ABSTRACT
Iron artifacts are common among the findings of archaeological excavations. The corrosion layer formed on these objects requires stabilization after their recovery, without which the destruction of the item due to physicochemical damage is likely. Current technologies for stabilizing the corrosion layer are lengthy and generate hazardous waste products. Therefore, there is a pressing need for an alternative method for stabilizing the corrosion layer on iron objects. The aim of this study was to evaluate an alternative conservation-restoration method using bacteria. For this, anaerobic iron reduction leading to the formation of stable iron minerals in the presence of chlorine was investigated for two strains of Desulfitobacterium hafniense (strains TCE1 and LBE). Iron reduction was observed for soluble Fe(III) phases as well as for akaganeite, the most troublesome iron compound in the corrosion layer of archaeological iron objects. In terms of biogenic mineral production, differential efficiencies were observed in assays performed on corroded iron coupons. Strain TCE1 produced a homogeneous layer of vivianite covering 80% of the corroded surface, while on the coupons treated with strain LBE, only 10% of the surface was covered by the same mineral. Finally, an attempt to reduce iron on archaeological objects was performed with strain TCE1, which led to the formation of both biogenic vivianite and magnetite on the surface of the artifacts. These results demonstrate the potential of this biological treatment for stabilizing archaeological iron as a promising alternative to traditional conservation-restoration methods.
IMPORTANCE Since the Iron Age, iron has been a fundamental material for the building of objects used in everyday life. However, due to its reactivity, iron can be easily corroded, and the physical stability of the object built is at risk. This is particularly true for archaeological objects on which a potentially unstable corrosion layer is formed during the time the object is buried. After excavation, changes in environmental conditions (e.g., higher oxygen concentration or lower humidity) alter the stability of the corrosion layer and can lead to the total destruction of the object. In this study, we demonstrate the feasibility of an innovative treatment based on bacterial iron reduction and biogenic mineral formation to stabilize the corrosion layer and protect these objects.
KEYWORDS: akaganeite, biogenic minerals, Desulfitobacterium, vivianite, iron reduction
INTRODUCTION
Iron artifacts are among the most recurring items in archaeological findings. When buried, these objects develop a complex corrosion layer, the composition of which depends on the environmental conditions of the burial site (i.e., oxygen, humidity, salinity, pH, and presence of microorganisms). A stratified corrosion layer containing mainly hematite (Fe2O3), magnetite (Fe3O4), wustite (FeO), lepidocrocite [γ-FeO(OH)], and goethite [α-FeO(OH)] is normally found on archaeological iron objects. The presence of oxides and oxyhydroxides is problematic, as these molecules are three times more voluminous than elemental iron and their production can result in a dramatic mechanical damage of the object (1). In addition, deposits from the burial environment (clay, sand, and soil minerals) can be found mixed with the corrosion layer. Furthermore, if the burial site contains chlorine, an acidic FeCl2 solution is produced (2). The presence of chloride ions (Cl−), which are usually localized at the interface between the metal core and the corrosion layer, is one of the most problematic issues for the conservation of archaeological iron (3). Indeed, after excavation, the artifact is exposed to a higher oxygen concentration and a lower humidity level, both of which lead to drying of the acidic FeCl2 solution produced during the burial phase. This phenomenon produces cracks that cause a greater exposure of the metal core to oxygen. The second issue caused by chloride ions is the formation of akaganeite [FeO0.833(OH)1.167Cl0.167] (4–6). This mineral is produced after excavation by the oxidation and hydrolysis of the FeCl2 solution. Akaganeite is an unstable corrosion compound that can release chloride ions in the presence of humidity, which instigates further corrosion (2, 6). Therefore, archaeological iron objects require rapid treatment after excavation and, in particular, akaganeite has to be removed or converted into a more stable iron compound.
Three different methods have been typically used to stabilize archeological iron artifacts. The most common approach is the immersion of the objects in anoxic aqueous solutions of alkaline sulfide. The purpose of this treatment is to diffuse out the chloride ions (2, 3). During the treatment, the corrosion compounds are reduced, allowing a passive diffusion of the chloride ions out of the object by increasing the porosity of the corrosion layer. However, the process is slow and the alkaline sulfide solution needs to be changed regularly. Moreover, the spent solution requires specific waste neutralization, and the amount of chlorine extracted from the object is hard to assess (7). Another method, electrolytic reduction, exposes the surface of the original artifact which removes salts and reduces corrosion products (8). However, this treatment leads to a significant loss of matter, which is a concern in the context of archaeological artifacts, particularly, of small objects. Thus, electrolytic reduction is exploitable only for large marine findings (3). Additionally, as molecular hydrogen (H2) is produced during the process, it has to be carefully monitored. Lastly, plasma treatment is a method that causes cracks and fissures without removing chloride ions. As such, it is used mainly as a pretreatment before performing alkaline sulfide baths (3). Considering the limitations of these existing methods, there is a pressing need to develop novel technologies for stabilizing archaeological iron artifacts.
Microorganisms are currently being considered for the development of alternative treatments in conservation-restoration (9). For example, nitrate- and sulfate-reducing bacteria have been used to remove black crust on stone artworks (10–13). Endospore-forming bacteria of the genus Bacillus have been recently reported as self-healing agents in concrete structures (14), and biogenic carbonated phases have been employed for improving the durability of buildings (15). Likewise, Pseudomonas spp. have been reported to be more effective than enzymes in the removal of organic matter from frescoes (12). Finally, the fungal production of copper oxalates has been exploited to produce a passivation layer on copper-based alloys (16–18). Iron has not yet been the target of such a biotechnological approach, but the multitude of microbial metabolisms affecting iron opens up the possibility of a microbiological method for the stabilization of iron artifacts.
Iron reduction is a common metabolic reaction among anaerobic bacteria. It is important for the oxidation of organic matter in anoxic environments, and it directly impacts the production and weathering of minerals (19–23). The most studied example of microbial iron reduction is iron respiration, where Fe(III) is used as a terminal electron acceptor by anaerobic iron-reducing bacteria. These bacteria are generally able to reduce both soluble iron and solid iron phases, such as iron oxyhydroxides (19). While the reduction of soluble iron phases occurs inside a cell compartment (i.e., cytoplasm or periplasm), the reduction of solid iron phases implies the extracellular transfer of electrons. Different strategies have been described for model microorganisms such as Geobacter sulfurreducens PCA and Shewanella oneidensis MR-1 (19, 24). The first step in the electrons' outward movement is their transfer to the outer membrane via multiple c-type cytochromes (19, 25, 26). Once they are in the outer membrane, the electrons can reach a solid iron phase in three different ways: first, by direct contact of an external c-type cytochrome with the solid phase (19, 27–30); second, via an electron-conductive pilus; and third, by a soluble electron carrier (such as a flavin) able to transfer electrons to the solid phase (19, 31, 32). Iron reduction can also be the result of fermentation. A recent study has shown a mechanism by which Fe(III) reduction enables the use of Fe(III) as an electron sink during pyruvate fermentation in Desulfotomaculum reducens strain MI-1 (19).
