Abstract
Background
Islet transplantation is a promising therapeutic approach for restore the physical response to blood glucose in type 1 diabetes. Current chronic use of immunosuppressive reagents for preventing islet allograft rejection is associated with severe complications. In addition, many of the immunosuppressive drugs are diabetogenic. The induction of transplant tolerance to eliminate the dependency on immunosuppression is ideal, but remains challenging.
Methods
Addition of hepatic stellate cells allowed generation of myeloid-derived suppressor cells (MDSC) from precursors in mouse bone marrow. Migration of MDSC was examined in an islet allograft transplant model by tracking the systemic administered MDSC from CD45.1 congenic mice.
Results
The generated MDSC were expressed C-C chemokine receptor type 2 (CCR2), which was enhanced by exposure to interferon-γ. A single systemic administration of MDSC markedly prolonged survival of islet allografts without requirement of immunosuppression. Tracking the administered MDSC showed that they promptly migrated to the islet graft sites, at which point they exerted potent immune suppressive activity by inhibiting CD8+ T cells, enhancing T regulatory T cell activity. MDSC generated from CCR2−/− mice failed to be mobilized and lost tolerogenic activity in vivo, but sustained suppressive activity in vitro.
Conclusion
MDSC migration was dependent on expression of CCR2, while CCR2 does not directly participate in immune suppression. Expression of CCR2 needs to be closely monitored for quality control purpose when MDSC are generated in vitro for immune therapy.
Introduction
Type 1 diabetes, an autoimmune disease with impaired insulin producing beta cells in pancreatic islets requires multiple insulin injections for treatment. Hypoglycemia and chronic diabetic complications often occur (1). Islet transplantation, a relatively noninvasive procedure, is ideal to restore the physiological response to blood glucose. However, many patients require repeat islet transplantation due to poor engraftment and progressive graft failure despite development of optimized immunosuppression regimens (2–5). Life-long immunosuppression regimens are also associated with significant morbidity (4,5). Many clinicians reason that chronic use of immunosuppressive drugs leads to worse outcomes than insulin therapy (7). These facts have limited the application of islet transplantation, and promoted investigation into tolerance inducing therapies with the goal of achieving indefinite graft survival without dependency on long-term immunosuppression.
The establishment and maintenance of transplant tolerance is a highly regulated process. The induction of tolerance to cell transplants encounters even more challenges. Thus, organ transplantation has successfully been applied for decades, but the outcomes of cell transplantation, such as islets and hepatocytes, remain disappointing. This is mirrored in animal models; liver transplants crossing MHC barriers are spontaneously accepted in mice (8), but hepatocyte transplants in the same donor and recipient combinations are acutely rejected (9), strongly suggesting the immune regulatory activity of organ nonparenchymal cells (NPC). Lack of appropriate NPC protection may contribute to the poor outcomes of cell transplants. This hypothesis is supported by the data reported by us and others. Islet allografts achieved long-term survival when they were co-transplanted with NPC from the immune privilege organs, including hepatic stellate cells (HpSC) (10,11) and testicular Sertoli cells (7,12,13). This approach has great potential for clinical application. A hurdle is that HpSC have to be derived from the patient, because HpSC from allogeneic or third-party sources failed to protect islet allografts (10). Isolation of HpSC from the patients carries risks.
A solution emerged. We have documented that HpSC protect islet allografts through induction of myeloid-derived suppressor cells (MDSC), and HpSC are potent inducers of MDSC. Addition of small amounts of HpSC into dendritic cell (DC) culture induced generation of MDSC, which was mediated by soluble factors produced by HpSC (14–16). MDSC is a group of heterogeneous myeloid progenitor cells, which were initially found for their suppressive roles in cancer (17,18). In healthy conditions, the progenitors differentiate into mature myeloid cells, while in inflammatory situations, their differentiation blocked, resulting in expansion of MDSC (19). In mice, MDSC are commonly characterized by expression of CD11b and Gr-1, as well as Ly6C and Ly6G, etc., but none of them can be used as specific marker (20). Production of arginase-1 and inducible nitric oxide synthase (iNOS), as well as the ability to suppress T cell response, has also been used to identify MDSC. MDSC inhibit T cells using variety of mechanisms, including nitrosylation of T cell-associated molecules, interference with T cell homing, induction of induction of T regulatory (Treg) cells (20,21), deprivation of cysteine and cysteine (22) and the engagement of B7-H1 inducing T cell apoptosis (14,23,24).
We previously showed that MDSC mixed with islet allografts and transplanted under renal capsule inhibited CD8+ T effector cell infiltration and expanded Treg cells, translating to marked improvement of islet allograft survival (23,25). The local delivery approach does not make sense in clinical settings where islets are transplanted through portal vein injection. In this study, we showed that a single systemic administration of MDSC protected islet allografts as effective as local delivery. The administered MDSC promptly migrated to the islet graft, which is CCR2 mediated, as MDSC deficient in CCR2 almost totally lost their in vivo tolerogenic activity.
Materials and Methods
Mice
Male B6 (C57BL/6J, H-2b), B6 CD45.1 congenic, BALB/c (H-2d) and CCR2−/− mice (H-2b) were purchased from Jackson Laboratory (Bar Harbor, ME), and used at 8 to 12 weeks of age in accordance with the guidelines of NIH.
Generation of DC and MDSC
DC: BM cells (2 × 106/well) isolated from mouse tibias and femurs were cultured in PRMI-1640 medium with mouse GM-CSF (8 ng/ml) and IL-4 (1,000 U/ml, both from Schering-Plough, Kenilworth, NJ) for 5 days (14). MDSC: HpSC were added at the beginning of DC culture at a ratio of 1:40 as described (40). CD11b+ cells were purified by positive sorting with MACs microbeads (Miltenyi Biotec, Auburn, CA).
