ABSTRACT
In 1953, investigators at the Rocky Mountain Laboratories in Hamilton, MT, described the isolation of a spotted fever group Rickettsia (SFGR) species from Dermacentor parumapertus ticks collected from black-tailed jackrabbits (Lepus californicus) in northern Nevada. Several decades later, investigators characterized this SFGR (designated the parumapertus agent) by using mouse serotyping methods and determined that it represented a distinct rickettsial serotype closely related to Rickettsia parkeri; nonetheless, the parumapertus agent was not further characterized or studied. To our knowledge, no isolates of the parumapertus agent remain in any rickettsial culture collection, which precludes contemporary phylogenetic placement of this enigmatic SFGR. To rediscover the parumapertus agent, adult-stage D. parumapertus ticks were collected from black-tailed jackrabbits shot or encountered as roadkills in Arizona, Utah, or Texas from 2011 to 2016. A total of 339 ticks were collected and evaluated for infection with Rickettsia species. Of 112 D. parumapertus ticks collected in south Texas, 16 (14.3%) contained partial ompA sequences with the closest identity (99.6%) to Rickettsia sp. strain Atlantic rainforest Aa46, an SFGR that is closely related or identical to an SFGR species that causes a mild rickettsiosis in several states of Brazil. A pure isolate, designated strain Black Gap, was cultivated in Vero E6 cells, and sequence analysis of the rrs, gltA, sca0, sca5, and sca4 genes also revealed the closest genetic identity to Rickettsia sp. Atlantic rainforest Aa46. Phylogenetic analysis of the five concatenated rickettsial genes place Rickettsia sp. strain Black Gap and Rickettsia sp. Atlantic rainforest Aa46 with R. parkeri in a distinct and well-supported clade.
IMPORTANCE We suggest that Rickettsia sp. Black Gap and Rickettsia sp. Atlantic rainforest Aa46 represent nearly identical strains of R. parkeri and that Rickettsia sp. Black Gap or a very similar strain of R. parkeri represents the parumapertus agent. The close genetic relatedness among these taxa, as well as the response of guinea pigs infected with the Black Gap strain, suggests that R. parkeri Black Gap could cause disease in humans. The identification of this organism could also account, at least in part, for the remarkable differences in severity ascribed to Rocky Mountain spotted fever (RMSF) among various regions of the American West during the early 20th century. We suggest that the wide variation in case fatality rates attributed to RMSF could have occurred by the inadvertent inclusion of cases of milder disease caused by R. parkeri Black Gap.
KEYWORDS: Dermacentor, RMSF, Rickettsia, Rickettsia Atlantic rainforest, Rickettsia parkeri, tick-borne pathogens
INTRODUCTION
The earliest reports of Rocky Mountain spotted fever (RMSF), a tick-borne bacterial infection caused by Rickettsia rickettsii and among the deadliest of all infectious diseases in the Americas, also described a clinically similar but surprisingly less virulent form of the disease. In contrast to the 65% case fatality rate (CFR) documented in the Bitterroot Valley of western Montana, a far milder form of spotted fever was recognized in the valleys of the Snake River and its tributaries in southern Idaho, where the CFR was only approximately 5% (1). Low CFRs were also noted by physicians in adjacent western states (2):
…in Eastern Oregon and along the Idaho and Nevada borders, it would certainly seem we had an interesting climatic variant of the celebrated pathological curio of the Bitter Root Valley. Its chief difference from the latter is a most fortunate one, and lies in the fact that its mortality is exceedingly low.
The remarkably wide spectrum of virulence attributed to R. rickettsii remains an enduring mystery of RMSF. The recent identification of other pathogenic spotted fever group Rickettsia (SFGR) species as causes of rickettsioses in the United States (3, 4) suggests that some or many of the cases classified historically as RMSF were attributed to SFGR other than R. rickettsii (5).
In 1953, C. B. Philip and colleagues described the isolation of an SFGR species from pooled samples of Dermacentor parumapertus ticks (the rabbit dermacentor) (Fig. 1) collected from black-tailed jackrabbits (Lepus californicus) in northern Nevada (6). When inoculated intraperitoneally into male guinea pigs (Cavia porcellus), strains of this organism (designated the parumapertus agent) produced a self-limiting illness, characterized by 1 to 2 days of a temperature of ∼40°C that was followed by scrotal swelling, similar to the disease caused by SFGR species with lower levels of virulence than R. rickettsii (6, 7). Additional isolates of the parumapertus agent were subsequently obtained from pooled specimens of adult D. parumapertus ticks collected in the Great Salt Lake Desert of western Utah from 1954 to 1957 (8) and from an adult D. parumapertus tick collected in northern California in 1980 (9). By using a microimmunofluorescence serotyping technique, investigators determined that the parumapertus agent was a unique rickettsial serotype that was most closely related to and that formed a minor subgroup with Rickettsia parkeri and Rickettsia sibirica (10, 11). To our knowledge, no isolate of the parumapertus agent remains in any rickettsial culture collection, precluding molecular-based phylogenetic placement of this enigmatic SFGR species. Herein, we describe the likely rediscovery of the parumapertus agent and consider some potentialities of its past and present influence on the ecology and epidemiology of rickettsial diseases in the western United States.
FIG 1.
Specimens of adult female (left) and male (right) Dermacentor parumapertus ticks (the rabbit dermacentor). (Courtesy of James Gathany [CDC].)
RESULTS
Tick collections.
A total of 339 adult D. parumapertus ticks were collected from three western states from 2011 to 2016 (Table 1). These included 72 male and 28 female ticks collected from 10 black-tailed jackrabbits from Skull Valley in Tooele County, UT, from 2013 to 2016; 41 male and 24 female ticks collected from 10 black-tailed jackrabbits within and adjacent to Dugway Proving Ground in Tooele County, UT, from 2011 and 2016; 55 male and 57 female ticks collected from 18 black-tailed jackrabbits from multiple locations along Farm Road 2627 in Black Gap Wildlife Management Area in Brewster County, TX, from 2013 to 2015; 30 male and 11 female ticks collected from a black-tailed jackrabbit in Arivaca in Pima County, AZ, in 2016; and 16 male and 5 female ticks collected from a black-tailed jackrabbit on Ruby Road in Santa Cruz County, AZ, in 2016. Twenty-five (20%) of the female tick specimens were fully engorged. Most ticks were found attached to the inner and outer aspects of the pinnae.
TABLE 1.
Collection locations and rates of infection with Rickettsia species in Dermacentor parumapertus ticks collected from black-tailed jackrabbits (Lepus californicus) in Utah, Texas, and Arizona, 2011 to 2016
| Location | Yr | No. of ticks evaluated | No. (%) of ticks positive for a Rickettsia species |
||
|---|---|---|---|---|---|
| Rickettsia parkeri | Rickettsia bellii | Rickettsia rhipicephali | |||
| Dugway Proving Ground, Tooele County, UT | 2011 | 6 | 0 | 4 (67) | 1 (17) |
| 2016 | 59 | 0 | 52 (88) | 27 (46) | |
| Skull Valley, Tooele County, UT | 2013 | 70 | 0 | 53 (76) | 12 (17) |
| 2016 | 30 | 1 (3)a | 18 (60) | 5 (17) | |
| Black Gap Wildlife Management Area, Brewster County, TX | 2013 | 8 | 0 | 1 (12) | 0 |
| 2014 | 60 | 11 (18)b | 6 (10) | 0 | |
| 2015 | 44 | 5 (11)b | 4 (9) | 0 | |
| Arivaca, Pima County, AZ | 2016 | 41 | 0 | 34 (83) | 0 |
| Ruby Road, Santa Cruz County, AZ | 2016 | 21 | 2 (10)c | 21 (100) | 0 |
A partial (513 bp) ompA sequence 98.2% identical to the corresponding ompA sequence of R. parkeri Maculatum 20T, 99.0% identical to the corresponding ompA sequence of Rickettsia sp. Black Gap, and 99.4% identical to the corresponding ompA sequence of Rickettsia sp. Atlantic rainforest Aa46.
A partial (532 bp) ompA sequence 97.9% identical to the corresponding ompA sequence of R. parkeri Maculatum 20T and 99.6% identical to the corresponding ompA sequence of Rickettsia sp. Atlantic rainforest Aa46.
A partial (532 bp) ompA sequence 100% identical to the corresponding ompA sequence of R. parkeri Maculatum 20T.
Molecular evaluation of ticks.
