Abstract
The links between systemic insulin resistance (IR), brain-specific IR and Alzheimer’s disease (AD) has been an extremely productive area of current research. This review will cover the fundamentals and pathways leading to IR; its connection to AD via cellular mechanisms, the most prominent methods and models used to examine it, an introduction to the role of extracellular vesicles (EVs) as a source of biomarkers for IR and AD, and an overview of modern clinical studies on the subject. To provide additional context, we also present a novel analysis of the spatial correlation of gene expression in the brain with the aid of Allen Human Brain Atlas data. Ultimately, examining the relation between IR and AD can be seen as a means of advancing the understanding of both disease states, with IR being a promising target for therapeutic strategies in AD treatment. In conclusion, we highlight the therapeutic potential of targeting brain IR in AD and the main strategies to pursue this goal.
Normal insulin signaling and insulin resistance
Insulin is one of the key hormone regulators of metabolism throughout the body, through a variety of largely tissue-specific actions. Elevations in blood glucose and other nutrients after meals trigger the release of hormones which homeostatically regulate blood glucose levels, particularly insulin, which is secreted by the β cells of the pancreas [1], and the insulin-regulating incretins, glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP). Insulin exerts its actions through binding of the extracellular α subunit of the insulin receptor, which leads to a conformational change that autophosphorylates the intracellular β subunit of the receptor via tyrosine kinase activation [2]. This kinase activation leads to the recruitment and phosphorylation of the Insulin Receptor Substrates 1 and 2 (IRS1 and 2), which represent the first node in the insulin signaling cascade and exerts downstream effects on several key regulatory proteins of cell metabolism, cell survival, growth, and differentiation, including the mammalian target of rapamycin (mTOR), PKB, and glycogen synthase kinase 3 (GSK3) [3, 4]. The divergent branches of the insulin pathway largely converge downstream onto Akt activation, primarily through PI3K activation of PDK [5, 6].
A key physiological action of insulin is to increase glucose uptake into cells, especially in muscle and adipose tissue [1], by translocation of various insulin-dependent glucose transporters (GLUT) to the plasma membrane. Specifically, the insulin cascade leads to PI3K/ PDK/Akt activation, which in turn leads to inactivation of AS-160 [7–9], which, coupled with the activation of other Rab GTPases, is thought to stimulate the translocation of certain insulin-dependent GLUTs, such as GLUT 4, to the membrane [10–12]. Of particular interest for brain metabolism are GLUT1, GLUT4, and GLUT3. GLUT1 is present in nearly all cell types, whereas GLUT 4 is primarily expressed in skeletal myocytes and adipocytes [13–15]. While GLUT3 is the primary brain neuronal GLUT and is mainly expressed in axons and dendrites, GLUT 1 and 4 have also been detected in brain tissue [16, 17]. GLUT3 is unique in terms of its low Michaelis-Menten constant, allowing for continuous transport of glucose into neurons even under low extracellular concentrations thereby providing a consistent energy source [18]. Different isoforms of GLUT 1 mediate glucose uptake by astrocytes as well as the endothelial cells of the Blood Brain Barrier (BBB). The BBB contains insulin-independent GLUT1 and GLUT3 transporters that ensure a dynamic response of glucose transport to meet variable energy demands independent of insulin [19]. This dynamic responsiveness of the BBB is highlighted in a study that found that when glucose transport across the BBB was increased, the luminal expression of GLUT1 increased, whereas abluminal expression increased with decreased glucose transport [20]. On the other hand, BBB insulin receptor expression is reduced with prolonged peripheral hyperinsulemia [21] and in aging [22], whereas insulin levels in the brain of older individuals are also reduced [23]. The combined effects of aging and peripheral IR may lead to a substantial decrease in brain insulin and insulin receptors and a corresponding decrease in insulin-dependent glucose transport. The effects of decreased insulin signaling on glucose transport may also be differentially impacted upon different brain regions depending on the type of GLUTs they express. GLUT 4 mRNA co-localized with GLUT3, insulin and insulin receptor mRNAs have been identified in the nuclei of basal forebrain cholinergic neurons, which may function as nutrient sensors. This partial GLUT4 dependence may help explain the vulnerability of these cells in low energy conditions and Alzheimer’s disease (AD) [18, 24, 25].
In insulin resistant states such as type 2 Diabetes Mellitus (T2D), the ability of insulin to stimulate glucose uptake via insulin-dependent GLUT transporters is impaired, requiring higher than normal concentrations of extracellular insulin to maintain normal circulating glucose levels [26]. Early in the course of T2D, these higher insulin concentrations are maintained by β cell overstimulation [27]. Conditions of persistent insulin activation trigger the excessive autophosphorylation of various Ser/Thr residues on IRS family members [28–30]. This aberrantly phosphorylated IRS-1 has been implicated in several proposed mechanisms of insulin resistance (IR) based on different sites of hyperphosphorylation on Ser/Thr residues. In a feed-forward loop, adaptive signaling elements such as mTORc and SK61 have been shown to hyperphosphorylate S632 and S302/S522 residues, respectively. This results in reduced insulin binding sensitivity of the insulin receptor and subsequent cellular IR, as well as the translocation of the active portion of IRS from the membrane to the cytosol [31–34]. S337 phosphorylation on IRS-1 by GSK3-β has been shown to inhibit insulin signaling in humans [35], while, phosphorylation at S312 in humans stimulates uncoupling of IRS-1 and leads to its degradation [36]. Insufficient downstream signaling as a result of this degradation is another proposed hypothesis for IR [37].
Methods of measuring insulin resistance
The gold standard for measuring whole body IR is the euglycemic hyperinsulemic clamp technique. While this provides accurate, real time data, the technique is laborious and invasive, requiring intravenous injection of insulin and glucose, as well as continuous blood collection over multiple hours [38]. The Oral Glucose Tolerance Test (OGTT) has long been used to quantify glucose intolerance, but by nature cannot serve as an indicator of insulin resistance. The Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), developed over 30 years ago by Matthews et al., provides an estimate of insulin resistance and β cell function by combining fasting insulin and glucose levels in a single metric [39]. Advances in computer-based modeling have led to the updated HOMA2-IR metric, improving reliability by accounting for various physiological adjustments. Nevertheless, it should be noted that transient fluctuations can also affect fasting glucose and insulin levels limiting the reliability of these metrics. The Somogyi effect, a somewhat rare phenomenon in T2D patients, results in hyperglycemia after extended hypoglycemia, particularly in the early morning. Coupled with the more common dawn effect, which results to variations in insulin levels due to circadian hormone fluctuations, it produces significant variability in fasting glucose and insulin levels. Because of these factors, it is imperative to develop rapid and reliable diagnostic markers for systemic, as well as tissue specific IR.
Peripheral Insulin Resistance
Muscle
Chronic IR in skeletal muscle has long been considered a hallmark of T2D [40]. In an insulin resistant state, muscle glycogen synthesis is impaired due to decreased glucose uptake [41]. This is thought to be the result of GLUT-4 gene suppression due to excess free fatty acids [42, 43]. Fatty acid levels have been shown to negatively correlate with insulin activity in skeletal muscle [44], whereas high levels of saturated fatty acids can directly induce IR in skeletal muscle by inhibiting normal IRS1 Tyr-phopshorylation [45–48]. Recent studies investigating O-linked-β-N-acetylglucosamine (O-GlcNAc) protein modifications have shown aberrant modification of IRS Ser/Thr residues (mediated by O-GlcNAc transferase (OGT) and β-N-acetylglucosaminidase (OGA)) interfering with IRS-PI3K interaction[49, 50].
Liver
A vital function of the liver is to both produce and store glycogen as a readily available glucose reserve for the body. In the normal post-prandial state, glycogenolysis and hepatic glucose output is sufficient to meet the energy requirements of the brain and other organs [51, 52]. In an insulin resistant state, this output is increased contributing to the phenomenon of systemic IR [53]. Moreover, liver-specific IR has long been regarded as a leading contributor to the onset of Non-Alcoholic Fatty Liver Disease (NAFLD), one of the most common manifestations of chronic liver diseases [52, 54]. Liver adipose tissue secretes several proinflammatory factors, such as IL-6 and TNF-α, which play a role in inducing systemic IR. IL-6, which is also secreted by skeletal muscle and select immune cells, can target multiple regions of the body, including the brain [55]. Chronically elevated levels of IL-6 lead to reduced Tyr-phosphorylation of IRS-1, as well as decreased glycogen synthesis, in primary murine hepatocytes as well as the human hepatocarcinoma cell line HepG2 [56]. In AD, IL-6 has long been implicated in neuroinflammation, been shown to stimulate the formation of amyloid precursor protein (APP), and is often co-localized with beta-amyloid (Aβ) plaques in AD patients [57–59]. TNF-α; secreted by various immune cells, acts to impair hepatic insulin signaling via inhibiting autophosphorylation of insulin receptor after insulin binding, which subsequently reduces tyrosine kinase activation of IRS-1 [60]. Finally, ceramides produced by the liver have also been implicated as a link between peripheral and brain IR, as well as neurodegeneration [61].
Brain Insulin Resistance
The brain was long considered to be an insulin-independent tissue because of insulin’s inability to affect bulk glucose uptake in cortical tissue, until radioimmunoassays showed high levels of insulin in brain extracts [62–65]. Insulin in the brain is predominantly shuttled across the BBB from the periphery, where it is concentrated to levels 50x higher than in circulating plasma independently of peripheral hormonal states [62, 66, 67]. Insulin receptors on the BBB are capable of signal transduction across the BBB. In addition, peripherally produced insulin can be actively transported into the brain via an endocytic-exocytic mechanism [68]. However, insulin can also be produced de novo in brain regions with many pyramidal cells, such as the hippocampus, prefrontal cortex, olfactory bulb, and entorhinal cortex [69–73]. While the exact origin of brain insulin is still debated, recent studies show that functional insulin signaling components in forebrain regions may exert a neuroprotective role in areas responsible for various functions of memory [74, 75]. Downstream elements in the signaling pathway known as the “PI3K route” have been shown to both promote neuronal cell survival and facilitate synaptic plasticity, and as such are heavily implicated in the link between insulin resistance and AD [76].
