google Scholar]
- 131. Carmina E , Legro RS , Stamets K , Lowell J , Lobo RA. 2003. Difference in body weight between American and Italian women with polycystic ovary syndrome: influence of the diet. Hum Reprod 18:2289–2293 [DOI] [PubMed] [Google Scholar]
- 132. Dunaif A , Sorbara L , Delson R , Green G. 1993. Ethnicity and polycystic ovary syndrome are associated with independent and additive decreases in insulin action in Caribbean-Hispanic women. Diabetes 42:1462–1468 [DOI] [PubMed] [Google Scholar]
- 133. Moran LJ , Misso ML , Wild RA , Norman RJ. 2010. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 16:347–363 [DOI] [PubMed] [Google Scholar]
- 134. American Diabetes Association 2007. Diagnosis and classification of diabetes mellitus. Diabetes Care 30:S42–S47 [DOI] [PubMed] [Google Scholar]
- 135. Tomlinson J , Millward A , Stenhouse E , Pinkney J. 2010. Type 2 diabetes and cardiovascular disease in polycystic ovary syndrome: what are the risks and can they be reduced? Diabet Med 27:498–515 [DOI] [PubMed] [Google Scholar]
- 136. Roumain J , Charles MA , de Courten MP , Hanson RL , Brodie TD , Pettitt DJ , Knowler WC. 1998. The relationship of menstrual irregularity to type 2 diabetes in Pima Indian women. Diabetes Care 21:346–349 [DOI] [PubMed] [Google Scholar]
- 137. Solomon CG , Hu FB , Dunaif A , Rich-Edwards J , Willett WC , Hunter DJ , Colditz GA , Speizer FE , Manson JE. 2001. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA 286:2421–2426 [DOI] [PubMed] [Google Scholar]
- 138. Taponen S , Martikainen H , Järvelin MR , Laitinen J , Pouta A , Hartikainen AL , Sovio U , McCarthy MI , Franks S , Ruokonen A. 2003. Hormonal profile of women with self-reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. J Clin Endocrinol Metab 88:141–147 [DOI] [PubMed] [Google Scholar]
- 139. Adams J , Polson DW , Franks S. 1986. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed) 293:355–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. American Academy of Pediatrics Committee on Adolescence, American College of Obstetricians and Gynecologists Committee on Adolescent Health Care, Diaz A , Laufer MR , Breech LL. 2006. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Pediatrics 118:2245–2250 [DOI] [PubMed] [Google Scholar]
- 141. Taponen S , Martikainen H , Järvelin MR , Sovio U , Laitinen J , Pouta A , Hartikainen AL , McCarthy MI , Franks S , Paldanius M , Ruokonen A. 2004. Metabolic cardiovascular disease risk factors in women with self-reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. J Clin Endocrinol Metab 89:2114–2118 [DOI] [PubMed] [Google Scholar]
- 142. Cooper GS , Ephross SA , Sandler DP. 2000. Menstrual patterns and risk of adult-onset diabetes mellitus. J Clin Epidemiol 53:1170–1173 [DOI] [PubMed] [Google Scholar]
- 143. Gast GC , Grobbee DE , Smit HA , Bueno-de-Mesquita HB , Samsioe GN , van der Schouw YT. 2010. Menstrual cycle characteristics and risk of coronary heart disease and type 2 diabetes. Fertil Steril 94:2379–2381 [DOI] [PubMed] [Google Scholar]
- 144. Dahlgren E , Johansson S , Lindstedt G , Knutsson F , Odén A , Janson PO , Mattson LA , Crona N , Lundberg PA. 1992. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulating hormones. Fertil Steril 57:505–513 [DOI] [PubMed] [Google Scholar]
- 145. Pierpoint T , McKeigue PM , Isaacs AJ , Wild SH , Jacobs HS. 1998. Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol 51:581–586 [DOI] [PubMed] [Google Scholar]
- 146. Wild S , Pierpoint T , Jacobs H , McKeigue P. 2000. Long-term consequences of polycystic ovary syndrome: results of a 31 year follow-up study. Hum Fertil (Camb) 3:101–105 [DOI] [PubMed] [Google Scholar]
- 147. Wild S , Pierpoint T , McKeigue P , Jacobs H. 2000. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol (Oxf) 52:595–600 [DOI] [PubMed] [Google Scholar]
- 148. Margolin E , Zhornitzki T , Kopernik G , Kogan S , Schattner A , Knobler H. 2005. Polycystic ovary syndrome in post-menopausal women—marker of the metabolic syndrome. Maturitas 50:331–336 [DOI] [PubMed] [Google Scholar]
- 149. Legro RS , Gnatuk CL , Kunselman AR , Dunaif A. 2005. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab 90:3236–3242 [DOI] [PubMed] [Google Scholar]
- 150. Norman RJ , Masters L , Milner CR , Wang JX , Davies MJ. 2001. Relative risk of conversion from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum Reprod 16:1995–1998 [DOI] [PubMed] [Google Scholar]
- 151. Boudreaux MY , Talbott EO , Kip KE , Brooks MM , Witchel SF. 2006. Risk of T2DM and impaired fasting glucose among PCOS subjects: results of an 8-year follow-up. Curr Diab Rep 6:77–83 [DOI] [PubMed] [Google Scholar]
- 152. Pesant MH , Baillargeon JP. 2011. Clinically useful predictors of conversion to abnormal glucose tolerance in women with polycystic ovary syndrome. Fertil Steril 95:210–215 [DOI] [PubMed] [Google Scholar]
- 153. Tuomilehto J , Lindström J , Eriksson JG , Valle TT , Hämäläinen H , Ilanne-Parikka P , Keinänen-Kiukaanniemi S , Laakso M , Louheranta A , Rastas M , Salminen V , Uusitupa M. 2001. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 154. Chiasson JL , Josse RG , Gomis R , Hanefeld M , Karasik A , Laakso M. 2002. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 359:2072–2077 [DOI] [PubMed] [Google Scholar]
- 155. Knowler WC , Hamman RF , Edelstein SL , Barrett-Connor E , Ehrmann DA , Walker EA , Fowler SE , Nathan DM , Kahn SE. 2005. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 54:1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Rizza RA. 2010. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes 59:2697–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Salley KE , Wickham EP , Cheang KI , Essah PA , Karjane NW , Nestler JE. 2007. Glucose intolerance in polycystic ovary syndrome—a position statement of the Androgen Excess Society. J Clin Endocrinol Metab 92:4546–4556 [DOI] [PubMed] [Google Scholar]
- 158. American Diabetes Association 2010. Diagnosis and classification of diabetes mellitus. Diabetes Care 33:S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Velling Magnussen L , Mumm H , Andersen M , Glintborg D. 2011. Hemoglobin A1c as a tool for the diagnosis of type 2 diabetes in 208 premenopausal women with polycystic ovary syndrome. Fertil Steril 96:1275–1280 [DOI] [PubMed] [Google Scholar]
- 160. DeFronzo RA. 1988. Lilly lecture 1987. The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37:667–687 [DOI] [PubMed] [Google Scholar]
- 161. Bergman RN. 2007. Orchestration of glucose homeostasis: from a small acorn to the California oak. Diabetes 56:1489–1501 [DOI] [PubMed] [Google Scholar]
- 162. Groop LC , Bonadonna RC , Simonson DC , Petrides AS , Shank M , DeFronzo RA. 1992. Effect of insulin on oxidative and nonoxidative pathways of free fatty acid metabolism in human obesity. Am J Physiol 263:E79–E84 [DOI] [PubMed] [Google Scholar]
- 163. Bergman RN , Mittelman SD. 1998. Central role of the adipocyte in insulin resistance. J Basic Clin Physiol Pharmacol 9:205–221 [DOI] [PubMed] [Google Scholar]
- 164. Rebrin K , Steil GM , Getty L , Bergman RN. 1995. Free fatty acid as a link in the regulation of hepatic glucose output by peripheral insulin. Diabetes 44:1038–1045 [DOI] [PubMed] [Google Scholar]
- 165. Rebrin K , Steil GM , Mittelman SD , Bergman RN. 1996. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J Clin Invest 98:741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kahn CR. 1985. The molecular mechanism of insulin action. Annu Rev Med 36:429–451 [DOI] [PubMed] [Google Scholar]
- 167. Bergman RN , Finegood DT , Ader M. 1985. Assessment of insulin sensitivity in vivo. Endocr Rev 6:45–86 [DOI] [PubMed] [Google Scholar]
- 168. Belfiore A , Frasca F , Pandini G , Sciacca L , Vigneri R. 2009. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 30:586–623 [DOI] [PubMed] [Google Scholar]
- 169. Benito M. 2011. Tissue-specificity of insulin action and resistance. Arch Physiol Biochem 117:96–104 [DOI] [PubMed] [Google Scholar]
- 170. DeFronzo RA , Tobin JD , Andres R. 1979. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 171. Gerich JE. 2010. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med 27:136–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Aber GM , Morris LO , Housley E. 1966. Gluconeogenesis by the human kidney. Nature 212:1589–1590 [DOI] [PubMed] [Google Scholar]
- 173. Gastaldelli A , Coggan AR , Wolfe RR. 1999. Assessment of methods for improving tracer estimation of non-steady-state rate of appearance. J Appl Physiol 87:1813–1822 [DOI] [PubMed] [Google Scholar]
- 174. Wolfe RR. 1992. Radioactive and stable isotope tracers in biomedicine: principles and practice of kinetic analysis. New York: Wiley-Liss, Inc. [Google Scholar]
- 175. Bergman RN , Phillips LS , Cobelli C. 1981. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and β-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68:1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Vicini P , Sparacino G , Caumo A , Cobelli C. 1997. Estimation of endogenous glucose production after a glucose perturbation by nonparametric stochastic deconvolution. Comput Methods Programs Biomed 52:147–156 [DOI] [PubMed] [Google Scholar]
- 177. Vicini P , Zachwieja JJ , Yarasheski KE , Bier DM , Caumo A , Cobelli C. 1999. Glucose production during an IVGTT by deconvolution: validation with the tracer-to-tracee clamp technique. Am J Physiol 276:E285–E294 [DOI] [PubMed] [Google Scholar]
- 178. Bergman RN , Hope ID , Yang YJ , Watanabe RM , Meador MA , Youn JH , Ader M. 1989. Assessment of insulin sensitivity in vivo: a critical review. Diabetes Metab Rev 5:411–429 [DOI] [PubMed] [Google Scholar]
- 179. Welch S , Gebhart SS , Bergman RN , Phillips LS. 1990. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab 71:1508–1518 [DOI] [PubMed] [Google Scholar]
- 180. Mitrakou A , Vuorinen-Markkola H , Raptis G , Toft I , Mokan M , Strumph P , Pimenta W , Veneman T , Jenssen T , Bolli G. 1992. Simultaneous assessment of insulin secretion and insulin sensitivity using a hyperglycemic clamp. J Clin Endocrinol Metab 75:379–382 [DOI] [PubMed] [Google Scholar]
- 181. Matthews DR , Hosker JP , Rudenski AS , Naylor BA , Treacher DF , Turner RC. 1985. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- 182. Legro RS , Dunaif A. 1999. Fasting glucose to insulin ratio, or product of glucose and insulin? J Clin Endocrinol Metab 84:383. [DOI] [PubMed] [Google Scholar]
- 183. Hrebícek J , Janout V , Malincíková J , Horáková D , Cízek L. 2002. Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. J Clin Endocrinol Metab 87:144–147 [DOI] [PubMed] [Google Scholar]
- 184. Hücking K , Watanabe RM , Stefanovski D , Bergman RN. 2008. OGTT-derived measures of insulin sensitivity are confounded by factors other than insulin sensitivity itself. Obesity (Silver Spring) 16:1938–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Legro RS , Finegood D , Dunaif A. 1998. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab 83:2694–2698 [DOI] [PubMed] [Google Scholar]
- 186. Matsuda M , DeFronzo RA. 1999. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 187. Diamanti-Kandarakis E , Kouli C , Alexandraki K , Spina G. 2004. Failure of mathematical indices to accurately assess insulin resistance in lean, overweight, or obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 89:1273–1276 [DOI] [PubMed] [Google Scholar]
- 188. Legro RS. 2001. Diabetes prevalence and risk factors in polycystic ovary syndrome. Obstet Gynecol Clin North Am 28:99–109 [DOI] [PubMed] [Google Scholar]
- 189. Evans DJ , Hoffmann RG , Kalkhoff RK , Kissebah AH. 1983. Relationship of androgenic activity to body fat topography, fat cell morphology, and metabolic aberrations in premenopausal women. J Clin Endocrinol Metab 57:304–310 [DOI] [PubMed] [Google Scholar]
- 190. Lovejoy JC , Bray GA , Bourgeois MO , Macchiavelli R , Rood JC , Greeson C , Partington C. 1996. Exogenous androgens influence body composition and regional body fat distribution in obese postmenopausal women—a clinical research center study. J Clin Endocrinol Metab 81:2198–2203 [DOI] [PubMed] [Google Scholar]
- 191. Bagatell CJ , Bremner WJ. 1996. Androgens in men—uses and abuses. N Engl J Med 334:707–714 [DOI] [PubMed] [Google Scholar]
- 192. Dunaif A , Segal KR , Shelley DR , Green G , Dobrjansky A , Licholai T. 1992. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes 41:1257–1266 [DOI] [PubMed] [Google Scholar]
- 193. Kissebah AH. 1996. Intra-abdominal fat: is it a major factor in developing diabetes and coronary artery disease? Diabetes Res Clin Pract 30 Suppl:25–30 [DOI] [PubMed] [Google Scholar]
- 194. Pasquali R , Casimirri F , Cantobelli S , Labate AM , Venturoli S , Paradisi R , Zannarini L. 1993. Insulin and androgen relationships with abdominal body fat distribution in women with and without hyperandrogenism. Horm Res 39:179–187 [DOI] [PubMed] [Google Scholar]