Of 165 ticks collected from northwest Utah, 127 (77%) were infected by Rickettsia bellii, including 88 (78%) male and 39 (75%) female ticks (Table 1). Rickettsia rhipicephali was detected in 29 (26%) and 16 (31%) of the male and female tick specimens, respectively, of which 39 (85%) were coinfected with R. bellii. A partial ompA sequence 98.2% and 99.4% identical to the corresponding R. parkeri strain Maculatum 20T and Rickettsia sp. strain Atlantic rainforest Aa46 ompA sequences, respectively, was detected in one male tick specimen collected in 2016 that was also coinfected with R. bellii. Sixteen (14%) of 112 ticks collected from south Texas, comprising 7 male and 9 female tick specimens, contained partial ompA sequences that were identical to each other and 97.9% and 99.6% identical to the corresponding ompA segments of R. parkeri Maculatum 20T and Rickettsia sp. Atlantic rainforest Aa46, respectively. R. bellii was detected in 5 (9%) male and 6 (11%) female ticks. No infections with R. rhipicephali and no rickettsial coinfections were identified in any of the specimens from Texas. Of 62 specimens of D. parumapertus ticks obtained in southern Arizona, 55 (89%) were infected with R. bellii, including 41 (89%) male and 14 (88%) female ticks. No specimens were infected with R. rhipicephali; however, of the R. bellii-infected ticks, two male tick specimens also contained partial ompA sequences with 100% identity to the corresponding ompA segment of R. parkeri Maculatum 20T and one female tick specimen contained a partial ompA sequence with 100% identity to the corresponding ompA segments of “Candidatus Rickettsia andeanae.”
Isolation in cell culture.
Initial attempts to cultivate Rickettsia species from D. parumapertus ticks were hampered by repeated contamination with various environmental bacterial species, including Bacillus cereus, Microbacterium paraoxydans, Stenotrophomonas maltophilia, and a Lysinibacillus sp. The isolation protocol was therefore modified by excluding the tick exoskeleton from the primary inoculum by removing the internal organs with a 26-gauge hypodermic needle and by adding 5 μg/ml gentamicin sulfate to the culture medium for the first 48 h of incubation. One rickettsial isolate was subsequently obtained in pure culture from a male tick collected in Brewster County, TX, in 2015. Rare diplobacilli were identified by using acridine orange stain 5 days after the cells were inoculated with minced tick tissues. A rickettsial isolate, designated strain Black Gap, was established and successfully passed 6 times in Vero E6 cells. This isolate was deposited in the CDC Rickettsial Isolate Reference Collection (WDCM 1093; CRIRC RPA035). Two isolates of R. bellii were also obtained, including one from a male tick specimen collected from Utah (strain Skull Valley; CRIRC RBE007) and one from a male tick specimen collected in Texas (strain TX15-1; CRIRC RBE010). Three mixed isolates, each one containing both R. bellii and R. rhipicephali, were obtained from three ticks coinfected with these two organisms, collected in Tooele County, UT, and designated strains UT13-17, UT13-26, and UT13-34.
Molecular characterization of Rickettsia sp. strain Black Gap.
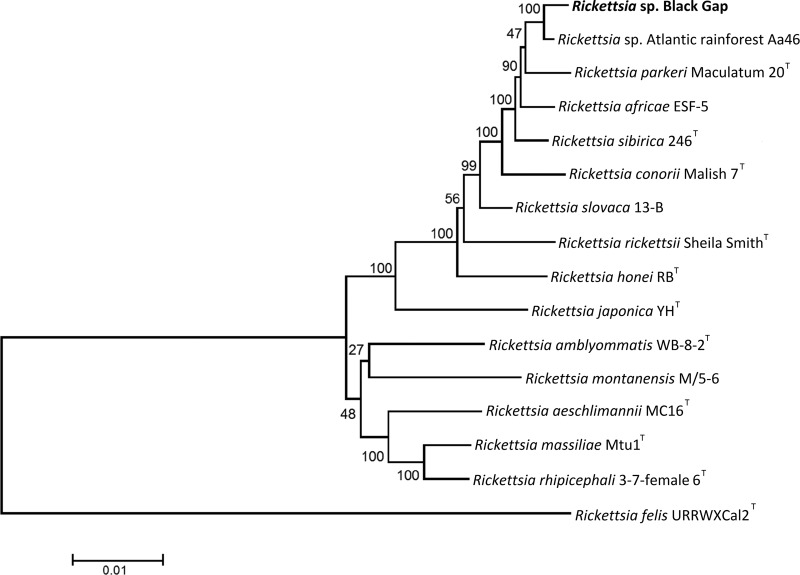
The complete sequences of five rickettsial genes (rrs, gltA, sca0, sca5, and sca4) were amplified and sequenced from DNA extracted from cell cultures for Rickettsia sp. Black Gap and Rickettsia sp. Atlantic rainforest Aa46. The resultant sequences were compared to the sequences of established Rickettsia species using BLASTn analysis (Table 2) and were also compared to each other and to the sequence of the type strain of R. parkeri (Table 3). To identify the relationship of Rickettsia sp. Black Gap and Rickettsia sp. Atlantic rainforest Aa46 to other established Rickettsia species, a phylogenetic analysis was performed using the concatenated sequences of the five sequenced loci. Maximum likelihood phylogenetic analysis (Fig. 2) confirmed the genetic comparison data and indicated that Rickettsia sp. Black Gap and Rickettsia sp. Atlantic rainforest Aa46 were most closely related to each other and to R. parkeri.
TABLE 2.
Comparisons of genetic identities of Rickettsia sp. Black Gap and Rickettsia sp. Atlantic rainforest Aa46 to established Rickettsia species in GenBank
| Locus and species (GenBank accession no.) | Established Rickettsia species with closest BLASTn result (GenBank accession no.) | No. of identical bases/no. of bases compared (% identity) |
|---|---|---|
| rrs | ||
| Rickettsia sp. Black Gap (KY124256) | R. parkeri Portsmouth (CP003341) | 1,387/1,390 (99.8) |
| Rickettsia sp. Atlantic rainforest Aa46 (KY113108) | R. parkeri Portsmouth (CP003341) | 1,353/1,356 (99.8) |
| gltA | ||
| Rickettsia sp. Black Gap (KY124257) | R. sibirica Novosibirsk (KU310587) | 1,047/1,048 (99.9) |
| Rickettsia sp. Atlantic rainforest Aa46 (KY113109) | R. sibirica Novosibirsk (KU310587) | 1,047/1,048 (99.9) |
| sca0 | ||
| Rickettsia sp. Black Gap (KY124258) | R. africae ESF-5 (CP001612) | 586/590 (99.3) |
| Rickettsia sp. Atlantic rainforest Aa46 (KY113110) | R. africae ESF-5 (CP001612) | 588/590 (99.7) |
| sca5 | ||
| Rickettsia sp. Black Gap (KY124259) | R. africae ESF-5 (CP001612) | 4,800/4,846 (99.1) |
| Rickettsia sp. Atlantic rainforest Aa46 (KY113111) | R. africae ESF-5 (CP001612) | 4,813/4,846 (99.3) |
| sca4 | ||
| Rickettsia sp. Black Gap (KY124260) | R. parkeri Portsmouth (CP003341) | 2,927/2,956 (99.0) |
| Rickettsia sp. Atlantic rainforest Aa46 (KY113112) | R. parkeri Portsmouth (CP003341) | 2,931/2,956 (99.2) |
TABLE 3.
Comparisons of selected genetic loci among Rickettsia sp. Black Gap, Rickettsia parkeri Maculatum 20T, and Rickettsia sp. Atlantic rainforest Aa46
| Locus | Sequence length (bp) | No. of base differences/% sequence identity |
||
|---|---|---|---|---|
| Rickettsia sp. Black Gap and Rickettsia sp. Atlantic rainforest Aa46 | Rickettsia sp. Black Gap and R. parkeri Maculatum 20T | R. parkeri Maculatum 20T and Rickettsia sp. Atlantic rainforest Aa46 | ||
| rrs | 1,390a | 1/99.9 | 3/99.8 | 3/99.8 |
| gltA | 1,048 | 2/99.8 | 2/99.8 | 2/99.8 |
| sca0 | 590 | 2/99.7 | 13/97.8 | 11/98.1 |
| sca5 | 4,846 | 27/99.4 | 53/98.9 | 42/99.1 |
| sca4 | 2,956 | 10/99.7 | 37/98.7 | 33/98.9 |
A total of 1,356 bp was amplified from Rickettsia sp. Atlantic rainforest Aa46.
FIG 2.
Phylogenetic relationship of Rickettsia sp. Black Gap to other spotted fever group Rickettsia taxa determined using concatenated sequences of the rrs, gltA, sca0, sca5, and sca4 genes. Homologous sequence data for validated species of SFGR and Rickettsia felis were obtained from GenBank (www.ncbi.nlm.nih.gov/GenBank). The evolutionary history was inferred using the maximum likelihood method. Positions containing gaps or missing data were eliminated, leaving a total of 10,466 nucleotides in the final data set. The scale bar is in units of the number of base substitutions per site. Bootstrap values for 1,000 replicates are displayed at the nodes.
Infection of guinea pigs.