Biogenic iron minerals can be produced as the result of iron reduction. Several studies reported the production of biogenic magnetite (Fe3O4), siderite (FeCO3), vivianite [Fe3(PO4)2·8H2O], and mackinawite (FeS) (23, 33, 34). The coupling of iron reduction with the biogenic formation of these mineral phases was evaluated in this study as an alternative method to stabilize the corrosion layer of archaeological iron artifacts. The anaerobic Firmicute Desulfitobacterium hafniense was selected due to the ability of the members of this genus to use a large spectrum of electron acceptors, including nitrate, sulfite, metals, humic acids, and halogenated organic compounds (35, 36). In addition, previous studies reported the ability of various members of the Desulfitobacterium genus to reduce ferric iron (35, 37–39). Finally, this bacterial species is not reported as a human pathogen (biosafety risk group 1 [40]).
In this study, we first evaluated iron reduction in the presence of chloride ions. Second, we assessed the potential of D. hafniense to reduce the corrosion layer of corroded iron coupons and archaeological iron artifacts containing chlorine. Finally, we characterized the biogenic minerals produced.
RESULTS AND DISCUSSION
The conservation-restoration methods for archaeological iron objects currently available present multiple disadvantages. To provide a sustainable and ecologically friendly alternative, we studied iron reduction in the presence of chlorine with D. hafniense strains TCE1 and LBE. We also examined biogenic mineral production as the result of iron reduction on archaeological objects.
Reduction of soluble iron phase in the presence of chlorine.
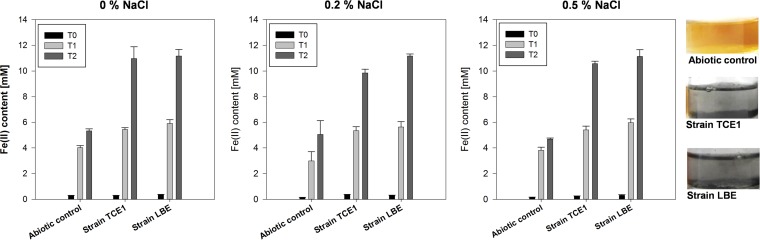
As chlorine is the most problematic compound present in the corrosion layer of archaeological objects (2), iron reduction was studied in liquid cultures amended with this element. Quantification of Fe(II) revealed some chemical reduction of iron in the abiotic controls (Fig. 1). This was probably due to the reductant (Na2S) added in the culture medium to remove oxygen, which likely reduced iron, as well. However, in addition to this abiotic reaction, both strains clearly displayed iron reduction abilities. In fact, the amounts of Fe(II) measured after 2 days of incubation were significantly higher in the TCE1 and LBE cultures than the amount of Fe(II) measured in the abiotic control. For example, in the experiment without additional chlorine, the amounts of Fe(II) measured in the cultures of strains TCE1 and LBE after 2 days of incubation (10.9 and 11.1 mM, respectively), corresponded to the reduction of the entire iron source added (when taking into account potential pipetting errors). By contrast, for the same incubation time, only 5.2 mM Fe(II) was measured in the abiotic control. Moreover, a change in the appearance of the cultures was observed in contrast to the abiotic control. In the latter, the solution remained orange, while in the cultures, a black precipitate was observed (Fig. 1). Overall, TCE1 and LBE strains performed similarly in terms of iron reduction.
FIG 1.
Reduction of iron citrate (10 mM initial concentration) in cultures of Desulfitobacterium hafniense strains TCE1 and LBE in the presence of chlorine. Three concentrations of NaCl (0, 0.2, and 0.5%) were compared. Iron reduction was evaluated by the production of Fe(II) at 0, 1, and 2 days (T0, T1, and T2, respectively) of incubation after the addition of the Fe(III) source. Abiotic controls were performed for each NaCl concentration tested. Fe(II) values are the averages and standard deviations from three independent replicates. A change in the appearance of the TCE1 and LBE cultures compared to the abiotic control is shown on the right-hand side of the figure (example for the cultures at 0% NaCl after 2 days of incubation).
In the case of the development of a novel treatment for stabilizing archaeological iron artifacts, iron reduction is the major prerequisite. However, the effect of chlorine on the bacterial activity must also be considered. In fact, a Cl− concentration of up to 50 mM (∼0.3% [wt/wt] in deionized water) has been reported for dissolved archaeological nails (7). In this study, the abilities of strains TCE1 and LBE to reduce iron in the presence of chlorine were evaluated at 0.2 and 0.5% NaCl. These values corresponded to the Cl− concentration measured in archaeological iron artifacts (7). The results showed that up to 0.5% of NaCl did not affect bacterial iron reduction, as Fe(III) was also fully reduced in the cultures amended with NaCl (Fig. 1).
Reduction of solid iron phase.
To evaluate the reductive dissolution of iron present as a solid phase, synthetic akaganeite was added to the cultures. Fe(II) concentration was measured at regular time intervals (Fig. 2). Within the first day, reductive dissolution was observed in the treatment with akaganeite. Fe(II) concentrations reached a maximum of 1.3 mM for strain TCE1 and 1.6 mM for strain LBE after 2 days of incubation. In contrast to the results obtained when reducing soluble Fe(III) phase, in the abiotic controls, Fe(II) increased only slightly and the maximal Fe(II) concentration measured was 0.4 mM (Fig. 3). During incubation with akaganeite, a change in color from yellow-green to black was also observed in the cultures. By contrast, the appearance of the abiotic controls remained unchanged (Fig. 2). With the observed production of Fe(II), we demonstrated that strains TCE1 and LBE reduced solid iron phases, particularly, akaganeite. As akaganeite represents the most problematic iron corrosion compound found on archaeological objects, its reduction by D. hafniense is very promising for the development of an alternative stabilization method to preserve iron.
FIG 2.
