Abstract
Rhythmic events in female reproductive physiology, including ovulation, are tightly controlled by the circadian timing system. The molecular clock, a feedback loop oscillator of clock gene transcription factors, dictates rhythms of gene expression in the hypothalamo-pituitary-ovarian axis. Circadian disruption due to environmental factors (eg, shift work) or genetic manipulation of the clock has negative impacts on fertility. Although the central pacemaker in the suprachiasmatic nucleus classically regulates the timing of ovulation, we have shown that this rhythm also depends on phasic sensitivity to LH. We hypothesized that this rhythm relies on clock function in a specific cellular compartment of the ovarian follicle. To test this hypothesis we generated mice with deletion of the Bmal1 locus in ovarian granulosa cells (GCs) (Granulosa Cell Bmal1 KO; GCKO) or theca cells (TCs) (Theca Cell Bmal1 KO; TCKO). Reproductive cycles, preovulatory LH secretion, ovarian morphology and behavior were not grossly altered in GCKO or TCKO mice. We detected phasic sensitivity to LH in wild-type littermate control (LC) and GCKO mice but not TCKO mice. This decline in sensitivity to LH is coincident with impaired fertility and altered patterns of LH receptor (Lhcgr) mRNA abundance in the ovary of TCKO mice. These data suggest that the TC is a pacemaker that contributes to the timing and amplitude of ovulation by modulating phasic sensitivity to LH. The TC clock may play a critical role in circadian disruption-mediated reproductive pathology and could be a target for chronobiotic management of infertility due to environmental circadian disruption and/or hormone-dependent reprogramming in women.
Precise timing of physiological events is critical for normal reproductive physiology. In female mammals rhythmic events in the reproductive tract are normally kept tightly entrained to (synchronized with) the light:dark (L:D) cycle (1, 2). A salient feature of the mammalian reproductive cycle is the preovulatory increase or “surge” in serum LH (3–6). In rats and mice, the LH surge occurs during the late afternoon and early evening of proestrus (3, 7). The daily pattern of ovulation is thought to be reliant on the timing of this gonadotropin surge (6, 8, 9). Rhythmic LH secretion depends on the activity of pacemaker neurons in the central clock or suprachiasmatic nucleus (SCN) (10, 11), GnRH neurons in the preoptic area and positive feedback from ovarian estradiol (12). Lesions that destroy the SCN or mutations that disrupt the activity of SCN neurons are known to affect both the timing and amplitude of the LH surge, block ovulation, and disrupt reproductive cycles (13, 14).
The substrate for circadian rhythms is a feedback loop of interacting transcriptional regulators or clock genes referred to collectively as the molecular clock. The core pacemaker includes the transcriptional activator BMAL1, its binding partner CLOCK and the repressors PERIOD (Per1–Per3) and CRYPTOCHROME (Cry1 and Cry2) (15). Rhythms of Bmal1 expression are maintained by the balanced activity of the transcriptional activator RORα and the repressor REV-ERBα, both regulated by the BMAL1:CLOCK complex (16, 17). Mutations altering or abolishing clock gene expression, especially Bmal1, have considerable negative impacts on fertility (1, 2, 18, 19). Although each tissue of the hypothalamo-pituitary-ovarian (HPO) axis is composed of cell-autonomous circadian oscillators, physiological function has been best described in the pituitary gland (20–22) and ovary (23). Clock function in the ovarian follicle has been linked to folliculogenesis, with rhythms of clock gene expression only appearing during the late stages of follicular maturation (preantral-antral; Ref. 24, 25). Rhythms of clock gene expression in the follicle are dependent on FSH and proliferation of gap junction proteins (26, 27) during follicular maturation. In granulosa cells (GCs), the clock drives rhythms of clock-controlled gene expression, including the LH receptor (LHR) (Lhcgr), prostaglandin synthase (Ptgs2 or Cox2), and steroidogenic factors like steroidogenic acute regulatory protein (StAR) and 3β-hydroxysteroid dehydrogenase (3β-HSD) (27–32). Less evidence has accrued for clock function in theca cells (TCs), although rhythms of clock gene expression have been reported. The ovarian clock is also critical for maintaining adequate progesterone (P4) secretion from luteal cells (18, 30) with both global (18) and targeted (30) deletion of the Bmal1 locus having been shown to impair uterine implantation in mice.
Growing evidence suggests that the ovarian clock plays a role in the timing of ovulation. We have reported phasic sensitivity to gonadotropins in the ovary (33) and that the timing of ovulation depends in part on the timing of this period of increased sensitivity (34). Equine LH (eLH), given to rats after suppression of endogenous hormone with a systemic GnRH receptor antagonist, produces a light-entrained circadian rhythm of oocyte release marked by a nighttime peak of sensitivity (34). Thus, the ovarian clock may dictate, either in concert with the SCN or independently, the timing of ovulation by regulating sensitivity of the preovulatory follicle to gonadotropins. However, these experiments failed to adequately define the influence of temporal cues that originate in the SCN. It is possible that rhythmic patterns of systemic hormone secretion (eg, corticosterone, leptin, etc.,) or inputs from descending autonomic nerves (parasympathetic nerves) convey timing cues to the ovary and singularly drive the rhythm of sensitivity to gonadotropins (35). To address this notion we developed transgenic mice with targeted deletion of the Bmal1 gene in specific cellular compartments of the ovarian follicle. Female mice expressing Cyp17-iCre (TCs) or Cyp19-Cre (GCs) transgenes were bred with mice homozygous for floxed alleles of the Bmal1 locus to generate ovary cell-specific conditional knockout (KO) mice (Cyp17-Cre;Bmal1flx/flx [TCKO] and Cyp19-Cre;Bmal1flx/flx [GCKO]). We hypothesized that animals with conditional KO of Bmal1 would be subfertile and display a marked change in the daily rhythm of sensitivity to gonadotropins. We predicted that this effect would be the result of circadian disruption within a specific cell-type in the ovary, most likely resulting in altered expression of clock-controlled genes important for phasic sensitivity to gonadotropins. Our results reveal that the clock in ovarian TCs regulates the timing of clock-controlled genes, including the LHR, and plays a considerable role in the timing of ovulation by maintaining a stable rhythm of ovarian sensitivity to gonadotropins.
Materials and Methods
Animal welfare assurance
All experiments and procedures were conducted according to the National Institutes of Health Guidelines for the Care and Use of Animals and were approved by the University Committee for Animal Resources at the University of Rochester School of Medicine and Dentistry.
Experimental animals
For our initial ovulation timing experiments, we used adult or juvenile (21–31 d old) female C57BL6/J mice. Ovarian cell type-specific Bmal1 knockout mice were developed in our laboratory by combining existing strains of cell type-specific CRE-expressing mice with mice carrying floxed alleles of the Bmal1 locus (generated by Dr Christopher Bradfield, University of Wisconsin-Madison) provided to us by Dr Joseph Takahashi (University of Texas Southwestern Medical Center). Briefly, Cyp17-iCRE (17α-hydroxylase-CRE; TC specific, provided by Dr Cheymong Ko, University of Illinois) (36, 37) and Cyp19-CRE (aromatase-CRE; GC specific, provided by Dr JoAnne S. Richards, Baylor College of Medicine) (38) males were bred with Bmal1flx/flx females to generate heterozygous Cyp17 or Cyp19-Cre+/−;Bmal1flx/− offspring. These mice were then bred to homozygosity for the floxed Bmal1 alleles. For the generation of experimental mice male carriers of the Cyp17/19-Cre transgenes that were homozygous for floxed Bmal1 were then bred to Cre null;Bmal1flx/flx females to generate confirmed Cyp17/19-Cre+/−;Bmal1flx/flx mice. For all experiments we used female Cyp17-Cre+/−;Bmal1flx/flx (referred to as TCKO mice), Cyp19-Cre+/−;Bmal1flx/flx (referred to as GCKO mice) or littermate controls (LCs) (Cre-null;Bmal1flx/flx; referred to as LC throughout). All mice were generated on a congenic C57BL6/J background.
Generation of transgenic fluorescent reporter mice and ovarian morphological analysis
To confirm the cell type specificity of the GCKO and TCKO mice both strains (GCKO [Cyp19cre/−;Bmal1flx/flx] and TCKO [Cyp17cre/−;Bmal1flx/flx]) were bred with B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J transgenic reporter mice (referred to as TOM-GFP mice) purchased from The Jackson Laboratory (stock number 007676). TOM-GFP mice possess floxed alleles of the membrane targeted tdTomato (mT) cassette and express red fluorescence in all cells and tissues. In cells expressing CRE recombinase the mT cassette is deleted allowing for free expression of the mG or eGFP locus just downstream. A combined ROSA and β-actin promoter drives the fluorescent reporters leading to enhanced membrane-associated fluorescent signal. For histological analyses tissues including the ovary, liver and pituitary gland from adult (3–4 mo old) transgenic mice (TCKO;TOM-GFP or GCKO;TOM-GFP) were recovered in 10% neutral-buffered formalin and fixed in the dark for 48 hours. Tissues were encased in low melting point agarose at 37°C (8% in HEPES-buffered sterile saline and sectioned with a vibratome at 500-μm thickness). Whole mounts of fixed tissue were placed on a slide with HEPES-buffered sterile saline, cover slipped, and imaged at ×5 with a Nikon C-DSD115 fluorescence compound microscope (Nikon USA).
