Abstract
Sex steroids affect the motivation to court mates, but less is known about how they influence motor movements associated with courtship behavior. Steroidal control of motor function may be especially important for species in which courtship requires superior strength, stamina, and neuromuscular coordination. Here we use the golden-collared manakin (Manacus vitellinus) to examine whether the neuromuscular circuitry that controls motoric aspects of courtship activity is sensitive to androgens. Males of this tropical species attract mates by rapidly jumping among branches in a courtship arena and using their wings to produce loud wing snaps. Testosterone activates this display via the androgen receptor (AR), and past work reveals that manakins injected with radio-labeled T (3H-T) accumulate radioactivity in the spinal cord. Thus, we used quantitative PCR to measure AR, estrogen receptor-α (ER-α) subtype, and aromatase (AROM) mRNA in spinal cords of male and female manakins and zebra finches. Expression of AR, but not ER-α or aromatase, was higher throughout the manakin spinal cord compared with the zebra finch. Next, we tested whether AR-expressing skeletal muscles are innervated by motor and sensory neurons that also express AR. To do this, we backfilled spinal neurons by injecting fluorescent tracers into select AR-sensitive wing and leg muscles of wild caught male and female manakins. We then removed these spinal cords and measured AR expression with in situ hybridization. Both sexes showed abundant AR mRNA in the cervical and lumbosacral spinal enlargements as well as in dorsal root ganglia attached to these enlargements. Together our findings suggest that androgens act widely on peripheral motor and sensory circuits in golden-collared manakins to influence wing snapping displays.
In many species, males perform elaborate courtship displays to attract mates (1), and sex steroids often mediate these displays by acting on intracellular steroid receptors found in the central nervous system (2). Most studies that explore steroidal control of masculine reproductive behavior focus on the hypothalamic and limbic systems of the brain, because nuclei in these areas are interconnected, express sex steroid receptors, and help activate sexual motivation and arousal (3–6). However, fewer studies have assessed how sex steroids mediate male reproductive behavior by acting on other levels of the central nervous system, such as the spinal cord and its circuits that govern movement.
The spinal cord is critical to male reproduction not only because its helps activate motor patterns performed during copulation and courtship, but also because it demonstrates sensitivity to sex steroids. Motoneurons control movement by projecting from the ventral portion of the spinal cord to skeletal muscles. The cervical and lumbosacral enlargements of the spinal cord are especially important for such motor skills because they house the abundant motoneurons that innervate the forelimbs and hindlimbs, respectively. In mammals, androgens modulate various functional properties of motoneurons that innervate the muscles controlling penile reflexes (7–11). Although testosterone (T) can be converted to estradiol by the aromatase (AROM) enzyme, most of the effects of T on lumbosacral motoneurons occur via signaling at the androgen receptor (AR) and not via signaling at the estrogen receptor (ER α- or β-subtype) (8, 12, 13). Despite this work, however, remarkably little is known about whether androgens similarly affect spinal motoneurons in non-mammalian species and whether these effects relate to sex behavior that involves highly complex movement, such as some courtship displays.
The golden collared manakin (Manacus vitellinus) is a model to investigate hormonal and neuromuscular control of complex courtship behavior (14–16). Males of this suboscine bird, which inhabit the rainforests of Panama, perform an acrobatic and athletic display to attract female mates (17). The display itself is characterized by rapid jumping between saplings surrounding a small arena, which the male clears of leaf litter. Males also forcefully flip their wings above their heads to produce firecracker-like sounds, called wing snaps or roll snaps [10 or more wing snaps at 50–70 Hz (wing snaps/sec)] (14–16, 18). Given the physically strenuous nature of this display, males have evolved physiological adaptations that permit them to display to females each day for the nearly 6-month breeding season (approximately January to July). Muscles that raise [i.e. the supracoracoideus (SC) and the scapulohumeralis caudalis (SH)] and retract [i.e. the pectoralis (PEC)] the wing are larger in mass and have a greater cross-sectional diameter in males compared with females (19). Such morphological adaptations are not evident in the muscles that guide jumping behavior [i.e. the gluteal, but called henceforth the M. Iliotibialis lateralis (ITB)] (19), although these muscles may be adapted to accommodate male display behavior in other ways.
At the endocrine level, experiments reveal that androgens are a primary activator of the male golden-collared manakin's courtship display. Males given T in the nonbreeding season (when circulating T is basal) begin wing snapping and roll snapping at frequencies observed in breeding males (20). Within a week of treatment with the AR antagonist flutamide, courtship activity of reproductively active males is substantially reduced (21). This latter study indicates that T activates display behavior via AR, although there is some evidence that the aromatization of T to estradiol and the activation of ER also play a role (L. Day and B.A. Schlinger, unpublished observations). Androgens likely act directly on manakin skeletal muscles that control wing snapping and jumping (i.e. SH, SC, PEC, ITB), given that manakins express high levels of AR in these tissues (22) and androgens regulate expression of known AR-dependent genes that enhance muscle growth and contractility in manakin muscles (M.J. Fuxjager and B.A. Schlinger, unpublished observations). The spinal cord is likely also a significant target of steroid action because breeding male manakins accumulate sex steroid in their spinal cords to a greater degree than female manakins (23). Nevertheless, the absence of significant information about sex steroid receptor expression and activity in the avian spinal cord limits our ability to conclude how and where steroids act on the male manakin spinal cord to regulate courtship performance. We hypothesize that, like skeletal muscles, AR is expressed at high levels in the manakin spinal cord especially in those spinal motor neurons innervating muscles involved in the physically elaborate male courtship display.
In the present study, we investigated the levels and expression patterns of sex steroid receptors and the enzyme AROM in the spinal cord of the golden-collared manakin. We first used quantitative PCR (qPCR) to compare the levels of AR, ER-α, and AROM expression in all regions of the spinal cord between both sexes of manakins and zebra finches (an oscine songbird that does not show an elaborate courtship display; see Ref. 24). Next, we injected fluorescent dyes into muscles that lift (SC and SH) and retract (PEC) the wings during wing snapping for retrograde transport to the spinal cord to map the distribution of their motoneurons. Finally, we performed an in situ hybridization on the spinal cord of these individuals to examine whether neurons innervating the SC, SH, PEC, and ITB express AR mRNA.
Materials and Methods
Animals and tissue preparation
The University of California, Los Angeles (UCLA; Los Angeles, CA) Chancellor's Animal Care Committee approved of all the procedures described below. Adult male and female golden-collared manakins were collected in Panama during February and March. Individuals were captured from actively breeding leks, using mist nets (21, 25). Adult male and female reproductively active zebra finches were collected from our breeding colony at UCLA. The birds were housed in free-flying aviaries (6 ft × 6 ft × 3 ft) under a 14-h light,10-h dark photoperiod to sustain breeding.
In the first study, we used qPCR to measure AR, ER-α, and AROM expression in the spinal cords of reproductively active male and female manakins and zebra finches. Spinal cords were collected from both species (golden-collared manakins: n = 4/sex; zebra finches: n = 4/sex) and immediately flash frozen on dry ice. Manakin tissues were transported (frozen) to UCLA and then stored at −80 C, whereas zebra finch tissues were stored at −80 C immediately after being flash frozen in the laboratory.