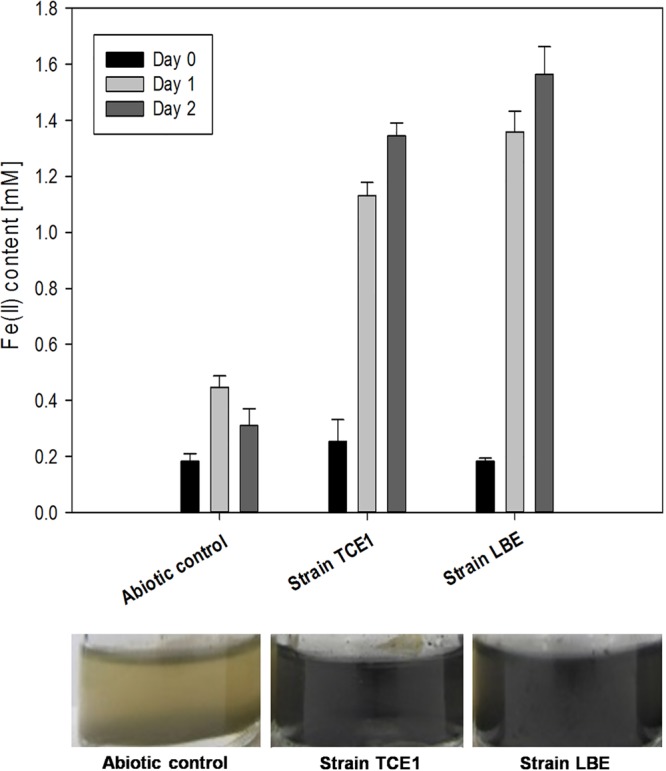
Reduction of akaganeite in cultures of Desulfitobacterium hafniense strains TCE1 and LBE. Reduction was measured by quantifying Fe(II) after 0, 1, and 2 days of incubation in the presence of akaganeite. Abiotic controls were also performed. Fe(II) values are the averages and standard deviations from three independent replicates. A change in the appearance of the TCE1 and LBE cultures compared to the abiotic control is shown at the bottom of the figure.
FIG 3.
Iron reduction tests on corroded iron coupons treated with cultures of Desulfitobacterium hafniense strains TCE1 and LBE. On the left, the appearance of the untreated coupon, the abiotic control, and the coupons treated with the strains TCE1 and LBE, are shown. On the right, the corresponding SEM images of the areas indicated by black squares inside the coupons are presented.
The role of biological reduction in the transformation of iron oxides such as akaganeite into crystalline Fe(II)-containing phases has already been studied using S. oneidensis strain MR1, Shewanella pealeana strain W3-7-1, and Thermoanaerobacter ethanolicus strain TOR-39 (41, 42). The results have underpinned the contribution of iron-reducing microorganisms in the geological formation of Fe(II)- and Fe(III)-bearing minerals in sediments and their role in the biogeochemical cycles of iron and carbon. Our results suggest that D. hafniense might be involved in the transformation of iron-bearing minerals in the environment. Regarding the mechanism of iron reduction, we hypothesize that Fe(III) acts as an electron sink, as described for the reduction of insoluble Fe(III) by D. reducens strain MI-1 during pyruvate fermentation (19).
Iron reduction on corroded coupons.
Although the reduction of akaganeite is promising, the composition of the corrosion layer of archaeological iron objects is not homogeneous and varies according to the burial environment. Therefore, to validate bacterial iron reduction and biogenic mineral production on real artifacts, naturally corroded iron coupons were used. The corrosion layer was composed of a mixture of iron oxides and oxyhydroxides. This composition corresponds to what it is commonly considered an urban corrosion layer.
The untreated coupons presented a homogeneous red-brown color (Fig. 3) and scanning electron microscopy coupled with energy dispersive X-ray spectroscopy (SEM-EDS) revealed that iron and oxygen were the main elements found, while silicon, carbon, aluminum, and calcium were found as secondary elements (Table 1). After a 7-day incubation period with the two strains, changes in the color, surface appearance, and its elemental composition were observed on the coupons (Fig. 3 and Table 1). The coupons used in the abiotic control turned to black-yellow, and spherical 1-μm aggregates covering 40% of the surface were observed. Besides iron and oxygen, carbon was also detected as a major element. In addition, several elements originating from the growth medium were detected as trace elements (Table 1). The coupons treated with strain TCE1 turned gray, and rosette-like crystals composed of iron, oxygen, and carbon (as major elements), phosphorus, sulfur, magnesium, and calcium (as secondary elements), covered 80% of the surface. The same types of crystals were also detected on the coupons treated with strain LBE. However, the layer produced was not as homogeneous and covered only a small proportion of the surface. Indeed, the coupons presented a rusty appearance in contrast to the coupons treated with strain TCE1. Likewise, rosette-like crystals with a similar elemental composition were also observed on the coupons of the abiotic control; however, they covered a minor proportion of the surface (see Fig. S1 in the supplemental material).
TABLE 1.
Elemental compositions of the iron coupons
| Composition categorya | Elements identified |
|||
|---|---|---|---|---|
| Before treatment | After treatment |
|||
| Abiotic control | Strain TCE1 | Strain LBE | ||
| Main elements (>10%) | Fe, O | Fe, O, C | Fe, O, C | Fe, O, C |
| Secondary elements (between 10 and 1%) | Si, C, Al, Ca | Si, Al | P, S, Mg, Ca | P, S, Si, Ca, Al |
| Trace elements (<1%) | K, Mg, P | Ca, Na, K, Mg, S, P, Cl | Si, Al | Mg, K |
The percentages reported correspond to the atomic percentages.