To confirm normal ovarian morphology in transgenic mice ovaries were recovered from 3- to 4-month-old adult GCKO and TCKO mice, fixed in 10% Neutral Buffered Formalin, paraffin embedded, sectioned at 5-μm thickness, and stained with hematoxylin/eosin (H+E) (University of Rochester Pathology Core). Brightfield images of H+E-stained ovary tissue from GCKO and TCKO mice were taken at 5× magnification with a Zeiss Primo Star compound microscope (Carl Zeiss, Inc). To determine whether follicular development is altered in TCKO mice, we measured ovarian morphology in 4-week-old equine gonadotropin (eCG)-primed LC and TCKO mice as described in (39). Brightfield images of H+E-stained ovarian tissue (5-μm sections every 30 μm) from LC and TCKO mice were used to count structures including primordial follicles, primary follicles, preantral follicles, antral follicles, atretic follicles, and corpora lutea across the entire rostro-caudal extent of the tissue. A labeling system was developed in MS Paint (Microsoft) to track and record the structures and data were expressed as percentage of the total structures counted. By recording images and carefully examining the image sequence we were able to avoid duplicate counts. Total ovarian area was also estimated by setting the scale for ovary cross-section images in ImageJ after micrometer scope calibration. A polygon tool was used to trace each section and the area measurement was calculated by ImageJ and integrated into mm2. The distance between cross-sections and the number of cross-sections were multiplied by this value to approximate a prism model of the ovary.
Measuring ovarian sensitivity to gonadotropins in vivo with an acute ovulation induction assay
We measured ovarian sensitivity to LH with our acute ovulation induction assay as previously described with slight modification (34). This approach allowed us to measure the acute and time-dependent response of the ovarian follicles in vivo to exogenous LH with (adults) or without (juvenile mice) necessary suppression of the endogenous LH and FSH surges. Regardless of age and previous treatment this approach relies on examination of the oviducts 14–16 hours after acute treatment with a rupture-inducing injection of eLH. In this method, the animal is treated only once with eLH and euthanized for oocyte counting, thus precluding repeated measurements. Adult mice (>60 d old) were housed in running wheel cages and reproductive cycles were monitored by vaginal lavage for a minimum of 14 days before treatment (see Supplemental Materials and Methods). Mice received a single injection of the GnRH receptor antagonist Cetrotide (CET; AEterna Zentaris) (500 μg; im under light isoflurane anesthesia) at zeitgeber time (ZT)5 (ZT is in reference to the 12:12 L:D cycle, where ZT0 = time of lights on) on the morning of proestrus. After CET treatment, groups of mice were treated with a single injection of eLH (200 IU/mouse in 100-μL sterile saline, ip) or 100 μL of sterile saline vehicle every 3 hours from ZT12 (time of lights off) on proestrus to ZT9 on estrus (the next day). For experiments in constant darkness (DD) mice of the same age were released into DD and injected with CET at circadian time (CT)5 (CT is a measure dependent on the activity of the animal, CT12 = onset of the activity period) on proestrus followed by eLH at CT12, CT18, CT24/0, and CT6 (the midpoint of the inactivity period) the next day (estrus). In both experiments, mice were euthanized 14–16 hours after eLH treatment, and the oviducts were removed followed by recovery and counting of cumulus oocyte complexes (COCs).
Juvenile mice were weaned on postnatal day (PND)23 and transferred to a wheel running cage. For experiments in a 12:12 L:D cycle, animals were injected with eCG (5 IU ip in sterile saline; Sigma) between ZT11.5 and ZT12 on PND29. Mice were then injected 37 hours later at ZT0.5–ZT1 with CET (500 μg im under light isoflurane anesthesia) followed by eLH (200 IU ip) or saline vehicle every 3 hours from ZT12 on to ZT9 the next day. For experiments in DD, mice were released into DD on PND25, primed with eCG on PND29, and treated with CET (500 μg im) at CT0.5 on PND31. Mice were then treated with eLH on PND31 at CT12, CT18, CT24/0, and CT6.
For experiments with transgenic mice carrying cell type-specific deletion of Bmal1 in the ovary, we repeated the experiment in adult LC, GCKO, and TCKO mice maintained under a 12:12 L:D cycle. Mice were treated with CET at ZT5 on proestrus followed by eLH (200 IU) at ZT18 or ZT6 on the next day (estrus). As before, mice were euthanized and oviducts were recovered for COC counts 14–16 hours later. Because we determined that the rhythm of ovarian sensitivity occurred in eCG-primed juvenile mice with a similar phase as cycling adults on proestrus we conducted subsequent experiments on juvenile-primed LC, GCKO, and TCKO mice. Because prepubertal mice fail to produce a significant preovulatory LH or FSH surge, and we are providing these mice with exogenous chorionic gonadotropins to stimulate follicular growth, we opted not to provide CET-mediated suppression to this group. This approach allowed us to eliminate the confounding effect of attenuated gonadotrophic support. Juvenile (24–30 d old) LC, GCKO, and TCKO mice were primed with eCG at ZT11.5–ZT12 as above and treated with eLH (200 IU ip) beginning 36 hours later every 3 hours from ZT0 to ZT18. Mice were sacrificed 14–16 hours after treatment and COCs were recovered and counted.
Quantitative real-time PCR analysis of ovarian clock gene and clock-controlled gene mRNA abundance in the ovary
Reproductive cycles were monitored in adult TCKO, GCKO, and LC mice for a minimum of 17 days and only mice with 2 confirmed cycles were included in our analysis. Animals were euthanized on proestrus at ZT0, ZT6, ZT12, and ZT18 and on estrus at ZT24/0 (ZT0 = lights on). Ovarian tissue was recovered and frozen in liquid nitrogen. After extraction with TRIzol (Sigma) 250 ng of total RNA was used for cDNA synthesis using the Maxima First Strand cDNA Synthesis kit with DNase treatment (Thermo Scientific). Quantitative real-time PCR was carried out using the Luminaris Color HiGreen qPCR Master Mix (Thermo Scientific) according to the manufacturer's instructions. Samples were analyzed with gene-specific primers for core clock genes (Bmal1, Clock, Cry1, Per1, and Rev-erbα) and clock-controlled genes, including the LH (Lhcgr) and FSH receptor (Fshr) (see Supplemental Table 1 for primer sequences). β-Actin was included as a housekeeping gene. Primer sequences were drawn from the Harvard/Massachusetts General Hospital Center for Computational and Integrative Biology PrimerBank database (pga.mgh.harvard.edu) and were independently verified by NCBI BLAST sequence analysis. Data are presented as change in mRNA abundance over time and were analyzed using the ΔΔCT threshold cycle method. Data were normalized to the housekeeping gene β-actin and analyzed relative to the level of mRNA abundance in tissues from LC mice.
Data analysis
For in vivo ovarian sensitivity assays the number of COCs as a function of time were analyzed with one-factor ANOVA followed by Neuman-Keuls post hoc tests. Data were also analyzed as a function of time [times genotype and their interaction with a two-factor ANOVA followed by Bonferroni post hoc comparisons. Absolute levels of LH at ZT12 on proestrus after CET-mediated suppression were analyzed with an unpaired t test. The percentage of ovarian structures was analyzed as a function of genotype [times structure with two-factor ANOVA followed by Bonferroni post hoc tests. Ovarian tissue volume was analyzed as a function of genotype using an unpaired student t test. The dose-response effects of eLH on proestrus were analyzed as a function of dose × time with a two-factor ANOVA followed by Bonferroni post hoc tests. Rhythms of serum LH and P4 were analyzed as a function of time [times genotype and their interaction with a two-factor ANOVA followed by Bonferroni post hoc tests. Rhythms of relative mRNA abundance were analyzed with CircWave (R. A. Hut, University of Groningen, The Netherlands) nonlinear regression software to test for significant rhythmicity and determine the “center of gravity” or peak of mRNA abundance. CircWave is an F-tested forward harmonic regression process that automatically detects the number of suitable harmonics using F-test criterion and is a well-established method for statistical confirmation of rhythmicity (40). CircWave analyses were used as a statistical criterion for referring to a give data series as being rhythmic. Quantitative PCR data were also analyzed with two-factor ANOVA followed by Bonferroni post hoc tests for pairwise comparison with confirm between genotype effects. With the exception of CircWave all other plotting and statistics were done using GraphPad Prism software (GraphPad). All data are presented as mean ± SEM and the threshold for significance was set at P < .05.