A variety of factors underlie brain IR. Maternal glucose and insulin sensitivity have been shown to directly correlate with fetal brain responses to fluctuations in circulating glucose, suggesting that predisposition to brain IR may be present before birth[77]. Multiple genetic mutations have been associated with increased predisposition to brain IR, such as mutations in the obesity genes Fat Mass and Obesity-Associated Protein (FTO) and Melanocortin-4 Receptor (MC4R). Polymorphisms spanning across introns 1 and 2 of FTO, which is most highly expressed in the brain, exhibit strong effects on brain IR [78]. Carriers of the at risk FTO-AA allele who are also carriers of an APOE ε4 allele have a significantly increased risk for AD and dementia [79]. MC4R is expressed in specific brain regions that regulate systemic metabolism (such as the hypothalamus) and regulates synaptic plasticity [80]. Additionally, a single nucleotide polymorphism near the MC4R gene (rs17782313) has been directly linked to increases in brain IR [81]. Increased circulating free fatty acids may also play a role in establishing brain IR. High fat diet leads to rapid release of pro-inflammatory factors at the hypothalamus, and triggers the JNK pathway to increase activation of NF-kB, a factor that inhibits leptin and insulin signaling [82, 83].
Dysfunctional phosphorylation of IRS-1 has been extensively linked with brain IR, similar to other tissues. Total levels of insulin signaling proteins in the aforementioned “PI3K route” are not significantly different in the brains of AD patients versus cognitively normal controls, suggesting that the phosphorylated active states of these molecules may play a role in IR and AD pathogenesis [84]. Studies in human hippocampal tissue have shown that phosphorylation mediated by factors such as mTOR and GSK-3β, coupled with feed-forward inhibition from the JNK pathway, leads to specific increased phosphorylation on multiple Ser residues of IRS-1 (specifically, S312, S616 and S636) [34, 84, 85]. However, conflicting evidence exists showing that S307 phosphorylation in mice (human S312) may in fact increase insulin sensitivity and improve insulin signaling [86].
Animal Models of AD and IR
It would be an understatement to say that animal models have proven to be effective and informative in the study of AD in relation to brain IR. There is in fact little we would know about the mechanisms underlying this relationship without their contribution. Despite this, one should keep in mind that there is no definitive orthologous version of AD in animals; instead the induction of a neuropathologically similar state in the animal model is used to simulate the human disease. In this section, we will review newer induced and transgenic animal models that incorporate aspects of both AD and brain IR. In these models the metabolic connection between AD and IR is exploited to provide a more valid model for translation to human research.
Interference with normal insulin signaling is a favored method of creating an animal model with a metabolic state comparable to diabetes. As impairments in insulin signaling can also elicit neurodegenerative changes, this method can satisfy the requirements for an animal model showing symptoms common to AD and IR. The most intuitive method involves mimicking adult onset diabetes by placing mice or rats on a high fat diet (HFD) that promotes IR [87]. As this method can easily be used in both wild type and transgenic mice, it provides a means of observing the effects of IR in a variety of phenotypes. Neurodegeneration has also been reliably evoked via HFD, as well as tau hyperphosphorylation and Aβ burden [88, 89]. Intracerebroventricular Streptozotocin (icv-STZ) [90] is another prominent method in which a rat or mouse is intracerebrally injected with STZ to induce IR as well as neurodegeneration, spatial memory deficits, and a concomitant increase in Aβ and plaque formation [91, 92]. Peripherally, the cytotoxic and diabetogenic effect of STZ relies on its entry into pancreatic B-cells through the GLUT 2 transporter, causing oxidative stress, necrosis due to alkylation of DNA, and activation of poly-ADP-ribosylation [93]. Centrally, the mechanism of action is proposed to be the desensitization of brain insulin receptors [90], and the STZ-vulnerable GLUT2 has been found in circumventricular areas of the brain [94, 95]. However, no direct evidence of insulin receptor desensitization has been provided, and the current lack of a known mechanism of action remains a limitation of this method.
Transgenic animal models include rats or mice with gene knockouts or inactivations that elicit insulin resistance and neurodegeneration similar to that expected of AD and IR in humans. The ob/ob transgenic mouse expresses an inactive version of the protein leptin, and feeds excessively as a result [96, 97]. These animals are obese and exhibit high blood glucose and insulin levels, lower levels of IRS-1and 2, behavioral deficits, and tau hyperphosphorylation [98–101]. A similar transgenic model is the db/db mouse, which fails to respond to leptin and also reliably displays a phenotype of obesity, increased tau phosphorylation and IR accompanied by profound behavioral deficits in learning and memory [102–104].
IR and Oxidative Stress in AD
A recent and prominent concern has been the role of IR as a factor promoting oxidative stress in the pathogenetic cascade of AD [105, 106]. The term “oxidative stress” refers to an imbalanced biochemical state wherein the cell is producing more reactive oxygen species than its antioxidant activity can withstand [107]. The brain is particularly vulnerable to oxidative stress due to its high oxygen requirements; low antioxidant levels that only decrease further with age, and the sheer membrane lipid content available for destructive peroxidation [108–110]. Many researchers now implicate oxidative stress as a causative factor upstream of Aβ and tau [111, 112].
AD is increasingly viewed as a consequence of a dysfunctional metabolic state, which makes specific metabolic dysfunctions such as IR and oxidative stress obvious culprits in its progression. The process by which this occurs is by no means straightforward. Neurons become especially vulnerable to oxidative stress when insulin signaling is disrupted, and oxidative stress leads to further IR [113–116]. Both IR and oxidative stress independently lead to the accumulation of Aβ and phosphorylated tau [117, 118]. Oxidative stress also occurs as a result of metabolic syndrome and obesity [119]. This web of possibly inextricable connections firmly places IR, oxidative stress, and AD in a complex positive feedback system.
One direct method for severing this oxidative-stress/IR knot would be to address oxidative stress by way of antioxidants. This has been a focus of numerous basic and clinical studies, in which antioxidant supplements, such as the free-radical scavenging vitamins C and E, estrogen, statins, fish oil, and resveratrol have all shown some effect in decreasing the risk of AD [120–123]. Likewise, caloric restriction and exercise recruits a variety of antioxidant defenses with similar preventive effects [124]. The IR/AD/antioxidant issue has been investigated using animal models displaying high oxidative stress, as well as in humans using phenotypes or measured exposure to risk factors for oxidative stress due to lifestyle or environment [125, 126]. It is important to note that the evidence in support of antioxidant supplements in AD comes from animal and epidemiological studies, whereas clinical trials have generally been negative. Overall, it seems that oxidative stress has an upstream role in AD pathogenesis, and lifestyle changes leading to its decrease (diet, exercise) may be a better therapeutic strategy than antioxidant supplementation.
Brain IR and Aβ pathology
Aβ refers to several peptides between 39–43 amino acids in length that are formed by the cleavage of the larger APP molecule through actions of β and γ secretases, and are a product of normal cellular metabolism with a possible but yet unknown physiologic role. Aberrant oligomerization of certain Aβ peptides (such as Aβ42) and formation of extracellular plaques with Aβ fibrils at their center are histopathological hallmarks of AD in post mortem brain tissue [127–130]. In sporadic AD, Aβ oligomerization may partly occur because of reduced Aβ degradation and clearance from the brain [131, 132]. In normal conditions, stimulation by insulin accelerates Aβ clearance from the brain, preventing extracellular accumulation and eventual fibril and plaque formation [133]. In the AD brain, Aβ oligomers have been shown to increase activation of the JNK pathway, leading to increased IRS-1 pS616 as well as Tau pS422 [134]. Furthermore, Aβ is known to suppress cell surface distribution of insulin receptor as a mechanism of inhibiting insulin function and inducing/aggravating the IR state [135–137]. A feed-forward mechanism where Aβ oligomers aggravate brain IR via Ser-phosphorylation of IRS-1, which in turn decreases Aβ clearance and increases extracellular Aβ is plausible.
Aβ can be degraded by a variety of peptidases, such as Insulin Degrading Enzyme (IDE), neprilysin, and angiotensin converting enzyme, as well as multiple serine proteases (plasmin, urokinase-type and tissue-type plasminogen activators) [138, 139]. Because of IDE’s ability to degrade insulin, amylin, and Aβ42, it is thought to be a link connecting hyperinsulemia, IR and AD [140, 141]. IDE’s unique structure with two half-dome subunits connected by a linker limits its ability to cleave large Aβ subunits[142]. Therefore, IDE is thought to only cleave monomeric Aβ [138, 142]. In mice, insulin resistance leads to increased brain amyloidosis through an increase in gamma-secretase activity, as well as decreased IDE [143, 144]. Furthermore, in human AD patients with the APOE ε4 allele, IDE expression in areas such as the hippocampus is greatly reduced [145].
Brain IR and tau pathology
Tau is a member of a large group of proteins known as microtubule associated proteins (MAPs). In its native conformation, tau is a soluble and unfolded protein involved in microtubule stabilization and axonal outgrowth in neurons. However, hyperphosphorylated tau’s aggregative properties play a role in the pathogenesis of various neurodegenerative diseases. In AD, tau aggregates to intracellular neurofibrillary tangles, which alongside extracellular amyloid plaques constitute the two main histopathological hallmarks used to identify the disease in post-mortem brain tissue [146].
Several studies have implicated insulin and IR in the development of tau aggregates. Tau aggregation potential depends on its phosphorylation state, which is dependent on the local activity of kinases and phosphatases. Intravenous insulin administration exerts a biphasic effect on tau phosphorylation. Short-term administration of insulin to human neuroblastoma cells or rat primary cortical neurons leads to rapid hyperphosphorylation of tau at several Ser/Thr residues, whereas prolonged exposure resulted in decreased phosphorylation of these epitopes [147, 148]. This increase and subsequent decrease was mirrored by GSK-3β activity, widely considered to be the primary kinase responsible for the phosphorylation of Tau in vivo and is modulated by insulin via the PKB/Akt pathway [149–152]. Upstream of GSK-3β, PKB/Akt itself also functions as a Ser/Thr kinase and can phosphorylate Tau directly, at least in vitro [153, 154]. Conversely, inhibiting the Ser/Thr phosphatases responsible for tau dephosphorylation can also increase the overall phosphorylation of tau. PP2A is the primary tau phosphatase implicated in AD and T2D and is suppressed by insulin administration in both human animal studies [155–159]. This should not be interpreted as a simple activation/inhibition model; GSK-3β, Akt/PKB, and PP2A also show regulatory effects on each other and a differential preference for the many available tau epitopes [154]. In broad terms however, the combined effects of insulin on tau phosphorylation by GSK-3β and lack of de-phosphorylation by PP2A may be a basis for explaining the increased tendency for Tau aggregation in AD and brain IR. Moreover, increased cytosolic levels of IRS-1 pS312 and pS616 correlate with the presence of neurofibrillary tangles in the brains of AD patients, whereas IRS-1 pS312 is restricted to nuclear regions of the cell in cognitively normal controls. This finding suggests that IRS-1 phospho-species may have actions promoting tau pathology in AD beyond their role in the development of brain IR [160].
