Abstract
Background
Prior studies show that African-American patients have a higher risk of stroke compared with Caucasians.
Objectives
This study hypothesized addition of African-American ethnicity to CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke, vascular disease, age 65 to 74, and female sex) score might improve stroke prediction in patients with atrial fibrillation (AF).
Methods
Medicare claims from January 2010 to December 2012 identified patients with newly diagnosed AF. The CHA2DS2-VASc was calculated on the basis of diagnoses in claims incurred during 12 months before first AF diagnosis. Ethnicity was identified from the Beneficiary Summary File. CHA2DS2-VASc-R score was calculated by giving 1 additional point for African-American ethnicity. The primary outcome was stroke, defined by primary diagnosis on acute inpatient admissions after the initial AF diagnosis. We used proportional hazards regression to determine the relationship between stroke and the CHA2DS2-VASc or a revised CHA2DS2-VASc-R score.
Results
Of 460,417 patients with AF, 390,590 (85%) were non-Hispanic whites, 31,702 (7%) were non-Hispanic African Americans, and the remainder were other non-white ethnicities. Mean age was 79.2 ± 8.0 years, with 60% females. Overall, 16,703 stroke events occurred, and 151,441 (32.7%) patients died during a mean follow-up period of 18.0 months. Compared with CHA2DS2-VASc, CHA2DS2-VASc-R score improved the fit of the model significantly as measured by the log likelihood ratio statistic (p < 0.001). Among individual risk factors in CHA2DS2-VASc-R score, only prior stroke, age ≥75 years, and female sex had a stronger association with incident stroke than African-American ethnicity.
Conclusions
In patients >65 years of age with newly diagnosed AF, the addition of ethnicity to CHA2DS2-VASc score significantly improved stroke prediction.
Keywords: atrial fibrillation, ethnicity, risk score, stroke
Atrial fibrillation (AF) is associated with an increased risk of stroke and systemic embolism (1). It accounts for 15% of all strokes in the United States, 36% of strokes in patients >80 years of age, and up to 20% of cryptogenic strokes (2). Ischemic stroke with AF is associated with >20% mortality and a significantly higher disability, particularly in older patients. Hence, prevention of stroke related to AF is a cornerstone in the management of AF patients.
In several studies, African Americans have a higher incidence of stroke compared with whites (3–7). This has been attributed to increased risk factors such as hypertension, diabetes mellitus, and lower socioeco-nomic status (SES) in this ethnic group. However, in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort, the traditional risk factors and SES accounted for only one-half of the excess stroke incidence in African Americans, whereas the other one-half was attributed to other unknown factors and pathways (8).
Similar racial differences in the incidence of stroke have also been reported in patients with AF, where patients of African ancestry consistently have higher risk of stroke compared with whites (9–11). Our prior work hasshownthatinpatients>65 years of age, AF in African Americans is associated with a higher incidence of stroke compared with whites, even after adjustment of risk factors and anticoagulation (12). This suggests that African-American ethnicity may be an independent risk factor for predicting stroke in AF patients.
Recent guidelines have endorsed the CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke, vascular disease, age 65 to 74, and female sex) score for identification of AF patients at risk of stroke in order to guide anti-coagulation management (13,14). In this study, we hypothesized that addition of African-American ethnicity to the CHA2DS2-VASc score would improve the prediction of stroke in AF patients. We used the Medicare database to test this hypothesis.
Methods
Study Population
We used Centers for Medicare & Medicaid (CMS) data for years 2009 through 2012, including: 1) Beneficiary Summary File Base and Chronic Conditions segments; 2) Inpatient (Part A) and Carrier (Part B) Standard Analytic Files; and 3) Pharmacy Drug Event (Part D). We included patients if they had a new AF diagnosis during the period January 2010 through December 31, 2011. We defined new AF based on previously published algorithms (i.e., 1 inpatient claim or 2 outpatient claims within a year with International Classification of Diseases–Ninth Revision–Clinical Modification [ICD-9-CM] code 427.31 as primary or first secondary diagnosis, with no previous AF diagnoses during the prior 12 months) (15,16). We excluded patients if they were younger than 66 years at the time of diagnosis (to ensure at least 12 months of Medicare eligibility before diagnosis), were enrolled in a Medicare managed care during the observation period, or were not enrolled in a Part D drug prescription plan at the time of AF diagnosis. We also excluded 13,991 patients whose AF diagnosis occurred during the same hospitalization as open heart surgery or within 30 days, as these may represent temporary post-surgical complications.
Our primary outcome was stroke, as identified on inpatient Standard Analytic File claims for years 2010 through 2012, and included acute hospitalizations admitted as emergent or urgent with a primary diagnosis of cerebral infarction (ICD-9-CM codes 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91). We determined race or ethnicity of the patients using the race code developed by the Research Triangle Institute (RTI) that is available on the CMS Beneficiary Summary File. The RTI race code is an enhanced race/ethnicity designation based on first and last name algorithms. The RTI race code has excellent agreement with self-reported race, with kappa coefficients $0.80 for Hispanic beneficiaries and ≥0.90 for African American beneficiaries (17).