In the second study, we used in situ hybridization to document AR expression in spinal neurons that had been backfilled by retrograde fluorescent dyes, which were injected into wing and leg muscles that control male courtship. Captured male (n = 10) and female (n = 12) manakins were quickly transferred to individual cages at the Smithsonian Tropical Research Institute in Gamboa, Panama. Individuals were hand fed a mixture of papaya, grape, and strawberries every 30–60 min until self-feeding was observed (within 48 h). Once this occurred, the birds were held in outdoor aviaries (0.61 × 0.91 × 2.13 m or 1.22 × 0.91 × 2.13 m) that contained plants with dense foliage and were given the same food and water ad libitum. After 2 d, these birds were recaptured and anesthetized, using a combination of 0.04 ml equithesin and metofane. One of two retrogradely transported fluorescent traces [True Blue (TB; 2% in distilled H2O; Molecular Probes Inc., Eugene, OR) or hydroxystilbamadine methanesulfonate (HM; 2% in distilled H2O, Molecular Probes)] were injected into the SH, SC, PEC, and ITB with a 10-ml Hamilton syringe (30 gauge). Injections occurred as follows: 9 × 2 μl TB into the left SH, 9 × 3 μl HM into the left PEC, 9 × 2 μl HM into the right SC, and 9 × 2 μl TB into the right ITB. Injections were directed toward different parts of each muscle to reach as many endplates as possible and maximize the number of backfilled motoneurons. Injections into the SC, which lies deep to the PEC, were made perpendicular to the PEC's surface; thus, to reach the center of the SC and avoid the PEC, the needle was first inserted into the bird's keel and then retracted 1 mm before injection. Injections into the SH, PEC, and ITB were made at an oblique angle to the muscle's surface because this allowed us to avoid inserting the needle too deeply into these superficial muscles. Motor and sensory neurons innervating each muscle were clearly distinguished in the spinal cord by their fluorescence as well as their position (right vs. left).
Because captivity can suppress gonadal T secretion (26) and androgens can affect AR expression (27, 28), we were concerned that diminished circulating androgens in captive manakins might reduce spinal AR expression. To avoid this potential complication, we implanted four males and six females with 12 mm SILASTIC tubes (0.76 mm inner diameter, 1.65 mm outer diameterl Dow Corning, Midland, MI) that were filled with 10 mm crystalline T. Implants were placed sc in the lower back. The remaining six males and six females were unimplanted. When the animals were killed, approximately 200 μl of blood was collected from three implanted and three unimplanted females to assess plasma T and dihydrotestosterone (DHT) levels. We examined females to estimate the increase in circulating T induced by the implants in individuals that otherwise have basal T levels. Blood samples were centrifuged, and the plasma was frozen and transported to the United States. Plasma T and DHT were measured in females using a RIA, using methods validated for golden-collared manakins and described previously (29). Intraassay coefficients of variation were 4 and 16% for T and DHT, respectively. Levels of T and DHT for implanted females were 2.24 and 1.45 ng/ml, respectively. Levels of T and DHT for unimplanted females were 0.51 and 0.37 ng/ml, respectively. For comparison, plasma T in male golden-collared manakins at this time of year is about 1.01 ± 1.18 ng/ml (20).
Birds were kept in captivity for 5–7 d after injection and implantation. The individual birds were then deeply anesthetized with equithesin (0.09 ml) and perfused through the heart with 0.9% saline followed by 4% paraformaldehyde. At this time, we verified that all implants were intact and that tissues surrounding injection sites were free of residual discoloration and needle wounds from injections (indicating that injections were restricted to appropriate muscles). Spinal cords were removed and then postfixed for 2 h in 4% paraformaldehyde, after which vertebral columns were dissected out of the spinal cord and cryoprotected overnight in 30% sucrose. Next, spinal cords were cut coronally into thirds, flash frozen with dry ice, and mounted onto cork with Tissue-Tek (Andwin Scientific, Shaumburg, IL). All spinal cord samples were then transported to the United States on dry ice, where they were stored at −80 C until analysis. At UCLA, spinal cord samples were sectioned longitudinally at −21 C in a freezing cryostat, and sections were immediately mounted on Fisher Superfrost Plus slides (Fisher, Fair Lawn, NJ) in a three slide series. The first two spinal cord sections were at 14 μm and the third section was cut at 30 μm. Slides that held 14-μm sections were postfixed for an additional 15 min in 4% paraformaldehyde. They were then washed for 15 min in 0.9% PBS (pH 7.2), dried at 37 C, and stored at −80 C. To protect fluorescence, slides were kept in the dark when possible. Slides that held 30-μm sections were coverslipped immediately with glycerol.
Quantitative PCR
Detailed qPCR procedures are described elsewhere (22). Individual spinal cord samples were homogenized at medium speed by a rotor/stator homogenizer, and total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RNA concentrations were measured with a spectrophotometer, and RNA integrity was verified by gel electrophoresis. RNA was treated with deoxyribonuclease (Promega, Madison, WI) at 42 C for 50 min and 70 C for 15 min, and then 1 μg of RNA was converted to cDNA using Superscript II reverse transcriptase (Invitrogen). RT-PCR was first used to verify the presence of AR, ER-α, and AROM in the various regions of the spinal cord in both species (see 22).
After confirmation of receptor and enzyme expression in all tissues by sequencing PCR fragments, qPCR was used to measure the relative levels of AR, ER-α, and AROM mRNA in each sample. All qPCR were performed in an ABI 7300 sequence detection system, using a SYBR Green PCR master mix kit (Applied Biosystems, Foster City, CA). For each sample, 5 ng of template was used per reaction. AR primers (forward: 5′-ATGAGTACCGCATGCACAAA, reverse: 5′-AACTCCTGGGGTGTGATCTG), ER-α primers (forward: 5′-TGAAAGGTGGAATCCGAAAAGA; reverse: 5′-TTGGCGTTTTTGTTTCATCACT), and AROM primers (forward: 5′-GGATGAGCACATGGATTTTGC; reverse 5′-GCAGTCAGATCCCCTCTGTTC) were designed using the zebra finch genome. Amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal control (forward: 5′-TGACCTGCCGTCTGGAAAA; reverse, 5′-CCATCAGCAGCAGCCTTCA) and was similarly designed from the zebra finch. All primers have been used previously with success in golden-collared manakins and zebra finches (22, 30). To further validate the use of these AR primers, we performed a separate experiment using AR qPCR primers that were designed specifically from the manakin AR gene and designed to amplify a region of the manakin AR gene that differed from the from the region amplified by the zebra finch AR qPCR primers (forward: 5′-GAACGACTGCACCATCGAC; reverse: 5′-ACCCAGTTTCTTCAGCTTGC). Reactions were carried out at 50 C for 2 min, 95 C for 10 min, 40 cycles at 95 C for 15 sec and 60 C for 1 min, followed by 95 C for 15 sec, 60 C for 30 sec, and 95 C for 15 sec. Samples were run in duplicate. In each reaction, standard curves with correlation coefficients of greater than 0.97 were generated using known concentrations of cDNA. Because all reaction efficiencies (E =10−1/slope − 1) were nearly 100% (31), mRNA concentrations for genes of interest were calculated using the ΔCt method [2-(Ct,Gene of Interest − Ct,GAPDH) × 1000]. The data were natural log transformed [ln(x+1)] to achieve a normal distribution.
In situ hybridization
We used in situ hybridization to localize the cells in the manakin spinal cord that express AR. First, we validated and optimized in situ reactions by conducting pilot experiments on spinal cords of intact male and female manakins. We then performed two independent in situ reactions that were balanced with respect to the number of males and females used that were implanted or unimplanted with T. We transcribed 33P-labeled sense and antisense probes once and then divided this into two aliquots, each of which was used in the two sequential reactions. To control for nonspecific hybridization, we incubated representative sections from parts of the manakin spinal cord with the 33P-labeled sense probe. As a positive control, we incubated sections of the zebra finch brain with 33P-labeled antisense probes.
