Significance
Some epiphytes gain most of their nutrients from ants that nest in plant-provided cavities, accessible only through plant-formed entrance holes. We use a large clade of such epiphytes to study when mutualisms break down and how this affects the symbiont filtering system. Results support three theoretical predictions: (i) only generalist symbioses returned to a non–ant-associated state; (ii) evolutionary returns to an ant-free state occur where partners are rare, in our system at high altitudes; and (iii) the rate of hole-diameter evolution increases drastically after mutualism breakdown, suggesting release from stabilizing selection exerted by mutualistic ants. This highlights the importance of partner abundances in determining mutualistic strategy and explains the convergent evolution of ant/plant symbioses in ant-rich tropical lowlands.
Keywords: mutualism, symbiosis, morphology, comparative phylogenetic methods, ants
Abstract
Mutualisms that involve symbioses among specialized partners may be more stable than mutualisms among generalists, and theoretical models predict that in many mutualisms, partners exert reciprocal stabilizing selection on traits directly involved in the interaction. A corollary is that mutualism breakdown should increase morphological rates of evolution. We here use the largest ant-plant clade (Hydnophytinae), with different levels of specialization for mutualistic ant symbionts, to study the ecological context of mutualism breakdown and the response of a key symbiosis-related trait, domatium entrance hole size, which filters symbionts by size. Our analyses support three predictions from mutualism theory. First, all 12 losses apparently only occur from a generalist symbiotic state. Second, mutualism losses occurred where symbionts are scarce, in our system at high altitudes. Third, domatium entrance hole size barely changes in specialized symbiotic species, but evolves rapidly once symbiosis with ants has broken down, with a “morphorate map” revealing that hotspots of entrance hole evolution are clustered in high-altitude areas. Our study reveals that mutualistic strategy profoundly affects the pace of morphological change in traits involved in the interaction and suggests that shifts in partners’ relative abundances may frequently drive reversions of generalist mutualisms to autonomy.
Understanding how mutualisms arise, persist, or break down is a major focus in ecology and evolutionary biology (1–3). Symbiotic mutualisms can revert to the free-living state if the cost-to-benefit ratio shifts so that costs outweigh benefits. There are three main pathways through which mutualism can break down, namely, extinction of the partner, reversion to the free-living state, or shift to parasitism (2). Extinction of one partner in an obligate mutualism should entail the extinction of the other, whereas in a facultative mutualism, extinction might lead to a reversion to autonomy (4–8), but these predictions have limited support from empirical studies. Similarly, mutualism could also break down if one partner becomes scarce, which may be especially important in laterally transferred symbioses where partners disperse independently and the interaction needs to be reestablished at each generation, involving vulnerable stages for both. Mutualisms can also break down by shifting to parasitism. Such shifts are predicted by theory (9, 10) because reducing or stopping reciprocation increases the fitness of the cheating partner (11–14). Phylogenetically unrelated freeloaders may also disrupt a mutualism by exploiting it (15–18). Finally, mutualism can break down if benefits can be obtained cheaply or freely from the environment, for example, when plants involved in mycorrhizal or rhizobia symbioses grow in nutrient-rich soils (19, 20) or when antiherbivore defense by ant mutualists is no longer required (21). Most of these theoretical expectations about conditions favoring mutualism breakdown lack empirical support, partly because there are few tractable systems with multiple evolutionary gains and losses of mutualisms. Here we use a species-rich and diverse ant/plant interaction system to study the ecological context under which breakdowns of symbiotic mutualisms have occurred on a geologic timescale.
Ant/plant mutualisms are ubiquitous in tropical ecosystems and encompass a wide range of strategies (22–25). In Australasia, the majority of ant-plants are epiphytes and appear to be primarily involved in trophic mutualisms rather than defense mutualisms (22, 24). An epiphytic habit means uneven water and nutrient supplies (26), and mutualisms with plant-nesting ants that provide detritus and feces to their host (27–34) are thus common among epiphytes (22). These mutualistic symbioses range from facultative interactions involving many arboreal ant species to obligate interactions that can be species-specific (17, 28, 32, 34, 35).
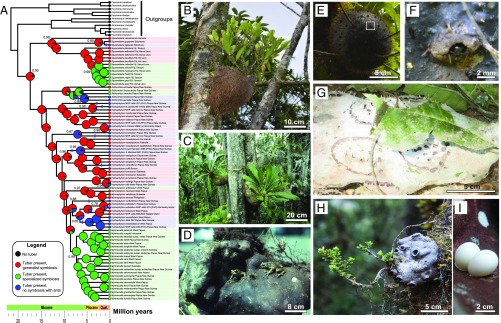
We here use the world’s most species-rich epiphytic ant-plant clade, the Hydnophytinae subtribe of the Rubiaceae (24), to study the occurrence and breakdown of mutualistic strategies and how this affects rates of morphological evolution. The Hydnophytinae comprise ∼100 epiphytic species in Australasia. They produce large characteristic ant-housing structures (domatia) that result from a modified hypocotyl with a network of galleries (Fig. 1 B–E and G –H). Three strategies are present: specialized ant-plants, where species associate consistently with one or a few species of ants (henceforth “specialized symbioses”), some of which are obligate (17, 34, 35); generalist ant-plants, where plants often, but not always, associate with generalist arboreal ants (hence also facultative); and, finally, species that form no association with ants. Theoretical models predict that in many mutualisms, partners exert stabilizing selection on each other, notably to maintain trait-matching phenotypes (36–39). A corollary is that loss of mutualistic interactions will lead to the relaxation of the stabilizing selection on traits previously involved in the interaction. To probe this expectation, we investigated the rate of morphological evolution of a pivotal mutualism-related trait: the diameter of domatium entrance holes. These holes filter the type of animal that can inhabit a domatium. Small holes prevent arthropods larger than ants from entering domatia, whereas large holes allow for the presence of a range of invertebrates, such as spiders or cockroaches, and even small vertebrates, such as geckos that lay eggs inside the domatium or frogs whose tadpoles develop in rainwater-filled domatia (Fig. 1 D–I). Ants provide direct nutritional benefits to their host plants (28, 34), but the contribution of other organisms is less clear and is likely limited for transient occupants.