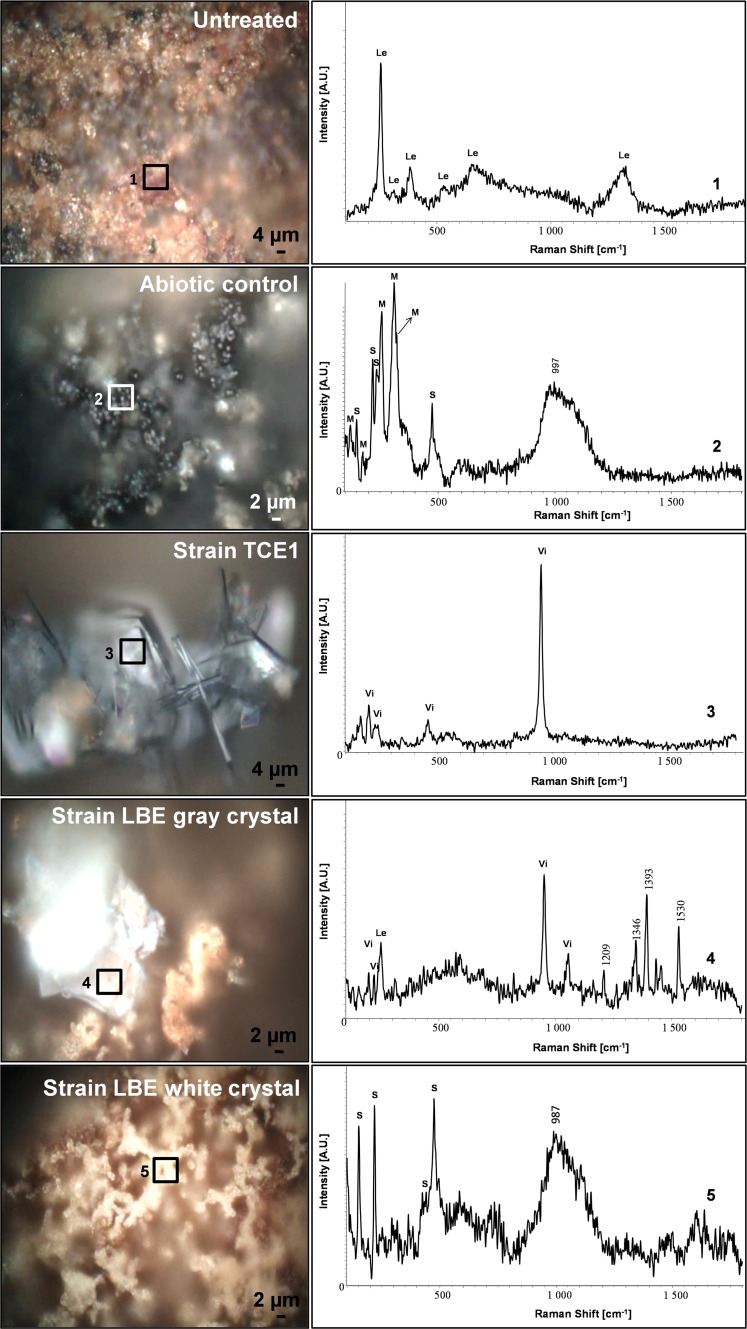
The biogenic minerals produced were identified by Raman spectroscopy. Before treatment, the corrosion layer of the coupons was mainly composed of lepidocrocite (Fig. 4) (43). After the treatment, new minerals were detected on all the coupons. On the coupons of the abiotic control, small aggregates were observed with SEM and identified as a mixture of mineral sulfur (α-S8) and poorly crystallized mackinawite [Fe(II)/Fe(III)S] (44). The additional large Raman shift between 980 and 1,100 cm−1 and the presence of carbon as a major element in the EDS spectrum suggested the formation of amorphous siderite. The crystals on the coupons treated with strain TCE1 were identified as mainly vivianite [Fe32+(PO4)2·8H2O] (45) (Fig. 4), as were the crystals on the coupons treated with strain LBE. Nevertheless, other corrosion compounds, such as lepidocrocite, were also detected. In addition, small white-yellow aggregates were found and identified as elemental sulfur (Fig. 4). Crystals found on the coupons used for the abiotic control were less abundant but similar to those on the coupons treated with strain TCE1 and also identified as vivianite (see Fig. S1). The partial abiotic reduction of the corrosion layer is likely the result of the reductant employed in the medium as indicated previously for the reduction of soluble Fe(III).
FIG 4.
Identification of the iron mineral phases produced on corroded iron coupons after treatment with strains TCE1 and LBE of D. hafniense. On the left are images of the areas analyzed by Raman spectroscopy (squares). On the right are the corresponding Raman spectra, identified as follows: 1, lepidocrocite (Le); 2, mixture of poorly crystallized mackinawite (M) and elemental sulfur (S); 3, vivianite (Vi); 4, mixture of vivianite (Vi) and lepidocrocite (Le); and 5, elemental sulfur (S).
On the basis of these results and regarding the treatment of a complex corrosion layer, we concluded that strains TCE1 and LBE have different efficiencies in terms of iron reduction on corroded coupons. With the aim of developing a biotechnological stabilization method for archaeological iron, it seems D. hafniense strain TCE1 would be more suitable as it produced a homogeneous layer of biogenic crystals of vivianite, a chemically stable iron phosphate compound.
Iron reduction on archaeological objects.
According to the above results on corroded iron coupons, only strain TCE1 was used for the evaluation of bacterial iron reduction on archaeological iron objects. First, the surfaces of nails were prepared by sandblasting according to the protocol traditionally employed for any conservation-restoration intervention. To ascertain the chemical composition of its corrosion layer, each object was characterized by noninvasive analytical techniques before and after bacterial treatment. Before treatment, the nails' surfaces presented a brown-red color with orange spots (Fig. 5). According to SEM-EDS analyses, crystals were not observed on the surfaces of the objects before incubation (Fig. 5). Oxygen and iron were detected as the main elements, while aluminum was found as a secondary element (Table 2). After 3 days of incubation, compared with the results for corroded coupons, the nails used for the abiotic control remained unchanged; neither a color change nor crystal formation were detected (Fig. 5). This is probably due to the difference, in terms of cohesion, between the corrosion layers on the coupons and the nails. In fact, the coupons presented a powdery corrosion layer that had been formed recently and hence, is much more reactive and unstable than the corrosion layer found on archaeological objects aged for centuries.
FIG 5.
Iron reduction on archaeological iron nails using strain TCE1. On the left, the appearance of the abiotic control and the nails treated with strain TCE1 before and after the treatment. On the right, the corresponding SEM images of the areas indicated by black squares inside the nails are presented.
TABLE 2.
Elemental compositions of the corroded iron nails
| Composition categorya | Element(s) identified |
|||
|---|---|---|---|---|
| Abiotic control |
Strain TCE1 |
|||
| Before treatment | After treatment | Before treatment | After treatment | |
| Main elements (>10%) | Fe, O | Fe, O | Fe, O | Fe, O, P |
| Secondary elements (between 10 and 1%) | Al | C | Al, Si | C |
| Trace elements (<1%) | Si, Ca | Al, Si, P, Ca | Ca, P | |
The percentages reported correspond to the atomic percentages.
The appearance of the nails treated with cultures of strain TCE1 changed dramatically during incubation and their surfaces turned dark-gray. Moreover, the formation of biogenic minerals presenting a bladed cluster habit was identified during SEM observations (Fig. 5). EDS analyses on biogenic mineral indicated the presence of oxygen, phosphorus, and iron as the main elements, and carbon as a secondary element. No phosphorus was detected on the surface of the nails used for the abiotic control (Table 2).