Islet Transplantation
Diabetes was induced through a single intraperitoneal injection of streptozotocin (STZ, Sigma-Aldrich, Milwaukee, WI). Only mice with nonfasting blood glucose greater than or equal to 350 mg/dL were used as recipients. 300 islets were isolated from donor mice were transplanted under the renal capsule of diabetic recipients. Transplantation was considered success when recipient blood glucose became ≤ 150 mg/dL. The first day of two consecutive blood glucose levels ≥ 250 mg/dL was defined as the date of rejection. To examine the influence of MDSC on survival of islet allografts, MDSC (2 × 106) were administered systemically by intravenously injected (i.v.) into recipients immediately after transplantation of islet allograft (systemic administration), or administered locally by direct co-transplantation with islet allografts under the renal capsule as previously described (23,25). To isolate the infiltrating cells, islet grafts were excised under a microscope to minimize contamination. Cells were minced and digested in collagenase IV (0.5 mg/ml, Sigma-Aldrich) at 37°C for 5 min, washed and passed through a loosely packed nylon wool column to clear the debris. Leukocytes were isolated through Percoll centrifugation. CD11b+ cells were purified via positive sorting with MACS micro-beads (Miltenyi Biotec, Auburn, CA) (14).
Flow Cytometry
All mAbs were purchased from BD PharMingen (San Diego, CA), except for anti-CCR2 (R&D Systems, Minneapolis, MN). Mouse Treg cell staining kit was purchased from eBioscience (San Diego, CA). The appropriate isotype-matched irrelevant Abs served as controls. For CFSE labeling, T cells (1×107 cells/mL) were incubated with 2 µM CFSE (Molecular Probes, Eugene, OR) for 10 minutes. For intracellular staining (Foxp3), cells were first permeabilized with 0.1% saponinb. Flow analyses were performed on a FACSCalibur flow cytometer (BD Biosciences) and the data was analyzed by FlowJo software.
Quantitative (q) PCR
Total RNA was extracted with TRIzol Reagent (Invitrogen, Carlsbad, CA) and complementary DNA was synthesized with SuperScript II reverse transcriptase (Invitrogen). The primers were CACGGCAGTGGCTTTAACCT/TGGCGCATTCACAGTCACTT for arginase 1 and TGGCCACCTTGTTCAGCTACG/GCCAAGGCCAAACACAGCATAC for iNOS. The messenger RNAs were quantified using Applied Biosystems 7500 Fast PCR System in duplicate. The expression levels were normalized to GAPDH messenger RNA.
Mixed Lymphocyte Reaction (MLR)
MLR was performed in triplicates in 96-well, round-bottom plates (Corning, NY). Nylon wool-eluted B6 splenic T cells (2 × 105/ well) labeled with CFSE were cultured with γ-irradiated (20Gy) syngeneic DC in the presence of BALB/c splenocyte lysates at T:DC ratio of 20:1 in RPMI-1640 complete medium in 5% CO2 for 3 days. For suppression assays, the irradiated suppressor cells were added at the beginning of the MLR culture at the suppressor-to-DC ratio of 2:1.
Immunohistochemistry
Insulin was stained with anti-insulin mAb (Santa Cruz Biotechnology, Santa Cruz, CA), and color developed by 3-amino-9-ethylcarbazole. Serial sections were made (7 µm thick). The insulin areas were analyzed using the image software in 10 most significant sections for each animal. The data were expressed as µm2/section. Treg cells were stained with anti-CD4 and -Foxp3 mAbs (BD PharMingen), and developed colors using fluorochrome-conjugated avidin-biotin system. The isotype-matched irrelevant antibodies served as controls. The positive cells were counted under a microscope. 20 high-power fields (hpf) were randomly selected in each group.
Statistics
Graft survival was analyzed using log-rank test. The parametric data were analyzed by Student’s t test (two-tailed). Bonferroni method was used for multicomparison correction. A p-value of <0.05 was considered statistically significant.
