Abstract
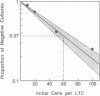
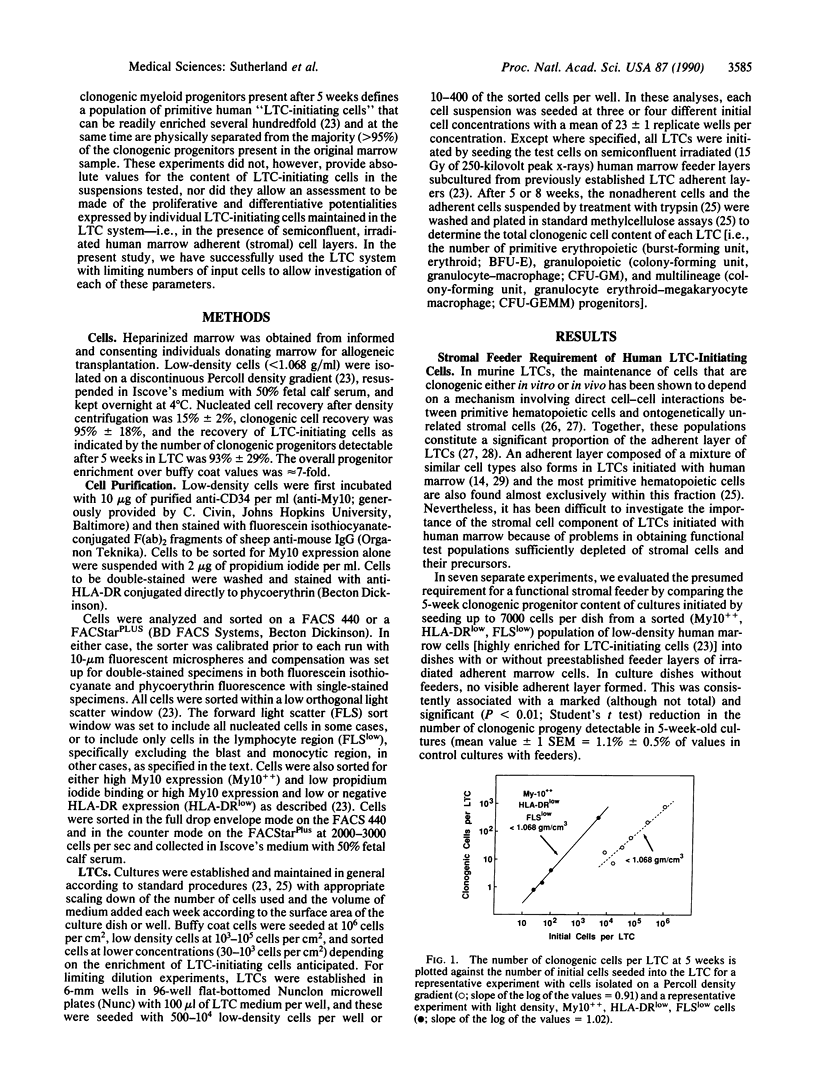
A major goal of current hematopoiesis research is to develop in vitro methods suitable for the measurement and characterization of stem cells with long-term in vivo repopulating potential. Previous studies from several centers have suggested the presence in normal human or murine marrow of a population of very primitive cells that are biologically, physically, and pharmacologically different from cells detectable by short-term colony assays and that can give rise to the latter in long-term cultures (LTCs) containing a competent stromal cell layer. In this report, we show that such cultures can be used to provide a quantitative assay for human "LTC-initiating cells" based on an assessment of the number of clonogenic cells present after 5-8 weeks. Production of derivative clonogenic cells is shown to be absolutely dependent on the presence of a stromal cell feeder. When this requirement is met, the clonogenic cell output (determined by assessment of 5-week-old cultures) is linearly related to the input cell number over a wide range of cell concentrations. Using limiting dilution analysis techniques, we have established the frequency of LTC-initiating cells in normal human marrow to be approximately 1 per 2 X 10(4) cells and in a highly purified CD34-positive subpopulation to be approximately 1 per 50-100 cells. The proliferative capacity exhibited by individual LTC-initiating cells cultured under apparently identical culture conditions was found to be highly variable. Values for the number of clonogenic cells per LTC-initiating cell in 5-week-old cultures ranged from 1 to 30 (the average being 4) with similar levels being detected in positive 8-week-old cultures. Some LTC-initiating cells are multipotent as evidenced by their generation of erythroid as well as granulopoietic progeny. The availability of a system for quantitative analysis of the proliferative and differentiative behavior of this newly defined compartment of primitive human hematopoietic cells should facilitate future studies of specific genetic or microenvironmental parameters involved in the regulation of these cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews R. G., Singer J. W., Bernstein I. D. Precursors of colony-forming cells in humans can be distinguished from colony-forming cells by expression of the CD33 and CD34 antigens and light scatter properties. J Exp Med. 1989 May 1;169(5):1721–1731. doi: 10.1084/jem.169.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley S. A. Close range cell:cell interaction required for stem cell maintenance in continuous bone marrow culture. Exp Hematol. 1981 Mar;9(3):308–312. [PubMed] [Google Scholar]
- Cashman J. D., Eaves A. C., Raines E. W., Ross R., Eaves C. J. Mechanisms that regulate the cell cycle status of very primitive hematopoietic cells in long-term human marrow cultures. I. Stimulatory role of a variety of mesenchymal cell activators and inhibitory role of TGF-beta. Blood. 1990 Jan 1;75(1):96–101. [PubMed] [Google Scholar]
- Cashman J., Eaves A. C., Eaves C. J. Regulated proliferation of primitive hematopoietic progenitor cells in long-term human marrow cultures. Blood. 1985 Oct;66(4):1002–1005. [PubMed] [Google Scholar]
- Cashman J., Eaves A. C., Eaves C. J. Regulated proliferation of primitive hematopoietic progenitor cells in long-term human marrow cultures. Blood. 1985 Oct;66(4):1002–1005. [PubMed] [Google Scholar]