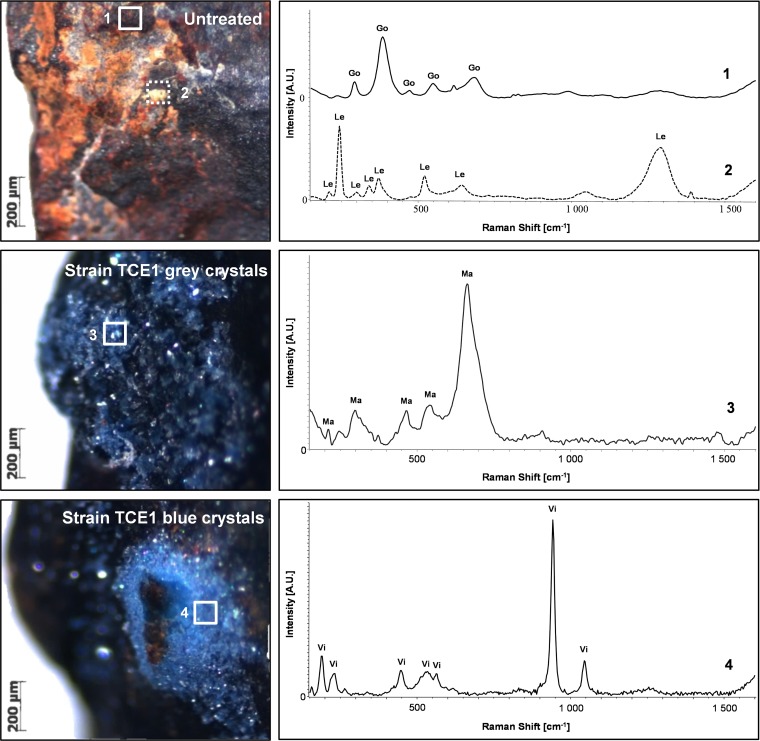
Raman spectroscopy was carried out to identify the newly formed biogenic crystals. The corrosion compounds present before treatment were characterized as a mixture of goethite α-FeOOH and lepidocrocite γ-FeOOH (Fig. 6). The same compounds were detected on the surface of the nails used for the abiotic control (see Fig. S2). Raman analysis on microsamples identified these biogenic minerals as magnetite (Fe2O4) and vivianite [Fe32+(PO4)2·8H2O) (Fig. 6). The ability of bacteria to produce vivianite is well documented (22, 46–48) and is generally linked to the activity of dissimilatory metal-reducing bacteria that couple organic carbon oxidation and Fe(III) reduction. However, in our study, vivianite production is not likely to be the consequence of iron respiration but rather of Fe(III) used as an electron sink. The production of iron phosphates might be the result of the presence of phosphate buffer in the culture medium, as it is known that the medium composition can affect the type of biogenic mineral formed (41, 49). The color and appearance of the artifact is also an important criterion in conservation-restoration, and the black appearance obtained with the formation of magnetite would be accepted according to conservation ethics. By contrast, the formation of blue stains related to the precipitation of vivianite should be avoided.
FIG 6.
Raman analysis of the archaeological nails before and after treatment with strain TCE1. On the left, stereoscope images of the areas sampled for the analysis. On the right are the corresponding Raman spectra, identified as follows: 1, goethite (Go); 2, lepidocrocite (Le); 3, magnetite (Ma); and 4, vivianite (Vi).
Currently, the negative aspects of the interaction between metallic objects and microbes have been highlighted in scientific research. Several studies have examined microbiologically induced corrosion of industrial materials such as steel (50–53). Indeed, the development of bacterial biofilms on the surface of such objects induces changes in the electrochemical conditions (50), which leads to the formation of corrosion layers and produces structural damage to the metal core. In this study, we demonstrated that bacteria can be used to convert an existing layer of unstable corrosion on archaeological iron objects into more stable compounds. These data represent a demonstration of the potential to use bacteria to reduce iron on archaeological objects for conservation-restoration purposes. Indeed, biogenic crystals were produced directly on the objects' surfaces, suggesting that the bacterial treatment converted the corrosion products present into more stable compounds, thereby stabilizing the objects. The production of chemically stable iron compounds displaying a lower molecular volume than the compounds originally present in the corrosion layer enhances the porosity of the latter, facilitating the removal of chlorine by diffusion. Additionally, SEM observations showed that the newly formed biogenic mineral layer was homogeneous and appeared to completely cover the original corroded surface. This homogeneous layer of stable crystals was produced after 3 to 7 days of incubation with D. hafniense strain TCE1. This represents an improvement with respect to conventional methods (such as alkaline sulfide baths) where immersion for a period of several weeks is required for objects of the same size (2). Further investigation on the long-term stability of the treatment and its efficiency on artifacts presenting different corrosion types should be carried out. However, the results obtained here are encouraging and represent a basis for the development and optimization of a sustainable and eco-friendly alternative conservation-restoration method for preserving archaeological iron.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
D. hafniense strains TCE1 (DSM 12704) and LBE were used in this study. Strain TCE1 was isolated from enriched cultures dechlorinating tetrachloroethene (37), while strain LBE was isolated as a contaminant from a Dehalobacter-containing culture at the Laboratory of Environmental Biotechnology (LBE), EPFL, Switzerland. To verify the identity of the strain, an average nucleotide identity analysis (ANI) was performed using the complete genome of D. hafniense strain DCB-2 as a reference (GenBank accession number NC_011830.1). The results show a mean ANI of 97.99% with the reference genome, confirming the identity of the LBE strain (see Fig. S3 in the supplemental material). Strains TCE1 and LBE were cultivated under anaerobic conditions (500-ml serum bottles capped with butyl rubber stoppers filled with 200 ml anaerobic medium) in a standard mineral medium as previously described (54). The exact composition of the medium is provided in the supplemental material. For the experiments, the medium contained 45 mM lactate and 20 mM fumarate as electron donor and acceptor, respectively. This standard medium was modified for the iron reduction experiments as described below. All the cultures were incubated at 30°C under agitation at 100 rpm until reaching an optical density at 600 nm (OD600) of 0.168 for strain TCE1 and 0.128 for strain LBE. The pH of the cultures was buffered at a value of 7.3 with a combination of phosphates (4 mM K2HPO4 and 1 mM NaH2PO4), carbonates (54 mM NaHCO3 and 6 mM NH4HCO3), and N2/CO2 (80%/20%) in the headspace.
Reduction of soluble iron phase in the presence of chlorine.
Soluble iron reduction was assessed by adding Fe(III)-citrate (final concentration 10 mM) (Fluka Analytical, Germany) to 20 ml of a bacterial culture incubated 1 day in standard medium at 30°C. To avoid changes in the pH of the cultures, the pH of the Fe(III)-citrate solution was adjusted to 7 by adding drops of 10 mM NaOH. To assess the influence of chlorine in iron reduction, cultures were also amended with 0.2% and 0.5% NaCl (Fluka Analytical, Germany). Abiotic controls without bacteria were also prepared. Aliquots of approximately 0.5 ml were collected at 0, 1, and 2 days after the addition of iron citrate. These samples contained the bacterial cells and the black precipitate produced during incubation. Iron reduction was evaluated by the quantifying Fe(II) with a ferrozine assay as previously described (55) with a few modifications. Briefly, to measure the total concentration of Fe(II), 450 μl of the previous sample was mixed with 50 μl of 5 M HCl to solubilize Fe(II) present in solid phase (black precipitates). Small volumes (10 μl) of this solution were transferred to a microplate containing 90 μl of 2 mM ferrozine reagent (Sigma-Aldrich, Germany) prepared in 100 mM HEPES solution (Sigma-Aldrich, United States). The absorbance was measured within 2 min at 562 nm with a UMV-340 microplate reader (Asis HiTech, Austria). The amount of Fe(II) was calculated using a calibration curve with known concentrations of ferrous ammonium sulfate as the Fe(II) standard. All the experiments were conducted in triplicates.