Results
Circadian rhythms of ovarian sensitivity to LH in adult C57BL6/J mice
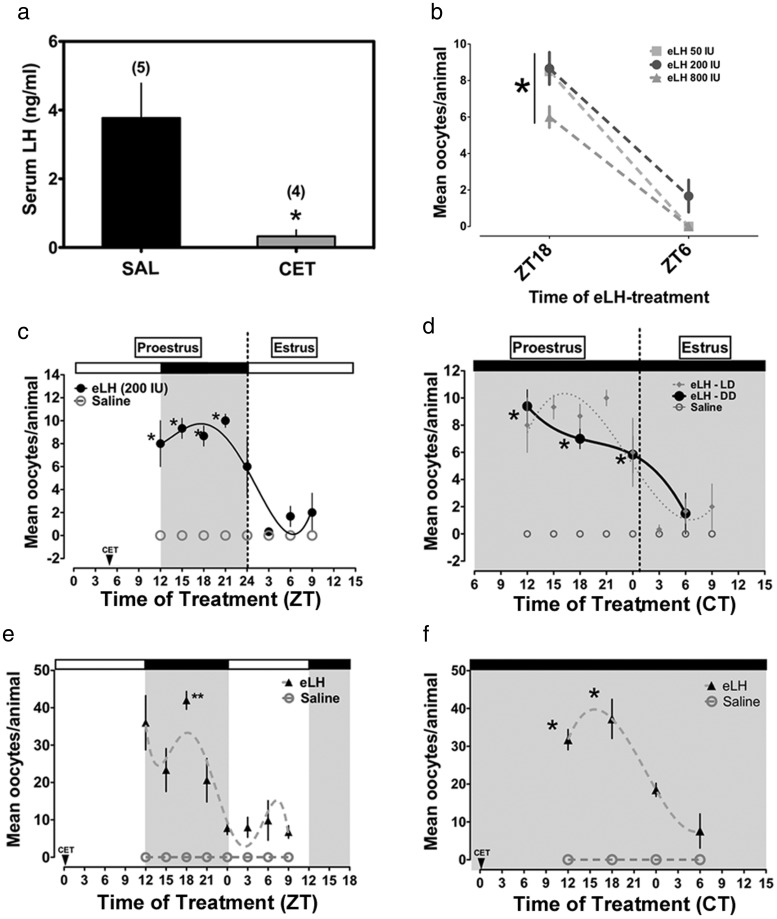
We have previously reported a light-entrained and free-running rhythm of ovarian sensitivity to eLH in female rats after suppression of endogenous LH (34). We observed an increased percentage of ovulating rats and COCs recovered during the night (ZT12–ZT24) and subjective night (CT12–CT24). We reasoned that this rhythm depends on the activity of the clock in cells of the ovarian follicle. To examine the role of the ovarian clock in the timing of ovulation in mice it was first necessary to confirm a rhythm of sensitivity to LH in cycling female mice. To that end we applied our acute ovulation induction assay with slight modification. As we have reported in rats, circulating LH levels were suppressed in mice after a single injection of Cetrorelix depot on the late morning of proestrus (Figure 1A). We next verified the dose-response to exogenous gonadotropins by injecting cycling, CET-treated mice with eLH (50, 200, or 800 IU) on proestrus at ZT18 or the next day at ZT6 (estrus). As expected, mice showed a greater response at ZT18 on proestrus, regardless of dose (Figure 1B).
Figure 1. Circadian rhythms of ovarian sensitivity to phasic eLH treatment in adult and juvenile eCG-primed C57BL6/J mice.
A, Serum levels of LH at ZT12 on proestrus were suppressed by treatment with CET (n = 4; *, P < .05 vs saline [n = 5]). B, Dose-response curve for eLH treatment. All 3 doses (50, 200, or 800 IU; n = 3–4 per treatment) stimulated more oocyte release at ZT18 (*, P < .001). C, Rhythm of ovarian sensitivity to eLH in adult C57BL6/J mice (n = 3–6 per treatment time; *, P < .05 vs ZT3, ZT6, and ZT9). D, Free-running circadian rhythm of sensitivity to LH in C57BL6/J mice (*, P < .05 vs ZT6; n = 3–6 per treatment time). E, A diurnal rhythm of sensitivity to eLH in juvenile eCG-primed mice (**, P < .001 vs ZT0–ZT9 on estrus, n = 3–6 per treatment time). F, A circadian rhythm of responsiveness to eLH in juvenile-eCG-primed mice housed in DD (*, P < .05 vs the nadir at CT6 on estrus, n = 3–4 per treatment time). In F, the time of CET injection (CT5) is not shown due to scaling of the x-axis. In C–F, saline failed to stimulate ovulation. Data are presented as mean ± SEM and fit to a nonlinear regression (see Materials and Methods). The shaded areas in C–F indicate the dark phase.
As in female rats, adult female C57BL6/J mice displayed significant diurnal rhythms of ovulation in response to phasic eLH (Figure 1D). One-factor ANOVA confirmed a significant effect of time (F = 7.07, df = 7, P < .001) with peak oocyte release in response to eLH given between ZT12 and ZT21 (P < .05 vs ZT3–ZT9). This peak was followed by a precipitous decline in oocyte release in response to eLH by ZT3 on estrus (Figure 1C). Although not reaching significance, there appears to be a slight increase in the response to eLH at ZT9 (Figure 1C). We also observed a significant rhythm of induced ovulation in mice maintained in DD for at least one full cycle (F = 6.43, df = 3, P < .05). Similar to the data from entrained mice, those free-running in DD showed a peak ovulatory response to eLH between CT12–CT24 followed by a considerable decline by CT6 the next day (estrus) (Figure 1D).
Circadian rhythms of sensitivity to eLH in juvenile gonadotropin-primed C57BL6/J mice
Evidence suggests that clock function depends on gonadotropin-stimulated follicular maturation (24, 26). Thus, we hypothesized that the timing of ovarian sensitivity to gonadotropins set by the ovarian clock is an innate property of the mature ovarian follicle. To address this hypothesis we examined the rhythm of induced ovulation in juvenile eCG-primed female mice. As in adult cycling mice, we observed a robust rhythm of ovarian sensitivity to eLH (F = 8.53, df = 7, P < .0001). This rhythm peaked during the dark phase at ZT18 (P < .05 when compared with ZT0–ZT9 on estrus) (Figure 1E). We also observed a rhythm of ovarian sensitivity to eLH in juvenile eCG-primed mice maintained in DD for several days before eLH treatment (F = 12.10, df = 3, P < .001) (Figure 1F). As in adult cycling mice, oocyte release in response to eLH peaked during the subjective night between CT12 and CT18 in juvenile eCG-primed mice (P < .05 when compared with CT0 and CT6 the next day) (Figure 1F).
Conditional KO of Bmal1 in ovarian TCs abolishes the rhythm of ovarian sensitivity to eLH
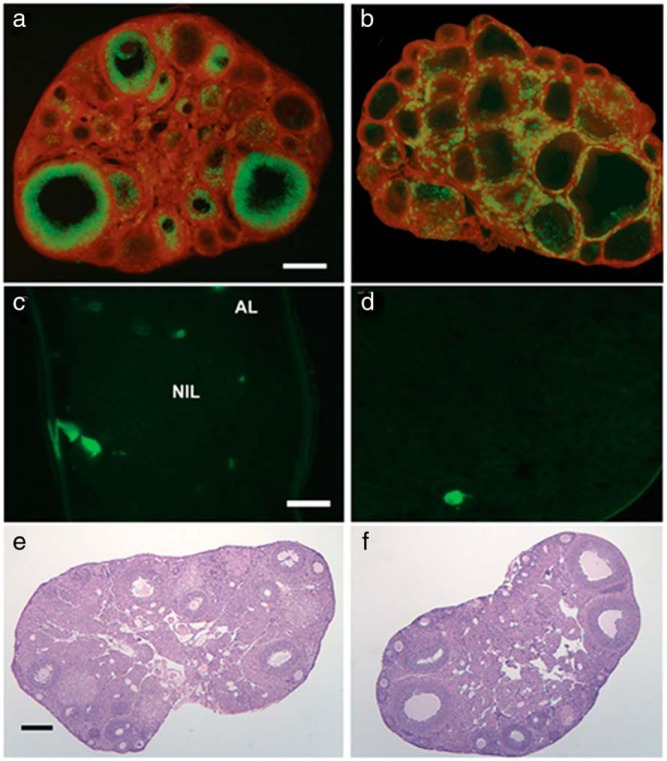
Our data suggest that the ovarian clock may play a role in the timing of ovulation by setting a window of sensitivity to gonadotropins. We hypothesized that this critical period of sensitivity depends on rhythms of clock-controlled gene expression and enzyme activity in one (or both) of the primary endocrine cell compartments of the follicle (23). Admittedly, it is also possible that this window of sensitivity is determined by inputs from the SCN, of either a neural or humoral nature (23). In an effort to address this hypothesis we generated ovarian cell type-specific conditional Bmal1 KO mice to selectively disturb clock function in the 2 primary endocrine cell compartments of the ovarian follicle (GCs and TCs). The cell-type specificity of Bmal1 deletion was confirmed by breeding GCKO and TCKO mice with a fluorescent reporter strain wherein every cell normally expresses membrane associated tdTomato but only express enhanced Green Fluorescent Protein in the presence of CRE recombinase (TOM-GFP mice). As shown in Figure 2, expression of CRE-driven Green Fluorescent Protein was limited to GCs (both mural and cumulus) lining the inside of the follicle in GCKO mice (Figure 2A) and TC/stromal cells (SCs) on the outside border of the follicle and the interstitial space in TCKO mice (Figure 2B). Signal in TCKO mice appears sparse due to the less compact and defined distribution of TCs/SCs in the ovary (37). Imaging of tissue sections from the pituitary gland (Figure 2C) and the liver (Figure 2D) confirm that CRE expression was largely confined to the ovary, although some cells did express CRE recombinase in these tissues (<5% of those observed; GCKO tissues shown). This pattern of expression is nearly identical to that originally reported in both Cyp17-iCre and Cyp19-Cre mice (36–38). These data suggest that CRE-driven deletion of the floxed Bmal1 alleles occurred only in the TC/SC or GC compartments of the ovarian follicle.
Figure 2. Targeted deletion of Bmal1 in specific cellular compartments in the ovary of GCKO and TCKO mice.
Targeted deletion of Bmal1 in granulosa (GCKO) and theca/stromal (TCKO) cells of the ovary using the CRE:LOX system. Transgenic GCKO (Cyp19-Cre;Bmal1flx/flx) and TCKO (Cyp17-Cre;Bmal1flx/flx) mice were crossed with TOM-GFP mice to produce TCKO-TOM-GFP and GCKO-TOM-GFP transgenic mice (see Supplemental Methods). As shown in A, CRE-driven eGFP expression in GCKO-TOM-GFP is nearly completely limited to the mural and cumulus GC layer with little to any GFP expression detected in the TC/SC compartment or the ovarian surface epithelium (OSE). B, In TCKO-TOM-GFP mice, CRE-driven GFP is more dispersed and spotty in the interstitial cells and limited the TC layer of larger antral follicles with no signal detected in the GC compartment or surface epithelium. Representative images of whole mount (C) pituitary gland and (D) liver from a GCKO mouse. A small number of cells in these tissues from both transgenic strains were positive for GFP (<5%). Scale bars: 0.25 μm shown in A (A and B), 0.05 mm shown in C (C and D), and 0.25 mm shown in E (E and F). Images are representative of 3–4 mice per genotype (LC, GCKO, and TCKO).
