Abstract
It has been suggested a role of fatty acid ethanolamides in control of feeding behavior. Among these, palmitoylethanolamide (PEA) has not been directly implicated in appetite regulation and weight gain. The aim of this study was to investigate the effect of PEA on food intake and body weight and the interaction between PEA and hypothalamic leptin signaling in ovariectomized rats. Ovariectomy produced hyperphagia and increased weight gain, making it an useful model of mild obesity. Ovariectomized rats were treated with PEA (30 mg/kg sc) for 5 weeks. Then, blood was collected, and hypothalamus and adipose tissue were removed for histological, cellular, and molecular measurements. We showed that PEA caused a reduction of food intake, body weight, and fat mass. The mechanisms underlying PEA effects involved an improvement in hypothalamic leptin signaling, through a raise in signal transducer and activator of transcription 3 phosphorylation. We also reported that PEA reduced AMP-activated protein kinase-α phosphorylation and modulated transcription of anorectic and orexigenic neuropeptides in the hypothalamus. Moreover, PEA increased AMP-activated protein kinase-α phosphorylation and carnitine palmitoyltransferase 1 transcription in adipose tissue, suggesting an increase in ATP-producing catabolic pathway. PEA also polarized adipose tissue macrophages to M2 lean phenotype, associated to a reduction of inflammatory cytokines/adipokines. To demonstrate the direct effect of PEA on leptin sensitivity without interference of adiposity loss, we obtained consistent data in PEA-treated sham-operated animals and in vitro in SH-SY5Y neuroblastoma cell line. Therefore, our data provide a rationale for the therapeutic use of PEA in obese postmenopausal woman.
N-palmitoylethanolamide (PEA) is a fully saturated, bioactive, and endogenous N-acylethanolamine, first identified half a century ago in lipid extracts from various tissues (1). Among its pathophysiological roles, PEA exerts antiinflammatory and analgesic effects mediated by peroxisome proliferator-activated receptor α (2–5). This receptor is distributed at peripheral and central level and functions as a regulator of fatty acid oxidation, lipid metabolism, and inflammation, affecting transcription of numerous genes (6–8). The involvement of N-acylethanolamines in appetite regulation has been demonstrated (9). In particular, the anorectic effect of oleoylethanolamide (OEA) has been recognized in 2001 by Rodríguez de Fonseca et al (10). Later, the effect on satiety and reduction of body weight by OEA was ascribed to the activation of peroxisome proliferator-activated receptor α (11, 12). Leptin contributes to body weight homeostasis by regulating food intake and energy expenditure (13), promoting fat mass loss and body weight reduction. This adipokine exerts metabolic effects in peripheral tissues directly and indirectly through the central nervous system (14, 15). In fact, leptin modulates AMP-activated protein kinase (AMPK) activity and orexigenic/anorexigenic neuropeptides in discrete hypothalamic regions (14, 16–18), leading to a reduction of appetite and to an overall negative energy balance. Moreover, obesity is often accompanied by leptin resistance. This latter phenomenon is defined as decreased sensitivity to the anorexigenic or weight loss effects of leptin accompanied by increased serum level of the hormone. We have previously demonstrated that ovariectomy in rat is associated with increased circulating leptin levels and decreased expression of hypothalamic leptin receptor (19). A number of studies have demonstrated that loss of ovarian function is associated with increased appetite, body weight, and visceral adiposity and reduced energy expenditure, leading to metabolic pathologies, such as insulin resistance (IR), type 2 diabetes, and cardiovascular disease (20, 21).
An important role in systemic metabolic dysfunction associated with obesity-linked disorders is the chronic low-grade inflammatory state (22, 23). There is accumulating interest in the role of adipose tissue macrophages (ATMs) as inflammatory players in obesity (24). ATMs, accumulated in adipose tissue, consist of at least 2 different phenotypes: classically activated M1 macrophages and alternatively activated M2 macrophages (25). Lumeng et al (26) reported that ATM isolated from lean animals expressed hallmarks of polarization toward M2 macrophages with IL-10 and arginase expression, whereas in obese animals with IR, a shift to M1-polarized ATM with increased TNF-α and IL-6. These cytokines can block insulin action in adipocytes via autocrine/paracrine signaling and cause systemic IR via endocrine signaling, providing a potential link between inflammation and IR (27–30).
Ovariectomy-induced obesity in rats mimics obesity induced by estrogen deficiency in humans, making it an useful model of mild obesity for studying the metabolic consequences of the loss of ovarian function. The aim of the present study was to investigate, in this animal model, the effect of PEA treatment on food intake and body weight and the interaction between PEA and hypothalamic leptin signaling. Moreover, we investigated PEA effect on adipose tissue and polarization of macrophages toward an M2 phenotype, reducing inflammatory cytokines and recovering the metabolic dysfunctions associated with obesity-linked disorders.
Materials and Methods
Materials and reagents
DMEM, fetal bovine serum, cell culture media, and supplements were purchased from Cambrex Bio Science Verviers (B-800). Protease and phosphatase inhibitors (leupeptin, trypsin inhibitor, phenylmethylsulfonylfluoride, and Na3VO4) were purchased from Sigma-Aldrich. PEA was purchased from Tocris Bioscience, and leptin was obtained from Tebu-Bio/Peprotech.
Animals
Female Wistar Han rats (Harlan) were housed in stainless steel cages in a room kept at 22 ± 1°C with a 12-hour light, 12-hour dark cycle. The animals were acclimated to their environment for 1 week and had ad libitum access to tap water and rodent standard diet. All procedures involving the animals were carried out in accordance with the Institutional Guidelines and complied with the Italian Decreto Legge no. 116 of January 27, 1992 of Ministero della Salute and associated guidelines in the European Communities Council Directive of November 24, 1986 (86/609/ECC). All animal procedures reported herein were approved by the Institutional Animal Care and Use Committee (Centro Servizi Veterinari) of University of Naples “Federico II” under protocol no. 2011-0129170.
Ovariectomy and drug treatment
At the onset of the study, female rats (mean body weight of the cohort, 197 ± 2 g) were bilaterally ovariectomized (OVX) under anesthesia (100-mg/kg ketamine plus 5-mg/kg xylazine ip). The sham-operated animals (SHAM) were subjected to the same general surgical procedure as OVX groups except that ovarian excision. Each experimental group was composed of 12 rats.
Five weeks after surgery, a group of OVX rats was treated with PEA at a dose of 30 mg/kg/d sc (at 8 am) in a volume of 100 μL per 100 g body weight, as previously described (31). PEA (Tocris Cookson Ltd) was dissolved in polyethylene glycol400 and Tween 80 2:1 (Sigma-Aldrich) and kept overnight under gentle agitation with a micro stirring bar. Before injection, sterile saline was added so that the final concentrations of polyethylene glycol400 and Tween 80 were 20% and 10% vol/vol, respectively. SHAM and OVX rats were treated with the vehicle. All treatments continued for 5 weeks. Last administration was performed 2 hours (8 am) before killing (10 am). Preliminary experiments were performed on SHAM rats administered PEA at the same dose and treatment protocol as OVX (30 mg/kg/d sc in a volume of 100 μL per 100 g body weight), to confirm its effect even in the physiological state (without estrogen loss or adiposity alteration) (see Supplemental data, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Body weight, food intake, and fat mass
Throughout the experimental period, body weight was assessed 2 times per week, whereas food intake was evaluated every day, and 2 animals were allocated each cage. Lastly, food intakes were cumulated. Before killing, bioelectrical impedance analysis was applied to body composition assessment using a BIA 101 analyzer, modified for the rat (Akern). Fat-free mass was calculated using the bioelectrical impedance analysis (50 kHz) prediction equation of Ilagan et al (32), and fat mass content was determined as the difference between body weight and fat-free mass.