Reduction of solid iron phase.
Considering that the majority of iron compounds found in the corrosion layer of archaeological iron objects are not soluble in aqueous solutions, iron reduction was also studied by analyzing solid iron phases. For this, the standard medium described above was amended with synthetic akaganeite [FeO0.833(OH)1.167Cl0.167] at a concentration of approximately 10 mM. Synthetic akaganeite was prepared following a published procedure (56). To confirm the purity of the synthetic akaganeite, Raman spectroscopy was conducted on the mineral before the experiment (see Fig. S4). Abiotic controls without bacteria but containing akaganeite were also prepared. Akaganeite-containing solution was added to 20 ml of a 1-day bacterial culture obtained in standard medium. Homogeneous samples of the culture, containing bacterial cells and the black precipitate produced during incubation, were collected after 0, 1, and 2 days of incubation, and the Fe(II) concentration was determined with the ferrozine assay as described in the previous section. All the experiments were conducted in triplicates.
Iron reduction on corroded coupons.
To evaluate iron reduction of a real object, steel coupons presenting a natural corrosion layer produced after outdoor exposure in the city of Zurich were used as an iron source. Before the incubation, the coupons were sterilized by spraying a solution of ethanol (70% [wt/wt] in deionized water) and exposed to UV radiation (20 min each side). Next, each sample was placed in an anaerobic serum bottle. Anoxic conditions were obtained by replacing the aerobic atmosphere with N2. After a second sterilization by autoclaving (120°C for 20 min), 20 ml of the cultures of strains TCE1 and LBE, as well as the abiotic control, were added to the serum bottle containing the sterile coupons. All the experiments were conducted in triplicates.
Cultures containing the coupons were incubated at 30°C for 7 days. After the incubation, coupons were washed with a solution of ethanol (70% [wt/wt] in deionized water) and exposed to UV radiation (20 min each side) to remove remaining bacteria. SEM-EDS was carried out to evaluate the habit (size and shape of the crystals), the distribution, and the elemental composition of the newly formed biogenic minerals. Coupons were directly analyzed simply by positioning them inside the microscope chamber without preparation. A Philips ESEM XL30 FEG environmental scanning electron microscope equipped with an energy-dispersive X-ray analyzer was used. The samples were observed in secondary electrons mode at an acceleration potential of 10 to 25 keV and a working distance of 10 mm. The homogeneity of the biogenic layer was evaluated by an estimation of the surface covering by stereoscopic observations (Nikon SM218; Nikon, Japan). To study the composition of the corrosion layer before and after the bacterial treatment, nondestructive Raman spectroscopy was performed directly on the surfaces of the coupons. The analysis was carried out with a Horiba-Jobin Yvon Labram Aramis microscope equipped with a Nd:YAG laser of 532 nm at a power lower than 1 mW (600 g/mm). The spectral interval was 100 and 1,600 cm−1 and the measurement conditions were 1,000-μm hole, 100-μm slit, and 5 accumulations of 100 s. The spectra recorded were corrected (automatic baseline correction) using LabSpec NGS spectral software. Reference spectra were used for identifying the compounds present.
Iron reduction on archaeological objects.
Archaeological iron nails provided by a metal conservator-restorer were used for the treatment of real objects. These nails were recovered from the Mediterranean Sea and presented a typical submarine corrosion layer. After sandblasting, the artifacts were prepared as described above for the iron coupons. After autoclaving, 20 ml of 1-day cultures of strain TCE1 and 20 ml of medium without bacteria as an abiotic control were added to the anaerobic flasks containing the nails. The cultures were incubated for 3 additional days. The sterilization process after the bacterial treatment was performed as described above for the iron coupons. All the experiments were conducted in triplicates. Nail surfaces were studied before and after the treatment by SEM-EDS and Raman spectroscopy as described for the iron coupons. Microsampling of the crystals was required for the Raman analyses.
Accession number(s).
The draft genome of strain LBE is available in the repository of the DOE Joint Genome Institute (http://genome.jgi.doe.gov/DesspLBE_FD/DesspLBE_FD.info.html). The entire 16S rRNA gene sequence of strain LBE has been submitted to GenBank under accession number KY554954.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this work was provided by the Ambizione grant MAIA (Microbes for Archaeological Iron Artworks) PZ00P2_142514 2013-2015 from the Swiss National Science Foundation.
We are grateful to Olivier Berger, AMC Art Metal Conservation S.A.R.L., for providing the archaeological iron nails used in this study. We also acknowledge the Archaeological Park and Museum Latenium for technical assistance during the sandblasting of those nails (Christian Cevey). We thank the research conservation laboratory of the Swiss National Museum (Marie Wörle and Tiziana Lombardo) for their help with conducting Raman investigations and for providing the steel plate used in the experiments.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.03478-16.