Spatial Co-Expression of AD & IR Related Genes
The advent of microarray techniques has brought with it the ability to sample massive amounts of genetic transcript data from human brain specimens. The Allen Human Brain Atlas (AHBA) is a groundbreaking online bioinformatics resource that enables users to freely examine the multi-sample microarray data from six normal human post-mortem brain specimens [161]. As this data contains a well-distributed set of approximately 500–1000 regional samples across each brain, it has the resolution needed to compare the spatial pattern of gene expression across different genes and brain regions. This information can be used to examine the similarity of gene expression across the brain, positing that “co-expressed” genes that share common spatial patterns of expression may also be related functionally [162]. For this analysis, AHBA expression data was downloaded from the Allen Brain Atlas data portal (www.brain-map.org) for each gene of interest. When multiple probes for the same gene existed, the expression z-scores were averaged within samples. Within each subject, the expression z-scores for genes of interest were correlated with each other, resulting r-scores were then z-transformed and averaged across subjects, then inverse z-transformed back to r using a custom MATLAB script. The resulting mean r-scores were displayed in heat map format for ease of comparison.
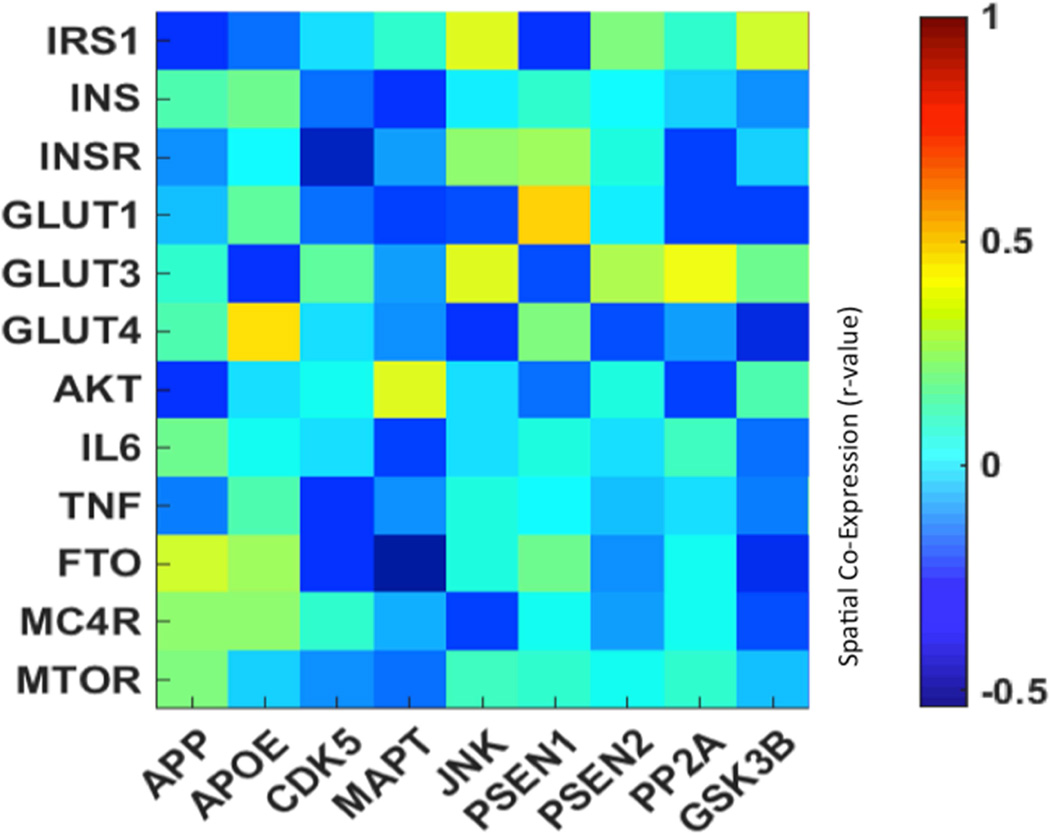
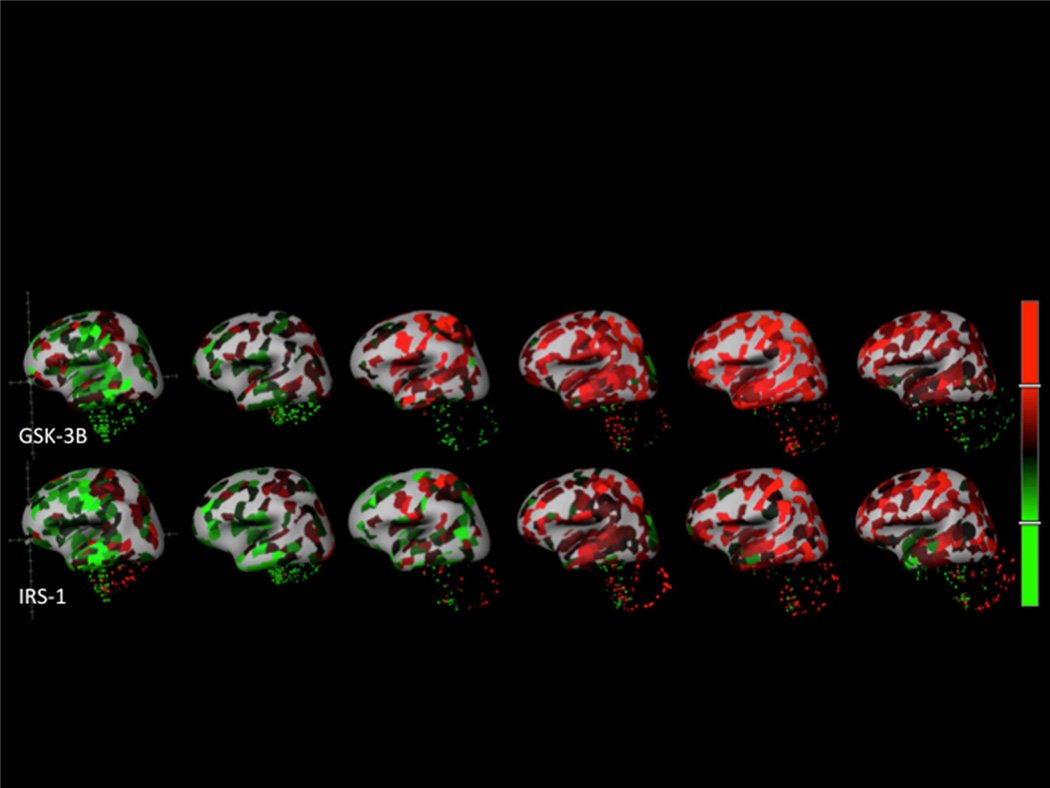
The spatial co-expression analysis shows substantial overlap between many of the AD and IR genes examined in this manuscript. Earlier, Tau hyperphosphorylation was tied to insulin regulation and an interaction between GSK-3β, while MAPT/Tau is likewise strongly associated with Akt in the described pathway. In the heat map (figure 1) and brain map images (figure 2) we can see positive correlations between IRS-1 and GSK-3β as an example, indicating strong spatial co-expression of these genes. The relation between IRS-1 and JNK can also be seen in figure 1; the two genes show spatial co-expression as well as with GLUT-3 and INSR. Uncorrelated or negatively correlated genes of interest likely have effects that are less dependent on regional co-expression.
Figure 1.
Heat map of the Pearson correlation r between a selection of probes associated with IR y-axis (top) and AD (x-axis). Heat maps highlight the relation between AD-related and IR-related gene expression levels. Squares with higher r-values (green to yellow) indicate genes that are co-expressed; squares with lower r-values (blue to dark blue) indicate genes with little to no spatial overlap.
Figure 2.
AHBA sampling locations with expression values superimposed on 3D representations of the six human brain specimens as z-scores (red indicates positive, green indicates negative). The top row is GSK-3B (probe: CUST_14455_PI416261804) and the bottom row is IRS-1 (probe: A_24_P225679). Image credit: Allen Brain Atlas.
Clinical studies on IR and AD
Neuroimaging studies on IR and AD
Advances in neuroimaging techniques are behind some of the most groundbreaking recent findings on this topic allowing researchers to detect a variety of AD/IR-related brain changes in-vivo, which is crucial for human research, and even longitudinal animal studies. Fluorodeoxyglucose Positron emission tomography (FDG-PET) imaging has a lengthy background as a means of probing both AD and brain metabolism. FDG is an analog of glucose that gathers in tissue undergoing glucose metabolism, providing a reliable marker of the cerebral metabolic rate for glucose (CMRGlc). As has been shown as early as 1989 [163] and confirmed repeatedly ever since [164, 165], in AD, CMRGlc is drastically decreased in a characteristic regional pattern including posterior cingulate, precuneus, parietotemporal, and frontal cortices. Intriguingly, the same pattern of relative hypometabolism was shown in relation to HOMA-IR in cognitively normal older adults with prediabetes/T2D [166] or at higher risk for AD given their parental history [167]. In a study of patients with MCI and AD, we showed that HOMA-IR is negatively associated with glucose metabolism in brain areas vulnerable to AD pathology, but not in areas typically unaffected by AD [168]. In addition, we showed that HOMA-IR is associated with a maladaptive increase in metabolism at the hippocampus in MCI patients who are going to progress to AD dementia [168].
PET is a flexible technique and other useful radiotracers have been developed to address different aspects of AD, particularly Pittsburgh compound B (PiB) and Florbetapir (F18-AV-45) for marking Aβ accumulations[169, 170], and most recently Flortaucepir (18F-AV-1451) and other tracers for tau. Conflicting findings exist on the relationship between Aβ deposition and peripheral IR, with some studies showing no such relationship [171, 172] and others indicating a relationship for normoglycemic but not hyperglycemic cognitively normal older adults [173]. Tau-PET imaging has attracted a surge of interest due to recent findings that it presents a stronger relation to neurodegeneration and cognitive decline than Aβ [174, 175], but being a very recent development there are no published results to report on the relation of tau distribution to IR.