The CHA2DS2-VASc is based on a point system reflecting the presence of congestive heart failure (1 point), hypertension (1 point), age ≥75 years (2 points), diabetes (1 point), previous stroke (2 points), vascular disease (1 point), age 65 to 74 (1 point), and female sex (1 point). The presence of heart failure, hypertension, diabetes, previous stroke, and vascular disease were identified by ICD-9-CM diagnoses in inpatient and outpatient claims during the 12 months before the first AF diagnosis (15,16). Age and sex were identified from Medicare enrollment data. We identified the date of first oral anticoagulant prescription fill after initial AF diagnosis in Part D event data in order to control for potential confounding due to differences in the use of oral anticoagulants by race/ethnicity. (Anticoagulants approved for stroke prevention in AF during the study time frame included warfarin, dabigatran, or rivaroxaban.) Additional patient characteristics were identified in the CMS enrollment and encounter data. Dual enrollment in Medicaid at the time of AF diagnosis was identified from the CMS Beneficiary Enrollment Summary. Other comorbid conditions were identified in inpatient and outpatient claims during the 12 months prior to the first AF diagnosis date and were defined using algorithms originally developed by Elixhauser et al. (18). Previous cerebrovascular events and prior bleeding episodes were identified using previously published algorithms (19,20).
Statistical Analysis
Data analysis encompassed bivariable and multivariable methods. First, characteristics of patients as of the date of first AF diagnosis were compared by race and ethnicity. Categorical variables (e.g., presence of hypertension) were compared using the chi-square statistic, whereas continuous or interval variables (e.g., age) were compared using analysis of variance. Subsequently, we created for each patient a longitudinal record that included the date of first AF diagnosis, pre-existing patient characteristics and comorbidities, oral anticoagulant use, and number of days to first stroke.
Time-to-event models were estimated using proportional hazards regression to determine the relationship between CHA2DS2-VASc, race, and stroke. To determine the appropriate weight to give race/ethnicity in a revised CHA2DS2-VASc-R score, we first estimated the incremental risk of stroke associated with 1 additional point on the CHA2DS2-VASc scale using proportional hazards regression in which the only predictor variable was the CHA2DS2-VASc. Because our data include patients who received anticoagulants after AF diagnosis, and this impacts stroke risk, models were estimate alternatively using: 1) 252,722 patients who did not initiate anticoagulants within 90 days of AF; 2) 207,695 patients who did initiate anticoagulants; and 3) all patients regardless of anticoagulant use, with anticoagulant use treated as a time-dependent covariate and an interaction to separately estimate the relative association of CHA2DS2-VASc to stroke risk in patients who did or did not initiate anticoagulants. We then re-estimated the models while further including indicators for patient ethnicity of African American, Hispanic, Asian and Pacific Islanders, and Native American, along with the CHA2DS2-VASc score. We also evaluated the interaction of race/ethnicity and CHA2DS2-VASc. On the basis of the results of these analyses, the CHA2DS2-VASc-R was calculated by adding 1 additional point to the stroke risk score for patients of African-American ethnicity.
Discrimination for models based on the CHA2DS2-VASc and modified CHA2DS2-VASc-R was compared using a C-statistic adapted to survival data by considering not only predicted and observed outcomes, but also the timing of the outcomes. A limitation to the C-statistic for measuring improvements in model performance is that the addition of an important risk marker to a standard set of risk markers often results in only small improvements in the C-statistic (21). Thus, we have included 2 additional measures to evaluate the improvement of stroke prediction with the addition of African-American ethnicity to the CHA2DS2-VASc score: net reclassification improvement (NRI), and integrated discrimination improvement (IDI) (22,23). These measures were estimated using an adaptation appropriate for proportional hazards regression (24). With the NRI, an increase in the predicted risk of stroke at baseline in patients who experienced a stroke implies improved performance, whereas a decrease in the predicted risk of stroke in patients with stroke implies worse performance. The interpretation is opposite for patients who do not experience stroke. Thus, for patients with stroke, the NRI is calculated as the percentage of patients whose predicted stroke risk increased using the CHA2DS2-VASc-R score rather than the CHA2DS2-VASc score, minus the percentage whose predicted stroke risk decreased. For patients who do not experience stroke, NRI is calculated as the percentage of patients whose stroke risk decreased, minus the percentage whose stroke risk increased. The total NRI index is summed for all patients. A value >0 indicates an overall improvement in risk prediction that is clinically meaningful. The IDI can be viewed as a continuous version of the NRI, with probability differences used instead of simple categories representing direction of change. The IDI quantifies the increase in the ability of the model to separate patient with and without strokes. It is calculated as the mean change in the predicted probability of stroke with the addition of African-American ethnicity to the CHA2DS2-VASc among patients who did experience stroke minus the mean change among patients who do not experience stroke. The relative IDI is the ratio of the change in predicted probability of stroke, among patients with stroke relative to patients without stroke. Because we expected the revised score to impact the predicted risk of stroke more for African-American than non– African-American patients, the IDI and the relative IDI were calculated separately for African-American and non–African-American patients.
We also assessed model calibration using plots and the Hosmer-Lemeshow (HL) statistic (25). Predicted risk of stroke was estimated for each patient based on the CHA2DS2-VASc score. Patients were then placed into 10 categories using the ranked predicted risk of stroke. The mean predicted risk of stroke based on the CHA2DS2-VASc, the CHA2DS2-VASc-R, and the actual observed stroke rate within each category was calculated separately for African-American and non-African-American patients.
Finally, we evaluated the relative importance of each patient factor in the CHA2DS2-VASc-R score by examining the chi-square associated with each factor in proportional hazards regression models for stroke. The chi-square for each patient factor was calculated as the difference between the –2 times the log likelihood ratio in a model with no covariates, and –2 times the log likelihood ratio in a model with a single patient factor.