- 195. Pasquali R , Casimirri F , Venturoli S , Antonio M , Morselli L , Reho S , Pezzoli A , Paradisi R. 1994. Body fat distribution has weight-independent effects on clinical, hormonal, and metabolic features of women with polycystic ovary syndrome. Metabolism 43:706–713 [DOI] [PubMed] [Google Scholar]
- 196. Pasquali R , Vicennati V. 2000. The abdominal obesity phenotype and insulin resistance are associated with abnormalities of the hypothalamic-pituitary-adrenal axis in humans. Horm Metab Res 32:521–525 [DOI] [PubMed] [Google Scholar]
- 197. Mannerås-Holm L , Leonhardt H , Kullberg J , Jennische E , Odén A , Holm G , Hellström M , Lönn L , Olivecrona G , Stener-Victorin E , Lönn M. 2011. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab 96:E304–E311 [DOI] [PubMed] [Google Scholar]
- 198. Barber TM , Golding SJ , Alvey C , Wass JA , Karpe F , Franks S , McCarthy MI. 2008. Global adiposity rather than abnormal regional fat distribution characterises women with polycystic ovary syndrome. J Clin Endocrinol Metab 93:999–1004 [DOI] [PubMed] [Google Scholar]
- 199. Yalamanchi SK , Sam S , Cardenas MO , Holaday LW , Urbanek M , Dunaif A. 2012. Association of fibrillin-3 and transcription factor-7-like 2 gene variants with metabolic phenotypes in PCOS. Obesity (Silver Spring) 20:1273–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Bonadonna RC , Groop L , Kraemer N , Ferrannini E , Del Prato S , DeFronzo RA. 1990. Obesity and insulin resistance in humans: a dose-response study. Metabolism 39:452–459 [DOI] [PubMed] [Google Scholar]
- 201. Holte J , Bergh T , Berne C , Berglund L , Lithell H. 1994. Enhanced early insulin response to glucose in relation to insulin resistance in women with polycystic ovary syndrome and normal glucose tolerance. J Clin Endocrinol Metab 78:1052–1058 [DOI] [PubMed] [Google Scholar]
- 202. Morales AJ , Laughlin GA , Bützow T , Maheshwari H , Baumann G , Yen SS. 1996. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab 81:2854–2864 [DOI] [PubMed] [Google Scholar]
- 203. Diamanti-Kandarakis E , Kouli C , Tsianateli T , Bergiele A. 1998. Therapeutic effects of metformin on insulin resistance and hyperandrogenism in polycystic ovary syndrome. Eur J Endocrinol 138:269–274 [DOI] [PubMed] [Google Scholar]
- 204. Morin-Papunen LC , Vauhkonen I , Koivunen RM , Ruokonen A , Martikainen HK , Tapanainen JS. 2000. Endocrine and metabolic effects of metformin versus ethinyl estradiol-cyproterone acetate in obese women with polycystic ovary syndrome: a randomized study. J Clin Endocrinol Metab 85:3161–3168 [DOI] [PubMed] [Google Scholar]
- 205. Ciampelli M , Fulghesu AM , Cucinelli F , Pavone V , Caruso A , Mancuso S , Lanzone A. 1997. Heterogeneity in β cell activity, hepatic insulin clearance and peripheral insulin sensitivity in women with polycystic ovary syndrome. Hum Reprod 12:1897–1901 [DOI] [PubMed] [Google Scholar]
- 206. Ciaraldi TP , Aroda V , Mudaliar S , Chang RJ , Henry RR. 2009. Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance. J Clin Endocrinol Metab 94:157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Arslanian SA , Lewy V , Danadian K , Saad R. 2002. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab 87:1555–1559 [DOI] [PubMed] [Google Scholar]
- 208. Glintborg D , Hermann AP , Andersen M , Hagen C , Beck-Nielsen H , Veldhuis JD , Henriksen JE. 2006. Effect of pioglitazone on glucose metabolism and luteinizing hormone secretion in women with polycystic ovary syndrome. Fertil Steril 86:385–397 [DOI] [PubMed] [Google Scholar]
- 209. Palomba S , Falbo A , Russo T , Manguso F , Tolino A , Zullo F , De Feo P , Orio F. 2007. Insulin sensitivity after metformin suspension in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab 92:3128–3135 [DOI] [PubMed] [Google Scholar]
- 210. Ovesen P , Moller J , Ingerslev HJ , Jørgensen JO , Mengel A , Schmitz O , Alberti KG , Moller N. 1993. Normal basal and insulin-stimulated fuel metabolism in lean women with the polycystic ovary syndrome. J Clin Endocrinol Metab 77:1636–1640 [DOI] [PubMed] [Google Scholar]
- 211. Vrbíková J , Cibula D , Dvoráková K , Stanická S , Sindelka G , Hill M , Fanta M , Vondra K , Skrha J. 2004. Insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab 89:2942–2945 [DOI] [PubMed] [Google Scholar]
- 212. de Paula Martins W , Santana LF , Nastri CO , Ferriani FA , de Sa MF , Dos Reis RM. 2007. Agreement among insulin sensitivity indexes on the diagnosis of insulin resistance in polycystic ovary syndrome and ovulatory women. Eur J Obstet Gynecol Reprod Biol 133:203–207 [DOI] [PubMed] [Google Scholar]
- 213. Ducluzeau PH , Cousin P , Malvoisin E , Bornet H , Vidal H , Laville M , Pugeat M. 2003. Glucose-to-insulin ratio rather than sex hormone-binding globulin and adiponectin levels is the best predictor of insulin resistance in nonobese women with polycystic ovary syndrome. J Clin Endocrinol Metab 88:3626–3631 [DOI] [PubMed] [Google Scholar]
- 214. Vigil P , Contreras P , Alvarado JL , Godoy A , Salgado AM , Cortés ME. 2007. Evidence of subpopulations with different levels of insulin resistance in women with polycystic ovary syndrome. Hum Reprod 22:2974–2980 [DOI] [PubMed] [Google Scholar]
- 215. Fulghesu AM , Angioni S , Portoghese E , Milano F , Batetta B , Paoletti AM , Melis GB. 2006. Failure of the homeostatic model assessment calculation score for detecting metabolic deterioration in young patients with polycystic ovary syndrome. Fertil Steril 86:398–404 [DOI] [PubMed] [Google Scholar]
- 216. Ciampelli M , Leoni F , Cucinelli F , Mancuso S , Panunzi S , De Gaetano A , Lanzone A. 2005. Assessment of insulin sensitivity from measurements in the fasting state and during an oral glucose tolerance test in polycystic ovary syndrome and menopausal patients. J Clin Endocrinol Metab 90:1398–1406 [DOI] [PubMed] [Google Scholar]
- 217. Carmina E , Wong L , Chang L , Paulson RJ , Sauer MV , Stanczyk FZ , Lobo RA. 1997. Endocrine abnormalities in ovulatory women with polycystic ovaries on ultrasound. Hum Reprod 12:905–909 [DOI] [PubMed] [Google Scholar]
- 218. Baillargeon JP , Nestler JE. 2006. Polycystic ovary syndrome: a syndrome of ovarian hypersensitivity to insulin? J Clin Endocrinol Metab 91:22–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Mansfield R , Galea R , Brincat M , Hole D , Mason H. 2003. Metformin has direct effects on human ovarian steroidogenesis. Fertil Steril 79:956–962 [DOI] [PubMed] [Google Scholar]
- 220. Saltiel AR , Kahn CR. 2001. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806 [DOI] [PubMed] [Google Scholar]
- 221. Kahn CR. 1994. Insulin action, diabetogenes, and the cause of type 2 diabetes. Diabetes 43:1066–1084 [DOI] [PubMed] [Google Scholar]
- 222. Cheatham B , Kahn CR. 1995. Insulin action and the insulin signaling network. Endocr Rev 16:117–142 [DOI] [PubMed] [Google Scholar]
- 223. Kasuga M , Hedo JA , Yamada KM , Kahn CR. 1982. The structure of insulin receptor and its subunits. Evidence for multiple nonreduced forms and a 210,000 possible proreceptor. J Biol Chem 257:10392–10399 [PubMed] [Google Scholar]
- 224. Ullrich A , Bell JR , Chen EY , Herrera R , Petruzzelli LM , Dull TJ , Gray A , Coussens L , Liao YC , Tsubokawa M. 1985. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature 313:756–761 [DOI] [PubMed] [Google Scholar]
- 225. Ebina Y , Ellis L , Jarnagin K , Edery M , Graf L , Clauser E , Ou JH , Masiarz F , Kan YW , Goldfine ID. 1985. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell 40:747–758 [DOI] [PubMed] [Google Scholar]
- 226. Kasuga M , Karlsson FA , Kahn CR. 1982. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science 215:185–187 [DOI] [PubMed] [Google Scholar]
- 227. Ullrich A , Schlessinger J. 1990. Signal transduction by receptors with tyrosine kinase activity. Cell 61:203–212 [DOI] [PubMed] [Google Scholar]
- 228. Kasuga M , Zick Y , Blith DL , Karlsson FA , Häring HU , Kahn CR. 1982. Insulin stimulation of phosphorylation of the β subunit of the insulin receptor. Formation of both phosphoserine and phosphotyrosine. J Biol Chem 257:9891–9894 [PubMed] [Google Scholar]
- 229. Cobb MH , Sang BC , Gonzalez R , Goldsmith E , Ellis L. 1989. Autophosphorylation activates the soluble cytoplasmic domain of the insulin receptor in an intermolecular reaction. J Biol Chem 264:18701–18706 [PubMed] [Google Scholar]
- 230. Shoelson SE , Boni-Schnetzler M , Pilch PF , Kahn CR. 1991. Autophosphorylation within insulin receptor β-subunits can occur as an intramolecular process. Biochemistry 30:7740–7746 [DOI] [PubMed] [Google Scholar]
- 231. Myers MG , Sun XJ , White MF. 1994. The IRS-1 signaling system. Trends Biochem Sci 19:289–293 [DOI] [PubMed] [Google Scholar]
- 232. Choi K , Kim YB. 2010. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J Intern Med 25:119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Sun XJ , Rothenberg P , Kahn CR , Backer JM , Araki E , Wilden PA , Cahill DA , Goldstein BJ , White MF. 1991. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 352:73–77 [DOI] [PubMed] [Google Scholar]
- 234. Lee J , Kim MS. 2007. The role of GSK3 in glucose homeostasis and the development of insulin resistance. Diabetes Res Clin Pract 77(Suppl 1):S49–S57 [DOI] [PubMed] [Google Scholar]
- 235. Pearson G , Robinson F , Beers Gibson T , Xu BE , Karandikar M , Berman K , Cobb MH. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–183 [DOI] [PubMed] [Google Scholar]
- 236. Flier JS , Moller DE , Moses AC , O'Rahilly S , Chaiken RL , Grigorescu F , Elahi D , Kahn BB , Weinreb JE , Eastman R. 1993. Insulin-mediated pseudoacromegaly: clinical and biochemical characterization of a syndrome of selective insulin resistance. J Clin Endocrinol Metab 76:1533–1541 [DOI] [PubMed] [Google Scholar]
- 237. Sasaoka T , Rose DW , Jhun BH , Saltiel AR , Draznin B , Olefsky JM. 1994. Evidence for a functional role of Shc proteins in mitogenic signaling induced by insulin, insulin-like growth factor-1, and epidermal growth factor. J Biol Chem 269:13689–13694 [PubMed] [Google Scholar]
- 238. Berhanu P , Anderson C , Hickman M , Ciaraldi TP. 1997. Insulin signal transduction by a mutant human insulin receptor lacking the NPEY sequence. Evidence for an alternate mitogenic signaling pathway that is independent of Shc phosphorylation. J Biol Chem 272:22884–22890 [DOI] [PubMed] [Google Scholar]
- 239. Podskalny JM , Kahn CR. 1982. Cell culture studies on patients with extreme insulin resistance. II. Abnormal biological responses in cultured fibroblasts. J Clin Endocrinol Metab 54:269–275 [DOI] [PubMed] [Google Scholar]
- 240. Cusi K , Maezono K , Osman A , Pendergrass M , Patti ME , Pratipanawatr T , DeFronzo RA , Kahn CR , Mandarino LJ. 2000. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest 105:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Cheng Z , Tseng Y , White MF. 2010. Insulin signaling meets mitochondria in metabolism. Trends Endocrinol Metab 21:589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Cantley LC , Neel BG. 1999. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA 96:4240–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Litherland GJ , Hajduch E , Hundal HS. 2001. Intracellular signalling mechanisms regulating glucose transport in insulin-sensitive tissues (Review). Mol Membr Biol 18:195–204 [DOI] [PubMed] [Google Scholar]
- 244. Bollag GE , Roth RA , Beaudoin J , Mochly-Rosen D , Koshland DE. 1986. Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc Natl Acad Sci USA 83:5822–5824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Takayama S , White MF , Kahn CR. 1988. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem 263:3440–3447 [PubMed] [Google Scholar]
- 246. Draznin B. 2006. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85α. Diabetes 55:2392–2397 [DOI] [PubMed] [Google Scholar]
- 247. Kruszynska YT , Olefsky JM. 1996. Cellular and molecular mechanisms of non-insulin dependent diabetes mellitus. J Investig Med 44:413–428 [PubMed] [Google Scholar]
- 248. Müller HK , Kellerer M , Ermel B , Mühlhöfer A , Obermaier-Kusser B , Vogt B , Häring HU. 1991. Prevention by protein kinase C inhibitors of glucose-induced insulin-receptor tyrosine kinase resistance in rat fat cells. Diabetes 40:1440–1448 [DOI] [PubMed] [Google Scholar]
- 249. Hotamisligil GS , Peraldi P , Budavari A , Ellis R , White MF , Spiegelman BM. 1996. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science 271:665–668 [DOI] [PubMed] [Google Scholar]
- 250. Podskalny JM , Kahn CR. 1982. Cell culture studies on patients with extreme insulin resistance. I. Receptor defects on cultured fibroblasts. J Clin Endocrinol Metab 54:261–268 [DOI] [PubMed] [Google Scholar]
- 251. Ciaraldi TP , el-Roeiy A , Madar Z , Reichart D , Olefsky JM , Yen SS. 1992. Cellular mechanisms of insulin resistance in polycystic ovarian syndrome. J Clin Endocrinol Metab 75:577–583 [DOI] [PubMed] [Google Scholar]
- 252. Seow KM , Juan CC , Hsu YP , Hwang JL , Huang LW , Ho LT. 2007. Amelioration of insulin resistance in women with PCOS via reduced insulin receptor substrate-1 Ser312 phosphorylation following laparoscopic ovarian electrocautery. Hum Reprod 22:1003–1010 [DOI] [PubMed] [Google Scholar]