Oral glucose tolerance test and IR assessment
One week before killing, overnight fasted rats received glucose (2 g/kg, per os), and glycaemia was measured at 0, 30, 60, 90, and 120 minutes after glucose administration. The animals received PEA or vehicle 2 hours before glucose. The area under the curve (AUC) was calculated from time zero, as the integrated and cumulative measure of glycemia up to 120 minutes for all animals. Glucose levels were measured by the glucometer One Touch UltraSmart (Lifescan).
Tissue collection and hematic parameters
SHAM (at random stages of the estrous cycle) and OVX rats, treated or not with PEA for 5 weeks, were killed after overnight fasting. Blood collected by cardiac puncture was centrifuged at 1500g at 4°C for 15 minutes, and sera were stored at −70°C for later biochemical and hormonal determinations.
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured in serum samples by standard automated procedures, according to manufacturer's protocols (AST Flex reagent cartridge, ALT Flex reagent cartridge; Dade Behring, Inc). Glucose, triglycerides (TGLs), and cholesterol were also quantified (TGL Flex reagent cartridge, CHOL Flex reagent cartridge; Dade Behring, Inc). Leptin and insulin levels were measured by RIA kits for rats according to the manufacturer's instruction (Linco Research, Inc and Millipore Corp, respectively). As index of IR, homeostasis model assessment (HOMA)-IR was calculated, using the formula [HOMA = fasting glucose (mmol/L) × fasting insulin (μU/mL)/22.5].
Whole hypothalamus and sc adipose tissue were excised and immediately frozen in liquid nitrogen.
Western blot analysis
Hypothalamus and white adipose tissue (WAT) obtained from each animal were disrupted by homogenization on ice in lysis buffer (20mM Tris-HCl [pH 7.5], 10mM NaF, 150mM NaCl, 1% Nonidet P-40, 1mM phenylmethylsulfonylfluoride, 1mM Na3VO4, leupeptin and trypsin inhibitor 10 microg/ml). After 1 hour, tissue lysates were obtained by centrifugation at 100 000g for 15 minutes at 4°C. Protein concentrations were estimated by the Bio-Rad protein assay using bovine serum albumin as standard.
For Western blot analysis, tissue lysates were dissolved in Laemmli sample buffer, boiled for 5 minutes, and subjected to SDS-PAGE. The blot was performed by transferring proteins from a slab gel to nitrocellulose membrane at 240 mA for 40 minutes at room temperature. The filter was then blocked with 1× PBS and 5% nonfat dried milk for 40 minutes at room temperature and probed with rabbit polyclonal antibody against the long form of leptin receptor (Ob-Rb) or antiprotein-tyrosine phosphatase 1B (PTP1B) antibody (dilution 1:1000; Santa Cruz Biotechnology, Inc), or antiphospho-AMPKα or anti-AMPK (dilution 1:1000; Cell Signaling Technology), or anti-suppressor of cytokine signaling 3 (SOCS3) (dilution 1:500; Santa Cruz Biotechnology, Inc), or phosphorylatedsignal transducer and activator of transcription 3 (pSTAT3) or STAT3 (dilution 1:1000; Cell Signaling Technology) in 1 PBS, 5% nonfat dried milk, and 0.1% Tween 20 at 4°C overnight. The secondary antibody (IgG-horseradish peroxidase conjugate; 1:2000 dilution) was incubated for 1 hour at room temperature. Subsequently, the blot was extensively washed with PBS, developed using enhanced chemiluminescence detection reagents (Amersham Pharmacia Biotech) according to the manufacturer's instructions, and the immune complex visualized by Imag Quant.
The protein bands were scanned and densitometrically analyzed with a model GS-700 imaging densitometer (Bio-Rad Laboratories). To ascertain that blots were loaded with equal amounts of protein lysates, they were also incubated in the presence of the antibody against the β-actin protein (Sigma-Aldrich).
Real-time semiquantitative PCR
Hypothalamus and WAT were excised and immediately frozen in RNA Later (QIAGEN). Total RNA was extracted using TRIzol reagent (Invitrogen Biotechnologies), according to the manufacturer's instructions. cDNA was synthesized using a reverse transcription kit (Maxima First Strand cDNA Synthesized kit; Fermentas) from 2-μg total RNA. PCRs were performed with a Bio-Rad CFX96 Connect Real-Time PCR System instrument and software (Bio-Rad Laboratories). The primer sequences are reported in Table 1. The PCR conditions were 10 minutes at 95°C followed by 40 cycles of 2-step PCR denaturation at 95°C for 15 seconds and annealing extension at 60°C for 60 seconds. Each sample contained 1- to 100-ng cDNA in 2× Power SYBR Green PCR Master Mix (Applied Biosystems) and 200 nmol/L of each primer (Eurofins MWG Operon) in a final volume of 25 μL. The relative expression of each studied mRNA was normalized to appropriate housekeeping gene for hypothalamus and adipose tissue, respectively), and the data were analyzed according to the 2−delta delta cycle threshold method.
Table 1.
Real-Time PCR Primer Sequences
| Target Gene | Forward Primer (5′→3′) | Reverse Primer (3′→5′) | Accession Number |
|---|---|---|---|
| POMC | CAAGAAGCGGCGCCCTGTGA | GCTGCTCGCCTTCCAGCTCC | NM_139326.2 |
| AgRP | AGAGTTCTCAGGTCTAAGTCT | TTGAAGAAGCGGCAGTAGCACGT | NM_033650.1 |
| RPLP0 | GCAGGTGTTTGACAACGGCAG | GATGATGGAGTGTGGCAC | NM_022402.2 |
| CPT1A | CGCTCATGGTCAACAGCAACTACT | CTCACGGTCTAATGTGCGACGA | NM_031559.2 |
| F4/80 | CCAGCTTATGCCACCTGCA | TCCAGGCCCTGGAACATTGG | NM_001007557.1 |
| CD11c | CCTGAGGGTGGGCTGGATGCCA | GCCAATTTCCTCCGGACA | NM_001037780.2 |
| TNF-α | GCTACGGGCTTGTCACTC | CCACGCTCTTCTGTCTACTG | NM_012675.3 |
| IL-6 | TCCTACCCCAACTTCCAATGCTCT | TTGGATGGTCTTGGTCCTTAGCC | NM_012589.2 |
| Leptin (Ob) | GACATTTCACACACGCAGTCG | GAGGAGGTCTCGCAGGTT | NM_013076.3 |
| Arginase-1 | GGCAATTGGAAGCATCTCTGGC | CTGTGATGTAGAGACCTTCTC | NM_017134.3 |
| Adiponectin (ACRP-30) | AATCCTGCCCAGTCATGAAG | TCTCCAGGAGTGCCATCTCT | NM_144744.3 |
| Rn18s | CGCGGTTCTATTTTGTTGGT | AGTCGGCATCGTTTATGGTC | NR_046237.1 |
Histological and morphometric analysis of WAT
WAT was isolated and fixed with 4% paraformaldehyde for 24–30 hours at room temperature and embedded in paraffin; 5-μm thick paraffin-embedded tissue sections were then deparaffinized and rehydrated in graduated alcohol in distilled water. Digital images of adipose tissue sections were capture using a light microscope (Olympus) at ×40 magnification. For each group, 5 fields from each of 5 different hematoxylin-eosin-stained section per animal were analyzed. Adipocyte areas was measured with the open-source image analysis software ImageJ v1.46r (Rasband, WS, ImageJ; National Institutes of Health).