The following reaction procedures are described in detail elsewhere (32). The AR probe we used was a generous gift from Dr. Manfred Gahr (Max Planck Institute for Omithology, Seewiesen, Germany) and was made from a zebra finch whole-brain cDNA library (33). The probe consists of a 759-bp fragment that extends from the receptor's DNA binding domain to its steroid binding domain. The probe has a 96.4% homology to canary AR. Riboprobes were generated from the entire linearized pOEM −7Zf(+/−) plasmid to produce antisense (SP6 polymerase; Promega) or sense (T7 polymerase; Promega) probes. 33P-labeled probes were prepared by in vitro transcription in a 10 μl solution containing 5× transcription buffer (Promega); 40 U RNasin (Promega); 10 mm dithiothreitol; 500 μm each of ATP, CTP, and GTP; 500 ng of linearized template cDNA; 6.25 μl 33P-uridine 5-triphosphate (2000 Ci/mmol; NEN Life Science Products, Boston, MA); and 1 μl of the appropriate RNA polymerase (SP6 or T7; Promega). SP6 and T7 transcription reactions were performed for 2 h at 40 C and 37 C, respectively. The cDNA template was then removed after a 15-min incubation at 37 C with 1 U/μl ribonuclease-free deoxyribonuclease (Promega), and 85 μl of 10 mm Tris-HCl per dithiothreitol (pH 7.5) was added to each reaction before quenching on ice. Unincorporated nucleotides were then removed by centrifugation through a G-50 Sephadex column (Boehringer Manneheim, Indianapolis, IN). Before hybridization, tissue sections were heated at 42 C for 30 min, cooled to room temperature for 15 min, and then washed three times in PBS (5 min/wash). To reduce nonspecific hybridization, basic residues were acetylated by treating the slides with 0.25% (vol/vol) acetic anhydride in 0.1 m triethanolamine (pH 8.0) for 10 min at room temperature. After a brief rinse in PBS, sections were dehydrated through a graded series of ethanols and air dried for 30 min. Prehybridization was carried out for 1–2 h at 48 C in a solution containing hybridization buffer (600 mm NaCl; 4 mm EDTA; 80 mm Tris-HCl, pH 7.8), Denhardt's medium, 0.2% Sheared Herring Sperm DNA, 250 μg/ml tRNA, 25 μg/ml polyA RNA, l μg/ml RNasin (Promega), and 50% formamide. Prehybridization buffer was aspirated from sections and the hybridization buffer that contained either sense or antisense riboprobe (at a concentration of 1–10 × 106 cpm/∼liter) was mixed with 10% dextran-sulfate and 50% formamide and subsequently placed on the tissue sections. Slides were then sealed under coverslips and incubated overnight at 48 C. On the next day, slides were washed in following posthybridization solutions: 4× saline sodium citrate (SSC) for 10 min at 50 C to remove coverslips, 2× SSC for 1 h, ribonuclease A (∼20 g/ml) for 30 min at 37 C, and finally 0.5× SSC for 1 h at 60 C. Slides were dehydrated in a series of washes of increasing ethanol concentrations that contained 300 mm ammonium acetate. Slides were then dried at room temperature for at least 2 h, and the tissue sections were exposed to film to estimate the length of exposure needed by subsequent emulsion autoradiography.
For autoradiography, the slides were dipped in emulsion (Kodak NTB-2; Eastman Kodak, Rochester, NY) at 42 C and then stored in light-proof, desiccated boxes at 4 C. After an exposure time of 3–4 wk, autoradiograms were developed (Eastman Kodak D-19) and fixed for 10 min (Eastman Kodak Fixer) at 15 C and then coverslipped.
Visualization of florescence and hybridization
Slides were examined under fluorescence, light-, and dark-field microscopy to determine the presence and distribution of silver grains and fluorescent-labeled cells. Backfilled neurons were visualized under UV light, using a Axioskop microscope and a Sony charge-coupled device video camera linked to a PowerMac 6600 (Carl Zeiss, New York, NY). TB-labeled cells were visualized using filters that excite at 360 nm wavelengths and pass emitted light at 460 nm. HM-labeled cells were visualized using filters that excite at 360 nm wavelengths and pass emitted light above 515 nm. To assist in accurately defining spinal levels in creating maps, all spinal cords were marked with a felt tip pen at levels 7–8 (defined below) upon dissection. With this mark as a reference, nerve roots could then be counted to determine cellular position within the rostrocaudal plane. Additionally, one spinal cord was left partially intact to provide an external view from which we counted nerve roots to verify the starting and ending of the enlargements for comparison. Other major landmarks of the spinal cord were used, including the lateral border of the gray matter and the central canal to determine position within the mediolateral plane, and morphologically distinct lamina IX motoneurons to determine position within the dorsoventral plane (34). Because no standard exists for the naming of vertebral levels in manakins or other passeriforms, spinal segments were numbered consecutively rostrocaudally, as in the chick (35).
Cells expressing AR mRNA were clearly distinguished by the concentration of silver grains clustered over the cell. To evaluate the degree of hybridization, we subjectively identified cells that showed light, medium, or heavy hybridization. We counted the number of silver grains over 30 representative cells from each group and also over a similar area of adjacent neuropil and divided the latter into the former. Cells in the lightly labeled group averaged 4.6 ± 1.4 times above background, cells showing medium hybridization averaged 7.3 ± 1.8 times above background, and cells exhibiting heavy hybridization averaged 11.4 ± 3.8 times above background. Cells subjectively categorized as lightly labeled fell below a 5 × background criteria [see Schultz and Schlinger (23)]. These cells, therefore, were excluded from our counts. The high concentration of silver grains over cells with medium and heavy hybridization made these AR-expressing cells readily apparent and these cells were used in creating the map.
The percentage of cells within a given motoneuron pool was determined by first counting the total number of fluorescently labeled cells to determine the total number of cells in that pool to create a denominator, and then dividing this into the total number of cells that were both fluorescently labeled and also positive for a concentration of silver grains at least 5 times the background silver grain concentration. Means were then calculated for each muscle.
Results
Sex-steroid receptor expression in the spinal cord
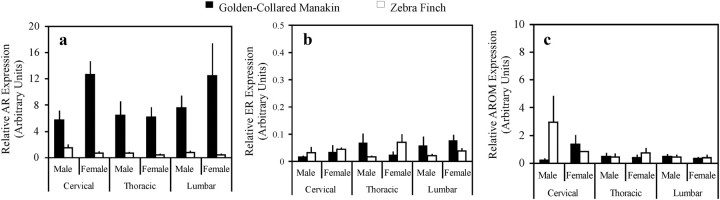
We find that, on average, male and female golden-collared manakins express more AR mRNA in the spinal cord, compared with male and female zebra finches (Fig. 1a; F1,32 = 202.55, P < 0.001). Although we detected no other main effects of sex or spinal cord region on AR expression (sex: F1,32 = 0.54, P = 0.47; spinal cord region: F2,32 = 2.49, P = 0.10), we did find a significant interaction effect between sex and species (F1,32 = 8.70, P = 0.006). Post hoc analysis of this interaction showed that female manakins express more AR in the spinal cord, compared with male manakins (F1,32 = 6.47, P = 0.016). This relationship, however, is not evident between female and male zebra finches (F1,32 = 2.58, P = 0.12). We found no other significant interactions in the statistical model related to AR expression (species by spinal cord region: F2,32 = 1.11, P = 0.34; sex by spinal cord region: F2,32 = 0.40, P = 0.68; species by sex by spinal cord region: F2,32 = 1.08, P = 0.351).
Fig. 1.
Relative expression (ΔCt levels) of AR (a), ER-α (b), and AROM (c) mRNA in the cervical, thoracic, and lumbar regions of the spinal cord in male and female golden-collared manakins and zebra finches. Spinal AR expression was higher in manakins than in zebra finches. Within manakins, spinal AR expression was higher in females than in males. Data represent mean ± sem.