- 253. Chang W , Goodarzi MO , Williams H , Magoffin DA , Pall M , Azziz R. 2008. Adipocytes from women with polycystic ovary syndrome demonstrate altered phosphorylation and activity of glycogen synthase kinase 3. Fertil Steril 90:2291–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Rosenbaum D , Haber RS , Dunaif A. 1993. Insulin resistance in polycystic ovary syndrome: decreased expression of GLUT-4 glucose transporters in adipocytes. Am J Physiol 264:E197–E202 [DOI] [PubMed] [Google Scholar]
- 255. Caro JF , Dohm LG , Pories WJ , Sinha MK. 1989. Cellular alterations in liver, skeletal muscle, and adipose tissue responsible for insulin resistance in obesity and type II diabetes. Diabetes Metab Rev 5:665–689 [DOI] [PubMed] [Google Scholar]
- 256. Caro JF. 1991. Clinical review 26: insulin resistance in obese and nonobese man. J Clin Endocrinol Metab 73:691–695 [DOI] [PubMed] [Google Scholar]
- 257. Freidenberg GR , Reichart D , Olefsky JM , Henry RR. 1988. Reversibility of defective adipocyte insulin receptor kinase activity in non-insulin-dependent diabetes mellitus. Effect of weight loss. J Clin Invest 82:1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Kim YB , Nikoulina SE , Ciaraldi TP , Henry RR , Kahn BB. 1999. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J Clin Invest 104:733–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Dunaif A , Xia J , Book CB , Schenker E , Tang Z. 1995. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. J Clin Invest 96:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Li M , Youngren JF , Dunaif A , Goldfine ID , Maddux BA , Zhang BB , Evans JL. 2002. Decreased insulin receptor (IR) autophosphorylation in fibroblasts from patients with PCOS: effects of serine kinase inhibitors and IR activators. J Clin Endocrinol Metab 87:4088–4093 [DOI] [PubMed] [Google Scholar]
- 261. Dunaif A , Wu X , Lee A , Diamanti-Kandarakis E. 2001. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS). Am J Physiol Endocrinol Metab 281:E392–E399 [DOI] [PubMed] [Google Scholar]
- 262. Højlund K , Glintborg D , Andersen NR , Birk JB , Treebak JT , Frøsig C , Beck-Nielsen H , Wojtaszewski JF. 2008. Impaired insulin-stimulated phosphorylation of Akt and AS160 in skeletal muscle of women with polycystic ovary syndrome is reversed by pioglitazone treatment. Diabetes 57:357–366 [DOI] [PubMed] [Google Scholar]
- 263. Dunaif A , Wu X , Lee A , Diamanti-Kandarakis E. 2001. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS). Am J Physiol Endocrinol Metab 281:E392–E399 [DOI] [PubMed] [Google Scholar]
- 264. Sarabia V , Lam L , Burdett E , Leiter LA , Klip A. 1992. Glucose transport in human skeletal muscle cells in culture. Stimulation by insulin and metformin. J Clin Invest 90:1386–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Henry RR , Abrams L , Nikoulina S , Ciaraldi TP. 1995. Insulin action and glucose metabolism in nondiabetic control and NIDDM subjects. Comparison using human skeletal muscle cell cultures. Diabetes 44:936–946 [DOI] [PubMed] [Google Scholar]
- 266. Thompson DB , Pratley R , Ossowski V. 1996. Human primary myoblast cell cultures from non-diabetic insulin resistant subjects retain defects in insulin action. J Clin Invest 98:2346–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Corbould A , Kim YB , Youngren JF , Pender C , Kahn BB , Lee A , Dunaif A. 2005. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab 288:E1047–E1054 [DOI] [PubMed] [Google Scholar]
- 268. Corbould A , Zhao H , Mirzoeva S , Aird F , Dunaif A. 2006. Enhanced mitogenic signaling in skeletal muscle of women with polycystic ovary syndrome. Diabetes 55:751–759 [DOI] [PubMed] [Google Scholar]
- 269. Eriksen MB , Minet AD , Glintborg D , Gaster M. 2011. Intact primary mitochondrial function in myotubes established from women with PCOS. J Clin Endocrinol Metab 96:E1298–E1302 [DOI] [PubMed] [Google Scholar]
- 270. Eriksen M , Pørneki AD , Skov V , Burns JS , Beck-Nielsen H , Glintborg D , Gaster M. 2010. Insulin resistance is not conserved in myotubes established from women with PCOS. PloS One 5:e14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Lowell BB , Shulman GI. 2005. Mitochondrial dysfunction and type 2 diabetes. Science 307:384–387 [DOI] [PubMed] [Google Scholar]
- 272. Skov V , Glintborg D , Knudsen S , Jensen T , Kruse TA , Tan Q , Brusgaard K , Beck-Nielsen H , Højlund K. 2007. Reduced expression of nuclear-encoded genes involved in mitochondrial oxidative metabolism in skeletal muscle of insulin resistant women with polycystic ovary syndrome. Diabetes 56:2349–2355 [DOI] [PubMed] [Google Scholar]
- 273. Skov V , Glintborg D , Knudsen S , Tan Q , Jensen T , Kruse TA , Beck-Nielsen H , Højlund K. 2008. Pioglitazone enhances mitochondrial biogenesis and ribosomal protein biosynthesis in skeletal muscle in polycystic ovary syndrome. PloS One 3:e2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Holte J , Bergh T , Berne C , Wide L , Lithell H. 1995. Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 80:2586–2593 [DOI] [PubMed] [Google Scholar]
- 275. Ek I , Arner P , Bergqvist A , Carlström K , Wahrenberg H. 1997. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J Clin Endocrinol Metab 82:1147–1153 [DOI] [PubMed] [Google Scholar]
- 276. Ek I , Arner P , Rydén M , Holm C , Thörne A , Hoffstedt J , Wahrenberg H. 2002. A unique defect in the regulation of visceral fat cell lipolysis in the polycystic ovary syndrome as an early link to insulin resistance. Diabetes 51:484–492 [DOI] [PubMed] [Google Scholar]
- 277. Book CB , Dunaif A. 1999. Selective insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab 84:3110–3116 [DOI] [PubMed] [Google Scholar]
- 278. Rajkhowa M , Brett S , Cuthbertson DJ , Lipina C , Ruiz-Alcaraz AJ , Thomas GE , Logie L , Petrie JR , Sutherland C. 2009. Insulin resistance in polycystic ovary syndrome is associated with defective regulation of ERK1/2 by insulin in skeletal muscle in vivo. Biochem J 418:665–671 [DOI] [PubMed] [Google Scholar]
- 279. Bergman RN , Yang YJ , Hope ID , Ader M. 1990. The role of the transcapillary insulin transport in the efficiency of insulin action: studies with glucose clamps and the minimal model. Horm Metab Res Suppl 24:49–56 [PubMed] [Google Scholar]
- 280. Flier JS , Minaker KL , Landsberg L , Young JB , Pallotta J , Rowe JW. 1982. Impaired in vivo insulin clearance in patients with severe target-cell resistance to insulin. Diabetes 31:132–135 [DOI] [PubMed] [Google Scholar]
- 281. Cryer PE , Davis SN , Shamoon H. 2003. Hypoglycemia in diabetes. Diabetes Care 26:1902–1912 [DOI] [PubMed] [Google Scholar]
- 282. Rice S , Christoforidis N , Gadd C , Nikolaou D , Seyani L , Donaldson A , Margara R , Hardy K , Franks S. 2005. Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum Reprod 20:373–381 [DOI] [PubMed] [Google Scholar]
- 283. Zhang LH , Rodriguez H , Ohno S , Miller WL. 1995. Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci USA 92:10619–10623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Sam S , Dunaif A. 2003. Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab 14:365–370 [DOI] [PubMed] [Google Scholar]
- 285. Bremer AA , Miller WL. 2008. The serine phosphorylation hypothesis of polycystic ovary syndrome: a unifying mechanism for hyperandrogenemia and insulin resistance. Fertil Steril 89:1039–1048 [DOI] [PubMed] [Google Scholar]
- 286. Tee MK , Dong Q , Miller WL. 2008. Pathways leading to phosphorylation of P450c17 and to the posttranslational regulation of androgen biosynthesis. Endocrinology 149:2667–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Nelson-Degrave VL , Wickenheisser JK , Hendricks KL , Asano T , Fujishiro M , Legro RS , Kimball SR , Strauss JF , McAllister JM. 2005. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol 19:379–390 [DOI] [PubMed] [Google Scholar]
- 288. Begum N , Sandu OA , Ito M , Lohmann SM , Smolenski A. 2002. Active Rho kinase (ROK-α) associates with insulin receptor substrate-1 and inhibits insulin signaling in vascular smooth muscle cells. J Biol Chem 277:6214–6222 [DOI] [PubMed] [Google Scholar]
- 289. Martens JW , Geller DH , Arlt W , Auchus RJ , Ossovskaya VS , Rodriguez H , Dunaif A , Miller WL. 2000. Enzymatic activities of P450c17 stably expressed in fibroblasts from patients with the polycystic ovary syndrome. J Clin Endocrinol Metab 85:4338–4346 [DOI] [PubMed] [Google Scholar]
- 290. Bergman RN , Finegood DT , Kahn SE. 2002. The evolution of β-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest 32(Suppl 3):35–45 [DOI] [PubMed] [Google Scholar]
- 291. Kahn BB. 1998. Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell 92:593–596 [DOI] [PubMed] [Google Scholar]
- 292. Kahn SE , Prigeon RL , McCulloch DK , Boyko EJ , Bergman RN , Schwartz MW , Neifing JL , Ward WK , Beard JC , Palmer JP. 1993. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 293. Watanabe RM , Valle T , Hauser ER , Ghosh S , Eriksson J , Kohtamäki K , Ehnholm C , Tuomilehto J , Collins FS , Bergman RN , Boehnke M. 1999. Familiality of quantitative metabolic traits in Finnish families with non-insulin-dependent diabetes mellitus. Finland-United States Investigation of NIDDM Genetics (FUSION) Study investigators. Hum Hered 49:159–168 [DOI] [PubMed] [Google Scholar]
- 294. Lyssenko V , Jonsson A , Almgren P , Pulizzi N , Isomaa B , Tuomi T , Berglund G , Altshuler D , Nilsson P , Groop L. 2008. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med 359:2220–2232 [DOI] [PubMed] [Google Scholar]
- 295. Boesgaard TW , Grarup N , Jørgensen T , Borch-Johnsen K , Hansen T , Pedersen O. 2010. Variants at DGKB/TMEM195, ADRA2A, GLIS3 and C2CD4B loci are associated with reduced glucose-stimulated β cell function in middle-aged Danish people. Diabetologia 53:1647–1655 [DOI] [PubMed] [Google Scholar]
- 296. Lorenzo C , Wagenknecht LE , Rewers MJ , Karter AJ , Bergman RN , Hanley AJ , Haffner SM. 2010. Disposition index, glucose effectiveness, and conversion to type 2 diabetes. Diabetes Care 33:2098–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Weyer C , Bogardus C , Mott DM , Pratley RE. 1999. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Ehrmann DA , Sturis J , Byrne MM , Karrison T , Rosenfield RL , Polonsky KS. 1995. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest 96:520–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. O'Meara NM , Blackman JD , Ehrmann DA , Barnes RB , Jaspan JB , Rosenfield RL , Polonsky KS. 1993. Defects in β-cell function in functional ovarian hyperandrogenism. J Clin Endocrinol Metab 76:1241–1247 [DOI] [PubMed] [Google Scholar]
- 300. Fehmann HC , Göke R , Göke B. 1995. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr Rev 16:390–410 [DOI] [PubMed] [Google Scholar]
- 301. Dunaif A , Finegood DT. 1996. β-Cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab 81:942–947 [DOI] [PubMed] [Google Scholar]
- 302. Arslanian SA , Lewy VD , Danadian K. 2001. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and β-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab 86:66–71 [DOI] [PubMed] [Google Scholar]
- 303. Weber RF , Pache TD , Jacobs ML , Docter R , Loriaux DL , Fauser BC , Birkenhäger JC. 1993. The relation between clinical manifestations of polycystic ovary syndrome and β-cell function. Clin Endocrinol (Oxf) 38:295–300 [DOI] [PubMed] [Google Scholar]
- 304. Ciampelli M , Fulghesu AM , Murgia F , Guido M , Cucinelli F , Apa R , Caruso A , Lanzone A. 1998. Acute insulin response to intravenous glucagon in polycystic ovary syndrome. Hum Reprod 13:847–851 [DOI] [PubMed] [Google Scholar]
- 305. Goodarzi MO , Erickson S , Port SC , Jennrich RI , Korenman SG. 2005. β-Cell function: a key pathological determinant in polycystic ovary syndrome. J Clin Endocrinol Metab 90:310–315 [DOI] [PubMed] [Google Scholar]
- 306. Wallace TM , Levy JC , Matthews DR. 2004. Use and abuse of HOMA modeling. Diabetes Care 27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 307. Grimmichova T , Vrbikova J , Matucha P , Vondra K , Veldhuis PP , Johnson ML. 2008. Fasting insulin pulsatile secretion in lean women with polycystic ovary syndrome. Physiol Res 57(Suppl 1):S91–S98 [DOI] [PubMed] [Google Scholar]
- 308. Liu S , Navarro G , Mauvais-Jarvis F. 2010. Androgen excess produces systemic oxidative stress and predisposes to β-cell failure in female mice. PloS One 5:e11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Diamond MP , Grainger D , Diamond MC , Sherwin RS , Defronzo RA. 1998. Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab 83:4420–4425 [DOI] [PubMed] [Google Scholar]
- 310. Biyasheva A , Legro RS , Dunaif A , Urbanek M. 2009. Evidence for association between polycystic ovary syndrome (PCOS) and TCF7L2 and glucose intolerance in women with PCOS and TCF7L2. J Clin Endocrinol Metab 94:2617–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Colilla S , Cox NJ , Ehrmann DA. 2001. Heritability of insulin secretion and insulin action in women with polycystic ovary syndrome and their first degree relatives. J Clin Endocrinol Metab 86:2027–2031 [DOI] [PubMed] [Google Scholar]