The relative adipocyte cell number was estimated using DNA content from 100-mg tissue. The genomic DNA was isolated from adipose tissue samples after proteinase K digestion, by means of isopropanol precipitation (33). Briefly, 100-mg adipose tissues were lysed in 0.5 mL of lysis buffer (100mM Tris-HCl [pH 8.5], 5mM EDTA, 0.2% sodium dodecyl sulfate, 200mM NaCl, and 100-μg proteinase K/mL) and incubated at 55°C with agitation for 5 hours. One volume of isopropanol was added to the lysate, and samples were mixed or swirled until precipitation was completed. Precipitated DNA was recovered and resuspended in 50 μL of 10mM Tris-HCl containing 0.1mM EDTA. DNA concentration was measured using the NanoDrop spectrophotometer (Thermo Scientific).
Cell culture and treatment
The human neuroblastoma cell line SH-SY5Y was grown in DMEM supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. Cells were passaged at confluence using a solution of 0.025% trypsin and 0.01% EDTA.
SH-SY5Y human neuroblastoma cell line (300 000/P60 dish) were incubated in serum-free DMEM at 37°C before each experiment. Sixteen hours after serum starvation, the cells were pretreated with PEA (10μM) for 8 hours. Then cells were treated with PEA alone or in combination with 30nM leptin (Tebu-Bio/Peprotech) for 1 hour. The vehicle control for PEA was 0.1% dimethylsulphoxide. For the preparation of whole-cell lysates, cells were washed with ice-cold PBS, harvested, and resuspended in 20mM Tris-HCl (pH 7.5), 10mM NaF, 150mM NaCl, 1% Nonidet P-40, 1mM phenylmethylsulphonyl fluoride, 1mM Na3VO4, leupeptin, and trypsin inhibitor (10 μg/mL). After 1 hour, cell lysates were obtained by centrifugation at 20 000g for 15 minutes at 4°C. Protein concentrations were estimated by the Bio-Rad protein assay using bovine serum albumin as standard. For Western blot analysis, cell lysates were dissolved in Laemmli sample buffer, boiled for 5 minutes, and subjected to SDS-PAGE. The blot was performed as reported above.
Statistical analysis
All data were presented as mean ± SEM. Statistical analysis was performed by ANOVA test for multiple comparisons, followed by Bonferroni's test. Statistical significance was set at P < .05.
Results
Effects of PEA on serum parameters
The effects of ovariectomy or PEA treatment on biochemical serum variables are presented in Table 2. Either OVX or PEA treatment did not modify ALT levels, whereas a significant increase in serum AST was shown in OVX compared with SHAM animals. A trend to decrease for AST level was shown in PEA-treated OVX. Total cholesterol significantly increased in OVX animals, and PEA treatment showed a significant hypocholesterolemic activity. No significant change in serum TGL or glucose concentrations was observed among groups. Conversely, a trend of increase in insulin level was shown in OVX animals, in parallel with an increase in HOMA-IR index. Both parameters were weakly lowered by PEA treatment. Serum leptin levels were significantly increased in OVX rats and significantly decreased by PEA.
Table 2.
Serum Parameters After 5 Weeks of Treatment With PEA
| Serum Parameters | SHAM | OVX | OVX+PEA |
|---|---|---|---|
| Serum ALT (U/L) | 38.17 ± 1.72 | 36.14 ± 2.76 | 37.43 ± 2.70 |
| Serum AST (U/L) | 166.7 ± 23.19 | 251.7 ± 14.68a | 237.4 ± 13.08a |
| Total cholesterol (mg/dL) | 46.40 ± 4.82 | 82.88 ± 4.81b | 65.25 ± 4.31a,c |
| Serum TGL (mg/dL) | 53.67 ± 5.28 | 52.75 ± 4.16 | 52.78 ± 2.60 |
| Fasting glycemia (mg/dL) | 89.56 ± 2.99 | 88.85 ± 2.75 | 88.08 ± 2.98 |
| Fasting insulinemia (ng/mL) | 0.16 ± 0.01 | 0.35 ± 0.11 | 0.31 ± 0.05 |
| HOMA index | 0.77 ± 0.11 | 2.08 ± 0.68 | 1.65 ± 0.28 |
| Leptinemia (ng/mL) | 1.07 ± 0.30 | 3.97 ± 0.76a | 2.01 ± 0.65c |
Values are means ± SEM.
P < 0.05 vs SHAM.
P < 0.001 vs SHAM.
P < 0.05 vs OVX.
Effects of PEA on body weight, food intake, and fat mass in OVX rats
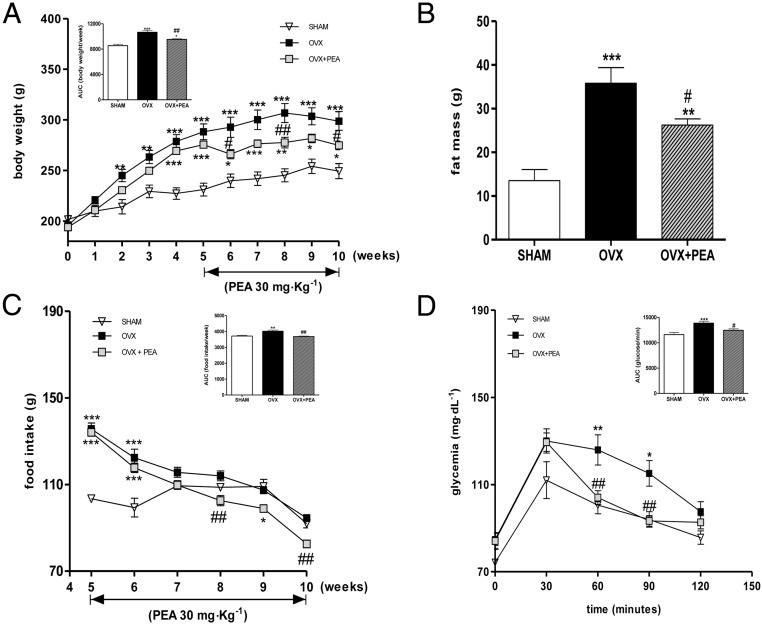
The mean body weights of the rats of each group, recorded during the experimental period, are shown in Figure 1A. Hypoestrogenism clearly led to a significant increase in body weight throughout the experimental period; PEA treatment, started at the 5th week, lowered body weight gain, already significantly after 3 weeks of treatment. After 5 weeks, body weight gain of PEA-treated rats was reduced by 30% compared with OVX animals. The AUC of body weight from 5th to 10th week was also performed showing an strong increase induced by ovariectomy and its significant reduction by PEA treatment.
Figure 1.