Magnetic Resonance Imaging (MRI) has also been used to study IR in relation to AD. Structural MRI has been used to find an association between the duration of T2D and the presence of AD-like neurodegenerative lacunae and hippocampal atrophy in older individuals [176]. In addition, HOMA-IR has been negatively associated with gray matter volume in late middle-aged, cognitively healthy individuals in a pattern typical of AD atrophy [177]. Brain iron concentration can be measured using T2* relaxation sequences; recent evidence has shown a relation between brain iron overload in the hippocampus and other areas with both IR and deficits in cognitive performance [178]. Advanced two-dimensional (2D MRS) methods currently gaining favor are capable of detecting glucose concentrations within specific regions of the brain [179] and may be used some day to study IR in relation to AD. Diffusion MRI has revealed deficits in the microstructural integrity of grey and white matter in AD [180–183] and T2D [184–186] that are associated with impaired cognitive performance. Functional MRI (fMRI) has been used to demonstrate that insulin infusion enhances neuronal activity in the medial temporal lobe [187], that patients with T2D showed connectivity decreases in regions associated with AD [188, 189], and that higher peripheral insulin levels are associated with less cognitive decline and atrophy in AD patients [190].
As a general comment to all neuroimaging studies to date, since no good biomarker of brain IR existed, the field had to rely on the assumption that some peripheral IR measure can be used as a surrogate of brain IR. With the discovery of IRS-1 phospho-peptides in neural-origin plasma extracellular vesicles (EVs) [191] we have introduced a unique biomarker for brain IR. We hope that future neuroimaging studies will take advantage of this novel biomarker and examine more brain-specific associations.
CSF Insulin and Glucose
Cerebrospinal fluid (CSF) has long been known to carry appreciable concentrations of glucose and insulin, which partially reflect blood levels [192]. In humans, the transfer of blood insulin into the CSF has been validated during intravenous injections of insulin [193]. Interestingly, in obesity, the CSF/plasma insulin ratio is decreased. Similarly, the CSF/plasma ratios for leptin and adiponectin are also decreased [194, 195]. IR as measured by HOMA-IR has been shown to correlate with increased CSF levels of AD biomarkers such as soluble amyloid precursor protein β (sAPPβ), P-tau181 and Aβ42, with ApoE ε4 carriers showing even higher levels of these proteins [144, 196]. Additionally, increased CSF levels of insulin correlate with decreased cognitive performance in patients with diabetes and AD [197].
Extracellular Vesicles as biomarkers for IR and AD
EVs (a subtype of which are exosomes, 30–150 nm in size), are membranous particles and are secreted from nearly every cell type throughout the body. A hot topic in recent research, these small vesicles are thought to serve a variety of functions in both healthy and disease states, from cellular waste removal to shuttles of various proteins, and are most recently being utilized as a soucres of biomarkers. A role for EVs in AD was first suggested by studies where EVs extracted directly from murine brain tissue were shown to contain full length APP, as well as various fragment length APP cleavage products, including Aβ [198, 199] Moreover, secreted EVs have been shown to contain hyperphosphorylated tau as well as Aβ, the latter through sorting into multivesicular bodies (MVB’s), a canonical step in the endosomal-exosomal formation pathway[200]. Moreover, astrocytic derived EV’s have been shown to mediate apoptosis leading to brain cell loss [201]. Lastly, there is evidence to support that tau overexpression selectively recruits mitochondrial proteins implicated in neurodegeneration to exosomes, providing a novel link between tau exosome secretion and AD pathology [202]. However, there are also findings that support a neuroprotective role of exosomes in the face of neurodegenerative diseases. For instance, EVs are also known to contain proteolytically active IDE which may be degrading extracellular Aβ [203]. Additionally, it is thought that EVs released by astrocytes may serve to regulate synaptic transmission, as well as synaptic regeneration following injury, supporting a neuroprotective role [204].
One of the most interesting and exciting aspects of EV research is the potential for them to be used as source for biomarkers for neurodegenerative diseases. For instance, EVs are highly enriched in miRNA, small noncoding RNA that function in RNA silencing and regulation of gene expression. Several miRNAs associated with neuronal development (miR-29, miR -128, miR-137), differentiation (miR-9, miR-107, miR-124, miR-128), apoptosis (miR-29b), and oxidative stress (miR-128) are downregulated in AD [205]. Our team has been a pioneer in pursuing protein biomarkers for AD using plasma EVs enriched for neuronal origin. These neuronal origin-enriched EV biomarkers include pathogenic proteins (total tau, p-tau, and Aβ42), but also intracellular signaling molecules normally not detectable in the soluble phase of plasma, such as phosphorylated IRS-1 species, Cathepsin-D, REST, LRP6, and others [191, 206–208].
Of particular interest are our findings concerning IRS-1. In plasma EVs enriched them for neuronal origin, we measured total IRS-1, pSer312-IRS-1 and p-PanY-IRS-1 in a clinical cohort of AD patients and cognitively normal (CN) older control subjects (as well as patients with Frontotemporal Dementia, as a neurodegenerative disease control, and cognitively normal patients with T2D, as a metabolic disease control. We showed that these two phospho-species, as well as their ratio, were highly significantly different in AD patients vs. all control groups. Interestingly, subjects with T2D had intermediate values between AD patients and CN controls, suggesting that the peripheral IR that characterizes T2D is linked to some degree to brain IR and corroborating the extensive body of literature suggesting that IR and T2D are risk factors for AD, but by no means obligatory causative factors. Furthermore, IRS-1 phospho-species achieved remarkable classification accuracy for AD patients vs. controls and, in a separate smaller cohort, were already abnormal up to 10 years before clinical onset of AD[191]. These findings not only further establish the links between IR and AD, but provide hope for a blood-based diagnostic assay to diagnose AD preclinically. With the discovery of IRS-1 peptides in neural-origin plasma EVs we have introduced a unique biomarker for brain IR. Importantly, since interventions that aim to reverse brain IR in AD are being subjected to clinical trials (e.g. intranasal insulin, exenatide), using these biomarkers we may be able to demonstrate target engagement and follow response to treatment.
Targeting IR as a therapeutic strategy for AD
Overcoming brain IR by increasing brain availability of insulin
There have been several studies investigating ways to directly administer insulin to the brain to increase its local availability, while avoiding systemic insulin effects and hypoglycemia. Intranasal insulin administration involves bulk flow through the olfactory bulb into the brain without affecting systemic insulin and glucose levels. In humans, increased CSF insulin levels are present as soon as 60 minutes after intranasal administration[209]. In a Phase II clinical trial, intranasal insulin at low doses (20 IU) over the course of four months improved cognitive (especially memory) performance, but this effect is not seen at higher doses (40 IU) [210]. Interestingly, carriers of the APOE ε4 allele did not exhibit any memory improvement [211]. A recent follow up study showed gender specific differences in patients’s responses and supported modifying effects for APOE ε4 [212].
Early results investigating the long-acting intranasal insulin analog detemir are also promising. Higher brain IR is a predictor of treatment efficacy, with highly resistant patients showing improvements in verbal and visuospatial working memory with 40 IU. Detemir uniquely showed cognitive improvement for APOE ε4 carriers on the 40 IU dosing regimen [213].
Insulin Sensitization (PPAR-gamma)
Thiazolidinediones (TZDs/Glitazones) constitute a group of diabetes drugs that improve whole body insulin sensitivity. While their mode of action is still largely unknown, it is thought to be through the activation of the perioxisome proliferator-activated receptor gamma (PPAR-gamma), a nuclear hormone receptor and transcriptional-level regulator in a variety of tissues throughout the body. The final effect is increased expression of GLUT-4, suppression of neuroinflammation, and increased Aβ clearance [214]. Two drugs, rosiglitazone and pioglitazone, are currently being investigated as therapeutic agents for AD. Rosiglitazone potentiates the protective effects of insulin on cultured neurons and inhibits the production of Aβ42 in mice, but evidence in human trials is inconclusive [135, 215, 216]. Additionally, rosiglitazone has been shown to have anti-inflammatory effects decreasing levels of NFκB [217]. Pioglitazone acts in a similar fashion to Rosiglitazone as an anti-diabetic drug. In mice, pioglitazone improves learning, reduces tau and Aβ deposits in the hippocampus, and improves neuronal plasticity [218]. In humans, consistent pioglitazone administration has been associated with decreased incidence of dementia, but clinical trials are lacking [219].
GLP-1 agonists/Exenatide
GLP-1 is a 30–amino acid peptide primarily produced by intestinal endocrine epithelial L-cells in response to food in order to stimulate insulin release from the pancreas [220]. Peripherally produced GLP1 crosses the blood brain barrier, although it is also produced in the brain [220]. A variety of therapeutics have been developed that greatly extend the normally rapid half life of native GLP-1, such as exenatide, liraglutide, lixisenatide, and albiglutide (a GLP-1 dimer fused to a human albumin) [221]. GLP-1 agonists have been shown to engage multiple targets in the pathogenesis of AD (offer neuroprotection [222, 223], reverse brain IR [224, 225], decrease Aβ and tau levels and deposits [226, 227], decrease tau hyper-phosphorylation [228], among multiple actions) in multiple cellular and animal models of AD. Most notably, exenatide, the synthetic version of exendin-4, which is found in Heloderma lizard venom that shares a 53% homology with human GLP-1 [229], has been shown to alleviate brain IR in AD [224] and be neuroprotective against a variety of neurodegenerative diseases and insults to the brain besides AD [230–232]. Importantly, exenatide has demonstrated clinical effectiveness for Parkinson disease, in terms of motor, but also cognitive performance measures [233]. Based on this evidence, our team conducts a Phase II clinical trial of exenatide in MCI/early AD (NCT01255163).
Conclusions
This review attempted to disentangle the complex mechanisms underlying brain-specific IR vis-à-vis systemic IR and highlight (proven or plausible) links to the AD pathogenic cascade. Given unanswered questions about the sequence of events leading to AD, the picture that emerges is far from being complete. Nevertheless, the amount and diverse sources of evidence make it certain that brain IR plays a major role in AD pathogenesis, which is generally compatible with the prevailing “amyloid hypothesis”. Ultimately, the proof of this hypothesis, as for any competing hypothesis, rests on demonstrating effectiveness in clinical trials, a goal that only recently started being pursued.
Acknowledgments
All authors have read the journal’s authorship agreement. This research was supported entirely by the Intramural Research Program of the National Institute on Aging, NIH.
Abbreviations
- AD
Alzheimer’s disease
- IR
insulin resistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: All authors have read the journal’s policy on disclosure of potential conflicts of interest and have none to declare.