To confirm the species difference in spinal cord AR expression, we conducted two additional tests. First, we verified that elevated AR expression in the manakin spinal cord does not reflect a more general increase in AR expression throughout the entire manakin body. To do this, we compared AR levels in the testes of male manakins and zebra finches because testes are established androgen targets. We found that the amount of AR mRNA did not differ in this tissue between species (data not illustrated, manakin > zebra finch; t test: t8 = 1.85, P = 0.10). This result is consistent with the idea that AR is not, in general, expressed more robustly in manakin tissues compared with zebra finch tissues. Second, we used an alternate pair of AR qPCR primers (see Materials and Methods for primer details) to measure spinal cord AR mRNA in a subset of tissue samples (male manakins vs. female zebra finches), We found the same species-level relationship in AR expression, with manakins showing more AR expression in all regions of the spinal cord than zebra finches (data not illustrated; two-way ANOVA, species effect: F1,12 = 29.53, P < 0.001).
We detected no significant effects of species, sex, or spinal cord region on spinal ER-α expression (Fig. 1b; species: F1,32 = 0.90, P = 0.35; sex: F1,32 = 1.61, P = 0.22; spinal cord region: F2,32 = 1.02, P = 0.37), nor did we find interactive effects of these factors on spinal ER-α expression (species by sex: F1,32 = 1.62, P = 0.21; species by spinal cord region: F2,32 = 2.02, P = 0.15; sex by spinal cord region: F2,32 = 0.17, P = 0.85; species by sex by spinal cord region: F2,32 = 2.12, P = 0.14). Similarly, we found no effects of species, sex, spinal cord region, or interactions among these factors on spinal AROM expression (Fig. 1c; species: F1,32 = 1.25, P = 0.27; sex: F1,32 = 0.08, P = 0.78; spinal cord region: F2,32 = 3.05, P = 0.062; species by sex: F1,32 = 1.15, P = 0.29; species by spinal cord region: F2,32 = 0.56, P = 0.58; sex by spinal cord region: F2,32 = 0.18, P = 0.83; species by sex by spinal cord region: F2,32 = 2.70, P = 0.083).
Distribution of AR mRNA in manakin spinal tissue
There is good evidence that the zebra finch antisense AR probe hybridized specifically to AR mRNA in manakin tissues. First, past studies show that the same antisense probe hybridizes specifically to AR mRNA in a number of bird species (36), and we confirm that the use of this probe hybridizes to AR mRNA in the brain regions that form the avian song control system and readily express AR (36). Second, there was no evidence of hybridization in manakin spinal cords when the sense probe was used (Fig. 2).
Fig. 2.
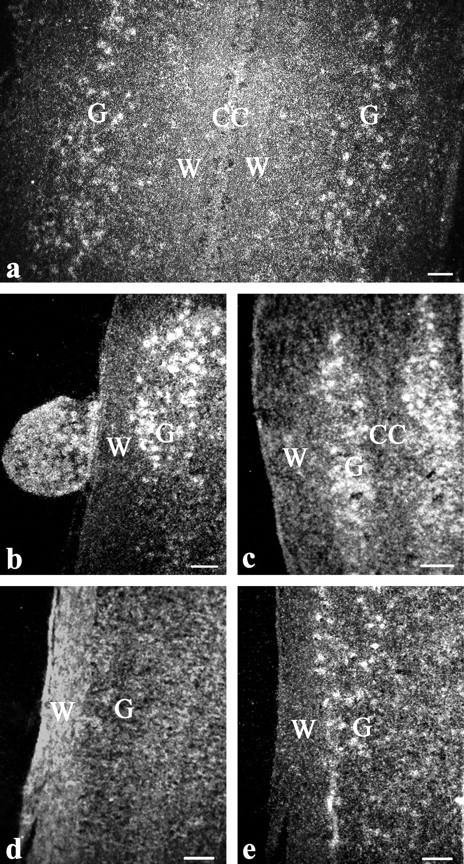
Darkfield photomicrographs of cells in the spinal cord that express AR mRNA. a, Cervical enlargement of a male implanted with T. b, Rostral cervical enlargement and attached DRG of a nonimplanted female. c, Thoracic region well outside the cervical and lumbosacral enlargements in a male implanted with T. d and e, Consecutive sections of the lumbosacral enlargement showing hybridization using the 33P-labeled AR sense probe (d) and the 33P-labeled antisense probe (e). CC, Position of the central canal; W, region of white matter; G, position of gray matter. Reference bar, 40 μm.
We found that cells expressing AR were distributed extensively throughout the spinal cords of both male and female manakins (Fig. 2). We noted variation among individuals in the absolute number of these cells; however, this variation did not relate to either sex or T treatment. This result is therefore consistent with our qPCR data, in that it suggests that male and female manakins express constitutively high levels of AR in the spinal cord. Furthermore, this result is consistent with the notion that potential suppression of the gonads in response to captivity and/or a bolus dose of T have little effect on spinal AR expression.
AR-expressing cells were most evident in the ventral horns of the cervical and lumbosacral spinal enlargements (Fig. 2, a and d). Moreover, it was clear that AR-expressing cells were mainly organized into columns along the lateral edge of the ventral horns, with only a few randomly dispersed AR-expressing cells medial to this column (Fig. 2e). Virtually no AR-expressing cells were detected in the gray matter adjacent to the central canal of the spinal cord (Fig. 2a).
Interestingly, the organizational distribution of AR-expressing cells changed throughout the cervical enlargement but not the lumbosacral enlargement. In particular, we found that AR-expressing cells that fill the lateral column of the ventral horns shift medially at the ends of the cervical enlargement. However, this does not occur in the lumbosacral enlargement; that is, AR-expressing cells fill the lateral column of the ventral horns throughout the entire region of the lumbosacral enlargement (levels 19–23) and at no point shift medially.
Using the information described above, we mapped the distributions of AR expressing cells in the cervical and lumbosacral enlargements. We should note that we discovered only a few AR-expressing cells in the ventral spinal cord outside the enlargements and in the central and dorsal spinal cord throughout the rostrocaudal extent. All of these cell groups were omitted from our map because they were very low in density and not organized into obvious pools.
In several instances, dorsal root ganglia (DRG) remained attached to the spinal cord and therefore were included in the in situ hybridization reactions (Fig. 2b). Many of the DRG that connected to the cervical enlargement (found in 10 birds) and the lumbosacral enlargement (found in seven birds) contain numerous AR-expressing cells. From six of the birds, we found 11 total DRG that remained connected to the spinal cord outside of the cervical and lumbosacral enlargements. Of these, AR-expressing cells were found in four DRG rostral to the cervical enlargement and seven DRG in the midthoracic spinal cord. Finally, four birds had at least one DRG outside the cervical and lumbosacral enlargements that showed no AR mRNA hybridization. Although these results are compelling, they should not be overinterpreted because not all DRG adhered to the collected cords upon dissection; thus, the degree to which the DRG results accurately and systematically depict the AR expression patterns in sensory afferent fibers is not completely clear.
Map of motoneurons that control manakin wing and leg muscles
Inspection of all tracer-injected muscles in each bird confirmed accurate placement of the injection sites. When we injected tracer into the SC, it was necessary to penetrate the PEC. Because of this and the fact that the PEC and SC are close to each other, we cannot be absolutely certain whether some tracer diffused between the muscles and caused inappropriate labeling. Despite this issue with these two muscles, we found clear back labeling of motoneurons in the spinal cord that innervate the SH, SC, PEC, and ITB (Fig. 3). Moreover, the distributions of these motoneuron pools closely resemble the positions reported previously in chicks and pigeons (Fig. 4) (35, 37, 38).
Fig. 3.