REFERENCES
- 1.Jain KK. 2009. Iron artifacts: history, metallurgy, corrosion and conservation. Agam Kala Prakashan, Delhi, India. [Google Scholar]
- 2.Selwyn L. 2004. Overview of archaeological iron: the corrosion problem, key factors affecting treatment, and gaps in current knowledge, p. 294–306. In Proceedings of Metal 2004. National Museum of Australia, Canberra, ACT, Australia. [Google Scholar]
- 3.Scott DA, Eggert G. 2009. Iron and steel in art: corrosion, colorants, conservation. Archetype Publications, London, UK. [Google Scholar]
- 4.Turgoose S. 1982. Post-excavation changes in iron antiquities. Stud Conserv 27:97–101. doi: 10.2307/1506144. [DOI] [Google Scholar]
- 5.Turgoose S. 1985. The corrosion of archaeological iron during burial and treatment. Stud Conserv 30:13–18. doi: 10.2307/1506129. [DOI] [Google Scholar]
- 6.Ståhl K, Nielsen K, Jiang J, Lebech B, Hanson JC, Norby P, van Lanschot J. 2003. On the akaganéite crystal structure, phase transformations and possible role in post-excavational corrosion of iron artifacts. Corros Sci 45:2563–2575. doi: 10.1016/S0010-938X(03)00078-7. [DOI] [Google Scholar]
- 7.Rimmer M, Watkinson D, Wang Q. 2012. The efficiency of chloride extraction from archaeological iron objects using deoxygenated alkaline solutions. Stud Conserv 57:29–41. doi: 10.1179/2047058411Y.0000000005. [DOI] [Google Scholar]
- 8.Carlin W, Keith D, Rodriguez J. 2001. Less is more: measure of chloride removal rate from wrought iron artifacts during electrolysis. Stud Conserv 46:68–76. doi: 10.1179/sic.2001.46.1.68. [DOI] [Google Scholar]
- 9.Bosch-Roig P, Ranalli G. 2014. The safety of biocleaning technologies for cultural heritage. Front Microbiol 5:155. doi: 10.3389/fmicb.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappitelli F, Toniolo L, Sansonetti A, Gulotta D, Ranalli G, Zanardini E, Sorlini C. 2007. Advantages of using microbial technology over traditional chemical technology in removal of black crusts from stone surfaces of historical monuments. Appl Environ Microbiol 73:5671–5675. doi: 10.1128/AEM.00394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cappitelli F, Zanardini E, Ranalli G, Mello E, Daffonchio D, Sorlini C. 2006. Improved methodology for bioremoval of black crusts on historical stone artworks by use of sulfate-reducing bacteria. Appl Environ Microbiol 72:3733–3737. doi: 10.1128/AEM.72.5.3733-3737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranalli G, Alfano G, Belli C, Lustrato G, Colombini M, Bonaduce I, Zanardini E, Abbruscato P, Cappitelli F, Sorlini C. 2005. Biotechnology applied to cultural heritage: biorestoration of frescoes using viable bacterial cells and enzymes. J Appl Microbiol 98:73–83. doi: 10.1111/j.1365-2672.2004.02429.x. [DOI] [PubMed] [Google Scholar]
- 13.Alfano G, Lustrato G, Belli C, Zanardini E, Cappitelli F, Mello E, Sorlini C, Ranalli G. 2011. The bioremoval of nitrate and sulfate alterations on artistic stonework: the case-study of Matera Cathedral after six years from the treatment. Int Biodeterior Biodegradation 65:1004–1011. doi: 10.1016/j.ibiod.2011.07.010. [DOI] [Google Scholar]
- 14.Jonkers H. 2011. Bacteria-based self-healing concrete. Heron 56:1–12. [Google Scholar]
- 15.Dhami NK, Reddy MS, Mukherjee A. 2013. Biomineralization of calcium carbonates and their engineered applications: a review. Front Microbiol 4:314. doi: 10.3389/fmicb.2013.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph E, Cario S, Simon A, Wörle M, Mazzeo R, Junier P, Job D. 2012. Protection of metal artifacts with the formation of metal-oxalates complexes by Beauveria bassiana. Front Microbiol 2:270. doi: 10.3389/fmicb.2011.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph E, Simon A, Mazzeo R, Job D, Wörle M. 2012. Spectroscopic characterization of an innovative biological treatment for corroded metal artifacts. J Raman Spectrosc 43:1612–1616. doi: 10.1002/jrs.4164. [DOI] [Google Scholar]
- 18.Joseph E, Letardi P, Comensoli L, Simon A, Junier P, Job D, Wörle M. 2013. Assesment of a biological approach for the protection of copper allosy artifacts. Proceedings of the International Council of Museums Committee for Conservation, Edinburgh, Scotland. [Google Scholar]
- 19.Dalla Vecchia E, Suvorova E, Maillard J, Bernier-Latmani R. 2014. Fe(III) reduction during pyruvate fermentation by Desulfotomaculum reducens strain MI-1. Geobiology 12:48–61. doi: 10.1111/gbi.12067. [DOI] [PubMed] [Google Scholar]
- 20.Lovley DR. 1991. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev 55:259–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canfield D, Jorgensen B, Fossing H, Glud R, Gundersen J, Ramsing N, Thamdrup B, Hansen J, Nielsen L, Hall P. 1993. Pathways of organic-carbon oxidation in 3 continental-margin sediments. Mar Geol 113:27–40. [DOI] [PubMed] [Google Scholar]
- 22.Fredrickson JK, Zachara JM, Kennedy DW, Dong H, Onstott TC, Hinman NW, Li S-M. 1998. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim Cosmochim Acta 62:3239–3257. doi: 10.1016/S0016-7037(98)00243-9. [DOI] [Google Scholar]
- 23.Frankel RB, Bazylinski DA. 2003. Biologically induced mineralization by bacteria. Rev Mineral Geochem 54:95–114. doi: 10.2113/0540095. [DOI] [Google Scholar]
- 24.Weber KA, Achenbach LA, Coates JD. 2006. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4:752–764. doi: 10.1038/nrmicro1490. [DOI] [PubMed] [Google Scholar]
- 25.Schröder I, Johnson E, de Vries S. 2003. Microbial ferric iron reductases. FEMS Microbiol Rev 27:427–447. doi: 10.1016/S0168-6445(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 26.Shi L, Squier TC, Zachara JM, Fredrickson JK. 2007. Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes. Mol Microbiol 65:12–20. doi: 10.1111/j.1365-2958.2007.05783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lower SK, Hochella MF, Beveridge TJ. 2001. Bacterial recognition of mineral surfaces: nanoscale interactions between Shewanella and α-FeOOH. Science 292:1360–1363. doi: 10.1126/science.1059567. [DOI] [PubMed] [Google Scholar]
- 28.Gralnick JA, Newman DK. 2007. Extracellular respiration. Mol Microbiol 65:1–11. doi: 10.1111/j.1365-2958.2007.05778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi L, Richardson DJ, Wang Z, Kerisit SN, Rosso KM, Zachara JM, Fredrickson JK. 2009. The roles of outer membrane cytochromes of Shewanella and Geobacter in extracellular electron transfer. Environ Microbiol Rep 1:220–227. doi: 10.1111/j.1758-2229.2009.00035.x. [DOI] [PubMed] [Google Scholar]