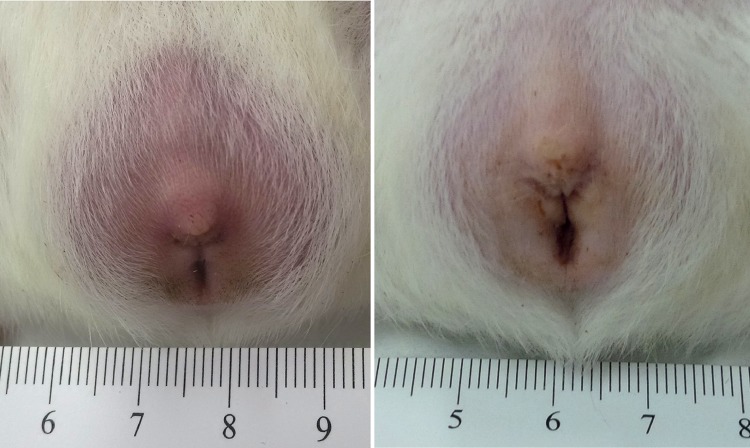
A total of eight guinea pigs were inoculated intraperitoneally with Rickettsia sp. Black Gap in two separate experiments. In the first experiment, two guinea pigs were each inoculated with 0.25 ml of the rickettsial suspension (3.5 × 107 genomic copies) and two other guinea pigs were each inoculated with 2.5 ml (3.5 × 108 genomic copies). Three of these animals developed a fever (maximum temperature [Tmax] = 39.8 to 40.3°C) that began on day 3 or 4 postinoculation and that persisted for 1 to 2 days, and three animals developed scrotal swelling and erythema that began on day 4 postinoculation and that persisted for 3 to 5 days. All animals remained active, and none died during the study period. In the second experiment, all four animals inoculated with 1 ml (1.9 × 108 genomic copies) of Rickettsia sp. Black Gap developed a febrile response (Tmax = 39.7 to 40.3°C) within 3 to 8 days postinoculation. Two animals were febrile for 1 day, one animal was febrile for 2 consecutive days, and one animal demonstrated an intermittent febrile response on days 3, 8, and 10 postinoculation. Mild to moderate scrotal swelling and erythema of the overlying skin were identified in each of the infected animals beginning on days 4 to 6 postinoculation and persisted for 1 to 5 days (Fig. 3). There was no evidence of fever or scrotal swelling in the guinea pigs inoculated with noninfected Vero E6 cells (Fig. 3). The mean percent weight gains for the infected and control animals were 21.5 and 21.0%, respectively. Infected animals remained active, and none died during the study period.
FIG 3.
(Right) Scrotal swelling and erythema of a male guinea pig (Cavia porcellus) 6 days following intraperitoneal inoculation with Rickettsia sp. Black Gap. (Left) The normal appearance of the scrotum of control guinea pig inoculated with noninfected Vero E6 cells.
Histopathological and immunohistochemical evaluation.
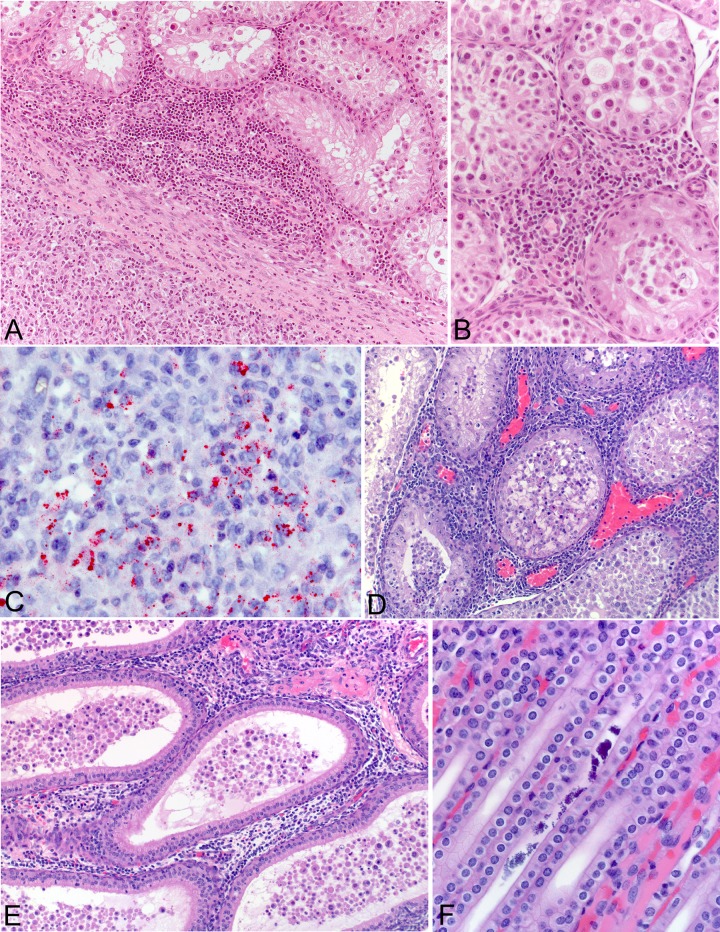
Histological examination of tissues from guinea pigs euthanized at day 8 postinoculation revealed extensive and focally transmural lymphocytic and histiocytic inflammatory cell infiltrates involving the fibrous and vascular tissues of the tunica vaginalis, tunica albuginea, and tunica vasculosa of the testes in each of the four infected animals (Fig. 4A). In some animals, the inflammatory infiltrates included moderate numbers of heterophils and were accompanied by foci of hemorrhage, thrombosis, and vasculitis. Multifocal lymphohistiocytic infiltrates were additionally observed in the interstitium of the testes in two animals (Fig. 4B). Other pathological lesions common to all infected animals included multifocal intraluminal deposits of mineralized material in collecting tubules in the medullary rays of the kidneys and small, multifocal, perivascular foci of mixed inflammatory cell infiltrates in the dermis of skin sampled from the pinnae. By using an immunohistochemical stain, SFGR were identified in abundance in the inflamed tunics surrounding the testes of each infected animal (Fig. 4C) and to a lesser extent within the testicular interstitium of two animals. In two animals, SFGR were also identified within the inflammatory lesions in the dermis of the pinnae. In one animal, rare immunohistochemical staining was observed in a single focus in the lung, but immunohistochemical staining was otherwise absent from the spleen, liver, kidney, and heart tissues of all infected animals.
FIG 4.
Histological appearance of guinea pig tissues at day 8 or 14 following intraperitoneal inoculation with Rickettsia sp. Black Gap. (A) Abundant lymphocytic and histiocytic inflammatory cell infiltrates involving the fibrous and vascular tissues of the tunica vaginalis, tunica albuginea, and tunica vasculosa in the testis of a guinea pig at day 8 postinfection (hematoxylin and eosin stain). Magnification, ×20. (B) Interstitial mononuclear inflammatory cell infiltrate in the testis of a guinea pig at day 8 postinfection (hematoxylin and eosin stain). Magnification, ×40. (C) Immunohistochemical localization of rickettsial antigens in the inflamed tunics of the testis of a guinea pig at day 8 postinfection (immunoalkaline phosphatase with naphthol fast-red and hematoxylin counterstain). Magnification, ×100. (D and E) Abundant interstitial inflammatory cell infiltrates involving a testis (D) and an epididymis (E) of guinea pigs at 14 days postinfection (hematoxylin and eosin stain). Magnifications, ×20. (F) Intraluminal mineralized debris in a renal tubule of a guinea pig at 14 days postinfection. Hematoxylin and eosin stain; magnification, ×100.
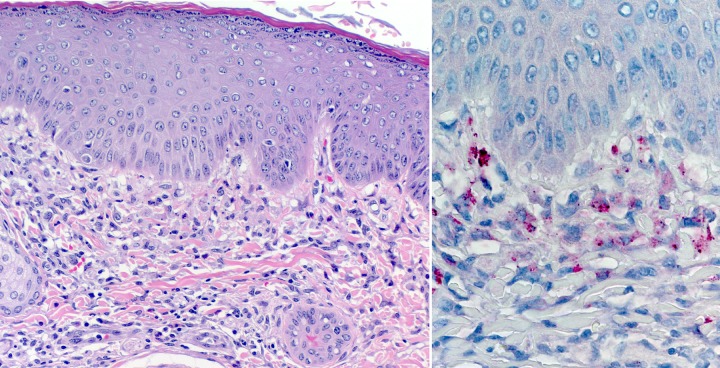
Histological examination of tissues from guinea pigs euthanized at day 14 postinoculation revealed mild to moderate lymphohistiocytic infiltrates of the tunics surrounding the testes in each of the four infected animals, but the amount and extent of inflammation were markedly reduced compared to the amount and extent of inflammation observed at day 8. Focally abundant interstitial lymphohistiocytic infiltrates were also observed in a testis of one animal (Fig. 4D) and an epididymis of another animal (Fig. 4E). In each infected animal, mineralized debris was observed in the collecting tubules of the kidneys (Fig. 4F), and small collections of predominantly lymphohistiocytic infiltrates were seen in the dermis of the pinnae (Fig. 5). These histopathological changes were absent from the tissues of the control animals. Immunohistochemical staining revealed sparse SFGR in the infiltrates around the testes of each infected animal, as well as the interstitial infiltrates in a testis of one animal and in an epididymis of another animal. SFGR were also evident by immunohistochemical staining in multiple foci in the dermal infiltrates of the pinnae of two infected animals (Fig. 5). No SFGR were identified by immunohistochemical staining in the lungs, spleen, liver, kidneys, and heart tissues of any infected animal or in any of the tissues of the control guinea pigs. At the time of necropsy, the spleens of infected animals were enlarged by approximately 25% in comparison to those of the control animals, with the mean spleen/body weight percentages being 0.16 and 0.12% for infected and noninfected guinea pigs, respectively.