- 312. Marshall S. 1985. Kinetics of insulin receptor internalization and recycling in adipocytes. Shunting of receptors to a degradative pathway by inhibitors of recycling. J Biol Chem 260:4136–4144 [PubMed] [Google Scholar]
- 313. Pasquali R , Venturoli S , Paradisi R , Capelli M , Parenti M , Melchionda N. 1982. Insulin and C-peptide levels in obese patients with polycystic ovaries. Horm Metab Res 14:284–287 [DOI] [PubMed] [Google Scholar]
- 314. Mahabeer S , Jialal I , Norman RJ , Naidoo C , Reddi K , Joubert SM. 1989. Insulin and C-peptide secretion in non-obese patients with polycystic ovarian disease. Horm Metab Res 21:502–506 [DOI] [PubMed] [Google Scholar]
- 315. Peiris AN , Mueller RA , Struve MF , Smith GA , Kissebah AH. 1987. Relationship of androgenic activity to splanchnic insulin metabolism and peripheral glucose utilization in premenopausal women. J Clin Endocrinol Metab 64:162–169 [DOI] [PubMed] [Google Scholar]
- 316. Carmina E , Napoli N , Longo RA , Rini GB , Lobo RA. 2006. Metabolic syndrome in polycystic ovary syndrome (PCOS): lower prevalence in southern Italy than in the USA and the influence of criteria for the diagnosis of PCOS. Eur J Endocrinol 154:141–145 [DOI] [PubMed] [Google Scholar]
- 317. Alvarez-Blasco F , Botella-Carretero JI , San Millán JL , Escobar-Morreale HF. 2006. Prevalence and characteristics of the polycystic ovary syndrome in overweight and obese women. Arch Intern Med 166:2081–2086 [DOI] [PubMed] [Google Scholar]
- 318. Gambineri A , Pelusi C , Vicennati V , Pagotto U , Pasquali R. 2002. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord 26:883–896 [DOI] [PubMed] [Google Scholar]
- 319. Escobar-Morreale HF , San Millán JL. 2007. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol Metab 18:266–272 [DOI] [PubMed] [Google Scholar]
- 320. Faulds G , Rydén M , Ek I , Wahrenberg H , Arner P. 2003. Mechanisms behind lipolytic catecholamine resistance of subcutaneous fat cells in the polycystic ovarian syndrome. J Clin Endocrinol Metab 88:2269–2273 [DOI] [PubMed] [Google Scholar]
- 321. Bergman RN , Kim SP , Hsu IR , Catalano KJ , Chiu JD , Kabir M , Richey JM , Ader M. 2007. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med 120(2 Suppl 1):S3–S8; discussion S29–S32 [DOI] [PubMed] [Google Scholar]
- 322. Toulis KA , Goulis DG , Farmakiotis D , Georgopoulos NA , Katsikis I , Tarlatzis BC , Papadimas I , Panidis D. 2009. Adiponectin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Hum Reprod Update 15:297–307 [DOI] [PubMed] [Google Scholar]
- 323. Berg AH , Combs TP , Du X , Brownlee M , Scherer PE. 2001. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7:947–953 [DOI] [PubMed] [Google Scholar]
- 324. Attie AD , Scherer PE. 2009. Adipocyte metabolism and obesity. J Lipid Res 50 Suppl:S395–S399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Hotamisligil GS , Spiegelman BM. 1994. Tumor necrosis factor α: a key component of the obesity-diabetes link. Diabetes 43:1271–1278 [DOI] [PubMed] [Google Scholar]
- 326. Wellen KE , Hotamisligil GS. 2005. Inflammation, stress, and diabetes. J Clin Invest 115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Gonzalez F , Thusu K , Abdel-Rahman E , Prabhala A , Tomani M , Dandona P. 1999. Elevated serum levels of tumor necrosis factor α in normal-weight women with polycystic ovary syndrome. Metabolism 48:437–441 [DOI] [PubMed] [Google Scholar]
- 328. Diamanti-Kandarakis E , Paterakis T , Kandarakis HA. 2006. Indices of low-grade inflammation in polycystic ovary syndrome. Ann NY Acad Sci 1092:175–186 [DOI] [PubMed] [Google Scholar]
- 329. Duleba AJ , Dokras A. 2012. Is PCOS an inflammatory process? Fertil Steril 97:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Chazenbalk G , Chen YH , Heneidi S , Lee JM , Pall M , Chen YD , Azziz R. 2012. Abnormal expression of genes involved in inflammation, lipid metabolism, and Wnt signaling in the adipose tissue of polycystic ovary syndrome. J Clin Endocrinol Metab 97:E765–E770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Pasquali R , Gambineri A , Pagotto U. 2006. The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG 113:1148–1159 [DOI] [PubMed] [Google Scholar]
- 332. Pasquali R. 2006. Obesity and androgens: facts and perspectives. Fertil Steril 85:1319–1340 [DOI] [PubMed] [Google Scholar]
- 333. Azziz R. 1989. Reproductive endocrinologic alterations in female asymptomatic obesity. Fertil Steril 52:703–725 [DOI] [PubMed] [Google Scholar]
- 334. Robinson S , Chan SP , Spacey S , Anyaoku V , Johnston DG , Franks S. 1992. Postprandial thermogenesis is reduced in polycystic ovary syndrome and is associated with increased insulin resistance. Clin Endocrinol (Oxf) 36:537–543 [DOI] [PubMed] [Google Scholar]
- 335. Segal KR , Dunaif A. 1990. Resting metabolic rate and postprandial thermogenesis in polycystic ovarian syndrome. Int J Obes 14:559–567 [PubMed] [Google Scholar]
- 336. Mantzoros CS , Dunaif A , Flier JS. 1997. Leptin concentrations in the polycystic ovary syndrome. J Clin Endocrinol Metab 82:1687–1691 [DOI] [PubMed] [Google Scholar]
- 337. Shi H , Clegg DJ. 2009. Sex differences in the regulation of body weight. Physiol Behav 97:199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Vrbikova J , Hill M , Bendlova B , Grimmichova T , Dvorakova K , Vondra K , Pacini G. 2008. Incretin levels in polycystic ovary syndrome. Eur J Endocrinol 159:121–127 [DOI] [PubMed] [Google Scholar]
- 339. Hirschberg AL , Naessén S , Stridsberg M , Byström B , Holtet J. 2004. Impaired cholecystokinin secretion and disturbed appetite regulation in women with polycystic ovary syndrome. Gynecol Endocrinol 19:79–87 [DOI] [PubMed] [Google Scholar]
- 340. Moran LJ , Noakes M , Clifton PM , Wittert GA , Tomlinson L , Galletly C , Luscombe ND , Norman RJ. 2004. Ghrelin and measures of satiety are altered in polycystic ovary syndrome but not differentially affected by diet composition. J Clin Endocrinol Metab 89:3337–3344 [DOI] [PubMed] [Google Scholar]
- 341. Moran LJ , Noakes M , Clifton PM , Wittert GA , Le Roux CW , Ghatei MA , Bloom SR , Norman RJ. 2007. Postprandial ghrelin, cholecystokinin, peptide YY, and appetite before and after weight loss in overweight women with and without polycystic ovary syndrome. Am J Clin Nutr 86:1603–1610 [DOI] [PubMed] [Google Scholar]
- 342. Sundblad C , Landén M , Eriksson T , Bergman L , Eriksson E. 2005. Effects of the androgen antagonist flutamide and the serotonin reuptake inhibitor citalopram in bulimia nervosa: a placebo-controlled pilot study. J Clin Psychopharmacol 25:85–88 [DOI] [PubMed] [Google Scholar]
- 343. Naessén S , Carlström K , Garoff L , Glant R , Hirschberg AL. 2006. Polycystic ovary syndrome in bulimic women—an evaluation based on the new diagnostic criteria. Gynecol Endocrinol 22:388–394 [DOI] [PubMed] [Google Scholar]
- 344. Naessén S , Carlström K , Byström B , Pierre Y , Hirschberg AL. 2007. Effects of an antiandrogenic oral contraceptive on appetite and eating behavior in bulimic women. Psychoneuroendocrinology 32:548–554 [DOI] [PubMed] [Google Scholar]
- 345. Poretsky L , Smith D , Seibel M , Pazianos A , Moses AC , Flier JS. 1984. Specific insulin binding sites in human ovary. J Clin Endocrinol Metab 59:809–811 [DOI] [PubMed] [Google Scholar]
- 346. el-Roeiy A , Chen X , Roberts VJ , LeRoith D , Roberts CT , Yen SS. 1993. Expression of insulin-like growth factor-I (IGF-I) and IGF-II and the IGF-I, IGF-II, and insulin receptor genes and localization of the gene products in the human ovary. J Clin Endocrinol Metab 77:1411–1418 [DOI] [PubMed] [Google Scholar]
- 347. el-Roeiy A , Chen X , Roberts VJ , Shimasakai S , Ling N , LeRoith D , Roberts CT , Yen SS. 1994. Expression of the genes encoding the insulin-like growth factors (IGF-I and II), the IGF and insulin receptors, and IGF-binding proteins-1–6 and the localization of their gene products in normal and polycystic ovary syndrome ovaries. J Clin Endocrinol Metab 78:1488–1496 [DOI] [PubMed] [Google Scholar]
- 348. Czech MP. 1982. Structural and functional homologies in the receptors for insulin and the insulin-like growth factors. Cell 31:8–10 [DOI] [PubMed] [Google Scholar]
- 349. Froesch ER , Zapf J. 1985. Insulin-like growth factors and insulin: comparative aspects. Diabetologia 28:485–493 [DOI] [PubMed] [Google Scholar]
- 350. LeRoith D , Werner H , Beitner-Johnson D , Roberts CT. 1995. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev 16:143–163 [DOI] [PubMed] [Google Scholar]
- 351. Moxham CP , Jacobs S. 1992. Insulin/IGF-I receptor hybrids: a mechanism for increasing receptor diversity. J Cell Biochem 48:136–140 [DOI] [PubMed] [Google Scholar]
- 352. Poretsky L. 1991. On the paradox of insulin-induced hyperandrogenism in insulin-resistant states. Endocr Rev 12:3–13 [DOI] [PubMed] [Google Scholar]
- 353. Willis D , Franks S. 1995. Insulin action in human granulosa cells from normal and polycystic ovaries is mediated by the insulin receptor and not the type-I insulin-like growth factor receptor. J Clin Endocrinol Metab 80:3788–3790 [DOI] [PubMed] [Google Scholar]
- 354. Nestler JE , Jakubowicz DJ , de Vargas AF , Brik C , Quintero N , Medina F. 1998. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab 83:2001–2005 [DOI] [PubMed] [Google Scholar]
- 355. Munir I , Yen HW , Geller DH , Torbati D , Bierden RM , Weitsman SR , Agarwal SK , Magoffin DA. 2004. Insulin augmentation of 17α-hydroxylase activity is mediated by phosphatidyl inositol 3-kinase but not extracellular signal-regulated kinase-1/2 in human ovarian theca cells. Endocrinology 145:175–183 [DOI] [PubMed] [Google Scholar]
- 356. Willis D , Mason H , Gilling-Smith C , Franks S. 1996. Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. J Clin Endocrinol Metab 81:302–309 [DOI] [PubMed] [Google Scholar]
- 357. Jakimiuk AJ , Weitsman SR , Navab A , Magoffin DA. 2001. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab 86:1318–1323 [DOI] [PubMed] [Google Scholar]
- 358. Wu XK , Zhou SY , Liu JX , Pöllänen P , Sallinen K , Mäkinen M , Erkkola R. 2003. Selective ovary resistance to insulin signaling in women with polycystic ovary syndrome. Fertil Steril 80:954–965 [DOI] [PubMed] [Google Scholar]
- 359. Yen HW , Jakimiuk AJ , Munir I , Magoffin DA. 2004. Selective alterations in insulin receptor substrates-1, -2 and -4 in theca but not granulosa cells from polycystic ovaries. Mol Hum Reprod 10:473–479 [DOI] [PubMed] [Google Scholar]
- 360. Barbieri RL , Makris A , Ryan KJ. 1983. Effects of insulin on steroidogenesis in cultured porcine ovarian theca. Fertil Steril 40:237–241 [PubMed] [Google Scholar]
- 361. Nestler JE , Strauss JF. 1991. Insulin as an effector of human ovarian and adrenal steroid metabolism. Endocrinol Metab Clin North Am 20:807–823 [PubMed] [Google Scholar]
- 362. Franks S , Gilling-Smith C , Watson H , Willis D. 1999. Insulin action in the normal and polycystic ovary. Endocrinol Metab Clin North Am 28:361–378 [DOI] [PubMed] [Google Scholar]
- 363. Adashi EY , Hsueh AJ , Yen SS. 1981. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology 108:1441–1449 [DOI] [PubMed] [Google Scholar]
- 364. Brothers KJ , Wu S , DiVall SA , Messmer MR , Kahn CR , Miller RS , Radovick S , Wondisford FE , Wolfe A. 2010. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metabolism 12:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Micic D , Popovic V , Nesovic M , Sumarac M , Dragasevic M , Kendereski A , Markovic D , Djordjevic P , Manojlovic D , Micic J. 1988. Androgen levels during sequential insulin euglycemic clamp studies in patients with polycystic ovary disease. J Steroid Biochem 31:995–999 [DOI] [PubMed] [Google Scholar]
- 366. Dunaif A , Graf M. 1989. Insulin administration alters gonadal steroid metabolism independent of changes in gonadotropin secretion in insulin-resistant women with the polycystic ovary syndrome. J Clin Invest 83:23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Tosi F , Negri C , Perrone F , Dorizzi R , Castello R , Bonora E , Moghetti P. 2012. Hyperinsulinemia amplifies GnRH agonist stimulated ovarian steroid secretion in women with polycystic ovary syndrome. J Clin Endocrinol Metab 97:1712–1719 [DOI] [PubMed] [Google Scholar]
- 368. Nestler JE , Barlascini CO , Matt DW , Steingold KA , Plymate SR , Clore JN , Blackard WG. 1989. Suppression of serum insulin by diazoxide reduces serum testosterone levels in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 68:1027–1032 [DOI] [PubMed] [Google Scholar]
- 369. Plymate SR , Jones RE , Matej LA , Friedl KE. 1988. Regulation of sex hormone binding globulin (SHBG) production in Hep G2 cells by insulin. Steroids 52:339–340 [DOI] [PubMed] [Google Scholar]
- 370. Nestler JE. 1993. Sex hormone-binding globulin: a marker for hyperinsulinemia and/or insulin resistance? J Clin Endocrinol Metab 76:273–274 [DOI] [PubMed] [Google Scholar]