Photomicrographs of labeled motoneurons in the spinal cord after the im injection of the retrograde transport fluorescent tracer TB or HM. a, Motoneurons after TB was injected into the SH of a T-implanted male. b, HM was injected into the SC of a nonimplanted female. c, HM was injected into the PEC of a nonimplanted female. d, TB was injected into the ITB of a T-implanted female. Reference bar, 40 μm.
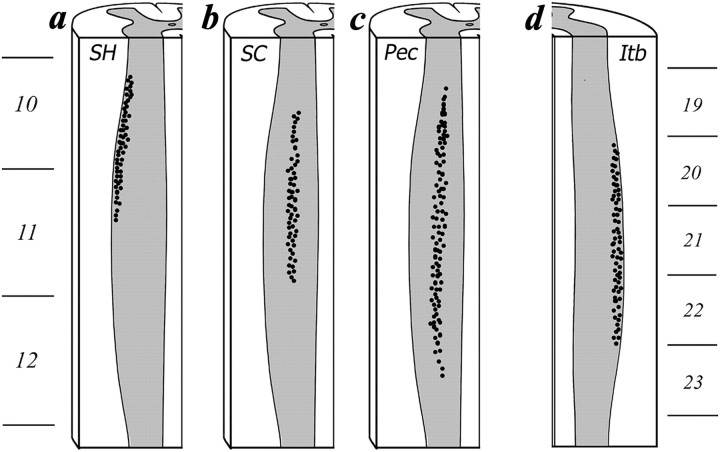
Fig. 4.
Schematic showing the rostrocaudal distribution of the motoneurons within spinal column that innervate the SH (a), SC (b), PEC (c), and ITB (d). The ventral third of the spinal cord is illustrated.
In the SH, injections of TB tracer revealed labeled motoneurons in a column at the ventrolateral margin of the gray matter within the cervical enlargement (Figs. 3a and 4a). This motoneuron pool extends from the rostral portion of spinal segment 10 through the middle of spinal segment 11. On average, 44 motoneurons per bird were backfilled by these injections.
In the SC, injection of HM tracer also showed labeled motoneurons in a discrete column in the ventrolateral portion of the gray matter within the cervical enlargement. However, this motoneuron pool was medial to the motoneurons that were backfilled by injections of TB into the SH (Figs. 3b and 4b). We observed labeled cells midway from the central and lateral border of the spinal cord, and these extended from the middle of spinal segment 10 through the caudal portion of spinal segment 11. On average, each bird showed 62 motoneurons backfilled by these injections.
In the PEC, injections of HM revealed labeled motoneurons in a column in the ventrolateral gray matter of the cervical enlargement (Figs. 3c and 4c). These motoneurons were distributed broadly across the lateral half of the gray matter and more widely than those innervating the SC. We observed backfilled motoneurons from injections in the PEC from the middle of spinal segment 10 through the caudal portion of spinal segment 12. We found that each bird had, on average, 109 motoneurons backfilled by HM injections into the PEC.
Finally, in the ITB, injections of TB showed backfilled motoneurons in a column along the ventrolateral margin of the gray matter of the lumbosacral enlargement (Figs. 3d and 4d). These motoneurons extended rostrocaudally from the beginning of spinal segment 20 to the end of spinal segment 22. We found that an average of 57 motoneurons per bird were backfilled from TB injections into the ITB.
Colocalization of hybridization and fluorescence
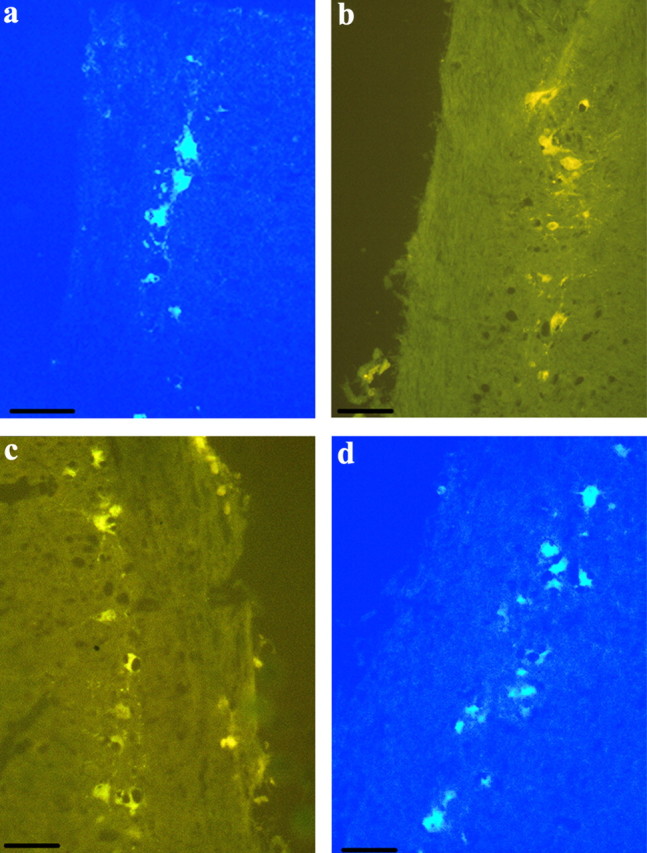
Nearly all of the motoneurons innervating the SH, SC, PEC, and ITB expressed AR (Fig. 5). In fact, our analysis indicates that 99.6% of backfilled motoneurons in the SH, 97.0% of backfilled motoneurons in the SC, 98.9% of backfilled motoneurons in the PEC, and 82.7% of backfilled motoneurons in the ITB readily express AR mRNA. However, although virtually all of the fluorescing motoneurons that innervate the wing muscles expressed AR, ITB motoneurons exhibited much more variability in regard to whether they expressed AR. For example, one bird in our study had as few as 29% of ITB motoneurons expressing AR mRNA.
Fig. 5.
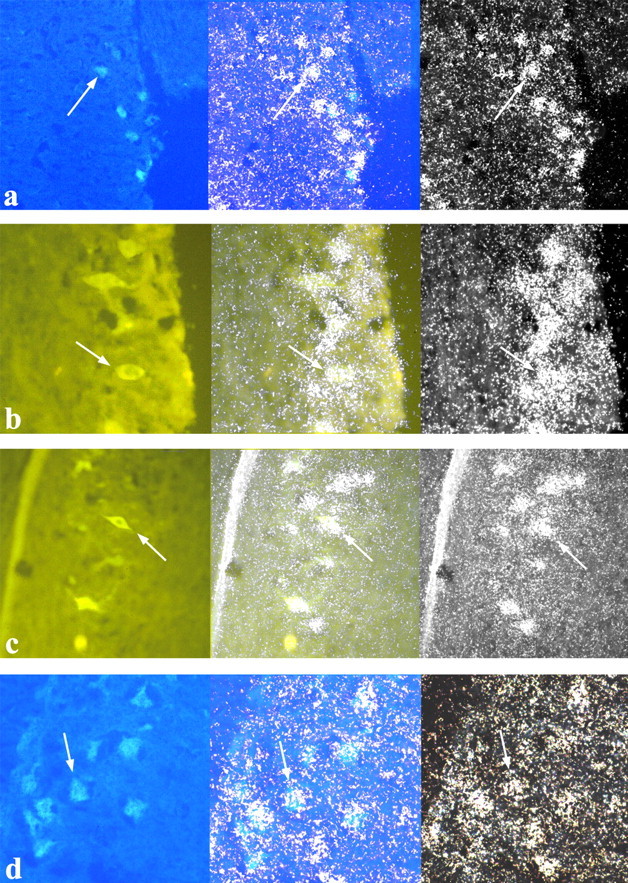
Photomicrographs of spinal motoneurons that innervate the SH (a), SC (b), PEC (c), and ITB (d). Photomicrographs in the left column show cells labeled with fluorescent tracers under UV illumination. Photomicrographs in the right column show the same tissue but viewed under dark-field illumination to illustrate the AR probe hybridization. Photomicrographs in the center column show an overlay of the left and right photomicrographs, demonstrating that fluorescent cells readily express AR mRNA. Arrows point to a representative cell that shows both florescence and silver grain accumulation.