Results
Interferon (IFN)-γ enhances expression of CCR2 on MDSC
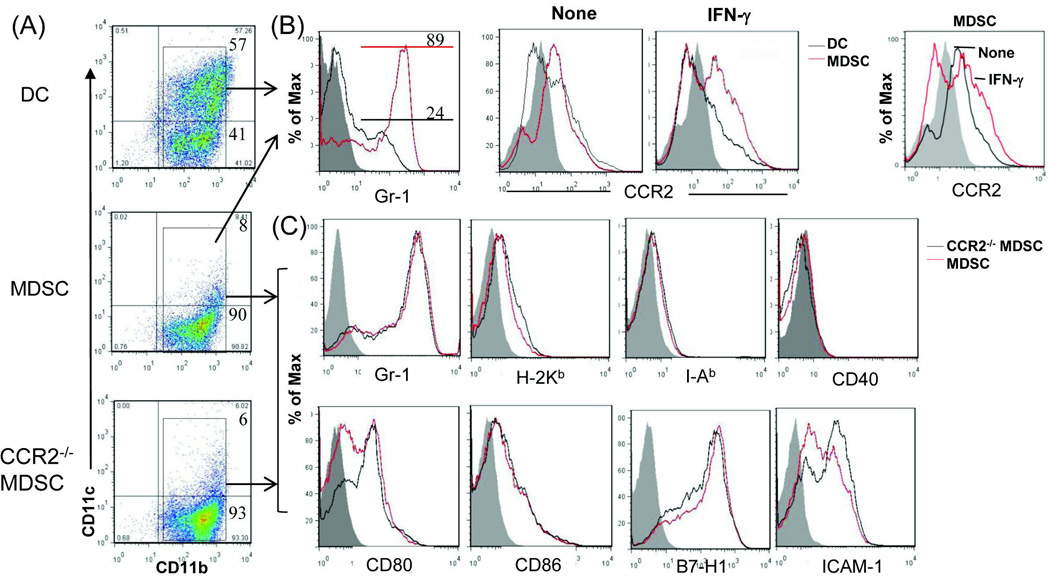
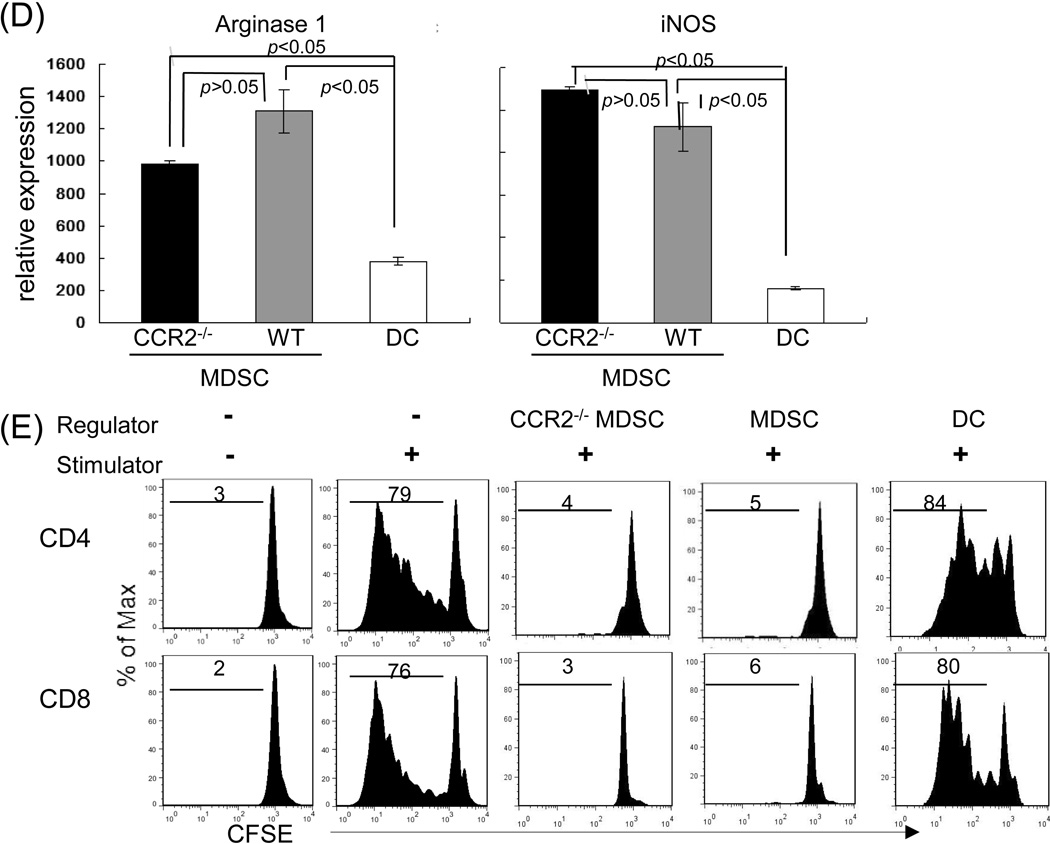
The MDSC used in this study were generated in vitro by addition of B6 HpSC at the beginning into a DC culture in which B6 bone marrow (BM) cells were cultured at an HpSC : BM ratio of 1:40 in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 for 5 days, as described (14). BM cells cultured without addition of HpSC served as controls (DC). The floating cells were harvested for phenotypic studies by staining with monoclonal antibodies (mAbs) against the key cell surface molecules and analyzed by flow cytometry. The cells in the cultures without HpSC contained ~57% CD11c+ cells, while those cultured with HpSC generated only ~8% CD11c+ cells (Fig. 1A), indicating the presence of HpSC inhibits DC propagation, while promotes generation of CD11b+CD11c− cells. ~89% of these CD11b+CD11c− cells were Gr-1+ (Fig. 1B, left panel), CD11b+Gr-1+ has been used for identification of mouse MDSC (19). We reported that addition of HpSC into DC culture promoted generation of MDSC (14,15). Flow analysis gated on CD11b+ populations showed that both DC and MDSC constitutively expressed CCR2 (Fig. 1B, middle panels), a receptor of chemokines which specifically mediates monocyte chemotaxis (26). Exposure to inflammatory cytokine interferon (IFN)-γ markedly upregulated CCR2 expression on MDSC (Fig. 1B, right panel), reflecting the response of MDSC to inflammatory stimulation. To determine the role of CCR2 expressed in MDSC immunological activity, we generated MDSC from CCR2−/− mice. CCR2−/− MDSC expressed the key surface molecules comparable to wild-type (WT) controls (Fig. 1A and C). Compared to DC, MDSC were expressed significantly higher arginase 1 and iNOS mRNA (Fig. 1D). Arginase-1 and iNOS enhance L-arginine catabolism, leading to inhibition of T cell function (27). CCR2 deficiency did not affect expression of arginase-1 and iNOS (Fig. 1D) in MDSC. The ability of MDSC to suppress T cell response was tested in vitro by addition of MDSC (WT or CCR2−/−) or DC into a MLR culture in which CFSE-labeled T cells were stimulated by allogeneic antigens. Compared to DC, addition of MDSC markedly inhibited the proliferative response [carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution assay] in both CD4+ and CD8+ T cells. Deficiency in CCR2 did not alter their in vitro inhibitory activity (Fig. 1E).