Results
Of 460,417 patients with AF, 390,590 (85.0%) were non-Hispanic whites, 31,702 (6.9%) were non-Hispanic African Americans, 24,977 (5.4%) were Hispanics, 1,518 (0.3%) were Native Americans, and 11,530 (2.5%) were Asian/Pacific Islanders. Patient baseline demographics by race/ethnicity are presented in Table 1. All differences are statistically significant (p < 0.01); thus, we have not included p values in Table 1. Although the mean age was similar across groups, the prevalence of several co-morbid conditions was significantly higher for African Americans and Hispanics compared with whites. The mean CHA2DS2-VASc score was higher in African Americans compared with whites, indicating higher risk of stroke. White patients were more likely to receive oral anticoagulants within 180 days of the initial AF diagnosis, compared with African Americans, Hispanics, Native Americans, or Asian/Pacific Islanders (46% vs. 39%, 40%, 38%, and 38%, respectively; p < 0.001).
Table 1. Patient Demographics.
| Whites (n = 390,590) | African Americans (n = 31,702) | Hispanics (n = 24,977) | Native Americans (n = 1,518) | Asians/Pacific Islanders (n = 11,630) | |
|---|---|---|---|---|---|
| Age, yrs | 79.3 ± 8.0 | 78.8 ± 8.3 | 79.3 ±7.8 | 77.6 ± 7.2 | 79.9 ± 7.7 |
|
| |||||
| Components of CHA2DS2-VASc score | |||||
| Females | 59.7 | 65.9 | 60.5 | 59.8 | 56.0 |
| Chronic heart failure | 34.2 | 50.6 | 45.1 | 38.5 | 38.0 |
| Hypertension | 82.5 | 92.5 | 88.9 | 82.1 | 87.4 |
| Age ≥75 yrs | 67.1 | 63.9 | 68.0 | 62.4 | 72.3 |
| Diabetes | 33.2 | 52.4 | 55.5 | 45.8 | 48.3 |
| Previous stroke | 16.3 | 28.5 | 22.7 | 17.4 | 20.4 |
| Vascular disease | 53.1 | 60.7 | 62.0 | 51.7 | 53.5 |
|
| |||||
| CHA2DS2-VASc score | 4.9 ± 1.8 | 5.6 ± 1.8 | 5.5 ± 1.8 | 5.0 ± 1.8 | 5.2 ± 1.8 |
| 1 | 2 | 1 | 1 | 1 | |
| 2–4 | 42 | 27 | 29 | 42 | 3 |
| 5–9 | 56 | 72 | 70 | 57 | 6 |
Values are mean ± SD or %.
CHA2DS2-VASc = congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke, vascular disease, age 65 to 74, and female sex.
A total of 16,703 stroke events were noted during a mean follow-up period of 18.0 months per patient (Table 2). The rate of stroke per 1,000 patient years of follow-up was the highest in African Americans, followed by Native Americans, Hispanics, whites, and Asians/Pacific Islanders. Overall, 151,441 (32.7%) of patients died during follow-up.
Table 2. Number of Initial Strokes and Initial Strokes per 1,000 Person-Years of Follow-Up After Initial AF Diagnosis.
| Whites (n = 390,590) | African Americans (n = 31,702) | Hispanics (n = 24,977) | Native Americans (n = 1,518) | Asians/PacificIslanders (n = 11,630) | |
|---|---|---|---|---|---|
| Strokes | 13,616 | 1,607 | 1,014 | 62 | 404 |
| Mean follow-up (months) | 18.2 | 15.9 | 17.5 | 17.0 | 18.2 |
| Strokes per 1,000 patient-yrs | 23.0 | 38.0 | 27.8 | 28.7 | 22.8 |
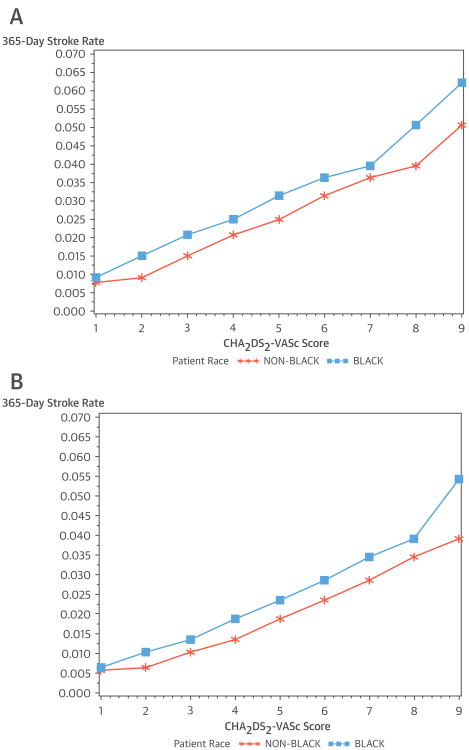
The Central Illustration and Figure 1 show: 1) stroke rate by race/ethnicity and CHA2DS2-VASc score for all patients; 2) stroke rate by race/ethnicity and CHA2DS2-VASc among patients who did not receive oral anticoagulants within 90 days of new AF; and 3) stroke rate by race/ethnicity and CHA2DS2-VASc among patients who received oral anticoagulants within 90 days of new AF. In these graphs, we excluded patients who died within 90 days of AF diagnosis, as we were not able to treat the receipt of anticoagulants as a time-dependent covariate in graphs. African-American ethnicity was associated with a higher rate of stroke irrespective of oral anticoagulant use across all CHA2DS2-VASc scores.