Fig. 1.
The evolution of mutualistic strategies in the Hydnophytinae. (A) Ancestral state reconstruction of mutualistic strategies from 1,000 simulations of character states on a dated phylogeny and a reverse-jump MCMC approach on 1,000 trees (probability shown at key nodes) with 75% of all Hydnophytinae. (B–D) Examples of the three mutualistic strategies. (B) Squamellaria wilkinsonii [G. Chomicki, J. Aroles, A. Naikatini 45 (M)], a generalist ant-plant from Fiji. (C) Myrmecodia alata, a specialized ant-plant from Indonesian Papua. (D) Hydnophytum myrtifolium [M.H.P. Jebb 322 (K)], a species from the highlands of Papua New Guinea that is not associated with ants, but instead accumulates rainwater where the frog, Cophixalus riparius, breeds. (D–I) Diversity of entrance holes in Hydnophytinae. (D) Frog-inhabited Hydnophytum myrtifolium, Papua New Guinea. (E and F) Squamellaria wilsonii, Taveuni, Fiji, with tiny entrance holes fitting the size of the ant partner, Philidris nagasau. (F) Detail of one entrance hole shown in E. (G) Specialized ant-plant, Myrmecodia tuberosa (form “versteegii” sensu Huxley and Jebb, 1993), Papua New Guinea. (H) Hydnophytum spec. nov. [same as Lam 1969 (L)], Papua New Guinea. (I) Eggs of Lepidodactylus buleli, a gecko endemic from Espiritu Santo Island, Vanuatu, inside a Squamellaria vanuatuensis domatium. Photographic credits: (B, E, and F): G. Chomicki; (C and D): M.H.P. Jebb; (G): M. Janda, (H): U. Bauer; (I): J. Orivel. SI Appendix, Fig. S1, gives statistical support.
The size of the Hydnophytinae clade and array of domatium types and symbioses make it suitable for investigating shifts between strategies. Specifically, we address three questions: (i) Are mutualism losses associated with particular ancestral states (such as generalist or specialized symbiosis)? (ii) Are losses associated with a particular ecological context, for example, shifts to habitats where partners are scarce or where nutritional resources are freely available? (iii) Given the role of the domatium entrance holes as a filter for “permitted” mutualists, how do shifts in strategies affect the rate of change in the size of these holes?
Results and Discussion
Recurrent Mutualism Breakdown in Generalist Symbioses.
Our matrix of six plastid and nuclear markers (ndhF, trnH-psbA, trnL intron, trnL-trnF spacer, ITS, and ETS) includes 75% of the 105 species of Hydnophytinae and yields a statistically strongly supported tree in both maximum likelihood (ML) and Bayesian analyses (SI Appendix, Fig. S1). Consistent with our previous analyses (17, 34, 35), we found a sister relationship between two clades: a Pacific clade comprising all 12 species of Squamellaria and an Australasian clade of species in the genera Anthorrhiza, Myrmephytum, and Myrmecodia, together nested within the paraphyletic genus Hydnophytum. We used ML stochastic mapping and Bayesian ancestral state reconstructions (Materials and Methods) to test the hypothesis that the absence of symbiosis with ants is the ancestral state. Contrary to our expectation, we found that generalist symbiosis with ants is the ancestral condition in our study clade, and it was subsequently lost independently 12 times (11.09 ± 1.82, Fig. 1A). To further probe that absence of mutualism is a secondary reversal, we forced the most recent common ancestor (MRCA) of Hydnophytinae to lack symbiosis with ants and compared it to an unconstrained run and a model where generalist symbioses are enforced. Bayes factor (BF) rejected the absence of symbiosis as ancestral (BF = 5.81). We also inferred four to five subsequent specializations (4.65 ± 2.12, Fig. 1A) of the symbioses in Squamellaria, Anthorrhiza (1 or 2), and Myrmephytum and Myrmecodia, involving preferential interactions with the dolichoderine genera, Philidris and Anonychomyrma (28, 34, 35, 40–43).
Eleven of the twelve losses of symbiosis with ants (including 17 of the 23 species that lack association with ants) evolved from generalist ant-plant ancestors (Fig. 1A, blue rectangles), the exception being Anthorrhiza, where the ancestral state is unresolved, perhaps due to our sampling of just one of the four generalist species of Anthorrhiza.
Loss of Mutualism with Ants Coincides with Shifts to Montane Habitats.