Even though most of the DRG attached to the cervical and lumbosacral enlargements showed substantial AR expression, not all of these cells contained fluorescence. Nevertheless, we did find some individuals (both male and female) that exhibited fluorescent DRG cells with positive AR hybridization (Fig. 6), and this occurred after tracer injections into the SH, SC, PEC, and ITB. All observed fluorescent-labeled DRG cells expressed AR. As discussed above regarding the DRG dissections, these latter results should not be overinterpreted.
Fig. 6.
Photomicrographs of a DRG containing fluorescently labeled neurons as well as hybridization with the AR probe. The photomicrograph to the left shows cells labeled with fluorescent traces under UV illumination. The photomicrograph to the right shows the same tissue viewed under dark-field illumination to illustrate the AR probe hybridization. The photomicrograph in the center shows an overlay of the left and right photomicrographs to demonstrate that fluorescent cells express AR mRNA. Arrows point to a representative cell that shows both florescence and silver grain accumulation. Reference bar, 40 μm.
Discussion
Here we document the distribution of AR throughout the spinal cord of the golden-collared manakin. From a comparative standpoint, we find that AR is expressed more abundantly in the spinal cords of male and female manakins, compared with male and female zebra finches. This effect is specific to AR because we did not detect differences in the spinal cord expression of ER or AROM between manakins and zebra finches. In additional studies in male and female manakins, we found that AR expression is localized to motor neurons in the ventral horns of the cervical and lumbosacral enlargements as well as the DRG that are connected to these enlargements. Nearly all of these AR-producing cells innervate wing and leg muscles, which appear to control the complex limb movements during male acrobatic courtship displays (19).
Spinal cord AR and manakin courtship displays
Prior work has suggested that androgen action in the spinal cord is important for male manakin display behavior. In particular, Schultz and Schlinger (23) found that male manakins injected with radio-labeled testosterone (3H-T) accumulate radioactivity in the cervical and lumbosacral spinal enlargements. This study, however, was not able to distinguish whether the accumulated 3H-hormone was actually 3H-T bound to AR or 3H-E (the aromatized product of 3H-T) bound to ER. Our results clarify this issue by suggesting that the accumulated radioactivity was 3H-T (or 3H-dihydrotestosterone) and not 3H-E because we found that these same spinal enlargements contain abundant AR and little ERα and AROM. Thus, when our results are considered alongside those of Schultz and Schlinger (23), they suggest androgens are indeed acting on motoneurons and DRG cells in the manakin spinal cord and that this might contribute to male displays. This idea that androgen action at the level of the spinal column is integral to neuromuscular control of male courtship is consistent with other works that shows that androgens activate wing snap and roll snap behavior (20, 21, 25).
Equally interesting is that Schultz and Schlinger (23) found that female manakins given the same treatment of 3H-T showed lower radioactivity in the spinal cord; however, we found here that female manakins have slightly more AR in the spinal cord than males. Although these data may at first seem counterintuitive, we ultimately suspect that they are explained by sex differences in androgen metabolism. For example, the spinal cord of male manakins might have higher levels of 5α-reductase compared with females, which in principle would increase the potency of T in male spinal cords by converting T into DHT (39). Studies of the rat spinal cord have shown similar sex differences in accumulated radioactivity after injections of 3H-T, and this work attributed such variation to sex differences in androgen metabolism (40). This conclusion is supported by subsequent measures of spinal cord 5α-reductase, which show that males have more of this enzyme in their spinal cord than do females (41). At the same time, however, it is possible that female manakins express more spinal AR but accumulate less radioactivity after 3H-T injections because they differ from males in rates of AR mRNA translation or in their AR protein half-lives.
Elevated androgen sensitivity in the spinal cord may be an adaptive physiological phenotype that facilitates performance of male displays. We found that manakins express more AR in the spinal cord than zebra finches, which are not known to engage in courtship behavior that requires superfast movement of the wings and legs like male manakins (24). Male manakin courtship appears to be under particularly strong selection pressure because females prefer to mate with males that are faster and more agile while displaying (42). This result therefore implies that males with more adept neuromuscular coordination acquire a greater number of copulations with females and thus sire more offspring. If androgen signaling via AR in the spinal cord enhances such neuromuscular ability, then presumably selection favors mechanisms that promote AR expression in these tissues. This idea is bolstered by the fact that our data suggest that species differences in spinal AR are not completely attributed to species differences in circulating T. If this were the case, then we would expect to see similar sex differences in spinal cord AR expression between manakins and zebra finches. This result, however, was not borne out. Of course, the hypothesis that elevated expression of AR in the spinal cord is associated with physical courtship requires further investigation, possibly by conducting a broader phylogenetic comparison of spinal AR expression among manakins and other related passeriformes that lack complex courtship displays.
We expected to find greater AR expression in the spinal cords of male manakins compared with female manakins because previous results show that retention of injected 3H-sex-steroid is sexually dimorphic (23) and that male courtship is androgen dependent (20, 21, 25). This expectation, however, was not borne out because spinal AR is expressed at higher levels in females than in males. Similar sexually monomorphic expression of AR was seen in manakin skeletal muscles (22). We ultimately suspect that circulating androgens drive sex differences in courtship behavior, rather than relative levels of AR expression in the spinal motoneurons or skeletal muscles. This idea is supported by studies showing that females given T implants begin wing snapping and roll snapping like breeding males (25).
Functional significance of AR in the manakin spinal cord
Although the functional role that AR plays in the manakin spinal cord is not yet understood, there is little doubt that androgen action in this part of the nervous system helps maintain spinal motoneurons that innervate the SH, SC, PEC, and ITB. In mammals, there is a large body of work that shows that androgens modulate the soma size, dendritic arborization, synaptic connectivity, and gap junction abundance of motoneurons in the lumbar spinal nucleus of the bulbocavernosus (SNB) (8–13). Some of these effects occur as a result of androgen action at SNB target muscles (43), making the functional importance of AR in the SNB less clear per se. Regardless, the relationship between androgens and the SNB is critical for reproduction because the SNB innervates the muscles that control penile reflexes required for successful insemination (44). Although research in this area is much more limited in avian species, studies suggest that AR in the avian spinal cord helps rescue motoneurons from injury-induced cell death (45). Taken together, this work in mammals and birds is consistent with our overall hypothesis that AR in the golden-collared manakin spinal column mediates the health and functional connectivity of motoneurons that guide motoric output for male sexual displays.
Of course, this notion that AR in the spinal cord helps maintain motoneuron health and functionality does not necessarily explain why AR is expressed so abundantly in spinal tissues in the first place. One possible answer to this question is that high AR expression in the manakin spinal cord allows males to decrease circulating T but still maintaining an enhanced ability to detect and respond to androgens in the spinal cord. This idea is consistent with the fact that circulating T in male manakins increases at the onset of the breeding season but then drops to near-basal levels for the remainder of the year (14, 21). Decreasing T in this manner is relatively common in many temperate bird species (26, 46) because T itself is known to impose physiological and behavioral costs (47–49). Given the limitations to studying the effects of spinal cord androgen action in wild birds, it may be difficult to ultimately resolve why manakins express such high levels of spinal AR. Nevertheless, a more detailed assessment of how AR affects spinal motoneurons throughout the course of the breeding season might shed some light on the issue.