- 371. Nestler JE , Singh R , Matt DW , Clore JN , Blackard WG. 1990. Suppression of serum insulin level by diazoxide does not alter serum testosterone or sex hormone-binding globulin levels in healthy, nonobese women. Am J Obstet Gynecol 163:1243–1246 [DOI] [PubMed] [Google Scholar]
- 372. Velazquez EM , Mendoza S , Hamer T , Sosa F , Glueck CJ. 1994. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism 43:647–654 [DOI] [PubMed] [Google Scholar]
- 373. Dunaif A , Scott D , Finegood D , Quintana B , Whitcomb R. 1996. The insulin-sensitizing agent troglitazone improves metabolic and reproductive abnormalities in the polycystic ovary syndrome. J Clin Endocrinol Metab 81:3299–3306 [DOI] [PubMed] [Google Scholar]
- 374. Dunaif A. 2008. Drug insight: insulin-sensitizing drugs in the treatment of polycystic ovary syndrome—a reappraisal. Nat Clin Pract Endocrinol Metab 4:272–283 [DOI] [PubMed] [Google Scholar]
- 375. Palomba S , Falbo A , Zullo F , Orio F. 2009. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev 30:1–50 [DOI] [PubMed] [Google Scholar]
- 376. Practice Committee of American Society for Reproductive Medicine 2008. Use of insulin-sensitizing agents in the treatment of polycystic ovary syndrome. Fertil Steril 90:S69–S73 [DOI] [PubMed] [Google Scholar]
- 377. Nestler JE. 2008. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med 358:47–54 [DOI] [PubMed] [Google Scholar]
- 378. Tang T , Lord JM , Norman RJ , Yasmin E , Balen AH. 2009. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev 1:CD003053. [DOI] [PubMed] [Google Scholar]
- 379. Nestler JE , Jakubowicz DJ. 1996. Decreases in ovarian cytochrome P450c17 α activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med 335:617–623 [DOI] [PubMed] [Google Scholar]
- 380. Arlt W , Auchus RJ , Miller WL. 2001. Thiazolidinediones but not metformin directly inhibit the steroidogenic enzymes P450c17 and 3β -hydroxysteroid dehydrogenase. J Biol Chem 276:16767–16771 [DOI] [PubMed] [Google Scholar]
- 381. Arlt W , Neogi P , Gross C , Miller WL. 2004. Cinnamic acid based thiazolidinediones inhibit human P450c17 and 3β-hydroxysteroid dehydrogenase and improve insulin sensitivity independent of PPAR-γ agonist activity. J Mol Endocrinol 32:425–436 [DOI] [PubMed] [Google Scholar]
- 382. Schoppee PD , Garmey JC , Veldhuis JD. 2002. Putative activation of the peroxisome proliferator-activated receptor γ impairs androgen and enhances progesterone biosynthesis in primary cultures of porcine theca cells. Biol Reprod 66:190–198 [DOI] [PubMed] [Google Scholar]
- 383. Attia GR , Rainey WE , Carr BR. 2001. Metformin directly inhibits androgen production in human thecal cells. Fertil Steril 76:517–524 [DOI] [PubMed] [Google Scholar]
- 384. Qu J , Wang Y , Wu X , Gao L , Hou L , Erkkola R. 2009. Insulin resistance directly contributes to androgenic potential within ovarian theca cells. Fertil Steril 91:1990–1997 [DOI] [PubMed] [Google Scholar]
- 385. Nestler JE , Usiskin KS , Barlascini CO , Welty DF , Clore JN , Blackard WG. 1989. Suppression of serum dehydroepiandrosterone sulfate levels by insulin: an evaluation of possible mechanisms. J Clin Endocrinol Metab 69:1040–1046 [DOI] [PubMed] [Google Scholar]
- 386. Beer NA , Jakubowicz DJ , Beer RM , Nestler JE. 1994. Disparate effects of insulin reduction with diltiazem on serum dehydroepiandrosterone sulfate levels in obese hypertensive men and women. J Clin Endocrinol Metab 79:1077–1081 [DOI] [PubMed] [Google Scholar]
- 387. Nestler JE , Kahwash Z. 1994. Sex-specific action of insulin to acutely increase the metabolic clearance rate of dehydroepiandrosterone in humans. J Clin Invest 94:1484–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Azziz R , Ehrmann DA , Legro RS , Fereshetian AG , O'Keefe M , Ghazzi MN , Group PCTS. 2003. Troglitazone decreases adrenal androgen levels in women with polycystic ovary syndrome. Fertil Steril 79:932–937 [DOI] [PubMed] [Google Scholar]
- 389. Moghetti P , Castello R , Negri C , Tosi F , Spiazzi GG , Brun E , Balducci R , Toscano V , Muggeo M. 1996. Insulin infusion amplifies 17 α-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: apparent relative impairment of 17,20-lyase activity. J Clin Endocrinol Metab 81:881–886 [DOI] [PubMed] [Google Scholar]
- 390. Romualdi D , Giuliani M , Draisci G , Costantini B , Cristello F , Lanzone A , Guido M. 2007. Pioglitazone reduces the adrenal androgen response to corticotropin-releasing factor without changes in ACTH release in hyperinsulinemic women with polycystic ovary syndrome. Fertil Steril 88:131–138 [DOI] [PubMed] [Google Scholar]
- 391. Lawson MA , Jain S , Sun S , Patel K , Malcolm PJ , Chang RJ. 2008. Evidence for insulin suppression of baseline luteinizing hormone in women with polycystic ovarian syndrome and normal women. J Clin Endocrinol Metab 93:2089–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Azziz R , Ehrmann D , Legro RS , Whitcomb RW , Hanley R , Fereshetian AG , O'Keefe M , Ghazzi MN, PCOS/Troglitazone Study Group 2001. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab 86:1626–1632 [DOI] [PubMed] [Google Scholar]
- 393. Eagleson CA , Bellows AB , Hu K , Gingrich MB , Marshall JC. 2003. Obese patients with polycystic ovary syndrome: evidence that metformin does not restore sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by ovarian steroids. J Clin Endocrinol Metab 88:5158–5162 [DOI] [PubMed] [Google Scholar]
- 394. Lord JM , Flight IH , Norman RJ. 2003. Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, D-chiro-inositol) for polycystic ovary syndrome. Cochrane Database Syst Rev 3:CD003053. [DOI] [PubMed] [Google Scholar]
- 395. Burks DJ , Font de Mora J , Schubert M , Withers DJ , Myers MG , Towery HH , Altamuro SL , Flint CL , White MF. 2000. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 407:377–382 [DOI] [PubMed] [Google Scholar]
- 396. Brüning JC , Gautam D , Burks DJ , Gillette J , Schubert M , Orban PC , Klein R , Krone W , Müller-Wieland D , Kahn CR. 2000. Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125 [DOI] [PubMed] [Google Scholar]
- 397. Hill JW , Elias CF , Fukuda M , Williams KW , Berglund ED , Holland WL , Cho YR , Chuang JC , Xu Y , Choi M , Lauzon D , Lee CE , Coppari R , Richardson JA , Zigman JM , Chua S , Scherer PE , Lowell BB , Brüning JC , Elmquist JK. 2010. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metabolism 11:286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Nandi A , Wang X , Accili D , Wolgemuth DJ. 2010. The effect of insulin signaling on female reproductive function independent of adiposity and hyperglycemia. Endocrinology 151:1863–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Yki-Järvinen H. 1984. Sex and insulin sensitivity. Metabolism 33:1011–1015 [DOI] [PubMed] [Google Scholar]
- 400. Nuutila P , Knuuti MJ , Mäki M , Laine H , Ruotsalainen U , Teräs M , Haaparanta M , Solin O , Yki-Järvinen H. 1995. Gender and insulin sensitivity in the heart and in skeletal muscles. Studies using positron emission tomography. Diabetes 44:31–36 [DOI] [PubMed] [Google Scholar]
- 401. Foley JE , Kashiwagi A , Chang H , Huecksteadt TP , Lillioja S , Verso MA , Reaven G. 1984. Sex difference in insulin-stimulated glucose transport in rat and human adipocytes. Am J Physiol 246:E211–E215 [DOI] [PubMed] [Google Scholar]
- 402. Polderman KH , Gooren LJ , Asscheman H , Bakker A , Heine RJ. 1994. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab 79:265–271 [DOI] [PubMed] [Google Scholar]
- 403. Elbers JM , Giltay EJ , Teerlink T , Scheffer PG , Asscheman H , Seidell JC , Gooren LJ. 2003. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol (Oxf) 58:562–571 [DOI] [PubMed] [Google Scholar]
- 404. Mortola JF , Yen SS. 1990. The effects of oral dehydroepiandrosterone on endocrine-metabolic parameters in postmenopausal women. J Clin Endocrinol Metab 71:696–704 [DOI] [PubMed] [Google Scholar]
- 405. Nestler JE , Barlascini CO , Clore JN , Blackard WG. 1988. Dehydroepiandrosterone reduces serum low density lipoprotein levels and body fat but does not alter insulin sensitivity in normal men. J Clin Endocrinol Metab 66:57–61 [DOI] [PubMed] [Google Scholar]
- 406. Holmäng A , Larsson BM , Brzezinska Z , Björntorp P. 1992. Effects of short-term testosterone exposure on insulin sensitivity of muscles in female rats. Am J Physiol 262:E851–E855 [DOI] [PubMed] [Google Scholar]
- 407. Rincon J , Holmäng A , Wahlström EO , Lönnroth P , Björntorp P , Zierath JR , Wallberg-Henriksson H. 1996. Mechanisms behind insulin resistance in rat skeletal muscle after oophorectomy and additional testosterone treatment. Diabetes 45:615–621 [DOI] [PubMed] [Google Scholar]
- 408. Allemand MC , Irving BA , Asmann YW , Klaus KA , Tatpati L , Coddington CC , Nair KS. 2009. Effect of testosterone on insulin stimulated IRS-1 Ser phosphorylation in primary rat myotubes—a potential model for PCOS-related insulin resistance. PloS One 4:e4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Mannerås L , Cajander S , Holmäng A , Seleskovic Z , Lystig T , Lönn M , Stener-Victorin E. 2007. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 148:3781–3791 [DOI] [PubMed] [Google Scholar]
- 410. Corbould A. 2007. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol 192:585–594 [DOI] [PubMed] [Google Scholar]
- 411. Dicker A , Rydén M , Näslund E , Muehlen IE , Wirén M , Lafontan M , Arner P. 2004. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia 47:420–428 [DOI] [PubMed] [Google Scholar]
- 412. Arner P. 2005. Effects of testosterone on fat cell lipolysis. Species differences and possible role in polycystic ovarian syndrome. Biochimie 87:39–43 [DOI] [PubMed] [Google Scholar]
- 413. Xu A , Chan KW , Hoo RL , Wang Y , Tan KC , Zhang J , Chen B , Lam MC , Tse C , Cooper GJ , Lam KS. 2005. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem 280:18073–18080 [DOI] [PubMed] [Google Scholar]
- 414. Mårin P , Krotkiewski M , Björntorp P. 1992. Androgen treatment of middle-aged, obese men: effects on metabolism, muscle and adipose tissues. Eur J Med 1:329–336 [PubMed] [Google Scholar]
- 415. Schroeder ET , Zheng L , Ong MD , Martinez C , Flores C , Stewart Y , Azen C , Sattler FR. 2004. Effects of androgen therapy on adipose tissue and metabolism in older men. J Clin Endocrinol Metab 89:4863–4872 [DOI] [PubMed] [Google Scholar]
- 416. Elbers JM , Asscheman H , Seidell JC , Megens JA , Gooren LJ. 1997. Long-term testosterone administration increases visceral fat in female to male transsexuals. J Clin Endocrinol Metab 82:2044–2047 [DOI] [PubMed] [Google Scholar]
- 417. Woodhouse LJ , Gupta N , Bhasin M , Singh AB , Ross R , Phillips J , Bhasin S. 2004. Dose-dependent effects of testosterone on regional adipose tissue distribution in healthy young men. J Clin Endocrinol Metab 89:718–726 [DOI] [PubMed] [Google Scholar]
- 418. Dunaif A , Green G , Futterweit W , Dobrjansky A. 1990. Suppression of hyperandrogenism does not improve peripheral or hepatic insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab 70:699–704 [DOI] [PubMed] [Google Scholar]
- 419. Lemieux S , Lewis GF , Ben-Chetrit A , Steiner G , Greenblatt EM. 1999. Correction of hyperandrogenemia by laparoscopic ovarian cautery in women with polycystic ovarian syndrome is not accompanied by improved insulin sensitivity or lipid-lipoprotein levels. J Clin Endocrinol Metab 84:4278–4282 [DOI] [PubMed] [Google Scholar]
- 420. Diamanti-Kandarakis E , Mitrakou A , Hennes MM , Platanissiotis D , Kaklas N , Spina J , Georgiadou E , Hoffmann RG , Kissebah AH , Raptis S. 1995. Insulin sensitivity and antiandrogenic therapy in women with polycystic ovary syndrome. Metabolism 44:525–531 [DOI] [PubMed] [Google Scholar]
- 421. Elkind-Hirsch KE , Valdes CT , Malinak LR. 1993. Insulin resistance improves in hyperandrogenic women treated with Lupron. Fertil Steril 60:634–641 [DOI] [PubMed] [Google Scholar]
- 422. Moghetti P , Tosi F , Castello R , Magnani CM , Negri C , Brun E , Furlani L , Caputo M , Muggeo M. 1996. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J Clin Endocrinol Metab 81:952–960 [DOI] [PubMed] [Google Scholar]
- 423. Cagnacci A , Paoletti AM , Arangino S , Melis GB , Volpe A. 1999. Effect of ovarian suppression on glucose metabolism of young lean women with and without ovarian hyperandrogenism. Hum Reprod 14:893–897 [DOI] [PubMed] [Google Scholar]
- 424. Gambineri A , Pelusi C , Genghini S , Morselli-Labate AM , Cacciari M , Pagotto U , Pasquali R. 2004. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 60:241–249 [DOI] [PubMed] [Google Scholar]
- 425. Gambineri A , Patton L , Vaccina A , Cacciari M , Morselli-Labate AM , Cavazza C , Pagotto U , Pasquali R. 2006. Treatment with flutamide, metformin and their combination added to hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. J Clin Endocrinol Metab 91:3970–3980 [DOI] [PubMed] [Google Scholar]
- 426. Judd HL , Scully RE , Herbst AL , Yen SS , Ingersol FM , Kliman B. 1973. Familial hyperthecosis: comparison of endocrinologic and histologic findings with polycystic ovarian disease. Am J Obstet Gynecol 117:976–982 [DOI] [PubMed] [Google Scholar]