The inferred minimally 12 losses of ant symbiosis—involving single species or the ancestors of clades comprising two to four species—prompted us to study their ecological context. Of the 23 species lacking symbiosis with ants, 17 are highland species from Papua New Guinea that occur at altitudes between 1,500 and over 3,500 m of elevation. Ant species’ richness and abundance both decrease with increasing elevation in the tropics (44–48), likely because of the decreasing temperature (49), and this pattern has also been found in New Guinea (Maurice Leponce, personal communication to G. Chomicki, June 2016). This suggests that mutualism breakdown could occur via shifts from ant-rich lowlands to ant-limited montane habitats in New Guinea, probably driven by bird dispersal (34). Three of the species that lost mutualism with ants have domatia that are filled with rainwater and then harbor the Cophixalus riparius frog at 1,900–2,600 m (40, 50; Figs. 1D). This frog is endemic in Papua New Guinea, where it occurs above 1,900 m (51). Six of the twenty-three species that have lost mutualistic interactions may obtain nutrients more cheaply from soil, but two specialized ant-plant species (Myrmecodia lamii and M. brassii) also often grow terrestrially and still have obligately occupied domatia (28, 43). If cheaply obtained nutrients drove mutualism breakdown, we would expect nonmutualists to be terrestrial and also to live in ant-occupied areas. However, this is not the case. Altogether, this suggests that mutualism loss was not driven by return to the terrestrial habit but instead by the scarcity of ants at high altitudes.
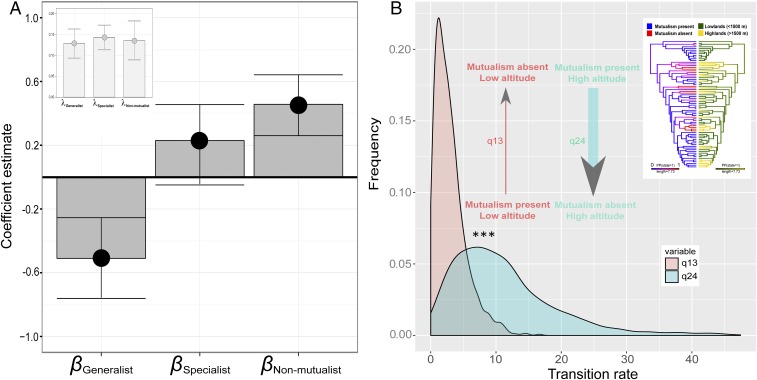
Generalist Hydnophytinae ant-plants are inhabited by >25 unrelated species of arboreal ants (SI Appendix, Table S1), whereas specialists are mainly inhabited by one or two species of Dolichoderinae ant species in the genera, Philidris and Anonychomyrma, some of which inhabit lowlands, whereas others live in highlands above 1,500 m (28, 40). Thus, under the hypothesis that altitude is the main driver of mutualism breakdown, we expected that (i) generalists occur predominantly in the lowlands, (ii) nonmutualists occur mostly in high-altitude environments, and (iii) specialists occur at all altitudes. To test these predictions, we first recorded the mean and the maximum altitude for each species and asked whether there were significant differences between the three mutualism categories (generalist, specialized, and nonmutualist) found in Hydnophytinae. Using a hierarchical Bayesian modeling approach accounting for phylogenetic autocorrelation, we verified our three predictions (Fig. 2A). We further confirmed these results using a phylogenetic ANOVA (F = 7.42, P < 0.05), followed by a post hoc test, which again revealed that species that lost mutualism grew at significantly higher altitude than species with generalist mutualisms (post hoc test, P = 0.003) from which they evolved (Fig. 1A). Our results were further confirmed by a multinomial logistical approach (SI Appendix, Figs. S2–S3 and SI Appendix, SI Materials and Methods).
Fig. 2.
Breakdown of mutualisms coincides with shifts to high altitudes. (A) Estimated coefficient values from a Bayesian hierarchical model testing the effect of altitude on mutualistic strategy, showing means and 95% confidence intervals (CI). The models control for phylogenetic autocorrelation, and a detailed description of regression components is presented in SI Appendix, SI Materials and Methods. Values reflect standardized data and can be interpreted as relative effect sizes. (Inset) Fitted values of phylogenetic signal (Pagel’s λ, mean, and 95% CI). (B) Frequencies of mutualism breakdown in the lowlands versus at high altitudes inferred in a correlated Bayesian analysis (SI Appendix, SI Materials and Methods). (Inset) Stochastic mapping reconstructions of mutualism and altitude. All transition states are shown in SI Appendix, Fig. S8.
To better characterize the niches of our three mutualistic strategies, we determined the niche space occupied using over 1,100 herbarium records (SI Appendix, Dataset S1) and nonmetric multidimensional scaling (NMDS). We first performed an analysis using all 105 Hydnophytinae species, which revealed that the 95% confidence clusters of generalist versus nonmutualist species do not overlap and that both are nested within the larger cluster of specialized ant-plants, as expected (SI Appendix, Fig. S4). We next repeated the analysis using the species sampled in our tree (∼75%), plotted in a phylomorphospace (Materials and Methods), which showed the same pattern (SI Appendix, Fig. S5). The larger climatic niche space for specialized species results from their principal ant partners occupying different niches. Philidris ants occupy mostly plants in lowland to midelevation savannah or disturbed forest, whereas Anonychomyrma species occupy mostly lowland to midelevation rainforest or montane habitats (28, 40, 43). These analyses all indicated that species that lost mutualism with ants inhabit high-altitude environments, whereas generalists are predominantly found in the lowland. Because nonmutualists evolved from generalists (Fig. 1), we next hypothesized that mutualism breakdown followed shifts to ant-limited, high-altitude environments.