We also found that many DRG in the cervical and lumbosacral spinal enlargements expressed AR. This result was surprising, given the relative dearth of research that explored the presence and/or effects of androgen action in these cells. DRG are involved in processing somatosensory information; thus, it is possible that the large somas that showed florescence under UV light were primary sensory afferent neurons that innervate proprioceptive receptors in muscle spindles. In rats, primary sensory afferents that innervate sexually dimorphic muscles sometimes show AR immunoreactivity (50), and these afferents can also be sexually dimorphic in terms of cell size and number (44, 51). Presumably the complexity and speed of manakin courtship behavior requires extraordinary integration of sensory information about the spatial position of limbs and the contractile state of multiple muscle systems. Based on our results, this sensory information can travel through androgen-dependent circuits, which makes it interesting to speculate that androgen action at these circuits fine-tunes neuronal communication in a manner similar to that which potentially occurs with motoneurons. It is interesting to note that some DRG do not express AR, implying that androgen action might be limited to only a select subset of sensory afferent neurons.
It is important to mention that our studies concentrate on only a small subset of muscles that are hypothesized to control male wing snapping and jumping behavior. There is no doubt that other wing and leg muscles similarly play an important role in the ability to execute this behavior, and they may also be innervated by motoneurons that express abundant AR. We consistently found AR-expressing cells that were not backfilled by our fluorescent tracer dyes but that were morphologically indistinguishable from those motoneurons that were indeed backfilled and located in the ventrolateral spinal cord.
Finally, our work presented here does not allow us to rule out or conclude that other sex steroid receptors in the spinal cord are unimportant to male manakin displays. We focus mostly on the expression of AR along the spinal column not only because androgen action plays a prominent role in activating the male display, but also because manakins appear to express relatively more AR throughout their entire spinal cord than zebra finches. However, ER-α and AROM are present in the manakin (and zebra finch) spinal cord, suggesting that they may have some effect on neuromuscular physiology. Studies in adult quail also describe the presence of ER-α and AROM immunoreactivity in the dorsal spinal cord and point to their functional importance (52–54). Moreover, there is even a specific population of ER-immunoreactive cells in the spinal nucleus that innervates cloacal muscles used during copulation (54). Future research into the effects of estrogens on male manakin courtship behavior is clearly warranted.
Conclusions
In summary, our results suggest that androgens have widespread actions on motor and sensory circuits in the manakin spinal cord, including those circuits that control three sexually dimorphic wing muscles involved in courtship. Together with our evidence for elevated AR expression in manakin skeletal muscles (19), our data suggest that golden-collared manakins possess a unique androgen-sensitive neuromuscular phenotype that may be an adaptation to facilitate the acrobatic courtship display males use to attract female mates.
Acknowledgments
We thank Sorina Igreti and Dr. Fritz Hertel for assistance capturing and acclimatizing the birds. We thank Dr. Manfred Gahr for the AR probe and Dr. Fred Freking for technical assistance. Finally, we thank Yulian Lin for performing RIA and the STRI and IN.RE.NA.RE. for assistance and permission to conduct the research in Panama.
This work was supported by National Science Foundation Grant IBN-9874619 (to B.A.S.) as well as funds from the University of California, Los Angeles, Academic Senate.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- AR
- Androgen receptor
- AROM
- aromatase
- DHT
- dihydrotestosterone
- DRG
- dorsal root ganglia
- ER
- estrogen receptor
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- HM
- hydroxystilbamadine methanesulfonate
- ITB
- M. Iliotibialis lateralis
- PEC
- pectoralis
- qPCR
- quantitative PCR
- SC
- supracoracoideus
- SH
- scapulohumeralis caudalis
- SNB
- spinal nucleus of the bulbocavernosus
- SSC
- saline sodium citrate
- T
- testosterone
- TB
- True Blue
- UCLA
- University of California, Los Angeles.
References
- 1. Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 2. Adkins-Regan E. 2005. Hormones and animal social behavior. Princeton, NJ: Princeton University Press [Google Scholar]
- 3. Sipos ML , Nyby JG. 1996. Concurent androgenic stimulation of the ventral tegmental area and medial preoptic area: synergistic effects on male-typical reproductive behaviors in house mice. Brain Res 729:29–44 [PubMed] [Google Scholar]
- 4. Goodson JL , Evans AK , Lindberg L , Allen CD. 2005. Neuro-evolutionary patterning of sociality. Proc Biol Sci 272:227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DiMeo AN , Wood RI. 2006. ICV testosterone induces fos in male Syrian hamster brain. Psychoneuroendocrinol 31:237–249 [DOI] [PubMed] [Google Scholar]
- 6. Francis RC , Soma K , Fernald RD. 1993. Social regulation of the brain-pituitary-gonadal axis. Proc Natl Acad Sci USA 90:7794–7798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hart BL , Haugen CM. 1968. Activation of sexual reflexes in male rats by spinal implantation of testosterone. Physiol Behav 3:735–738 [Google Scholar]
- 8. Breedlove SM , Arnold AP. 1981. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen insensitive rats. Brain Res 225:297–307 [DOI] [PubMed] [Google Scholar]
- 9. Kurz EM , Sengelaub DR , Arnold AP. 1986. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science 232:395–398 [DOI] [PubMed] [Google Scholar]
- 10. Matsumoto A , Arnold AP , Zampighi GA , Micevych PE. 1988. Androgenic regulation of gap junctions between motoneurons in the rat spinal cord. J Neurosci 8:4177–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leedy MG , Beattie MS , Bresnahan JC. 1987. Testosterone-induced plasticity of synaptic inputs to adult mammalian motoneurons. Brain Res 424:386–390 [DOI] [PubMed] [Google Scholar]
- 12. Holmes MM , Musa M , Lonstein JS , Monks DA. 2009. Sexual dimorphism and hormone responsiveness in the spinal cord of the socially monogamous prairie vole (Microtus ochrogaster). J Comp Neurol 516:117–124 [DOI] [PubMed] [Google Scholar]
- 13. Fraley GS , Ulibarri CM. 2002. Long-term castration effects motoneuron size but not number in the spinal nucleus of the bulbocavernosus in the adult male Mongolian gerbil. Brain Res 953:265–271 [DOI] [PubMed] [Google Scholar]
- 14. Schlinger BA , Day LB , Fusani L. 2008. Behavior, natural history and neuroendocrinology of a tropical bird. Gen Comp Endocrinol 157:254–258 [DOI] [PubMed] [Google Scholar]
- 15. Schlinger BA , Fusani L , Day L. 2008. Hormonal control of courtship in male Golden-collared manakins (Manacus vitellinus). Ornitologia Neotropical 19:229–239 [Google Scholar]
- 16. Schlinger BA , Schultz JD , Hertel F. 2001. Neuromuscular and endocrine control of an avian courtship behavior. Horm Behav 40:276–280 [DOI] [PubMed] [Google Scholar]
- 17. Chapman FM. 1935. The courtship of Gould's manakin (Manacus vitellinus vitellinus) on Barro Colorado Island, Canal Zone. Bull Am Museum Nat Hist 68:472–521 [Google Scholar]