- 427. Cohen PN , Givens JR , Wiser WL , Wilroy RS , Summitt RL , Coleman SA , Andersen RN. 1975. Polycystic ovarian disease, maturation arrest of spermiogenesis, and Klinefelter's syndrome in siblings of a family with familial hirsutism. Fertil Steril 26:1228–1238 [PubMed] [Google Scholar]
- 428. Givens JR. 1988. Familial polycystic ovarian disease. Endocrinol Metab Clin North Am 17:771–783 [PubMed] [Google Scholar]
- 429. Stuart CA , Peters EJ , Prince MJ , Richards G , Cavallo A , Meyer WJ. 1986. Insulin resistance with acanthosis nigricans: the roles of obesity and androgen excess. Metabolism 35:197–205 [DOI] [PubMed] [Google Scholar]
- 430. Nelson VL , Qin KN , Rosenfield RL , Wood JR , Penning TM , Legro RS , Strauss JF , McAllister JM. 2001. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab 86:5925–5933 [DOI] [PubMed] [Google Scholar]
- 431. Franks S , Gharani N , Waterworth D , Batty S , White D , Williamson R , McCarthy M. 1997. The genetic basis of polycystic ovary syndrome. Hum Reprod 12:2641–2648 [DOI] [PubMed] [Google Scholar]
- 432. Legro RS , Driscoll D , Strauss JF , Fox J , Dunaif A. 1998. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA 95:14956–14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Franks S , McCarthy MI , Hardy K. 2006. Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl 29:278–285; discussion 286–290 [DOI] [PubMed] [Google Scholar]
- 434. Urbanek M. 2007. The genetics of the polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab 3:103–111 [DOI] [PubMed] [Google Scholar]
- 435. Vink JM , Sadrzadeh S , Lambalk CB , Boomsma DI. 2006. Heritability of polycystic ovary syndrome (PCOS) in a Dutch twin-family study. J Clin Endocrinol Metab 91:2100–2104 [DOI] [PubMed] [Google Scholar]
- 436. Carey AH , Chan KL , Short F , White D , Williamson R , Franks S. 1993. Evidence for a single gene effect causing polycystic ovaries and male pattern baldness. Clin Endocrinol (Oxf) 38:653–658 [DOI] [PubMed] [Google Scholar]
- 437. Kashar-Miller M , Azziz R. 1999. Heritability and the risk of developing androgen excess. J Steroid Biochem Mol Biol 69:261–268 [DOI] [PubMed] [Google Scholar]
- 438. Lander ES , Schork NJ. 1994. Genetic dissection of complex traits. Science 265:2037–2048 [DOI] [PubMed] [Google Scholar]
- 439. Franks S , Gharani N , McCarthy M. 2001. Candidate genes in polycystic ovary syndrome. Hum Reprod Update 7:405–410 [DOI] [PubMed] [Google Scholar]
- 440. Legro RS , Kunselman AR , Demers L , Wang SC , Bentley-Lewis R , Dunaif A. 2002. Elevated dehydroepiandrosterone sulfate levels as the reproductive phenotype in the brothers of women with polycystic ovary syndrome. J Clin Endocrinol Metab 87:2134–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Veldhuis JD , Rogol AD , Samojlik E , Ertel NH. 1984. Role of endogenous opiates in the expression of negative feedback actions of androgen and estrogen on pulsatile properties of luteinizing hormone secretion in man. J Clin Invest 74:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Yildiz BO , Yarali H , Oguz H , Bayraktar M. 2003. Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab 88:2031–2036 [DOI] [PubMed] [Google Scholar]
- 443. Legro RS , Bentley-Lewis R , Driscoll D , Wang SC , Dunaif A. 2002. Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab 87:2128–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Sam S , Legro RS , Bentley-Lewis R , Dunaif A. 2005. Dyslipidemia and metabolic syndrome in the sisters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 90:4797–4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445. Sam S , Legro RS , Essah PA , Apridonidze T , Dunaif A. 2006. Evidence for metabolic and reproductive phenotypes in mothers of women with polycystic ovary syndrome. Proc Nat Acad Sci USA 103:7030–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Sam S , Sung YA , Legro RS , Dunaif A. 2008. Evidence for pancreatic β-cell dysfunction in brothers of women with polycystic ovary syndrome. Metabolism 57:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Sam S , Coviello AD , Sung YA , Legro RS , Dunaif A. 2008. Metabolic phenotype in the brothers of women with polycystic ovary syndrome. Diabetes Care 31:1237–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Talbot JA , Bicknell EJ , Rajkhowa M , Krook A , O'Rahilly S , Clayton RN. 1996. Molecular scanning of the insulin receptor gene in women with polycystic ovarian syndrome. J Clin Endocrinol Metab 81:1979–1983 [DOI] [PubMed] [Google Scholar]
- 449. Lee EJ , Yoo KJ , Kim SJ , Lee SH , Cha KY , Baek KH. 2006. Single nucleotide polymorphism in exon 17 of the insulin receptor gene is not associated with polycystic ovary syndrome in a Korean population. Fertil Steril 86:380–384 [DOI] [PubMed] [Google Scholar]
- 450. Conway GS , Avey C , Rumsby G. 1994. The tyrosine kinase domain of the insulin receptor gene is normal in women with hyperinsulinaemia and polycystic ovary syndrome. Hum Reprod 9:1681–1683 [DOI] [PubMed] [Google Scholar]
- 451. Mukherjee S , Shaikh N , Khavale S , Shinde G , Meherji P , Shah N , Maitra A. 2009. Genetic variation in exon 17 of INSR is associated with insulin resistance and hyperandrogenemia among lean Indian women with polycystic ovary syndrome. Eur J Endocrinol 160:855–862 [DOI] [PubMed] [Google Scholar]
- 452. Siegel S , Futterweit W , Davies TF , Concepcion ES , Greenberg DA , Villanueva R , Tomer Y. 2002. A C/T single nucleotide polymorphism at the tyrosine kinase domain of the insulin receptor gene is associated with polycystic ovary syndrome. Fertil Steril 78:1240–1243 [DOI] [PubMed] [Google Scholar]
- 453. Barber TM , Bennett AJ , Groves CJ , Sovio U , Ruokonen A , Martikainen H , Pouta A , Hartikainen AL , Elliott P , Wass JA , Järvelin MR , Zeggini E , Franks S , McCarthy MI. 2007. Disparate genetic influences on polycystic ovary syndrome (PCOS) and type 2 diabetes revealed by a lack of association between common variants within the TCF7L2 gene and PCOS. Diabetologia 50:2318–2322 [DOI] [PubMed] [Google Scholar]
- 454. Ewens KG , Jones MR , Ankener W , Stewart DR , Urbanek M , Dunaif A , Legro RS , Chua A , Azziz R , Spielman RS , Goodarzi MO , Strauss JF. 2011. Type 2 diabetes susceptibility single-nucleotide polymorphisms are not associated with polycystic ovary syndrome. Fertil Steril 95:2538–2541.e1–e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455. Hirschhorn JN , Daly MJ. 2005. Genome-wide association studies for common diseases and complex traits. Nat Rev Genet 6:95–108 [DOI] [PubMed] [Google Scholar]
- 456. Ioannidis A , Ikonomi E , Dimou NL , Douma L , Bagos PG. 2010. Polymorphisms of the insulin receptor and the insulin receptor substrates genes in polycystic ovary syndrome: a Mendelian randomization meta-analysis. Mol Genet Metab 99:174–183 [DOI] [PubMed] [Google Scholar]
- 457. Goodarzi MO , Louwers YV , Taylor KD , Jones MR , Cui J , Kwon S , Chen YD , Guo X , Stolk L , Uitterlinden AG , Laven JS , Azziz R. 2011. Replication of association of a novel insulin receptor gene polymorphism with polycystic ovary syndrome. Fertil Steril 95:1736–1741.e1–e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458. Legro RS , Spielman R , Urbanek M , Driscoll D , Strauss JF , Dunaif A. 1998. Phenotype and genotype in polycystic ovary syndrome. Recent Prog Horm Res 53:217–256 [PubMed] [Google Scholar]
- 459. Gharani N , Waterworth DM , Batty S , White D , Gilling-Smith C , Conway GS , McCarthy M , Franks S , Williamson R. 1997. Association of the steroid synthesis gene CYP11a with polycystic ovary syndrome and hyperandrogenism. Hum Mol Genet 6:397–402 [DOI] [PubMed] [Google Scholar]
- 460. Waterworth DM , Bennett ST , Gharani N , McCarthy MI , Hague S , Batty S , Conway GS , White D , Todd JA , Franks S , Williamson R. 1997. Linkage and association of insulin gene VNTR regulatory polymorphism with polycystic ovary syndrome. Lancet 349:986–990 [DOI] [PubMed] [Google Scholar]
- 461. Gaasenbeek M , Powell BL , Sovio U , Haddad L , Gharani N , Bennett A , Groves CJ , Rush K , Goh MJ , Conway GS , Ruokonen A , Martikainen H , Pouta A , Taponen S , Hartikainen AL , Halford S , Järvelin MR , Franks S , McCarthy MI. 2004. Large-scale analysis of the relationship between CYP11A promoter variation, polycystic ovarian syndrome, and serum testosterone. J Clin Endocrinol Metab 89:2408–2413 [DOI] [PubMed] [Google Scholar]
- 462. Bennett A , Sovio U , Ruokonen A , Martikainen H , Pouta A , Taponen S , Hartikainen AL , Franks S , Peltonen L , Elliott P , Järvelin MR , McCarthy MI. 2005. No association between insulin gene variation and adult metabolic phenotypes in a large Finnish birth cohort. Diabetologia 48:886–891 [DOI] [PubMed] [Google Scholar]
- 463. Strachan T , Read AP. 1999. Genetic mapping of complex characters. In: , Strachan T , Read AP, eds. Human molecular genetics 2. 2nd ed Oxford, UK: John Wiley, Sons, Inc.-BIOS Scientific Publishers Ltd.; 283–294 [Google Scholar]
- 464. Urbanek M , Legro RS , Driscoll DA , Azziz R , Ehrmann DA , Norman RJ , Strauss JF , Spielman RS , Dunaif A. 1999. Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci USA 96:8573–8578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Ewens KG , Stewart DR , Ankener W , Urbanek M , McAllister JM , Chen C , Baig KM , Parker SC , Margulies EH , Legro RS , Dunaif A , Strauss JF , Spielman RS. 2010. Family-based analysis of candidate genes for polycystic ovary syndrome. J Clin Endocrinol Metab 95:2306–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466. Stewart DR , Dombroski BA , Urbanek M , Ankener W , Ewens KG , Wood JR , Legro RS , Strauss JF , Dunaif A , Spielman RS. 2006. Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab 91:4112–4117 [DOI] [PubMed] [Google Scholar]
- 467. Tucci S , Futterweit W , Concepcion ES , Greenberg DA , Villanueva R , Davies TF , Tomer Y. 2001. Evidence for association of polycystic ovary syndrome in Caucasian women with a marker at the insulin receptor gene locus. J Clin Endocrinol Metab 86:446–449 [DOI] [PubMed] [Google Scholar]
- 468. Villuendas G , Escobar-Morreale HF , Tosi F , Sancho J , Moghetti P , San Millán JL. 2003. Association between the D19S884 marker at the insulin receptor gene locus and polycystic ovary syndrome. Fertil Steril 79:219–220 [DOI] [PubMed] [Google Scholar]
- 469. Prodoehl MJ , Hatzirodos N , Irving-Rodgers HF , Zhao ZZ , Painter JN , Hickey TE , Gibson MA , Rainey WE , Carr BR , Mason HD , Norman RJ , Montgomery GW , Rodgers RJ. 2009. Genetic and gene expression analyses of the polycystic ovary syndrome candidate gene fibrillin-3 and other fibrillin family members in human ovaries. Mol Hum Reprod 15:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470. Urbanek M , Sam S , Legro RS , Dunaif A. 2007. Identification of a PCOS susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J Clin Endocrinol Metab 92:4191–4198 [DOI] [PubMed] [Google Scholar]
- 471. Charbonneau NL , Ono RN , Corson GM , Keene DR , Sakai LY. 2004. Fine tuning of growth factor signals depends on fibrillin microfibril networks. Birth Defects Res C Embryo Today 72:37–50 [DOI] [PubMed] [Google Scholar]
- 472. Dietz HC , Cutting GR , Pyeritz RE , Maslen CL , Sakai LY , Corson GM , Puffenberger EG , Hamosh A , Nanthakumar EJ , Curristin SM , Stetten G , Meyers DA , Francomano CA. 1991. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352:337–339 [DOI] [PubMed] [Google Scholar]
- 473. Handford PA , Downing AK , Reinhardt DP , Sakai LY. 2000. Fibrillin: from domain structure to supramolecular assembly. Matrix Biol 19:457–470 [DOI] [PubMed] [Google Scholar]
- 474. Corson GM , Charbonneau NL , Keene DR , Sakai LY. 2004. Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics 83:461–472 [DOI] [PubMed] [Google Scholar]
- 475. Ramirez F , Sakai LY , Dietz HC , Rifkin DB. 2004. Fibrillin microfibrils: multipurpose extracellular networks in organismal physiology. Physiol Genomics 19:151–154 [DOI] [PubMed] [Google Scholar]
- 476. Shi Y , Massagué J. 2003. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113:685–700 [DOI] [PubMed] [Google Scholar]
- 477. Derynck R , Zhang YE. 2003. Smad-dependent and Smad-independent pathways in TGFβ family signalling. Nature 425:577–584 [DOI] [PubMed] [Google Scholar]
- 478. Reinhardt DP , Chalberg SC , Sakai LY. 1995. The structure and function of fibrillin. Ciba Found Symp 192:128–143; discussion 143–127 [DOI] [PubMed] [Google Scholar]
- 479. Charbonneau NL , Dzamba BJ , Ono RN , Keene DR , Corson GM , Reinhardt DP , Sakai LY. 2003. Fibrillins can co-assemble in fibrils, but fibrillin fibril composition displays cell-specific differences. J Biol Chem 278:2740–2749 [DOI] [PubMed] [Google Scholar]
- 480. Findlay JK , Drummond AE , Dyson ML , Baillie AJ , Robertson DM , Ethier JF. 2002. Recruitment and development of the follicle; the roles of the transforming growth factor-β superfamily. Mol Cell Endocrinol 191:35–43 [DOI] [PubMed] [Google Scholar]
- 481. Thompson TB , Lerch TF , Cook RW , Woodruff TK , Jardetzky TS. 2005. The structure of the follistatin:activin complex reveals antagonism of both type I and type II receptor binding. Dev Cell 9:535–543 [DOI] [PubMed] [Google Scholar]
