Abstract
Objective: Type 2 diabetes is heterogeneous in its clinical features, pathogenesis, and predisposing or causal genetic factors. This report examines what is known and what needs to be learned about the potential to individualize glycemic therapies in type 2 diabetes, based on phenotypes and genotypes.
Participants: A 29-member international working group with expertise in diabetes epidemiology, physiology, genetics, clinical trials, and clinical care participated in formal presentations and discussions at a conference on April 16–17, 2009. A writing group subsequently prepared this summary and recommendations. The conference was coendorsed by The Endocrine Society and the American Diabetes Association and was supported by an unrestricted educational grant from Novo Nordisk.
Evidence: Participants reviewed and discussed published literature, plus their own unpublished data.
Consensus Process: The summary and recommendations were supported unanimously by the writing group as representing the majority or unanimous opinions of the working group.
Conclusions: Recent advances in genetics, such as the identification of Kir6.2 mutations and the responsible genes for several forms of maturity onset diabetes of the young (MODY), have established precedents linking specifically effective therapies to defined diabetes subtypes. The recent increase in identified polygenic factors related to type 2 diabetes and our understanding of the pathogenesis of diabetes provide potential opportunities to individualize therapy. To further this process, we recommend expanded analysis of existing data sources and the development of new basic and clinical research studies, including a greater focus on identifying type 2 diabetes subtypes, their response to different therapies, and quantitation of cost-effectiveness.
Current knowledge of diabetes heterogeneity establishes subgroups of patients who benefit from specific therapies, and also defines a need for further studies targeted to identifying additional type 2 diabetes subgroups and testing their responsiveness to specific therapeutic options.
Recent reports show that diabetes affects nearly 24 million people in the United States—more than 8% of the U.S. population—and close to 250 million people worldwide. In the United States, the number of people with diabetes has increased by more than 3 million over the last 2 yr, with type 2 diabetes mellitus (T2DM) representing most of this increase and the vast majority of cases overall. Another 57 million have a prediabetic state that places them at high risk for progression to diabetes (1) (http://www.diabetesatlas.org). The overarching and unifying theme in the treatment of diabetes is to lower glycemic levels as close to the nondiabetic range as safely possible to reduce the development and progression of long-term complications. Such tight control has been definitively shown to reduce the development and progression of associated eye, kidney, and nerve complications of the disease (2, 3, 4). Failure to initiate or intensify treatment in a timely manner may foster further β-cell functional decline and thus decrease the likelihood of achieving control once therapy is introduced (5). Moreover, current therapies often fail to maintain glycemic control, particularly in the long-term, and may not affect the underlying pathophysiology (6, 7). Only slightly more than half of patients diagnosed with diabetes reach the glycosylated hemoglobin (HbA1c) target of less than 7%, leaving a substantial population exposed to prolonged periods of damaging hyperglycemia (8).
Although T2DM is considered to be heterogeneous with regard to its clinical presentation, features, and pathogenesis, including pathophysiology and underlying genetic risk factors, patients are generally treated similarly, independent of the underlying differences that might affect therapeutic response. Further insight into the heterogeneity of the T2DM patient population might help explain and, ultimately, reduce the high rate of treatment failure. The usual course of glycemia-lowering therapy for all T2DM patients includes the step-wise addition of medications to lifestyle interventions aimed at weight loss and increasing activity levels. The medications usually begin with a single oral agent and advance to combination oral medications, followed by the addition or substitution of insulin, based on the progressive failure of the medications to maintain target levels of glycemia. The uniformity of this approach is hardly surprising, given that clinical trials examining the glycemia-lowering treatments of T2DM are rarely designed or analyzed to determine the relative effectiveness of interventions among specific subgroups of patients. Furthermore, regulatory agencies do not label medications with regard to subsets of diabetes patients most likely to benefit from a specific intervention, and algorithms to guide therapy make few if any distinctions regarding the selection of interventions based on clinical, pathophysiological, or genetic subpopulations. As a result, most patients with T2DM are treated as if they all have the same disease and will respond identically to available interventions.
The accelerating pace of identifying diabetes-associated genes has added to our understanding of diabetes pathogenesis and heterogeneity. The goal of this expert working group was to examine known clinical, demographic, and physiological phenotypes, in the context of known genetic variation, as defining features of T2DM heterogeneity and to consider the potential for improving patient care by facilitating interventions tailored to specific subpopulations. The participants (Table 1) also were asked to identify areas of future research likely to improve understanding of phenotypes and genotypes in T2DM, particularly as they relate to the control of glycemia. While acknowledging other important treatment elements shown to improve outcomes, such as strategies for modifying lipid levels and lowering blood pressure, the meeting focused on glycemic control because of its intimate association with and causal role in the development of long-term complications. Time limitations precluded consideration of the genetics of complications not specifically related to glycemia, as well as the interactive effects of behavioral, adherence, socioeconomic status, and healthcare system issues, although these also are recognized as significant factors in the heterogeneity of T2DM and response to therapy.
TABLE 1.