References
- 1.Leney SE, Tavare JM. The molecular basis of insulin-stimulated glucose uptake: signalling, trafficking and potential drug targets. J Endocrinol. 2009;203:1–18. doi: 10.1677/JOE-09-0037. [DOI] [PubMed] [Google Scholar]
- 2.Draznin B. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: the two sides of a coin. Diabetes. 2006;55:2392–2397. doi: 10.2337/db06-0391. [DOI] [PubMed] [Google Scholar]
- 3.Tzatsos A. Raptor binds the SAIN (Shc and IRS-1 NPXY binding) domain of insulin receptor substrate-1 (IRS-1) and regulates the phosphorylation of IRS-1 at Ser-636/639 by mTOR. J Biol Chem. 2009;284:22525–22534. doi: 10.1074/jbc.M109.027748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Liu F. Tissue-specific insulin signaling in the regulation of metabolism and aging. IUBMB Life. 2014;66:485–495. doi: 10.1002/iub.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106:165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, et al. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–272. doi: 10.1016/j.cmet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Decker RE, Chalif DJ. Progressive coma after the transsphenoidal decompression of a pituitary adenoma with marked suprasellar extension: report of two cases. Neurosurgery. 1991;28:154–157. doi: 10.1097/00006123-199101000-00023. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell. 2006;17:4484–4493. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sano H, Eguez L, Teruel MN, Fukuda M, Chuang TD, Chavez JA, et al. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007;5:293–303. doi: 10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Saltiel AR, Pessin JE. Insulin signaling in microdomains of the plasma membrane. Traffic. 2003;4:711–716. doi: 10.1034/j.1600-0854.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 12.Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121–138. doi: 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebeling P, Koistinen HA, Koivisto VA. Insulin-independent glucose transport regulates insulin sensitivity. FEBS Lett. 1998;436:301–303. doi: 10.1016/s0014-5793(98)01149-1. [DOI] [PubMed] [Google Scholar]
- 14.Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- 15.Vannucci SJ, Koehler-Stec EM, Li K, Reynolds TH, Clark R, Simpson IA. GLUT4 glucose transporter expression in rodent brain: effect of diabetes. Brain Res. 1998;797:1–11. doi: 10.1016/s0006-8993(98)00103-6. [DOI] [PubMed] [Google Scholar]
- 16.Maher F, Davies-Hill TM, Lysko PG, Henneberry RC, Simpson IA. Expression of two glucose transporters, GLUT1 and GLUT3, in cultured cerebellar neurons: Evidence for neuron-specific expression of GLUT3. Mol Cell Neurosci. 1991;2:351–360. doi: 10.1016/1044-7431(91)90066-w. [DOI] [PubMed] [Google Scholar]
- 17.Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008;295:E242–E253. doi: 10.1152/ajpendo.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duelli R, Kuschinsky W. Brain glucose transporters: relationship to local energy demand. News Physiol Sci. 2001;16:71–76. doi: 10.1152/physiologyonline.2001.16.2.71. [DOI] [PubMed] [Google Scholar]
- 19.Leybaert L, De Bock M, Van Moorhem M, Decrock E, De Vuyst E. Neurobarrier coupling in the brain: adjusting glucose entry with demand. J Neurosci Res. 2007;85:3213–3220. doi: 10.1002/jnr.21189. [DOI] [PubMed] [Google Scholar]
- 20.Cornford EM, Hyman S. Localization of brain endothelial luminal and abluminal transporters with immunogold electron microscopy. NeuroRx. 2005;2:27–43. doi: 10.1602/neurorx.2.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz MW, Figlewicz DF, Kahn SE, Baskin DG, Greenwood MR, Porte D., Jr Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides. 1990;11:467–472. doi: 10.1016/0196-9781(90)90044-6. [DOI] [PubMed] [Google Scholar]
- 22.Heni M, Kullmann S, Preissl H, Fritsche A, Haring HU. Impaired insulin action in the human brain: causes and metabolic consequences. Nat Rev Endocrinol. 2015;11:701–711. doi: 10.1038/nrendo.2015.173. [DOI] [PubMed] [Google Scholar]
- 23.Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm (Vienna) 1998;105:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- 24.Morgello S, Uson RR, Schwartz EJ, Haber RS. The human blood-brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia. 1995;14:43–54. doi: 10.1002/glia.440140107. [DOI] [PubMed] [Google Scholar]
- 25.Apelt J, Mehlhorn G, Schliebs R. Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J Neurosci Res. 1999;57:693–705. [PubMed] [Google Scholar]
- 26.Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S135–S148. doi: 10.1055/s-2001-18576. [DOI] [PubMed] [Google Scholar]
- 27.Leahy JL. Pathogenesis of type 2 diabetes mellitus. Arch Med Res. 2005;36:197–209. doi: 10.1016/j.arcmed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Czech MP, Klarlund JK, Yagaloff KA, Bradford AP, Lewis RE. Insulin receptor signaling. Activation of multiple serine kinases. J Biol Chem. 1988;263:11017–11020. [PubMed] [Google Scholar]
- 29.Tanti JF, Gremeaux T, van Obberghen E, Le Marchand-Brustel Y. Serine/threonine phosphorylation of insulin receptor substrate 1 modulates insulin receptor signaling. J Biol Chem. 1994;269:6051–6057. [PubMed] [Google Scholar]
- 30.Singh TJ. Insulin receptor serine kinase activation by casein kinase 2 and a membrane tyrosine kinase. Mol Cell Biochem. 1993;121:167–174. doi: 10.1007/BF00925976. [DOI] [PubMed] [Google Scholar]
- 31.Ryu J, Galan AK, Xin X, Dong F, Abdul-Ghani MA, Zhou L, et al. APPL1 potentiates insulin sensitivity by facilitating the binding of IRS1/2 to the insulin receptor. Cell Rep. 2014;7:1227–1238. doi: 10.1016/j.celrep.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 34.Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. 2009;296:E581–E591. doi: 10.1152/ajpendo.90437.2008. [DOI] [PubMed] [Google Scholar]
- 35.Eldar-Finkelman H, Krebs EG. Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc Natl Acad Sci U S A. 1997;94:9660–9664. doi: 10.1073/pnas.94.18.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun XJ, Goldberg JL, Qiao LY, Mitchell JJ. Insulin-induced insulin receptor substrate-1 degradation is mediated by the proteasome degradation pathway. Diabetes. 1999;48:1359–1364. doi: 10.2337/diabetes.48.7.1359. [DOI] [PubMed] [Google Scholar]
- 37.Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes. 2001;50:24–31. doi: 10.2337/diabetes.50.1.24. [DOI] [PubMed] [Google Scholar]
- 38.Le DS, Brookshire T, Krakoff J, Bunt JC. Repeatability and reproducibility of the hyperinsulinemic-euglycemic clamp and the tracer dilution technique in a controlled inpatient setting. Metabolism. 2009;58:304–310. doi: 10.1016/j.metabol.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 40.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. ix. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Hunter SJ, Garvey WT. Insulin action and insulin resistance: diseases involving defects in insulin receptors, signal transduction, and the glucose transport effector system. Am J Med. 1998;105:331–345. doi: 10.1016/s0002-9343(98)00300-3. [DOI] [PubMed] [Google Scholar]
- 42.Armoni M, Harel C, Bar-Yoseph F, Milo S, Karnieli E. Free fatty acids repress the GLUT4 gene expression in cardiac muscle via novel response elements. J Biol Chem. 2005;280:34786–34795. doi: 10.1074/jbc.M502740200. [DOI] [PubMed] [Google Scholar]
- 43.Turcotte LP, Fisher JS. Skeletal muscle insulin resistance: roles of fatty acid metabolism and exercise. Phys Ther. 2008;88:1279–1296. doi: 10.2522/ptj.20080018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 45.Martins AR, Nachbar RT, Gorjao R, Vinolo MA, Festuccia WT, Lambertucci RH, et al. Mechanisms underlying skeletal muscle insulin resistance induced by fatty acids: importance of the mitochondrial function. Lipids Health Dis. 2012;11:30. doi: 10.1186/1476-511X-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shulman GI. Cellular mechanisms of insulin resistance in humans. Am J Cardiol. 1999;84:3J–10J. doi: 10.1016/s0002-9149(99)00350-1. [DOI] [PubMed] [Google Scholar]
- 47.Roden M. How free fatty acids inhibit glucose utilization in human skeletal muscle. News Physiol Sci. 2004;19:92–96. doi: 10.1152/nips.01459.2003. [DOI] [PubMed] [Google Scholar]
- 48.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma J, Hart GW. Protein O-GlcNAcylation in diabetes and diabetic complications. Expert Rev Proteomics. 2013;10:365–380. doi: 10.1586/14789450.2013.820536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ball LE, Berkaw MN, Buse MG. Identification of the major site of O-linked beta-N-acetylglucosamine modification in the C terminus of insulin receptor substrate-1. Mol Cell Proteomics. 2006;5:313–323. doi: 10.1074/mcp.M500314-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 52.Bugianesi E, Moscatiello S, Ciaravella MF, Marchesini G. Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des. 2010;16:1941–1951. doi: 10.2174/138161210791208875. [DOI] [PubMed] [Google Scholar]
- 53.Beck-Nielsen H, Hother-Nielsen O, Staehr P. Is hepatic glucose production increased in type 2 diabetes mellitus? Curr Diab Rep. 2002;2:231–236. doi: 10.1007/s11892-002-0088-0. [DOI] [PubMed] [Google Scholar]
- 54.Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 55.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 56.Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–3399. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- 57.Ringheim GE, Szczepanik AM, Petko W, Burgher KL, Zhu SZ, Chao CC. Enhancement of beta-amyloid precursor protein transcription and expression by the soluble interleukin-6 receptor/interleukin-6 complex. Brain Res Mol Brain Res. 1998;55:35–44. doi: 10.1016/s0169-328x(97)00356-2. [DOI] [PubMed] [Google Scholar]
- 58.Bauer J, Strauss S, Schreiter-Gasser U, Ganter U, Schlegel P, Witt I, et al. Interleukin-6 and alpha-2-macroglobulin indicate an acute-phase state in Alzheimer’s disease cortices. FEBS Lett. 1991;285:111–114. doi: 10.1016/0014-5793(91)80737-n. [DOI] [PubMed] [Google Scholar]
- 59.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res. 2012;9:35–66. doi: 10.2174/156720512799015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blazquez E, Velazquez E, Hurtado-Carneiro V, Ruiz-Albusac JM. Insulin in the brain: its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front Endocrinol (Lausanne) 2014;5:161. doi: 10.3389/fendo.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Havrankova J, Schmechel D, Roth J, Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci U S A. 1978;75:5737–5741. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gray SM, Meijer RI, Barrett EJ. Insulin regulates brain function, but how does it get there? Diabetes. 2014;63:3992–3997. doi: 10.2337/db14-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seaquist ER, Damberg GS, Tkac I, Gruetter R. The effect of insulin on in vivo cerebral glucose concentrations and rates of glucose transport/metabolism in humans. Diabetes. 2001;50:2203–2209. doi: 10.2337/diabetes.50.10.2203. [DOI] [PubMed] [Google Scholar]