Body weight and related AUC (A) and fat mass (B) of SHAM and OVX treated or not with PEA are shown. Food intake, monitored once a week in all experimental groups and AUC of food consumption during the treatment period (from 5th to 10th wk) are also reported (C). Glucose tolerance test in all groups was performed and AUC evaluated (D). Data are expressed as mean ± SEM (n = 12 for each group). *, P < .05; **, P < .01; and ***, P < .001 vs SHAM; #, P < .05; ##, P < .01 vs OVX.
Fat mass was evaluated at the end of the study by bioelectrical impedance. As shown in Figure 1B, fat mass was significantly increased in the OVX rats, compared with the SHAM animals, whereas PEA treatment significantly reduced it. The food intake rates for all rats were measured once a week. Food intake in OVX group was significantly higher than that of SHAM animals in the 5-week period after the surgery (data not shown). As shown in Figure 1C, food intake in the next 5 weeks progressively lessens in PEA group until the end of the experiment, raising significance after 8 weeks. The evaluation of glycemic profile after glucose load (evaluated at 9th wk) showed that PEA administration caused a significant reduction of glycemia between 60 and 90 minutes after glucose load (Figure 1D). A marked and significant increase in AUC values was shown in OVX group, and this effect resulted inhibited by PEA. Preliminary experiment performed on SHAM rats treated or not with PEA showed no modification of body weight, fat mass, or food intake in PEA-treated SHAM animals, compared with SHAM rats (see Supplemental Figure 1); conversely, a reduction in glycemia was shown in PEA-treated SHAM animals after glucose load compared with SHAM animals (see Supplemental Figure 2).
Effect of PEA on hypothalamic leptin signaling
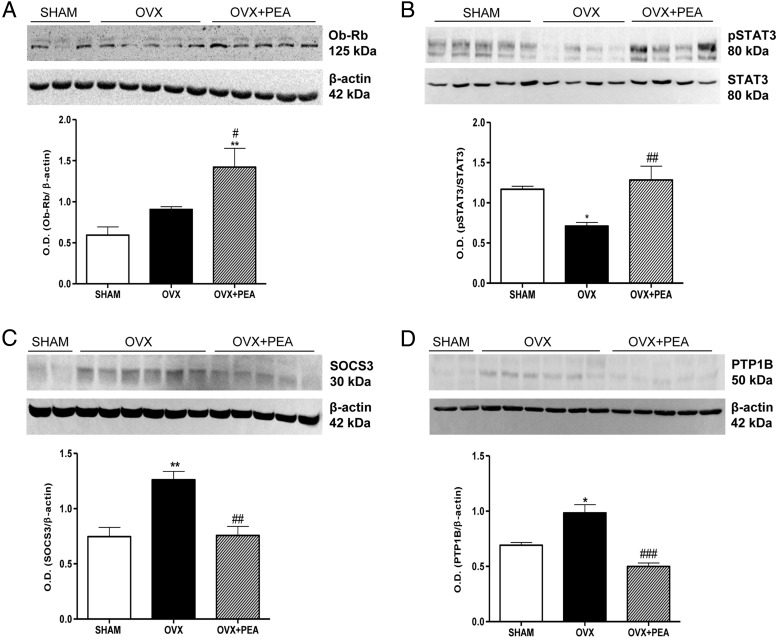
In order to evaluate leptin signaling sensitivity, we determined the hypothalamic expression of the functional isoform of leptin receptor Ob-Rb. As expected on the basis of our previous study (19), no significant modification of Ob-Rb expression was found in hypothalamus of OVX rats compared with that of SHAM animals; conversely, PEA treatment was able to induce a significant increase in the hypothalamic receptor expression (Figure 2A). Then, we evaluated the effectiveness of leptin signaling through the evaluation of STAT3 phosphorylated state and the expression of 2 important mediators of leptin resistance: SOCS3 (34) and PTP1B (35, 36). In particular, SOCS3 induced a reduction of leptin-induced STAT3 phosphorylation through the binding to Tyr985 of leptin receptor (37, 38), whereas PTP1B negatively regulates leptin signaling by dephosphorylating Janus kinase 2 (35, 36). As shown in Figure 2, B–D, PEA treatment significantly restored the blunted phosphorylation of STAT3 in OVX animals and reduced the expression of SOCS3 and PTP1B, suggesting the restoration of leptin signaling sensitivity.
Figure 2.
Changes in Ob-Rb (A), pSTAT3 (B), SOCS3 (C), and PTP1B (D) expression in hypothalamus obtained from SHAM, OVX, and OVX+PEA groups. These are representative blots of tissue lysates obtained from 6 animals in each group. Densitometric analysis performed for all groups is shown. *, P < .05 and **, P < .01 vs SHAM; #, P < .05; ##, P < .01; and ###, P < .001 vs OVX.
PEA modulation of leptin induced pSTAT3 in SH-SY5Y
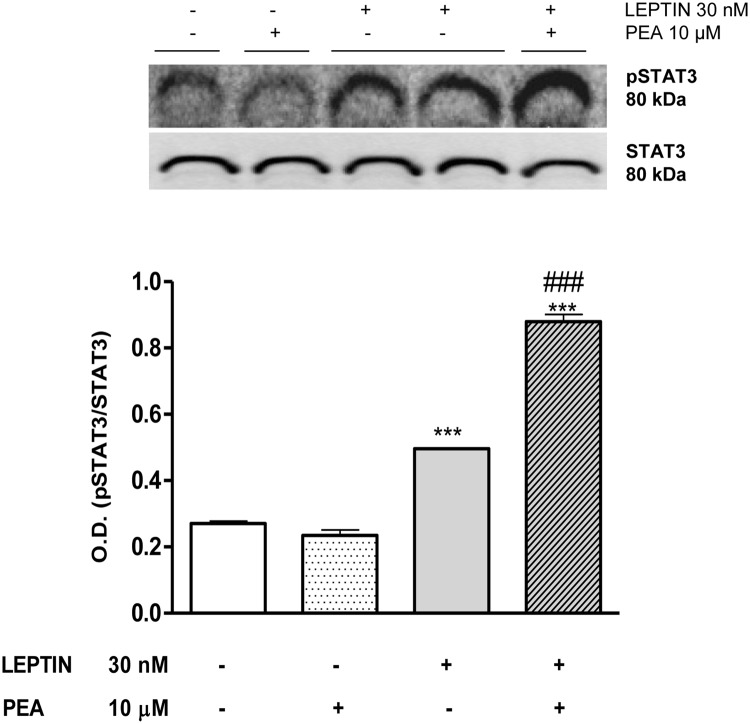
To test the direct effect of PEA on leptin signaling pathway, we examined the impact of PEA on STAT3 phosphorylation in response to leptin stimulation in SH-SY5Y neuroblastoma cell line, an in vitro model of neuronal leptin-STAT3 signaling. As shown in Figure 3, leptin-mediated STAT3 phosphorylation was significantly increased by cell pretreatment with PEA.
Figure 3.
Stimulation of SH-SY5Y cells with leptin (30nM) after 8 hours of pretreatment with PEA (10μM) acutely (∼1 h) increased STAT3 protein phosphorylation. Data are from triplicate experiments. ***, P < .001 vs untreated cells; ###, P < .001 vs leptin-stimulated cells.