Conference participants
| Co-Chairs |
| David M. Nathan, M.D., Harvard Medical School and Massachusetts General Hospital |
| Robert J. Smith, M.D., Division of Endocrinology and the Hallett Center for Diabetes and Endocrinology, Alpert Medical School of Brown University |
| Discussion leaders |
| Phillip Gorden, M.D., National Institute of Diabetes and Digestive and Kidney Diseases |
| Leif Groop, M.D., Ph.D., Lund University, Diabetes Centre |
| Robert A. Rizza, M.D., Mayo Clinic |
| Jerome I. Rotter, M.D., Cedars-Sinai Medical Center and University of California, Los Angeles |
| Speakers |
| Nancy J. Brown, M.D., Vanderbilt University School of Medicine |
| Pierre DeMeyts, M.D., Ph.D., Hagedorn Research Institute |
| Alessandro Doria, M.D., Ph.D., MPH, Joslin Diabetes Center and Harvard Medical School |
| Jose C. Florez, M.D., Ph.D., Massachusetts General Hospital, Broad Institute and Harvard Medical School |
| Francesco Giorgino, M.D., Ph.D., University of Bari School of Medicine |
| Andrew T. Hattersley, M.D., Peninsula Medical School |
| John L. Leahy, M.D., Endocrinology, Diabetes and Metabolism, University of Vermont |
| Christopher B. Newgard, Ph.D., Duke University Medical Center |
| Gerald I. Shulman, M.D., Ph.D., Yale University School of Medicine |
| Jaakko Tuomilehto, M.D., Ph.D., University of Helsinki |
| Other participants |
| Silva A. Arslanian, M.D., University of Pittsburgh School of Medicine and Children’s Hospital |
| Charlotte M. Boney, M.D., Hasbro Children’s Hospital and Alpert Medical School of Brown University |
| Samuel E. Dagogo-Jack, M.D., University of Tennessee Health Science Center |
| Mark N. Feinglos, M.D., CM, Duke University Medical Center |
| Judith E. Fradkin, M.D., National Institute of Diabetes and Digestive and Kidney Diseases |
| Allison B. Goldfine, M.D., Joslin Diabetes Center and Harvard Medical School |
| Jennifer L. Larsen, University of Nebraska Medical Center |
| Anthony L. McCall, M.D., Ph.D., University of Virginia Health System |
| Mary-Elizabeth Patti, M.D., Joslin Diabetes Center and Harvard Medical School |
| Louis H. Philipson, M.D., Ph.D., University of Chicago |
| Alvin C. Powers, M.D., Vanderbilt University |
| Christopher J. Rhodes, Ph.D., University of Chicago |
| Robert A. Vigersky, M.D., Diabetes Institute, Walter Reed Health Care System and Uniformed Services University of the Health Sciences |
Objectives
The conference participants specifically aimed to answer the following questions by reviewing present knowledge of both phenotypic and genotypic heterogeneity in T2DM: 1) What do the available data tell us about the heterogeneity of T2DM as it relates to response to glycemia-lowering therapy? 2) What can we conclude about the current potential to individualize therapy, based on our understanding of the heterogeneity of the disease? 3) What basic and clinical research questions need to be answered to gain further insight into individualizing therapy in T2DM? and 4) What resources are necessary to advance these areas of inquiry?
Meeting Summary
Heterogeneity of T2DM
T2DM has long been recognized to be a heterogeneous disorder with regard to the individual characteristics that determine the development of a prediabetic state and the progression from prediabetes to overt disease. The modern concept of heterogeneity can be traced to the early recognition of distinct adult- and juvenile-onset forms of diabetes and then, more than 50 yr ago, the emergence of data demonstrating individual variation in insulin resistance (9, 10) and insulin levels (11) in hyperglycemic patients. In his 1978 Banting Lecture, Stephen Fajans summarized then current thinking in his description of diabetes not as a single, specific disease but rather as a syndrome “comprised of a variety of diseases all characterized by hyperglycemia and tissue changes that result from heterogeneous etiological and pathogenetic factors” (12). New technologies subsequently have allowed recognition of a growing array of clinical, genetic, behavioral, environmental, and socioeconomic factors that may influence disease development, progression, and response to therapy.
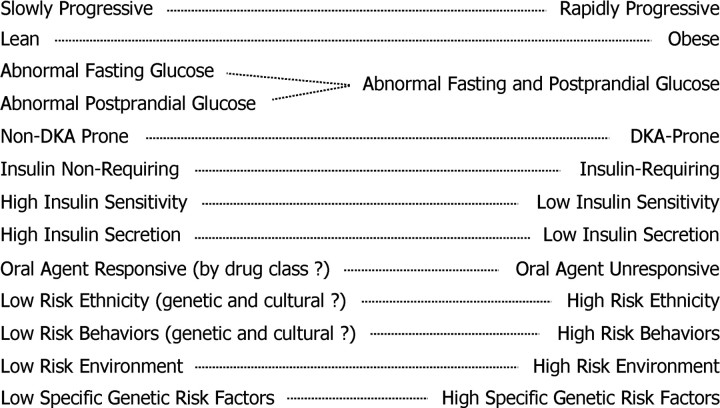
Figure 1 summarizes individual clinical factors that define this heterogeneity and may influence the course of T2DM. A similar spectrum of clinical features defines variations in individuals at high risk of developing diabetes. Among these variables, the presence or absence of obesity influences the emphasis on weight reduction as a component of therapy (13). States of hormone excess, such as hypercortisolemia, also are individually addressed as causes of secondary diabetes when recognized. However, most of the other clinical features listed in Fig. 1 do not routinely inform therapeutic strategies.
Fig. 1.
Heterogeneous clinical features of T2DM.