Figure 1. Deficiency in CCR2 expression does not alter MDSC phenotype and immunosuppressive activity in vitro.
The MDSC used in this study were generated in vitro by addition of HpSC (B6) at the beginning into the culture of BM cells that were isolated from WT or CCR2−/− B6 mice at a ratio of 1:50 in the presence of GM-CSF and IL-4 for 5 days. The floating cells were harvested. The BM cells cultured in the absence of served as controls (DC). Cells were stained for CD11b, CD11c and the indicated key surface molecules, and analyzed by flow cytometry. (A) Expression of CD11b and CD11c on DC, MDSC and CCR2−/− MDSC. The number is percentage of positive cells in whole cell populations. (B) Expression of Gr-1 and CCR2 on DC and MDSC. MDSC expressed high Gr-1 (left panel). For IFN-γ stimulation, the cells were exposed to IFN-γ (100U/ml) for last 18 hours of the cultures. Right panel shows an overlay of CCR2 expression on MDSC with or without exposure to IFN-γ. All data are displayed as histograms gated on CD11b+ populations. (C) Null of CCR2 expression in MDSC does not affect expression of the key surface molecules. Expression of the indicated molecules was analyzed on WT and CCR2−/− MDSC. The data are displayed as histograms gated on CD11b+ cells. (D) Deficiency in expression of CCR2 does not alter expression of arginase-1 and iNOS in MDSC. RNA was isolated from the magnetic beads purified CD11b+ cells. Expression of mRNA was determined by q-PCR, displayed as mean (n=3) relative expression ± SD, and analyzed by two-way t test with Bonferroni correction. (E) CCR2−/− MDSC demonstrate comparable T cell inhibitory activity in vitro. DC and MDSC generated from WT or CCR2−/− mice (B6) were used as regulators, and added into a 3-day MLR culture in which allogeneic T cells (BALB/c) were elicited by B6 DC as stimulators (T:DC = 20:1). T cell proliferation was determined by CFSE dilution assay, and expressed as histograms gated on CD4 and CD8 populations. The number is percentage of dividing CD4+ or CD8+ T cells. The data are representative of three separate experiments.
Systemic administration of CCR2−/− MDSC fails to protect islet allografts
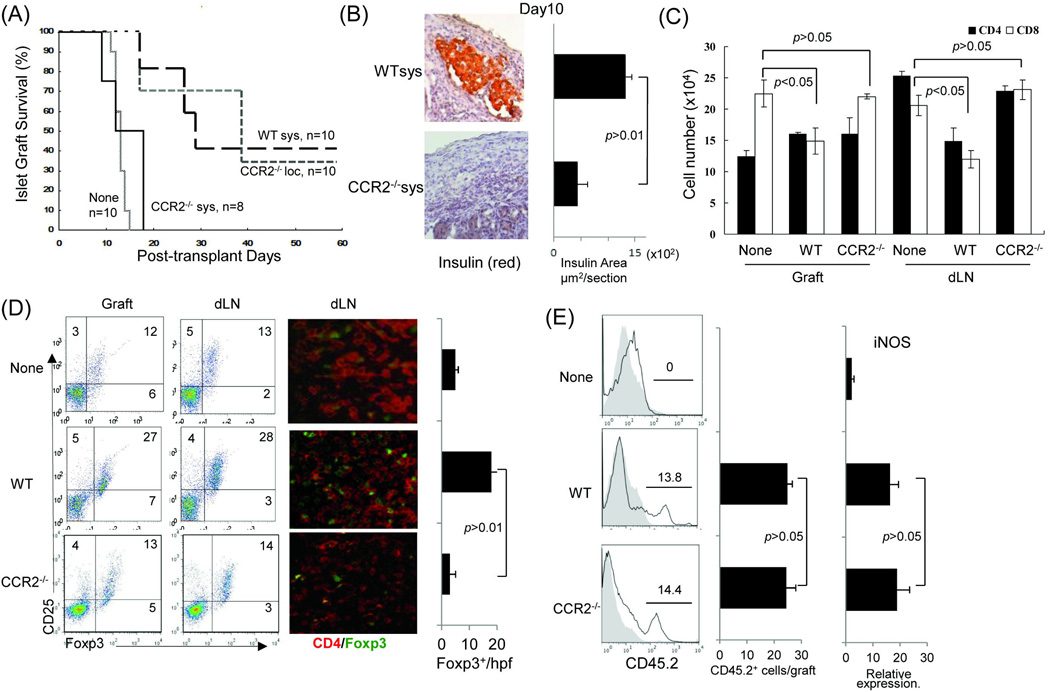
To determine the role of CCR2 expressed on MDSC in immunosuppressive activity in vivo, 2 × 106 MDSC generated from WT or CCR2−/− B6 mice were systemically (intravenously) injected immediately after transplantation of 300 islets (BALB/c) under renal capsule of diabetic B6 recipients. Islet allograft transplantation alone (without injection of MDSC) served as controls. For comparison, in the local delivery group, CCR2−/− MDSC were mixed with islet allografts and transplanted under renal capsule. Survival of the islet allografts were monitored by the blood glucose levels as described in the method. As shown in Fig 2A, none of islet allografts survived more than 15 days in the control group (None), while 40% islet allografts in WT MDSC group (WT sys) survived more than 60 days (p<0.05). Whereas, systemic administration of MDSC that were generated from CCR2−/− mice (CCR2−/− sys) lost their ability to protect islet allografts (p<0.05, WT Sys vs. CCR2−/− sys; p>0.05 CCR2−/− sys vs. None) (Fig. 2A). In contrast, local delivery of CCR2−/− MDSC markedly prolonged islet allograft survival comparable to systemic administration of WT MDSC (Fig. 2A, p<0.05, WT Sys vs. CCR2−/− loc). All recipients bearing islet grafts survived ≥60 days were routinely undergone nephrectomy (to remove islet grafts), which quickly restored hyperglycemia (Supplemental Data, Table 3), indicating that the normoglycocemia was maintained by the islet grafts. These data suggest that protection of islet allograft by systemically administration of MDSC is absolutely dependent on expression of CCR2. To clarify the underlying mechanisms, the recipient mice were sacrificed on post-operative day (POD) 10. The grafts sections were stained for insulin, and the insulin areas were quantitatively analyzed, showing the presence of functional islets in WT MDSC systemic administration group, while significantly fewer functional islets were identified in CCR2−/− group (Fig. 2B). Islet grafts were precisely peeled off under a microscope for isolation of the infiltrating lymphocytes. Lymphocytes isolated from the islet grafts and dLN were analyzed by flow cytometry following staining with anti-CD4, -CD8 mAbs. Systemic administration of