AMPKα phosphorylation and neuropeptide mRNA expression in hypothalamus from PEA-treated OVX rats
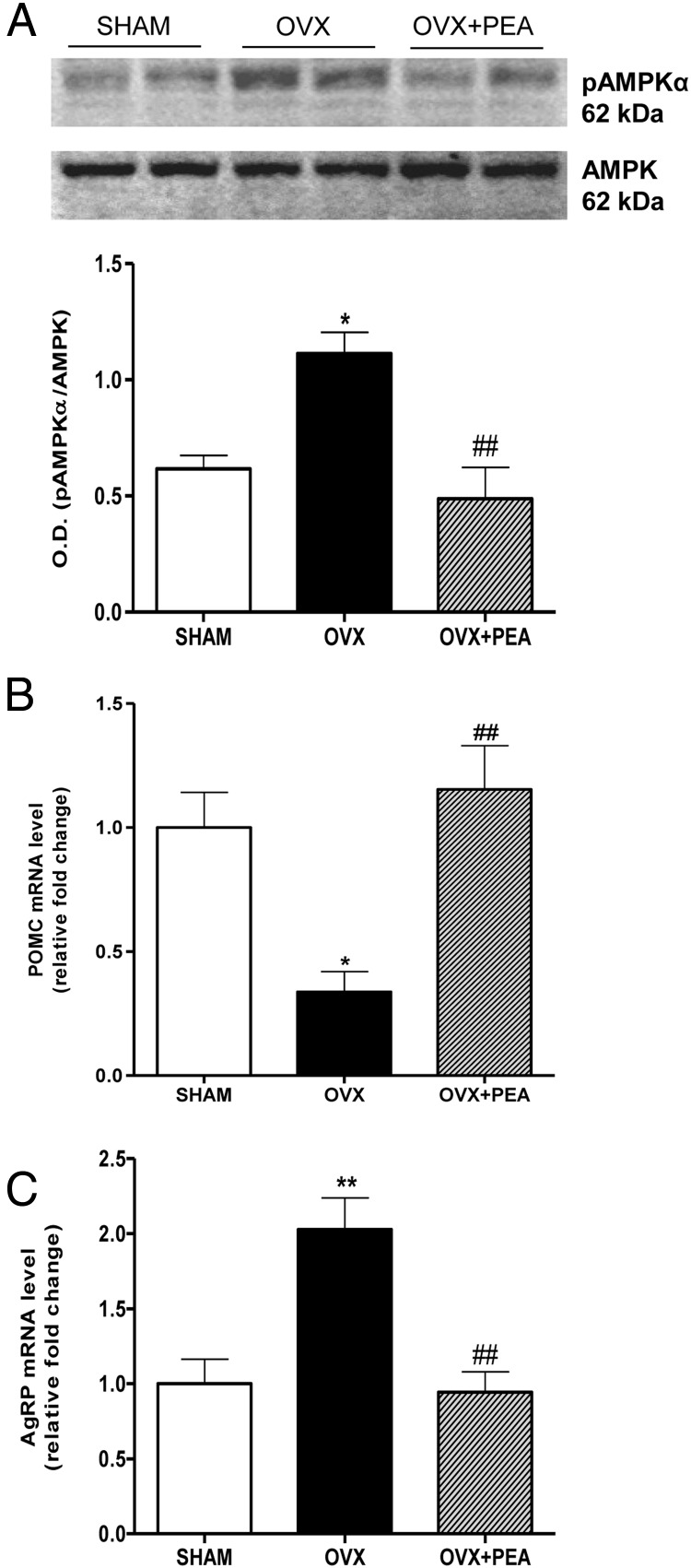
The effect of PEA on AMPK phosphorylation in hypothalamus was studied by immunoblotting analysis (Figure 4A). The pAMPKα/AMPK ratio level was increased in OVX rats compared with SHAM group; conversely, after PEA treatment, the accumulation of pAMPKα protein was significantly reversed, up to physiological level. Finally, the transcription of leptin-responsive anorectic proopiomelanocortine (POMC) and orexigenic Agouti-related protein (AgRP) neuropeptides was analyzed in the hypothalamus by RT-PCR. As shown in Figure 4B, the transcript encoding for POMC showed a significant decrease in OVX rats, which was prevented by PEA treatment; consistently, PEA normalized the up-regulation of hypothalamic AgRP mRNA levels induced by ovariectomy (Figure 4C).
Figure 4.
Levels of hypothalamic pAMPKα and neuropeptide transcription. A representative blot of pAMPKα and AMPK was shown. The ratio from densitometric analysis of bands from all samples are also shown (A). Hypothalamic POMC (B) and AgRP (C) mRNA (relative expression to SHAM) in the hypothalamus are reported. Data are the mean ± SEM of 6 rats per group. *, P < .05 and **, P < .01 vs SHAM; ##, P < .01 vs OVX.
Effects of PEA on adipocyte size and AMPKα phosphorylation in WAT
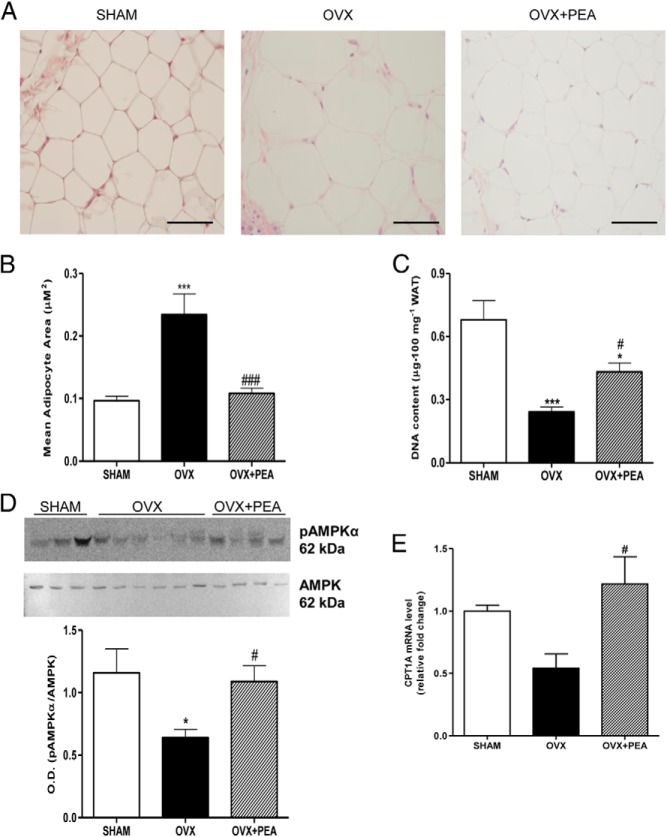
We examined adipocyte size of WAT by hematoxylin-eosin staining in all groups (Figure 5A). PEA treatment resulted in a decrease in adypocyte size compared with OVX animals (Figure 5B). These data are consistent with the partial increase of the number of adipocytes by PEA, indirectly measured by DNA content, which resulted deeply reduced in OVX rats (Figure 5C). In fact, the amount of DNA content is proportional to cell number and inversely related to lipidic amount in adipose tissue.
Figure 5.
Morphological hematoxylin-eosin of WAT (A) adipocyte size (B), and DNA content (C) of WAT from SHAM, OVX, and PEA-treated OVX rats are shown. Representative blot of pAMPKα and pAMPKα/AMPK ratio from densitometric analysis of bands of all samples are shown (D). CPT1A mRNA (relative expression to SHAM) in WAT is also reported (E). Data are the means ± SEM of 8 rats per group. Micrographs in panel A are representative pictures with original magnification ×40.*, P < .05 and ***, P < .01 vs SHAM; #, P < .05 and ###, P < .001 vs OVX.