Ovarian tissue from adult GCKO and TCKO mice failed to reveal any gross morphological abnormalities (Figure 2, E and F). We confirmed this conclusion with a more detailed analysis of ovarian morphology in juvenile eCG-primed LC and TCKO mice (Supplemental Figure 1). We detected a significant effect of structure analyzed (F = 172.9, P < .001) and an interaction between structure [times genotype (F = 5.55, P < .05), but we did not detect a significant effect of genotype alone (F = 3.04 × 10−7, P > .05). When compared with LCs, the percentage of primordial follicles (P < .05) was reduced and the percentage of preantral follicles (P < .01) was increased in TCKO mice. The remaining structures, including antral and atretic follicles, were not affected in TCKO mice. Most importantly, we did not detect a difference in the number of CL and the overall tissue volume between TCKO and LC mice (Supplemental Figure 1B).
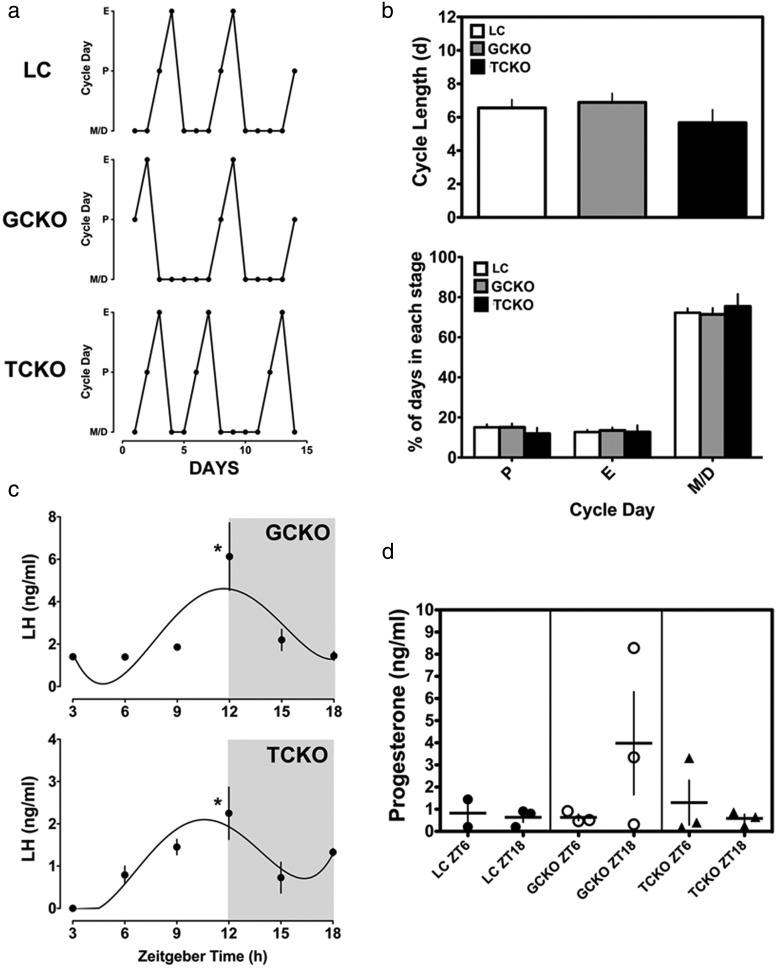
It is notable that we did not detect significant eGFP signal in the SCN of TCKO or GCKO mice, indicating that CRE-mediated deletion of Bmal1 did not disturb central clock function (data not shown). This assertion is supported by the observation that both GCKO and TCKO mice maintained normal, light entrained rhythms of locomotor activity when compared with LCs (Supplemental Figure 2A). Moreover, BMAL1 staining within SCN neurons was evident in both GCKO (Supplemental Figure 2B, top) and TCKO (Supplemental Figure 2B, bottom) mice. Also in agreement with the lack of an influence of the ovarian KO on central clock function, we found that reproductive cycles (Figure 3, A and B), serum LH levels (Figure 3C), and serum P4 levels (Figure 3D) on proestrus were normal in both GCKO and TCKO mice.
Figure 3. Reproductive cycles, serum LH, and serum P4 levels on proestrus are not altered by conditional Bmal1 deletion in the ovary of GCKO and TCKO mice.
A, Representative reproductive cycle graphs from LC, GCKO, and TCKO transgenic mice. B, Analysis of (top) cycle length and (bottom) percentage of time spent in each day of the cycle reveals no significant difference between LC, GCKO, and TCKO transgenic mice (n = 9 per group). C, Serum LH measured on proestrus from ZT3–ZT18 reveals that both GCKO and TCKO transgenic mice had appreciable surges of serum LH with both rhythms peaking at ZT12 (*, P < .05, n = 3–4 samples per time point). D, Levels of serum P4 at ZT6 and ZT18 on proestrus were not significantly affected by targeted deletion of Bmal1 in either GCKO or TCKO transgenic mice (n = 3 samples per time point except for ZT6 in LC mice [n = 2]). Data are labeled according to genotype and time of serum collection. All data are presented as mean ± SEM.
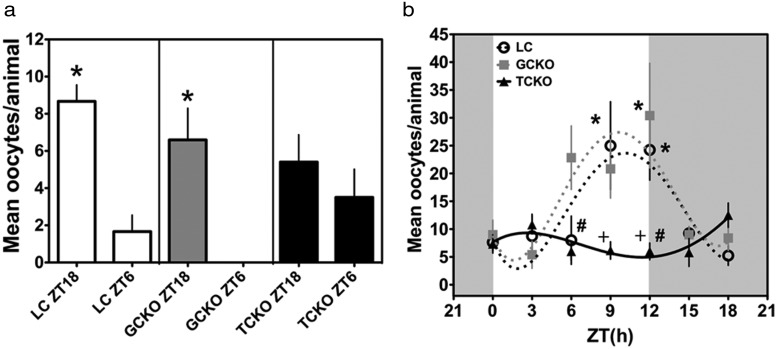
To determine the effects of targeted molecular clock disruption in ovarian follicular cells, we applied our acute ovulation induction assay to adult female GCKO, TCKO, and LCs. As shown for adult female C57BL6/J mice (see Figure 1C), we found a rhythm of ovarian sensitivity to eLH in LC mice characterized by greater oocyte release in response to eLH given at ZT18 when compared with a basal response at ZT6 (P < .05) (Figure 4A, left panel). We detected a similar pattern of ovarian sensitivity in GCKO mice, although the amplitude was diminished (P < .05 ZT18 vs ZT6) (Figure 4A, middle panel). Notably, we did not detect a day-night difference of oocyte release in response to eLH in TCKOs (P > .05 ZT18 vs ZT6) (Figure 4A, right panel). Analysis of ovarian sensitivity in juvenile eCG-primed mice provided additional confirmation of our finding in adult cycling mice. In both GCKO (F = 3.57, df = 6, P < .01) and LC (F = 3.77, df = 6, P < .01) mice we detected a significant diurnal rhythm of ovarian sensitivity to eLH with peaks at ZT12 and ZT9, respectively (Figure 4B). However, we did not observe a significant rhythm of ovarian sensitivity to eLH in TCKO mice (F = 2.02, df = 6, P = .09). Comparisons with two-factor ANOVA (time [times genotype; time, F = 4.99, df = 6, P < .001 and genotype, F = 6.62, df = 2, P < .01) reveal that peak oocyte release in response to exogenous LH was blunted in TCKOs relative to LCs (P < .05 at ZT9 and ZT12) and GCKO (P < .05 at ZT6 and ZT12) mice (Figure 4B). In agreement with the marked decline in rhythmic sensitivity to gonadotropins in TCKO mice, we found these mice to be subfertile relative to both GCKO mice and LCs (see Table 1). TCKO mice produced fewer viable litters (only 56% of crosses with a nonsibling Cre null:Bmal1flx/flx males) and approximately half as many pups per litter as LC and GCKO mice (TCKO 3.3 ± 1.8 vs GCKO 5.7 ± 2.2 and LCs 6.4 ± 1.8, P < .05 by ANOVA) (Table 1).
Figure 4. Phasic sensitivity to LH is disrupted in adult and juvenile eCG-primed conditional Bmal1 TCKO mice.
A, Rhythm of sensitivity to eLH in cycling adult LC (n = 3; both ZT18 and ZT6), GCKO (ZT18 n = 5, ZT6 n = 4), and TCKO (ZT18 n = 5, ZT6 n = 6) mice. Both LC and GCKO transgenic mice displayed strong diurnal rhythms of eLH sensitivity with peak responses to eLH treatment at ZT18 (*, P < .05 vs ZT6) for both. In contrast, adult cycling TCKO mice did not maintain a rhythm of sensitivity to eLH (P > .05 ZT18 vs ZT6). B, Diurnal rhythms of sensitivity to LH in juvenile-eCG-primed LC (n = 4–8 per time of treatment), GCKO (n = 5–6 per time of treatment), and TCKO (n = 4–6 per time of treatment) mice. Both LC (open circles) and GCKO (gray squares) mice displayed diurnal rhythms of responsiveness with significant peaks of responsiveness to eLH treatment at ZT9–ZT12 (*, P < .05 vs troughs at ZT0, ZT3, and ZT18). TCKO mice (black triangles) did not show a significant rhythm in response to phasic eLH. Data are presented as mean ± SEM and in B data are fit to a nonlinear regression (see Materials and Methods). In B, # indicates difference between TCKO and GCKO, and + indicates a significant difference between TCKO and LC mice.