CCR2−/− MDSC led to significantly increased infiltration of CD8+ T cells in islet grafts compared to WT controls. There were no significant differences in either CD4+ or CD8+ T cells in dLN (Fig. 2C), suggesting that inhibition of CD8+ T cell infiltration in islet allografts requires CCR2 expression on MDSC. Treg cell activity was examined by analyzing Foxp3 CD4 T cells in islet grafts and dLN by both flow cytometry and immunohistochemistry. The frequencies of CD25+Foxp3+ CD4 T cells were not enhanced in CCR2−/− MDSC group, compared to WT controls. This tendency was confirmed in immunohistochemical analysis. Significant more Foxp3+ CD4 T cells were seen in WT, not in CCR2−/− group (Fig. 2D). These results indicate that the rejection of islet allografts in CCR2−/− MDSC systemic administration group is associated with enhanced CD8+ T cell graft infiltration.
Figure 2. Systemically administered CCR2−/− MDSC lose ability to prolong survival of islet allografts.
Immediately after transplantation of 300 islets (BALB/c) under renal capsule of B6 diabetic STZ induced) recipient, 2 × 106 WT or CCR2−/− MDSC (B6) were intravenously injected (sys). For comparison purpose in a separate group, CCR2−/− MDSC were locally delivered (loc) by being mixed with islets, and then transplanted, as previously described (5). Islet transplantation alone (without MDSC treatment) served as control (None). For mechanistic studies, the recipients treated with systemic administration of WT or CCR2−/− MDSC were sacrificed on POD 10. The islet grafts and draining lymph node were harvested for sections and isolation of cells. (A) Survival of islet allografts. Systemic administration of WT MDSC or local treatment of CCR2−/− MDSC markedly prolonged survival of islet allografts (p<0.05, WT Sys or CCR2−/− Loc vs. None). Systemic administration of CCR2−/− MDSC failed to prolong islet allograft survival (p>0.05, CCR2−/− vs. none; p<0.05, CCR2−/− Sys vs. CCR2−/− Loc). (B) Islet allografts sections were stained with anti-insulin mAb (red). The pictures (left panels) show the presence of functional islets in the recipients receiving systemic administration of WT MDSC, but not in an animal (CCR2−/− MDSC group) with rejected islet grafts. Right panel shows the quantitative data for insulin areas analysis (n=10 in each group). The data were expressed as mean µm2/section ± SD. (C) Poor protection of islet allograft by systemic administration of CCR2−/− MDSC is associated with increased CD8+ T cells. Lymphocytes isolated from islet allografts and draining lymph node (dLN) from the islet allograft recipients receiving systemic administration of WT or CCR2−/− MDSC were stained with anti-CD4, -CD8 mAbs. Lymphocytes isolated from naïve animals served as the controls (None). CD4+ and CD8+ cell number was calculated based on flow analysis, and expressed as mean cell number ± SD (n=3). CD8+ cells, WT sys vs. CCDR2−/− sys, p<0.05 for two-way t test with Bonferroni correction. (D) Systemic administration of CCR2−/− MDSC is not associated with enhanced Treg cell activity. Treg cell activity was examined by flow cytometry for expression of CD25 and Foxp3 gated on CD4+ cells (left panels) or by immunohistochemistry where the cell suspensions were stained with anti-CD4 (red) and -Foxp3 (green) mAbs using fluorescent immunochemical protocol and examined by a microscope. The Foxp3+ cells were counted and expressed as mean Foxp3+ cells/high power field ± SD (n=3). p<0.05 for 2-way t test with Bonferroni correction. (E) Null of CCR2 does not affect MDSC stability in vivo. In a separate experiment, 2 × 106 MDSC propagated from WT (B6) or CCR2−/− mice (both CD45.2+) were mixed with allogeneic islets (BALB/c), and transplanted under the renal capsule of congenic B6 recipients (CD45.1+) (n=3). Islet allograft transplantation alone served as controls (None). On POD7, the grafts were peeled off under a microscope for leukocyte isolation. Cells were double stained with anti-CD11b and -CD45.2, analyzed by flow cytmetry gated on CD11b+ cells, and displayed as hystograms. The number is percentage of CD45.2+ cells (left panels). The absolute number of CD45.2+ cells were calculated based on the flow analysis (middle panel). The myeloid cells were purified using CD11b+ beads for isolation of mRNA. Expression of iNOS was determined by qPCR (right panel). The data were analyzed by t test (two-tailed) with Bonferroni correction.