- Coller H. A., Coller B. S. Poisson statistical analysis of repetitive subcloning by the limiting dilution technique as a way of assessing hybridoma monoclonality. Methods Enzymol. 1986;121:412–417. doi: 10.1016/0076-6879(86)21039-3. [DOI] [PubMed] [Google Scholar]
- Coulombel L., Eaves A. C., Eaves C. J. Enzymatic treatment of long-term human marrow cultures reveals the preferential location of primitive hemopoietic progenitors in the adherent layer. Blood. 1983 Aug;62(2):291–297. [PubMed] [Google Scholar]
- Dexter T. M., Spooncer E. Loss of immunoreactivity in long-term bone marrow culture. Nature. 1978 Sep 14;275(5676):135–136. doi: 10.1038/275135a0. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Spooncer E., Toksoz D., Lajtha L. G. The role of cells and their products in the regulation of in vitro stem cell proliferation and granulocyte development. J Supramol Struct. 1980;13(4):513–524. doi: 10.1002/jss.400130410. [DOI] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Dorshkind K., Phillips R. A. Characterization of early B lymphocyte precursors present in long-term bone marrow cultures. J Immunol. 1983 Nov;131(5):2240–2245. [PubMed] [Google Scholar]
- Eaves A. C., Cashman J. D., Gaboury L. A., Eaves C. J. Clinical significance of long-term cultures of myeloid blood cells. Crit Rev Oncol Hematol. 1987;7(2):125–138. doi: 10.1016/s1040-8428(87)80022-7. [DOI] [PubMed] [Google Scholar]
- Gordon M. Y., Dowding C. R., Riley G. P., Greaves M. F. Characterisation of stroma-dependent blast colony-forming cells in human marrow. J Cell Physiol. 1987 Jan;130(1):150–156. doi: 10.1002/jcp.1041300121. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Astle C. M. Loss of stem cell repopulating ability upon transplantation. Effects of donor age, cell number, and transplantation procedure. J Exp Med. 1982 Dec 1;156(6):1767–1779. doi: 10.1084/jem.156.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. J., Celano P., Sharkis S. J., Sensenbrenner L. L. Two phases of engraftment established by serial bone marrow transplantation in mice. Blood. 1989 Feb;73(2):397–401. [PubMed] [Google Scholar]
- LeBien T. W. Growing human B-cell precursors in vitro: the continuing challenge. Immunol Today. 1989 Sep;10(9):296–298. doi: 10.1016/0167-5699(89)90084-4. [DOI] [PubMed] [Google Scholar]
- Leary A. G., Ogawa M. Blast cell colony assay for umbilical cord blood and adult bone marrow progenitors. Blood. 1987 Mar;69(3):953–956. [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Magli M. C., Iscove N. N., Odartchenko N. Transient nature of early haematopoietic spleen colonies. Nature. 1982 Feb 11;295(5849):527–529. doi: 10.1038/295527a0. [DOI] [PubMed] [Google Scholar]
- Miyauchi J., Kelleher C. A., Yang Y. C., Wong G. G., Clark S. C., Minden M. D., Minkin S., McCulloch E. A. The effects of three recombinant growth factors, IL-3, GM-CSF, and G-CSF, on the blast cells of acute myeloblastic leukemia maintained in short-term suspension culture. Blood. 1987 Sep;70(3):657–663. [PubMed] [Google Scholar]
- PORTER E. H., BERRY R. J. THE EFFICIENT DESIGN OF TRANSPLANTABLE TUMOUR ASSAYS. Br J Cancer. 1963 Dec;17:583–595. doi: 10.1038/bjc.1963.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S., Fleischman R. A. Hematopoietic microenvironment. Origin, lineage, and transplantability of the stromal cells in long-term bone marrow cultures from chimeric mice. J Clin Invest. 1988 Apr;81(4):1072–1080. doi: 10.1172/JCI113419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploemacher R. E., Brons N. H. Isolation of hemopoietic stem cell subsets from murine bone marrow: II. Evidence for an early precursor of day-12 CFU-S and cells associated with radioprotective ability. Exp Hematol. 1988 Jan;16(1):27–32. [PubMed] [Google Scholar]