- 66.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Havrankova J, Roth J, Brownstein MJ. Concentrations of insulin and insulin receptors in the brain are independent of peripheral insulin levels. Studies of obese and streptozotocin-treated rodents. J Clin Invest. 1979;64:636–642. doi: 10.1172/JCI109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier insulin receptor. J Neurochem. 1985;44:1771–1778. doi: 10.1111/j.1471-4159.1985.tb07167.x. [DOI] [PubMed] [Google Scholar]
- 69.Mehran AE, Templeman NM, Brigidi GS, Lim GE, Chu KY, Hu X, et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012;16:723–737. doi: 10.1016/j.cmet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 70.Devaskar SU, Giddings SJ, Rajakumar PA, Carnaghi LR, Menon RK, Zahm DS. Insulin gene expression and insulin synthesis in mammalian neuronal cells. J Biol Chem. 1994;269:8445–8454. [PubMed] [Google Scholar]
- 71.Marks JL, King MG, Baskin DG. Localization of insulin and type 1 IGF receptors in rat brain by in vitro autoradiography and in situ hybridization. Adv Exp Med Biol. 1991;293:459–470. doi: 10.1007/978-1-4684-5949-4_41. [DOI] [PubMed] [Google Scholar]
- 72.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- 73.Heidenreich KA, Gilmore PR. Structural and functional characteristics of insulin receptors in rat neuroblastoma cells. J Neurochem. 1985;45:1642–1648. doi: 10.1111/j.1471-4159.1985.tb07237.x. [DOI] [PubMed] [Google Scholar]
- 74.McNay EC, Recknagel AK. Brain insulin signaling: a key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol Learn Mem. 2011;96:432–442. doi: 10.1016/j.nlm.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Felice FG, Lourenco MV, Ferreira ST. How does brain insulin resistance develop in Alzheimer’s disease? Alzheimers Dement. 2014;10:S26–S32. doi: 10.1016/j.jalz.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 76.van der Heide LP, Ramakers GM, Smidt MP. Insulin signaling in the central nervous system: learning to survive. Prog Neurobiol. 2006;79:205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Linder K, Schleger F, Ketterer C, Fritsche L, Kiefer-Schmidt I, Hennige A, et al. Maternal insulin sensitivity is associated with oral glucose-induced changes in fetal brain activity. Diabetologia. 2014;57:1192–1198. doi: 10.1007/s00125-014-3217-9. [DOI] [PubMed] [Google Scholar]
- 78.Reitz C, Tosto G, Mayeux R, Luchsinger JA Group N-LNFS, Alzheimer’s Disease Neuroimaging I. Genetic variants in the Fat and Obesity Associated (FTO) gene and risk of Alzheimer’s disease. PLoS One. 2012;7:e50354. doi: 10.1371/journal.pone.0050354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keller L, Xu W, Wang HX, Winblad B, Fratiglioni L, Graff C. The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer’s disease risk: a prospective cohort study. J Alzheimers Dis. 2011;23:461–469. doi: 10.3233/JAD-2010-101068. [DOI] [PubMed] [Google Scholar]
- 80.Shen Y, Fu WY, Cheng EY, Fu AK, Ip NY. Melanocortin-4 receptor regulates hippocampal synaptic plasticity through a protein kinase A-dependent mechanism. J Neurosci. 2013;33:464–472. doi: 10.1523/JNEUROSCI.3282-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tschritter O, Haupt A, Preissl H, Ketterer C, Hennige AM, Sartorius T, et al. An Obesity Risk SNP (rs17782313) near the MC4R Gene Is Associated with Cerebrocortical Insulin Resistance in Humans. J Obes. 2011;2011:283153. doi: 10.1155/2011/283153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakano H. Signaling crosstalk between NF-kappaB and JNK. Trends Immunol. 2004;25:402–405. doi: 10.1016/j.it.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 83.Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015;14:121. doi: 10.1186/s12944-015-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frojdo S, Vidal H, Pirola L. Alterations of insulin signaling in type 2 diabetes: a review of the current evidence from humans. Biochim Biophys Acta. 2009;1792:83–92. doi: 10.1016/j.bbadis.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 86.Copps KD, Hancer NJ, Opare-Ado L, Qiu W, Walsh C, White MF. Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metab. 2010;11:84–92. doi: 10.1016/j.cmet.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Scholmerich J, et al. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol. 2006;36:485–501. doi: 10.1677/jme.1.01909. [DOI] [PubMed] [Google Scholar]
- 88.Hiltunen M, Khandelwal VK, Yaluri N, Tiilikainen T, Tusa M, Koivisto H, et al. Contribution of genetic and dietary insulin resistance to Alzheimer phenotype in APP/PS1 transgenic mice. J Cell Mol Med. 2012;16:1206–1222. doi: 10.1111/j.1582-4934.2011.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Julien C, Tremblay C, Phivilay A, Berthiaume L, Emond V, Julien P, et al. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol Aging. 2010;31:1516–1531. doi: 10.1016/j.neurobiolaging.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 90.Hoyer S, Muller D, Plaschke K. Desensitization of brain insulin receptor. Effect on glucose/energy and related metabolism. J Neural Transm Suppl. 1994;44:259–268. doi: 10.1007/978-3-7091-9350-1_20. [DOI] [PubMed] [Google Scholar]
- 91.Plaschke K, Kopitz J, Siegelin M, Schliebs R, Salkovic-Petrisic M, Riederer P, et al. Insulin-resistant brain state after intracerebroventricular streptozotocin injection exacerbates Alzheimer-like changes in Tg2576 AbetaPP-overexpressing mice. J Alzheimers Dis. 2010;19:691–704. doi: 10.3233/JAD-2010-1270. [DOI] [PubMed] [Google Scholar]
- 92.Wang X, Zheng W, Xie JW, Wang T, Wang SL, Teng WP, et al. Insulin deficiency exacerbates cerebral amyloidosis and behavioral deficits in an Alzheimer transgenic mouse model. Mol Neurodegener. 2010;5:46. doi: 10.1186/1750-1326-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 94.Brant AM, Jess TJ, Milligan G, Brown CM, Gould GW. Immunological analysis of glucose transporters expressed in different regions of the rat brain and central nervous system. Biochem Biophys Res Commun. 1993;192:1297–1302. doi: 10.1006/bbrc.1993.1557. [DOI] [PubMed] [Google Scholar]
- 95.Grieb P. Intracerebroventricular Streptozotocin Injections as a Model of Alzheimer’s Disease: in Search of a Relevant Mechanism. Mol Neurobiol. 2016;53:1741–1752. doi: 10.1007/s12035-015-9132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 98.Asakawa A, Inui A, Inui T, Katsuura G, Fujino MA, Kasuga M. Leptin treatment ameliorates anxiety in ob/ob obese mice. J Diabetes Complications. 2003;17:105–107. doi: 10.1016/s1056-8727(02)00185-x. [DOI] [PubMed] [Google Scholar]
- 99.Kerouz NJ, Horsch D, Pons S, Kahn CR. Differential regulation of insulin receptor substrates-1 and -2 (IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in liver and muscle of the obese diabetic (ob/ob) mouse. J Clin Invest. 1997;100:3164–3172. doi: 10.1172/JCI119872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim B, Backus C, Oh S, Feldman EL. Hyperglycemia-induced tau cleavage in vitro and in vivo: a possible link between diabetes and Alzheimer’s disease. J Alzheimers Dis. 2013;34:727–739. doi: 10.3233/JAD-121669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Porter WD, Flatt PR, Holscher C, Gault VA. Liraglutide improves hippocampal synaptic plasticity associated with increased expression of Mash1 in ob/ob mice. Int J Obes (Lond) 2013;37:678–684. doi: 10.1038/ijo.2012.91. [DOI] [PubMed] [Google Scholar]
- 102.Dinel AL, Andre C, Aubert A, Ferreira G, Laye S, Castanon N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS One. 2011;6:e24325. doi: 10.1371/journal.pone.0024325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharma AN, Elased KM, Garrett TL, Lucot JB. Neurobehavioral deficits in db/db diabetic mice. Physiol Behav. 2010;101:381–388. doi: 10.1016/j.physbeh.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim B, Backus C, Oh S, Hayes JM, Feldman EL. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150:5294–5301. doi: 10.1210/en.2009-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee S, Tong M, Hang S, Deochand C, de la Monte S. CSF and Brain Indices of Insulin Resistance, Oxidative Stress and Neuro-Inflammation in Early versus Late Alzheimer’s Disease. J Alzheimers Dis Parkinsonism. 2013;3:128. doi: 10.4172/2161-0460.1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de la Monte SM, Re E, Longato L, Tong M. Dysfunctional pro-ceramide, ER stress, and insulin/IGF signaling networks with progression of Alzheimer’s disease. J Alzheimers Dis. 2012;30(Suppl 2):S217–S229. doi: 10.3233/JAD-2012-111728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Betteridge DJ. What is oxidative stress? Metabolism. 2000;49:3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- 108.Mattson MP, Chan SL, Duan W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev. 2002;82:637–672. doi: 10.1152/physrev.00004.2002. [DOI] [PubMed] [Google Scholar]
- 109.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 110.Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 111.Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:631–641. doi: 10.1097/01.jnen.0000228136.58062.bf. [DOI] [PubMed] [Google Scholar]
- 112.Gu F, Zhu M, Shi J, Hu Y, Zhao Z. Enhanced oxidative stress is an early event during development of Alzheimer-like pathologies in presenilin conditional knock-out mice. Neurosci Lett. 2008;440:44–48. doi: 10.1016/j.neulet.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 113.Duarte AI, Santos MS, Oliveira CR, Rego AC. Insulin neuroprotection against oxidative stress in cortical neurons--involvement of uric acid and glutathione antioxidant defenses. Free Radic Biol Med. 2005;39:876–889. doi: 10.1016/j.freeradbiomed.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 114.Yu XR, Jia GR, Gao GD, Wang SH, Han Y, Cao W. Neuroprotection of insulin against oxidative stress-induced apoptosis in cultured retinal neurons: involvement of phosphoinositide 3-kinase/Akt signal pathway. Acta Biochim Biophys Sin (Shanghai) 2006;38:241–248. doi: 10.1111/j.1745-7270.2006.00152.x. [DOI] [PubMed] [Google Scholar]
- 115.Park K, Gross M, Lee DH, Holvoet P, Himes JH, Shikany JM, et al. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. 2009;32:1302–1307. doi: 10.2337/dc09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7:1040–1052. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 117.Grunblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem. 2007;101:757–770. doi: 10.1111/j.1471-4159.2006.04368.x. [DOI] [PubMed] [Google Scholar]
- 118.Chen GJ, Xu J, Lahousse SA, Caggiano NL, de la Monte SM. Transient hypoxia causes Alzheimer-type molecular and biochemical abnormalities in cortical neurons: potential strategies for neuroprotection. J Alzheimers Dis. 2003;5:209–228. doi: 10.3233/jad-2003-5305. [DOI] [PubMed] [Google Scholar]