FIG 5.
(Left) Histological appearance of guinea pig pinna 14 days following intraperitoneal inoculation with Rickettsia sp. Black Gap, demonstrating predominantly mononuclear inflammatory infiltrates in the superficial dermis (hematoxylin and eosin stain). Magnification, ×50. (Right) Immunohistochemical staining of Rickettsia sp. Black Gap antigens within mononuclear cells in the same inflammatory focus (immunoalkaline phosphatase with naphthol fast-red and hematoxylin counterstain). Magnification, ×100.
Serology.
No antibodies reactive with any of the five rickettsial antigens were detected in the serum samples from the infected guinea pigs at day 0 or 7 postinoculation. By day 14 postinoculation, each of the guinea pig serum samples reacted with each of the five antigens at a reciprocal titer of ≥64. The geometric mean titers of antibodies to antigens of R. africae, R. sibirica, R. parkeri, Rickettsia sp. Atlantic rainforest Aa46, and Rickettsia sp. Black Gap were 108 (range, 64 to 128), 108 (range, 64 to 256), 181 (range, 128 to 512), 362 (range, 128 to 1,024), and 512 (256 to 1,024), respectively. Neither of the control guinea pigs demonstrated antibodies reactive with any of the rickettsial antigens at any time point.
DISCUSSION
In the course of these investigations, we identified and characterized a unique SFGR associated with D. parumapertus ticks that we suggest represents the organism designated the parumapertus agent by rickettsiologists more than 60 years ago. Furthermore, this bacterium shares the closest genetic identity with an SFGR responsible for a relatively mild rickettsiosis in Brazil, previously referred to as Rickettsia sp. Atlantic rainforest (12–15). The absence of an extant isolate of the parumapertus agent precludes genetic comparison with Rickettsia sp. Black Gap; nonetheless, multiple lines of evidence support the likely identity between these SFGR, including a direct association with D. parumapertus ticks, the similar clinical responses of guinea pigs following intraperitoneal inoculation, and similarities between historical and contemporary serological data determined from animal infection studies.
Only one molecular algorithm to validate a rickettsial species using partial or complete sequences of various rickettsial genes has been proposed (16). By this method, isolates are not considered to represent a novel species if two or more of the rrs, gltA, sca0, sca5, and sca4 gene sequences have identities equal to or greater than 99.8, 99.9, 98.8, 99.2, and 99.3%, respectively, to the sequences of the genes from another validated rickettsial species. By these criteria, neither Rickettsia sp. Black Gap nor Rickettsia sp. Atlantic rainforest Aa46 represents a novel species. Rickettsia sp. Black Gap is 99.8% identical to R. parkeri, 99.9% identical to R. sibirica, and 99.3% identical to R. africae for the rrs, gltA, and sca0 genes, respectively. Rickettsia sp. Atlantic rainforest Aa46 is 99.8% identical to R. parkeri, 99.9% identical to R. sibirica, 99.7% identical to R. africae, and 99.3% identical to R. africae for the rrs, gltA, sca0, and sca5 genes, respectively. Phylogenetic analysis using the concatenated sequences of these genes places Rickettsia sp. Black Gap and Rickettsia sp. Atlantic rainforest Aa46 with R. parkeri in a well-supported clade that is distinct from other SFGR species.
Philip and coworkers found that mice infected with the parumapertus agent produced antibodies with a reciprocal titer to R. parkeri that was 25% of the titer of antibodies to the homologous antigen (11). In the present study, IgG antibodies of guinea pigs convalescing from infection from Rickettsia sp. Black Gap reacted with antigens of R. africae, R. sibirica, R. parkeri, and Rickettsia sp. Atlantic rainforest Aa46 at geometric mean titers that were approximately 20, 20, 35, and 70%, respectively, of the geometric mean titer of antibodies to the homologous antigen. The composite serological and molecular data from this investigation indicate that Rickettsia sp. Black Gap is nearly identical to Rickettsia sp. Atlantic rainforest Aa46 and represents a unique strain of R. parkeri. Many other rickettsial taxa closely related to R. parkeri and associated predominantly or exclusively with Amblyomma species ticks have been identified during the past decade. These include other rickettsial strains identical or nearly identical to Rickettsia sp. Atlantic rainforest Aa46 (17–20), as well as several other SFGR of undetermined pathogenicity (21–26).
The response of male guinea pigs to intraperitoneal inoculation with R. parkeri Black Gap closely resembles the clinical characteristics elicited by the parumapertus agent, as described by investigators during the 1950s (6, 8). Interestingly, the characteristics of this nonlethal infection, manifested in most animals by a mild febrile period lasting a few days and followed by mild to moderate swelling and erythema of the scrotum for several days after the temperature has returned to normal, also resemble the characteristics described after intraperitoneal inoculation of guinea pigs with R. parkeri (27). In this study, infected animals also developed splenomegaly and prominent lymphohistiocytic periorchitis that occasionally progressed to orchitis and epididymitis. Evidence of the systemic dissemination of R. parkeri Black Gap was identified by the detection of multifocal aggregates of SFGR in mononuclear inflammatory cells in the pinnae of most infected guinea pigs. Surprisingly, rickettsiae persisted in this location for at least 14 days following intraperitoneal inoculation of the organism. Investigators previously noted the presence of rickettsial DNA in the pinnae of rodents collected in the field (28) and in guinea pigs experimentally infected by tick bite or intraperitoneal inoculation with R. parkeri, R. rickettsii, or Rickettsia slovaca (29). The observation of rickettsial aggregates in the dermis of the pinnae also complements the propensity of SFGR to multiply preferentially at temperatures slightly below normal body temperatures (30), such as those observed in the skin of rabbit pinnae (31). As noted in this study, the pinnae are also preferred sites of attachment by D. parumapertus ticks. Collectively, these results suggest that pinnae could represent an important and physiologically relevant site for the acquisition of SFGR by ticks and could provide a convenient source of samples that could be collected by nonlethal means from wildlife species for surveys of active infection with SFGR.
We initially focused out collection efforts in Tooele County in western Utah on the basis of historical records documenting the frequent occurrence of SFGR in specimens of D. parumapertus ticks collected from this region. Studies of black-tailed jackrabbits collected in western Utah from 1954 to 1957 identified serological reactivity to SFGR in serum samples from 193 (25%) of 773 jackrabbits collected on or near Dugway Proving Ground. Forty-six (14.7%) of 312 Ord's kangaroo rats (Dipodomys ordii) and 10 (9.6%) of 104 white-tailed antelope ground squirrels (Ammospermophilus [Citellus] leucurus) collected from the same location had antibodies reactive with SFGR. From the same investigation, SFGR were detected in 97 (24%) of 398 pooled specimens of 19,717 adult D. parumapertus ticks collected from black-tailed jackrabbits (8). In another study, serum samples from 21 to 40% of black-tailed jackrabbits from collection sites in the Great Salt Lake Desert in Utah from 1964 to 1973 had antibodies reactive with SFGR species (32). Longitudinal studies conducted in the same region from 1967 to 1971 identified serological reactivity to SFGR in 13 to 31% of 5,828 black-tailed jackrabbits, and the species D. parumapertus accounted for 2,657 (94%) of 2,834 ticks collected from 605 black-tailed jackrabbits. The causative agent was not isolated in culture, and investigators speculated that serological reactivity was most likely attributable to an SFGR other than a virulent strain of R. rickettsii (33).
We found only one specimen of D. parumapertus infected with an SFGR closely related to R. parkeri Black Gap among 165 adult ticks collected from 26 black-tailed jackrabbits encountered from multiple locations in Tooele County from 2011 to 2016. Surprisingly, we identified infections with R. bellii in 77% of adult D. parumapertus ticks collected in the same region of western Utah. These data suggest a shift in the predominant rickettsial species within D. parumapertus ticks following the last period of evaluation in this region during the 1970s. Similarly, we identified R. bellii in 89% of 62 D. parumapertus tick specimens from Arizona, where only 2 ticks contained the DNA of R. parkeri. Several laboratory investigations have shown that ticks cannot simultaneously maintain separate species of Rickettsia by vertical transmission, as demonstrated by the exclusion of transovarial transmission of R. rickettsii by Rickettsia peacockii in Dermacentor andersoni ticks (34), R. rhipicephali by Rickettsia montanensis in Dermacentor variabilis ticks (35), and R. rickettsii by R. bellii in Amblyomma dubitatum ticks (36). This process, known as rickettsial interference, can alter the composition of the predominant SFGR species in tick populations and is likely a dynamic occurrence. The decreased prevalence of R. rickettsii and a simultaneous predominance of R. bellii were noted in D. andersoni ticks from the western side of the Bitterroot Valley of Montana during the latter part of the 20th century (37, 38). In this context, the high rate of infection with R. bellii observed in the Utah and Arizona specimens of D. parumapertus ticks may account for the relative absence of R. parkeri in these populations of ticks. Interestingly, coinfections of ticks with R. bellii and R. rhipicephali were noted in approximately 24% of the Utah D. parumapertus tick specimens. Culture and molecularly confirmed coinfections of Dermacentor occidentalis ticks with these agents have been described previously, albeit rarely (9, 39), and culture-confirmed coinfection of Amblyomma ovale ticks with R. bellii and Rickettsia sp. Atlantic rainforest has been reported in Brazil (17).