Table 1.
Fertility of Targeted Bmal1 KO Mice
| Genotype | Dams | Average Matings per Dama | % Viable Litters (Total) | Mean (SEM) Pups/Litter |
|---|---|---|---|---|
| LC | 12 | 2 | 88% (24) | 6.4 (1.8) |
| GCKO | 12 | 1.6 | 94% (18) | 5.7 (2.2) |
| TCKO | 12 | 1.2 | 56% (16) | 3.3 (1.8)b |
Mated with Cre null;Bmal1flx/flx non-sibling male.
P < .05 by ANOVA vs LC and GCKO.
Altered rhythms of clock gene and clock-controlled mRNA abundance in the ovaries of conditional Bmal1 knockout mice
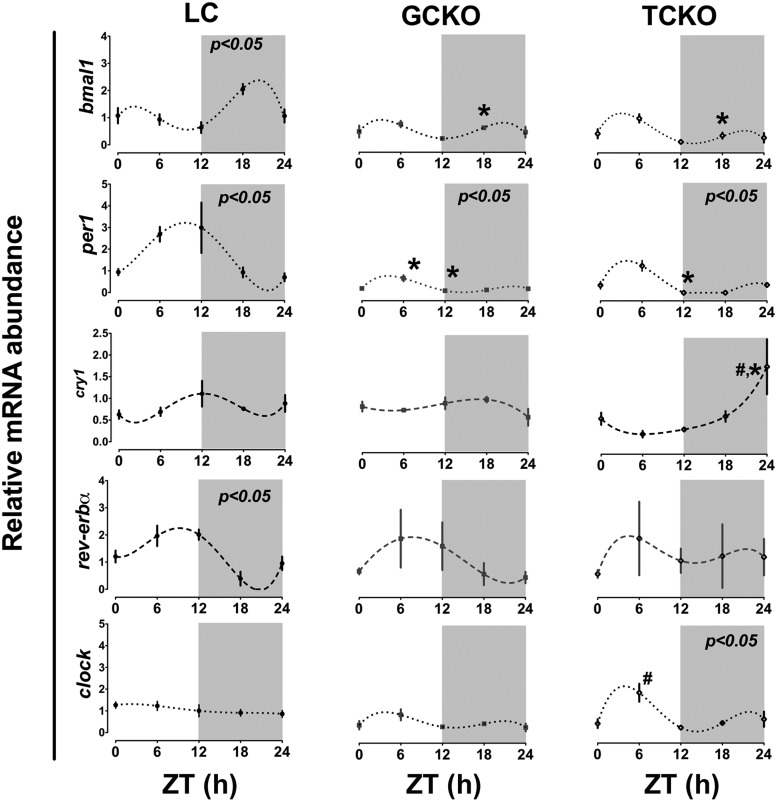
We hypothesized that the timing of ovarian sensitivity to gonadotropins depends on rhythms of CCG expression in follicular cells. In particular we had predicted, based on the literature and on our own work that GCs were the primary “gonadotropin-sensitive” oscillator in the follicle (23). Thus, we predicted that clock-controlled gene expression in GCs, including the rhythmic expression of LHRs, was critical for the maintenance of phasic sensitivity to gonadotropins. To our surprise, our data reveal that the timing of ovulation may depend more on clock function in the ovarian TC (Figure 4 and Table 1). To better define the role of the molecular clock in each ovarian cell type, we measured the abundance of clock and clock-controlled gene mRNA in ovaries recovered from GCKO, TCKO, and LC mice every 6 hours from ZT0 to ZT24 on proestrus. As shown in Figure 5, we detected diurnal rhythms of clock gene mRNA abundance, including Bmal1, Per1, and Rev-erbα (all P < .05 by CircWave as denoted in Figure 5) in ovaries from LC mice. In LC mice Bmal1 mRNA levels peaked in the late portion of the dark phase (ZT21.55 ± 1.6). This rhythm of Bmal1 expression was attenuated in both GCKO and TCKO mice (both P > .05 by CircWave) (Figure 5). Two-factor ANOVA (genotype × time; genotype F = 26.35, df = 2, P < .0001 and time F = 6.87, df = 4, P < .001) confirmed a significant decline in Bmal1 expression at ZT18 in both GCKO (P < .001) and TCKO (P < .001) mice compared with the same time in LCs. Further, the center of gravity or “peak” of Bmal1 mRNA abundance was delayed in the ovaries of both GCKO (ZT0.74 ± 1.6) and TCKO (ZT3.25 ± 1.5) mice. Per1 mRNA abundance was also rhythmic in the ovary from LCs, with a peak in the middle of the light phase (ZT8.49 ± 1.67). This rhythm persisted (P < .05 by CircWave) in targeted KO mice although the rhythm was slightly phase advanced and attenuated relative to LCs (GCKO mice, ZT4.13 ± 1.4; TCKO mice, ZT4.44 ± 0.96) (Figure 5). Again, two-factor ANOVA (genotype F = 25.92, df = 2, P < .001 × time F = 6.71, df = 4, P < .001) confirmed a significant decline in ovarian Per1 mRNA levels in both GCKO and TCKO mice near the peak (ZT6–ZT12) of Per1 mRNA abundance in LC mice (P < .05 for both). CircWave analysis did not detect a significant rhythm of Cry1 mRNA abundance in our LC or KO mice. Like Per1, Rev-erbα mRNA abundance was also rhythmic in LC mice with a peak at midday (ZT5.64 ± 1.7) followed by a nadir in the latter portion of the dark phase as determined by CircWave. As expected, the peak of Rev-erbα mRNA level preceded (and was nearly antiphase to) the peak of Bmal1 abundance. We did not detect a statistically significant effect of genotype (F = 0.23, df = 2, P = .80) or time (F = 1.96, df = 4, P = .13) on Rev-erbα mRNA abundance, due largely to an apparent increase in variance across time points in TCKO and GCKO mice (Figure 5). Analysis with CircWave confirmed results of ANOVA, indicating an absence of rhythmicity in both conditional KO strains. We also did not see an effect of targeted Bmal1 KO on the phase of Rev-erbα mRNA abundance. Two-factor ANOVA (genotype F = 7.59, df = 2, P < .01 and time F = 6.99, df = 4, P < .001) and CircWave (P < .05) confirmed a significant rhythm of Clock mRNA abundance in TCKO mice peaking during the middle of the subjective day (ZT4.83 ± 1.51). We did not detect similar rhythms in the ovary of LC or GCKO mice.
Figure 5. Rhythms of clock gene mRNA abundance in whole ovary are disrupted in both GCKO and TCKO transgenic mice.
Abundance of clock gene mRNA in ovaries recovered from LC (n = 3), GCKO (n = 3), and TCKO (n = 3) mice on proestrus. LC mice displayed diurnal rhythms of Bmal1, Per1, and Rev-erbα mRNA abundance (P < .05 indicated in top right of each panel). Rhythms of Bmal1 and Rev-erbα mRNA abundance were disrupted in both GCKO and TCKO mice. Although still rhythmic, the absolute level of Per1 mRNA was suppressed in both GCKO and TCKO mice. Although not significant by CircWave analysis, Cry1 mRNA abundance oscillated at low amplitude with a peak near midday in LC mice and was altered in both GCKO and TCKO mice. Clock mRNA displayed a low amplitude rhythm in TCKO mice that was not detected in LC or GCKO mice. Data are presented as mean ± SEM; n = 3 samples per time point except for ZT24 in TCKO mice (n = 2). (#, P < .05 GCKO vs TCKO; *, P < .05 LC vs GCKO or TCKO). Values for target mRNA abundance were calculated using the ΔΔCT method as described in Materials and Methods with β-actin as the reference gene. Data were fit to a nonlinear regression (see Materials and Methods).