- 119.Davi G, Guagnano MT, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, et al. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288:2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 120.Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 121.Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 122.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 123.Stoll LL, McCormick ML, Denning GM, Weintraub NL. Antioxidant effects of statins. Timely Top Med Cardiovasc Dis. 2005;9:E1. [PubMed] [Google Scholar]
- 124.Mattson MP, Duan W, Guo Z. Meal size and frequency affect neuronal plasticity and vulnerability to disease: cellular and molecular mechanisms. J Neurochem. 2003;84:417–431. doi: 10.1046/j.1471-4159.2003.01586.x. [DOI] [PubMed] [Google Scholar]
- 125.Ott A, Slooter AJ, Hofman A, van Harskamp F, Witteman JC, Van Broeckhoven C, et al. Smoking and risk of dementia and Alzheimer’s disease in a population-based cohort study: the Rotterdam Study. Lancet. 1998;351:1840–1843. doi: 10.1016/s0140-6736(97)07541-7. [DOI] [PubMed] [Google Scholar]
- 126.Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59:1258–1263. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- 127.Greenwald J, Riek R. Biology of amyloid: structure, function, and regulation. Structure. 2010;18:1244–1260. doi: 10.1016/j.str.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 128.Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1:213–2125. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pearson HA, Peers C. Physiological roles for amyloid beta peptides. J Physiol. 2006;575:5–10. doi: 10.1113/jphysiol.2006.111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 131.Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer’s Abeta peptide: the many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 132.Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2009;8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Watson GS, Peskind ER, Asthana S, Purganan K, Wait C, Chapman D, et al. Insulin increases CSF Abeta42 levels in normal older adults. Neurology. 2003;60:1899–1903. doi: 10.1212/01.wnl.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]
- 134.Yoon SO, Park DJ, Ryu JC, Ozer HG, Tep C, Shin YJ, et al. JNK3 perpetuates metabolic stress induced by Abeta peptides. Neuron. 2012;75:824–837. doi: 10.1016/j.neuron.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, et al. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A. 2009;106:1971–1916. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, Martins R. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci. 2002;22:RC221. doi: 10.1523/JNEUROSCI.22-10-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, et al. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22:246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- 138.Saido T, Leissring MA. Proteolytic degradation of amyloid beta-protein. Cold Spring Harb Perspect Med. 2012;2:a006379. doi: 10.1101/cshperspect.a006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang DS, Dickson DW, Malter JS. beta-Amyloid degradation and Alzheimer’s disease. J Biomed Biotechnol. 2006;2006:58406. doi: 10.1155/JBB/2006/58406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qiu WQ, Folstein MF. Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer’s disease: review and hypothesis. Neurobiol Aging. 2006;27:190–198. doi: 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 141.Authier F, Posner BI, Bergeron JJ. Insulin-degrading enzyme. Clin Invest Med. 1996;19:149–160. [PubMed] [Google Scholar]
- 142.Hulse RE, Ralat LA, Wei-Jen T. Structure, function, and regulation of insulin-degrading enzyme. Vitam Horm. 2009;80:635–648. doi: 10.1016/S0083-6729(08)00622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 144.Starks EJ, Patrick O’Grady J, Hoscheidt SM, Racine AM, Carlsson CM, Zetterberg H, et al. Insulin Resistance is Associated with Higher Cerebrospinal Fluid Tau Levels in Asymptomatic APOEvarepsilon4 Carriers. J Alzheimers Dis. 2015;46:525–533. doi: 10.3233/JAD-150072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Edland SD. Insulin-degrading enzyme, apolipoprotein E, and Alzheimer’s disease. J Mol Neurosci. 2004;23:213–217. doi: 10.1385/JMN:23:3:213. [DOI] [PubMed] [Google Scholar]
- 146.Brandt R, Leschik J. Functional interactions of tau and their relevance for Alzheimer’s disease. Curr Alzheimer Res. 2004;1:255–269. doi: 10.2174/1567205043332054. [DOI] [PubMed] [Google Scholar]
- 147.Lesort M, Jope RS, Johnson GV. Insulin transiently increases tau phosphorylation: involvement of glycogen synthase kinase-3beta and Fyn tyrosine kinase. J Neurochem. 1999;72:576–584. doi: 10.1046/j.1471-4159.1999.0720576.x. [DOI] [PubMed] [Google Scholar]
- 148.Lesort M, Johnson GV. Insulin-like growth factor-1 and insulin mediate transient site-selective increases in tau phosphorylation in primary cortical neurons. Neuroscience. 2000;99:305–316. doi: 10.1016/s0306-4522(00)00200-1. [DOI] [PubMed] [Google Scholar]
- 149.Planel E, Sun XY, Takashima A. Role of GSK-3 beta in Alzheimer’s disease pathology. Drug Develop Res. 2002;56:491–510. [Google Scholar]
- 150.Llorens-Martin M, Jurado J, Hernandez F, Avila J. GSK-3beta, a pivotal kinase in Alzheimer disease. Front Mol Neurosci. 2014;7:46. doi: 10.3389/fnmol.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Welsh GI, Proud CG. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J. 1993;294(Pt 3):625–629. doi: 10.1042/bj2940625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem. 1997;272:19547–19553. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- 153.Ksiezak-Reding H, Pyo HK, Feinstein B, Pasinetti GM. Akt/PKB kinase phosphorylates separately Thr212 and Ser214 of tau protein in vitro. Biochim Biophys Acta. 2003;1639:159–168. doi: 10.1016/j.bbadis.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 154.Zhou XW, Winblad B, Guan Z, Pei JJ. Interactions between glycogen synthase kinase 3beta, protein kinase B, and protein phosphatase 2A in tau phosphorylation in mouse N2a neuroblastoma cells. J Alzheimers Dis. 2009;17:929–937. doi: 10.3233/JAD-2009-1113. [DOI] [PubMed] [Google Scholar]
- 155.Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem. 1995;65:732–738. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- 156.Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, Lee VM. PP2A mRNA expression is quantitatively decreased in Alzheimer’s disease hippocampus. Exp Neurol. 2001;168:402–412. doi: 10.1006/exnr.2001.7630. [DOI] [PubMed] [Google Scholar]
- 157.Papon MA, El Khoury NB, Marcouiller F, Julien C, Morin F, Bretteville A, et al. Deregulation of protein phosphatase 2A and hyperphosphorylation of tau protein following onset of diabetes in NOD mice. Diabetes. 2013;62:609–617. doi: 10.2337/db12-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kins S, Crameri A, Evans DR, Hemmings BA, Nitsch RM, Gotz J. Reduced protein phosphatase 2A activity induces hyperphosphorylation and altered compartmentalization of tau in transgenic mice. J Biol Chem. 2001;276:38193–38200. doi: 10.1074/jbc.M102621200. [DOI] [PubMed] [Google Scholar]
- 159.Clodfelder-Miller BJ, Zmijewska AA, Johnson GV, Jope RS. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes. 2006;55:3320–3325. doi: 10.2337/db06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31:224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 161.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hawrylycz M, Ng L, Page D, Morris J, Lau C, Faber S, et al. Multi-scale correlation structure of gene expression in the brain. Neural Netw. 2011;24:933–942. doi: 10.1016/j.neunet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 163.Friedland RP, Jagust WJ, Huesman RH, Koss E, Knittel B, Mathis CA, et al. Regional cerebral glucose transport and utilization in Alzheimer’s disease. Neurology. 1989;39:1427–1434. doi: 10.1212/wnl.39.11.1427. [DOI] [PubMed] [Google Scholar]
- 164.Herholz K, Salmon E, Perani D, Baron JC, Holthoff V, Frolich L, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17:302–316. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- 165.Langbaum JB, Chen K, Lee W, Reschke C, Bandy D, Fleisher AS, et al. Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Neuroimage. 2009;45:1107–1116. doi: 10.1016/j.neuroimage.2008.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Willette AA, Bendlin BB, Starks EJ, Birdsill AC, Johnson SC, Christian BT, et al. Association of Insulin Resistance With Cerebral Glucose Uptake in Late Middle-Aged Adults at Risk for Alzheimer Disease. JAMA Neurol. 2015;72:1013–1020. doi: 10.1001/jamaneurol.2015.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Willette AA, Modanlo N, Kapogiannis D Alzheimer’s Disease Neuroimaging I. Insulin resistance predicts medial temporal hypermetabolism in mild cognitive impairment conversion to Alzheimer disease. Diabetes. 2015;64:1933–1940. doi: 10.2337/db14-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 170.Johnson KA, Sperling RA, Gidicsin CM, Carmasin JS, Maye JE, Coleman RE, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer’s disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9:S72–S83. doi: 10.1016/j.jalz.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thambisetty M, Jeffrey Metter E, Yang A, Dolan H, Marano C, Zonderman AB, et al. Glucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2013;70:1167–1172. doi: 10.1001/jamaneurol.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Edison P, Archer HA, Hinz R, Hammers A, Pavese N, Tai YF, et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68:501–508. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 173.Willette AA, Johnson SC, Birdsill AC, Sager MA, Christian B, Baker LD, et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement. 2015;11:504–510. e1. doi: 10.1016/j.jalz.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sarazin M, Lagarde J, Bottlaender M. Distinct tau PET imaging patterns in typical and atypical Alzheimer’s disease. Brain. 2016;139:1321–1324. doi: 10.1093/brain/aww041. [DOI] [PubMed] [Google Scholar]
- 175.Thal DR, Vandenberghe R. Monitoring the progression of Alzheimer’s disease with tau-PET. Brain. 2016;139:1318–1320. doi: 10.1093/brain/aww057. [DOI] [PubMed] [Google Scholar]
- 176.Korf ES, White LR, Scheltens P, Launer LJ. Brain aging in very old men with type 2 diabetes: the Honolulu-Asia Aging Study. Diabetes Care. 2006;29:2268–2274. doi: 10.2337/dc06-0243. [DOI] [PubMed] [Google Scholar]
- 177.Willette AA, Xu G, Johnson SC, Birdsill AC, Jonaitis EM, Sager MA, et al. Insulin resistance, brain atrophy, and cognitive performance in late middle-aged adults. Diabetes Care. 2013;36:443–449. doi: 10.2337/dc12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Blasco G, Puig J, Daunis IEJ, Molina X, Xifra G, Fernandez-Aranda F, et al. Brain iron overload, insulin resistance, and cognitive performance in obese subjects: a preliminary MRI case-control study. Diabetes Care. 2014;37:3076–3083. doi: 10.2337/dc14-0664. [DOI] [PubMed] [Google Scholar]