The evaluation of AMPKα activation in adipose tissue revealed a reduction in the phosphorylated protein in OVX rats, which was prevented by PEA treatment (Figure 5D). This finding was consistent with carnitine palmitoyltransferase (CPT)1 expression level, whose transcription was up-regulated by PEA treatment, suggesting an increase in the oxidation of fatty acids (Figure 5E).
Effect of PEA on cytokine expression and M1/M2 polarization in adipose tissue
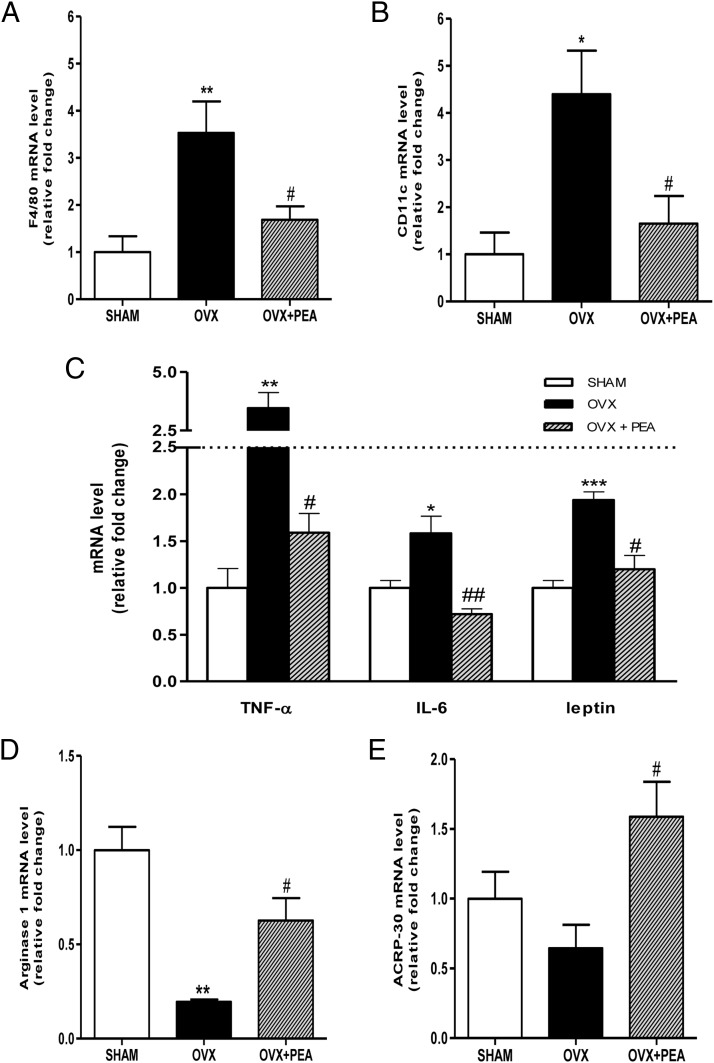
To evaluate macrophage infiltration in WAT, we analyzed the transcription of the macrophage markers EGF-like module-containing mucin-like hormone receptor-like 1, also known as cell surface glycoprotein F4/80, Integrin, alpha X (CD11c), and arginase-1 for M1 or M2 phenotype by RT-PCR. Moreover, we have also analyzed the cytokine expression pattern in WAT. The results showed that the ovariectomy induced an increase in F4/80 and CD11c expression, which was prevented by PEA treatment (Figure 6, A and B). Consistently, gene expression of TNF-α, IL-6, and leptin was up-regulated in adipose tissue from OVX rats and significantly reduced by PEA treatment (Figure 6C). Moreover, RT-PCR from adipose tissue extracts revealed a decrease in arginase-1 gene transcription in OVX animals, which was restored by PEA treatment (Figure 6D). These findings support the notion that PEA treatment promotes macrophage polarization toward the M2 state in WAT, reducing M1 inflammatory phenotype and increasing adiponectin transcription (Figure 6E).
Figure 6.
Effects of PEA on hallmarks of adipose tissue inflammation. mRNA expression of F4/80 (A), CD11c (B), TNF-α, IL-6, and leptin (C) (relative expression to SHAM) from WAT are shown. Arginase-1, as M2 phenotype marker, and adiponectin (ACRP-30) RT-PCR are also shown (D and E, respectively). Data are the means ± SEM of 8 rats per group. *, P < .05; **, P < .01; and ***, P < .001 vs SHAM; #, P < .05 and ##, P < .01 vs OVX.
Discussion
The current study demonstrates anorexic and fat-losing effects of a chronic treatment with PEA in ovariectomy-induced model of mild obesity. The recovery of the impairment of leptin and its receptor signaling and the increase of glucose tolerance after PEA treatment in OVX rats supports our finding and is consistent with the correction of leptin resistance in OVX-induced obesity in rats.
In a previous study by Rodríguez de Fonseca et al (10), the effect of NAEs on feeding behavior has been evaluated, demonstrating a profound anorexic effect of OEA and, although to a lesser extent, a reduction of food intake by PEA. However, the mechanism underlying PEA effect on feeding behavior was not elucidated. Here, we showed that PEA not only induced a decrease in food intake and body weight, but it also reduced fat mass, effects which may be in part mediated through the restoration of central and peripheral leptin sensitivity. It is well known that leptin is secreted by WAT in proportion to fat stores, in fact an increase in leptinemia, proportional to an increased fat storage, has been shown in obese phenotype, where the sensitivity to the anorexigenic or weight loss effects of the hormone are lacking. Beyond hyperleptinemia, a further hallmark of this condition, known as leptin resistance, is the impairment of the signal transduction of the functional isoform of leptin receptor, contributing to increase in food intake and body weight. Our findings strongly strengthened the idea of a pivotal role of leptin system restoration in PEA effects for different reasons. First, we showed a reduction of leptin gene transcription in adipose tissue accompanied by a decline in serum leptin level in PEA-treated OVX rats. Furthermore, we found an increase in the functional isoform of leptin receptor expression in the hypothalamus. Leptin signal transduction is regulated by SOCS3 and PTP1B, whose increase is another feature of leptin resistance, and involves the phosphorylation of STAT3, which is associated to the modulation of orexigenic/anorexigenic neuropeptides in the hypothalamus. Here, we found that both inhibitors of leptin signaling, SOCS3 and PTP1B, were down-regulated by PEA treatment, consistently with a restoration of leptin receptor sensitivity, showed by the increase in the hypothalamic pSTAT3 level. The basal level of SOCS expression in cells is generally very low but is induced in a transient manner upon cytokine stimulation both in vitro and in vivo (39). The cytokine-induced SOCS expression is STAT dependent, and as the SOCS proteins, once induced, act to down-regulate cytokine signaling, they appear to work in a classical negative-feedback loop (40). However, several studies also suggest that cross talk between different cytokine-induced signaling pathways can be mediated by the SOCS proteins (41). Here, we demonstrate SOCS3 levels are down-regulated in PEA-treated OVX rats. It is possible that the reduction of inflammatory cytokines (ie, IL-6, TNF-α, and leptin itself), observed in our experimental conditions, may be responsible of SOCS3 reduction, related to an improvement of leptin receptor signaling.