The frequency and distribution of R. parkeri Black Gap in the western United States are unknown. In the present study, the prevalence of R. parkeri Black Gap infections in D. parumapertus ticks collected over several years at a single location in southwestern Texas ranged from 0 to 18%, although more extensive evaluations are needed to better determine the infection prevalence across the range of D. parumapertus ticks. From one study of D. parumapertus ticks collected in west Texas, SFGR were detected in 4 (36.3%) of 11 adult ticks by using a nonspecific hemolymph test (40). The role of black-tailed jackrabbits in the distribution and maintenance of R. parkeri Black Gap is similarly unknown, although antibodies reactive with antigens of SFGR have been detected in approximately 5 to 40% of black-tailed jackrabbits sampled in California, Kansas, Texas, and Utah (40–43).
Dermacentor parumapertus ticks are distributed broadly across arid and semiarid regions of the western United States, and they extend also into several northern and central states of Mexico (44, 45). Adult D. parumapertus ticks predominantly parasitize rabbits and hares, especially the black-tailed jackrabbit, although these ticks occasionally attach to larger mammals, including coyotes (Canis latrans), cattle (Bos taurus), and humans (46–48). Immature D. parumapertus ticks have a more eclectic host range that includes at least 16 species of small mammals, although Ord's kangaroo rats are the host most commonly parasitized by larvae and nymphs (49–54).
In 1908, Maxey (55) noted that ∼40% of the cases of RMSF in Idaho occurred among sheepherders in the sagebrush plains, valleys, and foothills in the southern third of the state, a region that approximates almost identically the distribution of black-tailed jackrabbits in Idaho (56). From 1909 to 1914, approximately 200 to 400 cases of RMSF were reported each year from Idaho. The number of annual cases plummeted to fewer than 50 by 1930, attributed predominantly to changing agricultural practices in the Snake River Valley that included extensive replacement of sagebrush environments by cultivated lands and aggressive control campaigns that specifically targeted and markedly reduced the ground squirrel and jackrabbit populations in this region (57). While there are no confirmed cases of disease in humans attributable to infection with R. parkeri Black Gap, some published and anecdotal data document a disease in the western United States similar to R. parkeri rickettsiosis. One early report of a mild case of RMSF in a California patient described an illness that occurred approximately 1 week after the individual was exposed to the bite of an unknown arthropod while cutting sagebrush in northern Nevada (58). The illness started with the formation of a small pustule at the bite site and was followed a few days later by a fever consisting of a temperature that rose to 103°F, intense headache, myalgia, arthralgia, nausea, and a generalized petechial and maculopapular rash. Blood from the patient was inoculated into male guinea pigs, and the animals developed scrotal swelling but only a slight increase in temperature (58). More recently, cases of a rickettsial disease characterized by multiple eschars, fever, rash, headache, arthralgia, and myalgia have been identified sporadically in a few patients following travel to mountainous regions of Colorado and New Mexico (CDC, unpublished data).
The identification of R. parkeri Black Gap allows one possible explanation for the dramatically different CFRs of RMSF observed in the western United States during the early 20th century. From 1911 to 1914, Nevada reported 21 cases of RMSF with no deaths (59), and in southern Idaho from 1926 to 1927, there were 70 cases of spotted fever with only three deaths, for a CFR of 4.3%. By comparison, 41 (74.5%) of 55 cases of RMSF reported in western Montana from 1915 to 1924 ended in death (60). More than 50 cases of R. parkeri rickettsiosis have been documented in the United States, Argentina, and Uruguay since the initial identification of its role as a pathogen of humans in 2004 (3, 61–64). Since the discovery in 2010 of a rickettsial disease caused by an SFGR identical or very similar to Rickettsia sp. Atlantic rainforest Aa46, there have been four confirmed cases (12–15), and hundreds more are suspected (15, 20, 65) in several states in Brazil. Although U.S. and South American strains of R. parkeri produce infections that are clinically similar to and frequently misdiagnosed as RMSF, there are no known deaths attributed to infection with R. parkeri. In this context, the remarkable disparity of CFRs among regions of the American West during the early 20th century could have been caused, at least in part, by the inclusion of cases of disease caused by R. parkeri Black Gap that produced a nonlethal infection clinically similar to RMSF.
MATERIALS AND METHODS
Tick collection and processing.
Adult D. parumapertus ticks were collected from black-tailed jackrabbits shot or encountered as roadkills from multiple locations in Tooele County, UT, from 2011 to 2016, the Black Gap Wildlife Management Area in Brewster County, TX, from 2013 to 2015, and Santa Cruz and Pima Counties, AZ, during 2016. Shooting of black-tailed jackrabbits was performed by experienced hunters and wildlife biologists. These procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the Centers for Disease Control and Prevention (CDC; 2532PADRABX and 2824PADRABX). Live ticks were removed from the jackrabbit carcasses by forceps and identified to the species level by using a standard morphological key for Dermacentor species of the western United States (66). The ticks were placed directly into 70% ethanol or stored as live specimens for subsequent analyses. Voucher specimens from collection localities were deposited in the Mississippi Entomological Museum, Mississippi State University, Starkville, MS (voucher numbers UT13-71011, 80-2t, AZ16-711X1, and AZ16-711X2). Live specimens were transported to CDC, where these were washed sequentially for 5 min each in 5% MicroChem Plus, 1.7% sodium hypochlorite, and 3% hydrogen peroxide and received a final rinse in sterile distilled water. Individual ticks were transferred to a sterile petri dish and bisected longitudinally using a sterile scalpel blade. One half was placed in 0.2 ml of sterile-filtered Eagle's minimum essential medium with Earle's salts and 5% fetal bovine serum and then frozen at −80°C, and the other half was processed for DNA extraction.
Molecular evaluation of ticks.
Genomic DNA was extracted from frozen or ethanol-preserved tick specimens by using a DNA minikit (Qiagen, Valencia, CA) and eluted in a final volume of 100 μl. Four microliters from each extract was used as the template in each of two real-time assays designed to amplify segments of the gltA gene of SFGR species (67, 68) and R. bellii (17, 69), as well as a heminested PCR assay targeting a 532-bp segment of the ompA gene (70, 71) (Table 4). Amplicons obtained from the ompA assay were sequenced in both directions by using a BigDye Terminator (v3.1) kit and an ABI 3130xl genetic analyzer (Applied Biosystems, Carlsbad, CA). The sequences were assembled using the Sequencher (v5.1) program (Gene Codes, Ann Arbor, MI), aligned using the MEGA (v6.05) program (72), and compared with existing sequences in GenBank using BLAST analysis.
TABLE 4.
Loci, primers, and probes used in molecular assays in this study
| Locus | PCR assay | Primer or probe sequencea (5′ → 3′) | Amplicon size (bp) | Reference |
|---|---|---|---|---|
| gltA | Real-time | TCG CAA ATG TTC ACG GTA CTT T | 74 | 67 |
| TCG TGC ATT TCT TTC CAT TGT G | 67 | |||
| FAM-TGC AAT AGC AAG AAC CGT AGG CTG GAT G-BHQ1 | 68 | |||
| gltA | Real-time | ATC CTG ATT TGC TGA ATT TTT T | 338 | 17 |
| TGC AAT ACC AGT ACT GAC G | 17 | |||
| FAM-ATG ATG TTT GCC ACA CCT TGT GAA AA-BHQ1 | 69 | |||
| sca0 | Conventional | ATG GCG AAT ATT TCT CCA AAA | 590 | 70 |
| GTT CCG TTA ATG GCA GCA TCT | 71 | |||
| Heminested | AGT GCA GCA TTC GCT CCC CCT | 532 | 70 | |
| rrs | Conventional | AGA GTT TGA TCC TGG CTC AG | 1,462 | 75 |
| ACG GCT ACC TTG TTA CGA CTT | 75 | |||
| Conventional | CGA AAG CCT GAT CCA GCA AT | 812 | 75 | |
| TAA GGG CCA TGA TGA CTT GA | 75 | |||
| gltA | Conventional | ATG ACT AAT GGC AAT AAT AA | 1,047 | 76 |
| CAT AAC CAG TGT AAA GCT G | 76 | |||
| sca5 | Conventional | CCG CAG GGT TGG TAA CTG C | 2,929 | 77 |
| CCG GCT ATA CCG CCT GTA GT | 77 | |||
| Conventional | AAA CAA TAA TCA AGG TAC TGT | 2,091 | 77 | |
| TTA GAA GTT TAC ACG GAC TTT T | 77 | |||
| sca4 | Conventional | ATG AGT AAA GAC GGT AAC CT | 1,876 | 78 |
| TAG TTT GTT CTG CCA TAA TC | 78 | |||
| Conventional | CGA TGG TAG CAT TAA AAG CT | 2,302 | 78 | |
| TCA GCG TTG TGG AGG GGA AG | 78 |
FAM, 6-carboxyfluorescein; BHQ1, black hole quencher 1.
Cell culture isolation.