Central Illustration. CHA2DS2-VASc Score for Stroke Prediction in AF: Stroke Rates for All Patients.
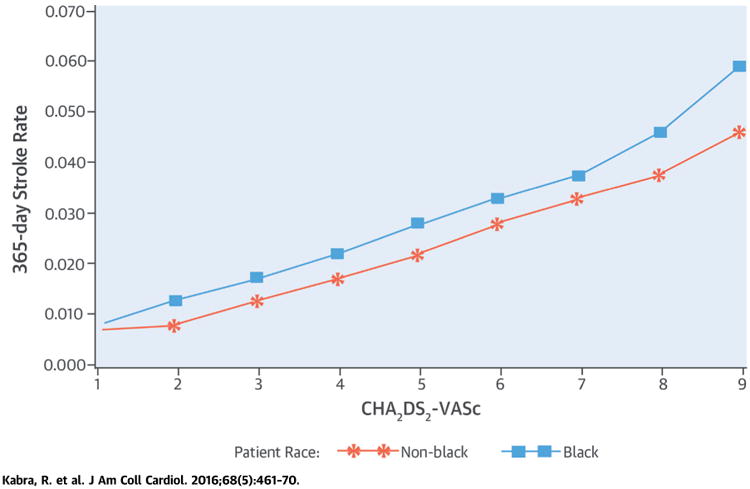
Stroke rate at 365 days after atrial fibrillation (AF) diagnosis, by race/ethnicity and CHA2DS2-VASc score for all patients. CHA2DS2-VASc = congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke, vascular disease, age 65 to 74, and female sex.
Figure 1. Stroke Rate at 365 Days After AF Diagnosis.
Stroke rate at 365 days after atrial fibrillation (AF) diagnosis, by race/ethnicity and CHA2DS2-VASc score for patients not taking oral anticoagulants within 90 days of AF diagnosis (A). Stroke rate at 365 days after AF diagnosis, by race/ethnicity and CHA2DS2-VASc score for patients taking oral anticoagulants within 90 days of AF diagnosis (B). CHA2DS2-VASc = congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke, vascular disease, age 65 to 74, and female sex.
Table 3 shows the relationship between race/ethnicity and risk of stroke after controlling for CHA2DS2-VASc score and provides the basis for the CHA2DS2-VASc-R score. Models are shown for patients who did not receive anticoagulants after AF diagnosis (Model 1), in patients who did receive anticoagulants (Model 2), and in all patients while controlling for anticoagulation (Model 3). Resulting coefficients associated with CHA2DS2-VASc were remarkably similar regardless of anticoagulant use. One additional CHA2DS2-VASc point increased the relative hazard of stroke by 1.24 to 1.25 (p < 0.001) in models that included patients without or with anticoagulant use, respectively. As expected, anticoagulant use (Model 3) was associated with decreased risk of stroke (hazard ratio: 0.74; p < 0.01), and there was no interaction between anticoagulant use and CHA2DS2-VASc score, suggesting that the incremental hazard of stroke with a 1-point increase in CHA2DS2-VASc score was not modified by use of anticoagulation. The coefficient for African-American ethnicity was statistically significant, and suggested a relative hazard of 1.30 for African-American ethnicity relative to whites in patients who did not receive anticoagulants, and 1.47 for African-American ethnicity relative to whites in patients who did receive anticoagulants. Regardless of the use of anticoagulants, the coefficient for African-American ethnicity suggested that African-American ethnicity is associated with an increase in stroke risk that is greater than the increase in risk associated with 1 additional point on the CHA2DS2-VASc score (i.e., >1.24 increase in risk in patients without anticoagulant use and >1.25 increase in patients with anticoagulant use) but less than the risk associated with 2 additional CHA2DS2-VASc points (i.e., <1.54 in patients without anticoagulant use and <1.56 in patients with anticoagulant use). Thus, the precise weight to assign to African-American ethnicity in a revised CHA2DS2-VASc-R score falls somewhere between 1 and 2. In keeping with the simplicity of the existing the CHA2DS2-VASc score, the CHA2DS2-VASc-R score was calculated by adding 1 point to the CHA2DS2-VASc for patients of African-American ethnicity. Coefficients associated with patients of Hispanic, Native American, or Asian/ Pacific Island descent were either small in magnitude or not statistically significant. Thus, no additional points on the CHA2DS2-VASc scale were warranted for those patients.