- Schrader J. W., Schrader S. In vitro studies on lymphocyte differentiation. I. Long term in vitro culture of cells giving rise to functional lymphocytes in irradiated mice. J Exp Med. 1978 Sep 1;148(3):823–828. doi: 10.1084/jem.148.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siena S., Castro-Malaspina H., Gulati S. C., Lu L., Colvin M. O., Clarkson B. D., O'Reilly R. J., Moore M. A. Effects of in vitro purging with 4-hydroperoxycyclophosphamide on the hematopoietic and microenvironmental elements of human bone marrow. Blood. 1985 Mar;65(3):655–662. [PubMed] [Google Scholar]
- Simmons P. J., Przepiorka D., Thomas E. D., Torok-Storb B. Host origin of marrow stromal cells following allogeneic bone marrow transplantation. 1987 Jul 30-Aug 5Nature. 328(6129):429–432. doi: 10.1038/328429a0. [DOI] [PubMed] [Google Scholar]
- Slovick F. T., Abboud C. N., Brennan J. K., Lichtman M. A. Survival of granulocytic progenitors in the nonadherent and adherent compartments of human long-term marrow cultures. Exp Hematol. 1984 Jun;12(5):327–338. [PubMed] [Google Scholar]
- Sutherland H. J., Eaves C. J., Eaves A. C., Dragowska W., Lansdorp P. M. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood. 1989 Oct;74(5):1563–1570. [PubMed] [Google Scholar]
- Szilvassy S. J., Fraser C. C., Eaves C. J., Lansdorp P. M., Eaves A. C., Humphries R. K. Retrovirus-mediated gene transfer to purified hemopoietic stem cells with long-term lympho-myelopoietic repopulating ability. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8798–8802. doi: 10.1073/pnas.86.22.8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilvassy S. J., Lansdorp P. M., Humphries R. K., Eaves A. C., Eaves C. J. Isolation in a single step of a highly enriched murine hematopoietic stem cell population with competitive long-term repopulating ability. Blood. 1989 Aug 15;74(3):930–939. [PubMed] [Google Scholar]
- TILL J. E., MCCULLOCH E. A., SIMINOVITCH L. A STOCHASTIC MODEL OF STEM CELL PROLIFERATION, BASED ON THE GROWTH OF SPLEEN COLONY-FORMING CELLS. Proc Natl Acad Sci U S A. 1964 Jan;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Turhan A. G., Humphries R. K., Phillips G. L., Eaves A. C., Eaves C. J. Clonal hematopoiesis demonstrated by X-linked DNA polymorphisms after allogeneic bone marrow transplantation. N Engl J Med. 1989 Jun 22;320(25):1655–1661. doi: 10.1056/NEJM198906223202504. [DOI] [PubMed] [Google Scholar]
- Winton E. F., Colenda K. W. Use of long-term human marrow cultures to demonstrate progenitor cell precursors in marrow treated with 4-hydroperoxycyclophosphamide. Exp Hematol. 1987 Jul;15(6):710–714. [PubMed] [Google Scholar]
- Wu A. M., Till J. E., Siminovitch L., McCulloch E. A. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968 Mar 1;127(3):455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager A. M., Kaizer H., Santos G. W., Saral R., Colvin O. M., Stuart R. K., Braine H. G., Burke P. J., Ambinder R. F., Burns W. H. Autologous bone marrow transplantation in patients with acute nonlymphocytic leukemia, using ex vivo marrow treatment with 4-hydroperoxycyclophosphamide. N Engl J Med. 1986 Jul 17;315(3):141–147. doi: 10.1056/NEJM198607173150301. [DOI] [PubMed] [Google Scholar]