Pathophysiological heterogeneity
Diabetes mellitus results when there is inadequate insulin secretion to meet the body’s needs. However, a variety of pathways may contribute to this inadequacy. Diabetes may stem from absolute or relative deficits in insulin secretion, defects in insulin action or, as in most cases of T2DM, a combination of both. Within the T2DM population, highly variable degrees of insulin resistance and deficiency underlie the possible existence of phenotypically heterogeneous subgroups with specific pathophysiological characteristics. The increasing prevalence of obesity with attendant insulin resistance in the type 1 diabetes mellitus (T1DM) population, which has historically been viewed as an insulin-deficient state, suggests that T1DM is becoming an increasingly heterogeneous disorder as well.
Abnormalities that often precede the development of T2DM, such as impaired fasting glucose and impaired glucose tolerance, are also heterogeneous, with impaired fasting glucose resulting largely from insulin resistance in the liver and impaired glucose tolerance from insulin resistance in muscle (14, 15). This suggests that heterogeneity in the pathogenesis of T2DM occurs early in the disease process. The subsequent worsening of hyperglycemia and development of diabetes is associated with insulin secretory failure (16, 17, 18) and is also highly variable, further exacerbated by alterations in glucose effectiveness, impaired incretin action, and relatively high glucagon levels (19).
Abundant data support the existence of pathophysiological subgroups within T2DM, including evidence that the prevalence of diabetes and its precursor states may vary significantly by gender, ethnicity, and age (20). In addition, the fact that nearly 25% of all patients diagnosed with T2DM are not insulin resistant at diagnosis strongly supports distinct pathophysiological phenotypes with potentially different therapeutic requirements. Although current data suggest that T2DM in children and adolescents is similar to T2DM in adults (21), the increasing rates of obesity in children with T1DM complicate the clinical and pathophysiological distinction between obese T1DM and T2DM despite their distinct underlying pathogeneses (22). In contrast to T2DM in adults, children diagnosed with T2DM are more likely to be female, and disease progression in these children occurs much more rapidly than in their adult counterparts (23, 24).
The degree to which alterations in specific aspects of glucose homeostasis differ between individuals, and the variable effects of current therapies on the molecular and physiological pathways involved, currently make it difficult to predict how any one individual will respond to therapy. More targeted studies and sophisticated tools will be required to recognize and diagnose additional specific subtypes that may, in turn, be linked to specific pathophysiologies and responses to individual and combined therapies. More appropriate and efficient screening and diagnostic tools to differentiate particular phenotypes in clinical practice must also be developed. Reaching these goals may involve identifying specific proteins and metabolites associated with pathophysiological subgroups, as well as validating markers of β-cell function that will allow easier individual phenotyping.
Genetics and the molecular basis of T2DM heterogeneity
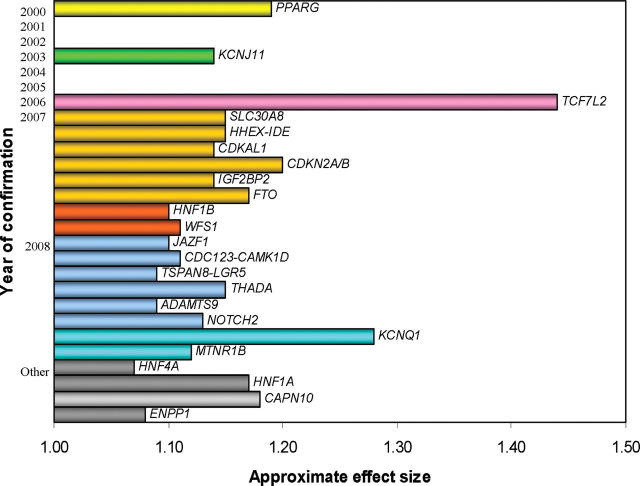
Insulin secretion and cellular responses to insulin are mediated by numerous receptors, transporters, ion channels, enzymes, functional regulators, transcription modifiers, and structural elements that have been identified over the past several decades. Variations in expression or function of these proteins and lipids undoubtedly represent important determinants of T2DM heterogeneity via effects on β-cell growth and development, insulin secretion, or changes in insulin responsiveness in specific or multiple tissues. Direct measures of these factors in patients with T2DM require samples of β-cells or insulin target tissues, which unfortunately cannot be routinely obtained from T2DM patients. As an alternative, genes encoding these proteins can be readily identified in DNA extracts from peripheral blood samples and other readily accessible tissues, such as buccal mucosa. This has made possible rapid advances in understanding of genetic determinants of T2DM. With improvements in genotyping technology and the introduction of genome-wide scanning strategies combining DNA polymorphism data from multiple studies, more than 20 loci associated with T2DM at a population level now have been identified (Fig. 2) (25, 26, 27, 28). It appears that many of these loci influence β-cell function rather than insulin resistance (28).
Fig. 2.
Genetic loci associated with T2DM plotted by publication year and approximate effect size. Genes in gray (designated “other”) are implicated in T2DM by substantial functional and genetic evidence but do not have established statistically significant genome-wide association. [Adapted from Florez et al. (28 ).]
More than 25 disorders also have been described in which alteration of a single gene results in abnormal glucose homeostasis with clinical features that overlap with typical T2DM (Table 2) (29). Most of these monogenic forms of diabetes are rare, and currently identified syndromes in aggregate account for no more than 5% of diabetes cases. However, they demonstrate the broad spectrum of affected pathways and the impact of genetic factors as causes of diabetes. Some of these disorders also illustrate ways in which specific genetic data can direct evidence-based decisions on choice of glycemia-lowering therapy in diabetes, as will be discussed in the next section.
TABLE 2.