Table 3. Hazard of Stroke by Race/Ethnicity, Relative to White Patients, After Controlling for CHA2DS2-VASc Score (Model 1) and CHA2DS2-VASc Score Plus Anticoagulant Use (Model 2).
| Model 1: CHA2DS2-VASc With Race/Ethnicity in Patients With No Anticoagulant Use in 180 Days (n = 252,722) | Model 2: CHA2DS2-VASc With Race/Ethnicity in Patients With Anticoagulant Use in 180 Days (n = 207,695) | Model 3: CHA2DS2-VASc With Race/Ethnicity in Models With All Patients, and Indicator for Anticoagulant Use (n = 460,417) | |
|---|---|---|---|
| CHA2DS2-VASc | 1.24 (1.22–1.25) (p < 0.001) | 1.25 (1.24–1.26) (p < 0.001) | 1.25 (1.23–1.26) (p < 0.001) |
| Race | |||
| African American | 1.30 (1.21–1.39) (p < 0.001) | 1.47 (1.36–1.59) (p < 0.001) | 1.36 (1.29–1.43) (p < 0.001) |
| Hispanic | 0.97 (0.89–1.05) (p = 0.43) | 1.14 (1.04–1.26) (p = 0.008) | 1.03 (0.97–1.10) (p = 0.36) |
| Native American | 1.17 (0.85–1.61) (p = 0.34) | 1.24 (0.84–1.84) (p = 0.28) | 1.19 (0.92–1.52) (p = 0.17) |
| Asian/Pacific Islanders | 0.79 (0.69–0.90) (p < 0.001) | 1.10 (0.95–1.27) (p = 0.21) | 0.89 (0.84–0.95) (p = 0.03) |
|
| |||
| Anticoagulant use | – | – | 0.74 (0.72–0.76) (p < 0.001) |
Values are hazard ratios from proportional hazards regression models (95% confidence intervals). CHA2DS2-VASc = congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke, vascular disease, age 65 to 74, and female sex.
As compared with CHA2DS2-VASc, CHA2DS2-VASc-R score improved the fit of the model significantly as measured by the log likelihood ratio statistic (p < 0.001) for stroke (Table 4). The model with the original CHA2DS2-VASc had a C-statistic of 0.60 (95% confidence interval: 0.59 to 0.61), compared with a C-statistic of 0.61 (95% confidence interval: 0.60 to 0.62) for the model with CHA2DS2-VASc-R. The calculated NRI was 7.6% (p < 0.001), suggesting that the net change in predicted stroke risk with the addition of African-American ethnicity to the CHA2DS2-VASc score was in the “correct” direction 7.6% more often than in the “incorrect” direction. The IDI was .012 for African-American patients, suggesting that the predicted hazard of stroke is, on average, 1.2% closer to the “correct” prediction using the CHA2DS2-VASc-R score rather than the CHA2DS2-VASc. The IDI for white patients was very small (<0.001), suggesting very little change in predicted probabilities for white patients. The interaction between CHA2DS2-VASc and race/ethnicity was not statistically significant, suggesting similar relative increase in stroke risk by race/ethnicity as the CHA2DS2-VASc increases.
Table 4. Comparison of CHA2DS2-VASc and CHA2DS2-VASc-R Scores in Predicting Stroke: Relative Hazard Associated With 1-Point Increase in Stroke Risk Score, and C-Statistic.
| CHA2DS2-VASc | CHA2DS2-VASc-R | |
|---|---|---|
| Hazard ratio | 1.24 (1.23–1.25) (p < 0.001) | 1.25 (1.24–1.26) (p < 0.001) |
| C-statistic | 0.60 (0.59–0.61) | 0.61 (0.60–0.62) |
Values in parentheses are 95% confidence intervals, unless otherwise indicated. CHA2DS2-VASc = congestive heart failure, hypertension, age ≥ 75 years, diabetes, previous stroke, vascular disease, age 65 to 74, and female sex. CHA2DS2-VASc-R = congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke, vascular disease, age 65 to 74, female sex, and African American ethnicity.
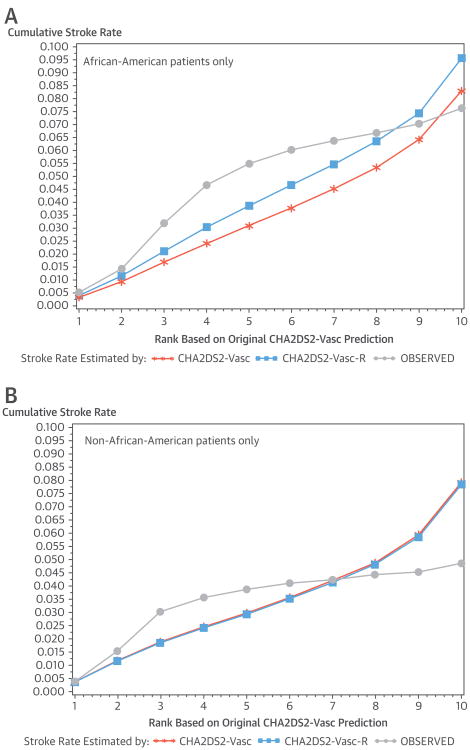
Figures 2A and 2B show calibration plots by race/ethnicity. For African-American patients, Figure 2A demonstrates substantially better model fit based on the CHADS2-VASc-R compared with the CHADS2-VASc. However, in Figure 2B, the predicted risk of stroke changes very little for non–African-American patients, resulting in little improvement in model calibration. HL statistics were large and statistically significant for both African-American and non–African-American patient segments (HL: 1,529 for non– African-American patients and 148 for African-American patients, on the basis of the difference between observed and predicted stroke by the CHADS2-VASc score). However, a statistically significant HL statistic is not informative with respect to calibration when using large samples (25). Nevertheless, we note that the HL statistic decreased only modestly for non–African-American patients with the use of CHA2DS2-VASc-R (from 1,529 to 1,503), but was reduced by one-half for African-American patients (from 148 to 72).