- 30.Inoue K, Leang C, Franks AE, Woodard TL, Nevin KP, Lovley DR. 2011. Specific localization of the c-type cytochrome OmcZ at the anode surface in current-producing biofilms of Geobacter sulfurreducens. Environ Microbiol Rep 3:211–217. doi: 10.1111/j.1758-2229.2010.00210.x. [DOI] [PubMed] [Google Scholar]
- 31.Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- 32.Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS. 2006. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci U S A 103:11358–11363. doi: 10.1073/pnas.0604517103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moskowitz BM, Frankel RB, Bazylinski DA, Jannasch HW, Lovley D. 1989. A comparison of magnetite particles produced anaerobically by magnetotactic and dissimilatory iron-reducing bacteria. Geophys Res Lett 16:665–668. doi: 10.1029/GL016i007p00665. [DOI] [Google Scholar]
- 34.Bazylinski DA, Frankel RB. 2000. Magnetic iron oxide and iron sulfide minerals within microorganisms, p 25–46. In Bauerlein E. (ed), Biomineralization: from biology to biotechnology and medical application. Wiley, Chichester, UK. [Google Scholar]
- 35.Villemur R, Lanthier M, Beaudet R, Lépine F. 2006. The Desulfitobacterium genus. FEMS Microbiol Rev 30:706–733. doi: 10.1111/j.1574-6976.2006.00029.x. [DOI] [PubMed] [Google Scholar]
- 36.Futagami T, Furukawa K. 2016. The genus Desulfitobacterium, p 173–207. In Adrian L, Löffler FE (ed), Organohalide-respiring bacteria. Springer, Berlin, Heidelberg. [Google Scholar]
- 37.Christiansen N, Ahring BK. 1996. Desulfitobacterium hafniense sp. nov., an anaerobic, reductively dechlorinating bacterium. Int J Syst Evol Microbiol 46:442–448. [Google Scholar]
- 38.Gerritse J, Drzyzga O, Kloetstra G, Keijmel M, Wiersum LP, Hutson R, Collins MD, Gottschal JC. 1999. Influence of different electron donors and acceptors on dehalorespiration of tetrachloroethene by Desulfitobacterium frappieri TCE1. Appl Environ Microbiol 65:5212–5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niggemyer A, Spring S, Stackebrandt E, Rosenzweig RF. 2001. Isolation and characterization of a novel As(V)-reducing bacterium: implications for arsenic mobilization and the genus Desulfitobacterium. Appl Environ Microbiol 67:5568–5580. doi: 10.1128/AEM.67.12.5568-5580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Federal Institute for Occupational Safety and Health. 2016. Technical rules for biological agents. TRBA 466. Federal Institute for Occupational Safety and Health, Dortmund, Germany: http://www.baua.de/en/Topics-from-A-to-Z/Biological-Agents/TRBA/TRBA-466.html;jsessionid=CB37D8B822BC6D05361657592621E6A2.s2t1. [Google Scholar]
- 41.Roh Y, Zhang C-L, Vali H, Lauf RJ, Zhou J, Phelps TJ. 2003. Biogeochemical and environmental factors in Fe Biomineralisation: magentite and siderite formation. Clays Clay Miner 51:83–95. doi: 10.1346/CCMN.2003.510110. [DOI] [Google Scholar]
- 42.Emmerich M, Bhansali A, Lösekann-Behrens T, Schröder C, Kappler A, Behrens S. 2012. Abundance, distribution, and activity of Fe(II)-oxidizing and Fe(III)-reducing microorganisms in hypersaline sediments of Lake Kasin, southern Russia. Appl Environ Microbiol 78:4386–4399. doi: 10.1128/AEM.07637-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monnier J, Bellot-Gurlet L, Baron D, Neff D, Guillot I, Dillmann P. 2011. A methodology for Raman structural quantification imaging and its application to iron indoor atmospheric corrosion products. J Raman Spectrosc 42:773–781. doi: 10.1002/jrs.2765. [DOI] [Google Scholar]
- 44.Rémazeilles C, Tran K, Guilminot E, Conforto E, Refait P. 2013. Study of Fe(II) sulphides in waterlogged archaeological wood. Stud Conserv 58:297–307. doi: 10.1179/2047058412Y.0000000071. [DOI] [Google Scholar]
- 45.Frost RL, Martens W, Williams P, Kloprogge JT. 2002. Raman and infrared spectroscopic study of the vivianite-group phosphates vivianite, baricite and bobierrite. Mineral Mag 66:1063–1073. doi: 10.1180/0026461026660077. [DOI] [Google Scholar]
- 46.Rothe M, Kleeberg A, Hupfer M. 2016. The occurrence, identification and environmental relevance of vivianite in waterlogged soils and aquatic sediments. Earth Sci Rev 158:51–64. doi: 10.1016/j.earscirev.2016.04.008. [DOI] [Google Scholar]
- 47.Glasauer S, Weidler PG, Langley S, Beveridge TJ. 2003. Controls on Fe reduction and mineral formation by a subsurface bacterium. Geochim Cosmochim Acta 67:1277–1288. doi: 10.1016/S0016-7037(02)01199-7. [DOI] [Google Scholar]
- 48.O'Loughlin EJ, Boyanov MI, Flynn TM, Gorski CA, Hofmann SM, McCormick ML, Scherer MM, Kemner KM. 2013. Effects of bound phosphate on the bioreduction of lepidocrocite (γ-FeOOH) and maghemite (γ-Fe2O3) and formation of secondary minerals. Environ Sci Technol 47:9157–9166. doi: 10.1021/es400627j. [DOI] [PubMed] [Google Scholar]
- 49.Bell PE, Mills AL, Herman JS. 1987. Biogeochemical conditions favoring magnetite formation during anaerobic iron reduction. Appl Environ Microbiol 53:2610–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Videla HA, Herrera LK. 2005. Microbiologically influenced corrosion: looking to the future. Int Microbiol 8:169. [PubMed] [Google Scholar]
- 51.Valencia-Cantero E, Peña-Cabriales JJ. 2014. Effects of iron-reducing bacteria on carbon steel corrosion induced by thermophilic sulfate-reducing consortia. J Microbiol Biotechnol 24:280–286. doi: 10.4014/jmb.1310.10002. [DOI] [PubMed] [Google Scholar]
- 52.Li S, Kim Y, Jeon K, Kho Y, Kang T. 2001. Microbiologically influenced corrosion of carbon steel exposed to anaerobic soil. Corrosion 57:815–828. doi: 10.5006/1.3280616. [DOI] [Google Scholar]
- 53.Xu D, Li Y, Song F, Gu T. 2013. Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis. Corros Sci 77:385–390. doi: 10.1016/j.corsci.2013.07.044. [DOI] [Google Scholar]
- 54.Prat L, Maillard J, Grimaud R, Holliger C. 2011. Physiological adaptation of Desulfitobacterium hafniense strain TCE1 to tetrachloroethene respiration. Appl Environ Microbiol 77:3853–3859. doi: 10.1128/AEM.02471-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stookey LL. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem 42:779–781. doi: 10.1021/ac60289a016. [DOI] [Google Scholar]
- 56.Schwertmann U, Cornell RM. 2008. Iron oxides in the laboratory: preparation and characterization. John Wiley & Sons, Chichester, UK. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.