Rare monogenic syndromes of abnormal glucose homeostasis
| Insulin secretion defect |
| MODY (HNF4α, glucokinase, HNF1α, IPF1, HNF1β, NeuroD1) |
| Neonatal diabetes (KCNJ11, ABCC8, INS) |
| Wolcott-Rallison syndrome (EIF2AK3) |
| Wolfram syndrome (WFS1, ZCD2) |
| Werner syndrome (RECQL2) |
| Friedreich’s ataxia (FRDA1) |
| Hemochromatosis (HFE) |
| Thiamine-responsive megaloblastic anemia syndrome (SLC19A2) |
| Insulin resistance |
| Lipodystrophies |
| Generalized (AGPAT2, BSCL2, CAV1) |
| Partial (LMNA, LMNB2, ZMPSTE24, PPARG, AKT2) |
| Type A insulin resistance syndrome (INSR) |
| Rabson-Mendenhall syndrome (INSR) |
| Donohue syndrome (INSR) |
The rapid advance in knowledge of genetic factors in T2DM has opened up many unresolved questions and issues in the realms of both basic science and clinical research. In addition to determining the degree to which common genetic variants increase the risk of diabetes and the value of genetic information in predicting individual risk, we must still determine whether and to what extent knowledge of genetic risk may affect individualization of therapy and outcomes (25, 26). Moreover, the cost-effectiveness of genetic screening, which continues to be a moving target as the price of genotyping falls, needs to be examined from a public health perspective. Appropriate and cost-effective tools for diagnosing the monogenic minority within the T1DM/T2DM majority must be developed as well. Building a systems-based understanding of diabetes that encompasses the full array of metabolic perturbances associated with its various forms will require continued efforts to identify both rare, disease-linked variants with strong effects and common genetic variants with weak effects. This approach will need to be linked to an examination of prevalence of the rare variants and the specific mechanisms by which they affect glycemic control. Meanwhile, efforts must continue to identify structural genetic variants (e.g. copy number variations), characterize the contribution of rare variants, elucidate epigenomic mechanisms and environmental triggers, and fine-map the newly discovered loci to identify the causal variants via sequencing and functional studies. Ultimately, it will be necessary to examine the interactive effects of multiple factors that may be operative in single individuals.
Implications for individualizing therapies
Clearly, not all existing therapies for T2DM provide similar control for every affected patient. With a few notable exceptions, how different responses to therapy relate to specific variations within identifiable subgroups of patients remains largely unexplored. The failure to appreciate the interindividual differences that may underlie differential response to therapy stems in part from the design of most clinical trials with their goals of maximizing adherence, minimizing confounders, and reducing variability that may affect responsiveness. In addition, clinical studies are generally powered for main effects and, thus, often don’t stipulate subgroup analyses as secondary outcomes. Even when subgroup analyses are included in a priori design, they are rarely reported. Finally, from a commercial point of view, most manufacturers are not motivated to restrict the potential market for their products. None of the currently available glucose-lowering medications includes specific recommendations for use other than T1DM vs. T2DM and safety.
Screening for autoimmunity, which has identified a subcohort of patients variously labeled as latent autoimmune diabetes in adults, slow-onset type 1, or type 1.5 diabetes shows the potential benefits of screening for diabetes subtypes. In the UK Prospective Diabetes Study (UKPDS), 12% of newly diagnosed T2DM patients tested positive for autoantibodies to GAD65 and/or insulinoma-associated antigen-2A and were managed as if they had T2DM according to the UKPDS protocol (30). This subgroup of patients, which was characterized, compared with the rest of the T2DM cohort, by relatively low body mass index, modest insulin resistance, low C-peptide levels, and absence of T2DM family history, responded better to initial insulin therapy than to sulfonylureas and other oral agents (31). A similar situation exists in children, where 10–75% of patients clinically diagnosed with T2DM have evidence of islet-cell autoimmunity (22).
Currently, the most powerful demonstration of the value of using specific genetic subtypes to individualize therapy comes from identification of monogenic forms of diabetes. Subgroups of diabetes defined by mutations in specific genes have been shown to be extremely responsive to some glucose-lowering therapies, but not to others. New knowledge of the genes underlying neonatal diabetes, for example, together with HLA analysis, has shown that infants under 6 months of age diagnosed with diabetes have a monogenic etiology rather than autoimmune type 1 diabetes (32, 33). Furthermore, studies of children and adults with the most common cause of permanent neonatal diabetes mellitus, Kir6.2 mutations, show that over 90% of these patients can be transferred to oral sulfonylureas, even after many years of insulin therapy and being C-peptide negative, with a resulting improvement in HbA1c levels (34, 35).