Figure 2. Observed Stroke Rate and Rate Predicted by CHA2DS2-VASc and CHA2DS2-VASc-R for African Americans and Non-African Americans.
(A) Observed stroke rate and rate predicted by CHA2DS2-VASc and CHA2DS2-VASc-R: for African-American patients only. (B) Observed stroke rate and rate predicted by CHA2DS2-VASc and CHA2DS2-VASc-R: for non-African-American patients only. CHA2DS2-VASc = congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke, vascular disease, age 65 to 74, and female sex; CHA2DS2-VASc-R = congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke, vascular disease, age 65 to 74, female sex, and African American ethnicity.
Finally, Table 5 also shows the relative importance of each factor in the CHA2DS2-VASc-R score in predicting stroke, as measured by the magnitude of the chi-square statistic in univariable and multivariable Cox regression models. We found that compared with the 7 existing variables in the CHA2DS2-VASc-R score, African-American ethnicity was a stronger predictor than heart failure, hypertension, diabetes mellitus, and history of vascular disease. Only prior history of stroke, age $75 years, and female sex were more important than African-American ethnicity in prediction of stroke in this population.
Table 5. Relative Contribution of Each Risk Factor of CHA2DS2-VASc-R Score in Stroke Prediction.
| Order of Importance | CHA2DS2-VASc-R Factor | Univariable Models | Multivariable Models With All Patient Factors | ||
|---|---|---|---|---|---|
|
|
|
||||
| Chi-Square | Relative Hazard | Chi-Square | Relative Hazard | ||
| 1 | History of stroke | 1,959.99 | 1.42 (p < 0.001) | 1,387.48 | 1.35 (p < 0.001) |
| 2 | Age ≥75 yrs | 1,296.73 | 1.95 (p < 0.001) | 906.27 | 1.77 (p < 0.001) |
| 3 | Female | 579.34 | 1.50 (p < 0.001) | 319.98 | 1.36 (p < 0.001) |
| 4 | African American ethnicity | 323.74 | 1.60 (p < 0.001) | 197.18 | 1.45 (p < 0.001) |
| 5 | Hypertension | 243.87 | 1.44 (p < 0.001) | 23.80 | 1.13 (p < 0.001) |
| 6 | Heart failure | 227.37 | 1.27 (p < 0.001) | 40.86 | 1.11 (p < 0.001) |
| 7 | Other vascular disease | 173.28 | 1.23 (p < 0.001) | 1.30 | 1.02 (p = 0.25) |
| 8 | Diabetes | 118.63 | 1.19 (p < 0.001) | 40.45 | 1.11 (p < 0.001) |
CHA2DS2-VASc-R = congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke, vascular disease, age 65 to 74, female sex, and African American ethnicity.
Discussion
Our study demonstrates that addition of African-American ethnicity to the CHA2DS2-VASc risk scoring system improves the stroke prediction in AF patients >65 years of age. This improvement in stroke prediction persisted across all CHA2DS2-VASc scores even after adjustment for anticoagulation use. Moreover, in analysis of individual patient factors in the CHA2DS2-VASc-R score, we found that only 3 factors (history of stroke, age ≥75 years, and female sex) were stronger predictors of stroke than African-American ethnicity.
African-American ethnicity has been shown to be a risk factor for stroke in AF patients. A recent observational study using Medicare population from 2010 showed that the risk of stroke was higher in African Americans (29.3 per 1,000 patient-years) versus whites (14.8 per 1,000 patient-years)—a finding similar to what we report (37.9 per 1,000 patient-years in African Americans and 22.7 per 1,000 patient-years in whites) (9). The differences in actual numbers are likely due to differences in sampling of the study population. Although we studied all patients with newly diagnosed AF, this study included a random sample of 5% all Medicare patients and included any AF diagnosis.
In our prior work using a similar Medicare database, we studied the racial/ethnic differences in mortality and stroke outcomes in 517,941 patients >65 years of age with newly diagnosed AF (12). We noted a 46% higher incidence of mortality and 66% higher incidence of stroke in African Americans compared with the white population. However, after adjustment of the pre-existing comorbidities, the higher risk of mortality in African Americans was eliminated, but they continued to have 46% higher risk of having stroke, compared with whites, even after adjusting for anticoagulation status. The increased risk of stroke in African Americans compared with whites was noted even when the groups were compared by the CHA2DS2-VASc score. The risk of stroke was also noted to be higher in Hispanics compared with whites. However, in our current study, none of the other racial/ethnic groups, including Hispanics, Native Americans, or Asian/Pacific Islanders, improved stroke prediction beyond the CHA2DS2-VASc score.
Despite higher prevalence of each CHA2DS2-VASc risk factor among African Americans, the strong association between African-American ethnicity and stroke was independent of them, as noted in our multivariable analyses. Moreover, addition of African-American ethnicity to the CHA2DS2-VASc risk score not only improved the model discrimination, but also led to the appropriate reclassification of patients to higher and lower risk categories. There are several possible explanations why addition of African-American ethnicity to CHA2DS2-VASc score improves stroke prediction in AF patients. There may be racial/ethnic differences in the impact of risk factors, with the presence of risk factors having a larger impact on African Americans than whites (26). The current risk score does not address the duration and severity of the risk factors. Several studies suggest that the control of hypertension, diabetes, and congestive heart failure may be poorer in African-American patients compared to whites and may thereby contribute to higher stroke risk (27–29). It is also possible that there may be other nontraditional risk factors of stroke in African Americans such as genetic factors or systemic inflammation, which are not accounted for in CHA2DS2-VASc score. Furthermore, there may be differences in the protective effects of anticoagulant therapy across different races/ ethnicities due to genetic polymorphisms that influence the pharmacokinetics and pharmacodynamics of the anticoagulants (30). Finally, evidence suggests that the prevalence of AF in African Americans is significantly lower than for whites. Although the reasons for this are unclear, possible explanations include genetics and smaller left atrial sizes in African Americans that protect against AF (31,32). AF development and progression may therefore follow different patterns and consequences in African American than white patients. Although there is a need to systematically study and address each of these possibilities that potentially increase the risk of stroke in African Americans with AF, there is enough evidence to suggest that African-American ethnicity is a predictor of stroke in AF patients.