Individual tick halves that matched the corresponding PCR-positive segments were thawed, transferred to a sterile petri dish, and minced with a sterile scalpel blade. The inoculum was subsequently placed onto a semiconfluent monolayer of Vero E6 cells in a T25 tissue culture flask containing 5 ml Eagle's minimum essential medium with Earle's salts, 500 mmol l-glutamine, and 10% heat-inactivated fetal bovine serum with 10 units/ml penicillin, 10 μg/ml streptomycin sulfate, and 0.25 μg/ml amphotericin B. The cultures were incubated at 32°C in a humidified 5% CO2-in-air atmosphere. The medium was replaced after 24 h with 5 ml of fresh medium containing the same antibiotics indicated above and again 24 h later with medium without antibiotics. The cell culture medium was thereafter replaced approximately every 5 days. Cultures were monitored for evidence of infection by examining cytospin preparations of cells fixed in methanol and stained with a 0.1-g/liter solution of acridine orange (Becton, Dickinson and Company, Sparks, MD).
Molecular characterization of rickettsial isolates.
The complete sequences of the 16S rRNA (rrs), gltA, ompA (sca0), ompB (sca5), and gene D (sca4) genes were amplified from DNA extracted from the cell culture isolate, as well as from an isolate of Rickettsia sp. Atlantic rainforest Aa46 (18), kindly provided by Marcelo Labruna, using the primers described in Table 4 and the thermocycler conditions described previously (16), with the exception of the gltA PCR, which was modified to use an annealing temperature of 55°C. The PCR amplicons were sequenced, assembled, and aligned as described above. Nucleotide sequences were concatenated in the following order: rrs, gltA, sca0, sca5, and sca4. Homologous sequences from validated SFGR species and strains were obtained from NCBI (www.ncbi.nlm.nih.gov/). Gaps were removed using the simple indel coding method (73), and the MEGA (v6.05) program was utilized to infer the evolutionary history by the maximum likelihood method with the subtree-pruning-regrafting algorithm. A total of 10,466 nucleotides were included in the final data set, and bootstrap values were calculated for 1,000 replicates.
Infection of guinea pigs.
Isolates from the fourth passage of R. parkeri Black Gap were cultivated in Vero E6 cells and pelleted by centrifugation at 1,455 × g for 20 min. The supernatant was discarded, and the cells were resuspended in sterile Snyder I medium. The inoculum was quantified as the rickettsial copy number per milliliter by using serial dilutions of a quantified plasmid to calculate a standard curve for a real-time PCR assay targeting the 23S rRNA gene of Rickettsia species (74). In two separate experiments, 2- to 3-month-old male Hartley guinea pigs (Charles River Laboratories, Wilmington, MA) were injected intraperitoneally with resuspended rickettsiae and monitored for 8 or 14 days. Noninfected Vero E6 cells were processed in an identical manner, and a volume of the cell suspension equal to the infectious inoculum was injected intraperitoneally into male guinea pigs of the same age. Animals were provided food and water ad libitum and housed in a biosafety level 3 containment room maintained at 20 to 21°C and 30 to 50% relative humidity with a 12-h light and a 12-h dark photoperiod. Rectal temperatures and scrotal appearance were monitored daily. A rectal temperature of ≥39.7°C was considered a febrile response. Animals were euthanized, using CO2 gas, at 8 or 14 days postinfection. All procedures were approved by the Institutional Animal Care and Use Committee at CDC (LEVGUEC2555).
Histopathological and immunohistochemical evaluation of tissues.
Representative segments of the lung, heart, liver, spleen, kidney, testes, and pinnae were placed in 10% neutral buffered formalin. Fixed tissues were embedded in paraffin, and sections were cut at 4 μm. Sections were stained by hematoxylin and eosin and by an immunoalkaline phosphatase technique using a hyperimmune polyclonal rabbit antiserum sample diluted 1/500. This antibody cross-reacts with multiple species of SFGR in formalin-fixed tissues (68).
Serology.
In the second guinea pig experiment, serum samples were collected from each animal 1 day prior to inoculation and at 7 and 14 days postinoculation. All samples were tested for antibodies reactive with antigens of R. parkeri Black Gap as well as those of R. parkeri Maculatum 20T, Rickettsia sp. Atlantic rainforest Aa46, R. sibirica 246T, and Rickettsia africae Z9-HuT by using an indirect immunofluorescence antibody technique. Antigens were prepared from rickettsiae cultivated in L929 cells and pelleted by centrifugation at 1,455 × g for 20 min. The supernatant was discarded, and the cells were resuspended in phosphate-buffered saline at pH 7.38 containing 1% bovine serum albumin and 0.01% thimerosal. Infected cells were irradiated with 1 ×106 rads and stored at −70°C until used. Thawed cell suspensions were dotted onto glass slides, air dried, desiccated for 2 h, and fixed in acetone before storage at −70°C. Serum samples were preabsorbed with noninfected L929 cells for 45 min at room temperature. Samples were screened at an initial dilution of 1/16 and serially diluted to the endpoint. A fluorescein isothiocyanate-labeled anti-guinea pig conjugate reactive with the heavy and light chains of immunoglobulin G (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) was used at 1/100. Serum from guinea pigs immunized with R. rickettsii and Coxiella burnetii served as positive and negative controls, respectively.
Accession number(s).
The GenBank accession numbers assigned to the sequences determined in this study include KY124256 for rrs of R. parkeri Black Gap, KY124257 for gltA of R. parkeri Black Gap, KY124258 for sca0 of R. parkeri Black Gap, KY124259 for sca5 of R. parkeri Black Gap, KY124260 for sca4 of R. parkeri Black Gap, KY113108 for rrs of Rickettsia sp. Atlantic rainforest Aa46, KY113109 for gltA of Rickettsia sp. Atlantic rainforest Aa46, KY113110 for sca0 of Rickettsia sp. Atlantic rainforest Aa46, KY113111 for sca5 of Rickettsia sp. Atlantic rainforest Aa46, KY113112 for sca4 of Rickettsia sp. Atlantic rainforest Aa46, and KY271186 for ompA of Rickettsia parkeri UT16-13.
ACKNOWLEDGMENTS
We thank Anthony Sanchez (CDC) for guidance in developing the jackrabbit protocols, James Gathany (CDC) for the images of D. parumapertus ticks, Rosella Goddard for assistance with collecting black-tailed jackrabbits from Utah, Lauren Schumacher (CDC) for assistance with the guinea pig studies, Kim Slater (CDC) for assistance with antigen irradiation and the maintenance of Vero E6 cells, and Marcelo Labruna (Universidade de São Paulo) for providing the isolate of Rickettsia sp. Atlantic rainforest Aa46 used in this study.
The findings and conclusions are those of the authors and do not necessarily reflect the views of the U.S. Department of Health and Human Services or the U.S. Army.