To examine the effect of null of CCR2 on survival of MDSC in vivo, MDSC propagated from B6 (WT or CCR2−/−) mice (CD45.2+) were co-transplanted with islet allografts under renal capsule of the congenic B6 recipients (CD45.1+) as described in Methods. On POD 7, leucocytes were isolated from the islet grafts, and analyzed by flow cytometry gated on CD11b+ (myeloid) population. Fig. 2E shows that comparable frequency and absolute number of CD45.2+CD11b+ cells were identified in CCR2−/− MDSC co-transplantation group compared to WT controls. In addition, similar high expression of iNOS (an immune suppressive factor produced by MDSC) (20) was detected in CD11b+ cells isolated from islet allografts that were co-transplanted with CCR2−/− or WT MDSC. These data indicate that absence of CCR2 expression is unlikely to affect the stability of MDSC in vivo
CCR2 drives the migration of the administered MDSC to islet allografts
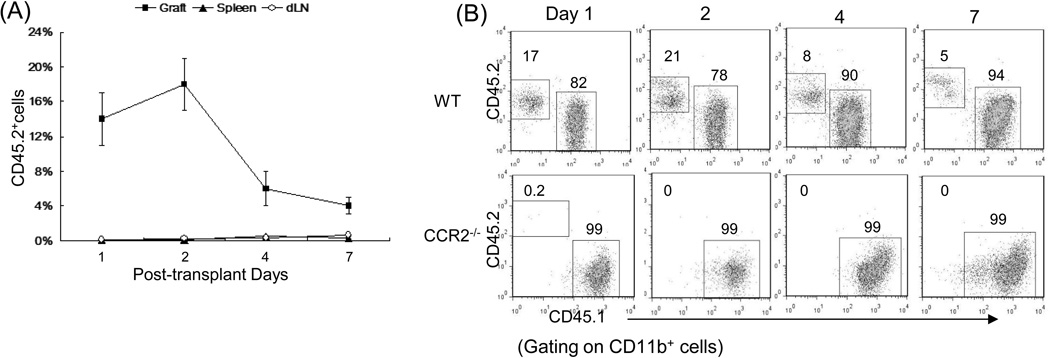
To investigate the migration pattern of administered MDSC following islet transplantation, MDSC propagated from WT or CCR2−/− B6 mice (CD45.2+) were i.v. injected into the congenic CD45.1+ diabetic recipients immediately after islet allograft (BALB/c) transplantation. Migration of the MDSC was tracked using anti-CD45.2 mAbs. A nice anti-CD45.2 mAb for immunohistichemical staining could not found. Alternativly, flow analysis was used. The leukocytes were isolated from islet grafts, draining lymphoid node (dLN) and spleen on POD 1, 2, 4 and 7, and stained with anti-CD11b, -CD45.1 and -CD45.2 mAbs. Flow analyses were performed gated on myeloid (CD11b+) cell population. Very few CD45.2+ myeloid cells were detected in dLN and spleen, while almost all the administered WT MDSC (CD45.2+) were promptly migrated to islet allografts, peaking at POD 2, and gradually reduced thereafter (Fig. 3A). Whereas, the systemically administered CCR2−/− MDSC almost totally lost their ability to migrate to islet allografts (Fig. 3B), resulting in the failure to prolong survival of islet allografts (Fig. 2A). Absolute numbers for CD45.2 cells (shown in Supplemental Data Tables 1 and 2) confirmed these observations.
Figure 3. Migration pattern of the systemically administered MDSC.
(A) MDSC preferably migrate to islet allografts. 2 × 106 MDSC generated from normal B6 mice (CD45.2) mice were i.v. injected into the congenic mice (CD45.1) immediately after transplantation of BALB/c islets. Leukocytes were isolated on POD 1, 2, 4 and 7 from islet allografts, draining lymph node (dLN) and spleen, and stained for CD11b and CD45.2 for flow analysis gated on CD11b+ population. The data show mean percentage of CD45.2+ cells ± SD (n=3) (p<0.05, graft vs. spleen or dLN at all time points). (B) Migration of MDSC to islet allografts requires CCR2. In a separate study, MDSC were generated from CCR2−/− mice, instead of WT mice, and i.v. injected into the congenic mice (CD45.1) immediately after transplantation of BALB/c islets. Leukocytes were isolated on POD 1, 2, 4 and 7 from islet allografts, and stained with anti-CD11b, CD45.1 and CD45.2 mAbs for low analysis. The number is percentage of CD45.1+ or CD45.2+ cells gated on CD11b+ cell population. The data are representative of three separate experiments.
Discussion
We find that a single systemic administration of in vitro-generated MDSC reduced graft infiltration of CD8+ T cells and promoted expansion of Treg cells, resulting in marked improvement of islet allograft survival, which was comparable to the local delivery approach where MDSC are mixed with islet allografts and transplanted under renal capsule, (Fig. 2A). Systemic administration of MDSC is a more feasible approach in clinical practice, as human islets are transplanted through portal vein injection. The results are in agreement with a previous report that systemic administration of cytokine-induced MDSC prolonged survival of allogeneic islets, which however, required three consecutive treatments (28). Islet exposure to blood triggers an instant blood mediated inflammatory reaction (IBMIR), leading to islet damage. IBMIR is an innate immune reaction (29). MDSC inhibit both innate and acquired immune responses (30), therefore, attenuate IBMIR.