To probe this hypothesis, we first performed ML ancestral state reconstruction of species’ altitudinal niches, coding both maximum and mean altitude based on the same species’ distribution data as before (SI Appendix, Fig. S6) as well as ancestral biome reconstruction (SI Appendix, Fig. S7), using stochastic mapping (Materials and Methods). Results from both approaches confirmed that mutualism loss was associated with occurrence at higher elevations, with 8 of the 12 losses of ant mutualisms coinciding with a shift to the montane biome (SI Appendix, Fig. S7). The four losses that do not coincide with shift to highlands all occurred outside mainland New Guinea, on islands where elevation is much more limited (Squamellaria kajewskii: Solomon Islands; Anthorrhiza areolata and A. bracteosa: D’Entrecasteaux Islands; Hydnophytum L.J. Brass 25652 (Leiden, L): D’Entrecasteaux Islands; H. petiolatum M.H.P. Jebb 379 (Oxford, FHO): Normanby Island). Only a single New Guinea mainland species appears to be nonassociated with ants and occurs in the lowlands: Hydnophytum R. Schlechter 18430 (British Museum, BM). Altogether, these approaches suggested that loss of mutualism with ants is associated with shifts to higher elevations. To statistically probe these observed correlations, we used models of independent (M0) and dependent evolution (M1), which revealed that shifts to high altitudes were strongly correlated with mutualism losses (Bayes factor = 14.81). Analyzing the transition rates of mutualism loss in the lowlands (q12) and mutualism losses at high elevations (q24) revealed a strong and significant bias toward the latter (Fig. 2B; Kolmogorov–Smirnov test, D = 0.617, P < 0.0001; see also SI Appendix, Fig. S8). To further probe our finding, we designed a third model (M3) consisting of the dependent model but forcing the MRCA of nonmutualist clades to have occurred in the lowlands (singletons could not be constrained; see SI Appendix). This model was rejected in favor of M2 (BF = 6.77).
Partner rarity is thought to lead to the local extinction of specialized mutualistic partnerships and the reversion to the free-living state in facultative ones (4, 5, 7, 8). Our results strongly suggest that shifts of generalist symbioses to partner-depleted high-altitude environments drove recurrent mutualism breakdown, thus providing support of this prediction with comparative phylogenetic data.
Relaxed Selective Constraints on Entrance Diameter after Mutualism Breakdown.
Theoretical studies predict that specialized mutualisms comaintain interaction-related traits via stabilizing selection (36–39). Conversely, mutualistic breakdown to the free-living state should relax selection on traits previously involved in the interaction. A glimpse at the diversity of Hydnophytinae domatia reveals a high morphological disparity, especially of entrance holes that can vary from millimeter-sized holes to over 5-cm-wide openings (Fig. 1 D–I). Because entrance holes have a pivotal role as “filters” of domatium partners (Fig. 1 D–I), this diversity raises the question of whether shift in mutualism strategy affects the rate of change in entrance hole size. We tested the hypothesis that mutualism strategy affects the rate of entrance hole-size evolution, with the expectations that (i) specialists should have the lowest rates, reflecting strong stabilizing selection; (ii) nonmutualists should have a high rate of morphological evolution, reflecting relaxed selection pressure; and (iii) generalists should have intermediate morphological rates.
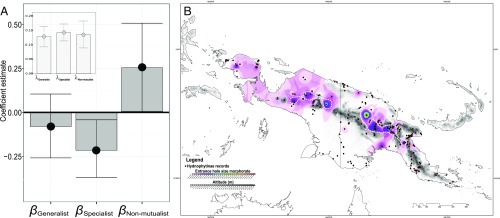
Ancestral state reconstruction for entrance hole diameter showed that the loss of ant mutualism is frequently accompanied by increases in entrance hole size (SI Appendix, Fig. S9), highlighting that many nonmutualists have large entrance holes (Fig. 1 D and H) and are inhabited by a range of invertebrates (cockroaches, millipedes, Peripatus worms, spiders, slugs, and leeches) and even small vertebrates (frogs, skinks, and geckos; Figs. 1F and 2E). We used BAMM (52) to investigate the rates of morphological evolution (morphorate) (SI Appendix, Figs. S10 and S11). As expected, we found that nonmutualists have the highest morphorates, whereas specialized species have the lowest morphorates, with generalist ones in between [x̅Morphorate Nonmutualists = 0.1 ± 0.02; x̅Morphorate Generalists = 0.04 ± 0.02; x̅Morphorate Specialists = 0.01 ± 0.004 (mean ± SE)]. To confirm these results when accounting for phylogenetic autocorrelation, we relied on a hierarchical Bayesian modeling approach verifying our three predictions (Fig. 3A). Morphorate through-time analyses showed that specialized species (Myrmecodia) have low morphological change rates compared with the rest of the Hydnophytinae (SI Appendix, Fig. S12). See also SI Appendix, Figs. S13 and S14 for a multinomial logistic approach and a macroevolutionary cohort matrix further illustrating these results.
Fig. 3.
Entrance hole evolution correlates with mutualistic strategy. (A) Estimated coefficient values from a Bayesian hierarchical model testing the effect of the evolutionary rate of entrance hole-size change (morphorate) on mutualistic strategy, showing means and 95% CI. The models control for phylogenetic autocorrelation, and a detailed description of regression components is presented in SI Appendix, SI Materials and Methods. Values reflect standardized data and can be interpreted as relative effect sizes. (Inset) Fitted values of phylogenetic signal (Pagel’s λ, mean, and 95% CI). (B) Map showing the inferred morphological rate of entrance hole-size (morphorate) evolution linked to species’ distribution in mainland New Guinea. Black dots with red circles show Hydnophytinae occurrences. Dotted lines show the entrance hole-size morphorate “hotspots,” concentrated in high-altitude areas.