REFERENCES
- 1.Maxey EE. 1899. Some observations of the so-called “spotted fever” of Idaho. Med Sentinel 7:433–438. [Google Scholar]
- 2.Anonymous. 1903. Spotted fever in Oregon. Med Sentinel 11:389–390. [Google Scholar]
- 3.Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA, McLellan SL, Goddard J, Holman RC, Openshaw JJ, Sumner JW, Zaki SR, Eremeeva ME. 2008. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis 47:1188–1196. doi: 10.1086/592254. [DOI] [PubMed] [Google Scholar]
- 4.Padgett KA, Bonilla D, Eremeeva ME, Glaser C, Lane RS, Porse CC, Castro MB, Messenger S, Espinosa A, Hacker J, Kjemtrup A, Ryan B, Scott JJ, Hu R, Yoshimizu MH, Dasch GA, Kramer V. 2016. The eco-epidemiology of Pacific Coast fever in California. PLoS Negl Trop Dis 10:e0005020. doi: 10.1371/journal.pntd.0005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paddock CD. 2009. The science and fiction of emerging rickettsioses. Ann N Y Acad Sci 1166:133–143. doi: 10.1111/j.1749-6632.2009.04529.x. [DOI] [PubMed] [Google Scholar]
- 6.Philip CB, Hughes LE. 1953. Disease agents found in the rabbit tick, Dermacentor parumapertus, in the southwestern United States. Atti VI Congr Int Microbiol 5:541–548. [Google Scholar]
- 7.Philip CB, Bell JF, Larson CL. 1955. Evidence of infectious diseases and parasites in a peak population of black-tailed jack rabbits in Nevada. J Wildl Management 19:225–233. doi: 10.2307/3796856. [DOI] [Google Scholar]
- 8.Stoenner HG, Holdenried R, Lackman D, Osborn JS Jr. 1959. The occurrence of Coxiella burnetii, Brucella, and other pathogens among fauna of the Great Salt Lake Desert in Utah. Am J Trop Med Hyg 8:590–596. [DOI] [PubMed] [Google Scholar]
- 9.Lane RS, Philip RN, Capser EA. 1981. Ecology of tick-borne agents in California. II. Further observations on rickettsiae, p 575–583. In Burgdorfer W, Anacker RL (ed), Rickettsiae and rickettsial diseases. Academic Press, Inc, New York, NY. [Google Scholar]
- 10.Philip RN, Casper EA, Burgdorfer W, Gerloff RK, Hughes LE, Bell EJ. 1978. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol 121:1961–1968. [PubMed] [Google Scholar]
- 11.Philip RN, Casper EA, Burgdorfer W. 1978. Current knowledge of the distribution of serotypes of spotted fever-group rickettsiae in the United States as determined by microimmunofluorescence. VII Int Cong Infect Parasit Dis 1:500–509. [Google Scholar]
- 12.Spolidorio MG, Labruna MB, Mantovani E, Brandão PE, Richtzenhain LJ, Yoshinari NH. 2010. Novel spotted fever group rickettsiosis, Brazil. Emerg Infect Dis 16:521–523. doi: 10.3201/eid1603.091338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva N, Eremeeva ME, Rozental T, Ribeiro GS, Paddock CD, Ramos EA, Favacho AR, Reis MG, Dasch GA, de Lemos ER, Ko AI. 2011. Eschar-associated spotted fever rickettsiosis, Bahia, Brazil. Emerg Infect Dis 17:275–278. doi: 10.3201/eid1702.100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonoldi VLN, Marangoni RG, Gauditano G, Moraes-Filho J, Labruna MB, Yoshinari NH. 2014. First report of mild Brazilian spotted fever associated to arthritis. Rev Bras Reumatol 54:237–240. doi: 10.1016/j.rbr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Krawczak FS, Muñoz-Leal S, Guztzazky AC, Oliveira SV, Santos FCP, Angerami RN, Moraes-Filho J, de Souza JC Jr, Labruna MB. 2016. Rickettsia sp. strain Atlantic rainforest infection in a patient from a spotted fever-endemic area in southern Brazil. Am J Trop Med Hyg 95:551–553. doi: 10.4269/ajtmh.16-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fournier PE, Dumler JS, Geub G, Zhang J, Wu Y, Raoult D. 2003. Gene sequence-based criteria for identification of new Rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol 41:5456–5465. doi: 10.1128/JCM.41.12.5456-5465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabó MPJ, Nieri-Bastos FA, Spolidorio MG, Martins TF, Barbieri AM, Labruna MB. 2013. In vitro isolation from Amblyomma ovale (Acari: Ixodidae) and ecological aspects of the Atlantic rainforest Rickettsia, the causative agent of a novel spotted fever rickettsiosis in Brazil. Parasitology 140:719–728. doi: 10.1017/S0031182012002065. [DOI] [PubMed] [Google Scholar]
- 18.Barbieri ARM, Filho JM, Nieri-Bastos FA, Souza JC Jr, Szabó MPJ, Labruna MB. 2014. Epidemiology of Rickettsia sp. strain Atlantic rainforest in a spotted fever-endemic area of southern Brazil. Ticks Tick Borne Dis 5:848–853. doi: 10.1016/j.ttbdis.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Figueiredo Voizzoni V, Silva AB, Cardoso KM, Santos FB, Stennzel B, Amorim M, Vilges de Oliveria S, Gazêta GS. 2016. Genetic identification of Rickettsia sp. strain Atlantic rainforest in an endemic area of mild spotted fever in Rio Grande do Sul State, southern Brazil. Acta Trop 162:142–145. doi: 10.1016/j.actatropica.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Moerbeck L, Vizzoni VF, Machado-Ferreira E, Cavalcante RC, Oliveira SV, Soares CAG, Amorim M, Gazêta GS. 2016. Rickettsia (Rickettsiales: Rickettsiaceae) vector biodiversity in high altitude Atlantic forest fragments within a semiarid climate: a new endemic area of spotted-fever in Brazil. J Med Entomol 53:1458–1466. doi: 10.1093/jme/tjw121. [DOI] [PubMed] [Google Scholar]
- 21.Ogrzewalska M, Pacheco RC, Uezu A, Richtzenhain LJ, Ferreira F, Labruna MB. 2009. Rickettsial infection in Amblyomma nodosum ticks (Acari: Ixodidae) from Brazil. Ann Trop Med Parasitol 103:413–425. doi: 10.1179/136485909X451744. [DOI] [PubMed] [Google Scholar]
- 22.Ogrzewalska M, Martins T, Capek M, Literak I, Labruna MB. 2013. A Rickettsia parkeri-like agent infecting Amblyomma calcaratum nymphs from wild birds in Mato Grosso do Sul, Brazil. Ticks Tick Borne Dis 4:145–147. doi: 10.1016/j.ttbdis.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Ogrzewalska M, Nieri-Bastos FA, Marcili A, Nava S, González-Acuña D, Muñoz-Leal S, Ruiz-Arrondo I, Venzal JM, Mangold A, Labruna MB. 2016. A novel spotted fever group Rickettsia infecting Amblyomma parvitarsum (Acari: Ixodidae) in highlands of Argentina and Chile. Ticks Tick Borne Dis 7:439–442. doi: 10.1016/j.ttbdis.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Pacheco RC, Arzua M, Nieri-Bastos FA, Moraes-Filho J, Marcili A, Richtzenhain LJ, Barros-Battesti DM, Labruna MB. 2012. Rickettsial infection in ticks collected on birds in southern Brazil. J Med Entomol 49:710–716. doi: 10.1603/ME11217. [DOI] [PubMed] [Google Scholar]
- 25.Matias J, Garcia MV, Cunha RC, Aguirre AAR, Barros JC, Csordas BG, Andreotti R. 2015. Spotted fever group Rickettsia in Amblyomma dubitatum tick from the urban area of Campo Grande, Mato Grosso do Sul, Brazil. Ticks Tick Borne Dis 6:107–110. doi: 10.1016/j.ttbdis.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Zemtsova GE, Gleim E, Yabsley MJ, Conner LM, Mann T, Brown MD, Wendland L, Levin ML. 2012. Detection of a novel spotted fever group Rickettsia in the gophertortoise tick. J Med Entomol 49:783–786. doi: 10.1603/ME11264. [DOI] [PubMed] [Google Scholar]
- 27.Parker RR, Kohls GM, Cox GW, Davis GE. 1939. Observations on an infectious agent from Amblyomma maculatum. Public Health Rep 54:1482–1484. doi: 10.2307/4582985. [DOI] [Google Scholar]
- 28.Schex S, Dobler G, Riehm J, Müller J, Essbauer S. 2011. Rickettsia spp. in wild small mammals in lower Bavaria, south-eastern Germany. Vector Borne Zoonotic Dis 5:493–502. doi: 10.1089/vbz.2010.0060. [DOI] [PubMed] [Google Scholar]
- 29.Levin ML, Snellgrove AN, Zemstova GE. 2016. Comparative value of blood and skin samples for diagnosis of spotted fever group rickettsial infection in model animals. Ticks Tick Borne Dis 7:1029–1034. doi: 10.1016/j.ttbdis.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoenner HG, Lackman DB, Bell EJ. 1962. Factors affecting the growth of rickettsias of the spotted fever group in fertile hens' eggs. J Infect Dis 110:121–128. doi: 10.1093/infdis/110.2.121. [DOI] [PubMed] [Google Scholar]
- 31.Honda N, Carlson LD, Judy WV. 1963. Skin temperature and blood flow in the rabbit ear. Am J Physiol 204:615–618. [DOI] [PubMed] [Google Scholar]
- 32.Eberhardt LE, Van Vorris P. 1986. Historical wildlife dynamics on Dugway Proving Ground: population and disease trends in jack rabbits over two decades, p 1–56. Pacific Northwest Laboratory, Richland, WA. [Google Scholar]
- 33.Anonymous. 1971. Ecology studies in Western Utah, series 72-1, p 1–102. EcoDynamics Inc, Salt Lake City, UT. [Google Scholar]
- 34.Burgdorfer W, Hayes SF, Mavros AJ. 1981. Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsii, p 585–594. In Burgdorfer W, Anacker RL (ed), Rickettsiae and rickettsial diseases. Academic Press, Inc, New York, NY. [Google Scholar]
- 35.Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF. 2002. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J Med Entomol 39:809–813. doi: 10.1603/0022-2585-39.6.809. [DOI] [PubMed] [Google Scholar]