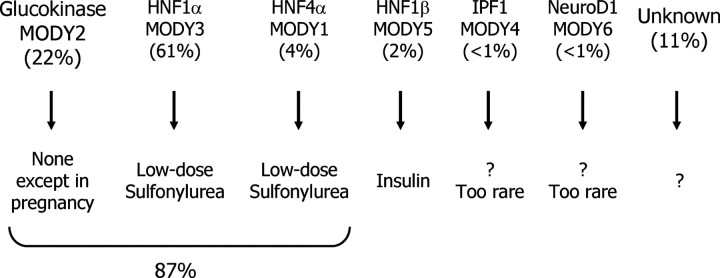
Maturity onset diabetes of the young (MODY) provides another example in which the identification of monogenic subgroups has already been used to define optimal treatment. Once confused with cases of T2DM that developed during childhood and adolescence, and sometimes with gestational diabetes or T1DM, MODY has been recognized for decades as a dominantly inherited form of monogenic diabetes (36, 37). Today, we know that this group of patients includes a variety of genetic subtypes, all of which appear to be characterized by defects in insulin secretion. However, among these monogenic forms of diabetes, the specific genetic variant determines clinical presentation and treatment response (38) (Fig. 3). Patients with glucokinase mutations (MODY 2) have an altered glucose set point for β-cell insulin secretion, which leads to modest elevations of fasting and postprandial blood glucose levels from birth that progress little with advancing age. Affected individuals do not appear to develop severe long-term diabetes complications, consistent with their near-normal glycemia. Most importantly, average blood glucose levels as reflected by HbA1c are similar whether patients receive insulin, sulfonylureas, or no glucose-lowering therapy. Thus, most individuals with MODY 2 can be taken off all glycemic therapy, with the exception of a potential need for vigorous insulin therapy in pregnant women to prevent a non-MODY 2 fetus from developing increased insulin-mediated growth in response to the mild maternal hyperglycemia. Patients with HNF4α and HNF1α mutations (MODY 1 and 3, respectively) often exhibit high sensitivity to sulfonylureas, even many years after the diagnosis of diabetes. Many of these individuals are treated with insulin but, when identified by genotyping, can be shifted to sulfonylureas with often improved glycemia (39). These three forms of MODY diabetes account for more than 80% of all MODY cases (Fig. 3).
Fig. 3.
MODY subtype prevalence and treatment. [Adapted from McCarthy et al. (38 ).]
With MODY and other forms of monogenic diabetes, the challenge lies not in treatment paradigms, which are clear once the diagnosis has been made, but rather in identifying these subtypes clinically. Monogenic diabetes is still vastly underdiagnosed. In the United Kingdom, 90% of genetically confirmed monogenic diabetes is initially diagnosed as T1DM or T2DM. Preliminary data from the SEARCH study show that only 8% of U.S. children with HNF1α mutations had been correctly diagnosed as having MODY, and few had received the most appropriate treatment (sulfonylurea) instead of insulin therapy or metformin (Hattersley, A.T., personal communication).
Although these monogenic syndromes offer a paradigm for the role of genotyping in driving the selection of the most effective therapy, the role of genotyping in the more common polygenic forms of T2DM is just being explored. Patients with the risk genotype for TCF7L2 appear to respond more poorly to sulfonylureas (40). On the other hand, those with the risk genotype at the gene that encodes the sulfonylurea receptor (ABCC8 A1369S), which is highly correlated with the risk genotype at the KCNJ11 E23K locus, seem to respond better to a sulfonylurea (41). Patients with a specific polymorphism of the multidrug and toxin extrusion 1 transporter protein may have augmented responsiveness to metformin (42). Future prospective studies must be conducted to confirm whether these and other genetic variants prove useful in disease prediction or choice of therapy.
The identification of subtypes of diabetes within T2DM, whether by specific genetic markers or other clinical features, remains a challenge. For example, whether an accurate separation of patients with T2DM into those with predominant insulin resistance vs. those with predominant insulin deficiency would aid the selection of the most effective therapies is unknown. Nor is it known whether further subtyping based on the locus of resistance (e.g. muscle vs. liver) would improve outcomes. These are all testable hypotheses that have been largely unexplored.
Recommendations
Current understanding of heterogeneity has not yet reached a point at which it can guide individualized therapy decisions regarding glycemia-lowering interventions for the majority of patients with T2DM. The progress already apparent, particularly in the realm of monogenic forms of diabetes, has stemmed from combining new knowledge of specific genetic susceptibilities with subsequent clinical observations. As we move forward, we should continue to incorporate these and additional clinical observations with new data on the physiology and genetics of diabetes to assess the determinants of glycemic response to treatments in the larger T2DM population. In addition, taking a number of specific steps, summarized in Table 3, may bring us considerably closer to the goal of individualizing therapy and improving treatment response.
TABLE 3.
Recommendations
| Analyze existing data sets/sources to determine associations and predictive power of phenotypic and genotypic measures with respect to glycemic control. |
| Expand existing and design new diabetes registries. |
| Systematically collect phenotypic and genotypic measures of heterogeneity, including materials for future biomarker and genetic analyses, in all existing and new registries. |
| Design data registries to allow examination of heterogeneity in relation to glycemic lowering of various therapies and in relation to specific hypotheses. |
| Develop new clinical trials to address heterogeneous response. |
| Design and conduct all new clinical trials to collect phenotypic and genetic measures of heterogeneity systematically, including materials for future biomarker and genetic analyses. |
| Design new trials to address heterogeneity in relation to response to therapies and in relation to specific hypotheses. |
| Include assessments of cost-effectiveness in trials where this is possible. |
| Develop more accurate and efficient technologies to measure markers of T2DM heterogeneity and heterogeneous response to treatment. |
| Expand basic research. |
| Explore biological correlates of metabolome and proteome with response to drug therapy. |
| Develop and validate a systems biology approach, including creation of in silico models, to predict heterogenic response to therapy more precisely. |
| Expand collaboration between geneticists and physiologists to determine how newly identified genetic variants influence therapy. |
Analyze existing data and data sources
There are already a plethora of data and data sources (Tables 4 and 5) that could be potentially valuable in individualizing therapy; however, to date, these have been largely underutilized. For example, measures of phenotypic heterogeneity, including relatively simple demographic variables such as age, sex, and race; clinical variables such as body mass and fat distribution; and even more sophisticated physiological variables, such as degree of baseline insulin resistance and secretion, are available in numerous clinical trials. Still other trials have genotyped their cohorts or have stored DNA. Pooled analyses or meta-analyses of such data may provide important insights into the relative effectiveness of specific interventions in subgroups of T2DM and advance our understanding of individualized therapy. Similarly, biomarkers from plasma/serum and urine that are already available or obtainable from clinical trials or large epidemiological studies could be used to assess established indices of glucose homeostasis and genomics. Mining these existing sources to determine the specific implications of T2DM heterogeneity as it affects the therapy of hyperglycemia is a logical next step.