To independently validate these results, we used the noncensored rate (ML) approach developed by O’Meara (53), which allows fitting distinct evolutionary rates for continuous characters. A strategy-specific rate model (three-rate) was strongly supported over a one-rate model (Likelihood Ratio Test = 138.52, critical Χ2 value = 5.99), confirming the results obtained with BAMM. We confirmed these results by analyzing the entrance hole-size disparity through time (54, 55), using taxon exclusion experiments (SI Appendix, SI Materials and Methods, and SI Appendix, Fig. S15). Taxon exclusion experiments revealed that (i) removing specialists has little effect on the overall disparity pattern, (ii) removing nonmutualists massively lowers disparity so that it falls in the range of that expected under pure Brownian motion, and (iii) the specialist clade, Myrmecodia, shows low disparity over time (SI Appendix, Fig. S15).
Finally, to specifically ask whether the rate of entrance hole-size evolution increased following mutualism loss, we coded both characters as binary and used Bayesian models of independent evolution (M1), dependent evolution (M2), and dependent evolution where nonmutualistic clades are forced to have a low entrance hole morphorate (M3). Bayes factors strongly favored the dependent model (BF = 42.83), but M3 was rejected in favor of M2 (BF = 12.45), consistent with our expectation that mutualism breakdown was followed by increase in entrance hole morphorate.
Altogether, these results provide strong support for the prediction that traits involved in the studied mutualisms are under stabilizing selection exerted by ant partners (36–39), and for our corollary expectation that mutualism breakdown leads to relaxation of that selection. However, high evolutionary rates leading to larger holes could also reflect selection exerted by alternative partners (other invertebrates and small vertebrates) with much larger body sizes. Further field data are needed to resolve whether large domatium entrance holes, such as present in the three species housing the frog Cophixalus riparius are the result of directional selection to house larger partners or a by-product of the relaxation of ant-driven selection to keep small entrance holes, notably by quantifying the extent to which they benefit their host nutritionally. However, the clearer benefits from ants (28, 34) than from other invertebrates or vertebrates suggest that relaxed selection constraint is more important than directional selection by larger organisms.
Because mutualism losses in mainland New Guinea correlate with shifts to high altitudes (Fig. 2B and SI Appendix, Figs. S6 and S7), we expected hotspots of entrance hole morphorate evolution in these areas. To investigate this, we developed a method that couples species-based morphorates inferred from BAMM with georeferenced specimen-based species distribution (Materials and Methods). As expected, the resulting “morphorate map” reveals that hotspots of entrance hole evolution are strongly clustered along the New Guinean Central Cordillera (Fig. 3B).
Morphological evolution can accelerate when selection on a trait is removed and the body plan is free to change if developmental constraints are limited (56, 57), or it can slow down when ecological opportunity diminishes simultaneously (58). Here we showed that mutualistic strategy profoundly affects the pace of morphological change in traits involved in the interaction.
The developmental genetic rules that govern morphological change are becoming well understood (59). The rules governing macroevolutionary changes in morphology under changing abiotic or biotic conditions are less clear. Our study provides support for one such rule, namely, that mutualism strategy affects the pace of change in interaction-related traits, consistent with a recent study of a pollination mutualism (57). Further empirical studies of traits are needed to develop a macroevolutionary theory of morphological evolution.
Conclusion
In symbiotic mutualisms, symbiont abundance is known to reciprocally affect host and symbiont fitnesses (8, 60–62). The Hydnophytinae provide an example for how a decrease in partners’ abundances at higher altitudes, over evolutionary timescales, has driven repeated losses of symbiosis and how this affected the macroevolutionary rate of trait change, here domatium entrance hole size. Mutualism theory has long focused on how mutualism prevents shifting to parasitism (1, 9, 10), yet empirical evidence shows that such shifts are rare (2, 18, 63). Our findings instead suggest that returns from mutualism to a nonmutalistic state (in symbioses, a free-living state) may be common, at least as long as mutualisms have not become too specialized.
Materials and Methods
Taxon Sampling, DNA Extraction, Phylogenetic Analyses, and Molecular Clock Dating.
We generated a matrix of six markers (nuclear ITS and ETS and plastid ndhF, psbA-trnH, trnL intron, and trnL-trnF spacer), sampling 76 species out of ∼106 Hydnophytinae species. A sampling of outgroups (in the tribe Psychotrieae) was selected on the basis of ref. 64. Voucher information is reported in SI Appendix, Table S2. DNA extraction, PCR, sequencing, alignments, and phylogenetic analyses were performed as previously described (25) and are detailed in the SI Appendix, SI Materials and Methods.
Molecular dating analyses relied on BEAST v. 2 (65) and uncorrelated lognormal relaxed clock models. We used the GTR + G substitution model with four rate categories, a Yule tree prior, and an MCMC chain of 40 million generations, with parameters and trees sampled every 10,000 generations. We used Tracer v. 1.6 (66) to check that the effective sample size of all parameters was >200, indicating that runs had converged. After discarding 10% of the trees as burn-in, trees were summarized in TreeAnnotator v. 1.8 (part of the BEAST package). More details are given in the SI Appendix, including the calibration scheme.
Phylogenetic Comparative Methods.