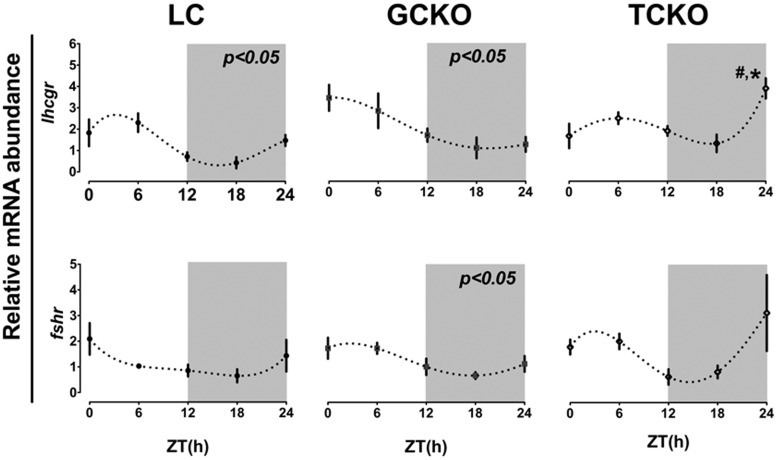
We next determined whether the altered rhythms of clock gene mRNA abundance we observed in TCKO mice resulted in irregular patterns of clock-controlled gene expression in the ovary. We measured the level of LH (Lhcgr) and FSH (Fshr) receptor mRNAs in whole ovaries collected every 6 hours from ZT0–ZT24 on proestrus. As shown in Figure 6, we detected a significant diurnal rhythm of Lhcgr mRNA abundance in LC mice that peaked during the early portion of the light phase (ZT2.4 ± 1.4) (Figure 6). Unlike the rhythm of Bmal1 mRNA abundance, the rhythm of Lhcgr expression persisted in GCKO mice with a peak close to lights on (ZT1.98 ± 1.59) (Figure 6). This rhythm was abolished in TCKO mice, as confirmed by both CircWave (P > .05) and two-factor ANOVA (genotype, F = 5.63, df = 2, P < .01 × time, F = 6.53, df = 4, P < .001). Dampening of this rhythm was associated with a decline of the daytime peak and a spike of Lhcgr mRNA levels at ZT24 in TCKO mice, which was not detected in GCKO mice or LCs (P < .05 vs both) (Figure 6). We did not detect a significant phase shift of Lhcgr expression in either GCKO or TCKO mice (when compared with LCs). Although Fshr mRNA appeared to fluctuate across the day in all 3 strains, we could only confirm a rhythm in GCKO mice (P < .05 by CircWave). As with Lhcgr, we did not detect a significant effect of genotype on Fshr mRNA abundance in the ovary in any strain at any of the time points examined (Figure 6). This result was also confirmed by two-factor ANOVA (genotype, F = 1.28, df = 2, P = .29 × time, F = 3.80, df = 4, P < .05).
Figure 6. Conditional KO of Bmal1 in TCs alters rhythms of LHR mRNA abundance in the ovary.
Rhythms of Lhcgr and Fshr expression in whole ovary tissue recovered from LC (n = 3), GCKO (n = 3), and TCKO (n = 3) transgenic mice. In both LC and GCKO mice we detected a significant diurnal rhythm of Lhcgr mRNA abundance (P < .05 for both) that was abolished in TCKO transgenic mice. There was a marked increase in Lhcgr expression at ZT24 in TCKO mice (#, P < .05) when compared with the same time point in both LC and GCKO mice. A low amplitude oscillation of Fshr mRNA expression was detected in GCKO transgenic mice with a peak in the morning (ZT1.99). Daily variation of Fshr mRNA abundance was not significant in either LC or TCKO transgenic mice. Data are presented as mean ± SEM. n = 3 ovaries from 3 different mice per time point. #, P < .05 GCKO vs TCKO; *, P < .05 LC vs GCKO or TCKO. Only those rhythms labeled directly with a P value were considered significant by CircWave analyses. Values for target mRNA abundance were calculated using the ΔΔCT method as described in Materials and Methods, with β-actin as the reference gene. Data were fit to a nonlinear regression (see Materials and Methods).
Discussion
The influence of the timing system on female reproductive physiology is well known, with the SCN playing a pivotal role in the timing of events in the hypothalamo-pituitary-ovarian axis (10, 41). Evidence has also accumulated in support of a contribution from peripheral oscillators including the ovary (42). We have previously reported a circadian rhythm of ovarian sensitivity to gonadotropins in rats which is independent of the timing of the endogenous LH surge (34). This rhythm, not surprisingly, peaks during the night. These data suggest that the ovarian clock may contribute to the timing of ovulation by setting a window of LH sensitivity. Although the follicle receives multiple timing cues from the SCN (23), logic dictates that a critical physiological system (eg, the reproductive axis) with strict temporal requirements might rely on redundant activity of central and peripheral oscillators. However, the weight of these contributions remains largely unknown (43). We applied our ovulation induction assay to mice and confirmed rhythmic sensitivity to LH, with a peak response also limited to the early/middle portion of the night when mating is likely to occur (44). These data extend our previous results and support our general hypothesis. It has been suggested that LH sensitivity is programmed during puberty and that the rhythm we observed depends almost entirely on the persistence (or absence) of gonadotrophic support. It is true that treatment with CET leads to a precipitous decline in gonadotrophic support for the developing follicle (45, 46). To address this issue we measured the timing of ovarian sensitivity in juvenile (<31 d old) mice primed with eCG. Chorionic gonadotropin has a long half-life in rodent serum ranging from 40–72 hours (47) and is used to stimulate folliculogenesis 48–72 hours before a “trigger” injection of LH (48, 49). Because gonadotropin secretion is low in the juvenile mouse this CET treatment was unnecessary, but initially included to control for the effects of blocking endogenous LH (3, 50). Juvenile eCG-primed mice displayed a rhythm of sensitivity to eLH that declined in the face of persistent gonadotrophic support for the ovarian follicles (ZT0–ZT6 on the second day after priming). Moreover, this rhythm showed peak and trough phases akin to those detected in adults, indicating that the phase of sensitivity is established early in development and may be an innate property of the mature follicle.
To identify the ovarian pacemaker responsible for this rhythm, we used the CRE-LOX system to generate ovarian cell type-specific conditional Bmal1 knockout mice. BMAL1 is a core component of the oscillator and the only single locus whose deletion leads to near complete clock disruption (17). We used CRE-driven fluorescent reporter mice to verify that CRE expression was largely restricted to the intended ovarian cell type and that BMAL1 expression in the SCN and rhythms of behavior were not affected in GCKO or TCKO mice. We found very limited expression of CRE-driven GFP outside of the ovary, including the anterior pituitary, liver (see Figure 2) and oviduct (not shown). Our findings are in agreement with the initial reports describing the development of both the Cyp17-iCre and Cyp19-Cre transgenic lines (36, 38). We found that TCKO mice were subfertile when compared with both GCKO and LC mice marked by mating difficulties and fewer offspring per litter, although most reproductive functions (eg, estrous cycles, P4, and LH levels) were unaffected. A limitation of our approach is that P4 levels were assessed on proestrous rather than metestrus or diestrus. Thus, it is possible that there is an undetected drop in P4 levels during the brief luteal phase in our TCKO and GCKO mice. Because we did not measure implantation rate (30), we cannot make strong conclusions regarding luteal P4 levels. However, the decline in offspring we observed matches the reduction in response to exogenous LH we detected in cycling TCKO mice, again supporting the conclusion that conditional Bmal1 KO in TCs affects ovulation but not luteal P4 levels or implantation.
Although we measured serum LH in both TCKO and GCKO mice, we did not directly measure this hormone in our Bmal1flx/flx; Cre null LC mice. However, the timing and amplitude of LH secretion during the mouse estrous cycle is well known and has recently been described in the literature (3, 50–52). Because we saw no gross deficits in fertility among our LC mice (Table 1) we can safely assume they produce a normal LH surge on proestrus. We also did not detect any gross deficits in folliculogenesis among TCKO mice. We did observe a slight decline in the number of primordial follicles and a modest increase in the percentage of preantral follicles. It is possible that an undetected increase in early atresia in TCKO mice or more rapid progression to the preantral stage accounts for this apparent decline in follicular reserve. Nonetheless, this does not appear to account for the decline in fertility we observed. Although able to reproduce, TCKO KO mice were less successful and produced on average half as many offspring. To directly assess the role of the clock in TCs we measured ovarian sensitivity to eLH across the 24-hour day. Both LC and GCKO mice displayed rhythmic sensitivity to eLH peaking at ZT18, but this rhythm was blunted in TCKO mice. To eliminate the confounding influences of CET treatment and a mature HPO axis we examined this rhythm in juvenile eCG-primed mice. In this experiment CET treatment was avoided to eliminate any confounding effects of the drug on Cyp19 expression in GCs (53). Juvenile eCG-primed LC and GCKO mice also displayed rhythmic sensitivity to eLH. We did not detect a rhythm of LH sensitivity in TCKO mice, suggesting that the oscillator in TCs is necessary for phasic responsiveness to LH. Because clock gene expression rhythms in the ovarian follicle appear to be linked to follicular development (ie, only more mature preantral and antral follicles express a rhythm of clock gene expression), we cannot feasibly dissociate the timing of ovarian sensitivity from the development of the follicle either across the cycle or in response to eCG-priming. Our results simply support the conclusion that the clock in the ovarian follicle, at that time when said follicles reach their peak of maturity and are ready to respond, will do so in a rhythmic fashion that is independent of any temporal cues from the SCN. This conclusion is drawn largely from our data in juvenile eCG-primed LC mice that failed to ovulate after approximately 40 hours of priming (ZT0–ZT3) and the dampened rhythm in TCKO mice primed with eCG. Further, the acute drop in ovulation in response to eLH at ZT15–ZT18 in these mice, notably only 51–54 hours after eCG priming when mature follicles are certainly still able to respond, supports our assertions.