TABLE 4.
Available data and data sources
| Phenotypic |
| Age |
| Gender |
| Duration of diabetes |
| Diabetes medications |
| Other medications |
| Clinical factors affecting choice of therapy (e.g. creatinine clearance, liver function tests) |
| Family history |
| History of gestational diabetes |
| Secondary causes (e.g. pancreatitis) and associated conditions (e.g. polycystic ovary syndrome) |
| Body composition (e.g. adiposity, fat distribution) |
| Birth weight |
| Lipids |
| Blood pressure levels |
| Autoantibodies |
| C-peptide levels |
| Ethnicity |
| Sociocultural factors (e.g. lifestyle, diet, adherence, socioeconomic status) |
| Biobank |
| Serum/plasma |
| DNA/cell lines |
| Tissue banks |
| Genetic |
| Candidate gene to genome-wide scans |
TABLE 5.
Data obtainable with existing technology
| Established measures and effectors of glucose homeostasis |
| Insulin secretion |
| Insulin action |
| Glucose effectiveness |
| Glucagon levels |
| Incretin levels |
| Genomics |
| Genotyping: candidate gene to genome-wide association studies |
| Sequencing: candidate gene to whole genome and cDNA |
| Transcriptome/microarrays |
| Micro RNA, long-range RNA (linc-RNA) |
| Epigenomics |
| Additional biomarkers (from plasma/serum, urine) |
| Proteins/proteomics (e.g. cytokines) |
| Metabolites/metabolomics |
Expand existing or develop new data registries
All new and existing diabetes registries (including drug postmarketing surveys) should systematically collect data to address phenotypic and genetic heterogeneity measures. Not only should these registries collect material for future biomarker and genetic analysis, but new registries should be designed to address specifically the heterogeneity of T2DM with hypotheses generated by examining existing data. Both new and existing registries should be designed or expanded to address the heterogeneity of T2DM because it may influence the relative glycemic-lowering effects of specific therapies as a function of identified and putative predictors of responsiveness.
Develop new clinical trials
Addressing the many phenotypic and genetic heterogeneity measures hypothesized to influence therapeutic response calls for new clinical trials. Future randomized studies of T2DM therapies should, by design, collect phenotypic information relevant to response to therapy. Moreover, material for future biomarker and genetic analyses should be made accessible from future trials. Because analyses of information from previous clinical trials and observational studies identify putative factors that may influence response to interventions, new trials should be designed that specifically address the resulting hypotheses.
Develop new technologies
Targeting therapy toward more appropriate subgroups of patients will require increasingly accurate and efficient methods to measure markers of specific pathophysiological mechanisms and T2DM heterogeneity on large numbers of patient samples. These markers might include those for insulin secretion, β-cell mass, glucose effectiveness, incretin secretion and action, glucagon secretion and action, and liver, muscle, and visceral fat levels.
Develop basic research to dissect heterogeneous response to therapy
We critically need basic research to explore numerous fundamental issues that underlie the heterogeneous response to T2DM therapy. One very promising area involves determining the relationship between metabolomic and proteomic variation and response to drug therapy and exploring ways in which genetic variation may influence this response. The development and validation of a systems biology approach that predicts variable response to therapy is another promising area, as is the creation of in silico models of β-cell function and insulin action that incorporate heterogeneity of glucose homeostasis in humans. To determine basic mechanisms underlying the influence of newly identified genetic variants on therapy, expanding collaboration between geneticists, physiologists, and bioinformatics scientists will be essential.
Summary
Permanent neonatal diabetes represents a diabetes subtype for which there is compelling evidence supporting cost-effectiveness of definitive diagnosis and resulting specific decisions on therapeutic management. Patients with this diabetes subtype can be readily identified by the onset of sustained hyperglycemia under 6 months of age, and the genetic diagnosis can be made with high efficiency (Kir6.2 mutations in approximately 50% of such patients). Confirmation by genetic analysis often enables a shift from insulin to sulfonylurea therapy and the resulting achievement of lower HbA1c levels. Although formal cost-effectiveness data have not been published, it can be convincingly argued that the costs of gene sequencing in this clinically identifiable subset of patients is favorably offset by the lower cost of sulfonylurea therapy and the downstream benefits of better glycemic control. For the majority of patients with MODY, a correct genetic diagnosis has similar implications for improved therapy (greater responsiveness to sulfonylureas in many patients with MODY 1 and 3; no therapy in MODY 2). However, the greater difficulty in identifying candidate patients by clinical features and the greater cost of sequencing multiple MODY genes results in less certainty about the cost-effectiveness of MODY screening. Improvement in screening methods and efforts to obtain cost-effectiveness data thus represent important goals for future work on the MODY subtypes of diabetes. These monogenic forms of diabetes provide tangible models that define needed directions for future work on the more common polygenic subtypes of T2DM.