- 179.Thomas MA, Hattori N, Umeda M, Sawada T, Naruse S. Evaluation of two-dimensional L-COSY and JPRESS using a 3 T MRI scanner: from phantoms to human brain in vivo. NMR Biomed. 2003;16:245–251. doi: 10.1002/nbm.825. [DOI] [PubMed] [Google Scholar]
- 180.Weston PS, Simpson IJ, Ryan NS, Ourselin S, Fox NC. Diffusion imaging changes in grey matter in Alzheimer’s disease: a potential marker of early neurodegeneration. Alzheimers Res Ther. 2015;7:47. doi: 10.1186/s13195-015-0132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Molinuevo JL, Ripolles P, Simo M, Llado A, Olives J, Balasa M, et al. White matter changes in preclinical Alzheimer’s disease: a magnetic resonance imaging-diffusion tensor imaging study on cognitively normal older people with positive amyloid beta protein 42 levels. Neurobiol Aging. 2014;35:2671–2680. doi: 10.1016/j.neurobiolaging.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 182.Meng JZ, Guo LW, Cheng H, Chen YJ, Fang L, Qi M, et al. Correlation between cognitive function and the association fibers in patients with Alzheimer’s disease using diffusion tensor imaging. J Clin Neurosci. 2012;19:1659–1663. doi: 10.1016/j.jocn.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 183.Hong YJ, Yoon B, Lim SC, Shim YS, Kim JY, Ahn KJ, et al. Microstructural changes in the hippocampus and posterior cingulate in mild cognitive impairment and Alzheimer’s disease: a diffusion tensor imaging study. Neurol Sci. 2013;34:1215–1221. doi: 10.1007/s10072-012-1225-4. [DOI] [PubMed] [Google Scholar]
- 184.Hsu JL, Chen YL, Leu JG, Jaw FS, Lee CH, Tsai YF, et al. Microstructural white matter abnormalities in type 2 diabetes mellitus: a diffusion tensor imaging study. Neuroimage. 2012;59:1098–1105. doi: 10.1016/j.neuroimage.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 185.Reijmer YD, Brundel M, de Bresser J, Kappelle LJ, Leemans A, Biessels GJ, et al. Microstructural white matter abnormalities and cognitive functioning in type 2 diabetes: a diffusion tensor imaging study. Diabetes Care. 2013;36:137–144. doi: 10.2337/dc12-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xiong Y, Sui Y, Xu Z, Zhang Q, Karaman MM, Cai K, et al. A Diffusion Tensor Imaging Study on White Matter Abnormalities in Patients with Type 2 Diabetes Using Tract-Based Spatial Statistics. AJNR Am J Neuroradiol. 2016 doi: 10.3174/ajnr.A4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim Biophys Acta. 2009;1792:482–496. doi: 10.1016/j.bbadis.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 188.Chen YC, Jiao Y, Cui Y, Shang SA, Ding J, Feng Y, et al. Aberrant brain functional connectivity related to insulin resistance in type 2 diabetes: a resting-state fMRI study. Diabetes Care. 2014;37:1689–1696. doi: 10.2337/dc13-2127. [DOI] [PubMed] [Google Scholar]
- 189.Musen G, Jacobson AM, Bolo NR, Simonson DC, Shenton ME, McCartney RL, et al. Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes. 2012;61:2375–2379. doi: 10.2337/db11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Burns JM, Honea RA, Vidoni ED, Hutfles LJ, Brooks WM, Swerdlow RH. Insulin is differentially related to cognitive decline and atrophy in Alzheimer’s disease and aging. Biochim Biophys Acta. 2012;1822:333–339. doi: 10.1016/j.bbadis.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2015;29:589–596. doi: 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Woods SC, Porte D., Jr Relationship between plasma and cerebrospinal fluid insulin levels of dogs. Am J Physiol. 1977;233:E331–E434. doi: 10.1152/ajpendo.1977.233.4.E331. [DOI] [PubMed] [Google Scholar]
- 193.Wallum BJ, Taborsky GJ, Jr, Porte D, Jr, Figlewicz DP, Jacobson L, Beard JC, et al. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab. 1987;64:190–194. doi: 10.1210/jcem-64-1-190. [DOI] [PubMed] [Google Scholar]
- 194.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 195.Kos K, Harte AL, da Silva NF, Tonchev A, Chaldakov G, James S, et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab. 2007;92:1129–1136. doi: 10.1210/jc.2006-1841. [DOI] [PubMed] [Google Scholar]
- 196.Hoscheidt SM, Starks EJ, Oh JM, Zetterberg H, Blennow K, Krause RA, et al. Insulin Resistance is Associated with Increased Levels of Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease and Reduced Memory Function in At-Risk Healthy Middle-Aged Adults. J Alzheimers Dis. 2016;52:1373–1383. doi: 10.3233/JAD-160110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Duarte AI, Moreira PI, Oliveira CR. Insulin in central nervous system: more than just a peripheral hormone. J Aging Res. 2012;2012:384017. doi: 10.1155/2012/384017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bellingham SA, Guo BB, Coleman BM, Hill AF. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol. 2012;3:124. doi: 10.3389/fphys.2012.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E. The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J Biol Chem. 2012;287:43108–43115. doi: 10.1074/jbc.M112.404467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–1177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang G, Dinkins M, He Q, Zhu G, Poirier C, Campbell A, et al. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD) J Biol Chem. 2012;287:21384–21395. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Saman S, Lee NC, Inoyo I, Jin J, Li Z, Doyle T, et al. Proteins recruited to exosomes by tau overexpression implicate novel cellular mechanisms linking tau secretion with Alzheimer’s disease. J Alzheimers Dis. 2014;40 Suppl 1:S47–S70. doi: 10.3233/JAD-132135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bulloj A, Leal MC, Xu H, Castano EM, Morelli L. Insulin-degrading enzyme sorting in exosomes: a secretory pathway for a key brain amyloid-beta degrading protease. J Alzheimers Dis. 2010;19:79–95. [Google Scholar]
- 204.Gupta A, Pulliam L. Exosomes as mediators of neuroinflammation. J Neuroinflammation. 2014;11:68. doi: 10.1186/1742-2094-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Van Giau V, An SS. Emergence of exosomal miRNAs as a diagnostic biomarker for Alzheimer’s disease. J Neurol Sci. 2016;360:141–152. doi: 10.1016/j.jns.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 206.Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, et al. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology. 2015;85:40–47. doi: 10.1212/WNL.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, et al. Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Ann Clin Transl Neurol. 2015;2:769–773. doi: 10.1002/acn3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2015;11:600–607. e1. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 210.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13:323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Claxton A, Baker LD, Wilkinson CW, Trittschuh EH, Chapman D, Watson GS, et al. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s disease. J Alzheimers Dis. 2013;35:789–797. doi: 10.3233/JAD-122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, et al. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis. 2015;44:897–906. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- 214.Hauner H. The mode of action of thiazolidinediones. Diabetes Metab Res Rev. 2002;18 Suppl 2:S10–S15. doi: 10.1002/dmrr.249. [DOI] [PubMed] [Google Scholar]
- 215.Miller BW, Willett KC, Desilets AR. Rosiglitazone and pioglitazone for the treatment of Alzheimer’s disease. Ann Pharmacother. 2011;45:1416–1424. doi: 10.1345/aph.1Q238. [DOI] [PubMed] [Google Scholar]
- 216.Landreth G, Jiang Q, Mandrekar S, Heneka M. PPARgamma agonists as therapeutics for the treatment of Alzheimer’s disease. Neurotherapeutics. 2008;5:481–489. doi: 10.1016/j.nurt.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Remels AH, Langen RC, Gosker HR, Russell AP, Spaapen F, Voncken JW, et al. PPARgamma inhibits NF-kappaB-dependent transcriptional activation in skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297:E174–E183. doi: 10.1152/ajpendo.90632.2008. [DOI] [PubMed] [Google Scholar]
- 218.Searcy JL, Phelps JT, Pancani T, Kadish I, Popovic J, Anderson KL, et al. Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2012;30:943–961. doi: 10.3233/JAD-2012-111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Heneka MT, Fink A, Doblhammer G. Effect of pioglitazone medication on the incidence of dementia. Ann Neurol. 2015;78:284–294. doi: 10.1002/ana.24439. [DOI] [PubMed] [Google Scholar]
- 220.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 221.Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6:19–28. doi: 10.1177/2042018814559725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther. 2002;302:881–888. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- 223.Perry T, Holloway HW, Weerasuriya A, Mouton PR, Duffy K, Mattison JA, et al. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Experimental neurology. 2007;203:293–301. doi: 10.1016/j.expneurol.2006.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Abeta oligomers. J Clin Invest. 2012;122:1339–1353. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Talbot K, Wang HY. The nature, significance, and glucagon-like peptide-1 analog treatment of brain insulin resistance in Alzheimer’s disease. Alzheimers Dement. 2014;10:S12–S25. doi: 10.1016/j.jalz.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J Alzheimers Dis. 2010;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.McClean PL, Parthsarathy V, Faivre E, Holscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci. 2011;31:6587–6594. doi: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Xu W, Yang Y, Yuan G, Zhu W, Ma D, Hu S. Exendin-4, a glucagon-like peptide-1 receptor agonist, reduces Alzheimer disease-associated tau hyperphosphorylation in the hippocampus of rats with type 2 diabetes. J Investig Med. 2015;63:267–272. doi: 10.1097/JIM.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 229.Malhotra R, Singh L, Eng J, Raufman JP. Exendin-4, a new peptide from Heloderma suspectum venom, potentiates cholecystokinin-induced amylase release from rat pancreatic acini. Regul Pept. 1992;41:149–156. doi: 10.1016/0167-0115(92)90044-u. [DOI] [PubMed] [Google Scholar]
- 230.Tweedie D, Rachmany L, Rubovitch V, Lehrmann E, Zhang Y, Becker KG, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Experimental neurology. 2012;239C:170–182. doi: 10.1016/j.expneurol.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Martin B, Golden E, Carlson OD, Pistell P, Zhou J, Kim W, et al. Exendin-4 Improves Glycemic Control, Ameliorates Brain and Pancreatic Pathologies and Extends Survival in a Mouse Model of Huntington’s Disease. Diabetes. 2008 doi: 10.2337/db08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123:2730–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]