Preliminary experiment on PEA-treated SHAM rats, where no hormonal modifications or alterations of weight gain or adiposity were shown, confirmed the intrinsic capability of PEA in modulating leptin signaling. As shown in Supplemental data, PEA treatment induced an increase in STAT3 phosphorylation and a reduction in SOCS3 hypothalamic expression compared with SHAM rats, confirming that the reversal of leptin resistance was due to PEA effect and not secondary to weight loss (Supplemental Figure 3). The direct effect of PEA on leptin signaling has been also addressed in human SH-SY5Y neuroblastoma cell line (42, 43). PEA increased in leptin-mediated STAT3 phosphorylation in response to hormone stimulation. This in vitro model, used for studying neuronal leptin-STAT3 signaling, allowed to exclude the influence of other hormones or factors able to indirectly modulate leptin or PEA effect, including the influence of adiposity/weight variations in in vivo models.
PEA reduction of body weight together with the reversion in leptin resistance might be related to the suppression of food intake and fat accumulation by central modulation of AMPK activity and neuropeptide synthesis. In fact, the restoration of leptin sensitivity at hypothalamic level is consistent with the increase in POMC and the reduction of AgRP.
Beyond the central hypothalamic effect of PEA, our data support a peripheral effect of this ethanolamide on WAT. As is well known, WAT has an endocrine function in addition to its key role in metabolism, secreting a large number of adipocytokines, including leptin, that influence systemic and local metabolism in addition to inflammatory pathways. Dysfunctional metabolism and a chronic low-grade proinflammatory environment are observed in WAT, typical in obesity, which some authors have named “metabolically” triggered inflammation (44).
During a prolonged period of energy surplus, a condition occurring in OVX animals and reproduced in our model, lipid excess in adipose tissue results in increased adipocyte size (hypertrophy), tightly associated with changes in adipokine secretion by adipocytes. PEA treatment normalized cell size and increased the number of adipocytes, suggesting a decrease in lipid content in adipocytes and predisposing these cells to the lean phenotype. This morphological modification is considered to be healthy, because smaller adipocytes are capable to produce adiponectin and to be insulin sensitive (45).
Beyond adipocytes size modification induced by ovariectomy, in adipose tissue, leptin receptors progressively decline, and despite an increase in leptin synthesis, leptin resistance develops (19). The increased secretion of leptin (and/or decreased production of adiponectin) by adipocytes may also contribute to macrophage adhesion to endothelial cells (46) and their accumulation by stimulating transport of macrophages to the adipose tissue (47). In fact, besides adipocytes, secreting leptin and adiponectin, other nonadipocytic cells, especially macrophages, are responsible of adipose tissue inflammation, producing most inflammatory cytokines, ie, TNF-α, IL-6, and monocyte chemoattractant protein-1, and contributing to the development of systemic IR (48). The interplay between adipocytes and macrophages is central to the development of inflammation within tissue. Macrophages have been reported to be recruited and form crown-like structures surrounding necrotic adipocytes (49–51). In fact, after migration into adipose tissue in response to different stimuli, macrophages undergo a shift, switching from an antiinflammatory M2 state to an “activated” M1 proinflammatory state (48).
Various studies have reported that activation of AMPK can inhibit the synthesis of proinflammatory cytokines, such as TNF-α, and IL-6 both in macrophages (52, 53), and in adipocytes (54). Here, PEA, inducing an increase in AMPK activation in adipose tissue, inhibits cytokine synthesis, reducing the activated state of macrophages. Therefore, it is conceivable that PEA-induced activation of AMPK in adipose tissue drives antiinflammatory polarization of macrophages to M2 phenotype, reverting the reduction in AMPK activation of OVX animals and the enhanced mRNA expression of TNF-α and IL-6 and increasing arginase-1 and adiponectin synthesis. Our data confirm the well-known antiinflammatory role of this acylethanolamide and evidence its novel and interesting metabolic effect at central and peripheral level.
In fact, pAMPK increases fatty acid oxidation through phosphorylation of acetyl-coenzyme A carboxylase resulting in a decrease in the intracellular malonyl-coenzyme A levels and an increase in CPT1, the rate-limiting enzyme of mitochondrial fatty acid oxidation (55). Accordingly, PEA not only increase pAMPK but also normalized CPT1, suggesting its role in the modulation of this catabolic pathways.
Therefore, we hypothesized that 2 separate but converging mechanisms could contribute to the fat loosing effect of PEA, a peripheral effect on adipose tissue through the normalization of leptin synthesis and the increase of lipid catabolic pathway, and in addition at central level the normalization of leptin sensitivity and the modulation of POMC and AgRP, which negatively regulate food consumption.
The reduction of weight gain induced by PEA is also accompanied by a reduced inflammatory state of the macrophagic infiltrate in WAT and cytokine production responsible of the metabolic dysfunctions associated with obesity-linked disorders. Considering that PEA is currently available as a food supplement for several indications, our findings support its potential beneficial effects in the management of early detrimental symptoms of menopause.
It is worthy to note that estrogen reduces food intake and body weight via a direct action within the hypothalamus by enhancing leptin sensitivity, and the female brain shows a relatively higher sensitivity to the catabolic action of leptin (56, 57). On this basis, PEA treatment in ovarian function loss would be useful in correcting the alterations due to estrogen deficiency and restoring leptin sensitivity, alternatively to hormonal replacement therapy, which differently from PEA, has been related to several adverse effects (58, 59).
Acknowledgments
We thank Mr Giovanni Esposito and Mr Angelo Russo for animal care and assistance.
This work was supported by Ministero della Università e della Ricerca Progetti di Ricerca di Interesse Nazionale 2009 Italy, by National Institutes of Health Grants DK084065 and DK097566, and by the American Diabetes Association Research Award 7-11-BS-33.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by Ministero della Università e della Ricerca Progetti di Ricerca di Interesse Nazionale 2009 Italy, by National Institutes of Health Grants DK084065 and DK097566, and by the American Diabetes Association Research Award 7-11-BS-33.
Footnotes
- ACRP-30
- adiponectin
- AgRP
- Agouti-related protein
- ALT
- alanine aminotransferase
- AMPK
- AMP-activated protein kinase
- AST
- aspartate aminotransferase
- ATM
- adipose tissue macrophage
- AUC
- area under the curve
- CD11c
- integrin alpha X
- CPT
- carnitine palmitoyltransferase
- F4/80
- EGF-like module-containing mucin-like hormone receptor-like 1
- HOMA
- homeostasis model assessment
- IR
- insulin resistance
- Ob-Rb
- long form of leptin receptor
- OEA
- oleoylethanolamide
- OVX
- ovariectomized
- PEA
- palmitoylethanolamide
- POMC
- proopiomelanocortine
- pSTAT3
- phosphorylatedsignal transducer and activator of transcription 3
- PTP1B
- protein-tyrosine phosphatase 1B
- SHAM
- sham operated animals
- SOCS3
- suppressor of cytokine signaling 3
- TGL
- triglyceride
- WAT
- white adipose tissue.