The key question that must be addressed in elucidating the heterogeneity of the broad mix of T2DM patients is not whether all therapies provide similar control but whether there are differences in therapeutic efficacy within distinguishable subgroups. Although this question has enormous implications for long-term outcomes and cost-effectiveness of care, it has had little exploration. In addition to a dearth of head-to-head comparison studies, our current knowledge base consists primarily of crude or deficient baseline phenotyping and genotyping together with inadequate subset analyses. As a result, current models of treatment for T2DM remain nonspecific. The rapidly expanding understanding of the genetic and pathophysiological heterogeneity of T2DM, however, suggests the strong potential of and need for additional research and new technologies to define more precisely specific phenotypes and genotypes that relate to glycemic control. Further insight into these subgroups should not only help elucidate the pathogenesis of T2DM but also facilitate more individualized treatment to improve glycemic control, thus maximizing individual benefit, minimizing risk, and providing reductions in global health cost.
Acknowledgments
The authors are grateful for the contributions of session co-chairs, speakers, and participants in the April 16–29, 2009 meeting, who are listed, together with their affiliations, in Table 1. We also acknowledge the help provided by Drs. Andrew Hattersley, Alessandro Doria, and Jose Florez in reviewing the figures and tables, as well as the editorial assistance of Dr. Terra Ziporyn.
Footnotes
This paper is based on a conference jointly sponsored by The Endocrine Society and the American Diabetes Association, with the financial support of an unrestricted educational grant to The Endocrine Society from Novo Nordisk.
Disclosure Summary: The authors have nothing to declare.
First Published Online March 1, 2010
Abbreviations: HbA1c, Glycosylated hemoglobin; MODY, maturity onset diabetes of the young; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
References
- 1.Centers for Disease Control and Prevention 2008. Diabetes Public Health Resource. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Accessed February 18, 2009
- 2.The Diabetes Control and Complications Trial Research Group 1993. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986 [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group 1998. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853 [PubMed] [Google Scholar]
- 4.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA 2008. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 5.Brown JB, Nichols GA, Perry A 2004. The burden of treatment failure in type 2 diabetes. Diabetes Care 27:1535–1540 [DOI] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study (UKPDS) Group 1998. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352:854–865 [PubMed] [Google Scholar]
- 7.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G 2006. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 8.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB 2008. Is glycemic control improving in U.S. adults? Diabetes Care 31:81–86 [DOI] [PubMed] [Google Scholar]
- 9.Falta W, Boller R 1931. Insularer und insulinresistenter diabetes. Klin Wochenschr 10:438–443 [Google Scholar]
- 10.Himsworth HP 1936. Diabetes mellitus: its differentiation into insulin-sensitive and insulin-insensitive types. Lancet 1:127–130 [DOI] [PubMed] [Google Scholar]
- 11.Yalow RS, Berson SA 1960. Immunoassay of endogenous plasma insulin in man. J Clin Invest 39:1157–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fajans SS, Cloutier MC, Crowther RL 1978. The Banting Memorial Lecture 1978. Clinical and etiologic heterogeneity of idiopathic diabetes mellitus. Diabetes 27:1112–1125 [DOI] [PubMed] [Google Scholar]
- 13.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI 2005. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54:603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, Landau BR, Rizza RA 2007. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes 56:1703–1711 [DOI] [PubMed] [Google Scholar]
- 15.Defronzo RA 2009. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leahy JL, Bonner-Weir S, Weir GC 1992. β-Cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care 15:442–455 [DOI] [PubMed] [Google Scholar]
- 17.Knowler WC, Saad MF, Pettitt DJ, Nelson RG, Bennett PH 1993. Determinants of diabetes mellitus in the Pima Indians. Diabetes Care 16:216–227 [DOI] [PubMed] [Google Scholar]
- 18.Brunzell JD, Robertson RP, Lerner RL, Hazzard WR, Ensinck JW, Bierman EL, Porte Jr D 1976. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab 42:222–229 [DOI] [PubMed] [Google Scholar]
- 19.Leahy JL 2005. Pathogenesis of type 2 diabetes mellitus. Arch Med Res 36:197–209 [DOI] [PubMed] [Google Scholar]
- 20.Carter JS, Pugh JA, Monterrosa A 1996. Non-insulin-dependent diabetes mellitus in minorities in the United States. Ann Intern Med 125:221–232 [DOI] [PubMed] [Google Scholar]