- 36.Sakai RK, Costa FB, Ueno TE, Ramirez DG, Soares JF, Fonseca AH, Labruna MB, Barros-Battesti DM. 2014. Experimental infection with Rickettsia rickettsii in an Amblyomma dubitatum tick colony, naturally infected with Rickettsia bellii. Ticks Tick Borne Dis 5:917–923. doi: 10.1016/j.ttbdis.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Philip RN, Casper EA. 1981. Serotypes of spotted fever group rickettsiae isolated from Dermacentor andersoni ticks in western Montana. Am J Trop Med Hyg 30:230–238. [DOI] [PubMed] [Google Scholar]
- 38.Gage KL, Schrumpf ME, Karstens RH, Burgdorfer W, Schwan TG. 1994. DNA typing of rickettsiae in naturally infected ticks using a polymerase chain reaction/restriction fragment length polymorphism system. Am J Trop Med Hyg 50:247–260. [DOI] [PubMed] [Google Scholar]
- 39.Wikswo ME, Hu R, Dasch GA, Krueger L, Arugay A, Jones K, Hess B, Bennett S, Kramer V, Eremeeva ME. 2008. Detection and identification of spotted fever group rickettsiae in Dermacentor species from southern California. J Med Entomol 45:509–516. [DOI] [PubMed] [Google Scholar]
- 40.Henke SE, Pence DB, Demaris S, Johnson JR. 1990. Serologic survey of selected zoonotic disease agents in black-tailed jack rabbits from western Texas. J Wildl Dis 26:107–111. doi: 10.7589/0090-3558-26.1.107. [DOI] [PubMed] [Google Scholar]
- 41.Lechleitner RR. 1959. Some parasites and infectious diseases in a black-tailed jackrabbit population in the Sacramento Valley, California. Calif Fish Game 45:83–91. [Google Scholar]
- 42.Pagan EF, McMahon KJ, Bowen RE. 1961. Complement-fixing antibodies for R. rickettsii in serums of black-tailed jack rabbits. Public Health Rep 76:1120–1122. doi: 10.2307/4591389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lane RS, Emmons RW, Dondero DV, Nelson BC. 1981. Ecology of tick-borne agents in California. I. Spotted fever group rickettsiae. Am J Trop Med Hyg 30:239–252. [DOI] [PubMed] [Google Scholar]
- 44.Cooley RA. 1938. The genera Dermacentor and Otocentor (Ixodidae) in the United States. Nat Inst Bull Health 171:49–54. [Google Scholar]
- 45.Guzmán-Cornejo C, Robbins RG, Guglielmone AA, Montiel-Parra G, Rivas G, Pérez TM. 2016. The Dermacentor (Acari, Ixodida, Ixodidae) of Mexico: hosts, geographical distribution, and new records. Zookeys 569:1–22. doi: 10.3897/zookeys.569.7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roscoe EJ. 1956. A rabbit tick, Dermacentor parumapertus, attached to man. J Parasitol 42:527. doi: 10.2307/3274452. [DOI] [PubMed] [Google Scholar]
- 47.Strickland RK, Gerrish RR. 1965. Collections of Dermacentor parumapertus from cattle. J Parasitol 51:1000. doi: 10.2307/3275892. [DOI] [PubMed] [Google Scholar]
- 48.Johnson DE. 1966. Ticks of Dugway Proving Ground and vicinity and their host associations. Utah Acad Sci Arts Lett 43:49–66. [Google Scholar]
- 49.Pfaffenberger GS, Valencia VB. 1988. Ectoparasites of sympatric cottontails (Sylvilagus audobonii Nelson) and jack rabbits (Lepus californicus Mearns) from the high plains of eastern New Mexico. J Parasitol 74:842–846. doi: 10.2307/3282264. [DOI] [PubMed] [Google Scholar]
- 50.Gastfriend A. 1955. New host records for the immature stages of the tick Dermacentor parumapertus. J Parasitol 41:63–65. doi: 10.2307/3273999. [DOI] [PubMed] [Google Scholar]
- 51.Eads RB, Menzies GC, Hightower BG. 1956. The ticks of Texas, with notes on their medical significance. Texas J Sci 8:7–24. [Google Scholar]
- 52.Beck DE, Allred DM, Brinton EP. 1963. Ticks of the Nevada test site. Brigham Young Univ Sci Bull 4:1–10. [Google Scholar]
- 53.Beck DE. 1955. Distributional studies of parasitic arthropods in Utah, determined as actual and potential vectors of Rocky Mountain spotted fever and plague, with notes on host-vector relationships. Brigham Young Univ Sci Bull 1:38–64. [Google Scholar]
- 54.Rotramel GL, Schwan TG, Doty RE. 1976. Distribution of suspected tick vectors and reported cases of Rocky Mountain spotted fever in California. Am J Epidemiol 104:287–293. [PubMed] [Google Scholar]
- 55.Maxey EE. 1908. Rocky Mountain spotted [tick] fever; with special reference to casual factors, mortality and geographical distribution in Idaho. Med Sentinel 16:666–678. [Google Scholar]
- 56.Johnson DR, Peek JM. 1984. The black-tailed jackrabbit in Idaho. Life history, population dynamics and control. Univ Idaho Coop Ext Serv Bull 637:1–16. [Google Scholar]
- 57.Parker RR. 1935. Rocky Mountain spotted fever. Epidemiology with particular reference to distribution and prevalence in the western United States. Northwest Med 34:111–121. [Google Scholar]
- 58.Cumming JG. 1917. Rocky Mountain spotted fever in California. J Infect Dis 21:509–514. doi: 10.1093/infdis/21.5.509. [DOI] [Google Scholar]
- 59.Michie HC, Parson HH. 1916. Rocky Mountain spotted (tick) fever. Report of an investigation in the Bitter Root Valley of Montana. Med Rec 89:265–277. [Google Scholar]
- 60.Parker RR. 1939. Rocky Mountain spotted fever: results of fifteen years' prophylactic vaccination. Proc Sixth Pac Sci Congr Pac Sci Assoc 5:589–596. [Google Scholar]
- 61.Romer Y, Seijo A, Crudo F, Nicholson WL, Varela-Stokes A, Lash RR, Paddock CD. 2011. Rickettsia parkeri rickettsiosis in Argentina. Emerg Infect Dis 17:1169–1173. doi: 10.3201/eid1707.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romer Y, Nava S, Govedic F, Cicuttin G, Denison AM, Singleton J, Kelly AJ, Kato CY, Paddock CD. 2014. Rickettsia parkeri in different ecological regions of Argentina and its association with Amblyomma tigrinum as a potential vector. Am J Trop Med Hyg 91:1156–1160. doi: 10.4269/ajtmh.14-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Portillo A, García-García C, Sanz MM, Santibáñez S, Venzal JM, Oteo JA. 2013. Case report: a confirmed case of Rickettsia parkeri infection in a traveler from Uruguay. Am J Trop Med Hyg 89:1203–1205. doi: 10.4269/ajtmh.13-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Straily A, Feldpausch A, Ulbrich C, Schell K, Casillas S, Zaki SR, Denison AM, Condit M, Gabel J, Paddock CD. 2016. Rickettsia parkeri rickettsiosis— Georgia, 2012-2014. MMWR Morb Mortal Wkly Rep 65:718–719. doi: 10.15585/mmwr.mm6528a3. [DOI] [PubMed] [Google Scholar]
- 65.Angerami RN, da Silva AM, Nascimento EM, Colombo S, Wada MY, dos Santos FC, Mancini DM, de Oliveira RC, Katz G, Martins EC, da Silva LJ. 2009. Brazilian spotted fever: two faces of a same disease? A comparative study of clinical aspects between an old and a new endemic area in Brazil. Clin Microbiol 15(Suppl 2):207–208. doi: 10.1111/j.1469-0691.2008.02160.x. [DOI] [PubMed] [Google Scholar]
- 66.Brinton EP, Beck DE, Allred DM. 1965. Identification of the adults, nymphs, and larvae of ticks of the genus Dermacentor Koch (Ixodidae) in the western United States. Brigham Young Univ Sci Bull 5:1–44. [Google Scholar]
- 67.Stenos J, Graves SR, Unsworth NB. 2005. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group rickettsiae. Am J Trop Med Hyg 73:1083–1085. [PubMed] [Google Scholar]
- 68.Denison AM, Amin BD, Nicholson WL, Paddock CD. 2014. Detection of Rickettsia rickettsii, Rickettsia parkeri, and Rickettsia akari in skin biopsy specimens using a multiplex real-time polymerase chain reaction assay. Clin Infect Dis 59:635–642. doi: 10.1093/cid/ciu358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hecht JA, Allerdice MEJ, Krawczak FS, Labruna MB, Paddock CD, Karpathy SE. 2016. Development of a Rickettsia bellii-specific TaqMan assay targeting the citrate synthase gene. J Med Entomol 53:1492–1495. doi: 10.1093/jme/tjw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Regnery RL, Spruill CL, Plikaytis BD. 1991. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol 173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roux V, Fournier PE, Raoult D. 1996. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol 34:2058–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogden TH, Rosenberg MS. 2007. Alignment and topological accuracy of the direct optimization approach via POY and traditional phylogenetics via ClustalW + PAUP*. Syst Biol 56:182–193. doi: 10.1080/10635150701281102. [DOI] [PubMed] [Google Scholar]
- 74.Kato CY, Chung IH, Robinson LK, Austin AL, Dasch GA, Massung RF. 2013. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J Clin Microbiol 51:314–317. doi: 10.1128/JCM.01723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roux V, Rydkina E, Eremeeva M, Raoult D. 1997. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int J Syst Bacteriol 47:252–261. doi: 10.1099/00207713-47-2-252. [DOI] [PubMed] [Google Scholar]
- 77.Roux V, Raoult D. 2000. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int J Syst Evol Microbiol 50:1449–1455. doi: 10.1099/00207713-50-4-1449. [DOI] [PubMed] [Google Scholar]
- 78.Sekeyova Z, Roux V, Raoult D. 2001. Phylogeny of Rickettsia spp. inferred by comparing sequences of ‘gene D,’ which encodes an intracytoplasmic protein. Int J Syst Evol Microbiol 51:1353–1360. doi: 10.1099/00207713-51-4-1353. [DOI] [PubMed] [Google Scholar]