LH, acting through its G protein-coupled receptor, initiates a cascade of cellular signaling events leading to cellular differentiation and altered gene expression (54). Previous studies have examined the daily variation in LHR gene (Lhcgr) expression in the ovary and isolated GCs (27, 30, 55). Combined, these data indicate that the clock drives rhythmic Lhcgr expression in luteinized GCs. We hypothesized that the blunted rhythm of sensitivity to LH we observed in TCKO mice was due to an attenuated rhythm of clock-controlled gene expression in TCs. We observed significant diurnal rhythms of Bmal1, Per1, and Rev-erbα mRNA abundance in ovaries from LC mice. These rhythms were suppressed in GCKO and TCKO mice, indicating that deletion of Bmal1 leads to altered or even abolished rhythms of clock gene expression in both GC and TC. We next examined the influence of Bmal1 KO on the timing of Lhcgr and Fshr mRNA abundance in the ovary. In both LC and GCKO mice we observed significant diurnal rhythms of Lhcgr mRNA abundance with peaks at ZT2.4 and ZT1.98, respectively. This rhythm was altered in TCKO transgenic mice that displayed a large increase in Lhcgr mRNA at ZT24. The altered rhythm of Lhcgr mRNA abundance we have observed in TCKO mice is likely due to the suppression of Bmal1 mRNA in these cells. Because we did not analyze gene expression directly in isolated TCs or theca interna tissue it is possible that the rhythm of Lhcgr and Fshr mRNA abundance we measured in TCKO mice is generated by GCs that are indirectly influenced by clock disruption in TCs. Further targeted analysis of the effects of Bmal1 KO in isolated GCs and TCs is warranted. In mammals the TC produces androgens that are critical for normal folliculogenesis (39, 56). The LHR is expressed at high levels in the TC wherein LH stimulates steroidogenesis through up-regulation of steroid acute regulatory protein (StAR) and Cyp17 gene expression (57, 58). LH enhances follicular development through its impacts on TC androgen secretion (59). Data from bovine and rodent models confirm considerable changes in gene expression across multiple loci in the TC after exposure to LH (60, 61). The expression of LHR is under the direct control of the molecular clock, as treatment with PER2 or CLOCK siRNA leads to down-regulation of Lhcgr expression (62). Conversely, LH and FSH can alter the timing of clock gene expression in the ovary (for review see Ref. 23). Our data suggest that suppression of ovarian sensitivity to LH in TCKO is the result of abnormal rhythms of Lhcgr expression in TCs. Although this is certainly a reasonable interpretation, there are several possible alternatives. Because we did not measure ovarian sensitivity across the entire 24-hour day or in DD it is possible that TCKO dramatically shifts the peak of sensitivity to a fine window in the late night. It is also possible, as previously alluded to, that deletion of the clock in TCs leads to indirect deficits in GC function which in turn leads to the decline in sensitivity in TCKO mice.
Although both novel and significant, our results conflict with a recent study indicating that deletion of Bmal1 in steroid-producing cells of the ovary using a steroidogenic factor 1 (SF-1)-Cre;Bmal1flx/flx transgenic model leads to marked reduction in P4 secretion and implantation failure (30). Their findings leave little doubt that deficits in implantation are due to a decline of ovarian P4 secretion. How then can we explain this discrepancy? It may be resolved by understanding differences in temporal/spatial expression of the ovary-targeted CRE drivers used. Both Cyp19 and Cyp17 mRNA expression levels vary during folliculogenesis (63, 64). Cyp19 and Cyp17 expression levels increase nearly 40-fold during follicular development, starting low in primary follicles and reaching a peak in large preovulatory follicles (63, 65). Further, evidence indicates that both Cyp17 and Cyp19 expression is dramatically down-regulated after the LH surge (66, 67). In contrast, SF-1 expression is more ubiquitously expressed with significant levels reported in primary, secondary and tertiary follicles (68–70). Hinshelwood et al reported weak SF-1 expression limited to the TC/SC compartment and exceedingly low levels of SF-1 in luteal cells, suggesting that SF-1 is not the primary regulator of P4 secretion during pregnancy (70). This finding is supported by genome wide studies showing a near 3-fold decline in SF-1 expression after luteinization (60). In contrast, others have recorded significant SF-1 signal in both ovarian cell types and confirmed that SF-1 regulates estradiol synthesis in GCs (69, 71). These data lead us to conclude that deletion of Bmal1 in ovarian follicles is enhanced in our transgenic mice during late folliculogenesis, resulting in robust circadian disruption in preovulatory follicles that is potentially silenced after luteinization.
Our data reveal, for the first time, that circadian clock function in the TC appears to be necessary and sufficient for a normal rhythm of ovarian sensitivity to gonadotropins and thus precise timing of ovulation in mice. Moreover, we have determined that this rhythm of sensitivity may be directly linked to rhythmic expression of LHR mRNA in the TC. A change in the diurnal rhythm of Lhcgr mRNA abundance is associated with an attenuated rhythm of sensitivity and an overall decline in fertility in TCKO mice. The TC is the primary androgen-producing cell in the ovary and androgen secretion from TCs represents a tipping point in the balance between normal folliculogenesis (low levels of androgen) and abnormal follicular growth (excess androgen). Our own data reveal that excess androgen exposure during sexual development, a treatment that produces a Polycystic Ovary Syndrome phenotype in mice, leads to considerable circadian disruption, irregular reproductive cycles and infertility (72, 73). Here we reveal that TCs, acting as the ovarian pacemaker for gonadotropin sensitivity, could play a critical part in the etiology of infertility due to environmental and/or genetic influences.
Acknowledgments
We thank Londyn Cullifer, Drew Phillips, and Lindsay Marchetti for technical assistance.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CET
- Cetrotide
- COC
- cumulus oocyte complex
- CT
- circadian time
- DD
- constant darkness
- eCG
- equine gonadotropin
- eLH
- equine LH
- Fshr
- FSH receptor
- GC
- granulosa cell
- H+E
- hematoxylin/eosin
- HPO
- hypothalamo-pituitary-ovarian
- KO
- knockout
- LC
- littermate control
- L:D
- light:dark
- LHR
- LH receptor
- P4
- progesterone
- PND
- postnatal day
- SC
- stromal cell
- SCN
- suprachiasmatic nucleus
- SF-1
- steroidogenic factor 1
- TC
- theca cell
- ZT
- zeitgeber time.
References
- 1. Kennaway DJ. The role of circadian rhythmicity in reproduction. Hum Reprod Update. 2005;11:91–101. [DOI] [PubMed] [Google Scholar]
- 2. Boden MJ, Kennaway DJ. Circadian rhythms and reproduction. Reproduction. 2006;132:379–392. [DOI] [PubMed] [Google Scholar]
- 3. Bronson FH, Vom Saal FS. Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology. 1979;104:1247–1255. [DOI] [PubMed] [Google Scholar]
- 4. Goldman BD. The circadian timing system and reproduction in mammals. Steroids. 1999;64:679–685. [DOI] [PubMed] [Google Scholar]
- 5. Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96:57–62. [DOI] [PubMed] [Google Scholar]
- 6. Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24:79–93. [DOI] [PubMed] [Google Scholar]
- 7. Everett JW, Sawyer CH. A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47:198–218. [DOI] [PubMed] [Google Scholar]
- 8. Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: timing is everything. Horm Behav. 2006;49:557–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM. Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod. 1997;56:303–309. [DOI] [PubMed] [Google Scholar]
- 10. de la Iglesia HO, Schwartz WJ. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147:1148–1153. [DOI] [PubMed] [Google Scholar]
- 11. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96:50–56. [DOI] [PubMed] [Google Scholar]
- 13. Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS. Vasopressin regulation of the proestrous luteinizing hormone surge in wildtype and clock mutant mice. Biol Reprod. 2006;75:778–784. [DOI] [PubMed] [Google Scholar]
- 14. Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31:147–157. [DOI] [PubMed] [Google Scholar]
- 15. Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74:246–260. [DOI] [PubMed] [Google Scholar]
- 16. Sato TK, Panda S, Miraglia LJ, et al. . A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–537. [DOI] [PubMed] [Google Scholar]
- 17. Bunger MK, Wilsbacher LD, Moran SM, et al. . Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ratajczak CK, Boehle KL, Muglia LJ. Impaired steroidogenesis and implantation failure in Bmal1−/− mice. Endocrinology. 2009;150:1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ratajczak CK, Asada M, Allen GC, et al. . Generation of myometrium-specific Bmal1 knockout mice for parturition analysis. Reprod Fertil Dev. 2012;24:759–767. [DOI] [PubMed] [Google Scholar]
- 20. Olcese J, Sikes HE, Resuehr D. Induction of PER1 mRNA expression in immortalized gonadotropes by gonadotropin-releasing hormone (GnRH): involvement of protein kinase C and MAP kinase signaling. Chronobiol Int. 2006;23:143–150. [DOI] [PubMed] [Google Scholar]
- 21. Resuehr D, Wildemann U, Sikes H, Olcese J. E-box regulation of gonadotropin-releasing hormone (GnRH) receptor expression in immortalized gonadotrope cells. Mol Cell Endocrinol. 2007;278:36–43. [DOI] [PubMed] [Google Scholar]
- 22. Resuehr HE, Resuehr D, Olcese J. Induction of mPer1 expression by GnRH in pituitary gonadotrope cells involves EGR-1. Mol Cell Endocrinol. 2009;311:120–125. [DOI] [PubMed] [Google Scholar]
- 23. Sellix MT. Circadian clock function in the mammalian ovary. J Biol Rhythms. 2015;30:7–19. [DOI] [PubMed] [Google Scholar]