In a meta-analysis consisting of 8 studies, the C-statistic ranged from 0.60 to 0.80 (median 0.683) for CHADS2 score compared with 0.64 to 0.79 (median 0.673) for the CHA2DS2-VASc score (33). This is similar in magnitude to our results comparing CHA2DS2-VASc to CHA2DS2-VASc-R (C-statistic of 0.60 vs. 0.61,respectively). According to the current guidelines, anticoagulation with warfarin or the novel anticoagulants is recommended for AF patients with a CHA2DS2-VASc score of ≥2 and may be considered for a score of 1 (14). On the basis of the results of our current study, we propose a new scheme CHA2DS2-VASc-R by adding 1 point for African-American ethnicity. In our study, this would extend the use of anticoagulation strategy in all African-American patients over the age of 65 years with AF, because they have all have CHA2DS2-VASc score of 1 or above.
Study Limitations
This was a retrospective study that studied a >65-year-old population, which has a higher prevalence of AF. Hence, all the patients had a CHA2DS2-VASc score of ≥1. Because anticoagulation is currently indicated for patients with a CHA2DS2-VASc score of 2 or more, the number of patients who will be clinically impacted by a change in CHA2DS2-VASc-R risk score from 1 to 2 in this study is small (1%). Future studies that include patients <65 years of age would help address the role of CHA2DS2-VASc-R risk score in patients with CHA2DS2-VASc score of 0 to 1. Nevertheless, there are several patients with AF with a high CHA2DS2-VASc score who are not anticoagulated for different reasons. Among these patients, it is important to recognize that African-American ethnicity is an independent risk factor for stroke, and our study helps to focus attention on this important risk factor. Hence, the data may not be extrapolated to the younger patient population. Diagnosis of new AF was dependent on the accuracy of ICD-9-CM diagnosis codes. However, this has been done with reasonable accuracy in prior studies. The assessment of stroke also depends on the accuracy of the ICD-9-CM diagnosis codes, which have also shown to have good positive predictive value in administrative data. In addition, administrative data generally do not contain important prognostic indicators such as laboratory test results. Nevertheless, our analysis incorporates multiple widely used risk scores (e.g., CHA2DS2-VASc) that have been shown to predict outcomes, as well as important indicators of comorbidities. Finally, the C-statistic that we used to assess model fit is often less sensitive to improvements in prediction than measures based on the likelihood or other global measures of model fit (34). Nevertheless, our analyses using the NRI index, and the IDI, suggest that use of the CHA2DS2-VASc-R model compared with CHA2DS2-VASc alone was associated with a net improvement in risk prediction for stroke. We also noted significant improvement in the likelihood ratio for Cox regression models using the CHA2DS2-VASc-R rather than CHA2DS2-VASc. We also note that, in general, the C-statistic found in this study is comparable to those reported elsewhere (34).
Conclusions
Our study demonstrates that in patients >65 years of age, addition of African-American ethnicity to CHA2DS2-VASc score significantly improved the stroke prediction in patients with AF. Of the individual risk factors in CHA2DS2-VASc-R score, only prior stroke, age ≥75 years, and female sex were more important predictors of stroke than African-American ethnicity.
Perspectives.
Competency In Patient Care And Procedural Skills
Patients of African-American ethnicity are at higher risk of stroke than whites. Addition of African-American ethnicity improves the predictive value of the to the CHA2DS2-VASc score in patients over age 65 years with AF.
Translational Outlook
Further studies are needed to assess the impact of African-American ethnicity on stroke risk in younger individuals with AF and to clarify the mechanisms linking race/ethnicity to clinical outcomes.
Acknowledgments
Dr. Girotra is supported by a career development award (K08 HL122527) from the National Heart, Lung, and Blood Institute at the National Institutes of Health. Dr. Vaughan Sarrazin was supported by an award from the Agency for Healthcare Research and Quality (R01-HS023104) and by the Health Services Research and Development Service of the Department of Veterans Affairs.