We coded mutualistic strategies, entrance hole diameter, elevation, and biome based on literature (28, 34, 35, 40–43, 50, 67) and new data (details in SI Appendix, SI Materials and Methods and Table S1). Ancestral state reconstructions were performed on the maximum clade credibility tree from BEAST or alternatively on 1,000 trees from the end of the MCMC chain to take phylogenetic uncertainty into account. We used the stochastic mapping approach and an ML approach for continuous characters implemented in the phytools package (68), and the reverse-jump MCMC approach implemented in BayesTraits v. 2 (69). Bayesian correlations were also performed in BayesTraits v. 2 (69). Further details are provided in SI Appendix, SI Materials and Methods. Niche space analysis, including filtering of autocorrelated bioclim variables, NMDS, and NMDS-based phylomorphospace, were performed as described in detail in SI Appendix, SI Materials and Methods. After log-transformation of the data, we analyzed the rate of entrance hole-diameter evolution using three approaches: (i) a Bayesian time-dependent model implemented in BAMM v.2.5.0 (52); (ii) an ML-based noncensored rate test (53) that employs Brownian motion models under a one-rate model and under a three-rate (strategy-specific) model, implemented in phytools (68); and (iii) disparity-through-time (DTT) analysis (54) as implemented in the package Geiger (55) (see SI Appendix, SI Materials and Methods for details). We tested for the significance of associations between mutualistic strategy, elevation, and entrance hole morphorate by accounting for phylogenetic autocorrelation in a hierarchical Bayesian modeling approach (see SI Appendix, SI Materials and Methods for details). Finally, to identify hotspots of morphological evolutionary rate, we developed a method to link (i) the morphological rate analysis in BAMM (52) and (ii) a matrix of GPS coordinates for all occurrences of the species sampled in the BAMM analysis. To do so, we retrieved morphorate from each tip from the BAMM analyses using the function “GetTipsRates” in BAMMtools v.2.1 (70). Rates were interpolated to a polygon representing mainland New Guinea using the inverse distance weight method implemented in the software ArcMap v.9.3 (71).
Supplementary Material
Acknowledgments
We thank Jeremy Aroles for proofreading the manuscript; Camilla Huxley-Lambrick, Maurice Leponce, and Matthew Jebb for discussion; Andreas Wistuba for cultivated plant material; Oscar Pérez for help with the map; Constantin Zohner for help with the Bayesian hierarchical model; Joel Sachs for critical comments on the manuscript; Daniel Rabosky and Robert Ricklefs for statistical advice; and the curators of the Oxford, British Museum, Leiden, and Sydney herbaria for allowing us to sample valuable specimens. This work was supported by a grant from the German Research Foundation (DFG), RE 603/20, and grants from the Society of Systematic Biologists and the American Association of Plant Taxonomy (to G.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: All GenBank accession numbers are provided in the SI Appendix.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616837114/-/DCSupplemental.
References
- 1.Sachs JL, Mueller UG, Wilcox TP, Bull JJ. The evolution of cooperation. Q Rev Biol. 2004;79:135–160. doi: 10.1086/383541. [DOI] [PubMed] [Google Scholar]
- 2.Sachs JL, Simms EL. Pathways to mutualism breakdown. Trends Ecol Evol. 2006;21:585–592. doi: 10.1016/j.tree.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Frederickson ME. Rethinking mutualism stability: Cheaters and the evolution of sanctions. Q Rev Biol. 2013;88:269–295. doi: 10.1086/673757. [DOI] [PubMed] [Google Scholar]
- 4.Vandermeer JH, Boucher DH. Varieties of mutualistic interaction in population models. J Theor Biol. 1978;74:549–558. doi: 10.1016/0022-5193(78)90241-2. [DOI] [PubMed] [Google Scholar]
- 5.Keeler KH. Cost:benefit models of mutualism. In: Boucher DH, editor. The Biology of Mutualism, Ecology and Evolution. Oxford Univ Press; Oxford, UK: 1985. pp. 100–127. [Google Scholar]
- 6.Schemske DW, Lande R. The evolution of self-fertilization and inbreeding depression in plants. II. Empirical observations. Evolution. 1985;39:41–52. doi: 10.1111/j.1558-5646.1985.tb04078.x. [DOI] [PubMed] [Google Scholar]
- 7.Holland JN, DeAngelis DL, Schultz ST. Evolutionary stability of mutualism: Interspecific population regulation as an evolutionarily stable strategy. Proc Biol Sci. 2004;271:1807–1814. doi: 10.1098/rspb.2004.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster KR, Wenseleers T. A general model for the evolution of mutualisms. J Evol Biol. 2006;19:1283–1293. doi: 10.1111/j.1420-9101.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- 9.Trivers RL. The evolution of reciprocal altruism. Q Rev Biol. 1971;46:35–57. [Google Scholar]
- 10.Axelrod R, Hamilton WD. The evolution of cooperation. Science. 1981;211:1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- 11.Pellmyr O, Leebens-Mack J, Huth CJ. Non-mutualistic yucca moths and their evolutionary consequences. Nature. 1996;380:155–156. doi: 10.1038/380155a0. [DOI] [PubMed] [Google Scholar]
- 12.Bidartondo MI, Bruns TD. Extreme specificity in epiparasitic Monotropoideae (Ericaceae): Widespread phylogenetic and geographical structure. Mol Ecol. 2001;10:2285–2295. doi: 10.1046/j.1365-294x.2001.01358.x. [DOI] [PubMed] [Google Scholar]