Lacking of reliable source has been a drawback of the initiatives to develop MDSC-based therapeutic strategies (31). MDSC used in this study were generated in vitro by addition of HpSC into BM cell culture with GM-CSF and IL-4. The generated MDSC exhibited CD11b+Gr-1+ phenotype, expressed high arginase-1 and iNOS, and suppressed T cell proliferation in vitro (Fig. 1). The idea of using HpSC to generate MDSC was inspired by our previous observation. MDSC were accumulated in islet allografts when they were cotransplanted with HpSC (14). Induction of MDSC by HpSC is mediated by soluble factors produced by HpSC (14), such as iC3b (15) and retinoic acid (16). We previously reported that induction of MDSC by HpSC in vitro was not MHC restricted (14), therefore, in clinical setting, MDSC can be generated by co-culture of the patient blood monocytes (containing myeloid progenitor cells) with any available HpSC. There are other protocols reported in the literature for generation of MDSC in vitro using GM-CSF, G-CSF, IL-6, IL-17 or tumor cells (28,32).
Our data reveal that the administered MDSC promptly migrate to islet allografts where MDSC contributed to elimination of CD8+ T cells and enhancement of Treg activity, as evidenced by the increases in Foxp3+ CD4 T cells. We also show that the migration of MDSC was absolutely requires CCR2 expression on MDSC, since MDSC deficient in CCR2 almost totally lost their ability to induce Treg and inhibit Tef cell response, resulting in failure to protect islet allografts. We ruled out the possibility that null of CCR2 expression may shorten the survival of MDSC in vivo, since the stability of CCR2−/− MDSC that were locally delivered in islet allografts was similar to WT MDSC controls. CCR2 is a chemokine receptor. Chemokines are a group of secreted proteins that regulate the trafficking of leukocytes to sites of inflammation and injury, among which monocyte chemoattractant proteins (MCP) are the best characterized. MCP attract monocytes through ligation with their cognate receptor, CCR2 is expressed on monocytes/macrophages (33), and specifically mediates the directed migration of mature monocyte/macrophages to areas of inflammation and injury (34,35). Here we report that CCR2 was expressed on HpSC-induced MDSC. Expression of CCR2 on MDSC was markedly enhanced by exposure to IFN-γ, a key inflammatory cytokine, mainly produced by Tef cells during allo-immune response. It was previously reported that the IFN-γ-primed macrophages exhibited increased CCR2 (36). We noted that although CCR2 was expressed on both DC and MDSC, exposure to IFN-γ further increased expression of CCR2 on MDSC, but not on DC. MDSC appear to be more responsive to inflammation-oriented migration, and actively participate in regulating the already established inflammation, such as in allogeneic grafts (Fig. 3). Although CD45.1+ congenic mice are powerful tools for the migration study, we could not find a nice anti-CD45.2 mAb for immunohistochmistry, and alternatively used flow analysis, which might not be sensitive enough identify the injected CD45.2 cells in naïve CD45.1 mice and sygeneic islet graft recipientss (data not shown). In tumor models, CCR2 on MDSC also induced their migration to sites of early tumor cell metastases to promote tumor spread out (37). Based on these observations, we strongly recommend monitoring of CCR2 expression as a quality control measurement when MDSC are generated in vitro for immune-therapies. It is interesting to know whether the MDSC behave similarly in a nontransplant model. We have shown the reverse of disease progress in experimental autoimmune myasthenia gravis by systemic administration of MDSC (38). The investigation of their migration pattern will be included in our coming studies.
MDSC migrated to islet allografts appeared to more effectively suppress CD8+ than CD4+ T cells. Thus, systemic administration of CCR2−/− MDSC failed to migrate to islet allografts and led to heavier infiltration of CD8+ T cells, compared to WT controls. There were no significant differences for CD4+ T cells (Fig. 2C). Preferential inhibition of CD8 functions by MDSC might be due to their higher expression of MHC class I then class II (Fig. 1C). MHC involve in antigen up-take and presentation, as well as the interaction with antigen-specific T cells. It was reported that MDSC isolated from tumor bearing mice showed antigen-dependent suppression in that they blocked T cells response to class I specific peptides but failed to inhibit the response to MHC class II-specific peptides. Masking MHC class I molecules on MDSC abrogated the immune suppressive activity (39).
Supplementary Material
Acknowledgments
Funding
This study was supported in part by grants from NIH: DK084192 (to LL) and AI090468 (to SQ).
Abbreviations
- BM
bone marrow
- CCR2
C-C chemokine receptor type 2
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- DC
dendritic cells
- dLN
draining lymph node
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HpSC
hepatic stellate cells
- hpf
high-power fields
- IFN
interferon
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IBMIR
instant blood mediated inflammatory reaction
- i.v.
intravenously
- MLR
mixed leukocyte reaction
- mAb
monocloncal antibody
- MDSC
myeloid-derived suppressor cells
- NPC
non-parenchymal cells
- POD
post-operative day
- qPCR
quantitative polymerase chain reaction
- STZ
streptozotocin
- Treg
T regulatory (cells)
- WT
wild type
Footnotes
Author's Specific Contributions
J.Q. and Y.A. did the bulk of the experimental work, data analysis; M.M. participated in some experiments; J.J.F. senior discussant and contributed to experimental design; S.Q. and L.L. participated in research design, wrote and finalized the manuscript, sponsored the project.
Disclosures
The authors declare no conflict of interest.