Abbreviations and Acronyms
- AF
atrial fibrillation
- CMS
Centers for Medicare & Medicaid
- HL
Hosmer-Lemeshow
- ICD-9-CM
International Classification of Diseases–Ninth Revision–Clinical Modification
- IDI
integrated discrimination improvement
- NRI
net reclassification improvement
- RTI
Research Triangle Institute
Footnotes
Dr. Kabra has reported that he has no relationships relevant to the contents of this paper to disclose.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–43. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 4.Kissela B, Schneider A, Kleindorfer D, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–31. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 5.Kleindorfer DO, Khoury J, Moomaw CJ, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 2010;41:1326–31. doi: 10.1161/STROKEAHA.109.575043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacco RL, Boden-Albala B, Abel G, et al. Race-ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke. 2001;32:1725–31. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 7.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–27. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard G, Cushman M, Kissela BM, et al. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half full (empty?) glass. Stroke. 2011;42:3369–75. doi: 10.1161/STROKEAHA.111.625277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shroff GR, Solid CA, Herzog CA. Atrial fibrillation, stroke, and anticoagulation in Medicare beneficiaries: trends by age, sex, and race, 1992-2010. J Am Heart Assoc. 2014;3:e000756. doi: 10.1161/JAHA.113.000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birman-Deych E, Radford MJ, Nilasena DS, Gage BF. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke. 2006;37:1070–4. doi: 10.1161/01.STR.0000208294.46968.a4. [DOI] [PubMed] [Google Scholar]
- 11.Shen AY, Yao JF, Brar SS, Jorgensen MB, Wang X, Chen W. Racial/ethnic differences in ischemic stroke rates and the efficacy of warfarin among patients with atrial fibrillation. Stroke. 2008;39:2736–43. doi: 10.1161/STROKEAHA.107.508580. [DOI] [PubMed] [Google Scholar]
- 12.Kabra R, Cram P, Girotra S, Vaughan Sarrazin M. Effect of race on outcomes (stroke and death) in patients > 65 years of age with atrial fibrillation. Am J Cardiol. 2015;116:230–5. doi: 10.1016/j.amjcard.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 14.January CT, Wann L, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Ellis ER, Culler SD, Simon AW, Reynolds MR. Trends in utilization and complications of catheter ablation for atrial fibrillation in Medicare beneficiaries. Heart Rhythm. 2009;6:1267–73. doi: 10.1016/j.hrthm.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gage BF, Boechler M, Doggette AL, et al. Adverse outcomes and predictors of underuse of antithrombotic therapy in Medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31:822–7. doi: 10.1161/01.str.31.4.822. [DOI] [PubMed] [Google Scholar]
- 17.Bonito AJ, Bann C, Eicheldinger C, Carpenter L. Rockville, MD: Agency for Healthcare Research and Quality; Jan, 2008. [Accessed September 1, 2015]. Creation of new race-ethnicity codes and socio-economic status (SES) indicators for Medicare beneficiaries RTI International report for the Agency for Healthcare Research and Quality (AHRQ) and the Centers for Medicare and Medicaid Services (CMS) under CMS contract No 500-00-0024. Available at: http://archive.ahrq.gov/research/findings/final-reports/medicareindicators/medicareindicators.pdf. [Google Scholar]
- 18.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Suh DC, Nelson WW, Choi JC, Choi I. Risk of hemorrhage and treatment costs associated with warfarin drug interactions in patients with atrial fibrillation. Clin Ther. 2012;34:1569–82. doi: 10.1016/j.clinthera.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Rothendler JA, Rose AJ, Reisman JI, Berlowitz DR, Kazis LE. Choices in the use of ICD-9 codes to identify stroke risk factors can affect the apparent population-level risk factor prevalence and distribution of CHADS2 scores. Am J Car-diovasc Dis. 2012;2:184–91. [PMC free article] [PubMed] [Google Scholar]
- 21.Baker SG, Schuit E, Steyerberg EW, et al. How to interpret a small increase in AUC with an additional risk prediction marker: decision analysis comes through. Stat Med. 2014;33:3946–59. doi: 10.1002/sim.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pencina MJ, D'Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. 2010;48:1703–11. doi: 10.1515/CCLM.2010.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 24.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new bio-markers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prabasaj P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat Med. 2013;32:67–80. doi: 10.1002/sim.5525. [DOI] [PubMed] [Google Scholar]
- 26.Howard VJ. Reasons underlying racial differences in stroke incidence and mortality. Stroke. 2013;44(1):S126–8. doi: 10.1161/STROKEAHA.111.000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong KL, Cheung BMY, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 28.Parrinello CM, Rastegar I, Godino JG, Miedema MD, Matsushita K, Selvin E. Prevalence of and racial disparities with risk factor control in older adults with diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2015;38:1290–8. doi: 10.2337/dc15-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickson VV, Knafl GJ, Wald J, Riegel B. Racial differences in clinical treatment and self-care behaviors of adults with chronic heart failure. J Am Heart Assoc. 2015;4:e001561. doi: 10.1161/JAHA.114.001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen AY, Chen W, Yao JF, Brar SS, Wang X, Go AS. Effect of race/ethnicity on the efficacy of warfarin: potential implications of stroke in patients with atrial fibrillation. CNS Drugs. 2008;22:815–25. doi: 10.2165/00023210-200822100-00003. [DOI] [PubMed] [Google Scholar]
- 31.Marcus GM, Alonso A, Peralta CA, et al. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122:2009–15. doi: 10.1161/CIRCULATIONAHA.110.958306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcus GM, Olgin JE, Whooley M, et al. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123:375.e1–7. doi: 10.1016/j.amjmed.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JY, Zhang AD, Lu HY, Guo J, Wang FF, Li ZC. CHADS2 versus CHA2DS2-VASc score in assessing the stroke and thromboembolism risk stratification in patients with atrial fibrillation: a systematic review and meta-analysis. J Geriatr Cardiol. 2013;10:258–66. doi: 10.3969/j.issn.1671-5411.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–35. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]