- 482. Urbanek M , Wu X , Vickery KR , Kao LC , Christenson LK , Schneyer A , Legro RS , Driscoll DA , Strauss JF , Dunaif A , Spielman RS. 2000. Allelic variants of the follistatin gene in polycystic ovary syndrome. J Clin Endocrinol Metab 85:4455–4461 [DOI] [PubMed] [Google Scholar]
- 483. Jones MR , Wilson SG , Mullin BH , Mead R , Watts GF , Stuckey BGA. 2007. Polymorphism of the follistatin gene in polycystic ovary syndrome. Mol Hum Reprod 13:237–241 [DOI] [PubMed] [Google Scholar]
- 484. Massagué J , Andres J , Attisano L , Cheifetz S , López-Casillas F , Ohtsuki M , Wrana JL. 1992. TGF-β receptors. Mol Reprod Dev 32:99–104 [DOI] [PubMed] [Google Scholar]
- 485. McNatty KP , Fidler AE , Juengel JL , Quirke LD , Smith PR , Heath DA , Lundy T , O'Connell A , Tisdall DJ. 2000. Growth and paracrine factors regulating follicular formation and cellular function. Mol Cell Endocrinol 163:11–20 [DOI] [PubMed] [Google Scholar]
- 486. Chang H , Brown CW , Matzuk MM. 2002. Genetic analysis of the mammalian transforming growth factor-β superfamily. Endocr Rev 23:787–823 [DOI] [PubMed] [Google Scholar]
- 487. Mehra A , Wrana JL. 2002. TGF-β and the Smad signal transduction pathway. Biochem Cell Biol 80:605–622 [DOI] [PubMed] [Google Scholar]
- 488. Tsuchida K , Sunada Y , Noji S , Murakami T , Uezumi A , Nakatani M. 2006. Inhibitors of the TGF-β superfamily and their clinical applications. Mini Rev Med Chem 6:1255–1261 [DOI] [PubMed] [Google Scholar]
- 489. Guo Q , Kumar TR , Woodruff T , Hadsell LA , DeMayo FJ , Matzuk MM. 1998. Overexpression of mouse follistatin causes reproductive defects in transgenic mice. Mol Endocrinol 12:96–106 [DOI] [PubMed] [Google Scholar]
- 490. Erickson GF , Chung DG , Sit A , DePaolo LV , Shimasaki S , Ling N. 1995. Follistatin concentrations in follicular fluid of normal and polycystic ovaries. Hum Reprod 10:2120–2124 [DOI] [PubMed] [Google Scholar]
- 491. Ogawa K , Ono K , Kurohmaru M , Hayashi Y. 1995. Effect of streptozotocin injection on expression of immunoreactive follistatin and β A and β B subunits of inhibin/activin in rat pancreatic islets. Eur J Endocrinol 132:363–369 [DOI] [PubMed] [Google Scholar]
- 492. Miralles F , Czernichow P , Scharfmann R. 1998. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development 125:1017–1024 [DOI] [PubMed] [Google Scholar]
- 493. Yamaoka T , Idehara C , Yano M , Matsushita T , Yamada T , Ii S , Moritani M , Hata J , Sugino H , Noji S , Itakura M. 1998. Hypoplasia of pancreatic islets in transgenic mice expressing activin receptor mutants. J Clin Invest 102:294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494. Lee SJ. 2004. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol 20:61–86 [DOI] [PubMed] [Google Scholar]
- 495. Elkasrawy MN , Hamrick MW. 2010. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact 10:56–63 [PMC free article] [PubMed] [Google Scholar]
- 496. Gonzalez-Cadavid NF , Bhasin S. 2004. Role of myostatin in metabolism. Curr Opin Clin Nutr Metab Care 7:451–457 [DOI] [PubMed] [Google Scholar]
- 497. Mitchell MD , Osepchook CC , Leung KC , McMahon CD , Bass JJ. 2006. Myostatin is a human placental product that regulates glucose uptake. J Clin Endocrinol Metab 91:1434–1437 [DOI] [PubMed] [Google Scholar]
- 498. Rodgers BD , Garikipati DK. 2008. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev 29:513–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499. Flanagan JN , Linder K , Mejhert N , Dungner E , Wahlen K , Decaunes P , Rydén M , Björklund P , Arver S , Bhasin S , Bouloumie A , Arner P , Dahlman I. 2009. Role of follistatin in promoting adipogenesis in women. J Clin Endocrinol Metab 94:3003–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500. Tsuchida K , Nakatani M , Uezumi A , Murakami T , Cui X. 2008. Signal transduction pathway through activin receptors as a therapeutic target of musculoskeletal diseases and cancer. Endocr J 55:11–21 [DOI] [PubMed] [Google Scholar]
- 501. Kollias HD , McDermott JC. 2008. Transforming growth factor-β and myostatin signaling in skeletal muscle. J Appl Physiol 104:579–587 [DOI] [PubMed] [Google Scholar]
- 502. Rodino-Klapac LR , Haidet AM , Kota J , Handy C , Kaspar BK , Mendell JR. 2009. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve 39:283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Mukherjee A , Sidis Y , Mahan A , Raher MJ , Xia Y , Rosen ED , Bloch KD , Thomas MK , Schneyer AL. 2007. FSTL3 deletion reveals roles for TGF-β family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci USA 104:1348–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504. Jordan CD , Bohling SD , Charbonneau NL , Sakai LY. 2010. Fibrillins in adult human ovary and polycystic ovary syndrome: is fibrillin-3 affected in PCOS? J Histochem Cytochem 58:903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505. Hatzirodos N , Bayne RA , Irving-Rodgers HF , Hummitzsch K , Sabatier L , Lee S , Bonner W , Gibson MA , Rainey WE , Carr BR , Mason HD , Reinhardt DP , Anderson RA , Rodgers RJ. 2011. Linkage of regulators of TGF-β activity in the fetal ovary to polycystic ovary syndrome. FASEB J 25:2256–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506. Klein RJ , Zeiss C , Chew EY , Tsai JY , Sackler RS , Haynes C , Henning AK , SanGiovanni JP , Mane SM , Mayne ST , Bracken MB , Ferris FL , Ott J , Barnstable C , Hoh J. 2005. Complement factor H polymorphism in age-related macular degeneration. Science 308:385–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507. Amos CI. 2007. Successful design and conduct of genome-wide association studies. Hum Mol Genet 16 Spec No. 2:R220–R225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508. Duerr RH , Taylor KD , Brant SR , Rioux JD , Silverberg MS , Daly MJ , Steinhart AH , Abraham C , Regueiro M , Griffiths A , Dassopoulos T , Bitton A , Yang H , Targan S , Datta LW , Kistner EO , Schumm LP , Lee AT , Gregersen PK , Barmada MM , Rotter JI , Nicolae DL , Cho JH. 2006. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314:1461–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509. Frayling TM , Timpson NJ , Weedon MN , Zeggini E , Freathy RM , Lindgren CM , Perry JR , Elliott KS , Lango H , Rayner NW , Shields B , Harries LW , Barrett JC , Ellard S , Groves CJ , Knight B , Patch AM , Ness AR , Ebrahim S , Lawlor DA , Ring SM , Ben-Shlomo Y , Jarvelin MR , Sovio U , Bennett AJ , Melzer D , Ferrucci L , Loos RJ , Barroso I , Wareham NJ , Karpe F , Owen KR , Cardon LR , Walker M , Hitman GA , Palmer CN , Doney AS , Morris AD , Smith GD , Hattersley AT , McCarthy MI. 2007. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316:889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510. McCarthy MI , Zeggini E. 2007. Genome-wide association scans for type 2 diabetes: new insights into biology and therapy. Trends Pharmacol Sci 28:598–601 [DOI] [PubMed] [Google Scholar]
- 511. Rioux JD , Xavier RJ , Taylor KD , Silverberg MS , Goyette P , Huett A , Green T , Kuballa P , Barmada MM , Datta LW , Shugart YY , Griffiths AM , Targan SR , Ippoliti AF , Bernard EJ , Mei L , Nicolae DL , Regueiro M , Schumm LP , Steinhart AH , Rotter JI , Duerr RH , Cho JH , Daly MJ , Brant SR. 2007. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet 39:596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Zeggini E , Scott LJ , Saxena R , Voight BF , Marchini JL , Hu T , de Bakker PI , Abecasis GR , Almgren P , Andersen G , Ardlie K , Boström KB , Bergman RN , Bonnycastle LL , Borch-Johnsen K , Burtt NP , Chen H , Chines PS , Daly MJ , Deodhar P , Ding CJ , Doney AS , Duren WL , Elliott KS , Erdos MR , Frayling TM , Freathy RM , Gianniny L , Grallert H , Grarup N , Groves CJ , Guiducci C , Hansen T , Herder C , Hitman GA , Hughes TE , Isomaa B , Jackson AU , Jørgensen T , Kong A , Kubalanza K , Kuruvilla FG , Kuusisto J , Langenberg C , Lango H , Lauritzen T , Li Y , Lindgren CM , Lyssenko V , Marvelle AF , Meisinger C , Midthjell K , Mohlke KL , Morken MA , Morris AD , Narisu N , Nilsson P , Owen KR , Palmer CN , Payne F , Perry JR , Pettersen E , Platou C , Prokopenko I , Qi L , Qin L , Rayner NW , Rees M , Roix JJ , Sandbaek A , Shields B , Sjögren M , Steinthorsdottir V , Stringham HM , Swift AJ , Thorleifsson G , Thorsteinsdottir U , Timpson NJ , Tuomi T , Tuomilehto J , Walker M , Watanabe RM , Weedon MN , Willer CJ , Illig T , Hveem K , Hu FB , Laakso M , Stefansson K , Pedersen O , Wareham NJ , Barroso I , Hattersley AT , Collins FS , Groop L , McCarthy MI , Boehnke M , Altshuler D. 2008. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 40:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513. Carlson CS , Eberle MA , Kruglyak L , Nickerson DA. 2004. Mapping complex disease loci in whole-genome association studies. Nature 429:446–452 [DOI] [PubMed] [Google Scholar]
- 514. de Bakker PI , Yelensky R , Pe'er I , Gabriel SB , Daly MJ , Altshuler D. 2005. Efficiency and power in genetic association studies. Nat Genet 37:1217–1223 [DOI] [PubMed] [Google Scholar]
- 515. Wang WY , Barratt BJ , Clayton DG , Todd JA. 2005. Genome-wide association studies: theoretical and practical concerns. Nat Rev Genet 6:109–118 [DOI] [PubMed] [Google Scholar]
- 516. Marchini J , Cardon LR , Phillips MS , Donnelly P. 2004. The effects of human population structure on large genetic association studies. Nat Genet 36:512–517 [DOI] [PubMed] [Google Scholar]
- 517. Price AL , Butler J , Patterson N , Capelli C , Pascali VL , Scarnicci F , Ruiz-Linares A , Groop L , Saetta AA , Korkolopoulou P , Seligsohn U , Waliszewska A , Schirmer C , Ardlie K , Ramos A , Nemesh J , Arbeitman L , Goldstein DB , Reich D , Hirschhorn JN. 2008. Discerning the ancestry of European Americans in genetic association studies. PLoS Genet 4:e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518. McCarthy MI , Abecasis GR , Cardon LR , Goldstein DB , Little J , Ioannidis JP , Hirschhorn JN. 2008. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet 9:356–369 [DOI] [PubMed] [Google Scholar]
- 519. Need AC , Goldstein DB. 2010. Whole genome association studies in complex diseases: where do we stand? Dialogues Clin Neurosci 12:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Manolio TA , Collins FS , Cox NJ , Goldstein DB , Hindorff LA , Hunter DJ , McCarthy MI , Ramos EM , Cardon LR , Chakravarti A , Cho JH , Guttmacher AE , Kong A , Kruglyak L , Mardis E , Rotimi CN , Slatkin M , Valle D , Whittemore AS , Boehnke M , Clark AG , Eichler EE , Gibson G , Haines JL , Mackay TF , McCarroll SA , Visscher PM. 2009. Finding the missing heritability of complex diseases. Nature 461:747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521. Goldstein DB. 2009. Common genetic variation and human traits. N Engl J Med 360:1696–1698 [DOI] [PubMed] [Google Scholar]
- 522. Cirulli ET , Goldstein DB. 2010. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet 11:415–425 [DOI] [PubMed] [Google Scholar]
- 523. Hirschhorn JN. 2009. Genomewide association studies—illuminating biologic pathways. N Engl J Med 360:1699–1701 [DOI] [PubMed] [Google Scholar]
- 524. Wellcome Trust Case Control Consortium 2007. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447:661–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525. Hoggart CJ , Clark TG , De Iorio M , Whittaker JC , Balding DJ. 2008. Genome-wide significance for dense SNP and resequencing data. Genet Epidemiol 32:179–185 [DOI] [PubMed] [Google Scholar]
- 526. Huang M , Wang H , Li J , Zhou Z , Du Y , Lin M , Sha J. 2006. Involvement of ALF in human spermatogenesis and male infertility. Int J Mol Med 17:599–604 [PubMed] [Google Scholar]
- 527. Drieschner N , Belge G , Rippe V , Meiboom M , Loeschke S , Bullerdiek J. 2006. Evidence for a 3p25 breakpoint hot spot region in thyroid tumors of follicular origin. Thyroid 16:1091–1096 [DOI] [PubMed] [Google Scholar]
- 528. Del Villar K , Miller CA. 2004. Down-regulation of DENN/MADD, a TNF receptor binding protein, correlates with neuronal cell death in Alzheimer's disease brain and hippocampal neurons. Proc Natl Acad Sci USA 101:4210–4215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529. Gronau I , Hubisz MJ , Gulko B , Danko CG , Siepel A. 2011. Bayesian inference of ancient human demography from individual genome sequences. Nat Genet 43:1031–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530. Liu H , Prugnolle F , Manica A , Balloux F. 2006. A geographically explicit genetic model of worldwide human-settlement history. Am J Hum Genet 79:230–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531. Li H , Durbin R. 2011. Inference of human population history from individual whole-genome sequences. Nature 475:493–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Dumesic DA , Abbott DH , Padmanabhan V. 2007. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord 8:127–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533. Dumesic DA , Schramm RD , Abbott DH. 2005. Early origins of polycystic ovary syndrome. Reprod Fertil Dev 17:349–360 [DOI] [PubMed] [Google Scholar]