- 24. Gräs S, Georg B, Jorgensen HL, Fahrenkrug J. Expression of the clock genes Per1 and Bmal1 during follicle development in the rat ovary. Effects of gonadotropin stimulation and hypophysectomy. Cell Tissue Res. 2012;350:539–548. [DOI] [PubMed] [Google Scholar]
- 25. Fahrenkrug J, Georg B, Hannibal J, Hindersson P, Gräs S. Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology. 2006;147:3769–3776. [DOI] [PubMed] [Google Scholar]
- 26. Chen H, Zhao L, Chu G, et al. . FSH induces the development of circadian clockwork in rat granulosa cells via a gap junction protein Cx43-dependent pathway. Am J Physiol Endocrinol Metab. 2013;304:E566–E575. [DOI] [PubMed] [Google Scholar]
- 27. Chen H, Zhao L, Kumazawa M, et al. . Downregulation of core clock gene Bmal1 attenuates expression of progesterone and prostaglandin biosynthesis-related genes in rat luteinizing granulosa cells. Am J Physiol Cell Physiol. 2013;304:C1131–C1140. [DOI] [PubMed] [Google Scholar]
- 28. Chen H, Chu G, Zhao L, Yamauchi N, Shigeyoshi Y, Hashimoto S, Hattori MA. Rev-erbα regulates circadian rhythms and StAR expression in rat granulosa cells as identified by the agonist GSK4112. Biochem Biophys Res Commun. 2012;420:374–379. [DOI] [PubMed] [Google Scholar]
- 29. Chu G, Misawa I, Chen H, et al. . Contribution of FSH and triiodothyronine to the development of circadian clocks during granulosa cell maturation. Am J Physiol Endocrinol Metab. 2012;302:E645–E653. [DOI] [PubMed] [Google Scholar]
- 30. Liu Y, Johnson BP, Shen AL, et al. . Loss of BMAL1 in ovarian steroidogenic cells results in implantation failure in female mice. Proc Natl Acad Sci USA. 2014;111:14295–14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alvarez JD, Sehgal A. The thymus is similar to the testis in its pattern of circadian clock gene expression. J Biol Rhythms. 2005;20:111–121. [DOI] [PubMed] [Google Scholar]
- 32. Alvarez JD, Hansen A, Ord T, et al. . The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms. 2008;23:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoshikawa T, Sellix M, Pezuk P, Menaker M. Timing of the ovarian circadian clock is regulated by gonadotrophins. Endocrinology. 2009;150:4338–4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sellix MT, Yoshikawa T, Menaker M. A circadian egg timer gates ovulation. Curr Biol. 2010;20:R266–R267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Menaker M, Murphy ZC, Sellix MT. Central control of peripheral circadian oscillators. Curr Opin Neurobiol. 2013;23:741–746. [DOI] [PubMed] [Google Scholar]
- 36. Bridges PJ, Koo Y, Kang DW, et al. . Generation of Cyp17iCre transgenic mice and their application to conditionally delete estrogen receptor α (Esr1) from the ovary and testis. Genesis. 2008;46:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee S, Kang DW, Hudgins-Spivey S, et al. . Theca-specific estrogen receptor-α knockout mice lose fertility prematurely. Endocrinology. 2009;150:3855–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fan HY, Liu Z, Cahill N, Richards JS. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol. 2008;22:2128–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24:1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hwang JW, Sundar IK, Yao H, Sellix MT, Rahman I. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J. 2014;28:176–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boden MJ, Varcoe TJ, Kennaway DJ. Circadian regulation of reproduction: from gamete to offspring. Prog Biophys Mol Biol. 2013;113:387–397. [DOI] [PubMed] [Google Scholar]
- 42. Sellix MT. Clocks underneath: the role of peripheral clocks in the timing of female reproductive physiology. Front Endocrinol (Lausanne). 2013;4:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. [DOI] [PubMed] [Google Scholar]
- 44. Davidson AJ, Menaker M. Birds of a feather clock together–sometimes: social synchronization of circadian rhythms. Curr Opin Neurobiol. 2003;13:765–769. [DOI] [PubMed] [Google Scholar]
- 45. Reissmann T, Schally AV, Bouchard P, Riethmiiller H, Engel J. The LHRH antagonist cetrorelix: a review. Hum Reprod Update. 2000;6:322–331. [DOI] [PubMed] [Google Scholar]
- 46. Dierich A, Sairam MR, Monaco L, et al. . Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95:13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith PL, Bousfield GR, Kumar S, Fiete D, Baenziger JU. Equine lutropin and chorionic gonadotropin bear oligosaccharides terminating with SO4–4-GalNAc and Sia α 2,3Gal, respectively. J Biol Chem. 1993;268:795–802. [PubMed] [Google Scholar]
- 48. Montgomery V, Loutradis D, Tulchinsky D, Kiessling A. FSH-induced ovulation in intact and hypophysectomized mice. J Reprod Fertil. 1988;84:1–6. [DOI] [PubMed] [Google Scholar]
- 49. Pakarainen T, Zhang FP, Nurmi L, Poutanen M, Huhtaniemi I. Knockout of luteinizing hormone receptor abolishes the effects of follicle-stimulating hormone on preovulatory maturation and ovulation of mouse graafian follicles. Mol Endocrinol. 2005;19:2591–2602. [DOI] [PubMed] [Google Scholar]
- 50. Bronson FH. The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology. 1981;108:506–516. [DOI] [PubMed] [Google Scholar]
- 51. Turgeon JL, Waring DW. Luteinizing hormone secretion from wild-type and progesterone receptor knockout mouse anterior pituitary cells. Endocrinology. 2001;142:3108–3115. [DOI] [PubMed] [Google Scholar]
- 52. Jayes FL, Burns KA, Rodriguez KF, Kissling GE, Korach KS. The naturally occurring luteinizing hormone surge is diminished in mice lacking estrogen receptor β in the ovary. Biol Reprod. 2014;90:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Winkler N, Bukulmez O, Hardy DB, Carr BR. Gonadotropin releasing hormone antagonists suppress aromatase and anti-Mullerian hormone expression in human granulosa cells. Fertil Steril. 2010;94:1832–1839. [DOI] [PubMed] [Google Scholar]
- 54. Espey LL, Richards JS. Ovulation. In: Neill J, ed. Physiology of Reproduction. vol 1 3rd ed St. Louis, MO: Elsevier Academic Press; 2006:425–474. [Google Scholar]
- 55. Chu G, Yoshida K, Narahara S, et al. . Alterations of circadian clockworks during differentiation and apoptosis of rat ovarian cells. Chronobiol Int. 2011;28:477–487. [DOI] [PubMed] [Google Scholar]
- 56. Leung PCK, Adashi EY. The Ovary. 2nd ed Amsterdam, Boston: Elsevier; 2004. [Google Scholar]
- 57. Orisaka M, Hattori K, Fukuda S, et al. . Dysregulation of ovarian follicular development in female rat: LH decreases FSH sensitivity during preantral-early antral transition. Endocrinology. 2013;154:2870–2880. [DOI] [PubMed] [Google Scholar]
- 58. Murayama C, Miyazaki H, Miyamoto A, Shimizu T. Luteinizing hormone (LH) regulates production of androstenedione and progesterone via control of histone acetylation of StAR and CYP17 promoters in ovarian theca cells. Mol Cell Endocrinol. 2012;350:1–9. [DOI] [PubMed] [Google Scholar]
- 59. Sen A, Prizant H, Light A, et al. . Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci USA. 2014;111:3008–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Christenson LK, Gunewardena S, Hong X, Spitschak M, Baufeld A, Vanselow J. Research resource: preovulatory LH surge effects on follicular theca and granulosa transcriptomes. Mol Endocrinol. 2013;27:1153–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Richards JS, Russell DL, Robker RL, Dajee M, Alliston TN. Molecular mechanisms of ovulation and luteinization. Mol Cell Endocrinol. 1998;145:47–54. [DOI] [PubMed] [Google Scholar]
- 62. Shimizu T, Hirai Y, Murayama C, Miyamoto A, Miyazaki H, Miyazaki K. Circadian clock genes Per2 and clock regulate steroid production, cell proliferation, and luteinizing hormone receptor transcription in ovarian granulosa cells. Biochem Biophys Res Commun. 2011;412:132–135. [DOI] [PubMed] [Google Scholar]
- 63. West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK, Shea LD. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol Reprod. 2009;80:432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hunzicker-Dunn M, Maizels ET. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal. 2006;18:1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Parakh TN, Hernandez JA, Grammer JC, et al. . Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires β-catenin. Proc Natl Acad Sci USA. 2006;103:12435–12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nimz M, Spitschak M, Fürbass R, Vanselow J. The pre-ovulatory luteinizing hormone surge is followed by down-regulation of CYP19A1, HSD3B1, and CYP17A1 and chromatin condensation of the corresponding promoters in bovine follicles. Mol Reprod Dev. 2010;77:1040–1048. [DOI] [PubMed] [Google Scholar]
- 67. Vanselow J, Spitschak M, Nimz M, Fürbass R. DNA methylation is not involved in preovulatory down-regulation of CYP11A1, HSD3B1, and CYP19A1 in bovine follicles but may have a role in permanent silencing of CYP19A1 in large granulosa lutein cells. Biol Reprod. 2010;82:289–298. [DOI] [PubMed] [Google Scholar]
- 68. Caron KM, Clark BJ, Ikeda Y, Parker KL. Steroidogenic factor 1 acts at all levels of the reproductive axis. Steroids. 1997;62:53–56. [DOI] [PubMed] [Google Scholar]
- 69. Logan KA, Juengel JL, McNatty KP. Onset of steroidogenic enzyme gene expression during ovarian follicular development in sheep. Biol Reprod. 2002;66:906–916. [DOI] [PubMed] [Google Scholar]
- 70. Hinshelwood MM, Repa JJ, Shelton JM, Richardson JA, Mangelsdorf DJ, Mendelson CR. Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Mol Cell Endocrinol. 2003;207:39–45. [DOI] [PubMed] [Google Scholar]
- 71. Falender AE, Lanz R, Malenfant D, Belanger L, Richards JS. Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology. 2003;144:3598–3610. [DOI] [PubMed] [Google Scholar]
- 72. Sellix MT, Murphy ZC, Menaker M. Excess androgen during puberty disrupts circadian organization in female rats. Endocrinology. 2013;154:1636–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mereness AL, Murphy ZC, Sellix MT. Developmental programming by androgen affects the circadian timing system in female mice. Biol Reprod. 2015;92:88. [DOI] [PMC free article] [PubMed] [Google Scholar]