- 21.Gungor N, Bacha F, Saad R, Janosky J, Arslanian S 2005. Youth type 2 diabetes: insulin resistance, β-cell failure, or both? Diabetes Care 28:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tfayli H, Bacha F, Gungor N, Arslanian S 2009. Phenotypic type 2 diabetes in obese youth: insulin sensitivity and secretion in islet cell antibody-negative versus -positive patients. Diabetes 58:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman FR 2002. Type 2 diabetes mellitus in children and youth: a new epidemic. J Pediatr Endocrinol Metab 15(Suppl 2): 737–744 [DOI] [PubMed]
- 24.Fagot-Campagna A 2000. Emergence of type 2 diabetes mellitus in children: epidemiological evidence. J Pediatr Endocrinol Metab 13(Suppl 6):1395–1402 [DOI] [PubMed]
- 25.Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, Berglund G, Altshuler D, Nilsson P, Groop L 2008. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med 359:2220–2232 [DOI] [PubMed] [Google Scholar]
- 26.Meigs JB, Shrader P, Sullivan LM, McAteer JB, Fox CS, Dupuis J, Manning AK, Florez JC, Wilson PW, D'Agostino Sr RB, Cupples LA 2008. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med 359:2208–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, de Bakker PI, Abecasis GR, Almgren P, Andersen G, Ardlie K, Boström KB, Bergman RN, Bonnycastle LL, Borch-Johnsen K, Burtt NP, Chen H, Chines PS, Daly MJ, Deodhar P, Ding CJ, Doney AS, Duren WL, Elliott KS, Erdos MR, Frayling TM, Freathy RM, Gianniny L, Grallert H, Grarup N, Groves CJ, Guiducci C, Hansen T, Herder C, Hitman GA, Hughes TE, Isomaa B, Jackson AU, Jørgensen T, Kong A, Kubalanza K, Kuruvilla FG, Kuusisto J, Langenberg C, Lango H, Lauritzen T, Li Y, Lindgren CM, Lyssenko V, Marvelle AF, Meisinger C, Midthjell K, Mohlke KL, Morken MA, Morris AD, Narisu N, Nilsson P, Owen KR, Palmer CN, Payne F, Perry JR, Pettersen E, Platou C, Prokopenko I, Qi L, Qin L, Rayner NW, Rees M, Roix JJ, Sandbaek A, Shields B, Sjögren M, Steinthorsdottir V, Stringham HM, Swift AJ, Thorleifsson G, Thorsteinsdottir U, Timpson NJ, Tuomi T, Tuomilehto J, Walker M, Watanabe RM, Weedon MN, Willer CJ, Illig T, Hveem K, Hu FB, Laakso M, Stefansson K, Pedersen O, Wareham NJ, Barroso I, Hattersley AT, Collins FS, Groop L, McCarthy MI, Boehnke M, Altshuler D 2008. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 40:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Florez JC 2008. Clinical review: the genetics of type 2 diabetes: a realistic appraisal in 2008. J Clin Endocrinol Metab 93:4633–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doria A, Patti ME, Kahn CR 2008. The emerging genetic architecture of type 2 diabetes. Cell Metab 8:186–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai M, Clark A 2008. Autoimmune diabetes in adults: lessons from the UKPDS. Diabet Med 25(Suppl 2):30–34 [DOI] [PubMed]
- 31.Maruyama T, Tanaka S, Shimada A, Funae O, Kasuga A, Kanatsuka A, Takei I, Yamada S, Harii N, Shimura H, Kobayashi T 2008. Insulin intervention in slowly progressive insulin-dependent (type 1) diabetes mellitus. J Clin Endocrinol Metab 93:2115–2121 [DOI] [PubMed] [Google Scholar]
- 32.Edghill EL, Dix RJ, Flanagan SE, Bingley PJ, Hattersley AT, Ellard S, Gillespie KM 2006. HLA genotyping supports a nonautoimmune etiology in patients diagnosed with diabetes under the age of 6 months. Diabetes 55:1895–1898 [DOI] [PubMed] [Google Scholar]
- 33.Hattersley AT 2005. Molecular genetics goes to the diabetes clinic. Clin Med 5:476–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearson ER, Flechtner I, Njølstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert JJ, Holst JJ, Clark PM, Ellard S, Søvik O, Polak M, Hattersley AT 2006. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med 355:467–477 [DOI] [PubMed] [Google Scholar]
- 35.Zung A, Glaser B, Nimri R, Zadik Z 2004. Glibenclamide treatment in permanent neonatal diabetes mellitus due to an activating mutation in Kir6.2. J Clin Endocrinol Metab 89:5504–5507 [DOI] [PubMed] [Google Scholar]
- 36.Tattersall RB 1974. Mild familial diabetes with dominant inheritance. Q J Med 43:339–357 [PubMed] [Google Scholar]
- 37.Fajans SS, Conn JW 1960. Tolbutamide-induced improvement in carbohydrate tolerance of young people with mild diabetes mellitus. Diabetes 9:83–88 [DOI] [PubMed] [Google Scholar]
- 38.McCarthy MI, Hattersley AT 2008. Learning from molecular genetics: novel insights arising from the definition of genes for monogenic and type 2 diabetes. Diabetes 57:2889–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shepherd M, Shields B, Ellard S, Rubio-Cabezas O, Hattersley AT 2009. A genetic diagnosis of HNF1A diabetes alters treatment and improves glycaemic control in the majority of insulin-treated patients. Diabet Med 26:437–441 [DOI] [PubMed] [Google Scholar]
- 40.Pearson ER, Donnelly LA, Kimber C, Whitley A, Doney AS, McCarthy MI, Hattersley AT, Morris AD, Palmer CN 2007. Variation in TCF7L2 influences therapeutic response to sulfonylureas: a GoDARTs study. Diabetes 56:2178–2182 [DOI] [PubMed] [Google Scholar]
- 41.Feng Y, Mao G, Ren X, Xing H, Tang G, Li Q, Li X, Sun L, Yang J, Ma W, Wang X, Xu X 2008. Ser1369Ala variant in sulfonylurea receptor gene ABCC8 is associated with antidiabetic efficacy of gliclazide in Chinese type 2 diabetic patients. Diabetes Care 31:1939–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH 2009. Genetic variation in the multidrug and toxin extrusion 1 transporter protein influences the glucose-lowering effect of metformin in patients with diabetes: a preliminary study. Diabetes 58:745–749 [DOI] [PMC free article] [PubMed] [Google Scholar]