- 13.Machado CA, Jousselin E, Kjellberg F, Compton SG, Herre EA. Phylogenetic relationships, historical biogeography and character evolution of fig-pollinating wasps. Proc Biol Sci. 2001;268:685–694. doi: 10.1098/rspb.2000.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Als TD, et al. The evolution of alternative parasitic life histories in large blue butterflies. Nature. 2004;432:386–390. doi: 10.1038/nature03020. [DOI] [PubMed] [Google Scholar]
- 15.Bronstein JL, Wilson WG, Morris WF. Ecological dynamics of mutualist/antagonist communities. Am Nat. 2003;162(Suppl):S24–S39. doi: 10.1086/378645. [DOI] [PubMed] [Google Scholar]
- 16.Wilson WG, Morris WF, Bronstein JL. Coexistence of mutualists and exploiters on spatial landscapes. Ecol Monogr. 2003;73:397–413. [Google Scholar]
- 17.Chomicki G, Staedler YM, Schönenberger J, Renner SS. Partner choice through concealed floral sugar rewards evolved with the specialization of ant-plant mutualisms. New Phytol. 2016;211:1358–1370. doi: 10.1111/nph.13990. [DOI] [PubMed] [Google Scholar]
- 18.Sachs JL. 2015. The exploitation of mutualism. Mutualism, ed Bronstein JL (Oxford Univ Press, Oxford, UK)
- 19.Allen MF. The Ecology of Mycorrhizae. Cambridge Univ Press; Cambridge, UK: 1991. [Google Scholar]
- 20.Sprent JI. Nodulation in Legumes. Royal Botanic Gardens; Kew, UK: 2001. [Google Scholar]
- 21.Palmer TM, et al. Breakdown of an ant-plant mutualism follows the loss of large herbivores from an African savanna. Science. 2008;319:192–195. doi: 10.1126/science.1151579. [DOI] [PubMed] [Google Scholar]
- 22.Davidson DW, Epstein WW. Vascular plants as epiphytes. Springer; Berlin: 1989. Epiphytic associations with ants; pp. 200–233. [Google Scholar]
- 23.Davidson DW, McKey D. The evolutionary ecology of symbiotic ant/plant relationships. J Hymenopt Res. 1993;2:13–83. [Google Scholar]
- 24.Chomicki G, Renner SS. Phylogenetics and molecular clocks reveal the repeated evolution of ant-plants after the late Miocene in Africa and the early Miocene in Australasia and the Neotropics. New Phytol. 2015;207:411–424. doi: 10.1111/nph.13271. [DOI] [PubMed] [Google Scholar]
- 25. Chomicki G, Ward PS, Renner SS (2015) Macroevolutionary assembly of ant/plant symbioses: Pseudomyrmex ants and their ant-housing plants in the neotropics. Proc R Soc B 282:20152200. [DOI] [PMC free article] [PubMed]
- 26.Laube S, Zotz G. Which abiotic factors limit vegetative growth in a vascular epiphyte? Funct Ecol. 2003;17:598–604. [Google Scholar]
- 27.Benzing DH, Burt KM. Foliar permeability among twenty species of the Bromeliaceae. Bull Torrey Bot Club. 1970;97:269–279. [Google Scholar]
- 28.Huxley CR. The ant-plants Myrmecodia and Hydnophytum (Rubiaceae), and the relationships between their morphology, ant occupants, physiology and ecology. New Phytol. 1978;80:231–268. [Google Scholar]
- 29.Rickson FR. Absorption of animal tissue breakdown products into a plant stem—The feeding of a plant by ants. Am J Bot. 1979;66:87–90. [Google Scholar]
- 30.Rico-Gray V, Barber JT, Thien LB, Ellgaard EG, Toney JJ. An unusual animal-plant interaction: Feeding of Schomburgkia tibicinis (Orchidaceae) by ants. Am J Bot. 1989;76:603–608. [Google Scholar]
- 31.Gay H. Animal‐fed plants: An investigation into the uptake of ant‐derived nutrients by the far‐eastern epiphytic fern Lecanopteris Reinw. (Polypodiaceae) Biol J Linn Soc Lond. 1993;50:221–233. [Google Scholar]
- 32.Treseder KK, Davidson DW, Ehleringer JR. Absorption of ant-provided carbon dioxide and nitrogen by a tropical epiphyte. Nature. 1995;375:137–139. [Google Scholar]
- 33.Gegenbauer C, Mayer VE, Zotz G, Richter A. Uptake of ant-derived nitrogen in the myrmecophytic orchid Caularthron bilamellatum. Ann Bot (Lond) 2012;110:757–766. doi: 10.1093/aob/mcs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chomicki G, Renner SS. Obligate plant farming by a specialized ant. Nat Plants. 2016;2:16181. doi: 10.1038/nplants.2016.181. [DOI] [PubMed] [Google Scholar]
- 35.Chomicki G, Renner SS. Evolutionary relationships and biogeography of the ant-epiphytic genus Squamellaria (Rubiaceae: Psychotrieae) and their taxonomic implications. PLoS One. 2016;11:e0151317. doi: 10.1371/journal.pone.0151317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson JN. The Geographic Mosaic of Coevolution. Univ of Chicago Press; Chicago: 2005. [Google Scholar]
- 37.Kopp M, Gavrilets S. Multilocus genetics and the coevolution of quantitative traits. Evolution. 2006;60:1321–1336. [PubMed] [Google Scholar]
- 38.Yoder JB, Nuismer SL. When does coevolution promote diversification? Am Nat. 2010;176:802–817. doi: 10.1086/657048. [DOI] [PubMed] [Google Scholar]
- 39.Raimundo RL, Gibert JP, Hembry DH, Guimarães PR., Jr Conflicting selection in the course of adaptive diversification: The interplay between mutualism and intraspecific competition. Am Nat. 2014;183:363–375. doi: 10.1086/674965. [DOI] [PubMed] [Google Scholar]
- 40.Jebb MHP. 1985. Taxonomy and tuber morphology of the rubiaceous ant-plants. doctoral dissertation (University of Oxford, Oxford, UK)
- 41.Huxley CR, Jebb MHP. The tuberous epiphytes of the Rubiaceae 3: A revision of Myrmephytum to include Myrmedoma. Blumea. 1991;36:43–52. [Google Scholar]