- 18. Fusani L , Giordano M , Day LB , Schlinger BA. 2007. High-speed video analysis reveals individual variability in the courtship displays of male golden-collared manakins. Ethology 113:964–972 [Google Scholar]
- 19. Schultz JD , Hertel F , Bauch M , Schlinger BA. 2001. Adaptations for rapid and forceful contraction in wing muscles of the male golden-collared manakin: sex and species comparisons. J Comp Physiol A 187:677–684 [DOI] [PubMed] [Google Scholar]
- 20. Day LB , McBroom JT , Schlinger BA. 2006. Testosterone increases display behaviors but does not stimulate growth of adult plumage in male golden-collared manakins (Manacus vitellinus). Horm Behav 49:223–232 [DOI] [PubMed] [Google Scholar]
- 21. Fusani L , Day LB , Canoine V , Reinemann D , Hernandez E , Schlinger BA. 2007. Androgen and the elaborate courtship behavior of a tropical lekking bird. Horm Behav 51:62–68 [DOI] [PubMed] [Google Scholar]
- 22. Feng NY , Katz A , Day LB , Barske J , Schlinger BA. 2010. Limb muscles are androgen targets in an acrobatic tropical bird. Endocrinology 151:1042–1049 [DOI] [PubMed] [Google Scholar]
- 23. Schultz JD , Schlinger BA. 1999. Widespread accumulation of [H-3]testosterone in the spinal cord of a wild bird with an elaborate courtship display. Proc Natl Acad Sci USA 96:10428–10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tomaszycki ML , Banerjee SB , Adkins-Regan E. 2006. The role of steroids in courtship, pairing and pairing behaviors in the socially monogamous zebra finch. Horm Behav 50:141–147 [DOI] [PubMed] [Google Scholar]
- 25. Day LB , Fusani L , Hernandez E , Billo TJ , Sheldon KS , Wise PM , Schlinger BA. 2007. Testosterone and its effects on courtship in golden-collared manakins (Manacus vitellinus): seasonal, sex, and age differences. Horm Behav 51:69–76 [DOI] [PubMed] [Google Scholar]
- 26. Wingfield JC , Hegner RE , Dufty AM , Ball GF. 1990. The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat 136:829–846 [Google Scholar]
- 27. Lu SF , McKenna SE , Cologer-Clifford A , Nau EA , Simon NG. 1998. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology 139:1594–1601 [DOI] [PubMed] [Google Scholar]
- 28. Lu S , Simon NG , Wang Y , Hu S. 1999. Neural androgen receptor regulation: effects of androgen and antiandrogen. J Neurobiol 41:505–512 [DOI] [PubMed] [Google Scholar]
- 29. Hau M , Wikelski M , Soma KK , Wingfield JC. 2000. Testosterone and year round territorial aggression in a tropical bird. Gen Comp Endocrinol 117:20–33 [DOI] [PubMed] [Google Scholar]
- 30. Remage-Healey L , Oyama RK , Schlinger BA. 2009. Elevated aromatase activity in forebrain synaptic terminals during song. J Neuroendocrinol 21:191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klein D , Janda P , Steinborn R , Müller M , Salmons B , Günzburg WH. 1999. Proviral load determination of different feline immunodeficiency virus isolates using real-time polymerase chain reaction: influence of mismatches on quantification. Electrophoresis 20:291–299 [DOI] [PubMed] [Google Scholar]
- 32. Jacobs EC , Arnold AP , Campagnoni AT. 1996. Zebra finch estrogen receptor cDNA: cloning and mRNA expression. J Steroid Biochem Mol Biol 59:135–145 [DOI] [PubMed] [Google Scholar]
- 33. Gahr M , Metzdorf R , Aschenbrenner S. 1996. The ontogeny of the canary HVC revealed by the expression of androgen and oestrogen receptors. Neuroreport 8:311–315 [DOI] [PubMed] [Google Scholar]
- 34. Leonard RB , Cohen DH. 1975. Cytoarchitectonic analysis of the spinal cord of the pigeon (Columba livia). J Comp Neurol 163:159–180 [DOI] [PubMed] [Google Scholar]
- 35. Hollyday M. 1980. Organization of motor pools in the chick lumbar lateral motor column. J Comp Neurol 194:143–170 [DOI] [PubMed] [Google Scholar]
- 36. Metzdorf R , Gahr M , Fusani L. 1999. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol 407:115–129 [PubMed] [Google Scholar]
- 37. Hollyday M , Jacobson RD. 1990. Location of motor pools innervating chick wing. J Comp Neurol 302:575–588 [DOI] [PubMed] [Google Scholar]
- 38. Sokoloff A , Deacon T , Goslow GE. 1989. Musculotopic innervation of the primary flight muscles, the pectoralis (pars throacicus) and supracoracoideus of the pigeon (Columba livia); a WGA-HRP study. Anatom Rec 225:35–40 [DOI] [PubMed] [Google Scholar]
- 39. Zoppi S , Cocconi M , Lechuga MJ , Messi E , Zanisi M , Motta M. 1988. Anti-hormonal activities of 5-α-reductase and aromatase inhibitors. J Steroid Biochem 31:677–683 [DOI] [PubMed] [Google Scholar]
- 40. Breedlove SM , Arnold AP. 1983. Sex differences in the pattern of steroid accumulation by motoneurons of the rat lumbar spinal cord. J Comp Neurol 215:211–216 [DOI] [PubMed] [Google Scholar]
- 41. Jurman ME , Erulkar SD , Krieger NR. 1982. Testosterone 5-α-reductase in the spinal cord of Xenopus laevis. J Neurochem 38:657–661 [DOI] [PubMed] [Google Scholar]
- 42. Barske J , Schlinger BA , Wikelski M , Fusani L. 2011. Female choice for male motor skills. Proc Biol Sci 278:3523–3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rand MN , Breedlove SM. 1995. Androgen alters the dentritic arbors of SNB motoneurons by acting upon their target muscles. J Neurosci 15:4408–4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mills AC , Sengelaub DR. 1993. Sexually dimorphic neuron number in lumbosacral dorsal root ganglia of the rat: development and steroid regulation. J Neurobiol 24:1543–1553 [DOI] [PubMed] [Google Scholar]
- 45. Gould TW , Burek MJ , Ishihara R , Lo AC , Prevette D , Oppenheim RW. 1999. Androgens rescue avian embryonic lumbar spinal motoneurons from injury-induced but not naturally occurring cell death. J Neurobiol 41:585–595 [DOI] [PubMed] [Google Scholar]
- 46. Hirschenhauser K , Winkler H , Oliveira RF. 2003. Comparative analysis of male androgen responsiveness to social environment in birds: the effects of mating system and paternal incubation. Horm Behav 43:508–519 [DOI] [PubMed] [Google Scholar]
- 47. Marler CA , Moore MC. 1988. Evolutionary costs of aggression revealed by testosterone manipulations in free-living male lizards. Behav Ecol Sociobiol 23:21–26 [Google Scholar]
- 48. Duckworth RA , Mendonça MT , Hill GE. 2001. A condition dependent link between testosterone and disease resistance in the house finch. Proc Biol Sci 268:2467–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grear DA , Perkins SE , Hudson PJ. 2009. Does elevated testosterone result in increased exposure and transmission of parasites? Ecol Lett 12:528–537 [DOI] [PubMed] [Google Scholar]
- 50. Keast JR , Gleeson RJ. 1998. Androgen receptor immunoreactivity is present in primary sensory neurons of male rats. Neuroreport 9:4137–4140 [DOI] [PubMed] [Google Scholar]
- 51. McKenna KE , Nadelhaft I. 1986. The organization of the pudenal nerve in the male and female rat. J Comp Neurol 248:532–549 [DOI] [PubMed] [Google Scholar]
- 52. Evrard HC , Balthazart J. 2004. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. J Neurosci 24:7225–7229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Evrard HC , Balthazart J. 2003. Aromatase (estrogen synthase) activity in the dorsal horn of the spinal cord: functional implications. Ann NY Acad Sci 1007:263–271 [DOI] [PubMed] [Google Scholar]
- 54. Evrard HC , Balthazart J. 2002. Localization of oestrogen receptors in the sensory and motor areas of the spinal cord in Japanese quail (Coturnix japonica). J Neuroendocrinol 14:894–903 [DOI] [PubMed] [Google Scholar]