References
- 1. Bachur NR, Masek K, Melmon KL, Undenfriend S. Fatty acid amides of ethanolamine in mammalian tissues. J Biol Chem. 1965;240:1019–1024. [PubMed] [Google Scholar]
- 2. Lo Verme J, Fu J, Astarita G, et al. The nuclear receptor peroxisome proliferator-activated receptor-α mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67:15–19. [DOI] [PubMed] [Google Scholar]
- 3. LoVerme J, Russo R, La Rana G, et al. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-α. J Pharmacol Exp Ther. 2006;319:1051–1061. [DOI] [PubMed] [Google Scholar]
- 4. D'Agostino G, La Rana G, Russo R, et al. Acute intracerebroventricular administration of palmitoylethanolamide, an endogenous peroxisome proliferator-activated receptor-α agonist, modulates carrageenan-induced paw edema in mice. J Pharmacol Exp Ther. 2007;322:1137–1143. [DOI] [PubMed] [Google Scholar]
- 5. D'Agostino G, La Rana G, Russo R, et al. Central administration of palmitoylethanolamide reduces hyperalgesia in mice via inhibition of NF-κB nuclear signalling in dorsal root ganglia. Eur J Pharmacol. 2009;613:54–59. [DOI] [PubMed] [Google Scholar]
- 6. Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR α in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cullingford TE, Bhakoo K, Peuchen S, Dolphin CT, Patel R, Clark JB. Distribution of mRNAs encoding the peroxisome proliferator-activated receptor α, β, and γ and the retinoid X receptor α, β, and γ in rat central nervous system. J Neurochem. 1998;70:1366–1375. [DOI] [PubMed] [Google Scholar]
- 8. Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 2011;1812:1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansen HS, Diep TA. N-acylethanolamines, anandamide and food intake. Biochem Pharmacol. 2009;78:553–560. [DOI] [PubMed] [Google Scholar]
- 10. Rodríguez de Fonseca F, Navarro M, Gómez R, et al. An anorexic lipid mediator regulated by feeding. Nature. 2001;414:209–212. [DOI] [PubMed] [Google Scholar]
- 11. Fu J, Gaetani S, Oveisi F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature. 2003;425:90–93. [DOI] [PubMed] [Google Scholar]
- 12. Lee SS, Pineau T, Drago J, et al. Targeted disruption of the α isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995;15:3012–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94:8878–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. [DOI] [PubMed] [Google Scholar]
- 15. Muoio DM, Lynis Dohm G. Peripheral metabolic actions of leptin. Best Pract Res Clin Endocrinol Metab. 2002;16:653–666. [DOI] [PubMed] [Google Scholar]
- 16. Minokoshi Y, Alquier T, Furukawa N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. [DOI] [PubMed] [Google Scholar]
- 17. Andersson U, Filipsson K, Abbott CR, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. [DOI] [PubMed] [Google Scholar]
- 18. Mountjoy PD, Bailey SJ, Rutter GA. Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity. Diabetologia. 2007;50:168–177. [DOI] [PubMed] [Google Scholar]
- 19. Meli R, Pacilio M, Raso GM, et al. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology. 2004;145:3115–3121. [DOI] [PubMed] [Google Scholar]
- 20. Abeles Ed, Cordeiro LM, Martins Ade S, et al. Estrogen therapy attenuates adiposity markers in spontaneously hypertensive rats. Metabolism. 2012;61:1100–1107. [DOI] [PubMed] [Google Scholar]
- 21. Qiu J, Bosch MA, Tobias SC, et al. G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation-mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol. 2012;32:1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. [DOI] [PubMed] [Google Scholar]
- 25. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. [DOI] [PubMed] [Google Scholar]
- 29. Mohamed-Ali V, Flower L, Sethi J, et al. β-Adrenergic regulation of IL-6 release from adipose tissue: in vivo and in vitro studies. J Clin Endocrinol Metab. 2001;86:5864–5869. [DOI] [PubMed] [Google Scholar]
- 30. Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006;116:33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mattace Raso G, Simeoli R, Russo R, et al. N-palmitoylethanolamide protects the kidney from hypertensive injury in spontaneously hypertensive rats via inhibition of oxidative stress. Pharmacol Res. 2013;76:67–76. [DOI] [PubMed] [Google Scholar]
- 32. Ilagan J, Bhutani V, Archer P, Lin PK, Jen KL. Estimation of body composition changes during weight cycling by bioelectrical impedance analysis in rats. J Appl Physiol. 1993;74:2092–2098. [DOI] [PubMed] [Google Scholar]
- 33. Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns. A simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bjørbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. [DOI] [PubMed] [Google Scholar]
- 35. Cheng A, Uetani N, Simoncic PD, et al. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2:497–503. [DOI] [PubMed] [Google Scholar]
- 36. Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, et al. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489–495. [DOI] [PubMed] [Google Scholar]
- 37. Bjørbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–30065. [DOI] [PubMed] [Google Scholar]
- 38. Bjorbak C, Lavery HJ, Bates SH, et al. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275:40649–40657. [DOI] [PubMed] [Google Scholar]
- 39. Münzberg H, Myers MG Jr. Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–570. [DOI] [PubMed] [Google Scholar]
- 40. Starr R, Willson TA, Viney EM, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917-921. [DOI] [PubMed] [Google Scholar]
- 41. Wang J, Campbell IL. Cytokine signaling in the brain: putting a SOCS in it? J Neurosci Res. 2002;67:423–427. [DOI] [PubMed] [Google Scholar]
- 42. Wang R, Swick AG. Identification and characterization of a leptin-responsive neuroblastoma cell line. Biochem Biophys Res Commun. 2009;379:835–839. [DOI] [PubMed] [Google Scholar]
- 43. Jang EH, Park CS, Lee SK, Pie JE, Kang JH. Excessive nitric oxide attenuates leptin-mediated signal transducer and activator of transcription 3 activation. Life Sci. 2007;80:609–617. [DOI] [PubMed] [Google Scholar]
- 44. Monteiro R, Azevedo I. Chronic inflammation in obesity and metabolic syndrome. Mediators Inflamm. 2010;2010; ID289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. [DOI] [PubMed] [Google Scholar]
- 47. Sierra-Honigmann MR, Nath AK, Murakami C, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. [DOI] [PubMed] [Google Scholar]
- 48. Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17:332–341. [DOI] [PubMed] [Google Scholar]
- 50. Nishimura S, Manabe I, Nagasaki M, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. [DOI] [PubMed] [Google Scholar]
- 51. Nishimura S, Manabe I, Nagasaki M, et al. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118:710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage α1 AMP-activated protein kinase (α1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051–10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lihn AS, Pedersen SB, Lund S, Richelsen B. The anti-diabetic AMPK activator AICAR reduces IL-6 and IL-8 in human adipose tissue and skeletal muscle cells. Mol Cell Endocrinol. 2008;292:36–41. [DOI] [PubMed] [Google Scholar]
- 55. Unger RH. The hyperleptinemia of obesity-regulator of caloric surpluses. Cell. 2004;117:145–146. [DOI] [PubMed] [Google Scholar]
- 56. Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. [DOI] [PubMed] [Google Scholar]
- 57. Clegg DJ, Benoit S, Barrera J, Woods SC. Estrogen mediates body fat distribution and brain sensitivity to adiposity signals. Diabetes. 2003;52(suppl 1):A24 (Abstract). [Google Scholar]
- 58. Hulka BS. Epidemiologic analysis of breast and gynecologic cancers. Prog Clin Biol Res. 1997;396:17–29. [PubMed] [Google Scholar]
- 59. Lindenfeld J, Ghali JK, Krause-Steinrauf HJ, et al. Hormone replacement therapy is associated with improved survival in woman with advanced heart failure. J Am Coll Cardiol. 2003;42:1238–1245. [DOI] [PubMed] [Google Scholar]