References
- 1.Barr CC. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive insulin therapy, by The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N. Engl. J. Med. 2000;342:381–389. [Google Scholar]
- 2.Harlan DM, Kenyon NS, Korsgren O, Roep BO. Current advances and travails in islet transplantation. Diabetes. 2009;58:2175–2184. doi: 10.2337/db09-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senior P, Kin T, Shapior J, Koh A. Islet transplantation at the university of alberta: Status update and review of progress over the last decade. Can J Diabetes. 2012;36:32–37. [Google Scholar]
- 4.Pepper AR, Gala-Lopez B, Ziff O, Shapiro AJ. Current status of clinical islet transplantation. World J Transplant. 2013;3:48–53. doi: 10.5500/wjt.v3.i4.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gala-Lopez B, Pepper AR, Shapiro AM. Biologic agents in islet transplantation. Curr Diab Rep. 2013;13:713–722. doi: 10.1007/s11892-013-0414-8. [DOI] [PubMed] [Google Scholar]
- 6.Wéclawiak H, Kamar N, Ould-Mohamed A, Cardeau-Desangles I, Rostaing L. Biological agents in kidney transplantation: belatacept is entering the field. Expert Opin Biol Ther. 2010;10:1501–1508. doi: 10.1517/14712598.2010.514901. [DOI] [PubMed] [Google Scholar]
- 7.Rocordi C, Strom TB. Clinical islet transplantation: Advances and immunological challenges. Nat Rev Immonol. 2004;4:259–268. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 8.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: Tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bumgardner GL, Heininger M, Li J, Xia D, Parker-Thornburg J, Ferguson RM, Orosz CG. A functional model of hepatocyte transplantation for in vivo immunologic studies. Transplantation. 1998;65:53–61. doi: 10.1097/00007890-199801150-00011. [DOI] [PubMed] [Google Scholar]
- 10.Chen C-H, Kuo L-M, Chang Y, et al. In vivo immune modulatory activity of mouse hepatic stellate cells. Hepatology. 2006;44:1171–1181. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]
- 11.Yang HR, Chou HS, Gu X, et al. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: a critical role of interferon-γ signaling. Hepatology. 2009;50:1981–1991. doi: 10.1002/hep.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suarez-Pinzon W, Korbutt GS, Power R, Hooton J, Rajotte RV, Rabinovitch A. Testicular sertoli cells protect islet beta-cells from autoimmune destruction in NOD mice by a transforming growth factor-beta-1-dependent mechanism. Diabetes. 2000;49:1810–1818. doi: 10.2337/diabetes.49.11.1810. [DOI] [PubMed] [Google Scholar]
- 13.Korbutt GS, Elliott JF, Rajotte RV. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes. 1997;46:317–322. doi: 10.2337/diab.46.2.317. [DOI] [PubMed] [Google Scholar]
- 14.Chou HS, Hsieh CC, Yang HR, et al. Hepatic stellate cells regulate immune response by way of induction of myeloid suppressor cells in mice. Hepatology. 2011;53:1007–1019. doi: 10.1002/hep.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh C-C, Chou HS, Yang H-R, et al. The Role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood. 2013;121:1760–1768. doi: 10.1182/blood-2012-06-440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatt S, Qin J, Bennett C, et al. All-trans retinoic acid induces argenase-1 and inducible nitric oxide synthase-producing dendritic cells with T cell inhibitory function. J Immunol. 2014;192:5098–5108. doi: 10.4049/jimmunol.1303073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 18.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and oxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 58:49–59. doi: 10.1007/s00262-008-0523-4. 12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peranzoni E, Zilio S, Marigo I, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opinn Immunol. 2010. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by timor. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bronte V, Zanovello P. Regulation of immune responses by Larginine metabolism. Nat. Rev. Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou H-S, Hsieh C-C, Charles R, et al. Myeloid-derived suppressor cells protect islet transplants via B7-H1 mediated enhancement of T regulatory cells. Transplantation. 2012;93:272–282. doi: 10.1097/TP.0b013e31823ffd39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arakawa Y, Qin J, Chou H-S, et al. Co-transplantation with myeloid-derived suppressor cells protects cell transplants: A Crucial Role of Inducible Nitric Oxide Synthase. Transplantation. 2014;97:740–747. doi: 10.1097/01.TP.0000442504.23885.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsou C-L, Peters W, Si Yue, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invst. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Can Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marigo I, Bosio E, Solito S, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPb transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson B, Ekdahl KN, Korsgren O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Current Opinion in Organ Transplantation. 2011;16:620–626. doi: 10.1097/MOT.0b013e32834c2393. [DOI] [PubMed] [Google Scholar]
- 30.Monu NR, Fre AB. Myeloid-derived suppressor cells and anti-tumor T cells: a complex relationship. Immunol Invest. 2012;41:595–613. doi: 10.3109/08820139.2012.673191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boros P, Ochando JC, Chen SH, Bromberg JS. Myeloid-derived suppressor cells: natural regulators for transplant tolerance. Hum Immunol. 2010;71:1061–1066. doi: 10.1016/j.humimm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charo I, Myers S, Herman A, Fanci C, Connolly A, Coughlin S. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci U S A. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 35.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 36.Hu X, Park-Min K-H, Ho HH, Ivashkiv LB. IFN-γ-Primed Macrophages Exhibit Increased CCR2-Dependent Migration and Altered IFN-γ Responses Mediated by Stat1. J Immunol. 2005;175:3637–3647. doi: 10.4049/jimmunol.175.6.3637. [DOI] [PubMed] [Google Scholar]
- 37.Toh B, Wang X, Keeble J, Sim WJ, Khoo K, et al. Mesenchymal transition and dissemination of celcer cells in driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 2011;9:e1001162. doi: 10.1371/journal.pbio.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Tu Z, Qian S, et al. Myeloid suppressor cells as a potential therapy for experimental autoimmune myasthenia gravis. J Immunol. 2014;193:2127–2134. doi: 10.4049/jimmunol.1400857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166:5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 40.Yu M-C, Chen C-H, Liang X, et al. Inhibition of T cell responses by hepatic stellate cells via B7-H1 mediated T cell apoptosis. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.