- 42.Huxley CR, Jebb MHP. The tuberous epiphytes of the Rubiaceae 2: The new genus Anthorrhiza. Blumea. 1991;36:21–41. [Google Scholar]
- 43.Huxley CR, Jebb MHP. The tuberous epiphytes of the Rubiaceae 5. A revision of Myrmecodia. Blumea. 1993;37:271–334. [Google Scholar]
- 44.Janzen DH. Sweep samples of tropical foliage insects: Effects of seasons, vegetation types, elevation, time of day, and insularity. Ecology. 1973;54:687–708. [Google Scholar]
- 45.Bentley BL. The protective function of ants visiting the extrafloral nectaries of Bixa orellana (Bixaceae) J Ecol. 1977;65:27–38. [Google Scholar]
- 46.Koptur S. Alternative defenses against herbivores in Inga (Fabaceae: Mimosoideae) over an elevational gradient. Ecology. 1985;66:1639–1650. [Google Scholar]
- 47.Longino JT, Branstetter MG, Colwell RK. How ants drop out: Ant abundance on tropical mountains. PLoS One. 2014;9:e104030. doi: 10.1371/journal.pone.0104030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gillette PN, Ennis KK, Domínguez Martínez G, Philpott SM. Changes in species richness, abundance, and composition of arboreal twig-nesting ants along an elevational gradient in coffee landscapes. Biotropica. 2015;47:712–722. [Google Scholar]
- 49.Sanders NJ, Lessard JP, Fitzpatrick MC, Dunn RR. Temperature, but not productivity or geometry, predicts elevational diversity gradients in ants across spatial grains. Glob Ecol Biogeogr. 2007;16:640–649. [Google Scholar]
- 50.Jebb MHP. Cavity structure and function in the tuberous Rubiaceae. In: Huxley CR, Cutler DF, editors. Ant-plant interactions. Oxford Univ Press; Oxford, UK: 1991. pp. 374–390. [Google Scholar]
- 51.International Union for Conservation of Nature 2015 The IUCN Red List of Threatened Species. Available at www.iucnredlist.org/details/57785/0.
- 52.Rabosky DL. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS One. 2014;9:e89543. doi: 10.1371/journal.pone.0089543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Meara BC, Ané C, Sanderson MJ, Wainwright PC. Testing for different rates of continuous trait evolution using likelihood. Evolution. 2006;60:922–933. [PubMed] [Google Scholar]
- 54.Harmon LJ, Schulte JA, 2nd, Larson A, Losos JB. Tempo and mode of evolutionary radiation in iguanian lizards. Science. 2003;301:961–964. doi: 10.1126/science.1084786. [DOI] [PubMed] [Google Scholar]
- 55.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. GEIGER: Investigating evolutionary radiations. Bioinformatics. 2008;24:129–131. doi: 10.1093/bioinformatics/btm538. [DOI] [PubMed] [Google Scholar]
- 56.Barkman TJ, et al. Accelerated rates of floral evolution at the upper size limit for flowers. Curr Biol. 2008;18:1508–1513. doi: 10.1016/j.cub.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 57.Davis CC, et al. Long-term morphological stasis maintained by a plant-pollinator mutualism. Proc Natl Acad Sci USA. 2014;111:5914–5919. doi: 10.1073/pnas.1403157111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahler DL, Revell LJ, Glor RE, Losos JB. Ecological opportunity and the rate of morphological evolution in the diversification of Greater Antillean anoles. Evolution. 2010;64:2731–2745. doi: 10.1111/j.1558-5646.2010.01026.x. [DOI] [PubMed] [Google Scholar]
- 59.Carroll SB. Evo-devo and an expanding evolutionary synthesis: A genetic theory of morphological evolution. Cell. 2008;134:25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 60.Agrawal AA, Karban R. Domatia mediate plant-arthropod mutualism. Nature. 1997;387:562–563. [Google Scholar]
- 61.Strack D, Fester T, Hause B, Schliemann W, Walter MH. Arbuscular mycorrhiza: biological, chemical, and molecular aspects. J Chem Ecol. 2003;29:1955–1979. doi: 10.1023/a:1025695032113. [DOI] [PubMed] [Google Scholar]
- 62.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 63.Hibbett DS, Gilbert LB, Donoghue MJ. Evolutionary instability of ectomycorrhizal symbioses in basidiomycetes. Nature. 2000;407:506–508. doi: 10.1038/35035065. [DOI] [PubMed] [Google Scholar]
- 64.Barrabé L, et al. New Caledonian lineages of Psychotria (Rubiaceae) reveal different evolutionary histories and the largest documented plant radiation for the archipelago. Mol Phylogenet Evol. 2014;71:15–35. doi: 10.1016/j.ympev.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 65.Bouckaert R, et al. BEAST 2: A software platform for Bayesian evolutionary analysis. PLOS Comput Biol. 2014;10:e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rambaut A, Suchard M, Xie W, Drummond A. 2014. Tracer (Institute of Evolutionary Biology, University of Edinburgh), Version 1.6.
- 67.Huxley CR, Jebb MHP. The tuberous epiphytes of the Rubiaceae 1: A new subtribe, the Hydnophytinae. Blumea. 1991;36:1–20. [Google Scholar]
- 68.Revell LJ. phytools: An R package for phylogenetic comparative biology (and other things) Methods Ecol Evol. 2012;3:217–223. [Google Scholar]
- 69.Pagel M, Meade A. 2013 BayesTraits (University of Reading, Berkshire, UK), Version 2. Available at www.evolution.rdg.ac.uk.
- 70.Rabosky DL, et al. BAMMtools: An R package for the analysis of evolutionary dynamics on phylogenetic trees. Methods Ecol Evol. 2014;5:701–707. [Google Scholar]
- 71.Environmental Systems Research Institute 2008. ArcGIS Desktop 9, ArcView 9.3, (Environmental Systems Research Institute, Redlands, CA)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.