- 534. Abbott DH , Tarantal AF , Dumesic DA. 2009. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol 71:776–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535. Abbott DH , Barnett DK , Bruns CM , Dumesic DA. 2005. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update 11:357–374 [DOI] [PubMed] [Google Scholar]
- 536. Demissie M , Lazic M , Foecking EM , Aird F , Dunaif A , Levine JE. 2008. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. Am J Physiol Endocrinol Metab 295:E262–E268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537. Foecking EM , Szabo M , Schwartz NB , Levine JE. 2005. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod 72:1475–1483 [DOI] [PubMed] [Google Scholar]
- 538. Abbott DH , Barnett DK , Levine JE , Padmanabhan V , Dumesic DA , Jacoris S , Tarantal AF. 2008. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biol Reprod 79:154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539. Lazic M , Aird F , Levine JE , Dunaif A. 2011. Prenatal androgen treatment alters body composition and glucose homeostasis in male rats. J Endocrinol 208:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540. Recabarren SE , Rojas-García PP , Recabarren MP , Alfaro VH , Smith R , Padmanabhan V , Sir-Petermann T. 2008. Prenatal testosterone excess reduces sperm count and motility. Endocrinology 149:6444–6448 [DOI] [PubMed] [Google Scholar]
- 541. Recabarren SE , Lobos A , Figueroa Y , Padmanabhan V , Foster DL , Sir-Petermann T. 2007. Prenatal testosterone treatment alters LH and testosterone responsiveness to GnRH agonist in male sheep. Biol Res 40:329–338 [PubMed] [Google Scholar]
- 542. Bruns CM , Baum ST , Colman RJ , Eisner JR , Kemnitz JW , Weindruch R , Abbott DH. 2004. Insulin resistance and impaired insulin secretion in prenatally androgenized male rhesus monkeys. J Clin Endocrinol Metab 89:6218–6223 [DOI] [PubMed] [Google Scholar]
- 543. Recabarren SE , Smith R , Rios R , Maliqueo M , Echiburú B , Codner E , Cassorla F , Rojas P , Sir-Petermann T. 2008. Metabolic profile in sons of women with polycystic ovary syndrome. J Clin Endocrinol Metab 93:1820–1826 [DOI] [PubMed] [Google Scholar]
- 544. Barnes RB , Rosenfield RL , Ehrmann DA , Cara JF , Cuttler L , Levitsky LL , Rosenthal IM. 1994. Ovarian hyperandrogenism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab 79:1328–1333 [DOI] [PubMed] [Google Scholar]
- 545. Sir-Petermann T , Maliqueo M , Angel B , Lara HE , Pérez-Bravo F , Recabarren SE. 2002. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod 17:2573–2579 [DOI] [PubMed] [Google Scholar]
- 546. Nagamani M , McDonough PG , Ellegood JO , Mahesh VB. 1979. Maternal and amniotic fluid steroids throughout human pregnancy. Am J Obstet Gynecol 134:674–680 [DOI] [PubMed] [Google Scholar]
- 547. Anderson H , Fogel N , Grebe SK , Singh RJ , Taylor RL , Dunaif A. 2010. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J Clin Endocrinol Metab 95:2180–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548. Cole B , Hensinger K , Maciel GA , Chang RJ , Erickson GF. 2006. Human fetal ovary development involves the spatiotemporal expression of p450c17 protein. J Clin Endocrinol Metab 91:3654–3661 [DOI] [PubMed] [Google Scholar]
- 549. Barry JA , Kay AR , Navaratnarajah R , Iqbal S , Bamfo JE , David AL , Hines M , Hardiman PJ. 2010. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol 30:444–446 [DOI] [PubMed] [Google Scholar]
- 550. Hickey M , Sloboda DM , Atkinson HC , Doherty DA , Franks S , Norman RJ , Newnham JP , Hart R. 2009. The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. J Clin Endocrinol Metab 94:3714–3720 [DOI] [PubMed] [Google Scholar]
- 551. Hales CN , Barker DJ. 1992. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35:595–601 [DOI] [PubMed] [Google Scholar]
- 552. Barker DJ. 1995. Intrauterine programming of adult disease. Mol Med Today 1:418–423 [DOI] [PubMed] [Google Scholar]
- 553. Godfrey KM. 1998. Maternal regulation of fetal development and health in adult life. Eur J Obstet Gynecol Reprod Biol 78:141–150 [DOI] [PubMed] [Google Scholar]
- 554. Godfrey KM , Barker DJ. 2001. Fetal programming and adult health. Public Health Nutr 4:611–624 [DOI] [PubMed] [Google Scholar]
- 555. Breier BH , Vickers MH , Ikenasio BA , Chan KY , Wong WP. 2001. Fetal programming of appetite and obesity. Mol Cell Endocrinol 185:73–79 [DOI] [PubMed] [Google Scholar]
- 556. Jones RH , Ozanne SE. 2009. Fetal programming of glucose-insulin metabolism. Mol Cell Endocrinol 297:4–9 [DOI] [PubMed] [Google Scholar]
- 557. Hales CN , Desai M , Ozanne SE. 1997. The thrifty phenotype hypothesis: how does it look after 5 years? Diabet Med 14:189–195 [DOI] [PubMed] [Google Scholar]
- 558. Simmons R. 2005. Developmental origins of adult metabolic disease: concepts and controversies. Trends Endocrinol Metab 16:390–394 [DOI] [PubMed] [Google Scholar]
- 559. Godfrey K , Robinson S. 1998. Maternal nutrition, placental growth and fetal programming. Proc Nutr Soc 57:105–111 [DOI] [PubMed] [Google Scholar]
- 560. Susser M , Levin B. 1999. Ordeals for the fetal programming hypothesis. The hypothesis largely survives one ordeal but not another. BMJ 318:885–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561. Silverman BL , Rizzo T , Green OC , Cho NH , Winter RJ , Ogata ES , Richards GE , Metzger BE. 1991. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes 40(Suppl 2):121–125 [DOI] [PubMed] [Google Scholar]
- 562. Silverman BL , Metzger BE , Cho NH , Loeb CA. 1995. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care 18:611–617 [DOI] [PubMed] [Google Scholar]
- 563. Silverman BL , Purdy LP , Metzger BE. 1996. The intrauterine environment: implications for the offspring of diabetic mothers. Diabetes Rev 4:21–35 [Google Scholar]
- 564. Silverman BL , Rizzo TA , Cho NH , Metzger BE. 1998. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care 21(Suppl 2):B142–B149 [PubMed] [Google Scholar]
- 565. Armitage JA , Taylor PD , Poston L. 2005. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol (Lond) 565:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566. Cresswell JL , Barker DJ , Osmond C , Egger P , Phillips DI , Fraser RB. 1997. Fetal growth, length of gestation, and polycystic ovaries in adult life. Lancet 350:1131–1135 [DOI] [PubMed] [Google Scholar]
- 567. Ibáñez L , de Zegher F , Potau N. 1998. Premature pubarche, ovarian hyperandrogenism, hyperinsulinism and the polycystic ovary syndrome: from a complex constellation to a simple sequence of prenatal onset. J Endocrinol Invest 21:558–566 [DOI] [PubMed] [Google Scholar]
- 568. Carlsen SM , Jacobsen G , Romundstad P. 2006. Maternal testosterone levels during pregnancy are associated with offspring size at birth. Eur J Endocrinol 155:365–370 [DOI] [PubMed] [Google Scholar]
- 569. Sir-Petermann T , Hitchsfeld C , Maliqueo M , Codner E , Echiburú B , Gazitúa R , Recabarren S , Cassorla F. 2005. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod 20:2122–2126 [DOI] [PubMed] [Google Scholar]
- 570. Rosenfield RL. 2007. Clinical review: identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab 92:787–796 [DOI] [PubMed] [Google Scholar]
- 571. Ibáñez L , Dimartino-Nardi J , Potau N , Saenger P. 2000. Premature adrenarche—normal variant or forerunner of adult disease? Endocr Rev 21:671–696 [DOI] [PubMed] [Google Scholar]
- 572. Ibañez L , Potau N , Virdis R , Zampolli M , Terzi C , Gussinyé M , Carrascosa A , Vicens-Calvet E. 1993. Postpubertal outcome in girls diagnosed of premature pubarche during childhood: increased frequency of functional ovarian hyperandrogenism. J Clin Endocrinol Metab 76:1599–1603 [DOI] [PubMed] [Google Scholar]
- 573. Ibáñez L , Potau N , Francois I , de Zegher F. 1998. Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J Clin Endocrinol Metab 83:3558–3562 [DOI] [PubMed] [Google Scholar]
- 574. Pandolfi C , Zugaro A , Lattanzio F , Necozione S , Barbonetti A , Colangeli MS , Francavilla S , Francavilla F. 2008. Low birth weight and later development of insulin resistance and biochemical/clinical features of polycystic ovary syndrome. Metabolism 57:999–1004 [DOI] [PubMed] [Google Scholar]
- 575. Melo AS , Vieira CS , Barbieri MA , Rosa-E-Silva AC , Silva AA , Cardoso VC , Reis RM , Ferriani RA , Silva-de-Sá MF , Bettiol H. 2010. High prevalence of polycystic ovary syndrome in women born small for gestational age. Hum Reprod 25:2124–2131 [DOI] [PubMed] [Google Scholar]
- 576. Laitinen J , Taponen S , Martikainen H , Pouta A , Millwood I , Hartikainen AL , Ruokonen A , Sovio U , McCarthy MI , Franks S , Järvelin MR. 2003. Body size from birth to adulthood as a predictor of self-reported polycystic ovary syndrome symptoms. Int J Obes Relat Metab Disord 27:710–715 [DOI] [PubMed] [Google Scholar]
- 577. Jaquet D , Leger J , Chevenne D , Czernichow P , Levy-Marchal C. 1999. Intrauterine growth retardation predisposes to insulin resistance but not to hyperandrogenism in young women. J Clin Endocrinol Metab 84:3945–3949 [DOI] [PubMed] [Google Scholar]
- 578. Sadrzadeh S , Klip WA , Broekmans FJ , Schats R , Willemsen WN , Burger CW , Van Leeuwen FE , Lambalk CB. 2003. Birth weight and age at menarche in patients with polycystic ovary syndrome or diminished ovarian reserve, in a retrospective cohort. Hum Reprod 18:2225–2230 [DOI] [PubMed] [Google Scholar]
- 579. Legro RS , Roller RL , Dodson WC , Stetter CM , Kunselman AR , Dunaif A. 2010. Associations of birthweight and gestational age with reproductive and metabolic phenotypes in women with polycystic ovarian syndrome and their first-degree relatives. J Clin Endocrinol Metab 95:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580. Sir-Petermann T , Maliqueo M , Codner E , Echiburú B , Crisosto N , Pérez V , Pérez-Bravo F , Cassorla F. 2007. Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 92:4637–4642 [DOI] [PubMed] [Google Scholar]
- 581. Sir-Petermann T , Codner E , Pérez V , Echiburú B , Maliqueo M , Ladrón de Guevara A , Preisler J , Crisosto N , Sánchez F , Cassorla F , Bhasin S. 2009. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 94:1923–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582. Pigny P , Merlen E , Robert Y , Cortet-Rudelli C , Decanter C , Jonard S , Dewailly D. 2003. Elevated serum level of anti-Mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab 88:5957–5962 [DOI] [PubMed] [Google Scholar]
- 583. Crisosto N , Codner E , Maliqueo M , Echiburú B , Sánchez F , Cassorla F , Sir-Petermann T. 2007. Anti-Mullerian hormone levels in peripubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 92:2739–2743 [DOI] [PubMed] [Google Scholar]
- 584. Maliqueo M , Sir-Petermann T , Pérez V , Echiburú B , de Guevara AL , Gálvez C , Crisosto N , Azziz R. 2009. Adrenal function during childhood and puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 94:3282–3288 [DOI] [PubMed] [Google Scholar]
- 585. Maliqueo M , Echiburú B , Crisosto N , Amigo P , Aranda P , Sánchez F , Sir-Petermann T. 2009. Metabolic parameters in cord blood of newborns of women with polycystic ovary syndrome. Fertil Steril 92:277–282 [DOI] [PubMed] [Google Scholar]
- 586. Kent SC , Gnatuk CL , Kunselman AR , Demers LM , Lee PA , Legro RS. 2008. Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab 93:1662–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587. Randeva HS , Tan BK , Weickert MO , Lois K , Nestler JE , Sattar N , Lehnert H. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev 24 July 2012. doi:10.1210/er.2012-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588. Essah PA , Wickham EP , Nestler JE. 2007. The metabolic syndrome in polycystic ovary syndrome. Clin Obstet Gynecol 50:205–225 [DOI] [PubMed] [Google Scholar]
- 589. Ehrmann DA , Liljenquist DR , Kasza K , Azziz R , Legro RS , Ghazzi MN, for the PCOS/Troglitazone Group 2006. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 91:48–53 [DOI] [PubMed] [Google Scholar]
- 590. Paradisi G , Steinberg HO , Hempfling A , Cronin J , Hook G , Shepard MK , Baron AD. 2001. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation 103:1410–1415 [DOI] [PubMed] [Google Scholar]
- 591. Gambarin-Gelwan M , Kinkhabwala SV , Schiano TD , Bodian C , Yeh HC , Futterweit W. 2007. Prevalence of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Clin Gastroenterol Hepatol 5:496–501 [DOI] [PubMed] [Google Scholar]
- 592. Boomsma CM , Eijkemans MJ , Hughes EG , Visser GH , Fauser BC , Macklon NS. 2006. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 12:673–683 [DOI] [PubMed] [Google Scholar]
- 593. Legro RS. 2007. Pregnancy considerations in women with polycystic ovary syndrome. Clin Obstet Gynecol 50:295–304 [DOI] [PubMed] [Google Scholar]
- 594. Gambineri A , Patton L , Altieri P , Pagotto U , Pizzi C , Manzoli L , Pasquali R. 2012. Polycystic syndrome is a risk factor for type 2 diabetes: results from a long-term prospective study. Diabetes 61:2369–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]